Contents
- What is ventricular tachycardia
- Ventricular tachycardia symptoms
- Ventricular tachycardia causes
- Ventricular tachycardia diagnosis
- Ventricular tachycardia treatment
- Ventricular tachycardia prognosis
- Catecholaminergic polymorphic ventricular tachycardia
- Catecholaminergic polymorphic ventricular tachycardia causes
- Catecholaminergic polymorphic ventricular tachycardia diagnosis
- Catecholaminergic polymorphic ventricular tachycardia treatment
- Treatment of manifestations
- Prevention of primary manifestations
- Prevention of secondary complications
- Surveillance
- Agents/circumstances to avoid
- Can catecholaminergic polymorphic ventricular tachycardia go into remission?
- Do all people with catecholaminergic polymorphic ventricular tachycardia require treatment?
- Can people with catecholaminergic polymorphic ventricular tachycardia play sports?
What is ventricular tachycardia
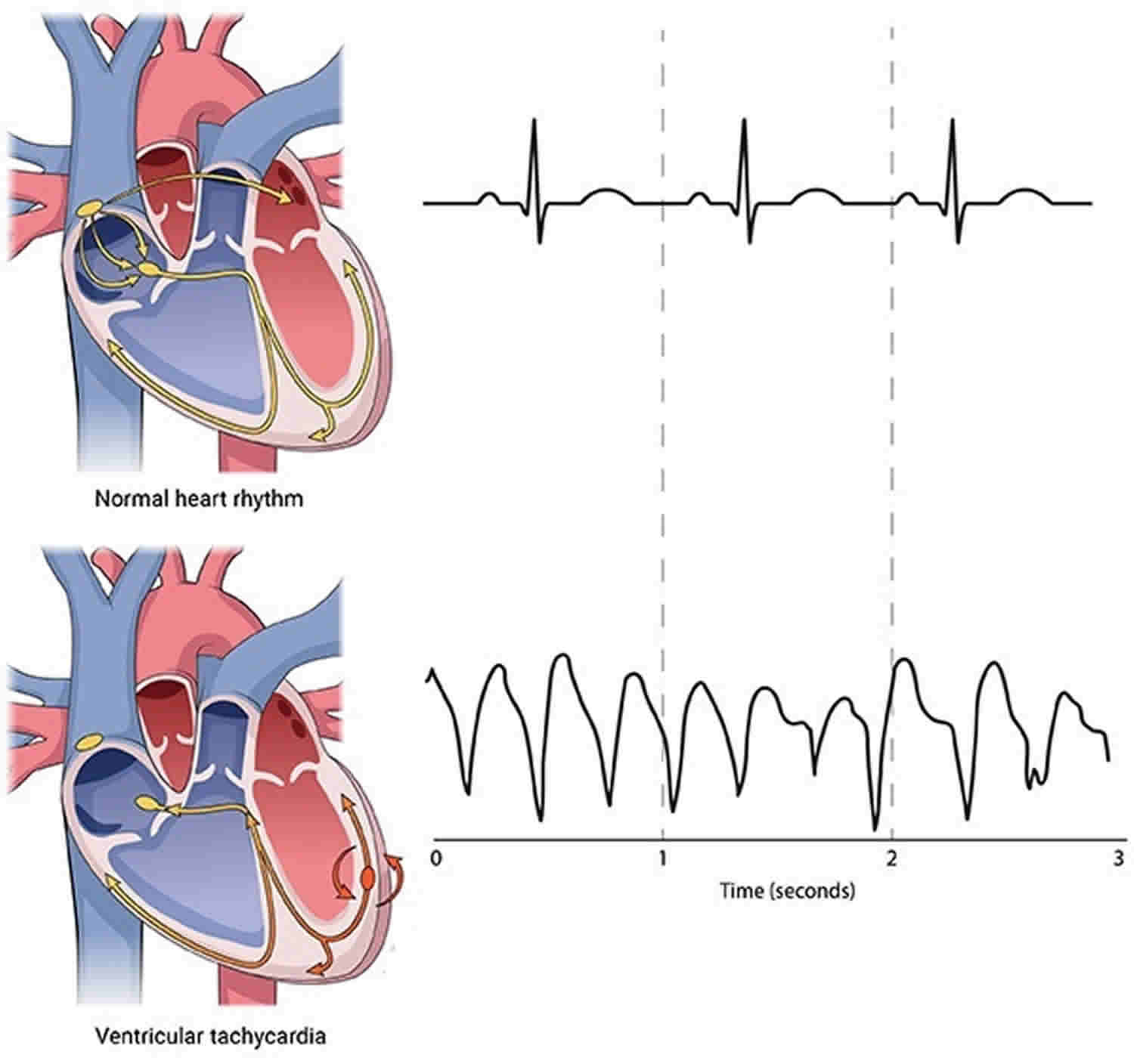
Ventricular tachycardia is a fast abnormal heart rhythm disorder (arrhythmia) that starts in the lower chambers of your heart (ventricles), the abnormal electrical signals in the ventricles cause the heart to beat faster than normal, usually 100 or more beats a minute that is out of sync with the upper chambers 1. Some experts define ventricular tachycardia as ≥ 3 consecutive ventricular beats at a rate ≥ 120 beats/min 2. Other experts use a cutoff rate of ≥ 100 beats/min with at least 3 irregular heartbeats in a row for ventricular tachycardia 3.
A healthy heart normally beats about 60 to 100 times a minute when at rest and is defined by signals that originate in the upper chambers of the heart (atria).
In ventricular tachycardia, abnormal electrical signals in the ventricles cause the heart to beat faster than normal, usually 100 or more beats a minute, out of sync with the upper chambers. When that happens, your heart may not be able to pump enough blood to your body and lungs because the chambers are beating so fast or out of sync with each other that they don’t have time to fill properly.
Ventricular tachycardia may be brief, lasting for only a few seconds, and perhaps not cause any symptoms. Or it can last for much longer and cause symptoms such as dizziness, lightheadedness, palpitations or even loss of consciousness. Episodes that last for more than a few seconds can be dangerous. Ventricular tachycardia can turn into other more serious arrhythmias, such as ventricular fibrillation (VF).
In some cases, ventricular tachycardia can cause your heart to stop (sudden cardiac arrest), which is a life-threatening medical emergency. This condition usually occurs in people with other heart conditions, such as those who have had a previous heart attack or other structural heart disease (cardiomyopathy).
The outcome of ventricular tachycardia depends on the heart condition and symptoms.
Among patients younger than 35 years, the most common cardiac causes of sudden death, and presumably of ventricular tachycardia, include the following 4:
- Hypertrophic cardiomyopathy
- Right ventricular cardiomyopathy (arrhythmogenic right ventricular dysplasia)
- Myocarditis
- Long QT syndrome
- Congenital coronary artery abnormalities
Ventricular tachycardia is unusual in children but may occur in the postoperative cardiac setting or in patients with associated congenital heart disease. Tachydysrhythmias in children are more commonly due to paroxysmal supraventricular tachycardias (PSVTs) 5. The incidence of ischemic ventricular tachycardia increases with age, regardless of sex, as the prevalence of coronary artery disease increases. Ventricular tachycardia rates peak in the middle decades of life, in concert with the incidence of structural heart disease. Idiopathic ventricular tachycardia can be observed at any age.
Ventricular tachycardia can be classified as sustained or nonsustained, with a generally accepted cutoff of 30 seconds.
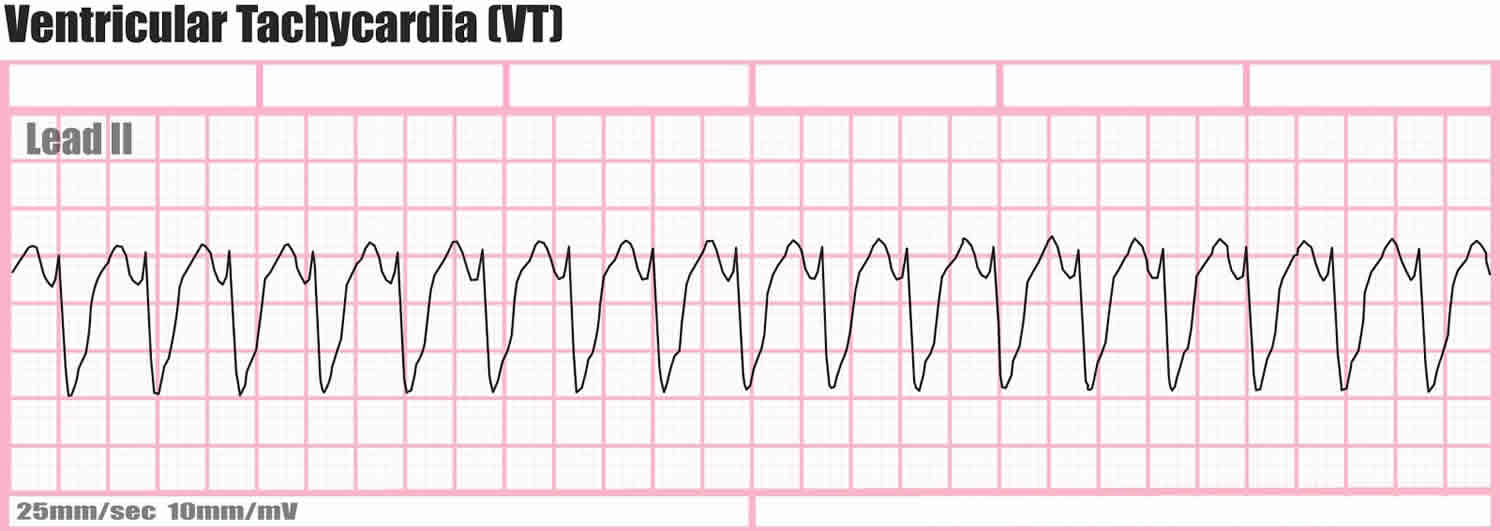
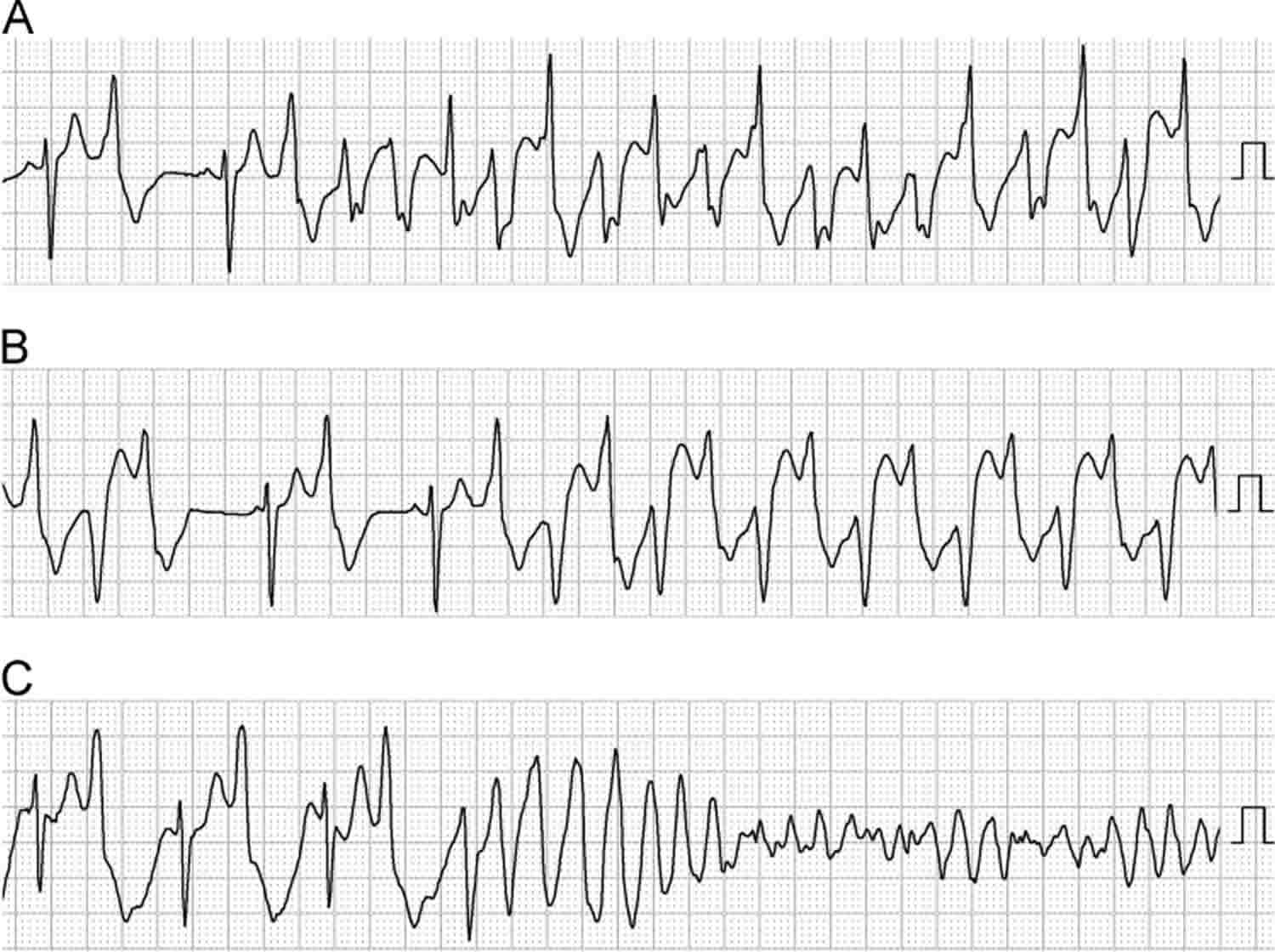
Ventricular tachycardia is further classified according to the electrocardiographic (ECG) appearance. If the QRS complex remains identical from beat to beat, as occurs when ventricular tachycardia originates from a single focus or circuit, it is classified as monomorphic (Figure 1 below). Monomorphic ventricular tachycardia means that the appearance of all the heart beats match each other in each lead of a surface electrocardiogram (ECG). Scar-related monomorphic ventricular tachycardia is the most common type and a frequent cause of death in patients having survived a heart attack or myocardial infarction, especially if they have weak heart muscle.
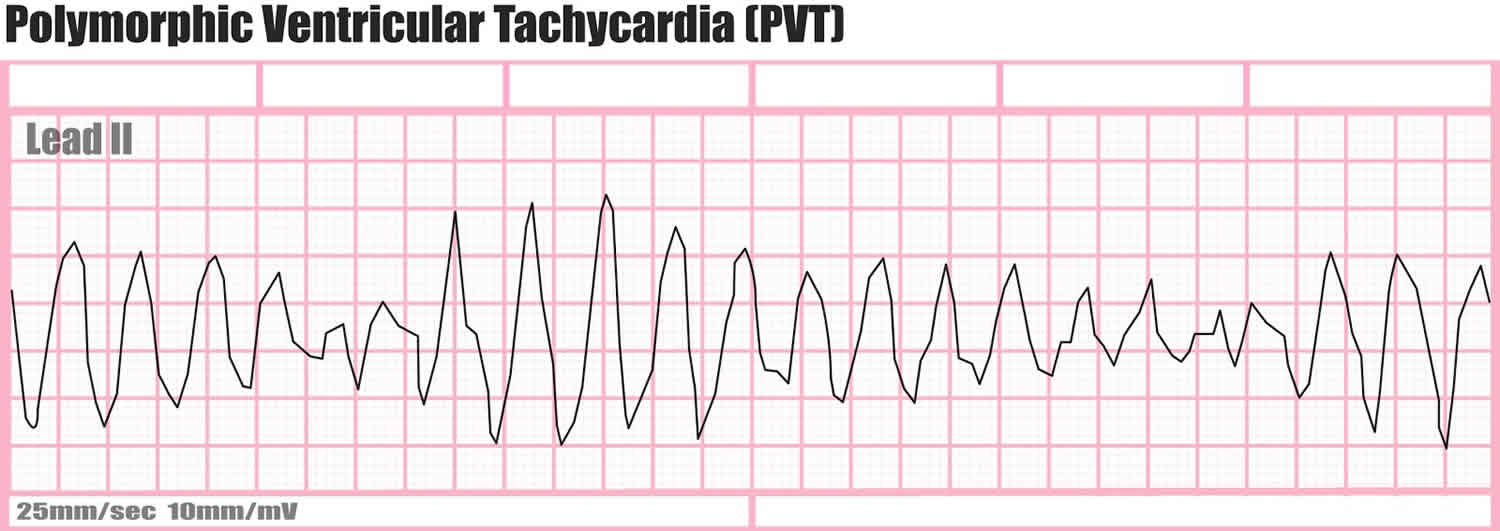
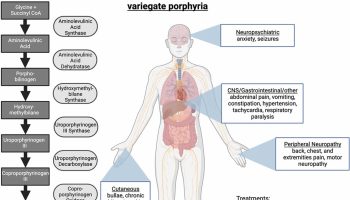
If the QRS morphology changes from beat to beat, the ventricular tachycardia is classified as polymorphic ventricular tachycardia (Figure 2 below). Further classification can be made on the basis of the substrate and the location of the earliest activation.
Figure 1. Ventricular tachycardia ECG
Figure 2. Polymorphic ventricular tachycardia
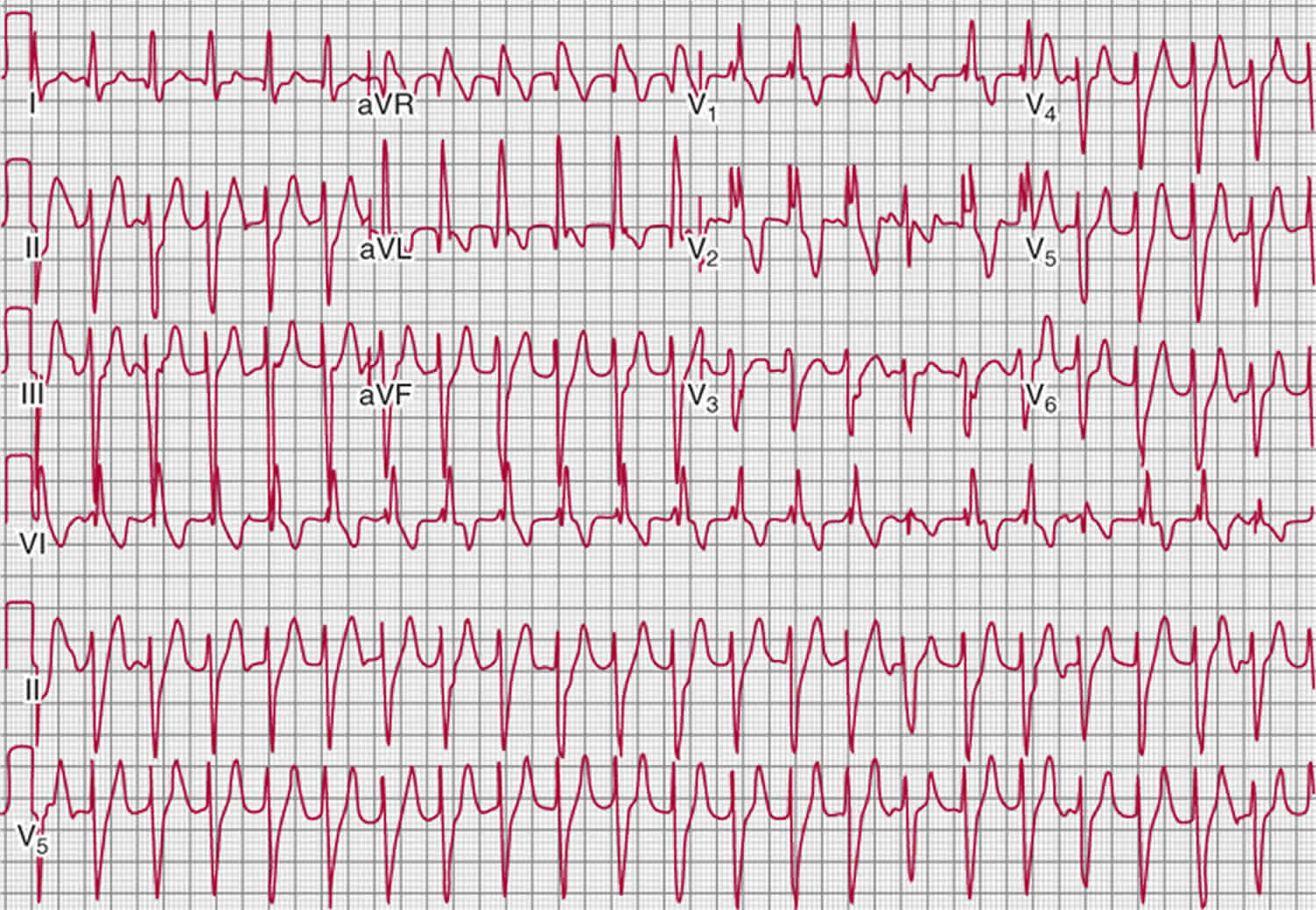
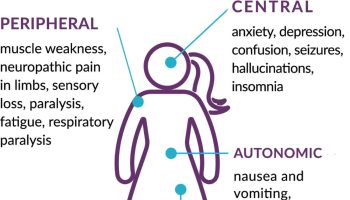
Figure 3. Catecholaminergic polymorphic ventricular tachycardia
Footnote: Typical features of ventricular tachycardia in a patient with catecholaminergic polymorphic ventricular tachycardia. (A) Polymorphic ventricular tachycardia. (B) Bidirectional ventricular tachycardia. (C) Rapid polymorphic ventricular tachycardia deteriorating into ventricular fibrillation. These electrocardiograms were recorded by Holter monitoring in the CM3 lead in the same patient.
[Source 6]In the United States, the most common setting for ventricular tachycardia is ischemic heart disease, in which myocardial scar tissue is the substrate for electrical reentry. ventricular tachycardia can also be seen in other conditions that create a myocardial scar, such as the following 7:
- Dilated cardiomyopathy
- Hypertrophic cardiomyopathy
- Arrhythmogenic right ventricular dysplasia or cardiomyopathy
- Chagas disease 8
- Surgical incisions in the ventricle
Ventricular tachycardia may also occur in the absence of structural heart disease. Ventricular tachycardia in this setting may result from enhanced automaticity, which most commonly originates in the right ventricular outflow tract or from the fascicles of the cardiac conduction system. Bundle-branch reentrant ventricular tachycardia occurs in patients with conduction system disease distal to the bundle of His. Finally, functional reentrant ventricular tachycardias can occur in structurally normal hearts, in patients with inherited channelopathies 9. The ventricular tachycardia morphology can provide a guide to the anatomic likely site of origin in the heart 10.
Ventricular tachycardia can also be triggered by the following 7:
- Electrolyte deficiencies (eg, hypokalemia, hypocalcemia, hypomagnesemia)
- Systemic diseases that affect the myocardium (eg, sarcoidosis, amyloidosis, systemic lupus erythematosus, hemochromatosis, rheumatoid arthritis)
- Sympathomimetic agents, including intravenous (IV) inotropes and illicit drugs such as methamphetamine or cocaine
- Digitalis toxicity, which can lead to biventricular tachycardia
- Drugs that prolong the QT interval (class IA and class III antiarrhythmics, azithromycin, levofloxacin, and many others); these may cause torsade de pointes
- Drugs that slow conduction velocity, particularly when an underlying myocardial scar is present (eg, halothane and class IA and IC antiarrhythmics)
Clinically, ventricular tachycardia may be reflected in symptoms such as syncope, palpitations, and dyspnea. Ventricular tachycardia is often, but not always, associated with hemodynamic compromise, particularly if the left ventricle is impaired or the heart rate is especially fast. With some exceptions, ventricular tachycardia is associated with increased risk of sudden death 11.
The ECG diagnosis of ventricular tachycardia is generally straightforward, but it does require that this condition be distinguished from aberrantly conducted supraventricular tachycardia (SVT), which has a similar ECG pattern. ECG criteria for confirming the presence of a ventricular tachycardia mechanism for a wide-complex tachycardia include the following:
- Presence of atrioventricular (AV)—technically, ventriculoatrial—dissociation (in which the ventricles fire at a faster rate than the atria)
- Fusion beats
- Capture beats
Because AV dissociation, fusion, and capture beats occur in only a minority of ventricular tachycardia tracings, additional 12-lead ECG criteria [the Brugada criteria 12 and the Vereckei criteria 13] have been derived to facilitate discrimination between ventricular tachycardia and aberrantly conducted supraventricular tachycardia (SVT).
Accelerated idioventricular rhythm, sometimes termed slow ventricular tachycardia, is a variant of ventricular tachycardia that produces a rate of 60-120 beats/min. It typically occurs in patients with underlying heart disease (ischemic or structural), is transient, and only rarely is associated with hemodynamic compromise or collapse. Treatment of the dysrhythmia itself usually is not required unless significant hemodynamic impairment develops.
Patients with frank hemodynamic compromise from acute ventricular tachycardia require emergency management with electrical cardioversion. Although cardioversion treats ventricular tachycardia, it does not prevent recurrence of ventricular tachycardia, and patients may experience repeated episodes of recurrent ventricular tachycardia after cardioversion; this phenomenon is termed ventricular tachycardia storm. These patients additionally require acute antiarrhythmic therapy, ablation therapy, or both.
The mainstays of long-term treatment for clinically stable patients with ventricular tachycardia are the various antiarrhythmic drugs. However, cardiologists are increasingly making use of interventional therapy with devices and ablation procedures designed to abort ventricular tachycardia or to destroy arrhythmogenic tissue in the heart.
Ventricular tachycardia symptoms
Ventricular tachycardia may not cause symptoms in some people. However, ventricular tachycardia can be deadly, especially after degeneration to ventricular fibrillation (VF) 14. Patients in whom this occurs may first present with syncope (fainting). Ventricular tachycardia is a major cause of sudden cardiac death.
You may have symptoms if the heart rate during a ventricular tachycardia episode is very fast or lasts longer than a few seconds. Symptoms may include:
- Chest discomfort (angina)
- Fainting (syncope)
- Lightheadedness or dizziness
- Sensation of feeling the heart beat (palpitations)
- Shortness of breath
Symptoms may start and stop suddenly. In some cases, there are no symptoms.
During ventricular tachycardia, the following may be observed:
- Hypotension (low blood pressure)
- Tachypnea (abnormally rapid breathing)
- Signs of diminished perfusion, including a diminished level of consciousness, pallor, and diaphoresis
- High jugular venous pressure
- Cannon A waves (if the atria are in sinus rhythm)
- Variation in intensity of first heart sound (S1), caused by loss of atrioventricular (AV) synchrony
After cardioversion, physical findings during normal sinus rhythm are related to any underlying structural heart disease.
Ventricular tachycardia causes
Ventricular tachycardia is a pulse rate of more than 100 beats per minute, with at least 3 irregular heartbeats in a row.
Causes of ventricular tachycardia (VT) include the following 9:
- Ischemic heart disease (most common)
- Structural heart disease with disruption of normal conduction patterns (e.g., nonischemic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy or cardiomyopathy, hypertrophic cardiomyopathy)
- Congenital structural cardiac disorders (eg, tetralogy of Fallot) and associated surgical scar
- Acquired channelopathies, most commonly from drugs that prolong the QT interval (eg, class IA and class III antiarrhythmics, phenothiazines, methadone, many others); drugs that slow myocardial conduction (eg, flecainide, propafenone, halothane) may also promote reentrant ventricular tachycardia
- Inherited channelopathies (eg, long QT syndrome, short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia)
- Electrolyte imbalances (eg, hypokalemia, hypocalcemia, hypomagnesemia)
- Sympathomimetic agents, including intravenous (IV) inotropes and illicit drugs such as methamphetamine or cocaine
- Digitalis toxicity, which can lead to biventricular tachycardia
- Systemic diseases causing infiltrative cardiomyopathy or scar (eg, sarcoidosis, amyloidosis, systemic lupus erythematosus, hemochromatosis, rheumatoid arthritis)
Ventricular tachycardia can develop as an early or late complication of a heart attack. It may also occur in people with:
- Cardiomyopathy
- Heart failure
- Heart surgery
- Myocarditis
- Valvular heart disease
Scar tissue may form in the muscle of the ventricles days, months, or years after a heart attack. This can lead to ventricular tachycardia.
Ventricular tachycardia can also occur without heart disease.
Ventricular tachycardia can also be caused by:
- Anti-arrhythmic drugs (used to treat an abnormal heart rhythm)
- Changes in blood chemistry (such as a low potassium level)
- Changes in pH (acid-base)
- Lack of enough oxygen
“Torsade de pointes” is a form of ventricular tachycardia. It is often due to congenital heart disease or the use of certain medicines.
Ventricular tachycardia diagnosis
Electrocardiography (ECG) is the criterion standard for the diagnosis of ventricular tachycardia. If the clinical situation permits, a 12-lead ECG should be obtained before conversion of the rhythm. In a patient who is hemodynamically unstable or unconscious, however, the diagnosis of ventricular tachycardia is made from the physical findings and ECG rhythm strip only.
The health care provider will look for:
- Absent pulse
- Loss of consciousness
- Normal or low blood pressure
- Rapid pulse
Tests that may be used to detect ventricular tachycardia include:
- Holter monitor
- ECG
- Intracardiac electrophysiology study (EPS)
- Rhythm monitoring with a loop recorder or device
You may also have blood chemistries and other tests.
Blood test to assess levels of serum electrolytes, including the following, in all patients with ventricular tachycardia:
- Calcium (ionized calcium levels are preferred to total serum calcium levels)
- Magnesium
- Phosphate
Hypokalemia, hypomagnesemia, and hypocalcemia may predispose patients to either monomorphic ventricular tachycardia or torsade de pointes.
Laboratory studies can also include the following:
- Levels of therapeutic drugs (e.g., digoxin)
- Toxicology screens (potentially helpful in cases related to recreational or therapeutic drug use, such as cocaine or methadone)
- Serum cardiac troponin I or T levels or other cardiac markers (to evaluate for myocardial ischemia or heart attack)
Postconversion ventricular tachycardia
In patients with ventricular tachycardia after conversion, the diagnostic workup proceeds as follows:
- Repeat the ECG after termination of ventricular tachycardia
- Include electrolyte levels in an acute evaluation; the hyperadrenergic state or hemodynamic compromise often associated with ventricular tachycardia may affect the subsequently obtained laboratory values
- Perform toxicology screens for cocaine metabolites and tricyclic antidepressants, in accordance with the patient’s clinical history
- Check cardiac enzyme levels if clinical symptoms or signs of ischemia are present
- Perform echocardiography and coronary angiography after conversion to sinus rhythm to assess for structural and ischemic heart disease
Electrophysiologic study
Diagnostic electrophysiologic study (EPS) requires placement of electrode catheters in the ventricle, followed by programmed ventricular stimulation using progressive pacing protocols. Electrophysiologic study is particularly relevant in patients considered to be at high risk for sudden death as a result of significant underlying structural heart disease.
Ventricular tachycardia treatment
Treatment depends on the symptoms, and the type of heart disorder.
If someone with ventricular tachycardia is in distress, they may require:
- CPR (cardiopulmonary resuscitation)
- Cardioversion (electric shock)
- Medicines (such as lidocaine, procainamide, sotalol, or amiodarone) given through a vein
Unstable patients with monomorphic ventricular tachycardia should be immediately treated with synchronized direct current (DC) cardioversion, usually at a starting energy dose of 100 J (monophasic). Unstable polymorphic ventricular tachycardia is treated with immediate defibrillation. Please refer to the most current Adult Advanced Cardiovascular Life Support guidelines, which are subject to periodic revision here (https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-7-adult-advanced-cardiovascular-life-support/).
Ventricular tachycardia medication
- In stable patients with monomorphic ventricular tachycardia and normal left ventricular function, restoration of sinus rhythm is typically achieved with intravenous (IV) procainamide, amiodarone, sotalol, or lidocaine 15.
- IV lidocaine is effective at suppressing peri-infarction ventricular tachycardia but may have common and limiting side effects and, consequently, increase the overall mortality risk 15.
- In torsade de pointes, magnesium sulfate may be effective if a long QT interval is present at baseline 15.
- For long-term treatment of most patients with left ventricular dysfunction, current clinical practice favors class III antiarrhythmics (eg, amiodarone, sotalol) 15.
- In patients with heart failure, the best proven antiarrhythmic drug strategies include the use of beta receptor–blocking drugs (eg, carvedilol, metoprolol, bisoprolol); angiotensin-converting enzyme (ACE) inhibitors; and aldosterone antagonists 15.
Implantable cardioverter-defibrillators
Multisociety guidelines recommend implantable cardioverter-defibrillator (ICD) therapy to augment medical management for the following 16:
- Most patients with hemodynamically unstable ventricular tachycardia
- Most patients with prior heart attack and hemodynamically stable sustained ventricular tachycardia
- Most cardiomyopathy patients with unexplained syncope (an arrhythmia is presumed)
- Most patients with genetic sudden death syndromes when unexplained syncope is noted
Radiofrequency ablation
Radiofrequency ablation (RFA) via endocardial or epicardial catheter placement can be used to treat ventricular tachycardia in patients who have the conditions noted in the following bulleted list. For patients with structural heart disease, it is currently uncertain whether ventricular tachycardia ablation obviates other therapies, such as an implantable cardioverter-defibrillator (ICD) 17.
- Left ventricular dysfunction from prior heart attack (myocardial infarction)
- Cardiomyopathy
- Bundle-branch reentry
- Various forms of idiopathic ventricular tachycardia
Ventricular tachycardia treatment guidelines
2010 and 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care — Adult Advanced Cardiovascular Life Support guidelines can be found here (https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-7-adult-advanced-cardiovascular-life-support/).
Ventricular tachycardia prognosis
The prognosis in patients with ventricular tachycardia varies with the specific cardiac process, but it is predicted best by left ventricular function. Patients with ventricular tachycardia may suffer heart failure and its attendant morbidity as a result of hemodynamic compromise. In patients with ischemic cardiomyopathy and nonsustained ventricular tachycardia, sudden-death mortality approaches 30% in 2 years. In patients with idiopathic ventricular tachycardia, the prognosis is excellent, with the major risk being injury incurred during syncopal spells.
Data from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial suggest that ventricular tachycardia or ventricular fibrillation occurring before coronary angiography and revascularization in the setting of ST-segment elevation myocardial infarction has a strong association with increased 3-year rates of death and stent thrombosis 18.
Appropriate treatment can significantly improve the prognosis in selected patients. Beta-blocker therapy can reduce the risk of sudden cardiac death from ventricular tachycardia, and implantable cardioverter-defibrillators can terminate malignant arrhythmias 19.
The prognosis does not always correlate with left ventricular function. Patients with long QT syndrome, right ventricular dysplasia, or hypertrophic cardiomyopathy may be at increased risk for sudden death despite relatively well preserved left ventricular function. These possibilities should be considered in any patient with a strong family history of premature sudden death.
Catecholaminergic polymorphic ventricular tachycardia
Catecholaminergic polymorphic ventricular tachycardia is a genetic disorder that causes an abnormally fast and irregular heart rhythm in response to physical activity or emotional stress 20. Catecholaminergic polymorphic ventricular tachycardia is characterized by episodic syncope occurring during exercise or acute emotion in individuals without structural cardiac abnormalities 21. The underlying cause of these episodes is the onset of fast ventricular tachycardia (bidirectional or polymorphic). Spontaneous recovery may occur when these arrhythmias self-terminate 21. In other instances, ventricular tachycardia may degenerate into ventricular fibrillation and cause sudden death if cardiopulmonary resuscitation is not readily available 21. The mean age of onset of symptoms (usually a syncopal episode) is between age seven and twelve years; onset as late as the fourth decade of life has been reported 21. If untreated, catecholaminergic polymorphic ventricular tachycardia is highly lethal, as approximately 30% of affected individuals experience at least one cardiac arrest and up to 80% one or more syncopal spells 21. Sudden death may be the first manifestation of catecholaminergic polymorphic ventricular tachycardia disease 21.
Signs and symptoms of catecholaminergic polymorphic ventricular tachycardia include light-headedness, dizziness, and fainting. Symptoms most often develop between 7 to 9 years of age. If untreated catecholaminergic polymorphic ventricular tachycardia can cause a heart attack and death.
The prevalence of catecholaminergic polymorphic ventricular tachycardia is estimated to be about 1 in 10,000 people 22. However, the true prevalence of this condition is unknown.
Catecholaminergic polymorphic ventricular tachycardia is caused by mutations in the RYR2 or CASQ2 genes 22. RYR2 gene mutations cause about half of all cases, while mutations in the CASQ2 gene account for 1 percent to 2 percent of cases. In people without an identified mutation in one of these genes, the genetic cause of catecholaminergic polymorphic ventricular tachycardia is unknown.
Treatment of adults with catecholaminergic polymorphic ventricular tachycardia related arrhythmias typically involves the use of beta blockers. An implantable cardioverter-defibrillator (ICD) and beta blocker treatment may be considered for people with catecholaminergic polymorphic ventricular tachycardia who have experienced a heart attack, fainting spells, or sustained abnormal heart rhythms on beta blockers alone 20. If these individuals still experience heart rhythm abnormalities flecainide and/or verapamil may also be recommended. People whose condition remains resistant to therapy may be counseled regarding further treatment options, such as left sympathetic denervation 23.
Catecholaminergic polymorphic ventricular tachycardia causes
Catecholaminergic polymorphic ventricular tachycardia can result from mutations in two genes, RYR2 and CASQ2. RYR2 gene mutations cause about half of all cases, while mutations in the CASQ2 gene account for 1 percent to 2 percent of cases. In people without an identified mutation in one of these genes, the genetic cause of the disorder is unknown.
The RYR2 and CASQ2 genes provide instructions for making proteins that help maintain a regular heartbeat. For the heart to beat normally, heart muscle cells called myocytes must tense (contract) and relax in a coordinated way. Both the RYR2 and CASQ2 proteins are involved in handling calcium within myocytes, which is critical for the regular contraction of these cells.
Mutations in either the RYR2 or CASQ2 gene disrupt the handling of calcium within myocytes. During exercise or emotional stress, impaired calcium regulation in the heart can lead to ventricular tachycardia in people with catecholaminergic polymorphic ventricular tachycardia.
When catecholaminergic polymorphic ventricular tachycardia results from mutations in the RYR2 gene, it has an autosomal dominant pattern of inheritance. Autosomal dominant inheritance means that one copy of the altered gene in each cell is sufficient to cause the disorder. In about half of cases, an affected person inherits an RYR2 gene mutation from one affected parent. The remaining cases result from new mutations in the RYR2 gene and occur in people with no history of the disorder in their family.
When catecholaminergic polymorphic ventricular tachycardia is caused by mutations in the CASQ2 gene, the condition has an autosomal recessive pattern of inheritance. Autosomal recessive inheritance means that both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
Genetic counseling
Autosomal dominant catecholaminergic polymorphic ventricular tachycardia
CALM1- and RYR2-related catecholaminergic polymorphic ventricular tachycardia are inherited in an autosomal dominant manner. Each child of an individual with autosomal dominant catecholaminergic polymorphic ventricular tachycardia has a 50% chance of inheriting the pathogenic variant.
Autosomal recessive catecholaminergic polymorphic ventricular tachycardia
CASQ2- and TRDN-related catecholaminergic polymorphic ventricular tachycardia are inherited in an autosomal recessive manner. The parents of an affected child are obligate heterozygotes (i.e., carriers of one pathogenic variant). Minor abnormalities (rare and benign arrhythmias) have been reported in heterozygotes in anecdotal cases. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being heterozygous, and a 25% chance of being unaffected and not a heterozygote.
Once the catecholaminergic polymorphic ventricular tachycardia-related pathogenic variant(s) have been identified in an affected family member, prenatal testing for a pregnancy at increased risk and preimplantation genetic diagnosis are possible options.
Evaluation of relatives at risk
Because treatment and surveillance are available to reduce morbidity and mortality, first-degree relatives should be offered clinical work up and molecular genetic testing if the family-specific pathogenic variant(s) are known. Indeed, the availability of effective preventive therapies can reduce the number of fatal arrhythmic events if individuals with pathogenic variants are diagnosed early.
If the family-specific pathogenic variant(s) are not known, all first-degree relatives of an affected individual should be evaluated with resting ECG, Holter monitoring, and – most importantly – exercise stress testing.
Catecholaminergic polymorphic ventricular tachycardia diagnosis
Making a diagnosis for a genetic or rare disease can often be challenging. Healthcare professionals typically look at a person’s medical history, symptoms, physical exam, and laboratory test results in order to make a diagnosis.
Catecholaminergic polymorphic ventricular tachycardia should be suspected in individuals who have one or more of the following 24:
- Syncope occurring during physical activity or acute emotion; mean onset age seven to 12 years. Less frequently, first manifestations may occur later in life; individuals with first event up to age 40 years are reported.
- History of exercise- or emotion-related palpitations and dizziness in some individuals
- Sudden unexpected cardiac death triggered by acute emotional stress or exercise
- Family history of juvenile sudden cardiac death triggered by exercise or acute emotion
- Exercise-induced polymorphic ventricular arrhythmias
- ECG during a graded exercise (exercise stress test)* allows ventricular arrhythmias to be reproducibly elicited in the majority of affected individuals. Typically, the onset of ventricular arrhythmias is 100-120 beats/min.
- With increase in workload, the complexity of arrhythmias progressively increases from isolated premature beats to bigeminy and runs of non-sustained ventricular tachycardia (VT). If the affected individual continues exercising, the duration of the runs of ventricular tachycardia progressively increases and ventricular tachycardia may become sustained.
- An alternating 180°-QRS axis on a beat-to-beat basis, so-called bidirectional ventricular tachycardia, is often the distinguishing presentation of catecholaminergic polymorphic ventricular tachycardia arrhythmias.
- Some individuals with catecholaminergic polymorphic ventricular tachycardia may also present with irregular polymorphic ventricular tachycardia without a “stable” QRS vector alternans 25.
- Exercise-induced supraventricular arrhythmias (supraventricular tachycardia and atrial fibrillation) are common 26.
- Ventricular fibrillation occurring in the setting of acute stress
- Absence of structural cardiac abnormalities
*Note: The resting ECG of individuals with catecholaminergic polymorphic ventricular tachycardia is usually normal, although some authors have reported a lower-than-normal resting heart rate 27 and others have observed a high incidence of prominent U waves 28. Overall these features are inconsistent and not sufficiently specific to allow diagnosis. Therefore, in many instances the origin of the syncope may be erroneously attributed to a neurologic disorder. The exercise stress test is the single most important diagnostic test. In the present authors’ series, the mean time interval to diagnosis after the first symptom was 2±0.8 years 25.
Establishing the Diagnosis
According to the most recent version of the International Guidelines on sudden cardiac death 29, the diagnosis of catecholaminergic polymorphic ventricular tachycardia is established:
- In the presence of a structurally normal heart, normal resting ECG, and exercise- or emotion-induced bidirectional or polymorphic ventricular tachycardia;
OR - In individuals who have a heterozygous pathogenic variant in RYR2 or CALM1 or biallelic pathogenic variants in CASQ2 or TRDN.
Molecular testing approaches can include serial single-gene testing, use of a multigene panel, and more comprehensive genomic testing:
Serial single-gene testing
- Sequence analysis of RYR2 can be performed first and followed by sequence analysis of CASQ2 if no pathogenic variant is found. If no pathogenic variant in CASQ2 is found, sequence analysis of CALM1 and TRDN should be performed next, keeping in mind that pathogenic variants in CALM1 and TRDN are extremely rare causes of catecholaminergic polymorphic ventricular tachycardia.
- Gene-targeted deletion/duplication analysis of RYR2 can be performed next if a pathogenic variant in RYR2 or CALM1 or biallelic pathogenic variants in CASQ2 or TRDN have not been identified (see Table 1).
A multigene panel that includes CALM1, CASQ2, RYR2, and TRDN and other genes of interest may also be considered. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
More comprehensive genomic testing (when available) including exome sequencing and genome sequencing may be considered if serial single-gene testing (and/or use of a multigene panel that includes CALM1, CASQ2, RYR2, and TRDN) fails to confirm a diagnosis in an individual with features of catecholaminergic polymorphic ventricular tachycardia. Such testing may provide or suggest a diagnosis not previously considered (e.g., mutation of a different gene or genes that results in a similar clinical presentation).
Catecholaminergic polymorphic ventricular tachycardia treatment
Treatment of manifestations
The use of beta-blockers is the mainstay of catecholaminergic polymorphic ventricular tachycardia therapy 25. Although there are no comparative studies, the majority of international referral centers use nadolol (1-2.5 mg/kg/day divided into 2 doses per day) or propranolol (2-4 mg/kg/day divided into 3-4 doses per day). Non-selective beta-blockers are recommended in all individuals in the absence of contraindications (e.g., asthma). Reproducible induction of arrhythmia during exercise allows titration and monitoring of the dose of beta-blockers. When there is evidence of incomplete protection (recurrence of syncope or complex arrhythmias during exercise) with beta blockers, flecainide (100-300 mg/day) should be added. Beta-blockers and flecainide are also indicated for affected individuals who have experienced a previous aborted sudden death. An implantable cardioverter defibrillator (ICD) may be necessary for those with recurrent cardiac arrest while on beta-blocker therapy or for those unable to take beta-blockers. Pharmacologic therapy should be maintained/optimized even in individuals with an implantable cardioverter defibrillator in order to reduce the probability of implantable cardioverter defibrillator firing. Left cardiac sympathetic denervation (LCSD) can be considered in those who are refractory to other therapies or in those who are intolerant of or have contraindications to beta-blocking therapy; however, given the side effects and recurrence of cardiac events associated with left cardiac sympathetic denervation, pharmacologic therapy should always be optimized prior to considering left cardiac sympathetic denervation.
Prevention of primary manifestations
Beta-blockers are indicated for all clinically affected individuals, and for individuals with pathogenic variants in one of the genes associated with catecholaminergic polymorphic ventricular tachycardia with a negative exercise stress test, since sudden death can be the first manifestation of the disease. Flecainide can be added for primary prevention of a cardiac arrest when beta-blockers alone cannot control the onset of arrhythmias during exercise stress test.
Prevention of secondary complications
To avoid exacerbation of allergic asthma, a cardiac-specific beta-blocker, metoprolol, may be used; the dose should be individualized. Anticoagulation may be necessary for some persons with an implantable cardioverter defibrillator.
Surveillance
Regular follow-up visits to the cardiologist every six to twelve months (depending on the severity of clinical manifestations) are required in order to monitor therapy efficacy. These visits should include the following:
- Resting ECG
- Exercise stress test, performed at the maximal age-predicted heart rate. For individuals on beta-blocker therapy (in whom maximal heart rate cannot be reached), the test should be performed at the highest tolerated workload.
- Holter monitoring
- Echo and MRI at least every two years
The limit for any allowed physical activity can be defined on the basis of exercise stress test done in the hospital setting; the use of commercially available heart rate monitoring devices for sports participation can be helpful in keeping the heart rate in a safe range during physical activity but should not be considered as an alternative to medical follow-up visits.
Agents/circumstances to avoid
Competitive sports and other strenuous exercise; digitalis.
Competitive sports and other strenuous exercise are always contraindicated 21. All individuals showing exercise-induced arrhythmias should avoid physical activity, with the exception of light training for those individuals showing good suppression of arrhythmias on exercise stress testing while on therapy. It is important to note that efficacy needs to be periodically retested 30. The risk for arrhythmias during sports in individuals who have pathogenic variants in genes associated with catecholaminergic polymorphic ventricular tachycardia but no clinical phenotype (no exercise-induced arrhythmias) is not known; thus it may be safest for these individuals to refrain from intense physical activities.
Digitalis favors the onset of cardiac arrhythmias due to delayed afterdepolarization and triggered activity; therefore, digitalis should be avoided in all individuals with catecholaminergic polymorphic ventricular tachycardia.
Can catecholaminergic polymorphic ventricular tachycardia go into remission?
In medicine the word “remission” is often used to refer to a condition where there is no evidence of ongoing disease activity. The signs and symptoms of catecholaminergic polymorphic ventricular tachycardia (catecholaminergic polymorphic ventricular tachycardia) are typically not constant, but serious concern is warranted for sudden catecholaminergic polymorphic ventricular tachycardia events that may be triggered by activity or emotional stress. It has been estimated that 83% of people with catecholaminergic polymorphic ventricular tachycardia will experience catecholaminergic polymorphic ventricular tachycardia related symptoms. Symptoms of catecholaminergic polymorphic ventricular tachycardia often present in childhood (75% by age 20) 31, however catecholaminergic polymorphic ventricular tachycardia has presented in older adults as well 21.
Do all people with catecholaminergic polymorphic ventricular tachycardia require treatment?
It has been recommended that all people clinically diagnosed with catecholaminergic polymorphic ventricular tachycardia receive treatment. Some individuals who have never had or demonstrated symptoms of catecholaminergic polymorphic ventricular tachycardia, for example asymptomatic family members with CASQ2 gene mutations, may still benefit from treatment 23. We recommend that you speak with your healthcare provider regarding your treatment options.
Can people with catecholaminergic polymorphic ventricular tachycardia play sports?
Competitive sports and other strenuous exercise are always contraindicated 21. All individuals showing exercise-induced arrhythmias should avoid physical activity, with the exception of light training for those individuals showing good suppression of arrhythmias on exercise stress testing while on therapy. It is important to note that efficacy needs to be periodically retested 30. The risk for arrhythmias during sports in individuals who have pathogenic variants in genes associated with catecholaminergic polymorphic ventricular tachycardia but no clinical phenotype (no exercise-induced arrhythmias) is not known; thus it may be safest for these individuals to refrain from intense physical activities.
- Ventricular tachycardia. https://www.mayoclinic.org/diseases-conditions/ventricular-tachycardia/symptoms-causes/syc-20355138[↩]
- Ventricular Tachycardia (VT). https://www.msdmanuals.com/professional/cardiovascular-disorders/arrhythmias-and-conduction-disorders/ventricular-tachycardia-vt[↩]
- Ventricular tachycardia. https://medlineplus.gov/ency/article/000187.htm[↩]
- Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996 Apr 18. 334 (16):1039-44.[↩]
- Garson A Jr, Gillette PC, McNamara DG. Supraventricular tachycardia in children: clinical features, response to treatment, and long-term follow-up in 217 patients. J Pediatr. 1981 Jun. 98 (6):875-82.[↩]
- Sumitomo N. Current topics in catecholaminergic polymorphic ventricular tachycardia. Journal of Arrhythmia. 2016;32(5):344-351. doi:10.1016/j.joa.2015.09.008. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5063269/[↩]
- Ventricular tachycardia. https://emedicine.medscape.com/article/159075-overview[↩][↩]
- Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007 Mar 6. 115 (9):1101-8.[↩]
- Brugada J, Brugada R, Brugada P. Channelopathies: a new category of diseases causing sudden death. Herz. 2007 May. 32 (3):185-91.[↩][↩]
- Haqqani HM, Morton JB, Kalman JM. Using the 12-lead ECG to localize the origin of atrial and ventricular tachycardias: part 2–ventricular tachycardia. J Cardiovasc Electrophysiol. 2009 Jul. 20 (7):825-32.[↩]
- Tilz RR, Lenarczyk R, Scherr D, et al. Management of ventricular tachycardia in the ablation era: results of the European Heart Rhythm Association Survey. Europace. 2017 Nov 23.[↩]
- Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991 May. 83 (5):1649-59 [↩]
- Vereckei A, Duray G, Szénási G, Altemose GT, Miller JM. New algorithm using only lead aVR for differential diagnosis of wide QRS complex tachycardia. Heart Rhythm. 2008 Jan. 5 (1):89-98.[↩]
- Baher A, Valderrabano M. Management of Ventricular Tachycardia in Heart Failure. Methodist DeBakey Cardiovascular Journal. 2013;9(1):20-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3600880/[↩]
- Ventricular Tachycardia Medication. https://emedicine.medscape.com/article/159075-medication[↩][↩][↩][↩][↩]
- Guideline] Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovas… J Am Coll Cardiol. 2013 Mar 26. 61(12):1318-68.[↩]
- Tung R, Vaseghi M, Frankel DS, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015 Sep. 12 (9):1997-2007.[↩]
- Kosmidou I, Embacher M, McAndrew T, et al. Early ventricular tachycardia or fibrillation in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention and impact on mortality and stent thrombosis (from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol. 2017 Nov 15. 120 (10):1755-60.[↩]
- Turakhia M, Tseng ZH. Sudden cardiac death: epidemiology, mechanisms, and therapy. Curr Probl Cardiol. 2007 Sep. 32 (9):501-46.[↩]
- Catecholaminergic polymorphic ventricular tachycardia. https://rarediseases.info.nih.gov/diseases/4421/catecholaminergic-polymorphic-ventricular-tachycardia[↩][↩]
- Napolitano C, Priori SG, Bloise R. Catecholaminergic Polymorphic Ventricular Tachycardia. 2004 Oct 14 [Updated 2016 Oct 13]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1289[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Catecholaminergic polymorphic ventricular tachycardia. https://ghr.nlm.nih.gov/condition/catecholaminergic-polymorphic-ventricular-tachycardia[↩][↩]
- Buxton A. Catecholaminergic polymorphic ventricular tachycardia and other polymorphic ventricular tachycardias with a normal QT interval. In: Basow, DS (Ed). UpToDate. Waltham, MA: UpToDate; 2012[↩][↩]
- Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013a;10:1932–63.[↩]
- Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74.[↩][↩][↩]
- Fisher JD, Krikler D, Hallidie-Smith KA. Familial polymorphic ventricular arrhythmias: a quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J Am Coll Cardiol. 1999;34:2015–22.[↩]
- Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–70.[↩]
- Aizawa Y, Komura S, Okada S, Chinushi M, Aizawa Y, Morita H, Ohe T. Distinct U wave changes in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT). Int Heart J. 2006;47:381–9.[↩]
- Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Available online. 2015. Accessed 2-8-17.[↩]
- Heidbüchel H, Corrado D, Biffi A, Hoffmann E, Panhuyzen-Goedkoop N, Hoogsteen J, Delise P, Hoff PI, Pelliccia A. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part II: ventricular arrhythmias, channelopathies and implantable defibrillators. Eur J Cardiovasc Prev Rehabil. 2006;13:676–86.[↩][↩]
- Pflaumer A, Davis AM. Guidelines for the diagnosis and management of catecholaminergic polymorphic ventricular tachycardia. Heart, Lung and Circulation. February 2012;21(2):96-100; http://www.ncbi.nlm.nih.gov/pubmed/22119737[↩]