Contents
What is Mohs surgery
Mohs surgery also called Mohs micrographic surgery, is a precise surgical procedure to remove skin cancer lesion in several steps whilst sparing the healthy tissue as much as possible 1. With Mohs micrographic surgery, the smallest amount of tissue possible is removed. You will have a smaller scar than you might have with other treatment options. First, a thin layer of cancerous tissue is removed. Then, a second thin layer of tissue is removed and viewed under a microscope to check for cancer cells. More layers are removed one at a time until the tissue viewed under a microscope shows no remaining cancer. Mohs surgery has a 99% cure rate in treating certain skin cancer. Mohs micrographic surgery is a surgical approach that offers high cure rates for the treatment of a variety of skin cancers, including basal cell carcinomas (BCC) and squamous cell carcinomas (SCC). The main advantage of Mohs surgery is that it offers precise microscopic control of the entire tumor margin while maximizing conservation of healthy tissue.
Mohs surgery is used to remove as little normal tissue as possible and is often used to remove skin cancers on the face. Mohs micrographic surgery is the treatment of choice for skin tumors in critical sites, large or recurrent tumors, tumors in sites of radiation therapy, and tumors with aggressive histologic features.
During the Mohs surgery procedure, the surgeon removes the cancer in layers until all the cancer has been removed. The surgeon will:
- Numb your skin where cancer is so you do not feel any pain. You stay awake for the procedure.
- Remove the visible tumor along with a thin layer of tissue next to the tumor.
- Look at the tissue under a microscope.
- Check for cancer. If there is still cancer in that layer, the doctor will take out another layer and look at that under the microscope.
- Keep repeating this procedure until there is no cancer found in a layer. Each round takes about 1 hour. The surgery takes 20 to 30 minutes and looking at the layer under the microscope takes 30 minutes.
- Do about 2 to 3 rounds to get all of the cancer. Deep tumors may need more layers.
- Stop any bleeding by applying a pressure dressing, using a small probe to heat the skin (electrocautery), or giving you a stitch.
Mohs surgery is appropriate for skin cancers with a high risk of recurrence and when tissue conservation is essential. Mohs surgery is performed by removing a thin margin of tissue circumferentially around and deep to the clinical margins of a skin tumor. The specimen is typically removed with a 45-degree bevel to facilitate tissue processing. It is then rapidly frozen and sectioned in a cryostat microtome, allowing for quick tissue processing (about 15 to 30 minutes). Sectioning the tissue in a horizontal direction allows virtually 100% of the tissue margin (peripheral and deep margins) to be examined under the microscope. The process is repeated until the tumor has negative histologic margins.
Mohs surgery may be preferred when the skin cancer is on an area where:
- It is important to remove as little tissue as possible, such as the eyelids, nose, ears, lips, or hands
- Your doctor needs to be certain the entire tumor is removed before stitching you up
- There is a scar or prior radiation treatment was used
- There is a higher chance the tumor will come back, such as on the ears, lip, nose, eyelids, or temples
Mohs surgery may also be preferred when:
- The skin cancer was already treated, and it was not completely removed or it came back
- The skin cancer is large, or the edges of the skin cancer are not clear
- Your immune system is not working well due to cancer, cancer treatments, or medicines you are taking
- The tumor is deeper
The tissue-sparing properties of Mohs micrographic surgery make it particularly useful in areas of functional and aesthetic importance such as the head and neck area, anogenital area, hands, and feet.
In 1941, Frederick Mohs 2 described a surgical technique he had developed for the staged removal of skin cancer using in situ fixation of cutaneous tissue. After fixation, Mohs excised the cancer and cut horizontal sections from the undersurface of the tissue sample for microscopic viewing. The horizontal sectioning allowed for complete examination of the peripheral tumor margins, as opposed to the standard vertical sectioning, which did not produce continuity of a 360-degree circumferential margin.
Although many refinements have been made to Mohs’ original technique, the two main objectives are the same: to remove all of the tumor roots as accurately as possible by histologically confirming negative margins, and to create the smallest possible defect by sparing tissue uninvolved by the tumor 3. Mohs micrographic surgery minimizes tumor recurrence rates; reduces the size of surgical defects; allows for cosmesis; and preserves the function of the eyelids, the nares (nostrils), the commissure of the mouth, and the ears.
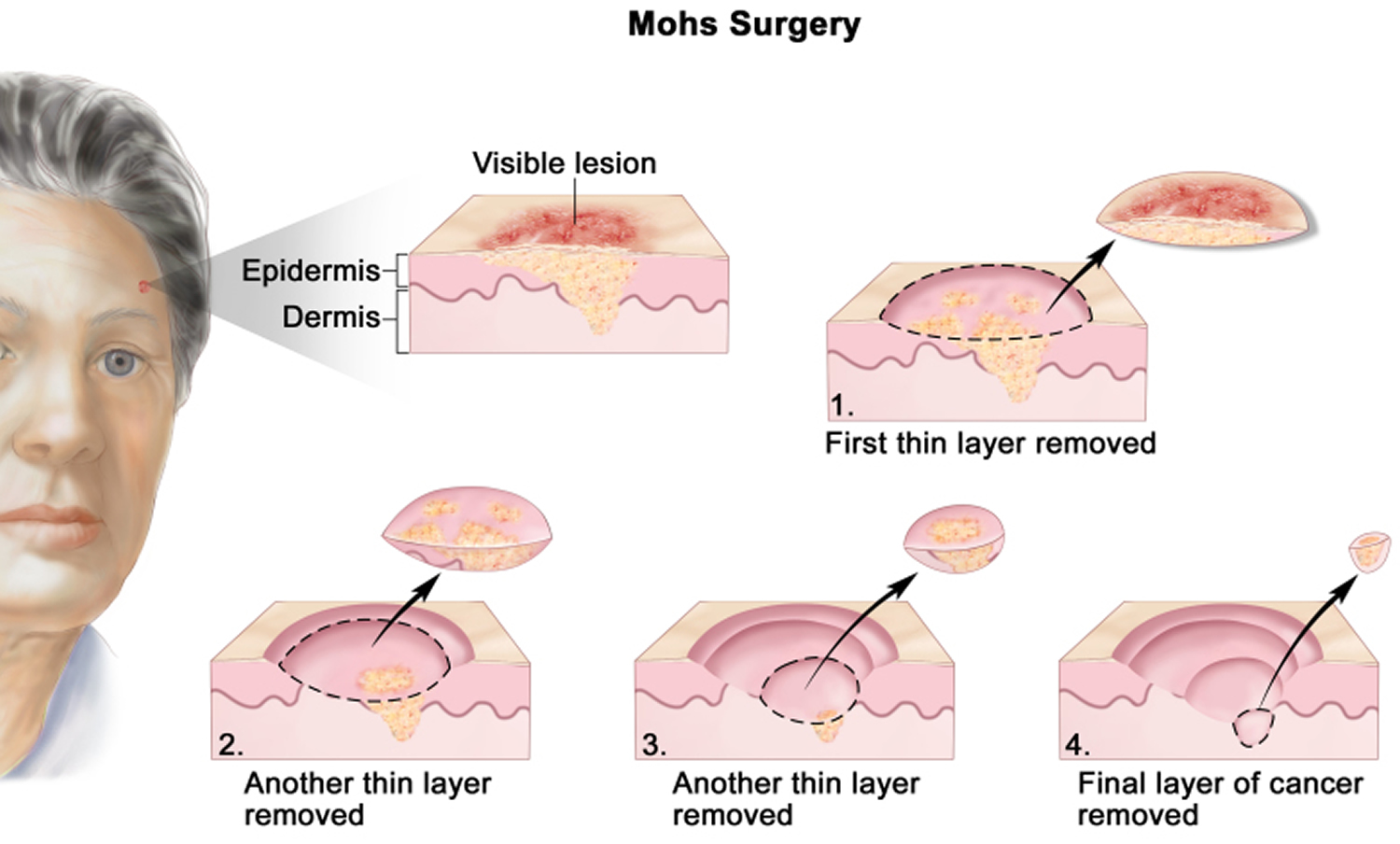
Figure 1. Mohs surgery – drawing shows a patient with skin cancer on the face. The pullout shows a block of skin with cancer in the epidermis (outer layer of the skin) and the dermis (inner layer of the skin). A visible lesion is shown on the skin’s surface. Four numbered blocks show the removal of thin layers of the skin one at a time until all the cancer is removed.
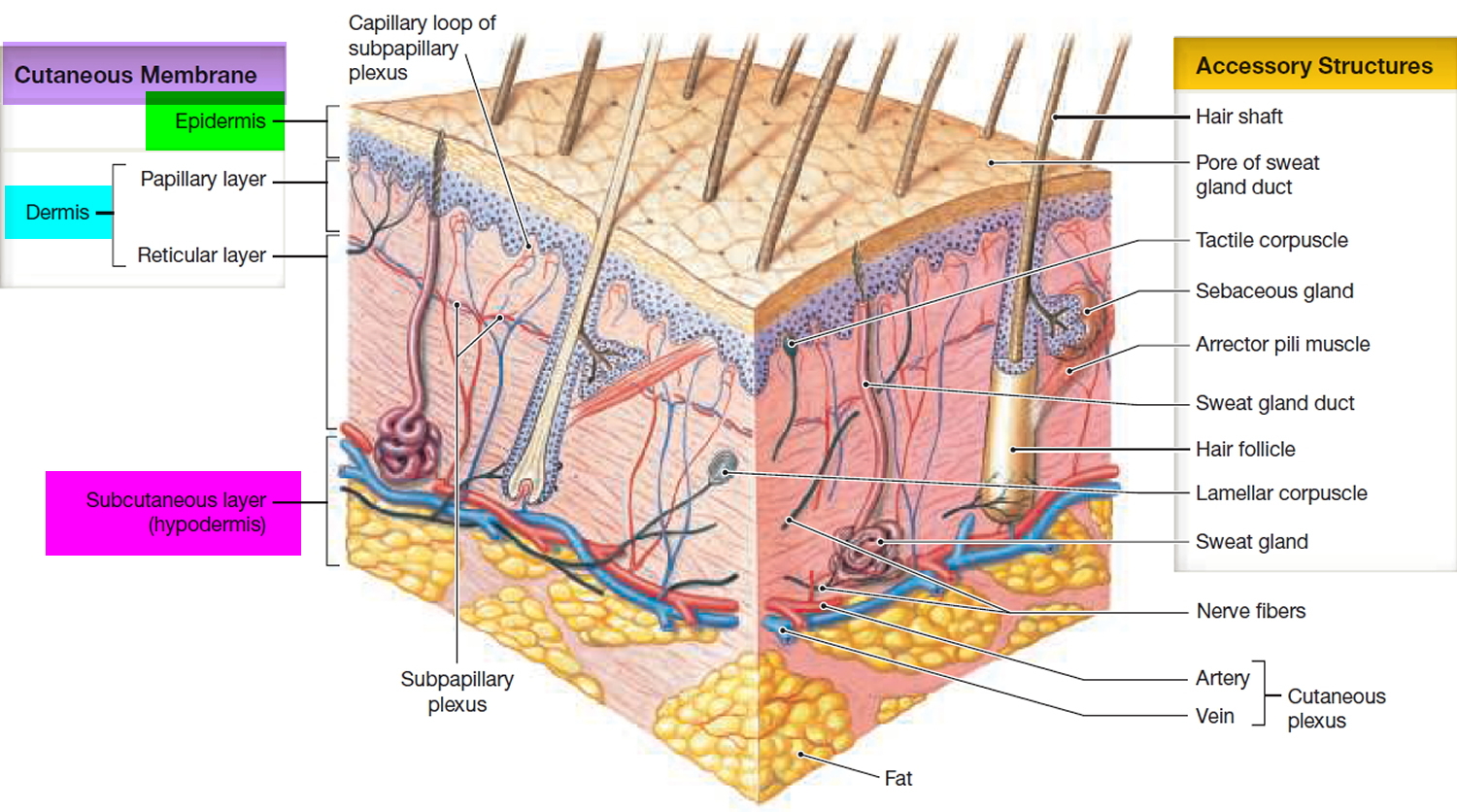
Figure 2. Skin structure
Mohs micrographic surgery procedure
Most patients who are referred for Mohs micrographic surgery are treated on an outpatient basis with local anesthetic. Occasionally, oral sedation is added.
Mohs surgery procedure requires the surgeon and at least one assistant in the surgical suite. In addition, at least one histotechnician is needed in the Mohs laboratory for tissue processing.
The Mohs surgery technique is as follows:
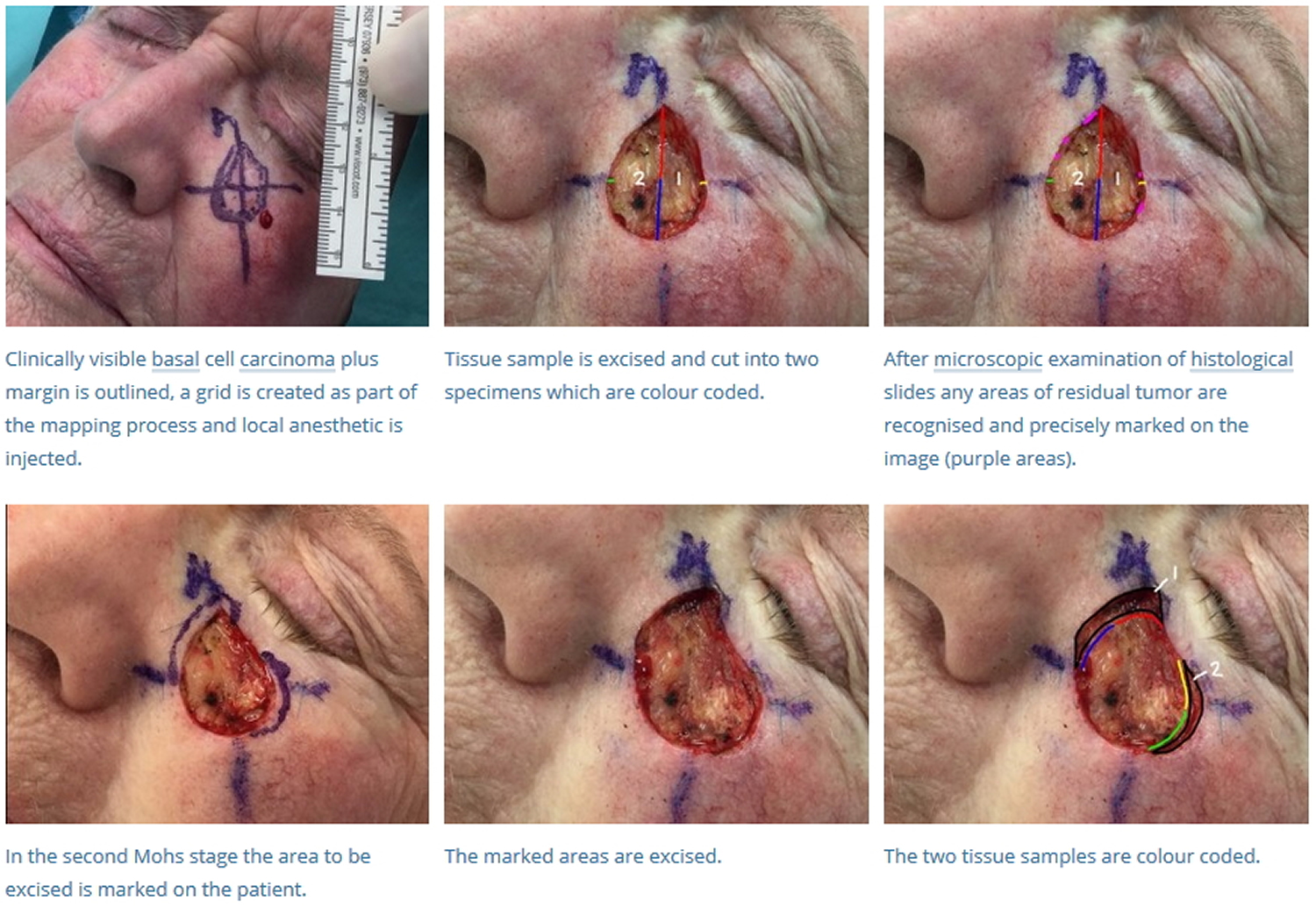
- The tumor is first outlined prior to injection with a local anesthetic. After anesthetized, any visible tumor is removed or “debulked,” with a curette, flexible blade, or scalpel.
- Prior to removal, the tissue layer is carefully oriented by placing small superficial etch marks with a scalpel (often at 3 o’clock, 6 o’clock, 9 o’clock, and noon) around the tissue layer and corresponding in-situ skin.
- A thin margin of tissue is then removed circumferentially around and deep to the debulked tumor defect. This “layer” of tissue is removed with a beveled angle of approximately 45 degrees, which facilitates tissue processing (see below).
- Once removed, the tissue layer is often cut into halves or quadrants and then marked with colored dyes to facilitate precise mapping of the tumor. The tissue is then pressed flat, so the epidermal edge occupies the same tissue plane as the deep margin. The “beveled” edge acquired tissue removal facilitates this flattening process.
- The tissue is then cut and processed in a horizontal direction so that virtually 100% of the peripheral and deep margin can be examined on the same tissue section under the microscope. This is in contrast to the traditional vertical, or “breadloafed,” tissue processing which examines only a small portion of the tumor margin.
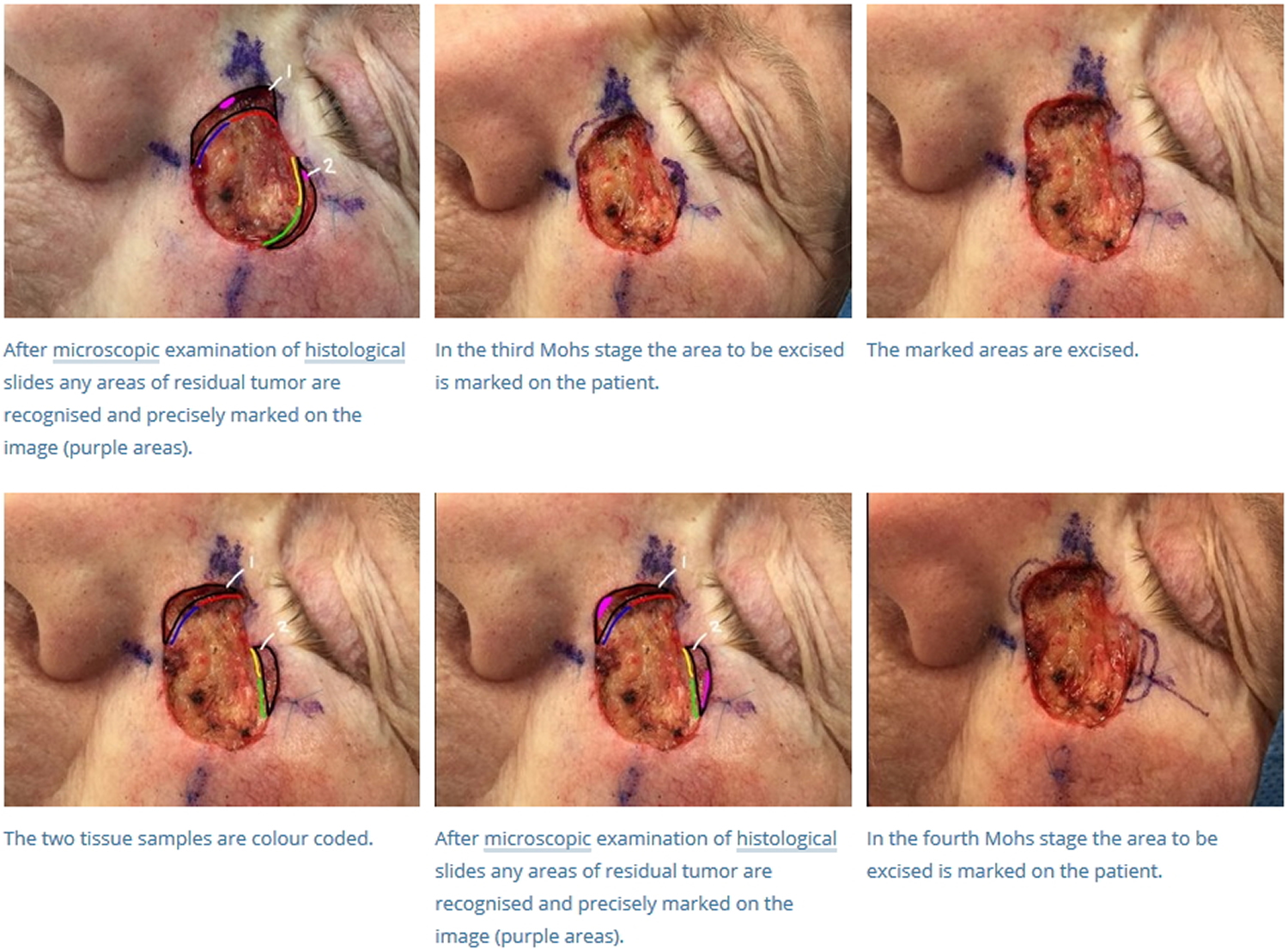
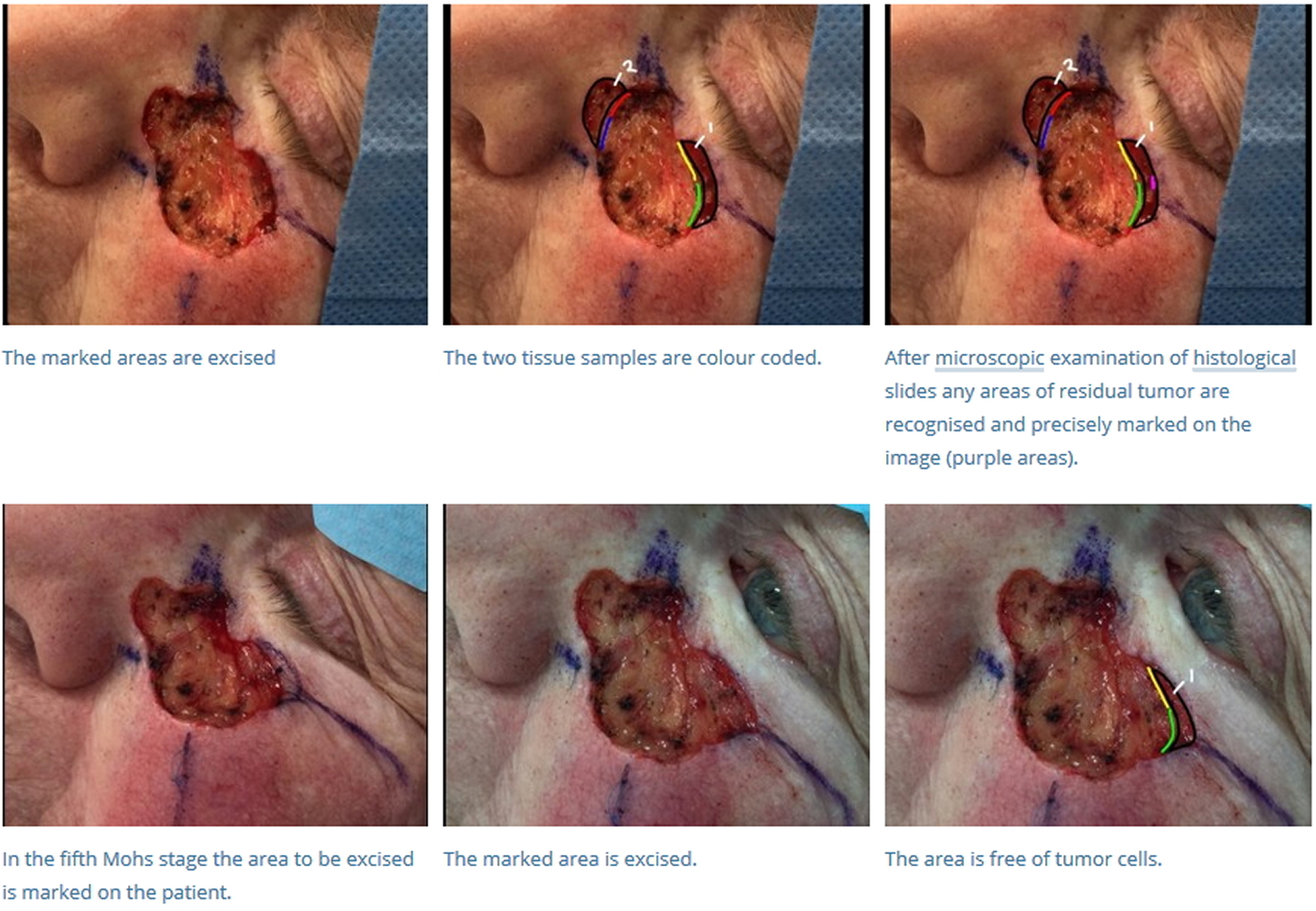
- If residual tumor is identified under the microscope, then the Mohs map is marked and the corresponding in-situ tissue is precisely removed from the patient in that portion that was found to still have tumor. This process is repeated until the tumor is histologically negative, thus ensuring complete tumor removal with maximum conservation of healthy tissue.
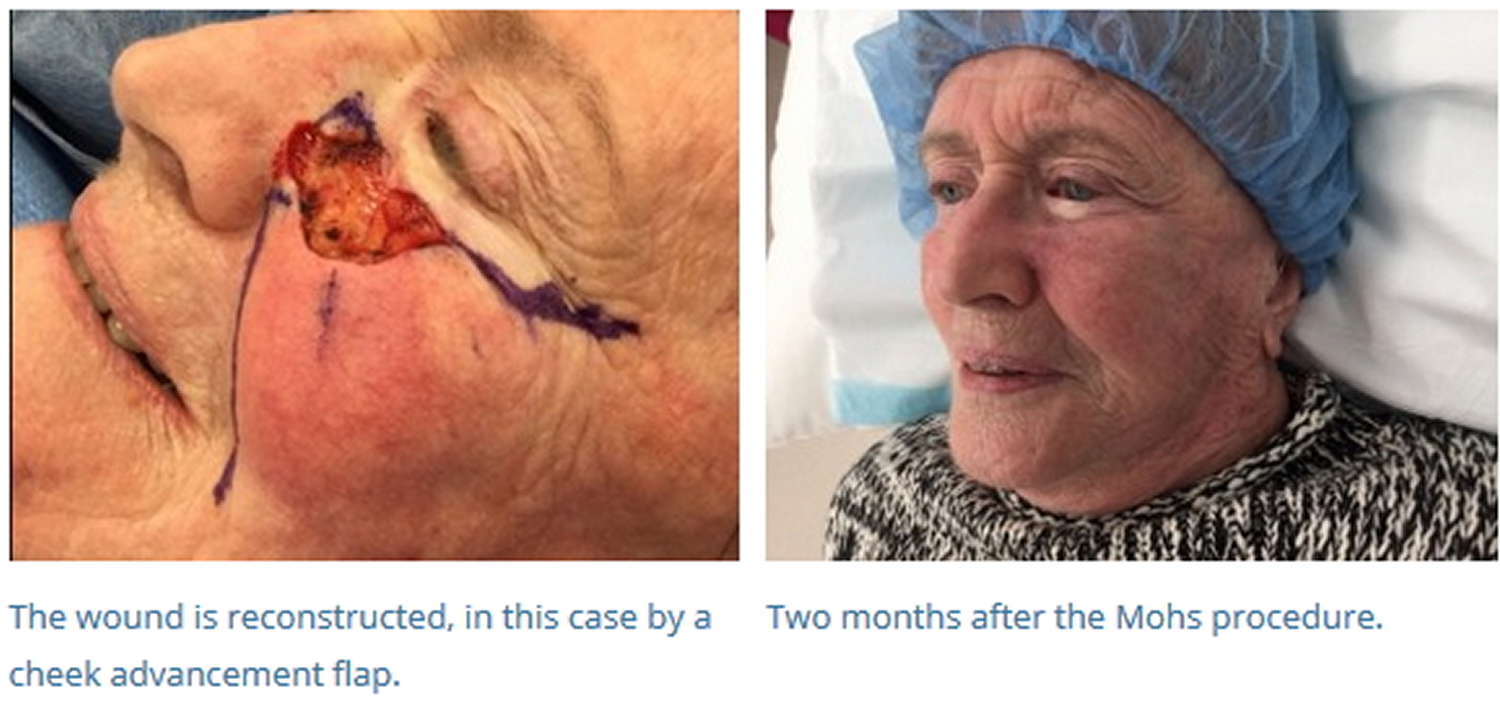
- Once the tumor has been removed, a variety of techniques are used to close the defect, including primary closure, flaps, grafts, and second intention healing. A recent tabulation of Mohs stages per case for experienced Mohs surgeons showed a median of about 1.7 stages per tumor to clear. Obviously, that number can be much higher for more complicated cases. Many defects are larger and deep enough that, without repair, functional impairment may result (e.g., retraction of an eyelid, elevation of a nasal ala, distortion of a commissure of the mouth). In these cases, surgeons have many reconstructive options from which to choose, including primary closure, local flaps or grafts 3. In some cases, the defects are severe enough that the patient must be referred for reconstructive surgery performed under general anesthesia.
Tissue stains most commonly used for Mohs surgery are hematoxylin and eosin (H&E) and toluidine blue. While the majority of Mohs surgeons use hematoxylin and eosin (H&E) routinely, a significant minority prefer toluidine blue for processing basal cell carcinoma (BCC), since mucopolysaccharides and hyaluronic acid that are associated with basal cell carcinoma (BCC) stain metachromatically with a magenta coloration.
The Mohs procedure depends upon the presence of continuous tumor growth (no “skip” areas) to be maximally effective. Fortunately, this characteristic is present in most cancers that occur on the skin.
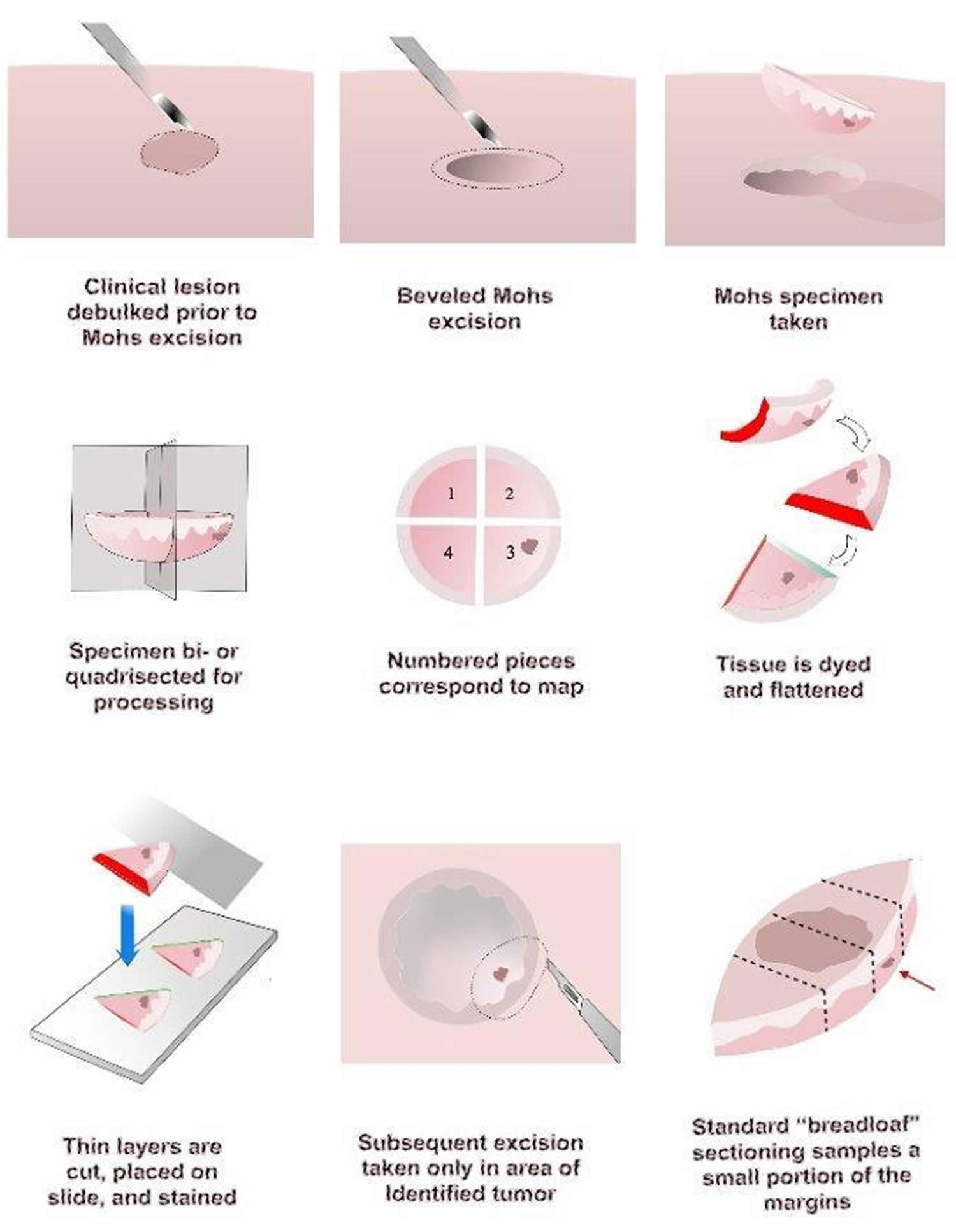
Figure 3. Mohs surgery technique
Notes: The process begins with curettage of any residual tumor that may have persisted after the biopsy. Residual tumor generally is soft and evokes little resistance to the curette. Preoperative borders can be better predicted with this simple initial step. The visible tumor is then excised, usually with 2-mm margins of normal skin. Any specimens are marked with colored dye to specify orientation. By convention, the 12 o’clock position usually is cephalad and the 6 o’clock position is caudad. Hemostasis is obtained with electrocautery, and a temporary dressing is applied.
Mohs micrographic surgery equipment
Mohs micrographic surgery requires equipment for the operating room as well as for the lab in which tissue is processed and examined microscopically. The operating room requires good lighting and an adjustable table to provide optimal visualization and access to the tumor. Surgical equipment is relatively simple, consisting of a scalpel, fine forceps, scissors, gauze, and an electrosurgical device for coagulation. Reconstruction can be achieved with an expanded tray that includes needle holders, scissors, fine forceps, skin hooks, and a scalpel.
The Mohs histology laboratory consists of microtomes that freeze tissue and then allow cutting of very thin slices of tissue to mount on glass slides. The slides are then placed in an automated stainer or may be stained by hand. This process may require a vent hood to minimize exposure to chemicals involved in the staining process. Completed slides are then read by the Mohs surgeon under light microscopy to determine if tumor remains in the tissue. Many Mohs labs also have special stainers and reagents to allow immunohistochemical staining of tissue.
Mohs surgery recovery
Taking proper care of your wound after surgery will help your skin look its best. Your doctor will talk with you about your options:
- Let a small wound heal itself. Most small wounds heal well on their own.
- Use stitches to close the wound.
- Use skin grafts. The doctor covers the wound using skin from another part of your body.
- Use skin flaps. The doctor covers the wound with the skin next to your wound. Skin near your wound matches in color and texture.
What is the difference between Mohs surgery and standard excision?
In standard excision, the tissue sample is sent off for histological processing while the wound is closed. The processing takes a number of days during which cross sections (or vertical sections) are created at various distances through the sample and are microscopically assessed by a pathologist. The pathologist looks for skin cancer at the margins of each section, but these are only a fraction of the actual excision margin.
In Mohs surgery, the histological processing takes place on the day of surgery and the wound is only closed after it has been confirmed that the entire cancer has been removed. The excision margin is examined by an embedding technique that allows horizontal sections to be cut involving all the deep and radial excision margins. If any tumor is visible in these sections, it means that the excision is incomplete and the patient requires a further Mohs stage.
A mapping process and color coding system is used during Mohs surgery to precisely localize any remaining cancer, and tissue is only removed if it contains cancer. This process preserves healthy tissue.
Mohs surgery yields higher clearance rates than standard excision, and smaller wounds — therefore better cosmetic results.
Mohs surgery complications
Potential complications from Mohs micrographic surgery are similar to those expected in the outpatient surgical setting (i.e., scarring, postoperative pain, bleeding, hematoma, flap or graft necrosis, and wound infection) 3. Scarring can be minimized by selecting a repair technique appropriate for the size of the wound and the anatomic site. Most pain after Mohs micrographic surgery is minimal and can be controlled with oral analgesics.
Bleeding and hematoma can occur, particularly with local flaps and grafts. Bleeding complications are minimized by assiduous intraoperative hemostasis and occasionally by the placement of a temporary drain under larger flaps. For patients receiving anticoagulants, perioperative discontinuation of these medications is not indicated 4, but patients must be counseled to restrict physical activity during the first 48 hours after surgery to minimize the risk of hematoma.
Flap necrosis is rare but can occur with poor flap design, with excessive wound tension, or as a consequence of bleeding and hematoma. Flap necrosis also can occur in persons who smoke heavily. Skin grafts often are delayed for a few weeks in smokers to allow the formation of granulation tissue at the wound bed before repair. Wound infection develops in less than 3 percent of patients 5, generally occurs about 48 hours after surgery, and usually can be managed with oral antibiotics.
Mohs surgery advantages
There are several advantages to taking a Mohs micrographic surgical approach to the treatment of skin cancer. First, Mohs micrographic surgery is the most effective method of eradicating the most common type of skin cancer, basal cell carcinoma 6, with a five-year cure rate of 99 percent 7. Having the same physician perform the dual function of surgeon and pathologist increases the accuracy of tumor localization and intraoperative interpretation of risk based on histologic subtypes encountered during surgery. Indications of more aggressive tumor behavior, such as perineural invasion, may lead the surgeon to take more generous margins at specific sites.
Another benefit is that Mohs micrographic surgery spares tissue. Because the majority of skin cancers occur on the head and neck, preserving uninvolved tissue is of paramount importance, especially around the eyes, nose, ears, and mouth.
Finally, compared with other surgical techniques involving postoperative repair, the cost of Mohs micrographic surgery is similar to that of simple excision in the office with permanent section postoperative margin control. Mohs micrographic surgery is less expensive than excisions with intraoperative margin control with frozen sections performed in a private office or in an outpatient surgical facility 8.
Mohs surgery has had a high degree of clinical success
- Mohs surgery reports excellent 5-year cure rates for non-melanoma skin cancers, in particular basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Examples of 5-year cure rates include: Primary basal cell carcinoma (BCC) (99%), recurrent basal cell carcinoma (94.4%), primary squamous cell carcinoma (SCC) (92-99%), and recurrent squamous cell carcinoma (SCC) (90%).
- Mohs surgery also can be used to treat other less common tumors, including dermatofibrosarcoma protuberans, microcystic adnexal carcinoma, extramammary Paget disease, Merkel cell carcinoma, and sebaceous carcinoma.
How effective and cost effective is Mohs surgery?
Mohs surgery leads to fewer tumor recurrences than standard excision of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Recurrence rates for Mohs are typically reported to be 1–5%, depending on type of tumor and length of follow-up.
In a randomized clinical trial with 10-year follow-up, recurrence rates were 9:
- 4.4% for Mohs surgery and 12.2% for standard excision for high risk primary basal cell carcinoma
- 3.9% for Mohs and 13.5% for standard excision for high risk recurrent basal cell carcinoma.
Several studies have also found that Mohs is more cost effective than standard excision. The main reason for this is that there are fewer costly operations for recurrent tumors compared to standard excision 10.
Mohs surgery cost
To compare costs associated with removal of skin cancers using Mohs micrographic surgery with that using standard surgical excision with frozen or permanent margin control in the office or an ambulatory surgery center. Four hundred six tumors were included in this study on cost analysis comparing Mohs micrographic surgery with standard surgical excision 11. An average tumor was cleared in 1.6 stages. Mohs micrographic surgery was the least expensive surgical procedure evaluated, at $805 per tumor. Standard surgical excision with permanent margins ($1,026) was more expensive than Mohs micrographic surgery but less expensive than standard surgical excision with frozen margins ($1,200) and ambulatory surgery center- standard surgical excision with frozen margins ($2,507). Adjusted for inflation, the cost of Mohs micrographic surgery, inclusive of initial examination, biopsy, and 5-year follow-up, in 2009 ($1,376) was lower than in 1998 ($1,635) 11.
Mohs surgery for skin cancer guidelines
Mohs surgery is appropriate for skin cancers with a high risk of recurrence and when tissue conservation is essential. The Mohs Appropriate Use Criteria guidelines were developed to assist clinicians in determining if a specific tumor would be appropriately managed by Mohs surgery 1. A Mohs AUC mobile phone app is available for download to mobile devices. These criteria were based on areas of the body, patient characteristics, and tumor characteristics.
Mohs surgery is particularly suitable for areas of the body in the “H” area:
- Central face, eyelids/canthi, eyebrows, nose, lips, chin, ear, and periauricular area
- Genitalia
- Hands, feet, ankles, and nail units
- Nipples/areola
Higher-risk patient characteristics include:
- Immunocompromised
- Genetic syndromes (basal cell nevus syndrome, xeroderma pigmentosum)
- Prior radiated skin
- Patient with history of high-risk tumors
Tumor characteristics include:
- Positive margin on recent excision
Aggressive features that are high risk for recurrence of basal cell carcinomas (BCC):
- Aggressive histologic subtype: morpheaform, infiltrating, micronodular
- Perineural involvement
- Metatypical/keratotic
Aggressive features of squamous cell carcinomas (SCC):
- Poorly or undifferentiated (characterized by a high degree of nuclear polymorphism, high mitotic rate, or low degree of keratinization)
- Perineural/perivascular
- Spindle cell
- Breslow depth 2 mm or greater
- Clark level IV or greater
While the Mohs Appropriate Use Criteria can be helpful in determining if a specific lesion is appropriately managed with Mohs surgery, it does not exclude the validity of alternate modalities in treating the same lesion (e.g. curettage, electrodesiccation and curettage, or excision).
Types of Skin Cancers Treated with Mohs Micrographic Surgery
Mohs surgery is widely accepted as treatment of first choice for high risk basal cell carcinoma and squamous cell carcinoma. Although different criteria are used across the globe, the main reason to perform Mohs is to minimize the risk of incomplete excision. This reduces burden to the patient and may avoid large and costly re-excisions later.
Mohs surgery has greatest benefit for a tumor at high risk of incomplete excision, such as a:
- Recurrent or incompletely excised tumor
- Tumor arising in skin previously exposed to radiotherapy
- Large tumor, especially in the head and neck area
- Tumor that has poorly defined clinical borders
- Basal cell carcinoma with an aggressive growth pattern on histology (infiltrative, micronodular or with perineural invasion)
- Squamous cell carcinoma at higher risk of metastasis (e.g.,, located on ear, lip; with perineural invasion; or in a patient that is immune suppressed)
Mohs may also be appropriate when a large reconstruction is needed to close the defect or when the tumor is located in a cosmetically sensitive area.
In 2012, a joint effort by various medical organizations in the USA led to the development of appropriate use criteria for Mohs surgery. These criteria may be used as guidance when considering Mohs surgery although they may not apply in all jurisdictions 12.
Mohs for other types of skin cancer
There is plenty of evidence that Mohs is the best form of surgery for high risk basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Large trials comparing Mohs to standard excision for other types of skin cancer are lacking.
In Mohs surgery, tumor cells must be accurately identified on microscopic examination of frozen sections. This can be challenging in some skin cancers, such as:
- Atypical fibroxanthoma
- Very poorly differentiated squamous cell carcinoma
- Dermatofibrosarcoma protuberans
- Microcystic adnexal carcinoma
- Lentigo maligna/melanoma in situ
- Extramammary Paget disease
For these tumors, variations of Mohs surgery may be applied that follow the basic principles of Mohs surgery (microscopic margin control, horizontal embedding, and mapping and colour coding of tissue) but use paraffin embedded sections instead of frozen sections. This allows the use of immunohistochemical markers to help identify tumor cells.
Such techniques are sometimes collectively referred to as slow Mohs. They include the ‘Tuebingen Cake’, and ‘Muffin’ techniques 13.
Mohs micrographic surgery is not commonly used to treat melanoma because melanocytic atypia is difficult to assess with frozen sections 3. Permanent paraffin-embedded sections yield superior histologic resolution for assessment of melanocytic atypia 3. However, more recently, with the availability of reliable immunohistochemical stains, Mohs micrographic has also shown great usefulness in treating some forms of malignant melanoma, including lentigo maligna, lentigo maligna melanoma, and thin melanomas 1.
In 2001, nonmelanoma skin cancers accounted for an estimated 1 million cancers in the United States 14. Skin cancer accounts for more than 50 percent of all cancers in the United States. Patients with basal cell carcinomas (BCCs) receive the most referrals for Mohs micrographic surgery, followed by persons with squamous cell carcinomas (SCCs). Mohs micrographic surgery is an excellent approach to the management of a variety of other less common skin tumors, including Merkel cell carcinoma and neoplasms arising from the sebaceous gland and hair follicle unit or from the sweat coils and ducts.
After a biopsy has been performed and the presence of a carcinoma has been confirmed, the physician needs to classify the tumor as low risk or high risk. Low-risk tumors usually can be treated with simple techniques such as electrodesiccation and curettage, cryosurgery, or fusiform excision.
Features of high-risk basal cell and squamous cell carcinomas that qualify for Mohs micrographic surgery can be grouped into five broad categories 15:
- Tumors in critical sites,
- Tumors of large size,
- Tumors with aggressive histology,
- Tumors in the immunosuppressed patient, and
- Tumors with postoperative involved margins or clinically ambiguous borders
Table 1. Indications for Mohs Micrographic Surgery in Patients with High-Risk Cancers
High-risk anatomic location (eyelids, nose, ears, lips, genitalia, fingers) |
Large tumors (20 mm or more in diameter) on the torso and extremities |
Recurrent tumors after previous excision or destruction |
Tumors occurring in previous sites of radiation therapy |
Tumors with aggressive histologic patterns (small-strand, infiltrative, or morphea-like growth in basal cell carcinomas; perineural invasion; or poorly differentiated histology or deep invasion in squamous cell carcinomas) |
Tumors in immunosuppressed patients |
Tumors with involved borders or vague clinical margins, or incompletely excised tumors (positive histologic margins after resection) |
In critical sites such as the eyelids, nose, ears, lips, fingers, and genitalia, where tissue sparing is of paramount importance, tumors generally are treated best with Mohs micrographic surgery 3. The risk of recurrence and perineural invasion increases with tumor size 16 and the more simple therapeutic approaches often are ineffective for larger tumors. The size at which a tumor is considered at high risk of recurrence is relative to its location: a tumor of 6 mm or more in diameter on the central face is considered high risk, as is a tumor measuring 20 mm or more on the back (Table 1) 17. Tumors in some locations, such as the eyelids, nose, ears, or lips, are candidates for Mohs micrographic surgery regardless of size.
Tumors with aggressive histology include sclerosing patterned basal cell carcinomas, which are characterized by thin tumor strands disseminated throughout a fibrous stroma. A sclerosing pattern arises in basal cell carcinomas from three causes: spontaneous evolution, a previous surgical or destructive procedure, and radiation fibrosis. Other features of aggressive histology are perineural or perivascular invasion, areas of squamous differentiation within a basal cell carcinoma, and poor differentiation in a squamous cell carcinoma. Immunosuppressed patients tend to develop more aggressive tumors with higher recurrence rates, particularly squamous cell carcinomas 18. Mohs micrographic surgery is always a consideration in this population.
Inadequate margin control, another feature of high-risk tumors, implies that the physician is having trouble defining the tumor borders clinically or that the tumor has been excised and the margins are histologically positive.
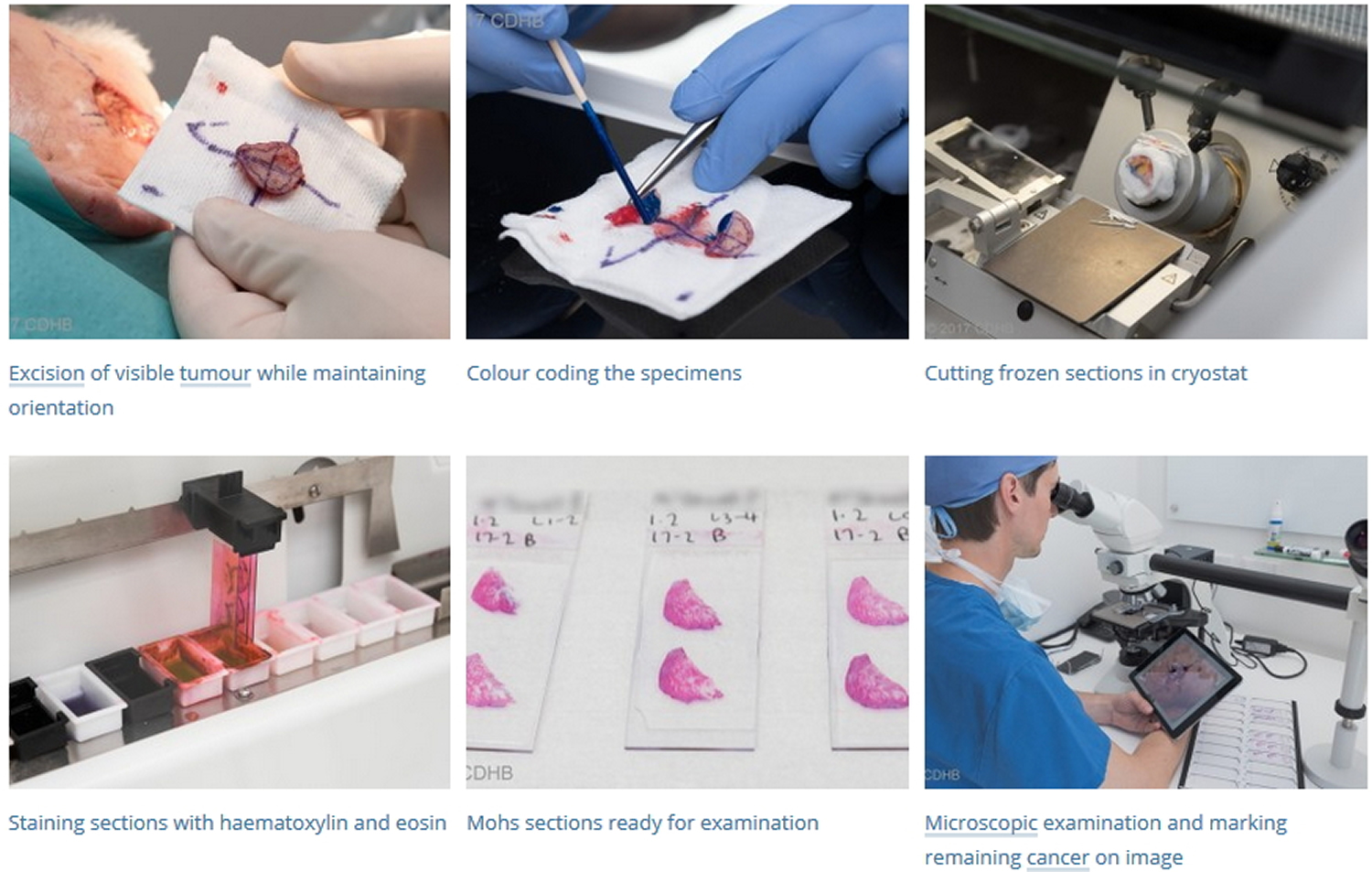
Figure 4. Mohs surgery for infiltrative basal cell carcinoma
[Source 19]Mohs surgery for melanoma
Not all doctors agree on using Mohs surgery for melanoma 20. Mohs micrographic surgery is not commonly used to treat melanoma because melanocytic atypia is difficult to assess with frozen sections 3. Permanent paraffin-embedded sections yield superior histologic resolution for assessment of melanocytic atypia 3.
Melanoma Skin Cancer Stages
After someone is diagnosed with melanoma, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics.
The earliest stage melanomas are called stage 0 (carcinoma in situ), and then range from stages I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
How is the melanoma stage determined?
The staging system most often used for melanoma is the American Joint Committee on Cancer (AJCC) TNM system, which is based on 3 key pieces of information:
The extent of the tumor (T): How deep has the cancer grown into the skin? Is the cancer ulcerated?
- Tumor thickness: The thickness of the melanoma is called the Breslow measurement. In general, melanomas less than 1 millimeter (mm) thick (about 1/25 of an inch) have a very small chance of spreading. As the melanoma becomes thicker, it has a greater chance of spreading.
- Ulceration: Ulceration is a breakdown of the skin over the melanoma. Melanomas that are ulcerated tend to have a worse outlook.
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or distant organs such as the lungs or brain?
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
The staging system in the table below uses the pathologic stage (also called the surgical stage). It is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away or at all, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests. The clinical stage will be used to help plan treatment. Sometimes, though, the cancer has spread further than the clinical stage estimates, and may not predict the patient’s outlook as accurately as a pathologic stage.
There are both clinical and pathologic staging systems for melanoma. Since most cancers are staged with the pathologic stage, we have included that staging system below. If your cancer has been clinically staged, it is best to talk to your doctor about your specific stage.
The table below is a simplified version of the TNM system. It is based on the most recent AJCC system, effective January 2018. It’s important to know that melanoma cancer staging can be complex. If you have any questions about the stage of your cancer or what it means, please ask your doctor to explain it to you in a way you understand.
Table 2. Melanoma Stage description
| AJCC Stage | Melanoma Stage description | |
| 0 | The cancer is confined to the epidermis, the outermost skin layer. It has not spread to nearby lymph nodes or distant sites. This stage is also known as melanoma in situ. | |
| I
| The cancer is no more than 2mm (2/25 of an inch) thick and might or might not be ulcerated. It has not spread to nearby lymph nodes or to distant sites. | |
| II | The cancer is at least 1.01 mm and may be thicker than 4.0 mm. It might or might not be ulcerated. It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
| |
| IIIA | The cancer is no more than 2.0 mm thick. It might or might not be ulcerated. It has spread to 3 or less lymph node(s), but it is so small that it is only seen under the microscope. It has not spread to distant sites. | |
| IIIB
| There is no sign of the primary cancer AND:
It has not spread to distant sites. | |
| OR | ||
The cancer is no more than 4.0 mm thick. It might or might not be ulcerated AND:
It has not spread to distant sites. | ||
| IIIC | There is no sign of the primary cancer AND:
It has not spread to distant sites. | |
| OR | ||
The cancer is no more than 4.0 mm thick. It might or might not be ulcerated AND:
It has not spread to distant sites. | ||
| OR | ||
The cancer is between 2.1 and 4.0mm OR thicker than 4.0 mm. It might or might not be ulcerated AND:
It has not spread to distant sites. | ||
| OR | ||
The cancer is thicker than 4.0 mm and is ulcerated AND:
It has not spread to distant sites. | ||
| IIID | The cancer is thicker than 4.0 mm and is ulcerated AND:
It has not spread to distant sites. | |
| IV | The cancer can be any thickness and might or might not be ulcerated. It might or might not have spread to nearby lymph nodes. It has spread to distant lymph nodes or organs such as the lungs, liver or brain. | |
Survival rates for melanoma 22
The following survival rates are based on nearly 60,000 patients who were part of the 2008 American Joint Committee on Cancer (AJCC) Melanoma Staging Database. These survival rates include some people diagnosed with melanoma who may have died later from other causes, such as heart disease. Therefore, the percentage of people surviving the melanoma itself may be higher.
- Stage IA: The 5-year survival rate is around 97%. The 10-year survival is around 95%.
- Stage IB: The 5-year survival rate is around 92%. The 10-year survival is around 86%.
- Stage IIA: The 5-year survival rate is around 81%. The 10-year survival is around 67%.
- Stage IIB: The 5-year survival rate is around 70%. The 10-year survival is around 57%.
- Stage IIC: The 5-year survival rate is around 53%. The 10-year survival is around 40%.
- Stage IIIA: The 5-year survival rate is around 78%. The 10-year survival is around 68%.*
- Stage IIIB: The 5-year survival rate is around 59%. The 10-year survival is around 43%.
- Stage IIIC: The 5-year survival rate is around 40%. The 10-year survival is around 24%.
- Stage IV: The 5-year survival rate is about 15% to 20%. The 10-year survival is about 10% to 15%. The outlook is better if the spread is only to distant parts of the skin or distant lymph nodes rather than to other organs, and if the blood level of lactate dehydrogenase (LDH) is normal.
*The survival rate is higher for stage IIIA cancers than for some stage II cancers. This is likely because the main (primary) tumor is often less advanced for IIIA cancers, although this is not clear.
- Remember, these survival rates are only estimates – they can’t predict what will happen to any individual. We understand that these statistics can be confusing and might lead you to have more questions. Talk to your doctor to better understand your specific situation.
Other factors affecting survival
Factors other than stage can also affect survival. For example:
- Older people generally have shorter survival times than younger people, regardless of stage.
- Melanoma is uncommon among African Americans, but when it does occur, survival times tend to be shorter than when it occurs in whites. Some studies have found that melanoma tends to be more serious if it occurs on the sole of the foot or palm of the hand, or if it is in a nail bed. (Cancers in these areas make up a larger portion of melanomas in African Americans than in whites.)
- People with melanoma who have weakened immune systems, such as people who have had organ transplants or who are infected with HIV, also are at greater risk of dying from their melanoma.
The National Comprehensive Cancer Network Guidelines for Melanoma Treatment 23
- Stage 0 (melanoma in-situ) and Stage 1A (<0.8mm thick, no ulceration): Wide excision + follow up care
- Wide excision: Is a fairly minor operation will cure most thin melanomas. Local anesthesia is injected into the area to numb it before the excision. The site of the tumor is then cut out, along with a small amount of normal skin at the edges. The normal, healthy skin around the edges of the cancer is called the margin. The wound is carefully stitched back together afterward. This will leave a scar. The removed sample is then viewed with a microscope to make sure that no cancer cells were left behind at the edges of the skin that was removed. Wide excision differs from an excisional biopsy. The margins are wider because the diagnosis is already known. The recommended margins vary depending on the thickness of the tumor. Thicker tumors need larger margins (both at the edges and in the depth of the excision). The margins can also vary based on where the melanoma is on the body and other factors. For example, if the melanoma is on the face, the margins may be smaller to avoid large scars or other problems. Smaller margins might increase the risk of the cancer coming back, so be sure to discuss the options with your doctor.
- Stage 1B (T1b [<0.8mm thick with ulceration OR 0.8-1.0mm thick +/- ulceration): Wide excision + follow up care OR Wide excision with sentinel lymph node biopsy + follow up care
- Stage 1B (T2a) or Stage 2 [>1.0mm thick, any feature, N0): Wide excision + follow up care OR Wide excision with sentinel lymph node biopsy + interferon alpha + follow up care
Treating stage 0 melanoma
Stage 0 melanomas have not grown deeper than the top layer of the skin (the epidermis). They are usually treated by surgery (wide excision) to remove the melanoma and a small margin of normal skin around it. The removed sample is then sent to a lab to be looked at with a microscope. If cancer cells are seen at the edges of the sample, a repeat excision of the area may be done.
Some doctors may consider the use of imiquimod cream (Zyclara) or radiation therapy instead of surgery, although not all doctors agree with this.
For melanomas in sensitive areas on the face, some doctors may use Mohs surgery or even imiquimod cream if surgery might be disfiguring, although not all doctors agree with these uses.
Treating stage I melanoma
Stage I melanoma is treated by wide excision (surgery to remove the melanoma as well as a margin of normal skin around it). The margin of normal skin removed depends on the thickness and location of the melanoma.
Some doctors may recommend a sentinel lymph node biopsy, especially if the melanoma is stage IB or has other characteristics that make it more likely to have spread to the lymph nodes. You and your doctor should discuss this option.
If cancer cells are found on the sentinel lymph node biopsy, a lymph node dissection (removal of all lymph nodes near the cancer) is often recommended, but it’s not clear if this improves survival. Some doctors may recommend adjuvant (additional) treatment with interferon after the lymph node surgery. Other drugs or perhaps vaccines might be options as part of a clinical trial to try to lower the chance the melanoma will come back.
Treating stage II melanoma
Wide excision (surgery to remove the melanoma and a margin of normal skin around it) is the standard treatment for stage II melanoma. The amount of normal skin removed depends on the thickness and location of the melanoma.
Because the melanoma may have spread to lymph nodes near the melanoma, many doctors recommend a sentinel lymph node biopsy as well. This is an option that you and your doctor should discuss. If it is done and the sentinel node contains cancer cells, then a lymph node dissection (where all the lymph nodes in that area are surgically removed) will probably be done at a later date.
For some patients (such as those with lymph nodes containing cancer), doctors may advise treatment with interferon after surgery (adjuvant therapy). Other drugs or perhaps vaccines may also be recommended as part of a clinical trial to try to lower the chance the melanoma will come back.
Treating stage III melanoma
These cancers have already reached the lymph nodes when the melanoma is first diagnosed. Surgical treatment for stage III melanoma usually requires wide excision of the primary tumor as in earlier stages, along with lymph node dissection.
After surgery, adjuvant treatment with immunotherapy (such as nivolumab [Opdivo], ipilimumab [Yervoy], or interferon) or targeted therapy (for cancers with BRAF gene changes) may help lower the risk of the melanoma coming back. Other drugs or perhaps vaccines may also be recommended as part of a clinical trial to try to reduce the chance the melanoma will come back. Another option is to give radiation therapy to the areas where the lymph nodes were removed, especially if many of the nodes contain cancer.
If melanomas are found in nearby lymph vessels in or just under the skin (known as in-transit tumors), they should all be removed, if possible. Other options include injections of the T-VEC vaccine (Imlygic), Bacille Calmette-Guerin (BCG) vaccine, interferon, or interleukin-2 (IL-2) directly into the melanoma; radiation therapy; or applying imiquimod cream. For melanomas on an arm or leg, another option might be isolated limb perfusion (infusing the limb with a heated solution of chemotherapy). Other possible treatments might include targeted therapy, immunotherapy, chemotherapy, or a combination of immunotherapy and chemotherapy (biochemotherapy).
Some patients might benefit from newer treatments being tested in clinical trials. Many patients with stage III melanoma might not be cured with current treatments, so they may want to think about taking part in a clinical trial.
Treating stage IV melanoma
Stage IV melanomas are often hard to cure, as they have already spread to distant lymph nodes or other areas of the body. Skin tumors or enlarged lymph nodes causing symptoms can often be removed by surgery or treated with radiation therapy.
Metastases in internal organs are sometimes removed, depending on how many there are, where they are, and how likely they are to cause symptoms. Metastases that cause symptoms but cannot be removed may be treated with radiation, immunotherapy, targeted therapy, or chemotherapy.
The treatment of widespread melanomas has changed in recent years as newer forms of immunotherapy and targeted drugs have been shown to be more effective than chemotherapy.
Immunotherapy drugs called checkpoint inhibitors such as pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy) have been shown to help some people with advanced melanoma live longer. These drugs can sometimes have serious side effects, so patients who get them need to be watched closely. Other types of immunotherapy might also help, but these are only available through clinical trials.
In about half of all melanomas, the cancer cells have changes in the BRAF gene. If this gene change is found, treatment with newer targeted therapy drugs such as vemurafenib (Zelboraf), dabrafenib (Tafinlar), trametinib (Mekinist), and cobimetinib (Cotellic) might be helpful. They might be tried before or after the newer immunotherapy drugs, but they aren’t used at the same time. Like the checkpoint inhibitors, these drugs can help some people live longer, although they haven’t been shown to cure these melanomas.
A small portion of melanomas have changes in the C-KIT gene. These melanomas might be helped by targeted drugs such as imatinib (Gleevec) and nilotinib (Tasigna), although, again, these drugs aren’t known to cure these melanomas.
Immunotherapy using interferon or interleukin-2 can help a small number of people with stage IV melanoma live longer. Higher doses of these drugs seem to be more effective, but they can also have more severe side effects, so they might need to be given in the hospital.
Chemotherapy can help some people with stage IV melanoma, but other treatments are usually tried first. Dacarbazine (DTIC) and temozolomide (Temodar) are the chemo drugs used most often, either by themselves or combined with other drugs. Even when chemotherapy shrinks these cancers, the cancer usually starts growing again within several months.
Some doctors may recommend biochemotherapy, which is a combination of chemotherapy and either interleukin-2, interferon, or both. This can often shrink tumors, which might make patients feel better, although it has not been shown to help patients live longer.
It’s important to carefully consider the possible benefits and side effects of any recommended treatment before starting it.
Because stage IV melanoma is hard to cure with current treatments, patients may want to think about taking part in a clinical trial. Many studies are now looking at new targeted drugs, immunotherapies, chemotherapy drugs, and combinations of different types of treatments.
Even though stage IV melanoma is often hard to cure, a small portion of people respond very well to treatment and survive for many years after diagnosis.
Treating recurrent melanoma
Treatment of melanoma that comes back after initial treatment depends on the stage of the original melanoma, what treatments a person has already had, where the melanoma comes back, and other factors.
Melanoma might come back in the skin near the site of the original tumor, sometimes even in the scar from the surgery. In general, these local (skin) recurrences are treated with surgery similar to what would be recommended for a primary melanoma. This might include a sentinel lymph node biopsy. Depending on the thickness and location of the tumor, other treatments may be considered, such as isolated limb perfusion chemotherapy; radiation therapy; or local immunotherapy treatments such as tumor injection with the T-VEC vaccine (Imlygic), BCG vaccine, interferon, or interleukin-2. Systemic treatments such as immunotherapy, targeted therapy, or chemotherapy might also be options.
If nearby lymph nodes weren’t removed during the initial treatment, the melanoma might come back in these lymph nodes. Lymph node recurrence is treated by lymph node dissection if it can be done, sometimes followed by treatments such as interferon or radiation therapy. If surgery is not an option, radiation therapy or systemic treatment (immunotherapy, targeted therapy, or chemo) can be used.
Melanoma can also come back in distant parts of the body. Almost any organ can be affected. Most often, the melanoma will come back in the lungs, bones, liver, or brain. Treatment for these recurrences is generally the same as for stage IV melanoma (see above). Melanomas that recur on an arm or leg may be treated with isolated limb perfusion chemotherapy.
Melanoma that comes back in the brain can be hard to treat. Single tumors can sometimes be removed by surgery. Radiation therapy to the brain (stereotactic radiosurgery or whole brain radiation therapy) may help as well. Systemic treatments (immunotherapy, targeted therapy, or chemo) might also be tried.
As with other stages of melanoma, people with recurrent melanoma may want to think about taking part in a clinical trial.
- Prickett KA, Ramsey ML. Mohs Micrographic Surgery. [Updated 2017 Oct 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441833[↩][↩][↩]
- Mohs FE. Chemosurgery: a microscopically controlled method of cancer excision. Arch Surg. 1941;42:279–95.[↩]
- Mohs Micrographic Surgery. Am Fam Physician. 2005 Sep 1;72(5):845-848. https://www.aafp.org/afp/2005/0901/p845.html[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kovich O, Otley CC. Thrombotic complications related to discontinuation of warfarin and aspirin therapy perioperatively for cutaneous operation. J Am Acad Dermatol. 2003;48:233–7.[↩]
- Griego RD, Zitelli JA. Intra-incisional prophylactic antibiotics for dermatologic surgery. Arch Dermatol. 1998;134:688–92.[↩]
- Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177–83.[↩]
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–28.[↩]
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5 pt 1):698–703.[↩]
- Van Loo, Mosterd K, Krekels GA. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face. Eur J Cancer. 2014; 50(17):3011-20. https://www.ejcancer.com/article/S0959-8049(14)00913-7/fulltext[↩]
- Kauvar AN, Cronin T Jr, Roenigk R. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015; 41(5):550-71. https://www.ncbi.nlm.nih.gov/pubmed/25868035[↩]
- Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012 Apr;38(4):585-94. doi: 10.1111/j.1524-4725.2012.02341.x. Epub 2012 Mar 22. https://www.ncbi.nlm.nih.gov/pubmed/22443180[↩][↩]
- Ad Hoc Task Force1 Connolly SM, Baker DR. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2012; 67(4):531-50. https://www.jaad.org/article/S0190-9622(12)00667-6/fulltext[↩]
- Möhrle M, Breuninger H. The Muffin technique-an alternative to Mohs’ micrographic surgery. J Dtsch Dermatol Ges. 2006; 4: 1080-4. http://esms-mohs.cap-partner.eu/skin-cancer-information/treatments/tuebingen-torte-and-muffin-technique/[↩]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001 [published correction appears in CA Cancer J Clin 2001;51:144]. CA Cancer J Clin. 2001;51:15–36.[↩]
- Shriner DL, McCoy DK, Goldberg DJ, Wagner RF Jr. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79–97.[↩]
- Lang PG Jr, Osguthorpe JD. Indications and limitations of Mohs micrographic surgery. Dermatol Clin. 1989;7:627–44.[↩]
- The complete library of NCCN clinical practice guidelines in oncology. Rockledge, Pa.: National Comprehensive Cancer Network[↩]
- Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004;77:574–9.[↩]
- Mohs surgery images. https://www.dermnetnz.org/topics/mohs-surgery-images/[↩]
- Surgery for Melanoma Skin Cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/treating/surgery.html[↩]
- Melanoma Skin Cancer Stages. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/melanoma-skin-cancer-stages.html[↩]
- Survival Rates for Melanoma Skin Cancer, by Stage. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html[↩]
- National Comprehensive Cancer Network https://www.nccn.org/patients/guidelines/melanoma/index.html[↩]