Contents
- What is acute lymphocytic leukemia
- Acute lymphocytic leukemia causes
- Acute lymphoblastic leukemia prevention
- Acute lymphocytic leukemia symptoms
- Acute lymphoblastic leukemia complications
- Acute lymphocytic leukemia diagnosis
- Acute lymphocytic leukemia subtypes and prognostic factors
- Acute lymphocytic leukemia survival rate
- Acute lymphocytic leukemia prognosis
- Acute lymphocytic leukemia treatment
What is acute lymphocytic leukemia
Acute lymphoblastic leukemia (ALL) is also known as acute lymphocytic leukemia, is a cancer of the blood and bone marrow — in which the bone marrow makes too many lymphocytes (a type of white blood cell). Acute lymphoblastic leukemia (ALL) cancer usually gets worse quickly if it is not treated.
The word “acute” in acute lymphocytic leukemia comes from the fact that the disease progresses rapidly and creates immature blood cells, rather than mature ones. The word “lymphocytic” in acute lymphocytic leukemia refers to the white blood cells called lymphocytes, which acute lymphoblastic leukemia (ALL) affects.
Acute lymphocytic leukemia (acute lymphoblastic leukemia) is the most common type of cancer in children, and treatments result in a good chance for a cure. Acute lymphocytic leukemia can also occur in adults, though the chance of a cure is greatly reduced.
Acute lymphocytic leukemia (acute lymphoblastic leukemia) starts in the bone marrow (the soft inner part of certain bones, where new blood cells are made). Most often, the leukemia cells invade the blood fairly quickly. They can also sometimes spread to other parts of the body, including the lymph nodes, liver, spleen, central nervous system (brain and spinal cord), and testicles (in males).
Other types of cancer that start in lymphocytes are known as lymphomas (either non-Hodgkin lymphoma or Hodgkin lymphoma). While leukemias like acute lymphocytic leukemia (acute lymphoblastic leukemia) mainly affect the bone marrow and the blood, lymphomas mainly affect the lymph nodes or other organs (but may also involve the bone marrow). Sometimes it can be hard to tell if a cancer of lymphocytes is a leukemia or a lymphoma. Usually, if at least 20% of the bone marrow is made up of cancerous lymphocytes (called lymphoblasts, or just blasts), the disease is considered leukemia.
Normal bone marrow, blood, and lymph tissue
To understand leukemia, it helps to know about the blood and lymph systems.
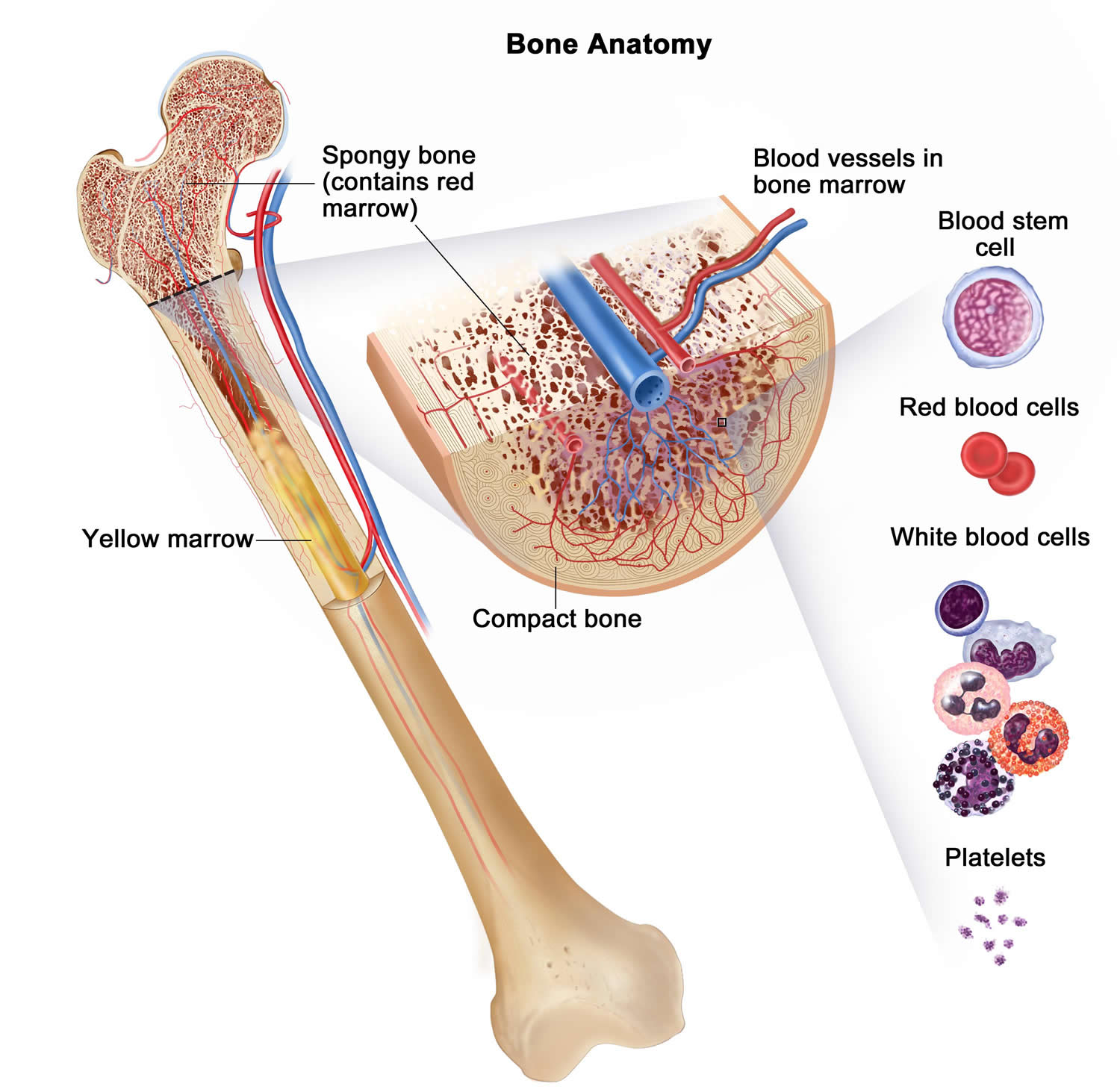
Bone marrow
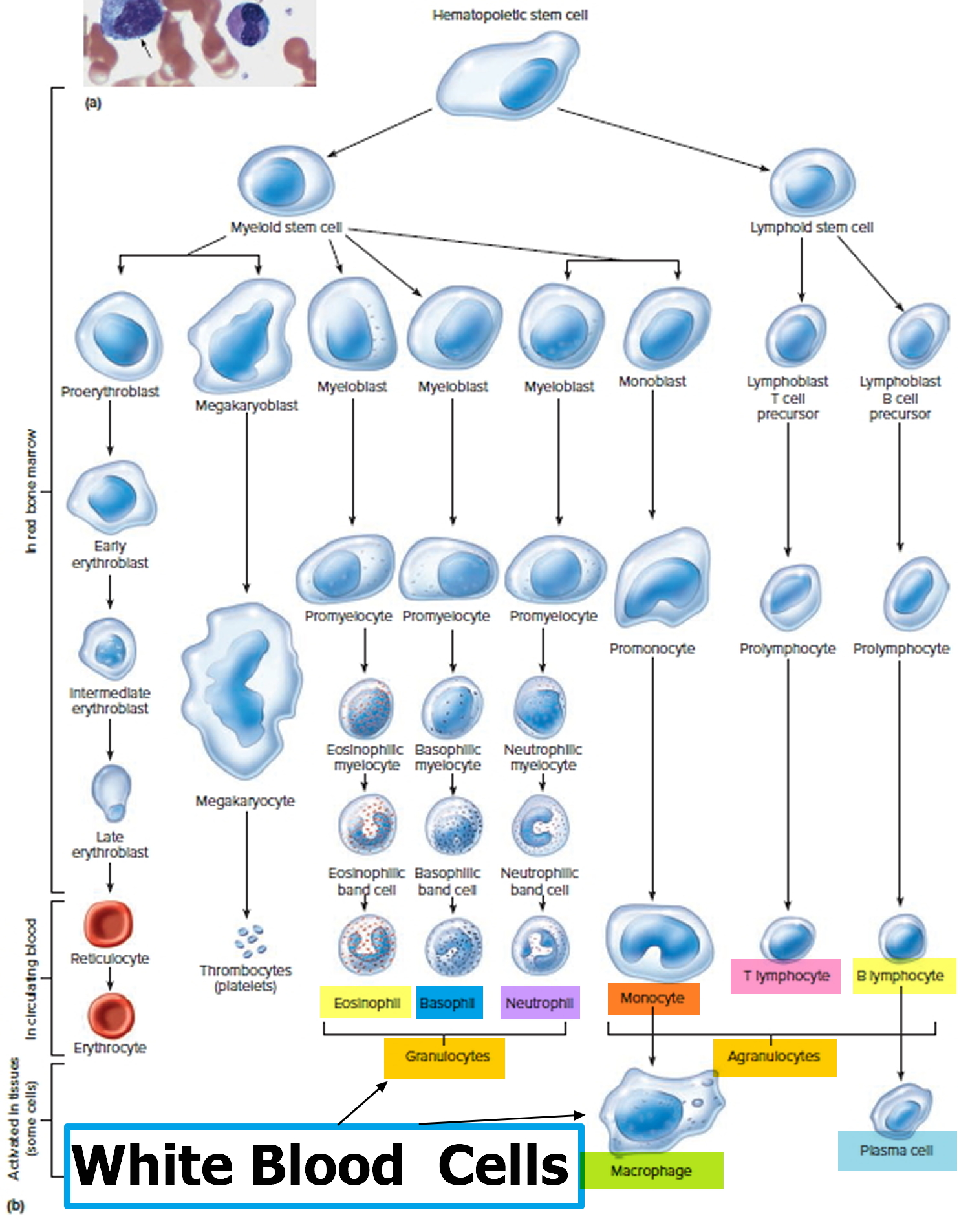
Bone marrow is the soft inner part of certain bones. It is made up of blood-forming cells, fat cells, and supporting tissues. A small fraction of the blood-forming cells are blood stem cells.
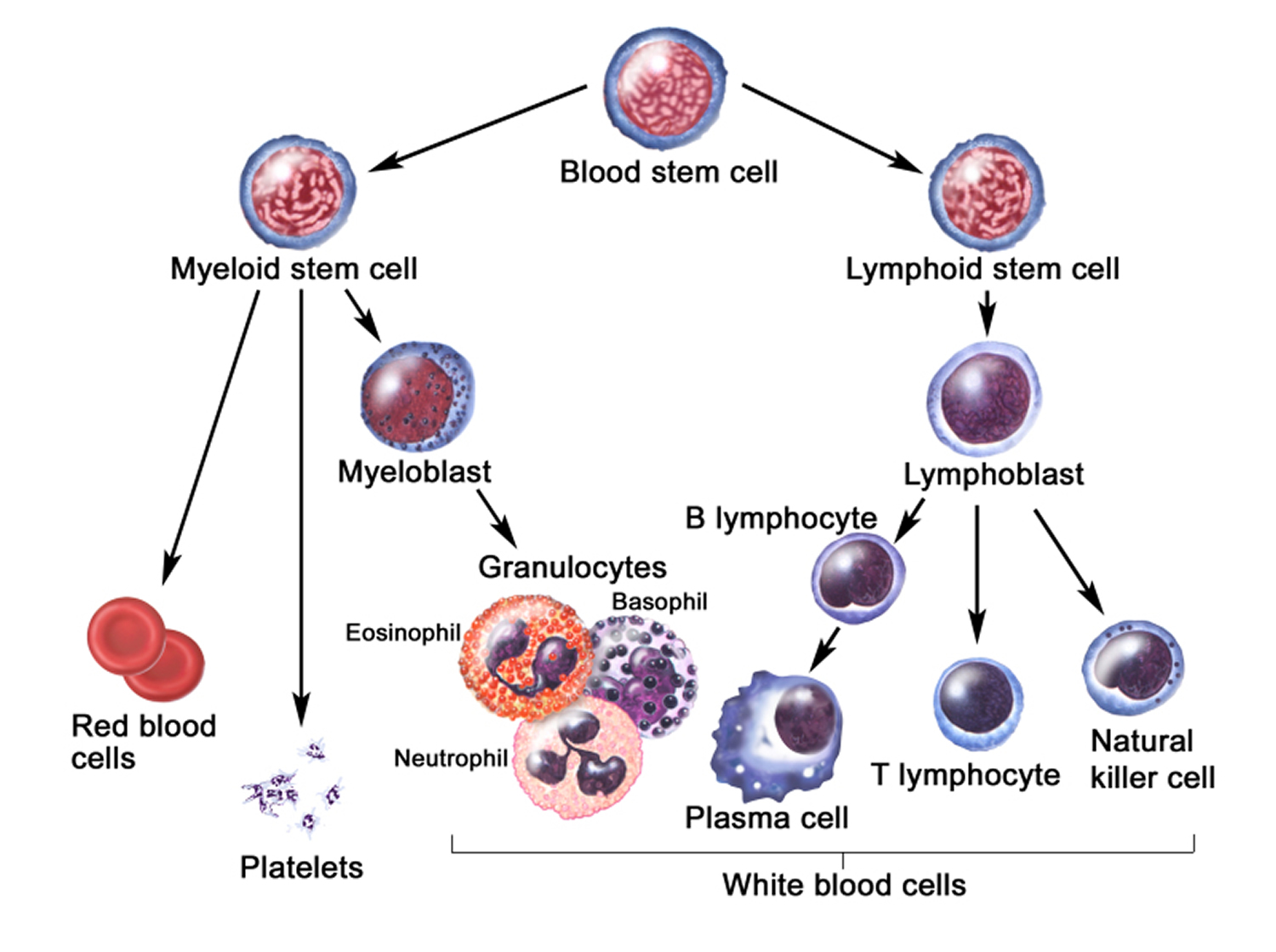
Inside the bone marrow, blood stem cells develop into new blood cells. During this process, the cells become either lymphocytes (a kind of white blood cell) or other blood-forming cells, which are types of myeloid cells. Myeloid cells can develop into red blood cells, white blood cells (other than lymphocytes), or platelets. These myeloid cells are the ones that are abnormal in acute myeloid leukemia.
Types of blood cells
There are 3 main types of blood cells:
- Red blood cells (RBCs) carry oxygen from the lungs to all other tissues in the body, and take carbon dioxide back to the lungs to be removed.
- Platelets are actually cell fragments made by a type of bone marrow cell called the megakaryocyte. Platelets are important in stopping bleeding. They help plug up holes in blood vessels caused by cuts or bruises.
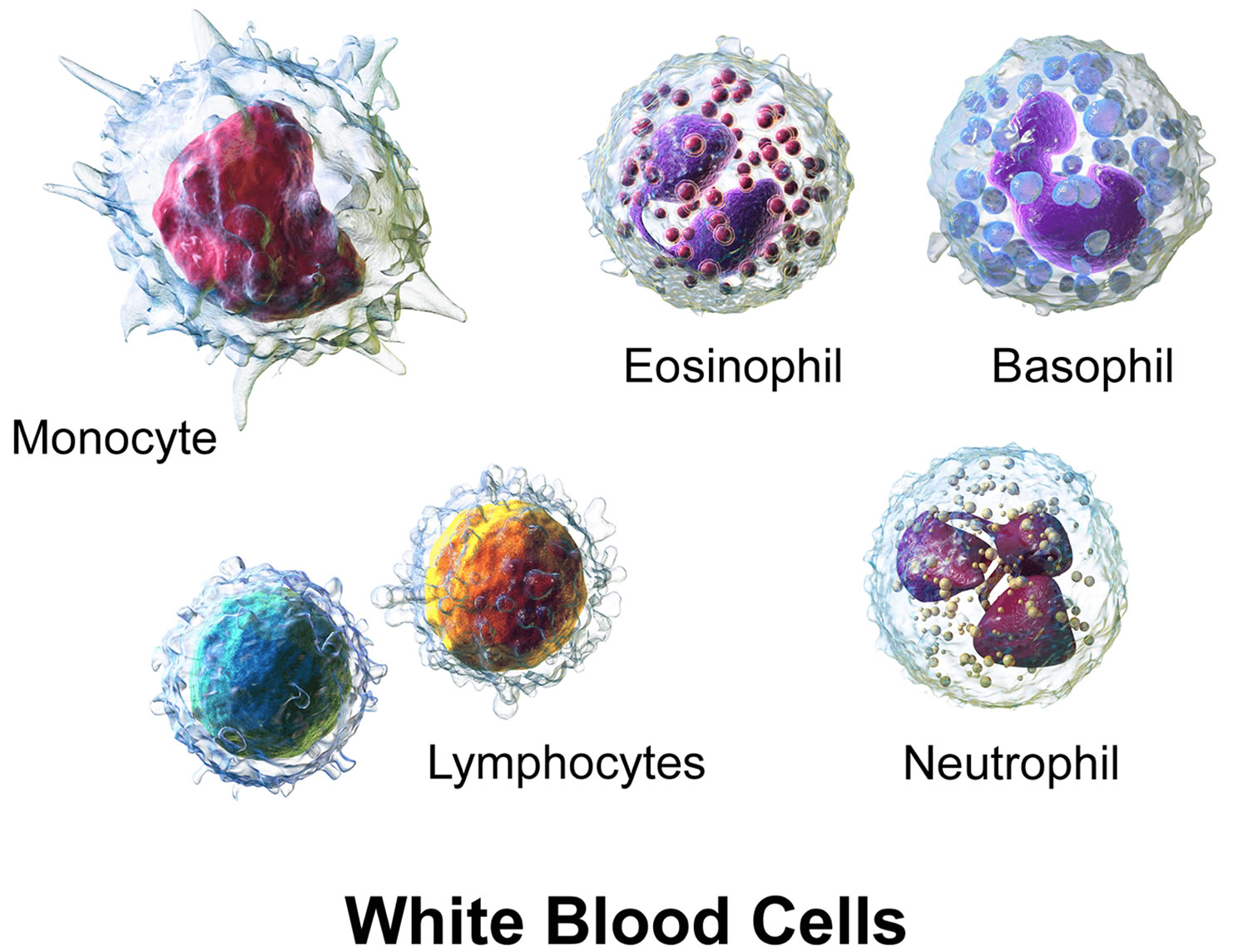
- White blood cells (WBCs) help the body fight infections.
There are different types of white blood cells:
- Granulocytes are mature white blood cells that develop from myeloblasts, a type of blood-forming cell in the bone marrow. Granulocytes have granules that show up as spots under the microscope. These granules contain enzymes and other substances that can destroy germs, such as bacteria. The 3 types of granulocytes – neutrophils, basophils, and eosinophils – are distinguished by the size and color of their granules.
- Monocytes are white blood cells that develop from blood-forming monoblasts in the bone marrow. After circulating in the bloodstream for about a day, monocytes enter body tissues to become macrophages, which can destroy some germs by surrounding and digesting them. Macrophages also help lymphocytes recognize germs and make antibodies to fight them.
- Lymphocytes are mature white blood cells that develop from lymphoblasts in the bone marrow. Lymphocytes are the main cells that make up lymph tissue, a major part of the immune system. Lymph tissue is found in lymph nodes, the thymus (a small organ behind the breast bone), the spleen, the tonsils and adenoids, and is scattered throughout the digestive and respiratory systems and the bone marrow. The 2 main types of lymphocytes are B cell and T cells.
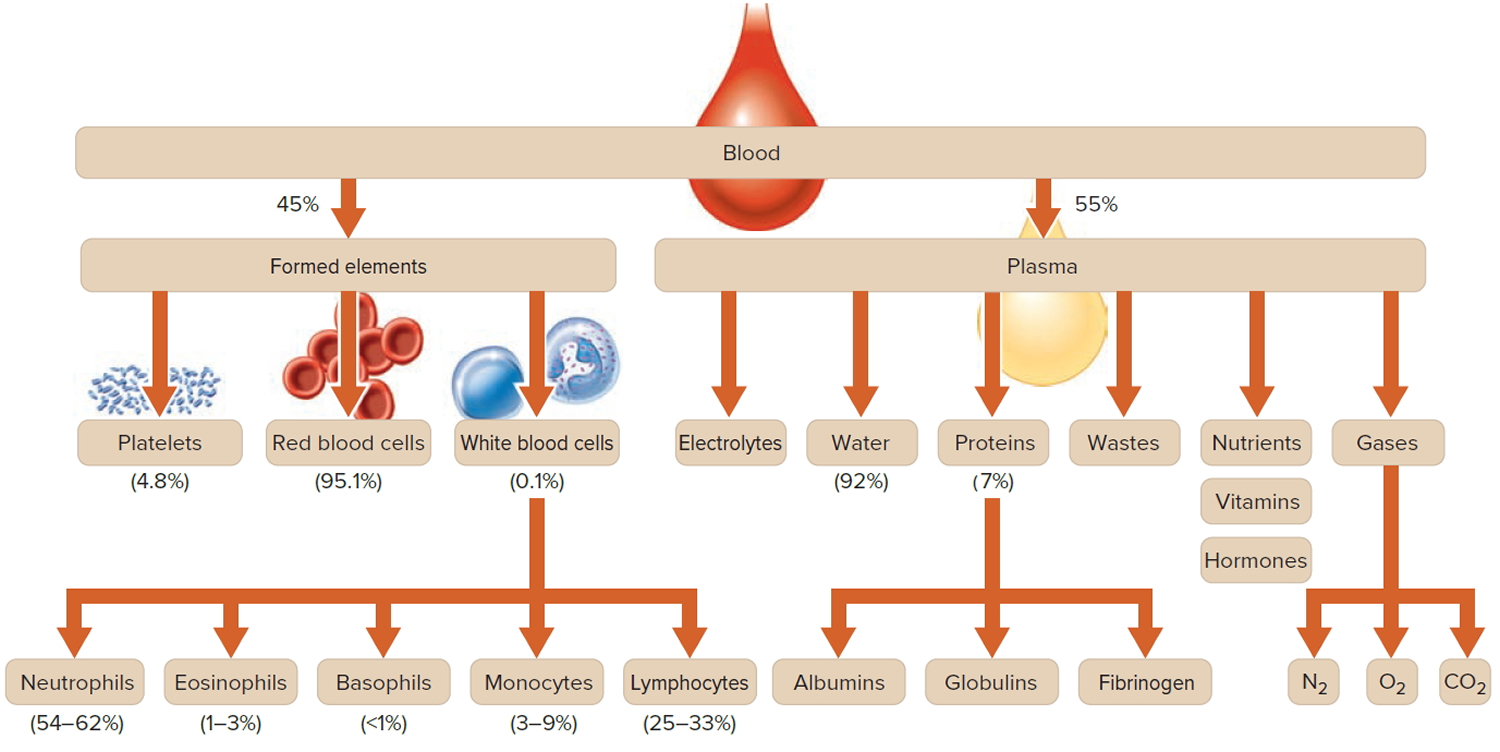
Figure 1. Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 2. Bone marrow anatomy
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
Figure 5. White blood cells
Acute lymphocytic leukemia causes
Acute lymphocytic leukemia (acute lymphoblastic leukemia) occurs when a bone marrow cell develops errors in its DNA. The errors tell the cell to continue growing and dividing, when a healthy cell would normally stop dividing and eventually die. When this happens, blood cell production becomes abnormal. The bone marrow produces immature cells that develop into leukemic white blood cells called lymphoblasts. These abnormal cells are unable to function properly, and they can build up and crowd out healthy cells.
It’s not clear what causes the DNA mutations that can lead to acute lymphocytic leukemia. But doctors have found that most cases of acute lymphocytic leukemia (acute lymphoblastic leukemia) aren’t inherited.
Great progress has been made in understanding how certain changes in the DNA in normal bone marrow cells can cause them to become leukemia cells. The DNA inside your cells makes up your genes, which control how your cells function.
Some genes control when your cells grow, divide to make new cells, and die at the right time:
- Certain genes that help cells grow, divide, or stay alive are called oncogenes.
- Genes that keep cell growth and division under control or make cells die at the right time are called tumor suppressor genes.
Each time a cell divides into 2 new cells, it must make a new copy of its chromosomes (long strands of DNA). This process isn’t perfect, and errors can occur that can affect genes within the chromosomes. Cancers (including acute lymphocytic leukemia) can be caused by mutations (changes) that turn on oncogenes or turn off tumor suppressor genes. These types of changes can stop bone marrow cells from maturing the way they normally would, or help the cells grow out of control.
Mutations in many different genes can be found in acute lymphocytic leukemia cells, but larger changes in one or more chromosomes are also common. Even though these changes involve larger pieces of DNA, their effects are still likely to be due to changes in just one or a few genes that are on that part of the chromosome.
Several types of chromosome changes may be found in acute lymphocytic leukemia cells:
Translocations are the most common type of chomosome change that can lead to leukemia. A translocation means that DNA from one chromosome breaks off and becomes attached to a different chromosome. The point on the chromosome where the break occurs can affect nearby genes – for example, it can turn on oncogenes or turn off genes that would normally help a cell mature.
The most common translocation in acute lymphocytic leukemia in adults is known as the Philadelphia chromosome, which is a swap of DNA between chromosomes 9 and 22, abbreviated as t(9;22). Many other, less common translocations, can occur as well, including those between chromosomes 4 and 11, t(4;11), or 8 and 14, t(8;14).
Other chromosome changes such as deletions (the loss of part of a chromosome) and inversions (the rearrangement of the DNA within part of a chromosome) are also sometimes found in acute lymphocytic leukemia cells, although they are less common. In many cases of acute lymphocytic leukemia, the gene changes that lead to the leukemia are not known.
Doctors are trying to figure out why these changes occur and how each of them might lead to leukemia. But there are different subtypes of acute lymphocytic leukemia, and even within a subtypes, not all cases of acute lymphocytic leukemia have the same gene or chromosome changes. Some changes are more common than others, and some seem to have more of an effect on a person’s prognosis (outlook) than others.
Risk factors for acute lymphoblastic leukemia
A risk factor is something that increases your chance of getting a disease such as cancer. But having a risk factor, or even several risk factors, does not mean that you will definitely get the disease. And many people who get the disease may have few or no known risk factors.
There are only a handful of known risk factors for acute lymphocytic leukemia (acute lymphoblastic leukemia).
Factors that may increase the risk of acute lymphocytic leukemia include:
- Age: Acute lymphocytic leukemia is more likely to occur in children and in adults over the age of 50.
- Previous cancer treatment. Children and adults who’ve had certain types of chemotherapy and radiation therapy for other kinds of cancer may have an increased risk of developing acute lymphocytic leukemia.
- Exposure to radiation. People exposed to very high levels of radiation, such as survivors of a nuclear reactor accident, have an increased risk of developing acute lymphocytic leukemia and acute myeloid leukemia (AML). Treating cancer with radiation therapy also increases the risk of leukemia, although more for acute myeloid leukemia than acute lymphocytic leukemia (ALL). The risk seems to be higher if chemotherapy and radiation are both used in treatment. The possible risks of leukemia from being exposed to lower levels of radiation, such as from medical imaging tests like x-rays or CT scans, are not well understood. Exposure to such radiation, especially very early in life, may carry an increased risk of leukemia, but this is not clear. If there is an increased risk it is likely to be small, but to be safe, most doctors try to limit radiation exposure from these tests as much as possible, especially in children and pregnant women.
- Genetic disorders. Acute lymphocytic leukemia itself doesn’t appear to have a strong inherited component. That is, it doesn’t seem to run in families, so a person’s risk is not increased if a family member (other than an identical twin – see below) has the disease. But there are some genetic syndromes (some of which can be inherited from a parent) that seem to raise the risk of acute lymphocytic leukemia. These include:
- Down syndrome
- Klinefelter syndrome
- Fanconi anemia
- Bloom syndrome
- Ataxia-telangiectasia
- Neurofibromatosis
- Li-Fraumeni syndrome
- Having a brother or sister with acute lymphocytic leukemia. People who have a sibling, including a twin, with acute lymphocytic leukemia have an increased risk of acute lymphocytic leukemia.
- Certain chemical exposures: The risk of acute lymphocytic leukemia may be increased by exposure to certain chemotherapy drugs and certain other chemicals, including benzene. Benzene is used in many industries to make other products, and is also in cigarette smoke, as well as some glues, cleaning products, detergents, art supplies, and paint strippers. Chemical exposure is more strongly linked to an increased risk of acute myeloid leukemia (AML) than to acute lymphocytic leukemia.
- Certain viral infections: Infection with the human T-cell lymphoma/leukemia virus-1 (HTLV-1) can cause a rare type of T-cell acute lymphocytic leukemia. Most cases occur in Japan and the Caribbean area. This disease is not common in the United States. In Africa, the Epstein-Barr virus (EBV) has been linked to Burkitt lymphoma, as well as to a form of acute lymphocytic leukemia. In the United States, Epstein-Barr virus (EBV) most often causes infectious mononucleosis (“mono”). Epstein-Barr virus (EBV) has also been linked with a type of lymphoma that can occur after a stem cell transplant (known as post-transplant lymphoproliferative disorder, or PTLD).
- Smoking – smokers are much more likely to develop acute leukemia than non-smokers, and studies have shown that parents who smoke in the home may increase the risk of leukemia in their children.
- Being very overweight (obese) – some studies have shown that people who are very overweight have a slightly higher risk of developing leukemia than those who are a normal weight.
- Having a weakened immune system – people with lowered immunity (as a result of having HIV or AIDS or taking immunosuppressants) have an increased risk of developing leukemia
- Race/ethnicity: Acute lymphocytic leukemia is more common in whites than in African Americans, but the reasons for this are not clear.
- Gender: Acute lymphocytic leukemia is slightly more common in males than in females. The reason for this is unknown.
- Having an identical twin with acute lymphocytic leukemia: Someone who has an identical twin who develops acute lymphocytic leukemia in the first year of life has an increased risk of getting acute lymphocytic leukemia.
Uncertain, unproven or controversial risk factors
Other factors that have been studied for a possible link to acute lymphocytic leukemia include:
- Exposure to electromagnetic fields (such as living near power lines or using cell phones)
- Workplace exposure to diesel, gasoline, pesticides, and certain other chemicals
- Smoking
- Exposure to hair dyes
So far, none of these factors has been linked conclusively to acute lymphocytic leukemia, but research in these areas continues.
Acute lymphoblastic leukemia prevention
There is no known way to prevent most cases of leukemia at this time. Most people who get acute lymphocytic leukemia have no known risk factors, so there is no way to prevent these leukemias from developing.
Acute lymphocytic leukemia symptoms
Acute lymphocytic leukemia (acute lymphoblastic leukemia) can cause many different signs and symptoms. Most of these occur in all kinds of acute lymphocytic leukemia, but some are more common with certain subtypes of acute lymphocytic leukemia.
Acute lymphoblastic leukaemia usually starts slowly before rapidly becoming severe as the number of immature white blood cells in your blood increases.
Most of the symptoms are caused by the lack of healthy blood cells in your blood supply.
Symptoms caused by low numbers of blood cells
Most signs and symptoms of acute lymphocytic leukemia are the result of shortages of normal blood cells, which happen when the leukemia cells crowd out the normal blood-making cells in the bone marrow. These shortages show up on blood tests, but they can also cause symptoms, including:
- Feeling tired
- Feeling weak
- Feeling dizzy or lightheaded
- Shortness of breath
- Pale skin
- Infections that don’t go away or keep coming back
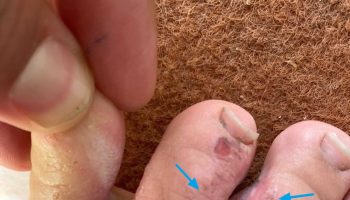
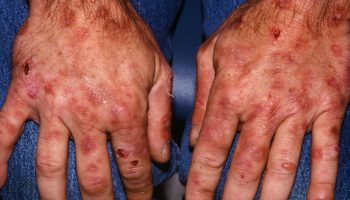
- Bruises (or small red or purple spots) on the skin
- Bleeding, such as frequent or severe nosebleeds, bleeding gums, or heavy menstrual bleeding in women
General symptoms
Patients with acute lymphocytic leukemia also often have several non-specific symptoms. These can include:
- Weight loss
- Fever
- Night sweats
- Loss of appetite
Of course, these are not just symptoms of acute lymphocytic leukemia and are more often caused by something other than leukemia.
Swelling in the abdomen
Leukemia cells may build up in the liver and spleen, making them larger. This might be noticed as a fullness or swelling of the belly, or feeling full after eating only a small amount. The lower ribs usually cover these organs, but when the organs are enlarged the doctor can feel them.
Enlarged lymph nodes
Acute lymphocytic leukemia that has spread to lymph nodes close to the surface of the body (such as on the sides of the neck, in the groin, or in underarm areas), might be noticed as lumps under the skin. Lymph nodes inside the chest or abdomen may also swell, but these can be detected only by imaging tests such as CT or MRI scans.
Bone or joint pain
Sometimes leukemia cells build up near the surface of the bone or inside the joint, which can lead to bone or joint pain.
Spread to other organs
Less often, acute lymphocytic leukemia spreads to other organs:
- If acute lymphocytic leukemia spreads to the brain and spinal cord it can cause headaches, weakness, seizures, vomiting, trouble with balance, facial muscle weakness or numbness, or blurred vision.
- Acute lymphocytic leukemia may spread inside the chest, where it can cause fluid buildup and trouble breathing.
- Rarely, acute lymphocytic leukemia may spread to the skin, eyes, testicles, ovaries, kidneys, or other organs.
Symptoms from an enlarged thymus
The T-cell subtype of acute lymphocytic leukemia often affects the thymus, which is a small organ in the middle of the chest behind the sternum (breastbone) and in front of the trachea (windpipe). An enlarged thymus can press on the trachea, which can lead to coughing or trouble breathing.
The superior vena cava, a large vein that carries blood from the head and arms back to the heart, passes next to the thymus. If the thymus is enlarged, it may press on the superior vena cava, causing the blood to “back up” in the veins. This is known as superior vena cava syndrome. It can cause:
- Swelling in the face, neck, arms, and upper chest (sometimes with a bluish-red color)
- Headaches
- Dizziness
- Change in consciousness if it affects the brain
The superior vena cava syndrome can be life-threatening, and needs to be treated right away.
Acute lymphoblastic leukemia complications
Being immunocompromised (having a weakened immune system) is a possible complication for some people with acute leukemia.
There are two reasons for this:
- the lack of healthy white blood cells means your immune system is less able to fight infection
- many of the medicines used to treat acute leukemia can weaken the immune system
This makes you more vulnerable to developing an infection, and any infection you do have is more likely to cause serious complications.
You may be advised to take regular doses of antibiotics to prevent infections occurring. You should report any possible symptoms of an infection immediately to your doctor or care team because prompt treatment may be needed to prevent serious complications.
Symptoms of infection include:
- high temperature (fever) of 101.4 °F (38 °C) or above
- headache
- aching muscles
- diarrhea
- tiredness
Avoid contact with anyone known to have an infection, even if it’s a type of infection that you were previously immune to, such as chickenpox or measles. This is because your previous immunity to these conditions will probably be lower.
It’s important to go outside on a regular basis, both for exercise and for your wellbeing, but you should avoid visiting crowded places and using public transport during rush hour.
Also, make sure all of your vaccinations are up-to-date. Your doctor or care team will be able to advise you about this. You’ll be unable to have any vaccine containing activated particles of viruses or bacteria such as the:
- mumps, measles and rubella (MMR) vaccine
- polio vaccine
- oral typhoid vaccine
- BCG vaccine (used to vaccinate against tuberculosis)
- yellow fever vaccine
Bleeding
If you have acute leukemia, you’ll bleed and bruise more easily because of the low levels of platelets (clot-forming cells) in your blood.
Although major bleeding is uncommon, you need to be aware of the related symptoms that can occur in different parts of the body.
Bleeding can occur:
- inside the skull (intracranial hemorrhage)
- inside the lungs (pulmonary hemorrhage)
- inside the stomach (gastrointestinal hemorrhage)
The symptoms of an intracranial hemorrhage include:
- severe headache
- stiff neck
- vomiting
- change in mental state, such as confusion
The most common symptoms of a pulmonary hemorrhage are:
- coughing up blood from your nose and mouth
- breathing difficulties
- a bluish skin tone (cyanosis)
The two most common symptoms of a gastrointestinal hemorrhage are:
- vomiting blood
- passing stools (feces) that are very dark or tar-like
All three types of hemorrhages should be regarded as medical emergencies. Call your local emergency services number for an ambulance if you suspect that you or your child is experiencing a hemorrhage.
Infertility
Many of the treatments used to treat acute leukemia can cause infertility. Infertility is often temporary, although in some cases it may be permanent.
People who are particularly at risk of becoming infertile are those who’ve received high doses of chemotherapy and radiotherapy in preparation for stem cell and bone marrow transplants.
It may be possible to guard against any risk of infertility before you begin your treatment. For example, men can store sperm samples. Similarly, women can have fertilised embryos stored, which can be put back into their womb following treatment.
Psychological effects of leukemia
Being diagnosed with acute lymphocytic leukemia (acute lymphoblastic leukemia) can be very distressing, particularly if a cure is unlikely. At first, the news may be difficult to take in. It can be particularly difficult if you don’t currently have any leukemia symptoms, but you know that it could present a serious problem later on. Having to wait many years to see how the leukemia develops can be very stressful and can trigger feelings of anxiety and depression.
If you’ve been diagnosed with acute lymphocytic leukemia (acute lymphoblastic leukemia), talking to a counselor or psychiatrist (a doctor who specializes in treating mental health conditions) may help you combat feelings of depression and anxiety. Antidepressants or medicines that help reduce feelings of anxiety may also help you cope better.
You may find it useful to talk to other people living with leukemia. Your doctor or multidisciplinary team may be able to provide you with details of local support groups.
Acute lymphocytic leukemia diagnosis
Certain signs and symptoms can suggest that a person might have acute lymphocytic leukemia (acute lymphoblastic leukemia), but tests are needed to confirm the diagnosis.
Medical history and physical exam
If you have signs and symptoms that suggest you might have leukemia, the doctor will want to get a thorough medical history, including how long you have had symptoms and if you have possibly been exposed to anything considered a risk factor.
During the physical exam, the doctor will probably focus on any enlarged lymph nodes, areas of bleeding or bruising, or possible signs of infection. The eyes, mouth, and skin will be looked at carefully, and a thorough nervous system exam may be done. Your abdomen will be felt for spleen or liver enlargement.
If there is reason to think low levels of blood cells might be causing your symptoms (anemia, infections, bleeding or bruising, etc.), the doctor will most likely order blood tests to check your blood cell counts. You might also be referred to a hematologist, a doctor who specializes in diseases of the blood (including leukemia).
Tests used to diagnose and classify acute lymphocytic leukemia
If your doctor thinks you might have leukemia, he or she will need to check samples of cells from your blood and bone marrow to be sure. Other tissue and cell samples may also be taken to help guide treatment.
Blood tests
Blood samples for acute lymphocytic leukemia tests are generally taken from a vein in the arm.
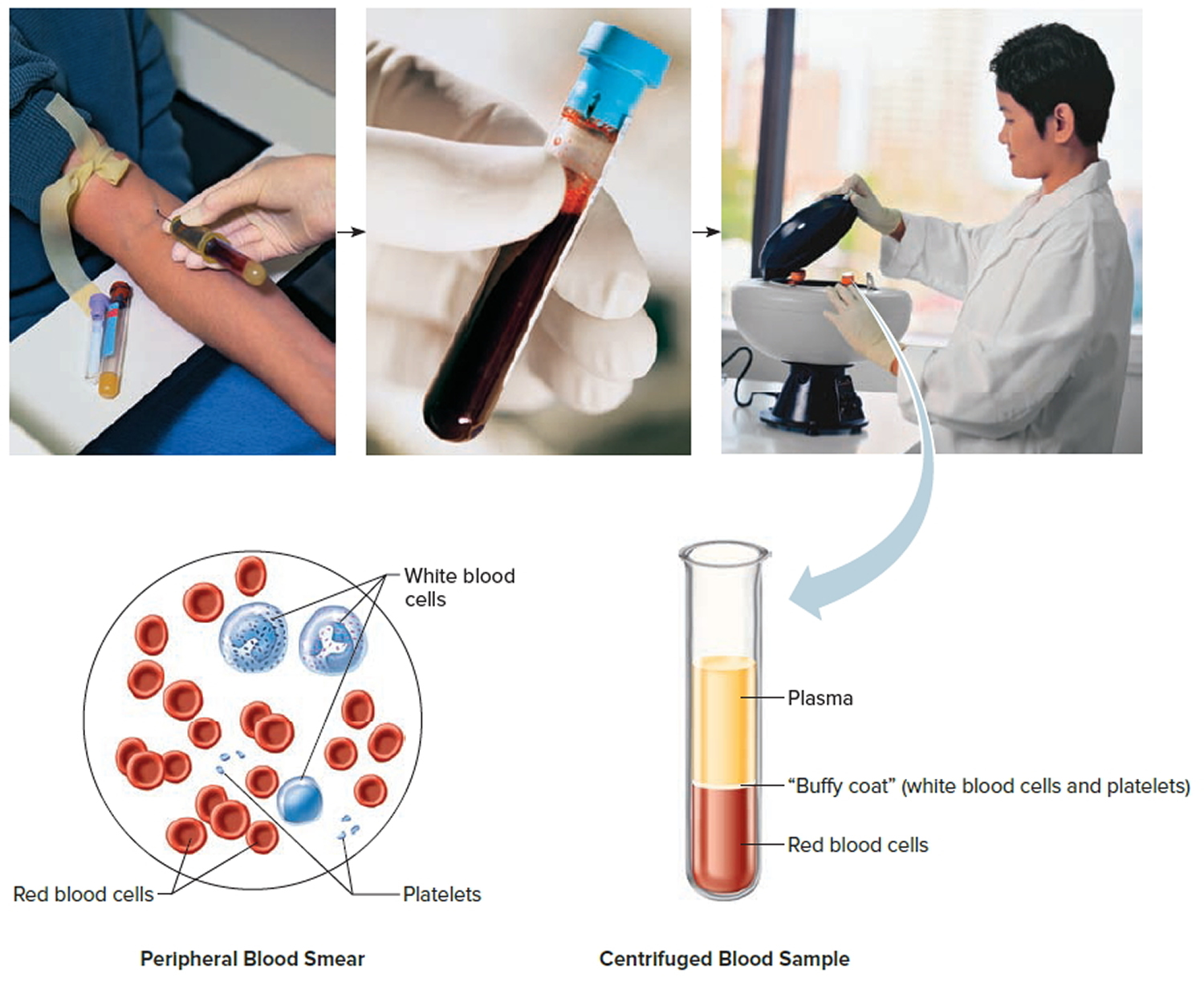
Complete blood count (CBC) and peripheral blood smear
The complete blood count (CBC) measures the numbers of red blood cells, white blood cells, and platelets. This test is often done along with a differential (or diff) which looks at the numbers of the different types of white blood cells. These tests are often the first ones done on patients with a suspected blood problem.
For the peripheral blood smear (sometimes just called a smear), a drop of blood is smeared across a slide and then looked at under a microscope to see how the cells look. Changes in the numbers and the appearance of the cells often help diagnose leukemia.
Most patients with acute lymphocytic leukemia have too many immature white cells called lymphoblasts (or just blasts) in their blood, and not enough red blood cells or platelets. Lymphoblasts are not normally found in the blood, and they don’t function like normal, mature white blood cells.
Even though these findings may suggest leukemia, the disease usually is not diagnosed without looking at a sample of bone marrow cells.
Blood chemistry tests
Blood chemistry tests measure the amounts of certain chemicals in the blood, but they are not used to diagnose leukemia. In patients already known to have acute lymphocytic leukemia, these tests can help detect liver or kidney problems caused by spreading leukemia cells or the side effects of certain chemotherapy drugs. These tests also help determine if treatment is needed to correct low or high blood levels of certain minerals.
Coagulation tests
Blood coagulation tests may be done to make sure the blood is clotting properly.
Bone marrow tests
Leukemia starts in the bone marrow, so checking the bone marrow for leukemia cells is a key part of testing for it.
Bone marrow aspiration and biopsy
Bone marrow samples are obtained by bone marrow aspiration and biopsy – tests usually done at the same time. The samples are usually taken from the back of the pelvic (hip) bone, although in some cases they may be taken from the sternum (breastbone) or other bones.
In bone marrow aspiration, you lie on a table (either on your side or on your belly). After cleaning the skin over the hip, the doctor numbs the skin and the surface of the bone by injecting a local anesthetic, which may cause a brief stinging or burning sensation. A thin, hollow needle is then inserted into the bone and a syringe is used to suck out a small amount of liquid bone marrow. Even with the anesthetic, most patients still have some brief pain when the marrow is removed.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed down into the bone. With local anesthetic, most patients just feel some pressure and tugging from the biopsy, but some may feel a brief pain. Once the biopsy is done, pressure will be applied to the site to help prevent bleeding.
These bone marrow tests are used to help diagnose leukemia. They may also be done again later to tell if the leukemia is responding to treatment.
Lab tests used to diagnose and classify acute lymphocytic leukemia
One or more of the following lab tests may be done on the samples to diagnose acute myeloid leukemia (AML) and/or to determine the specific subtype of acute lymphocytic leukemia.
Routine exams with a microscope
The bone marrow (and sometimes blood) samples are looked at with a microscope by a pathologist (a doctor specializing in lab tests) and may be reviewed by the patient’s hematologist/oncologist (a doctor specializing in cancer and blood diseases).
The doctors will look at the size, shape, and other traits of the white blood cells in the samples to classify them into specific types. A key factor is whether the cells look mature (like normal blood cells), or immature (lacking features of normal blood cells). The most immature cells are called lymphoblasts (or just blasts).
Determining what percentage of cells in the bone marrow are blasts is particularly important. A diagnosis of acute lymphocytic leukemia generally requires that at least 20% of the cells in the bone marrow are blasts. Under normal circumstances, blasts don’t make up more than 5% of bone marrow cells. Sometimes just counting and looking at the cells doesn’t provide a definite diagnosis, and other lab tests are needed.
Cytochemistry
In cytochemistry tests, cells are put on a slide and exposed to chemical stains (dyes) that react only with some types of leukemia cells. These stains cause color changes that can be seen under a microscope, which can help the doctor determine what types of cells are present. For instance, one stain will turn parts of acute myeloid leukemia (AML) cells black, but has no effect on acute lymphocytic leukemia cells.
Flow cytometry and immunohistochemistry
For both flow cytometry and immunohistochemistry, samples of cells are treated with antibodies, which are proteins that stick only to certain other proteins on cells. For immunohistochemistry, the cells are examined under a microscope to see if the antibodies stuck to them (meaning they have those proteins), while for flow cytometry a special machine is used.
These tests are used for immunophenotyping – classifying leukemia cells according to proteins on or in the cells. This kind of testing is very helpful in determining the exact type of leukemia. For diagnosing leukemia, it is most often done on cells from bone marrow, but it can also be done on cells from the blood, lymph nodes, and other body fluids.
For acute lymphocytic leukemia, these tests are most often used to help determine the exact subtype of in someone already thought to have acute lymphocytic leukemia based on other tests.
Chromosome tests
These tests look at the chromosomes (long strands of DNA) inside the cells. Normal human cells contain 23 pairs of chromosomes (bundles of DNA). In acute lymphocytic leukemia, the cells sometimes have chromosome changes. Recognizing these changes can help identify certain types of acute lymphocytic leukemia, and it can be important in determining a patient’s outlook and likely response to some treatments. For this reason, chromosome testing is a standard part of the work-up for acute lymphocytic leukemia.
The most common chromosome change in acute lymphocytic leukemia is a translocation, in which, 2 chromosomes swap some of their DNA, so that part of one chromosome becomes attached to part of a different chromosome. The most common chromosome change in adult acute lymphocytic leukemia is a translocation that results in a shortened chromosome 22 (called the Philadelphia chromosome). About 1 out of 4 adults with acute lymphocytic leukemia have this abnormality in their leukemia cells. This change is especially important because it can be targeted with certain drugs.
Cytogenetics
For this test, the cells are grown in lab dishes until they start dividing. Then the chromosomes are looked at under a microscope to detect any changes. Because it takes time for the cells to start dividing, cytogenetic testing often takes about 2 to 3 weeks. Not all chromosome changes can be seen under a microscope. Other lab tests can often help find these changes.
Fluorescent in situ hybridization (FISH)
This is another way to look at chromosomes and genes. It uses special fluorescent dyes that only attach to specific genes or parts of particular chromosomes. Fluorescent in situ hybridization (FISH) can find most chromosome changes (such as translocations) that are visible under a microscope in standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing.
Fluorescent in situ hybridization (FISH) can be used on regular blood or bone marrow samples. Because the cells don’t have to be able to divide for this test, it can also be used to look at cells from other tissues, like lymph node samples. It is very accurate and can usually provide results within a couple of days. But because fluorescent in situ hybridization (FISH) only tests for certain gene changes (and doesn’t look at the chromosomes overall), it is best for looking for the changes that are important based on the kind of leukemia a person has.
Polymerase chain reaction (PCR)
This is a very sensitive DNA test that can also find certain gene and chromosome changes too small to be seen with a microscope, even if very few leukemia cells are present in a sample. Like fluorescent in situ hybridization (FISH), it is used to find particular gene changes and not to look at the chromosomes overall.
If the leukemia cells have a particular gene (or chromosome) change, polymerase chain reaction (PCR) can be used after treatment to try to find small numbers of leukemia cells that may not be visible with a microscope.
Other molecular and genetic tests
Other, newer types of lab tests can also be done on the samples to look for specific gene or other changes in the leukemia cells.
Lumbar puncture (spinal tap)
Acute lymphocytic leukemia can spread to the area around the brain and spinal cord. To check for this spread, doctors remove a sample of the fluid from that area (cerebrospinal fluid or CSF) for testing. You may lay on your side or sit up for this test. The doctor first numbs an area in the lower part of the back over the spine. A small, hollow needle is then placed between the bones of the spine and into the area around the spinal cord to collect some fluid.
A lumbar puncture can also be used to put chemotherapy drugs into the CSF to try to prevent or treat the spread of leukemia to the spinal cord and brain.
Lymph node biopsy
A lymph node or part of a lymph node is often removed to help diagnose lymphomas, but this is only rarely needed with leukemia because the diagnosis is usually made looking at blood and bone marrow. In this procedure, a surgeon cuts through the skin to remove all or part of a lymph node. If the node is just under the skin, this is a simple operation that can often be done with local anesthesia, but if the node is inside the chest or abdomen, general anesthesia is used to keep you asleep during the biopsy.
When the entire lymph node is removed, it is called an excisional lymph node biopsy. If only part of the lymph node is removed, it is called an incisional lymph node biopsy.
Imaging tests
Imaging tests use x-rays, sound waves, magnetic fields, or radioactive particles to create pictures of the inside of the body. Leukemia does not usually form tumors, so imaging tests aren’t as useful as they are for other types of cancer. Imaging tests might be done in people with acute lymphocytic leukemia to help determine the extent of the disease, if it is thought to have spread beyond the bone marrow and blood. They might also be done to look for infections or other problems. .
X-rays
Chest x-rays may be done if the doctor suspects a lung infection. They may also be done to look for enlarged lymph nodes in the chest.
Computed tomography (CT) scan
The CT scan uses x-rays to make detailed, cross-sectional images of your body. The CT scan can show if any lymph nodes or organs in your body are enlarged. It isn’t usually needed to diagnose acute lymphocytic leukemia, but it may be done if your doctor suspects leukemia cells are growing in an organ, like your spleen.
Sometimes a test that combines the CT scan with a PET (positron emission tomography) scan (PET/CT scan) is done. This is not often needed for patients with acute lymphocytic leukemia.
Magnetic resonance imaging (MRI) scan
MRI scans make detailed images of the body using radio waves and strong magnets instead of x-rays. They are very helpful in looking at the brain and spinal cord. This test might be done if a lumbar puncture finds leukemia cells in the cerebrospinal fluid (CSF), or if a person is having symptoms that could mean the acute lymphocytic leukemia has spread to the area around the brain.
Ultrasound
This is an easy test to have, and it uses no radiation. Ultrasound can be used to look at lymph nodes near the surface of the body or to look for enlarged organs inside the abdomen such as the kidneys, liver, and spleen. It can also be used to look at the testicles, if needed.
Acute lymphocytic leukemia subtypes and prognostic factors
For most types of cancer, determining the stage (extent) of the cancer is very important. The stage is based on the size of the tumor and how far the cancer has spread. This can be helpful in predicting a person’s outlook and deciding on treatment.
Acute lymphocytic leukemia (acute lymphoblastic leukemia), on the other hand, does not usually form tumors. It generally affects all of the bone marrow in the body and, in some cases, has already spread to other organs, such as the liver, spleen, and lymph nodes, by the time it is found. Therefore acute lymphoblastic leukemia is not staged like most other cancers. The outlook for a person with acute lymphoblastic leukemia depends on other information, such as the subtype of acute lymphoblastic leukemia (determined by lab tests), the patient’s age, and other lab test results.
Subtypes of acute lymphocytic leukemia (acute lymphoblastic leukemia)
Different systems have been used to classify acute lymphocytic leukemia into subtypes.
In the 1970s, a group of French, American, and British (FAB) leukemia experts divided acute lymphocytic leukemia into 3 subtypes (L1, L2, and L3), based on the way the leukemia cells looked under the microscope after routine staining. This system, known as the FAB classification, has largely been replaced, as newer lab tests now allow doctors to classify acute lymphocytic leukemia more accurately.
Doctors have found that cytogenetic tests, flow cytometry, and other lab tests provide more detailed information about the subtype of acute lymphocytic leukemia and the patient’s prognosis. These tests help divide acute lymphocytic leukemia into groups based on the gene and chromosome changes in the leukemia cells.
The World Health Organization (WHO) system, most recently updated in 2016, includes some of these factors to try to better classify acute lymphocytic leukemia. The WHO system divides acute lymphocytic leukemia into several groups:
B-cell acute lymphocytic leukemia
- B-cell acute lymphocytic leukemia with certain genetic abnormalities (gene or chromosome changes)
- B-cell acute lymphocytic leukemia with hypodiploidy (the leukemia cells have fewer than 44 chromosomes [normal cells have 46])
- B-cell acute lymphocytic leukemia with hyperdiploidy (the leukemia cells have more than 50 chromosomes)
- B-cell acute lymphocytic leukemia with a translocation between chromosomes 9 and 22 [t(9;22)] (the Philadelphia chromosome, which creates the BCR-ABL1 fusion gene)
- B-cell acute lymphocytic leukemia with a translocation between chromosome 11 and another chromosome
- B-cell acute lymphocytic leukemia with a translocation between chromosomes 12 and 21 [t(12;21)]
- B-cell acute lymphocytic leukemia with a translocation between chromosomes 1 and 19 [t(1;19]
- B-cell acute lymphocytic leukemia with a translocation between chromosomes 5 and 14 [t(5;14)]
- B-cell acute lymphocytic leukemia with amplification (too many copies) of a portion of chromosome 21 (iAMP21)*
- B-cell acute lymphocytic leukemia with translocations involving certain tyrosine kinases or cytokine receptors (also known as “BCR-ABL1–like acute lymphocytic leukemia”)*
- B-cell acute lymphocytic leukemia, not otherwise specified
T-cell acute lymphocytic leukemia
- Early T-cell precursor lymphoblastic leukemia. It’s not yet clear if there’s enough evidence that it’s a unique group (meaning it is still a “provisional entity”)
Mixed lineage acute leukemias
A small number of acute leukemias have both lymphocytic and myeloid features. Sometimes the leukemia cells have both myeloid and lymphocytic traits in the same cells. In other cases, a person may have some leukemia cells with myeloid features and others with lymphocytic features. These types of leukemias may be called mixed lineage leukemia, acute undifferentiated leukemia, or, or mixed phenotype acute leukemia.
Most studies suggest these leukemias tend to have a poorer outlook than standard subtypes of acute lymphocytic leukemia or acute myeloid leukemia. Not all doctors agree on the best way to treat them. Intensive treatment (such as a stem cell transplant) is often used when possible, as there is a high risk of recurrence after treatment.
Prognostic factors for acute lymphocytic leukemia
As leukemia treatment has improved over the years, research has focused on why some people have a better chance for cure than others. Different factors that affect a person’s prognosis (outlook) are called prognostic factors. They can help doctors decide if people with a certain type of leukemia should get more or less treatment.
Age
Among adults, younger patients tend to have a better prognosis than older patients. There is no set cutoff for this, but generally those younger than 50 do better than those in their 50s, while people in their 50s do better than those in their 60s or older.
Some of this might be because older patients are more likely to have unfavorable chromosome abnormalities (see below). Older patients are also more likely to have other medical conditions that can make it harder to treat them with more intense chemotherapy regimens.
Initial white blood cell count
People with a lower white blood cell count (less than 30,000 for B-cell acute lymphocytic leukemia and less than 100,000 for T-cell acute lymphocytic leukemia) when they are first diagnosed tend to have a better prognosis.
Gene or chromosome abnormalities
Whether the leukemia cells have certain changes in their genes or chromosomes can affect prognosis. For example, patients tend to have a poorer outcome if the leukemia cells have:
- The Philadelphia chromosome (a translocation between chromosomes 9 and 22), although this outlook has improved with modern targeted therapy drugs
- A translocation between chromosomes 4 and 11
- A translocation involving chromosome 14
- Amplification (too many copies) of part of chromosome 21
- Fewer than 44 chromosomes (hypodiploidy)
- 5 or more chromosome changes (complex karyotype)
On the other hand, people tend to have a better outlook if the leukemia cells have:
- A translocation between chromosomes 12 and 21
- More than 50 chromosomes (hyperdiploidy)
Response to chemotherapy
Patients who go into a complete remission (no visible leukemia in the bone marrow – see below) within 4 to 5 weeks of starting treatment tend to have a better prognosis than those for whom this takes longer. Patients who don’t achieve a complete remission at all have a poorer outlook. The presence of minimal residual disease (described below) after initial treatment also seems to affect prognosis, although this is still being studied.
Status of acute lymphocytic leukemia during and after treatment
How well leukemia responds to treatment affects the patient’s long-term chance for recovery.
Remission
A remission (complete remission) is usually defined as having no evidence of leukemia after treatment. This means the bone marrow contains fewer than 5% blast cells, the blood cell counts are within normal limits, and there are no signs or symptoms of the disease. A complete molecular remission means there is no evidence of leukemia cells in the bone marrow, even when using very sensitive lab tests, such as polymerase chain reaction (PCR). Even when leukemia is in remission, this does not always mean that it has been cured.
Minimal residual disease
Minimal residual disease is a term used after treatment when leukemia cells can’t be found in the bone marrow using standard lab tests (such as looking at cells under a microscope), but they can still be detected with more sensitive tests (such as flow cytometry or PCR).
Patients with minimal residual disease after treatment are more likely to have the leukemia relapse (come back after treatment) and overall have a poorer outlook than those who achieve a complete remission. Doctors are studying if these patients could benefit from further or more intensive treatment.
Active disease
Active disease means that either there is evidence that the leukemia is still present during treatment or that the disease has relapsed (come back) after treatment. For a patient to be in relapse, more than 5% of the bone marrow must be made up of blast cells.
Acute lymphocytic leukemia survival rate
Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain length of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they might help give you a better understanding about how likely it is that your treatment will be successful.
Statistics on the outlook for people with a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer. For example, a 5-year survival rate of 90% means that an estimated 90 out of 100 people who have that cancer are still alive 5 years after being diagnosed.
Relative survival rates are often a more accurate way to estimate the effect of cancer on survival. These rates compare people with adrenal cancer to people in the overall population. For example, if the 5-year relative survival rate for a specific type and stage of cancer is 90%, it means that people who have that cancer are, on average, about 90% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
But remember, the 5-year relative survival rates are estimates – your outlook can vary based on a number of factors specific to you.
Cancer survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with adrenal cancer may have a better outlook than these statistics show.
- These statistics are based on the stage of the cancer when it was first diagnosed. They do not apply to cancers that come back later or spread, for example.
- Besides the cancer stage, many other factors can affect a person’s outlook, such as age and overall health, and how well the cancer responds to treatment.
Your doctor can tell you how these numbers may apply to you, as he or she is familiar with your situation.
Generally for people with acute lymphocytic leukemia:
- around 70 out of 100 people (70%) will survive their leukemia for 5 years or more after they are diagnosed
This is for people of all ages. Younger people tend to do much better than older people:
- in those aged 14 or younger, more than 90 out of 100 (more than 90%) will survive their leukemia for 5 years or more after they are diagnosed
- in those aged between 15 and 24, almost 70 out of 100 (almost 70%) will survive their leukemia for 5 years or more after diagnosis
- in those aged between 25 and 64, almost 40 out of 100 (almost 40%) will survive their leukemia for 5 years or more after they are diagnosed
- in those aged 65 or older, almost 15 out of 100 (almost 15%) will survive their leukemia for 5 years or more after diagnosis
What affects survival
Your age affects outlook. Younger people have a better prognosis.
Outlook depends on the specific type of white blood cell the acute lymphoblastic leukemia affects. It is also affected by changes in your chromosomes or genes. These are called cytogenetic tests. Some specific genetic abnormalities in your leukemia cells may make the leukemia harder to treat successfully.
Survival is affected by how advanced the acute lymphocytic leukemia is at diagnosis. If you have a high number of white blood cells in the blood at diagnosis, the outlook is poorer. Your outlook is also worse if there are leukemia cells in your brain or spinal fluid when you are diagnosed.
Your outlook is also affected by how well the acute lymphocytic leukemia responds to treatment and how long it takes to get into remission. Remission means the acute lymphocytic leukemia is not active and doctors can’t find any sign of it.
If the acute lymphocytic leukemia comes back (relapses) after treatment, it is sometimes possible to have a second remission with more chemotherapy.
Acute lymphocytic leukemia prognosis
The prognosis for children with acute lymphocytic leukemia is usually good. Almost all children will achieve remission (a period of time where they’re free from symptoms), and 85% will be completely cured.
The outlook for adults with acute lymphocytic leukemia is less promising. Around 40% of people aged between 25 and 64 will live for five years or more after receiving their diagnosis. In those aged 65 or over, around 15% will live for five years or more after being diagnosed.
Acute lymphocytic leukemia treatment
In general, treatment for acute lymphocytic leukemia falls into separate phases:
- Induction therapy. The purpose of the first phase of treatment is to kill most of the leukemia cells in the blood and bone marrow and to restore normal blood cell production.
- Consolidation therapy. Also called post-remission therapy, this phase of treatment is aimed at destroying any remaining leukemia in the body, such as in the brain or spinal cord.
- Maintenance therapy. The third phase of treatment prevents leukemia cells from regrowing. The treatments used in this stage are often given at much lower doses over a long period of time, often years.
- Preventive treatment to the spinal cord. During each phase of therapy, people with acute lymphocytic leukemia may receive additional treatment to kill leukemia cells located in the central nervous system. In this type of treatment, chemotherapy drugs are often injected directly into the fluid that covers the spinal cord.
Depending on your situation, the phases of treatment for acute lymphocytic leukemia can span two to three years.
Treatments may include:
- Chemotherapy. Chemotherapy, which uses drugs to kill cancer cells, is typically used as an induction therapy for children and adults with acute lymphocytic leukemia. Chemotherapy drugs can also be used in the consolidation and maintenance phases.
- Targeted therapy. Targeted drugs attack specific abnormalities present in cancer cells that help them grow and thrive. A certain abnormality called the Philadelphia chromosome is found in some people with acute lymphocytic leukemia. For these people, targeted drugs may be used to attack cells that contain that abnormality. Targeted therapy may be used during or after chemotherapy.
- Radiation therapy. Radiation therapy uses high-powered beams, such as X-rays or protons, to kill cancer cells. If the cancer cells have spread to the central nervous system, your doctor may recommend radiation therapy.
- Bone marrow transplant. A bone marrow transplant, also known as a stem cell transplant, may be used as consolidation therapy in people at high risk of relapse or for treating relapse when it occurs. This procedure allows someone with leukemia to re-establish healthy bone marrow by replacing leukemic bone marrow with leukemia-free marrow from a healthy person. A bone marrow transplant begins with high doses of chemotherapy or radiation to destroy any leukemia-producing bone marrow. The marrow is then replaced by bone marrow from a compatible donor (allogeneic transplant).
- Clinical trials. Clinical trials are experiments to test new cancer treatments and new ways of using existing treatments. While clinical trials give you or your child a chance to try the latest cancer treatment, treatment benefits and risks may be uncertain. Discuss the benefits and risks of clinical trials with your doctor.
Induction therapy
The induction stage of treatment is carried out in hospital or a specialist center. This is because you’ll probably need regular blood transfusions as your blood won’t contain enough healthy blood cells. You’ll also be vulnerable to infection, so it’s important you’re in a sterile environment where your health can be carefully monitored and any infection that develops can be treated quickly. Antibiotics may also be prescribed to help prevent further infection.
Chemotherapy
You’ll have chemotherapy to kill the leukemia cells in your bone marrow. Although some medications may be given as pills, you’ll need more than 1 medication given as an injection. To make things easier and avoid repeated injections, they can all be given through a flexible tube (a central line) that goes into a vein in your chest.
Some chemotherapy medication may also be directly administered into your cerebrospinal fluid (CSF) to kill any leukemia cells that may have spread to your nervous system and brain.
The type of chemotherapy medication used is called methotrexate. It’s given as an injection into your spine, in a similar way to a lumbar puncture.
After you have had the injection, you’ll have to lie flat for a few hours with your head positioned slightly lower than your feet. You may have a headache or feel sick afterwards.
Methotrexate is also given directly into a vein (intravenously) in adults with acute lymphoblastic leukemia after induction therapy and before consolidation.
Other common side effects following chemotherapy include:
- nausea
- vomiting
- diarrhea
- loss of appetite
- mouth ulcers
- tiredness
- skin rashes
- infertility
- hair loss
The side effects should resolve once treatment has finished. Your hair will usually take between 3 and 6 months to grow back.
Steroid therapy
You may also be given corticosteroid injections or tablets to help improve the effectiveness of chemotherapy.
Imatinib
If you have a type of leukemia known as Philadelphia chromosome-positive acute lymphoblastic leukemia, you’ll also be given a medicine called imatinib.
Imatinib is what’s known as a targeted therapy, which works by blocking the signals in the cancerous cells that cause them to grow and reproduce. This kills the cancerous cells. Imatinib is taken orally (as a tablet). The side effects of imatinib are usually mild and should improve over time.
The side effects of imatinib include:
- nausea
- vomiting
- swelling in the face and lower legs
- muscle cramps
- rash
- diarrhea
Depending on how well you respond to treatment, the induction phase can last from 2 weeks to several months.
In some cases, you or your child may be able to leave hospital and receive treatment on an outpatient basis if your symptoms improve.
Consolidation therapy
Leukemia can return if just 1 cancerous cell remains in your body. The aim of consolidation treatment is to ensure that any remaining leukemia cells are killed.
Treatment involves receiving regular injections of chemotherapy medication. This is usually done on an outpatient basis, which means you won’t have to stay in hospital overnight. But you may need some short stays in hospital if your symptoms suddenly get worse or you develop an infection.
The consolidation phase of treatment lasts several months.
Maintenance therapy
The maintenance phase is designed to act as further insurance against the possibility of the leukemia returning. It involves taking regular doses of chemotherapy tablets while having regular check-ups to monitor the effectiveness of your treatment.
The maintenance phase can often last for 2 years.
Other treatments
As well as chemotherapy and imatinib, other treatments are used in some circumstances. These are described below.
Targeted therapies
If other treatments don’t work, your cancer comes back or you have a certain type of acute lymphoblastic leukemia, you may be offered another kind of targeted therapy. There are several types of targeted therapy. Your doctors and specialists will talk to you about these if they think they might work for you.
Radiotherapy
Radiotherapy is where high doses of controlled radiation are used to kill cancerous cells.
It’s usually used to treat acute lymphocytic leukemia in the following 2 situations:
- to treat advanced cases of acute lymphoblastic leukemia that have spread to the nervous system or brain
- to prepare the body for a bone marrow transplant
Side effects include:
- hair loss
- nausea
- fatigue
These side effects should pass after your course of radiotherapy has been completed. But your skin may be very sensitive to the effects of light for several months after treatment has finished. If this is the case, avoid sunbathing or exposure to sources of artificial light, such as sunbeds, for several months.
Many younger children treated with radiotherapy will go on to have restricted physical growth during puberty.
A small number of people develop cataracts several years after having radiotherapy. Cataracts are cloudy patches in the transparent structure at the front of the eye (the lens) that can make your vision blurred or misty. They can usually be successfully treated using cataract surgery.
Stem cell and bone marrow transplants
A stem cell and bone marrow transplant is a possible alternative treatment option if you or your child don’t respond to chemotherapy.
Transplantations are more successful if the donor has the same tissue type as you, so the ideal donor is usually a brother or sister. Before transplantation can take place, the person receiving the transplant will need to have aggressive high-dose chemotherapy and radiotherapy to destroy any cancerous cells in their body.
This can put a big strain on the body, so transplantations are usually only successful when they’re carried out in:
- children and young people
- older people in good health
- when there’s a suitable donor, such as a brother or sister
Recent research has shown it’s possible for people over the age of 40 to have a reduced intensity stem cell transplant.
This is where lower than normal doses of chemotherapy and radiotherapy are used before the transplant, which places less strain on the body.
Clinical trials
In the US, clinical trials are currently being carried out to find the best way of treating acute lymphocytic leukemia. These studies are using innovative new techniques to see how well they work in treating and possibly curing acute lymphocytic leukemia.
It’s important to be aware of new studies so you can choose which treatments to have. But there’s no guarantee the techniques being studied in the clinical trial will be more effective than current treatments.
If you would like to learn more about clinical trials that might be right for you, start by asking your doctor if your clinic or hospital conducts clinical trials, and ask about the pros and cons of enrolling in one of them. See Clinical Trials (https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials.html) to learn more.
Acute lymphocytic leukemia in older adults
Older adults, such as those older than 60, tend to experience more complications from acute lymphocytic leukemia treatments. And older adults generally have a worse prognosis than children who are treated for acute lymphocytic leukemia.
Discuss your options with your doctor. Based on your overall health and your goals and preferences, you may decide to undergo treatment for your acute lymphocytic leukemia.
Some people may choose to forgo treatment for the cancer, instead focusing on treatments that improve their symptoms and help them make the most of the time they have remaining.
Alternative medicine
No alternative treatments have been proved to cure acute lymphocytic leukemia. But some alternative therapies may help ease the side effects of cancer treatment and make you or your child more comfortable. Discuss your options with your doctor, as some alternative treatments could interfere with cancer treatments, such as chemotherapy.
Alternative treatments that may ease symptoms include:
- Acupuncture
- Aromatherapy
- Massage
- Meditation
- Relaxation exercises
Coping and support
Although treatment for acute lymphocytic leukemia is typically very successful, it can be a long road. Treatment often lasts two to three years, although the first three to six months are the most intense.
During maintenance phases, children can usually live a relatively normal life and go back to school. And adults may be able to continue working. To help you cope, try to:
- Learn enough about leukemia to feel comfortable making treatment decisions. Ask your doctor to write down as much information about your specific disease as possible. Then narrow your search for information accordingly.
- Write down questions for your doctor before each appointment, and look for information in your local library and on the internet. Good sources include the National Cancer Institute (https://www.cancer.gov), the American Cancer Society (https://www.cancer.org/) and the Leukemia & Lymphoma Society (https://www.lls.org/).
- Lean on your whole health care team. At major medical centers and pediatric cancer centers, your health care team may include psychologists, psychiatrists, recreation therapists, child-life workers, teachers, dietitians, chaplains and social workers. These professionals can help with a whole host of issues, including explaining procedures to children, finding financial assistance and arranging for housing during treatment. Don’t hesitate to rely on their expertise.
- Explore programs for children with cancer. Major medical centers and nonprofit groups offer numerous activities and services specifically for children with cancer and their families. Examples include summer camps, support groups for siblings and wish-granting programs. Ask your health care team about programs in your area.
- Help family and friends understand your situation. Set up a free, personalized webpage at the nonprofit website CaringBridge (https://www.caringbridge.org/). This allows you to tell the whole family about appointments, treatments, setbacks and reasons to celebrate — without the stress of calling everyone every time there’s something new to report.