Contents
- Riboflavin
- Riboflavin Function
- Benefits of Riboflavin on Health
- How much riboflavin do I need?
- What foods provide riboflavin?
- Riboflavin supplements
- Am I getting enough riboflavin?
- Riboflavin deficiency
- Health Risks from Excessive Riboflavin
Riboflavin
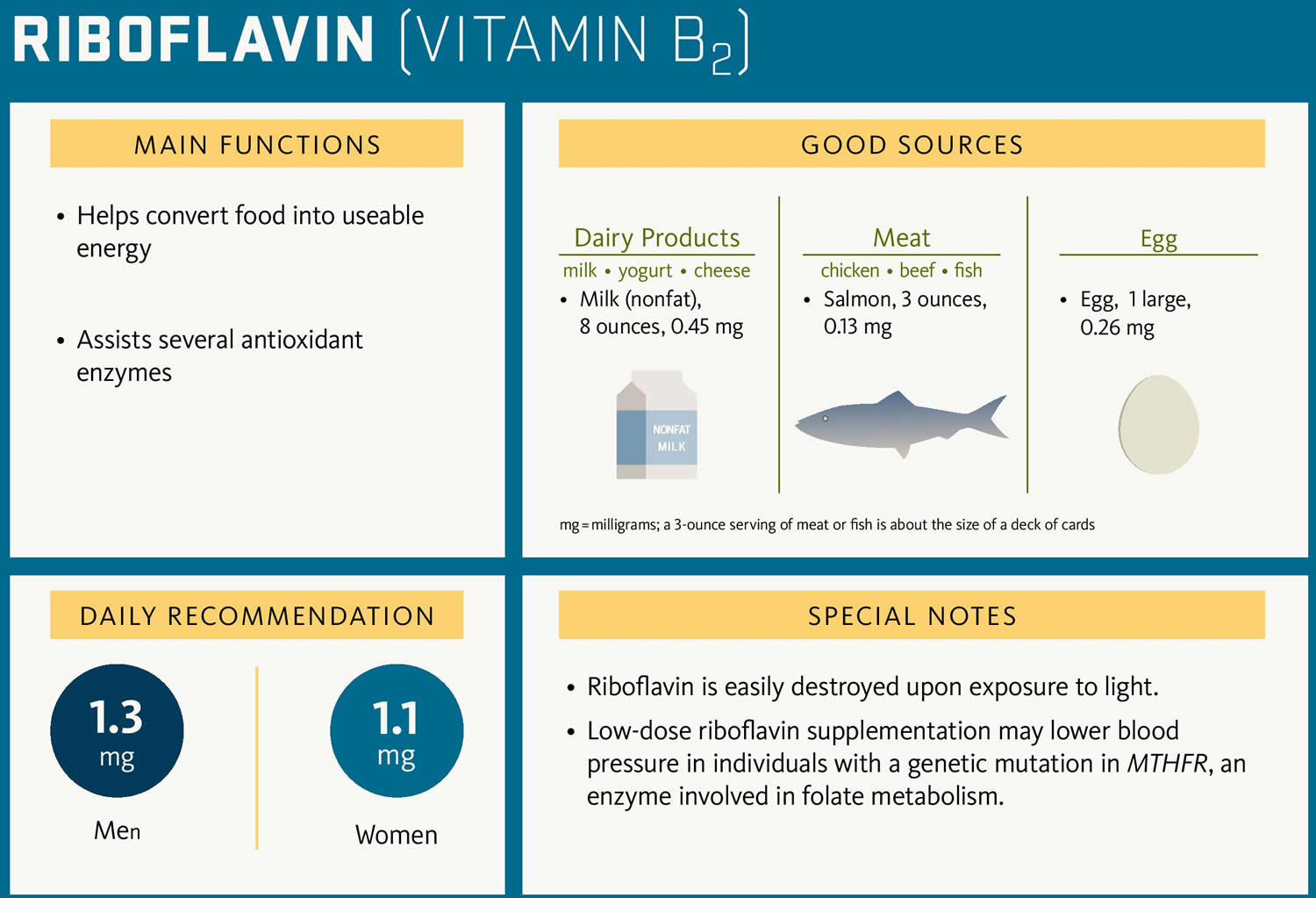
Riboflavin also called vitamin B2 is one of the B vitamins, which are all water soluble and it’s important for the growth, development, and function of the cells in your body. Riboflavin also helps turn the food you eat into the energy you need. Riboflavin or vitamin B2 is naturally present in some foods, added to some food products, and available as a dietary supplement. You can get recommended amounts of riboflavin by eating a variety of foods, including the following 1:
- Eggs, organ meats (such as kidneys and liver), lean meats, and low-fat milk
- Some vegetables (such as mushrooms and spinach)
- Fortified cereals, bread, and grain products.
The Recommended Dietary Allowance (RDA) of riboflavin is 1.3 mg for men and 1.1 mg for women 2.
More than 90% of dietary riboflavin is in the form of two major coenzymes, flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN); the remaining 10% is comprised of the free form and glycosides or esters 3, 4. Coenzymes derived from riboflavin are termed flavocoenzymes, and enzymes that use a flavocoenzyme are called flavoproteins 5. The two major riboflavin coenzymes, flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN), act as electron carriers in a number of oxidation-reduction (redox) reactions involved in energy production; cellular antioxidant function, growth, and development; and in metabolism of fats, drugs, and steroids 6, 3, 7, 8. The conversion of the amino acid tryptophan to niacin (sometimes referred to as vitamin B3) requires flavin adenine dinucleotide (FAD) 7. Similarly, the conversion of vitamin B6 (Pyridoxine) to the coenzyme pyridoxal 5’-phosphate (the active form of vitamin B6) needs flavin mononucleotide (FMN). Riboflavin (as FAD or FMN) is also required for the metabolism of iron and in the synthesis of niacin from tryptophan 9. Riboflavin also plays an essential role in folate (vitamin B9) and related one-carbon metabolism, where FAD is required as a cofactor for methylenetetrahydrofolate reductase (MTHFR), a key folate-metabolizing enzyme 9. In addition, riboflavin helps maintain normal levels of homocysteine, an amino acid in the blood 6.
Most riboflavin or vitamin B2 is absorbed in the proximal small intestine via a rapid, active and saturable transport system 10. Riboflavin is absorbed from the gastrointestinal tract predominantly by riboflavin transporter 3 (RFVT3) 11. Inside the gastrointestinal cells, riboflavin can either be further metabolized to flavin mononucleotide (FMN) by riboflavin kinase (RFK) or to flavin adenine dinucleotide (FAD) by FAD synthase (FADS) or transported to the bloodstream by riboflavin transporter 1 (RFVT1) and riboflavin transporter 2 (RFVT2) 10. Riboflavin is absorbed from the gastrointestinal tract predominantly by riboflavin transporter 3 (RFVT3) 11. Riboflavin is distributed via the bloodstream to its destination cells. In addition to being expressed in the gastrointestinal system, RFVT1 is expressed in the placenta, where it carries riboflavin from maternal bloodstream to fetal bloodstream 10. Riboflavin is absorbed from the gastrointestinal tract predominantly by riboflavin transporter 3 (RFVT3) 11. RFVT2 is expressed all over the body and highly expressed in the brain, endocrine organs, such as pancreas, but also in the liver and muscle tissue 10. Riboflavin is absorbed from the gastrointestinal tract predominantly by riboflavin transporter 3 (RFVT3) 11. Inside the destination cells, riboflavin is used directly or transformed into either flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN), which are used as cofactors for several processes.
The rate of riboflavin absorption is proportional to intake, and it increases when riboflavin is ingested along with other foods and in the presence of bile salts. The body absorbs little riboflavin from single doses beyond 27 mg and stores only small amounts of riboflavin in the liver, heart, and kidneys 2. When excess amounts are consumed, they are either not absorbed or the small amount that is absorbed is excreted in urine 4.
Bacteria in the large intestine produce free riboflavin that can be absorbed by the large intestine in amounts that depend on your diet. More riboflavin is produced after ingestion of vegetable-based than meat-based foods 3. A small amount of riboflavin circulates via the enterohepatic system 10. Malabsorption from conditions such as celiac disease, malignancies, and alcoholism can promote deficiency of riboflavin.
Riboflavin is yellow and naturally fluorescent when exposed to ultraviolet light 6. Moreover, ultraviolet and visible light can rapidly inactivate riboflavin and its derivatives 2. Because of this sensitivity, lengthy light therapy to treat jaundice in newborns or skin disorders can lead to riboflavin deficiency 2. The risk of riboflavin loss from exposure to light is the reason why milk is not typically stored in glass containers 4, 12.
Several factors can affect human riboflavin status, of which diet has the largest impact in the general population. However, other factors such as pregnancy, exercise, aging, infections—and in rare cases genetic variations—can also affect riboflavin status 11.
Riboflavin status is not routinely measured in healthy people 2. A stable and sensitive measure of riboflavin deficiency is the red blood cell glutathione reductase activity coefficient (erythrocyte glutathione reductase activity coefficient or EGRAC), which is based on the ratio between this enzyme’s in vitro activity in the presence of FAD to that without added FAD 6, 13, 14. The most appropriate erythrocyte glutathione reductase activity coefficient (EGRAC) thresholds for indicating normal or abnormal riboflavin status are uncertain 13. An EGRAC of 1.2 or less is usually used to indicate adequate riboflavin status, 1.2–1.4 to indicate marginal deficiency, and greater than 1.4 to indicate riboflavin deficiency 6, 13. However, a higher EGRAC does not necessarily correlate with degree of riboflavin deficiency. Furthermore, the EGRAC cannot be used in people with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is present in about 10% of African Americans 15.
Another widely used measure of riboflavin status is fluorometric measurement of urinary excretion over 24 hours (expressed as total amount of riboflavin excreted or in relation to the amount of creatinine excreted) 3. Because the body can store only small amounts of riboflavin, urinary excretion reflects dietary intake until tissues are saturated 13. Total riboflavin excretion in healthy, riboflavin-replete adults is at least 120 mcg/day; a rate of less than 40 mcg/day indicates deficiency 6, 13. This technique is less accurate for reflecting long-term riboflavin status than EGRAC 6, 13. Also, urinary excretion levels can decrease with age and increase with exposure to stress and certain drugs, and the amount excreted strongly reflects recent intake 6.
Riboflavin Function
Riboflavin or vitamin B2 is important for energy production, enzyme function, and normal fatty acid and amino acid synthesis. In addition to producing energy for the body, riboflavin works as an antioxidant and is necessary for the reproduction of glutathione, a free radical scavenger. Additionally, riboflavin or vitamin B2 is essential for normal development, growth, reproduction, lactation, physical performance, and well-being.
Living organisms derive most of their energy from redox reactions (oxidation-reduction reaction), which are reactions in which electrons are removed from one molecule or atom and transferred to another molecule or atom. In such a reaction one substance is oxidized (loses electrons) while the other is reduced (gains electrons) 9. Riboflavin or vitamin B2 is an essential component of two major coenzymes, flavin mononucleotide (FMN; also known as riboflavin-5’-phosphate) and flavin adenine dinucleotide (FAD). These coenzymes play major roles in energy production; cellular function, growth, and development; and metabolism of fats, drugs, and steroids 6, 3, 4. The conversion of the amino acid tryptophan to niacin (vitamin B3) requires FAD 4. Similarly, the conversion of vitamin B6 (Pyridoxine) to the coenzyme pyridoxal 5’-phosphate (the active form of vitamin B6) needs flavin mononucleotide (FMN). Riboflavin (as FAD or FMN) is also required for the metabolism of iron and in the synthesis of niacin from tryptophan 9. Riboflavin also plays an essential role in folate (vitamin B9) and related one-carbon metabolism, where FAD is required as a cofactor for methylenetetrahydrofolate reductase (MTHFR), a key folate-metabolizing enzyme 9. In addition, riboflavin helps maintain normal levels of homocysteine, an amino acid in the blood 6.
Antioxidant functions
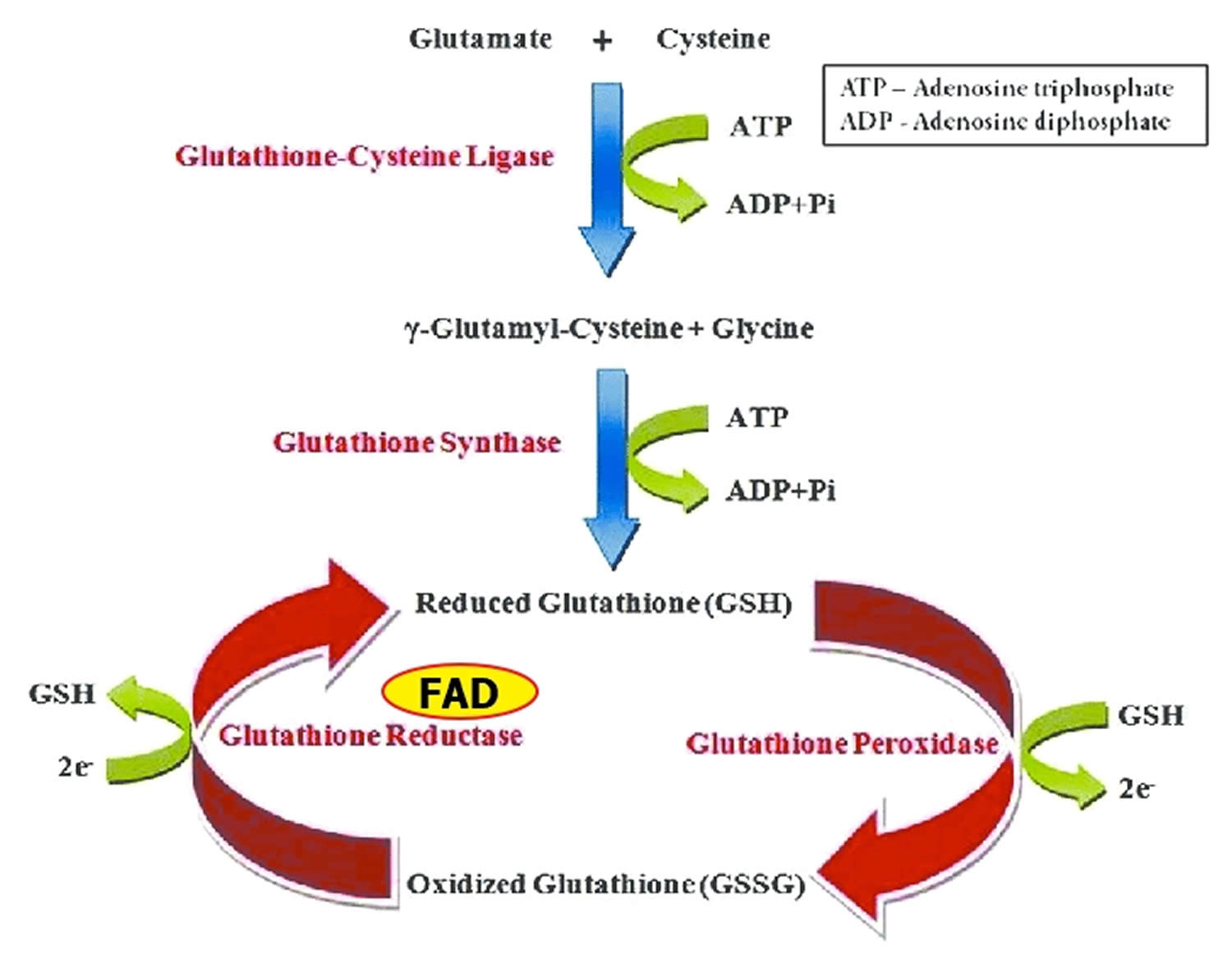
Glutathione reductase is a FAD-dependent enzyme that participates in the glutathione oxidation-reduction (redox) cycle (Figure 1) 9. The glutathione oxidation-reduction (redox) cycle plays a major role in protecting organisms from reactive oxygen species (ROS), such as hydroperoxides. Glutathione reductase requires FAD to regenerate two molecules of reduced glutathione from oxidized glutathione. Riboflavin deficiency has been associated with increased oxidative stress 16. Measurement of glutathione reductase activity in red blood cells is commonly used to assess riboflavin nutritional status 17.
Glutathione peroxidases are selenium-containing enzymes that require two molecules of reduced glutathione (GSH) to break down hydroperoxides. Glutathione peroxidase are involved in the glutathione oxidation-reduction (redox) cycle (Figure 1).
Xanthine oxidase, another FAD-dependent enzyme, catalyzes the oxidation of hypoxanthine and xanthine to uric acid. Uric acid is one of the most effective water-soluble antioxidants in the blood. Riboflavin deficiency can result in decreased xanthine oxidase activity, reducing blood uric acid levels 18.
Figure 1. Glutathione oxidation-reduction (redox) cycle
Footnotes: One molecule of hydrogen peroxide (H2O2) is reduced to two molecules of water (H2O), while two molecules of glutathione (GSH) are oxidized in a reaction catalyzed by the selenoenzymes, glutathione peroxidase. Oxidized glutathione (GSSG) may be reduced by the flavin adenine dinucleotide (FAD) dependent enzyme, glutathione reductase.
[Source 9 ]Metabolism of other vitamins
Flavoproteins are involved in the metabolism of several other vitamins: vitamin B6 (pyridoxine), niacin (vitamin B3), vitamin B12 (Cobalamin), and folate (vitamin B9) 9. Therefore, low and deficient riboflavin status can affect several enzyme systems. The conversion of vitamin B6 (pyridoxine) to its active coenzyme form in tissues, pyridoxal 5′-phosphate (PLP), requires the FMN-dependent enzyme, pyridoxine 5′-phosphate oxidase (PPO) 19. Human studies have provided evidence of the metabolic dependency of vitamin B6 (pyridoxine) on riboflavin status in older and younger adults 20, 21, 22. The synthesis of the niacin (vitamin B3)-containing coenzymes, NAD and NADP, from the amino acid tryptophan, requires the FAD-dependent enzyme, kynurenine 3-monooxygenase. Severe riboflavin deficiency can thus decrease the conversion of tryptophan to NAD and NADP, increasing the risk of niacin deficiency 8.
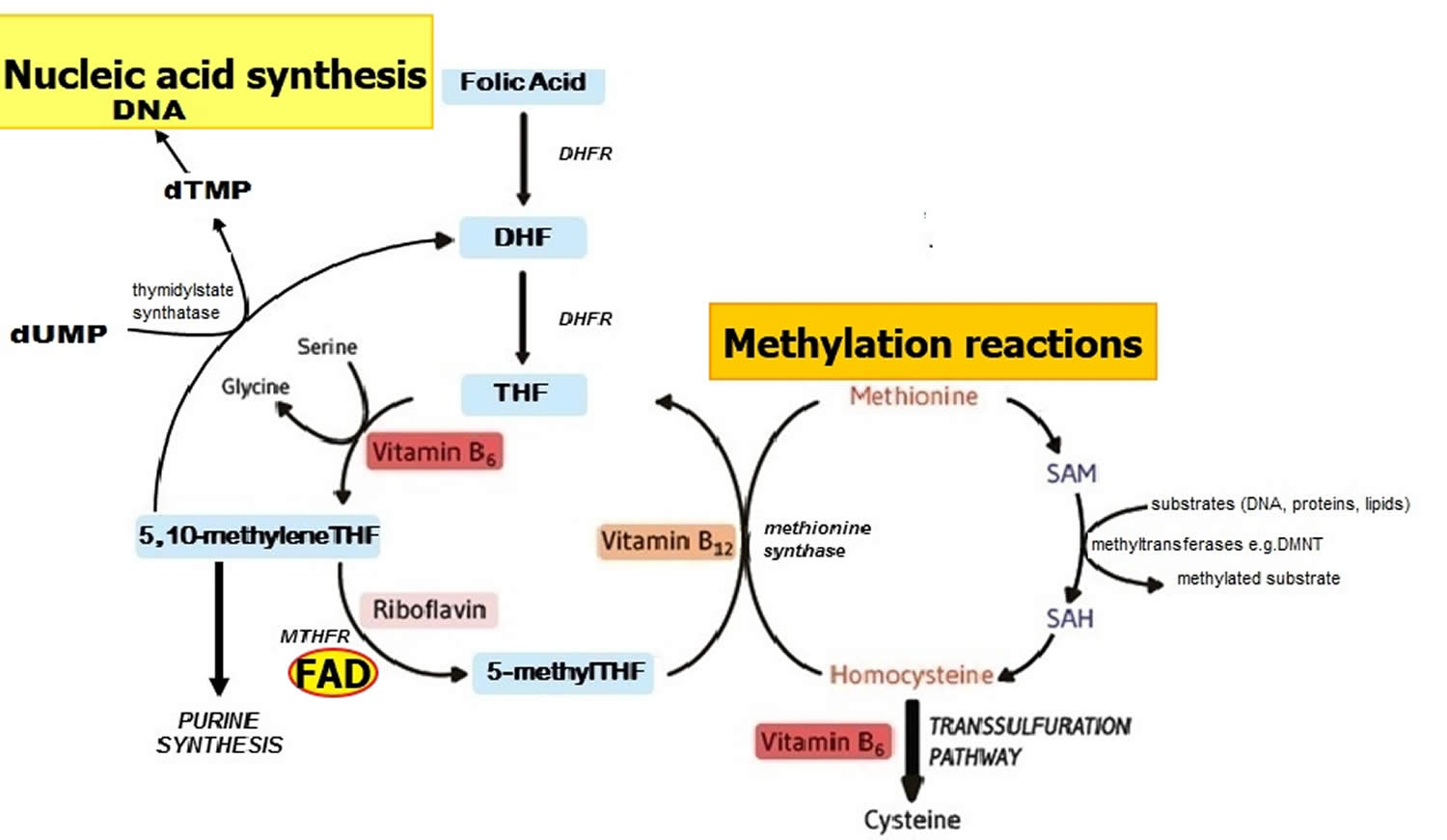
Methylenetetrahydrofolate reductase (MTHFR) is an FAD-dependent enzyme that plays a key role in one-carbon metabolism by catalyzing the reduction of 5,10 methyleneTHF to 5 methylTHF (Figure 2) 9. Once formed, 5 methylTHF is used by methionine synthase for the vitamin B12-dependent conversion of homocysteine to methionine and the formation of tetrahydrofolate (THF) (Figure 2). Both flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) are coenzymes for the enzyme methionine synthase reductase, which is responsible for the regeneration of methylcobalamin, the biologically active form of vitamin B12 acting as a coenzyme for methionine synthase 23. Along with other B vitamins (folate, vitamin B12, and vitamin B6), higher dietary riboflavin intakes have been associated with lower plasma concentrations of homocysteine 24. In individuals homozygous for the C677T polymorphism in the MTHFR gene, low riboflavin status is associated with elevated plasma homocysteine, and in turn linked with a higher risk of cardiovascular disease and other chronic diseases 25, 26. Furthermore, supplementation with riboflavin results in marked lowering of homocysteine concentrations specifically in individuals with the variant MTHFR 677TT genotype 27. Such results illustrate that chronic disease risk may be influenced by complex interactions between genetic and dietary factors 9.
Figure 2. Folate and nucleic acid synthesis
Footnote: Overview of folate and related B vitamins in nucleic acid synthesis.
Abbreviations: DHF = dihydrofolate; DHFR = dihydrofolate reductase; DMNT = DNA methyltransferase; dTMP = deoxythymidine monophosphate; dUMP = deoxyuridine monophosphate; MTHFR = methylenetetrahydrofolate reductase; SAH = S-adenosylhomocysteine; SAM = S-adenosylmethionine; THF = tetrahydrofolate.
[Source 9 ]Immune functions and responses
Riboflavin, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) play a key role in immune functions and responses as described recently by Suwannasom et al. 28. FMN and FAD are important cofactors for the human energy metabolism that is closely connected to the cellular immune responses. The immune response requires tremendous amounts of ATP since a large number of cells need to either be differentiated, proliferated or activated in order to perform their function. Both FMN and FAD are exceedingly involved in the production of ATP, as cofactors for crucial flavoenzymes in the oxidation of fatty acids and branched-chain amino acids, in the Krebs cycle and in the electron transport chain 11. Recently, riboflavin was shown to have an important role in macrophage function, and that riboflavin deficiency causes disruption in the activation of macrophages that ultimately leads to a decreasing recognition of pathogens and a failed activation of immune responses 29. Additionally, the key producers of ROS in the immune response, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), require binding of FAD. The production of ROS is crucial for destroying pathogen DNA, RNA and proteins, and ROS have an important role as cellular signaling molecules of immune cell function 30, 31.
Several studies have investigated the functions of riboflavin in the immune responses, and riboflavin has been reported to have anti-inflammatory effects by lowering several proinflammatory cytokines, namely TNF-α, IL-1ß and IL-6 32, 33. Moreover, riboflavin can reduce mortality in mice with septic shock, and it has been suggested that riboflavin treatment in septic shock could be potentially useful also in humans 32. Using riboflavin as a treatment in disease is not new, and in the field of inborn errors of metabolism such as multiple acyl-CoA dehydrogenation deficiency (MADD) and riboflavin transporter deficiencies, riboflavin treatment has been used extensively and shown extraordinary results leading to a significant clinical improvement or stabilization in the majority of patients 11.
Benefits of Riboflavin on Health
Scientists are studying riboflavin to better understand how it affects health. Here is an example of what this research has shown.
Migraine headaches
Migraine headaches typically produce intense pulsing or throbbing pain in one area of the head 34. These headaches are sometimes preceded or accompanied by aura (transient focal neurological symptoms before or during the headaches). Mitochondrial dysfunction is thought to play a causal role in some types of migraine 35. Because riboflavin is required for mitochondrial function, researchers are studying the potential use of riboflavin to prevent or treat migraine headaches 36.
Some, but not all, of the few small studies conducted to date have found evidence of a beneficial effect of riboflavin supplements on migraine headaches in adults and children 2. In a randomized trial in 55 adults with migraine, 400 mg/day riboflavin reduced the frequency of migraine attacks by two per month compared to placebo 37. In a retrospective study in 41 children (mean age 13 years) in Italy, 200 or 400 mg/day riboflavin for 3 to 6 months significantly reduced the frequency (from 21.7 ± 13.7 to 13.2 ± 11.8 migraine attacks over a 3-month period) and intensity of migraine headaches during treatment 38. The beneficial effects lasted throughout the 1.5-year follow-up period after treatment ended. However, two small randomized studies in children found that 50 to 200 mg/day riboflavin did not reduce the number of migraine headaches or headache severity compared to placebo 39, 40.
The Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society concluded that riboflavin is probably effective for preventing migraine headaches and recommended offering it for this purpose 41. The Canadian Headache Society recommends 400 mg/day riboflavin for migraine headache prevention, noting that although the evidence supporting this recommendation is of low quality, there is some evidence for benefit and side effects (such as discolored urine) are minimal 42.
Cataracts
Age-related cataracts are the leading cause of visual disability in the US and other developed countries. Research has focused on the role of nutritional antioxidants because of evidence that light-induced oxidative damage of lens proteins may lead to the development of age-related cataracts. A case-control study found significantly decreased risk of age-related cataracts (33% to 51%) in men and women in the highest quintile of dietary riboflavin intake (median of 1.6 to 2.2 mg/day) compared to those in the lowest quintile (median of 0.08 mg/day in both men and women) 43. Another case-control study reported that individuals in the highest quintile of riboflavin status, as measured by red blood cell glutathione reductase activity, had approximately one-half the occurrence of age-related cataract as those in the lowest quintile of riboflavin status, though the results were not statistically significant 44. A cross-sectional study of 2,900 Australian men and women, 49 years of age and older, found that those in the highest quintile of riboflavin intake were 50% less likely to have cataracts than those in the lowest quintile 45. A prospective cohort study of more than 50,000 women did not observe a difference between rates of cataract extraction between women in the highest quintile of riboflavin intake (median of 1.5 mg/day) and women in the lowest quintile (median of 1.2 mg/day) 46. However, the range between the highest and lowest quintiles was small, and median intake levels for both quintiles were above the RDA for riboflavin. A study in 408 women found that higher dietary intakes of riboflavin were inversely associated with a five-year change in lens opacification 47. A randomized controlled trial using a fractional factorial design showed that compared with placebo, the combined supplementation with riboflavin (3 mg/day) and niacin (40 mg/day) for five to six years reduced the prevalence of nuclear cataract but increased the progression of posterior subcapsular cataracts in population affected by multiple nutrient deficiency living in rural China 48. Of note is that the results of this trial are somewhat conflicting, and the study design does not allow the effects of riboflavin and niacin to be differentiated. In summary, there is some evidence predominantly from observational studies, that suggests higher riboflavin status might be beneficial; however, more evidence from well-designed, randomized controlled trials is needed to confirm a role for riboflavin in the prevention of cataracts.
Cancer prevention
Experts have theorized that riboflavin might help prevent the DNA damage caused by many carcinogens by acting as a coenzyme with several different cytochrome P450 enzymes 6. However, data on the relationship between riboflavin and cancer prevention or treatment are limited and study findings are mixed.
As mentioned above, riboflavin intake is a determinant of homocysteine concentration. This suggests that riboflavin status can influence methylenetetrahydrofolate reductase (MTHFR) activity and the metabolism of folate, thereby affecting cancer risk 49. In a randomized, double-blind, placebo-controlled study, 93 subjects with colorectal polyps and 86 healthy subjects were given a placebo, folic acid (400 or 1,200 mcg/day), or folic acid (400 mcg/day) plus riboflavin (5 mg/day) for 45 days. These interventions significantly improved folate and riboflavin status in vitamin-supplemented individuals compared to those taking the placebo 50. Interestingly, riboflavin enhanced the effect of 400 μg folic acid on circulating 5-methyltetrahydrofolate (5-MTHF) specifically in the polyp patients with the C677T genetic variant 50. This suggests that riboflavin may improve the response to folic acid supplementation in individuals with a reduced MTHFR activity. Additionally, a prospective cohort study of 88,045 postmenopausal women found total (dietary plus supplemental) intake of riboflavin to be inversely correlated with colorectal cancer risk when comparing the highest (>3.97 mg) and lowest (<1.80 mg) quartiles of daily intake 51; intake in the reference group was well above the RDA for riboflavin of 1.1 mg/day. The subjects in this study were not prescreened to identify those with the variant MTHFR 677TT genotype, and the association between this polymorphism and colorectal cancer remains unclear, with some reports suggesting a reduction in cancer risk with the T allele 52. Two meta-analyses have found inverse associations between riboflavin intake and risk of colorectal cancer 53, 54. The most recent of these was a dose-response meta-analysis that pooled results from five prospective cohort studies, nine case-control studies, and two studies reporting blood concentrations of riboflavin. This analysis found that higher intakes of riboflavin were associated with a significantly lower risk of colorectal cancer; inverse associations were observed for both dietary riboflavin intake and total daily intake from the diet and supplements 54.
Associations between riboflavin intake and cancer risk have also been evaluated in other types of cancer. A seven-year intervention study evaluated the use of riboflavin-fortified salt in 22,093 individuals at high risk for esophageal cancer in China 55. Riboflavin status and esophageal pathology (percent normal, dysplastic, and cancerous tissues) improved in the intervention group compared to the control group, but the lower incidence of esophageal cancer found in the intervention group was not statistically significant 55. Additionally, a 25-year follow up of an intervention trial in patients at high risk for gastric cancer found that dietary supplementation with riboflavin (3.2 mg/day) and niacin (40 mg/day) for five years decreased the risk of mortality from esophageal cancer by 8% but had no effect on mortality from gastric cancer 56.
A few large observational studies have produced conflicting results on the relationship between riboflavin intakes and lung cancer risk. A prospective study followed 41,514 men and women current, former, and never smokers in the Melbourne Collaborative Cohort Study for 15 years, on average 57, 58. The average riboflavin intake among all participants was 2.5 mg/day. The results showed a significant inverse association between dietary riboflavin intake and lung cancer risk in current smokers (fifth versus first quintile) but not former or never smokers 57 and breast cancer 58; no association of riboflavin intake with prostate cancer was observed in this cohort 59. However, another cohort study in 385,747 current, former, and never smokers who were followed for up to 12 years in the European Prospective Investigation into Cancer and Nutrition found no association between riboflavin intakes and colorectal cancer risk in any of the three groups 60.
A 2017 meta-analysis of 10 observational studies found an overall inverse association of riboflavin intake and breast cancer incidence and reported a 6% lower risk with each 1 mg/day increment of riboflavin intake 61. Moreover, the prospective Canadian National Breast Screening Study showed no association between dietary intakes or serum levels of riboflavin and lung cancer risk in 89,835 women aged 40-59 from the general population over 16.3 years, on average 62. Furthermore, studies to date have not found riboflavin intake or measures of riboflavin status to be associated with renal cell carcinoma, as reviewed in a recent meta-analysis 63.
Observational studies on the relationship between riboflavin intakes and colorectal cancer risk have not yielded conclusive results either. An analysis of data on 88,045 postmenopausal women in the Women’s Health Initiative Observational Study showed that total intakes of riboflavin from both foods and supplements were associated with a lower risk of colorectal cancer 51. A study that followed 2,349 individuals with cancer and 4,168 individuals without cancer participating in the Netherlands Cohort Study on Diet and Cancer for 13 years found no significant association between riboflavin and proximal colon cancer risk among women 64.
Future studies, including clinical trials, are needed to clarify the relationship between riboflavin intakes and various types of cancer and determine whether riboflavin supplements might reduce cancer risk.
Hypertension
Hypertension in adulthood is recognized as the leading risk factor contributing to mortality worldwide primarily from cardiovascular disease, while hypertension in pregnancy leads to serious adverse fetal and maternal outcomes. A number of risk factors are recognized to contribute to the development of hypertension. In recent years, evidence has emerged from genetic and clinical studies pointing to the role of one-carbon metabolism in blood pressure 65. The common methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, affecting 1 in 10 adults globally, is associated with higher blood pressure, although this is much less well recognized compared with the phenotype of elevated homocysteine concentrations that was established at the time of discovery of this polymorphism and its link with cardiovascular disease 66. Meta-analyses show that this polymorphism is associated with an increased risk of hypertension by up to 87% and of heart disease and stroke by up to 40% 67. The MTHFR C677T polymorphism is also associated with a significantly higher risk of hypertension in pregnancy 68 and with preeclampsia 69.
Since FAD is required as a cofactor for the methylenetetrahydrofolate reductase (MTHFR) enzyme and the MTHFR C677T polymorphism results in decreased MTHFR activity, studies have investigated whether affected individuals may benefit from riboflavin supplementation. In an initial randomized controlled trial in 77 healthy young adults stratified by MTHFR genotype, riboflavin supplementation at dietary levels (1.6 mg/day for 12 weeks) resulted in marked lowering of homocysteine concentrations in the MTHFR 677TT genotype group, but not in the 677CC or 677CT genotype groups who exhibited normal plasma homocysteine at baseline 27. Three randomized controlled trials subsequently investigated the effect of riboflavin on blood pressure in patients with hypertension with or without overt cardiovascular disease 66, 70, 71. The results of these trials showed that supplementation with low-dose riboflavin (1.6 mg/day for 16 weeks) resulted in significant lowering of blood pressure and reduction in incidence of hypertension specifically in those patients with the variant MTHFR 677TT genotype 66, 70, 71. Riboflavin intervention reduced mean systolic/diastolic blood pressure in those with the TT genotype from 144/87 to 131/80 mm Hg, with no response observed in those without the genetic variant (i.e., the CT or CC genotypes) 72. Notably, the 13 mm Hg decrease in systolic blood pressure occurred even though over 80% of the patients were taking one or more antihypertensive drugs at recruitment, and the addition of supplemental riboflavin was shown to greatly enhance the achievement of goal blood pressure with routine antihypertensive drugs 72, 66. Furthermore, the magnitude of blood pressure response achieved with riboflavin in these trials compares very favorably with typical decreases from other interventions, such as dietary salt reductions of 3 g/day (3.6/1.9 mm Hg) and 6 g/day (7.1/3.9 mm Hg). The trial findings therefore suggest that the excess risk of hypertension linked to this genetic polymorphism can be overcome by low-dose riboflavin supplementation. Also, analysis of plasma samples from individuals participating in these trials showed lower concentrations of S-adenosylmethionine (SAM), an important methyl group donor for methylation reactions, in those with the MTHFR 677TT genotype versus the CC genotype 73. However, riboflavin supplementation (1.6 mg/day) for 12 weeks was shown to increase plasma concentrations of SAM and another one-carbon metabolite, cystathionine 73, and thus may have potential in correcting the altered one-carbon metabolism arising with the variant TT genotype.
In summary, studies to date indicate that riboflavin supplementation may have benefits in lowering blood pressure and reducing hypertension in individuals (and sub-populations) affected by the common MTHFR C677T polymorphism. However, the mechanisms explaining the blood pressure phenotype and its responsiveness to riboflavin remain unclear. Future studies examining the effects of riboflavin supplementation on one-carbon metabolism may help to elucidate the biological mechanisms involved. Interestingly, a recent randomized controlled trial found that riboflavin supplementation in those with the variant MTHFR 677TT genotype resulted in altered DNA methylation of certain genes known to be involved in blood pressure regulation 74.
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of unknown cause that is characterized by the progressive destruction of myelin and nerve fibers in the central nervous system, causing neurological symptoms in affected individuals 75. Riboflavin appears to have a role in the formation of myelin 76, and oxidative stress has been implicated in the pathogenesis of multiple sclerosis (MS); thus, riboflavin may be helpful in treatment of the disease. A strong inverse association between dietary riboflavin intake and risk for MS was initially observed in a case-control study 77. In a mouse model of MS (i.e., experimental autoimmune encephalomyelitis), riboflavin supplementation improved clinical measures of the disease 78. However, a randomized, double-blind, placebo-controlled pilot study in 29 patients with MS found that supplementation with 10 mg/day of riboflavin for six months had no effect on MS-related disability, assessed by the Expanded Disability Status Scale 79. Large-scale randomized, placebo-controlled trials are needed to determine whether riboflavin supplementation has a beneficial effect in the treatment of MS.
How much riboflavin do I need?
The amount of riboflavin you need depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg) (Table 1).
Intake recommendations for riboflavin and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies 4. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA; intake at this level is assumed to ensure nutritional adequacy.
- Estimated Average Requirement (EAR): average daily level of intake estimated to meet the requirements of 50% of healthy individuals. It is usually used to assess the adequacy of nutrient intakes in populations but not individuals.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects.
Table 1 lists the current Recommended Dietary Allowance (RDA) for riboflavin or vitamin B2 4. For infants from birth to 12 months, the Food and Nutrition Board established an Adequate Intake (AI) for riboflavin that is equivalent to the mean intake of riboflavin in healthy, breastfed infants.
Table 1. Recommended Dietary Allowances (RDAs) for Riboflavin
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 0.3 mg |
| Infants 7–12 months* | 0.4 mg |
| Children 1–3 years | 0.5 mg |
| Children 4–8 years | 0.6 mg |
| Children 9–13 years | 0.9 mg |
| Teen boys 14–18 years | 1.3 mg |
| Teen girls 14–18 years | 1.0 mg |
| Men | 1.3 mg |
| Women | 1.1 mg |
| Pregnant teens and women | 1.4 mg |
| Breastfeeding teens and women | 1.6 mg |
Footnote: *Adequate Intake (AI) is the intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
[Source 4 ]What foods provide riboflavin?
Riboflavin or vitamin B2 is found naturally in some foods and is added to many fortified foods. Several food sources of riboflavin are listed in Table 2. You can get recommended amounts of riboflavin by eating a variety of foods, including the following 1:
- Eggs, organ meats (such as kidneys and liver), lean meats, and low-fat milk
- Some vegetables (such as mushrooms and spinach)
- Fortified cereals, bread, and grain products
Foods that are particularly rich in riboflavin include eggs, organ meats (kidneys and liver), lean meats, and milk 3, 10. Green vegetables also contain riboflavin. Grains and cereals are fortified with riboflavin in the United States and many other countries 10. The largest dietary contributors of total riboflavin intake in U.S. men and women are milk and milk drinks, bread and bread products, mixed foods whose main ingredient is meat, ready-to-eat cereals, and mixed foods whose main ingredient is grain 4. The riboflavin in most foods is in the form of flavin adenine dinucleotide (FAD), although the main form in eggs and milk is free riboflavin 80.
About 95% of riboflavin in the form of flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) from food is bioavailable up to a maximum of about 27 mg of riboflavin per meal or dose 4. The bioavailability of free riboflavin is similar to that of FAD and FMN 80, 81. Because riboflavin is soluble in water, about twice as much riboflavin content is lost in cooking water when foods are boiled as when they are prepared in other ways, such as by steaming or microwaving 82.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing riboflavin arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Riboflavin-Content.pdf) and food name (https://ods.od.nih.gov/pubs/usdandb/Riboflavin-Food.pdf).
Table 2. Selected Food Sources of Riboflavin
| Food | Milligrams (mg) per serving | Percent Daily Value (DV)* |
|---|---|---|
| Beef liver, pan fried, 3 ounces | 2.9 | 223 |
| Breakfast cereals, fortified with 100% of the DV for riboflavin, 1 serving | 1.3 | 100 |
| Oats, instant, fortified, cooked with water, 1 cup | 1.1 | 85 |
| Yogurt, plain, fat free, 1 cup | 0.6 | 46 |
| Milk, 2% fat, 1 cup | 0.5 | 38 |
| Beef, tenderloin steak, boneless, trimmed of fat, grilled, 3 ounces | 0.4 | 31 |
| Clams, mixed species, cooked, moist heat, 3 ounces | 0.4 | 31 |
| Almonds, dry roasted, 1 ounce | 0.3 | 23 |
| Cheese, Swiss, 3 ounces | 0.3 | 23 |
| Mushrooms, portabella, sliced, grilled, ½ cup | 0.2 | 15 |
| Rotisserie chicken, breast meat only, 3 ounces | 0.2 | 15 |
| Egg, whole, scrambled, 1 large | 0.2 | 15 |
| Quinoa, cooked, 1 cup | 0.2 | 15 |
| Bagel, plain, enriched, 1 medium (3½”–4” diameter) | 0.2 | 15 |
| Salmon, pink, canned, 3 ounces | 0.2 | 15 |
| Spinach, raw, 1 cup | 0.1 | 8 |
| Apple, with skin, 1 large | 0.1 | 8 |
| Kidney beans, canned, 1 cup | 0.1 | 8 |
| Macaroni, elbow shaped, whole wheat, cooked, 1 cup | 0.1 | 8 |
| Bread, whole wheat, 1 slice | 0.1 | 8 |
| Cod, Atlantic, cooked, dry heat, 3 ounces | 0.1 | 8 |
| Sunflower seeds, toasted, 1 ounce | 0.1 | 8 |
| Tomatoes, crushed, canned, ½ cup | 0.1 | 8 |
| Rice, white, enriched, long grain, cooked, ½ cup | 0.1 | 8 |
| Rice, brown, long grain, cooked, ½ cup | 0 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for riboflavin is 1.3 mg for adults and children aged 4 years and older 2. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 83]Riboflavin supplements
Riboflavin is available in many dietary supplements. Multivitamin/multimineral supplements with riboflavin commonly provide 1.3 mg riboflavin (100% of the DV) 84. Supplements containing riboflavin only or B-complex vitamins (that include riboflavin) are also available. In most supplements, riboflavin is in the free form, but some supplements have riboflavin 5’-phosphate.
To date, there are no reported complications associated with riboflavin supplementation, even when supplied in very high doses 85, 86.
Am I getting enough riboflavin?
Most people in the United States consume the recommended amounts of riboflavin 2. An analysis of data from the 2003-2006 National Health and Nutrition Examination Survey showed that less than 6% of the U.S. population has an intake of riboflavin from foods and supplements below the Estimated Average Requirement (EAR) 87. An analysis of self-reported data from the 1999–2004 National Health and Nutrition Examination Survey found that intakes of riboflavin were higher in lacto-ovo vegetarians (2.3 mg/day) than nonvegetarians (2.1 mg/day) 88.
Among children and teens, the average daily riboflavin intake from foods is 1.8 mg for ages 2–5 years, 1.9 mg for ages 6–11, and 2.1 mg for ages 12–19 89. In adults, the average daily riboflavin intake from foods is 2.5 mg in men and 1.8 mg in women. The average daily riboflavin intake from foods and supplements in children and teens is 2.1 mg for ages 2–5 years, 2.2 mg for ages 6–11, and 2.3 mg for ages 12–19. In adults aged 20 and older, the average daily riboflavin intake from foods and supplements is 4.5 mg in men and 4.7 mg in women.
Most people in America get enough riboflavin from the foods they eat and deficiencies are very rare. However, certain groups of people are more likely than others to have trouble getting enough riboflavin:
- Athletes who are vegetarians (especially strict vegetarians who avoid dairy foods and eggs)
- Pregnant women and breastfeeding women and their babies
- People who are vegan
- People who do not eat dairy foods
- People with a genetic disorder that causes riboflavin deficiency (such as infantile Brown-Vialetto-Van Laere syndrome)
What happens if I don’t get enough riboflavin?
You can develop riboflavin deficiency if you don’t get enough riboflavin in the foods you eat, or if you have certain diseases or hormone disorders.
Riboflavin deficiency can cause skin disorders, sores at the corners of your mouth, swollen and cracked lips, hair loss, sore throat, liver disorders, and problems with your reproductive and nervous systems.
Severe, long-term riboflavin deficiency causes a shortage of red blood cells (anemia), which makes you feel weak and tired. It also causes clouding of the lens in your eyes (cataracts), which affects your vision.
Riboflavin deficiency
Riboflavin deficiency, also known as ariboflavinosis, is extremely rare in the United States because of fortification of many foods, including grains and cereals 2. Fortification is the practice of deliberately increasing the content of one or more micronutrients (i.e., vitamins and minerals) in a food or condiment to improve the nutritional quality of the food supply and provide a public health benefit with minimal risk to health 90. Riboflavin deficiency usually occurs with other B vitamin deficiencies 91. Riboflavin deficiency can occur with a diet deficient in riboflavin-rich foods such as eggs, organ meats such as kidneys and liver, lean meats, low-fat milk, mushrooms, spinach, almonds, green leafy vegetables, legumes, fortified cereals, bread, and whole grain products 92. Additionally, glass milk containers promote degradation of riboflavin from exposure to light. Daily consumption of breakfast cereal and milk would be expected to provide an adequate intake of riboflavin 93.
Riboflavin deficiency is more commonly seen in persons with such risk factors as pregnancy 94, lactation, phototherapy for hyperbilirubinemia (in premature infants), advanced age 95, 96, low income, and/or depression 97, 98, 99, 100, 101
In addition to inadequate intake, causes of riboflavin deficiency can include endocrine abnormalities such as thyroid hormone insufficiency (hypothyroidism) and malabsorption conditions such as celiac disease, malignancies, and alcoholism 6.
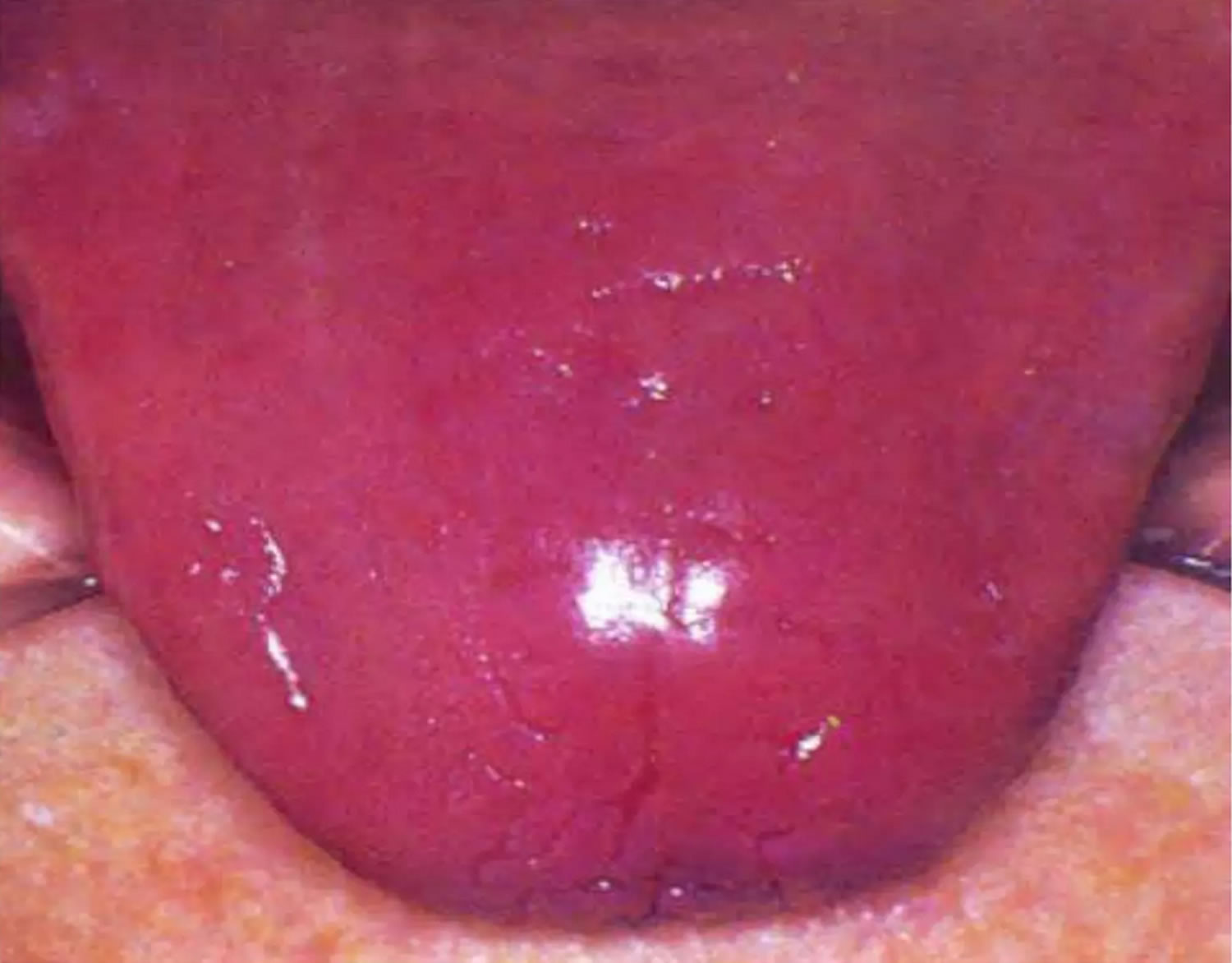
Clinical symptoms of riboflavin deficiency appear only after several months of insufficient riboflavin intake, and vary from milder symptoms as sore throat, hair loss, and scaly skin inflammation (seborrheic dermatitis), to severe symptoms as sore throat, redness and swelling of the lining of the mouth and throat, cracks or sores on the outsides of the lips (cheliosis) and at the corners of the mouth (angular stomatitis), inflammation and redness of the tongue (magenta tongue), hyperemia (excess blood), reproductive problems, itchy and red eyes (conjunctivitis), and degeneration of the liver and nervous system 102, 7, 15.
Other signs may involve the formation of blood vessels in the clear covering of the eye (vascularization of the cornea) and decreased red blood cell count in which the existing red blood cells contain normal levels of hemoglobin and are of normal size (normochromic-normocytic anemia) 8, 7.
The earlier changes associated with riboflavin deficiency are easily reversed. However, riboflavin supplements rarely reverse later anatomical changes such as formation of cataracts 6.
Subclinical riboflavin deficiency (low status of riboflavin) without clinical signs may be widespread, including in high-income countries, but usually goes undetected because riboflavin biomarkers are very rarely measured in human studies 9. Low or deficient riboflavin status may result in decreased conversion of vitamin B6 (Pyridoxine) to its active coenzyme form (pyridoxal 5’-phosphate) and decreased conversion of tryptophan to niacin (vitamin B3) 9.
People with riboflavin deficiency typically have deficiencies of other nutrients, so some of these signs and symptoms might reflect these other deficiencies 2. Severe riboflavin deficiency can impair the metabolism of other nutrients, especially other B vitamins, through diminished levels of flavin coenzymes 7. Anemia and cataracts can develop if riboflavin deficiency is severe and prolonged 6.
Although these symptoms are rarely seen in non-developing countries and well-nourished societies, dietary insufficiency and subclinical riboflavin deficiency is detected in remarkably large groups in the population 103, 104, 105, 106. Several population studies of vitamin status report on riboflavin insufficiency in children and young adults, especially in young women 107. A national survey performed in the United Kingdom, investigating the biochemical riboflavin status in 2127 schoolchildren, revealed a poor riboflavin status that increased with age. In boys, from 59% insufficient riboflavin intake in 4–6-year-olds, to 78% in 7–10-year-olds, but the largest group with riboflavin insufficiency were the 15–18-year-old girls 107. The survey revealed that 95% of the 15–18-year-old girls had an insufficient intake of riboflavin and an increasing risk of developing riboflavin deficiency 107. The increase of riboflavin insufficiency in both boys and girls is comparable to a declined consumption of milk, from 25% of the daily riboflavin intake in the 4–6-year-olds to only 10% of the daily riboflavin intake in the 15–18-year-olds 11. The implications for this riboflavin insufficiency, especially in young girls, are not fully known, but it has been shown that subclinical riboflavin deficiency could influence iron handling and that a daily supplement with riboflavin (2 or 4 mg) for 8 weeks significantly improves the hematologic status, with an increase in circulating red blood cells and hemoglobin concentrations in young women, even without an additional iron supplementation 105.
Most of the reported population studies performed on riboflavin status are older studies and the recent year’s changes in lifestyle, especially in well-nourished societies, with diets based on less dairy and meat products in combination with more exercise, could potentially increase the risk of riboflavin deficiency 11. In this context, studies on the dietary intake of riboflavin in well-nourished countries amongst people that follow a vegan diet without meat, dairy products and eggs, have shown that up to 48% have lower than the recommended daily intake of riboflavin, and thereby an increasing risk for developing riboflavin deficiency 108, 109, 110.
Preeclampsia is defined as the presence of elevated blood pressure, protein in the urine (proteinuria), and edema (significant swelling) during pregnancy. About 5% of women with preeclampsia progress to eclampsia, a significant cause of maternal and fetal death. Eclampsia is characterized by seizures, in addition to high blood pressure and increased risk of hemorrhage (severe bleeding) 111. A study in 154 pregnant women at increased risk of preeclampsia found that those who were riboflavin deficient were 4.7 times more likely to develop preeclampsia than those who had adequate riboflavin nutritional status 112. The cause of preeclampsia-eclampsia is not known 113. Decreased intracellular levels of flavocoenzymes could cause mitochondrial dysfunction, increase oxidative stress, and interfere with nitric oxide release and thus blood vessel dilation – all of these changes have been associated with preeclampsia 112.
A 2015 meta-analysis of 54 case-control studies found that the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism was associated with an increased risk of preeclampsia, especially in Caucasian and Asian populations 69. The reduction in the flavoprotein MTHFR activity observed in individuals with the variant MTHFR 677TT genotype leads to an increase in plasma homocysteine 26; higher homocysteine concentrations have been associated with preeclampsia 114. One small randomized controlled trial in 450 pregnant women in West Africa, without specified MTHFR genotype but at high risk for preeclampsia, found that supplementation with 15 mg of riboflavin daily was not effective in preventing the condition 115, but the study was likely underpowered to detect a significant effect. Further studies are needed to assess the potential benefit of riboflavin supplementation in reducing perinatal complications generally and specifically in preeclamptic women with the MTHFR 677TT genotype 9.
Riboflavin deficiency alters iron metabolism 9. Although the mechanism is not clear, research in animals suggests that riboflavin deficiency may impair iron absorption, increase intestinal loss of iron, and/or impair iron utilization for the synthesis of hemoglobin 116. In humans, low dietary intake of riboflavin has been associated with an increased risk for anemia 117, and improving riboflavin nutritional status has been found to increase circulating hemoglobin levels 118. Correction of riboflavin deficiency in individuals who are both riboflavin and iron deficient improves the response of iron-deficiency anemia to iron therapy 119. Anemia during pregnancy, a worldwide public health problem, is responsible for considerable perinatal morbidity and mortality 120, 121. The management of maternal anemia typically involves supplementation with iron alone or iron in combination with folic acid 122. It is possible that the inclusion of riboflavin could enhance the effects of iron-folic acid supplementation in treating maternal anemia, but the evidence is limited 9. There are, however, randomized, double-blind intervention trials conducted in pregnant women with anemia in Southeast Asia showing that a combination of folic acid, iron, vitamin A, and riboflavin (vitamin B2) improved hemoglobin levels and decreased anemia prevalence compared to iron-folic acid supplementation alone 123, 94.
Riboflavin deficiency causes
Primary riboflavin deficiency results from inadequate intake of the following:
- Fortified cereals
- Milk
- Other animal products
Secondary riboflavin deficiency is most commonly caused by the following:
- Chronic diarrhea
- Malabsorption syndromes
- Liver disorders
- Hemodialysis
- Peritoneal dialysis
- Long-term use of barbiturates
- Chronic alcoholism
- Endocrine abnormalities such as thyroid hormone insufficiency.
Most riboflavin or vitamin B2 is absorbed in the proximal small intestine by the human riboflavin transporter 1 (RFVT1) and riboflavin transporter 3 (RFVT3) 10. The rate of riboflavin absorption is proportional to intake, and it increases when riboflavin is ingested along with other foods and in the presence of bile salts. The body absorbs little riboflavin from single doses beyond 27 mg and stores only small amounts of riboflavin in the liver, heart, and kidneys 2. When excess amounts are consumed, they are either not absorbed or the small amount that is absorbed is excreted in urine 4.
Bacteria in the large intestine produce free riboflavin that can be absorbed by the large intestine in amounts that depend on your diet. More riboflavin is produced after ingestion of vegetable-based than meat-based foods 3. A small amount of riboflavin circulates via the enterohepatic system 10. Malabsorption from conditions such as celiac disease, malignancies, and alcoholism can promote deficiency of riboflavin.
A third riboflavin transporter (RFVT2) is expressed in the brain. Mutations in the riboflavin transporter genes SLC52A2 (coding for RFVT2) and SLC52A3 (coding for RFVT3) cause riboflavin transporter deficiency, a neurodegenerative disorder formerly known as Brown-Vialetto-Van Laere syndrome 124, 125. The only report of riboflavin deficiency caused by the RFVT1 transporter was in an infant of a mother with one mutation in the RFVT1 gene 126.
Alcoholics are at an increased risk of riboflavin deficiency, likely due to decreased dietary intake, decreased absorption, and/or impaired utilization of riboflavin 9. Interestingly, the elevated blood homocysteine concentrations associated with riboflavin deficiency rapidly decline during alcohol withdrawal 127. Additionally, people with anorexia rarely consume adequate dietary riboflavin, and those who are lactose intolerant are unlikely to meet requirements due to the avoidance of dairy products, the major dietary sources of riboflavin 9. The conversion of riboflavin into the active cofactor forms FAD and FMN is impaired in underactive thyroid (hypothyroidism) and adrenal insufficiency 8, 16. Furthermore, people who are very active physically (athletes, laborers) may have slightly increased riboflavin requirements. However, riboflavin supplementation has not generally been found to increase exercise tolerance or performance 128 unless the individuals are riboflavin deficient 129.
Groups at Risk of Riboflavin deficiency
The following groups are among those most likely to have riboflavin deficiency.
Vegetarian athletes
Athletes and people with high physical activity could be at risk of developing riboflavin deficiency. Exercise produces stress in the metabolic pathways that use riboflavin 130. Studies in healthy men with a moderate activity level and biochemical signs of riboflavin deficiency have shown that even short periods with increased physical activity deteriorate riboflavin levels further 131, 132. The deterioration in riboflavin is caused by the metabolic stress that occurs during periods of increased physical activity 131, 132. The Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine state that vegetarian athletes are at risk of riboflavin deficiency because of their increased need for this nutrient and because some vegetarians exclude all animal products (including milk, yogurt, cheese, and eggs), which tend to be good sources of riboflavin, from their diets 133. The Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine recommend that vegetarian athletes consult a sports dietitian to avoid this potential problem 133.
Pregnant and lactating women and their infants
Pregnant or lactating women who rarely consume meats or dairy products (such as those living in developing countries and some vegetarians in the United States) are at risk of riboflavin deficiency, which can have adverse effects on the health of both mothers and their infants 3.
In 1943, the first connections between women’s diet and their pregnancy were reported by Burke et al. 134. They observed that maternal nutritional status affected the infant’s condition at birth 134. Today, the connection between maternal nutritional status and normal fetus development and growth is well described, and it is known that many vitamins are of huge importance during pregnancy, including riboflavin. Riboflavin is essential for normal fetal development, and animal studies have shown that severe riboflavin deficiency in pregnant mice and chicken leads to abnormal fetal development and termination of pregnancy 135, 136.
In humans, most studies documenting riboflavin deficiency have been performed in societies with low riboflavin intake. The studies have shown that the risk of riboflavin deficiency in pregnant women is especially high during the third trimester, approaching parturition and during lactation. During pregnancy, the metabolic needs increase, with an average of 230 kcal per day, which for most women is more than 10% of their total daily kcal intake. In the first two trimesters the anabolism is dominating, there is an increased insulin sensitivity, and the maternal fat deposits increase 137. At the beginning of the third trimester, hormones from the placenta cause an increasing insulin resistance and catabolism in the mother, that enable nutrients for fetal growth, and an increasing need for energy rich nutrients, such as fatty acids and vitamins, including riboflavin, to ensure mitochondrial energy metabolism 138, 139. The increased need of nutrients and supporting vitamins, such as riboflavin, continues during parturition and postpartum. During parturition, riboflavin has an important antioxidant function, based on FAD being an essential cofactor for glutathione. Glutathione is crucial for counteracting the peroxidation reactions triggered by the rapid change from a hypoxic to a hyperoxic environment during birth 18. Riboflavin is important also postpartum. Studies have shown that light therapy, that is frequently used for treating hyperbilirubinemia in infants shortly after birth, can cause riboflavin deficiency and lowers riboflavin levels to 50% within hours 140. Moreover, maternal nutrient and riboflavin status is of great importance during breastfeeding. For infants that are exclusively breastfed, maternal milk is the only source of riboflavin. Riboflavin is mostly present as FAD in human milk and maternal riboflavin deficiency is rapidly reflected in low flavin concentration in the milk 141. The postpartum breastfeeding of the infant is of great importance to ensure nutrient needs, but also to develop the infant’s immune response.
Riboflavin deficiency during pregnancy, for example, can increase the risk of preeclampsia (a condition in pregnancy characterized by high blood pressure, sometimes with fluid retention and protein in the urine [proteinuria]) 112. The limited evidence on the benefits of riboflavin supplements during pregnancy in both developed and developing countries is mixed 142, 143, 123.
Riboflavin intakes during pregnancy have a positive association with infant birth weight and length 144. Infants of mothers with riboflavin deficiency or low dietary intakes (less than 1.2 mg/day) during pregnancy have a higher risk of riboflavin deficiency and of certain birth defects such as outflow tract defects of the heart 143, 145. However, maternal riboflavin intake has no association with the risk of orofacial clefts in infants 146.
In well-nourished women, riboflavin concentrations in breast milk range from 180 to 800 mcg/L and concentrations of riboflavin in breast milk increase over time 147, 148. In developing countries, in contrast, riboflavin levels in breast milk range from 160 to 220 mcg/L 148.
People who are vegan and/or consume little milk
In people who eat meat and dairy products, these foods contribute a substantial proportion of riboflavin in the diet. For this reason, people who live in developing countries and have limited intakes of meat and dairy products have an increased risk of riboflavin deficiency 149, 97. Vegans and those who consume little milk in developed countries are also at risk of riboflavin inadequacy 118, 150, 151, 152, 153.
Elderly population
Feneral riboflavin insufficiency has been described in the elderly population. Studies in the United Kingdom and the United States indicate that 10–41% of the elderly population have an insufficient riboflavin intake 154, 155 and are at risk of developing riboflavin deficiency, based on dietary reports 156. Riboflavin deficiency observed in the elderly population in these studies can partly be explained by a decreased intake of milk and other dairy products. However, the most plausible explanation is that the elderly population displays a reduced efficiency in the absorption of riboflavin that increases with aging 85, 157. In addition, riboflavin deficiency and deficiency of other B vitamins in the elderly have been linked to depression and changes in cognitive function, and it has been shown that riboflavin supplementation in elderly people could work as a neuroprotective agent and prevent disorders such as dementia, Parkinson’s disease and Alzheimer’s disease 158, 159, 160, 161, 162.
People with infantile Brown-Vialetto-Van Laere syndrome (riboflavin transporter deficiency)
Infantile Brown-Vialetto-Van Laere syndrome also known as riboflavin transporter deficiency is a very rare autosomal recessive neurological disorder that can begin at any age (usually in infancy or in childhood before age 8 years) and is associated with deafness, bulbar palsy (a motor-neuron disease), axial and appendicular weakness, sensory neuronopathy, gait ataxia, optic atrophy, facial weakness and respiratory difficulties 124, 125, 163. Riboflavin transporter deficiency disease is caused by mutations in the SLC52A3 or SLC52A2 genes, which encode riboflavin transporters 164, 163. As a result, these patients cannot properly absorb and transport riboflavin, so they develop riboflavin deficiency. Although no cure exists for riboflavin transporter deficiency, high-dose riboflavin supplementation can be a life-saving treatment in this population, especially when it is initiated soon after symptom onset 2. If untreated, it can be fatal 163.
Riboflavin deficiency symptoms
The Recommended Dietary Allowance (RDA) of riboflavin is 1.3 milligrams (mg) for men and 1.1 milligrams (mg) for women 2. Clinical signs of riboflavin deficiency in humans appear at intakes of less than 0.5 to 0.6 mg/day 9. People with riboflavin deficiency typically have deficiencies of other nutrients, so some of these signs and symptoms might reflect these other deficiencies. Severe riboflavin deficiency can impair the metabolism of other nutrients, especially other B vitamins, through diminished levels of flavin coenzymes 4. Anemia and cataracts can develop if riboflavin deficiency is severe and prolonged 6.
Symptoms of a severe riboflavin deficiency include:
- Anemia
- Mouth or lip sores
- Skin complaints
- Sore throat
- Swelling of mucous membranes
Signs and symptoms of riboflavin deficiency may also include the following 165:
- Red, itchy eyes
- Night blindness 166
- Cataracts
- Migraines
- Peripheral neuropathy
- Anemia (secondary to interference with iron absorption)
- Fatigue
- Cancer (esophageal and cervical dysplasia)
Riboflavin deficiency can be associated with developmental abnormalities, such as the following 165:
- Cleft lip and palate deformities
- Growth retardation in infants and children: Results from the National Birth Defects Prevention Study, which included an investigation of 324 infants with transverse limb deficiency, indicated that low maternal dietary intake of riboflavin is a risk factor for transverse limb deficiency 167
- Congenital heart defects: A study from the Netherlands indicated that a maternal diet that is high in saturated fats and low in riboflavin and nicotinamide may increase the risk for congenital heart defects 143
The signs and symptoms of riboflavin deficiency include skin disorders, hyperemia (excess blood) and edema of the mouth and throat, angular stomatitis (lesions at the corners of the mouth), cheilosis (swollen, cracked lips), hair loss, reproductive problems, sore throat, itchy and red eyes, and degeneration of the liver and nervous system 6, 3, 4, 102, 15.
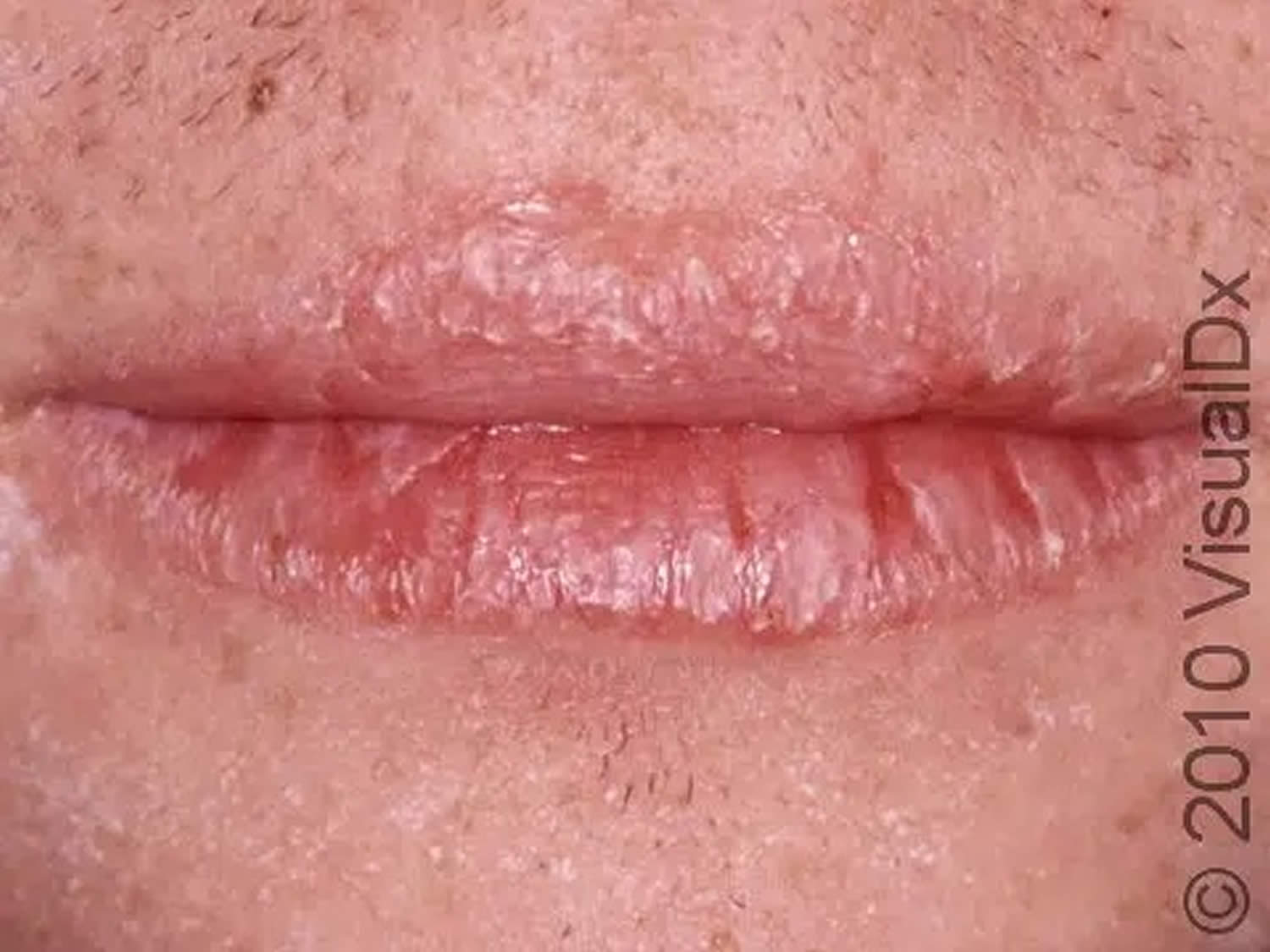
The most common signs of riboflavin deficiency are pallor and maceration of the mucosa at the angles of the mouth (angular stomatitis) and vermilion surfaces of the lips (cheilosis), eventually replaced by superficial linear fissures (see the image below). The fissures can become infected with Candida albicans, causing grayish white lesions (perlèche). A sore, red tongue that may appear magenta 102.
Seborrheic dermatitis develops, usually affecting the nasolabial folds, ears, eyelids, and scrotum or labia majora 163. These areas become red, scaly, and greasy.
Rarely, neovascularization and keratitis of the cornea occur, causing lacrimation and photophobia.
The earlier changes associated with riboflavin deficiency are easily reversed. However, riboflavin supplements rarely reverse later anatomical changes such as formation of cataracts 6.
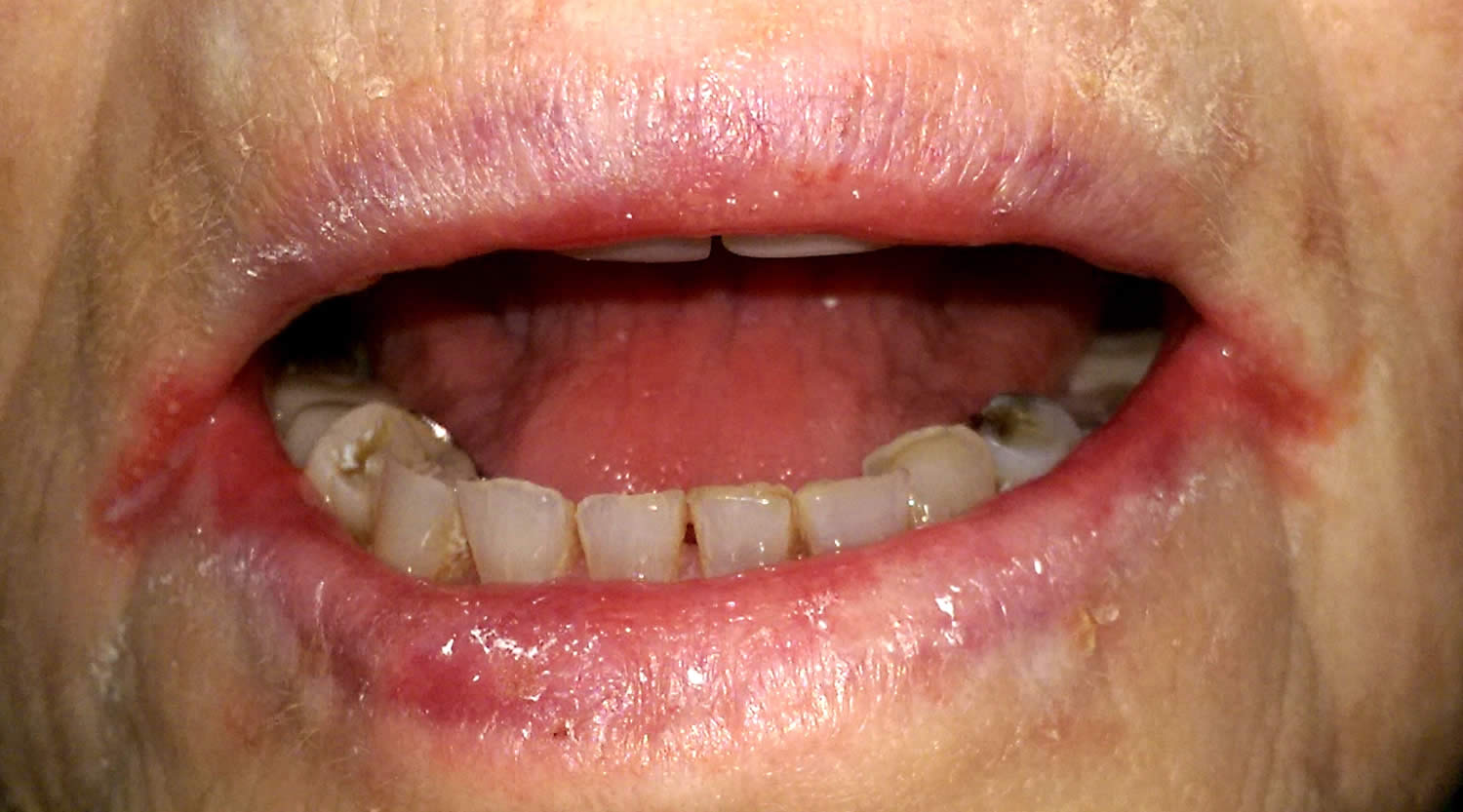
Figure 3. Riboflavin deficiency cheilitis (chapping and fissuring of the lips)
Footnote: Chapped lips (cheilitis) are lips that appear dry, scaly, and may have one or more small cracks (fissures). Often, the lips are sensitive, and there may or may not be redness (erythema) and swelling (edema) present.
Figure 4. Riboflavin deficiency angular cheilitis (inflammation of one or both corners of the mouth)
Figure 5. Riboflavin deficiency magenta tongue
Riboflavin deficiency diagnosis
Riboflavin deficiency diagnosis is usually clinical.
Measurement of red blood cell glutathione reductase activity may help in the detection of riboflavin deficiency 14. An increase in the stimulation of this enzymatic reaction confirms a low level of riboflavin.
Riboflavin can cause false elevations of urinary catecholamines and false-positive urine urobilinogen reactions (Ehrlich test).
The diagnosis of riboflavin transporter deficiency is based on clinical, neurophysiologic, neuroimaging, and laboratory findings as well as the identification of pathogenic variants in either SLC52A2 or SLC52A3 on molecular genetic testing 164.
Riboflavin deficiency treatment
Treatment of riboflavin deficiency consists of riboflavin replenishment, with care taken not to overlook coexisting B-complex deficiencies 168. Treatment consists of oral or, if needed, intramuscular (IM) riboflavin. Riboflavin 5 to 10 mg orally once/day is given until recovery. Other water-soluble vitamins should also be given. Multivitamins have no documented role, because the physician must establish the presence of individual vitamin deficiencies and correct them appropriately 168. This prevents toxicities and masking of the clinical picture 169, 126.
Except in malabsorption syndromes, riboflavin is readily absorbed from the upper gastrointestinal tract. The extent of gastrointestinal absorption is increased when riboflavin is administered with food and is decreased in patients with hepatitis, cirrhosis, and biliary obstruction 168.
Riboflavin is a water-soluble vitamin, is considered nontoxic, and has no known adverse effects. Riboflavin should be taken with food, because only about 15% is absorbed when taken alone on an empty stomach; excess riboflavin is excreted in urine, giving the urine a fluorescent yellow-green tint 170.
Dosages of riboflavin for deficiency treatment are as follows 168:
- Age < 3 years: not established
- Age 3-12 years: 3-10 mg oral divided daily
- Age >12 years: Administer as in adults (see below)
- Adult dose: 6-30 mg oral divided daily for replacement when deficiency is suspected
The biologic half-life of riboflavin is about 66-84 minutes following oral or intramuscular administration of a single large dose in healthy individuals. Only about 9% of the riboflavin is excreted unchanged. Excretion appears to involve renal tubular secretion as well as glomerular filtration. Amounts in excess of the body’s needs are excreted in urine.
As a photosynthesizing agent, riboflavin is destroyed by light. A combination of light, oxygen, and riboflavin can lead to formation of free radicals and, consequently, cataracts; patients with cataracts are advised to take no more than 10 mg of riboflavin daily 168.
Females who have riboflavin transporter deficiency (Brown-Vialetto-Van Laere syndrome) or are carriers of a pathogenic variant in SLC52A2 or SLC52A3 should have riboflavin supplements before and during pregnancy and when breast feeding to avoid inducing riboflavin deficiency in the baby 164.
For patients with riboflavin transporter deficiency (Brown-Vialetto-Van Laere syndrome), high-dose oral supplementation of riboflavin between 10 mg and 50 mg/kg/day improves symptoms, objective testing (vital capacity, brain stem evoked potentials, nerve conduction studies), and normalizes acylcarnitine levels. The optimal dose is as yet unknown. Although some patients show improvement within days of riboflavin supplementation, others with more severe symptoms have a more gradual recovery over months. Because oral riboflavin supplementation has been shown to decrease mortality, it should begin as soon as a riboflavin transporter deficiency (Brown-Vialetto-Van Laere syndrome) is suspected and be continued even in the absence of initial treatment response 171.
Riboflavin treatment has been used extensively in the field of inborn errors of metabolism. It is an established therapy in multiple acyl-CoA dehydrogenation deficiency (MADD) and riboflavin transporter deficiencies, with significant clinical improvement or stabilization in a majority of patients 11. Numerous inborn errors of flavin metabolism and flavoenzyme function have been described, and supplementation with riboflavin has in many cases been shown to be lifesaving or to mitigate symptoms 11.
For patients with riboflavin transporter deficiency (Brown-Vialetto-Van Laere syndrome), supportive care includes the following 164:
- Respiratory support
- Physiotherapy to avoid contractures
- Occupational therapy to support activities of daily living
- Orthotics for limb and trunk bracing
- Speech and language therapy to avoid choking and respiratory problems
- Wheel chair as needed
- Low vision aids as needed
- Routine management of scoliosis to avoid long-term respiratory problems
- Routine management of depression
At 3 months and 6 months after initiation of riboflavin supplementation, follow-up physical and neurologic examinations, and measurement of blood riboflavin/FAD/FMN and acylcarnitine analysis should be conducted 164. Thereafter, follow up should be biannual in older individuals and more frequent in younger children 164.
Health Risks from Excessive Riboflavin
Intakes of riboflavin from food that are many times the RDA have no observable toxicity, possibly because riboflavin’s solubility and capacity to be absorbed in the gastrointestinal tract are limited 6, 4. Because adverse effects from high riboflavin intakes from foods or supplements (400 mg/day for at least 3 months) have not been reported, the Food and Nutrition Board did not establish Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) for riboflavin 4. The limited data available on riboflavin’s adverse effects do not mean, however, that high intakes have no adverse effects, and the Food and Nutrition Board urges people to be cautious about consuming excessive amounts of riboflavin 4.
- Riboflavin. https://ods.od.nih.gov/factsheets/Riboflavin-Consumer[↩][↩]
- Riboflavin. https://ods.od.nih.gov/factsheets/Riboflavin-HealthProfessional[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Said HM, Ross AC. Riboflavin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:325-30.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline 1998. https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Brody T. Nutritional Biochemistry. 2nd ed. San Diego: Academic Press; 1999.[↩]
- Rivlin RS. Riboflavin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:691-9.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. https://nap.nationalacademies.org/read/6015/chapter/1[↩][↩][↩][↩][↩]
- McCormick D. Riboflavin. In: Shils M, Olson J, Shike M, Ross A, eds. Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:391-399.[↩][↩][↩][↩]
- Riboflavin. https://lpi.oregonstate.edu/mic/vitamins/riboflavin[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- McCormick DB. Riboflavin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:280-92.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int J Mol Sci. 2020 May 28;21(11):3847. doi: 10.3390/ijms21113847[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Gaylord AM, Warthesen JJ, Smith DE. Influence of milk fat, milk solids, and light intensity on the light stability of vitamin A and riboflavin in lowfat milk. J Dairy Sci. 1986 Nov;69(11):2779-84. doi: 10.3168/jds.S0022-0302(86)80729-9[↩]
- Gibson RS. Assessment of the Status of Thiamin, Riboflavin, and Niacin. In: Principles of Nutritional Assessment. 2nd ed. New York: Oxford University Press; 2005:545-68.[↩][↩][↩][↩][↩][↩]
- Hoey L, McNulty H, Strain JJ. Studies of biomarker responses to intervention with riboflavin: a systematic review. Am J Clin Nutr. 2009 Jun;89(6):1960S-1980S. doi: 10.3945/ajcn.2009.27230B[↩][↩]
- Donald B McCormick, Vitamin/mineral supplements: of questionable benefit for the general population, Nutrition Reviews, Volume 68, Issue 4, 1 April 2010, Pages 207–213, https://doi.org/10.1111/j.1753-4887.2010.00279.x[↩][↩][↩]
- Powers HJ. Current knowledge concerning optimum nutritional status of riboflavin, niacin and pyridoxine. Proc Nutr Soc. 1999 May;58(2):435-40. doi: 10.1017/s0029665199000579[↩][↩]
- Rivlin R. Riboflavin. In: Ziegler E, Filer L, eds. Present Knowledge in Nutrition. 7th ed. Washington D.C.: ILSI Press; 1996:167-173.[↩]
- Böhles H. Antioxidative vitamins in prematurely and maturely born infants. Int J Vitam Nutr Res. 1997;67(5):321-8.[↩][↩]
- McCormick DB. Two interconnected B vitamins: riboflavin and pyridoxine. Physiol Rev. 1989 Oct;69(4):1170-98. doi: 10.1152/physrev.1989.69.4.1170[↩]
- Madigan SM, Tracey F, McNulty H, Eaton-Evans J, Coulter J, McCartney H, Strain JJ. Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. Am J Clin Nutr. 1998 Aug;68(2):389-95. doi: 10.1093/ajcn/68.2.389[↩]
- Löwik MR, van den Berg H, Kistemaker C, Brants HA, Brussaard JH. Interrelationships between riboflavin and vitamin B6 among elderly people (Dutch Nutrition Surveillance System). Int J Vitam Nutr Res. 1994;64(3):198-203.[↩]
- Jungert A, McNulty H, Hoey L, Ward M, Strain JJ, Hughes CF, McAnena L, Neuhäuser-Berthold M, Pentieva K. Riboflavin Is an Important Determinant of Vitamin B-6 Status in Healthy Adults. J Nutr. 2020 Oct 12;150(10):2699-2706. doi: 10.1093/jn/nxaa225[↩]
- Wolthers, K.R. and Scrutton, N.S. (2009), Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase–methionine synthase reductase complex. The FEBS Journal, 276: 1942-1951. https://doi.org/10.1111/j.1742-4658.2009.06919.x[↩]
- Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001 Mar;73(3):613-21. doi: 10.1093/ajcn/73.3.613[↩]
- Jacques PF, Kalmbach R, Bagley PJ, Russo GT, Rogers G, Wilson PW, Rosenberg IH, Selhub J. The relationship between riboflavin and plasma total homocysteine in the Framingham Offspring cohort is influenced by folate status and the C677T transition in the methylenetetrahydrofolate reductase gene. J Nutr. 2002 Feb;132(2):283-8. doi: 10.1093/jn/132.2.283[↩]
- McNulty H, McKinley MC, Wilson B, McPartlin J, Strain JJ, Weir DG, Scott JM. Impaired functioning of thermolabile methylenetetrahydrofolate reductase is dependent on riboflavin status: implications for riboflavin requirements. Am J Clin Nutr. 2002 Aug;76(2):436-41. doi: 10.1093/ajcn/76.2.436[↩][↩]
- McNulty H, Dowey le RC, Strain JJ, Dunne A, Ward M, Molloy AM, McAnena LB, Hughes JP, Hannon-Fletcher M, Scott JM. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C->T polymorphism. Circulation. 2006 Jan 3;113(1):74-80. doi: 10.1161/CIRCULATIONAHA.105.580332[↩][↩]
- Suwannasom N., Kao I., Pruss A., Georgieva R., Baumler H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020;21:950. doi: 10.3390/ijms21030950[↩]
- Mazur-Bialy AI, Pochec E, Plytycz B. Immunomodulatory effect of riboflavin deficiency and enrichment – reversible pathological response versus silencing of inflammatory activation. J Physiol Pharmacol. 2015 Dec;66(6):793-802. https://www.jpp.krakow.pl/journal/archive/12_15/pdf/793_12_15_article.pdf[↩]
- Angajala A., Lim S., Phillips J.B., Kim J.-H., Yates C., You Z., Tan M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front. Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605[↩]
- Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89[↩]
- Toyosawa T., Suzuki M., Kodama K., Araki S. Effects of intravenous infusion of highly purified vitamin B2 on lipopolysaccharide-induced shock and bacterial infection in mice. Eur. J. Pharmacol. 2004;492:273–280. doi: 10.1016/j.ejphar.2004.04.004[↩][↩]
- Toyosawa T., Suzuki M., Kodama K., Araki S. Potentiation by amino acid of the therapeutic effect of highly purified vitamin B2 in mice with lipopolysaccharide-induced shock. Eur. J. Pharmacol. 2004;493:177–182. doi: 10.1016/j.ejphar.2004.04.019[↩]
- Migraine. https://www.ninds.nih.gov/health-information/disorders/migraine[↩]
- Yorns WR Jr, Hardison HH. Mitochondrial dysfunction in migraine. Semin Pediatr Neurol. 2013 Sep;20(3):188-93. doi: 10.1016/j.spen.2013.09.002[↩]
- Di Lorenzo C, Pierelli F, Coppola G, Grieco GS, Rengo C, Ciccolella M, Magis D, Bolla M, Casali C, Santorelli FM, Schoenen J. Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology. 2009 May 5;72(18):1588-94. doi: 10.1212/WNL.0b013e3181a41269[↩]
- Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998 Feb;50(2):466-70. doi: 10.1212/wnl.50.2.466[↩]
- Condò M, Posar A, Arbizzani A, Parmeggiani A. Riboflavin prophylaxis in pediatric and adolescent migraine. J Headache Pain. 2009 Oct;10(5):361-5. doi: 10.1007/s10194-009-0142-2[↩]
- Bruijn J, Duivenvoorden H, Passchier J, Locher H, Dijkstra N, Arts WF. Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia. 2010 Dec;30(12):1426-34. doi: 10.1177/0333102410365106[↩]
- MacLennan SC, Wade FM, Forrest KM, Ratanayake PD, Fagan E, Antony J. High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. J Child Neurol. 2008 Nov;23(11):1300-4. doi: 10.1177/0883073808318053[↩]
- Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012 Apr 24;78(17):1346-53. doi: 10.1212/WNL.0b013e3182535d0c[↩]
- Canadian Headache Society Guideline for Migraine Prophylaxis: Supplement 2. (2012). Canadian Journal of Neurological Sciences, 39(S2), I-63. doi:10.1017/S0317167100015109[↩]
- Julie A. Mares-Perlman and others, Diet and Nuclear Lens Opacities, American Journal of Epidemiology, Volume 141, Issue 4, 15 February 1995, Pages 322–334, https://doi.org/10.1093/aje/141.4.322[↩]
- Leske MC, Wu SY, Hyman L, Sperduto R, Underwood B, Chylack LT, Milton RC, Srivastava S, Ansari N. Biochemical factors in the lens opacities. Case-control study. The Lens Opacities Case-Control Study Group. Arch Ophthalmol. 1995 Sep;113(9):1113-9. doi: 10.1001/archopht.1995.01100090039020[↩]
- Cumming RG, Mitchell P, Smith W. Diet and cataract: the Blue Mountains Eye Study. Ophthalmology. 2000 Mar;107(3):450-6. doi: 10.1016/s0161-6420(99)00024-x[↩]
- Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, Willett WC. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992 Aug 8;305(6849):335-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1882980/pdf/bmj00086-0017.pdf[↩]
- Jacques PF, Taylor A, Moeller S, Hankinson SE, Rogers G, Tung W, Ludovico J, Willett WC, Chylack LT Jr. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Ophthalmol. 2005 Apr;123(4):517-26. doi: 10.1001/archopht.123.4.517[↩]
- Sperduto RD, Hu TS, Milton RC, Zhao JL, Everett DF, Cheng QF, Blot WJ, Bing L, Taylor PR, Li JY, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993 Sep;111(9):1246-53. doi: 10.1001/archopht.1993.01090090098027[↩]
- Wen YY, Yang SJ, Zhang JX, Chen XY. Methylenetetrahydrofolate reductase genetic polymorphisms and esophageal squamous cell carcinoma susceptibility: a meta-analysis of case-control studies. Asian Pac J Cancer Prev. 2013;14(1):21-5. doi: 10.7314/apjcp.2013.14.1.21[↩]
- Powers HJ, Hill MH, Welfare M, Spiers A, Bal W, Russell J, Duckworth Y, Gibney E, Williams EA, Mathers JC. Responses of biomarkers of folate and riboflavin status to folate and riboflavin supplementation in healthy and colorectal polyp patients (the FAB2 Study). Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):2128-35. doi: 10.1158/1055-9965.EPI-07-0208[↩][↩]
- Zschäbitz S, Cheng TY, Neuhouser ML, Zheng Y, Ray RM, Miller JW, Song X, Maneval DR, Beresford SA, Lane D, Shikany JM, Ulrich CM. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013 Feb;97(2):332-43. doi: 10.3945/ajcn.112.034736[↩][↩]
- Kennedy DA, Stern SJ, Matok I, Moretti ME, Sarkar M, Adams-Webber T, Koren G. Folate Intake, MTHFR Polymorphisms, and the Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. J Cancer Epidemiol. 2012;2012:952508. doi: 10.1155/2012/952508[↩]
- Liu Y, Yu QY, Zhu ZL, Tang PY, Li K. Vitamin B2 intake and the risk of colorectal cancer: a meta-analysis of observational studies. Asian Pac J Cancer Prev. 2015;16(3):909-13. doi: 10.7314/apjcp.2015.16.3.909[↩]
- Ben S, Du M, Ma G, Qu J, Zhu L, Chu H, Zhang Z, Wu Y, Gu D, Wang M. Vitamin B2 intake reduces the risk for colorectal cancer: a dose-response analysis. Eur J Nutr. 2019 Jun;58(4):1591-1602. doi: 10.1007/s00394-018-1702-5[↩][↩]
- He Y, Ye L, Shan B, Song G, Meng F, Wang S. Effect of riboflavin-fortified salt nutrition intervention on esophageal squamous cell carcinoma in a high incidence area, China. Asian Pac J Cancer Prev. 2009 Oct-Dec;10(4):619-22. http://journal.waocp.org/article_24976_7c27c46f4eaabcb1326f82b42d795002.pdf[↩][↩]
- Wang SM, Taylor PR, Fan JH, Pfeiffer RM, Gail MH, Liang H, Murphy GA, Dawsey SM, Qiao YL, Abnet CC. Effects of Nutrition Intervention on Total and Cancer Mortality: 25-Year Post-trial Follow-up of the 5.25-Year Linxian Nutrition Intervention Trial. J Natl Cancer Inst. 2018 Nov 1;110(11):1229-1238. doi: 10.1093/jnci/djy043[↩]
- Bassett JK, Hodge AM, English DR, Baglietto L, Hopper JL, Giles GG, Severi G. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012 Feb;66(2):182-7. doi: 10.1038/ejcn.2011.157[↩][↩]
- Bassett JK, Baglietto L, Hodge AM, Severi G, Hopper JL, English DR, Giles GG. Dietary intake of B vitamins and methionine and breast cancer risk. Cancer Causes Control. 2013 Aug;24(8):1555-63. doi: 10.1007/s10552-013-0232-y[↩][↩]
- Bassett JK, Severi G, Hodge AM, Baglietto L, Hopper JL, English DR, Giles GG. Dietary intake of B vitamins and methionine and prostate cancer incidence and mortality. Cancer Causes Control. 2012 Jun;23(6):855-63. doi: 10.1007/s10552-012-9954-5[↩]
- Johansson M, Relton C, Ueland PM, Vollset SE, Midttun Ø, Nygård O, Slimani N, Boffetta P, Jenab M, Clavel-Chapelon F, Boutron-Ruault MC, Fagherazzi G, Kaaks R, Rohrmann S, Boeing H, Weikert C, Bueno-de-Mesquita HB, Ros MM, van Gils CH, Peeters PH, Agudo A, Barricarte A, Navarro C, Rodríguez L, Sánchez MJ, Larrañaga N, Khaw KT, Wareham N, Allen NE, Crowe F, Gallo V, Norat T, Krogh V, Masala G, Panico S, Sacerdote C, Tumino R, Trichopoulou A, Lagiou P, Trichopoulos D, Rasmuson T, Hallmans G, Riboli E, Vineis P, Brennan P. Serum B vitamin levels and risk of lung cancer. JAMA. 2010 Jun 16;303(23):2377-85. doi: 10.1001/jama.2010.808[↩]
- Yu L, Tan Y, Zhu L. Dietary vitamin B2 intake and breast cancer risk: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017 Mar;295(3):721-729. doi: 10.1007/s00404-016-4278-4[↩]
- Kabat GC, Miller AB, Jain M, Rohan TE. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008 Sep 2;99(5):816-21. doi: 10.1038/sj.bjc.6604540[↩]
- Clasen JL, Heath AK, Scelo G, Muller DC. Components of one-carbon metabolism and renal cell carcinoma: a systematic review and meta-analysis. Eur J Nutr. 2020 Dec;59(8):3801-3813. doi: 10.1007/s00394-020-02211-6[↩]
- de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008 Dec;138(12):2372-8. doi: 10.3945/jn.108.091157[↩]
- McNulty H, Strain JJ, Hughes CF, Ward M. Riboflavin, MTHFR genotype and blood pressure: A personalized approach to prevention and treatment of hypertension. Mol Aspects Med. 2017 Feb;53:2-9. doi: 10.1016/j.mam.2016.10.002[↩]
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 May;10(1):111-3. doi: 10.1038/ng0595-111[↩][↩][↩][↩]
- McNulty H, Strain JJ, Hughes CF, Pentieva K, Ward M. Evidence of a Role for One-Carbon Metabolism in Blood Pressure: Can B Vitamin Intervention Address the Genetic Risk of Hypertension Owing to a Common Folate Polymorphism? Curr Dev Nutr. 2019 Sep 16;4(1):nzz102. doi: 10.1093/cdn/nzz102[↩]
- Yang B, Fan S, Zhi X, Li Y, Liu Y, Wang D, He M, Hou Y, Zheng Q, Sun G. Associations of MTHFR gene polymorphisms with hypertension and hypertension in pregnancy: a meta-analysis from 114 studies with 15411 cases and 21970 controls. PLoS One. 2014 Feb 5;9(2):e87497. doi: 10.1371/journal.pone.0087497[↩]
- Wu X, Yang K, Tang X, Sa Y, Zhou R, Liu J, Luo Y, Tang W. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: a meta-analysis. J Assist Reprod Genet. 2015 May;32(5):797-805. doi: 10.1007/s10815-014-0408-8[↩][↩]
- Horigan G, McNulty H, Ward M, Strain JJ, Purvis J, Scott JM. Riboflavin lowers blood pressure in cardiovascular disease patients homozygous for the 677C–>T polymorphism in MTHFR. J Hypertens. 2010 Mar;28(3):478-86. doi: 10.1097/HJH.0b013e328334c126[↩][↩]
- Wilson CP, Ward M, McNulty H, Strain JJ, Trouton TG, Horigan G, Purvis J, Scott JM. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: a 4-y follow-up. Am J Clin Nutr. 2012 Mar;95(3):766-72. doi: 10.3945/ajcn.111.026245[↩][↩]
- Scimone, C, Alibrandi, S, Donato, L, et al. Antiretroviral treatment leading to secondary trimethylaminuria: Genetic associations and successful management with riboflavin. J Clin Pharm Ther. 2021; 46: 304– 309. https://doi.org/10.1111/jcpt.13315[↩][↩]
- Rooney M, Bottiglieri T, Wasek-Patterson B, McMahon A, Hughes CF, McCann A, Horigan G, Strain JJ, McNulty H, Ward M. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie. 2020 Jun;173:91-99. doi: 10.1016/j.biochi.2020.04.004[↩][↩]
- Amenyah SD, Ward M, McMahon A, Deane J, McNulty H, Hughes C, Strain JJ, Horigan G, Purvis J, Walsh CP, Lees-Murdock DJ. DNA methylation of hypertension-related genes and effect of riboflavin supplementation in adults stratified by genotype for the MTHFR C677T polymorphism. Int J Cardiol. 2021 Jan 1;322:233-239. doi: 10.1016/j.ijcard.2020.09.011[↩]
- Definition of Multiple Sclerosis. https://www.nationalmssociety.org/What-is-MS/Definition-of-MS[↩]
- Parks NE, Jackson-Tarlton CS, Vacchi L, Merdad R, Johnston BC. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst Rev. 2020 May 19;5(5):CD004192. doi: 10.1002/14651858.CD004192.pub4[↩]
- Parviz Ghadirian and others, Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada, International Journal of Epidemiology, Volume 27, Issue 5, October 1998, Pages 845–852, https://doi.org/10.1093/ije/27.5.845[↩]
- Naghashpour M, Amani R, Sarkaki A, Ghadiri A, Samarbafzadeh A, Jafarirad S, Malehi AS. Brain-derived neurotrophic and immunologic factors: beneficial effects of riboflavin on motor disability in murine model of multiple sclerosis. Iran J Basic Med Sci. 2016 Apr;19(4):439-48. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4887718[↩]
- Naghashpour M, Majdinasab N, Shakerinejad G, Kouchak M, Haghighizadeh MH, Jarvandi F, Hajinajaf S. Riboflavin supplementation to patients with multiple sclerosis does not improve disability status nor is riboflavin supplementation correlated to homocysteine. Int J Vitam Nutr Res. 2013;83(5):281-90. doi: 10.1024/0300-9831/a000170[↩]
- Dainty JR, Bullock NR, Hart DJ, Hewson AT, Turner R, Finglas PM, Powers HJ. Quantification of the bioavailability of riboflavin from foods by use of stable-isotope labels and kinetic modeling. Am J Clin Nutr. 2007 Jun;85(6):1557-64. doi: 10.1093/ajcn/85.6.1557[↩][↩]
- Gregory JF 3rd. Accounting for differences in the bioactivity and bioavailability of vitamers. Food Nutr Res. 2012;56. doi: 10.3402/fnr.v56i0.5809[↩]
- Agte V, Tarwadi K, Mengale S, Hinge A, Chiplonkar S. Vitamin profile of cooked foods: how healthy is the practice of ready-to-eat foods? Int J Food Sci Nutr. 2002 May;53(3):197-208. doi: 10.1080/09637480220132814[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/[↩]
- National Institutes of Health. The Dietary Supplement Label Database. https://dsld.nlm.nih.gov/dsld/[↩]
- Powers H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003;77:1352–1360. doi: 10.1093/ajcn/77.6.1352[↩][↩]
- Schoenen J., Lenaerts M., Bastings E. High-dose riboflavin as a prophylactic treatment of migraine: Results of an open pilot study. Cephalalgia. 1994;14:328–329. doi: 10.1046/j.1468-2982.1994.1405328.x[↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩]
- Farmer B, Larson BT, Fulgoni VL 3rd, Rainville AJ, Liepa GU. A vegetarian dietary pattern as a nutrient-dense approach to weight management: an analysis of the national health and nutrition examination survey 1999-2004. J Am Diet Assoc. 2011 Jun;111(6):819-27. doi: 10.1016/j.jada.2011.03.012[↩]
- U.S. Department of Agriculture, Agricultural Research Service. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/[↩]
- Food fortification. https://www.who.int/health-topics/food-fortification#tab=tab_1[↩]
- McNulty H, Scott JM. Intake and status of folate and related B-vitamins: considerations and challenges in achieving optimal status. Br J Nutr. 2008 Jun;99 Suppl 3:S48-54. doi: 10.1017/S0007114508006855[↩]
- LeBlanc J.G., Laino J.E., del Valle M.J., Vannini V., van Sinderen D., Taranto M.P., de Valdez G.F., de Giori G.S., Sesma F. B-group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011;111:1297–1309. doi: 10.1111/j.1365-2672.2011.05157.x[↩]
- Riboflavin Deficiency. https://emedicine.medscape.com/article/125193-overview[↩]
- Ma AG, Schouten EG, Zhang FZ, Kok FJ, Yang F, Jiang DC, Sun YY, Han XX. Retinol and riboflavin supplementation decreases the prevalence of anemia in Chinese pregnant women taking iron and folic Acid supplements. J Nutr. 2008 Oct;138(10):1946-50. doi: 10.1093/jn/138.10.1946[↩][↩]
- Porter K, Hoey L, Hughes CF, Ward M, McNulty H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients. 2016 Nov 16;8(11):725. doi: 10.3390/nu8110725[↩]
- Yazdanpanah, N., Uitterlinden, A.G., Zillikens, M.C., Jhamai, M., Rivadeneira, F., Hofman, A., de Jonge, R., Lindemans, J., Pols, H.A. and van Meurs, J.B. (2008), Low Dietary Riboflavin but Not Folate Predicts Increased Fracture Risk in Postmenopausal Women Homozygous for the MTHFR 677 T Allele. J Bone Miner Res, 23: 86-94. https://doi.org/10.1359/jbmr.070812[↩]
- Nichols EK, Talley LE, Birungi N, McClelland A, Madraa E, Chandia AB, Nivet J, Flores-Ayala R, Serdula MK. Suspected outbreak of riboflavin deficiency among populations reliant on food assistance: a case study of drought-stricken Karamoja, Uganda, 2009-2010. PLoS One. 2013 May 2;8(5):e62976. doi: 10.1371/journal.pone.0062976[↩][↩]
- Naghashpour M, Amani R, Nutr R, Nematpour S, Haghighizadeh MH. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011 Oct;30(5):340-7. doi: 10.1080/07315724.2011.10719977[↩]
- Whitfield KC, Karakochuk CD, Liu Y, McCann A, Talukder A, Kroeun H, Ward M, McNulty H, Lynd LD, Kitts DD, Li-Chan EC, McLean J, Green TJ. Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J Nutr. 2015 Mar;145(3):628-33. doi: 10.3945/jn.114.203604 Erratum in: J Nutr. 2016 Jan;146(1):147-8.[↩]
- Choi JY, Kim YN, Cho YO. Evaluation of riboflavin intakes and status of 20-64-year-old adults in South Korea. Nutrients. 2014 Dec 31;7(1):253-64. doi: 10.3390/nu7010253[↩]
- Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum vitamin B-12 and folate concentrations and low thiamin and riboflavin intakes are inversely associated with greater adiposity in Mexican American children. J Nutr. 2014 Dec;144(12):2027-33. doi: 10.3945/jn.114.201202[↩]
- Merck Sharp & Dohme Corp., Merck Manual. Riboflavin. https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/riboflavin[↩][↩][↩]
- O’Brien M.M., Kiely M., Harrington K.E., Robson P.J., Strain J.J., Flynn A. The North/South Ireland Food Consumption Survey: vitamin intakes in 18-64-year-old adults. Public Health Nutr. 2001;4:1069–1079. doi: 10.1079/PHN2001188[↩]
- Anderson J.J.B., Suchindran C.M., Roggenkamp K.J. Micronutrient intakes in two US populations of older adults: lipid research clinics program prevalence study findings. J. Nutr. Health Aging. 2009;13:595–600. doi: 10.1007/s12603-009-0169-8[↩]
- Powers H.J., Hill M.H., Mushtaq S., Dainty J.R., Majsak-Newman G., Williams E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM) Am. J. Clin. Nutr. 2011;93:1274–1284. doi: 10.3945/ajcn.110.008409[↩][↩]
- Preziosi P., Galan P., Deheeger M., Yacoub N., Drewnowski A., Hercberg S. Breakfast type, daily nutrient intakes and vitamin and mineral status of French children, adolescents, and adults. J. Am. Coll. Nutr. 1999;18:171–178. doi: 10.1080/07315724.1999.10718846[↩]
- Smithers G., Gregory J.R., Bates C.J., Prentice A., Jackson L.V., Wenlock R. The National Diet and Nutrition Survey: young people aged 4–18 years. Nutr. Bull. 2000;25:105–111. doi: 10.1046/j.1467-3010.2000.00027.x[↩][↩][↩]
- Waldmann A., Koschizke J.W., Leitzmann C., Hahn A. Dietary intakes and lifestyle factors of a vegan population in Germany: results from the German Vegan Study. Eur. J. Clin. Nutr. 2003;57:947–955. doi: 10.1038/sj.ejcn.1601629[↩]
- Larsson C.L., Johansson G.K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 2002;76:100–106. doi: 10.1093/ajcn/76.1.100[↩]
- Majchrzak D., Singer I., Manner M., Rust P., Genser D., Wagner K.-H., Elmadfa I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann. Nutr. Metab. 2006;50:485–491. doi: 10.1159/000095828[↩]
- Crombleholme W. Obstetrics. In: Tierney L, McPhee S, Papadakis M, eds. Current Medical Treatment and Diagnosis. Stamford: Appleton and Lange; 1998:731-734.[↩]
- Wacker J, Frühauf J, Schulz M, Chiwora FM, Volz J, Becker K. Riboflavin deficiency and preeclampsia. Obstet Gynecol. 2000 Jul;96(1):38-44. doi: 10.1016/s0029-7844(00)00847-4[↩][↩][↩]
- Preeclampsia. https://medlineplus.gov/genetics/condition/preeclampsia[↩]
- Braekke K, Ueland PM, Harsem NK, Karlsen A, Blomhoff R, Staff AC. Homocysteine, cysteine, and related metabolites in maternal and fetal plasma in preeclampsia. Pediatr Res. 2007 Sep;62(3):319-24. doi: 10.1203/PDR.0b013e318123fba2[↩]
- Neugebauer, J., Zanré, Y. and Wacker, J. (2006), Riboflavin supplementation and preeclampsia. International Journal of Gynecology & Obstetrics, 93: 136-137. https://doi.org/10.1016/j.ijgo.2006.01.007[↩]
- Powers HJ, Weaver LT, Austin S, Beresford JK. A proposed intestinal mechanism for the effect of riboflavin deficiency on iron loss in the rat. Br J Nutr. 1993 Mar;69(2):553-61. doi: 10.1079/bjn19930055[↩]
- Shi Z, Zhen S, Wittert GA, Yuan B, Zuo H, Taylor AW. Inadequate riboflavin intake and anemia risk in a Chinese population: five-year follow up of the Jiangsu Nutrition Study. PLoS One. 2014 Feb 12;9(2):e88862. doi: 10.1371/journal.pone.0088862[↩]
- Powers HJ, Hill MH, Mushtaq S, Dainty JR, Majsak-Newman G, Williams EA. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am J Clin Nutr. 2011 Jun;93(6):1274-84. doi: 10.3945/ajcn.110.008409[↩][↩]
- Powers HJ. Riboflavin-iron interactions with particular emphasis on the gastrointestinal tract. Proc Nutr Soc. 1995 Jul;54(2):509-17. doi: 10.1079/pns19950019[↩]
- Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. de Benoist B, McLean E, Egli I, Cogswell M, eds. 2008; World Health Organization Press. https://www.who.int/publications/i/item/9789241596657[↩]
- Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009 Nov;130(5):627-33.[↩]
- Pena-Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004736. doi: 10.1002/14651858.CD004736.pub2. Update in: Cochrane Database Syst Rev. 2009;(4):CD004736[↩]
- Suprapto B, Widardo, Suhanantyo. Effect of low-dosage vitamin A and riboflavin on iron-folate supplementation in anaemic pregnant women. Asia Pac J Clin Nutr. 2002;11(4):263-7. doi: 10.1046/j.1440-6047.2002.00310.x[↩][↩]
- Jaeger B, Bosch AM. Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience. J Inherit Metab Dis. 2016 Jul;39(4):559-64. doi: 10.1007/s10545-016-9924-2[↩][↩]
- Bosch AM, Stroek K, Abeling NG, Waterham HR, Ijlst L, Wanders RJ. The Brown-Vialetto-Van Laere and Fazio Londe syndrome revisited: natural history, genetics, treatment and future perspectives. Orphanet J Rare Dis. 2012 Oct 29;7:83. doi: 10.1186/1750-1172-7-83[↩][↩]
- Russell, RM. Vitamin and trace mineral deficiency and excess. Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005. 403-11.[↩][↩]
- Peter Heese and others, Alterations of Homocysteine Serum Levels during Alcohol Withdrawal Are Influenced by Folate and Riboflavin: Results from the German Investigation on Neurobiology in Alcoholism (GINA), Alcohol and Alcoholism, Volume 47, Issue 5, September/October 2012, Pages 497–500, https://doi.org/10.1093/alcalc/ags058[↩]
- Soares MJ, Satyanarayana K, Bamji MS, Jacob CM, Ramana YV, Rao SS. The effect of exercise on the riboflavin status of adult men. Br J Nutr. 1993 Mar;69(2):541-51. doi: 10.1079/bjn19930054[↩]
- Suboticanec K, Stavljenić A, Schalch W, Buzina R. Effects of pyridoxine and riboflavin supplementation on physical fitness in young adolescents. Int J Vitam Nutr Res. 1990;60(1):81-8.[↩]
- Manore MM. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr. 2000 Aug;72(2 Suppl):598S-606S. doi: 10.1093/ajcn/72.2.598S[↩]
- Soares M.J., Satyanarayana K., Bamji M.S., Jacob C.M., Ramana Y.V., Rao S.S. The effect of exercise on the riboflavin status of adult men. Br. J. Nutr. 1993;69:541–551. doi: 10.1079/BJN1993005[↩][↩]
- Rodriguez N.R., Di Marco N.M., Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009;41:709–731. doi: 10.1249/MSS.0b013e31890eb86[↩][↩]
- American Dietetic Association; Dietitians of Canada; American College of Sports Medicine; Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009 Mar;41(3):709-31. doi: 10.1249/MSS.0b013e31890eb86[↩][↩]
- Burke B.S., Beal V.A., Kirkwood S.B., Stuart H.C. The Influence of Nutrition During Pregnancy Upon the Condition of the Infant at Birth. J. Nutr. 1943;26:569–583. doi: 10.1093/jn/26.6.569[↩][↩]
- Natraj U., Kumar R A., Kadam P. Termination of pregnancy in mice with antiserum to chicken riboflavin-carrier protein. Biol. Reprod. 1987;36:677–685. doi: 10.1095/biolreprod36.3.677[↩]
- White H.B., Merrill A.H.J. Riboflavin-binding proteins. Annu. Rev. Nutr. 1988;8:279–299. doi: 10.1146/annurev.nu.08.070188.001431[↩]
- King J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000;71:1218S–1225S. doi: 10.1093/ajcn/71.5.1218s[↩]
- Herrera E., Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 2016;26:109–127. doi: 10.1515/hmbci-2015-0025[↩]
- Bates C.J., Prentice A.M., Paul A.A., Sutcliffe B.A., Watkinson M., Whitehead R.G. Riboflavin status in Gambian pregnant and lactating women and its implications for Recommended Dietary Allowances. Am. J. Clin. Nutr. 1981;34:928–935. doi: 10.1093/ajcn/34.5.928[↩]
- Gromisch D.S., Lopez R., Cole H.S., Cooperman J.M. Light (phototherapy)-induced riboflavin deficiency in the neonate. J. Pediatr. 1977;90:118–122. doi: 10.1016/S0022-3476(77)80784-1[↩]
- Allen L.H. B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012;3:362–369. doi: 10.3945/an.111.001172[↩]
- Borghetti M, Benelli G, Cannatelli G, Iori M, Panzeri ML. Agobiopsia percutanea ecoguidata di lesioni epatiche sospette per epatocarcinoma. Valutazione su 104 pazienti [Ultrasound-guided percutaneous needle biopsy in hepatic lesions suspected of hepatocarcinoma. Evaluation of 104 patients]. Radiol Med. 1991 Jul-Aug;82(1-2):73-8. Italian.[↩]
- Smedts HP, Rakhshandehroo M, Verkleij-Hagoort AC, de Vries JH, Ottenkamp J, Steegers EA, Steegers-Theunissen RP. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur J Nutr. 2008 Oct;47(7):357-65. doi: 10.1007/s00394-008-0735-6[↩][↩][↩]
- Badart-Smook A, van Houwelingen AC, Al MD, Kester AD, Hornstra G. Fetal growth is associated positively with maternal intake of riboflavin and negatively with maternal intake of linoleic acid. J Am Diet Assoc. 1997 Aug;97(8):867-70. doi: 10.1016/s0002-8223(97)00211-3[↩]
- Sánchez DJ, Murphy MM, Bosch-Sabater J, Fernández-Ballart J. Enzymic evaluation of thiamin, riboflavin and pyridoxine status of parturient mothers and their newborn infants in a Mediterranean area of Spain. Eur J Clin Nutr. 1999 Jan;53(1):27-38. doi: 10.1038/sj.ejcn.1600674[↩]
- Vujkovic M, Steegers EA, van Meurs J, Yazdanpanah N, van Rooij IA, Uitterlinden AG, Steegers-Theunissen RP. The maternal homocysteine pathway is influenced by riboflavin intake and MTHFR polymorphisms without affecting the risk of orofacial clefts in the offspring. Eur J Clin Nutr. 2010 Mar;64(3):266-73. doi: 10.1038/ejcn.2009.138[↩]
- Sakurai T, Furukawa M, Asoh M, Kanno T, Kojima T, Yonekubo A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J Nutr Sci Vitaminol (Tokyo). 2005 Aug;51(4):239-47. doi: 10.3177/jnsv.51.239[↩]
- Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr. 2012 May 1;3(3):362-9. doi: 10.3945/an.111.001172[↩][↩]
- Murphy SP, Allen LH. Nutritional importance of animal source foods. J Nutr. 2003 Nov;133(11 Suppl 2):3932S-3935S. doi: 10.1093/jn/133.11.3932S[↩]
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002 Jul;76(1):100-6. doi: 10.1093/ajcn/76.1.100[↩]
- Waldmann A, Koschizke JW, Leitzmann C, Hahn A. Dietary intakes and lifestyle factors of a vegan population in Germany: results from the German Vegan Study. Eur J Clin Nutr. 2003 Aug;57(8):947-55. doi: 10.1038/sj.ejcn.1601629[↩]
- Majchrzak D, Singer I, Männer M, Rust P, Genser D, Wagner KH, Elmadfa I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann Nutr Metab. 2006;50(6):485-91. doi: 10.1159/000095828[↩]
- Whitfield KC, Karakochuk CD, Liu Y, McCann A, Talukder A, Kroeun H, Ward M, McNulty H, Lynd LD, Kitts DD, Li-Chan EC, McLean J, Green TJ. Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J Nutr. 2015 Mar;145(3):628-33. doi: 10.3945/jn.114.203604[↩]
- Fanelli M.T., Woteki C.E. Nutrient intakes and health status of older Americans. Data from the NHANES II. Ann. N. Y. Acad. Sci. 1989;561:94–103. doi: 10.1111/j.1749-6632.1989.tb20973.x[↩]
- Bates C.J., Prentice A., Cole T.J., van der Pols J.C., Doyle W., Finch S., Smithers G., Clarke P.C. Micronutrients: highlights and research challenges from the 1994-5 National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 1999;82:7–15. doi: 10.1017/S0007114599001063[↩]
- Flynn A., Moreiras O., Stehle P., Fletcher R.J., Muller D.J.G., Rolland V. Vitamins and minerals: A model for safe addition to foods. Eur. J. Nutr. 2003;42:118–130. doi: 10.1007/s00394-003-0391-9[↩]
- Boisvert W.A., Mendoza I., Castaneda C., De Portocarrero L., Solomons N.W., Gershoff S.N., Russell R.M. Riboflavin requirement of healthy elderly humans and its relationship to macronutrient composition of the diet. J. Nutr. 1993;123:915–925. doi: 10.1093/jn/123.5.915[↩]
- Mikkelsen K., Stojanovska L., Tangalakis K., Bosevski M., Apostolopoulos V. Cognitive decline: A vitamin B perspective. Maturitas. 2016;93:108–113. doi: 10.1016/j.maturitas.2016.08.001[↩]
- Mikkelsen K., Stojanovska L., Apostolopoulos V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016;23:4317–4337. doi: 10.2174/0929867323666160920110810[↩]
- Seidl S.E., Santiago J.A., Bilyk H., Potashkin J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014;6:36. doi: 10.3389/fnagi.2014.00036[↩]
- Synofzik M., Schule R. Overcoming the divide between ataxias and spastic paraplegias: Shared phenotypes, genes, and pathways. Mov. Disord. 2017;32:332–345. doi: 10.1002/mds.26944[↩]
- Udhayabanu T., Manole A., Rajeshwari M., Varalakshmi P., Houlden H., Ashokkumar B. Riboflavin Responsive Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Clin. Med. 2017;6:52. doi: 10.3390/jcm6050052[↩]
- Menezes MP, Farrar MA, Webster R, Antony J, O’Brien K, Ouvrier R, Kiernan MC, Burns J, Vucic S. Pathophysiology of motor dysfunction in a childhood motor neuron disease caused by mutations in the riboflavin transporter. Clin Neurophysiol. 2016 Jan;127(1):911-918. doi: 10.1016/j.clinph.2015.05.012[↩][↩][↩][↩]
- Cali E, Dominik N, Manole A, et al. Riboflavin Transporter Deficiency. 2015 Jun 11 [Updated 2021 Apr 8]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK299312[↩][↩][↩][↩][↩][↩]
- Riboflavin Deficiency. https://emedicine.medscape.com/article/125193-overview#a3[↩][↩]
- Zhao X, Tebbe L, Naash MI, Al-Ubaidi MR. The Neuroprotective Role of Retbindin, a Metabolic Regulator in the Neural Retina. Front Pharmacol. 2022 Jul 6;13:919667. doi: 10.3389/fphar.2022.919667[↩]
- Robitaille, J., Carmichael, S.L., Shaw, G.M. and Olney, R.S. (2009), Maternal nutrient intake and risks for transverse and longitudinal limb deficiencies: Data from the National Birth Defects Prevention Study, 1997–2003 . Birth Defects Research Part A: Clinical and Molecular Teratology, 85: 773-779. https://doi.org/10.1002/bdra.20587[↩]
- Riboflavin Deficiency. https://emedicine.medscape.com/article/125193-overview#a5[↩][↩][↩][↩][↩]
- Schoenen J, Lenaerts M, Bastings E. High-dose riboflavin as a prophylactic treatment of migraine: results of an open pilot study. Cephalalgia. 1994 Oct;14(5):328-9. doi: 10.1046/j.1468-2982.1994.1405328.x[↩]
- Moriyama Y. Riboflavin transporter is finally identified. J Biochem. 2011 Oct;150(4):341-3. doi: 10.1093/jb/mvr095[↩]
- Thulasi V, Veerapandiyan A, Pletcher BA, Tong CM, Ming X. A Case of Brown-Vialetto-Van Laere Syndrome Due To a Novel Mutation in SLC52A3 Gene: Clinical Course and Response to Riboflavin. Child Neurol Open. 2017 Aug 22;4:2329048X17725610. doi: 10.1177/2329048X17725610[↩]