Contents
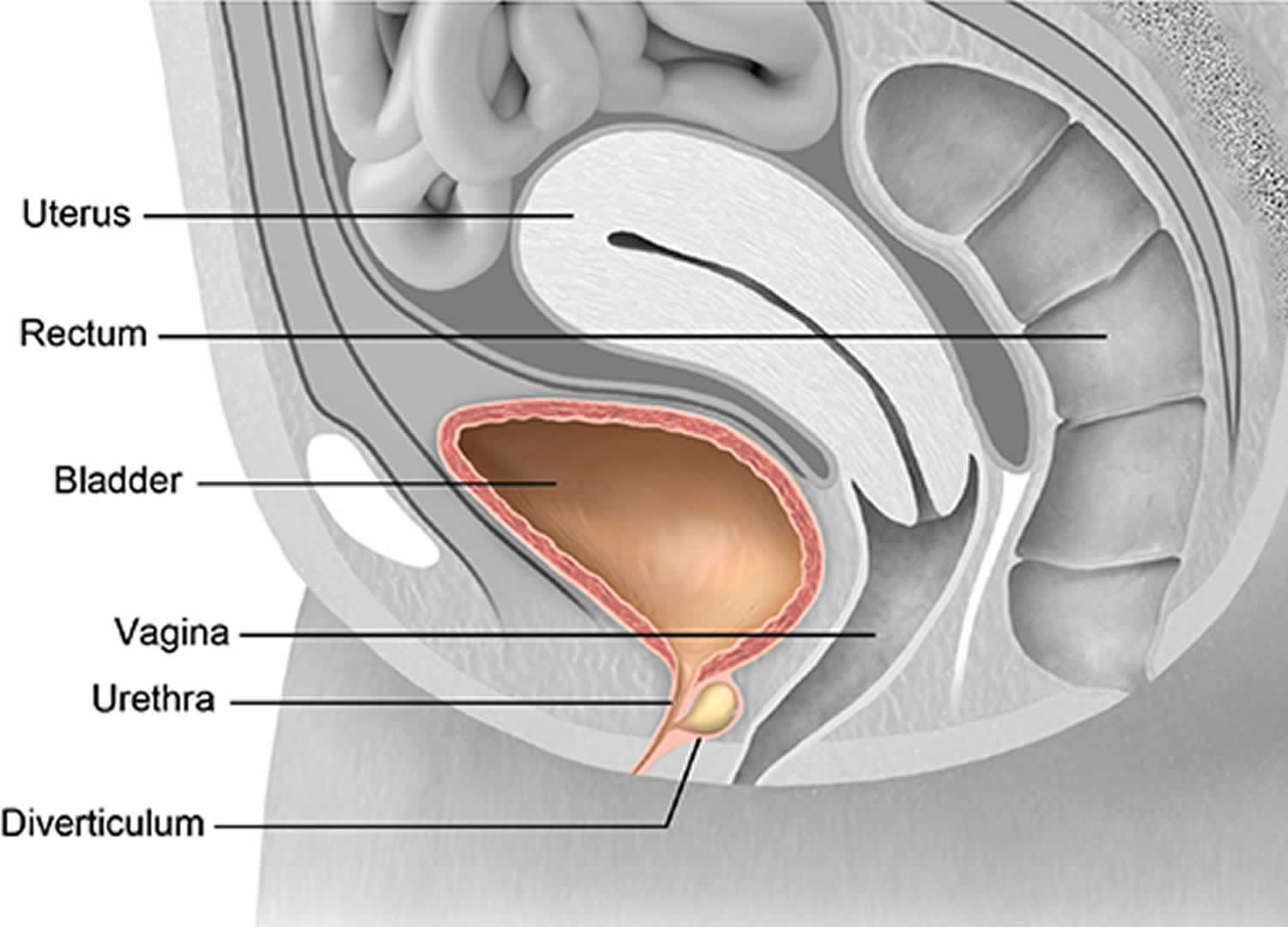
What is urethral diverticulum
Urethral diverticulum is a epithelium-lined pocket or pouch that forms along the urethra. Because of its location, urethral diverticulum can be filled with urine and lead to infections. Urethral diverticulum have historically been described with the classic triad of three D’s e.g. Dysuria (painful urination), Dysparunia (pain with sex), and Dribbling, which are present only in about one third of cases 1. They can be asymptomatic and incidentally detected or may present with symptoms like painful vaginal mass, chronic pelvic pain, refractory lower urinary tract symptoms, and recurrent urinary tract infections (UTI) 2. Because of the varied symptomatology, urethral diverticulum poses a challenge to the treating clinician.
Urethral diverticulum can cause:
- A painful vaginal mass
- Ongoing pelvic pain
- Many urinary tract infections (UTIs)
Urethral diverticulum is rare, but much more common in females than in males. Urethral diverticulum is more common in women between age 40 and 70 with overall incidence of 1 –6% 2. Children are not usually affected, unless they’ve had urethral surgery 3.
The rare cases reported in males generally have been associated with lower urinary tract congenital anomalies or surgical trauma. In a series of 108 female patients from the Mayo Clinic, the age range was reported as 10-76 years, with the disorder observed most commonly in women aged 30-50 years 4.
Very rarely, periurethral cystic masses have been reported in newborns. Whether these masses represent a congenital form of urethral diverticulum or some type of genitourinary congenital remnant remains uncertain 4.
With better imaging, more urethral diverticulums have been found and treated. Still, many cases are missed or misdiagnosed simply because no one considered it.
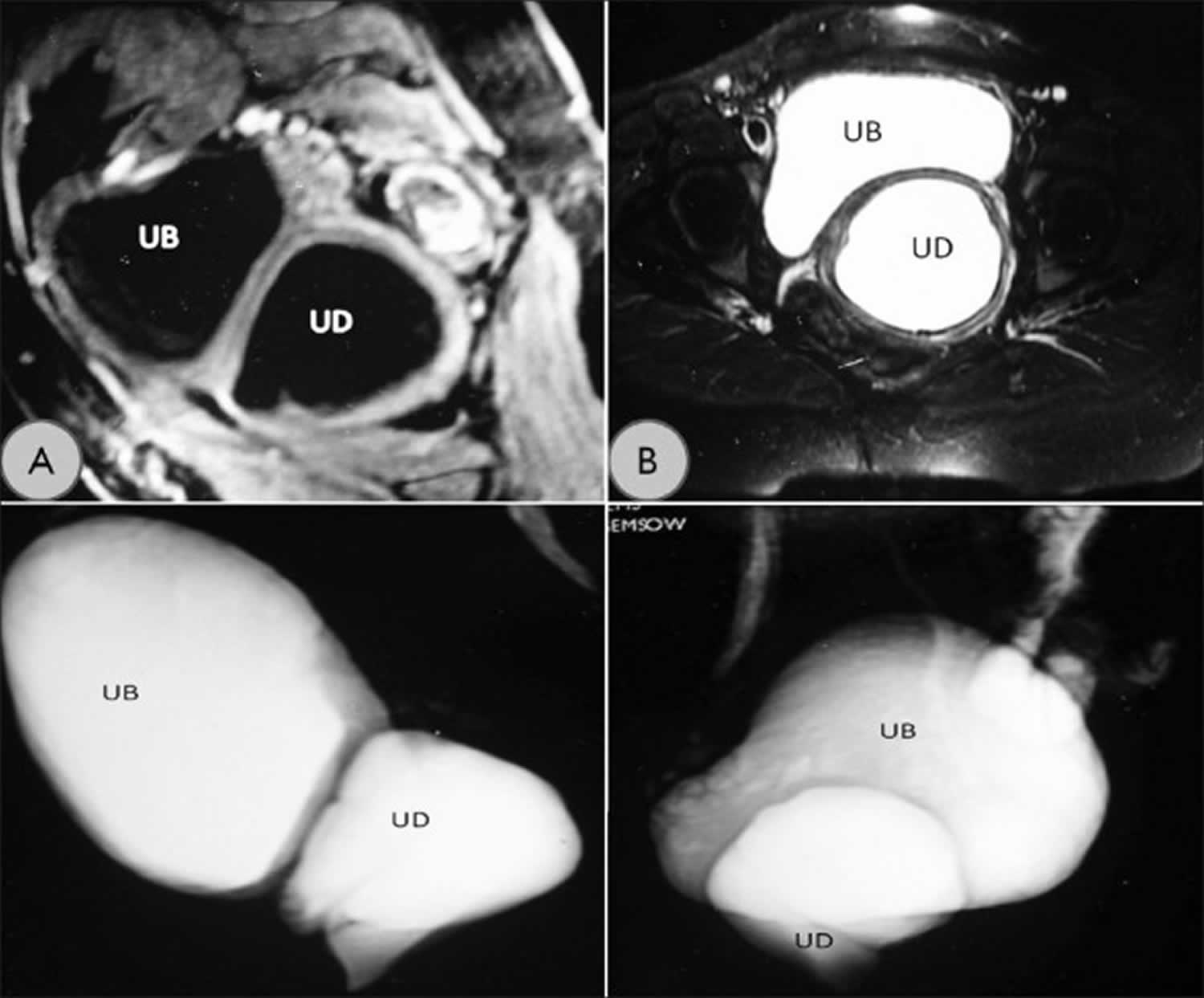
Figure 1. Urethral diverticulum MRI
Footnote: (a) T1-weighted MRI image showing the urethral diverticulum (UD) and urinary bladder (UB) in the sagittal plane. (b) T2-weighted cross-sectional image showing the diverticulum. (c, d) Reconstructed T2-weighted MRI images showing relationship of urethral diverticulum with urinary bladder.
[Source 1 ]Urethral diverticulum female
Urethral diverticulum in females is a localized outpouching of the urethra into the anterior vaginal wall 5. Most often present in the mid or distal urethra, urethral diverticula result from enlargement of obstructed periurethral glands 1. Urethral diverticulum is thought to be derived from the periurethral glands as a result of recurrent infections. Although urethral diverticulum is often difficult to diagnose, it has been identified with increasing frequency over the past several decades because of increased physician awareness of the condition.
The most common symptoms associated with urethral diverticula include urinary frequency, urgency, urinary incontinence, recurrent urinary tract infections (UTIs), dysuria (painful or difficult urination) and dyspareunia (difficult or painful sexual intercourse). In some cases, urethral carcinoma and calculi are also present. Despite the increased awareness in recent years, this entity continues to be overlooked during routine evaluation of women with voiding problems. Accurate diagnosis and treatment of urethral diverticula require a high index of suspicion and appropriate radiologic and endoscopic evaluations.
The adult female urethra is approximately 4 cm long and extends from the bladder neck to the external meatus. The mucosa of the female urethra is lined by transitional cell epithelium that gradually changes to nonkeratinizing squamous epithelium from the bladder neck to the external urethral meatus.
Small periurethral secretory glands interdigitate the wall of the urethra to produce lubrication for the inner mucosa. These periurethral glands converge at the distal urethra as Skene glands and empty through 2 small ducts on either side of the external meatus.
Repeated bouts of infection and occlusion of the periurethral glands lead to formation of suburethral cysts. These suburethral cysts enlarge and eventually rupture into the urethral lumen. During urination, constant pooling of urine within these cysts gives rise to urethral diverticula.
Treatment options for urethral diverticulum can range from conservative management to extensive surgery, most commonly diverticulectomy 6. Patients with pre-existing stress incontinence may receive concomitant autologous pubovaginal sling placement at the time of diverticulectomy 7.
Urethral diverticulum causes
The cause of a urethral diverticulum is not clear, although several theories exist. It is often linked to repeat infections causing weakness in the urethra wall. A block in the glands near the urethra may also cause it. Or, earlier studies point to a birth defect or trauma during childbirth. Although this mechanism may account for a small number of urethral diverticula, causation is difficult to prove. Also, 15% or more of all urethral diverticula occur in nulliparous females with no known history of urethral trauma.
Some suburethral cysts may be congenital, as evidenced by the fact that they have been reported in newborns. Many of these cysts have epithelial linings, which indicate that they arise from structures that are not the paraurethral glands or ducts. Examples include cloacogenic rests lined with colonic epithelium, Gartner duct cysts, and Müllerian remnants. In the opinion of this author, these cysts should not be classified as urethral diverticula.
Rarely, a diverticulum may be associated with an anomalous accessory urethra. Such diverticula are generally recognized at birth, presenting as a large fluid-filled mass occupying space between the main urethra and the clitoris. The external meatus of the accessory urethra is stenotic and drains poorly, resulting in the accumulation of urine in the diverticular sac. Associated genitourinary anomalies, such as absence of the perineum, absence of the labia minora, a multicystic kidney, and hydronephrosis can be encountered. The enlarged accessory urethra and diverticulum can have phallic appearance and, as such, this congenital abnormality is considered by many experts to be a form of pseudohermaphroditism.
A recently published case report describes the use of urography and genitography to aid in defining the anatomy. At 2 days of age, the stenotic meatus was incised to promote drainage. Definitive surgery was undertaken at 5 months of age including excision of the accessory urethra and diverticulum, and indicated genital reconstruction with attention paid to preserving the clitoris and associated.
Urethral diverticulum pathophysiology
The pathophysiology of most cases of urethral diverticula appears to revolve around obstruction of and infection within the paraurethral glands. The glands are thought to become enlarged and inflamed, eventually forming a retention cyst and then an abscess, which ruptures back into the urethra. In 1890, Routh first described this pathophysiologic mechanism.
In 1953, Telinde suggested that gonococcal infection in the paraurethral glands was an important initiating factor in the pathogenesis of urethral diverticula. In a subsequent small series in 1975, 10 of 31 patients had proven gonorrhea and another 7 had histories suggestive of gonococcal infection. Bacteruria is a common finding in individuals with urethral diverticula. Typical urinary tract pathogens predominate. Bacteruria and recurrent UTIs are thought to result from bacterial growth in the stagnant urine within the diverticulum and reflux of infected material into the bladder. Little else apparently is known regarding the bacteriology of this disorder.
Urethral diverticula may be associated with variable degrees of peridiverticulitis. Tancer and Ravski 8 state that this may result from recurring infection within the diverticulum. They argue that signs and symptoms, such as urethral tenderness and dyspareunia, appear or become more severe upon development of peridiverticulitis.
On occasion, severe, recurrent infection in and around the diverticulum may result in rupture through the periurethral connective tissue and into the space between this tissue and the vaginal wall. Leng and McGuire 9 proposed this phenomenon in their description of urethral diverticulum subtypes. The author has observed this phenomenon on 2 occasions, including 1 case in which the rupture may have occurred at the time of double balloon positive-pressure urethrography.
In 1.5 to 10% of cases, stones may form within the diverticular sac. Stones may be singular or multiple. Most are calcium oxalate or calcium phosphate stones. Stagnation of urine with crystal formation in the presence of chronic infection probably is the main etiologic factor. Stones are more common in men, especially if some degree of associated obstruction is present 10. In rare instances, giant calculi have been reported to occur in urethral diverticula.
Due to the presence of chronic inflammation, mucosal changes within the diverticulum often resemble chronic cystitis. Cystitis glandularis, glandular metaplasia, and focal hyperplasia have been reported. Chronic mucosal injury may cause hyperplastic and neoplastic changes within the diverticulum. Rarely, carcinoma develops within a diverticulum. These cases represent 5% of all urethral carcinomas. For unknown reasons, carcinomas appear to be more common in blacks with urethral diverticula. Among diverticulum-associated cancers, about 60% are adenocarcinomas, 30% are transitional cell carcinomas, and 10% are squamous cell cancers. Traditionally, the squamous cell variety was thought to have worse prognosis. More recent information suggests that tumor grade may be more important than cell type as an indicator of prognosis.
In 1998, Leng and McGuire 9 proposed a simple classification system of urethral diverticula partly based on pathophysiologic findings and proposed etiologies. In their series of patients, they observed some cases in which the mucosal lining of the urethra was observed to be extruding through a defect in the periurethral connective tissue. This finding was at variance with the more typical intraoperative finding of intact periurethral connective tissue surrounding the diverticular sac. They found this subtype to be associated more commonly with previous periurethral surgery. They called this subtype a pseudodiverticulum, although the described lesion closely approximates the medical definition of a true diverticulum.
In the series reported by Leng and McGuire, the pseudodiverticulum often was observed following suture bladder neck suspension procedures. They hypothesized that traction on these sutures during increases in intra-abdominal pressure may have torn the connective tissue, leaving a gaping defect. Most often, this type of lesion had a broad-based ostium, which was easily identifiable on urethroscopy. Also, these patients tended to have fewer chronic lower urinary tract symptoms, with the exception of stress incontinence, which was more common. In this series of 18 patients, 12 had true diverticula, 5 had pseudodiverticula, and 1 patient had both subtypes.
Three cases of urethral diverticulum following synthetic tension-free midurethral sling have recently come to light 11. These cases were probably related to surgical disruption of the subepithelial connective tissue or erosion of the sling material through this layer. These diverticula have responded to transvaginal diverticulectomy with and without excision of the sling.
Urethral diverticulum symptoms
Up to 20% of patients with urethral diverticulum may not have clear signs. Symptoms are different for everyone, but the most common are:
- Bladder or urinary tract infections (UTIs) that return
- Pelvic pain
- Lower urinary tract symptoms (similar to an overactive bladder)
- Nocturia (feeling the need to urinate several times at night)
- Pain with sex
- Dribbling
- Blood in the urine
- Vaginal discharge
- Urinary blockage
- Trouble emptying the bladder
- Accidental loss of urine (incontinence)
A recent review reported the most common symptoms as follows:
- Urinary frequency and urgency (40-100%)
- Dysuria (30-70%)
- Recurrent UTI (30-50%)
- Postmicturition urinary dribbling (10-30%)
- Dyspareunia (10-25%)
- Hematuria (10-25%)
In a series of 120 patients conducted in Taiwan, 100% of the patients presented with the classic triad of postmicturition dribbling, pain with sex (dyspareunia) and painful urination (dysuria). The next most common presenting symptoms were incomplete voiding, urgency, and frequency, reported in 38%, 21%, and 18% of the patients, respectively 12.
Some women have a tender area or mass at the front vaginal wall. With a gentle press, urine or pus may show through the urethral opening.
It is important to note that the size of the urethral diverticulum doesn’t matter. In some cases, a very large urethral diverticulum may cause only minor symptoms. Or a small urethral diverticulum may still cause pain. Symptoms can also go away and come back.
Urethral diverticulum diagnosis
Because urethral diverticulum does not have clear signs, they can be found during an exam or imaging test. In some people, it can be years before the correct diagnosis is made. Patients are often misdiagnosed and treated for other things first.
A proper diagnosis can be made with:
- An in-depth health history
- Physical exam
- Urine studies
- Direct exam of the bladder and urethra (with an endoscope, or tube-like test with a light)
- Imaging tests, such as an MRI or Ultrasound
Physical Exam
When a urethral diverticulum is found, the urologist may “milk” the sac to try to remove pus or urine. In women, the front vaginal wall may be felt for masses and soreness.
Imaging
Many imaging tests can be used to find urethral diverticulum. No single test is best. Each has pros and cons. The final choice often depends on:
- Whether the test is available
- How much it costs
- The skill of the radiologist
Magnetic Resonance Imaging (MRI)
This type of test uses radio waves and a magnetic field to look closely at the urinary tract. It has the best record of finding a urethral diverticulum.
Ultrasound
Using sound waves, this test may show a urethral diverticulum, but would need a follow-up MRI to be sure.
Urodynamic Studies
These tests measure lower urinary tract function. They may find stress urinary incontinence (accidental loss of urine caused by pressure on the bladder) from a urethral diverticulum.
Videourodynamic studies (that add imaging) may be able to tell why stress urinary incontinence is happening.
Urethral diverticulum treatment
Surgery is the main way to treat urethral diverticulum. Still, not all cases call for surgery. Some patients may not want it, or be able to have surgery.
Not much is known about untreated urethral diverticulum. It is not known if the pockets will become larger or if symptoms will get worse. Some people prefer to wait until symptoms get worse before doing anything. In rare cases there have been reports of cancers growing in people with urethral diverticulum.
If you prefer not to have surgery, counseling and follow-up visits with your doctor is important.
If you choose not to have surgery, you should still see your urologist for follow-up care.
Urethral diverticulum surgery
Surgical excision is the treatment of choice. It should be performed with care with an experienced urologist. The urethral diverticulum sac may be attached to the urethral opening. If the sac is not removed carefully, it could damage the urethra. This would lead to a major surgical repair.
Surgical options are:
- Cutting into the sac neck
- Creating a permanent opening of the sac into the vagina
- Removing the sac
Other key issues in surgery
- The diverticular neck (the connection to the urethral opening) should be closed.
- The lining of the diverticular sac should be fully removed to prevent the urethral diverticulum from coming back.
- A closure with many layers is needed so a new opening doesn’t form between the urethra and vagina.
- If you have stress urinary incontinence, a procedure to fix the leaking may be done at the same time as fixing the diverticulum.
The choice of surgical procedure depends largely on the location of the diverticulum along the urethra. Simple marsupialization may be appropriate for diverticula with ostia emptying into the distal one third of the urethra, although not all authorities advocate this approach. If the ostium of the diverticulum lies distal to the urethral rhabdosphincter, then incision into the posterior urethral wall to achieve exteriorization of the diverticular sac is thought to have no adverse effect on continence 13.
Diverticula involving the middle or proximal one third of the urethra are treated most effectively with either total excision or partial ablation. Each procedure has advocates; however, partial ablation generally is considered simpler and less likely to result in urethrovaginal fistula. This especially may be true if significant peridiverticular inflammation is present, if the sac is adherent to the posterior urethra, or if tissue planes are obscured. A third, more conservative approach has been described for use under these adverse surgical conditions. This technique involves a small transvaginal incision into the sac of the diverticulum followed by copious irrigation with an antiseptic solution and packing of the diverticular lumen with Oxycel. The resultant extensive fibrosis is thought to close the diverticulum from within.
With either total excision or partial ablation, the diverticulum is approached surgically through a vaginal incision. Transverse or midline vertical vaginal incisions both have been used with good results. Some surgeons have advocated an inverted horseshoe-shaped incision, with the line of the incision away from the anticipated underlying suture lines that will close the urethra and periurethral connective tissue. Avoiding superimposed suture lines is thought to lower the incidence of postoperative fistula formation; however, this concept never has been proven. Probably more important than the choice of vaginal incision are the basic principles of tension-free meticulous layered closure and good hemostasis. Finally, a semilunar submeatal incision has been described. With this technique, the diverticular neck and sac are approached by way of dissection beneath and parallel to the urethra. Experience with this incision is limited, but reported results have been favorable.
Optimal surgical treatment of patients with diverticula associated with malignancy has yet to be determined. In patients with the diagnosis made prior to surgery, treatment can be wide local excision (urethrectomy) followed by local radiation. Anterior exenteration is reserved for local recurrences. Some patients have been treated primarily with anterior exenteration and diversion. Cure rates at 0.5-2 years of follow-up have been high (87%) but at the cost of high morbidity. If the diagnosis is made after diverticulectomy, postoperative radiation therapy is an option versus anterior exenteration and urinary diversion.
Difficulties with surgical excision can arise in a variety of circumstances. Surgical planes can be obliterated by the sequelae of chronic and acute infection. Bleeding may obscure visualization. Rupture of the diverticular sac or inability to keep the sac distended can be a significant impediment to complete dissection and excision. Friability of the diverticular wall may impair dissection further. Unexpected intraoperative findings, such as multiple or complex diverticula, can prolong and complicate any procedure. The surgeon must be patient, meticulous, and resourceful in order to achieve the best possible outcomes.
Urethral diverticulum surgery recovery
Postoperative care of patients surgically treated for urethral diverticula may differ from case to case depending on the specifics of the procedure(s) performed. Generally, vaginal packing is avoided or limited to one day. Stool softeners may be prescribed in order to avoid straining.
With simple marsupialization procedures, urinary catheterization usually is not needed, and most patients resume voiding promptly. Tancer recommends urinary drainage for 5-7 days by either the transurethral or suprapubic route following partial ablation. Similarly, many surgeons drain the bladder for 5-7 days following diverticulectomy; however, some authors have recommended drainage for as long as 5 weeks via a transurethral catheter. No definitive reports exist in the literature to guide catheter management. One author suggested that longer periods of drainage should be considered if more than one diverticulum was excised or the diverticulum was located at the bladder neck. Also, concomitant surgery for stress incontinence may affect catheter management decisions.
Most patients with uncomplicated repairs are ready for discharge in 24-48 hours. Patients with more complicated surgeries or who require additional procedures may have more prolonged hospital stays.
If you have surgery:
- You will have antibiotics for at least 24 hours.
- You will be sent home with a catheter in place for 2-3 weeks.
- You may have bladder spasms, which can be managed with drugs.
Two to 3 weeks after surgery, a voiding cystourethrogram (VCUG, an x-ray using dye) will be done.
- If there is no fluid leaking, the catheter will be removed.
- If fluid is seen, repeat voiding cystourethrogram (VCUG) will be done weekly until leaking ends.
- In most cases the leaking will end in a few weeks.
Common issues after surgery are:
- UTIs
- Accidental loss of urine
- Urethral diverticulum that comes back
- Ongoing symptoms
- Urethrovaginal fistula (an abnormal passage between the urethra and vagina, which is a serious problem that needs treatment)
If urethral diverticulum returns, it may be due to a few things. For example: the pouching is not completely removed, the opening is not completely sealed, remaining dead space, or other technical issues. Repeat surgery can be difficult. This surgery requires a high level of technical skill.
Urethral diverticulum surgery complications
The types of complications observed following surgery for urethral diverticulum are partly related to the type of lesion treated and the particular operation utilized. For example, postoperative stress incontinence appears to be more common after surgery for proximal and bladder neck diverticula. Urethrovaginal fistula occurs more commonly after complete diverticulectomy than after partial ablation. Overall complication rates for surgical treatment of urethral diverticula have been reported to range from 5-46%. Common complications reported following surgery for urethral diverticulum include the following:
- Recurrence or persistence of diverticula
- Urethrovaginal fistula
- Vesicovaginal fistula
- Urethral stricture
- Urethral pain syndrome
- New-onset or persistent stress incontinence
- Missed carcinoma
- UTI
- Hemorrhage or hematoma formation
In one series of 70 patients undergoing mostly diverticulectomy from 1955-1979 14, the cases were divided into 3 groups based on the location of the lesion along the urethra. With diverticula of the proximal one third of the urethra, the main complication was recurrence (33%), indicating difficulty with complete sac excision. Mid urethral diverticula excision resulted in fistula formation in 4 of 26 cases (15.4%). Recurrence was observed in 31% and stricture in 3.8% of cases. Distal urethral diverticula had the lowest postoperative complication rates, with no fistulas and 2 out of 17 cases recurring 1-6 years after surgery 14.
One hundred and eight patients at the Mayo Clinic were treated over 15 years with complete diverticulectomy. Low complication rates in this series can be attributed to meticulous surgical technique. Reported complications included urethrovaginal fistula (0.9%), urethral pain syndrome (1.8%), recurrent UTIs (0.9%), immediate postoperative urinary incontinence (1.8%), delayed (>2 y) urinary incontinence (13%), recurrent diverticulum in less than 1 year (3.7%), and recurrent diverticulum after longer than 1 year (5.6%). Of note, the author found that most recurrences were at the same location as the original diverticulum. This finding suggests that most recurrences are the result of incomplete excision of the sac or remaining local weakness in the urethral wall rather than the formation of entirely new lesions.
In 1994, in series of 63 patients over 10 years 15, complication rates for complete diverticulectomy were less than those previously reported. Urethrovaginal fistulas and recurrences were observed in 1.6% and 3.2% of the cases, respectively. No cases of vesicovaginal fistula were reported. UTIs occurred postoperatively in 9.5% of cases. Twenty-two percent of women who had genuine stress incontinence and urethral diverticulum had persistent stress incontinence despite treatment with bladder neck suspension at the same surgery. The degree of incontinence was reported as requiring less than 2 pads per day. About 10% of patients undergoing diverticulectomy alone developed mild de novo stress incontinence in this series.
In another retrospective review of 50 cases without preoperative stress incontinence or concurrent incontinence surgery, 50% developed stress incontinence after diverticulum repair. Most of these were mild cases and only 10% sought subsequent surgery for stress incontinence.
In a series of 25 patients 16, 16% developed postoperative de novo stress incontinence. The cases were described as mild with only 1 out of 4 requiring surgical treatment. Risk factors for the development of de novo stress incontinence in this series were a diverticulum larger than 30 mm and proximal urethral location.
Tancer et al 17 reported no cases of recurrence, fistula formation, or stress incontinence in their series of 34 patients treated with partial ablation. Follow-up was from 4 months to 10 years. This group attributed most of the serious complications from excision procedures to overzealous attempts to completely remove the diverticular sac and neck.
Marsupialization procedures, if limited to treatment of distal diverticula only, should have low complication rates. In a review of 17 cases treated in this manner, no cases of fistula formation, recurrence, stricture, or recurrent UTIs occurred. One case of mild, new-onset stress incontinence was noted.
Urethral diverticulum prognosis
Surgical outcomes with regard to urethral diverticula generally have been good; however, surgical outcomes have been reported in an inconsistent manner in the literature. Several issues create the inconsistency. First, outcome can be considered in terms of resolution of individual symptoms or in terms of recurrence of the diverticulum itself. Additionally, that recurrences can be found years after primary excision is well documented. Some recurrences may have not yet occurred at the time of case series publication. Whether or not these recurrences are due to incomplete initial treatment or the formation of completely new diverticula by reinfection and obstruction of paraurethral glands is uncertain. Finally, publication bias may be a problem. Occasionally, some urethral surgeons may have lower cure rates and might be less likely to publish results.
Given these uncertainties, an overall cure rate of 70% for symptomatic patients was cited in a recent review article 18. Successful excision by diverticulectomy can be expected in 80-90% of cases as evidenced by recurrence rates of 10-20%.
Marsupialization in properly selected patients has yielded symptomatic cure in essentially 100% of patients; however, published numbers are small. In general, treatment of distal diverticula is highly successful in terms of high cure rates and minimal complications regardless of surgical approach.
Tancer et al 17 reported symptomatic relief and no recurrences in all of 34 patients treated with partial ablation. Follow-up was from 4 months to 10 years.
- Pradhan MR, Ranjan P, Kapoor R. Female urethral diverticulum presenting with acute urinary retention: Reporting the largest diverticulum with review of literature. Indian J Urol. 2012;28(2):216–218. doi:10.4103/0970-1591.98473 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3424907[↩][↩][↩]
- Rovner ES. Urethral Diverticula. In: Raz S, Rodriguez L, editors. Female Urology. 3rd ed. Philadelphia: Saunders; 2008. pp. 815–34.[↩][↩]
- Neonatal female urethral diverticulum. Glassman TA, Weinerth JL, Glenn JF. Urology. 1975 Feb; 5(2):249-51.[↩]
- Urethral Diverticulum. https://emedicine.medscape.com/article/269493-overview[↩][↩]
- Urethral Diverticula. https://emedicine.medscape.com/article/443296-overview[↩]
- El-Nashar SA, Singh R, Bacon MM, Kim-Fine S, Occhino JA, Gebhart JB, et al. Female Urethral Diverticulum: Presentation, Diagnosis, and Predictors of Outcomes After Surgery. Female Pelvic Med Reconstr Surg. 2016 Nov/Dec. 22 (6):447-452.[↩]
- Greiman A, Rittenberg L, Freilich D, Rames R, El-Zawahry A, Koski M, et al. Outcomes of treatment of stress urinary incontinence associated with female urethral diverticula: A selective approach. Neurourol Urodyn. 2018 Jan. 37 (1):478-484.[↩]
- Tancer ML, Ravski NA. Suburethral diverticulum. Clin Obstet Gynecol. 1982 Dec. 25(4):831-7.[↩]
- Leng WW, McGuire EJ. Management of female urethral diverticula: a new classification. J Urol. 1998 Oct. 160(4):1297-300.[↩][↩]
- Shim JS, Oh MM, Kang JI, Ahn ST, Moon du G, Lee JG. Calculi in a female urethral diverticulum. Int Neurourol J. 2011 Mar. 15(1):55-7.[↩]
- Mahdy A, Elmissiry M, Ghoniem GM. Urethral diverticulum after tension-free vaginal tape procedure: case report. Urology. 2008 Aug. 72(2):461.e5-6.[↩]
- Wang AC, Wang CR. Radiologic diagnosis and surgical treatment of urethral diverticulum in women. A reappraisal of voiding cystourethrography and positive pressure urethrography. J Reprod Med. 2000 May. 45(5):377-82.[↩]
- Blaivas JG, Santos JA, Tsui JF, Deibert CM, Rutman MP, Purohit RS, et al. Management of urethral stricture in women. J Urol. 2012 Nov. 188(5):1778-82.[↩]
- Ginsburg D, Genadry R. Suburethral diverticulum: classification and therapeutic considerations. Obstet Gynecol. 1983 Jun. 61(6):685-8.[↩][↩]
- Ganabathi K, Leach GE, Zimmern PE. Experience with the management of urethral diverticulum in 63 women. J Urol. 1994 Nov. 152(5 Pt 1):1445-52.[↩]
- Stav K, Dwyer PL, Rosamilia A, Chao F. Urinary symptoms before and after female urethral diverticulectomy–can we predict de novo stress urinary incontinence?. J Urol. 2008 Nov. 180(5):2088-90.[↩]
- Tancer ML, Mooppan MM, Pierre-Louis C. Suburethral diverticulum treatment by partial ablation. Obstet Gynecol. 1983 Oct. 62(4):511-3.[↩][↩]
- Bennett SJ. Urethral diverticula. Eur J Obstet Gynecol Reprod Biol. 2000 Apr. 89(2):135-9.[↩]