Contents
- Potassium
- Potassium function
- How much potassium do you need?
- Foods high in Potassium
- Potassium supplements
- Potassium Toxicity
- Potassium Health Benefits
- High Potassium (Hyperkalemia)
- Low Potassium (Hypokalemia)
Potassium
Potassium (K+) is a mineral that is vital to cell metabolism. Potassium is a major intracellular cation (positively charged ion) and a type of electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance 1, 2, 3. Potassium is present in all body tissues and is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients 4, 5. Potassium helps transport nutrients into cells and removes waste products out of cells. Potassium is essential for the proper functioning of the heart, kidneys, muscles, nerves, and digestive system. Potassium is also important in muscle function, helping to transmit messages between nerves and muscles 4.
Potassium (K+) is a positively charged ion (cation), which is present throughout your body in both intracellular and extracellular fluids. The majority of body potassium, > 90%, are intracellular. It moves freely from intracellular fluid (ICF) to extracellular fluid (ECF) and vice versa when adenosine triphosphate (ATP) increases the permeability of the cell membrane. Potassium (K+) is mainly replaced inside or outside the cells by another cation, sodium (Na+). The movement of potassium into or out of the cells is linked to certain body hormones and also to certain physiological states. Standard laboratory tests measure extracellular fluid (ECF) potassium. Potassium enters the body rapidly during food ingestion. Insulin is produced when a meal is eaten; this causes the temporary movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). Over the ensuing hours, the kidneys excrete the ingested potassium and homeostasis is returned. In the critically ill patient, suffering from high potassium level or hyperkalemia, this mechanism can be manipulated beneficially by administering high concentration (50%) intravenous glucose. Insulin can be added to the glucose, but glucose alone will stimulate insulin production and cause movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). The stimulation of alpha receptors causes increased movement of potassium from intracellular fluid (ICF) to extracellular fluid (ECF). A noradrenaline infusion can elevate serum potassium levels. An adrenaline infusion, or elevated adrenaline levels, can lower serum potassium levels. Metabolic acidosis causes a rise in extracellular potassium levels (hyperkalemia). In this situation, excess of hydrogen ions (H+) are exchanged for intracellular potassium ions, probably as a result of the cellular response to a falling blood pH. Metabolic alkalosis causes the opposite effect, with potassium moving into the cells 6.
Potassium (K+), along with other electrolytes such as sodium (Na+), chloride (Cl–), and bicarbonate (HCO3–), helps regulate the amount of fluid in your body and maintains a stable acid-base balance. Potassium is present in all body fluids, but most potassium is found within the cells (intracellularly). Only a small amount is present in fluids outside the cells and in the liquid part of the blood (called serum or plasma).
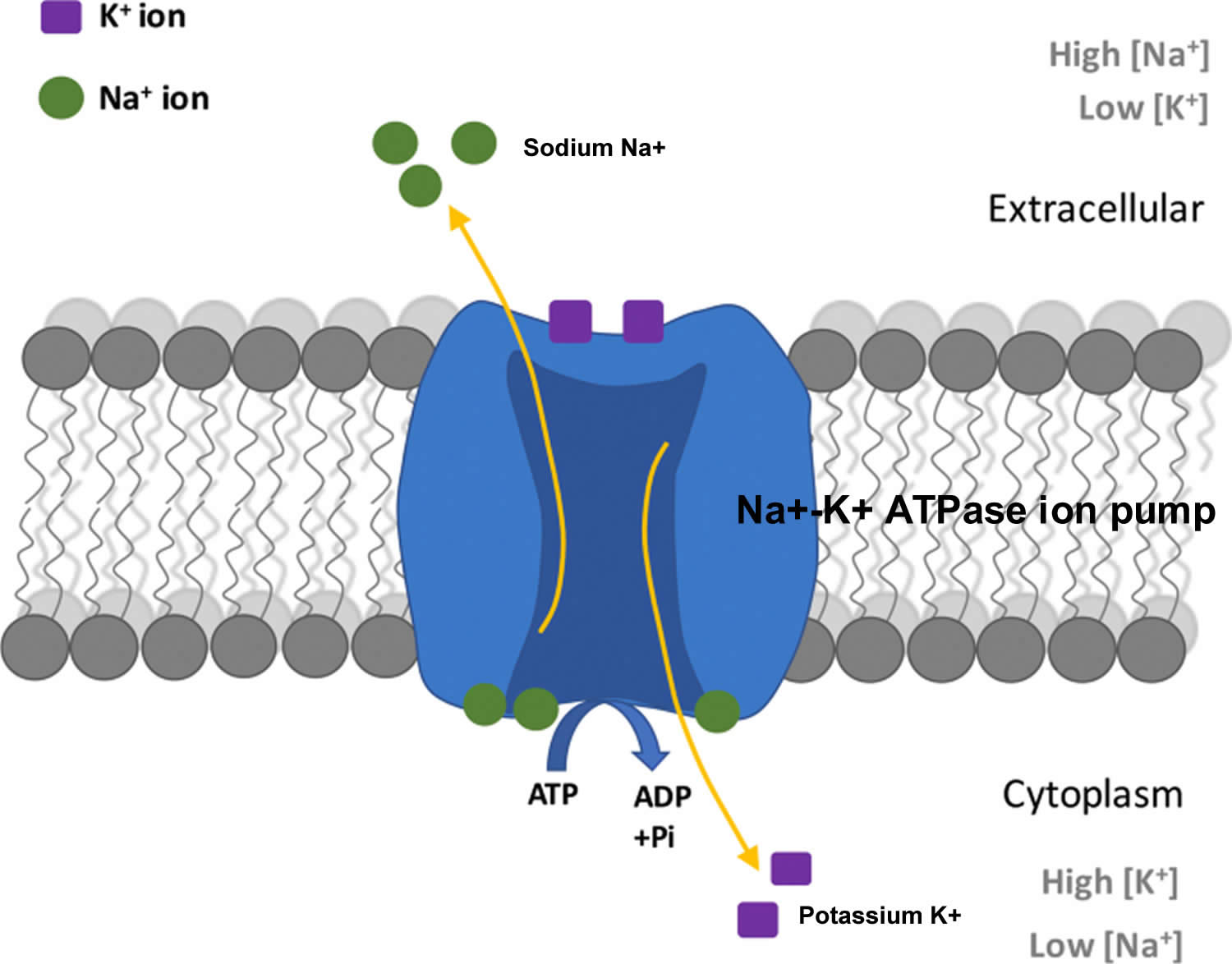
The total amount of potassium (K+) in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 millimole [mmol] = 1 milliequivalent [mEq] = 39.1 mg of potassium) 7. Most potassium are found within the cells (intracellularly) and a small amount is in extracellular fluid. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps) 8. When activated, the sodium-potassium ATPase pump (Na+-K+ ATPase ion pumps) exchanges 2 extracellular potassium (K+) ions for 3 intracellular sodium (Na+) ions, influencing membrane potential based on physiological excitation or inhibition. These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) are partially responsible, along with the sodium-potassium-chloride (Na+-K+-2Cl) co-transporter and sodium-calcium (Ca) exchanger, for maintaining the potential difference across the resting cell membrane as well. In addition to maintaining cellular tonicity, this gradient is required for proper nerve transmission, muscle contraction, and kidney function 4, 9, 10.
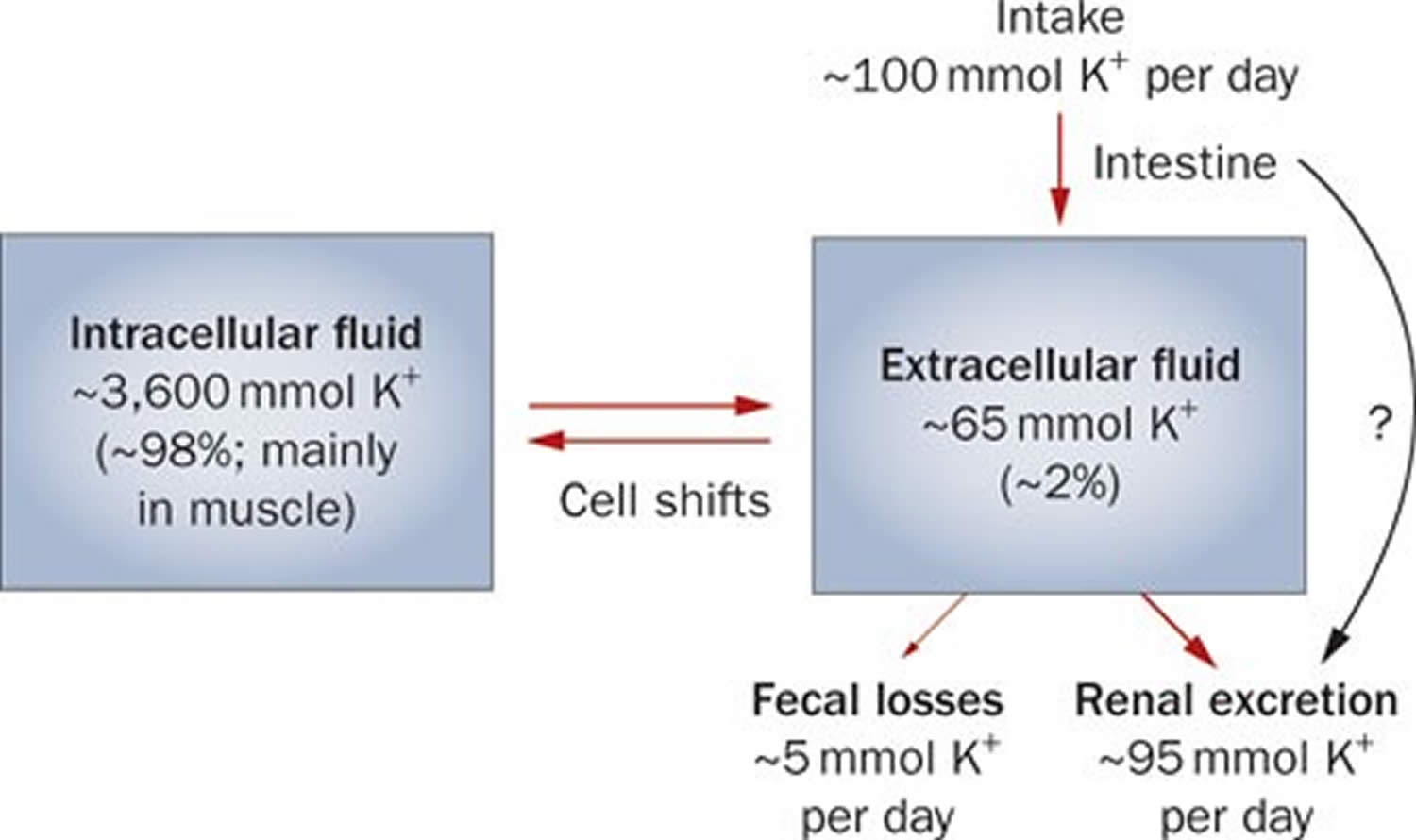
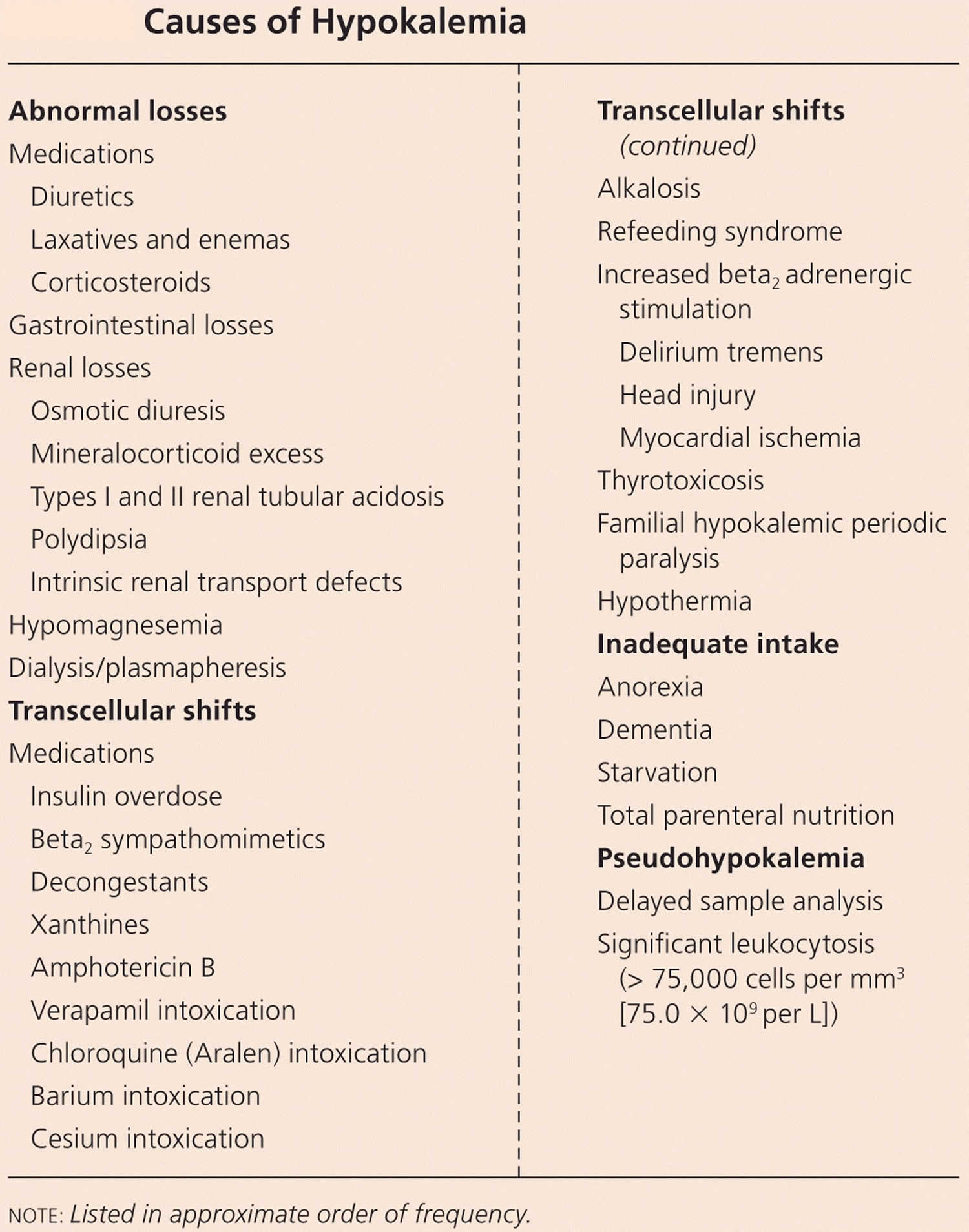
Potassium (K+) homeostasis depends on external balance (dietary intake [typically 100 mmol per day] versus excretion [95% via the kidney; 5% via the colon]) and internal balance (the distribution of potassium (K+) between intracellular and extracellular fluid compartments). The uneven distribution of potassium (K+) across cell membranes means that a mere 1% shift in its distribution can cause a 50% change in plasma potassium (K+) concentration 3. Hormonal mechanisms involving insulin, beta-adrenergic agonists and aldosterone modulate potassium (K+) distribution by promoting rapid transfer of potassium (K+) across the plasma membrane 11. Your body uses what potassium (K+) it requires and your kidneys eliminate the rest in the urine. Your body tries to keep the blood potassium level within a very narrow range. Levels are mainly controlled by aldosterone, a hormone produced by the adrenal glands in the kidneys. Extrarenal potassium (K+) losses from the body are usually small, but can be marked in individuals with chronic diarrhea, severe burns or prolonged sweating 3, 11. Under normal circumstances, the kidney’s distal nephron secretes potassium (K+) and determines final urinary excretion. In patients with low potassium levels or hypokalemia (plasma K+ concentration <3.5 mmol/l), after the exclusion of extrarenal causes, alterations in sodium ion (Na+) delivery to the distal nephron, aldosterone status, or a specific inherited or acquired defect in distal nephron function (each of which affects distal nephron K+ secretion), should be considered 3.
Figure 1. Potassium physiology
Because most potassium (K+) ions are found within the cells (a major intracellular cation), it is widely distributed in foods once derived from living tissues. Potassium concentration is higher in fruits and vegetables than in cereals and meat. You get most of the potassium you need from the foods that you eat and most people have an adequate intake of potassium. Recommended adequate intakes for potassium were set by the Food and Nutrition Board of the Institute of Medicine at 4700 mg/day 5. However it should be noted that the Food and Nutrition Board of the Institute of Medicine Recommended adequate intakes (AIs) for potassium at 4700 mg/day for adults is substantially higher than the World Health Organization’s (WHO) guidelines, which recommend 3150 mg/day for adults 12. The National Health and Nutrition Examination Survey (NHANES) data indicates that 99.2% of potassium in the US diet is naturally occurring, with the remaining 0.8% coming from fortified foods 13. These naturally occurring potassium sources include milk and other non-alcoholic beverages, as well as potatoes and fruit, which rank highest as sources of potassium intake among American adults 14. In addition, Western dietary practices with higher consumption of cereal, low nutrient density processed foods and lower consumption of fruits and vegetables has led to a diet lower in potassium and higher in sodium in recent decades 5. Salting foods and discarding the liquid induces sodium (Na+) for potassium (K+) exchange and reduces the potassium content of foods. Few Americans meet the recommended intakes; the average intake is 2591 ± 9 mg/day 13. This large gap between potassium intakes and recommended intakes led to potassium being called a shortfall nutrient in the Dietary Guidelines for Americans 15.
Actual potassium requirements would vary with an individual’s genetics, blood pressure (BP) status, and sodium intake 4. Blood pressure is currently the primary criterion for determining potassium requirements, with African Americans being more vulnerable to high blood pressure (hypertension) and more responsive to potassium supplementation than whites; individuals with high blood pressure (hypertension) are more responsive to increasing potassium intakes than individuals with normal blood pressure, and potassium having a greater benefit for those consuming a high salt diet 16. Other benefits of increasing potassium consumption may include improved blood sugar (glucose) control, glucose intolerance and insulin resistance becoming a concern for individuals with high blood pressure (hypertension) prescribed potassium wasting diuretics (water pills) 17. These differences support personalized nutrition approaches. Understanding movement of potassium within the body may help to improve these health outcomes.
Potassium is absorbed via passive diffusion, primarily in the small intestine 8. About 90% of ingested potassium is absorbed and used to maintain its normal intracellular and extracellular concentrations 18. There is around 50 mEq/kg of potassium (K+) in the body such that total body potassium (K+) in a 70-kg person is 3,500 mEq. Around 98% of potassium (K+) is found mainly within cells, and about 2% of the bodies’ potassium (K+) is in the extracellular fluid. The normal concentration of potassium (K+) in the extracellular fluid is 3.5–5.3 mEq/L. Large deviations from these values are not compatible with life.
Approximately 90% of the daily potassium (K+) intake is excreted in the urine, whereas a smaller percentage (10%) is excreted by the gastrointestinal tract in the stool and a very small amount is lost in sweat 19, 11, 20. Therefore, within the body, the kidney is the major organ responsible for potassium (K+) homeostasis. The kidneys control potassium excretion in response to changes in dietary intakes, and potassium excretion increases rapidly in healthy people after potassium consumption, unless body stores are depleted 4. The kidney facilitates potassium (K+) homeostasis by adjusting renal potassium (K+) excretion over several hours in response to a potassium load. Initial changes in extracellular potassium (K+) concentration are buffered by movement of potassium (K+) into or out of skeletal muscle cells. Internal potassium (K+) balance is a term used to refer to regulation of potassium (K+) distribution between the intracellular and extracellular space. Insulin, catecholamines, and, to a lesser extent, aldosterone are critical factors responsible for maintaining the normal internal distribution of potassium (K+) 11, 20.
The kidneys can adapt to variable potassium intakes in healthy individuals even in the setting of high dietary intake, but a minimum of 5 mmol (about 195 mg) potassium is excreted daily in urine 7. To demonstrate this, studies have shown potassium (K+) levels are kept within the normal range even when there are increases to ~15 g daily of dietary potassium (K+) intake sustained for 20 days 21, 22. Recent findings have identified the presence of an enteric potassium (K+) sensing mechanism that initiates the renal secretory process upon K+ entry into the gastrointestinal tract 20. The distal convoluted tubule has been identified as a site critical for potassium (K+) homeostasis, where it acts as a potassium (K+) sensor capable of initiating potassium (K+) excretion independent of mineralocorticoid activity 20. Combined with other obligatory losses, potassium balance cannot be achieved with intakes less than about 400–800 mg/day 11, 20.
Assessing potassium status is not routinely done in clinical practice, and it is difficult to do because most potassium in the body is inside cells 23. Although blood potassium levels can provide some indication of potassium status, they often correlate poorly with tissue potassium stores 7, 24, 25. Other methods to measure potassium status include collecting balance data (measuring net potassium retention and loss); measuring the total amount of potassium or the total amount of exchangeable potassium in the body; and conducting tissue analyses (e.g., muscle biopsies), but all have limitations 24.
Normal serum concentrations of potassium range from about 3.6 to 5.0 mmol/L and are regulated by a variety of mechanisms 7, 26. Diarrhea, vomiting, kidney disease, use of certain medications, and other conditions that alter potassium excretion or cause transcellular potassium shifts can cause low potassium level also called hypokalemia (serum potassium levels below 3.6 mmol/L) or high potassium level also called hyperkalemia (serum potassium levels above 5.0 mmol/L) 26. Otherwise, in healthy individuals with normal kidney function, abnormally low or high blood levels of potassium are rare.
Because the blood concentration of potassium is so small, minor changes can have significant consequences. If potassium levels are too low (serum potassium levels below 3.6 mmol/L) or too high (serum potassium levels above 5.0 mmol/L), there can be serious health consequences; a person may be at risk for developing shock, respiratory failure, or heart rhythm disturbances. An abnormal potassium level can alter the function of the nerves and muscles; for example, the heart muscle may lose its ability to contract.
Your body needs potassium to:
- Build proteins
- Break down and use carbohydrates
- Build muscle
- Maintain normal body growth
- Control the electrical activity of the heart
- Control the acid-base balance
Reduced potassium consumption has been associated with hypertension and cardiovascular diseases, and appropriate consumption levels could be protective against these conditions 27. A recent meta-analysis including 11 cohort studies reported an inverse association between potassium intake and risk of stroke 28. Additionally, two meta-analyses of trials comparing increased potassium to lower potassium intake found that increased potassium intake lowers blood pressure 29, 30. These results were further supported by a systematic review without a meta-analysis, which concluded that increased potassium intake results in decreased blood pressure in adults 31. Thus, a public health intervention aimed at increasing potassium intake from food could be a cost-effective strategy to reduce the burden of cardiovascular morbidity and mortality. Moreover, increasing potassium consumption from food in the population is safe; in individuals without renal impairment caused by medical conditions or drug therapy, the body is able to efficiently adapt and excrete excess potassium via the urine when consumption 32.
The American Heart Association recommended potassium intake for an average adult is 4,700 milligrams (mg) per day. Most of us aren’t getting nearly that much. On average, adult males eat almost 3,200 mg/day, and adult females eat about 2,400 mg/day 33. Remember that potassium is only part of an overall heart-healthy eating pattern. Other dietary factors that may affect blood pressure include amount and type of dietary fat; cholesterol; protein, sugar and fiber; calcium and magnesium, and of course, sodium.
For example, the DASH (Dietary Approaches to Stop Hypertension) diet study found that a diet rich in fruits, vegetables, fat-free or low-fat (1 percent) milk and milk products, whole-grain foods, fish, poultry, beans, seeds and unsalted nuts reduced blood pressure compared to a typical American diet. The DASH eating plan also had less sodium; sweets, added sugars and sugar-containing beverages; saturated and trans fats; and red meats than the typical American diet.
People with kidney problems, especially those on dialysis, should not eat too many potassium-rich foods. Your health care provider will recommend a special diet.
What are normal potassium levels?
Normal serum potassium values are between 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L) 1, 34, 35, 36. However, there can be slight variation between laboratories and for this reason, it is important to look for the specific reference interval listed on your test report. Potassium levels outside this range, 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L), are not compatible with life with increased rates of death from several causes 37, 38.
Your health care provider may order a potassium blood test as part of your regular checkup or to monitor an existing condition, such as diabetes, kidney disease, or adrenal gland disorders. You may also need this test if you take medicines that could affect your potassium levels or if you have symptoms of having too much or too little potassium.
Interpretation of a potassium test requires carefully considering the result, the laboratory reference range, and your health situation. Because potassium is frequently measured with other electrolytes, levels may be evaluated together. For a blood test, the report should list the amount of potassium measured in either milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L). The test report will also show a reference range, which the laboratory considers an expected range for potassium levels.
What are high potassium levels?
High potassium levels also known as hyperkalemia is defined as serum potassium level greater than 5 mEq/L or greater than 5 mmol/L (Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html)).
If your potassium levels are too high or hyperkalemia, your symptoms may include 39:
- Arrhythmia (a problem with the rate or rhythm of your heartbeat)
- Fatigue
- Muscle weakness
- Nausea
- Numbness or tingling
Too much potassium in the blood or hyperkalemia is often the result of two or more causes. High potassium levels may be a sign of 39:
- Kidney disease. Your kidneys remove extra potassium from your body. Too much potassium may mean your kidneys aren’t working well.
- Addison disease also called adrenal insufficiency, is a disorder in which your immune system mistakenly attacks your adrenal glands, damaging the adrenal cortex. Other causes include infections and cancer. The damage causes the adrenal glands cortex not to make enough of the hormone cortisol and sometimes the hormone aldosterone.
- Injuries, burns, or surgery that can cause your cells to release extra potassium into your blood
- Type 1 diabetes that is not well controlled
- The side effects of certain medicines, such as diuretics (water pills) or antibiotics
- A diet too high in potassium (not common). Bananas, apricots, green leafy vegetables, avocados and many other foods are good sources of potassium that are part of a healthy diet. But eating very large amounts of potassium-rich foods or taking potassium supplements can lead to health problems.
What are low potassium levels?
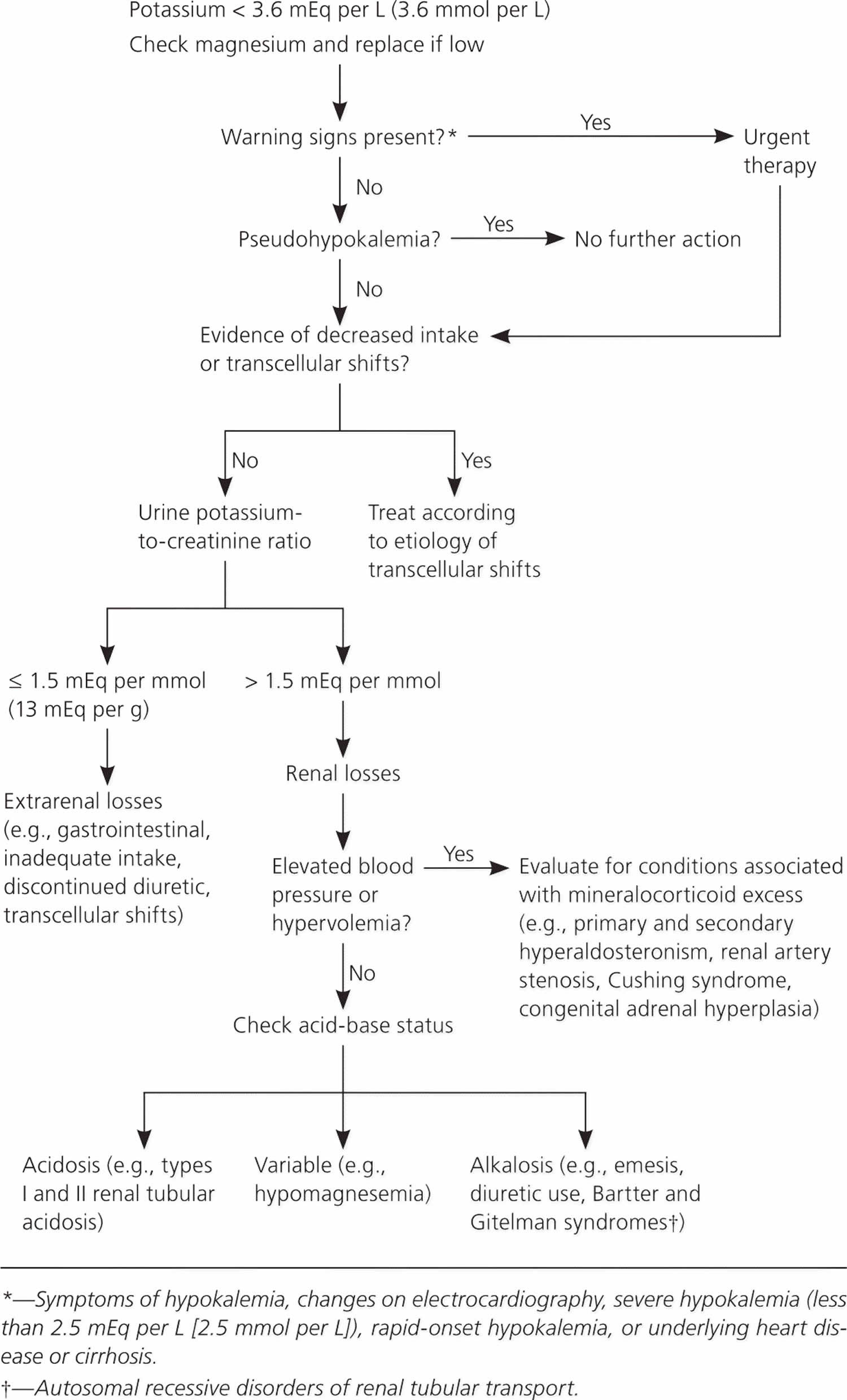
Low potassium levels also known as hypokalemia is defined as serum potassium level less than 3.6 mEq/L or less than 3.6 mmol/L (Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html)).
If your potassium levels are too low or hypokalemia, your symptoms may include 39:
- Irregular heartbeat
- Muscle cramps
- Weak or twitching muscles
- Fatigue
- Nausea
- Constipation
- Severe hypokalemia may result in muscular paralysis or abnormal heart rhythms (cardiac arrhythmias) that can be fatal 40, 41
- Chronic low potassium levels (chronic hypokalemia) is associated with high blood pressure (hypertension) and kidney stone formation.
Too little potassium in the blood or hypokalemia may be a sign of 39:
- Use of prescription diuretics (water pills)
- Fluid loss from diarrhea, vomiting, or heavy sweating
- Using too many laxatives
- Adrenal gland disorders, including Cushing’s syndrome and aldosteronism
- Kidney disease
- Alcohol use disorder
- Eating a lot of real licorice, which comes from licorice plants. Most licorice products sold in the U.S. don’t contain any real licorice. Check the package ingredient label to be sure.
- A diet too low in potassium (not common). Bananas, apricots, green leafy vegetables, avocados and many other foods are good sources of potassium that are part of a healthy diet.
Potassium function
Potassium (K+) is the principal positively charged ion (cation) in the fluid inside of cells, while sodium (Na+) is the principal cation in the extracellular fluid. Potassium (K+) concentrations are about 30 times higher inside than outside cells, while sodium (Na+) concentrations are more than 10 times lower inside than outside cells 42. The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. A cell’s membrane potential is maintained by ion pumps in the cell membrane, especially the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps). These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) use ATP (energy) to pump sodium (Na+) of the cell and potassium (K+) into the cell, leading to a potassium (K+) gradient across the cell membrane [potassium (K+) in > potassium (K+) out], which is partially responsible for maintaining the cell membrane potential (Figure 2). The sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) activity has been estimated to account for 20%-40% of the resting energy consumption in a typical adult 42. The large proportion of energy dedicated to maintaining sodium/potassium concentration gradients emphasizes the importance of this function in sustaining life 42. The cell membrane potential created by potassium and sodium ions allows the cell generate an action potential–a “spike” of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as nerve impulse transmission, muscle contraction, and heart function 43, 44, 45.
Potassium is also an essential mineral needed to regulate water balance, blood pressure and levels of acidity 46. The more potassium you eat, the more sodium you pass out of the body through urine. Increased potassium intake has no adverse effect on blood lipid concentration, catecholamine concentrations, or renal function in apparently healthy adults without impaired renal handling of potassium 33. The largest benefit was detected when sodium intake was more than 4 g/day, which is the intake of most populations globally 47, so increased potassium intake should benefit most people in most countries. However, the authors also found a statistically significant decrease in blood pressure with increased potassium when sodium intake was 2-4 g/day. Therefore, increased potassium can continue to be beneficial in terms of blood pressure even as individuals and populations decrease their sodium intake. Studies examining both nutrients simultaneously support this concept, showing an increased benefit with simultaneous reduction in sodium and increase in potassium compared with changes in one nutrient individually 48, 49.
Potassium also helps relax blood vessel walls, which helps lower blood pressure 33.
World Health Organization recommends an increase in potassium intake from food to reduce blood pressure and risk of cardiovascular disease, stroke and coronary heart disease in adults. World Health Organization suggests a potassium intake of at least 90 mmol/day (3510 mg/day) for adults (conditional recommendation) 32.
Potassium also acts as a cofactor for some enzymes activity. For example, the activation of Na+/K+-ATPase requires the presence of sodium and potassium. The presence of potassium is also required for the activity of pyruvate kinase, an important enzyme in carbohydrate metabolism 50.
Figure 2. Sodium-Potassium ATPase pump
How much potassium do you need?
The amount of potassium you need each day depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg). Table 1 lists the current Adequate Intakes (AIs) for potassium for healthy individuals. Intake recommendations for potassium and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine 5. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
When the Food and Nutrition Board evaluated the available data in 2005, it found the data insufficient to derive an Estimated Average Requirement (average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) for potassium, so the board established Adequate Intake (intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance) for all ages based on potassium intakes in healthy populations 51. In 2019, a National Academies of Sciences, Engineering, and Medicine committee updated the Dietary Reference Intake (DRI) for potassium and sodium 5. The committee found the data insufficient to derive an Estimated Average Requirement (EAR) for potassium. Therefore, they established Adequate Intakes (AIs) for all ages based on the highest median potassium intakes in healthy children and adults, and on estimates of potassium intakes from breast milk and complementary foods in infats. The National Academies of Sciences, Engineering, and Medicine committee also used an expanded Dietary Reference Intake (DRI) model to include a recommended intake level for a nutrient to reduce the risk of chronic disease, what they termed the chronic disease risk reduction intake 51. In 2019, a National Academies of Sciences, Engineering, and Medicine committee updated the Dietary Reference Intake (DRI) for potassium and sodium 5. According to the model, a chronic disease risk reduction intake might be set for a nutrient like potassium when there is a causal relationship between a certain level of intake and a reduced risk of chronic disease based on evidence of at least moderate strength. However, the committee found the evidence to be insufficient to derive a chronic disease risk reduction intake for potassium 5.
[Source 52 ]Table 1. Average daily recommended intake for Potassium
| Birth to 6 months | 400 mg |
|---|---|
| Infants 7–12 months | 860 mg |
| Children 1–3 years | 2,000 mg |
| Children 4–8 years | 2,300 mg |
| Children 9–13 years (boys) | 2,500 mg |
| Children 9–13 years (girls) | 2,300 mg |
| Teens 14–18 years (boys) | 3,000 mg |
| Teens 14–18 years (girls) | 2,300 mg |
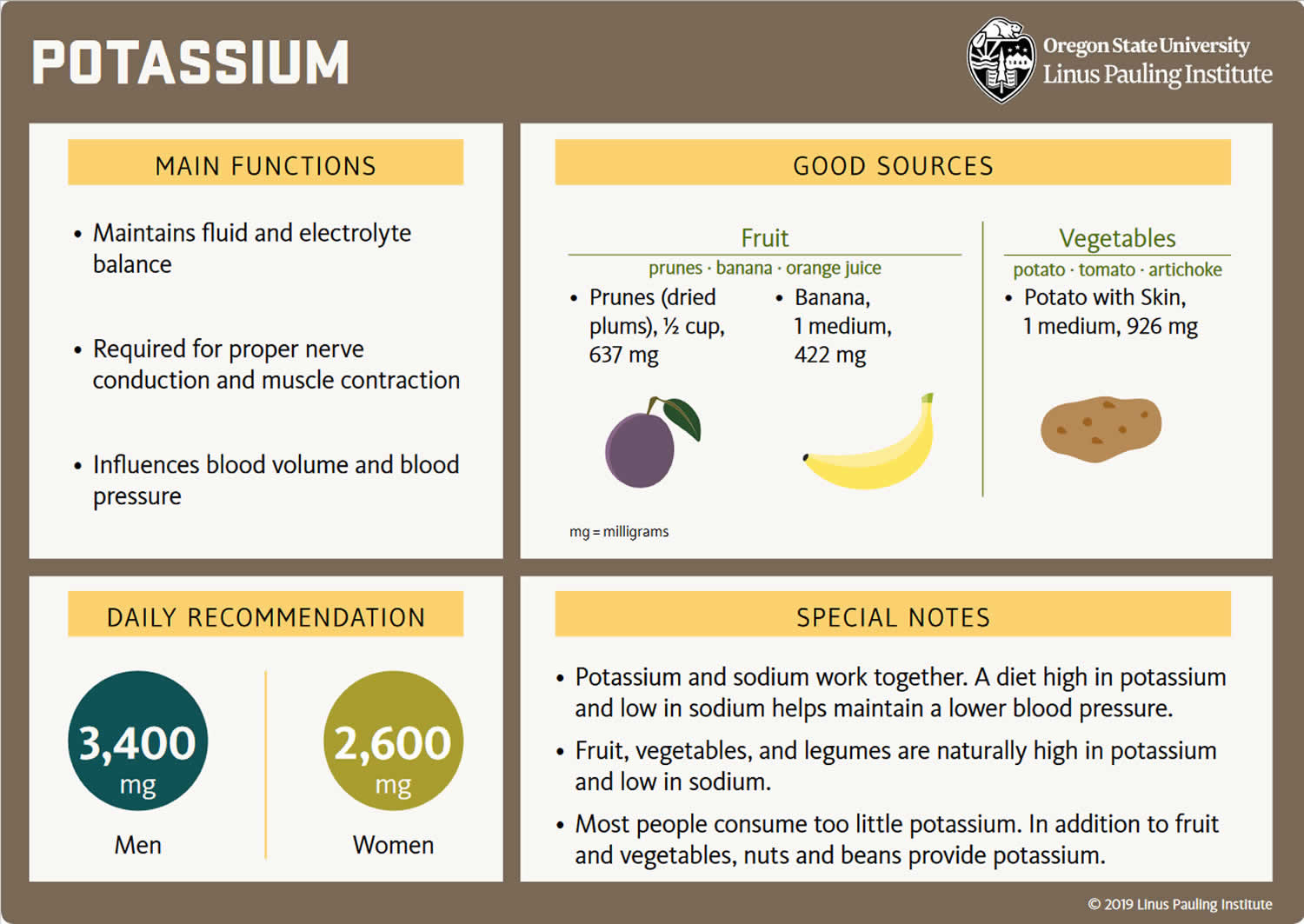
| Adults 19+ years (men) | 3,400 mg |
| Adults 19+ years (women) | 2,600 mg |
| Pregnant teens | 2,600 mg |
| Pregnant women | 2,900 mg |
| Breastfeeding teens | 2,500 mg |
| Breastfeeding women | 2,800 mg |
Footnote: *Adequate Intakes (AIs) do not apply to individuals with impaired potassium excretion because of medical conditions (e.g., kidney disease) or the use of medications that impair potassium excretion.
[Source 23 ]Potassium Intakes and Status of Americans
Dietary surveys consistently show that people in the United States consume substantially less potassium than recommended, which is why the 2015–2020 Dietary Guidelines for Americans identifies potassium as a “nutrient of public health concern” 53. According to data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES), the average daily potassium intake from foods is 2,423 mg for males aged 2–19, and 1,888 mg for females aged 2–19 54. In adults aged 20 and over, the average daily potassium intake from foods is 3,016 mg for men and 2,320 mg for women.
Average potassium intakes vary by race. Non-Hispanic blacks aged 20 and older consume an average of 2,449 mg potassium per day. Average daily intakes are 2,695 mg for Hispanic whites and 2,697 mg for non-Hispanic whites 54.
Use of potassium-containing dietary supplements does not significantly increase total potassium intakes among U.S. adults 55, probably because most potassium-containing dietary supplements provide no more than 99 mg potassium per serving 56. Data from NHANES 2013–2014 indicate that 12% of children and adults aged 2 and over use supplements containing potassium, and among those who do, supplement use adds a mean of only 87 mg to total daily potassium intakes 54.
Foods high in Potassium
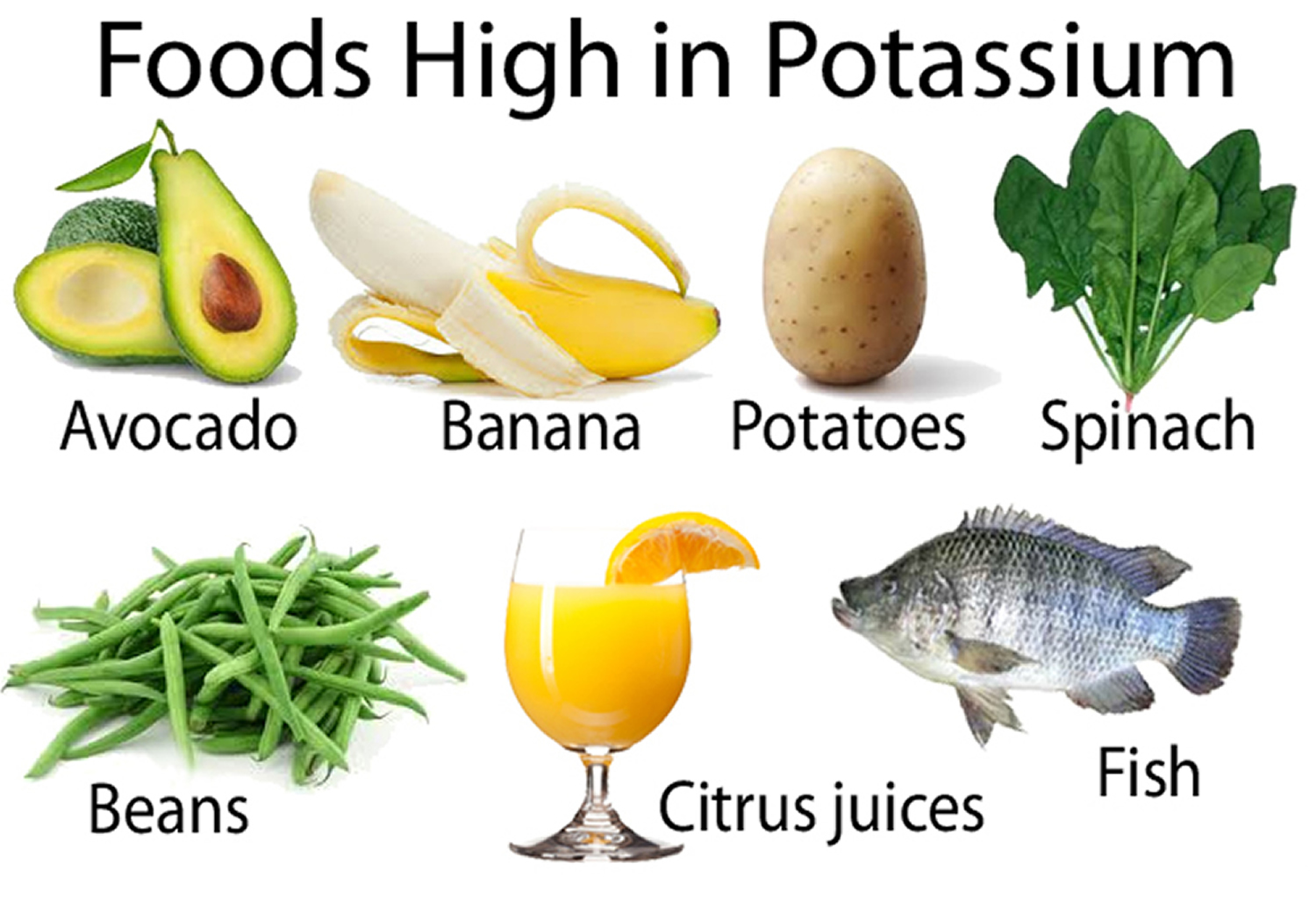
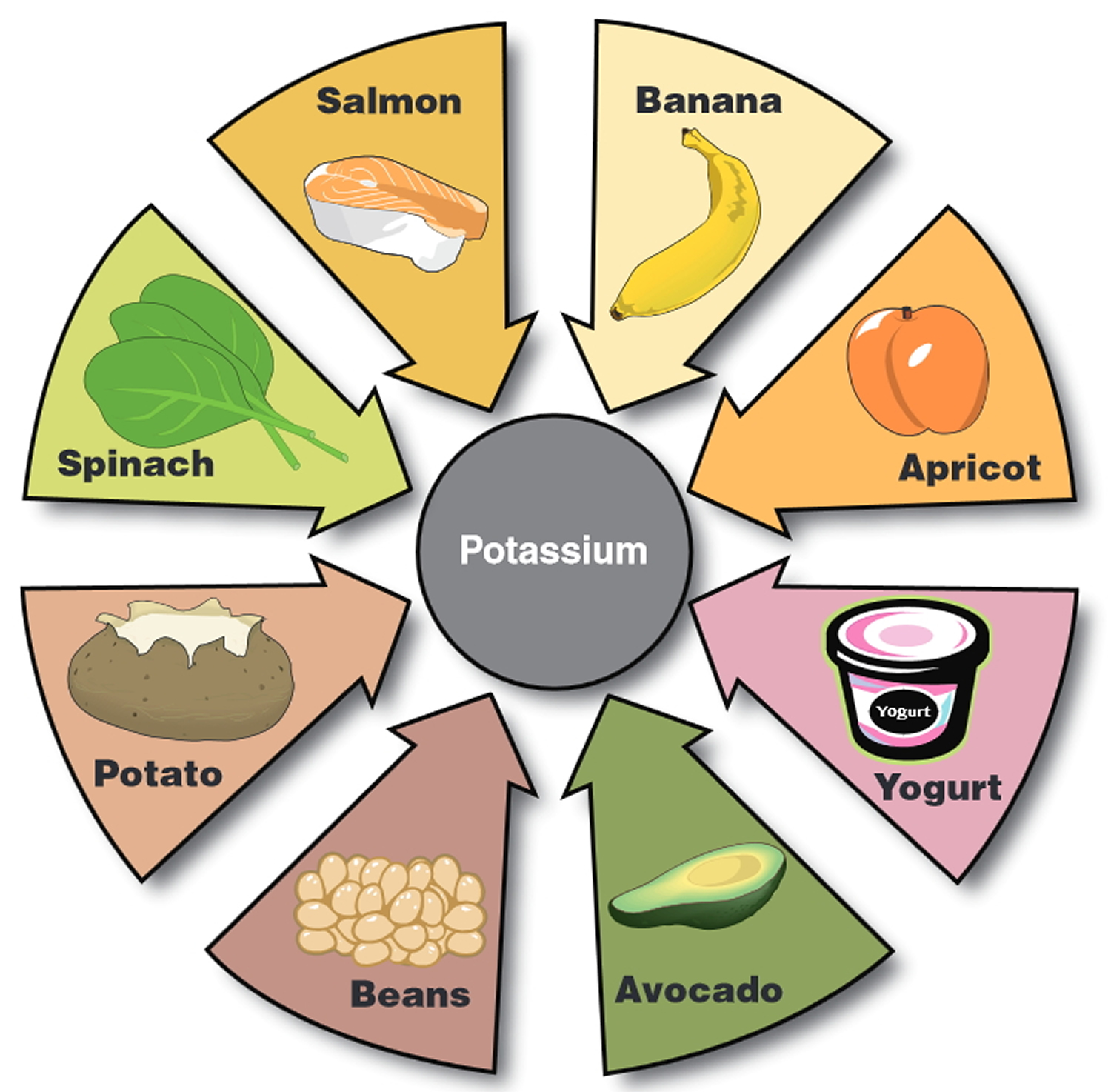
Potassium is found in many foods. You can get recommended amounts of potassium by eating a variety of foods, including the following 57:
- Fruits, such as dried apricots, prunes, raisins, orange juice, and bananas
- Vegetables, such as acorn squash, potatoes, spinach, tomatoes, and broccoli
- Lentils, kidney beans, soybeans, and nuts
- Milk and yogurt
- Meats, poultry, and fish
Potassium is found in a wide variety of plant and animal foods and in beverages. Many fruits and vegetables are excellent sources, as are some legumes (e.g., soybeans) and potatoes. Meats, poultry, fish, milk, yogurt, and nuts also contain potassium 18. Among starchy foods, whole-wheat flour and brown rice are much higher in potassium than their refined counterparts, white wheat flour and white rice 58. Selected food sources of potassium are listed in Table 2. People with kidney problems, especially those on dialysis, should not eat too many potassium-rich foods. Your health care provider will recommend a special diet low in potassium.
Milk, coffee, tea, other nonalcoholic beverages, and potatoes are the top sources of potassium in the diets of American adults 59. Among children in the United States, milk, fruit juice, potatoes, and fruit are the top sources 60.
It is estimated that the body absorbs about 85%–90% of dietary potassium 4. The forms of potassium in fruits and vegetables include potassium phosphate, sulfate, citrate, and others, but not potassium chloride 61.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing potassium ordered by nutrient content (https://www.nal.usda.gov/sites/www.nal.usda.gov/files/potassium.pdf). The 2015–2020 Dietary Guidelines for Americans provides a a list of foods containing potassium 62.
Table 2. Food Sources of Potassium
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Apricots, dried, ½ cup | 755 | 16 |
| Lentils, cooked, 1 cup | 731 | 16 |
| Squash, acorn, mashed, 1 cup | 644 | 14 |
| Prunes, dried, ½ cup | 635 | 14 |
| Raisins, ½ cup | 618 | 13 |
| Potato, baked, flesh only, 1 medium | 610 | 13 |
| Kidney beans, canned, 1 cup | 607 | 13 |
| Orange juice, 1 cup | 496 | 11 |
| Soybeans, mature seeds, boiled, ½ cup | 443 | 9 |
| Banana, 1 medium | 422 | 9 |
| Milk, 1%, 1 cup | 366 | 8 |
| Spinach, raw, 2 cups | 334 | 7 |
| Chicken breast, boneless, grilled, 3 ounces | 332 | 7 |
| Yogurt, fruit variety, nonfat, 6 ounces | 330 | 7 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 326 | 7 |
| Beef, top sirloin, grilled, 3 ounces | 315 | 7 |
| Molasses, 1 tablespoon | 308 | 7 |
| Tomato, raw, 1 medium | 292 | 6 |
| Soymilk, 1 cup | 287 | 6 |
| Yogurt, Greek, plain, nonfat, 6 ounces | 240 | 5 |
| Broccoli, cooked, chopped, ½ cup | 229 | 5 |
| Cantaloupe, cubed, ½ cup | 214 | 5 |
| Turkey breast, roasted, 3 ounces | 212 | 5 |
| Asparagus, cooked, ½ cup | 202 | 4 |
| Apple, with skin, 1 medium | 195 | 4 |
| Cashew nuts, 1 ounce | 187 | 4 |
| Rice, brown, medium-grain, cooked, 1 cup | 154 | 3 |
| Tuna, light, canned in water, drained, 3 ounces | 153 | 3 |
| Coffee, brewed, 1 cup | 116 | 2 |
| Lettuce, iceberg, shredded, 1 cup | 102 | 2 |
| Peanut butter, 1 tablespoon | 90 | 2 |
| Tea, black, brewed, 1 cup | 88 | 2 |
| Flaxseed, whole, 1 tablespoon | 84 | 2 |
| Bread, whole-wheat, 1 slice | 81 | 2 |
| Egg, 1 large | 69 | 1 |
| Rice, white, medium-grain, cooked, 1 cup | 54 | 1 |
| Bread, white, 1 slice | 37 | 1 |
| Cheese, mozzarella, part skim, 1½ ounces | 36 | 1 |
| Oil (olive, corn, canola, or soybean), 1 tablespoon | 0 | 0 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs (Daily Values) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value (DV) for potassium is 4,700 mg for adults and children age 4 years and older 63. FDA requires the new food labels to list potassium content. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 58 ] [Source 64 ]Potassium supplements
In dietary supplements, potassium is often present as potassium chloride, but many other different supplemental forms including potassium citrate, potassium gluconate, potassium bicarbonate, potassium aspartate, potassium orotate and potassium phosphate are also used 65. The Supplement Facts panel on a dietary supplement label declares the amount of elemental potassium in the product, not the weight of the entire potassium-containing compound. Some dietary supplements contain potassium iodide in microgram amounts, but this ingredient serves as a form of the mineral iodine, not potassium. Potassium iodide is used to protect your thyroid gland from taking in radioactive iodine that may be released during a nuclear radiation emergency 66. Radioactive iodine can damage your thyroid gland. You should only take potassium iodide if there is a nuclear radiation emergency and public officials tell you that you should take it 66. Potassium iodide is in a class of medications called anti-thyroid medications. It works by blocking radioactive iodine from entering your thyroid gland. Potassium iodide is also sometimes used to treat overactive thyroid gland and sporotrichosis (a skin infection caused by a fungus) 66. Talk to your doctor about the risks of using potassium iodide for your condition. Many salt substitutes contain potassium chloride, and acesulfame potassium (Ace-K) is an FDA-approved general purpose sweetener.
Not all multivitamin/mineral supplements contain potassium, but those that do typically provide about 80 mg potassium 65. Potassium-only supplements are also available, and most contain up to 99 mg potassium 67. Information on many dietary supplements that contain potassium is available in the Dietary Supplement Label Database 65 from the National Institutes of Health, which contains label information from tens of thousands of dietary supplement products on the market. Because of the potential for serious side effects from potassium supplements, you should seek medical advice before deciding to use a potassium supplement. The best way to increase your potassium intake is by increasing the consumption of potassium-rich food and beverages 67.
Many dietary supplement manufacturers and distributors limit the amount of potassium in their products to 99 mg (which is only about 3% of the DV) because of two concerns related to potassium-containing drugs. First, the FDA has ruled that some oral drug products that contain potassium chloride and provide more than 99 mg potassium are not safe because they have been associated with small-bowel lesions 68. Second, the FDA requires some potassium salts containing more than 99 mg potassium per tablet to be labeled with a warning about the reports of small-bowel lesions 69. In accordance with a ruling by Congress, the FDA may not limit the amount of any nutrient, including potassium, in a dietary supplement, except for safety-related reasons 70. However, the FDA has not issued a ruling about whether dietary supplements containing more than 99 mg potassium must carry a warning label 69.
Higher doses of supplemental potassium are generally prescribed to prevent and treat potassium depletion and hypokalemia 42. The use of more potent potassium supplements in potassium deficiency requires close monitoring of serum potassium concentrations 42.
Only a few studies have examined how well the various forms of potassium in dietary supplements are absorbed. A 2016 dose-response trial found that humans absorb about 94% of potassium gluconate in supplements, and this absorption rate is similar to that of potassium from potatoes 71. According to an older study, liquid forms of potassium chloride (used as drugs to treat conditions such as digitalis intoxication or arrhythmias due to hypokalemia) are absorbed within a few hours 72. Enteric coated tablet forms of potassium chloride (designed to prevent dissolution in the stomach but allow it in the small intestine) are not absorbed as rapidly as liquid forms 73.
Salt substitutes
Many salt substitutes contain potassium chloride as a replacement for some or all of the sodium chloride in salt. The potassium content of these products varies widely, from about 440 mg to 2,800 mg potassium per teaspoon 51. Some people, such as those with kidney disease or who are taking certain medications, should consult their healthcare provider before taking salt substitutes because of the risk of hyperkalemia posed by the high levels of potassium in these products.
Adverse reactions to potassium supplements
Gastrointestinal symptoms are the most common side effects of potassium supplements, including nausea, vomiting, abdominal discomfort, and diarrhea 74. Intestinal ulceration has been reported after the use of enteric-coated potassium chloride tablets. The use of potassium salts in certain medications has been associated with small-bowel lesions, causing obstruction, hemorrhage, and perforation 69, 75. For this reason, the FDA requires some oral drugs providing more than 99 mg of potassium to be labeled with a warning. Taking potassium with meals or taking a microencapsulated form of potassium may reduce gastrointestinal side effects 67. Rashes may occasionally occur. The most serious adverse reaction to potassium supplementation is hyperkalemia (elevated serum potassium levels), yet is rare in subjects with normal kidney function (see Potassium Toxicity). Chronic ingestion of doses of potassium supplements (e.g., up to 15,600 mg for 5 days) in healthy people can increase plasma levels of potassium, but not beyond the normal range 76. However, very high amounts of potassium supplements or salt substitutes that contain potassium could exceed the kidney’s capacity to excrete potassium, causing acute hyperkalemia even in healthy individuals. Furthermore, individuals with abnormal kidney function and those on potassium-sparing medications should be monitored closely to prevent hyperkalemia 67, 77.
Drug interactions
Several types of medications have the potential to affect potassium status in ways that could be dangerous. Table 3 lists the classes of medications known to increase the risk of hyperkalemia (elevated serum potassium) in patients who also use potassium supplements 67, 78, 79. People taking these and other medications should discuss their potassium intakes and status with their health care providers.
Several classes of medications are known to induce hypokalemia (low serum potassium) see Table 4 below. In the absence of treatment, hypokalemia can have serious complications and even be fatal (see Potassium Deficiency). Various mechanisms explain how certain medications can lead to potassium depletion. For example, both loop and thiazide diuretics increase the urinary excretion of potassium. Corticoids cause sodium retention that leads to a compensatory increase in urinary potassium excretion. Penicillins formulated as sodium salts also stimulate potassium excretion. Several medications, including aminoglycosides, anti-fungal agents (amphotericin-B, fluconazole), and cisplatin, can damage the renal tubular epithelium and lead to severe potassium loss. Outdated tetracycline antibiotics have been linked to electrolyte disturbances.
Table 3. Medications associated with Hyperkalemia (high potassium levels)
| Medication Family | Specific Medications |
|---|---|
| Angiotensin converting enzyme (ACE) inhibitors | captopril (Capoten), enalapril (Vasotec), fosinopril (Monopril), ramipril (Altace) |
| Angiotensin receptor blockers | Losartan (Cozaar), valsartan (Diovan), irbesartan (Avapro), candesartan (Atacand) |
| Anticoagulant | Heparin |
| Anti-hypertensive agents | Beta-blockers, alpha-blockers |
| Anti-infective agents | Trimethoprim-sulfamethoxazole, pentamidine |
| Cardiac glycoside | Digitalis |
| Nonsteroidal anti-inflammatory agents (NSAID) | Indomethacin, ibuprofen, ketorolac |
| Potassium-sparing diuretics | Spironolactone (Aldactone), triamterene (Dyrenium), amiloride (Midamor) |
ACE inhibitors and angiotensin receptor blockers (ARBs)
ACE inhibitors, such as benazepril (Lotensin®), and ARBs such as losartan (Cozaar®), are used to treat hypertension and heart failure, slow progression of kidney disease in patients with chronic kidney disease and type 2 diabetes, and decrease morbidity and mortality after myocardial infarction 80, 81. These medications reduce urinary potassium excretion, which can lead to hyperkalemia. Experts recommend monitoring potassium status in people taking ACE inhibitors or angiotensin receptor blockers (ARBs), especially if they have other risk factors for hyperkalemia, such as impaired kidney function 80.
Potassium sparing diuretics
Potassium-sparing diuretics, such as amiloride (Midamor®) and spironolactone (Aldactone®), reduce the excretion of potassium in the urine and can cause hyperkalemia 82. Experts recommend monitoring potassium status in people taking these medications, especially if they have impaired kidney function or other risk factors for hyperkalemia 82.
Loop and thiazide diuretics
Treatment with loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®), and thiazide diuretics, such as chlorothiazide (Diuril®) and metolazone (Zaroxolyn®), increases urinary potassium excretion and can lead to hypokalemia 82. Experts recommend monitoring potassium status in people taking these medications, and initiating potassium supplementation if warranted 82.
Table 4. Medications associated with Hypokalemia (low serum potassium)
| Medication Family | Specific Medications |
|---|---|
| Aminoglycosides | amikacin (Amikin), gentamicin (Garamycin), kanamycin (Kantrex), tobramycin (Nebcyn), streptomycin |
| Antibiotics | Penicillins: penicillin G sodium (Pfizerpen), mezlocillin (Mezlin), carbenicillin (Geocillin), ticarcillin (Ticar) Tetracyclines (when outdated) |
| Anti-cancer agent | cisplatin (Platinol-AQ) |
| Anti-fungal agents | amphotericin B (Abelcet, Amphotec, AmBisome, Amphocin, Fungizone), fluconazole (Diflucan) |
| Beta-adrenergic agonists | albuterol (Salbutamol, Ventolin), bitolterol (Tornalate), metaproterenol (Alupent) |
| Diuretics | Loop diuretics: bumetanide (Bumex), ethacrynic acid (Edecrin), furosemide (Lasix), torsemide (Demadex) Thiazide diuretics: Acetazolamide, thiazides, chlorthalidone (Hygroton), indapamide (Lozol), metolazone (Zaroxolyn), chlorothiazide (Diuril) |
| Mineralocorticoids | fludrocortisone (Florinef), hydrocortisone (Cortef), cortisone (Cortone), prednisone (Deltasone) Substances with mineralocorticoid effects: licorice, carbenoxolone, gossypol |
| Other | methylxanthines (e.g., theophylline), sodium polystyrene sulfonate, sodium phosphates, caffeine |
Potassium Toxicity
Abnormally elevated serum potassium concentrations are referred to as hyperkalemia. Hyperkalemia occurs when potassium intake exceeds the capacity of the kidneys to eliminate it. Acute or chronic kidney failure, the use of potassium-sparing diuretics, and insufficient aldosterone secretion (hypoaldosteronism) may result in the accumulation of potassium due to a decreased urinary potassium excretion. Oral doses of potassium >18 g taken at one time in individuals not accustomed to high intakes may lead to severe hyperkalemia, even in those with normal kidney function 83, 67. Hyperkalemia may also result from a shift of intracellular potassium into the circulation, which may occur with the rupture of red blood cells (hemolysis) or tissue damage (e.g., trauma or severe burns). Symptoms of hyperkalemia may include tingling of the hands and feet, muscular weakness, and temporary paralysis. The most serious complication of hyperkalemia is the development of an abnormal heart rhythm (cardiac arrhythmia), which can lead to cardiac arrest 78. A meta-analysis of randomized controlled studies showed that heart rate in healthy adults was unlikely to be affected by the chronic use of supplemental potassium doses of 2 to 3 g/day 84.
Potassium Health Benefits
There is substantial evidence suggesting that a diet high in potassium-rich food and beverages may be associated with lower risks of stroke, hypertension, kidney stones, and possibly osteoporosis 42. The relative deficiency of dietary potassium in the modern diet and a higher sodium-to-potassium ratio may contribute to the development of some chronic diseases 42. However, currently there is insufficient evidence to establish a causal relationship between potassium intakes and the risk of these chronic conditions 85. As a consequence, median potassium intakes observed in apparently healthy people were used to set adequate intakes (AI) by age/life stage in the recent revision of the Dietary Reference Intakes (DRIs) for potassium. The revised adequate intakes (AI) values are 2600 mg/day for women and 3400 mg/day for men 5.
Fruit and vegetables are among the richest sources of dietary potassium, and a large body of evidence supports the association of increased fruit and vegetable intakes with reduced risk of cardiovascular disease. Health experts recommends the consumption of a diet high in potassium-rich foods, especially fruit, vegetables, nuts, and dairy products to ensure adequate potassium intakes.
A diet rich in fruit and vegetables that supplies 2600 to 3400 mg/day of potassium should contribute to maintaining a low risk of chronic disease in generally healthy older adults. This recommendation does not apply to individuals who have been advised to limit potassium consumption by a health care professional.
High blood pressure
High blood pressure or hypertension is a major risk factor for heart disease and stroke, affects almost a third of Americans 4, 86. According to an extensive body of literature, low potassium intakes increase the risk of hypertension, especially when combined with high sodium intakes 87. Higher potassium intakes, in contrast, may help decrease blood pressure, in part by widening of blood vessels (vasodilation) and urinary sodium excretion, which in turn reduces plasma volume 51; this effect may be most pronounced in salt-sensitive individuals 4.
Forty-five percent of adult Americans have hypertension (blood pressure levels ≥130/80 mm Hg) 88. Chronic hypertension damages the heart, blood vessels, and kidneys, thereby increasing the risk of heart disease and stroke, as well as hypertensive kidney disease 89, 90. Modern diets, which are high in sodium and low in potassium, are recognized as largely contributing to the high prevalence of hypertension. Unlike 24-hour dietary recalls, 24-hour urine collections provide accurate estimates of dietary intakes of sodium and potassium 91. An analysis of the 2014 US National Health and Nutrition Examination Survey (NHANES) showed an increase in systolic blood pressure with increasing sodium excretion and increasing sodium-to-potassium ratio in the urine 92. In this study, the highest versus lowest quartile of urinary potassium excretion (mid-values, 3,043 mg/day versus 1,484 mg/day) was associated with a 62% lower risk of hypertension 92.
The Dietary Approaches to Stop Hypertension (DASH) eating pattern, which emphasizes potassium from fruits, vegetables, modestly higher in protein, and lower in total fat, saturated fat, cholesterol, red meat, sweets, and sugar-containing beverages, lowers systolic blood pressure by an average of 5.5 mmHg and diastolic blood pressure by 3.0 mmHg 93. The DASH eating pattern provides three times more potassium than the average American diet 94. Compared to the control diet providing only 3.5 servings/day of fruit and vegetables and 1,700 mg/day of potassium, adherence to the DASH diet that included 8.5 servings/day of fruit and vegetables and 4,100 mg/day of potassium lowered systolic/diastolic blood pressures by an average 11.4/5.5 mm Hg in people with hypertension and 3.5/2.1 mm Hg in those without hypertension 94. A 2014 meta-analysis of 17 randomized controlled trials that examined the effect of the DASH diet compared to a control diet in a total of 2,561 adults found overall reductions in systolic and diastolic blood pressure by 6.7 mm Hg and 3.5 mm Hg, respectively 95. However effective the DASH diet is, the blood pressure-lowering effects can hardly be solely attributed to potassium intakes, because the DASH diet also increases intakes of other nutrients, such as magnesium and calcium, that are also associated with reductions in blood pressure 96.
Results from most clinical trials suggest that potassium supplementation reduces blood pressure. A 2017 meta-analysis of 25 randomized controlled trials in 1,163 participants with hypertension found significant reductions in systolic blood pressure by 4.48 mm Hg and diastolic blood pressure by 2.96 mmHg with potassium supplementation, mostly as potassium chloride at 30–120 mmol/day potassium (1,173–4,692 mg), for 4–15 weeks 97. Another meta-analysis of 15 randomized controlled trials found that potassium supplements (mostly containing potassium chloride at 60–65 mEq/day potassium [2,346–2,541 mg]) for 4–24 weeks in 917 patients with normal blood pressure or hypertension who were not taking antihypertensive medications significantly reduced both systolic and diastolic blood pressure 98. The supplements had the greatest effect in patients with hypertension, reducing systolic blood pressure by a mean of 6.8 mmHg and diastolic blood pressure by 4.6 mmHg. Two earlier meta-analyses of 19 trials 99 and 33 trials 100 had similar findings. However, a Cochrane review of six of the highest-quality trials found nonsignificant reductions in systolic and diastolic blood pressure with potassium supplementation 101.
In 2018, the Agency for Healthcare Research and Quality (AHRQ) published a systematic review of the effects of sodium and potassium intakes on chronic disease outcomes and their risk factors 74. The authors concluded that, based on observational studies, the associations between dietary potassium intakes and lower blood pressure in adults were inconsistent 74. They also found no evidence for an association between potassium intakes and the risk of hypertension 74. The authors did report, however, that potassium supplements (mostly containing potassium chloride) in doses ranging from 20 to 120 mmol/day (782 to 4,692 mg/day) for 1 to 36 months lowered both systolic and diastolic blood pressure compared to placebo 74. A similar analysis conducted by the National Academies of Sciences, Engineering, and Medicine committee that included 16 trials found that potassium supplements significantly lowered systolic blood pressure by a mean of 6.87 mmHg and diastolic blood pressure by 3.57 mmHg 102. However, the effects were stronger among studies including participants with hypertension; for studies including only participants without hypertension, the effects were not statistically significant 102. Based on 13 randomized controlled trials that primarily enrolled patients with hypertension, the Agency for Healthcare Research and Quality (AHRQ) review found that the use of potassium-containing salt substitutes in place of sodium chloride significantly reduced systolic blood pressure in adults by a mean of 5.58 mmHg and diastolic blood pressure by 2.88 mmHg 74. However, reducing sodium intake decreased both systolic and diastolic blood pressure in adults, and increasing potassium intake via food or supplements did not reduce blood pressure any further. This finding suggests that at least some of the beneficial effects of potassium salt substitutes on blood pressure may be due to the accompanying reduction in sodium intake, rather than the increase in potassium intake 23.
Supplemental potassium can help lower blood pressure, but potassium supplements should only be used in consultation with a medical provider 42. Increasing potassium intake to recommended levels (see Table 1) by consuming a diet rich in fruit and vegetables (see Table 2) can help lower blood pressure and may have additional benefits to health 42. Blood pressure is a reliable cardiovascular diseases (CVDs) risk marker 103. Yet, although reducing sodium consumption while increasing potassium intake helps with lowering blood pressure 104, current evidence suggests that dietary advice and support interventions may not be sufficient to deliver long-term cardiovascular benefits in individuals with hypertension 105.
Stroke
Higher potassium intakes have been associated with a decreased risk of stroke and possibly other cardiovascular diseases (CVDs) 106. Observational studies have consistently reported an increased risk of cardiovascular disease with elevated dietary sodium intakes 107, 108. Several prospective cohort studies have also found an inverse association between potassium intake and risk of stroke. A meta-analysis of 11 prospective cohort studies in 247,510 adults found that a 1,640 mg per day higher potassium intake was associated with a significant 21% lower risk of stroke as well as nonsignificant lower risks of coronary heart disease and total cardiovascular disease 109. Similarly, the authors of a meta-analysis of 9 cohort studies reported a significant 30% lower risk of stroke with daily potassium intakes ranging between 3,510 mg and 4,680 mg and a nonsignificant reduction in coronary heart disease and cardiovascular disease (CVD) risk 110. However, the Agency for Healthcare Research and Quality (AHRQ) review found inconsistent relationships between potassium intakes and risk of stroke based on 15 observational studies 74.
In a more recent meta-analysis of 16 studies, the highest versus lowest dietary potassium intake was found to be associated with a 13% lower risk of stroke after multiple adjustments (including for blood pressure) 111. The lowest risk of stroke corresponded to daily potassium intakes around 3,500 mg. Subgroup analyses showed a reduced risk of ischemic stroke, but not hemorrhagic stroke. Finally, in a recent meta-analysis of 16 observational studies, each 1-unit increase in the dietary sodium-to-potassium ratio was found to be associated with a 22% higher risk of stroke 86.
Any beneficial effect of potassium on cardiovascular disease (CVD) is likely due to its antihypertensive effects. However, some research shows a benefit even when blood pressure is accounted for. For example, a 2016 meta-analysis of 16 cohort studies with a total of 639,440 participants found that those with the highest potassium intakes (median 103 mmol [4,027 mg] per day) had a 15% lower risk of stroke than those with the lowest potassium intakes (median 52.5 mmol [2,053 mg] per day) 111. In addition, participants who consumed 90 mmol potassium/day (approximately 3,500 mg) had the lowest risk of stroke 111. However, even when blood pressure was accounted for, higher potassium intakes still produced a significant 13% lower risk of stroke. These findings suggest that other mechanisms (e.g., improved endothelial function and reduced free radical formation) may be involved 86.
The FDA has approved the following health claim: “Diets containing foods that are a good source of potassium and that are low in sodium may reduce the risk of high blood pressure and stroke” 112. Overall, the evidence suggests that consuming more potassium might have a favorable effect on blood pressure and stroke, and it might also help prevent other forms of cardiovascular disease. However, more research on both dietary and supplemental potassium is needed before firm conclusions can be drawn.
Kidney stones
Kidney stones are most common in people aged 40 to 60 113. Stones containing calcium in the form of calcium oxalate or calcium phosphate are the most common type of kidney stone. Low potassium intakes impair calcium reabsorption within the kidney, increasing urinary calcium excretion and potentially causing abnormally high urinary calcium (hypercalciuria) and kidney stones 61, 86. Low urinary levels of citrate also contribute to kidney stone development.
In individuals with a history of developing calcium-containing kidney stones, increased dietary acid load has been significantly associated with increased urinary calcium excretion 114. Increasing dietary potassium (and alkali) intake by increasing fruit and vegetable intake or by taking potassium bicarbonate (KHCO3) supplements has been found to decrease urinary calcium excretion 115, 116. Conversely, potassium deprivation has been found to increase urinary calcium excretion 115, 116.
Observational studies show inverse associations between dietary potassium intakes and risk of kidney stones. In a cohort of 45,619 men aged 40 to 75 years with no history of kidney stones, those with the highest potassium intakes (≥4,042 mg/day on average) had a 51% lower risk of kidney stones over 4 years of follow-up than those with the lowest intakes (≤2,895 mg/day) 117. Similarly, in over 90,000 women aged 34–59 who participated in the Nurses’ Health Study and had no history of kidney stones, those who consumed an average of over 4,099 mg of potassium per day had a 35% lower risk of kidney stones over a 12-year follow-up period than those who averaged less than 2,407 mg of potassium per day 118.
Three large US prospective cohort studies — the Health Professionals Follow-up Study and the Nurses’ Health Studies I and II — which included 193,676 participants, have examined dietary potassium intake and animal protein-to-potassium ratio (a marker of dietary acid load) in the diet in relation to the risk of developing kidney stones 119. In all three cohorts, dietary potassium intake was derived almost entirely from potassium-rich foods, such as fruit and vegetables. Across the three cohorts, individuals in the highest quintile of potassium intake were found to be 33%-56% less likely to develop symptomatic kidney stones than those in the lowest quintile of intake 119. Additionally, a pooled analysis of the data from all three cohorts showed that those with the highest versus lowest animal protein-to-potassium ratio were 41% more likely to develop kidney stones 119.
Some research suggests that supplementation with potassium citrate reduces hypercalciuria as well as the risk of kidney stone formation and growth 113. In a clinical trial of 57 patients with at least two kidney stones (either calcium oxalate or calcium oxalate plus calcium phosphate) over the previous 2 years and hypocitraturia (low urinary citrate levels), supplementation with 30–60 mEq potassium citrate (providing 1,173 to 2,346 mg potassium) for 3 years significantly reduced kidney stone formation compared with placebo 120. This study was included in a 2015 Cochrane review of seven studies that examined the effects of potassium citrate, potassium-sodium citrate, and potassium-magnesium citrate supplementation on the prevention and treatment of calcium-containing kidney stones in a total of 477 participants, most of whom had calcium oxalate stones 113. The potassium citrate salts significantly reduced the risk of new stones and reduced stone size. However, the proposed mechanism involves citrate, not potassium per se; citrate forms complexes with urinary calcium and increases urine pH, inhibiting the formation of calcium oxalate crystals 113, 121. Urinary alkalinization with supplemental potassium citrate is used in kidney stone formers to reduce the risk of recurrent kidney stone formation 122. However, potassium citrate therapy should only be initiated under the supervision of a medical provider.
The authors of the Agency for Healthcare Research and Quality (AHRQ) review concluded that observational studies suggest an association between higher potassium intakes and lower risk of kidney stones 74. However, they also found the evidence insufficient to determine whether potassium supplements are effective because only one trial that addressed this question 120 met their inclusion criteria.
Additional research is needed to fully understand the potential link between dietary and supplemental potassium and the risk of kidney stones.
Bone health
Observational studies suggest that increased consumption of potassium from fruits and vegetables is associated with increased bone mineral density (BMD) 123. This evidence, combined with evidence from metabolic studies and a few clinical trials, suggests that dietary potassium may improve bone health.
In a 2015 case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk study, which included 5,319 individuals, dietary intakes of potassium (alone or combined with intakes of magnesium) were found to be inversely associated with heel bone (calcaneus) broadband ultrasound attenuation (BUA) measurements (a predictor of the risk of incidental fracture) and risk of hip fracture in women but not in men 124. More recently, a cross-sectional study in older Korean adults reported higher total hip and femur neck bone mineral density (BMD) in those in the top versus bottom tertile of potassium intakes 125. Although these observational studies suggest a link between potassium intakes and bone health, they cannot establish whether there is a cause-and-effect relationship 42.
The underlying mechanisms by which potassium might influence bone health are poorly understood, but one hypothesis is that potassium helps protect bone through its effect on acid-base balance 86. Western diets tend to be relatively low in sources of alkali (fruit and vegetables) and high in sources of acid (fish, meat, and cheese) 126. When the quantity of bicarbonate ions is insufficient to maintain normal pH, the body is capable of mobilizing alkaline calcium salts from bone in order to neutralize acids consumed in the diet or generated by metabolism 127. Because fruit and vegetables are rich in both potassium and precursors to bicarbonate ions, increasing their consumption might help reduce the net acid content of the diet and preserve calcium in bones, which might otherwise be mobilized to maintain normal pH.
Diets that are high in acid-forming foods, such as meats and cereal grains, contribute to metabolic acidosis and might have an adverse effect on bone. Alkaline components in the form of potassium salts (potassium bicarbonate or citrate, but not potassium chloride) from food or potassium supplements might counter this effect and help preserve bone tissue. In the Framingham Heart Study for example, higher potassium intake was associated with significantly greater bone mineral density in 628 elderly men and women 128. In another study, the DASH eating pattern significantly reduced biochemical markers of bone turnover 129. The DASH eating pattern has a lower acid load than typical Western diets and is also high in calcium and magnesium, in addition to potassium, so any independent contribution of potassium cannot be determined 23.
Only a few clinical trials have examined the effects of potassium supplements on markers of bone health. One trial found that supplementation with potassium citrate at either 60 mmol/day (2,346 mg potassium) or 90 mmol/day (3,519 mg potassium) for 6 months significantly reduced urinary calcium excretion compared with placebo in 52 healthy men and women older than 55 years 130. In another clinical trial, 201 healthy adults aged 65 years or older received daily supplementation with 60 mEq potassium citrate (providing 2,346 mg potassium) or placebo as well as 500 mg/day calcium (as calcium carbonate) and 400 IU/day vitamin D3 for 2 years 131. Potassium supplementation significantly increased bone mineral density at the lumbar spine and bone microarchitecture compared with placebo. In a similar clinical trial among older adults, supplemental potassium bicarbonate (mean doses of 2,893 or 4,340 mg/day potassium) for 84 days significantly reduced biochemical markers of bone turnover and urinary calcium excretion 132. Conversely, a clinical trial in 276 postmenopausal women aged 55–65 years found that supplementation with potassium citrate at either 18.5 mEq/day (providing 723 mg potassium) or 55.5 mEq/day (2,170 mg potassium) for 2 years did not significantly reduce bone turnover or increase bone mineral density at the hip or lumbar spine compared with placebo 133.
Overall, higher intakes of potassium from diets that emphasize fruits and vegetables might improve bone health. However, more research is needed to elucidate the underlying mechanisms and tease out potassium’s individual contribution 23.
Blood glucose control and type 2 diabetes
Type 2 diabetes is a growing public health concern that currently affects almost 12% of U.S. adults 134. Although obesity is the primary risk factor for type 2 diabetes, other metabolic factors also play a role. Because potassium is needed for insulin secretion from pancreatic cells, hypokalemia impairs insulin secretion and could lead to glucose intolerance 4. This effect has been observed mainly with long-term use of diuretics (particularly those containing thiazides) or hyperaldosteronism (excessive aldosterone production), which both increase urinary potassium losses, but it can occur in healthy individuals as well 4, 25, 61, 135.
Numerous observational studies of adults have found associations between lower potassium intakes or lower serum or urinary potassium levels and increased rates of fasting glucose, insulin resistance, and type 2 diabetes 136, 137, 138, 139, 140, 141, 142. These associations might be stronger in African Americans, who tend to have lower potassium intakes, than in whites 138, 141. For example, one study of 1,066 adults aged 18–30 years without diabetes found that those with urinary potassium levels in the lowest quintile were more than twice as likely to develop type 2 diabetes over 15 years of follow-up than those in the highest quintile 138. Among 4,754 participants from the same study with potassium intake data, African Americans with lower potassium intakes had a significantly greater risk of type 2 diabetes over 20 years of follow-up than those with higher intakes, but this association was not found in whites 138.
In another observational study, which analyzed data from 84,360 women aged 34–59 years participating in the Nurses’ Health Study, those in the highest quintile of potassium intake had a 38% lower risk of developing type 2 diabetes over 6 years of follow-up than those in the lowest quintile 136. Serum potassium levels were inversely associated with fasting glucose levels in 5,415 participants aged 45–84 years from the Multi-Ethnic Study of Atherosclerosis, but these levels had no significant association with diabetes risk over 8 years of follow-up 140.
Although observational studies suggest that potassium status is linked to blood glucose control and type 2 diabetes, this association has not been adequately evaluated in clinical trials. In a small clinical trial in 29 African American adults with prediabetes and low to normal serum potassium levels (3.3–4.0 mmol/L), supplementation with 40 mEq (1,564 mg) potassium (as potassium chloride) for 3 months significantly lowered fasting glucose levels, but it did not affect glucose or insulin measures during an oral glucose tolerance test 143.
The findings from studies conducted to date are promising. But more research, including randomized controlled trials, is needed before potassium’s link with blood glucose control and type 2 diabetes can be confirmed 23.
High Potassium (Hyperkalemia)
Hyperkalemia is the medical term that describes a potassium level in your blood that is greater than 5 mEq/L or greater than 5 mmol/L (Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html)), 144. Your blood potassium level is normally between 3.5 to 5.0 millimoles per liter (mmol/L) or 3.5 to 5.0 milliequivalent per liter (mEq/L) 1, 34, 35, 36, 145.
Potassium is a nutrient that is critical to the function of nerve and muscle cells, including those in your heart 146.
In healthy people with normal kidney function, high dietary potassium intakes do not pose a health risk because the kidneys eliminate excess potassium in the urine 51. Although case reports indicate that very large doses of potassium supplements can cause heart abnormalities and death, the National Academies of Sciences, Engineering, and Medicine committee concluded that these reports do not provide sufficient evidence to set a Tolerable Upper Intake Level (UL) 102. In addition, there is no evidence that high intakes of potassium cause hyperkalemia in adults with normal kidney function or other adverse effects. Therefore, the committee did not set a Tolerable Upper Intake Level (UL) for potassium 102.
However, in people with impaired urinary potassium excretion due to chronic kidney disease or the use of certain medications, such as angiotensin converting enzyme (ACE) inhibitors or potassium-sparing diuretics, even dietary potassium intakes below the Adequate Intake (see Table 1) can cause hyperkalemia 102. Hyperkalemia can also occur in people with type 1 diabetes, congestive heart failure, adrenal insufficiency, or liver disease 26, 147. Individuals at risk of hyperkalemia should consult a physician or registered dietitian about appropriate potassium intakes from all sources.
Often a report of high blood potassium isn’t true hyperkalemia also known as pseudohyperkalemia. Instead, pseudohyperkalemia (false elevation in measured potassium) may be caused by the rupture of blood cells in the blood sample during or shortly after the blood draw due to specimen collection, handling, or other causes 148, 149. The ruptured cells leak their potassium into the sample. This falsely raises the amount of potassium in the blood sample, even though the potassium level in your body is actually normal. When this is suspected, a repeat blood sample is done 149.
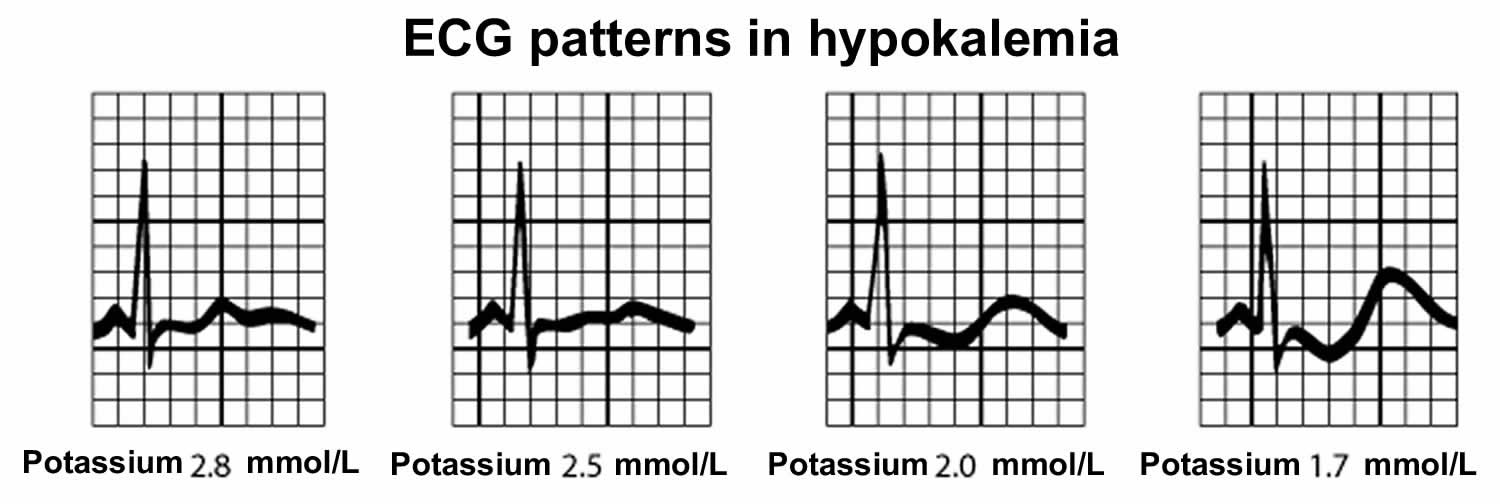
While mild hyperkalemia is usually asymptomatic, very high potassium levels (blood potassium level higher than 6.0 mmol/L) may cause life-threatening cardiac arrhythmias, heart palpitations, muscle weakness, paralysis and paresthesias (a burning or prickling sensation in the extremities) and requires immediate treatment 148, 26, 147, 150. Symptoms usually develop at higher levels, 6.5 mEq/L to 7 mEq/L, but the rate of change is more important than the numerical value 148.
Patients with chronic hyperkalemia may be asymptomatic at increased levels, while patients with dramatic, acute potassium shifts may develop severe symptoms at lower ones 148. Infants have higher baseline levels than children and adults 148.
Talk to your doctor about what your results mean. Hyperkalemia should always be confirmed before aggressive treatment in cases where the serum potassium is elevated without explanation 148. True hyperkalemia may be caused by increased potassium intake, transcellular movement of intracellular potassium into the extracellular space, and decreased renal excretion. The urgency of therapy depends on symptoms, serum levels, and causes of hyperkalemia 151, 152. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your high potassium level. Treatment of high potassium is often directed at the underlying cause. In some instances, you may need emergency medications or dialysis.
High potassium symptoms
There are often no symptoms with a high level of potassium.
High potassium is usually found when your doctor has ordered blood tests to help diagnose a condition you’re already experiencing or to monitor medications you’re taking. It’s usually not discovered by chance.
If you have symptoms of hyperkalemia, particularly if you have kidney disease or are taking medications that raise your potassium level, call your doctor immediately. Hyperkalemia is a serious and potentially life-threatening disorder. It can cause:
- Muscle fatigue
- Weakness
- Paralysis
- Difficulty breathing
- Abnormal heart rhythms (arrhythmias) – slow, weak, or irregular pulse
- Palpitations
- Nausea or vomiting
- Chest pain
- Sudden collapse, when the heartbeat gets too slow or even stops
Talk to your doctor about what your results mean. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your high potassium level 153. Treatment of high potassium is often directed at the underlying cause. In some instances, you may need emergency medications or dialysis.
If you have symptoms of hyperkalemia and have reason to think your potassium level might be high, call your doctor immediately.
Hyperkalemia (high potassium) causes
The most common cause of high blood potassium or hyperkalemia is pseudohyperkalemia, which is not reflective of the true serum potassium levels 148. Instead, it may be caused by the rupture of blood cells in the blood sample (hemolysis of the sample) during or shortly after the blood draw. The ruptured blood cells leak their potassium into the sample. This falsely raises the amount of potassium in the blood sample (pseudohyperkalemia), even though the potassium level in your body is actually normal. When this is suspected, a repeat blood sample is done. Hemolysis is more common when a syringe is used than a vacuum device. Using tourniquets and excessive fist-pumping during the blood draw also increase the risk. Specimens drawn from patients with leukocytosis or thrombocytosis are also frequently associated with falsely elevated potassium concentrations 148.
The most common cause of genuinely high potassium (hyperkalemia) is related to your kidneys, such as 154:
- Acute kidney failure also called acute renal failure or acute kidney injury occurs when your kidneys suddenly become unable to filter waste products from your blood. Acute kidney failure can occur when 155:
- You have a condition that slows blood flow to your kidneys. Diseases and conditions that may slow blood flow to the kidneys and lead to kidney injury include:
- Blood or fluid loss
- Blood pressure medications
- Heart attack
- Heart disease
- Infection
- Liver failure
- Use of aspirin, ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve, others) or related drugs
- Severe allergic reaction (anaphylaxis)
- Severe burns
- Severe dehydration
- You experience direct damage to your kidneys. Diseases, conditions and agents that may damage the kidneys and lead to acute kidney failure include:
- Blood clots in the veins and arteries in and around the kidneys
- Cholesterol deposits that block blood flow in the kidneys
- Glomerulonephritis, inflammation of the tiny filters in the kidneys (glomeruli)
- Hemolytic uremic syndrome, a condition that results from premature destruction of red blood cells
- Infection, such as with the virus that causes coronavirus disease 2019 (COVID-19)
- Lupus, an immune system disorder causing glomerulonephritis
- Medications, such as certain chemotherapy drugs, antibiotics and dyes used during imaging tests
- Scleroderma, a group of rare diseases affecting the skin and connective tissues
- Thrombotic thrombocytopenic purpura, a rare blood disorder
- Toxins, such as alcohol, heavy metals and cocaine
- Muscle tissue breakdown (rhabdomyolysis) that leads to kidney damage caused by toxins from muscle tissue destruction
- Breakdown of tumor cells (tumor lysis syndrome), which leads to the release of toxins that can cause kidney injury
- Your kidneys’ urine drainage tubes (ureters) become blocked and wastes can’t leave your body through your urine. Diseases and conditions that block the passage of urine out of the body (urinary obstructions) and can lead to acute kidney injury include:
- Bladder cancer
- Blood clots in the urinary tract
- Cervical cancer
- Colon cancer
- Enlarged prostate
- Kidney stones
- Nerve damage involving the nerves that control the bladder
- Prostate cancer
- You have a condition that slows blood flow to your kidneys. Diseases and conditions that may slow blood flow to the kidneys and lead to kidney injury include:
- Chronic kidney disease also called chronic kidney failure, involves a gradual loss of kidney function. Chronic kidney disease occurs when a disease or condition impairs kidney function, causing kidney damage to worsen over several months or years. Diseases and conditions that cause chronic kidney disease include 156:
- Type 1 or type 2 diabetes
- High blood pressure
- Glomerulonephritis, an inflammation of the kidney’s filtering units (glomeruli)
- Interstitial nephritis, an inflammation of the kidney’s tubules and surrounding structures
- Polycystic kidney disease or other inherited kidney diseases
- Prolonged obstruction of the urinary tract, from conditions such as enlarged prostate, kidney stones and some cancers
- Vesicoureteral reflux, a condition that causes urine to back up into your kidneys
- Recurrent kidney infection, also called pyelonephritis
Glomerular filtration rate (GFR) is a measure of how well your kidneys filter blood and it is equal to the total of the filtration rates of the functioning nephrons in the kidney 157. Normal GFR varies according to age, sex, and body size; in young adults, it is approximately 120 mL/minute/1.73 m² and declines with age. Acute or chronic kidney disease hyperkalemia is usually not seen until the glomerular filtration rate (GFR) falls below 30 mL/min/1.73 m² 148. This is commonly due to primary kidney dysfunction but may be due to acute volume depletion from dehydration or bleeding or decreased circulating blood volume due to congestive heart failure or cirrhosis. Tubular dysfunction due to aldosterone deficiency or insensitivity can also cause hyperkalemia.
Other causes of hyperkalemia include:
- Addison’s disease (adrenal failure). Disease in which the adrenal glands do not make enough aldosterone hormone (and cortisol), reducing the kidneys’ ability to remove potassium from the body
- Alcoholism or heavy drug use that causes rhabdomyolysis, a breakdown of muscle fibers that results in the release of potassium into the bloodstream
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin 2 receptor blockers (ARBs)
- Beta blockers
- Dehydration (which is when the body doesn’t have enough water and other fluids to work properly)
- Destruction of red blood cells due to severe injury or burns
- Excessive use of potassium supplements
- Type 1 diabetes
ACE inhibitors and angiotensin receptor blockers (ARBs)
ACE inhibitors, such as benazepril (Lotensin®), and angiotensin 2 receptor blockers (ARBs) such as losartan (Cozaar®), are used to treat hypertension and heart failure, slow progression of kidney disease in patients with chronic kidney disease and type 2 diabetes, and decrease morbidity and mortality after myocardial infarction 80. These medications reduce urinary potassium excretion, which can lead to hyperkalemia. Experts recommend monitoring potassium status in people taking ACE inhibitors or angiotensin receptor blockers (ARBs), especially if they have other risk factors for hyperkalemia, such as impaired kidney function 80.
Potassium sparing diuretics
Potassium-sparing diuretics, such as amiloride (Midamor®) and spironolactone (Aldactone®), reduce the excretion of potassium in the urine and can cause hyperkalemia 82. Experts recommend monitoring potassium status in people taking these medications, especially if they have impaired kidney function or other risk factors for hyperkalemia 82.
Loop and thiazide diuretics
Treatment with loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®), and thiazide diuretics, such as chlorothiazide (Diuril®) and metolazone (Zaroxolyn®), increases urinary potassium excretion and can lead to hypokalemia 82. Experts recommend monitoring potassium status in people taking these medications, and initiating potassium supplementation if warranted.
Intracellular potassium shifts
Cellular injury can release large quantities of intracellular potassium into the extracellular space. This can be due to rhabdomyolysis from a crush injury, excessive exercise, or other hemolytic processes. Metabolic acidosis may cause intracellular potassium to shift into the extracellular space without red cell injury. Metabolic acidosis is most frequently caused by decreased, effective circulating arterial blood volume. Sepsis or dehydration may lead to hypotension and decreased tissue perfusion leading to metabolic acidosis with subsequent potassium elevation.
Insulin deficiency and diabetic ketoacidosis may cause dramatic extracellular shifts causing measured serum potassium to be elevated in the setting of whole-body potassium depletion. Certain medications, such as succinylcholine, may cause severe, acute potassium elevations in patients with up-regulation of receptors, particularly in subacute neuromuscular disease. Tumor lysis syndrome, particularly in patients receiving chemotherapy for hematogenous malignancy, may cause acute hyperkalemia due to massive cancer cell death 158.
Hyperkalemic periodic paralysis (hyperPP or hyperKPP) is a rare, autosomal dominant condition caused by a mutation in the SCN4A gene that codes for voltage-gated sodium channel causing potassium to shift into the extracellular space due to impaired sodium channel function in skeletal muscle 159, 160. Hyperkalemic periodic paralysis (hyperPP) is characterized by attacks of flaccid limb weakness (which may also include weakness of the muscles of the eyes, throat, breathing muscles, and trunk), hyperkalemia (serum potassium concentration greater than 5 mmol/L) or an increase of serum potassium concentration of at least 1.5 mmol/L during an attack of weakness and/or provoking/worsening of an attack by oral potassium intake, normal serum potassium between attacks, and onset before age 20 years 161, 160. In approximately half of affected individuals, attacks of flaccid muscle weakness begin in the first decade of life, with 25% reporting their first attack at age ten years or older 160. Initially infrequent, the attacks then increase in frequency and severity over time until approximately age 50 years, after which the frequency of attacks declines considerably 160. The major attack trigger is eating potassium-rich foods; other triggers include: cold environment; rest after exercise, stress, or fatigue; alcohol; hunger; and changes in activity level. A spontaneous attack commonly starts in the morning before breakfast, lasts for 15 minutes to one hour, and then passes. Individuals with hyperPP frequently have myotonia (muscle stiffness), especially around the time of an episode of weakness. Paramyotonia (muscle stiffness aggravated by cold and exercise) is present in about 45% of affected individuals. More than 80% of individuals with hyperkalemic periodic paralysis (hyperPP) older than age 40 years report permanent muscle weakness and about one third develop a chronic progressive myopathy 160. Hyperkalemic periodic paralysis (hyperPP) is caused by a mutation in the SCN4A gene that codes for voltage-gated sodium channel Na1.4 159. Several diagnostic modalities exist in assisting diagnosis, such as genetic testing, although they are not always definitive. In case of diagnostic uncertainty, a provocative test can be employed, although the availability of genetic testing and electrophysiologic studies largely obviates the need for such dangerous tests. Treatment for hyperkalemic periodic paralysis (hyperPP) is both proactive and reactive, with avoidance of triggers being the mainstay of therapy. At the onset of weakness, attacks may be prevented or aborted with mild exercise and/or oral ingestion of carbohydrates, intravenously injected glucocorticoids, inhalation of salbutamol, or intravenous calcium gluconate 160. Hyperkalemic attacks of weakness can be prevented by frequent meals rich in carbohydrates; continuous use of a thiazide diuretic or a carbonic anhydrase inhibitor; and avoidance of potassium-rich medications and foods, fasting, strenuous work, and exposure to cold 160.
Increased potassium intake
Increased potassium intake from food is a very uncommon cause of hyperkalemia in adult patients with normal kidney function but can be an important cause in those with kidney disease. Foods with high potassium content include dried fruits, seaweed, nuts, molasses, avocados, and Lima beans. Many vegetables that are also high in potassium include spinach, potatoes, tomatoes, broccoli, beets, carrots, and squash. High-potassium-containing fruits include kiwis, mangoes, oranges, bananas, and cantaloupe. Red meats are also rich in potassium. While generally safe to consume even in large quantities by patients with normal potassium homeostasis, these foods should be avoided in patients with severe renal disease or other underlying conditions or medications predisposing them to hyperkalemia. Intravenous intake through high potassium-containing fluids, particularly total parenteral nutrition, medications with high potassium content, and massive blood transfusions can significantly elevate serum potassium levels.
Can hyperkalemia be prevented?
Dietary changes can help prevent and treat high potassium levels. Talk to your doctor to understand any risk you might have for hyperkalemia. Your doctor may recommend foods that you may need to limit or avoid. These may include:
- asparagus, avocados, potatoes, tomatoes or tomato sauce, winter squash, pumpkin, cooked spinach
- oranges and orange juice, nectarines, kiwifruit, bananas, cantaloupe, honeydew, prunes and raisins or other dried fruit.
If you are on a low-salt diet, avoid taking salt substitutes 162.
Hyperkalemia diagnosis
Your health care provider will perform a physical exam and ask about your symptoms. Most patients with mild and even moderate hyperkalemia are relatively asymptomatic 148. High potassium is often discovered on screening blood tests done in patients with nonspecific complaints, to monitor medications you’re taking or those with suspected electrolyte abnormalities due to infection, dehydration, or hypoperfusion. High potassium is usually not discovered by chance. Causes include renal disease, diabetes, chemotherapy, major trauma, crush injury, or muscle pain suggestive of rhabdomyolysis. Medications that may predispose to the development of hyperkalemia include digoxin, potassium-sparing diuretics, non-steroidal anti-inflammatory drugs, ace-inhibitors or recent intravenous (IV) potassium, total parenteral nutrition, potassium penicillin, or succinylcholine. Patients may complain of weakness, fatigue, palpitations, or syncope.
Physical exam findings may include hypertension and edema in the setting of kidney disease. There may also be signs of hypoperfusion. Muscle tenderness may be present in patients with rhabdomyolysis. Jaundice may be seen in patients with hemolytic conditions. Patients may have muscle weakness, flaccid paralysis, or depressed deep tendon reflexes.
Tests that may be ordered include:
- Electrocardiogram (ECG). The first test that should be ordered in a patient with suspected hyperkalemia is an ECG since the most lethal complication of hyperkalemia is cardiac condition abnormalities which can lead to abnormal heart rhythms (arrhythmias) and death.
- Potassium level
Elevated potassium causes ECG changes in a dose-dependent manner 148:
- Potassium level = 5.5 to 6.5 mEq/L ECG will show tall, peaked T-waves
- Potassium level = 6.5 to 7.5 mEq/L ECG will show loss of P-waves
- Potassium level = 7 to 8 ECG mEq/L will show widening of the QRS complex
- Potassium level = 8 to 10 mEq/L will produce cardiac arrhythmias, sine wave pattern, and asystole
It should be noted that the rate of rising serum potassium is a greater factor than the potassium level 148. Patients with chronic hyperkalemia may have relatively normal EGCs even at high levels, and significant ECG changes may be present at much lower levels in patients with sudden spikes in serum potassium 148.
ECG features of hyperkalemia include 148:
- Small or absent P wave
- Prolonged PR interval
- Augmented R wave
- Wide QRS
- Peaked T waves
Since pseudohyperkalemia is so common, confirmation should be obtained in asymptomatic patients without typical ECG changes before initiating aggressive therapy 148.
Your doctor will also likely check your blood potassium level and do kidney blood tests on a regular basis if you:
- Have been prescribed extra potassium
- Have chronic kidney disease
- Take medicines to treat heart disease or high blood pressure
- Use salt substitutes
Additional laboratory testing should include serum blood urea nitrogen (BUN) and creatinine to assess renal function and urinalysis to screen for renal disease. Urine potassium, sodium, and osmolality may also help evaluate the cause. In patients with renal disease, the serum calcium level should also be checked because hypocalcemia may exacerbate the cardiac effects of hyperkalemia. A complete blood count to screen for leukocytosis or thrombocytosis may also be helpful. Serum glucose and blood gas analysis should be ordered in diabetics and patients with suspected acidosis. Lactate dehydrogenase should be ordered in patients with suspected hemolysis. Creatinine phosphokinases and urine myoglobin should be ordered in patients with suspected rhabdomyolysis. Uric acid and phosphorus should be ordered in patients with suspected tumor lysis syndrome. Digoxin toxicity may cause hyperkalemia, so serum levels should be checked in patients on digoxin. If no other cause is found, consider cortisol and aldosterone levels to assess for mineralocorticoid deficiency.
Hyperkalemia treatment
The urgency with which hyperkalemia should be managed depends on how rapidly the condition develops, the absolute serum potassium level, the degree of symptoms, and the cause 163, 164, 165. If your potassium level is very high (more than 5.5 mEq/L in patients at risk for ongoing hyperkalemia or confirmed hyperkalemia of 6.5 mEq/L), or if there are dangerous indications such as changes in an electrocardiogram (ECG), neuromuscular weakness or paralysis, emergency treatment is needed. That may involve supplying calcium to the body through an intravenous to treat the effects on muscles and the heart or administering glucose and insulin through an intravenous to decrease potassium levels long enough to correct the cause. There are also medicines that help remove the potassium from your intestines and in some cases, a diuretic may be given.
Emergency treatment may also include kidney dialysis if kidney function is deteriorating; medication to help remove potassium from the intestines before absorption; sodium bicarbonate if acidosis is the cause; and water pills, or diuretics.
A doctor may also advise stopping or reducing potassium supplements and stopping or changing the doses of certain medicines for heart disease and high blood pressure. Always follow your health provider’s instructions about taking or stopping medicines.
Hyperkalemia treatment is usually prescribed in the following manner 148:
- Exogenous sources of potassium should be immediately discontinued.
- Treatment of the reversible cause should begin along with the management of hyperkalemia.
- Calcium given into your veins (IV) to treat the muscle and heart effects of high potassium levels. Calcium therapy will stabilize the cardiac response to hyperkalemia and should be initiated first in the setting of cardiac toxicity. Calcium does not alter the serum concentration of potassium but is a first-line therapy in hyperkalemia-related arrhythmias and ECG changes. Calcium chloride contains three times more elemental calcium than calcium gluconate but is more irritating to peripheral vessels and more likely to cause tissue necrosis with extravasation, so it is usually only given through central venous lines or peripherally in cardiac arrest. Thus, calcium gluconate is the usual initial drug of choice in patients with evidence of cardiac toxicity 166. As a precaution, calcium should never be given in bicarbonate-containing fluids, as it can cause the precipitation of calcium carbonate.
- Insulin and glucose given into your veins (IV) to help lower potassium levels long enough to correct the cause or insulin alone in hyperglycemic patients, will drive the potassium back into the cells, effectively lowering serum potassium 167. A common regimen is ten units of regular insulin given with 50 ml of a 50% dextrose solution (D50). Patients should be monitored closely for the development of hypoglycemia (low blood sugar level). A 10% dextrose infusion at 50 to 75 ml/hour is associated with less hypoglycemia than bolus dosing with 50% dextrose solution (D50).
- Beta-2 adrenergic agents such as albuterol will also shift potassium intracellularly 168. To be effective, beta-2 agonists are given in much higher doses than those commonly used for bronchodilation. Intravenous epinephrine, however, should not be used to manage hyperkalemia due to an increased risk of causing angina.
- Sodium bicarbonate infusion may be helpful in patients with metabolic acidosis. Bolus dosing of sodium bicarbonate is less effective.
- Loop or thiazide diuretics may help enhance potassium excretion. They may be used in non-oliguric, volume-overloaded patients but should not be used as monotherapy in symptomatic patients. In hypervolemic patients with preserved kidney function (e.g., patients with congestive cardiac failure), 40 mg of intravenous furosemide is administered every 12 hours or may be given as a continuous infusion. In euvolemic or hypovolemic patients with preserved kidney function, an isotonic saline infusion is given before as needed to the patient before administering 40 mg of intravenous furosemide every 12 hours or a continuous furosemide infusion.
- Medicines that help remove potassium from the intestines before it is absorbed such as patiromer may be helpful, particularly in patients with renal insufficiency who cannot receive immediate dialysis. Sodium polystyrene sulfonate, though commonly used, is falling out of favor due to lack of effectiveness and adverse effects, particularly bowel necrosis in elderly patients. If used due to a lack of alternatives, it should not be given with sorbitol, which increases toxicity 169.
- Kidney dialysis should be performed in patients with end-stage renal disease or severe renal impairment.
Complications of hyperkalemia treatment 148:
- Hypokalemia
- Inability to control hyperkalemia
- Hypocalcemia as a result of bicarbonate infusion
- Hypoglycemia due to insulin
- Metabolic alkalosis from bicarbonate therapy
- Volume depletion from diuresis.
Changes in your diet can help both prevent and treat high potassium levels. You may be asked to:
- Limit or avoid asparagus, avocados, potatoes, tomatoes or tomato sauce, winter squash, pumpkin, and cooked spinach
- Limit or avoid oranges and orange juice, nectarines, kiwifruit, raisins, or other dried fruit, bananas, cantaloupe, honeydew, prunes, and nectarines
- Avoid taking salt substitutes if you are asked to eat a low-salt diet
Your doctor may make the following changes to your medicines:
- Reduce or stop potassium supplements
- Stop or change the doses of medicines you are taking, such as ones for heart disease and high blood pressure
- Take a certain type of water pill to reduce potassium and fluid levels if you have chronic kidney failure
Follow your doctor’s directions when taking your medicines:
- DO NOT stop or start taking medicines without first talking to your provider
- Take your medicines on time
- Tell your provider about any other medicines, vitamins, or supplements you are taking
For people with heart failure
There are some drugs that heart failure patients take that are associated with hyperkalemia. These are: diuretics, beta-blockers and angiotension converting enzyme inhibitors (ACE inhibitors). For patients with heart failure on these drugs, if any symptoms are experienced as above, you should tell your doctor to make sure that the symptoms are not related to hyperkalemia.
If your potassium level is too high, you may need to cut back on certain foods (see Table 5). These tips can also help:
- Soak or boil vegetables and fruits to leach out some of the potassium.
- Avoid foods that list potassium or K, KCl, or K+ — chemical symbols for potassium or related compounds — as ingredients on the label.
- Stay away from salt substitutes. Many are high in potassium. Read the ingredient lists carefully and check with your doctor before using one of these preparations.
- Avoid canned, salted, pickled, corned, spiced, or smoked meat and fish.
- Avoid imitation meat products containing soy or vegetable protein.
- Limit high-potassium fruits such as bananas, citrus fruits, and avocados.
- Avoid baked potatoes and baked acorn and butternut squash.
- Don’t use vegetables or meats prepared with sweet or salted sauces.
- Avoid all types of peas and beans, which are naturally high in potassium.
Table 5. Potassium levels in common foods
| Foods | High potassium | Medium potassium | Low potassium | No potassium |
|---|---|---|---|---|
| Fruits and vegetables | Artichokes, avocados, bananas, broccoli, coconut, dried fruits, leafy greens, kiwis, nectarines, oranges, papayas, potatoes, prunes, spinach, tomatoes, winter squash, yams | Apples, apricots, asparagus, carrots, cherries, corn, eggplant, peaches, pears, peppers, pineapple juice, radishes | Blueberries, cauliflower, cucumbers, grapefruit, grapes, green beans, lettuce, strawberries | |
| Meat and protein | Dried beans and peas, imitation bacon bits, nuts, soy products | Beef, eggs, fish, peanut butter, poultry, pork, veal | ||
| Dairy | Milk, yogurt | Sour cream | ||
| Grains and processed foods | Plain bagel, plain pasta, oatmeal, white bread, white rice | Bran muffins and cereals, corn tortillas, whole-wheat bread | Fruit punches, jelly beans, nondairy topping, nondairy creamers |
Hyperkalemia prognosis
The prognosis is excellent for patients with mild transient hyperkalemia due to too much potassium in the diet if the inciting cause is addressed and treated. Sudden onset, extreme hyperkalemia can cause cardiac arrhythmias that can be deadly in up to two-thirds of cases if not rapidly treated 148. Hyperkalemia is an independent risk factor for death in hospitalized patients 148.
Low Potassium (Hypokalemia)
Low potassium levels also known as hypokalemia is defined as serum potassium level less than 3.6 mEq/L or less than 3.6 mmol/L (Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html)). Severe potassium deficiency or hypokalemia is most common in hospitalized patients, affecting up to 21% of hospitalized patients, usually because of the use of water pills (e.g., loop diuretics and thiazide diuretics) and other medications that cause your body to excrete too much potassium, but it is rare among healthy people with normal kidney function 171, 172. Because your kidneys work to maintain normal blood levels of potassium by flushing out excess amounts through urine. Potassium can also be lost through stool and sweat. Hypokalemia is also seen in people with inflammatory bowel diseases (Crohn’s disease, ulcerative colitis) that may cause diarrhea and malabsorption of nutrients.
Your body needs potassium for the contraction of muscles (including the heart), and for the functioning of many complicated proteins (enzymes). Potassium is found primarily in the skeletal muscle and bone, and participates with sodium to contribute to the normal flow of body fluids between the cells in the body. Potassium is predominantly intracellular (within a cell) where it is the most abundant cation (positive charged ion) and involved in cell regulation and several cellular processes. The fraction of potassium in the extracellular fluid is small. Therefore, plasma or serum potassium levels are not a reliable indicator of total body potassium stores. The normal concentration of potassium in the body is maintained through a combination of adjustments in acute cellular shifts between the extracellular and intracellular fluid compartments, regulated by your kidneys through the excretion of urine and, to a lesser extent, gastrointestinal losses. When your kidneys are functioning normally, the amount of potassium in your diet is sufficient for use by your body and the excess is usually excreted through urine and sweat. Body chemicals and hormones such as aldosterone also regulate potassium balance. Secretion of the hormone insulin, which is normally stimulated by food, prevents a temporary diet-induced hypokalemia by increasing cell absorption of potassium. When hypokalemia occurs, there is an imbalance resulting from a dysfunction in this normal process, or the rapid loss of urine or sweat without replacement of sufficient potassium.
Potassium deficiency or hypokalemia is rarely caused by low dietary potassium intake alone because potassium is found in so many foods (see Table 2) 23, 42, 173. However, insufficient dietary potassium in patients at risk of hypokalemia can precipitate hypokalemia 40. In rare cases, habitual consumption of large amounts of black licorice has resulted in hypokalemia 174, 175. Licorice contains a compound called glycyrrhizic acid that has similar physiologic effects to those of aldosterone, a hormone that increases urinary excretion of potassium.
Too little potassium in your blood or hypokalemia may be a sign of 39:
- Use of prescription diuretics (water pills)
- Fluid loss from diarrhea, vomiting, or heavy sweating
- Using too many laxatives
- Adrenal gland disorders, including Cushing’s syndrome and aldosteronism
- Kidney disease
- Alcohol use disorder
- Eating a lot of real licorice, which comes from licorice plants. Most licorice products sold in the U.S. don’t contain any real licorice. Check the package ingredient label to be sure.
- A diet too low in potassium (not common). Bananas, apricots, green leafy vegetables, avocados and many other foods are good sources of potassium that are part of a healthy diet.
Potassium deficiency or hypokalemia is most commonly a result of excessive loss of potassium from prolonged vomiting or diarrhea, use of some diuretics, laxative abuse, eating clay (a type of pica), heavy sweating, dialysis, using certain medications, some forms of kidney disease, inflammatory bowel disease (Crohn’s disease or ulcerative colitis)or metabolic disturbances. Hypokalemia can also be caused by refeeding syndrome (the metabolic response to initial refeeding after a starvation period) because of potassium’s movement into cells 7, 18, 26, 176, 177.
Magnesium deficiency also known as hypomagnesemia can also contribute to hypokalemia by increasing urinary potassium losses 41, 178, 179. Magnesium deficiency can also increase the risk of cardiac arrhythmias by decreasing intracellular potassium concentrations. More than 50% of individuals with clinically significant hypokalemia might have magnesium deficiency 179. In people with hypomagnesemia and hypokalemia, both should be treated concurrently 26.
In general, hypokalemia is associated with diagnoses of cardiac disease, kidney failure, malnutrition, and shock 180. Hypothermia (occurs when your body loses heat faster than it can produce heat and your body temperature falls below 95°F (35°C)) and increased blood cell production (for example, leukemia) are additional risk factors for developing hypokalemia 180. There are subsets of patients that are susceptible to the development of hypokalemia. For instance, psychiatric patients are at risk for hypokalemia due to their drug therapy 180. Hypokalemia is also prevalent in hospitalized patients, in particular, children, those who have a fever and those who are critically ill 180. Additionally, in developing countries, an increased risk of death is observed in children when severe hypokalemia is associated with diarrhea and severe malnutrition 180.
The symptoms of hypokalemia are related to alterations in membrane potential and cellular metabolism 40. Mild hypokalemia (serum potassium level is 3 to 3.4 mmol/L) is characterized by fatigue, muscle weakness and cramps, not feeling well, tiredness, and intestinal paralysis, which may lead to bloating, constipation, and abdominal pain 7, 42. Moderate to severe hypokalemia (serum potassium level less than about 2.5 mmol/L) can cause increased urination (polyuria or large volume of dilute urine); decreased brain function (encephalopathy) in patients with kidney disease; high blood sugar levels or glucose intolerance; muscle paralysis; difficulty breathing; and cardiac arrhythmias (irregular heartbeats), especially in individuals with underlying heart disease 41, 7, 52. Severe hypokalemia (serum potassium level less than about 2.5 mmol/L) may result in muscular paralysis or abnormal heart rhythms (cardiac arrhythmias) that can be life threatening 40, 41.
Getting too little potassium can increase blood pressure (hypertension), deplete calcium in bones, and increase the risk of kidney stones formation 42, 23. In the absence of treatment, hypokalemia can have serious complications and even be fatal.
Treatment of low potassium or hypokalemia is directed at the underlying cause and may include potassium supplements. Don’t start taking potassium supplements without talking to your doctor first. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your low potassium level.
Hypokalemia (low potassium) causes
Low potassium (hypokalemia) has many causes. Hypokalemia is always a symptom of another disorder, rather than a disease that occurs by itself. The most common cause is excessive loss of potassium through the urine (kaliuresis) due to prescription water or fluid pills (diuretics). Water pills or diuretics medications are often prescribed for people who have high blood pressure or heart disease.
The excessive excretion of potassium in the urine (kaliuresis) may also result from a deficiency of magnesium in the blood (hypomagnesemia), excessive mineralocorticoids such as aldosterone in the blood (hyperaldosteronism) which affect the electrolyte and fluid balance in the body (usually caused by endocrine diseases), kidney disorders, or from the use of high doses of penicillin.
Typically, the potassium level becomes low because too much potassium is lost from the digestive tract (gastrointestinal losses) due to prolonged diarrhea or vomiting, chronic laxative abuse, inadequate dietary intake of potassium, intestinal obstruction or infections such as fistulas in the intestines which continually drain intestinal fluids. Additionally, excessive perspiration due to hot weather or exercise can cause hypokalemia. Hypokalemia is rarely caused by consuming too little potassium because many foods (such as beans, dark leafy greens, potatoes, fish, and bananas) contain potassium.
In many adrenal disorders, such as Cushing syndrome, the adrenal glands produce too much aldosterone, a hormone that causes the kidneys to excrete large amounts of potassium.
Certain drugs (such as insulin, albuterol, and terbutaline) cause more potassium to move from blood into cells and can result in hypokalemia. However, these drugs usually cause temporary hypokalemia, unless another condition is also causing potassium to be lost.
Hypokalemia sometimes occurs with or is caused by a low level of magnesium in the blood (hypomagnesemia).
Causes of potassium loss leading to low potassium include:
- Excessive alcohol use
- Chronic kidney disease
- Diabetic ketoacidosis
- Diarrhea (causing anal irritation)
- Diuretics (water retention relievers)
- Excessive laxative use
- Excessive sweating
- Folic acid deficiency
- Prescription water or fluid pills (diuretics) use
- Primary aldosteronism
- Some antibiotic use
- Vomiting
Gastrointestinal tract losses
Gastrointestinal potassium losses are another common cause of hypokalemia, particularly among hospitalized patients 181. Abnormal gastrointestinal potassium losses occur in all of the following 182:
- Chronic diarrhea, including chronic laxative abuse and bowel diversion
- Clay (bentonite) ingestion, which binds potassium and greatly decreases absorption
- Rarely, villous adenoma of the colon, which causes massive potassium secretion
As a portion of daily potassium is excreted in the colon, lower gastrointestinal losses in the form of persistent diarrhea can also result in hypokalemia and may be accompanied by hyperchloremic acidosis 77.
Protracted vomiting or gastric suction (which removes volume and hydrochloric acid) causes renal potassium losses due to metabolic alkalosis and stimulation of aldosterone due to volume depletion; aldosterone and metabolic alkalosis both cause the kidneys to excrete potassium 182.
Renal potassium losses
Various disorders can increase renal potassium excretion.
Diuretic use is a common cause of renally mediated hypokalemia 183. When given in the same dosage, chlorthalidone is more likely to induce hypokalemia than hydrochlorothiazide, which is more often implicated because of its widespread use 184, 185. Diuretic-induced hypokalemia is dose-dependent and tends to be mild (3 to 3.5 mEq per L [3 to 3.5 mmol per L]), although it can be more severe when accompanied by other causes (e.g., gastrointestinal losses) 186.
Excess mineralocorticoid (ie, aldosterone) effect can directly increase potassium secretion by the distal nephrons and occurs in any of the following 182:
- Adrenal steroid excess that is due to Cushing syndrome, primary hyperaldosteronism, rare renin-secreting tumors, glucocorticoid-remediable aldosteronism (a rare inherited disorder involving abnormal aldosterone metabolism), and congenital adrenal hyperplasia.
- Bartter syndrome, an uncommon genetic disorder that is characterized by renal potassium and sodium wasting, excessive production of renin and aldosterone, and normotension. Bartter syndrome is caused by mutations in a loop diuretic–sensitive ion transport mechanism in the loop of Henle.
- Gitelman syndrome is an uncommon genetic disorder characterized by renal potassium and sodium wasting, excessive production of renin and aldosterone, and normotension. Gitelman syndrome is caused by loss of function mutations in a thiazide-sensitive ion transport mechanism in the distal nephron.
- Ingestion of substances such as glycyrrhizin (present in natural licorice and used in the manufacture of chewing tobacco), which inhibits the enzyme 11 beta-hydroxysteroid dehydrogenase (11β-HSDH), preventing the conversion of cortisol, which has some mineralocorticoid activity, to cortisone, which does not, resulting in high circulating concentrations of cortisol and renal potassium wasting.
- Liddle syndrome, a rare autosomal dominant disorder caused by unrestrained sodium reabsorption in the distal nephron due to one of several mutations found in genes encoding for epithelial sodium channel subunits. Inappropriately high reabsorption of sodium results in both severe hypertension and renal potassium wasting, resulting in hypokalemia.
Renal potassium wasting can also be caused by numerous congenital and acquired renal tubular diseases, such as the renal tubular acidoses and Fanconi syndrome, an unusual syndrome resulting in renal wasting of potassium, glucose, phosphate, uric acid, and amino acids 182.
Hypomagnesemia is a common correlate of hypokalemia. Much of this correlation is attributable to common causes (ie, diuretics, diarrhea), but hypomagnesemia itself may also result in increased renal potassium losses 182.
Intracellular shift
The transcellular shift of potassium into cells may also cause hypokalemia. This shift can occur in any of the following 182:
- After administration of insulin
- Familial periodic paralysis
- Glycogenesis during total parenteral nutrition or enteral hyperalimentation (stimulating insulin release)
- Stimulation of the sympathetic nervous system, particularly with beta 2-agonists (eg, albuterol, terbutaline), which may increase cellular potassium uptake
- Thyrotoxicosis (occasionally) due to excessive beta-sympathetic stimulation (hypokalemic thyrotoxic periodic paralysis)
Familial periodic paralysis is a rare autosomal dominant disorder characterized by transient episodes of profound hypokalemia thought to be due to sudden abnormal shifts of potassium into cells. Episodes frequently involve varying degrees of paralysis. They are typically precipitated by a large carbohydrate meal or strenuous exercise.
Drug interactions
Several classes of medications are known to induce low serum potassium or hypokalemia see Table 1 below.
Diuretics are by far the most commonly used drugs that cause hypokalemia. Potassium-wasting diuretics that block sodium reabsorption proximal to the distal nephron include 182, 82:
- Loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®)
- Osmotic diuretics
- Thiazide diuretics, such as chlorothiazide (Diuril®) and metolazone (Zaroxolyn®)
Experts recommend monitoring potassium status in people taking these medications, and initiating potassium supplementation if warranted 82.
By inducing diarrhea, laxatives, especially when abused, can cause hypokalemia. Secret diuretic or laxative use or both is a frequent cause of persistent hypokalemia, particularly among patients preoccupied with weight loss and among health care practitioners with access to prescription drugs 182.
Other drugs that can cause hypokalemia include 182:
- Aminoglycosides
- Anti-fungal agents (amphotericin-B, fluconazole)
- Antipseudomonal penicillins (eg, carbenicillin)
- Cisplatin. Cisplatin can damage the renal tubular epithelium and lead to severe potassium loss.
- Corticoids. Corticoids cause sodium retention that leads to a compensatory increase in urinary potassium excretion 42.
- Penicillin in high doses. Penicillins formulated as sodium salts also stimulate potassium excretion 42.
- Theophylline (both acute and chronic intoxication)
Outdated tetracycline antibiotics have been linked to electrolyte disturbances 42.
Table 4. Medications associated with Hypokalemia (low serum potassium)
| Medication Family | Specific Medications |
|---|---|
| Aminoglycosides | amikacin (Amikin), gentamicin (Garamycin), kanamycin (Kantrex), tobramycin (Nebcyn), streptomycin |
| Antibiotics | Penicillins: penicillin G sodium (Pfizerpen), mezlocillin (Mezlin), carbenicillin (Geocillin), ticarcillin (Ticar) Tetracyclines (when outdated) |
| Anti-cancer agent | cisplatin (Platinol-AQ) |
| Anti-fungal agents | amphotericin B (Abelcet, Amphotec, AmBisome, Amphocin, Fungizone), fluconazole (Diflucan) |
| Beta-adrenergic agonists | albuterol (Salbutamol, Ventolin), bitolterol (Tornalate), metaproterenol (Alupent) |
| Diuretics | Loop diuretics: bumetanide (Bumex), ethacrynic acid (Edecrin), furosemide (Lasix), torsemide (Demadex) Thiazide diuretics: Acetazolamide, thiazides, chlorthalidone (Hygroton), indapamide (Lozol), metolazone (Zaroxolyn), chlorothiazide (Diuril) |
| Mineralocorticoids | fludrocortisone (Florinef), hydrocortisone (Cortef), cortisone (Cortone), prednisone (Deltasone) Substances with mineralocorticoid effects: licorice, carbenoxolone, gossypol |
| Other | methylxanthines (e.g., theophylline), sodium polystyrene sulfonate, sodium phosphates, caffeine |
Groups at Risk of Potassium Deficiency
Potassium deficiency can occur with intakes that are below the Adequate Intake (AI) [intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA)] but above the amount required to prevent hypokalemia. The following groups are more likely than others to have poor potassium status.
People with inflammatory bowel diseases
Potassium is secreted within the colon, and this process is normally balanced by absorption 187. However, in inflammatory bowel disease (including Crohn’s disease and ulcerative colitis), potassium secretion increases, which can lead to poor potassium status. Inflammatory bowel diseases are also characterized by chronic diarrhea, which can further increase potassium excretion 188.
People who use certain medications, including diuretics and laxatives
Certain diuretics (e.g., thiazide diuretics, loop diuretics) that are commonly used to treat high blood pressure increase urinary potassium excretion and can cause hypokalemia 26, 77. Potassium- sparing diuretics, however, do not increase potassium excretion and can actually cause hyperkalemia. Large doses of laxatives and repeated use of enemas can also cause hypokalemia because they increase losses of potassium in stool 23.
People with pica
Pica is the persistent eating of non-nutritive substances, such as clay. When consumed, clay binds potassium in the gastrointestinal tract, which can increase potassium excretion and lead to hypokalemia 18, 176, 177. Cessation of pica combined with potassium supplementation can restore potassium status and resolve symptoms of potassium deficiency.
Potassium deficiency prevention
Routine potassium replacement is not necessary in most patients receiving diuretics. However, serum potassium should be monitored during diuretic use when risk of hypokalemia or of its complications is high. Risk is high in 182:
- Patients with decreased left ventricular function
- Patients taking digoxin
- Patients with diabetes (in whom insulin concentrations can fluctuate)
- Patients with asthma who are taking beta 2-agonists
Triamterene 100 mg orally once a day or spironolactone 25 mg orally 4 times a day does not increase potassium excretion and may be useful in patients who become hypokalemic but must use diuretics. When hypokalemia develops, potassium supplementation, usually with oral potassium chloride, is indicated.
Hypokalemia may also be minimized by dietary restriction of salt (sodium) since high rates of sodium excretion promote urinary potassium losses. People who participate in vigorous sports or exercise in warm weather should be sure to replace potassium that is lost through excessive sweating. This can be accomplished through dietary planning.
Hypokalemia (low potassium) symptoms
Most often, a slight decrease in the potassium level in blood or mild hypokalemia (serum potassium 3 to 3.5 mEq/L [3 to 3.5 mmol/L]) usually causes no symptoms or asymptomatic. In most cases, low potassium is found by a blood test that is done because of an illness, or because you are taking diuretics (water pills). Hypokalemia affects up to 21% of hospitalized patients, usually because of the use of diuretics and other medications 171, 172, 189. It is rare for low potassium to cause isolated symptoms such as muscle cramps if you are feeling well in other respects.
Low potassium symptoms may include 39:
- Muscle weakness
- Weak or twitching muscles
- Nausea and vomiting
- Fatigue
- Muscle cramps
- Constipation
- Feeling of skipped heart beats or palpitations
- Tingling or numbness
Serum potassium less than 3 mEq/L (less than 3 mmol/L) generally causes muscle weakness and may lead to muscular paralysis and possibly respiratory failure. Other muscular dysfunction includes cramping, muscle twitches (fasciculations), paralysis of the bowel (paralytic ileus), hypoventilation, low blood pressure (hypotension), tetany (involuntary contraction of muscles), and rhabdomyolysis. Severe hypokalemia may also lead to disruption of skeletal muscle cells, particularly during exercise. The normal physical response to exercise requires the local release of potassium from muscle. In potassium depleted muscle, the lack of potassium prevents adequate widening of blood vessels, resulting in decreased muscle blood flow, cramps and the destruction of skeletal muscle. Other symptoms may include loss of appetite, nausea and vomiting, confusion, distention of the abdomen and a decrease in mental activity.
Persistent hypokalemia may also impair the ability of the kidneys to concentrate urine, resulting in excessive urination (polyuria) with secondary excessive thirst (polydipsia).
A large drop in potassium level (serum potassium level less than about 2.5 mmol/L) may lead to abnormal heart rhythms (arrhythmias), especially in people with underlying heart disease that can be fatal and requires urgent medical attention 40, 41. This can cause you to feel lightheaded or faint. Abnormal heart rhythms (arrhythmias) are the most worrisome complication of very low potassium levels, particularly in people with underlying heart disease. A very low potassium level can even cause your heart to stop.
Chronic low potassium levels (chronic hypokalemia) is associated with high blood pressure (hypertension), kidney stone formation, increased bone turnover, urinary calcium excretion, and salt sensitivity (meaning that changes in sodium intakes affect blood pressure to a greater than normal extent).
Potassium deficiency diagnosis
Hypokalemia is often asymptomatic. The diagnosis of hypokalemia (serum potassium < 3.5 mEq/L [< 3.5 mmol/L]) may be found during routine serum electrolyte measurement. Doctors then try to identify what is causing the potassium level to decrease (see Figure 3) 190, 191. The cause may be clear based on your symptoms (such as vomiting) or use of drugs or other substances. If the cause is not clear, further investigation is warranted and doctors measure how much potassium is excreted in urine to determine whether excess excretion is the cause. Because low potassium levels can cause abnormal heart rhythms, doctors usually do electrocardiography (ECG) to check for abnormal heart rhythms.
Evaluation begins with a search for warning signs or symptoms warranting urgent treatment 26. These include weakness or palpitations, changes on electrocardiography (ECG), severe hypokalemia (less than 2.5 mEq per L [2.5 mmol per L]), rapid-onset hypokalemia, or underlying heart disease or cirrhosis 190, 192. Most cases of hypokalemia-induced rhythm disturbances occur in individuals with underlying heart disease 183. Early identification of potassium transcellular shifts is important because management may differ. Identification and treatment of concurrent hypomagnesemia are also important because magnesium depletion impedes potassium repletion and can exacerbate hypokalemia-induced rhythm disturbances 193, 194.
It should be suspected in patients with typical changes on an ECG or who have muscular symptoms and risk factors and confirmed by blood testing.
Figure 3. Potassium deficiency diagnostic algorithm
[Source 26 ]History and physical examination
A focused history includes evaluation for possible gastrointestinal losses, review of medications, and assessment for underlying cardiac comorbidities 26. A history of paralysis, hyperthyroidism, or use of insulin or beta agonists suggests possible transcellular shifts leading to redistributive hypokalemia. The physical examination should focus on identifying cardiac arrhythmias and neurologic manifestations, which range from generalized weakness to ascending paralysis.
Electrocardiography (ECG)
Electrocardiography (ECG) should be done on patients with hypokalemia. Cardiac effects of hypokalemia are usually minimal until serum potassium concentrations are less than 3 mEq/L (< 3 mmol/L). Hypokalemia causes sagging of the ST segment, depression of the T wave (decreased T-wave amplitude), and elevation of the U wave (see Figure 4) 182. With marked hypokalemia can lead to PR-interval prolongation, ST-interval depression, the T wave becomes progressively smaller or T-wave inversions and the U wave becomes increasingly larger 182. Sometimes, a flat or positive T wave merges with a positive U wave, which may be confused with QT prolongation (see figure ECG patterns in hypokalemia). Hypokalemia may cause sinus bradycardia, premature ventricular beats and premature atrial contractions, ventricular tachycardia or fibrillation and supraventricular tachyarrhythmias, torsade de pointes and 2nd- or 3rd-degree atrioventricular block 195, 182. Such arrhythmias become more severe with increasingly severe hypokalemia; eventually, ventricular fibrillation may occur 182. Although the risk of ECG changes and arrhythmias increases as serum potassium concentration decreases, these findings are not reliable because some patients with severe hypokalemia do not have ECG changes 196. Patients with significant preexisting heart disease and patients receiving digoxin are at risk of cardiac conduction abnormalities as a result of even mild hypokalemia.
Figure 4. ECG patterns in hypokalemia
[Source 182 ]Laboratory and urine tests
Potassium deficiency or hypokalemia diagnosis should be confirmed with a repeat serum potassium measurement. Other laboratory tests include serum glucose and magnesium levels, urine electrolyte and creatinine levels, and acid-base balance. After acidosis and other causes of intracellular potassium shift (increased beta-adrenergic effect, hyperinsulinemia) have been eliminated, 24-hour urinary potassium and serum magnesium concentrations are measured.
The most accurate method for evaluating urinary potassium excretion is a 24-hour timed urine potassium collection; normal kidneys excrete no more than 15 to 30 mEq per L (15 to 30 mmol per L) of potassium per day in response to hypokalemia. In hypokalemia, potassium secretion is normally less than 15 mEq/L (< 15 mmol/L) 182. Extrarenal gastrointestinal potassium loss or decreased potassium ingestion is suspected in chronic unexplained hypokalemia when renal potassium secretion is less than 15 mEq/L (< 15 mmol/L). Secretion of more than 15 mEq/L (> 15 mmol/L) suggests a renal cause for potassium loss 182. A more practical approach is calculation of the urine potassium-to-creatinine ratio from a spot urine specimen; a ratio greater than 1.5 mEq per mmol (13 mEq per g) of creatinine is indicative of renal potassium wasting 197, 180.
Unexplained hypokalemia with increased renal potassium secretion and hypertension suggests an aldosterone-secreting tumor or Liddle syndrome 182. Unexplained hypokalemia with increased renal potassium loss and normal blood pressure suggests Bartter syndrome or Gitelman syndrome, but hypomagnesemia, surreptitious vomiting, and diuretic abuse are more common and should also be considered 182.
If no cause is identified with the initial workup, assessment of thyroid and adrenal function should be considered.
Hypokalemia Treatment
Treatment of low potassium is directed at the underlying cause and may include potassium supplements. If your condition is mild, your doctor will likely prescribe oral potassium pills. If your condition is severe, you may need to get potassium through a vein (IV). Treatment urgency depends on the severity of your hypokalemia, the existence of comorbid conditions and the rate of decline of serum potassium levels 180. Rapid correction is possible with oral potassium; the fastest results are likely best achieved by combining oral (e.g., 20 to 40 mmol) and intravenous administration 198.
The goal of potassium replacement in the context of kidney or gastrointestinal losses is to immediately raise serum potassium concentration to a safe level and then replace the remaining deficit over days to weeks. A potassium-sparing diuretic should also be considered when the cause of hypokalemia involves renal potassium wasting as potassium replacement therapy alone may not be enough.
The immediate goal of treatment is the prevention of potentially life-threatening cardiac conduction disturbances and neuromuscular dysfunction by raising serum potassium to a safe level 26. Further replenishment can proceed more slowly, and attention can turn to the diagnosis and management of the underlying disorder 192. Patients with a history of congestive heart failure or heart attack (myocardial infarction) should maintain a serum potassium concentration of at least 4 mEq per L (4 mmol per L), based on expert opinion 192.
For hypokalemia associated with diuretic use, stopping the diuretic or reducing its dosage may be effective 192. Another strategy, if otherwise indicated to treat a comorbid condition, is use of an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), beta blocker, or potassium-sparing diuretic because each of these drugs is associated with an elevation in serum potassium 26.
It is appropriate to increase dietary potassium in patients with low-normal and mild hypokalemia, particularly in those with a history of hypertension or heart disease 192. The effectiveness of increased dietary potassium is limited, however, because most of the potassium contained in foods is coupled with phosphate, whereas most cases of hypokalemia involve chloride depletion and respond best to supplemental potassium chloride 192, 77.
Eating foods rich in potassium can help treat and prevent low level of potassium. These foods include:
- Avocados
- Baked potato
- Bananas
- Bran
- Carrots
- Cooked lean beef
- Milk
- Oranges
- Peanut butter
- Peas and beans
- Salmon
- Seaweed
- Spinach
- Tomatoes
- Wheat germ
Many oral potassium supplements are available. Nonurgent hypokalemia is treated with 40 to 100 mmol of oral potassium per day over days to weeks. For the prevention of hypokalemia in patients with persistent losses, as with ongoing diuretic therapy or hyperaldosteronism, 20 mmol per day is usually sufficient 199. Because high single doses can cause gastrointestinal irritation and occasional bleeding, potassium deficiency are usually replaced in divided doses. Liquid potassium chloride given orally elevates concentrations within 1 to 2 hours but has a bitter taste and is tolerated particularly poorly in doses greater than 25 to 50 mEq. (> 25 to 50 mmol) 182. Wax-impregnated potassium chloride preparations are safe and better tolerated. GI bleeding may be even less common with microencapsulated potassium chloride preparations. Several of these preparations contain 8 or 10 mEq/capsule. Because a decrease in serum potassium of 1 mEq/L (1 mmol/L) correlates with about a 200- to 400-mEq (200 to 400 mmol) deficit in total body potassium stores, total deficit can be estimated and replaced over a number of days at 20 to 80 mEq (20 to 80 mmol)/day.
When hypokalemia is severe (eg, with ECG changes or severe symptoms), is unresponsive to oral therapy, or occurs in hospitalized patients who are taking digoxin or who have significant heart disease or ongoing losses, potassium must be replaced intravenously (IV) 182. Because use of intravenous (IV) potassium increases the risk of hyperkalemia (a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L) and can cause pain and vein inflammation (phlebitis), intravenous potassium should be reserved for patients with severe hypokalemia, hypokalemic ECG changes, or physical signs or symptoms of hypokalemia, or for those unable to tolerate the oral form 26.
When intravenous (IV) potassium is used, standard administration is 20 to 40 mmol of potassium in 1 L of normal saline [the concentration should not exceed 40 mEq/L (40 mmol/L)] 26. Correction typically should not exceed 20 mmol per hour, although higher rates using central venous catheters have been successful in emergency situations 198. Continuous cardiac monitoring is indicated if the rate exceeds 10 mmol per hour. In children, dosing is 0.5 to 1.0 mmol per L per kg over one hour (maximum of 40 mmol) 200. Potassium should not be given in dextrose-containing solutions because dextrose-stimulated insulin secretion can exacerbate hypokalemia 26.
In hypokalemia-induced arrhythmia, intravenous (IV) potassium chloride must be given more rapidly, usually through a central vein or using multiple peripheral veins simultaneously. Infusion of 40 mEq (40 mmol) potassium chloride/hour can be undertaken but only with continuous cardiac monitoring and hourly serum potassium determinations. Glucose solutions are avoided because elevation in the serum insulin concentrations could result in transient worsening of hypokalemia.
Even when potassium deficits are severe, it is rarely necessary to give > 100 to 120 mEq (> 100 to 120 mmol) of potassium in a 24-hour period unless potassium loss is ongoing. In potassium deficit with high serum potassium concentration, as in diabetic ketoacidosis, intravenous (IV) potassium is deferred until the serum potassium starts to fall. When hypokalemia occurs with hypomagnesemia, both the potassium and magnesium deficiencies must be corrected to stop ongoing renal potassium wasting.
Careful monitoring during treatment is essential because supplemental potassium is a common cause of hyperkalemia (a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L) in hospitalized patients 201. The risk of rebound hyperkalemia is higher when treating redistributive hypokalemia. Because serum potassium concentration drops approximately 0.3 mEq per L (0.3 mmol per L) for every 100-mEq (100-mmol) reduction in total body potassium, the approximate potassium deficit can be estimated in patients with abnormal losses and decreased intake 26. For example, a decline in serum potassium from 3.8 to 2.9 mEq per L (3.8 to 2.9 mmol per L) roughly corresponds to a 300-mEq (300-mmol) reduction in total body potassium 26. Additional potassium will be required if losses are ongoing. Concomitant hypomagnesemia should be treated concurrently.
Potassium deficiency prognosis
The prognosis for patients with hypokalemia depends entirely on the underlying cause of hypokalemia 202. For example, a patient with an acute episode of hypokalemia resulting from diarrhea has an excellent prognosis. Hypokalemia due to a congenital disorder such as Bartter syndrome has a poor to nonexistent potential for resolution 202.
Hypokalemia is associated with increased mortality for patients with diabetes, chronic kidney disease, myocardial infarction, heart failure and COVID-19 pneumonia 203, 204, 205, 206.
- Sur M, Mohiuddin SS. Potassium. [Updated 2022 Dec 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539791[↩][↩][↩]
- Potassium. https://pubchem.ncbi.nlm.nih.gov/compound/potassium[↩]
- Unwin, R., Luft, F. & Shirley, D. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 7, 75–84 (2011). https://doi.org/10.1038/nrneph.2010.175[↩][↩][↩][↩]
- Stone MS, Martyn L, Weaver CM. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients. 2016 Jul 22;8(7):444. doi: 10.3390/nu8070444[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria M, Harrison M, Stallings VA, editors. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019 Mar 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538102[↩][↩][↩][↩][↩][↩][↩][↩]
- Brooks G. Potassium additive algorithm for use in continuous renal replacement therapy. Nurs Crit Care. 2006 Nov-Dec;11(6):273-80. doi: 10.1111/j.1478-5153.2006.00185.x[↩]
- Preuss HG, Clouatre DL. Sodium, chloride, and potassium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:475-92.[↩][↩][↩][↩][↩][↩][↩]
- Hinderling PH. The Pharmacokinetics of Potassium in Humans Is Unusual. J Clin Pharmacol. 2016 Oct;56(10):1212-20. doi: 10.1002/jcph.713[↩][↩]
- Palmer B.F. Regulation of potassium homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813[↩]
- Unwin R.J., Luft F.C., Shirley D.G. Pathophysiology and management of hypokalemia: A clinical perspective. Nat. Rev. Nephrol. 2011;7:75–84. doi: 10.1038/nrneph.2010.175[↩]
- Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019 Nov;74(5):682-695. doi: 10.1053/j.ajkd.2019.03.427. Erratum in: Am J Kidney Dis. 2022 Nov;80(5):690.[↩][↩][↩][↩][↩]
- World Health Organisation (WHO) Guideline: Potassium Intake for Adults and Children. WHO; Geneva, Switzerland: 2012.[↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩][↩]
- O’Neil C.E., Keast D.R., Fulgoni V.L., Nicklas T.A. Food sources of energy and nutrients among adults in the us: Nhanes 2003–2006. Nutrients. 2012;4:2097–2120. doi: 10.3390/nu4122097[↩]
- DeSalvo K.B., Olson R., Casavale K.O. Dietary guidelines for americans. JAMA. 2016;315:457–458. doi: 10.1001/jama.2015.18396[↩]
- Weaver C.M. Potassium and health. Adv. Nutr. (Bethesda Md.) 2013;4:S368–S377. doi: 10.3945/an.112.003533[↩]
- He F.J., MacGregor G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008;133:725–735. doi: 10.1111/j.1399-3054.2007.01033.x[↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid-based metabolism. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:102-32.[↩][↩][↩][↩]
- Shils M.E., Shike M. Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006.[↩]
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016 Dec;40(4):480-490. doi: 10.1152/advan.00121.2016[↩][↩][↩][↩][↩]
- Hené RJ, Koomans HA, Boer P, Dorhout Mees EJ. Adaptation to chronic potassium loading in normal man. Miner Electrolyte Metab. 1986;12(3):165-72.[↩]
- Rabelink TJ, Koomans HA, Hené RJ, Dorhout Mees EJ. Early and late adjustment to potassium loading in humans. Kidney Int. 1990 Nov;38(5):942-7. doi: 10.1038/ki.1990.295[↩]
- Potassium. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Patrick J. Assessment of body potassium stores. Kidney Int 1977;11:476-90. https://www.kidney-international.org/article/S0085-2538(15)31757-9/pdf[↩][↩]
- Chatterjee R, Yeh HC, Edelman D, Brancati F. Potassium and risk of Type 2 diabetes. Expert Rev Endocrinol Metab. 2011 Sep;6(5):665-672. doi: 10.1586/eem.11.60[↩][↩]
- Viera AJ, Wouk N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2015 Sep 15;92(6):487-95. https://www.aafp.org/pubs/afp/issues/2015/0915/p487.html[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- WHO. Diet, nutrition and the prevention of chronic disease. Report of a Joint WHO/FAO Expert Consultation. Geneva, World Health Organization (WHO), 2003. http://whqlibdoc.who.int/trs/WHO_TRS_916.pdf [↩]
- D’Elia L, Barba G, Cappuccio FP et al. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. Journal of the American College of Cardiology, 2011, 57(10):1210–1219. http://www.ncbi.nlm.nih.gov/pubmed/21371638[↩]
- Whelton PK, He J, Cutler JA et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. Journal of the American Medical Association, 1997, 277(20):1624–1632. http://www.ncbi.nlm.nih.gov/pubmed/9168293[↩]
- Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. Journal of Human Hypertension, 2003, 17(7):471–480. http://www.ncbi.nlm.nih.gov/pubmed/12821954[↩]
- Dietary Guidelines Advisory Committee. The report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. Washington, D.C., Department of Health and Human Services and Department of Agriculture, 2005. http://www.health.gov/dietaryguidelines/dga2005/report/default.htm[↩]
- World Health Organization. Guideline: Potassium intake for adults and children. http://apps.who.int/iris/bitstream/10665/77986/1/9789241504829_eng.pdf[↩][↩]
- American Heart Association. A Primer on Potassium. https://sodiumbreakup.heart.org/a_primer_on_potassium?utm_source=SRI&utm_medium=HeartOrg&utm_term=Website&utm_content=SodiumAndSalt&utm_campaign=SodiumBreakup[↩][↩][↩]
- Weiss JN, Qu Z, Shivkumar K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol. 2017 Mar;10(3):e004667. doi: 10.1161/CIRCEP.116.004667[↩][↩]
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8: advanced challenges in resuscitation: section 1: life-threatening electrolyte abnormalities. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000 Aug 22;102(8 Suppl):I217-22.[↩][↩]
- Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018 Jul 1;4(3):180-188. doi: 10.1093/ehjcvp/pvy015[↩][↩]
- Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012 Jan 11;307(2):157-64. doi: 10.1001/jama.2011.1967[↩]
- Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM; ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014 Dec;86(6):1205-12. doi: 10.1038/ki.2014.214[↩]
- Potassium Blood Test. https://medlineplus.gov/lab-tests/potassium-blood-test[↩][↩][↩][↩][↩][↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid — Base Metabolism In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease: Lippincott Williams & Wilkins; 2014:102-132.[↩][↩][↩][↩][↩]
- Food and Nutrition Board, Institute of Medicine. Potassium. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, D.C.: National Academies Press; 2005:186-268. https://nap.nationalacademies.org/read/10925/chapter/7[↩][↩][↩][↩][↩]
- Potassium. https://lpi.oregonstate.edu/mic/minerals/potassium[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Clausen T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol. 2013 Oct;142(4):327-45. doi: 10.1085/jgp.201310980[↩]
- Larsen BR, Stoica A, MacAulay N. Managing Brain Extracellular K(+) during Neuronal Activity: The Physiological Role of the Na(+)/K(+)-ATPase Subunit Isoforms. Front Physiol. 2016 Apr 22;7:141. doi: 10.3389/fphys.2016.00141[↩]
- Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, Goldhaber J, Kaplan J, Lingrel JB, Pavlovic D, Philipson K, Sipido KR, Xie ZJ. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015 Mar 15;593(6):1361-82. doi: 10.1113/jphysiol.2014.282319[↩]
- DrugBank. Potassium. http://www.drugbank.ca/drugs/DB01345[↩]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol2009;38:791-813.[↩]
- Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium-reduced, potassium- and magnesium-enriched mineral salt in subjects with mild essential hypertension. Hypertens Res1998;21:235-43. https://www.ncbi.nlm.nih.gov/pubmed/9877516?access_num=9877516&link_type=MED&dopt=Abstract[↩]
- Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr2006;83:1289-96. http://ajcn.nutrition.org/content/83/6/1289?ijkey=52dc92efe22c9a7ff1bd4e7366b4fcecb7f45e7e&keytype2=tf_ipsecsha[↩]
- Sheng H-W. Sodium, chloride and potassium. In: Stipanuk M, ed. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: W.B. Saunders Company; 2000:686-710.[↩]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC; 2005.[↩][↩][↩][↩][↩]
- Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html[↩][↩]
- U.S. Department of Health and Human Services, U.S. Department of Agriculture. https://health.gov/dietaryguidelines/2015/guidelines/[↩]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2013-2014. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/[↩][↩][↩]
- Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr 2011;94:1376-81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3192481/[↩]
- Council for Responsible Nutrition. Re: Docket No. FDA-2012-N-1210; Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.crnusa.org/sites/default/files/pdfs/comments-pdfs/CRN_Comments_FDA_ProposedRule-RevisionNutritionSupplementFactsLabels080114.pdf[↩]
- Potassium. https://ods.od.nih.gov/factsheets/Potassium-Consumer[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. https://ndb.nal.usda.gov/ndb/[↩][↩]
- O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012 Dec 19;4(12):2097-120. doi: 10.3390/nu4122097[↩]
- Keast DR, Fulgoni VL 3rd, Nicklas TA, O’Neil CE. Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients. 2013 Jan 22;5(1):283-301. doi: 10.3390/nu5010283[↩]
- He FJ, MacGregor GA. Beneficial effects of potassium on human health. Physiol Plant. 2008 Aug;133(4):725-35. doi: 10.1111/j.1399-3054.2007.01033.x[↩][↩][↩]
- 2015-2020 Dietary Guidelines. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015[↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels[↩]
- United States Department of Agriculture (USDA). Foods rich in potassium. https://www.ars.usda.gov/is/br/potassium/potassium.pdf[↩]
- National Institutes of Health. Dietary Supplement Label Database. http://www.dsld.nlm.nih.gov/dsld/[↩][↩][↩]
- Potassium Iodide. https://medlineplus.gov/druginfo/meds/a611043.html[↩][↩][↩]
- Hendler SS, Rorvik DR. PDR for Nutrititional Supplements. Montvale: Thomson Reuters; 2008.[↩][↩][↩][↩][↩][↩]
- U.S. Food and Drug Administration. List of Drug Products That Have Been Withdrawn or Removed from the Market for Reasons of Safety or Effectiveness. https://www.gpo.gov/fdsys/pkg/FR-1999-03-08/pdf/99-5517.pdf[↩]
- U.S. Food and Drug Administration. Code of Federal Regulations Title 21. 21CFR201.306. Potassium salt preparations intended for oral ingestion by man. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.306[↩][↩][↩]
- Commission on Dietary Supplement Labels. Report of the Commission 0n Dietary Supplement Labels. https://health.gov/dietsupp/cover.htm[↩]
- Macdonald-Clarke CJ, Martin BR, McCabe LD, McCabe GP, Lachcik PJ, Wastney M, et al. Bioavailability of potassium from potatoes and potassium gluconate: a randomized dose response trial. Am J Clin Nutr 2016;104:346-53. https://www.ncbi.nlm.nih.gov/pubmed/27413123[↩]
- Levene DL. Potassium chloride: absorption and excretion. Can Med Assoc J 1973;108:853-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1941311/pdf/canmedaj01665-0044.pdf[↩]
- Levene DL. The absorption of potassium chloride–liquid vs. tablet. Can Med Assoc J 1973;108:1480 passim. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1941543/pdf/canmedaj01670-0017.pdf[↩]
- Agency for Healthcare Research and Quality. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks. Rockville, MD; 2018. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-206-report-sodium-potassium-update.pdf[↩][↩][↩][↩][↩][↩][↩][↩]
- ALLEN AC, BOLEY SJ, SCHULTZ L, SCHWARTZ S. POTASSIUM-INDUCED LESIONS OF THE SMALL BOWEL. II. PATHOLOGY AND PATHOGENESIS. JAMA. 1965 Sep 20;193:1001-6. doi: 10.1001/jama.1965.03090120009003[↩]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC; 2005. https://nap.nationalacademies.org/read/10925/chapter/1[↩]
- Gennari FJ. Hypokalemia. N Engl J Med. 1998 Aug 13;339(7):451-8. doi: 10.1056/NEJM199808133390707[↩][↩][↩][↩]
- Mandal AK. Hypokalemia and hyperkalemia. Med Clin North Am. 1997 May;81(3):611-39. doi: 10.1016/s0025-7125(05)70536-8[↩][↩]
- Natural Medicines. Potassium/Professional Monograph/Nutrient Depletion. https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=851[↩]
- Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012 Jun;30(3):e156-66. doi: 10.1111/j.1755-5922.2010.00258.x[↩][↩][↩][↩]
- Epstein M. Hyperkalemia as a constraint to therapy with combination Renin-Angiotensin system blockade: the elephant in the room. J Clin Hypertens (Greenwich). 2009 Feb;11(2):55-60. doi: 10.1111/j.1751-7176.2008.00071.x[↩]
- Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf. 2010 Mar;9(2):259-73. doi: 10.1517/14740330903499257[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Food and Nutrition Board, Institute of Medicine. Potassium. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, D.C.: National Academies Press; 2005:186-268. https://nap.nationalacademies.org/read/10925/chapter/1[↩]
- Gijsbers L, Mölenberg FJ, Bakker SJ, Geleijnse JM. Potassium supplementation and heart rate: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2016 Aug;26(8):674-82. doi: 10.1016/j.numecd.2016.05.003[↩]
- Newberry SJ, Chung M, Anderson CAM, et al. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Jun. (Comparative Effectiveness Review, No. 206.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK519328[↩]
- Weaver CM. Potassium and health. Adv Nutr. 2013 May 1;4(3):368S-77S. doi: 10.3945/an.112.003533[↩][↩][↩][↩][↩]
- He FJ, MacGregor GA. Beneficial effects of potassium on human health. Physiol Plant 2008;133:725-35. https://www.ncbi.nlm.nih.gov/pubmed/18724413[↩]
- Facts About Hypertension. https://www.cdc.gov/bloodpressure/facts.htm[↩]
- Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S; PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016 Jul 30;388(10043):465-75. doi: 10.1016/S0140-6736(16)30467-6. Epub 2016 May 20. Erratum in: Lancet. 2021 Apr 10;397(10282):1350.[↩]
- Sanghavi S, Vassalotti JA. Dietary sodium: a therapeutic target in the treatment of hypertension and CKD. J Ren Nutr. 2013 May;23(3):223-7. doi: 10.1053/j.jrn.2013.01.027[↩]
- Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS, Appel LJ. Estimated 24-Hour Urinary Sodium and Potassium Excretion in US Adults. JAMA. 2018 Mar 27;319(12):1209-1220. doi: 10.1001/jama.2018.1156[↩]
- Jackson SL, Cogswell ME, Zhao L, Terry AL, Wang CY, Wright J, Coleman King SM, Bowman B, Chen TC, Merritt R, Loria CM. Association Between Urinary Sodium and Potassium Excretion and Blood Pressure Among Adults in the United States: National Health and Nutrition Examination Survey, 2014. Circulation. 2018 Jan 16;137(3):237-246. doi: 10.1161/CIRCULATIONAHA.117.029193[↩][↩]
- Champagne CM. Dietary interventions on blood pressure: the Dietary Approaches to Stop Hypertension (DASH) trials. Nutr Rev. 2006 Feb;64(2 Pt 2):S53-6. doi: 10.1111/j.1753-4887.2006.tb00234.x[↩]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997 Apr 17;336(16):1117-24. doi: 10.1056/NEJM199704173361601[↩][↩]
- Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014 Dec;24(12):1253-61. doi: 10.1016/j.numecd.2014.06.008[↩]
- Weaver CM, Stone MS, Lobene AJ, Cladis DP, Hodges JK. What Is the Evidence Base for a Potassium Requirement? Nutr Today. 2018 Sep-Oct;53(5):184-195. doi: 10.1097/NT.0000000000000298[↩]
- Filippini T, Violi F, D’Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol. 2017 Mar 1;230:127-135. doi: 10.1016/j.ijcard.2016.12.048[↩]
- Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2015 Aug;33(8):1509-20. doi: 10.1097/HJH.0000000000000611[↩]
- Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens. 1991 May;9(5):465-73. doi: 10.1097/00004872-199105000-00011[↩]
- Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997 May 28;277(20):1624-32. doi: 10.1001/jama.1997.03540440058033[↩]
- Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004641. doi: 10.1002/14651858.CD004641.pub2[↩]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria M, Harrison M, Stallings VA, editors. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019 Mar 5. 4, Potassium: Dietary Reference Intakes for Adequacy. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545428[↩][↩][↩][↩][↩]
- Viera AJ. Screening for Hypertension and Lowering Blood Pressure for Prevention of Cardiovascular Disease Events. Med Clin North Am. 2017 Jul;101(4):701-712. doi: 10.1016/j.mcna.2017.03.003[↩]
- Appel LJ, Giles TD, Black HR, Izzo JL Jr, Materson BJ, Oparil S, Weber MA. ASH position paper: dietary approaches to lower blood pressure. J Am Soc Hypertens. 2010 Mar-Apr;4(2):79-89. doi: 10.1016/j.jash.2010.03.004[↩]
- Adler AJ, Taylor F, Martin N, Gottlieb S, Taylor RS, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014 Dec 18;2014(12):CD009217. doi: 10.1002/14651858.CD009217.pub3[↩]
- Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013 Sep;88(9):987-95. doi: 10.1016/j.mayocp.2013.06.005[↩]
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013 Apr 3;346:f1326. doi: 10.1136/bmj.f1326[↩]
- Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin Nutr. 2019 Jun;38(3):1092-1100. doi: 10.1016/j.clnu.2018.05.017[↩]
- D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011 Mar 8;57(10):1210-9. doi: 10.1016/j.jacc.2010.09.070[↩]
- Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013 Apr 3;346:f1378. doi: 10.1136/bmj.f1378[↩]
- Vinceti M, Filippini T, Crippa A, de Sesmaisons A, Wise LA, Orsini N. Meta-Analysis of Potassium Intake and the Risk of Stroke. J Am Heart Assoc. 2016 Oct 6;5(10):e004210. doi: 10.1161/JAHA.116.004210[↩][↩][↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that[↩]
- Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev 2015:CD010057. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD010057.pub2/full[↩][↩][↩][↩]
- Trinchieri A, Zanetti G, Currò A, Lizzano R. Effect of potential renal acid load of foods on calcium metabolism of renal calcium stone formers. Eur Urol. 2001 Jan;39 Suppl 2:33-6; discussion 36-7. doi: 10.1159/000052556[↩]
- Morris RC Jr, Schmidlin O, Tanaka M, Forman A, Frassetto L, Sebastian A. Differing effects of supplemental KCl and KHCO3: pathophysiological and clinical implications. Semin Nephrol. 1999 Sep;19(5):487-93.[↩][↩]
- Lemann J Jr, Pleuss JA, Gray RW. Potassium causes calcium retention in healthy adults. J Nutr. 1993 Sep;123(9):1623-6. doi: 10.1093/jn/123.9.1623[↩][↩]
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328:833-8. https://www.nejm.org/doi/10.1056/NEJM199303253281203[↩]
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997 Apr 1;126(7):497-504. doi: 10.7326/0003-4819-126-7-199704010-00001[↩]
- Ferraro PM, Mandel EI, Curhan GC, Gambaro G, Taylor EN. Dietary Protein and Potassium, Diet-Dependent Net Acid Load, and Risk of Incident Kidney Stones. Clin J Am Soc Nephrol. 2016 Oct 7;11(10):1834-1844. doi: 10.2215/CJN.01520216[↩][↩][↩]
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993 Dec;150(6):1761-4. doi: 10.1016/s0022-5347(17)35888-3[↩][↩]
- Maalouf NM, Moe OW, Adams-Huet B, Sakhaee K. Hypercalciuria associated with high dietary protein intake is not due to acid load. J Clin Endocrinol Metab. 2011 Dec;96(12):3733-40. doi: 10.1210/jc.2011-1531[↩]
- Suarez M, Youssef RF. Potassium citrate: treatment and prevention of recurrent calcium nephrolithiasis. J Clin Nephrol Res. 2015;2(1). https://www.jscimedcentral.com/public/assets/articles/nephrology-2-1015.pdf[↩]
- Hanley DA, Whiting SJ. Does a high dietary acid content cause bone loss, and can bone loss be prevented with an alkaline diet? J Clin Densitom. 2013 Oct-Dec;16(4):420-5. doi: 10.1016/j.jocd.2013.08.014[↩]
- Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015 Aug;102(2):376-84. doi: 10.3945/ajcn.114.102723[↩]
- Kong SH, Kim JH, Hong AR, Lee JH, Kim SW, Shin CS. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES) (2008-2011). Osteoporos Int. 2017 May;28(5):1577-1585. doi: 10.1007/s00198-017-3908-4[↩]
- Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr. 2008 Oct;88(4):1159-66. doi: 10.1093/ajcn/88.4.1159[↩]
- Morris RC, Jr., Frassetto LA, Schmidlin O, Forman A, Sabastian A. Expression of osteoporosis as determined by diet-disordered electrolyte and acid-base metabolism. In: Burckhardt P, Dawson-Hughes B, Heaney R, eds. Nutritional Aspects of Osteoporosis. San Diego: Academic Press; 2001:357-378.[↩]
- Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999 Apr;69(4):727-36. doi: 10.1093/ajcn/69.4.727[↩]
- Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003 Oct;133(10):3130-6. doi: 10.1093/jn/133.10.3130[↩]
- Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013 Mar;28(3):497-504. doi: 10.1002/jbmr.1764[↩]
- Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013 Jan;98(1):207-17. doi: 10.1210/jc.2012-3099[↩]
- Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, Ceglia L. Potassium Bicarbonate Supplementation Lowers Bone Turnover and Calcium Excretion in Older Men and Women: A Randomized Dose-Finding Trial. J Bone Miner Res. 2015 Nov;30(11):2103-11. doi: 10.1002/jbmr.2554[↩]
- Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, Hardcastle AC, Lanham New SA, Fraser WD, Reid DM. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr. 2008 Aug;88(2):465-74. doi: 10.1093/ajcn/88.2.465[↩]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf[↩]
- Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006 Aug;48(2):219-24. doi: 10.1161/01.HYP.0000231552.10054.aa[↩]
- Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992 May;55(5):1018-23. doi: 10.1093/ajcn/55.5.1018[↩][↩]
- Chatterjee R, Biggs ML, de Boer IH, Brancati FL, Svetkey LP, Barzilay J, Djoussé L, Ix JH, Kizer JR, Siscovick DS, Mozaffarian D, Edelman D, Mukamal KJ. Potassium and glucose measures in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2015 Feb;70(2):255-61. doi: 10.1093/gerona/glu071[↩]
- Chatterjee R, Colangelo LA, Yeh HC, Anderson CA, Daviglus ML, Liu K, Brancati FL. Potassium intake and risk of incident type 2 diabetes mellitus: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetologia. 2012 May;55(5):1295-303. doi: 10.1007/s00125-012-2487-3[↩][↩][↩][↩]
- Chatterjee R, Yeh HC, Shafi T, Selvin E, Anderson C, Pankow JS, Miller E, Brancati F. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2010 Oct 25;170(19):1745-51. doi: 10.1001/archinternmed.2010.36[↩]
- Chatterjee R, Davenport CA, Svetkey LP, Batch BC, Lin PH, Ramachandran VS, Fox ER, Harman J, Yeh HC, Selvin E, Correa A, Butler K, Edelman D. Serum potassium is a predictor of incident diabetes in African Americans with normal aldosterone: the Jackson Heart Study. Am J Clin Nutr. 2017 Feb;105(2):442-449. doi: 10.3945/ajcn.116.143255[↩][↩]
- Chatterjee R, Yeh HC, Shafi T, Anderson C, Pankow JS, Miller ER, Levine D, Selvin E, Brancati FL. Serum potassium and the racial disparity in diabetes risk: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2011 May;93(5):1087-91. doi: 10.3945/ajcn.110.007286[↩][↩]
- Chatterjee R, Zelnick L, Mukamal KJ, Nettleton JA, Kestenbaum BR, Siscovick DS, Ix JH, Tracy R, Hoofnagle AN, Svetkey LP, Edelman D, de Boer IH. Potassium Measures and Their Associations with Glucose and Diabetes Risk: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2016 Jun 9;11(6):e0157252. doi: 10.1371/journal.pone.0157252[↩]
- Chatterjee R, Slentz C, Davenport CA, Johnson J, Lin PH, Muehlbauer M, D’Alessio D, Svetkey LP, Edelman D. Effects of potassium supplements on glucose metabolism in African Americans with prediabetes: a pilot trial. Am J Clin Nutr. 2017 Dec;106(6):1431-1438. doi: 10.3945/ajcn.117.161570[↩]
- Hyperkalemia. University of Maryland Medical Center. http://umm.edu/health/medical/altmed/condition/hyperkalemia. Accessed Oct. 1, 2014.[↩]
- Potassium, serum. Mayo Medical Laboratories. http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/81390. Accessed Oct. 1, 2014.[↩]
- Mayo Foundation for Medical Education and Research, MayoClinic. High potassium (hyperkalemia) Symptoms. http://www.mayoclinic.org/symptoms/hyperkalemia/basics/definition/SYM-20050776?p=1[↩]
- Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70. https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html[↩][↩]
- Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470284[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- High potassium (hyperkalemia). https://www.mayoclinic.org/symptoms/hyperkalemia/basics/causes/sym-20050776[↩][↩]
- High potassium (hyperkalemia). https://www.mayoclinic.org/symptoms/hyperkalemia/basics/definition/sym-20050776[↩]
- Lytvyn Y, Godoy LC, Scholtes RA, van Raalte DH, Cherney DZ. Mineralocorticoid Antagonism and Diabetic Kidney Disease. Curr Diab Rep. 2019 Jan 23;19(1):4. doi: 10.1007/s11892-019-1123-8[↩]
- Flury G. Das «gefährliche» EKG [The ‘Dangerous’ ECG]. Praxis (Bern 1994). 2019 Jan;108(1):45-52. German. doi: 10.1024/1661-8157/a003155[↩]
- Rose BD. Causes of hyperkalemia. http://www.uptodate.com/home. Accessed Oct. 1, 2014.[↩]
- MayoClinic. Causes of Hyperkalemia. https://www.mayoclinic.org/symptoms/hyperkalemia/basics/causes/sym-20050776[↩]
- Acute kidney failure. https://www.mayoclinic.org/diseases-conditions/kidney-failure/symptoms-causes/syc-20369048[↩]
- Chronic kidney disease. https://www.mayoclinic.org/diseases-conditions/chronic-kidney-disease/symptoms-causes/syc-20354521[↩]
- Frequently asked questions about GFR estimates. https://www.kidney.org/sites/default/files/441-8491_2202_faqs_aboutgfr_v5.pdf[↩]
- Williams SM, Killeen AA. Tumor Lysis Syndrome. Arch Pathol Lab Med. 2019 Mar;143(3):386-393. doi: 10.5858/arpa.2017-0278-RS[↩]
- Sekhon DS, Vaqar S, Gupta V. Hyperkalemic Periodic Paralysis. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564319[↩][↩]
- Weber F. Hyperkalemic Periodic Paralysis. 2003 Jul 18 [Updated 2021 Jul 1]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1496[↩][↩][↩][↩][↩][↩][↩]
- Statland JM, Fontaine B, Hanna MG, Johnson NE, Kissel JT, Sansone VA, Shieh PB, Tawil RN, Trivedi J, Cannon SC, Griggs RC. Review of the Diagnosis and Treatment of Periodic Paralysis. Muscle Nerve. 2018 Apr;57(4):522-530. doi: 10.1002/mus.26009[↩]
- American Heart Association. Hyperkalemia (High Potassium). http://www.heart.org/HEARTORG/Conditions/HeartFailure/TreatmentOptionsForHeartFailure/Hyperkalemia-High-Potassium_UCM_488806_Article.jsp[↩]
- Campbell CA, Lam Q, Horvath AR. An evidence- and risk-based approach to a harmonized laboratory alert list in Australia and New Zealand. Clin Chem Lab Med. 2018 Dec 19;57(1):89-94. doi: 10.1515/cclm-2017-1114[↩]
- Butler J, Vijayakumar S, Pitt B. Revisiting hyperkalaemia guidelines: rebuttal. Eur J Heart Fail. 2018 Sep;20(9):1255. doi: 10.1002/ejhf.1249[↩]
- Butler J, Vijayakumar S, Pitt B. Need to revisit heart failure treatment guidelines for hyperkalaemia management during the use of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018 Sep;20(9):1247-1251. doi: 10.1002/ejhf.1217[↩]
- Long B, Warix JR, Koyfman A. Controversies in Management of Hyperkalemia. J Emerg Med. 2018 Aug;55(2):192-205. doi: 10.1016/j.jemermed.2018.04.004[↩]
- Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016 Mar;89(3):546-54. doi: 10.1016/j.kint.2015.11.018[↩]
- Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for beta-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol. 2002 Oct;283(4):C1025-32. doi: 10.1152/ajpcell.00096.2002[↩]
- Mistry M, Shea A, Giguère P, Nguyen ML. Evaluation of Sodium Polystyrene Sulfonate Dosing Strategies in the Inpatient Management of Hyperkalemia. Ann Pharmacother. 2016 Jun;50(6):455-62. doi: 10.1177/1060028016641427[↩]
- Harvard University. Harvard Health Publications. Heart failure and potassium. http://www.health.harvard.edu/heart-health/heart-failure-and-potassium[↩]
- Paice BJ, Paterson KR, Onyanga-Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J. 1986 Mar;62(725):187-91. doi: 10.1136/pgmj.62.725.187[↩][↩]
- Lippi G, Favaloro EJ, Montagnana M, Guidi GC. Prevalence of hypokalaemia: the experience of a large academic hospital. Intern Med J. 2010 Apr;40(4):315-6. doi: 10.1111/j.1445-5994.2009.02146.x[↩][↩]
- Potassium. https://www.hsph.harvard.edu/nutritionsource/potassium[↩]
- Mumoli N, Cei M. Licorice-induced hypokalemia. Int J Cardiol. 2008 Mar 14;124(3):e42-4. doi: 10.1016/j.ijcard.2006.11.190[↩]
- Walker BR, Edwards CR. Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol Metab Clin North Am. 1994 Jun;23(2):359-77.[↩]
- Ukaonu C, Hill DA, Christensen F. Hypokalemic myopathy in pregnancy caused by clay ingestion. Obstet Gynecol. 2003 Nov;102(5 Pt 2):1169-71. doi: 10.1016/s0029-7844(03)00705-1[↩][↩]
- McKenna D. Myopathy, hypokalaemia and pica (geophagia) in pregnancy. Ulster Med J. 2006 May;75(2):159-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1891740[↩][↩]
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:527-37.[↩]
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007 Oct;18(10):2649-52. doi: 10.1681/ASN.2007070792[↩][↩]
- Castro D, Sharma S. Hypokalemia. [Updated 2023 Mar 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482465[↩][↩][↩][↩][↩][↩][↩]
- Reid A, Jones G, Isles C. Hypokalaemia: common things occur commonly – a retrospective survey. JRSM Short Rep. 2012 Nov;3(11):80. doi: 10.1258/shorts.2012.011179[↩]
- Hypokalemia. https://www.msdmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypokalemia[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Schulman M, Narins RG. Hypokalemia and cardiovascular disease. Am J Cardiol. 1990 Mar 6;65(10):4E-9E; discussion 22E-23E. doi: 10.1016/0002-9149(90)90244-u[↩][↩]
- Greenberg A. Diuretic complications. Am J Med Sci. 2000 Jan;319(1):10-24. https://doi.org/10.1016/S0002-9629(15)40676-7[↩]
- Dhalla IA, Gomes T, Yao Z, Nagge J, Persaud N, Hellings C, Mamdani MM, Juurlink DN. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013 Mar 19;158(6):447-55. doi: 10.7326/0003-4819-158-6-201303190-00004[↩]
- Morgan DB, Davidson C. Hypokalaemia and diuretics: an analysis of publications. Br Med J. 1980 Mar 29;280(6218):905-8. doi: 10.1136/bmj.280.6218.905[↩]
- Barkas F, Liberopoulos E, Kei A, Elisaf M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol. 2013;26(1):23-28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959504[↩]
- Musto D, Rispo A, Testa A, Sasso F, Castiglione F. Hypokalemic myopathy in inflammatory bowel diseases. J Crohns Colitis. 2013 Sep;7(8):680. doi: 10.1016/j.crohns.2013.01.005[↩]
- Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013 Mar;126(3):256-63. doi: 10.1016/j.amjmed.2012.06.037[↩]
- Weiner ID, Wingo CS. Hypokalemia–consequences, causes, and correction. J Am Soc Nephrol. 1997 Jul;8(7):1179-88. doi: 10.1681/ASN.V871179[↩][↩]
- Mount DB, Zandi-Nejad K. Disorders of potassium balance. In: Taal MW, Chertow GM, Marsden PA, Brenner BM, Rector FC, eds. Brenner and Rector’s The Kidney. Philadelphia, Pa.: Elsevier/Saunders; 2012.[↩]
- Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004 Jan 21;43(2):155-61. doi: 10.1016/j.jacc.2003.06.021[↩][↩][↩][↩][↩][↩]
- Whang R, Whang DD, Ryan MP. Refractory Potassium Repletion: A Consequence of Magnesium Deficiency. Arch Intern Med. 1992;152(1):40–45. doi:10.1001/archinte.1992.00400130066006[↩]
- Millane TA, Ward DE, Camm AJ. Is hypomagnesemia arrhythmogenic? Clin Cardiol. 1992 Feb;15(2):103-8. doi: 10.1002/clc.4960150210[↩]
- Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004 Aug;27(2):153-60. doi: 10.1016/j.jemermed.2004.04.006[↩]
- WEAVER WF, BURCHELL HB. Serum potassium and the electrocardiogram in hypokalemia. Circulation. 1960 Apr;21:505-21. doi: 10.1161/01.cir.21.4.505[↩]
- Kamel KS, Ethier JH, Richardson RM, Bear RA, Halperin ML. Urine electrolytes and osmolality: when and how to use them. Am J Nephrol. 1990;10(2):89-102. doi: 10.1159/000168062[↩]
- Kim GH, Han JS. Therapeutic approach to hypokalemia. Nephron. 2002;92 Suppl 1:28-32. doi: 10.1159/00006537[↩][↩]
- Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004 Jan 21;43(2):155-61. doi: 10.1016/j.jacc.2003.06.02[↩]
- Ingram TC, Olsson JM. In brief: hypokalemia. Pediatr Rev. 2008 Sep;29(9):e50-1. doi: 10.1542/pir.29-9-e50[↩]
- Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007 Dec;22(12):3471-7. doi: 10.1093/ndt/gfm471[↩]
- Hypokalemia. https://emedicine.medscape.com/article/242008-overview#a2[↩][↩]
- Coregliano-Ring L, Goia-Nishide K, Rangel ÉB. Hypokalemia in Diabetes Mellitus Setting. Medicina (Kaunas). 2022 Mar 16;58(3):431. doi: 10.3390/medicina58030431[↩]
- Cunningham JW, Mehra MR. Hypokalemia in heart failure: A low or a high point? Eur J Prev Cardiol. 2021 Apr 23;28(3):313-315. doi: 10.1177/2047487320914745[↩]
- Makinouchi R, Machida S, Matsui K, Shibagaki Y, Imai N. Severe hypokalemia in the emergency department: A retrospective, single-center study. Health Sci Rep. 2022 Apr 14;5(3):e594. doi: 10.1002/hsr2.594[↩]
- Chen D, Li X, Song Q, Hu C, Su F, Dai J, Ye Y, Huang J, Zhang X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw Open. 2020 Jun 1;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122[↩]