Contents
What are electrolytes
Electrolytes are minerals that are found in your body tissues and blood in the form of dissolved salts that have an electric charge. As electrically charged particles, electrolytes help move nutrients into and wastes out of the body’s cells, maintain a healthy water balance, and help stabilize the body’s acid/base (pH) level.
Electrolytes are in your blood, urine, tissues, and other body fluids. Electrolytes are important because they help
- Balance the amount of water in your body
- Balance your body’s acid/base (pH) level
- Move nutrients into your cells
- Move wastes out of your cells
- Make sure that your nerves, muscles, the heart, and the brain work the way they should
Sodium (Na+), calcium (Ca2+), potassium (K+), chloride (Cl−), bicarbonate (HCO3−, sometimes reported as total CO2), phosphate (PO4 3−), and magnesium (Mg2+) are all electrolytes. You get them from the foods you eat and the fluids you drink.
A person’s diet provides sodium, potassium, and chloride. The kidneys help maintain proper levels by reabsorption or by elimination into the urine. The lungs provide oxygen and regulate CO2. The CO2 is produced by the body and is in balance with bicarbonate bicarbonate (HCO3−). The overall balance of these chemicals is an indication of the functional well-being of several basic body functions. They are important in maintaining a wide range of body functions, including heart and skeletal muscle contraction and nerve impulse conduction.
Any disease or condition that affects the amount of fluid in the body, such as dehydration, or affects the lungs, kidneys, metabolism, or breathing has the potential to cause a fluid, electrolyte, or pH imbalance (acidosis or alkalosis). Normal pH must be maintained within a narrow range of 7.35-7.45 and electrolytes must be in balance to ensure the proper functioning of metabolic processes and the delivery of the right amount of oxygen to tissues.
The levels of electrolytes in your body can become too low or too high. This can happen when the amount of water in your body changes. The amount of water that you take in should equal the amount you lose. If something upsets this balance, you may have too little water (dehydration) or too much water (overhydration). Some medicines, vomiting, diarrhea, sweating, and liver or kidney problems can all upset your water balance. For more on this, see the article on what are symptoms and signs of dehydration.
Treatment helps you to manage the electrolyte imbalance. It also involves identifying and treating what caused the electrolyte imbalance.
Fluid and electrolytes
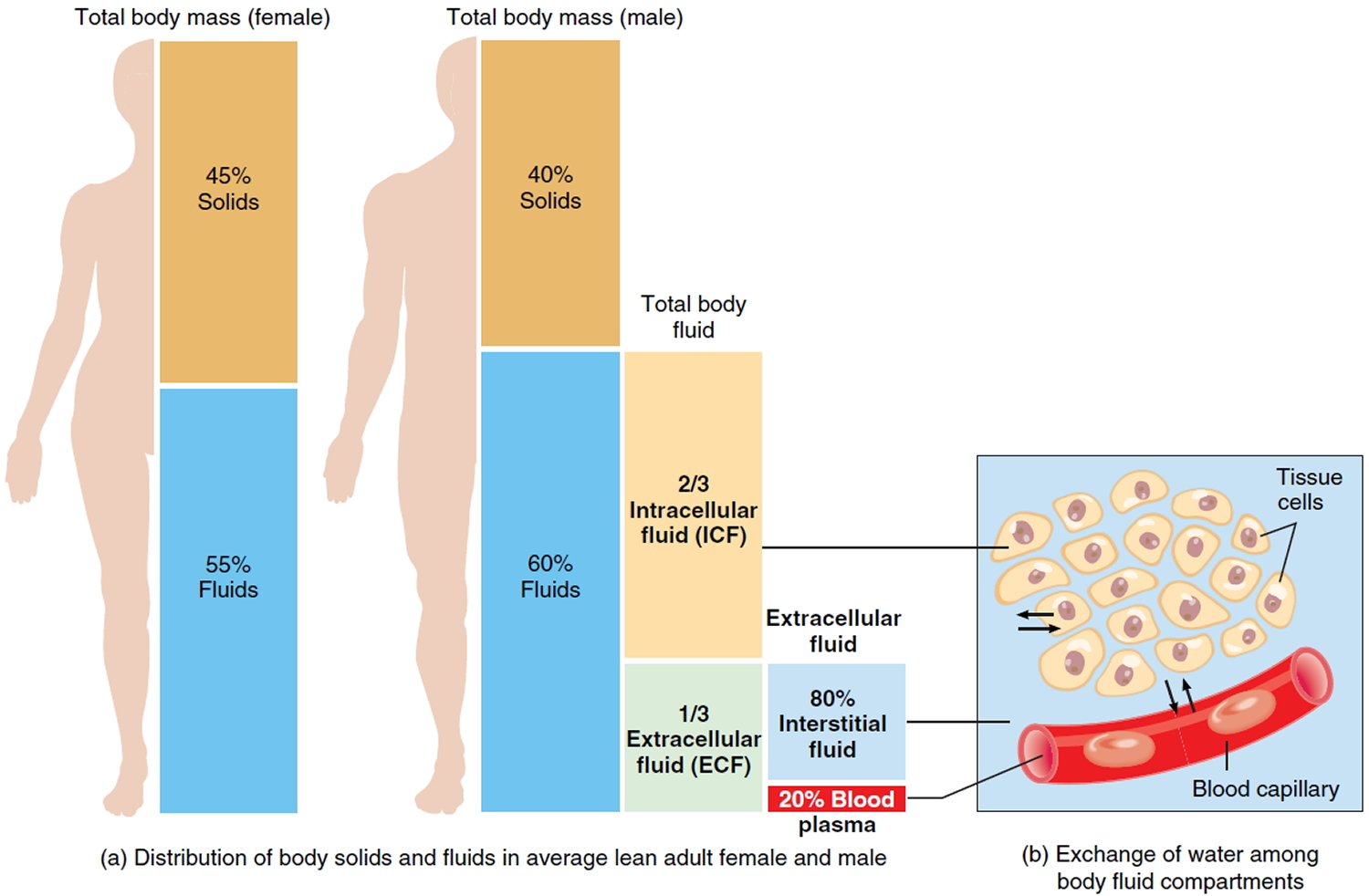
Water makes up at least two-thirds of the human body 1. About 60% of our body weight is made of water 2. The body of an average adult female is about 52-55% water by weight and that of an average male is about 60-63% water by weight. The reason for this difference is that females generally have more adipose tissue, which contains little water 3. Males generally have proportionately more muscle tissue, which contains a great deal of water. Water in the adult human body (about 40 liters), with its dissolved electrolytes, is distributed into two major compartments: an intracellular fluid compartment and an extracellular fluid compartment. This water content varies with body composition (lean and fat mass) 4. In infants and young children, water as a percentage of body weight is higher than in adults. This is mainly due to higher water content in the extracellular compartment, whereas the water content in the intracellular compartment is lower in infants than in older children and adults 2. Body composition changes rapidly during the first year of life, with a decrease in the water content of the fat-free mass and an increase in the content of protein and minerals 2.
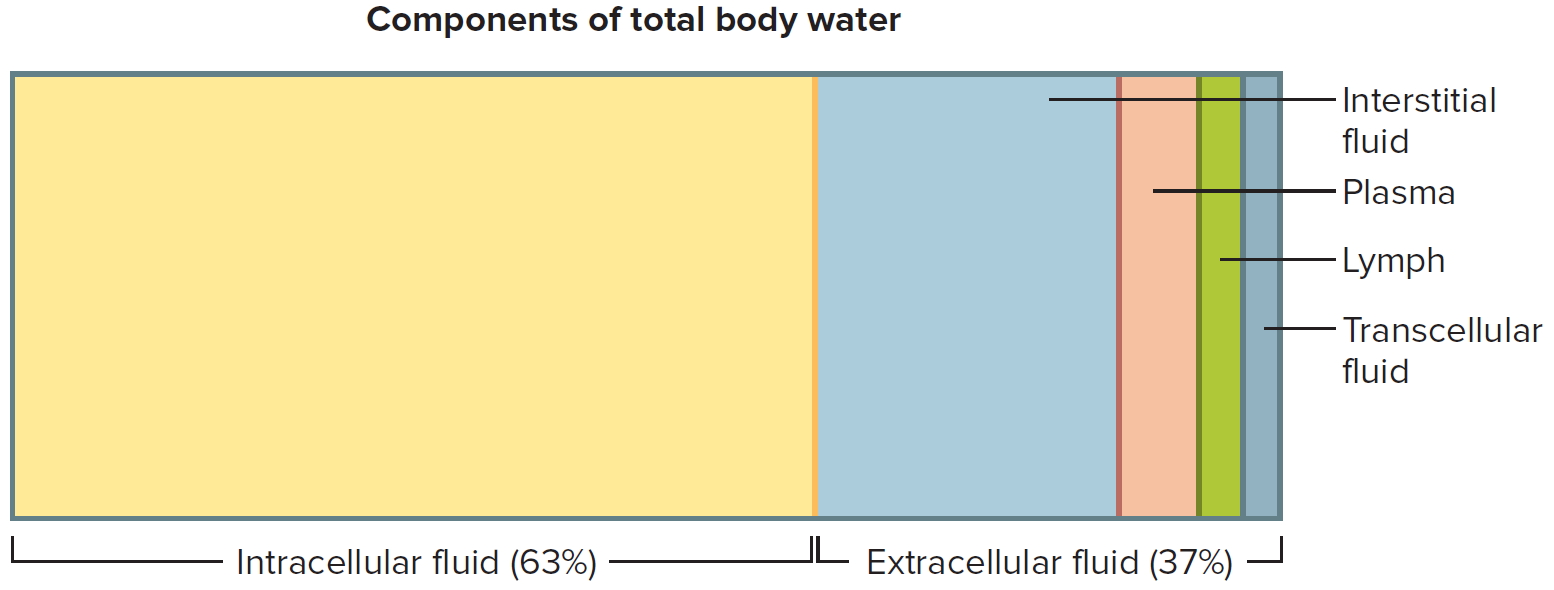
In adults, about two-thirds of total water is in the intracellular space, whereas one-third is extracellular water 2. The intracellular fluid compartment includes all the water and electrolytes that cell membranes enclose. In other words, intracellular fluid is the fluid inside cells. In an adult it accounts for about 63% by volume of total body water.
The extracellular fluid compartment includes all the fluid outside of cells—in tissue spaces (interstitial fluid), blood vessels (plasma), and lymphatic vessels (lymph). Transcellular fluid, a type of extracellular fluid, is found in cavities separated from other extracellular fluids by epithelial or connective tissue membranes. Transcellular fluid includes cerebrospinal fluid of the central nervous system, aqueous and vitreous humors of the eyes, synovial fluid of the joints, and serous fluid in the body cavities. The fluids of the extracellular compartment constitute about 37% by volume of total body water.
A 70kg human has about 42 liters of total body water, of which 28 liters is intracellular water and 14 liters is extracellular fluid (ECF) 5. Of the latter, 3 liters is in blood plasma, 1 liter is the transcellular fluid (cerebrospinal fluid, ocular, pleural, peritoneal and synovial fluids) and 10 liters is the interstitial fluid, including lymph, which provides an aqueous medium surrounding cells 2.
Figure 1. Body fluid compartments
Body Fluid Composition
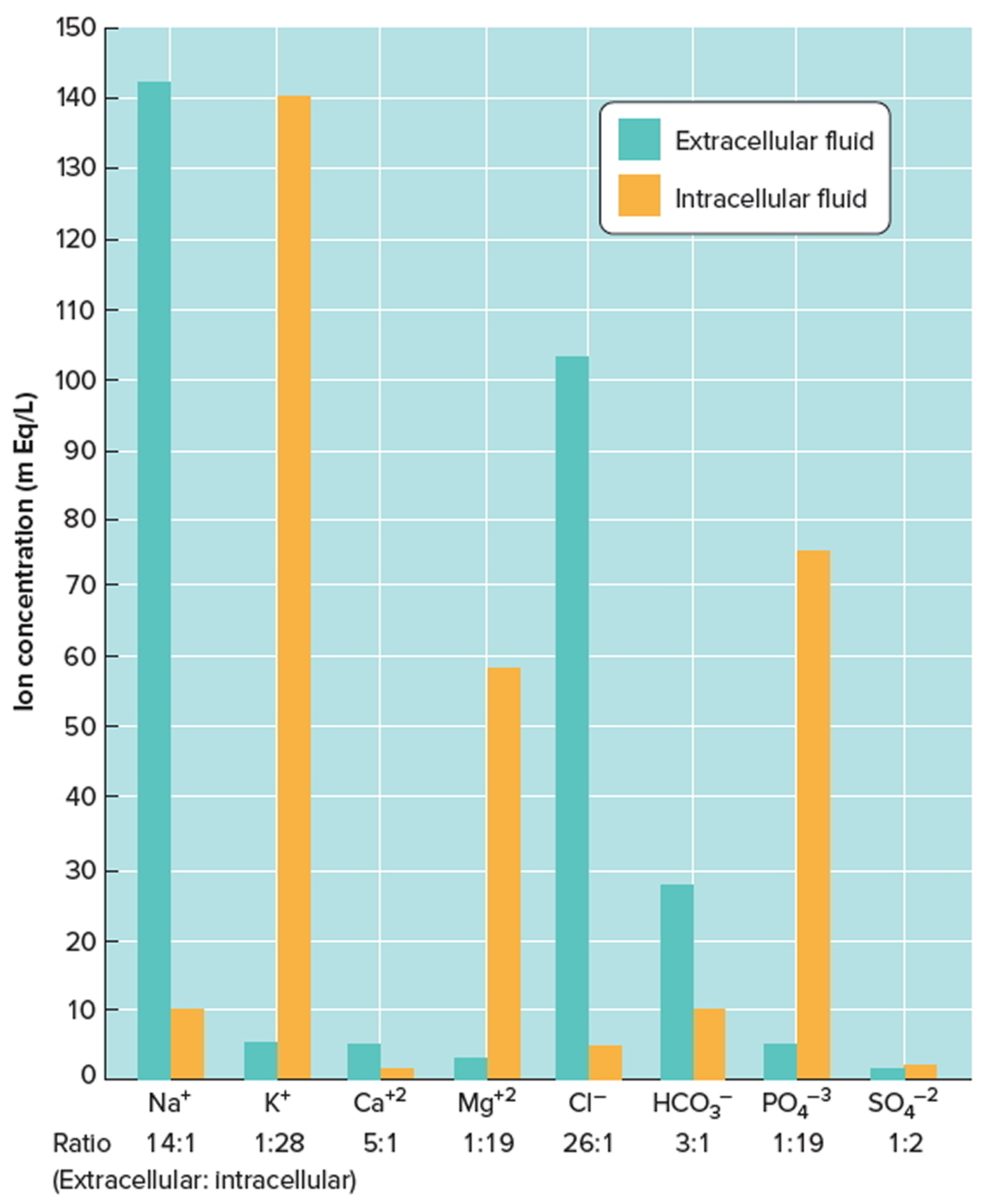
- Extracellular fluids (ECF): The other third, called extracellular fluid (ECF) (extra- = outside), is outside cells and includes all other body fluids. Extracellular fluid (ECF) generally are similar in composition, including high concentrations of sodium, chloride, calcium, and bicarbonate ions and lesser concentrations of potassium, magnesium, phosphate, and sulfate ions. The blood plasma portion of extracellular fluid has considerably more protein than does either interstitial fluid or lymph. About 80% of the extracellular fluid (ECF) is interstitial fluid (inter- = between), which occupies the microscopic spaces between tissue cells, and 20% of the extracellular fluid (ECF) is blood plasma, the liquid portion of the blood. Other extracellular fluids that are grouped with interstitial fluid include lymph in lymphatic vessels; cerebrospinal fluid in the nervous system; synovial fluid in joints; aqueous humor and vitreous body in the eyes; endolymph and perilymph in the ears; and pleural, pericardial, and peritoneal fluids between serous membranes.
- Intracellular fluid (ICF): About two-thirds of body fluid is intracellular fluid (intra- = within) or cytosol, the fluid within cells has high concentrations of potassium, phosphate, and magnesium ions. It includes a greater concentration of sulfate ions and lesser concentrations of sodium, chloride, calcium, and bicarbonate ions than does extracellular fluid. Intracellular fluid also has a greater concentration of protein than does plasma.
Figures 2 and 3 show these relative concentrations.
Figure 2. Components of total body water
Figure 3. Intracellular and extracellular fluid electrolyte concentrations
Note: Extracellular fluids have relatively high concentrations of sodium (Na+), calcium (Ca+2), chloride (Cl−), and bicarbonate (HCO3−) ions. Intracellular fluid has relatively high concentrations of potassium (K+), magnesium (Mg+2), phosphate (PO4−3), and sulfate (SO4−2) ions.
[Source 3]The constancy of the amount and composition of extracellular fluid (ECF) is a necessity for the function of cells. This constancy is due to the homeostatic mechanisms that monitor and regulate its composition, osmotic pressure, pH and temperature 6. These mechanisms rely on the function of the main systems of the body, such as the circulatory, respiratory, renal and alimentary systems. The monitoring and regulation of these systems are coordinated by the nervous and endocrine systems. The composition of the intracellular fluid is maintained by solute movement across the cell membrane by passive or active transports 6.
An average person on an average day needs about 3 liters of water. But if you’re out in the hot sun, you’ll need a lot more than that. Most healthy bodies are very good at regulating water. Elderly people, young children and some special cases – like people taking certain medications – need to be a little more careful.
Water plays a large part in your normal body functions, drinking enough water is essential for physiological processes such as circulation, metabolism, temperature regulation, and waste removal 7. Water is the main constituent of cells, tissues and organs and is vital for life 8. Water as a vital nutrient, a multifunctional constituent of the human body. Every day you lose water through your breath, perspiration, urine and bowel movements.
Water is essential for cellular homeostasis because it transports nutrients to cells and removes wastes from cells 9. It is the medium in which all transport systems function, allowing exchanges between cells, interstitial fluid and capillaries 10. Water maintains the vascular volume and allows blood circulation, which is essential for the function of all organs and tissues of the body 11. Thus, the cardiovascular and respiratory systems, the digestive tract, the reproductive system, the kidney and liver, the brain and the peripheral nervous system, all depend on adequate hydration to function effectively 9. Severe dehydration therefore affects the function of many systems and is a life-threatening condition 12.
For your body to function properly, you must replenish its water supply by consuming beverages and foods that contain water. Although excessive dehydration is associated with serious health problems, even mild dehydration can cause issues, including headaches, irritability, poorer physical performance, and reduced cognitive functioning. Once the water in your body is reduced, it needs to be replaced because an imbalance between the salts and sugar in your body can affect the way you will perform. Dehydration has been classically referred to the excessive loss of body water through conditions such as diarrhea, sweating, or urinary losses, but among the lay public dehydration may also refer to the loss of both water and salt leading to a hypovolemic state 13.
How you use water to keep every system in your body functioning properly
- carrying nutrients and oxygen to your cells
- flushing bacteria from your bladder
- aiding digestion
- preventing constipation
- normalizing blood pressure
- stabilizing the heartbeat
- cushioning joints
- protecting organs and tissues
- regulating body temperature
- maintaining electrolyte (sodium) balance.
Giving your body enough fluids to carry out those tasks means that you’re staying hydrated.
If you don’t drink enough water, you risk becoming dehydrated. Warning signs of dehydration include weakness, low blood pressure, dizziness, confusion, or urine that’s dark in color.
How much water should you drink each day ? It’s a simple question with no easy answers. Studies have produced varying recommendations over the years, but in truth, your water needs depend on many factors, including your health, how active you are and where you live.
Although no single formula fits everyone, knowing more about your body’s need for fluids will help you estimate how much water to drink each day.
If your body has lost one to two percent of its entire water content, you will feel thirsty. Thirst is normally just the brain’s way of warning that you’re dehydrated because you’re not drinking enough fluid. But excessive and persistent thirst (known as polydipsia) could be a sign of an underlying problem such as diabetes.
Dehydration happens when you’ve lost too much water in your body without replacing it, preventing your body to perform its normal functions. Mild dehydration can easily be treated but if it reaches extreme levels, it can be life-threatening and will require immediate medical attention.
It’s particularly important to stay well hydrated during hot weather, while exercising and while you’re unwell with vomiting and diarrhea.
Distribution of Body Fluids
Body fluids are not uniformly distributed. Instead, they occupy regions, or compartments, of different volumes that contain fluids of varying compositions. The movement of water and electrolytes between these compartments is regulated to stabilize both the distribution and the composition of body fluids.
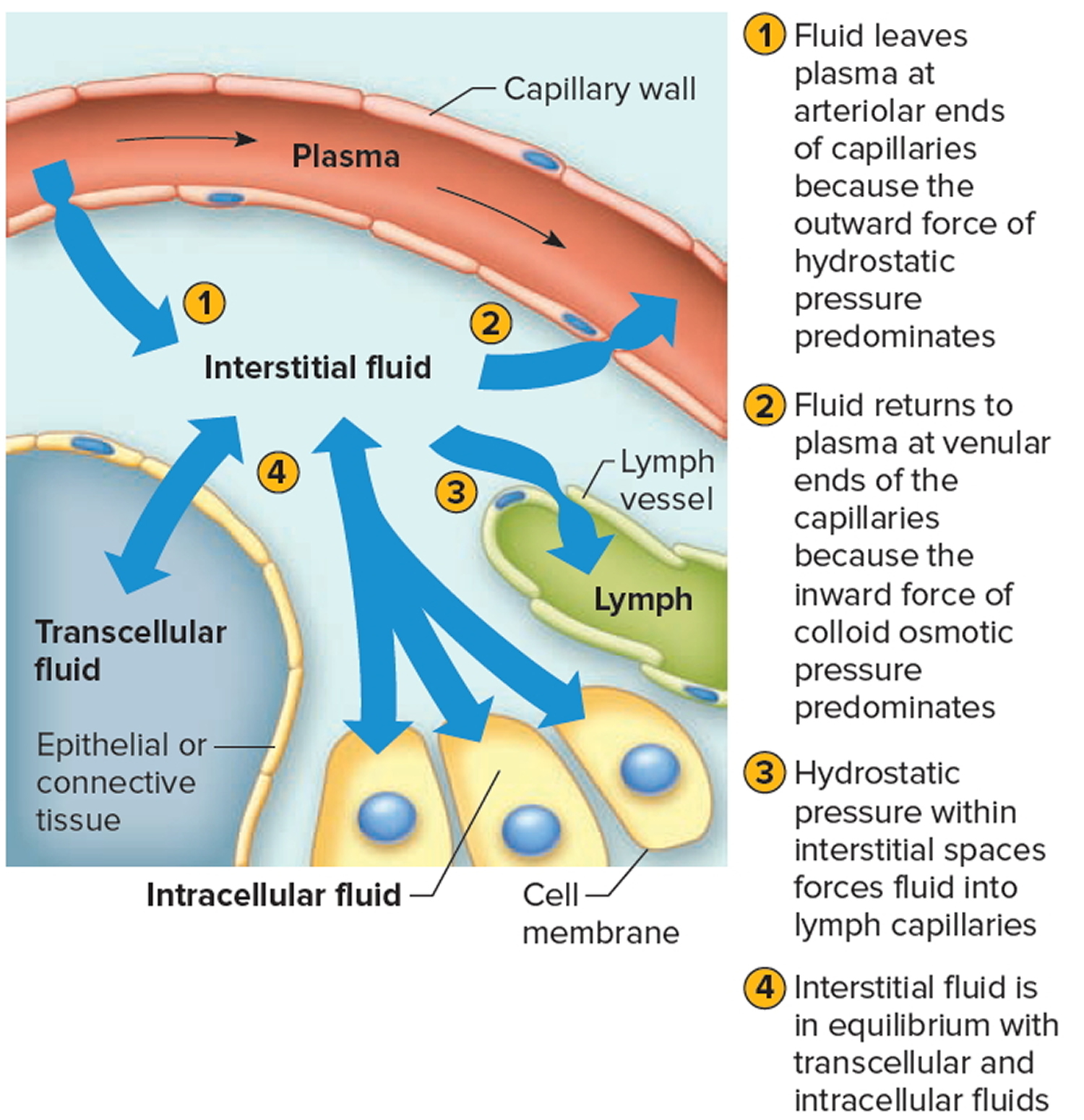
Movement of Fluid Between Compartments
Two major factors contribute to the movement of fluid from one compartment to another: hydrostatic pressure and osmotic pressure (Figure 4). For example, blood vessels, fluid leaves the plasma at the arteriolar ends of capillaries and enters the interstitial spaces because of the net outward force of hydrostatic pressure (blood pressure). Fluid returns to the plasma from the interstitial spaces at the venular ends of capillaries because of the net inward force of colloid osmotic pressure due to the plasma proteins. Likewise, tissue fluid and lymph, fluid leaves the interstitial spaces and enters the lymph capillaries due to the hydrostatic pressure of the interstitial fluid. The circulation of lymph returns interstitial fluid to the plasma.
Hydrostatic pressure in the cells and surrounding interstitial fluid is ordinarily equal and remains stable. Therefore, any net fluid movement is likely to be the result of changes in osmotic pressure.
The total solute concentration in extracellular and intracellular fluids is normally equal. However, a decrease in extracellular sodium ion concentration causes a net movement of water from the extracellular compartment into the intracellular compartment by osmosis. The cells swell. Conversely, if the extracellular sodium ion concentration increases, cells shrink as they lose water by osmosis.
Figure 4. Net movements of fluids between compartments result from differences in hydrostatic and osmotic pressures
[Source 3]Water Balance
Dehydration, water intoxication, and water retention (edema) are among the more common disorders that involve a water imbalance in body fluids.
For homeostasis, water balance and electrolyte balance must be maintained; that is, the quantities entering the body must equal the quantities leaving it 3. In other word, homeostasis requires control of both water intake and water output. Water balance exists when water intake equals water output. Therefore, the body requires mechanisms to:
- Replace lost water and electrolytes, and
- Excrete any excess water and electrolytes.
Levels of electrolytes in your body can become too low or too high. That can happen when the amount of water in your body changes, causing dehydration or overhydration. Causes include some medicines, vomiting, diarrhea, sweating or kidney problems 14. Problems most often occur with levels of sodium, potassium or calcium.
Water balance and electrolyte balance are interdependent because electrolytes are dissolved in the water of body fluids. Consequently, anything that alters the concentrations of the electrolytes will alter the concentration of the water, either by adding solutes to it or by removing solutes from it. Likewise, anything that changes the concentration of water will change concentrations of the electrolytes by concentrating or diluting them.
- Water Intake
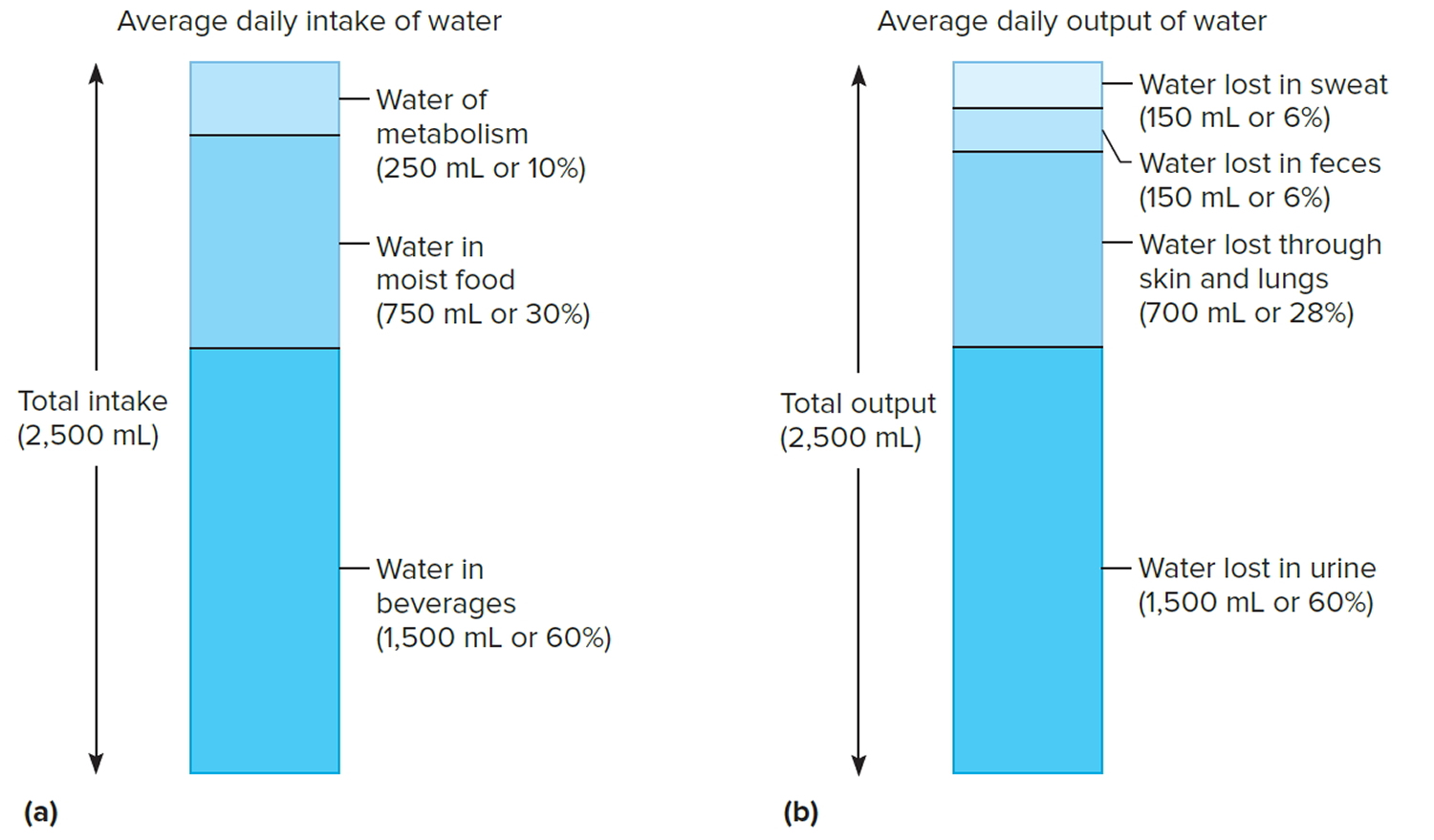
The volume of water gained each day varies among individuals. An average adult living in a moderate environment takes in about 2.5 liters. Probably 60% is obtained from drinking water or beverages, and another 30% comes from moist foods. The remaining 10% is a by-product of the oxidative metabolism of nutrients, called water of metabolism.
The primary regulator of water intake is thirst. The intense feeling of thirst derives from the osmotic pressure of extracellular fluids and both neural and hormonal input to the brain. As the body loses water, the osmotic pressure of extracellular fluids increases. Such a change stimulates osmoreceptors in the hypothalamus, and in response, the hypothalamus causes the person to feel thirsty and to seek water.
Thirst is a homeostatic mechanism, normally triggered when total body water decreases by as little as 1%. The act of drinking and the resulting distension of the stomach wall trigger impulses that inhibit the thirst mechanism. In this way, drinking stops even before the swallowed water is absorbed, preventing the person from drinking more than is required to replace the volume lost, avoiding development of an imbalance.
- Water Output
Water normally enters the body only through the mouth, but it can be lost by a variety of routes. These include obvious losses in urine, feces, and sweat (sensible perspiration), as well as evaporation of water from the skin (insensible perspiration) and from the lungs during breathing.
If an average adult takes in 2.5 liters of water each day, then 2.5 liters must be eliminated to maintain water balance. Of this volume, perhaps 60% is lost in urine, 6% in feces, and 6% in sweat. About 28% is lost by evaporation from the skin and lungs. These percentages vary with such environmental factors as temperature
and relative humidity and with physical exercise.
The primary means of regulating water output is urine production. The renal distal convoluted tubules of the nephrons and collecting ducts are the effectors of the mechanism that regulates urine volume. The epithelial linings in these structures remain relatively impermeable to water unless antidiuretic hormone (ADH) is present. Antidiuretic hormone (ADH) increases the permeability of the distal convoluted tubule and collecting duct, thereby increasing water reabsorption and reducing urine production. In the absence of antidiuretic hormone (ADH), less water is reabsorbed and more urine is produced.
Figure 5. Water balance – Sources of daily water gain and loss under normal conditions. Numbers are average volumes for adults.
Note: Water balance. (a) Major sources of body water. (b) Routes by which the body loses water.
[Source 3]Electrolyte balance
Electrolyte balance exists when the quantities of electrolytes the body gains equal those lost. Homeostatic mechanisms maintain electrolyte balance. This involves keeping the associated ions in appropriate concentrations within the plasma and the interstitial fluid.
- Electrolyte Intake
The electrolytes of greatest importance to cellular functions dissociate to release sodium, potassium, calcium, magnesium, chloride, sulfate, phosphate, bicarbonate, and hydrogen ions. These electrolytes are primarily obtained from foods, but they may also be found in drinking water and other beverages. In addition, some electrolytes are by-products of metabolic reactions.
Ordinarily, a person obtains sufficient electrolytes by responding to hunger and thirst. However, a severe electrolyte deficiency may cause salt craving, which is a strong desire to eat salty foods.
- Electrolyte Output
The body loses some electrolytes by perspiring, with more lost in sweat on warmer days and during strenuous exercise. Varying amounts of electrolytes are lost in the feces. The greatest electrolyte output occurs as a result of kidney function and urine production. The kidneys alter electrolyte output to maintain the proper composition of body fluids, thereby promoting homeostasis.
Precise concentrations of positively charged ions, such as sodium (Na+), potassium (K+), and calcium (Ca2+), are required for impulse conduction along an axon, muscle fiber contraction, and maintenance of cell membrane potential. Sodium ions account for nearly 90% of positively charged ions in extracellular fluids. The kidneys and the hormone aldosterone regulate these ions. Aldosterone, which the adrenal cortex secretes, increases sodium ion reabsorption in the distal convoluted tubules of the kidneys’ nephrons and in the collecting ducts.
Aldosterone also regulates potassium ion concentration. A rising potassium ion concentration directly stimulates cells of the adrenal cortex to secrete aldosterone. This hormone enhances tubular secretion of potassium ions at the same time that it causes tubular reabsorption of sodium ions.
The calcium ion concentration dropping below normal directly stimulates the parathyroid glands to secrete parathyroid hormone. This hormone returns the concentration of calcium in extracellular fluids toward normal.
Generally the regulatory mechanisms that control positively charged ions secondarily control the concentrations of negatively charged ions. For example, chloride ions (Cl−), the most abundant negatively charged ions in extracellular fluids, are passively reabsorbed in response to the active tubular reabsorption of sodium ions. That is, the negatively charged chloride ions are electrically attracted to positively charged sodium ions and accompany them as they are reabsorbed. Water is then reabsorbed by osmosis.
Active transport mechanisms with limited transport capacities partially regulate some negatively charged ions, such as phosphate ions (PO4 3−) and sulfate ions (SO42−). Therefore, if the extracellular phosphate ion concentration is low, renal tubules reabsorb phosphate ions. On the other hand, if the renal plasma threshold is exceeded, excess phosphate is excreted in urine.
Regulation of Electrolyte and Water Loss
Even though the loss of water and electrolytes through sweating and exhalation increases during exercise, elimination of excess body water or electrolytes occurs mainly by control of their loss in urine. The extent of urinary salt (NaCl) loss is the main factor that determines body fluid volume. The reason for this is that “water follows electrolytes” in osmosis, and the two main electrolytes in extracellular fluid (and in urine) are sodium ions (Na+) and chloride ions (Cl−). In a similar way, the main factor that determines body fluid osmolarity is the extent of urinary water loss.
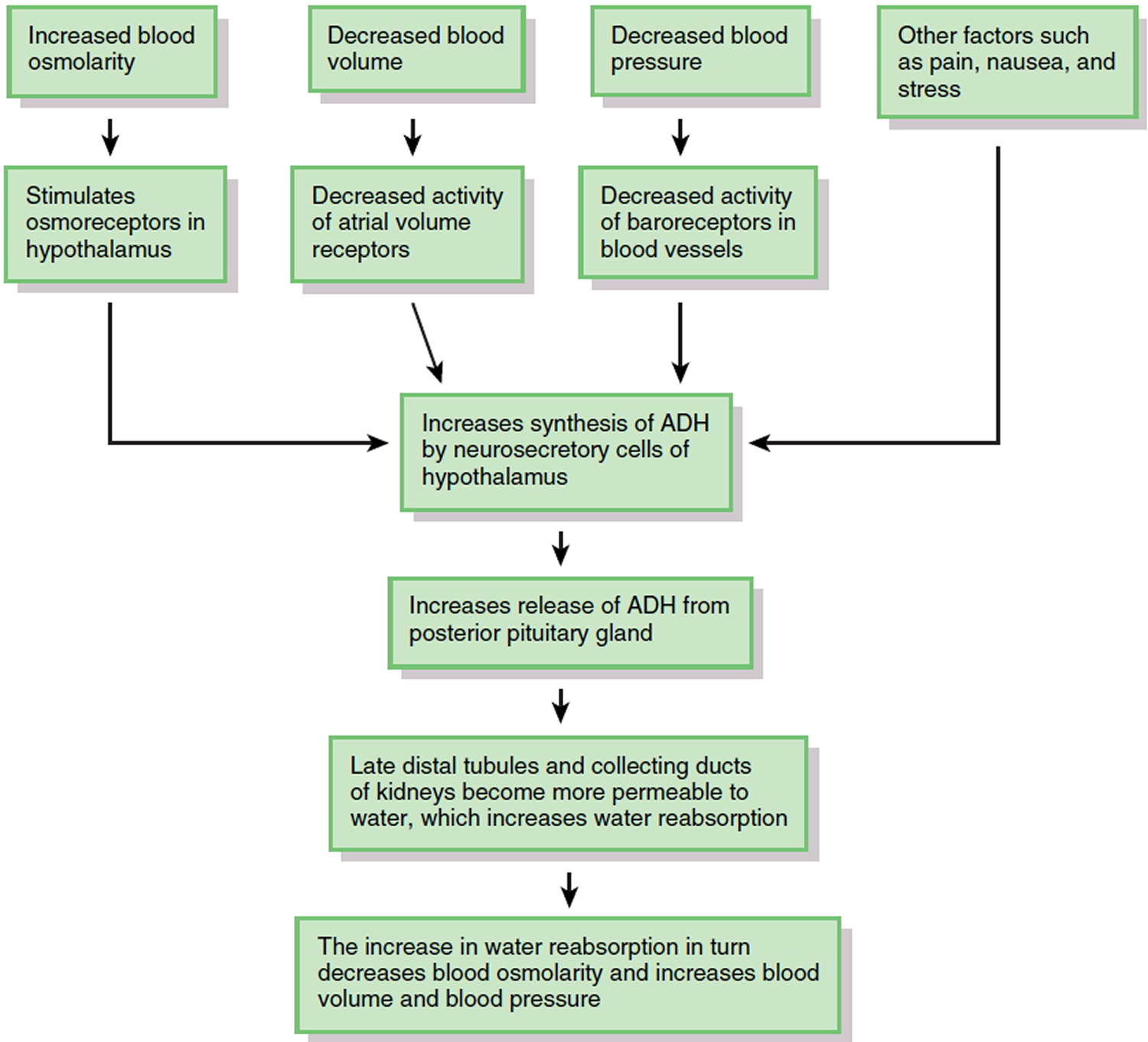
The major hormone that regulates water loss is antidiuretic hormone (ADH). This hormone, also known as vasopressin, is produced by neurosecretory cells in the hypothalamus and stored in the posterior pituitary gland. When the osmolarity of body fluids increases, osmoreceptors in the hypothalamus not only stimulate thirst; they also increase the synthesis and release of ADH (Figure 6). ADH promotes the insertion of water-channel proteins (aquaporin-2) into the apical membranes of principal cells in the late distal tubules and collecting ducts of the kidneys. As a result, the permeability of these cells to water increases. Water molecules move by osmosis from the renal tubular fluid into the cells and then from the cells into the bloodstream. This results in a decrease in blood osmolarity, an increase in blood volume and blood pressure, and the production of a small volume of concentrated urine. Once the body has adequate water, the ADH level in the bloodstream decreases. As the amount of ADH in the blood declines, some of the aquaporin-2 channels are removed from the apical membrane via endocytosis. Consequently, the water permeability of the principal cells decreases and more water is lost in the urine.
Factors other than blood osmolarity influence ADH secretion (Figure 6). A decrease in blood volume or blood pressure also stimulates ADH release. Atrial volume receptors detect the decrease in blood volume, and baroreceptors in blood vessels detect the decrease in blood pressure. ADH release is also stimulated by factors that are unrelated to water balance, such as pain, nausea, and stress. Secretion of ADH is inhibited by alcohol, which is why consumption of alcoholic beverages promotes diuresis (voiding large amounts of urine).
Figure 6. Role of Antidiuretic Hormone (ADH) in water balance
Because your daily diet contains a highly variable amount of NaCl (salt), urinary excretion of sodium (Na+) and chloride ions (Cl−) must also vary to maintain homeostasis. Hormones regulate the urinary loss of Na+ ions, Cl− ions usually follow Na+ ions because of electrical attraction or because they are transported along with Na+ ions via symporters. The two most important hormones that regulate the extent of renal Na+ reabsorption (and thus how much is lost in the urine) are aldosterone and atrial natriuretic peptide.
- Aldosterone. When there is a decrease in blood pressure, which occurs in response to a decrease in blood volume, or when there is a deficiency of Na+ in the plasma, the kidneys release renin, which activates the renin-angiotensin-aldosterone pathway. Once aldosterone is formed, it increases Na+ reabsorption in the late distal tubules and collecting ducts of the kidneys, which relieves the Na+ deficiency in the plasma. Because antidiuretic hormone (ADH) is also released when blood pressure is low, water reabsorption accompanies Na+ reabsorption via osmosis. This conserves the volume of body fluids by reducing urinary loss of water.
- Atrial natriuretic peptide. An increase in blood volume, as might occur after you finish one or more supersized drinks, stretches the atria of the heart and promotes release of atrial natriuretic peptide (ANP). Atrial natriuretic peptide promotes natriuresis, elevated excretion of Na+ into the urine. The osmotic consequence of excreting more Na+ is loss of more water in urine, which decreases blood volume and blood pressure. In addition to stimulating the release of ANP, an increase in blood volume also slows the release of renin from the kidneys. When the renin level declines, less aldosterone is formed, which causes reabsorption of filtered Na+ to slow in the late distal tubules and collecting ducts of the kidneys. More filtered Na+ and water (due to osmosis) thus remain in the tubular fluid to be excreted in the urine.
Table 1. Summary of Factors That Maintain Body Water Balance
FACTOR | MECHANISM | EFFECT |
| Thirst center in hypothalamus | Stimulates desire to drink fluids. | Water gained if thirst is quenched. |
| Antidiuretic hormone (ADH), also known as vasopressin | Promotes insertion of water-channel proteins (aquaporin-2) into apical membranes of principal cells in collecting ducts of kidneys. As a result, water permeability of these cells increases and more water is reabsorbed. | Reduces loss of water in urine. |
| Aldosterone | By promoting urinary reabsorption of Na+, increases water reabsorption via osmosis. | Reduces loss of water in urine. |
| Atrial natriuretic peptide (ANP) | Promotes natriuresis, elevated urinary excretion of Na+, accompanied by water. | Increases loss of water in urine. |
What do electrolytes do
Sodium
Sodium is an electrolyte present in all body fluids and is vital to normal body function, including nerve and muscle function. Sodium and sodium chloride — known commonly as salt — occur naturally in foods, usually in small amounts. Salt and other sodium-containing ingredients are often used in food processing. Some people add salt and salty sauces, such as soy sauce, to their food at the table, but most dietary sodium or salt comes from foods to which salt has already been added during processing or preparation. Although many people add salt to enhance the taste of foods. In the body, sodium plays an essential role in regulation of fluids and blood pressure. Many studies in diverse populations have shown that a high sodium intake is associated with higher blood pressure. Most evidence suggests that many people at risk for high blood pressure reduce their chances of developing this condition by consuming less salt or sodium.
Sodium, along with other electrolytes such as potassium, chloride, and bicarbonate (or total CO2), helps cells function normally and helps regulate the amount of fluid in the body. While sodium is present in all body fluids, it is found in the highest concentration in the blood and in the fluid outside of the body’s cells. This extracellular sodium, as well as all body water, is regulated by the kidneys.
You get sodium in your diet, from table salt (sodium chloride or NaCl), and to some degree from most of the foods that you eat. Most people have an adequate intake of sodium. The body uses what it requires and the kidneys eliminate the rest in the urine. The body tries to keep the blood sodium within a very narrow concentration range. It does this by:
- Producing hormones that can increase (atrial natriuretic peptide [ANP]) or decrease (aldosterone) the amount of sodium eliminated in urine
- Producing a hormone that prevents water losses (antidiuretic hormone, ADH, sometimes called vasopressin)
- Controlling thirst; even a 1% increase in blood sodium will make a person thirsty and cause that person to drink water, returning the sodium level to normal.
Abnormal blood sodium is usually due to some problem with one of these systems. When the level of sodium in the blood changes, the water content in the body also changes. These changes can be associated with too little fluid (dehydration) or with too much fluid (edema), often resulting in swelling in the legs.
A low level of blood sodium (hyponatremia) may be due to:
- Losing too much sodium, most commonly from conditions such as diarrhea, vomiting, excessive sweating, use of diuretics, kidney disease or low levels of cortisol, aldosterone and sex hormones (Addison disease)
- Drinking too much water as might occur during exercise
- Excess fluid accumulation in the body (edema) caused by heart failure, cirrhosis, and kidney diseases that cause protein loss (nephrotic syndrome) or malnutrition. In a number of diseases, particularly those involving the brain and the lungs, many kinds of cancer, and with some drugs, the body makes too much anti-diuretic hormone (ADH), causing a person to keep too much water in the body.
Low blood sodium is rarely due to decreased sodium intake (deficient dietary intake or deficient sodium in IV fluids).
A high blood sodium level (hypernatremia) is almost always caused by losing too much water (dehydration) without drinking enough water. In rare cases, it may be due to increased salt intake without enough water, Cushing syndrome, or a condition caused by too little anti-diuretic hormone (ADH) called diabetes insipidus.
Sodium urine concentrations must be evaluated in association with blood levels. The body normally elimiates excess sodium, so the concentration in the urine may be elevated because it is elevated in the blood. It may also be elevated in the urine when the body is losing too much sodium; in this case, the blood level would be normal to low. If blood sodium levels are low due to insufficient intake, then urine concentrations will also be low.
- Decreased urinary sodium levels may indicate dehydration, congestive heart failure, liver disease, or nephrotic syndrome.
- Increased urinary sodium levels may indicate diuretic use or Addison disease.
Sodium levels are often evaluated in relation to other electrolytes and can be used to calculate a quantity termed anion gap. The anion gap is useful in identifying the presence of unknown substances such as toxins in the blood.
What is the recommended dietary salt intake?
The Dietary Guidelines for Americans 15 recommends a sodium intake of less than 2,300 mg per day (1 teaspoon of salt a day) for adults. People normally obtain adequate amounts of sodium in their daily diet, but it is important not to exceed this recommended maximum amount. According to the Dietary Guidelines for Americans 15, average intakes of sodium are high across the U.S. population. Average intakes for those ages 1 year and older is 3,440 mg per day. Average intakes are generally higher for men than women. For all adult men, the average intake is 4,240 mg, and for adult women, the average is 2,980 mg per day. Only a small proportion of total sodium intake is from sodium inherent in foods or from salt added in home cooking or at the table. Most sodium consumed in the United States comes from salts added during commercial food processing and preparation.
The American Heart Association recommends you consume no more than 2,300 milligrams (mgs) a day of salt (sodium) and an ideal limit of no more than 1,500 mg per day for most adults 16.
Common dietary sources of sodium are often processed food to which salt is added during preparation, such as cheeses, soups, pickles, and pretzels. Additionally, other processed, commercially prepared, or restaurant foods are generally high in sodium.
For people who are sodium-sensitive or have hypertension, reducing sodium intake can lead to markedly beneficial health effects. But even if you don’t have high blood pressure, limiting sodium as part of a healthy diet may decrease your risk of developing blood pressure problems and heart disease.
Your taste for salt is both acquired and reversible. As you use less salt, your preference for it will lessen.
Why Limit Salt (Sodium) ?
Because it is the body’s main source of sodium. You need a little bit of sodium to transmit nerve impulses, to contract muscle fibers, and, along with potassium, to balance fluid levels in all of your cells. But getting too much sodium and too little potassium can have a major impact on overall health. It can increase blood pressure and the risk of heart disease and stroke.
- Sodium, potassium together influence heart health 17
A new report from the National Health and Nutrition Examination Survey 18 suggests that this imbalance — more sodium than potassium — contributes to heart disease and premature death. Here are some foods rich in potassium and low in sodium. Note the huge differences between unprocessed foods and the processed or salted foods marked in italics. A more extensive list of potassium in foods, ranked high potassium content to low, is available from the US Department of Agriculture 19.
Table 2. Sodium and potassium content of foods
| Food | Amount | Potassium (mg) | Sodium (mg) | Potassium to sodium ratio |
| Banana, raw | 1 medium | 422 | 1 | 422:1 |
| Black beans, cooked without salt | 1/2 cup | 305 | 1 | 305:1 |
| Orange | 1 medium | 232 | 1 | 232:1 |
| Orange juice | 3/4 cup | 357 | 2 | 178:1 |
| Grapefruit juice | 3/4 cup | 252 | 2 | 126:1 |
| Peanuts, dry roasted, no salt | 1 1/2 ounces | 280 | 3 | 93:1 |
| Peanuts, dry roasted, with salt | 1 1/2 ounces | 280 | 346 | 0.8:1 |
| Avocado | 1/2 medium | 487 | 7 | 69:1 |
| Raisins | 1/2 cup | 543 | 8 | 68:1 |
| Prune juice | 3/4 cup | 530 | 8 | 66:1 |
| Baked potato, plain, with skin | 1 medium | 926 | 17 | 54:1 |
| Fast-food French fries | 1 medium order | 655 | 266 | 2.5:1 |
| Peanut butter, without salt | 2 tablespoons | 208 | 5 | 42:1 |
| Peanut butter, with salt | 2 tablespoons | 208 | 147 | 1.4:1 |
| Brussels sprouts, steamed | 1/2 cup | 248 | 7 | 35:1 |
| Applesauce (jar), no salt | 1/2 cup | 92 | 3 | 31:1 |
| Applesauce (jar), with salt | 1/2 cup | 78 | 36 | 2.2:1 |
| Oatmeal, regular | 1 cup | 164 | 9 | 18:1 |
| Quaker’s Instant Oatmeal | 1 packet | 116 | 249 | 0.5:1 |
| Cantaloupe | 1/4 medium | 368 | 22 | 17:1 |
| Halibut, baked | 3 ounces | 490 | 59 | 8:1 |
| Spinach, boiled | 1/2 cup | 420 | 63 | 7:1 |
| Salmon, baked | 3 ounces | 244 | 39 | 6:1 |
| Salmon, canned | 3 ounces/em> | 311 | 399 | 0.8:1 |
| V8, low-sodium | 1 cup | 820 | 140 | 6:1 |
| V8, regular | 1 cup | 470 | 480 | 1:1 |
| Carrots, raw | 1/2 cup | 205 | 44 | 5:1 |
| Beet greens | 1/2 cup | 655 | 173 | 4:1 |
| Milk, 1% | 1 cup | 366 | 107 | 3:1 |
| Cheerios | 1 cup | 171 | 186 | 0.9:1 |
| Marinara sauce, prepared | 1/2 cup | 406 | 527 | 0.8:1 |
| Pork and beans, canned | 1 cup | 726 | 1075 | 0.7:1 |
| Fast-food cheeseburger | 1 regular | 444 | 1176 | 0.4:1 |
| French bread | 1 medium slice | 82 | 416 | 0.2:1 |
| Cornflakes | 1 cup | 33 | 266 | 0.1:1 |
| Source: USDA National Nutrient Database 19 | ||||
Three new studies in BMJ (the British Medical Journal) once again confirm the relationship between salt intake and health problems 20. The first of these studies combined information from 34 earlier studies. This technique is called meta-analysis. It showed that, over the long term, a reduction in salt intake led to a drop in systolic blood pressure (the top number of a blood pressure reading) of 4 millimeters of mercury (mmHg). There was also an average 2 mmHg drop in systolic blood pressure. Eating less salt was also linked to lower risks of heart attack, stroke and heart failure.
A second meta-analysis 21 combined information from 56 studies. Its findings were similar to the first meta-analysis: reduced salt intake reduces blood pressure and has no adverse effect on blood lipids, catecholamine levels, or renal function, and moderate quality evidence in children shows that a reduction in sodium intake reduces blood pressure. Lower sodium intake is also associated with a reduced risk of stroke and fatal coronary heart disease in adults. The totality of evidence suggests that most people will likely benefit from reducing sodium intake.
The third study 22 looked at potassium intake. Potassium is needed for many normal body functions, including heart and muscle function. In this review of 33 studies that included more than 128,000 people, consuming more potassium was linked to lower blood pressure and lower risk of stroke. High quality evidence shows that increased potassium intake reduces blood pressure in people with hypertension and has no adverse effect on blood lipid concentrations, catecholamine concentrations, or renal function in adults. Higher potassium intake was associated with a 24% lower risk of stroke (moderate quality evidence). These results suggest that increased potassium intake is potentially beneficial to most people without impaired renal handling of potassium for the prevention and control of elevated blood pressure and stroke.
Putting it into practice
Current dietary guidelines recommend that Americans get no more than 1 teaspoon of salt a day. That’s the equivalent of 2,300 milligrams (mg) of sodium a day. Those with high blood pressure or other conditions such as kidney disease or heart failure and anyone over age 50 years are urged to limit sodium to 1,500 mg a day.
Only about 10% to 20% of the salt that most people take in comes from the salt shaker at the table or in the kitchen. Most of the sodium we get is added to processed foods like canned soups and vegetables; breads, crackers, and other prepared baked goods; deli and other processed meats; chips and other snacks; and restaurant foods.
Here are some tips for limiting your salt intake:
- Know how much salt is in your diet. Pay attention to labels. Some soup labels are misleading and will have two servings per can, with a large amount of sodium in each serving.
- Avoid processed and prepared foods. These tend to have a lot of sodium. When in doubt, eat fresh food or food you have prepared yourself.
- Look for labels that say “low sodium” or “no added sodium.” But be sure to read labels carefully. These products will tend to have less salt, but can still contain a fair amount of sodium.
- Use other seasonings in place of salt. Herbs and spices, for example, can add flavor to your food.
What about potassium ? Most healthy Americans consume less than half of the recommended amount of potassium (4,700 mg per day). Getting more of this mineral may help lower blood pressure. But too much potassium can be dangerous, especially for people with kidney problems. So the right amount is critical.
It’s best to get potassium from food, especially fruits and vegetables. Green leafy vegetables, beans, and bananas have a lot of potassium. The USDA has published a list of potassium in hundreds of foods.
Low sodium level
Low sodium level also called hyponatremia, occurs when the concentration of sodium in your blood is abnormally low. Sodium is an electrolyte, and it helps regulate the amount of water that’s in and around your cells. Normal blood sodium range is 135 to 145 mEq/L, or 135 to 145 nmol/L. Hyponatremia occurs when the sodium in your blood falls below 135 mEq/L.
With low sodium level (hyponatremia), the imbalance of water to sodium is caused by one of three conditions:
- Euvolemic hyponatremia — total body water increases, but the body’s sodium content stays the same
- Hypervolemic hyponatremia — both sodium and water content in the body increase, but the water gain is greater
- Hypovolemic hyponatremia — water and sodium are both lost from the body, but the sodium loss is greater
Lab tests that can confirm and help diagnose low sodium include:
- Comprehensive metabolic panel (includes blood sodium)
- Osmolality blood test
- Urine osmolality
- Urine sodium (normal level is 20 mEq/L in a random urine sample, and 40 to 220 mEq/L for a 24-hour urine test)
In hyponatremia (low sodium level), one or more factors — ranging from an underlying medical condition to drinking too much water — cause the sodium in your body to become diluted. When this happens, your body’s water levels rise, and your cells begin to swell. This swelling can cause many health problems, from mild to life-threatening.
Hyponatremia treatment is aimed at resolving the underlying condition. Depending on the cause of hyponatremia, you may simply need to cut back on how much you drink. In other cases of hyponatremia, you may need intravenous electrolyte solutions and medications.
Low sodium level symptoms
Common symptoms may include:
- Confusion, irritability and restlessness
- Convulsions or seizures
- Coma
- Fatigue
- Headache
- Loss of appetite
- Muscle weakness, spasms, or cramps
- Nausea, vomiting
- Loss of energy, drowsiness and fatigue
Causes of low sodium
Sodium plays a key role in your body. It helps maintain normal blood pressure, supports the work of your nerves and muscles, and regulates your body’s fluid balance.
Many possible conditions and lifestyle factors can lead to hyponatremia, including:
- Certain medications. Some medications, such as some water pills (diuretics), antidepressants and pain medications, can interfere with the normal hormonal and kidney processes that keep sodium concentrations within the healthy normal range.
- Heart, kidney and liver problems. Congestive heart failure and certain diseases affecting the kidneys or liver can cause fluids to accumulate in your body, which dilutes the sodium in your body, lowering the overall level.
- Syndrome of inappropriate anti-diuretic hormone (SIADH). In this condition, high levels of the anti-diuretic hormone (ADH) are produced, causing your body to retain water instead of excreting it normally in your urine.
- Chronic, severe vomiting or diarrhea and other causes of dehydration. This causes your body to lose electrolytes, such as sodium, and also increases ADH levels.
- Drinking too much water. Drinking excessive amounts of water can cause low sodium by overwhelming the kidneys’ ability to excrete water. Because you lose sodium through sweat, drinking too much water during endurance activities, such as marathons and triathlons, can also dilute the sodium content of your blood.
- Hormonal changes. Adrenal gland insufficiency (Addison’s disease) affects your adrenal glands’ ability to produce hormones that help maintain your body’s balance of sodium, potassium and water. Low levels of thyroid hormone also can cause a low blood-sodium level.
- The recreational drug Ecstasy. This amphetamine increases the risk of severe and even fatal cases of hyponatremia.
- Burns that affect a large area of the body
- Sweating
Risk factors for low sodium
The following factors may increase your risk of hyponatremia:
- Age. Older adults may have more contributing factors for hyponatremia, including age-related changes, taking certain medications and a greater likelihood of developing a chronic disease that alters the body’s sodium balance.
- Certain drugs. Medications that increase your risk of hyponatremia include thiazide diuretics as well as some antidepressants and pain medications. In addition, the recreational drug Ecstasy has been linked to fatal cases of hyponatremia.
- Conditions that decrease your body’s water excretion. Medical conditions that may increase your risk of hyponatremia include kidney disease, syndrome of inappropriate anti-diuretic hormone (SIADH) and heart failure, among others.
- Intensive physical activities. People who drink too much water while taking part in marathons, ultramarathons, triathlons and other long-distance, high-intensity activities are at an increased risk of hyponatremia.
Low sodium complications
In chronic hyponatremia, sodium levels drop gradually over 48 hours or longer — and symptoms and complications are typically more moderate.
In acute hyponatremia, sodium levels drop rapidly — resulting in potentially dangerous effects, such as rapid brain swelling, which can result in a coma and death.
Premenopausal women appear to be at the greatest risk of hyponatremia-related brain damage. This may be related to the effect of women’s sex hormones on the body’s ability to balance sodium levels.
Low sodium prevention
The following measures may help you prevent hyponatremia:
- Treat associated conditions. Getting treatment for conditions that contribute to hyponatremia, such as adrenal gland insufficiency, can help prevent low blood sodium.
- Educate yourself. If you have a medical condition that increases your risk of hyponatremia or you take diuretic medications, be aware of the signs and symptoms of low blood sodium. Always talk with your doctor about the risks of a new medication.
- Take precautions during high-intensity activities. Athletes should drink only as much fluid as they lose due to sweating during a race. Thirst is generally a good guide to how much water or other fluids you need.
- Consider drinking sports beverages during demanding activities. Ask your doctor about replacing water with sports beverages that contain electrolytes when participating in endurance events such as marathons, triathlons and other demanding activities.
- Drink water in moderation. Drinking water is vital for your health, so make sure you drink enough fluids. But don’t overdo it. Thirst and the color of your urine are usually the best indications of how much water you need. If you’re not thirsty and your urine is pale yellow, you are likely getting enough water.
Low sodium treatment
Hyponatremia treatment is aimed at addressing the underlying cause, if possible.
If you have moderate, chronic hyponatremia due to your diet, diuretics or drinking too much water, your doctor may recommend temporarily cutting back on fluids. He or she may also suggest adjusting your diuretic use to increase the level of sodium in your blood.
If you have severe, acute hyponatremia, you’ll need more-aggressive treatment. Options include:
- Intravenous fluids. Your doctor may recommend IV sodium solution to slowly raise the sodium levels in your blood. This requires a stay in the hospital for frequent monitoring of sodium levels as too rapid of a correction is dangerous.
- Medications. You may take medications to manage the signs and symptoms of hyponatremia, such as headaches, nausea and seizures.
- Limiting water intake
Potassium
Potassium is an electrolyte that is vital to cell metabolism. Potassium is present in all body tissues and is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients 23. Potassium helps transport nutrients into cells and removes waste products out of cells. Potassium is also important in muscle function, helping to transmit messages between nerves and muscles.
Potassium, along with other electrolytes such as sodium, chloride, and bicarbonate (total CO2), helps regulate the amount of fluid in the body and maintains a stable acid-base balance. Potassium is present in all body fluids, but most potassium is found within the cells. Only a small amount is present in fluids outside the cells and in the liquid part of the blood (called serum or plasma).
The total amount of potassium in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 mmol = 1 milliequivalent [mEq] or 39.1 mg potassium) 24. Most potassium resides intracellularly, and a small amount is in extracellular fluid. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the sodium-potassium (Na+/K+) ATPase transporter 25. In addition to maintaining cellular tonicity, this gradient is required for proper nerve transmission, muscle contraction, and kidney function.
You get most of the potassium you need from the foods that you eat and most people have an adequate intake of potassium. The body uses what it requires and the kidneys eliminate the rest in the urine. The body tries to keep the blood potassium level within a very narrow range. Levels are mainly controlled by aldosterone, a hormone produced by the adrenal glands in the kidneys.
Potassium is absorbed via passive diffusion, primarily in the small intestine 25. About 90% of ingested potassium is absorbed and used to maintain its normal intracellular and extracellular concentrations 26. Potassium is excreted primarily in the urine, some is excreted in the stool, and a very small amount is lost in sweat. The kidneys control potassium excretion in response to changes in dietary intakes, and potassium excretion increases rapidly in healthy people after potassium consumption, unless body stores are depleted 23. The kidneys can adapt to variable potassium intakes in healthy individuals, but a minimum of 5 mmol (about 195 mg) potassium is excreted daily in urine 24. This, combined with other obligatory losses, suggests that potassium balance cannot be achieved with intakes less than about 400–800 mg/day.
Assessing potassium status is not routinely done in clinical practice, and it is difficult to do because most potassium in the body is inside cells. Although blood potassium levels can provide some indication of potassium status, they often correlate poorly with tissue potassium stores 24. Other methods to measure potassium status include collecting balance data (measuring net potassium retention and loss); measuring the total amount of potassium or the total amount of exchangeable potassium in the body; and conducting tissue analyses (e.g., muscle biopsies), but all have limitations 27.
Normal serum concentrations of potassium range from about 3.6 to 5.0 mmol/L and are regulated by a variety of mechanisms 24. Diarrhea, vomiting, kidney disease, use of certain medications, and other conditions that alter potassium excretion or cause transcellular potassium shifts can cause hypokalemia (serum levels below 3.6 mmol/L) or hyperkalemia (serum levels above 5.0 mmol/L) 28. Otherwise, in healthy individuals with normal kidney function, abnormally low or high blood levels of potassium are rare.
Because the blood concentration of potassium is so small, minor changes can have significant consequences. If potassium levels are too low or too high, there can be serious health consequences; a person may be at risk for developing shock, respiratory failure, or heart rhythm disturbances. An abnormal potassium level can alter the function of the nerves and muscles; for example, the heart muscle may lose its ability to contract.
Potassium Deficiency
Insufficient potassium intakes can increase blood pressure, kidney stone risk, bone turnover, urinary calcium excretion, and salt sensitivity (meaning that changes in sodium intakes affect blood pressure to a greater than normal extent).
Severe potassium deficiency can cause hypokalemia, (serum potassium level less than about 3.6 mmol/L). Hypokalemia affects up to 21% of hospitalized patients, usually because of the use of diuretics and other medications, but it is rare among healthy people with normal kidney function.
Mild hypokalemia is characterized by constipation, fatigue, muscle weakness, and malaise 24. Moderate to severe hypokalemia (serum potassium level less than about 2.5 mmol/L) can cause polyuria (large volume of dilute urine); encephalopathy in patients with kidney disease; glucose intolerance; muscular paralysis; poor respiration; and cardiac arrhythmias, especially in individuals with underlying heart disease 24. Severe hypokalemia can be life threatening because of its effects on muscle contraction and, hence, cardiac function 26.
Hypokalemia is rarely caused by low dietary potassium intake alone, but it can result from diarrhea due to potassium losses in the stool. It can also result from vomiting, which produces metabolic alkalosis, leading to potassium losses in the kidneys. Hypokalemia can also be caused by refeeding syndrome (the metabolic response to initial refeeding after a starvation period) because of potassium’s movement into cells; laxative abuse; diuretic use; eating clay (a type of pica); heavy sweating; or dialysis 29.
Magnesium depletion can contribute to hypokalemia by increasing urinary potassium losses 30. It can also increase the risk of cardiac arrhythmias by decreasing intracellular potassium concentrations. More than 50% of individuals with clinically significant hypokalemia might have magnesium deficiency 31. In people with hypomagnesemia and hypokalemia, both should be treated concurrently 28.
Low potassium symptoms may include:
- Weakness
- Fatigue
- Muscle cramps
- Constipation
- In severe cases, life-threatening paralysis may develop, such as with hypokalemic periodic paralysis.
Abnormal heart rhythms (arrhythmias) are the most worrisome complication of very low potassium levels, particularly in people with underlying heart disease.
In most cases, low potassium is found by a blood test that is done because of an illness, or because you are taking diuretics. It is rare for low potassium to cause isolated symptoms such as muscle cramps if you are feeling well in other respects.
Talk to your doctor about what your results mean. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your low potassium level.
Treatment of low potassium is directed at the underlying cause and may include potassium supplements. Don’t start taking potassium supplements without talking to your doctor first.
Causes of Hypokalemia (low potassium)
Low potassium (hypokalemia) has many causes. The most common cause is excessive potassium loss in urine due to prescription water or fluid pills (diuretics). Vomiting or diarrhea or both can result in excessive potassium loss from the digestive tract. Only rarely is low potassium caused by not getting enough potassium in your diet.
Causes of potassium loss leading to low potassium include:
- Chronic kidney disease
- Diabetic ketoacidosis
- Diarrhea (causing anal irritation)
- Excessive alcohol use
- Excessive laxative use
- Excessive sweating
- Folic acid deficiency
- Prescription water or fluid pills (diuretics) use
- Primary aldosteronism
- Vomiting
- Some antibiotic use
Groups at Risk of Potassium Inadequacy
Potassium inadequacy can occur with intakes that are below the Adequate Intake (AI) [intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA)] but above the amount required to prevent hypokalemia. The following groups are more likely than others to have poor potassium status.
People with inflammatory bowel diseases
Potassium is secreted within the colon, and this process is normally balanced by absorption 32. However, in inflammatory bowel disease (including Crohn’s disease and ulcerative colitis), potassium secretion increases, which can lead to poor potassium status. Inflammatory bowel diseases are also characterized by chronic diarrhea, which can further increase potassium excretion 33.
People who use certain medications, including diuretics and laxatives
Certain diuretics (e.g., thiazide diuretics) that are commonly used to treat high blood pressure increase urinary potassium excretion and can cause hypokalemia 28. Potassium- sparing diuretics, however, do not increase potassium excretion and can actually cause hyperkalemia. Large doses of laxatives and repeated use of enemas can also cause hypokalemia because they increase losses of potassium in stool.
People with pica
Pica is the persistent eating of non-nutritive substances, such as clay. When consumed, clay binds potassium in the gastrointestinal tract, which can increase potassium excretion and lead to hypokalemia 26. Cessation of pica combined with potassium supplementation can restore potassium status and resolve symptoms of potassium deficiency.
Hypokalemia Treatment
If your condition is mild, your provider will likely prescribe oral potassium pills. If your condition is severe, you may need to get potassium through a vein (IV).
If you need diuretics, your provider may:
- Switch you to a form that keeps potassium in the body. This type of diuretic is called potassium-sparing.
- Prescribe extra potassium for you to take every day.
Eating foods rich in potassium can help treat and prevent low level of potassium. These foods include:
- Avocados
- Baked potato
- Bananas
- Bran
- Carrots
- Cooked lean beef
- Milk
- Oranges
- Peanut butter
- Peas and beans
- Salmon
- Seaweed
- Spinach
- Tomatoes
- Wheat germ
Outlook (Prognosis)
Taking potassium supplements can usually correct the problem. In severe cases, without proper treatment, a severe drop in potassium level can lead to serious heart rhythm problems that can be fatal.
Potassium and Health
Because of potassium’s wide-ranging roles in the body, low intakes can increase the risk of illness. This section focuses on four diseases and disorders in which potassium might be involved: hypertension and stroke; kidney stones; bone health; and blood glucose control and type 2 diabetes.
Hypertension and stroke
Hypertension, a major risk factor for heart disease and stroke, affects almost a third of Americans. According to an extensive body of literature, low potassium intakes increase the risk of hypertension, especially when combined with high sodium intakes 34. Higher potassium intakes, in contrast, may help decrease blood pressure, in part by increasing vasodilation and urinary sodium excretion, which in turn reduces plasma volume 35; this effect may be most pronounced in salt-sensitive individuals 23.
The Dietary Approaches to Stop Hypertension (DASH) eating pattern, which emphasizes potassium from fruits, vegetables, and low-fat dairy products, lowers systolic blood pressure by an average of 5.5 mmHg and diastolic blood pressure by 3.0 mmHg 36. The DASH eating pattern provides three times more potassium than the average American diet. However, it also increases intakes of other nutrients, such as magnesium and calcium, that are also associated with reductions in blood pressure, so potassium’s independent contribution cannot be determined.
Results from most clinical trials suggest that potassium supplementation reduces blood pressure. A 2017 meta-analysis of 25 randomized controlled trials in 1,163 participants with hypertension found significant reductions in systolic blood pressure (by 4.48 mm Hg) and diastolic blood pressure (by 2.96 mmHg) with potassium supplementation, mostly as potassium chloride at 30–120 mmol/day potassium (1,173–4,692 mg), for 4–15 weeks 37. Another meta-analysis of 15 randomized controlled trials found that potassium supplements (mostly containing potassium chloride at 60–65 mEq/day potassium [2,346–2,541 mg]) for 4–24 weeks in 917 patients with normal blood pressure or hypertension who were not taking antihypertensive medications significantly reduced both systolic and diastolic blood pressure 38. The supplements had the greatest effect in patients with hypertension, reducing systolic blood pressure by a mean of 6.8 mmHg and diastolic blood pressure by 4.6 mmHg. Two earlier meta-analyses of 19 trials 39 and 33 trials 40 had similar findings. However, a Cochrane review of six of the highest-quality trials found nonsignificant reductions in systolic and diastolic blood pressure with potassium supplementation 41.
In 2017, the Agency for Healthcare Research and Quality (AHRQ) published a draft systematic review of the effects of sodium and potassium intakes on chronic disease outcomes and their risk factors 42. The authors concluded that, based on observational studies, higher potassium intakes were not associated with lower blood pressure in adults or with the risk of hypertension 42. The authors did report, however, that potassium supplements (mostly containing potassium chloride) in doses ranging from 20 to 129 mmol/day (782 to 5,044 mg/day) for 1 to 36 months significantly lowered systolic blood pressure by a mean of 6.04 mmHg and diastolic blood pressure by 3.43 mmHg in adults, most of whom had hypertension 42. Based on 13 randomized controlled trials that primarily enrolled patients with hypertension, the review found that the use of potassium-containing salt substitutes in place of sodium chloride significantly reduced systolic blood pressure in adults by a mean of 5.34 mmHg and diastolic blood pressure by 2.62 mmHg. However, reducing sodium intake decreased both systolic and diastolic blood pressure in adults, and increasing potassium intake via food or supplements did not reduce blood pressure any further. This finding suggests that at least some of the beneficial effects of potassium salt substitutes on blood pressure may be due to the accompanying reduction in sodium intake, rather than the increase in potassium intake 42.
Higher potassium intakes have been associated with a decreased risk of stroke and possibly other cardiovascular diseases (CVDs) 43. A meta-analysis of 11 prospective cohort studies in 247,510 adults found that a 1,640 mg per day higher potassium intake was associated with a significant 21% lower risk of stroke as well as nonsignificant lower risks of coronary heart disease and total CVD 44. Similarly, the authors of a meta-analysis of 9 cohort studies reported a significant 24% lower risk of stroke with higher potassium intakes and a nonsignificant reduction in coronary heart disease and cardiovascular disease (CVD) risk 45. However, a draft Agency for Healthcare Research and Quality report found inconsistent relationships between dietary potassium intakes and risk of stroke based on 1 case-cohort and 10 prospective cohort studies 42.
Any beneficial effect of potassium on cardiovascular disease (CVD) is likely due to its antihypertensive effects. However, some research shows a benefit even when blood pressure is accounted for. For example, a 2016 meta-analysis of 16 cohort studies with a total of 639,440 participants found that those with the highest potassium intakes (median 103 mmol [4,027 mg] per day) had a 15% lower risk of stroke than those with the lowest potassium intakes (median 52.5 mmol [2,053 mg] per day). In addition, participants who consumed 90 mmol potassium/day (approximately 3,500 mg) had the lowest risk of stroke 46. However, even when blood pressure was accounted for, higher potassium intakes still produced a significant 13% lower risk of stroke. These findings suggest that other mechanisms (e.g., improved endothelial function and reduced free radical formation) may be involved 47.
The FDA has approved the following health claim: “Diets containing foods that are a good source of potassium and that are low in sodium may reduce the risk of high blood pressure and stroke” 48. Overall, the evidence suggests that consuming more potassium might have a favorable effect on blood pressure and stroke, and it might also help prevent other forms of cardiovascular disease. However, more research on both dietary and supplemental potassium is needed before firm conclusions can be drawn.
Kidney stones
Kidney stones are most common in people aged 40 to 60 49. Stones containing calcium—in the form of calcium oxalate or calcium phosphate—are the most common type of kidney stone. Low potassium intakes impair calcium reabsorption within the kidney, increasing urinary calcium excretion and potentially causing hypercalciuria and kidney stones 47. Low urinary levels of citrate also contribute to kidney stone development.
Observational studies show inverse associations between dietary potassium intakes and risk of kidney stones. In a cohort of 45,619 men aged 40 to 75 years with no history of kidney stones, those with the highest potassium intakes (≥4,042 mg/day on average) had a 51% lower risk of kidney stones over 4 years of follow-up than those with the lowest intakes (≤2,895 mg/day) 50. Similarly, in over 90,000 women aged 34–59 who participated in the Nurses’ Health Study and had no history of kidney stones, those who consumed an average of over 4,099 mg of potassium per day had a 35% lower risk of kidney stones over a 12-year follow-up period than those who averaged less than 2,407 mg of potassium per day 51.
Some research suggests that supplementation with potassium citrate reduces hypercalciuria as well as the risk of kidney stone formation and growth 49. In a clinical trial of 57 patients with at least two kidney stones (either calcium oxalate or calcium oxalate plus calcium phosphate) over the previous 2 years and hypocitraturia (low urinary citrate levels), supplementation with 30–60 mEq potassium citrate (providing 1,173 to 2,346 mg potassium) for 3 years significantly reduced kidney stone formation compared with placebo 52. This study was included in a 2015 Cochrane review of seven studies that examined the effects of potassium citrate, potassium-sodium citrate, and potassium-magnesium citrate supplementation on the prevention and treatment of calcium-containing kidney stones in a total of 477 participants, most of whom had calcium oxalate stones 49. The potassium citrate salts significantly reduced the risk of new stones and reduced stone size. However, the proposed mechanism involves citrate, not potassium per se; citrate forms complexes with urinary calcium and increases urine pH, inhibiting the formation of calcium oxalate crystals 49. The authors of a draft Agency for Healthcare Research and Quality report 42 concluded that observational studies suggest an association between higher potassium intakes and lower risk of kidney stones. However, they also found the evidence insufficient to determine whether potassium supplements are effective because only one trial that addressed this question 52 met their inclusion criteria.
Additional research is needed to fully understand the potential link between dietary and supplemental potassium and the risk of kidney stones.
Bone health
Observational studies suggest that increased consumption of potassium from fruits and vegetables is associated with increased bone mineral density 53. This evidence, combined with evidence from metabolic studies and a few clinical trials, suggests that dietary potassium may improve bone health.
The underlying mechanisms are unclear, but one hypothesis is that potassium helps protect bone through its effect on acid-base balance 47. Diets that are high in acid-forming foods, such as meats and cereal grains, contribute to metabolic acidosis and might have an adverse effect on bone. Alkaline components in the form of potassium salts (potassium bicarbonate or citrate, but not potassium chloride) from food or potassium supplements might counter this effect and help preserve bone tissue. In the Framingham Heart Study for example, higher potassium intake was associated with significantly greater bone mineral density in 628 elderly men and women 54. In another study, the DASH eating pattern significantly reduced biochemical markers of bone turnover 55. This eating pattern has a lower acid load than typical Western diets and is also high in calcium and magnesium, in addition to potassium, so any independent contribution of potassium cannot be determined.
Only a few clinical trials have examined the effects of potassium supplements on markers of bone health. One trial found that supplementation with potassium citrate at either 60 mmol/day (2,346 mg potassium) or 90 mmol/day (3,519 mg potassium) for 6 months significantly reduced urinary calcium excretion compared with placebo in 52 healthy men and women older than 55 years 56. In another clinical trial, 201 healthy adults aged 65 years or older received daily supplementation with 60 mEq potassium citrate (providing 2,346 mg potassium) or placebo as well as 500 mg/day calcium (as calcium carbonate) and 400 IU/day vitamin D3 for 2 years 57. Potassium supplementation significantly increased bone mineral density at the lumbar spine and bone microarchitecture compared with placebo. In a similar clinical trial among older adults, supplemental potassium bicarbonate (mean doses of 2,893 or 4,340 mg/day potassium) for 84 days significantly reduced biochemical markers of bone turnover and urinary calcium excretion 58. Conversely, a clinical trial in 276 postmenopausal women aged 55–65 years found that supplementation with potassium citrate at either 18.5 mEq/day (providing 723 mg potassium) or 55.5 mEq/day (2,170 mg potassium) for 2 years did not significantly reduce bone turnover or increase bone mineral density at the hip or lumbar spine compared with placebo 59.
Overall, higher intakes of potassium from diets that emphasize fruits and vegetables might improve bone health. However, more research is needed to elucidate the underlying mechanisms and tease out potassium’s individual contribution.
Blood glucose control and type 2 diabetes
Type 2 diabetes is a growing public health concern that currently affects almost 12% of U.S. adults 60. Although obesity is the primary risk factor for type 2 diabetes, other metabolic factors also play a role. Because potassium is needed for insulin secretion from pancreatic cells, hypokalemia impairs insulin secretion and could lead to glucose intolerance 23. This effect has been observed mainly with long-term use of diuretics (particularly those containing thiazides) or hyperaldosteronism (excessive aldosterone production), which both increase urinary potassium losses, but it can occur in healthy individuals as well 23.
Numerous observational studies of adults have found associations between lower potassium intakes or lower serum or urinary potassium levels and increased rates of fasting glucose, insulin resistance, and type 2 diabetes 61. These associations might be stronger in African Americans, who tend to have lower potassium intakes, than in whites 62. For example, one study of 1,066 adults aged 18–30 years without diabetes found that those with urinary potassium levels in the lowest quintile were more than twice as likely to develop type 2 diabetes over 15 years of follow-up than those in the highest quintile 62. Among 4,754 participants from the same study with potassium intake data, African Americans with lower potassium intakes had a significantly greater risk of type 2 diabetes over 20 years of follow-up than those with higher intakes, but this association was not found in whites.
In another observational study, which analyzed data from 84,360 women aged 34–59 years participating in the Nurses’ Health Study, those in the highest quintile of potassium intake had a 38% lower risk of developing type 2 diabetes over 6 years of follow-up than those in the lowest quintile 63. Serum potassium levels were inversely associated with fasting glucose levels in 5,415 participants aged 45–84 years from the Multi-Ethnic Study of Atherosclerosis, but these levels had no significant association with diabetes risk over 8 years of follow-up 61.
Although observational studies suggest that potassium status is linked to blood glucose control and type 2 diabetes, this association has not been adequately evaluated in clinical trials. In a small clinical trial in 29 African American adults with prediabetes and low to normal serum potassium levels (3.3–4.0 mmol/L), supplementation with 40 mEq (1,564 mg) potassium (as potassium chloride) for 3 months significantly lowered fasting glucose levels, but it did not affect glucose or insulin measures during an oral glucose tolerance test 64.
The findings from studies conducted to date are promising. But more research, including randomized controlled trials, is needed before potassium’s link with blood glucose control and type 2 diabetes can be confirmed.
High potassium (hyperkalemia)
Hyperkalemia is the medical term that describes a potassium level in your blood that’s higher than normal. Potassium is a chemical that is critical to the function of nerve and muscle cells, including those in your heart.
Your blood potassium level is normally 3.6 to 5.2 millimoles per liter (mmol/L). Having a blood potassium level higher than 6.0 mmol/L can be dangerous and usually requires immediate treatment.
High potassium symptoms
There are often no symptoms with a high level of potassium.
High potassium is usually found when your doctor has ordered blood tests to help diagnose a condition you’re already experiencing or to monitor medications you’re taking. It’s usually not discovered by chance.
Talk to your doctor about what your results mean. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your high potassium level. Treatment of high potassium is often directed at the underlying cause. In some instances, you may need emergency medications or dialysis.
If you have symptoms of hyperkalemia, particularly if you have kidney disease or are taking medications that raise your potassium level, call your doctor immediately. Hyperkalemia is a serious and potentially life-threatening disorder. It can cause:
- Muscle fatigue
- Weakness
- Paralysis
- Abnormal heart rhythms (arrhythmias) – slow, weak, or irregular pulse
- Nausea
High potassium level causes
Often a report of high blood potassium isn’t true hyperkalemia. Instead, it may be caused by the rupture of blood cells in the blood sample during or shortly after the blood draw. The ruptured cells leak their potassium into the sample. This falsely raises the amount of potassium in the blood sample, even though the potassium level in your body is actually normal. When this is suspected, a repeat blood sample is done.
The most common cause of genuinely high potassium (hyperkalemia) is related to your kidneys, such as:
- Acute kidney failure
- Chronic kidney disease
If your kidneys are not working well, they may not be able to remove the proper amount of potassium. As a result, potassium can build up in the blood.
Other causes of hyperkalemia include:
- Addison’s disease (adrenal insufficiency)
- Angiotensin II receptor blockers
- Angiotensin-converting enzyme (ACE) inhibitors
- Beta blockers
- Dehydration
- Destruction of red blood cells due to severe injury or burns
- Excessive use of potassium supplements
- Type 1 diabetes
Exams and Tests
The health care provider will perform a physical exam and ask about your symptoms.
Tests that may be ordered include:
- Electrocardiogram (ECG)
- Potassium level
Your provider will likely check your blood potassium level and do kidney blood tests on a regular basis if you:
- Have been prescribed extra potassium
- Have chronic kidney disease
- Take medicines to treat heart disease or high blood pressure
- Use salt substitutes
Treatment for high potassium (hyperkalemia)
You will need emergency treatment if your potassium level is very high, or if you have danger signs, such as changes in an ECG.
Emergency treatment may include:
- Calcium given into your veins (IV) to treat the muscle and heart effects of high potassium levels
- Glucose and insulin given into your veins (IV) to help lower potassium levels long enough to correct the cause
- Kidney dialysis if your kidney function is poor
- Medicines that help remove potassium from the intestines before it is absorbed
- Sodium bicarbonate if the problem is caused by acidosis
- Some water pills (diuretics)
Changes in your diet can help both prevent and treat high potassium levels. You may be asked to:
- Limit or avoid asparagus, avocados, potatoes, tomatoes or tomato sauce, winter squash, pumpkin, and cooked spinach
- Limit or avoid oranges and orange juice, nectarines, kiwifruit, raisins, or other dried fruit, bananas, cantaloupe, honeydew, prunes, and nectarines
- Avoid taking salt substitutes if you are asked to eat a low-salt diet
Your provider may make the following changes to your medicines:
- Reduce or stop potassium supplements
- Stop or change the doses of medicines you are taking, such as ones for heart disease and high blood pressure
- Take a certain type of water pill to reduce potassium and fluid levels if you have chronic kidney failure
Follow your provider’s directions when taking your medicines:
- DO NOT stop or start taking medicines without first talking to your provider
- Take your medicines on time
- Tell your provider about any other medicines, vitamins, or supplements you are taking
What is the recommended dietary potassium intake?
Intake recommendations for potassium and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine 35. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
When the Food and Nutrition Board evaluated the available data in 2005, it found the data insufficient to derive an EAR for potassium, so the board established AIs for all ages based on potassium intakes in healthy populations 35. Table 2 lists the current AIs for potassium for healthy individuals. The Food and Nutrition Board is reevaluating the DRIs for potassium and expects to release a new report with its findings in 2019.
Table 3: Adequate Intakes (AIs) for Potassium*
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | 400 mg | 400 mg | ||
| 7–12 months | 700 mg | 700 mg | ||
| 1–3 years | 3,000 mg | 3,000 mg | ||
| 4–8 years | 3,800 mg | 3,800 mg | ||
| 9–13 years | 4,500 mg | 4,500 mg | ||
| 14–18 years | 4,700 mg | 4,700 mg | 4,700 mg | 5,100 mg |
| 19–50 years | 4,700 mg | 4,700 mg | 4,700 mg | 5,100 mg |
| 51+ years | 4,700 mg | 4,700 mg |
*The AIs do not apply to individuals with impaired potassium excretion because of medical conditions (e.g., kidney disease) or the use of medications that impair potassium excretion.
[Source 35]Potassium Intakes and Status of Americans
Dietary surveys consistently show that people in the United States consume substantially less potassium than recommended, which is why the 2015–2020 Dietary Guidelines for Americans identifies potassium as a “nutrient of public health concern” 65. According to data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES), the average daily potassium intake from foods is 2,423 mg for males aged 2–19, and 1,888 mg for females aged 2–19 66. In adults aged 20 and over, the average daily potassium intake from foods is 3,016 mg for men and 2,320 mg for women.
Average potassium intakes vary by race. Non-Hispanic blacks aged 20 and older consume an average of 2,449 mg potassium per day. Average daily intakes are 2,695 mg for Hispanic whites and 2,697 mg for non-Hispanic whites 66.
Use of potassium-containing dietary supplements does not significantly increase total potassium intakes among U.S. adults 67, probably because most potassium-containing dietary supplements provide no more than 99 mg potassium per serving 68. Data from NHANES 2013–2014 indicate that 12% of children and adults aged 2 and over use supplements containing potassium, and among those who do, supplement use adds a mean of only 87 mg to total daily potassium intakes 66.
Food sources of potassium
Potassium is found in a wide variety of plant and animal foods and in beverages. Many fruits and vegetables are excellent sources, as are some legumes (e.g., soybeans) and potatoes. Meats, poultry, fish, milk, yogurt, and nuts also contain potassium 26. Among starchy foods, whole-wheat flour and brown rice are much higher in potassium than their refined counterparts, white wheat flour and white rice 69.
Milk, coffee, tea, other nonalcoholic beverages, and potatoes are the top sources of potassium in the diets of U.S. adults 70. Among children in the United States, milk, fruit juice, potatoes, and fruit are the top sources 71.
It is estimated that the body absorbs about 85%–90% of dietary potassium 23. The forms of potassium in fruits and vegetables include potassium phosphate, sulfate, citrate, and others, but not potassium chloride 34.
Selected food sources of potassium are listed in Table 4.
Table 4: Selected Food Sources of Potassium
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Apricots, dried, ½ cup | 1,101 | 31 |
| Lentils, cooked, 1 cup | 731 | 21 |
| Prunes, dried, ½ cup | 699 | 20 |
| Squash, acorn, mashed, 1 cup | 644 | 18 |
| Raisins, ½ cup | 618 | 18 |
| Potato, baked, flesh only, 1 medium | 610 | 17 |
| Kidney beans, canned, 1 cup | 607 | 17 |
| Orange juice, 1 cup | 496 | 14 |
| Soybeans, mature seeds, boiled, ½ cup | 443 | 13 |
| Banana, 1 medium | 422 | 12 |
| Milk, 1%, 1 cup | 366 | 10 |
| Spinach, raw, 2 cups | 334 | 10 |
| Chicken breast, boneless, grilled, 3 ounces | 332 | 9 |
| Yogurt, fruit variety, nonfat, 6 ounces | 330 | 9 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 326 | 9 |
| Beef, top sirloin, grilled, 3 ounces | 315 | 9 |
| Molasses, 1 tablespoon | 308 | 9 |
| Tomato, raw, 1 medium | 292 | 8 |
| Soymilk, 1 cup | 287 | 8 |
| Yogurt, Greek, plain, nonfat, 6 ounces | 240 | 7 |
| Broccoli, cooked, chopped, ½ cup | 229 | 7 |
| Cantaloupe, cubed, ½ cup | 214 | 6 |
| Turkey breast, roasted, 3 ounces | 212 | 6 |
| Asparagus, cooked, ½ cup | 202 | 6 |
| Apple, with skin, 1 medium | 195 | 6 |
| Cashew nuts, 1 ounce | 187 | 5 |
| Rice, brown, medium-grain, cooked, 1 cup | 154 | 4 |
| Tuna, light, canned in water, drained, 3 ounces | 153 | 4 |
| Coffee, brewed, 1 cup | 116 | 3 |
| Lettuce, iceberg, shredded, 1 cup | 102 | 3 |
| Peanut butter, 1 tablespoon | 90 | 3 |
| Tea, black, brewed, 1 cup | 88 | 3 |
| Flaxseed, whole, 1 tablespoon | 84 | 2 |
| Bread, whole-wheat, 1 slice | 81 | 2 |
| Egg, 1 large | 69 | 2 |
| Rice, white, medium-grain, cooked, 1 cup | 54 | 2 |
| Bread, white, 1 slice | 37 | 1 |
| Cheese, mozzarella, part skim, 1½ ounces | 36 | 1 |
| Oil (olive, corn, canola, or soybean), 1 tablespoon | 0 | 0 |
*DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for potassium used as the basis for the values in Table 2 is 3,500 mg for adults and children aged 4 and older, but the DV will increase to 4,700 mg when the updated Nutrition and Supplement Facts labels are implemented 72)). The updated labels and DVs must appear on food products and dietary supplements beginning in January 2020, but they can be used now 48. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 69]The U.S. Department of Agriculture’s Nutrient Database 69 lists the nutrient content of many foods and provides a comprehensive list of foods containing potassium ordered by food name 73 and by nutrient content 74. The 2015–2020 Dietary Guidelines for Americans 15 provides a list of foods with at least 5% of the DV for potassium per serving.
Chloride
Chloride is an electrolyte. It is a negatively charged ion that works with other electrolytes, such as potassium, sodium, and bicarbonate, to help regulate the amount of fluid in the body and maintain the acid-base balance (pH).
Chloride is present in all body fluids but is found in the highest concentration in the blood and in the fluid outside of the body’s cells. Most of the time, chloride concentrations mirror those of sodium, increasing and decreasing for the same reasons and in direct relationship to sodium. When there is an acid-base imbalance, however, blood chloride levels can change independently of sodium levels as chloride acts as a buffer. It helps to maintain electrical neutrality at the cellular level by moving into or out of the cells as needed.
You get chloride in your diet through food and table salt (NaCl), which is made up of sodium and chloride ions. Most of the chloride is absorbed by the digestive tract, and the excess is eliminated in urine. The normal blood level remains steady, with a slight drop after meals (because the stomach produces acid after eating, using chloride from blood).
A typical normal chloride range is 96 to 106 milliequivalents per liter (mEq/L) or 96 to 106 millimoles per liter (millimol/L).
Normal value ranges may vary slightly among different laboratories. Talk to your provider about the meaning of your specific test results.
The example above shows the common measurement range for results for these tests. Some laboratories use different measurements or may test different specimens.
The blood chloride test is almost never ordered by itself. It is usually ordered as part of an electrolyte panel, a basic metabolic panel, or a comprehensive metabolic panel, which are ordered frequently as part of a routine physical.
Chloride, as part of an electrolyte or metabolic panel, may be ordered when acidosis or alkalosis is suspected or when someone has an acute condition with symptoms that may include the following:
- Prolonged vomiting and/or diarrhea
- Weakness, fatigue
- Difficulty breathing (respiratory distress)
Electrolytes may be ordered at regular intervals when a person has a disease or condition or is taking a medication that can cause an electrolyte imbalance. Electrolyte panels or basic metabolic panels are commonly used to monitor treatment of certain problems, including high blood pressure (hypertension), heart failure, and liver and kidney disease.
A urine chloride test may be performed along with a blood or urine sodium when evaluating the cause of low or high blood chloride levels.
- An increased level of urine chloride can indicate dehydration, starvation, Addison disease, or increased salt intake. If both chloride and sodium levels are high in a person on a restricted salt diet, the person is not complying with the diet.
- A decreased level of urine chloride can be seen with Cushing syndrome, Conn syndrome, congestive heart failure, malabsorption syndrome, and diarrhea.
A healthcare practitioner will look at whether the chloride measurement changes mirror those of the sodium. This helps the healthcare practitioner determine if there is also an acid-base imbalance and helps to guide treatment.
What Abnormal Chloride Results Mean
Drugs that affect sodium blood levels will also cause changes in chloride. In addition, swallowing large amounts of baking soda or substantially more than the recommended dosage of antacids can also cause low blood chloride.
A greater-than-normal level of chloride is called hyperchloremia. It may be due to:
An increased level of blood chloride (called hyperchloremia) usually indicates dehydration, but can also occur with other problems that cause high blood sodium, such as Cushing syndrome or kidney disease. Hyperchloremia also occurs when too much base is lost from the body (producing metabolic acidosis) or when a person hyperventilates (causing respiratory alkalosis).
- Carbonic anhydrase inhibitors (used to treat glaucoma)
- Diarrhea
- Metabolic acidosis
- Respiratory alkalosis (compensated)
- Renal tubular acidosis
A lower-than-normal level of chloride is called hypochloremia. It may be due to:
A decreased level of blood chloride (called hypochloremia) occurs with any disorder that causes low blood sodium. Hypochloremia also occurs with congestive heart failure, prolonged vomiting or gastric suction, Addison disease, emphysema or other chronic lung diseases (causing respiratory acidosis), and with loss of acid from the body (called metabolic alkalosis).
- Addison disease
- Bartter syndrome
- Burns
- Congestive heart failure
- Dehydration
- Excessive sweating
- Hyperaldosteronism
- Metabolic alkalosis
- Respiratory acidosis (compensated)
- Syndrome of inappropriate diuretic hormone secretion (SIADH)
- Vomiting
This test may also be done to help rule out or diagnose:
- Multiple endocrine neoplasia (MEN) II
- Primary hyperparathyroidism
What treatment is prescribed for abnormal chloride levels?
The same treatment used to treat sodium imbalances (diuretics, fluid replacement) may be used to treat chloride imbalances.
Are there dietary recommendations for chloride?
Yes. The Food and Nutrition Board at the Institute of Medicine recommends that adolescents and adults ages 14 to 50 years consume 2.3 g/day. The recommendations vary based on age, sex, and other factors. Chloride is readily available in the food supply and most Americans probably consume more than necessary, in the form of table salt and salt in prepared foods. It is also found in many vegetables and in foods such as salted meats, butter, tomatoes, lettuce, celery, and olives.
Bicarbonate
Bicarbonate (HCO3−, sometimes reported as total CO2) is an electrolyte, a negatively charged ion that is used by the body to help maintain the body’s acid-base (pH) balance. It also works with the other electrolytes (sodium, potassium, and chloride) to maintain electrical neutrality at the cellular level. This test measures the total amount of carbon dioxide (CO2) in the blood, which occurs mostly in the form of bicarbonate (HCO3-). The CO2 is mainly a by-product of various metabolic processes.
Measuring bicarbonate as part of an electrolyte or metabolic panel may help diagnose an electrolyte imbalance or acidosis or alkalosis. Acidosis and alkalosis describe the abnormal conditions that result from an imbalance in the pH of the blood caused by an excess of acid or alkali (base). This imbalance is typically caused by some underlying condition or disease.
The body’s maintenance of a healthy pH range for blood and tissues that is slightly basic (pH between 7.35 – 7.45). This balance is achieved through the use of systems in the blood (which help to minimize pH changes) and by the lungs and kidneys, which eliminate excess amounts of acids or bases from the body.
The lungs and kidneys are the major organs involved in regulating blood pH through the removal of excess bicarbonate.
- The lungs flush acid out of the body by exhaling CO2. Raising and lowering the respiratory rate alters the amount of CO2 that is breathed out, and this can affect blood pH within minutes.
- The kidneys eliminate acids in the urine and they regulate the concentration of bicarbonate (HCO3−, a base) in blood. Acid-base changes due to increases or decreases in HCO3− concentration occur more slowly than changes in CO2, taking hours or days.
Any disease or condition that affects the lungs, kidneys, metabolism, or breathing has the potential to cause acidosis or alkalosis.
The bicarbonate test gives a healthcare practitioner a rough estimate of a patient’s acid-base balance. This is usually sufficient, but measurements of gases dissolved in the blood (blood gases) may be done if more information is needed. Bicarbonate is typically measured along with sodium, potassium, and possibly chloride in an electrolyte panel as it is the balance of these molecules that gives the healthcare practitioner the most information.
The bicarbonate (or total CO2) test is usually ordered along with sodium, potassium, and chloride as part of an electrolyte panel. The electrolyte panel is used to help detect, evaluate, and monitor electrolyte imbalances and/or acid-base (pH) imbalances (acidosis or alkalosis). It may be ordered as part of a routine exam or to help evaluate a variety of chronic or acute illnesses.
This testing may be ordered when acidosis or alkalosis is suspected or when someone has an acute condition with symptoms that may include the following:
- Prolonged vomiting and/or diarrhea
- Weakness, fatigue
- Difficulty breathing (respiratory distress)
Electrolytes may be ordered at regular intervals when a person has a disease or condition or is taking a medication that can cause an electrolyte imbalance. Electrolyte panels or basic metabolic panels are commonly used to monitor treatment of certain problems, including high blood pressure (hypertension), heart failure, and liver and kidney disease.
An electrolyte panel may be used to help monitor conditions, such as kidney disease, lung disorders, and high blood pressure (hypertension). When acidosis or alkalosis is identified, bicarbonate (as part of the electrolyte panel) and blood gases may be ordered to evaluate the severity of the pH imbalance. These tests help determine whether it is primarily respiratory (due to an imbalance between the amount of oxygen coming in and CO2 being released) or metabolic (due to increased or decreased amounts of bicarbonate in the blood). They also help monitor treatment until acid-base balance is restored.
What does the test bicarbonate result mean?
When bicarbonate levels are higher or lower than normal, it suggests that the body is having trouble maintaining its acid-base balance, either by failing to remove carbon dioxide through the lungs or the kidneys or perhaps because of an electrolyte imbalance, particularly a deficiency of potassium. Both of these imbalances may be due to a wide range of conditions.
Examples of causes of a low bicarbonate level include:
- Addison disease
- Chronic diarrhea
- Diabetic ketoacidosis
- Metabolic acidosis
- Respiratory alkalosis, which can be caused by hyperventilation
- Shock
- Kidney disease
- Ethylene glycol or methanol poisoning
- Salicylate (aspirin) overdose
- Drugs that may decrease bicarbonate levels include methicillin, nitrofurantoin, tetracycline, thiazide diuretics, and triamterene.
Examples of causes of a high bicarbonate level include:
- Severe, prolonged vomiting and/or diarrhea
- Lung diseases, including COPD
- Cushing syndrome
- Conn syndrome
- Metabolic alkalosis
- Some drugs may increase bicarbonate levels including fludrocortisone, barbiturates, bicarbonates, hydrocortisone, loop diuretics, and steroids.
If bicarbonate levels are too high or low, what treatments can help?
If your bicarbonate is high or low, your healthcare practitioner will identify and treat the underlying cause. For example, high bicarbonate may be caused by emphysema, which may be treated with oxygen therapy and medications, or by severe diarrhea or vomiting, which would be addressed by treating the cause of the diarrhea or vomiting. Low bicarbonate may be caused by diabetic ketoacidosis, for example, which can be addressed in part by fluid and electrolyte replacement and insulin therapy.
If I’ve had a bicarbonate (total CO2) test, why does my doctor want to test my blood gases?
Blood gas tests, in which blood is drawn from an artery instead of a vein, can give your healthcare practitioner more information about your acid-base balance. They can tell your provider whether your lungs are working properly to keep oxygen and carbon dioxide at healthy levels.
- NHS. Dehydration – Introduction. http://www.nhs.uk/Conditions/Dehydration/Pages/Introduction.aspx[↩]
- Water as an essential nutrient: the physiological basis of hydration. European Journal of Clinical Nutrition (2010) 64, 115–123; doi:10.1038/ejcn.2009.111; published online 2 September 2009. http://www.nature.com/ejcn/journal/v64/n2/full/ejcn2009111a.html[↩][↩][↩][↩][↩]
- Hole’s Essentials of Human Anatomy and Physiology 13th edition. Published by McGraw-Hill Education, 2 Penn Plaza, New York, NY 10121.[↩][↩][↩][↩][↩]
- Dietary Reference Intakes (2006). The Essential Guide to Nutrients Requirements. Institute of Medicine of the National Academies: Washington DC, 543 pp.[↩]
- Wang ZM, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB (1999). Hydration of fat-free body mass: a review and critique of a classic body-composition constant. Am J Clin Nutr 69, 833–841. https://www.ncbi.nlm.nih.gov/pubmed/10232621[↩]
- Ganong WF (2005). Review of Medical Physiology, 22nd edn, LANGE-Science: New York.[↩][↩]
- Jequier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64(2):115–123. http://www.nature.com/ejcn/journal/v64/n2/full/ejcn2009111a.html[↩]
- Lang F, Waldegger S (1997). Regulating cell volume. Am Scientist 85, 456–463.[↩]
- Häussinger D (1996). The role of cellular hydration in the regulation of cell function. Biochem J 313, 697–710. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1216967/[↩][↩]
- Grandjean AC, Campbell SM (2004). Hydration: Fluids for Life. A monograph by the North American Branch of the International Life Science Institute. ILSI North America: Washington DC.[↩]
- Ritz P, Berrut G (2005). The importance of good hydration for day-to-day health. Nutr Rev 63, S6–S13. https://academic.oup.com/nutritionreviews/article-abstract/63/suppl_1/S6/1927737/The-Importance-of-Good-Hydration-for-Day-to-Day[↩]
- Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U (2005). Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol 289, R275–R280. http://ajpregu.physiology.org/content/289/1/R275.long[↩]
- Parikh C, Berl T: Disorders of Water Metabolism. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology. 4th edition ed. Philadelphia, Elsevier, 2010.[↩]
- U.S. National Library of Medicine. Fluid and Electrolyte Balance. https://medlineplus.gov/fluidandelectrolytebalance.html[↩]
- Dietary Guidelines for Americans. https://health.gov/dietaryguidelines/[↩][↩][↩]
- American Heart Association. How much sodium should I eat per day ? https://sodiumbreakup.heart.org/how_much_sodium_should_i_eat[↩]
- Harvard University. Harvard Health Publications. Sodium, potassium together influence heart health. Published: September, 2011. http://www.health.harvard.edu/heart-health/sodium-potassium-together-influence-heart-health[↩]
- Journal of the American Medical Association (JAMA) July 11, 2011. Sodium and Potassium Intake and Mortality Among US Adults. Prospective Data From the Third National Health and Nutrition Examination Survey. http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/1106080[↩]
- USDA National Nutrient Database. www.nal.usda.gov/fnic/foodcomp/search/[↩][↩]
- BMJ 2013; 346 doi: https://doi.org/10.1136/bmj.f1325 (Published 04 April 2013). Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. http://www.bmj.com/content/346/bmj.f1325[↩]
- BMJ 2013; 346 doi: https://doi.org/10.1136/bmj.f1326 (Published 04 April 2013). Effect of lower sodium intake on health: systematic review and meta-analyses. http://www.bmj.com/content/346/bmj.f1326[↩]
- BMJ 2013; 346 doi: https://doi.org/10.1136/bmj.f1378 (Published 04 April 2013). Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. http://www.bmj.com/content/346/bmj.f1378[↩]
- Stone MS, Martyn L, Weaver CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients 2016;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4963920/[↩][↩][↩][↩][↩][↩]
- Preuss HG, Clouatre DL. Sodium, chloride, and potassium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:475-92.[↩][↩][↩][↩][↩][↩]
- Hinderling PH. The pharmacokinetics of potassium in humans is unusual. J Clin Pharmacol 2016;56:1212-20 https://www.ncbi.nlm.nih.gov/pubmed/26854277[↩][↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid-based metabolism. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:102-32.[↩][↩][↩][↩]
- Patrick J. Assessment of body potassium stores. Kidney Int 1977;11:476-90. https://www.kidney-international.org/article/S0085-2538(15)31757-9/pdf[↩]
- Viera AJ, Wouk N. Potassium disorders: Hypokalemia and hyperkalemia. Am Fam Physician 2015;92:487-95. https://www.aafp.org/afp/2015/0915/p487.html[↩][↩][↩]
- McKenna D. Myopathy, hypokalaemia and pica (geophagia) in pregnancy. Ulster Med J 2006;75:159-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1891740/[↩]
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:527-37.[↩]
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007;18:2649-52. http://jasn.asnjournals.org/content/18/10/2649.long[↩]
- Barkas F, Liberopoulos E, Kei A, Elisaf M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol 2013;26:23-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959504/[↩]
- Musto D, Rispo A, Testa A, Sasso F, Castiglione F. Hypokalemic myopathy in inflammatory bowel diseases. J Crohns Colitis 2013;7:680. https://www.ncbi.nlm.nih.gov/pubmed/23339931[↩]
- He FJ, MacGregor GA. Beneficial effects of potassium on human health. Physiol Plant 2008;133:725-35. https://www.ncbi.nlm.nih.gov/pubmed/18724413[↩][↩]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC; 2005.[↩][↩][↩][↩]
- Champagne CM. Dietary interventions on blood pressure: the Dietary Approaches to Stop Hypertension (DASH) trials. Nutr Rev 2006;64:S53-6. https://www.ncbi.nlm.nih.gov/pubmed/16532899[↩]
- Filippini T, Violi F, D’Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol 2017;230:127-35. https://www.ncbi.nlm.nih.gov/pubmed/28024910[↩]
- Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 2015;33:1509-20. https://www.ncbi.nlm.nih.gov/pubmed/26039623[↩]
- Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens 1991;9:465-73. https://www.ncbi.nlm.nih.gov/pubmed/1649867[↩]
- Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997;277:1624-32. https://www.ncbi.nlm.nih.gov/pubmed/9168293[↩]
- Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev 2006:CD004641. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004641.pub2/full[↩]
- Agency for Healthcare Research and Quality. Effects of Dietary Sodium and Potassium Intake on Chronic Disease Outcomes and Related Risk Factors. https://effectivehealthcare.ahrq.gov/topics/sodium-potassium/research-protocol[↩][↩][↩][↩][↩][↩]
- Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 2013;88:987-95. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3833247/[↩]
- D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol 2011;57:1210-9. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0049398/[↩]
- Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4816263/[↩]
- Vinceti M, Filippini T, Crippa A, de Sesmaisons A, Wise LA, Orsini N. Meta-Analysis of Potassium Intake and the Risk of Stroke. J Am Heart Assoc 2016;5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5121516/[↩]
- Weaver CM. Potassium and health. Adv Nutr 2013;4:368S-77S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3650509/[↩][↩][↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that[↩][↩]
- Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev 2015:CD010057. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD010057.pub2/full[↩][↩][↩][↩]
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328:833-8. https://www.nejm.org/doi/10.1056/NEJM199303253281203[↩]
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997;126:497-504. https://www.ncbi.nlm.nih.gov/pubmed/9092314[↩]
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 1993;150:1761-4. https://www.ncbi.nlm.nih.gov/pubmed/8230497[↩][↩]
- Hanley DA, Whiting SJ. Does a high dietary acid content cause bone loss, and can bone loss be prevented with an alkaline diet? J Clin Densitom 2013;16:420-5. https://www.ncbi.nlm.nih.gov/pubmed/24094472[↩]
- Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999;69:727-36. https://www.ncbi.nlm.nih.gov/pubmed/10197575[↩]
- Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 2003;133:3130-6. https://www.ncbi.nlm.nih.gov/pubmed/14519796[↩]
- Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res 2013;28:497-504. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578058/[↩]
- Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2013;98:207-17. https://academic.oup.com/jcem/article/98/1/207/2823184[↩]
- Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, et al. Potassium bicarbonate supplementation lowers bone turnover and calcium excretion in older men and women: A randomized dose-finding trial. J Bone Miner Res 2015;30:2103-11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4817273/[↩]
- Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, et al. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr 2008;88:465-74. https://www.ncbi.nlm.nih.gov/pubmed/18689384[↩]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf[↩]
- Chatterjee R, Davenport CA, Svetkey LP, Batch BC, Lin PH, Ramachandran VS, et al. Serum potassium is a predictor of incident diabetes in African Americans with normal aldosterone: the Jackson Heart Study. Am J Clin Nutr 2017;105:442-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5267306/[↩][↩]
- Chatterjee R, Colangelo LA, Yeh HC, Anderson CA, Daviglus ML, Liu K, et al. Potassium intake and risk of incident type 2 diabetes mellitus: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetologia 2012;55:1295-303 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3934349/[↩][↩]
- Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992;55:1018-23. https://www.ncbi.nlm.nih.gov/pubmed/1315120[↩]
- Chatterjee R, Slentz C, Davenport CA, Johnson J, Lin PH, Muehlbauer M, et al. Effects of potassium supplements on glucose metabolism in African Americans with prediabetes: a pilot trial. Am J Clin Nutr 2017;106:1431-8. https://www.ncbi.nlm.nih.gov/pubmed/29092881[↩]
- U.S. Department of Health and Human Services, U.S. Department of Agriculture. https://health.gov/dietaryguidelines/2015/guidelines/[↩]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2013-2014. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/[↩][↩][↩]
- Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr 2011;94:1376-81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3192481/[↩]
- Council for Responsible Nutrition. Re: Docket No. FDA-2012-N-1210; Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.crnusa.org/sites/default/files/pdfs/comments-pdfs/CRN_Comments_FDA_ProposedRule-RevisionNutritionSupplementFactsLabels080114.pdf[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. https://ndb.nal.usda.gov/ndb/[↩][↩][↩]
- O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003-2006. Nutrients 2012;4:2097-120. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3546624/[↩]
- Keast DR, Fulgoni VL, 3rd, Nicklas TA, O’Neil CE. Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003-2006. Nutrients 2013;5:283-301. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3571649/[↩]
- ((U.S. Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels.external link disclaimer Federal Register 81(103):33894-33895. 2016.[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=306&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=306&nutrient2=&nutrient3=&subset=0&fg=&sort=c&measureby=m[↩]