Contents
What is antiemetic
Antiemetic is a drug to relieve nausea and vomiting. Antiemetics are used for the symptomatic management of nausea and vomiting that can be caused by a variety of medical conditions and situations, including acute gastroenteritis, pregnancy, surgery, anesthesia, opioids and chemotherapy.
Antiemetics can be categorized based upon their mechanism of action, their receptor activity or their chemical structure. Antihistamines have mild antiemetic activity and are commonly used for motion sickness and the transient nausea that might accompany a viral illness or gastrointestinal infection or disorder. Antihistamines that are commonly used for nausea include cyclizine, chlorcyclizine, diphenhydramine, dimenhydrinate, doxylamine, meclizine, hydroxyzine and promethazine.
The anticholinergics also have antiemetic activity, although their other side effects often outweigh any relief they might provide for nausea. Nevertheless, some anticholinergics are used alone or in combination with other agents for mild nausea and vomiting, including scopolamine and hyoscyamine.
Phenothiazines, which are typically used as antipsychotic medications, also have antiemetic effects and several minor phenothiazines are used to treat transient nausea and vomiting associated with viral infections, surgery or gastrointestinal illnesses.
More potent antiemetics used in restricted situations include cannabinoid agonists, serotonin receptor (5-HT3) antagonists and antagonists of the neurokinin 1 (NK-1) receptor for substance P. Cannabinoid agonists have potent activity against nausea and can also stimulate appetite. The cannabinoids are generally reserved for patients with recurrent nausea, particularly if accompanied by cachexia as might occur with cancer chemotherapy. Cannabinoids in current use for nausea and cachexia include tetrahydrocannabinol (THC), nabilone and dronabinol as well as cannabis itself, often referred to as medical marijuana. The serotonin type 3 (5-HT3) receptor antagonists have potent activity both in the central nervous system and the gastrointestinal tract and are used to treat postoperative and chemotherapy induced nausea and vomiting. They typically can also cause diarrhea or constipation, dizziness and fatigue and are usually give for a limited time in persons with severe temporary nausea and vomiting. Antagonists of the neurokinin 1 receptor for substance P are very potent inhibitors of nausea and vomiting, and particularly the delayed (>24 hours) nausea and vomiting after cyclic cancer chemotherapy. These agents include aprepitant, fosaprepitant and rolapitant and are generally given for a short time only during cyclic, highly emetigenic cancer chemotherapy.
Finally, there are miscellaneous antiemetics that may have other major activities, the effect on nausea and vomiting being a secondary effect. These agents include trimethobenzamide, metoclopramide, tegaserod and octreotide.
Antiemetic OTC
Several over-the-counter (OTC) medicines are used as antiemetics. These include:
- Bismuth subsalicylate (2 brand names: Kaopectate, Pepto-Bismol). It may help treat some types of nausea and vomiting, such as from gastroenteritis (stomach flu). It’s also used for upset stomach and as an antidiarrheal (medicine to treat diarrhea). Bismuth subsalicylate works by protecting the stomach lining.
- Antihistamines. Certain types may help prevent nausea and vomiting caused by motion sickness. These include dimenhydrinate (brand name: Dramamine) and meclizine hydrochloride (brand name: Dramamine Less Drowsy). Antihistamines appear to dull the inner ear’s ability to sense motion. They block messages to the part of the brain that controls nausea and vomiting. This is why they work best if you take them before you start feeling motion sickness.
Before you take an OTC antiemetic medication, read the directions on the drug facts label. This will tell you how much medicine to take and how often to take it. If you have any questions, call your family doctor or pharmacist. Keep a record of which OTC medications you are using and when you take them. If you need to go to the doctor, take this list with you.
Follow these tips to make sure you are taking the right amount of medicine:
- Take only the amount recommended on the medicine’s label. Don’t assume that more medicine will work better or quicker. Taking more than the recommended amount can be dangerous.
- If you are taking a prescription medicine, ask your doctor if it’s okay to also take an OTC antiemetic medication.
- Don’t use more than 1 kind of OTC antiemetic medication at a time unless your doctor says it’s okay. They may have similar active ingredients. These could add up to be too much medicine.
Antihistamines
Histamine is an important mediator of immediate hypersensitivity reactions acting locally and causing smooth muscle contraction, vasodilation, increased vascular permeability, edema and inflammation. Histamine acts through specific cellular receptors which have been categorized into four types, H1 through H4. Antihistamines represent a class of medications that block the histamine type 1 (H1) receptors. Importantly, antihistamines do not block or decrease the release of histamine, but rather ameliorate its local actions. Agents that specially block other H2 receptors are generally referred to as H2 blockers rather than antihistamines.
H1 receptors are widely distributed and are particularly common on smooth muscle of the bronchi, gastrointestinal tract, uterus and large blood vessels. H1 receptors are also found in the central nervous system. The antihistamines are widely used to treat symptoms of allergic conditions including itching, nasal stuffiness, runny nose, teary eyes, urticaria, dizziness, nausea and cough. Their most common use alone or in combination with other agents is for symptoms of upper respiratory illnesses such as the common cold. The central nervous system effects of antihistamines include sedation and decrease in anxiety, tension and adventitious movements.
Antihistamines are typically separated into sedating (first generation) and nonsedating (second generation) forms, based upon their central nervous system effects, the nonsedating agents being less likely to cross the blood-brain barrier. In addition, some antihistamines have additional anticholinergic, antimuscarinic or other actions. The antihistamines are some of the most commonly used drugs in medicine, and most are available in multiple forms, both by prescription and in over-the-counter products, alone or combined with analgesics or sympathomimetic agents. Common uses include short term treatment of symptoms of the common cold, seasonal allergic rhinitis (hay fever), motion sickness, nausea, vertigo, cough, urticaria, pruritus and anaphylaxis. The sedating antihistamines are also used as mild sleeping aids and to alleviate tension and anxiety. Many antihistamines are also available in topical forms, as creams, nasal sprays and eye drops for local use in alleviating allergic symptoms. The nonsedating antihistamines are typically used in extended or long term treatment of allergic disorders, including allergic rhinitis (hay fever), sinusitis, atopic dermatitis, and chronic urticaria.
The antihistamines have several adverse side effects which are related to their antihistaminic actions. Side effects are, however, usually mild and rapidly reversed with stopping therapy or decreasing the dose. These common side effects include sedation, impaired motor function, dizziness, dry mouth and throat, blurred vision, urinary retention and constipation. Antihistamines can worsen urinary retention and narrow angle glaucoma.
Cyclizine and Chlorcyclizine
Cyclizine and chlorcyclizine are first generation antihistamines and antiemetics that are used largely to treat or prevent nausea, vomiting and dizziness associated with motion sickness. Cyclizine has been linked to very rare instances of clinically apparent acute liver injury.
Cyclizine and chlorcyclizine are also used to treat symptoms of allergic rhinitis and the common cold, particularly in combination with sympathomimetic agents such as phenylephrine. Cyclizine was first approved for use in the United States in 1966. It is still widely used and is available as tablets or capsules of 25 mg in multiple generic forms and under the trade name Cyclivert and Bonine in both prescription and nonprescription forms. The typical dose is 1 or 2 tablets one hour before travel. Chlorcyclizine is used in a similar dose regimen. Both cyclizine and chlorcyclizine are also available as chewable tablets and in oral liquid formulations as well as combinations with sympathomimetic agents (pseudoephedrine) for the treatment of symptoms of the common cold and nasal congestion.
Cyclizine and chlorcyclizine common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Dimenhydrinate
Dimenhydrinate is the 8-chlorotheophylline salt of diphenhydramine and, thus, combines a first generation antihistamine with a xanthine that is added to counteract the drowsiness caused by diphenhydramine. Dimenhydrinate was approved as an over-the-counter agent in 2004 and is used largely for prevention of motion sickness or symptoms of nausea and dizziness.
Dimenhydrinate is available in multiple generic forms as tablets 25 or 50 mg or oral solutions, most of which are available without prescription, a common brand name being Dramamine. A liquid formulation for intramuscular use is also available. The typical adult oral dose is 50 mg taken an hour before expected travel and up to three or four times daily.
Dimenhydrinate common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Hydroxyzine
Hydroxyzine is a first generation antihistamine that is used largely for symptoms of itching, nausea, anxiety and tension. Because of its sedating effects, hydroxyzine is also used for anxiety, tension and as a mild sleeping aid. Hydroxyzine belongs to the piperazine class of antihistamines (with cyclizine and meclizine) which are more commonly used for itching, nausea and anxiety than for their effects on symptoms of allergic rhinitis or coryza. Hydroxyzine was approved for use in the United States in 1957 and continues to be widely used.
Hydroxyzine is available as tablets or capsules of 10, 25, 50 and 100 mg in multiple generic forms and under the trade names Atarax and Vistaril. Hydroxyzine is also available as an oral suspension or syrup and as a liquid for injection. Most formulations are available without prescription. The recommended adult oral dose ranges from 25 to 100 mg three to four times daily. Doses used for itching are generally lower than those for anxiety and tension.
Hydroxyzine common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Meclizine
Meclizine is a first generation antihistamine that is used largely for its antiemetic and sedative effects to treat vertigo and symptoms of nausea, vomiting and dizziness associated with motion sickness. Meclizine was first approved for use in the United States in 1957 and is available in tablets and capsules of 12.5, 25 and 50 mg in multiple generic forms and under the trade name Antivert. Meclizine is available in both prescription and nonprescription forms. The recommended adult oral dose for motion sickness is 25 to 50 mg taken one hour before travel. The recommended dose for vertigo is 25 to 100 mg daily in divided doses.
Meclizine common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Who shouldn’t take OTC antiemetic medications?
Some people are allergic to aspirin or other salicylate medicines. They should not take bismuth subsalicylate. Don’t give bismuth subsalicylate to children 12 years of age or younger. Don’t give it to children or teenagers who may have the flu or chickenpox. This increases their risk for Reye syndrome. This is a serious illness that can lead to death.
Before taking an antihistamine, talk to your doctor if you have any of the following problems:
- Glaucoma
- Trouble urinating (from an enlarged prostate gland)
- Breathing problems, such as asthma, emphysema, or chronic bronchitis
- Thyroid disease
- Heart disease
- High blood pressure
Antiemetic drug interactions
Bismuth subsalicylate may affect how well some medicines work. It also may cause side effects if combined with other medicines. Ask your doctor before taking bismuth subsalicylate if you also take:
- Blood-thinning medicines
- Medicines for gout
- Medicines for arthritis
- Medicines for diabetes
Ask your doctor before taking bismuth subsalicylate if you take pain relievers or cold medicines. These medicines may contain aspirin, which is a salicylate. You may get too much salicylate if you take more than 1 of these medicines at a time.
Talk to your doctor before taking an antihistamine if you take sleeping pills, sedatives, or muscle relaxants. Many OTC cold and allergy medicines contain antihistamines. If you use more than 1 of these medicines, you may get more antihistamine than you intend. Some prescription medicines have side effects similar to the side effects of antihistamines. These could include dry mouth and drowsiness. Talk with your doctor before taking these medicines at the same time.
Antiemetic side effects
Healthy adults usually don’t experience side effects from antiemetic medicines. Side effects can be a concern for older adults or people who have health problems.
The most common side effects of bismuth subsalicylate are:
- Darkened stools or tongue
- Constipation
- Ringing sound in the ears (tinnitus)
These are short-term side effects.
Antihistamines may make you feel sleepy. This can affect your ability to drive or operate machines. It may be hard for you to think clearly. Alcohol can increase the drowsiness caused by antihistamines. Antihistamines may also cause your mouth and eyes to feel dry.
Antiemetic in pregnancy
Morning sickness also called nausea and vomiting of pregnancy, is when you have nausea (feeling sick to your stomach) and vomiting during pregnancy. Even though it’s called morning sickness, it can happen any time of day. Morning sickness usually starts at about 6 weeks of pregnancy and goes away in the second trimester. Mild morning sickness doesn’t harm you or your baby. But if nausea and vomiting becomes severe (called hyperemesis gravidarum), it can cause serious problems during pregnancy, and you may need to stay in the hospital for treatment. Hyperemesis gravidarum is severe nausea and vomiting that needs treatment (sometimes in a hospital) to help you get better.
Lots of pregnant women have morning sickness. It usually doesn’t cause harm to you or your baby. At least 7 in 10 pregnant women (70 percent) have morning sickness in the first trimester (first 3 months or the first 12 weeks) of pregnancy. It usually starts at about 6 weeks of pregnancy and is at its worst at about 9 weeks. Most women feel better in their second trimester, but some have morning sickness throughout pregnancy. If you have morning sickness, tell your health care provider.
If your morning sickness is severe or if it goes into your fourth month of pregnancy, tell your health care provider right away.
Here’s what you can do to help you feel better and even prevent morning sickness:
- Take a prenatal vitamin before you get pregnant. Talk to your provider about which one to take. Sometimes vitamins can upset your stomach, so take it with a snack.
- Keep snacks by your bed. Eat a few crackers before you get up in the morning to help settle your stomach.
- Eat five or six small meals each day instead of three larger meals.
- Eat foods that are low in fat and easy to digest, like cereal, rice and bananas. Don’t eat spicy or fatty foods.
- Eat healthy snacks between meals. This can help keep your stomach from being empty and helps prevent nausea. Try snacks that are high in protein, like milk or yogurt.
- Drink plenty of fluids, especially water.
- Avoid smells that upset your stomach.
You may have heard about these ways to prevent or relieve morning sickness. Talk to your provider before trying any of these:
- Acupressure and acustimulation (also called electrical nerve stimulation) wristbands. These involve putting pressure on or stimulating certain points of the body (called pressure points) to help prevent nausea.
- Acupuncture. This is a kind of treatment in which thin needles are put into your skin. If you’re thinking about acupuncture to help with morning sickness, tell your provider and find an acupuncturist who is trained to work with pregnant women.
- Ginger. Ginger is an herb (plant) used in cooking and medicine. Ginger ale, tea or candies may help relieve morning sickness.
Even if it’s legal where you live for either personal or medical use, it’s not safe to use marijuana to treat morning sickness. No amount of marijuana has been proven safe to use during pregnancy. If you’re thinking of using marijuana to help with morning sickness, talk to your provider about other treatments that are safer for your baby.
If you can’t relieve morning sickness on your own or if you have severe nausea and vomiting of pregnancy, your doctor may treat you with these medicines:
- Vitamin B6 and doxylamine. Your provider may treat you with these medicines separately or together. You can get vitamin B6 and doxylamine over-the-counter (OTC), which means you don’t need a prescription (order) for them from your provider. Doxylamine is found in some OTC sleep aids (medicines that help you sleep). Or your provider may prescribe you a medicine that combines them.
- Antiemetic drugs. These are drugs that help prevent vomiting. If Vitamin B6 and doxylamine don’t work, your provider may prescribe an antiemetic drug for you. Not all are safe to use during pregnancy, so talk to your provider to make sure the medicine is a good choice for you.
Talk to your doctor before you take any medicine during pregnancy, even medicine to help treat morning sickness.
Prescription antiemetic drugs
Anticholinergic Agents
Anticholinergics are agents that decrease or block the actions of acetylcholine on its parasympathetic nervous system receptors on smooth muscle cells, glands and the central nervous system. Cholinergic receptors are usually categorized as nicotinic or muscarinic. Anticholinergics often demonstrate differential antagonism for different receptors types and subtypes, accounting in part for their variety of actions and clinical usefulness for different conditions.
The anticholinergics in clinical use include natural, semisynthetic and synthetic compounds that demonstrate a multitude of actions on smooth muscle cells and the parasympathetic nervous system. Anticholinergics have antisecretory activities and decrease nasal and bronchial secretions, salivation, lacrimation, sweating and gastric acid production, and can be used to decrease secretions in allergic and inflammatory diseases. Anticholinergics relax smooth muscle in the gastrointestinal tract, bladder and lung and can be used for gastrointestinal, urological or respiratory conditions associated with spasm and dysmotility. Some anticholinergics have antiemetic properties and are used to prevent nausea and vomiting from motion sickness or during the perioperative period. Anticholinergics increase heart rate and can be used to treat bradycardia. They are also used to reverse cholinergic overstimulation caused by cholinesterase inhibitors and neuromuscular blockers in anesthesia.
The common side effects of anticholinergic agents are largely those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating and hyperthermia, headache, visual blurring, constipation, urinary retention, impotence, tachycardia and palpitations, anxiety, restlessness and in some instances agitation and delusions. Anticholinergics rarely cause liver injury. Their relative safety probably relates to their use in low doses for short periods of time only. Most anticholinergics are metabolized in the liver via the cytochrome P450 system.
Hyoscyamine
Hyoscyamine as a natural plant alkaloid derivative and anticholinergic that is used to treat mild to moderate nausea, motion sickness, hyperactive bladder and allergic rhinitis. Hyoscyamine has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury.
Hyoscyamine is a derivative of natural alkaloid found in plants of the Solanacea family such as henbane (Hyoscyamus niger, for which it is named), jimson weed (Datura stramonium), tomatoes (Soanum lycopersicum) and deadly nightshade (Atropa belladonna). Hyoscyamine is the levorotary isomer of atropine and has potent anticholinergic, antimuscarinic activity. Hyoscyamine has been used for decades as an antiemetic, antisecretory and antispasmotic agent in the treatment of nausea, motion sickness, allergic rhinitis, gastrointestinal spasm and hypermotility, functional bowel syndrome and hyperactive bladder. Despite having been used in clinical medicine for decades, hyoscyamine has not been formally approved for many of its common uses in the United States.
Hyoscyamine is used to control symptoms associated with disorders of the gastrointestinal (GI) tract. It works by decreasing the motion of the stomach and intestines and the secretion of stomach fluids, including acid. Hyoscyamine is also used in the treatment of bladder spasms, peptic ulcer disease, diverticulitis, colic, irritable bowel syndrome, cystitis, and pancreatitis. Hyoscyamine may also be used to treat certain heart conditions, to control the symptoms of Parkinson’s disease and rhinitis (runny nose), and to reduce excess saliva production.
Hyoscyamine is available as tablets, capsules, liquids, elixirs, powders, and solutions for injection in both prescription and over-the-counter forms. Common brand names for products that include hyoscyamine are Belladonna Alkaloids, Donnatal, Hyomax, Urogesic, and Cyclospaz. The recommended adult oral dose varies, but is generally 0.125 to 0.25 mg two to four times daily. The tablets and liquid are usually taken three or four times a day.
Hyoscyamine extended-release capsules are usually taken twice a day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take hyoscyamine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Swallow the extended-release tablets whole; do not split, chew, or crush them.
Hyoscyamine controls symptoms associated with disorders of the GI tract, but it does not cure the disorders. Continue to take hyoscyamine even if you feel well. Do not stop taking hyoscyamine without talking to your doctor.
Common side effects of hyoscyamine are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, urinary retention, impotence, tachycardia and palpitations, anxiety, restlessness and in some instances agitation and hallucinations. Anticholinergic agents can precipitate acute narrow angle glaucoma and acute urinary retention.
Hyoscyamine special precautions
Before taking hyoscyamine:
- tell your doctor and pharmacist if you are allergic to hyoscyamine, any other medications, or any of the ingredients in hyoscyamine tablets, capsules, or liquid. Ask your doctor or pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking pr plan to take. Be sure to mention any of the following: amantadine (Symadine, Symmetrel), amitriptyline (Elavil), chlorpromazine (Thorazine), clomipramine (Anafranil), desipramine (Norpramin), doxepin (Sinequan), fluphenazine (Prolixin), haloperidol (Haldol), imipramine (Tofranil), medications containing belladonna (Donnatal), mesoridazine (Serentil), nortriptyline (Pamelor), perphenazine (Trilafon), phenelzine (Nardil), prochlorperazine (Compazine), promazine (Sparine), promethazine (Phenergan), protriptyline (Vivactil), thioridazine (Mellaril), tranylcypromine (Parnate), trifluoperazine (Stelazine), triflupromazine (Vesprin), trimeprazine (Temaril), and trimipramine (Surmontil). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- be aware that antacids may interfere with hyoscyamine, making it less effective. Take hyoscyamine 1 hour before or 2 hours after antacids.
- tell your doctor if you have or have ever had glaucoma; heart, lung, liver, or kidney disease; a urinary tract or intestinal obstruction; an enlarged prostate; ulcerative colitis (a condition which causes swelling and sores in the lining of the colon [large intestine] and rectum); or myasthenia gravis.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking hyoscyamine, call your doctor.
- talk to your doctor about the risks and benefits of taking hyoscyamine if you are 65 years of age or older. Older adults should not usually take hyoscyamine because it is not as safe and may not be as effective as other medications that can be used to treat the same condition.
- if you are having surgery, including dental surgery, tell your doctor or dentist that you take hyoscyamine.
- you should know that this medication may make you drowsy. Do not drive a car or operate machinery until you know how hyoscyamine affects you.
- ask your doctor about the safe use of alcohol during your treatment with hyoscyamine. Alcohol can make the side effects of this medication worse.
Hyoscyamine side effects
Hyoscyamine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- drowsiness
- dizziness or lightheadedness
- headache
- blurred vision
- flushing (feeling of warmth)
- dry mouth
- constipation
- difficulty urinating
- increased sensitivity to light
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- diarrhea
- skin rash
- eye pain
- fast or irregular heartbeat
Hyoscyamine may cause other side effects. Call your doctor if you have any unusual problems while you are taking hyoscyamine.
Scopolamine
Scopolamine as a natural plant alkaloid that has potent anticholinergic effects and is used to treat mild to moderate nausea, motion sickness, during surgery and allergic rhinitis. Scopolamine works by blocking the effects of a certain natural substance (acetylcholine) on the central nervous system.
Scopolamine is a derivative of natural alkaloid found in plants of the nightshade family (henbane, jimson weed). Scopolamine has potent anticholinergic activity and broadly and nonspecifically inhibits muscarinic transmission (antimuscarinic). Scopolamine has been used for decades as an antiemetic, antisecretory and antispasmotic agent in the treatment of nausea, motion sickness, allergic rhinitis, duodenal ulcer disease, gastrointestinal upset and spasm, functional bowel syndrome and hyperactive bladder. Semisynthetic derivatives include methscopolamine which is a quaternary ammonium derivative, which makes it less like to cross the blood brain barrier. Scopolamine has been used in clinical medicine for decades, but many commercial forms have not been formally approved for many of their common indications in the United States.
Scopolamine transdermal patch to be placed on the skin behind your ear, is available for prevention of perioperative nausea and vomiting (1.5 mg: Transderm Scop). When used to help prevent nausea and vomiting caused by motion sickness, apply the patch at least 4 hours before its effects will be needed and leave in place for up to 3 days. If treatment is needed for longer than 3 days to help prevent nausea and vomiting caused by motion sickness, remove the current patch and apply a new patch behind the other ear. When used to prevent nausea and vomiting from medications used with surgery, apply the patch as directed by your doctor and leave it in place for 24 hours after your surgery. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Use the scopolamine patch exactly as directed.
To apply the scopolamine patch, follow the directions:
- After washing the area behind the ear, wipe the area with a clean, dry tissue to ensure that the area is dry. Avoid placing on areas of your skin that have cuts, pain, or tenderness.
- Remove the patch from its protective pouch. Peel off the clear plastic protective strip and discard it. Don’t touch the exposed adhesive layer with your fingers.
- Place the adhesive side against the skin.
- After you have placed the patch behind your ear, wash your hands thoroughly with soap and water.
When the scopolamine patch is no longer needed, remove the patch and dispose of it. Wrap the patch in tissue or paper to avoid exposing anyone else to the remaining medication. Wash your hands and the area behind your ear thoroughly with soap and water to remove any traces of scopolamine from the area. If a new patch needs to be applied, place a fresh patch on the hairless area behind your other ear.
Do not cut the patch.
Limit contact with water while swimming and bathing because the patch may fall off.
Common side effects are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, urinary retention, impotence, tachycardia and palpitations, anxiety, restlessness and in some instances agitation and delusions (scopolamine madness). Anticholinergic agents can precipitate acute narrow angle glaucoma and acute urinary retention.
Scopolamine patch special precautions
Before using scopolamine patches:
- tell your doctor and pharmacist if you are allergic to scopolamine, other belladonna alkaloids, any other medications, or any of the ingredients in scopolamine patches. Ask your doctor or pharmacist or check the package label for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: antihistamines; muscle relaxants; sedatives; or tricyclic antidepressants such as desipramine (Norpramin), clomipramine (Anafranil), imipramine (Tofranil), trimipramine (Surmontil), and tranquilizers.
tell your doctor if you have angle-closure glaucoma (a condition where the fluid is suddenly blocked and unable to flow out of the eye causing a quick, severe increase in eye pressure which may lead to a loss of vision). Your doctor will probably tell you not to use scopolamine patch.
tell your doctor if you have or have ever had open-angle glaucoma (increase in internal eye pressure that damages the optic nerve); seizures; psychotic disorders (conditions that cause difficulty telling the difference between things or ideas that are real and things or ideas that are not real); stomach or intestinal obstruction; difficulty urinating; or heart, liver, or kidney disease. - tell your doctor if you are pregnant, plan to become pregnant, or are breastfeeding. If you become pregnant while using scopolamine patches, call your doctor immediately.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are using scopolamine patches.
- you should know that this drug may make you drowsy. Do not drive a car or operate machinery until you know how scopolamine patches will affect you. If you participate in water sports, use caution because this medication can have disorienting effects.
- talk to your doctor about the safe use of alcohol while taking this drug. Alcohol increases the side effects caused by scopolamine patches.
- talk to your doctor about the risks and benefits of using scopolamine if you are 65 years of age or older. Older adults should not usually use scopolamine because it is not as safe or effective as other medications that can be used to treat the same condition.
Scopolamine patch side effects
Scopolamine patches may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- disorientation
- dry mouth
- vision changes
- confusion
- agitation
- difficulty speaking
- difficulty urinating
- dizziness
- sweating
Some side effects can be serious. If you experience any of the following symptoms, remove the patch and call your doctor immediately:
- rash
- hallucinations
- confusion
- delusions
Scopolamine patches may cause other side effects. Call your doctor if you have any unusual problems while you are using scopolamine.
Antihistamine
Promethazine
Promethazine is first generation antihistamine that belongs to the phenothiazine class and is used predominantly as an antiemetic to treat nausea and vomiting and prevent motion sickness and as a mild sedative. Because of its sedating effects, promethazine is also used for anxiety, tension and as a mild sleeping aid. Promethazine is also used to relieve the symptoms of allergic reactions such as allergic rhinitis (runny nose and watery eyes caused by allergy to pollen, mold or dust), allergic conjunctivitis (red, watery eyes caused by allergies), allergic skin reactions, and allergic reactions to blood or plasma products. Promethazine is used with other medications to treat anaphylaxis (sudden, severe allergic reactions) and the symptoms of the common cold such as sneezing, cough, and runny nose. Promethazine is also used to relax and sedate patients before and after surgery, during labor, and at other times. Promethazine is also used to prevent and control nausea and vomiting that may occur after surgery, and with other medications to help relieve pain after surgery. Promethazine works by blocking the action of a certain natural substance in the body.
Promethazine was approved for use in the United States in 1957 and continues to be widely used with more than 10 million prescriptions filled yearly.
Promethazine is available in multiple forms including tablets, capsules, syrups, suppositories and liquid solution for injection generically and under the brand name Phenergan. Unlike other antihistamines, promethazine is not available over-the-counter. Current indications include nausea, preoperative sedation, nighttime sedation, rhinitis and allergic symptoms, cough and respiratory symptoms, and treatment and prevention of motion sickness.
When promethazine is used to treat motion sickness, it is taken 30 to 60 minutes before travel and again after 8 to 12 hours if needed. On longer trips, promethazine is usually taken in the morning and before the evening meal on each day of travel. When promethazine is used to treat or prevent nausea and vomiting it is usually taken every 4 to 6 hours as needed. Promethazine may also be taken at bedtime the night before surgery to relieve anxiety and produce quiet sleep. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take promethazine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Promethazine suppositories are for rectal use only. Do not try to swallow the suppositories or insert in any other part of your body.
If you are taking promethazine liquid, do not use a household spoon to measure your dose. Use the measuring spoon or cup that came with the medication or use a spoon made especially for measuring medication.
To insert a promethazine suppository, follow these steps:
- If the suppository feels soft, hold it under cold, running water for 1 minute. Remove the wrapper.
- Dip the tip of the suppository in water.
- Lie down on your left side and raise your right knee to your chest. (A left-handed person should lie on the right side and raise the left knee.)
- Using your finger, insert the suppository into the rectum, about 1/2 to 1 inch (1.25 to 2.5 centimeters) in children who are 2 years of age older and 1 inch (2.5 centimeters) in adults. Hold it in place for a few moments.
- Stand up after about 15 minutes. Wash your hands thoroughly and resume your normal activities.
Promethazine common side effects include excessive sedation, impairment of motor function, confusion, dizziness, extrapyramidal symptoms, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Promethazine may cause breathing to slow or stop, and may cause death in children. Promethazine should not be given to babies or children who are younger than 2 years old and should be given with caution to children who are 2 years of age or older. Combination products containing promethazine and codeine should not be given to children younger than 16 years old. Promethazine should not routinely be used to treat vomiting in children; it should only be used in specific cases when a doctor decides that it is needed. Tell your child’s doctor if your child has any condition that affects his/her breathing such as lung disease, asthma, or sleep apnea (stops breathing for short periods of time during sleep). Tell your doctor or pharmacist about all the medications your child is taking, especially barbiturates such as phenobarbital (Luminal), medications for anxiety, narcotic medications for pain, sedatives, sleeping pills, and tranquilizers. Call your child’s doctor immediately and get emergency medical treatment if your child has difficulty breathing, wheezes, slows or pauses in breathing, or stops breathing.
Talk to your doctor about the risks of giving promethazine to your child.
Promethazine special precautions
Before taking promethazine:
- tell your doctor and pharmacist if you are allergic to promethazine, other phenothiazines (certain medications used to treat mental illness, nausea, vomiting, severe hiccups, and other conditions) or any other medications. Also tell your doctor and pharmacist if you have ever had an unusual or unexpected reaction when you took promethazine, another phenothiazine, or any other medication. Ask your doctor or pharmacist if you do not know if a medication you are allergic to is a phenothiazine.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements and herbal products you are taking or plan to take. Be sure to mention any of the following: antidepressants (‘mood elevators’) such as amitriptyline (Elavil), amoxapine (Asendin), clomipramine (Anafranil), desipramine (Norpramin), doxepin (Adapin, Sinequan), imipramine (Tofranil), nortriptyline (Aventyl, Pamelor), protriptyline (Vivactil), and trimipramine (Surmontil); antihistamines; azathioprine (Imuran);barbiturates such as phenobarbital (Luminal); cancer chemotherapy; epinephrine (Epipen); ipratropium (Atrovent)medications for anxiety, irritable bowel disease, mental illness, motion sickness, Parkinson’s disease, seizures, ulcers, or urinary problems; monoamine oxidase (MAO) inhibitors such as isocarboxazid (Marplan), phenelzine (Nardil), tranylcypromine (Parnate), and selegiline (Eldepryl, Emsam, Zelapar); narcotics and other pain medication; sedatives; sleeping pills;and tranquilizers. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have or have ever had an enlarged prostate (a male reproductive gland); glaucoma (a condition in which increased pressure in the eye can lead to gradual loss of vision); seizures; ulcers; blockage in the passage between the stomach and intestine; blockage in the bladder; asthma or other lung disease; sleep apnea; cancer;any condition that affects the production of blood cells in your bone marrow; or heart or liver disease. If you will be giving promethazine to a child, also tell the child’s doctor if the child has any of the following symptoms before he or she receives the medication: vomiting, listlessness, drowsiness, confusion, aggression, seizures, yellowing of the skin or eyes, weakness, or flu-like symptoms. Also tell the child’s doctor if the child has not been drinking normally, has had excessive vomiting or diarrhea, or appears dehydrated.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking promethazine, call your doctor.
- talk to your doctor about the risks and benefits of taking promethazine if you are 65 years of age or older. Older adults should not usually take promethazine because it is not as safe as other medications that can be used to treat the same conditions.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking promethazine.
- you should know that this medication may make you drowsy. Do not drive a car or operate machinery until you know how this medication affects you. If you are giving promethazine to a child, watch the child to be sure he or she does not get hurt while riding a bike or participating in other activities that could be dangerous.
- ask your doctor about the safe use of alcohol while you are taking this medication. Alcohol can make the side effects of promethazine worse.
- plan to avoid unnecessary or prolonged exposure to sunlight and to wear protective clothing, sunglasses, and sunscreen. Promethazine may make your skin sensitive to sunlight.
Promethazine side effects
Promethazine can cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- dry mouth
- drowsiness
- listlessness
- difficulty falling asleep or staying asleep
- nightmares
- dizziness
- ringing in ears
- blurred or double vision
- loss of coordination
- nausea
- vomiting
- nervousness
- restlessness
- hyperactivity
- abnormally happy mood
- stuffy nose
- itching
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- wheezing
- slowed breathing
- breathing stops for a short time
- fever
- sweating
- stiff muscles
- decreased alertness
- fast or irregular pulse or heartbeat
- faintness
- abnormal or uncontrollable movements
- hallucinations (seeing things or hearing voices that do not exist)
- confusion
- overwhelming or unmanageable fear or emotion
- seizures
- uncontrollable shaking of a part of the body
- unusual bruising or bleeding
- sore throat, fever, chills, and other signs of infection
- uncontrolled eye movements
- tongue sticking out
- abnormal neck position
- inability to respond to people around you
- yellowing of the skin or eyes
- rash
- hives
- swelling of the face, eyes, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- hoarseness
- difficulty breathing or swallowing
Promethazine may cause other side effects. Call your doctor if you have any unusual problems while you are using promethazine.
Symptoms of overdose may include:
- difficulty breathing
- slowed or stopped breathing
- dizziness
- lightheadedness
- fainting
- loss of consciousness
- fast heartbeat
- tight muscles that are difficult to move
- loss of coordination
- continuous twisting movements of the hands and feet
- dry mouth
- wide pupils (black circles in the middle of the eyes)
- flushing
- nausea
- constipation
- abnormal excitement or agitation
- nightmares
Antipsychotic agents
Chlorpromazine
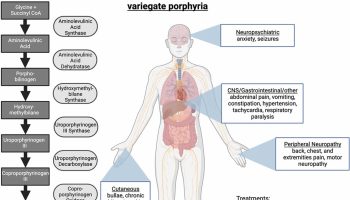
Chlorpromazine is a conventional antipsychotic called tricyclic aliphatic phenothiazine that acts by postsynaptic inhibition of dopamine receptors. Chlorpromazine was once the most commonly prescribed antipsychotic agent, but that is now rarely used. In recent years, chlorpromazine has been replaced in large part by the atypical antipsychotics, which have fewer extrapyramidal and hepatic side effects. Chlorpromazine current indications include psychotic disorders, schizophrenia, nausea and vomiting, acute intermittent porphyria and intractable hiccups.
Chlorpromazine is used to treat the symptoms of schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions) and other psychotic disorders (conditions that cause difficulty telling the difference between things or ideas that are real and things or ideas that are not real) and to treat the symptoms of mania (frenzied, abnormally excited mood) in people who have bipolar disorder (manic depressive disorder; a condition that causes episodes of mania, episodes of depression, and other abnormal moods). Chlorpromazine is also used to treat severe behavior problems such as explosive, aggressive behavior and hyperactivity in children 1 to 12 years of age. Chlorpromazine is also used to control nausea and vomiting, to relieve hiccups that have lasted one month or longer, and to relieve restlessness and nervousness that may occur just before surgery. Chlorpromazine is also used to treat acute intermittent porphyria (condition in which certain natural substances build up in the body and cause stomach pain, changes in thinking and behavior, and other symptoms). Chlorpromazine is also used along with other medications to treat tetanus (a serious infection that may cause tightening of the muscles, especially the jaw muscle).
Chlorpromazine is available in multiple generic forms as tablets of 10, 25, 50, 100 and 200 mg, as extended release capsules of 200 and 300 mg, and as syrup in various concentrations. Parenteral forms are also available. Chlorpromazine was formerly available under the brand names Thorazine and Largactil. The typical maintenance dose of chlorpromazine is 100 to 200 mg daily.
Chlorpromazine comes as a tablet to take by mouth. When chlorpromazine is used to control nausea and vomiting, it is usually taken every 4-6 hours as needed. When chlorpromazine is used to relieve nervousness before surgery, it is usually taken 2-3 hours before surgery. When chlorpromazine is used to relieve hiccups, it is usually taken 3-4 times a day for up to 3 days or until the hiccups stop. If the hiccups do not stop after 3 days of treatment, a different medication should be used. If you are taking chlorpromazine on a regular schedule, take it at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take chlorpromazine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Your doctor may start you on a low dose of chlorpromazine and gradually increase your dose. Your doctor may decrease your dose once your condition is controlled. Be sure to tell your doctor how you are feeling during your treatment with chlorpromazine.
Chlorpromazine common side effects include drowsiness, dizziness, headache, blurred vision, dry mouth, constipation, tremor, restlessness, muscle spasms and weight gain. Chlorpromazine can cause mild and transient serum enzyme elevations and is also a well known cause of clinically apparent acute and chronic cholestatic liver injury.
Studies have shown that older adults with dementia (a brain disorder that affects the ability to remember, think clearly, communicate, and perform daily activities and that may cause changes in mood and personality) who take antipsychotics (medications for mental illness) such as chlorpromazine have an increased chance of death during treatment.
Chlorpromazine is not approved by the Food and Drug Administration (FDA) for the treatment of behavior problems in older adults with dementia. Talk to the doctor who prescribed this medication if you, a family member, or someone you care for has dementia and is taking chlorpromazine. For more information, visit the FDA website: https://www.fda.gov/Drugs/DrugSafety/ucm085729.htm
Chlorpromazine special precautions
Before taking chlorpromazine:
- tell your doctor and pharmacist if you are allergic to chlorpromazine; other phenothiazines such as fluphenazine, perphenazine, prochlorperazine (Compazine), promethazine (Phenergan), thioridazine, and trifluoperazine; or any other medications.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: anticoagulants (blood thinners) such as warfarin (Coumadin); antidepressants; antihistamines; atropine (in Motofen, in Lomotil, in Lonox); barbiturates such as pentobarbital (Nembutal), phenobarbital (Luminal), and secobarbital (Seconal); cancer chemotherapy; diuretics (water pills); epinephrine (Epipen); guanethidine (not available in the US); ipratropium (Atrovent); lithium (Eskalith, Lithobid); medications for anxiety, irritable bowel disease, mental illness, motion sickness, Parkinson’s disease, ulcers, or urinary problems; medications for seizures such as phenytoin (Dilantin); narcotic medications for pain; propranolol (Inderal); sedatives; sleeping pills; and tranquilizers. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have or have ever had asthma; emphysema (a lung disease that causes shortness of breath); an infection in your lungs or bronchial tubes (tubes that bring air to the lungs); trouble keeping your balance; glaucoma (condition in which increased pressure in the eye can lead to gradual loss of vision); breast cancer; pheochromocytoma (tumor on a small gland near the kidneys); seizures; an abnormal electroencephalogram (EEG; test that records electrical activity in the brain); any condition that affects the production of blood cells by your bone marrow; or heart, liver, or kidney disease. Also
- tell your doctor if you have ever had to stop taking a medication for mental illness due to severe side effects or if you plan to work with organophosphorus insecticides (a type of chemical used to kill insects).
- if you will be using chlorpromazine to treat nausea and vomiting, it is important to tell your doctor about any other symptoms you are experiencing, especially listlessness; drowsiness; confusion; aggression; seizures; headaches; problems with vision, hearing, speech, or balance; stomach pain or cramps; or constipation. Nausea and vomiting that is experienced along with these symptoms may be a sign of a more serious condition that should not be treated with chlorpromazine.
- tell your doctor if you are pregnant, especially if you are in the last few months of your pregnancy, or if you plan to become pregnant or are breast-feeding. If you become pregnant while taking chlorpromazine, call your doctor. Chlorpromazine may cause problems in newborns following delivery if it is taken during the last months of pregnancy.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking chlorpromazine.
- if you are having a myelogram (x-ray examination of the spine), tell your doctor and the radiographer that you are taking chlorpromazine. Your doctor will probably tell you not to take chlorpromazine for 2 days before the myelogram and for one day after the myelogram.
- you should know that this medication may make you drowsy and may affect your thinking and movements. Do not drive a car or operate machinery until you know how this medication affects you.
- ask your doctor about the safe use of alcohol during your treatment with chlorpromazine. Alcohol can make the side effects of chlorpromazine worse.
- plan to avoid unnecessary or prolonged exposure to sunlight and to wear protective clothing, sunglasses, and sunscreen. Chlorpromazine may make your skin sensitive to sunlight.
- you should know that chlorpromazine may cause dizziness, lightheadedness, fast heartbeat, and fainting, especially when you get up too quickly from a lying position. This is most common at the beginning of treatment with chlorpromazine, especially after the first dose. To avoid this problem, get out of bed slowly, resting your feet on the floor for a few minutes before standing up.
- you should know that chlorpromazine may make it harder for your body to cool down when it gets very hot. Tell your doctor if you plan to do vigorous exercise or be exposed to extreme heat.
Chlorpromazine side effects
Chlorpromazine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- dizziness, feeling unsteady, or having trouble keeping your balance
- blank facial expression
- shuffling walk
- restlessness
- agitation
- nervousness
- unusual, slowed, or uncontrollable movements of any part of the body
- difficulty falling asleep or staying asleep
- increased appetite
- weight gain
- breast milk production
- breast enlargement
- missed menstrual periods
- decreased sexual ability
- changes in skin color
- dry mouth
- stuffed nose
- difficulty urinating
- widening or narrowing of the pupils (black circles in the middle of the eyes)
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- fever
- muscle stiffness
- falling
- confusion
- fast or irregular heartbeat
- sweating
- yellowing of the skin or eyes
- flu-like symptoms
- sore throat, chills, and other signs of infection
- unusual bleeding or bruising
- neck cramps
- tongue that sticks out of the mouth
- tightness in the throat
- difficulty breathing or swallowing
- fine, worm-like tongue movements
- uncontrollable, rhythmic face, mouth, or jaw movements
- seizures
- blisters
- rash
- hives
- itching
- swelling of the eyes, face, mouth, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- vision loss, especially at night
- seeing everything with a brown tint
Chlorpromazine may cause other side effects. Call your doctor if you have any unusual problems while you are taking chlorpromazine.
Symptoms of chlorpromazine overdose may include:
- sleepiness
- loss of consciousness
- unusual, slowed, or uncontrollable movements of any part of the body
- agitation
- restlessness
- fever
- seizures
- dry mouth
- irregular heartbeat
Prochlorperazine
Prochlorperazine suppositories and tablets is a tricyclic aliphatic phenothiazine which acts by postsynaptic inhibition of dopamine receptors used primarily as an antiemetic agent to control severe nausea and vomiting. Prochlorperazine tablets are also used to treat the symptoms of schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions). Prochlorperazine tablets are also used on a short-term basis to treat anxiety that could not be controlled by other medications. Prochlorperazine should not be used to treat any condition in children who are younger than 2 years old or who weigh less than 20 pounds (about 9 kilograms). Prochlorperazine is in a class of medications called conventional antipsychotics. It works by decreasing abnormal excitement in the brain.
Prochlorperazine was approved for use in the United States in 1956 and is still widely used in therapy of nausea and vomiting. Prochlorperazine is available in generic forms as tablets of 5, 10 and 25 mg, in long acting capsules of 15 mg, as an oral solution of 5 mg/ 5 mL, as suppositories of 2.5, 5 and 25 mg, and in parenteral forms. Prochlorperazine is also available under the brand names of Compazine and Compro. Typical doses for nausea are 5 to 10 mg three to four times daily.
Prochlorperazine suppositories are usually inserted twice a day. Use prochlorperazine at around the same time(s) every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Use prochlorperazine exactly as directed. Do not use more or less of it or use it more often than prescribed by your doctor.
Your doctor may start you on a low dose of prochlorperazine and gradually increase your dose, not more often than once every 2-3 days.
Prochlorperazine common side effects are similar to other phenothiazines and include drowsiness, dizziness, headache, blurred vision, dry mouth, constipation, tremor, restlessness, muscle spasms and weight gain.
Studies have shown that older adults with dementia (a brain disorder that affects the ability to remember, think clearly, communicate, and perform daily activities and that may cause changes in mood and personality) who take antipsychotics (medications for mental illness) such as prochlorperazine have an increased chance of death during treatment.
Prochlorperazine is not approved by the Food and Drug Administration (FDA) for the treatment of behavior problems in older adults with dementia. Talk to the doctor who prescribed this medication if you, a family member, or someone you care for has dementia and is taking prochlorperazine. For more information, visit the FDA website: http://www.fda.gov/Drugs
Prochlorperazine special precautions
Before using prochlorperazine:
- tell your doctor and pharmacist if you are allergic to prochlorperazine, other phenothiazines such as chlorpromazine, fluphenazine, perphenazine, promethazine (Phenergan), thioridazine, and trifluoperazine; or any other medications. If you will be taking prochlorperazine tablets, also tell your doctor if you are allergic to tartrazine (a yellow dye found in some foods and medications) or aspirin.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements and herbal products you are taking or plan to take. Be sure to mention any of the following: anticoagulants (‘blood thinners’) such as warfarin (Coumadin); antidepressants; antihistamines; atropine (in Motofen, in Lomotil, in Lonox); barbiturates such as pentobarbital (Nembutal), phenobarbital (Luminal), and secobarbital (Seconal); diuretics (‘water pills’); epinephrine (Epipen); guanethidine (not available in the US); ipratropium (Atrovent); lithium (Eskalith, Lithobid), medications for anxiety, irritable bowel disease, mental illness, Parkinson’s disease, motion sickness, ulcers, or urinary problems; medications for seizures such as phenytoin (Dilantin); narcotic medications for pain; propranolol (Inderal); sedatives; sleeping pills; and tranquilizers. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have or have ever had glaucoma (condition in which increased pressure in the eye can lead to gradual loss of vision), trouble keeping your balance, seizures, an abnormal electroencephalogram (EEG; test that measures electrical activity in the brain), brain damage, pheochromocytoma (tumor on a small gland near the kidneys), breast cancer, any condition that affects the production of blood cells by your bone marrow, or heart disease. Also tell your doctor if you have ever had to stop taking a medication for mental illness due to severe side effects and if you plan to work with organophosphorus insecticides (a type of chemical used to kill insects).
- if you will be giving prochlorperazine to a child, tell the child’s doctor if the child has chickenpox, measles, a stomach virus, or an infection of the brain or spinal cord. Also tell the child’s doctor if the child has any of the following symptoms: vomiting, listlessness, drowsiness, confusion, aggression, seizures, yellowing of the skin or eyes, weakness, or flu-like symptoms. Be sure to tell the child’s doctor if the child has not been drinking normally, has excessive diarrhea, or appears dehydrated.
- if you will be using prochlorperazine to treat nausea and vomiting, it is important to tell your doctor about any other symptoms you are experiencing, especially listlessness; drowsiness; confusion; aggression; seizures; headaches; problems with vision, hearing, speech, or balance; stomach pain or cramps; or constipation. Nausea and vomiting that is experienced along with these symptoms may be a sign of a more serious condition that should not be treated with prochlorperazine.
- tell your doctor if you are pregnant, especially if you are in the last few months of your pregnancy, or if you plan to become pregnant or are breast-feeding. If you become pregnant while taking prochlorperazine, call your doctor. Ptrochlorperazine may cause problems in newborns following delivery if it is taken during the last months of pregnancy.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are using prochlorperazine.
- if you will be having a myelogram (x-ray examination of the spine), tell your doctor and the radiographer that you are taking prochlorperazine. Your doctor will probably tell you not to take prochlorperazine for 2 days before the myelogram and for one day after the myelogram.
- you should know that this medication may make you drowsy and may affect your thinking and movements, especially at the beginning of your treatment. Do not drive a car or operate machinery until you know how this medication affects you.
- ask your doctor about the safe use of alcohol during your treatment with prochlorperazine. Alcohol can make the side effects of prochlorperazine worse.
- you should know that prochlorperazine may cause dizziness, especially when you get up from a lying position. To avoid this problem, get out of bed slowly, resting your feet on the floor for a few minutes before standing up.
- you should know that prochlorperazine may make it harder for your body to cool down when it gets very hot. Tell your doctor if you plan to do vigorous exercise or be exposed to extreme heat.
Prochlorperazine side effects
Prochlorperazine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- dizziness, feeling unsteady, or having trouble keeping your balance
- blurred vision
- dry mouth
- stuffed nose
- headache
- nausea
- constipation
- difficulty urinating
- widening or narrowing of the pupils (black circles in the center of the eyes)
- increased appetite
- weight gain
- agitation
- jitteriness
- difficulty falling asleep or staying asleep
- blank facial expression
- drooling
- uncontrollable shaking of a part of the body
- shuffling walk
- breast enlargement
- breast milk production
- missed menstrual periods
- decreased sexual ability in men
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- fever
- muscle stiffness
- falling
- confusion
- fast or irregular heartbeat
- sweating
- yellowing of the skin or eyes
- flu-like symptoms
- sore throat, chills, and other signs of infection
- neck cramps
- tongue that sticks out of the mouth
- tightness in the throat
- difficulty breathing or swallowing
- fine, worm-like tongue movements
- uncontrollable, rhythmic face, mouth, or jaw movements
- seizures
- rash
- hives
- itching
- swelling of the eyes, face, mouth, lips, tongue, throat, arms, hands, feet, ankles, or lower legs
- vision loss, especially at night
- seeing everything with a brown tint
- coma (loss of consciousness for a period of time)
- erection that lasts for hours
Prochlorperazine may cause other side effects. Tell your doctor if you have any unusual problems while you are taking prochlorperazine.
Symptoms of prochlorperazine overdose may include:
- agitation
- jitteriness
- difficulty falling asleep or staying asleep
- blank facial expression
- drooling
- uncontrollable shaking of a part of the body
- shuffling walk
- sleepiness
- coma (loss of consciousness for a period of time)
- seizures
- irregular heartbeat
- fever
- dry mouth
- constipation
Serotonin 5-HT3 Receptor Antagonists
5-hydroxytryptamine-3 (5-HT3) antagonists also called serotonin type 3 receptor antagonists or serotonin blockers, block serotonin both peripherally, on gastrointestinal (GI) vagal nerve terminals, and centrally in the chemoreceptor trigger zone 1. This results in powerful antiemetic effects. The serotonin type 3 (5-HT3) receptor antagonists are potent antiemetics used for prevention of postsurgical or chemotherapy induced nausea and vomiting and for some agents as therapy of diarrhea-predominant irritable bowel syndrome. 5-HT3 antagonists are not effective for motion sickness. They are sometimes used off-label to treat severe nausea and vomiting including that from hyperemesis gravidarum and acute gastroenteritis. These agents are generally given intravenously before or at the time of chemotherapy or surgery, but are also available as oral tablets, capsules and solutions, but are usually given for 1 to 3 days only. Common side effects include headache, fatigue, dizziness and constipation.
Currently, there are four 5-HT3 receptor antagonists on the market, which are FDA-approved for the prevention of nausea and vomiting in children and adults related to chemotherapy use, radiation therapy, and the effects of postoperative anesthesia. Four 5-HT3 receptor antagonists are currently approved for use in the United States: ondansetron, granisetron, dolasetron, and palonosetron.
Dolasetron is also FDA-approved for the treatment of postoperative nausea and vomiting in adults and children. There are a number of formulations providing clinicians and patients many options for effective dose administration. The most common adverse effects include headaches, fatigue, and constipation. Rarely, 5-HT3 receptor antagonists can cause QT prolongation if administered concomitantly with other QT-prolonging medications or in patients with a history of congenital, long-QT syndrome. There are also documented cases of serotonin syndrome in susceptible patient populations. There is also the concern for masking symptoms of bowel obstruction in the elderly or postoperative patient. An overdose of 5-HT3 receptor antagonists is rare and no fatal dose has been established. Treatment is largely supportive in these cases. Overall, select serotonin receptor antagonists are an effective antiemetic class with a wide therapeutic index and mild side effect profile 2.
Adult FDA-Approved Indications:
- Prevention of chemotherapy-induced nausea and vomiting
- Prevention of radiation therapy-induced nausea and vomiting
- Prevention of postoperative nausea and vomiting
- Treatment of postoperative nausea and vomiting (dolasetron)
Adult Non-FDA-Approved Indications:
- Treatment of postoperative nausea and vomiting
- Nausea and vomiting during pregnancy, severe or refractory
Pediatric FDA-Approved Indications:
- Prevention of chemotherapy-induced nausea and vomiting
- Prevention of postoperative nausea and vomiting
- Treatment of postoperative nausea and vomiting (dolasetron)
Serotonin 5-HT3 receptor antagonists special precautions
Pregnancy
- 5-HT3 receptor antagonists are FDA pregnancy category B. Available human studies examining early pregnancy conclude there is not a high risk of congenital malformations. There is a small increased risk of septal defects and cleft palate. Animal studies show no increased risk during early pregnancy. It is not known if 5-HT3 receptor blockers are present in breast milk.
Monitoring
- Obtain baseline ECG in at-risk patient populations, along with monitoring sodium, potassium, calcium, and magnesium.
Arrhythmia
5-HT3 receptor antagonists have been associated with many dose-dependent increases in ECG intervals (PR, QRS, QT/QTc, JT), usually occurring 1 to 2 hours after intravenous (IV) administration. When used in conjunction with other interval prolonging agents, there is the risk of arrhythmia development. Healthcare providers should observe caution with patients with a history of congenital long QT syndrome, ventricular arrhythmias, cardiac disease, electrolyte abnormalities, or with those who are receiving concomitant cardiotoxic chemotherapy.
Serotonin Syndrome
Fatal case reports of serotonin syndrome have been reported with 5-HT3 receptor antagonists, most often in the post-anesthesia setting. This is thought to be from the concomitant use of serotonergic medications, such as SSRIs, selective norepinephrine serotonin reuptake inhibitors (SNRIs), lithium, fentanyl, and mirtazapine. Patients should be monitored for signs of serotonin syndrome, including mental status changes, autonomic instability, neuromuscular changes, GI symptoms, and/or seizures. If serotonin syndrome occurs, 5-HT3 receptor antagonists should be discontinued, and supportive management should be initiated.
Constipation
Constipation is a commonly reported adverse effect with all formulations of 5-HT3 receptor antagonists, especially the use of tablets or extended-release subcutaneous injection. This can be compounded during pregnancy or in patient populations at risk for constipation. There is concern regarding the masking of symptoms of bowel obstruction with 5-HT3 receptor antagonist use and should not be used as a substitute for nasogastric suctioning. Exercise caution in patients with recent abdominal surgery.
Other
Some dosage forms of 5-HT3 receptor antagonists may contain sodium benzoate/benzoic acid, which is a metabolite of benzyl alcohol. Large amounts of benzyl alcohol (greater than 99 mg/kg per day) have been associated with fatal neonatal “gasping syndrome,” which consists of metabolic acidosis, respiratory distress, gasping respirations, central nervous system (CNS) depression, renal failure, and hypotension. Caution should be taken in patients with phenylketonuria, as orally disintegrating tablets contain phenylalanine. The dosing of 5-HT3 receptor antagonists should be decreased in patients with hepatic impairment, due to the altered hepatic clearance and prolonged drug half-life.
Alosetron
Alosetron is a 5-HT3 receptor blocker, but was developed and used largely for management of diarrhea-predominant irritable bowel syndrome rather than as an antiemetic. Its introduction in the 1990s however, was followed by cases of severe, even life-threatening, constipation which led to its withdrawal in 2000. Alosetron was reintroduced in 2002 with restrictions on its availability and limitation to women with severe diarrhea-predominant irritable bowel syndrome. Alosetron is available as 0.5 and 1.0 mg tablets generically and under the brand name Lotronex. The typical initial dose in adults is 0.5 mg twice daily, which can be increased to 1 mg twice daily if it is well tolerated.
Alosetron side effects include abdominal pain and constipation, which can be severe resulting in intestinal obstruction, ileus, impaction and ischemic colitis. Patients treated with alosetron must be enrolled in a prescribing program and have regular monitoring for side effects.
Dolasetron
Dolasetron is a 5-HT3 receptor blocker used as an antiemetic to prevent nausea and vomiting postoperatively or after cancer chemotherapy. It was first approved in 1997 and is available as tablets of 50 and 100 mg and as a solution for injection in single or multidose vials (20 mg/mL) under the brand name Anzemet. As with most 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea.
Dolasetron side effects include headache, fatigue, diarrhea and dizziness. Dolasetron can prolong the QTc interval and is susceptible to drug-drug interactions.
Granisetron
Granisetron is a 5-HT3 receptor blocker used as an antiemetic to prevent nausea and vomiting postoperatively or after cancer chemotherapy. Introduced in 1993, granisetron is available as tablets of 1 mg and as a solution for injection in single or multidose vials (1 mg/mL) generically and under the brand name Kytril. A transdermal patch of granisetron for prevention of chemotherapy associated nausea and vomiting is also available (Sancuso). As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea.
Granisetron side effects can include headache, fatigue, diarrhea and dizziness.
Ondansetron
Ondansetron is a 5-HT3 receptor blocker that was developed as an antiemetic and was introduced in 1993, the first of this class of anti-emetic agents. The current indications are for prevention of nausea and vomiting postoperatively or after cancer chemotherapy. Ondansetron is available as tablets of 4, 8, 16 and 24 mg and as a solution for injection in single or multidose vials (2 mg/mL) in multiple generic forms and under the brand name Zofran. As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea.
Ondansetron side effects include headache, fatigue, diarrhea and dizziness. Ondansetron can prolong the QTc interval.
Palonosetron
Palonosetron is a 5-HT3 receptor blocker that was developed as an antiemetic and introduced in 2003. The current indications are prevention of nausea and vomiting postoperatively or after cancer chemotherapy. Palonosetron is available as capsules of 0.5 mg and as a solution for injection in single or multidose vials (0.05 mg/mL) generically and under the brand name Aloxi. As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea.
Palonosetron side effects include headache, fatigue, diarrhea and dizziness. Palonosetron has not been shown to prolong the QTc interval.
Serotonin 5-HT3 receptor antagonists side effects
Most common adverse reactions:
- Headache (9% to 27%)
- Fatigue (9% to 13%)
- Malaise (9% to 13%)
- Constipation (6% to 11%)
One percent to 10%:
- Drowsiness, sedation, dizziness, agitation, anxiety, paresthesia, the sensation of cold, pruritus, skin rash, diarrhea, gynecologic disease, urinary retention, transient increase (greater than 2 times) serum aminotransferases, injection site reaction, hypoxia, fever
Less than 1%:
- Abdominal pain, accommodation disturbance, anaphylactoid reaction, anaphylaxis, angina pectoris, angioedema, atrial fibrillation, bradycardia, bronchospasm, bullous skin disease, cardiac arrhythmia, cardiorespiratory arrest, chest pain, chills, depression of ST segment on ECG, dyspnea, dystonic reaction, ECG changes, extrapyramidal reaction, flushing, hepatic failure, hiccups, hypersensitivity reaction, hypokalemia, hypotension, ischemic heart disease, laryngeal edema, laryngospasm, liver enzyme disorder, mucosal tissue reaction, myocardial infarction, neuroleptic malignant syndrome, oculogyric crisis, palpitations, positive lymphocyte transformation test, prolonged Q-T interval on ECG (dose-dependent), second-degree atrioventricular block, serotonin syndrome, shock, Stevens-Johnson syndrome, stridor, supraventricular tachycardia, syncope, tachycardia, tonic-clonic seizures, torsades de pointes, toxic epidermal necrolysis, transient blindness, transient blurred vision, urticaria, vascular occlusive events, ventricular premature contractions, ventricular tachycardia, weakness, xerostomia
Cannabinoid Receptor Agonists
There are currently three cannabinoids available on the pharmaceutical market. Dronabinol and Nabilone are both synthetic tetrahydrocannabinol (THC) which the FDA has approved for treatment of chemotherapy-induced nausea and vomiting after the failure of a trial of first-line anti-emetics 3. Both are also FDA approved to treat anorexia associated with AIDS. Recently, the FDA has also approved a cannabidiol (CBD) product to treat seizures associated with Lennox-Gastaut Syndrome and Dravel Syndrome in pediatric patients. However, there is no FDA approved indication for its use as an anti-emetic 4. Independently produced cannabidiol extracts are being used increasingly in the general population for many non-FDA approved indications, frequently including nausea and emesis. In states that have decriminalized marijuana, both in recreational and medicinal contexts, products with varying ratios of cannabidiol and THC are also used for their anti-emetic properties 4.
Cannabinoids and their anti-emetic potential are still under research, and many of the intricacies behind their mechanisms are still unknown or lack universal consensus. Cannabinoids exert their anti-emetic properties through interactions with the centrally located CB1 receptors and 5-HT3 receptors in the dorsal vagal complex, which mediates emesis. A significant contributor to emesis is believed to be via activation of 5-HT3 receptors in the dorsal vagal complex, specifically in the area postrema. Studies in animal models have shown that anandamide, an endogenous cannabinoid, tetrahydrocannabinol (THC), and several synthetic cannabinoids have been shown to have allosteric inhibitor effects on the 5-HT3 receptors in the dorsal vagal complex, providing a mechanism through which emesis control occurs. Animal models have also shown that cannabinoids may work on pre-synaptic CB1 to decrease the release of serotonin into the synapse, thus inhibiting the nausea/emesis response.
Animal models have also indicated cannabidiol to have an allosteric inhibitory effect on the 5-HT3 receptor. However, this effect is thought to be largely through its activation of the 5-HT1A receptor. Through activation of the 5-HT1A receptor, there is ultimately a reduction in the amount of serotonin released, and thus a lower potential to trigger emesis. Cannabidiol is also thought to activate the CB1 receptors in the gastrointestinal tract, through their G-protein-coupled receptor inhibitory effect, leading to decreased gastrointestinal motility. Tetrahydrocannabinol (THC) and cannabidiol were once credited with inhibiting fatty acid amide hydrolase, leading to an increased concentration of anandamide that could exert its anti-emetic properties at a higher intensity, but recent studies into this mechanism have been equivocal 5.
Dronabinol
Dronabinol is an orally available cannabinoid agonist that is used to treat nausea and vomiting and to stimulate appetite, particularly in patients with wasting disease or cachexia. Dronabinol is associated with a minimal rate of serum enzyme elevations during therapy and has not been linked to cases of clinically apparent liver injury with jaundice.
Dronabinol is the main isomer of tetrahydrocannabinol, the principal psychoactive constituent of the marijuana plant (Cannabis sativa). Dronabinol is a partial agonist of the cannabinoid receptors which are found in the central nervous system (CB1 receptor), but also peripherally (largely CB2 receptors). Activation of CB receptors results in effects on appetite, mood, cognition, memory and perception. Dronabinol therapy has been shown to improve in patients with AIDS related weight loss and to decrease the nausea and vomiting associated with cancer chemotherapy. Dronabinol was approved for use in the United States in 1985 and current indications include treatment of anorexia associated with weight loss in patients with AIDS, and prevention of cancer chemotherapy associated nausea and vomiting.
Dronabinol is available as 2.5, 5 and 10 mg capsules generically and under the brand name Marinol. The typical adult oral dose is 2.5 mg twice daily, which can be increased based upon tolerance and effect to a maximum of 20 mg/day.
Dronabinol common side effects include fatigue, somnolence, dizziness, euphoria, abnormal thinking, paranoid reactions, conjunctivitis, diarrhea, nausea, vomiting and abdominal pain. Rare side effects include hallucinations and seizures. Dronabinol is classified as a Schedule III drug, indicating that it has mild potential for physical and psychological dependency and abuse.
Nabilone
Nabilone is an orally available synthetic cannabinoid agonist that is used to treat nausea and vomiting and to stimulate appetite, particularly in patients with wasting disease or cachexia.
Nabilone is a synthetic cannabinoid which is similar to the principal psychoactive constituent of the marijuana plant (Cannabis sativa). Nabilone is a partial agonist of the cannabinoid receptors which are found in the central nervous system (CB1 receptor), but also peripherally (largely CB2 receptors). Activation of CB receptors results in effects on appetite, mood, cognition, memory and perception. Nabilone therapy has been shown to decrease nausea and vomiting in patients undergoing cancer chemotherapy. Nabilone was approved for use in the United States in 1985 and current indications are prevention of cancer chemotherapy associated nausea and vomiting.
Nabilone is available as 1 mg capsules under the brand name Cesamet. The typical adult oral dose is 1 to 2 mg twice daily, the initial dose being 1 to 3 hours before the chemotherapeutic agent is given.
Nabilone common side effects include fatigue, somnolence, dizziness, euphoria, abnormal thinking, paranoid reactions, conjunctivitis, diarrhea, nausea, vomiting and abdominal pain. Rare side effects include hallucinations and seizures. Nabilone is classified as a Schedule II drug, indicating that it has clear potential for physical and psychological dependency and abuse.
Neurokinin 1 Receptor Antagonists
Neurokinin-1 (NK-1) receptor antagonists are a new class of antiemetic drugs which possess unique anxiolytic, antidepressant, and antiemetic properties 6. The discovery of Neurokinin-1 (NK-1) receptor blockers was a crucial point in the prevention of emesis associated with cancer chemotherapy 7.
Neurokinin-1 receptor inhibitors is one of the seven classes of drugs used to suppress nausea and vomiting in patients associated with cancer chemotherapy. The classes of antiemetic drugs are: serotonin (5-HT) receptor anatgonists, dopamine (D) receptor antagonists, histamine (H) receptor antagonists, Neurokinin-1 Receptor (NK-1) antagonists, antimuscarinics, cannabinoids, and corticosteroids. Patients’ development of nausea, vomiting, and vertigo may be due to pregnancy, during cancer chemotherapy, and motion sickness in a car, boat or ship cruise. Some examples of the neurokinin-1 receptor inhibitor drug class are: aprepitant, rolapitant, casopitant, netupitant, maropitant, and fosaprepitant. A new, highly selective NK-1 receptor antagonist, rolapitant, has an exceptionally long plasma half-life, T1/2 (180 hr) and it was FDA approved in September 2015 for the prevention of chemotherapy-induced delayed emesis.
Clinical uses of Neurokinin-1 Receptor Antagonists:
Neurokinin-1 receptor (NK-1) antagonists such as aprepitant, rolapitant, casopitant, fosaprepitant, netupitant, and maropitant are effective to treat postsurgical nausea and vomiting, and cancer chemotherapy-induced nausea and vomiting. Fosaprepitant is administered an intravenous (IV) formulation that is converted within 30 minutes to aprepitant after infusion. Aprepitant is administered orally or IV that is available in two strengths. There is a combination netupitant/palonosetron as an oral formulation. Casopitant is an investigational medication in development. Maropitant is used in the United States as antiemetic in dogs and cats.
Off-Label Uses of Neurokinin-1 Receptor Antagonists:
No off-label uses for Neurokinin-1 receptor antagonist are reported.
Neurokinin 1 receptor antagonists special precautions
Contraindications
Patients taking pimozide should not take neurokinin-1 (NK-1) receptor antagonists. Inhibition of CYP3A4 enzyme by neurokinin-1 (NK-1) receptor antagonists, e.g., aprepitant, could precipitate elevated plasma concentrations of pimozide, which is a CYP3A4 substrate. Drug interaction between pimozide and aprepitant could cause serious or life-threatening reactions, such as QTc prolongation which is a known adverse reaction of pimozide. Neurokinin-1 (NK-1) receptor antagonists, e.g., aprepitant are also contraindicated with the following drugs:
- Astemizole
- Cisapride
- Flibanserin
- Lomitapide
- Pimozide
- Terfenadine
- Ivabradine
Do not take Neurokinin-1 (NK-1) receptor antagonists if pregnant or planning to get pregnant or breastfeeding. During treatment with neurokinin-1 (NK-1) receptor antagonist, if a patient is on birth control medication, the patient should use an additional method of birth control in order to avoid unplanned or unwanted pregnancy. Additionally, the patient should continue with other pregnancy protection for one month after treatment with neurokinin-1 (NK-1) receptor antagonists, e.g., aprepitant.
Neurokinin-1 (NK-1) receptor antagonist is also FDA classified as Pregnancy Class B: Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women.
Pregnancy
Do not take Neurokinin-1 (NK-1) receptor antagonists if pregnant or planning to get pregnant or breastfeeding. During treatment with Neurokinin-1 (NK-1) receptor antagonists, if a patient is on birth control drugs, the patient should use an additional method of birth control in order to avoid unplanned or unwanted pregnancy. Additionally, the patient should continue with other pregnancy protection for one month after treatment with Neurokinin-1 (NK-1) receptor antagonists, e.g., aprepitant. It is FDA classified as Pregnancy Class B.
Drug Interactions
Potential drug interactions can occur with neurokinin-1 (NK-1) receptor antagonists with concomitant medications because of changes in drug metabolism, e.g., CYP3A4 inhibition. This can occur in patients taking prescription, nonprescription medications, as well as herbal and nutritional supplements. The following is a list of potential drug interactions that may require drug monitoring or avoid combination: warfarin (monitor therapy); ivabradine (avoid combination); antifungals such as ketoconazole and itraconazole; lansoprazole; clarithromycin; diltiazem; carbamazepine; HIV protease inhibitors, such as ritonavir, and nelfinavir; hormonal contraceptives; nefazodone; oral steroids such as dexamethasone and methylprednisolone; paroxetine; phenytoin; cancer chemotherapy medications such as docetaxel, etoposide, ifosfamide, imatinib, irinotecan, paclitaxel , tamoxifen, vinblastine, vincristine, and vinorelbine; rifampin; tolbutamide; and troleandomycin; benzodiazepines such as alprazolam, diazepam, midazolam, and triazolam.
Monitoring
- Central Nervous System Effects: There is no known monitoring requirement for neurokinin-1 (NK-1) receptor antagonists.
- Cardiovascular Effects: There is no known monitoring requirement for neurokinin-1 (NK-1) receptor antagonists.
- Respiratory Effects: There is no known monitoring requirement for neurokinin-1 (NK-1) receptor antagonists.
- Additional Monitoring Requirements/Precautions: There is no known additional monitoring requirement for neurokinin-1 (NK-1) receptor antagonists.
Aprepitant
Aprepitant is an orally available antiemetic agent that is used to prevent postoperative or cancer chemotherapy related nausea and vomiting. Aprepitant is a complex molecule with a central morpholine core and two ring carbons and fluorinated phenyl groups. Aprepitant acts as a substance P antagonist blocking the neurokinin 1 (NK1) receptor, which is found in the central nervous system and induces the vomiting reflex when activated by substance P.
Aprepitant has been shown to inhibit both acute and delayed nausea and vomiting associated with cancer chemotherapy and surgical procedures. It appears to act synergistically with serotonin type 3 (5-HT3) receptor blockers. Aprepitant was approved for use in the United States in 2003 and current indications include prevention of postoperative and chemotherapy associated nausea and vomiting. Aprepitant is available as 40, 80 and 125 mg capsules under the brand name Emend. The typical adult oral dose for postoperative nausea and vomiting is 40 mg within four hours of anesthesia induction. The dose for preventing nausea and vomiting due to chemotherapy is usually 125 mg one hour before chemotherapy given in combination with dexamethasone and a 5-HT3 receptor blocker, followed by 80 mg of aprepitant and dexamethasone orally on days 2 and 3.
Fosaprepitant is a prodrug of aprepitant and is available as a solution for injection in single use vials of 115 and 150 mg.
Common side effects of oral aprepitant include fatigue, drowsiness, dizziness, headache, diarrhea and abdominal discomfort.
Rolapitant
Rolapitant is an orally available antiemetic agent that is used to prevent cancer chemotherapy related nausea and vomiting. Rolapitant has been used off label to treat postoperative nausea and vomiting.
Rolapitant is a substance P/neurokinin 1 (NK-1) receptor antagonist which has potent and prolonged antiemetic activity. Rolapitant acts as a substance P antagonist blocking the neurokinin 1 (NK1) receptor, which is found in the central nervous system and induces the vomiting reflex when activated by substance P. Rolapitant has been shown to inhibit both acute and delayed nausea and vomiting associated with cancer chemotherapy and surgical procedures, and appears to act synergistically with serotonin type 3 (5-HT3) receptor blockers. Because of its delayed half-life, rolapitant is particularly potent in preventing delayed (>24 hours after chemotherapy) nausea and vomiting. Rolapitant was approved for use in the United States in 2015 and current indications are in combination with other antiemetic agents in adults for prevention of delayed chemotherapy associated nausea and vomiting.
Rolapitant is available as tablets of 90 mg under the brand name Varubi. The typical adult oral dose is 90 mg on day one of each emetogenic chemotherapy cycle, generally in combination with a 5-HT3 receptor blocker and dexamethasone.
Rolapitant side effects are uncommon, but can include anorexia, headache, neutropenia and dizziness.
Neurokinin 1 receptor antagonists side effects
Adverse Reactions: Severe
- Hives and rash
- Stevens-Johnson Syndrome
Adverse Reactions: Mild
- Sleepiness
- Diarrhea
- Weakness
- Tiredness
- Constipation
- Gas
- Stomach pain
- Heartburn
- Nausea
- Hiccups
- Loss of appetite
- Headache
- Fever
- Itching
- Hair loss
Symptoms of an overdose of Neurokinin-1 (NK-1) receptor antagonists are drowsiness and headache. Aprepitant is associated with serum liver enzyme increases during therapy, but cases of clinically specific liver injury with jaundice have not been categorically linked.
Serum aminotransferase elevations during aprepitant therapy occurred in 6% of treated patients versus 4.3% in controls receiving cancer chemotherapy. The aminotransferase elevations were found to transient, mild to moderate in severity in some cases, and may not be associated with symptoms or jaundice. No convincing cases of clinically significant liver injury attributable to aprepitant have been published in the literature. Therefore, significant liver injury from aprepitant, or fosaprepitant would be exceeding rare.
Miscellaneous
Metoclopramide
Metoclopramide is an oral prokinetic and antiemetic agent used in the therapy of gastroesophageal reflux disease (GERD), gastroparesis and severe chemotherapy induced nausea and vomiting. Metoclopramide is also sometimes used to treat the symptoms of slowed stomach emptying in people who are recovering from certain types of surgery. Ask your doctor about the risks of using this medication to treat your condition.
Metoclopramide is a benzamide (a para-aminobenzoic acid derivative) which acts as a prokinetic agent on the gastrointestinal tract and an antiemetic, particularly in persons with gastrointestinal dysmotility. Its mechanism of action is uncertain; it is a serotonin type 4 (5-HT4) receptor agonist, but also has antagonist activity against vagal and central 5HT3 receptors and dopamine type 2 (D2) receptors. It increases the tone and amplitude of gastric contractions, relaxes the pyloric sphincter and increases peristalsis in the duodenum and jejunum, resulting in improved gastrointestinal motility and accelerated gastric emptying. Metoclopramide was approved for use in the United States in 2005 and is widely used in therapy of nausea, vomiting and gastrointestinal motility disorders including gastroparesis, with more than 6 million prescriptions being filled yearly. Metoclopramide is available as 5 and 10 mg tablets in generic forms and under the brand name Reglan.
Metoclopramide is also available as a liquid solution for oral use and in injectable forms. The typical oral dose for gastroesophageal reflux and gastroparesis in adults is 10 to 15 mg taken 30 minutes before meals. Intravenous and intramuscular formulations are used for therapy of postoperative or chemotherapy induced nausea and vomiting.
If you are taking the orally disintegrating tablet, use dry hands to remove the tablet from the package just before you take your dose. If the tablet breaks or crumbles, dispose of it and remove a new tablet from the package. Gently remove the tablet and immediately place it on the top of your tongue. The tablet will usually dissolve in about one minute and can be swallowed with saliva.
If you are taking metoclopramide to treat the symptoms of slow stomach emptying caused by diabetes, you should know that your symptoms will not improve all at once. You may notice that your nausea improves early in your treatment and continues to improve over the next 3 weeks. Your vomiting and loss of appetite may also improve early in your treatment, but it may take longer for your feeling of fullness to go away.
Continue to take metoclopramide even if you feel well. Do not stop taking metoclopramide without talking to your doctor. You may experience withdrawal symptoms such as dizziness, nervousness, and headaches when you stop taking metoclopramide.
Metoclopramide common side effects include restlessness, drowsiness, dizziness, headache, fatigue, diarrhea and polyuria. Chronic therapy has been associated with episodes of dystonia, oculogyric crises and extrapyramidal syndromes including tardive dyskinesia and Parkinson like symptoms for which it has a “black box” warning.
Taking metoclopramide may cause you to develop a muscle problem called tardive dyskinesia. If you develop tardive dyskinesia, you will move your muscles, especially the muscles in your face in unusual ways. You will not be able to control or stop these movements. Tardive dyskinesia may not go away even after you stop taking metoclopramide. The longer you take metoclopramide, the greater the risk that you will develop tardive dyskinesia. Therefore, your doctor will probably tell you not to take metoclopramide for longer than 12 weeks. The risk that you will develop tardive dyskinesia is also greater if you are taking medications for mental illness, if you have diabetes, or if you are elderly, especially if you are a woman. Call your doctor immediately if you develop any uncontrollable body movements, especially lip smacking, mouth puckering, chewing, frowning, scowling, sticking out your tongue, blinking, eye movements, or shaking arms or legs.
Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide) when you begin treatment with metoclopramide and each time you refill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm) or the manufacturer’s website to obtain the Medication Guide.
Talk to your doctor about the risks of taking metoclopramide.
Metoclopramide special precautions
Before taking metoclopramide:
- tell your doctor and pharmacist if you are allergic to metoclopramide, any other medications, or any of the ingredients in metoclopramide tablets or solution. Ask your doctor or pharmacist or check the Medication Guide for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements and herbal products you are taking or plan to take. Be sure to mention any of the following: acetaminophen (Tylenol, others); antihistamines; aspirin; atropine (in Lonox, in Lomotil); cyclosporine (Gengraf, Neoral, Sandimmune); barbiturates such as pentobarbital (Nembutal), phenobarbital (Luminal), and secobarbital (Seconal); digoxin (Lanoxicaps, Lanoxin); haloperidol (Haldol);insulin; ipratropium (Atrovent); lithium (Eskalith, Lithobid); levodopa (in Sinemet, in Stalevo); medications for anxiety, blood pressure, irritable bowel disease, motion sickness, nausea, Parkinson’s disease, ulcers, or urinary problems; monoamine oxidase (MAO) inhibitors, including isocarboxazid (Marplan), phenelzine (Nardil), selegiline (Eldepryl, Emsam, Zelapar), and tranylcypromine (Parnate); narcotic medications for pain; sedatives; sleeping pills; tetracycline (Bristacycline, Sumycin); or tranquilizers. Your doctor may need to change the doses of your medications or monitor you more carefully for side effects.
- tell your doctor if you have or have ever had blockage, bleeding, or a tear in your stomach or intestines; pheochromocytoma (tumor on a small gland near the kidneys); or seizures. Your doctor will probably tell you not to take metoclopramide.
- tell your doctor if you have or have ever had Parkinson’s disease (PD; a disorder of the nervous system that causes difficulties with movement, muscle control, and balance); high blood pressure; depression; breast cancer; asthma;glucose-6-phosphate dehydrogenase (G-6PD) deficiency (an inherited blood disorder); NADH cytochrome B5 reductase deficiency (an inherited blood disorder); or heart, liver, or kidney disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breastfeeding. If you become pregnant while taking metoclopramide, call your doctor.
- talk to your doctor about the risks and benefits of taking metoclopramide if you are 65 years of age or older. Older adults should not usually take metoclopramide, unless it is used to treat slow stomach emptying, because it is not as safe or effective as other medications that can be used to treat those conditions.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking metoclopramide.
- you should know that this medication may make you drowsy. Do not drive a car or operate machinery until you know how this medication affects you.
- ask your doctor about the safe use of alcohol while you are taking this medication. Alcohol can make the side effects of metoclopramide worse.
Metoclopramide side effects
Metoclopramide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- drowsiness
- excessive tiredness
- weakness
- headache
- dizziness
- diarrhea
- nausea
- vomiting
- breast enlargement or discharge
- missed menstrual period
- decreased sexual ability
- frequent urination
- inability to control urination
Some side effects can be serious. If you experience any of the following symptoms, or those mentioned in the IMPORTANT WARNING section, call your doctor immediately:
- tightening of the muscles, especially in the jaw or neck
- speech problems
- depression
- thinking about harming or killing yourself
- fever
- muscle stiffness
- confusion
- fast, slow, or irregular heartbeat
- sweating
- restlessness
- nervousness or jitteriness
- agitation
- difficulty falling asleep or staying asleep
- pacing
- foot tapping
- slow or stiff movements
- blank facial expression
- uncontrollable shaking of a part of the body
- difficulty keeping your balance
- rash
- hives
- swelling of the eyes, face, lips, tongue, mouth, throat, arms, hands, feet, ankles, or lower legs
- sudden weight gain
- difficulty breathing or swallowing
- high-pitched sounds while breathing
- vision problems
Metoclopramide may cause other side effects. Call your doctor if you have any unusual problems while you are taking metoclopramide.
Symptoms of metoclopramide overdose may include the following:
- drowsiness
- confusion
- seizures
- unusual, uncontrollable movements
- lack of energy
- bluish coloring of the skin
- headache
- shortness of breath
Trimethobenzamide
Trimethobenzamide is an orally available, antiemetic agent used in the therapy of nausea and vomiting associated with medications, after surgery and gastroenteritis (‘stomach flu’; a virus that may cause nausea, vomiting, and diarrhea).
Trimethobenzamide is in a class of medications called antihistamines. Trimethobenzamide may work by decreasing activity in the area of the brain that causes nausea and vomiting. Its mechanism of action is uncertain, but it is believed to act directly on the central chemoreceptor nausea trigger zone of the medulla oblongata of the brain. It has weak antihistaminic activity, but does not appear to act via serotonin or dopamine pathways. Trimethobenzamide was approved for use in the United States in 1974 and is widely used in therapy of nausea, vomiting caused by gastroenteritis, medications and other illnesses.
Trimethobenzamide is available as 300 mg capsules in generic forms and under the brand name Tigan. It is also available as a solution for injection (100 mg/mL). Trimethobenzamide used to be available also as suppositories, but this formulation was discontinued due to lack of efficacy.
The typical oral dose of trimethobenzamide for in adults is 300 mg three or four times a day as needed, usually for short periods only. Intravenous formulations are used for therapy of postoperative nausea and vomiting.
Trimethobenzamide common side effects include drowsiness, dizziness, headache, fatigue, diarrhea and polyuria. Rare side effects include hypersensitivity reactions, disorientation coma and seizures.
In April 2007, the Food and Drug Administration (FDA) announced that suppositories containing trimethobenzamide may no longer be marketed in the United States. The FDA made this decision because trimethobenzamide suppositories have not been shown to work to treat nausea and vomiting. If you are currently using trimethobenzamide suppositories, you should call your doctor or other healthcare professional to talk about switching to another treatment.
Trimethobenzamide special precautions
Before taking trimethobenzamide:
- tell your doctor and pharmacist if you are allergic to trimethobenzamide or any other medications.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements and herbal products you are taking or plan to take. Be sure to mention any of the following: antidepressants; antihistamines; barbiturates such as phenobarbital (Luminal); belladonna alkaloids (Donnatal); medications for anxiety, mental illness, pain and seizures; other medications for nausea and vomiting; sedatives; sleeping pills; and tranquilizers. Your doctor may need to change the doses of your medications or monitor you more carefully for side effects.
tell your doctor if you have Reye’s Syndrome (a condition affecting the brain and liver that can happen after a viral illness), encephalitis (inflammation of the brain), or a high fever, or if you have or have ever had liver disease. If you will be giving trimethobenzamide to a child, also tell the child’s doctor if the child has any of the following symptoms before he or she receives the medication: vomiting, listlessness, drowsiness, confusion, aggression, seizures, yellowing of the skin or eyes, weakness, or flu-like symptoms. Also tell the child’s doctor if the child has not been drinking normally, has had excessive vomiting or diarrhea, or appears dehydrated. - tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking trimethobenzamide, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking trimethobenzamide.
- you should know that this medication may make you drowsy. Do not drive a car or operate machinery until you know how this medication affects you.
- ask your doctor about the safe use of alcoholic beverages while you are taking trimethobenzamide. Alcohol can make the side effects from trimethobenzamide worse.
Trimethobenzamide side effects
Trimethobenzamide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- drowsiness
- dizziness
- headache
- depression
- diarrhea
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- rash
- backward arching of the head, neck, and back
- muscle cramps
- uncontrollable shaking of a part of the body
- slow, jerking movements
- shuffling walk
- slow speech
- confusion
- blurred vision
- yellowing of the skin or eyes
- seizures
- coma (loss of consciousness for a period of time)
Trimethobenzamide may cause other side effects. Call your doctor if you have any unusual problems while you are taking trimethobenzamide.
- Theriot J, Ashurst JV. Antiemetic Serotonin-5-HT3 Receptor Blockers. [Updated 2019 Jan 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513318[↩]
- Ahmed YM, Messiha BA, Abo-Saif AA. Granisetron and carvedilol can protect experimental rats againstadjuvant-induced arthritis. Immunopharmacol Immunotoxicol. 2017 Apr;39(2):97-104[↩]
- Taylor BN, Sauls RS. Cannaboinoid Antiemetic Therapy. [Updated 2019 Mar 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535430[↩]
- Corroon J, Kight R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018;3(1):190-194.[↩][↩]
- Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J, McGregor I, Olver I, Allsop DJ, Gedye C, Kirby AC, Morton RL, Fox P, Clarke S, Briscoe K, Aghmesheh M, Wong N, Walsh A, Hahn C, Grimison P. Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ Open. 2018 Sep 12;8(9):e020745[↩]
- Ibrahim MA, Preuss CV. Antiemetic Neurokinin-1 Receptor Blockers. [Updated 2019 Mar 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470394[↩]
- IV aprepitant (Cinvanti) for chemotherapy-induced nausea and vomiting. Med Lett Drugs Ther. 2018 Dec 03;60(1561):e200-e201[↩]