Contents
What is Coenzyme Q10
Coenzyme Q10 (CoQ10) also called ubiquinone, ubidecarenone or vitamin Q10 is a benzoquinone compound (a fat-soluble vitamin-like molecule) that is naturally present in the human body in most body tissues, with the highest levels in the heart, liver, kidneys, and pancreas 1. The lowest amounts are found in the lungs 2. CoQ10 decreases in the body as people get older due to increased requirements, decreased production or insufficient intake of the chemical precursors needed for coenzyme Q10 synthesis 2, 3. Your body uses Coenzyme Q10 to make energy needed for the cells to grow and stay healthy. Your body also uses Coenzyme Q10 as an antioxidant 4, 5, 6, 7, 8. An antioxidant is a substance that protects cells from free radicals, which are highly reactive chemicals, often containing oxygen atoms, capable of damaging important cellular components such as DNA and lipids. In addition, the plasma level of coenzyme Q10 has been used in studies as a measure of oxidative stress 9, 10. Conditions such as fibromyalgia, diabetes, cancer, heart failure, and neurodegenerative, mitochondrial, and muscular diseases are associated with decreased circulating levels of Coenzyme Q10 (CoQ10) 11, 12, 13, 14, 15, 16. Several studies have been conducted to determine whether increasing systemic CoQ10 levels would enhance bodily function 17, 18, 19.
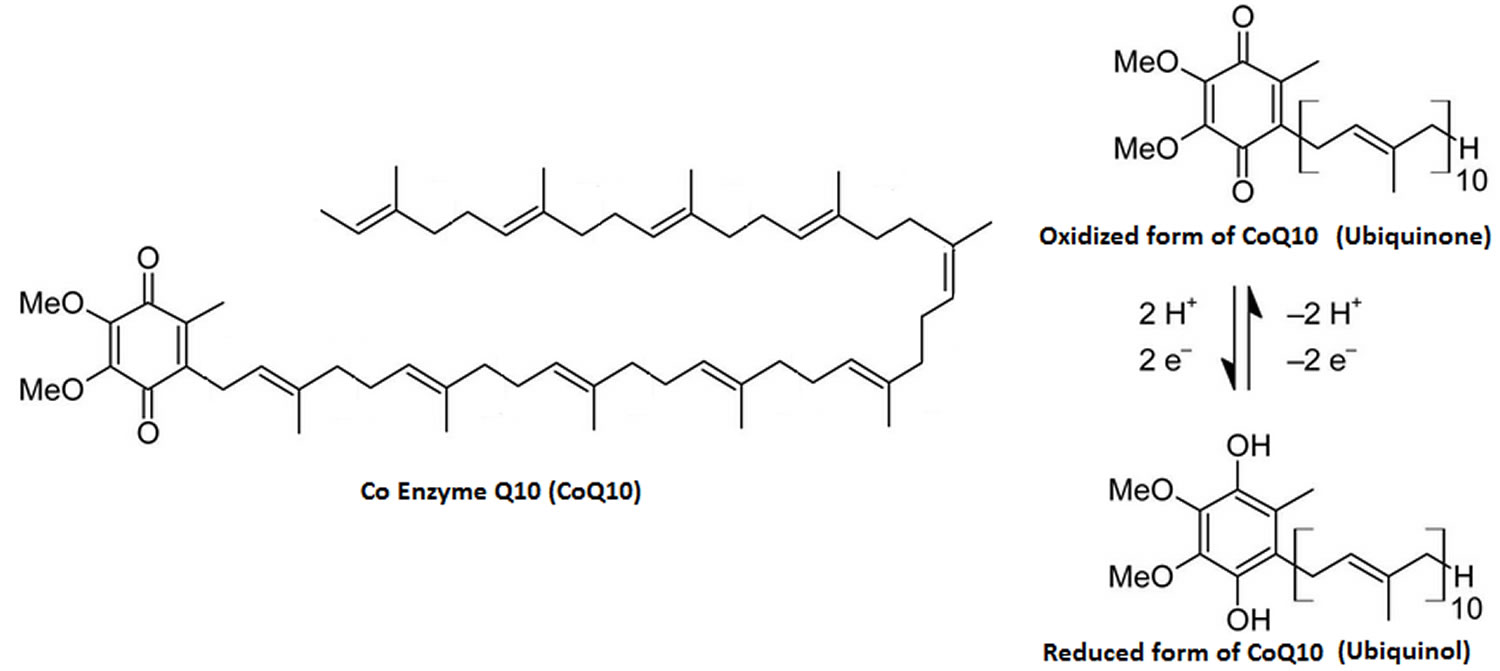
Coenzyme Q10 is a lipid-soluble benzoquinone that has 10 isoprenyl units in its side chain and is a key component of the mitochondrial respiratory chain for adenosine triphosphate (ATP) synthesis by acting as an electron carrier in mitochondria and as a co-enzyme for mitochondrial enzymes 20, 21. Studies has indicated that coenzyme Q10 is an intracellular antioxidant can protects membrane phospholipids, mitochondrial membrane protein, and low density lipoprotein-cholesterol (LDL-C) from free radical-induced oxidative damage 22. In vitro or in vivo studies have demonstrated that coenzyme Q10 not only plays an antioxidant, but also has anti-inflammation effects 23 by modulating the expression of cyclooxygenase-2 and nuclear factor-κB (NF-κB) in the liver tissue of rats with hepatocellular carcinoma 24.
The “Q” and the “10” in Coenzyme Q10 refer to the quinone chemical group and the 10 isoprenyl subunits that are part of this compound’s structure 25. The term “coenzyme” denotes it as an organic (meaning it contains carbon atoms) nonprotein molecule necessary for the proper functioning of its protein partner (an enzyme or an enzyme complex) 25.
Coenzyme Q10 is used by cells of your body in a process known variously as 25:
- Aerobic respiration: A chemical process in which oxygen is used to make energy from carbohydrates (sugars). Also called aerobic metabolism, cell respiration, and oxidative metabolism.
- Aerobic metabolism: A chemical process in which oxygen is used to make energy from carbohydrates (sugars). Also called aerobic respiration, cell respiration, and oxidative metabolism.
- Oxidative metabolism: A chemical process in which oxygen is used to make energy from carbohydrates (sugars). Also called aerobic metabolism, aerobic respiration, and cell respiration
- Cell respiration: A chemical process in which oxygen is used to make energy from carbohydrates (sugars). Also called aerobic metabolism, aerobic respiration, and oxidative metabolism..
Coenzyme Q10 is crucial for efficiently transferring electrons within the mitochondrial oxidative respiratory chain and producing adenosine triphosphate (ATP), which is a packet of energy for cell growth and maintenance 26, 4, 5, 27, 28. CoQ10 can potentially increase the production of vital antioxidants, such as superoxide dismutase, an enzyme that effectively reduces vascular oxidative stress in individuals with high blood pressure 29. In addition, CoQ10 lowers lipid peroxidation levels by diminishing pro-oxidative compounds 30. Furthermore, CoQ10 can improve blood flow and safeguard blood vessels by preserving nitric oxide.
Figure 1. Coenzyme Q10
A coenzyme Q10 deficiency occurs with age; however, certain drugs can cause depletion of coenzyme Q10 levels, particularly hydroxy-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins 31. Statins are prescribed to reduce cholesterol levels and work by inhibiting HMG-CoA reductase and the mevalonate metabolic pathway 32. Mevalonate is used to synthesize cholesterol as well as coenzyme Q10 33, therefore, when statin drugs lower cholesterol levels they simultaneously lower coenzyme Q10 levels. Statins are known to block coenzyme Q10 biosynthesis and reduce serum concentrations of coenzyme Q10 by up to 40% 34. Furthermore, statin use is often associated with a variety of muscle-related symptoms or myopathies. Research has suggested that coenzyme Q10 supplementation may decrease muscle pain associated with statin treatment 35.
Coenzyme Q10 is sold in the United States as a dietary supplement. Supplementary oral administration of coenzyme Q10 has been shown to increase coenzyme Q10 levels in plasma, platelets, and white blood cells 36. Because CoQ10 has important functions in the body and because people with some diseases have reduced levels of this substance, researchers have been interested in finding out whether CoQ10 supplements might have health benefits. Studies suggest that CoQ10 deficiency may be associated with a multitude of diseases as diverse as coronary artery disease and congestive heart failure, Parkinson’s disease, diabetes, and breast cancer, as well as the risk factor, hypertension 36. It has been suggested that Coenzyme Q10 has the potential to lower blood pressure without significant adverse events in hypertensive patients 37.
There are also a number of ways that Coenzyme Q10 could act favorably to reduce blood pressure. Coenzyme Q10 could act directly on vascular endothelium and decrease total peripheral resistance by acting as an antagonist of vascular superoxide, by either scavenging it, or suppressing its synthesis 38. Further to this, a recent meta-analysis has associated CoQ10 supplementation with a significant improvement in arterial endothelial function in patients with and without cardiovascular disease 39. Coenzyme Q10’s antioxidant properties may also result in the quenching of free radicals that cause inactivation of endothelium-derived relaxing factor or fibrosis of arteriolar smooth muscle, or both 40. In addition, CoQ10 has been found to decrease blood viscosity and improve blood flow to cardiac muscle in patients with ischemic heart disease; therefore it may reduce blood pressure 41.
Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 (the reduced form of coenzyme Q10) within circulating lipoproteins. In its reduced form the coenzyme Q10 molecule acts as a powerful intracellular antioxidant due to its ability to hold electrons rather loosely, and will quite easily give up one or both electrons. The antioxidant and free radical scavenger effects of coenzyme Q10 can therefore help to prevent lipid peroxidation and thus the progression of atherosclerosis 34. Furthermore, coenzyme Q10 has also been found to modulate the amount of ß-integrin levels on the surface of blood monocytes, strongly suggesting that the anti-atherogenic effects of coenzyme Q10 are mediated by other mechanisms beside its antioxidant properties 42.
According to 2022 American College of Cardiology/American Heart Association/The Heart Failure Society of America guidelines for the Management of Heart Failure recommended supplementation with coenzyme Q10 effectively reduced vascular mortality, all-cause mortality, and hospital stays for heart failure at 2 years 43. However, long-term supplementation is needed 43.
A recently published systematic review showed that supplementation with coenzyme Q10, in addition to standard therapy in patients with moderate-to-severe heart failure, is associated with symptom reduction and reduction of major adverse cardiovascular events 18, 44. CoQ10 may improve functional capacity, endothelial function, and left ventricle contractility in congestive heart failure patients 18, 45.
Coenzyme Q10 supplementation shows promising results in improving endothelial function in several subsets of patients. CoQ10 can improve endothelial function in patients with ischemic left ventricular systolic dysfunction and heart failure 46, 47. Likewise, compared with placebo, CoQ10 improves endothelial function in the peripheral circulation of patients with type 2 diabetes and hyperlipidemia 48. However, routine use of CoQ10 in patients with coronary artery disease apart from congestive heart failure is still inconclusive 46, 49.
There is also evidence that combined with selenium, CoQ10 supplementation in healthy older patients and older patients with diabetes, hypertension, and ischemic heart disease may decrease cardiovascular mortality risk 50. Data are conflicting on whether CoQ10 may play a role in treating high blood pressure 51. Coenzyme Q10 shows the potential to decrease pain, fatigue, and morning tiredness compared to a placebo in patients with fibromyalgia 52, 53. Some data suggest that supplementation with moderate-to-high dose coenzyme Q10 may influence bicycle exercise aerobic capacity in patients with mitochondrial disorders 54.
Supplementation with Coenzyme Q10 300 mg daily for 24 weeks in men with Peyronie disease may decrease penile plaque size, reduce penile curvature, and improve erectile function 55. Statin drugs inhibit the production of an intermediate in the mevalonate pathway—a biochemical route leading to CoQ10 synthesis 56. Therefore, many researchers theorize that statin drugs may contribute to coenzyme Q10 depletion in the body. Given that muscle pain and cramping are frequent side effects of statins, they attribute these symptoms to the diminished levels of coenzyme Q10 57.
Although most studies have used patients with preexisting medical conditions, one study of healthy participants did show that oral coenzyme Q10 supplementation improved fatigue and physical performance during bicycle exercise routines 58.
Coenzyme Q10 has also shown promise in migraine prevention 13. A cohort study of 1550 children and adolescents with headaches found that this population has low coenzyme Q10 levels 59. Coenzyme Q10 supplementation appeared to decrease headache frequency 13 A recent study indicated that CoQ10 is beneficial for the prophylactic treatment of migraine headaches in children without significant adverse effects 60. A double-blind, randomized controlled trial showed Coenzyme Q10 300 mg daily to be safe and superior to a placebo for migraine prevention 61. Another randomized, double-blind, placebo-controlled trial in adult women showed that Coenzyme Q10 400 mg of supplementation decreased migraine frequency, severity, and duration 13. One study showed that only Coenzyme Q10 100 mg daily reduced the severity of headaches and the number of headaches per month in migraine sufferers 62.
Interestingly, CoQ10 levels may be decreased in those with acute influenza infection 63. However, studies on coenzyme Q10 supplementation in patients with acute influenza have yet to be done.
Coenzyme Q10 supplemented alongside standard psychiatric medical therapy appears to lessen symptoms of depression in patients with bipolar disorder 64. In patients with polycystic ovary syndrome (PCOS), Coenzyme Q10 supplementation may improve fasting blood glucose, insulin levels, and total testosterone levels 65.
Primary Coenzyme Q10 deficiency (COQ10D6) is a rare autosomal recessive disorder caused by COQ6 gene mutations involved in coenzymeQ biosynthesis with clinical features of steroid-resistant nephrotic syndrome (SRNS), optic atrophy, retinopathy, and encephalopathy 66, 67. Coenzyme Q10 replacement therapy is indicated for this rare genetic disorder 66, 67. In patients with primary Coenzyme Q10 deficiency (COQ10D6), early treatment with high-dose Coenzyme Q10 supplementation (ranging from 5 to 50 mg/kg/day) can limit disease progression 66. A recent study of CoQ10 supplementation (20 mg/kg) demonstrated promising results in patients with primary CoQ10 deficiency with nephrotic syndrome and steroid-resistant nephrotic syndrome (SRNS) 66. Children with severe multisystem CoQ10 deficiency respond poorly to treatment and generally die within the neonatal period or in the first year of life 66.
Coenzyme Q10 (CoQ10) key facts
- Coenzyme Q10 (CoQ10) has not been shown to be of value in treating cancer, but it may reduce the risk of heart damage caused by cancer chemotherapy drug doxorubicin. It has been postulated that doxorubicin interferes with energy-generating biochemical reactions that involve coenzyme Q10 in heart muscle mitochondria and that this interference can be overcome by coenzyme Q10 supplementation 25. Studies with adults and children have confirmed the decrease in cardiac toxicity observed in animal studies 68, 69, 70, 71. A randomized trial of 20 patients tested the ability of coenzyme Q10 to reduce cardiotoxicity caused by chemotherapy drug doxorubicin 5, 6, 7.
- CoQ10 supplements might be beneficial for treating congestive heart failure 18. The 2022 American College of Cardiology/American Heart Association/The Heart Failure Society of America guidelines for the Management of Heart Failure recommended supplementation with coenzyme Q10 effectively reduced vascular mortality, all-cause mortality, and hospital stays for heart failure at 2 years 43. However, long-term supplementation is needed 43.
- Although findings are mixed, CoQ10 might help reduce blood pressure 51. Some research also suggests that when combined with other nutrients, CoQ10 might aid recovery in people who’ve had bypass and heart valve surgeries.
- Although more studies are needed, some research suggests that CoQ10 may help reduce low-density lipoprotein (LDL) cholesterol and total cholesterol levels in people with diabetes, lowering their risk of heart disease.
- Some research suggests that CoQ10 might help ease the muscle weakness and pain sometimes associated with taking statins. Supplementation with 50 mg twice daily has decreased statin-related mild-to-moderate myalgias, resulting in an increased ability to perform daily activities 57. The meta-analysis of randomized controlled trials indicated that CoQ10 supplementation (100 to 600 mg/day) decreased the Statin-Associated Muscle Symptoms (SAMS) 72.
- Recent research suggests that even high doses of CoQ10 don’t seem to improve symptoms in people with Parkinson’s disease. A major study showed that coenzyme Q10, even in higher-than-usual doses, didn’t improve symptoms in patients with early Parkinson’s disease. A 2017 evaluation of this study and several other, smaller studies concluded that coenzyme Q10 is not helpful for Parkinson’s symptoms.
- Guidelines from the American Academy of Neurology and the American Headache Society say that coenzyme Q10 is “possibly effective” in preventing migraines, but this conclusion is based on limited evidence. Some research suggests that CoQ10 might decrease the frequency of these headaches. A double-blind, randomized controlled trial showed Coenzyme Q10 300 mg daily to be safe and superior to a placebo for migraine prevention 61. Another randomized, double-blind, placebo-controlled trial in adult women showed that Coenzyme Q10 400 mg of supplementation decreased migraine frequency, severity, and duration 13. One study showed that only Coenzyme Q10 100 mg daily reduced the severity of headaches and the number of headaches per month in migraine sufferers 62.
- Because CoQ10 is involved in energy production, it’s believed that this supplement might improve your physical performance. However, research in this area has produced mixed results.
- Coenzyme Q10 (CoQ10) has also been studied for a variety of other conditions, including amyotrophic lateral sclerosis (Lou Gehrig’s disease), Down syndrome, Huntington’s disease, and male infertility, but the research is too limited for any conclusions to be drawn.
Coenzyme Q10 (CoQ10) supplement benefits
The U.S. Food and Drug Administration (FDA) does not approve dietary supplements as safe or effective. The company that makes the dietary supplements is responsible for making sure that they are safe and that the claims on the label are true and do not mislead the patient. The way that supplements are made is not regulated, so all batches and brands of coenzyme q10 supplements may not be the same.
Coenzyme Q10 for cancer
There have been few clinical trials on the use of coenzyme Q10 to prevent side effects of cancer treatment, treat side effects of cancer treatment, and/or as a treatment for cancer 73, 25. A trial of 236 breast cancer patients were randomized to receive either Coenzyme q10 or placebo, each combined with vitamin E, for 24 weeks. The study found that levels of fatigue and quality of life were not improved in patients who received Coenzyme q10 compared to patients who received the placebo 73.
A randomized trial of 20 children treated for acute lymphoblastic leukemia or non-Hodgkin lymphoma looked at whether Coenzyme q10 would protect the heart from the damage caused by doxorubicin. The results reported that CoQ10 decreased the harmful effects of doxorubicin on the heart 73. It has been postulated that doxorubicin interferes with energy-generating biochemical reactions that involve coenzyme Q10 in heart muscle mitochondria and that this interference can be overcome by coenzyme Q10 supplementation 5, 27, 28. Studies with adults and children, including the aforementioned randomized trial, have confirmed the decrease in heart toxicity observed in animal studies 5, 7, 74, 6. A randomized trial 7 of 20 patients tested the ability of coenzyme Q10 to reduce cardiotoxicity caused by anthracycline drugs.
Anecdotal reports of coenzyme Q10 lengthening the survival of patients with pancreatic, lung, rectal, laryngeal, colon, and prostate cancers also exist in the peer-reviewed scientific literature 6. The patients described in these reports also received therapies other than coenzyme Q10, including chemotherapy, radiation therapy, and surgery.
A recent observational study conducted with 1,134 patients with breast cancer enrolled in an National Cancer Institute multi-institution clinical trial (SWOG S0221) suggested that the use of antioxidant supplements, including coenzyme Q10, prior to and during cancer treatment may be associated with increased recurrence rates and decreased survival 75.
Reversal of statin-induced myopathy
Statins (HMG-CoA reductase inhibitors) deplete circulating coenzyme Q10 levels by interfering with its biosynthesis 76. Most studies indicate a correlation between the decrease in serum coenzyme Q10 and decreases of total and low-density lipoprotein cholesterol levels. This effect may be particularly important in elderly patients, in whom coenzyme Q10 levels are already compromised, and is also associated with higher dosages (lower dosages do not seem to affect intramuscular levels of coenzyme Q10) 77. The use of ezetimibe alone or in combination with a statin does not offer protection against depletion of coenzyme Q10 77. No correlation has been established for decreased serum coenzyme Q10 and cardiovascular events 77. Supplemental coenzyme Q10 increased circulating levels of the compound. However, results from randomized clinical trials are inconsistent in showing an effect on statin-associated myopathy 78, 77.
Neurological disorders
The case for coenzyme Q10 as a treatment option in neurological (mitochondrial-related) disease is not as strong 79. The role of coenzyme Q10 in Parkinson, Alzheimer, and Huntington diseases; amyotrophic lateral sclerosis; and Friedreich ataxia is postulated but not established 80.
Studies in Friedreich and non-Friedreich ataxia have largely shown a continued worsening of disease, as measured by the International Cooperative Ataxia Group rating scale, irrespective of coenzyme q10 use (5 mg/kg/day) 81.
A link between mitochondrial dysfunction and Parkinson disease has been established, but the relationship with coenzyme Q10 has not 82. A multicenter clinical trial found a decrease in worsening of symptoms in patients with early Parkinson disease receiving coenzyme Q10 1,200 mg/day, but not at lower dosages 83. Effects were not apparent at 1 month, but were evident at 8 months. Changes in daily living factors were more pronounced than clinical disease progression changes 84. Increases in plasma coenzyme Q10 were recorded. A larger trial using higher dosages (coenzyme Q10 600 mg chewable wafers 4 times a day) found a mean change in total rating score high enough to warrant a phase 3 trial 85; however, the trial was not designed to evaluate efficacy 85. A multicenter trial of patients receiving anti-Parkinson medication found no difference in symptoms over placebo 86.
The role of mitochondrial stress in Alzheimer disease led to more studies of coenzyme Q10 81. Multicenter clinical trials using idebenone dosages of up to 360 mg 3 times a day found no effect on the rate of decline over placebo. Analyses using various rating scales showed some differences that were not considered clinically important, mirroring other older trials 87. Similarly, no slowing of decline was noted in Huntington disease 88.
Other uses
Coenzyme Q10 has been evaluated in migraine versus placebo in small trials. Decreases in attack frequency, days with headache, and days with nausea were found for a daily dose of 300 mg 89. The coadministration of ubiquinone with tamoxifen mitigated the hyperlipidemia associated with tamoxifen, and tumor marker levels indicated an antiangiogenesis effect 90. An Agency for Healthcare Research and Quality review of clinical trials reported no evidence to support the use of ubiquinone in the prevention or treatment of cancer 91.
Deficiencies of coenzyme Q10 have been described, predominantly affecting children, in a spectrum of diseases including infantile-onset, multisystem diseases, as well as adult-onset cerebellar ataxia and pure myopathies 80. Lymphocyte and platelet coenzyme Q10 levels were lower in Down syndrome 92, while lowered serum levels are associated with phenylketonuria and mevalonic aciduria 93.
In infants with Prader-Willi syndrome, coenzyme Q10 had no effect on lean mass versus growth hormone 94.
Coenzyme Q10 for blood pressure and cardiovascular disease
Considering the key role of coenzyme Q10 in cellular energy production, and the high energy requirements of cardiac cells, coenzyme Q10 has a potential role in the prevention and treatment of heart ailments by improving cardiac bioenergetics 34.
Studies have shown that a coenzyme Q10 deficiency is associated with cardiovascular disease 34; however, it is uncertain whether a coenzyme Q10 deficiency is the cause or the effect of disease, especially in observational studies 20. Patients with ischemic heart disease (coronary heart disease) and dilated cardiomyopathy have been found to have significantly lower levels of coenzyme Q10 compared to healthy controls 95. In addition, the concentrations of coenzyme Q10 in blood and heart tissue decline with increasing severity of heart disease 96. Coenzyme Q10 deficiency has also been observed in patients with hypertension; enzymatic deficiency of coenzyme Q10 has been reported in 39% of hypertensive patients compared with only 6% of healthy controls 34.
Congestive heart failure
Much of the research on coenzyme Q10 is related to the secondary prevention of cardiovascular disease and results of clinical trials support the use of coenzyme Q10 in the treatment of congestive heart failure 97. Several meta-analyses and systematic reviews of clinical trials in congestive heart failure have been published, with results generally being more consistent for congestive heart failure than with other disease states. The inclusion of 2 trials in which coenzyme Q10 failed to show an effect greater than placebo in these analyses, results in only a trend in favor of coenzyme Q10 in improving cardiac function (an increase in resting ejection fraction of 1.9% 98. In a meta-analysis that included trials with a crossover or parallel-arm design, a 3.7% absolute difference in resting ejection fraction was found for coenzyme Q10 99. In other trials, coenzyme Q10 has been used in combination with other micronutrients 100. The studies, however, either do not evaluate or are underpowered to evaluate mortality outcomes 101. Because differing Coenzyme Q10 preparations were used in the studies, both the bioavailability of the compound 102 and the adequacy of dosing to reach sufficient plasma coenzyme Q10 levels for effect have been questioned 103.
Hypertension
In primary prevention, a meta-analysis of observational studies and clinical trials (12 studies, total of 362 patients) has shown that coenzyme Q10 supplementation reduces blood pressure 104. Rosenfeldt et al. conducted a meta-analysis, comprising three randomized trials 105, 106, 107, one randomized crossover study 108 and eight open-label studies 109, 110, 111 in 362 hypertensive patients, most of whom had essential hypertension or isolated systolic hypertension 112. They reported that coenzyme Q10 therapy had the potential to reduce BP by up to 17/10 mm Hg 113. The meta-analysis was however, limited by the inclusion of studies which were open-labeled and not placebo-controlled. Furthermore, there were considerable differences in patient populations with respect to age, underlying disease and comorbidities, coenzyme Q10 dose and duration, and use of concomitant antihypertensive therapy between the trials. Finally, the meta-analysis did not make use of individual patient data from the component studies, which would have provided a more robust assessment of any effect of coenzyme Q10 on arterial pressure.
On the other hand, a recent 2016 Cochrane systematic review of randomized controlled trials in participants with primary hypertension dispute these findings 114. The 2016 Cochrane Review provides moderate-quality evidence that coenzyme Q10 does not have a clinically significant effect on blood pressure. Due to the small number of individuals and studies available for analysis, more well-conducted trials are needed 115.
The additional findings from Young et al. 116, showing no effect of coenzyme q10 on the 24-hour ambulatory monitoring systolic BP, diastolic BP, or heart rate compared to placebo provide supporting evidence for the conclusion that coenzyme Q10 has no clinically significant effect on BP (blood pressure). The 24-hour measurements are done using an automatic machine, and thus are free from observer bias that could occur with clinic measurements done by a physician or nurse.
These findings concur with another double-blind, placebo-controlled intervention trial by Mori et al. 117 who found 8 weeks of coenzyme Q10 administration had no effect on 24-h ambulatory BP in patients with chronic kidney disease. In that study, treated BP levels were 125/73 mm Hg before randomization. As noted above, however, any antihypertensive action of coenzyme Q10 is likely to be less obvious the lower the baseline level of BP. In this regard, it has been shown that coenzyme Q10 does not have vasodilatory effects in normotensive animals or humans 113.
In conclusion, randomized controlled studies demonstrated that compared with placebo, coenzyme Q10 does not result in clinically significant reductions in systolic or diastolic 24-h ambulatory BP or heart rate in patients with the metabolic syndrome and inadequately treated hypertension, although there was a significant reduction in daytime diastolic BP loads. Coenzyme Q10 was well tolerated and was not associated with any clinically relevant changes in safety parameters.
Cardiac surgery and cardiac arrest
The use of coenzyme Q10 in improving mitochondrial function has been evaluated in cardiac surgery. A review was published of 8 studies, in which improvements in contractility of the myocardial tissue were demonstrated in association with increases in serum coenzyme Q10 99. Doses of coenzyme Q10 300 mg daily for 2 weeks prior to surgery were evaluated versus placebo 118. A randomized, placebo-controlled trial evaluated coenzyme Q10 450 mg in divided doses in conjunction with hypothermia after cardiac arrest. Increased survival was shown for the coenzyme Q10 group 119.
Coenzyme Q10 dosage
Several dosage forms exist, including compressed and chewable tablets, powder-filled and gel-filled capsules, liquid syrups, and newer solubilized formulations. The reduced form of coenzyme Q10, ubiquinol, is also commercially available. It may also be given by injection into a vein (IV).
Pharmacokinetic studies suggest split dosing is superior to single daily dosing; for tissue uptake and crossing the blood-brain barrier, plasma coenzyme Q10 levels should be higher than normal 120.
Usual adult dose of Coenzyme-Q10 is 30 to 200 mg/day oral.
In observational studies and clinical trials (12 studies, total of 362 patients) has shown that coenzyme Q10 supplementation reduces blood pressure 104. Dose of coenzyme Q10 ranged between 34 and 225 mg/day and duration of individual studies from one to 56 weeks.
Cardiovascular and neurologic trials predominately use coenzyme Q10 dosages of 300 mg/day or coenzyme Q10 dosages of 5 mg/kg/day.
High-dose coenzyme Q10 (1,200 mg/day) was used in patients with early Parkinson disease 83, while dosages of 2,700 to 3,000 mg/day were used in amyotrophic lateral sclerosis trials 121. An open-label study that included children evaluated tolerability of high-dose coenzyme Q10. Daily dosages of 60 mg/kg given in 3 divided doses were used for 1 month 122.
Coenzyme Q10 Contraindications
Patients receiving chemotherapeutic drugs should also avoid CoQ10, as there is insufficient data on its interaction with these drugs. As CoQ10 lowers fasting blood glucose in some patients, it should be used cautiously in those with diabetes and patients prone to hypoglycemic episodes 1. CoQ10 is contraindicated in patients with known hypersensitivity reactions to CoQ10 or excipients. Some supplements contain silicon dioxide, which may be responsible for hypersensitivity reactions 123.
Coenzyme Q10 side effects
No serious side effects of CoQ10 have been reported. Mild side effects such as insomnia or digestive upsets may occur.
Reported side effects from the use of Coenzyme-Q10 include the following:
- High levels of liver enzymes.
- Nausea.
- Heartburn.
- Headache.
- Pain in the upper part of the abdomen.
- Dizziness.
- Rashes.
- Unable to fall sleep or stay asleep.
- Feeling very tired.
- Feeling irritable.
- Sensitive to light.
It is important to check with health care providers to find out if CoQ10 can be safely used with other drugs. Certain drugs, such as those that are used to lower cholesterol, blood pressure, or blood sugar levels, may decrease the effects of Coenzyme-Q10. CoQ10 may change the way the body uses warfarin (a drug that prevents the blood from clotting) and insulin.
Coenzyme-Q10 may interact with the anticoagulant (blood thinner) warfarin and the diabetes drug insulin, and it may not be compatible with some types of cancer treatment.
Coenzyme Q10 Overdose
Coenzyme-Q10 toxicity is unlikely up to a daily intake of 1200 mg/day, although typical dosages have been 100 to 200 mg/day 124. In preclinical studies, ubiquinol’s No-Observed-Adverse-Effect Level (NOAEL) is 300 to 600 mg/kg (Sprague Dawley rats).
The human supplementation dose of CoQ10 is generally 100 to 300 mg/day. Assuming the human dose is 300 mg/day (5 mg/kg body weight), the safety factor is 60 to -120 times. The study indicated that chronic use of ubiquinol as a dietary supplement in humans is safe 125.
What other drugs will affect Coenzyme Q10?
Do not take Coenzyme Q10 without medical advice if you are using any of the following medications:
- omega-3 fatty acids;
- vitamins (especially A, C, E, or K);
- blood pressure medicine;
- cancer medicine; or
- warfarin (Coumadin, Jantoven).
This list is not complete. Other drugs may affect Coenzyme Q10, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
Toxicology
A review of animal experiments and clinical trials has estimated an acceptable daily intake for coenzyme Q10 to be 12 mg/kg (ie, 720 mg/day for a 60 kg person) based on a no-observed-adverse-effect level in rats of 1,200 mg/kg/day. An observed safety level based on clinical data is given as 1,200 mg/day. No accumulation in plasma or tissue following cessation of coenzyme Q10 consumption was noted and endogenous biosynthesis was not affected 126.
Coenzyme Q10 are a class of lipid-soluble benzoquinones that are involved in mitochondrial electron transport. They are found in the majority of aerobic organisms, from bacteria to mammals, hence the name ubiquinone (“ubiquitous quinone”).
- Sood B, Patel P, Keenaghan M. Coenzyme Q10. [Updated 2024 Jan 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531491[↩][↩]
- Ernster L, Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71(8 Suppl):S60-5. doi: 10.1007/BF00226842[↩][↩]
- Folkers K. The potential of coenzyme Q 10 (NSC-140865) in cancer treatment. Cancer Chemother Rep 2. 1974 Dec;4(4):19-22.[↩]
- Ambrosone CB, Zirpoli GR, Hutson AD, et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol. 2020 Mar 10;38(8):804-814. doi: 10.1200/JCO.19.01203[↩][↩]
- Cortes EP, Gupta M, Chou C, Amin VC, Folkers K. Adriamycin cardiotoxicity: early detection by systolic time interval and possible prevention by coenzyme Q10. Cancer Treat Rep. 1978 Jun;62(6):887-91.[↩][↩][↩][↩][↩]
- Folkers K, Brown R, Judy WV, Morita M. Survival of cancer patients on therapy with coenzyme Q10. Biochem Biophys Res Commun. 1993 Apr 15;192(1):241-5. doi: 10.1006/bbrc.1993.1405[↩][↩][↩][↩]
- Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, Indolfi P, Iacono A. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15 Suppl:s207-12. doi: 10.1016/0098-2997(94)90030-2[↩][↩][↩][↩]
- Lesser GJ, Case D, Stark N, Williford S, Giguere J, Garino LA, Naughton MJ, Vitolins MZ, Lively MO, Shaw EG; Wake Forest University Community Clinical Oncology Program Research Base. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013 Mar;11(1):31-42. doi: 10.1016/j.suponc.2012.03.003[↩]
- Iwase S, Kawaguchi T, Yotsumoto D, Doi T, Miyara K, Odagiri H, Kitamura K, Ariyoshi K, Miyaji T, Ishiki H, Inoue K, Tsutsumi C, Sagara Y, Yamaguchi T. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support Care Cancer. 2016 Feb;24(2):637-646. doi: 10.1007/s00520-015-2824-4[↩]
- Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 1997 May 19;234(2):296-9. doi: 10.1006/bbrc.1997.6522[↩]
- Du J, Wang T, Huang P, Cui S, Gao C, Lin Y, Fu R, Shen J, He Y, Tan Y, Chen S. Clinical correlates of decreased plasma coenzyme Q10 levels in patients with multiple system atrophy. Parkinsonism Relat Disord. 2018 Dec;57:58-62. doi: 10.1016/j.parkreldis.2018.07.017[↩]
- Chang PS, Chou HH, Lai TJ, Yen CH, Pan JC, Lin PT. Investigation of coenzyme Q10 status, serum amyloid-β, and tau protein in patients with dementia. Front Aging Neurosci. 2022 Jul 25;14:910289. doi: 10.3389/fnagi.2022.910289[↩]
- Dahri M, Tarighat-Esfanjani A, Asghari-Jafarabadi M, Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr Neurosci. 2019 Sep;22(9):607-615. doi: 10.1080/1028415X.2017.1421039[↩][↩][↩][↩][↩]
- Al Saadi T, Assaf Y, Farwati M, Turkmani K, Al-Mouakeh A, Shebli B, Khoja M, Essali A, Madmani ME. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2021 Feb 3;(2)(2):CD008684. doi: 10.1002/14651858.CD008684.pub3[↩]
- Rauchová H. Coenzyme Q10 effects in neurological diseases. Physiol Res. 2021 Dec 30;70(Suppl4):S683-S714. doi: 10.33549/physiolres.934712[↩]
- Ho CC, Tseng CY, Chen HW, Chiu YW, Tsai MC, Chang PS, Lin PT. Coenzyme Q10 status, glucose parameters, and antioxidative capacity in college athletes. J Int Soc Sports Nutr. 2020 Jan 10;17(1):5. doi: 10.1186/s12970-020-0334-3[↩]
- Cordero MD, Santos-García R, Bermejo-Jover D, Sánchez-Domínguez B, Jaramillo-Santos MR, Bullón P. Coenzyme Q10 in salivary cells correlate with blood cells in Fibromyalgia: improvement in clinical and biochemical parameter after oral treatment. Clin Biochem. 2012 Apr;45(6):509-11. doi: 10.1016/j.clinbiochem.2012.02.001[↩]
- Jafari M, Mousavi SM, Asgharzadeh A, Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018 Jul;70 Suppl 1(Suppl 1):S111-S117. doi: 10.1016/j.ihj.2018.01.031[↩][↩][↩][↩]
- Garrido-Maraver J, Cordero MD, Oropesa-Ávila M, Fernández Vega A, de la Mata M, Delgado Pavón A, de Miguel M, Pérez Calero C, Villanueva Paz M, Cotán D, Sánchez-Alcázar JA. Coenzyme q10 therapy. Mol Syndromol. 2014 Jul;5(3-4):187-97. doi: 10.1159/000360101[↩]
- Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. International Journal of Biological Sciences 2007;3(4):257-62.[↩][↩]
- Bhagavan H.N., Chopra R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006;40:445–453. doi: 10.1080/10715760600617843[↩]
- Flowers N., Hartley L., Todkill D., Stranges S., Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014;12:CD010405 https://www.ncbi.nlm.nih.gov/pubmed/25474484[↩]
- Yoneda T., Tomofuji T., Kawabata Y., Ekuni D., Azuma T., Kataoka K., Kunitomo M., Morita M. Application of coenzyme Q10 for accelerating soft tissue wound healing after tooth extraction in rats. Nutrients. 2014;6:5756–5769. doi: 10.3390/nu6125756[↩]
- Fouad A.A., Al-Mulhim A.S., Jresat I. Therapeutic effect of coenzyme Q10 against experimentally-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2013;35:100–108. doi: 10.1016/j.etap.2012.11.016[↩]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Coenzyme Q10 (PDQ®): Health Professional Version. 2024 Apr 5. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65890[↩][↩][↩][↩][↩]
- Schniertshauer D, Müller S, Mayr T, Sonntag T, Gebhard D, Bergemann J. Accelerated Regeneration of ATP Level after Irradiation in Human Skin Fibroblasts by Coenzyme Q10. Photochem Photobiol. 2016 May;92(3):488-94. doi: 10.1111/php.12583[↩]
- Usui T, Ishikura H, Izumi Y, Konishi H, Dohmae N, Sawada H, Uchino H, Matsuda H, Konishi T. Possible prevention from the progression of cardiotoxicity in adriamycin-treated rabbits by coenzyme Q10. Toxicol Lett. 1982 Jun;12(1):75-82. doi: 10.1016/0378-4274(82)90201-6[↩][↩]
- Iwamoto Y, Hansen IL, Porter TH, Folkers K. Inhibition of coenzyme Q10-enzymes, succinoxidase and NADH-oxidase, by adriamycin and other quinones having antitumor activity. Biochem Biophys Res Commun. 1974 Jun 4;58(3):633-8. doi: 10.1016/s0006-291x(74)80465-1[↩][↩]
- Kędziora-Kornatowska K, Czuczejko J, Motyl J, Szewczyk-Golec K, Kozakiewicz M, Pawluk H, Kędziora J, Błaszczak R, Banach M, Rysz J. Effects of coenzyme Q10 supplementation on activities of selected antioxidative enzymes and lipid peroxidation in hypertensive patients treated with indapamide. A pilot study. Arch Med Sci. 2010 Aug 30;6(4):513-8. doi: 10.5114/aoms.2010.14461[↩]
- Cordero MD, Cano-García FJ, Alcocer-Gómez E, De Miguel M, Sánchez-Alcázar JA. Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q₁₀ effect on clinical improvement. PLoS One. 2012;7(4):e35677. doi: 10.1371/journal.pone.0035677[↩]
- Flowers N, Hartley L, Todkill D, Stranges S, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No.: CD010405. DOI: 10.1002/14651858.CD010405.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD010405.pub2/full[↩]
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, et al. Lovastatin decreases coenzyme Q levels in humans. Proceedings of the National Academy of Sciences of the United States of America 1990;87:8931-4.[↩]
- Schaars CF, Stalenhoef AF. Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Current Opinion in Lipidology 2008;19:553-7.[↩]
- Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacology and Therapeutics 2009;124(3):259-68.[↩][↩][↩][↩][↩]
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. American Journal of Cardiology 2007;99(10):1409-12.[↩]
- Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defence against oxidative damage. International Journal of Biological Sciences 2007;3(4):257-62.[↩][↩]
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong J-Y, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. Journal of Human Hypertension 2007;21:297–306.[↩]
- McCarty MF. Coenzyme Q versus hypertension: does CoQ decrease endothelial superoxide generation?. Medical Hypotheses 1999;53(4):300-4.[↩]
- Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis 2012;221(2):311-6.[↩]
- Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circulation Research 1989;65:1-21.[↩]
- Kato T, Yoneda S. Reduction in blood viscosity by treatment with coenzyme Q10 in patients with ischemic heart disease. International Journal of Clinical Pharmacology, Therapy and Toxicology 1990;28(3):123-6.[↩]
- Turunen M, Wehlin L, Sjoberg M, Lundahl J, Dallner G, Brismar K, et al. Beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochemical and Biophysical Research Communications 2002;296:255-60.[↩]
- Correction to: 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Sep 27;146(13):e185. doi: 10.1161/CIR.0000000000001097. Epub 2022 Sep 26. Erratum for: Circulation. 2022 May 3;145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063[↩][↩][↩][↩]
- Bhatt KN, Butler J. Myocardial Energetics and Heart Failure: a Review of Recent Therapeutic Trials. Curr Heart Fail Rep. 2018 Jun;15(3):191-197. doi: 10.1007/s11897-018-0386-8[↩]
- Belardinelli R, Muçaj A, Lacalaprice F, Solenghi M, Seddaiu G, Principi F, Tiano L, Littarru GP. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006 Nov;27(22):2675-81. doi: 10.1093/eurheartj/ehl158[↩]
- Dai YL, Luk TH, Yiu KH, Wang M, Yip PM, Lee SW, Li SW, Tam S, Fong B, Lau CP, Siu CW, Tse HF. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011 Jun;216(2):395-401. doi: 10.1016/j.atherosclerosis.2011.02.013[↩][↩]
- Zozina VI, Covantev S, Goroshko OA, Krasnykh LM, Kukes VG. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr Cardiol Rev. 2018;14(3):164-174. doi: 10.2174/1573403X14666180416115428[↩]
- Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia. 2002 Mar;45(3):420-6. doi: 10.1007/s00125-001-0760-y[↩]
- Ayers J, Cook J, Koenig RA, Sisson EM, Dixon DL. Recent Developments in the Role of Coenzyme Q10 for Coronary Heart Disease: a Systematic Review. Curr Atheroscler Rep. 2018 May 16;20(6):29. doi: 10.1007/s11883-018-0730-1[↩]
- Alehagen U, Aaseth J, Alexander J, Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS One. 2018 Apr 11;13(4):e0193120. doi: 10.1371/journal.pone.0193120[↩]
- Wong AP, Kassab YW, Mohamed AL, Abdul Qader AM. Review: Beyond conventional therapies: Complementary and alternative medicine in the management of hypertension: An evidence-based review. Pak J Pharm Sci. 2018 Jan;31(1):237-244.[↩][↩]
- Cordero MD, Alcocer-Gómez E, de Miguel M, Culic O, Carrión AM, Alvarez-Suarez JM, Bullón P, Battino M, Fernández-Rodríguez A, Sánchez-Alcazar JA. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal. 2013 Oct 20;19(12):1356-61. doi: 10.1089/ars.2013.5260[↩]
- Cordero MD, Alcocer-Gómez E, de Miguel M, Cano-García FJ, Luque CM, Fernández-Riejo P, Fernández AM, Sánchez-Alcazar JA. Coenzyme Q(10): a novel therapeutic approach for Fibromyalgia? case series with 5 patients. Mitochondrion. 2011 Jul;11(4):623-5. doi: 10.1016/j.mito.2011.03.122[↩]
- Glover EI, Martin J, Maher A, Thornhill RE, Moran GR, Tarnopolsky MA. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010 Nov;42(5):739-48. doi: 10.1002/mus.21758[↩]
- Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie’s disease: a double-blind, placebo-controlled randomized study. Int J Impot Res. 2010 Sep-Oct;22(5):298-309. doi: 10.1038/ijir.2010.20[↩]
- Zaleski AL, Taylor BA, Thompson PD. Coenzyme Q10 as Treatment for Statin-Associated Muscle Symptoms-A Good Idea, but…. Adv Nutr. 2018 Jul 1;9(4):519S-523S. doi: 10.1093/advances/nmy010[↩]
- Skarlovnik A, Janić M, Lunder M, Turk M, Šabovič M. Coenzyme Q10 supplementation decreases statin-related mild-to-moderate muscle symptoms: a randomized clinical study. Med Sci Monit. 2014 Nov 6;20:2183-8. doi: 10.12659/MSM.890777[↩][↩]
- Mizuno K, Tanaka M, Nozaki S, Mizuma H, Ataka S, Tahara T, Sugino T, Shirai T, Kajimoto Y, Kuratsune H, Kajimoto O, Watanabe Y. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008 Apr;24(4):293-9. doi: 10.1016/j.nut.2007.12.007. Epub 2008 Feb 13. Erratum in: Nutrition. 2008 Jun;24(6):616.[↩]
- Hershey AD, Powers SW, Vockell AL, Lecates SL, Ellinor PL, Segers A, Burdine D, Manning P, Kabbouche MA. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007 Jan;47(1):73-80. doi: 10.1111/j.1526-4610.2007.00652.x[↩]
- Yaghini O, Hoseini N, Ghazavi MR, Mansouri V, Nasiri J, Moosavian T, Salehi MM. A Comparative Study on the Efficacy of Coenzyme Q10 and Amitriptyline in the Prophylactic Treatment of Migraine Headaches in Children: A Randomized Controlled Trial. Adv Biomed Res. 2022 May 30;11:43. doi: 10.4103/abr.abr_235_20[↩]
- Sándor PS, Di Clemente L, Coppola G, Saenger U, Fumal A, Magis D, Seidel L, Agosti RM, Schoenen J. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005 Feb 22;64(4):713-5. doi: 10.1212/01.WNL.0000151975.03598.ED[↩][↩]
- Shoeibi A, Olfati N, Soltani Sabi M, Salehi M, Mali S, Akbari Oryani M. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: an open-label, add-on, controlled trial. Acta Neurol Belg. 2017 Mar;117(1):103-109. doi: 10.1007/s13760-016-0697-z[↩][↩]
- Chase M, Cocchi MN, Liu X, Andersen LW, Holmberg MJ, Donnino MW. Coenzyme Q10 in acute influenza. Influenza Other Respir Viruses. 2019 Jan;13(1):64-70. doi: 10.1111/irv.12608[↩]
- Mehrpooya M, Yasrebifar F, Haghighi M, Mohammadi Y, Jahangard L. Evaluating the Effect of Coenzyme Q10 Augmentation on Treatment of Bipolar Depression: A Double-Blind Controlled Clinical Trial. J Clin Psychopharmacol. 2018 Oct;38(5):460-466. doi: 10.1097/JCP.0000000000000938[↩]
- Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, Gargari BP. Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019 Feb 1;104(2):319-327. doi: 10.1210/jc.2018-01221[↩]
- Salviati L, Trevisson E, Agosto C, et al. Primary Coenzyme Q10 Deficiency Overview. 2017 Jan 26 [Updated 2023 Jun 8]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK410087[↩][↩][↩][↩][↩]
- Yuruk Yildirim Z, Toksoy G, Uyguner O, Nayir A, Yavuz S, Altunoglu U, Turkkan ON, Sevinc B, Gokcay G, Kurkcu Gunes D, Kiyak A, Yilmaz A. Primary coenzyme Q10 Deficiency-6 (COQ10D6): Two siblings with variable expressivity of the renal phenotype. Eur J Med Genet. 2020 Jan;63(1):103621. doi: 10.1016/j.ejmg.2019.01.011[↩][↩]
- Beyer RE, Nordenbrand K, Ernster L: The role of coenzyme Q as a mitochondrial antioxidant: a short review. In: Folkers K, Yamamura Y, eds.: Biomedical and Clinical Aspects of Coenzyme Q. Vol 5. Elsevier Science Publishers B V (Biomedical Division), 1986, pp 17-24.[↩]
- Pepping J. Coenzyme Q10. Am J Health Syst Pharm. 1999 Mar 15;56(6):519-21. doi: 10.1093/ajhp/56.6.519[↩]
- Palazzoni G, Pucello D, Littarru GP, Nardone L, Marin AW, Romagnoli A. Coenzyme Q10 and colorectal neoplasms in aged patients. Rays. 1997 Jan-Mar;22(1 Suppl):73-6.[↩]
- Gordon M. Dietary antioxidants in disease prevention. Nat Prod Rep. 1996 Aug;13(4):265-73. doi: 10.1039/np9961300265[↩]
- Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018 Oct 2;7(19):e009835. doi: 10.1161/JAHA.118.009835[↩]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Coenzyme Q10 (PDQ®): Patient Version. 2024 May 28. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65828[↩][↩][↩]
- Folkers K, Wolaniuk A. Research on coenzyme Q10 in clinical medicine and in immunomodulation. Drugs Exp Clin Res. 1985;11(8):539-45.[↩]
- Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, Kelly KM, Cannioto R, Sucheston-Campbell LE, Hershman DL, Unger JM, Moore HCF, Stewart JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Budd GT, Albain KS. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol. 2020 Mar 10;38(8):804-814. doi: 10.1200/JCO.19.01203[↩]
- Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Curr Opin Clin Nutr Metab Care . 2005;8(6):641-646.[↩]
- Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol . 2007;49(23):2231-2237.[↩][↩][↩][↩]
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol . 2007;99(10):1409-1412.[↩]
- Marriage B, Clandinin MT, Glerum DM. Nutritional cofactor treatment in mitochondrial disorders. J Am Diet Assoc . 2003;103(8):1029-1038.[↩]
- Quinzii CM, López LC, Naini A, DiMauro S, Hirano M. Human CoQ10 deficiencies. Biofactors . 2008;32(1-4):113-118.[↩][↩]
- Schapira AH. Mitochondrial disease. Lancet . 2006;368(9529):70-82.[↩][↩]
- Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA . 2004;291(3):358-364.[↩]
- Shults CW, Oakes D, Kieburtz K, et al; Parkinson Study Group. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol . 2002;59(10):1541-1550.[↩][↩]
- LeWitt PA. Clinical trials of neuroprotection for Parkinson’s disease. Neurology . 2004;63(7 suppl 2):S23-S31.[↩]
- NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology . 2007;68(1):20-28.[↩][↩]
- Storch A, Jost WH, Vieregge P, et al; German Coenzyme Q(10) Study Group. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol . 2007;64(7):938-944.[↩]
- Steele PE, Tang PH, DeGrauw AJ, Miles MV. Clinical laboratory monitoring of coenzyme Q10 use in neurologic and muscular diseases. Am J Clin Pathol . 2004;121(suppl):S113-S120.[↩]
- Thal LJ, Grundman M, Berg J, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology . 2003;61(11):1498-1502.[↩]
- Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology . 2005;64(4):713-715.[↩]
- Sachdanandam P. Antiangiogenic and hypolipidemic activity of coenzyme Q10 supplementation to breast cancer patients undergoing tamoxifen therapy. Biofactors . 2008;32(1-4):151-159.[↩]
- Coulter I, Hardy M, Shekelle P, et al. Effect of the Supplemental Use of Antioxidants Vitamin C, Vitamin E, and Coenzyme Q10 for the Prevention and Treatment of Cancer . Evidence Report/Technology Assessment Number 75 (Prepared by Southern California Evidence-based Practice Center under Contract No. 290-97-0001). AHRQ Publication No. 03-E047. Rockville, MD[↩]
- Tiano L, Padella L, Carnevali P, et al. Coenzyme Q10 and oxidative imbalance in Down syndrome: biochemical and clinical aspects. Biofactors . 2008;32(1-4):161-167.[↩]
- Hargreaves IP. Coenzyme Q10 in phenylketonuria and mevalonic aciduria. Mitochondrion . 2007;7(suppl 1):S175-S180.[↩]
- Eiholzer U, L’allemand D, Schlumpf M, Rousson V, Gasser T, Fusch C. Growth hormone and body composition in children younger than 2 years with Prader-Willi syndrome. J Pediatr . 2004;144(6):753-758.[↩]
- Langsjoen PH. A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10. International Journal of Tissue Reactions 1990;12(3):169-71.[↩]
- Littarru GP, Ho L, Folkers K. Deficiency of coenzyme Q10 in human heart disease. Part I. International Journal for Vitamin and Nutrition Research 1972;42:291-305.[↩]
- Hofman-Bang C, Rehnquist N, Swedberg K, Wiklund I, Astrom H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 study group. Journal of Cardiac Failure 1995;1:101-7.[↩]
- Vogel JH, Bolling SF, Costello RB, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Integrating complementary medicine into cardiovascular medicine. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine). J Am Coll Cardiol . 2005;46(1):184-221.[↩]
- Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion . 2007;7(suppl):S154-S167.[↩][↩]
- Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal of Cardiology 2013;167:1860-6.[↩]
- Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (Coenzyme Q10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors . 2008;32(1-4):145-149.[↩]
- Young JM, Florkowski CM, Molyneux SL, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol . 2007;100(9):1400-1403.[↩]
- Sinatra ST. Metabolic cardiology: an integrative strategy in the treatment of congestive heart failure. Altern Ther Health Med . 2009;15(3):44-52[↩]
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong J-Y, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. Journal of Human Hypertension 2007;21:297-306.[↩][↩]
- Yamagami T, Takagi M, Akagami H, Kubo SH, Toyama S, Okamoto T. Effect of coenzyme Q10 on essential hypertension: a double blind controlled study. in: Folkers K, Yamamura Y (eds). Biomedical & Clinical Aspects of Coenzyme Q. Elsevier Science Publishers BV: Amsterdam, North Holland :: Amsterdam, North Holland, 1986, pp. 337–343.[↩]
- Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens ; 1999;13:203–208.[↩]
- Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J ; 2001;94:1112–1117.[↩]
- Digiesi V, Cantini F, Brodbeck B. Effect of coenzyme Q10 on essential arterial hypertension. Curr Ther Res ; 1990;47:841–845.[↩]
- Yamagami T, Shibata N, Folkers K. Bioenergetics in clinical medicine. VIII. Adminstration of coenzyme Q10 to patients with essential hypertension. Res Commun Chem Pathol Pharmacol ; 1976;14:721–727.[↩]
- Folkers K, Drzewoski J, Richardson PC, Ellis J, Shizukuishi S, Baker L. Bioenergetics in clinical medicine. XVI. Reduction of hypertension in patients by therapy with coenzyme Q10. Res Commun Chem Pathol Pharmacol ; 1981;31:129–140.[↩]
- Langsjoen P, Langsjoen P, Willis R, Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol Aspects Med ; 1994;15 Suppl:S265–S272.[↩]
- [↩]
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens ; 2007;21:297–306.[↩][↩]
- Ho MJ, Li ECK, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No.: CD007435. DOI: 10.1002/14651858.CD007435.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007435.pub3/full[↩]
- Ho MJ, Li ECK, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No.: CD007435. DOI:10.1002/14651858.CD007435.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007435.pub3/full[↩]
- Joanna M. Young, Christopher M. Florkowski, Sarah L. Molyneux, Roberta G. McEwan, Christopher M. Frampton, M. Gary Nicholls, Russell S. Scott, Peter M. George; A Randomized, Double-Blind, Placebo-Controlled Crossover Study of Coenzyme Q10 Therapy in Hypertensive Patients With the Metabolic Syndrome, American Journal of Hypertension, Volume 25, Issue 2, 1 February 2012, Pages 261–270, https://doi.org/10.1038/ajh.2011.209[↩]
- Mori TA, Burke V, Puddey I, Irish A, Cowpland CA, Beilin L, Dogra G, Watts GF. The effects of [omega]3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: a randomized controlled trial. J Hypertens ; 2009;27:1863–1872.[↩]
- Rosenfeldt F, Marasco S, Lyon W, et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg . 2005;129(1):25-32.[↩]
- Damian MS, Ellenberg D, Gildemeister R, et al. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: a preliminary study. Circulation . 2004;110(19):3011-3016.[↩]
- Bhagavan HN, Chopra RK. Potential role of ubiquinone (coenzyme Q10) in pediatric cardiomyopathy. Clin Nutr . 2005;24(3):331-338.[↩]
- Levy G, Kaufmann P, Buchsbaum R, et al. A two-stage design for a phase II clinical trial of coenzyme Q10 in ALS. Neurology . 2006;66(5):660-663.[↩]
- Di Prospero NA, Sumner CJ, Penzak SR, Ravina B, Fischbeck KH, Taylor JP. Safety, tolerability, and pharmacokinetics of high-dose idebenone in patients with Friedreich ataxia. Arch Neurol . 2007;64(6):803-808.[↩]
- Ben Fredj N, Ben Fadhel N, Chaabane A, Chadly Z, Ben Romdhane H, Boughattas A, Aouam K. Colloidal silica-induced hypersensitivity: myth or reality. Int J Clin Pharm. 2016 Feb;38(1):7-9. doi: 10.1007/s11096-015-0225-x[↩]
- Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors. 2008;32(1-4):199-208. doi: 10.1002/biof.5520320124[↩]
- Deshmukh G, Venkataramaiah SB, Doreswamy CM, Umesh MC, Subbanna RB, Pradhan BK, Seekallu S, Sekar R, Prabhu K, Sadagopan S, Arumugam SN, Sharma S, Gavara G, Balaraman S, Sambasivam G, Chandrappa RK, Flynn S, Shivarudraiah P. Safety Assessment of Ubiquinol Acetate: Subchronic Toxicity and Genotoxicity Studies. J Toxicol. 2019 Apr 1;2019:3680757. doi: 10.1155/2019/3680757[↩]
- Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors . 2008;32(1-4):199-208.[↩]