Contents
What is a teratoma
Teratoma is a type of germ cell tumor that may contain several different types of tissue, such as hair, muscle, and bone. Teratomas may be mature or immature, based on how normal the cells look under a microscope. Sometimes teratomas are a mix of mature and immature cells. Teratomas usually occur in the ovaries in women, the testicles in men, and the tailbone in children. They may also occur in the central nervous system (brain or spinal cord), chest, or abdomen. Teratomas may be benign (not cancer) or malignant (cancer).
Teratomas are made up of a variety of parenchymal cell types representing more than 1 germ layer and often all 3. Arising from totipotential cells, these tumors typically are midline or paraxial 1.
The most common location is sacrococcygeal (57%). Because they arise from totipotential cells, teratomas are encountered commonly in the gonads (29%). By far the most common gonadal location is the ovary, although they also occur somewhat less frequently in the testes. Cystic teratomas occasionally occur in sequestered midline embryonic cell rests and can be mediastinal (7%), retroperitoneal (4%), cervical (3%), and intracranial (3%) 2.
Cells differentiate along various germ lines, essentially recapitulating any tissue of the body. Examples include hair, teeth, fat, skin, muscle, and endocrine tissue.
The parthenogenic theory, which suggests an origin from the primordial germ cells, is now the most widely accepted. This theory is bolstered by the anatomic distribution of the tumors along lines of migration of the primordial germ cells from the yolk sac to the primitive gonads 3. Additional support came from Linder and associates’ studies of mature cystic teratomas of the ovaries. They used sophisticated cytogenetic techniques to demonstrate that these tumors are of germ cell origin and arise from a single germ cell after the first meiotic division 4.
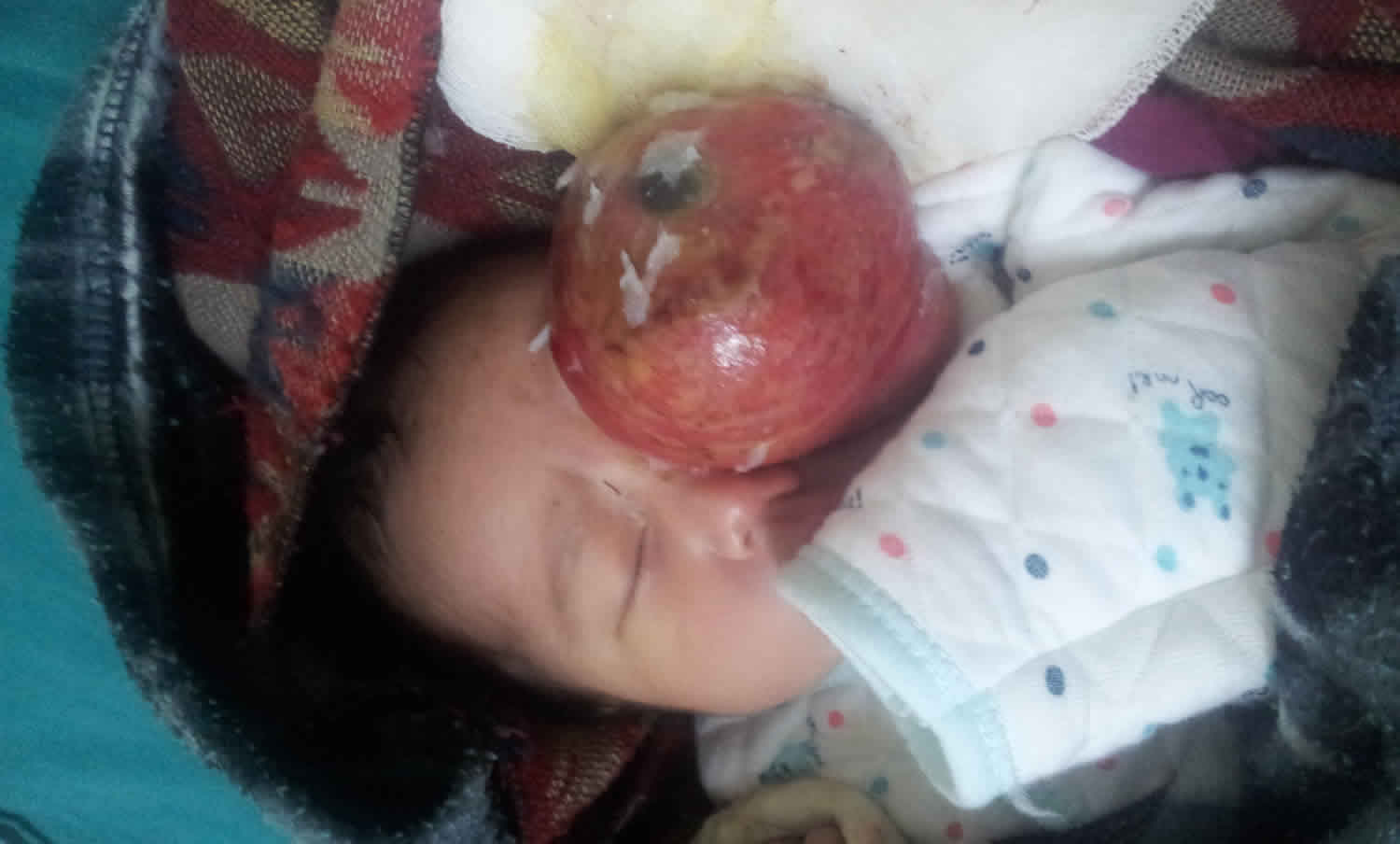
Sacrococcygeal teratoma
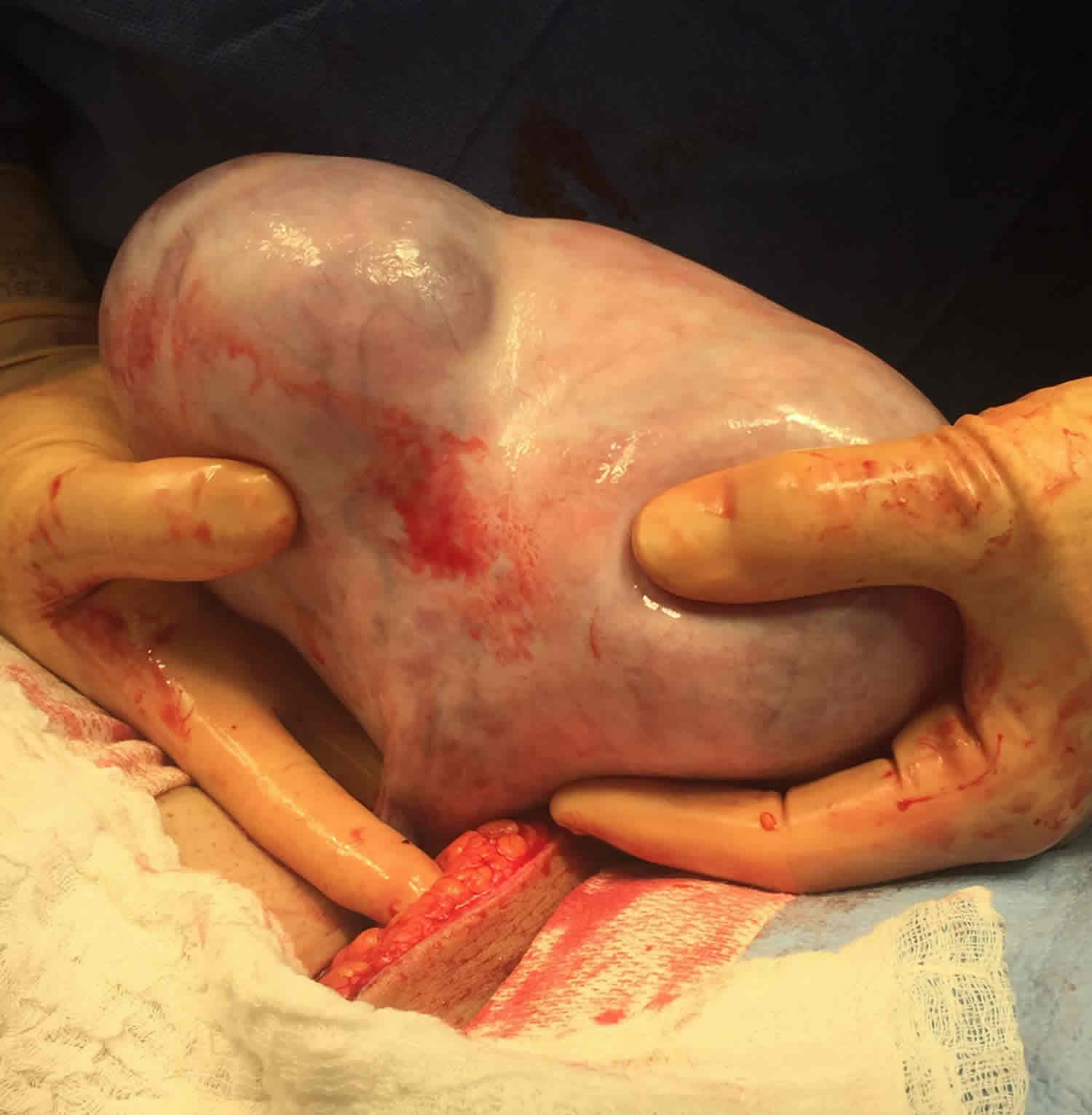
A sacrococcygeal teratoma is a tumor that grows at the base of the developing baby’s coccyx or tailbone 5. Sacrococcygeal teratoma is the most common tumor found in newborns, occurring in 1 out of every 35,000 to 40,000 newborns and girls are four times more likely to be affected than boys. Though sacrococcygeal teratoma is usually benign (non-cancerous), there is a possibility that sacrococcygeal teratoma could become malignant. As such, the recommended treatment of a sacrococcygeal teratoma is complete removal of the tumor by surgery, performed soon after the birth. If not all of the sacrococcygeal teratoma tumor is removed during the initial surgery, the sacrococcygeal teratoma may grow back (recur) and additional surgeries may be needed 6. Studies have found that sacrococcygeal teratomas recur in up to 22% of cases 7.
It is likely that all sacrococcygeal teratomas are present at birth (congenital) and most are discovered before birth by a routine prenatal ultrasound examination or an exam indicated for a uterus too large for dates. In extremely rare cases, sacrococcygeal tumors may be seen in adults. Most of these represent slow growing tumors that originated prenatally. In the majority of these cases, the tumor is benign, but may cause lower back pain and genitourinary and gastrointestinal symptoms.
Sacrococcygeal teratoma tumor is usually covered with skin, but may be covered by a thin, transparent tissue called a membrane. Most tumors have many blood vessels coming through them. Sacrococcygeal teratomas come in many different sizes, and sometimes they may grow outward from the back or toward your child’s stomach.
Sacrococcygeal teratomas are classified according to the American Academy of Pediatrics Surgical Section. Sacrococcygeal teratoma tumors are categorized according to their location and severity:
- Type 1 tumors are external (outside the body) tumors and are attached to the tailbone. Type 1 is rarely associated with malignancy.
- Type 2 tumors have both internal (internal extension into the presacral space) and external parts.
- Type 3 tumor is visible externally, but is predominantly located in your child’s pelvic area with some extension into the abdomen.
- Type 4 tumors, the most serious tumors, can’t been seen from the outside. They are located in the presacral space. Type 4 has the highest rate of malignancy.
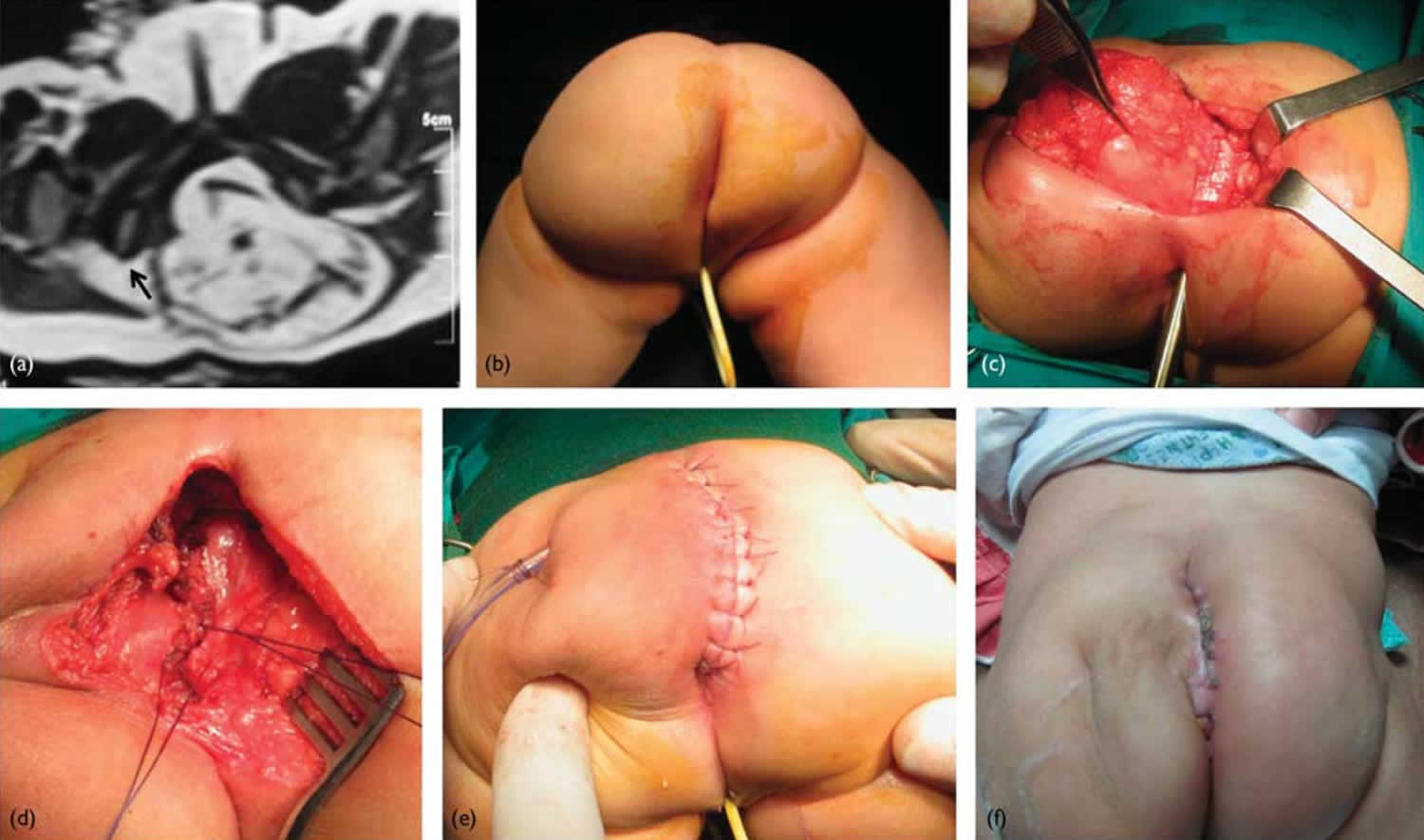
Figure 1. Sacrococcygeal teratoma
Footnote: 5-month-old female patient with a relatively small sacrococcygeal teratoma. (a) Pelvic MRI (axial T2WI) showing the mass displacing the anorectum (black arrow). (b) The patient placed in the prone (face down) position. (c) Dissection of the mass off the rectum. (d) After excision of the mass, the pelvic floor muscles are closed vertically in the midline from below upwards. (e) Vertical midline skin closure. (f) Follow-up at 3 weeks postoperatively.
[Source 8 ]Figure 2. Closure of skin flaps after sacrococcygeal teratoma resection
Sacrococcygeal teratoma causes
The cause of sacrococcygeal teratomas is unknown. Sacrococcygeal teratomas are germ cell tumors. Germ cells are the cells that develop into the embryo and later on become the cells that make up the reproductive system of men and women. Most germ cell tumors occur in the testes or ovaries (gonads) or the lower back. When these tumors occur outside of the gonads, they are known as extragonadal tumors. Researchers do not know how extragonadal germ cell tumors form. One theory suggests that germ cells accidentally migrate during to unusual locations early during the development of the embryo (embryogenesis). Normally, such misplaced germ cells degenerate and die, but in cases of extragonadal teratomas researchers speculate that these cells continue to undergo mitosis, the process where cells divide and multiply, eventually forming a teratoma.
Sacrococcygeal teratomas are thought to arise from an area under the coccyx called “Henson’s Node”. This is an area where primitive cells persist (germ cells) that can give rise to cells of the three major tissue layers of an embryo: ectoderm, endoderm, and mesoderm. These embryonic layers eventually give rise to the various cells and structures of the body. Sacrococcygeal teratomas can contain mature tissue that looks like any tissue in the body, or immature tissue resembling embryonic tissues.
Sacrococcygeal teratoma signs and symptoms
The symptoms that occur with sacrococcygeal teratomas vary widely depending upon the size and specific location of the tumor. Small tumors often do not cause any symptoms (asymptomatic) and can usually be removed surgically after birth without difficulty.
However, larger sacrococcygeal tumors can cause a variety of complications before and after birth. Sacrococcygeal teratomas can grow rapidly in the fetus and require very high blood flow resulting in fetal heart failure, a condition known as hydrops. This is manifest as dilation of the heart, and the collection of fluid in tissues of the body, including the skin and body cavities such as around the lungs (pleural effusion), around the heart (pericardial effusion), and/or in the abdominal cavity (ascites). If neglected, hydrops can also be dangerous for the mother resulting in similar symptoms of swelling, hypertension, and fluid on the lungs with shortness of breath. In addition to hydrops, which can occur in approximately 15% of very large fetal sacrococcygeal teratomas, these tumors can cause polyhydramnios (too much amniotic fluid), fetal urinary obstruction (hydronephrosis), bleeding into the tumor or rupture of the tumor with bleeding into the amniotic space, or dystocia (a condition where the fetus cannot be delivered due to the size of the tumor. It is very important to have very close monitoring during pregnancy to recognize these symptoms as early as possible.
In adults, sacrococcygeal teratomas may not cause symptoms (asymptomatic). In some cases, they may cause progressive lower back pain, weakness, and abnormalities due to obstruction of the genitourinary and gastrointestinal tracts. Such symptoms include constipation and increased frequency of stools or urinary tract infections. In rare cases, sacrococcygeal tumors cause partial paralysis (paresis) of the legs and tingling or numbness (paresthesia).
Sacrococcygeal teratoma diagnosis
The signs and symptoms of sacrococcygeal teratoma depend largely on the size and location of the tumor. Some tumors can be diagnosed by ultrasound before your child is born. An abnormally sized uterus is typically the first sign that your baby may have a sacrococcygeal teratoma tumor. The size discrepancy can be due to a massive tumor or to polyhydramnios (excess amniotic fluid). Less common presentations include maternal preeclampsia. Fetal sacrococcygeal teratoma may be cystic, solid or mixed in its sonographic appearance. The heterogeneous appearance of the mass may be due to mixed areas of tumor necrosis, cystic degeneration, hemorrhage or calcification.
If your baby is prenatally diagnosed with fetal sacrococcygeal teratoma, your doctor will continue to monitor your pregnancy to watch for any growth of the tumor or changes in your baby’s condition that may require intervention. Your baby may need fetal surgery to remove the sacrococcygeal teratoma if the size and severity of the tumor cause complications such as fetal hydrops that put you or your baby at risk.
Other tumors may not be visible until after your baby is born. After delivery, your child may have symptoms that indicate a possible sacrococcygeal teratoma, such as being unable to urinate or have a bowel movement because the tumor is pressing on their bladder or rectum. Some children have no symptoms at all.
Monitoring and delivery
There are several factors your doctor will consider when predicting the likelihood of complications including hydrops in prenatal cases of sacrococcygeal teratoma.
Factors considered might include:
- Tumor size
- Tumor composition (whether the tissue is solid, fluid-filled, or both)
- Rate of tumor growth
- Degree of vascularization (blood flow to the tumor)
- Signs of impaired cardiac function (including hydrops)
- Development and timing of polyhydramnios (too much amniotic fluid) 9
Many of these factors need to be followed over time with routine ultrasound, MRI, and echocardiogram. Given the complicated nature of the development of sacrococcygeal teratoma and the multiple factors involved in determining prognosis, doctors cannot predict the likelihood of hydrops fetalis 10.
If your baby’s condition is stable with no high output cardiac failure (hydrops fetalis), your pregnancy will be followed with regular ultrasound monitoring. If the sacrococcygeal teratoma is small, a vaginal delivery at term may be planned.
If the sacrococcygeal teratoma is large or if there is an excess of amniotic fluid (polyhydramnios), an early cesarean section is planned to avoid tumor rupture as well as the risks of preterm labor and premature delivery.
If hydrops fetalis develops, you may be a candidate for fetal surgery.
Sacrococcygeal teratoma risks and complications
When a prenatally diagnosed sacrococcygeal teratoma is associated with fetal hydrops, the tumor can become life-threatening to both mother and baby.
In severe cases, the tumor “steals” blood from fetal circulation, causing the heart to work extra hard and making cardiac failure possible. Cardiac failure exhibits as fetal hydrops, a massive accumulation of fluid in the body of the fetus. In our experience, fetal hydrops associated with sacrococcygeal teratoma is rapidly progressive and nearly always fatal.
For the mother, there is the risk of “maternal mirror syndrome” in which the mom’s condition parallels that of the sick fetus. When fetal hydrops is present, the mother may “mirror” the sick fetus, becoming ill with signs of preeclampsia. Preeclampsia, also called toxemia, is a condition characterized by pregnancy-induced high blood pressure, protein in the urine, and swelling due to fluid retention.
Sacrococcygeal teratoma treatment
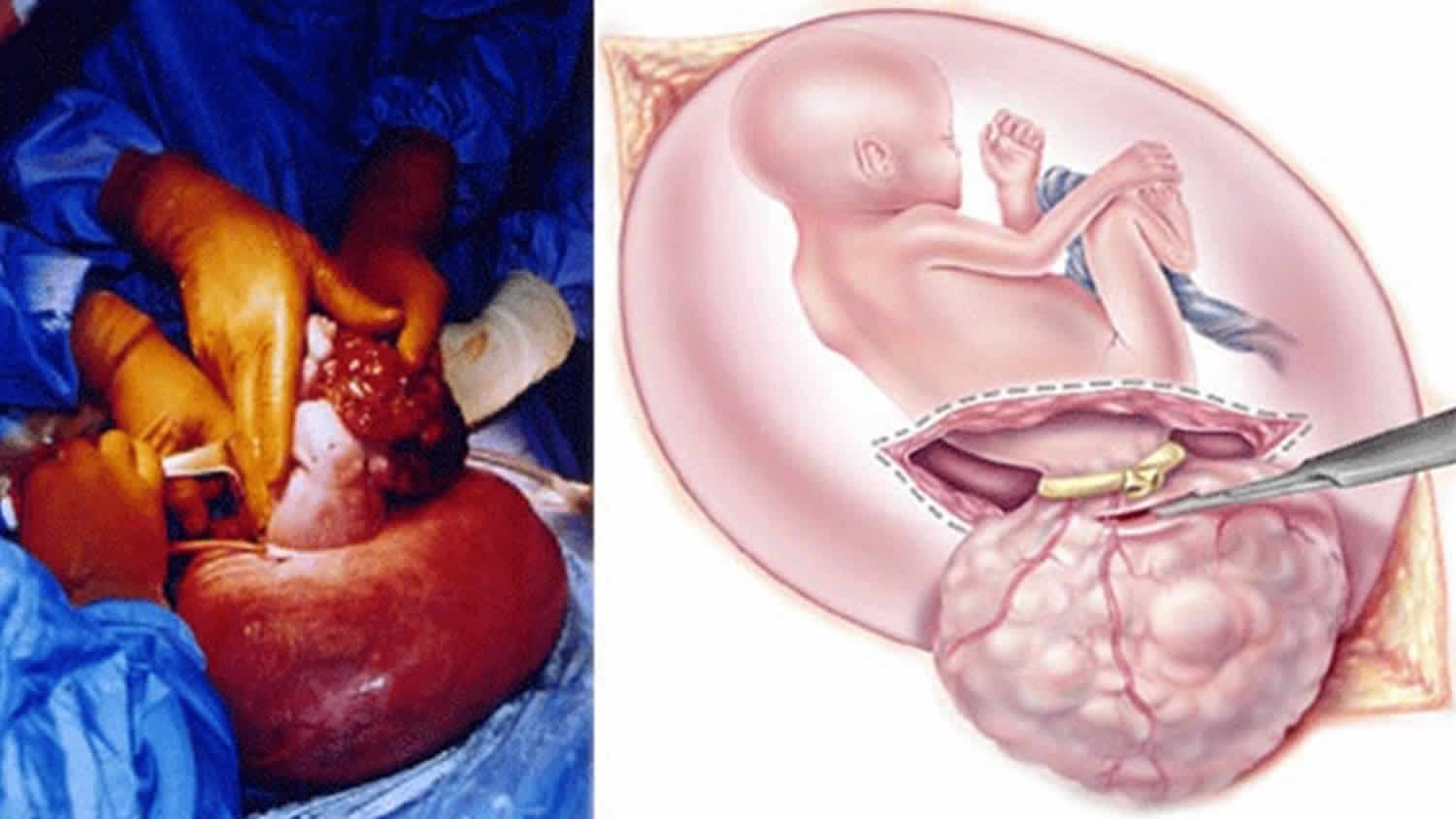
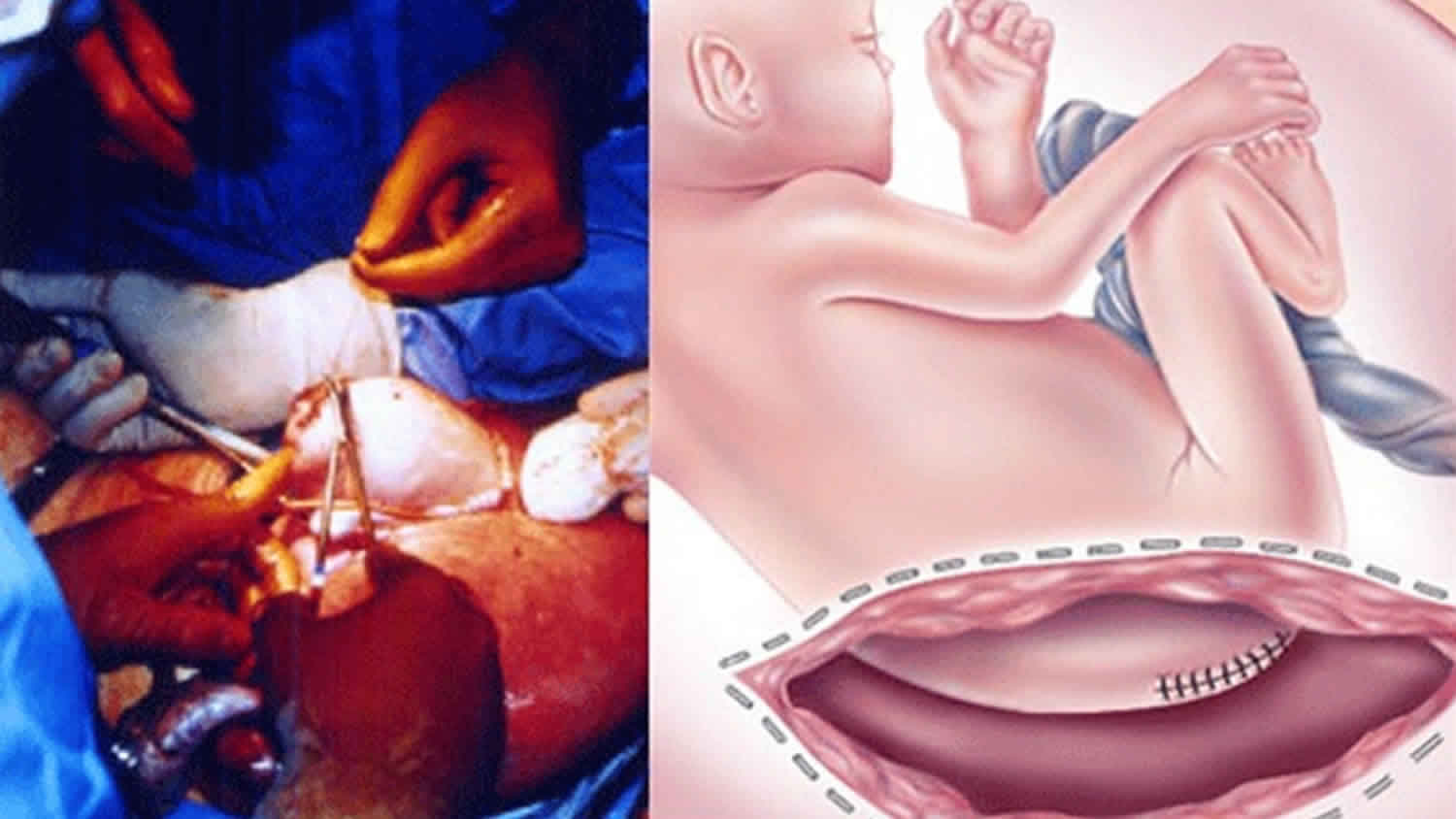
The treatment for sacrococcygeal teratoma typically involves surgery to remove the tumor. Surgery occurs either in the prenatal period or shortly after delivery. The timing is dependent on the size of the tumor and the associated symptoms 11.
If your baby with sacrococcygeal teratoma develops hydrops fetalis and his condition puts him in jeopardy, immediate intervention is recommended. If your baby is mature, your doctor will perform an emergency cesarean section. If your baby are not ready to be born, fetal surgery to remove the sacrococcygeal teratoma will be recommended.
In fetal surgery for sacrococcygeal teratoma, you will receive general anesthesia to provide complete pain relief. Although general anesthesia will alleviate most of the pain of the fetus, additional anesthesia is provided to your baby directly during the operation.
Once you are asleep, your fetal surgeon will make an incision similar to that of a cesarean section and will lift the uterus partly out of the abdomen. This procedure is called a hysterotomy. Next, the surgical team will open the uterus and expose your baby’s tumor. You fetal surgeon will remove the sacrococcygeal teratoma tumor and close the wound in your baby’s back. The uterus is then closed, placed back inside your abdomen, and the abdominal incision repaired. Mothers recover in the hospital for several days.
Sacrococcygeal teratoma prognosis
Most fetal sacrococcygeal teratomas are not likely to be malignant, and the prognosis tends to be good after resection. However, because a sacrococcygeal teratoma occurs when a fetus is developing and surgery is needed to remove it, there can be long-term effects of this tumor. The causes of long-term effects are thought to be the sacrococcygeal teratoma pressing on nerves and tissues thereby altering the normal development of these body structures, or the nerves and tissues may be disturbed during surgery to remove the sacrococcygeal teratoma 6. The effects may include difficulty controlling the bowels and urination, weakness in the legs, and issues with the spine 12. These symptoms may not become apparent until many years after surgery to remove the teratoma. For example, one study found that 22% of individuals who were previously diagnosed with a sacrococcygeal teratoma experienced lower back pain and stiffness beginning in adolescence 13.
Ovarian teratoma
Ovarian teratoma is a type of germ cell tumor with areas that, when seen under the microscope, look like each of the 3 layers of a developing embryo: the endoderm (innermost layer), mesoderm (middle layer), and ectoderm (outer layer). This germ cell tumor has a benign form called mature teratoma and a cancerous form called immature teratoma. Cancerous teratomas (immature teratomas) are rare, and usually affect girls and young women up to their early 20s 14. Teratomas are
Germ cell tumors begin in egg cells in women or sperm cells in men. There are 2 main types of ovarian teratoma:
- Mature teratoma, which is non cancerous (benign)
- Immature teratoma, which is cancerous
Figure 3. Ovarian teratoma
Mature teratoma
The mature teratoma is by far the most common type of ovarian germ cell tumor. It is most often diagnosed in women during their reproductive years (from teens to forties).
The mature teratoma is a benign tumor that usually affects women of reproductive age (teens through forties). Mature teratoma is often called a dermoid cyst because its lining is made up of tissue similar to skin (dermis). These tumors or cysts can contain different kinds of benign tissues including, bone, hair, and teeth. The patient is cured by surgical removal of the cyst, but sometimes a new cyst develops later in the other ovary.
Mature teratoma is removed with surgery and the condition is then cured.
Immature teratoma
Immature teratomas are a type of cancer. These are rare cancers that contain cells that look like those from embryonic or fetal tissues such as connective tissue, respiratory passages, and brain. They are called immature because the cancer cells are at a very early stage of development. Immature teratomas occur in girls and young women up to their early 20s, usually younger than 18. Tumors that are relatively more mature (called grade 1 immature teratoma) and haven’t spread beyond the ovary are treated by surgical removal of the ovary. When they have spread beyond the ovary and/or much of the tumor has a very immature appearance (grade 2 or 3 immature teratomas), chemotherapy is recommended in addition to surgery.
Most immature teratomas of the ovary are cured, even if they are diagnosed at an advanced stage.
Grading and staging ovarian teratoma
The grade and stage of your cancer is very important because they help your specialist to decide what treatment you need. The grade describes how the cells look under a microscope.
The less developed the cells look, the higher the grade. Higher grade cancers grow more quickly than low grade.
There are 3 different grades of immature teratoma. Generally, grade 1 teratomas are the slowest growing and least likely to spread.
The stage of a cancer tells you how far it has grown. In ovarian teratoma there are 4 stages, from 1 to 4:
- Stage 1 means the cancer is only in the ovary (or both ovaries)
- Stage 2 means the cancer has spread into the fallopian tube, womb, or elsewhere in the area circled by your hip bones (your pelvis)
- Stage 3 means the cancer has spread to the lymph nodes or to the tissues lining the abdomen (called the peritoneum)
- Stage 4 means the cancer has spread to another body organ some distance away, for example the lungs
Ovarian teratoma symptoms
Ovarian cancer may cause several signs and symptoms. Women are more likely to have symptoms if the disease has spread, but even early-stage ovarian cancer can cause them. The most common symptoms include:
- Bloating
- Pelvic or abdominal (belly) pain
- Trouble eating or feeling full quickly
- Urinary symptoms such as urgency (always feeling like you have to go) or frequency (having to go often)
These symptoms are also commonly caused by benign (non-cancerous) diseases and by cancers of other organs. When they are caused by ovarian cancer, they tend to be persistent and a change from normal − for example, they occur more often or are more severe. These symptoms are more likely to be caused by other conditions, and most of them occur just about as often in women who don’t have ovarian cancer. But if you have these symptoms more than 12 times a month, see your doctor so the problem can be found and treated if necessary.
Others symptoms of ovarian cancer can include:
- Fatigue (extreme tiredness)
- Upset stomach
- Back pain
- Pain during sex
- Constipation
- Changes in a woman’s period, such as heavier bleeding than normal or irregular bleeding
- Abdominal (belly) swelling with weight loss
Ovarian teratoma treatment
Surgery and chemotherapy are the treatments doctors most often use for immature teratoma of the ovary.
Surgery
During your surgery, the surgeon (gynecological oncologist) will remove the affected ovary but your other ovary will be left. As most women with ovarian teratoma are young, doctors are aware that they may want to have children in the future and so will remove as little tissue as possible.
During the operation, the surgeon examines the inside of your abdomen and your abdominal organs for signs of cancer. They may take biopsies and send them to the lab to look for cancer cells. Your surgeon will also wash out the inside of your abdomen and send the fluid to be checked for cancer cells. This all helps to make absolutely sure that the cancer hasn’t spread.
If you have a grade 1 immature teratoma that has not spread outside the ovary (stage 1) then surgery is likely to cure it and you may not need chemotherapy.
If your cancer has spread beyond the ovary, your surgeon will remove some or all of the tumour, depending on where it is. It is not uncommon to have tumour left behind.
Chemotherapy
You have chemotherapy once you have recovered from your surgery. If there is any cancer left after you have had chemotherapy, you may have more surgery to remove it then. Immature teratomas of the ovary can often be cured with a combination of surgery and chemotherapy, even if they have spread when they are diagnosed.
Your exact treatment is decided by your own specialist, but the most common combination of drugs used is BEP – bleomycin, etoposide and cisplatin. Doctors use this combination because it is very good at preventing the teratoma from coming back.
You have this type of chemotherapy as several cycles of treatment. Each cycle lasts 3 weeks. You have several days of chemotherapy at the beginning of the cycle and then a break until the start of the next cycle.
Follow up
After you’ve finished your treatment, your specialist will see you regularly.
When you were first diagnosed, you would have had blood tests that may have shown up chemicals released by the cancer cells. These are called markers. The markers produced by most immature teratomas are called human chorionic gonadotropin (hCG) and alpha-fetoprotein (AFP).
Not everyone with a teratoma has raised markers. But if you did when you were diagnosed, your specialist can use them to monitor your health at your follow up appointments. If they show up in your blood test, this could mean that the cancer has come back.
At your follow up appointments, your specialist will also examine you and ask how you are feeling. You may have CT scans from time to time, but not everyone needs these.
Teratoma eye
Teratoma eye is commonly called orbital teratoma. Teratoma eye is a rare, but typical, location in which primary extragonadal germ cell tumors arise 15.
Teratomas, the most common of orbital germ cell tumors, probably arise from pluripotential embryonic stem cells that are carried to the orbit by blood circulation and escape regulatory influences, or from primordial germ cells that aim toward the pineal gland, even though other theories have also been proposed 16. Orbital teratomas reported so far have been unilateral, and tumors cited as bilateral seem to have been limbal dermoids 17.
Teratomas account for 6.6% of childhood tumors, most commonly occurring in the testes, ovaries, and retroperitoneum; the orbit is a very rare site. A review of the literature showed that females are affected more than males in an approximately 2:1 ratio, with a propensity of the tumor to occur more frequently in the left than in the right orbit 18. Most cases reported in the literature are cytologically benign, however in some cases, tumors may grow rapidly, and severe complications may ensue 19.
Figure 4. Teratoma eye (huge orbital teratoma with compressed eyeball in a two day old female neonate)
Eye teratoma classification
Classification of orbital teratomas by Mizuo 20 and Duke-Elder 21;
- A complete fetus implanted in the orbit (orbitopagus parasiticus).
- A portion of a second fetus in the orbit.
- A tumor consisting of all three germinal layers.
- Tumors containing representatives of two germinal cell layers only.
- Tumors containing representatives of one layer only (dermoid cysts, osteomata, chondromata, etc.).
This classical system of classification has been modified by Damato 21 where the last sub-category was removed and it includes only four categories.
Teratoma eye diagnosis
Clinical presentation
Extreme unilateral proptosis in an otherwise healthy newborn with marked stretching of the eyelids over a tense, fluctuating mass, elongation of the palpebral fissure, normally developed eye that may exhibit degenerative changes secondary to the displacement by the teratoma, and transillumination of all or part of the orbital mass 22. Most tumors are intraconal and stretch the four recti, which results in a quadrangular shape and axial proptosis. The eye might be buried within the tumor itself, and only the cornea or a narrow rim of the sclera visible. Exposure keratopathy, ulceration, keratoiritis, and even spontaneous perforation occur secondarily. The proptosis may be axial or vertical 23.
The eye is usually normally formed, but displaced superiorly and forward. In rare cases there is no organized eye, with only remnants of ocular tissues being present 24. The persistent enlargement of this neoplasm is attributed to mucus secretion from the embryonic intestinal tissue 25. The optic nerve may be encased or adherent to the tumor, leading to secondary atrophy and poor pupil reaction 26. Commonly the eye is normally developed but often vision is not preserved either due to optic atrophy or corneal exposure, probably to the amniotic fluid.
Other features include; no family history of congenital deformities with non-consanguineous parents and normal siblings, normal pregnancy and delivery, no history of teratogenic influences to the mother 27.
Radiological finding
Upon imaging, benign orbital teratomas usually reveals multiloculated, cystic masses with an admixture of tissues including calcification, fat and ossification 28. Radiologic examinations are essential for a prompt diagnosis: MRI is the criterion standard and is usually co-related with a CT scan.
Histopathological findings
Histologically, teratomas are composed of tissues derived from the three germinal layers 29. The predominant germ cell types observed in orbital teratoma are surface ectoderm producing squamous epithelium-lined cyst, hair follicles, and sweat glands. Neuroectodermal tissues include primitive neural tubes, choroidal plexus, and ganglia. Mesoderm is the next most common cell layer represented by the muscle, bone, cartilage, and fat. Endoderm is the least common and may produce gastrointestinal tissue cysts lined by respiratory-type psuedostratified columnar epithelium. Cystic spaces lined by glandular epithelium are responsible for the rapid enlargement of the lesions 27.
Eye teratoma treatment
The clinical management of these lesions is unclear, due in part to their low incidence and to an incomplete understanding of their natural history 30. Complete surgical excision of tumor is the only accepted modality of treatment.
Surgery
Early surgery is mandatory to avoid permanent complications. The treatment is complete tumor excision with sparing of the eye, if possible. In the past, many surgeons preferred orbital exenteration because of possibility of malignancy 31. It is hard to recommend a fixed management plan, however a common agreement in the management objectives should be to save the eye, retain some vision, encourage normal orbitofacial development, and maintain good cosmetic result 27.
Malignant teratomas and intracranial extension
Most of the orbital teratomas are benign. Only few cases of orbital teratomas with malignant changes have been reported 31. Histopathological examination revealed a malignant sarcomatous changes in these variant 31. Teratoma is considered malignant when the tissue is embryonal or immature in nature 32. There is controversy on the diagnostic value of serum alpha-fetoprotein for mature teratomas 33, especially in the newborn period, being normally high as a result of fetal production 34. However, elevated serum alpha-fetoprotein (AFP) levels may signal the presence of regional recurrence or metastatic disease in the setting of malignant teratoma 35. Usually orbital teratoma is limited within the orbital cavity but there is also a reported instances with cranial extension termed as orbito-cranial teratoma 18.
Follow up and prognosis
Orbital teratomas have been known to recur and may undergo malignant degeneration. Therefore, close follow-up is necessary 25. Follow up is necessary not only to ascertain recurrence but also to encourage normal orbito facial development and maintain cosmesis.
The prognosis of the orbital teratoma is related to several factors including the age, site, and grade of immaturity, and is usually good if complete excision is performed early in neonatal life.
Testicular teratoma
Testicular teratomas occur in children and adults, but their incidence and natural history contrast sharply. Pure teratomas comprise 38% of germ cell tumors in infants and children but only 3% after puberty. In children, they behave as a benign tumor, whereas in adults and adolescents they are known to metastasize 36. With no documented cases of metastasis, morbidity from prepubertal testicular teratomas is largely limited to surgical or postoperative complications.
During and after puberty, all teratomas are regarded as malignant because even mature teratomas (composed of entirely mature histologic elements) can metastasize to retroperitoneal lymph nodes or to other systems. Reported rates of metastasis vary from 29-76%. Morbidity is associated with growth of the tumor, which may invade or obstruct local structures and become unresectable. Approximately 20% of patients relapse during surveillance 37.
Testicular teratoma symptoms
Testicular teratomas most often present as a painless scrotal mass, except in the case of torsion. In most cases, the masses are firm or hard, nontender, and do not transilluminate. Testicular pain and scrotal swelling are occasionally reported with teratomas, but this is nonspecific and simply indicates torsion until proven otherwise. Hydrocele is frequently associated with teratoma in childhood. On examination, the testis is diffusely enlarged, rather than nodular, although a discreet nodule in the upper or lower pole sometimes can be appreciated 38.
Testicular teratoma treatment
Testicular teratomas traditionally have been treated by simple or radical orchiectomy. More recently, conservative excision by enucleation also has been recommended for prepubertal teratomas of the testis 39. Several studies have failed to demonstrate negative sequelae for prepubertal testicular teratomas, so testis-sparing procedures are appropriate 40. Patients should be counseled regarding the following risks 36:
- Inadequate sampling
- Incorrect diagnosis by frozen section
- Tumor spillage and seeding
- Unidentified microinvasive disease
The risk of malignancy increases with maturation of the testes, and this is a significant concern in children at or near puberty. In this group, areas of normal surrounding testicular tissue should be excised and sent for frozen section. If frozen section reveals areas of maturity, proceeding to orchiectomy is recommended. Enucleation or partial orchiectomy for teratoma in pubertal or adult males is not recommended 36.
Mediastinal teratoma
Mature teratomas of the mediastinum, the most common mediastinal germ cell tumor, are benign lesions. They do not have the metastatic potential observed in testicular teratoma and are cured by surgical resection alone. Because of their anatomic location, intraoperative and postoperative complications are the only significant source of morbidity, as other intrathoracic structures are often intimately involved with the tumor 41.
Mediastinal teratoma symptoms
Mediastinal teratomas are often asymptomatic. When symptoms are present, they relate to mechanical effects and include chest pain, cough, dyspnea, or symptoms related to recurrent pneumonitis. Many patients present with respiratory findings, and the pathognomonic finding of trichoptysis (cough productive of hair or sebaceous material) may result if a communication develops between the mass and the tracheobronchial tree. Other serious presentations are superior vena cava syndrome or lipoid pneumonia. Mediastinal teratomas are occasionally discovered incidentally on chest radiographsa 42.
Mediastinal teratoma treatment
Mature teratomas of the mediastinum should be completely surgically resected. The tumor may be adherent to surrounding structures, necessitating resection of the pericardium, pleura, or lung. When complete resection is achieved, it results in excellent long-term cure rates with little chance of recurrence. When complete resection is impossible, partial resection often leads to symptom relief, frequently without relapse 43.
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2005. 7th ed.[↩]
- Grosfeld JL, Billmire DF. Teratomas in infancy and childhood. Curr Probl Cancer. 1985 Sep. 9(9):1-53.[↩]
- Ulbright TM. Gonadal teratomas: a review and speculation. Adv Anat Pathol. 2004 Jan. 11(1):10-23.[↩]
- Linder D, McCaw BK, Hecht F. Parthenogenic origin of benign ovarian teratomas. N Engl J Med. 1975 Jan 9. 292(2):63-6.[↩]
- Sacrococcygeal Teratoma. https://rarediseases.info.nih.gov/diseases/319/sacrococcygeal-teratoma[↩]
- Schmidt B, Haberlik A, Uray E, Ratschek M, Lackner H, Höllwarth ME. Sacrococcygeal teratoma: clinical course and prognosis with a special view to long-term functional results. Pediatric Surgery International. 1999; 15:573-576.[↩][↩]
- Tailor J, Roy PG, Hitchcock R, Grant H, Johnson P, Joseph VT, Lakhoo K. Long-term functional outcome of sacrococcygeal teratoma in a UK regional center (1993 to 2006). Journal of Pediatric Hematology/oncology. 2009; 31:183-186.[↩]
- Sacrococcygeal teratoma excision: a vertical rather than transverse wound closure. Annals of Pediatric Surgery 2017, 13:207–212 https://www.ajol.info/index.php/aps/article/viewFile/167055/156492[↩]
- Leonardo Gucciardo, Anne Uyttebroek, Ivo De Wever, Marleen Renard, Filip Claus, Roland Devlieger, Liesbeth Lewi, Luc De Catte, Jan Deprest. Prenatal assessment and management of sacrococcygeal teratoma. Prenatal Diagnosis. June 8, 2011; 31(7):678-688.[↩]
- Sacrococcygeal Teratoma. https://rarediseases.org/rare-diseases/sacrococcygeal-teratoma[↩]
- About Fetal Surgery for Sacrococcygeal Teratoma (SCT). https://www.chop.edu/treatments/fetal-surgery-sacrococcygeal-teratoma-sct/about[↩]
- Zaccara A, Iacobelli BD, Adorisio O, Petrarca M, Di Rosa G, Pierro MM, Bagolan P. Gait analysis in patients operated on for sacrococcygeal teratoma. Journal of Pediatric Surgery. 2004; 39:947-952.[↩]
- Lahdenne P, Heikinheimo M, Jääskeläinen J, Merikanto J, Heikkilä J, Siimes MA. Vertebral abnormalities associated with congenital sacrococcygeal teratoma. Journal of pediatric orthopedics. 1991; 11:603-607.[↩]
- Teratoma of the ovary. https://www.cancerresearchuk.org/about-cancer/ovarian-cancer/types/teratoma[↩]
- Duke-Elder S: System of Ophthalmology, Vol. 3, Normal and Abnormal Development, Part 2. London, Henry Kimpton, 1964, pp 966-975.[↩]
- Gonzalez-Crussi F: Extragonadal Teratomas, in Hartmann WH, Cowan WR (ed): Atlas of Tumor Pathology. Washington, Armed Forces Institute of Pathologv, 1982, 2nd Seryes, 18th Fast, pp l-49, 100-108.[↩]
- Gemolotto G, Gaipa M: Quadro bilaterale di teratoma epibulbare associate a malformazioni palpebrali. Arch Ottalmol,1956, 60:161-170.[↩]
- Sesenna E, Ferri A, Thai E, Magri AS. Huge orbital teratoma with intracranial extension: a case report. Journal of pediatric surgery. 2010 May 1;45(5):e27-31.[↩][↩]
- Bilgic S, Dayanir V, Kiratli H, Güngen Y. Congenital orbital teratoma: a clinicopathologic case report. Ophthalmic plastic and reconstructive surgery. 1997 Jun;13(2):142-6.[↩]
- Mizuo G. Eine seltene Form von Teratoma orbitae (Foetus in Orbita: Orbitopagus parasiticus). Arch Augenheilkd. 1910;65:365-83.[↩]
- Damato PJ, Damato FJ. Neonatal orbital teratoma. The British journal of ophthalmology. 1962 Nov;46(11):685.[↩][↩]
- Hoyt WF, Joe S. Congenital teratoid cyst of the orbit. Arch Ophthalmol. 1962;68(19620):1.[↩]
- Kivelä T, Tarkkanen A. Orbital germ cell tumors revisited: a clinicopathological approach to classification. Survey of ophthalmology. 1994 May 1;38(6):541-54.[↩]
- Sreenan C, Johnson R, Russell L, Bhargava R, Osiovich H. Congenital orbital teratoma. American journal of perinatology. 1999;16(05):251-5.[↩]
- Patel B: Benign orbital tumors: teratoma; in Perry J, Singh A (eds): Clinical Ophthalmic Oncology: Orbital Tumors, vol 1. Berlin/Heidelberg/New York/Dordrecht/London,Springer, 2014, pp 83–84.[↩][↩]
- Pellerano F, Guillermo E, Garrido G, Berges P. Congenital Orbital Teratoma. Ocular oncology and pathology. 2017;3(1):11-6.[↩]
- Gnanaraj L, Skibell BC, Coret-Simon J, Halliday W, Forrest C, DeAngelis DD. Massive congenital orbital teratoma. Ophthalmic Plastic & Reconstructive Surgery. 2005 Nov 1;21(6):445-7.[↩][↩][↩]
- Herman TE, Vachharajani A, Siegel MJ. Massive congenital orbital teratoma. Journal of Perinatology. 2009 May;29(5):396.[↩]
- Mamalis N, Garland PE, Argyle JC, Apple DJ. Congenital orbital teratoma: a review and report of two cases. Survey of ophthalmology. 1985 Jul 1;30(1):41-6.[↩]
- Lee GA, Sullivan TJ, Tsikleas GP, Davis NG. Congenital orbital teratoma. Aust NZJ Ophthalmol 1997; 25: 63-6.[↩]
- Mahesh L, Krishnakumar S, Subramanian N, et al. Malignant teratoma of the orbit: a clinicopathological study of a case. Orbit 2003;22: 305-9.[↩][↩][↩]
- Hann LE, Borden S, Weber AL. Orbital teratoma in the newborn A case report. Pediatric radiology. 1977 Sep 1;5(3):172-4.[↩]
- Tsuchida Y, Hasegawa H. The diagnostic value of alpha-fetoprotein in infants and children with teratomas: a questionnaire survey in Japan. Journal of pediatric surgery. 1983 Apr 1;18(2):152-5.[↩]
- Heerema-McKenney A, Bowen J, Hill DA, Suster S, Qualman SJ. Protocol for the examination of specimens from pediatric and adult patients with extragonadal germ cell tumors. Archives of pathology & laboratory medicine. 2011 May;135(5):630-9.[↩]
- Frazier AL, Brodsky JR, Kanwar VS, Stafford LM, Rahbar R. Germ cell tumors/teratoma. InPediatric Head and Neck Tumors 2014 (pp. 153-163). Springer, New York, NY.[↩]
- Walsh C, Rushton HG. Diagnosis and management of teratomas and epidermoid cysts. Urol Clin North Am. 2000 Aug. 27(3):509-18.[↩][↩][↩]
- Carver BS, Al-Ahmadie H, Sheinfeld J. Adult and pediatric testicular teratoma. Urol Clin North Am. 2007 May. 34(2):245-51; abstract x.[↩]
- Garrett JE, Cartwright PC, Snow BW, Coffin CM. Cystic testicular lesions in the pediatric population. J Urol. 2000 Mar. 163(3):928-36.[↩]
- Ross JH, Kay R. Prepubertal testis tumors. Rev Urol. 2004 Winter. 6(1):11-8.[↩]
- Shukla AR, Woodard C, Carr MC, Huff DS, Canning DA, Zderic SA. Experience with testis sparing surgery for testicular teratoma. J Urol. 2004 Jan. 171(1):161-3.[↩]
- Dulmet EM, Macchiarini P, Suc B, Verley JM. Germ cell tumors of the mediastinum. A 30-year experience. Cancer. 1993 Sep 15. 72(6):1894-901.[↩]
- Billmire DF, Grosfeld JL. Teratomas in childhood: analysis of 142 cases. J Pediatr Surg. 1986 Jun. 21(6):548-51.[↩]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005 Oct. 128(4):2893-909.[↩]