Contents
- What is cooking oil ?
- What are bad fats and oil ?
- Healthy cooking oil
- Types of cooking oil
- Table 3. Canola oil nutritional facts
- Table 4. Corn oil nutritional facts
- Table 5. Olive oil nutritional facts
- Table 6. Peanut oil nutritional facts
- Table 7. Rice bran oil nutritional facts
- Table 8. Safflower oil nutritional facts
- Table 9. Sesame oil nutritional facts
- Table 10. Soybean oil nutritional facts
- Table 11. Sunflower oil nutritional facts
- Cooking Oil and Deep Frying
- Figure 1. Outline of the oil degradation during frying and the consequences on the product quality
- Figure 2. Typical dynamics during deep frying fatty foods
- Oil Degradation in Frying
- Figure 3. Average oxidative stability of mainstream cooking oils based on analysis performed on standard supermarket products by ISO 17025 accredited laboratory
- Frying factors
- Vacuum frying
- Health implications of dietary oxidised oils
- Acrylamide
- How acrylamide is formed
- Potential health effects of acrylamide
- Are consumers at risk of developing cancer from acrylamide in food ?
- Are there other health risks besides cancer ?
- Foods high in acrylamide
- What can consumers do to reduce the risk from acrylamide in food ?
- What happens to acrylamide in the body ?
- What is high-temperature cooking ?
- How to reduce acrylamide in food at home
- TAG polymers
- Cooking oil smoke points
- Types of cooking oil
What is cooking oil ?
Oils are fats that are liquid at room temperature, like the vegetable oils used in cooking or deep frying. Oils come from many different plants and from fish. Oils are NOT a food group, but they provide essential nutrients. Fats and oils are part of a healthy diet and play many important roles in your body. Fat provides energy and is a carrier of essential nutrients such as vitamins A, D, E, K, and carotenoids. But, fat can impact the health of your heart and arteries in a positive or negative way, depending on how much you eat and the types of fat you eat.
You need some fat/oil in your diet but not too much. Fats give you energy and help your body absorb vitamins. Dietary fat also plays a major role in your cholesterol levels. Therefore, oils are included in the U.S. Department of Health and Human Services’ Dietary Guidelines for Americans 1.
Some commonly eaten oils include: canola oil, corn oil, cottonseed oil, olive oil, safflower oil, soybean oil, and sunflower oil. Some oils are used mainly as flavorings, such as walnut oil and sesame oil. A number of foods are naturally high in oils, like nuts, olives, some fish, and avocados.
Most foods contain several different kinds of fat. Some are better for your health than others. It is wise to choose healthier types of fat, and enjoy them in moderation. Keep in mind that even healthier fats contain calories and should be used sparingly for weight management. Here is some information about healthy and harmful dietary fats.
The four major types of fats 2 are:
- Monounsaturated fats (Good)
- Polyunsaturated fats (Good)
- Saturated fats (Bad)
- Trans fats (Very Bad)
Monounsaturated and polyunsaturated fats are known as “healthy fats” because they are good for your heart, cholesterol levels, and overall health. Monounsaturated and polyunsaturated fats do not raise LDL “bad” cholesterol and are beneficial when consumed in moderation. These fats tend to be “liquid” at room temperature. Olive oil and canola oil are examples of a type of oil that contains monounsaturated fats.
Monounsaturated fats (MUFAs) can help reduce bad cholesterol levels in your blood which can lower your risk of heart disease and stroke 3. They also provide nutrients to help develop and maintain your body’s cells. Oils rich in monounsaturated fats also contribute vitamin E to the diet, an antioxidant vitamin most Americans need more of.
Polyunsaturated fats (PUFAs) are simply fat molecules that have more than one unsaturated carbon bond in the molecule, this is also called a double bond. Oils that contain polyunsaturated fats are typically liquid at room temperature but start to turn solid when chilled. Soybean oil and flax seed oil are examples of a type of oil that contains polyunsaturated fats. Polyunsaturated fats can help reduce bad cholesterol levels in your blood which can lower your risk of heart disease and stroke 4. They also provide nutrients to help develop and maintain your body’s cells. Oils rich in polyunsaturated fats also contribute vitamin E to the diet, an antioxidant vitamin most Americans need more of.
Oils rich in polyunsaturated fats also provide essential fats that your body needs but can’t produce itself – such as omega-6 and omega-3 fatty acids. You must get essential fats through food. Omega-6 and omega-3 fatty acids are important for many functions in the body.
For good health, the majority of the fats that you eat should be monounsaturated or polyunsaturated. Consider eating more of the beneficial polyunsaturated fats containing Omega-3 fatty acids found in fatty fish, flaxseed, chia seeds and walnuts 4. Eat foods containing monounsaturated fats and/or polyunsaturated fats instead of foods that contain saturated fats and/or trans fats.
The majority of saturated fat comes from animal products such as beef, lamb, pork, poultry with skin, butter, cream, cheese and other dairy products made from whole or 2 percent milk. All of these foods also contain dietary cholesterol. Foods from plants that contain saturated fat include coconut, coconut oil, palm oil and palm kernel oil (often called tropical oils) and cocoa butter. Replacing foods that are high in saturated fat with healthier monounsaturated and polyunsaturated fats (oils) can lower your blood cholesterol levels and improve your blood lipid profiles 5. The American Heart Association recommends you don’t eat more than 5% to 6% of calories from saturated fat 5. For example, if you need about 2,000 calories a day, no more than 120 of them should come from saturated fat. That’s about 13 grams of saturated fat per day.
Trans fats (or trans fatty acids) are created in an industrial process that adds hydrogen to liquid vegetable oils to make them more solid. Another name for trans fats is “partially hydrogenated oils.” Partially hydrogenated oils (trans fats) are used by food manufacturers to improve the texture, shelf life and flavor stability of foods. Partially hydrogenated oils should not be confused with “fully hydrogenated oils,” or saturated fats which are solid fats that contain very low levels of trans fat. Trans fats are found in many fried foods and baked goods such as pastries, pizza dough, pie crust, cookies and crackers. And trans fat are also formed naturally and is found in small amounts in some animal products, such as meats and dairy products 6.
Trans fats raise your bad (LDL) cholesterol levels and lower your good (HDL) cholesterol levels 6. These changes are associated with a higher risk of heart disease.
There are two sources of trans fat:
- Trans fat formed naturally – this type of trans fat is produced in the gut of some grazing animals (such as cattle and sheep).
- Trans fat formed artificially during food processing – this type of trans fat is created during a process called “partial hydrogenation” in which hydrogen is added to liquid vegetable oil to make it more solid, and therefore more resistant to becoming spoiled or rancid. The process generally does not make the oil completely solid, resulting in “partially” hydrogenated oils.
Since 2006, the U.S. Food and Drug Administration (FDA) has required trans fat content to be listed on the Nutrition Facts panel of packaged foods 7. In recent years, many major national fast-food chains and casual-dining restaurant chains have announced they will no longer use trans fats to fry or deep-fry foods. Many smaller local and regional restaurant chains have made similar announcements.
On June 16, 2015, the U.S. Food and Drug Administration (FDA) took action that will significantly reduce the use of partially hydrogenated oils, the major source of artificial trans fats in the food supply. The FDA announcement to eliminate trans fats from processed foods and their action is expected to reduce cardiovascular disease and prevent thousands of fatal heart attacks each year in the U.S. 8. According to the FDA, “On June 18, 2018, manufacturers must ensure that their products no longer contain partially hydrogenated oils (trans fats) for uses that have not been otherwise authorized by FDA.”
To find the amount of trans fats in a particular packaged food, look at the Nutrition Facts panel. Companies must list any measurable amount of trans fat (0.5 grams or more per serving) in a separate line in the “Total Fat” section of the panel, directly beneath the line for “Saturated Fat.” This means if a food package states 0 gram of trans fats, it might still have some trans fats if the amount per serving is less than 0.5 g. Make sure to check the ingredients list for “partially hydrogenated oil.”
What are bad fats and oil ?
Bad fats are often called solid fats because they are fats that are solid at room temperature, like beef fat, butter, ghee, lard, coconut oil and shortening. Solid fats are bad because they contain high amount of saturated fats and/or cholesterol and trans fats.
Saturated fats and trans fats are known as the “harmful fats.” They increase your risk of heart disease and elevate cholesterol. Saturated fats tend to be solid at room temperature, but they are also found in liquid tropical oils (palm oil and coconut oil). Trans fats (partially hydrogenated or hydrogenated fats) are oils that have been modified for longer shelf life. Trans fats are very bad for you 2. No amount of trans fats is healthy. Eating foods containing saturated fat and trans fat causes your body to produce even more bad LDL cholesterol, raising your blood cholesterol level.
In a 5 year study conducted by the government in the Republic of Mauritius, an island nation in the Indian Ocean about 2,000 kilometres off the southeast coast of the African continent, to find out if reducing saturated cooking oil – palm oil – polyunsaturated soybean oil 9. In 1987, as part of public health intervention the government changed the composition of the commonly used cooking oil from being mostly palm oil (high in saturated fatty acids) to being wholly soya bean oil (high in unsaturated fatty acids). From 1987 to 1992 total cholesterol concentrations fell significantly by 0.79 mmol/l in men and 0.82 mmol/l in women 9. The estimated intake of saturated fatty acids decreased by 3.5% of energy intake in men and by 3.6% in women, and the intake of polyunsaturated fatty acids increased by 5.5% and 5.6% of energy intake, respectively. These changes were reflected in changes in the fatty acid composition of serum phospholipids. Conclusion: Dietary changes that entailed a reduction in the saturated fat content of a ubiquitous cooking oil (palm oil) explained most of the observed decrease in serum cholesterol concentration over five years in the population of Mauritius. The key messages are: the saturated fatty acid intake of Mauritians was reduced by modifying the composition of the widely used cooking oil; mean serum cholesterol concentration decreased by 15% after the cooking oil (replacing palm oil with soybean oil) intervention was introduced nationwide and effective, low cost prevention programmes for chronic disease are needed in less industrialised countries. Simple action in dietary policy could be one such strategy 9.
According to the new American Heart Association advisory 10, replacing saturated fats with healthier poly-unsaturated fats and mono-unsaturated fats found in some vegetable oils can reduce cholesterol levels and heart disease risk as much as statins (a cholesterol lowering medication). In summary, randomized controlled trials that involve people lowering their intake of dietary saturated fat and replaced it with polyunsaturated vegetable oil reduced their cardiovascular disease by ≈30%, similar to the reduction achieved by statin treatment 10. Poly-unsaturated fats are found in corn, soybean and peanut oils. Mono-unsaturated fats are found in oils such as olive, canola, safflower and avocado.
Saturated fats are bad for your health in several ways:
- Heart disease risk. Your body needs healthy fats for energy and other functions. But too much saturated fat can cause cholesterol to build up in your arteries (blood vessels). Saturated fats raise your LDL, or bad, cholesterol. High LDL cholesterol increases your risk for heart disease and stroke.
- Weight gain. Many high-fat foods such as pizza, baked goods, and fried foods have a lot of saturated fat. Eating too much fat can cause you to gain weight. All fats contain 9 calories per gram of fat. This is more than twice the amount found in carbohydrates and protein.
Cutting out high-fat foods can help keep your weight in check and keep your heart healthy. Staying at a healthy weight can reduce your risk of diabetes, heart disease, and other health problems.
You should try to avoid:
- Saturated fats such as butter, solid shortening, and lard
- Trans fats. These are found in vegetable shortenings, some margarines, crackers, cookies, snack foods, and other foods made with or fried in partially hydrogenated oils. By 2018, most U.S. companies will not be allowed to add partially hydrogenated oils to food.
Solid fats mainly come from animal foods and can also be made from vegetable oils through a process called hydrogenation. Some common solid fats are:
- butter
- ghee
- milk fat
- beef fat (tallow, suet)
- chicken fat
- cream
- pork fat (lard)
- stick margarine
- shortening
- hydrogenated and partially hydrogenated oils*
- coconut oil*
- palm and palm kernel oils*
* The starred items are called “oils” because they come from plant sources (plant oils) like coconut oil, palm oil, and palm kernel oil. Even though they are called “oils,” they are considered to be solid fats because they are high in saturated or trans fatty acids, and for nutritional purposes should be considered to be solid fats and they are bad.
Table 1. Eat LESS than 10% of your calories from saturated fat per day
| Total Calorie Intake | Limit on Saturated Fat Intake a |
|---|---|
| 1,600 | 18 grams or less |
| 2,000 | 20 grams or less |
| 2,200 | 24 grams or less |
| 2,500 | 25 grams or less |
| 2,800 | 31 grams or less |
aAim to consume less than 10% of total calories from saturated fat. For people who need to lower their cholesterol, the American Heart Association recommends reducing saturated fat to no more than 5 to 6 percent of total daily calories 12.
Eating too much saturated fat, the type of fat that is solid at room temperature, will increase your risk of heart disease. Similarly, eating too much trans fat, which is made when liquid vegetable oil is processed to become solid, also may increase risk of heart disease. And, eating too much cholesterol, a fatty substance found only in animal-based products, may clog arteries. It is important to eat less than 10% of your calories from saturated fat.
For example, if you aim to eat 2,000 calories a day, your daily allowance of saturated fat would be less than 200 calories or 20 grams—which equals 100 percent Daily Value (% DV) for saturated fat. The table below shows the saturated fat limits for people with various calorie needs. Furthermore, you should keep trans fat as low as possible and eat less than 300 milligrams of cholesterol each day.
Foods with Saturated Fats
- High-fat cuts of meat (beef, lamb, pork)
- Chicken with the skin
- Whole-fat dairy products (cream/milk)
- Butter
- Palm and coconut oil (snack foods, non-dairy creamers, whipped toppings)
- Ice cream
- Cheese
- Lard
- Coconut oil
Foods with Trans Fats
- Commercially baked pastries, cookies, doughnuts, muffins, cakes, pizza dough, pie crusts
- Packaged snack foods (crackers, microwave popcorn, chips)
- Stick margarine
- Vegetable shortening
- Fried foods (French fries, fried chicken, chicken nuggets, breaded fish)
- Candy bars
- Pre-mixed products (cake mix, pancake mix, chocolate drink mix)
- Coffee creamer
- Ready-to-use frostings
- Fast food
- Frozen pizza
It is fine to treat yourself to these types of foods once in a while. But, it is best to limit how often you eat them and limit portion sizes when you do.
Tips for decreasing harmful fats in your diet:
- Read food labels and avoid trans fats and hydrogenated/partially hydrogenated oils.
- Avoid fried products.
- Avoid fast food.
- When eating out, ask that foods be prepared with olive, canola, olive, safflower, sesame, peanut or sunflower oil.
What are healthy fats and oil ?
Healthy oils are high in monounsaturated or polyunsaturated fats, and low in saturated fats. Oils from plant sources (vegetable and nut oils) do not contain any cholesterol. In fact, no plant foods contain cholesterol except for the ones listed in the saturated and bad fats section e.g. coconut oil, palm oil.
Table 2. Common food sources of Healthy Fats
| Monounsaturated | Polyunsaturated Omega-6 | Polyunsaturated Omega-3 | |
|---|---|---|---|
| almonds, peanuts, hazelnuts, macadamia nuts, pecans, cashews) Avocados Olives Peanut butter (without added sugar or salt) | Vegetable oils: Soybean oil Corn oil Safflower oil | Certain fish: Salmon Trout Herring | Vegetable oils: Soybean oil Canola oil Walnuts oil Flaxseed oil |
Healthy cooking oil
Replacing bad fats (saturated and trans) with healthier fats (monounsaturated and polyunsaturated) is better for your heart.
One way you can do this is by choosing healthier nontropical vegetable oils for cooking and preparing food.
Use these oils instead of solid fats (including butter, shortening, lard and hard stick margarine) and tropical oils (including palm and coconut oil), which can have a lot of saturated fat.
- Use liquid vegetable oils in place of solid fats
- Liquid vegetable oils such as canola, safflower, sunflower, soybean and olive can often be used instead of solid fats such as butter, lard or shortening. If you must use margarine, try the soft or liquid kind.
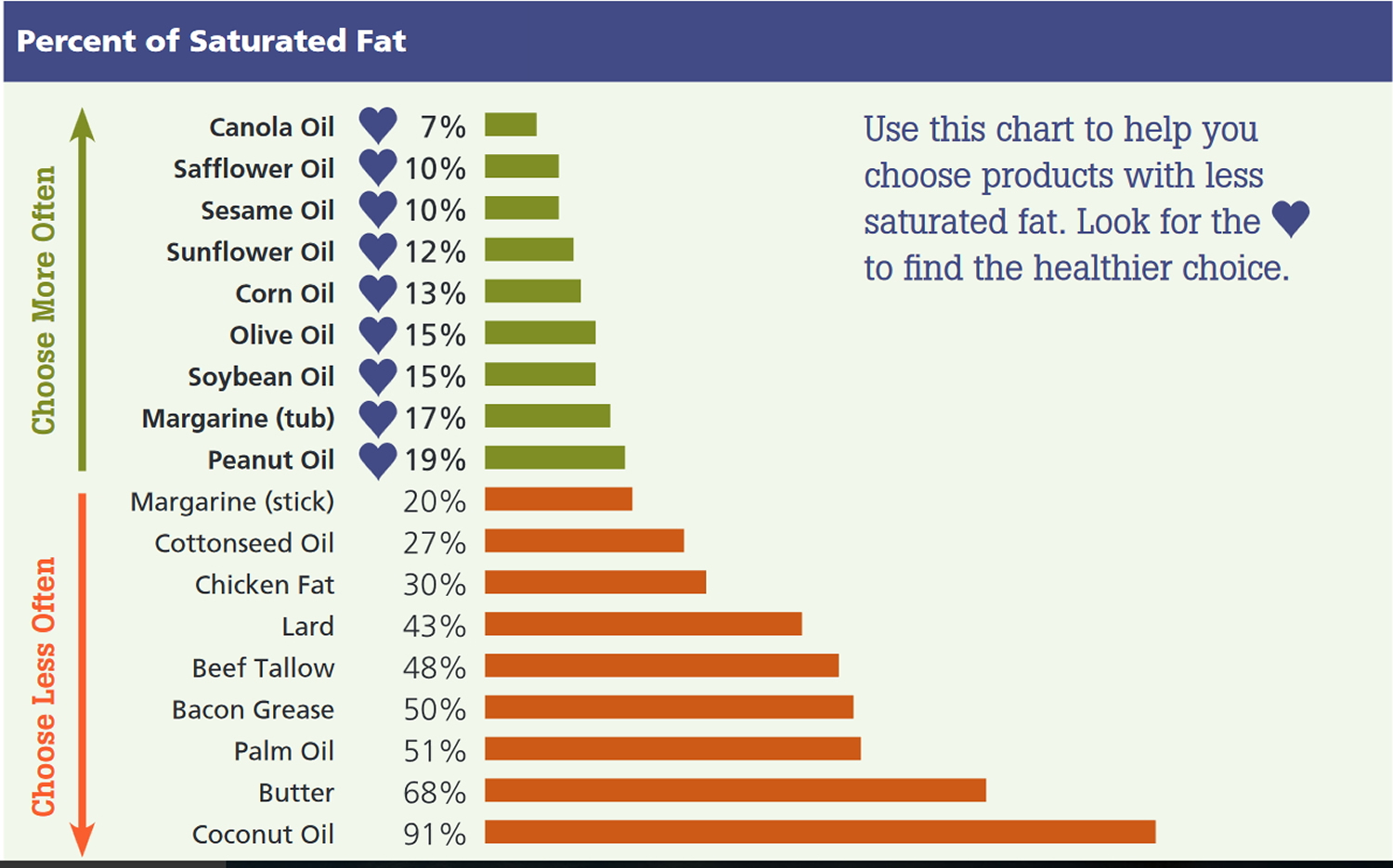
Here’s an alphabetical list 15 of common cooking oils that contain more of the “better-for-you” fats and less saturated fat.
- Canola oil
- Corn oil
- Olive oil
- Peanut oil
- Safflower oil
- Soybean oil
- Sunflower oil
Blends or combinations of these oils, often sold under the name “vegetable oil,” and cooking sprays made from these oils are also good choices. Some specialty oils, like avocado oil, grapeseed oil, rice bran oil and sesame oil, can be healthy choices too, but may cost a bit more or be harder to find.
In general, choose oils with less than 4 grams of saturated fat per tablespoon, and no partially hydrogenated oils or trans fats.
You may find that some oils have distinctive flavors, so try different types to discover which ones you like. Also, some oils are better for certain types of cooking than others, so you may want to have more than one type in your pantry.
You can usually use cooking oils just like solid cooking fats. For example, use them to:
- Make your own salad dressings, marinades, dips and sauces.
- Grill, sauté, stir fry, bake or roast foods.
- Coat pans to keep food from sticking.
- Spread or drizzle on foods for flavor.
- “Season” cast-iron cookware.
- Substitute for butter, margarine or solid fats in recipes.
Types of cooking oil
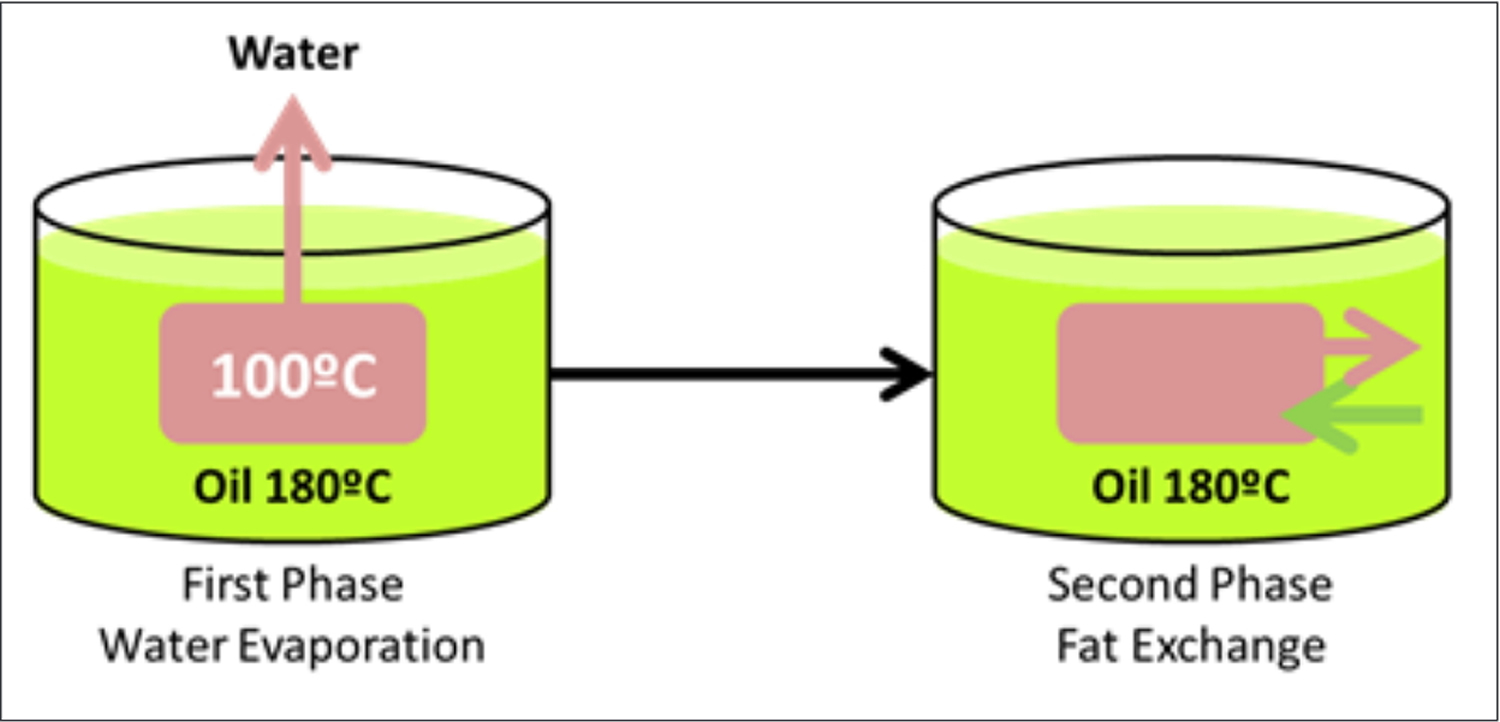
During any cooking process, when oil is the heating medium, two phases typically occur (see Figure 2). The first phase is where water evaporates from the food being cooked, and this commences once the oil reaches 100ºC, and continues until most of the food moisture has evaporated. The second phase is that during which the food absorbs and releases (in the case of fatty foods) fat. This second process determines the changes in fatty acid composition of the food which occurs during the cooking process (see Figure 2). It has been well documented that the fatty acid profile of the food after deep frying is closer to that of the oil used to fry the food than that of the raw food itself 16, 17.
Watch out for manufacturer’s added antioxidants in oils like butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), and tert-butylhydroquinone (TBHQ), which are suppose to slow down the oxidation of oil at room temperature. It’s best you stay clear from any cooking oils with any added antioxidants and/or preservatives, and cook with only 100% pure vegetables oil e.g. canola oil, olive oil, peanut oil, etc. Some cooking oils like soybean oil and sesame oil contain natural antioxidants like tocopherols in soybean and lignan compounds (sesamol, sesamin, and sesamolin) in sesame oil, are stable during heating and contribute to high oxidative stability of the oil during heating at high temperature.
Canola Oil
Canola oil is extracted from the seeds of the canola plant, which was developed through crossbreeding with the rapeseed plant. Canola is a healthy oil that’s low in saturated fat and a good source of monounsaturated and polyunsaturated fats, including omega-3s. (Note: Canola oil is not the same thing as rapeseed oil, which contains erucic acid that can be harmful to humans in large quantities.)
How should you use canola oil ?
Canola oil has a light flavor, which makes it versatile in cooking. Replace solid fats such as butter or margarine with canola oil when cooking or baking. Canola oil works well for sautéing and stir-frying. It also is good for coating pots, pans and your grill.
Table 3. Canola oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 1.000 | 7.140 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 9.001 | 64.290 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 4.000 | 28.570 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Table 4. Corn oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 2.001 | 14.290 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 4.000 | 28.570 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 8.000 | 57.140 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Olive Oil
Olive oil is high in monounsaturated fatty acids (MUFAs), which may help reduce one’s risk of heart disease. MUFAs lower LDL (“bad”) cholesterol and raise HDL (“good”) blood cholesterol. Olive oil is often sold as “virgin” or “extra virgin.” Extra-virgin olive oil has less acid and a fruitier flavor and stronger aroma than pure or virgin olive oil, so a little goes a long way. Olive oil labeled as “light” is often lighter in hue or flavor, but it’s not lighter in calories.
Table 5. Olive oil nutritional facts
Nutrient | Unit | 15 ml | Value per 100 ml | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 800 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 93.33 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 1.999 | 13.330 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 10.500 | 70.000 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 1.500 | 10.000 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Peanut Oil
Peanut oil is a source of phytosterols, which benefit the heart by preventing cholesterol absorption in the intestines. Although, it’s difficult to get enough phytosterols from peanut oil or any other food unless it’s a product fortified with added sterols. Peanut oil is also a common monounsaturated fat, and contains vitamin E — an antioxidant.
How should you use it ?
Peanut oil is often used in deep frying because of the high temperature it can reach. Because of its nutty flavor, use this oil in stir-fries and ginger dressing.
Table 6. Peanut oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 2.500 | 17.860 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 8.000 | 57.140 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 3.000 | 21.430 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Table 7. Rice bran oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 2.500 | 17.860 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 6.201 | 44.290 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 5.300 | 37.860 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Table 8. Safflower oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 1.000 | 7.140 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 11.000 | 78.570 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 2.001 | 14.290 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Sesame Oil
Sesame oil is rich in mono- and polyunsaturated acids (PUFAs) — the good kind of fat that cuts cholesterol. Sesame oil contains linoleic acid, which is a type of omega-6 fatty acid that may promote heart health by reducing LDL cholesterol.
Table 9. Sesame oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 130 | 929 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 2.001 | 14.290 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 6.000 | 42.860 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 6.000 | 42.860 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
Table 10. Soybean oil nutritional facts
Nutrient | Unit | 14 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 120 | 857 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 100.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Potassium, K | mg | 0 | 0 | ||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 2.001 | 14.290 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 3.000 | 21.430 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 9.001 | 64.290 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Table 11. Sunflower oil nutritional facts
Nutrient | Unit | 15 ml | Value per 100 ml | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 130 | 867 | ||||||||||||||||
| Protein | g | 0.00 | 0.00 | ||||||||||||||||
| Total lipid (fat) | g | 14.00 | 93.33 | ||||||||||||||||
| Carbohydrate, by difference | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Sodium, Na | mg | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 1.999 | 13.330 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 4.000 | 26.670 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 7.999 | 53.330 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Cooking Oil and Deep Frying
Deep-fat frying is a multi-functional operation of food transformation. This process may be defined as cooking food by immersion in edible oil or fat at a temperature above the boiling point of water 19. The major use of cooking oil is in frying, where it functions as a heat transfer medium and contributes flavor and texture to foods 20. One requirement of a cooking oil is that it be stable under the very abusive conditions of deep-fat frying, namely, high temperatures and moisture. In general, oil should be kept at a maximum temperature of 180°C during frying. Frying food at a temperature which is too low results in increased fat uptake. However, oil uptake is a complex phenomenon resulting from interactions between oil and products that undergo numerous physical, chemical, and structural transformations during frying 21. Water, which is contributed by the foods that are fried in an oil enhances the breakdown of fatty acids which occurs during heating. Hydrolysis results in a poor-quality oil that has a reduced smoke point, darkened color and altered flavor. During heating, oils also polymerized, creating a viscous oil that is readily absorbed by foods and that produces a greasy product 20. The more saturated (solid) the oil, the more stable it is to oxidative and hydrolytic breakdown, and the less likely it is to polymerize 20.
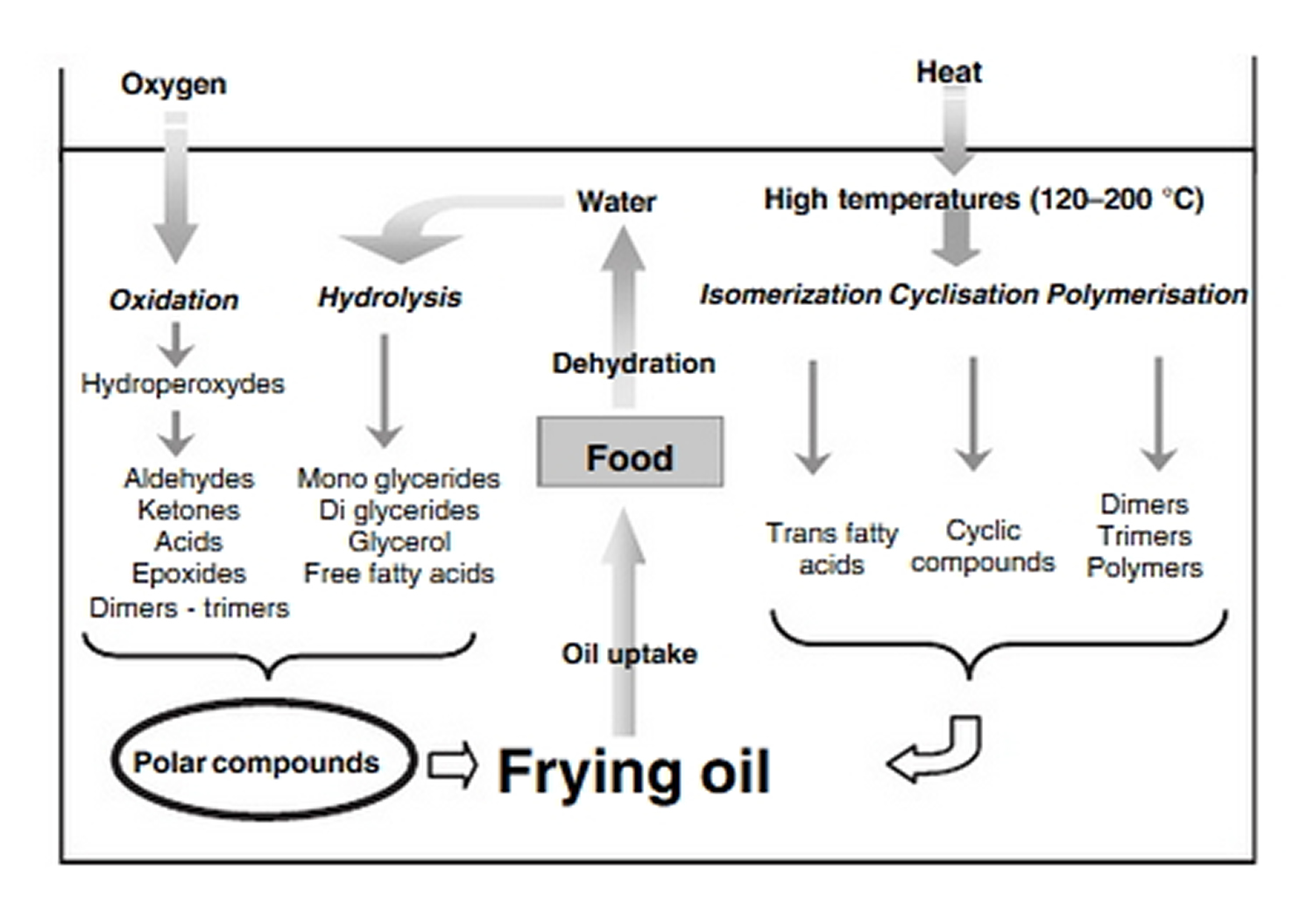
Figure 1. Outline of the oil degradation during frying and the consequences on the product quality
[Source 21]During frying, water escapes from the food while oil migrates into the food providing nutrients and flavours. Frying oils thus have the original property of being both a heat transfer medium and an ingredient of the final product representing up to 40% of the total mass in products like chips 21. In addition, the fatty acid composition of the oil present in the fried product does not differ from the fatty acid composition of the frying medium. Hence, the choice of suitable oil for frying is very important, not only for its potential nutritional value but also for its ability to support frying conditions. Indeed, during frying, oil deteriorates due to heat, water, and oxygen exposure 22 as shown in Figure 1. The result of these complex chemical reactions is an increasing amount of lipid degradation compounds in the oil bath as free fatty acid, mono and diglycerides, polymers, potentially toxic carbonyl compounds and so on, that can be further absorbed by the product, lowering its nutritional value 23. Even if a frying oil regulation is established limiting polar compounds to 25% and polymer content to 12%, potentially toxic compounds can appear in the oil bath. The main hazardous compounds presently identified are potentially carcinogenic molecules such as carbonyl compounds or mono epoxides and some aldehydes produced from linoleic acid, e.g. 4-hydroxy-2-trans-nonenal that has been proven to be cytotoxic 24, 25.
Figure 2. Typical dynamics during deep frying fatty foods
[Source 26]The total deterioration of oil when frying can be measured by both sensory (i.e. changes in color, smell and taste) and laboratory values (i.e. measuring FFAs and the formation of total polar compounds 27. Overall, there are two major properties of cooking oils which dictate the behavior of that oil, and subsequent safety when exposed to high cooking temperatures – smoke point and, most importantly, oxidative stability. Some products of decomposition in used oil have been identified to have adverse effects on human health 28, 29, 30, as they may have a higher chance of absorption into the fried foods 21. Therefore, it is important to understand the factors affecting the deterioration of frying oil and to monitor the quantity of products of decomposition for ensuring the quality of fried foods.
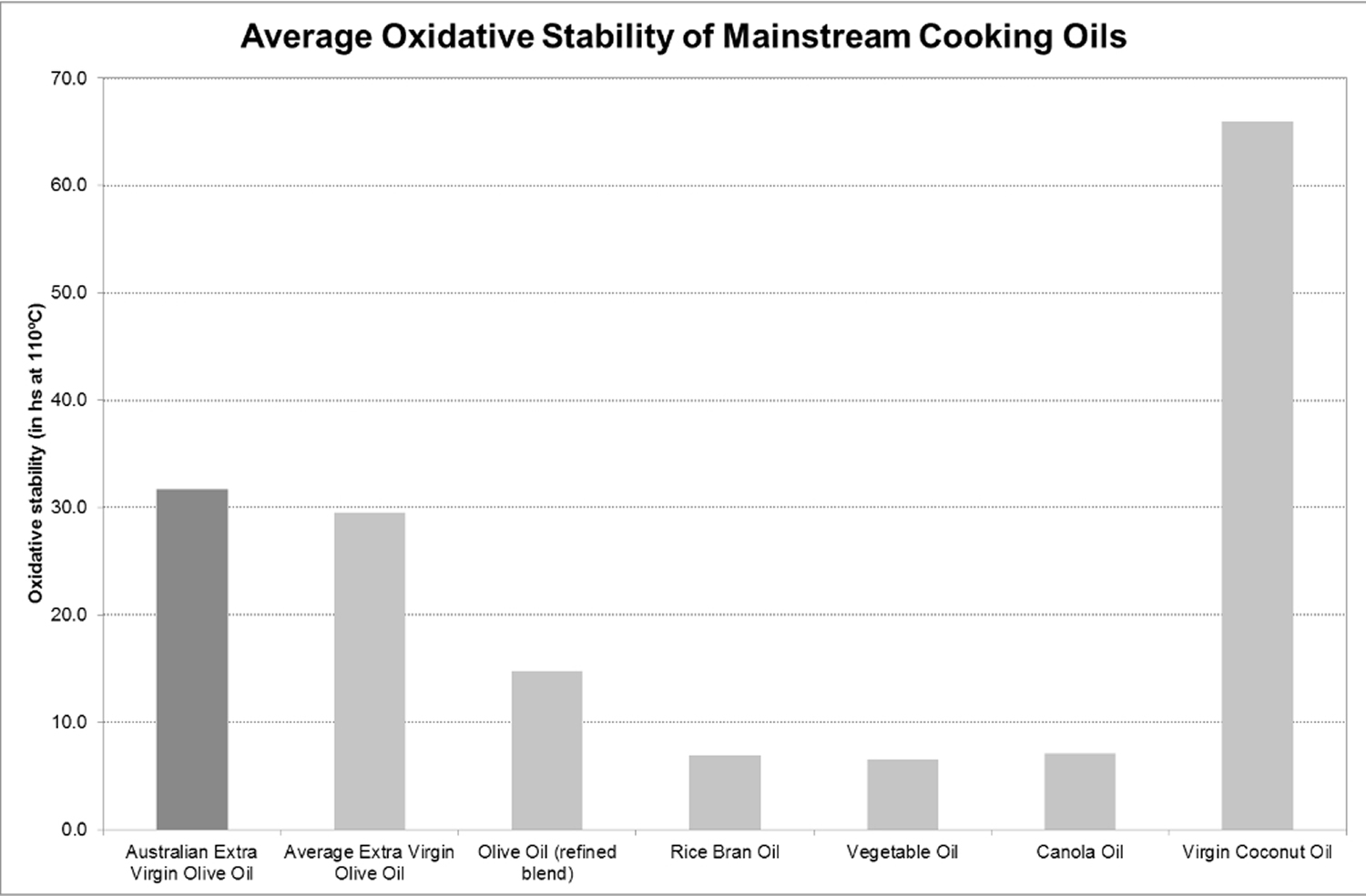
Hydrolysis and oxidation are the two primary degradation processes that occur in an oil during cooking 31. Oxidative stability is how resistant an oil is to reacting with oxygen, breaking down and potentially producing harmful compounds while exposed to continuous heat. Oxidative stability is the best predictor of how an oil behaves during cooking 32, 33, 34. The Rancimat method is one of the most common methods for testing oxidative stability in oils 34. This determination entails speeding up the oxidation process in the oil (under heat and forced air), which enables the oils’ stability to be evaluated by monitoring volatile substances associated with rancidity. It is measured as “Induction time” and recorded as total hours before the oil breaks down. Canola oil requires 7.5 hours, for example, whereas extra virgin olive oil and virgin coconut oil will last over a day at 110 °C of continuous heat 26. The differing stabilities correlates with lower levels of polyunsaturated fatty acids, which are more prone to oxidation. Extra virgin olive oil is high in monounsaturated fatty acids and antioxidants, conferring stability 26.
The content of total polar compounds and acid value are the most predominant indicators for oil quality and are widely used in many international regulations 35, 36. For public health concerns, the content of total polar compounds and acid value in frying oil are regulated at not more than 25% and 2.0 mg KOH/g, respectively, in Taiwan
37. Determination of total polar compounds in frying oil provides a more robust measurement on the extent of deterioration in most situations 35 due to its higher accuracy and reproducibility. The contents of free fatty acid and total polar compounds were commonly used for initial oil quality assurance and after-use frying oil quality assessment, respectively 37. Nevertheless, the standard analytical procedure for oil quality evaluation needs to be done in a laboratory with proper equipment by skilled technicians 38. It is not suitable for a small industry or even households to use on site.
Oil Degradation in Frying
The mechanism of thermal degradation of frying oil is complicated. Variables involved in the process include frying conditions, replenishment of fresh oil, original oil quality, food materials, and fryer type 39. Cooking oils with more saturated fatty acids such as lard and palm oil are usually more stable for frying 40. On the other hand, soybean oil with more unsaturated fatty acids is less stable, and decomposes easily at high frying temperature. In Taiwan, soybean oil is one of the most commonly used cooking oils at home and is also used by many small snack vendors for frying 40. Palm oil is mostly used commercially to prepare fried potato foods 27.
Among frying oils, those with high oleic acid content such as sunflower oil and palm olein 27, 41 have better health profile and heat stability. The rate of total polar compounds generation in palm olein was slower than that in soybean oil during frying. However, the content of total polar compounds in palm olein reached the official limit of 25% faster than soybean oil because the fresh palm olein contained more total polar compounds than soybean oil. Moisture in foods induces and accelerates oxidation with the hydrolytic compounds. Foods with high water content like potato and foods with breading or battering materials cause faster hydrolysis of frying oil. During frying, the fat of chicken or pork that is released into frying oil alters the fatty acid composition and increases the degradation rate of the oil 42. Oils rich in linolenic acid, such as soybean and canola oils, are particularly susceptible to these undesirable changes. When soybean oil is partially hydrogenated to reduce the linolenic acid from about 8 percent to below 3 percent, it is a relatively stable frying oil and it is used in processed fried foods, pan and griddle frying, and sauces. Stability is increased by using cottonseed, corn oil, palm oil or palmolein or by more hydrogenation of soybean oil.
Foods that are fried and stored before eating, for example, snack products, require an even more stable oil. More saturated oils improve stability, however, if the frying fat is solid at room temperature it will produce a dry dull surface that is undesirable on some fried products. When oils are used continuously, as in restaurants, a frying fat which can withstand very heavy use is needed. In these cases, more solid shortenings are used to maximize the stability of the fat for many hours of frying.
Frying oils made from sunflower and safflower have lower stability because of their high polyunsaturated fatty acids and low tocopherol content; however, high-oleic safflower and sunflower oils that have been genetically developed are suitable frying oils.
Frying with extra-virgin olive oil can also improve the nutritional properties of the food. A common myth is that heating extra-virgin olive oil converts the mono-unsaturated fats to trans fats. It is important to understand that trans-fats form when any edible oil is subjected to an industrial process such as refining or hydrogenation (which is designed to turn liquid oil into an edible fat that is solid at room temperature (i.e. margarine)) 43. The vast majority of trans-fats in the average person’s diet arise from fast food, inexpensive margarines, or more commonly commercially baked products. Extra-virgin olive oil is naturally trans-fat free and cooking with extra-virgin olive oil will ensure that undesirable trans-fats are not added to the diet. Contrary to popular myths, high quality extra-virgin olive oil is an excellent choice for cooking 26. High quality extra-virgin olive oil has a smoke point well above the standard temperatures required for cooking, and its resistance to oxidation is higher than most cooking oils due to the antioxidant and mono-unsaturated fat content 26. Therefore, it is ideal for both hot and cold cooking and there is less chance that harmful substances will be formed upon the application of heat through cooking 26. Furthermore, although the heating process will reduce the natural antioxidant content of extra-virgin olive oil there is scientific evidence to demonstrate that a substantial amount of antioxidants can still be found in the prepared meal 44).
For optimal use of cooking oils, it is necessary to distinguish between different frying conditions. The most important parameters to be monitored are duration of use and nature of the foods to be fried. If food fats enter the frying oil, food components could destabilize the oil and the water content of the material could influence the frying operation. Whether the use is continuous or intermittent is relevant, continuous use provides a protective water vapour blanket that protects against oxidation. Finally, temperature must be considered.
Industrial use of fats and oils is usually well-monitored. The continuous operation (implying the constant addition of fresh oil) and quality requirements for the products normally ensure good quality control of the oil. In homes, where oils are normally used for much shorter periods of time and are discarded after being used once or twice, stability problems play a lesser role. Stability of frying oils is a more important factor in catering operations, where heating is intermittent and oils may be used for long periods.
The release of amino acids from food into oil can prevent oil degradation during deep frying, but the starch has not been observed to have a similar effect 45. However, only very few researches related to the influences of oil and food types on frying oil quality can be found.
Figure 3. Average oxidative stability of mainstream cooking oils based on analysis performed on standard supermarket products by ISO 17025 accredited laboratory
[Source 26]Note: The combination of a high content of MUFA (and low PUFA content), together with the antioxidant components found in extra-virgin olive oil, make it highly resistant to oxidation, and therefore there is less chance that harmful substances will be formed upon the application of heat, through cooking. This also means that extra-virgin olive oil will last longer in storage 46, 47, 48, 49. Furthermore, despite the impact that heating has in reducing the antioxidant content in extra-virgin olive oil, a significant amount of polyphenols, tocopherols, sterols and squalene still remain in EVOO after heating, and they are absorbed by the cooked food.
Frying factors
The quality of products is the result of the deep-fat frying conditions which determine oil distribution in the product, its structure, and its flavour properties 50. Oil temperature and frying time couple. The frying temperature depends on the type of product, its size and components, and varies from 120°C to 190°C. High oil temperatures (160–190°C) enable rapid heat transfer, rapid browning, and short frying time. For this reason, putting too large an amount of cold food in to hot fat is detrimental to product quality and process efficiency because it causes a dramatic decrease in oil temperature and longer cooking time. At the industrial scale, a good food to oil ratio is generally 1/6 51. An increase in oil temperature triggers an increase in dehydration and coupled reaction speeds. Therefore, high temperatures limit frying time. However, for the same residual content, the effect of frying temperature is marginal and some authors even argue that temperatures between 140°C and 190°C have no influence on oil absorption 52. However, the incitation of limiting oil temperature in order to limit its degradation motivated the study of frying temperatures below 140°C. These works showed that a temperature such as 120°C resulted in longer frying time and higher oil uptake for the same residual water content 53, 54. This phenomenon can be explained by the higher oil uptake during frying caused by the longer frying time and the weaker opposite water flows or by the development of different crust structures (i.e. a different porosity more likely to enhance oil uptake). Indeed, during frying at a very low oil temperature, such as 120°C, crusts exhibit a low level of firmness that could let the oil penetrate easily into the product 55.
Vacuum frying
Vacuum frying makes it possible to fry at a lower oxygen concentration and at a lower frying temperature because of the decrease in the water boiling point. This process can therefore preserve natural colors and flavors of a product and limit oil degradation. In addition, Garayo & Moreira 56 have demonstrated that the final oil content is lower for chips fried under vacuum pressure than for those fried at atmospheric pressure while enabling the same dehydration time. More recently, Liu-Ping et al. 57 have stated that the rate of moisture loss and oil uptake increases while the degree of vacuum increases for carrot chips and Tan & Mittal 58 have found that oil uptake is higher in donuts fried under vacuum than those fried at atmospheric pressure for the same final moisture content. In conclusion, vacuum frying has been poorly studied and its effect on oil uptake is not clearly recognised.
Health implications of dietary oxidised oils
Physiological and nutritional effects of frying oils have been the subject of intensive investigations since the 1950s. A detailed review of the distinct aspects studied and of the difficulties to reach thorough conclusions has been published recently 59. The main reason for the difference in the results obtained is the composition of the oils used. On the one hand, those researchers who found high levels of toxicity used abusive heating conditions in an attempt to generate sufficient amounts of degradation products, but the level and structures of the compounds thus formed are not representative of those encountered in oils subjected to normal culinary practices. On the other hand, some researchers applied very soft conditions when heating oils disregarding that the use of good practices in the frying process is obviously safe.
Used frying fats and oils
With regard to epidemiological studies, researchers have so far not found any direct link between used frying oils and health problems. In fact, fried foods are an important component of the Mediterranean diet, which is strongly associated with a reduced risk of cardiovascular events 60, 61, 61. Nevertheless, a number of studies have been conducted in human subjects to unravel the possible associations between the consumption of fried foods and the incidence of prevalent diseases, mainly cancer 62, 63, 64, 65, metabolic syndrome 66, 67, 68, 69, 70 and coronary heart disease 71, 72, 73. Few studies have found an increased risk of cancer in association with consumption of deep-fried foods, specifically in prostate 62, breast 63, oral/pharyngeal 64, oesophageal 64 and laryngeal 65 cancers. Nevertheless, in all these studies, the intake of frying oil was not defined and the associations reported were not attributed to degradation compounds in the frying oil but to the heterocyclic amines or polycyclic aromatic hydrocarbons formed from meat, or acrylamide formed in carbohydrate-rich foods.
Of special interest are the recent studies regarding the incidence of metabolic syndrome 66, 67, 68, 69, 70 and the risk of coronary heart disease 70, 74, 73. Variable results were obtained including association with a higher prevalence of arterial hypertension 66, obesity 67, 68, or lower HDL-cholesterol levels and a larger waist circumference 69. Also, a null association with the incidence of metabolic syndrome has been reported in the case of a moderate consumption of fried foods 70. With regard to the studies evaluating the effect of fried foods on the risk of coronary heart disease, either positive 71 or null association 72, 73 has been found. Concerning these studies, it is important to remark two aspects. On the one hand, most of the studies have been conducted in Mediterranean countries where olive oil, less prone to degradation than other edible oils, is commonly used for domestic frying. Therefore, research needs to be extended to other communities consuming preferentially polyunsaturated oils or solid fats. On the other hand, information provided on the oxidation of frying oils or fried foods tested was too scarce. Hence, one important variable contributing to the differences between the results obtained in different studies could be the level of oxidation compounds present in the diet. In fact, a significant association between consumption of fried foods and some of the components of metabolic syndrome was found when the amounts of oxidation compounds in the diet increased. In this respect, two examples are worthy to comment. First, in Soriguer et al.’s 66 study, oil samples were taken from the kitchens of a random subset of 538 participants and 10 % of the oils collected contained over 20 % polar compounds. A strong association was found between the consumption of such oils and the risk of hypertension, even after inclusion in the models of variables influencing hypertension, such as age, sex and obesity. Also, Sayon-Orea et al. 70, in the SUN cohort study, classified 8289 participants in three groups according to their frequency of fried food consumption and found that those participants who consumed fried foods >4 times/week had a higher risk to develop two out of five components of metabolic syndrome: central adiposity and high blood pressure, compared with those who consumed ≤ 2 times/week.
Paradoxically, feeding experiments in animals have consistently demonstrated that thermally oxidised oils improves the blood lipid profile, i.e. a reduction in TAG and cholesterol levels in plasma and VLDL, attributed to the activation of hepatic PPARα. Even more, it has also been postulated that PPARα activation in the vasculature would inhibit pro-atherogenic events such as monocyte recruitment and smooth muscle cells proliferation and migration. The authors suggest that some of the multiple components found in thermally oxidised oils may exhibit potent regulatory activity on lipid metabolism. Nevertheless, thermally oxidised oils also cause oxidative stress in animals probably due to the depletion of antioxidants such as tocopherols in serum and tissues. Hence, the possible atheroprotective effect due to activation of PPARα is probably compromised by the simultaneous induction of intense oxidative stress 75.
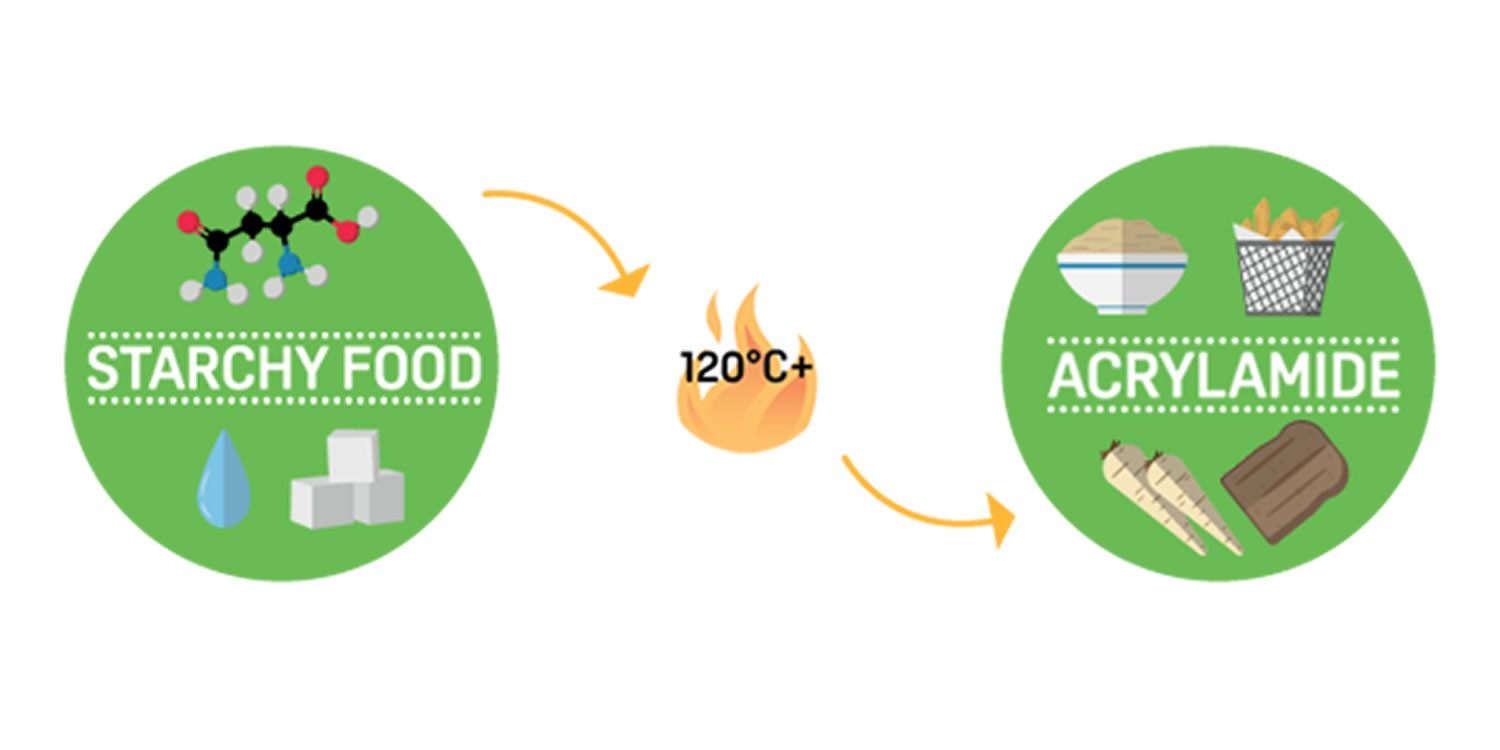
Acrylamide
Acrylamide is a chemical that naturally forms in starchy food products during high-temperature cooking, including frying, baking, roasting and also industrial processing, at +120°C and low moisture 76. Acrylamide is a formed by a reaction between amino acids and sugars. Acrylamide forms from sugars and amino acids (mainly one called asparagine) that are naturally present in many foods 76. Acrylamide typically occurs when foods with high starch content such as potatoes, root vegetables and bread, are cooked at high temperatures (over 120°C) in a process of frying, roasting or baking 77.
Acrylamide is not deliberately added to foods, it is a natural by-product of the cooking process and has always been present in our food.
Acrylamide is also a chemical used in many industrial processes, such as the production of paper, dyes, and plastics, and in the treatment of drinking water and wastewater, including sewage 78 and is present in tobacco smoke 76. High levels of acrylamide in the workplace have been shown to cause neurological damage, e.g., among workers using acrylamide polymers to clarify water in coal preparation plants 79.
The U.S. Environmental Protection Agency (EPA) regulates acrylamide in drinking water. The EPA established an acceptable level of acrylamide exposure, set low enough to account for any uncertainty in the data relating acrylamide to cancer and neurotoxic effects. The U.S. Food and Drug Administration regulates the amount of residual acrylamide in a variety of materials that come in contact with food, but there are currently no guidelines governing the presence of acrylamide in food itself.
How acrylamide is formed
During high temperature cooking, a process called the Maillard Reaction occurs 80, it is the same reaction that ‘browns’ food and affects its taste. The naturally present water, sugar and amino acids combine to create a food’s characteristic flavour, texture, colour and smell. This process can also produce acrylamide.
The duration and temperature of cooking determines the amount of acrylamide produced: long durations and higher temperatures form more acrylamide than short durations and lower temperatures.
Potential health effects of acrylamide
Laboratory tests show that acrylamide in the diet causes cancer in animals 81, 82, 83. While evidence from human studies on the impact of acrylamide in the diet is inconclusive, scientists agree that acrylamide in food has the potential to cause cancer in humans as well and it would be prudent to reduce exposure.
While the evidence from human studies is still incomplete. The National Toxicology Program and the International Agency for Research on Cancer consider acrylamide to be a “probable human carcinogen,” based on studies in laboratory animals given acrylamide in drinking water. However, toxicology studies have shown differences in acrylamide absorption rates between humans and rodents 84.
A series of case-control studies have investigated the relationship between dietary intake of acrylamide and the risk of developing cancers of the oral cavity, pharynx, esophagus, larynx, large bowel, kidney, breast, and ovary. These studies generally found no excess of tumors associated with acrylamide intake 85, 85, 86, 87, 87. In the studies, however, not all acrylamide-containing foods were included in estimating exposures. In addition, information in case-control studies about exposures is often based on interviews (personal or through questionnaires) with the case and control subjects, and these groups may differ in the accuracy of their recall about exposures. One factor that might influence recall accuracy in cancer-related dietary studies is that diets are often altered after receiving a diagnosis of cancer.
To avoid such limitations in accurately determining acrylamide exposure, biomarkers of exposure were recently used in a Danish cohort study designed to evaluate the subsequent risk of breast cancer in postmenopausal women 88. Among women with higher levels of acrylamide bound to the hemoglobin in their blood, there was a statistically significant increase in risk of estrogen receptor-positive breast cancer. This finding suggests an endocrine hormone-related effect, which would be consistent with the results of a questionnaire-based cohort study in the Netherlands that found an excess of endometrial and ovarian cancer—but not of postmenopausal breast cancer—associated with higher levels of acrylamide exposure 89. Another cohort study from the Netherlands suggested a positive association between dietary acrylamide and the risk of renal cell cancer, but not of prostate or bladder cancer 90.
In 2002, Swedish studies 91 revealed that high levels of acrylamide formed during the frying or baking of potato and cereal products. This raised worldwide public concern because studies in laboratory animals suggested acrylamide had the potential to cause cancer in humans. Subsequent assessment by organisations including the World Health Organisation, the European Food Safety Authority (EFSA) and UK scientific advisory committees also suggests that acrylamide is a human carcinogen which has the potential to cause cancer by interacting with the genetic material (DNA) in cells. Most recently, in 2015, the EFSA published its first full risk assessment of acrylamide in food 76, which confirms that acrylamide levels found in food potentially increases the risk of cancer for all age groups. This means that acrylamide might contribute to your lifetime risk of developing cancer; although it is not possible to estimate how big this contribution may be.
On 4 June 2015, EFSA published its first full risk assessment of acrylamide in food. Experts from EFSA’s Panel on Contaminants in the Food Chain (CONTAM) reconfirmed previous evaluations that acrylamide in food potentially increases the risk of developing cancer for consumers in all age groups 76.
Evidence from animal studies shows that acrylamide and its metabolite glycidamide are genotoxic and carcinogenic: they damage DNA and cause cancer 76. Evidence from human studies that dietary exposure to acrylamide causes cancer is currently limited and inconclusive 76.
Since acrylamide is present in a wide range of everyday foods, this health concern applies to all consumers but children are the most exposed age group on a body weight basis. The most important food groups contributing to acrylamide exposure are fried potato products, coffee, biscuits, crackers, crisp bread and soft bread.
Are consumers at risk of developing cancer from acrylamide in food ?
Currently, studies on human subjects have provided limited and inconsistent evidence of increased risk of developing cancer. However, studies on laboratory animals have shown that exposure to acrylamide through the diet increased the likelihood of developing gene mutations and tumours in various organs.
Based on these animal studies, European Food Safety Authority’s experts agree with previous evaluations that acrylamide in food potentially increases the risk of developing cancer for consumers in all age groups. While this applies to all consumers, on a body weight basis, children are the most exposed age group.
Are there other health risks besides cancer ?
European Food Safety Authority’s experts have considered possible harmful effects of acrylamide on the nervous system, pre- and post-natal development and on male reproduction. These effects were not considered to be a concern, based on current levels of dietary exposure.
Foods high in acrylamide
Acrylamide is found in wide range of foods including roasted potatoes and root vegetables, potato crisps, French fries, chips, toast, cakes, biscuits, cereals and coffee.
- Fried potato products (including French fries, croquettes and roasted potatoes) and coffee/coffee substitutes are the most important dietary source of acrylamide for adults, followed by soft bread, biscuits, crackers and crisp breads.
For most children, fried potato products account for up to half of all dietary exposure to acrylamide with soft bread, breakfast cereals, biscuits, crackers and crisp breads amongst the other contributors.
- Baby food (mainly rusks and biscuits) is the most important source for infants.
Some other food categories such as potato crisps and snacks contain relatively high levels of acrylamide but their overall contribution to dietary exposure is more limited (based on a normal/varied diet).
What can consumers do to reduce the risk from acrylamide in food ?
Generally, since it is practically impossible to eliminate acrylamide entirely from the diet, most public advice for the consumer aims at more selective home cooking habits and more variety in the diet.
Since acrylamide levels are directly related to the browning of these foods, some countries recommend to consumers: “Don’t burn it, lightly brown it”. Varying cooking practices and finding a better balance, e.g. boiling, steaming, sautéing as well as frying or roasting, could also help reduce overall consumer exposure.
A balanced diet generally reduces the risk of exposure to potential food risks. Balancing the diet with a wider variety of foods, e.g. meat, fish, vegetables, fruit as well as the starchy foods that can contain acrylamide, could help consumers to reduce their acrylamide intake.
What happens to acrylamide in the body ?
Acrylamide consumed orally is absorbed from the gastrointestinal tract, distributed to all organs and extensively metabolised. Glycidamide is one of the main metabolites from this process, and is the most likely cause of the gene mutations and tumours seen in animals.
What is high-temperature cooking ?
Typically, this means cooking at temperatures above 120°C with low moisture, including frying, baking and roasting, and also processing by industry. While this applies to commercial food preparation, including catering and food manufacturing, European Food Safety Authority’s opinion states clearly that home-cooking choices can have a substantial impact on the level of acrylamide humans are exposed to through the diet.
How to reduce acrylamide in food at home
The following is a summary of the EFSA’s 2015 scientific review, however, it is important to note that EFSA has not evaluated the validity of these findings.
(Note: µg or mg/kg = micrograms or milligrams per kilogram)
Choice of ingredients:
- Coffee substitutes made from chicory generally contained on average six times more acrylamide (3 mg/kg) than cereal-based coffee substitutes (0.5 mg/kg)
- Fried products made from potato dough (including crisps and snacks) generally contained 20% less acrylamide (338 µg/kg) than those made from fresh potato (392 µg/kg)
- Potatoes grown in sulphur-deficient soil usually accumulate less asparagine, reducing acrylamide formation during heating
Storage method
- Storage of potatoes at below 8° C generally increases sugar levels in potatoes, potentially leading to higher acrylamide levels following cooking
- Soaking potato slices in water or citric acid solution can reduce acrylamide levels in crisps by up to 40% or 75%, respectively.
Processing (temperature and duration)
- Lighter coffee roasts generally contained more acrylamide than medium and dark roasts (which are roasted for longer), potentially increasing average exposure by 14%
- Tests by industry and consumer organisations indicate hot-air fryers generally produce 30-40% more acrylamide than conventional deep oil fryers
- Temperature generally increases acrylamide levels in French fries more than cooking time; frying above 175°C can lead to greatly increased levels.
Home cooking
- Consumer preferences for crispy and brown French fries and other fried potato products may increase average dietary exposure by 64% (for high consumers, by 80%)
- Toasting bread for five minutes instead of three minutes can increase the acrylamide content from 31µg/kg up to 118µg/kg, depending on the bread type and temperature of the toaster. Consumption of well-toasted bread, however, only increases overall average dietary exposure by 2.4%.
- Go for gold: As a general rule of thumb, aim for a golden yellow colour or lighter when frying, baking, toasting or roasting starchy foods like potatoes, root vegetables and bread.
- Check the pack
Check for cooking instructions on the pack and follow carefully when frying or oven-cooking packaged food products such as chips, roast potatoes and parsnips. The on-pack instructions are designed to cook the product correctly. This ensures that you aren’t cooking starchy foods for too long or at temperatures which are too high.
- Don’t keep raw potatoes in the fridge
Don’t store raw potatoes in the fridge if you intend to cook them at high temperatures (e.g. roasting or frying). Storing raw potatoes in the fridge may lead to the formation of more free sugars in the potatoes (a process sometimes referred to as ‘cold sweetening’) and can increase overall acrylamide levels especially if the potatoes are then fried, roasted or baked. Raw potatoes should ideally be stored in a dark, cool place at temperatures above 8°C.
- Eat a varied and balanced diet
Acrylamide levels in food vary widely depending on the manufacturer, the cooking time, and the method and temperature of the cooking process 92, 93. The best advice at this time is to follow established dietary guidelines and eat a healthy, balanced diet that is low in fat and rich in high-fiber grains, fruits, and vegetables.
TAG polymers
Polymerisation reactions are accelerated by the high temperatures used in frying but the identification of specific polymeric structures formed is very difficult. Consequently, there are no relevant studies on their effects on metabolic pathways with the exception of those concerning their absorption and digestibility.
Even though complexity of dimers and polymers is a major handicap for nutritional studies, two key points support further analytical and nutritional research. First, TAG polymers constitute the major fraction in used frying oils and fats, and second, the low absorption of the dimeric and polymeric fatty acids released does not necessarily means lack of health risk. In fact, it involves increased levels of non-digested, non-absorbed lipids throughout the gastrointestinal tract that might potentially affect epithelial cells and microflora metabolism. In connection with this subject, it has been reported that both unabsorbed lipids and bile acids secreted in response to a high fat intake might injure the intestinal mucose by their detergent activity, and metabolites of bile acids formed by intestinal bacteria (secondary bile acids) act as tumour promoters 94. Also, an interesting aspect is the potential contribution of intestinal flora to the production of mutagens from the oxidation of faecal lipids and the effect of vitamin E as a chemopreventive agent 95.
Cooking oil smoke points
The smoke point of an oil or fat is the temperature at which, under specific and defined conditions, an oil begins to produce a continuous bluish smoke that becomes clearly visible 96. Smoke point values can vary greatly, depending on factors such as the volume of oil utilized, the size of the container, the presence of air currents, the type and source of light as well as the quality of the oil and its acidity content, otherwise known as free fatty acid content 97. The higher free fatty acid in the oil to begin with, the quicker it will break down and start smoking 97, 98. The higher in quality and the lower in free fatty acid content, the higher the smoke point 99. It is important to consider, however, that the free fatty acid only represents typically less than 1% of the total oil and consequently renders smoke point a poor indicator of the capacity of a fat or oil to withstand heat 99, 100, 101.
The smoke point of an oil correlates with their level of refinement 102, 103. Many cooking oils have smoke points above standard home cooking temperatures:
Standard Home Cooking Temperatures 26
- Pan frying (sauté) on stove top heat: 120 °C (248 °F)
- Deep frying: 160 – 180 °C (320 °F – 356 °F)
- Oven baking: Average of 180 °C (356 °F)
Smoke point decreases at different pace in different oils 31.
Considerably above the temperature of the smoke point is the flash point, the point at which the vapors from the oil can ignite in air, given an ignition source.
Table 12. Smoke points and oxidative stability of various fats and cooking oils
| Fat | Quality | Smoke Point | |
|---|---|---|---|
| Almond oil | 221°C | 430°F | |
| Avocado oil | Refined | 270°C | 520°F |
| Mustard oil | 250°C | 480°F | |
| Butter | 150°C | 302°F | |
| Canola oil | 220-230°C | 428–446°F | |
| Canola oil (Rapeseed) | Expeller press | 190-232°C | 375-450°F |
| Canola oil (Rapeseed) | Refined | 204°C | 400°F |
| Canola oil (Rapeseed) | Unrefined | 107°C | 225°F |
| Castor oil | Refined | 200°C | 392°F |
| Coconut oil | Refined, dry | 204°C | 400°F |
| Coconut oil | Unrefined, dry expeller pressed, virgin | 177°C | 350°F |
| Corn oil | 230-238°C | 446-460°F | |
| Corn oil | Unrefined | 178°C | 352°F |
| Cottonseed oil | Refined, bleached, deodorized | 220-230°C | 428–446 °F |
| Flaxseed oil | Unrefined | 107°C | 225°F |
| Lard | 190°C | 374°F | |
| Olive oil | Refined | 199-243°C | 390-470°F |
| Olive oil | Virgin | 210°C | 410°F |
| Olive oil | Extra virgin, low acidity, high quality | 207°C | 405°F |
| Olive oil | Extra virgin | 190°C | 374°F |
| Olive oil | Extra virgin | 160°C | 320°F |
| Palm oil | Difractionated | 235°C | 455°F |
| Peanut oil | Refined | 232°C | 450°F |
| Peanut oil | 229°C | 445°F | |
| Peanut oil | 227°C | 441°F | |
| Peanut oil | Unrefined | 160°C | 320°F |
| Rice bran oil | Refined | 213°C | 415°F |
| Sesame oil | Unrefined | 177°C | 350°F |
| Sesame oil | Semirefined | 232°C | 450°F |
| Soybean oil | 234°C | 453°F | |
| Sunflower oil | Neutralized, dewaxed, bleached & deodorized | 252-254°C | 486–489°F |
| Sunflower oil | Semirefined | 232°C | 450°F |
| Sunflower oil | 227°C | 441°F | |
| Sunflower oil | Unrefined, first cold-pressed, raw | 107°C | 225°F |
| Sunflower oil, high oleic | Refined | 232°C | 450°F |
| Sunflower oil, high oleic | Unrefined | 160°C | 320°F |
| Vegetable oil blend | Refined | 220°C | 428°F |
Summary
For cooking and deep-frying foods the healthiest oils are those that are high in monounsaturated and polyunsaturated fats, such as vegetable oils like canola oil, olive oil, peanut oil sunflower oil, rice bran, soybean oil, safflower oil etc. These types of cooking oils can help lower your risk of heart disease when used instead of saturated and trans fats. And the key to choosing the type of oil to use is what type of flavor you want to obtain from the oil and its smoke point.
Some oils can handle the heat, and some can’t. An oil’s smoke point is the temperature at which it will start to smoke and break down. When cooking oil starts to smoke, it can lose some of its nutritional value and can give food an unpleasant taste. Oils with high smoke points, such as corn oil, soybean oil, peanut oil and sesame oil, are good for high-heat frying and stir-frying. Olive oil, canola oil and grapeseed oil have moderately high smoke points, making them good for sauteing over medium-high heat.
Oils with low smoke points, such as flaxseed oil and walnut oil, are best saved for use in salad dressings and dips.
Deep-fat frying causes the hydrolysis, oxidation, and polymerization of the oil. Hydrolysis increases the amount of free fatty acids, mono- and diacylglycerols, and glycerols in oils. Oxidation occurs at a greater rate than hydrolysis during deep-fat frying. Oxidation produces hydroperoxides and then low molecular volatile compounds such as aldehydes, ketones, carboxylic acids, and short-chain alkanes and alkenes. Dimers and polymers are also formed in oil by radical and Diels-Alder reactions during deep-fat frying. However, from the literature reviewed, there is general agreement that a moderate consumption of used frying oils under normal culinary practices is safe, but it is also evident that some compounds formed can impair their nutritional value or be potentially harmful.
High consumption of fried foods is probably related to a higher risk of weight gain, metabolic syndrome and hypertension 104.
The myth that frying foods is generally associated with a higher risk of cardiovascular disease is not supported by the available evidence 104.
Extra-virgin olive oil significantly reduces the risk of cardiovascular disease clinical events and weight gain, based on the results of a large randomised trial that included as part of the intervention the recommendation to use large amounts of extra-virgin olive oil for culinary purposes, also for frying foods. But the whole Mediterranean dietary pattern plays a more significant role rather than just the supplemental extra-virgin olive oil alone 105.
Replenishment of fresh oil, frying conditions, quality of frying oil, food materials, fryer, antioxidants, and oxygen concentration affect the quality and flavor of oil during deep-fat frying.
- U.S. Department of Health and Human Services. The Dietary Guidelines for Americans. https://health.gov/dietaryguidelines/[↩]
- U.S. Department of Health and Human Services. What Are the Types of Fat ? https://www.move.va.gov/docs/NewHandouts/Nutrition/N09_WhatAreTheTypesOfFat.pdf[↩][↩]
- American Heart Association. Monounsaturated Fat. https://healthyforgood.heart.org/Eat-smart/Articles/Monounsaturated-Fats[↩]
- American Heart Association. Polyunsaturated Fat. https://healthyforgood.heart.org/Eat-smart/Articles/Polyunsaturated-Fats[↩][↩]
- American Heart Association. Saturated Fat. https://healthyforgood.heart.org/Eat-smart/Articles/Saturated-Fats[↩][↩]
- U.S. Food and Drug Administration. Trans Fat. https://www.accessdata.fda.gov/scripts/interactivenutritionfactslabel/trans-fat.html[↩][↩]
- U.S. Food and Drug Administration. Trans Fat Now Listed With Saturated Fat and Cholesterol. https://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm274590.htm[↩]
- U.S. Food and Drug Administration. Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat). https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm449162.htm[↩]
- Uusitalo U, Feskens EJ, Tuomilehto J, Dowse GK, Haw U, Fareed D, Hemraj F, Gareebo H, Alberti KG, Zimmet P. Fall in total cholesterol concentration over five years in association with changes in fatty acid composition of cooking oil in Mauritius: cross sectional survey. BMJ. 1996; 313: 1044–1046. http://www.bmj.com/content/313/7064/1044[↩][↩][↩]
- Circulation August 15, 2017, Volume 136, Issue 7. https://doi.org/10.1161/CIR.0000000000000510. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. http://circ.ahajournals.org/content/early/2017/06/15/CIR.0000000000000510[↩][↩]
- U.S. Department of Health and Human Services. Know Your Fats. https://health.gov/dietaryguidelines/dga2005/toolkit/healthfacts/fats.htm[↩]
- American Heart Association. The Skinny on Fats. http://www.heart.org/HEARTORG/Conditions/Cholesterol/PreventionTreatmentofHighCholesterol/Know-Your-Fats_UCM_305628_Article.jsp[↩]
- U.S. Department of Health and Human Services. Know Your Fats. https://health.gov/dietaryguidelines/dga2005/toolkit/olderadults/oafats.htm[↩]
- U.S. Department of Health and Human Services. National Heart, Lung, and Blood Institute. Cooking with Healthier Fats and Oils. https://www.nhlbi.nih.gov/health/educational/wecan/downloads/tip-fats-and-oils.pdf[↩]
- American Heart Association. Healthy Cooking Oils. https://recipes.heart.org/Articles/1013/Healthy-Cooking-Oils[↩]
- Nwosu, V, Boyd, L. Oxidative Stability of various oils as determined by Rancimat Method”. North Carolina State University. Department of Food Science.[↩]
- Gomez-Alonso S, Fregapane G, Desampardos Salvador M, Gordon M. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J Agric Food Chem. 2003;51:667–72.[↩]
- Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hubbard, L.J. & Farkas, B.E. (2000). Influence of oil temperature onheat transfer during immersion frying. Journal of Food Processingand Preservation, 24, 143–162.[↩]
- Food and Agriculture Organizations of the United Nations. Chapter 6 : Selected uses of fats and oils in food. http://www.fao.org/docrep/v4700e/V4700E0b.htm[↩][↩][↩]
- Ziaiifar, A. M., Achir, N., Courtois, F., Trezzani, I. and Trystram, G. 2008. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 43: 1410-1423.[↩][↩][↩][↩]
- Achir, N., Kara, W., Chipeaux, C., Trezzani, I. & Cuvelier, M.E.(2006). Effect of energy transfer conditions on the chemicaldegradation of frying oil. European Journal of Lipid Science and Technology, 108, 999–1006.[↩]
- Gertz, C. (2005). Quality control and safety of cooking oils. Frankfurt:Workshop Deep-Frying.[↩]
- Seppanen, C.M. & Saari Csallany, A. (2002). Formation of 4-hydroxynonenal, a toxic aldehyde, in soybean oil at frying temperature. Journal of the American Oil Chemists’ Society, 79, 1033–1038.[↩]
- Matthaus, B. & Wohrmann, D. (2006). Formation of 4-hydroxy-2-trans-nonenal during heating of edible oils. 4th Euro Fed Lipid Congress: Oils, Fats and Lipids for a Healthier Future, Madrid, Spain.[↩]
- ACNEM Journal June 2015; volume 34(2):8–12. “Cooking with extra virgin olive oil” http://acnem.org/members/journals/ACNEM_Journal_June_2015.pdf[↩][↩][↩][↩][↩][↩][↩][↩]
- Tabee, E., Jägerstad, M. and Dutta, P. C. 2009. Frying quality characteristics of French fries prepared in refined olive oil and palm olein. J. Am. Oil Chem. Soc. 86: 885-893.[↩][↩][↩]
- Boatella-Riera, J., Codony, R., Rafecas, M. and Guardiola, F. 2000. Recycled cooking oils: assessment of risks for public health. European Parliament. PE 289.889/Fin.St.[↩]
- Seppanen, C. M. and Sarri-Csallany, A. 2002. Formation of 4-hydroxynonenal, a toxic aldehyde, in soybean oil at frying temperature. J. Am. Oil Chem. Soc. 79: 1033-1038.[↩]
- Romero, A., Bastida, S. and Sanchez-Muniz, F. J. 2006. Cyclic fatty acid monomer formation in domestic frying of frozen foods in sunflower oil and high oleic acid sunflower oil without oil replenishment. Food Chem. Toxicol. 44: 1674-1681.[↩]
- Monoj K. Gupta, Kathleen Warner, Pamela J. White (2004). Frying technology and Practices. AOCS Press, Champaign, Illinois.[↩][↩]
- Fats and oils in human nutrition. Food and Agriculture Organization of the United Nations and the World Health Organization. 1994. ISBN 92-5-103621-7.[↩]
- Nwosu, V., et al,. Oxidative Stability of various oils as determined by Rancimat Method. Department of Food Science.: North Carolina State University.[↩]
- Methrom. “Oxidative stability of oils and fats – Rancimat method”. Application Bulletin. 204/2 e.[↩][↩]
- Fritch, C. W. 1981. Measurements of frying fat deterioration: a brief review. J. Am. Oil Chem. Soc. 58: 272-274.[↩][↩]
- Firestone, D. 2007. Regulation of frying fat and oil, In “Deep Frying: Chemistry, Nutrition, and Practical Applications”. 2nd ed. pp. 373-385. Erickson, M. D. ed. AOCS Press, Urbana, USA.[↩]
- Lee, C. H. 2009. The optimum maintain of frying oil quality and the rapid measurements of acid value and total polar compounds. Taiwan Food News 234: 70-78.[↩][↩]
- Bansal, G., Zhou, W., Barlow, P. J., Joshi, P., Neo, F. L. and Lo, H. L. 2010. Evaluation of commercially available rapid test kits for the determination of oil quality in deep-frying operations. Food Chem. 121: 621-626.[↩]
- Paul, S. and Mittal, G. S. 1997. Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit. Rev. Food Sci. Nutr. 37: 635-662.[↩]
- Journal of Food and Drug Analysis, Vol. 21, No. 1, 2013, Pages 58-65. doi:10.6227/jfda.2013210107. Total Polar Compounds and Acid Values of Repeatedly Used Frying Oils Measured by Standard and Rapid Methods. http://www.fda.gov.tw/upload/133/Content/2013050913435287631.pdf[↩][↩]
- Lee, C. H. 2009. How to manage the frying oil quality. Taiwan Food News 232: 38-42.[↩]
- Akoh, C. C. and Min, D. B. 2002. Food Lipids. Marcel Dekker, New York.[↩]
- Moreno d, L ́opez-berenguer c, Garcia-viguera c. Effects of Stir-Fry Cooking with Different Edible Oils on the Phytochemical Composition of Broccoli. J Food Sci. 2007;72.[↩]
- Verela, G., Ruiz-Roso, B. “Some effects of frying on dietary fat intake”. Nutrition Reviews; 50: 256-
262. (1992[↩] - Choe, E. and Min, D. B. 2007. Chemistry of deep-fat frying oils. J. Food Sci. 72: R77-R86.[↩]
- Casal S, Malhero R, Sendas A, Oliveira O, Pereira J. Olive oil stability under deep-frying conditions.
Food Chem Toxicol. 2010;48:2972–9.[↩] - Moncel, B. “Functions of Fat in Food”. About Food.com[↩]
- Fats and Oils in human nutrition. FAO. Chapter 6. Cooking Oils.[↩]
- Methrom. Oxidation stability of oils and fats – Rancimat method. Application Bulletin 204/2 e.[↩]
- Rajkumar, V., Moreira, R. & Barrufet, M. (2003). Modeling thestructural changes of tortilla chips during frying. Journal of Food Engineering, 60, 167–175.[↩]
- Mehta, U. & Swinburn, B. (2001). A review of factors affecting fatabsorption in hot chips. Critical Reviews in Food Science and Nutrition, 41, 133–154.[↩]
- Moreira, R.G., Sun, X. & Chen, Y. (1997). Factors affecting oil uptakein tortilla chips in deep-fat frying. Journal of Food Engineering, 31,485–498.[↩]
- Pedreschi, F., Hernandez, P., Figueroa, C. & Moyano, P. (2005).Modeling water loss during frying of potato slices. International Journal of Food Properties, 8, 289–299.[↩]
- Rojas, J., Avallone, S., Brat, P., Trystram, G. & Bohuon, P. (2006). Effect of deep-fat frying on ascorbic acid, carotenoids and potassium contents of plantain cylinders. International Journal of Food Sciences and Nutrition, 57, 123–136.[↩]
- Blumenthal, M.M. & Stier, R.F. (1991). Optimization of deep-fatfrying operations. Trends in Food Science and Technology, 2, 144– 148.[↩]
- Garayo, J. & Moreira, R. (2002). Vacuum frying of potato chips. Journal of Food Engineering, 55, 181–191.[↩]
- Liu-Ping, F., Min, Z. & Mujumdar, A.S. (2005). Vacuum frying of carrot chips. Drying Technology, 23, 645–656.[↩]
- Tan, K.J. & Mittal, G.S. (2006). Physicochemical properties changes of donuts during vacuum frying. International Journal of Food Prop-erties, 9, 85–98.[↩]
- G Márquez-Ruiz & MC Dobarganes (2007) Nutritional and physiological effects of used frying fats. In Deep Frying: Chemistry, Nutrition and Practical Applications, 2nd ed., pp. 173–203 [ MD Erickson , editor]. Champaign, IL: AOCS Press.[↩]
- R Estruch , E Ros , J Salas-Salvadó , et al. (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368, 1279–1290.[↩]
- A Vallverdú-Queralt , JF Rinaldi de Alvarenga , R Estruch , et al. (2013) Bioactive compounds present in the Mediterranean sofrito. Food Chem 141, 3365–3372.[↩][↩]
- M Stott-Miller , ML Neuhouser & JL Stanford (2013) Consumption of deep-fried foods and risk of prostate cancer. Prostate 73, 960–969.[↩][↩]
- Q Dai , XO Shu , F Jin , et al. (2002) Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 11, 801–808.[↩][↩]
- C Galeone , C Pelucchi , R Talamini , et al. (2005) Role of fried foods on oral/pharyngeal and oesophageal cancers. Br J Cancer 92, 2065–2069.[↩][↩][↩]
- C Bosetti , R Talamini , F Levi , et al. (2002) Fried foods: a risk factor for laryngeal cancer? Br J Cancer 87, 1230–1233.[↩][↩]
- F Soriguer , G Rojo-Martínez , MC Dobarganes , et al. (2003) Hypertension is related to the degradation of dietary frying oils. Am J Clin Nutr 78, 1092–1097.[↩][↩][↩][↩]
- P Guallar-Castillón , F Rodríguez-Artalejo , E Lopez-García , et al. (2007) Intake of fried foods is associated with obesity in the cohort of Spanish adults from the European prospective investigation into cancer and nutrition. Am J Clin Nutr 86, 198–205.[↩][↩][↩]
- C Sayon-Orea , M Bes-Rastrollo , FJ Basterra-Gortari , et al. (2013) Consumption of fried foods and weight gain in a Mediterranean cohort: the SUN project. Nutr Metab Cardiovasc Dis 23, 144–150.[↩][↩][↩]
- C Donfrancesco , NC Lo , O Brignoli , et al. (2008) Italian network for obesity and cardiovascular disease surveillance: a pilot project. BMC Fam Pract 9, 53.[↩][↩][↩]
- C Sayon-Orea , MA Martínez-González , A Gea , et al. (2014) Consumption of fried foods and risk of metabolic syndrome: the SUN cohort study. Clin Nutr 33, 545–549.[↩][↩][↩][↩][↩]
- R Iqbal , S Anand , S Ounpuu , et al. (2008) Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 118, 1929–1937.[↩][↩]
- EK Kabagambe , A Baylin , X Siles , et al. (2003) Individual saturated fatty acids and nonfatal acute myocardial infarction in Costa Rica. Eur J Clin Nutr 57, 1447–1457.[↩][↩]
- P Guallar-Castillon , F Rodríguez-Artalejo , E Lopez-García , et al. (2012) Consumption of fried foods and risk of coronary heart disease: Spanish cohort of the European prospective investigation into cancer and nutrition study. BMJ 344, e363.[↩][↩][↩]
- EK Kabagambe , A Baylin , X Siles , et al. (2003) Individual saturated fatty acids and nonfatal acute myocardial infarction in Costa Rica. Eur J Clin Nutr 57, 1447–1457[↩]
- E Ringseis , A Gutgesell , C Dathe , et al. (2007) Feeding oxidized fat during pregnancy up-regulates expression of PPARα-responsive genes in the liver of rat foetuses. Lipids Health Dis 6, 6[↩]
- European Food Safety Authority. Acrylamide. https://www.efsa.europa.eu/en/topics/topic/acrylamide[↩][↩][↩][↩][↩][↩][↩]
- Food Standards Agency UK. Acrylamide. https://www.food.gov.uk/science/acrylamide-0[↩]
- National Cancer Institute, National Institutes of Health. Acrylamide in Food and Cancer Risk. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/acrylamide-fact-sheet[↩]
- Mulloy KB. Two case reports of neurological disease in coal mine preparation plant workers. American Journal of Industrial Medicine 1996; 30(1):56–61.[↩]
- Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature 2002; 419(6906):448–449.[↩]
- Dearfield KL, Abernathy CO, Ottley MS, Brantner JH, Hayes PF. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutation Research 1988; 195(1):45–77.[↩]
- Dearfield KL, Douglas GR, Ehling UH, et al. Acrylamide: A review of its genotoxicity and an assessment of heritable genetic risk. Mutation Research 1995; 330(1–2):71–99.[↩]
- Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. Journal of Agricultural and Food Chemistry 2003; 51(16):4504–4526.[↩]
- Fuhr U, Boettcher MI, Kinzig-Schippers M, et al. Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiology Biomarkers and Prevention 2006; 15(2):266–271.[↩]
- Pelucchi C, Galeone C, Levi F, et al. Dietary acrylamide and human cancer. International Journal of Cancer 2006; 118(2):467–471.[↩][↩]
- Mucci LA, Lindblad P, Steineck G, Adami HO. Dietary acrylamide and risk of renal cell cancer. International Journal of Cancer 2004; 109(5):774–776.[↩]
- Mucci LA, Adami HO, Wolk A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. International Journal of Cancer 2006; 118(1):169–173.[↩][↩]
- Olesen PT, Olsen A, Frandsen H, et al. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study. International Journal of Cancer 2008; 122(9):2094–2100.[↩]
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiology Biomarkers and Prevention 2007; 16(11):2304–2313.[↩]
- Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. American Journal of Clinical Nutrition 2008; 87(5):1428–1438.[↩]
- Stadler RH, Blank I, Varga N, et al. Acrylamide from Maillard reaction products. Nature 2002; 419(6906):449–450.[↩]
- Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry 2002; 50(17):4998–5006.[↩]
- Mojska H, Gielecinska I, Szponar L. Acrylamide content in heat-treated carbohydrate-rich foods in Poland. Roczniki Panstwowego Zakladu Higieny 2007; 58(1):345–349.[↩]
- RJ Vonk , M Kalivianakis , DM Minich , et al. (1997) The metabolic importance of unabsorbed dietary lipids in the colon. Scand J Gastroenterol 32, 65–67.[↩]
- S Campbell , WL Stone & S Whaley (2003) Development of gamma (γ)-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol 47, 249–259.[↩]
- Wikipedia. Smoke point. https://en.wikipedia.org/wiki/Smoke_point[↩][↩]
- Thomas, Alfred (2002). Fats and Fatty Oils. Ullmann’s Encyclopedia of Industrial Chemistry. Wenheim: Wiley-VCH. ISBN 3-527-30673-0.[↩][↩]
- Bastida, SS; et al. (2001). “Thermal oxidation of olive oil, sunflower oil and a mix of both oils during forty continuous domestic fryings of different foods”. Food Sci Tech Int. 7: 15–21. doi:10.1106/1898-plw3-6y6h-8k22[↩]
- Gennaro, L. et al., (1998). “Effect of biophenols on olive oil stability evaluated by thermogravimetric analysis.”. Journal of Agricultural and Food Chemistry. 46: 4465–4469. doi:10.1021/jf980562q.[↩][↩]
- Gomez-Alonso, S., et al, (2003). “Changes in phenolic composition and antioxidant activity of virgin olive oil during frying”. J Agric Food Chem. 51: 667–72. PMID 12537439. doi:10.1021/jf025932w.[↩]
- Chen, W., et al, (2013). “Total polar compounds and acid values of repeatedly used frying oils measured by standard and rapid methods” (PDF). J Food Drug Anal. 21 (1): 85-85. http://www.fda.gov.tw/upload/133/Content/2013050913435287631.pdf[↩]
- Boickish, Michael (1998). Fats and oils handbook. Champaign, IL: AOCS Press. pp. 95–96. ISBN 0-935315-82-9.[↩]
- Morgan, D.A. (1642). “Smoke, fire, and flash points of cottonseed, peanut, and other vegetable oils”. Oil & Soap. 19 (11): 193–198. doi:10.1007/BF02545481.[↩]
- British Journal of Nutrition, Volume 113, Issue S2, April 2015 , pp. S36-S48. https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/does-cooking-with-vegetable-oils-increase-the-risk-of-chronic-diseases-a-systematic-review/AF44097B0E9C4FF9BD44F1D55CD353D6/core-reader[↩][↩]
- Guasch-Ferré M, Hu FB, Martínez-González MA, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Medicine. 2014;12:78. doi:10.1186/1741-7015-12-78. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4030221/[↩]