Contents
- What is alpha lipoic acid
- Alpha lipoic acid functions
- Alpha lipoic acid foods
- Alpha lipoic acid supplements
- What is alpha lipoic acid used for?
- Alpha lipoic acid dosage
- Alpha lipoic acid side effects
What is alpha lipoic acid
Alpha-lipoic acid also called lipoic acid, thioctic acid or 1,2-dithiolane-3-pentanoic acid (pentanoic acid), is a naturally occurring short chain fatty acid which contains a sulfur (thiol) bond, synthesized in small amounts by plants and animals including humans, with antioxidant and potential chemopreventive activities 1, 2, 3, 4. Alpha lipoic acid is a common ingredient in multivitamin tablets and in dietary supplements and is even included in many pet foods 5, 6, 7. Food sources of alpha-lipoic acid include red meat, beets, carrots, potatoes, spinach, and broccoli.
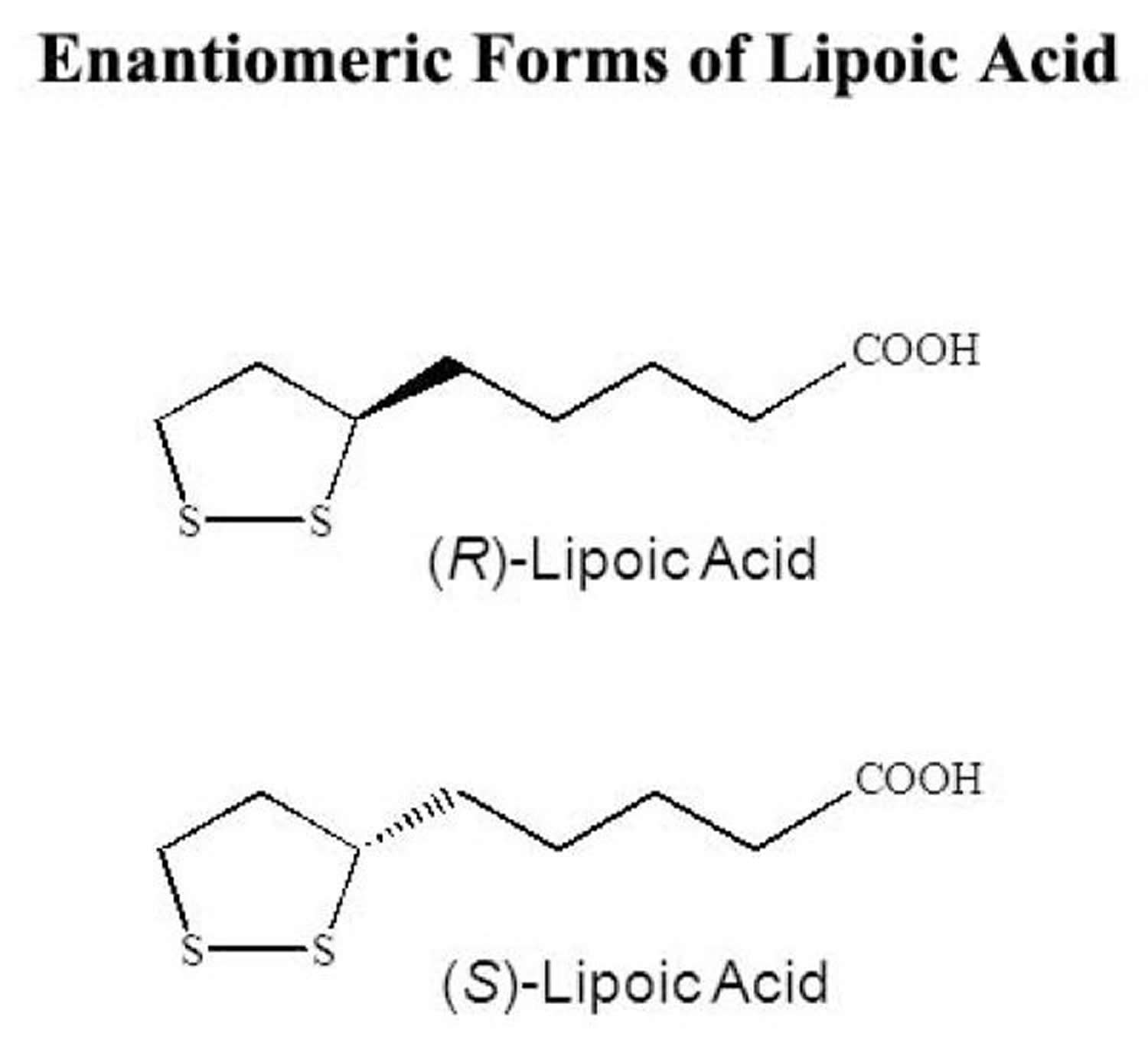
Chemically, alpha lipoic acid is a short-chain fatty acid with a disulfide group in its dithiolane ring and a chiral carbon resulting in R and S enantiomers (see Figure 1 below). Alpha-lipoic acid is available naturally in foods as the R-alpha-lipoic acid and is bound to lysine in proteins 8, 9. Alpha lipoic acid in supplements is available as a racemic mixture of R-alpha lipoic acid (the R form) and S-alpha lipoic acid (the S form) also referred to as d,l-lipoic acid and is not bonded to protein 9. R-alpha-lipoic acid (the R form) is the biologically active form that is endogenously produced by your body, while the S form (S-alpha-lipoic acid) is produced from chemical manufacture and is not biologically active 10. Only R-alpha-lipoic acid is bound to a specific lysine residue in at least one of the proteins in each multienzyme complex, thus making R-alpha-lipoic acid essential as a cofactor (a compound that is essential for the activity of an enzyme) for several mitochondrial multienzyme complexes that catalyze critical reactions related to the catabolism (breakdown) of amino acids and the production of energy 11, 12, 13. Alpha lipoic acid, when exogenously administered, is readily absorbed from the gut and has been clinically used in Europe for the treatment of diabetic neuropathy (nerve damage caused by diabetes) 14.
Though in body synthesis appears to supply all the necessary alpha lipoic acid needed for its role in intermediary metabolism, alpha lipoic acid can also be absorbed intact from dietary sources and can be found in almost all foods, but slightly more so in organ meats (kidney, heart, liver), spinach, broccoli, peas, brussel sprouts, and rice bran 15. While the direct roles of alpha lipoic acid as a cofactor (a compound that is essential for the activity of an enzyme) are well understood, less is known about the precise metabolic functions of orally supplied alpha lipoic acid. Alpha-lipoic acid can also be made in the laboratory. Alpha-lipoic acid is biosynthesized by cleavage of linoleic acid and is a coenzyme of oxoglutarate dehydrogenase (ketoglutarate dehydrogenase complex).
An upper limit for alpha lipoic acid consumption in humans has not been conclusively established 5. However, a dosage of 20–50 mg daily is recommended for general antioxidant support 16. The use of alpha lipoic acid is currently approved for the treatment of diabetic neuropathy in Germany and is available by prescription 17, 18. It is important to note that many of the studies that examined the efficacy of alpha lipoic acid in the treatment of diabetic neuropathy have been primarily conducted by one German research group and funded by the manufacturer of alpha lipoic acid in Germany 19. The recommended dosage of alpha lipoic acid for the treatment of diabetes is 300–600 mg daily. Alpha lipoic acid is available as an over the counter dietary supplement in the United States 20.
The amounts of alpha lipoic acid available in dietary supplements vary between 50–600 mg and could be as much as 1000 times higher than the amounts consumed from food intake 5. Taking alpha lipoic acid with food is known to decrease its bioavailability; hence it is recommended that alpha lipoic acid be taken half an hour to an hour prior to a meal 8, 21. Alpha lipoic acid in food does not cause detectable increase of free alpha lipoic acid in human plasma or in cells 22, 8. However, high oral doses (≥50 mg) of free alpha lipoic acid increase levels of free lipoic acid in human plasma and cells 8, 23. Human studies have shown that approximately 30–40% of an orally administered racemic mixture of R- and S-alph lipoic acid is absorbed 8, 23. All published human clinical trials involving alpha lipoic acid supplementation have used racemic mixture of R-alpha lipoic acid (the R form) and S-alpha lipoic acid (the S form) 24, 25, 26, 27, 28, 29, 30, 21, 31, 23. Following oral administration with racemic alpha lipoic acid, the peak concentrations of R-alpha lipoic acid in plasma were found to be 40–50% more than those of S-alpha lipoic acid, indicating that R-alpha lipoic acid may be more efficiently absorbed than S-alpha lipoic acid, but both isomers were metabolized and eliminated rapidly 8, 32, 33. A study involving 19 healthy adults suggests that the bioavailability of the R and S-isoforms of alpha lipoic acid may vary with age and gender 34. Concentrations of alpha-lipoic acid in plasma typically peak within an hour and then decline rapidly 8, 23, 33. Alpha lipoic acid is swiftly reduced to dihydrolipoic acid (DHLA) in cells, and in vitro studies show that dihydrolipoic acid (DHLA) is then quickly exported from cells 34.
Two forms of alpha-lipoic acid are oxidized lipoic acid and reduced dihydrolipoic acid (DHLA) 35. Both are capable of scavenging a variety of reactive oxygen species (ROS). At the cellular level, alpha lipoic acid is reduced to dihydrolipoic acid (DHLA), which is a potent antioxidant that can neutralize free radicals. Dihydrolipoic acid (DHLA) has a number of cellular actions including free radical scavenging and modulating oxidative stress and inflammatory pathways 36, 37, 10, 38, 39, 40. Furthermore, alpha-lipoic acid simultaneously regenerates other antioxidant factors, such as vitamins C and E, increasing glutathione synthesis 41. Alpha-lipoic acid is generally involved in keto acid oxidative decarboxylation processes and is a growth factor for some organisms 42.
Alpha-lipoic acid acts as a free radical scavenger and assists in repairing oxidative damage and regenerates endogenous antioxidants, including vitamins C and E and glutathione. Alpha lipoic acid also promotes glutathione synthesis. In addition, alpha-lipoic acid exerts metal chelating capacities and functions as a cofactor for energy production in the mitochondria in various mitochondrial enzyme complexes involved in the decarboxylation of alpha-keto acids and thus serves a critical role in mitochondrial energy metabolism 43.
Alpha lipoic acid is marketed in the US as an over-the-counter nutritional antioxidant supplement, alone or in combination with other antioxidants. In medicine, alpha lipoic acid has been shown to reduce symptoms of diabetic polyneuropathy, and several clinical trials established some efficacy and an excellent safety profile in this patient population 44. Meta-analyses of randomized controlled trials suggest that infusion of 300 to 600 mg/day of alpha lipoic acid for two to four weeks significantly reduced the symptoms of diabetic neuropathy to a clinically meaningful degree 45, 46. Regarding the efficacy of oral alpha lipoic acid supplementation, an initial short-term study in 24 patients with type 2 diabetes mellitus found that the symptoms of peripheral neuropathy improved in those who took 600 mg of alpha lipoic acid three times a day for three weeks compared to those who took a placebo 47. A larger clinical trial randomly assigned more than 500 patients with type 2 diabetes and symptomatic peripheral neuropathy to one of the following treatments: (i) 600 mg/day of intravenous alpha lipoic acid for three weeks followed by 1,800 mg/day of oral lipoic acid for six months, (ii) 600 mg/day of intravenous alpha lipoic acid for three weeks followed by oral placebo for six months, or (iii) intravenous placebo for three weeks followed by oral placebo for six months 48. Evidence of improvements in sensory and motor deficits — assessed by physicians — could be observed after three weeks of intravenous alpha lipoic acid therapy, yet not at the end of six months of oral alpha lipoic acid therapy. However, another randomized, double-blind, placebo-controlled trial in 181 patients with diabetic neuropathy found that oral supplementation with either 600 mg/day, 1,200 mg/day, or 1,800 mg/day of alpha lipoic acid for five weeks significantly improved neuropathic symptoms 49. In this study, the 600 mg/day dose was as effective as the higher doses. Finally, a four-year, multicenter, clinical trial in 421 diabetic patients with distal symmetric sensorimotor polyneuropathy found no difference between oral administration of 600 mg/day of alpha lipoic acid and placebo on the primary endpoint, a composite score that assessed neuropathic impairment of the lower limbs and nerve conduction 50. Yet, measures of specific neuropathic impairments (secondary outcomes) improved with alpha lipoic acid supplementation 50. A post-hoc analysis suggested that oral alpha lipoic acid supplementation may reduce neuropathic symptoms particularly in subjects with a high burden of cardiovascular disease, diabetes, and neuropathy yet with normal body mass index (BMI) and blood pressure 51. Overall, the available research suggests that treatment with intravenous or oral alpha lipoic acid may help reduce symptoms of diabetic peripheral neuropathy.
Alpha-lipoic acid is a type of antioxidant and chemoprotective agent. Alpha-lipoic acid is an antioxidant — a substance the body can use to prevent or manage a tissue-damaging process called oxidative stress. Oxidative stress is a part of the diabetic neuropathy disease process. Alpha-lipoic acid also has been shown to reduce blood sugar levels. Alpha lipoic acid is being studied for its ability to protect normal cells from the side effects of chemotherapy and prevent peripheral neuropathy (numbness, tingling, burning, and weakness in the hands or feet) 52. A few small clinical trials have tested the treatment effect of alpha-lipoic acid given either as a supplement or intravenously. People with diabetic neuropathy had reduced pain, improvements in nerve function tests and improvements in other clinical measures of diabetic neuropathy (Han T, Bai J, Liu W, et al. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. European Journal of Endocrinology. 2012;167(4):465-471.)). But long-term studies are needed.
Alpha-lipoic acid is being studied for its effect on complications of diabetes, including diabetic macular edema (an eye condition that can cause vision loss) and diabetic neuropathy (nerve damage caused by diabetes).
- In a 2011 study of 235 people with type 2 diabetes, 2 years of supplementation with alpha-lipoic acid did not help to prevent macular edema 53.
- A 2016 assessment of treatments for symptoms of diabetic neuropathy that included 2 studies of oral alpha-lipoic acid, with a total of 205 participants, indicated that alpha-lipoic acid may be helpful 54.
Currently, alpha lipoic acid is available in tablets and capsules of 50 to 600 mg and the recommended dosage has ranged from 100 to 600 mg once or twice daily. Alpha lipoic acid is usually well tolerated when taken as recommended but side effects at higher doses can include abdominal discomfort, heartburn, constipation or diarrhea, nausea, dizziness, and headache. Rare, potentially severe adverse effects reported after single large overdoses include confusion, stupor, seizures, lactic acidosis, rhabdomyolysis, coma, and multiorgan failure that can be fatal. Don’t use alpha-lipoic acid if you’re a heavy user of alcohol.
Alpha lipoic acid deficiency has been described in rare cases of inherited mutations in the lipoic acid biosynthetic pathway. Mutations identified in patients with defective alpha lipoic acid metabolism affect genes involved in the synthesis of iron-sulfur clusters and genes coding for lipoic acid synthetase (LIAS), lipoyl transferase 1 (LIPT1), and dihydrolipoamide dehydrogenase (E3 component of alpha-ketoacid dehydrogenase complexes; DLD) 55, 56, 57.
Figure 1. Alpha lipoic acid R and S enantiomers
Figure 2. Alpha lipoic acid functions
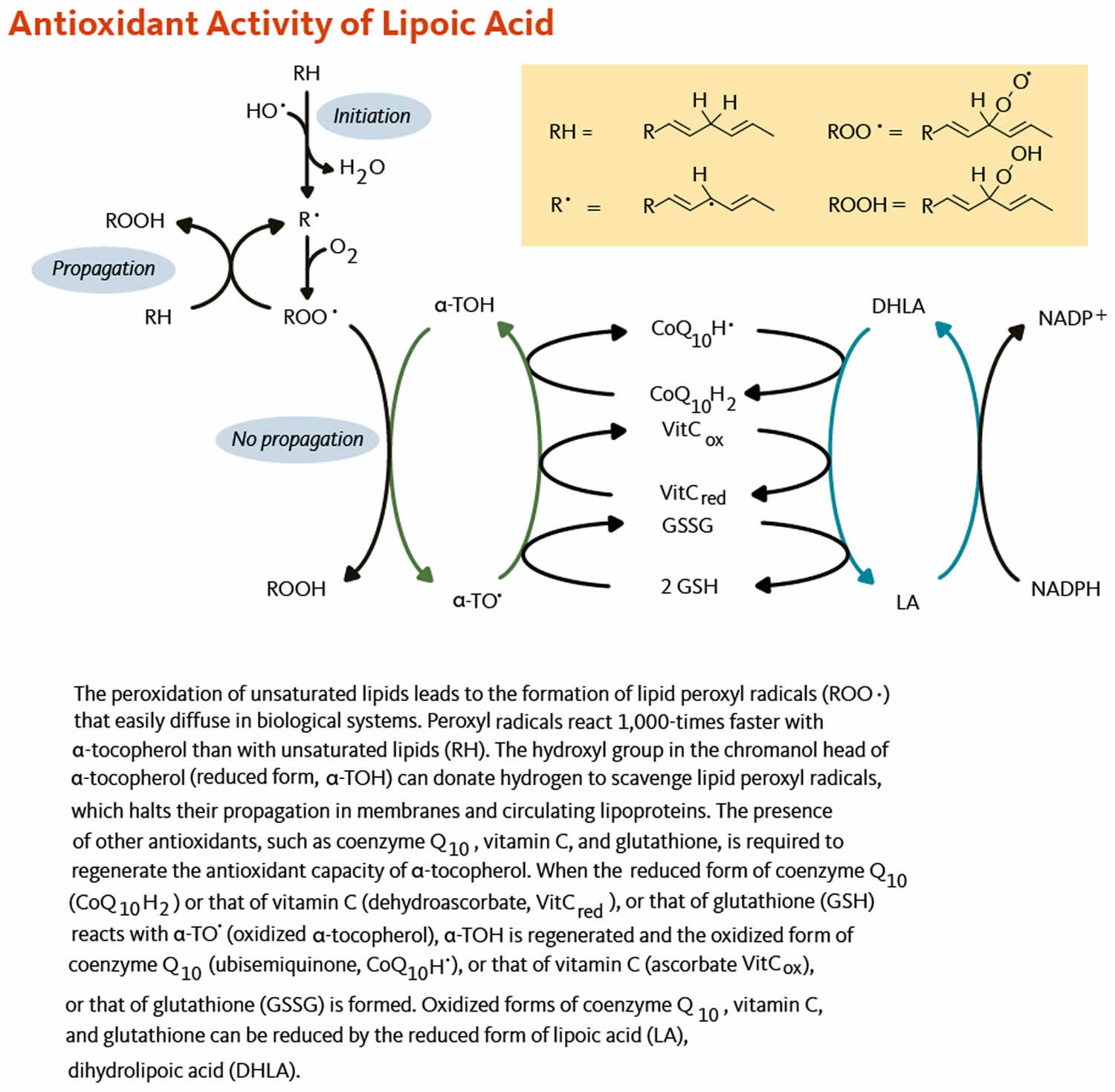
[Source 58 ]Figure 3. Alpha lipoic acid antioxidant activity
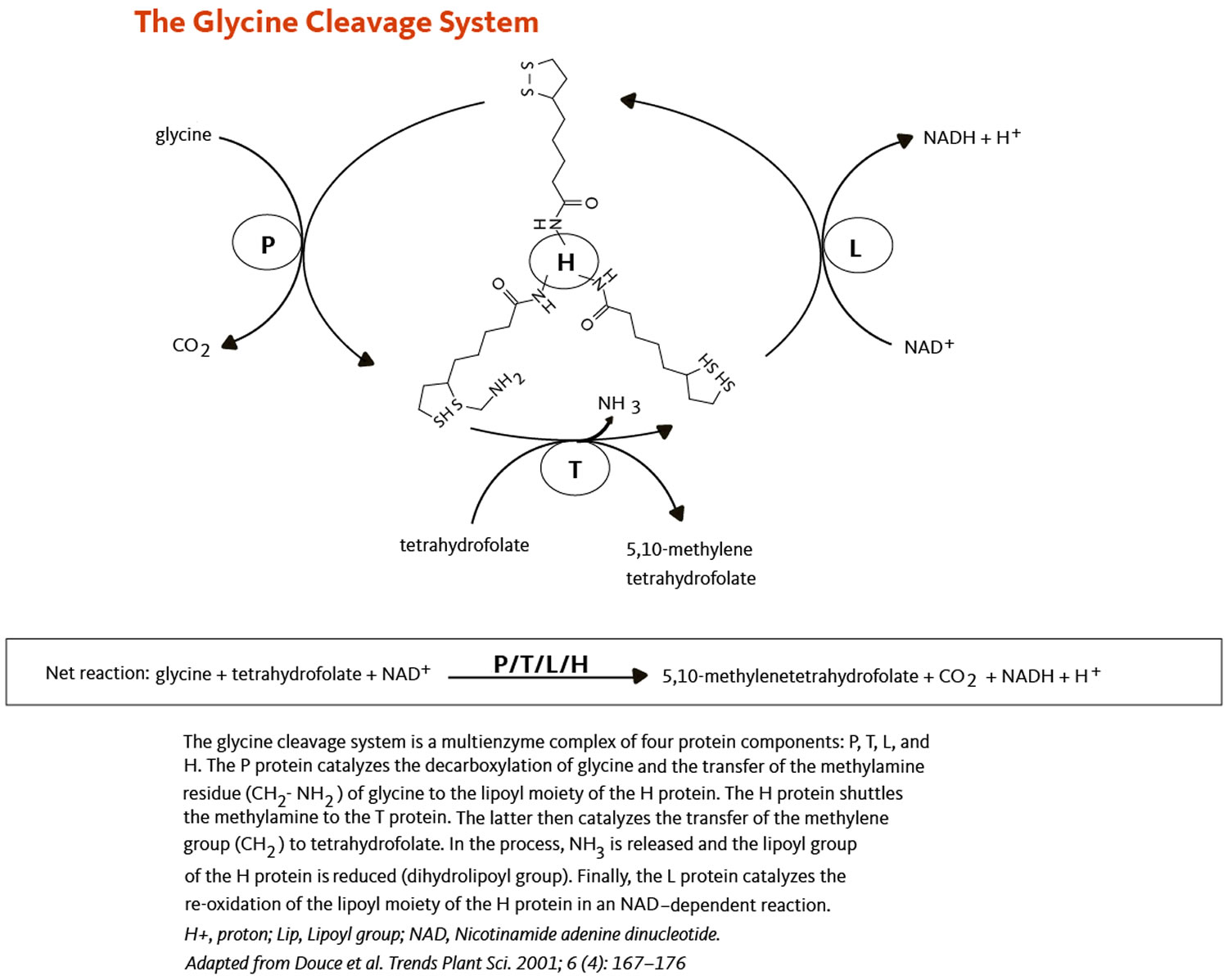
[Source 59 ]Figure 4. Alpha lipoic acid in the glycine cleavage system
[Source 59 ]Figure 5. Alpha lipoic acid in alpha-ketoacid-dehydrogenase multienzyme complexes
[Source 59 ]Alpha lipoic acid functions
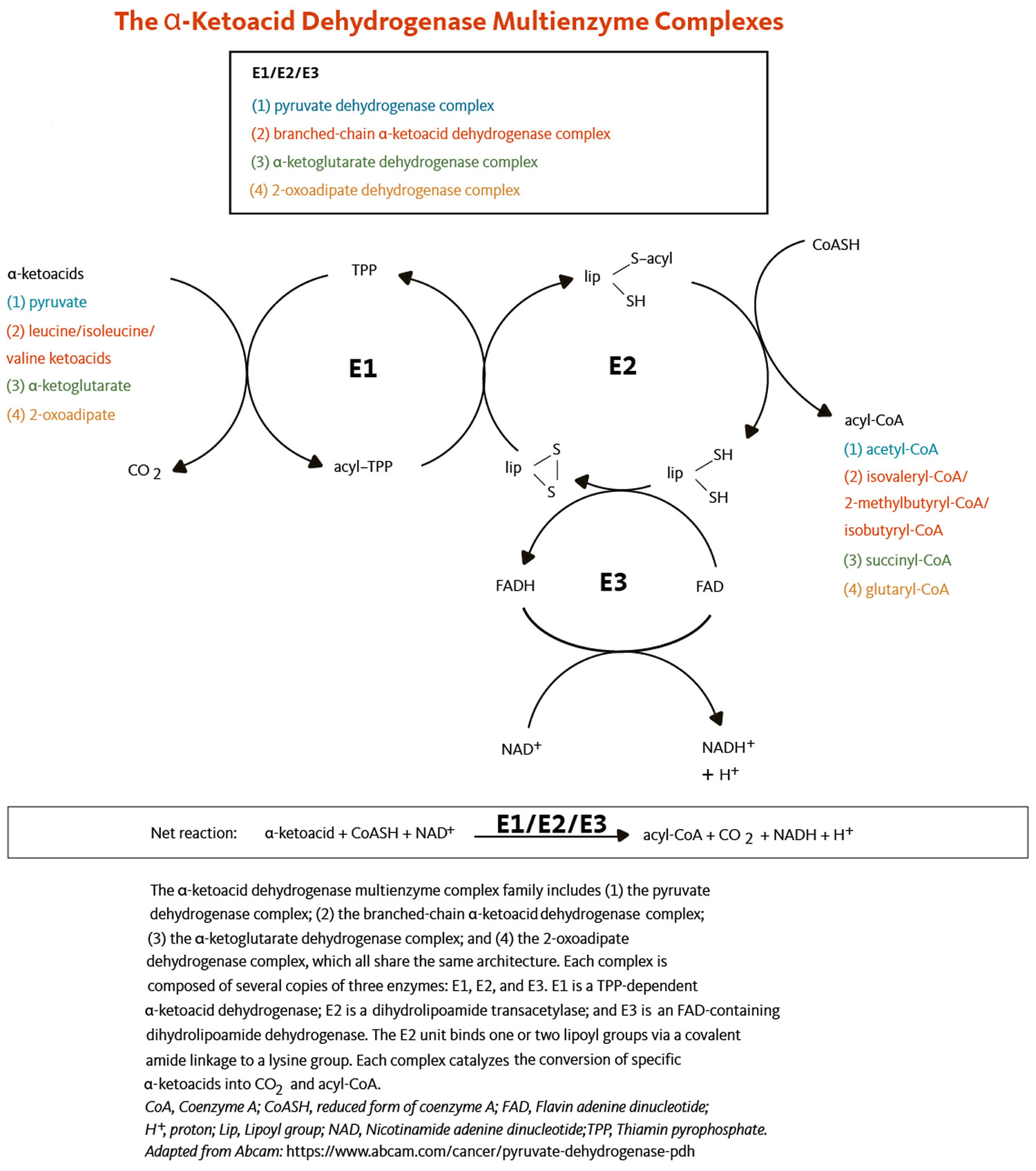
R-alpha lipoic acid is an essential cofactor (a compound that is essential for the activity of an enzyme) for several mitochondrial multienzyme complexes that catalyze critical reactions related to the catabolism (breakdown) of amino acids and the production of energy 11, 12, 13. R-alpha lipoic acid is covalently bound to a specific lysine residue in at least one of the proteins in each multienzyme complex. Such a non-protein cofactor is known as a “prosthetic group”. R-alpha lipoic acid functions as a “prosthetic group” for the biological activity of the following multienzyme complexes:
- The glycine cleavage system that catalyzes the decarboxylation of glycine coupled with the addition of a methylene group (-CH2) to tetrahydrofolate to form 5,10-methylene tetrahydrofolate, an important cofactor in the synthesis of DNA (deoxyribonucleic acid) (Figure 3). Within the glycine cleavage system, R-alpha lipoic acid is covalently bound to a conserved lysine of the H protein (Figures 3 and 4).
- Four alpha-ketoacid dehydrogenase complexes including:
- the pyruvate dehydrogenase complex that catalyzes the conversion of pyruvate to acetyl-coenzyme A (CoA), an important substrate for energy production via the citric acid cycle;
- the α-ketoglutarate dehydrogenase complex that catalyzes the conversion of α-ketoglutarate to succinyl CoA, another important intermediate of the citric acid cycle;
- the branched-chain α-ketoacid dehydrogenase complex that is involved in the decarboxylation of ketoacids in the catabolic pathway of the branched-chain amino acids, namely leucine, isoleucine, and valine;
- the 2-oxoadipate dehydrogenase complex that catalyzes the decarboxylation of 2-oxoadipate to glutaryl-CoA in the catabolic pathway of lysine, hydroxylysine, and tryptophan.
All four alpha-ketoacid dehydrogenase complexes contain three enzymatic activities, namely E1, E2, and E3. E1 is a thiamin pyrophosphate (TPP)-dependent alpha-ketoacid dehydrogenase, R-alpha-lipoic acid functions as a prosthetic group essential for E2 transacetylase activity, and E3 is a flavin adenine dinucleotide (FAD)-dependent dihydrolipoamide dehydrogenase (Figure 4). R-alpha-lipoic acid is also found in the E3-binding protein (protein X component) of the pyruvate dehydrogenase complex 55.
Antioxidant activities
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are highly reactive compounds with the potential to damage DNA, proteins, and lipids in cell membranes. Alpha-lipoic acid exerts its antioxidant activity through multiple mechanisms, including radical scavenging, the regeneration of other antioxidants, metal chelation, and signalling regulation. The sulfur atoms within the alpha-lipoic acid ring play a crucial role in exerting antioxidant activity. The oxidized alpha-lipoic acid and its reduced derivative (dihydrolipoic acid, DHLA) function as a potent redox couple, participating in different aspects of the antioxidant defence system by directly scavenging (neutralizing) physiologically relevant reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the test tube, thereby reducing oxidative stress and damage 60. Different mechanisms have been proposed for the free-radical scavenging activity of alpha-lipoic acid 61. Alpha lipoic acid was suggested to work through a proton-loss electron transfer mechanism, while dihydrolipoic acid (DHLA) exhibits its activity by single electron transfer followed by proton transfer, or hydrogen atom transfer. In dihydrolipoic acid (DHLA), the ideal spacing between the sulfhydryl and carboxylate groups, determined by the optimal length of the alkyl chain, facilitates the efficient trapping of radicals and the transfer of hydrogen atoms 62.
One distinctive feature of alpha-lipoic acid is its ability to regenerate the reduced form of either non-enzymatic antioxidants, such as vitamins C and E, glutathione and cysteine, or enzymatic antioxidants, like the coenzyme Q10 63. By recycling other antioxidants, alpha-lipoic acid actively contributes to maintaining the antioxidant network and the cellular redox equilibrium.
Furthermore, alpha-lipoic acid acts as a modulator of proteins involved in cell signalling and transcription through the thiol/disulfide exchange mechanism 64, 65, 66. As a result, it has been proposed to influence different aspects, including cell growth and death regulation, inflammatory responses, and glucose and lipid metabolism.
The highest tissue concentrations of free alpha lipoic acid likely to be achieved through oral supplementation are at least 10 times lower than those of other intracellular antioxidants, such as vitamin C and glutathione. Moreover, free alpha lipoic acid is rapidly eliminated from cells, so any increases in direct free radical scavenging activity are unlikely to be sustained 59.
Regeneration of other antioxidants
When an antioxidant scavenges a free radical, it becomes oxidized itself and is not able to scavenge additional ROS or RNS until it has been reduced. In the test tube, dihydrolipoic acid (DHLA) is a potent reducing agent with the capacity to reduce the oxidized forms of several important antioxidants, including coenzyme Q10, vitamin C, and glutathione 67, 68. Dihydrolipoic acid (DHLA) may also reduce the oxidized form of alpha-tocopherol (vitamin E) directly or indirectly through regenerating oxidized vitamin C 69 or oxidized coenzyme Q10 70. Whether dihydrolipoic acid (DHLA) effectively regenerates antioxidants under physiological conditions is unclear 60.
Metal chelation
Redox-active metal ions, such as free iron and copper, can induce oxidative damage by catalyzing reactions that generate highly reactive free radicals 71. Compounds that chelate free metal ions in a way that prevents them from generating free radicals offer promise in the treatment of neurodegenerative diseases and other chronic diseases in which metal-induced oxidative damage may play a pathogenic role 72. Both alpha lipoic acid and dihydrolipoic acid (DHLA) have been found to inhibit copper- and iron-mediated oxidative damage in the test tube 73, 74 and to inhibit excess iron and copper accumulation in animal models 74, 75. Alpha lipoic acid may also be helpful as an add-on treatment against heavy metal toxicity. No clinical trial has examined the use of alpha lipoic acid as a chelating agent in mercury toxicity, yet it has proven to be effective in several mammalian species 76, 77.
Activation of antioxidant signaling pathways
Glutathione is an important intracellular antioxidant that also plays a role in the detoxification and elimination of potential carcinogens and toxins. Reductions in glutathione synthesis and tissue glutathione concentrations in aged animals compared to younger ones are suggestive of a potentially lower ability to respond to oxidative stress or toxin exposure 78. Alpha lipoic acid has been found to increase glutathione concentrations in cultured cells and in the tissues of aged animals fed alpha lipoic acid 79, 80. Alpha lipoic acid might be able to increase glutathione synthesis in aged rats by up-regulating the expression of gamma-glutamylcysteine ligase (γ-GCL), the rate-limiting enzyme in glutathione synthesis 81 and by increasing cellular uptake of cysteine, an amino acid required for glutathione synthesis 82. Alpha lipoic acid was found to upregulate the expression of gamma-glutamylcysteine ligase (γ-GCL) and other antioxidant enzymes via the activation of the nuclear factor E2-related factor 2 (Nrf2)-dependent pathway 81, 83.
Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor that is bound to the protein Kelch-like ECH-associated protein 1 (Keap1) in the cytosol. Keap1 responds to oxidative stress signals by freeing Nrf2. Upon release, nuclear factor E2-related factor 2 (Nrf2) translocates to the nucleus where it can bind to the antioxidant response element (ARE) located in the promoter region of genes coding for antioxidant enzymes and scavengers. Alpha lipoic acid but not dihydrolipoic acid (DHLA) can react with specific sulfhydryl residues of Keap1, causing the release of Nrf2 84. Nrf2/antioxidant response element (ARE) target genes code for several mediators of the antioxidant response, including gamma-glutamylcysteine ligase (γ-GCL), nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1 (NQO-1), heme oxygenase-1 (HO-1), catalase, and superoxide dismutase (SOD). For example, the upregulation of the nuclear factor E2-related factor 2 (Nrf2) pathway by lipoic acid in cultured hepatocytes and in the liver of obese or diabetic rats prevented lipid overload-induced steatosis 85 and cell death 86. Alpha lipoic acid also protected liver from oxidative stress-induced liver injury in methotrexate-treated rats through the activation of Nrf-2 pathway and other anti-inflammatory pathways 87. Pre-treatment and post-treatment with alpha-lipoic acid prevented and reversed lipopolysaccharide (LPS)-induced lung histopathological alterations in rats through Nrf2-mediated HO-1 upregulation 88.
Inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is a plasma membrane-bound enzymatic complex that catalyzes the production of superoxide from oxygen and NADPH and has been involved in innate immune defense against microbes 89. Alpha lipoic acid prevented NOX-induced superoxide production in a rat model of cerebral ischemia and limited infarct volume and neurological deficiencies through upregulating the insulin-phosphatidylinositide-3 kinase (PI3K)-protein kinase B (PKB/Akt) signaling pathway 90. Treatment of gastric cancer cells with alpha lipoic acid limited NOX-generated reactive oxygen species (ROS) production and reduced cancer cell proliferation induced by Helicobacter pylori (H. pylori) infection 91.
Regulation of cellular glucose uptake
The binding of insulin to the insulin receptor stimulates a cascade of protein phosphorylations leading to the translocation of glucose transporters 4 (GLUT4) to the cell membrane and an increased cellular uptake of glucose 92. Alpha lipoic acid has been found to activate the insulin signaling cascade in cultured cells 92, 93, increase glucose transporters 4 (GLUT4) translocation to cell membranes, and increase glucose uptake in cultured adipose and muscle cells 94, 95. A computer modeling study suggested that alpha lipoic acid might bind to the intracellular tyrosine kinase domain of the insulin receptor and stabilize the active form of the enzyme 93.
Regulation of other signaling pathways
In addition to nuclear factor E2-related factor 2 (Nrf2) and insulin signaling pathways, alpha lipoic acid was found to target other cell-signaling molecules thereby affecting a variety of cellular processes, including metabolism, stress responses, proliferation, and survival. For example, in cultured endothelial cells, alpha lipoic acid was found to inhibit IKK-β, an enzyme that promotes the translocation of redox-sensitive and pro-inflammatory transcription factor, nuclear factor-kappa B (NFκB) from the cytosol to the nucleus 96. Alpha lipoic acid has also been shown to improve nitric oxide (NO)-dependent vasodilation in aged rats by increasing PKB/Akt-dependent phosphorylation of endothelial NO synthase (eNOS) and eNOS-catalyzed nitric oxide (NO) production 97. Additionally, alpha lipoic acid increased mitochondrial biogenesis through triggering AMP-activated protein kinase (AMPK)-induced transcription factor PGC-1α activation in skeletal muscle of aged mice 98. Several reviews of the literature have described pathways that are potential targets of alpha lipoic acid in various models and under different experimental conditions 99, 11, 100, 41.
Alpha lipoic acid foods
Almost all foods contain alpha lipoic acid, but slightly more so in organ meats (kidney, heart, liver), spinach, broccoli, peas, brussel sprouts, and rice bran. R-alpha lipoic acid occurs naturally in food covalently bound to lysine in proteins (lipoyllysine). Animal tissues with high alpha lipoic acid lysine content (~1-3 μg/g dry weight) include kidney, heart, and liver, while lipoyllysine-rich vegetables include spinach and broccoli 101. Somewhat lower amounts of alpha lipoic acid lysine (~0.5 μg/g dry weight) have been measured in tomatoes, peas, and Brussels sprouts. Consumption of alpha lipoic acid from food has not yet been found to result in detectable increases of free alpha lipoic acid in human plasma or cells 102, 60.
Alpha lipoic acid supplements
Unlike alpha lipoic acid in foods covalently bound to lysine in proteins (lipoyllysine), alpha lipoic acid in supplements is not bound to protein. Moreover, the amounts of alpha lipoic acid available in dietary supplements (50-600 mg) are likely as much as 1,000 times greater than the amounts that could be obtained from foods. In Germany, alpha lipoic acid is approved for the treatment of diabetic neuropathy and retinopathy and is available by prescription 17. Alpha lipoic acid is available as a dietary supplement without a prescription in the US. Most alpha lipoic acid supplements contain a racemic mixture of R-alpha lipoic acid and S-alpha lipoic acid (sometimes noted d,l-alpha lipoic acid). Supplements that claim to contain only R-alpha lipoic acid are usually more expensive, and information regarding their purity is not publicly available 103. Since taking alpha lipoic acid with a meal decreases its bioavailability, it is generally recommended that alpha lipoic acid be taken 30 minutes prior to a meal (see also Metabolism and Bioavailability) 104.
High oral doses of free alpha lipoic acid (≥50 mg) significantly, yet transiently, increase the concentration of free alpha lipoic acid in plasma and cells. Pharmacokinetic studies in humans have found that about 30%-40% of an oral dose of a racemic mixture of R-alpha lipoic acid and S-alpha lipoic acid is absorbed 102, 105. Oral alpha lipoic acid supplements are better absorbed on an empty stomach than with food: taking alpha lipoic acid with food versus without food decreased peak plasma alpha lipoic acid concentrations by about 30% and total plasma alpha lipoic acid concentrations by about 20% 104. Oral liquid form of R-alpha lipoic acid was found to be better absorbed and more stable in the plasma, suggesting that it might be more efficacious than the solid form R-alpha lipoic acid in the management of a condition like diabetic neuropathic pain 106, 107.
There may also be differences in bioavailability of the two isomers of alpha lipoic acid. Following single oral doses R,S-alpha lipoic acid (racemic mixture), peak plasma concentrations of R-alpha lipoic acid were found to be 40%-50% higher than S-alpha lipoic acid, suggesting a differential absorption in favor of the R-alpha lipoic acid 102, 104, 108. Yet, following oral ingestion, both R,S-alpha lipoic acid (racemic mixture) are rapidly broken down (metabolized) and excreted. Plasma alpha lipoic acid concentrations generally peak within one hour or less and decline rapidly 102, 108, 109. In cells, alpha lipoic acid is swiftly reduced to dihydrolipoic acid (DHLA) and test tube studies indicate that dihydrolipoic acid (DHLA) is then rapidly exported from cells 60. Moreover, a pilot study in 19 healthy adults suggested that the bioavailability of R,S-alpha lipoic acid and R-alpha lipoic acid may vary with age and gender 110.
In rats, R-alpha lipoic acid was more effective than S-alpha lipoic acid in enhancing insulin-stimulated glucose transport and metabolism in skeletal muscle 111 and R-alpha lipoic acid was more effective than R,S-alpha lipoic acid and S-alpha lipoic acid in preventing cataracts in newborn rats 112. However, all of the published human studies have used R,S-alpha lipoic acid (racemic mixture). It has been suggested that the presence of S-alpha lipoic acid in the racemic mixture may limit the polymerization of R-alpha lipoic acid and enhance its bioavailability 41. At present, it remains unclear which supplemental form is best to use in clinical trials.
Finally, there is no evidence in humans that exogenous alpha lipoic acid can be ‘activated’ with ATP or GTP and incorporated into lipoic acid-dependent enzymes by a lipoyl transferase 113. As a consequence, a loss of alpha lipoic acid-dependent enzymatic activity caused by defects in endogenous alpha lipoic acid synthesis cannot be rescued by the provision alpha lipoic acid supplement 55.
What is alpha lipoic acid used for?
Alpha lipoic acid have antioxidant activity in scavenging free radicals and restoring glutathione levels, as well as antidiabetes activities in normalizing glucose and insulin activity and antiaging and as chelator for heavy metals like mercury, iron, cadmium, lead, and copper 114, 115, 116. Alpha-lipoic acid has recently gained a reputation as an antioxidant. In the reduced form, dihydrolipoate reacts and neutralizes reactive oxygen species (ROS), such as superoxide radicals, singlet oxygen, and hydroxyl radicals. Theref0re, alpha-lipoic acid is highly beneficial in several oxidative-stress-associated conditions such as ischemia-reperfusion or radiation injury 117. Multiple studies of alpha lipoic acid in patients with diabetic polyneuropathy, arthritis, diabetes, fibromyalgia, multiple sclerosis, osteoarthritis and other conditions have yielded variable results. So far, alpha-lipoic acid has the most substantial evidence of the therapeutic effect in diabetic neuropathy and oxidative stress conditions. There is still a need for more studies on the benefits of other conditions such as HIV/AIDS, liver disease, multiple sclerosis and weight loss. However, alpha lipoic acid has not been approved for use by the FDA for any medical disease or condition.
Numerous studies have strongly supported the role of alpha-lipoic acid in treating diabetic neuropathy. Alpha-lipoic acid does so by enhancing nitric oxide-mediated endothelium-dependent vasodilation, improving microcirculation in patients with diabetic polyneuropathy 118. In placebo controlled clinical trials in patients with diabetes and peripheral neuropathy, alpha lipoic acid was associated with mild improvements in markers of neuropathy but was not shown to reduce symptoms or progression of peripheral neuropathy. Additionally, when taken with avocado or soybean unsaponifiable compounds, alpha-lipoic acid is shown to significantly suppress prostaglandin E-2 production, a key cytokine in the pathogenesis of inflammation 119.
Alpha-lipoic acid possesses an excellent iron-chelation property. The thiol groups in alpha-lipoic acid are responsible for chelating irons. By increasing the glutathione levels inside the cells, alpha-lipoic acid and dihydrolipoate excrete toxins, especially toxic metals, into the body. Alpha-lipoic acid preferentially binds to zinc, lead, and copper. On the other hand, dihydrolipoate forms complexes with iron, zinc, mercury, lead, and copper 41.
Diabetic peripheral neuropathy
People with both types of diabetes develop multisystem complications 120, one of the most frequent being diabetic peripheral neuropathy. Diabetic peripheral neuropathy has an estimated prevalence in the diabetic population of between 10% and 100% depending upon the data source and ascertainment methodology 121.
Diabetic peripheral neuropathy is defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” 122. Diabetic peripheral neuropathy may be asymptomatic and insidious at onset. The most common symptom of diabetic peripheral neuropathy is neuropathic pain, which occurs in up to 50% of people with diabetic peripheral neuropathy and is the most frequent reason for seeking medical care 123. Painful symptoms are varied and include pain, tingling, burning sensations, paresthesia, shooting or lancinating pains, aching, and contact pain (allodynia) provoked by clothing 124.
Diabetic peripheral neuropathy can be classified clinically as either focal or diffuse. Diffuse disease can affect the sensorimotor or the autonomic nervous systems or both. Sensorimotor disease can involve large or small nerve fibers 125, is usually predominantly sensory, and may be painful.
Distal symmetrical sensorimotor polyneuropathy is the most common form of diabetic peripheral neuropathy, with a reported prevalence in diabetes mellitus ranging from 28.5% to 45%, increasing with age and disease duration 126. Distal symmetrical sensorimotor polyneuropathy represents a major cause of morbidity and the leading source of diabetes-related hospitalizations and non-traumatic amputations. It is also accountable for considerable physical disability, altered quality of life, and increased mortality 124.
Diabetic peripheral neuropathy complications are also a major threat to the general well-being and quality of life of people with diabetes. Numbness caused by diabetic peripheral neuropathy, along with retinopathy and vestibular dysfunction, increase the risk of falls two- to three-fold compared to people without diabetic peripheral neuropathy 127. People with diabetic peripheral neuropathy are also seven times more likely to develop foot ulcerations 128. Foot ulcerations further predispose to active or passive soft tissue infection, which can progress to bone infection and subsequent lower extremity amputation 129. Diabetic peripheral neuropathy, peripheral vascular disease, and soft tissue and bone deformity are serious complications that make diabetes the leading cause of lower extremity amputation 130.
Diabetic peripheral neuropathy symptoms are usually assessed using patient-reported outcome measures that quantify discomfort, sleep disturbances, and quality of life 123.
The pathophysiology of diabetic peripheral neuropathy is not fully understood, and very likely to be multifactorial (genetic, environmental, behavioral, metabolic, neurotrophic, and vascular) 131. Oxidative stress generated by excess free-radical formation or errors in antioxidant protection, or both, is thought to be important in the pathogenesis 132. Good glycemic control reduces the risk of developing diabetic peripheral neuropathy, but glycemic control is not always achievable and is usually not sufficient to halt diabetic peripheral neuropathy progression 131.
Diabetic peripheral neuropathy pathophysiology can mainly be explained as neural dysfunction caused by the interplay of decreased blood flow to nerves as a result of hyperglycaemia, and increased oxidative stress, which induces local inflammatory reactions through reactive oxygen species (ROS) 133. Prolonged hyperglycemia simultaneously activates multiple pathways. It promotes the following:
- Activation of polyol and protein kinase pathways that leads to reduced nicotinamide adenine dinucleotide phosphate (NADPH) and subsequent depletion of glutathione and nitric oxide 134.
- Angiogenesis driven by the vascular endothelial growth factor pathway.
- Basement membrane thickening and endothelial proliferation (via transforming growth factor-β and nuclear factor – kappa B), which cause altered capillary permeability and local hypoxia.
- Activation of the hexosamine pathway and shunting of fructose-6-phosphate from the glycolytic pathway.
- Modified gene expression for glucose transporters and glucokinase 135.
Generation of reactive oxygen species and advanced glycosylation end-products activates the same NFkB pathway, which increases oxidative stress with additional nicotinamide adenine dinucleotide phosphate (NADPH) depletion. Oxidative stress also induces poly (ADP-ribose) polymerase activation, which sequentially results in supplementary nicotinamide adenine dinucleotide depletion, positive loop activation of the protein kinase pathway, and promotes inflammation 136. All these pathways promote mitochondrial dysfunction, which in turn is followed by apoptosis, axonal degeneration, and axonal death. Local pro-inflammatory cytokines induced by oxidative stress promote macrophage recruitment with subsequent glial failure, myelin breakdown, and impaired nerve regeneration 137.
The clinical consequences of this hyperglycemia-induced inflammatory and oxidative state are axonal dystrophy, decreased nerve conduction velocity, diminished neurovascular flow and, ultimately, small- and large-fiber neuropathy 125.
Current management of diabetic peripheral neuropathy consists of three therapeutic approaches. The main target is prevention, through control of fasting and postprandial glucose 138. Medications that target symptoms and disease-modifying treatments are used in the treatment of people with diagnosed diabetic peripheral neuropathy. Symptomatic treatments target pain; they include anticonvulsants, tricyclic antidepressants 139, serotonin and noradrenaline reuptake inhibitors 140, opioids and opioid-like drugs 141, systemic local anaesthetics 142, nonsteroidal anti-inflammatory agents 141, and non-drug therapies such as transcutaneous electrical nerve stimulation (TENS), pulsed radiofrequency sympathectomy 143, and acupuncture 144.
Disease-modifying treatments aim to prevent, slow, or reverse diabetic peripheral neuropathy progression by reduction of oxidative stress and inhibition of the polyol, hexosamine, protein kinase, advanced glycosylation product, and poly (ADP-ribose) polymerase pathways.
Alpha lipoic acid acts as a scavenger of reactive oxygen species and has antioxidant properties that could block the oxidative stress–inflammation pathways activated in diabetic peripheral neuropathy 145. Alpha lipoic acid could therefore be useful both in prevention and treatment of diabetic peripheral neuropathy 145.
Early in test tube studies showed that alpha lipoic acid and its reduced form, dihydrolipoic acid (DHLA), scavenge reactive oxygen species, including hydroxyl radicals, hypochlorous acid, and singlet oxygen 146. In animal studies also indicated that alpha lipoic acid decreases oxidative stress 147, participates in restoring endogenous cellular antioxidant levels and reducing pro-inflammatory pathways 148 and may influence the regeneration of vitamins C and E 145.
The benefit of alpha lipoic acid in people with diabetes could range beyond antioxidant and anti-inflammatory effects. The therapeutic properties of alpha lipoic acid might include the ability to restore glucose availability and increase insulin-stimulated glucose transport and non-oxidative and oxidative glucose metabolism in insulin-resistant muscle cells 149. Alpha lipoic acid has therefore been a candidate for clinical study in diabetic peripheral neuropathy. The efficacy of lipoic acid, administered either intravenously or orally, in the management of neuropathic symptoms has been examined in patients with diabetes.
The interaction of alpha lipoic acid with regulatory components of the insulin signaling cascade has proved functionally beneficial to skeletal muscle glucose uptake, whole-body glucose tolerance, and helpful against insulin resistance in animal models 150. Improvements in glucose disposal were also observed in human patients with type 2 diabetes receiving alpha lipoic acid either intravenously or orally 151. Several clinical trials have been conducted to measure the efficacy of racemic alpha lipoic acid in decreasing symptoms of diabetic polyneuropathies; these are the “alpha-lipoic acid in diabetic neuropathy” (ALADIN) trials and the “symptoms of diabetic polyneuropathy” (SYDNEY) trials. Alpha lipoic acid was given orally or intravenously (i.v.) with oral follow-up. A meta-analysis of four clinical trials using i.v. alpha lipoic acid, including ALADIN, SYDNEY, and the first 3 weeks of ALADIN III, showed a significant improvement in diabetic polyneuropathies of the feet and lower limbs in patients infused with alpha lipoic acid 600 mg/day, for three weeks 152. Diabetic patients in the ALADIN II trial were administered alpha lipoic acid i.v. at 600 or 1200 mg/day for 5 days, then oral alpha lipoic acid for 2 years, resulting in improved indices of neuropathy 153. Patients in the ALADIN III study received alpha lipoic acid (600 mg/day i.v.) or placebo for three weeks, followed by oral alpha lipoic acid (600 mg t.i.d.) or placebo for 6 months. The oral phase of this trial, however, was without clinically significant benefits 154. One possible conclusion from these studies was that alpha lipoic acid administered intravenously was more efficacious than oral alpha lipoic acid, which may be due to either greater bioavailability or poor solubility of the medication in the stomach acid. However, some additional studies have found that oral alpha lipoic acid is very effective. For example, the oral pilot (ORPIL) study showed a reduction in diabetic polyneuropathic symptoms after three weeks with 600 mg alpha lipoic acid three times daily 155. While the first SYDNEY trial used i.v. alpha lipoic acid 156, the SYDNEY II study used oral alpha lipoic acid at 600, 1200, or 1800 mg once daily for 5 weeks 157; consequently, both studies showed significant improvements in neuropathic endpoints.
A 2022 review that evaluated 8 studies (1,500 participants) indicated inconsistent findings of alpha-lipoic acid’s effectiveness in treating diabetic neuropathy: 3 studies found improvements in symptoms, and 5 studies did not 158. All 8 studies found alpha-lipoic acid to be safe, with no reported adverse effects 158. Another 2022 review of 9 studies (2,062 participants) found that alpha-lipoic acid might help reduce pain in people with diabetic neuropathy 159. A 2022 review of 12 studies (653 participants) found that alpha-lipoic acid supplementation did not improve kidney dysfunction in people with diabetes (diabetic nephropathy) 160. Some of the studies evaluated alpha-lipoic acid on its own, and the other studies looked at alpha-lipoic acid combined with pharmaceuticals or vitamin supplementation. The authors indicated that the evidence was limited because of the small number of studies and participants 160.
Cardiac autonomic neuropathy
Another neuropathic complication of diabetes mellitus is cardiac autonomic neuropathy, which occurs in as many as 25% of diabetic patients 46. Cardiac autonomic neuropathy is characterized by damage to the nerve fibers that innervate the heart and blood vessels, leading to reduced heart rate variability (variability in the time interval between heartbeats) and increased risk of mortality 161. In a randomized controlled trial of 72 patients with type 2 diabetes and reduced heart rate variability, oral supplementation with 800 mg/day of alpha lipoic acid for four months resulted in significant improvement in two out of four measures of heart rate variability compared to placebo 162.
Diabetes mellitus treatment
Alpha-lipoic acid has been studied for its effect on improving blood sugar and lipid (fat) levels in people with diabetes as well as on complications of diabetes, including diabetic macular edema (an eye condition that can cause vision loss), diabetic neuropathy (nerve damage caused by diabetes), and diabetic nephropathy (kidney damage caused by diabetes).
The effect of high-dose alpha lipoic acid on glucose utilization has been primarily examined in individuals with type 2 diabetes. An early clinical trial in 13 patients with type 2 diabetes found that a single intravenous infusion of 1,000 mg of alpha lipoic acid improved insulin sensitivity (insulin-stimulated glucose disposal) by 50% compared to a placebo infusion 163. A placebo-controlled study of 72 patients with type 2 diabetes found that oral administration of alpha lipoic acid at doses of 600 mg/day, 1,200 mg/day or 1,800 mg/day improved insulin sensitivity by 25% after four weeks of treatment 164. There were no significant differences among the three doses of alpha lipoic acid, suggesting that 600 mg/day may be the maximum effective alpha lipoic acid dose 46. However, in a more recent randomized placebo-controlled study in 102 subjects, daily supplementation with 600 mg of alpha lipoic acid (+/- 800 mg of vitamin E [α-tocopherol]) for 16 weeks had no effect on fasting blood glucose, fasting blood insulin, or a measure of insulin resistance called the homeostatic model assessment of insulin resistance (HOMA-IR) index 165. A 2018 systematic review and meta-analysis identified 20 randomized controlled trials (published between 2007 and 2017) that examined the effect of supplemental alpha lipoic acid on markers of glucose utilization in 1,245 subjects with metabolic disorders (not limited to type 2 diabetes) 166. Administration of alpha lipoic acid (200 to 1,800 mg/day for 2 weeks to 1 year), alone or together with other nutrients, was found to lower fasting blood glucose and insulin concentrations, insulin resistance, and blood HbA1c concentration — a marker of glycemic control over the past few months 166. A 2019 review of 10 studies (553 participants) showed that alpha-lipoic acid was no better than placebo at reducing levels of blood sugar, cholesterol, or triglycerides 167.
Diabetic retinopathy
Chronic hyperglycemia can damage blood vessels in the retina and cause a potentially sight-threatening condition called diabetic retinopathy. One placebo-controlled study examined the effect of alpha lipoic acid on the visual capability of 80 participants of whom 12 had type 1 diabetes, 48 had type 2 diabetes, and 20 were diabetes-free 168. The result showed that daily oral administration of 300 mg of alpha lipoic acid for three months prevented the deterioration of contrast sensitivity in patients with diabetes and improved it in healthy patients compared to placebo 168.
Alpha lipoic acid and weight loss
Previous studies have suggested anti-obesity properties of alpha lipoic acid 169, 170. In animal studies, it was shown that alpha lipoic acid supplementation promotes the reduction of body weight and fat mass by decreasing food intake and enhancing energy expenditure, possibly by suppressing hypothalamic AMP-activated protein kinase (AMPK) activity 171. However, studies in humans with alpha lipoic acid supplementation are limited, and the results have been inconsistent. Some clinical trials have shown that alpha lipoic acid supplementation may help overweight or obese individuals lose weight 172, while other studies have observed no effects of alpha lipoic acid on weight 173, 174. Nevertheless, alpha lipoic acid appears to have a wide range of beneficial effects on obesity related conditions such as insulin resistance, metabolic syndrome, and type II diabetes, including their complications such as vascular damage 175.
Cumulative results in a 2017 meta-analysis showed significant reduction of body weight and BMI (body mass index) with alpha lipoic acid treatment compared to placebo, regardless if it was used for weight loss or other purposes 176. Small but significant reduction of body weight with alpha lipoic acid intervention is in line with previous open label studies that have well documented the effectiveness of alpha lipoic acid on weight loss in overweight and obese individuals 172, 177 and randomized studies 178, 179, 180, 181. These results conclude that alpha lipoic acid supplementation with diet intervention may provide more beneficial effects on body weight management in overweight and obese individuals.
Studies in the 2017 meta-analysis explored various doses of alpha lipoic acid intervention (300 mg/day to 1800 mg/day) on different intervention durations (8 weeks to 52 weeks). Only one placebo controlled study compared the effectiveness of different doses of alpha lipoic acid on body weight 170. Koh et al. 170 explored the effects of 1200 mg/day and 1800 mg/day alpha lipoic acid intervention on body weight loss. Koh et al. 170 found that the higher dose of alpha lipoic acid resulted in significant weight loss and BMI reduction throughout the study compared to placebo. The lower dose of alpha lipoic acid led to significant weight loss in the first weeks of this study, however this effect was not sustainable through the entire duration of the study. From these findings, it can argue that the effect of alpha lipoic acid on body weight is limited to the short term, especially when it is used at lower doses with an adaptation mechanism taking over later. This may have implications for future study designs, for example phasic use of the medication may be tried 182.
A 2018 meta-analysis of randomized, placebo-controlled trials found that alpha lipoic acid supplementation in those with high body mass index (BMI) resulted in significant, yet modest, reductions in weight (9 studies) and BMI (11 studies) in the absence of caloric restriction (except in one study) 183. Subgroup analyses revealed that weight loss was greater in overweight versus obese participants, in unhealthy versus healthy participants, with daily doses ≤600 mg, and for intervention period shorter than 10 weeks 183. There was no reduction in waist circumference with supplemental alpha lipoic acid (5 studies) 183.
In summary, findings from 2017 176 and 2018 meta-analyses 184, 183 suggest that alpha lipoic acid may be a useful supplementation for weight loss in overweight and obese individuals in reducing body weight and BMI, but has no significant effect on waist circumference. The benefits of alpha lipoic acid compared to placebo appear smaller than that of available prescription weight loss medications 185. However, alpha lipoic acid can be considered in clinical practice due to its benign side-effect profile, other beneficial effects such as in diabetic neuropathy, and lower side effects comparing to the available weight loss medications. Further research is needed to examine the effect of different doses and the long-term benefits of alpha lipoic acid on metabolic parameter in unhealthy obese individuals.
Alpha lipoic acid on endothelial function
Vascular endothelial cells, which line the blood vessel lumen, form the physical interface between the blood and the vessel wall, preventing platelet adhesion and regulating blood vessel patency. The elasticity of the vessel wall is regulated by nitric oxide (NO), a gas produced by endothelial nitric oxide synthase (eNOS). Loss of endothelial nitric oxide synthase (eNOS) activity causes endothelial dysfunction due to nitric oxide (NO) limitation, and is characterized by reduced vasodilation, a proinflammatory milieu, and a prothrombic state. Oxidative stress has been implicated in endothelial dysfunction on the basis that antioxidants, such as ascorbate and alpha lipoic acid, improve the redox state of the plasma and endothelium-dependent nitric oxide (NO)-mediated vasodilation 186. Intra-arterial infusion of alpha lipoic acid was found to improve endothelium-dependent vasodilation in 39 subjects with type 2 diabetes but not in 11 healthy controls 187. A more recent randomized, double-blind, placebo-controlled study in 30 patients with type 2 diabetes found that intravenous infusion of 600 mg of alpha lipoic acid improved the response to the endothelium-dependent vasodilator acetylcholine but not to the endothelium-independent vasodilator, glycerol trinitrate 188. Another noninvasive technique using ultrasound to measure flow-mediated vasodilation was used in two additional studies conducted by Xiang et al. 189, 190. The results of these randomized, placebo-controlled studies showed that intravenous alpha lipoic acid could improve endothelial function in patients with impaired fasting glucose 189 or impaired glucose tolerance 190.
One randomized placebo-controlled trial (the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study) that assessed the effect of oral alpha lipoic acid supplementation in 58 patients diagnosed with metabolic syndrome, a condition characterized by abnormal glucose and lipid metabolism, showed that flow-mediated vasodilation improved by 44% with 300 mg/day of lipoic acid for four weeks 191.
Alpha lipoic acid as a hypotensive agent
Hypertension is a risk factor for stroke, heart attack and arterial aneurysm, and a leading cause of chronic kidney failure. Even moderate elevation in arterial blood pressure correlates with shortened life expectancy. The rationale for the therapeutic use of alpha lipoic acid against hypertension stemmed from its ability to increase tissue GSH levels and prevent deleterious sulfhydryl group modification in Ca2+ channels. Feeding alpha lipoic acid to hypertensive rats normalized systolic blood pressure and cytosolic free Ca2+, and attenuated adverse renal vascular changes 192. The role of alpha lipoic acid in regenerating reduced GSH was further put forth by El Midaoui and de Champlain 193 who associated the restoration of glutathione peroxidase activity seen in alpha lipoic acid-fed rats with the normalization of aortic superoxide production and blood pressure. It was also suggested that dietary alpha lipoic acid inhibits renal and vascular overproduction of endothelin-1, a vasoconstrictor secreted by the endothelium 194. Because NO is the main vasodilator in conduit arteries and the recent finding that alpha lipoic acid improves endothelial NO synthesis 195, pharmacologists have a new rationale to investigate the role of alpha lipoic acid and high blood pressure. Clinically, alpha lipoic acid administration (in combination with acetyl-L-carnitine) showed some promise as an antihypertensive therapy by decreasing systolic pressure in high blood pressure patients and subjects with the metabolic syndrome 196. In contrast, the administration of alpha lipoic acid (300 mg/day for 4 weeks) to patients with the metabolic syndrome had no significant effect on blood pressure compared to placebo group 197.
Alpha lipoic acid as an anti-inflammatory agent
Inflammation results from the innate biological response of vascular tissues to harmful agents, such as pathogens or irritants. It is an attempt by the organism to remove the injurious stimuli, protect the surrounding tissue, and initiate the healing process. However, unabated chronic inflammation also contributes to a host of diseases, such as atherosclerosis, asthma, and rheumatoid arthritis. Elevated levels of oxidative stress play an important role in chronic inflammation. Oxidative stress-associated inflammation is thought to provoke early vascular events in atherogenesis, including the upregulation of vascular adhesion molecules and matrix metalloproteinase activity. These events require the activation of NF-kappaB, a transcription factor that induces expression of many genes involved in inflammation and endothelial cell migration. Given the oxidative nature of inflammation, therapeutic strategies aimed at mitigating oxidant production and oxidative damage have been investigated for decades in various models of inflammation.
In keeping with this strategy, alpha lipoic acid has been studied for its antioxidant properties in cytokine-induced inflammation; it is also widely known as an inhibitor of NF-kappaB 198. Results show that alpha lipoic acid lowers expression of vascular cell adhesion molecule-1 (VCAM-1) and endothelial adhesion of human monocytes 199, and inhibits NF-kappaB-dependent expression of metalloproteinase-9 in vitro 200. Similarly, alpha lipoic acid (25-100 μg/ml = 122-486 μM) prevents the upregulation of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in spinal cords and in TNF-alpha stimulated cultured brain endothelial cells 201. Collagen-induced arthritis was attenuated by alpha lipoic acid (10-100 mg/kg i.p.) in DBA/1 mice by reduction of inflammatory cytokines like TNF-alpha, and partial inhibition of NF-kappaB binding to DNA 202. In this study, alpha lipoic acid also inhibited osteoclast formation, suggesting that alpha lipoic acid may be useful in the prevention of bone erosion and joint destruction in rheumatoid arthritis. In another study, pretreatment of collagen sheets with alpha lipoic acid (2 mg) prior to implantation decreased TNF-alpha-induced bone resorption in ICR mice 202. In experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis) alpha lipoic acid-treated mice showed marked improvement in central nervous system infiltrating T-cells and macrophages, decreased demyelination and spinal cord expression of adhesion molecules (ICAM-1 and VCAM-1) 203. The downregulation of surface CD4 seen in alpha lipoic acid-treated blood mononuclear cells was proposed to account, at least in part, for the modulation of inflammatory cell infiltration into the central nervous system 204. This is because co-receptor CD4 amplifies the signal generated at the T-cell receptor by recruiting lymphocyte protein kinase Lck, which in turn triggers a cascade of events leading to T-cell activation. Interestingly, DHalpha lipoic acid did not downregulate CD4 from the surface of human peripheral blood mononuclear cells 204. As an alternative or in addition to CD4 downregulation, the immunomodulatory properties of alpha lipoic acid may involve the upregulation of cAMP in T-cells and natural killer cells 205. Cell migration and neovascularization were also inhibited by alpha lipoic acid (86 μg/day in drinking water) in c57/black mice injected with Kaposi’s sarcoma in a matrigel sponge, as well as in nude mice injected with KS cells 206. In a mouse model of bronchial asthma, dietary alpha lipoic acid significantly attenuated airway hyper-responsiveness, lowered the eosinophil count among bronchoalveolar lavage cells, and significantly improved pathologic lesion scores of the lungs 207. alpha lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells via a mechanism seemingly distinct from antioxidants, such as ascorbate or reduced GSH, but consistent with the workings of a metal chelator 208. Recently, the inhibition of endotoxin-induced acute inflammation by alpha lipoic acid was associated with the stimulation of the PI3K/Akt pathway 209.
To date, the anti-inflammatory properties of alpha lipoic acid have rarely been investigated in humans. The ISalpha lipoic acidND (ISLAND) trial showed a 15% significant decrease in serum interleukin-6 levels following 4 weeks of supplementation with alpha lipoic acid (300 mg/day) 210. This finding may prove important to human health because interleukin-6 is a recognized marker of inflammation in coronary atherosclerotic plaques, and also regulates the expression of other inflammatory cytokines, such as interleukin-1 and TNF-alpha 211. However, the body of evidence is currently too limited to be conclusive.
Multiple sclerosis
Multiple sclerosis is an autoimmune disease of unknown cause that is characterized by the progressive destruction of myelin and nerve fibers in the central nervous system, causing neurological symptoms in affected individuals 212. There are four main types of multiple sclerosis defined according to the disease course: (i) clinically isolated syndrome, (ii) relapsing-remitting multiple sclerosis, (iii) secondary progressive multiple sclerosis, and (iv) primary progressive multiple sclerosis 213. Alpha lipoic acid was found to effectively slow multiple sclerosis disease progression in mice with experimental autoimmune encephalomyelitis (a model of multiple sclerosis) when administered either orally 214, intraperitoneally 215 or subcutaneously 216. Test tube and animal studies have found that alpha lipoic acid exhibits immunomodulatory properties through mechanisms that stimulate the production of cyclic AMP (cAMP) a central regulator of innate immune functions 217, 218 and inhibits the migration of immune cells into the brain and spinal cord 219, possibly by decreasing endothelial expression of cell adhesion molecules, inhibiting expression of enzymes like matrix metalloproteinases (MMP), and/or reducing the permeability of the blood-brain barrier 220, 221, 214, 216.
Only a few studies have examined alpha lipoic acid supplementation in humans. A small pilot study designed to evaluate the safety of alpha lipoic acid in 30 people with relapsing or progressive multiple sclerosis found that treatment with 1,200 to 2,400 mg/day of oral alpha lipoic acid for two weeks was generally well tolerated 222. In this study 222, higher serum concentrations of alpha lipoic acid were associated with the lowest serum concentrations of MMP-9 — a marker of inflammation. Another study suggested that an oral dose of 1,200 mg of alpha lipoic acid in subjects with multiple sclerosis could help achieve serum alpha lipoic acid concentrations similar to those found to be therapeutic in mice 223. A randomized, placebo-controlled study in 52 subjects (mean age, 30 years) with relapsing-remitting multiple sclerosis found an increase in total antioxidant capacity in blood with alpha lipoic acid supplementation (1,200 mg/day for 12 weeks) yet not in the activity of specific antioxidant enzymes (superoxide dismutase and glutathione peroxidase) 224. Supplemental alpha lipoic acid also decreased the serum concentrations of some (IFN-γ, ICAM-1, TGF-γ, IL-4), but not all markers (TNF-γ, IL-6, MMP-9), cytokines and other inflammation 225. In addition, alpha lipoic acid supplementation did not reduce the severity of multiple sclerosis symptoms, as assessed by the Expanded Disability Status Scale (EDSS) scoring system 225, 226.
A two-year clinical trial designed to assess the effect of alpha lipoic acid (1,200 mg/day) on loss of mobility and changes in brain volume in patients with progressive multiple sclerosis is ongoing.
Cognitive impairments and dementia
Studies in animal models of aging and neurodegenerative disease have indicated that alpha lipoic acid administration might improve measures of spatial memory, learning capacity, and/or motor function 227. It is not known whether oral alpha lipoic acid supplementation can slow cognitive decline related to aging or pathological conditions in humans. An uncontrolled, open-label trial in nine patients with probable Alzheimer’s disease and related dementias, who were also taking acetylcholinesterase inhibitors, reported that oral supplementation with 600 mg/day of alpha lipoic acid appeared to stabilize cognitive function over a one-year period 228. A subsequent study that followed 43 patients for up to four years found those with mild dementia or moderate-early dementia who took alpha lipoic acid 600 mg/day, in addition to acetylcholinesterase inhibitors, experienced slower cognitive decline compared to the typical cognitive decline of Alzheimer’s patients as reported in the literature 229. However, the significance of these findings is difficult to assess without a control group for comparison. The results of another randomized trial in 39 patients with Alzheimer’s disease suggested that supplementation with fish oil concentrate (high in omega-3 fatty acids) with or without alpha lipoic acid 600 mg/day for one year could delay the progression in cognitive and functional impairments assessed by the Instrumental Activities of Daily Living (IADL) scoring system compared to placebo 230. Interestingly, patients who took fish oil concentrate together with alpha lipoic acid showed no worsening of global cognitive function (as assessed by the Mini-Mental State Examination [MMSE] score system) over 12 months as opposed to those who took either the fish oil concentrate alone or a placebo 230. A randomized controlled trial found that oral supplementation with 1,200 mg/day of alpha lipoic acid for 10 weeks was of no benefit in treating HIV-associated cognitive impairment 231. Larger trials are needed to confirm these preliminary findings and further evaluate the usefulness of supplemental alpha lipoic acid in the prevention and/or management of neurodegenerative diseases.
Alpha lipoic acid dosage
Clinical trials have used different forms of administration and treatment durations. Alpha lipoic acid dosage ranges from 200 mg/day to 1800 mg/day, administered intravenously or orally. Although the maximum dose of alpha lipoic acid has not been defined, previous studies have shown that alpha lipoic acid can be used safely up to as high as 1800 mg/day 170.
However, despite the evidence attesting to its safety in moderate doses, precautions for the oral intake of alpha lipoic acid have also been voiced. Cakatay et al. 232 conducted a series of experiments in aged rats with intraperitoneal administration of racemic alpha lipoic acid (100 mg/kg body weight per day for 2 weeks) and showed that this high chronic dose (the equivalent of 5 to 10 grams per day in humans) increased plasma lipid hydroperoxide levels and oxidative protein damage 232. Alpha lipoic acid-mediated protein damage was noted in rat heart 233 and brain 234, but lipid hydroperoxide levels were beneficially decreased in both these organs. Apparently in keeping with its metal chelating abilities, this group noted that alpha lipoic acid lowered selenium levels in the serum, heart, brain, and muscle; manganese was lowered only in the heart, but increased in the brain and muscle 235. Thus, while intake of moderate doses of alpha lipoic acid have relatively few adverse side-effects, alpha lipoic acid may mediate oxidative insult at higher doses or when administered intraperitoneally. More research is therefore warranted regarding both the safety and optimal dose of alpha lipoic acid.
Alpha lipoic acid side effects
In general, high-dose alpha lipoic acid administration has been found to have few serious side effects. The most commonly reported side effects that were related with alpha lipoic acid in these two studies 236, 237 were gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, and diarrhea 238 and dermatological symptoms, such as urticaria and itching sensation. One subject in the 1800 mg/d alpha-lipoic acid group and 3 subjects in the 1200 mg/d alpha-lipoic acid group withdrew because of itching sensation 236. The most frequently reported side effects of oral alpha lipoic acid supplementation are allergic reactions affecting the skin, including rashes, hives, and itching. Abdominal pain, nausea, vomiting, diarrhea, and vertigo have also been reported, and one trial found that the incidence of nausea, vomiting, and vertigo was dose-dependent 49. Furthermore, malodorous urine has been noted by people taking 1,200 mg/day of alpha lipoic acid orally 222.
Intravenous (IV) administration of alpha lipoic acid at doses of 600 mg/day for three weeks 239 and oral alpha lipoic acid at doses as high as 1,800 mg/day for six months 240 and 1,200 mg/day for two years 48 did not result in serious adverse effects when used to treat diabetic peripheral neuropathy. There was no significant difference in the incidence of adverse events and serious adverse events in patients with diabetic neuropathy who took 600 mg/day of alpha lipoic acid for four years compared to those in the placebo group 50. Oral intake of alpha lipoic acid 2,400 mg/day for two weeks was also found to be safe in a pilot study that included participants with multiple sclerosis 222. Two mild anaphylactoid reactions and one severe anaphylactic reaction (severe allergic reaction), including laryngospasm, were reported after intravenous alpha lipoic acid administration 46.
Pregnancy and lactation
A retrospective observational study reported that daily oral supplementation with 600 mg of alpha lipoic acid (racemic mixture) during pregnancy and without interruption from a period spanning between week 10 and week 30 of gestation and until the end of week 37 was not associated with any adverse effect in mothers and their newborns 241. In absence of further evidence, alpha lipoic acid supplementation during pregnancy should only be considered under strict medical supervision. The safety of alpha lipoic acid supplements in breastfeeding women has not been established and should be discouraged.
Children
A case of intoxication was reported in a 20-month old child (10.5 kg body weight) after the accidental ingestion of four 600-mg tablets of alpha lipoic acid 242. The child was admitted to hospital with seizure, acidosis, and unconsciousness. Symptomatic management and rapid elimination of alpha lipoic acid led to a full recovery without complications within five days 242. The non-accidental ingestion of a very high dose of alpha lipoic acid led to multi-organ failure and subsequent death of an adolescent girl 243.
Drug interactions
In theory, because alpha lipoic acid supplementation may improve insulin-mediated glucose utilization, there is a potential risk of hypoglycemia in diabetic patients using insulin or oral anti-diabetic agents. Consequently, blood glucose concentrations should be monitored closely when alpha lipoic acid supplementation is added to diabetes treatment regimens. Yet, one study in 24 healthy volunteers reported no significant drug interactions with the co-administration of a single oral dose of alpha lipoic acid (600 mg) and the oral anti-diabetic agents, glyburide (glybenclamide) or acarbose (Precose/Prandase/Glucobay) 244.
Nutrient interactions
The chemical structure of biotin is similar to that of alpha lipoic acid, and there is some evidence that high concentrations of alpha lipoic acid can compete with biotin for transport across cell membranes 245, 246. The administration of high doses of alpha lipoic acid by injection to rats decreased the activity of two biotin-dependent enzymes by about 30%-35% 247, but it is not known whether oral or intravenous alpha lipoic acid supplementation substantially increases the requirement for biotin in humans 248.
- Nguyen H, Pellegrini MV, Gupta V. Alpha-Lipoic Acid. [Updated 2024 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564301[↩]
- Alpha-lipoic acid: a multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Evans JL, Goldfine ID. Diabetes Technol Ther. 2000 Autumn; 2(3):401-13. https://www.ncbi.nlm.nih.gov/pubmed/11467343/[↩]
- Reed LJ. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J Biol Chem. 2001 Oct 19;276(42):38329-36. doi: 10.1074/jbc.R100026200[↩]
- Carreau JP. Biosynthesis of lipoic acid via unsaturated fatty acids. Methods Enzymol. 1979;62:152-8. doi: 10.1016/0076-6879(79)62212-7[↩]
- Anthony RM, MacLeay JM, Gross KL. Alpha-Lipoic Acid as a Nutritive Supplement for Humans and Animals: An Overview of Its Use in Dog Food. Animals (Basel). 2021 May 19;11(5):1454. doi: 10.3390/ani11051454[↩][↩][↩]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. BBA-General Subjects. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026[↩]
- Lee SJ, Kang JG, Ryu OH, Kim CS, Ihm SH, Choi MG, Yoo HJ, Kim DS, Kim TW. Effects of α-lipoic acid on transforming growth factor β1-p38 mitogen-activated protein kinase-fibronectin pathway in diabetic nephropathy. Metabolism. 2009;58:616–623. doi: 10.1016/j.metabol.2008.12.006[↩]
- Hermann R., Niebch G., Borbe H.O., Fieger-Buschges H., Ruus P., Nowak H., Riethmuller H., Peukert M., Blume H. Enantioselective pharmacokinetics and bioavailability of different racemic alpha-lipoic formulations in healthy volunteers. Eur. J. Pharm. Sci. 1996;4:167–174. doi: 10.1016/0928-0987(95)00045-3[↩][↩][↩][↩][↩][↩][↩]
- Uchida R, Okamoto H, Ikuta N, Terao K, Hirota T. Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats. Int J Mol Sci. 2015 Sep 21;16(9):22781-94. doi: 10.3390/ijms160922781[↩][↩]
- Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Biochim Biophys Acta. 2009 Oct; 1790(10):1149-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2756298/[↩][↩]
- Packer L, Cadenas E. Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J Clin Biochem Nutr. 2011 Jan;48(1):26-32. doi: 10.3164/jcbn.11-005FR[↩][↩][↩]
- Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998 Apr;24(6):1023-39. doi: 10.1016/s0891-5849(97)00371-7[↩][↩]
- Reed L. Multienzyme complexes. Accts Chem Res. 1974;7:40–46.[↩][↩]
- The pharmacology of the antioxidant lipoic acid. Biewenga GP, Haenen GR, Bast A. Gen Pharmacol. 1997 Sep; 29(3):315-31. https://www.ncbi.nlm.nih.gov/pubmed/9378235/[↩]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochimica et biophysica acta. 2009;1790(10):1149-1160. doi:10.1016/j.bbagen.2009.07.026. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2756298/[↩]
- Ross S.M. Clinical applications of lipoic acid in type II diabetes mellitus. Holist. Nurs. Pract. 2006;20:305–306. doi: 10.1097/00004650-200611000-00009[↩]
- Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997 Sep;29(3):315-31. doi: 10.1016/s0306-3623(96)00474-0[↩][↩]
- Kramer K, Packer L. R-alpha-lipoic acid. In: Kramer K, Hoppe P, Packer L, eds. Nutraceuticals in Health and Disease Prevention. New York: Marcel Dekker, Inc.; 2001:129-164.[↩]
- Nguyen N, Takemoto JK. A Case for Alpha-Lipoic Acid as an Alternative Treatment for Diabetic Polyneuropathy. J Pharm Pharm Sci. 2018;21(1s):177s-191s. doi: 10.18433/jpps30100[↩]
- Hendler S.S., Rorvik D.R. PDR for Nutritional Supplements. Medical Economics Company, Inc.; Montvale, NJ, USA: 2001.[↩]
- Ziegler D., Ametov A., Barinov A., Dyck P.J., Gurieva I., Low P.A., Munzel U., Yakhno N., Raz I., Novosadova M., et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216[↩][↩]
- Smith A.R., Shenvi S.V., Widlansky M., Suh J.H., Hagen T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387[↩]
- Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998 Dec;36(12):625-8.[↩][↩][↩][↩]
- Bernkop-Schnurch A., Reich-Rohrwig E., Marschutz M., Schuhbauer H., Kratzel M. Development of a sustained release dosage form for alpha-lipoic acid. II. Evaluation in human volunteers. Drug Dev. Ind. Pharm. 2004;30:35–42. doi: 10.1081/DDC-120027509[↩]
- Mignini F., Streccioni V., Tomassoni D., Traini E., Amenta F. Comparative crossover, randomized, open-label bioequivalence study on the bioequivalence of two formulations of thioctic acid in healthy volunteers. Clin. Exp. Hypertens. 2007;29:575–586. doi: 10.1080/10641960701744111[↩]
- Amenta F., Traini E., Tomassoni D., Mignini F. Pharmacokinetics of different formulations of tioctic (alpha-lipoic) acid in healthy volunteers. Clin. Exp. Hypertens. 2008;30:767–775. doi: 10.1080/10641960802563568[↩]
- Ziegler D., Nowak H., Kempler P., Vargha P., Low P.A. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A meta-analysis. Diabet. Med. 2004;21:114–121. doi: 10.1111/j.1464-5491.2004.01109.x[↩]
- Ziegler D., Hanefeld M., Ruhnau K.J., Hasche H., Lobisch M., Schutte K., Kerum G., Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-lipoic acid in diabetic neuropathy. Diabetes Care. 1999;22:1296–1301. doi: 10.2337/diacare.22.8.1296[↩]
- Ziegler D., Low P.A., Litchy W.J., Boulton A.J.M., Vinik A.I., Freeman R., Samigullin R., Tritschler H., Munzel U., Maus J., et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: The NATHAN 1 trial. Diabetes Care. 2011;34:2054–2060. doi: 10.2337/dc11-0503[↩]
- Yadav V., Marracci G., Lovera J., Woodward W., Bogardus K., Marquardt W., Shinto L., Morris C., Bourdette D. Lipoic acid in multiple sclerosis: A pilot study. Mult. Scler. 2005;11:159–165. doi: 10.1191/1352458505ms1143oa[↩]
- Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007 Dec;12(4):343-51.[↩]
- Gleiter C.H., Schug B.S., Hermann R., Elze M., Blume H.H., Gundert-Remy U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Clin. Pharmacol. 1996;50:513–514. doi: 10.1007/s002280050151[↩]
- Breithaupt-Grogler K., Niebch G., Schneider E., Erb K., Hermann R., Blume H.H., Schug B.S., Belz G.G. Dose-proportionality of oral thioctic acid–coincidence of assessments via pooled plasma and individual data. Eur. J. Pharm. Sci. 1999;8:57–65. doi: 10.1016/S0928-0987(98)00061-X[↩][↩]
- Keith D.J., Butler J.A., Bemer B., Dixon B., Johnson S., Garrard M., Sudakin D.L., Christensen J.M., Pereira C., Hagen T.M. Age and gender dependent bioavailability of R- and R,S-alpha-lipoic acid: A pilot study. Pharmacol. Res. 2012;66:199–206. doi: 10.1016/j.phrs.2012.05.002[↩][↩]
- Reed L.J. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J. Biol. Chem. 2001;276:38329–38336. doi: 10.1074/jbc.R100026200[↩]
- Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol. 2011 Nov 17;2:69. doi: 10.3389/fphar.2011.00069[↩]
- Petersen Shay K., Moreau R. F., Smith E. J., Hagen T. M. (2008). Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 60, 362–367 10.1002/iub.40[↩]
- Gorąca A., Huk-Kolega H., Piechota A., Kleniewska P., Ciejka E., Skibska B. Lipoic acid-biological activity and therapeutic potential. Pharm. Rep. 2011;63:849–858. doi: 10.1016/S1734-1140(11)70600-4[↩]
- Biewenga G.P., Haenen G.R., Bast A. The Pharmacology of the Antioxidant Lipoic Acid. Gen. Pharmac. 1997;29:315–331. doi: 10.1016/S0306-3623(96)00474-0[↩]
- Spector A., Huang R.-R.C., Yan G.-Z., Wang R.-R. Thioredoxin fragment 31–36 is reduced by dihydrolipoamide and reduces oxidized protein. Biochem. Biophys. Res. Commun. 1988;150:156–162. doi: 10.1016/0006-291X(88)90499-8[↩]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009 Oct;1790(10):1149-60. doi: 10.1016/j.bbagen.2009.07.026[↩][↩][↩][↩]
- Park S, Karunakaran U, Jeoung NH, Jeon JH, Lee IK. Physiological effect and therapeutic application of alpha lipoic acid. Curr Med Chem. 2014;21(32):3636-45. doi: 10.2174/0929867321666140706141806[↩]
- Molecular aspects of lipoic acid in the prevention of diabetes complications. Packer L, Kraemer K, Rimbach G. Nutrition. 2001 Oct; 17(10):888-95.[↩]
- Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370.[↩]
- Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012 Oct;167(4):465-71. doi: 10.1530/EJE-12-0555[↩]
- Ziegler D. Thioctic acid for patients with symptomatic diabetic polyneuropathy: a critical review. Treat Endocrinol. 2004;3(3):173-89. doi: 10.2165/00024677-200403030-00005[↩][↩][↩][↩]
- Ruhnau KJ, Meissner HP, Finn JR, Reljanovic M, Lobisch M, Schütte K, Nehrdich D, Tritschler HJ, Mehnert H, Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999 Dec;16(12):1040-3. doi: 10.1046/j.1464-5491.1999.00190.x[↩]
- Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schütte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999 Aug;22(8):1296-301. doi: 10.2337/diacare.22.8.1296[↩][↩]
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006 Nov;29(11):2365-70. doi: 10.2337/dc06-1216[↩][↩]
- Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schütte K, Dyck PJ. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011 Sep;34(9):2054-60. doi: 10.2337/dc11-0503[↩][↩][↩]
- Ziegler D, Low PA, Freeman R, Tritschler H, Vinik AI. Predictors of improvement and progression of diabetic polyneuropathy following treatment with α-lipoic acid for 4 years in the NATHAN 1 trial. J Diabetes Complications. 2016 Mar;30(2):350-6. doi: 10.1016/j.jdiacomp.2015.10.018[↩]
- Alpha-Lipoic Acid. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C61595[↩]
- Haritoglou C, Gerss J, Hammes HP, et al. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica. 2011;226(3):127-137. https://www.ncbi.nlm.nih.gov/pubmed/21811051[↩]
- Çakici N, Fakkel TM, van Neck JW, et al. Systematic review of treatments for diabetic peripheral neuropathy. Diabetic Medicine. 2016;33(11):1466-1476.[↩]
- Mayr JA, Feichtinger RG, Tort F, Ribes A, Sperl W. Lipoic acid biosynthesis defects. J Inherit Metab Dis. 2014 Jul;37(4):553-63. doi: 10.1007/s10545-014-9705-8[↩][↩][↩]
- Mayr JA, Zimmermann FA, Fauth C, Bergheim C, Meierhofer D, Radmayr D, Zschocke J, Koch J, Sperl W. Lipoic acid synthetase deficiency causes neonatal-onset epilepsy, defective mitochondrial energy metabolism, and glycine elevation. Am J Hum Genet. 2011 Dec 9;89(6):792-7. doi: 10.1016/j.ajhg.2011.11.011[↩]
- Tort F, Ferrer-Cortès X, Thió M, Navarro-Sastre A, Matalonga L, Quintana E, Bujan N, Arias A, García-Villoria J, Acquaviva C, Vianey-Saban C, Artuch R, García-Cazorla À, Briones P, Ribes A. Mutations in the lipoyltransferase LIPT1 gene cause a fatal disease associated with a specific lipoylation defect of the 2-ketoacid dehydrogenase complexes. Hum Mol Genet. 2014 Apr 1;23(7):1907-15. doi: 10.1093/hmg/ddt585[↩]
- Tripathi AK, Ray AK, Mishra SK, Bishen SM, Mishra H, Khurana A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev Bras Farmacogn. 2023;33(2):272-287. doi: 10.1007/s43450-023-00370-1[↩]
- Lipoic Acid. https://lpi.oregonstate.edu/mic/dietary-factors/lipoic-acid#biological-activities[↩][↩][↩][↩]
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004 May;11(9):1135-46. doi: 10.2174/0929867043365387[↩][↩][↩][↩]
- Tripathi A.K., Ray A.K., Mishra S.K., Bishen S.M., Mishra H., Khurana A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023;33:272–287. doi: 10.1007/s43450-023-00370-1[↩]
- Castañeda-Arriaga R., Alvarez-Idaboy J.R. Lipoic Acid and Dihydrolipoic Acid. A Comprehensive Theoretical Study of Their Antioxidant Activity Supported by Available Experimental Kinetic Data. J. Chem. Inf. Model. 2014;54:1642–1652. doi: 10.1021/ci500213p[↩]
- Gorąca A., Huk-Kolega H., Piechota A., Kleniewska P., Ciejka E., Skibska B. Lipoic Acid—Biological Activity and Therapeutic Potential. Pharmacol. Rep. 2011;63:849–858. doi: 10.1016/S1734-1140(11)70600-4[↩]
- Packer L., Cadenas E. Lipoic Acid: Energy Metabolism and Redox Regulation of Transcription and Cell Signaling. J. Clin. Biochem. Nutr. 2010;48:26–32. doi: 10.3164/jcbn.11-005FR[↩]
- Smith A.R., Shenvi S.V., Widlansky M., Suh J.H., Hagen T.M. Lipoic Acid as a Potential Therapy for Chronic Diseases Associated with Oxidative Stress. Curr. Med. Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387[↩]
- Tibullo D., Li Volti G., Giallongo C., Grasso S., Tomassoni D., Anfuso C.D., Lupo G., Amenta F., Avola R., Bramanti V. Biochemical and Clinical Relevance of Alpha Lipoic Acid: Antioxidant and Anti-Inflammatory Activity, Molecular Pathways and Therapeutic Potential. Inflamm. Res. 2017;66:947–959. doi: 10.1007/s00011-017-1079-6[↩]
- Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002 Jul 1;33(1):83-93. doi: 10.1016/s0891-5849(02)00862-6[↩]
- Kozlov AV, Gille L, Staniek K, Nohl H. Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubiquinone and one-electron reduction of ubisemiquinone. Arch Biochem Biophys. 1999 Mar 1;363(1):148-54. doi: 10.1006/abbi.1998.1064[↩]
- May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998 Jan 15;349(2):281-9. doi: 10.1006/abbi.1997.0473[↩]
- Upston JM, Terentis AC, Stocker R. Tocopherol-mediated peroxidation of lipoproteins: implications for vitamin E as a potential antiatherogenic supplement. FASEB J. 1999 Jun;13(9):977-94. doi: 10.1096/fasebj.13.9.977[↩]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161-208. doi: 10.2174/0929867053764635[↩]
- Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 2004 Jul;3(7):431-4. doi: 10.1016/S1474-4422(04)00809-9[↩]
- Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995 Jun 29;50(1):123-6. doi: 10.1016/0006-2952(95)00116-h[↩]
- Suh JH, Moreau R, Heath SH, Hagen TM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10(1):52-60. doi: 10.1179/135100005X21624[↩][↩]
- Yamamoto H, Watanabe T, Mizuno H, Endo K, Fukushige J, Hosokawa T, Kazusaka A, Fujita S. The antioxidant effect of DL-alpha-lipoic acid on copper-induced acute hepatitis in Long-Evans Cinnamon (LEC) rats. Free Radic Res. 2001 Jan;34(1):69-80. doi: 10.1080/10715760100300071[↩]
- Patrick L. Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev. 2002 Dec;7(6):456-71.[↩]
- Rooney JP. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology. 2007 May 20;234(3):145-56. doi: 10.1016/j.tox.2007.02.016. Epub 2007 Mar 1. Erratum in: Toxicology. 2007 Sep 5;238(2-3):216.[↩]
- Hagen TM, Vinarsky V, Wehr CM, Ames BN. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid Redox Signal. 2000 Fall;2(3):473-83. doi: 10.1089/15230860050192251[↩]
- Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung. 1992 Jun;42(6):829-31.[↩]
- Monette JS, Gómez LA, Moreau RF, Dunn KC, Butler JA, Finlay LA, Michels AJ, Shay KP, Smith EJ, Hagen TM. (R)-α-Lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol Res. 2011 Jan;63(1):23-9. doi: 10.1016/j.phrs.2010.09.007[↩]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3381-6. doi: 10.1073/pnas.0400282101[↩][↩]
- Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004 Mar 1;423(1):126-35. doi: 10.1016/j.abb.2003.12.020[↩]
- Zhang J, Zhou X, Wu W, Wang J, Xie H, Wu Z. Regeneration of glutathione by α-lipoic acid via Nrf2/ARE signaling pathway alleviates cadmium-induced HepG2 cell toxicity. Environ Toxicol Pharmacol. 2017 Apr;51:30-37. doi: 10.1016/j.etap.2017.02.022[↩]
- Fratantonio D, Speciale A, Molonia MS, Bashllari R, Palumbo M, Saija A, Cimino F, Monastra G, Virgili F. Alpha-lipoic acid, but not di-hydrolipoic acid, activates Nrf2 response in primary human umbilical-vein endothelial cells and protects against TNF-α induced endothelium dysfunction. Arch Biochem Biophys. 2018 Oct 1;655:18-25. doi: 10.1016/j.abb.2018.08.003[↩]
- Sena CM, Cipriano MA, Botelho MF, Seiça RM. Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation. Int J Mol Sci. 2018 Sep 11;19(9):2706. doi: 10.3390/ijms19092706[↩]
- Pilar Valdecantos M, Prieto-Hontoria PL, Pardo V, Módol T, Santamaría B, Weber M, Herrero L, Serra D, Muntané J, Cuadrado A, Moreno-Aliaga MJ, Alfredo Martínez J, Valverde ÁM. Essential role of Nrf2 in the protective effect of lipoic acid against lipoapoptosis in hepatocytes. Free Radic Biol Med. 2015 Jul;84:263-278. doi: 10.1016/j.freeradbiomed.2015.03.019[↩]
- Fayez AM, Zakaria S, Moustafa D. Alpha lipoic acid exerts antioxidant effect via Nrf2/HO-1 pathway activation and suppresses hepatic stellate cells activation induced by methotrexate in rats. Biomed Pharmacother. 2018 Sep;105:428-433. doi: 10.1016/j.biopha.2018.05.145[↩]
- Lin YC, Lai YS, Chou TC. The protective effect of alpha-lipoic Acid in lipopolysaccharide-induced acute lung injury is mediated by heme oxygenase-1. Evid Based Complement Alternat Med. 2013;2013:590363. doi: 10.1155/2013/590363[↩]
- Segal AW. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol. 2008;40(4):604-18. doi: 10.1016/j.biocel.2007.10.003[↩]
- Dong Y, Wang H, Chen Z. Alpha-Lipoic Acid Attenuates Cerebral Ischemia and Reperfusion Injury via Insulin Receptor and PI3K/Akt-Dependent Inhibition of NADPH Oxidase. Int J Endocrinol. 2015;2015:903186. doi: 10.1155/2015/903186[↩]
- Byun E, Lim JW, Kim JM, Kim H. α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells. Mediators Inflamm. 2014;2014:380830. doi: 10.1155/2014/380830[↩]
- Konrad D. Utilization of the insulin-signaling network in the metabolic actions of alpha-lipoic acid-reduction or oxidation? Antioxid Redox Signal. 2005 Jul-Aug;7(7-8):1032-9. doi: 10.1089/ars.2005.7.1032[↩][↩]
- Diesel B, Kulhanek-Heinze S, Höltje M, Brandt B, Höltje HD, Vollmar AM, Kiemer AK. Alpha-lipoic acid as a directly binding activator of the insulin receptor: protection from hepatocyte apoptosis. Biochemistry. 2007 Feb 27;46(8):2146-55. doi: 10.1021/bi602547m[↩][↩]
- Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996 Dec;45(12):1798-804. doi: 10.2337/diab.45.12.1798[↩]
- Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000 Mar;43(3):294-303. doi: 10.1007/s001250050047[↩]
- Ying Z, Kampfrath T, Sun Q, Parthasarathy S, Rajagopalan S. Evidence that α-lipoic acid inhibits NF-κB activation independent of its antioxidant function. Inflamm Res. 2011 Mar;60(3):219-25. doi: 10.1007/s00011-010-0256-7[↩]
- Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003 Dec;31(Pt 6):1447-9. doi: 10.1042/bst0311447[↩]
- Wang Y, Li X, Guo Y, Chan L, Guan X. alpha-Lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice. Metabolism. 2010 Jul;59(7):967-76. doi: 10.1016/j.metabol.2009.10.018[↩]
- Moura FA, de Andrade KQ, dos Santos JC, Goulart MO. Lipoic Acid: its antioxidant and anti-inflammatory role and clinical applications. Curr Top Med Chem. 2015;15(5):458-83. doi: 10.2174/1568026615666150114161358[↩]
- Rochette L, Ghibu S, Richard C, Zeller M, Cottin Y, Vergely C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol Nutr Food Res. 2013 Jan;57(1):114-25. doi: 10.1002/mnfr.201200608[↩]
- Lodge JK, Youn HD, Handelman GJ, et al. Natural sources of lipic acid: determination of lipoyllysine released from protease-digested tissues by high performance liquid chromatography incorporating electrochemical detection. J Appl Nutr. 1997;49(1 & 2):3-11.[↩]
- Hermann R, Niebch G, Borbe H, et al. Enantioselective pharmacokinetics and bioavailability of different racemic alpha-lipoic acid formulations in healthy volunteers. Eur J Pharm Sci. 1996;4(3):167-174.[↩][↩][↩][↩]
- Alpha-Lipoic Acid Supplements Review. https://www.consumerlab.com/reviews/alpha-lipoic-acid-supplements/alphalipoic[↩]
- Gleiter CH, Schug BS, Hermann R, Elze M, Blume HH, Gundert-Remy U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur J Clin Pharmacol. 1996;50(6):513-4. doi: 10.1007/s002280050151[↩][↩][↩]
- Teichert J, Hermann R, Ruus P, Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J Clin Pharmacol. 2003 Nov;43(11):1257-67. doi: 10.1177/0091270003258654[↩]
- Brufani M, Figliola R. (R)-α-lipoic acid oral liquid formulation: pharmacokinetic parameters and therapeutic efficacy. Acta Biomed. 2014 Aug 20;85(2):108-15.[↩]
- Maglione E, Marrese C, Migliaro E, Marcuccio F, Panico C, Salvati C, Citro G, Quercio M, Roncagliolo F, Torello C, Brufani M. Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain. Acta Biomed. 2015 Dec 14;86(3):226-33.[↩]
- Breithaupt-Grögler K, Niebch G, Schneider E, Erb K, Hermann R, Blume HH, Schug BS, Belz GG. Dose-proportionality of oral thioctic acid–coincidence of assessments via pooled plasma and individual data. Eur J Pharm Sci. 1999 Apr;8(1):57-65. doi: 10.1016/s0928-0987(98)00061-x[↩][↩]
- Evans JL, Heymann CJ, Goldfine ID, Gavin LA. Pharmacokinetics, tolerability, and fructosamine-lowering effect of a novel, controlled-release formulation of alpha-lipoic acid. Endocr Pract. 2002 Jan-Feb;8(1):29-35. doi: 10.4158/EP.8.1.29[↩]
- Keith DJ, Butler JA, Bemer B, Dixon B, Johnson S, Garrard M, Sudakin DL, Christensen JM, Pereira C, Hagen TM. Age and gender dependent bioavailability of R- and R,S-α-lipoic acid: a pilot study. Pharmacol Res. 2012 Sep;66(3):199-206. doi: 10.1016/j.phrs.2012.05.002[↩]
- Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol. 1997 Jul;273(1 Pt 1):E185-91. doi: 10.1152/ajpendo.1997.273.1.E185[↩]
- Maitra I, Serbinova E, Tritschler HJ, Packer L. Stereospecific effects of R-lipoic acid on buthionine sulfoximine-induced cataract formation in newborn rats. Biochem Biophys Res Commun. 1996 Apr 16;221(2):422-9. doi: 10.1006/bbrc.1996.0611[↩]
- Hiltunen JK, Autio KJ, Schonauer MS, Kursu VA, Dieckmann CL, Kastaniotis AJ. Mitochondrial fatty acid synthesis and respiration. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1195-202. doi: 10.1016/j.bbabio.2010.03.006[↩]
- Bjørklund G., Aaseth J., Crisponi G., Rahman M., Chirumbolo S. Insights on Alpha Lipoic and Dihydrolipoic Acids as Promising Scavengers of Oxidative Stress and Possible Chelators in Mercury Toxicology. J. Inorg. Biochem. 2019;195:111–119. doi: 10.1016/j.jinorgbio.2019.03.019[↩]
- Gurer H., Ozgunes H., Oztezcan S., Ercal N. Antioxidant Role of α-Lipoic Acid in Lead Toxicity. Free Radic. Biol. Med. 1999;27:75–81. doi: 10.1016/S0891-5849(99)00036-2[↩]
- Ou P., Tritschler H.J., Wolff S.P. Thioctic (Lipoic) Acid: A Therapeutic Metal-Chelating Antioxidant? Biochem. Pharmacol. 1995;50:123–126. doi: 10.1016/0006-2952(95)00116-H[↩]
- Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995 Aug;19(2):227-50. doi: 10.1016/0891-5849(95)00017-r[↩]
- Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic Acid and diabetic neuropathy. Rev Diabet Stud. 2009 Winter;6(4):230-6. doi: 10.1900/RDS.2009.6.230[↩]
- Frondoza CG, Fortuno LV, Grzanna MW, Ownby SL, Au AY, Rashmir-Raven AM. α-Lipoic Acid Potentiates the Anti-Inflammatory Activity of Avocado/Soybean Unsaponifiables in Chondrocyte Cultures. Cartilage. 2018 Jul;9(3):304-312. doi: 10.1177/1947603516686146[↩]
- World Health Organization. Global Report on Diabetes. 2016. www.who.int/diabetes/global-report/en/[↩]
- Feldman E. Epidemiology and classification of diabetic neuropathy. UpToDate®.[↩]
- Boulton AJM, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management diabetic peripheral neuropathy. Diabetic Medicine 1998;15(6):508-14.[↩]
- Bredfeldt C, Altschuler A, Adams AS, Ports J, Bayliss E. Patient reported outcomes for diabetic peripheral neuropathy. Journal of Diabetes and Its Complications 2015;29(8):1112-8.[↩][↩]
- Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes/Metabolism Research and Reviews 2011;27(7):629-38.[↩][↩]
- Edwards JL, Vincent AM Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacology & Therapeutics 2008;120(1):1-34.[↩][↩]
- Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the US population. Diabetes Care 1993;16(11):1446–52.[↩]
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otology & Neurotology 2010;31(9):1445-50.[↩]
- Amin N, Doupis J. Diabetic foot disease: From the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World Journal of Diabetes 2016;7(7):153-64.[↩]
- Kim PJ, Steinberg JS. Complications of the diabetic foot. Endocrinology and Metabolism Clinics of North America 2013;42(4):833-47.[↩]
- Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurology 2012;11(6):521-34.[↩]
- Chen W, Zhang Y, Li X, Yang G, Liu JP. Chinese herbal medicine for diabetic peripheral neuropathy. Cochrane Database of Systematic Reviews 2013, Issue 10.[↩][↩]
- Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 1997;46(Suppl 2):S38-42.[↩]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54(6):1615–25.[↩]
- Uehara K, Yamagishi S, Otsuki S, Chin S, Yagihashi S. Effects of polyol pathway hyperactivity on protein kinase C activity, nociceptive peptide expression, and neuronal structure in dorsal root ganglia in diabetic mice. Diabetes 2004;53(12):3239–47.[↩]
- Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. Journal of Clinical Investigation 1998;101(1):160-9.[↩]
- Vinik AI, Mehrabyan A. Diabetic neuropathies. Medical Clinics of North America 2004;88(4):947-99.[↩]
- Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA. Enhanced inflammatory response via activation of NF-kappaB in acute experimental diabetic neuropathy subjected to ischemia reperfusion injury. Journal of the Neurological Sciences 2006;247(1):47-52.[↩]
- Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews 2012, Issue 6.[↩]
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1.[↩]
- Allen R, Sharma U, Barlas S. Clinical experience with desvenlafaxine in treatment of pain associated with diabetic peripheral neuropathy. Journal of Pain Research 2014;7:339-51.[↩]
- Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Practice 2014;14(2):167-84.[↩][↩]
- Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anaesthetic agents to relieve neuropathic pain. Cochrane Database of Systematic Reviews 2005, Issue 4.[↩]
- Naderi Nabi B, Sedighinejad A, Haghighi M, Biazar G, Hashemi M, Haddadi S, et al. Comparison of transcutaneous electrical nerve stimulation and pulsed radiofrequency sympathectomy for treating painful diabetic neuropathy. Anesthesiology and Pain Medicine 2015;5(5):e29280.[↩]
- Zhang C, Ma YX, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. Chung i Tsa Chih Ying Wen Pan [Journal of Traditional Chinese Medicine] 2010;30(1):13-4.[↩]
- Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Canadian Journal of Physiology and Pharmacology 2015;93(12):1021-7.[↩][↩][↩]
- Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radical Biology & Medicine 1995;19(2):227-50.[↩]
- Marangon K, Devaraj S, Tirosh O, Packer L, Jialal I. Comparison of the effect of alpha-lipoic acid and alpha-tocopherol supplementation on measures of oxidative stress. Free Radical Biology & Medicine 1999;27(9-10):1114-21.[↩]
- Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 2008;60(6):362-7.[↩]
- Khanna S, Roy S, Packer L, Sen CK. Cytokine-induced glucose uptake in skeletal muscle: redox regulation and the role of alpha-lipoic acid. American Journal of Physiology 1999;276(5 Pt 2):R1327-33.[↩]
- Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol. 1997;273:E185–91.[↩]
- Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–7.[↩]
- Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21:114–21. https://www.ncbi.nlm.nih.gov/pubmed/14984445[↩]
- Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Moller W, Tritschler HJ, Mehnert H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic Res. 1999;31:171–9. https://www.ncbi.nlm.nih.gov/pubmed/10499773[↩]
- Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999;22:1296–301. https://www.ncbi.nlm.nih.gov/pubmed/10480774[↩]
- Ruhnau KJ, Meissner HP, Finn JR, Reljanovic M, Lobisch M, Schutte K, Nehrdich D, Tritschler HJ, Mehnert H, Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999;16:1040–3. https://www.ncbi.nlm.nih.gov/pubmed/10656234[↩]
- Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, Low PA, Nehrdich D, Novosadova M, O’Brien PC, Reljanovic M, Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003;26:770–6. https://www.ncbi.nlm.nih.gov/pubmed/12610036[↩]
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–70. https://www.ncbi.nlm.nih.gov/pubmed/17065669[↩]
- Abubaker SA, Alonazy AM, Abdulrahman A. Effect of Alpha-Lipoic Acid in the Treatment of Diabetic Neuropathy: A Systematic Review. Cureus. 2022 Jun 8;14(6):e25750. doi: 10.7759/cureus.25750[↩][↩]
- Cassanego G, Rodrigues P, De Freitas Bauermann L, Trevisan G. Evaluation of the analgesic effect of ɑ-lipoic acid in treating pain disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2022 Mar;177:106075. doi: 10.1016/j.phrs.2022.106075[↩]
- Vakali E, Rigopoulos D, Carrillo AE, Flouris AD, Dinas PC. Effects of Alpha-lipoic Acid Supplementation on Human Diabetic Nephropathy: A Systematic Review and Meta-analysis. Curr Diabetes Rev. 2022;18(6):e140921196457. doi: 10.2174/1573399817666210914103329[↩][↩]
- Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015 Feb 15;6(1):80-91. doi: 10.4239/wjd.v6.i1.80[↩]
- Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997 Mar;20(3):369-73. doi: 10.2337/diacare.20.3.369[↩]
- Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ, Jung WI, Augustin HJ, Dietze GJ. Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung. 1995 Aug;45(8):872-4.[↩]
- Jacob S, Rett K, Henriksen EJ, Häring HU. Thioctic acid–effects on insulin sensitivity and glucose-metabolism. Biofactors. 1999;10(2-3):169-74. doi: 10.1002/biof.5520100212[↩]
- de Oliveira AM, Rondó PH, Luzia LA, D’Abronzo FH, Illison VK. The effects of lipoic acid and α-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Res Clin Pract. 2011 May;92(2):253-60. doi: 10.1016/j.diabres.2011.02.010[↩]
- Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Khatibi SR, Asemi Z. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metabolism. 2018 Oct;87:56-69. doi: 10.1016/j.metabol.2018.07.002[↩][↩]
- Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, Tolo CU, Ogwang PE. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res Clin Pract. 2019 Oct;156:107815. doi: 10.1016/j.diabres.2019.107815[↩]
- Gębka A, Serkies-Minuth E, Raczyńska D. Effect of the administration of alpha-lipoic acid on contrast sensitivity in patients with type 1 and type 2 diabetes. Mediators Inflamm. 2014;2014:131538. doi: 10.1155/2014/131538[↩][↩]
- Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. 2015;23:313–321.[↩]
- Koh EH, Lee WJ, Lee SA, et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am J Med. 2011;124:85.e1–8.[↩][↩][↩][↩][↩]
- Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Martinez JA, Moreno-Aliaga MJ. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. Eur J Nutr. 2013;52:779–787[↩]
- Carbonelli MG, Di Renzo L, Bigioni M, Di Daniele N, De Lorenzo A, Fusco MA. Alpha-lipoic acid supplementation: a tool for obesity therapy? Curr Pharm Des. 2010;16:840–846.[↩][↩]
- Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.[↩]
- McNeilly AM, Davison GW, Murphy MH, et al. Effect of alpha-lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Health Dis. 2011;10:217.[↩]
- Ziegler D. Thioctic acid for patients with symptomatic diabetic polyneuropathy: a critical review. Treat Endocrinol. 2004;3:173–189.[↩]
- Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-Lipoic Acid (ALA) as a supplementation for weight loss: Results from a Meta-Analysis of Randomized Controlled Trials. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(5):594-601. doi:10.1111/obr.12528. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523816/[↩][↩]
- Ratliff JC, Palmese LB, Reutenauer EL, Tek C. An open-label pilot trial of alpha-lipoic acid for weight loss in patients with schizophrenia without diabetes. Clin Schizophr Relat Psychoses. 2013;8:196–200.[↩]
- Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. 2015;23:313–321[↩]
- Koh EH, Lee WJ, Lee SA, et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am J Med. 2011;124:85.e1–8[↩]
- Kim NW, Song YM, Kim E, et al. Adjunctive alpha-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: a double-blind randomized placebo-controlled study. Int Clin Psychopharmacol. 2016;31:265–274.[↩]
- Mohammadi V, Khalili M, Eghtesadi S, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: a clinical trial. Spinal Cord. 2015;53:646.[↩]
- Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-Lipoic Acid (ALA) as a supplementation for weight loss: Results from a Meta-Analysis of Randomized Controlled Trials. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(5):594-601. doi:10.1111/obr.12528 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523816/[↩]
- Namazi N, Larijani B, Azadbakht L. Alpha-lipoic acid supplement in obesity treatment: A systematic review and meta-analysis of clinical trials. Clin Nutr. 2018 Apr;37(2):419-428. doi: 10.1016/j.clnu.2017.06.002[↩][↩][↩][↩]
- Alpha-lipoic acid supplement in obesity treatment: A systematic review and meta-analysis of clinical trials. Clinical Nutrition April 2018; Volume 37, Issue 2, Pages 419–428. https://www.clinicalnutritionjournal.com/article/S0261-5614(17)30212-1/fulltext[↩]
- Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016;315:2424–2434.[↩]
- Sena CM, Nunes E, Louro T, Proenca T, Fernandes R, Boarder MR, Seica RM. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol. 2007[↩]
- Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001 Jul 1;31(1):53-61. doi: 10.1016/s0891-5849(01)00551-2[↩]
- Heinisch BB, Francesconi M, Mittermayer F, Schaller G, Gouya G, Wolzt M, Pleiner J. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial. Eur J Clin Invest. 2010 Feb;40(2):148-54. doi: 10.1111/j.1365-2362.2009.02236.x[↩]
- Xiang G, Pu J, Yue L, Hou J, Sun H. α-lipoic acid can improve endothelial dysfunction in subjects with impaired fasting glucose. Metabolism. 2011 Apr;60(4):480-5. doi: 10.1016/j.metabol.2010.04.011[↩][↩]
- Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Clin Endocrinol (Oxf). 2008 May;68(5):716-23. doi: 10.1111/j.1365-2265.2007.03099.x[↩][↩]
- Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005 Jan 25;111(3):343-8. doi: 10.1161/01.CIR.0000153272.48711.B9[↩]
- Vasdev S, Gill V, Longerich L, Parai S, Gadag V. Salt-induced hypertension in WKY rats: prevention by alpha-lipoic acid supplementation. Mol Cell Biochem. 2003;254:319–26.[↩]
- El Midaoui A, de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension. 2002;39:303–7.[↩]
- Takaoka M, Kobayashi Y, Yuba M, Ohkita M, Matsumura Y. Effects of alpha-lipoic acid on deoxycorticosterone acetate-salt-induced hypertension in rats. Eur J Pharmacol. 2001;424:121–9.[↩]
- Shay K. Petersen, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–7.[↩]
- McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, Gokce N, Hagen TM, Keaney JF, Jr., Vita JA. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9:249–55.[↩]
- Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–8.[↩]
- Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–50.[↩]
- Kunt T, Forst T, Wilhelm A, Tritschler H, Pfuetzner A, Harzer O, Engelbach M, Zschaebitz A, Stofft E, Beyer J. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond) 1999;96:75–82.[↩]
- Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 2007;39:106–13.[↩]
- Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96.[↩]
- Lee EY, Lee CK, Lee KU, Park JY, Cho KJ, Cho YS, Lee HR, Moon SH, Moon HB, Yoo B. Alpha-lipoic acid suppresses the development of collagen-induced arthritis and protects against bone destruction in mice. Rheumatol Int. 2007;27:225–33.[↩][↩]
- Morini M, Roccatagliata L, Dell’Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–53[↩]
- Marracci GH, Marquardt WE, Strehlow A, McKeon GP, Gross J, Buck DC, Kozell LB, Bourdette DN. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck) Biochem Biophys Res Commun. 2006;344:963–71[↩][↩]
- Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun. 2007;354:259–64[↩]
- Larghero P, Vene R, Minghelli S, Travaini G, Morini M, Ferrari N, Pfeffer U, Noonan DM, Albini A, Benelli R. Biological assays and genomic analysis reveal lipoic acid modulation of endothelial cell behavior and gene expression. Carcinogenesis. 2007;28:1008–20[↩]
- Cho YS, Lee J, Lee TH, Lee EY, Lee KU, Park JY, Moon HB. alpha-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:429–35.[↩]
- Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. Faseb J. 2001;15:2423–32.[↩]
- Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–82[↩]
- Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–8[↩]
- Ikeda U, Ito T, Shimada K. Interleukin-6 and acute coronary syndrome. Clin Cardiol. 2001;24:701–4.[↩]
- What Is Multiple Sclerosis? https://www.nationalmssociety.org/understanding-ms/what-is-ms[↩]
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014 Jul 15;83(3):278-86. doi: 10.1212/WNL.0000000000000560[↩]
- Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002 Oct;131(1-2):104-14. doi: 10.1016/s0165-5728(02)00269-2[↩][↩]
- Morini M, Roccatagliata L, Dell’Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004 Mar;148(1-2):146-53. doi: 10.1016/j.jneuroim.2003.11.021[↩]
- Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol SM, Hendrikx EM, Döpp ED, Dijkstra CD, Drukarch B, de Vries HE. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006 Aug 15;177(4):2630-7. doi: 10.4049/jimmunol.177.4.2630[↩][↩]
- Salinthone S, Schillace RV, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production via the EP2 and EP4 prostanoid receptors and inhibits IFN gamma synthesis and cellular cytotoxicity in NK cells. J Neuroimmunol. 2008 Aug 13;199(1-2):46-55. doi: 10.1016/j.jneuroim.2008.05.003[↩]
- Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun. 2007 Mar 2;354(1):259-64. doi: 10.1016/j.bbrc.2006.12.195[↩]
- George JD, Kim E, Spain R, Bourdette D, Salinthone S. Effects of lipoic acid on migration of human B cells and monocyte-enriched peripheral blood mononuclear cells in relapsing remitting multiple sclerosis. J Neuroimmunol. 2018 Feb 15;315:24-27. doi: 10.1016/j.jneuroim.2017.12.009[↩]
- Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006 Jun;175(1-2):87-96. doi: 10.1016/j.jneuroim.2006.03.007[↩]
- Marracci GH, McKeon GP, Marquardt WE, Winter RW, Riscoe MK, Bourdette DN. Alpha lipoic acid inhibits human T-cell migration: implications for multiple sclerosis. J Neurosci Res. 2004 Nov 1;78(3):362-70. doi: 10.1002/jnr.20255[↩]
- Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005 Apr;11(2):159-65. doi: 10.1191/1352458505ms1143oa[↩][↩][↩][↩]
- Yadav V, Marracci GH, Munar MY, Cherala G, Stuber LE, Alvarez L, Shinto L, Koop DR, Bourdette DN. Pharmacokinetic study of lipoic acid in multiple sclerosis: comparing mice and human pharmacokinetic parameters. Mult Scler. 2010 Apr;16(4):387-97. doi: 10.1177/1352458509359722[↩]
- Khalili M, Eghtesadi S, Mirshafiey A, Eskandari G, Sanoobar M, Sahraian MA, Motevalian A, Norouzi A, Moftakhar S, Azimi A. Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: a randomized controlled clinical trial. Nutr Neurosci. 2014 Jan;17(1):16-20. doi: 10.1179/1476830513Y.0000000060[↩]
- Khalili M, Azimi A, Izadi V, Eghtesadi S, Mirshafiey A, Sahraian MA, Motevalian A, Norouzi A, Sanoobar M, Eskandari G, Farhoudi M, Amani F. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-6. doi: 10.1159/000356145[↩][↩]
- Khalili M, Soltani M, Moghadam SA, Dehghan P, Azimi A, Abbaszadeh O. Effect of alpha-lipoic acid on asymmetric dimethylarginine and disability in multiple sclerosis patients: A randomized clinical trial. Electron Physician. 2017 Jul 25;9(7):4899-4905. doi: 10.19082/4899[↩]
- Molz P, Schröder N. Potential Therapeutic Effects of Lipoic Acid on Memory Deficits Related to Aging and Neurodegeneration. Front Pharmacol. 2017 Dec 12;8:849. doi: 10.3389/fphar.2017.00849[↩]
- Hager K, Marahrens A, Kenklies M, Riederer P, Münch G. Alpha-lipoic acid as a new treatment option for Alzheimer [corrected] type dementia. Arch Gerontol Geriatr. 2001 Jun;32(3):275-82. doi: 10.1016/s0167-4943(01)00104-2. Erratum in: Arch Gerontol Geriatr. 2010 Jul-Aug;51(1):110.[↩]
- Hager K, Kenklies M, McAfoose J, Engel J, Münch G. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease–a 48 months follow-up analysis. J Neural Transm Suppl. 2007;(72):189-93. doi: 10.1007/978-3-211-73574-9_24[↩]
- Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, Waichunas D, Bumgarner L, Bourdette D, Silbert L, Kaye J. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis. 2014;38(1):111-20. doi: 10.3233/JAD-130722[↩][↩]
- A randomized, double-blind, placebo-controlled trial of deprenyl and thioctic acid in human immunodeficiency virus-associated cognitive impairment. Dana Consortium on the Therapy of HIV Dementia and Related Cognitive Disorders. Neurology. 1998 Mar;50(3):645-51. doi: 10.1212/wnl.50.3.645[↩]
- Plasma protein oxidation in aging rats after alpha-lipoic acid administration. Cakatay U, Kayali R. Biogerontology. 2005; 6(2):87-93. https://www.ncbi.nlm.nih.gov/pubmed/16034676/[↩][↩]
- Prooxidant activities of alpha-lipoic acid on oxidative protein damage in the aging rat heart muscle. Cakatay U, Kayali R, Sivas A, Tekeli F. Arch Gerontol Geriatr. 2005 May-Jun; 40(3):231-40. https://www.ncbi.nlm.nih.gov/pubmed/15814157/[↩]
- Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Kayali R, Cakatay U, Akçay T, Altuğ T. Cell Biochem Funct. 2006 Jan-Feb; 24(1):79-85. https://www.ncbi.nlm.nih.gov/pubmed/15532093/[↩]
- Postmitotic tissue selenium and manganese levels in alpha-lipoic acid-supplemented aged rats. Cakatay U, Kayali R, Kiziler AR, Aydemir B. Chem Biol Interact. 2008 Feb 15; 171(3):306-11. https://www.ncbi.nlm.nih.gov/pubmed/17996229/[↩]
- Koh EH, Lee WJ, Lee SA, et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am J Med. 2011;124:85.e1–8. https://www.amjmed.com/article/S0002-9343(10)00743-6/fulltext[↩][↩]
- Kim NW, Song YM, Kim E, et al. Adjunctive alpha-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: a double-blind randomized placebo-controlled study. Int Clin Psychopharmacol. 2016;31:265–274. https://www.ncbi.nlm.nih.gov/pubmed/27276401[↩]
- Ziegler, D., Ametov, A., Barinov, A. et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006; 29: 2365–2370[↩]
- Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004 Feb;21(2):114-21. doi: 10.1111/j.1464-5491.2004.01109.x[↩]
- Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Möller W, Tritschler HJ, Mehnert H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic Res. 1999 Sep;31(3):171-9. doi: 10.1080/10715769900300721[↩]
- Parente E, Colannino G, Picconi O, Monastra G. Safety of oral alpha-lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci. 2017 Sep;21(18):4219-4227.[↩]
- Karaarslan U, İşgüder R, Bağ Ö, Kışla M, Ağın H, Ünal N. Alpha lipoic acid intoxication, treatment and outcome. Clin Toxicol (Phila). 2013 Jul;51(6):522. doi: 10.3109/15563650.2013.801983[↩][↩]
- Hadzik B, Grass H, Mayatepek E, Daldrup T, Hoehn T. Fatal non-accidental alpha-lipoic acid intoxication in an adolescent girl. Klin Padiatr. 2014 Sep;226(5):292-4. doi: 10.1055/s-0034-1372622[↩]
- Gleiter CH, Schreeb KH, Freudenthaler S, Thomas M, Elze M, Fieger-Büschges H, Potthast H, Schneider E, Schug BS, Blume HH, Hermann R. Lack of interaction between thioctic acid, glibenclamide and acarbose. Br J Clin Pharmacol. 1999 Dec;48(6):819-25. doi: 10.1046/j.1365-2125.1999.00097.x[↩]
- Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys. 1999 Jun 1;366(1):95-106. doi: 10.1006/abbi.1999.1213[↩]
- Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol. 2005 Apr;288(4):F823-31. doi: 10.1152/ajprenal.00375.2004[↩]
- Zempleni J, Trusty TA, Mock DM. Lipoic acid reduces the activities of biotin-dependent carboxylases in rat liver. J Nutr. 1997 Sep;127(9):1776-81. doi: 10.1093/jn/127.9.1776[↩]
- Zempleni J, Mock DM. Biotin biochemistry and human requirements. J Nutr Biochem. 1999 Mar;10(3):128-38. doi: 10.1016/s0955-2863(98)00095-3[↩]