Contents
What are lip fillers
Lip augmentation consists of the reshaping and/or enlargement of the visible portion of the lip, the vermilion. Lip augmentation with the use of injectable fillers achieves quick results with minimal downtime. Alteration of the shape of the Cupid’s bow and of the relationship between the vermilion and the skin underlying the nasal columella also falls within the category of lip augmentation. It is also important to consider the relationship between lip height and incisor shown in the anatomic analysis, to evaluate possible maxillary hypoplasia and protrusion, and to consider the patient’s occlusion status.
Lip augmentation with fillers can be performed by injecting the material in any or all of the anatomic parts of the lip, allowing for a very controlled and predictable result. Precautions regarding mode of injection and quantity of the substance injected vary widely with patient profiles. At times, budgetary constraints decide treatments, but that should be avoided, and you need to be told about the optimum filler requirements.
Various fillers, temporary and permanent, have been tried in shaping the lips, with gratifying results. Disasters in the form of granulomas have been reported with both temporary and permanent fillers, more often with the latter.
Hyaluronic acid (HA) and polyacrylamide (PA) are the two main fillers widely available. Hyaluronic acid (HA) is the predominant one, with polyacrylamide (PA) being virtually out of the race because of fears of granuloma. Worldwide usage and published reports clearly confirm the efficacy and safety of HA fillers. They are one of the key components of successful combination treatments of the aging face and lips 1.
Common commercial preparations of hyaluronic acid (HA) that are widely available include the Anteis range (Fortélis® and Esthélis®, Switzerland), Galderma range, USA (Restylane and Perlane), and the Allergan range, Irvine, CA 92612 (Juvéderm Ultra® and Juvéderm Ultra Plus®). Other brands such as Revanesse®, Prollenium Medical Technologies Inc., Canada and Amazing Fill® are also available 2.
Significant ease of use, “off-the-shelf” availability, and widespread acceptance by the public make hyaluronic acid (HA) one of the most commonly used fillers.
Optimizing the aesthetic outcome of lip augmentation with dermal fillers, such as HA, requires skillful application of a suitable injection technique. Moreover, achieving aesthetic goals with minimal risk for adverse events requires knowledge of lip anatomy and function, clinical experience in the use of various injection techniques, and an individualized treatment approach.
How long do lip fillers last?
That depends on the type of lip fillers you are having. For in depth detail discussion see types of dermal lip fillers below.
Here is a table of dermal fillers and their duration
| Temporary biodegradable (< 1 year) | Semi-permanent biodegradable (1 – 2 years) | Permanent non-biodegradable (> 2 years) |
| Autologous fat transfer | Calcium hydroxylapatite (CaHA) | Collagen implant – porcine |
| Acellular dermis – cadaver | Dextran particles (DEAE) | Silicone oil (polydimethylsiloxane oil) (LIS) |
| Collagen – human, bovine, porcine | Hydroxyethylmethacrylate (HEMA) | Polyacrylamide gel (PAAG) |
| Cultured fibroblasts – human | Polylactic acid (PLA) | Polymethylmethacrylate (PMMA) |
| Fascia autograft – human | Polyvinyl alcohol (PVA) | Expanded polytetrafluoroethylene (ePTFE) |
| Hyaluronic acid (HA) – avian, bacterial | Polytetrafluoroethylene (PTFE) |
- ALWAYS work with a licensed health care provider who uses properly labeled, sealed vials for treatments. You also can ask to confirm that you are receiving an FDA-approved filler. And never get injectable fillers from unlicensed providers or in non-medical settings like hotels or private homes.
- ALWAYS request and read the patient labeling information on U.S. Food and Drug Administration (FDA)-approved injectable wrinkle fillers from your licensed health care provider.
- ALWAYS know the type of product to be injected and all of its possible side effects. Know where each product used is to be injected. Talk to your licensed health care provider if you have any questions.
- NEVER buy dermal fillers on the Internet. They may be fake, contaminated, and/or harmful.
- NEVER get any type of filler or liquid silicone injected for body contouring. This means you should never get breast fillers, “butt” fillers, or fillers for spaces between your muscles. These products, which include certain types of injectable silicone, can be dangerous and can cause serious injury and even death.
Are lip injections safe
These days, people across the country are seeking treatments to smooth smile lines and crow’s feet and to plump up their lips and cheeks. But injectable dermal fillers are not for everyone and may not be indicated for people with certain conditions (such as bleeding disorders or certain allergies) 3. If your health care provider confirms that dermal fillers are an option for you, know that all products have benefits and risks. The U.S. Food and Drug Administration (FDA) advises you to work with a licensed health care provider and to understand all of the risks and benefits before receiving treatment. In May 2015, the FDA issued a warning to healthcare providers and the public about serious complications that can occur if dermal fillers are inadvertently injected into blood vessels in the face. The complications could possibly include vision impairment, blindness, stroke, and damage and/or necrosis of the skin and underlying facial structures. Caution should be used to ensure proper placement of the filler material, and patients should be informed about the potential adverse effects and how to recognize symptoms of impending serious complications.
The US Food and Drug Administration (FDA) is warning healthcare providers and the public about the possibility of rare, but serious, injuries that may occur as a result of unintentional injection of a soft tissue filler into blood vessels in the face. Unintentional injection can block blood vessels and restrict blood supply to tissues and lead to embolization, which could cause vision impairment, blindness, stroke, and damage and/or necrosis of the skin and underlying facial structures, the agency says in a Safety Communication.
Unintentional injections into blood vessels may occur with injection sites anywhere on the face, but the FDA’s review of the literature and submitted adverse event reports pinpoints certain injection locations at which blood vessel blockages have been reported more often. These include the skin between the eyebrows and nose (glabella), in and around the nose, the forehead, and around the eyes (periorbital region), the FDA says.
Two separate but related reports from 2017 and 2018 have reaffirmed the risks, some very serious—including strokes and blindness—that can be associated with dermal fillers 4. A 3-year study (2014-2016) reported swelling, infection, blindness (8 cases), and tissue death as the most common problems. A 10-year analysis (2007-2017) reported nodule formation, infection, inflammation, allergic complications, and vascular complications as the most common problems.
Risks of FDA-approved fillers
Remember to work with a licensed health care provider to ask what you can expect for FDA-approved fillers. Then contact your health care provider if you are concerned about a particular side effect.
The occurrence of adverse reactions relates to both the inherent properties of the product and inappropriate delivery or dilution of the filler, which may lead to harmful sequelae 5.
Underscoring the need for product-specific training, uneven distribution of injected products, due to poor technique, can also lead to postinjection lumps and nodules 6. This is of particular concern with more permanent products because the undesired results are also long-lasting. Overaggressive injection may lead to irregularity or lumpiness, whereas if the product is placed too superficially, beading can occur 7.
The most common side effects include 3:
- bruising
- redness
- swelling
- pain
- itching
Additional side effects less commonly reported include 3:
- infections
- lumps and bumps
- discoloration or change in pigmentation
Rare but serious risks include 3:
- scarring, blurred vision, partial vision loss, and blindness if the dermal filler is inadvertently injected into a blood vessel. It is recommended that health care providers take care to avoid injection into blood vessels (especially around the forehead, nose and eye area) for these reasons.
- allergic reaction that may lead to a severe reaction (anaphylactic shock) that requires emergency medical help.
Most side effects occur shortly after injection and go away within two weeks. In some cases, side effects may emerge weeks, months, or years later. Talk to your licensed health care provider if you have questions or concerns.
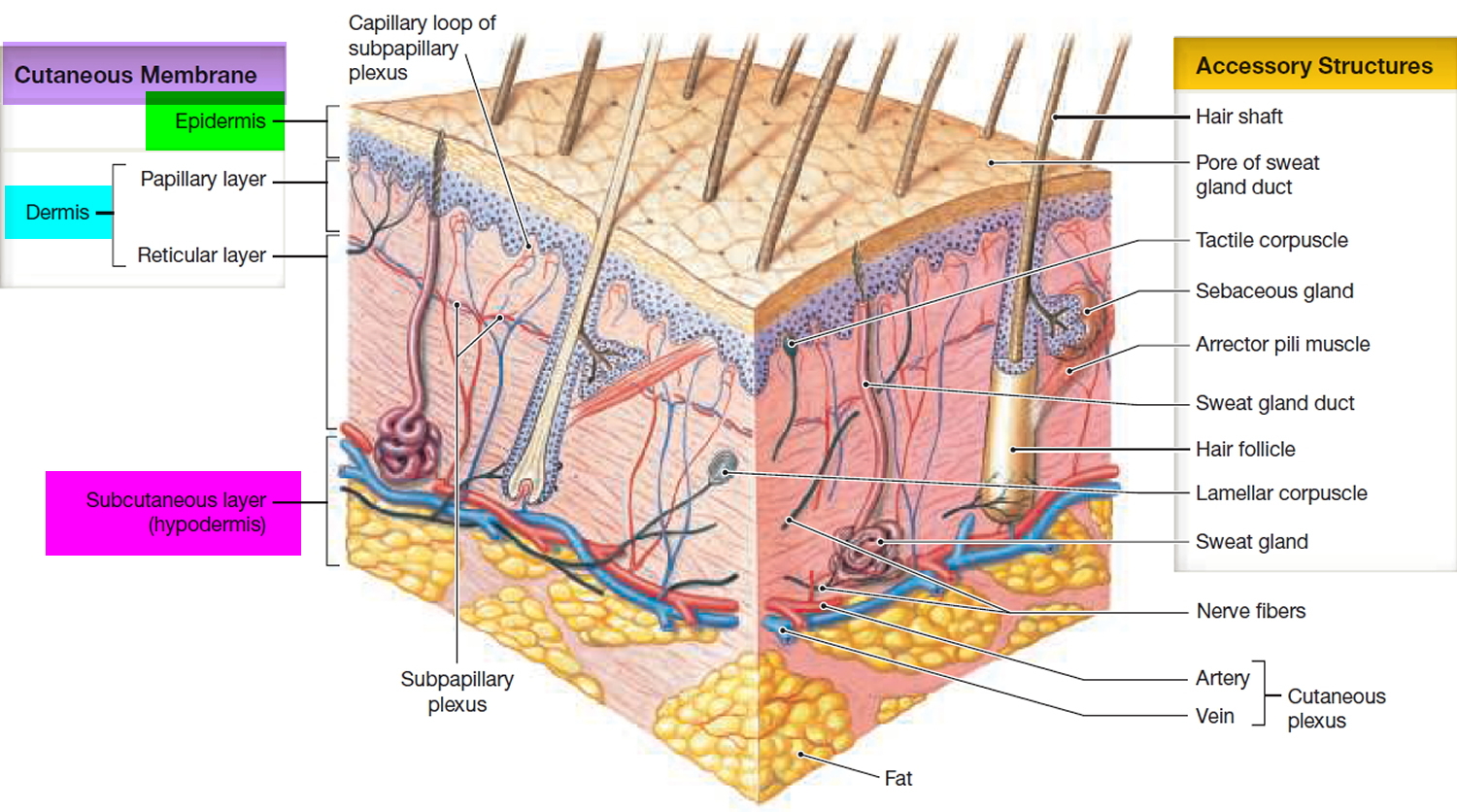
The skin
The skin (integument) is the body’s largest and heaviest organ. In adults, the skin covers an area of 1.5 to 2.0 m2 and accounts for about 15% of the body weight.
The skin consists of two layers:
- a stratified squamous epithelium called the Epidermis (Figure 2) and
- a deeper connective tissue layer called the Dermis (Figure 1).
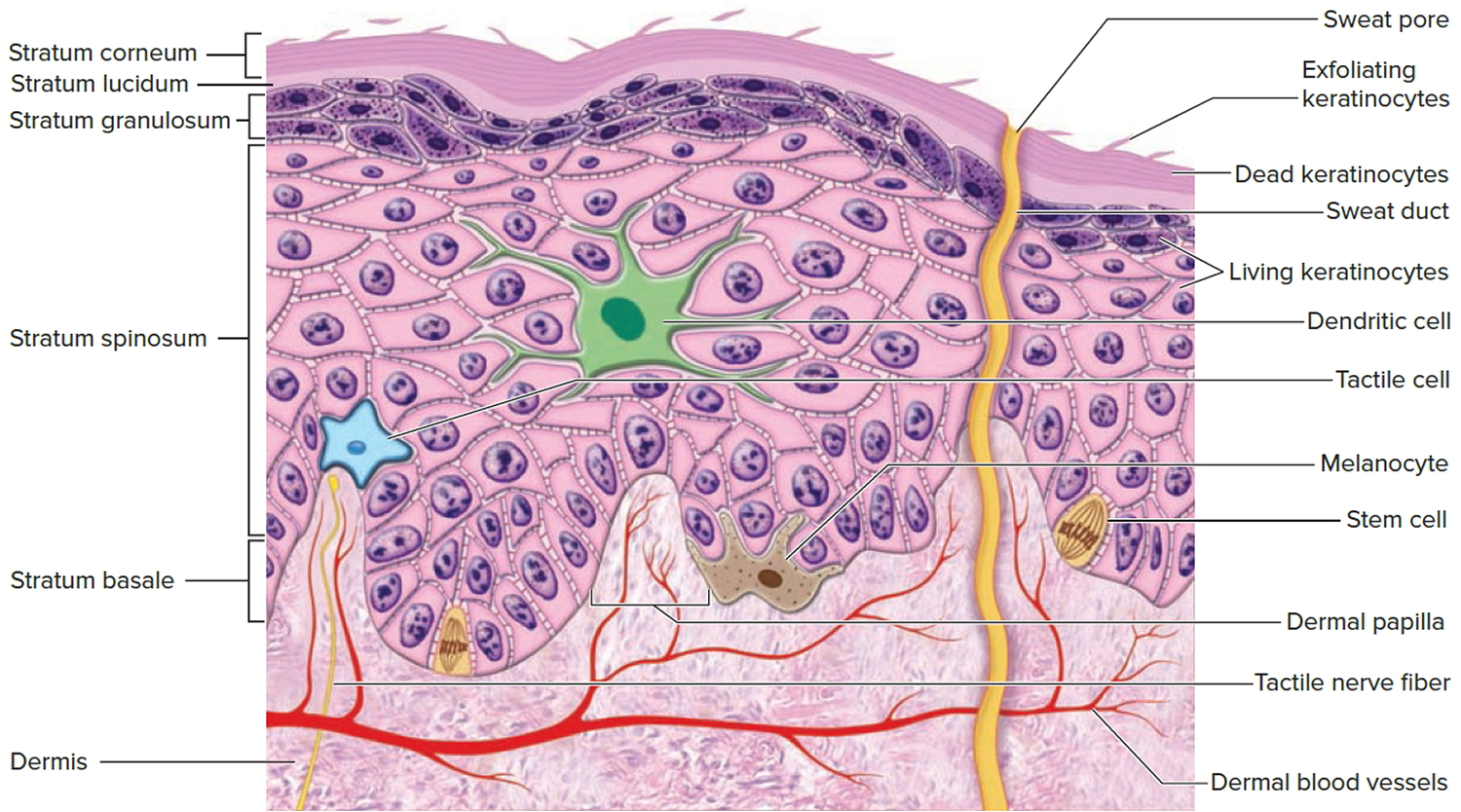
The epidermis gives rise to the outer barrier layer of dead cells, the stratum corneum, through terminal differentiation of keratinocytes, which are the predominant cell type. Below the epidermis is the dermis. This layer contains the blood vessels, lymphatics, nerves, and deeper portions of the hair follicles and glands that originate from the epidermis. The dermis is composed largely of extracellular matrix and gives skin its strength and elasticity. Below the dermis is a subcutaneous layer of fatty tissue that gives contour to the skin.
Figure 1. Skin structure
Below the dermis is another connective tissue layer, the hypodermis, which is not part of the skin but is customarily studied in conjunction with it. Most of the skin is 1 to 2 mm thick, but it ranges from less than 0.5 mm on the eyelids to 6 mm between the shoulder blades. The difference is due mainly to variation in thickness of the dermis, although skin is classified as thick or thin based on the relative thickness of the epidermis alone.
Thick skin covers the palms, soles, and corresponding surfaces of the fingers and toes. Its epidermis alone is about 0.5 mm thick, due to a very thick surface layer of dead cells called the stratum corneum (see Figure 2). Thick skin has sweat glands but no hair follicles or sebaceous (oil) glands. The rest of the body is covered with thin skin, which has an epidermis about 0.1 mm thick, with a thin stratum corneum. It possesses hair follicles, sebaceous glands, and sweat glands.
The accessory structures include hair, nails, and a variety of multicellular exocrine glands. These structures are located in the dermis and protrude through the epidermis to the surface.
Figure 2. Structure and skin cells of the Epidermis
The ageing process
The process of ageing leads to visible changes in facial contour as there is a loss of subcutaneous volume. Diminished dermal vasculature and blood flow, combined with a reduction of dermal extracellular matrix (including collagen, hyaluronic acid and elastin), lead to progressive thinning of the dermis. This results in a loss of elasticity and underlying atrophy of the natural fat pads. The soft tissue that encircles bony skeleton slowly collapses so that these bony landmarks are more visible 8. This is exacerbated by the effects of gravity, which results in sagging of the soft tissues relative to their substructure 9.
The full fibrous tissue associated with youthful appearance is replaced by less dense, less supple and less supportive tissue 8.
Among the most noticeable changes in the face as it ages, is the development of wrinkles (rhytids) and furrows, and the increased visibility of blood vessels. Critical changes in the perioral area can include vertical rhytids, increased prominence of nasolabial folds (crease that runs from nose to the corner of the mouth), ptosis of the oral commissures (sagging of lines from the corners of the mouth), thinning of the lips, and flattening of the upper lip with less definition of the Cupid’s bow 8. Transverse forehead lines become prominent and can be accompanied by a lowering of the eyebrows.
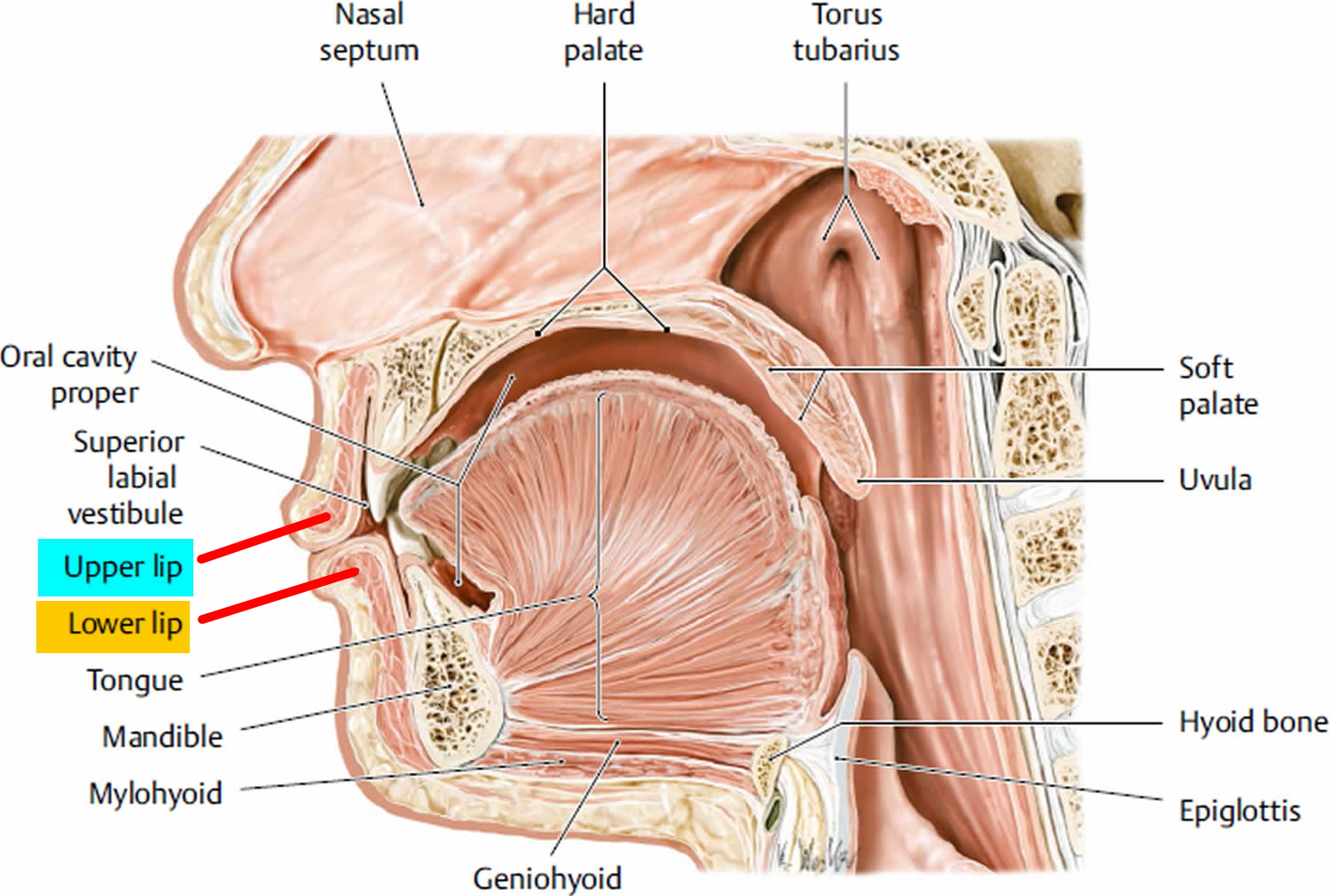
Lips Anatomy
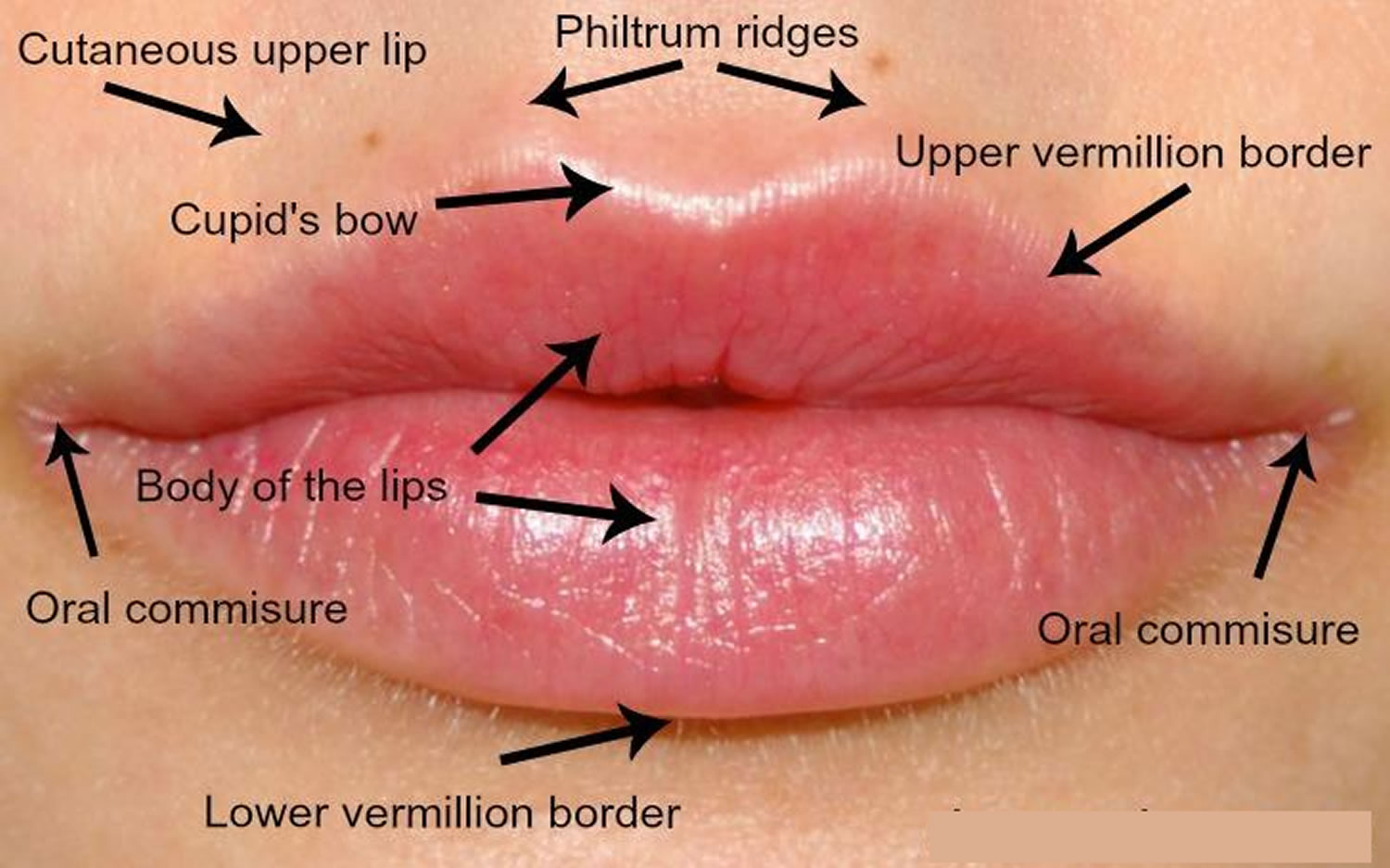
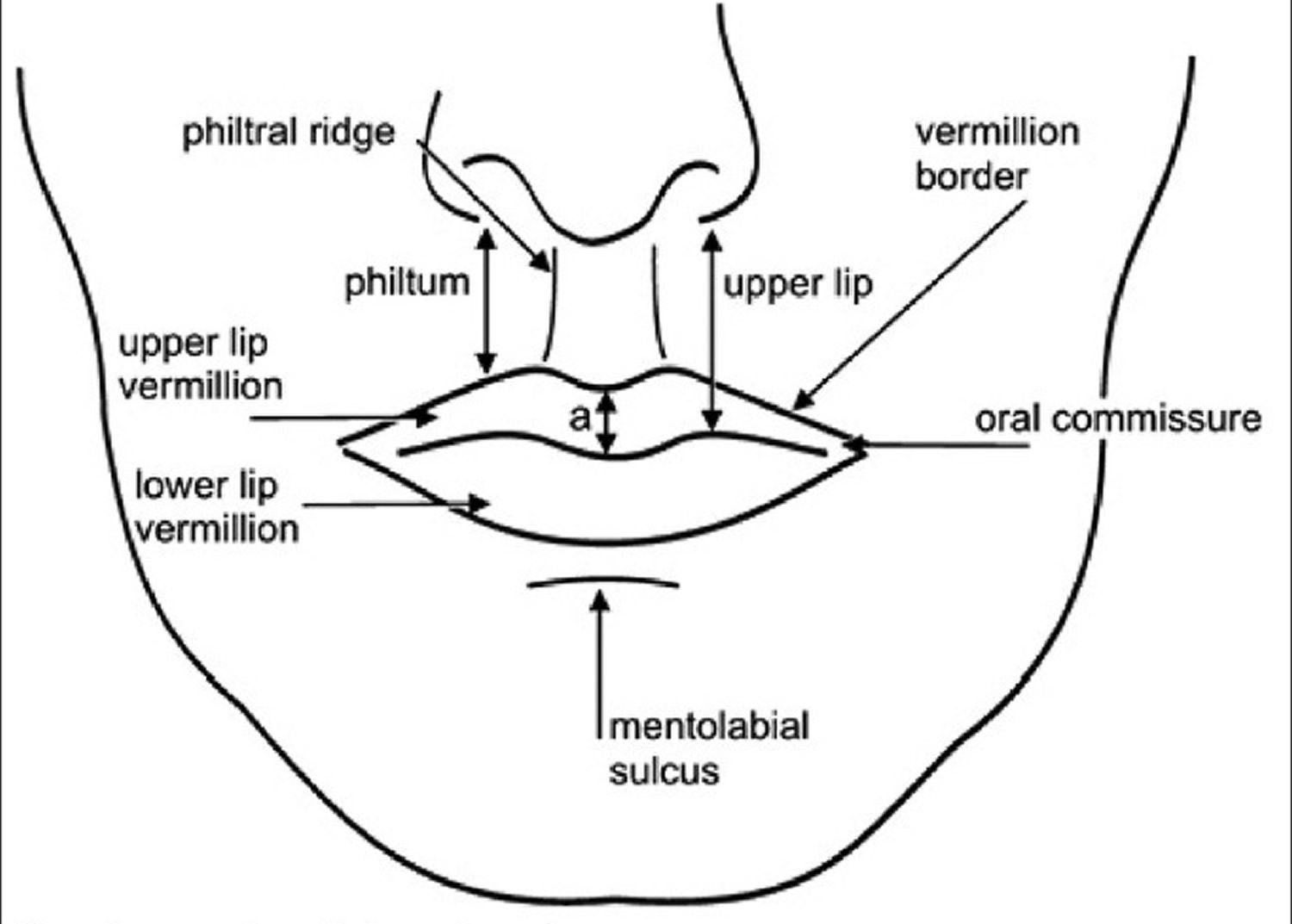
The upper lip extends from the base of the nose superiorly to the nasolabial folds laterally and to the free edge of the vermilion border inferiorly. The lower lip extends from the superior free vermilion edge superiorly, to the commissures laterally, and to the mandible inferiorly. Around the circumferential vermilion/skin border, a fine line of pale skin accentuates the color difference between the vermilion and normal skin. Along the upper vermilion/skin border, two paramedian elevations of the vermilion form the Cupid’s bow. Two raised vertical columns of tissue form a midline depression called the philtrum.
Female lips are, on average, a little fuller than male lips 10. They bulge forward more than male lips – in other words, they are slightly more “pouty.” Female lips are not noticeably bigger when you see them from the front but they do bulge forward more as seen from the side. You need to keep this in mind while receiving treatment for male and female lips. Over-volumization of the male lip can result in feminization of the area.
With the passage of time, photodamage, hereditary factors, and smoking contribute to loss of lip volume, perioral rhytides, and prominence of mentolabial folds. Genetically thin lips and cosmetic asymmetries of the lips are also issues that can be dealt with similarly, that is, by soft tissue augmentations using fillers. Successful rejuvenation of the perioral region requires sophistication in using a combination of technologies and injectables.
A deflating vermilion (the red part) is the most common complaint, followed by drooping angles of the mouth 10. These two together complete the picture of an aged face. Lips that have good volume can be highlighted by defining them and injecting into the white margins (the vermilion border) (Figures 3). Pouts can be created by injecting the filler below the muscle (Figure 5). Typically, the upper lip is treated more often than the lower lip. The best approach to lip augmentation depends on the nature of the defect and the subject’s aesthetic desires. For genetically thin lips, structural augmentation with a deeper-placed filler followed by volume correction with a superficial filler is ideal. For pure cosmetic enhancement of lips, a superficially placed filler with emphasis on the white roll and expansion of the vermilion is ideal 11.
Figure 3. Lips anatomy for lip dermal filler injections
Figure 4. Lips anatomy
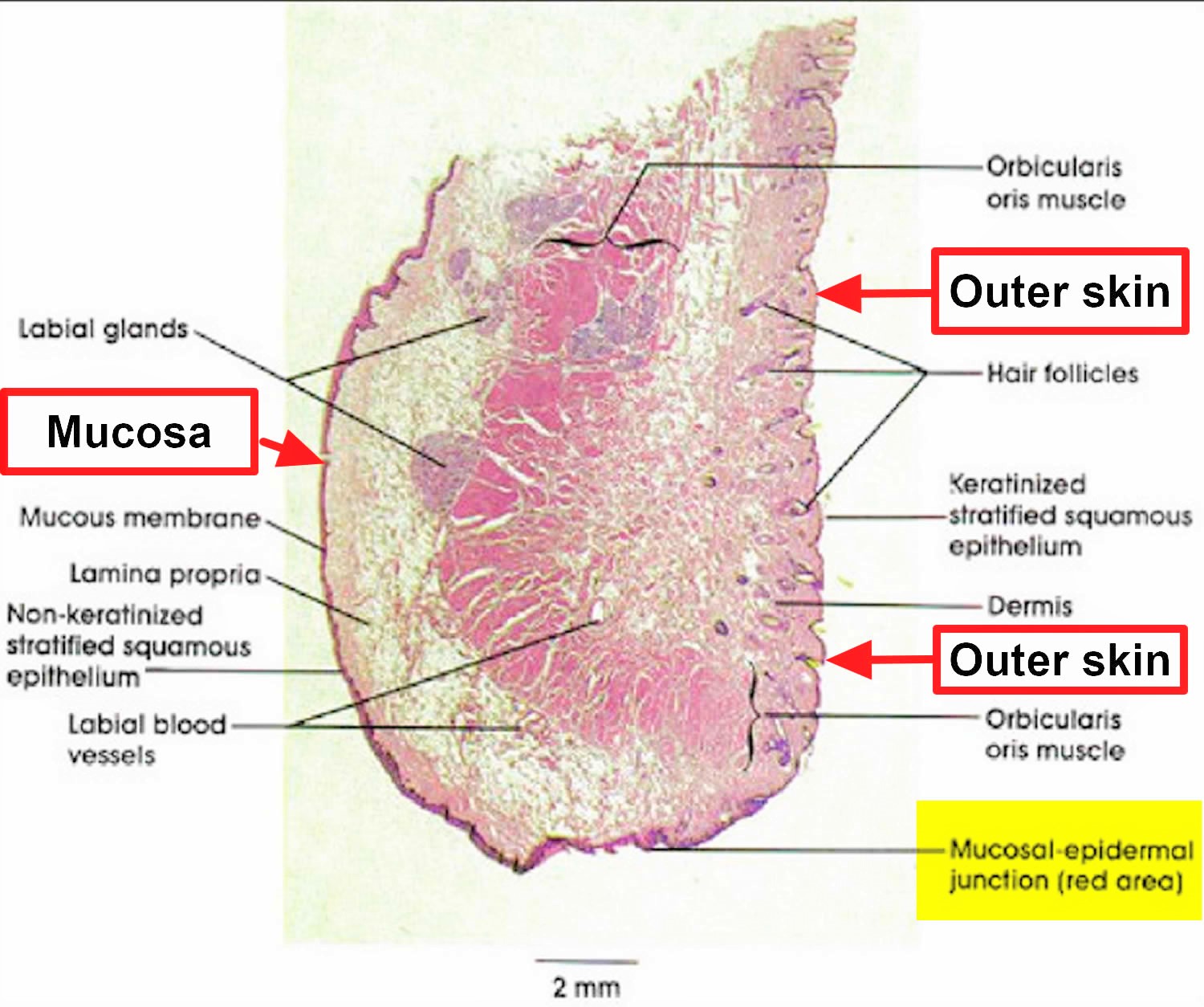
Figure 5. Lips histology
Dermal lip fillers
The perfect dermal filler would be inexpensive, safe, painless to inject, hypoallergenic, and long lasting. In addition, it should have consistent and predictable results, feel natural under the skin, take little time to inject, be ready-to-use, exert no downtime on the patient, and have a low risk of complications. With the increasing desire for people to achieve a more youthful appearance, the aging baby boomer population, and the increased demand for “lunch-time procedures,” the pharmaceutical market has responded by providing the cosmetic surgeon with an increasing number of options to meet the demands of the cosmetic patient. Thus, this segment of cosmetic surgery has been the fastest growing for the past decade.
By definition, a dermal filler is a product that is injected or placed into the dermis. Patients are instructed to not manipulate the treated areas, because the product may shift. The best way to reduce inflammation is to immediately apply a cool pack to the areas that were treated. In current practice, several dermal fillers are available for use in the United States, in addition to subdermal fillers, or those that are placed underneath the dermis in the subcutis.
Injectable dermal fillers and synthetic dermal implants have been developed to address volume loss and contour defects and appear to be useful in the lower third of the face 12. Dermal fillers address volume loss to give the face a fuller, more youthful appearance. Dermal fillers are injected or inserted into the skin to give the face a fuller look, as opposed to the pulled, flat, two-dimensional look that is sometimes associated with facelift surgery (rhytidectomy) 13. Facelifts, which eliminate loose skin folds in the neck and wrinkles (rhytids) in the cheeks by the removing tissue and tightening or ‘lifting’ of the skin, do not augment or ‘fill’ the skin 14.
Dermal fillers have become a popular means of addressing volume loss and contour defects resulting from ageing, photo-damage, disease, trauma and/or scarification 15. Fillers that are biodegradable are resorbed over time by the tissues and require repeat applications. Semi-permanent and permanent dermal fillers have emerged as alternatives, to give patients a longer lasting or permanent effect. However, the potential side effects and complications associated with these fillers have not been well documented, may be difficult to manage, and can last as long as the filler.
Dermal fillers can complement surgical and topical treatments to give a greater and longer lasting aesthetic result 13. A combination of filler materials can be injected or inserted to give a natural contour to the face 16.
Also know that the safety of these products is unknown for use in pregnant or breastfeeding women or in patients under 18 years of age. The safety also is unknown if used with Botox or other wrinkle therapies.
You should discuss the different types of FDA-approved dermal fillers and the results you want to achieve with your licensed health care provider, who can refer you to a licensed dermatologist or plastic surgeon. You may want to contact the American Academy of Dermatology (https://www.aad.org/), the American Society of Plastic Surgeons (https://www.plasticsurgery.org/) or the American Society for Aesthetic Plastic Surgery (https://www.surgery.org/).
Dermal Fillers Approved by the FDA
FDA approval is based on the review of data collected from controlled clinical studies that evaluated the safe and effective use of the wrinkle fillers when injected into specified areas of facial tissue.
Most dermal fillers have a temporary effect, because they contain materials that are absorbed by the body over time. The FDA has approved only one product made from a material that remains in the body and is not absorbed. Some dermal fillers also contain lidocaine, which is intended to decrease pain or discomfort related to the injection.
The materials used in dermal fillers include:
Absorbable (temporary) materials
- Collagen: Collagen is a type of protein that is a major part of skin and other tissues in the body. Sources of purified collagen used in soft tissue fillers can be from cow (bovine) or human cells. The effects of collagen fillers generally last for 3-4 months. They are the shortest lasting of injectable filler materials.
- Hyaluronic acid: Hyaluronic acid is a type of sugar (polysaccharide) that is present in body tissues, such as in skin and cartilage. It is able to combine with water and swell when in gel form, causing a smoothing/filling effect. Sources of hyaluronic acid used in dermal fillers can be from bacteria or rooster combs (avian). In some cases, hyaluronic acid used in dermal fillers is chemically modified (crosslinked) to make it last longer in the body. The effects of this material last approximately 6 – 12 months.
- Calcium hydroxylapatite (CaHA): Calcium hydroxylapatite (CaHA) is a type of mineral that is commonly found in human teeth and bones. For wrinkle filling in the face or for the hand, calcium hydroxylapatite particles are suspended in a gel-like solution and then injected into the wrinkle in the face or under the skin in the back of the hand. The effects of this material last approximately 18 months. While in the body, calcium hydroxylapatite will be visible in x-rays and may obscure underlying features.
- Polylactic acid (PLA): Polylactic acid (PLA) is a biodegradable, biocompatible man-made polymer. This material has wide uses in absorbable stitches and bone screws. Polylactic acid (PLA) is a long lasting filler material that is given in a series of injections over a period of several months. The effects of polylactic acid (PLA) generally become increasingly apparent over time (over a period of several weeks) and its effects may last up to 2 years.
Non-absorbable (permanent) materials
- Polymethylmethacrylate beads (PMMA microspheres): Polymethylmethacrylate beads (PMMA microspheres) is a non-biodegradable, biocompatible, man-made polymer. This material is used in other medical devices, such as bone cement and intraocular lenses. Polymethylmethacrylate beads (PMMA microspheres) are tiny, round, smooth particles that are not absorbed by the body. When used as a soft tissue filler, polymethylmethacrylate beads (PMMA microspheres) are suspended in a gel-like solution that contains cow (bovine) collagen and injected into the face.
Table 1. FDA Approved Dermal Fillers
| Trade Name (with link to additional information) | Material | Applicant | PMA Number | Decision Date | Approved For |
|---|---|---|---|---|---|
| Restylane, Refyne, Restylane Defyne | Sodium Hyaluronate | Q-Med AB | P140029 | 12/9/2016 | Restylane Refyne is indicated for injection into the mid-to-deep dermis for the correction of moderate to severe facial wrinkles and folds (such as nasolabial fold) in patients over the age of 21. Restylane Defyne is indicated for injection into the mid-to-deep dermis for the correction of moderate to severe deep facial wrinkles and folds (such as nasolabial fold) in patients over the age of 21. |
| JUVEDERM VOLLURE XC | Hyaluronic Acid | Allergan | P110033/S020 | 3/17/2017 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds) in adults over the age of 21. |
| JUVEDERM VOLBELLA XC | Hyaluronic Acid with Lidocaine | Allergan | P110033/S018 | 5/31/2016 | Injection into the lips for lip augmentation and for correction of perioral rhytids in adults over the age of 21. |
| RESTYLANE LYFT WITH LIDOCAINE | Hylauronic acid with lidocaine | Galderma Laboratories | P040024 S073 | 7/1/2015 | Moderate to severe facial folds and wrinkles or in patients over the age of 21 who have age-related volume loss. |
| RADIESSE | Hydroxylapatite | Bioform Medical, Inc. | P050052/S049 | 6/4/2015 | Subdermal implantation for hand augmentation to correct volume loss in the dorsum of the hands. |
| RESTYLANE SILK | Hyaluronic Acid with Lidocaine | Valeant Pharmaceuticals North America LLC/Medicis | P040024 S072 | 6/13/2014 | Indicated for lip augmentation and dermal implantation for correction of perioral rhytids (wrinkles around the lips) in patients over the age of 21. |
| JUVEDERM VOLUMA XC | Hyaluronic Acid with Lidocaine | Allergan | P110033 | 10/22/2013 | Deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid-face in adults over the age of 21. |
| RESTYLANE-L INJECTABLE GEL | Hyaluronic Acid with Lidocaine | Medicis Aesthetics Holdings, Inc. | P040024 S056 | 8/30/2012 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles/folds (such as nasolabial folds) and for lip augmentation in those over the age of 21 years. |
| BELOTERO BALANCE | Hyaluronic Acid | Merz Pharmaceuticals | P090016 | 11/14/2011 | Injection into facial tissue to smooth wrinkles and folds, especially around the nose and mouth (nasolabial folds). |
| RESTYLANE INJECTABLE GEL | Hyaluronic Acid | Medicis Aesthetics Holdings, Inc | P040024 S051 | 10/11/2011 | Lip augmentation in those over the age of 21 years. |
| JUVEDERM ULTRA XC AND JUVEDERM ULTRA PLUS XC | Hyaluronic Acid with Lidocaine | Allergan | P050047 | 1/7/2010 | The addition of 0.3% Lidocaine into Juvederm Ultra and Juvederm Ultra Plus. |
| SCULPTRA AESTHETIC | Poly-L-Lactic Acid (PLLA) | Sanofi Aventis U.S. | P030050 S002 | 7/28/2009 | Use in shallow to deep nasolabial fold contour deficiencies and other facial wrinkles. |
| EVOLENCE COLLAGEN FILLER | Collagen | Colbar Lifescience l | P070013 | 6/27/2008 | The correction of moderate to deep facial wrinkles and folds (such as nasolabial folds). |
| PREVELLE SILK | Hyaluronic Acid with Lidocaine | Genzyme Biosurgery | P030032 S007 | 2/26/2008 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| RADIESSE 1.3CC AND 0.3CC | Hydroxylapatite | Bioform Medical, Inc | P050037 | 12/22/2006 | Restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with HIV. |
| RADIESSE 1.3CC AND 0.3CC | Hydroxylapatite | Bioform Medical, Inc | P050052 | 12/22/2006 | Subdermal implantation for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| ELEVESS | Hyaluronic Acid with Lidocaine | Anika Therapeutics | P050033 | 12/20/2006 | Use in mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| ARTEFILL | Polymethylmethacrylate Beads, Collagen and Lidocaine. | Suneva Medical, Inc. | P020012 | 10/27/2006 | Use in facial tissue around the mouth (i.e., nasolabial folds). |
| JUVEDERM 24HV, JUVEDERM 30, and JUVEDERM 30HV | Hyaluronic Acid | Allergan | P050047 | 6/2/2006 | Use in mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| RESTYLANE INJECTABLE GEL | Hyaluronic Acid | Medicis Aesthetics Holdings, Inc | P040024 | 3/25/2005 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| CAPTIQUE INJECTABLE GEL | Hyaluronic Acid | Genzyme Biosurgery | P030032 S002 | 11/12/2004 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| SCULPTRA | Poly-Lactic Acid (PLA) | Sanofi Aventis U.S. | P030050 | 8/3/2004 | Restoration and/or correction of the signs of facial fat loss (facial lipoatrophy) in people with Human Immunodeficiency Virus (HIV). |
| HYLAFORM (HYLAN B GEL) | Modified hyaluronic acid derived from a bird (avian) source | Genzyme Biosurgery | P030032 | 4/22/2004 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| RESTYLANE INJECTABLE GEL | Hyaluronic Acid | Q-med Ab | P020023 | 12/12/2003 | Injection into the mid to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). |
| COSMODERM 1 HUMAN-BASED C | Collagen | Inamed Corporation | P800022 S050 | 3/11/2003 | Injection into the superficial papillary dermis for correction of soft tissue contour deficiencies, such as wrinkles and acne scars. |
| FIBREL | Collagen | Serono Laboratories | P850053 | 2/26/1988 | The correction of depressed cutaneous scars which are distendable by manual stretching of the scar borders. |
| ZYPLAST(R) | Collagen | Collagen Corp. | P800022 S011 | 6/24/1985 | Use in mid to deep dermal tissues for correction of contour deficiencies. |
| ZYDERM COLLAGEN IMPLANT | Collagen | Allergan | P800022 | 9/18/1981 | Use in the dermis for correction of contour deficiencies of this soft tissue. |
Hyaluronic Acid
Hyaluronic acid is the most prominent glycosaminoglycan in the skin. Hyaluronic acid potently binds to water and, when injected into the skin, volumizes, softens, and hydrates the skin. In addition to these benefits, it plays a role in cell growth, membrane receptor function, and adhesion.
Hyaluronic acid stabilizes intercellular structures and produces the viscoelastic network for collagen and elastin fibers to bind together. As seen with photoaging, these connections fail, thus resulting in disorganized clumps of collagen and elastin. These benefits make hyaluronic acid an excellent dermal filling agent 18. In February 2003, the FDA approved Restylane, a cross-linked, nonanimal source hyaluronic acid. This dermal filler was quickly found to be relatively long lasting, have minimal adverse effects, was easy to use, was ready to use out of the box, did not require refrigeration, was cost effective, and did not require skin testing prior to treatment.
Because hyaluronic acid is identical in all species, the risk of allergy is remote. Hyaluronic acid has a heparinlike effect, thus resulting in a greater incidence of bruising than is seen with collagen fillers 19. Until 2010, nearly every FDA-approved hyaluronic acid product in the United States did not contain lidocaine, thus significantly increasing the discomfort experienced with injection of these dermal fillers compared with the bovine- and human-derived collagen fillers. Despite these shortcomings, hyaluronic acid fillers still emerged as the leader of dermal filling agents for soft tissue augmentation, owing to their superior cosmetic results.
In the uncommon circumstance when an undesirable outcome occurs with hyaluronic acid, correction is possible with the injection of commercially available hyaluronidase, which breaks down the unwanted hyaluronic acid dermal filler. The use of hyaluronidase for this purpose is not FDA approved and is considered an off-label use. In many cases, 10-30 units of unpreserved hyaluronidase is sufficient to achieve the desired correction. Local site reactions may occur in up to 25% of persons, although they are typically transient and mild. Initial treatment with as little as 5-10 units is commonly recommended and is often effective, although some treat with as much as 75 units with few adverse effects. Additional corrections can be performed, although full correction may take up to 4 weeks to fully appreciate. Some preparations are bovine derived, and skin testing should be considered prior to treatment with these dermal fillers 20.
Hyaluronidase preparations are clear, concentrated liquids that are stored in a refrigerated vial. To reconstitute these dermal fillers, physicians typically add normal saline or lidocaine (with or without epinephrine). When using Amphadase, reconstitution using 3 mL of 1% lidocaine with 1:100,000 epinephrine has been commonly used with great success. After mixing, the vial is gently swirled. Prior to treatment, a skin test can be performed by injecting 3-5 units (0.06-0.1 mL) of the reconstituted solution into the superficial dermis at the antecubital fossa. A positive hypersensitivity reaction consists of a wheal appearing within 5 minutes and lasting 20-30 minutes, accompanied by local itching 21.
Restylane
- Restylane and Restylane-L
Restylane was approved by the FDA in 2003 for the treatment of nasolabial folds. This dermal filler is produced by fermentation in bacterial cultures of equine streptococci. This dermal filler contains approximately 100,000 particles per mL (20 mg/mL). These particles are approximately 300 mm and are highly cross-linked (single cross-linked) using an ether bond, making these dermal fillers one of the stiffest hyaluronic acid fillers. This dermal filler has been used for correction of the nasolabial folds, marionette lines, tear troughs, and glabellar frown lines, in addition to lip enhancement and cheek augmentation. Other clinical uses include correction of the jowls and nasal deformities. In general, most patients can expect 6 months of correction, if not longer 22. In 2010, Restylane-L became available, which contains lidocaine to reduce pain upon injection.
- Restylane Lyft with lidocaine
This dermal filler was originally marketed as Perlane-L, but had a name change in 2015. Restylane Lyft is identical to Restylane except that it consists of larger gel particles. This dermal filler is suitable for the correction of deeper folds, such as the nasolabial folds, and works well for cheek enhancement. Additionally, some experienced physicians prefer this dermal filler over Restylane for lip augmentation. Most patients can expect 6-12 months of correction with Perlane. In 2010, Perlane-L became available, which contains lidocaine to reduce pain upon injection.
- Restylane Silk
This dermal filler was FDA approved in 2014 for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytids in patients older than 21 years. It has the same concentration of hyaluronic acid and a of pH 7.0 as the other Restylane products, but the molecules are smaller. This product is intended to be used for fine perioral lines, although other areas on the face can be treated as well.
- Precautions
Use of products that contain lidocaine (Restylane-L, Restylane Lyft, and Restylane Silk) reduces pain upon injection and is recommended, unless contraindications to the use of lidocaine are present. Erythema and edema are common after treatment and typically last a few days. When injected too superficially, a bluish Tyndall effect can be seen, which represents visible hyaluronic acid seen through the translucent epidermis. Fortunately, these bluish cysts are easily corrected by nicking the skin with a small-gauge needle (30 gauge) or No. 11 blade and expressing the superficial, unwanted dermal filler 23.
Occasionally, palpable nodules can be felt under the skin, which often occurs when the multiple-puncture technique is used or depot injections are performed to place the dermal filler. For this reason, linear threading is now commonly used, which minimizes the risk of nodules. Some prefer using a small (30-gauge) blunt-tipped cannula to inject instead of the 30-gauge needle that comes with the product. As with other fillers, patients should avoid all blood thinners for 10 days prior to treatment 24.
Juvederm (Juvederm Ultra/Juvederm Ultra Plus)
In 2006, the FDA approved Juvederm, which is also a nonanimal stabilized hyaluronic dermal filler. In the United States, three types of Juvederm dermal fillers are FDA approved. Both Juvederm Ultra and Juvederm Ultra Plus contain 24 mg/mL of hyaluronic acid, but Juvederm Ultra Plus has a higher proportion of cross-linking than Juvederm Ultra. Juvederm is a homologous gel with the highest degree of cross-linking of any of the hyaluronic acid fillers and thus has a smooth consistency 25. Juvederm Ultra and Juvederm Ultra Plus have indications similar to those of Restylane and Perlane, respectively, and also do not require refrigeration or skin tests prior to use. In 2010, both Juvederm Ultra and Juvederm Ultra Plus became available with lidocaine, to reduce pain with injection, both labeled as Juvederm (R).
- Precautions
The adverse effects of Juvederm are similar to those seen with Restylane and Perlane 26. Like all of the hyaluronic acid filling agents that are FDA approved, Juvederm does not contain lidocaine. Therefore, in addition to discomfort with injection, one may see erythema, swelling, and bruising. If injected too superficially, a bluish Tyndall effect and nodules may appear 27.
Hylaform/Hylaform Plus
A sterile, nonpyrogenic, viscoelastic, clear gel implant composed of cross-linked molecules of hyaluronic acid derived from an avian (bird) source. Hylaform contains 5.5 mg/mL of medium-sized particles of hylan B. Hylaform Plus contains larger molecules of the same concentration of Hylaform. The indications for Hylaform and Hylaform Plus are similar to Juvederm Ultra and Juvederm Ultra Plus, respectively 24.
- Precautions
These dermal fillers have precautions similar to those of Restylane and Perlane. Additionally, because this dermal filler is derived from an avian (bird) source, persons with a known allergy to avian proteins should not be treated with these dermal fillers. The Hylaform dermal fillers are stored at room temperature and no skin test is required before use.
Captique
This dermal filler is identical to Hylaform except that it is derived from a bacterial source through fermentation. This dermal filler is slightly stiffer than Hylaform 25. Captique is stored at room temperature and no skin test is required before use.
Puragen
This dermal filler contains 20 mg/mL of bacterially derived hyaluronic acid. The particle size is approximately 200 mm, which is slightly smaller than Restylane, and is double–cross-linked with both ether and ester bonds. Ester bond linkages make this dermal filler more stable, protecting the ether bonds, and they are hydrophobic, thus making the product less susceptible to breakdown by hyaluronidase 28. Pilot studies have shown that this dermal filler may be degraded more slowly than Restylane, although larger studies are needed to confirm this. This dermal filler has indications and precautions similar to those of Restylane.
Prevelle Silk
This dermal filler was FDA approved for use in the United States in 2008 and was the first hyaluronic acid dermal filler to contain lidocaine, which reduces pain upon injection. Prevelle Silk is indicated for the treatment of moderate-to-severe facial wrinkles, although it may be better suited for fine lines. Injection into the mid-to-deep dermis is recommended by Mentor, the manufacturer. Similar to other hyaluronic dermal fillers, the most common adverse effects include temporary injection site reactions such as swelling, pain, tenderness, redness, lumps, and bumps.
Polymethylmethacrylate (PMMA) With Bovine Collagen
Artefill (now called Bellafill) was FDA approved for use in the United States in 2007. This dermal filler is composed of nonresorbable polymethylmethacrylate (PMMA) microspheres, which are 30-50 μ m in diameter, suspended in a water-based carrier gel composed of 3.5% bovine collagen, 92.6% buffered isotonic water, 0.3% lidocaine, 2.7% phosphate buffer, and 0.9% sodium chloride 29. Because this dermal filler contains lidocaine, the injection is less painful compared with other dermal fillers that do not contain lidocaine. This dermal filler is indicated for the correction of the nasolabial folds, although it has been used for acne scars and forehead furrows. Using a 26-gauge needle, Bellafill should be injected directly beneath the skin fold into the deep dermis and not into the subcutis. Most practitioners prefer a threading injection technique. Unlike the other dermal fillers, this dermal filler should be considered a permanent dermal filler 30.
- Precautions
Because this product contains bovine collagen, skin testing must be performed prior to treatment, as would be indicated with Zyderm or Zyplast (see Collagen). Because results are permanent, it is often best not to try to achieve full correction in one session, but to accomplish the desired result over several treatment sessions. In addition to allergy, other adverse effects may include lumpiness, persistent swelling or redness, and increased sensitivity at the injection site 31. The product must be refrigerated.
Poly-L-Lactic Acid (PLA)
This novel product (Sculptra) differs from all other agents in several aspects. Poly-L-lactic acid is a synthetic, biodegradable, biocompatible, immunologically inert peptide polymer that is believed to stimulate fibroblasts to produce more collagen, thus increasing facial volume. In the United States, poly-L-lactic acid is only FDA approved for the treatment of HIV-associated lipoatrophy; and it is used for the correction of skin folds (ie, nasolabial folds) and other facial wrinkles in immune-competent persons 32.
Although poly-L-lactic acid is nearly always injected subdermally, dermal neocollagenesis occurs, thus it is a dermal stimulating agent, not a true dermal filling agent. Several limitations have prevented poly-L-lactic acid from becoming as popular as other products. Poly-L-lactic acid must be premixed prior to use, making immediate treatment impossible. Unlike dermal fillers, results are not appreciated for 4 or more weeks. Lastly, most patients require 2-3 treatment sessions that are at least 4-6 weeks apart 33.
- Precautions
Because poly-lactic acid is not a true filler, but relies on neocollagenesis to achieve clinical improvement, the clinical results from this agent are less predictable than the true dermal fillers. Patients should be properly educated that results take 4-6 weeks to be appreciated. Dermal nodules have been reported after treatment and often take 7 months or much longer to develop 34. When treating the face, these nodules can often be felt, but not seen. Reconstitution of the product with 6 or more milliliters of sterile water, in addition to vigorous posttreatment massage, is believed to reduce the incidence of nodule formation.
Use of this product on the hands has increased; however, unfortunately, the incidence of nodules in this location may be as high as 10% and the nodules are often visible, unsightly, and difficult to treat. In this location, treatment with Sculptra reconstituted with as much as 10 mL of sterile water has still resulted in nodule formation, which may take 1-3 years to appear after treatment 35. Additional long-term studies are needed to fully assess the safety of poly-L-lactic acid for the treatment of the hands in an immune-competent individual.
No skin test is required prior to treatment. The product is stored at room temperature, although it must be reconstituted prior to treatment.
Calcium Hydroxylapatite (CaHA)
This novel filler, Radiesse, was FDA approved in December 2006 for the correction of facial wrinkles and folds and for the correction of HIV-associated facial atrophy. In 2009, it received FDA approval for cosmetic use in non-HIV patients as well. The subdermal filler is composed of 30% calcium hydroxylapatite and 70% carrier gel 36. The clinical results may last as long as 12 months or longer, although the carrier gel lasts no longer than 6 months, thus often resulting in a slight decrease in correction by that time.
Radiesse is nearly always injected subdermally at the dermal-subcutaneous junction or just above the periosteum; thus, this product is not a true dermal filler. Studies from 2008 suggest that calcium hydroxylapatite may induce neocollagenesis, although further research is needed 37. Most commonly, Radiesse is used for the correction of nasolabial folds, atrophic cheeks, and temporal wasting.
Because of the pain associated with injection, some practitioners added lidocaine to the syringe, without a clinical appreciable decrease in effect. Mariano Busso 38, had treated numerous patients by adding 1 drop of 10% lidocaine to the previously available 1.3-mL syringe of Radiesse to decrease the discomfort associated with injection. In 2010, Radiesse with lidocaine was released to reduce pain upon injection and no premixing is needed.
Treatment of the hands has been accomplished with the addition of 0.15-0.23 mL of 2% lidocaine per 1.3-mL syringe of Radiesse. This can be accomplished using a nose-to-nose (female-to-female) Luer-lock connector to connect the syringe of Radiesse to a 3-mL syringe containing the lidocaine. At least 10 passes of the product back and forth between the two syringes is recommended to achieve adequate and even distribution of the lidocaine. This makes the consistency of the Radiesse slightly thinner, thus making it easier to spread when using a bolus injection technique. Similarly, this mixture is often preferred for the treatment of the temples.
- Precautions
Injection into the dermis may result in nodule formation and should be avoided. Extreme care must be taken to avoid injection while withdrawing the needle out of the skin, which will result in the deposition of material into the dermis. Treatment of the lips has resulted in cyst formation containing the carrier gel 29. For this reason, most cosmetic surgeons avoid treating the lips with Radiesse.
No skin test is required prior to treatment, and the product is stored at room temperature.
Collagen replacement therapy
Collagen is a natural substance that is found in skin, muscle, tendons and bones and provides structural support. In the dermis (the mid-layer of skin), collagen is made by fibroblast cells. It forms a fibrous network on which new cells can grow. Through the natural processes of aging, collagen in the dermis is gradually lost and contributes to the formation facial lines.
Injectable bovine collagen is made of sterile, purified collagen from cow skin. Human collagen implants are highly purified and isolated from human skin grown in a laboratory. The cells have been grown for the last ten years or so primarily to manufacture living skin-equivalents to treat burns and ulcers.
When injected into the body’s skin both forms of collagen are accepted as if they were the body’s own collagen, forming a network of collagen fibres.
Zyderm® and Zyplast® are popular brands of injectable bovine collagen. Others include Resoplast®. Human collagen brands include Dermalogen®, Cymetra™, CosmoDerm® and CosmoPlast®.
Zyderm® and Cosmoderm® are used for fine lines and Zyplast® and CosmoDerm® are used for deeper furrows.
There are also products derived from porcine collagen, including Evolence™, and Fibrel®. Fibrel is injected with the patient’s own serum. The injections are said to be rather painful and may cause allergic reactions.
A person’s own skin may be used to produce fibroblast cultures (Isolagen®) or a suspension of collagen (Autologen®). It can also be mixed with synthetic PMMA beads (Artecoll®).
How long does the collagen implant last?
Collagen implants are not permanent. Because collagen is a natural protein it slowly breaks down into amino acids that are then absorbed by the body. In most cases, implants last anywhere between one to six months, although in some people one implant may be sufficient for up to two years. Repeat treatments will be necessary to maintain the results.
The longevity of the implant will depend on its location and individual response. Muscular activity such as smiling and frowning will reduce how long it lasts. Conversely, it may last longer than expected in scars because these are not caused by facial muscle movement.
Precautions
People with severe allergies (anaphylaxis) or allergy to injected local anaesthetics should not be treated with bovine, porcine or human collagen.
Since injectable bovine collagen is derived from cows it may cause allergic reactions. Approximately 3% of the population is allergic to bovine collagen; these individuals should not receive these implants. Human collagen is a better choice in the following circumstances:
- Allergies to foods, especially meat products
- Family or personal history of severe allergies (including asthma, hay fever and atopic dermatitis)
- Any previous reaction to a test dose of collagen.
To see if you are eligible for bovine collagen replacement therapy you will require one or two skin tests. No skin test is necessary for human collagen as allergic reactions are very unlikely.
Procedure for collagen skin test
- Test dose of bovine collagen injected just below the skin’s surface on forearm
- Observe the site closely for at least 4 weeks for any of the following signs:
- redness
- swelling
- hardness
- itching
- tenderness
- Most reactions will occur within the first 3 days, but can happen at anytime within this timeframe.
- If it appears that you have a very mild reaction, the doctor may need you to have a second skin test on the other arm to confirm sensitivity.
- Report all reactions to your doctor. After the 4-week observation period (or 8 weeks if second test is required) your doctor will advise whether or not you can proceed with bovine collagen replacement therapy.
Collagen replacement therapy side effects
At the time of treatment most patients report minor discomfort. This is minimized by the addition of lignocaine (lidocaine) to the collagen preparation. This is local anaesthetic to numb the treatmnet area, and is known as ‘lidocaine’ in America.
Immediately after treatment the area may be red, swollen and tender; this usually improves over the following days. Temporary bruising and discolouration may also occur. The collagen is sometimes visible for a while in the form of small white bumps at the treatment site. This generally smoothes out within a few weeks.
Very rarely, if a blood vessel is accidentally blocked by the collagen injection, a small area of skin may die resulting in an ulcer that scabs. This reaction may leave a permanent scar. It has most often been reported in the glabellar area.
Injections through the skin carry a risk of bacterial infection (impetigo). This is more likely if inflammation is present in the treated area such as acne or rosacea spots. Injections in and around the lips may also provoke cold sores in those prone to them.
Allergic reaction to bovine collagen is usually recognized after one or two test doses. In rare cases, allergic reactions may occur during the course of treatment even though there was no reaction to the test dose. They may occur rapidly after treatment or arise weeks to months afterwards. The reaction may clear up in a few days or persist for months. See your doctor immediately if you have an allergic reaction.
Allergic reactions may include:
- Shortness of breath, low blood pressure, and chest pain.
- Urticaria (hives)
- Red, itchy or sore lumps at injection sites
- Scarring when the lumps have resolved
- Shorter lasting effect of collagen injections
People with connective tissue diseases such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis and dermatomyositis may be more likely to experience an allergic reaction to bovine collagen, and the effect of the treatment might not last as long. There have also been reports of connective tissue disease arising for the first time after bovine collagen injections. However a connection between the injections and the connective tissue disease has not been established.
The safety of collagen implants in pregnancy or in children has not been established.
Autologous Cell Therapy
Azficel-T (laViv) is an autologous aesthetic cell therapy indicated to improve the appearance of moderate-to-severe nasolabial fold wrinkles in adults. The product is available from Fibrocell Science, Inc, a company focused on developing personalized cell therapies for aesthetic, medical, and scientific applications.
According to the company, creating azficel-T involves a patented technology whereby fibroblasts are extracted from behind the patient’s ear and sent to the Fibrocell Science laboratory, where they are multiplied for about 3 months and are then frozen until needed. Only physicians who complete a Fibrocell-approved training program will be able to administer it.
This is a biological that works over time that provides gradual and natural results.
The FDA approval was based largely on two identical phase 3 multicenter, randomized, double-blind, placebo-controlled studies involving 421 patients who underwent 3 treatment sessions approximately 5 weeks apart 39.
On the basis of investigators’ and patients’ assessments, a significantly greater proportion of patients demonstrated a positive response to treatment with azficel-T than with placebo. The treatment improved the appearance of nasolabial fold wrinkles for the 6 months of patient follow-up after the last treatment. The company said studies are ongoing, looking at how long beyond 6 months after the last treatment the effect may last.
In clinical trials, the most common adverse events were mild-to-moderate injection-site reactions that usually resolved within 1 week.
Unapproved Dermal Fillers
The FDA is aware that unapproved versions of Juvederm, such as Juvederm Ultra 2, 3, and 4 are being sold and distributed in the U.S., including by online retailers 17. Juvederm is a prescription device that should only be injected and sold by or on the prescription of a licensed health care provider. The FDA is warning health care providers and patients not to use any Juvederm Ultra 2, 3 or 4, because these products are not approved for use in the U.S. As such, the safety and effectiveness of these products cannot be assured. A list of approved products and indications for use in the U.S. can be found in the table below.
The FDA has NOT approved liquid silicone or silicone gel for injection to fill wrinkles or augment tissues anywhere in the body.
Types of lip fillers
Dermal fillers can be temporary, semi-permanent or permanent and can be classed on the basis of biodegradability or their mechanism of action (Table 2). Permanence of fillers refers to a lack of degradation of the in vivo material over time rather than to a permanent cosmetic result. Permanent aesthetic results are seldom possible because of continued tissue volume loss and other factors associated with the ageing face.
The longevity of the effects of dermal filler products vary and are generally based on their composition and level of biodegradability within the tissues. Permanent and semi-permanent dermal fillers were developed as an alternative to biodegradable dermal fillers to increase the durability of the aesthetic effect, with a lower risk, less expense and a faster recovery time 40.
Fillers that are placed superficially can be injected without anaesthesia, whereas deeper implants, such as those used for lip augmentation, usually require nerve blockade 12. Autologous fat transfer requires local or general anaesthesia or deep sedation depending on the volume of fat harvested and the harvesting location. Harvesting is done using cannulae and syringes under negative pressure. Other materials, such as expanded polytetrafluoroethylene (ePTFE), are inserted in the tissues through small incisions under local anaesthesia.
Table 2. Classification of dermal fillers by composition
| Temporary biodegradable (< 1 year) | Semi-permanent biodegradable (1 – 2 years) | Permanent non-biodegradable (> 2 years) |
| Autologous fat transfer | Calcium hydroxylapatite (CaHA) | Collagen implant – porcine |
| Acellular dermis – cadaver | Dextran particles (DEAE) | Silicone oil (polydimethylsiloxane oil) (LIS) |
| Collagen – human, bovine, porcine | Hydroxyethylmethacrylate (HEMA) | Polyacrylamide gel (PAAG) |
| Cultured fibroblasts – human | Polylactic acid (PLA) | Polymethylmethacrylate (PMMA) |
| Fascia autograft – human | Polyvinyl alcohol (PVA) | Expanded polytetrafluoroethylene (ePTFE) |
| Hyaluronic acid (HA) – avian, bacterial | Polytetrafluoroethylene (PTFE) |
Table 3. Summary and taxonomy of the semi-permanent and permanent dermal fillers available in the US.
| Type of Filler | Product Name | Mode of Action | Longevity |
SUSPENSION OF INSOLUBLE POLYMER FRAGMENTS OR MICROSPHERES AND A RESORBABLE LIQUID | |||
| SILICONE PARTICLES 100 – 600 μm irregular shaped, solid silicone particles suspended in a polyvinylpyrrolidone (PVP) carrier | Bioplastique™ Bioplasty Inc, St Paul, MN, USA | Injected into subcutaneous tissue (below dermis). Collagen encapsulates and localises the particles. Particles remain in tissue and produce a local foreign body host response, ending in fibrosis, which contributes to the filling effect. Not for fine wrinkles. No allergy test needed. | Permanent |
| POLYMETHYLMETHACRYLATE (PMMA) MICROSPHERES 30-42 μm smooth particles of PMMA suspended in bovine collagen | Arte-Fill® Artes Medical, Inc, San Diego, CA, USA. Superceeded products: Artecoll & Arteplast | Injected into sub-dermis (at junction of dermis and subcutaneous fat). Collagen encapsulates and localises the particles. Collagen degraded in 1-3 months and replaced by body’s own connective tissue. Particles remain in tissue and produce a local foreign body host response. Final result takes up to 1 – 2 years. Not for fine wrinkles. Allergy test recommended. | Permanent |
| POLYVINYLHYDROXIDE (PVOH) PARTICLES IN POLYACRYLAMIDE GEL (PAAG) 5 – 80 μm porous PVOH microspheres suspended in PAAG | Evolution™ ProCytech Labs, Bordeaux, France | Gel is phagocytosed. Microparticles surrounded by fibrous capsule. After 9 months implant is totally infiltrated by macrophages, fibroblasts and giant cells. No allergy test needed. | Permanent |
| HYDROXYETHYLMETHACRYLATE (HEMA) AND ETHYLMETHACRYLATE (EMA) PARTICLES IN HYALURONIC ACID (HA) GEL Smooth, non-spherical acrylic hydrogel (HA) particles (copolymer of hydroxyethylmethacrylate (HEMA) and ethylmethacrylate (EMA)) and crosslinked hyaluronic acid | DermaLive® 45 – 65 μm DermaDeep® 80 – 110 μm Dermatech, Paris, France | DermaLive injected into deeper layers of dermis (junction of dermis and hypodermis). DermaDeep injected into subperiosteal layer or hypodermis. Hyaluronic acid is completely broken down over months. Particles produce a local foreign body host response. No skin test required. | Semi-permanent |
SUSPENSION OF SLOWY DEGRADABLE POLYMER MICROSPHERES AND A RESORBABLE LIQUID | |||
| POLYLACTIC ACID (PLA) MICROSPHERES 1 – 50 μm PLA microspheres in mannitol/ carbomethoxycellulose gel | Sculptra® Dermik Laboratories, Berwyn, PA (Also known as New-Fill™) | Injected into deep dermis or subcutaneous space. Local foreign body host response. Polylactic acid is completely broken down within months. New collagen growth can last for up to 2 years or longer. No skin test necessary. | Semi-permanent |
| CALCIUM HYDROXYLAPATITE (CaHA) PARTICLES 25 – 45 μm calcium hydroxylapatite (CaHA) microspheres suspended in cellulose gel. | Radiesse® BioForm Medical, Inc. Franksville, Wisconsin (formerly Radiance FN) | Injected subcutaneously (sub-dermis). Product incorporated by tissues and then replaced by collagen. CaHA broken down into calcium and phosphate. Good for deeper folds. No skin test necessary. | Semi-permanent |
| DEXTRAN PARTICLES IN HA | |||
| 40 – 60 μm dextran micro beads suspended HA gel | Reviderm® Intra Rofil Medical International, Breda, The Netherlands | After injection, there is in an initial macrophage response followed by fibroblast proliferation and new collagen formation. | Semi-permanent |
| 80-120 μm micro particles with a positively charged surface suspended in cross linked hyaluronic acid | Matridex® BioPolymer GmbH & Co. KG | Stimulates new collagen production. | Semi-permanent |
| Biodegradable cross-linked HA gel with positively charged dextranomer beads | ReDexis® (formerly HylaDex) Prollenium Medical Technologies Inc, ON, Canada | Positively charged dextranomer beads attract body’s own collagen and elastin to injection site. Results in filling through cross-linked HA and collagen regeneration. HA broken down, but microspheres remain permanently. | Semi-permanent |
HOMOGENOUSLY BUILT POLYMER GEL (SILICONE GEL , POLYACRYLAMIDE HYDROGEL – NO PARTICLES/SPHERES) | |||
| LIQUID SILICONE Injectable liquid silicone -poly dimethylsiloxane | Silikon™ 1000, Alcon Laboratories Inc, Fort Worth, TX, USA PMS 350®, Vikomed, Meinerzhagen,Germany VitreSil® 1000 & SilSkin® 1000, RJ Development Corp. Peabody, MA, USA Adatosil® 5000, Bausch & Lomb Inc | Injected into sub-dermis. Each micro-droplet induces its own fibroblastic response resulting in augmentation by forming a fibrous capsule around each silicone particle. No skin test needed. | Permanent |
| POLYACRYLAMIDE GEL (PAAG) Polyacrylamide gel: 95% sterile water and 5% hydrophilic, cross-linked polyacrylamide polymer | Aquamid® Contura, Soeberg, Denmark (aka Formacryl (Russia), Interfall (Ukraine), Royamid (Sweden), BioFormacryl, Argiform, Kosmogel) Phigel® Outline™, Original (2 years), Ultra (5 years) & Fine Line (1 year), ProCytech Labs, Bordeaux, France Beautical 2® (2 years) & Beautical 5® (5 years), Rofil Medical International, Breda, The Netherlands Eutrophill®, Mediform | Aquamid injected subcutaneously. Permanent filler where the volume of the implant is given by water not from a solid product. Body accepts the gel readily and forms a thin membrane around the implant which helps to keep it in place; as the gel is very elastic it moves with all facial expressions. Aquamid marketed under different names in different countries. Similar products available with different polyacrylamide/water ratio. | Permanent |
| Polyalkylimide gel (based on polyacrylamide gel): 96% sterile water and 4% hydrophilic, cross-linked polymer (poly-Alkyl-Imide) | Bio-Alcamid® Polymekon, Biotech Industrie, Milan, Italy | Injected into subcutaneous tissue. Product becomes covered by thin collagen capsule which completely surrounds the gel, isolating it from the host tissues. Does not become integrated into surrounding tissues and can be removed. Volume of the implant given by water not from a solid product. | Permanent |
| Based on polyacrylamide gel: 92% sterile water and 8% polyvinyl alcohol | Bioinblue® Polymekon, Biotech Industrie, Milan, Italy | As for polyacrylamide gel | Semi-permanent |
Temporary fillers
Most biological materials such as collagen, hyaluronic acid (HA) gels and humanderived products last temporarily, persisting generally for less than 12 months before being resorbed into the tissues 41. To maintain the visible filling effect produced by these materials, patients must receive regular reapplications. Temporary fillers can be appealing to patients who want to experiment with soft tissue augmentation, but do not necessarily want to commit to their new look.
Semi-permanent fillers
To achieve a longer visible filling effect, slowly resorbable substances such as polylactic acid (PLA), calcium hydroxylapatite (CaHA), hydroxyethylmethacrylate
(HEMA) and dextran (DEAE) have been used 42. These substances slowly biodegrade and typically persist for one to two years in the tissues. In some cases, multiple injections may initially be required to get the desired effect. Over time, this may need to be repeated because the visible effect deteriorates as the filler breaks down.
Permanent fillers
To address the issue of materials being resorbed over time, non-biodegradable materials have emerged as dermal filler materials. Liquid injectable silicone (LIS),
certain polyacrylamide gels (PAAG), polymethylmethacrylate (PMMA) and polytetrafluoroethylene (PTFE) are non-resorbable and remain in the tissues permanently 41. Used correctly, permanent fillers offer certain advantages over non-permanent ones, particularly as they do not require touch-up sessions once the expected result has been achieved 43. However, permanent dermal fillers are much more technique sensitive than temporary fillers.
How do lip injections work
Dermal fillers can be classed by their mechanism of action as either volumisers (fillers) or stimulators (sculptors) 44. When injected into the skin, volumizers increase facial volume and fill out the skin directly. Volumizers are normally of minimal viscosity and are injected into the upper layers of the dermis to fill superficial lines 41. They do not initiate a chronic immune response and hence cause little foreign body reaction in the tissues. Volumizers include silicone, collagen, certain polyacrylamide gels (PAAGs) and hyaluronic acid (HA).
Stimulators can also directly create volume, but primarily, they stimulate the tissues to create a foreign body reaction over a limited time. This stimulates long-term or permanent collagen deposition. Stimulators include polylactic acid (PLA), dextran particles (DEAE), calcium hydroxylapatite (CaHA), hydroxyethylmethacrylate (HEMA) and polymethylmethacrylate (PMMA). Fillers such as polylactic acid (PLA) and dextran particles (DEAE) cause a foreign body reaction for a limited time before they are absorbed, whereas polymethylmethacrylate (PMMA) is non-biodegradable and stimulates collagen deposition indefinitely 41. Stimulators are intended for deeper lines and furrows, and are generally injected into the deep dermis or upper subcutaneous tissue 44. Some fillers contain both volumizing and stimulating components and fall into both categories.
The majority of stimulators consist of microparticles suspended in a carrier gel. The carrier gel provides some distance between the particles and holds them in place so they do not move during the initial phases of wound healing. According to their chemical composition, size, shape, surface structure and surface charge, different microparticles demonstrate different biocompatibility 45. According to the chemical composition and surface charge of the microparticle, the tissue reacts either with protein attachment and consequent encapsulation with fibrous tissue, or with an attempt to phagocytose the particle 42. Microparticle size determines whether it is phagocytosable, and hence biodegradable. Small particles (< 60 μm) are phagocytosed, whereas larger particles remain in the tissue 46. Macrophages containing phagocytosed particles may migrate toward distant organs such as the spleen, the lymph nodes or liver 42.
Smooth-walled microparticles become enveloped by a fibrous capsule that holds them in place. A monolayer of macrophages then surrounds the surface of the microparticle. Fibroplasia is initiated and results in fibroblast adhesion and collagen synthesis, resulting in augmentation 47. There is no chronic inflammation, and the microparticle becomes embedded in the ‘new’ collagen.
Microparticles with rough surfaces and irregular shape encourage tissue ingrowth and create a foreign body reaction 48. Activated macrophages and tissue monocytes release cytokines, such as angiogenic growth factors, to induce the invasion of capillaries into the granulation tissue. Collagen is then synthesized, which forms a scaffold for cellular reconstruction and remodelling in this tissue.
Lip augmentation surgery patient selection and outcomes
Careful patient selection, history, and a detailed consultation outlining the benefits, limitations, and adverse events of lip reshaping go a long way toward providing the desired results. Discussing the immediate aftermath of a lip augmentation, that is, swelling and bruising, is a key component to counselling as often patients tend to be secretive about these procedures and do not wish to disclose any treatment taken. The existence of downtime, varying from 2 days to 2 weeks, needs to be emphasized; the recent use of cannulae instead of needles has helped to reduce it. What the patient may desire may not be realistically possible and it is crucial to align them on what to expect (Figure 6).
Patient alignment checklist
- Synchronization of patient and physician expectations
- Information on immediate and late postprocedure outcomes
- Number of syringes and cost
- Longevity of product used
- Repetitive and temporary nature of treatment
- Adverse events
- Postprocedure care
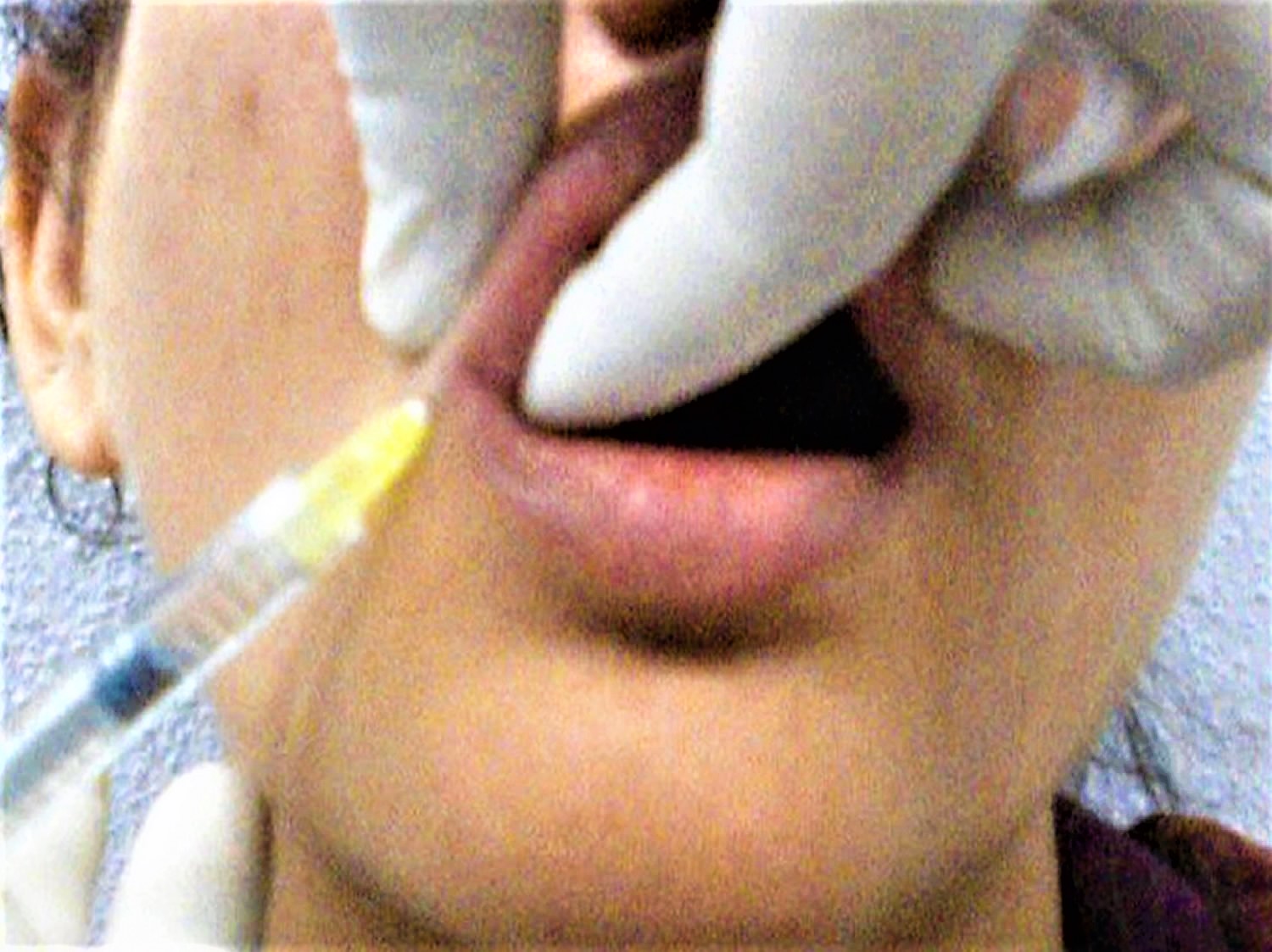
Figure 6. Swelling of the lips immediately after HA injection with needles
Lip augmentation treatment techniques
Anesthesia is first administered with either a eutectic mixture of lidocaine and prilocaine or regional blocks (infraorbital for the upper lip and mental for the lower lip). It is critical not to distort the shape of the lips. Using premixed hyaluronic acid (HA) with lidocaine as an add-on reduces the pain of the injection. Some patients may experience anxiety over the amount of swelling and bruising, and may require ice soaks, nonsteroidal anti-inflammatory drugs (NSAIDs; not in the first 6 hours as that might mask any signs of vascular compromise), and even prednisolone 11.
Medium-depth fillers such as Restylane®, Juvéderm Ultra®, and Esthélis Basic® are preferred, using either a 30-G needle or a 27-G cannula.
Expected postprocedure outcomes of lip augmentation include edema, bruising, and ecchymosis. Complications are extremely rare and include nodules and lumps, which can be massaged in or dissolved with hyaluronidase injections. Intravascular injections may result in immediate blanching, but the collateral circulation of the lips is highly forgiving. Warm compresses, use of hyaluronidase, and topical nitroglycerin help. Herpetic reactivation can be prevented with oral antivirals (acyclovir, famciclovir or valaciclovir).
Overcorrection is not indicated. Simultaneous vermilion and vermilion border augmentations result in a complete effect.
The techniques for injection of HA for lip augmentation have included serial puncture and linear threading, which may be antegrade or retrograde. The choice of one technique over another or a combination of techniques may be influenced by aesthetic goals and patient factors.
The use of cannulae has cut down the downtime involved in this procedure and increased the percentage of patients returning for repeat augmentations [Figure 7]. Only one point each on either side at the oral commissure is utilized to reach both the upper and the lower lip. This technique needs getting accustomed to and is difficult for a beginner. The author advises using needles initially but ultimately moving to a cannula–it pays off in the long run with better results and less downtime [Figure 8] 10.
Figure 7. Lip augmentation with a cannula
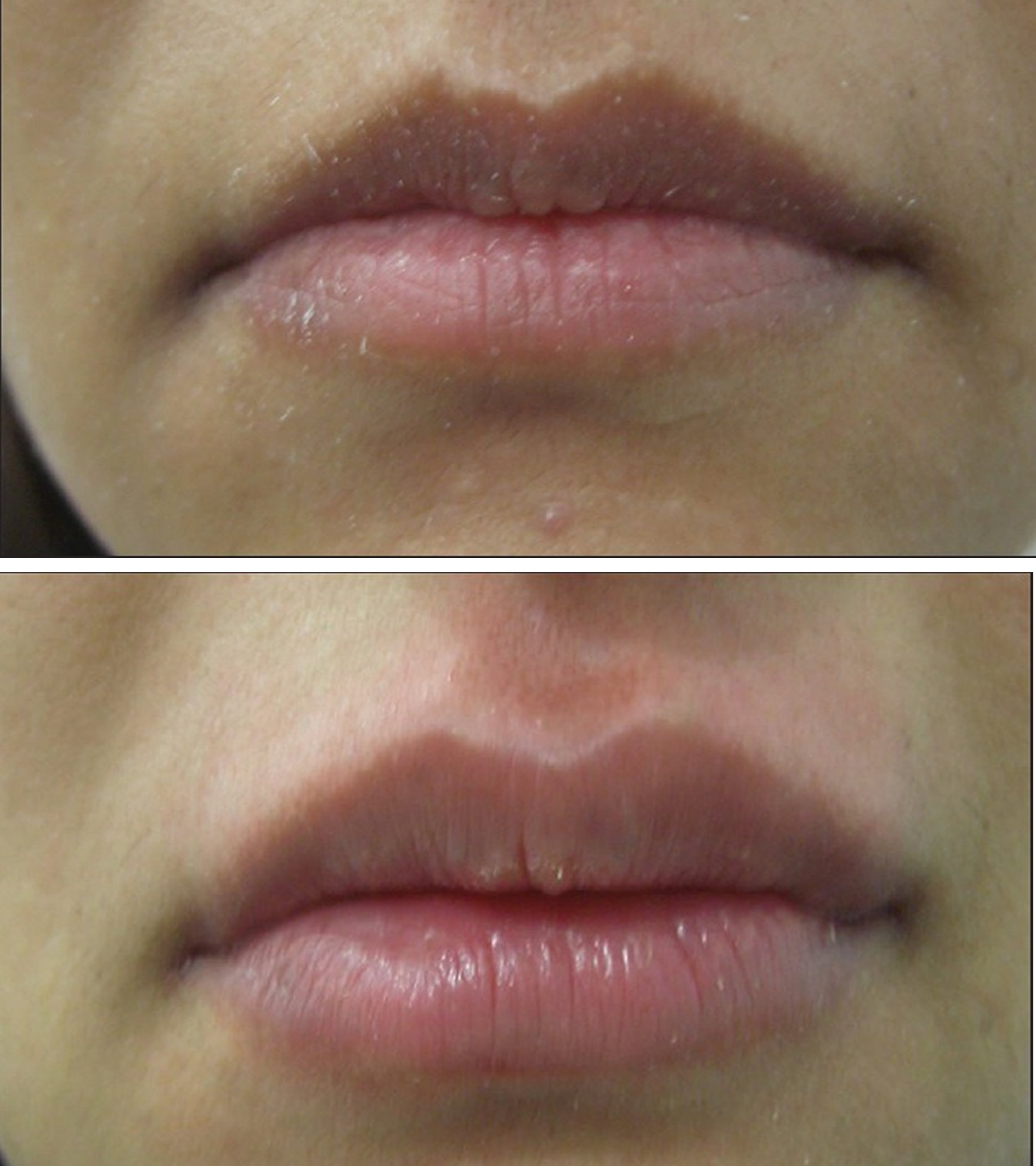
Figure 8. Lip augmentation before and after – shaping of upper and lower lips with 1 mL of HA
- Lupo PM. Hyaluronic acid fillers in facial rejuvenation. Semin Cutan Med Surg. 2006;25:122–6. https://www.ncbi.nlm.nih.gov/pubmed/17055390[↩]
- Luthra A, Kumar V. Fillers and autologus fat. In: Khunger M, editor. Step by Step Treatment of Acne Scars. India: Jaypee Brothers; 2014. pp. 153–71.[↩]
- Filling in Wrinkles Safely. https://www.fda.gov/forconsumers/consumerupdates/ucm049349.htm[↩][↩][↩][↩]
- Doheny K. Dermal Fillers: The Risks to Eliminating Wrinkles. Medscape News & Perspectives. https://www.medscape.com/viewarticle/894549#vp_1[↩]
- Lowe NJ. Temporary dermal fillers — European experiences. In: Lowe NJ, editor. Textbook of Facial Rejuvenation. London: Martin Dunitz/Taylor and Francis; 2002. pp. 177–88.[↩]
- Rudolph CM, Soyer HP, Sculler-Petrovic S, Kerl H. Foreign body granulomas due to injectable aesthetic microimplants. Am J Surg Pathol. 1999;23:113–7. https://www.ncbi.nlm.nih.gov/pubmed/9888711[↩]
- Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with Artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003;29:573–87. https://www.ncbi.nlm.nih.gov/pubmed/12786699[↩]
- Chisholm BB. Facial implants: Facial augmentation and volume restoration. Oral & Maxillofacial Surgery Clinics of North America 2005; 17(1): 77-84.[↩][↩][↩]
- Mandeville JT and Rubin PA. Injectable Agents for Facial Rejuvenation: Botulinum Toxin and Dermal Filling Agents. International Ophthalmology Clinics 2004; 44(1):189-212.[↩]
- Luthra A. Shaping Lips with Fillers. Journal of Cutaneous and Aesthetic Surgery. 2015;8(3):139-142. doi:10.4103/0974-2077.167269. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4645142/[↩][↩][↩]
- Carruthers J, Narurkar VA. Management of the lips and mouth corners. In: Carruthers J, Carruthers A, editors. Soft Tissue Augmentation. Philadelphia: Saunders; 2006. pp. 109–19.[↩][↩]
- Narins RS & Bowman PH. Injectable skin fillers. Clinics in Plastic Surgery 2005; 32(2):151-162.[↩][↩]
- de Maio M. The minimal approach: an innovation in facial cosmetic procedures. Aesthetic Plastic Surgery 2004; 28(5): 295–300.[↩][↩]
- Dermal Fillers. https://emedicine.medscape.com/article/1125066-overview[↩]
- Dermal fillers. https://emedicine.medscape.com/article/1125066-overview[↩]
- Godin MS, Majmundar MV, Chrzanowski DS, Dodson KM. Use of Radiesse in combination with Restylane for facial augmentation. Archives of Facial Plastic Surgery 2006; 8(2): 92–97.[↩]
- Dermal Fillers Approved by the Center for Devices and Radiological Health. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm[↩][↩]
- Sundaram H, Cassuto D. Biophysical Characteristics of Hyaluronic Acid Soft-Tissue Fillers and Their Relevance to Aesthetic Applications. Plast Reconstr Surg. 2013 Oct. 132(4S-2 Clinical Introduction to the Hyaluronic Acid Dermal Filler Using Cohesive Polydensified Matrix Technology):5S-21S.[↩]
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004 Feb. 20(1):21-9.[↩]
- Cassuto D, Sundaram H. A Problem-Oriented Approach to Nodular Complications from Hyaluronic Acid and Calcium Hydroxylapatite Fillers: Classification and Recommendations for Treatment. Plast Reconstr Surg. 2013 Oct. 132(4S-2 Clinical Introduction to the Hyaluronic Acid Dermal Filler Using Cohesive Polydensified Matrix Technology):48S-58S.[↩]
- Lambros V. The use of hyaluronidase to reverse the effects of hyaluronic acid filler. Plast Reconstr Surg. 2004 Jul. 114(1):277[↩]
- Dover JS, Carruthers A, Carruthers J, Alam M. Clinical Use of RESTYLANE. Skin Therapy Lett. 2005 Feb. 10(1):5-7.[↩]
- Hirsch RJ, Narurkar V, Carruthers J. Management of injected hyaluronic acid induced Tyndall effects. Lasers Surg Med. 2006 Mar. 38(3):202-4.[↩]
- Rao J, Chi GC, Goldman MP. Clinical comparison between two hyaluronic acid-derived fillers in the treatment of nasolabial folds: hylaform versus restylane. Dermatol Surg. 2005 Nov. 31(11 Pt 2):1587-90.[↩][↩]
- Monheit GD, Prather CL. Juvéderm: a hyaluronic acid dermal filler. J Drugs Dermatol. 2007 Nov. 6(11):1091-5.[↩][↩]
- Flynn TC, Thompson DH, Hyun SH. Molecular Weight Analyses and Enzymatic Degradation Profiles of the Soft-Tissue Fillers Belotero Balance, Restylane, and Juvéderm Ultra. Plast Reconstr Surg. 2013 Oct. 132(4S-2 Clinical Introduction to the Hyaluronic Acid Dermal Filler Using Cohesive Polydensified Matrix Technology):22S-32S.[↩]
- Ballin AC, Cazzaniga A, Brandt FS. Long-term efficacy, safety and durability of Juvéderm® XC. Clin Cosmet Investig Dermatol. 2013. 6:183-9.[↩]
- Kono T, Kinney BM, Groff WF, Chan HH, Ercocen AR, Nozaki M. Randomized, evaluator-blind, split-face comparison study of single cross-linked versus double cross-linked hyaluronic acid in the treatment of glabellar lines. Dermatol Surg. 2008 Jun. 34 Suppl 1:S25-30.[↩]
- Alam M, Gladstone H, Kramer EM, Murphy JP Jr, Nouri K, Neuhaus IM, et al. ASDS guidelines of care: injectable fillers. Dermatol Surg. 2008 Jun. 34 Suppl 1:S115-48.[↩][↩]
- ArteFill – Instructions for Use [package insert]. San Diego, Calif: Artes Medical, Inc. 2008.[↩]
- Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003 Jun. 29(6):573-87; discussion 587.[↩]
- Butterwick K. Understanding injectable poly-L-lactic acid. Cosmet Dermatol. 2007. 20:388-92.[↩]
- Vleggaar D. Soft-tissue augmentation and the role of poly-L-lactic acid. Plast Reconstr Surg. 2006 Sep. 118(3 Suppl):46S-54S.[↩]
- Narins RS, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg. 2008 Jun. 34 Suppl 1:S31-9.[↩]
- Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008 Jun. 34 Suppl 1:S92-9.[↩]
- Coleman KM, Voigts R, DeVore DP, Termin P, Coleman WP 3rd. Neocollagenesis after injection of calcium hydroxylapatite composition in a canine model. Dermatol Surg. 2008 Jun. 34 Suppl 1:S53-5.[↩]
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008 Jun. 34 Suppl 1:S64-7.[↩]
- Busso M, Voigts R. An investigation of changes in physical properties of injectable calcium hydroxylapatite in a carrier gel when mixed with lidocaine and with lidocaine/epinephrine. Dermatol Surg. 2008 Jun. 34 Suppl 1:S16-23; discussion S24.[↩]
- azficel-T (laViv) [package insert]. Fibrocell Science, Inc.[↩]
- Dermal Fillers https://emedicine.medscape.com/article/1125066-overview[↩]
- Carruthers J. Review of long-lasting dermal fillers. Aesthetic Buyers Guide Supplement 2006; 2(1): 1-28.[↩][↩][↩][↩]
- Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatologic Surgery 2002; 28(6): 484-490.[↩][↩][↩]
- Haneke E. Polymethyl methacrylate microspheres in collagen. Seminars in Cutaneous Medicine & Surgery 2004; 23(4): 227-232.[↩]
- Thioly-Bensoussan D. A new option for volumetric restoration: Poly-l-lactic acid. Journal of the European Academy of Dermatology & Venereology 2006; 20(Suppl 1):12-16.[↩][↩]
- Marler JJ, Guha A, Rowley T, Koka R, Mooney D, Vacanti JP. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plastic & Reconstructive Surgery 2000; 105(6): 2059-2058.[↩]
- Ersek RA & Beisang AA, III. Bioplastique: a new textured copolymer microparticle promises permanence in soft tissue augmentation. Plastic & Reconstructive Surgery 1991; 87(4): 693-702.[↩]
- Laeschke K. Biocompatibility of microparticles into soft tissue fillers. Seminars in Cutaneous Medicine & Surgery 2004; 23(4): 214-217.[↩]
- Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plastic & Reconstructive Surgery 2004; 113(5): 1380-1390.[↩]