Contents
What is carrageenan
Carrageenans or carrageenins are a family of linear sulfated galactose polymer (linear sulfated polysaccharides) that are obtained from certain members of the class Rhodophyceae (red seaweeds) 1. Carrageenan is extracted from seaweeds in two ways. In native extraction, the seaweed is made into an aqueous solution, and the residue is filtered, leaving nearly pure carrageenan. The alkaline-modified method is less expensive and easier. The seaweed is mixed in an alkali solution, leaving a mixture of carrageenan and cellulose that can be sold as semirefined carrageenan. Carrageenan is yellowish or tan to white, coarse to fine powder that is practically odorless.
Indonesia, the Philippines, and Chile are three main sources of raw material and extracted carrageenan.
The use of dried carrageenan-containing seaweeds to produce puddings from milk reportedly goes back at least two centuries, and the Irish are reported to have used a seaweed in foods and medicines for about six hundred years 2. Carrageenan was first extracted only in 1837; the structure was not investigated until the 1840s and was not clarified until the mid-1950s. Commercial production of carrageenan started in 1937; it remained an almost exclusively US industry until the 1950s when it began to be carried out on a large scale in European countries 3.
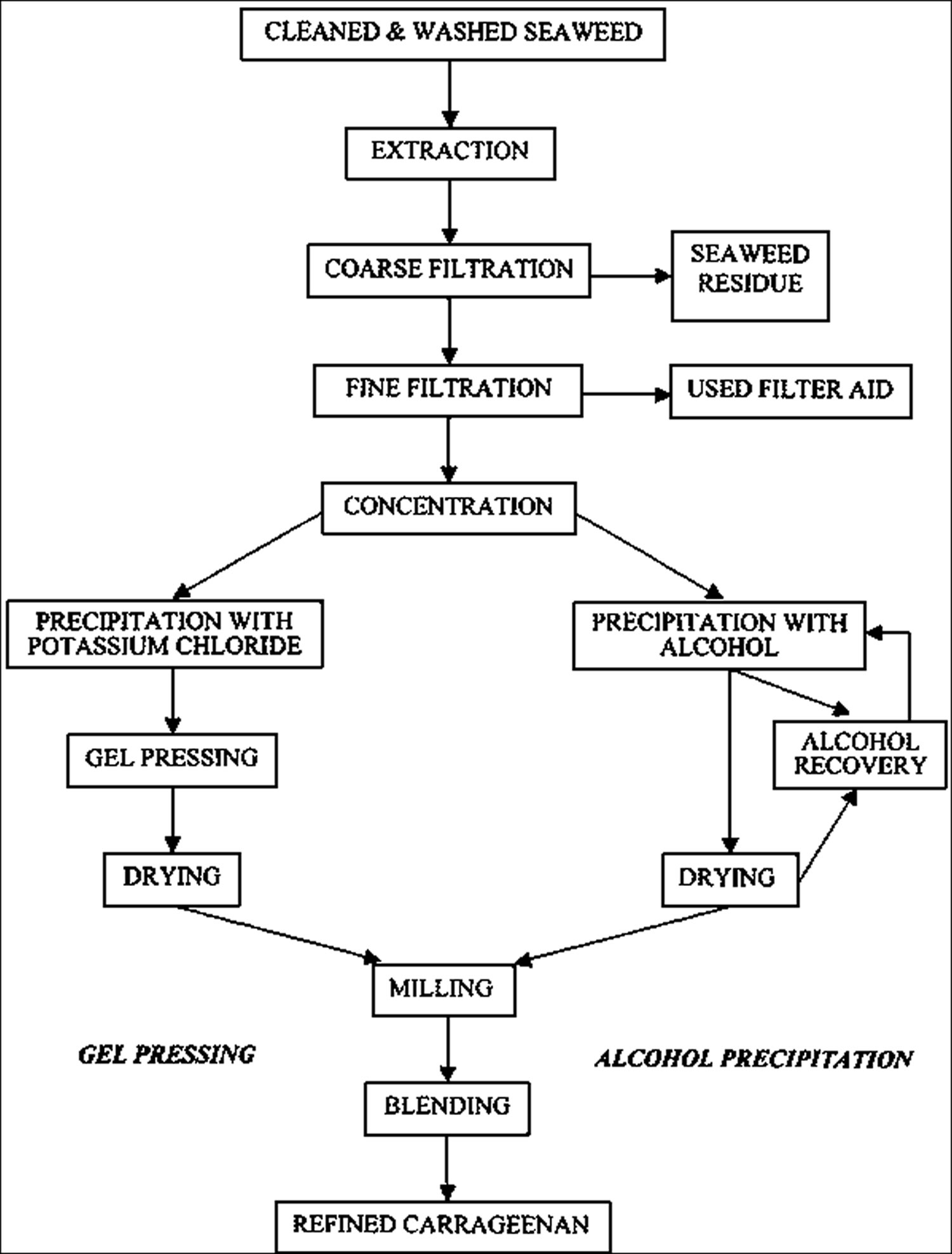
Carrageenan is recovered commercially from Chondrus seaweed in the US and from Chondrus and Gigartina seaweed in Europe 4. Processing steps used to recover carrageenan vary considerably and are closely-guarded trade secrets. Cottrell and Baird 3 state that patent literature indicates that the process generally includes the following steps: The seaweed is washed to remove soluble salts and debris before being extracted with slightly alkaline hot water. The extract is concentrated to about 3% carrageenan before alcohol is added to precipitate it; alternatively, the extract can be drum dried to produce a less pure product.
Figure 1. Red seaweeds (source of Carrageenans)

Carrageenan is a hydrocolloid consisting mainly of the ammonium, calcium, magnesium, potassium and sodium sulfate esters of galactose and 3,6-anhydrogalactose polysaccharides. This gives them the ability to form a variety of different gels at room temperature.
- Carrageenans are widely used in the food and other industries as thickening and stabilizing agents. Their main application of carrageenan is in dairy and meat products, due to their strong binding to food proteins.
- When used in food products, carrageenan has the EU additive E-number E407 or E407 5.
Carrageenan is a vegetarian and vegan alternative to gelatin in some applications or may be used to replace gelatin in confectionery.
Carrageenans are used in a variety of commercial applications as gelling, thickening, and stabilizing agents, especially in food products such as frozen desserts, chocolate milk, cottage cheese, whipped cream, instant products, yogurt, jellies, pet foods, and sauces. Aside from these functions, carrageenans are used in pharmaceutical formulations, cosmetics, and industrial applications such as mining.
There are three main varieties of carrageenan, which differ in their degree of sulphation.
- Kappa-carrageenan has one sulphate group per disaccharide. Kappa forms strong, rigid gels in the presence of potassium ions; it reacts with dairy proteins. It is sourced mainly from Kappaphycus alvarezii (the elkhorn sea moss, which is a species of red algae) 6.
- Iota-carrageenan has two sulphate group per disaccharide. Iota forms soft gels in the presence of calcium ions. It is produced mainly from Eucheuma denticulatum (a species of red algae) 7.
- Lambda-carrageenan has three sulphate group per disaccharide. Lambda does not gel, and is used to thicken dairy products.
All carrageenans are soluble in hot water. Lambda carrageenans are soluble in cold water, but kappa and iota carrageenans are soluble in cold water only as their sodium salts.
Table 1. The carrageenan composition in red seaweeds differs from one species to another
| Chondrus crispus | mixture of kappa and lambda. |
| Kappaphycus alvarezii | mainly kappa. |
| Eucheuma denticulatum | mainly iota. |
| Gigartina skottsbergii | mainly kappa, some lambda. |
| Sarcothalia crispata | mixture of kappa and lambda. |
Carrageenan production methods
There are two different methods of producing carrageenan, based on different principles.
- In the original method – the only one used until the late 1970s-early 1980s – the carrageenan is extracted from the seaweed into an aqueous solution, the seaweed residue is removed by filtration and then the carrageenan is recovered from the solution, eventually as a dry solid containing little else than carrageenan. This recovery process is difficult and expensive relative to the costs of the second method.
- In the second method, the carrageenan is never actually extracted from the seaweed. Rather the principle is to wash everything out of the seaweed that will dissolve in alkali and water, leaving the carrageenan and other insoluble matter behind. This insoluble residue, consisting largely of carrageenan and cellulose, is then dried and sold as semi-refined carrageean. Because the carrageenan does not need to be recovered from solution, the process is much shorter and cheaper.
Figure 2. Carrageenan production method of refined Carrageenan
There are two basic grades of carrageenan:
- Refined carrageenan. Refined carrageenan is the original carrageenan and until the late 1970s-early 1980s was simply called carrageenan. It is now sometimes called filtered carrageenan. It was first made from Chondrus crispus, but now the process is applied to all of the above algae. The seaweed is washed to remove sand, salts and other foreign matter. It is then heated with water containing an alkali, such as sodium hydroxide, for several hours, with the time depending on the seaweeds being extracted and determined by prior small-scale trials, or experience. Alkali is used because it causes a chemical change that leads to increased gel strength in the final product. In chemical terms, it removes some of the sulphate groups from the molecules and increases the formation of 3,6-AG: the more of the latter, the better the gel strength. The seaweed that does not dissolve is removed by centrifugation or a coarse filtration, or a combination. The solution is then filtered again, in a pressure filter using a filter aid that helps to prevent the filter cloth becoming blocked by fine, gelatinous particles. At this stage, the solution contains 1-2 percent carrageenan and this is usually concentrated to 2-3 percent by vacuum distillation and ultrafiltration.
- Semi-refined carrageenan.
In the United States both grades are labeled as carrageenan. In the European Union, refined carrageenan is designated by the E number E-407, and semi-refined carrageenan as E-407a 5. Refined carrageenan has a 2% maximum for acid insoluble material and is produced through an alcohol precipitation process or potassium chloride gel press process. Semi-refined carrageenan contains a much higher level of cellulosic content and is produced in a less complex process.
Regulatory status of carrageenan
In the U.S., carrageenan is allowed under the U.S. Food and Drug Administration (FDA) regulations “Carrageenan” (21 CFR 172.620) as a direct food additive and is considered safe 9 when used in the amount necessary as an emulsifier, stabilizer, or thickener in foods, except those standardized foods that do not provide for such use. FDA also reviewed carrageenan safety for infant formula. The European Food Safety Authority concluded “there is no evidence of any adverse effects in humans from exposure to food-grade carrageenan, or that exposure to degraded carrageenan from use of food-grade carrageenan is occurring” 10, Furthermore, the Joint FAO/WHO expert committee on food additives stated in a July 2014 review of carrageenan “that the use of carrageenan in infant formula or formula for special medical purposes at concentrations up to 1000 mg/L is not of concern” 11.
In the most recent review by an independent panel, the Joint Expert Committee of the Food and Agriculture Organization of the United Nations and World Health Organization on Food Additives released a technical report in 2015 on the use of carrageenan in infant formula and found that the additive was ‘not of concern’ in infant formula as food for special medical purposes at concentrations up to 1000 milligrams per liter 12. The use of carrageenan in infant formula, organic or otherwise, is prohibited in the EU for precautionary reasons, but is permitted in other food items.
Carrageenan Foods and Other Uses
- Desserts, ice cream, cream, milkshakes, yogurts, salad dressings, sweetened condensed milks
- Sauces: to increase viscosity
- Beer: clarifier to remove haze-causing proteins
- Pâtés and processed meats (e.g., ham): substitute for fat, increase water retention, increase volume, or improve slicing
- Toothpaste: stabilizer to prevent constituents separating
- Fruit Gushers: ingredient in the encapsulated gel
- Fire fighting foam: thickener to cause foam to become sticky
- Shampoo and cosmetic creams: thickener
- Air freshener gels
- Marbling: the ancient art of paper and fabric marbling uses a carrageenan mixture on which to float paints or inks; the paper or fabric is then laid on it, absorbing the colours
- Shoe polish: to increase viscosity
- Biotechnology: to immobilize cells and enzymes
- Pharmaceuticals: used as an inactive excipient in pills and tablets
- Soy milk and other plant milks: to thicken
- Diet sodas: to enhance texture and suspend flavours
- Pet food
- Personal lubricants
- Vegetarian hot dogs
Clinical studies in infants
Carrageenan is currently used in some cow milk– and soy-based formulas. These include both powders and liquids. The predominant type of carrageenan used in infant formula is κ-carrageenan. The typical level of carrageenan used in reconstituted powdered and liquid cow milk– and soy-based formulas is 0.009–0.1 g/100 mL (90–1000 mg/L), with the higher levels being used in formulas containing hydrolysed proteins.
There are brief reports of two studies in human infants given formula containing carrageenan.
In one study, 1269 full-term infants given liquid formula containing carrageenan at 300 mg/L (0.03%) for the first 6 months of life were compared with 149 infants given powder-based formula not containing carrageenan for frequency of symptomatic upper respiratory tract infection. There were no statistically significant differences between the two groups; a slightly higher proportion of infants given formula containing carrageenan were illness-free during the first 6 months of life. The authors concluded that carrageenan-containing liquid infant formula is not immunosuppressive 13, 14.
In a masked, randomized study on healthy newborn infants aged 0–9 days at enrolment in the study, 95 infants were fed powdered casein hydrolysate–based formula that did not contain carrageenan, and 100 infants were fed liquid ready-to-feed casein hydrolysate–based formula containing carrageenan at 1000 mg/L (0.1%). This is at the high end of carrageenan concentrations used in formulas for special medical purposes. One hundred and thirty-seven infants completed the study; no information was given on the numbers in each of the two groups at the end of the study, but intolerance to the formulas accounted for dropout for 21 infants in the group fed formula containing carrageenan and 16 in the group fed formula not containing carrageenan. Intake, stool patterns and anthropometric measurements were monitored at entry and on days 14, 28, 56, 84 and 112 of the study. There were no differences between the two groups in weight gain, length, head circumference or tolerance to the formulas. Infants on powdered formula had significantly lower intakes of formula and passed significantly fewer stools per day. Stool consistencies were similar except for liquid formula infants, who had firmer stools on entry 15.
Carrageenan side effects
Studies in a variety of species of experimental animals, including rats, guinea pigs and primates, have shown that there is negligible absorption of food grade carrageenan from the gastrointestinal tract 16, 12.
Carrageen an has been extensively evaluated for genotoxicity (agents that damages the DNA) using a range of in vitro and in vivo assays, including the bacterial reverse mutation assay ± S9 fraction, sister chromatid exchange assays, cytogenetic assays, rec-assay in Bacillus subtilis, mouse micronucleus assay, host-mediated assays and dominant lethal assays in rats. Results of these various assays have been negative 17.
There is no robust evidence of allergic reactions to ingested carrageenan 18.
In the toxicity studies, in addition to a wide range of toxicological parameters, a detailed examination of the histology of all segments of the gastrointestinal tract and quantification of mast cells along the gastrointestinal tract were undertaken in both the minipig and pig. In the pig, an appropriate array of serum and gut cytokines was also assessed, together with blood leukocyte immunophenotyping. From these new investigations, there was no evidence of any inflammation in the gut or any effects on immune parameters. A no-observed-adverse-effect-level (NOAEL) of 430 mg/kg body weight per day, which was the highest dose tested, was derived from the neonatal pig study. The no-observed-adverse-effect-level (NOAEL) of 430 mg/kg body weight per day from the neonatal pig study is almost identical to that from the earlier infant baboon study of 432 mg/kg body weight per day 12.
In the 10-day neonatal minipig study, animals were given infant formula containing carrageenan at concentrations up to 3000 mg/kg (0.3%). Concentrations of carrageenan above approximately 2500 mg/kg (0.25%) become highly viscous, and this appears to have adversely affected palatability and growth in the minipigs. Accordingly, the amount of carrageenan added to the formula fed to piglets in the main study was reduced to 2250 mg/kg (0.225%). As a consequence of this limitation, the margins of exposure (MOEs) between the no-observed-adverse-effect-level (NOAEL) from the pig study and human infant exposures at 2–4 weeks of age range from 2 to 12 on a body weight basis and from 2 to 8 on a concentration basis 12.
The Joint Food and Agriculture Organization of the United Nation (FAO) and the World Health Organization (WHO) Food Additives Committee noted that although the margins of exposure (MOEs) are small in magnitude, they are derived from a neonatal pig study in which the highest dose tested was without adverse effects on the gut or on immune parameters, supported by a neonatal minipig study. The neonatal pig and minipig are appropriate models for the young human infant up to at least 12 weeks of age, for whom infant formula may be the sole source of nutrition. These new studies allay the earlier concerns that carrageenan, which is unlikely to be absorbed, may have a direct effect on the immature gut. The Joint FAO/WHO Expert Committee on Food Additives also took account of the previous toxicological database on carrageenan, which did not indicate other toxicological concerns.
The FAO and WHO Committee concluded that the use of carrageenan in infant formula or formula for special medical purposes at concentrations up to 1000 mg/L is not of concern 12. The Committee recognizes that there is variability in medical conditions among infants requiring formulas for special medical purposes that contain the higher levels of carrageenan, and the Committee notes that these infants would normally be under medical supervision 12.
- http://www.fao.org/ag/agn/jecfa-additives/specs/Monograph1/Additive-117.pdf[↩]
- https://monographs.iarc.fr/ENG/Monographs/vol1-42/mono31.pdf[↩]
- Cottrell, LW. & Baird, J.K. (1980) Gums. ln: Kirk, RE. & Othmer, D.F., eds, Encyelopedia of Chemical Technology, 3rd ed., Vol. 12, New York, John Wiley & Sons, pp. 51-53,64-66.[↩][↩]
- Informatics, Inc. (1972) GRAS (Generally Recognized As Safe) Food Ingredients: Carrageenan, PB-221206. Prepared for US Food & Drug Administration, Springfield, V A, National Technicallnformation Service, pp. 9-11, 30, 37[↩]
- Current EU approved additives and their E Numbers. https://www.food.gov.uk/science/additives/enumberlist[↩][↩]
- Kappaphycus alvarezii. Wikipedia. https://en.wikipedia.org/wiki/Kappaphycus_alvarezii[↩]
- Eucheuma denticulatum. Wikipedia. https://en.wikipedia.org/wiki/Eucheuma_denticulatum[↩]
- CARRAGEENAN. http://www.fao.org/docrep/006/y4765e/y4765e0a.htm[↩]
- Guidance for Industry: Assessing the Effects of Significant Manufacturing Process Changes, Including Emerging Technologies, on the Safety and Regulatory Status of Food Ingredients and Food Contact Substances, Including Food Ingredients that Are Color Additives. https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/ucm300661.htm[↩]
- https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_reports_35.pdf[↩]
- http://www.fao.org/fileadmin/user_upload/agns/news_events/JECFA%2079%20Summary%20Version%20Final.pdf[↩]
- Safety evaluation of certain food additives. WHO Food Additives Series:70. Prepared by the Seventy-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA).2015. http://apps.who.int/iris/bitstream/10665/171781/3/9789240693982_eng.pdf[↩][↩][↩][↩][↩][↩]
- Sherry B, Flewelling A, Smith AL (1993). Carrageenan: an asset or a detriment in infant formula? Am J Clin Nutr. 58:715.[↩]
- Sherry B, Flewelling A, Smith AL (1999). Erratum for “Carrageenan: an asset or a detriment in infant formula? Am J Clin Nutr. 58:715”. Am J Clin Nutr. 69:1293.[↩]
- Borsches MW, Barrett-Reis B, Baggs GE, Williams TA (2002). Growth of healthy term infants fed a powdered casein hydrolysate–based formula (CHF). FASEB J. 16:A66 (Abstract 497.7[↩]
- Weiner ML (2014). Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Critical Reviews in Toxicology 44: 244–269[↩]
- Cohen SM and Ito N (2002). A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract. Critical Reviews in Toxicology 32:413–444[↩]
- World Health Organisation (2008) Safety evaluation of certain food additives / prepared by the sixty-eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO food additives series, 59.[↩]