Contents
What is quercetin
Extracts of quercetin have been purported to be beneficial to health, improving metabolic function, modulating inflammation and decreasing serum cholesterol. Animal, test tube and human studies suggest a long list of desirable effects result from consuming quercetin in the diet. These include antioxidative, anti-inflammatory, antibacterial, immunomodulatory, anticarcinogenic, and cardioprotective actions 11 and of the food mutagen 2-amino-3-methylimadazo[4,5-f]-quinoline (IQ) in lymphocytes from patients with inflammatory bowel disease (IBD). Mutagenesis. 2009;(5):405-411.)), 12, 13, 14, 15, 16. High quercetin intake via diet is associated with decreased rates of colorectal, kidney, pancreatic, prostate, and lung cancer; cardiovascular disease; and diabetes 17, 18, 19, 20, 21, 22, 23, 24, 25.
Quercetin has been purported to improve athletic performance and reduce postexercise inflammation and oxidative stress. The diverse potential beneficial effects of quercetin have led it to be marketed in multiple dietary supplements with many health claims. Quercetin supplements have been used to treat or prevent diverse conditions including cardiovascular disease, high cholesterol (hypercholesterolemia), rheumatic diseases, infections and cancer but have not been shown to be effective in clinical trials for any medical condition.
The beneficial effects of quercetin have been suggested by several studies of human nutrition which have shown decreases in illness and death associated with higher intake of quercetin-rich foods such as fruits and green vegetables. Flavonoids have antioxidant effects that may protect against cell injury. In test tube study, antioxidants such as quercetin have been shown to decrease oxidation of cholesterol esters which appears to play a role in atherosclerosis. However, it is quite difficult to demonstrate that specific food components in isolation such as flavonoids have biologically important antioxidant effects in the human body. Quercetin also has anti-inflammatory activity inhibiting cyclooxygenase (COX) enzymes, synthesis of leukotrienes and prostaglandins and inhibiting histamine release. In cell culture and animal models, quercetin appears to have anti-cancer effects which have led to its evaluation in several forms of cancer, although some studies have used concentrations of the flavonoids that are 100 times or higher than achieved by eating diets high in flavonoid-containing foods. To put the concentrations used in typical cell culture studies into perspective, it was estimated that blood concentrations of quercetin ranging between about 50 nmol/L and about 140 nmol/L are reached for persons consuming their habitual diets and diets high in vegetables, fruits, and berries, respectively 26. Nieman et al. 27 reported achieving levels of 1,158 µg/L, or about 3.8 µmol/L, in test subjects receiving 1,000 mg of quercetin daily for several weeks. These blood levels are substantially below those of most test tube trials. It is not known whether concentrations of quercetin higher than plasma levels might be achieved in other tissues after consuming quercetin from diet. However, it appears that the majority of test tube studies have involved quercetin levels that are not achieved even with high-dose dietary supplements. The applicability of the findings of these high-dose test tube studies to human health and safety is questionable.
In a double-blind, randomized, crossover study, MacRae and Mefferd 28 investigated whether 6 weeks of supplementation with an antioxidant would enhance the performance of elite male cyclists in 30-km time trials. The supplement contained a variety of nutrient and nonnutrient antioxidants, and included a total of 600 mg of quercetin. The results showed that the supplement improved the time trial performance and enhanced power output 28. These findings could not be attributed to quercetin, however, as there was no quercetin-only supplement. Nieman et al. 27 tested whether 1,000 mg/day of quercetin would have an effect on upper respiratory tract infections (URTI) and exercise-induced changes in immune function in trained male cyclists (n=40). Participants were randomized to receive quercetin or placebo supplements twice daily under double-blind conditions for 3 weeks prior to, during, and 2 weeks following a 3-day period in which subjects cycled at high output for 3 hours per day 27. The results of this trial showed no effects on natural killer cell activity, PHA-stimulated lymphocyte proliferation, polymorphonuclear oxidative burst activity, or salivary immunoglobulin A (IgA) output. However, the incidence of upper respiratory tract infections (URTI) during the 2-week postexercise period differed significantly between groups, with quercetin resulting in only one versus nine episodes of URTI in the placebo group. Interestingly, plasma quercetin was increased from 113 µg/L in the placebo group to 1,158 µg/L in supplemented groups. The authors concluded that even in the absence of demonstrated effects of the supplement on multiple measures of immune function, quercetin may have a direct antiviral mechanism 27.
Davis et al. 29 evaluated the effects of 7 days of an oral gavage of quercetin (either 12.5 or 25 mg/kg body weight) on tissue mitochondrial enzymes and performance on a treadmill in previously sedentary mice. Both dose levels of quercetin were associated with significant increases in mitochondrial content in skeletal muscle and brain cells as well as increased endurance capacity in the mice.
| Foods containing quercetin | Quercetin (mg/100g of edible portion) |
|---|---|
| capers, raw | 234 |
| capers, canned | 173 |
| lovage | 170 |
| dock like sorrel | 86 |
| radish leaves | 70 |
| carob fiber | 58 |
| dill | 55 (48-110) |
| cilantro | 53 |
| Hungarian wax pepper | 51 |
| fennel leaves | 48.8 |
| onion, red | 32 |
| radicchio | 31.5 |
| watercress | 30 |
| buckwheat | 23 |
| kale | 23 |
| chokeberry | 19 |
| cranberry | 15 |
| lingonberry | 13 |
| plums, black | 12 |
| cow peas | 11 |
| sweet potato | 10 |
| blueberry, cultivated | 8 |
| sea buckthorn berry | 8 |
| rowanberry | 7 |
| crowberry | 5 |
| prickly pear cactus fruits | 5 |
| apples, Red Delicious | 4 |
| broccoli | 3 |
| bilberry | 3 |
| tea, black or green Camellia sinensis | 2 |
| red kidney beans, raw (powdered) | 0.0603 +/- 0.0307 |
Quercetin acutally belongs to a type of flavonols, which is a class of flavonoids that have the 3-hydroxyflavone backbone (3-hydroxy-2-phenylchromen-4-one) and present in a wide variety of herbs including licorice 30, 31. Quercetin is a licorice flavonoid and its IUPAC name is 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one with a molecular weight of 302.24 g/mol and a melting point of 316 °C, and its molecular formula is C15H10O7. Quercetin consists of five hydroxyl groups, which determine its biological activity 32, 33. Naturally, quercetin is distributed in the form of derivatives, either in glycosidic form, mainly attached to the C-3 carbon with glucose, rhamnose, and rutinose, or linked to others, and it is very rarely distributed as an aglycone 34. On the other hand, quercetin in its aglycone form is a bright lemon-yellow powder, insoluble in water but soluble in alcohol and lipids. In addition, the aglycone has been reported to have a minor effect in vivo and poor bioavailability in human plasma after oral intake of quercetin 35, 36. The most common form of quercetin is rutin (quercetin glycoside), which is usually glycosylated, formed by the addition of a glycoside group (a sugar, such as glucose, rhamnose, or rutin) instead of an -OH group (usually at the 3-position) 6.
Several studies demonstrated that quercetin had diverse bioactive effects such as antiviral activity, anti-asthmatic activity, anti-oxidant, monoamine-oxidase inhibitor, anti-tumor and anti-inflammatory activity and quercetin has been studied extensively as a chemoprevention agent in several cancer models 37, 38, 39. Quercetin has been used in medicine to decrease capillary fragility. It has also been used in dyes and as a veterinary drug 40. However, there is no reliable clinical evidence that quercetin has a protective effect on human cancer.
Quercetin itself (aglycone quercetin), as opposed to quercetin glycosides, is not a normal dietary component. Quercitin glycosides are converted to phenolic acids as they pass through the gastrointestinal tract. Quercetin has neither been confirmed scientifically as a specific therapeutic for any condition nor been approved by any regulatory agency. The U. S. Food and Drug Administration has not approved any health claims for quercetin. Nevertheless, the interest in dietary flavonoids has grown after the publication of several epidemiological studies showing an inverse correlation between dietary consumption of flavonols and flavones and reduced incidence and mortality from cardiovascular disease and cancer. In recent years, a large amount of experimental and some clinical data have accumulated regarding the effects of flavonoids on the endothelium under physiological and pathological conditions. The meta-analysis of seven prospective cohort studies concluded that the individuals in the top third of dietary flavonol intake are associated with a reduced risk of mortality from coronary heart disease as compared with those in the bottom third, after adjustment for known risk factors and other dietary components. A limited number of intervention studies with flavonoids and flavonoid containing foods and extracts has been performed in several pathological conditions.
Flavonoids are a large group of compounds (>10,000) that occur naturally in fruits, vegetables, nuts, seeds, flowers and other plant matter and as such they are an integral part of the human diet 41. They all share a common three-ring structure but are subdivided into flavonols, flavons, flavanols and flavanons according to their substituents 42. Epidemiological studies indicate that diets rich in flavonoids are associated with reduced incidences of several chronic diseases including cardiovascular disease, asthma, type II diabetes and certain types of cancer 43. The cardioprotective properties of flavonoids are multi-faceted involving antioxidant, anti-hypercholesterolaemia, anti-inflammatory and inhibition of platelet aggregation effects 43. The antioxidant property of flavonoids was thought, until relatively recently, to underlie the majority of their protective cellular effects. However, it is becoming increasingly apparent that flavonoids also influence cellular function by modulating the activity of many enzymes including the inhibition of protein kinases and lipid kinases 44.
The inhibitory effect of flavonoids on protein kinase activity is primarily due to their ability to function as competitive inhibitors of the ATP-binding domain located in the active site of these enzymes 44. Protein kinases directly inhibited by flavonoids include protein kinase C (PKC), myosin light chain kinase (MLCK) and MEK1 44. MEK1 is an upstream kinase responsible for the activation of extracellular signal-regulated kinase 1/2 (ERK1/2), a member of the mitogen-activated protein kinase (MAPK) family of protein kinases 45. This family also includes p38 MAPK and JNK signalling pathways, both of which are targets for modulation by flavonoids 46. It is also notable that flavonoids such as quercetin also inhibit (via competitive inhibition of the ATP-binding site) the lipid kinase PI-3Kγ 47, which plays a prominent role in the activation of protein kinase B (PKB; also known as Akt).
The MEK/ERK1/2 and the PI-3K/PKB pathways are important signalling pathways which mediate cell survival and cardioprotection against ischaemia/reperfusion injury 48. In contrast, p38 MAPK and JNK cascades are typically associated with inflammation and cell death; however, there is also evidence that they mediate cardioprotection 48. Hence, the cardioprotective effects of flavonoids may relate to their ability to modulate MAPK and PI-3K/PKB signalling cascades. Indeed, recent studies have shown that grape seed proanthocyanidin extract protects cardiomyocytes from ischaemia/reperfusion injury via PKB activation 49. Similarly, quercetin (the most abundant dietary flavonoid) protected H9c2 cardiomyoblasts from H2O2-induced cell death via enhancement of ERK1/2 and PI-3K/PKB signalling 50. In contrast, isorhamnetin (a flavonol isolated from sea buckthorn) prevented H2O2-induced activation of apoptosis in H9c2 cells through ERK1/2 inactivation 51.
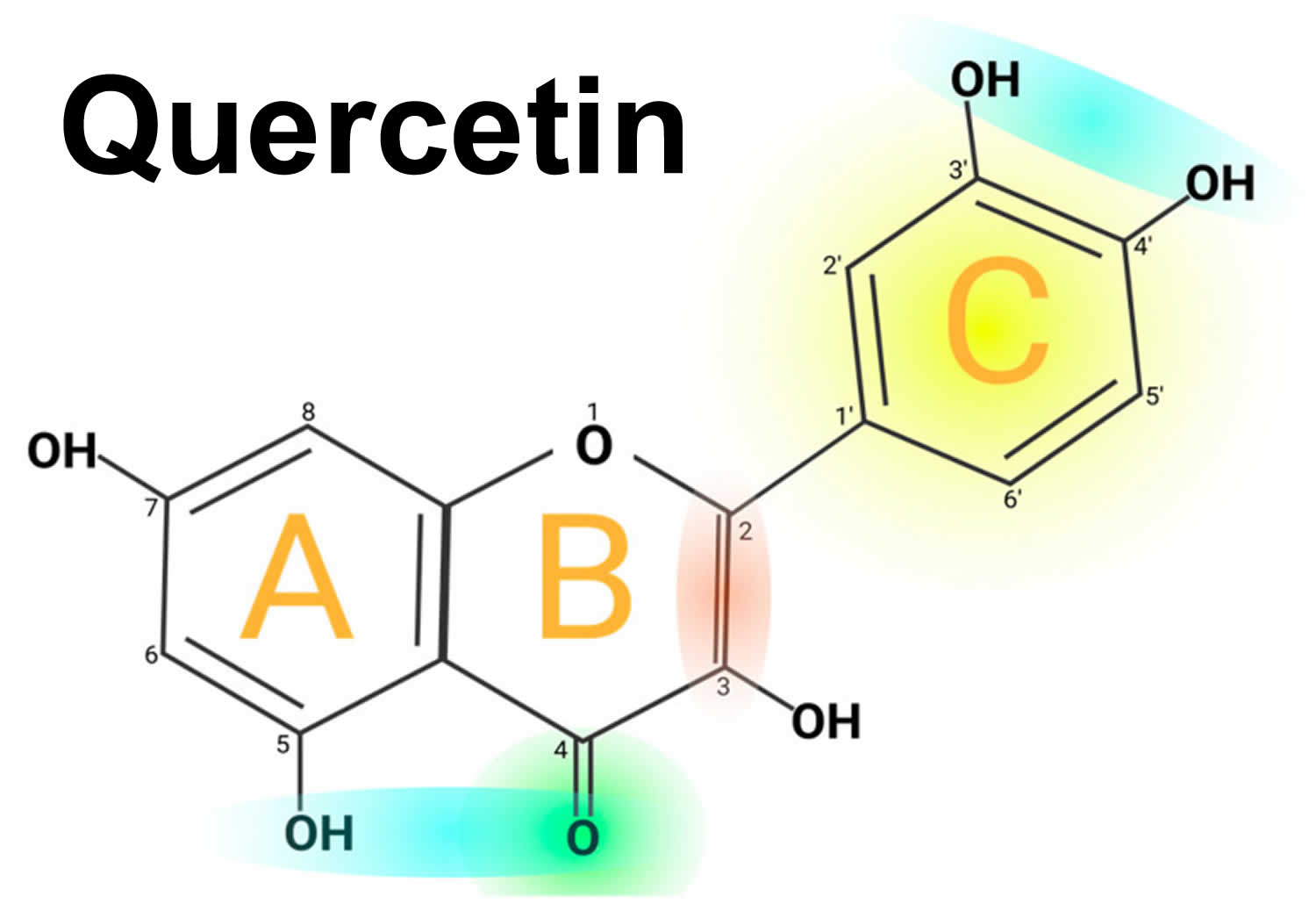
Figure 1. Quercetin chemical structure
Footnotes: Quercetin chemical structure. The figure shows the chemical characteristics that give Quercetin its antioxidant capacity, the most important of which is the catechol or dehydroxylated B-ring structure (yellow). Other important characteristics are the presence of unsaturation in the C-ring (red) and the presence of a 4-oxo in the C-ring (green); the oxygen in position 4 and the hydroxyl in position 5 allow it the capacity to chelate ions such as Cu++ and Fe++ (blue). On the other hand, in total, quercetin has 5 functional hydroxyl groups with the potential to be conjugated and that differ in their chemical reactivity following the order of reactivity 3 > 7 > 3′ > 4′ >> 5. The phenolic hydroxyl groups of quercetin act as donors of electrons, and these are responsible for the capture activity of free radicals, in particular the catechol structure with 2 hydroxyl groups in the neighboring positions, which is notably superior to other arrangements in electron donation.
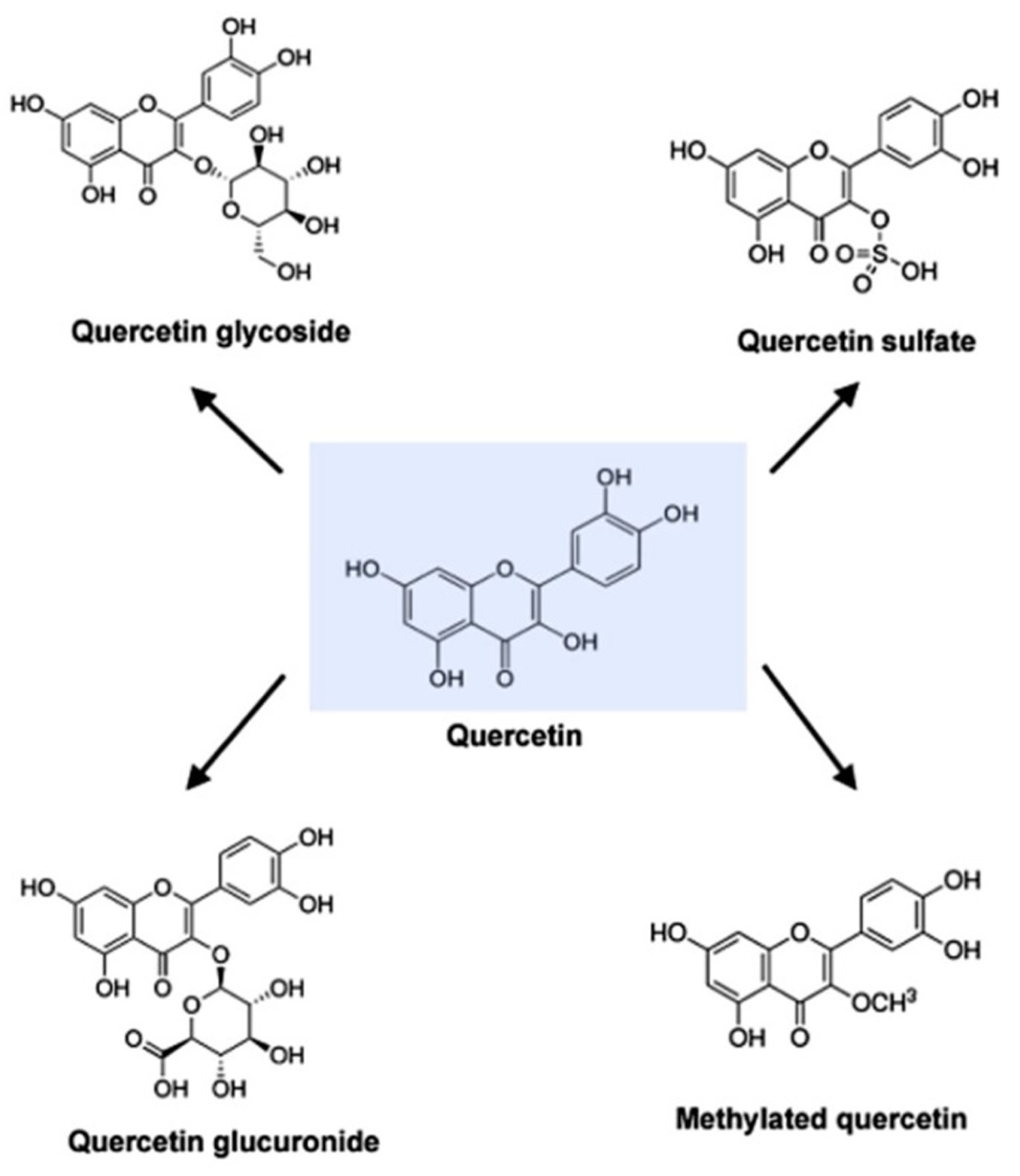
[Source 6 ]Figure 2. Molecular structure of quercetin, quercetin glycoside, quercetin glucuronide, quercetin sulfate and methylated quercetin
Note:
Quercetin (C15H10O7) is an aglycone, lacking an attached sugar. It is a brilliant citron yellow needle crystal and entirely insoluble in cold water, poorly soluble in hot water, but quite soluble in alcohol and lipids. A quercetin glycoside is formed by attaching a glycosyl group (a sugar such as glucose, rhamnose, or rutinose) as a replacement for one of the OH groups (commonly at position 3). The attached glycosyl group can change the solubility, absorption, and in vivo effects. As a general rule of thumb, the presence of a glycosyl group (quercetin glycoside) results in increased water solubility compared to quercetin aglycone 52.
A quercetin glycoside is unique by the attached glycosyl group. Generally, the term quercetin should be used to describe the aglycone only; however, the name is occasionally used to refer to quercetin-type molecules, including its glycosides in research and the supplement industry.
Quercetin Absorption
Quercetin glycosides might be differently absorbed based on the type of sugar attached 53, 54, 55. Available evidence indicates that quercetin glucosides (like those found predominantly in onion or shallot flesh) are far better absorbed than its rutinosides (the major quercetin glycoside in tea). The glucosides are efficiently hydrolyzed in the small intestine by beta-glucosidases to the aglycone form, much of which is then absorbed 56. Quercetin glucuronic acid and its sulfuric acid derivatives were more easily absorbed than quercetin 56. Thereafter, its absorption is affected by differences in its glycosylation, the food matrix from which it is consumed, and the co-administration of dietary components such as fiber and fat 57. Thus different sugar types and sugar group conjugation sites will result in absorption variation. For example, Ullah H. et al. 58 determined the absorption of various forms of quercetin in human volunteers using healthy subjects with ileostomy to avoid bacterial degradation in the colon. Their results showed that quercetin absorption was 52% for an onion-rich meal, 24% for quercetin supplementation, and 17% for routine supplementation 58, 59. Once ingested, quercetin glycosides are rapidly hydrolyzed by the enzyme beta-glucosidase in the epithelial cells of the upper small intestine, much of which is subsequently absorbed.
Similarly, the glucuronic acid in quercetin and its sulfuric acid derivatives are more readily absorbed than quercetin. However, it has not been reported whether quercetin and its derivatives are stable in gastric acid; therefore, there are no reports on whether they can be absorbed in the stomach 60. In addition, purified quercetin glycosides can interact with sodium-dependent glucose transport receptors in the mucosal epithelium and, therefore, can be absorbed by the small intestine 61.
However, rutin and other quercetin glycosides bound to oligosaccharides or polysaccharides are absorbed in the lower part of the digestive tract (large intestine) in the form of aglycones through deglycosylation by enterobacteria (Eubacterium ramulus, Clostridium orbiscindens, Eubacterium oxidoreducens, Butyrovibrio spp.) 6. In contrast, quercetin monoglucosides such as isoquercitrin and quercetin-4’-glucoside (Qu4’G) are absorbed in the upper part of the intestine (small intestine) after enzymatic hydrolysis by β-glucosidase and/or lactase-phlorizin hydrolase (LPH) in the intestinal mucosa derived from intestinal microbiota 6. Some of the quercetin monoglucosides can be taken up by sodium-glucose cotransporter 1 (SGLT-1). These metabolites enter the gastrointestinal tract via multidrug resistance-associated protein 2 (MRP2) and are subsequently transported through the blood vessels to the liver, where they are subjected to secondary metabolism 62. After absorption, quercetin binds to albumin and is transported primarily to the liver via the portal vein and to other organs, including the small intestine, colon, and kidney 6. In the liver, it undergoes O-methylation, glucuronidation, and/or sulfation to form its conjugates quercetin-3-glucuronide, quercetin-30-sulfate, and isorhamnetin-3-glucuronide 63. Conjugation reactions with glucuronic acid and/or sulfate appear to be the most common type of flavonoid metabolic pathway 64, 65. For example, glucuronidation of flavanols occurs in human microsomes and the liver, and uridine diphosphate (UDP)- glucuronosyltransferase 1A9 (UGT-1A9) plays a vital role in this process 6. Kuhnle et al. 66 reported that glucuronidation, O-methylation, and O-methyl-glucuronidation are part of flavonoid metabolism in the small intestine.
Quercetin foods
Quercetin-type flavonols (primarily as quercetin glycosides), the most abundant of the flavonoid molecules, are widely distributed in plants. They are found in a variety of foods including apples, berries, Brassica vegetables, capers, grapes, onions, shallots, tea, and tomatoes, as well as many seeds, nuts, flowers, barks, and leaves. Quercetin is also found in medicinal botanicals, including Ginkgo biloba, Hypericum perforatum, and Sambucus canadensis 67, 68, 69. In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration of quercetin glycosides 70. Up to 10% of an onion’s dry weight can comprise quercetin in various forms 71. One study found that organically grown tomatoes had 79% more quercetin than chemically grown fruit 72. Quercetin is present in various kinds of honey from different plant sources 73. Food-based sources of quercetin include vegetables, fruits, berries, nuts, beverages and other products of plant origin 74. In the determined food, the highest concentration is 234 mg/100 g of edible portion in capers (raw), the lowest concentration is 2 mg/100 g of edible portion in black or green tea (Camellia sinensis) 75.
Dietary intake of quercetin was different in several countries. The estimated flavonoid intake ranges from 50 to 800 mg/day (quercetin accounts for 75%), mostly depending on the consumption of fruits and vegetables and the intake of tea 76. In the Suihua area of northern China, quercetin intake was reported to be 4.37 mg/day, where the main food sources of flavonol was apples (7.4%), followed by potatoes (3.9%), lettuce (3.8%) and oranges (3.8%) 77, whereas the average quercetin intake was 4.43 mg/day, with apple (3.7%), potato (2.5%), celery (2.2%), eggplant (2.2%), and actinidia (1.6%) being the main food sources of quercetin in Harbin, China 78. The most recent study showed that quercetin intake is about 18 mg/day for Chinese healthy young males. In the USA, flavonol intake is about 13 mg/day for U.S. adults, while quercetin represents three-quarters of this amount. The mean quercetin intake was approximately 14.90 to 16.39 mg per day. Onions, tea, and apples contained high amounts of quercetin 79. In Japan, the average and median quercetin intakes were 16.2 and 15.5 mg/day, respectively; the quercetin intake by men was lower than that by women; and the quercetin intakes showed a low correlation with age in both men and women. The estimated quercetin intake was similar during summer and winter. Quercetin was mainly ingested from onions and green tea, both in summer and in winter. Vegetables, such as asparagus, green pepper, tomatoes, and red leaf lettuce, were good sources of quercetin in summer 80. In Australia, black and green teas were the dominant sources of quercetin. Other sources included onion, broccoli, apple, grape, and beans 81. Analysis of the 24-h recall data indicated an average adult intake of total flavonoids (>18 years) of 454 mg/day. Apple was the most important source of quercetin until age 16–18 years, after which onion became an increasingly important prominent source 81. In Spain, the average daily intake of quercetin is 18.48 mg/day, which is significantly higher than that in the United States (9.75 mg/day), based on sources like tea, citrus fruits and juice, beers and ales, wines, melon, apples, onions, berries and bananas 82.
Table 1. Foods containing quercetin
| Food Source | Quercetin Content (mg/100 g) |
|---|---|
| FRUITS | |
| Apple with skin (Malus domestica) | 4.42 |
| Acerola (Malpighia emarginata) | 4.74 |
| Arctic bramble (Rubus arcticus) | 9.1 |
| Blueberry (Vaccinium caesariense) | 7.67 |
| Cranberry (Vaccinium macrocarpon) | 14.84 |
| Elderberry (Sambucus spp.) | 26.77 |
| Fig (Ficus carica) | 5.47 |
| Plum (Prunus domestica) | 12.45 |
| Sea buckthorn (Hippophae rhamnoides) | 7.4 |
| Wolfberry (Lycium barbarum) | 13.6 |
| Common juniper (Juniperus communis) | 46.61 |
| Prickly pear (Opuntia spp.) | 4.86 |
| VEGETABLES | |
| Sowthistle (Sonchus oleraceus) | 16 |
| Arugula (Eruca sativa) | 7.92 |
| Sparrow grass (Asparagus officinalis) | 13.98 |
| Swiss chard (Beta vulgaris) | 7.5 |
| Green chicory (Cichorium intybus) | 6.49 |
| Coriander (Coriandrum sativum) | 52.9 |
| Golden poppy (Eschscholzia californica) | 26.3 |
| Drumstick tree (Moringa oleifera) | 16.65 |
| Fennel (Foeniculum vulgare) | 48.8 |
| Leaf cabbage (Brassica oleracea) | 7.71 |
| Red lettuce (Lactuca sativa) | 7.61 |
| Mustard greens (Brassica juncea) | 8.8 |
| Okra (Abelmoschus esculentus) | 20.97 |
| Onions (Allium cepa) | 20.3 |
| Perennial wall-rocket (Diplotaxis tenuifolia) | 66.19 |
| New Mexico chile (Capsicum annuum) | 15 |
| Sweet potato (Ipomoea batatas) | 16.94 |
| SPICES AND HERBS | |
| Caper bush (Capparis spinosa) | 180.77 |
| Dill (Anethum graveolens) | 55.15 |
| Oregano (Origanum vulgare) | 7.3 |
| Tarragon (Artemisia dracunculus) | 11 |
| Turmeric (Curcuma longa) | 4.92 |
| Buckwheat (Fagopyrum esculentum) | 15.38 |
Quercetin health benefits
Pharmacological reports have demonstrated that quercetin has pronounced pharmaceutical effects, including anti-inflammatory, antioxidant, antihypertensive (decrease in ventricular hypertrophy), anti-cancer, anti-apoptotic, anti-aging, immunomodulatory, anti-allergy and antiviral (against human immunodeficiency virus [HIV], hepatitis B virus [HBV], and herpes simplex virus [HSV]) properties 83, 84, 85, 86, 87, 88, 89, 90. In addition, quercetin is included in a novel class of chemotherapeutic drugs for the treatment of various cancers 91 and can also be combined with ultrasonic pretreatment to increase the concentration of quercetin to inhibit the growth of the prostate and skin cancer cell lines 92. However, quercetin has low aqueous solubility, poor absorption, and rapid metabolism (bioavailability approximately 1%–5%) 93, all of which can generate in vivo (animal & human study) results that differ from the powerful in vitro (test tube study) efficacy of quercetin. Most of the studies which have shown the antitumor activity of quercetin were performed with a high concentration of quercetin, rangin from 25 μM to 200 μM. But pharmacokinetic research reveals that the peak concentration of quercetin in blood after food uptake reaches to a peak of around 10 μM 94.
Quercetin has been shown to inhibit the proliferation of a wide range of cancers such as prostate, cervical, lung, breast, and colon. Recent studies have revealed that quercetin inhibits cell proliferation by causing apoptosis and/or cell cycle arrest 95. It has been shown that quercetin treatment causes cell cycle arrests such as G2/M arrest or G1 arrest in different cell types 96. Moreover, quercetin-mediated apoptosis may result from the induction of stress proteins, disruption of microtubules and mitochondria, release of cytochrome c, and activation of caspases 97. Quercetin is a strong antioxidant because it can chelate metals, scavenge oxygen free radicals 98 and inhibit xanthine oxidase and lipid peroxidation in vitro 99.
Quercetin has also been shown to impair an oncoviral replication. Quercetin inhibited HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis B e antigen) secretion in the HBV (Hepatitis virus B)-producing 2.2.15 cells 100. In addition, quercetin appeared to be the most effective inhibitor for HCV (hepatitis C virus) replication among all flavonoids, demonstrating a strong anti-hepatitis C virus activity in hepatitis C virus replicon-containing cells when combined with interferon (IFN)α 101. Quercetin and its analog quercetin showed stronger inhibition on HIV-1 reverse transcriptase, all with IC50 values of 60 μM than HIV-1 protease and α-Glucosidase 102. Quercetin was shown to inhibit other three reverse transcriptases from avian myeloblastosis, Rous-associated virus-2 and Maloney murine leukemia virus when poly(rA)oligo(dT)12-18 or rabbit globin mRNA were used as template 103. Several studies investigated potential use for quercetin as anti-cancer agent 104. A couple of cell culture studies showed that quercetin has an anti-cancer activity due to its antioxidant or anti-inflammatory properties 105. Quercetin also inhibits the growth of cancer cells and helps induction of apoptosis 106. Some studies using animal models have shown that quercetin could protect against colon cancer 107.
Quercetin Clinical Studies
Diet supplementation with combinations of resveratrol, pterostilbene, morin hydrate, quercetin, δ-tocotrienol, riboflavin, and nicotinic acid reduces cardiovascular risk factors in humans when used as nutritional supplements with, or without, other dietary changes in healthy seniors and hypercholesterolemic subjects 108.
In a randomized, double-blinded, placebo-controlled trial, 1002 subjects took 500 or 1000 mg/day quercetin or a placebo for 12 weeks. For the group as a whole, quercetin supplementation had no significant influence on rates of upper respiratory tract infections (URTI) compared to placebo. In a subgroup of subjects age 40 or older who self-rated themselves as physically fit, 1000 mg/day quercetin resulted in a statistically significant reduction in total sick days and symptom severity associated with URTI 109. Female subjects were supplemented with 500 or 1000 mg/day quercetin or placebo for 12 weeks. While quercetin supplementation significantly increased plasma quercetin levels, it had no influence on measure of immune function 110. Quercetin (100 mg/day) did not alter exercise-induced changes in several measures of immune function following three days of intense exercise in trained athletes, but it significantly reduced URTI incidence (1 of 20 subjects in active versus 9 of 20 in placebo group) during the two-week post-exercise period 111. A similar lack of effect on strenuous exercise-induced immune system perturbation was found in subjects who took 1000 mg/day of quercetin for three weeks before, during, and continuing for two weeks after the 160-km Western States Endurance Run. In this study, however, there were no differences in the post-race illness rates between quercetin and placebo groups 112.
There are several studies in humans investigating the correlation of quercetin and its immunomodulatory effects. Quercetin does indeed reduce illness after intensive exercise. Again, under double-blind conditions, Nieman et al. showed that a supplement of 1000 mg of quercetin alone three weeks before, during and two weeks after a three-day period of 3 h of cycling in the winter resulted in a markedly lower incidence of URTI in well-trained subjects in the two weeks after the intensified training, but had no effect on exercise-induced immune dysfunction, inflammation and oxidative stress 113.
The literature is supportive of the anti-pathogenic capacities of quercetin when it is cultured with target cells and a broad spectrum of pathogens including URTI-related rhinoviruses, adenoviruses and coronaviruses. The impact of the co-ingestion of two or more flavonoids increases their bioavailability and the outcomes on immunity. Nieman et al. determined the influence of two weeks of 1000 mg/day quercetin compared with placebo supplementation on exercise performance and skeletal muscle mitochondrial biogenesis in untrained, young adult males. It resulted in significantly reduced post-exercise measures for both inflammation and oxidative stress, with a chronic augmentation of granulocyte oxidative burst activity 114. When taken together, quercetin showed a successful reduction in the illness rates of exercise-stressed athletes as well as a chronic augmentation of their innate immune function.
Most in vitro research suggests that quercetin possesses anti-inflammation and immunological improvement. However, the results from a double-blinded, placebo-controlled, randomized trial indicated that quercetin supplementation at 500 and 1000 mg/day for 12 weeks significantly increased plasma quercetin levels but had no influence on measures of innate immune function or inflammation in community-dwelling adult females 110.
The main action of quercetin on inflammation and immune function in vivo is summarized in the Table 2.
- These results suggest that quercetin studies in human did not totally support these results from cells and animals. The effect, in which quercetin acts as an immune booster in humans, needs to be further verified for future broad application.
Table 2. Summary of the main effects of quercetin on inflammation and immune function in human
| Dosage | Subjects | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Human | ||||
| 50 and 100 mg/person | Elderly Human subject | Anti-inflammatory properties | Inhibition of proteasome (nitric oxide, C-reactive protein, γ-glutamyltransferase) activity | 115 |
| 500 and 1000 mg/day | Human subject | Reduction of upper respiratory tract infection and total sick days; Improvement in 12-min treadmill time trial performance | No effect | 116 |
| 1000 mg/day | Human in treadmill | No effect | 117 | |
| 500 and 1000 mg/day | Human subject | No effect on innate immune function or inflammation, illness rates | No effect | 118 |
| 1000 mg/day | Human cyclist | No effect | 119 | |
| 1000 mg/day | Human runner | No effect | 120 | |
| 1000 mg/day | Human cyclist | No effect | 121 | |
Antioxidant activity
The imbalance between oxidative and antioxidant capacity in the organism leads to oxidative stress, which tends to oxidize and cause excessive production of highly reactive molecules, such as reactive oxygen radicals (ROS) and reactive nitrogen radicals (RNS), leading to tissue damage. Reactive oxygen radicals (ROS) include hydroxyl radicals (OH−), hydrogen peroxide (H2O2), superoxide anion (O2−), and nitric oxide (NO), which are highly unstable. Therefore, scavenging oxygen-containing radicals is an important process of antioxidants. Quercetin, a natural antioxidant flavonoid provides protection mostly via the extinction of free radicals formed during cellular metabolism 122. Significant characteristics for quercetin antioxidant activity include dispersion in the membrane lipid bilayer, orientation, and affinity 123. The mechanism of action of quercetin has been attributed to its binding to or interference with enzymes, receptors, transporters, and signal transduction systems because quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a polyphenolic component that possess broad biological activities primarily due to its antioxidant property 123. Given that the cell membrane concentrates the available quercetin molecules, investigating the biophysical characteristics connected to the variety of lipid compositions of cell membranes may be the key to understanding the involvement of cell membrane in these activities. These significant mechanisms primarily happen in membrane environments, inside, and through lipid bilayers 124.
Anti-inflammatory effect
Inflammation is an adaptive response that underlies a variety of physiological and pathological processes triggered by noxious condition. Many studies have shown that quercetin can provide potent anti-inflammatory effects through inhibiting inflammatory factors and inflammatory signaling pathways. Quercetin has been shown to inhibit the expression of proinflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin (IL)-6, monocyte chemotactic protein-1 (MCP-1), IL-10, etc.) in lipopolysaccharide-stimulated neutrophils 125, macrophages 126 and liver tissue from tripterygium-induced liver injury 127 and epididymal adipose tissue from high-fat-fed mice 128. In addition, quercetin decreased the number of M1 macrophages and increased the number of M2 macrophages in epididymal adipose tissue 128, and decreased the gene expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) 126, which are enzymes involved in the inflammatory response. Quercetin also inhibited the activation of transcription factors NF-κB and activator protein 1 (AP-1) in TNF-α-treated human umbilical vein endothelial cells 129. Furthermore, in diabetic nephropathy rats, quercetin reduced renal inflammation by decreasing NOD-like receptor thermal protein domain associated protein 3 (NLRP3) activation 130. Multiple studies have shown that mast cells are involved in the pathogenesis of several inflammatory diseases. Activated mast cells produce inflammatory and chemotactic factors such as TNF-α, IL-1β, IL-6, IL-8, IL-4, IL-13, and transforming growth factor-β (TGF-β) 131. In addition, quercetin has numerous effects on reducing mast cell recruitment 128, maintaining mast cells stability as well as inhibiting the release of mast cell-like trypsin and histamine, which may be related to the inhibition of intracellular calcium influx and calcium-insensitive protein kinase C theta (PKCθ) 132; meanwhile, quercetin blocked the activation of p38 mitogen-activated protein kinase (p38MAPK) and NF-κB in mast cells, thereby attenuating the expression of pro-inflammatory cytokines such as TNF-α, IL-6β, IL-8, and IL-1 131.
Anti-cancer effect
Cancer remains a significant challenge worldwide, both in developed and developing countries. One possible avenue for cancer treatment is using plant secondary metabolites, which have shown promise in treating various diseases. Therefore, researchers are actively searching for new plant species and new compounds that can serve as effective treatments against multiple forms of cancer. As research on quercetin continues, many researchers have found that quercetin produces anticancer effects against a wide range of tumors under both in vivo and in vitro conditions. Quercetin has been shown to have an antiproliferative and proapoptotic effect on various cell lines 133. The anticancer effect of quercetin is closely related to its regulation of apoptosis. Quercetin can promote tumor cell apoptosis through both intrinsic and extrinsic apoptotic pathways. Quercetin can: directly bind to the BH3 structural domain of BCL-2 and B-cell lymphoma-extra large (BCL-XL)proteins 134, thereby inhibiting their anti-apoptotic activity; stimulate the expression of pro-apoptotic genes such as BAX, BCL-2 associated agonist of cell death (BAD), and apoptotic protease activating factor 1(Apaf-1) 135; increase the release of cytochrome C from the mitochondria into the cytoplasm 136; and promote the activation of caspase-9 and caspase-3 137 from the mitochondria-induced apoptosis pathway to induce apoptosis in tumor cells. Meanwhile, quercetin increased the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), caspase-8, Fas, Fas-L, and Fas-associated protein with death domain (FADD), which enhanced apoptosis in the death receptor pathway 138. In addition to inducing apoptosis in cancer cells, many studies have shown that quercetin can also exert a fundamental role for cell cycle regulation in the chemosensitivity of cancer cells. Quercetin down-regulates the expression of cyclin D, cyclin E, E2F1, E2F2, and cell cycle protein-dependent kinase 1 (CDK1), and induces p21 expression in a checkpoint kinase 2 (CHK2)-dependent manner 139, 140, leading to cell cycle arrest in G1 and G2/M phases in human breast cancer cell lines (SKBR3 cells and MDA-MB-453 cells) and human leukemia U937 cells; moreover, in human liver cancer cells (HepG2), quercetin-3-O-glucoside was found to inhibit DNA topoisomerase II activity, affecting DNA replication and transcription, and increase S-phase cell populations and cell cycle arrest, ultimately leading to cell death 141.
The carcinostatic effect of quercetin can be explained in part by its interaction with some of the main intracellular signaling pathways involved in cancer, which activate the transcription of proteins necessary for cell cycle progression and are therefore considered potential therapeutic targets: LY294002, a chemical compound, and PI3-kinase inhibitor, which has been modeled on the structure of quercetin and binds to the ATP-binding site of the enzyme in several different orientations, are currently available. Apparently, the number of -OH groups in the B-ring and the degree of C2–C3 instauration are essential determinants of quercetin’s particular bioactivity 142.
Another pathway susceptible to quercetin action is the protein kinase C (PKC) pathway, which is also downregulated 143; by blocking the diacylglycerol (DAG) precursor of PKC, inhibition of this pathway leads to blocking the formation of phosphatidylinositol (3,4,5)-trisphosphate, which activates the entry of extracellular calcium 144. Studies with human leukemia 60 (HL-60) cells report that quercetin concentrations between 20 and 30 µM are sufficient to exert an inhibitory effect on cytosolic protein kinase C (PKC) activity and membrane tyrosine protein kinase (TPK) activity. Within the same study, it is noted that quercetin concentration of 80 µM is sufficient to block the activity of phosphatidylinositol (4,5) bisphosphate 145. On the other hand, it has been shown that quercetin induces the inactivation of the AKT protein, an antiapoptotic protein, by decreasing its phosphorylation and also directly inactivates procaspase 9, thus blocking cellular apoptosis 146. In addition, exposure of cells to quercetin concentration of 50 µM resulted in the blockade of the extracellular signal-regulated kinases (ERK1/2) pathway. It showed better effects than when higher quercetin doses (75–100 µM) were used, as these doses reduced the expression of proapoptotic factors such as Bcl-2-associated X protein (Bax) and caspases 3 and 9 147.
Moreover, studies in human leukemia cells K562 show that the action of quercetin is not only focused on the inhibition of cell proliferation but also is able to induce apoptosis at concentrations of 80 µM and also causes a downregulation of cellular myelocytomatosis (c-myc) and Kirsten RAt sarcoma (K-ras) oncogenes 148. The proapoptotic effect of quercetin can be partly explained because the compound’s antioxidative effect changes entirely to a prooxidant effect at high concentrations, which induces selective cytotoxicity 149. In a different study, the selectivity of these processes was proven since quercetin was able to decrease cell viability in human glioma cultures of the U-118 MG cell line as well as an increase in death by apoptosis and cell arrest at the G2 checkpoint of the cell cycle. On the other hand, when noncancerous cells are exposed to quercetin, it exerts cytoprotective effects; in hippocampal cell cultures, quercetin is protective against ischemic damage 150. Quercetin also interacts directly with mitochondria by modulating the mitochondrial transition pore (mPT), which controls the release of cytochrome C, second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (SMAC/DIABLO), and the mPT, which possesses a benzodiazepine binding site where quercetin binds and influences the opening and release of cytochrome C 151.
Cardiovascular protection
Several studies have shown that quercetin has positive effects on cardiovascular diseases. Fikriah et al. 152 evaluated the impact on vasoconstrictor and vasodilator reactions in the porcine heart, reporting that quercetin increased both cyclic guanosine mono-phosphate (cGMP) content and cGMP-dependent relaxations to glyceryl trinitrate (GTN) and sodium nitroprusside (SNP), as well as porcine receptor-mediated restrained contractions. Also, a study with rats 153 showed that quercetin (0.1–100 μM) relaxed the contraction induced by pretreatment with five mM norepinephrine in a concentration-dependent manner. It was concluded that quercetin induces Ca++ elevation, leading to nitric oxide (NO) production and activation of the endothelial cell’s Ca++-activated K+ channel (KCa) 153. Quercetin showed anti-inflammatory properties in patients with coronary artery disease (CAD) through a decrease in nuclear factor kappa B (NF-Κb) transcriptional activity. Chekalina et al. 154 determined the effect of quercetin in patients with coronary artery disease to test this effect. Eighty-five patients with coronary artery disease participated in the study 154. Thirty patients received quercetin at a daily dose of 120 mg for two months, while the remaining fifty-five patients considered as a control group received β-blockers, statins, and aspirin. Increased levels of interleukin 1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-10 were detected in the serum of coronary artery disease patients. Under the influence of quercetin, the levels of interleukin 10 (IL-10), IL-1β, and TNF-α were reduced. Also, quercetin decreased the expression of the βα kappa inhibitor (Ikβα) relative to the control group 154.
Antihypertensive effect
High blood pressure is a condition in which the blood pressure in the arteries is constantly high. High blood pressure can be dangerous because it can increase the risk of cardiovascular disease and stroke. Quercetin has been shown to benefit hypertension by lowering blood pressure in animal and human studies 155. For example, Abdelghffar et al. 156 found a decrease in systolic, diastolic, and mean blood pressure, a reduction in ventricular hypertrophy, and less damage to the renal and vascular parenchyma in a model of hypertensive rats. These findings are due in part to the vasodilator property of quercetin, which in turn is explained by quercetin’s ability to scavenge free radicals, which would usually activate the secretion of 8-iso-prostaglandin F2α, a potent vasoconstrictor hormone 156. Likewise, a study published in the British Journal of Nutrition found that daily supplementation with quercetin (730 mg) for 12 weeks significantly reduced blood pressure in obese and overweight adults with prehypertension and stage 1 hypertension 157. In addition, some underlying molecular mechanisms by which quercetin may be acting have been investigated. One theory is that quercetin causes endothelial nitric oxide synthase (eNOS) to be activated, producing nitric oxide (NO), a vasodilator that relaxes blood vessels and reduces blood pressure. According to several studies, quercetin increases eNOS activity and nitric oxide (NO) generation 158. Another mechanism by which quercetin may exert its antihypertensive effect is the inhibition of angiotensin converting enzyme (ACE). Angiotensin 2, a potent vasoconstrictor that raises blood pressure, is created by the ACE enzyme, and quercetin has been shown to inhibit ACE activity, reducing blood pressure 159.
Mental activity
Quercetin can play a significant role in mental health diseases such as depression and anxiety 160. Mice studies have demonstrated that some natural products including quercetin possess anxiolytic properties when administered orally. Moreover, they are unlikely to have side effects serious enough to prevent their pharmacological utility, so they could constitute the starting point for the development of more selective anxiolytic agents 161. Due to its antioxidant activity, quercetin can lower nitric oxide (NO) and some other compounds that are vital for these diseases. According to SwissADME, quercetin can inhibit CYP isoenzymes. As a result, it can protect the organism from pathogenic factors.
Alzheimer’s disease
It has also been proposed that quercetin may have therapeutic effects on Alzheimer’s disease through several molecular mechanisms. Oxidative stress and neuroinflammation play a crucial role in the pathogenesis of Alzheimer’s disease, and quercetin can scavenge free radicals and inhibit the production of inflammatory cytokines, thereby reducing oxidative stress and inflammation in the brain 162. Another mechanism is quercetin’s ability to modulate the enzyme activity in clearing amyloid-beta (Aβ) plaques, a hallmark of Alzheimer’s disease pathology. Quercetin can increase the activity of neprilysin and insulin-degrading enzymes responsible for degrading amyloid beta (Aβ) plaques in the brain, thereby reducing their accumulation and toxicity 163. Quercetin can also modulate the activity of kinases involved in tau protein phosphorylation, another hallmark of Alzheimer’s disease pathology.
Furthermore, quercetin can inhibit the activity of glycogen synthase kinase 3β, responsible for abnormal tau protein phosphorylation, thus reducing tau aggregation and neurofibrillary tangles in the brain 163. Many in vivo studies demonstrated that the hydroxy-functional groups in the B-ring of quercetin are essential in inhibiting Aβ aggregation and altering mature fibrils by forming hydrogen bonds with the β-sheet structure. In addition, other studies have shown that quercetin increased the survival of neuronal cultures in vivo. Likewise, quercetin at 100 μM showed considerable inhibition of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) in an in vivo system by 11.85% 164. The other characteristic pathological implication of Alzheimer’s disease is the appearance of neurofibrillary tangles with tau being the central protein. Several studies demonstrated that quercetin effectively inhibited tau accumulation through different mechanisms, such as a reduction in tau protein hyperphosphorylation, through the inhibition of glycogen synthase kinase 3β (GSK3β) activity 162.
Several studies have suggested that quercetin may have antiviral effects against a wide variety of viruses, including hepatitis C virus (HCV), influenza A virus (IAV), Chikungunya virus (CHIKV), and severe acute respiratory syndrome coronavirus 2 (COVID-19). Inhibition of viral replication, host cell entry, and host immune response are some of the mechanisms hypothesized for the antiviral effect of quercetin. The main molecular mechanisms of quercetin’s antiviral effects are inhibition of viral neuraminidase, proteases, deoxyribonucleic acid (DNA)/ribonucleic acid (RNA) polymerases, and modification of several viral proteins. It has also been documented to suppress hepatitis C virus (HCV) by binding to and inactivating the viral NS3 protease 35. Rahman et al. 165 performed molecular docking in which they found that the significant interconnected nodes in the protein–protein network were protein kinase B (AKT1) (serine/threonine protein kinase), proto-oncogene tyrosine protein kinase sarcoma (SRC), epidermal growth factor receptor (EGFR), matrix metalloprotein (MMP9), kinase insert domain receptor (KDR), MMP2, insulin-like growth factor 1 receptor (IGF1R), protein tyrosine kinase 2 (PTK2), breast cancer resistance protein (BCRP), ATP-binding cassette super-family G (ABCG2), and mesenchymal–epithelial transition (MET). Quercetin inhibits viral retrotranscriptase, as is the case with Rauscher murine leukemia virus (RMLV), human immunodeficiency virus (HIV), and hepatitis B virus (HBV) 166, 167. Some published articles suggest that quercetin’s antiviral activity is due in part to a nonspecific protein denaturation mechanism that results in viral inactivation; however, some others offer the possibility that quercetin may bind to the surface receptors that viruses use to enter cells, thus blocking their infective capacity 168.
Diabetes
There is evidence to suggest that quercetin may have beneficial effects on diabetes 169. Several mechanisms have been proposed for the antihyperglycemic action of quercetin, including increasing insulin sensitivity, promoting glycogen synthesis, and improving insulin resistance. In addition to that, quercetin promotes insulin sensitization by stimulating pancreatic β-cell proliferation, which improves glucose metabolism and insulin secretion 170. It has also been reported that quercetin is an inhibitor of α-glucosidase and α-amylase. Furthermore, quercetin improved plasma insulin levels and lowered blood glucose in streptozotocin (STZ)-induced diabetes model by maintaining β-cells, thereby enhancing the effect of serum insulin 171. In addition, quercetin has been shown to stimulate glucose uptake in isolated cells by promoting GLUT4 expression and endogenous GLUT4 translocation through upregulation of estrogen receptor-α, subsequently increasing the phosphorylation of the phosphatidylinositol-3-kinase/Akt (PI3K/Akt) signaling pathways 172.
Arthritis
Arthritis is associated with decreased mobility, and its symptoms often include swelling, pain, stiffness, and redness. There are over 100 types of arthritis, the most common being osteoarthritis (OA), gout, and rheumatoid arthritis. Genetic, hormonal, and environmental factors have been linked to arthritis. Treatment of arthritis includes pharmacological steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and/or surgery 173. Quercetin reduces pain and inflammation associated with arthritis, as evidenced in a mouse study where it inhibited mechanical knee joint hyperalgesia, edema, and leukocyte recruitment 174. Mechanisms of quercetin included the inhibition of oxidative stress, production of cytokines such as cyclooxygenase-2 (COX-2) and proteoglycan degradation, and activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) (Nrf2/HO-1) signaling pathway 174. Guo H. et al. 175 demonstrated that quercetin activated the P110α/AKT/mammalian target of rapamycin (p110α/AKT/mTOR) signaling pathway by targeting p110α, revealing its promising potential to delay the osteoarthritis process by inhibiting cartilage ECM degradation and increasing chondrocyte proliferation. For this, a rat model was established to simulate osteoarthritis in vivo. It was concluded that quercetin exerts an anti-osteoarthritis effect by inhibiting MMP13 expression and increasing collagen deposition 175. In addition, quercetin has been reported as a potential drug for treating rheumatoid arthritis, as quercetin administration alone was more effective than methotrexate in reducing joint inflammation in mice. Mechanisms included decreased levels of TNF-α, IL-1β, IL-17, and monocyte chemoattractant protein-1 (MCP-1) 176.
Liver protection activity
Another essential aspect of quercetin is its liver protective function. This function has been demonstrated in several animal models, specifically in models of chronic liver damage. Chronic liver diseases, in general, progress in a similar way; they start with a process of oxidative stress in the organ caused by a foreign agent that can be toxic to a virus, which in turn leads to an inflammatory state, which activates the processes of fibrogenesis. If the damage persists, then the disease will progress to what is known as liver cirrhosis characterized mainly by thick bundles of extracellular matrix, regeneration nodules, and necrosis 177.
Quercetin side effects
There is inadequate evidence in humans for the carcinogenicity of quercetin. There is limited evidence in experimental animals for the carcinogenicity of quercetin 178. Groups of 50 male and 50 female Fischer 344/DuCrj rats, six weeks of age, were fed quercetin (purity, at least 99.4%) in the diet at concentrations of 0 (control), 1.25 or 5% for 104 weeks and were maintained for a further eight weeks without quercetin. The high dose was the maximum tolerated. At the end of the 112-week study, the survival rates for males were 56, 66 and 68% and those for females were 66, 62 and 72% for the three groups, respectively. No statistically significant increase in incidence of tumors was seen, but males at the high dose showed a significant increase in the incidence of non-neoplastic hyperplastic polyps of the cecum, and one adenoma and two adenocarcinomas of
the cecum were found in males at this dose and two adenomas of the colon occurred in females 179.
Groups of 70 male and 70 female Fischer 344/N rats, seven weeks of age, were given quercetin (purity, > 95%; ellagic acid was the predominant impurity at 1.1–2.6%) in the diet at concentrations of 0, 1000, 10 000 or 40 000 mg/kg (ppm) for 104 weeks. Ten animals per group were killed at 6 and 15 months. The high dose approached the maximum tolerated. Treatment did not affect the survival of either male or female rats, but the decreased body-weight gain of animals at the high dose was attributed to quercetin. Males at the high-dose had an increased incidence of renal tubular tumours (three adenomas and one adenocarcinoma) with none in control males, but the increase did not achieve statistical significance. After step-sectioning, a total of nine renal tubular tumours were found in these animals, and the increase was statistically significant. The severity of spontaneous progressive nephropathy was exacerbated in male rats by exposure to quercetin 180.
Overall, Quercetin is not classifiable as to its carcinogenicity to humans (Group 3) 178.
Oral supplementation with quercetin glycosides at doses ranging between 3 mg/day to 1,000 mg/day for up to three months has not resulted in significant adverse effects in clinical studies 181. A randomized, placebo-controlled study in 30 patients with chronic prostatitis reported one case of headache and another of tingling of the extremities associated with supplemental quercetin (1,000 mg/day for one month); both issues resolved after the study ended 182. In a phase 1 clinical trial in cancer patients unresponsive to standard treatments, administration of quercetin via intravenous infusion resulted in symptoms of nausea, vomiting, sweating, flushing, and dyspnea (difficulty breathing) at doses ≥10.5 mg/kg body weight (~756 mg of quercetin for a 70 kg individual) 183. Higher doses of quercetin up to 51.3 mg/kg body weight (~3,591 mg of quercetin) were associated with kidney toxicity, yet without evidence of nephritis, infection, or obstructive uropathy 181. Okamoto 184 concluded that it is unlikely that administration of quercetin at a typical dosage could cause any adverse effect. There do not appear to be any safety concerns about quercetin supplements at doses of 1,000 mg daily or less. However, most dietary supplements currently on the market contain mixtures of compounds, not just quercetin in isolation. There are no clear interactions of quercetin with other dietary supplements or medications.
- Terao J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants. 2023;12:258. doi: 10.3390/antiox12020258[↩]
- Georgiou N, Kakava MG, Routsi EA, Petsas E, Stavridis N, Freris C, Zoupanou N, Moschovou K, Kiriakidi S, Mavromoustakos T. Quercetin: A Potential Polydynamic Drug. Molecules. 2023 Dec 17;28(24):8141. doi: 10.3390/molecules28248141[↩]
- Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, Shu G, Tian Y, Zhao X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022 Jul 22;13:943321. doi: 10.3389/fimmu.2022.943321[↩]
- Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M, Shams MH, Baghbadorani PZ, Eskandari N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front Immunol. 2023 Feb 28;14:1077531. doi: 10.3389/fimmu.2023.1077531[↩]
- Zhang X, Tang Y, Lu G, Gu J. Pharmacological Activity of Flavonoid Quercetin and Its Therapeutic Potential in Testicular Injury. Nutrients. 2023 May 8;15(9):2231. doi: 10.3390/nu15092231[↩]
- Carrillo-Martinez EJ, Flores-Hernández FY, Salazar-Montes AM, Nario-Chaidez HF, Hernández-Ortega LD. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules. 2024 Feb 25;29(5):1000. doi: 10.3390/molecules29051000[↩][↩][↩][↩][↩][↩][↩][↩]
- Hosseini A., Razavi B.M., Banach M., Hosseinzadeh H. Quercetin and metabolic syndrome: A review. Phytother. Res. PTR. 2021;35:5352–5364. doi: 10.1002/ptr.7144[↩]
- Shen P., Lin W., Deng X., Ba X., Han L., Chen Z., Qin K., Huang Y., Tu S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021;12:689044. doi: 10.3389/fimmu.2021.689044[↩]
- Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radic Res. 2006 Oct;40(10):1054-65. https://www.ncbi.nlm.nih.gov/pubmed/17015250[↩]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19-34. doi: 10.1146/annurev.nutr.22.111401.144957[↩]
- Najafzadeh M, Reynolds PD, Baumgartner A, Anderson D. Flavonoids inhibit the genotoxicity of hydrogen peroxide (H(2)O(2[↩]
- Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol. 2006 Mar;13(3):319-28. doi: 10.1128/CVI.13.3.319-328.2006[↩]
- Razavi SM, Zahri S, Zarrini G, Nazemiyeh H, Mohammadi S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Bioorg Khim. 2009 May-Jun;35(3):414-6. doi: 10.1134/s1068162009030133[↩]
- Kempuraj D, Castellani ML, Petrarca C, Frydas S, Conti P, Theoharides TC, Vecchiet J. Inhibitory effect of quercetin on tryptase and interleukin-6 release, and histidine decarboxylase mRNA transcription by human mast cell-1 cell line. Clin Exp Med. 2006 Dec;6(4):150-6. doi: 10.1007/s10238-006-0114-7[↩]
- Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: molecular and histological evidences. J Exp Clin Cancer Res. 2009 Jun 11;28(1):80. doi: 10.1186/1756-9966-28-80[↩]
- Annapurna A, Reddy CS, Akondi RB, Rao SR. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009 Oct;61(10):1365-74. doi: 10.1211/jpp/61.10.0014[↩]
- Kyle JA, Sharp L, Little J, Duthie GG, McNeill G. Dietary flavonoid intake and colorectal cancer: a case-control study. Br J Nutr. 2010 Feb;103(3):429-36. doi: 10.1017/S0007114509991784[↩]
- Wilson RT, Wang J, Chinchilli V, Richie JP, Virtamo J, Moore LE, Albanes D. Fish, vitamin D, and flavonoids in relation to renal cell cancer among smokers. Am J Epidemiol. 2009 Sep 15;170(6):717-29. doi: 10.1093/aje/kwp178[↩]
- Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J, Stolzenberg-Solomon RZ. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland). Cancer Epidemiol Biomarkers Prev. 2008 Mar;17(3):553-62. doi: 10.1158/1055-9965.EPI-07-2523[↩]
- McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, Wilkinson GS, Graham S. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53(1):33-41. doi: 10.1207/s15327914nc5301_4[↩]
- Vijayababu MR, Arunkumar A, Kanagaraj P, Arunakaran J. Effects of quercetin on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) secretion and induction of apoptosis in human prostate cancer cells. J Carcinog. 2006 Apr 6;5:10. doi: 10.1186/1477-3163-5-10[↩]
- Lam TK, Rotunno M, Lubin JH, Wacholder S, Consonni D, Pesatori AC, Bertazzi PA, Chanock SJ, Burdette L, Goldstein AM, Tucker MA, Caporaso NE, Subar AF, Landi MT. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis. 2010 Apr;31(4):634-42. doi: 10.1093/carcin/bgp334[↩]
- Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, Cozen W, Mack TM, Lu QY, Zhang ZF. Dietary flavonoid intake and lung cancer–a population-based case-control study. Cancer. 2008 May 15;112(10):2241-8. doi: 10.1002/cncr.23398[↩]
- Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, Wolffram S, Müller MJ. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009 Oct;102(7):1065-74. doi: 10.1017/S0007114509359127[↩]
- Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J Agric Food Chem. 2009 Dec 23;57(24):11463-8. doi: 10.1021/jf903083h[↩]
- Iris Erlund, Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research, Volume 24, Issue 10, 2004, Pages 851-874, ISSN 0271-5317, https://doi.org/10.1016/j.nutres.2004.07.005[↩]
- Nieman DC, Henson DA, Gross SJ, Jenkins DP, Davis JM, Murphy EA, Carmichael MD, Dumke CL, Utter AC, McAnulty SR, McAnulty LS, Mayer EP. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007 Sep;39(9):1561-9. doi: 10.1249/mss.0b013e318076b566[↩][↩][↩][↩]
- MacRae HS, Mefferd KM. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int J Sport Nutr Exerc Metab. 2006 Aug;16(4):405-19. doi: 10.1123/ijsnem.16.4.405[↩][↩]
- Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R1071-7. doi: 10.1152/ajpregu.90925.2008[↩]
- Committee of Pharmacognosy Publication. Pharmacognosy. Pharmacognosy. Seoul Korea: Dong Myoung; 2013. pp. 102–105.[↩]
- Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, Inflammation and Immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167[↩]
- Mutha R.E., Tatiya A.U., Surana S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021;7:25. doi: 10.1186/s43094-020-00161-8[↩]
- Roy A., Khan A., Ahmad I., Alghamdi S., Rajab B.S., Babalghith A.O., Alshahrani M.Y., Islam S., Islam M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res. Int. 2022;2022:5445291. doi: 10.1155/2022/5445291[↩]
- Tronina T., Łużny M., Dymarska M., Urbaniak M., Kozłowska E., Piegza M., Stępień Ł., Janeczko T. Glycosylation of Quercetin by Selected Entomopathogenic Filamentous Fungi and Prediction of Its Products’ Bioactivity. Int. J. Mol. Sci. 2023;24:11857. doi: 10.3390/ijms241411857[↩]
- Shorobi F.M., Nisa F.Y., Saha S., Chowdhury M.A.H., Srisuphanunt M., Hossain K.H., Rahman M.A. Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules. 2023;28:938. doi: 10.3390/molecules28030938[↩][↩]
- Marrelli M., Russo C., Statti G., Argentieri M.P., Meleleo D., Mallamaci R., Avato P., Conforti F. Phytochemical and biological characterization of dry outer scales extract from Tropea red onion (Allium cepa L. var. Tropea)–A promising inhibitor of pancreatic lipase. Phytomed. Plus. 2022;2:100235. doi: 10.1016/j.phyplu.2022.100235[↩]
- Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Hertog MG, Hollman PC, Katan MB, Kromhout D. Nutr Cancer. 1993; 20(1):21-9. https://www.ncbi.nlm.nih.gov/pubmed/8415127/[↩]
- Effects of the dietary flavonoid quercetin upon performance and health. Davis JM, Murphy EA, Carmichael MD. Curr Sports Med Rep. 2009 Jul-Aug; 8(4):206-13. https://www.ncbi.nlm.nih.gov/pubmed/19584608/[↩]
- Aguirre L., Arias N., Macarulla M.T., Gracia A., Portillo M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011;4:189–198.[↩]
- National Toxicology Program (1991) NTP Chemical Repository Data Sheet: Quercetin , Research Triangle Park, NC.[↩]
- Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 2000;52:673–751.[↩]
- Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 2003;133:3248S–54S.[↩]
- Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92.[↩][↩]
- Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol 2012;83:6–15.[↩][↩][↩]
- Roskosko R Jr. ERK1/2 MAP kinases: structure, function and regulation. Pharmacol Res 2012;66:105–43.[↩]
- Gutiérrez-Venegas G, Bando-Campos CG. The flavonoids luteolin and quercetagetin inhibit lipoteichoic acid actions on H9c2 cardiomyocytes. Int Immunopharmacol 2010;10:1003–9.[↩]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 2000;6:909–19.[↩]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther 2007;114:208–21.[↩][↩]
- Shao Z-H, Wojcik KR, Dossumbekova A, Hsu C, Mehendale SR, Li C-Q et al. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J Cell Biochem 2009;107:697–705.[↩]
- Angeloni C, Spencer JPE, Leoncini E, Biagi PL, Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie 2007;89:73–82.[↩]
- Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu Y et al. Isorhamnetin inhibits H2O2-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem 2012;113:473–85.[↩]
- Dietary flavonoids: bioavailability, metabolic effects, and safety. Ross JA, Kasum CM. Annu Rev Nutr. 2002; 22():19-34. https://www.ncbi.nlm.nih.gov/pubmed/12055336/[↩]
- Owczarek-Januszkiewicz A., Magiera A., Olszewska M.A. Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. Int. J. Mol. Sci. 2022;23:14784. doi: 10.3390/ijms232314784[↩]
- Billowria K., Ali R., Rangra N.K., Kumar R., Chawla P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022:1–5. doi: 10.1080/10408347.2022.2105641[↩]
- Scholz S., Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007;77:224–235. doi: 10.1024/0300-9831.77.3.224. https://www.ncbi.nlm.nih.gov/pubmed/18214024[↩]
- Ader P., Wessmann A., Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic. Biol. Med. 2000;28:1056–1067. doi: 10.1016/S0891-5849(00)00195-7.[↩][↩]
- Guo Y., Mah E., Davis C.G., Jalili T., Ferruzzi M.G., Chun O.K., Bruno R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013;57:896–905. doi: 10.1002/mnfr.201200619.[↩]
- Ullah H., Minno A.D., Santarcangelo C., Tantipongpiradet A., Dacrema M., Matteo R.D., El-Seedi H.R., Khalifa S.A.M., Baldi A., Rossi A., et al. In Vitro Bioaccessibility and Anti-Inflammatory Activity of a Chemically Characterized Allium cepa L. Extract Rich in Quercetin Derivatives Optimized by the Design of Experiments. Molecules. 2022;27:9065. doi: 10.3390/molecules27249065[↩][↩]
- Rha C.-S., Choi J.-M., Jung Y.S., Kim E.-R., Ko M.J., Seo D.-H., Kim D.-O., Park C.-S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzym. Microb. Technol. 2019;120:84–90. doi: 10.1016/j.enzmictec.2018.10.006[↩]
- Kandemir K., Tomas M., McClements D.J., Capanoglu E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022;119:192–200. doi: 10.1016/j.tifs.2021.11.032[↩]
- Zhang Y., Mu T., Deng X., Guo R., Xia B., Jiang L., Wu Z., Liu M. New Insights of Biological Functions of Natural Polyphenols in Inflammatory Intestinal Diseases. Int. J. Mol. Sci. 2023;24:9581. doi: 10.3390/ijms24119581[↩]
- Oh J.-H., Lee J.H., Lee Y.-J. Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models. Asian J. Pharm. Sci. 2019;14:621–630. doi: 10.1016/j.ajps.2018.12.003[↩]
- Moghaddam A.H., Eslami A., Jelodar S.k., Ranjbar M., Hasantabar V. Preventive effect of quercetin-Loaded nanophytosome against autistic-like damage in maternal separation model: The possible role of Caspase-3, Bax/Bcl-2 and Nrf2. Behav. Brain Res. 2023;441:114300. doi: 10.1016/j.bbr.2023.114300[↩]
- Hedayati N., Yaghoobi A., Salami M., Gholinezhad Y., Aghadavood F., Eshraghi R., Aarabi M.H., Homayoonfal M., Asemi Z., Mirzaei H., et al. Impact of polyphenols on heart failure and cardiac hypertrophy: Clinical effects and molecular mechanisms. Front. Cardiovasc. Med. 2023;10:1174816. doi: 10.3389/fcvm.2023.1174816[↩]
- Bešlo D., Golubić N., Rastija V., Agić D., Karnaš M., Šubarić D., Lučić B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants. 2023;12:1141. doi: 10.3390/antiox12061141[↩]
- Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem Biophys Res Commun. 2000 Oct 22;277(2):507-12. doi: 10.1006/bbrc.2000.3701[↩]
- Wiczkowski W., Romaszko J., Bucinski A., Szawara-Nowak D., Honke J., Zielinski H., Piskula M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008;138:885–888. http://jn.nutrition.org/content/138/5/885.long[↩]
- Häkkinen S.H., Kärenlampi S.O., Heinonen I.M., Mykkänen H.M., Törrönen A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999;47:2274–2279. doi: 10.1021/jf9811065.[↩]
- Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81(Suppl. S1):243S–255S.[↩]
- Smith C., Lombard K.A., Peffley E.B., Liu W. Genetic analysis of quercetin in onion (Allium cepa L.) Lady Raider. Tex. J. Agric. Natl. Resour. 2003;16:24–28.[↩]
- Ulusoy H.G., Sanlier N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020;60:3290–3303. doi: 10.1080/10408398.2019.1683810[↩]
- Mitchell A.E., Hong Y.J., Koh E., Barrett D.M., Bryant D.E., Denison R.F., Kaffka S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007;55:6154–6159. doi: 10.1021/jf070344+.[↩]
- Petrus K., Schwartz H., Sontag G. Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2011;400:2555–2563. doi: 10.1007/s00216-010-4614-7.[↩]
- Tutelian V.A., Lashneva N.V. Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sources and consumption. Vopr. Pitan. 2013;82:4–22.[↩]
- Bhagwat S., Haytowits D.B., Holden J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3. U.S. Department of Agriculture; Beltsville, MD, USA: 2011.[↩]
- Chun O.K., Chung S.J., Song W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007;137:1244–1252.[↩]
- Sun C., Wang H., Wang D., Chen Y., Zhao Y., Xia W. Using an FFQ to assess intakes of dietary flavonols and flavones among female adolescents in the Suihua area of northern China. Public Health Nutr. 2015;18:632–639. doi: 10.1017/S1368980014000780.[↩]
- Zhang Y., Li Y., Cao C., Cao J., Chen W., Zhang Y., Wang C., Wang J., Zhang X., Zhao X. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutr. Cancer. 2010;62:1120–1127. doi: 10.1080/01635581.2010.513800.[↩]
- Sampson L., Rimm E., Hollman P.C., de Vries J.H., Katan M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002;102:1414–1420. doi: 10.1016/S0002-8223(02)90314-7.[↩]
- Nishimuro H., Ohnishi H., Sato M., Ohnishi-Kameyama M., Matsunaga I., Naito S., Ippoushi K., Oike H., Nagata T., Akasaka H., et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients. 2015;7:2345–2358. doi: 10.3390/nu7042345.[↩]
- Somerset S.M., Johannot L. Dietary flavonoid sources in Australian adults. Nutr. Cancer. 2008;60:442–449. doi: 10.1080/01635580802143836.[↩][↩]
- Zamora-Ros R., Andres-Lacueva C., Lamuela-Raventos R.M., Berenguer T., Jakszyn P., Barricarte A., Ardanaz E., Amiano P., Dorronsoro M., Larrañaga N., et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain) J. Am. Diet. Assoc. 2010;110:390–398. doi: 10.1016/j.jada.2009.11.024.[↩]
- Murakami A., Ashida H., Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046[↩]
- Cui Z., Zhao X., Amevor F.K., Du X., Wang Y., Li D., Shu G., Tian Y., Zhao X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022;13:943321. doi: 10.3389/fimmu.2022.943321[↩]
- Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to m2 macrophages. Free Radical Bio Med (2019) 145:146–60. doi: 10.1016/j.freeradbiomed.2019.09.024[↩]
- Shabir I., Kumar Pandey V., Shams R., Dar A.H., Dash K.K., Khan S.A., Bashir I., Jeevarathinam G., Rusu A.V., Esatbeyoglu T., et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022;9:999752. doi: 10.3389/fnut.2022.999752[↩]
- Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G, et al.. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between Nlrp3 inflammasome and mitophagy. Redox Biol (2021) 44:102010. doi: 10.1016/j.redox.2021.102010[↩]
- Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, et al.. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res (2018) 62(1):1700447. doi: 10.1002/mnfr.201700447[↩]
- Cheng S.C., Huang W.C., JH S.P., Wu Y.H., Cheng C.Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019;20:2957. doi: 10.3390/ijms20122957[↩]
- Kaushik D, O’Fallon K, Clarkson PM, Dunne CP, Conca KR, Michniak-Kohn B. Comparison of quercetin pharmacokinetics following oral supplementation in humans. J Food Sci. 2012;77(11):H231–H238.[↩]
- Lee SM, Moon J, Chung JH, Cha YJ, Shin MJ. Effect of quercetin-rich onion peel extracts on arterial thrombosis in rats. Food Chem Toxicol. 2013;57:99–105.[↩]
- Paliwal S, Sundaram J, Mitragotri S. Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound. Br J Cancer. 2005;92(3):499–502.[↩]
- Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104(2):198–203.[↩]
- Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. FEBS Lett. 1997 Nov 24; 418(1-2):152-6.[↩]
- Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Lee TJ, Kim OH, Kim YH, Lim JH, Kim S, Park JW, Kwon TK. Cancer Lett. 2006 Aug 28; 240(2):234-42. https://www.ncbi.nlm.nih.gov/pubmed/16274926/[↩]
- Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Ong CS, Tran E, Nguyen TT, Ong CK, Lee SK, Lee JJ, Ng CP, Leong C, Huynh H. Oncol Rep. 2004 Mar; 11(3):727-33. https://www.ncbi.nlm.nih.gov/pubmed/14767529/[↩]
- Quercetin inhibits Shc- and phosphatidylinositol 3-kinase-mediated c-Jun N-terminal kinase activation by angiotensin II in cultured rat aortic smooth muscle cells. Yoshizumi M, Tsuchiya K, Kirima K, Kyaw M, Suzaki Y, Tamaki T. Mol Pharmacol. 2001 Oct; 60(4):656-65.[↩]
- Free radical scavenging and antioxidant activity of plant flavonoids. Kandaswami C, Middleton E Jr. Adv Exp Med Biol. 1994; 366():351-76. https://www.ncbi.nlm.nih.gov/pubmed/7771265/[↩]
- Inhibition of the metmyoglobin-induced peroxidation of linoleic acid by dietary antioxidants: Action in the aqueous vs. lipid phase. Vulcain E, Goupy P, Caris-Veyrat C, Dangles O. Free Radic Res. 2005 May; 39(5):547-63.[↩]
- In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Wu LL, Yang XB, Huang ZM, Liu HZ, Wu GX. Acta Pharmacol Sin. 2007 Mar; 28(3):404-9.[↩]
- Modulation of PI3K-LXRα-dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin. Pisonero-Vaquero S, García-Mediavilla MV, Jorquera F, Majano PL, Benet M, Jover R, González-Gallego J, Sánchez-Campos S. Lab Invest. 2014 Mar; 94(3):262-74.[↩]
- Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Yu YB, Miyashiro H, Nakamura N, Hattori M, Park JC. Arch Pharm Res. 2007 Jul; 30(7):820-6.[↩]
- Inhibition of reverse transcriptases by flavonoids. Spedding G, Ratty A, Middleton E Jr. Antiviral Res. 1989 Sep; 12(2):99-110.[↩]
- Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer chemotherapy and pharmacology. 2011;68:1449–1457.[↩]
- Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. Journal of ethnopharmacology. 2012;143:383–396.[↩]
- Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin’s effects on intestinal polyp multiplicity and macrophage number in the Apc(Min/+) mouse. Nutrition and cancer. 2011;63:421–426.[↩]
- Sekine-Osajima Y, Sakamoto N, Nakagawa M, Itsui Y, Tasaka M, Nishimura-Sakurai Y, Chen CH, Suda G, Mishima K, Onuki Y, Yamamoto M, Maekawa S, Enomoto N, Kanai T, Tsuchiya K, Watanabe M. Two flavonoids extracts from Glycyrrhizae radix inhibit in vitro hepatitis C virus replication. Hepatology research : the official journal of the Japan Society of Hepatology. 2009;39:60–69.[↩]
- Qureshi A.A., Khan D.A., Mahjabeen W., Papasian C.J., Qureshi N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J. Clin. Exp. Cardiol. 2012;S5:167. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3486425/[↩]
- Heinz S.A., Henson D.A., Austin M.D., Jin F., Nieman D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010;62:237–242. doi: 10.1016/j.phrs.2010.05.001. https://www.ncbi.nlm.nih.gov/pubmed/20478383[↩]
- Heinz S.A., Henson D.A., Nieman D.C., Austin M.D., Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010;104:849–857. doi: 10.1017/S000711451000156X. https://www.ncbi.nlm.nih.gov/pubmed/20500927[↩][↩]
- Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. https://www.ncbi.nlm.nih.gov/pubmed/17805089[↩]
- Henson D., Nieman D., Davis J.M., Dumke C., Gross S., Murphy A., Carmichael M., Jenkins D.P., Quindry J., McAnulty S., et al. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008;29:856–863. doi: 10.1055/s-2007-989424. https://www.ncbi.nlm.nih.gov/pubmed/18213545[↩]
- Nieman D.C., Henson D.A., Maxwell K.R., Williams A.S., McAnulty S.R., Jin F., Shanely R.A., Lines T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009;41:1467–1475. doi: 10.1249/MSS.0b013e318199491f. https://www.ncbi.nlm.nih.gov/pubmed/19516153[↩]
- Nieman D.C., Williams A.S., Shanely R.A., Jin F., McAnulty S.R., Triplett N.T., Austin M.D., Henson D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010;42:338–345. doi: 10.1249/MSS.0b013e3181b18fa3. https://www.ncbi.nlm.nih.gov/pubmed/19927026[↩]
- Qureshi A.A., Khan D.A., Mahjabeen W., Papasian C.J., Qureshi N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J. Clin. Exp. Cardiol. 2012;S5:167.[↩]
- Heinz S.A., Henson D.A., Austin M.D., Jin F., Nieman D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010;62:237–242. doi: 10.1016/j.phrs.2010.05.001.[↩]
- Nieman D.C., Henson D.A., Maxwell K.R., Williams A.S., McAnulty S.R., Jin F., Shanely R.A., Lines T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009;41:1467–1475. doi: 10.1249/MSS.0b013e318199491f.[↩]
- Heinz S.A., Henson D.A., Nieman D.C., Austin M.D., Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010;104:849–857. doi: 10.1017/S000711451000156X.[↩]
- Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566.[↩]
- Henson D., Nieman D., Davis J.M., Dumke C., Gross S., Murphy A., Carmichael M., Jenkins D.P., Quindry J., McAnulty S., et al. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008;29:856–863. doi: 10.1055/s-2007-989424.[↩]
- Nieman D.C., Williams A.S., Shanely R.A., Jin F., McAnulty S.R., Triplett N.T., Austin M.D., Henson D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010;42:338–345. doi: 10.1249/MSS.0b013e3181b18fa3.[↩]
- Alugoju P, Narsimulu D, Bhanu JU, Satyanarayana N, Periyasamy L. Role of quercetin and caloric restriction on the biomolecular composition of aged rat cerebral cortex: an FTIR study. Spectrochim Acta A Mol Biomol Spectrosc. (2019) 220:117128. 10.1016/j.saa.2019.05.033[↩]
- Shabir I, Kumar Pandey V, Shams R, Dar AH, Dash KK, Khan SA, Bashir I, Jeevarathinam G, Rusu AV, Esatbeyoglu T, Pandiselvam R. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front Nutr. 2022 Nov 30;9:999752. doi: 10.3389/fnut.2022.999752[↩][↩]
- Leite NB, Martins DB, Alvares DS, dos Santos Cabrera MP. Quercetin induces lipid domain-dependent permeability. Chem Phys Lipids. (2022) 242:105160. 10.1016/j.chemphyslip.2021.105160[↩]
- Liu J, Li X, Yue Y, Li J, He T, He Y. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell Mol Immunol. 2005 Dec;2(6):455-60.[↩]
- Chansiw N., Champakam S., Chusri P., Pangjit K., Srichairatanakool S. Quercetin-Rich Ethanolic Extract of Polygonum odoratum var Pakphai Leaves Decreased Gene Expression and Secretion of Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Murine RAW264.7 Macrophages. Molecules. 2022;27:3657. doi: 10.3390/molecules27123657[↩][↩]
- Wang J., Miao M., Zhang Y., Liu R., Li X., Cui Y., Qu L. Quercetin ameliorates liver injury induced with Tripterygium glycosides by reducing oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2015;93:427–433. doi: 10.1139/cjpp-2015-0038[↩]
- Dong J., Zhang X., Zhang L., Bian H.-X., Xu N., Bao B., Liu J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKα1/SIRT1. J. Lipid Res. 2014;55:363–374. doi: 10.1194/jlr.M038786[↩][↩][↩]
- Chen T., Zhang X., Zhu G., Liu H., Chen J., Wang Y., He X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine. 2020;99:e22241. doi: 10.1097/MD.0000000000022241[↩]
- Wang C., Pan Y., Zhang Q.-Y., Wang F.-M., Kong L.-D. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE. 2012;7:e38285. doi: 10.1371/journal.pone.0038285[↩]
- Min Y.D., Choi C.H., Bark H., Son H.Y., Park H.H., Lee S., Park J.W., Park E.K., Shin H.I., Kim S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007;56:210–215. doi: 10.1007/s00011-007-6172-9[↩][↩]
- Kempuraj D., Madhappan B., Christodoulou S., Boucher W., Cao J., Papadopoulou N., Cetrulo C.L., Theoharides T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246[↩]
- Patra S., Pradhan B., Nayak R., Behera C., Das S., Patra S.K., Efferth T., Jena M., Bhutia S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine. 2021;90:153554. doi: 10.1016/j.phymed.2021.153554[↩]
- Primikyri A., Chatziathanasiadou M.V., Karali E., Kostaras E., Mantzaris M.D., Hatzimichael E., Shin J.-S., Chi S.-W., Briasoulis E., Kolettas E., et al. Direct binding of Bcl-2 family proteins by quercetin triggers its pro-apoptotic activity. ACS Chem. Biol. 2014;9:2737–2741. doi: 10.1021/cb500259e[↩]
- Luo C.-L., Liu Y.-Q., Wang P., Song C.-H., Wang K.-J., Dai L.-P., Zhang J.-Y., Ye H. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed. Pharmacother. 2016;82:595–605. doi: 10.1016/j.biopha.2016.05.029[↩]
- Zhu Y., Jiang Y., Shi L., Du L., Xu X., Wang E., Sun Y., Guo X., Zou B., Wang H., et al. 7-O-Geranylquercetin induces apoptosis in gastric cancer cells via ROS-MAPK mediated mitochondrial signaling pathway activation. Biomed. Pharmacother. 2017;87:527–538. doi: 10.1016/j.biopha.2016.12.095[↩]
- Teekaraman D., Elayapillai S.P., Viswanathan M.P., Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019;300:91–100. doi: 10.1016/j.cbi.2019.01.008[↩]
- Ma Y.S., Yao C.N., Liu H.C., Yu F.S., Lin J.J., Lu K.W., Liao C.L., Chueh F.S., Chung J.G. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol. Lett. 2018;15:9663–9672. doi: 10.3892/ol.2018.8584[↩]
- Jeong J.-H., An J.Y., Kwon Y.T., Rhee J.G., Lee Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977[↩]
- Lee T.-J., Kim O.H., Kim Y.H., Lim J.H., Kim S., Park J.-W., Kwon T.K. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006;240:234–242. doi: 10.1016/j.canlet.2005.09.013[↩]
- Sudan S, Rupasinghe HP. Quercetin-3-O-glucoside induces human DNA topoisomerase II inhibition, cell cycle arrest and apoptosis in hepatocellular carcinoma cells. Anticancer Res. 2014 Apr;34(4):1691-9.[↩]
- Joyner P.M. Protein Adducts and Protein Oxidation as Molecular Mechanisms of Flavonoid Bioactivity. Molecules. 2021;26:5102. doi: 10.3390/molecules26165102[↩]
- Shah S., Narang R., Singh V.J., Pilli G., Nayak S.K. A Review on Anticancer Profile of Flavonoids: Sources, Chemistry, Mechanisms, Structure-activity Relationship and Anticancer Activity. Curr. Drug Res. Rev. Former. Curr. Drug Abus. Rev. 2023;15:122–148. doi: 10.2174/2589977515666230120144852[↩]
- Zhu S, Yu W, Bi L, Qin F, Li J, Zeng H, Lu L. [Quercetin induces apoptosis of human breast cancer cells by activiting PTEN and inhibiting PI3K/AKT and JNK signaling pathways]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022 Aug;38(8):714-720. Chinese.[↩]
- Mohammadinejad S., Jafari-Gharabaghlou D., Zarghami N. Development of PEGylated PLGA Nanoparticles Co-Loaded with Bioactive Compounds: Potential Anticancer Effect on Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2022;23:4063–4072. doi: 10.31557/APJCP.2022.23.12.4063[↩]
- Svitina H., Hamman J.H., Gouws C. Molecular mechanisms and associated cell signalling pathways underlying the anticancer properties of phytochemical compounds from Aloe species (Review) Exp. Ther. Med. 2021;22:852. doi: 10.3892/etm.2021.10284[↩]
- Akhtar M.F., Saleem A., Rasul A., Faran Ashraf Baig M.M., Bin-Jumah M., Abdel Daim M.M. Anticancer natural medicines: An overview of cell signaling and other targets of anticancer phytochemicals. Eur. J. Pharmacol. 2020;888:173488. doi: 10.1016/j.ejphar.2020.173488[↩]
- Mumtaz P.T., Bashir S.M., Rather M.A., Dar K.B., Taban Q., Sajood S., Ali A., Rather Z.A., Amin I., Dar M.A. Antiproliferative and Apoptotic Activities of Natural Honey. In: Rehman M.U., Majid S., editors. Therapeutic Applications of Honey and its Phytochemicals. Volume 1. Springer; Singapore: 2020. pp. 345–360.[↩]
- Boccellino M., Quagliuolo L., D’Angelo S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Curr. Nutr. Food Sci. 2021;17:111–120. doi: 10.2174/1573401316999200504093028[↩]
- Almatroodi S.A., Alsahli M.A., Almatroudi A., Verma A.K., Aloliqi A., Allemailem K.S., Khan A.A., Rahmani A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules. 2021;26:1315. doi: 10.3390/molecules26051315[↩]
- Kicinska A., Jarmuszkiewicz W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules. 2020;25:3060. doi: 10.3390/molecules25133060[↩]
- Fikriah I., Ismail S., Kosala K. In vitro evaluation of the vasodilatory activity of ethanol extracts of Eleutherine bulbosa bulbs and leaves. J. Appl. Pharm. Sci. 2021;11:135–140.[↩]
- Solfaine R., Muniroh L., Irawan A. Roles of averrhoa bilimbi extract in increasing serum nitric oxide concentration and vascular dilatation of ethanol-induced hypertensive rats. Prev. Nutr. Food Sci. 2021;26:186. doi: 10.3746/pnf.2021.26.2.186[↩][↩]
- Chekalina N., Burmak Y., Petrov Y., Borisova Z., Manusha Y., Kazakov Y., Kaidashev I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018;70:593–597. doi: 10.1016/j.ihj.2018.04.006[↩][↩][↩]
- Da Rocha E.V., Falchetti F., Pernomian L., De Mello M.M.B., Parente J.M., Nogueira R.C., Gomes B.Q., Bertozi G., Sanches-Lopes J.M., Tanus-Santos J.E. Quercetin decreases cardiac hypertrophic mediators and maladaptive coronary arterial remodeling in renovascular hypertensive rats without improving cardiac function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023;396:939–949. doi: 10.1007/s00210-022-02349-6[↩]
- Abdelghffar E.A., Obaid W.A., Elgamal A.M., Daoud R., Sobeh M., El Raey M.A. Pea (Pisum sativum) peel extract attenuates DOX-induced oxidative myocardial injury. Biomed. Pharmacother. 2021;143:112120. doi: 10.1016/j.biopha.2021.112120[↩][↩]
- Popiolek-Kalisz J., Fornal E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022;47:101350. doi: 10.1016/j.cpcardiol.2022.101350[↩]
- Das M., Devi K.P., Belwal T., Devkota H.P., Tewari D., Sahebnasagh A., Nabavi S.F., Khayat Kashani H.R., Rasekhian M., Xu S., et al. Harnessing polyphenol power by targeting eNOS for vascular diseases. Crit. Rev. Food Sci. Nutr. 2023;63:2093–2118. doi: 10.1080/10408398.2021.1971153[↩]
- Ra J.-E., Woo S.-Y., Jin H., Lee M.J., Kim H.Y., Ham H., Chung I.-M., Seo W.D. Evaluation of antihypertensive polyphenols of barley (Hordeum vulgare L.) seedlings via their effects on angiotensin-converting enzyme (ACE) inhibition. Appl. Biol. Chem. 2020;63:1–9. doi: 10.1186/s13765-020-00519-9[↩]
- Hosseini A., Razavi B.M., Banach M., Hosseinzadeh H. Quercetin and Metabolic Syndrome: A Review. Phyther. Res. 2021;35:5352–5364. doi: 10.1002/ptr.7144[↩]
- Yoon B.H., Jung J.W., Lee J.-J., Cho Y.-W., Jang C.-G., Jin C., Oh T.H., Ryu J.H. Anxiolytic-like Effects of Sinapic Acid in Mice. Life Sci. 2007;81:234–240. doi: 10.1016/j.lfs.2007.05.007[↩]
- Zhao Y., Zhang Y., Zhang J., Zhang X., Yang G. Molecular mechanism of autophagy: Its role in the therapy of Alzheimer’s disease. Curr. Neuropharmacol. 2020;18:720–739. doi: 10.2174/1570159X18666200114163636[↩][↩]
- Guo M., Zhu F., Qiu W., Qiao G., Law B.Y.-K., Yu L., Wu J., Tang Y., Yu C., Qin D., et al. High-throughput screening for amyloid-β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC−DAD-Q/TOF-MS/MS. Acta Pharm. Sin. B. 2022;12:1723–1739. doi: 10.1016/j.apsb.2021.08.030[↩][↩]
- Zaplatic E., Bule M., Shah S.Z.A., Uddin M.S., Niaz K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019;224:109–119. doi: 10.1016/j.lfs.2019.03.055[↩]
- Rahman M.A., Shorobi F.M., Uddin M.N., Saha S., Hossain M.A. Quercetin attenuates viral infections by interacting with target proteins and linked genes in chemicobiological models. Silico Pharmacol. 2022;10:17. doi: 10.1007/s40203-022-00132-2[↩]
- Parvez M.K., Al-Dosari M.S., Arbab A.H., Al-Rehaily A.J., Abdelwahid M.A.S. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm. J. 2020;28:550–559. doi: 10.1016/j.jsps.2020.03.006[↩]
- Silva C.C.F.d., Salatino A., Motta L.B.d., Negri G., Salatino M.L.F. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará state. Rev. Bras. Farmacogn. 2019;29:309–318. doi: 10.1016/j.bjp.2019.04.001[↩]
- Upadhyay R., Tiwari K.N. The antiviral potential of Phyllanthus species: A systematic review. Arch. Virol. 2023;168:177. doi: 10.1007/s00705-023-05802-w[↩]
- Jaishree V, Narsimha S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed Pharmacother. (2020) 130:110561. 10.1016/j.biopha.2020.110561[↩]
- Ansari P., Choudhury S.T., Seidel V., Rahman A.B., Aziz M.A., Richi A.E., Rahman A., Jafrin U.H., Hannan J.M.A., Abdel-Wahab Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life. 2022;12:1146. doi: 10.3390/life12081146[↩]
- Shi G.-J., Li Y., Cao Q.-H., Wu H.-X., Tang X.-Y., Gao X.-H., Yu J.-Q., Chen Z., Yang Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019;109:1085–1099. doi: 10.1016/j.biopha.2018.10.130[↩]
- Hamilton K.E., Rekman J.F., Gunnink L.K., Busscher B.M., Scott J.L., Tidball A.M., Stehouwer N.R., Johnecheck G.N., Looyenga B.D., Louters L.L. Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie. 2018;151:107–114. doi: 10.1016/j.biochi.2018.05.012[↩]
- ASalehi B., Machin L., Monzote L., Sharifi-Rad J., Ezzat S.M., Salem M.A., Merghany R.M., El Mahdy N.M., Kılıç C.S., Sytar O., et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020;5:11849–11872. doi: 10.1021/acsomega.0c01818[↩]
- Borghi S.M., Mizokami S.S., Pinho-Ribeiro F.A., Fattori V., Crespigio J., Clemente-Napimoga J.T., Napimoga M.H., Pitol D.L., Issa J.P., Fukada S.Y. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018;53:81–95. doi: 10.1016/j.jnutbio.2017.10.010[↩][↩]
- Guo H., Yin W., Zou Z., Zhang C., Sun M., Min L., Yang L., Kong L. Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J. Adv. Res. 2021;28:255–267. doi: 10.1016/j.jare.2020.06.020[↩][↩]
- Córdoba-Moreno M.O., Mendes M.T., Markus R.P., Fernandes P.A. Rat resistance to rheumatoid arthritis induction as a function of the early-phase adrenal–pineal crosstalk. J. Physiol. 2023;601:535–549. doi: 10.1113/JP283456[↩]
- Shao C., Xu H., Sun X., Huang Y., Guo W., He Y., Ye L., Wang Z., Huang J., Liang X., et al. New Perspectives on Chinese Medicine in Treating Hepatic Fibrosis: Lipid Droplets in Hepatic Stellate Cells. Am. J. Chin. Med. 2023;51:1413–1429. doi: 10.1142/S0192415X23500647[↩]
- https://monographs.iarc.fr/ENG/Monographs/vol73/mono73-23.pdf[↩][↩]
- Ito, N., Hagiwara, A., Tamano, S., Kagawa, M., Shibata, M.-A., Kurata, Y. & Fukushima, S.(1989) Lack of carcinogenicity of quercetin in F344/DuCrj rats. Jpn. J. Cancer Res .,80,317–325.[↩]
- National Toxicology Program (1992). Toxicology and Carcinogenesis Studies of Quercetin (CASNo. 117-39-5) in F344/N Rats (Feed Studies) (Tech. Rep. Ser. No. 409; NIH Publ. No. 92-3140), Research Triangle Park, NC.[↩]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007 Nov;45(11):2179-205. doi: 10.1016/j.fct.2007.05.015[↩][↩]
- Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999 Dec;54(6):960-3. doi: 10.1016/s0090-4295(99)00358-1[↩]
- Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996 Apr;2(4):659-68.[↩]
- Okamoto T. Safety of quercetin for clinical application (Review). Int J Mol Med. 2005 Aug;16(2):275-8.[↩]