Contents
What is casein
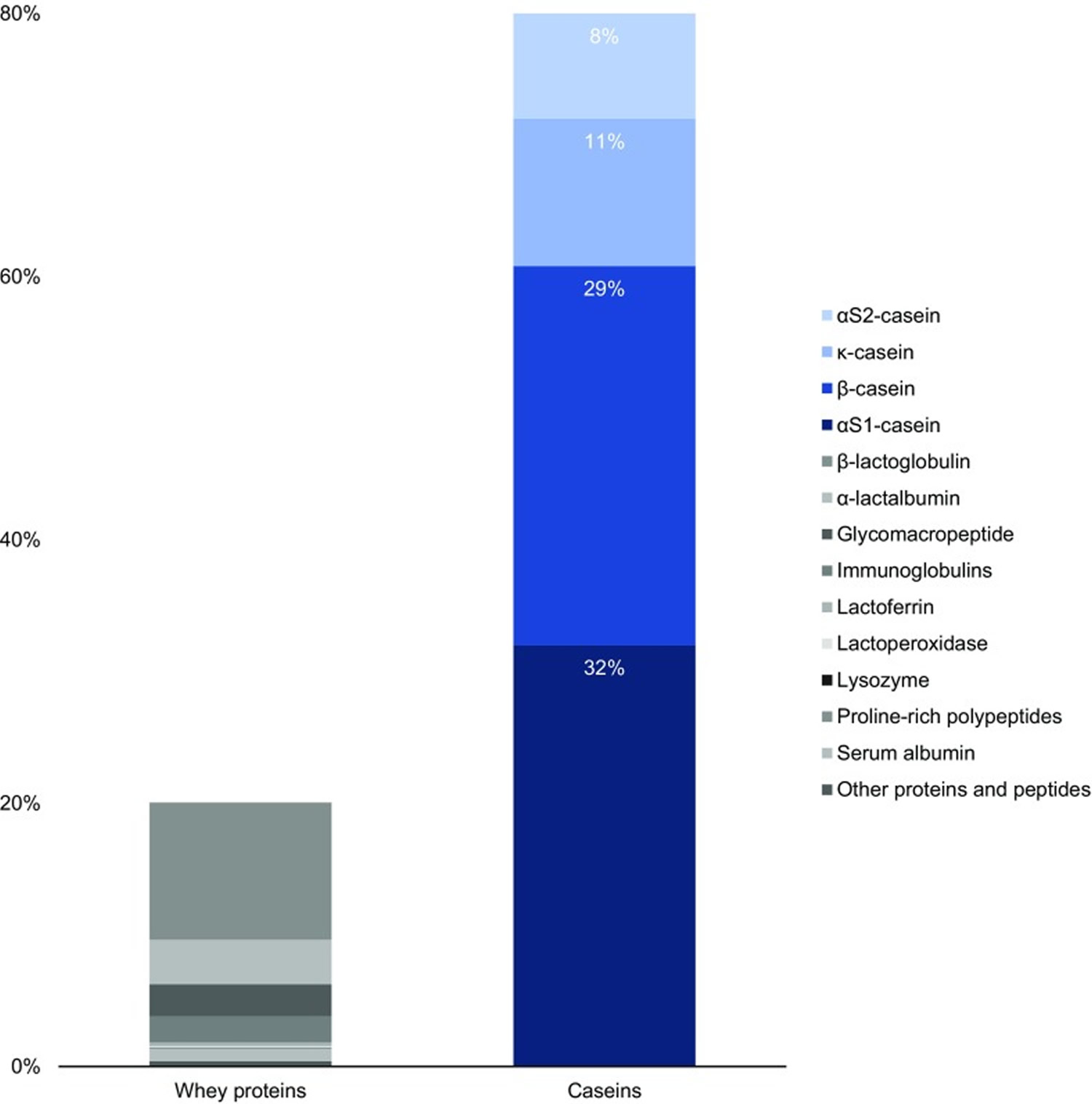
Casein is the collective name for a family of milk proteins. Caseins, in contrast to the second milk protein fraction, i.e. whey proteins, are insoluble and account for 80 % of total bovine milk proteins 1, which translates to 2.75 % of total milk components (Figure 1). Caseins, representing about 80% of the protein content in cow’s milk, are isolated from milk by acid or by rennet precipitation. The acid or isoelectric, precipitation is performed at pH 4.6, where the caseins precipitate and the whey proteins remain soluble. Caseins are flexible and heat stable proteins.
In bovine milk, casein comprises four peptides: αS1 casein (40%), αS2 casein (10%), β casein (36%) and κ casein (14%) differing in their amino acid, phosphorus and carbohydrate content but similar in their amphiphilic (having both hydrophilic and hydrophobic parts) character.
Milk proteins have long been known for their nutritional and technological value. Proteins are important constituents of the human diet, since they comprise a principal source of nitrogen and essential amino acids. Milk proteins have high nutritional value compared to other proteins because of their relatively high content of essential amino acids and good digestibility 2. This has led to the use of cow’s milk proteins as an essential ingredient in the manufacturing of specialized foods in the pharmaceutical and food industries 3.
Beta-casein has consistently demonstrated important biological activities on immune, cardiovascular, gastrointestinal and central nervous systems 4. Beta-casein is the second most abundant protein in cow’s milk representing 30% of total protein 5 . It is encoded by the CSN2 gene mapped on chromosome 6q31 and consists of 209-amino-acid single polypeptide chain, and molecular mass of about 24 kDa 6.
Beta-casein proteins may be present as one of two major genetic variants: A1 and A2 7. The distinguishing structure between these 2 forms of β-casein is the presence of either histidine (His67) in A1 or proline (Pro67) in A2 at position 67 of this 209–amino acid protein, with A1 being consequential to a point mutation from Pro67 to His67 occurring in ancestors to modern European-type cattle 8. A2 beta-casein is recognized as the original beta-casein variant because it existed before a proline67 to histidine67 point mutation caused the appearance of A1 beta-casein in some European herds some 5000–10,000 years ago 9.
In human milk, beta-casein (~68% of total casein) is of the A2 type, with a proline at the equivalent position on the beta-casein protein chain 10. Human bioactive opioid peptide beta-casomorphin-7 (BCM-7) has a different amino acid sequence to bovine bioactive opioid peptide beta-casomorphin-7 (BCM-7), with homology in five of seven amino acids (differing amino acids at positions four and five) 11 and considerably weaker opioid activity 12. Wada and Lonnerdal 11 examined non-digested and in vitro-digested human milk, and reported the presence of human BCM-9 (which has a proline at position eight), but not human BCM-7 or BCM-5 (i.e., BCM-5 is the truncated form of BCM-7). However, Jarmolowska et al. reported the presence of both human BCM-5 and BCM-7 in colostrum (averaging 5 and 3 μg/mL respectively), but at 2 months into the lactation period, the authors reported much lower quantities 13. It has been postulated that casomorphin functionality in neonates may relate to maternal bonding, gastrointestinal function, mucosal development and sleep induction 13.
Figure 1. Standard protein content in bovine milk
A1 beta-casein has only been found in cattle of European origin. Purebred Asian and African cattle produce milk containing only the A2 beta-casein type, although some cattle presenting phenotypically as Asian or African cattle may produce A1 beta-casein as a consequence of crossbred ancestry. The relative prevalence of A1 and A2 beta-casein in cattle is breed-dependent, with Northern European breeds generally having higher levels of A1 beta-casein than Southern European breeds. Guernsey and Fleckvieh breeds are generally considered to have a particularly high A2 allele frequency. However, within any specific herd, basing the estimation of allele frequency on breed category is not reliable. In the herds in many Western countries, the ratio of A1:A2 is approximately 1:1 14. Herd testing for beta-casein alleles can be undertaken using DNA analysis, which is available commercially in some countries. Converting a specific herd by selective breeding to eliminate all A1 beta-casein from the milk can be achieved within 4 years using intensive methods of animal selection that incorporate the use of sex-selected semen, but more typically this will take 5–8 years or longer 15.
There is increasing evidence that A1 beta-casein, a protein produced by a major proportion of European-origin cattle but not purebred Asian or African cattle, is also associated with cows’ milk intolerance 16. In humans, digestion of bovine A1 beta-casein, but not the alternative A2 beta-casein, releases beta-casomorphin-7 (BCM-7), which activates μ-opioid receptors expressed throughout the gastrointestinal tract and body. Studies in rodents show that milk containing A1 beta-casein significantly increases gastrointestinal transit time, production of dipeptidyl peptidase-4 (DPP4) and the inflammatory marker myeloperoxidase compared with milk containing A2 beta-casein 16. Co-administration of the opioid receptor antagonist naloxone blocks the myeloperoxidase and gastrointestinal motility effects, indicating opioid signaling pathway involvement. In humans, a double-blind, randomized cross-over study showed that participants consuming A1 beta-casein type cows’ milk experienced statistically significantly higher Bristol stool values compared with those receiving A2 beta-casein milk. The Bristol stool scale, is a diagnostic medical tool designed to classify the form of human faeces into seven categories. It is used in both clinical and experimental fields. Additionally, a statistically significant positive association between abdominal pain and stool consistency was observed when participants consumed the A1 but not the A2 diet. However, further studies of the role of A1 beta-casein in milk intolerance are needed.
There is wide-ranging evidence for both inflammatory and immune responses to casomorphins within the gastrointestinal system. However, the overall implications of these responses are not fully understood. It has been shown in both rats 17 and mice 18 that A1 beta-casein is associated with increased levels of the inflammatory marker myeloperoxidase in the colon. This effect is eliminated by administration of naloxone, indicating that it is an opioid-dependent response. Interestingly, intestinal inflammation enhances the potency of μ-opioid receptor agonists in inhibiting gastrointestinal transit, and increases the expression of μ-opioid receptors in the mouse intestine 19. It has also been shown in rats that A1 beta-casein stimulates the production of the enzyme dipeptidyl peptidase 4 (DPP4) in the jejunum 17. However, this effect is not attenuated by naloxone administration, indicating a non-opioid effect of A1 beta-casein on DPP4. The full implications of this are not understood, but it is notable that DPP4 degrades the gut incretin hormones rapidly. In humans, incretin hormones modulate insulin and glucose metabolism 20 and affect antroduodenal motility 21. Dipeptidyl peptidase 4 (DPP4) inhibitors are now widely used in the management of type 2 diabetes mellitus.
The avoidance of A1 is feasible within dairy-based diets through the consumption of goat, sheep, and buffalo milk or through the consumption of bovine milk from the native Asian and African bovine breeds, or through the consumption of milk from genetically selected herds of European-type cattle that are certified free of the His67 mutation. Such herds are being developed in many countries. Bovine milk that is free of A1 is now available commercially in a range of countries, including Australia, the United Kingdom, the United States, New Zealand, and The Netherlands, and is widely promoted as beneficial for people who suffer from milk intolerances. Infant formula containing casein but free of A1 is now marketed widely in China and Australia and is promoted commercially as being more gentle on the infant digestive system.
Gastrointestinal Effects of A1 Compared with A2 Beta-Casein
The main constituent of milk protein is casein, which accounts for around 80% of its content, is classified as phosphoproteins and are characterized into 4 isoforms: alphaS1-casein (αS1-CN), alphaS2-casein (αS2-CN), beta-casein (β-CN) and kappa-casein (k-CN), organized in a micellar format according to their electrophobic interactions 22. Casein phosphoproteins are arranged together according to their hydrophobicity, electrostatic interactions and interact with minerals collectively; they are referred to as casein-calcium-phosphate (CCP) 23. Beta-casein (β-CN) represents over 30% of total casein and is gaining importance in nutrition science due to its potential to release bioactive peptides, some of which have opioid characteristics 24.
The beta-casein (β-CN) protein is a 209-amino-acid-long peptide chain, with thirteen identified genetic variants (A1, A2, A3, A4, B, C, D, E, F, G, H1, H2 and I), resulting from changes in the 209 amino acids in the sequence 22. These genetic variations have characteristics of codominance, which means that alleles may be present in either homozygous, i.e., A1A1 or A2A2, etc., or heterozygous, i.e., A1A2, A1B, etc., forms, where both alleles are expressed for each of the 13 variants 25. The A1A2 and A2A2 beta-casein genotypes are the most frequent, while other genotypes are considered rare or of very low prevalence, such as the A1A1 genotype 26.
Approximately 30% of the total protein in cow’s milk is beta-casein (β-CN), which is comprised of both A1 beta-casein protein and A2 beta-casein protein 27, 7. These beta-casein proteins differ by the amino acid residue present at position 67, where A1 beta-casein has a histidine residue at position 67 (His67), and A2 beta-casein retains a proline residue at position 67 (Pro67) 28, 29, 30. The presence of histidine amino acid at position 67 (His67) in the A1 beta-casein increases the protein’s susceptibility to enzymatic cleavage of the preceding seven amino acids in the small intestine by pepsin and leucine aminopeptidase, this results in higher opioid peptide beta-casomorphin-7 (BCM-7) levels with moderate activity on mu (μ) opioid receptors 22, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41. In contrast, A2 beta-casein peptide bond between isoleucine and proline has more enzymatic resistance, which makes it difficult for proteases to cut between positions 66 and 67, thus releasing much less and probably minimal amounts of the opioid peptide beta-casomorphin-7 (BCM-7) under normal gut conditions 14. In addition, in the A2 beta-casein, protein chain cleavage occurs in the nine amino acid long peptide known as β-Casomorphin-9 (BCM-9) (Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-Pro67-Asn68), which is considered a potentially bioactive peptide with antihypertensive and antioxidant properties 31, 42.
It has been reported that casein and its derivatives, particularly beta-casomorphin-7 (BCM-7), exert a variety of effects on gastrointestinal function in animal models, including reducing the frequency and amplitude of intestinal contractions, increasing mucus secretion, and suppressing lymphocyte proliferation 43, 44, 45, 46, 47, 48, 49, 50. Beta-casomorphin-7 (BCM-7) is classified as an exorphin because it is an exogenous opioid peptide, with the same classification as that of morphine 51. Since beta-casomorphin-7 (BCM-7) is released into the intestinal lumen in about 30 minute to 6 hour after ingestion of beta-casein and because it is an exorphin, numerous studies show concern about its physiological activity as an opioid agonist, potential to activate the mu (μ) opioid receptor 52. The mu (μ) opioid receptors are located in various body parts, such as in the central nervous system (brain and spinal cord), the gastrointestinal tract, bladder, and in the cells of the immune and endocrine system 28. The opioid system has the following functions: inhibition of pain stimuli, endocrine and autonomic nervous system functions, emotions and cognitive ability, learning and modulation of memory and gastrointestinal functions 53, 54. The main potential effect of beta-casomorphin-7 (BCM-7) is the modulation of motility that delays gastrointestinal transit time and increases mucus production in humans 55.
Some studies have been conducted in humans in order to investigate the possible effects of beta-casomorphin-7 (BCM-7). Beta-casomorphin-7 (BCM-7) results in gastrointestinal discomfort, pro-inflammatory responses, and reduced antioxidant glutathione (GSH) levels 35, 22, 56. In contrast, the A2-type beta-casein releases almost no beta-casomorphin-7 (BCM-7) and consumption of milk without A1-type β-casein (A1PF milk) results in relatively fewer gastrointestinal symptoms and inflammatory markers versus milk containing both A1-type and A2-type β-casein (conventional milk) 57, 58, 59, 60, 61, 62, 63.
There is increasing evidence in the literature that the effects of dairy products on gastrointestinal dysfunction may be at least be partially attributed to the proteolytic release of the bioactive peptide beta-casomorphin-7 (BCM-7) from beta-casein rather than lactose intolerance 64, 65, 57. Recent studies report that consumption of A1 beta-casein induces inflammation in the small intestine, which may potentiate lactose intolerance symptoms by downregulating lactase enzyme expression or activity 66, 67. The A2 variant of beta-casein, however, has a much slower rate of proteolytic digestion; therefore, its consumption results in a nil or much lower and not physiologically relevant BCM-7 level 68, 69.
Two recent studies concluded that in lactose-intolerant patients, after consuming A2A2 milk, gastrointestinal discomforts were reduced compared to the case of conventional milk (A1A2) consumption; and symptoms of pain and fecal urgency were the main findings 70, 71. He et al. 67 observed acute gastrointestinal symptoms associated with milk consumption, such as abdominal pain, bloating, stool frequency and consistency measured by Bristol Stool Scale after the consumption of conventional (A1A2) milk and A2A2 milk after 1 to 12 hour by lactose-intolerant individuals. Among the results, it was observed that abdominal pain, distension and stool frequency were reduced after the consumption of A2 milk for 12 hour by lactose-intolerant Chinese adults. Those studies found a correlation between improved gastrointestinal discomfort and reduced exposure to BCM-7 through the consumption of A2A2 milk, although the peptide was not quantified in these studies. These suggest that A2A2 milk may result in higher tolerance to dairy consumption. He et al. concluded that milk containing A2 beta-casein reduce acute gastrointestinal symptoms of milk intolerance in Chinese adults, while conventional milk containing A1 beta-casein reduced lactase activity and increased gastrointestinal symptoms compared with milk containing A2 beta-casein 67. Therefore, milk-related gastrointestinal symptoms may result from the ingestion of A1 beta-casein rather than lactose in some individuals 67. These studies suggest that A2A2 milk may result in higher tolerance to dairy consumption.
Ho et al. 72 also identified an increase in abdominal pain in healthy individuals after the consumption of conventional milk (A1A2) without lactose intolerance when compared to a group that consumed A2A2 milk. Significant changes in stool consistency were also observed between groups, as well as a significant positive correlation between abdominal pain and softer stool after the consumption of A1A2 milk, raising the hypothesis that these adverse factors were induced by proinflammatory cytokines 72. These preliminary results suggest differences in gastrointestinal responses in some adult humans consuming milk containing beta-casein of either the A1 or the A2 beta-casein type, but require confirmation in a larger study of participants with perceived intolerance to ordinary A1 beta-casein-containing milk.
In contrast, the study conducted by Crowley et al. 73 showed no significant difference in patients after the intake of 400 mL of conventional milk (A1A2 milk) and the same amount of A2A2 milk. A two week period was considered in children with chronic constipation, the measurements of which were performed by bowel movements in relation to transit time. Therefore, under these experimental conditions, the different milk genotypes, conventional milk (A1A2) and A2A2 milk, were not significantly associated with better resolution of chronic constipation symptoms 73.
A double-blind, randomized, crossover study in 45 Chinese individuals (lactose-intolerant and lactose-tolerant [control group]) by Sun et al 74 showed consumption of milk containing A1 beta-casein (A1A2 milk) was associated with increased gastrointestinal inflammation in the small intestine and stomach, worsening of post-dairy digestive discomfort symptoms, delayed transit, and decreased cognitive processing speed and accuracy. Because elimination of A1 beta-casein attenuated these effects, some symptoms of lactose intolerance may stem from inflammation it triggers (the systemic increase in serum biomarkers such as interleukin-4 [IL-4] and immunoglobulins [IgG, IgE and IgG1] in the lactose-intolerant group), and can be avoided by consuming milk containing only the A2 type of beta casein (A2A2 milk) 74.
Recently, another double-blind, randomized, crossover study involving Chinese children aged 5 to 6 years who consumed 300 mL/day of conventional milk (A1A2 milk) versus milk containing only A2 beta-casein (A2A2 milk) for five days 36. The study found that Chinese children aged 5 to 6 years who consumed milk containing only A2 beta-casein (A2A2 milk) had significantly less severe gastrointestinal symptoms as measured by visual analog scales, reduced stool frequency, and improvements in stool consistency, compared with subjects consuming conventional milk (A1A2 milk) 36. There were significant increases from baseline in serum immunologic and inflammatory biomarkers such as interleukin-4 (IL-4), immunoglobulins G (IgG), E (IgE), and G1 (IgG1), and beta-casomorphin-7 (BCM-7) coupled to lower glutathione levels, in subjects consuming conventional milk (A1A2 milk) compared with milk containing only A2 beta-casein (A2A2 milk). Subtle Cognitive Impairment Test analysis showed significant improvements in test accuracy after consumption of milk containing only A2 beta-casein (A2A2 milk). There were no severe adverse events related to consumption of either milk product 36.
Overall, the few human studies do not provide conclusive results that confirm the adverse effects of beta-casomorphin-7 (BCM-7) due to conventional milk (A1A2 milk) consumption. These human studies highlighted the potential effects of beta-casomorphin-7 (BCM-7) to promote greater gastrointestinal discomfort such as gastric disorders and abdominal discomfort similar to the effects caused by lactose intolerance, increased mucus secretion, reduced motility, pain and abdominal distention 22. Studies on the action of beta-casomorphin-7 (BCM-7) in the human central nervous system (brain and spinal cord) reported the activation of the gut–brain axis due to gastric discomfort, and not directly by the binding of BCM-7 to mu (µ) opioid receptors in the central nervous system. This relationship is evidenced in the study conducted by Sheng et al 36 where, after the consumption of A2A2 milk by school-aged children with lactose intolerance, the subtle cognitive impairment test showed improvements in response accuracy and greater processing efficiency. This association with the cognitive system was correlated with the reduction in the effects on the gastrointestinal system caused by BCM-7 released due to the consumption of conventional milk (A1A2 milk). This is a potentially important result, in which exposure to beta-casomorphin-7 (BCM-7) briefly causes side effects in the central nervous system (brain and spinal cord), since direct consequences of BCM-7 to the central nervous system (brain and spinal cord) or other organs are difficult to investigate. In addition, the potential of beta-casomorphin-7 (BCM-7) to increase levels of some immune–inflammatory markers and causing gastrointestinal inflammation condition, which can trigger secondary effects in the abdominal region such as pain, distension, flatulence, and diarrhea.
Another potential emerging field is the possibility of beta-casomorphin-7 (BCM-7) indirectly influencing the central nervous system (brain and spinal cord) through stress to the intestinal microbiota. The gut–brain axis theory was expanded to the microbiota–gut–brain axis to explain the influence of microorganisms on cognitive development and functioning 75. The intestinal microbiota also called gut microbiota, gut microbiome, or gut flora, are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts and this microbiota is critically involved in the communication of the intestine–brain axis, influencing the brain and behavior modulation, also presenting analgesia effects 76. Gut microbiome disorders may also be associated with excessive mucin production in the intestine triggered by BCM-7 77. Intestinal microbiota disorders can influence the central nervous system by the deficiency in the absorption of molecules used as a substrate for its proper functioning, for example, the absorption of tryptophan, precursor of serotonin, an important neurotransmitter in the central nervous system involved in the regulation of mood, appetite, memory, learning and sleep 78. Some diseases and disorders present as characteristics the imbalance of intestinal microorganisms, such as in gastrointestinal disorders, celiac disease, obesity, diabetes, as well as in mental disorders, eating disorders, autism spectrum disorders and mood disorders 78. These data reinforce the hypothesis raised by Osman et al 79, who observed changes in the microbiota of mice accompanied by changes in mood through gut–brain communication, suggesting that this effect was due to the effect of BCM-7 released from the consumption of conventional milk (A1A2 milk).
In summary, due to the few studies in humans, results from clinical trials are inconclusive 54. Although clinical trials have not yet been sufficient to establish a clear relationship of the adverse effects of beta-casomorphin-7 (BCM-7) on different physiological responses, the likelihood that this mechanism may initiate or exacerbate some gastrointestinal symptoms seems to be high, the evidence of which has been reported in most studies and explored in this review 22. Research is expected to continue to evolve as new studies are being developed and more results are released in the very near future.
Casein protein vs Whey protein
Casein protein
Caseins not only provide adequate amounts of essential amino acids, but peptides derived from caseins have been shown to express various biological effects. Bioactive peptides are produced by in vitro and in vivo enzymatic proteolysis of bovine and human caseins 80.
Casein derivatives were tested in treating dry mouth, dentin hypersensitivity and in caries prevention 81. In a randomized clinical trial with subjects with salivary gland dysfunction (radiotherapy and Sjögren’s syndrome patients) two mouthrinse solutions containing either casein derivatives with calcium phosphate or a 0.05% sodium fluoride (as control), used for 12 months, were compared 82. No statistically significant differences between the two groups regarding coronal caries were found. between the two.
Hydrolyzed casein was found effective in decreasing postprandial glucose concentrations in type 2 diabetes mellitus patients who continued their oral anti-diabetic medication 83. In a double-blind trial single meal replacements with proprietary casein hydrolysate (insuVidaTM; DSM Nutritional Products) alone or with addition of leucine, unhydrolyzed casein or placebo were used. Postprandial serum glucose, insulin and glucagon concentrations were measured after 4 h. The addition of leucine to casein hydrolysate caused the biggest increase in insulin (by 51.8%). All three treatments increased glucagon concentration compared to placebo. In addition, a single dose of casein hydrolysate with or without leucine significantly lowered plasma glucose content compared to placebo and intact casein. The results suggest a benefit of the studied nutrients in management of type 2 diabetes.
β-casein and its peptides, as well as αS-casein, possess antioxidant and antimicrobial activity 84. As yet, however, none of the above-mentioned peptides has been clinically tested.
Whey protein
From the nutritional point of view, milk whey proteins have been considered superior to casein in various aspects. They present amino acid profile superior to casein, being similar to human milk, is what recommends whey proteins for the formulation of humanized milk products for replacement of bovine milk in infant nutrition 85. Whey protein from cow’s milk is also a rich source of essential and branched chain amino acids 86. Some publications 87, 88 reported on important differential properties between caseins and the milk whey proteins. It was observed that the caseins undergo much lower digestion and absorption than the whey proteins.
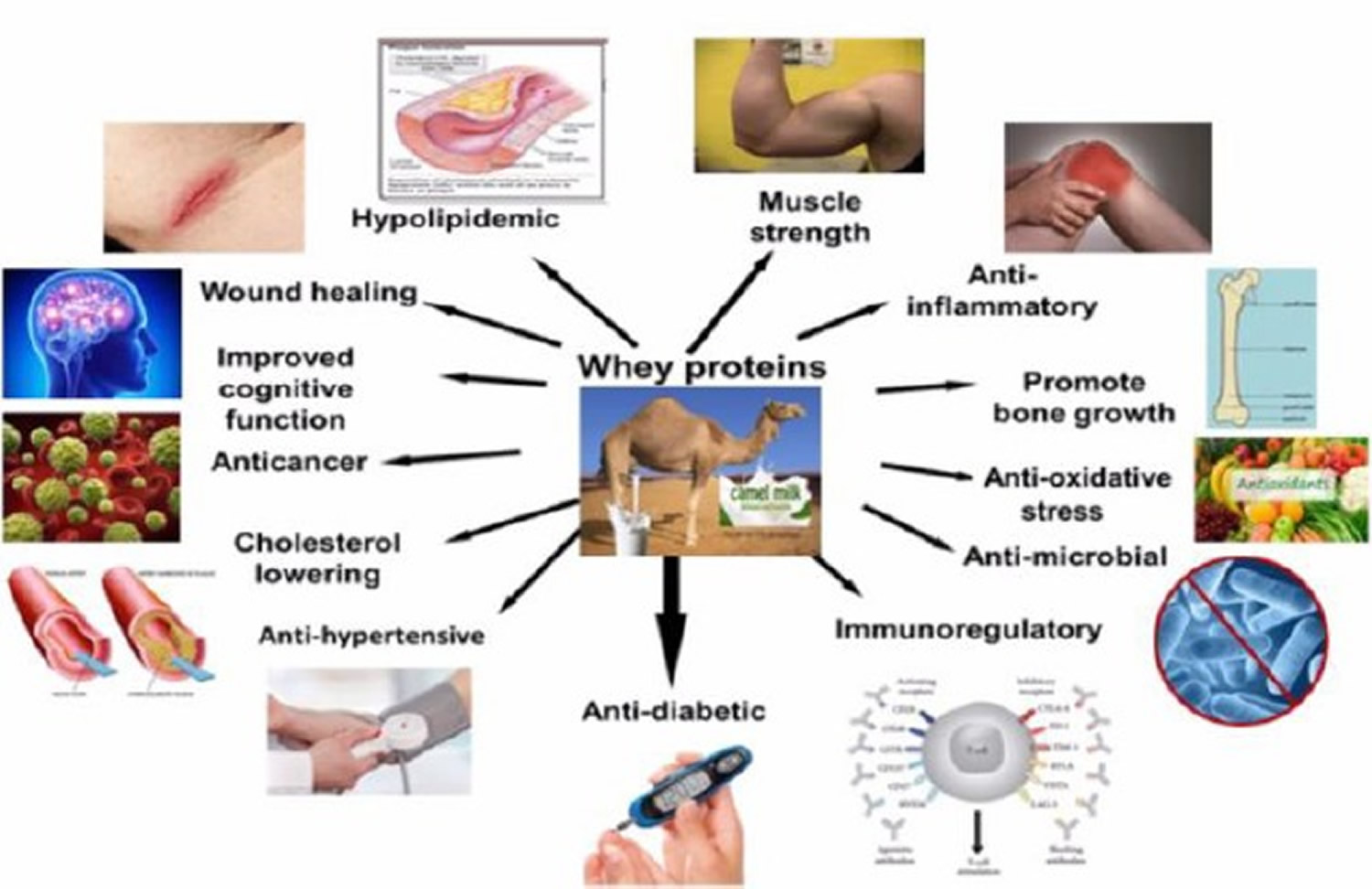
Whey protein is composed of several bioactive fractions including glycomacropeptide, β-lactoglobulin, α-lactalbumin and lactoferrin, with multiple health benefits against cancer, infection and inflammation 89. Dietary lactalbumin was found to suppress hunger in humans 90, decrease weight gain and adiposity in rats 91, mice 92 and minipigs 93, and improve glucose tolerance in diabetic rats 94 and minipigs 93. Further, lactalbumin and milk protein comparably decrease weight and fat mass in calorie restricted human subjects 95. Dietary supplementation with lactoferrin has been reported to modulate gut microbiota, decrease weight gain, reduce hepatic lipidosis, and improve glucose tolerance in mice 96 and produce greater weight and fat loss in calorie restricted mice 97. Others reported that lactoferrin decreases hepatic lipid content and mesenteric fat without altering food intake, weight gain and body composition in mice 98. Similarly, in humans, lactoferrin supplementation for 8 weeks 99, but not 4 weeks 100, has been shown to decrease visceral adiposity in overweight subjects without altering caloric intake. The reduction in caloric intake with whey-based diets is associated with increased circulating concentrations of satiety hormones of the lower gut including glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in humans 101, and GLP-1 in rodents. Despite producing hypophagia, lactalbumin does not alter GLP-1 in humans 102. However, it is unknown whether whey fractions differentially modulate the secretion of these and other satiety hormones. Although, lactalbumin was found to transiently increase energy expenditure in exercising rats, such thermogenic effects are not observed in calorie restricted humans 95. Thus, despite some evidence for the effects of lactalbumin and lactoferrin on body weight, the relative efficacies of these whey fractions in modulating food intake, energy expenditure and gut hormone secretion, and whether these effects are independent of caloric intake, remain largely unknown.
In a study using diet induced obese rats 103, randomized to isocaloric diets: Control, Whey, Lactalbumin, Lactoferrin, or pair-fed to lactoferrin. Whey and lactalbumin produced transient hypophagia (suppression of caloric intake in animals), whereas lactoferrin caused prolonged hypophagia. Lactalbumin decreased weight and fat gain. Notably, lactoferrin produced sustained weight and fat loss, and attenuated the reduction in energy expenditure associated with calorie restriction. Lactalbumin and lactoferrin decreased plasma leptin and insulin, and lactalbumin increased peptide YY. Whey’s lactalbumin and lactoferrin improved glucose clearance partly through differential upregulation of glucoregulatory transcripts in the liver and skeletal muscle. Interestingly, lactalbumin and lactoferrin decreased hepatic lipidosis (disorder of lipid metabolism in the body tissues) partly through downregulation of lipogenic and/or upregulation of β-oxidation transcripts, and differentially modulated cecal bacterial populations. The findings of that study 103 demonstrated that protein quantity and quality are important for improving energy balance. Dietary lactalbumin and lactoferrin improved energy balance and metabolism, and decreased adiposity, with the effects of lactoferrin being partly independent of caloric intake.
Figure 2. Whey protein health benefits
Table 1. Whey proteins component and their biological activities
| Components of whey protein | Biological Function | Species | References |
|---|---|---|---|
| α-lactalbumin | Enhancement of antibody response to systematic antigen stimulation and used in manufacturing of infant food | Camel, bovine, and human | 104 |
| Lactoferrin | Antimicrobial activities against microorganisms, anticancer, anti- inflammatory | Camel, bovine, and human | 105 |
| β-lactoglobulin | Source of essential and branched chain amino acid, responsible for child allergy | Bovine, buffalo, caprine, and equine | 106 |
| Lysozymes | Antibacterial protein present in milk, tears, and saliva, and thus plays an important role in enhancing innate immunity | Camel and bovine | 107 |
| Immunoglobulin | Enhances immune functions | Camel, bovine, and human | 108 |
| Lactoperoxidase | Suppression of bacterial growth | Camel and bovine | 109 |
| Glycomacropeptide | Has an inhibitory effect on acid gastric secretion and modifies the concentration of blood which regulates digestive peptides | Camel and bovine | 110 |
Milk Allergy
Milk allergy is an immune system response to proteins (whey and casein) in cow’s milk, causing symptoms like hives (a red, raised, and itchy rash), swelling, vomiting, and diarrhea, which can range from mild or moderate allergic reactions such as swelling of lips, face eyes, hives (a red, raised, and itchy rash) or welts on the skin, stomach (abdominal) pain, vomiting and diarrhea to a life-threatening reaction called anaphylaxis that include noisy breathing or wheeze, swelling or tightness in throat, or young children may be pale and floppy. Milk allergy is one of the most common food allergies in children and babies, affecting more than 2% of babies 112. Cow’s milk is the usual cause of milk allergy, but milk from sheep, goats, buffalo and other mammals also can cause milk allergy. A milk allergy is different from lactose intolerance, which is an inability to digest the sugar lactose, not the proteins in milk. A milk allergy can be deadly. If you have severe allergic reaction symptoms, such as trouble breathing, call your local emergency number or go to your nearest emergency room (ER) immediately. Milk allergy diagnosis is made by an allergy specialist using tests like skin prick or oral challenges, and management involves strict milk avoidance and carrying an adrenaline autoinjector for severe reactions.
Cow’s milk contains more than 20 protein fractions. There are 2 main proteins in cow’s milk that can cause an allergic reaction 113, 114, 115:
- Casein (alpha-s1-, alpha-s2-, beta-, and kappa-casein) found in the solid part (curd) of milk that curdles.
- Whey (alpha-lactalbumin and beta-lactoglobulin) found in the liquid part of milk that remains after milk curdles.
You or your child may be allergic to only one milk protein or to both. Most individuals with cow’s milk allergies have a sensitivity to both caseins and whey proteins 116. These milk proteins may be hard to avoid because they’re also in some processed foods. And most people who react to cow’s milk will react to sheep, goat and buffalo milk.
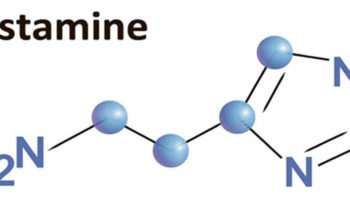
In all allergies, the immune system reacts to triggers, also known as allergens. Your immune system produces antibodies that detect the allergen, causing inflammatory reactions and the release of chemicals, including histamine, which cause allergic symptoms. In the case of milk, the triggers are milk proteins including whey and casein. You or your child may be allergic to either one of these proteins, or both.
If you have milk allergy, your immune system identifies certain milk proteins as harmful, triggering the production of immunoglobulin E (IgE) antibodies to neutralize the protein (allergen) 114. The next time you come in contact with these proteins, IgE (immunoglobulin E) antibodies recognize them and signal your immune system to release histamine and other immune mediators. This release of chemicals causes the signs and symptoms of cow’s milk allergy.
The release of these chemicals can cause someone to have the following problems:
- wheezing
- trouble breathing
- coughing
- hoarseness
- throat tightness
- stomachache
- vomiting
- diarrhea
- itchy, watery, or swollen eyes
- hives
- red spots
- swelling
- a drop in blood pressure.
Children who do not have symptoms shortly after they have milk can have a different type of reaction that causes problems hours to days later, such as:
- loose stools (possibly with blood)
- vomiting or gagging
- refusal to eat
- irritability or colic
- skin rashes, like dermatitis.
An allergic reaction to milk can be rapid usually happens soon after you or your child consumes milk, appearing within minutes to two hours after consumption, or delayed, occurring several hours or even days later. Symptoms of milk allergy range from mild to severe and can include wheezing, vomiting, hives and digestive problems. Milk allergy also can cause anaphylaxis, a severe, life-threatening reaction.
Although it’s not common, milk allergies can cause a severe reaction called anaphylaxis. Anaphylaxis may begin with some of the same symptoms as a less severe reaction, but then quickly worsen. A person might have trouble breathing, feel lightheaded, or pass out. If it’s not treated, anaphylaxis can be life-threatening.
Anaphylaxis symptoms start soon after milk consumption and can include:
- Constriction of airways, including a swollen throat that makes it difficult to breathe.
- Chest tightness
- Shortness of breath (dyspnea).
- Difficulty breathing.
- Wheezing.
- Difficulty swallowing (dysphagia)
- Rash.
- Facial flushing.
- Itching.
- Dizziness.
- Shock, with a marked drop in blood pressure (hypotension).
- Loss of consciousness (syncope).
Milk allergy is often confused with lactose intolerance because people can have the same kinds of things happening to them (like stomach pains or bloating, for example) with both conditions. But they’re not related:
- Milk allergy is a problem involving the immune system.
- Lactose intolerance involves the digestive system (which doesn’t produce enough of the enzyme needed to break down the sugar in milk).
Certain factors may increase your risk of developing milk allergy:
- Other allergies. Many children who are allergic to milk also have other allergies. Milk allergy may develop before other allergies.
- Atopic dermatitis also known as eczema, is a common, chronic, and non-contagious skin condition that causes dry, itchy, and inflamed skin that can sometimes crack and ooze. Children who have atopic dermatitis, a common, chronic inflammation of the skin, are much more likely to develop a food allergy. It is often genetic and linked to other atopic conditions like asthma and hay fever, and its severity can be managed with moisturizers and topical corticosteroids, although there is no cure. Treatment focuses on managing symptoms, preventing flares, and avoiding triggers, with management similar for both adults and children.
- Family history. A person’s risk of a food allergy increases if one or both parents have a food allergy or another type of allergy or allergic disease, including hay fever, asthma, hives or eczema.
- Age. Milk allergy is more common in children. As they age, their digestive systems mature, and their bodies are less likely to react to milk.
Diagnosis of allergic reactions is usually obvious if symptoms occur soon after your child has consumed cow’s milk or other dairy foods. This can be confirmed by your doctor after taking a medical history and using allergy tests. Diagnosis should be made in consultation with a allergy specialist and/or specialist pediatrician. This usually involves excluding cow’s milk and other dairy foods from the diet for a trial period of one to four weeks to check for a clear improvement. A planned reintroduction of cow’s milk and other dairy foods should occur to confirm diagnosis before longer term exclusion is advised. Allergy tests (skin tests or blood tests), that measure allergen specific antibodies called immunoglobulin E (IgE), to cow’s milk are usually positive for rapid onset reactions. This is known as IgE-mediated cow’s milk allergy.
Milk allergy diagnostic algorithm 114:
- If there are signs of anaphylaxis or immediate reaction, then diet elimination is recommended, and testing for serum IgE should follow. If serum-specific IgE is positive, then the child is diagnosed with a cow’s milk allergy. If IgE is negative and symptoms improve after diet elimination, an oral challenge should be next. If the symptoms reoccur, the diagnosis is confirmed. If the symptoms do not reoccur, then the diagnosis of cow’s milk allergy is excluded 117.
- If the symptoms are not consistent with anaphylaxis or immediate reaction, then an elimination diet is recommended. If symptoms improve, then an oral challenge should be done, and if symptoms reoccur, the diagnosis is confirmed. If the symptoms do not reoccur, it excludes the diagnosis of cow’s milk allergy 117, 118.
- If symptoms do not improve after the elimination diet, this eliminates the diagnosis of cow’s milk allergy, and further evaluation should be done to assess the patient 117.
The only way to prevent an allergic reaction is to avoid milk and milk products and it is the primary treatment for milk allergy. This can be difficult because milk is a common ingredient in many foods. Also, some people with milk allergy can tolerate milk in some forms, such as milk that’s heated in baked goods (muffins, cakes or biscuits), or in some processed foods, such as yogurt. However, unless you are already certain that cooked or baked cow’s milk is tolerated, you should discuss this with your allergy specialist before introducing these foods at home. Talk to your allergy specialist about what to avoid. People with cow’s milk (dairy) allergy must avoid medicated toothpastes, chewing gums and any other dental products containing Recaldent™ which is made from cow’s milk protein.
Sources of allergy-causing milk proteins are found in dairy products, including:
- Whole milk, low-fat milk, skim milk and buttermilk.
- Butter.
- Yogurt.
- Ice cream, gelato.
- Cheese and anything that contains cheese.
- Half-and-half.
Milk can be harder to identify when it’s used as an ingredient in processed foods, including baked goods and processed meats.
Hidden sources of milk include:
- Whey.
- Casein.
- Ingredients spelled with the prefix “lact” — such as lactose and lactate.
- Candies, such as chocolate, nougat and caramel.
- Protein powders.
- Artificial butter flavor.
- Artificial cheese flavor.
- Hydrolysates.
Even if a food is labeled “milk-free” or “nondairy”, it may contain allergy-causing milk proteins. It’s important to read the label carefully. When in doubt, contact the manufacturer to be sure a product doesn’t contain milk ingredients.
Milk substitutes such as sheep’s and goat’s milk generally are not acceptable because of a high degree of cross-reactivity with cow’s milk protein. However, research shows that there have been decreased incidents of cross-reactivity to camel’s milk 119.
When eating out, ask how foods have been prepared. Does your steak have melted butter on it? Was your seafood dipped in milk before cooking?
As many as 50% of children affected by cow’s milk protein intolerance also develop soy protein intolerance if fed with soy-based formulas. Therefore, soy-based formulas are not generally a viable option for the treatment of cow’s milk protein intolerance 120, 121.
If you or your child has a serious allergic reaction (anaphylaxis), talk to your allergy specialist about carrying and using emergency epinephrine (adrenaline) 122, 123. Have your doctor or pharmacist demonstrate how to use injectable epinephrine (EpiPen, Adrenaclick, others) device so that you’re prepared for an emergency. If you have already had a severe reaction, wear a medical alert bracelet or necklace that lets others know you have a food allergy.
Oral immunotherapy may also be an option for some people with milk allergy. Oral immunotherapy is a special treatment program sometimes offered and supervised by allergy specialists. Speak with your allergy specialist about whether oral immunotherapy may be an option for you.
Fortunately, around 80% of children will outgrow their cow’s milk allergy by the age of three to five years and by the age of 6, the prevalence of cow’s milk allergy has fallen to less than 1% 124. Those who don’t outgrow it may need to continue to avoid milk products. Your doctor should advise if further allergy testing and food allergen challenges are needed. These are usually performed in hospital clinics and supervised by a allergy specialist.
How Can Doctors Tell It’s a Milk Allergy ?
If your doctor suspects you might have a milk allergy, he or she will probably refer you to an allergist or allergy specialist for more testing. The allergy specialist will ask you questions — like how often you have the reaction, the time it takes between eating a particular food and the start of the symptoms, and whether any family members have allergies or conditions like eczema and asthma.
The allergy specialist may do a skin test on you. This involves placing liquid extracts of milk protein on your forearm or back, pricking the skin a tiny bit, and waiting to see if a reddish, raised spot forms, indicating an allergic reaction.
You may need to stop taking anti-allergy medications (such as over-the-counter antihistamines) or prescription medicine 5 to 7 days before the skin test because they can affect the results. Most cold medicines and some antidepressants also may affect skin testing. Check with the allergist’s office if you are unsure about what medications need to be stopped and for how long.
The doctor also might take a blood sample and send it to a lab, where it will be mixed with some of the suspected allergen and checked for IgE antibodies.
These types of tests are used for diagnosing what doctors call a fast-onset type of milk allergy. But for people whose allergic reactions to milk develop more slowly, skin and blood tests are not as helpful.
In these cases, doctors try to diagnose the person using a food challenge. The person is told not to eat or drink anything made with milk for a period of time — usually a few weeks. Then, during the challenge, the person eats foods containing milk under a doctor’s close supervision. If symptoms come back after eating milk products, it’s a pretty sure bet the person has a milk allergy.
- Skin prick test. In this test, the skin is pricked and exposed to small amounts of the proteins found in milk. If someone has an allergy, a raised bump called a hive forms at the test location on the skin. Allergy specialists usually are best equipped to perform and interpret allergy skin tests. Keep in mind that this type of test isn’t completely accurate for detecting milk allergy.
- Blood test. A blood test can measure the immune system’s response to milk by measuring the amount of immunoglobulin E (IgE) antibodies in the blood. But this test isn’t completely accurate in identifying milk allergy.
- Diet elimination. If suspected, an infant should receive a diet free of cow’s milk protein for a month. If symptoms improve following elimination of the suspected food, then an oral food challenge is the gold standard test 125.
- Oral food challenge. If your examination and test results can’t confirm milk allergy, a allergy specialist might administer an oral food challenge. In this test, you are fed different foods that may or may not contain milk in increasing amounts to see if you react to the ones that contain milk. It’s a good idea to have allergy tests administered by an allergist who’s been trained to manage serious immunoglobulin E (IgE) mediated reaction. Patients should undergo reevaluation every 6 to 12 months to determine if they have developed a tolerance to cow’s milk protein 120.
If your allergy specialist suspects that your symptoms are caused by something other than a food allergy, you may need other tests to identify or rule out other medical problems.
There is no place in the diagnosis of cow’s milk allergy for non evidence-based tests such as cytotoxic food testing, kinesiology, hair analysis, vega testing (electro-diagnostic), electrodermal testing, pulse testing, reflexology, Bryan’s or Alcat tests, and Immunoglobulin G (IgG) to foods.
How Milk Allergy is Treated
To treat a milk allergy, the person who is allergic needs to completely avoid any foods that contain milk or milk products.
Avoiding milk involves more than just leaving the cheese off your sandwich. If you are allergic to milk, you need to read food labels carefully and not eat anything that you’re not sure about. It’s a good idea to work with a registered dietitian to develop an eating plan that provides all the nutrients you need while avoiding things you can’t eat.
If you have a severe milk allergy — or any kind of serious allergy — your doctor may want you to carry a shot of epinephrine with you in case of an emergency. Epinephrine comes in an easy-to-carry container about the size of a large marker. It’s easy to use — your doctor will show you how.
If you accidentally eat something with milk in it and start having serious allergic symptoms — like swelling inside your mouth, chest pain, or difficulty breathing — give yourself the shot right away to counteract the reaction while you’re waiting for medical help. Always call for emergency help when using epinephrine. You should make sure your school and even good friends’ houses keep injectable epinephrine on hand, too.
Keeping epinephrine with you at all times should be just part of your action plan for living with a milk allergy. It’s also a good idea to carry an over-the-counter antihistamine, which can help ease allergy symptoms in some people. But antihistamines should be used in addition to the epinephrine, not as a replacement for the shot.
If you’ve had to take an epinephrine shot because of an allergic reaction, go immediately to a medical facility or hospital emergency room so they can give you additional treatment if you need it. Sometimes, anaphylactic reactions are followed by a second wave of symptoms a few hours later. So you might need to be watched in a clinic or hospital for 4 to 8 hours following the reaction.
Living With a Milk Allergy
It can be challenging to eliminate milk from your diet, but it’s not impossible. Because most people don’t get enough calcium in their diets even if they do drink milk, many other foods are now enriched with calcium, such as juices, cereals, and rice and soy beverages. But before you eat or drink anything calcium-enriched, make sure it’s also dairy-free.
Milk and milk products can lurk in strange places, such as processed lunchmeats, margarine, baked goods, artificial butter flavor, and non-dairy products. Chocolate is another product that may contain dairy — so be sure to check the label before you eat it.
Manufacturers of foods sold in the United States must list on their labels whether a food contains any of the most common allergens. This means that you should be able to find the word “milk” stated plainly in the ingredients list, in parentheses in the ingredients list, or somewhere on the label with a statement like: “Contains milk.”
It is optional, however, for food manufacturers to use “may contain” statements. The U.S. Food and Drug Administration does not control whether companies can say things like “Processed in a facility that also processes milk products” or “May contain milk.” So call the manufacturer to be sure if you see statements like this on a food label.
New labeling requirements make it a little easier than reading the ingredients list — instead of needing to know that the ingredient “hydrolyzed casein” comes from milk protein, you should be able to tell at a glance which foods to avoid. But it’s still a good idea to get to know the “code words” for milk products when you see them in the ingredients of a food.
Some ingredients and foods that contain milk are:
- casein, calcium casein, casein hydrolysate, magenesium casein, potassium casein, rennet casein, sodium casein
- dairy products like cheese, yogurt, milk, pudding, sour cream, and cottage cheese
- butter, butter flavoring (such as diacetyl), butter fat, butter oil, ghee
- lactalbumin, lactoalbumin phosphate, lactaglobulin, lactose, lactoferrin, lactulose
- non-dairy creamers
- whey, whey hydrolysate
Vegan foods are made without animal products, such as eggs or milk. You can buy vegan products at health food stores. Be careful to read the labels of soy cheeses, though. They may say “milk-free” but could contain milk protein.
For your sweet tooth, soy- or rice-based frozen desserts, sorbets, and puddings are good substitutes for ice cream (as long as you’re not allergic to soy), as are ice pops. For baking, milk substitutes work as well as milk and some come out better. Dairy-free margarine works as well as butter for recipes and spreading on your bagel.
Try to avoid fried foods or foods with batter on them. Even if the batter doesn’t contain milk products, the oil used to fry the foods may have been used to fry something that contains milk.
People are usually understanding when it comes to food allergies — nobody wants to risk your health. When dining out, tell the waitstaff about anything you’re allergic to. Order the simplest foods and ask the waitstaff detailed questions about menu items. At a friend’s house, explain your situation and don’t be embarrassed to ask questions if you’re staying for a meal.
Having a milk allergy doesn’t mean you can’t still enjoy eating. In fact, some people think that some of the milk substitutes — like vanilla soy milk — taste better than regular cow’s milk. As with any specialized diet, you’ll probably find that avoiding milk gives you the chance to explore and discover some great foods that you’d never have found otherwise.
Milk alternatives for infants
Some research suggests that breast-feeding during the first four to six months of a baby’s life instead of giving a standard cow’s milk formula can help prevent milk allergy. In children who are allergic to milk, breast-feeding and use of hypoallergenic formula can prevent allergic reactions.
Breast-feeding is the best source of nutrition for your child. Breast-feeding for at least the first four to six months of life if possible is recommended, especially if your infant is at high risk of developing a milk allergy.
Hypoallergenic formulas are produced by using enzymes to break down (hydrolyze) milk proteins, such as casein or whey. Further processing can include heat and filtering. Depending on the level of processing, products are classified as either partially or extensively hydrolyzed. Or they may also be called elemental formulas.
Some hypoallergenic formulas aren’t milk based, but instead contain amino acids. Besides extensively hydrolyzed products, amino-acid-based formulas are the least likely to cause an allergic reaction.
Soy-based formulas are based on soy protein instead of milk. Soy formulas are fortified to be nutritionally complete — but, unfortunately, some children with a milk allergy also develop an allergy to soy.
If you’re breast-feeding and your child has a milk allergy, cow’s milk proteins passed through your breast milk may cause an allergic reaction. Then you may need to exclude all products that contain milk from your diet. Talk to your doctor if you know — or suspect — your child has a milk allergy and develops allergy signs and symptoms after breast-feeding.
If you or your child is on a milk-free diet, your doctor or dietitian can help you plan nutritionally balanced meals. You or your child may need to take supplements to replace calcium and nutrients found in milk, such as vitamin D and riboflavin.
- Milk nutritional composition and its role in human health. Pereira PC. Nutrition. 2014 Jun; 30(6):619-27. https://www.ncbi.nlm.nih.gov/pubmed/24800664/[↩]
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Applied Science; 1992. pp. 457–490.[↩]
- Mulvihill D.M., Ennis M.P. Functional milk proteins: Production and utilization. In: Fox P.F., McSweeney P.L.H., editors. Advanced Dairy Chemistry Proteins. 3rd ed. Volume 1. Springer International Publishing AG; New York, NY, USA: 2003. pp. 1175–1228.[↩]
- Raies M.H., Kapila R., Shandilya U.K., Kapila S. Impact of milk derived β-casomorphins on physiological functions and trends in research: A review. Int. J. Food Prop. 2014;17:1726–1741.[↩]
- Phelan M., Aherne A., FitzGerald R.J., O’Brien N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009;19:643–654. doi: 10.1016/j.idairyj.2009.06.001.[↩]
- Swaisgood H.E. Chemistry of the caseins. In: Fox P.F, editor. Advanced Dairy Chemistry. 1st ed. Volume 1. Elsevier Applied Science; London, UK: 1992. pp. 63–110.[↩]
- Formaggioni P., Summer A., Malacarne M., Mariani P. Milk protein polymorphism: Detection and diffusion of the genetic variants in Bos genus. Ann. Fac. Med. Vet. Univ. Parma. 1999;19:127–165.[↩][↩]
- Kamiński S, Cieslińska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. J Appl Genet 2007;48:189–98. https://www.ncbi.nlm.nih.gov/pubmed/17666771[↩]
- Ng-Kwai-Hang K.F., Grosclaude F. Genetic polymorphism of milk proteins. In: Fox P.F., McSweeney P.L.H., editors. Advanced Dairy Chemistry: Volume 1: Proteins, Parts A & B. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. pp. 739–816.[↩]
- Hamosh M., Hong H., Hamosh P. Beta-Casomorphins: Milk-β-casein derived opioid peptides. In: Lebenthal E., editor. Textbook of Gastroenterology and Nutrition in Infancy. 2nd ed. Raven Press; New York, NY, USA: 1989. pp. 143–150.[↩]
- Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Wada Y, Lönnerdal B. Pediatr Res. 2015 Apr; 77(4):546-53. https://www.ncbi.nlm.nih.gov/pubmed/25580741/[↩][↩]
- Novel opioid peptides derived from human beta-casein: human beta-casomorphins. Brantl V. Eur J Pharmacol. 1984 Oct 30; 106(1):213-4. https://www.ncbi.nlm.nih.gov/pubmed/6529969/[↩]
- Changes of beta-casomorphin content in human milk during lactation. Jarmołowska B, Sidor K, Iwan M, Bielikowicz K, Kaczmarski M, Kostyra E, Kostyra H. Peptides. 2007 Oct; 28(10):1982-6. https://www.ncbi.nlm.nih.gov/pubmed/17869380/[↩][↩]
- De Noni R.J., FitzGerald H.J.T., Korhonen Y., Le Roux C.T., Livesey I., Thorsdottir D., Tomé R.W. Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009;231:1–107.[↩][↩]
- Mencarini I.R., Woodford K.B., Old K.M. Comparing herd selection strategies for A2 beta-casein. Proc. N. Z. Soc. Anim. Prod. 2013;73:149–154.[↩]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015;7(9):7285-7297. doi:10.3390/nu7095339. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4586534/[↩][↩]
- Barnett M.P., McNabb W.C., Roy N.C., Woodford K.B., Clarke A.J. Dietary A1 β-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 β-casein in Wistar rats. Int. J. Food Sci. Nutr. 2014;65:720–727. doi: 10.3109/09637486.2014.898260. https://www.ncbi.nlm.nih.gov/pubmed/24649921[↩][↩]
- Ul Haq M.R., Kapila R., Sharma R., Saliganti V., Kapila S. Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur. J. Nutr. 2014;53:1039–1049. doi: 10.1007/s00394-013-0606-7. https://www.ncbi.nlm.nih.gov/pubmed/24166511[↩]
- Pol O, Sasaki M, Jiménez N, Dawson VL, Dawson TM, Puig MM. The involvement of nitric oxide in the enhanced expression of μ-opioid receptors during intestinal inflammation in mice. British Journal of Pharmacology. 2005;145(6):758-766. doi:10.1038/sj.bjp.0706227. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1576189/[↩]
- Holst J.J., Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. http://ajpendo.physiology.org/content/287/2/E199.long[↩]
- Schirra J, Nicolaus M, Roggel R, et al. Endogenous glucagon‐like peptide 1 controls endocrine pancreatic secretion and antro‐pyloro‐duodenal motility in humans. Gut. 2006;55(2):243-251. doi:10.1136/gut.2004.059741. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856508/[↩]
- de Vasconcelos ML, Oliveira LMFS, Hill JP, Vidal AMC. Difficulties in Establishing the Adverse Effects of β-Casomorphin-7 Released from β-Casein Variants-A Review. Foods. 2023 Aug 22;12(17):3151. doi: 10.3390/foods12173151[↩][↩][↩][↩][↩][↩]
- Uzma S., Gill H., Chandrapala J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods. 2021;10:1965. doi: 10.3390/foods10081965[↩]
- Hannelore D., Vohwinkel M., Rehner G. Effect of Casein and β-Casomorphins on Gastrointestinal Motility in Rats. J. Nutr. 1990;120:252–257. doi: 10.1093/jn/120.3.252[↩]
- Shiven P., Shah T., Sabara P., Bhatia D., Panchal K., Italiya J., Koringa P., Rank D.N. Understanding Functional Implication of β-Casein Gene Variants in Four Cattle Breeds Characterized Using AmpliSeq Approach. 3 Biotech. 2020;10:414. doi: 10.1007/s13205-020-02410-2[↩]
- Nan G., Uniacke-Lowe T., O’Regan J., Faulkner H., Alan, Kelly L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods. 2021;10:2409. doi: 10.3390/foods10102409[↩]
- Phelan M., Aherne A., FitzGerald R.J., O’Brien N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009;19:643–654. doi: 10.1016/j.idairyj.2009.06.001[↩]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015 Aug 31;7(9):7285-97. doi: 10.3390/nu7095339[↩][↩]
- Jaiswal K., De S., Sarsavan A. Detection of Single Nucleotide Polymorphism by T-ARMS PCR of Cross Bred Cattle Karan Fries for A1, A2 Beta Casein Types. Int. J. Sci. Res. Biol. Sci. 2014;1:18–22.[↩]
- Nguyen H.T., Schwendel H., Harland D., Day L. Differences in the Yoghurt Gel Microstructure and Physicochemical Properties of Bovine Milk Containing A1A1 and A2A2 β-Casein Phenotypes. Food Res. Int. 2018;112:217–224. doi: 10.1016/j.foodres.2018.06.043[↩]
- Teagan E., Krista, Dawson L., Jacqueline, Keenan I., Andrew, Day S. A Simple Method to Generate β-Casomorphin-7 by in Vitro Digestion of Casein from Bovine Milk. J. Funct. Foods. 2021;85:104631. doi: 10.1016/j.jff.2021.104631[↩][↩]
- Cieslinska A, Kostyra E, Kostyra H, Olenski K, Fiedorowicz E, Kaminski S. Milk from cows of different beta-casein genotypes as a source of beta-casomorphin-7. Int J Food Sci Nutr. 2012;63:426–430. doi: 10.3109/09637486.2011.634785[↩]
- Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides. 1999;20:957–962. doi: 10.1016/S0196-9781(99)00088-1[↩]
- Noni ID. Release of beta-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine beta-casein variants and milk-based infant formulas. Food Chem. 2008;110:897–903. doi: 10.1016/j.foodchem.2008.02.077[↩]
- Asledottir T, Le TT, Poulsen NA, Devold TG, Larsen LB, Vegarud GE. Release of β-casomorphin-7 from bovine milk of different β-casein variants after exvivo gastrointestinal digestion. Int Dairy J. 2018; 81: 8-11. doi:10.1016/j.idairyj.2017.12.014[↩][↩]
- Sheng X, Li Z, Ni J, Yelland G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J Pediatr Gastroenterol Nutr. 2019 Sep;69(3):375-382. doi: 10.1097/MPG.0000000000002437[↩][↩][↩][↩][↩]
- Asledottir T, Le TT, Petrat-Melin B, Devold TG, Larsen LB, Vegarud GE. Identification of bioactive peptides and quantification of β-casomorphin-7 from bovine β-casein A1, A2 and I after ex vivo gastrointestinal digestion. Int Dairy J. 2017; 71: 98-106. doi:10.1016/j.idairyj.2017.03.008[↩]
- Boutrou R., Gaudichon C., Dupont D., Jardin J., Airinei G., Marsset-Baglieri A., Benamouzig R., Tomé D., Leonil J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am. J. Clin. Nutr. 2013;97:1314–1323. doi: 10.3945/ajcn.112.055202[↩]
- De Noni I., Cattaneo S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem. 2010;119:560–566. doi: 10.1016/j.foodchem.2009.06.058[↩]
- De Noni I. Release of β-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine β-casein variants and milk-based infant formulas. Food Chem. 2008;110:897–903. doi: 10.1016/j.foodchem.2008.02.077[↩]
- Ul Haq M.R., Kapila R., Kapila S. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries) Food Chem. 2015;168:70–79. doi: 10.1016/j.foodchem.2014.07.024[↩]
- Woodford K.B. Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. Int. J. Environ. Res. Public Health. 2021;18:7911. doi: 10.3390/ijerph18157911[↩]
- Defilippi C, Gomez E, Charlin V, Silva C. Inhibition of small intestinal motility by casein: a role of beta casomorphins? Nutrition. 1995 Nov-Dec;11(6):751-4.[↩]
- Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J Nutr. 1990 Mar;120(3):252-7. doi: 10.1093/jn/120.3.252[↩]
- Becker A, Hempel G, Grecksch G, Matthies H. Effects of beta-casomorphin derivatives on gastrointestinal transit in mice. Biomed Biochim Acta. 1990;49(11):1203-7.[↩]
- Claustre J, Toumi F, Trompette A, Jourdan G, Guignard H, Chayvialle JA, et al. Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum. Am J Physiol Gastrointest Liver Physiol. 2002;283:G521–8. doi: 10.1152/ajpgi.00535.2001[↩]
- Trompette A, Claustre J, Caillon F, Jourdan G, Chayvialle JA, Plaisancie P. Milk bioactive peptides and beta-casomorphins induce mucus release in rat jejunum. J Nutr. 2003;133:3499–503. doi: 10.1093/jn/133.11.3499[↩]
- Zoghbi S, Trompette A, Claustre J, El Homsi M, Garzon J, Jourdan G, et al. beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1105–13. doi: 10.1152/ajpgi.00455.2005[↩]
- Elitsur Y, Luk GD. Beta-casomorphin (BCM) and human colonic lamina propria lymphocyte proliferation. Clin Exp Immunol. 1991;85:493–7. doi: 10.1111/j.1365-2249.1991.tb05755.x[↩]
- Kayser H, Meisel H. Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett. 1996;383:18–20. doi: 10.1016/0014-5793(96)00207-4[↩]
- Richard D., Clarke A., Ni J., Trivedi M. Clinical Evaluation of Glutathione Concentrations after Consumption of Milk Containing Different Subtypes of β-Casein: Results from a Randomized, Cross-over Clinical Trial. Nutr. J. 2015;15:82. doi: 10.1186/s12937-016-0201-x[↩]
- Boutrou R., Gaudichon C., Dupont D., Jardin J., Airinei G., Marsset-Baglieri A., Benamouzig R., Tomé D., Leonil J. Sequential Release of Milk Protein-Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013;97:1314–1323. doi: 10.3945/ajcn.112.055202[↩]
- Sheng X., Li Z., Ni J., Yelland G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J. Pediatr. Gastroenterol. Nutr. 2019;69:375–382. doi: 10.1097/MPG.0000000000002437[↩]
- Andrea S., Di Frangia F., Marsan P.A., De Noni I., Malacarne M. Occurrence, Biological Properties and Potential Effects on Human Health of β-Casomorphin 7: Current Knowledge and Concerns. Crit. Rev. Food Sci. Nutr. 2020;60:3705–3723. doi: 10.1080/10408398.2019.1707157[↩][↩]
- Sebely P., Woodford K., Kukuljan S., Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015;7:7285–7297. doi: 10.3390/nu7095339[↩]
- Morris D, Khurasany M, Nguyen T, et al. Glutathione and infection. Biochim Biophys Acta. 2013; 1830(5): 3329-3349. doi:10.1016/j.bbagen.2012.10.012[↩]
- Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides. 1999;20(8):957-62. doi: 10.1016/s0196-9781(99)00088-1[↩][↩]
- Sheng X, Li Z, Ni J, Yelland G. Effects of conventional milk versus milk containing only A2 β-casein on digestion in Chinese children: a randomized study. J Pediatr Gastroenterol Nutr. 2019; 69(3): 375-382. doi:10.1097/MPG.0000000000002437[↩]
- Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. 2016; 15: 35. doi:10.1186/s12937-016-0147-z[↩]
- Duarte-Vázquez M, García-Ugalde C, Villegas-Gutiérrez L, García-Almendárez B, Rosado J. Production of cow’s milk free from beta-casein A1 and its application in the manufacturing of specialized foods for early infant nutrition. Foods. 2017; 6(7):50. doi:10.3390/foods6070050[↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. 2017; 16: 72. doi:10.1186/s12937-017-0275-0[↩]
- Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr. 2014; 68(9): 994-1000. doi:10.1038/ejcn.2014.127[↩]
- Park YW, Haenlein GFW. A2 bovine milk and caprine milk as a means of remedy for milk protein allergy. Dairy. 2021; 2(2): 191-201. doi:10.3390/dairy2020017[↩]
- Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice–myths and realities. Aliment Pharmacol Ther. 2008 Jan 15;27(2):93-103. doi: 10.1111/j.1365-2036.2007.03557.x[↩]
- Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr. 2006 Apr;136(4):1107-13. doi: 10.1093/jn/136.4.1107[↩]
- Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. 2016 Apr 2;15:35. doi: 10.1186/s12937-016-0147-z. Erratum in: Nutr J. 2016 Apr 29;15(1):45. doi: 10.1186/s12937-016-0164-y[↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. 2017 Oct 25;16(1):72. doi: 10.1186/s12937-017-0275-0[↩][↩][↩][↩]
- Boutrou R, Gaudichon C, Dupont D, Jardin J, Airinei G, Marsset-Baglieri A, Benamouzig R, Tomé D, Leonil J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr. 2013 Jun;97(6):1314-23. doi: 10.3945/ajcn.112.055202[↩]
- Ul Haq MR, Kapila R, Kapila S. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries). Food Chem. 2015 Feb 1;168:70-9. doi: 10.1016/j.foodchem.2014.07.024[↩]
- Ramakrishnan M., Eaton T.K., Sermet O.M., Savaiano D.A. Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients. 2020;12:3855. doi: 10.3390/nu12123855[↩]
- Milan A.M., Shrestha A., Karlström H.J., Martinsson J.A., Nilsson N.J., Perry J.K., Day L., Barnett M.P.G., Cameron-Smith D. Comparison of the Impact of Bovine Milk β-Casein Variants on Digestive Comfort in Females Self-Reporting Dairy Intolerance: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020;111:149–160. doi: 10.1093/ajcn/nqz279[↩]
- Ho S., Woodford K., Kukuljan S., Pal S. Comparative Effects of A1 versus A2 Beta-Casein on Gastrointestinal Measures: A Blinded Randomised Cross-over Pilot Study. Eur. J. Clin. Nutr. 2014;68:994–1000. doi: 10.1038/ejcn.2014.127[↩][↩]
- Crowley E., Williams L., Roberts T., Dunstan R., Jones P. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients. 2013;5:253–266. doi: 10.3390/nu5010253[↩][↩]
- Jianqin S., Leiming X., Lu X., Yelland G.W., Ni J., Clarke A.J. Effects of Milk Containing Only A2 Beta Casein versus Milk Containing Both A1 and A2 Beta Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2015;15:35. doi: 10.1186/s12937-016-0147-z[↩][↩]
- Elisa K., Zukerman R., Eshraghi R.S., Mittal J., Deth R.C., Castejon A.M., Trivedi M., Mittal R., Eshraghi A.A. Nutritional Interventions for Autism Spectrum Disorder. Nutr. Rev. 2020;78:515–531. doi: 10.1093/nutrit/nuz092[↩]
- Cryan J.F., O’Riordan K.J., Cowan C.S., Sandhu K.V., Bastiaanssen T.F., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018[↩]
- Kay SI S., Delgado S., Mittal J., Eshraghi R.S., Mittal R., Eshraghi A.A. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J. Nutr. 2021;151:1061–1072. doi: 10.1093/jn/nxaa454[↩]
- Boushra D., Van Oudenhove L., Vervliet B., Verbeke K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3[↩][↩]
- Aya O., Zuffa S., Walton G., Fagbodun E., Zanos P., Georgiou P., Kitchen I., Swann J., Bailey A. Post-Weaning A1/A2 β-Casein Milk Intake Modulates Depressive-like Behavior, Brain μ-Opioid Receptors, and the Metabolome of Rats. IScience. 2021;24:103048. doi: 10.1016/j.isci.2021.103048[↩]
- Shah N.P.: Effects of milk-derived bioactives: an overview. Br. J. Nutr., 2000; 84: S3-S10[↩]
- Azarpazhooh A., Limeback H.: Clinical efficacy of casein derivatives: a systematic review of the literature. J. Am. Dent. Assoc., 2008; 139: 915-924.[↩]
- Hay K.D., Thomson W.M.: A clinical trial of the anticaries efficacy of casein derivatives complexed with calcium phosphate in patients with salivary gland dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 2002; 93: 271-275[↩]
- Geerts B.F., van Dongen M.G., Flameling B., Moerland M.M., de Kam M.L., Cohen A.F., Romijn J.A., Gerhardt C.C, Kloek J., Burggraaf J.: Hydrolyzed casein decreases postprandial glucose concentrations in T2DM patients irrespective of leucine content. J. Diet. Suppl., 2011; 8: 280-292[↩]
- Darewicz M., Dziuba B., Minkiewicz P., Dziuba J.: The preventive potential of milk and colostrum proteins and protein fragments. Food Rev. Int., 2011; 27: 357-388.[↩]
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Developments in Dairy Chemistry–1: Proteins. London: Applied Science Publishers; 1982. pp. 289–313.[↩]
- Piccolomini AF, Kubow S, Lands LC. Clinical Potential of Hyperbaric Pressure-Treated Whey Protein. Samman S, Darnton-Hill I, eds. Healthcare. 2015;3(2):452-465. doi:10.3390/healthcare3020452. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939533/[↩]
- Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14930-14935. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC25140/[↩]
- Protein metabolism. Slow and fast dietary proteins. Frühbeck G. Nature. 1998 Feb 26; 391(6670):843, 845. https://www.ncbi.nlm.nih.gov/pubmed/9495333/[↩]
- Emerging health properties of whey proteins and their clinical implications. Krissansen GW. J Am Coll Nutr. 2007 Dec; 26(6):713S-23S. https://www.ncbi.nlm.nih.gov/pubmed/18187438/[↩]
- Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J. Am. Coll. Nutr. 2007;26(6):713S–23S. doi: 10.1080/07315724.2007.10719652. https://www.ncbi.nlm.nih.gov/pubmed/18187438[↩]
- Hamad EM, Taha SH, Abou Dawood AG, Sitohy MZ, Abdel-Hamid M. Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids Health Dis. 2011;10 doi: 10.1186/1476-511X-10-57 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096574/[↩]
- Tauriainen E, et al. Skeletal muscle gene expression profile is modified by dietary protein source and calcium during energy restriction. J. Nutrigenet. Nutrigenomics. 2011;4(1):49–62. doi: 10.1159/000327132. https://www.ncbi.nlm.nih.gov/pubmed/21525773[↩]
- Blat S, et al. Dietary alpha-lactalbumin supplementation alleviates normocaloric western diet-induced glucose intolerance in Gottingen minipigs. Obesity. 2015;23(2):415–21. doi: 10.1002/oby.20990. https://www.ncbi.nlm.nih.gov/pubmed/25594308[↩][↩]
- Yamaguchi M, Takai S. Chronic administration of bovine milk-derived alpha-lactalbumin improves glucose tolerance via enhancement of adiponectin in Goto-Kakizaki rats with type 2 diabetes. Biol. Pharm. Bull. 2014;37(3):404–8. doi: 10.1248/bpb.b13-00762. https://www.ncbi.nlm.nih.gov/pubmed/24583859[↩]
- Soenen S, Hochstenbach-Waelen A, Westerterp-Plantenga MS. Efficacy of alpha-lactalbumin and milk protein on weight loss and body composition during energy restriction. Obesity. 2011;19(2):370–9. doi: 10.1038/oby.2010.146. https://www.ncbi.nlm.nih.gov/pubmed/20577225[↩][↩]
- Sun J, Ren F, Xiong L, Zhao L, Gao H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods. 2016;22:189–200. doi: 10.1016/j.jff.2016.01.022.[↩]
- Shi J, et al. Metabolic effects of lactoferrin during energy restriction and weight regain in diet induced obese mice. J. Funct. Foods. 2012;4(1):66–78. doi: 10.1016/j.jff.2011.08.001.[↩]
- Morishita S, et al. Bovine lactoferrin reduces visceral fat and liver triglycerides in ICR mice. J. Oleo. Sci. 2013;62(2):97–103. doi: 10.5650/jos.62.97. https://www.ncbi.nlm.nih.gov/pubmed/23391533[↩]
- Ono T, et al. Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010;104(11):1688–95. doi: 10.1017/S0007114510002734. https://www.ncbi.nlm.nih.gov/pubmed/20691130[↩]
- Cox AJ, et al. Effects of short-term supplementation with bovine lactoferrin and/or immunoglobulins on body mass and metabolic measures: a randomised controlled trial. Int. J. Food Sci. Nutr. 2017;68(2):219–226. doi: 10.1080/09637486.2016.1224230. https://www.ncbi.nlm.nih.gov/pubmed/27592680[↩]
- Chungchunlam SM, Henare SJ, Ganesh S, Moughan PJ. Dietary whey protein influences plasma satiety-related hormones and plasma amino acids in normal-weight adult women. Eur. J. Clin. Nutr. 2015;69(2):179–86. doi: 10.1038/ejcn.2014.266. https://www.ncbi.nlm.nih.gov/pubmed/25563737[↩]
- Veldhorst MA, et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin. Nutr. 2009;28(2):147–55. doi: 10.1016/j.clnu.2008.12.003. https://www.ncbi.nlm.nih.gov/pubmed/19185957[↩]
- Zapata RC, Singh A, Pezeshki A, Nibber T, Chelikani PK. Whey Protein Components – Lactalbumin and Lactoferrin – Improve Energy Balance and Metabolism. Scientific Reports. 2017;7:9917. doi:10.1038/s41598-017-09781-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577213[↩][↩]
- Bounous G, Batist G, Gold P. Immunoenhancing property of dietary whey protein in mice: role of glutathione. Clin Invest Med. 1989 Jun;12(3):154-61.[↩]
- Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. In Bioactive components of milk. Springer; 2008. pp. 163–194.[↩]
- Guimont C, Marchall E, Girardet JM, Linden G. Biologically active factors in bovine milk and dairy byproducts: influence on cell culture. Crit Rev Food Sci Nutr. 1997 Jun;37(4):393-410. doi: 10.1080/10408399709527780[↩]
- El-Agamy EI, Nawar M, Shamsia SM, Awad S, Haenlein GF. Are camel milk proteins convenient to the nutrition of cow milk allergic children?Small Rumin Res. 2009;82:1–6.[↩]
- Laleye LC, Jobe B, Wasesa AA. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J Dairy Sci. 2008 Dec;91(12):4527-34. doi: 10.3168/jds.2008-1446[↩]
- Konuspayeva G, Faye B, Loiseau G, Levieux D. Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J Dairy Sci. 2007 Jan;90(1):38-46. doi: 10.3168/jds.S0022-0302(07)72606-1[↩]
- El-Hatmi H, Girardet JM, Gaillard JL, Yahyaoui MH, Attia H. Characterisation of whey proteins of camel (camelus dromedarius) milk and colostrum. SmallRumin Res. 2007;70:267–271.[↩]
- Badr G, Ramadan NK, Sayed LH, Badr BM, Omar HM, Selamoglu Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iranian Journal of Basic Medical Sciences. 2017;20(4):338-349. doi:10.22038/IJBMS.2017.8573. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5425915/[↩]
- Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015 Feb;174(2):141-50. doi: 10.1007/s00431-014-2422-3[↩]
- Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, Henning AK, Mayer L, Burks AW, Grishin A, Stablein D, Sampson HA. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013 Mar;131(3):805-12. doi: 10.1016/j.jaci.2012.10.060[↩]
- Edwards CW, Younus MA. Cow Milk Allergy. [Updated 2024 Oct 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542243[↩][↩][↩]
- Milk allergy. https://www.mayoclinic.org/diseases-conditions/milk-allergy/symptoms-causes/syc-20375101[↩]
- Bartuzi Z, Cocco RR, Muraro A, Nowak-Węgrzyn A. Contribution of Molecular Allergen Analysis in Diagnosis of Milk Allergy. Curr Allergy Asthma Rep. 2017 Jul;17(7):46. doi: 10.1007/s11882-017-0716-z[↩]
- Kansu A, Yüce A, Dalgıç B, Şekerel BE, Çullu-Çokuğraş F, Çokuğraş H. Consensus statement on diagnosis, treatment and follow-up of cow’s milk protein allergy among infants and children in Turkey. Turk J Pediatr. 2016;58(1):1-11. doi: 10.24953/turkjped.2016.01.001[↩][↩][↩]
- Pensabene L, Salvatore S, D’Auria E, Parisi F, Concolino D, Borrelli O, Thapar N, Staiano A, Vandenplas Y, Saps M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients. 2018 Nov 9;10(11):1716. doi: 10.3390/nu10111716[↩]
- Restani P, Gaiaschi A, Plebani A, Beretta B, Cavagni G, Fiocchi A, Poiesi C, Velonà T, Ugazio AG, Galli CL. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy. 1999 Jul;29(7):997-1004. doi: 10.1046/j.1365-2222.1999.00563.x[↩]
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9. doi: 10.1097/MPG.0b013e31825c9482[↩][↩]
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM; NIAID-Sponsored Expert Panel. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010 Dec;126(6):1105-18. doi: 10.1016/j.jaci.2010.10.008[↩]
- Yue D, Ciccolini A, Avilla E, Waserman S. Food allergy and anaphylaxis. J Asthma Allergy. 2018 Jun 20;11:111-120. doi: 10.2147/JAA.S162456[↩]
- Anagnostou K. Anaphylaxis in Children: Epidemiology, Risk Factors and Management. Curr Pediatr Rev. 2018;14(3):180-186. doi: 10.2174/1573396314666180507115115[↩]
- Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(s15):23-8. doi: 10.1034/j.1399-3038.13.s.15.7.x[↩]
- Caffarelli C, Ricò S, Rinaldi L, Povesi Dascola C, Terzi C, Bernasconi S. Blood pressure monitoring in children undergoing food challenge: association with anaphylaxis. Ann Allergy Asthma Immunol. 2012 Apr;108(4):285-6. doi: 10.1016/j.anai.2012.02.001[↩]