Contents
What is trypsin
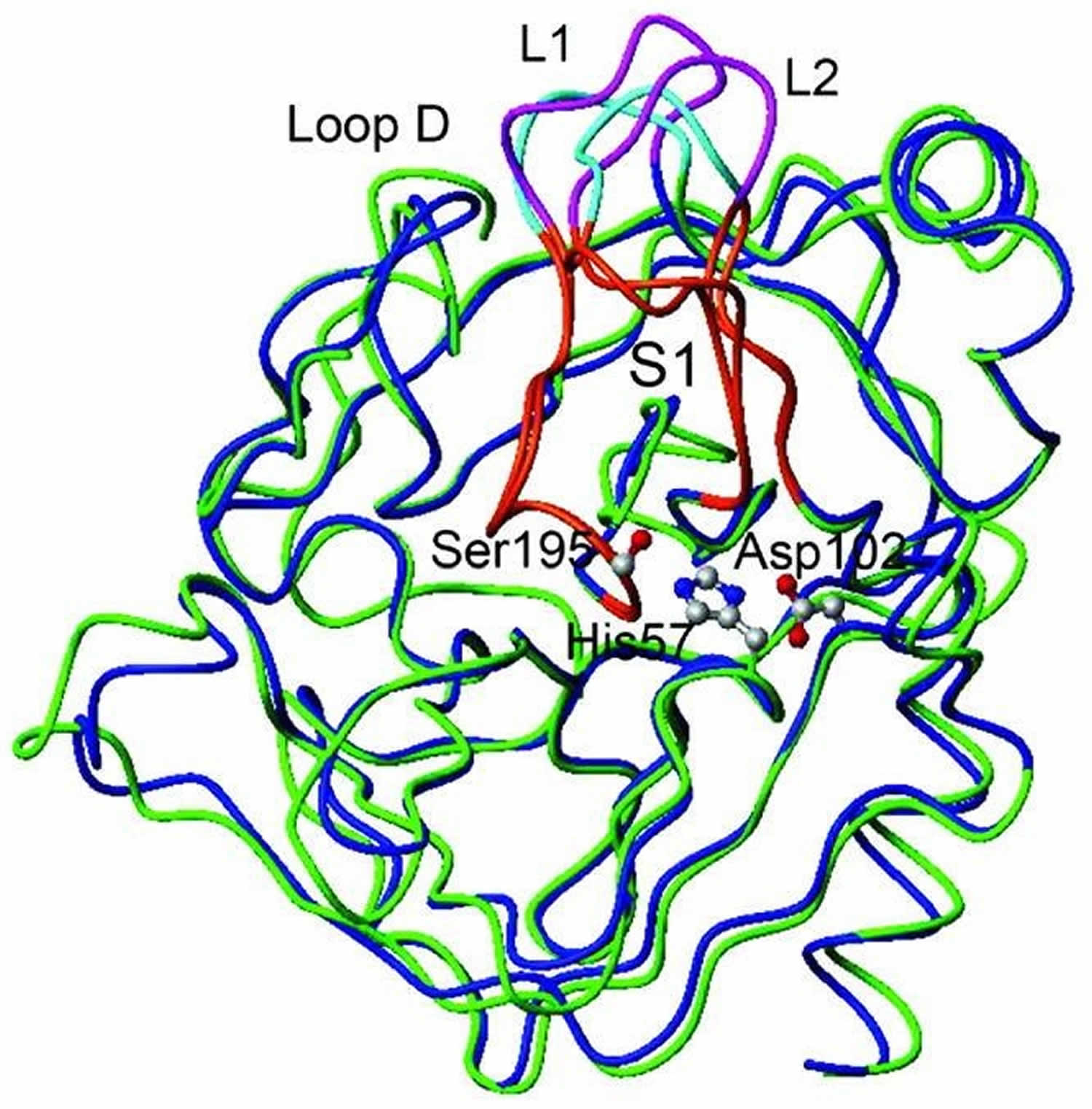
Trypsin is a serine protease enzyme which is secreted by the pancreas and trypsin plays an essential role in protein hydrolysis and absorption in mammals. Trypsin is most active in the pH range between 7 and 9 at 37°C. When converted from its zymogen trypsinogen (inactive trypsin), trypsin is available as an active peptide hydrolase form to cleave peptide bonds mainly between the carboxylic acid group of lysine or arginine and the amino group of the adjacent amino acid residue. Trypsin contains a nucleophilic residue Ser in the enzyme active site which attacks the carbonyl moiety of the substrate peptide bond to form an acyl-enzyme intermediate. This nucleophilic attack is facilitated by the catalytic triad consisting of histidine-57, aspartate-102, and serine-195. Trypsin also contains an oxyanion hole that stabilizes the charge negative charge on the carbonyl oxygen atom formed from the cleavage of peptide bonds. Therapeutic forms of trypsin is obtained from purified extracts of porcine or bovine pancreas and is intended to aid in digestion when administered orally.
Your body needs a steady supply of amino acids for use in growth and repairs. Each day, a typical adult needs something in the range of 35-90 grams of protein, depending on their weight. Quite surprisingly, a large fraction of this may come from inside. A typical North American diet may contain 70-100 grams of protein each day. But your body also secretes 20-30 grams of digestive proteins, which are themselves digested when they finish their duties. Dead intestinal cells and proteins leaking out of blood vessels are also digested and reabsorbed as amino acids, showing that our bodies are experts at recycling.
Trypsin uses a special serine amino acid in its protein-cutting reaction, and is consequently known as a serine protease. The serine proteases are a diverse family of enzymes, all of which use similar enzymatic machinery. In digestion, trypsin, chymotrypsin and elastase work together to chop up proteins. Each has a particular taste for protein chains: trypsin cuts next to lysine and arginine, chymotrypsin cuts next to phenylalanine and other large amino acids, and elastase likes chains with small amino acids like alanine. Trypsin-like enzymes are also found in many other places in the body. Some of these are highly specific, cleaving only a specific target protein. For instance, thrombin is designed to make a specific cut in fibrinogen, creating a blood clot.
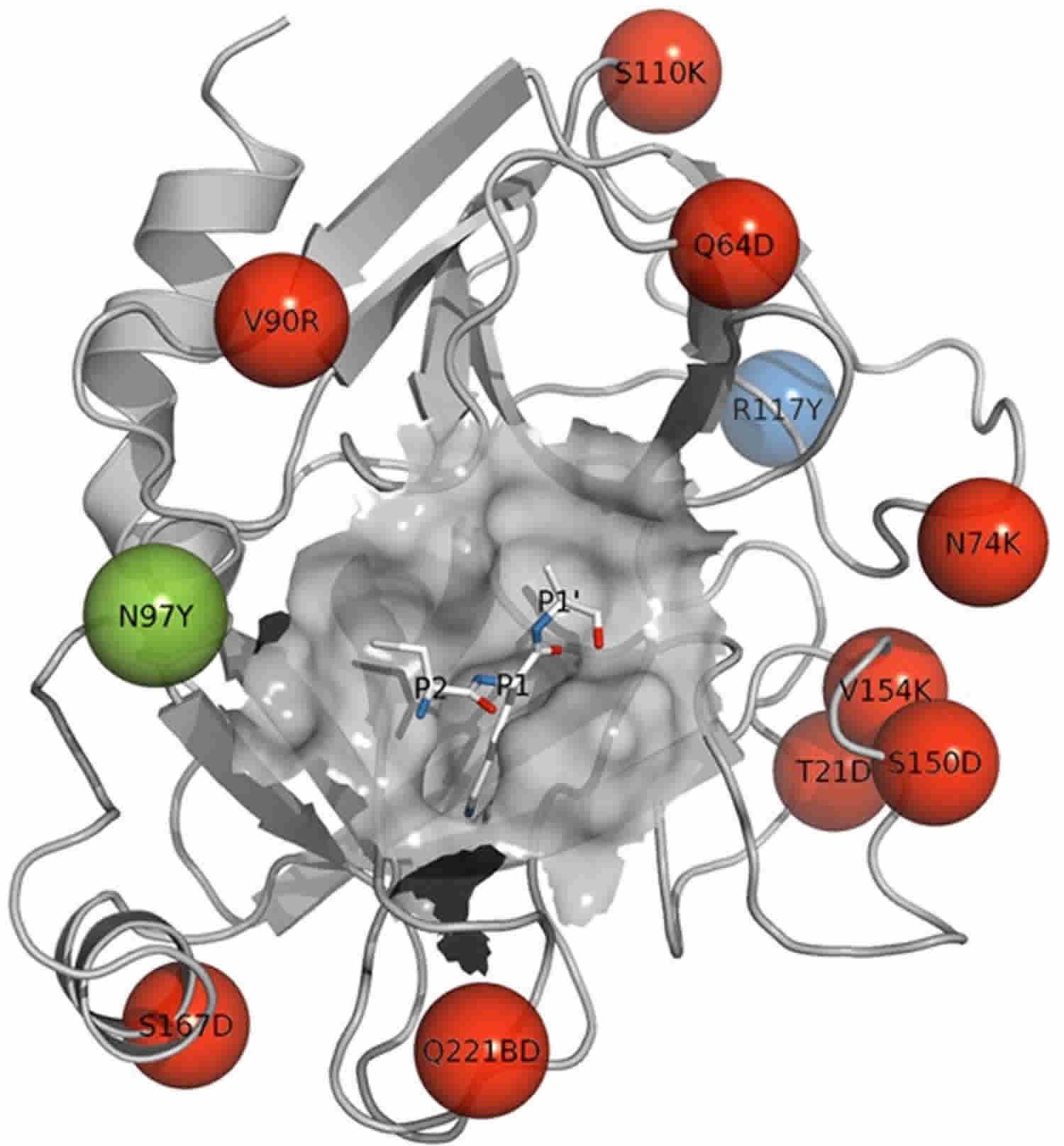
Figure 1. Trypsin enzyme
Where is trypsin produced?
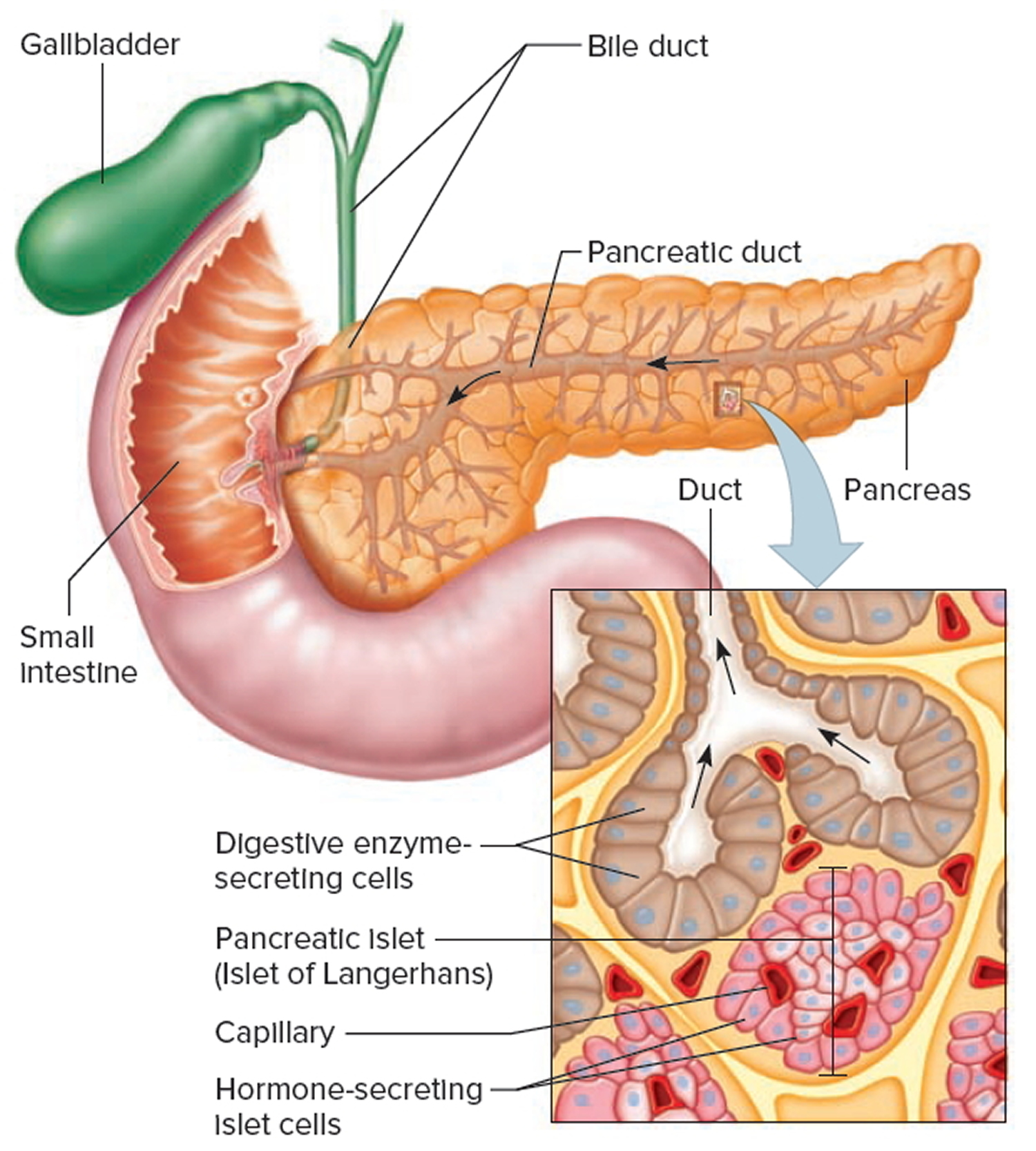
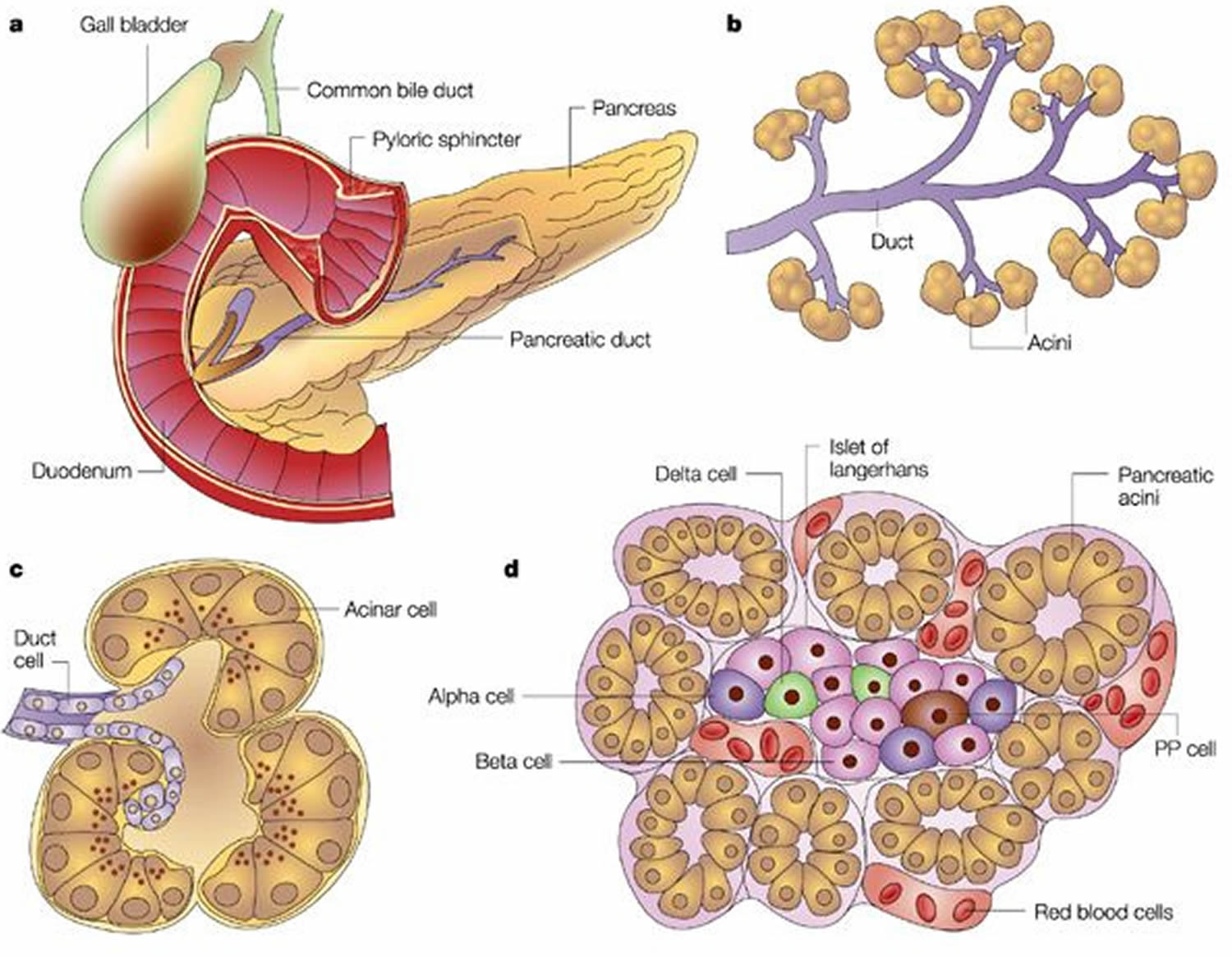
Trypsin is synthesized as an inactive precursor called trypsinogen in the pancreas. Trypsinogen is made by the acinar cells of the exocrine pancreas. After secretion into the small intestine, trypsinogen is converted into its active form trypsin by enteropeptidase an enzyme on the brush border of the duodenum, whereas ectopic activation inside the pancreas occurs through trypsin-mediated trypsinogen activation commonly referred to as autoactivation 1.
Figure 2. Pancreas anatomy
Trypsin function
Trypsin, as one of important proteases, that is specific for catalyzing the hydrolysis of peptide and ester bonds containing lysine and arginine amino acids at the carboxyl-terminus. Trypsin is also an autocatalytic—it converts trypsinogen into still more trypsin. Trypsin also converts the other two zymogens into chymotrypsin and carboxypeptidase, in addition to its primary role of digesting dietary protein.
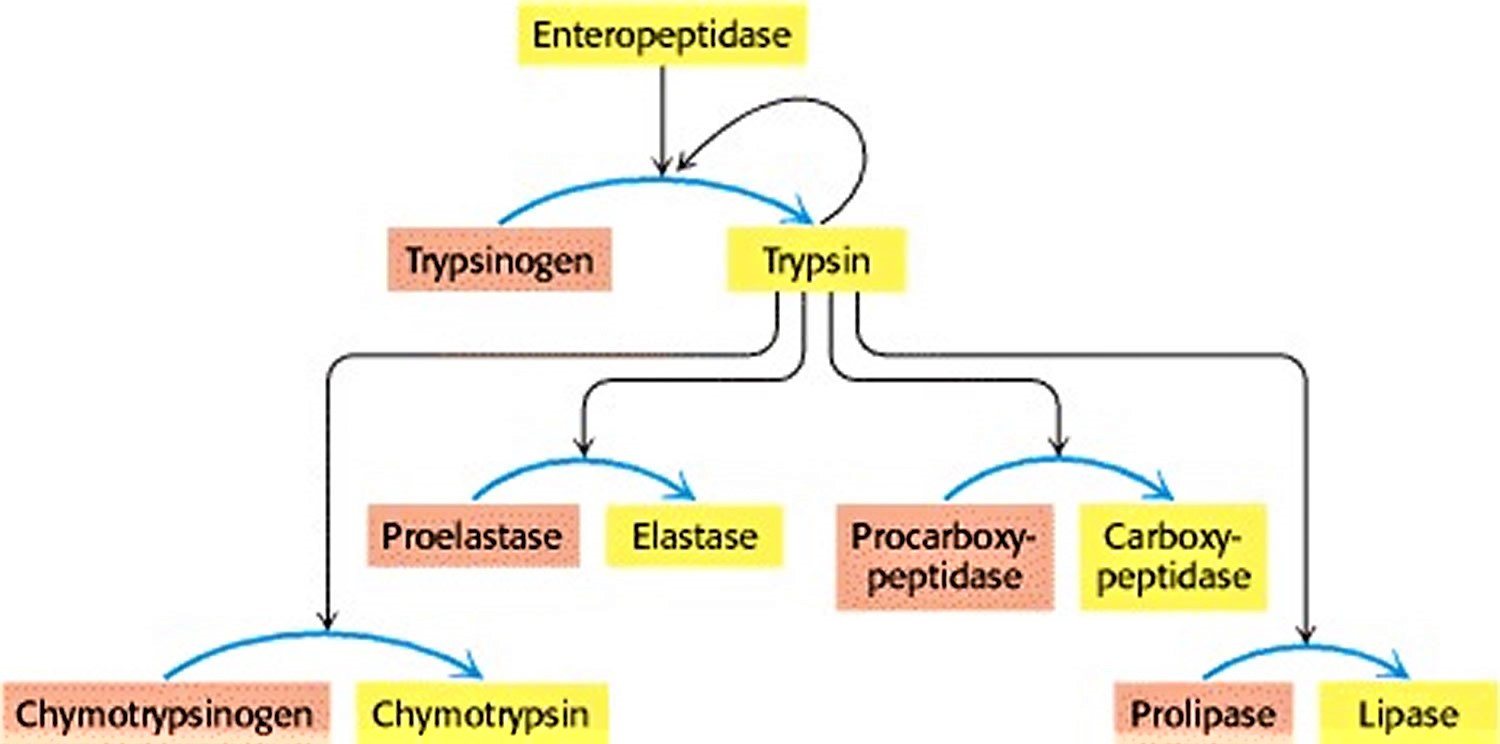
The digestion of proteins in the duodenum requires the concurrent action of several proteolytic enzymes, because each is specific for a limited number of side chains. Thus, the zymogens must be switched on at the same time. Coordinated control is achieved by the action of trypsin as the common activator of all the pancreatic zymogens—trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidase, and prolipase, a lipid degrading enzyme. To produce active trypsin, the cells that line the duodenum secrete an enzyme, enteropeptidase, that hydrolyzes a unique lysine-isoleucine peptide bond in trypsinogen as the zymogen enters the duodenum from the pancreas. The small amount of trypsin produced in this way activates more trypsinogen and the other zymogens (Figure 4). Thus, the formation of trypsin by enteropeptidase is the master activation step.
Figure 4. Zymogen activation by Trypsin
Footnote: Enteropeptidase initiates the activation of the pancreatic zymogens by activating trypsin, which then activates other zymogens. Active enzymes are shown in yellow; zymogens are shown in orange.
[Source 2 ]Where does trypsin cleave?
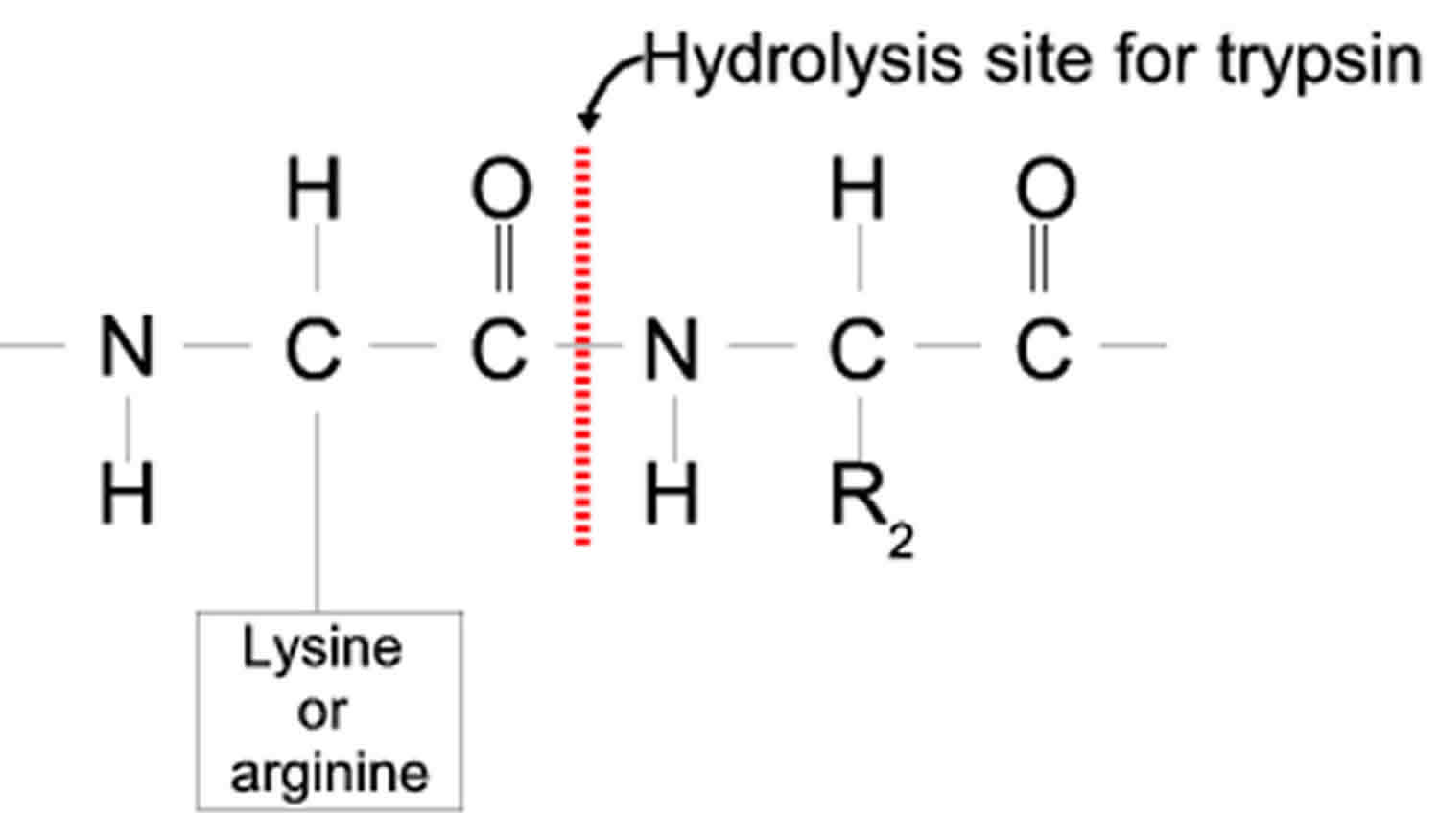
Trypsin cleaves peptide bonds mainly between the carboxylic acid group of lysine or arginine and the amino group of the adjacent amino acid residue.
Figure 5. Hydrolysis site of trypsin
 Trypsin inhibitor
Trypsin inhibitor
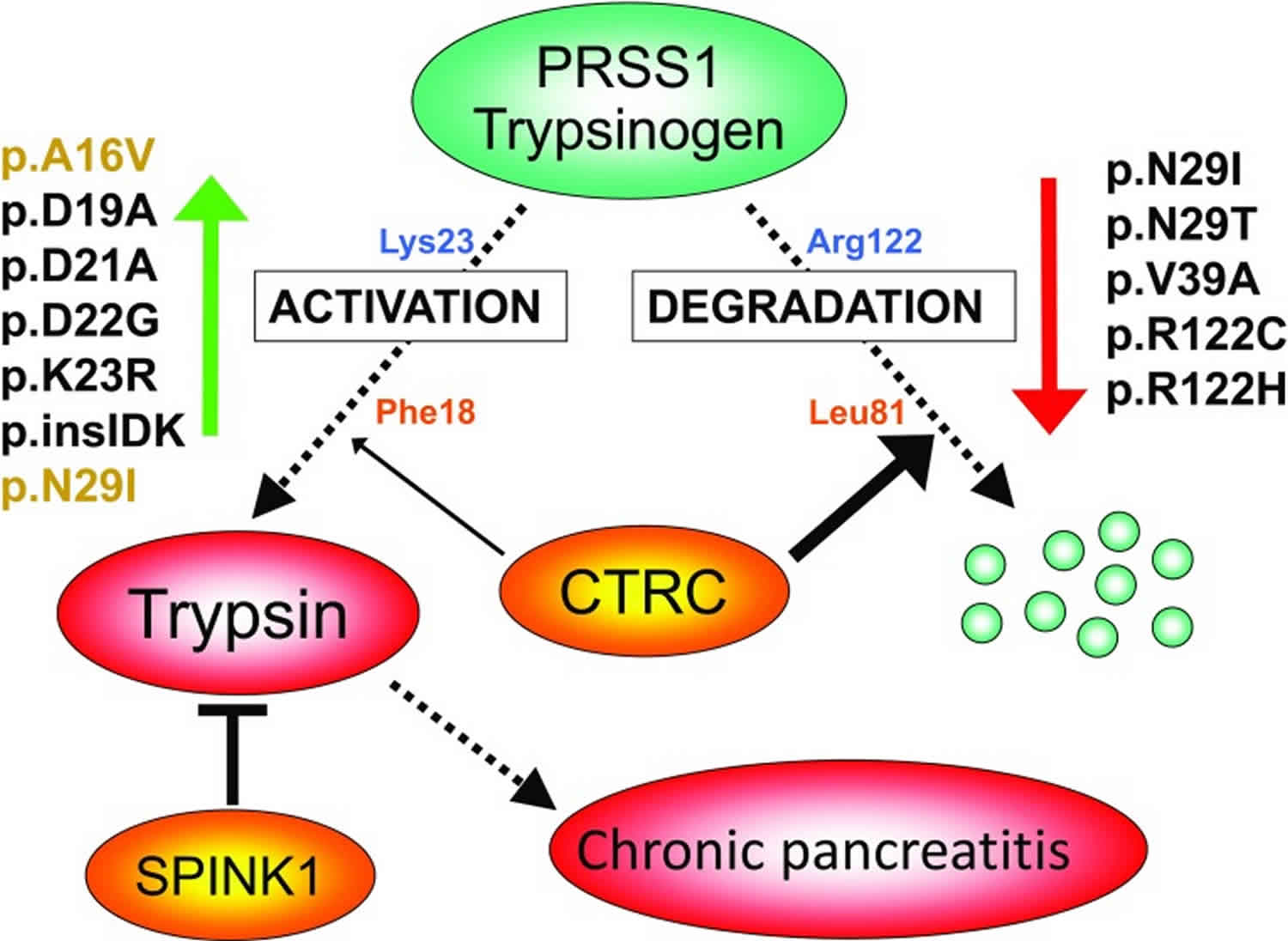
The conversion of a zymogen into a protease by cleavage of a single peptide bond is a precise means of switching on enzymatic activity. However, this activation step is irreversible, and so a different mechanism is needed to stop proteolysis. Specific protease inhibitors accomplish this task. Protective mechanisms in the pancreas that curtail trypsinogen activation and therefore reduce trypsin activity involve either trypsin inhibition or trypsinogen degradation (Figure 6). For example, serine protease inhibitor Kazal type 1 (SPINK1, also known as pancreatic secretory trypsin inhibitor) is a 6.2-kDa protein secreted by the pancreatic acinar cells that can potently inhibit trypsin. Levels in the pancreatic juice amount to about 0.1–0.8% of total protein 3 which, assuming that about 25% of the juice proteins is trypsinogen 4 and after correction for the molecular mass difference, should translate to serine protease inhibitor Kazal type 1 (SPINK1) concentrations that can inhibit 2–13% of the potential trypsin content. Rinderknecht et al. 5 reported average SPINK1 concentrations that can inhibit 13% of the potential trypsin content in the pancreatic juice of healthy volunteers and 5% in chronic alcoholics. During autoactivation of trypsinogen, the newly generated trypsin reacts with SPINK1 and becomes unavailable to catalyze further trypsinogen activation. In time, however, serine protease inhibitor Kazal type 1 (SPINK1) reserves become depleted and autoactivation can freely proceed. Thus, the protective role of SPINK1 is to delay trypsinogen autoactivation. This delay is important as it allows for the digestive proteases to transit from the pancreas to the duodenum in an inactive form. Consequently, a decrease in serine protease inhibitor Kazal type 1 (SPINK1) levels or reduced ductal fluid flow can impair this protective mechanism and increase the risk of premature, intra-pancreatic trypsinogen autoactivation 6.
Figure 6. Protective mechanisms in the pancreas that curtail trypsinogen activation
Footnote: Activation of serine protease 1 (PRSS1) trypsinogen to active trypsin in the pancreas is responsible for disease onset and progression. Protective mechanisms to control trypsinogen activation include trypsin inhibition by SPINK1 and trypsinogen degradation by chymotrypsin C (CTRC) and trypsin. Chymotrypsin C (CTRC) cleaves the Leu81-Glu82 peptide bond, and trypsin cleaves the Arg122-Val123 peptide bond; the combination of these two cleavages results in irreversible trypsinogen degradation. Chymotrypsin C (CTRC) also stimulates autoactivation of cationic trypsinogen by cleaving the Phe18-Asp19 peptide bond in the activation peptide. The shortened activation peptide is more susceptible to trypsin-mediated activation at the Lys23-Ile24 peptide bond. The indicated hereditary pancreatitis-associated serine protease 1 (PRSS1) mutations increase trypsinogen autoactivation by inhibition of chymotrypsin C (CTRC)-dependent trypsinogen degradation (red arrow) or by increasing chymotrypsin C (CTRC)-dependent stimulation of autoactivation (green arrow, mutations in orange type). Activation peptide mutations directly stimulate autoactivation independently of chymotrypsin C (CTRC) function (green arrow, mutations in black type). Loss-of-function mutations in SPINK1 reduce inhibitor expression and thus compromise trypsin inhibition. Loss-of function mutations in chymotrypsin C (CTRC) reduce secretion, block zymogen activation, diminish catalytic activity or promote degradation by trypsin, and therefore impair protective trypsinogen degradation.
[Source 6 ]Why does trypsin inhibitor exist?
Recall that trypsin activates other zymogens (see Figure 4). Consequently, it is vital that even small amounts of trypsin be prevented from initiating the cascade prematurely. Trypsin molecules activated in the pancreas or pancreatic ducts could severely damage those tissues, leading to acute pancreatitis. Tissue necrosis may result from the activation of proteolytic enzymes (as well as prolipases) by trypsin, and hemorrhaging may result from its activation of elastase. We see here the physiological need for the tight binding of the inhibitor to trypsin.
Pancreatic trypsin inhibitor is not the only important protease inhibitor. α1-Antitrypsin (also called α1-antiproteinase), a 53-kd plasma protein, protects tissues from digestion by elastase, a secretory product of neutrophils (white blood cells that engulf bacteria) 2. Antielastase would be a more accurate name for this inhibitor, because it blocks elastase much more effectively than it blocks trypsin. Like pancreatic trypsin inhibitor, α1-antitrypsin blocks the action of target enzymes by binding nearly irreversibly to their active sites. Genetic disorders leading to a deficiency of α1-antitrypsin show that this inhibitor is physiologically important. For example, the substitution of lysine for glutamate at residue 53 in the type Z mutant slows the secretion of this inhibitor from liver cells. Serum levels of the inhibitor are about 15% of normal in people homozygous for this defect. The consequence is that excess elastase destroys alveolar walls in the lungs by digesting elastic fibers and other connective-tissue proteins.
The resulting clinical condition is called emphysema (also known as destructive lung disease). People with emphysema must breathe much harder than normal people to exchange the same volume of air, because their alveoli are much less resilient than normal. Cigarette smoking markedly increases the likelihood that even a type Z heterozygote will develop emphysema. The reason is that smoke oxidizes methionine 358 of the inhibitor, a residue essential for binding elastase. Indeed, this methionine side chain is the bait that selectively traps elastase. The methionine sulfoxide oxidation product, in contrast, does not lure elastase, a striking consequence of the insertion of just one oxygen atom into a protein.
- Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Rinderknecht H. Dig Dis Sci. 1986 Mar; 31(3):314-21.[↩]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 10.5, Many Enzymes Are Activated by Specific Proteolytic Cleavage. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22589[↩][↩]
- Trypsin inhibitor from human pancreas and pancreatic juice. Pubols MH, Bartelt DC, Greene LJ. J Biol Chem. 1974 Apr 10; 249(7):2235-42.[↩]
- Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Scheele G, Bartelt D, Bieger W. Gastroenterology. 1981 Mar; 80(3):461-73.[↩]
- Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Rinderknecht H, Stace NH, Renner IG. Dig Dis Sci. 1985 Jan; 30(1):65-71.[↩]
- Hegyi E, Sahin-Tóth M. Genetic Risk in Chronic Pancreatitis: The Trypsin-Dependent Pathway. Dig Dis Sci. 2017;62(7):1692-1701. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5487703/[↩][↩]