Contents
- Genitourinary syndrome of menopause
- Genitourinary syndrome of menopause cause
- Genitourinary syndrome of menopause pathophysiology
- Genitourinary syndrome of menopause prevention
- Genitourinary syndrome of menopause signs and symptoms
- Genitourinary syndrome of menopause complications
- Genitourinary syndrome of menopause diagnosis
- Genitourinary syndrome of menopause differential diagnosis
- Treatment of genitourinary syndrome of menopause
- Table 2. Nonhormonal treatment for genitourinary syndrome of menopause
- Over-the-counter water-based vaginal lubricants and vaginal moisturizers
- Table 3. Hormonal therapy for management of genitourinary syndrome of menopause
- Topical estrogen
- Ospemifene (Osphena)
- Intravaginal dehydroepiandrosterone (DHEA) – Prasterone (Intrarosa)
- Systemic estrogen therapy
- Vaginal dilators
- Topical lidocaine
- Systemic or local testosterone
- Women with breast cancer
- Vaginal laser therapy
- Radiofrequency therapy
- Education
- Alternative medicine
- Genitourinary syndrome of menopause prognosis
Genitourinary syndrome of menopause
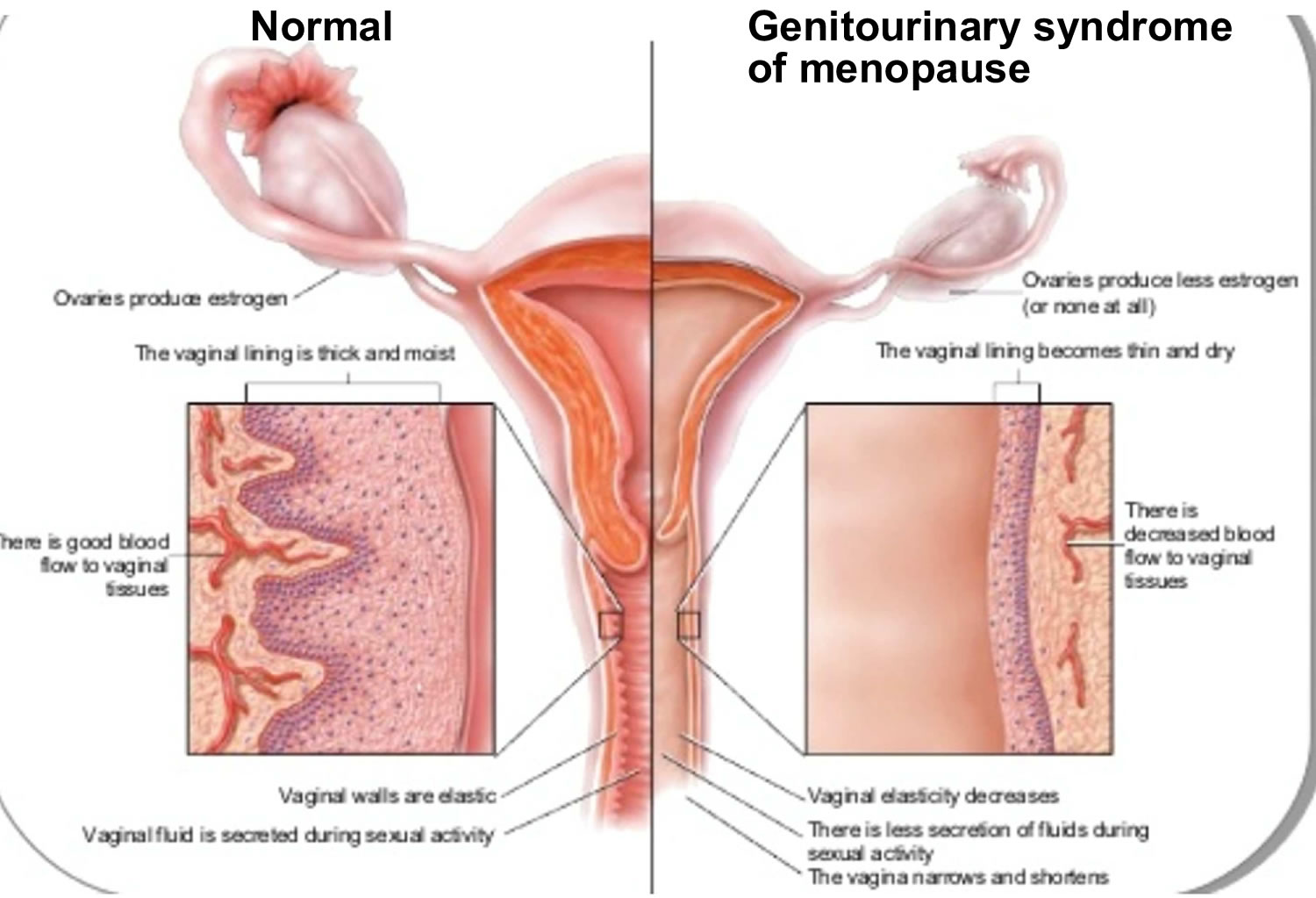
Genitourinary syndrome of menopause or GSM previously known as vaginal atrophy, vulvovaginal atrophy, atrophic vaginitis or urogenital atrophy, is a chronic, progressive vulvovaginal, sexual, and lower urinary tract condition characterized by a collection of symptoms that frequently affects more than 50% of postmenopausal women and 15% of hypoestrogenic premenopausal women where the tissues of the urinary tract (urethra, and bladder) and the female genital tract (labia majora, labia minora, clitoris, vestibule or introitus, vagina) gets drier and thinner from a lack of estrogen and other sex hormones 1, 2, 3, 4, 5. During menopause, your body makes less estrogen (95% reduction in estrogen production). Without estrogen, the lining of your vagina can become thinner and less stretchy. Your vaginal canal can also narrow and shorten. Less estrogen also lowers the amount of normal vaginal fluids and changes the acid balance in your vagina. All of these factors make your vaginal tissue more delicate and more likely to become irritated.
Your body can also produce less estrogen during events other than menopause. People who are breastfeeding, receiving treatment for cancer or who have had both ovaries (bilateral oophorectomy) removed, primary ovarian insufficiency, ovarian failure due to radiation or arterial embolization, hypothalamic-pituitary disorders and take anti-estrogen medications such as leuprolide or danazol used commonly for endometriosis, can also experience vaginal atrophy due to lack of estrogen 6, 3. Also at risk are breast cancer survivors suffering from consequences from treatment such as chemotherapy or aromatase inhibitors 2, 7, 4. Aromatase inhibitors lower estrogen levels by stopping an enzyme in fat tissue called aromatase from changing other hormones into estrogen 8. Aromatase inhibitors don’t stop the ovaries from making estrogen. They only lower estrogen levels in women whose ovaries aren’t making estrogen (such as women who have already gone through menopause). Because of this, they are used mainly in women who have gone through menopause already.
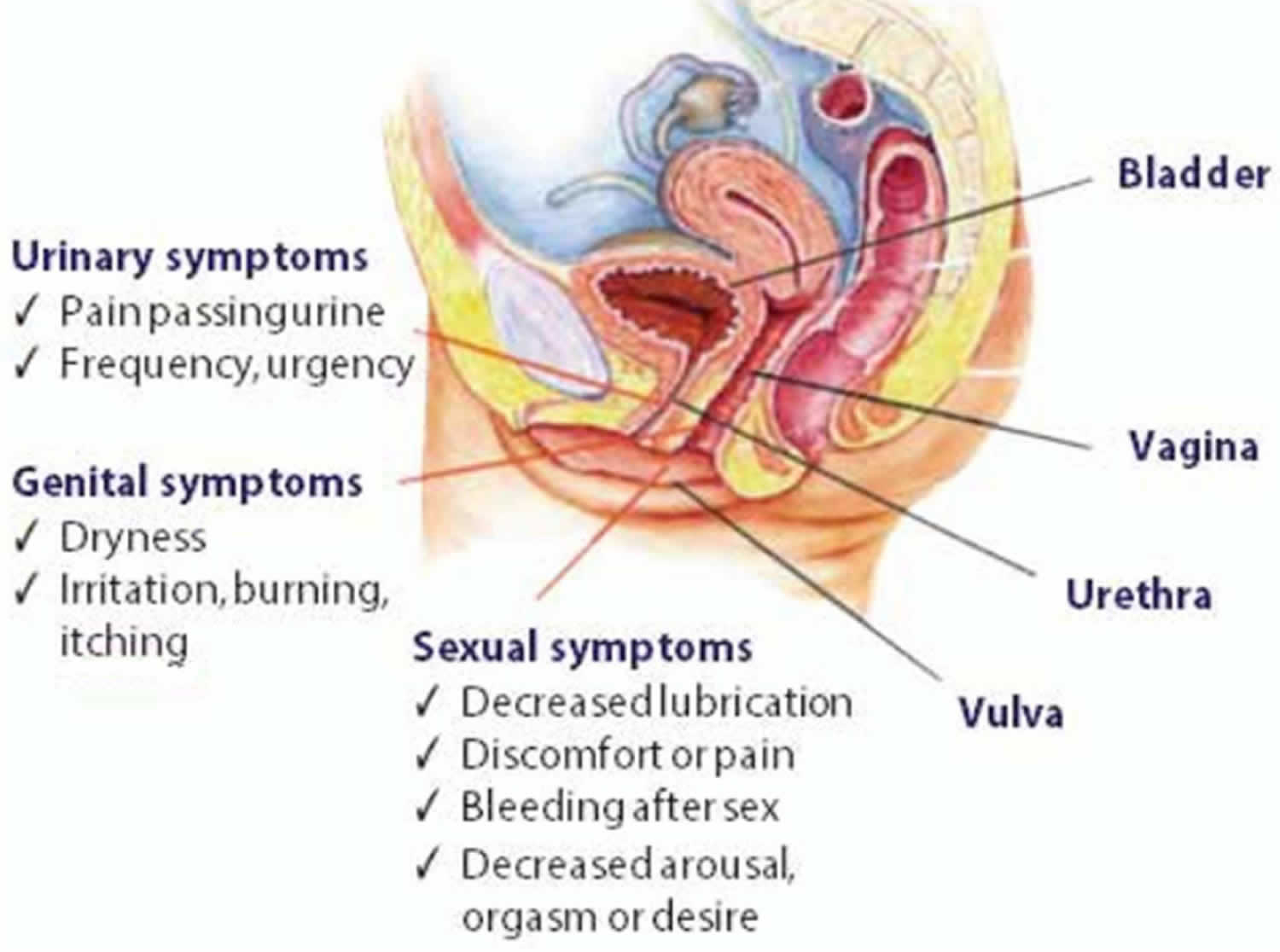
In 2014, the new term Genitourinary Syndrome of Menopause (GSM) was introduced by the International Society for the Study of Women’s Sexual Health and the North American Menopause Society 9. This term encompasses all of the atrophic symptoms patients may have in the vulvovaginal and bladder-urethral areas from loss of estrogen that occurs with menopause 10. Genitourinary syndrome of menopause symptoms can vary between patients; however, common GSM symptoms include sexual symptoms (lack of lubrication, discomfort or pain with sex, and impaired function during sex), genital symptoms (dryness, burning, and irritation of the vulva and vagina) and urinary symptoms (frequent urination, urgency, burning urination, and recurrent urinary tract infections). Genitourinary syndrome of menopause (GSM) signs and symptoms may begin to bother you during the years leading up to menopause, or they may not become a problem until several years into menopause. Although the condition is common, not all menopausal women experience GSM. Regular sexual activity, with or without a partner, can help you maintain healthy vaginal tissues.
Genitourinary syndrome of menopause can be a common issue for all women and can impair your quality of life, your sexual function, and your relationships with partners. Many postmenopausal women experience GSM. Women are often reluctant or embarrassed to discuss or receive treatment for their symptoms. They believe the GSM signs and symptoms are expected and part of the aging process. This not only results in underdiagnosing but also undertreatment of this condition. Reports show that 15% of the female population experiences symptoms of vaginal atrophy before menopause, whereas 40% to 54% of postmenopausal women have symptoms 4, 5.
Due to sexual embarrassment and the sensitive nature of discussing symptoms, genitourinary syndrome of menopause is greatly underdiagnosed. Approximately 70% of women with genitourinary syndrome of menopause (GSM) signs and symptoms do not discuss the issue with their doctor 11. According to Nappi et al 12, only 54% of menopausal women are willing to discuss their sexual health with a specialist and 33% of patients even refuse to answer questions. Most are even not aware of treatment modalities. Therefore, less than 25% of women with GSM do not receive care, and their doctors do not screen for symptoms of vaginal atrophy 3.
Clinically, genitourinary syndrome of menopause is manifested by dryness in the vagina, painful sexual intercourse (dyspareunia), vaginal discharge, itching, and pain 13, 14. Painful sexual intercourse (dyspareunia) leads to a decrease in sex drive and fear of sexual intercourse. As the frequency of coitus diminishes, vaginal lubrication declines further 1. Some women may already have narrowing of the vagina or manifestations of vaginismus (involuntary muscle spasm interferes with vaginal intercourse or other penetration of the vagina), limiting the penetration into the vagina. However, in some women with mild-to-moderate severity, genitourinary syndrome of menopause occurs asymptomatically and verification of diagnosis is possible only with vaginal examination 15.
The spectrum of genitourinary syndrome of menopause (GSM) signs and symptoms makes long-term treatment essential in many patients, not only for relief of symptoms, but also for the more troublesome problems that may occur, such as light bleeding after intercourse (postcoital bleeding) and recurrent urinary tract infections. This in turn can complicate the process of sexual arousal and achievement of orgasm, therefore leading to sexual dysfunction 16. But few women seek treatment. Women may be embarrassed to discuss their symptoms with their doctor and may resign themselves to living with these symptoms. Simple, effective treatments for genitourinary syndrome of menopause (GSM) are available 2. Make an appointment with your doctor if you have any unexplained vaginal spotting or bleeding, unusual discharge, burning, or soreness. Also make an appointment to see your doctor if you experience painful intercourse that’s not resolved by using a vaginal moisturizer (K-Y Liquibeads, Replens, Sliquid, others) or water-based lubricant (Astroglide, K-Y Jelly, Sliquid, others).
The diagnosis of genitourinary syndrome of menopause (GSM) is made difficult by the low awareness of women about the pathological manifestations of the postmenopausal period and unwillingness/embarrassment to discuss the symptoms of an intimate character with their doctor. In their study, Nappi and Kokot-Kierepa 17 noted that only 4% of respondents associated their symptoms with the manifestations of menopause. Unfortunately, 75% of patients with the clinical manifestations of genitourinary syndrome of menopause (GSM) do not seek help from gynecologists 4.
Diagnosis of genitourinary syndrome of menopause (GSM) may involve:
- Pelvic exam: In a pelvic exam, your health care provider inserts two gloved fingers inside your vagina. Pressing down on your abdomen at the same time, your doctor can examine your uterus, ovaries and other organs.
- Urine test, which involves collecting and testing your urine, if you have urinary symptoms.
- Vaginal pH test, which involves taking a sample of vaginal fluids or placing a paper indicator strip in your vagina to test its acid balance. Vaginal pH measurement is useful if pH>5 in the absence of any infections or discharges.
A healthcare provider can diagnose vaginal atrophy based on your symptoms and a pelvic exam to look at your vagina and cervix. The diagnosis of genitourinary syndrome of menopause (GSM) is confirmed by vaginal examination and colposcopy 7.
Classic signs of vaginal atrophy during a pelvic exam include:
- A shortened or narrowed vagina.
- Dryness, redness and swelling.
- Loss of stretchiness.
- Whitish discoloration to your vagina.
- Vulvar skin conditions, vulvar lesions and/or vulvar patch redness.
- Minor cuts (lacerations) near your vaginal opening.
- Decrease in size of the labia.
A pale, dry, smooth, shiny, and inflammation changes such as patchy erythema or petechiae or increased visibility of blood vessels are all classical findings of atrophy. There may also be friability, bleeding, and discharge 18.
Laboratory tests such as urinalysis and culture, urine antigen for sexually transmitted infections, and pelvic cultures are usually used to rule out genitourinary infections 3. Measurement of estrogen in the form of serum estradiol is inaccurate, and current assays are not sufficiently sensitive to detect accurately 19. Currently, research is the Maturation Index Test. The lining cells of the vaginal wall are measured with atrophy demonstrating shifting and loss of superficial cells to basal cells.
Treatment of genitourinary syndrome of menopause depends on the severity of the symptoms of the disease and on the preferences and expectations of women.
To treat genitourinary syndrome of menopause, your doctor may first recommend over-the-counter treatment options, including:
- Vaginal moisturizers. Try a vaginal moisturizer (K-Y Liquibeads, Replens, Sliquid, others) to restore some moisture to your vaginal area. You may have to apply the moisturizer every few days. The effects of a moisturizer generally last a bit longer than those of a lubricant.
- Water-based lubricants. These water-based lubricants (Astroglide, K-Y Jelly, Sliquid, others) are applied just before sexual activity and can reduce discomfort during intercourse. Choose products that don’t contain glycerin or warming properties because women who are sensitive to these substances may experience irritation. Avoid petroleum jelly or other petroleum-based products for lubrication if you’re also using condoms, because petroleum can break down latex condoms on contact.
- When choosing lubricants and moisturizers, it is important that the product is similar to vaginal secretion in terms of osmolality, pH, and composition 20.
- Vaginal lubricants and moisturizers can be used as needed in combination with other genitourinary syndrome of menopause treatments.
- Allow time to become aroused during intercourse. The vaginal lubrication that results from sexual arousal can help reduce symptoms of dryness or burning.
If those options don’t ease your symptoms, your doctor may recommend:
- Topical estrogen. Vaginal estrogen has the advantage of being effective at lower doses and limiting your overall exposure to estrogen because less reaches your bloodstream. Vaginal estrogen may also provide better direct relief of symptoms than oral estrogen does. Vaginal estrogen therapy comes in a number of forms. Because they all seem to work equally well, you and your doctor can decide which one is best for you.
- Vaginal estrogen cream (Estrace, Premarin). You insert this cream directly into your vagina with an applicator, usually at bedtime. Typically women use it daily for one to three weeks and then one to three times a week thereafter, but your doctor will let you know how much cream to use and how often to insert it.
- Vaginal estrogen suppositories (Imvexxy). These low-dose estrogen suppositories are inserted about 2 inches into the vaginal canal daily for weeks. Then, the suppositories only need to be inserted twice a week.
- Vaginal estrogen ring (Estring, Femring). You or your doctor inserts a soft, flexible ring into the upper part of the vagina. The ring releases a consistent dose of estrogen while in place and needs to be replaced about every three months. Many women like the convenience this offers. A different, higher dose ring is considered a systemic rather than topical treatment.
- Vaginal estrogen tablet (Vagifem). You use a disposable applicator to place a vaginal estrogen tablet in your vagina. Your doctor will let you know how often to insert the tablet. You might, for instance, use it daily for the first two weeks and then twice a week thereafter.
- Ospemifene (Osphena). Taken daily, this pill can help relieve painful sex symptoms in women with moderate to severe genitourinary syndrome of menopause (GSM). It is not approved in women who’ve had breast cancer or who have a high risk of developing breast cancer.
- Prasterone (Intrarosa). These vaginal inserts deliver the hormone dehydroepiandrosterone (DHEA) directly to the vagina to help ease painful sex. DHEA is a steroid precursor hormone (prohormone) with weak androgenic effects that is converted into male sex hormones (androgens) and/or female sex hormones (estrogens) in peripheral tissues in your body to exert their effects 21, 22. Prasterone or DHEA is used nightly for moderate to severe vaginal atrophy.
- Systemic estrogen therapy. If vaginal dryness is associated with other symptoms of menopause, such as moderate or severe hot flashes, your doctor may suggest estrogen pills, patches or gel, or a higher dose estrogen ring. Estrogen taken by mouth enters your entire system. Ask your doctor to explain the risks versus the benefits of oral estrogen, and whether or not you would also need to take another hormone called progestin along with estrogen.
- Vaginal dilators. You may use vaginal dilators as a nonhormonal treatment option. Vaginal dilators may also be used in addition to estrogen therapy. These devices stimulate and stretch the vaginal muscles to reverse narrowing of the vagina. If painful sex is a concern, vaginal dilators may relieve vaginal discomfort by stretching the vagina. They are available without a prescription, but if your symptoms are severe, your doctor may recommend pelvic floor physical therapy and vaginal dilators. Your health care provider or a pelvic physical therapist can teach you how to use vaginal dilators.
- Topical lidocaine. Available as a prescription ointment or gel, topical lidocaine (topical local anesthetic) can be used to lessen discomfort associated with sexual activity. Apply it five to 10 minutes before you begin sexual activity.
If you have a history of breast cancer, tell your doctor and consider these options:
- Nonhormonal treatments. Try moisturizers and lubricants as a first choice.
- Vaginal dilators. Vaginal dilators are a nonhormonal option that can stimulate and stretch the vaginal muscles. This helps to reverse narrowing of the vagina.
- Vaginal estrogen. In consultation with your cancer specialist (oncologist), your doctor might recommend low-dose vaginal estrogen if nonhormonal treatments don’t help your symptoms. However, there’s some concern that vaginal estrogen might increase your risk of the cancer coming back, especially if your breast cancer was hormonally sensitive.
- Systemic estrogen therapy. Systemic estrogen treatment generally isn’t recommended, especially if your breast cancer was hormonally sensitive.
Figure 1. Genitourinary syndrome of menopause
Figure 2. Genitourinary syndrome of menopause pathophysiology
[Source 7 ]What’s the difference between genitourinary syndrome of menopause and a yeast infection?
Both genitourinary syndrome of menopause and yeast infections can have symptoms of dryness, itching, redness and pain. However, lack of estrogen causes vaginal atrophy while a fungal infection causes a vaginal yeast infection. Consult with your doctor regarding symptoms so that, together, you can determine what condition you have.
Genitourinary syndrome of menopause cause
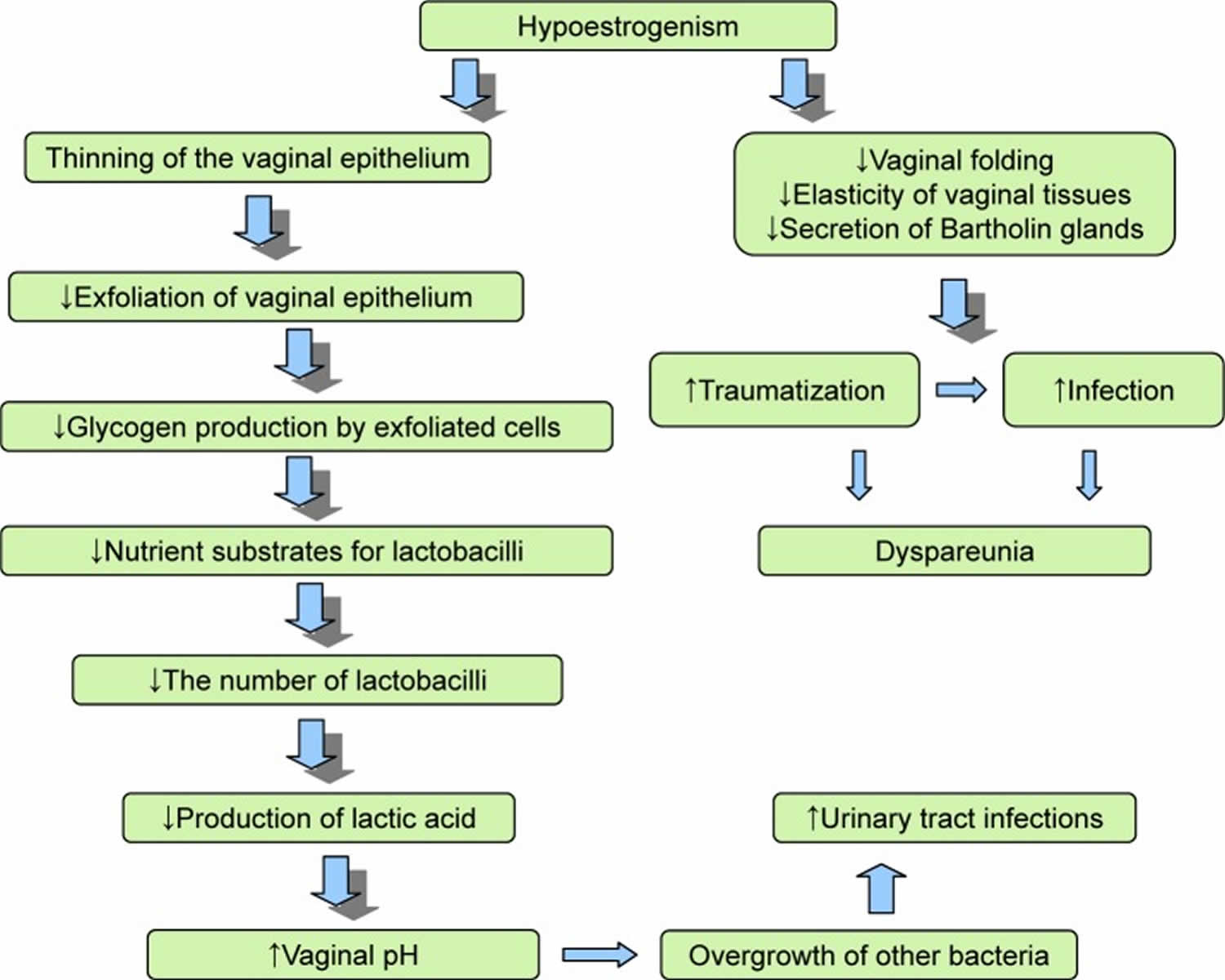
Genitourinary syndrome of menopause is caused by a decrease in estrogen production. Vaginal atrophy is most commonly caused by a decreasing or lower estrogen state. As production and amount of total body estrogen decreases, secretions diminish, and the genitourinary tissues become atrophic, causing the many symptoms associated with genitourinary syndrome of menopause. There are receptors in the vagina, vulva, urethra, and trigone of the bladder that respond to estrogen stimulation to maintain normal blood flow, tissue thickness, tissue folding, tissue elasticity, and keeps the epithelial surfaces moist 3. This healthy tissue is rich in glycogen, which the normal flora Lactobacilli convert to lactic acid. This creates an acidic environment with a pH of 3.5 to 5.0, which the lactobacilli continue to thrive and protect from vaginal and urinary tract infections. Concurrently, sexual activities promote epithelial turnover and elevated vaginal lubrication.
Less estrogen makes your vaginal tissues thinner, drier, its barrier function is lost, the vaginal folding decreases, the elasticity of the tissues decreases, and more fragile and the secretory activity of the Bartholin glands decreases, which lead to traumatization of the vaginal mucosa and painful sensations 4, 23, 24. In addition, under conditions of estrogen deficiency, the balance of the vaginal microbiota is disrupted, the pathogenic gram-negative fecal flora and other bacteria prevail in its composition, and the vagina develops a less acidic pH, that is, from 5.5 to 6.8 25, 26. In studies by Brotman et al 27, a correlation between the nature of the vaginal microbiota, the low Lactobacillus content, and the development of vulvovaginal atrophy was noticed. The data obtained by the authors demonstrate differences in the vaginal microbiota of pre-, peri-, and postmenopausal women. In the proposed hypothesis, the predominance of anaerobic flora in the vaginal environment plays a role in the development of vulvovaginal atrophy symptoms 27.
A drop in estrogen levels may occur:
- After menopause
- During the years leading up to menopause (perimenopause)
- After surgical removal of both ovaries (surgical menopause)
- During breast-feeding (lactation)
- While taking medications that can affect estrogen levels, such as some birth control pills
- After pelvic radiation therapy for cancer
- After chemotherapy for cancer
- As a side effect of breast cancer hormonal treatment
Genitourinary syndrome of menopause (GSM) also occurs in other low-estrogen states, such as postpartum, during breastfeeding (lactation), women who have surgical removal of both ovaries (bilateral oophorectomy), primary ovarian insufficiency, ovarian failure due to radiation or arterial embolization, hypothalamic-pituitary disorders and take anti-estrogen medications such as leuprolide or danazol used commonly for endometriosis 6, 3, 2, 28. Also at risk are breast cancer survivors suffering from consequences from treatment such as chemotherapy or aromatase inhibitors 2, 7, 4.
Risk factors for genitourinary syndrome of menopause
Certain factors may contribute to genitourinary syndrome of menopause, such as:
- Smoking. Cigarette smoking affects your blood circulation, and may lessen the flow of blood and oxygen to the vagina and other nearby areas. Smoking also reduces the effects of naturally occurring estrogens in your body.
- No vaginal births. Researchers have observed that women who have never given birth vaginally are more likely to develop GSM symptoms than women who have had vaginal deliveries.
- No sexual activity. Sexual activity, with or without a partner, increases blood flow and makes your vaginal tissues more elastic. People who have penetrative sexual activity less often — with or without a partner or partners — may also have a higher risk of moderate to severe vaginal atrophy. Studies show that people who have sex more often tend to have milder cases of atrophy than those who stop having sex. This is because sexual stimulation increases blood flow to your vagina and makes your vaginal tissue more elastic.
- Decreased ovarian functioning due to chemotherapy or radiation therapy.
- Medications that contain antiestrogen properties including tamoxifen, medroxyprogesterone and nafarelin.
- Oophorectomy (removal of your ovaries).
- Some birth control pills.
- Immune disorders.
- Breastfeeding.
Genitourinary syndrome of menopause pathophysiology
Vaginal atrophy occurs in conditions causing a diminished estrogen state. Estrogen typically stimulates exfoliation of vaginal epithelial cells, causing increased levels of glycogen in which vaginal flora lactobacilli convert into lactic acid. This process allows for the replacement of older vaginal epithelium, keeping the typical acidic pH of the vaginal canal. As estrogen levels decrease, this process is hindered, and the vaginal epithelium becomes atrophic with diminished secretions and a less acidic environment (pH >5) 7, 29. This, in turn, increases the risk of vaginal and urinary tract infections.
Pathophysiology of genitourinary syndrome of menopause 30:
- Estrogen deficiency
- Loss of labial and vulval thickness
- Decreased collagen, elasticity, and blood flow
- Reduced vaginal discharge
- Reduced pubic hair, subcutaneous fat of labia majora
- Reduced labia minora and hymenal remnants
- Decreased vaginal cells glucogen => change vaginal microbiome => increased pH
- Decreased pelvic floor strength and control
- Dry and thin epithelium
- Short and narrow vagina
- Prolapse (vaginal vault, pelvic organs, urethral)
- Decreased bladder capacity and sensation
- Vaginal hypersensitivity or decreased feeling
Genitourinary syndrome of menopause prevention
Losing estrogen is part of your body’s natural aging process. However, there are ways to keep vaginal atrophy from getting worse. Avoid vaginal irritants such as perfumes, dye, shampoo, detergents and douching.
Regular sexual activity, either with or without a partner, may help prevent genitourinary syndrome of menopause. Sexual activity increases blood flow to your vagina, which helps keep vaginal tissues healthy.
Genitourinary syndrome of menopause signs and symptoms
The tissue that lines the wall of your vagina becomes thin, dry and inflamed when you have vaginal atrophy. Often, the first sign is less lubrication (vaginal dryness), which you may notice during sex. Other signs and symptoms of genitourinary syndrome of menopause may include 30, 31:
- Genital signs and symptoms
- Vaginal burning
Vaginal discharge - Genital itching or vulvar itching (itching around your external genitals)
- Irritation/burning/itching
- Leukorrhea
- Thinning/graying pubic hair
- Vaginal/pelvic pain and pressure
- Shortening and tightening of the vaginal canal
- Vaginal vault prolapse
- Vaginal burning
- Sexual symptoms
- Discomfort with intercourse (dyspareunia)
- Decreased vaginal lubrication during sexual activity
- Light bleeding after intercourse (post-coital bleeding)
- Decreased arousal, orgasm, desire
- Loss of libido, arousal
- Dysorgasmia
- Urinary signs and symptoms
- Burning with urination (dysuria)
- Urgency with urination
- Frequent urination
- Blood in your urine (hematuria)
- Urinary incontinence
- Stress/urgency incontinence
- Recurrent urinary tract infections (UTIs)
- Urethral prolapse
- Ischemia of vesical trigone
Urinary symptoms of GSM may include frequency, dysuria, and increased risk for urinary tract infections 6. For some women, symptoms can be severe enough to preclude penetrative sexual activity and to cause discomfort even with sitting or wiping. In women taking aromatase inhibitors, symptoms are more common and may be particularly severe 6.
In a large cohort surveys in western populations, 45-63% of postmenopausal women experienced vulvovaginal symptoms, most commonly vaginal dryness 32. In a survey on 1578 women with vaginal discomfort, 83% had vaginal dryness 33. Similarly, the frequency of vaginal dryness in Iranian menopause women has reported 41.1% and it is one of the most three frequent symptoms 34. In the postmenopausal survivors of breast cancer, 70% are affected by vaginal atrophic symptoms 33.
Genitourinary syndrome of menopause complications
If not identified, and treated early, genitourinary syndrome of menopause will cause recurrent genitourinary infections and much vaginal/pelvic discomfort and pain.
Genitourinary syndrome of menopause increases your risk of:
- Vaginal infections. Changes in the acid balance of your vagina make vaginal infections more likely.
- Urinary problems. Urinary changes associated with GSM can contribute to urinary problems. You might experience increased frequency or urgency of urination or burning with urination. Some women experience more urinary tract infections or urine leakage (incontinence).
Genitourinary syndrome of menopause diagnosis
Genitourinary syndrome of menopause is a clinical diagnosis, and laboratory testing is usually unnecessary 2, 35, 36. A healthcare provider can diagnose vaginal atrophy based on your symptoms and a pelvic exam to look at your vagina and cervix. Although some women with mild GSM remain asymptomatic, many women report symptoms such as vaginal dryness, burning, irritation, decreased lubrication with sexual activity, and painful sex (dyspareunia) with resultant sexual dysfunction 6. Changes on examination include scant pubic hair, loss of the labial fat pad, thinning and resorption of the labia minora, narrowing of the introitus, and increased vaginal pH 6. Internal examination findings include reduced vaginal caliber; smooth, shiny, pale mucosa with loss of folds; and a cervix flush with the vaginal vault. With inflammation, the vagina may appear erythematous, develop petechiae or increased visibility of blood vessels and bleed easily 6. There may also be friability, bleeding, and discharge 18.
Diagnosis of genitourinary syndrome of menopause (GSM) may involve:
- Pelvic exam: In a pelvic exam, your health care provider inserts two gloved fingers inside your vagina. Pressing down on your abdomen at the same time, your doctor can examine your uterus, ovaries and other organs. A pelvic examination can be helpful to exclude other vulvar and vaginal conditions that may present with symptoms similar to those of GSM, including irritant, infectious, or inflammatory vaginitis; dermatoses; and neoplasia 6.
- Urine test, which involves collecting and testing your urine, if you have urinary symptoms.
- Vaginal pH test, which involves taking a sample of vaginal fluids or placing a paper indicator strip in your vagina to test its acid balance. Vaginal pH measurement is useful if pH>5 in the absence of any infections or discharges.
Laboratory tests such as urinalysis and culture, urine antigen for sexually transmitted infections, and pelvic cultures are usually used to rule out genitourinary infections 3. Measurement of estrogen in the form of serum estradiol is inaccurate, and current assays are not sufficiently sensitive to detect accurately 19. Currently, research is the Maturation Index Test. The lining cells of the vaginal wall are measured with atrophy demonstrating shifting and loss of superficial cells to basal cells.
Genitourinary syndrome of menopause differential diagnosis
Differential diagnoses must exclude the presence of vulvovaginitis, including candidiasis, bacterial vaginosis, trichomoniasis. Sexually transmitted infections, such as gonorrhea and chlamydia. Dermatologic conditions including lichen planus, lichen sclerosis, or inflammation secondary to an irritant. Symptoms and signs may also mimic urinary tract infections or malignancy 28. Genitourinary syndrome of menopause differential diagnosis is summarized in Table 1. Although these differential diagnoses must be ruled out, their presence does not exclude the diagnosis, and simply may be recurring symptoms/infections secondary to ongoing vaginal atrophy.
Table 1. Genitourinary syndrome of menopause differential diagnosis
| Condition | Main symptoms and signs |
|---|---|
| Vaginal infections | Vaginal discharge (leukorrhea, xanthorrhea), pruritus, bad smell |
| Allergic reactions | Redness, swelling, pruritus, occasionally blistering, and pain |
| Lichen planus | Painful, red plaques or erosions with white or violaceous borders; may extend into vagina |
| Lichen sclerosis | Coalescing ivory and pink plaques in butterfly of crinkled, wax-like tissue. May result in labial and clitoral hood agglutination |
| Ulcers and cracks in the external genitalia associated with systemic diseases: Behçet | There are intensely painful, punched-out ulcers, which are often bilateral, with a yellow-white base and red borders |
| Ulcers and cracks in the external genitalia associated with systemic diseases: Crohn | Mixed inflammatory lesions, fissures, and “knife-cut” ulcers of variable severity. May progress into fistulae; most commonly, in perianal or rectovaginal sites. Marked painless vulval edema may occur |
| Acute vulvar ulcers or Lipschütz ulcers | Acute painful genital ulcerations of the vulva or lower vagina. May appear in nonsexually active adolescent girls or young women |
| Other vulvovaginal ulcers | Gluten enteropathy, systemic lupus erythematosus, Stevens–Johnson syndrome, pyoderma, pemphigus vulgaris, pemphigoid, and so on |
| Tumors of the urogenital tract | Presented as multifocal red, white, or dark raised or eroded neoplasm lesions, or as solitary ulcer with raised or indurated edge |
| Extramammary Paget disease | Brick red, scaly, eczematoid plaque with sharply demarcated border and sometimes a roughened surface |
Treatment of genitourinary syndrome of menopause
Genitourinary syndrome of menopause has a few different treatment modalities. It can be classified into non-hormonal and hormonal options.
Non-hormonal treatment with vaginal moisturizers and lubricants is considered first-line. Lubricants provide short-term relief for patients and are typically used for vaginal dryness during intercourse, while moisturizers have a longer-lasting effect and may be used daily to every 2-3 days per week. Patients are also encouraged to continue safe, regular sexual activity. The use of moisturizers, lubricants, and regular safe sexual activities helps in maintaining the health and integrity of the vaginal epithelium and flora.
As the environment of the vagina is altered with the progression of vaginal atrophy, the pH and osmolality of the moisturizer or lubricant are also of importance. Your doctor should be prescribing topical vaginal moisturizers and lubricants which are closest to the physiological conditions of the your natural vaginal environment. This not only assists in symptomatic relief but may also decrease the incidence of promoting vaginal infections 3.
Hormonal therapy would be an additional or second-line treatment as it focuses on treating the root cause of the symptoms. Moisturizers and lubricants are still considered the first line, but if symptoms persist, hormonal therapy may be necessary. Hormonal replacement therapy may be systemic (oral estrogen replacement) or localized (intravaginal/topical estrogen, estrogen intravaginal releasing ring, and vaginal dehydroepiandrosterone (DHEA, testosterone). Studies show that systemic hormonal replacement therapy alleviates symptoms in 75% of cases, whereas local therapy does so in 80%-90% of cases 3. Side effects are comparable but local hormonal replacement therapy is considered much safer than systemic hormonal replacement therapy 3.
Hormonal therapy risks should be evaluated thoroughly and factors such as age, duration of use, dose, type of treatment, route, histologic type of cancer and prior exposure should be considered before the prescription of such regimen in survivors of gynecological cancer 37. Breast cancer is a hormone-sensitive cancer in many cases; hence, systemic hormonal therapy is not usually recommended for women with breast cancer 37, 38. As far as endometrial cancer survivors are concerned, the data, although limited, suggest that hormonal therapy is considered relatively safe in low-risk subtypes (i.e., early-stage, low-grade, or type 1), but should not be employed in high-risk types 37, 39. Similarly, in survivors of epithelial ovarian cancer, hormonal treatment could be considered in selected cases, since evidence suggests a neutral effect in survival; but it should be avoided in certain histologic types, such as advanced serous and endometrioid and other estrogen-sensitive tumor types 37, 39.
Table 2. Nonhormonal treatment for genitourinary syndrome of menopause
| Treatment strategy | Specific therapy | Typical use | Comments |
|---|---|---|---|
| Education | Provide education on potential vulvar and vaginal changes associated with menopause or other low-estrogen states Offer therapy as indicated | General patient education | Education should be offered to women regardless of partner status Regular, painless sexual activity or vaginal stimulation can help maintain sexual function |
| Lubricants | Examples of lubricants: YES, JO, Good Clean Love, Pink, and Uberlube | Used as needed for sexual activity | Used to increase comfort and pleasure Avoid irritants (eg, glycerin, parabens, and propylene glycol) Can be used with other therapies (hormonal and nonhormonal) |
| Moisturizers | Examples of moisturizers: Replens, RepHresh, Sliquid Satin, and Hyalo Gyn | Used daily or every few days on a regular basis to maintain vulvar and vaginal moisture | Mimic normal vaginal secretions Do not reverse cellular and pH changes of GSM Can be used with other therapies (hormonal and nonhormonal) |
| Use of dilators and vibrators | Multiple types available | Used as needed | Gently stimulate and stretch the vulvar and vaginal tissues to maintain function |
| Pelvic floor physical therapy | Provide education as indicated on kinesthetic awareness, pelvic floor muscle relaxation, manual therapies, and biofeedback | Used as needed for nonrelaxing pelvic floor muscle dysfunction | Find a physical therapist who specializes in pelvic floor disorders (https://www.aptapelvichealth.org) |
| Topical lidocaine | 4% aqueous lidocaine | Applied to the vestibule a few minutes before sexual activity | Can be used as an adjunct to other therapies, including lubricants, moisturizers, and physical therapy |
| Laser therapy | Carbon dioxide laser Erbium:YAG laser | Administered as a series of 3 treatments a few weeks apart | Use is limited by a lack of studies examining long-term safety and efficacy and comparing laser therapy with estrogen therapy and sham control |
Over-the-counter water-based vaginal lubricants and vaginal moisturizers
According to the generally accepted international standards, the first-line recommendations for the treatment of mild and moderate manifestations of genitourinary syndrome of menopause are nonhormonal vaginal lubricants that should be used before intercourse and vaginal moisturizers with a long-term effect that are used regularly (several times a week); in such cases, regular sexual activity is important in maintaining the health and integrity of the vaginal epithelium and flora 7, 3. This treatment option is also recommended for women for whom the use of vaginal estrogen preparations is unacceptable 20. When choosing lubricants and moisturizers, it is important that the product is similar to vaginal secretion in terms of osmolality, pH, and composition 20. Vaginal lubricants and moisturizers can be used as needed in combination with other genitourinary syndrome of menopause treatments.
To treat genitourinary syndrome of menopause, your doctor may first recommend over-the-counter treatment options, including:
- Vaginal moisturizers. Try a vaginal moisturizer (K-Y Liquibeads, Replens, Sliquid, others) to restore some moisture to your vaginal area and reduce the pH. You may have to apply the moisturizer daily or every 2–3 days, depending on the extent of your symptoms 20. The effects of a moisturizer generally last a bit longer than those of a lubricant. According to Chen et al 40, the use of hyaluronic acid vaginal gel every 3 days causes improvement in symptoms of vaginal dryness, comparable with the effect of topical estrogen therapy.
- Water-based lubricants. These water-based lubricants (Astroglide, K-Y Jelly, Sliquid, others) are applied just before sexual activity and can reduce discomfort during intercourse. Vaginal water-based lubricants provide a short-term relief from vaginal dryness and discomfort during sexual intercourse. Choose products that don’t contain glycerin or warming properties because women who are sensitive to these substances may experience irritation. Avoid petroleum jelly or other petroleum-based products for lubrication if you’re also using condoms, because petroleum can break down latex condoms on contact. Lubricants with a water base have fewer side effects, which is an advantage compared to lubricants based on silicone 20. The literature contains sparse data on the safety of lubricants based on oils, the effect on motility of spermatozoa, and the properties of condoms. There is also evidence of an increased risk of developing bacterial vaginosis and vaginal candidiasis with the use of these medications 41, 42, 43.
- Allow time to become aroused during intercourse. The vaginal lubrication that results from sexual arousal can help reduce symptoms of dryness or burning.
If those options don’t ease your symptoms, your doctor may recommend:
Table 3. Hormonal therapy for management of genitourinary syndrome of menopause
| Treatment | Product | Dosage | Comments | |
|---|---|---|---|---|
| Initial | Maintenance | |||
| Vaginal cream | ||||
| Estradiol-17β | Estrace | 0.5-1 g daily for 2 wk | 0.5-1 g 1-3 times weekly | FDA-approved dose is higher loading dose (2-4 g daily; maintenance dose, 1 g 1-3 times weekly) |
| Conjugated estrogens | Premarin | 0.5-1 g daily for 2 wk | 0.5-1 g 1-3 times weekly | FDA-approved dose is higher and administration is cyclical (for genitourinary syndrome of menopause: 0.5-2 g daily for 21 d and then off for 7 d; for dyspareunia: 0.5 g daily for 21 d and then off for 7 d or 0.5 g twice weekly) |
| Vaginal insert | ||||
| Estradiol hemihydrate | Vagifem, Yuvafem | 10-μg insert once daily for 2 wk | 1 twice weekly | … |
| Estradiol-17β softgel capsules | TX-004HR | 4, 10, or 25 μg daily for 2 wk | 1 twice weekly | This product is not yet FDA approved |
| DHEA (prasterone) | Intrarosa | 6.5 mg once daily | 6.5 mg once daily | … |
| Vaginal ring | ||||
| Estradiol-17β | Estring | Insert for 90 d (2 mg releases approximately 7.5 μg daily) | Change every 90 d | … |
| Estradiol acetate | Femring | Insert for 90 d (12.4 mg or 24.8 mg releases 0.05 mg or 0.1 mg daily, respectively) | Change every 90 d | This product is delivered vaginally but it provides systemic hormone levels to treat vasomotor symptoms and genitourinary syndrome of menopause |
| Selective estrogen receptor modulator (SERM) | ||||
| Ospemifene | Osphena | 60 mg daily | 60 mg daily | FDA approved for dyspareunia |
Topical estrogen
Vaginal estrogen has the advantage of being effective at lower doses and limiting your overall exposure to estrogen because less reaches your bloodstream. Vaginal estrogen may also provide better direct relief of symptoms than oral estrogen does.
Vaginal estrogen therapy comes in a number of forms. Because they all seem to work equally well, you and your doctor can decide which one is best for you 44, 45.
- Vaginal estrogen cream (Estrace, Premarin). You insert this cream directly into your vagina with an applicator, usually at bedtime. Typically women use it daily for one to three weeks and then one to three times a week thereafter, but your doctor will let you know how much cream to use and how often to insert it.
- Vaginal estrogen suppositories (Imvexxy). These low-dose estrogen suppositories are inserted about 2 inches into the vaginal canal daily for weeks. Then, the suppositories only need to be inserted twice a week.
- Vaginal estrogen ring (Estring, Femring). You or your doctor inserts a soft, flexible ring into the upper part of the vagina. The ring releases a consistent dose of estrogen while in place and needs to be replaced about every three months. Many women like the convenience this offers. A different, higher dose ring is considered a systemic rather than topical treatment.
- Vaginal estrogen tablet (Vagifem). You use a disposable applicator to place a vaginal estrogen tablet in your vagina. Your doctor will let you know how often to insert the tablet. You might, for instance, use it daily for the first two weeks and then twice a week thereafter.
According to the North American menopausal community, when taking low-dose vaginal estrogen preparations, the following occur: a decrease in the rugae of the vagina, an increase in the number of lactobacilli, and an improvement in the state of the vaginal epithelium and the epithelium of the urethra 13. When administering local estrogen preparations, it is necessary to remember the side effects of drugs associated with systemic adsorption. Excess estrogen levels in postmenopausal women are associated with an increased risk of heart disease, breast cancer, thromboembolic complications, and cerebrovascular diseases 46. Preference should be given to low-dose local estrogens, especially if the therapy is prescribed to aged patients.
The most commonly used form of local estrogen therapy is vaginal creams based on conjugated equine estrogens and 17b estradiol; they provide a good moisturizing effect. However, the amount of cream administered can vary, exceeding the recommended daily dosage, which is particularly undesirable for patients at high risk. According to data of Kingsberg et al 47, several women experience discomfort when using vaginal cream, finding it messy.
If more controlled dosing of estrogen is required, the drug of choice may be vaginal tablets containing 10 mg of estradiol.
During the first 2 weeks of use, the daily use of the above-mentioned drug groups is recommended, followed by a transition to maintenance therapy with a dose of two to three times a week 1.
Sustained-release estradiol vaginal rings are preferred for women for whom daily use of drugs is unacceptable. Vaginal rings are inserted for up to 90 days and can be independently installed and removed by the patient. However, depending on the daily dose of estrogens, such systems can facilitate not only urogenital symptoms, but also vasomotor symptoms of menopause 6.
The use of vaginal rings is not recommended in women with prolapse of the genitals. It is also necessary to warn a woman about the possible expulsion of the vaginal ring.
In the studies of Constantine et al 48, Simon et al 49, and Pickar et al 50, a good result regarding the rapid relief from the genitourinary syndrome of menopause symptoms was noted when using gel capsules with estradiol, released at a dose of 4, 10, and 25 µg. At the same time, the absorption of the drug into the systemic circulation and the maximum concentration of estradiol in the serum were significantly lower in comparison with the use of vaginal tablets. The authors noted relief from the main symptoms of genitourinary syndrome of menopause for a few weeks after the use of vaginal capsules, but in most cases, it took up to 12 weeks of therapy to completely relieve the symptoms. However, clinical observations of the use of this group of low-dose estrogens are limited to 1 year.
Several clinical studies have also noted improvements in urinary symptoms, such as urgency, frequency, nocturia, and stress and urgency urinary incontinence 51.
Very interesting are the data published by Santen 52, where various variants of local estrogen therapy (low-, intermediate-, and high-dose) and their adsorption into the systemic circulation are considered. The author compared the peak concentrations of estrogens and the “chronic concentration” of estrogens in the blood plasma as a result of adsorption with prolonged use. According to data analysis of more than 30 studies, the use of low-dose vaginal estrogens (7.5 µg silastic vaginal ring and a 10 µg estradiol tablet [eg, VagifemR]) showed adsorption to the systemic circulation, but during long-term administration the estradiol levels were <20 pg/mL 52.
Absorption of low-dose vaginal estrogen preparations into the systemic blood stream is minimal and serum estradiol level does not exceed the physiological value for the postmenopausal period 6. Such a route of administration of drugs allows to avoid hepatic metabolism, which minimizes the risk of side effects inherent in systemic estrogen therapy and excludes the effects on the endometrium and breast tissue, and thus, there is no need to prescribe progesterone preparations for the prevention of endometrial hyperplasia and adenocarcinoma. Local estrogen therapy allows to quickly eliminate the symptoms of genitourinary syndrome of menopause, but it does not alleviate the vasomotor symptoms and reduce the risk of osteoporosis 7. However, according to several authors, against the background of long-term use of low-dose local estrogens, systemic effects on bone resorption and serum lipid were observed 53, 54.
Ospemifene (Osphena)
Selective estrogen receptor modulators (SERMs) are another option for genitourinary syndrome of menopause treatment among women in whom estrogen preparations are contraindicated. Selective estrogen receptor modulators (SERMs) are structurally different and interact with intracellular estrogen receptors in the target organs as agonists or antagonists of estrogens.
In 2013, Ospemifene (Osphena) was approved for use by the US Food and Drug Administration (FDA) and is now successfully used in USA and Europe. The literature has already accumulated sufficient reports on the use of oral ospemifene. Taken daily, oral ospemifene 60 mg daily can help relieve painful sex symptoms in women with moderate to severe genitourinary syndrome of menopause (GSM). It is not approved in women who’ve had breast cancer or who have a high risk of developing breast cancer.
Ospemifene has relatively weak estrogenic and antiestrogenic effects in classical hormonal tests, has an anti-osteoporosis effect, and reduces the level of total cholesterol.
A comparable effect with vaginal estrogens has been noted regarding alleviating the symptoms of vaginal atrophy and dyspareunia, improving the vaginal epithelium and pH of the vagina 55. The results of the studies with ospemifene show significant increase in the percentage of surface cells of the vaginal epithelium and a decrease in the percentage of parabasal cells and a decrease in vaginal pH 56.
Constantine et al 57, in their double-blind, placebo-controlled study, found the efficacy and safety within 1 year of using ospemifene. No cases of endometrial cancer were reported and only <1% of patients had endometrial hyperplasia. The incidence of thromboembolism associated with ospemifene in the pivotal studies was comparable to placebo; however, as a SERM, the class effect regarding the increased risk of venous thrombosis should be considered and this drug should be avoided in patients with an increased risk of venous thrombosis.
Lasofoxifene is new third-generation SERM that binds to both estrogen receptor types and is currently not approved for use by the FDA 58. Lasofoxifene has a pronounced positive effect on the state of the vaginal epithelium and pH and provides relief from the main symptoms of genitourinary syndrome of menopause in comparison to taking placebo 56. A number of studies have shown the high efficacy of lasofoxifene in improving bone mineral density, as well as reducing the risk of coronary heart disease and stroke and alleviating the symptoms of genitourinary syndrome of menopause 59, 60, 61, 62.
A tissue-specific estrogen complex is now being developed, including a combination of SERM (bazedoxifene) with conjugated estrogens. In the literature, there is evidence of the drug causing significant relief from the symptoms of genitourinary syndrome without the development of endometrial hyperplasia 63, 64.
The effect of tamoxifen in the vaginal epithelium is not well established; however, most of the data demonstrated that tamoxifen exerts antiestrogenic effect on the vaginal epithelium. Raloxifene slightly increased the percentage of vaginal superficial cells and decreased the percentage of parabasal cells; however, raloxifene did not improve the symptom of painful sex (dyspareunia) 65.
Intravaginal dehydroepiandrosterone (DHEA) – Prasterone (Intrarosa)
These vaginal inserts deliver the hormone dehydroepiandrosterone (DHEA) directly to the vagina to help ease painful sex (dyspareunia). For the daily vaginal use of DHEA, Intrarosa (prasterone) (6.5 mg) was recently approved by the FDA for use in the treatment of painful sex (dyspareunia). DHEA is a steroid precursor hormone (prohormone) with weak androgenic effects that is converted into male sex hormone (testosterone) and/or female sex hormone (estrogens) in peripheral tissues in your body to exert their effects 21, 22. Prasterone or DHEA is used nightly for moderate to severe vaginal atrophy.
The vaginal metabolism of DHEA into estrogens/testosterone leads to the activation of estrogen and androgen receptors in the three layers of the vaginal wall, including the fibers of the basal membrane collagen and the muscle wall, but the absence of aromatase in the normal endometrium does not lead to its stimulation 66. The levels of estradiol and testosterone in the serum may have minimal increases, without clinical significance presumably because of local inactivation. There is a slight increase in the serum concentration of DHEA sulfate (DHEAS).
In a new prospective, randomized, double-blind clinical trial, Labrie et al 67 confirmed the local beneficial effect of intravaginal DHEA (prasterone) on the symptoms of mild or severe dyspareunia, the most frequent manifestation of genitourinary syndrome in postmenopausal women.
Systemic estrogen therapy
If vaginal dryness is associated with other symptoms of menopause, such as moderate or severe hot flashes, your doctor may suggest estrogen pills, patches or gel, or a higher dose estrogen ring. Estrogen taken by mouth enters your entire body. Ask your doctor to explain the risks versus the benefits of oral estrogen, and whether or not you would also need to take another hormone called progestin along with estrogen. Estrogen therapy is prescribed after a clinical examination, identifying the risk factors for possible complications, and explaining the information to the patient. Low doses of estrogens are preferred for use, since the use of high doses of estrogen in menopausal age is associated with a high risk of hyperproliferative endometrial and adenocarcinoma 1.
Studies have shown that systemic estrogen therapy eliminates the symptoms of vaginal atrophy in 75% of cases, while local therapy does so in 80%–90% of cases 26.
Vaginal dilators
You may use vaginal dilators as a nonhormonal treatment option. Vaginal dilators may also be used in addition to estrogen therapy. Lubricated vibrator or vaginal dilator stimulate and stretch the vaginal muscles to reverse narrowing of the vagina. In women who experience vaginismus (ie, the involuntary contraction of the muscles surrounding the vagina), the progressive use of vaginal dilators with relaxation and mindfulness exercises can facilitate reinitiation of painless penetrative sexual activity 68. If painful sex is a concern, vaginal dilators may relieve vaginal discomfort by stretching your vagina. They are available without a prescription, but if your symptoms are severe, your doctor may recommend pelvic floor physical therapy and vaginal dilators. Pelvic floor physical therapy, ideally provided by a physical therapist with specialized training in pelvic floor disorders, may be useful for treatment of women with nonrelaxing or high-tone pelvic floor muscle dysfunction triggered by painful sexual activity related to genitourinary syndrome of menopause 69. Your health care provider or a pelvic physical therapist can teach you how to use vaginal dilators. Use this link to find a physical therapist who specializes in pelvic floor disorders (https://www.aptapelvichealth.org)
Topical lidocaine
Available as a prescription ointment or gel, topical lidocaine (topical local anesthetic) can be used to lessen discomfort associated with sexual activity. Apply it five to 10 minutes before you begin sexual activity.
Topical lidocaine applied to the entrance of the vagina a few minutes before sexual activity has reduced pain with sexual activity in breast cancer survivors and may serve as an adjunct to other therapies (eg, vaginal moisturizers, lubricants, and physical therapy) for management of GSM 70.
Systemic or local testosterone
Studies on the effects of androgens on the genitourinary system have mainly been conducted in animals 71. Several studies have demonstrated that androgens play an important role in the human urogenital system 72, but data so far are scarce. In a study by Salinger 73, menopausal women aged 54 to 85 years were administered intramuscular testosterone propionate daily or every other day to a total of 125 mg over 1 week. Subsequently, vaginal tissue biopsy was performed to evaluate the hormonal effect—vaginal superficial cells, epithelial intermediates, and glycogen deposition were increased 73. However, current studies of systemic transdermal testosterone therapy, alone or in combination with estrogen treatment, have not proven its effects on vaginal health 74, 75.
A study of postmenopausal women using intravaginal testosterone showed decreased vaginal pH and increased vaginal lactobacilli compared to the group using lubricating gel 76. Another study compared the group using intravaginal testosterone (5 mg/dose) in combination with conjugated equine estrogen with the group using conjugated equine estrogen alone. However, as conjugated equine estrogen was administered to both groups, there was a limitation in elucidating the effect of vaginal testosterone alone 77.
According to the 2020 position statement of the North American Menopause Society, to date, there is not enough data to confirm the safety and efficacy of systemic local testosterone therapy for the treatment of GSM 78. To date, hypoactive sexual desire disorder/dysfunction is the only evidence-based indication for testosterone use in postmenopausal women 79.
Women with breast cancer
If you have a history of breast cancer, tell your doctor and consider these options:
- Nonhormonal treatments. Try moisturizers and lubricants as a first choice.
- Vaginal dilators. Vaginal dilators are a nonhormonal option that can stimulate and stretch the vaginal muscles. This helps to reverse narrowing of the vagina.
- Vaginal estrogen. In consultation with your cancer specialist (oncologist), your doctor might recommend low-dose vaginal estrogen if nonhormonal treatments don’t help your symptoms. However, there’s some concern that vaginal estrogen might increase your risk of the cancer coming back, especially if your breast cancer was hormonally sensitive.
- Systemic estrogen therapy. Systemic estrogen treatment generally isn’t recommended, especially if your breast cancer was hormonally sensitive.
More than 60% of postmenopausal women with breast cancer report symptoms of GSM 80. Management of women with breast cancer, particularly those with estrogen receptor (ER) positive breast cancer, poses a challenge. These women often experience profound symptoms of GSM, but treatment options are limited. Women at high risk for breast cancer or those with estrogen receptor (ER)-positive breast cancers who are taking tamoxifen with persistent or severe symptoms not responding to non-hormonal therapies may be offered low-dose vaginal estrogen therapies, provided they have factors indicating a low risk of recurrence 81. Most oncologists are opposed to the use of even vaginal estrogen in women taking aromatase inhibitors; however, if symptoms are profound following discussion with their oncologist and there is recognition that even a small amount of estrogen absorbed may impact the effectiveness of the aromatase inhibitor on some occasions, low-dose vaginal estrogen therapy may be considered 82. The use of vaginal estrogen in women with a history of triple-negative disease is theoretically reasonable, but data are lacking 83.
Vaginal laser therapy
Recently introduced in the treatment of genitourinary syndrome of menopause, laser vaginal therapy has demonstrated effectiveness as well as high satisfaction among patients and health care providers 84, 85, 86, 87, 88, 89, 90, 91. Although the carbon dioxide laser has been cleared by the US Food and Drug Administration (FDA) for “incision, excision, ablation, vaporization, and coagulation of body soft tissues in medical specialties, including aesthetic (dermatology and plastic surgery), podiatry, otolaryngology (ENT), gynaecology” and other specialties and the YAG laser has been cleared for “incision, excision, ablation, vaporization of soft tissue for General Dermatology, Dermatologic and General Surgical procedures for coagulation and hemostasis,” vulvovaginal atrophy or genitourinary syndrome of menopause is not specifically listed as an indication for treatment 92, 93, 94. On July 30, 2018, the FDA released an FDA Safety Communication1 with the goal of alerting patients and healthcare providers to the fact that the use of energy-based devices (such as radiofrequency or laser) approved to treat gynecologic conditions but are also being used for various vaginal procedures such as vaginal “rejuvenation,” vaginal cosmetic procedures, and procedures intended to treat vaginal conditions and symptoms related to menopause, as well as for urinary incontinence or sexual function may be associated with serious adverse events 95, 96. Longer-term safety and efficacy studies using sham controls are needed before laser treatments can be recommended as a standard therapy for genitourinary syndrome of menopause 97, 2. Based on the available scientific evidence, with no supporting long term follow-up data, the use of laser should, at present, not be recommended for the treatment of vaginal atrophy, vulvodynia or lichen sclerosus 84, 97, 98. The data for the role of laser for stress urinary incontinence and vaginal laxity are inadequate to draw any conclusions or safe practice recommendations. Therefore based on the available scientific evidence and on the lack of long term follow-up, the use of laser should not be recommended for the treatment of vaginal atrophy, vulvodynia, lichen sclerosus, stress urinary incontinence, vaginal prolapse, or vaginal laxity 97.
Laser therapy improves the vascularization of the vaginal mucosa, stimulates the synthesis of new collagen and matrix basic substance in the connective tissue, thickens the vaginal epithelium with the formation of new papillae, replenishes glycogen in the vaginal epithelium, allows restoring the balance of the mucosa and therefore improves the symptoms of atrophy caused by a lack of estrogen 99, 100, 33.
Salvatore et al 100 also noted a significant improvement in the quality of life and sexual activity when laser therapy was used in women with genitourinary syndrome of menopause. In the study of Salvatore et al 100, 85% of women who were previously not sexually active due to genitourinary syndrome of menopause symptoms regained a normal sexual life at 12 weeks following therapy. The positive effect in the treatment of women with genitourinary syndrome of menopause can be achieved by combining hormonal and non-hormonal methods of treatment. It is important not to forget about the positive effect of sexual intercourse on the improvement of vaginal health. For the women who do not have regular sexual intercourse or have vaginal narrowing, the phenomenon of vaginismus, gradual careful stretching of the vagina with special vaginal dilators using lubricants is recommended. It can play an important role in restoring and maintaining the vaginal function. Then, the resumption of regular sexual activity will help to maintain vaginal health. Many women with these disorders benefit from the exercises that strengthen the muscles of the pelvic floor. In those patients, the use of vaginal estrogens before and after the expansion of the vagina and/or therapy to strengthen the pelvic muscles may be useful.
Radiofrequency therapy
With high-frequency alternating current, radiofrequency can temporarily increase the intracellular temperature, leading to cellular membrane rupture. This leads to neo-collagenesis of the vaginal mucosal wall; this is a mechanism similar to that of laser treatment. A pilot study showed that 14 women with genitourinary syndrome of menopause, treated with microablative fractional radiofrequency 3 times with a 1-month interval, experienced improvement in symptom severity and sexual satisfaction 101. However, this energy-based device is also not FDA approved for the treatment of genitourinary syndrome of menopause, and more studies are needed 71.
Education
Education is important so that women know about the genitourinary changes that occur with the loss of estrogen associated with menopause. Patients should be advised that genitourinary syndrome of menopause symptoms are unlikely to improve without treatment, and counseling should include a review of treatment options, both nonhormonal and hormonal 2. Women who are sexually active are often aware of these gradual changes, which may cause discomfort with sexual activity, but sexually inactive women who have genitourinary syndrome of menopause and who resume sexual activity may be surprised that it is painful (or not even possible) 6. Regular, painless sexual activity can help maintain vaginal health. A vibrator can be used therapeutically to stimulate blood flow and maintain vaginal function in women with or without a partner 6.
Alternative medicine
Some alternative medicines such as oral vitamin D, vaginal vitamin E, and probiotics are used to treat vaginal dryness and irritation associated with menopause, but few approaches are backed by sufficient evidence from clinical trials 102, 103, 104, 105. Interest in complementary and alternative medicine is growing, and researchers are working to determine the benefits and risks of various alternative treatments for genitourinary syndrome of menopause.
Talk with your doctor before taking any herbal or dietary supplements for perimenopausal or menopausal symptoms. The Food and Drug Administration (FDA) doesn’t regulate herbal products, and some may interact with other medications you take, putting your health at risk.
Genitourinary syndrome of menopause prognosis
Women suffering from genitourinary syndrome of menopause are able to get significant symptomatic relief with treatment if diagnosed and educated. Women who do not undergo management will, unfortunately, continue to experience ongoing symptoms, which can increase frustration, poor sexual lifestyle, recurrent infections, and overall lead to a decreased quality of life 3. Without treatment, your vaginal atrophy may get worse. Occasionally, vaginal atrophy can become so severe that it can significantly narrow your vaginal opening. This may make it harder to treat the vaginal atrophy if treatment is started too late.
Living with genitourinary syndrome of menopause
Vaginal atrophy can seriously affect your quality of life in general, not just your sex life 106. The pain, dryness, burning/itching, spotting, bleeding, urinary problems, urinary tract infections (UTIs) and discharge can make you very uncomfortable and interfere with your daily living. One in 4 people report that vaginal atrophy has had a negative impact on other areas of their lives including their sleep, sexual health and general happiness.
Can genitourinary syndrome of menopause be reversed?
Genitourinary syndrome of menopause can’t be cured, but you don’t have to live with the discomfort. With proper diagnosis and treatment, the symptoms can be managed. Women who do not undergo management will, unfortunately, continue to experience ongoing symptoms, which can increase frustration, poor sexual lifestyle, recurrent infections, and overall lead to a decreased quality of life 3. Without treatment, your vaginal atrophy may get worse. Occasionally, vaginal atrophy can become so severe that it can significantly narrow your vaginal opening. This may make it harder to treat the vaginal atrophy if treatment is started too late.
- Gandhi J, Chen A, Dagur G, Suh Y, Smith N, Cali B, Khan SA. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016 Dec;215(6):704-711. https://doi.org/10.1016/j.ajog.2016.07.045[↩][↩][↩][↩]
- Faubion SS, Sood R, Kapoor E. Genitourinary Syndrome of Menopause: Management Strategies for the Clinician. Mayo Clin Proc. 2017 Dec;92(12):1842-1849. https://doi.org/10.1016/j.mayocp.2017.08.019[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Bleibel B, Nguyen H. Vaginal Atrophy. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559297[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Palacios S. Managing urogenital atrophy. Maturitas. 2009 Aug 20;63(4):315-8. doi: 10.1016/j.maturitas.2009.04.009[↩][↩][↩][↩][↩][↩]
- DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The Association Between Vulvovaginal Atrophy Symptoms and Quality of Life Among Postmenopausal Women in the United States and Western Europe. J Womens Health (Larchmt). 2015 Sep;24(9):713-22. doi: 10.1089/jwh.2014.5177[↩][↩]
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013 Sep;20(9):888-902; quiz 903-4. doi: 10.1097/GME.0b013e3182a122c2[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Naumova I, Castelo-Branco C. Current treatment options for postmenopausal vaginal atrophy. Int J Womens Health. 2018 Jul 31;10:387-395. doi: 10.2147/IJWH.S158913[↩][↩][↩][↩][↩][↩][↩][↩]
- Aromatase Inhibitors for Lowering Breast Cancer Risk. https://www.cancer.org/cancer/types/breast-cancer/risk-and-prevention/aromatase-inhibitors-for-lowering-breast-cancer-risk.html[↩]
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014 Oct;21(10):1063-8. doi: 10.1097/GME.0000000000000329[↩]
- Lubián López DM. Management of genitourinary syndrome of menopause in breast cancer survivors: An update. World J Clin Oncol. 2022 Feb 24;13(2):71-100. doi: 10.5306/wjco.v13.i2.71[↩]
- Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997 Oct;314(4):228-31. doi: 10.1097/00000441-199710000-00004[↩]
- Nappi RE, Panay N, Bruyniks N, Castelo-Branco C, De Villiers TJ, Simon JA. The clinical relevance of the effect of ospemifene on symptoms of vulvar and vaginal atrophy. Climacteric. 2015 Apr;18(2):233-40. doi: 10.3109/13697137.2014.975199[↩]
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007 May-Jun;14(3 Pt 1):355-69; quiz 370-1. doi: 10.1097/gme.0b013e31805170eb[↩][↩]
- Wines N, Willsteed E. Menopause and the skin. Australas J Dermatol. 2001 Aug;42(3):149-8; quiz 159. doi: 10.1046/j.1440-0960.2001.00524.x[↩]
- Davila GW, Singh A, Karapanagiotou I, Woodhouse S, Huber K, Zimberg S, Seiler J, Kopka SL. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003 Feb;188(2):382-8. doi: 10.1067/mob.2003.23[↩]
- Burich R, Degregorio M. Currenttreatment options for vulvovaginal atrophy. Expert Rev Obstet Gynecol. 2011;6:141–151.[↩]
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) – results from an international survey. Climacteric. 2012 Feb;15(1):36-44. doi: 10.3109/13697137.2011.647840[↩]
- Balica AC, Cooper AM, McKevitt MK, Schertz K, Wald-Spielman D, Egan S, Bachmann GA. Dyspareunia Related to GSM: Association of Total Vaginal Thickness via Transabdominal Ultrasound. J Sex Med. 2019 Dec;16(12):2038-2042. doi: 10.1016/j.jsxm.2019.08.019[↩][↩]
- Demers LM, Hankinson SE, Haymond S, Key T, Rosner W, Santen RJ, Stanczyk FZ, Vesper HW, Ziegler RG; Endocrine Society; PATH (Partnership for Accurate Testing of Hormones); AACC (American Association for Clinical Chemistry). Measuring Estrogen Exposure and Metabolism: Workshop Recommendations on Clinical Issues. J Clin Endocrinol Metab. 2015 Jun;100(6):2165-70. doi: 10.1210/jc.2015-1040[↩][↩]
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016 Apr;19(2):151-61. doi: 10.3109/13697137.2015.1124259[↩][↩][↩][↩][↩]
- Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005 Nov;187(2):169-96. https://joe.bioscientifica.com/view/journals/joe/187/2/1870169.xml[↩][↩]
- Papadopoulou-Marketou N, Kassi E, Chrousos GP. Adrenal Androgens and Aging. [Updated 2023 Jan 18]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279006[↩][↩]
- Sturdee DW, Panay N; International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010 Dec;13(6):509-22. doi: 10.3109/13697137.2010.522875[↩]
- Basaran M, Kosif R, Bayar U, Civelek B. Characteristics of external genitalia in pre- and postmenopausal women. Climacteric. 2008 Oct;11(5):416-21. doi: 10.1080/13697130802366670[↩]
- Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018 Jun;34(6):451-455. doi: 10.1080/09513590.2017.1407753[↩]
- Goldstein I. Recognizing and treating urogenital atrophy in postmenopausal women. J Womens Health (Larchmt). 2010 Mar;19(3):425-32. doi: 10.1089/jwh.2009.1384[↩][↩]
- Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J, Gravitt PE. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014 May;21(5):450-8. doi: 10.1097/GME.0b013e3182a4690b[↩][↩]
- Woelber L, Prieske K, Mendling W, Schmalfeldt B, Tietz HJ, Jaeger A. Vulvar pruritus-Causes, Diagnosis and Therapeutic Approach. Dtsch Arztebl Int. 2020 Feb 21;116(8):126-133. doi: 10.3238/arztebl.2020.0126[↩][↩]
- Lindahl SH. Reviewing the options for local estrogen treatment of vaginal atrophy. Int J Womens Health. 2014 Mar 13;6:307-12. doi: 10.2147/IJWH.S52555[↩]
- Angelou K, Grigoriadis T, Diakosavvas M, Zacharakis D, Athanasiou S. The Genitourinary Syndrome of Menopause: An Overview of the Recent Data. Cureus. 2020 Apr 8;12(4):e7586. doi: 10.7759/cureus.7586[↩][↩]
- Vaginal atrophy. https://www.mayoclinic.org/diseases-conditions/vaginal-atrophy/symptoms-causes/syc-20352288[↩]
- Kim HK, Kang SY, Chung YJ, Kim JH, Kim MR. The Recent Review of the Genitourinary Syndrome of Menopause. J Menopausal Med. 2015 Aug;21(2):65-71. doi: 10.6118/jmm.2015.21.2.65[↩]
- Hutchinson-Colas J, Segal S. Genitourinary syndrome of menopause and the use of laser therapy. Maturitas. 2015 Dec;82(4):342-5. doi: 10.1016/j.maturitas.2015.08.001[↩][↩][↩]
- Asadi M, Jouyandeh Z, Nayebzadeh F. Prevalence of menopause symptoms among Iranian women. Journal of Family and Reproductive Health. 2012;6:1–3.[↩]
- Phillips NA, Bachmann GA. Genitourinary syndrome of menopause: Common problem, effective treatments. Cleve Clin J Med. 2018 May;85(5):390-398. doi: 10.3949/ccjm.85a.15081[↩]
- Shifren JL. Genitourinary Syndrome of Menopause. Clin Obstet Gynecol. 2018 Sep;61(3):508-516. doi: 10.1097/GRF.0000000000000380[↩]
- Harris BS, Bishop KC, Kuller JA, Ford AC, Muasher LC, Cantrell SE, Price TM. Hormonal management of menopausal symptoms in women with a history of gynecologic malignancy. Menopause. 2020 Feb;27(2):243-248. doi: 10.1097/GME.0000000000001447[↩][↩][↩][↩]
- Temkin SM, Mallen A, Bellavance E, Rubinsak L, Wenham RM. The role of menopausal hormone therapy in women with or at risk of ovarian and breast cancers: Misconceptions and current directions. Cancer. 2019 Feb 15;125(4):499-514. doi: 10.1002/cncr.31911[↩]
- Kapoor E, Benrubi D, Faubion SS. Menopausal Hormone Therapy in Gynecologic Cancer Survivors: A Review of the Evidence and Practice Recommendations. Clin Obstet Gynecol. 2018 Sep;61(3):488-495. doi: 10.1097/GRF.0000000000000381[↩][↩]
- Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013 Jun;10(6):1575-84. doi: 10.1111/jsm.12125[↩]
- Sandhu RS, Wong TH, Kling CA, Chohan KR. In vitro effects of coital lubricants and synthetic and natural oils on sperm motility. Fertil Steril. 2014 Apr;101(4):941-4. doi: 10.1016/j.fertnstert.2013.12.024[↩]
- Voeller B, Coulson AH, Bernstein GS, Nakamura RM. Mineral oil lubricants cause rapid deterioration of latex condoms. Contraception. 1989 Jan;39(1):95-102. doi: 10.1016/0010-7824(89)90018-8[↩]
- Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol. 2013 Apr;121(4):773-780. doi: 10.1097/AOG.0b013e31828786f8[↩]
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005 Nov 15;52 Suppl 1:S46-52. doi: 10.1016/j.maturitas.2005.06.014[↩]
- Pickar JH. Emerging therapies for postmenopausal vaginal atrophy. Maturitas. 2013 May;75(1):3-6. doi: 10.1016/j.maturitas.2013.01.020[↩]
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013 Oct 2;310(13):1353-68. doi: 10.1001/jama.2013.278040[↩]
- Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women’s EMPOWER Survey: Identifying Women’s Perceptions on Vulvar and Vaginal Atrophy and Its Treatment. J Sex Med. 2017 Mar;14(3):413-424. doi: 10.1016/j.jsxm.2017.01.010[↩]
- Constantine GD, Simon JA, Pickar JH, Archer DF, Kushner H, Bernick B, Gasper G, Graham S, Mirkin S; REJOICE Study Group. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause. 2017 Apr;24(4):409-416. doi: 10.1097/GME.0000000000000786[↩]
- Simon JA, Archer DF, Constantine GD, Pickar JH, Amadio JM, Bernick B, Graham S, Mirkin S. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: Efficacy and pharmacokinetic data review. Maturitas. 2017 May;99:51-58. doi: 10.1016/j.maturitas.2017.02.008[↩]
- Pickar JH, Amadio JM, Bernick BA, Mirkin S. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric. 2016 Apr;19(2):181-7. doi: 10.3109/13697137.2015.1136926[↩]
- Rahn DD, Carberry C, Sanses TV, Mamik MM, Ward RM, Meriwether KV, Olivera CK, Abed H, Balk EM, Murphy M; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014 Dec;124(6):1147-1156. doi: 10.1097/AOG.0000000000000526[↩]
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric. 2015 Apr;18(2):121-34. doi: 10.3109/13697137.2014.947254[↩][↩]
- Naessen T, Berglund L, Ulmsten U. Bone loss in elderly women prevented by ultralow doses of parenteral 17beta-estradiol. Am J Obstet Gynecol. 1997 Jul;177(1):115-9. doi: 10.1016/s0002-9378(97)70448-4[↩]
- Naessen T, Rodriguez-Macias K, Lithell H. Serum lipid profile improved by ultra-low doses of 17 beta-estradiol in elderly women. J Clin Endocrinol Metab. 2001 Jun;86(6):2757-62. doi: 10.1210/jcem.86.6.7524[↩]
- Paton DM. Ospemifene for the treatment of dyspareunia in postmenopausal women. Drugs Today (Barc). 2014 May;50(5):357-64. doi: 10.1358/dot.2014.50.5.2134451[↩]
- Mirkin S, Pickar JH. Selective estrogen receptor modulators (SERMs): a review of clinical data. Maturitas. 2015 Jan;80(1):52-7. doi: 10.1016/j.maturitas.2014.10.010[↩][↩]
- Constantine GD, Goldstein SR, Archer DF. Endometrial safety of ospemifene: results of the phase 2/3 clinical development program. Menopause. 2015 Jan;22(1):36-43. doi: 10.1097/GME.0000000000000275[↩]
- Pinkerton JV, Stanczyk FZ. Clinical effects of selective estrogen receptor modulators on vulvar and vaginal atrophy. Menopause. 2014 Mar;21(3):309-19. doi: 10.1097/GME.0b013e31829755ed[↩]
- Moffett A, Ettinger M, Bolognese M, et al. Lasofoxifene, a next generation SERM, is effective in preventing loss of BMD and reducing LDLC in postmenopausal women. J Bone Miner Res. 2004;19:96.[↩]
- McClung MR, Siris E, Cummings S, Bolognese M, Ettinger M, Moffett A, Emkey R, Day W, Somayaji V, Lee A. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006 May-Jun;13(3):377-86. doi: 10.1097/01.gme.0000188736.69617.4f[↩]
- Gennari L. Lasofoxifene, a new selective estrogen receptor modulator for the treatment of osteoporosis and vaginal atrophy. Expert Opin Pharmacother. 2009 Sep;10(13):2209-20. doi: 10.1517/14656560903127241[↩]
- Cummings S, Eastell R, Ensrud K. The effects of lasofoxifene on fractures and breast cancer: 3 year results from the PEARL trial. J Bone Miner Res. 2008;23:81.[↩]
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010 Mar;17(2):281-9. doi: 10.1097/GME.0b013e3181b7c65f[↩]
- Lobo RA, Pinkerton JV, Gass MLS, Dorin MH, Ronkin S, Pickar JH, Constantine G. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009 Sep;92(3):1025-1038. doi: 10.1016/j.fertnstert.2009.03.113[↩]
- Komm BS, Mirkin S. Evolution of the tissue selective estrogen complex (TSEC). J Cell Physiol. 2013 Jul;228(7):1423-7. doi: 10.1002/jcp.24324[↩]
- Portman DJ, Labrie F, Archer DF, Bouchard C, Cusan L, Girard G, Ayotte N, Koltun W, Blouin F, Young D, Wade A, Martel C, Dubé R; other participating members of VVA Prasterone Group. Lack of effect of intravaginal dehydroepiandrosterone (DHEA, prasterone) on the endometrium in postmenopausal women. Menopause. 2015 Dec;22(12):1289-95. doi: 10.1097/GME.0000000000000470[↩]
- Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016 Mar;23(3):243-56. doi: 10.1097/GME.0000000000000571[↩]
- Zarski AC, Berking M, Fackiner C, Rosenau C, Ebert DD. Internet-Based Guided Self-Help for Vaginal Penetration Difficulties: Results of a Randomized Controlled Pilot Trial. J Sex Med. 2017 Feb;14(2):238-254. doi: 10.1016/j.jsxm.2016.12.232[↩]
- Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc. 2012 Feb;87(2):187-93. doi: 10.1016/j.mayocp.2011.09.004[↩]
- Goetsch MF, Lim JY, Caughey AB. A Practical Solution for Dyspareunia in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol. 2015 Oct 20;33(30):3394-400. doi: 10.1200/JCO.2014.60.7366[↩]
- Shim S, Park KM, Chung YJ, Kim MR. Updates on Therapeutic Alternatives for Genitourinary Syndrome of Menopause: Hormonal and Non-Hormonal Managements. J Menopausal Med. 2021 Apr;27(1):1-7. doi: 10.6118/jmm.20034[↩][↩]
- Traish AM, Vignozzi L, Simon JA, Goldstein I, Kim NN. Role of Androgens in Female Genitourinary Tissue Structure and Function: Implications in the Genitourinary Syndrome of Menopause. Sex Med Rev. 2018 Oct;6(4):558-571. doi: 10.1016/j.sxmr.2018.03.005[↩]
- SALINGER SL. Proliferative effect of testosterone propionate on human vaginal epithelium. Acta Endocrinol (Copenh). 1950;4(3):265-84. doi: 10.1530/acta.0.0040265[↩][↩]
- Panay N, Al-Azzawi F, Bouchard C, Davis SR, Eden J, Lodhi I, Rees M, Rodenberg CA, Rymer J, Schwenkhagen A, Sturdee DW. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric. 2010 Apr;13(2):121-31. doi: 10.3109/13697131003675922[↩]
- Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Bouchard C, Derzko C, Buch A, Rodenberg C, Lucas J, Davis S. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005 Sep;90(9):5226-33. doi: 10.1210/jc.2004-1747[↩]
- Bell RJ, Rizvi F, Islam RM, Davis SR. A systematic review of intravaginal testosterone for the treatment of vulvovaginal atrophy. Menopause. 2018 Jun;25(6):704-709. doi: 10.1097/GME.0000000000001052[↩]
- Raghunandan C, Agrawal S, Dubey P, Choudhury M, Jain A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J Sex Med. 2010 Mar;7(3):1284-90. doi: 10.1111/j.1743-6109.2009.01667.x[↩]
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020 Sep;27(9):976-992. doi: 10.1097/GME.0000000000001609[↩]
- Davis SR, Baber R, Panay N, Bitzer J, Cerdas Perez S, Islam RM, Kaunitz AM, Kingsberg SA, Lambrinoudaki I, Liu J, Parish SJ, Pinkerton J, Rymer J, Simon JA, Vignozzi L, Wierman ME. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. Climacteric. 2019 Oct;22(5):429-434. doi: 10.1080/13697137.2019.1637079. Epub 2019 Sep 2. Erratum in: Climacteric. 2019 Dec;22(6):637.[↩]
- Kingsberg SA, Larkin L, Krychman M, et al.: WISDOM survey: Attitudes and behaviors of physicians toward vulvar and vaginal atrophy (VVA) treatment in women including those with breast cancer history. Menopause. 2019; 26(2): 124–31. 10.1097/GME.0000000000001194[↩]
- Faubion SS, Larkin LC, Stuenkel CA, et al.: Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause. 2018; 25(6): 596–608. 10.1097/GME.0000000000001121[↩]
- Pitkin J, British Menopause Society medical advisory council: BMS – Consensus statement. Post Reprod Health. 2018; 24(3): 133–8. 10.1177/2053369118795349[↩]
- Da Silva AS, Baines G, Araklitis G, Robinson D, Cardozo L. Modern management of genitourinary syndrome of menopause. Fac Rev. 2021 Mar 3;10:25. doi: 10.12703/r/10-25[↩]
- Cucinella L, Tiranini L, Cassani C, Martella S, Nappi RE. Genitourinary Syndrome of Menopause in Breast Cancer Survivors: Current Perspectives on the Role of Laser Therapy. Int J Womens Health. 2023 Aug 8;15:1261-1282. doi: 10.2147/IJWH.S414509[↩][↩]
- Athanasiou S, Pitsouni E, Falagas ME, Salvatore S, Grigoriadis T. CO2-laser for the genitourinary syndrome of menopause. How many laser sessions? Maturitas. 2017 Oct;104:24-28. doi: 10.1016/j.maturitas.2017.07.007[↩]
- Anger JT. CO2 Laser Treatment is Effective for Symptoms of Vaginal Atrophy. J Urol. 2017 Dec;198(6):1228-1229. doi: 10.1016/j.juro.2017.09.003[↩]
- Li J, Li H, Zhou Y, Xie M, Miao Y, Wang L, Zhao Y, Ying T, Hu Y, Chen Y, Chen Y, Sun X, Wang J. The Fractional CO2 Laser for the Treatment of Genitourinary Syndrome of Menopause: A Prospective Multicenter Cohort Study. Lasers Surg Med. 2021 Jul;53(5):647-653. doi: 10.1002/lsm.23346[↩]
- Enemchukwu EA. CO2 Laser Treatment is Effective for Symptoms of Vaginal Atrophy: No. J Urol. 2017 Dec;198(6):1228-1229. doi: 10.1016/j.juro.2017.09.004[↩]
- https://urology.stanford.edu/content/dam/sm/urology/JJimages/publications/CO2-Laser-Treatment-is-Effective-for-Symptoms-of-Vaginal-Atrophy-No.pdf[↩]
- Arunkalaivanan A, Kaur H, Onuma O. Laser therapy as a treatment modality for genitourinary syndrome of menopause: a critical appraisal of evidence. Int Urogynecol J. 2017 May;28(5):681-685. doi: 10.1007/s00192-017-3282-y[↩]
- Ghanbari Z, Sohbati S, Eftekhar T, Sahebi L, Darvish S, Alasiri S, Deldar Pasikhani M. Fractional CO2 Laser for Treatment of Vulvovaginal Atrophy: A Short Time Follow-up. J Family Reprod Health. 2020 Jun;14(2):68-73. doi: 10.18502/jfrh.v14i2.4347[↩]
- The American College of Obstetricians and Gynecologists. Fractional Laser Treatment of Vulvovaginal Atrophy and US Food and Drug Administration Clearance: Position Statement. May 2016.[↩]
- https://www.accessdata.fda.gov/cdrh_docs/pdf10/K101904.pdf[↩]
- https://www.accessdata.fda.gov/cdrh_docs/pdf11/K110304.pdf[↩]
- https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf[↩]
- Ahluwalia J, Avram MM, Ortiz AE. Lasers and energy-based devices marketed for vaginal rejuvenation: A cross-sectional analysis of the MAUDE database. Lasers Surg Med. 2019 Oct;51(8):671-677. doi: 10.1002/lsm.23084[↩]
- Preti M, Vieira-Baptista P, Digesu GA, Bretschneider CE, Damaser M, Demirkesen O, Heller DS, Mangir N, Marchitelli C, Mourad S, Moyal-Barracco M, Peremateu S, Tailor V, Tarcan T, De EJB, Stockdale CK. The Clinical Role of LASER for Vulvar and Vaginal Treatments in Gynecology and Female Urology: An ICS/ISSVD Best Practice Consensus Document. J Low Genit Tract Dis. 2019 Apr;23(2):151-160. doi: 10.1097/LGT.0000000000000462[↩][↩][↩]
- Palacios S, Combalia J, Emsellem C, Gaslain Y, Khorsandi D. Therapies for the management of genitourinary syndrome of menopause. Post Reprod Health. 2020 Mar;26(1):32-42. doi: 10.1177/2053369119866341[↩]
- Abrahamse H. Regenerative medicine, stem cells, and low-level laser therapy: future directives. Photomed Laser Surg. 2012 Dec;30(12):681-2. doi: 10.1089/pho.2012.9881[↩]
- Salvatore S, Athanasiou S, Candiani M. The use of pulsed CO2 lasers for the treatment of vulvovaginal atrophy. Curr Opin Obstet Gynecol. 2015 Dec;27(6):504-8. doi: 10.1097/GCO.0000000000000230. Erratum in: Curr Opin Obstet Gynecol. 2017 Aug;29(4):282.[↩][↩][↩]
- Kamilos MF, Borrelli CL. New therapeutic option in genitourinary syndrome of menopause: pilot study using microablative fractional radiofrequency. Einstein (Sao Paulo). 2017 Oct-Dec;15(4):445-451. doi: 10.1590/S1679-45082017AO4051[↩]
- Caruso S, Cianci S, Fava V, Rapisarda AMC, Cutello S, Cianci A. Vaginal health of postmenopausal women on nutraceutical containing equol. Menopause. 2018 Apr;25(4):430-435. doi: 10.1097/GME.0000000000001061[↩]
- Yildirim B, Kaleli B, Düzcan E, Topuz O. The effects of postmenopausal Vitamin D treatment on vaginal atrophy. Maturitas. 2004 Dec 10;49(4):334-7. doi: 10.1016/j.maturitas.2004.02.008[↩]
- Costantino D, Guaraldi C. Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: an open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 2008 Nov-Dec;12(6):411-6.[↩]
- Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016 Sep;91:42-50. doi: 10.1016/j.maturitas.2016.05.015[↩]
- Simon JA, Davis SR, Althof SE, et al. Sexual well-being after menopause: an International Menopause Society White Paper. Climacteric. 2018;21(5):415–427. doi: 10.1080/13697137.2018.1482647[↩]