Contents
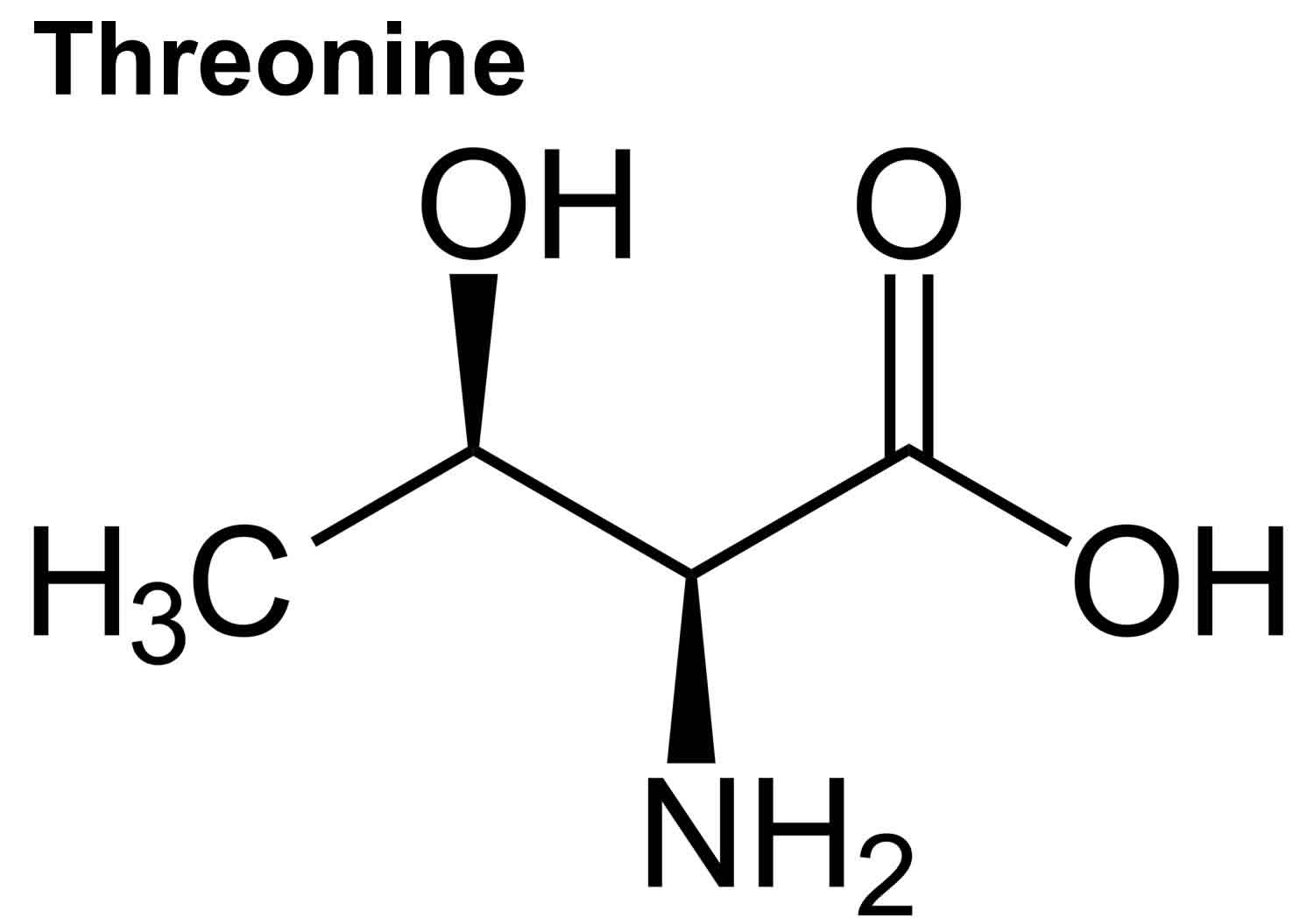
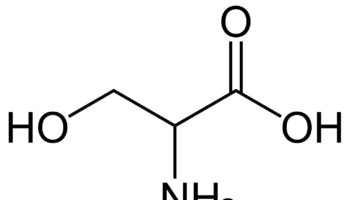
What is Threonine
Threonine (Thr) is an essential amino acid that cannot be synthesized by your body and need to be acquired from your diet 1, 2. The other essential amino acids include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tryptophan, and valine 3. Threonine is naturally in the L-threonine form, which is the active form of threonine. Threonine is found in eggs, milk, gelatin, and other proteins. Currently, the industrial production of L-threonine primarily relies on free-cell fermentation of E. coli for L-threonine production due to its well-defined genetic background and shorter fermentation period 4, 5, 6. Animal and human studies have shown threonine is of great importance for the maintenance of intestinal health 7, 8, 9, 10. Threonine deficiency and excessive intake of threonine can compromise the gut barrier integrity 9. Animal studies have shown limited threonine intake causes diarrhea, reduced mucin synthesis, changed goblet cell morphology, villous atrophy, and increased paracellular permeability 11, 12, 13, 14, 15. In contrast, excessive threonine intake was also reported to reduce mucin synthesis and to result in changes in the villus architecture 9. Furthermore, increasing the threonine plasma concentrations leads to accumulation of threonine and glycine in the brain. Such accumulation affects the neurotransmitter balance which may have consequences for the brain development during early infant life. Therefore, excess dietary threonine is suggested to be neurotoxic because of a consequent elevation of brain glycine concentrations 16. Excessive threonine intake during infant feeding should be avoided 16. The present threonine amino acid recommendations of formula-fed infants is 68 mg/kg/day for threonine in the first month of life, based on the amino acid profile of human milk and the average intake of breast-fed infants 17, 18, 19, 1. In intravenously fed, postsurgical infants, the threonine requirement was estimated to be 32.8 mg/kg/day using the indicator amino acid oxidation (IAAO) method 20, 21, 22. Adult humans require about 16 to 24 mg/kg/day of threonine (26 mg/kg/day for pregnant women and 30 mg/kg/day for breastfeeding mothers) 23. Based on distribution data from the 1988–1994 Third National Health and Nutrition Examination Survey (NHANES 3), the mean daily intake for all life stage and gender groups of threonine from food and supplements is 3 g/day 23. Men 51 through 70 years of age had the highest intakes at the 99th percentile of 7.1 g/day 23.
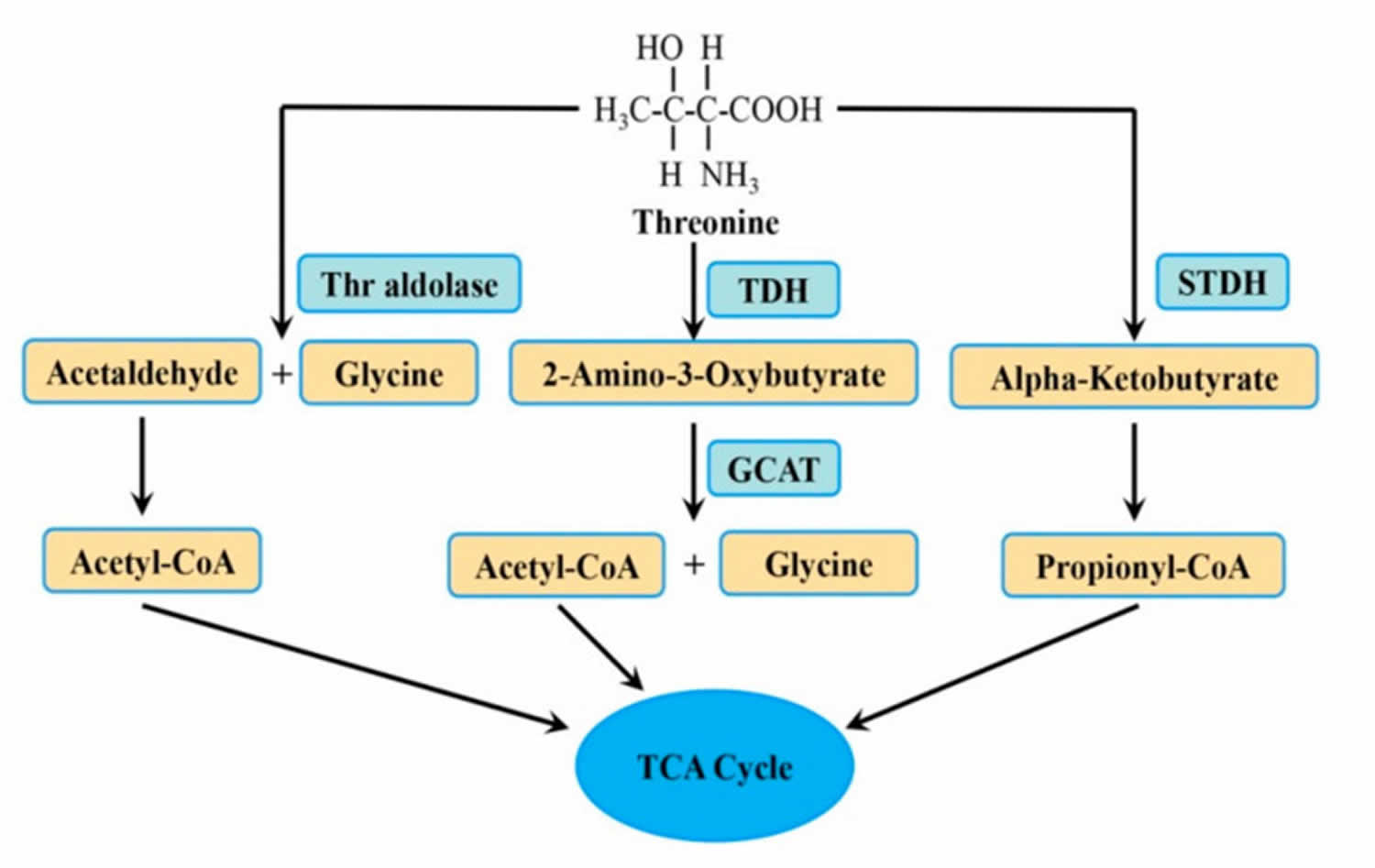
Threonine breakdown in mammals appears to be due primarily (70-80%) to the activity of threonine dehydrogenase that oxidizes threonine to 2-amino-3-oxobutyrate, which forms glycine and acetyl CoA, whereas threonine dehydratase that catabolizes threonine into 2-oxobutyrate and ammonia, is significantly less active. The degradation of threonine is impaired in the following metabolic diseases:
- Combined malonic and methylmalonic aciduria also called “CMAMMA” is an inherited condition in which malonic acid (MA) and methylmalonic acid (MMA) accumulate in the blood and urine of affected individuals 24, 25, 26, 27, 28, 29, 30. Combined malonic and methylmalonic aciduria (CMAMMA) is biochemically characterized by elevated concentration of the diagnostic compounds malonic acid (MA) and methylmalonic acid (MMA) in both urine and plasma. A distinguishing feature of combined malonic and methylmalonic aciduria (CMAMMA) is higher levels of methylmalonic acid (MMA) than malonic acid (MA) in the urine, although both are elevated. Methylmalonic acid (MMA) excretion in patients with CMAMMA has been reported to be 8–194 times normal, malonic acid (MA) excretion is typically present at relatively lower levels 3–91 times normal excretion 31, 26. Plasma malonic acid (MA)/methylmalonic acid (MMA) ratio can serve as fast diagnostic tool for detection of CMAMMA 32.
- Methylmalonic acidemia is a group of inherited disorders that prevent the body from breaking down proteins and fats (lipids) properly 33. Mutations in the MMUT, MMAA, MMAB, MMADHC, and MCEE genes cause methylmalonic acidemia. The long-term effects of methylmalonic acidemia depend on which gene is altered and the severity of the variant. The effects of methylmalonic acidemia, which usually appear in early infancy, vary from mild to life-threatening. Affected infants can experience vomiting, dehydration, weak muscle tone (hypotonia), developmental delays, excessive tiredness (lethargy), an enlarged liver (hepatomegaly), and failure to gain weight and grow at the expected rate (failure to thrive). Long-term complications can include feeding problems, intellectual disabilities, movement problems, chronic kidney disease, and inflammation of the pancreas (pancreatitis). People with methylmalonic acidemia can have frequent episodes of excess acid in the blood (metabolic acidosis) that cause serious health complications. Without treatment, methylmalonic acidemia can lead to coma and death in some cases.
- Propionic acidemia is a rare metabolic disorder affecting from 1/20,000 to 1/250,000 individuals in various regions of the world 34. Propionic acidemia is characterized by deficiency of propionyl-CoA carboxylase enzyme that is involved in the breakdown (catabolism) of amino acids isoleucine, valine, threonine, and methionine. Symptoms most commonly become apparent during the first weeks of life and may include abnormally diminished muscle tone (hypotonia), poor feeding, vomiting, listlessness (lethargy), dehydration and seizures. Without appropriate treatment, coma and death may result. Long-term treatment includes administration of a low-protein diet, possibly in combination with medical formula (medical foods) that are low in certain amino acids (i.e., amino acids which give rise to propionate, e.g., isoleucine, valine, threonine, and methionine). Infants and children with the disorder may develop secondary deficiency of carnitine, a substance that plays a role in metabolism and the proper use of fatty acids. In such cases, therapy includes administration of L-carnitine (carnitine or levocarnitine). Antibiotic therapy with metronidazole can reduce the burden of propionyl-CoA in the body, as this chemical is produced by some bacteria during fermentation of carbohydrates in your gut.
Figure 1. Threonine metabolic pathway
Footnote: Threonine (Thr) is broken down (metabolized) to different intermediates and end products by three enzymes.
Abbreviations: Thr = threonine; GCAT = 2-amino-3-oxobutyrate CoA ligase; STDH = serine/threonine dehydratase; TCA = tricarboxylic acid cycle; TDH = threonine dehydrogenase.
[Source 35 ]Threonine function
Threonine possesses a number of different functions within the human body:
- Threonine represents an essential component in the formation of connective tissues such as enamel, elastin, and collagen.
- Threonine is essential for lipid metabolism and helps to prevent the build up of fats within the liver.
- Threonine maintains the integrity of intestinal mucosa, thereby improving both digestion and immune function.
- Threonine is needed to produce proteins, as well as two amino acids- glycine and serine, which are also important to the formation of connective tissue.
- Threonine helps with muscle function by decreasing muscle spasticity and involuntary muscle contractions.
- Dietary threonine promotes sleep and facilitates sleep onset in Drosophila flies 36
Severe deficiency of threonine causes neurological dysfunction and lameness in experimental animals. Threonine is an immunostimulant which promotes the growth of thymus gland. It also can probably promote cell immune defense function. Threonine has been useful in the treatment of genetic spasticity disorders and multiple sclerosis at a dose of 1 gram daily.
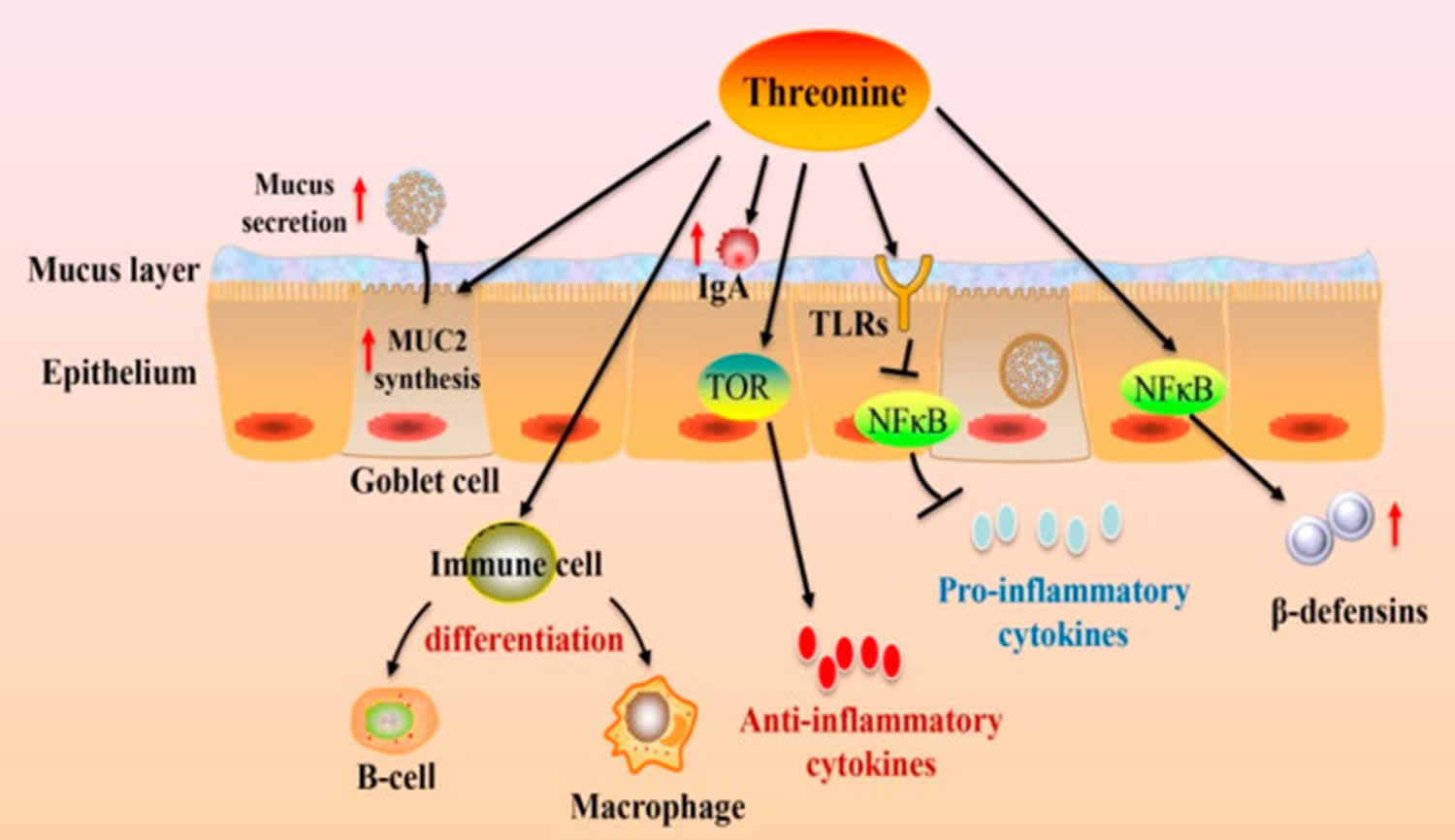
Figure 2. Threonine intestinal immune function
Footnote: Dietary threonine modulates the intestinal immune function. Dietary threonine affects goblet cell differentiation, which, in turn, stimulates MUC2 synthesis. threonine regulates immune cell differentiation and immunoglobulins (Ig) production. Meanwhile, threonine can modulate the release of cytokines via the TOR and NF-κB pathways.
Abbreviations: IgA = immunoglobulins A; MUC2 = mucin-2; TLRs = toll-like receptors; TOR = the target of rapamycin; NF-κB = nuclear factor κ-light-chain-enhancer of activated B cells.
[Source 35 ]Lipid metabolism
Essential amino acids have a close connection with the fat (lipid) metabolism, whereby certain amino acids (methionine, leucine, and isoleucine) can affect lipid deposition 37. Similarly, Threonine takes part in regulating lipid metabolism and deposition 38. Threonine supplementation in rats has been shown to enhance liver fat (lipid) metabolism, while threonine deficiency may induce liver triglycerides accumulation 39. Research on Pekin ducks found that dietary threonine shortage increased the expression of genes encoding 3-oxoacyl-ACP synthase (OXSM), very-long-chain fatty acids protein 7 (ELOVL7), and long-chain fatty acyl-CoA ligase (ACSBG2), which were related to fatty acid and triglyceride synthesis. However, genes participating in fatty acid oxidation, such as acyl-CoA dehydrogenase, acyl-CoA dehydrogenase family member 11 (ACAD11) and cytochrome P450 (CYP4B), as well as genes associated with fatty acid and triglyceride transport, acyl-CoA binding protein (DBI), apolipoprotein D (APOD), and microsomal triglyceride transfer protein (MTTP), were downregulated under threonine deficiency in Pekin ducks 38. The equilibrium between the synthesis of fatty acids (lipogenesis) and the breakdown of fatty acids (lipolysis) determines lipid homeostasis 40. Lipogenesis is the process by which preadipocytes develop into mature adipocytes (mature fat cells), which is modulated by adipogenic transcription factors including peroxisome proliferator-activated receptors (PPARs) 41. Threonine supplementation in a high-fat diet may decrease lipid deposition by regulating the PPARγ signaling pathway 42. Uncoupling protein-1 (UCP1) is a thermogenetic-related gene expressed in brown adipose tissue, which can disperse energy as heat to fight obesity 43. Uncoupling protein-1 (UCP1) expression was decreased in the brown adipose tissue of obese mice, but this was restored by supplementation with threonine in a high-fat diet 42. The disruption of lipid metabolism can contribute to cardiovascular disease. Threonine may be a positive modulator of lipid metabolic disorders. One study discovered that plasma threonine level is linked to a lower risk of having an atherogenic lipid profile in humans, an obvious negative correlation between threonine and levels of small dense low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) 44.
However, emerging evidence suggests that moderate dietary protein restriction is beneficial for metabolism in animals and humans 45, 46. Many studies have indicated that restriction of essential amino acids and non-essential amino acids can promote lipid metabolism and resist obesity through various pathways 47, 48. One study found that dietary threonine restriction helps to prevent the metabolic disorders associated with obesity 49. Dietary threonine restriction can improve metabolism by regulating hormone liver fibroblast growth factor 21 (FGF21) 49. FGF21 increases lipolysis in white adipose tissue and substrate consumption in the liver, making it an effective modulator of fatty acid oxidation and lipid metabolism 50.
Protein synthesis
Threonine is required for the synthesis of threonine-rich proteins in epithelial tissues, such as mucins (glycoprotein constituent of mucus) 51. Mucin synthesis is quite sensitive to dietary threonine concentration, where threonine deficiency or excessive dietary threonine reduces the production of gut mucosal protein and mucins in animals 52. Apart from intestinal mucin, threonine also has beneficial effects on protein synthesis in other tissues such as skeletal muscles. Studies on different aquatic animals and livestock have reported that threonine can promote growth and enhance the protein synthesis of skeletal muscle 52, 53. Threonine not only serves as a precursor for protein synthesis but also as a signaling molecule that can regulate the protein synthesis pathway. Insulin-like growth factor I (IGF-I), an upstream activator of mammalian target of rapamycin (mTOR) in skeletal muscle, is essential for muscle development and regeneration 54. A recent publication reported that dietary threonine promoted muscle protein synthesis through activating the PI3K/AKT/TOR signaling cascade via IGF-1 in fish 55. As a precursor of glycine, threonine is degraded by threonine dehydrogenase (TDH) to generate glycine. Glycine modulates protein synthesis by activating mTORC1 in a PI3K/Akt-dependent manner and suppressing proteolysis 56.
Nutrient digestion and absorption
The gut has various roles, including nutrient digestion and absorption as well as immune defense against pathogens and toxins 57, 58. The integrity of the intestinal structure is the foundation for the intestinal function of nutrient uptake and absorption. Adequate threonine is taken and utilized by the intestinal mucosa, and contributes to the maintainance of mucosa integrity. In the small intestine of broilers, dietary threonine supplementation has a positive effect on villus height, crypt depth, epithelial thickness, and the quantity of goblet cells 59, 60. Deficient or excessive dietary threonine reduces villous area, induces villous atrophy, and increases the apoptosis rate of intestinal epithelial cells in weaned piglets 61, 62. The higher villus contributes to providing more surface area for nutrient intake 60. In addition, the threonine demand for amylase synthesis is particularly high, accounting for around 11% of total protein. Studies have discovered that a lack of threonine in the diet reduces the synthesis of digestive enzymes and the activity of brush border enzymes in the animal gut 53, 63. It is therefore possible that threonine may aid in enhancing intestinal digestive and absorptive capacity.
Intestinal barrier function
The mucus layer that covers the intestinal epithelium is a vital aspect of the intestinal barrier function 64. The mucus layer is a physical layer that contains mucins, immunoglobulins, salts, antimicrobial substance, etc., which is secreted by different glands. The mucus layer serves as the frontline for preventing damage caused by digestive enzymes, microbes, and pathogens 65. Mucin-2 (MUC2) is the major constituent of intestinal mucus, which is produced primarily by goblet cells. Mucin-2 (MUC2) is a highly glycosylated glycoprotein with a central protein backbone rich in threonine and serine, and is connected to multiple O-linked oligosaccharide side chains, leading to high resistance to proteolysis 66. Threonine is the main factor to regulate intestinal mucin secretion. Compared with adequate threonine (0.89%), both a lack and excess of (0.37% and 1.11%) threonine significantly affects the amount and type of intestinal mucin in piglets 61. However, it is not clear how excessive dietary threonine reduces the synthesis of intestinal mucosal protein and mucins. One possible explanation is that neutral amino acids (including branched-chain amino acids) share the same transport systems with threonine, which restricts its intestinal intake, thus limiting the protein synthesis 52, 67. This possible explanation requires further study. Increasing evidence has shown that goblet cell differentiation and mucin production are sensitive to dietary threonine concentration 51, 68. When dietary threonine supplementation was higher than growth requirements, goblet cell density and MUC2 mRNA expression were enhanced in gut 69, 70. One study indicated that dietary threonine could affect goblet cell differentiation through modulating the expression of genes associated in the Notch-Hes1-Math1 pathway, which promoted the MUC2 synthesis 71. Mucin-2 (MUC2) has the dual functions of a physical barrier and immune regulation, and can interact with epithelium, microbiota, and the host immune system to maintain intestinal homeostasis. Moreover, the effect of threonine on mucin synthesis is related to the age and physiological state. It was found that 45% of the dietary threonine requirement was needed to maintain intestinal mucosa in 3-day-old pigs 72. Research on animals has shown that under conditions of disease and stress, such as ileitis, sepsis, inflammation, and intrauterine growth retardation, the threonine requirement in the intestine is greatly raised in order to promote mucin production 71. This suggests that intestinal inflammation increases the production of mucus to protect the gut. In conclusion, dietary threonine supplementation stimulates the synthesis of Mucin-2 (MUC2), making MUC2 exert a stronger effect on the gut barrier and immune function, and maintain intestinal homeostasis.
Gut microbiota
Dietary threonine has a positive impact on gut microbiota. High dietary threonine reduced Salmonella and Escherichia coli (E. coli) colonies, while increasing Lactobacillus in broiler chickens 69. In addition, studies have shown that when under stress, dietary threonine reequilibrates the gut microbiota of animals. A low crude protein diet supplemented with threonine (at 0.3%) recovered the microbial diversity and significantly enhanced the abundance of potentially beneficial microorganisms in laying hens 73. An amino acid cocktail containing L-threonine increased the frequency of beneficial populations of Enterobacteria, Lactobacillus, Bacteroides, and Enterococci in rats challenged with dextran sodium sulfate 74. One of the explanations for this effect might be that threonine promotes mucin secretion, because mucins cannot be digested in the small intestine and, therefore, arrive to the large intestine, where they serve as the substrates for saccharolytic bacteria 75. Koo et al. 76 found that the supplementation of threonine 15% higher than the nutrient requirements recommendation can interact with feed composition to alter gut microbial fermentation. The data also imply that threonine has beneficial effects on intestinal microbiota which might be related to mucin synthesis and immunoglobulin production as a result of threonine addition 74.
Immune function
Numerous reports have shown that dietary threonine is essential for maintaining gut immune function (Figure 2) 77, 78. Threonine is of great importance in maintaining the immune system because it is a major amino acid component of mucins and immunoglobulin (Ig) 79. The immune function depends on its innate immunity and adaptive immunity response in the gut. The adaptive immunity depends on lymphocytes proliferation and immunoglobulins (Ig) production. In vitro, threonine was proven to be used by lymphocytes to support their proliferation and antibody secretion 80. K203 is a CC chemokine that attracts macrophages and monocytes 81. C-C chemokine receptor type 9 (CCR9) is found mostly on gut-homing T lymphocytes and induces the formation of gut lymphoid tissue 82. C-X-C chemokine receptor type 5 (CXCR5) is expressed on B cells and B-helper T cells and is necessary for intestinal immunity 83. Threonine deficiency leads to enhanced K203 and CXCR5 mRNA expression, and reduced CCR9 mRNA expression, showing that immune cells tend to transfer to B cells and macrophages instead of T cells 80. Moreover, threonine is also a vital element of immunoglobulin (Ig) also known as antibody, taking up to 7–11% of total amino acids, especially immunoglobulin A (IgA). The polymeric immunoglobulin receptor (pIgR) is present on the basolateral surface of intestinal epithelium and is responsible for transporting IgA to the lumen through epithelial cells 84. Threonine can regulate polymeric immunoglobulin receptor (pIgR) concentration by NF-κB activation to influence IgA production under stress 80, 77.
Threonine not only regulates lymphocytes and immunoglobulin A (IgA) secretion but also modulates the expression of inflammatory cytokines. In the animal intestine, threonine supplementation up-regulates inflammatory factors interleukin (IL)-6 gene expression in piglets 71, down-regulates the expression of inflammatory mediators interferon γ (IFN-γ) and IL-12 in the broilers 69, 85 and tumor necrosis factor alpha (TNF-α) in fish 86. The levels of inflammatory factors, such as IL-8, IL-6, IL-1β, inducible nitric oxide synthase (iNOS), TNF-α, and IFN-γ are significantly decreased in stress-related mucosal disease of rats pretreated with threonine 87. When fish are infected with bacteria, threonine deficiency will aggravate an intestinal inflammatory response by promoting pro-inflammatory cytokine TNF-α, IL-1β, IFN-γ2, IL-6, IL-8, and IL-17 gene expression through the NF-κB signaling cascade, and inhibiting the expression of anti-inflammatory cytokines TGF-β1, TGF-β2, IL-4/13A, and IL-10 via the TOR complex signal pathway 86, 88. Futhermore, threonine supplementation may suppress the inflammatory cytokine synthesis by inhibiting the TLR4 signal pathway to improve intestinal immune function 89. Antimicrobial peptides in the gut also contribute to the intestinal immune response 90. A recent study found that L-threonine at the concentration of 1mM upregulates β-defensin (pBD-1, pBD-2, and pBD-3) expression by activating NF-κB signaling and inhibiting sirtuin-1 (SIRT1) expression in porcine intestinal epithelial cells 91. In addition, the interaction between threonine and fiber can enhance the immune function. Research showed that threonine and pectin had a synergistic action on intestinal immune response in broilers infected with coccidiosis 85. Moreover, dietary threonine supplementation with the addition of sugar beet pulp drove the antibody titer to a high level 92.
Threonine benefits
Threonine possesses a number of different functions within the human body:
- Threonine represents an essential component in the formation of connective tissues such as enamel, elastin, and collagen.
- Threonine is essential for lipid metabolism and helps to prevent the build up of fats within the liver.
- Threonine maintains the integrity of intestinal mucosa, thereby improving both digestion and immune function.
- Threonine is needed to produce proteins, as well as two amino acids- glycine and serine, which are also important to the formation of connective tissue.
- Threonine helps with muscle function by decreasing muscle spasticity and involuntary muscle contractions 93, 94, 95.
Threonine uses
L-threonine is authorized for use in food, cosmetics and as a veterinary medicinal product 96, 97, 98, 99.
Threonine food sources
Foods high in threonine include eggs, milk, cottage cheese, poultry, fish, meat, wheat germ, lentils, gelatin, and other proteins.
Threonine side effects
No data were found on healthy humans given oral L-threonoine supplements 23. However, L-threonine has been used clinically with the aim of increasing glycine concentrations in the cerebral spinal fluid of patients with spasticity 93. When given in amounts of 4.5 to 6.0 g/day for 14 days, no adverse clinical effects were noted in these patients 93. Threonine also has been studied in low birth weight infants. In a study of 163 low birth weight infants, threonine serum concentrations were directly related to the threonine concentrations of the formula 100. The authors suggested that threonine intakes should not exceed about 140 mg/kg body weight/day for premature infants 100. Excess dietary threonine is suggested to be neurotoxic because of a consequent elevation of brain glycine concentrations 16. Excessive threonine intake during infant feeding should be avoided 16. The present threonine amino acid recommendations of formula-fed infants is 68 mg/kg/day for threonine in the first month of life, based on the amino acid profile of human milk and the average intake of breast-fed infants 17, 18, 19, 1. In intravenously fed, postsurgical infants, the threonine requirement was estimated to be 32.8 mg/kg/day using the indicator amino acid oxidation (IAAO) method 20, 21, 22.
- Hogewind-Schoonenboom JE, Huang L, de Groof F, Zhu L, Voortman GJ, Schierbeek H, Vermes A, Chen C, Huang Y, van Goudoever JB. Threonine Requirement of the Enterally Fed Term Infant in the First Month of Life. J Pediatr Gastroenterol Nutr. 2015 Sep;61(3):373-9. doi: 10.1097/MPG.0000000000000807[↩][↩][↩]
- L-Threonine. http://www.t3db.ca/toxins/T3D4368[↩]
- Reeds PJ. Dispensable and indispensable amino acids for humans. J Nutr. 2000 Jul;130(7):1835S-40S. doi: 10.1093/jn/130.7.1835S[↩]
- Sun W, Shi S, Chen J, Zhao W, Chen T, Li G, Zhang K, Yu B, Liu D, Chen Y, Ying H, Ouyang P. Blue Light Signaling Regulates Escherichia coli W1688 Biofilm Formation and l-Threonine Production. Microbiol Spectr. 2022 Oct 26;10(5):e0246022. doi: 10.1128/spectrum.02460-22[↩]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific opinion on the safety of L-threonine produced by fermentation using Escherichia coli CGMCC 3703, for all animal species based on a dossier submitted by GBT Europe GmbH. EFSA Journal 2016; 14(1):4344, 13 pp. doi:10.2903/j.efsa.2016.4344[↩]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis, V, Azimonti, G, Bastos, ML, Christensen, H, Dusemund, B, Kouba, M, Kos Durjava, M, López-Alonso, M, López Puente, S, Marcon, F, Mayo, B, Pechová, A, Petkova, M, Sanz, Y, Villa, RE, Woutersen, R, Costa, L, Dierick, N, Flachowsky, G, Mantovani, A, Wallace, RJ, Tarrés-Call, J and Ramos, F, 2019. Scientific Opinion on the safety and efficacy of l-threonine produced by fermentation with Corynebacterium glutamicum KCCM 80117 for all animal species. EFSA Journal 2019;17(2):5602, 13 pp. https://doi.org/10.2903/j.efsa.2019.5602[↩]
- Rhodes JM. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut. 1989 Dec;30(12):1660-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1434463/pdf/gut00225-0008.pdf[↩]
- Fuller MF, Milne A, Harris CI, Reid TM, Keenan R. Amino acid losses in ileostomy fluid on a protein-free diet. Am J Clin Nutr. 1994 Jan;59(1):70-3. doi: 10.1093/ajcn/59.1.70[↩]
- Wang W, Zeng X, Mao X, Wu G, Qiao S. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J Nutr. 2010 May;140(5):981-6. doi: 10.3945/jn.109.118497[↩][↩][↩]
- Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr. 1998 Mar;128(3):606-14. doi: 10.1093/jn/128.3.606[↩]
- Hamard A, Sève B, Le Floc’h N. Intestinal development and growth performance of early-weaned piglets fed a low-threonine diet. Animal. 2007 Sep;1(8):1134-42. doi: 10.1017/S1751731107000560[↩]
- Hamard A, Mazurais D, Boudry G, Le Huërou-Luron I, Sève B, Le Floc’h N. A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J Nutr Biochem. 2010 Oct;21(10):914-21. doi: 10.1016/j.jnutbio.2009.07.004[↩]
- Law GK, Bertolo RF, Adjiri-Awere A, Pencharz PB, Ball RO. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1293-301. doi: 10.1152/ajpgi.00221.2006[↩]
- Faure M, Moënnoz D, Montigon F, Mettraux C, Breuillé D, Ballèvre O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J Nutr. 2005 Mar;135(3):486-91. doi: 10.1093/jn/135.3.486[↩]
- Nichols NL, Bertolo RF. Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets. J Nutr. 2008 Jul;138(7):1298-303. doi: 10.1093/jn/138.7.1298[↩]
- Boehm G, Cervantes H, Georgi G, Jelinek J, Sawatzki G, Wermuth B, Colombo JP. Effect of increasing dietary threonine intakes on amino acid metabolism of the central nervous system and peripheral tissues in growing rats. Pediatr Res. 1998 Dec;44(6):900-6. doi: 10.1203/00006450-199812000-00013[↩][↩][↩][↩]
- Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. 2007;(935):1-265.[↩][↩]
- Agostoni C, Domellöf M. Infant formulae: from ESPGAN recommendations towards ESPGHAN-coordinated global standards. J Pediatr Gastroenterol Nutr. 2005 Nov;41(5):580-3. doi: 10.1097/01.mpg.0000188741.46380.24[↩][↩]
- Stam J, Sauer PJ, Boehm G. Can we define an infant’s need from the composition of human milk? Am J Clin Nutr. 2013 Aug;98(2):521S-8S. doi: 10.3945/ajcn.112.044370[↩][↩]
- Chapman KP, Courtney-Martin G, Moore AM, Ball RO, Pencharz PB. Threonine requirement of parenterally fed postsurgical human neonates. Am J Clin Nutr. 2009 Jan;89(1):134-41. doi: 10.3945/ajcn.2008.26654[↩][↩]
- Elango R, Ball RO, Pencharz PB. Indicator amino acid oxidation: concept and application. J Nutr. 2008 Feb;138(2):243-6. doi: 10.1093/jn/138.2.243[↩][↩]
- Hayamizu K, Aoki Y, Izumo N, Nakano M. Estimation of inter-individual variability of protein requirement by indicator amino acid oxidation method. J Clin Biochem Nutr. 2021 Jan;68(1):32-36. doi: 10.3164/jcbn.20-79[↩][↩]
- Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. https://nap.nationalacademies.org/download/10490[↩][↩][↩][↩]
- Tucci S. Brain metabolism and neurological symptoms in combined malonic and methylmalonic aciduria. Orphanet J Rare Dis. 2020 Jan 22;15(1):27. doi: 10.1186/s13023-020-1299-7[↩]
- Gabriel, M.C., Rice, S.M., Sloan, J.L., Mossayebi, M.H., Venditti, C.P. and Al-Kouatly, H.B. (2021), Considerations of expanded carrier screening: Lessons learned from combined malonic and methylmalonic aciduria. Mol. Genet. Genomic Med., 9: e1621. https://doi.org/10.1002/mgg3.1621[↩]
- Sloan JL, Johnston JJ, Manoli I, Chandler RJ, Krause C, Carrillo-Carrasco N, Chandrasekaran SD, Sysol JR, O’Brien K, Hauser NS, Sapp JC, Dorward HM, Huizing M; NIH Intramural Sequencing Center Group; Barshop BA, Berry SA, James PM, Champaigne NL, de Lonlay P, Valayannopoulos V, Geschwind MD, Gavrilov DK, Nyhan WL, Biesecker LG, Venditti CP. Exome sequencing identifies ACSF3 as a cause of combined malonic and methylmalonic aciduria. Nat Genet. 2011 Aug 14;43(9):883-6. doi: 10.1038/ng.908[↩][↩]
- Levtova A, Waters PJ, Buhas D, Lévesque S, Auray-Blais C, Clarke JTR, Laframboise R, Maranda B, Mitchell GA, Brunel-Guitton C, Braverman NE. Combined malonic and methylmalonic aciduria due to ACSF3 mutations: Benign clinical course in an unselected cohort. J Inherit Metab Dis. 2019 Jan;42(1):107-116. doi: 10.1002/jimd.12032[↩]
- Combined malonic and methylmalonic aciduria. https://medlineplus.gov/genetics/condition/combined-malonic-and-methylmalonic-aciduria[↩]
- Combined malonic and methylmalonic acidemia. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=289504[↩]
- Combined malonic and methylmalonic acidemia. https://rarediseases.info.nih.gov/diseases/10818/combined-malonic-and-methylmalonic-aciduria[↩]
- Alfares A, Nunez LD, Al-Thihli K, et al. Combined malonic and methylmalonic aciduria: exome sequencing reveals mutations in the ACSF3 gene in patients with a non-classic phenotype. J Med Genet. 2011;48:602–605. doi: 10.1136/jmedgenet-2011-100230[↩]
- de Sain-van der Velden MG, van der Ham M, Jans JJ, Visser G, Prinsen HC, Verhoeven-Duif NM, van Gassen KL, van Hasselt PM. A New Approach for Fast Metabolic Diagnostics in CMAMMA. JIMD Rep. 2016;30:15-22. doi: 10.1007/8904_2016_531[↩]
- Methylmalonic acidemia. https://medlineplus.gov/genetics/condition/methylmalonic-acidemia[↩]
- Propionic Acidemia. https://rarediseases.org/rare-diseases/propionic-acidemia[↩]
- Tang Q, Tan P, Ma N, Ma X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients. 2021 Jul 28;13(8):2592. doi: 10.3390/nu13082592[↩][↩]
- Ki Y, Lim C. Sleep-promoting effects of threonine link amino acid metabolism in Drosophila neuron to GABAergic control of sleep drive. Elife. 2019 Jul 17;8:e40593. doi: 10.7554/eLife.40593[↩]
- Nie C., He T., Zhang W., Zhang G., Ma X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018;19:954. doi: 10.3390/ijms19040954[↩]
- Jiang Y., Xie M., Fan W., Xue J., Zhou Z., Tang J., Chen G., Hou S. Transcriptome Analysis Reveals Differential Expression of Genes Regulating Hepatic Triglyceride Metabolism in Pekin Ducks During Dietary Threonine Deficiency. Front. Genet. 2019;10:710. doi: 10.3389/fgene.2019.00710[↩][↩]
- Ross-Inta C.M., Zhang Y.F., Almendares A., Giulivi C. Threonine-deficient diets induced changes in hepatic bioenergetics. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1130–G1139. doi: 10.1152/ajpgi.90545.2008[↩]
- Wu Y., Ma N., Song P., He T., Levesque C., Bai Y., Zhang A., Ma X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019;149:1523–1532. doi: 10.1093/jn/nxz102[↩]
- Lasar D., Rosenwald M., Kiehlmann E., Balaz M., Tall B., Opitz L., Lidell M.E., Zamboni N., Krznar P., Sun W., et al. Peroxisome Proliferator Activated Receptor Gamma Controls Mature Brown Adipocyte Inducibility through Glycerol Kinase. Cell Rep. 2018;22:760–773. doi: 10.1016/j.celrep.2017.12.067[↩]
- Ma Q., Zhou X., Sun Y., Hu L., Zhu J., Shao C., Meng Q., Shan A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020;68:4876–4883. doi: 10.1021/acs.jafc.0c01023[↩][↩]
- Seale P., Kajimura S., Yang W., Chin S., Rohas L.M., Uldry M., Tavernier G., Langin D., Spiegelman B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001[↩]
- Wang F.H., Liu J., Deng Q.J., Qi Y., Wang M., Wang Y., Zhang X.G., Zhao D. Association between plasma essential amino acids and atherogenic lipid profile in a Chinese population: A cross-sectional study. Atherosclerosis. 2019;286:7–13. doi: 10.1016/j.atherosclerosis.2019.04.225[↩]
- Piper M.D.W., Soultoukis G.A., Blanc E., Mesaros A., Herbert S.L., Juricic P., He X., Atanassov I., Salmonowicz H., Yang M., et al. Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan. Cell Metab. 2017;25:1206. doi: 10.1016/j.cmet.2017.04.020[↩]
- Sjøberg K.A., Schmoll D., Piper M.D.W., Kiens B., Rose A.J. Effects of Short-Term Dietary Protein Restriction on Blood Amino Acid Levels in Young Men. Nutrients. 2020;12:2195. doi: 10.3390/nu12082195[↩]
- Maida A., Zota A., Sjøberg K.A., Schumacher J., Sijmonsma T.P., Pfenninger A., Christensen M.M., Gantert T., Fuhrmeister J., Rothermel U., et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Investig. 2016;126:3263–3278. doi: 10.1172/JCI85946[↩]
- Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A., Cava E., Spelta F., Tosti V., Syed F.A., et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092[↩]
- Yap Y.W., Rusu P.M., Chan A.Y., Fam B.C., Jungmann A., Solon-Biet S.M., Barlow C.K., Creek D.J., Huang C., Schittenhelm R.B., et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 2020;11:2894. doi: 10.1038/s41467-020-16568-z[↩][↩]
- Green C.L., Lamming D.W. Regulation of metabolic health by essential dietary amino acids. Mech. Ageing Dev. 2019;177:186–200. doi: 10.1016/j.mad.2018.07.004[↩]
- Faure M., Moënnoz D., Montigon F., Mettraux C., Breuillé D., Ballèvre O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J. Nutr. 2005;135:486–491. doi: 10.1093/jn/135.3.486[↩][↩]
- Wang X., Qiao S., Yin Y., Yue L., Wang Z., Wu G. A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. J. Nutr. 2007;137:1442–1446. doi: 10.1093/jn/137.6.1442[↩][↩][↩]
- Hong Y., Jiang W., Kuang S., Hu K., Tang L., Liu Y., Jiang J., Zhang Y., Zhou X., Feng L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of sub-adult grass carp Ctenopharyngodonidella fed graded levels of dietary threonine. J. Anim. Sci. Biotechnol. 2015;6:34. doi: 10.1186/s40104-015-0032-1[↩][↩]
- Rabinovsky E.D., Gelir E., Gelir S., Lui H., Kattash M., DeMayo F.J., Shenaq S.M., Schwartz R.J. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003;17:53–55. doi: 10.1096/fj.02-0183fje[↩]
- Zhao Y., Jiang Q., Zhou X.Q., Xu S.X., Feng L., Liu Y., Jiang W.D., Wu P., Zhao J., Jiang J. Effect of dietary threonine on growth performance and muscle growth, protein synthesis and antioxidant-related signalling pathways of hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Br. J. Nutr. 2020;123:121–134. doi: 10.1017/S0007114519002599[↩]
- Wang W., Wu Z., Lin G., Hu S., Wang B., Dai Z., Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014;144:1540–1548. doi: 10.3945/jn.114.194001[↩]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653[↩]
- Wu J., Ma N., Johnston L.J., Ma X. Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression. Adv. Nutr. 2020;11:92–102. doi: 10.1093/advances/nmz057[↩]
- Zaefarian F., Zaghari M., Shivazad M. The Threonine Requirements and its Effects on Growth Performance and Gut Morphology of Broiler Chicken Fed Different Levels of Protein. Int. J. Poult. Sci. 2008;12:1207–1215. doi: 10.3923/ijps.2008.1207.1215[↩]
- Min Y.N., Liu S.G., Qu Z.X., Meng G.H., Gao Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 2017;96:1290–1297. doi: 10.3382/ps/pew393[↩][↩]
- Wang W., Zeng X., Mao X., Wu G., Qiao S. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J. Nutr. 2010;140:981–986. doi: 10.3945/jn.109.118497[↩][↩]
- Hamard A., Mazurais D., Boudry G., Le Huërou-Luron I., Sève B., Le Floc’h N. A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J. Nutr. Biochem. 2010;21:914–921. doi: 10.1016/j.jnutbio.2009.07.004[↩]
- Dozier W.A., III, Moran E.T., Jr., Kidd M.T. Male and female broiler responses to low and adequate dietary threonine on nitrogen and energy balance. Poult. Sci. 2001;80:926–930. doi: 10.1093/ps/80.7.926[↩]
- Ma N., Guo P., Zhang J., He T., Kim S.W., Zhang G., Ma X. Nutrients Mediate Intestinal Bacteria-Mucosal Immune Crosstalk. Front. Immunol. 2018;9:5. doi: 10.3389/fimmu.2018.00005[↩]
- McAuley J.L., Linden S.K., Png C.W., King R.M., Pennington H.L., Gendler S.J., Florin T.H., Hill G.R., Korolik V., McGuckin M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007;117:2313–2324. doi: 10.1172/JCI26705[↩]
- Montagne L., Piel C., Lallès J.P. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr. Rev. 2004;62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x[↩]
- Wu G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249[↩]
- Law G.K., Bertolo R.F., Adjiri-Awere A., Pencharz P.B., Ball R.O. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1293–G1301. doi: 10.1152/ajpgi.00221.2006[↩]
- Chen Y.P., Cheng Y.F., Li X.H., Yang W.L., Wen C., Zhuang S., Zhou Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2017;96:405–413. doi: 10.3382/ps/pew240[↩][↩][↩]
- Azzam M.M., Dong X.Y., Xie P., Zou X.T. Influence of L-threonine supplementation on goblet cell numbers, histological structure and antioxidant enzyme activities of laying hens reared in a hot and humid climate. Br. Poult. Sci. 2012;53:640–645. doi: 10.1080/00071668.2012.726707[↩]
- Zhang H., Chen Y., Li Y., Zhang T., Ying Z., Su W., Zhang L., Wang T. l-Threonine improves intestinal mucin synthesis and immune function of intrauterine growth-retarded weanling piglets. Nutrition. 2019;59:182–187. doi: 10.1016/j.nut.2018.07.114[↩][↩][↩]
- Bertolo R.F., Chen C.Z., Law G., Pencharz P.B., Ball R.O. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. J. Nutr. 1998;128:1752–1759. doi: 10.1093/jn/128.10.1752[↩]
- Dong X.Y., Azzam M.M.M., Zou X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017;96:3654–3663. doi: 10.3382/ps/pex185[↩]
- Faure M., Mettraux C., Moennoz D., Godin J.P., Vuichoud J., Rochat F., Breuillé D., Obled C., Corthésy-Theulaz I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 2006;136:1558–1564. doi: 10.1093/jn/136.6.1558[↩][↩]
- Liao S.F. Invited Review: Maintain or Improve Piglet Gut Health around Weanling: The Fundamental Effects of Dietary Amino Acids. Animals. 2021;11:1110. doi: 10.3390/ani11041110[↩]
- Koo B., Choi J., Yang C., Nyachoti C.M. Diet complexity and l-threonine supplementation: Effects on growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa278.181[↩]
- Zhang Q., Chen X., Eicher S.D., Ajuwon K.M., Applegate T.J. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 2017;96:3043–3051. doi: 10.3382/ps/pex111[↩][↩]
- Zhang Q., Zeng Q.F., Cotter P., Applegate T.J. Dietary threonine response of Pekin ducks from hatch to 14 d of age based on performance, serology, and intestinal mucin secretion. Poult. Sci. 2016;95:1348–1355. doi: 10.3382/ps/pew032[↩]
- Yin Y. L., Li D., Kim S. W., and Wu G.. . 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X[↩]
- Zhang Q., Chen X., Eicher S.D., Ajuwon K.M., Applegate T.J. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 2016;116:2030–2043. doi: 10.1017/S0007114516003238[↩][↩][↩]
- Kaňková Z., Zeman M., Schwarz S., Kaspers B. Preliminary results on intersexual differences in gene expression of chemokine K203 in mononuclear cells of chicken. Acta Vet. Hung. 2016;64:56–64. doi: 10.1556/004.2016.006[↩]
- Papadakis K.A., Prehn J., Nelson V., Cheng L., Binder S.W., Ponath P.D., Andrew D.P., Targan S.R. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069[↩]
- Ebert L.M., Schaerli P., Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040[↩]
- Kaetzel C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x[↩]
- Wils-Plotz E.L., Jenkins M.C., Dilger R.N. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult. Sci. 2013;92:735–745. doi: 10.3382/ps.2012-02755[↩][↩]
- Habte-Tsion H.M., Ge X., Liu B., Xie J., Ren M., Zhou Q., Miao L., Pan L., Chen R. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala) Fish Shellfish Immunol. 2015;42:439–446. doi: 10.1016/j.fsi.2014.11.021[↩][↩]
- An J.M., Kang E.A., Han Y.M., Kim Y.S., Hong Y.G., Hah B.S., Hong S.P., Hahm K.B. Dietary threonine prevented stress-related mucosal diseases in rats. J. Physiol. Pharmacol. 2019;70:467–478. doi: 10.26402/jpp.2019.3.14[↩]
- Dong Y.W., Jiang W.D., Liu Y., Wu P., Jiang J., Kuang S.Y., Tang L., Tang W.N., Zhang Y.A., Zhou X.Q., et al. Threonine deficiency decreased intestinal immunity and aggravated inflammation associated with NF-κB and target of rapamycin signalling pathways in juvenile grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Br. J. Nutr. 2017;118:92–108. doi: 10.1017/S0007114517001830[↩]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740[↩]
- Tan P., Fu H.Y., Ma X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today. 2021;39 doi: 10.1016/j.nantod.2021.101229[↩]
- Wang C., Yang Y., Gao N., Lan J., Dou X., Li J., Shan A. L-Threonine upregulates the expression of β-defensins by activating the NF-κB signaling pathway and suppressing SIRT1 expression in porcine intestinal epithelial cells. Food Funct. 2021;12:5821–5836. doi: 10.1039/D1FO00269D[↩]
- Saadatmand N., Toghyani M., Gheisari A. Effects of dietary fiber and threonine on performance, intestinal morphology and immune responses in broiler chickens. Anim. Nutr. 2019;5:248–255. doi: 10.1016/j.aninu.2019.06.001[↩]
- Growdon JH, Nader TM, Schoenfeld J, Wurtman RJ. L-threonine in the treatment of spasticity. Clin Neuropharmacol. 1991 Oct;14(5):403-12. doi: 10.1097/00002826-199110000-00003[↩][↩][↩]
- Hauser SL, Doolittle TH, Lopez-Bresnahan M, Shahani B, Schoenfeld D, Shih VE, Growdon J, Lehrich JR. An antispasticity effect of threonine in multiple sclerosis. Arch Neurol. 1992 Sep;49(9):923-6. doi: 10.1001/archneur.1992.00530330045014[↩]
- Lee A, Patterson V. A double-blind study of L-threonine in patients with spinal spasticity. Acta Neurol Scand. 1993 Nov;88(5):334-8. doi: 10.1111/j.1600-0404.1993.tb05353.x[↩]
- Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009, OJ L 181, 29.6.2013, p. 35.[↩]
- Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.[↩]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.[↩]
- Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OL L 152, 16.6.2009, p. 11.[↩]
- Rigo J, Senterre J. Optimal threonine intake for preterm infants fed on oral or parenteral nutrition. JPEN J Parenter Enteral Nutr. 1980 Jan-Feb;4(1):15-7. doi: 10.1177/014860718000400105[↩][↩]