Contents
What is hyaluronic acid
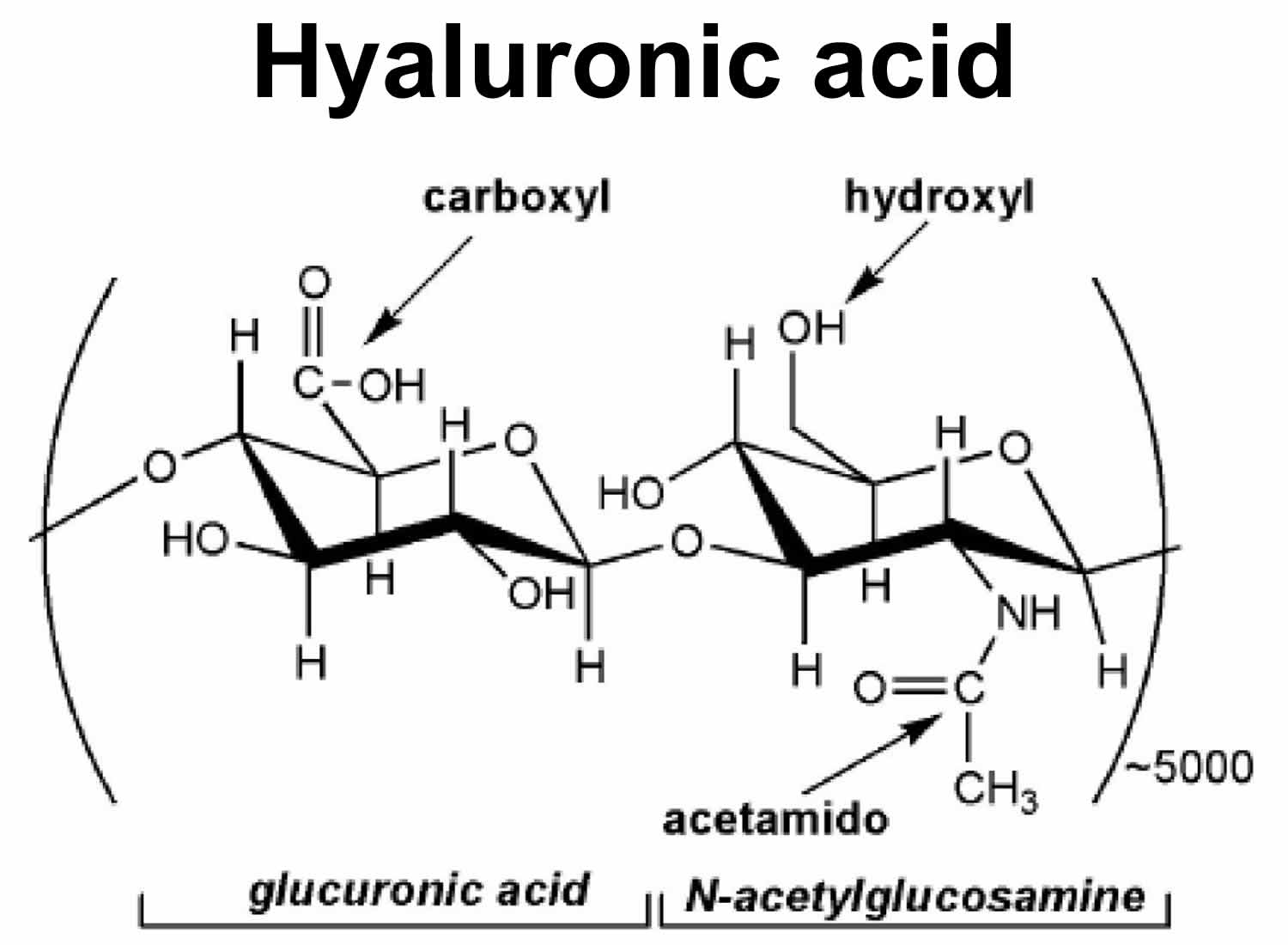
Hyaluronic acid is a non-sulphated glycosaminoglycan and is composed of repeating polymeric disaccharide repeats of D-glucuronic acid (GlcUA) and N-acetylglucosamine (GlcNAc) joined alternately by β-1, 3 and β-1, 4 glycosidic bonds (Figure 1) with a molecular weight up to 6 million Daltons 1. Traditionally hyaluronic acid was extracted from rooster combs, and now it is mainly produced via streptococcal fermentation with lower production costs and less environmental pollution 2. Hyaluronic acid has been successfully produced on an industrial scale with Streptococcus species bacteria as the main producer. Nevertheless, the production of hyaluronic acid from Streptococcus species bacteria is facing a growing concern due to the fact that streptococci are pathogenic 3. In this background, the recombinant hyaluronic acid production has attracted an increasing interest, and Novozymes has produced hyaluronic acid with recombinant Bacillus subtilis on an industrial scale 3.
Hyaluronic acid is also synthesized by your body by specific enzymes called hyaluronic acid synthases (HAS). These are membrane bound enzymes that synthesize hyaluronic acid on the inner surface of the plasma membrane 4 and then hyaluronic acid is extruded through pore-like structures into the extracellular space 5. There are three mammalian enzymes HAS -1, -2 and -3, which exhibit distinct enzymatic properties and synthesize hyaluronic acid chains of various length 6. In aqueous solutions hyaluronic acid forms specific stable tertiary structures 7. Hyaluronic acid is traditionally regarded as a biological ‘goo’ that participates in lubricating joints or holding together gel-like connective tissues. Although these are common physiological roles of hyaluronic acid in adult organisms, hyaluronic acid also functions as a microenvironmental cue that co-regulates cell behavior during embryonic development, healing processes, inflammation and tumor development 8. Hyaluronic acid polymers occur in a vast number of configurations and shapes, depending on their size, salt concentration, pH, and associated cations 9. Hyaluronic acid encompasses a large volume of water giving solutions high viscosity, even at low concentrations 10.
Molecular weight is an important quality parameter for a commercial hyaluronic acid product, as it determines the hyaluronic acid’s rheological properties, affects physiological response, and defines suitable applications 11. Hyaluronic acid with a high molecular weight (greater than 10 kDa) has good viscoelasticity, moisture retention, and mucoadhesion, — qualities desirable in the areas of ophthalmology, orthopedics, wound healing, and cosmetics. Whereas, hyaluronic acid with a relatively low molecular weight (2-3.5 kDa) or hyaluronic acid oligosaccharides (10-20 sugars in length) have shown to promote angiogenesis, induce expression of inflammatory mediators, and inhibit tumor growth 12.
Figure 1. Hyaluronic acid
In humans, hyaluronic acid is most abundant in the skin 13, accounting for 50% of the total body hyaluronic acid 14, the vitreous of the eye 15, the umbilical cord 16, and synovial fluid 17, but it is also present in all tissues and fluids of the body, such as skeletal tissues 18, heart valves 19, the lung 20, the aorta 21, the prostate 22, tunica albuginea, corpora cavernosa and corpus spongiosum of the penis 23. Hyaluronic acid is produced primarily by mesenchymal cells but also by other cell types 24.
What does hyaluronic acid do
Functions of hyaluronic acid include the following: hydration, lubrication of joints, a space filling capacity, and the framework through which cells migrate 25. The synthesis of hyaluronic acid increases during tissue injury and wound healing 26 and hyaluronic acid regulates several aspects of tissue repair, including activation of inflammatory cells to enhance immune response 27 and the response to injury of fibroblasts 28 and epithelial cells 29. Hyaluronic acid also provides the framework for blood vessel formation 30 and fibroblast migration 31, that may be involved in tumor progression 32. The correlation of hyaluronic acid levels on the cell surface of cancer cells with the aggressiveness of tumors has also been reported 33.
The size of hyaluronic acid appears to be of critical importance for its various functions described above 34. Hyaluronic acid of high molecular size, usually in excess of 1,000 kDa, is present in intact tissues and is antiangiogenic and immunosuppressive, whereas smaller polymers of hyaluronic acid are distress signals and potent inducers of inflammation and angiogenesis 24.
Hyaluronic acid receptors
There is a variety of proteins that bind hyaluronic acid, called hyaladherins, which are widely distributed in the extracellular matrix, the cell surface, the cytoplasm and the nucleus 35. Those that attach hyaluronic acid to the cell surface constitute hyaluronic acid receptors. The most prominent among these receptors is the transmembrane glycoprotein “cluster of differentiation 44” (CD44) that occurs in many isoforms, which are the products of a single gene with variable exon expression 36. CD44 is found on virtually all cells, except red blood cells, and regulates cell adhesion, migration, lymphocyte activation and homing, and cancer metastasis.
The receptor for hyaluronic acid-mediated motility (RHAMM) is another major receptor for hyaluronic acid, and it is expressed in various isoforms 37. RHAMM is a functional receptor in many cell types, including endothelial cells 38 and in smooth muscle cells from human pulmonary arteries 39 and airways 24. The interactions of hyaluronic acid with RHAMM control cell growth and migration by a complex network of signal transduction events and interactions with the cytoskeleton 40. Transforming growth factor (TGF)-β1, which is a potent stimulator of cell motility, elicits the synthesis and expression of RHAMM and hyaluronic acid, and thus initiates locomotion 41.
Degradation of hyaluronic acid
Hyaluronic acid has a dynamic turnover rate. Hyaluronic acid has a half-life of 3 to 5 min in the blood, less than a day in the skin and 1 to 3 weeks in the cartilage 42. Hyaluronic acid is degraded into fragments of varying size by hyaluronidases (HYAL) by hydrolyzing the hexosaminidic β (1–4) linkages between N-acetyl-D-glucosamine and D-glucuronic acid residues in hyaluronic acid. In humans, six hyaluronidases (HYAL) have been identified so far: HYAL-1, -2, -3, -4, PH-20 and HYALP1 43. The family of hyaluronidase (HYAL) enzymes received little attention until recently 44, because they are found at extremely low concentrations and they are difficult to purify, characterize and measure their activity, which is high but unstable 45. New procedures have now enabled the isolation and characterization of HYAL. HYAL-1 is the major HYAL in serum 46. Mutations in the HYAL-1 gene are associated with HYAL deficiency and mucopolysaccharidosis type IX 47. HYAL-2 has very low activity in comparison to plasma HYAL-1 and it hydrolyzes specifically hyaluronic acid of high molecular weight, yielding hyaluronic acid fragments of approximately 20 kDa, which are further degraded to small oligosaccharides by PH-20 48. HYAL-3 is mainly expressed in bone marrow and testis 44, but also in other organs, such as the human lung 24. The role of HYAL-3 in the catabolism of hyaluronic acid is not clear and it is suggested that it may contribute to hyaluronic acid degradation by enhancing the activity of HYAL-1 49.
Hyaluronic acid can also be degraded non-enzymatically by a free-radical mechanism 50 in the presence of reducing agents such as ascorbic acid, thiols, ferrous, or cuprous ions, a process that requires the presence of molecular oxygen. Thus, agents that could delay the free-radical-catalyzed degradation of hyaluronic acid may be useful in maintaining the integrity of dermal hyaluronic acid and its moisturizing properties 45.
Hyaluronic acid for skin
Human skin aging is a complex biological process, not yet fully understood. It is the result of two biologically independent processes. The first is intrinsic or innate aging, an unpreventable process, which affects the skin in the same pattern as it affects all internal organs. The second is extrinsic aging, which is the result of exposure to external factors, mainly ultraviolet (UV) irradiation, that is also referred to as photoaging 51. Intrinsic skin aging is influenced by hormonal changes that occur with age 52, such as the gradual decreased production of sex hormones from the mid-twenties and the diminution of estrogens and progesterone associated with menopause. It is well established that the deficiency in estrogens and androgens results in collagen degradation, dryness, loss of elasticity, epidermal atrophy and wrinkling of the skin 53.
Even though intrinsic and extrinsic skin aging are distinctive processes, they share similarities in molecular mechanisms. For example, reactive oxygen species (ROS), arising from oxidative cell metabolism, play a major role in both processes 54. Reactive oxygen species (ROS) in extrinsic or intrinsic skin aging induce the transcription factor c-Jun via mitogen-activated protein kinases (MAPK), leading to overexpression of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-9 and prevention of the expression of procollagen-1 55. Therefore, elevated levels of degraded collagen and reduced collagen synthesis are pathologies occurring in intrinsically aged as well as photoaged skin.
Skin aging is also associated with loss of skin moisture. The key molecule involved in skin moisture is hyaluronic acid (hyaluronic acid), a glycosaminoglycan with a unique capacity to bind and retain water molecules 56. Hyaluronic acid belongs to the extracellular matrix (ECM) molecules. During the past decades the constituents of the skin have been well characterized. In the beginning, most of the studies focused on the cells that comprise the skin layers, such as the epidermis, the dermis and the underlying subcutis. Recently, it is appreciated that extracellular matrix (ECM) molecules that lie between cells, in addition to providing a constructive framework, they exert major effects on cellular function. These extracellular matrix (ECM) molecules, although they appear amorphous by light microscopy, they form a highly organized structure, comprising mainly of glycosaminoglycan, proteoglycans, growth factors and structural proteins such as collagens. Yet, the predominant component of the skin extracellular matrix (ECM) is hyaluronic acid.
As mentioned above, skin hyaluronic acid accounts for most of 50% of total body hyaluronic acid 14. The hyaluronic acid content of the dermis is significantly higher than that of the epidermis, while papillary dermis has much greater levels of hyaluronic acid than reticular dermis 57. The hyaluronic acid of the dermis is in continuity with the lymphatic and vascular systems. Hyaluronic acid in the dermis regulates water balance, osmotic pressure and ion flow and functions as a sieve, excluding certain molecules, enhancing the extracellular domain of cell surfaces and stabilizes skin structures by electrostatic interactions 45. Elevated levels of hyaluronic acid are synthesized during scar-free fetal tissue repair and the prolonged presence of hyaluronic acid assures such scar-free tissue repair 58. Dermal fibroblasts provide the synthetic machinery for dermal hyaluronic acid and should be the target for pharmacologic attempts to enhance skin hydration. Unfortunately, exogenous hyaluronic acid is cleared from the dermis and is rapidly degraded 59.
Hyaluronic acid and skin aging
The most dramatic histochemical change observed in aged skin is the marked disappearance of epidermal hyaluronic acid, while hyaluronic acid is still present in the dermis 57. The reasons for this change in hyaluronic acid homeostasis with aging is unknown. As mentioned above, the synthesis of epidermal hyaluronic acid is influenced by the underlying dermis and is under separate controls from the synthesis of dermal hyaluronic acid 45. Progressive reduction of the size of the hyaluronic acid polymers in skin as a result of aging has also been reported 60. Thus, the epidermis loses the principle molecule responsible for binding and retaining water molecules, resulting in loss of skin moisture. In the dermis, the major age-related change is the increasing avidity of hyaluronic acid with tissue structures with the concomitant loss of hyaluronic acid extractability. This parallels the progressive cross-linking of collagen and the steady loss of collagen extractability with age 45. All of the above age related phenomena contribute to the apparent dehydration, atrophy and loss of elasticity that characterizes aged skin.
Premature aging of skin is the result of repeated and extended exposure to UV (ultraviolet) radiation 61. Approximately 80% of facial skin aging is attributed to UV-exposure 62. UV radiation damage causes initially a mild form of wound healing and is associated at first with an increase of dermal hyaluronic acid. As little as 5 minute of UV exposure in nude mice caused enhanced deposition of hyaluronic acid, indicating that UV radiation induced skin damage is an extremely rapid event 63. The initial redness of the skin following exposure to UV (ultraviolet) radiation may be due to a mild edematous reaction induced by the enhanced hyaluronic acid deposition and histamine release. Repeated and extensive exposures to UV ultimately simulate a typical wound healing response with deposition of scarlike type I collagen, rather than the usual types I and III collagen mixture that gives skin resilience and pliability 63.
In the skin, photoaging results in abnormal glycosaminoglycan content and distribution compared with that found in scars, or in the wound healing response, with diminished hyaluronic acid and increased levels of chondroitin sulfate proteoglycans 64. In dermal fibroblasts this reduction in hyaluronic acid synthesis was attributed to collagen fragments, which activate αvβ3-integrins and in turn inhibit Rho kinase signaling and nuclear translocation of phosphoERK, resulting in reduced hyaluronic acid synthase-2 (HAS-2) expression 65. We have recently unraveled some of the biochemical changes that may distinguish photoaging and natural aging. Using photoexposed and photoprotected human skin tissue specimens, obtained from the same patient, we have shown a significant increase in the expression of hyaluronic acid of lower molecular mass in photoexposed skin, as compared with photoprotected skin. This increase of degraded hyaluronic acid was associated with a significant decrease in the expression of HAS-1 (hyaluronic acid synthase-1) and an increased expression of hyaluronidase-1, -2 and -3. Furthermore, the expression of hyaluronic acid receptors CD44 (cluster of differentiation 44) and RHAMM (receptor for hyaluronic acid-mediated motility) was significantly downregulated in photoexposed, as compared with photoprotected skin. These findings indicate that photoexposed skin, and therefore extrinsic skin aging, is characterized by distinct homeostasis of hyaluronic acid 13. Scientists have also assessed photoprotected skin tissue specimens from adults and juvenile patients and observed that intrinsic skin aging was associated with a significant reduction in the content of hyaluronic acid and downregulation of HAS-1, HAS -2, CD44 and RHAMM 66. Similar results for photoprotected skin have also been reported for both genders for hyaluronic acid, HAS-2 and CD44 67.
Conclusion
The available data suggest that hyaluronic acid homeostasis exhibits a distinct profile in intrinsic skin aging (innate or biological aging), which is totally different of that in extrinsic skin aging (photoaging due to exposure to external factors, mainly ultraviolet (UV) irradiation). Additional insight needs to be gained in understanding the metabolism of hyaluronic acid in skin layers and the interactions of hyaluronic acid with other skin components. Such information will facilitate the ability to modulate skin moisture in a rational manner and may contribute to the refinement of current drugs and the development of novel treatments for skin aging 34.
Hyaluronic acid fillers for facial volume restoration and contouring
Loss of appropriate facial fullness and youthful appearance occurs in most areas of the face to varying degrees, including the periorbital, malar, forehead, temporal, glabellar, mandibular, mental and perioral zones 68. A recent study determined that facial subcutaneous fat is partitioned into multiple, independent anatomical compartments, which may age independently, resulting in abrupt contour changes between them 69.
Re-establishing the integrity of these foundation structures through appropriate volume restoration and contouring has proven to be a highly effective means of harmonizing features, contours, proportions and balance associated with the youthful face 68.
The products chosen most frequently by some experts for volume restoration and contouring were a high-viscosity, low-molecular-weight hyaluronic acid (LMWHA) for structural support and high molecular weight hyaluronic acids for lines, grooves and furrows 70.
That the recent addition of a high-viscosity, low molecular weight hyaluronic acid (LMWHA) to the volumizing product has resulted in significantly improved patient outcomes. This is a smooth consistency gel that is uniquely composed of a mix of low and high molecular weight hyaluronic acid. Compared to hyaluronic acid fillers with 100% high molecular weight, the LMWHA (low molecular weight hyaluronic acid) allows a combination of high cohesivity and viscosity. This property enables the LMWHA (low molecular weight hyaluronic acid) to retain its structure following a deep injection and makes it particularly well suited for facial volumizing and contouring 71.
As a point of interest, while the term ‘dermal filler’ is still widely used, it is arguably not anatomically accurate. Facial fillers are frequently used at multiple levels beneath the dermis – sub-muscularly above the periosteum and in the subcutaneous plane 70.
For the purposes of treatment discussion, the face is divided into three fundamental, but overlapping sections: Mid, upper and lower. However, the global, whole-face and three-dimensional approach must always be the physician’s primary consideration. Treatment is always carried out in the context of how it will integrate with the rest of the face, both at the moment of implementation and over time, taking changing facial dynamics into consideration.
It should be noted that these are recently developed procedures representing the collective experience and best practices of the authors at the time of writing and should not be construed as absolute, validated directives. It is important that each individual be treated uniquely while keeping these general principles in mind.
Table 1. Five categories of facial filler
| Category | Agent | Durationa |
|---|---|---|
| High-viscosity, low molecular weight hyaluronic acid (LMWHA) | Voluma (Allergan, Inc) | Long duration (up to 18 months) |
| Hyaluronic acid (HA) | Juvéderm (Allergan, Inc) | Intermediate duration (~12 mo) |
| Restylane (Medicis Aesthetics) | Intermediate duration (~6 mo) | |
| Perlane (Medicis Aesthetics) | ||
| Teosyal (Clarion Medical Technologies) | ||
| Esthelis (Anteis) | ||
| Prevelle (Mentor) | ||
| Revanesse (Prollenium Medical) | ||
| Calcium hydroxylapatite | Radiesse (Merz Aesthetics) | Intermediate duration (~1 yr) |
| Poly-L-lactic acid (PLLA) | Sculptra (Valeant) | Long duration (up to 18 months) |
| Polymethyl methacrylate (PMMA) | Artefill (Artes Medical/suneva) | Permanent (~10 years) |
| Platelet-Rich Fibrin Matrix (PRFM) | Selphyl (Canderm) | Long duration (up to 18 months) |
Footnote:
a) Some authors have seen duration of action which is longer than the amounts selphyl (Canderm) listed above.
[Source 70]Table 2. Strategies for volume restoration and contouring in the upper face with hyaluronic acid
| Treated area | Preferred product | Technique | Dosinga (per degree of severity of deficit) | Needles/cannulae (gauge and length) |
|---|---|---|---|---|
| Temporal hollow | Hyaluronic acid |
| ||
| Hyaluronic acid Mild: 0.5 cc/side Moderate: 1–2 cc/side Severe: 3+ cc/side | Hyaluronic acid 27 gauge (deep) 30 gauge (superficial) 22 or 25 g cannula | ||
| TIPS/PEARLS |
| |||
| Brow | Hyaluronic acid |
| 0.2–0.5 cc/side | 27 gauge 30 gauge |
| TIPS/PEARLS |
| |||
| Upper eyelid hollow | Hyaluronic acid |
| Use small aliquots (<0.25 cc) Rarely need more than 1 cc/side | 30–31 gauge ½”–1” 30 g microcannula |
| TIPS/PEARLS |
| |||
Footnote:
a) General dosing range based on consensus group experience.
Abbreviations: HA = hyaluronic acid; LMWHA = low-molecular-weight hyaluronic acid; Poly-L = lactic acid.
[Source 70]Table 3. Strategies for volume restoration and contouring in the mid-face with hyaluronic acid
| Treated area | Preferred product | Technique | Dosinga (per degree of severity of deficit) | Needles/cannulae (gauge and length) |
|---|---|---|---|---|
| Cheek | HA LMWHA |
| Mild: 0.5–1 cc/side Medium: 1–2 cc/side Severe: 3+ cc/side | Needle: 25–27–29 UTW gauge ½” Cannula: 25 g 30 mm/22 g 40 mm |
| TIPS/PEARLS |
| |||
| Submalar cheek | Hyaluronic acid |
| Hyaluronic acid Mild: 1 cc/side Medium: 2 cc/side Severe: 3 cc/side | 27–30 gauge needle 25 g or 27 g microcannula |
| TIPS/PEARLS |
| |||
| Tear Trough/Infra-orbital hollows | Hyaluronic acid |
| Use small aliquots (<0.25 cc) | 30–31 gauge ½”–1” or BD tuberculin syringe (backloaded) or 30 g microcannula |
| Rarely need more than 1 cc/side | |||
| TIPS/PEARLS |
| |||
| Nasolabial folds (NLF) | Hyaluronic acid |
| Mild: <0.5 cc/side Medium: 0.5–1 cc/side Severe: 1+ cc/side | 27–31 gauge 3/8”–½” 25 g cannula, 30 mm 22 g cannula, 40 mm |
| TIPS/PEARLS |
| |||
Footnote:
a) General dosing range based on consensus group experience.
Abbreviations: HA = hyaluronic acid; LMWHA = low-molecular-weight hyaluronic acid; NLF = nasolabial fold.
[Source 70]Table 4. Strategies for volume restoration and contouring in the lower face with hyaluronic acid
| Treated area | Preferred product | Technique | Dosinga (per degree of severity of deficit) | Needles/cannulae (gauge and length) |
|---|---|---|---|---|
| Chin | LMWHA/HA |
| Hyaluronic acid Mild: 1 cc Moderate: 2 cc Severe: 3 cc | 27–30 gauge ½” 22 or 25 g cannula |
| TIPS/PEARLS |
| |||
| Pre-jowl sulcus/marionette | LMWHA/HA |
| Hyaluronic acid Mild: 0.5 cc/side Moderate: 1 cc/side Severe: 2+ cc/side | 27–30 gauge ½”–1” 22 or 25 g cannula |
| TIPS/PEARLS |
| |||
| Post jowl sulcus, mandibular angle and preauricular | LMWHA/HA |
| Hyaluronic acid Mild: 1–2 cc/side Moderate: 2–3 cc/side Severe: 3–4 cc/side | 27 gauge ½”–1½” |
| TIPS/PEARLS |
| |||
Footnote:
a) General dosing range based on consensus group experience.
Abbreviations: HA = hyaluronic acid; LMWHA = low-molecular-weight hyaluronic acid.
[Source 70]Summary
Low-molecular-weight hyaluronic acid (LMWHA) has made it possible for physicians to achieve new levels of excellence in facial aesthetics. In clinical practice, physicians have demonstrated that high- viscosity LMWHA has advantages when used for volume restoration and contouring, including versatility of use (different facial planes), high malleability, minimal swelling, limited downtime, and immediate patient satisfaction 70.
Combination treatment of fillers, neuromodulators and energy devices to create optimal contours is forecast to remain the mainstay of non-surgical facial aesthetics. Further advances are expected to standardize techniques, increase reproducibility and consistency of results, and augment patient experiences by minimizing bruising and downtime.
Serum hyaluronic acid
Serum hyaluronic acid (sHA) is a serum biomarker for knee osteoarthritis (OA). Serum hyaluronic acid concentration is elevated in patients with knee osteoarthritis and the concentration of serum hyaluronic acid was positively correlated with the severity of radiographic knee osteoarthritis 72. Several previous cross-sectional studies have reported that measuring serum hyaluronic acid concentration may be useful for not only diagnosing knee osteoarthritis, but also identifying disease duration, severity, and the extent of osteoarthritis-related knee pain 73. Furthermore, over 5 years, higher serum hyaluronic acid concentrations were positively correlated with the progression of Kellgren–Lawrence grade in those with knee osteoarthritis and of joint space narrowing in those both with and without knee osteoarthritis. These results suggest that elevated serum hyaluronic acid concentration can be used as a predictor of knee osteoarthritis progression. Thus, serum hyaluronic acid concentration may be useful as a prognostic marker for predicting joint space narrowing, though not for predicting osteophyte formation (Sasaki E, Tsuda E, Yamamoto Y, et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis – a five-year prospective cohort study. Arthritis Research & Therapy. 2015;17:283. doi:10.1186/s13075-015-0793-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600294/)). However, serum hyaluronic acid concentration increases with age because of an impaired ability to metabolize hyaluronic acid in the elderly 74. Hyaluronic acid is actively secreted into joint fluid by the surrounding synovial lining cells 75. When intra-articular fluid pressure is increased, hyaluronic acid is also distributed to the liver (90 %), kidney (9 %), and spleen (1 %) via the lymphatic and capillary systems 74. The increase in serum hyaluronic acid concentration with age may even be a consequence of progressive age-related hepatic and renal impairment 76. serum hyaluronic acid concentration is reported to be influenced by other factors, including renal failure 77, liver failure 78, rheumatoid arthritis 79, malignancy 80, and physical activity 81.
The mechanisms underpinning the phenomenon of elevated serum hyaluronic acid concentration in progressive knee osteoarthritis are thought to be mechanical stress and synovitis with cartilage degeneration 82. Osteoarthritis is characterized by focal damage to the articular cartilage centered on the load-bearing areas, osteophyte formation at the joint margins, subchondral bone changes, and synovitis 83. Synovitis is present at the onset of osteoarthritis 84 and results in the production of hyaluronic acid (HA) and pro-inflammatory cytokines 85. In addition, synovitis activates fibroblasts, which promote the production of other pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β 83. These cytokines promote matrix metalloproteinase production by fibroblasts, which degrade the articular cartilage matrix 86. Thus, the presence of synovitis is thought to contribute to the progression of knee osteoarthritis. Ayral et al. 87 suggested that synovitis was also predictive of subsequent chondropathy.
Hyaluronic acid for joints
Hyaluronic acid is a substance already in the fluid of your knee. It helps lubricate the joint. When you have arthritis, the hyaluronic acid in your joint becomes thinner and less effective. Your doctor can inject a form of hyaluronic acid into your joint to help lubricate and protect it. This is sometimes called artificial joint fluid, or viscosupplementation. Hyaluronic acid injections are one treatment option doctors may offer when a patient is no longer able to control osteoarthritis pain with ibuprofen or other nonsteroidal anti-inflammatory drugs (NSAIDs), or the patient can’t tolerate these drugs (which can cause side effects such as stomach bleeding and kidney problems). The treatment regimen for hyaluronic acid usually involves receiving one injection in the affected joint per week for three to five weeks. Many patients appear to get at least some relief – eventually.
Hyaluronic acid injections are approved by the U.S. Food and Drug Administration (FDA) for treating osteoarthritis of the knee, though some doctors have used the therapy on other joints, such as the hip and ankle. While studies of hyaluronic acid injections have occasionally yielded disappointing results, many doctors who treat osteoarthritis say that the weight of scientific evidence and their own clinical experience – suggests that hyaluronic acid injection in the knee can produce significant relief for some patients. Furthermore, lab and clinical research hints that hyaluronic acid may do much more than simply re-grease a creaky joint.
In 2006, a team led by Nicholas Bellamy, MD, of the University of Queensland in Brisbane, Australia, reviewed 76 studies examining the use of hyaluronic acid for treating knee osteoarthritis. The review, the largest and most comprehensive of its kind, found that pain levels in the average patient who receives these injections are reduced by 28 to 54 percent. That’s roughly what a patient might expect from taking NSAIDs, the authors concluded. Meanwhile, hyaluronic acid improved the ability to move about and perform daily activities by 9 to 32 percent.
As a patient soon learns, though, hyaluronic acid is no quick fix. According to Bellamy’s review (which was conducted on behalf of the Cochrane Collaboration, an international consortium that reviews scientific evidence for medical treatments), it takes about five weeks, on average, before a patient experiences the full benefits of hyaluronic acid. By contrast, corticosteroid injections – the other primary treatment choice when NSAIDs aren’t an option – provide significant relief within a few days. However, pain relief from corticosteroids diminishes markedly within a month or so. What’s more, overuse of corticosteroids can have a catabolic effect – that is, it could cause cartilage to break down and deteriorate further. Meanwhile, the Cochrane review found that pain-relieving benefits of hyaluronic acid persist at peak levels for about three months, on average. Some doctors sometimes give patients a double shot in the knee – one injection each of hyaluronic acid and corticosteroids – for quick-acting, long-lasting relief.
About 30 percent of people who undergo hyaluronic acid injections become virtually pain free, and symptom relief may last up to two years. Yet, another 20 percent of patients experience no benefit. Unfortunately, experts don’t know how to pick out those people who are going to have an outstanding response versus a modest response versus no response at all. Researchers have tried to identify ways to predict how a patient will respond to hyaluronic acid, but so far have come up empty.
However, hyaluronic acid isn’t universally approved. In June 2013, the American Academy of Orthopedic Surgeons (AAOS) issued a new set of recommendations for the treatment of knee osteoarthritis. Based on a review of 14 studies, the organization determined hyaluronic acid did not meet the minimum clinically important improvement measures.
With five brands available in the United States, it’s natural to ask which is most effective. There have been relatively few head-to-head comparisons of the various products in clinical trials. Experts believe that they all work about the same. Likewise, the risk of side effects is similar among the different products, the most common being pain and swelling at the injection site that fades within a few days.
Beyond the question of how well hyaluronic acid injections (viscosupplements) work lies another intriguing area of inquiry: How do they work? Hyaluronic acid may act as a lubricant and shock absorber. Hyaluronic acid has a lot of other activities in the joint. For example, research suggests that hyaluronic acid interfere with prostaglandins and cytokines, naturally occurring compounds that promote inflammation.
What’s more, studies indicate that injecting supplemental hyaluronic acid may coax the joint into increasing its own production of this important substance, which may in turn help to preserve cartilage. There’s a lot of data to suggest that it can slow the disease down. However, as pointed out above, hyaluronic acid is not a magic pill, but it has a definite role in the armamentarium for treating osteoarthritis of the knee.
Hyaluronic acid injection
Hyaluronic acid injection is used to treat knee pain caused by osteoarthritis (OA) in patients who have already been treated with pain relievers (e.g., acetaminophen) and other treatments that did not work well.
Hyaluronic acid is similar to a substance that occurs naturally in the joints. It works by acting like a lubricant and shock absorber in the joints and helps the joints to work properly.
Hyaluronic acid injection is to be administered only by or under the immediate supervision of your doctor. How often you have the hyaluronic acid injections depends on the type of hyaluronan (hyaluronic acid) preparation used. You’ll probably be given a course of 3–5 injections, each separated by 1–3 weeks. Some doctors give the first two injections close together and then extend the period between those remaining. You probably won’t need to rest the joint after the hyaluronic acid injections. However, some doctors recommend you avoid activities such as jogging, soccer, tennis, heavy lifting, or standing on your feet for a long time for two days after the injection.
Hyaluronic acid injection is available in the following dosage forms:
- Solution
- Gel/Jelly
Before having hyaluronic acid injection
In deciding to use hyaluronic acid injection, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
- Allergies: Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
- Pediatric: Appropriate studies have not been performed on the relationship of age to the effects of hyaluronic acid injection in the pediatric population. Safety and efficacy have not been established.
- Geriatric: No information is available on the relationship of age to the effects of hyaluronic acid injection in geriatric patients.
- Breastfeeding: There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Drug Interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. Tell your healthcare professional if you are taking any other prescription or nonprescription (over-the-counter [OTC]) medicine.
Other Interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of hyaluronic acid injection. Make sure you tell your doctor if you have any other medical problems, especially:
- Allergy to bacterial proteins, gram positive or
- Allergy to hyaluronate preparations or
- Skin or knee joint infections or other problems at the place where the injection is to be given—Should not be given in patients with these conditions.
- Joint effusion (too much fluid in the knees)—Patients with this condition should be treated first before receiving hyaluronic acid injection.
How long do they take to work?
The hyaluronic acid injections should work within days if they’re effective and they’ll probably last for a few months. Sometimes your doctor may recommend you have repeat courses.
The particles in hyaluronan are quite large, so they’ll stay in your joint for some time before they’re absorbed into your blood stream. Some of the hyaluronic acid preparations available contain significantly larger particles than others and this can influence your doctor’s choice.
Side-effects and risks of hyaluronic acid injection
Hyaluronan injections have very few side-effects, although some people have a slight allergic reaction, which causes temporary pain and swelling in their joint after the injection. There’s also a small risk of infection. If the injection is done in slightly the wrong place, it won’t damage your skin or muscle.
Temporary pain or swelling in the knee joint may occur after receiving hyaluronic acid injection. See your doctor if the pain or swelling in the knee persists or becomes worse after receiving hyaluronic acid injection.
Do not use hyaluronic acid injection with disinfectants containing quaternary ammonium salts (e.g., benzalkonium chloride). This may prevent hyaluronic acid injection from working properly.
More common side effects
- Difficulty with moving
- Muscle pain or stiffness
- Pain in the joints
Less common
- Swelling or redness in the joints
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
Less common
- Bleeding, blistering, burning, coldness, discoloration of the skin, feeling of pressure, hives, infection, inflammation, itching, lumps, numbness, pain, rash, redness, scarring, soreness, stinging, swelling, tenderness, tingling, ulceration, or warmth at the injection site
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
- Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microbial Cell Factories201110:99 https://doi.org/10.1186/1475-2859-10-99[↩]
- Chien LJ, Lee CK: Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin. Biotechnol Prog. 2007, 23: 1017-1022.[↩]
- Widner B, Behr R, Von Dollen S: Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol. 2005, 71: 3747-3752. 10.1128/AEM.71.7.3747-3752.2005[↩][↩]
- Hyaluronate is synthesized at plasma membranes. Prehm P. Biochem J. 1984 Jun 1; 220(2):597-600.[↩]
- Molecular identification of a putative human hyaluronan synthase. Watanabe K, Yamaguchi Y. J Biol Chem. 1996 Sep 20; 271(38):22945-8.[↩]
- Mammalian hyaluronan synthases. Itano N, Kimata K. IUBMB Life. 2002 Oct; 54(4):195-9.[↩]
- Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Scott JE, Heatley F. Proc Natl Acad Sci U S A. 1999 Apr 27; 96(9):4850-5.[↩]
- Hyaluronan: from extracellular glue to pericellular cue. Nature Reviews Cancer volume 4, pages 528–539 (2004). https://www.nature.com/articles/nrc1391[↩]
- Laurent TC. Structure of hyaluronic acid. In: Balazs, EA, ed. Chemistry and Molecular Biology of the Intercellular Matrix, Academic Press: New York, 1970:p. 703.[↩]
- Hyaluronan in respiratory injury and repair. Turino GM, Cantor JO. Am J Respir Crit Care Med. 2003 May 1; 167(9):1169-75.[↩]
- Blank LM, Hugenholtz P, Nielsen LK: Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic streptococci. J Mol Evol. 2008, 67: 13-22. 10.1007/s00239-008-9117-1[↩]
- Sheng JZ, Ling PX, Zhu XQ, Guo XP, Zhang TM, He YL, Wang FS: Use of induction promoters to regulate hyaluronan synthase and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer. J Appl Microbiol. 2009, 107: 136-144. 10.1111/j.1365-2672.2009.04185.x[↩]
- Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Tzellos TG, Klagas I, Vahtsevanos K, Triaridis S, Printza A, Kyrgidis A, Karakiulakis G, Zouboulis CC, Papakonstantinou E. Exp Dermatol. 2009 Dec; 18(12):1028-35.[↩][↩]
- Hyaluronan in the rat with special reference to the skin. Reed RK, Lilja K, Laurent TC. Acta Physiol Scand. 1988 Nov; 134(3):405-11.[↩][↩]
- Meyer K, Palmer JW. The Polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–34.[↩]
- Weissmann B, Meyer K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J Am Chem Soc. 1954;76:1753–7. doi: 10.1021/ja01636a010[↩]
- Hyaluronate in normal human synovial fluid. HAMERMAN D, SCHUSTER H. J Clin Invest. 1958 Jan; 37(1):57-64.[↩]
- Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Armstrong SE, Bell DR. Am J Physiol Heart Circ Physiol. 2002 Dec; 283(6):H2485-94.[↩]
- Hyaluronan: from extracellular glue to pericellular cue. Toole BP. Nat Rev Cancer. 2004 Jul; 4(7):528-39.[↩]
- The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Papakonstantinou E, Karakiulakis G. Br J Pharmacol. 2009 Aug; 157(7):1111-27.[↩]
- A 340 kDa hyaluronic acid secreted by human vascular smooth muscle cells regulates their proliferation and migration. Papakonstantinou E, Karakiulakis G, Eickelberg O, Perruchoud AP, Block LH, Roth M. Glycobiology. 1998 Aug; 8(8):821-30.[↩]
- Benign hyperplasia of the human prostate is associated with tissue enrichment in chondroitin sulphate of wide size distribution. Goulas A, Hatzichristou DG, Karakiulakis G, Mirtsou-Fidani V, Kalinderis A, Papakonstantinou E. Prostate. 2000 Jul 1; 44(2):104-10.[↩]
- Tissue structure-specific distribution of glycosaminoglycans in the human penis. Goulas A, Papakonstantinou E, Karakiulakis G, Mirtsou-Fidani V, Kalinderis A, Hatzichristou DG. Int J Biochem Cell Biol. 2000 Sep; 32(9):975-82.[↩]
- Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Eur Respir J. 2009 Sep; 34(3):616-28.[↩][↩][↩][↩]
- Hyaluronan: from extracellular glue to pericellular cue. Toole BP. Nat Rev Cancer. 2004 Jul; 4(7):528-39. https://www.ncbi.nlm.nih.gov/pubmed/15229478/[↩]
- Hyaluronan in skin. Juhlin L. J Intern Med. 1997 Jul; 242(1):61-6. https://onlinelibrary.wiley.com/doi/pdf/10.1046/j.1365-2796.1997.00175.x[↩]
- Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, Lowe E, Mahoney DJ, Tammi MI, Kahmann JD, Campbell ID, Day AJ, Jackson DG. Mol Cell. 2004 Feb 27; 13(4):483-96.[↩]
- The role of hyaluronan synthase 3 in ventilator-induced lung injury. Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, Quinn DA. Am J Respir Crit Care Med. 2005 Jul 1; 172(1):92-8.[↩]
- The role of Toll-like receptors in non-infectious lung injury. Jiang D, Liang J, Li Y, Noble PW. Cell Res. 2006 Aug; 16(8):693-701.[↩]
- Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Matrix Biol. 2007 Jan; 26(1):58-68.[↩]
- Inhibition of platelet-derived growth factor-BB-induced receptor activation and fibroblast migration by hyaluronan activation of CD44. Li L, Heldin CH, Heldin P. J Biol Chem. 2006 Sep 8; 281(36):26512-9.[↩]
- Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Knudson W. Am J Pathol. 1996 Jun; 148(6):1721-6.[↩]
- Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Zhang L, Underhill CB, Chen L. Cancer Res. 1995 Jan 15; 55(2):428-33.[↩]
- Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology. 2012;4(3):253-258. doi:10.4161/derm.21923. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583886/[↩][↩]
- Hyaluronan as an immune regulator in human diseases. Jiang D, Liang J, Noble PW. Physiol Rev. 2011 Jan; 91(1):221-64.[↩]
- Hyaluronan-binding proteins in development, tissue homeostasis, and disease. Knudson CB, Knudson W. FASEB J. 1993 Oct; 7(13):1233-41.[↩]
- Hyaluronan and cell locomotion. Turley EA. Cancer Metastasis Rev. 1992 Mar; 11(1):21-30.[↩]
- Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. Lokeshwar VB, Selzer MG. J Biol Chem. 2000 Sep 8; 275(36):27641-9[↩]
- Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Papakonstantinou E, Kouri FM, Karakiulakis G, Klagas I, Eickelberg O. Eur Respir J. 2008 Dec; 32(6):1504-12.[↩]
- Soluble hyaluronan receptor RHAMM induces mitotic arrest by suppressing Cdc2 and cyclin B1 expression. Mohapatra S, Yang X, Wright JA, Turley EA, Greenberg AH. J Exp Med. 1996 Apr 1; 183(4):1663-8.[↩]
- TGF-beta 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. Samuel SK, Hurta RA, Spearman MA, Wright JA, Turley EA, Greenberg AH. J Cell Biol. 1993 Nov; 123(3):749-58.[↩]
- Catabolism of hyaluronan in rabbit skin takes place locally, in lymph nodes and liver. Laurent UB, Dahl LB, Reed RK. Exp Physiol. 1991 Sep; 76(5):695-703.[↩]
- Hyaluronidases: their genomics, structures, and mechanisms of action. Stern R, Jedrzejas MJ. Chem Rev. 2006 Mar; 106(3):818-39.[↩]
- The six hyaluronidase-like genes in the human and mouse genomes. Csoka AB, Frost GI, Stern R. Matrix Biol. 2001 Dec; 20(8):499-508.[↩][↩]
- Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Stern R, Maibach HI. Clin Dermatol. 2008 Mar-Apr; 26(2):106-22.[↩][↩][↩][↩][↩]
- Assay of serum hyaluronic acid in clinical application. Chichibu K, Matsuura T, Shichijo S, Yokoyama MM. Clin Chim Acta. 1989 May 31; 181(3):317-23.[↩]
- Clinical and biochemical manifestations of hyaluronidase deficiency. Natowicz MR, Short MP, Wang Y, Dickersin GR, Gebhardt MC, Rosenthal DI, Sims KB, Rosenberg AE. N Engl J Med. 1996 Oct 3; 335(14):1029-33.[↩]
- HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. Lepperdinger G, Strobl B, Kreil G. J Biol Chem. 1998 Aug 28; 273(35):22466-70.[↩]
- Mouse Hyal3 encodes a 45- to 56-kDa glycoprotein whose overexpression increases hyaluronidase 1 activity in cultured cells. Hemming R, Martin DC, Slominski E, Nagy JI, Halayko AJ, Pind S, Triggs-Raine B. Glycobiology. 2008 Apr; 18(4):280-9.[↩]
- Photodegradation of hyaluronic acid: EPR and size exclusion chromatography study. Lapcík L Jr, Chabrecek P, Stasko A. Biopolymers. 1991 Oct 15; 31(12):1429-35.[↩]
- How best to halt and/or revert UV-induced skin ageing: strategies, facts and fiction. Berneburg M, Trelles M, Friguet B, Ogden S, Esrefoglu M, Kaya G, Goldberg DJ, Mordon S, Calderhead RG, Griffiths CE, Saurat JH, Thappa DM. Exp Dermatol. 2008 Mar; 17(3):228-40.[↩]
- Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Makrantonaki E, Adjaye J, Herwig R, Brink TC, Groth D, Hultschig C, Lehrach H, Zouboulis CC. Aging Cell. 2006 Aug; 5(4):331-44.[↩]
- Brincat MP. Hormone replacement therapy and the skin. Maturitas 2000; 35:107–117. 9 Makrantonaki E, Zouboulis CC. Androgens and aging of the skin. Curr Opin Endocrinol Diabetes Obes. 2009;16:240–5[↩]
- Mechanisms of photoaging and chronological skin aging. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Arch Dermatol. 2002 Nov; 138(11):1462-70.[↩]
- Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. Chung JH, Kang S, Varani J, Lin J, Fisher GJ, Voorhees JJ. J Invest Dermatol. 2000 Aug; 115(2):177-82.[↩]
- Skin ageing and its treatment. Baumann L. J Pathol. 2007 Jan; 211(2):241-51.[↩]
- Age-dependent changes of hyaluronan in human skin. Meyer LJ, Stern R. J Invest Dermatol. 1994 Mar; 102(3):385-9.[↩][↩]
- Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Ann Surg. 1991 Apr; 213(4):292-6.[↩]
- Removal rate of [3H]hyaluronan injected subcutaneously in rabbits. Reed RK, Laurent UB, Fraser JR, Laurent TC. Am J Physiol. 1990 Aug; 259(2 Pt 2):H532-5.[↩]
- Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Longas MO, Russell CS, He XY. Carbohydr Res. 1987 Jan 15; 159(1):127-36.[↩]
- A review of skin ageing and its medical therapy. Gilchrest BA. Br J Dermatol. 1996 Dec; 135(6):867-75.[↩]
- Understanding premature skin aging. Uitto J. N Engl J Med. 1997 Nov 13; 337(20):1463-5.[↩]
- Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Stern R, Maibach HI. Clin Dermatol. 2008 Mar-Apr; 26(2):106-22[↩][↩]
- Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Bernstein EF, Underhill CB, Hahn PJ, Brown DB, Uitto J. Br J Dermatol. 1996 Aug; 135(2):255-62.[↩]
- Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): new insights into mechanisms of matrix remodeling. Röck K, Grandoch M, Majora M, Krutmann J, Fischer JW. J Biol Chem. 2011 May 20; 286(20):18268-76.[↩]
- Differential hyaluronan homeostasis and expression of proteoglycans in juvenile and adult human skin. Tzellos TG, Sinopidis X, Kyrgidis A, Vahtsevanos K, Triaridis S, Printza A, Klagas I, Karakiulakis G, Papakonstantinou E. J Dermatol Sci. 2011 Jan; 61(1):69-72.[↩]
- Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Oh JH, Kim YK, Jung JY, Shin JE, Chung JH. Exp Dermatol. 2011 May; 20(5):454-6.[↩]
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9[↩][↩]
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119(7):2219–2227[↩]
- Muhn C, Rosen N, Solish N, et al. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: a Canadian overview. Clinical, Cosmetic and Investigational Dermatology. 2012;5:147-158. doi:10.2147/CCID.S30794. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3469309/[↩][↩][↩][↩][↩][↩][↩]
- Raspaldo H. Volumizing effect of a new hyaluronic acid sub–dermal facial filler: a retrospective analysis based on 102 cases. J Cosmet Laser Ther. 2008;10(3):134–142.[↩]
- Sasaki E, Tsuda E, Yamamoto Y, et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis – a five-year prospective cohort study. Arthritis Research & Therapy. 2015;17:283. doi:10.1186/s13075-015-0793-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600294/[↩]
- Serum hyaluronan levels in patients with knee osteoarthritis. Turan Y, Bal S, Gurgan A, Topac H, Koseoglu M. Clin Rheumatol. 2007 Aug; 26(8):1293-8.[↩]
- Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Association between age and knee structural change: a cross sectional MRI based study. Ann Rheum Dis. 2005;64:549–555. doi: 10.1136/ard.2004.023069.[↩][↩]
- Fraser JRE, Laurent TC, Laurent UBG. Hyluronan: its nature, distribution, fuuctions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x[↩]
- Engström-Laurent A, Laurent UB, Lilja K, Laurent TC. Concentration of sodium hyaluronate in serum. Scand J Clin Lab Invest. 1985;45:497–504. doi: 10.3109/00365518509155249[↩]
- Hällgren R, Engström-Laurent A, Nisbeth U. Circulating hyaluronate: A potential marker of altered metabolism of the connective tissue in uremia. Nephron. 1987;46:150–154. doi: 10.1159/000184331[↩]
- Hartley JL, Brown RM, Tybulewicz A, Hayes P, Wilson DC, Gillett P, et al. Hyaluronic acid predicts hepatic fibrosis in children with hepatic disease. J Pediatr Gastroenterol Nutr. 2006;43:217–221. doi: 10.1097/01.mpg.0000228121.44606.9f[↩]
- Engström-Laurent A, Hällgren R. Circulating hyaluronate in rheumatoid arthritis: relationship to inflammatory activity and the effect of corticosteroid therapy. Ann Rheum Dis. 1985;44:83–88. doi: 10.1136/ard.44.2.83.[↩]
- Xing RD, Chang SM, Li JH, Li H, Han ZX. Serum hyaluronan levels in oral cancer patients. Chin Med J. 2008;121:327–330.[↩]
- Rössler A, László Z, Kvas E, Hinghofer-Szalkay HG. Plasma hyaluronan concentration: no circadian rhythm but large effect of food intake in humans. Eur J Appl Physiol Occup Physiol. 1998;78:573–577. doi: 10.1007/s004210050463[↩]
- Shimura Y, Kurosawa H, Sugawara Y, Tsuchiya M, Sawa M, Kaneko H, et al. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: a cross-sectional study. Osteoarthritis Cartilage. 2013;21:1179–1184. doi: 10.1016/j.joca.2013.05.014[↩]
- Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290[↩][↩]
- Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373[↩]
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159[↩]
- Marini S, Fasciglione GF, Monteleone G, Maiotti M, Tarantino U, Coletta M. A correlation between knee cartilage degradation observed by arthroscopy and synovial proteinases activities. Clin Biochem. 2003;36:295–304. doi: 10.1016/S0009-9120(03)00029-8[↩]
- Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis e results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005[↩]