Contents
Hexyl acetate in food
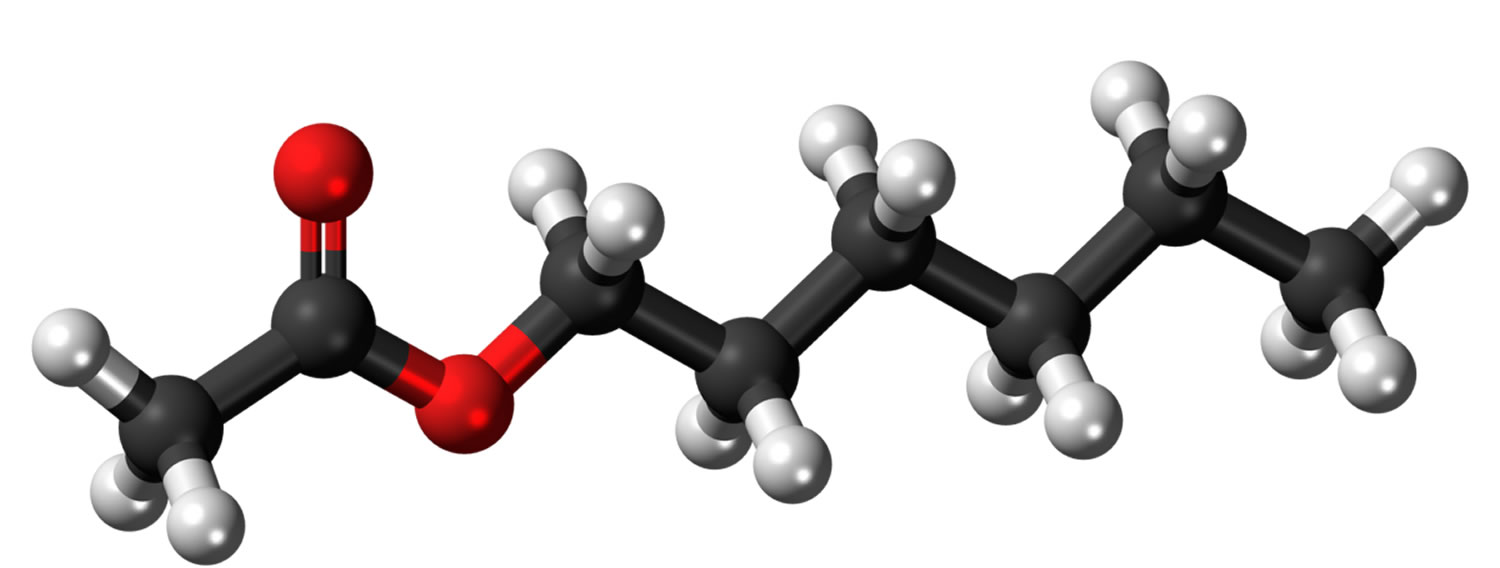
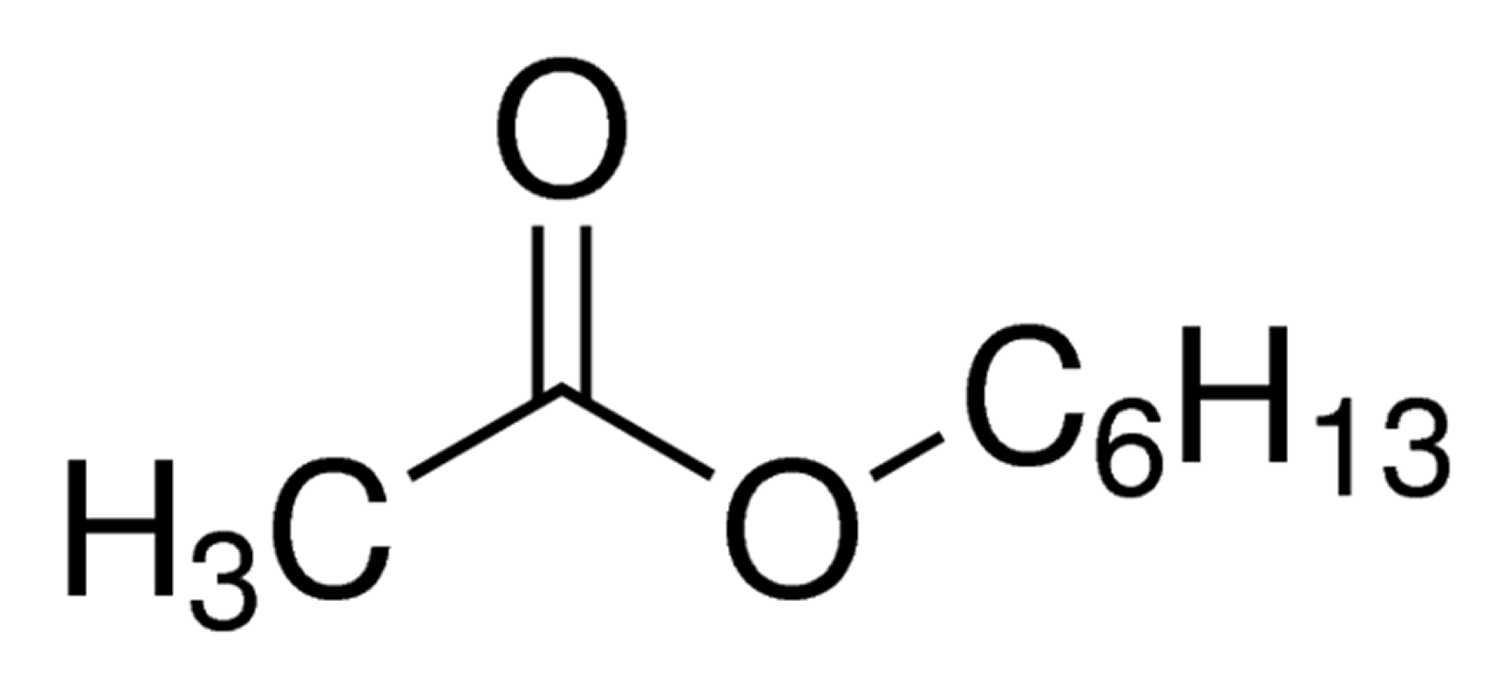
Hexyl acetate is an ester with the molecular formula C8H16O2. Hexyl acetate is found in alcoholic beverages. Hexyl acetate is used in fruit essences and fruit aroma concentrates. Hexyl acetate is present in wines, black tea, soya bean and other food. Hexyl acetate, produces a characteristic apple, banana, grass, herb and pear aroma. Intake estimates USA ~ 160 µg/person per day and Europe ~ 3200 µg/person per day. The component hexyl alcohol is oxidized to hexanoic acid which is endogenous as an intermediary metabolite in the fatty acid pathway and acetate is a component of the tricarboxylic acid cycle. In the opinion of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the endogenous levels of these two metabolites would not give rise to perturbations outside the physiological range. Therefore, hexyl acetate was also determined to be of no safety concern based on its structural class and known metabolism 1. No safety concern at current levels of intake when used as a flavoring agent 2.
Hexyl acetate is mainly used as a solvent for resins, polymers, fats and oils. It is also used as a paint additive to improve its dispersion on a surface.
Hexyl acetate is a colorless liquid with a mild sweet odor. Flash point 113°F (45 °C). Hexyl acetate is a moderate fire risk. Inhalation of hexyl acetate is may cause adverse effects.
Hexyl acetate is insoluble in water and very soluble in alcohols and ethers. When heated to high temperatures emits acrid smoke and fumes. Used as a solvent and as a propellant in aerosols. Hexyl acetate has a boiling point of 334.4 to 338° F (168-170 °C) at 760 mm Hg and melting point of -112° F (−80 °C).
Figure 1. Hexyl acetate chemical structure
Toxicological data
- LD50 oral rat. Lethal Dose 50% (LD50) or median lethal dose is the amount of the substance required (usually per body weight) to kill 50% of the test population.
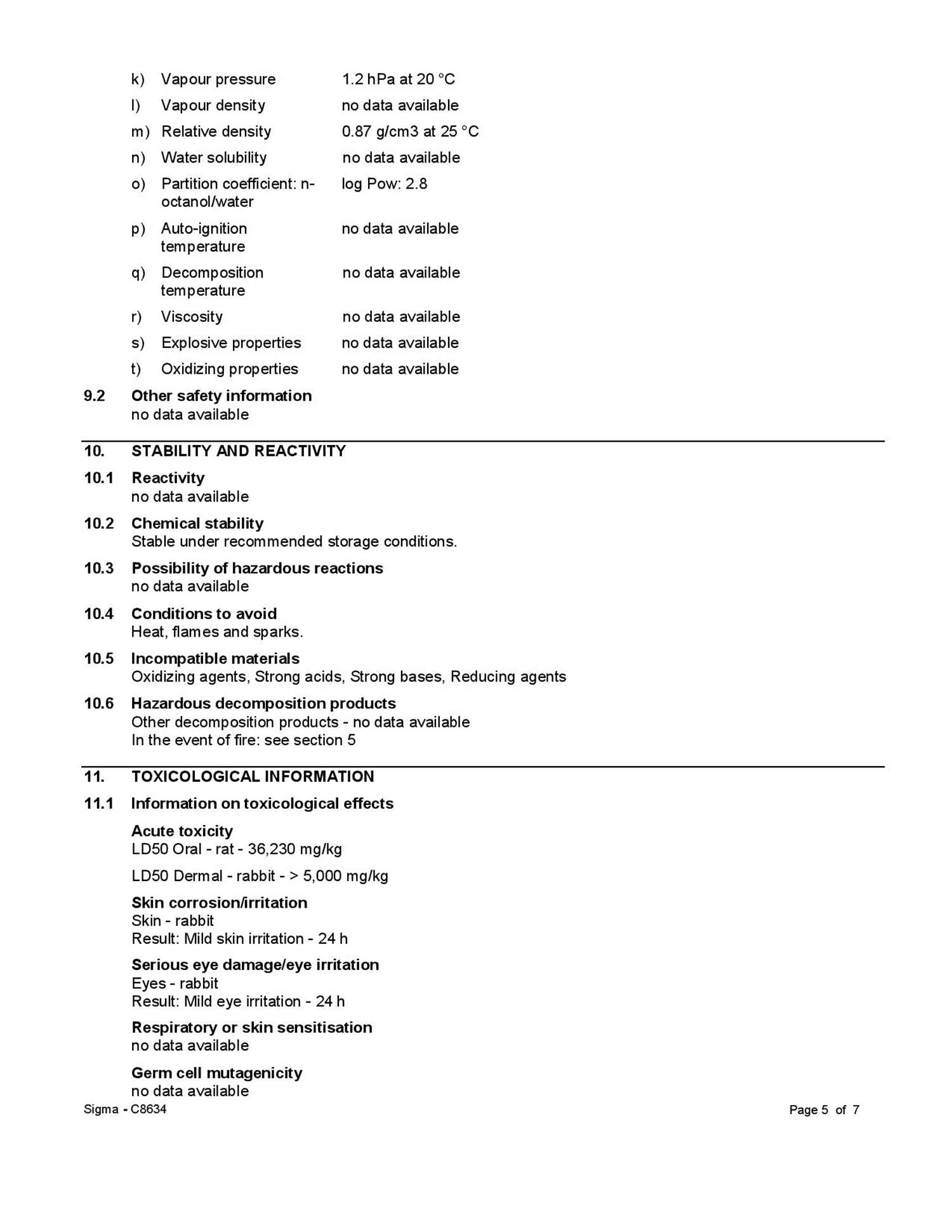
Value: 36,100 mg/kg body weight 3 - LD50 dermal rabbit. Value: > 5000 mg/kg body weight 4
Acute toxicity
The acute potential of hexyl acetate was examined in series of animal experiments. Since the results imply the typical active profile of alkyl acetates with comparable chain lengths, relevant general experiences with this substance group can be used as a supplement.
In a test on the eye-irritating potential on rabbit eyes, undiluted hexyl acetate (0.5 ml) triggered only minor irritations (irritation index 1 of max. 10). Labeling of the substance as eye-irritating proved to be unnecessary 5.
Undiluted hexyl acetate caused minor irritations (irritation index 3 of max. 10) in a trial on rabbit skin. Based on this finding hexyl acetate was classified as a weakly skin-irritating substance 5.
The skin-sensitizing potential was investigated in a test on probands (Repeat Insult Patch Test on 25 test persons) with 4% hexyl acetate in Vaseline. Skin reactions were not reported. Nor did a trial (Freund’s complete adjuvant test) of the ester on the skin of guinea pigs reveal any skin reactions. Although this test does not count among the standard methods, the result was considered sufficient to assess hexyl acetate as a non-skin irritating substance 5.
The dermal toxicity of the substance proved to be minor in older tests on rabbits. One of ten rabbits died after dermal application of undiluted hexyl acetate doses of 5,000 mg per kg of body weight; details were not provided 5. The other animals did not exhibit any toxicity symptoms during and after the exposure; the follow-up monitoring period amounted to 14 days. Other researchers did not observe any fatalities after the application of 20 ml per kg of body weight (approx. 17 g per kg of body weight) in a screening test on rabbits 5.
In humans, exposures to the vapors of alkyl acetates of comparable chain lengths (particularly the pentyl acetates) entailed chiefly irritations of the upper respiratory tract in humans, and, to a minor extent, eye irritations; exposures to very high concentrations also triggered CNS disorders (CNS-depressive effects) 5.
Corresponding effects are expected after exposure to hexyl acetate, but relevant data pertaining to the dose-response relation are scant:
An RD50 value determined for hexyl acetate in mice amounted to 740 ppm (The RD50 concentration is that concentration which reduces respiratory rate by 50%. RD50 gauge is used for measuring the sensory irritation: Decrease in the respiratory rate by 50%). It was determined that this concentration is not tolerable for humans due to the irritative effects on the eyes and the nose and throat region. Exposures to 74 ppm are expected to entail minor irritations 5. This assessment corresponds with the observation that 15-minute exposures to concentrations of 100 ppm of the isomer 1,3-dimethylbutyl acetate (sec-hexyl acetate) triggered irritations of the eyes and the upper respiratory tract in probands.
Systemic effects appear to occur only after exposures to much higher concentrations:
In a screening test rats tolerated 8-hour exposures to an atmosphere saturated with hexyl acetate vapors (approx. 1,450 ppm) without the occurrence of fatalities.
A more recent test available for the homologous 1PAcetate yielded similar results: Six-hour exposures to 3,000 ppm entailed neither toxicity symptoms nor the death of any rats.
In an animal experiment orally applied hexyl acetate proved to be almost non-toxic. Intragastral administration of the undiluted substance yielded an LD50 value of 41.5 ml per kg of body weight (approx. 36 g per kg of body weight). Symptoms or findings were not reported 5.
In humans the ingestion of large substance doses is expected to trigger irritations in the mouth and the throat as well as disorders of the CNS (depressive effects) 5.
Chronic toxicity
Specific experience reports on the industrial handling of the substance or results of long-term animal experiments are not available for hexyl acetate. Analogous to comparable alkyl acetates, repeated skin contact is expected to result in dehydration and chapped skin, followed by inflammations (dermatitis) 5.
Data from the industrial handling of pentyl acetates indicated that long-term exposures to very high vapour concentrations (> 3,500 ppm) triggered damage to the visual nerve in addition to pronounced eye irritations in individual exposed persons 5.
More recent long-term animal experiments with hexyl acetate homologues did not demonstrate any pronounced chronic systemic potential. Manufacturers refer to a series of subchronic inhalation studies performed with 1-butyl acetate in which rats had tolerated substance concentrations of 500 or 750 ppm 5.
A 13-week study performed with 1PA in which exposures of rats to concentrations of 600 or 1,200 ppm entailed decreased activities of the animals only in the first weeks appears to be of particular relevance. In the further development, exposures were tolerated without toxicity symptoms or other toxic effects. Nor did special tests on the neurotoxicity of the substance reveal corresponding dysfunctions in the animals; the dissection did not demonstrate changes in the nerve tissues 5.
With regard to the determination of work-place threshold values, it is assumed that irritative effects in the respiratory tract present the critical effect produced by the pentyl acetates. An analogous estimation is also available for the hexyl acetate isomer 1,3-dimethyl butyl acetate (sec-hexyl acetate) 5.
Reproductive toxicity, Mutagenicity and Carcinogenicity
Reproductive toxicity
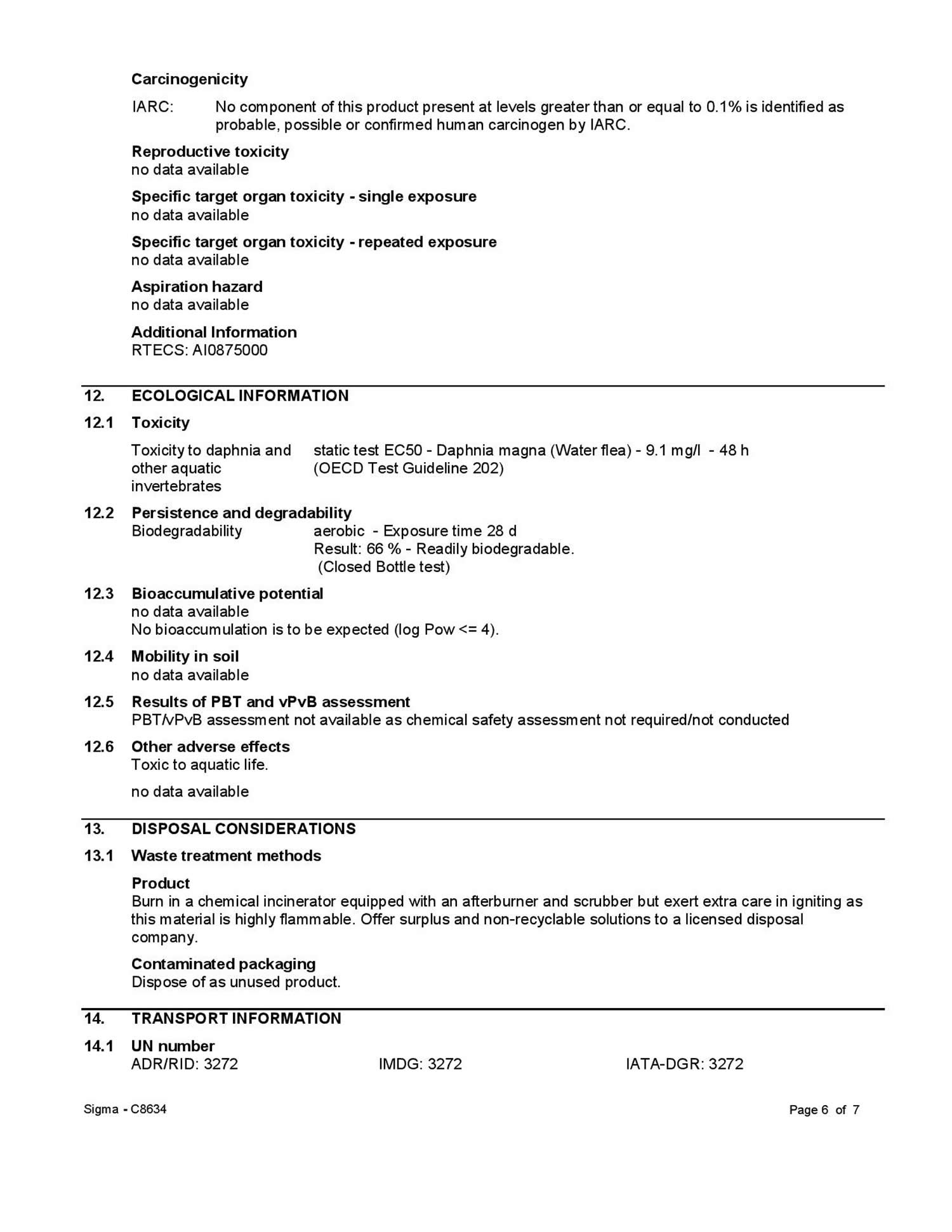
Test results pertaining to hexyl acetate are not available 5. With regard to the developmental toxicity manufacturers refer to results of studies performed with 1-octyl acetate and 1-butyl acetate on rats that exhibited slight foetotoxic effects only after exposures to maternal toxic doses. In a study on rats n-hexanol, the hydrolysis product of hexyl acetate, triggered slight foetotoxic effects only after exposures to very high, maternal toxic doses. Suspicion of a specific developmental toxic potential of hexyl acetate could not be derived from these findings 5.
Relevant studies investigating the impact of the substance on fertility are not available 5.
Mutagenicity
The available information is insufficient 5. In a series of microbiological tests performed with hexyl acetate, 1 of 5 test systems yielded a positive result pointing to a mutagenic effect. However, this test result could not be reproduced in a later, analogous trial 5. Other in-vitro or in-vivo test results are lacking 5.
Carcinogenic potential
Information is not available 5.
Biotransformation and Excretion
Hexyl acetate is rapidly hydrolysed to 1-hexanol and acetic acid in the organism. In an in-vitro study on the metabolism of alkyl acetates (including hexyl acetate) performed with tissue preparations of nose, liver, lungs and trachea, the highest hydrolytic activities were found in the liver, followed by the nasal tissue. In acetates of straight-chain alcohols the hydrolyse process increased up to the pentyl residue but decreased with further increasing chain lengths. Hexyl acetate thus belongs to the very rapidly hydrolysed acetates, considerable portions of which are expected to be hydrolysed as early as in the respiratory tract 5. In the organism, hydrolytically generated 1-hexanol can be transformed to valeric acid via oxidation. Hexanoic acid, like the hydrolysis product acetic acid, is included in the physiological metabolism, since both metabolites are also formed endogenously 5.
Hexyl acetate uses
Hexyl acetate is present in wines, black tea, soya bean and other food. Hexyl acetate is used as a flavoring agent because it produces a characteristic apple, banana, grass, herb and pear aroma.
Hexyl acetate is mainly used as a solvent for resins, polymers, fats and oils. It is also used as a paint additive to improve its dispersion on a surface.
Hexyl acetate msds



- Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO FOOD ADDITIVES SERIES 40. World Health Organization, Geneva 1998. http://www.inchem.org/documents/jecfa/jecmono/v040je14.htm[↩]
- http://www.inchem.org/documents/jecfa/jeceval/jec_1044.htm[↩]
- Toxicology and Applied Pharmacology. Vol. 28, Pg. 313, 1974[↩]
- Food and Cosmetics Toxicology. Vol. 12, Pg. 913, 1974.[↩]
- http://gestis-en.itrust.de/nxt/gateway.dll/gestis_en/570141.xml?f=templates$fn=default.htm$3.0[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]