Contents
What is vitreous hemorrhage
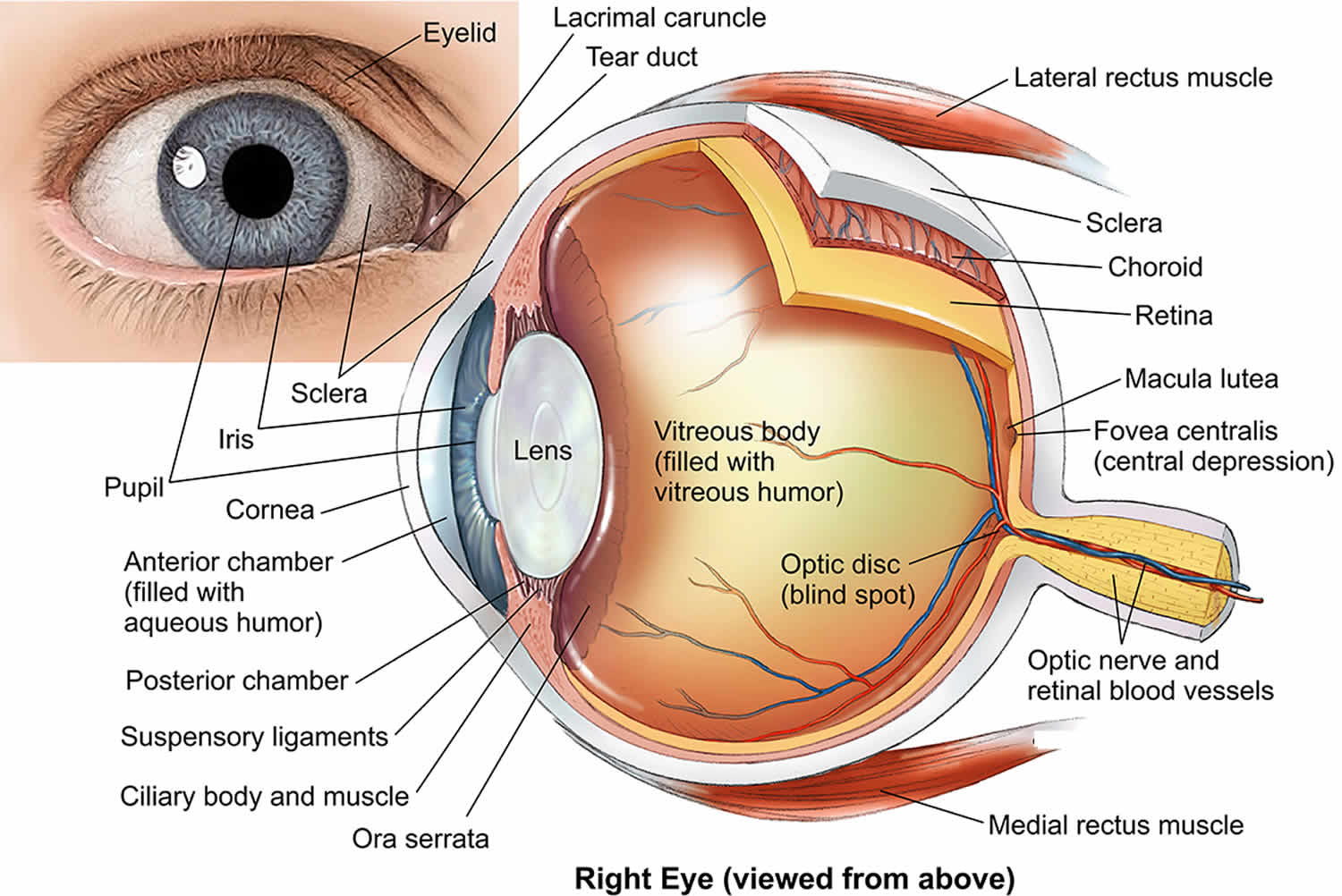
Vitreous hemorrhage is defined as the presence of blood within the vitreous cavity, which is the space lined posteriorly by the retina and anteriorly by the ciliary body, zonular fibers, and posterior lens capsule (Figure 1). Vitreous hemorrhage is a relatively common cause of rapid onset painless vision loss, having an incidence of approximately of 7 cases per 100,000 1. Sudden, painless visual loss or haze is a common presentation. Patients may describe a red hue to their vision. Patients may describe new onset floaters, shadows, or “cobwebs”. Symptoms may be worse in the morning if blood settles on the macula during the night. History of diabetes, hypertension, sickle cell disease, trauma, previous retinal conditions or ocular surgery may help lead to the diagnosis.
Although the diagnosis of vitreous hemorrhage is often straightforward to make on fundoscopic examination or ultrasonography, further investigation may be required to determine the underlying cause. The diagnosis of vitreous hemorrhage alone is not specific and the list of causes of vitreous hemorrhage is extensive. Fortunately, three conditions cause 59 to 88.5% of vitreous hemorrhage cases: proliferative diabetic retinopathy, posterior vitreous detachment and ocular trauma 1. In most circumstances, normal vessels only bleed in the setting of trauma or posterior vitreous detachment. Conversely, patients with a history of chronic retinal ischemia (e.g., diabetic retinopathy) can develop abnormal fragile vessels (i.e., neovascularization) with a propensity to leak.
Vitreous hemorrhage clears slowly, on the order of 1% per day 1. Conservative measures, such as elevation of the head of the bed, are often taken to treat vitreous hemorrhage. Patients with a low risk of recurrent bleeding (e.g., posterior vitreous detachment) have a good prognosis for resolution of symptoms and regaining baseline visual acuity. Patients with vitreous hemorrhage secondary to diabetic retinopathy or age-related macular degeneration (AMD) have the worst prognosis for clearing vitreous hemorrhage and regaining baseline visual acuity 1. Treatment beyond conservative measures is guided by disease and severity. Intravitreal anti-VEGF drug injections are the first line treatment for proliferative retinopathies and age-related macular degeneration (AMD) with neovascularization. Panretinal photocoagulation is used in proliferative diabetic retinopathy to reduce the amount of angiogenesis. Laser treatment may also be pursued in the setting of retinal break with impending retinal detachment. The definitive treatment for non-clearing vitreous hemorrhage is pars plana vitrectomy, in which the vitreous is surgically removed and replaced with saline. Most ophthalmologists will observe vitreous hemorrhage for at least six months before proceeding with vitrectomy.
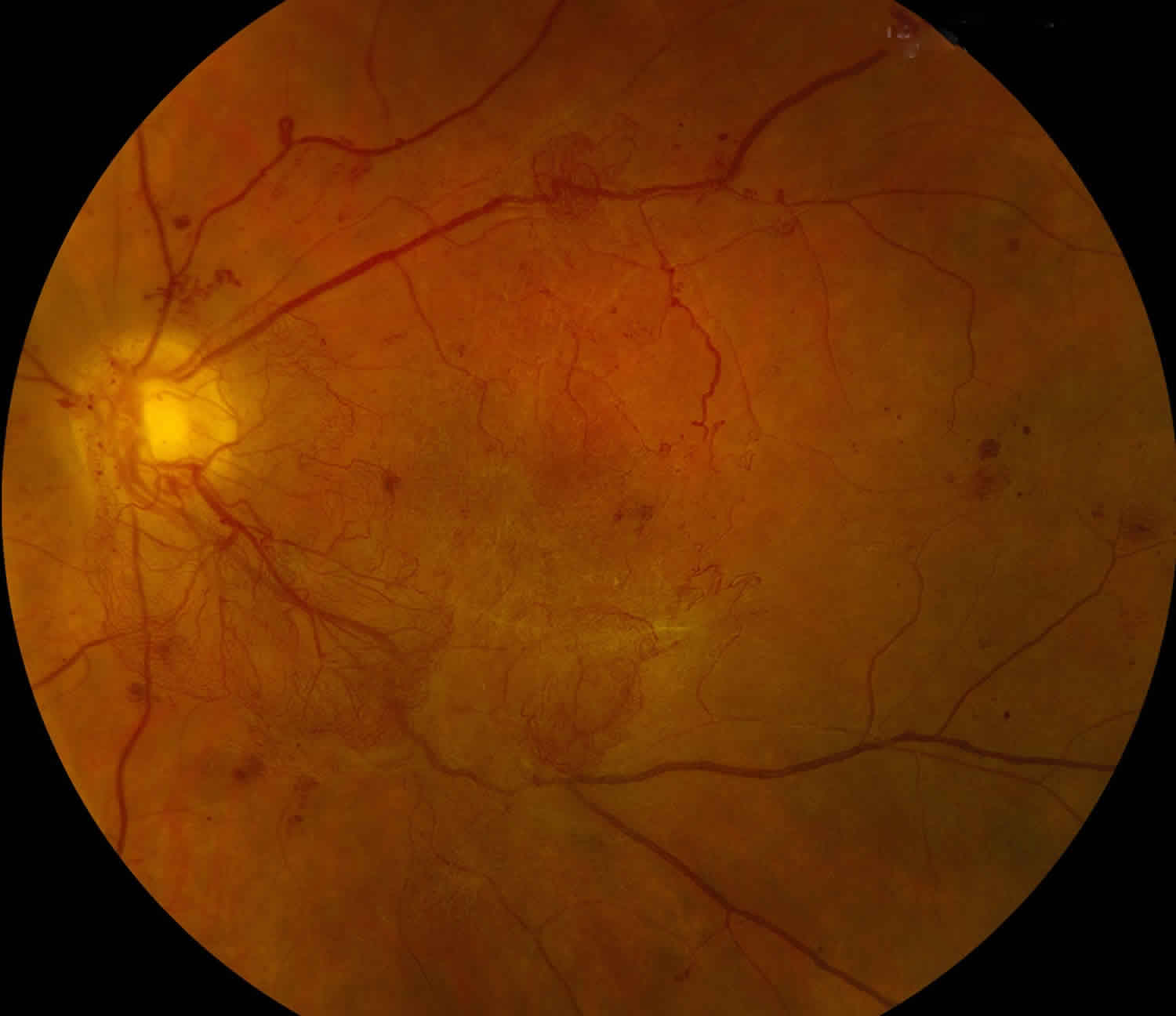
Figure 1. Vitreous hemorrhage (Diabetic retinopathy)
Vitreous anatomy
The vitreous humor is 99 percent water. The remaining 1 percent is made up of collagen and hyaluronic acid, giving it a gelatinous consistency and optical clarity. The vitreous body is defined by the internal limiting membrane of the retina posterolaterally, by the nonpigmented epithelium of the ciliary body anterolaterally, and by the posterior lens capsule and lens zonular fibers anteriorly. This space represents 80 percent of the eye and has a volume of approximately 4 ml. The vitreous is firmly attached to the retina in three places: the strongest attachment is anteriorly at the vitreous base, followed by the optic nerve head and retinal vasculature.
Figure 2. Human eye anatomy
Vitreous hemorrhage causes
The frequency of the causes of vitreous hemorrhage is variable according to the characteristics of the study population. The three most common causes, which account for 59-88.5% of all cases include 1:
- Proliferative diabetic retinopathy (see Figure 1): Diabetic retinopathy is a common cause of vitreous hemorrhage. Diabetic retinopathy affects 5.3 million Americans and is the most common cause of vitreous hemorrhage, constituting 31-54% of all vitreous hemorrhage cases 2. In diabetic retinopathy, chronic hyperglycemia causes endothelial damage and basement membrane thickening, leading to vessel weakening and obstruction. Vessel damage in diabetic retinopathy incites chronic ischemia, which promotes cells to release angiogenic factors and leads to new blood vessel growth (i.e., neovascularization) or proliferative diabetic retinopathy. Unfortunately, the new blood vessels are fragile and likely to bleed (Figure 1) 3. Due to the high prevalence of diabetes and diabetic retinopathy, it should remain high on the differential for vitreous hemorrhage.
- Posterior vitreous detachment (PVD) with or without retinal tear: The vitreous undergoes syneresis as you age, affording the opportunity to peel away from attachments in a process known as a posterior vitreous detachment (PVD). When vitreous becomes completely separated from the optic nerve head, a Weiss ring (Figure 4) may be visible on fundus exam. A posterior vitreous detachment can create enough traction to tear or detach the retina. Patients presenting with posterior vitreous detachment without retinal involvement can still have a vitreous hemorrhage (4-12% of patients), although vitreous hemorrhage is more likely in the setting of retinal tear (11-44%) 4.
- Ocular trauma.
Less common causes of vitreous hemorrhage include retinal vein occlusion (Figure 3), proliferative sickle cell retinopathy, retinal macroanuerysm, subarachnoid hemorrhage (Terson syndrome), and neovascular age-related macular degeneration (AMD).
Vitreous hemorrhage causes 5:
- Vascular
- Proliferative diabetic retinopathy (32%)
- Central or branch retinal artery occlusion (11%)
- Proliferative sickle cell retinopathy (2%)
- Age-related macular degeneration (“wet” AMD) (2%)
- Coat’s disease
- Retinopathy of prematurity
- Ocular ischemic syndrome
- Macroaneurysm (2%)
- Hypertensive retinopathy
- Venous stasis retinopathy
- Familial retinal arteriolar tortuosity
- Vitreoretinal
- Posterior vitreous detachment (8%)
- Retinal break or tear (30%)
- Retinal detachment
- Complications of surgical procedure of retina
- Traumatic
- Direct eye trauma
- Open or closed globe injury
- Foreign body
- Non-accidental trauma
- Terson syndrome
- Anterior Segment
- Cataract extraction with or without intraocular lens (IOL)
- Secondary intraocular lens (IOL) implantation
- Prolapsed posterior chamber intraocular lens (IOL)
- Trabeculectomy
- Uveitis-glaucoma-hyphema syndrome
- Fuch’s iridocyclitis
- Swan syndrome (cataract wound neovascularization)
- Inflammatory
- Vasculitis
- Infectious choroidopathy
- Behcet’s disease
- Eale’s disease
- Endophthalmitis
- Uveitis
- Syphilitic retinitis
- Indirect Mechanisms
- Idiopathic intracranial hypertension
- Valsalva retinopathy
- Blood Disorders
- Anticoagulation
- Thrombocytopenia
- Leukemia
- Hemophilia
- Von Willebrand disease
- Tumor
- Choroidal malignant melanoma
- Retinoblastoma
- Cavernous hemangioma
Mechanisms of vitreous hemorrhage
The mechanisms of vitreous hemorrhage fall into three main categories:
- Abnormal vessels that are prone to bleeding:
- Diabetic retinopathy (31–54 percent) of vitreous hemorrhages are caused by diabetes
- Neovascularization from branch or central retinal vein occlusion (4–16 percent)
- Sickle cell retinopathy (0.2–6 percent)
- Normal vessels that rupture under stress:
- Retinal tear (11–44 percent)
- Trauma (12–19 percent)
- Posterior vitreous detachment with retinal vascular tear (4–12 percent)
- Retinal detachment (7–10 percent)
- Terson’s syndrome (0.5–1 percent)
- Extension of blood from an adjacent source:
- Macroaneurysm (0.6–7 percent)
- Age-related macular degeneration (0.6–4 percent)
Abnormal vessels
Abnormal retinal blood vessels are typically the result of neovascularization due to ischemia in diseases such as diabetic retinopathy, sickle cell retinopathy, retinal vein occlusion, retinopathy of prematurity or ocular ischemic syndrome. As the retina experiences inadequate oxygen supply, caused by conditions such as diabetic retinopathy, retinal vein occlusion, sickle cell retinopathy, and retinopathy of prematurity promote the growth of new vessels (neovascularization) via the elaboration of angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and insulinlike growth factor. These newly formed vessels grow into the vitreous and are fragile due to their lack endothelial tight junctions, which predispose them to spontaneous bleeding. They also coexist with a fibrous component that often contracts, putting additional stress on already fragile vessels. Also, normal vitreous traction with eye movement can lead to rupture of these vessels.
Retinal vein occlusion
Central retinal vein occlusion and branch retinal vein occlusion (Figure 3), account for 4-16% of all vitreous hemorrhages 1. It is the second most common retinal vascular disorder after diabetic retinopathy. Retinal vein occlusion pathogenesis is multifactorial, including arterial disease, mechanical compression, degeneration of venous walls, and hypercoagulability leading to venous occlusion 6. Acutely, venous stasis can damage venules allowing extravasation of blood into the vitreous. After an occlusive event, the retina is subject to chronic ischemia leading to compensatory angiogenesis and increased risk of vitreous hemorrhage.
Figure 3. Branch retinal vein occlusion
Figure 4. Posterior vitreous detachment (Weiss ring seen in complete posterior vitreous detachment)
Rupture of normal retinal blood vessels through mechanical force
Normal retinal blood vessels can rupture when sufficient mechanical force that overcomes the structural integrity of the retinal blood vessel. During a posterior vitreous detachment, vitreous traction on the retinal vasculature may compromise a blood vessel, especially at the firm attachments mentioned above. This may happen with or without a retinal tear or detachment. However, vitreous hemorrhage in the setting of an acute symptomatic posterior vitreous detachment should alert the clinician that the risk of a concurrent retinal break is quite high (70–95 percent).
Blunt or perforating trauma can injure intact vessels directly and is the leading cause of vitreous hemorrhage in people younger than 40.
- Closed globe injury from blunt trauma: Compression of the globe in an anterior-posterior direction causes the equator of the globe to bulge in a coronal plane. Especially in a young patient with formed vitreous and strong adherence of the vitreous to the retina, this bulging in the coronal plane causes inward-directed traction exerted by the vitreous on the retina. The fact that the vitreous base, an area of especially strong vitreous attachment, is near the equator contributes to the tractional force the vitreous exerts on the retina in this area. This inward-directed tractional force on the retina by the vitreous can cause a retinal dialysis, but it can also result in retinal tears and vitreous hemorrhage as retinal vessels may be ruptured. Blunt trauma may also rupture blood vessels associated with the iris and ciliary body, causing hyphema and spillover hemorrhage into vitreous.
- Open globe injury: Blunt or sharp trauma causing a full-thickness defect in the eyewall may cause hemorrhage in all layers of the eye, including vitreous hemorrhage.
- Shaken baby syndrome: the trauma associated with shaken baby syndrome may cause hemorrhage in all ocular layers, including vitreous hemorrhage. It has been suggested that vitreous hemorrhage in this context may portend a worse prognosis 7.
- Acute posterior vitreous detachment: Vitreous hemorrhage in the setting of acute posterior vitreous detachment is associated with a retinal tear or break in 70-95% of cases 1 and should invoke an exhaustive search for retinal breaks.
- Terson syndrome: Terson syndrome which refers to an extravasation of blood into the vitreous due to a subarachnoid hemorrhage or intracerebral hemorrhage, is a rare cause of vitreous hemorrhage. The vitreous hemorrhage is not a direct extension of subarachnoid hemorrhage into the eye via the optic nerve sheath. Rather, increased intracranial pressure causes increased pressure in retinal venules, causing them to rupture.
Figure 5. Terson Syndrome
Blood from an adjacent source
Pathology adjacent to the vitreous can also cause vitreous hemorrhage. Hemorrhage from retinal macroaneurysms and occluded retinal venules in retinal vein occlusion may rupture, causing vitreous hemorrhage. Choroidal tumors and choroidal neovascularization secondary to conditions such as age-related macular degeneration (AMD) can cause “break-through” bleeding through the retina and extend through the internal limiting membrane into the vitreous.
Risk factor for vitreous hemorrhage
In the setting of vitreous hemorrhage, there may be contributing factors, such as medications or other medical conditions. The use of medications, particularly anticoagulants, may affect a patient’s risk for vitreous hemorrhage. Witmer, et al. 8 found that patients on aspirin, clopidogrel, or warfarin presenting with signs and symptoms of acute posterior vitreous detachment had vitreous hemorrhage 43% of the time, while controls had an incidence of 31%. However, the Early Treatment Diabetic Retinopathy Study Number 7 9 did not find an increased risk of vitreous hemorrhage in patients taking aspirin. Supra-therapeutic levels of anticoagulation, thrombocytopenia, or other hematologic disorders may cause vitreous hemorrhage, although these cases are rare 1. In patients on anticoagulation who are diagnosed with vitreous hemorrhage, most physicians will not discontinue anticoagulation in order to aid in resolution. The risks of stopping this medication could be life-threatening (e.g., stroke) and outweigh the benefits (e.g., faster recovery of vision). Concerns arising about a patient’s anticoagulation status after vitreous hemorrhage would be best addressed through a discussion with the prescribing physician and ophthalmology.
Patients with systemic coagulation disorders and blood dyscrasias such as leukemia and thrombocytopenia may have an increased risk of vitreous hemorrhage, but these cases are rare.
Vitreous hemorrhage pathophysiology
Hemorrhage into the vitreous body results in rapid clot formation and clears at a rate of approximately 1% per day 1. Erythrocytes exit through the trabecular meshwork, undergo hemolysis, phagocytosis, or persist within the vitreous for many months. The cellular response to erythrocytes in the vitreous is unusual because there is no early polymorphonuclear cell response and the ensuing inflammatory response is instead more similar to a “low-turnover” granuloma. The muted inflammatory response in the immunologically privileged eye serves to mitigate ocular tissue damage and promote clarity of the visual axis.
Vitreous hemorrhage prevention
Vitreous hemorrhage prevention should be directed at controlling risk factors for systemic vascular disease such as diabetes, hypertension, and smoking. Frequent dilated fundus exams can reveal advanced retinopathy in high risk populations and provide the opportunity for therapeutic intervention. Proper eye protection should be worn during activities likely to cause eye trauma (e.g. hammering or grinding metal, using firearms, playing sports with high-speed balls such as racquetball).
Vitreous hemorrhage symptoms
The symptoms of vitreous hemorrhage are varied but usually include painless unilateral floaters and/or visual loss. Early or mild hemorrhage may be described as floaters, cobwebs, haze, shadows or a red hue. More significant hemorrhage limits visual acuity and visual fields or can cause scotomas. Patients often say vision is worse in the morning as blood has settled to the back of the eye, covering the macula.
Patients should be questioned regarding a history of trauma, ocular surgery, diabetes, sickle cell anemia, leukemia, carotid artery disease and high myopia.
Complete examination consists of indirect ophthalmoscopy with scleral depression, gonioscopy to evaluate neovascularization of the angle, IOP and B-scan ultrasonography if complete view of the posterior pole is obscured by blood. Dilated examination of the contralateral eye can help provide clues to the etiology of the vitreous hemorrhage, such as proliferative diabetic retinopathy.
The presence of vitreous hemorrhage is not hard to detect. At the slit lamp, red blood cells may be seen just posterior to the lens with the slit beam set “off-axis” and the microscope on the highest power. In nondispersed hemorrhage, a view to the retina may be possible and the location and source of the vitreous hemorrhage may be determined. Vitreous hemorrhage present in the subhyaloid space is also known as preretinal hemorrhage. Such a hemorrhage is often boat-shaped as it is trapped in the potential space between the posterior hyaloid and the internal limiting membrane, and settles out like a hyphema. Dispersed vitreous hemorrhage into the body of vitreous has no defined border and can range from a few small distinct red blood cells to total obscuration of the posterior pole.
Vitreous hemorrhage diagnosis
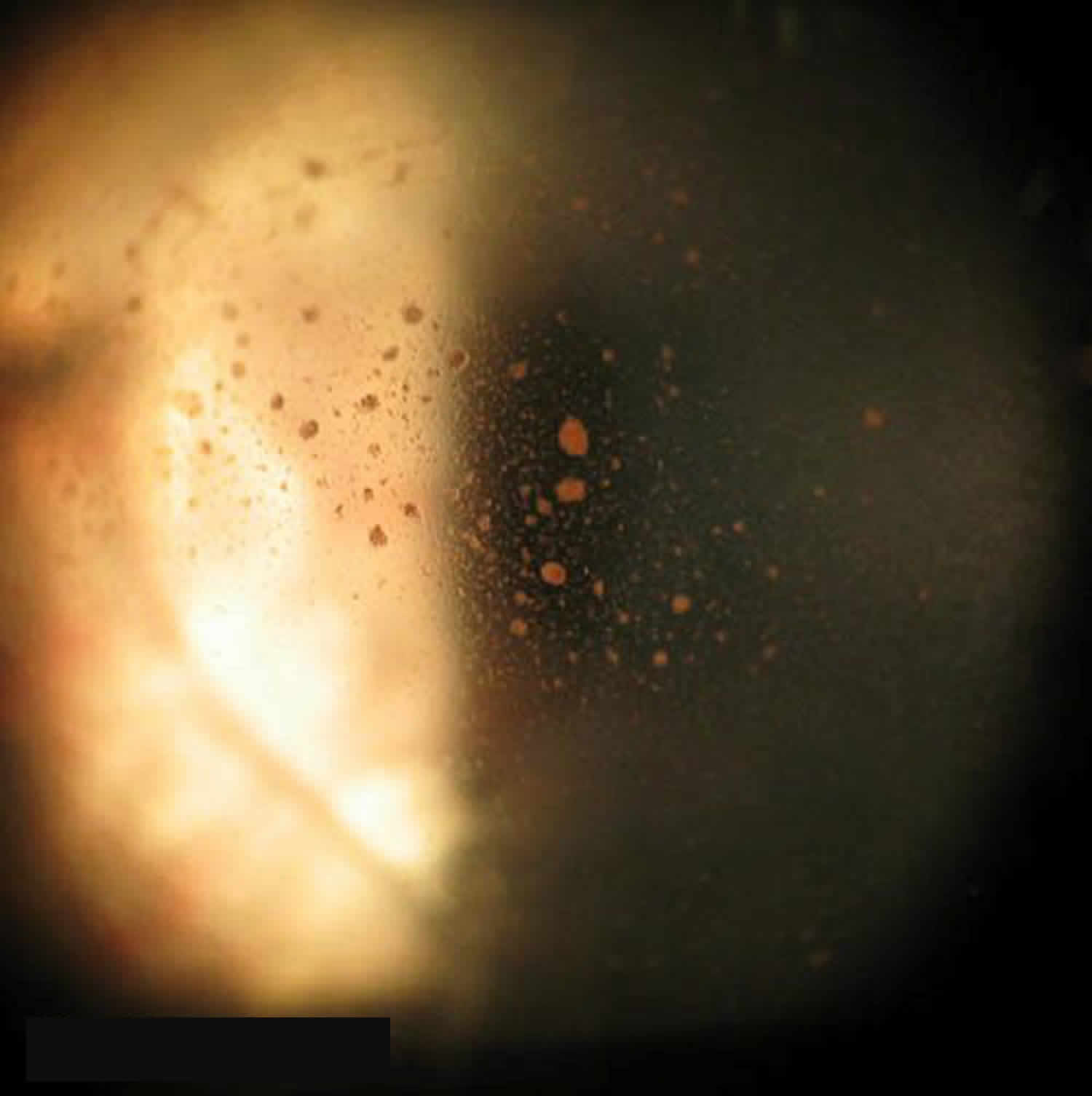
All patients with suspected vitreous hemorrhage should undergo visual acuity, slit lamp biomicroscopy, and dilated fundus examinations. It is crucial to rule out retinal tear or retinal detachment in all vitreous hemorrhage cases with either scleral depression or ultrasound (B-scan) if the view of the retina is obstructed by hemorrhage. Even patients with a history of proliferative diabetic retinopathy can develop posterior vitreous detachment or retinal tear. In severe vitreous hemorrhages, there may be a loss of red reflex or a relative afferent pupillary defect. Schaffer’s sign (Figure 6) is the presence of pigment granules in the anterior vitreous. Tanner, et al. 10 found that a positive Schaffer’s sign had a sensitivity of 95.8% and a specificity of 100% for retinal break. In patients with a history of proliferative retinopathy (e.g., diabetic retinopathy), examining the fellow eye will help assess the degree of retinopathy and likelihood of vitreous hemorrhage from a proliferative retinopathy. If there is high suspicion for intraocular foreign body, a B-scan or CT scan should be considered. There is utility in fluorescein angiography in cases of indolent bleeds of unclear origin, neovascularization, or venous occlusion. vitreous hemorrhage from the anterior segment can generally be detected on slit lamp or gonioscopy examination.
Figure 6. Schaffer’s sign
Physical examination
Visual acuity is variable based on the location, size, and degree of vitreous hemorrhage. In severe cases, patients may have dramatically reduced visual acuity and/or visual field defects. Slit lamp examination usually reveals red blood cells in the anterior vitreous. Presence of iris rubeosis and intraocular pressure should be noted. Gonioscopy should be used to detect neovascularization of the angle. Dilated fundus exam may reveal hemorrhage diffusely spread throughout the vitreous cavity, or the blood may conform to the anatomy of the vitreous. For example, hemorrhage in the subhyaloid space may result in a scaphoid (boat-shaped) hemorrhage. Occasionally, a detached posterior hyaloid face will have blood adherent to its posterior surface. If vitreous hemorrhage is associated with acute posterior vitreous detachment, retinal tear or detachment should be ruled out using scleral depression. It is important to thoroughly examine the fellow eye, as it will often reveal clues as to the etiology. In chronic vitreous hemorrhage, the red blood cells become dehemoglobinized and the hemorrhage takes on a khaki color.
Diagnostic procedures
If the vitreous hemorrhage obscures a complete view to the retina, B-scan ultrasonography can detect vitreous hemorrhage, posterior vitreous detachment, retinal tears, retinal detachment, tractional membranes, intraocular tumors, and foreign bodies. Fluorescein angiography may be useful in the setting of mild to moderate vitreous hemorrhage to help identify neovascularization. If an open globe injury is suspected, orbital CT scan is indicated to characterize the orbital bony structures, assess the integrity of the eyewall, and rule out intraocular foreign body. Blood pressure should also be checked. If familial exudative vitreoretinopathy is suspected, examination of family members is helpful.
Laboratory test
Laboratory testing is used according to clinical suspicion to diagnose diabetes, sickle cell disease, leukemia, thrombocytopenia, and other hematologic abnormalities.
Vitreous hemorrhage treatment
The management and prognosis of vitreous hemorrhage depends on the underlying pathology. As always, individual situations, patient wishes, and surgeon judgment are paramount.
- If the retina can be adequately visualized and the underlying pathology of the vitreous hemorrhage can be determined, treatment of the underlying cause may be attempted, if needed.
- If the retina can be adequately visualized and the underlying cause of the vitreous hemorrhage can be determined, but the vitreous hemorrhage does not permit safe treatment of the underlying cause, pars plana vitrectomy is indicated. A short period of cautious observation for vitreous clearing may be reasonable. For example, occasionally a retinal tear associated with vitreous hemorrhage can be seen with a bright indirect ophthalmoscope, but adequate uptake of laser spots to the posterior margin of the break cannot be achieved. Cryotherapy can be considered in these cases, but the dual risk of cryotherapy and vitreous hemorrhage potentially leading to proliferative vitreoretinopathy should be considered compared to the risk of pars plana vitrectomy with endolaser 11.
- If the retina cannot be adequately visualized in 360 degrees and the underlying pathology of the vitreous hemorrhage is unknown, prompt pars plana vitrectomy is indicated. Again, a short period of cautious observation for vitreous clearing may be reasonable. For example, in a case of a superior retinal tear with dense vitreous hemorrhage, adequate treatment of the superior retinal tear may be achieved. However, undetected inferior retinal breaks may be present. Between office visits, retinal detachment may progress underneath vitreous hemorrhage without a change in the patient’s symptoms. Therefore, if the view is not clearing briskly, pars plana vitrectomy is indicated. Neovascularization of the iris or angle in the setting of new dense vitreous hemorrhage would prompt earlier surgical intervention.
Diabetics frequently present with vitreous hemorrhage, and in general the same principles apply as outlined above. Of note, new vitreous hemorrhage in diabetics cannot always be assumed to be secondary to diabetic retinopathy if there is an inadequate view; diabetics are susceptible to retinal tears and detachments like the general population. The severity of the fellow eye may give a clue as to the etiology of the vitreous hemorrhage, but asymmetric levels of retinopathy are relatively common. In any case, diabetic patients with proliferative diabetic retinopathy, new vitreous hemorrhage prohibiting adequate panretinal photocoagulation, and no history of panretinal photocoagulation benefit from pars plana vitrectomy with intra-operative panretinal photocoagulation or intravitreal anti-vascular endothelial growth factor (anti-VEGF) injection. In this scenario, if the vitreous hemorrhage does not clear in about one month, many surgeons will perform pars plana vitrectomy. However, in the case of an established patient with known proliferative diabetic retinopathy and known panretinal photocoagulation, a new or recurrent vitreous hemorrhage is far more likely to be secondary to diabetes than a retinal tear. Longer periods of observation (3 to 6 months) before considering pars plana vitrectomy may be reasonable in these situations.
Medical therapy
Patients are instructed to minimize strenuous activity, as an increase in blood pressure may disrupt the newly formed clot and cause new active bleeding. Patients are also instructed to keep their head of bed elevated to allow settling of the blood, improving their vision and permitting more complete fundoscopic examination. Bilateral patching and bedrest may facilitate settling of blood. However, the patches must be removed immediately before examination or treatment, as normal eye movements quickly disperse the hemorrhage again. For this reason and its inconvenience to patients, bilateral patching is infrequently attempted.
Neovascularization from proliferative retinopathy, associated with diabetes or otherwise, is often treated with panretinal photocoagulation if the view is adequate. This will cause regression of neovascularization and help reduce the risk of further hemorrhage.
Intravitreal injection of anti-VEGF agents may be used to cause regression of neovascularization in proliferative retinopathies, particularly if there is no view to perform panretinal photocoagulation 12. Bhavsar et al 13 compared the use of ranibizumab with saline injected intravitreally for patients with proliferative diabetic retinopathy-associated vitreous hemorrhage in a randomized trial, and found that there seemed to be no difference in vitrectomy rates at 16 weeks between the two groups. Of note, the recent Protocol S data 14 from the Diabetic Retinopathy Clinical Research Network compared the use of panretinal photocoagulation versus intravitreal ranibizumab for proliferative diabetic retinopathy; at five years, the rates of vitreous hemorrhage were similar (nearly 50%) in both groups. Additionally, there is anecdotal evidence that anti-VEGF injection may worsen tractional retinal detachment as neovascular membranes contract, so the the potential risks and benefits should be considered in this setting.
Many surgeons use pre-operative anti-VEGF agents 1 to 7 days before pars plana vitrectomy for vitreous hemorrhage in diabetics, as regression of neovascular membranes reduces intra- and post-operative bleeding. Several small studies support this belief 15, although other small studies refute it 16. There is concern, however, that these patients frequently fail their pre-anesthesia testing and that their surgery may be cancelled after the anti-VEGF agent has been given, potentially exacerbating tractional retinal detachment. For this reason, many surgeons wait until the patient is medically cleared for surgery before giving the anti-VEGF agent.
Intravitreal injection of an anti-VEGF agent is usually indicated when the cause of vitreous hemorrhage is neovascular age-related macular degeneration (AMD).
The Early Treatment of Diabetic Retinopathy Study 17 showed that aspirin did not increase risk of vitreous hemorrhage, and no anticoagulant has been definitively shown to increase risk of vitreous hemorrhage. One report showed that patients taking aspirin, clopidogrel, or warfarin who develop an acute posterior vitreous detachment are more likely to develop a vitreous hemorrhage, although the difference was small 18. Most clinicians do not recommend discontinuation of anticoagulation with the goal of resolving a vitreous hemorrhage, especially when the anticoagulation is medically indicated.
Medical Follow-Up
Patients should be followed periodically to monitor for clearing of the vitreous hemorrhage. If the patient has systemic disease, such as diabetes, follow-up with a primary care provider should also be recommended. If an adequate view to the posterior pole is not possible, patients should be reevaluated every two or three weeks with B-scan ultrasonagraphy to exclude a retinal break or detachment. In the event of recurrent vitreous hemorrhage, referral to a retinal specialist for possible vitrectomy is warranted.
Vitreous hemorrhage surgery
Pars plana vitrectomy is indicated for vitreous hemorrhage accompanied by retinal detachment or break seen on B-scan, nonclearing vitreous hemorrhage, many cases of intraocular foreign body, vitreous hemorrhage with iris neovascularization or associated with hemolytic or ghost-cell glaucoma. Pars plana vitrectomy is also indicated in cases of dense vitreous hemorrhage of unknown cause. In these cases, pars plana vitrectomy can be both diagnostic and therapeutic. Depending on the etiology of vitreous hemorrhage, endolaser may also be placed intraoperatively.
Some surgeons will also perform a concurrent air-fluid exchange after the vitrectomy or may even inject intravitreal anti-VEGF at the conclusion of case, but there is no substantial evidence that this prevents post-operative recurrent vitreous hemorrhage, a frustrating occurrence seen in some patients, especially diabetic patients.
In cases of open globe injury with vitreous hemorrhage but without intraocular foreign body, most surgeons close the eyewall first and address the vitreous hemorrhage as a second stage procedure.
Surgical follow up
Frequency of follow up is specific to the surgical indication.
Vitreous hemorrhage recovery time
The blood is typically cleared from within the vitreous hemorrhage at a rate of approximately 1 percent per day. Blood outside the formed vitreous resolves more quickly. Vitreous hemorrhage is cleared more quickly in syneretic and vitrectomized eyes, and more slowly in younger eyes with well-formed vitreous. The natural history of vitreous hemorrhage depends on the underlying pathology with the worst prognoses for diabetics and age-related macular degeneration (AMD) patients.
With the exception of proliferative vitreoretinopathy, complications of vitreous hemorrhage typically occur if blood has been present for more than one year.
Hemosiderosis bulbi is a serious complication thought to be caused by iron toxicity as hemoglobin is broken down. Since hemolysis occurs slowly, the iron-binding capacity of proteins in the vitreous usually outpaces the slow rate of hemolysis, thereby avoiding hemosiderosis bulbi.
Proliferative vitreoretinopathy. After vitreous hemorrhage, proliferative vitreoretinopathy can occur. It is thought that macrophages and chemotactic factors induce fibrovascular proliferation, which can lead to scarring and subsequent retinal detachment.
Ghost cell glaucoma. Ghost cells are spherical, rigid, khaki-colored red blood cells filled with denatured hemoglobin present in long-standing vitreous hemorrhage. If these cells gain access to the anterior chamber, their shape and rigidity can block the trabecular meshwork, resulting in ghost cell glaucoma.
Hemolytic glaucoma. In hemolytic glaucoma, free hemoglobin, hemoglobin-laden macrophages and red-blood cell debris can block the trabecular meshwork.
Vitreous hemorrhage prognosis
Vitreous hemorrhage prognosis is variable according to the underlying pathology and macular involvement. For example, patients with vitreous hemorrhage secondary to proliferative diabetic retinopathy or age-related macular degeneration will have a more guarded prognosis compared to those with vitreous hemorrhage resulting from posterior vitreous detachment. Thus, it is important to counsel the patient pre-operatively that prognosis might be guarded, especially since a thorough assessment is precluded while the vitreous hemorrhage is present.
- Spraul, Christoph W, Grossniklaus HE. “Vitreous hemorrhage.” Survey of Ophthalmology 42.1 (1997): 3-39.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- QuickStats: Age-Adjusted Percentage of Adults Aged ≥18 Years Reporting Diabetic Retinopathy Among Those with Prediabetes or Diagnosed Diabetes, by Age Group — National Health Interview Survey, 2016–2017. MMWR Morb Mortal Wkly Rep 2019;68:485. DOI: http://dx.doi.org/10.15585/mmwr.mm6821a5[↩]
- American Academy of Ophthalmology. Diabetic Retinopathy, Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology, 2017[↩]
- Bond-Taylor, Martin, Jakobsson G, Zetterberg M. “Posterior vitreous detachment–prevalence of and risk factors for retinal tears.” Clinical Ophthalmology (Auckland, NZ) 11 (2017): 1689.[↩]
- Causes and treatment of vitreous hemorrhage. Compr Ophthalmol Update. 2006 May-Jun;7(3):97-111. https://www.ncbi.nlm.nih.gov/pubmed/16882398[↩]
- Jaulim, Adil, et al. “Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature.” Retina 33.5 (2013): 901-910[↩]
- Matthews GP, Das A. Dense vitreous hemorrhages predict poor visual and neurological prognosis in infants with shaken baby syndrome. J Pediatr Ophthalmol Strabismus. 1996 Jul-Aug;33(4):260-5.[↩]
- Witmer, Matthew T., and Steven M. Cohen. “Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment.” Retina 33.3 (2013): 621-626.[↩]
- Early Treatment Diabetic Retinopathy Study Research Group. “Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7.” Ophthalmology 98.5 (1991): 741-756.[↩]
- Tanner, Vaughan, et al. “Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology.” British journal of ophthalmology 84.11 (2000): 1264-1268.[↩]
- Wickham L, Bunce C, Wong D, Charteris DG. Retinal detachment repair by vitrectomy: simplified formulae to estimate the risk of failure. Br J Ophthalmol. 2011 Sep;95(9):1239-44.[↩]
- Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009 Oct;116(10):1943-8.[↩]
- Bhavsar AR, Torres K, Beck, RW, et al.; Diabetic Retinopathy Clinical Research Network Study Investigators. Randomized Clinical Trial Evaluating Intravitreal Ranibizumab or Saline for Vitreous Hemorrhage from Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2013 Mar; 131(3): 283–293.[↩]
- Gross JG, Glassman AR, Liu D, et al.;Diabetic Retinopathy Clinical Research Network. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018 Oct 1;136(10):1138-1148[↩]
- Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. RANDOMIZED CONTROLLED STUDY OF INTRAVITREAL BEVACIZUMAB 0.16 MG INJECTED ONE DAY BEFORE SURGERY FOR PROLIFERATIVE DIABETIC RETINOPATHY. Retina. 2015 Apr 29[↩]
- Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina. 2011 Jul-Aug;31(7):1254-60.[↩]
- Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991 May;98(5Suppl):741-56.[↩]
- Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013 Mar;33(3):621-6.[↩]