What is molybdenum
Molybdenum is a heavy metallic element found naturally throughout the environment and is also used by industries to manufacture a wide range of common products. Molybdenum is widely distributed in nature, the abundance in the earth‟s crust being about 1-1.5 mg molybdenum/kg 1. Molybdenum is ubiquitous in food and water as soluble molybdates (Mo(VI)O42-). Molybdenum is required as a component of enzymes involved in the catabolism of sulphur amino acids and heterocyclic compounds, as well as in the metabolism of aromatic aldehydes. Because of its role in metabolism, molybdenum is considered an essential dietary element for mammals, though clinical signs of dietary molybdenum deficiency in otherwise healthy humans have not been described 2. Molybdenum is a refractory metallic element used principally as an alloying agent in steel, cast iron, and superalloys to enhance hardenability, strength, toughness, and wear and corrosion resistance 3. To achieve desired metallurgical properties, molybdenum, primarily in the form of molybdic oxide or ferromolybdenum, is frequently used in combination with or added to chromium, manganese, niobium, nickel, tungsten, or other alloy metals. The versatility of molybdenum in enhancing a variety of alloy properties has ensured it a significant role in contemporary industrial technology, which increasingly requires materials that are serviceable under high stress, expanded temperature ranges, and highly corrosive environments. Moreover, molybdenum finds significant usage as a refractory metal in numerous chemical applications, including catalysts, lubricants, and pigments 3. There is little substitution for molybdenum in its major application in steels and cast irons. In fact, because of the availability and versatility of molybdenum, industry has sought to develop new materials that benefit from its alloying properties.
In humans, molybdenum is also an essential trace element, being a component of the enzymes xanthine oxidoreductase, sulphite oxidase, aldehyde oxidase, nitrate reductase and mitochondrial amidoxime reducing component require molybdenum linked with a pterin (molybdopterin) as the cofactor 4. These enzymes are involved in the metabolism of aromatic aldehydes and the catabolism of sulphur-containing amino acids and heterocyclic compounds, including purines, pyrimidines, pteridins and pyridines.
Molybdenum-containing enzymes catalyse redox reactions and are found in many plants and animal organisms. As a consequence of the easy interconvertibility of different oxidation states (Mo4+/Mo6+), molybdenum-containing enzymes have the ability to provide electron transfer pathways. In addition to molybdenum, they also contain other prosthetic groups such as flavin adenine dinucleotide or haeme 5.
Molybdenum cofactor is synthesized in the cytosol by a conserved biosynthetic pathway that can be divided into four main steps. In the final step of molybdenum cofactor biosynthesis, a single molybdenum ion is bound to one or two molybdopterin dithiolates. After completion of biosynthesis, mature molybdenum cofactor has to be inserted into molybdoenzymes. A molybdenum cofactor carrier protein has been described in the green alga Chlamydomonas rheinhardtii, but information is lacking for other eukaryotes 6. The formation of active molybdoenzymes depends not only on the availability of molybdenum but also on the presence of iron, zinc and copper 7.
The US Institute of Medicine 8 derived an average requirement based on a molybdenum balance study with four young males by Turnlund et al. 9. Average molybdenum balance was achieved with an intake of 22 μg/day, and no clinical signs of deficiency or biochemical changes associated with molybdenum deficiency were observed. The average minimum molybdenum requirement for maintaining adequate molybdenum status was estimated to be 22 μg/day, to which an additional 3 μg/day was added to allow for miscellaneous losses. In addition, it was assumed that molybdenum bioavailability from some diets may be lower than from the diet provided in the study. Thus, an average bioavailability of 75 % was used to set an Estimated Average Requirement (EAR) of 34 μg/day. Because of the use of only two different molybdenum intake levels and the small size of the study, US Institute of Medicine 8 used a coefficient of variation of 15 % and derived a Recommended Dietary Allowance (RDA) of 45 μg/day as the Estimated Average Requirement (EAR) plus twice the coefficient of variation to cover the needs of 97 to 98 % of the individuals in the group. As no data on which to base an Estimated Average Requirement (EAR) were found for women or older adults, the same values were given for these population groups 8.
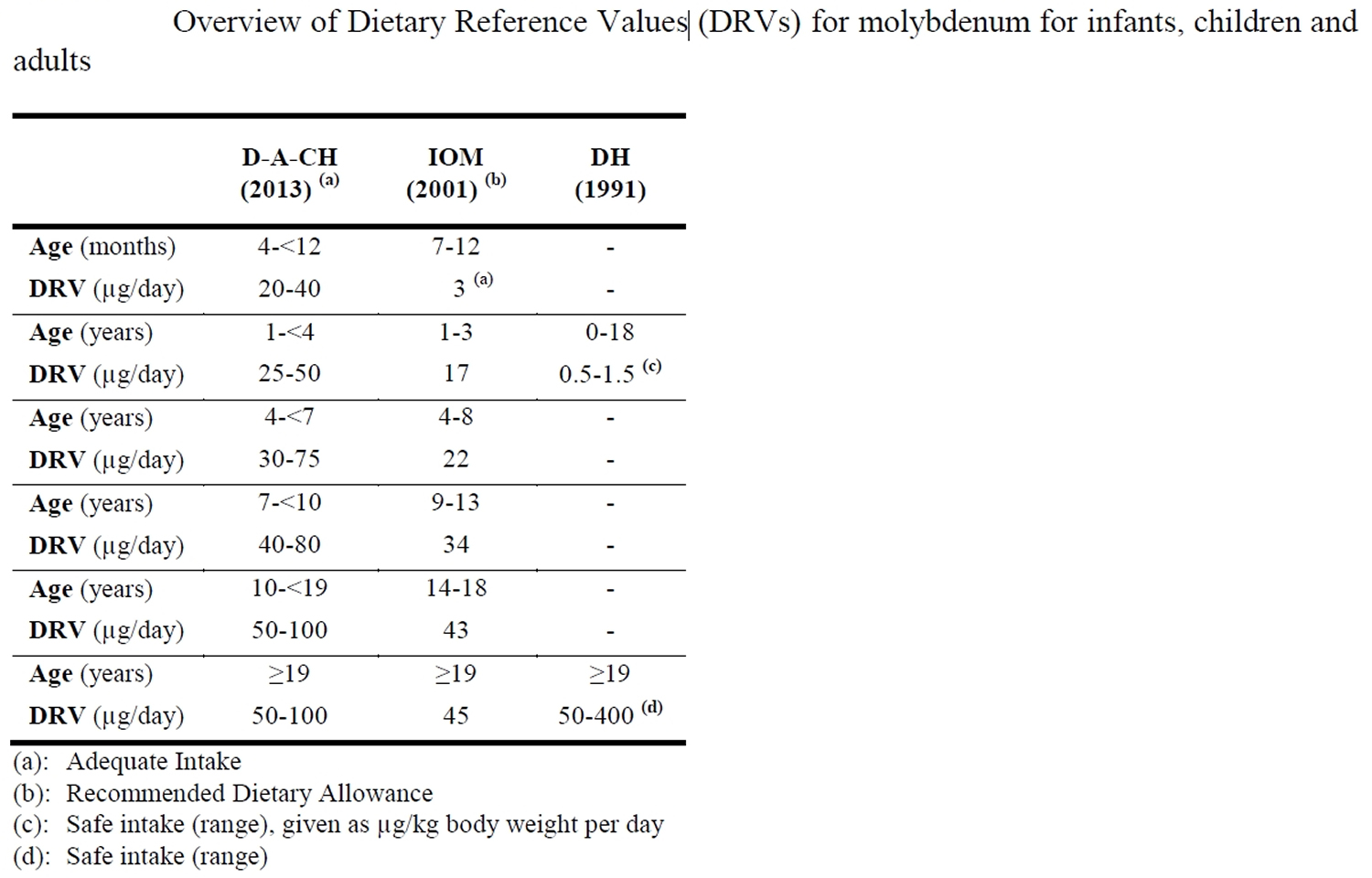
Table 1. Molybdenum dietary reference values
Abbreviations: COMA = UK Committee on Medical Aspects of Food Policy; IOM = US Institute of Medicine; DACH = German-speaking countries
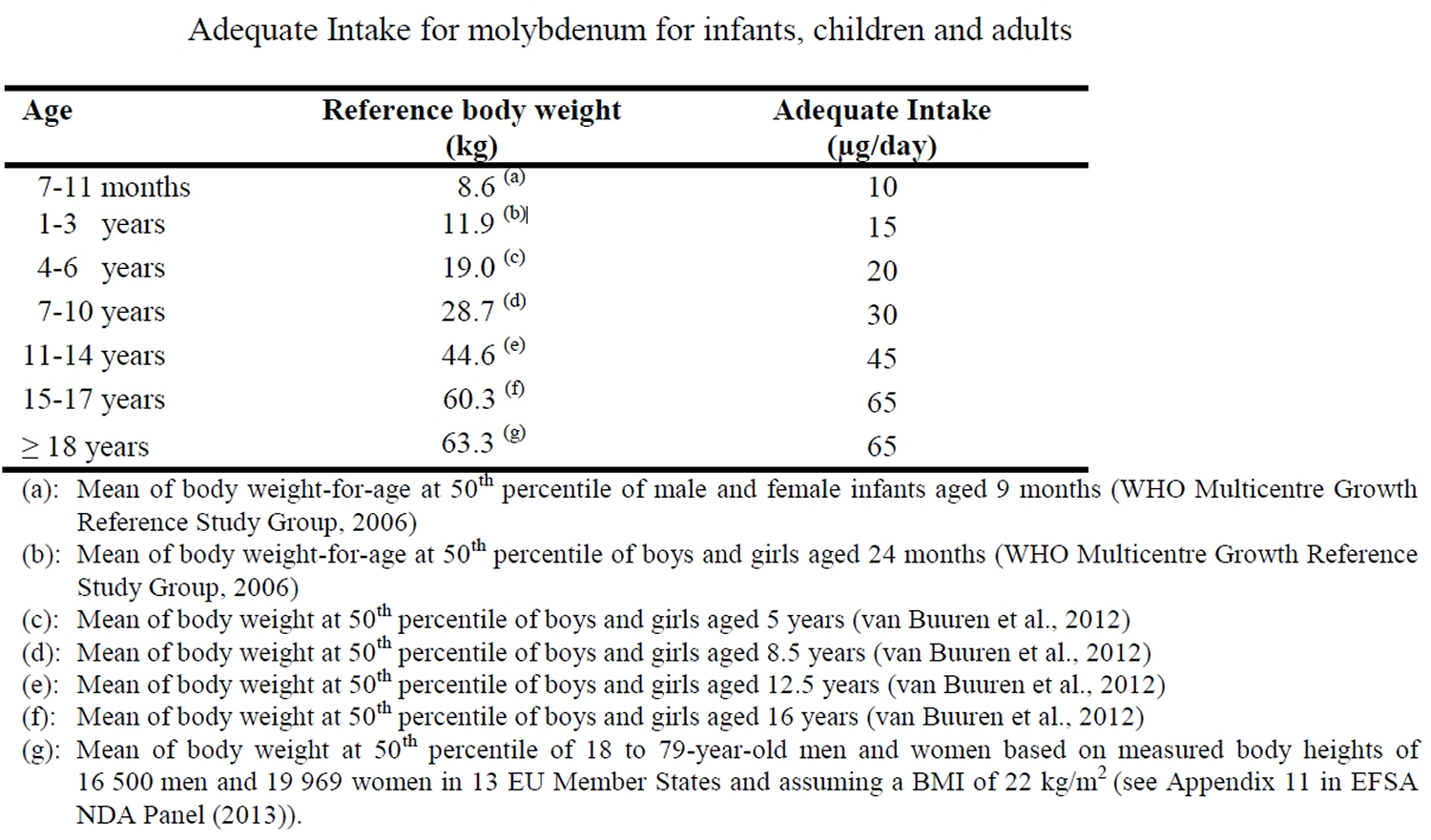
[Source 2]Table 2. Molybdenum adequate intake
The Scientific Committee on Food has set a Tolerable Upper Intake Level (UL) based on adverse effects of molybdenum on reproduction, particularly fetal development, shown in studies with rats and mice, from which a No Observed Adverse Effect Level (NOAEL) of 0.9 mg/kg body weight per day was derived. Using an uncertainty factor of 100, a UL of 0.01 mg/kg body weight per day, equivalent to 0.6 mg/day, was derived for adults, including pregnant and lactating women. For children from one year of age onwards, the UL was extrapolated from the adult UL on a body weight basis, and was set at between 0.1 and 0.5 mg/day 10.
Molybdenum is relatively nontoxic, although high levels may be a cause of high uric acid levels and an increased incidence of gout. Liver toxicity from molybdenum has not been described 11. However,
Molybdenum foods
Molybdenum is present in nearly all foods in trace amounts as soluble molybdates.
Foods high in molybdenum are:
- Pulses, cereal grains and grain products, offal (liver, kidney) and nuts
- Organ meats, whole grains, green leafy vegetables, milk, beans
The molybdenum content in plant-based foods varies greatly and depends on the properties of the soil where the foods are grown; molybdenum uptake by plants is promoted by neutral or alkaline soils 12. Molybdenum concentrations in drinking water are usually below 10 μg/L, although concentrations as high as 200 μg/L have been reported in areas near mining sites 13. Currently, potassium molybdate (molybdenum VI) may be added to food supplements 14, whereas ammonium molybdate (molybdenum VI) and sodium molybdate (molybdenum VI) may be added to both foods8 and food supplements 14.
Cereals and cereal-based products including bread are the major food contributors to the dietary molybdenum intake of adults 2. Mean molybdenum intakes, as assessed in duplicate diet or food portion studies, total diet studies and market basket studies, vary over a wide range, i.e. 58 μg/day to 157 μg/day, for adults in various European countries. Mean intakes are at or above 100 μg/day in five of the eight European countries for which data are available. Molybdenum intakes of children are only available from two European countries.
Infant and follow-on formula: In a report on the essential requirements of infant and follow-on formulae, the SCF did not define a minimum or maximum content of molybdenum for either type of formulae 15. Compared to mature human milk, cow‟s milk has a higher molybdenum concentration [34 μg/kg as reported by Rose et al. 16, mean of 46 μg/kg as reported by Anses 17]. Hence, the molybdenum content of cow’s milk based-infant formula is higher compared to mature human milk. For 81 powdered cow’s milk-based or soy-based infant formulae from the US and Canada, molybdenum concentrations ranged from 15.4 to 80.3 μg/L (mean ± SE, 37.7 ± 1.7 μg/L) 18.
Water-soluble molybdates are efficiently and rapidly absorbed from the digestive tract at a wide range of intakes, and the body is able to adapt to this wide intake range by regulating excretion via the urine. At doses up to about 1 mg, molybdenum dissolved in water is completely absorbed into the systemic circulation. Molybdenum absorption in the presence of solid foods (cress, green salad, tomatoes, bean soup) is lower compared to administration with water 19. When added to a beverage containing starch, dextrimaltose, oil, sucrose, α-cellulose and minerals, the absorption efficiency of increasing doses of molybdenum ranging from 24 to 1 378 μg was between 90 and 94 % in healthy men 20. Black tea has been shown to considerably reduce molybdenum absorption upon ingestion of relatively high amounts of molybdenum (0.5-1 mg as a single dose of stable isotope) 19. In ten premature infants, absorption of the stable isotope molybdenum from infant formula was 97.5 % (96.3-99.1 %) after receiving 25 μg molybdenum/kg body weight 21.
Studies using kale or soy intrinsically labeled with stable isotopes of molybdenum have shown that molybdenum absorption was 86.1 % and 56.7 %, respectively, from meals with either kale or soy casseroles containing about 100 μg molybdenum. Molybdenum absorption from an extrinsic label also added to the meals was 87.5 %. When the molybdenum content of the meal was increased to about 310 μg in a subsequent study, molybdenum absorption from soy amounted to 58.3 %, and molybdenum absorption from the extrinsic label was 92.8% 22.
Using a compartmental model based on a molybdenum depletion-repletion study in four men, the mean bioavailability of molybdenum from the experimental diet was predicted to be 76 % 23. A slightly higher bioavailability of 83 % for food-bound molybdenum was predicted with the compartmental model, based on a study which gave the same three-day rotating diet regimen but with five different molybdenum contents consecutively for 24 days each to four men 20.
Little is known about the mechanism of molybdenum absorption and the site of absorption in the gastrointestinal tract. In animals, molybdenum (VI) but not molybdenum (IV) is readily absorbed from the duodenum and proximal jejunum 10. Recently, a family of proteins probably related to molybdate transport in animals and humans has been described, though the exact location of this high-affinity transporter within the cell has not yet been identified 24. It is assumed that in addition to a possible high-affinity uptake system, molybdate may also enter the cell nonspecifically through the sulphate uptake system, which has been shown to be present in plants 25.
Tungsten is known to inhibit molybdenum uptake, and this inhibitory effect has been used in animal studies to induce molybdenum deficiency, but it is not considered relevant for humans because of the rare occurrence of tungsten in the environment and consequently in the food chain 26. In sheep and rats, high sulphate intakes have been shown to inhibit molybdenum absorption, suggesting that both sulphate and molybdenum share a common transport mechanism 26. An interaction with copper has been observed leading to copper deficiency in sheep exposed to high molybdenum intake. In ruminants, excessive intakes of molybdenum lead to formation of thiomolybdate in the sulphide-rich environment of the rumen; thiomolybdate (a molecule where sulphur groups surround a molybdenum centre) is a chelator of copper ions, thereby inhibiting copper absorption 27. By contrast, in humans, clinical symptoms of copper deficiency are largely confined to individuals with rare genetic defects in copper metabolism 28. In four adult males on two sorghum diets providing daily 2.4 mg of copper and 166 μg or 540 μg of molybdenum, respectively, fecal copper excretion was comparable and apparent copper absorption unaffected by molybdenum intake 29.
The highest molybdenum concentrations are found in the liver and kidney. In adults, the liver contains 1.3-2.9 mg molybdenum/kg dry matter, the kidney 1.6 mg/kg dry matter, the lung 0.15 mg/kg dry matter, the brain and muscle 0.14 mg/kg dry matter 12, and for hair concentrations of 0.03 mg/kg (Ochi et al., 2011) have been reported. Total body molybdenum of a “standard man” was calculated to be about 2.3 mg after analysis of tissues from 150 accidental deaths 30, and about 2.2 mg with the use of a compartmental model and fractional transfer coefficients observed at a molybdenum intake of 121 μg/day given for 24 days, and which was considered to be in line with the habitual molybdenum intake of participants prior to the study 20.
Storage of molybdenum in mammals is low, and most tissue molybdenum is thought to be associated with molybdoenzymes, as indicated by the reported absence of detectable molybdenum in the liver tissue of molybdenum cofactor-deficient patients 5. In the liver of fetuses (age: 23 weeks of gestation to term), molybdenum concentrations were more than seven-fold lower compared to adults 31, and such differences have subsequently been interpreted as the absence of molybdenum stores and a low fetal molybdenum requirement 18.
In order to fulfill its biological role, molybdenum must enter the cell and be assembled into a molybdenum cofactor. In eukaryotes, the molybdate transport process and the proteins involved are not fully understood 7.
There are no suitable biomarkers of molybdenum status. Biochemical changes observed in subjects with molybdopterin cofactor deficiency, a genetic disorder, or in the one subject reported with possible molybdenum deficiency, have not been observed in healthy individuals on varying levels of molybdenum intake. Low activity of molybdoenzymes in tissues, or changes in substrate/product relationships, are considered as insufficiently specific to be used as biomarkers of status.
In 1993, the Scientific Committee for Food did not publish Dietary Reference Values for molybdenum. More recently, other authorities have set Dietary Reference Values for molybdenum and these are based on the maintenance of molybdenum homeostasis as measured in balance studies, taking into account molybdenum bioavailability from various food sources, or are based on observed molybdenum intakes with a mixed diet.
Various balance studies have been performed to establish molybdenum requirements. However, only one balance study in adults was considered to be of sufficient duration, and was performed with a constant diet and under controlled conditions. In this study carried out in four men, balance was reported to be near zero from day 49 until day 102 of the depletion period when intakes were as low as 22 μg/day 2. Biochemical changes or symptoms suggestive of molybdenum deficiency were not observed and the possibility that humans may be able to achieve molybdenum balance at even lower intakes cannot be excluded 2. Results of two balance studies with some methodological limitations were reported in children, but these studies cannot be used to derive an average molybdenum requirement for children. Data on molybdenum intakes and health outcomes were unavailable for the setting of DRVs for molybdenum.
As the evidence to derive an Average Requirement and thus a Population Reference Intake, was considered insufficient, an Adequate Intake (AI) is proposed. An Adequate Intake (AI) of 65 μg/day is proposed for adult men and women based on mean molybdenum intakes at the lower end of the wide range of observed intakes from mixed diets in Europe 2. Given the scarcity of data on molybdenum intakes in pregnant and lactating women, it is suggested that the adult Adequate Intake (AI) also applies to pregnant and lactating women 2. For infants from seven months and children, it was decided that an Average Requirement could not be established, and an Adequate Intake (AI) is proposed based on extrapolation from the adult AI using isometric scaling and reference body weights of the respective age groups. The respective AIs vary between 10 μg/day in infants aged 7-11 months and 65 μg/day in adolescent boys and girls 2.
Molybdenum deficiency
Molybdenum is found in many foods and deficiencies are rare. Molybdenum deficiency has been described in animals and molybdenum deficiency in otherwise healthy humans has not been observed and there are no biomarkers of molybdenum status 2. Various metabolic balance studies have been performed to establish molybdenum requirements.
In humans, only a single case report of a syndrome suggestive of dietary molybdenum deficiency in a patient on total parenteral nutrition for several months has been reported 2. A 24-year-old male patient with Crohn’s disease and short bowel syndrome was on total parenteral nutrition (TPN) lacking in molybdenum for 12 months, at which point he developed a syndrome characterized by tachycardia, tachypnea, severe headache, nausea and vomiting, night blindness, and central scotomas, which progressed to edema, lethargy, disorientation and coma 2. These symptoms were associated with high plasma methionine and low serum uric acid concentrations, as well as reduced urinary concentrations of sulphate, thiosulphate, and uric acid. Whilst modification of the total parenteral nutrition solution by lowering the sulphur load was ineffective, treatment with ammonium molybdate (300 μg/day) resulted in considerable improvement of the clinical symptoms and progressive reversal of the biochemical abnormalities within 30 days 32. Clinical signs of molybdenum deficiency in otherwise healthy humans have not been observed 2.
A distinct molybdenum deficiency syndrome has not been observed in animals when subjected to molybdenum restriction, despite considerable reduction in the activity of molybdoenzymes.
Molybdenum cofactor deficiency 33, a rare autosomal recessive syndrome with a defective hepatic synthesis of molybdenum cofactor, results in deficiency of all molybdoenzymes in humans. This genetic defect, for which three subtypes are known according to the gene affected, has been found in a variety of ethnic groups and all over the world 4. Molybdenum cofactor deficiency is characterized by brain dysfunction (encephalopathy) that worsens over time and usually death at an early age 33. Babies with this condition appear normal at birth, but within a week they have feeding difficulties and develop seizures that do not improve with treatment (intractable seizures). Brain abnormalities, including deterioration (atrophy) of brain tissue, lead to severe developmental delay; affected individuals usually do not learn to sit unassisted or to speak. A small percentage of affected individuals have an exaggerated startle reaction (hyperekplexia) to unexpected stimuli such as loud noises. Other features of molybdenum cofactor deficiency can include a small head size (microcephaly) and facial features that are described as “coarse.” The successful treatment of one affected child with molybdenum cofactor deficiency type A using the first detectable intermediate substance in the biosynthesis pathway of molybdenum cofactor has recently been reported 24. In untreated patients, tests reveal that affected individuals have high levels of chemicals called sulfite, S-sulfocysteine, xanthine, and hypoxanthine in the urine and low levels of a chemical called uric acid in the blood. Because of the serious health problems caused by molybdenum cofactor deficiency, affected individuals usually do not survive past early childhood.
Acute molybdenum toxicity data
Animal data: No fatalities were reported among animals that ingested amounts of molybdenum disulfide in doses as great as 6,000 mg molybdenum/kg 34. No changes were observed in rats over a 4-week period following inhalation exposures to metallic molybdenum at 25,000 to 30,000 mg/m3 or to molybdenum dioxide at 10,000 to 12,000 mg/m3 for 1 hour 35.
Human data: Mining and metallurgy workers chronically exposed to 60 to 600 mg molybdenum/m3 reported an increased incidence of nonspecific symptoms that included weakness, fatigue, headache, anorexia, and joint and muscle pain 36.
Immediately Dangerous to Life or Health Concentrations: 5,000 mg molybdenum/m3
The available toxicological data contain no evidence that an acute exposure to a high concentration of insoluble molybdenum compounds would impede escape or cause any irreversible health effects within 30 minutes. However, the revised Immediately Dangerous to Life or Health Concentrations for insoluble molybdenum compounds is 5,000 mg Mo/m3 based on being 500 times the Occupational Safety and Health Administration permissible exposure limit (legal limit in the United States for exposure of an employee to a chemical substance) of 10 mg molybdenum/m3 (500 is an assigned protection factor for respirators and was used arbitrarily during the Standards Completion Program for deciding when the “most protective” respirators should be used for particulates).
- Eckhert CD, 2006. Other trace elements. In: Modern nutrition in health and disease. Eds Shils ME, Shike M, Ross AC, Caballero B, Cousins R. Lippincott Williams & Wilkins, Philadelphia, USA, 338-350. [↩]
- Scientific Opinion on Dietary Reference Values for molybdenum. EFSA Journal 2013;11(8):3333. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2013.3333[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Molybdenum https://minerals.usgs.gov/minerals/pubs/commodity/molybdenum[↩][↩]
- Reiss J and Hahnewald R, 2011. Molybdenum cofactor deficiency: Mutations in GPHN, MOCS1, and MOCS2. Human Mutation, 32, 10-18.[↩][↩]
- Rajagopalan KV, 1988. Molybdenum: an essential trace element in human nutrition. Annual Review of Nutrition, 8, 401-427.[↩][↩]
- Llamas A, Otte T, Multhaup G, Mendel RR and Schwarz G, 2006. The Mechanism of nucleotide-assisted molybdenum insertion into molybdopterin. A novel route toward metal cofactor assembly. Journal of Biological Chemistry, 281, 18343-18350.[↩]
- Llamas A, Tejada-Jimenez M, Fernandez E and Galvan A, 2011. Molybdenum metabolism in the alga Chlamydomonas stands at the crossroad of those in Arabidopsis and humans. Metallomics, 3, 578-590.[↩][↩]
- Institute of Medicine, 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press, Washington, D.C., USA, 797 pp.[↩][↩][↩]
- Turnlund JR, Keyes WR, Peiffer GL and Chiang G, 1995a. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men during depletion and repletion. American Journal of Clinical Nutrition, 61, 1102-1109.[↩]
- Scientific Committee on Food, 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of molybdenum. 15 pp.[↩][↩]
- TRACE ELEMENTS AND METALS. https://livertox.nlm.nih.gov/TraceElementsAndMetals.htm[↩]
- World Health Organization, 1996. Trace elements in human nutrition and health. 343 pp.[↩][↩]
- World Health Organization, 2008. Guidelines for drinking-water quality. Volume 1: Recommendations, 410 pp.[↩]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements, OJ L 183, 12.7.2002, p. 51.[↩][↩]
- Scientific Committee on Food, 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-on Formulae. 211 pp.[↩]
- Rose M, Baxter M, Brereton N and Baskaran C, 2010. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Additives and Contaminants: Part A, 27, 1380-1404.[↩]
- Anses (Agence nationale de sécurité sanitaire de l‟alimentation, de l‟environnement et du travail), 2011. Étude de l‟alimentation totale française 2 (EAT 2). Tome 1: Contaminants inorganiques, minéraux, polluants organiques persistants, mycotoxines, phyto-estrogènes. 305 pp.[↩]
- Abramovich M, Miller A, Yang H and Friel JK, 2011. Molybdenum content of Canadian and US infant formulas. Biological Trace Element Research, 143, 844-853.[↩][↩]
- Giussani A, Cantone MC, Hollriegl V, Oeh U, Tavola F and Veronese I, 2007. Modelling urinary excretion of molybdenum after oral and intravenous administration of stable tracers. Radiation Protection Dosimetry, 127, 136-139.[↩][↩]
- Novotny JA and Turnlund JR, 2007. Molybdenum intake influences molybdenum kinetics in men. Journal of Nutrition, 137, 37-42.[↩][↩][↩]
- Sievers E, Dorner K, Garbe-Schonberg D and Schaub J, 2001. Molybdenum metabolism: stable isotope studies in infancy. Journal of Trace Elements in Medicine and Biology, 15, 185-191.[↩]
- Turnlund JR, Weaver CM, Kim SK, Keyes WR, Gizaw Y, Thompson KH and Peiffer GL, 1999. Molybdenum absorption and utilization in humans from soy and kale intrinsically labeled with stable isotopes of molybdenum. American Journal of Clinical Nutrition, 69, 1217-1223.[↩]
- Novotny JA and Turnlund JR, 2006. Molybdenum kinetics in men differ during molybdenum depletion and repletion. Journal of Nutrition, 136, 953-957.[↩]
- Mendel RR and Kruse T, 2012. Cell biology of molybdenum in plants and humans. Biochimica et Biophysica Acta, 1823, 1568-1579.[↩][↩]
- Fitzpatrick KL, Tyerman SD and Kaiser BN, 2008. Molybdate transport through the plant sulfate transporter SHST1. FEBS Letters, 582, 1508-1513.[↩]
- Eckhert CD, 2006. Other trace elements. In: Modern nutrition in health and disease. Eds Shils ME, Shike M, Ross AC, Caballero B, Cousins R. Lippincott Williams & Wilkins, Philadelphia, USA, 338-350.[↩][↩]
- Nederbragt H, van den Ingh TS and Wensvoort P, 1984. Pathobiology of copper toxicity. Veterinary Quarterly, 6, 179-185, 235.[↩]
- Suttle NF, 2012. Copper imbalances in ruminants and humans: unexpected common ground. Advances in Nutrition, 3, 666-674.[↩]
- Deosthale YG and Gopalan C, 1974. The effect of molybdenum levels in sorghum (Sorghum vulgare Pers.) on uric acid and copper excretion in man. British Journal of Nutrition, 31, 351-355.[↩]
- Schroeder HA, Balassa JJ and Tipton IH, 1970. Essential trace metals in man: molybdenum. Journal of Chronic Diseases, 23, 481-499.[↩]
- Meinel B, Bode JC, Koenig W and Richter FW, 1979. Contents of trace elements in the human liver before birth. Biology of the Neonate, 36, 225-232.[↩]
- Abumrad NN, Schneider AJ, Steel D and Rogers LS, 1981. Amino acid intolerance during prolonged total parenteral nutrition reversed by molybdate therapy. American Journal of Clinical Nutrition, 34, 2551-2559.[↩]
- Molybdenum cofactor deficiency. https://ghr.nlm.nih.gov/condition/molybdenum-cofactor-deficiency[↩][↩]
- Fairhall LT, Dunn RC, Sharpless NE, Pritchard EA 1945. The toxicity of molybdenum. Public Health Bulletin 293:1-36,40-41.[↩]
- FDA 1975. Toxicity of essential minerals. Information pertinent to establishing appropriate levels of single-mineral dietary supplements. Washington, DC: U.S. Food and Drug Administration.[↩]
- Lener J, Bibr B 1984. Effects of molybdenum on the organism: a review. J Hyg Epidemiol Microbiol Immunol 29:405-419.[↩]