Contents
- Systemic lupus erythematosus
- Systemic lupus erythematosus and pregnancy
- Systemic lupus erythematosus causes
- Systemic lupus erythematosus pathophysiology
- Systemic lupus erythematosus prevention
- Systemic lupus erythematosus signs and symptoms
- Systemic lupus erythematosus complications
- Systemic lupus erythematosus diagnosis
- Table 2. The American College of Rheumatology and the European League Against Rheumatism Criteria for the Classification of Systemic Lupus Erythematosus
- Systemic Lupus International Collaborating Clinics (SLICC) criteria for diagnosing systemic lupus erythematosus
- Blood and urine tests
- Imaging tests
- Biopsy
- Photoprovocation tests
- Other tests
- Systemic lupus erythematosus differential diagnosis
- Systemic lupus erythematosus treatment
- Systemic lupus erythematosus prognosis
- Systemic lupus erythematosus life expectancy
Systemic lupus erythematosus
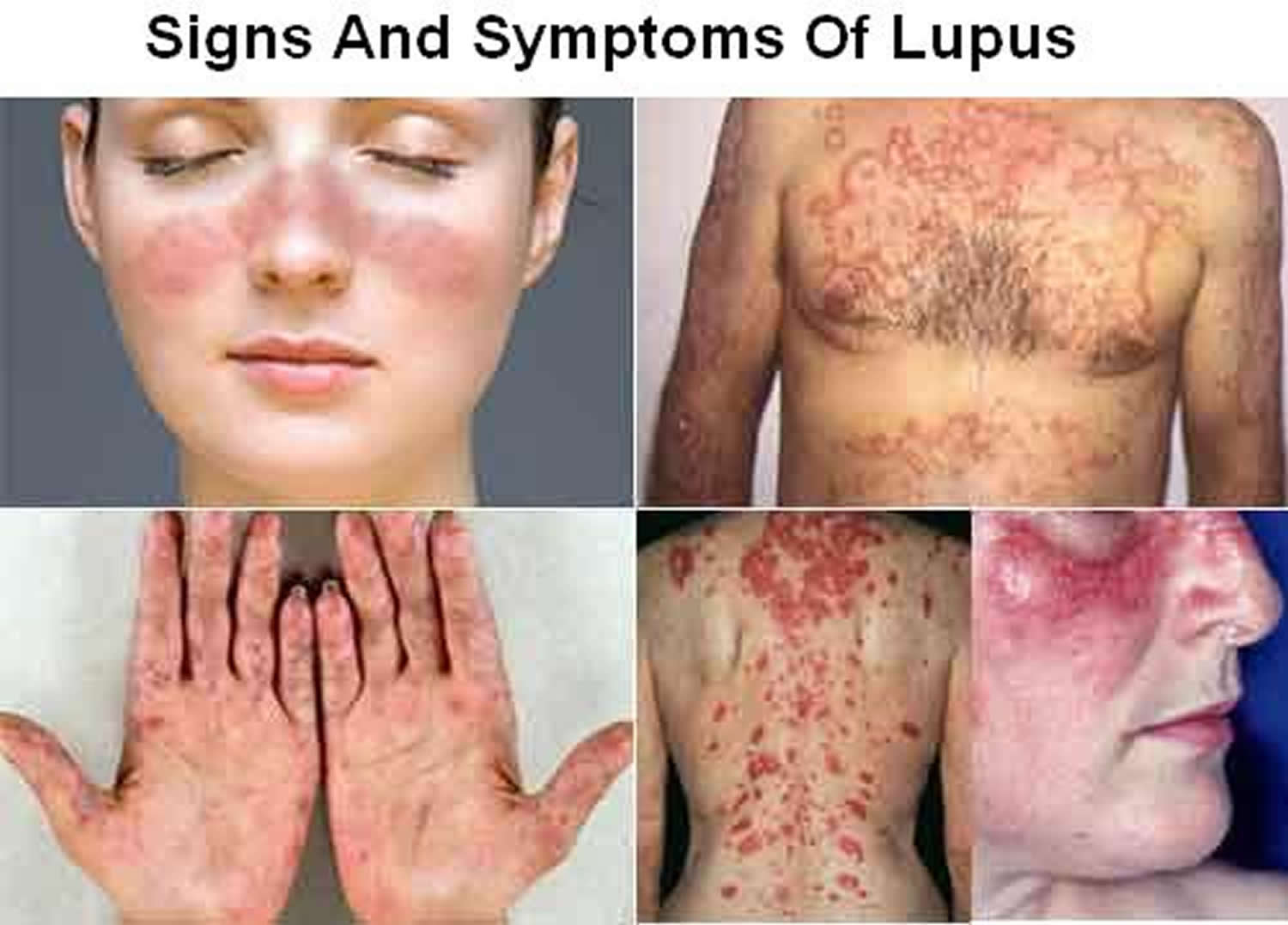
Systemic lupus erythematosus (SLE) also called lupus, is a chronic (long-term) inflammatory disease that can affect almost any part of your body, especially your skin, joints, kidneys, heart (pericardium), the tissue lining your lungs (pleura), bones, blood, or brain 1, 2. Systemic lupus erythematosus is considered an autoimmune disorder, meaning that a person’s own immune system attacks his or her own healthy cells and tissues, causing inflammation and damage 3. Many patients experience fatigue, weight loss, and fever. Systemic lupus erythematosus (SLE) is sometimes called acute lupus erythematosus and the cutaneous features may be described as acute cutaneous lupus or cutaneous lupus erythematosus (CLE). Skin involvement or cutaneous lupus (CLE) affects 80% of patients with SLE and it is the first sign of SLE in about one-quarter of them. Systemic lupus erythematosus (SLE) can present as lupus erythematosus-specific or lupus erythematosus-nonspecific manifestations. Lupus erythematosus-specific lesions tend to be induced or aggravated by exposure to ultraviolet (UV) radiation and are localized in sun-exposed sites such as the face, neck, V of the neck and upper back.
Because systemic lupus erythematosus (SLE) can affect any organ system, no two people have identical forms of the disease. However, most people with systemic lupus erythematosus (SLE) report periods of time in which their symptoms seem to be mild or absent (remission) and other periods of time when the inflammation is more severe (flare or relapse).
The causes of SLE are unknown, but are believed to be linked to environmental, genetic, and hormonal factors. Some people are born with a tendency toward developing systemic lupus erythematosus (SLE), which may be triggered by infections, certain drugs or even sunlight.
Risk factors leading to systemic lupus erythematosus (SLE) include:
- Genetic predisposition, including haplotype HLA-B8, -DR3
- Exposure to sunlight ultraviolet (UV) radiation
- Viral infection, particularly Epstein-Barr virus
- Hormones
- Toxins such as cigarette smoke. SLE is more prevalent and more severe in smokers. Smoking also reduces the effectiveness of antimalarials and other therapies.
- Drugs in drug-induced lupus erythematosus
- Emotional upset.
Systemic lupus erythematosus (SLE) affects women more than men, at a ratio of 9 to 1 4. SLE is diagnosed most often in women in the first to fourth decades 5, the so called ‘child‐bearing years’ 6. In men, diagnosis is most common after age 59 4. Most recent studies have confirmed that females have higher incidence and prevalence regardless of age or ethnic origin 7. Gender differences in the clinical presentation of SLE have been reported 8. Rees et al 7 noted differences based on ethnicity, reporting that for either gender, prevalence of SLE was highest among those of black ethnicity, with white ethnic groups reporting the lowest prevalence, and Asian and Hispanic groups an intermediate prevalence.
Systemic lupus erythematosus (SLE) flares vary from mild to serious. Most patients have times when the disease is active, followed by times when the disease is mostly quiet – referred to as a remission. Yet, there is much reason for hope. Improvements in treatment have greatly improved these patients’ quality of life and increased their lifespan.
Systemic lupus erythematosus (SLE) can be difficult to diagnose because its signs and symptoms often mimic those of other ailments. Systemic lupus erythematosus can affect so many different organ systems, its symptoms can come and go, and no 2 people have exactly the same form of the disease. However, the most distinctive sign of systemic lupus erythematosus (SLE) is a facial rash that resembles the wings of a butterfly unfolding across both cheeks (systemic lupus erythematosus butterfly rash), which occurs in many (90% of cases) but not all cases of lupus. No one test can diagnose systemic lupus erythematosus. The combination of blood tests, urinalysis, chest X-ray, or an electrocardiogram (ECG), signs and symptoms, and physical examination findings leads to the diagnosis of lupus.
Even with a confirmed diagnosis of systemic lupus erythematosus, treatments vary as much as the disease itself. Treatments depend greatly on which organs are affected and how severe your symptoms are. In general, however, the following oral medications are frequently used for systemic lupus erythematosus:
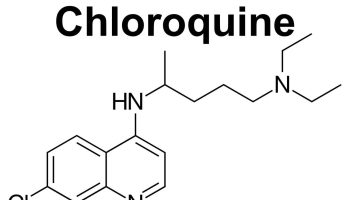
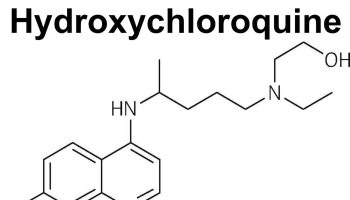
- Anti-malarial drugs such as hydroxychloroquine, chloroquine, or quinacrine
- Corticosteroids
- Anti-inflammatory medications such as aspirin, ibuprofen, naproxen, or indomethacin
- Immune-suppressing medications including azathioprine, cyclophosphamide, methotrexate, cyclosporine, chlorambucil, or mycophenolate mofetil
Systemic lupus erythematosus (SLE) key points:
- Systemic lupus erythematosus is a chronic disease, with flares and remissions.
- Systemic lupus erythematosus is not contagious.
- Systemic lupus erythematosus (SLE) affects 7.5 million people worldwide 9. Every year about 2–7 new systemic lupus erythematosus (SLE) cases are diagnosed in a population of 100,000 people.
- Systemic lupus erythematosus (SLE) occurs ten times more often in women than in men and onset is most often between the ages of 15 and 45 years. SLE is typically more prevalent in young women of childbearing age 10.
- Systemic lupus erythematosus can affect many different areas of the body.
- There is no cure for SLE. However, there are treatments designed to improve symptoms.
- Systemic lupus erythematosus (SLE) treatment depends on the organs involved. A special doctor called a rheumatologist can help you find the right treatment plan and refer you to other types of doctors to treat specific symptoms.
- Involvement of the kidneys or/and the brain is the most serious manifestation of systemic lupus erythematosus.
- People can live well with systemic lupus erythematosus (SLE) if they actively work toward good health.
- Sun exposure can lead to lupus flares.
- Carefully plan your pregnancies; systemic lupus erythematosus can flare during pregnancy and can affect its outcome.
Figure 1. Systemic lupus erythematosus rash (systemic lupus erythematosus butterfly rash)
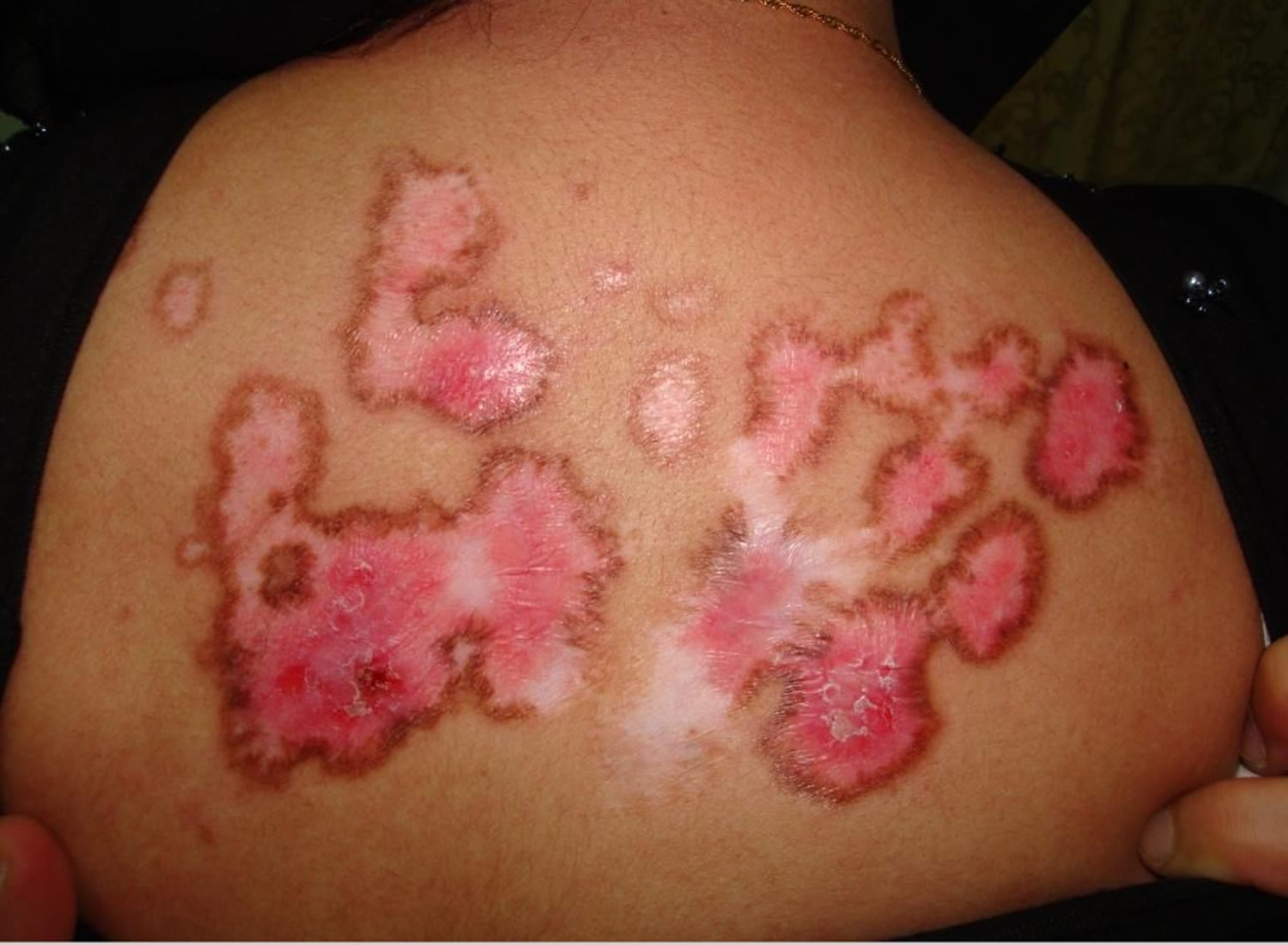
Figure 2. Systemic lupus erythematosus rash on back
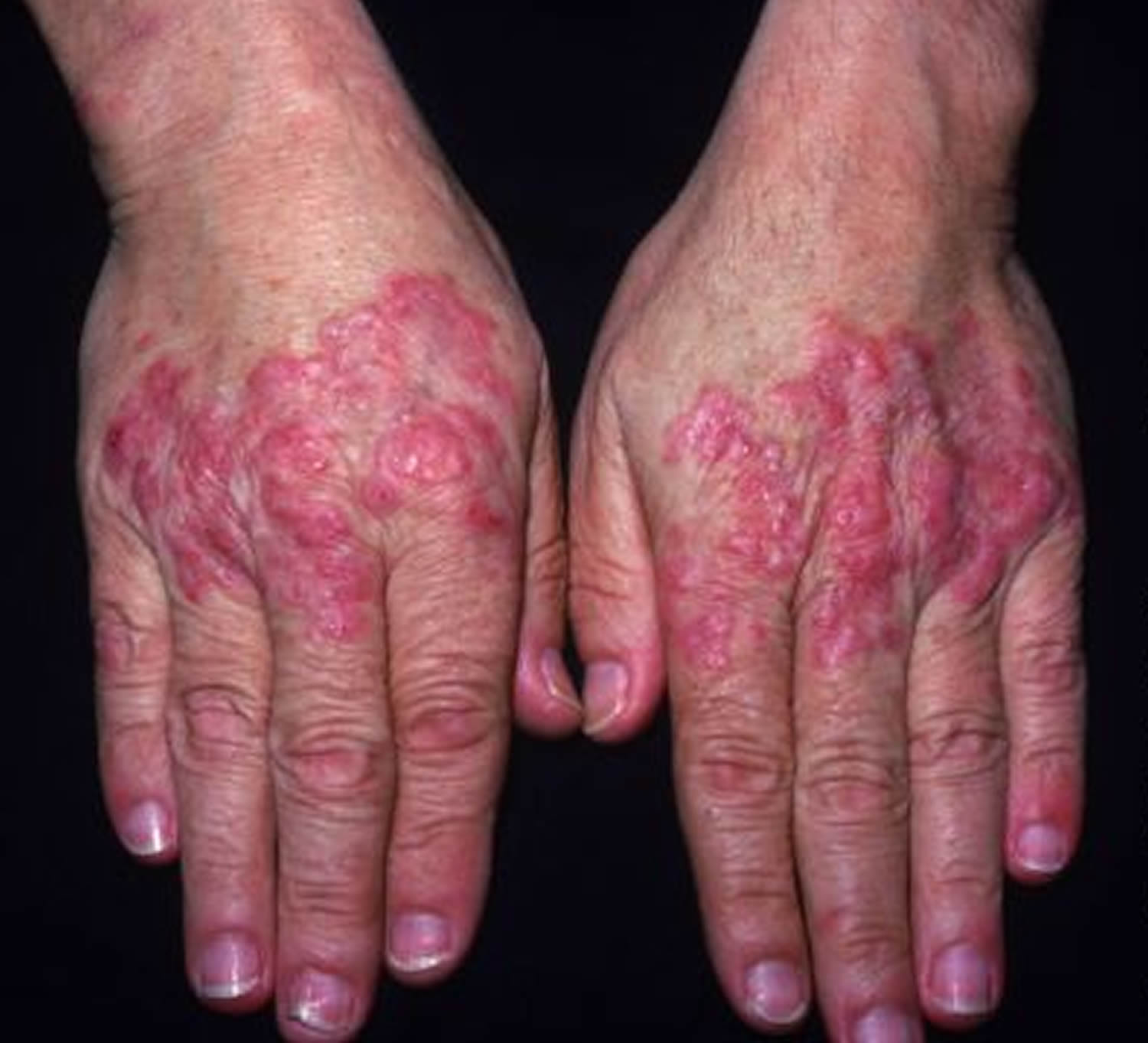
Figure 3. Systemic lupus erythematosus rash hand
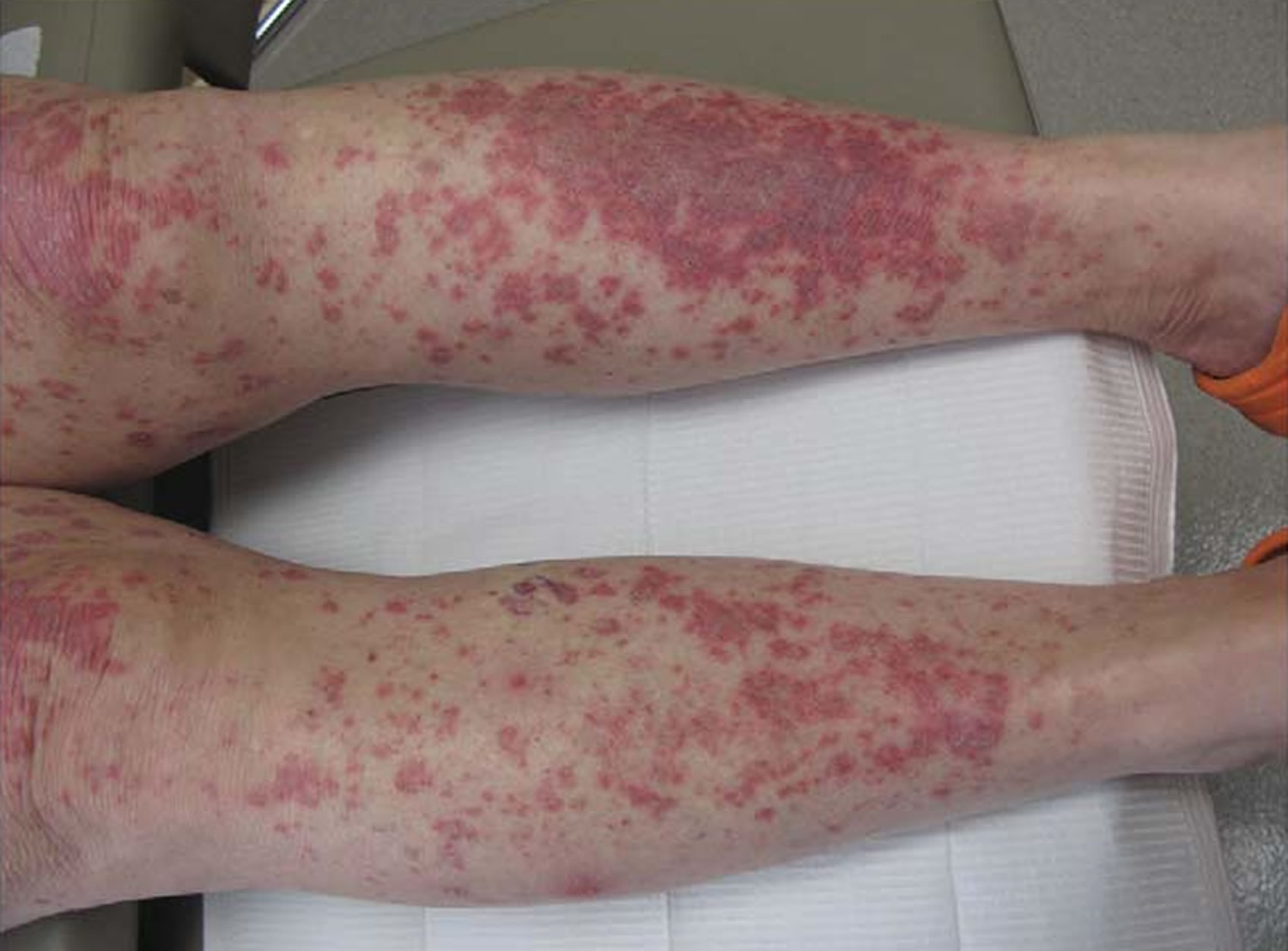
Figure 4. Lupus vasculitis
Footnotes: Lupus vasculitis. Palpable purpuric papules and plaques on the lower extremities in a patient with flaring systemic lupus erythematosus. Skin biopsy showed leukocytoclastic vasculitis in addition to specific features of cutaneous lupus erythematosus.
[Source 11 ]See your doctor if you:
- develop an unexplained skin rashes – often over the nose and cheeks
- ongoing fever,
- persistent aching or fatigue,
- joint pain and stiffness,
- extreme tiredness that won’t go away no matter how much you rest
As well as the main symptoms, you might also have:
- weight loss
- swollen glands
- sensitivity to light (causing rashes on uncovered skin)
- poor circulation in fingers and toes (Raynaud’s phenomenon)
What are the types of lupus?
When people talk about lupus, they’re usually talking about systemic lupus erythematosus (SLE). But there are many clinical variants grouped under the name ‘lupus erythematosus’ 12:
- Systemic lupus erythematosus (SLE), the most common form of lupus
- Cutaneous lupus erythematosus (CLE), a form of lupus that is limited to the skin (including subacute cutaneous lupus erythematosus [SCLE] and discoid lupus erythematosus [DLE]). Cutaneous lupus erythematosus (CLE) may occur with or without systemic involvement 13.
- Drug‐induced lupus erythematosus (DILE), a lupus-like disease caused by certain prescription drugs. Drug‐induced lupus erythematosus (DILE) has characteristics that distinguish it from classical (also known as idiopathic) SLE. For example, DILE develops in parallel with drug exposure and stops once treatment is complete 14.
- Neonatal lupus erythematosus, a rare condition that affects infants of women who have lupus. Infants born to women with lupus can also be affected by lupus, so called ‘neonatal lupus’. Neonatal lupus occurs when maternal anti–Sjögren’s syndrome‐related antigen A, also called anti‐Ro (SSA/Ro), or anti–Sjögren’s syndrome‐related antigen B, also called anti‐La (SSB/La), antibodies pass via the placenta and trigger the neonatal lupus syndrome characterised by congenital heart block, photosensitivity rash, cytopenia, and liver abnormalities 15.
- Child‐onset lupus erythematosus or juvenile-onset systemic lupus erythematosus (JSLE). Children may also affected by SLE, so called “child‐onset lupus” or “juvenile-onset systemic lupus” 16, 17. Because there are some clinical differences between child‐onset and typical adult‐onset SLE, it has been divided into a separate subcategory.
Who’s at risk of systemic lupus erythematosus?
Systemic lupus erythematosus (SLE) can occur in people of all ages, all races, and both sexes. However, systemic lupus erythematosus is far more common in women, especially those between 15–45 years old. Women are at ten times more risk of developing SLE than men, and the risk of SLE is 14 times more in Klinefelter syndrome (47, XXY) 18, 19. This suggests an association with genes on the X-chromosome. However, despite several studies, the exact genes have not been identified 20. In America, systemic lupus erythematosus (SLE) is also more commonly seen in people with darker skin than in light-skinned people. The Georgia and Michigan lupus registries reported prevalence of 72.1 to 74.4 per 100,000 persons and incidence rates of 5.6 per 100,000 person-years in primarily Caucasian and African-American populations. African-Americans have the highest rates, which are higher among Asian and Hispanic populations than Caucasians. The disease tends to have an earlier age of onset and is more severe in African-Americans 20.
Although it is not directly inherited, systemic lupus erythematosus (SLE) and other autoimmune diseases may run in families. Inheriting certain genes may make some people more susceptible to developing systemic lupus erythematosus (SLE).
In addition, certain environmental factors may trigger lupus in those who have a family (genetic) tendency toward systemic lupus erythematosus (SLE), including:
- Ultraviolet light, especially sunlight
- Certain medications, especially hydralazine and procainamide
- Infections
- Antibiotics, especially penicillins or sulfa-containing medicines
- Stress
- Hormonal changes, especially related to pregnancy and menstrual cycles 21.
Can I die from systemic lupus erythematosus?
Yes, systemic lupus erythematosus (SLE) can cause death. But, thanks to new and better treatments, most people with systemic lupus erythematosus (SLE) can expect to live long, healthy lives. The leading causes of death in people with lupus are health problems that are related to lupus, such as kidney disease, infections, and heart disease 22, 23.
Work with your doctor to manage lupus. Take your medicine as your doctor tells you to and make healthy choices, such as not smoking, eating healthy foods, getting regular physical activity, and managing your weight.
What are systemic lupus erythematosus flares?
The times when your symptoms get worse and you feel sick are called flares. Flares can come and go. You may have swelling and rashes one week and no symptoms the next. Sometimes flares happen without clear symptoms and are seen only with laboratory tests. Some flares are mild, but others are serious and require medical care.
What are some triggers for systemic lupus erythematosus flares?
Common triggers for systemic lupus erythematosus flares include:
- Overwork and not enough rest
- Being out in the sun or having close exposure to fluorescent or halogen light
- Infection
- Injury
- Stopping your lupus medicines
- Other types of medicines
Even if you take medicine for lupus, you may find that some things trigger a flare. For instance, your symptoms may still flare after you’ve been out in the sun or after a hard day at work, even if you are taking your lupus medicine.
How can I tell if a systemic lupus erythematosus flare is coming?
Systemic lupus erythematosus (SLE) flares most often have warning signs. You can help prevent flares or make them less severe if you can spot the warning signs and get treatment quickly. Before a flare, your symptoms might get worse, or you might get new signs and symptoms, such as:
- Feeling more tired
- Pain
- Rash
- Fever
- Stomach ache
- Severe headache
- Dizziness
There is no way to know if a flare will be mild or serious. Mild or moderate flares may cause only a rash or more joint pain. But severe flares can damage organs in the body, including fluid buildup around your heart and kidney disease.
See your doctor if you get the warning signs of a flare. Your doctor may want to adjust your medicine or treatment plan.
Systemic lupus erythematosus and pregnancy
Systemic lupus erythematosus (SLE) may first appear during pregnancy; women who have had an unexplained 2nd-trimester stillbirth, a fetus with growth restriction, preterm delivery, or recurrent spontaneous abortions are often later diagnosed with SLE.
The course of preexisting SLE during pregnancy cannot be predicted, but SLE may worsen, particularly immediately postpartum. Outcomes are better if conception can be delayed until the disorder has been inactive for at least 6 months, the drug regimen has been adjusted in advance, and blood pressure and renal function are normal.
Complications of preexisting SLE during pregnancy may include:
- Fetal growth restriction
- Preterm delivery due to preeclampsia
- Congenital heart block due to maternal antibodies that cross the placenta
If you are a young woman with systemic lupus erythematosus (SLE) and wish to have a baby, carefully plan your pregnancy. With your doctor’s guidance, time your pregnancy for when your systemic lupus erythematosus (SLE) activity is low. While pregnant, avoid medications that can harm your baby. These include cyclophosphamide, cyclosporine, and mycophenolate mofetil. If you must take any of these medicines, or your disease is very active, use birth control.
The relationship between systemic lupus erythematosus (SLE) activity and pregnancy is more debated. In general, there is a tendency for mild to moderate flares, especially during the second half of pregnancy and the post-partum period. However, most of these flares do not endanger the mother’s or the baby’s life, nor do they substantially alter the long-term prognosis of systemic lupus erythematosus (SLE). Being in clinical remission for three – six months prior to getting pregnant decreases the chance that a flare will occur during the pregnancy.
Patients who have or have had kidney disease, due to lupus, generally have an increased risk of severe hypertension and pre-eclampsia. Diffuse nephritis, hypertension, or the presence of circulating antiphospholipid antibodies (usually anticardiolipin antibody or lupus anticoagulant) increases risk of perinatal mortality. Neonates may have anemia, thrombocytopenia, or leukopenia; these disorders tend to resolve during the first weeks after birth when maternal antibodies disappear.
If kidney function and blood pressure prior to pregnancy are normal and the systemic lupus erythematosus (SLE) is inactive at the time of conception for a period of at least six months, the outcome is likely to be good. Women with severely impaired kidney function, uncontrolled hypertension (high blood pressure), and/or active systemic lupus erythematosus (SLE) disease flares are advised not to get pregnant.
Facts
- Each woman’s systemic lupus erythematosus (SLE) disease should be well under control for a period of at least three – six months before attempting pregnancy. As long as your medicines are not harmful to the fetus, you should remain on your medicines to prevent risk of a disease flare. Any changes should be discussed in advance with your rheumatologist.
- Women with a low-risk profile can be managed with usual visits to the rheumatologist as a precaution. Those with a high-risk profile should be managed by both the rheumatologist and obstetric team with experience in high-risk pregnancies
Rheumatologists have long been concerned that the female hormone estrogen or treatment with estrogen may cause or worsen systemic lupus erythematosus (SLE). Recent research showed that estrogen therapy can trigger some mild or moderate flares of systemic lupus erythematosus (SLE), but does not cause symptoms to get much worse. Yet, estrogen can raise the risk of blood clots. Thus, you should not take estrogen if your blood tests show antiphospholipid antibodies (meaning you already have a high risk of blood clots).
Management of pregnancy in women with systemic lupus erythematosus
All women with systemic lupus erythematosus (SLE) should undergo counseling about their specific risks if they are thinking about having a baby. During that discussion with your doctor, you can review specific concerns of pregnancy and learn what pregnancy complications can occur.
Here are a few things that make a pregnancy “high risk.”
- Previous pregnancy with complications
- Underlying kidney disease
- Underlying heart disease
- Underlying lung disease (including pulmonary hypertension)
- Flare of a lupus disease
- A history of previous blood clot
- Presence of anti-Ro (SSA) and anti-La (SSB) antibodies
- IVF (in vitro fertilization)
- Pregnancy with twins, triplets, etc.
- Mother being over 40
Each woman’s systemic lupus erythematosus (SLE) disease should be well under control for a period of at least three – six months before attempting pregnancy. As long as your medicines are not harmful to the fetus, you should remain on your medicines to prevent risk of a disease flare. SLE flares are usually treated with low-dose prednisone, IV pulse methylprednisolone, hydroxychloroquine, and/or azathioprine. High-dose prednisone and cyclophosphamide increase obstetric risks and are thus reserved for severe lupus complications. Prednisone should be used at doses below 10 mg/day whenever possible, due to the risk of associated complications such as high blood pressure, diabetes, excessive weight gain, risk of infections, and premature rupture of membranes (PROM). Hydroxychloroquine is an extremely safe drug for both the mother and the fetus and should not be stopped before, during, or after pregnancy. High blood pressure should be managed using medicines that are safe during pregnancy. Captopril and enalapril are safe drugs during breastfeeding.
Women with a low-risk profile should include in their usual treatment plan regular three-month visits to the rheumatologist, as a precaution. However, those with a high-risk profile should be managed by a combined medical and obstetric team with experience in high-risk pregnancies. Visits should be more frequent as pregnancy advances (weekly during the late third trimester), and include monitoring of fetal and maternal well-being. Blood pressure measurements and urine testing should be frequently performed to assure the early detection and treatment of pre-eclampsia.
Use of rheumatic medications during pregnancy and lactation
During pregnancy, the effects of inflammation when systemic lupus erythematosus (SLE) becomes active and medications used to treat rheumatic disease can cause problems. Information on the safety of many drugs in pregnant women is incomplete and difficult to obtain. Based on the information available, most rheumatologists generally recommend the following:
Table 1: Acceptable medications during pregnancy and lactation
| Pregnancy | Lactation | |
|---|---|---|
| NSAID | Yes (avoid after 32 weeks) | Yes |
| Sulfasalazine | Yes | Yes |
| Antimalarials | Yes | Yes |
| Corticosteroids | Yes | Yes |
| Cyclosporine | Yes | Probably yes |
| Azathioprine | Yes | Probably yes |
| Mycophenolate | No | No |
| Methotrexate | No | No |
| Cyclophosphamide | No | No |
| Anti-tumor necrosis factor (TNF) | Yes | Yes |
| Rituximab | No | No |
| Warfarin | No (with caution, only after first trimester) | Yes |
| Heparin | Yes | Yes |
This list should only be considered a general guide and may not apply in all situations. Women who are pregnant or considering pregnancy should discuss their medications with both their rheumatologist and their obstetrician. Many women would prefer to take no medication during pregnancy and nursing. However, the consequences of not being on medicine and the risk of their rheumatic disease flaring are important considerations that should be discussed with both the rheumatologist and obstetrician.
Several drugs (particularly methotrexate and cyclophosphamide have effects on sperm cells in men. It is recommended that these medications be stopped for three months before a man fathers a child.
Systemic lupus erythematosus causes
The causes of systemic lupus erythematosus (SLE) are unknown, but are believed to be linked to your genetics (a genetic predisposition to have an overactive immune system), your environment (e.g., a virus, sun, or drug reactions) and hormonal factors 25. As an autoimmune disease, systemic lupus erythematosus (SLE) occurs when your immune system attacks healthy tissue in your body.
It appears that people with an inherited predisposition for lupus may develop the disease when they come into contact with something in the environment that can trigger lupus. The cause of systemic lupus erythematosus (SLE) in most cases, however, is unknown. Some potential triggers include:
- Sunlight. Exposure to the sun may bring on lupus skin lesions or trigger an internal response in susceptible people.
- Infections. Having an infection can initiate lupus or cause a relapse in some people.
- Medications. Lupus can be triggered by certain types of blood pressure medications, anti-seizure medications and antibiotics. People who have drug-induced lupus usually get better when they stop taking the medication. Rarely, symptoms may persist even after the drug is stopped.
Female sex and hormonal influence are significant risk factors for SLE. Estrogen stimulates CD8+ and CD4+ T cells, B cells, macrophages, thymocytes, the release of some specific cytokines (e.g., IL-1), and the expression of HLA and endothelial cell adhesion molecules (VCAM, ICAM) 20. In addition, estrogens and prolactin promote autoimmunity, increase the B-cell activation factor production, and modulate lymphocyte and plasmacytoid dendritic cells (pDC) activation. The use of estrogen-containing contraceptives and postmenopausal hormone replacement therapy can cause flares in patients with SLE and have been associated with a higher incidence of SLE. In addition, elevated levels of prolactin are seen in patients with SLE. Androgens, on the other hand, are immunosuppressive 26.
Several environmental triggers of SLE have been identified. Several drugs have been implicated in causing a lupus-like phenomenon by causing demethylation of DNA and alteration of self-antigens. While procainamide and hydralazine have the highest incidence of causing drug-induced lupus, more than 100 drugs have been associated with drug-induced lupus 20. Furthermore, several drugs such as the sulfa-drugs are well known to cause flares in patients with SLE. Ultraviolet rays and sun exposure lead to increased cell apoptosis and are well-known triggers for SLE. Several viral infections have been implicated, and the underlying mechanism is thought to be molecular mimicry. Antibodies against Epstein-Barr virus (EBV) are more prevalent in children and adults with SLE compared to the general population 20. Smoking is also thought to be a risk, with a dose-response. Other potential risk factors include silica exposure, other viral infections, vitamin D deficiency, alfalfa sprouts, and foods containing canavanine 27.
Familial segregation and high concordance rates in identical twins suggest a strong genetic contribution in SLE, although there is no obvious inheritance pattern 20. Concordant rates for identical twins have been reported as high as 50%. To date, about 100 SLE susceptibility gene loci with polymorphisms (or, rarely, copy numbers or mutations) have been identified, mostly in European and Asian populations, explaining around 30% of the inheritability of lupus 28. And more than 30 genes causing monogenic forms of SLE or SLE-like phenotype have been identified 20. These genes are associated with activation of the immune system in response to foreign antigens, self-antigen generation, and activation of innate and adaptive immune systems. Some gene mutations that are rare but are considered very high risk for the development of SLE include deficiencies of early complement components C1q, C1r, C1s (>90% risk), C4 (50%), C2 (20%), and TREX1. Some of the other genes associated include HLA-DRB1, HLA-DR2, HLA-DR3, HLA-DRX, TNFAIP3, STAT-4, STAT-1, TLR-7, IRAK1/MECP2, IRF5-TNPO3, ITGAM, etc. The most common genetic predisposition is located at the major histocompatibility (MHC) locus. The MHC contains genes for antigen-presenting molecules (class 1 human leukocyte antigens [HLA-A, -B, and -C] and class 2 HLA molecules [HLA-DR, -DQ, and -DP]) 29.
Risk factors for developing systemic lupus erythematosus
Factors that may increase your risk of systemic lupus erythematosus include:
- Your sex. Systemic lupus erythematosus (SLE) is more common in women.
- Age. Although systemic lupus erythematosus (SLE) affects people of all ages, it’s most often diagnosed between the ages of 15 and 45.
- Race. Systemic lupus erythematosus (SLE) is more common in African Americans, Hispanics and Asian Americans.
Systemic lupus erythematosus pathophysiology
SLE is an autoimmune disorder characterized by multisystem inflammation with the generation of autoantibodies. The pathogenesis of systemic lupus erythematosus (SLE) is complex, and the understanding of SLE pathogenesis is constantly evolving 20. A break in the tolerance in genetically susceptible individuals on exposure to environmental factors leads to the activation of autoimmunity. Cell damage caused by infectious and other environmental factors exposes the immune system to self-antigens leading to activation of T and B cells, which become self-sustained by a chronic self-aimed immune response. Cytokine release, complement activation, and autoantibody production lead to organ damage.
Both innate and adaptive immune systems play a role in the pathogenesis of systemic lupus erythematosus (SLE). The innate immune system activation is either Toll-like receptor (TLR) dependent or independent. The cell membrane-bound TLRs (TLR 2, 4, 6) are activated on exposure to the extracellular DNA and RNA from dying cells, which leads to downstream activation of the interferon regulatory family (IRF-3), NF-κB, and MAP-kinases, which serve as transcription factors for the production of proinflammatory mediators such as IFN-b. The endosomal TLRs (TLR 7, 9) are activated by single-stranded RNA and demethylated DNA, leading to interferon-alpha production and RNA binding autoantibodies such as antibodies against Ro La, Sm, and RNP. The TLR-independent pathway is activated by intracytoplasmic RNA sensors (RIG-1, MDA-5) and DNA sensors (IFI16, DAI) and leads to activation of IRF3 and NF-κB. Both self DNA/RNA and foreign DNA/RNA, such as from viruses, can lead to this activation. NETosis has recently gained attention in the pathogenesis of SLE. On activation by various factors such as cytokines, activated platelets, and vascular endothelial cells, neutrophils systematically release their nuclear aggregates in the extracellular environment. These nuclear aggregates can then promote Interferon-alpha production by the dendritic cells, mediate thrombosis and vascular damage and serve as self-antigens for T-lymphocytes.
T-lymphocytes and B-lymphocytes play a significant role in the pathogenesis of systemic lupus erythematosus (SLE). Apoptotic and damaged cell-derived antigens are presented to T-cells by antigen-presenting cells. T-cells in systemic lupus erythematosus (SLE) display a distorted gene expression leading to the production of several cytokines. These T-cells produce less IL-2, which leads to altered regulatory T-cell production. Increased IL-6, IL-10, IL-12, and IL-23 increase mononuclear cell production while increased IL-17 and IL-21 lead to increased T-cel production. Increased Interfern-γ leads to defective T-cell production. T-cells lead to the activation of autoreactive B-cells by CD40L and cytokine production, leading to autoantibody production, a hallmark of systemic lupus erythematosus (SLE). Toll-like receptors on interaction with DNA and RNA lead to activation of these B-cells, and the nucleic acid and protein-containing intranuclear complexes are the most prominent antigens leading to B-cel activation. These autoantibodies are pathogenic and cause organ damage by immune complex deposition, complement, and neutrophil activation, altering cell function leading to apoptosis and cytokine production 27, 30.
Furthermore, the autoreactive B-cells in SLE, stimulated by self-antigens, are not readily eliminated due to a deficiency of the process involved in the functional neutralization of autoreactive B cells. The B-cells can also serve as antigen-presenting cells and activate T-cells by presenting internalized soluble antigens to T-cells. This creates a loop where both B and T cells activate each other, leading to more autoimmunity 31.
Systemic lupus erythematosus prevention
Because the cause of systemic lupus erythematosus (SLE) is unknown, no one knows how to prevent it. Flares of lupus may be reduced by avoiding sun exposure (wearing strong sunscreen, hats, long-sleeved shirts in the sun), getting adequate sleep, and taking recommended medications. Risk of osteoporosis may be reduced by taking calcium and vitamin D supplements.
Systemic lupus erythematosus signs and symptoms
The clinical features of SLE are highly variable and may overlap with other diseases and conditions. No two cases of systemic lupus erythematosus are exactly alike. Signs and symptoms may come on suddenly or develop slowly, may be mild or severe, and may be temporary or permanent. Most people with systemic lupus erythematosus have mild disease characterized by episodes — called flares — when signs and symptoms get worse for a while, then improve or even disappear completely for a time.
The signs and symptoms of systemic lupus erythematosus (SLE) that you experience will depend on which body systems are affected by the disease. More than 90% of people with systemic lupus erythematosus have skin symptoms. The most common locations for the skin lesions of systemic lupus erythematosus include:
- Face, especially cheeks and nose
- Sun-exposed skin on arms, backs of hands, upper chest, and upper back due to increased sensitivity to sunlight (photosensitivity)
- Fingers and fingernails
- Mouth or nose
- Scalp
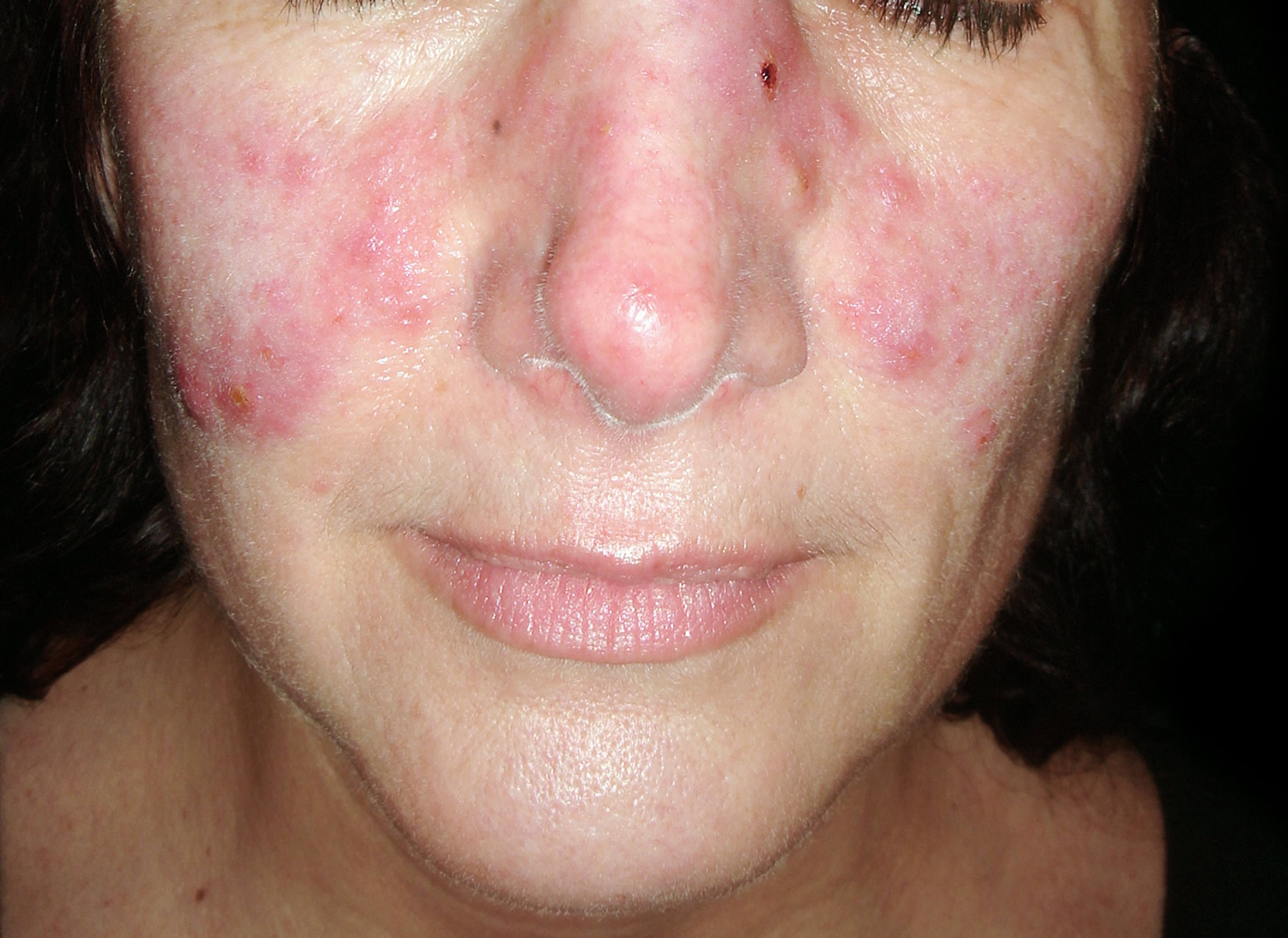
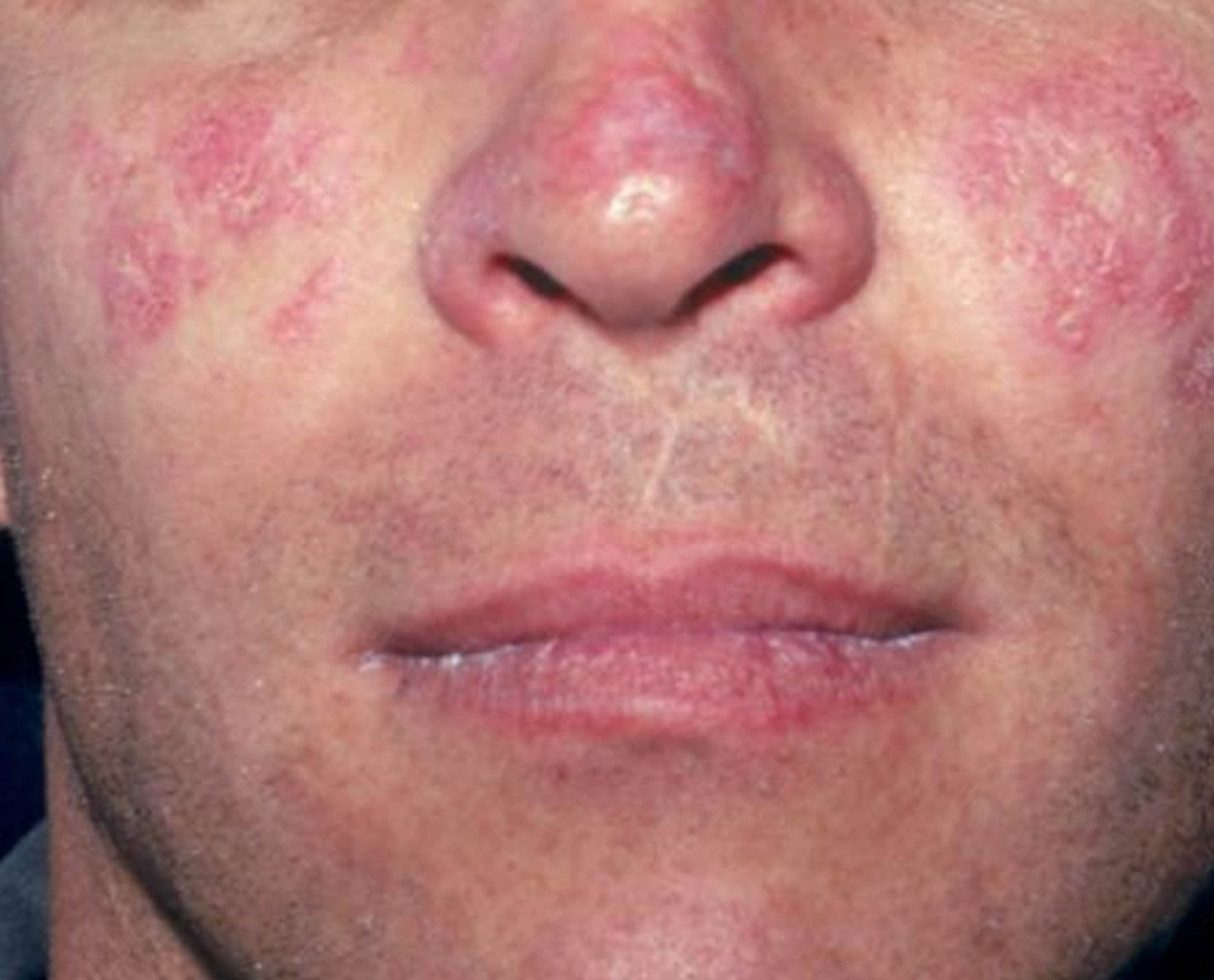
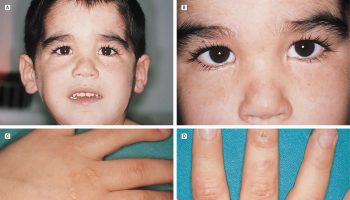
The classic skin finding in systemic lupus erythematosus is the butterfly rash (malar blush) (Figure 1). Redness across the cheeks and bridge of the nose can occur after sun exposure and may appear as much as several weeks before other symptoms develop.
A rash can develop in sun-exposed skin (photo-distribution), especially on the backs of the hands and fingers. This rash, which appears as red, scaly patches, can also affect the arms and trunk.
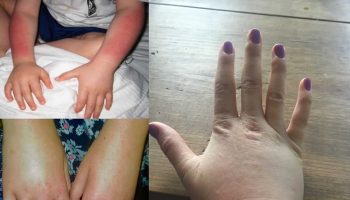
The skin around fingernails (nail folds) can be red and inflamed, and tiny, dilated blood vessels (telangiectasia) may be seen. In addition, people may develop Raynaud phenomenon, in which the fingers and sometimes toes turn pale and numb after exposure to cold temperatures or during stressful periods.
Small, painless ulcers can develop in the nose or, more commonly, in the mouth, especially on the roof of the mouth.
When lupus affects the scalp skin, you may notice hair loss. It may be patchy, or there may be thinning across the scalp, especially at the temples.
In addition to the skin lesions of lupus, people may have:
- Joint pain, stiffness and swelling, especially in hands, wrists, and knees (arthritis or synovitis causing swelling, pain and morning stiffness)
- Blood problems reduced numbers of white cells (neutropenia) and platelets (thrombocytopenia), anemia (reduced numbers of red cells) and clotting disorders
- Kidney disorders (protein in urine, casts in urine, glomerulonephritis)
- Dry eyes
- Lung problems, such as painful breathing or shortness of breath (pleurisy or pleural effusions)
- Chest pain (pericarditis or pericardial effusions)
- Seizures, psychosis, confusion or other brain disorders
- Headaches and memory loss
- Swollen lymph glands
- Fever
- Fatigue
- Nervous system: mononeuritis multiplex, myelitis, peripheral neuropathy
Cutaneous features of systemic lupus erythematosus
More than 90% of people with systemic lupus erythematosus have skin involvement (cutaneous lupus erythematosus), and it is the first sign of SLE in about one-quarter of them. It can present as lupus erythematosus-specific or lupus erythematosus-nonspecific manifestations. Lupus erythematosus-specific lesions tend to be induced or aggravated by exposure to ultraviolet radiation and are localized in sun-exposed sites such as the face, neck, V of the neck and upper back.
Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)
The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) was developed in an attempt to classify the severity of cutaneous lupus erythematosus 32. A score of activity and damage due to the disease is calculated in each of 12 anatomical locations 32.
The total activity score is made up of:
- The degree of redness (0–3) and scale (0–2)
- Mucous membrane involvement (0–1)
- Recent hair loss (0–1), nonscarring alopecia (0–3)
Total damage score is made up of:
- The degree of dyspigmentation (0–2), and scarring (0–2)
- Persistence of dyspigmentation more than 12 months doubles the dyspigmentation score
- Scalp scarring (0,3,4,5,6)
Specific cutaneous SLE
Cutaneous lupus erythematosus (CLE) has specific acute, subacute and chronic manifestations.
- Typically, SLE presents with acute cutaneous lupus erythematosus (CLE).
- About half of patients with subacute cutaneous lupus erythematosus develop mild SLE
- Only 5% of patients with chronic cutaneous lupus erythematosus (CLE) have SLE, as cutaneous lupus erythematosus (CLE) presents as a skin problem without the involvement of other organs.
Acute cutaneous lupus erythematosus
- Central face malar or “butterfly” violaceous erythema with a sharp cutoff at lateral margins, resolves without scarring (may result in persistent telangiectasia)
- Bullous systemic lupus erythematosus: a blistering rash, if severe, this may resemble toxic epidermal necrolysis
- A maculopapular rash resembling morbilliform drug eruption
- Mucosal erosions and ulcerations (lips, nose, mouth, genitals)
- Photosensitivity: lupus rashes are mainly on sun-exposed sites. Photosensitivity can be mild to very severe with the rash appearing after minimal light exposure.
- Diffuse hair loss (nonscarring alopecia) with brittle hair shafts
Subacute cutaneous lupus erythematosus
- Flat, scaly patches resembling psoriasis, often in a network pattern
- Annular (ring-shaped) polycyclic (overlapping circular) lesions
- Lesions resolve with minimal scarring
- Affects trunk and arms
- Flares on exposure to the sun, but usually spares face and hands
Chronic cutaneous lupus erythematosus
- Chronic cutaneous lupus erythematosus (CLE) affects 25% of patients with SLE
- Classic discoid lupus is most common: indurated hyperpigmented plaques
- Localized (above the neck in 80%) or generalized (above and below the neck in 20%)
- Hypertrophic (warty) lupus
- Tumid lupus
- Lupus panniculitis/profundus
- Mucosal lupus (lips, nose, mouth, genitals)
- Chilblain lupus erythematosus
- Discoid lupus/lichen planus overlap
- Discoid lesions and panniculitis resolve with scarring
Nonspecific cutaneous SLE
Nonspecific cutaneous SLE refers to features relating to underlying illness rather than an autoimmune attack. These features may occur in other connective tissue and autoimmune diseases.
- Nail fold capillary telangiectasia
- Raynaud phenomenon (white fingers and toes on exposure to the cold)
- Vasculopathy of tips of digits (occlusion of small blood vessels by thrombus)
- Diffuse hair thinning without brittle hair
- Urticaria, which may be neutrophilic on biopsy
- Cutaneous vasculitis: palpable purpura or urticarial vasculitis
- Livedo reticularis (a network pattern of blood vessels) in 20–30% patients with SLE
- Papular mucinosis (deposits of mucin in the skin)
- Calcinosis cutis (deposits of calcium in the skin)
Systemic lupus erythematosus complications
Complications in patients with SLE may occur either due to organ damage by the disease or due to the adverse effects of the medications.
Inflammation caused by systemic lupus erythematosus (SLE) can affect many areas of your body, including your:
- Kidneys. Lupus can cause serious kidney damage (lupus nephritis) and kidney failure is one of the leading causes of death among people with lupus.
- Brain and central nervous system. If your brain is affected by lupus, you may experience headaches, dizziness, behavior changes, vision problems, and even strokes or seizures. Many people with lupus experience memory problems and may have difficulty expressing their thoughts.
- Blood and blood vessels. Lupus may lead to blood problems, including a reduced number of healthy red blood cells (anemia) and an increased risk of bleeding or blood clotting. It can also cause inflammation of the blood vessels (vasculitis).
- Lungs. Having lupus increases your chances of developing an inflammation of the chest cavity lining, which can make breathing painful. Bleeding into lungs and pneumonia also are possible.
- Heart. Lupus can cause inflammation of your heart muscle (myocarditis), your arteries (arteritis) or heart membrane (pericarditis). The risk of cardiovascular disease and heart attacks increases greatly as well.
Having systemic lupus erythematosus also increases your risk of:
- Infection. People with lupus are more vulnerable to infection because both the disease and its treatments can weaken the immune system.
- Cancer. Having lupus appears to increase your risk of cancer; however, the risk is small.
- Bone tissue death (osteonecrosis). This occurs when the blood supply to a bone declines, often leading to tiny breaks in the bone and eventually to the bone’s collapse.
- Pregnancy complications. Women with lupus have an increased risk of miscarriage. Lupus increases the risk of high blood pressure during pregnancy (preeclampsia) and preterm birth. To reduce the risk of these complications, doctors often recommend delaying pregnancy until your disease has been under control for at least six months.
Lupus nephritis
Lupus nephritis can involve the glomeruli, interstitium, tubules, and vessels with immune complex deposition in all four compartments. The World Health Organization (WHO) classification criteria for lupus nephritis describes six classes of lupus nephritis, all with distinct pathological features and significant differences in clinical outcomes 33. This has led to a different treatment approach for each class and knowing the class of lupus nephritis before initiating treatment is vital.
- Class 1: Minimal mesangial lupus nephritis
- Class 2: Mesangial proliferative lupus nephritis
- Class 3: Focal lupus nephritis
- Class 4: Diffuse segmental or Diffuse global lupus nephritis
- Class 5: Membranous lupus nephritis
- Class 6: Advanced sclerosing lupus nephritis.
Lupus pneumonitis
Lupus pneumonitis can be seen in up to 10% of lupus patients. Interstitial pneumonitis, alveolitis, alveolar wall injury, and edema and hemorrhage are commonly seen in these patients. Immunoglobulin and complement deposition is seen in the vessel wall. Chronic interstitial lung disease can occur in up to 50% of these patients and is characterized by interstitial lymphoid aggregates and fibrosis, septal thickening, and type-2 pneumocyte hyperplasia. Medial hypertrophy and intimal fibrosis involving the pulmonary artery branches lead to pulmonary hypertension in SLE. Again, immunoglobulin and complement deposition can be seen in the vessel wall.
Heart complications
Heart pathology may include valvular involvement leading to Libman-Sacks endocarditis which is sterile verrucous endocarditis. It tends to involve the mitral valve, most commonly with vegetations seen on the forward flow side of the valve. Pathology reveals platelet thrombi, necrotic cell debris, proteinaceous deposits, and mononuclear cells. Pericarditis with fibrinous exudate is common, and pathology reveals mononuclear cells’ fibrinoid necrosis and perivascular infiltration. Myocarditis can be seen as well. SLE poses a very high risk for atherosclerotic coronary artery disease, and vasculitis, immune complex deposition in addition to corticosteroid use, and hypertension are thought to be contributory 34.
Vasculitis
Vasculitis is common in SLE, and vascular lesions may demonstrate various pathologies. Immune complex deposition with an inflammatory response is the most common lesion, although it may be seen without a significant inflammatory response. Small and large vessel necrotizing vasculitis with fibrinoid necrosis is less common but can be seen and differentiated from other vasculitides by immune complex deposition in the vessel wall. Thrombotic microangiopathy can present in patients with SLE and antiphospholipid antibody syndrome 35.
Systemic lupus erythematosus diagnosis
Systemic lupus erythematosus (SLE) can be difficult to diagnose at times because of the great variety of presentations of the disease, and the presence of similar symptoms in people that do not have the disease. Furthermore, signs and symptoms of systemic lupus erythematosus may change over time and overlap with those of many other disorders. Several attempts have been made to help clinicians reach the diagnosis, including the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria 36. In September 2019, the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) published new criteria for the classification of SLE 37. The 2019 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) criteria have a specificity of 93.4% and sensitivity of 96.1% 20. However, no one test can diagnose systemic lupus erythematosus (SLE). The combination of blood and urine tests, signs and symptoms, and physical examination findings, skin and kidney biopsy can assist in the diagnosis.
Table 2. The American College of Rheumatology and the European League Against Rheumatism Criteria for the Classification of Systemic Lupus Erythematosus
| Clinical domains and criteria [a] [b] | Weight [c] |
|---|---|
Constitutional:
| 2 |
Hematologic:
| 3 |
Neuropsychiatric:
| 2 |
Mucocutaneous:
| 2 |
Serosal:
| 5 |
Musculoskeletal:
| 6 |
Renal:
| 4 |
| Immunologic domains and criteria | |
Antiphospholipid antibodies:
| 2 |
Complement proteins:
| 3 |
SLE-specific antibodies:
| 6 |
| Total score: Classify as systemic lupus erythematosus (SLE) with a score of 10 or more if entry criterion fulfilled. | |
Footnotes:
[a] Patients are eligible for these criteria only if they have a positive ANA ≥ 1:80.
[b] Criteria do not need to occur simultaneously. Only the highest-weighted criterion score within a single domain should be used. SLE must be the most likely explanation for each criterion.
[c] Each criterion is assigned a weight of 2 to 10. If the patient’s score is 10 or more, and at least one clinical criterion is fulfilled, disease is classified as SLE.
[d] Evidence of autoimmune hemolysis (such as the presence of reticulocytosis, low haptoglobin, elevated indirect bilirubin, elevated lactate dehydrogenase) and a positive direct antiglobulin (direct Coombs) test.
[e] This criterion may be noted during physical examination or review of a photo.
[f] Joint involvement is defined as either synovitis involving ≥ 2 joints characterized by swelling or effusion or tenderness in ≥ 2 joints and at least 30 minutes of morning stiffness.
Abbreviations: ANA = antinuclear antibodies; anti-dsDNA = anti–double-stranded (ds) DNA; EULAR/ACR = European League Against Rheumatism/American College of Rheumatology; SLE = systemic lupus erythematosus.
[Source 37 ]Table 3. Definitions of SLE classification criteria (American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) criteria)
| Criteria | Definition |
|---|---|
| Antinuclear antibodies (ANA) | Antinuclear antibodies (ANA) at a titer of ≥1:80 on HEp-2 cells or an equivalent positive test at least once. Testing by immunofluorescence on HEp-2 cells or a solid phase ANA screening immunoassay with at least equivalent performance is highly recommended. |
| Fever | Temperature >38.3° Celsius. |
| Leukopenia | White blood cell count <4,000/mm³. |
| Thrombocytopenia | Platelet count <100,000/mm³. |
| Autoimmune hemolysis | Evidence of hemolysis, such as reticulocytosis, low haptoglobin, elevated indirect bilirubin, elevated LDH AND positive Coomb’s (direct antiglobulin) test. |
| Delirium | Characterized by (1) change in consciousness or level of arousal with reduced ability to focus, and (2) symptom development over hours to <2 days, and (3) symptom fluctuation throughout the day, and (4) either (4a) acute/subacute change in cognition (e.g. memory deficit or disorientation), or (4b) change in behavior, mood, or affect (e.g. restlessness, reversal of sleep/wake cycle). |
| Psychosis | Characterized by (1) delusions and/or hallucinations without insight and (2) absence of delirium. |
| Seizure | Primary generalized seizure or partial/focal seizure. |
| Non-scarring alopecia | Non-scarring alopecia observed by a clinician*. |
| Oral ulcers | Oral ulcers observed by a clinician*. |
| Subacute cutaneous or discoid lupus | Subacute cutaneous lupus erythematosus observed by a clinician*: Annular or papulosquamous (psoriasiform) cutaneous eruption, usually photodistributed. Discoid lupus erythematosus observed by a clinician*: Erythematous-violaceous cutaneous lesions with secondary changes of atrophic scarring, dyspigmentation, often follicular hyperkeratosis/ plugging (scalp), leading to scarring alopecia on the scalp. If skin biopsy is performed, typical changes must be present. Subacute cutaneous lupus: interface vacuolar dermatitis consisting of a perivascular lymphohistiocytic infiltrate, often with dermal mucin noted. Discoid lupus: interface vacuolar dermatitis consisting of a perivascular and/or periappendageal lymphohistiocytic infiltrate. In the scalp, follicular keratin plugs may be seen. In longstanding lesions, mucin deposition and basement membrane thickening may be noted. |
| Acute cutaneous lupus | Malar rash or generalized maculopapular rash observed by a clinician*. If skin biopsy is performed, typical changes must be present (Acute cutaneous lupus: interface vacuolar dermatitis consisting of a perivascular lymphohistiocytic infiltrate, often with dermal mucin noted. Perivascular neutrophilic infiltrate may be present early in the course. |
| Pleural or pericardial effusion | Imaging evidence (such as ultrasound, x-ray, CT scan, MRI) of pleural or pericardial effusion, or both. |
| Acute pericarditis | ≥2 of (1) pericardial chest pain (typically sharp, worse with inspiration, improved by leaning forward), (2) pericardial rub, (3) EKG with new widespread ST-elevation or PR depression, (4) new or worsened pericardial effusion on imaging (such as ultrasound, x-ray, CT scan, MRI). |
| Joint involvement | EITHER (1) synovitis involving 2 or more joints characterized by swelling or effusion OR (2) tenderness in 2 or more joints and at least 30 minutes of morning stiffness. |
| Proteinuria >0.5g/24 hours | Proteinuria >0.5g/24h by 24 hour urine or equivalent spot urine protein-to-creatinine ratio. |
| Class II or V lupus nephritis on renal biopsy according to International Society of Nephrology/Renal Pathology Society 2003 classification. | Class II: Mesangial proliferative lupus nephritis: Purely mesangial hypercellularity of any degree or mesangial matrix expansion by light microscopy, with mesangial immune deposit. A few isolated subepithelial or subendothelial deposits may be visible by immune-fluorescence or electron microscopy, but not by light microscopy. Class V: Membranous lupus nephritis: Global or segmental subepithelial immune deposits or their morphologic sequelae by light microscopy and by immunofluorescence or electron microscopy, with or without mesangial alterations. |
| Class III or IV lupus nephritis on renal biopsy according to International Society of Nephrology/Renal Pathology Society 2003. | Class III: Focal lupus nephritis: Active or inactive focal, segmental or global endo- or extracapillary glomerulonephritis involving <50% of all glomeruli, typically with focal subendothelial immune deposits, with or without mesangial alterations. Class IV: Diffuse lupus nephritis: Active or inactive diffuse, segmental or global endo- or extracapillary glomerulonephritis involving ≥50% of all glomeruli, typically with diffuse subendothelial immune deposits, with or without mesangial alterations. This class includes cases with diffuse wire loop deposits but with little or no glomerular proliferation. |
| Positive anti-phospholipid antibodies | Anti-Cardiolipin antibodies (IgA, IgG, or IgM) at medium or high titer (>40 APL, GPL or MPL, or >the 99th percentile) or positive anti-β2GP1 antibodies (IgA, IgG, or IgM) or positive lupus anticoagulant. |
| Low C3 OR low C4 | C3 OR C4 below the lower limit of normal. |
| Low C3 AND low C4 | Both C3 AND C4 below their lower limits of normal. |
| Anti-dsDNA antibodies OR Anti-Smith (Sm) antibodies. | Anti-dsDNA antibodies in an immunoassay with demonstrated ≥ 90% specificity for SLE against relevant disease controls OR Anti-Smith (Sm) antibodies. |
Footnotes: *This may include physical examination or review of a photograph.
Abbreviations: ANA = antinuclear antibodies; anti-dsDNA = anti–double-stranded (ds) DNA; EULAR/ACR = European League Against Rheumatism/American College of Rheumatology; SLE = systemic lupus erythematosus.
[Source 37 ]Systemic Lupus International Collaborating Clinics (SLICC) criteria for diagnosing systemic lupus erythematosus
Using the Systemic Lupus International Collaborating Clinics (SLICC) criteria, SLE is diagnosed if the patient has either of the following over time 36:
- Four criteria including ≥ one clinical criterion and ≥ one immunological criterion
- Biopsy-proven lupus nephritis and antinuclear antibodies (ANA) or anti-double-stranded DNA (anti-dsDNA) antibodies
The Systemic Lupus International Collaborating Clinics (SLICC) criteria depend on history, clinical examination, exclusion of other causes of the symptoms, and the results of investigations—including blood tests and biopsy of the affected tissue 36. Four of the 17 Systemic Lupus International Collaborating Clinics (SLICC) criteria relate to the skin.
Clinical criteria
- Acute or subacute cutaneous lupus
- Chronic cutaneous lupus
- Oral ulcers
- Nonscarring alopecia
- Synovitis involving 2 or more joints
- Serositis involving lungs or heart
- Renal involvement
- Neurological involvement
- Hemolytic anemia
- Leukopenia or lymphopenia
- Thrombocytopenia
Immunological criteria
- Raised antinuclear antibody (ANA) level
- A raised anti-double-stranded DNA (anti-dsDNA) antibody level
- Presence of anti-Sm
- Positive antiphospholipid antibody (lupus anticoagulant, false positive rapid plasma reagin, high-titer anticardiolipin antibody, positive anti–2-glycoprotein 1)
- Low complement levels
- Positive direct Coombs’ test.
Blood and urine tests
Blood and urine tests may include:
- Complete blood count. This test measures the number of red blood cells, white blood cells and platelets as well as the amount of hemoglobin, a protein in red blood cells. Results may indicate you have anemia, which commonly occurs in lupus. A low white blood cell or platelet count may occur in lupus as well.
- Erythrocyte sedimentation rate (ESR). Erythrocyte sedimentation rate (ESR) blood test determines the rate at which red blood cells settle to the bottom of a tube in an hour. A faster than normal rate may indicate a systemic disease, such as lupus. The sedimentation rate isn’t specific for any one disease. It may be elevated if you have lupus, an infection, another inflammatory condition or cancer.
- C-reactive protein (CRP), immunoglobulins and rheumatoid factor (RF). Markers of inflammation such as C-reactive protein may be elevated.
- Complements C3 and C4 shall be checked in patients with SLE or suspicion of SLE, and low complement levels indicate complement consumption and may correlate with disease activity. Low serum complement in SLE has been associated with urticarial vasculitis and renal disease.
- Kidney and liver assessment. Blood tests can assess how well your kidneys and liver are functioning. Lupus can affect these organs.
- Urinalysis. An examination of a sample of your urine may show an increased protein level or red blood cells in the urine, which may occur if lupus has affected your kidneys.
- Antinuclear antibody (ANA) test. A positive test for the presence of antinuclear antibody (ANA) — produced by your immune system — indicates a stimulated immune system. The fluorescent test for antinuclear antibody (ANA) is the best initial test for SLE in patients who have compatible symptoms and signs; positive ANA tests (usually in high titer: > 1:80) occur in > 98% of people with SLE. However, most people with a positive ANA do not have lupus. Positive ANA tests can also occur in rheumatoid arthritis other connective tissue disorders, autoimmune thyroid disease, cancers, and even in the general population. The false-positive rate varies from about 3% with ANA titers of 1:320 to about 30% for ANA titers of 1:40 among healthy controls. Drugs such as hydralazine, procainamide, and tumor necrosis factor-alpha antagonists can produce positive ANA results as well as a lupus-like syndrome; the ANA eventually becomes negative if the drug is stopped. If you test positive for ANA, your doctor order more specific testing such as anti-dsDNA antibodies; high titers of anti-dsDNA are highly specific for SLE but occur in < 70% of people with SLE. The ANA test is very sensitive, but it is not specific for SLE; thus, evidence of other autoantibodies is used to aid in diagnosis. They include Ro (SSA), La (SSB), Smith (Sm), ribonucleoprotein (RNP), and dsDNA. Ro is predominantly cytoplasmic; anti-Ro antibodies are occasionally present in ANA-negative SLE patients presenting with chronic cutaneous lupus. Anti-Ro is the causal antibody for neonatal lupus and congenital heart block. Anti-Sm is highly specific for SLE but, like anti-dsDNA, is not sensitive. Anti-RNP occurs in patients with SLE, mixed connective tissue disease, and occasionally other systemic autoimmune disorders and systemic sclerosis.
- Antibodies to deoxyribonucleic acid (DNA) can be primarily divided into two groups: those reactive with denatured, single-stranded DNA (anti-ssDNA) and those identifying native, double-stranded DNA (anti-dsDNA). Notably, anti-ssDNA (single-stranded DNA) antibodies are considered non-specific and may be seen either as a laboratory error or in the healthy population. Anti-double-stranded deoxyribonucleic acid (anti-dsDNA) antibodies have more than 95% specificity for SLE but are found in only about 60% to 70% of SLE patients. Thus a negative anti dsDNA does not rule out the diagnosis of SLE. The Farr radioimmunoassay test is considered the gold standard for detecting anti-dsDNA antibodies, although it is not frequently used. ELISA tests are available, but they have a high risk of giving a false positive test. The immunofluorescence test by using the Crithidia luciliae method can confirm the presence of anti-Ds-DNA antibodies. Anti-dsDNA antibodies can also be seen in drug-induced lupus, primarily secondary to anti-TNF agents and interferon-alpha. Rarely low titers of anti-dsDNA antibodies have been reported in rheumatoid arthritis and Sjogren syndrome. In SLE, anti-dsDNA antibodies can correlate with disease activity and the development of lupus nephritis. However, this may not always be true as some patients have elevated anti-dsDNA antibodies in the setting of minimally active or inactive lupus.
- Anti-Ro (also called anti-SSA) and anti-La (also called anti-SSB) antibodies target ribonucleoprotein particles. Anti-Ro and Anti-La antibodies are seen in up to 90% of cases of Sjögren syndrome but can be seen in SLE as well (anti-Ro in up to 50% and anti-La in up to 20% of the cases). In SLE, they may be associated with secondary Sjogren syndrome and keratoconjunctivitis sicca, subacute cutaneous lupus, photosensitivity, congenital heart block, and neonatal lupus 38.
- Anti-Smith antibodies are seen in less than 30% of SLE patients but have 99% specificity for SLE. They are observed more in African-American patients with SLE. Anti-Smith antibodies in SLE are usually always associated with Anti-U1-RNP antibodies, which are present in up to 30% of SLE patients. Anti-U1-RNP antibodies can also be seen in mixed connective tissue disease (MCTD), although in MCTD, a disorder that is closely related to SLE. Anti-ribosomal-P antibodies are very specific for SLE, although their prevalence in SLE is less than 5%, and they may correlate with neuropsychiatric manifestations of SLE. Anti-histone antibodies are not specific for drug-induced lupus and can be seen in 50% to 70% of cases of SLE. Anti-centromere and anti-topoisomerase-I (SCL70) antibodies are seen in systemic sclerosis and rarely in SLE (less than 5%). Anti-histidyl-tRNA-synthetase antibodies are seen in myositis. Patients with SLE may also have antiphospholipid antibodies (lupus anticoagulants, anti-cardiolipin, and anti-beta-2-glycoprotein I antibodies) and are associated with more thrombotic events and adverse pregnancy-related outcomes.
- Anti Ro/La is also associated with and risk of neonatal lupus erythematosus.
- Antiphospholipid antibodies are associated with livedo reticularis, thrombosis and pregnancy complications (antiphospholipid syndrome).
- Anti-annexin 1 antibodies may be a diagnostic marker for discoid cutaneous lupus erythematosus
Imaging tests
If your doctor suspects that lupus is affecting your lungs or heart, he or she may suggest:
- Chest X-ray. An image of your chest may reveal abnormal shadows that suggest fluid or inflammation in your lungs.
- Echocardiogram. This test uses sound waves to produce real-time images of your beating heart. It can check for problems with your valves and other portions of your heart.
Biopsy
Systemic lupus erythematosus can harm your kidneys in many different ways, and treatments can vary, depending on the type of damage that occurs. In some cases, it’s necessary to test a small sample of kidney tissue (kidney biopsy) to determine what the best treatment might be. The sample can be obtained with a needle or through a small incision.
Skin biopsy is sometimes performed to confirm a diagnosis of lupus affecting the skin (cutaneous lupus erythematosus).
- Acute cutaneous lupus erythematosus: nonspecific dermatitis.
- Subacute cutaneous lupus erythematosus: features of lupus noted in the epidermis and superficial dermis
- Chronic discoid cutaneous lupus erythematosus: typical features of lupus with atrophy and scarring
- Direct immunofluorescence is positive in sun-protected healthy skin in SLE.
Photoprovocation tests
Photoprovocation tests are sometimes carried out to confirm that a skin eruption is precipitated by exposure to particular wavelengths of ultraviolet or visible radiation.
Other tests
Other tests depend on which organ is affected. They may, for example, include:
- Urine tests for hyaline casts, creatinine, protein and blood
- Blood pressure
- Ultrasound, CT and MRI scans
- Electrocardiograph (ECG) and echocardiography
- Nerve and muscle testing
- Ophthalmological examination
- Endoscopy of the gastrointestinal tract.
Systemic lupus erythematosus differential diagnosis
Systemic lupus erythematosus is a systemic disease with multiorgan involvement, and several other diseases can mimic SLE.
Other autoimmune diseases
- Rheumatoid arthritis (RA) can present with several extra-articular manifestations in addition to the classic polyarticular inflammatory arthritis and may be difficult to differentiate from SLE. Positive ANA, Anti-Ro, and Anti-La can also be seen in rheumatoid arthritis, although other SLE-specific autoantibodies and hypocomplementemia are rare. SLE can be associated with a positive rheumatoid factor, but the Anti-CCP is negative in SLE
- Drug-induced lupus may be difficult to differentiate from SLE, especially due to a significant overlap in the clinical and serological features. Drug-induced lupus is characterized by the resolution of symptoms after drug withdrawal and lack of more severe manifestations, although the autoantibodies may remain positive for several years.
- Adult-onset Still disease characterized by arthralgia, fever, lymphadenopathy, and splenomegaly but no malar rash or other organ manifestations and lacks the SLE specific autoantibodies.
- Behcet disease presents with aphthous ulcers, uveitis, and arthralgia but lacks the other systemic and serological features of SLE.
- Sarcoidosis presents with fever, cough, dyspnea, fatigue, night sweats, rash, and uveitis. It shows non-caseating granuloma on chest radiography and bilateral adenopathy, which is rarely present in SLE 39.
Infections
- Several viral infections can mimic SLE. Parvovirus B19 infection can cause fever, rash, inflammatory arthritis, and cytopenias. ANA and rheumatoid factor have been reported. Hepatitis B and C can be associated with arthralgia/inflammatory arthritis and positive ANA and rheumatoid factor. Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections can cause fever, fatigue, cytopenias, and transaminitis. Human immunodeficiency virus (HIV) can cause fever, fatigue, oral ulcers, and cytopenias. More specific autoantibodies and systemic manifestations of SLE are absent in these viral infections. Further, positive viral serologies may help make the right diagnosis.
- Infectious endocarditis characterized by fever, arterial emboli, arthralgia, myalgia, and a heart murmur; may be confused with cardiac manifestations of SLE but can be differentiated by the absence of specific SLE associated autoantibodies and positive blood cultures 40.
Cancers
- Lymphomas, especially non-Hodgkins lymphoma, can present with fatigue, weight loss, fever, arthralgia, cytopenia, lymphadenopathy, and a positive ANA. The more specific SLE-associated autoantibodies are absent. In elderly patients presenting with lupus-like symptoms, malignancy shall be ruled out by cancer screening.
Systemic lupus erythematosus treatment
Treatment for lupus depends on your signs and symptoms. Determining whether you should be treated and what medications to use requires a careful discussion of the benefits and risks with your doctor.
As your signs and symptoms flare and subside, you and your doctor may find that you’ll need to change medications or dosages. The medications most commonly used to control lupus include:
- Nonsteroidal anti-inflammatory drugs (NSAIDs). Over-the-counter NSAIDs, such as naproxen sodium (Aleve) and ibuprofen (Advil, Motrin IB, others), may be used to treat pain, swelling and fever associated with lupus. Stronger NSAIDs are available by prescription. Side effects of NSAIDs may include stomach bleeding, kidney problems and an increased risk of heart problems.
- Antimalarial drugs. Medications commonly used to treat malaria, such as hydroxychloroquine (Plaquenil), affect the immune system and can help decrease the risk of lupus flares. Hydroxychloroquine is indicated for all patients with SLE regardless of disease severity because it decreases disease flares and decreases mortality 41; however, hydroxychloroquine is not used in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency because it can cause hemolysis. Side effects can include stomach upset and, very rarely, damage to the retina of the eye. Regular eye exams are recommended when taking these medications.
- Corticosteroids. Prednisone and other types of corticosteroids can counter the inflammation of lupus. High doses of steroids such as methylprednisolone (Medrol) are often used to control serious disease that involves the kidneys and brain. Side effects include weight gain, easy bruising, thinning bones, high blood pressure, diabetes and increased risk of infection. The risk of side effects increases with higher doses and longer term therapy.
- Immunosuppressants. Drugs that suppress the immune system may be helpful in severe cases of lupus, when lupus affects major organs and other treatments do not work. Examples include azathioprine (Imuran, Azasan), mycophenolate (Cellcept), methotrexate (Trexall, Xatmep, others), cyclosporine (Sandimmune, Neoral, Gengraf) and leflunomide (Arava). Potential side effects may include an increased risk of infection, liver damage, decreased fertility and an increased risk of cancer.
- Biologics. Biological therapies limit the amount of abnormal B cells (cells in the immune system that create antibodies) found in people with lupus. Belimumab (Benlysta) is a class of medications called monoclonal antibodies. It works by blocking the activity of a certain protein in people with SLE and lupus nephritis. Belimumab administered intravenously reduces lupus symptoms in some people. Belimumab is also used with other medications to treat lupus nephritis (an autoimmune disease in which the immune system attacks the kidneys) in adults. Side effects include nausea, diarrhea and infections. Rarely, worsening of depression can occur. Rituximab (Rituxan, Truxima) may be beneficial for some people in whom other medications haven’t helped. Side effects include allergic reaction to the intravenous infusion and infections.
- Other medicines. You may need other medicines to treat illnesses or diseases that are linked to your lupus — such as high blood pressure or osteoporosis. Many people with lupus are also at risk for blood clots, which can cause a stroke or heart attack. Your doctor may prescribe anticoagulants (“blood thinners”), such as warfarin or heparin, to prevent your blood from clotting too easily. You cannot take warfarin during pregnancy.
In clinical trials, voclosporin has been shown to be effective in treating lupus.
Other potential drugs to treat lupus are currently being studied, including abatacept (Orencia), anifrolumab and others.
Cutaneous lupus erythematosus
Mild cutaneous lupus erythematosus can usually be treated with topical corticosteroids or topical calcineurin inhibitors such as tacrolimus. Hydroxychloroquine is the drug of choice for most cutaneous manifestations and is very efficacious. Quinacrine can be used if intolerance or adverse effects of hydroxychloroquine. Methotrexate can be used if no response to hydroxychloroquine. For severe or resistant disease, systemic corticosteroids, mycophenolate mofetil (dual-benefit with underlying lupus nephritis), and belimumab can be considered. Other alternatives include thalidomide, cyclophosphamide, intravenous immunoglobulin (IVIG), dapsone, azathioprine, and rituximab 42.
Musculoskeletal signs and symptoms
Hydroxychloroquine is the initial drug of choice for lupus arthritis. If no response, methotrexate or leflunomide can be considered. Belimumab and rituximab can be considered in refractory cases 43.
Hematological signs and symptoms
Drug-induced cytopenias shall be excluded. Mild cytopenias usually require no treatment. For moderate to severe cytopenias, corticosteroids are the mainstay of treatment, and azathioprine or cyclosporine-A can be used as a steroid-sparing agent. Severe refractory cytopenias may require intravenous pulse dose steroids, mycophenolate mofetil, rituximab, cyclophosphamide, plasmapheresis, recombinant G-CSF, or splenectomy 40.
Cardiopulmonary complications
Serositis usually responds to nonsteroidal anti-inflammatory drugs (NSAIDs) or moderate to high dose oral corticosteroids. Hydroxychloroquine and methotrexate can be considered as steroid-sparing agents. Acute lupus pneumonitis requires high dose IV pulse corticosteroids, while plasmaphereses and/or cyclophosphamide may be needed if the diffuse alveolar hemorrhage is present. Interstitial lung disease can e managed by low to moderate dose corticosteroids with immunosuppressive agents such as azathioprine or mycophenolate mofetil. Pulmonary arterial hypertension requires vasodilator therapy, while thrombotic complications such as pulmonary embolism require anticoagulation. Therefore, high-dose corticosteroids are necessary to manage myocarditis and coronary arteritis.
SLE affecting the brain
Accurate diagnosis and ruling out other potential causes is critical before initiating treatment for neuropsychiatric manifestations of SLE. High-dose corticosteroids with immunosuppressive agents such as cyclophosphamide, azathioprine, or rituximab are used for inflammation-related neuropsychiatric manifestations such as optic neuritis, aseptic meningitis, demyelinating disease, etc. Lifelong warfarin is indicated in cases of thromboembolic CNS events associated with antiphospholipid antibody syndrome. High-dose corticosteroids can be used in cognitive impairment, although there is no robust data on this.
Lupus nephritis
Lupus nephritis shall be confirmed with a biopsy, which confirms the diagnosis, rules out other causes and helps to classify the disease. Class 1 and 2 lupus nephritis shall be treated with the Renin-angiotensin-aldosterone system blockade. Immunosuppression with high-dose corticosteroids followed by azathioprine is indicated only if proteinuria is more than 1 gram/day. Membranous lupus nephritis (class 5) shall also be treated with the Renin-angiotensin-aldosterone system blockade. If proteinuria of more than 1 gram/day is present (which is frequent in Class 5 lupus nephritis), induction therapy with high dose corticosteroids and azathioprine (mild disease) or tacrolimus/cyclosporine-A/mycophenolate mofetil/IV cyclophosphamide (moderate to severe disease) followed by maintenance therapy with azathioprine, mycophenolate mofetil, cyclosporine-A or tacrolimus shall be used.
Corticosteroids shall be gradually tapered during maintenance therapy. Proliferative lupus nephritis (class 3 or 4) requires more aggressive therapy. Induction therapy is with IV pulse dose methylprednisolone followed by high dose oral steroids combined with mycophenolate mofetil, IV cyclophosphamide, or azathioprine (only in mild disease in whites). Maintenance therapy with mycophenolate mofetil or azathioprine shall be continued for at least three years. IV pulse cyclophosphamide for one year can be considered maintenance therapy for severe disease. Lupus nephritis patients need very close monitoring of their renal function and proteinuria in addition to other SLE disease activity markers. Flares and incomplete remission are common. Renal replacement therapy and transplant may be needed in some patients 30.
Maintenance therapy
Chronic disease should be treated with the lowest dose of corticosteroids (eg, oral prednisone ≤ 7.5 mg once a day or its equivalent) and other drugs that control inflammation (eg, antimalarials, immunosuppressants [mycophenolate mofetil or azathioprine]) to maintain remission 44. Treatment should be guided by clinical features primarily, although anti-dsDNA antibody titers or serum complement levels may be followed, particularly if they have correlated with disease activity in the past. However, anti-dsDNA antibody titers or serum complement levels may not parallel nonrenal disease flares. Other pertinent blood and urine tests may be used to assess specific organ involvement.
Calcium, vitamin D, and bisphosphonate therapy (for the prevention of osteoporosis) should be considered in patients taking corticosteroids long term.
Systemic lupus erythematosus lifestyle and home remedies
Simple measures can help you prevent systemic lupus erythematosus (SLE) flares and, should they occur, better cope with the signs and symptoms you experience. Try to:
- See your doctor regularly. Having regular checkups instead of only seeing your doctor when your symptoms worsen may help your doctor prevent flares, and can be useful in addressing routine health concerns, such as stress, diet and exercise that can be helpful in preventing systemic lupus erythematosus (SLE) complications.
- Be sun smart and avoid intense sun exposure. Because ultraviolet (UV) light can trigger a flare, wear protective clothing — such as a hat, long-sleeved shirt and long pants — and use broad-spectrum (UV-A and UV-B) suncreens with a sun protection factor (SPF) of at least 50+ every time you go outside.
- Get regular exercise. Exercise can help keep your bones strong, reduce your risk of heart attack and promote general well-being.
- Don’t smoke. Smoking increases your risk of cardiovascular disease and can worsen the effects of systemic lupus erythematosus (SLE) on your heart and blood vessels.
- Eat a healthy diet. According to the Dietary Guidelines for Americans 2020–2025 45, a healthy diet:
- Emphasizes fruits, vegetables, whole grains, and fat-free or low-fat milk and milk products
- Includes a variety of protein foods such as seafood, lean meats and poultry, eggs, legumes (beans and peas), soy products, nuts, and seeds.
- Is low in added sugars, sodium, saturated fats, trans fats, and cholesterol.
- Stays within your daily calorie needs. Sometimes you may have dietary restrictions, especially if you have high blood pressure, kidney damage or gastrointestinal problems.
- Reduce stress.
- Ask your doctor if you need vitamin D and calcium supplements. There is some evidence to suggest that people with systemic lupus erythematosus (SLE) may benefit from supplemental vitamin D. A calcium supplement can help you meet the daily recommended dietary allowance of 1,000 milligrams to 1,200 milligrams — depending on your age — to help keep your bones healthy.
Systemic lupus erythematosus diet
You may have to change what you eat based on your symptoms or treatment plan. Ask your doctor if you need to eat special foods or limit other foods because of your systemic lupus erythematosus (SLE) 46.
- If you develop hyperlipidemia (high level of fats in the blood) because of your systemic lupus erythematosus (SLE), you may need to follow a low-fat eating plan.
- If steroids and other medicines cause you to gain weight, you may want to follow a low-calorie eating plan.
- Because people with systemic lupus erythematosus (SLE) need to avoid the sun, you may lack vitamin D 47. Your doctor may advise you to take vitamin D.
Alternative medicine
Sometimes people with systemic lupus erythematosus seek alternative or complementary medicine. There aren’t any alternative therapies that have been shown to alter the course of lupus, although some may help ease symptoms of the disease. Discuss these treatments with your doctor before initiating them on your own. Your doctor can help you weigh the benefits and risks and tell you if the treatments will interfere adversely with your current lupus medications.
Complementary and alternative treatments for systemic lupus erythematosus include:
- Dehydroepiandrosterone (DHEA). Taking supplements containing this hormone along with conventional treatment may help reduce lupus flares. DHEA may lead to acne in women.
- Fish oil. Fish oil supplements contain omega-3 fatty acids that may be beneficial for people with lupus. Preliminary studies have found some promise, though more study is needed. Side effects of fish oil supplements can include nausea, belching and a fishy taste in the mouth.
- Acupuncture. This therapy uses tiny needles inserted just under the skin. It may help ease the muscle pain associated with lupus.
Systemic lupus erythematosus prognosis
Systemic lupus erythematosus course is usually chronic, relapsing, and unpredictable. Remissions may last for years. If the initial acute phase is controlled, even if very severe (e.g, with cerebral thrombosis or severe nephritis), the long-term prognosis is usually good. Systemic lupus erythematosus disease course is milder and the survival rate higher in persons with isolated skin and musculoskeletal involvement than in those with kidney disease 48 and central nervous system (brain and spinal cord) disease 49. The 10-year survival of systemic lupus erythematosus in most developed countries is > 95%. Ten-year survival rates in Asia and Africa are significantly lower than those in the United States, ranging from 60-70% 50, 51. However, that may reflect detection bias of severe cases only. Improved prognosis is in part due to earlier diagnosis and more effective therapies. Complications include infection from immunosuppression or osteoporosis from long-term corticosteroid use. Increased risk of coronary artery disease can contribute to premature death.
Systemic lupus erythematosus life expectancy
Although historically, systemic lupus erythematosus was associated with a reduced life expectancy, mortality in patients with SLE has decreased over the past few decades 52. Prior to 1955, the 5-year survival rate in SLE was less than 50%; currently, the average 10-year survival rate exceeds 90% 53, 49 and the 15-year survival rate is approximately 80% 54. Previously, mortality was due to the disease itself; currently, mortality is often a result of medication side effects (eg, fatal infections in individuals receiving potent immunosuppressive medications) or cardiovascular events. Leading causes of death include cardiovascular disease, infections, and renal disease. Early diagnosis with therapy to prevent organ damage, monitoring and screening patients for cardiovascular disease and infections with early intervention may improve these outcomes.
According to the Centers for Disease Control and Prevention (CDC), however, 35% of SLE-related deaths in the United States occur in patients younger than 45 years, making this a serious issue despite declining overall mortality rates 55.
Poor prognostic factors in SLE include African American ethnicity, renal disease (especially diffuse proliferative glomerulonephritis), male sex, young age, older age at presentation, hypertension, low socioeconomic status, antiphospholipid antibody syndrome, presence of antiphospholipid antibodies, and high overall disease activity 56.
The European League Against Rheumatism (EULAR) task force also identified the following comorbidities as increasing the risk of morbidity and mortality in patients with SLE 44:
- Infections
- Hypertension
- Lipid disorders (dyslipidemia), atherosclerosis, and coronary heart disease
- Diabetes mellitus
- Bone-related conditions: Osteoporosis; avascular bone necrosis
- Cancers (eg, non-Hodgkin lymphoma, lung cancer, hepatobiliary cancer).
Late deaths (after age 35 years) are generally from myocardial infarction or stroke secondary to accelerated atherosclerosis 57, 58. Inflammation is central to SLE pathogenesis and plays a major role in the development and accelerated progression of atherosclerosis. Manzi et al 57 reported that women aged 35-44 years with SLE were 50 times more likely to develop myocardial ischemia than healthy Framingham study control women. The presence of lupus nephritis may increase these risks 59. The presence of traditional and nontraditional risk factors increases the risk of cardiovascular (CVD) disease in patients with SLE.
In a study by Petri et al 60 that evaluated a large sample of SLE patients, the investigators reported that more than 50% of these patients had at least 3 classic cardiac risk factors, with the most common ones being a sedentary lifestyle, obesity, and hypercholesterolemia. In another study, Salmon et al 61 found that nontraditional cardiovascular disease risk factors in SLE patients included having higher homocysteine levels, kidney impairment, enhanced LDL oxidation, and chronic inflammation.
Causes of accelerated coronary artery disease in persons with SLE are likely multifactorial. They include endothelial dysfunction, inflammatory mediators, corticosteroid-induced atherogenesis, and dyslipidemia.
The influence of race on prognosis has been widely debated. The LUMINA study group reported that high disease activity and poverty predicted higher mortality in Black and Hispanic patients with lupus 62. In the Michigan Lupus Epidemiology and Surveillance program, the proportion of patients with kidney disease was 2.2-fold higher, and that of progression to end-stage kidney disease was 3.4-fold higher, in Black patients than in White patients 63. A 2022 study 64 reported that patients from the Black racial group faced 19-fold higher risk of cardiovascular disease, with peak events occurring in the second year of lupus diagnosis. This study highlighted discoid lupus as another unique predictor of cardiovascular disease in patients with SLE 64.
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008 Feb 28;358(9):929-39. doi: 10.1056/NEJMra071297[↩]
- Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol. 2013 Nov;9(11):687-94. doi: 10.1038/nrrheum.2013.103[↩]
- Lupus. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Lupus[↩]
- Jarukitsopa S, Hoganson DD, Crowson CS, Sokumbi O, Davis MD, Michet CJ Jr, Matteson EL, Maradit Kremers H, Chowdhary VR. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken). 2015 May;67(6):817-28. doi: 10.1002/acr.22502[↩][↩]
- Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev. 2017 Mar;16(3):258-268. doi: 10.1016/j.autrev.2017.01.007[↩]
- Feldman CH, Costenbader KH. Epidemiology and classification of systemic lupus erythematosus. Chapter 133. In: Hochberg MC, Gravallese EM, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors(s). Rheumatology. Seventh edition. Philadelphia PA, USA: Elsevier, 2019:1091-5.[↩]
- Frances Rees, Michael Doherty, Matthew J Grainge, Peter Lanyon, Weiya Zhang, The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies, Rheumatology, Volume 56, Issue 11, November 2017, Pages 1945–1961, https://doi.org/10.1093/rheumatology/kex260[↩][↩]
- Yacoub Wasef SZ. Gender differences in systemic lupus erythematosus. Gend Med. 2004 Aug;1(1):12-7. doi: 10.1016/s1550-8579(04)80006-8[↩]
- Hannon CW, McCourt C, Lima HC, Chen S, Bennett C. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021 Mar 9;3(3):CD007478. doi: 10.1002/14651858.CD007478.pub2[↩]
- Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016 Apr-Jun;52(2):205-12. doi: 10.4415/ANN_16_02_12[↩]
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365-81. doi: 10.2165/11310780-000000000-00000[↩]
- Yazdany J, Dall’Era M. Definition and Classification of Lupus and Lupus Related Disorders. Chapter 2. In: Wallace D, Hahn BH, editors(s). Dubois’ Lupus Erythematosus. Ninth edition. Elsevier Inc, 2019:15-22.[↩]
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous Manifestations of “Lupus”: Systemic Lupus Erythematosus and Beyond. Int J Rheumatol. 2021 May 18;2021:6610509. doi: 10.1155/2021/6610509[↩]
- Richardson BC. Drug-Induced Lupus Erythematosus. Chapter 31. In: Wallace DJ, Hahn BH, editors(s). Dubois’ Lupus Erythematosus and Related Syndromes. Ninth edition. Elsevier Inc, 2019:377-388.[↩]
- Miliaresis C, Izmirly PM, Buyon JP, Phoon CK, Friedman D. Neonatal Lupus Erythematosus: Pathogenesis and Clinical Approaches. Chapter 39. In: Wallace DJ, Hahn BH, editors(s). Dubois’ Lupus Erythematosus and Related Syndromes. Ninth edition. Elsevier Inc, 2019:486-98.[↩]
- Smith EMD, Lythgoe H, Hedrich CM. Vasculitis in Juvenile-Onset Systemic Lupus Erythematosus. Front Pediatr. 2019 May 9;7:149. doi: 10.3389/fped.2019.00149[↩]
- Livingston B, Bonner A, Pope J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus. 2011 Nov;20(13):1345-55. doi: 10.1177/0961203311416694[↩]
- Christou EAA, Banos A, Kosmara D, Bertsias GK, Boumpas DT. Sexual dimorphism in SLE: above and beyond sex hormones. Lupus. (2019) 28:3–10. 10.1177/0961203318815768[↩]
- Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:1983–9. 10.1136/annrheumdis-2014-205375[↩]
- Justiz Vaillant AA, Goyal A, Varacallo M. Systemic Lupus Erythematosus. [Updated 2022 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535405[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kim JW, Kim HA, Suh CH, Jung JY. Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus. Front Med (Lausanne). 2022 Aug 11;9:906475. doi: 10.3389/fmed.2022.906475[↩]
- Trends in Deaths from Systemic Lupus Erythematosus — United States, 1979–1998. MMWR;51(17):371–374. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5117a3.htm[↩]
- Zeller CB, Appenzeller S. Cardiovascular disease in systemic lupus erythematosus: the role of traditional and lupus related risk factors. Curr Cardiol Rev. 2008 May;4(2):116-22. doi: 10.2174/157340308784245775[↩]
- Pregnancy and Rheumatic Disease. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Living-Well-with-Rheumatic-Disease/Pregnancy-Rheumatic-Disease[↩]
- Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med. (2020) 172:Itc81–96. 10.7326/AITC202006020[↩]
- Losada-García A, Cortés-Ramírez SA, Cruz-Burgos M, Morales-Pacheco M, Cruz-Hernández CD, Gonzalez-Covarrubias V, Perez-Plascencia C, Cerbón MA, Rodríguez-Dorantes M. Hormone-Related Cancer and Autoimmune Diseases: A Complex Interplay to be Discovered. Front Genet. 2022 Jan 17;12:673180. doi: 10.3389/fgene.2021.673180[↩]
- Tayem MG, Shahin L, Shook J, Kesselman MM. A Review of Cardiac Manifestations in Patients With Systemic Lupus Erythematosus and Antiphospholipid Syndrome With Focus on Endocarditis. Cureus. 2022 Jan 28;14(1):e21698. doi: 10.7759/cureus.21698[↩][↩]
- Kwon YC, Chun S, Kim K, Mak A. Update on the Genetics of Systemic Lupus Erythematosus: Genome-Wide Association Studies and Beyond. Cells. 2019 Sep 30;8(10):1180. doi: 10.3390/cells8101180[↩]
- Selvaraja M, Too CL, Tan LK, Koay BT, Abdullah M, Shah AM, Arip M, Amin-Nordin S. Human leucocyte antigens profiling in Malay female patients with systemic lupus erythematosus: are we the same or different? Lupus Sci Med. 2022 Feb;9(1):e000554. doi: 10.1136/lupus-2021-000554[↩]
- Scheen M, Adedjouma A, Esteve E, Buob D, Abisror N, Planche V, Fain O, Boffa JJ, De Seigneux S, Mekinian A, Haidar F. Kidney disease in antiphospholipid antibody syndrome: Risk factors, pathophysiology and management. Autoimmun Rev. 2022 May;21(5):103072. doi: 10.1016/j.autrev.2022.103072[↩][↩]
- Takeshima Y, Iwasaki Y, Nakano M, Narushima Y, Ota M, Nagafuchi Y, Sumitomo S, Okamura T, Elkon K, Ishigaki K, Suzuki A, Kochi Y, Yamamoto K, Fujio K. Immune cell multiomics analysis reveals contribution of oxidative phosphorylation to B-cell functions and organ damage of lupus. Ann Rheum Dis. 2022 Jun;81(6):845-853. doi: 10.1136/annrheumdis-2021-221464[↩]
- Albrecht J, Werth VP. Clinical outcome measures for cutaneous lupus erythematosus. Lupus. 2010 Aug;19(9):1137-43. doi: 10.1177/0961203310370049[↩][↩]
- Liu T, Neuner R, Thompson A, Pottackal G, Petullo D, Liu J, Nikolov N, Sahajwalla C, Doddapaneni S, Chen J. Clinical pharmacology considerations for the approval of belimumab for the treatment of adult patients with active lupus nephritis: A regulatory perspective. Lupus. 2022 Apr;31(4):424-432. doi: 10.1177/09612033221079771[↩]
- Sinha A, Rivera AS, Chadha SA, Prasada S, Pawlowski AE, Thorp E, DeBerge M, Ramsey-Goldman R, Lee YC, Achenbach CJ, Lloyd-Jones DM, Feinstein MJ. Comparative Risk of Incident Coronary Heart Disease Across Chronic Inflammatory Diseases. Front Cardiovasc Med. 2021 Nov 10;8:757738. doi: 10.3389/fcvm.2021.757738[↩]
- Hsu T, Nguyen P, Petronic-Rosic V. A case of systemic lupus erythematosus with cutaneous granulomatous vasculitis. JAAD Case Rep. 2022 Jan 19;21:93-96. doi: 10.1016/j.jdcr.2022.01.006[↩]
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012 Aug;64(8):2677-86. doi: 10.1002/art.34473[↩][↩][↩]
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019 Sep;71(9):1400-1412. doi: 10.1002/art.40930[↩][↩][↩]
- Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J Autoimmun. 2022 Apr;128:102813. doi: 10.1016/j.jaut.2022.102813[↩]
- Sahoo RR, Wakhlu A, Agarwal V. Neglected tropical rheumatic diseases. Clin Rheumatol. 2022 May;41(5):1293-1304. doi: 10.1007/s10067-022-06090-6[↩]
- Wafa A, Hicham H, Naoufal R, Hajar K, Rachid R, Souad B, Mouna M, Zoubida MT, Mohamed A. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: a study of 20 Moroccan adult patients. Clin Rheumatol. 2022 Jul;41(7):2021-2033. doi: 10.1007/s10067-022-06055-9[↩][↩]
- Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alén J, Bastian HM, Vilá LM, Reveille JD; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007 Sep;66(9):1168-72. doi: 10.1136/ard.2006.068676[↩]
- Skudalski L, Shahriari N, Torre K, Santiago S, Bibb L, Kodomudi V, Grant-Kels JM, Lu J. Emerging therapeutics in the management of connective tissue disease. Part I. Lupus erythematosus and Sjögren syndrome. J Am Acad Dermatol. 2022 Jul;87(1):1-18. doi: 10.1016/j.jaad.2021.12.067[↩]
- Dima A, Jurcut C, Chasset F, Felten R, Arnaud L. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskelet Dis. 2022 Feb 14;14:1759720X211073001. doi: 10.1177/1759720X211073001[↩]
- Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, Houssiau F, Jayne D, Kouloumas M, Kuhn A, Larsen JL, Lerstrøm K, Moroni G, Mosca M, Schneider M, Smolen JS, Svenungsson E, Tesar V, Tincani A, Troldborg A, van Vollenhoven R, Wenzel J, Bertsias G, Boumpas DT. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019 Jun;78(6):736-745. doi: 10.1136/annrheumdis-2019-215089[↩][↩]
- Dietary Guidelines for Americans, 2020-2025. https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf[↩]
- Klack K, Bonfa E, Borba Neto EF. Diet and nutritional aspects in systemic lupus erythematosus. Rev Bras Reumatol. 2012 May-Jun;52(3):384-408. English, Portuguese.[↩]
- Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008 Sep;20(5):532-7. doi: 10.1097/BOR.0b013e32830a991b[↩]
- Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken). 2010 Jun;62(6):873-80. https://onlinelibrary.wiley.com/doi/epdf/10.1002/acr.20116[↩]
- Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore). 2006 May;85(3):147-156. doi: 10.1097/01.md.0000224709.70133.f7[↩][↩]
- Murali R, Jeyaseelan L, Rajaratnam S, John L, Ganesh A. Systemic lupus erythematosus in Indian patients: prognosis, survival and life expectancy. Natl Med J India. 1997 Jul-Aug;10(4):159-64.[↩]
- Wang F, Wang CL, Tan CT, Manivasagar M. Systemic lupus erythematosus in Malaysia: a study of 539 patients and comparison of prevalence and disease expression in different racial and gender groups. Lupus. 1997;6(3):248-53. doi: 10.1177/096120339700600306[↩]
- Ruiz-Irastorza G, Khamashta MA, Castellino G, Hughes GR. Systemic lupus erythematosus. Lancet. 2001 Mar 31;357(9261):1027-32. doi: 10.1016/S0140-6736(00)04239-2[↩]
- Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol. 2001 Sep;13(5):345-51. doi: 10.1097/00002281-200109000-00002[↩]
- Abu-Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. II. Predictor variables for mortality. J Rheumatol. 1995 Jul;22(7):1265-70.[↩]
- Systemic Lupus Erythematosus (SLE). https://www.cdc.gov/lupus/facts/detailed.html[↩]
- Kayser C, Dutra LA, Dos Reis-Neto ET, Castro CHM, Fritzler MJ, Andrade LEC. The Role of Autoantibody Testing in Modern Personalized Medicine. Clin Rev Allergy Immunol. 2022 Oct;63(2):251-288. doi: 10.1007/s12016-021-08918-6[↩]
- Susan Manzi, Elaine N Meilahn, Joan E Rairie, Claudia G. Conte, Thomas A. Medsger, Jr., Linda Jansen-McWilliams, Ralph B. D’Agostino, Lewis H. Kuller, Age-specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study, American Journal of Epidemiology, Volume 145, Issue 5, 1 March 1997, Pages 408–415, https://doi.org/10.1093/oxfordjournals.aje.a009122[↩][↩]
- Gladman DD, Urowitz MB. Prognosis, mortality and morbidity in systemic lupus erythematosus In: Wallace DJ, Hahn BH. Dubois’ lupus erythematosus. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2007:1333-53.[↩]
- Faurschou M, Mellemkjaer L, Starklint H, Kamper AL, Tarp U, Voss A, Jacobsen S. High risk of ischemic heart disease in patients with lupus nephritis. J Rheumatol. 2011 Nov;38(11):2400-5. doi: 10.3899/jrheum.110329[↩]
- Petri M, Spence D, Bone LR, Hochberg MC. Coronary artery disease risk factors in the Johns Hopkins Lupus Cohort: prevalence, recognition by patients, and preventive practices. Medicine (Baltimore). 1992 Sep;71(5):291-302. doi: 10.1097/00005792-199209000-00004[↩]
- Salmon JE, Roman MJ. Accelerated atherosclerosis in systemic lupus erythematosus: implications for patient management. Curr Opin Rheumatol. 2001 Sep;13(5):341-4. doi: 10.1097/00002281-200109000-00001[↩]
- Alarcón GS, McGwin G Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, Friedman AW, Reveille JD. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001 Apr;45(2):191-202. https://onlinelibrary.wiley.com/doi/epdf/10.1002/1529-0131%28200104%2945%3A2%3C191%3A%3AAID-ANR173%3E3.0.CO%3B2-2[↩]
- Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, Leisen J, Shaltis D, McCune WJ. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014 Feb;66(2):369-78. doi: 10.1002/art.38238[↩]
- Garg S, Bartels CM, Bao G, Helmick CG, Drenkard C, Lim SS. Timing and Predictors of Incident Cardiovascular Disease in Systemic Lupus Erythematosus: Risk Occurs Early and Highlights Racial Disparities. J Rheumatol. 2023 Jan;50(1):84-92. doi: 10.3899/jrheum.220279[↩][↩]