Butterbur
Butterbur is derived from the leaves and roots of Petasites hybridus a member of the Asteraceae family of plants that grows in Europe, northern Africa, south western Asia and North America 1. So far 18 species of Petasites have been identified 2. The name, butterbur, is attributed to the traditional use of its large leaves to wrap butter in warm weather to keep the butter clean and to help it last. Butterbur has large leaves, reaching 90 cm across. They appear in about March to April, around about the same time the plant produces pale pink to purplish spikes of flowers. Butterbur leaves resemble rhubarb leaves, perhaps slightly more heart-shaped. The scientific name for the butterbur genus Petasites originates from the Greek word “petasos” the name for a felt, leather or straw hat with a wide brim and worn by farmers in ancient Greece. Butterbur leaves do feel “felty” and would be large enough for a sun hat.
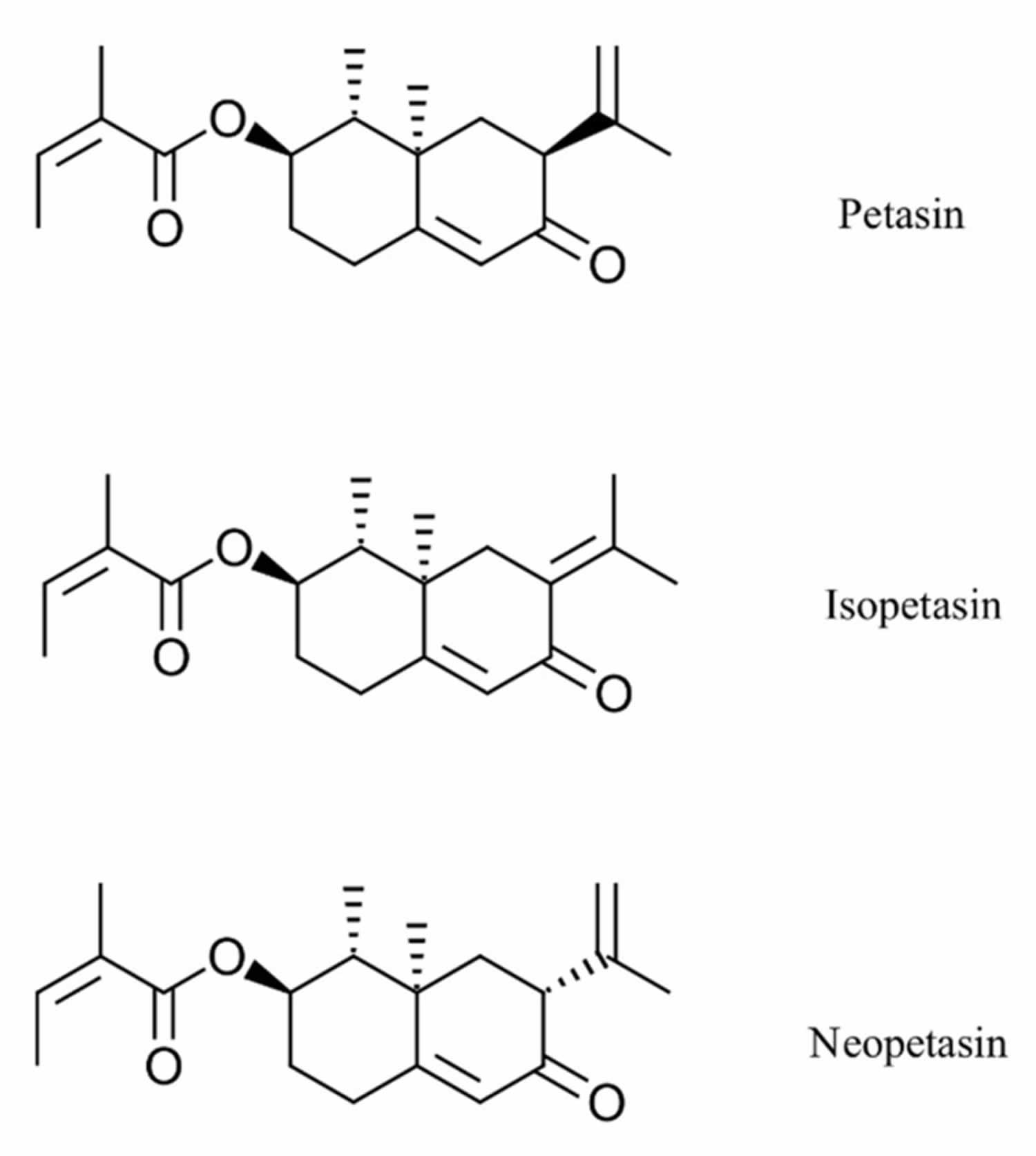
The leaves and roots of common butterbur (Petasites hybridus) contain a mixture of eremophilan type sesquiterpenes (i.e., esters of petasol and angelic acid) called “petasins”, that are thought to be the pharmacologically active ingredients of butterbur and may have an anti-inflammatory effect (see Figure 2) 3. Extracts of butterbur have been used traditionally in bronchial asthma, smooth muscle spasms, spasms of the gastrointestinal tract, stomach pain, allergic rhinitis, asthma, migraine and pain associated with tension headaches 4, 5, 6, 7, 8. Studies have shown that petasines inhibit the biosynthesis of leukotrienes, which may be associated with antispasmodic activity and anti-inflammatory action in type 1 hypersensitivity 9, 10, 11, 12. Type 1 hypersensitivity reaction is also known as an immediate allergic reaction and involves immunoglobulin E (IgE) mediated release of antibodies against the soluble antigen 13. Type 1 hypersensitivity reaction is where your body reacts to a stimulus, or allergen, leading to swelling of the airways, and cardiovascular and other organ system effects 14. Type 1 hypersensitivity causes some of the most common allergies such as dust mites, pollens, foods, and animal dander and anaphylaxis.
Butterbur appears to help reduce the frequency of migraines in adults and children 15, 16, 17, 18. In 2012, the American Academy of Neurology recommended its use for preventing migraines 19, 20. One major area of concern regarding butterbur safety is with pyrrolizidine alkaloids which are commonly found in the butterbur plant 21. Pyrrolizidine alkaloids can cause liver damage, lung damage, cancer, and blood clots. Fortunately, the commercial preparation process of butterbur typically removes these substances. Therefore, it is recommended for consumers of butterbur only buy brands that are free of alkaloids and other plant carcinogens 1.
Butterbur is an extract of the roots of the butterbur bush and the extract has to be processed carefully to eliminate harmful pyrrolizidine alkaloids that occur naturally in the plant. Butterbur preparations processed to remove pyrrolizidine alkaloids do not appear to cause liver injury, either in the form of serum enzyme elevations during treatment or clinically apparent acute liver injury. However, recent cases of clinically apparent liver injury have been reported with use of several commercial preparations of butterbur, suspected to be due to residual pyrrolizidine alkaloid contamination.
Many butterbur extracts or even drug powders are marketed in various countries, especially in the United States. However, those extracts are not comparable among each other. Only one product Petadolex a proprietary CO2-butterbur extract made from the root of Petasites hybridus was extensively studied in preclinical and clinical studies in migraine to demonstrate therapeutic effectiveness and safety. Therefore, published results of Petadolex are not transferable to any other butterbur extract 20. This special butterbur extract is mainly available in the United States and Canada and is the only butterbur extract in the market with a proven published clinical efficacy in placebo-controlled randomized clinical trials of migraine prevention 22, 23, 24. There is only one more well-characterized butterbur extract called Butterbur extract Ze339 with published preclinical and clinical data available among the numerous commercial butterbur products. Butterbur extract Ze339 is used for seasonal rhinitis and is made from leaves of Petasites hybridus 25, 26, 27, 28.
Given the many butterbur brands and uncertainties regarding their petasin content, the need for labeling of products remain an important topic. Furthermore, manufacturers should be requested to inform patients on potential harmful ingredients such as pyrrolizidine alkaloids. Based on information provided by the manufacturer as well as independent investigations Petadolex is essentially free of pyrrolizidine alkaloid contaminants. In regards to butterbur safety, the spontaneous reports on liver toxicity events in relation to Petadolex use have been evaluated (N = 48 cases over a period of > 30 years and an estimated 2.6 million patient month exposure) 29. The causality assessment showed most cases to be confounded by liver toxic co-medications especially during periods of pain. In clinical trials with migraine patients there was not a single case with clinically relevant liver function abnormalities.
Figure 1. Butterbur (Petasites hybridus)
Figure 2. Petasins chemical structure
Footnote: Chemical structures of the main petasins from butterbur (Petasites hybridus)
[Source 30 ]How does butterbur work?
Petasins are the pharmacologically active ingredients of butterbur and of therapeutic benefit in the treatment of migraine and tension headaches (Figure 2) 31. Petadolex, a special butterbur extract from Petasites hybridus, is a natural herbal product and the only butterbur extract with proven clinical efficacy in migraine prevention.
Petadolex (produced by Germany-based Weber & Weber) is produced from the rhizome (a rootlike horizontal underground plant stem) of common butterbur (Petasites hybridus) and is the only butterbur extract that was extensively studied for safety and clinical effectiveness in the preventive treatment of migraine 31. Petadolex was introduced 1972 in Germany and 1998 in the USA, and pharmacological research identified petasins as active ingredients.
Petadolex duration of treatment ranged from 3 to 4 months. The pivotal study by Lipton et al 32 evaluated 4 months of treatment with Petadolex in doses of 50 mg twice daily or 75 mg twice daily in a randomized, double-blind, placebo-controlled trial. The study included 233 patients with episodic migraine. Compared with placebo, the frequency of migraine attacks decreased in study participants receiving a daily dose of 150 mg Petadolex 32. However, the difference from placebo was not statistically significant for the 100 mg dose (48% reduction for 150 mg, 36% reduction for 100 mg and 26% in the placebo group) 32. The proportion of patients with a ≥50% reduction in attack frequency at 4 months was 68% for patients receiving Petadolex at 150 mg, 56% in the 100 mg arm and 49% for the placebo arm 32.
A second study, designed as a randomized, group-parallel, placebo-controlled, double-blind trial, investigated the therapeutic efficacy of Petadolex in 60 patients with episodic migraine 33. The patients received Petadolex at a dosage of 2 capsules twice daily over 12 weeks (each capsule contains 25 mg Petadolex). Inclusion criteria were a minimum of three attacks per month within the last 3 month prior to study enrolment in addition to a minimum of two attacks in the run-in phase, i.e., 4 weeks without trial medication. Subsequently, the patients were on butterbur (100 mg butterbur extract daily) for 12 weeks, and reductions in migraine attacks per 4 weeks defined the primary efficacy variable. Additionally, the patients reported the number of migraine days and the duration and intensity of migraine attacks. The clinical investigators reported high efficacy and excellent tolerance 33, and an independent reanalysis of the data by Diener et al 34 revealed the 100 mg dose of Petadolex to be effective. The ≥50% responder rate for migraine frequency was significantly higher in the Petadolex study arm as compared to the placebo group (45 vs. 15%).
A third study, designed as prospective, randomized, partly double-blinded, placebo-controlled and parallel-group, investigated the efficacy of Petadolex in 63 elementary school children with episodic migraine 35. The inclusion criteria were ages between 8 and 12 years, initial onset of migraine at least 1 year before study enrolment and an average of ≥2 attacks/month for the last 3 month. The study evaluated the efficacy of 50 to 150 mg daily doses (N = 19), music therapy (N = 20) or placebo (N = 19) for 12 weeks 35. Only music for therapy was superior to placebo, while during the follow-up period both music therapy and butterbur extract were superior to placebo 35. All groups showed a significant reduction in migraine frequency relative to baseline.
Mechanism of action for the treatment of Migraines
Petasites inhibit the opening of L-type voltage-gated calcium channels, decreasing vasoconstriction of vessels and excitation of neurons 17. The herb’s active components include Sesquiterpenes (Petasin and Isopetasin) which have been found to exhibit anti-inflammatory effects through the inhibition of COX-2. This leads to decreased leukotriene synthesis and prostaglandin E2 release 21.
Mechanism of action for the treatment of Asthma and Allergic Rhinitis
Petatewalide B, a derivative of Petasites, has also been found to exhibit anti-allergic activities 36. Specifically, the compound inhibits the activation of ß-hexosaminidase in RBL-2H3 mast cells. Petatewalide B also inhibits nitric oxide synthase, which decreases nitric oxide production in mouse peritoneal macrophages. The compound also decreases the concentration of eosinophils, macrophages, and lymphocytes in mouse bronchoalveolar lavage fluid 36.
Mechanism of action for the treatment of Alzheimer Disease
Derivatives of Petasites have been found to have antioxidant activity; the DPPH free radical-scavenging value and ferric ion reducing potential were both increased 37. In one study examining the cognitive effects of butterbur, the herb significantly decreased levels of reactive oxygen species (ROS) levels and increased the viability of HT22 mouse neuronal cells exposed to Aß plaques. The study found that the mechanism for the former was related to increased expression of heme oxygenase-1 (HO-1), NAD(P)H quinine dehydrogenase 1, and cyclic AMP response element-binding protein (CREB), which are all involved in antioxidant pathways 38. Similarly, in Aß plaque-injected mice, the administration of butterbur resulted in significantly decreased cyclic AMP response element-binding protein (CREB) expression in the dentate gyrus 38.
Butterbur health benefits
There are evidence suggesting that butterbur (Petasites) is effective in the treatment of migraine headaches and hay fever or seasonal allergies (seasonal rhinitis), however, it is not specifically approved by the U.S. Food and Drug Administration (FDA) for these uses in the United States 39, 26, 27, 40. Butterbur appears to help reduce the frequency of migraines in adults and children 41, 42, 43, 20.
Many butterbur extracts or even drug powders are marketed in various countries, especially in the United States. However, those extracts are not comparable among each other. Only one product Petadolex (produced by Germany-based Weber & Weber) a proprietary CO2-butterbur extract made from the root of Petasites hybridus was extensively studied in preclinical and clinical studies in migraine to demonstrate therapeutic effectiveness and safety. Therefore, published results of Petadolex are not transferable to any other butterbur extract 20. This special butterbur extract is mainly available in the United States and Canada and is the only butterbur extract in the market with a proven published clinical efficacy in placebo-controlled randomized clinical trials of migraine prevention 22, 23, 24. There is only one more well-characterized butterbur extract called Butterbur extract Ze339 with published preclinical and clinical data available among the numerous commercial butterbur products. Butterbur extract Ze339 is used for seasonal rhinitis and is made from leaves of Petasites hybridus 25, 26, 27, 28.
A specific proprietary butterbur extract called Petadolex (produced by Germany-based Weber & Weber), has been studied as a migraine preventative in several randomized clinical trials 33, 22. The first placebo-controlled, randomized clinical trial with butterbur extract Petadolex was conducted in 2001 33. A group of 33 adult subjects given a dose of 50 mg oral twice daily demonstrated a decrease in migraine frequency from a baseline of 3.4 per month to 1.8 per month after 12 weeks of treatment 33. No significant adverse effects, including elevation of liver function tests, were reported during the treatment period 33, 22. This was followed by a larger placebo-controlled randomized clinical trial in 2004 in which 233 adult subjects with episodic migraine were randomized to doses of 50 mg oral twice daily or 75 mg oral twice daily of Petadolex versus placebo over a 16-week treatment period 23. The 75 mg oral twice daily group saw a reduction of migraine frequency of 45% 23. The 50 mg oral twice daily group had a reduction of 32% and the placebo group 28%, both of which were not statistically significant 23. No significant adverse effects, including elevation of liver function tests, were reported during the treatment period, and the authors noted burping as the only unique adverse effect 23. They concluded that Petadolex at 75 mg oral twice daily was more effective than placebo in migraine prevention 23.
These two studies were together evaluated as part of the American Academy of Neurology evidence-based guideline update for non-steroidal anti-inflammatory drugs (NSAIDs) and other complementary treatments for episodic migraine prevention in adults in 2012 19. The review categorized the studies as class 1 trials, and Petasites received Level A classification, establishing it as effective for migraine prevention 19. In 2012, the American Academy of Neurology recommended the use of butterbur (Petasites) for preventing migraines 44, 45, 19. Petasites is a purified extract from the butterbur plant. However, the American Academy of Neurology has stopped recommending butterbur (Petasites) in 2015 because of serious concerns about possible liver damage.
Butterbur is available in a variety of formulations and the typical oral dosage is 100 to 150 mg per day in 2 to 3 divided doses. Side effects of butterbur are uncommon and mild, and include gastrointestinal upset, eructation, nausea, diarrhea, headache, dizziness, increased bleeding tendency and rash. In clinical trials, both serious and common side effects were often no more frequent with butterbur than placebo.
Migraine in Adults
The American Headache Society and the American Academy of Neurology: 2012 evidence-based guideline update indicated that Petasites (butterbur) demonstrates effectiveness for migraine prevention and is a valid option for patients with migraine to reduce both the severity and frequency of migraine attacks (Level A) 19. However, the American Academy of Neurology has stopped recommending butterbur (Petasites) in 2015 because of serious concerns about possible liver damage.
Butterbur (leaves of Petasites hybridus) has been found to be effective in the prevention of adult migraines in multiple studies 46. Based on these trials, the American Headache Society gave the herb a level A recommendation and declared it to be effective in the prevention of migraine headaches. Similarly, the Canadian Headache Society guidelines give butterbur a strong recommendation for use in migraine prevention 47. Although the American Academy of Neurology recommended butterbur, it has retracted its current guidelines on it 47. In the United Kingdom and Germany, butterbur is not authorized for official use due to safety concerns 20, 47. In one randomized parallel-group study from 2004, the group given butterbur had a response rate of 45%, whereas the group who used placebo had a response rate of 15% 1. In another randomized controlled trial from 2004, butterbur reduced the frequency of migraines by 48% in the experimental group, whereas the placebo reduced the frequency by 26% in the control group 1.
Migraine in Children
Similar to adult migraines, there is evidence for Petasites in the prevention of migraines in children 17. In one randomized controlled trial from 2005, butterbur reduced the frequency of attacks by at least 50% in 77% of the pediatric experimental group 46. In another randomized controlled trial from 2008, butterbur reduced the frequency of attacks by 59% in the experimental group, whereas the placebo reduced the frequency of attacks by 31% in the control group 48.
Allergic Rhinitis and Asthma
Butterbur has been used in countries in Asia as a herbal treatment of asthma and allergic diseases 49, 36. There is a limited body of evidence from randomized controlled trials that butterbur may be useful as a therapy for asthma and allergic rhinitis but not as effective as preventing migraine 46, 50.
Given its anti-inflammatory properties butterbur extracts were also evaluated in conditions of seasonal allergic rhinitis (hay fever). A clinical study investigated the effectiveness of butterbur extracts on nasal provocation testing in seasonal allergic rhinitis (hay fever) 51. Twenty patients with grass-pollen-sensitized seasonal allergic rhinitis were randomized in a double-blind, cross-over design to receive either 50 mg butterbur extract or placebo twice daily for 2 weeks during the grass pollen season 51. The study participants were challenged with a single dose of 400 mg/mL adenosine monophosphate (AMP), and the spontaneous recovery following AMP challenge defined the primary outcome. In this study butterbur significantly reduced the peak nasal inspiratory flow from baseline following AMP challenge 51. Therefore, butterbur exhibited protection against adenosine monophosphate (AMP)-induced nasal responsiveness during the grass pollen season in sensitized patients 51.
Additionally, sixteen patients with perennial allergic rhinitis and house dust mite sensitization were randomized in a double-blind, cross-over study 52. The study evaluated 50 mg butterbur bid, fexofenadine 180 mg once daily and placebo for 1 week 52. Both butterbur and fexofenadine were equally effective in attenuating the nasal response to adenosine monophosphate (AMP) and improved nasal symptoms significantly 52.
Moreover, a prospective, randomized, double-blind, placebo-controlled, parallel-group compared the efficacy of a high (N = 60 patients) and low dose (N = 65 patients) butterbur leaf extract Ze339 to placebo (N = 61 patients) 53. Established diagnostic criteria for intermittent allergic rhinitis were confirmed by skin allergy tests, and the extract was reported to be an effective treatment for intermittent allergic rhinitis and was well tolerated 53. However, butterbur failed to demonstrate clinical efficacy in intermittent allergic rhinitis in a smaller trial of 35 patients 54. Here, the patients received 50 mg butterbur, bid or placebo for 2 weeks, and the primary outcome variables were peak nasal inspiratory flow, nasal and eye symptoms and rhinoconjunctivitis-specific quality-of-life score 54. In a larger clinical trial of 330 patients designed as prospective, randomized, double-blind, parallel group comparison of butterbur leaf extract (butterbur leaf extract Ze339; 8 mg total petasin; one tablet thrice-daily) against fexofenadine (Telfast 180, one tablet once-daily) and placebo clearly established butterbur and fexofenadine as equally effective 55. Finally, butterbur conferred anti-inflammatory activity in atopic asthmatic patients maintained on inhaled corticosteroids 56.
Alzheimer’s Disease
Studies have found that butterbur may potentially be effective in treating Alzheimer’s disease due to its neuroprotective effect. However, only studies involving test tubes and animals models have been performed 38.
Anti-inflammatory Effects
The anti-inflammatory activity of butterbur extracts is attributed to its sesquiterpene ester components, such as petasin and isopetasin 31. Butterbur decreases the production of the inflammatory mediators prostaglandin E2 (PGE2), leukotriene B4 (LTB4) and cysteinyl-leukotrienes (LTs), in animal and human cellular system, as well as purified enzyme preparations 57. In addition, asthmatic patients were shown to benefit from butterbur’s anti-inflammatory activity whilst on steroids 56.
Petasin blocks intracellular calcium influx into neutrophils and eosinophils and inhibits leukotriene biosynthesis 57. Petasin, isopetasin and neopetasin inhibit leukotriene biosynthesis in human blood cells 58. Petasin blocks eosinophil cationic protein release from eosinophils and platelet-activating factor- and/ or complement factor 5a-induced increases in intracellular Ca2+. Petasin also inhibits phospholipase A2 activity and 5-lipoxygenase translocation from the cytosol to the nucleus in stimulated eosinophils, therefore suggesting that petasins may block different intracellular signaling molecules 57. This leads to the notion that petasins may block earlier signaling events initiated by G protein-coupled receptors in granulocytes, including phospholipase C activity 57. Consistent with the importance of the inflammatory response in migraine attacks, phospholipase C activity is increased in cerebrospinal fluid of migraine patients 59.
Additionally, butterbur inhibits cyclooxygenase-2 (COX-2) activity 60. The inhibition is independent of the petasin and isopetasin content. This suggests that the therapeutic effect of butterbur on cyclooxygenase inhibition may not result from a single constituent of the extract alone 60.
Anti-inflammatory effects of the butterbur extract Petadolex® were also demonstrated in various test tube studies, e.g., swine leucocytes 61, 62, with inhibition of 5-lipoxygenase and selective inhibition of COX-2 61, 63. Petasites root extracts inhibited dose-dependently lipopolysaccharide-induced PGE2 release and p42/44 MAPK activation in primary rat microglial cells. Repression of cyclooxygenase-2 (COX-2) expression was also confirmed at the mRNA and protein level 60. Petadolex inhibited the laurine sulfate-induced vascularization in the chicken embryo HET-CAM assay, again supporting an anti-inflammatory activity 64.
A murine macrophage study showed that petasin may antagonize the inhibition of isopetasin and oxopetasin on leukotriene E4 (LTE4) production 65.
Taken together, butterbur extracts are anti-inflammatory, and the various studies provide reasons for its traditional use 31.
Is butterbur safe?
Severe liver damage may be associated with butterbur use, but the evidence is unclear 1, 20, 66. From the World Health Organization’s Vigibase, one study reported 40 cases of liver damage, including two liver transplants associated with the use of Petasites formulations 47. However, these cases may be the result of the use of butterbur contaminated with pyrrolizidine alkaloids. Another article reported detecting levels of toxic alkaloids in seven out of 21 commercially available compounds 47. Therefore, at least some brands are inadequately removing pyrrolizidine alkaloids from their butterbur formulations 66. The mechanism by which some preparations of butterbur might cause liver injury is not known but is likely due to a contaminant or mislabeling of the product 66. Butterbur-drug interactions have not been defined 66. While pyrrolizidine alkaloids are mentioned as the possible cause of liver injury associated with butterbur use, the clinical features of cases occurring during treatment suggested unusual liver injury rather than direct sinusoidal cell damage.
Several studies, including a few studies of children and adolescents, have reported that pyrrolizidine alkaloid-free butterbur products seem to be safe when taken by mouth in recommended doses for up to 16 weeks. However, some products claiming to be pyrrolizidine alkaloid-free may not in fact be. For example, Petadolex is marketed as a pyrrolizidine alkaloid-free butterbur product, but it has been associated with liver damage in some people, suggesting that it may have had pyrrolizidine alkaloids. Also, the safety of longer-term use of butterbur has not been established.
Twelve reports of suspected adverse drug reactions involving the hepatobiliary system were reported in clinical trials of the special butterbur root extract Petadolex with a total of 397 patients 22, 23, 24. All of these cases concerned increased liver enzymes.
Three cases from the single-center double-blind randomized placebo-controlled trial with 60 migraine patients were categorized as not clinically relevant since liver enzyme elevations were well below a two-fold increase over the reference values 22. In the multicenter, double-blind, randomized placebo-controlled trial study of 229 patients, nine cases of increased liver enzymes were documented. Seven of them were assessed as possibly and two as unlikely related to the special butterbur root extract 23. No liver-related adverse events occurred in the open trial, with 108 children suffering from migraine. Liver enzymes were not analyzed in this noninterventional study 24.
Between 1972 and November 2015, a total number of 233 suspected adverse drug reactions have been reported outside of clinical trials 67; 198 of these spontaneous reports came from German patients/doctors, 20 reports from Switzerland, five reports from the United Kingdom, one report from Austria, and nine from the United States 67. Suspected adverse drug reactions reported with the highest frequency were mild gastrointestinal discomforts, such as nausea, burping or belching, and stomach pain 20. There were 50 nonserious suspected adverse drug reactions involving the hepatobiliary system reported. Of these cases, two were assessed as probably related to the butterbur root extract, 15 as possibly related, seven as unlikely related, and four as not being causally related to the butterbur root extract; 22 suspected adverse drug reactions could not be classified because requested data were not obtained by the physicians after repeated requests 20.
Of the 233 suspected adverse drug reactions reported outside of clinical trials, 12 were serious 20. Ten of them concerned the hepatobiliary system. The other two serious cases were believed to be unrelated to the proprietary butterbur extract: one was a case of pancreatitis and the other case was a status asthmaticus. Reports of suspected serious adverse hepatic drug reactions came from Germany (eight cases between 1998 and 2006), UK (one case in 2004) and Austria (one case in 2011) 20.
Nine of the 10 reported serious cases involving the hepatobiliary system have been previously evaluated by an independent expert group on 28 July 2008 20. The evaluation performed according to the WHO-UMC criteria, and results have been published by Evers 68. In the nine serious hepatobiliary suspected adverse drug reactions, only one reversible case of hepatitis with cholangitis was assessed according to WHO-UMC as likely related to the intake of the butterbur root extract Petadolex 20.
All 10 serious hepatobiliary suspected adverse drug reactions were reassessed in a recent publication using the RUCAM (Roussel Uclaf Causality Assessment Method) as updated in 2016 69. In contrast to the WHO-UMC method, the RUCAM test (previously called CIOMS) was developed and validated for drug-induced liver injury (DILI) or herb-induced liver injury (HILI) and does consider hepatotoxicity-related characteristics 70. Based on individual narratives, causality assessment by RUCAM including a search for alternative causes, the clinical assessment, confounding variables, and comparative evaluations, there is no evidence of liver injury by the butterbur root extract. Only two cases were found to be possibly related to Petadolex, five cases were found to be unlikely related, and three cases were not linked to the butterbur root extract at all. None of the cases was probably related to the butterbur root extract. Two cases of liver transplantations occurred in the 10 serious adverse events. One case was rated as unlikely by RUCAM as well as WHO-UMC and the other case was rated as unlikely by RUCAM and possible by WHO-UMC.
The authors of the article state that idiosyncratic herb-induced liver injury characteristics were not applicable to the 10 liver cases, as RUCAM-based causality for the butterbur root extract does not exist. There was also no evidence of an intrinsic herb-induced liver injury by the butterbur root extract because dose dependency was not evident; the liver diseases occurred with short- and long-term use 69. The reports of the 10 suspected cases of hepatotoxicity were mainly restricted to Germany. Not a single case of liver damage was reported in the United States, Canada, or Japan.
In summary, it is unclear if liver damage is due to alkaloids in the formulation or the butterbur itself.
To evaluate the liver toxic effects of butterbur more clearly, a future study can collect data on brands that claim to be alkaloid-free by thoroughly reviewing the literature and the Periodic Update Safety Report. The data can then be analyzed with the Roussel Uclaf Causality Assessment Method test to determine if butterbur carries an association with liver damage 71. Butterbur use is contraindicated in patients using anticholinergic medications. Also, there have not been studies establishing safety criteria in children under six or pregnant or lactating women. Therefore, butterbur use is not recommended in these groups 21. If you are considering using butterbur, discuss its risks and benefits with your health care provider. Furthermore, if you are taking butterbur and you develop unexplained symptoms such as fatigue, nausea, abdominal pain or dark urine, you should have routine liver function tests and discontinue the use of the herb if there are any abnormalities.
Pyrrolizidine alkaloids
Pyrrolizidine alkaloids are liver toxic substances. European Food Safety Authority considers a threshold of 0.007 μg pyrrolizidine alkaloid/kg body weight per day as acceptable exposure via foodstuffs based on lifelong exposure 72, 73, 74. This threshold corresponds to 0.35 μg pyrrolizidine alkaloid daily intake for a person weighing 50 kg. There are no publications issued by the U.S. Food and Drug Administration (FDA) limiting pyrrolizidine alkaloid intake. The National Center for Natural Products Research analyzed 21 commercial butterbur products available in the United States employing the latest chemical analysis techniques for the detection of petasins and pyrrolizidine alkaloids. The analysis included the proprietary butterbur root extract Petadolex 75. Several different companies market the proprietary butterbur root extract from the original manufacturer under different brand names. Products containing butterbur powder or an extract that is not identical or comparable with Petadolex are also sold. In 7 of the 21 butterbur dietary supplements analyzed, the amount of petasin was found to be within limits of label claim, and with no detectable levels of pyrrolizidine alkaloids. Three of those seven products (labeled #9807, #9808, and #9819) in the publication by Avula et al 75 were provided by the manufacturer and contain the butterbur extract Petadolex. Six products tested contained no detectable amounts of petasins at all. In seven products, pyrrolizidine alkaloid levels between 0.1 and 4.48 μg per tablet, capsule, or gel content were detected, and one product was a liquid with 8.43 μg of pyrrolizidine alkaloids per milliliter.
Toxicity studies in animals
A 6-month toxicity study of the butterbur root extract Petadolex was performed in a study with 40 rats per group 76. Dose ranges were from 45 mg/kg to 270 mg/kg, corresponding to a 10- to 60-fold greater amount of the maximal clinical human dose. Increases in alkaline phosphatase (ALP), gamma-glutamyl-transferase (GGT), and bilirubin were noticed early in the treatment, but also in the vehicle group at nearly the same frequency in all dose groups. All levels in all dose and vehicle groups returned to normal levels after 13 weeks. This normalization of gamma-glutamyl-transferase indicates an adaptive metabolic process rather than a toxic effect. aspartate aminotransferase (AST) and alanine aminotransferase (ALT) did not change during the 6-month treatment. Histological findings showed bile duct dilatations and biliary duct hyperplasia without liver cell damage in the two highest dose groups, which would be an expected response to the metabolic effects of large amounts of the very dense and lipophilic butterbur root extract Petadolex.
The special butterbur root extracts was recently tested in mini-pigs for 28 days 67. A butterbur extract, which contained only trace amounts of petasins, was used as control to study if petasins had a toxic effect on the animals. The extracts were applied at a dose of 720 mg/kg body weight, which corresponds to a human equivalent dose of 654 mg/kg. Since the maximal clinical dose is 3 mg/kg per day, the test dose represents a 218-fold increase beyond the clinical dose. No detrimental effect of petasins could be detected. All laboratory parameters, including hematology and clinical chemistry including liver parameters such as bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), gamma-glutamyl-transferase (GGT), glutamate dehydrogenase (GLDH), and 5-nucleotidase, showed normal values in all test groups at the end of the study at day 28 20. Histopathology of the liver did not reveal any drug-related changes suggesting liver toxicity, such as the formation of vacuoles 20.
Regulatory issues of the butterbur root extract Petadolex
In 1988 the manufacturer of Petadolex changed the extraction procedure from the solvent methylene chloride to CO2 to improve the removal of pyrrolizidine alkaloids 31, 20. Note, all published studies establishing clinical efficacy of Petadolex were performed with the CO2 extract as this is the sole extract marketed in the USA and Canada 31, 20. In 2002, the German regulatory authority BfArM assessed this modification in the extraction protocol and concluded that this change had altered the composition of active ingredients 31, 20. The German regulatory authority considered Petadolex not to be the original one for which market authorization was granted, and Petadolex was removed from the market. However, pyrrolizidine alkaloids do not contribute to the pharmacological activity of the product but are a safety concern 31, 20. Given that the specification of the active ingredient remained the same the decision to withdraw market authorization is perplexing. In addition, the manufacturer of Petadolex® applied for a new traditional license of the UK Medicines and Healthcare products Regulatory Agency (MHRA) which was declined because of safety concerns related to the distribution by general sale outlets. A product license for Petadolex was issued by Health Canada in 2021.
A further point of considerable concern is the lack of product label specifications among the different butterbur brands. Specifically, provisions of federal law in the US require nutrition labeling only but information on the amount of active ingredient is not a legal requirement nor are manufacturers required to supply information on potential hazardous impurities and their quantities. Importantly, an analysis of 21 commercial butterbur products by the University of Mississippi, National Center for Natural Products Research, revealed that the majority of these products do not specify the quantity of their ingredients but contain pyrrolizidine alkaloids which is an important safety concern 77. Therefore, regulators should request manufacturers to provide the specifications of toxic ingredients in butterbur extracts.
- Din L, Lui F. Butterbur. [Updated 2023 Jun 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537160[↩][↩][↩][↩][↩]
- Tys J, Szopa A, Lalak J, Chmielewska M, Serefko A, Poleszak E, et al.. botanical and pharmacological description of petasites species. Curr Issues Pharm Med Sci. (2015) 28:151–4. 10.1515/cipms-2015-0062[↩]
- Aydın AA, Zerbes V, Parlar H, Letzel T. The medical plant butterbur (Petasites): analytical and physiological (re)view. J Pharm Biomed Anal. 2013 Mar 5;75:220-9. doi: 10.1016/j.jpba.2012.11.028[↩]
- D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. (2014) 35:135–40. 10.1007/s10072-014-1757-x[↩]
- Sutherland A, Sweet BV. Butterbur: an alternative therapy for migraine prevention. Am J Health Syst Pharm. (2010) 67:705–11. 10.2146/ajhp090136[↩]
- Evans RW, Taylor FR. “Natural” or alternative medications for migraine prevention. Headache. (2006) 46:1012–8. 10.1111/j.1526-4610.2006.00473.x[↩]
- Laccourreye O, Werner A, Laccourreye L, Bonfils P. Benefits, pitfalls and risks of phytotherapy in clinical practice in otorhinolaryngology. Eur Ann Otorhinolaryngol Head Neck Dis. (2017) 134:95–9. 10.1016/j.anorl.2016.11.001[↩]
- Malone M, Tsai G. The evidence for herbal and botanical remedies, Part 2. J Fam Pract. 2018 Jan;67(1):E1-E9. https://www.mdedge.com/jfponline/article/155078/mental-health/evidence-herbal-and-botanical-remedies-part-2[↩]
- Meier B, Meier-Liebi M. Drogenmonographie Petasites. In: Hänsel R, Keller K, Rimpler H, Schneider G, eds. Hagers Handbuch der pharmazeutischen Praxis. 5th ed. Berlin: Springer Verlag, 1994:81-105.[↩]
- Brune K, Bickel D, Peskar BA. Gastro-protective effects by extracts of Petasites hybridus: the role of inhibition of peptido-leukotriene synthesis. Planta Med. 1993 Dec;59(6):494-6. doi: 10.1055/s-2006-959746[↩]
- Bickel D, Röder T, Bestmann HJ, Brune K. Identification and characterization of inhibitors of peptido-leukotriene-synthesis from Petasites hybridus. Planta Med. 1994 Aug;60(4):318-22. doi: 10.1055/s-2006-959492[↩]
- Thomet OA, Wiesmann UN, Schapowal A, Bizer C, Simon HU. Role of petasin in the potential anti-inflammatory activity of a plant extract of petasites hybridus. Biochem Pharmacol. 2001 Apr 15;61(8):1041-7. doi: 10.1016/s0006-2952(01)00552-4[↩]
- Abbas M, Moussa M, Akel H. Type I Hypersensitivity Reaction. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560561[↩]
- Allergies explained. https://dermnetnz.org/topics/allergies-explained[↩]
- Butterbur. https://www.nccih.nih.gov/health/butterbur[↩]
- Malone M, Tsai G. The evidence for herbal and botanical remedies, Part 1. J Fam Pract. 2018 Jan;67(1):10-16. https://www.mdedge.com/familymedicine/article/155077/pain/evidence-herbal-and-botanical-remedies-part-1[↩]
- Orr SL. The Evidence for the Role of Nutraceuticals in the Management of Pediatric Migraine: a Review. Curr Pain Headache Rep. 2018 Apr 4;22(5):37. doi: 10.1007/s11916-018-0692-6[↩][↩][↩]
- Wells RE, Beuthin J, Granetzke L. Complementary and Integrative Medicine for Episodic Migraine: an Update of Evidence from the Last 3 Years. Curr Pain Headache Rep. 2019 Feb 21;23(2):10. doi: 10.1007/s11916-019-0750-8[↩]
- Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012 Apr 24;78(17):1346-53. doi: 10.1212/WNL.0b013e3182535d0c[↩][↩][↩][↩][↩]
- Diener H, Freitag F, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. 2018;1. doi:10.1177/2515816318759304[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014 May;35 Suppl 1:135-40. doi: 10.1007/s10072-014-1757-x[↩][↩][↩]
- Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51(2):89-97. doi: 10.1159/000076535[↩][↩][↩][↩][↩][↩]
- Lipton RB, Göbel H, Einhäupl KM, Wilks K, Mauskop A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004 Dec 28;63(12):2240-4. doi: 10.1212/01.wnl.0000147290.68260.11[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005 Mar;45(3):196-203. doi: 10.1111/j.1526-4610.2005.05044.x[↩][↩][↩][↩]
- Schapowal A, Petasites Study Group. Butterbur Ze339 for the Treatment of Intermittent Allergic Rhinitis: Dose-Dependent Efficacy in a Prospective, Randomized, Double-blind, Placebo-Controlled Study. Arch Otolaryngol Head Neck Surg. 2004;130(12):1381–1386. doi:10.1001/archotol.130.12.1381[↩][↩]
- Schapowal A; Petasites Study Group. Randomised controlled trial of butterbur and cetirizine for treating seasonal allergic rhinitis. BMJ. 2002 Jan 19;324(7330):144-6. doi: 10.1136/bmj.324.7330.144[↩][↩][↩]
- Schapowal A; Petasites Study Group. Butterbur Ze339 for the treatment of intermittent allergic rhinitis: dose-dependent efficacy in a prospective, randomized, double-blind, placebo-controlled study. Arch Otolaryngol Head Neck Surg. 2004 Dec;130(12):1381-6. doi: 10.1001/archotol.130.12.1381[↩][↩][↩]
- Schapowal A; Study Group. Treating intermittent allergic rhinitis: a prospective, randomized, placebo and antihistamine-controlled study of Butterbur extract Ze 339. Phytother Res. 2005 Jun;19(6):530-7. https://doi.org/10.1002/ptr.1705[↩][↩]
- Anderson N, Borlak J. Hepatobiliary events in migraine therapy with herbs-the case of petadolex, a petasites hybridus extract. J Clin Med. (2019) 8:652. 10.3390/jcm8050652[↩]
- Urda L, Kreuter MH, Drewe J, Boonen G, Butterweck V, Klimkait T. The Petasites hybridus CO2 Extract (Ze 339) Blocks SARS-CoV-2 Replication In Vitro. Viruses. 2022 Jan 7;14(1):106. doi: 10.3390/v14010106[↩]
- Borlak J, Diener HC, Kleeberg-Hartmann J, Messlinger K, Silberstein S. Petasites for Migraine Prevention: New Data on Mode of Action, Pharmacology and Safety. A Narrative Review. Front Neurol. 2022 Apr 26;13:864689. doi: 10.3389/fneur.2022.864689[↩][↩][↩][↩][↩][↩][↩][↩]
- Lipton RB, Gobel H, Einhaupl KM, Wilks K, Mauskop A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. (2004) 63:2240–4. 10.1212/01.WNL.0000147290.68260.11[↩][↩][↩][↩]
- Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001 Jun;6(3):303-10.[↩][↩][↩][↩][↩][↩]
- Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. (2004) 51:89–97. 10.1159/000076535[↩]
- Oelkers-Ax R, Leins A, Parzer P, Hillecke T, Bolay HV, Fischer J, et al.. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain. (2008) 12:301–13. 10.1016/j.ejpain.2007.06.003[↩][↩][↩]
- Choi YW, Lee KP, Kim JM, Kang S, Park SJ, Lee JM, Moon HR, Jung JH, Lee YG, Im DS. Petatewalide B, a novel compound from Petasites japonicus with anti-allergic activity. J Ethnopharmacol. 2016 Feb 3;178:17-24. doi: 10.1016/j.jep.2015.12.010[↩][↩][↩]
- Ezzatzadeh E, Hossaini Z. Green synthesis and antioxidant activity of novel series of benzofurans from euparin extracted of Petasites hybridus. Nat Prod Res. 2019 Jun;33(11):1617-1623. doi: 10.1080/14786419.2018.1428598[↩]
- Kim N, Choi JG, Park S, Lee JK, Oh MS. Butterbur Leaves Attenuate Memory Impairment and Neuronal Cell Damage in Amyloid Beta-Induced Alzheimer’s Disease Models. Int J Mol Sci. 2018 Jun 1;19(6):1644. doi: 10.3390/ijms19061644[↩][↩][↩]
- Periodic Safety Update of Max Zeller Söhne AG, CH-8590 Romanshorn, 2015.[↩]
- Schapowal A; Study Group. Treating intermittent allergic rhinitis: a prospective, randomized, placebo and antihistamine-controlled study of Butterbur extract Ze 339. Phytother Res. 2005 Jun;19(6):530-7. doi: 10.1002/ptr.1705[↩]
- Silberstein SD. Preventive Migraine Treatment. Continuum (Minneap Minn). 2015 Aug;21(4 Headache):973-89. doi: 10.1212/CON.0000000000000199[↩]
- Wells, R.E., Beuthin, J. & Granetzke, L. Complementary and Integrative Medicine for Episodic Migraine: an Update of Evidence from the Last 3 Years. Curr Pain Headache Rep 23, 10 (2019). https://doi.org/10.1007/s11916-019-0750-8[↩]
- Rajapakse, T. and Pringsheim, T. (2016), Nutraceuticals in Migraine: A Summary of Existing Guidelines for Use. Headache, 56: 808-816. https://doi.org/10.1111/head.12789[↩]
- American Academy of Neurology New Guidelines: Treatments Can Help Prevent Migraine. https://www.aan.com/globals/axon/assets/9774.pdf[↩]
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012 Apr 24;78(17):1337-45. doi: 10.1212/WNL.0b013e3182535d20. Erratum in: Neurology. 2013 Feb 26;80(9):871.[↩]
- Malone M, Tsai G. The evidence for herbal and botanical remedies, Part 1. J Fam Pract. 2018 Jan;67(1):10-16. http://www.mdedge.com/jfponline/article/155077/pain/evidence-herbal-and-botanical-remedies-part-1[↩][↩][↩]
- Rajapakse T, Pringsheim T. Nutraceuticals in Migraine: A Summary of Existing Guidelines for Use. Headache. 2016 Apr;56(4):808-16. doi: 10.1111/head.12789[↩][↩][↩][↩][↩]
- Oelkers-Ax R, Leins A, Parzer P, Hillecke T, Bolay HV, Fischer J, Bender S, Hermanns U, Resch F. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain. 2008 Apr;12(3):301-13. doi: 10.1016/j.ejpain.2007.06.003[↩]
- Brattström A. A newly developed extract (Ze 339) from butterbur (Petasites hybridus L.) is clinically efficient in allergic rhinitis (hay fever) Phytomedicine. 2003;10:50–52. doi: 10.1078/1433-187X-00304[↩]
- Lee DK, Haggart K, Robb FM, Lipworth BJ. Butterbur, a herbal remedy, confers complementary anti-inflammatory activity in asthmatic patients receiving inhaled corticosteroids. Clin Exp Allergy. 2004 Jan;34(1):110-4. doi: 10.1111/j.1365-2222.2004.01838.x[↩]
- Lee DK, Carstairs IJ, Haggart K, Jackson CM, Currie GP, Lipworth BJ. Butterbur, a herbal remedy, attenuates adenosine monophosphate induced nasal responsiveness in seasonal allergic rhinitis. Clin Exp Allergy. (2003) 33:882–6. 10.1046/j.1365-2222.2003.01705.x[↩][↩][↩][↩]
- Lee DKC, Gray RD, Robb FM, Fujihara S, Lipworth BJ. A placebo-controlled evaluation of butterbur and fexofenadine on objective and subjective outcomes in perennial allergic rhinitis. Clin Exp Allergy. (2004) 34:646–9. 10.1111/j.1365-2222.2004.1903.x[↩][↩][↩]
- Schapowal A, Petasites Study Group. Butterbur Ze339 for the treatment of intermittent allergic rhinitis: dose-dependent efficacy in a prospective, randomized, double-blind, placebo-controlled study. Arch Otolaryngol Head Neck Surg. (2004) 130:1381–6. 10.1001/archotol.130.12.1381[↩][↩]
- Gray RD, Haggart K, Lee DKC, Cull S, Lipworth BJ. Effects of butterbur treatment in intermittent allergic rhinitis: a placebo-controlled evaluation. Ann Allergy Asthma Immunol. (2004) 93:56–60. 10.1016/S1081-1206(10)61447-0[↩][↩]
- Schapowal A. Treating intermittent allergic rhinitis: a prospective, randomized, placebo and antihistamine-controlled study of Butterbur extract Ze 339. Phytother Res. (2005) 19:530–7. 10.1002/ptr.1705[↩]
- Lee DK, Haggart K, Robb FM, Lipworth BJ. Butterbur, a herbal remedy, confers complementary anti-inflammatory activity in asthmatic patients receiving inhaled corticosteroids. Clin Exp Allergy. (2004) 34:110–4. 10.1111/j.1365-2222.2004.01838.x[↩][↩]
- Thomet OA, Wiesmann UN, Schapowal A, Bizer C, Simon HU. Role of petasin in the potential anti-inflammatory activity of a plant extract of petasites hybridus. Biochem Pharmacol. (2001) 61:1041–7. 10.1016/s0006-2952(01)00552-4[↩][↩][↩][↩]
- Thomet OA, Wiesmann UN, Blaser K, Simon HU. Differential inhibition of inflammatory effector functions by petasin, isopetasin and neopetasin in human eosinophils. Clin Exp Allergy. (2001) 31:1310–20. 10.1046/j.1365-2222.2001.01158.x[↩]
- Fonteh AN, Chung R, Sharma TL, Fisher RD, Pogoda JM, Cowan R, et al.. Cerebrospinal fluid phospholipase C activity increases in migraine. Cephalalgia. (2011) 31:456–62. 10.1177/0333102410383589[↩]
- Fiebich BL, Grozdeva M, Hess S, Hull M, Danesch U, Bodensieck A, et al.. Petasites hybridus extracts in vitro inhibit COX-2 and PGE2 release by direct interaction with the enzyme and by preventing p42/44 MAP kinase activation in rat primary microglial cells. Planta Med. (2005) 71:12–9. 10.1055/s-2005-837744[↩][↩][↩]
- Resch M, Heilmann J, Steigel A, Bauer R. Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med. (2001) 67:437–42. 10.1055/s-2001-15817[↩][↩]
- Bauer R. Inhibition of Different Extracts of Petasites Hybridus on 5-Lipoxygenase. University of Düsseldorf, Germany (Study Report) (2001).[↩]
- Bauer R. Inhibitory Effects of (different) Extracts of Petasites Hybridus on COX-1 and COX-2. University of Düsseldorf (Study report) (2002).[↩]
- Franz G. Effects of Petasites Hybridus Extracts in the HET-CAM-Assay (Study Report). University of Regensburg, Germany (1998).[↩]
- Bickel D, Roder T, Bestmann HJ, Brune K. Identification and characterization of inhibitors of peptido-leukotriene-synthesis from Petasites hybridus. Planta Med. (1994) 60:318–22. 10.1055/s-2006-959492[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Butterbur. [Updated 2019 Feb 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547997[↩][↩][↩][↩]
- Periodic Safety Update of Weber & Weber International GmbH & Co.KG, 2016.[↩][↩][↩]
- Evers S. Pestwurz in der Behandlung der Migräne. Nervenheilkunde 2009; 8: 548–552.[↩]
- Teschke R, Eickhoff A, Schulze J, et al. Petadolex®, a herbal extract for migraine prophylaxis with spontaneous case reports of disputed liver injury: Robust causality evaluation by RUCAM, the Roussel Uclaf Causality Assessment Method. EJPMR 2016; 3: 154–177.[↩][↩]
- Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015 Dec 24;17(1):14. doi: 10.3390/ijms17010014[↩]
- Chen CW. [Carefully reviewing the history of diagnostic scales and paying more attention to the diagnostic value of Roussel – Uclaf causality assessment method scale for drug – induced liver injury]. Zhonghua Gan Zang Bing Za Zhi. 2016 Nov 20;24(11):801-803. Chinese. doi: 10.3760/cma.j.issn.1007-3418.2016.11.001[↩]
- Allgaier C, Franz S. Risk assessment on the use of herbal medicinal products containing pyrrolizidine alkaloids. Regul Toxicol Pharmacol. 2015 Nov;73(2):494-500. doi: 10.1016/j.yrtph.2015.09.024[↩]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA Journal 2011; 9: 2406–2500. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2011.2406[↩]
- Committee on Herbal Medicinal Products (HMPC). Public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (PAs) including recommendations regarding contamination of herbal medicinal products with PAs. https://www.ema.europa.eu/en/documents/public-statement/public-statement-use-herbal-medicinal-products-containing-toxic-unsaturated-pyrrolizidine-alkaloids-pas-including-recommendations-regarding-contamination-herbal-medicinal-products-pyrrolizidine_en.pdf[↩]
- Avula B, Wang YH, Wang M, Smillie TJ, Khan IA. Simultaneous determination of sesquiterpenes and pyrrolizidine alkaloids from the rhizomes of Petasites hybridus (L.) G.M. et Sch. and dietary supplements using UPLC-UV and HPLC-TOF-MS methods. J Pharm Biomed Anal. 2012 Nov;70:53-63. doi: 10.1016/j.jpba.2012.05.021[↩][↩]
- Evers S. Pestwurz in der Behandlung der Migräne. Nervenheilkunde 2009; 8: 548–552. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0038-1628674[↩]
- Avula B, Wang YH, Wang M, Smillie TJ, Khan IA. Simultaneous determination of sesquiterpenes and pyrrolizidine alkaloids from the rhizomes of Petasites hybridus (L) GM et Sch and dietary supplements using UPLC-UV and HPLC-TOF-MS methods. J Pharm Biomed Anal. (2012) 70:53–63. 10.1016/j.jpba.2012.05.021[↩]