Contents
- Human chorionic gonadotropin
- Human chorionic gonadotropin function
- Human chorionic gonadotropin uses
- Human chorionic gonadotropin in Normal and Abnormal Pregnancies
- What does human chorionic gonadotropin test result mean?
- How does the test that I do at home myself compare with the results of a test done in a lab?
- When is a blood human chorionic gonadotropin test ordered instead of a urine human chorionic gonadotropin?
- How many days after a miscarriage would it take for a urine pregnancy test to show a negative result?
- What is an ectopic pregnancy?
- Human chorionic gonadotropin as a Potential Biomarker for Preeclampsia
- Human chorionic gonadotropin as Serum Marker for Down’s Syndrome Screening
- Human chorionic gonadotropin as tumor marker
- Human Chorionic Gonadotropin (hCG) in Female Infertility Treatment
- Human chorionic gonadotropin in Normal and Abnormal Pregnancies
- Human chorionic gonadotropin treatment
- Human chorionic gonadotropin treatment side effects
Human chorionic gonadotropin
Human chorionic gonadotropin (hCG) is “the hormone of pregnancy” produced primarily by syncytiotrophoblastic cells of the placenta of a pregnant woman 1, 2, 3. Smaller amounts of human chorionic gonadotropin (hCG) are also produced in the pituitary gland, the liver, and the colon 4. Human chorionic gonadotropin (hCG) plays an important role in synchronizing fetal and endometrial developments. Early in pregnancy, the level of human chorionic gonadotropin (hCG) increases in the blood and is eliminated in the urine. A pregnancy test detects human chorionic gonadotropin (hCG) in the blood or urine and confirms or rules out pregnancy 3. Throughout pregnancy, human chorionic gonadotropin (hCG) is also a marker of placental function. Human chorionic gonadotropin (hCG) can also be used in medical diagnostics to detect some cancers.
In everyday clinical practice, human chorionic gonadotropin (hCG) is mainly used to diagnose pregnancy and to supervise first trimester adverse pregnancy outcomes. Abnormalities in the production and the circulating levels of hCG during specific periods of gestation have been associated with a large array of pregnancy complications, such as miscarriages 5, fetal chromosomal anomalies 6, pre-eclampsia 7, 8, disturbances in fetal growth and development 9 and gestational trophoblastic diseases 10. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules 1, 11.
Nevertheless, the persistence of low hCG concentrations in a non-pregnant woman is not always malignant and can even be benign 12, 13. In addition, very high concentrations of hCG have been shown to have deleterious effects on fetal tissues, notably on fetal gonadal steroidogenesis 14. To avoid this, the human fetal tissue macrophages are thought to eliminate excess hCG. Katabuchi et al. 15 have shown that hCG induces the formation of vacuoles in human monocytes and hypothesized that these vacuoles would be involved in the protection of fetal tissues.
Multiple factors influence hCG levels during pregnancy. Among them, endocrine disruptive chemicals (EDCs), particularly bisphenol A and para-nonylphenol, can modulate hCG production and cause fetal damage as well as long-lasting consequences in adult life 16.
Human chorionic gonadotropin (hCG) stimulates the corpus luteum to produce progesterone to maintain the pregnancy. Human chorionic gonadotropin (hCG), is crucially involved in processes such as implantation and placentation, two milestones of pregnancy whose successful progress is a prerequisite for adequate fetal growth. Moreover, hCG determines fetal fate by regulating maternal innate and adaptive immune responses allowing the acceptance of the foreign fetal antigens 17. As one of the first signals provided by the embryo to its mother, human chorionic gonadotropin (hCG) has the potential to regulate very early pregnancy-driven immune responses, allowing the establishment and preservation of fetal tolerance.
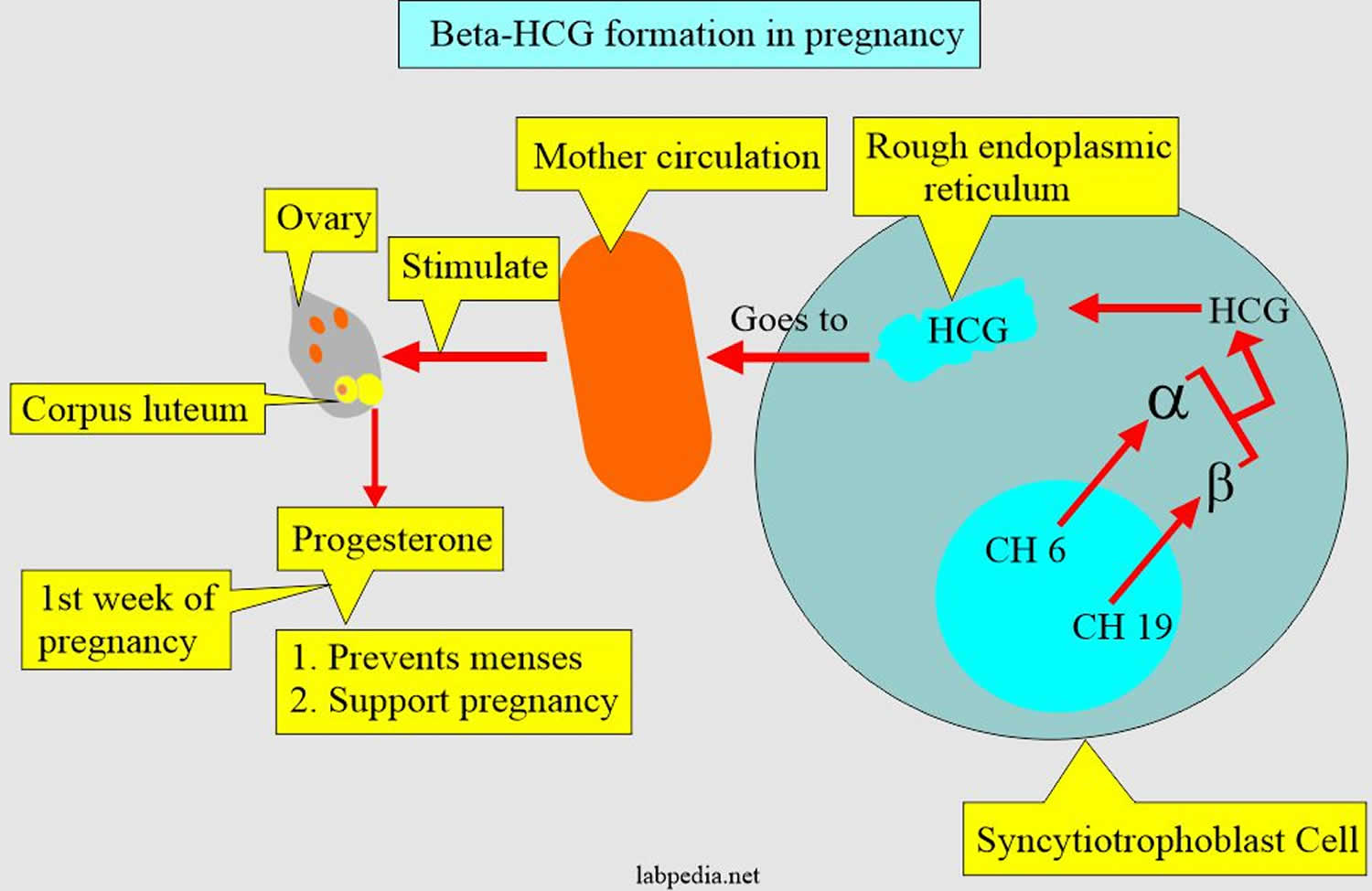
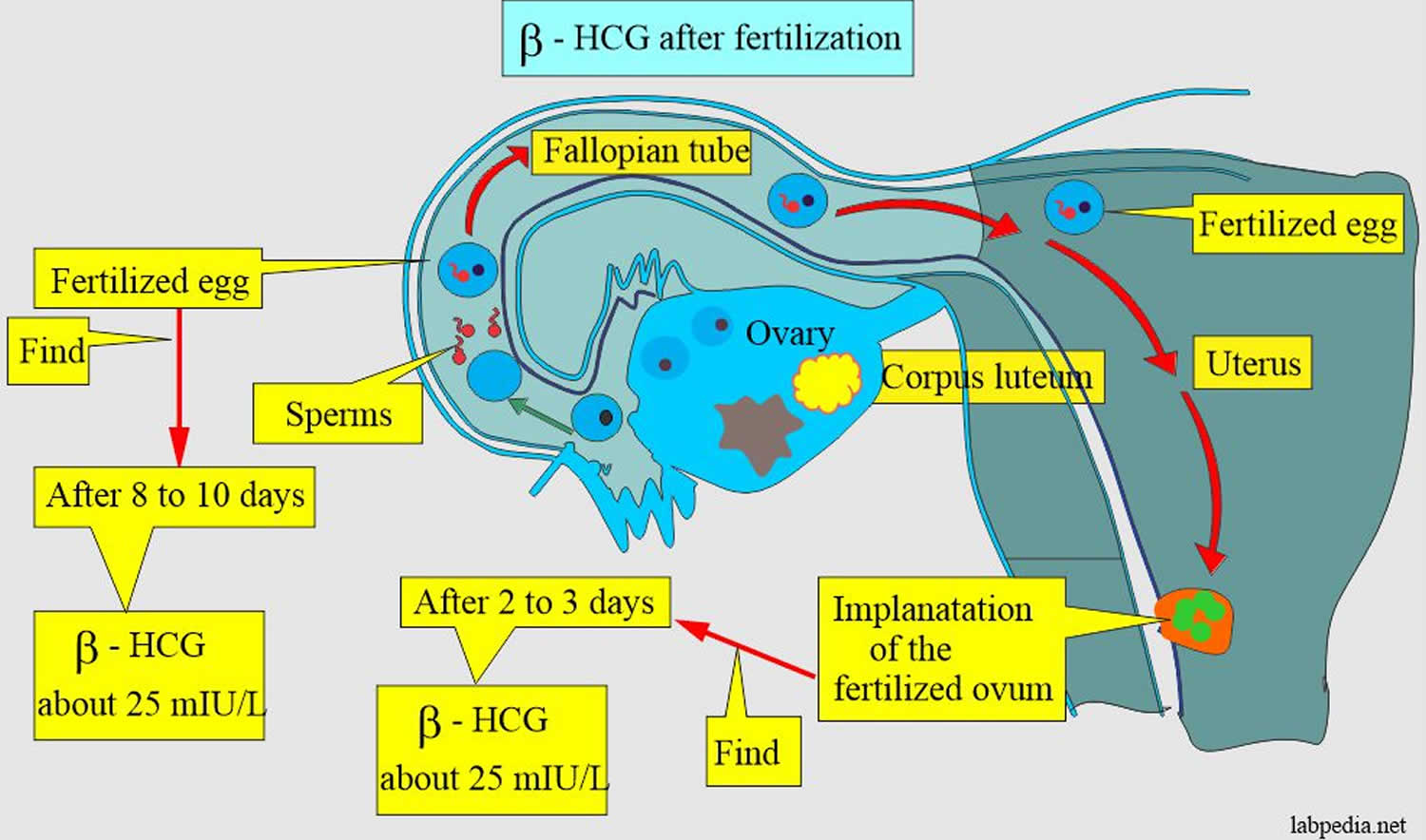
During the early weeks of pregnancy, human chorionic gonadotropin (hCG) is important in maintaining function of the corpus luteum. Circulating human chorionic gonadotropin (hCG) interacts with the luteinizing hormone receptors (LHRs) of the ovary, promoting the corpus luteum and its production of progesterone which is necessary to maintain pregnancy and support the growth of the fetus. Production of human chorionic gonadotropin (hCG) increases steadily during the first trimester (8-10 weeks) of a normal pregnancy, peaking around the 10th week after the last menstrual cycle. Levels then fall slowly during the remainder of the pregnancy. Human chorionic gonadotropin is no longer detectable within a few weeks after delivery.
When a pregnancy occurs outside of the uterus (ectopic pregnancy), the level of human chorionic gonadotropin (hCG) in the blood increases at a slower rate. When an ectopic pregnancy is suspected, measuring the level of human chorionic gonadotropin (hCG) in the blood (quantitative test) over time may be useful in helping to make a diagnosis of ectopic pregnancy.
Similarly, the human chorionic gonadotropin (hCG) blood level may be abnormal when the developing baby (fetus) has a chromosome defect such as Down syndrome. An human chorionic gonadotropin (hCG) test is used routinely in conjunction with a few other tests as part of screening for fetal abnormalities.
Injections of human chorionic gonadotropin (hCG) mimic the surge in luteinizing hormone (LH) that is necessary for ovulation and are used in the therapy of female infertility, in assisted reproduction techniques (ART). In clinical trials, hCG resulted in pregnancies in approximately 30% of women. Human chorionic gonadotropin (hCG) prepared from urine of pregnant women and was approved for use in the United States in 1967 as treatment of ovulatory dysfunction in women desiring pregnancy. Subsequently, recombinant forms of hCG have been developed and licensed for use. Currently, hCG is available as a powder or in solution generically and under trade names such as Novarel and Pregnyl. Recombinant hCG is available as Overle. The dose and regimen of hCG therapy varies by indication and it should be used only by physicians with expertise in the management of infertility and hypogonadism. Common side effects include headache, nausea, anorexia, and local injection reactions. Uncommon, but potentially severe adverse events include ovarian hyperstimulation syndrome.
Figure 1. Human chorionic gonadotropin (hCG)
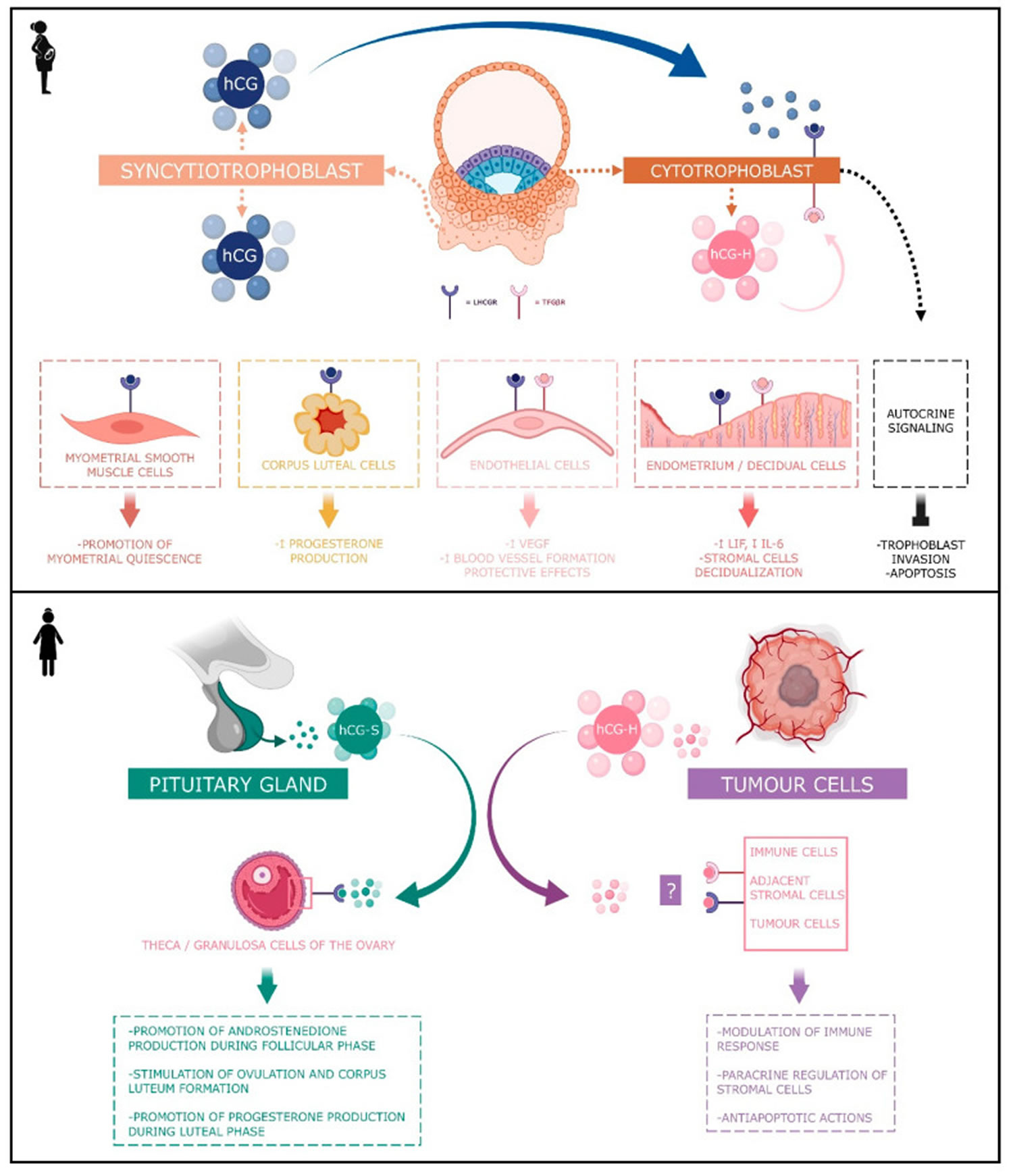
[Source 18 ]Figure 2. Human chorionic gonadotropin (hCG) actions during pregnancy and in non-pregnant woman
Footnotes: Human chorionic gonadotropin (hCG) has 4 major isoforms found in the serum and urine during pregnancy: classical hCG, hyperglycosylated hCG, free beta (β) subunit, and sulphated hCG 18. The classical form of hCG is schematized by a blue dot, the hyperglycosylated hCG by a pink dot and the sulphated form of hCG by a green dot. The blue receptor is the LH-hCG receptor (LHCGR) and the pink receptor is the transforming growth factor β receptor (TGFβR).
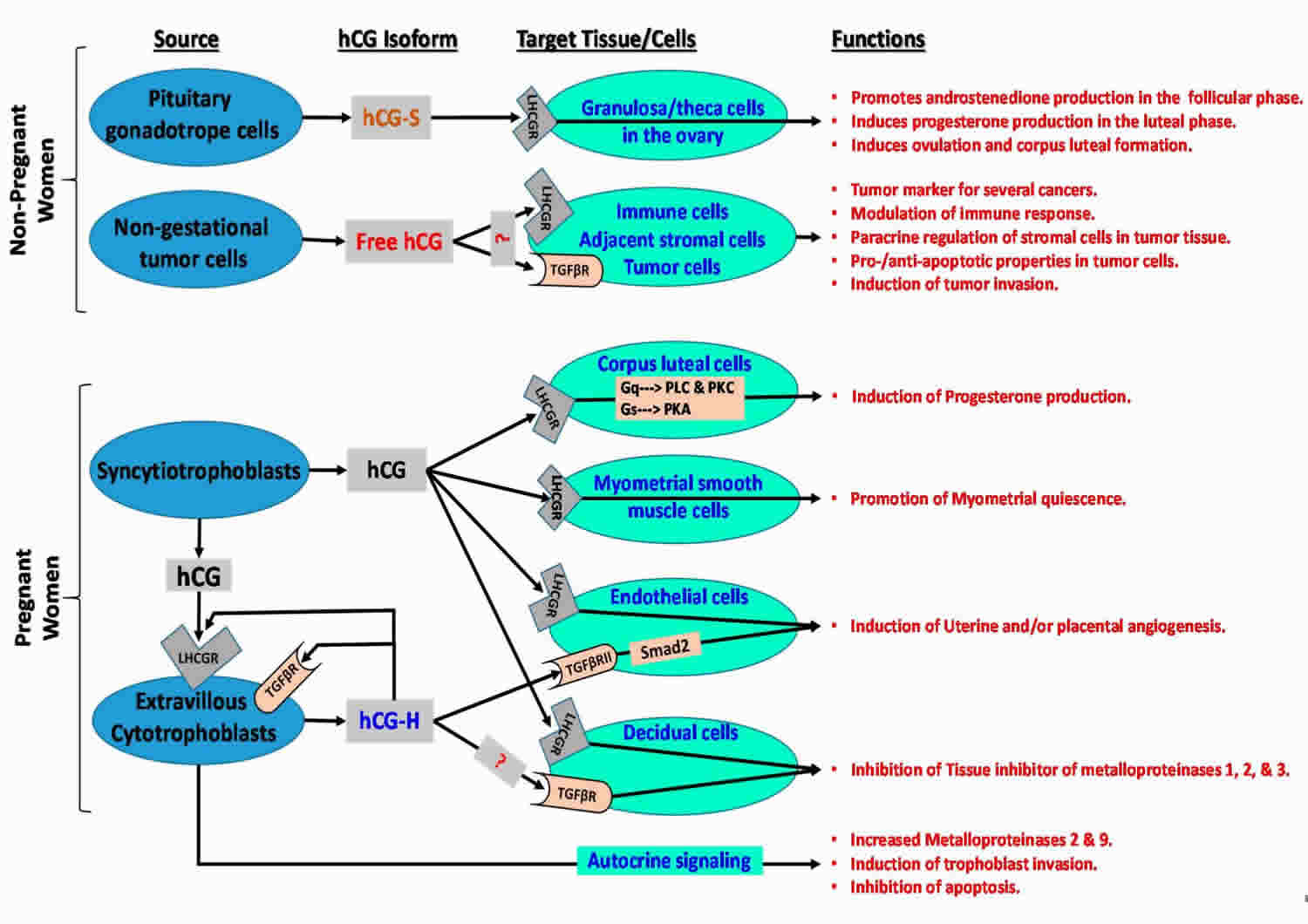
[Source 18 ]Figure 3. Human chorionic gonadotropin function
Footonotes: Human chorionic gonadotropin (hCG) isoforms function in non-pregnant and pregnant women.
Abbreviations: LHCGR = luteinizing hormone/choriogonadotropin receptor; TGFβR = transforming growth factor beta receptor; ?: hCG may bind to relevant receptor in target cells; Smad2 = similar to drosophila gene ‘mothers against decapentaplegic’ 2; Gq = heterotrimeric G protein subunit that activates phospholipase C (PLC)-associated protein kinase C (PKC); Gs = heterotrimeric G protein subunit that activates cAMP-dependent protein kinase A (PKA) signaling by activating adenylyl cyclase; hCH-S = sulfated hCG; hCG-H = hyperglycosylated hCG.
[Source 19 ]Figure 4. Human chorionic gonadotropin (hCG) formation in pregnancy
[Source 20 ]Figure 5. Human chorionic gonadotropin (hCG) in pregnancy
[Source 20 ]Human chorionic gonadotropin function
Human chorionic gonadotropin (hCG) has 4 major isoforms found in the serum and urine during pregnancy: classical hCG, hyperglycosylated hCG (H-hCG), free beta subunit (hCGβ), sulphated hCG, all of them with distinct biological functions 18, 21, 22, 23)):
- Classical human chorionic gonadotropin (hCG) is one of the first molecules secreted by the embryo 24. Its RNA is transcribed as early as the eight-cells stage 25 and the blastocyst produces the protein before implantation 26, 27. During the implantation, human chorionic gonadotropin (hCG) is mainly secreted by the syncytiotrophoblast and less by the cytotrophoblast 18. Human chorionic gonadotropin (hCG) can be detected in the maternal blood 10 days after ovulation. Its concentration reaches its top level around the 10th and 11th weeks of gestation 18. Afterwards, this level decreases and remains basal from the 12th week of gestation onwards until the end of the pregnancy. However, it remains significantly higher than in non-pregnant women (Betz D, Fane K. Human Chorionic Gonadotropin. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532950)), 28. By binding to its receptor called LH-hCG receptor (LHCGR), classical hCG acts on multiple types of cells: corpus luteum cells, myometrial smooth muscle cells, endothelial cells, and decidual cells. During the fifth and sixth days of embryogenesis, the blastocyst secretes hCG into the uterine cavity. This hormone binds to its LH-hCG receptor (LHCGR) on the deciduous surface. In response, the decidua prepares for implantation 29, 30, 31. Human chorionic gonadotropin (hCG) influences stromal cells by underpinning the decidualization and the prolactin secretion 32. D’Hauterive et al 30 have shown that the hCG–LHCGR complex also increases the secretion of leukemia inhibitory factor (LIF) and decreases the secretion of interleukine-6 (IL-6) by endometrial cells, factors affecting embryo implantation. This complex also promotes the differentiation of cytotrophoblasts into syncytiotrophoblasts 33. The hCG–LHCGR complex also regulates prostaglandin synthesis 34 and the formation of cAMP 35. Human chorionic gonadotropin (hCG) encourages trophoblast invasion and interstitial theca cell proliferation by over-modulating ERK and AKT signals 36, 37. Aside from this hCG-LHCGR complex, it has been shown that multiple hCG isoforms could stimulate trophoblastic invasion without regard to the LH-hCG receptor (LHCGR) 38.
- Hyperglycosylated hCG (H-hCG). Hyperglycosylated hCG β subunit has four oligosaccharide-linked instead of two in the classical form of the hCG β subunit 39. Hyperglycosylated hCG is massively produced during the first trimester of pregnancy, particularly by the extravillous cytotrophoblasts 40. Hyperglycosylated hCG represents the majority of the total hCG in the third week of gestation and 50% during the fourth week 18, 41. Then, it decreases rapidly until it completely disappears from the maternal blood circulation at the end of the first trimester 42. Hyperglycosylated hCG is useful for predicting pregnancy outcomes in women, with a first trimester suspicion of abortion. However, it is not considered as a better tool than the classical form of hCG 43. Hyperglycosylated hCG acts through autocrine instead of endocrine action. It decreases the apoptosis of trophoblast cells 44 and induces the implantation of the embryo 45 and trophoblastic invasion 46. Hyperglycosylated hCG is also massively secreted by choriocarcinomas and germ cell tumors 46, 47, 39. Its anti-apoptotic action would be achieved by its binding with the TGF-β receptor and independently of LH-hCG receptor (LHCGR). Hyperglycosylated hCG monitoring is useful in predicting Down’s syndrome 46, pre-eclampsia 48, and the therapeutic response to trophoblastic diseases, as well as in pregnancy predictions performed in in vitro fertilization (IVF) 49. The hyperglycosylated hCG (hCG-H) produced early in pregnancy and by various cancers contains more structurally-complex sialylated glycans than hyperglycosylated hCG (hCG-H) produced in mid and late pregnancy 50, 51, 52.
- Free beta subunit human chorionic gonadotropin (βhCG). Free beta subunit human chorionic gonadotropin (hCGβ) acts as an agonist of LH-hCG receptor (LHCGR) and an antagonist of the TGF-β receptor. High blood pressure during pregnancy (preeclampsia) could also be predicted by the abnormal rise in the circulating free β subunit of hCG (hCGβ). However, the association of beta subunit human chorionic gonadotropin (hCGβ) and inflammation, and oxidative stress in a pregnancy-caused hypertensive disorder, on the perinatal stage remains unclear. However, Wang et al. 53 demonstrated via a case–control study that the correlation of circulating free beta subunit human chorionic gonadotropin (hCGβ) levels with inflammatory and oxidative stress markers in patients with pregnancy-induced hypertension (preeclampsia) in perinatal stage was statistically significant. Like hyperglycosylated hCG (H-hCG), maternal serum free beta subunit human chorionic gonadotropin (hCGβ) is also used as a biomarker in first trimester screening for fetal Down’s syndrome 54. The free beta subunit human chorionic gonadotropin (hCGβ) also has a promotive action on cancer: germ cell malignancies, epithelial malignancies or carcinomas, adenocarcinomas, sarcomas, teratomas, blastomas, leukemias and lymphomas 55. For example, this action on the bladder carcinoma is exerted through the inhibition of apoptosis 56. According to Sirikunalai et al. 5, abnormally low (<0.5 MoM) or high (>2.0 MoM) free β subunit human chorionic gonadotropin (hCGβ) levels during gestation are generally associated with an increased risk of adverse pregnancy outcome (spontaneous abortion, preterm birth, low Apgar score, etc.).
- Sulphated human chorionic gonadotropin (hCG-S). Sulphated human chorionic gonadotropin (hCG-S) is produced by the pituitary gland in non-pregnant women and is secreted at the same time as LH during the cycle. Hence, its concentration ranges around one-fiftieth of the LH concentration 12, 57, 58, 59. While these levels are low, sulphated hCG is exactly 50 times more potent than LH 6 and acts the same way by stimulating androstenedione production during the follicular phase of the cycle as well as stimulating ovulation and corpus luteum formation. During the luteal phase, it may help stimulate progesterone production 12, 57, 58, 59. During the menstrual cycle, sulphated human chorionic gonadotropin (hCG-S) mediates several endocrine functions by inducing theca cell androstenedione production, corpus luteal progesterone production and by contributing to the process of ovulation. During pregnancy, syncytiotrophoblast derived hCG also induces corpus luteal cells to produce progesterone, whereas cytotrophoblast-derived hyperglycosylated hCG (hCG-H) appears to act as an autocrine and paracrine factor by activating the transforming growth factor-β receptor (TGFβR) mediated signaling 60, 61, 62, 63.
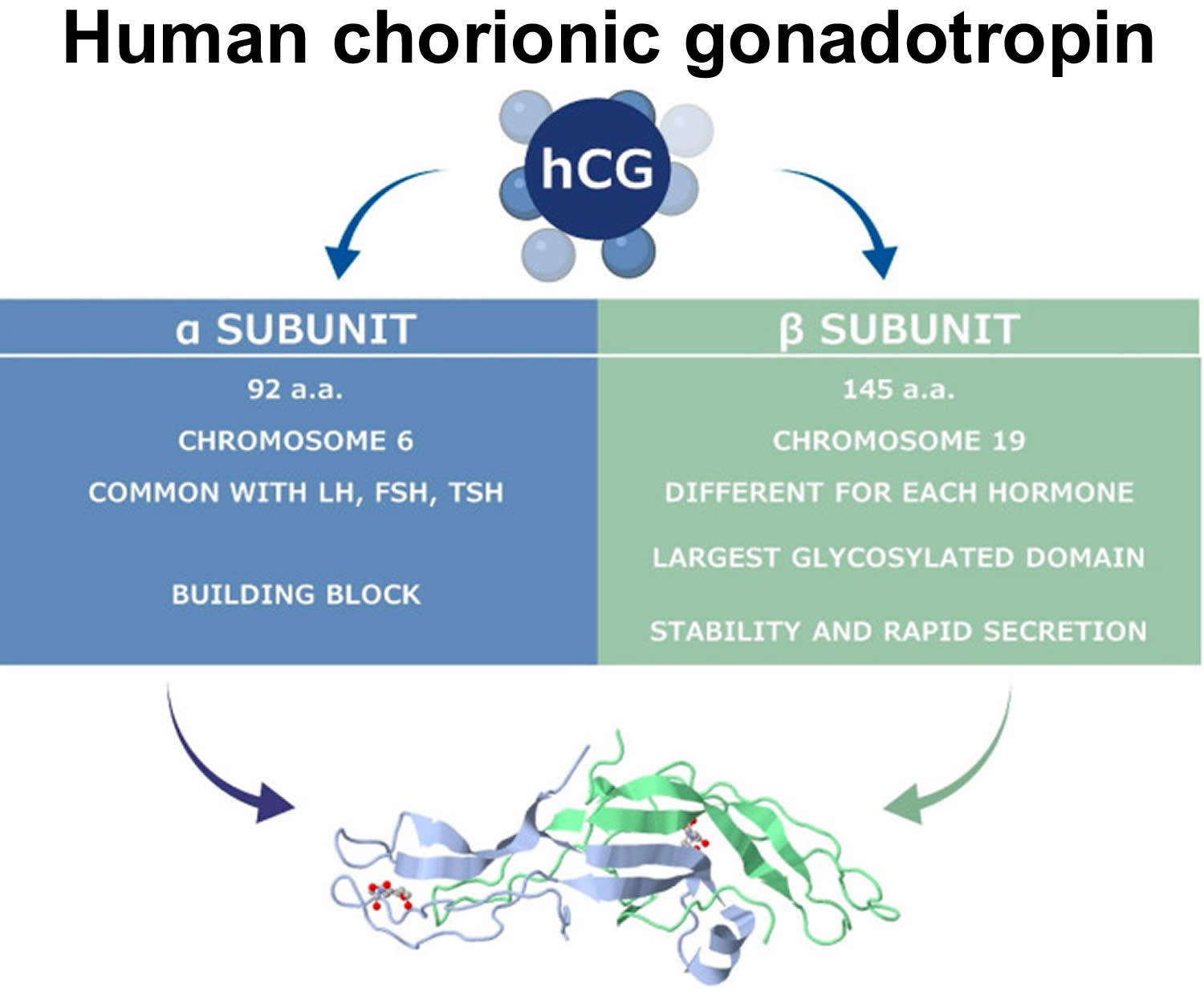
Human chorionic gonadotropin (hCG) is a glycoprotein composed of two subunits, the alpha (α) and beta (β) subunits 4. The alpha (α)-subunit of hCG is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH)50, 64, 65. The beta (β)-subunit of hCG, while structurally somewhat similar to the beta-subunit of luteinizing hormone (LH), differentiates hCG, hyperglycosylated hCG, and pituitary hCG from other molecules. Both human chorionic gonadotropin (hCG) and luteinizing hormone (LH) bind and function through a common luteinizing hormone/chorionic gonadotropin receptor (LHCGR) 65. The marked distinction between the human chorionic gonadotropin (hCG) and luteinizing hormone (LH) besides the absence of a beta subunit in LH is the difference in half-life. LH has a half-life of approximately 25 to 30 minutes 66, while human chorionic gonadotropin (hCG) has a much longer half-life at 24 to 37 hours 67, 68. This difference in half-life is critical to hCG’s function as a type of “super LH” during pregnancy to support the maintenance of an optimal intrauterine environment 69, 67, 70. As a result of human chorionic gonadotropin (hCG) structural homogeneity to luteinizing hormone (LH), hCG binds to luteinizing hormone/chorionic gonadotropin receptor (LHCGR) during the first 3–4 weeks of pregnancy, stimulating corpus luteal cells until the steroidogenic activity of the placenta produces sufficient progesterone to maintain pregnancy 71, 72, 73.
The hCG alpha (α)-subunit is encoded by a single CGA gene localized to chromosome 6q21.1-23 74, while the beta (β)-subunit is encoded by six non-allelic CGB1, 2, 3, 5, 7 and 8 genes localized to chromosome 19q13.3 75, 76. This gene cluster is widely accepted to have evolved as a result of duplication from the LHB gene 77. Expression of hCG genes is regulated by several hormones (corticosteroids, progesterone, GnRH), growth factors (placental growth hormone, leukemia inhibitory factor, vascular endothelial growth factor (VEGF)), cytokines (Interleukin (IL)-6, epidermal growth factors (EGF), tumor necrosis factor (TNF)-α), ligands of the nuclear receptor PPARγ and the homeobox gene (DLX3) 78, 79, 80, 81.
Human chorionic gonadotropin (hCG) is primarily metabolized by the liver with approximately 20% of circulating hCG excreted by the kidneys in the urine 82, 83. The beta-subunit is degraded in the kidney to make hCG β-core fragment (hCGβcf) which is measured by urine hCG tests 82, 84, 1. In early pregnancy, levels of beta-core fragment hCG (hCGβcf) in urine are low 50, while in the second trimester, approximately 80% of immunoreactive urinary hCG levels consists of hCGβcf 85.
The most well-known function of human chorionic gonadotropin (hCG) is the promotion of progesterone production during pregnancy. Human chorionic gonadotropin (hCG) stimulates ovarian corpus luteal cells to produce progesterone, therefore reinforcing the endometrial walls and preventing menstrual bleeding. This promotion of progesterone production is active in approximately 10% of the total length of the pregnancy or around 3 to 4 weeks following implantation. In a non-pregnant female, LH promotes progesterone production 65, 86, 87.
Studies done over recent years have shown hCG to be involved in a plethora of functions supporting the placenta, uterus, and fetus throughout pregnancy. These functions include the promotion of angiogenesis, immunosuppression, and blockage of phagocytosis of invading trophoblasts, promotion of growth and differentiation of fetal organs, and involvement in the adult brain and brainstem 88, 87.
Human chorionic gonadotropin (hCG) promotes the formation of new blood vessels (angiogenesis) and vasculogenesis through the upregulation of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) 89. Uterine spinal arteries have hCG receptors that, when acted upon by hCG, undergo growth, and support the adequate blood supply and nutrition to the placenta. Human chorionic gonadotropin (hCG) also promotes the fusion of cytotrophoblast cells and their subsequent differentiation into syncytiotrophoblasts 88, 65.
Human chorionic gonadotropin (hCG) achieves many of its functions through the regulation of the expression of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) and its receptors 89. The EG-VEGF receptors are G protein-coupled receptors (GPCRs), prokineticin 1 (PROKR1), and prokineticin 2. EG-VEGF is an angiogenic factor specific to endocrine tissues, including the placenta. EG-VEGF expression peaks around the same time as the peak of hCG concentration at approximately 8 to 11 weeks gestation 89. As an angiogenic factor, EG-VEGF expression increases in conditions of hypoxia. EG-VEGF and its receptors are regulators of both pathological and normal development of the fetus. EG-VEGF, PROKR1, and PROKR2 levels are significantly higher in fetal growth-restricted patients. Some have proposed that increases in EG-VEGF expression and its receptors brought on by increased levels of hCG are a form of compensation in fetal growth restriction 87, 90, 70, 86.
Maternal human chorionic gonadotropin (hCG) has implications in the development of fetal organs during development. There are hCG receptors in the fetal liver and kidney that are completely absent in adult organs. Human chorionic gonadotropin (hCG) has also been shown to support the growth and development of the umbilical cord 65, 70, 86.
Researchers have found hCG receptors in various areas of the adult female brain, including the hippocampus, hypothalamus, and brain stem. Speculation is that the presence of these receptors in the brain are involved in the pathophysiology of hyperemesis gravidarum. Other contributing factors may involve a combination of rising hormone levels overall, including estrogen, progesterone, and serum thyroxine, in addition to elevated hCG 65, 86, 91.
Human chorionic gonadotropin in Embryo Implantation and Placentation
Human chorionic gonadotropin (hCG) represents one of the first molecular messages send out by the pre-implanting embryo to regulate the implantation site and to ensure a timely initiation of the nidation process 92. Despite CGB gene expression was proven already in the 8-cell stage embryo 93, active secretion of human chorionic gonadotropin (hCG) starts at the blastocyst stage 94 and enables hCG detection in the maternal circulation 10 days after fertilization. Later on, human chorionic gonadotropin (hCG) is produced in high amounts by trophoblast cells 95 resulting in the highest hCG values between the 10th and 11th week of pregnancy. By the end of the first trimester, hCG levels decrease but remain elevated compared to non-pregnant individuals. A drop of hCG seems to be required for normal pregnancy progression 92. Blastocyst is an embryo that has formed a fluid-filled cavity and the cells have begun to form the early placenta and embryo, usually 5 days after ovulation or egg retrieval 96. A recent meta-analysis suggested that elevated hCG levels can be detected already at the end of the first trimester in women developing preterm preeclampsia (high blood pressure during pregnancy) 97 and hCG was suggested as a useful predictor for the development and severity of preeclampsia (high blood pressure during pregnancy) 98, 99.
Human chorionic gonadotropin angiogenic actions
Classical human chorionic gonadotropin (hCG) has angiogenic actions through the LHCGR (LH-hCG receptor) and achieves many of its functions through the regulation of the expression of endocrine gland-vascular endothelial growth factor (EG-VEGF) and its receptors 100, 101, 101, 2.
Human chorionic gonadotropin (hCG) increases blood vessel formation (angiogenesis) and the migration and maturation of pericytes in different in vitro and in vivo models. Through this action, the trophoblast can form plugs that prevent maternal blood from bleeding into the intervillous spaces during early pregnancy 2, 102, 103, 104, 105.
Human chorionic gonadotropin (hCG) also enhances the secretion of VEGF through the activation of NF-κB on angiogenesis during the luteal phase (Berndt S., D’Hauterive S.P., Blacher S., Pequeux C., Lorquet S., Munaut C., Applanat M., Hervé M.A., Lamandé N., Corvol P., et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje)), 106, 107. In addition, hCG shields vascular endothelial cells against oxidative stress through the inhibition of apoptosis, activation of cell survival signaling, and mitochondrial function retention 108. Jing et al. 109 have shown that the decreased production of the β subunit human chorionic gonadotropin in early pregnant women could act on the expression of VEGF-MEK/ERK signal pathway by down-regulating it. It reduces angiogenesis and eventually leads to the abnormal angiogenesis of the villosities, a mechanism which may be an important factor of missed abortion 109.
As hyperglycosylated hCG subunit still presents a potent angiogenic effect but is acting regardless of LHCGR signaling pathways 77, 110. Gallardo et al. 111 have suggested that the striking overlapping of hCG and Heme oxygenase-1 (HO-1) functions in pregnancy could indicate that hCG hormonal effects are mediated by HO-1 activity, which may be affected by a HMOX1polymorphism in humans.
Human chorionic gonadotropin (hCG) and its hyperglycosylated isoform are accordingly considered pro-angiogenic molecules granting adequate fetal perfusion and fetal-maternal exchanges.
Human chorionic gonadotropin immunological actions
The immunomodulatory properties of human chorionic gonadotropin (hCG) are various and important for maternal tolerance of the embryo, an essential mechanism for the embryonic implantation and development 101, 112, 113. Immune cells situated in the uterine cavity play a key role in the embryo implantation 114, 115.
Several studies have supported the function of hCG in the prevention of fetoplacental tissue rejection through inhibitory immune-mediated mechanisms 116, 117. Some groups have shown that an anti-macrophage inhibitory factor is upregulated by human chorionic gonadotropin (hCG) activity during pregnancy, thus reducing macrophage activity at the uterine-placental interface 118, 119, 120 Other studies support a more proximate mechanism of action in which hCG directly suppresses immune actions taken against the fetus 88, 87, 121.
Human chorionic gonadotropin uses
Human chorionic gonadotropin in Normal and Abnormal Pregnancies
Human chorionic gonadotropin (hCG) is a pregnancy supporting hormone and its clinical utility is primarily centered around its detection in early pregnancy, along with serial measurement during pregnancy and pregnancy-related complications. Early in pregnancy, the level of human chorionic gonadotropin (hCG) increases in the blood and is eliminated in the urine. A pregnancy test detects human chorionic gonadotropin (hCG) in the blood or urine and confirms or rules out pregnancy 3. Levels of hCG can vary widely between women with normal pregnancies. In viable pregnancies, a median hCG concentration of 126 IU/L is observed 12 days after embryo transfer, whereas levels below 76 IU/L are associated with early pregnancy loss 122. Typically, serum and urine concentrations of hCG rise exponentially in the first trimester of pregnancy, doubling about every 24 hours during the first 8 weeks 1. The peak is usually around 10 weeks of gestation and then levels decrease until about the 16th week of gestation where they remain fairly constant until term 123. Compared with an ongoing pregnancy, a failing pregnancy is generally associated with lower serum HCG levels, which gradually turns into a decrease 124. Patients who have hCG levels that plateau prior to 8 weeks or that fail to double commonly have a nonviable pregnancy, whether intra-uterine or extra-uterine 61, 125. Extra-uterine (ectopic) pregnancies usually have a rate-of-rise that is low without the typical doubling. However, given the large range of normal hCG levels and inconsistent rates-of-rise of this hormone, checking serum hCG levels is typically paired with ultrasound evaluation to improve sensitivity and specificity 126.
Qualitative human chorionic gonadotropin (hCG) testing detects the presence of human chorionic gonadotropin (hCG) and is routinely used to screen for a pregnancy. This test may be performed by a laboratory, at a doctor’s office, or at home using a home pregnancy test kit. Methods will vary slightly but for most, a test strip is dipped into a collected cup of urine or exposed to a woman’s urine stream. A colored line (or other color change) appears within the time allotted per instructions, usually about 5 minutes. For accurate test results, it is important to carefully follow the test directions. (See the article on Home Testing: Avoiding Errors for more on this.) If the test is negative, it is often repeated several days later. Since human chorionic gonadotropin (hCG) rises rapidly, an initial negative test can turn positive within this time period.
Quantitative human chorionic gonadotropin (hCG) testing, often called beta human chorionic gonadotropin (β-hCG), measures the amount of human chorionic gonadotropin present in the blood. It may be used to confirm a pregnancy. It may also be used, along with a progesterone test, to help diagnose an ectopic pregnancy, to help diagnose and monitor a pregnancy that may be failing, and/or to monitor a woman after a miscarriage.
Human chorionic gonadotropin (hCG) blood measurements may also be used, along with a few other tests, as part of screening for fetal abnormalities.
Occasionally, an human chorionic gonadotropin test is used to screen for pregnancy if a woman is to undergo a medical treatment, be placed on certain drugs, or have other testing, such as x-rays, that might harm the developing baby. This is usually done to help confirm that the woman is not pregnant. It has become standard practice at most institutions to screen all female patients for pregnancy using a urine or blood human chorionic gonadotropin test before a medical intervention, such as an operation, that could potentially harm a fetus.
Certain cancers can also produce either human chorionic gonadotropin (hCG) or hCG-related hormone. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules 1, 11. Detection of hCG is also useful in the evaluation of trophoblastic disease, including complete and partial hydatidiform mole, postmolar tumor, gestational choriocarcinoma, testicular choriocarcinoma, and placental site trophoblastic disease. All of these entities produce hCG, varying levels of which are reported on commercial assays. A total hCG level of greater than 100,000 mIU/mL in early pregnancy, for example, is highly suggestive of a complete hydatidiform mole, although many normal pregnancies may reach this level at their peak around weeks 8 to 11 of gestation 127. Precise hCG measurements are important to assess the tumor mass, the successful treatment of trophoblastic cancer and to test for recurrence or persistence of trophoblastic disease 128.
The development of molar pregnancy correlates with fluxes in the levels of free beta-subunit of hCG. Return of hCG to zero following delivery or termination of pregnancy ranges from 7 to 60 days 129. Trending the fall of hCG levels can be important in termination of molar pregnancies and also following the termination of normal or ectopic pregnancies to be assured that the therapy has been successful. In a complete molar pregnancy, it is not uncommon to see large theca-lutein cysts as a result of increased stimulation of the ovaries by excess free beta-subunit hCG 130, 131, 132.
A molar pregnancy or hydatidiform mole, is a tumor arising from the trophoblast, which surrounds a blastocyst and subsequently develops into the chorion and amnion 131, 132. Hydatidiform mole may manifest as a complete or partial molar pregnancy. A complete hydatidiform mole is usually diploid with a 46 XX karyotype. There is trophoblastic hyperplasia producing a mass of multiple vesicles with little evidence of fetal and embryonic development. A partial hydatidiform mole is usually triploid due to dispermic fertilization or fertilization with an unreduced diploid sperm. In contrast to the complete mole, there is usually evidence of fetal development with an enlarged placenta 87, 131.
Patients with a history of prior molar pregnancy are at a 10-fold greater risk of a second hydatidiform pregnancy compared to the general population. The recommendation is that these women have their hCG levels monitored throughout pregnancy, as well as undergo evaluation by early ultrasonography 131, 132, 3.
Abnormal levels of hCG are associated with adverse pregnancy outcomes such as molar pregnancies and fetal growth restrictions. The intrauterine conditions are dependent upon placental function as the placenta is the main source of fetal nourishment. Suboptimal conditions due to an atrophic placenta may contribute to the risk of low birth weight. Several studies support the correlation between low birth weight and the risk of developing chronic conditions such as diabetes and hypertension later in life 133, 130, 131, 65.
Several clinical studies support the association of hCG concentration abnormalities with adverse fetal outcomes. This association varies with gestational age as hCG levels fluctuate throughout the pregnancy 131, 132, 130.
In the first trimester, low levels of hCG have correlated with spontaneous abortion and preeclampsia. Some studies have shown an association between low hCG concentrations (especially of the free beta-subunit of hCG) during the latter half of the first trimester and low birth weight due to attenuated fetal growth. Interestingly, some studies show that higher maternal hCG concentrations at the end of the first trimester are associated with fetal growth acceleration only in female-sex fetuses 133, 130.
In the second trimester, high levels of hCG have associations with gestational hypertension, spontaneous abortion, preeclampsia, fetal growth restriction (low birth weight), and pre-term delivery; this is in contrast to the association of low levels of hCG and low birth weight observed in the first trimester of pregnancy 130, 134.
What does human chorionic gonadotropin test result mean?
- A negative human chorionic gonadotropin result means that it is unlikely that a woman is pregnant. However, tests performed too early in a pregnancy, before there is a significant human chorionic gonadotropin (hCG) level, may give false-negative results. The test may be repeated a few days later if there is a strong possibility of pregnancy.
- A positive human chorionic gonadotropin means that a woman is likely pregnant.
- However, blood or protein in the urine may cause false-positive pregnancy results. Urine human chorionic gonadotropin (hCG) tests may give a false-negative result if the urine is too diluted or if testing is done too soon in the pregnancy.
- Certain drugs such as diuretics and promethazine (an antihistamine) may cause false-negative urine results. Other drugs such as anti-convulsants, anti-parkinson drugs, hypnotics, and tranquilizers may cause false-positive results. The presence of protein in the urine (proteinuria), blood in the urine (hematuria), or excess pituitary gonadotropin may also cause a false positive.
- There are reports of false-positive blood human chorionic gonadotropin (hCG) results due to the presence of certain types of antibodies that some individuals produce or fragments of the human chorionic gonadotropin (hCG) molecule. Generally, if results are questionable, they may be confirmed by testing with a different method.
The blood level of human chorionic gonadotropin in a woman with an ectopic pregnancy usually rises at a slower rate than normal. Typically, human chorionic gonadotropin (hCG) levels double about every two days for the first four weeks of a normal pregnancy, then slow to every 31/2 days by six weeks. Those with failing pregnancies will also frequently have a longer doubling time early on or may even show falling human chorionic gonadotropin (hCG) concentrations during the doubling period. Human chorionic gonadotropin (hCG) concentrations will drop rapidly following a miscarriage. If human chorionic gonadotropin (hCG) does not fall to undetectable levels, it may indicate remaining human chorionic gonadotropin-producing tissue that will need to be removed (dilation and curettage – D&C).
How does the test that I do at home myself compare with the results of a test done in a lab?
Home pregnancy testing is very similar to qualitative urine human chorionic gonadotropin testing performed in the laboratory, but there are factors surrounding its use that are important to note.
- Home tests come with very specific directions that must be followed explicitly. If you are using a home test, follow the directions extremely carefully. There can be variability in sensitivity to detecting the presence of human chorionic gonadotropin with different brands of home pregnancy kits.
- Home tests are sometimes done too soon after the missed menstrual cycle to result in a positive test. It typically takes 10 days after a missed menstrual period before the presence of human chorionic gonadotropin can be detected by the urine test.
- All urine human chorionic gonadotropin tests should be done on a first morning urine sample, if possible. Urine becomes more dilute after ingestion of liquids (coffee, juice, water, etc.) and urine human chorionic gonadotropin concentrations may become too low to register as positive.
Generally, when used correctly, the home test should produce the same result as the urine human chorionic gonadotropin test done by your health practitioner. Blood testing for human chorionic gonadotropin is more sensitive than urine human chorionic gonadotropin testing, so sometimes a blood test will indicate pregnancy when the urine test is negative.
When is a blood human chorionic gonadotropin test ordered instead of a urine human chorionic gonadotropin?
Since human chorionic gonadotropin is not normally detected in the urine of a non-pregnant woman, a urine human chorionic gonadotropin is enough to confirm a pregnancy. This can also be done with a qualitative blood human chorionic gonadotropin test. Sometimes, however, it is important to know how much human chorionic gonadotropin is present to evaluate a suspected ectopic pregnancy or to monitor a woman following a miscarriage. In these circumstances, a health practitioner will order a quantitative blood human chorionic gonadotropin test.
How many days after a miscarriage would it take for a urine pregnancy test to show a negative result?
Urine human chorionic gonadotropin decreases at about the same rate as serum human chorionic gonadotropin, which can take anywhere from 9 to 35 days, with a median of 19 days. However, the timeframe for when an human chorionic gonadotropin result will be negative is dependent on what the human chorionic gonadotropin level was at the time of the miscarriage. Frequently, miscarriages are monitored with quantitative blood human chorionic gonadotropin testing. If the levels of human chorionic gonadotropin do not fall to undetectable levels, some human chorionic gonadotropin-producing tissue may remain and have to be removed.
What is an ectopic pregnancy?
An ectopic pregnancy occurs when the fertilized egg (ovum) implants somewhere other than in the uterus. This is a serious condition needing immediate treatment. Women with ectopic pregnancies often have abdominal pain and uterine bleeding. Usually, abnormally low levels of human chorionic gonadotropin are produced in ectopic pregnancies with slower-than-normal rates of increase.
Human chorionic gonadotropin as a Potential Biomarker for Preeclampsia
Pregnancies complicated by high blood pressure during pregnancy (preeclampsia) display an over-abundance of non-invasive syncytiotrophoblasts accompanied by inadequate cytotrophoblast invasion 135. Preeclampsia (high blood pressure during pregnancy) is frequently accompanied by low hyperglycosylated human chorionic gonadotrophin (hCG-H) serum levels during the first trimester of pregnancy (8–13 weeks) 136. A study by Kalkunte et al. 137 found higher hCG levels in serum from preeclamptic pregnancies at term compared with serum derived from normal pregnancies. The pathogenesis of preeclampsia is associated with altered glycosylation patterns and/or presence of sialyl Lewis antigens on hCG, which impairs the recruitment and/or expansion of tolerance-inducing immune cell types 7, 138. The combination of hyperglycosylated human chorionic gonadotrophin (hCG-H) together with pregnancy-associated plasma protein A (PAPP-A) levels, maternal mean arterial pressure and parity were shown to predict early onset preeclampsia 136. A meta-analysis of by Zhong et al. 139 evaluated first-trimester serum screening for 112,400 women in order to predict preeclampsia. Although the results showed no improvement in prediction, the detection rate of first trimester serum markers for early preeclampsia was observed to be better than that for late preeclampsia 136, 140, 141, 142.
Human chorionic gonadotropin as Serum Marker for Down’s Syndrome Screening
Measurements of free beta-human chorionic gonadotropin (hCGβ) levels in first trimester maternal serum proved to be extremely useful in screening for Down’s syndrome 19. Pregnancies complicated by Down’s syndrome are associated with elevated serum hCG and free beta-human chorionic gonadotropin (hCGβ) concentrations 19. Recommended screening for trisomy 21 (Down’s syndrome) includes a combination of maternal age, fetal nuchal translucency (NT) thickness, maternal serum hCGβ and PAPP-A at around 11–13 weeks gestation 143. However, for second trimester (15–22 weeks gestation) screening, hCG is combined with inhibin A, alpha-fetoprotein (AFP) and unconjugated estriol. To assess patient-specific risks for trisomy 21 or Down’s syndrome, a prior maternal age-related risk is multiplied by likelihood ratios determined from deviation of the measured markers from the respective expected levels 144. Furthermore, several markers of Down’s syndrome (β-core fragment hCG [hCGβcf], β human chorionic gonadotropin [hCGβ] and hyperglycosylated hCG [hCG-H]) can be detected in maternal urine 145. Among these, β-core fragment hCG (hCGβcf) is the major metabolic product of hCG in maternal urine with second trimester levels increasing in pregnancies complicated by Down’s syndrome 145. Urinary hyperglycosylated hCG (hCG-H) levels are also elevated in affected pregnancies although fewer cases have been tested for hCG-H compared to hCGβcf levels. Maternal serum hCG assays remain the preferred test for Down’s syndrome screening versus urine β-core fragment hCG (hCGβcf) or hyperglycosylated hCG (hCG-H) due to the wide standard deviation of the urine markers and the significant heterogeneity that exists among studies 145.
Human chorionic gonadotropin as tumor marker
Serum human chorionic gonadotropin (hCG) is also a tumor marker for certain types of testicular cancer (seminomas, nonseminomatous germ cell tumor and extragonadal tumors) in males and gestational trophoblastic disease (GTD) in females after pregnancy and germ cell tumors 146, 147, 148. Since hCG is not normally present in men or non-pregnant women, it is useful as a tumor marker. If the tumor or cancer is producing hCG, then the hCG test can be used to help detect and monitor tumor activity. If hCG is increased with these conditions, then the hCG test can be used as a diagnostic and monitoring tool. Tumor markers are substances, often proteins, that are produced by the cancer tissue itself or sometimes by the body in response to cancer growth. Because some of these substances can be detected in body samples such as blood, urine, and tissue, these markers may be used, along with other tests and procedures, to help detect and diagnose some types of cancer, predict and monitor a person’s response to certain treatments, and detect recurrence.
When testing hCG as a tumor marker, unlike detecting pregnancy, it is important to measure the intact (alpha + beta subunits) form of hCG. There may be some benefit to also measuring the beta-subunit of hCG (hCGβ) in certain tumors, and different labs will detect intact and beta human chorionic gonadotropin (hCGβ) differently. For this reason, it is very important to continue to use the same lab when monitoring testicular cancer, gestational trophoblastic disease or germ cell tumors.
Gestational trophoblastic disease (GTD) is a group of tumor types that develop in a woman’s uterus from the layer of cells surrounding an embryo that creates the placenta during a normal pregnancy (trophoblasts) and produces hCG 149. Gestational trophoblastic disease usually occurs at the beginning of pregnancy after an egg has been fertilized, but instead of supporting the growth of a fetus, the cells form abnormal tissue masses. This tissue is made of trophoblast cells and normally surrounds the fertilized egg in the uterus. Trophoblast cells help connect the fertilized egg to the wall of the uterus and form part of the placenta (the organ that passes nutrients from the mother to the fetus) 149. Sometimes there is a problem with the fertilized egg and trophoblast cells. Instead of a healthy fetus developing, a tumor forms. Until there are signs or symptoms of the tumor, the pregnancy will seem like a normal pregnancy. Most gestational trophoblastic disease is benign (not cancer) and does not spread, but some types become malignant (cancer) and spread to nearby tissues or distant parts of the body. According to the American Cancer Society, gestational trophoblastic disease occurs in about 1 in 1,000 pregnancies. It can also occur after a normal pregnancy or after a miscarriage or abortion.
The primary forms of gestational trophoblastic disease are 149:
- Hydatidiform mole also called a “molar pregnancy”, which may be complete (only tumor tissue) or a mixture of tumor and fetal tissue but does not develop into a viable baby; these are usually benign but must be surgically removed.
- Complete hydatidiform mole.
- Partial hydatidiform mole.
- Gestational Trophoblastic Neoplasia (GTN)
- Invasive mole – a hydatidiform mole that grows into the uterus wall; it must be surgically removed; however, the condition can persist if gestational trophoblastic disease tissue remains.
- Choriocarcinoma – a rare cancer that may develop from the gestational trophoblastic disease tumor tissue in about 2 to 7 per 100,000 pregnancies; these cancers can grow quickly and spread to other parts of the body.
- Placental-site trophoblastic tumors (PSTT; very rare). Placental site trophoblastic tumor arises at the site of placental attachment in the uterus. This tumor usually develops after a normal or aborted pregnancy but doesn’t often spread through the body.
- Epithelioid trophoblastic tumors (ETT; extremely rare). Epithelioid trophoblastic tumor is similar in nature to the choriocarcinoma but is now considered a separate disease. It may take many years after a pregnancy for this tumor to be detected and may have already spread to other parts of the body.
Testicular cancer is cancer that starts in the testicles. More than 90% of testicular cancers start in cells known as germ cells 150. These are the cells that make sperm. The main types of germ cell tumors in the testicles are seminomas and non-seminomas.
Seminomas is a slow-growing form of testicular cancer found in men in their 40s and 50s 150. Seminomas cancer is in the testes, but it can spread to the lymph nodes. Lymph node involvement is either treated with radiotherapy or chemotherapy. Seminomas are very sensitive to radiation therapy. Elevation of beta-human chorionic gonadotropin (hCGβ) is found in approximately 14% of patients with stage 1 pure seminomas before surgical procedure to remove one or both testicles (orchiectomy) and in about one-half of patients with metastatic seminomas 151, 152, 153. Stage 1 cancer has not spread beyond the testicle. Approximately 40% to 60% of men with nonseminomas have an elevated serum beta-hCG 154.
Non-seminomas, another type of testicular cancer, include embryonal carcinomas, teratomas, yolk sac carcinomas and choriocarcinomas 154, 150. Most nonseminomas are a mix of different types sometimes with seminoma cells too, but this doesn’t change the treatment of most non-seminoma cancers 150. Nonseminomas usually occur in men between their late teens and early 30s. For patients with nonseminomas, one of the most significant predictors of prognosis is the degree of tumor-marker elevation after the cancerous testicle has been removed 155. Alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (hCGβ), and lactase dehydrogenase (LDH) play an important role as serum tumor markers in the staging and monitoring of germ cell tumors and should be measured prior to removing the involved testicle 156. Elevated levels of serum tumor markers are often the earliest sign of relapse, making these markers useful for monitoring all stages of nonseminomas and metastatic seminomas. Tumors that appear to have a seminoma histology but are accompanied by an elevated serum level of alpha-fetoprotein (AFP) should be treated as nonseminomas because seminomas do not produce alpha-fetoprotein (AFP) 154.
- Embryonal carcinoma: These cells are found in about 40% of testicular tumors, but pure embryonal carcinomas occur only 3% to 4% of the time. When seen under a microscope, these tumors can look like tissues of very early embryos. This type of non-seminoma tends to grow rapidly and spread outside the testicle. Embryonal carcinoma can increase blood levels of a tumor marker protein called alpha-fetoprotein (AFP), as well as human chorionic gonadotropin (HCG).
- Choriocarcinoma: This is a very rare and fast-growing type of testicular cancer in adults. Pure choriocarcinoma is likely to spread rapidly to other parts of the body, including the lungs, bones, and brain. More often, choriocarcinoma cells are seen with other types of non-seminoma cells in a mixed germ cell tumor. These mixed tumors tend to have a somewhat better outlook than pure choriocarcinomas, although the presence of choriocarcinoma is always a worrisome finding. Choriocarcinoma increases blood levels of human chorionic gonadotropin (hCG).
- Teratoma: Teratomas are germ cell tumors with areas that, under a microscope, look like each of the 3 layers of a developing embryo: the endoderm (innermost layer), mesoderm (middle layer), and ectoderm (outer layer). Pure teratomas of the testicles are rare and do not increase alpha-fetoprotein (AFP) or human chorionic gonadotropin (hCG) levels. Most often, teratomas are seen as parts of mixed germ cell tumors.
An increased level of hCG is seen in some germ cell tumors (benign and cancerous).
Germ cell tumors and cancers occur primarily in the ovaries and testicles but can also rarely develop in other locations such as the chest.
- Germ cell tumors can occur in the egg-producing cells of the ovaries and are more often seen in younger women.
- Germ cell tumors can affect cells within the testicles that make sperm and account for more than 90% of testicular cancers.
Levels of hCG may also be elevated in other diseases such as liver, breast, lung, skin, and stomach cancers. Increased human chorionic gonadotropin (hCG) levels may also be seen in non-cancer conditions such as cirrhosis, duodenal ulcer, and inflammatory bowel disease.
Human Chorionic Gonadotropin (hCG) in Female Infertility Treatment
Human chorionic gonadotropin (hCG) is a pregnancy supporting hormone extracted from the urine of pregnant women and is an injectable hormone that is widely used as an ovulation trigger that helps with the final maturation of the eggs in the ovariain follicles and triggers the ovaries to release the mature eggs (ovulation) in women undergoing Assisted Reproductive Technology (ART) like intrauterine insemination (IUI), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) 157. Human chorionic gonadotropin (hCG) also stimulates the corpus luteum to secrete progesterone to prepare the lining of the uterus for implantation of the fertilized egg.
Human chorionic gonadotropin (hCG) is structurally similar to the luteinizing hormone (LH) that is produced by a woman’s anterior pituitary gland 158. Human chorionic gonadotropin (hCG) acts on the ovary in a manner similar to a woman’s own luteinizing hormone (LH). Human chorionic gonadotropin (hCG), like LH, stimulates the final maturation of the eggs in the follicle. Human chorionic gonadotropin (hCG) also stimulates progesterone production from the ovary after egg retrieval. This progesterone is important to prepare the uterus for implantation of the embryo.
Human chorionic gonadotropin (hCG) and luteinizing hormone (LH) share the same receptor the LHCGR (LH-hCG receptor).
Intrauterine administration of synthetic or natural hCG around the time of embryo transfer in in vitro fertilization (IVF) is a novel approach that has been suggested to improve the outcomes of assisted reproduction treatment (ART) based on the fundamental role of hCG in embryo implantation and the early stages of pregnancy 65. The intervention involves intrauterine administration of hCG via an embryo transfer catheter. The hCG can be released at different points inside the uterine cavity (close to the internal cervical os, mid‐cavity, or near the fundus) within minutes, hours, or days before the actual embryo transfer. Human chorionic gonadotropin (hCG) sources for medical treatments include extraction from the urine of pregnant women (natural) or from cell cultures using recombinant DNA technology (rhCG). This 2018 Cochrane review found moderate quality evidence that women undergoing cleavage‐stage embryo transfer using an intrauterine (intracavity) administration of human chorionic gonadotropin (hCG) dose ≥ 500 IU have an improved live birth rate 159. There is insufficient evidence for intrauterine (intracavity) human chorionic gonadotropin (hCG) treatment for blastocyst transfer. There should be further trials with live birth as the primary outcome to identify the groups of women who would benefit the most from this intervention. There was no evidence that miscarriage was reduced following intrauterine (intracavity) human chorionic gonadotropin (hCG) administration, irrespective of embryo stage at transfer or dose of intrauterine (intracavity) human chorionic gonadotropin (hCG). Events were too few to allow conclusions to be drawn with regard to other complications 159.
Human chorionic gonadotropin treatment
Human chorionic gonadotropin (hCG) is a hormone produced by the placenta following implantation of the fertilized egg that supports the normal development of an egg in a woman’s ovary, and stimulates the release of the egg during ovulation. Human chorionic gonadotropin (hCG) is either derived from the urine of pregnant women (uhCG) or synthetized via recombinant DNA technology also known as recombinant hCG (rhCG) 160. Currently, urine-derived human chorionic gonadotropin preparation (uhCG) is available as a powder or in solution generically and under trade names such as Novarel and Pregnyl. Recombinant hCG (rhCG) is available as Overle. The dose and regimen of hCG therapy varies by indication and it should be used only by physicians with expertise in the management of infertility and hypogonadism. Common side effects include headache, nausea, anorexia, and local injection reactions. Uncommon, but potentially severe adverse events include ovarian hyperstimulation syndrome.
Human chorionic gonadotropin (hCG) is used to cause ovulation and to treat infertility in women, and in men to treat hypogonadism, a condition in which the body doesn’t produce enough testosterone. Human chorionic gonadotropin (hCG) is also used in young boys when their testicles have not dropped down into the scrotum normally. This can be caused by a pituitary gland disorder. Human chorionic gonadotropin (hCG) is a hormone that is used to cause ovulation and to treat infertility in women. Human chorionic gonadotropin (hCG) is not effective in women with primary ovarian failure. Although hCG can help you become pregnant, it should not be used during pregnancy. Tell your doctor right away if you become pregnant during treatment.
The action of human chorionic gonadotropin (hCG) is virtually identical to that of pituitary luteinizing hormone (LH), although human chorionic gonadotropin (hCG) appears to have a small degree of follicle stimulating hormone (FSH) activity as well. Human chorionic gonadotropin (hCG) stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce testosterone and the corpus luteum of the ovary to produce progesterone. Testosterone stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. This descent is usually reversible when hCG is discontinued. During the normal menstrual cycle, luteinizing hormone (LH) participates with follicle stimulating hormone (FSH) in the development and maturation of the normal ovarian follicle, and the mid-cycle LH surge triggers ovulation. Human chorionic gonadotropin (hCG) can substitute for LH in this function. During a normal pregnancy, hCG secreted by the placenta maintains the corpus luteum after LH secretion decreases, supporting continued secretion of estrogen and progesterone and preventing menstruation.
Circulating human chorionic gonadotropin (hCG) interacts with the luteinizing hormone receptors (LHR) of the ovary, promoting the corpus luteum and its production of progesterone which is necessary to maintain pregnancy and support the growth of the fetus. Injections of hCG mimic the surge in LH that is necessary for ovulation and are used in the therapy of female infertility, in assisted reproduction techniques. In clinical trials, hCG resulted in pregnancies in approximately 30% of women. hCG prepared from urine of pregnant women and was approved for use in the United States in 1967 as treatment of ovulatory dysfunction in women desiring pregnancy. Subsequently, recombinant forms of hCG have been developed and licensed for use. Currently, hCG is available as a powder or in solution generically and under trade names such as Novarel and Pregnyl. Recombinant hCG is available as Overle. The dose and regimen of hCG therapy varies by indication and it should be used only by physicians with expertise in the management of infertility and hypogonadism. Common side effects include headache, nausea, anorexia, and local injection reactions. Uncommon, but potentially severe adverse events include ovarian hyperstimulation syndrome.
Human chorionic gonadotropin (hCG) Dosage and Administration
Human chorionic gonadotropin (hCG) medications are Profasi®, Ovidrel®, Novarel®,and Pregnyl® can be administered several different ways. The commonly administered dose is a single injection of 10,000 units. Once hCG is administered, ovulation usually occurs in approximately 36 to 40 hours. Therefore oocyte retrieval is routinely scheduled at 34-36 hours after hCG. This helps ensure maximal egg maturity, which is important for fertilization and embryo development. Occasionally, several doses of 2,500 units (usually every three days) are administered after egg retrieval to stimulate progesterone production. If your response to stimulation is particularly exuberant, the dose of hCG can be reduced to 5,000 units in an attempt to reduce the risk of ovarian hyperstimulation syndrome.
It typically takes 8-10 days for single injection of 10,000 units of hCG to be cleared from the blood stream. As hCG is the same hormone that is produced by a developing pregnancy, patients should not have a blood or urine pregnancy test sooner than 10 days following the hCG injection. If a pregnancy test is performed earlier, it may measure the hCG that was given by injection rather than measure hCG produced by a pregnancy.
Human chorionic gonadotropin (hCG) Therapeutic Uses in the Male
In the male human chorionic gonadotropin (hCG) hormone treatment uses:
- Hypogonadotrophic hypogonadism.
- Delayed puberty associated with insufficient gonadotrophic pituitary function.
- Cryptorchism, not due to an anatomic obstruction.
- Preoperative preparation of ectopic testes.
- Infertility, in selected cases of deficient spermatogenesis.
Hypogonadism is impaired testicular function; this can occur due to a problem with the testes (primary hypogonadism or hypergonadotropic hypogonadism) or due to a problem with the hypothalamic-pituitary-gonadal axis (secondary hypogonadism or hypogonadotropic hypogonadism). These two entities can be distinguished by measuring serum LH and FSH concentrations 161. Primary hypogonadism or hypergonadotropic hypogonadism is characterized by a low serum testosterone level and oligo- or azoospermia in the presence of elevated serum LH and FSH concentrations 161. In contrast, secondary hypogonadism or hypogonadotropic hypogonadism is diagnosed in the setting of a low testosterone level and sperm count in association with low or inappropriately normal serum LH and FSH concentrations 161.
Men with secondary hypogonadism or hypogonadotropic hypogonadism have low levels of androgens in the plasma as well as a lack or delay of sexual maturity, which can cause symptoms such as a lack of libido, depression, increase in adipose tissue, and diminished erectile function 162.
Patients with secondary hypogonadism or hypogonadotropic hypogonadism usually have an issue with gonadotropin-releasing hormone (GnRH) signaling, which then causes a decrease in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion. This decreased FSH and LH contributes to decreased androgen levels and reduced spermatogenesis. Studies have shown that giving these patients pulsatile GnRH or LH (or hCG) and FSH can help increase spermatogenesis and thus increase the sperm concentration in the ejaculate. Even then, most couples will need assisted reproductive technology (ART) to achieve pregnancy 163.
Testosterone therapy, whether by exogenous testosterone replacement or induction of endogenous testosterone production by human chorionic gonadotropin (hCG) is needed in all hypogonadotropic hypogonadism patients 161. Testosterone plays a number of important physiologic roles in the human and are required not only for virilization and normal sexual function, but also for maintenance of both muscle and bone mass, as well as normal mood and cognition.
While testosterone is the primary treatment modality used to induce and maintain secondary sexual characteristics and sexual function in men with hypogonadism, treatment with testosterone does not restore fertility. Therefore, in patients in whom fertility is the treatment goal, induction of gonadotropin secretion by pulsatile GnRH or exogenous gonadotropins is necessary. While hormone therapy is the mainstay of treatment for congenital hypogonadotropic hypogonadism; undescended testicle (cryptorchidism), if present, should be treated surgically with orchidopexy, ideally before the age of 2 years to improve fertility outcomes and reduce the risk of future testicular malignancy 164.
Exogenous Gonadotropin Therapy
The typical gonadotropin regimen for induction of spermatogenesis in men comprises human chorionic gonadotropin (hCG) in combination with follicle-stimulating hormone (FSH). Purified human chorionic gonadotropin (hCG) is an effective substitute for luteinizing hormone (LH) given the structural homology between these 2 hormones which act through the same receptor on Leydig cells 161. While a variety of FSH formulations are now available in different countries, there is little to choose between them in terms of therapeutic efficacy. Traditionally, FSH has been administered in the form of human menopausal gonadotropins (hMG) derived from the urine of postmenopausal women. Although human menopausal gonadotropins (hMG) has both FSH and LH activity, FSH activity predominates and LH activity is so low that combined administration with hCG is necessary to achieve fertility. Subsequently, highly purified urinary FSH preparations were developed, which have enhanced specific activity in comparison to hMG (10,000 IU/mg of protein vs 150 IU/mg of protein for hMG) 161. In the early 1990s, recombinant human follicle stimulating hormone (r-hFSH) formulations were developed, which have greater purity and specific activity than any of the urinary preparations and no intrinsic LH activity 165, 166. Recombinant human follicle stimulating hormone (r-hFSH) is produced in genetically engineered Chinese hamster ovary cells, in which the genes encoding the alpha and beta subunits have been introduced using recombinant DNA technology 165. Pharmacokinetic studies of r-hFSH indicate a half-life of 48 ± 5 hour and a dose-dependent increase in the serum level of FSH 165.
The subcutaneous route of administration is as effective as the intramuscular route for both gonadotropins and significantly increases patient compliance. Therapy is typically initiated with human chorionic gonadotropin (hCG) alone at a dose of 1,000 IU on alternate days and the dose titrated based on trough testosterone levels and testicular growth 167. Alternatively, recombinant human hCG can also be administered subcutaneously from a prefilled syringe. In some patients the dose of hCG can be decreased over time as testicular size increases. In the majority of patients with larger testes at baseline, spermatogenesis can be initiated with hCG alone most likely due to residual FSH secretion 168, 169. Once there is a plateau in the response to hCG which typically occurs at around 6 months, therapy with FSH (in one of the three forms described above) should be added at a dose of 75 IU on alternate days. If sperm output and testicular growth remain suboptimal the dose of FSH can be gradually increased to 150 U daily. Continuation of this combined regimen for 12-24 months induces testicular growth in almost all patients, spermatogenesis in approximately 80% and pregnancy rates in the range of 50% 170, 171, 172. In an Australian study of 75 men with hypogonadotropic hypogonadism treated with gonadotropins the median time for sperm to appear in the ejaculate was 7.1 months and for conception was just over 28 months 171. Similar data were reported in a compilation of clinical trial data from Asian, European, Australian and American patients 172. Factors predictive of better outcome include larger baseline testicular size and absence of cryptorchidism. The Australian study reported that prior androgen use is also a negative prognostic indicator of response 171. While the study investigators propose that gonadotropin therapy be considered to induce puberty based on their results, confirmation that such an approach is superior to the conventional practice of giving testosterone would require a large clinical trial the logistics of which would be challenging given the rarity of this patient population. Gynecomastia is the most common side effect of gonadotropin therapy and is due to human chorionic gonadotropin (hCG) stimulation of aromatase causing increased secretion of estradiol. This undesirable side effect can be prevented by using the lowest dose of human chorionic gonadotropin (hCG) capable of maintaining serum testosterone levels towards the lower end of the normal range.
In the majority of hypogonadotropic hypogonadism patients treated with gonadotropins sperm density remains below the normal range. However, failure to achieve a normal sperm density does not preclude fertility. Indeed, the median sperm concentrations reported at conception range from 5-8 million/mL 168, 171. While spermatogenesis can be initiated even in patients with very small testes 168, 173, a longer duration of therapy is typically required and it may take up to 24 months for spermatogenesis to be induced. Accordingly when discussing the issue of fertility with patients, experts recommend that they start treatment at least 6 to 12 months prior to the time at which fertility is desired. Once pregnancy is achieved, experts advise continuing therapy until at least the second trimester. If the couple plans to have another child in the near future, then hCG monotherapy should be continued. However, if a long interval is expected to elapse before the next pregnancy, it may be more convenient for the patient to resume testosterone therapy. Patients should also be offered the option of storing sperm for subsequent use in intrauterine insemination or intracytoplasmic sperm injection. In patients in whom the combination of hCG and FSH is required to induce spermatogenesis initially, treatment with hCG alone may be sufficient for subsequent pregnancies due to larger testicular size.
In patients with panhypopituitarism who fail to respond to gonadotropin therapy, the addition of recombinant growth hormone (rGH) therapy should be considered. It is thought that a direct effect of growth hormone on Leydig cells may play a role in the delayed puberty encountered commonly in patients with isolated growth hormone deficiency 174, 175. While small non-randomized studies suggest that recombinant growth hormone (rGH) may enhance the testosterone response to human chorionic gonadotropin (hCG) administration 174, larger, randomized studies are needed before a definitive decision about the benefit of recombinant growth hormone (rGH) in inducing spermatogenesis in men with hypopituitarism can be reached.
Human chorionic gonadotropin treatment side effects
Along with its needed effects, human chorionic gonadotropin (hCG) hormone treatment may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Some women using human chorionic gonadotropin (hCG) hormone treatment develop ovarian hyperstimulation syndrome (OHSS) (2-3%), a potentially life-threatening condition. Call your doctor right away if you have symptoms of ovarian hyperstimulation syndrome (OHSS):
- severe stomach pain or pelvic pain
- rapid weight gain, swelling around your waist, feeling short of breath
- severe nausea and vomiting, diarrhea
- little or no urination.
Human chorionic gonadotropin (hCG) hormone treatment may cause serious side effects. Call your doctor at once if you have:
- fluid build-up around the lungs (pleural effusion) or stomach (acites) causing rapid weight gain, stomach pain and bloating, pain when you breathe, feeling short of breath while lying down, cough with foamy mucus, rapid heartbeats;
- signs of a blood clot–sudden numbness or weakness on one side of the body, chest pain, problems with vision or speech, pain or swelling in one leg.
Common side effects of human chorionic gonadotropin (hCG) hormone may include:
- nausea (3%)
- vomiting (3%)
- headache, depression
- feeling restless or irritable
- swelling
- breast tenderness or swelling
- ovarian cyst (3%)
- pain where the medicine was injected or injection site inflammation (<2%).
Some boys and men using human chorionic gonadotropin (hCG) hormone treatment develop:
- early puberty in boys–enlarged testicles and penis, facial hair, a deepened voice.
- acne
- enlargement of penis and testes
- growth of pubic hair
- increase in height (rapid)
- chest tightness
- flushing of the skin
- hives or welts
- itching of the skin
- large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- pain in chest, groin, or legs, especially in the calves
- redness of the skin
- severe, sudden headache
- skin rash
- slurred speech
- sudden loss of coordination
- sudden, severe weakness or numbness in the arm or leg
- unusually warm skin
- vision changes.
Human chorionic gonadotropin (hCG) hormone treatment may cause other side effects. Call your doctor if you experience any unusual problems while you are taking this medication.
- Betz D, Fane K. Human Chorionic Gonadotropin. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532950[↩][↩][↩][↩][↩]
- Ogino MH, Tadi P. Physiology, Chorionic Gonadotropin. [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556118[↩][↩][↩]
- Cole LA, Laidler LL. Inherited human chorionic gonadotropin. J Reprod Med. 2010 Mar-Apr;55(3-4):99-102.[↩][↩][↩][↩]
- Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostics. Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. doi: 10.1016/j.cca.2011.05.025[↩][↩]
- Sirikunalai P., Wanapirak C., Sirichotiyakul S., Tongprasert F., Srisupundit K., Luewan S., Traisrisilp K., Tongsong T. Associations between maternal serum free beta human chorionic gonadotropin (β-hCG) levels and adverse pregnancy outcomes. J. Obstet. Gynaecol. 2016;36:178–182. doi: 10.3109/01443615.2015.1036400[↩][↩]
- Craig W.Y., Haddow J.E., Palomaki G.E., Roberson M. Major fetal abnormalities associated with positive screening tests for Smith-Lemli-Opitz syndrome (SLOS) Prenat. Diagn. 2007;27:409–414. doi: 10.1002/pd.1699[↩][↩]
- Norris W., Nevers T., Sharma S., Kalkunte S. Review: hCG, preeclampsia and regulatory T cells. Placenta. 2011;32:S182–S185. doi: 10.1016/j.placenta.2011.01.009[↩][↩]
- Barjaktarovic M., Korevaar T.I.M., Jaddoe V.W.V., de Rijke Y.B., Peeters R.P., Steegers E.A.P. Human chorionic gonadotropin and risk of pre-eclampsia: Prospective population-based cohort study. Ultrasound Obstet. Gynecol. 2019;54:477–483. doi: 10.1002/uog.20256[↩]
- Barjaktarovic M., Korevaar T.I., Jaddoe V.W., de Rijke Y.B., Visser T.J., Peeters R.P., Steegers E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017;32:135–144. doi: 10.1007/s10654-016-0201-3[↩]
- Stevens F.T., Katzorke N., Tempfer C., Kreimer U., Bizjak G.I., Fleisch M.C., Fehm T.N. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Und Frauenheilkd. 2015;75:1043–1050. doi: 10.1055/s-0035-1558054[↩]
- Cole LA, Butler S. Detection of hCG in trophoblastic disease. The USA hCG reference service experience. J Reprod Med. 2002 Jun;47(6):433-44.[↩][↩]
- Cole L.A. “Background” Human Chorionic Gonadotropin in Healthy, Nonpregnant Women. Clin. Chem. 2005;51:1765–1766. doi: 10.1373/clinchem.2005.056507[↩][↩][↩]
- De Backer B., Goffin F., Nisolle M., Minon J.M. Élévation faible d’hCG en dehors d’un contexte gravidique: À propos de deux cas et revue de la littérature [Persistent low hCG levels beyond pregnancy: Report of two cases and review of the literature] Ann. Biol. Clin. 2013;71:496–502. doi: 10.1684/abc.2013.0876[↩]
- Katabuchi H., Ohba T. Human chorionic villous macrophages as a fetal biological shield from maternal chorionic gonadotropin. Dev. Growth Differ. 2008;50:299–306. doi: 10.1111/j.1440-169X.2008.01030.x[↩]
- Yamaguchi M., Ohba T., Tashiro H., Yamada G., Katabuchi H. Human Chorionic Gonadotropin Induces Human Macrophages to Form Intracytoplasmic Vacuoles Mimicking Hofbauer Cells in Human Chorionic Villi. Cells Tissues Organs. 2013;197:127–135. doi: 10.1159/000342806[↩]
- Paulesu L., Rao C., Ietta F., Pietropolli A., Ticconi C. hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. Int. J. Mol. Sci. 2018;19:914. doi: 10.3390/ijms19030914[↩]
- Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18(10), 2166; doi:10.3390/ijms18102166 http://www.mdpi.com/1422-0067/18/10/2166/htm[↩]
- d’Hauterive SP, Close R, Gridelet V, Mawet M, Nisolle M, Geenen V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int J Mol Sci. 2022 Jan 26;23(3):1380. doi: 10.3390/ijms23031380[↩][↩][↩][↩][↩][↩][↩]
- Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: Biological Functions and Clinical Applications. Int J Mol Sci. 2017 Sep 22;18(10):2037. doi: 10.3390/ijms18102037[↩][↩][↩]
- Pregnancy test:- Part 1 – Normal Pregnancy, Beta-HCG, Human Chorionic Gonadotropin (HCG). https://labpedia.net/pregnancy-part1-normal-pregnancy-beta-hcg-human-chorionic-gonadotrophin-hcg[↩][↩]
- Cole L.A. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod. Biol. Endocrinol. 2009;7:8. doi: 10.1186/1477-7827-7-8[↩]
- Cole LA. hCG, five independent molecules. Clin Chim Acta. (2012) 413:48–65. 10.1016/j.cca.2011.09.037[↩]
- ((Fournier T, Guibourdenche J, Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta. 2015 Apr;36 Suppl 1:S60-5. doi: 10.1016/j.placenta.2015.02.002[↩]
- Jarvela IY, Ruokonen A, Tekay A. Effect of rising hCG levels on the human corpus luteum during early pregnancy. Hum Reprod. (2008) 23:2775–81. 10.1093/humrep/den299[↩]
- Jurisicova A., Antenos M., Kapasi K., Meriano J., Casper R.F. Variability in the expression of trophectodermal markers β-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific β-1 glycoprotein by the human blastocyst. Hum. Reprod. 1999;14:1852–1858. doi: 10.1093/humrep/14.7.1852[↩]
- Bonduelle M.-L., Dodd R., Liebaers I., Van Steirteghem A., Williamson R., Akhurst R. Chorionic gonadotrophin-β mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum. Reprod. 1988;3:909–914. doi: 10.1093/oxfordjournals.humrep.a136808[↩]
- Lopata A., Hay D.L. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum. Reprod. 1989;4:87–94. doi: 10.1093/humrep/4.suppl_1.87[↩]
- Braunstein G.D., Rasor J., Danzer H., Adler D., Wade M.E. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am. J. Obstet. Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4[↩]
- Ohlsson R., Larsson E., Nilsson O., Wahlström T., Sundström P. Blastocyst implantation precedes induction of insulin-like growth factor II gene expression in human trophoblasts. Development. 1989;106:555–559. doi: 10.1242/dev.106.3.555[↩]
- D’Hauterive S.P., Charlet-Renard C., Berndt S., Dubois M., Munaut C., Goffin F., Hagelstein M.-T., Noel A., Hazout A., Foidart J.-M., et al. Human chorionic gonadotropin and growth factors at the embryonic–endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum. Reprod. 2004;19:2633–2643. doi: 10.1093/humrep/deh450[↩][↩]
- Srisuparp S., Strakova Z., Fazleabas A.T. The Role of Chorionic Gonadotropin (CG) in Blastocyst Implantation. Arch. Med. Res. 2001;32:627–634. doi: 10.1016/S0188-4409(01)00330-7[↩]
- Lobo S.C., Srisuparp S., Peng X., Fazleabas A.T. Uterine Receptivity in the Baboon: Modulation by Chorionic Gonadotropin. Semin. Reprod. Med. 2001;19:069–074. doi: 10.1055/s-2001-13913[↩]
- Shi Q.J., Lei Z.M., Rao C.V., Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387–1395. doi: 10.1210/endo.132.3.7679981[↩]
- North R.A., Whitehead R., Larkins R.G. Stimulation by Human Chorionic Gonadotropin of Prostaglandin Synthesis by Early Human Placental Tissue. J. Clin. Endocrinol. Metab. 1991;73:60–70. doi: 10.1210/jcem-73-1-60[↩]
- Weedon-Fekjær M., Taskén K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta. 2012;33:S87–S91. doi: 10.1016/j.placenta.2011.11.003[↩]
- Prast J., Saleh L., Husslein H., E Sonderegger S., Helmer H., Knöfler M. Human Chorionic Gonadotropin Stimulates Trophoblast Invasion through Extracellularly Regulated Kinase and AKT Signaling. Endocrinology. 2007;149:979–987. doi: 10.1210/en.2007-1282[↩]
- Palaniappan M., Menon K. Human Chorionic Gonadotropin Stimulates Theca-Interstitial Cell Proliferation and Cell Cycle Regulatory Proteins by a cAMP-Dependent Activation of AKT/mTORC1 Signaling Pathway. Mol. Endocrinol. 2010;24:1782–1793. doi: 10.1210/me.2010-0044[↩]
- Lee C.-L., Chiu C.N., Hautala L., Salo T., Yeung S.B.W., Stenman U.-H., Koistinen H. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 2013;375:43–52. doi: 10.1016/j.mce.2013.05.009[↩]
- Cole LA, Butler SA. Hyperglycosylated human chorionic gonadotropin and human chorionic gonadotropin free beta-subunit: tumor markers and tumor promoters. J Reprod Med. 2008 Jul;53(7):499-512.[↩][↩]
- Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. (2008) 89:1781–6. 10.1016/j.fertnstert.2007.03.010[↩]
- Cole LA. Hyperglycosylated hCG, a review. Placenta. (2010) 31:653–64. 10.1016/j.placenta.2010.06.005[↩]
- Guibourdenche J., Handschuh K., Tsatsaris V., Gerbaud P., Leguy M.C., Müller F., Brion D.E., Fournier T. Hyperglycosylated hCG Is a Marker of Early Human Trophoblast Invasion. J. Clin. Endocrinol. Metab. 2010;95:E240–E244. doi: 10.1210/jc.2010-0138[↩]
- Salas A., Gastón B., Barrenetxea J., Sendino T., Jurado M., Alcázar J.L. Predictive value of hyperglycosylated human chorionic gonadotropin for pregnancy outcomes in threatened abortion in first-trimester viable pregnancies. An. Sist. Sanit. Navar. 2021;44:23–31. doi: 10.23938/assn.0933[↩]
- Hamada A.L., Nakabayashi K., Sato A., Kiyoshi K., Takamatsu Y., Laoag-Fernandez J.B., Ohara N., Maruo T. Transfection of Antisense Chorionic Gonadotropin β Gene into Choriocarcinoma Cells Suppresses the Cell Proliferation and Induces Apoptosis. J. Clin. Endocrinol. Metab. 2005;90:4873–4879. doi: 10.1210/jc.2004-2458[↩]
- Sasaki Y., Ladner D.G., Cole L.A. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil. Steril. 2008;89:1781–1786. doi: 10.1016/j.fertnstert.2007.03.010[↩]
- Cole L. Hyperglycosylated hCG. Placenta. 2007;28:977–986. doi: 10.1016/j.placenta.2007.01.011[↩][↩][↩]
- Cole L.A., Dai D., Butler S.A., Leslie K.K., Kohorn E.I. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol. Oncol. 2006;102:145–150. doi: 10.1016/j.ygyno.2005.12.047[↩]
- Kovalevskaya G., Kakuma T., Schlatterer J., O’Connor J.F. Hyperglycosylated HCG expression in pregnancy: Cellular origin and clinical applications. Mol. Cell. Endocrinol. 2007;260–262:237–243. doi: 10.1016/j.mce.2006.02.021[↩]
- Bersinger N.A., Wunder D.M., Nicolas M., Birkhäuser M.H., Porquet D., Guibourdenche J. Serum Hyperglycosylated Human Chorionic Gonadotropin to Predict the Gestational Outcome in in vitro Fertilization/Intracytoplasmic Sperm Injection Pregnancies. Fetal Diagn. Ther. 2008;24:74–78. doi: 10.1159/000132412[↩]
- Stenman U.H., Tiitinen A., Alfthan H., Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Update. 2006;12:769–784. doi: 10.1093/humupd/dml029[↩][↩][↩]
- Elliott M.M., Kardana A., Lustbader J.W., Cole L.A. Carbohydrate and peptide structure of the α- and β-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7:15–32. doi: 10.1007/BF02778058[↩]
- Birken S., Yershova O., Myers R.V., Bernard M.P., Moyle W. Analysis of human choriogonadotropin core 2 o-glycan isoforms. Mol. Cell. Endocrinol. 2003;204:21–30. doi: 10.1016/S0303-7207(03)00153-9[↩]
- Wang R., Chen L., Wang X., Liu Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc. Res. 2021;135:104130. doi: 10.1016/j.mvr.2020.104130[↩]
- Ballantyne A., Rashid L., Pattenden R. Stability of maternal serum free β-hCG following whole blood sample transit: First trimester Down’s syndrome screening in Scotland. Ann. Clin. Biochem. 2022;59:87–91. doi: 10.1177/00045632211045250[↩]
- Cole L.A., Butler S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer promoters. Mol. Cell. Endocrinol. 2012;349:232–238. doi: 10.1016/j.mce.2011.10.029[↩]
- Butler S.A., Ikram M.S., Mathieu S., Iles R.K. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br. J. Cancer. 2000;82:1553–1556. doi: 10.1054/bjoc.2000.1177[↩]
- Cole L.A., Laidler L.L., Muller C.Y. USA hCG reference service, 10-year report. Clin. Biochem. 2010;43:1013–1022. doi: 10.1016/j.clinbiochem.2010.05.006[↩][↩]
- Birken S., Maydelman Y., Gawinowicz M.A., Pound A., Liu Y., Hartree A.S. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology. 1996;137:1402–1411. doi: 10.1210/endo.137.4.8625917[↩][↩]
- Cole LA, Gutierrez JM. Production of human chorionic gonadotropin during the normal menstrual cycle. J Reprod Med. 2009 Apr;54(4):245-50.[↩][↩]
- Kovalevskaya G., Birken S., Kakuma T., Ozaki N., Sauer M., Lindheim S., Cohen M., Kelly A., Schlatterer J., O’Connor J.F. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: Preliminary characterization of the hyperglycosylated hCG epitope. J. Endocrinol. 2002;172:497–506. doi: 10.1677/joe.0.1720497[↩]
- Kovalevskaya G., Genbacev O., Fisher S.J., Caceres E., O’Connor J.F. Trophoblast origin of hCG isoforms: Cytotrophoblasts are the primary source of choriocarcinoma-like hCG. Mol. Cell. Endocrinol. 2002;194:147–155. doi: 10.1016/S0303-7207(02)00135-1[↩][↩]
- Cole L.A. hCG, five independent molecules. Clin. Chim. Acta Int. J. Clin. Chem. 2012;413:48–65. doi: 10.1016/j.cca.2011.09.037[↩]
- Berndt S., Blacher S., Munaut C., Detilleux J., Perrier d’Hauterive S., Huhtaniemi I., Evain-Brion D., Noel A., Fournier T., Foidart J.M. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013;27:1309–1321. doi: 10.1096/fj.12-213686[↩]
- Stenman U.H., Alfthan H. Determination of human chorionic gonadotropin. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:783–793. doi: 10.1016/j.beem.2013.10.005[↩]
- Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010 Aug 24;8:102. doi: 10.1186/1477-7827-8-102[↩][↩][↩][↩][↩][↩][↩][↩]
- Schalch DS, Parlow AF, Boon RC, Reichlin S. Measurement of human luteinizing hormone in plasma by radioimmunoassay. J Clin Invest. 1968 Mar;47(3):665-78. doi: 10.1172/JCI105762[↩]
- Faiman C, Ryan RJ, Zwirek SJ, Rubin ME. Serum FSH and HCG during human pregnancy and puerperium. J Clin Endocrinol Metab. 1968 Sep;28(9):1323-9. doi: 10.1210/jcem-28-9-1323[↩][↩]
- Rao CV. Differential properties of human chorionic gonadotrophin and human luteinizing hormone binding to plasma membranes of bovine corpora lutea. Acta Endocrinol (Copenh). 1979 Apr;90(4):696-710. doi: 10.1530/acta.0.0900696[↩]
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol. 2006 Aug;102(2):145-50. doi: 10.1016/j.ygyno.2005.12.047[↩]
- Strott CA, Yoshimi T, Ross GT, Lipsett MB. Ovarian physiology: relationship between plasma LH and steroidogenesis by the follicle and corpus luteum; effect of HCG. J Clin Endocrinol Metab. 1969 Sep;29(9):1157-67. doi: 10.1210/jcem-29-9-1157[↩][↩][↩]
- Cole L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012;10:24. doi: 10.1186/1477-7827-10-24[↩]
- Dufau M.L. The luteinizing hormone receptor. Ann. Rev. Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461[↩]
- Jameson JL, Hollenberg AN. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 1993 Apr;14(2):203-21. doi: 10.1210/edrv-14-2-203[↩]
- Fiddes JC, Goodman HM. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3-18.[↩]
- Boorstein W.R., Vamvakopoulos N.C., Fiddes J.C. Human chorionic gonadotropin β-subunit is encoded by at least eight genes arranged in tandem and inverted pairs. Nature. 1982;300:419–422. doi: 10.1038/300419a0[↩]
- Rull K., Hallast P., Uuskula L., Jackson J., Punab M., Salumets A., Campbell R.K., Laan M. Fine-scale quantification of HCG bata gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol. Hum. Reprod. 2008;14:23–31. doi: 10.1093/molehr/gam082[↩]
- Fournier T., Guibourdenche J., Evain-Brion D. Review: hCGs: Different sources of production, different glycoforms and functions. Placenta. 2015;36:S60–S65. doi: 10.1016/j.placenta.2015.02.002[↩][↩]
- Knofler M. What factors regulate HCG production in Down’s syndrome pregnancies? Regulation of HCG during normal gestation and in pregnancies affected by Down’s syndrome. Mol. Hum. Reprod. 1999;5:895–897. doi: 10.1093/molehr/5.10.895[↩]
- Handschuh K., Guibourdenche J., Cocquebert M., Tsatsaris V., Vidaud M., Evain-Brion D., Fournier T. Expression and regulation by PPARgamma of hCG α- and β-subunits: Comparison between villous and invasive extravillous trophoblastic cells. Placenta. 2009;30:1016–1022. doi: 10.1016/j.placenta.2009.09.006[↩]
- Handschuh K., Guibourdenche J., Tsatsaris V., Guesnon M., Laurendeau I., Evain-Brion D., Fournier T. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-γ Endocrinology. 2007;148:5011–5019. doi: 10.1210/en.2007-0286[↩]
- Murthi P., Kalionis B., Cocquebert M., Rajaraman G., Chui A., Keogh R.J., Evain-Brion D., Fournier T. Homeobox genes and down-stream transcription factor PPARgamma in normal and pathological human placental development. Placenta. 2013;34:299–309. doi: 10.1016/j.placenta.2013.01.005[↩]
- Montagnana M., Trenti T., Aloe R., Cervellin G., Lippi G. Human chorionic gonadotropin in pregnancy diagnostics. Clin. Chim. Acta. 2011;412:1515–1520. doi: 10.1016/j.cca.2011.05.025[↩][↩]
- Nisula B.C., Blithe D.L., Akar A., Lefort G., Wehmann R.E. Metabolic fate of human choriogonadotropin. J. Steroid Biochem. 1989;33:733–737. doi: 10.1016/0022-4731(89)90485-8[↩]
- Wehmann R.E., Nisula B.C. Characterization of a discrete degradation product of the human chorionic gonadotropin β-subunit in humans. J. Clin. Endocrinol. Metab. 1980;51:101–105. doi: 10.1210/jcem-51-1-101[↩]
- Norman RJ, Menabawey M, Lowings C, Buck RH, Chard T. Relationship between blood and urine concentrations of intact human chorionic gonadotropin and its free subunits in early pregnancy. Obstet Gynecol. 1987 Apr;69(4):590-3.[↩]
- Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors–a review. Reprod Biol. 2001 Nov;1(2):5-11.[↩][↩][↩][↩]
- Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007 Jan 2;260-262:264-70. doi: 10.1016/j.mce.2006.02.019[↩][↩][↩][↩][↩]
- Toth P, Li X, Rao CV, Lincoln SR, Sanfilippo JS, Spinnato JA 2nd, Yussman MA. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994 Jul;79(1):307-15. doi: 10.1210/jcem.79.1.8027246[↩][↩][↩]
- Berndt S, Blacher S, Perrier d’Hauterive S, Thiry M, Tsampalas M, Cruz A, Péqueux C, Lorquet S, Munaut C, Noël A, Foidart JM. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. 2009 Nov;94(11):4567-74. doi: 10.1210/jc.2009-0443[↩][↩][↩]
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000 May;82(9):1553-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363404/pdf/82-6691177a.pdf[↩]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993 May;132(5):2262-70. doi: 10.1210/endo.132.5.8477671[↩]
- Schumacher A, Zenclussen AC. Human Chorionic Gonadotropin-Mediated Immune Responses That Facilitate Embryo Implantation and Placentation. Front Immunol. 2019 Dec 10;10:2896. doi: 10.3389/fimmu.2019.02896[↩][↩]
- Bonduelle ML, Dodd R, Liebaers I, Van Steirteghem A, Williamson R, Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum Reprod. (1988) 3:909–14. 10.1093/oxfordjournals.humrep.a136808[↩]
- Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum Reprod. (1989) 4(8 Suppl.):87–94. 10.1093/humrep/4.suppl_1.87[↩]
- Jeschke U, Karsten U, Reimer T, Richter DU, Bergemann C, Briese V, et al.. Stimulation of hCG protein and mRNA in first trimester villous cytotrophoblast cells in vitro by glycodelin A. J Perinat Med. (2005) 33:212–8. 10.1515/JPM.2005.039[↩]
- Society for Assisted Reproductive Technology – Glossary and Terms. https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/patient-resources/glossary/[↩]
- Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, et al.. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol. (2018) 9:1661. 10.3389/fimmu.2018.01661[↩]
- Panwar M, Kumari A, Hp A, Arora R, Singh V, Bansiwal R. Raised neutrophil lymphocyte ratio and serum beta hCG level in early second trimester of pregnancy as predictors for development and severity of preeclampsia. Drug Discov Ther. (2019) 13:34–7. 10.5582/ddt.2019.01006[↩]
- Jensen F, Wallukat G, Herse F, Budner O, El-Mousleh T, Costa SD, et al.. CD19+CD5+ cells as indicators of preeclampsia. Hypertension. (2012) 59:861–8. 10.1161/HYPERTENSIONAHA.111.188276[↩]
- Tsampalas M., Gridelet V., Berndt S., Foidart J.-M., Geenen V., D’Hauterive S.P. Human chorionic gonadotropin: A hormone with immunological and angiogenic properties. J. Reprod. Immunol. 2010;85:93–98. doi: 10.1016/j.jri.2009.11.008[↩]
- Polese B., Gridelet V., Araklioti E., Martens H., Perrier d’Hauterive S., Geenen V. The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front. Endocrinol. 2014;5:106. doi: 10.3389/fendo.2014.00106[↩][↩][↩]
- Berndt S., D’Hauterive S.P., Blacher S., Pequeux C., Lorquet S., Munaut C., Applanat M., Hervé M.A., Lamandé N., Corvol P., et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje[↩]
- Berndt S., Blacher S., D’Hauterive S.P., Thiry M., Tsampalas M., Cruz A., Péqueux C., Lorquet S., Munaut C., Noël A., et al. Chorionic Gonadotropin Stimulation of Angiogenesis and Pericyte Recruitment. J. Clin. Endocrinol. Metab. 2009;94:4567–4574. doi: 10.1210/jc.2009-0443[↩]
- Herr F., Baal N., Reisinger K., Lorenz A., McKinnon T., Preissner K., Zygmunt M. hCG in the Regulation of Placental Angiogenesis. Results of an In Vitro Study. Placenta. 2007;28:S85–S93. doi: 10.1016/j.placenta.2007.02.002[↩]
- Bourdiec A., Bédard D., Rao C.V., Akoum A. Human Chorionic Gonadotropin Regulates Endothelial Cell Responsiveness to Interleukin 1 and Amplifies the Cytokine-Mediated Effect on Cell Proliferation, Migration and the Release of Angiogenic Factors. Am. J. Reprod. Immunol. 2013;70:127–138. doi: 10.1111/aji.12080[↩]
- Reisinger K., Baal N., McKinnon T., Münstedt K., Zygmunt M. The gonadotropins: Tissue-specific angiogenic factors? Mol. Cell. Endocrinol. 2007;269:65–80. doi: 10.1016/j.mce.2006.11.015[↩]
- Zhang Z., Huang Y., Zhang J., Liu Z., Lin Q., Wang Z. Activation of NF-κB signaling pathway during HCG-induced VEGF expression in luteal cells. Cell Biol. Int. 2019;43:344–349. doi: 10.1002/cbin.11090[↩]
- Surico D., Farruggio S., Marotta P., Raina G., Mary D., Surico N., Vacca G., Grossini E. Human Chorionic Gonadotropin Protects Vascular Endothelial Cells from Oxidative Stress by Apoptosis Inhibition, Cell Survival Signalling Activation and Mitochondrial Function Protection. Cell. Physiol. Biochem. 2015;36:2108–2120. doi: 10.1159/000430178[↩]
- Jing G., Yao J., Dang Y., Liang W., Xie L., Chen J., Li Z. The role of β-HCG and VEGF-MEK/ERK signaling pathway in villi angiogenesis in patients with missed abortion. Placenta. 2021;103:16–23. doi: 10.1016/j.placenta.2020.10.005[↩][↩]
- Berndt S., Blacher S., Munaut C., Detilleux J., D’Hauterive S.P., Huhtaniemi I., Evain-Brion D., Noël A., Fournier T., Foidart J. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013;27:1309–1321. doi: 10.1096/fj.12-213686[↩]
- Gallardo V., González M., Toledo F., Sobrevia L. Role of heme oxygenase 1 and human chorionic gonadotropin in pregnancy associated diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020;1866:165522. doi: 10.1016/j.bbadis.2019.07.016[↩]
- Raghupathy R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today. 1997;18:478–482. doi: 10.1016/S0167-5699(97)01127-4[↩]
- Schumacher A., Heinze K., Witte J., Poloski E., Linzke N., Woidacki K., Zenclussen A.C. Human Chorionic Gonadotropin as a Central Regulator of Pregnancy Immune Tolerance. J. Immunol. 2013;190:2650–2658. doi: 10.4049/jimmunol.1202698[↩]
- Lea R.G., Sandra O. Immunoendocrine aspects of endometrial function and implantation. Reproduction. 2007;134:389–404. doi: 10.1530/REP-07-0167[↩]
- Fujiwara H. Do circulating blood cells contribute to maternal tissue remodeling and embryo-maternal cross-talk around the implantation period? Mol. Hum. Reprod. 2009;15:335–343. doi: 10.1093/molehr/gap027[↩]
- Akoum A, Metz CN, Morin M. Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J Clin Endocrinol Metab. 2005 May;90(5):2904-10. doi: 10.1210/jc.2004-1900[↩]
- Herrmann-Lavoie C, Rao CV, Akoum A. Chorionic gonadotropin down-regulates the expression of the decoy inhibitory interleukin 1 receptor type II in human endometrial epithelial cells. Endocrinology. 2007 Nov;148(11):5377-84. doi: 10.1210/en.2007-0368[↩]
- Matsuura T, Sugimura M, Iwaki T, Ohashi R, Kanayama N, Nishihira J. Anti-macrophage inhibitory factor antibody inhibits PMSG-hCG-induced follicular growth and ovulation in mice. J Assist Reprod Genet. 2002 Dec;19(12):591-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3455831/pdf/10815_2004_Article_450330.pdf[↩]
- Kamada M, Ino H, Naka O, Irahara M, Daitoh T, Mori K, Maeda N, Maegawa M, Hirano K, Aono T. Immunosuppressive 30-kDa protein in urine of pregnant women and patients with trophoblastic diseases. Eur J Obstet Gynecol Reprod Biol. 1993 Aug;50(3):219-25. doi: 10.1016/0028-2243(93)90204-p[↩]
- Majumdar S, Bapna BC, Mapa MK, Gupta AN, Devi PK, Subrahmanyam D. Pregnancy specific proteins: suppression of in vitro blastogenic response to mitogen by these proteins. Int J Fertil. 1982;27(2):66-9.[↩]
- CEDARD L, VARANGOT J, YANNOTTI S. [The metabolism of estrogens in human placentas artificially maintained in survival by perfusion in vitro]. C R Hebd Seances Acad Sci. 1962 Mar 5;254:1870-1. French.[↩]
- Poikkeus P., Hiilesmaa V., Tiitinen A. Serum HCG 12 days after embryo transfer in predicting pregnancy outcome. Hum. Reprod. 2002;17:1901–1905. doi: 10.1093/humrep/17.7.1901[↩]
- Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem. 1997 Dec;43(12):2233-43.[↩]
- Korhonen J., Stenman U.H., Ylostalo P. Serum human chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil. Steril. 1994;61:632–636. doi: 10.1016/S0015-0282(16)56638-2[↩]
- O’Connor J.F., Ellish N., Kakuma T., Schlatterer J., Kovalevskaya G. Differential urinary gonadotrophin profiles in early pregnancy and early pregnancy loss. Prenat. Diagn. 1998;18:1232–1240. doi: 10.1002/(SICI)1097-0223(199812)18:12<1232::AID-PD439>3.0.CO;2-Z[↩]
- Davies S, Byrn F, Cole LA. Human chorionic gonadotropin testing for early pregnancy viability and complications. Clin Lab Med. 2003 Jun;23(2):257-64, vii. doi: 10.1016/s0272-2712(03)00026-x[↩]
- Menczer J, Modan M, Serr DM. Prospective follow-up of patients with hydatidiform mole. Obstet Gynecol. 1980 Mar;55(3):346-9. doi: 10.1097/00006250-198003000-00015[↩]
- Cole LA, Shahabi S, Butler SA, Mitchell H, Newlands ES, Behrman HR, Verrill HL. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases. Clin Chem. 2001 Feb;47(2):308-15.[↩]
- Butts SF, Guo W, Cary MS, Chung K, Takacs P, Sammel MD, Barnhart KT. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013 Aug;122(2 Pt 1):337-343. doi: 10.1097/AOG.0b013e31829c6ed6[↩]
- Barjaktarovic M, Korevaar TI, Jaddoe VW, de Rijke YB, Visser TJ, Peeters RP, Steegers EA. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur J Epidemiol. 2017 Feb;32(2):135-144. doi: 10.1007/s10654-016-0201-3[↩][↩][↩][↩][↩]
- Cavaliere A, Ermito S, Dinatale A, Pedata R. Management of molar pregnancy. J Prenat Med. 2009 Jan;3(1):15-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3279094[↩][↩][↩][↩][↩][↩]
- Brouillet S, Murthi P, Hoffmann P, Salomon A, Sergent F, De Mazancourt P, Dakouane-Giudicelli M, Dieudonné MN, Rozenberg P, Vaiman D, Barbaux S, Benharouga M, Feige JJ, Alfaidy N. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: case of fetal growth restriction (FGR). Cell Mol Life Sci. 2013 Feb;70(3):511-25. doi: 10.1007/s00018-012-1141-z[↩][↩][↩][↩]
- Visconti F, Quaresima P, Chiefari E, Caroleo P, Arcidiacono B, Puccio L, Mirabelli M, Foti DP, Di Carlo C, Vero R, Brunetti A. First Trimester Combined Test (FTCT) as a Predictor of Gestational Diabetes Mellitus. Int J Environ Res Public Health. 2019 Sep 28;16(19):3654. doi: 10.3390/ijerph16193654[↩][↩]
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000 May;82(9):1553-6. doi: 10.1054/bjoc.2000.1177[↩]
- Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607[↩]
- Keikkala E., Vuorela P., Laivuori H., Romppanen J., Heinonen S., Stenman U.H. First trimester hyperglycosylated human chorionic gonadotrophin in serum—A marker of early-onset preeclampsia. Placenta. 2013;34:1059–1065. doi: 10.1016/j.placenta.2013.08.006[↩][↩][↩]
- Kalkunte S., Navers T., Norris W., Banerjee P., Fazleabas A., Kuhn C., Jeschke U., Sharma S. Presence of non-functional hCG in preeclampsia and rescue of normal pregnancy by recombinant hCG. Placenta. 2010;31:A126.[↩]
- Kalkunte S., Boij R., Norris W., Friedman J., Lai Z., Kurtis J., Lim K.H., Padbury J.F., Matthiesen L., Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am. J. Pathol. 2010;177:2387–2398. doi: 10.2353/ajpath.2010.100475[↩]
- Zhong Y., Zhu F., Ding Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: Systematic review and meta-analysis. BMC Pregnancy Childbirth. 2015;15:191. doi: 10.1186/s12884-015-0608-y[↩]
- Ong C.Y., Liao A.W., Spencer K., Munim S., Nicolaides K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG Int. J. Obstet. Gynaecol. 2000;107:1265–1270. doi: 10.1111/j.1471-0528.2000.tb11618.x[↩]
- Smith G.C., Stenhouse E.J., Crossley J.A., Aitken D.A., Cameron A.D., Connor J.M. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002;87:1762–1767. doi: 10.1210/jcem.87.4.8430[↩]
- Ranta J.K., Raatikainen K., Romppanen J., Pulkki K., Heinonen S. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:48–52. doi: 10.1016/j.ejogrb.2011.03.004[↩]
- Kagan K.O., Wright D., Baker A., Sahota D., Nicolaides K.H. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008;31:618–624. doi: 10.1002/uog.5331[↩]
- Wright D., Spencer K., Kagan K.K., Torring N., Petersen O.B., Christou A., Kallikas J., Nicolaides K.H. First-trimester combined screening for trisomy 21 at 7–14 weeks’ gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010;36:404–411. doi: 10.1002/uog.7755[↩]
- Cuckle H. Biochemical screening for Down syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;92:97–101. doi: 10.1016/S0301-2115(00)00431-0[↩][↩][↩]
- Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem. 2004;37(7):549–61. 10.1016/j.clinbiochem.2004.05.008[↩]
- Bellet D, Lazar V, Bièche I, Paradis V, Giovangrandi Y, Paterlini P, Lidereau R, Bedossa P, Bidart JM, Vidaud M. Malignant transformation of nontrophoblastic cells is associated with the expression of chorionic gonadotropin beta genes normally transcribed in trophoblastic cells. Cancer Res. 1997 Feb 1;57(3):516-23.[↩]
- Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhommé C, Rougier P, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992 Jul 15;52(14):3901-7.[↩]
- Gestational Trophoblastic Disease Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/gestational-trophoblastic/patient/gtd-treatment-pdq[↩][↩][↩]
- What Is Testicular Cancer? https://www.cancer.org/cancer/types/testicular-cancer/about/what-is-testicular-cancer.html[↩][↩][↩][↩]
- Gholam D, Fizazi K, Terrier-Lacombe MJ, Jan P, Culine S, Theodore C. Advanced seminoma–treatment results and prognostic factors for survival after first-line, cisplatin-based chemotherapy and for patients with recurrent disease: a single-institution experience in 145 patients. Cancer. 2003 Aug 15;98(4):745-52. doi: 10.1002/cncr.11574[↩]
- Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ, Joffe JK, de Wit R, Aass N, Graham JD, Coleman R, Kirk SJ, Stenning SP; MRC TE19 collaborators and the EORTC 30982 collaborators. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005 Jul 23-29;366(9482):293-300. doi: 10.1016/S0140-6736(05)66984-X[↩]
- Weissbach L, Bussar-Maatz R, Mann K. The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur Urol. 1997;32(1):16-22.[↩]
- Testicular Cancer Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/testicular/hp/testicular-treatment-pdq[↩][↩][↩]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997 Feb;15(2):594-603. doi: 10.1200/JCO.1997.15.2.594[↩]
- Sturgeon CM, Duffy MJ, Stenman UH, et al. National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008 Dec;54(12):e11-79. doi: 10.1373/clinchem.2008.105601[↩]
- Loutradis D., Drakakis P., Vomvolaki E., Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. J. Assist. Reprod. Genet. 2007;24:597–611. doi: 10.1007/s10815-007-9181-2[↩]
- Cole L.A. Biological functions of hcg and hcg-related molecules. Reprod. Biol. Endocrinol. 2010;8:102–115. doi: 10.1186/1477-7827-8-102[↩]
- Craciunas L, Tsampras N, Raine-Fenning N, Coomarasamy A. Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction. Cochrane Database Syst Rev. 2018 Oct 20;10(10):CD011537. doi: 10.1002/14651858.CD011537.pub3[↩][↩]
- Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (profasi) for induction of final follicular maturation on in vitro fertilization-embryo transfer. Fertil Steril. 2001;76:67–74. doi:10.1016/S0015-0282(01)01851-9[↩]
- Hayes F, Dwyer A, Pitteloud N. Hypogonadotropic Hypogonadism (HH) and Gonadotropin Therapy. [Updated 2013 Nov 25]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279078[↩][↩][↩][↩][↩][↩]
- Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo). 2013;68 Suppl 1(Suppl 1):81-8. doi: 10.6061/clinics/2013(sup01)09[↩]
- Clavijo RI, Hsiao W. Update on male reproductive endocrinology. Transl Androl Urol. 2018 Jul;7(Suppl 3):S367-S372. doi: 10.21037/tau.2018.03.25[↩]
- Canavese F, Mussa A, Manenti M, Cortese MG, Ferrero L, Tuli G, Macchieraldo R, Lala R. Sperm count of young men surgically treated for cryptorchidism in the first and second year of life: fertility is better in children treated at a younger age. Eur J Pediatr Surg. 2009 Dec;19(6):388-91. doi: 10.1055/s-0029-1241171[↩]
- Recombinant follicle stimulating hormone: development of the first biotechnology product for the treatment of infertility. Recombinant Human FSH Product Development Group. Hum Reprod Update. 1998 Nov-Dec;4(6):862-81. doi: 10.1093/humupd/4.6.862[↩][↩][↩]
- Mannaerts B, Fauser B, Lahlou N, Harlin J, Shoham Z, Bennink HC, Bouchard P. Serum hormone concentrations during treatment with multiple rising doses of recombinant follicle stimulating hormone (Puregon) in men with hypogonadotropic hypogonadism. Fertil Steril. 1996 Feb;65(2):406-10. doi: 10.1016/s0015-0282(16)58108-4[↩]
- King TF, Hayes FJ. Long-term outcome of idiopathic hypogonadotropic hypogonadism. Curr Opin Endocrinol Diabetes Obes. 2012 Jun;19(3):204-10. doi: 10.1097/MED.0b013e328353565b[↩]
- Burris AS, Rodbard HW, Winters SJ, Sherins RJ. Gonadotropin therapy in men with isolated hypogonadotropic hypogonadism: the response to human chorionic gonadotropin is predicted by initial testicular size. J Clin Endocrinol Metab. 1988 Jun;66(6):1144-51. doi: 10.1210/jcem-66-6-1144[↩][↩][↩]
- Vicari E, Mongioì A, Calogero AE, Moncada ML, Sidoti G, Polosa P, D’Agata R. Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism–long-term follow-up. Int J Androl. 1992 Aug;15(4):320-9. doi: 10.1111/j.1365-2605.1992.tb01131.x[↩]
- Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998 Sep;139(3):298-303. doi: 10.1530/eje.0.1390298[↩]
- Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009 Mar;94(3):801-8. doi: 10.1210/jc.2008-1648[↩][↩][↩][↩]
- Warne DW, Decosterd G, Okada H, Yano Y, Koide N, Howles CM. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009 Aug;92(2):594-604. doi: 10.1016/j.fertnstert.2008.07.1720[↩][↩]
- Whitcomb RW, Crowley WF Jr. Clinical review 4: Diagnosis and treatment of isolated gonadotropin-releasing hormone deficiency in men. J Clin Endocrinol Metab. 1990 Jan;70(1):3-7. doi: 10.1210/jcem-70-1-3[↩]
- Kulin HE, Samojlik E, Santen R, Santner S. The effect of growth hormone on the Leydig cell response to chorionic gonadotrophin in boys with hypopituitarism. Clin Endocrinol (Oxf). 1981 Nov;15(5):463-72. doi: 10.1111/j.1365-2265.1981.tb00689.x[↩][↩]
- Chatelain PG, Sanchez P, Saez JM. Growth hormone and insulin-like growth factor I treatment increase testicular luteinizing hormone receptors and steroidogenic responsiveness of growth hormone deficient dwarf mice. Endocrinology. 1991 Apr;128(4):1857-62. doi: 10.1210/endo-128-4-1857[↩]