Contents
What is urethral stricture
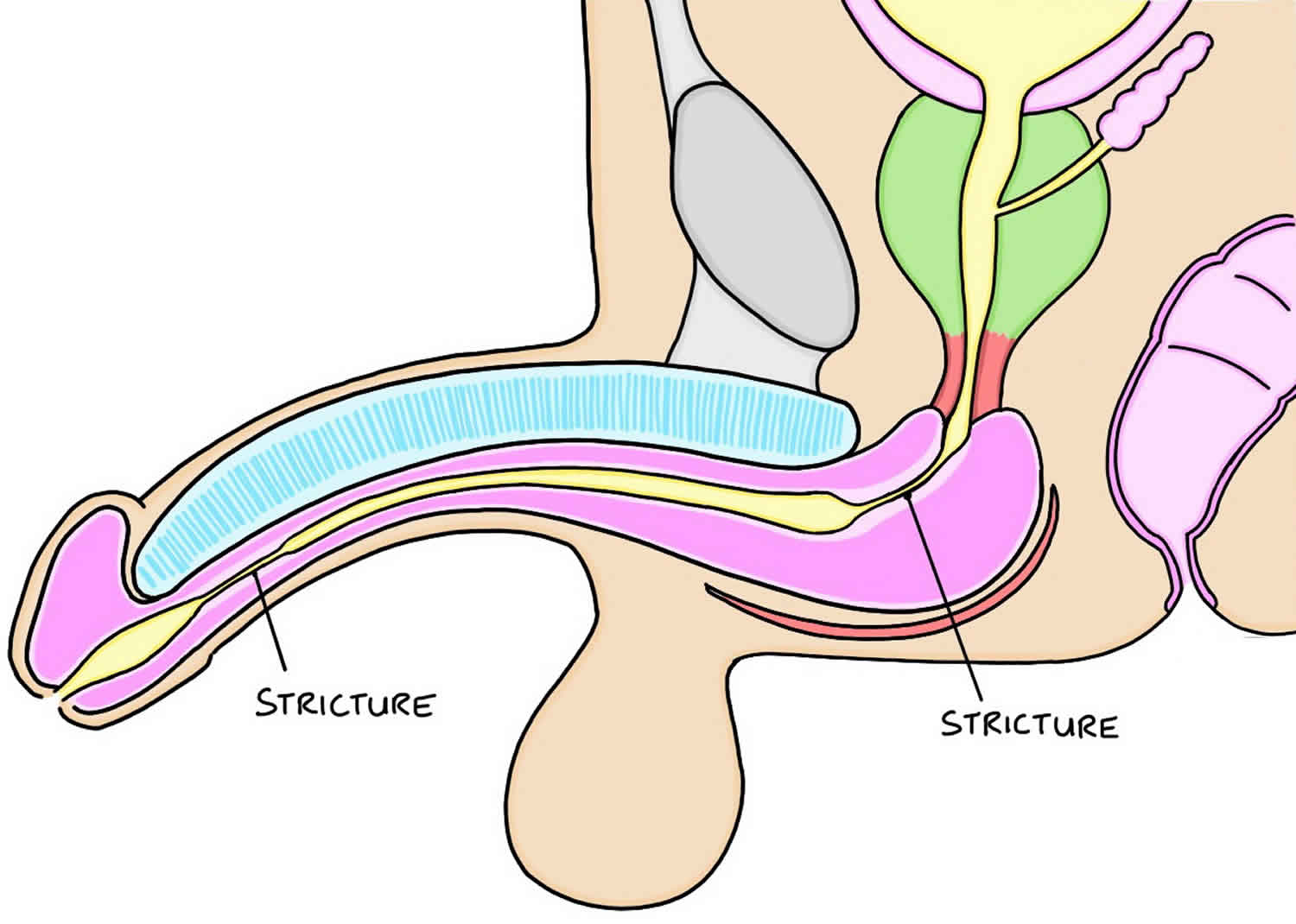
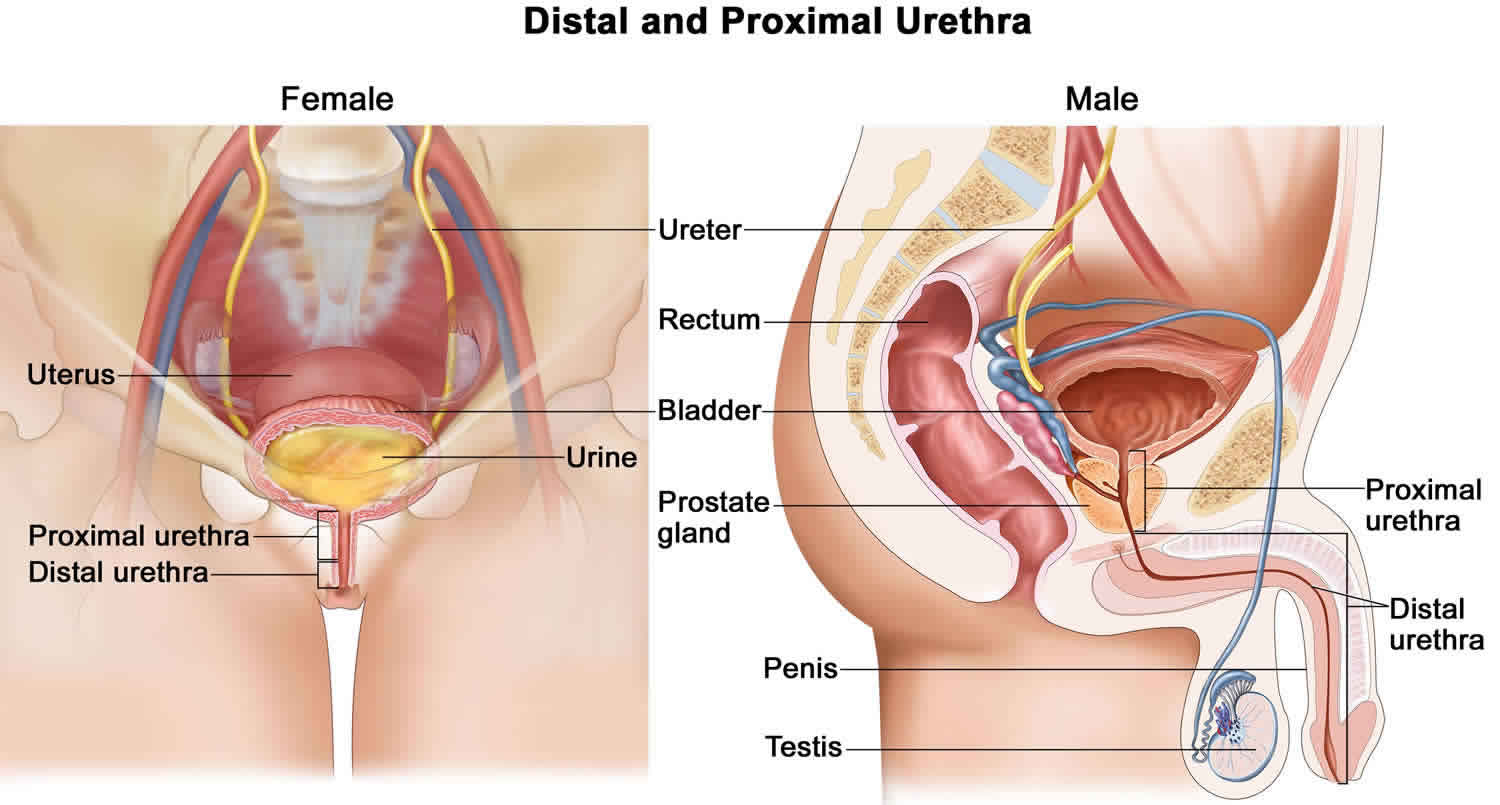
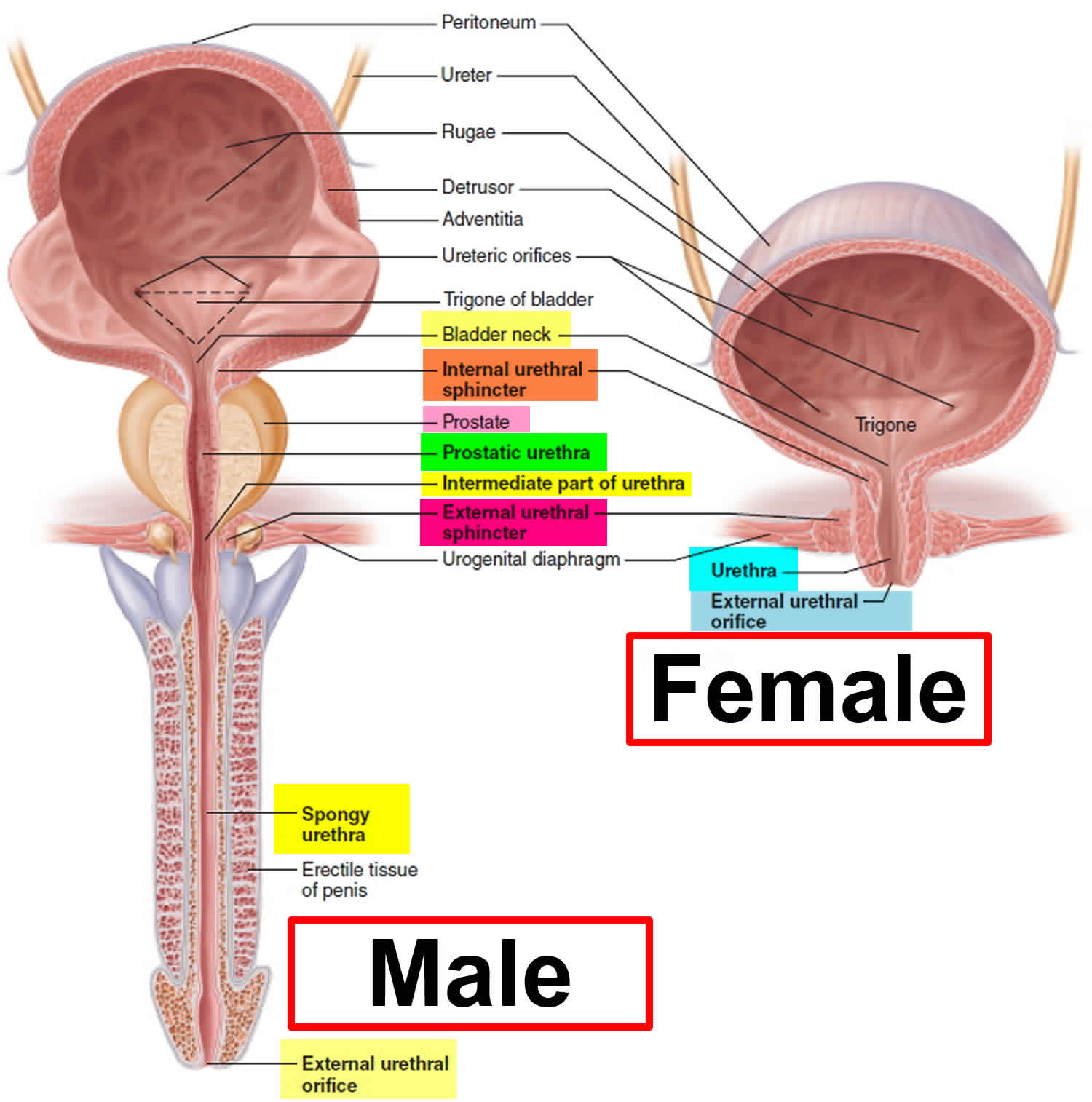
Urethral stricture is an abnormal narrowing of the urethra. Urethra is the tube that carries urine out of the body from the bladder to the external environment. No formal studies of urethral stricture disease incidence have been published. Urethral stricture occur predominantly in males for the male urethra is 6-7 times longer than the female urethra (see Figure 1), allowing both infection and surgical damage to occur more frequently.
Urethral strictures that are present at birth (congenital) are rare. Urethral stricture is also rare in women.
Urethral strictures can be divided into two main types, anterior and posterior, which differ not only in their location, but also in their underlying pathogenesis 1. In a retrospective analysis of all urethral strictures that had been reconstructed at a single institution, the vast majority of urethral strictures were anterior (92.2%), with most of these occurring in the bulbar urethra (46.9%), followed by penile (30.5%), penile and bulbar (9.9%), and panurethral (4.9%) strictures 2.
Current opinion holds that urethral stricture does not occur over the short term, but is a progressive condition caused by urine leaking into tissues around the urethra. The initial damage is done through trauma and/or infection, which leads to permanent changes of the urethral lining, and long-term leakage of urine into the tissues around the urethra. The urethral stricture will progressively become more severe, and partially or sometimes totally obstruct the passage of urine to the external environment. These changes may lead to complications of urinary incontinence and kidney problems if left untreated.
Figure 1. Male and female urethra anatomy
Urethral stricture causes
Urethral stricture may be caused by swelling or scar tissue from surgery. Urethral stricture can also occur after an infection or injury. Rarely, it may be caused by pressure from a growing tumor near the urethra.
Common causes of urethral strictures include:
- Infection
- Sexually transmitted infection (STI) e.g., gonococcal urethritis (more common) and
- Non-gonoccocal urethritis (less common)
- Repeated urethritis
- Inflammatory
- Balanitis xerotica obliterans (BXO)
- Injury to the pelvic area
- Straddle injury (most common)
- Pelvic fractures
- Iatrogenic: procedures that place a tube into the urethra (such as a catheter or cystoscope)
- instrumentation
- prolonged catheterisation
- transurethral resection of the prostate
- open radical prostatectomy
- urethra reconstruction (hypospadias/epispadias)
- Congenital (uncommon), not to be confused with posterior urethral valves
- Benign prostatic hyperplasia (BPH)
Although gonorrhea remains the most common sexually transmitted disease, urethral strictures are far less common than previously due to early treatment.
Instrumentation-related strictures usually occur in the bulbomembranous region and, less commonly, at the penoscrotal junction.
Risk factors for urethral stricture
There are a number of factors that predispose the development of urethral stricture.
Urethral stricture may occur due to infection or trauma of the urethral part of the urinary tract.
Trauma factors:
- Previous surgery upon the urinary tract.
- Presence of cancer in adjacent structures such as the prostate.
- Presence of a long-term urinary catheter.
Infection prediposing factors include:
- Other urinary tract abnormalities.
- Frequent participation is unprotected sexual intercourse.
- Poor functioning immune system.
Urethral stricture prevention
Practicing safer sex may decrease the risk of getting sexually transmitted infections (STIs) and urethral stricture.
Treating urethral stricture quickly may prevent kidney or bladder complications.
Urethral stricture symptoms
Men with symptomatic stricture disease will typically present with obstructive voiding symptoms such as straining, incomplete emptying, and a weak stream; they might also have a history of recurrent urinary tract infection (UTI), prostatitis (inflammation of the prostate), epididymitis (inflammation of the epidydimis), hematuria (blood in urine) or bladder stones.
Urethral stricture symptoms include:
- Blood in the semen
- Discharge from the urethra
- Bloody or dark urine
- Strong urge to urinate and frequent urination
- Inability to empty bladder (urinary retention)
- Painful urination or difficulty urinating
- Loss of bladder control
- Increased frequency or urgency to urinate
- Pain in the lower abdomen and pelvic area
- Slow urine stream (may develop suddenly or gradually) or spraying of urine
- Swelling of the penis
Urethral stricture complications
Complications of untreated urethral strictures 3:
- Thick-walled trabeculated bladder (85% incidence)
- Acute retention (60% incidence)
- Prostatitis (50% incidence)
- Epididymo-orchitis (25% incidence)
- Hydronephrosis (20% incidence)
- Periurethal abscess (15% incidence)
- Bladder or urethral stones (10% incidence)
Urethral stricture diagnosis
A physical exam may show the following:
- Decreased urinary stream
- Discharge from the urethra
- Enlarged bladder
- Enlarged or tender lymph nodes in the groin
- Enlarged or tender prostate
- Hardness on the under surface of the penis
- Redness or swelling of the penis
Sometimes, the exam reveals no abnormalities.
Tests include the following:
- Cystoscopy
- Postvoid residual (PVR) volume
- Retrograde urethrogram (RUG)
- Voiding cystourethrography (VCUG)
- Ultrasonography
- Tests for chlamydia and gonorrhea
- Urinalysis
- Urinary flow rate
- Urine culture
Retrograde urethrography (RUG) and voiding cystourethrography (VCUG) are used to determine the location, length, and severity of the urethral stricture. Typically, narrowing of the urethral lumen is evident at the site of the stricture, with dilation of the urethra proximal to the stricture. A cystoscopy can also be performed if the retrograde urethrogram and voiding cystourethrogram are inconclusive to give an idea as to the location of the stricture and the elasticity and appearance of the urethra. A cystoscopy can be performed either through the meatus (with a paediatric cystoscope or ureteroscope if necessary) or through a suprapubic cystostomy (with a flexible cystoscope) depending on the location and grade of stricture disease. Ultrasonography can be used as an adjunct to determine the length and degree of spongiofibrosis, and can influence the operative approach 4. Ultrasonography can be performed either preoperatively or intraoperatively. One advantage of intraoperative ultrasonography is that it can be performed with hydrodistension once the patient is anesthetized, enabling accurate evaluation of anterior strictures and avoiding an additional investigation during preoperative evaluation. This approach also assesses the stricture at the time of repair and, therefore, at the maximum severity of the stricture.
As urethral strictures may be caused by sexually transmitted infections (STIs), the patient may be tested for a number of STI’s that relate to the urethral stricture itself, and others which may occur in association with sexually transmitted infection. This will require blood samples to be taken from the patient. A urinary sample will also be required to test for the presence of the urinary tract infection.
Urethral stricture treatment
The most common treatment for urethral stricture is the use of dilators, to expand the narrowed segment of urethra. The urethra may be widened (dilated) during cystoscopy. Topical numbing medicine will be applied to the area before the procedure. Dilating rods are passed into the urethra to expand the narrow segment of urethra. This form of therapy provides good relief from symptoms in the short term, but the procedure will often need repeating as the stricture will commonly recur after this procedure.
You may be able to treat your stricture by learning to dilate the urethra at home.
If urethral dilation cannot correct the condition, you may need surgery. The type of surgery will depend on the location and length of the stricture. If the narrowed area is short and not near the muscles that control the exit from the bladder, the stricture may be cut or dilated.
An open urethroplasty may be done for longer strictures. This surgery involves removing the diseased area. The urethra is then rebuilt. The results vary, depending on the size and location of the stricture, the number of treatments you have had, and the surgeon’s experience.
In acute cases when you cannot pass urine, a suprapubic catheter may be placed. This is an emergency treatment. This allows the bladder to drain through the abdomen.
There are currently no drug treatments for urethral stricture.
If no other treatments work, a urinary diversion called an appendicovesicostomy (Mitrofanoff procedure) or another type of surgery may be done. This lets you drain your bladder through the wall of the abdomen using a catheter or a stoma bag.
Urethral stricture dilation
Several methods for urethral dilation exist, including dilation with a balloon, filiform and followers, urethral sounds, or self-dilation with catheters. Overall, studies have shown no difference in recurrence rates following urethral dilation versus internal urethrotomy 5. One prospective randomized controlled study of men with urethral stricture treated with filiform dilation or internal urethrotomy reported no significant difference in stricture-free rates at 3 years or in the median time to recurrence for these two approaches 6. Rates of complications and failure at the time of the procedure do not differ significantly between dilation and internal urethrotomy 7, although complications associated with urethral dilation might be more likely to occur in patients who present with urinary retention 7.
Internal urethrotomy
Direct vision internal urethrotomy is performed by making a cold-knife transurethral incision to release scar tissue, allowing the tissue to heal by secondary intention at a larger caliber and thereby increasing the size of the urethral lumen. Many studies have evaluated the benefit of placing a urethral catheter after urethrotomy, although no consensus has been reached to date on whether to leave a catheter and, if so, for what duration 8. Internal urethrotomy success rates vary widely, ranging from 8–80%, depending on patient selection, length of follow-up assessment, and methods of determining success and recurrence 9. Overall long-term success rates are estimated to be just 20–30% 10.
In general, recurrence is more likely with longer strictures; the risk of recurrence at 12 months is 40% for strictures shorter than 2 cm, 50% for strictures between 2–4 cm, and 80% for strictures longer than 4 cm 5. For each additional 1 cm of stricture, the risk of recurrence has been shown to increase by 1.22 5. Recurrence rates also vary according to stricture location; 58% of bulbar strictures will recur after urethrotomy, compared with 84% for penile strictures and 89% for membranous strictures 11. Risk of stricture recurrence is greatest at 6 months; however, if the stricture has not recurred by 1 year, the risk of recurrence is significantly decreased 5. Data from one study suggests if the stricture has not recurred within the first 3 months after a single dilation or urethrotomy, the stricture- free rate is 50–60% for up to 4 years of follow-up assessment 12. Repeat urethrotomy is known to be associated with progressively worse outcomes; in one study, the stricture-free rate for a second procedure was found to be 30–50% at 2 years, 0–40% at 4 years, and 0% at 2 years following a third procedure 13.
Overall, men with the highest success rates have strictures in the bulbar urethra that are primary strictures of <1.5 cm in length and are not associated with spongiofibrosis 13. Risk factors for recurrence include previous internal urethrotomy, strictures located within the penile or membranous urethra, strictures of >2 cm in length, multiple strictures, UTI at the time of procedure, and strictures associated with extensive periurethral spongiofibrosis 13. These data can be used to help predict which patients might be good candidates for urethrotomy or dilation.
Some urologists have suggested that self- catheterization following urethrotomy might decrease recurrence rates, reporting delayed time to recurrence and decreased rates of recurrence with self-catheterization 14. However, other studies have shown that self-dilation does not decrease recurrence rates (as it is required on a long-term basis to prevent recurrence), and that self-dilation is associated with significant long-term complications and high dropout rates 13.
The main complications following urethrotomy include recurrence, perineal hematoma, urethral haemorrhage, and extravastion of irrigation fluid into perispongiosal tissues. With deep incisions at the 10 o’clock and 2 o’clock positions, there is also a risk of entering the corpus cavernosum and creating fistulas between the corpus spongiosum and cavernosa, leading to erectile dysfunction 15. One meta-analysis of complications of cold-knife urethrotomy established an overall complication rate of 6.5%; the most common complications were erectile dysfunction (5%), urinary incontinence (4%), extravasation (3%), UTI (2%), haematuria (2%), epididymitis (0.5%), urinary retention (0.4%), and scrotal abscess (0.3%) 16. Of note, erectile dysfunction is particularly common in patients with long and dense strictures requiring extensive incision 17. In general, complications associated with internal urethrotomy are more likely to occur in men with a positive urine culture, a history of urethral trauma, multiple stricture segments, and long (>2 cm) strictures 7.
Injectable agents
Some studies have evaluated the efficacy of agents injected into the scar tissue at the time of internal urethrotomy to decrease recurrence rates. Mitomycin C has shown promise when used for both anterior urethral strictures and incision of bladder neck contractures 18. Studies evaluating the use of triamcinolone injection have shown a significant decrease in both time to recurrence and recurrence rate at 12 months without any significant increase in rates of perioperative complications 19.
Laser urethrotomy
In addition to cold-knife internal urethrotomy, studies have evaluated the use of lasers for urethrotomy 20. Many types of lasers have been utilized, including carbon dioxide, argon, potassium titanyl phosphate (KTP), neodymium- doped yttrium aluminium garnet (Nd:Yag), holmium:Yag, and excimer lasers. These lasers each use different technologies and offer differing depths of tissue penetration. As such, lasers pose distinctive risks depending on their mechanism of action, such as carbon dioxide embolus with the use of a gas cystoscope for the carbon dioxide laser, and peripheral tissue injury due to thermal necrosis with the use of the argon and Nd:Yag lasers. Overall, data seem to show equivalence in terms of both complication and success rates for these different lasers 16. Currently, there is no clear consensus on which laser or technique is best to use, but a survey conducted in 2011 showed that nearly 20% of urologists reported using laser urethrotomy to manage anterior urethral strictures 21.
A meta-analysis of complications associated with laser urethrotomy reported an overall complication rate of 12%, which compares unfavourably to the 6.5% incidence reported for cold-knife urethrotomy 16. Common complications included UTI (11% incidence), urinary retention (9% incidence), haematuria (5% incidence), dysuria (5% incidence), urinary extravasation (3% incidence), UTI (3% incidence), urinary incontinence (2% incidence), and urinary fistula (1.5% incidence).
Urethral stents
The use of urethral stents following dilation or internal urethrotomy has also been explored. Temporary stents such as the Spanner® stent (SRS Medical, USA) require exchange every 3–12 months depending on the type of stent, and are more suitable for men with posterior urethral obstruction 22. Permanent stents, such as the Urolume® (Endo Health Solutions, USA) and Memotherm® (Bard, Germany) stent, are placed into the bulbar urethra and incorporated into the wall of the urethra 23. However, use of these stents has been largely abandoned and, in some countries, these stents have been removed from the market because of limited use and high rates of complications such as perineal pain, stent migration, stent obstruction (owing to tissue hyperplasia or stone encrustation), incontinence, and infection 24.
Urethral stricture surgery
End-to-end anastomotic urethroplasty
The most commonly performed procedure is called an “anastamotic repair of the urethra.” Essentially the narrowed segment of the urethra is removed and the remaining ends attached together. This procedure will require admission to a hospital and the insertion of a catheter following the procedure to allow the urethra to heal and allow urine to pass easily, throughout the post-operative period.
An end-to-end anastomotic repair technique has traditionally been used for bulbar strictures that are <2 cm in length 25. Anastomotic urethroplasty scores highly for both objective and patient-centred subjective criteria, with most studies reporting success rates of between 90–95% 26. Anastomotic repair of the urethra has a 15-year recurrence and complication rates of 14% and 7%, respectively, in one series, and 5–10% recurrence rates in another series 27. Recurrent strictures in men who have undergone urethroplasty are generally treated with direct vision internal urethrotomy alone, resulting in good long-term success rates 28. Additionally, some clinicians have suggested that younger men might have better tissue compliance, increasing the chances of successful excision and primary re-anastomosis for fairly long strictures 29.
Urethral stricture surgery complications
Complications are rare, but include erectile and ejaculatory dysfunction, chordee, wound infections, UTIs, fistula development, neuropraxia, and incontinence. Given the dissection required by urethroplasty that inevitably injures corporal blood supply and innervation, it is unsurprising that one of the main complications is short-term erectile dysfunction. Erectile function has been shown to decrease at 3 months postoperatively but generally returns by 6 months. One year after surgical reconstruction, many studies have shown no significant difference in erectile function compared with preoperative function 30. In fact, some studies have shown improvement in ejaculation following urethroplasty 31. Data suggest that the risk of de novo dysfunction is very low, with an incidence of just 1% 32. Risk factors for the development of erectile dysfunction after repair include posterior urethral stenoses and an end-to-end anastomosis 30.
Urethral stricture prognosis
With surgical repair, the prognosis of this condition is good. Sometimes, treatment needs to be repeated to remove scar tissue.
Urethral stricture may totally block urine flow. This can cause sudden urinary retention. This condition must be treated quickly. Long-term blockage can lead to permanent bladder or kidney damage.
- Male urethral strictures and their management. Nat Rev Urol. 2014 Jan; 11(1): 43–50. doi: 10.1038/nrurol.2013.275 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4123447/[↩]
- Contemporary urethral stricture characteristics in the developed world. Palminteri E, Berdondini E, Verze P, De Nunzio C, Vitarelli A, Carmignani L. Urology. 2013 Jan; 81(1):191-6.[↩]
- Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107:6–26.[↩]
- Impact of urethral ultrasonography on decision-making in anterior urethroplasty. Buckley JC, Wu AK, McAninch JW. BJU Int. 2012 Feb; 109(3):438-42.[↩]
- Steenkamp JW, Heyns CF, De Kock M. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J. Urol. 1997;157:98–101.[↩][↩][↩][↩]
- Wong SSW, Narahari R, O’Riordan A, Pickard R. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database of Systematic Reviews. 2010:CD006934. Issue 1. Art. No.[↩]
- Steenkamp JW, Heyns CF, De Kock M. Outpatient treatment for male urethral strictures—dilatation versus internal urethrotomy. S. Afr. J. Surg. 1997;35:125–130.[↩][↩][↩]
- Naudé AM, Heyns CF. What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat. Clin. Pract. Urol. 2005;2:538–545.[↩]
- Greenwell TJ, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J. Urol. 2004;172:275–277[↩]
- Santucci RA, McAninch JW. Actuarial success rate of open urethral stricture repair in 369 patient open repairs, compared to 210 DIV or dilation; Presented at the 2001 AUA meeting.[↩]
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J. Urol. 1996;156:73–75.[↩]
- Heyns CF, Steenkamp JW, de Kock ML, Whitaker P. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J. Urol. 1998;160:356–358.[↩]
- Dubey D. The current role of direct vision internal urethrotomy and self-catheterization for anterior urethral strictures. Indian J. Urol. 2011;27:392.[↩][↩][↩][↩]
- Lauritzen M, et al. Intermittent self-dilatation after internal urethrotomy for primary urethral strictures: a case-control study. Scand. J. Urol. Nephrol. 2009;43:220–225.[↩]
- Naudé AM, Heyns CF. What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat. Clin. Pract. Urol. 2005;2:538–545[↩]
- Jin T, Li H, Jiang L-H, Wang L, Wang K-J. Safety and efficacy of laser and cold knife urethrotomy for urethral stricture. Chin. Med. J. 2010;123:1589–1595.[↩][↩][↩]
- Graversen PH, Rosenkilde P, Colstrup H. Erectile dysfunction following direct vision internal urethrotomy. Scand. J. Urol. Nephrol. 1991;25:175–178.[↩]
- Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin c for the management of recurrent bladder neck contractures. J. Urol. 2011;186:156–160.[↩]
- Tavakkoli Tabassi K, Yarmohamadi A, Mohammadi S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol. J. 2011;8:132–136.[↩]
- Dutkiewicz SA, Wroblewski M. Comparison of treatment results between holmium laser endourethrotomy and optical internal urethrotomy for urethral stricture. Int. Urol. Nephrol. 2012;44:717–724.[↩]
- Ferguson GG, Bullock TL, Anderson RE, Blalock RE, Brandes SB. Minimally invasive methods for bulbar urethral strictures: a survey of members of the American Urological Association. Urology. 2011;78:701–706.[↩]
- McKenzie P, Badlani G. Critical appraisal of the Spanner™ prostatic stent in the treatment of prostatic obstruction. Med. Devices (Auckl.) 2011;4:27–33.[↩]
- Sertcelik MN, Bozkurt IH, Yalcinkaya F, Zengin K. Long-term results of permanent urethral stent Memotherm implantation in the management of recurrent bulbar urethral stenosis. BJU Int. 2011;108:1839–1842.[↩]
- Choi EK, et al. Management of recurrent urethral strictures with covered retrievable expandable nitinol stents: long-term results. AJR Am. J. Roentgenol. 2007;189:1517–1522.[↩]
- Jordan GH, McCammon KA. Surgery of the Penis and Urethra. Vol. 1. Oxford: Elsevier Saunders; 2012. pp. 956–1000.[↩]
- Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. Cooperberg MR, McAninch JW, Alsikafi NF, Elliott SP. J Urol. 2007 Nov; 178(5):2006-10; discussion 2010.[↩]
- The long-term results of urethroplasty. Andrich DE, Dunglison N, Greenwell TJ, Mundy AR. J Urol. 2003 Jul; 170(1):90-2.[↩]
- Anastomotic urethroplasty for bulbar urethral stricture: analysis of 168 patients. Santucci RA, Mario LA, McAninch JW. J Urol. 2002 Apr; 167(4):1715-9.[↩]
- Proximal bulbar urethroplasty via extended anastomotic approach–what are the limits? Morey AF, Kizer WS. J Urol. 2006 Jun; 175(6):2145-9; discussion 2149.[↩]
- Xie H, et al. Evaluation of erectile function after urethral reconstruction: a prospective study. Asian J. Androl. 2009;11:209–214.[↩][↩]
- Sharma V, Kumar S, Mandal AK, Singh SK. A study on sexual function of men with anterior urethral stricture before and after treatment. Urol. Int. 2011;87:341–345.[↩]
- De novo erectile dysfunction after anterior urethroplasty: a systematic review and meta-analysis. Blaschko SD, Sanford MT, Cinman NM, McAninch JW, Breyer BN. BJU Int. 2013 Sep; 112(5):655-63.[↩]