What is cochineal
Cochineal is a red dye called carmine (E 120) or carminic acid that is obtained from the dried bodies of female cochineal insects (Dactylopius coccus Costa insects) 1. Cochineal extract [carmine (E 120) or carminic acid] is used directly in food and is also processed further to carmines. Specifications exist for cochineal extract and carmines, both of which contain carminic acid as the colouring principle. Cochineal, carminic acid and carmines (E 120) are red anthraquinone dyes authorized as food additives in the European Union (EU), in accordance with Annex II to Regulation (EC) No 1333/2008 2. Cochineal, carminic acid and carmine (E 120) have most recently been evaluated by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) in 2000 1, which set new specifications, and by the Scientific Committee for Food (SCF) in 1983 3. Both committees established an Acceptable Daily Intake (ADI) of 0–5 mg/kg body weight per day. The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) acceptable daily intake (ADI), established in 1982 for carmines (formerly cochineal, carmines and carminic acid), includes ammonium carmine or the equivalent calcium, potassium or sodium salts. For the Scientific Committee for Food (SCF), the acceptable daily intake (ADI) applies to cochineal (carmines), without further details being specified. The 1981 Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) evaluation specifically excluded the lithium salt, considering it as not acceptable for food additive use. An acceptable daily intake (ADI) is the maximum amount considered safe to consume each day over the course of your lifetime.
No studies on absorption, distribution, metabolism or excretion on cochineal extract, carminic acid or carmines were available for evaluation, but indirect evidence suggests that carmines are absorbed and distributed in the body. Both the ionisation properties of carminic acid and indirect evidence from toxicological studies suggest that these compounds can be absorbed to some extent as suggested by the accumulation of colour in tissues and the red colouring of urine reported in rats treated with ammonium carmine. Acute, short-term, subchronic, carcinogenicity, reproduction and developmental toxicity studies conducted in rats or mice did not show toxicological potential 2. Two long-term studies in rats and mice investigated the carcinogenic potential of carmine and cochineal extract, respectively. After considering all the available information, the Panel considered that the incidences of mammary hyperplasia reported in the rat study were not treatment related. Overall, the European Food Safety Authority Panel concluded that carmine is not carcinogenic 2. Consideration of the available information regarding genotoxicity indicated that carminic acid is not genotoxic 2. No adverse effects were reported in reproductive and developmental toxicity studies in rats and mice when tested at doses of up to 1 000 mg carmine/kg body weight per day or 3,000 mg cochineal extract/kg body weight per day. Overall, the European Food Safety Authority Panel considered that the available data suggest that cochineal extract and carmine do not show reproductive or developmental toxicity.
The European Food Safety Authority Panel concluded that the present dataset does not give reason to revise the acceptable daily intake (ADI) of 5 mg carmine (containing approximately 50 % carminic acid)/kg body weight, allocated by the Scientific Committee of Food in 1983 3. The European Food Safety Authority Panel concluded that this acceptable daily intake (ADI) of 0–5 mg/kg body weight should be expressed as carminic acid content, which would correspond to 2.5 mg carminic acid/kg body weight per day. The European Food Safety Authority Panel considered that, since no threshold dose can be established for allergic reactions, it is advisable that exposure to the eliciting allergens, such as proteinaceous compounds, in cochineal (E 120) is avoided by introducing appropriate purification steps in the manufacturing process. Refined exposure estimates show that exposure to cochineal (E 120) for the non-brand-loyal scenario, is below the acceptable daily intake (ADI) of 2.5 mg carminic acid/kg body weight per day for all population groups.
Specifications have been defined in Commission Regulation (EU) No 231/2012 and by JECFA in 2000. In the European Commission specifications, cochineal, carminic acid and carmine colours are defined as having not less than 2.0 % carminic acid in the extracts and not less than 50 % carminic acid in the chelates 2. The remaining material (50 to 80 %) is not precisely specified, being only described as cations that may be present in excess in the colour and also maybe containing proteinaceous material derived from the source insect, together with free carminate or a small residue of unbound aluminium cations. The European Food Safety Authority Panel noted thus that the specifications of carmines need to be updated with respect to the percentage of material not accounted for. The European Food Safety Authority Panel noted that the title of the EC specifications, “E 120, cochineal, carminic acid, carmines”, does not adequately correspond to the specified food colour. The European Food Safety Authority Panel also noted that the actual EC specifications for cochineal extract, carminic acid, carmines do not include limits for the protein content, total ash, residual solvents, or insoluble matter. The European Food Safety Authority Panel considered that further indication on the proportions or percentages of these components, particularly the protein content and the molecular weight of the key allergenic proteins, in the commercial product should be required. Furthermore, the European Food Safety Authority Panel considered that the maximum limits for toxic elements (arsenic, lead, mercury and cadmium) present as impurities in the EC specifications for E 120 should be revised in order to ensure that E 120 used as a food additive will not be a significant source of exposure to these toxic elements in food.
The European Food Safety Authority Panel noted that the term Cochineal per se is a description of the ground bodies of the female insect Dactylopius coccus Costa before extraction, and to the knowledge of the European Food Safety Authority Panel, this material is not used as a food colour. Furthermore, the composition of cochineal extracts is not well defined, and, as described further, the established acceptable daily intake (ADI) was based on studies using carmine with a defined amount of carminic acid as test material. Therefore, the European Food Safety Authority Panel proposes that the current title of the food additive (“E 120 cochineal, carminic acid, carmines”) should be revised to “E 120 cochineal extract, carminic acid and carmines” which would more accurately reflect the material used. Carmines should meet existing carmines EC specifications including those concerning the content of ≥ 50 % carminic acid.
Figure 1. Cochineal beetle
Figure 2. Cochineal bugs
Figure 3. Cochineal bugs in their natural state
Cochineal food colouring allergy
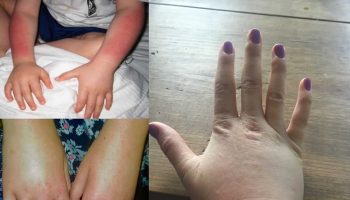
Allergic reactions have been associated with exposure to cochineal extract and carmines. Both substances are able to trigger acute hypersensitivity reactions, such as angioedema, dyspnoea and bronchospasm, in sensitised individuals, and can cause severe anaphylactic reactions. Angioedema is a skin reaction similar to hives or urticaria. Angioedema is most often characterized by an abrupt and short-lived swelling of the skin and mucous membranes. All parts of the body may be affected but swelling most often occurs around the eyes and lips. In severe cases the internal lining of the upper respiratory tract and intestines may also be affected. In addition, chronic hypersensitivity symptoms, such as rhinoconjuctivitis and asthma, have also been associated with occupational exposure to carmine. The reported effects are likely to be the consequence of allergic reactions involving an immunoglobulin E (IgE)-mediated mechanism, elicited by proteinaceous compounds in the food colour E 120.
Exposure via food and drink
A 34-year-old atopic woman had a severe anaphylactic reaction 15 min after drinking Campari with orange juice. After initial sneezing, rhinitis, and conjunctivitis, pruritus, urticaria, angioedema, dyspnoea, bronchospasm, chills, nausea, vomiting, and diarrhoea developed, requiring emergency treatment. The results of skin prick tests with Campari, the carmine dye (E120) used in the manufacture of Campari, and a commercially available carmine preparation were positive, but those of tests with extracts of common food allergens, including those from foods that the woman had eaten just before the anaphylactic reaction, were negative. She had previously experienced reactions to various carmine-containing cosmetic products, and the results of skin prick tests to these substances were positive. Specific IgE to carmine was not detected at the time of the anaphylactic reaction but only after 1 year, during which time she had suffered minor allergic episodes caused by the presence of undeclared colours in foodstuffs 4.
The same group subsequently reported this case and four others that had been referred to them over 2 years. All the new patients were female (aged 25-43) and had drunk Campari with orange or Campari Bitter before experiencing either acute urticaria and angioedema (two cases) or an anaphylactic reaction requiring emergency treatment (two cases). Two of the four new patients had a history of atopic disease. All had positive responses in skin prick tests with Campari or the carmine used in its manufacture, but only one patient reacted to a commercially available carmine preparation. The results of specific skin prick tests were positive for camomile and celeriac (two cases) or mugwort (one case). Class 1 or 2 reactions were found in a radioallergosorbent test to carmine; in only one case was a positive response found in a radioallergosorbent test to a carminic acid-human serum albumin conjugate 5.
A woman aged 35 presented to an emergency service with generalized urticaria, angioedema, and asthma that had begun 2 hours after she had eaten a yogurt containing carmine dye. She had experienced similar episodes after eating various red-coloured foods. The amount of carmine in the yoghurt was estimated to have been 1.3 mg. The results of skin prick tests with the same brand of yogurt and with carmine powder were positive, as was that of a test for the release of histamine from leukocytes, with a maximum of 18% release of histamine at a concentration of carmine of 0.1 mg /ml 6. The amount of carmine was estimated from information provided by the French food industry on the content of carmine of cochineal in a yogurt containing ‘forest fruits’ 7.
A 27-year old woman with a history of allergic rhinitis and positive responses to skin prick tests with various aeroallergens experienced an anaphylactic reaction requiring emergency treatment after eating a popsicle (iced lollipop) containing carmine. There was immediate onset of nausea, and pruritus, urticaria, and hypotension with tachycardia developed within 3 hour. She reported having had a pruritic, erythematous eruption after applying a cosmetic containing carmine directly to the skin, which did not occur if the cosmetic was applied over a foundation make-up. This reaction was not reproduced in the clinic. The results of skin prick tests with the popsicle and a carmine preparation used in the manufacture of the popsicle were positive in the patient and negative in her husband. Twenty additional control subjects gave negative reactions to skin prick tests with the carmine preparation. None of the other ingredients of the popsicle elicited positive reactions in either skin prick tests or open oral challenge of the patient. Passive sensitization (Prausnitz-Küstner test) with the husband as recipient was used to demonstrate the presence of carmine-specific IgE in the patient’s serum. Reactions did not occur at sites on the husband’s arm which had been injected with heat-treated serum but did occur at sites injected with untreated serum 8.
The same group reported the cases of two non-atopic women aged 27 and 42 who had experienced anaphylactic reactions requiring emergency treatment after ingestion of, respectively, a carmine-containing yoghurt and Campari. Both had experienced reactions to foodstuffs and carmine-containing cosmetics on previous occasions. Positive reactions in skin prick tests to a dilution of carmine and to a carmine filtrate containing materials of relative molecular mass < 3 kDa were reported in both women; the results of skin prick tests to common aeroallergens were negative. The patients refused a double-blind placebo-controlled food challenge. Forty-two control subjects, of whom 29 were considered to be atopic, did not react to a skin prick test with carmine, and 10 control subjects, who may have comprised a separate group, did not react to a skin prick test with the carmine filtrate 9.
The results of studies on the proteins in carmine that reacted with antibodies in the sera of three female patients who had experienced allergic reactions to carmine-containing foods were reported in an abstract. Two of these cases may not have been reported previously. Minced cochineal beetles (Dactylopius coccus insects) retained a red colour after dialysis; after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, several bands of relative molecular mass 23–88 kDa were recognized by sera from individual patients. The binding was inhibited by carmine. Similarly, three bands from dialysed carmine lake were detected by the sera of two of the three patients. The authors suggested that insect-derived proteins, possibly complexed with carminic acid, are responsible for allergy to carmine 10.
A 28-year old woman noted a disorder of the larynx 1 hour after drinking a Campari from an unspecified company. One hour later she had irritation and edema of the eyelids, which was followed by generalized urticaria, severe stomach ache, and diarrhea. Similar symptoms had occurred on three previous occasions after consumption of strawberry-flavored milk or red-coloured cocktails. The results of skin prick tests to the strawberry milk, the Campari, and a cochineal extract were positive 11.
A 35-year-old man who had developed asthma and rhinoconjunctivitis after handling carmine at work reported an asthmatic episode after ingestion of a red-coloured sweet containing carmine. The result of a double-blind oral challenge with E 120 was positive 12.
Conclusion
Adverse reactions to cochineal colors after occupational exposure, dermal contact, or consumption of colored food and drinks have been the subject of case reports. The reported effects were the consequence of allergic reactions, and the involvement of an immunologically mediated mechanism has been demonstrated. The nature of the adverse reactions, e.g. urticaria, rhinitis, diarrhea, and anaphylaxis, provides clear evidence that systemic reactions can follow exposure of a sensitized individual to cochineal colors. Some of the adverse reactions were severe and required emergency treatment. The weight of evidence suggests that proteins in the food colors are the allergenic species; however, the structures of the proteins and the role of protein-bound carminic acid in the allergic reaction are unknown.
The European Food Safety Authority Panel noted that cases of severe allergic reactions, occurring after the consumption of carmine-containing foodstuffs, have been reported, and indicated that the information provided to alert individuals allergic to these colours is not sufficiently acted upon. The European Food Safety Authority Panel considered that, since no threshold dose can be established for allergic reactions, it is advisable that exposure to the eliciting allergens, such as proteinaceous compounds, is avoided as much as possible. Therefore, the European Food Safety Authority Panel considered that it may be advisable to reduce the presence of these allergens as much as possible by introducing appropriate purification steps to the manufacturing process.
Recommendations
- The European Food Safety Authority Panel proposed that the current title of the food additive (“E 120 cochineal, carminic acid, carmines”) should be revised to “E 120 cochineal extract, carminic acid and carmines”, which would more accurately reflect the material used.
- The European Food Safety Authority Panel noted that the specifications for carmines need to be updated with respect to the percentage of material not accounted for, including 4-aminocarminic acid.
- The European Food Safety Authority Panel considered that the maximum limits for toxic elements (arsenic, lead, mercury and cadmium) present as impurities in the EC specifications for E 120, should be revised in order to ensure that E 120 used as a food additive will not be a significant source of exposure to these toxic elements in food.
- The European Food Safety Authority Panel considered that no threshold dose can be established for allergic reactions. Therefore, it is advisable that exposure to the eliciting allergens, such as proteinacous compounds, is avoided as much as possible. The European Food Safety Authority Panel considered that it may be advisable to reduce the presence of such compounds in E 120 by introducing appropriate purification steps in the manufacturing process.
- Because of potential allergic reactions in some people, carmine/cochineal extract are required to be identified by name on food labels. The European Food Safety Authority Panel supports an appropriate labeling of products containing E 120 (carmine/cochineal extract) to provide information regarding its presence and the potential risk of allergic reactions in susceptible individuals.
- WHO FOOD ADDITIVES SERIES 46:COCHINEAL EXTRACT, CARMINE, AND CARMINIC ACID. http://www.inchem.org/documents/jecfa/jecmono/v46je03.htm[↩][↩]
- Scientific Opinion on the re-evaluation of cochineal, carminic acid, carmines (E 120) as a food additive. EFSA Journal 2015;13(11):4288 https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2015.4288[↩][↩][↩][↩][↩]
- SCF (Scientific Committee for Food), 1983. Reports of the Scientific Committee for Food, fourteenth series. Opinion expressed 7 July 1983. https://ec.europa.eu/info/departments/health-and-food-safety_en[↩][↩]
- Kägi, M.K., Wüthrich, B. & Johansson, S.G.O. (1994). Campari-Orange anaphylaxis due to carmine allergy. Lancet, 344, 60–61.[↩]
- Wüthrich, B., Kägi, M.K. & Stücker, W. (1997) Anaphylactic reactions to ingested carmine (E120). Allergy, 52, 1133–1137.[↩]
- Beaudouin, E., Kanny, G., Lambert, H., Fremont, S. & Moneret-Vautrin, D.A. (1995) Food anaphylaxis following ingestion of carmine. Ann. Allergy Asthma Immunol., 74, 427–430.[↩]
- Moneret-Vautrin, D.-A. (2000) Unpublished information submitted to WHO by Professor D.A. Moneret-Vautrin, Service d’Immunologie Clinique et d’Allergologie, Hôpital Central, Nancy, France.[↩]
- Baldwin, J.L., Chou, A.H. & Solomon, W.R. (1997) Popsicle-induced anaphylaxis due to carmine dye allergy. Ann. Allergy Asthma Immunol., 79, 415–419.[↩]
- DiCello, M.C., Myc, A., Baker, J.R., Jr & Baldwin, J.L. (1999) Anaphylaxis after ingestion of carmine colored foods: Two case reports and a review of the literature. Allergy Asthma Proc., 20, 377–382.[↩]
- Chung, K., Chou, A., Baker, J., Jr & Baldwin, J. (2000) Identification of carmine allergens among three carmine allergy patients [Abstract]. J. Allergy Clin. Immunol., 105, S132.[↩]
- Kume, A., Fujimoto, M., Hino, N., Ueda, K. & Higashi, N. (1997) A case of Type I allergy due to cochineal extract [Abstract]. In: Program and Abstracts. The 22nd Annual Meeting of Japanese Society for Contact Dermatitis, 28–30 November 1997, Yokohama, Japan. Submitted to WHO by the National Food Colours Association, Basel, Switzerland.[↩]
- Acero, S., Tabar, A.I., Alvarez, M.J., Garcia, B.E., Olaguibel, J.M. & Moneo, I. (1998) Occupational asthma and food allergy due to carmine. Allergy, 53, 897–901.[↩]