Contents
- What is African sleeping sickness
- Human African sleeping sickness
- Who is at risk of trypanosoma sleeping sickness?
- What causes sleeping sickness
- African sleeping sickness life cycle
- Sleeping sickness prevention
- Sleeping sickness symptoms
- East African sleeping sickness signs and symptoms
- How is East African sleeping sickness spread?
- How soon after infection will I have symptoms of East African sleeping sickness?
- Is East African sleeping sickness a serious illness?
- Where can you become infected with East African sleeping sickness?
- What should I do if I think I may have African sleeping sickness?
- What is the treatment for East African sleeping sickness?
- Once infected, am I immune to East African sleeping sickness?
- Who is at risk for contracting East African sleeping sickness?
- Can I take a medication to prevent East African sleeping sickness?
- West African sleeping sickness signs and symptoms
- How soon after infection will I have symptoms of West African trypanosomiasis?
- How can I get West African trypanosomiasis?
- Who is at risk for contracting West African trypanosomiasis?
- Is West African trypanosomiasis a serious illness?
- Is treatment available for West African trypanosomiasis?
- Can I take medication to prevent West African trypanosomiasis?
- East African sleeping sickness signs and symptoms
- Sleeping sickness diagnosis
- African sleeping sickness treatment
What is African sleeping sickness
Sleeping sickness also called African sleeping sickness or African trypanosomiasis, is an infection caused by two types of parasites Trypanosoma brucei rhodesiense and Trypanosomoa brucei gambiense carried by the tsetse fly (genus Glossina). When an infected tsetse fly bites you, the infection spreads through your blood. Sleeping sickness results in swelling of the brain. Symptoms include fatigue, high fever, headaches, and muscle aches. If African sleeping sickness is not treated, it can cause death. Trypanosoma brucei rhodesiense causes the more acute and severe form of the sleeping sickness and can cause death in a matter of weeks or months. Trypanosomoa brucei gambiense has a chronic and protracted course and may last several years.
Risk factors for getting sleeping sickness include living in parts of Africa where the disease is found and being bitten by tsetse flies. The disease does not occur in the United States, but travelers who have visited or lived in Africa can be infected.
Trypanosomoa brucei gambiense is found in foci in large areas of West and Central Africa. The distribution of Trypanosoma brucei rhodesiense is much more limited, with the species found in East and Southeast Africa. The tsetse flies acquired their infection from human beings or from animals harboring human pathogenic parasites.
African sleeping sickness occurs in 3 stages. A trypanosomal chancre can develop on the site of inoculation. This is followed by a hemolymphatic stage with symptoms that include fever, lymphadenopathy, and pruritus. In the meningoencephalitic stage, invasion of the central nervous system can cause headaches, somnolence, abnormal behavior, and lead to loss of consciousness and coma. The course of infection is much more acute with Trypanosoma brucei rhodesiense than Trypanosomoa brucei gambiense.
Sleeping sickness key facts
- Sleeping sickness occurs in 36 sub-Saharan Africa countries where there are tsetse flies that transmit the disease.
- The people most exposed to the tsetse fly and therefore the disease live in rural areas and depend on agriculture, fishing, animal husbandry or hunting.
- Human African trypanosomiasis takes 2 forms, depending on the parasite involved 1:
- Trypanosoma brucei gambiense (West African sleeping sickness) accounts for more than 98% of reported cases of sleeping sickness and causes a chronic infection. Trypanosoma brucei gambiense is found in 24 countries in west and central Africa. A person can be infected for months or even years without major signs or symptoms of the disease. When more evident symptoms emerge, the patient is often already in an advanced disease stage where the central nervous system is affected. Humans are the important reservoir of West African sleeping sickness (Trypanosomoa brucei gambiense), although the parasite can sometimes be found in domestic animals (e.g., pigs, dogs, goats). Imported infection in the U.S. is extremely rare, and most cases have occurred in African nationals who have immigrated rather than in returning U.S. travelers. Over 95% of the cases of human infection are found in Democratic Republic of Congo, Angola, Sudan, Central African Republic, Chad, and northern Uganda. Epidemics of sleeping sickness have been a significant public health problem in the past, but the disease is reasonably well-controlled at present, with 7,000-10,000 cases reported annually in recent years.
- Trypanosoma brucei rhodesiense (East African sleeping sickness) is found in 13 countries in eastern and southern Africa. Nowadays, this form represents under 3% of reported cases and causes an acute infection. First signs and symptoms are observed a few months or weeks after infection. The disease develops rapidly and invades the central nervous system. Only Uganda presents both forms of the disease, but in separate zones. Animals are the primary reservoir of infection. Cattle have been implicated in the spread of East African sleeping sickness (Trypanosoma brucei rhodesiense) to new areas and in local outbreaks. A wild animal reservoir is thought to be responsible for sporadic transmission to hunters and visitors to game parks. Infection of international travelers is rare, but it occasionally occurs. In the U.S., one case per year, on average, is diagnosed. Most cases of sleeping sickness imported into the U.S. have been in travelers who were on safari in East Africa. Over 95% of the cases of human infection occur in Tanzania, Uganda, Malawi, and Zambia.
- Sustained control efforts have reduced the number of new cases. In 2009 the number reported dropped below 10,000 for the first time in 50 years, and in 2015 there were 2804 cases recorded.
- Diagnosis and treatment of African sleeping sickness is complex and requires specifically skilled staff.
Tsetse flies are found just in sub-Saharan Africa though only certain species transmit the disease. For reasons that are so far unexplained, in many regions where tsetse flies are found, sleeping sickness is not. Rural populations living in regions where transmission occurs and which depend on agriculture, fishing, animal husbandry or hunting are the most exposed to the tsetse fly and therefore to the disease. Sleeping sickness develops in areas ranging from a single village to an entire region. Within an infected area, the intensity of the disease can vary from one village to the next.
Figure 1. Tsetse fly
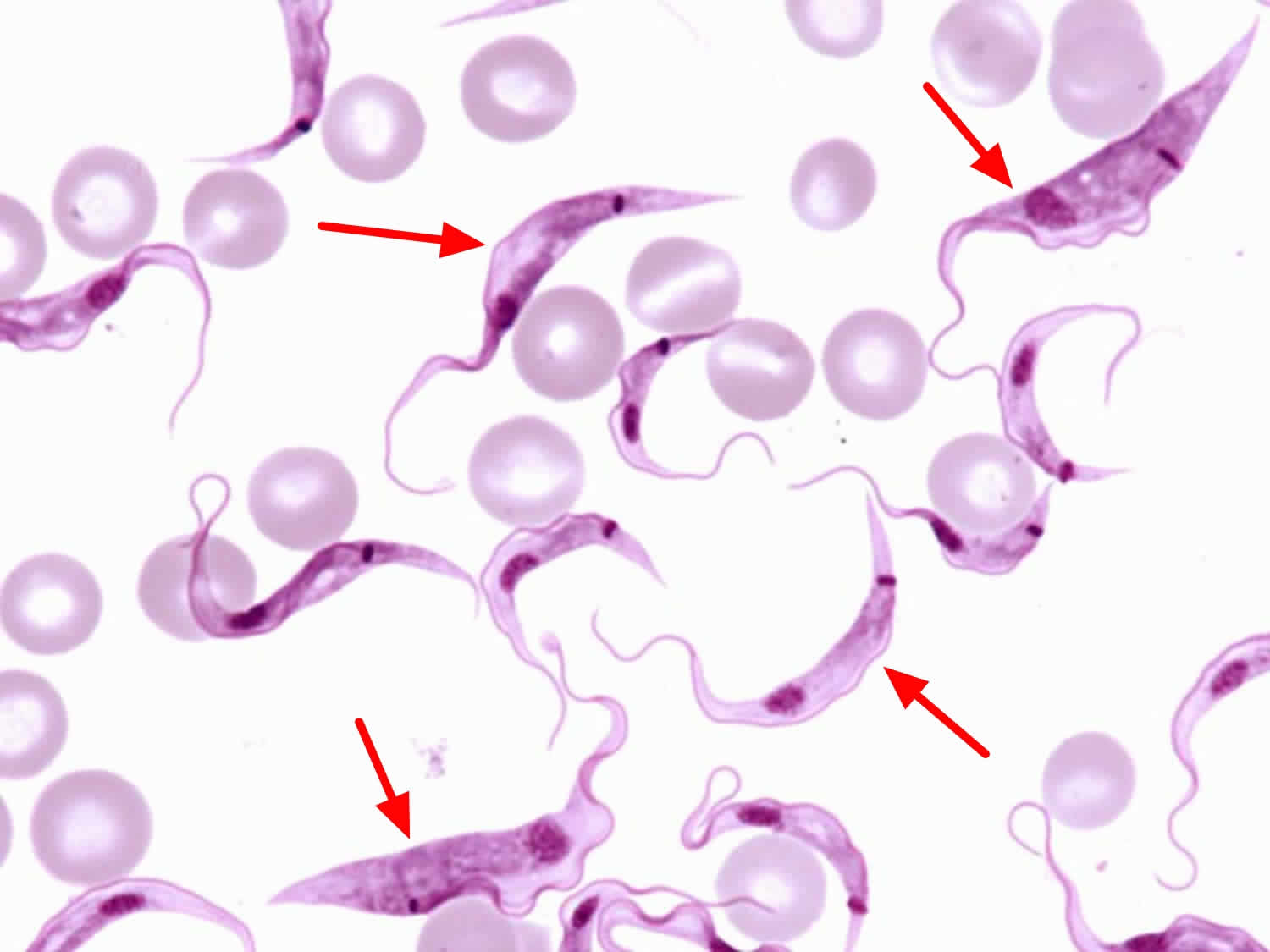
Figure 2. African sleeping sickness parasites (Trypanosoma brucei subspecies in a thin blood smear stained with Giemsa.)
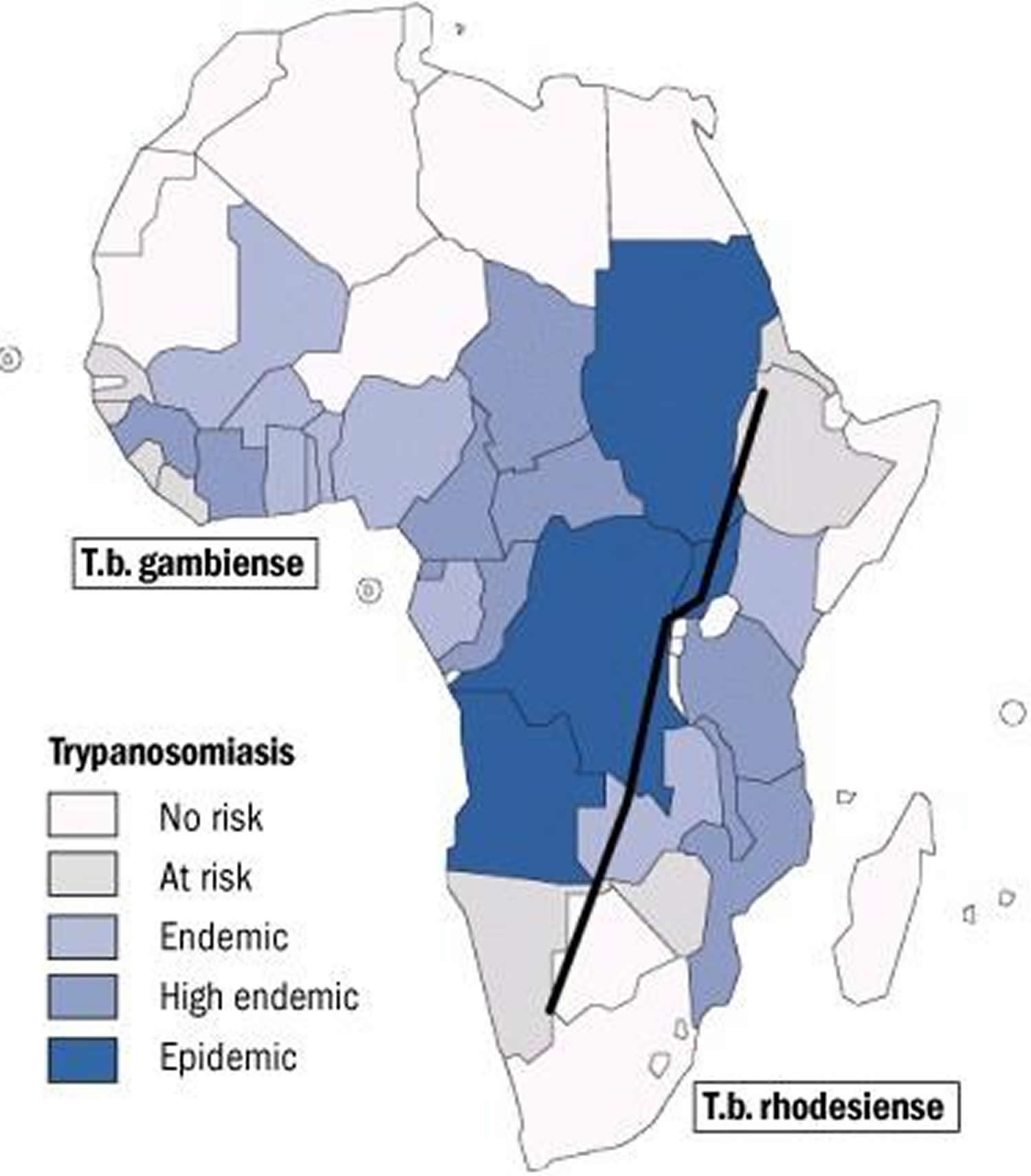
Figure 3. African sleeping sickness geographic distribution
Current sleeping sickness distribution
The disease incidence differs from one country to another as well as in different parts of a single country.
- In the last 10 years, over 70% of reported cases occurred in the Democratic Republic of the Congo (DRC).
- The Democratic Republic of the Congo (DRC) is the only country that currently reports more than 1000 new cases annually and accounts for 84% of the cases reported in 2015.
- Central African Republic is the only country that declared between 100 and 200 new cases in 2015.
- Countries such as Angola, Burkina Faso, Cameroon, Chad, Congo, Côte d’Ivoire, Equatorial Guinea, Gabon, Gjana, Guinea, Malawi, Nigeria, South Sudan, Uganda, United Republic of Tanzania, Zambia and Zimbabwe are reporting fewer than 100 new cases per year.
Countries like Benin, Botswana, Burundi, Ethiopia, Gambia, Guinea Bissau, Kenya, Liberia, Mali, Mozambique, Namibia, Niger, Rwanda, Senegal, Sierra Leone, Swaziland and Togo have not reported any new cases for over a decade. Transmission of the disease seems to have stopped in some of these countries but there are still some areas where it is difficult to assess the exact situation because the unstable social circumstances and/or difficult accessibility hinder surveillance and diagnostic activities.
Human African sleeping sickness
Human African sleeping sickness takes 2 forms, depending on the parasite involved 1:
- Trypanosoma brucei gambiense is found in 24 countries in west and central Africa. This form currently accounts for 97% of reported cases of sleeping sickness and causes a chronic infection. A person can be infected for months or even years without major signs or symptoms of the disease. When more evident symptoms emerge, the patient is often already in an advanced disease stage where the central nervous system is affected.
- Trypanosoma brucei rhodesiense is found in 13 countries in eastern and southern Africa. Nowadays, this form represents under 3% of reported cases and causes an acute infection. First signs and symptoms are observed a few months or weeks after infection. The disease develops rapidly and invades the central nervous system. Only Uganda presents both forms of the disease, but in separate zones.
Another form of trypanosomiasis occurs mainly in Latin America. It is known as American trypanosomiasis or Chagas disease. The causal organism belongs to a different Trypanosoma subgenus and is transmitted by a different vector.
What should I do if I think I have African sleeping sickness?
If you suspect that you may have West African trypanosomiasis, see your health care provider who will order several tests to look for the parasite. Common tests include examination of blood samples and a spinal tap. Your physician may also take a sample of fluid from swollen lymph nodes.
Who is at risk of trypanosoma sleeping sickness?
Travelers who go to sub-Saharan Africa are at risk (see map in Figure 3). Travelers who plan to spend a lot of time outdoors or who go to game parks are at increased risk.
How can I prevent African sleeping sickness and other insect bites?
There is no vaccine or medicine that prevents African trypanosomiasis. Travelers can protect themselves by preventing tsetse fly bites.
- Wear protective clothing, including long-sleeved shirts and pants. The tsetse fly can bite through thin fabrics, so clothing should be made of medium-weight material.
- Wear neutral-colored clothing. The tsetse fly is attracted to bright colors and very dark colors.
- Inspect vehicles for tsetse flies before entering. The tsetse fly is attracted to moving vehicles.
- Avoid bushes. The tsetse fly is less active during the hottest period of the day. It rests in bushes but will bite if disturbed.
- Use insect repellant. Though insect repellants have not proven effective in preventing tsetse fly bites, they are effective in preventing other insects from biting and causing illness.
What causes sleeping sickness
The bite of an infected tsetse fly (Glossina spp.) carrying the parasites Trypanosoma brucei rhodesiense and Trypanosomoa brucei gambiense. These two subspecies are morphologically indistinguishable, Trypanosomoa brucei gambiense causes West African sleeping sickness and Trypanosoma brucei rhodesiense causes East African sleeping sickness. A third member of the complex, Trypanosoma brucei brucei, under normal conditions does not infect humans. Bloodborne and congenital transmission are rare.
African sleeping sickness is mostly transmitted through the bite of an infected tsetse fly but there are other ways in which people are infected:
- Mother-to-child infection: the trypanosome can cross the placenta and infect the fetus.
- Mechanical transmission through other blood-sucking insects is possible, however, it is difficult to assess its epidemiological impact.
- Accidental infections have occurred in laboratories due to pricks with contaminated needles.
- Transmission of the parasite through sexual contact has been documented.
Both forms of sleeping sickness are transmitted by the bite of the tsetse fly (Glossina species). Tsetse flies inhabit rural areas, living in the woodlands and thickets that dot the East African savannah. In central and West Africa, they live in the forests and vegetation along streams. Tsetse flies bite during daylight hours. Both male and female flies can transmit the infection, but even in areas where the disease is endemic, only a very small percentage of flies are infected. Although the vast majority of infections are transmitted by the tsetse fly, other modes of transmission are possible. Occasionally, a pregnant woman can pass the infection to her unborn baby. In theory, the infection can also be transmitted by blood transfusion or sexual contact, but such cases have rarely been documented.
Animals can host the human pathogen parasites, especially Trypanosoma brucei rhodesiense, of which domestic and wild animals are an important reservoir. Animals can also be infected with Trypanosomoa brucei gambiense and act as a reservoir to a lesser extent. However the precise epidemiological role of the animal reservoir in the gambiense form of the disease is not yet well known.
African sleeping sickness life cycle
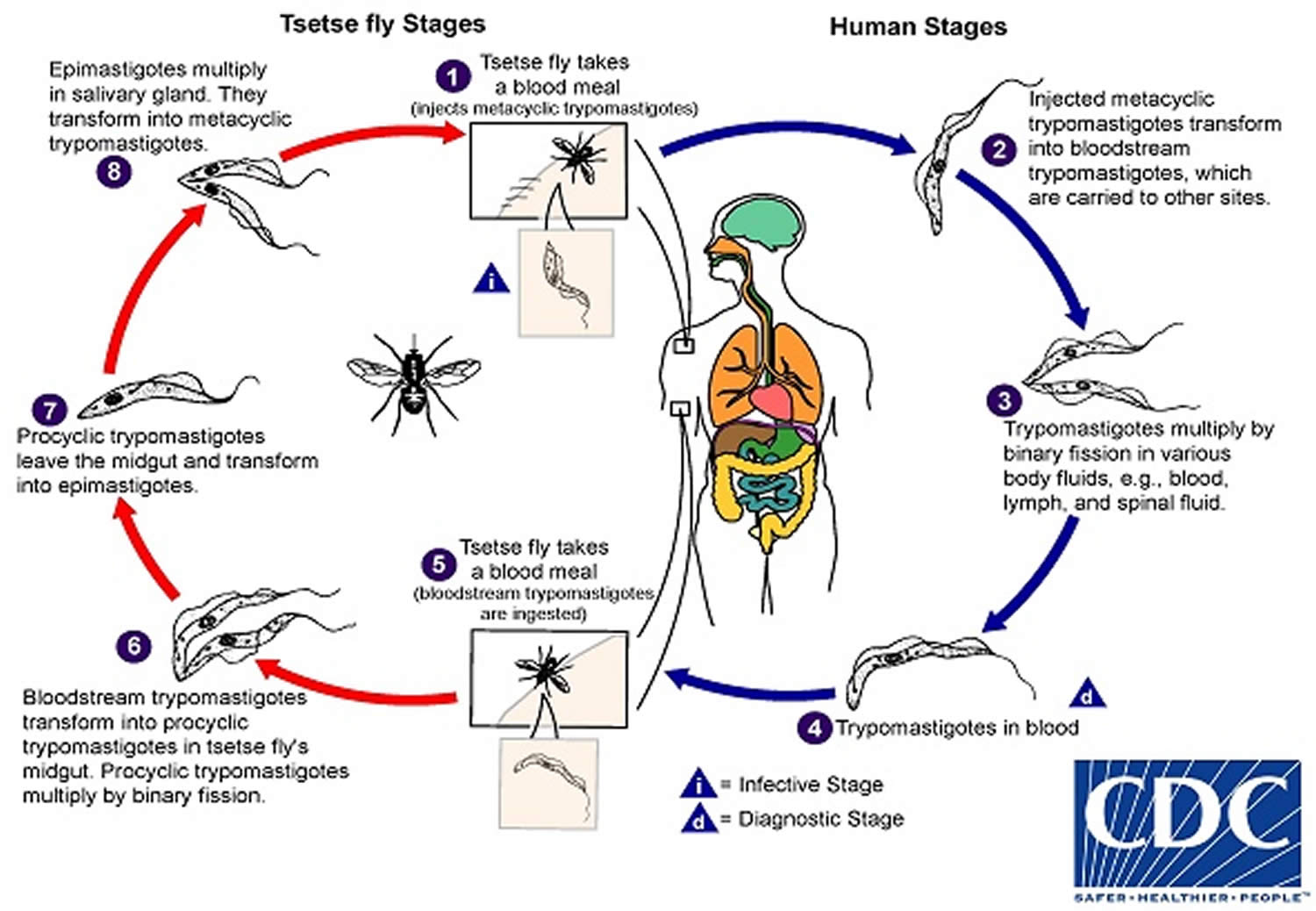
During a blood meal on the mammalian host, an infected tsetse fly (genus Glossina) injects metacyclic trypomastigotes into skin tissue. The parasites enter the lymphatic system and pass into the bloodstream (1). Inside the host, they transform into bloodstream trypomastigotes (2), are carried to other sites throughout the body, reach other body fluids (e.g., lymph, spinal fluid), and continue the replication by binary fission (3). The entire life cycle of African trypanosomes is represented by extracellular stages. The tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host (number 4 and 5). In the fly’s midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission (6), leave the midgut, and transform into epimastigotes (7) . The epimastigotes reach the fly’s salivary glands and continue multiplication by binary fission (8). The cycle in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma brucei gambiense, but this species can also be found in animals. Wild game animals are the main reservoir of Trypanosoma brucei rhodesiense.
Figure 4. African sleeping sickness life cycle
Sleeping sickness prevention
There is no vaccine or drug for prophylaxis against African trypanosomiasis. Preventive measures are aimed at minimizing contact with tsetse flies. Local residents are usually aware of the areas that are heavily infested and they can provide advice about places to avoid. Other helpful measures include:
- Wear long-sleeved shirts and pants of medium-weight material in neutral colors that blend with the background environment. Tsetse flies are attracted to bright or dark colors, and they can bite through lightweight clothing.
- Inspect vehicles before entering. The flies are attracted to the motion and dust from moving vehicles.
- Avoid bushes. The tsetse fly is less active during the hottest part of the day but will bite if disturbed.
- Use insect repellent. Permethrin-impregnated clothing and insect repellent have not been proved to be particularly effective against tsetse flies, but they will prevent other insect bites that can cause illness.
- Higher percentages of active ingredient provide longer protection. Use products with the following active ingredients:
- DEET (Products containing DEET include Off!, Cutter, Sawyer, and Ultrathon)
- Picaridin (also known as KBR 3023, Bayrepel, and icaridin products containing picaridin include Cutter Advanced, Skin So Soft Bug Guard Plus, and
- Autan [outside the US])
- Oil of lemon eucalyptus (OLE) or PMD (Products containing OLE include Repel and Off! Botanicals)
- IR3535 (Products containing IR3535 include Skin So Soft Bug Guard Plus Expedition and SkinSmart)
- Always follow product directions and reapply as directed:
- If you are also using sunscreen, apply sunscreen first and insect repellent second.
- Follow package directions when applying repellent on children. Avoid applying repellent to their hands, eyes, and mouth.
- Use permethrin-treated clothing and gear (such as boots, pants, socks, and tents). You can buy pre-treated clothing and gear or treat them yourself:
- Treated clothing remains protective after multiple washings. See the product information to find out how long the protection will last.
- If treating items yourself, follow the product instructions carefully.
- Do not use permethrin directly on skin.
- Stay and sleep in screened or air conditioned rooms.
Control of African sleeping sickness rests on two strategies:
- reducing the disease reservoir and
- controlling the tsetse fly vector.
Because humans are the significant disease reservoir for Trypanosomoa brucei gambiense, the main control strategy for this subspecies is active case-finding through population screening, followed by treatment of the infected persons that are identified. Tsetse fly traps are sometimes used as an adjunct. Reducing the reservoir of infection is more difficult for Trypanosoma brucei rhodesiense, since there are a variety of animal hosts. Vector control is the primary strategy in use. This is usually done with traps or screens, in combination with insecticides and odors that attract the flies.
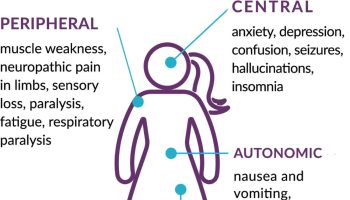
Sleeping sickness symptoms
The clinical course of human African sleeping sickness has two stages.
- In the first stage, the parasite is found in the peripheral circulation and multiply in subcutaneous tissues, blood and lymph, but it has not yet invaded the central nervous system. This is also called haemo-lymphatic stage, which entails bouts of fever, headaches, joint pains and itching.
- In the second stage the parasites cross the blood-brain barrier to infect the central nervous system. This is known as the neurological or meningo-encephalic stage. In general this is when more obvious signs and symptoms of the disease appear: changes of behavior, confusion, sensory disturbances and poor coordination. Disturbance of the sleep cycle, which gives the disease its name, is an important feature. Without treatment, sleeping sickness is considered fatal although cases of healthy carriers have been reported.
The subspecies that cause African trypanosomiasis have different rates of disease progression, and the clinical features depend on which form of the parasite (Trypanosoma brucei rhodesiense or Trypanosomoa brucei gambiense) is causing the infection. However, infection with either form will eventually lead to coma and death if not treated.
East African sleeping sickness signs and symptoms
Trypanosoma brucei rhodesiense infection (East African sleeping sickness) progresses rapidly. A bite by the tsetse fly is often painful and in some patients, a large red sore (a chancre) can develop at the site of the tsetse bite. Most patients develop fever, severe headaches, irritability, muscle and joint aches, extreme fatigue and swollen lymph nodes within 1-2 weeks of the infective bite. Some people develop a skin rash. After a few weeks of infection, the parasite invades the central nervous system and eventually causes mental deterioration, progressive confusion, personality changes and other neurologic problems. If left untreated, infection becomes worse and death will occur within months.
How is East African sleeping sickness spread?
A person will get East African trypanosomiasis if he or she is bitten by a tsetse fly infected with the Trypanosoma brucei rhodesiense parasite. The proportion of tsetse flies that are infected with this parasite is low. The tsetse fly is found only in rural Africa.
How soon after infection will I have symptoms of East African sleeping sickness?
Symptoms usually within 1 to 3 weeks after an infective tsetse fly bite.
Is East African sleeping sickness a serious illness?
Yes. If a person fails to receive medical treatment for East African trypanosomiasis, death will occur, usually within months.
Where can you become infected with East African sleeping sickness?
East African trypanosomiasis is found in parts of Eastern and Southeastern Africa. More than 95% of cases are reported from Uganda, Tanzania, Malawi, and Zambia.
What should I do if I think I may have African sleeping sickness?
If you suspect that you may have East African trypanosomiasis, immediately consult with your health care provider, who will order several tests to look for the parasite. A skin biopsy may be done if you have a chancre. Blood tests will be done and a spinal tap may also be performed.
What is the treatment for East African sleeping sickness?
Medical treatment of East African trypanosomiasis should begin as soon as possible and is based on the infected person’s laboratory results. Medication for the treatment of East African trypanosomiasis is available through CDC. Hospitalization for treatment is usually necessary. Periodic follow-up exams that include a spinal tap are required for 2 years.
Once infected, am I immune to East African sleeping sickness?
Even if you had the disease once, you can get re-infected.
Who is at risk for contracting East African sleeping sickness?
Tsetse flies are found in woodland and savannah areas and they bite during daylight hours. Travelers to urban areas are not at risk. The persons most likely to be exposed to the infection are tourists, hunters, and others working in or visiting game parks. Villagers with infected cattle herds are also at risk.
Can I take a medication to prevent East African sleeping sickness?
There is neither a vaccine nor recommended drug available to prevent East African sleeping sickness.
West African sleeping sickness signs and symptoms
Trypanosomoa brucei gambiense infection (West African sleeping sickness) progresses more slowly.
At first, there may be only mild symptoms. Occasionally, within 1 to 3 weeks, the infective tsetse fly bite develops into a red sore, also called a chancre. Several weeks to months later, other symptoms of sleeping sickness occur. These include intermittent fevers, rash, swelling of the face and hands, headaches, fatigue, aching muscles and joints, itching skin, and swollen lymph nodes. Weight loss occurs as the illness progresses. Usually, after 1-2 years, there is evidence of central nervous system involvement, with personality changes, daytime sleepiness with nighttime sleep disturbance, and progressive confusion. Other neurologic signs, such as partial paralysis or problems with balance or walking may occur, as well as hormonal imbalances. These symptoms become worse as the illness progresses. The course of untreated infection rarely lasts longer than 6-7 years and more often kills in about 3 years.
How soon after infection will I have symptoms of West African trypanosomiasis?
Symptoms may be minimal or intermittent during the first months of infection. They are usually apparent within a few months to a year after getting an infected tsetse fly bite.
How can I get West African trypanosomiasis?
A person gets West African trypanosomiasis through the bite of an infected tsetse fly. Occasionally a pregnant woman may pass the infection to her baby. In theory, the infection can be transmitted through a blood transfusion, but such cases rarely have been documented.
Who is at risk for contracting West African trypanosomiasis?
The tsetse flies that transmit West African trypanosomiasis are found only in rural areas. Travelers to urban areas are not at risk. The flies bite during daylight hours. They inhabit forests and areas of thick vegetation along rivers and waterholes. Even in areas where the disease is present, most flies are not infected with this parasite, so the risk of infection increases with the number of times a person is bitten by the tsetse fly. Therefore, tourists are not at great risk for contracting West African trypanosomiasis unless they are traveling and spending long periods of time in rural areas of central Africa where the disease is present.
Is West African trypanosomiasis a serious illness?
Yes. West African trypanosomiasis is eventually fatal if it is not treated.
Is treatment available for West African trypanosomiasis?
Medication for the treatment of West African trypanosomiasis is available. Treatment of West African trypanosomiasis should begin as soon as possible and is based on the infected person’s laboratory results. Hospitalization for treatment is usually necessary. Periodic follow-up exams that include a spinal tap are required for 2 years.
Can I take medication to prevent West African trypanosomiasis?
There is neither a vaccine nor recommended drug available to prevent West African trypanosomiasis.
Sleeping sickness diagnosis
The diagnosis of African Trypanosomiasis is made through laboratory methods, because the clinical features of infection are not sufficiently specific. The definitive diagnosis rests on finding the parasite in body fluid or tissue by microscopy. The parasite load in Trypanosoma brucei rhodesiense infection is substantially higher than the level in Trypanosomoa brucei gambiense infection.
Trypanosoma brucei rhodesiense parasites can easily be found in blood. They can also be found in lymph node fluid or in fluid or biopsy of a chancre. Serologic testing is not widely available and is not used in the diagnosis, since microscopic detection of the parasite is straightforward.
The classic method for diagnosing Trypanosomoa brucei gambiense infection is by microscopic examination of lymph node aspirate, usually from a posterior cervical node. It is often difficult to detect Trypanosomoa brucei gambiense in blood. Concentration techniques and serial examinations are frequently needed. Serologic testing is available outside the U.S. for Trypanosomoa brucei gambiense; however, it normally is used for screening purposes only and the definitive diagnosis rests on microscopic observation of the parasite.
All patients diagnosed with African trypanosomiasis must have their cerebrospinal fluid (CSF) examined to determine whether there is involvement of the central nervous system, since the choice of treatment drug(s) will depend on the disease stage. The World Health Organization criteria for central nervous system involvement include increased protein in cerebrospinal fluid and a white cell count of more than 5. Trypanosomes can often be observed in cerebrospinal fluid (CSF) in persons with second stage infection.
The level of parasitemia is relatively high, particularly in the first stage of disease, and trypanosomes can be found in blood. In centrifuged blood, the parasite sediments just above the white cells, and examination of buffy coat will increase sensitivity. Slides stained with Giemsa can be used, but it is easiest to find the parasite by microscopic examination of fresh wet preparations, because the trypanosomes are motile and attract the eye. Delay between sampling and microscopy should be minimized, because trypanosomes will lose motility within a few hours. Parasites can also be found in fluid expressed in trypanosomal chancres and in lymph node aspirates. Serologic testing is not used for the diagnosis of Trypanosoma brucei rhodesiense infection.
Detecting trypanosomes in Trypanosomoa brucei gambiense infection is more difficult. The card agglutination test for trypanosomiasis/Trypanosomoa brucei gambiense is a serologic screening test used for mass population screening in endemic areas of Africa. It is not available in the U.S., however, the Centers for Disease Control and Prevention (CDC) can provide information for testing in Europe. The test is not specific enough for confirmation of infection, but it is helpful in identifying suspect cases. For parasitologic confirmation, a posterior cervical lymph node (if present) is punctured and the fluid examined. The yield in lymph node examination varies from about 40% to 80%. Trypanosomes can also be found in blood, however, the yield is low, and concentration techniques (e.g. buffy coat examination, miniature anion-exchange centrifugation technique) are helpful. Serial examinations on consecutive days may be needed.
Treatment decisions are based on the stage of the disease. Every patient diagnosed with African trypanosomiasis must undergo a lumbar puncture for the examination of cerebrospinal fluid (CSF). The most widely used criteria for defining second stage disease are the observation of trypanosomes in cerebrospinal fluid (CSF) or a white cell count of 6 or higher. Other indications of second stage disease include elevated protein and an increase in nonspecific IgM in cerebrospinal fluid (CSF).
Laboratory Diagnosis
The diagnosis rests upon demonstrating trypanosomes by microscopic examination of chancre fluid, lymph node aspirates, blood, bone marrow, or, in the late stages of infection, cerebrospinal fluid. A wet preparation should be examined for the motile trypanosomes, and in addition a smear should be fixed, stained with Giemsa (or Field), and examined. Concentration techniques can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the buffy coat; mini anion-exchange/centrifugation; and the Quantitative Buffy Coat technique. For other samples such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment. Isolation of the parasite by inoculation of rats or mice is a sensitive method, but its use is limited to Trypanosoma brucei rhodesiense. Antibody detection has sensitivity and specificity that are too variable for clinical decisions. In addition, in infections with Trypanosoma brucei rhodesiense, seroconversion occurs after the onset of clinical symptoms and thus is of limited use.
The Centers for Disease Control and Prevention (CDC) currently does not offer any serologic or molecular tests for African trypanosomiasis.
African sleeping sickness treatment
Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis. Choice of therapy depends on the infecting subspecies of the parasite and on the disease stage. The drugs used in the first stage are safer and easier to administer than those for second stage. Also, the earlier the disease is identified, the better the prospect of a cure. The assessment of treatment outcome requires follow up of the patient up to 24 months and entails laboratory exams of body fluids including cerebrospinal fluid (CSF) obtained by lumbar puncture, as parasites may remain viable for long periods and reproduce the disease months after treatment.
Treatment success in the second stage depends on drugs that cross the blood-brain barrier to reach the parasite. Such drugs are toxic and complicated to administer.
In total 5 different drugs are used for the treatment of sleeping sickness.
Drugs used in first stage treatment:
- Pentamidine: discovered in 1940, used for the treatment of the first stage of Trypanosomoa brucei gambiense sleeping sickness. Despite non-negligible undesirable effects, it is in general well tolerated by patients.
- Suramin: discovered in 1920, used for the treatment of the first stage of Trypanosoma brucei rhodesiense. It provokes certain undesirable effects, including urinary tract and allergic reactions.
Drugs used in second stage treatment:
- Melarsoprol: discovered in 1949, it is used for the treatment of both gambiense and rhodesiense infections. It is derived from arsenic and has many undesirable side effects, the most dramatic of which is reactive encephalopathy (encephalopathic syndrome) which can be fatal (3% to 10%). An increase in resistance to the drug has been observed in several foci, particularly in central Africa. It is currently recommended as first-line treatment for the rhodesiense form, and as second-line for the gambiense form.
- Eflornithine: this molecule, less toxic than melarsoprol, was registered in 1990. It is only effective against Trypanosomoa brucei gambiense. The regimen is complex and difficult to apply.
- Nifurtimox: A combination treatment of nifurtimox and eflornithine was introduced in 2009. It simplifies the use of eflornithine by reducing the duration of treatment and the number of IV perfusions, but unfortunately it has not been studied for Trypanosoma brucei rhodesiense. Nifurtimox is registered for the treatment of American trypanosomiasis but not for human African trypanosomiasis. Nevertheless, after safety and efficacy data provided by clinical trials, its use in combination with eflornithine has been included in the “WHO List of Essential Medicines” and is currently recommended as first-line treatment for the gambiense form. Both drugs are provided free of charge by WHO to endemic countries with a kit containing all the material needed for its administration.
The first line drugs for both first and second stage disease are highly effective. Pentamidine, given by intravenous infusion over 2 hours or by intramuscular injection, is used to treat first stage Trypanosomoa brucei gambiense infection. It is generally well tolerated, but adverse reactions of hypoglycemia, injection site pain, diarrhea, nausea and vomiting occur. Suramin is used to treat first stage Trypanosoma brucei rhodesiense. Suramin is also effective against Trypanosomoa brucei gambiense, but it is not often used because severe reactions occur in persons who are co-infected with Onchocerca volvulus. Adverse reactions to suramin are frequent, but usually mild and reversible. These include drug rash, nephrotoxicity, and peripheral neuropathy. In rare instances, suramin administration results in a hypersensitivity reaction, and, for this reason, a small test dose is usually given prior to the full first dose.
Second stage Trypanosomoa brucei gambiense is treated with eflornithine, which is given in 4 intravenous infusions daily for 14 days. Adverse effects of eflornithine include bone marrow suppression, gastrointestinal symptoms, and seizures. Eflornithine is highly effective, but the difficulty in administering 4 infusions daily in rural African facilities has led to the use of eflornithine (dosed less frequently) in combination with nifurtimox. The efficacy of the combination regimen appears to be at least as high as eflornithine monotherapy. Eflornithine is not effective against Trypanosoma brucei rhodesiense and it is not recommended for treating the East African form of the disease. Melarsoprol, an organoarsenic compound, is the only drug available for treating second stage Trypanosoma brucei rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. An encephalopathic reaction occurs in 5-10% of patients with a case-fatality rate of approximately 50% when it occurs. Prednisolone is often given to patients who are being treated with melarsoprol to reduce the risk of encephalopathy. Other adverse reactions observed with melarsoprol include skin reactions, gastrointestinal upset, and peripheral neuropathy. Intravenous injections of melarsoprol are painful and can cause phlebitis. The drug is administered by use of lengthy and complicated dosing schedules, however, an abbreviated 10-day regimen appears promising.
Pentamidine is available for human use in the United States.
Eflornithine is available for human use in the United States through the Centers for Disease Control and Prevention (CDC).
There is no test of cure for African trypanosomiasis. Patients should be followed with a lumbar puncture every 6 months (or sooner, if symptoms return) for 2 years after treatment to detect a relapse should it occur.
For treatment advice and to obtain suramin, melarsoprol, or eflornithine, physicians should contact the Centers for Disease Control and Prevention (CDC) Division of Parasitic Diseases and Malaria (telephone, 404-718-4745; email, [email protected]).
Table 1. African sleeping sickness treatment
| Species | Drug of choice | Adult Dosage | Pediatric Dosage |
|---|---|---|---|
| Trypanosoma brucei rhodesiense, hemolymphatic stage | Suramin | 1 gm IV on days 1, 3, 7 ,14, and 21 | 20 mg/kg IV on days 1, 3, 7, 14, and 21 |
| Trypanosoma brucei rhodesiense, CNS involvement | Melarsoprol | 2-3.6 mg/kg/day IV x 3 days. After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. | 2-3.6 mg/kg/day IV x 3 days. After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. |
| Trypanosomoa brucei gambiense, Hemolymphatic stage | Pentamidine | 4 mg/kg/day IM or IV x 7-10 days | 4 mg/kg/day IM or IV x 7-10 days |
| Trypanosomoa brucei gambiense, CNS involvement | Eflornithine | 400 mg/kg/day in 4 doses x 14 days | 400 mg/kg/day in 4 doses x 14 days |
Footnote:
- Pentamidine is also effective against Trypanosoma brucei rhodesiense in the hemolymphatic stage, but suramin may have somewhat higher efficacy.
- Suramin is also effective against Trypanosomoa brucei gambiense in the hemolymphatic stage.
- Suramin adult dosage: A test dose of 100 mg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- Suramin pediatric dosage: A test dose of 2 mg/kg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- Corticosteroids have been used to prevent melarsoprol encephalopathy. The dose of melarsoprol is progressively increased during the first series.
- Eflornithine (400 mg/kg/d IV in 2 doses x 7 days) given in combination with oral nifurtimox (15 mg/kg/d x 10 days) is also highly effective against Trypanosomoa brucei gambiense with CNS involvement 3. Nifurtimox is not FDA-approved for this indication.
Eflornithine
- Treatment in Pregnancy: Data on the use of eflornithine in pregnant women are limited, and risk to the embryo-fetus is unknown. Eflornithine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Treatment During Breastfeeding: It is not known whether eflornithine is excreted in breast milk. Eflornithine should be used with caution in breast-feeding women.
- Treatment in Pediatric Patients: The safety of eflornithine in children has not been established. Eflornithine is not approved by the Food and Drug Administration (FDA) for use in pediatric patients. Eflornithine is listed for the treatment of 1st stage African trypanosomiasis in Trypanosoma brucei gambiense infection on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
Pentamidine
- Treatment in Pregnancy: Pentamidine is in pregnancy category C. Data on the use of pentamidine in pregnant women are limited, and risk to the embryo-fetus is unknown. Pentamidine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Treatment During Breastfeeding: It is not known whether pentamidine is excreted in breast milk. The World Health Organization (WHO) classifies pentamidine as compatible with breast-feeding, although data on the use of pentamidine during lactation are limited. Pentamidine should be used during lactation only if the potential benefit of therapy to the mother justifies the potential risk to the infant.
- Treatment in Pediatric Patients: Intravenous and intramuscular pentamidine have a similar safety profile in children age 4 months and older as in adults. Pentamidine is listed as a medicine for the treatment of 1st stage African trypanosomiasis infection (Trypanosoma brucei gambiense) on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
- Trypanosomiasis, human African (sleeping sickness). http://www.who.int/en/news-room/fact-sheets/detail/trypanosomiasis-human-african-sleeping-sickness[↩][↩]
- WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases – African trypanosomiasis. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1tryps/en/[↩]
- Priotto G et al. Lancet 2009:374; 56-64[↩]
- Sleeping Sickness Resources for Health Professionals. https://www.cdc.gov/parasites/sleepingsickness/health_professionals/index.html[↩]