Contents
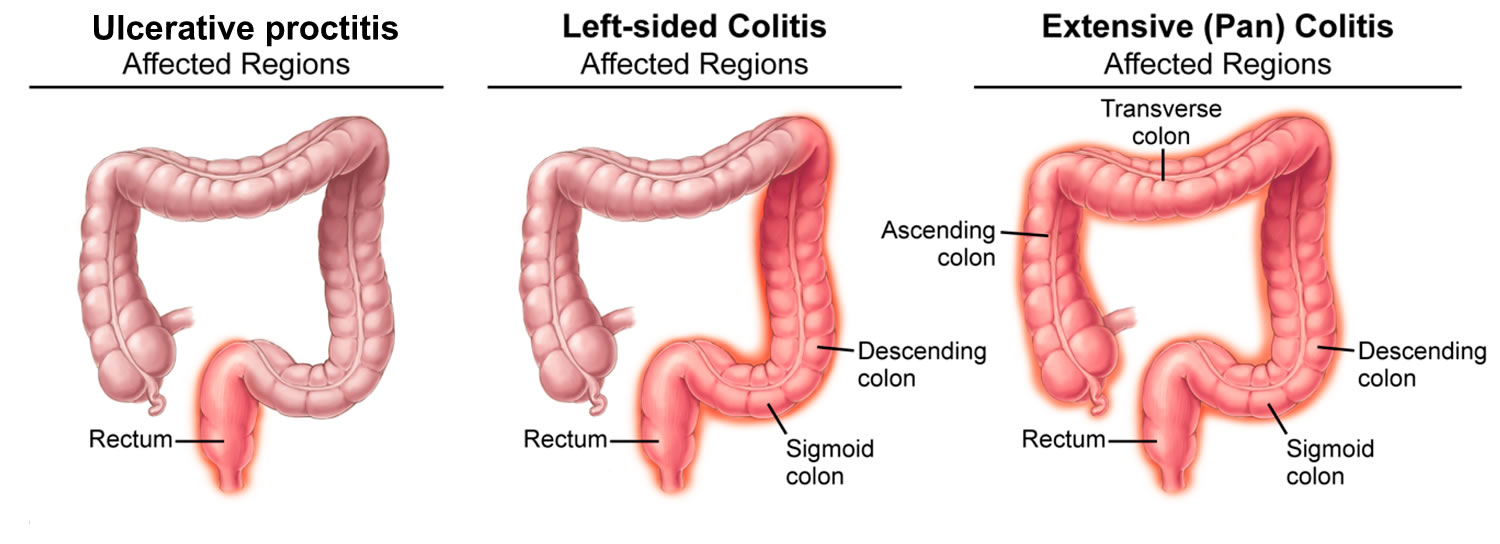
Ulcerative proctitis
Ulcerative proctitis is a form of ulcerative colitis (a type of inflammatory bowel disease [IBD]) where the inflammation and ulcers is limited to the rectum (see Table 1 below) 1, 2. Ulcerative proctitis typically affects less than six inches of the rectum, and it is not associated with an increased risk of cancer 3. American Gastroenterological Association (AGA) Institute Guidelines defined proctitis as an in inflammation limited to the rectum or less than 15–20 cm from anus. Although it is generally assumed that ulcerative proctitis represents the benign end of the spectrum of ulcerative colitis, it is responsible for many distressing symptoms. Patients with ulcerative proctitis have rectal bleeding, rectal pain, urgency in your bowel movements, and tenesmus (feeling that you need to pass stools, even though your bowels are already empty), and endoscopy typically shows diffuse inflammation of the rectum only 4, 5, 6, 7.
Ulcerative colitis is a chronic inflammatory disease of the large intestine (colon) and rectum. Ulcerative colitis is a chronic disease in which abnormal reactions of the immune system cause inflammation and ulcers on the inner lining of your large intestine. In population-based studies, 25%–55% of patients had ulcerative proctitis at diagnosis 7. The Montreal classification divides the distribution of ulcerative colitis into ulcerative proctitis (E1: limited to the rectum), left-sided colitis (E2: up to the splenic flexure) and extensive colitis (E3: beyond splenic flexure) 8.
Table 1. Montreal classification of extent of ulcerative colitis (UC)
| Extent | Anatomy | |
|---|---|---|
| E1 | Ulcerative proctitis | Involvement limited to the rectum (that is, proximal extent of inflammation is distal to the rectosigmoid junction) |
| E2 | Left sided ulcerative colitis (distal ulcerative colitis) | Involvement limited to a proportion of the colorectum distal to the splenic flexure |
| E3 | Extensive ulcerative colitis (pancolitis) | Involvement extends proximal to the splenic flexure |
A study found that 28 percent of ulcerative proctitis cases had proximal extension, which proceeded to left-sided or pancolitis during five years 9, 7, 10, 11, 12. Therefore effective and timely management of patients with ulcerative proctitis is important not only to control symptoms and improve quality of life, but also potentially to delay or prevent proximal extension of inflammation 12, 13, 14, 15, 16.
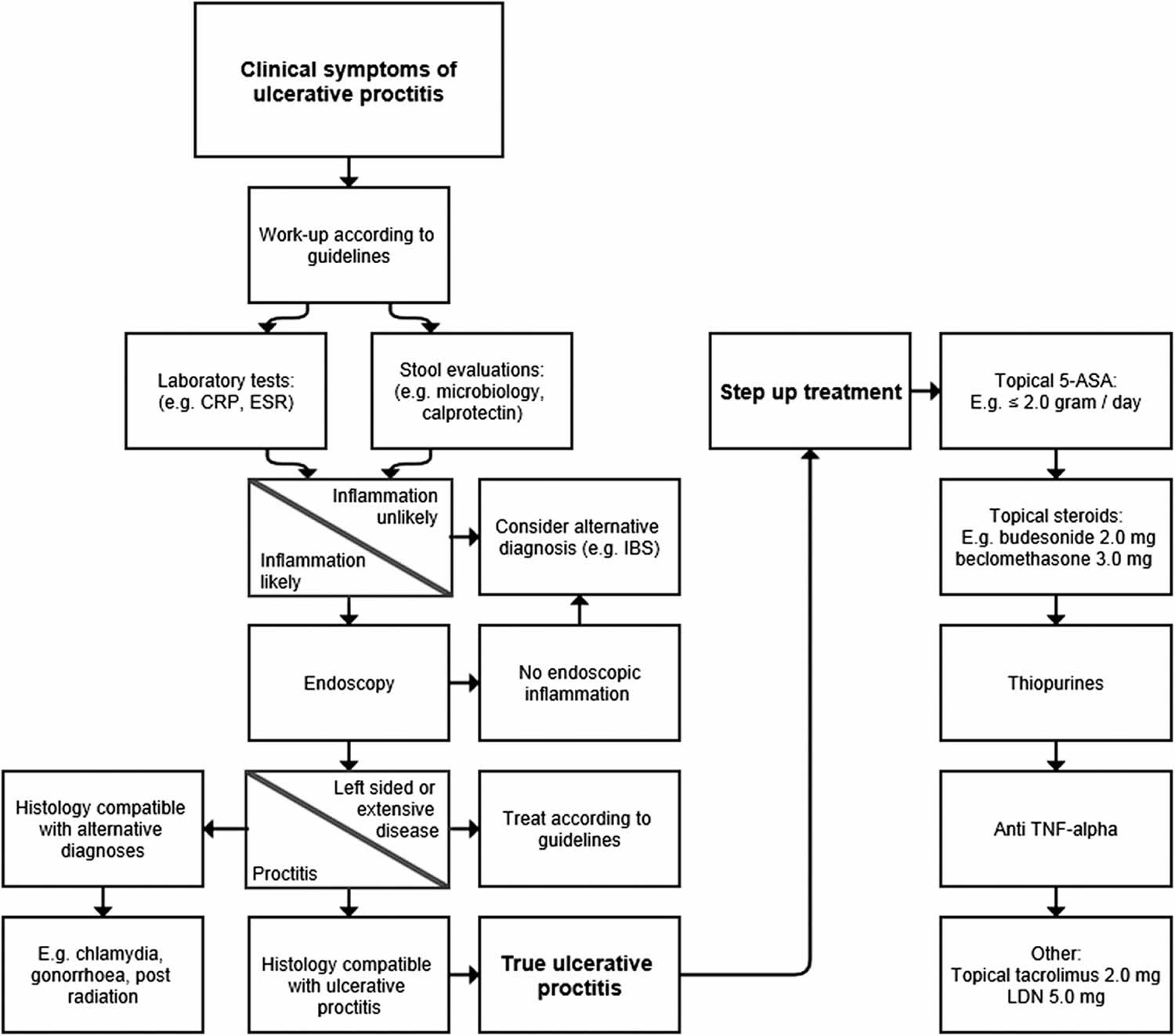
First-line treatment for mild or moderately active ulcerative proctitis is rectal therapy with 5-aminosalicylic acid (5-ASA) 12, 17, 18, 19, 20. Topical 5-aminosalicylic acid (5-ASA) is more effective than topical steroids and oral 5-ASA 17, 18. When there is insufficient response, the next step is to combine topical 5-ASA with topical corticosteroids and/or with oral 5-ASA 21.
In patients who fail to improve despite combination therapy, oral corticosteroids are often necessary 14, 17. Refractory ulcerative proctitis is defined as active ulcerative proctitis which fails rectal and oral therapy with 5-ASA and corticosteroids 14. Treatment of refractory ulcerative proctitis remains challenging because these patients are systematically excluded from randomized controlled trials with drugs with new modes of action 14. In the absence of controlled data, recommendations for the management of ulcerative proctitis are therefore often extrapolated from data in more extended ulcerative colitis or from small real-world evidence 14. Refractory ulcerative proctitis may require treatment with intravenous steroids, immunomodulators or biologicals 18. A small retrospective multicentre study assessed short- and long-term outcomes of refractory ulcerative proctitis treated with azathioprine. Only 20% had treatment success at last follow-up, with a median follow-up of 46 months 22. Another retrospective multicenter study analysed the efficacy of anti-tumour necrosis factor (anti-TNF) therapy in patients with refractory ulcerative proctitis 23. After a median follow-up of 24 months, 64% (67/104) of patients were in clinical remission 23. There are no data with other biologicals or small molecules in ulcerative proctitis. A small randomized, placebo controlled, double-blinded trial showed that topical tacrolimus was more effective than placebo for achievement of clinical remission and mucosal healing in patients with ulcerative proctitis 24.
Figure 1. Ulcerative proctitis
[Source 20 ]The anus
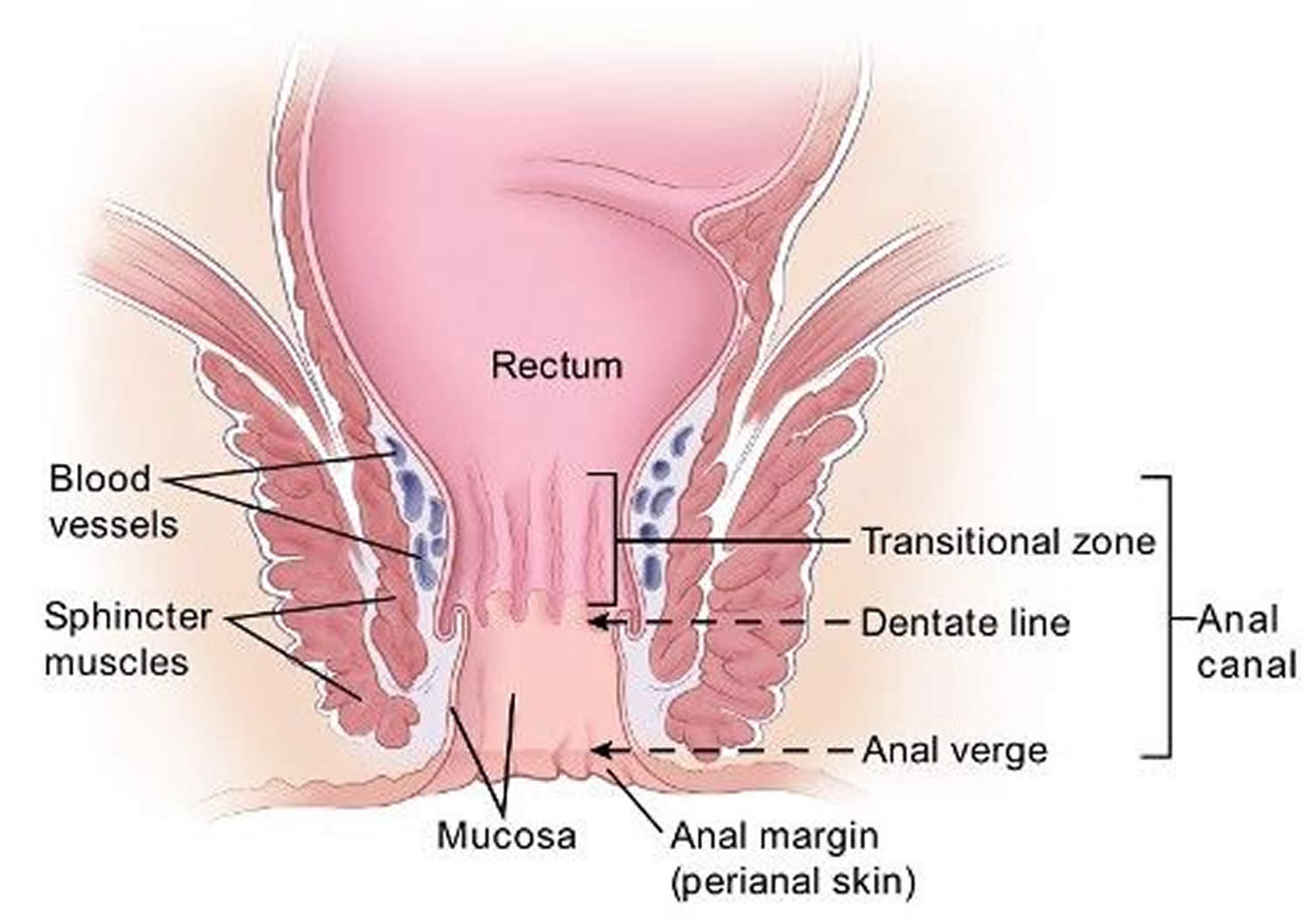
The anus is connected to the rectum by the anal canal. The anus is the continuation of the large intestine (the colon or large bowel) inferior to the rectum. It’s where the end of the intestines connect to the outside of the body.
The anal canal is about 1-1/2 inches (about 3 to 5 cm) long, it begins where the rectum passes through the levator ani (the muscle that forms the pelvic floor) and goes to the anal verge. The anal verge is where the anal canal connects to the outside skin at the anus. This skin around the anal verge is called the perianal skin (previously called the anal margin). The anal canal has two ring-shaped muscles (called sphincter muscles – an internal and external anal sphincter) that keep the anus closed and prevent stool from leaking out.
As food is digested, it passes from the stomach to the small intestine. It then moves from the small intestine into the main part of the large intestine (called the colon). The colon absorbs water and salt from the digested food. The waste matter that’s left after going through the colon is known as feces or stool. Stool is stored in the last part of the large intestine, called the rectum. From there, stool is passed out of the body through the anus as a bowel movement.
The inner lining of the anal canal is the mucosa. Most anal cancers start from cells in the mucosa. Glands and ducts (tubes leading from the glands) are found under the mucosa. The glands make mucus, which acts as a lubricating fluid. Anal cancers that start from cells in the glands are called adenocarcinomas.
The anal canal changes as it goes from the rectum to the anal verge. The parts of the anus include the:
- Cells above the anal canal (in the rectum) and in the part of the anal canal close to the rectum are shaped like tiny columns.
- Most cells near the middle of the anal canal are shaped like cubes and are called transitional cells. This area is called the transitional zone – this is where the rectum meets the anal canal.
- About midway down the anal canal is the dentate line, which is where most of the anal glands empty into the anus.
- Below the dentate line are flat (squamous) cells.
- At the anal verge, the squamous cells of the lower anal canal merge with the skin just outside the anus. This skin around the anal verge called the perianal skin or the anal margin, is also made up of squamous cells, but it also contains sweat glands and hair follicles, which are not found in the lining of the lower anal canal. The anal margin is the lower part of the anal canal and it contains muscles called the anal sphincters. You have an internal and external anal sphincter. They are the muscles that control your bowel movements.
Figure 3. Rectum
Figure 4. Anus anatomy
Ulcerative proctitis causes
The precise cause of ulcerative proctitis is unknown. Current theory holds that genetically susceptible individuals seem to have a dysregulated mucosal immune response to altered commensal gut flora or dysbiosis, resulting in chronic bowel inflammation 26, 2. Patients with different genetic mutations may be predisposed to certain clinical phenotypes of ulcerative colitis 27. For example, Toll-like receptor polymorphisms and human leukocyte antigen alleles have been shown to influence the disease extension in ulcerative colitis 28, 29. The demographics of ulcerative proctitis also mirror those of ulcerative colitis. A family history of inflammatory bowel disease (IBD) may be the most important risk factor for ulcerative colitis, including ulcerative proctitis 30. The risk is particularly high in the first-degree relatives: 5.7% to 15.5% of patients with ulcerative colitis were found to have the first-degree relative with the same disease 31, 32. People of Jewish descent have a rate of ulcerative colitis that is 3 to 5 times higher than non-Jews counterparts 33. Furthermore, monozygotic twins have concordance rates for ulcerative colitis of 6% to 13% 34, 35. Therefore, multiple lines of evidence suggest genetic links, and contributions from environmental factors in the cause of ulcerative proctitis 36.
Genes
Ulcerative colitis sometimes runs in families. Research suggests that certain genes increase the chance that a person will develop ulcerative colitis.
Abnormal immune reactions
Abnormal reactions of the immune system may play a role in causing ulcerative colitis. Abnormal immune reactions lead to inflammation in the large intestine.
Microbiome
The microbes in your digestive tract—including bacteria, viruses, and fungi—that help with digestion are called the microbiome. Studies have found differences between the microbiomes of people who have inflammatory bowel disease (IBD) and those who don’t. Researchers are still studying the relationship between the microbiome and inflammatory bowel disease (IBD).
Environment
Experts think a person’s environment—one’s surroundings and factors outside the body—may play a role in causing ulcerative colitis. Researchers are still studying how people’s environments interact with genes, the immune system, and the microbiome to affect the chance of developing ulcerative colitis.
Ulcerative proctitis symptoms
Ulcerative proctitis signs and symptoms may include:
- A frequent or continuous feeling that you need to have a bowel movement (tenesmus)
- Rectal bleeding
- Passing mucus through your rectum
- Rectal pain
- Pain on the left side of your abdomen
- A feeling of fullness in your rectum
- Diarrhea
- Pain with bowel movements.
Ulcerative proctitis complications
Proctitis that isn’t treated or that doesn’t respond to treatment may lead to complications, including:
- Anemia. Chronic bleeding from your rectum can cause anemia. With anemia, you don’t have enough red blood cells to carry adequate oxygen to your tissues. Anemia causes you to feel tired, and you may also experience dizziness, shortness of breath, headache, pale skin and irritability. Ulcerative colitis may lead to more than one type of anemia, including iron-deficiency anemia and anemia of inflammation or chronic disease.
- Ulcers. Chronic inflammation in the rectum can lead to open sores (ulcers) on the inside lining of the rectum.
- Fistulas. Sometimes ulcers extend completely through the intestinal wall, creating a fistula, an abnormal connection that can occur between different parts of your intestine, between your intestine and skin, or between your intestine and other organs, such as the bladder and vagina.
- Bone problems include low bone mass, such as osteopenia or osteoporosis, because ulcerative proctitis and corticosteroids used to treat the disease can affect the bones.
- Problems with growth and development in children, such as gaining less weight than normal, slowed growth, short stature, or delayed puberty.
Some people with ulcerative colitis also have inflammation in parts of the body other than the large intestine, including:
- joints, causing certain types of arthritis
- skin
- eyes
- liver and bile ducts, causing conditions such as primary sclerosing cholangitis
People with ulcerative colitis also have a higher risk of blood clots in their blood vessels.
Ulcerative proctitis diagnosis
Doctors will ask about your medical history, perform a physical exam, and order tests to diagnose proctitis and find the cause.
Tests and procedures used to diagnose proctitis include:
- Blood tests. These can detect blood loss or infections.
- Stool test. You may be asked to collect a stool sample for testing. A stool test may help determine if your proctitis is caused by a bacterial infection.
- Scope exam of the last portion of your colon (flexible sigmoidoscopy). During this test, your doctor uses a slender, flexible, lighted tube to examine the last part of your colon (sigmoid), as well as the rectum. During the procedure, your doctor can also take small samples of tissue (biopsy) for laboratory analysis.
- Scope exam of your entire colon (colonoscopy). A colonoscopy allows your doctor to view your entire colon using a thin, flexible, lighted tube with an attached camera. Your doctor can also take a biopsy during this test.
- Tests for sexually transmitted infections. These tests involve obtaining a sample of discharge from your rectum or from the tube that drains urine from your bladder (urethra).
Medical history
Your doctor will review your symptoms and ask about your medical history, including:
- current and past medical conditions
- family history of digestive diseases, such as inflammatory bowel disease (IBD)
- history of radiation therapy for cancer
- history of, or risk factors for, sexually transmitted diseases (STDs)
- history of travel to areas where some infections that cause proctitis are more common
- use of medicines, including antibiotics or nonsteroidal anti-inflammatory drugs (NSAIDs)
Physical exam
Your doctor will perform a physical exam, which may include a digital rectal exam, to check for signs of proctitis or other problems in the rectum.
Blood tests
A health care professional will take a blood sample from you and send it to a lab. Blood tests can show signs of infections or other conditions that may cause proctitis.
Stool tests
A doctor will give you a container for catching and storing stool. You will receive instructions on where to send or take the container for analysis. Stool tests may show signs of infections that can cause proctitis.
Rectal cultures
A doctor will use a cotton swab to collect a sample of the bacteria and other microbes inside your rectum. A rectal culture can show signs of infections that cause proctitis.
Endoscopy
A doctor uses an endoscope—a long, flexible, narrow tube with a light and tiny camera on one end—to view the lining of your anus, rectum, and colon. The doctor may pass a tool through the endoscope to take biopsies of the lining of your rectum and colon. A pathologist will examine the biopsied tissue under a microscope.
Common endoscopy procedures that doctors can use to diagnose, find the cause, and check for complications of proctitis include:
- Colonoscopy to view the lining of your rectum and your entire colon
- Flexible sigmoidoscopy to view the lining of your rectum and lower colon
Doctors may also view the inside of your anus or rectum with a shorter, rigid scope, such as a proctoscope or anoscope.
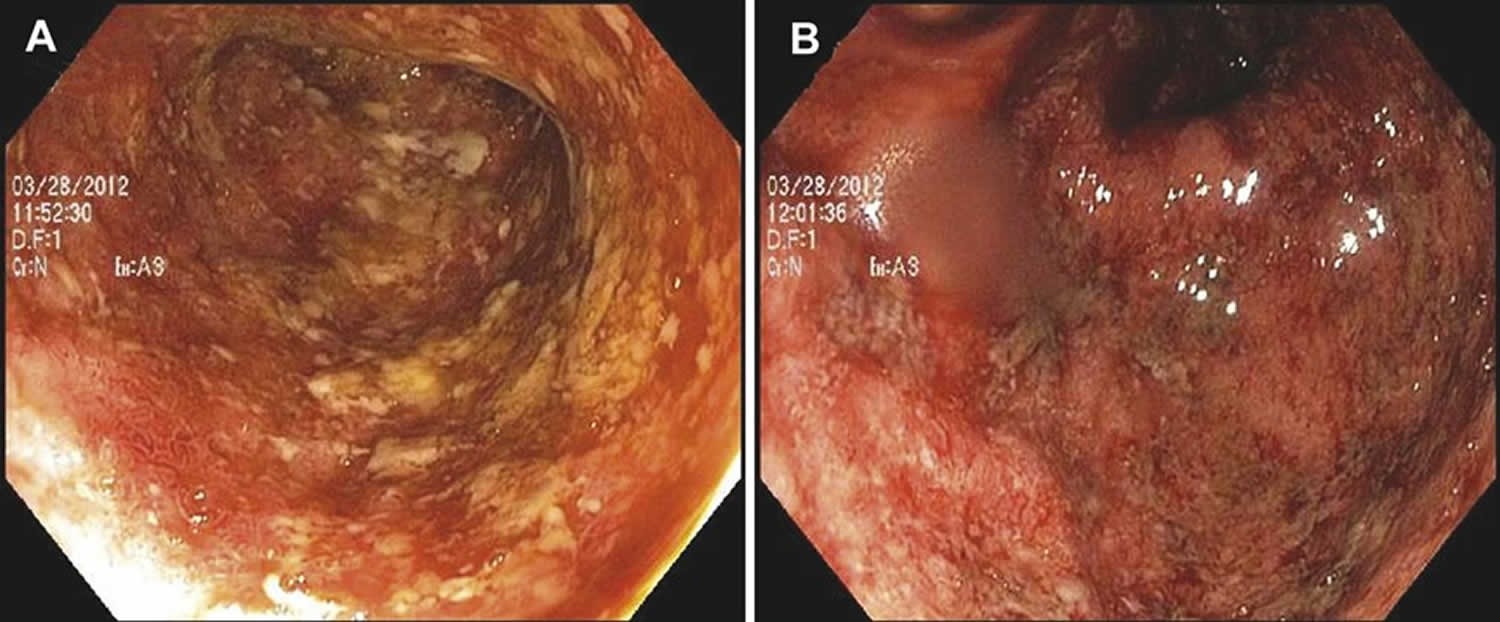
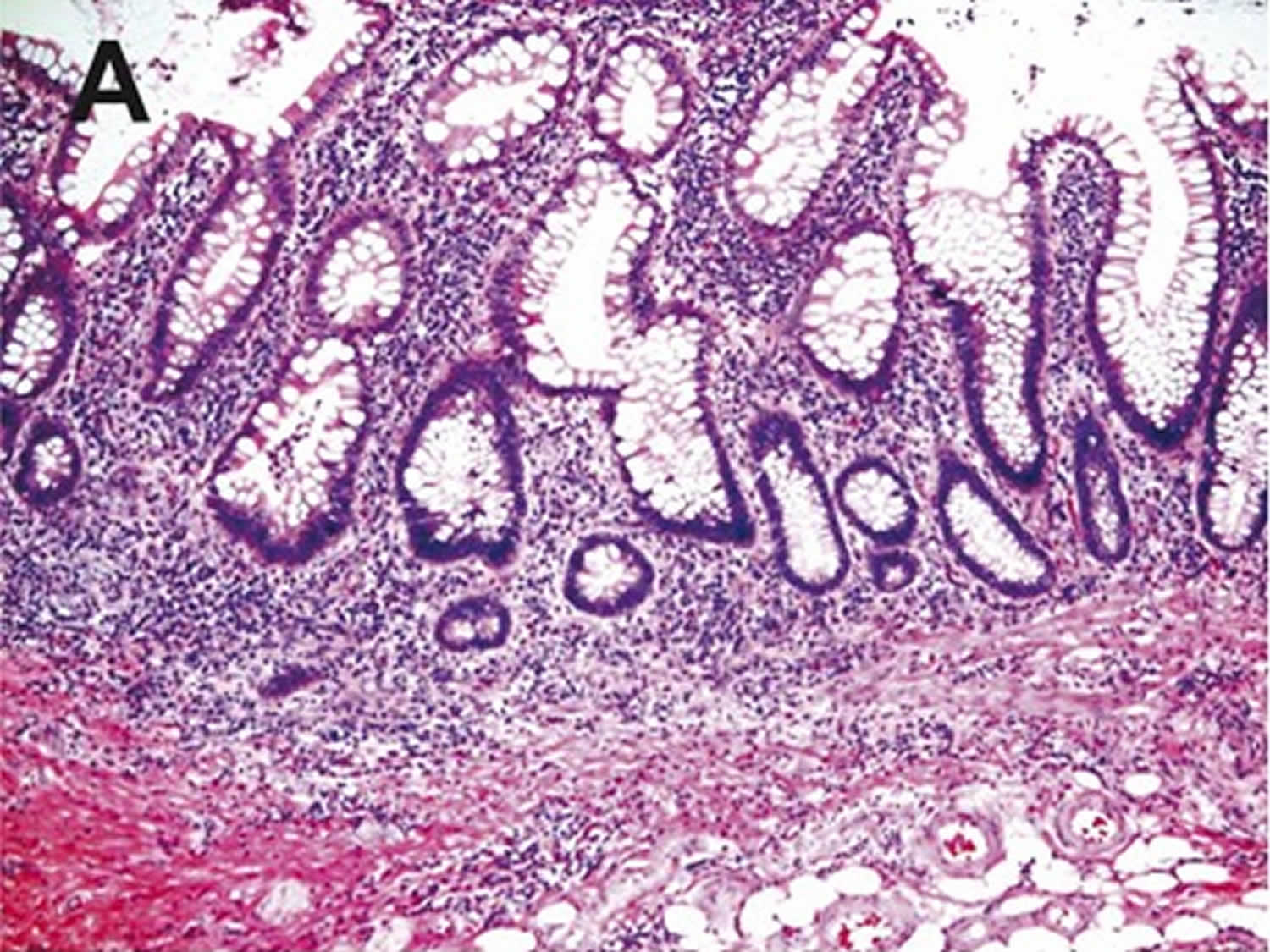
Endoscopic features of ulcerative proctitis include mucosal edema, erythema, friability, granularity, and loss of the typical vascular pattern. Spontaneous hemorrhage, mucopurulent exudates, and ulceration may also occur 37, 38, 39, 40. A sharp demarcation of diseased and nondiseased segments of the distal large bowel can be a specific feature, which helps to differentiate ulcerative proctitis from proctitis caused by other etiologies. On histology, there are epithelial injury (ranging from mucin depletion, cryptitis, crypt abscess, to erosion or ulceration), and features of chronicity, such as chronic inflammation including prominent basal lymphoplasmacytosis, crypt distortion, and Paneth cell metaplasia 41, 42, 43, 44.
Figure 5. Ulcerative proctitis endoscopic features
[Source 4 ]Figure 6. Ulcerative proctitis histology
Footnote: Ulcerative proctitis is characterized by a combination of cryptitis, crypt distortion, Paneth cell metaplasia, and basal lymphoplasmacytosis (H&E stain, ×100).
[Source 4 ]Ulcerative proctitis treatment
Doctors prescribe medicines to treat ulcerative proctitis due to inflammatory bowel disease (IBD)—ulcerative colitis. Medicines may reduce inflammation in the rectum and help bring on and maintain ulcerative proctitis remission—a time when your symptoms disappear.
Medicines to treat ulcerative proctitis include:
- aminosalicylates, also called 5-aminosalicylic acid (5-ASA)
- corticosteroids, also called steroids
- immunosuppressants
- biologics
Ulcerative proctitis is treated with topical mesalazine in the form of suppositories, enemas, foams and gels and in severe cases combined with oral mesalazine with topical steroids or systemic corticosteroids in more severe cases. For patients with mild to moderate ulcerative proctitis, guidelines from American College of Gastroenterology (ACG) and American Gastroenterological Association (AGA) recommend using rectal 5-aminosalicylic acid (5-ASA) mesalamine rather than oral mesalamine 45. 5-aminosalicylic acid (5-ASA) suppositories are more effective than enemas. For induction of remission, the dose is 1 g/day and this is to be continued at the same dose to maintain remission. In cases of intolerance, refractoriness, hypersensitivity to mesalamine suppositories, inability to retain rectal therapy, rectal corticosteroid therapy is suggested for induction of remission rather than no therapy, despite the superiority of rectal 5-ASA over rectal steroids 45. Corticosteroids are not recommended and are not effective in maintenance of remission secondary to side effects and long-term complications. Up to 46% of patients with ulcerative proctitis may develop extensive colitis. This should be especially suspected in patients refractory to topical treatment and follow-up is recommended 19, 46.
In steroid resistant ulcerative proctitis cases, the addition of cyclosporine or immunomodulators; thiopurines as azathioprine (AZA) and 6-mercaptopurine (6-MP) is considered. Other options include anti TNF-α (infliximab, adalimumab, and golimumab), anti-integrin antibodies as vedolizumab and etrolizumab, or oral tacrolimus 47, 48.
Doctors may recommend surgery to treat ulcerative proctitis if medicines don’t work or if you develop complications. To treat ulcerative proctitis, surgeons typically remove the colon and rectum and change how your body stores and passes stool. The most common types of surgery for ulcerative colitis are:
- ileoanal reservoir surgery. Surgeons create an internal reservoir, or pouch, from the end part of the small intestine, called the ileum. Surgeons attach the pouch to the anus. Ileoanal reservoir surgery most often requires two or three operations. After the operations, stool will collect in the internal pouch and pass through the anus during bowel movements.
- ileostomy. Surgeons attach the end of your ileum to an opening in your abdomen called a stoma. After an ileostomy, stool will pass through the stoma. You’ll use an ostomy pouch—a bag attached to the stoma and worn outside the body—to collect stool.
5-Aminosalicylates (5-ASA)
Rectally administered preparations of 5-aminosalicylic acid (5-ASA) should be considered as the first-line treatment for mild-to-moderate ulcerative proctitis 49, 41, 50, 51. Topical treatment offers the advantage of delivering a high dosage of the active compound directly to the site of inflammation, minimizing the systemic absorption of the drug and therefore limiting the frequency of systemic adverse effects. Several delivery forms of rectal 5-aminosalicylic acid (5-ASA) have been tested in clinical trials, including suppositories, enemas, foams, and gel. These differ not only in their chemical properties and dosage but also particularly in their potential proximal coverage 49, 52. Although no specific topical 5-aminosalicylic acid (5-ASA) formulations have demonstrated clinical superiority over the others in inducing remission in ulcerative proctitis, suppositories are generally better tolerated and preferred by patients 53.
The administration of rectal 5-ASA preparations have demonstrated efficacy in the induction and maintenance of remission in the distal colon and rectum. Results from 3 meta-analyses suggest that topical 5-ASA is superior to topical corticosteroids by all measures of remission, clinically, endoscopically, and histologically 54, 55, 56. Because topical 5-ASA drugs exert their therapeutic effects at a mucosal level, it would seem that an important aim of medication delivery would be to provide high topical concentrations of 5-ASA to areas of mucosal inflammation. However, there is no evidence supporting a dose-response effect for rectal 5-ASA therapy. There seem no differences in the efficacy and speed of onset of action between 5-ASA 500 mg enemas varying between 1 and 4 g/day 57, 58, 59.
Oral 5-ASA is also effective in the treatment of active distal colitis 60. Although the oral forms are often preferred for their convenience and compliance by some patients, topical mesalamine has been shown to be superior to oral 5-ASA in achieving clinical improvement in patients with mild-to-moderate distal ulcerative colitis and ulcerative proctitis 56, 61. This may be due to the asymmetric distribution of 5-ASA within the colon that is exaggerated in active left-sided disease resulting in proximal colonic stasis and fast colonic transit through inflamed colon 62. However, combination therapy with rectally and orally delivered 5-ASA may be more effective than either administration route used alone and may be useful in refractory patients, although this may be simply a dose-response effect 57, 63, 64.
Corticosteroids
Patients with ulcerative proctitis who fail the topical and/or oral 5-ASA therapies should be reevaluated to ensure proper diagnosis and to exclude a 5-ASA reaction or concurrent superimposed infections. Inadequate dosing, inappropriate type and duration of treatment, and noncompliance with therapy should also be taken into account 49, 41. After careful evaluation, the use of topical glucocorticoids can be considered, although it has been indicated to be less effective than topical 5-ASA therapy and holds the potential disadvantage of side effects, particularly regarding suppression of the pituitary–adrenal axis 54, 55, 56. New steroids with enhanced topical potency and less systemic activity, such as prednisolone–metasulphobenzoate, beclometasone dipropionate, tixocortol pivalate, fluticasone, and budesonide, may represent an even more valuable alternative 65. They have been shown to be significantly superior to placebo and to have an efficacy similar to systemic corticosteroids 66, 67. Once remission has been achieved, however, there is no evidence supporting that topical steroids are effective in maintaining remission 68.
Oral corticosteroids therapy may be used when patients lose response to or only have a suboptimal response to treatment with 5-ASA compounds and/or to topical steroids. Oral prednisone demonstrates a dose-response effect between 20 and 60 mg/day, with 60 mg/day being modestly more effective than 40 mg/day but at the expense of a greater risk for adverse effects 69, 70, 71. No randomized trials are available to evaluate corticosteroid-tapering schedules, but it is recommended to the use of 40 to 60 mg/day until a substantial clinical improvement has been obtained and then a dose taper of 5 to 10 mg weekly until a daily dose of 20 mg is reached 72.
Steroid-refractory or Steroid-dependent ulcerative proctitis
Patients who develop steroid-refractory or -dependent ulcerative proctitis present a treatment dilemma. There are limited evidence-based data available to guide the treatment of these patients. Immunomodulators (such as azathioprine, 6-mercaptopurine, and cyclosporine) and/or monoclonal antibodies to TNF-α (such as infliximab, adalimumab, and golimumab) or even antibody to integrin (vedolizumab) may be considered in these cases 64, 41, 26, 2. Should these treatments fail, surgical intervention with proctectomy or proctocolectomy with an ileostomy or colostomy may be required. Langholz et al 73 reported that 12% of patients with an initial diagnosis of ulcerative proctitis eventually underwent colectomy. The restoration of intestinal continuity with the construction of ileal pouch–anal anastomosis may remain an option for those whose resected rectum shows no evidence of Crohn’s disease 2.
Ulcerative proctitis diet
If you have ulcerative proctitis, you should eat a healthy, well-balanced diet. Talk with your doctor about a healthy eating plan.
Ulcerative proctitis symptoms may cause some people to lose their appetite and eat less, and they may not get enough nutrients. In children, a lack of nutrients may play a role in problems with growth and development.
Researchers have not found that specific foods cause ulcerative proctitis symptoms, although healthier diets appear to be associated with less risk of developing inflammatory bowel disease (IBD). Researchers have not found that specific foods worsen ulcerative proctitis. Talk with your doctor about any foods that seem to be related to your symptoms. Your doctor may suggest keeping a food diary to help identify foods that seem to make your symptoms worse.
Depending on your symptoms and the medicines you take, your doctor may recommend changes to your diet.
Ulcerative proctitis prognosis
The clinical course of ulcerative proctitis is unpredictable, and most patients experiencing alternating periods of exacerbation and remission 74, 75, 20. Long-term epidemiological studies have revealed that ulcerative proctitis often extends to more proximal colitis and even to total colitis. It was shown that nearly 22% of patients with ulcerative proctitis had disease progression within 12 to 24 months of initial diagnosis despite medical treatment 76. This is in line with other studies, showing proximal extension of ulcerative proctitis in 28% during 5 years of follow-up 77, 78, 10. The recognition of ulcerative proctitis and left-sided ulcerative colitis is important because it has been suggested that effective local treatment may prevent or delay proximal spread of the inflammation 15.
- Du L., Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterology Clinics of North America . 2020;49(4):643–654. doi: 10.1016/j.gtc.2020.07.005[↩]
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012 Nov 3;380(9853):1606-19. doi: 10.1016/S0140-6736(12)60150-0[↩][↩][↩][↩]
- Types of Ulcerative Colitis. https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis/types-of-ulcerative-colitis[↩]
- Xian-rui Wu, Xiu-li Liu, Seymour Katz, Bo Shen, Pathogenesis, Diagnosis, and Management of Ulcerative Proctitis, Chronic Radiation Proctopathy, and Diversion Proctitis, Inflammatory Bowel Diseases, Volume 21, Issue 3, 1 March 2015, Pages 703–715, https://doi.org/10.1097/MIB.0000000000000227[↩][↩][↩]
- Gecse KB, Lakatos PL. Ulcerative proctitis: an update on the pharmacotherapy and management. Expert Opin Pharmacother. 2014 Aug;15(11):1565-73. https://core.ac.uk/reader/50566389[↩]
- Caron B., Sandborn W. J., Schreiber S., Panaccione R., Danese S., Peyrin-Biroulet L. Drug development for ulcerative proctitis: current concepts. Gut . 2021;70(7):1203–1209. https://gut.bmj.com/content/70/7/1203.long[↩]
- Meucci G, Vecchi M, Astegiano M, Beretta L, Cesari P, Dizioli P, Ferraris L, Panelli MR, Prada A, Sostegni R, de Franchis R. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII). Am J Gastroenterol. 2000 Feb;95(2):469-73. doi: 10.1111/j.1572-0241.2000.t01-1-01770.x[↩][↩][↩]
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006 Jun;55(6):749-53. doi: 10.1136/gut.2005.082909[↩][↩]
- Ungar B., Kopylov U. Long-term outcome of ulcerative proctitis. United European Gastroenterology Journal . 2020;8(8):847–848. doi: 10.1177/2050640620948795[↩]
- Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, Langholz E, Thomsen OØ, Munkholm P. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007 Apr;13(4):481-9. doi: 10.1002/ibd.20036[↩][↩]
- Kim B, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Proximal disease extension and related predicting factors in ulcerative proctitis. Scand J Gastroenterol. 2014 Feb;49(2):177-83. doi: 10.3109/00365521.2013.867360[↩]
- Lie MR, Kanis SL, Hansen BE, van der Woude CJ. Drug therapies for ulcerative proctitis: systematic review and meta-analysis. Inflamm Bowel Dis. 2014 Nov;20(11):2157-78. doi: 10.1097/MIB.0000000000000141[↩][↩][↩]
- Gecse KB, Lakatos PL. Ulcerative proctitis: an update on the pharmacotherapy and management. Expert Opin Pharmacother. 2014 Aug;15(11):1565-73. doi: 10.1517/14656566.2014.920322[↩]
- Pineton de Chambrun G, Tassy B, Kollen L, Dufour G, Valats JC, Bismuth M, Funakoshi N, Panaro F, Blanc P. The treatment of refractory ulcerative colitis. Best Pract Res Clin Gastroenterol. 2018 Feb-Apr;32-33:49-57. doi: 10.1016/j.bpg.2018.05.009[↩][↩][↩][↩][↩]
- Lindgren S, Löfberg R, Bergholm L, Hellblom M, Carling L, Ung KA, Schiöler R, Unge P, Wallin C, Ström M, Persson T, Suhr OB. Effect of budesonide enema on remission and relapse rate in distal ulcerative colitis and proctitis. Scand J Gastroenterol. 2002 Jun;37(6):705-10. doi: 10.1080/00365520212512[↩][↩]
- Pica R, Paoluzi OA, Iacopini F, Marcheggiano A, Crispino P, Rivera M, Bella A, Consolazio A, Paoluzi P. Oral mesalazine (5-ASA) treatment may protect against proximal extension of mucosal inflammation in ulcerative proctitis. Inflamm Bowel Dis. 2004 Nov;10(6):731-6. doi: 10.1097/00054725-200411000-00006[↩]
- Calafat M, Lobatón T, Mañosa M, Marín L, Caballero N, Larraín M, Cabré E, Domènech E. Therapeutic requirements in active ulcerative proctitis: A single-centre study. Gastroenterol Hepatol. 2017 Dec;40(10):663-668. English, Spanish. doi: 10.1016/j.gastrohep.2017.05.006[↩][↩][↩]
- Marcus Harbord, Rami Eliakim, Dominik Bettenworth, Konstantinos Karmiris, Konstantinos Katsanos, Uri Kopylov, Torsten Kucharzik, Tamás Molnár, Tim Raine, Shaji Sebastian, Helena Tavares de Sousa, Axel Dignass, Franck Carbonnel, for the European Crohn’s and Colitis Organisation [ECCO], Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management, Journal of Crohn’s and Colitis, Volume 11, Issue 7, July 2017, Pages 769–784, https://doi.org/10.1093/ecco-jcc/jjx009[↩][↩][↩]
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019 Mar;114(3):384-413. doi: 10.14309/ajg.0000000000000152[↩][↩]
- Mitchell R. K. L. Lie, Shannon L. Kanis, Bettina E. Hansen, C. Janneke van der Woude, Drug Therapies for Ulcerative Proctitis: Systematic Review and Meta-analysis, Inflammatory Bowel Diseases, Volume 20, Issue 11, 1 November 2014, Pages 2157–2178, https://doi.org/10.1097/MIB.0000000000000141[↩][↩][↩]
- Dubois E, Moens A, Geelen R, Sabino J, Ferrante M, Vermeire S. Long-term outcomes of patients with ulcerative proctitis: Analysis from a large referral centre cohort. United European Gastroenterol J. 2020 Oct;8(8):933-941. doi: 10.1177/2050640620941345[↩]
- Mallet AL, Bouguen G, Conroy G, Roblin X, Delobel JB, Bretagne JF, Siproudhis L, Peyrin-Biroulet L. Azathioprine for refractory ulcerative proctitis: A retrospective multicenter study. Dig Liver Dis. 2017 Mar;49(3):280-285. doi: 10.1016/j.dld.2016.12.001[↩]
- Pineton de Chambrun G, Amiot A, Bouguen G, Viennot S, Altwegg R, Louis E, Collins M, Fumery M, Poullenot F, Armengol L, Buisson A, Abitbol V, Laharie D, Seksik P, Nancey S, Blanc P, Bouhnik Y, Pariente B, Peyrin-Biroulet L; PROTECT-GETAID study group. Efficacy of Tumor Necrosis Factor Antagonist Treatment in Patients With Refractory Ulcerative Proctitis. Clin Gastroenterol Hepatol. 2020 Mar;18(3):620-627.e1. https://doi.org/10.1016/j.cgh.2019.05.060[↩][↩]
- Lawrance IC, Baird A, Lightower D, Radford-Smith G, Andrews JM, Connor S. Efficacy of Rectal Tacrolimus for Induction Therapy in Patients With Resistant Ulcerative Proctitis. Clin Gastroenterol Hepatol. 2017 Aug;15(8):1248-1255. doi: 10.1016/j.cgh.2017.02.027[↩]
- Kayal M, Shah S. Ulcerative Colitis: Current and Emerging Treatment Strategies. J Clin Med. 2019 Dec 30;9(1):94. doi: 10.3390/jcm9010094[↩]
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009 Nov 19;361(21):2066-78. doi: 10.1056/NEJMra0804647[↩][↩]
- Thompson AI, Lees CW. Genetics of ulcerative colitis. Inflamm Bowel Dis. 2011 Mar;17(3):831-48. doi: 10.1002/ibd.21375[↩]
- Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006 Jan;12(1):1-8. doi: 10.1097/01.mib.0000195389.11645.ab[↩]
- Matsumura Y, Kinouchi Y, Nomura E, Negoro K, Kakuta Y, Endo K, Aizawa H, Takagi S, Takahashi S, Shimosegawa T. HLA-DRB1 alleles influence clinical phenotypes in Japanese patients with ulcerative colitis. Tissue Antigens. 2008 May;71(5):447-52. doi: 10.1111/j.1399-0039.2008.01031.x[↩]
- Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991 Jan 10;324(2):84-8. doi: 10.1056/NEJM199101103240203[↩]
- Monsén U, Broström O, Nordenvall B, Sörstad J, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with ulcerative colitis. Scand J Gastroenterol. 1987 Mar;22(2):214-8. doi: 10.3109/00365528708991882[↩]
- Farmer RG. Study of family history among patients with inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:64-5; discussion 66-8. doi: 10.3109/00365528909091355[↩]
- Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut. 1993 Apr;34(4):517-24. doi: 10.1136/gut.34.4.517[↩]
- Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988 Jul;29(7):990-6. doi: 10.1136/gut.29.7.990[↩]
- Orholm M, Binder V, Sørensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol. 2000 Oct;35(10):1075-81. doi: 10.1080/003655200451207[↩]
- Birkenfeld S, Zvidi I, Hazazi R, Niv Y. The prevalence of ulcerative colitis in Israel: a twenty-year survey. J Clin Gastroenterol. 2009 Sep;43(8):743-6. doi: 10.1097/MCG.0b013e31818b3a02[↩]
- Simpson P, Papadakis KA. Endoscopic evaluation of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008 Sep;14(9):1287-97. doi: 10.1002/ibd.20398[↩]
- Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D, Qureshi WA, Rajan E, Zuckerman MJ, VanGuilder T, Fanelli RD; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006 Apr;63(4):558-65. doi: 10.1016/j.gie.2006.02.005[↩]
- Fefferman DS, Farrell RJ. Endoscopy in inflammatory bowel disease: indications, surveillance, and use in clinical practice. Clin Gastroenterol Hepatol. 2005 Jan;3(1):11-24. doi: 10.1016/s1542-3565(04)00441-0[↩]
- Chen JC, Lin TM, Chen YL, Wang YH, Jin YT, Yue CT. RHD 1227A is an important genetic marker for RhD(el) individuals. Am J Clin Pathol. 2004 Aug;122(2):193-8. doi: 10.1309/3XMF-2NV5-707T-JE7X[↩]
- Regueiro MD. Diagnosis and treatment of ulcerative proctitis. J Clin Gastroenterol. 2004 Oct;38(9):733-40. doi: 10.1097/01.mcg.0000139178.33502.a3[↩][↩][↩][↩]
- Jenkins D, Balsitis M, Gallivan S, Dixon MF, Gilmour HM, Shepherd NA, Theodossi A, Williams GT. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol. 1997 Feb;50(2):93-105. doi: 10.1136/jcp.50.2.93[↩]
- Nostrant TT, Kumar NB, Appelman HD. Histopathology differentiates acute self-limited colitis from ulcerative colitis. Gastroenterology. 1987 Feb;92(2):318-28. doi: 10.1016/0016-5085(87)90124-7[↩]
- Dundas SA, Dutton J, Skipworth P. Reliability of rectal biopsy in distinguishing between chronic inflammatory bowel disease and acute self-limiting colitis. Histopathology. 1997 Jul;31(1):60-6. doi: 10.1046/j.1365-2559.1997.5810818.x[↩]
- Meseeha M, Attia M. Proctitis and Anusitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430892[↩][↩]
- Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology. 2019 Feb;156(3):748-764. doi: 10.1053/j.gastro.2018.12.009[↩]
- Kent A, Keshav S. Managing intractable proctitis and the problematic pouch. Dig Dis. 2014;32(4):427-37. doi: 10.1159/000358149[↩]
- Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, Koyanagi K, Hibi T. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012 May;18(5):803-8. doi: 10.1002/ibd.21853[↩]
- Gionchetti P, Rizzello F, Morselli C, Campieri M. Review article: problematic proctitis and distal colitis. Aliment Pharmacol Ther. 2004 Oct;20 Suppl 4:93-6. doi: 10.1111/j.1365-2036.2004.02049.x[↩][↩][↩]
- Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010 Mar;105(3):501-23; quiz 524. doi: 10.1038/ajg.2009.727. Epub 2010 Jan 12. Erratum in: Am J Gastroenterol. 2010 Mar;105(3):500.[↩]
- Lawrance IC. Topical agents for idiopathic distal colitis and proctitis. J Gastroenterol Hepatol. 2011 Jan;26(1):36-43. doi: 10.1111/j.1440-1746.2010.06497.x[↩]
- Campieri M, Paoluzi P, D’Albasio G, Brunetti G, Pera A, Barbara L. Better quality of therapy with 5-ASA colonic foam in active ulcerative colitis. A multicenter comparative trial with 5-ASA enema. Dig Dis Sci. 1993 Oct;38(10):1843-50. doi: 10.1007/BF01296108[↩]
- Gionchetti P, Ardizzone S, Benvenuti ME, Bianchi Porro G, Biasco G, Cesari P, D’albasio G, De Franchis R, Monteleone G, Pallone F, Ranzi T, Trallori G, Valpiani D, Vecchi M, Campieri M. A new mesalazine gel enema in the treatment of left-sided ulcerative colitis: a randomized controlled multicentre trial. Aliment Pharmacol Ther. 1999 Mar;13(3):381-8. doi: 10.1046/j.1365-2036.1999.00482.x[↩]
- Marshall JK, Irvine EJ. Rectal aminosalicylate therapy for distal ulcerative colitis: a meta-analysis. Aliment Pharmacol Ther. 1995 Jun;9(3):293-300. doi: 10.1111/j.1365-2036.1995.tb00384.x[↩][↩]
- Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis. Gut. 1997 Jun;40(6):775-81. doi: 10.1136/gut.40.6.775[↩][↩]
- Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am J Gastroenterol. 2000 May;95(5):1263-76. doi: 10.1111/j.1572-0241.2000.01940.x[↩][↩][↩]
- Marshall JK, Irvine EJ. Putting rectal 5-aminosalicylic acid in its place: the role in distal ulcerative colitis. Am J Gastroenterol. 2000 Jul;95(7):1628-36. doi: 10.1111/j.1572-0241.2000.02180.x[↩][↩]
- Campieri M, De Franchis R, Bianchi Porro G, Ranzi T, Brunetti G, Barbara L. Mesalazine (5-aminosalicylic acid) suppositories in the treatment of ulcerative proctitis or distal proctosigmoiditis. A randomized controlled trial. Scand J Gastroenterol. 1990 Jul;25(7):663-8. doi: 10.3109/00365529008997590[↩]
- D’Arienzo A, Panarese A, D’Armiento FP, Lancia C, Quattrone P, Giannattasio F, Boscaino A, Mazzacca G. 5-Aminosalicylic acid suppositories in the maintenance of remission in idiopathic proctitis or proctosigmoiditis: a double-blind placebo-controlled clinical trial. Am J Gastroenterol. 1990 Sep;85(9):1079-82.[↩]
- Sutherland LR, May GR, Shaffer EA. Sulfasalazine revisited: a meta-analysis of 5-aminosalicylic acid in the treatment of ulcerative colitis. Ann Intern Med. 1993 Apr 1;118(7):540-9. doi: 10.7326/0003-4819-118-7-199304010-00009[↩]
- Gionchetti P, Rizzello F, Venturi A, Ferretti M, Brignola C, Miglioli M, Campieri M. Comparison of oral with rectal mesalazine in the treatment of ulcerative proctitis. Dis Colon Rectum. 1998 Jan;41(1):93-7. doi: 10.1007/BF02236902[↩]
- Hebden JM, Blackshaw PE, Perkins AC, Wilson CG, Spiller RC. Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2000 Feb;14(2):155-61. doi: 10.1046/j.1365-2036.2000.00697.x[↩]
- Safdi M, DeMicco M, Sninsky C, Banks P, Wruble L, Deren J, Koval G, Nichols T, Targan S, Fleishman C, Wiita B. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997 Oct;92(10):1867-71.[↩]
- Regueiro M, Loftus EV Jr, Steinhart AH, Cohen RD. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006 Oct;12(10):979-94. doi: 10.1097/01.mib.0000231495.92013.5e[↩][↩]
- Thiesen A, Thomson AB. Review article: older systemic and newer topical glucocorticosteroids and the gastrointestinal tract. Aliment Pharmacol Ther. 1996 Aug;10(4):487-96. doi: 10.1046/j.1365-2036.1996.41183000.x[↩]
- Löfberg R, Ostergaard Thomsen O, Langholz E, Schiöler R, Danielsson A, Suhr O, Graffner H, Påhlman L, Matzen P, Møller-Petersen JF, et al. Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1994 Dec;8(6):623-9. doi: 10.1111/j.1365-2036.1994.tb00340.x. Erratum in: Aliment Pharmacol Ther 1995 Apr;9(2):213.[↩]
- Campieri M, Cottone M, Miglio F, Manenti F, Astegiano M, D’Arienzo A, Manguso F, D’Albasio G, Bonanomi A, Galeazzi R, Orlando A, Castiglione GN, Gionchetti P. Beclomethasone dipropionate enemas versus prednisolone sodium phosphate enemas in the treatment of distal ulcerative colitis. Aliment Pharmacol Ther. 1998 Apr;12(4):361-6. doi: 10.1046/j.1365-2036.1998.00299.x[↩]
- d’Albasio G, Pacini F, Camarri E, Messori A, Trallori G, Bonanomi AG, Bardazzi G, Milla M, Ferrero S, Biagini M, Quaranta S, Amorosi A. Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized double-blind study. Am J Gastroenterol. 1997 Jul;92(7):1143-7.[↩]
- LENNARD-JONES JE, LONGMORE AJ, NEWELL AC, WILSON CW, JONES FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut. 1960 Sep;1(3):217-22. doi: 10.1136/gut.1.3.217[↩]
- BARON JH, CONNELL AM, KANAGHINIS TG, LENNARD-JONES JE, JONES AF. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br Med J. 1962 Aug 18;2(5302):441-3. doi: 10.1136/bmj.2.5302.441[↩]
- TRUELOVE SC, WITTS LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041-8. doi: 10.1136/bmj.2.4947.1041[↩]
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006 Mar;130(3):940-87. doi: 10.1053/j.gastro.2006.01.048[↩]
- Langholz E, Munkholm P, Davidsen M, Nielsen OH, Binder V. Changes in extent of ulcerative colitis: a study on the course and prognostic factors. Scand J Gastroenterol. 1996 Mar;31(3):260-6. doi: 10.3109/00365529609004876[↩]
- Kornbluth AA, Salomon P, Sacks HS, Mitty R, Janowitz HD. Meta-analysis of the effectiveness of current drug therapy of ulcerative colitis. J Clin Gastroenterol. 1993 Apr;16(3):215-8. doi: 10.1097/00004836-199304000-00010[↩]
- Meyers S, Janowitz HD. The “natural history” of ulcerative colitis: an analysis of the placebo response. J Clin Gastroenterol. 1989 Feb;11(1):33-7. doi: 10.1097/00004836-198902000-00008[↩]
- Kruis W, Brandes JW, Schreiber S, Theuer D, Krakamp B, Schütz E, Otto P, Lorenz-Mayer H, Ewe K, Judmaier G. Olsalazine versus mesalazine in the treatment of mild to moderate ulcerative colitis. Aliment Pharmacol Ther. 1998 Aug;12(8):707-15. doi: 10.1046/j.1365-2036.1998.00360.x[↩]
- Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987 Jun;92(6):1894-8. doi: 10.1016/0016-5085(87)90621-4[↩]
- Prantera C, Viscido A, Biancone L, Francavilla A, Giglio L, Campieri M. A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis. 2005 May;11(5):421-7. doi: 10.1097/01.mib.0000158386.25660.1e[↩]