Contents
- What is Hepatitis B
- Hepatitis B worldwide
- Hepatitis B in the United States
- Who is more likely to get hepatitis B?
- What is acute (short-term) hepatitis B?
- When symptoms of acute hepatitis B occur, how long do they usually last?
- What is chronic (long-term) hepatitis B?
- Who is most likely to get chronic (long-term) hepatitis B?
- How common is chronic (long-term) hepatitis B infection in the United States?
- How many deaths can be attributed to chronic (long-term) hepatitis B infection?
- How long does hepatitis B virus survive outside the body?
- What should be used to remove hepatitis B virus from environmental surfaces?
- Can a person spread the hepatitis B virus and not know it?
- Can the hepatitis B virus be spread through sex?
- Can hepatitis B be spread through food?
- What is the incubation period for hepatitis B?
- What should I do if I think I have been exposed to the hepatitis B virus?
- What is hepatitis B immune globulin (HBIG)?
- If I have been infected with the hepatitis B virus in the past, can I get it again?
- What is the difference between hepatitis A, hepatitis B, and hepatitis C?
- What is Hepatitis B virus (HBV) and Human Immunodeficiency virus (HIV) coinfection?
- What is Hepatitis B virus (HBV) and Hepatitis D virus (HDV) coinfection or superinfection?
- What is Hepatitis B virus (HBV) and Hepatitis C virus (HCV) coinfection?
- Pregnancy and Hepatitis B
- Should I be screened for hepatitis B?
- What is Acute Hepatitis B vs. Chronic Hepatitis B?
- How many new hepatitis B virus infections occur annually in the United States?
- What causes hepatitis B?
- How is Hepatitis B spread?
- Prevention of Hepatitis B virus (HBV) infection
- Hepatitis B signs and symptoms
- Hepatitis B complications
- Hepatitis B virus (HBV) Diagnosis
- Hepatitis B Serology
- Hepatitis B surface antigen (HBsAg)
- Hepatitis B surface antibody (anti-HBs)
- Total hepatitis B core antibody (anti-HBc)
- IgM antibody to hepatitis B core antigen (anti-HBc IgM)
- IgG antibody to hepatitis B core antigen (anti-HBc IgG)
- Hepatitis B e antigen (HBeAg)
- Hepatitis B e antibody (HBeAb or anti-HBe)
- Hepatitis B Virus DNA Quantification (“viral load”)
- How do I interpret hepatitis B serologic test results?
- Hepatitis B treatment
- Hepatitis B prognosis
- Hepatitis B Vaccine
- Who should be vaccinated against hepatitis B ?
- Is hepatitis B vaccination recommended in certain settings ?
- Who should not receive hepatitis B vaccine ?
- Can hepatitis B vaccine be given during pregnancy or lactation?
- Can hepatitis B vaccine be given after exposure to Hepatitis B virus (HBV)?
- What are the hepatitis B vaccines licensed for use in the United States?
- Is it harmful to administer an extra dose(s) of Hepatitis A or hepatitis B vaccine or to repeat the entire vaccine series if documentation of vaccination history is unavailable ?
- If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted ?
- Can hepatitis B vaccine be administered concurrently with other vaccines?
- How long does protection from hepatitis B vaccine last?
- Why should an infant receive hepatitis B vaccine at birth before hospital discharge, even if the mother is negative for hepatitis B surface antigen (HBsAg)?
- Can hepatitis B vaccine be given to immunocompromised persons, such as persons on hemodialysis or persons with HIV infection ?
- Can a patient receive the first dose of hepatitis B vaccine from one manufacturer and subsequent doses from another manufacturer ?
- Is there any benefit or risk in vaccinating a person who has been infected with Hepatitis B virus (HBV) ?
- Should persons be tested for immunity to hepatitis B before being vaccinated?
- Who should receive post-vaccination testing?
- When should postvaccination testing be done?
- Are booster doses of hepatitis B vaccine recommended?
- Hepatitis B vaccine side effects
What is Hepatitis B
Hepatitis B is a serious liver infection caused by the Hepatitis B virus (HBV) 1. Hepatitis B is transmitted when blood, semen, or another body fluid from a person infected with the Hepatitis B virus enters the body of someone who is not infected. This can happen through sexual contact; sharing needles, syringes, or other drug-injection equipment; or from mother to baby during childbirth (maternal-to-newborn perinatal transmission or vertical transmission) 1. High-risk groups for hepatitis B virus (HBV) infection include intravenous drug users, infants born to infected mothers, males who have sexual intercourse with other males, hemodialysis patients (and workers), healthcare workers, household contacts of known patients with chronic hepatitis B virus (HBV). A majority of the global hepatitis B virus (HBV) disease burden is primarily through vertical transmission (maternal-to-newborn perinatal transmission) 2. For some people (more than 95% of immunocompetent adults), hepatitis B is an acute or short-term, illness but for others if it does not get better, hepatitis B can become a long-term, chronic hepatitis B infection, which lasts a lifetime. Risk for chronic hepatitis B infection is related to age at infection: approximately 90% of infected infants become chronically infected, compared with 2%–6% of adults. Chronic Hepatitis B can lead to serious health issues, like cirrhosis (scarring of the liver), liver failure or liver cancer. People with chronic hepatitis B may also develop kidney disease or inflammation of blood vessels.
Not all people with acute hepatitis B infection have symptoms. Acute hepatitis B infection symptoms can range from asymptomatic or mild disease to, rarely, severe life-threatening hepatitis (fulminant hepatitis). Symptoms of acute hepatitis B usually appear about 1 to 4 months after you’ve been infected, although you could see them as early as two weeks after you’re infected. Some people, usually infants, children under 5 years old, and immunosuppressed adults, may not have any symptoms (asymptomatic). People less than 30 years old are less likely to be symptomatic compared with persons aged 30 years and older 3. When present, signs and symptoms of acute hepatitis B infections can include:
- fever
- weakness and fatigue
- loss of appetite
- nausea
- vomiting
- abdominal pain
- dark urine
- clay-colored stool
- joint pain
- yellowing of the skin and the whites of the eyes (jaundice)
Most people with chronic HBV infection are asymptomatic and have no evidence of liver disease or injury. However, some people develop chronic hepatitis (elevation of liver enzymes alanine aminotransferase [ALT] or aspartate aminotransferase [AST]), cirrhosis, or hepatocellular carcinoma (i.e., primary liver cancer).
A vaccine against hepatitis B has been available since 1982 4. The vaccine is 95% effective in preventing infection and the development of chronic hepatitis B infection and liver cancer due to hepatitis B. The best way to prevent Hepatitis B is by getting vaccinated. It requires three shots. All babies should get the hepatitis B vaccine, but older children and adults can get it too. If you travel to countries where Hepatitis B is common, you should get the vaccine.
If you know you’ve been exposed to hepatitis B, contact your health care provider immediately. A preventive treatment may reduce your risk of infection if you receive the treatment within 24 hours of exposure to the virus. The mainstay of postexposure prophylaxis (PEP) is hepatitis B vaccine, but, in certain circumstances, hepatitis B immune globulin (HBIg) is recommended in addition to vaccine for added protection.
If you think you have symptoms of hepatitis B, contact your health care provider.
Hepatitis B worldwide
Hepatitis B affects approximately 296 million people, including over 6 million children under 5 worldwide 5. Prevalence areas are based on the percentage of the population with hepatitis B surface antigen (HBsAg) positivity 2. Hepatitis B prevalence is highest in the World Health Organization (WHO) Western Pacific Region and the World Health Organization (WHO) African Region, where 6.2% and 6.1% respectively of the adult population is infected. In the WHO Eastern Mediterranean Region, the WHO South-East Asia Region and the WHO European Region, an estimated 3.3%, 2.0% and 1.6%% of the general population is infected, respectively. 0.7% of the population of the WHO Region of the Americas is infected. While hepatitis B vaccines are available, limited access to healthcare and lack of proper health education contributes to the increasing global prevalence of hepatitis B. Lower incidence of hepatitis B in the United States compared to Asia and Africa is due to better access to healthcare and better use of vaccinations and other preventive measures.
Hepatitis B worldwide statistics:
- 350-400 million of the world population has chronic hepatitis B 6.
- The following population is known to have a higher prevalence: Asian Pacific Islanders, Alaskan Eskimos, and Australian aboriginees 7.
- The following geographic regions have higher prevalence: the Indian sub-continent, sub-Saharan Africa, and central Asia.
- The prevalence of hepatitis B is reduced after the initiation of the hepatitis B vaccination program.
- 10 genotypes (A-J) of hepatitis B have been identified 8.
Hepatitis B in the United States
In 2020, a total of 2,157 cases of acute (short-term) hepatitis B were reported to Centers for Disease Control and Prevention (CDC) 9. Since many people with hepatitis B may not have symptoms or don’t know they are infected, their illness is often not diagnosed so it isn’t reported or counted. The Centers for Disease Control and Prevention (CDC) estimates the actual number of acute hepatitis B cases was closer to 14,000 in 2020. Many more people (about 880,000) are estimated to be living with chronic, long-term hepatitis B. Asian Americans and African Americans have higher rates of chronic hepatitis B than other U.S. racial and ethnic groups 10. Researchers estimate that about half of the people living with chronic hepatitis B in the United States are Asian Americans and Pacific Islanders 11. Chronic hepatitis B is also more common among people born in other countries than among those born in the United States 12.
The hepatitis B vaccine has been available since the 1980s and, in 1991, doctors began recommending that children in the United States receive the hepatitis B vaccine. The annual rate of acute hepatitis B infections went down 88.5 percent between 1982 and 2015 13. In 2017, the annual number of hepatitis B infections rose in some states 14. Experts think the rise was related to increases in injection drug use. Injection drug use increases the risk of hepatitis B infection.
Who is more likely to get hepatitis B?
People are more likely to get hepatitis B if they are born to a mother who has hepatitis B (also known as vertical transmission). Hepatitis B virus can spread from mother to child during birth. For this reason, people are more likely to have hepatitis B if they 15:
- were born in a part of the world where 2 percent or more of the population has hepatitis B infection
- were born in the United States, didn’t receive the hepatitis B vaccine as an infant, and have parents who were born in an area where 8 percent or more of the population had hepatitis B infection.
People are also more likely to have hepatitis B if they:
- are infected with HIV, because hepatitis B and HIV spread in similar ways
- have lived with or had sex with someone who has hepatitis B
- have had more than one sex partner in the last 6 months or have a history of sexually transmitted disease (STD)
- are men who have sex with men
- are injection drug users
- work in a profession, such as health care, in which they have contact with blood, needles, or body fluids at work
- live or work in a care facility for people with developmental disabilities
- have diabetes
- have hepatitis C
- have lived in or travel often to parts of the world where hepatitis B is common
- have been on kidney dialysis
- live or work in a prison
- had a blood transfusion or organ transplant before the mid-1980s
In the United States, hepatitis B spreads among adults mainly through contact with infected blood through the skin, such as during injection drug use, and through sexual contact 13.
What is acute (short-term) hepatitis B?
Acute hepatitis B is a short-term illness that lasts less than 6 months after someone is exposed to the hepatitis B virus. Some people with acute hepatitis B have no symptoms at all or only mild illness. For others, acute hepatitis B can cause a more severe illness that requires hospitalization. Your immune system likely can clear acute hepatitis B from your body, and you should recover completely within a few months. Most people who get hepatitis B as adults have an acute infection, but it can lead to chronic infection.
When symptoms of acute hepatitis B occur, how long do they usually last?
Symptoms typically last for several weeks but can persist for up to 6 months 16, 17.
What is chronic (long-term) hepatitis B?
Some people, especially those who get infected with hepatitis B virus in adulthood, can fight the virus without treatment. For other people, acute hepatitis B leads to life-long infection known as chronic hepatitis B. Chronic hepatitis B infection lasts 6 months or longer. Chronic hepatitis B lingers because your immune system can’t fight off the infection. Chronic hepatitis B infection may last a lifetime, possibly leading to serious illnesses such as liver damage, cirrhosis, liver cancer, and even death. Some people with chronic hepatitis B may have no symptoms at all. Some may have ongoing fatigue and mild symptoms of acute hepatitis.
The younger you are when you get hepatitis B — particularly newborns or children younger than 5 — the higher your risk of the infection becoming chronic. Chronic infection may go undetected for decades until a person becomes seriously ill from liver disease.
Who is most likely to get chronic (long-term) hepatitis B?
Age plays a role in whether hepatitis B will become chronic. The younger a person is when infected with the hepatitis B virus, the greater the chance of developing chronic infection. About 9 in 10 infants who become infected go on to develop life-long, chronic infection. The risk goes down as a child gets older. About one in three children who get infected before age 6 will develop chronic hepatitis B. By contrast, almost all children 6 years old and older and adults who get infected with the hepatitis B virus recover completely and do not develop chronic infection.
How common is chronic (long-term) hepatitis B infection in the United States?
There is an estimated 580,000 to 1.17 million people with hepatitis B virus infection in the United States; two-thirds of whom may be unaware of their infection 18. Chronic hepatitis B disproportionately affects people born outside the United States; while accounting for only 14% of the US general population, non–US-born people account for 69% of the US population living with chronic HBV infection 18, 19.
How many deaths can be attributed to chronic (long-term) hepatitis B infection?
During 2020, a total of 1,752 hepatitis B-associated deaths among US residents were reported in the US Multiple Cause of Death data from the National Center for Health Statistics, which corresponds to an age-adjusted death rate of 0.45 cases per 100,000 population 20.
How long does hepatitis B virus survive outside the body?
Hepatitis B virus (HBV) can survive outside the body for at least 7 days 21. During that time, the hepatitis B virus is still capable of causing infection.
What should be used to remove hepatitis B virus from environmental surfaces?
Any blood spills — including dried blood, which can still be infectious — should be cleaned using 1:10 dilution of one part household bleach to 10 parts of water for disinfecting the area. Gloves should be used when cleaning up any blood spills.
Can a person spread the hepatitis B virus and not know it?
Yes. Many people with hepatitis B don’t know they are infected with the hepatitis B virus because they don’t feel or look sick. However, they can still spread the hepatitis B virus to others.
Can the hepatitis B virus be spread through sex?
Yes. The hepatitis B virus can be found in the blood, semen, and other body fluids of an infected person. A person who has sex with an infected partner can become infected with hepatitis B virus.
Can hepatitis B be spread through food?
No. Hepatitis B is not spread through food or water, unlike hepatitis A.
What is the incubation period for hepatitis B?
If symptoms occur, they begin an average of 90 days (range: 60–150 days) after exposure to hepatitis B virus 16, 17.
What should I do if I think I have been exposed to the hepatitis B virus?
If you are concerned that you might have been exposed to the hepatitis B virus, see your health care provider or your local health department immediately. Tell your doctor that you may have been infected and ask if the hepatitis B vaccine and/or a shot called “HBIG” (hepatitis B immune globulin) is right for you. These measures can prevent infection if a person gets treatment as soon as possible, ideally within 24 hours after you think you were exposed.
What is hepatitis B immune globulin (HBIG)?
Hepatitis B immune globulin (HBIg) is a substance made from human blood samples that contains antibodies that help fight against the hepatitis B virus. A doctor or healthcare provider can give this as a shot to people exposed to the hepatitis B virus to protect them from infection.
If I have been infected with the hepatitis B virus in the past, can I get it again?
No. If you have been infected with hepatitis B virus in the past, you can’t get infected again. However, some people who were infected in the past can become sick if the virus “reactivates”. Reactivation is rare but there is a risk of it happening if your immune system is suppressed. This can happen if you are starting chemotherapy, or your doctor puts you on other immune suppressive drugs. If you are going to start any of these treatments let your doctor know if you have had hepatitis B in the past.
Other people, especially those infected during early childhood, remain infected for life because they never cleared the virus from their bodies. These people are considered to have chronic infection and are at risk for developing severe liver disease.
What is the difference between hepatitis A, hepatitis B, and hepatitis C?
Hepatitis A, hepatitis B, and hepatitis C are all liver infections caused by three different viruses. Although each can cause similar symptoms, they are spread in different ways and can affect the liver differently. Hepatitis A is usually a short-term infection. Hepatitis B and hepatitis C can also begin as short-term infections but in some people, the virus remains in the body and causes chronic, or lifelong, infection. There are vaccines to prevent hepatitis A and hepatitis B; but there is no vaccine available for hepatitis C.
What is Hepatitis B virus (HBV) and Human Immunodeficiency virus (HIV) coinfection?
According to the World Health Organization (WHO) about 1% of persons living with hepatitis B virus (HBV) infection (2.7 million people) are also infected with Human Immunodeficiency Virus (HIV) 4. Conversely, the global prevalence of hepatitis B virus (HBV) infection in Human Immunodeficiency Virus (HIV)-infected persons is approximately 10%. This figure may approach 20% in Southeast Asia, and 5% in North America and Western Europe. Worldwide prevalence of hepatitis B virus (HBV) coinfection could be estimated to be 5%–10% in persons living with HIV infection. In the U.S., Western Europe and Australia, the prevalence of chronic hepatitis B was reported to be 5%-14% among HIV-positive individuals.
Since both HIV and the hepatitis B virus share similar transmission routes, it is not surprising that there is a high frequency of coinfection. Sexual activity and/or injection drug use are the most common routes of transmission of the hepatitis B virus among those also infected with HIV.
Conditions associated with hepatitis B and C are currently among the leading causes of hospital admission and death in the HIV-infected population. Therefore, the adequate management of hepatitis B and C is now being considered a priority in HIV-coinfected patients.
There are three main reasons for considering hepatitis B virus (HBV) therapy as a priority in hepatitis B virus (HBV)/HIV coinfected patients:
- First, liver disease may progress more rapidly in those patients co-infected with hepatitis B virus (HBV)/HIV and could lead to serious liver disease complications such as cirrhosis and liver cancer at younger ages.
- Second, there is a higher risk of developing hepatotoxicity following the initiation of antiretroviral therapy in HIV patients co-infected with hepatitis B virus (HBV) than in patients infected with HIV alone.
- Hepatitis B in HIV-infected patients is associated with a lower CD4 T-cell count than HIV-monoinfected people.
There is no evidence that hepatitis B affects HIV disease progression or that hepatitis B alters the response of HIV to antiretroviral therapy (ART). However, starting ART may be associated with an increased risk of liver inflammation in coinfected individuals, as evidenced by ALT (Alanine Aminotransferase) flares or rising liver enzymes. This may reflect both an immune response against hepatitis B and/or drug toxicity.
Since 2015, WHO has recommended treatment for everyone diagnosed with human immunodeficiency virus (HIV) infection, regardless of the stage of disease. Tenofovir, which is included in the treatment combinations recommended in first intention against Human Immunodeficiency Virus (HIV) infection, is also active against hepatitis B virus (HBV).
What is Hepatitis B virus (HBV) and Hepatitis D virus (HDV) coinfection or superinfection?
Hepatitis D and hepatitis B infections may occur together as a coinfection (acquired at the same time) or a superinfection (hepatitis D infection in a patient with chronic hepatitis B infection). People can only become infected with hepatitis D when they also have hepatitis B. Hepatitis D (a member of the delta virus family) has been long associated with hepatitis B virus (HBV) infections and cannot exert pathological influence without the presence of hepatitis B virus (HBV) infection 2. Superinfection tends to be more severe than coinfection. Due to the preexisting hepatitis B infection, anti-HBcAg IgM is undetectable in superinfection states but can be noted in coinfection.
Hepatitis B virus (HBV) and Hepatitis D virus (HDV) Coinfection
A coinfection occurs when you get both hepatitis D and hepatitis B infections at the same time. Coinfections usually cause acute, or short-term, hepatitis D and B infections. Coinfections may cause severe acute hepatitis. In most cases, people are able to recover from and fight off the acute hepatitis D and B infections and the viruses go away. However, in less than 5 percent of people with a coinfection, both infections become chronic and do not go away 22.
Hepatitis B virus (HBV) and Hepatitis D virus (HDV) Superinfection
A superinfection occurs if you already have chronic hepatitis B and then become infected with hepatitis D. When you get a superinfection, you may have severe acute hepatitis symptoms 23. Up to 90 percent of people with a superinfection are not able to fight off the hepatitis D virus and develop chronic hepatitis D 24. As a result, these people will have both chronic hepatitis D and chronic hepatitis B.
What is Hepatitis B virus (HBV) and Hepatitis C virus (HCV) coinfection?
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections account for a substantial proportion of liver diseases worldwide. Because the two viruses share similar modes of transmission, co-infection with the two viruses is not uncommon, though the vast majority of those coinfected with hepatitis C virus (HCV) and hepatitis B virus (HBV) acquired these viruses through intravenous drug use, unscreened blood products, or exposure to dirty needles and unsterilized medical equipment.
The exact number of patients co-infected with hepatitis C virus (HCV) and hepatitis B virus (HBV) is unknown and may be underestimated because the hepatitis C virus can become the “dominant” liver virus, and reduces hepatitis B virus levels to be nearly undetectable 25. In patients with chronic hepatitis B, estimates of the rates of hepatitis C virus (HCV) co-infection vary from 9% to 30% 25. The primary concern with hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infection is that it can lead to more severe liver disease and an increased risk for progression to liver cancer (hepatocellular carcinoma).
Treatment of hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfected patients can represent a challenge. The American Association for the Study of Liver Diseases recommends starting people with hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfection, who meet the criteria for treatment of active hepatitis B virus (HBV) infection, on therapy at the same time or before starting direct-acting antiviral (DAA) for hepatitis C virus (HCV) treatment 26. Patients with low or undetectable hepatitis B virus (HBV) DNA levels should be monitored at regular intervals during hepatitis C treatments. Those requiring treatment for hepatitis B virus (HBV) should be placed on therapy based on the American Association for the Study of Liver Diseases’ hepatitis B virus (HBV) treatment guidelines. Those with hepatitis C virus (HCV) who have resolved the hepatitis B virus (HBV) virus, whether spontaneously resolving the infection or following treatment, should be monitored for hepatitis B virus (HBV) reactivation while on direct-acting antiviral (DAA) therapy.
Pregnancy and Hepatitis B
Babies born to a mother with hepatitis B have a greater than 90% chance of developing chronic hepatitis B if they are not properly treated at birth 27. It is very important that pregnant women know their hepatitis B status in order to prevent passing the virus on to their newborn baby during delivery. If your doctor is aware that you have hepatitis B, he or she can ensure hepatitis B transmission to your baby is prevented by taking the right steps based on blood tests results and to make arrangements to have the proper medications in the delivery room to prevent your baby from being infected.
ALL pregnant women should be tested for hepatitis B 27. Testing is especially important for women who fall into high-risk groups such as health care workers, women from ethnic communities or countries where hepatitis B is common, spouses or partners living with an infected person, etc. If you are pregnant, be sure your doctor tests you for hepatitis B before your baby is born, ideally as early as possible during the first trimester.
If you test positive for hepatitis B infection, then your newborn must be given proper prevention immediately in the delivery room, clinic or bedside:
- first dose (called “birth dose”) of the hepatitis B vaccine
- one dose of the Hepatitis B Immune Globulin (HBIG).*
* Note: Hepatitis B Immune Globulin (HBIG) is recommended by U.S. CDC. HBIG is not recommended by WHO and may not be available in all countries. What is most important is to make sure the hepatitis B vaccine birth dose is given as soon as possible!
If these two medications are given correctly, a newborn born to a mother with hepatitis B has more than a 90% chance of being protected from a hepatitis B infection. You must make sure your baby receives the remaining shots of the vaccine series according to schedule to ensure complete protection.
Although the CDC states that the medications can be given within the first 12 hours of life and the WHO states vaccine birth dose can be given within 24 hours, there is no second chance to protect an infant once this window of opportunity is missed 27. Therefore, the Hepatitis B Foundation strongly recommends that health care professionals properly administer the birth dose of the hepatitis B vaccine immediately in the delivery room to avoid any delays or mistakes 27.
If you test positive for hepatitis B infection while pregnant, your doctor also should do a hepatitis B viral load blood test (hepatitis B virus (HBV) DNA) during your pregnancy. In some cases, the laboratory test results may show a very high viral load. In these cases, your physician may recommend that you take an oral antiviral drug in your third trimester to reduce the risk of infecting your newborn at birth. If the hepatitis B viral load test is not available, WHO recommends that pregnant women are tested for the hepatitis B e-antigen (HBeAg), and if positive, an antiviral is recommended during the last trimester. Regardless of viral load levels or HBeAg status, the hepatitis B vaccine birth dose and completion of the vaccine series is essential to protect your infant from infection with the hepatitis B virus.
Should I be screened for hepatitis B?
Screening is testing for a disease in people who have no symptoms. Doctors use blood tests to screen for hepatitis B. Many people who have hepatitis B don’t have symptoms and don’t know they are infected with hepatitis B. Screening tests can help doctors diagnose and treat hepatitis B, which can lower your chances of developing serious health problems.
Your doctor may recommend screening for hepatitis B if you 28:
- are pregnant
- were born in an area of the world where 2 percent or more of the population has hepatitis B infection, which includes Africa, Asia, and parts of the Middle East, Eastern Europe, and South America
- didn’t receive the hepatitis B vaccine as an infant and have parents who were born in an area where 8 percent or more of the population had hepatitis B infection, which includes sub-Saharan Africa and parts of Asia
- are HIV-positive
- have injected drugs
- are a man who has sex with men
- have lived with or had sex with a person who has hepatitis B
- have an increased chance of infection due to other factors
What is Acute Hepatitis B vs. Chronic Hepatitis B?
Hepatitis B infection may be either short-lived (Acute) or long lasting (Chronic).
- Acute hepatitis B infection lasts less than six months. Your immune system likely can clear acute hepatitis B from your body, and you should recover completely within a few months. Most people who get hepatitis B as adults have an acute infection, but it can lead to chronic infection.
- Chronic hepatitis B infection lasts six months or longer. It lingers because your immune system can’t fight off the infection. Chronic hepatitis B infection may last a lifetime, possibly leading to serious illnesses such as cirrhosis, liver failure and liver cancer.
The younger you are when you get hepatitis B — particularly newborns or children younger than 5 — the higher your risk of hepatitis B infection becoming chronic. Chronic hepatitis B infection may go undetected for decades until a person becomes seriously ill from liver disease.
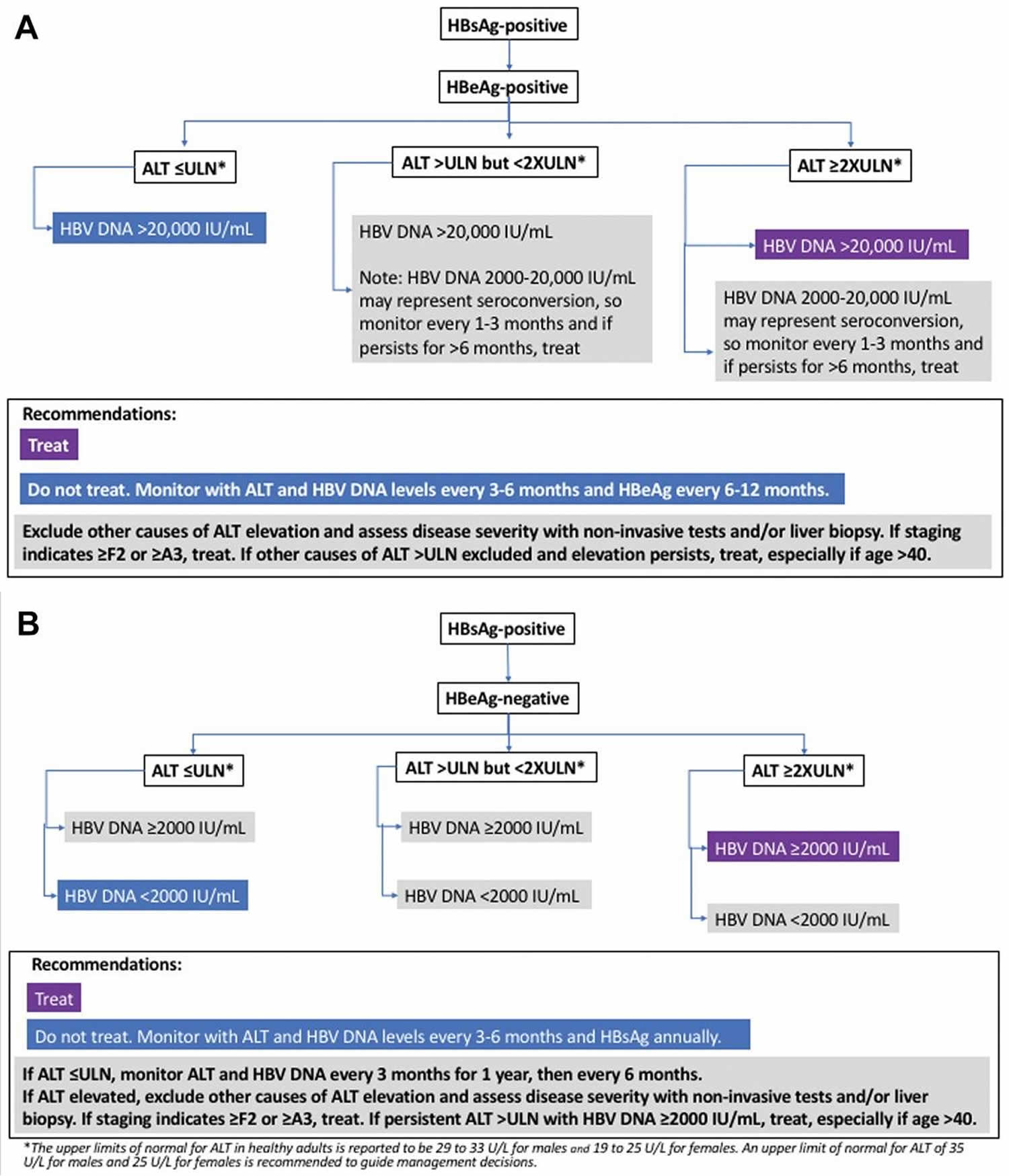
Figure 1. Chronic hepatitis B diagnostic and treatment algorithm
[Source 29 ]How serious is Acute hepatitis B infection ?
Acute hepatitis B is a short-term infection. Acute hepatitis B infection ranges from asymptomatic or mild disease to — rarely — fulminant hepatitis. Disease is more severe among adults aged >60 years. Some people have symptoms, which may last several weeks. In some cases, symptoms last up to 6 months. Sometimes the body is able to fight off the infection and the hepatitis B virus goes away. If the body isn’t able to fight off the hepatitis B virus, the virus does not go away, and chronic hepatitis B infection occurs.
Most healthy adults and children older than 5 years who have hepatitis B get better and do not develop a chronic hepatitis B infection 7. The fatality rate among acute hepatitis B cases reported to the Centers for Disease Control and Prevention (CDC) is 0.5%–1%.
How serious is Chronic hepatitis B virus (HBV) infection?
Chronic hepatitis B is a long-lasting infection. Your chance of developing chronic hepatitis B is greater if you were infected with the virus as a young child. About 90 percent of infants infected with hepatitis B develop a chronic infection. Approximately 25 to 50 percent of those who become chronically infected with hepatitis B virus during childhood (between the ages of 1 and 5 years) and 15% of those who become chronically infected after childhood die prematurely from cirrhosis or liver cancer, and the majority remain asymptomatic until onset of cirrhosis or end-stage liver disease. In the United States, chronic hepatitis B virus (HBV) infection results in an estimated 1,800 deaths per year. In 2018, a total of 1,649 U.S. death certificates had hepatitis B virus (HBV) recorded as an underlying or contributing cause of death 30. However, this is a conservative estimate.
How likely is hepatitis B virus (HBV) infection to become chronic?
The risk for chronic infection varies according to the age at infection and is greatest among young children. Approximately 90% of infants and 25%–50% of children aged 1–5 years will remain chronically infected with hepatitis B virus (HBV). By contrast, approximately 95% of adults recover completely from hepatitis B virus (HBV) infection and do not become chronically infected.
How many new hepatitis B virus infections occur annually in the United States?
In 2014, a total of 2,953 cases of acute hepatitis B were reported from 48 states to the Centers for Disease Control and Prevention (CDC). The overall incidence rate for 2014 was 0.9 cases per 100,000 population. After adjusting for under-ascertainment and under-reporting, an estimated 19,200 acute hepatitis B cases occurred in 2014. In 2017, the annual number of hepatitis B infections rose in some states 14. Experts think the rise was related to increases in injection drug use. Injection drug use increases the risk of hepatitis B infection.
U.S. Statistics
- Around 60,000 new cases of hepatitis B virus (HBV) infection annually 31
- 2 million or more people with chronic hepatitis B infection 31
- Prevalence is higher in black, Hispanic, and Asian populations compared to whites 32
- Prevalence is lower in people less than 12 years of age born in the U.S.
- Accounts for 5% to 10% of chronic end-stage liver disease and 10% to 15% of cases of hepatocellular cancer (liver cancer)
- Causes 5000 deaths annually
High-risk groups for hepatitis B virus (HBV) infection include intravenous drug users, infants born to infected mothers, males who have sexual intercourse with other males, hemodialysis patients (and workers), healthcare workers, household contacts of known patients with chronic hepatitis B virus (HBV). A majority of the global hepatitis B virus (HBV) disease burden is primarily through vertical transmission.
Figure 2. Acute Hepatitis B virus (HBV) incidence rate
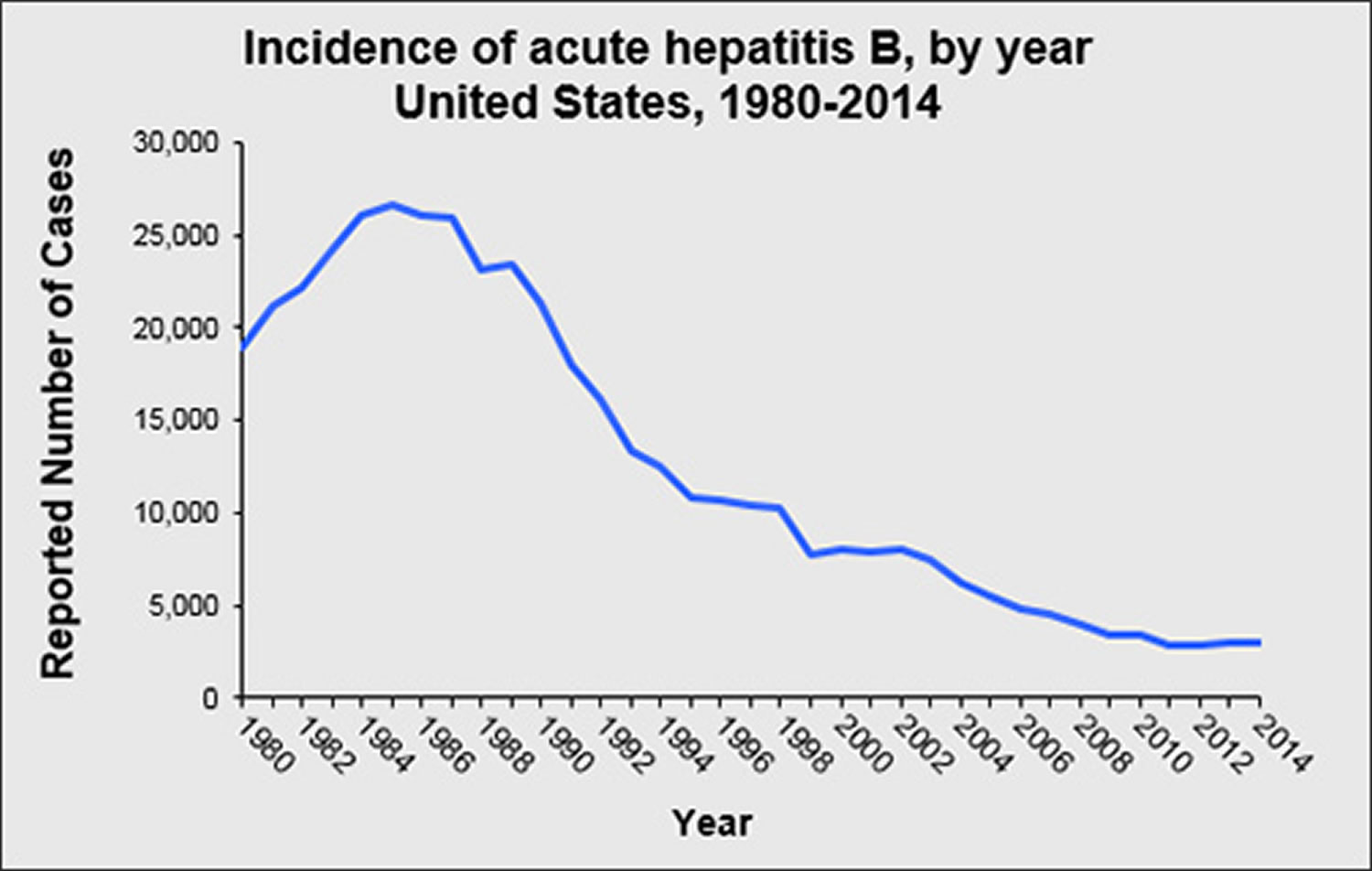
Has the rate of new hepatitis B virus infections in the United States declined ?
The rate of new hepatitis B virus infections has declined by approximately 82% since 1991, when a national strategy to eliminate hepatitis B virus infection was implemented in the United States. The decline has been greatest among children born since 1991, when routine vaccination of children was first recommended.
How common is Chronic Hepatitis B virus infection in the United States ?
An estimated 850,000–2.2 million persons in the United States have chronic hepatitis B virus infection. In 2016, an estimated 862,000 people were living with hepatitis B virus (HBV) infection 33. Chronic infection is an even greater problem globally, affecting approximately 240 million persons 34. An estimated 786,000 persons worldwide die from hepatitis B virus -related liver disease each year 35.
How many deaths can be attributed to chronic hepatitis B virus infection?
In 2018, a total of 1,649 U.S. death certificates had hepatitis B virus (HBV) recorded as an underlying or contributing cause of death 30. However, this is a conservative estimate.
What causes hepatitis B?
The hepatitis B virus (HBV) causes hepatitis B. The hepatitis B virus (HBV) spreads through contact with an infected person’s blood, semen, or other body fluids. Contact can occur by:
- being born to a mother with hepatitis B
- having unprotected sex with an infected person
- sharing drug needles or other drug materials with an infected person
- getting an accidental stick with a needle that was used on an infected person
- being tattooed or pierced with tools that were used on an infected person and weren’t properly sterilized, or cleaned in a way that destroys all viruses and other microbes
- having contact with the blood or open sores of an infected person
- using an infected person’s razor, toothbrush, or nail clippers
You can’t get hepatitis B from:
- being coughed on or sneezed on by an infected person
- drinking unclean water or untreated water that has not been boiled
- eating food that is unclean or has not been properly cooked
- hugging an infected person
- shaking hands or holding hands with an infected person
- sharing spoons, forks, and other eating utensils
- sitting next to an infected person
Mothers who have hepatitis B can safely breastfeed their babies. If a baby receives hepatitis B immune globulin (HBIg) and starts receiving the hepatitis B vaccine to prevent hepatitis B infection shortly after birth, hepatitis B is unlikely to spread from mother to child through breastfeeding 36.
Who is at risk for hepatitis B virus infection?
The following populations are at increased risk of becoming infected with hepatitis B virus:
- Infants born to infected mothers
- Sex partners of infected persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Men who have sex with men
- Injection drug users
- Household contacts of persons with chronic hepatitis B virus infection
- Health care and public safety workers at risk for occupational exposure to blood or blood-contaminated body fluids
- Hemodialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to countries with intermediate or high prevalence of hepatitis B virus infection
How is Hepatitis B spread?
Hepatitis B is spread when blood, semen, or other body fluids – even in microscopic amounts — from a person infected with the hepatitis B virus enters the body of someone who is not infected. Hepatitis B virus (HBV) is transmitted through activities that involve percutaneous (i.e., puncture through the skin) or mucosal contact with infectious blood and various body fluids, menstrual, vaginal, and seminal fluids. Major modes of transmission for hepatitis B virus are as follows:
- Sex with an infected partner. Sexual contact includes unprotected intercourse (vaginal, oral, or anal) and mucosal contact involves any contact involving an infected patient’s saliva, vaginal secretion, semen, and blood.
- Injection drug use that involves sharing needles, syringes, or drug-preparation equipment
- Birth to an infected mother (also known as vertical transmission). Pregnant women infected with hepatitis B virus (HBV) can pass the virus to their babies during childbirth 37. However, the newborn can be vaccinated to avoid getting infected in almost all cases. Talk to your doctor about being tested for hepatitis B if you are pregnant or want to become pregnant.
- Contact with blood or open sores of an infected person
- Needle sticks or sharp instrument exposures
- Sharing items such as razors or toothbrushes with an infected person
Sexual transmission of hepatitis B may occur, particularly in unvaccinated men who have sex with men and heterosexual persons with multiple sex partners or contact with sex workers.
Although the hepatitis B virus (HBV) can be found in saliva, it is not spread through kissing or sharing utensils. Hepatitis B is not spread through sneezing, coughing, hugging, breastfeeding or through food or water.
Is it safe for the hepatitis B virus (HBV)-positive mother to breastfeed if her nipples are cracked and bleeding?
Data are insufficient to say yes or no. However, hepatitis B virus (HBV) is spread by infected blood. Therefore, if the hepatitis B virus (HBV)-positive mother’s nipples and/or surrounding areola are cracked and bleeding, she should stop nursing temporarily. To maintain her milk supply while not breastfeeding, she can express and discard her breast milk until her nipples are healed. Once her nipples are no longer cracked or bleeding, the hepatitis B virus (HBV)-positive mother may fully resume breastfeeding. Providers may need to refer mothers for lactation support to learn how to maintain milk production and how to supplement with pasteurized donor human milk or formula while temporarily not breastfeeding.
Are international travelers at risk for hepatitis B virus infection?
The risk for hepatitis B virus infection in international travelers is generally low, except for certain travelers to regions where the prevalence of chronic hepatitis B virus infection is high or intermediate (i.e., hepatitis B surface antigen prevalence of ≥2%). Hepatitis B vaccination should be administered to unvaccinated persons traveling to those countries.
More information about hepatitis B and travel is available from CDC’s Travelers’ Health site 38.
Prevention of Hepatitis B virus (HBV) infection
The best way to prevent hepatitis B is by getting vaccinated. The hepatitis B vaccine is typically given as two injections separated by a month or three or four injections over six months, depending on which vaccine is given. You can’t get hepatitis B from the vaccine. You need to get all shots in the series to be fully protected. The Advisory Committee on Immunization Practices (ACIP) recommends hepatitis B (HepB) vaccination among all adults aged 19–59 years and adults >60 years with risk factors for hepatitis B or without identified risk factors but seeking protection 39. Talk to your health care provider or local health department about getting vaccinated. Some clinics offer free or low-cost hepatitis B vaccines.
The hepatitis B vaccine is recommended for:
- All infants
- All children and adolescents younger than 19 years of age who have not been vaccinated
- Adults aged 19 through 59 years
- Adults aged 60 years and older with risk factors for hepatitis B
- Those who work or live in a center for people who are developmentally disabled
- People who live with someone who has hepatitis B
- Health care workers, emergency workers and other people who come into contact with blood
- Anyone who has a sexually transmitted infection, including HIV
- Men who have sex with men
- People who have multiple sexual partners
- Sexual partners of someone who has hepatitis B
- People who inject illegal drugs or share needles and syringes
- People with chronic liver disease
- People with end-stage kidney disease
- Travelers planning to go to an area of the world with a high hepatitis B infection rate
Hepatitis B vaccines licensed for use in the United States
There are three single-antigen vaccines, one three-antigen vaccine, and three combination vaccines currently licensed in the United States.
- Single-antigen hepatitis B vaccines
- Engerix-B
- Recombivax HB
- Heplisav-B
- Three-antigen hepatitis B vaccines
- PreHevbrio (13)
- Combination vaccines
- Pediarix: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine
- Twinrix: Combined hepatitis A and hepatitis B vaccine
- Vaxelis: Combined DTaP, IPV, Haemophilus influenzae type b, and hepatitis B vaccine
Hepatitis B vaccination schedule most often used for children and adults is three intramuscular injections, the second and third doses administered at 1 and 6 months, respectively, after the first dose. Alternate hepatitis B vaccination schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved for two doses, 1 month apart.
Table 1. Recommended doses of currently licensed hepatitis B vaccine, by age group and vaccine type
| Hepatitis B Vaccine*, Age Group (yrs) | Dose (µg) | Vol (mL) | Schedule |
|---|---|---|---|
| Recombivax HB | |||
| Infants (<1 yr) | 5 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–10 yrs) | 5 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Adolescents (11–19 yrs) † | 5 | 0.5 | 3 doses at 0, 1, 6 mos† |
| Adults (≥20 yrs) | 10 | 1 | |
| Patients on hemodialysis and other immune-compromised persons, <20 yrs§ | 5 | 0.5 | |
| Patients on hemodialysis and other immune-compromised persons, ≥20 yrs | 40 | 1 | |
| Engerix-B | |||
| Infants (<1 yr) | 10 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–10 yrs) | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Adolescents (11–19yrs) | 10 | 0.5 | 3 doses at 0, 1, 6 mos |
| Adults (≥20 yrs) | 20 | 1 | |
| Patients on hemodialysis and other immune-compromised persons, <20 yrs§ | 10 | 0.5 | |
| Patients on hemodialysis and other immune-compromised persons, ≥20 yrs | 40 | 2 | 4 doses at 0, 1, 2, 6 mos ¶ |
| Heplisav-B | |||
| Adults (≥18 yrs**) | 20 | 0.5 | 2 doses at 0 and 1 mos |
| PreHevbrio (FDA-approved in 2021) | |||
| Adults (≥18 yrs**) | 10 | 1 | 3 doses at 0, 1, 6 mos |
| Pediarix (combination hepatitis B, diphtheria, tetanus, acellular pertussis, and inactivated poliovirus) | |||
| Infants (<1 yr) | 10 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–6 yrs) †† | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Vaxelis (combination diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, and hepatitis B) | |||
| Infants (<1 yr) | 10 | 0.5 | 3 doses at age 0, 1–2, 6–18 mos |
| Children (1–4 yrs) §§ | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Twinrix (combination hepatitis A-hepatitis B) ¶¶ | |||
| Adults (≥18 yrs) | 20 | 1 | 3 doses at 0, 1, 6 mos (standard) or 4 doses at 0, 7d, 21–30 d, 12 mos (accelerated) |
Footnotes:
* Refer to package inserts for further information. For all ages, when the hepatitis B vaccine schedule is interrupted, the vaccine series does not need to be restarted. If a 3-dose series is interrupted after the first dose, the second dose should be administered as soon as possible, and the second and third doses should be separated by an interval of at least 8 weeks. If only the third dose has been delayed, it should be administered as soon as possible. The final dose of a 3-dose series vaccine must be administered at least 8 weeks after the second dose and should follow the first dose by at least 16 weeks; the minimum interval between the first and second doses is 4 weeks. Inadequate doses of hepatitis B vaccine or doses received after a shorter-than-recommended dosing interval should be readministered, using the correct dosage or schedule. Vaccine doses administered ≤4 days before the minimum interval or age are considered valid. Because of the unique accelerated schedule for Twinrix, the 4-day guideline does not apply to the first three doses of this vaccine when administered on a 0-day, 7-day, 21–30-day, and 12-month schedule. PreHevbrio is a three-antigen HepB vaccine that was FDA approved in 2021.
† A 2-dose schedule of Recombivax HB adult formulation (10 µg) is licensed for adolescents aged 11 through 15 years. When scheduled to receive the second dose, adolescents aged 16 years or older should be switched to a 3-dose series, with doses 2 and 3 consisting of the pediatric formulation administered on an appropriate schedule.
§ Higher doses might be more immunogenic, but no specific recommendations have been made.
¶ Engerix-B for adults on hemodialysis: Administer series of 4 doses (2 mL each) as a single 2-mL dose or as two 1-mL doses on a 0-, 1-, 2-, 6-month schedule. Recombivax HB for adults on hemodialysis is a 3-dose series.
** Data on Heplisav-B and PreHevbrio are currently insufficient to inform vaccine-associated risks in pregnancy. Thus, providers should vaccinate pregnant people needing HepB vaccination with Engerix-B, Recombivax HB, or Twinrix.
†† Pediarix cannot be administered at birth, before age 6 weeks, or at age ≥7 years.
§§ Vaxelis is approved for use as a 3-dose series in children 6 weeks through 4 years of age.
¶¶ Twinrix is recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections.
[Source 40 ]Take precautions to avoid Hepatitis B virus (HBV)
Other ways to reduce your risk of Hepatitis B virus (HBV) include:
- Know the Hepatitis B virus (HBV) status of any sexual partner. Don’t engage in unprotected sex unless you’re absolutely certain your partner isn’t infected with hepatitis B virus or any other sexually transmitted infection.
- Use a new latex or polyurethane condom every time you have sex if you don’t know the health status of your partner. Remember that although condoms can reduce your risk of contracting hepatitis B virus, they don’t eliminate the risk.
- Don’t use illegal drugs. If you use illicit drugs, get help to stop. If you can’t stop, use a sterile needle each time you inject illicit drugs. Never share needles.
- Be cautious about body piercing and tattooing. If you get a piercing or tattoo, look for a reputable shop. Ask about how the equipment is cleaned. Make sure the employees use sterile needles. If you can’t get answers, look for another shop.
- Ask about the hepatitis B vaccine before you travel. If you’re traveling to a region where hepatitis B is common, ask your doctor about the hepatitis B vaccine in advance. It’s usually given in a series of three injections over a six-month period.
Screening healthy people for hepatitis B
Doctors sometimes test certain healthy people for hepatitis B infection because the virus can damage the liver before causing signs and symptoms. Talk to your doctor about screening for hepatitis B infection if you:
- Are pregnant
- Live with someone who has hepatitis B
- Have had many sexual partners
- Have had sex with someone who has hepatitis B
- Are a man who has sex with men
- Have a history of a sexually transmitted illness
- Have HIV or hepatitis C
- Have a liver enzyme test with unexplained abnormal results
- Receive kidney dialysis
- Take medications that suppress the immune system, such as those used to prevent rejection after an organ transplant
- Use illegal injected drugs
- Are in prison
- Were born in a country where hepatitis B is common, including Asia, the Pacific Islands, Africa and Eastern Europe
- Have parents or adopted children from places where hepatitis B is common, including Asia, the Pacific Islands, Africa and Eastern Europe
The CDC recommends the following screening practices for hepatitis B infection 41:
- Adults: Clinicians should screen all adults aged 18 years and older for hepatitis B virus infection at least once during their lifetime using the triple panel test which includes hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (anti-HBc).
- Infants: Clinicians should test all infants born to pregnant people who are HBsAg positive or have other evidence of hepatitis B virus infection for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B surface antigen (anti-HBs) seromarkers, at 9-12 months of age or 1-2 months after vaccine series completion if the series is delayed.
- Pregnant people: Clinicians should screen all pregnant people for hepatitis B surface antigen (HBsAg) during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing 42. Pregnant people with a history of appropriately timed triple panel screening and without subsequent risk for exposure to hepatitis B virus (i.e., no new hepatitis B virus exposures since triple panel screening) only need HBsAg screening.
Hepatitis B serologic test results:
- Hepatitis B surface antigen (HBsAg): A protein on the surface of hepatitis B virus that can be detected in high levels in serum during acute or chronic hepatitis B virus infection. The presence of HBsAg indicates that the person is infectious, except when it might be transiently positive within 30 days after a dose of hepatitis B vaccine (HepB). The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make HepB vaccine.
- Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from hepatitis B virus infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B. Among vaccine responders who completed a vaccine series, anti-HBs levels can decline over time, however the majority are still immune and will mount a response when exposed to HBV.
- Total antibody to hepatitis B core antigen (anti-HBc): Appears at the onset of symptoms in acute hepatitis B, is a measure of both IgM and IgG, and persists for life. The presence of total anti-HBc indicates previous or ongoing infection with hepatitis B virus in an undefined time frame. People who have immunity to hepatitis B from a vaccine do not develop anti-HBc.
- IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with hepatitis B virus (<6 months). Its presence indicates acute infection. IgM anti-HBc should be ordered only when acute HBV infection is a concern.
Table 2. Hepatitis B serologic test results interpretation
| Test and Result | Interpretation |
|---|---|
| HBsAg—Positive Total anti-HBc — Positive IgM anti-HBc — Positive Anti-HBs — Negative | Acute infection |
| HBsAg — Positive Total anti-HBc — Positive IgM anti-HBc — Negative 1 Anti-HBs — Negative | Chronic Infection |
| HBsAg — Negative Total anti-HBc — Positive Anti-HBs — Positive | Resolved Infection |
| HBsAg — Negative Total anti-HBc — Negative Anti-HBs — Positive 2 | Immune from receipt of prior vaccination (if documented complete series) |
| HBsAg — Negative Total anti-HBc — Positive Anti-HBs — Negative | Only core antibody is positive. See possible interpretations and corresponding actions: |
| Resolved infection where anti-HBs levels have waned | |
| Occult Infection | |
| Passive transfer of anti-HBc to an infant born to an HBsAg-positive gestational parent | |
| A false positive, thus patient is susceptible | |
| A mutant HBsAg strain that is not detectable by laboratory assay | |
| HBsAg — Negative Total anti-HBc — Negative Anti-HBs — Negative 3 | Susceptible, never infected (if no documentation of HepB vaccine series completion) |
Footnotes:
1 IgM anti-HBc also might be positive in persons with chronic infection during severe HBV infection flares or reactivation.
2 Immune if anti-HBs concentration is >10 mIU/mL after vaccine series completion.
3 Anti-HBs concentrations might wane over time among vaccine responders. People with a documented, complete HepB vaccine series typically do not need to be revaccinated, except for special populations like patients on hemodialysis or health care personnel.
[Source 43 ]Hepatitis B signs and symptoms
The presence of hepatitis B signs and symptoms varies by age. Most children under age 5 years and newly infected immunosuppressed adults are asymptomatic, whereas 30%–50% of persons aged ≥5 years have initial signs and symptoms. Some people with acute hepatitis B have symptoms 2 to 5 months after they come in contact with the virus 7.
When present, hepatitis B signs and symptoms can include:
- Fever
- Fatigue or feeling tired
- Loss of appetite
- Nausea
- Vomiting
- Abdominal pain
- Dark yellow urine
- Gray- or clay-colored stools
- Joint pain
- Jaundice: yellowing of your skin and the whites of your eyes
Persons with chronic hepatitis B virus infection might be asymptomatic, have no evidence of liver disease, or have a spectrum of disease ranging from chronic hepatitis to cirrhosis or hepatocellular carcinoma (a type of liver cancer). For this reason, hepatitis B screening is important, even if you have no symptoms.
Hepatitis B complications
Unlike hepatitis A and hepatitis E, in which there is no chronic state, hepatitis B virus (HBV) infection has the potential for the development of chronic hepatitis B. Chronic hepatitis B predisposes a patient to the development of portal hypertension, cirrhosis, and its complications or hepatocellular carcinoma (liver cancer). As such, patients with hepatitis B virus (HBV) infection should be monitored closely, and a referral to a specialist is highly recommended. Fulminant liver failure from hepatitis B virus (HBV) infection requires an emergency liver transplant evaluation at a liver transplant center.
Acute hepatitis B complications
In rare cases, acute hepatitis B can lead to acute liver failure, a condition in which the liver fails suddenly. People with acute liver failure may require a liver transplant.
Chronic hepatitis B complications
Chronic hepatitis B can lead to:
- Cirrhosis, a condition in which scar tissue replaces healthy liver tissue and prevents your liver from working normally. The inflammation associated with a hepatitis B infection can lead to extensive liver scarring (cirrhosis), which may impair the liver’s ability to function. Scar tissue also partly blocks the flow of blood through the liver. As cirrhosis gets worse, the liver begins to fail.
- Liver failure, in which your liver is badly damaged and stops working. Liver failure is also called end-stage liver disease. People with liver failure may require a liver transplant to stay alive.
- Liver cancer. People with chronic hepatitis B infection have an increased risk of liver cancer. Your doctor may suggest blood tests and an ultrasound or another type of imaging test to check for liver cancer. Finding cancer at an early stage improves the chance of curing the cancer.
- Reactivation of the hepatitis B virus. People with chronic hepatitis B who have suppression of their immune system are prone to reactivation of the hepatitis B virus. This can lead to significant liver damage or even liver failure. This includes people on immunosuppressive medications, such as high-dose corticosteroids or chemotherapy. Before taking these medications, you should be tested for hepatitis B. If you test positive for hepatitis B, you should be seen by a liver specialist (hepatologist) before starting these therapies.
- Other conditions. People with chronic hepatitis B may develop kidney disease or inflammation of blood vessels.
Reactivated hepatitis B
In people who have ever had hepatitis B, the virus may become active again, or reactivated, later in life. When hepatitis B is reactivated, it may start to damage the liver and cause symptoms. Reactivated hepatitis B can lead to acute liver failure.
People at risk for reactivated hepatitis B include those who:
- take medicines that reduce the activity of the immune system, such as:
- chemotherapy to treat cancer
- medicines prescribed to treat conditions that involve the immune system, such as inflammatory bowel disease, rheumatoid arthritis, and psoriasis
- medicines prescribed for people receiving an organ transplant or bone marrow transplant
- corticosteroids, if taken for more than a few weeks
- take hepatitis C medicines
- have HIV infection
Doctors may test for current or past hepatitis B infection in people at risk for reactivated hepatitis B.
Hepatitis B virus (HBV) Diagnosis
Your doctor will examine you and look for signs of liver damage, such as yellowing skin or belly pain. Tests that can help diagnose hepatitis B or its complications are:
- Blood tests and Hepatitis Serology. Blood tests can detect signs of the hepatitis B virus in your body and tell your doctor whether it’s acute or chronic. A simple blood test can also determine if you’re immune to the condition.
- Liver ultrasound. A special ultrasound called transient elastography can show the amount of liver damage.
- Liver biopsy. Your doctor might remove a small sample of your liver for testing (liver biopsy) to check for liver damage. During this test, your doctor inserts a thin needle through your skin and into your liver and removes a tissue sample for laboratory analysis.
Laboratory diagnosis of hepatitis B infection focuses on the detection of the hepatitis B surface antigen (HBsAg).
- Acute hepatitis B virus infection is characterized by the presence of hepatitis B surface antigen (HBsAg) and immunoglobulin M (IgM) antibody to the core antigen, HBcAg (anti-HBc IgM). During the initial phase of infection, patients are also seropositive for hepatitis B e antigen (HBeAg). Hepatitis B e antigen (HBeAg) is usually a marker of high levels of replication of the virus. The presence of HBeAg indicates that the blood and body fluids of the infected individual are highly infectious.
- Chronic hepatitis B virus infection is characterized by the persistence of hepatitis B surface antigen (HBsAg) for at least 6 months (with or without concurrent hepatitis B e antigen [HBeAg]). Persistence of hepatitis B surface antigen (HBsAg) is the principal marker of risk for developing chronic liver disease and liver cancer (hepatocellular carcinoma) later in life.
How long does it take for blood to test HBsAg-positive after exposure to Hepatitis B virus (HBV)?
Hepatitis B surface antigen (HBsAg) will be detected in an infected person’s blood an average of 4 weeks (range: 1–9 weeks) after exposure to the virus. About 1 of 2 patients will no longer be infectious by 7 weeks after onset of symptoms and all patients who do not remain chronically infected will be HBsAg-negative by 15 weeks after onset of symptoms.
Hepatitis B blood tests
Hepatitis B blood test includes three parts. All three test results are needed to fully understand whether a person is infected or not. Below is an explanation of the 3-part “Hepatitis B Panel” of blood test results.
- Hepatitis B surface antigen (HBsAg) – A “positive” or “reactive” hepatitis B surface antigen (HBsAg) test result means that the person is infected with hepatitis B. This test can detect the actual presence of the hepatitis B virus (called the “surface antigen”) in your blood. If a person tests “positive,” then further testing is needed to determine if this is a new “acute” infection or a “chronic” hepatitis B infection. A positive HBsAg test result means that you are infected and can spread the hepatitis B virus to others through your blood.
- Hepatitis B surface antibody (anti-HBs or HBsAb) – A “positive” or “reactive” hepatitis B surface antibody (anti-HBs or HBsAb) test result indicates that a person is protected against the hepatitis B virus. This protection can be the result of receiving the hepatitis B vaccine or successfully recovering from a past hepatitis B infection. This test is not routinely included in blood bank screenings. A positive hepatitis B surface antibody (anti-HBs or HBsAb) test result means you are “immune” and protected against the hepatitis B virus and cannot be infected. You are not infected and cannot spread hepatitis B to others.
- Hepatitis B core antibody (anti-HBc or HBcAb) – A “positive” or “reactive” hepatitis B core antibody (anti-HBc or HBcAb) test result indicates a past or current hepatitis B infection. The core antibody does not provide any protection against the hepatitis B virus (unlike the surface antibody described above). This test can only be fully understood by knowing the results of the first two tests ( hepatitis B surface antigen [HBsAg] and hepatitis B surface antibody [anti-HBs or HBsAb]). A positive hepatitis B core antibody (anti-HBc or HBcAb) test result requires talking to your health care provider for a complete explanation of your hepatitis B status.
The hepatitis B virus specifically attacks the liver, so health care providers will order blood tests to monitor the health of your liver. Some of the most common liver related blood tests are described below.
These blood tests measure potential liver damage (or liver inflammation). If a person is infected with the hepatitis B virus, the liver cells can be injured by the virus and then the liver enzymes can leak into the bloodstream. The higher the number, the greater the risk of potential liver damage.
- ALT (alanine aminotransferase) is found almost exclusively in the liver and is monitored most closely in a chronic hepatitis B infection. This test is useful in deciding whether a patient would benefit from treatment or for evaluating how well a person is responding to therapy. The upper limits of normal for ALT in healthy adults is 35 U/L for men and 25 U/L for women.
- AST (aspartate aminotransferase) is found in the liver, heart and muscle so is less accurate than the ALT in measuring liver damage. But this enzyme is often ordered to help monitor potential liver damage from the hepatitis B virus.
- AFP (Alpha-FetoProtein) – This is a normal protein produced in the developing fetus, thus, pregnant women will have elevated AFP. Other adults, however, should not have elevated AFP in their blood. This test is used to screen for primary liver cancer patients with chronic hepatitis B. Patients should have their AFP levels monitored at every visit since hepatitis B is the leading cause of liver cancer. If the AFP level is high, the health care provider will order more blood tests and imaging studies.
- Ferritin – Iron is stored in the liver in the form of ferritin. Increased levels of ferritin indicate that a high level of iron is being stored. This could result from an increased iron intake in the diet (vitamin supplements, food cooked in iron pots, etc.). For people living with chronic hepatitis B, a high level can indicate liver damage since ferritin is leaked into the bloodstream as liver cells are injured by the virus.
Diagnostic Criteria and Definitions for Chronic Hepatitis B
American Association for the Study of Liver Diseases (AASLD) Diagnostic Criteria and Definitions for Chronic Hepatitis B 29:
Chronic hepatitis B (CHB)
- HBsAg present for ≥6 months
- Serum HBV DNA varies from undetectable to several billion IU/mL
- Subdivided into HBeAg positive and negative. HBV DNA levels are typically >20,000 IU/mL in HBeAg-positive chronic hepatitis B, and lower values (2000–20,000 IU/mL) are often seen in HBeAg-negative chronic hepatitis B
- Normal or elevated ALT and/or AST levels
- Liver biopsy results showing chronic hepatitis with variable necroinflammation and/or fibrosis
Immune-Tolerant chronic hepatitis B
- HBsAg present for ≥6 months
- HBeAg positive
- HBV DNA levels are very high (typically >1 million IU/mL)
- Normal or minimally elevated ALT and/or AST
- Liver biopsy or noninvasive test results showing no fibrosis and minimal inflammation
Immune-Active chronic hepatitis B
- HBsAg present for ≥6 months
- Serum HBV DNA >20,000 IU/mL in HBeAg-positive chronic hepatitis B and >2000 IU/mL in HBeAg-negative chronic hepatitis B
- Intermittently or persistently elevated ALT and/or AST levels
- Liver biopsy or noninvasive test results showing chronic hepatitis with moderate or severe necroinflammation and with or without fibrosis
Inactive chronic hepatitis B
- HBsAg present for ≥6 months
- HBeAg negative, anti-HBe positive
- Serum HBV DNA <2000 IU/mL
- Persistently normal ALT and/or AST levels
- Liver biopsy confirms absence of significant necroinflammation. Biopsy or noninvasive testing show variable levels of fibrosis
Other Definitions
- HBV reactivation: loss of HBV immune control in HBsAg-positive, anti-HBc–positive or HBsAg-negative, anti-HBc–positive patients receiving immunosuppressive therapy for a concomitant medical condition; a rise in HBV DNA compared to baseline (or an absolute level of HBV DNA when a baseline is unavailable); and reverse seroconversion (seroreversion) from HBsAg-negative to HBsAg-positive for HBsAg-negative, anti-HBc–positive patients
- Hepatitis flare: ALT increase ≥3 times baseline and >100 U/L
- HBV-associated hepatitis: HBV reactivation and hepatitis flare
- HBeAg clearance: loss of HBeAg in a person who was previously HBeAg positive
- HBeAg seroconversion: loss of HBeAg and detection of anti-HBe in a person who was previously HBeAg positive and anti-HBe negative
- HBeAg seroreversion: reappearance of HBeAg in a person who was previously HBeAg negative
- Resolved chronic hepatitis B: sustained loss of HBsAg in person who was previously HBsAg positive, with undetectable HBV DNA levels and absence of clinical or histologic evidence of active viral infection
- Virologic breakthrough: >1 log10 (10-fold) increase in serum HBV DNA from nadir during treatment in a patient who had an initial virologic response and who is adherent
Hepatitis B Serology
What do the different hepatitis B serologic markers mean?
Hepatitis B surface antigen (HBsAg)
A protein on the surface of Hepatitis B virus (HBV); it can be detected in high levels in serum during acute or chronic Hepatitis B virus (HBV) infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
HBsAg Quantitative (quantitative hepatitis B surface antigen / qHBsAg)
HBsAg Quantitative (quantitative hepatitis B surface antigen / qHBsAg) blood test measures the actual amount of hepatitis B surface antigen in the blood. When used in combination with the Hepatitis B virus (HBV) DNA test, quantitative hepatitis B surface antigen can provide a liver specialist with additional insights to an individual’s hepatitis B virus infection. It can also be used in predicting and monitoring treatment response.
Hepatitis B surface antibody (anti-HBs)
The presence of anti-HBs is generally interpreted as indicating recovery and immunity from hepatitis B virus (HBV) infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B.
Total hepatitis B core antibody (anti-HBc)
Appears at the onset of symptoms in acute hepatitis B and persists for life. The presence of anti-HBc indicates previous or ongoing infection with hepatitis B virus in an undefined time frame.
IgM antibody to hepatitis B core antigen (anti-HBc IgM)
A positive/reactive anti-HBc IgM indicates recent infection with hepatitis B virus (≤6 months). Its presence indicates acute infection.
IgG antibody to hepatitis B core antigen (anti-HBc IgG)
A positive/reactive anti-HBc IgG test usually indicates a chronic infection. Hepatitis B core antibody (anti-HBc or HBcAb) remains positive indefinitely as a marker of past hepatitis B virus (HBV) infection.
- Note: These test results must be explained by your health care provider because they can be confusing. For example, sometimes the liver of a person who is chronically infected with hepatitis B may become more inflamed than usual (this is called a “liver flare”). So a chronically infected person could also test positive for the anti-HBc IgM blood test, although this usually indicates a new infection. Therefore, it is important to be seen by a health care provider who understands hepatitis B so you get the right diagnosis and the right care and follow-up.
Hepatitis B e antigen (HBeAg)
HBeAg (Hepatitis B e-Antigen) is a secreted product of the nucleocapsid gene of hepatitis B virus and is released from the infected liver cells into the blood that is found in serum during acute and chronic hepatitis B. Its presence indicates that the virus is replicating and the infected person has high levels of hepatitis B virus.
- A positive HBeAg indicates high levels of virus in the blood and a person is considered infectious.
- A negative HBeAg indicates very low to no virus in the blood and a person is usually considered less infectious; sometimes this can indicate a person has a mutant hepatitis B virus (see below).
HBeAg (Hepatitis B e-Antigen) test detects how much virus is in the blood as a result of very active viral replication. A negative test result indicates the virus may not be actively reproducing in the liver. In general, a person is considered very infectious when the HBeAg (Hepatitis B e-Antigen) test is positive, and less infectious when the test is negative. The loss of e-Antigen can occur naturally or as a result of drug treatment. Sometimes a negative HBeAg (Hepatitis B e-Antigen) test result can indicate a mutant hepatitis B virus is present. So, the absence of e-Antigen does not always mean there is little or no active viral replication. The doctor can confirm with additional tests.
The hepatitis B e-antigen test result is often used to monitor the effectiveness of many hepatitis B drug therapies that aim to change a chronically infected person’s e-antigen status from “positive” to “negative.” By achieving a “negative” e-antigen result, this means that the hepatitis B drug successfully stopped or slowed down the virus replication. Although this is not a cure, stopping or slowing down the virus will result in less damage to the liver, which decreases the risk of developing serious liver disease in the future.
Some people with chronic hepatitis B naturally lose e-antigen and develop e-antibody, even without treatment. To make things a bit more confusing, however, there are some chronically infected patients with a high viral load who are untreated and still test “negative” for the hepatitis B e-antigen. So, the absence of e-antigen does not always mean there is no active viral replication. Instead, these persons have a mutant hepatitis B virus that does not produce the e-antigen. As a result, treating someone who is e-antigen negative (but with a high viral load) is difficult because the mutant hepatitis B virus is more resistant to the current drugs. In addition, the absence of e-antigen makes it harder to evaluate whether a drug is working or not.
Hepatitis B e antibody (HBeAb or anti-HBe)
Hepatitis B e-Antibody (anti-HBe or HBeAb) is produced by the immune system temporarily during acute hepatitis B virus infection or consistently during or after a burst in viral replication. Hepatitis B e-Antibody (anti-HBe or HBeAb) is made in response to the hepatitis B e-antigen. This is not a protective antibody. Spontaneous conversion from e antigen to e antibody (a change known as seroconversion) is a predictor of long-term clearance of hepatitis B virus in patients undergoing antiviral therapy and indicates lower levels of hepatitis B virus.
Chronically infected individuals who stop producing e-antigen sometimes produce e-antibodies. The clinical significance of this result is not fully understood, but it is generally considered to be a good thing. For those with e-antigen negative chronic hepatitis B infections (meaning they have a mutant virus), the presence of anti-HBe may still be associated with active viral replication.
Hepatitis B Virus DNA Quantification (“viral load”) blood test measures the amount of hepatitis B virus DNA (or viral load) in the blood of chronically infected patients. The blood is tested using a Polymerase Chain Reaction (PCR) technique that is highly sophisticated and accurate. The hepatitis B “viral load” provides important information, but should only be considered in relation to other information such as your e-antigen status and liver enzymes test results (see below). The viral load is usually measured in “international units per milliliter” (IU/mL), but may also be measured in “copies per milliliter”(cp/ml). There are approximately 5 copies in one international unit.
How do I interpret hepatitis B serologic test results?
- Hepatitis B surface antigen (HBsAg): A protein on the surface of hepatitis B virus (HBV); it can be detected in high levels in serum during acute or chronic hepatitis B virus (HBV) infection. The presence of HBsAg indicates that the person is infectious. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make hepatitis B vaccine.
- Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs is generally interpreted as indicating recovery and immunity from hepatitis B virus (HBV) infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B.
- Total hepatitis B core antibody (anti-HBc): Appears at the onset of symptoms in acute hepatitis B and persists for life. The presence of anti-HBc indicates previous or ongoing infection with hepatitis B virus (HBV) in an undefined time frame.
- IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity indicates recent infection with hepatitis B virus (HBV) (≤6 months). Its presence indicates acute infection.
- Hepatitis B Drug Resistance, Genotype, and BCP/PreCore Mutation: This blood test is not commonly ordered. A liver specialist may order the test to determine a patient’s hepatitis B virus genotype (A-H) for research purposes and to detect a viral mutation that may be associated with resistance to current treatments. This is a Polymerase Chain Reaction (PCR) test, which again, is not readily available or used outside large teaching hospitals.
Table 3. Interpretation of Hepatitis B Serologic Test Results
| Tests | Results | Interpretation |
|---|---|---|
| HBsAg anti-HBc anti-HBs | negative negative negative | Susceptible |
| HBsAg anti-HBc anti-HBs | negative positive positive | Immune due to natural infection |
| HBsAg anti-HBc anti-HBs | negative negative positive | Immune due to hepatitis B vaccination |
| HBsAg anti-HBc IgM anti-HBc anti-HBs | positive positive positive negative | Acutely infected |
| HBsAg anti-HBc IgM anti-HBc anti-HBs | positive positive negative negative | Chronically infected |
| HBsAg anti-HBc anti-HBs | negative positive negative | Interpretation unclear; four possibilities: 1. Resolved infection (most common) |
Hepatitis B treatment
Treatment to prevent hepatitis B infection after exposure
If you know you’ve been exposed to the hepatitis B virus and aren’t sure if you’ve been vaccinated, call your doctor immediately. An injection of immunoglobulin (an antibody) given within 12 hours of exposure to the virus may help protect you from getting sick with hepatitis B. Because this treatment only provides short-term protection, you also should get the hepatitis B vaccine at the same time, if you never received it.
Treatment for acute hepatitis B infection
If your doctor determines your hepatitis B infection is acute — meaning it is short-lived and will go away on its own — you may not need treatment. Instead, your doctor might recommend rest, proper nutrition and plenty of fluids while your body fights the infection and including replacement of fluids lost from vomiting and diarrhea. In severe cases, antiviral drugs or a hospital stay is needed to prevent complications.
Patients with severe acute hepatitis B infection (2 of the 3: bilirubin more than 10 mg/dl, INR more than 1.6 and hepatic encephalopathy) and protracted acute severe disease (total bilirubin more than 3 mg/dl or direct bilirubin more than 1.5 mg/dl, INR more than 1.5, hepatic encephalopathy, or ascites) need antiviral treatment 45.
Treatment for chronic hepatitis B infection
In most people, the treatment does not cure hepatitis B infection, but only suppresses the replication of the virus. Therefore, most people with chronic hepatitis B infection must receive treatment for the rest of their lives. Treatment helps reduce the risk of liver disease and prevents you from passing the infection to others. Treatment for chronic hepatitis B may include 46:
- Antiviral medications. Several antiviral medicines — including entecavir (Baraclude), tenofovir (Viread), lamivudine (Epivir), adefovir (Hepsera) and telbivudine (Tyzeka) — can help fight the virus and slow its ability to damage your liver. These drugs are taken by mouth. Your doctor may recommend combining two of these medications or taking one of these medications with interferon to improve treatment response. Talk to your doctor about which medication might be right for you.
- Interferon injections. Interferon alfa-2b (Intron A) is a man-made version of a substance produced by the body to fight infection. It’s used mainly for young people with hepatitis B who wish to avoid long-term treatment or women who might want to get pregnant within a few years, after completing a finite course of therapy. Women should use contraception during interferon treatment. Interferon should not be used during pregnancy. Side effects may include nausea, vomiting, difficulty breathing and depression.
- Liver transplant. If your liver has been severely damaged, a liver transplant may be an option. During a liver transplant, the surgeon removes your damaged liver and replaces it with a healthy liver. Most transplanted livers come from deceased donors, though a small number come from living donors who donate a portion of their livers.
Other drugs to treat hepatitis B are being developed.
Hepatitis B prognosis
Acute hepatitis B virus infection can be treated symptomatically and in immunocompetent patients, can spontaneously resolve. Those that progress to the chronic state, however, are at increased risk for the development of hepatocellular carcinoma, cirrhosis, or fulminant liver failure 2. The likelihood of risk is dependent on the particular genotype, and the method of transmission as vertical transmission has a higher risk of long-term complications compared to horizontal 2.
Living with hepatitis B
If you test positive for the hepatitis B virus for longer than 6 months, this indicates that you have a chronic hepatitis B infection. All patients with chronic hepatitis B infections, including children and adults, should be monitored regularly since they are at increased risk for developing cirrhosis, liver failure, or liver cancer.
You should make an appointment with a hepatologist (liver specialist) or gastroenterologist familiar with hepatitis B. This specialist will order blood tests and possibly a liver ultrasound to evaluate your hepatitis B status and the health of your liver. Your doctor will probably want to see you at least once or twice a year to monitor your hepatitis B and determine if you would benefit from treatment.
Not everyone who tests positive for hepatitis B will require medication. Depending on your test results, you and your doctor might decide to wait and monitor your condition. If your test results indicate that you would be a good candidate for treatment, then your doctor will discuss the current treatment options with you. Whether you start treatment or not, your doctor will want to see you every six months, or at minimum once every year.
Before you start any treatment, make sure you research each treatment option, and ask your doctor to thoroughly explain each option, so that you are well informed. It also might be a good idea to get a second opinion from another doctor before starting any treatment, because more information is always better!
Once you are diagnosed with chronic hepatitis B, the virus will most likely stay in your blood and liver for a lifetime. It is important to know that you can pass the virus along to others, even if you don’t feel sick. This is why it’s so important that you make sure that all close household contacts and sex partners are tested and vaccinated against hepatitis B.
While living with hepatitis B can be difficult and scary at first, the more information and support that you have, the easier it gets. Many patients become such experts at managing their hepatitis B that they sometimes teach their health care providers about the latest research and information!
The most important thing to remember is that hepatitis B is a chronic medical condition (such as diabetes and high blood pressure) that can be successfully managed if you take good care of your health and your liver. You should expect to live a long, full life.
Children with Hepatitis B
Hepatitis B does not usually affect a child’s normal growth and development 47. Most children with chronic hepatitis B infections will enjoy long and healthy lives. Unlike other chronic medical conditions, there are generally no physical disabilities associated with hepatitis B, nor are there usually any physical restrictions for these children.
As a parent, you can take comfort from the fact that every child presents unique challenges. Therefore, your child with hepatitis B is just like any other child. The challenges of raising a child with hepatitis B are manageable if you are well informed and use common sense.
The Hepatitis B Foundation convened an Expert Pediatric Panel of nationally recognized pediatric liver specialists to create the first national recommendations for the screening, monitoring and treatment of children living with hepatitis B to ensure that they receive the best care possible.
- Hepatitis B Foundation’s Pediatric HBV Screening and Monitoring Recommendations (https://www.hepb.org/assets/Uploads/HBF-PedsPaper-Nov-2009.pdf)
- Hepatitis B Foundation’s Pediatric HBV Management and Treatment Recommendations (https://www.hepb.org/assets/Uploads/Jonas-2010-Treatment-of-children-w-chronic-HBV.pdf)
Parents of Kids with Infectious Diseases (PKIDs) Hepatitis Report
Parents of Kids with Infectious Diseases (PKIDs) is a national non-profit organization that supports families whose children have been affected by hepatitis B and C, HIV/AIDS and other infectious diseases. PKIDs published the first-ever comprehensive Pediatric Hepatitis Report to help parents, health care providers, school personnel, and public health officials understand the unique issues of children living with viral hepatitis, including hepatitis B and hepatitis C. The 530 page report can be downloaded for free at www.pkids.org.
Hepatitis B Vaccine
Who should be vaccinated against hepatitis B ?
The Advisory Committee on Immunization Practices recommends that the following persons be vaccinated against hepatitis B:
- All infants, beginning at birth
- All children aged <19 years who have not been vaccinated previously
- Susceptible sex partners of hepatitis B surface antigen (HBsAg)-positive persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., >1 sex partner during the previous 6 months)
- Persons seeking evaluation or treatment for a sexually transmitted disease
- Men who have sex with men
- Injection drug users
- Susceptible household contacts of HBsAg-positive persons
- Health care and public safety workers at risk for exposure to blood or blood-contaminated body fluids
- Persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients
- Residents and staff of facilities for developmentally disabled persons
- Travelers to regions with intermediate or high rates of endemic HBV infection
- Persons with chronic liver disease
- Persons with HIV infection
- Unvaccinated adults with diabetes mellitus who are aged 19 through 59 years (discretion of clinicians for unvaccinated adults with diabetes mellitus who are aged ≥60 years)
- All other persons seeking protection from HBV infection — acknowledgment of a specific risk factor is not a requirement for vaccination
Is hepatitis B vaccination recommended in certain settings ?
Yes. In certain health care, evaluation, or treatment settings, a high proportion of clients have known risk factors for Hepatitis B virus infection. The Advisory Committee on Immunization Practices recommends universal vaccination of adults who receive care in those settings, including:
- Sexually transmitted disease treatment facilities
- HIV testing and treatment facilities
- Facilities providing drug-abuse treatment and prevention services
- Health care settings targeting services to injection drug users
- Correctional facilities
- Health care settings targeting services to men who have sex with men
- Chronic hemodialysis facilities and end-stage renal disease programs
- Institutions and nonresidential day care facilities for developmentally disabled persons.
Who should not receive hepatitis B vaccine ?
Anyone who has had a serious allergic reaction to a prior dose of hepatitis B vaccine, a component of the hepatitis B vaccine, or yeast should not receive hepatitis B vaccine.
Can hepatitis B vaccine be given during pregnancy or lactation?
Yes. hepatitis B vaccine contains no live virus, so neither pregnancy nor lactation should be considered a contraindication to vaccination of women. On the basis of limited experience, there is no apparent risk of adverse effects to developing fetuses when hepatitis B vaccine is administered to pregnant women. Meanwhile, new Hepatitis B virus (HBV) infection in a pregnant woman might result in severe disease for the mother and chronic infection for the newborn.
Can hepatitis B vaccine be given after exposure to Hepatitis B virus (HBV)?
Yes. After a person has been exposed to hepatitis B virus, appropriate prophylaxis, given as soon as possible but preferably within 24 hours, can effectively prevent infection. The mainstay of postexposure immunoprophylaxis is hepatitis B vaccine, but in certain circumstances the addition of hepatitis B immune globulin (HBIG) will provide increased protection.
What are the hepatitis B vaccines licensed for use in the United States?
There are three single-antigen vaccines, one three-antigen vaccine, and three combination vaccines currently licensed in the United States.
- Single-antigen hepatitis B vaccines
- Engerix-B
- Recombivax HB
- Heplisav-B
- Three-antigen hepatitis B vaccines
- PreHevbrio (13)
- Combination vaccines
- Pediarix: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine
- Twinrix: Combined hepatitis A and hepatitis B vaccine
- Vaxelis: Combined DTaP, IPV, Haemophilus influenzae type b, and hepatitis B vaccine
Hepatitis B vaccination schedule most often used for children and adults is three intramuscular injections, the second and third doses administered at 1 and 6 months, respectively, after the first dose. Alternate hepatitis B vaccination schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved for two doses, 1 month apart.
Table 1. Recommended doses of currently licensed hepatitis B vaccine, by age group and vaccine type
| Hepatitis B Vaccine*, Age Group (yrs) | Dose (µg) | Vol (mL) | Schedule |
|---|---|---|---|
| Recombivax HB | |||
| Infants (<1 yr) | 5 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–10 yrs) | 5 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Adolescents (11–19 yrs) † | 5 | 0.5 | 3 doses at 0, 1, 6 mos† |
| Adults (≥20 yrs) | 10 | 1 | |
| Patients on hemodialysis and other immune-compromised persons, <20 yrs§ | 5 | 0.5 | |
| Patients on hemodialysis and other immune-compromised persons, ≥20 yrs | 40 | 1 | |
| Engerix-B | |||
| Infants (<1 yr) | 10 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–10 yrs) | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Adolescents (11–19yrs) | 10 | 0.5 | 3 doses at 0, 1, 6 mos |
| Adults (≥20 yrs) | 20 | 1 | |
| Patients on hemodialysis and other immune-compromised persons, <20 yrs§ | 10 | 0.5 | |
| Patients on hemodialysis and other immune-compromised persons, ≥20 yrs | 40 | 2 | 4 doses at 0, 1, 2, 6 mos ¶ |
| Heplisav-B | |||
| Adults (≥18 yrs**) | 20 | 0.5 | 2 doses at 0 and 1 mos |
| PreHevbrio (FDA-approved in 2021) | |||
| Adults (≥18 yrs**) | 10 | 1 | 3 doses at 0, 1, 6 mos |
| Pediarix (combination hepatitis B, diphtheria, tetanus, acellular pertussis, and inactivated poliovirus) | |||
| Infants (<1 yr) | 10 | 0.5 | 3-doses at age 0, 1–2, 6–18 mos |
| Children (1–6 yrs) †† | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Vaxelis (combination diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, and hepatitis B) | |||
| Infants (<1 yr) | 10 | 0.5 | 3 doses at age 0, 1–2, 6–18 mos |
| Children (1–4 yrs) §§ | 10 | 0.5 | 3 doses at 0, 1–2, 6 mos |
| Twinrix (combination hepatitis A-hepatitis B) ¶¶ | |||
| Adults (≥18 yrs) | 20 | 1 | 3 doses at 0, 1, 6 mos (standard) or 4 doses at 0, 7d, 21–30 d, 12 mos (accelerated) |
Footnotes:
* Refer to package inserts for further information. For all ages, when the hepatitis B vaccine schedule is interrupted, the vaccine series does not need to be restarted. If a 3-dose series is interrupted after the first dose, the second dose should be administered as soon as possible, and the second and third doses should be separated by an interval of at least 8 weeks. If only the third dose has been delayed, it should be administered as soon as possible. The final dose of a 3-dose series vaccine must be administered at least 8 weeks after the second dose and should follow the first dose by at least 16 weeks; the minimum interval between the first and second doses is 4 weeks. Inadequate doses of hepatitis B vaccine or doses received after a shorter-than-recommended dosing interval should be readministered, using the correct dosage or schedule. Vaccine doses administered ≤4 days before the minimum interval or age are considered valid. Because of the unique accelerated schedule for Twinrix, the 4-day guideline does not apply to the first three doses of this vaccine when administered on a 0-day, 7-day, 21–30-day, and 12-month schedule. PreHevbrio is a three-antigen HepB vaccine that was FDA approved in 2021.
† A 2-dose schedule of Recombivax HB adult formulation (10 µg) is licensed for adolescents aged 11 through 15 years. When scheduled to receive the second dose, adolescents aged 16 years or older should be switched to a 3-dose series, with doses 2 and 3 consisting of the pediatric formulation administered on an appropriate schedule.
§ Higher doses might be more immunogenic, but no specific recommendations have been made.
¶ Engerix-B for adults on hemodialysis: Administer series of 4 doses (2 mL each) as a single 2-mL dose or as two 1-mL doses on a 0-, 1-, 2-, 6-month schedule. Recombivax HB for adults on hemodialysis is a 3-dose series.
** Data on Heplisav-B and PreHevbrio are currently insufficient to inform vaccine-associated risks in pregnancy. Thus, providers should vaccinate pregnant people needing HepB vaccination with Engerix-B, Recombivax HB, or Twinrix.
†† Pediarix cannot be administered at birth, before age 6 weeks, or at age ≥7 years.
§§ Vaxelis is approved for use as a 3-dose series in children 6 weeks through 4 years of age.
¶¶ Twinrix is recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections.
[Source 40 ]No. If necessary, administering extra doses of Hepatitis A or hepatitis B vaccine is not harmful.
If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted ?
No, the series does not need to be restarted.
- If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible.
- The second and third doses should be separated by an interval of at least 8 weeks.
- If only the third dose is delayed, it should be administered as soon as possible.
Can hepatitis B vaccine be administered concurrently with other vaccines?
Yes. When hepatitis B vaccine has been administered at the same time as other vaccines, no interference with the antibody response of the other vaccines has been demonstrated. Separate body sites and syringes should be used for simultaneous administration of injectable vaccines.
How long does protection from hepatitis B vaccine last?
Studies indicate that immunologic memory remains intact for at least 20 years among healthy vaccinated individuals who initiated hepatitis B vaccination >6 months of age. The vaccine confers long-term protection against clinical illness and chronic hepatitis B virus infection. Cellular immunity appears to persist even though antibody levels might become low or decline below detectable levels.
Among vaccinated cohorts who initiated hepatitis B vaccination at birth, long-term follow-up studies are ongoing to determine the duration of vaccine-induced immunity.
Why should an infant receive hepatitis B vaccine at birth before hospital discharge, even if the mother is negative for hepatitis B surface antigen (HBsAg)?
Infants born to hepatitis B virus-infected mothers require hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth to protect them from infection. However, because errors or delays in documenting, testing, and reporting maternal HBsAg status can and do occur, administering the first dose of hepatitis B vaccine soon after birth to all infants acts as a safety net, reducing the risk for perinatal infection when maternal HBsAg status is either unknown or incorrectly documented at delivery. Also, initiating the hepatitis B vaccine series at birth has been shown to increase a child’s likelihood of completing the vaccine series on schedule.
Can hepatitis B vaccine be given to immunocompromised persons, such as persons on hemodialysis or persons with HIV infection ?
Yes, although a larger vaccine dose is required to induce protective antibody in hemodialysis patients. Larger doses or additional doses might also be necessary for other immunocompromised persons. Serologic testing of hemodialysis patients and other immunocompromised persons is recommended 1–2 months after administration of the final dose of the primary vaccine series to determine the need for revaccination. Detailed guidance on vaccination of hemodialysis patients and other immunocompromised persons is available from the Advisory Committee on Immunization Practices recommendations on adult hepatitis B vaccination [available at 48].
Can a patient receive the first dose of hepatitis B vaccine from one manufacturer and subsequent doses from another manufacturer ?
Yes. No differences in immune response are observed when vaccines from different manufacturers are used to complete the vaccine series.
Is there any benefit or risk in vaccinating a person who has been infected with Hepatitis B virus (HBV) ?
Persons who have already been infected with Hepatitis B virus (HBV) will receive no benefit from vaccination. However, there is no risk to a previously infected person who receives vaccination.
Should persons be tested for immunity to hepatitis B before being vaccinated?
Routine prevaccination testing for immunity has not been recommended because it has not generally been found to be cost-effective. However, in adult populations that have an expected high prevalence (>20%) of hepatitis B virus infection (e.g., IDUs and MSM, especially those in older age groups), conducting prevaccination serologic testing for susceptibility prior to or at the same time of the initial vaccine dose might be considered to be cost saving, by reducing the cost of the complete the vaccination series.
Prevaccination testing for susceptibility is recommended for foreign-born persons born in Africa, Asia, the Pacific Islands, and other regions with high endemicity of hepatitis B virus infection (HBsAg prevalence of ≥8%); unvaccinated household, sexual, and needle-sharing contacts of HBsAg-positive persons; and for HIV-infected persons. Serologic testing should not be a barrier to vaccination. The first vaccine dose should be administered immediately after collection of the blood sample for serologic testing. Vaccination of persons who are immune to hepatitis B virus infection because of current or previous infection or vaccination is not harmful and does not increase the risk for adverse events.
When a single test is used for prevaccination testing, anti-HBc is the test of choice. Persons who are anti-HBc–positive should be tested for HBsAg. If persons are determined to be HBsAg negative, no further action is required. Persons with positive HBsAg should be referred to a specialist in the management of hepatitis B infection and receive further serologic evaluation, prevention counseling, and evaluation for antiviral treatment.
Who should receive post-vaccination testing?
Testing for immunity is advised only for persons whose subsequent clinical management depends on knowledge of their immune status, including
- Infants born to HBsAg-positive mothers
- Health care workers and public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids
- Chronic hemodialysis patients, HIV-infected persons, and other immunocompromised persons (e.g., hematopoietic stem-cell transplant recipients or persons receiving chemotherapy)
- Sex partners of persons with chronic hepatitis B virus infection
When should postvaccination testing be done?
For infants born to HBsAg-positive mothers, postvaccination testing should be performed 1–2 months after completion of ≥3 doses of a licensed hepatitis B vaccine series (i.e., at age 9–12 months, generally at the next well-child visit). To avoid detection of anti-HBs from hepatitis B immune globulin administered during infancy and to maximize detection of late HBV infection, testing should not be performed before age 9 months nor within 4 weeks of the most recent vaccine dose.
Are booster doses of hepatitis B vaccine recommended?
Booster doses of hepatitis B vaccine are recommended only in certain circumstances:
- For hemodialysis patients, the need for booster doses should be assessed by annual testing for antibody to hepatitis B surface antigen (anti-HBs). A booster dose should be administered when anti-HBs levels decline to <10 mIU/mL.
- For other immunocompromised persons (e.g., HIV-infected persons, hematopoietic stem-cell transplant recipients, and persons receiving chemotherapy), the need for booster doses has not been determined. When anti-HBs levels decline to <10 mIU/mL, annual anti-HBs testing and booster doses should be considered for those with an ongoing risk for exposure.
For persons with normal immune status who have been vaccinated, booster doses are not recommended.
Hepatitis B vaccine side effects
On the basis of Vaccine Safety Datalink data, the estimated incidence of anaphylaxis among children and adolescents who received hepatitis B vaccine is one case per 1.1 million vaccine doses distributed 48.
- Hepatitis B FAQs for Health Professionals. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm[↩][↩]
- Tripathi N, Mousa OY. Hepatitis B. [Updated 2021 Jul 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555945[↩][↩][↩][↩][↩]
- McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985 Apr;151(4):599-603. doi: 10.1093/infdis/151.4.599[↩]
- Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en/[↩][↩]
- Hepatitis B Frequently Asked Questions for the Public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm#bFAQa01[↩]
- Nebbia, G., Peppa, D., & Maini, M. K. (2012). Hepatitis B infection: current concepts and future challenges. QJM : monthly journal of the Association of Physicians, 105(2), 109–113. https://doi.org/10.1093/qjmed/hcr270[↩]
- Hepatitis B Questions and Answers for Health Professionals. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm[↩][↩][↩]
- Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis. 2012 May;16(2):347-69. doi: 10.1016/j.cld.2012.03.003[↩]
- 2020 Viral Hepatitis Surveillance Report. https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm[↩]
- Kim HS, Rotundo L, Yang JD, et al. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. Journal of Viral Hepatitis. 2017;24(11):1052–1066. doi: 10.1111/jvh.12735[↩]
- People Born Outside of the United States and Viral Hepatitis. https://www.cdc.gov/hepatitis/populations/Born-Outside-United-States.htm[↩]
- Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011–2016. Clinical Infectious Diseases. 2019. [Epub ahead of print] doi: 10.1093/cid/ciz001[↩]
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendation Report. 2018;67(1):1–31. doi: 10.15585/mmwr.rr6701a1[↩][↩]
- Surveillance for Viral Hepatitis – United States, 2017. https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm[↩][↩]
- Hepatitis B. https://www.niddk.nih.gov/health-information/liver-disease/viral-hepatitis/hepatitis-b[↩]
- Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991 May;11(2):73-83. doi: 10.1055/s-2008-1040426[↩][↩]
- Krugman S, Overby LR, Mushahwar IK, Ling CM, Frösner GG, Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979 Jan 18;300(3):101-6. doi: 10.1056/NEJM197901183000301[↩][↩]
- Roberts H, Ly KN, Yin S, Hughes E, Teshale E, Jiles R. Prevalence of HBV Infection, Vaccine-Induced Immunity, and Susceptibility Among At-Risk Populations: US Households, 2013-2018. Hepatology. 2021 Nov;74(5):2353-2365. doi: 10.1002/hep.31991[↩][↩]
- Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, Kim WR, Kowdley KV, Lok AS, Tsai N, Ward J, Wong SS, Gish RG. An Updated Assessment of Chronic Hepatitis B Prevalence Among Foreign-Born Persons Living in the United States. Hepatology. 2021 Aug;74(2):607-626. doi: 10.1002/hep.31782[↩]
- Centers for Disease Control and Prevention National Center for Health Statistics. Multiple Cause of Death 1999–2020 CDC WONDER Online Database. https://wonder.cdc.gov[↩]
- Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981 Mar 7;1(8219):550-1. doi: 10.1016/s0140-6736(81)92877-4[↩]
- Hepatitis D. https://emedicine.medscape.com/article/178038-overview[↩]
- Farci P, Niro GA. Clinical features of hepatitis D. Seminars in Liver Disease. 2012;32(3):228‒236.[↩]
- Ahn J, Gish RG. Hepatitis D virus: a call to screening. Gastroenterology & Hepatology. 2014;10(10):647‒686.[↩]
- Hepatitis C Coinfection. https://www.hepb.org/what-is-hepatitis-b/hepatitis-c-co-infection[↩][↩]
- Initial Treatment of Adults with HCV Infection. https://www.hcvguidelines.org/treatment-naive[↩]
- Pregnancy and Hepatitis B. https://www.hepb.org/treatment-and-management/pregnancy-and-hbv[↩][↩][↩][↩]
- U.S. Preventive Services Task Force. Screening for hepatitis B virus infection in pregnant women: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322(4):349–354. doi: 10.1001/jama.2019.9365[↩]
- Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018 Apr;67(4):1560-1599. doi: 10.1002/hep.29800[↩][↩]
- Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991;11(2):73–83.[↩][↩]
- Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012 Aug;56(2):422-33. doi: 10.1002/hep.24804[↩][↩]
- Higgins DC, Kuncio DE, Johnson CC, Viner KM. Influence of birth origin and risk factor profile on hepatitis B mortality: Philadelphia, PA 2003-2013. Ann Epidemiol. 2018 Mar;28(3):169-174. doi: 10.1016/j.annepidem.2017.12.006[↩]
- LeFevre ML. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: US Preventive Services Task Force recommendation statement. Annals Internal Med. 2014;161(1):58–66.[↩]
- World Health Organization. Media Centre: hepatitis B. July 2013. http://www.who.int/mediacentre/factsheets/fs204/en/[↩]
- Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012; 380: 2095-128.[↩]
- Hepatitis B or C Infections. https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/maternal-or-infant-illnesses/hepatitis.html[↩]
- Alter MJ, Hadler SC, Margolis HS, Alexander WJ, Hu PY, Judson FN, Mares A, Miller JK, Moyer LA. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA. 1990 Mar 2;263(9):1218-22. doi:10.1001/jama.1990.03440090052025[↩]
- https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/hepatitis-b[↩]
- Weng MK, Doshani M, Khan MA, et al. Universal Hepatitis B Vaccination in Adults Aged 19–59 Years: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:477–483. DOI: http://dx.doi.org/10.15585/mmwr.mm7113a1[↩]
- Hepatitis B Frequently Asked Questions for Health Professionals. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm#C1[↩][↩]
- Conners EE, Panagiotakopoulos L, Hofmeister MG, Spradling PR, Hagan LM, Harris AM, Rogers-Brown JS, Wester C, Nelson NP; Contributors. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations – United States, 2023. MMWR Recomm Rep. 2023 Mar 10;72(1):1-25. doi: 10.15585/mmwr.rr7201a1[↩]
- Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018 Jan 12;67(1):1-31. doi: 10.15585/mmwr.rr6701a1[↩]
- Interpretation of Hepatitis B Serologic Test Results. https://www.cdc.gov/hepatitis/hbv/interpretationOfHepBSerologicResults.htm[↩]
- A Comprehensive Immunization Strategy to Eliminate Transmission of hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Part I: Immunization of Infants, Children, and Adolescents. MMWR 2005;54(No. RR-16).[↩]
- Tripathi N, Mousa OY. Hepatitis B. [Updated 2023 Jul 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555945[↩]
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016 Jan;63(1):261-83. doi: 10.1002/hep.28156[↩]
- Children Living with Hepatitis B. https://www.hepb.org/treatment-and-management/children-with-hepatitis-b[↩]
- https://www.cdc.gov/mmwr/PDF/rr/rr5516.pdf[↩][↩]
- Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med 1989;87(Suppl 3A):S14–20.[↩]
- Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. Pediatr Infect Dis J 1993;12:438–45.[↩]