Mesenteric artery anatomy
The mesentery is a translucent sheet that suspends the intestines and other abdominal viscera from the posterior body wall. The mesentery contains numerous arteries, veins, and lymphatic vessels that supply and drain the intestines. The arterial supply arises from the superior and inferior mesenteric arteries; numerous anastomoses between these ensure adequate collateral circulation to the intestines even if one route is temporarily obstructed.
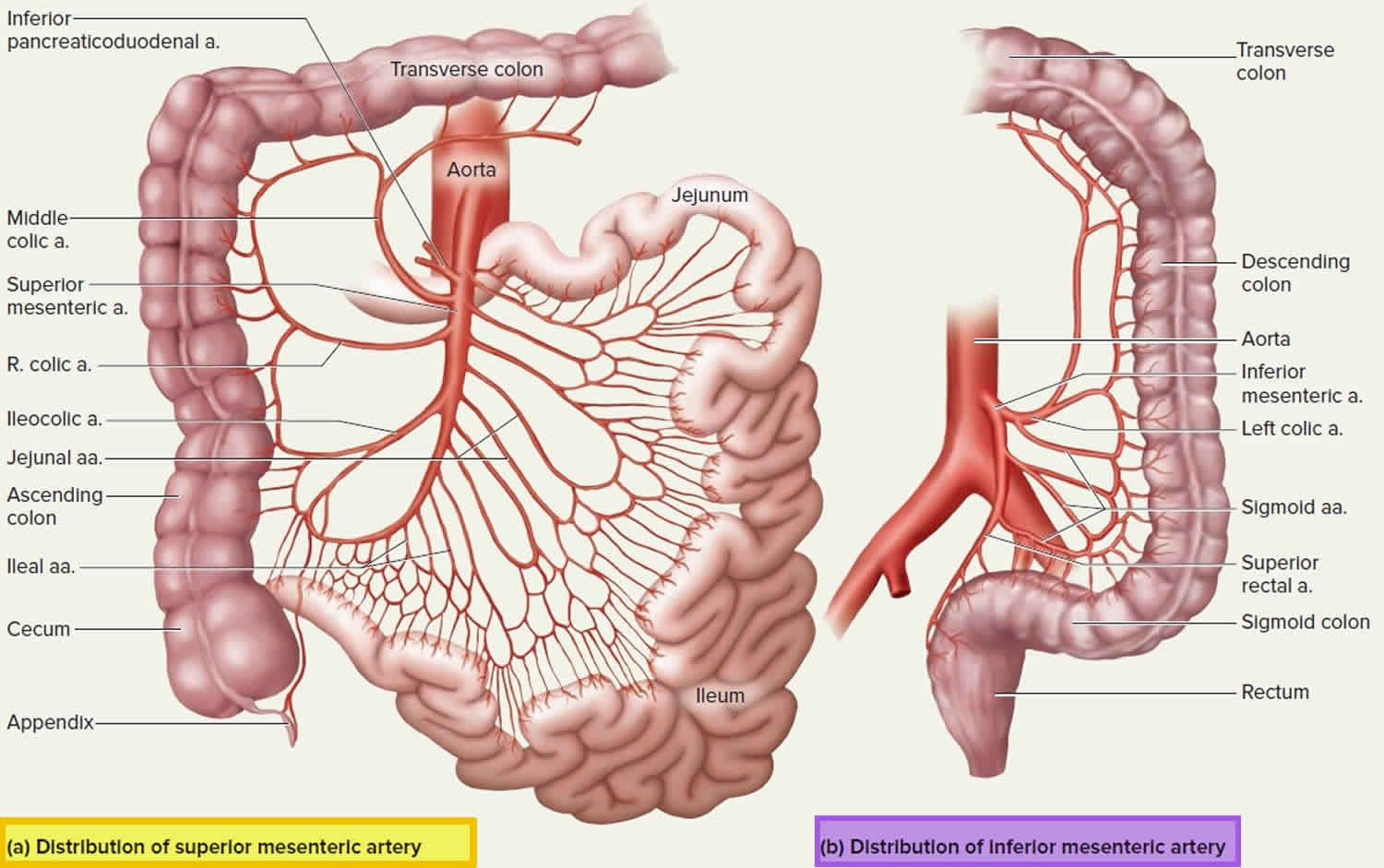
Figure 1. Mesenteric artery
Superior mesenteric artery
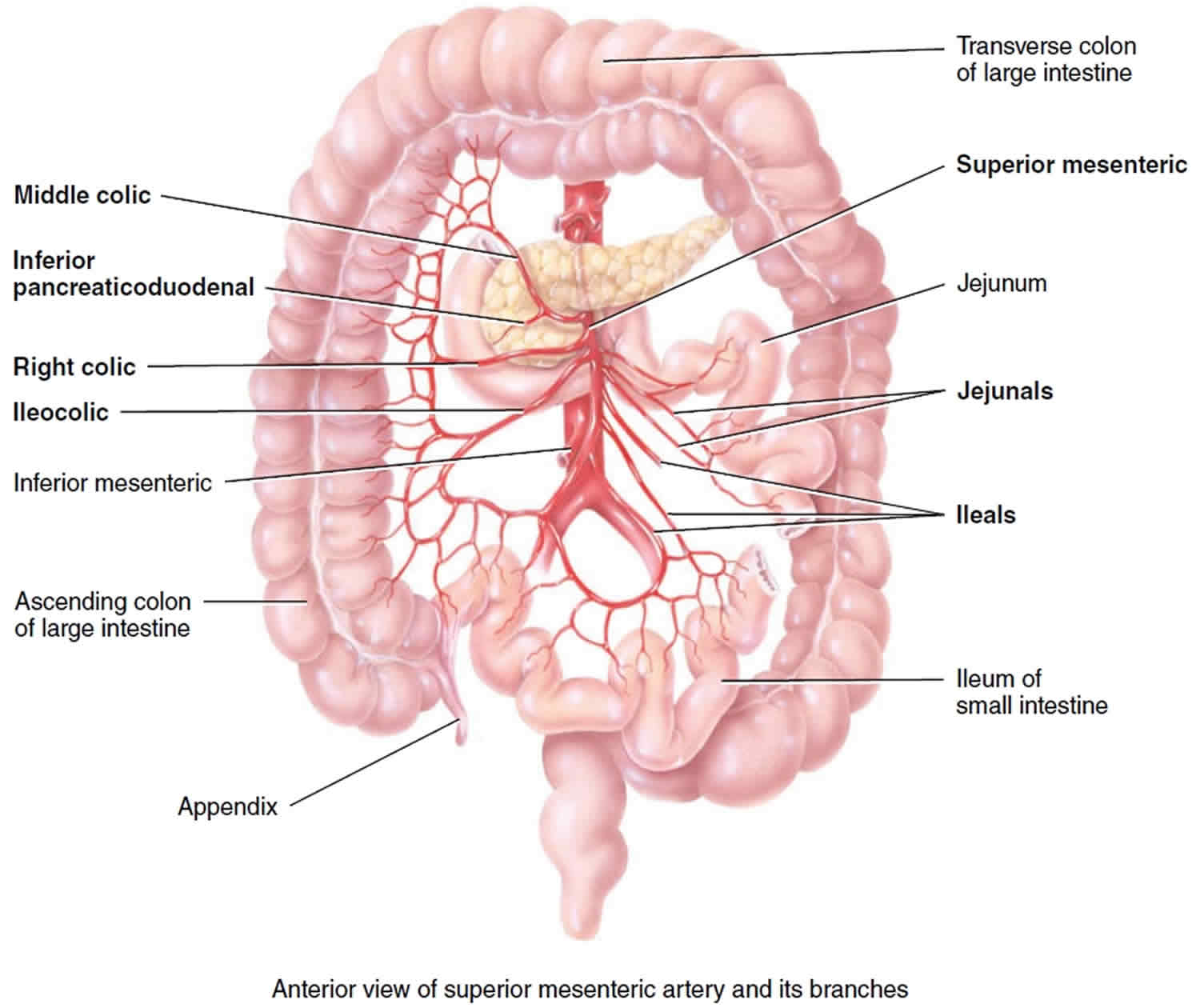
The superior mesenteric artery (Figure 1 a) is the most significant intestinal blood supply, serving nearly all of the small intestine, cecum, ascending and transverse colons, and pancreas.
The superior mesenteric artery arises from anterior surface of abdominal aorta about 1 cm inferior to celiac trunk at level of first lumbar vertebra and extends inferiorly and anteriorly between layers of mesentery (portion of peritoneum that attaches small intestine to posterior abdominal wall).
The superior mesenteric artery anastomoses extensively and gives off the following five branches.
Branches of superior mesenteric artery
- Inferior pancreaticoduodenal artery passes superiorly and to right toward head of pancreas and duodenum. The inferior pancreaticoduodenal artery branches to pass around the anterior and posterior sides of the pancreas and anastomose with the two branches of the superior pancreaticoduodenal artery. The inferior pancreaticoduodenal artery supplies blood to the pancreas and duodenum.
- Twelve to 15 jejunal and ileal arteries form a fanlike array that supplies nearly all of the small intestine (portions called the jejunum and ileum).
- Ileocolic artery supplies the ileum, appendix, and parts of the large intestine (cecum and ascending colon).
- Right colic artery also supplies the ascending colon.
- Middle colic artery supplies most of the transverse colon.
Figure 2. Superior mesenteric artery and its branches
Inferior mesenteric artery
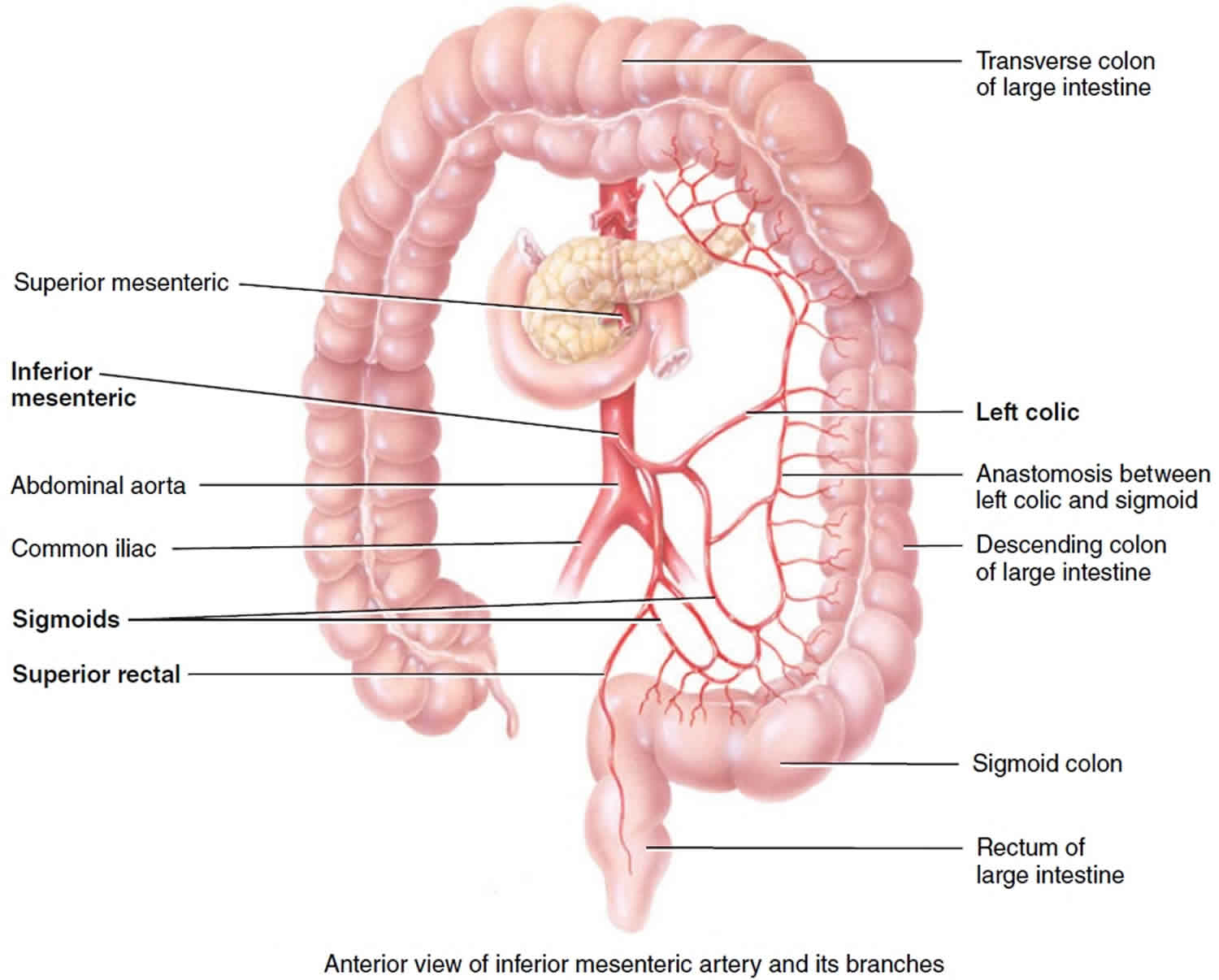
The inferior mesenteric artery arises from anterior aspect of the lower abdominal aorta at level of third lumbar vertebra and and then passes inferiorly to left of aorta. The inferior mesenteric artery anastomoses extensively and has three branches. The inferior mesenteric artery supplies blood to the distal part of the large intestine – the transverse, descending and sigmoid colons and rectum.
Branches of inferior mesenteric artery
- Left colic artery supplies the transverse and descending colon.
- Sigmoid arteries supply the descending and sigmoid colon.
- Superior rectal artery supplies the rectum.
Figure 3. Inferior mesenteric artery and its branches
Superior mesenteric artery syndrome
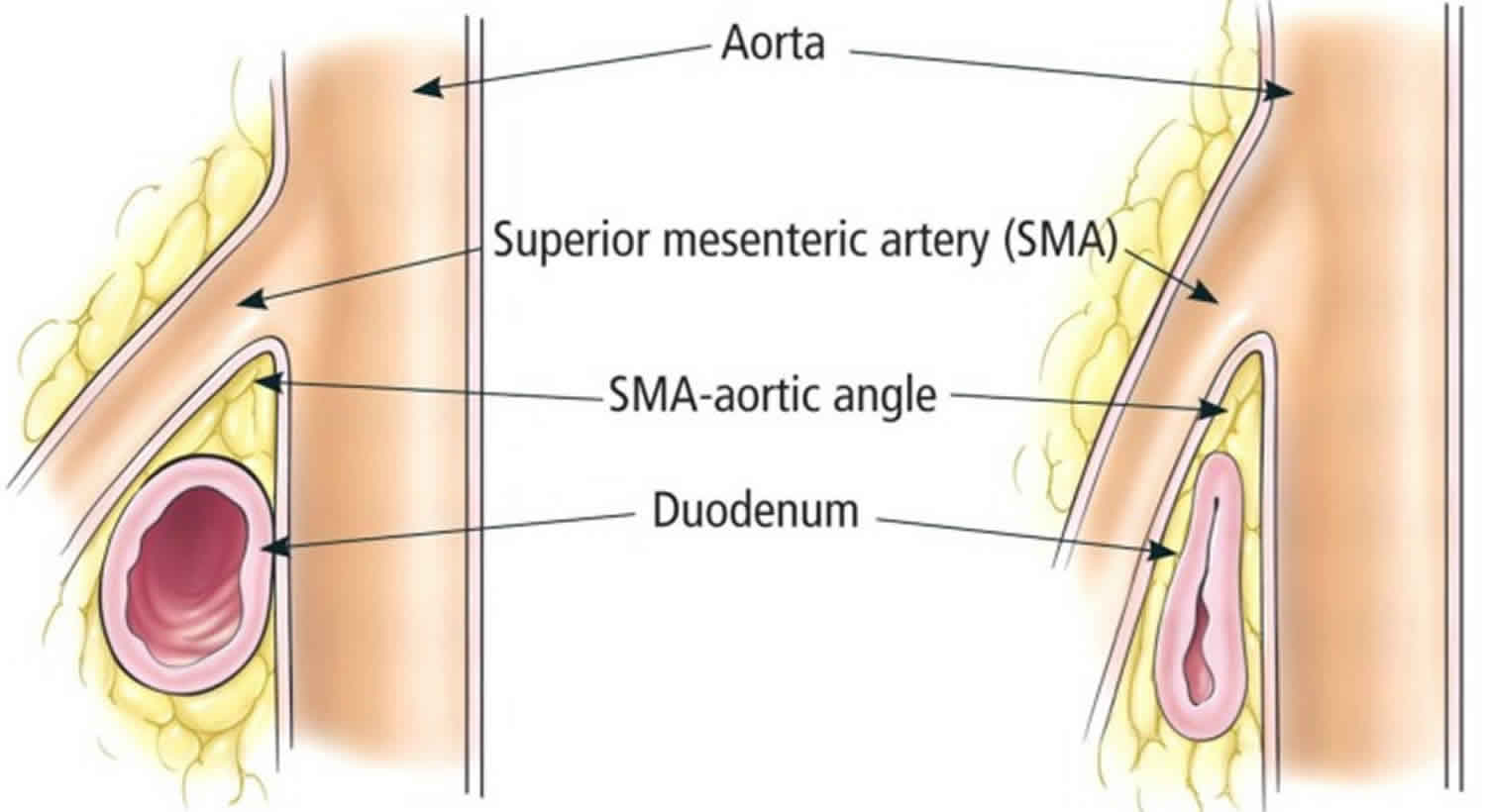
Superior mesenteric artery syndrome, also known as Wilkie’s syndrome 1, cast syndrome or aortomesenteric syndrome, is a digestive condition that occurs when the duodenum (the first part of the small intestine) is compressed between two arteries (the aorta and the superior mesenteric artery). This compression causes partial or complete blockage of the duodenum. Superior mesenteric artery syndrome is described as the loss of the intervening mesenteric fat pad (fatty tissue that surrounds the superior mesenteric artery) between the aorta and superior mesenteric artery, leading to narrowing of the angle between the two vessels, which in turn causes compression of the third portion of the duodenum 2. Superior mesenteric artery syndrome is a digestive condition that occurs when the duodenum (the first part of the small intestine) is compressed between two arteries (the aorta and the superior mesenteric artery). This compression causes partial or complete blockage of the duodenum 3.
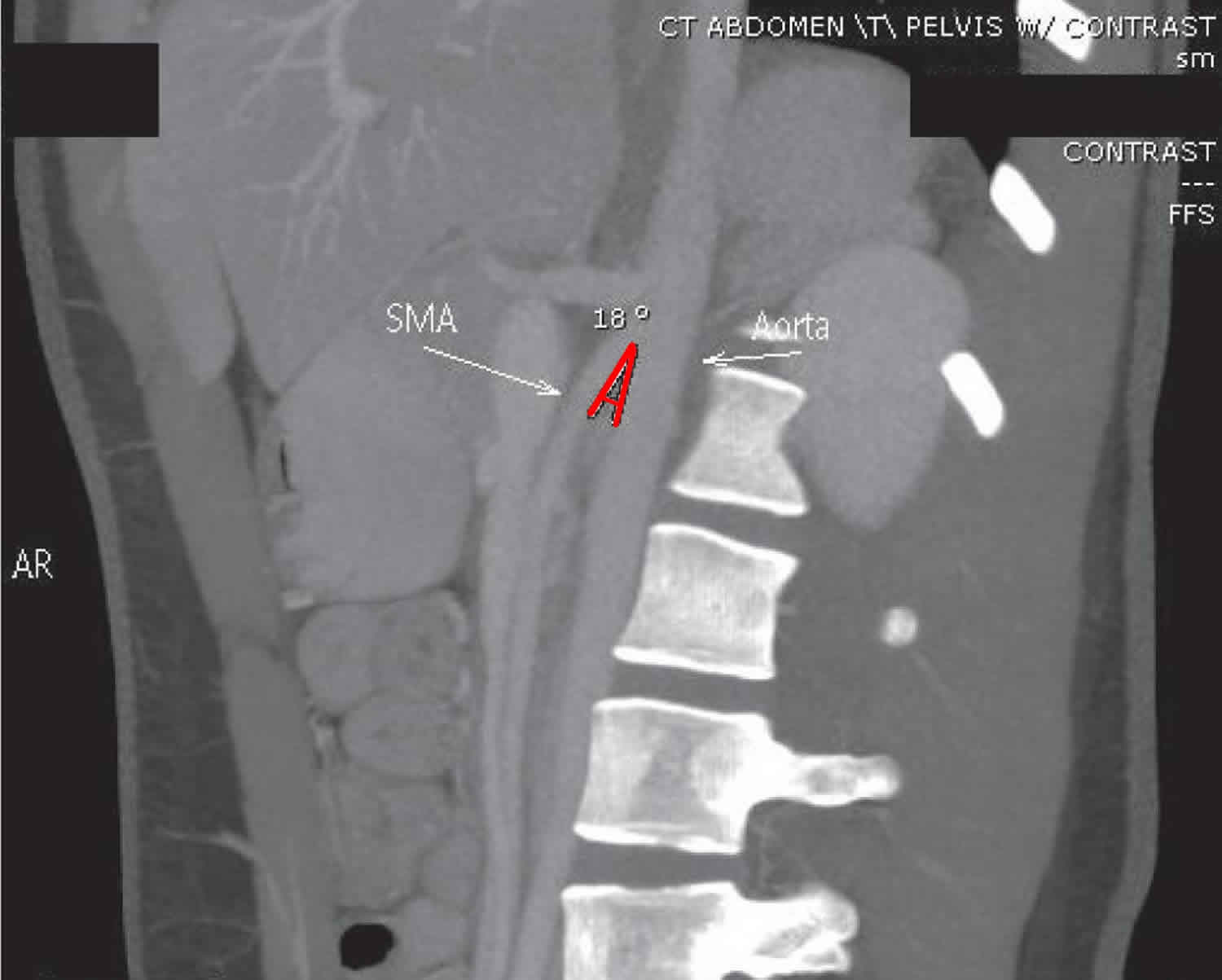
The superior mesenteric artery usually forms an angle of approximately 45° (range, 38-56°) with the abdominal aorta, and the third part of the duodenum crosses caudal to the origin of the superior mesenteric artery, coursing between the superior mesenteric artery and aorta 3. Any factor that sharply narrows the aorto-mesenteric angle to approximately 6-25° can cause entrapment and compression of the third part of the duodenum as it passes between the superior mesenteric artery and aorta, resulting in superior mesenteric artery syndrome.
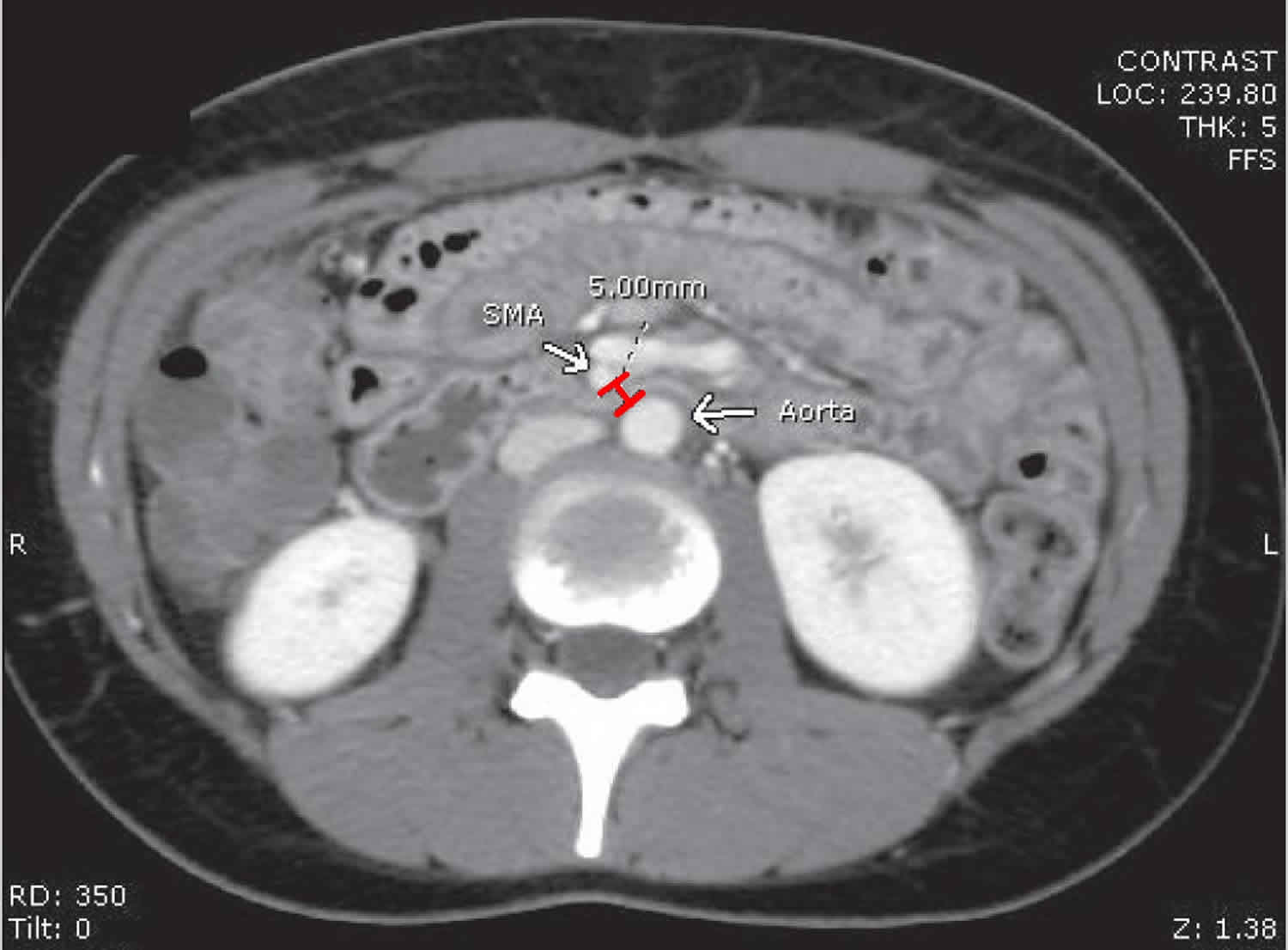
In addition, the aorto-mesenteric distance in superior mesenteric artery syndrome is decreased to 2-8 mm (normal is 10-20 mm). Alternatively, other causes implicated in superior mesenteric artery syndrome include high insertion of the duodenum at the ligament of Treitz, a low origin of the superior mesenteric artery, and compression of the duodenum due to peritoneal adhesions 4.
Virtually, any condition associated with weight loss may be followed by superior mesenteric artery syndrome 5. Tuberculosis 6, brucellosis 7, diabetes mellitus 8, anorexia nervosa 9, blunt abdominal trauma 10, and burns 11 are only few to mention. It has also been reported after spinal surgery 12, application of body casts 13, and bed confinement in the supine position 14. Moreover, superior mesenteric artery syndrome may complicate weight reduction following bariatric surgery 15, a pertinent fact to consider after the current surge of this type of surgery.
Superior mesenteric artery syndrome is a rarely diagnosed condition 5. Delay in the diagnosis of superior mesenteric artery syndrome can result in malnutrition, dehydration, electrolyte abnormalities, gastric pneumatosis and portal venous gas, formation of an obstructing duodenal bezoar, hypovolemia secondary to massive gastrointestinal hemorrhage, and even death secondary to gastric perforation 16. Keeping a high index of suspicion followed by the utilization of appropriate radiology is essential for its diagnosis. Although the clinical manifestations of superior mesenteric artery syndrome are shared with many other disease entities, it has unique radiological as well as endoscopic features, which enable a confident diagnosis to be made. Once diagnosed, conservative treatment with nutritional support (small feedings or a liquid diet) and positioning should be tried first. In case of unresponsiveness, surgery may give a lasting cure.
Figure 4. Superior mesenteric artery syndrome anatomy
[Source 17 ]Superior mesenteric artery syndrome symptoms
Superior mesenteric artery syndrome symptoms vary based on severity, but can be severely debilitating 18. Symptoms may include abdominal pain, fullness, nausea, vomiting, and/or weight loss 19.
The signs and symptoms of superior mesenteric artery syndrome vary but may include:
- Feeling full quickly when eating
- Bloating after meals
- Burping (belching)
- Nausea and vomiting of partially digested food or bile-like liquid
- Small bowel obstruction
- Weight loss
- Mid-abdominal “crampy” pain that may be relieved by the prone or knee-chest position or by lying on the left side
Superior mesenteric artery syndrome causes
The most common cause of superior mesenteric artery syndrome is severe weight loss including trauma, burns, anorexia nervosa and/or after prolonged bed rest 20. Other rare causes of superior mesenteric artery syndrome are surgical correction of scoliosis in children 21 and a congenitally short ligament of Treitz. Patients with superior mesenteric artery syndrome present with bloating sensation, epigastric pain, abdominal distension, nausea and vomiting. Symptoms may be relieved when patients are in the left lateral decubitus, prone or knee-chest position 1.
Superior mesenteric artery syndrome diagnosis
A comprehensive history should be obtained, especially weight loss, in patients in whom the clinical suspicion of superior mesenteric artery syndrome is high. The diagnosis of superior mesenteric artery syndrome is difficult. Confirmation usually requires radiographic studies, such as an upper GI series, hypotonic duodenography, and abdominal computed tomography (CT) scanning 22 with contrast to measure the aortomesenteric angle and aortomesenteric distance.
Figure 5. Superior mesenteric artery syndrome diagnosis – sagittal maximum intensity projection image of aorta and superior mesenteric artery (SMA) showing a narrow aorta-superior mesenteric artery angle of 18°
[Source 2 ]Figure 6. Superior mesenteric artery syndrome diagnosis – axial contrast-enhanced computed tomography scan showing a narrow aorta-superior mesenteric artery (SMA) distance of 5 mm
Superior mesenteric artery syndrome treatment
The treatment for superior mesenteric artery syndrome is correction of electrolyte imbalance, decompression of the obstruction via a nasogastric tube and nutritional support. Feeding in such patients is aided by a nasojejunal tube placed beyond the third portion of the duodenum. Nasogastric decompression (a tube passed through the nose into the stomach) and proper positioning after eating (such as lying in the left side or standing or sitting with a knee-to-chest position) may be recommended to alleviate symptoms.
In severe cases, intravenous (IV) nutritional support and/or a feeding tube may be needed to provide enough calories. Affected people can usually then be started on oral liquids, followed by slow and gradual introduction of small and frequent soft meals as tolerated. Then, regular solid foods may be introduced. Metoclopramide medication to avoid vomiting may be beneficial for some people.
After initial weeks, and once the patient begins gaining weight, oral feeding should be encouraged. If the weight gain does not improve the symptoms, surgical mobilization of the duodenum after the ligament of Treitz is required. The duodenum is placed to the right of the superior mesenteric artery so it does not lie in the acute angle between the superior mesenteric artery and the aorta 23. Surgery may be needed if other treatment strategies do not work. However, other treatment options should usually be tried for at least 4-6 weeks before considering surgery.
Surgery options are 19:
- Strong’s procedure: Where the duodenum is re-positioned to the right of the superior mesenteric artery
- Gastrojejunostomy: Where the jejunum (the part of the intestines that continues with the duodenum) is joined directly to the stomach
- Duodenojejunostomy with or without division or resection of the fourth part of the duodenum.
Superior mesenteric artery syndrome prognosis
Superior mesenteric artery syndrome can be severely debilitating and may require long term management, medications, costly parenteral nutrition (intravenous feeding) and rigorous follow-up 18. The long-term outlook (prognosis) can depend on whether the condition is diagnosed and treated in a timely manner. The prognosis may be excellent if it is diagnosed quickly and appropriate therapy is given. However, superior mesenteric artery syndrome may go unrecognized until a person experiences symptoms for a long time. In the past, deaths have been reported due to complications including progressive dehydration, hypokalemia (low potassium), and oliguria (producing too little urine). Most deaths have been reported in people in whom the diagnosis was delayed or missed 3.
Other complications that may arise in people with superior mesenteric artery syndrome include 3:
- Other electrolyte imbalances
- Malnutrition
- Hypotension
- Peptic ulcer disease
- Aspiration pneumonia
Mesenteric artery stenosis
Chronic mesenteric ischemia is usually related to progressive atherosclerotic narrowing of the mesenteric arteries 24. Due to the wide range of causes, mesenteric ischemia often goes undiagnosed and untreated, leading to high morbidity and mortality.
Mesenteric artery stenosis results in insufficient blood flow to the small intestine, causing intestinal ischemia. Chronic mesenteric ischemia is usually due to atherosclerosis, but is rarely caused by extensive fibromuscular disease or trauma. The celiac trunk, superior mesenteric artery, and inferior mesenteric artery usually have ostial disease and occlusions are typically found in the proximal few centimeters of these arteries. Chronic mesenteric ischemia results when at least two of the three major splanchnic arteries have severe stenosis. The superior mesenteric artery is almost always involved in symptomatic cases. At rest, patients have sufficient intestinal blood flow to maintain gut viability and prevent symptom development. However, the increased demand on mesenteric circulation after a meal may overwhelm the compensatory ability of the collateral circulation, thereby causing postprandial intestinal angina.
Patients with chronic mesenteric ischemia typically present in the fifth or sixth decades of life 25. They often have atherosclerotic disease elsewhere, such as peripheral vascular disease, coronary artery disease, and/or cerebrovascular disease. Females are more likely to be affected than males. Most patients complain of after meal abdominal pain. The classic description is crampy or colicky pain located in the epigastric area that begins 15–30 minutes following eating, lasts for 2–3 hours, and gradually subsides. The postprandial pain can result in fear of food (sitophobia) 26. Patients may compensate by eating smaller portions. Most patients with chronic mesenteric ischemia have marked weight loss. A physical examination often reveals weight loss and generalized signs of atherosclerosis. Rarely, patients have an abdominal bruit. Most often, patients initially receive a malignancy work-up.
Mesenteric artery stenosis diagnosis
Plain abdominal x-ray, CT scan, and endoscopy are insensitive in diagnosing chronic mesenteric ischemia, but can rule out other diseases (such as malignancy). Duplex ultrasound requires excellent technical skills and a well-prepared patient. Vessel tortuosity, respiratory motion, and the presence of bowel gas impede good visualization.
Velocity parameters used to determine the presence of ≥ 70% stenosis (peak systolic velocity >275 cm/s for the celiac artery and >200 cm/s for the superior mesenteric artery) have been reported with sensitivities and specificities of around 90% compared with angiography. Therefore, duplex ultrasound is fairly reliable in excluding the diagnosis of chronic mesenteric ischemia 24.
Gadolinium-enhanced MRA (magnetic resonance angiogram) has also been used in the diagnosis of mesenteric artery stenosis. Biplanar aortography, including selective engagement of the celiac trunk, superior mesenteric artery, and inferior mesenteric artery, remains the diagnostic test of choice. Lateral abdominal aortograms are optimal to visualize the origin and the proximal portion of the mesenteric arteries. In addition to defining the extent of the disease, angiography determines collateral flow. Chronic mesenteric ischemia requires flow-limiting stenosis or occlusion of at least two of the three mesenteric arteries. In general, large collaterals (such as the wandering artery of Drummond from the inferior mesenteric artery to the superior mesenteric artery in the case of superior mesenteric artery stenosis) are present and help to confirm the presence of lesions that are suspected to be flow limiting.
Mesenteric artery stenosis treatment
Patients with chronic mesenteric ischemia have traditionally been treated with mesenteric vascular surgical revascularization 27. Overall, the operative mortality remains high (approximately 7.5%–10%) 26. There are several surgical revascularization strategies, including visceral endarterectomy, antegrade supraceliac aorta to visceral bypass, and retrograde infrarenal aorta to visceral bypass. Before surgical revascularization, the patient may benefit from total parenteral nutrition (TPN). Although there is controversy regarding the length and benefit of total parenteral nutrition (TPN), improving the nutritional status of the patient prior to surgery seems rational.
Transaortic visceral endarterectomy is a technically challenging procedure that requires extensive retroperitoneal vascular exposure. This technique also has a greater risk of paraplegia because of supraceliac aortic cross-clamping. Endarterectomy may be particularly beneficial in cases where the patient has both visceral stenosis and renal artery stenosis. Endarterectomy is very difficult in patients with extensive aortic atherosclerosis and an alternative technique may be considered.
Retrograde superior mesenteric artery bypass is the most commonly performed visceral bypass procedure. The simplicity of the approach to the infrarenal aorta and infrapancreatic superior mesenteric artery makes this procedure attractive to surgeons. With retrograde bypass, care must be taken to configure a graft that will not kink. Antegrade bypass is the procedure of choice when there is marked infrarenal aortic atherosclerosis. Endarterectomy appears to have the lowest recurrence rate, followed by antegrade bypass reconstruction, and finally retrograde reconstruction.
Endovascular treatment is becoming the first-line therapy for chronic mesenteric ischemia at many experienced centers. Early studies found high restenosis rates; however, stenting has significantly improved outcomes. Visceral surgical revascularization in a malnourished patient, even in specialized centers, leads to significant morbidity and mortality in comparison with endovascular treatment. The endovascular approach is less invasive and compares favorably with surgery in terms of clinical success, complications, and long-term outcomes. Steimetz et al. demonstrated that endovascular therapy for mesenteric artery stenosis is efficient in both the short and long-term 28.
Intervention
Mesenteric artery revascularization can be performed using either the femoral or the brachial approach, with the femoral approach being preferred. Many of the concepts discussed in the section on renal artery intervention also apply to mesenteric artery revascularization, including the use of similar guide catheters, such as the Hockey Stick, Multipurpose, JR4, and RDC catheters in 6–8 Fr sizes. Most operators currently use a 6-inch Fr guide catheter and a 0.014-inch guidewire system 24.
Once an appropriate guide is seated in the ostium, unfractionated heparin is administered at 60 U/kg to achieve an activated clotting time of 200–250 seconds. The stenosis is crossed with a 0.014-inch guidewire and predilated with an appropriately sized balloon catheter (5–6 mm diameter for most mesenteric arteries). Mesenteric angioplasty has a good technical success rate but a high rate of restenosis 29)), and routine stenting is recommended. Following dilatation, an appropriately sized stent (typically 6–7 mm diameter) is advanced and deployed to cover the entire lesion, with 1–2 mm of the stent extending into the aorta to ensure complete coverage of the ostium.
A major consideration in the choice of the stent is that it has good radial strength to prevent recoil of the ostium for mesenteric artery stenting. The ostium is typically then postdilated with the stent balloon partially in the aorta at higher atmospheres to flare the ostium. Similarly to in renal stenting, care should be taken to align the balloon catheter coaxially during postdilatation to avoid creating aortic dissections. Potential complications of mesenteric artery stenting include atheroembolization, aortic dissections, and access site vascular complications. Postprocedure, patients should receive lifelong aspirin and clopidogrel for 1–12 months. In the future, emboli protection devices and drug-eluting stents may further improve the safety and durability of endovascular mesenteric artery revascularization.
- Gniftiths GJ, Whitehouse GH, Wilkie DP. Chronic duodenal ileus. Br J Surg. 1921;9:204.[↩][↩]
- Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3222767/[↩][↩][↩]
- Superior Mesenteric Artery Syndrome. https://emedicine.medscape.com/article/932220-overview[↩][↩][↩][↩]
- Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. 2009 Feb. 13(2):287-92.[↩]
- Rabie ME, Ogunbiyi O, Al Qahtani AS, Taha SB, El Hadad A, El Hakeem I. Superior Mesenteric Artery Syndrome: Clinical and Radiological Considerations. Surg Res Pract. 2015;2015:628705. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4549571/[↩][↩]
- Limaye C. S., Karande S. P., Aher S. P., Pati K. A. Superior mesenteric artery syndrome secondary to tuberculosis induced cachexia. The Journal of the Association of Physicians of India. 2011;59:670–671[↩]
- Prasad S., Lingadakai R., Chethan K., Abdul Z. Superior mesenteric artery syndrome secondary to brucellosis—a case report. Indian Journal of Surgery. 2010;72(3):265–267. doi: 10.1007/s12262-010-0066-8[↩]
- Wu M.-C., Wu I.-C., Wu J.-Y., Wu D.-C., Wang W.-M. Superior mesenteric artery syndrome in a diabetic patient with acute weight loss. World Journal of Gastroenterology. 2009;15(47):6004–6006. doi: 10.3748/wjg.15.6004[↩]
- Lee C. W., Park M. I., Park S. J., et al. A case of superior mesenteric artery syndrome caused by anorexia nervosa. The Korean Journal of Gastroenterology. 2011;58(5):280–283. doi: 10.4166/kjg.2011.58.5.280[↩]
- Falcone J. L., Garrett K. O. Superior mesenteric artery syndrome after blunt abdominal trauma: a case report. Vascular and Endovascular Surgery. 2010;44(5):410–412. doi: 10.1177/1538574410369390[↩]
- Lescher T. J., Sirinek K. R., Pruitt B. A., Jr. Superior mesenteric artery syndrome in thermally injured patients. Journal of Trauma. 1979;19(8):567–571. doi: 10.1097/00005373-197908000-00004[↩]
- Tsirikos A. I., Anakwe R. E., Baker A. D. L. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. Journal of Medical Case Reports. 2008;2, article 9 doi: 10.1186/1752-1947-2-9[↩]
- Hutchinson D. T., Bassett G. S. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clinical Orthopaedics and Related Research. 1990;(250):250–257[↩]
- Townsend C. M., Naoum J. J. Vascular compression of the duodenum. In: Fischer J. E., editor. Mastery of Surgery. 5th. Lippincott Williams & Wilkins; 2007. pp. 955–961.[↩]
- Goitein D., Gagné D. J., Papasavas P. K., et al. Superior mesenteric artery syndrome after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obesity Surgery. 2004;14(7):1008–1011. doi: 10.1381/0960892041719626[↩]
- Ko KH, Tsai SH, Yu CY, Huang GS, Liu CH, Chang WC. Unusual complication of superior mesenteric artery syndrome: spontaneous upper gastrointestinal bleeding with hypovolemic shock. J Chin Med Assoc. 2009 Jan. 72(1):45-7.[↩]
- Bernotavičius G, Saniukas K, Karmonaitė I, Zagorskis R. Superior mesenteric artery syndrome. Acta Med Litu. 2016;23(3):155-164. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5287987/[↩]
- FJ Bohanon, O Nunez Lopez, BM Graham, LW Griffin, and RS Radhakrishnan. A Case Series of Laparoscopic Duodenojejunostomy for the Treatment of Pediatric Superior Mesenteric Artery Syndrome. Int J Surg Res. April, 2016; https://www.ncbi.nlm.nih.gov/pubmed/27747293[↩][↩]
- Scovell S & Hamdan A. Superior Mesenteric Artery Syndrome. UpToDate. Waltham, MA: UpToDate; March 6, 2017; http://www.uptodate.com/contents/superior-mesenteric-artery-syndrome[↩][↩]
- Laffont I, Bensmail D, Rech C, Prigent G, Loubert G, Dizien O. Late superior mesenteric artery syndrome in paraplegia: Case report and review. Spinal Cord. 2002;40:88.[↩]
- Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27:E528.[↩]
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol. 1991;21:154[↩]
- Wilson-Storey D, MacKinlay GA. The superior mesenteric artery syndrome. J R Coll Surg (Edin) 1986;31:175[↩]
- Mukherjee D, Cho L. Mesenteric artery stenosis. In: Bhatt DL, editor. Guide to Peripheral and Cerebrovascular Intervention. London: Remedica; 2004. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27423[↩][↩][↩]
- Greenwald DA , Brandt LJ , Reinus JF . Ischemic bowel disease in the elderly. Gastroenterol Clin North Am. 2001;30:445–73[↩]
- Barkhordarian S , Gusberg R . Mesenteric ischemia: identification and treatment. ACC Curr J Rev. 2003;12:19–21.[↩][↩]
- Stanley JC . Mesenteric arterial occlusive and aneurysmal disease. Cardiol Clin. 2002;20:611–22. vii.[↩]
- Steinmetz E , Tatou E , Favier-Blavoux C . et al. Endovascular treatment as first choice in chronic intestinal ischemia. Ann Vasc Surg. 2002;16:693–9[↩]
- ((Odurny A , Sniderman KW , Colapinto RF . Intestinal angina: percutaneous transluminal angioplasty of the celiac and superior mesenteric arteries. Radiology. 1988;167:59–62.[↩]