Contents
- Wernicke’s encephalopathy
- What is Thiamine?
- Wernicke’s encephalopathy causes
- Wernicke’s encephalopathy pathophysiology
- Wernicke’s encephalopathy prevention
- Wernicke’s encephalopathy symptoms
- Wernicke’s encephalopathy complications
- Wernicke’s encephalopathy diagnosis
- Wernicke’s encephalopathy treatment
- Wernicke’s encephalopathy prognosis
- Wernicke-Korsakoff syndrome recovery
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening brain disease (encephalopathy) caused by acute thiamine (vitamin B1) deficiency 1, 2, 3. The maximum body store of thiamine or vitamin B1 is 30 mg, which is rapidly depleted within 2 weeks of beginning a thiamine-deficient diet 4. In the US, Wernicke’s encephalopathy is most commonly seen in people who drink a lot of alcohol and not getting enough of a nutrient called vitamin B1 or thiamine 5. People who drink too much alcohol often don’t get enough thiamine (vitamin B1), partly because they tend to have a poor diet, and partly because alcohol irritates the lining of the stomach and makes it harder to absorb certain vitamins. Other rare causes of Wernicke’s encephalopathy include severe malnutrition, severe and prolonged vomiting, hyperemesis gravidarum (severe nausea and vomiting during pregnancy), older age, diabetes, post-weight loss surgery, cancers that have spread throughout the body, cancer chemotherapy with poor dietary intake, gastrointestinal disease, severe anorexia nervosa, prolonged intravenous feeding or parenteral nutrition, immunodeficiency syndromes (e.g., HIV & AIDS), liver disease, hyperthyroidism (overactive thyroid), kidney failure on hemodialysis, lactation, fasting, starvation, use of unbalanced diets, malnourished patients with intestinal obstruction or malabsorption syndromes and systemic infections 6, 7, 8, 9, 10, 11, 12, 13, 14.
If not treated quickly, Wernicke’s encephalopathy can lead to permanent brain damage, a condition called Wernicke-Korsakoff syndrome 15, 16, 17, 18. The classic triad of Wernicke’s encephalopathy is altered mental status (i.e., confusion or dementia), nystagmus (or ophthalmoplegia), and ataxia 5. This is sometimes known ‘alcohol-related dementia’ because it causes symptoms that are similar to dementia.
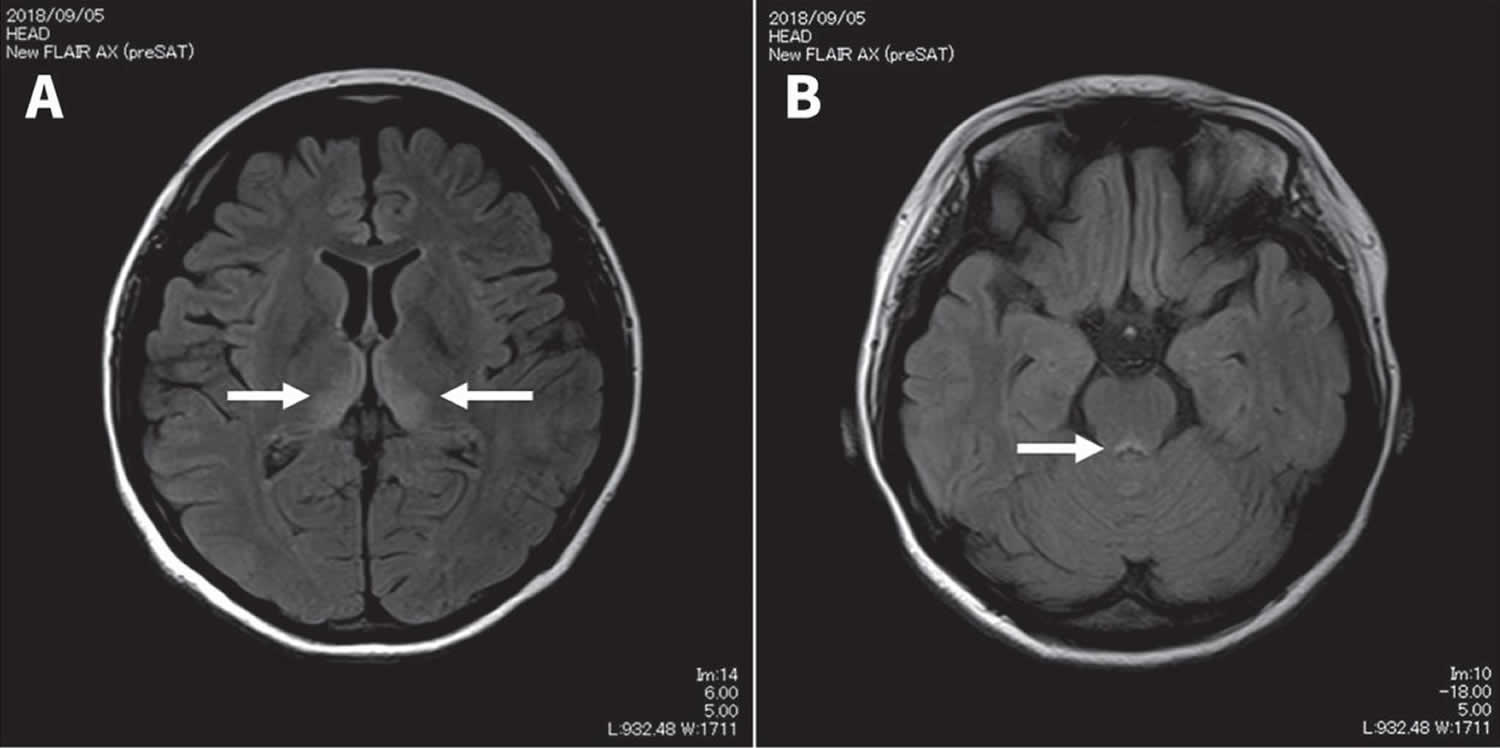
The term Wernicke-Korsakoff syndrome refers to two different syndromes, each representing a different stage of the same disorder (Figure 2). Wernicke’s encephalopathy represents the “acute” (short term) stage and Korsakoff’s syndrome (Korsakoff’s amnesic syndrome or Korsakoff psychosis) represents the “chronic” or long-lasting stage 19. Korsakoff syndrome or Korsakoff psychosis, tends to develop as Wernicke’s encephalopathy symptoms go away. Wernicke’s encephalopathy causes brain damage in lower parts of the brain called the thalamus and hypothalamus 20. Common findings in MRI of the brain for Wernicke encephalopathy are hyperintense signals in the dorsal medial thalamic nuclei, periaqueductal grey area and the third or fourth ventricles (Figure 1) 1, 21, 22. Korsakoff psychosis results from permanent damage to areas of the brain involved with memory. However, the term Wernicke-Korsakoff syndrome or Wernicke’s encephalopathy are sometimes used interchangeable in many sites.
Wernicke-Korsakoff syndrome and Wernicke encephalopathy are two separate syndromes 23, 24, 25:
- Wernicke’s encephalopathy causes brain damage in lower parts of the brain called the thalamus and hypothalamus 20. Symptoms of Wernicke encephalopathy include 20:
- Confusion and loss of mental activity that can progress to coma and death
- Loss of muscle coordination (ataxia) that can cause leg tremor
- Vision changes such as abnormal eye movements (back and forth movements called nystagmus), double vision, eyelid drooping
- Hypothermia
- Low blood pressure
- Alcohol withdrawal
- Wernicke encephalopathy may be identified by the presence of a delirium in malnourished alcoholic patients who have trouble walking 16. In these patients, the delirium is often caused by thiamine deficiency or in combination with thiamine deficiency, which may be erroneously diagnosed as alcohol withdrawal delirium 26.
- Wernicke encephalopathy symptoms are often reversible clinical features 27.
- Korsakoff syndrome also called Korsakoff’s amnesic syndrome or Korsakoff psychosis, tends to develop as Wernicke encephalopathy symptoms go away or accompanies Wernicke encephalopathy 20. Korsakoff’s syndrome or Korsakoff psychosis results from permanent damage to areas of the brain involved with memory 20. Korsakoff’s amnesic syndrome is a memory disorder that results from vitamin B1 deficiency due to permanent damages nerve cells and supporting cells in the brain and spinal cord, as well as the part of the brain involved with memory and is associated with alcoholism 28. Although the mechanisms of the cognitive dysfunction are still not fully understood, loss of function in the Papez and frontocerebellar circuits 29, 30 both including parts of the thalamus 31, may cause the impaired memory and executive functions that are the main characteristics of Korsakoff syndrome 32, 33. Some patients with Korsakoff syndrome suffer from additional damage in the cerebellum 34. Korsakoff syndrome main features are problems in acquiring new information or establishing new memories, and in retrieving previous memories. Korsakoff syndrome other symptoms include making up stories (confabulation), seeing or hearing things that are not really there (hallucinations), tremor, coma, disorientation and vision problems 27.
- Wernicke-Korsakoff syndrome is often characterized by irreversible clinical features including dementia and gait abnormalities. Both Wernicke-Korsakoff syndrome and Wernicke encephalopathy are due to brain damage caused by a lack of thiamine (vitamin B1).
The clinical presentation of thiamine deficiency includes loss of appetite, dizziness, tachycardia, and urinary bladder retention that can be attributed to anticholinergic autonomic dysfunction, as well as confusion or delirium 35, 36, which is part of the classic triad of Wernicke encephalopathy 37. These triad signs of Wernicke encephalopathy are described as ocular motility abnormalities: external ophthalmoplegia and/or nystagmus, ataxia affecting primarily the gait, and confusion or delirium 32.
Wernicke’s encephalopathy diagnosis is made based on clinical presentation, and a definitive diagnosis is complicated as the clinical triad may not be present in up to 90% of patients 1.
Caine and colleagues proposed four specific criteria (the Caine criteria) for the clinical identification of Wernicke encephalopathy in chronic alcoholics 38. When 2 out of these 4 criteria apply, the clinical diagnosis of Wernicke encephalopathy is made 39, 38:
- Dietary deficiencies
- Undernutrition (body mass index <2 SD below normal)
- A history of grossly impaired dietary intake
- An abnormal thiamine status
- Oculomotor abnormalities
- Ophthalmoplegia
- Nystagmus
- Gaze palsy
- Cerebellar dysfunction
- Unsteadiness or ataxia
- Abnormalities of past pointing
- Dysdiadokokinesia
- Impaired heel-shin testing
- Either an altered mental state OR mild memory impairment
- Altered mental status
- Disorientation in two of three fields
- Confused
- An abnormal digit span
- Comatose
- Mild memory impairment
- Failure to remember two or more words in the four-item memory test
- Impairment on more elaborate neuropsychological tests of memory function
- Altered mental status
Using any two of Caine’s criteria would greatly improve diagnostic sensitivity from 9 out of 40 patients (23%) to 34 out of 40 (85%). The Caine criteria have been described to have a sensitivity or 85% and specificity of 100% for Wernicke encephalopathy, in the alcoholic population 38, 39. Because of the strong overlap, the Caine criteria can be used to help screen for Korsakoff syndrome as well.
The diagnosis of Korsakoff syndrome can be made according to the DSM-5 criteria for major neurocognitive disorder of the confabulating amnestic type 40. In general, Korsakoff syndrome is characterized by severe anterograde and, to a lesser extent, retrograde amnesia for declarative knowledge 32. Patients with Korsakoff syndrome may also have difficulty in correctly identifying the temporal sequence of events 41. Moreover, many patients have executive function deficits, such as problems with initiating, planning, organizing, and regulating behavior 42. Patients themselves often do not recognize their problems in daily functioning because of limited awareness of their illness (anosognosia). Patients with Korsakoff syndrome can exhibit confabulations, although the intensity and frequency can vary per patient 43. Furthermore, Korsakoff syndrome is very often accompanied by a peripheral neuropathy 44.
If you have Wernicke’s encephalopathy, you will need to be treated with thiamine (vitamin B1) as quickly as possible. This is given through injection into your vein. Treatment also involves getting proper nutrition and hydration (enough water in your body). In some cases, medications might also be used.
Thiamine needs to be administered quickly and intravenously in both high doses and duration.
- The preferred dose of thiamine treatment for Wernicke encephalopathy may be as high as 500 mg intravenous thiamine given three times daily for 3 to 5 days, followed by intravenous thiamine, 250 mg/day for 3 to 5 days or until the symptoms disappear, and then further treatment with oral thiamine, 100 mg/day 45, 5. The rationale for using three times daily dosing of intravenous thiamine in acute presentations is based on the short half-life of intravenous thiamine (96 minutes) and the slow, carrier mediated process of thiamine transport across the blood–brain barrier 4. A single intravenous dose of thiamine is less likely to achieve sufficient brain tissue levels, and the bioavailability of oral thiamine hydrochloride is only 3.7% to 5.3% 45.
- For Korsakoff syndrome high dose thiamine at 500 mg to 1500 mg, IV, three times daily for at least 3 days 39.
If you are treated in time, most Wernicke’s encephalopathy symptoms can be reversed, although it can take a while for some symptoms to go away. If you aren’t treated in time, you could end up with permanent brain damage. Left untreated, about 80% of patients with Wernicke encephalopathy acquire Korsakoff syndrome, which is characterized by memory impairment associated with confabulation 1, 21. Victor et al. 46, in their innovative and comprehensive studies in Boston (USA), found that only 16% of Wernicke’s encephalopathy patients treated with inappropriately low parenteral doses of 50–100 mg of thiamine daily recovered fully, with a reported death rate of 17–20%, and 84% developed Korsakoff syndrome. Only 21% of these patients with Korsakoff syndrome showed complete recovery; 26% showed no improvement, 28% only slight improvement and 25% showed significant recovery from the amnesic state which may take from 2 months to 10 years. Twenty-five per cent of patients with Korsakoff syndrome require long-term institutionalization 47.
Prevalence data on Wernicke’s encephalopathy comes mainly from autopsy studies with rates ranging between 1% and 3%. Several studies indicate that prevalence rates via analysis of clinical records are lower in comparison to necropsy studies as the diagnosis is easily overlooked or missed. The incidence of Wernicke’s encephalopathy is believed to be higher in developing countries due to vitamin deficiencies and malnutrition. The female to male ratio for Wernicke encephalopathy is 1:1.7, and there are no studies that show a particular race predisposed to Wernicke’s encephalopathy 48.
Wernicke encephalopathy is mostly related to alcohol addiction (400/434 cases = 92.2%) 16. The mean age of these patients with Wernicke encephalopathy was 55.1 years and 50.7 years, respectively in alcohol related and non-alcohol related cases, and the gender ratio (male/female) was, respectively 6.0 and 0.7 49. Most patients with alcohol related Wernicke-Korsakoff syndrome (103/128 = 80.5%) are initially admitted following a state of confusion with impaired consciousness, or after a physical collapse 50, 51. Some patients with alcohol related Korsakoff syndrome (9/118 = 8%) show intermittent episodes of Wernicke encephalopathy over time 50. Alcoholic patients presented more frequently (368/434 = 84.8%) than non-alcoholic patients (23/34 = 68%) with cerebellar signs, but less frequently with ocular signs (65.7% versus 85%). Alcoholic patients had a significantly higher frequency of hyponatremia compared with non-alcoholics (105/425 = 24.7%, respectively 9% of patients) and lower platelet counts (mean 227 × 103/μL, respectively 281 × 103/μL). The median time from hospital admission to Wernicke encephalopathy diagnosis was 1 versus 4 days, respectively, in alcoholic and non-alcoholic patients 49. Vomiting (81.9% of patients) and weight loss (mean 18.3 kg weight loss) commonly characterized the onset of non-alcohol-related Wernicke encephalopathy 52. Complaints of blurred vision were reported in 24.3% of Wernicke cases in hyperemesis gravidarum and may be associated with optic neuropathy in thiamine deficiency 52. Cognitive deficits may be less prominent in non-alcoholic Wernicke encephalopathy, suggesting relatively lower susceptibility to confusion in this patient population. This is especially the case among younger patient groups or patients who seek treatment earlier than those struggling with alcoholism. For instance, in thiamine deficiency after weight loss surgery, patients presenting without mental status changes were on average 11 years younger than those with mental status changes (Mann–Whitney U test, U (66) = 262) 53.
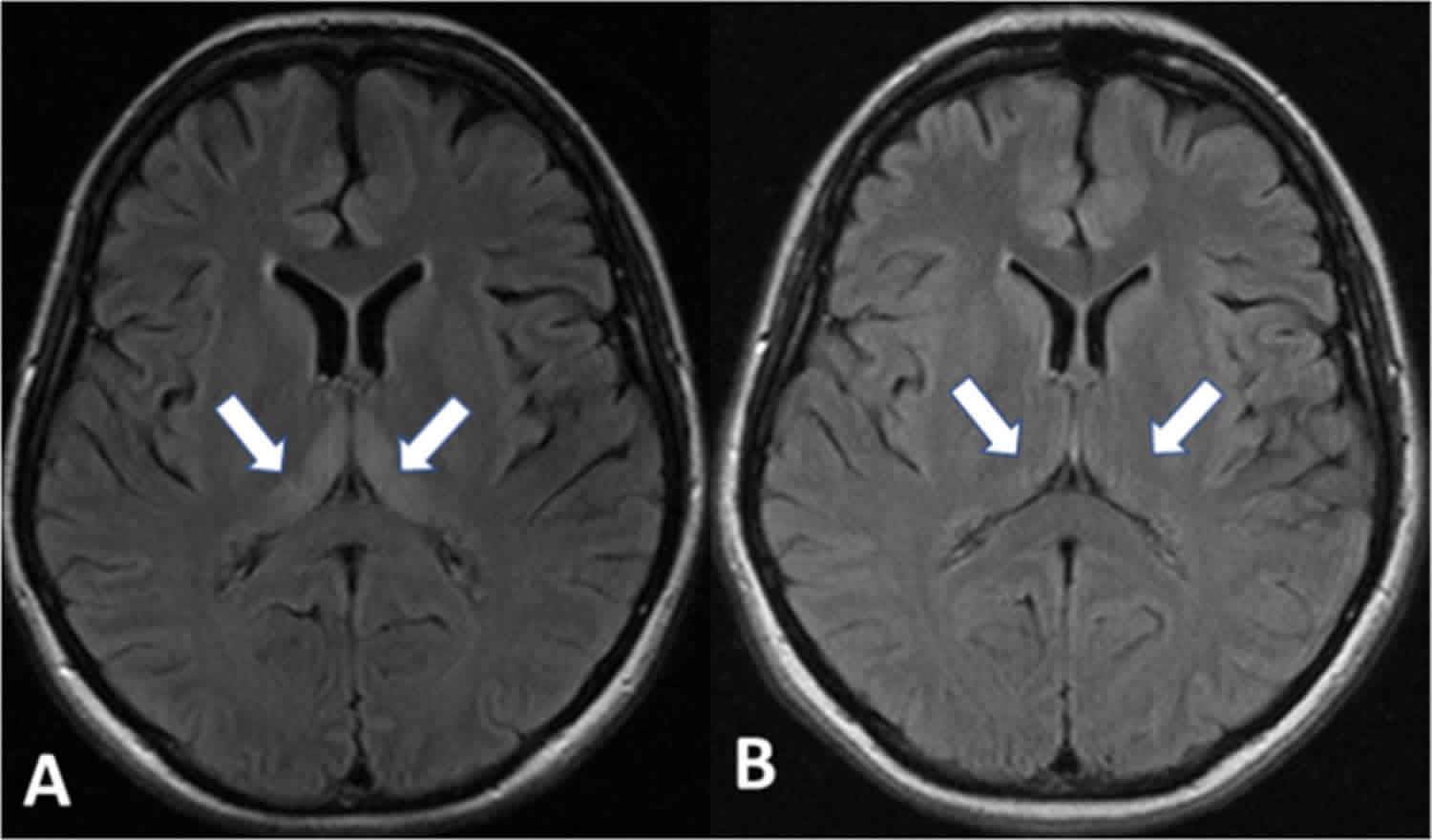
Figure 1. Wernicke’s encephalopathy brain MRI
Footnote: Fluid-attenuated inversion recovery magnetic resonance images of the brain of a 28-yearold woman with Wernicke encephalopathy showing hyperintense signals in the (A) bilateral medial thalami (arrows) and (B) in the periaqueductal grey matter (arrow).
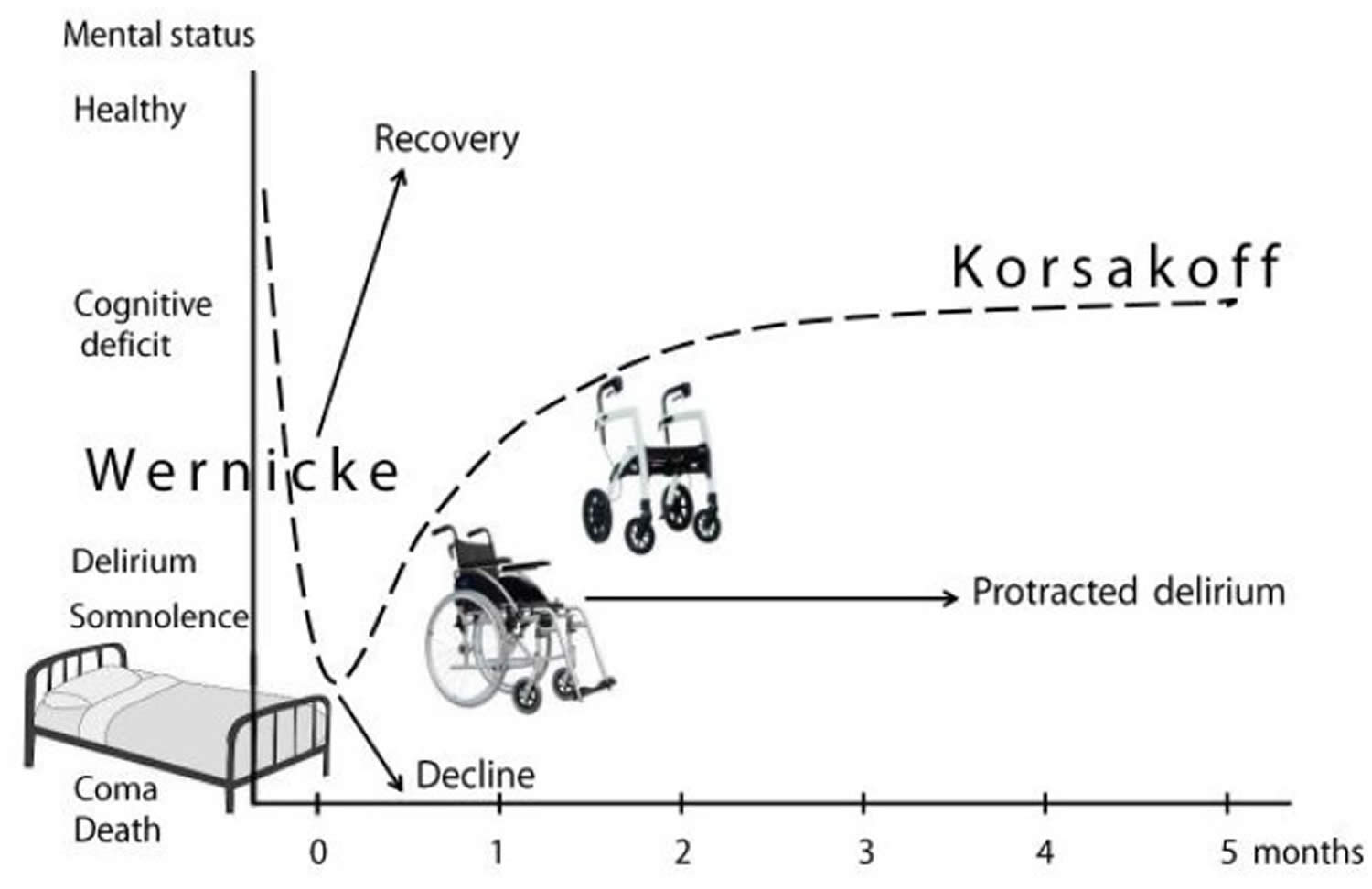
[Source 54 ]Figure 2. Wernicke-Korsakoff syndrome
Footnote: Mental and motor symptoms in Wernicke-Korsakoff syndrome. A patient’s mobility and evolving neuropsychiatric symptoms in Wernicke-Korsakoff syndrome (dashed line), starting with initial hospitalization at 0 months. Arrows depict alternative outcomes: further decline, full recovery, or protracted delirium.
[Source 16 ]If you have any signs of Wernicke’s encephalopathy, you need to seek medical help straight away. If you don’t, you run the risk of permanent brain damage.
If you have problems with alcohol, seek help before you get a condition like Wernicke’s encephalopathy. Talk to your doctor, or get help from an organization such as Alcoholics Anonymous.
If you are concerned about whether you are getting enough thiamine in your diet, talk to your doctor or see a dietician.
What is Thiamine?
Thiamine also known as vitamin B1 or thiamin, is one of the water-soluble B vitamins. Vitamin B1 or thiamine is naturally present in some foods, added to some food products, and available as a dietary supplement. Vitamin B1 or thiamine plays a critical role in energy metabolism and, therefore, in the growth, development, and function of cells 55. Foods rich in vitamin B1 or thiamine include whole grains, brown rice, lean pork, poultry, eggs, fish, soybean, nuts, dried beans, peas, and fortified or enriched grain products such as cereals, infant formulas, and bread 56, 57, 58. Multivitamins will provide an additional 1.5 mg of thiamine on top of a diet rich in thiamine 59.
Ingested vitamin B1 or thiamine from food and dietary supplements is absorbed by the small intestine through active transport at nutritional doses and by passive diffusion at pharmacologic doses 60, 55. Most dietary vitamin B1 or thiamine is in phosphorylated forms, and intestinal enzyme phosphatase hydrolyzes them to free thiamin before the vitamin is absorbed by the small intestine 55. The remaining dietary vitamin B1 or thiamine is in free (absorbable) form 55, 61. Thiamine, a water-soluble vitamin, cannot be stored in appreciable amounts 4. Humans store vitamin B1 or thiamine primarily in the liver, but in very small amounts (approximately 30 mg) 62. Vitamin B1 or thiamine has a short half‐life of 14 to 18 days and body stores are limited, so people require a continuous supply of thiamine from the diet 63, 60, 64.
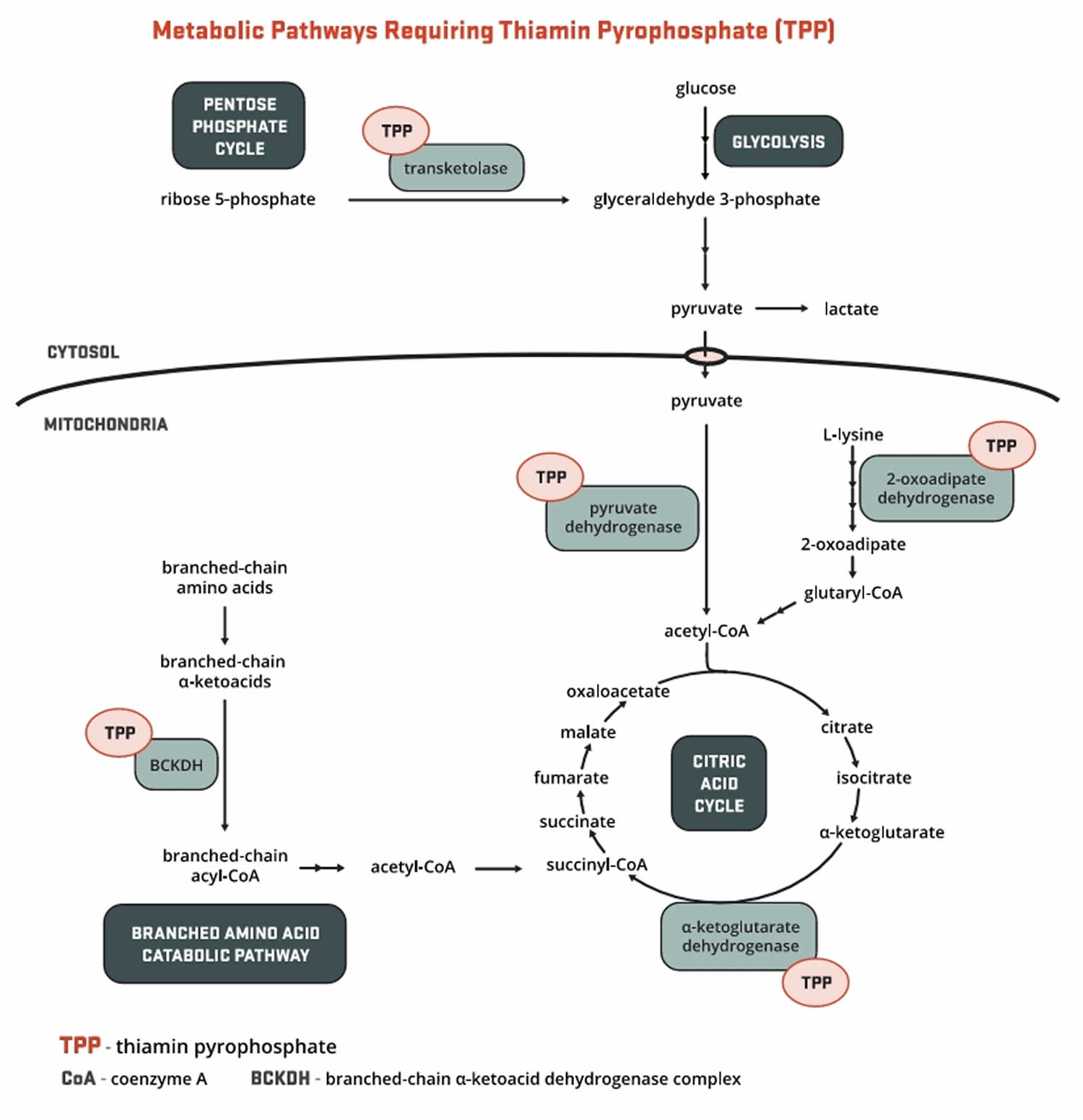
Vitamin B1 or thiamine is present in the body as free thiamine, as well as in several phosphorylated forms: thiamine monophosphate (TMP), thiamine diphosphate (TDP also known as thiamin pyrophosphate (TPP)), and thiamine triphosphate (TTP) 65, 66, 67. About 80% of the approximately 25–30 mg of vitamin B1 or thiamine in the adult human body is in the form of thiamin pyrophosphate (TPP) also known as thiamin diphosphate (TDP), the main metabolically active form of vitamin B1 or thiamine. Bacteria in the large intestine also synthesize free thiamin and thiamin pyrophosphate (TPP), but their contribution, if any, to thiamin nutrition is currently unknown 68. Thiamin pyrophosphate (TPP) serves as an essential cofactor for five enzymes involved in glucose, amino acid (proteins), and fatty acids (lipids) metabolism (Figure 3) 69, 55, 62.
Levels of vitamin B1 or thiamine in the blood are not reliable indicators of thiamin status. Thiamine status is often measured indirectly by assaying the activity of the transketolase enzyme, which depends on thiamin pyrophosphate (TPP), in red blood cell hemolysates in the presence and absence of added thiamin pyrophosphate (TPP) 62. The result, known as the “TPP effect,” reflects the extent of unsaturation of transketolase with TPP. The result is typically 0%–15% in healthy people, 15%–25% in those with marginal deficiency, and higher than 25% in people with thiamine deficiency. Another commonly used measure of vitamin B1 or thiamine status is urinary thiamine excretion, which provides data on dietary intakes but not tissue stores 70. For adults, excretion of less than 100 mcg/day vitamin B1 or thiamine in urine suggests insufficient thiamine intake, and less than 40 mcg/day indicates an extremely low intake 71.
Figure 3. Vitamin B1 (thiamine) function
Footnote: Major biochemical reactions requiring vitamin B1 or thiamine as a cofactor (a compound that is essential for the activity of an enzyme).
[Source 72 ]How much Thiamine do I need?
The amount of vitamin B1 or thiamine you need depends on your age and sex 73. Average daily recommended amounts are listed in Table 1 below in milligrams (mg). The Recommended Dietary Allowance (RDA) is average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals 74. Table 1 lists the current Recommended Dietary Allowance (RDA) for vitamin B1 or thiamine 74. For infants from birth to 12 months, the Food and Nutrition Board at the Institute of Medicine of the National Academies established an adequate intake (intake at this level is assumed to ensure nutritional adequacy; adequate intake is established when evidence is insufficient to develop an RDA). for vitamin B1 or thiamine that is equivalent to the mean intake of vitamin B1 or thiamine in healthy, breastfed infants 70.
Most people in the United States consume the recommended amounts of vitamin B1 or thiamine 70. An analysis of data from the 2003-2006 National Health and Nutrition Examination Survey showed that only 6% of the U.S. population has a usual intake below the estimated average requirement (average daily level of intake estimated to meet the requirements of 50% of healthy individuals. It is usually used to assess the adequacy of nutrient intakes in populations but not individuals) 57.
Among children and teens, the average daily vitamin B1 or thiamine intake from foods is 1.27 mg for ages 2–5 years, 1.54 mg for ages 6–11 years, and 1.68 mg for ages 12–19 years 75. In adults aged 20 and older, the average daily vitamin B1 or thiamine intake from foods is 1.95 mg in men and 1.39 mg in women. The average daily vitamin B1 or thiamine intake from foods and supplements in children and teens is 1.51 mg for ages 2–5 years, 1.76 mg for ages 6–11 years, and 1.95 mg for ages 12–19 years. In adults aged 20 and older, the average daily vitamin B1 or thiamine intake from foods and supplements is 4.89 mg in men and 4.90 mg in women 70.
No current data on rates of thiamine deficiency in the U.S. population are available 70.
Most people in the United States get enough vitamin B1 or thiamine from the foods they eat. Thiamine deficiency is rare in this country. However, certain groups of people are more likely than others to have trouble getting enough vitamin B1 or thiamine:
- People with alcohol dependence
- Older individuals
- People with HIV/AIDS
- People with diabetes
- People who have had weight loss surgery
Table 1. Recommended Dietary Allowances (RDAs) for vitamin B1 or thiamine
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 0.2 mg |
| Infants 7–12 months | 0.3 mg |
| Children 1–3 years | 0.5 mg |
| Children 4–8 years | 0.6 mg |
| Children 9–13 years | 0.9 mg |
| Teen boys 14–18 years | 1.2 mg |
| Teen girls 14–18 years | 1.0 mg |
| Men | 1.2 mg |
| Women | 1.1 mg |
| Pregnant teens and women | 1.4 mg |
| Breastfeeding teens and women | 1.4 mg |
What foods provide Thiamine or vitamin B1?
Vitamin B1 or thiamine is found naturally in many foods and is added to some fortified foods. You can get recommended amounts of vitamin B1 or thiamine by eating a variety of foods, including the following 61:
- Whole grains and fortified bread, cereal, pasta, and rice
- Meat (especially pork) and fish
- Legumes (such as black beans and soybeans), seeds, and nuts
Breads, cereals, and infant formulas in the United States and many other countries are fortified with thiamine 61. The most common sources of vitamin B1 or thiamine in the U.S. diet are cereals and bread 56. Pork is another major source of the vitamin. Dairy products and most fruits contain little vitamin B1 or thiamine 62. About half of the vitamin B1 or thiamine in the U.S. diet comes from foods that naturally contain thiamin; the remainder comes from foods to which thiamine has been added 57.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin B1 or thiamine arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Thiamin-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Thiamin-Food.pdf).
Heating foods containing vitamin B1 or thiamine can reduce their thiamin content. For example, bread has 20%–30% less vitamin B1 or thiamine than its raw ingredients, and milk pasteurization reduces thiamin content (which is very small to begin with) in milk by up to 20% 62. Because vitamin B1 or thiamine dissolves in water, a significant amount of the vitamin is lost when cooking water is thrown out 62. Processing also alters vitamin B1 or thiamine levels in foods; for example, unless white rice is enriched with thiamin, it has one tenth the amount of thiamin in unenriched brown rice 76.
Data on the bioavailability of vitamin B1 or thiamine from food are very limited 77. Some studies do show, however, that thiamin absorption increases when intakes are low 55.
Several food sources of thiamine are listed in Table 2.
Table 2. Vitamin B1 (thiamine) food sources
| Food | Milligrams (mg) per serving | Percent Daily Value (DV*) |
|---|---|---|
| Breakfast cereals, fortified with 100% of the Daily Value (DV) for thiamine, 1 serving | 1.2 | 100 |
| Egg noodles, enriched, cooked, 1 cup | 0.5 | 42 |
| Pork chop, bone-in, broiled, 3 ounces | 0.4 | 33 |
| Trout, cooked, dry heat, 3 ounces | 0.4 | 33 |
| Black beans, boiled, ½ cup | 0.4 | 33 |
| English muffin, plain, enriched, 1 muffin | 0.3 | 25 |
| Mussels, blue, cooked, moist heat, 3 ounces | 0.3 | 25 |
| Tuna, Bluefin, cooked, dry heat, 3 ounces | 0.2 | 17 |
| Macaroni, whole wheat, cooked, 1 cup | 0.2 | 17 |
| Acorn squash, cubed, baked, ½ cup | 0.2 | 17 |
| Rice, brown, long grain, not enriched, cooked, ½ cup | 0.2 | 17 |
| Rice, white, long grain, enriched, cooked, ½ cup | 0.1 | 8 |
| Bread, whole wheat, 1 slice | 0.1 | 8 |
| Orange juice, prepared from concentrate, 1 cup | 0.1 | 8 |
| Sunflower seeds, toasted, 1 ounce | 0.1 | 8 |
| Beef steak, bottom round, trimmed of fat, braised, 3 ounces | 0.1 | 8 |
| Yogurt, plain, low fat, 1 cup | 0.1 | 8 |
| Oatmeal, regular and quick, unenriched, cooked with water, ½ cup | 0.1 | 8 |
| Corn, yellow, boiled, 1 medium ear | 0.1 | 8 |
| Milk, 2%, 1 cup | 0.1 | 8 |
| Barley, pearled, cooked, 1 cup | 0.1 | 8 |
| Cheddar cheese, 1½ ounces | 0 | 0 |
| Chicken, meat and skin, roasted, 3 ounces | 0 | 0 |
| Apple, sliced, 1 cup | 0 | 0 |
Footnote: *DV = Daily Value. Daily Values were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for thiamine is 1.5 mg for adults and children age 4 and older. Foods providing 20% or more of the Daily Value are considered to be high sources of a nutrient.
[Source 78 ]Wernicke’s encephalopathy causes
Wernicke’s encephalopathy is caused by acute thiamine or vitamin B1 deficiency 79. Check with your doctor right away if you have confusion, drowsiness, double vision, diarrhea, nausea, vomiting, problems with muscle control or coordination, shakiness and unsteady walk, trembling, trouble remembering, and weight loss (leading to malnutrition and lower thiamine levels).
Thiamine deficiency or vitamin B1 deficiency may result from inadequate dietary thiamine intake, administration of total parenteral nutrition without adequate thiamine replacement, increased physiological requirements for thiamine, excessive loss of thiamine from the body, impaired intestinal absorption, small intestinal bacterial overgrowth (SIBO), consumption of anti-thiamine factors (ATF) in food, antacids, sulfites, or sodium bicarbonate food preservatives or a combination of these factors 80, 81, 82, 72. Several other risk factors may significantly contribute to thiamine deficiency, including infections, esophageal stenosis (Barrett esophagus), colitis, and importantly renal loss of thiamine in diabetes mellitus or nephropathy 83, 84. Loss of appetite and vomiting may be both a cause 85 and a complication of thiamine deficiency 86, 87.
The maximum body store of thiamine is 30 mg, which is rapidly depleted within 2 weeks of beginning a thiamine-deficient diet 4. Risk factors for decreased thiamine intake include protracted vomiting, weight loss surgery or gastrointestinal surgery, malnutrition, extreme nausea and vomiting during pregnancy (hyperemesis gravidarum), anorexia, and alcoholism 88.
Other conditions that may cause thiamine deficiency or vitamin B1 deficiency include:
- HIV/AIDS
- Cancers that have spread throughout the body
- Extreme nausea and vomiting during pregnancy (hyperemesis gravidarum)
- Heart failure (when treated with long-term diuretic therapy)
- Long periods of intravenous (IV) nutrition without receiving thiamine supplements
- Long-term dialysis
- Taking high doses of diuretics (water pills)
- Very high thyroid hormone levels (thyrotoxicosis)
- Breastfed infants whose mother is lacking in thiamine
- Infants fed unusual formulas that don’t have enough thiamine.
The most common social factor associated with Wernicke-Korsakoff syndrome is chronic alcohol abuse which leads to decreased absorption and utilization of thiamine. In the past, baby formula which was deficient in thiamine also led to Wernicke-Korsakoff syndrome. Wernicke-Korsakoff syndrome also can develop during the first trimester of pregnancy in women who develop hyperemesis gravidarum. Another common cause is weight loss surgery and malignancies of the gastrointestinal (GI) tract.
In rare cases, thiamine deficiency or vitamin B1 deficiency can be a genetic metabolic disease 89, 90. This condition is passed down through families. People with this condition lose the ability to absorb thiamine from foods. This can happen slowly over time. The symptoms occur when the person is an adult. However, this diagnosis is often missed. This is because health care providers may not consider thiamine deficiency in nonalcoholics 90.
Inadequate dietary thiamine or vitamin B1 intake
Inadequate consumption of thiamine or vitamin B1 is the main cause of thiamine deficiency in developing countries 91. Thiamine deficiency is common in low-income populations whose diets are high in carbohydrate and low in thiamine. Examples of foods that can lead to inadequate thiamine intake upon prolonged consumption include sago, cassava flour, unfortified white bread, or highly refined cereals such as polished white rice 4. The consumption of a diet composed mainly of refined carbohydrates or one that includes high alcohol intake also reduces body thiamine stores 4.
To attain maximal erythrocyte transketolase activity, at least 0.6 mg of thiamine per 1000 kcal of carbohydrate is required 4. Most humans will develop symptoms of thiamine deficiency when intake is below 0.2 mg of thiamine per 1000 kcal. Whole wheat flour contains 0.55 mg of thiamine per 100 g, brown rice 0.33 mg per 100 g, and highly milled white rice only 0.08 mg per 100 g 4. Adding baking powder (sodium bicarbonate) to wholemeal flour when baking bread reduces the thiamine content 4. Washing white rice in water prior to cooking reduces the thiamine content by half. Thiamine is heat labile, chlorine sensitive, and water soluble, so discarding the rice water after cooking or using chlorinated water for cooking or washing rice contributes to thiamine loss from the diet 80. Parboiling (partly cook food by boiling) of rice was originally developed in India but is not practiced in Southeast Asia or Japan, where milled white rice is preferred. Rice parboiling distributes the thiamine content from the bran and aluerone layer to the endosperm prior to milling. This is why beriberi is rare in India, where parboiled rice is the primary form of rice consumed 80.
After 1878, mechanical roller milling of wheat and polishing of rice became widespread 92. Consumption of mass-produced, polished white rice as the staple diet in East Asian countries led to epidemics of thiamine deficiency (beriberi) in the 1800s and 1900s 93, 92. Polished rice and milled wheat were also popular because removal of the oil-rich bran layer from rice or the wheat bran from wheat grains prolonged storage times, minimized rancidity, and reduced the susceptibility to weevils. Mandatory fortification of wheat flour with the thiamine mononitrate vitamer was introduced in Australia in 1991. Since then, Wernicke-Korsakoff syndrome has become very uncommon in Australia. The thiamine mononitrate vitamer is used because it is non-hygroscopic and more stable than thiamine hydrochloride 94. Mandatory fortification of bread with iodine and folate was commenced in Australia in 2009. Folate deficiency may indirectly contribute to thiamine deficiency, as folate is required for the regeneration of reduced nicotinamide adenine dinucleotide (NADH) by dihydrofolate reductase. NADH is necessary for the regeneration of TPP 95. Vitamin C may protect against the development of symptoms of thiamine deficiency 80.
Breast-fed infants whose mothers are thiamine deficient are vulnerable to developing infantile beriberi. Alcoholism, which is associated with low intake of thiamine among other nutrients, is the primary cause of thiamine deficiency in industrialized countries. Some of the non-alcoholic conditions associated with Wernicke-Korsakoff syndrome include anorexia nervosa, bariatric surgery (weight-loss surgery), gastrointestinal malignancies, and malabsorption syndromes 96, 97, 98, 99. Obese individuals may also be at heightened risk of thiamine deficiency 100, 25. Moreover, cases of Wernicke’s encephalopathy have been linked with hyperemesis gravidarum (severe nausea and vomiting during pregnancy) 101, 102, and with parenteral nutrition lacking vitamin supplementation 103, 104.
Increased thiamine or vitamin B1 requirement
Conditions resulting in an increased requirement for thiamine or vitamin B1 include strenuous physical exertion, fever, severe infection or sepsis, hyperthyroidism, pregnancy, breast-feeding, adolescent growth, major surgery, refeeding syndrome, or rapid growth of cancers 60, 105, 106, 107, 108, 109, 110, 111, 112. Such conditions place individuals with marginal thiamine intake at risk for developing symptomatic thiamine deficiency.
Fever can critically increase the requirement for thiamine, as a rise in core body temperature of 1°C will increase the basal metabolic rate by 10% 113. Consumption of a high-fat or a high-carbohydrate diet results in increased metabolic consumption of thiamine 95. This is particularly relevant in individuals with preexisting thiamine deficiency, such as refugees, prisoners of war, persons with alcoholism, oncology patients, and postoperative bariatric surgery or gastrectomy patients. Administering oral, enteral, or parenteral nutrition to these patients without concomitant thiamine supplementation can result in fulminant beriberi or acute refeeding syndrome 80, 114, 115, 116, 117.
Malaria patients in Southeast Asia were found to be thiamine deficient more frequently than non-infected individuals 118, 119. Malarial infection leads to a large increase in the metabolic demand for glucose. Because thiamine is required for enzymes involved in glucose metabolism, the stresses induced by malarial infection could exacerbate thiamin deficiency in predisposed individuals. HIV-infected individuals, whether or not they had developed AIDS, were also found to be at increased risk for thiamine deficiency 120. Furthermore, chronic alcohol abuse impairs intestinal absorption and utilization of thiamine 121; therefore, alcoholics have increased requirements for thiamine or vitamin B1. Thiamine deficiency is also observed as a complication of the refeeding syndrome: the introduction of carbohydrates in severely starved individuals leads to an increased demand for thiamine in glycolysis and the citric acid cycle that precipitates thiamine deficiency 122.
Anti-thiamin factors
The presence of anti-thiamin factors (ATF) in foods contributes to the risk of thiamin deficiency. Certain plants contain anti-thiamin factors (ATF), which react with thiamine to form an oxidized, inactive product. Consuming very large amounts of tea or coffee (including decaffeinated), as well as chewing tea leaves and betel nuts, might lower thiamin status due to the presence of anti-thiamin factors (ATF) 123, 124. Anti-thiamin factors (ATF) include mycotoxins (molds), thiaminases that break down thiamin in food, thiamine antagonists, and hemin 4. Individuals who habitually eat certain raw fresh-water fish, raw shellfish, or ferns are at higher risk of thiamine deficiency because these foods contain thiaminase that normally is inactivated by heat in cooking 121, 125, 126, 127, 128. In Nigeria, an acute, neurologic syndrome (seasonal ataxia) has been associated with thiamine deficiency precipitated by a thiaminase in African silkworms (Anaphe species), a traditional, high-protein food for some Nigerians 129.
Thiamine antagonists are found in tea and betel nuts (tannic acid), coffee (chlorogenic acid, caffeic acid), bracken fern (caffeic acid), and pigmented polyphenol-containing foods such as red cabbage, blueberries, red currants, and red beets 4. These cause oxidation of the thiazole ring of thiamine, forming nonabsorbable thiamine disulfide 80. For example, consumption of 1 g of dry tea leaves boiled in 100 mL of water for 5 minutes caused thiamine loss of 0.21 mg per hour 130. Vitamin C and cysteine can protect thiamine from degradation caused by organic acids and polyphenols 131. Outbreaks of beriberi in the wet season in some countries may be related to low availability of food, importation of poor-quality rice or milled white rice, seasonal variations in plant tannin levels, or the mycotoxin citreoviridin, produced by rice mold 132, 106, 133.
Food additives and thiamine
Thiamine is unstable under alkaline conditions produced by food additives such as preservatives and antacids (eg, sodium bicarbonate), which cause disruption of the thiamine methylene bridge 4. Sulfite-type food preservatives include sulfur dioxide, sodium sulfite, sodium and potassium bisulfite, and sodium and potassium metabisulfite 4. These are used extensively in the production and preservation of foods and beverages, including dried fruit, ready-to-eat salad vegetables, frozen fried potatoes, wine, beer, soft drinks, packaged fruit juices, shellfish, and pickled and pureed foods. Sulfites possess antioxidant and antimicrobial activities, which inhibit the enzymatic and nonenzymatic (browning) spoiling of food. This preserves the color, freshness, flavor, and crispness of food but substantially reduces the available thiamine. For example, respective thiamine loss from cabbage blanched with sulfite-treated water versus untreated water was 45% vs 15% 80. The use of sulfiting agents in foods recognized as important sources of thiamine is prohibited by the US Food and Drug Administration 134, 135, 136, 137.
Excessive loss of thiamine caused by thiamine-drug interactions
Excessive loss of thiamine may precipitate thiamine deficiency. Increased losses of ingested thiamine can be caused by drug-related polyuria or diarrhea, drug interactions, or chronic excessive alcohol consumption 4. Thiamine is a polar, water-soluble vitamin and is not protein bound, which allows it to be easily dialyzed or filtered in the glomerulus. Increased urine flow, loop diuretics, or dialysis can all cause thiamine deficiency. Thiamine deficiency is associated with drugs such as omeprazole, phenytoin, 5-fluorouracil, metformin, alcohol, antibiotics, furosemide, and thiazide diuretics 88. By increasing urinary flow, diuretics may prevent reabsorption of thiamine by the kidneys and increase its excretion in the urine 138, 139. The risk of thiamine deficiency is increased in diuretic-treated patients with marginal thiamine intake 140 and in individuals receiving long-term, diuretic therapy 141. Patients with congestive heart failure who receive chronic therapy with furosemide or thiazide diuretics can also develop thiamine deficiency. The prevalence of thiamine deficiency in this patient population varies from 21% to 98% 4. Supplementation with thiamine has been shown to improve left ventricular ejection fraction by 22%, New York Heart Association Functional Classification, and TPP effect (from 11.7% to 5.4%) in these patients 142. Mechanisms for exacerbation of heart failure by diuretics include increased thiamine loss in urine, furosemide-related inhibition of cardiac myocyte thiamine uptake, furosemide-induced anorexia, furosemide inhibition of intestinal absorption or cellular uptake of thiamine, and hypomagnesemia 88. Unrecognized thiamine deficiency in heart failure patients treated with long-term diuretic therapy may result in Shoshin beriberi 143.
Individuals with kidney failure requiring hemodialysis lose thiamine at an increased rate and are at risk for thiamine deficiency 144. Alcoholics who maintain a high fluid intake and high urine flow rate may also experience increased loss of thiamine, exacerbating the effects of low thiamine intake 145.
Omeprazole a proton pump inhibitor because of its azole analogue molecular structure may cause inactivation of pyruvate decarboxylase and human erythrocyte transketolase, resulting in antagonism of thiamine 146. Omeprazole may inhibit gastric proton pumps by competing with thiamine for binding to hydrogen/potassium adenosine triphosphatase 89, 146. Proton pump inhibitor drugs or Roux-en-Y gastric bypass surgery may also worsen subclinical thiamine deficiency by promoting small intestinal bacterial overgrowth, which alters luminal thiamine levels 147, 82. Hypomagnesemia induced by proton pump inhibitors can contribute to functional thiamine deficiency, as magnesium is a required cofactor for the formation of TPP and acetyl coenzyme A 147. 5-Fluorouracil decreases hepatic thiamine levels and thiamine-dependent transketolase activity. This is associated with an increase in the TPP effect in vitro and in whole blood 148. 5-Fluorouracil is catabolized to fluoroacetate, which blocks the Krebs cycle and ATP production, leading to neurotoxicity, ammonia formation, and encephalopathy 149, 150, 151. Metformin, a substrate and inhibitor of the human thiamine transporter 2, reduces both intestinal absorption of thiamine and levels of thiamine in tissues and liver 152, 153, 154. Alcohol decreases carrier-mediated thiamine transport in the brush border and basolateral membrane of enterocytes in the jejunum (thiamine transporter 1) and potentially decreases thiamine production by intestinal flora in the lumen 155
Small intestinal bacterial overgrowth can be treated specifically with certain oral antibiotics such as rifaximin, neomycin, and metronidazole, which can improve postoperative thiamine deficiency in Roux-en-y gastric bypass patients 156, 81, 82. The use of broad-spectrum antibiotics (penicillins, cephalosporins, aminoglycosides, tetracyclines, fluoroquinolones, sulfonamides, trimethoprim), however, can potentially cause thiamine deficit in some patients by reducing counts of normal intestinal bacteria that produce thiamine (eg, Escherichia coli, bifidobacteria, Lactobacillus spp) and by promoting the growth of pathogenic flora such as Clostridium spp, which produce thiaminases 157, 88. Metronidazole, a thiazole, has been shown to be a substrate for thiaminase 1. Formation of thiamine antimetabolites from metronidazole, which can occur particularly with high cumulative doses or prolonged use of metronidazole, can inhibit thiamine pyrophosphokinase, leading to irreversible, painful, peripheral neuropathy 131.
Table 3. Effects of drugs and antithiamine agents on thiamine
| Drug family/antithiamine agent | Drug | Effect on thiamine |
|---|---|---|
| Alcohol | Ethanol | Decreased intestinal thiamine transport |
| Antibiotic | Metronidazole | Production of thiamine antimetabolites |
| Antibiotics | β-lactams, aminoglycosides, trimethoprim, quinolones | Decreased production of thiamine by intestinal microbiota |
| Chemotherapy | 5-fluorouracil | Decreased production of hepatic thiamine, decreased TKT activity |
| Polyphenols (coffee, tea) | Caffeic acid, tannic acid | Oxidation of thiazole ring |
| Diuretics | Furosemide, thiazides | Increased renal excretion, decreased intestinal absorption |
| Flavonoids | Quercetin, rutin | Oxidation to thiamine disulfide |
| Food preservatives | Sulfites | Disruption of thiamine methylene bridge |
| Oral hypoglycemics (biguanide) | Metformin | THTR2 inhibitor, prevents active transport of thiamine |
| Proton pump inhibitors | Omeprazole | Inactivation of PDH, erythrocyte transketolase, and H/K ATPase |
Abbreviations: H/K ATPase = hydrogen/potassium adenosine triphosphatase; PDH = pyruvate dehydrogenase; TKT = transketolase; THTR2 = thiamine transporter 2.
[Source 4 ]Groups at risk of thiamine deficiency
The following groups are among those most likely to have inadequate thiamine status 70.
People with alcohol dependence
In highly industrialized countries, chronic alcohol use disorders appear to be the most common cause of thiamine deficiency 55. Up to 80% of people with chronic alcoholism develop thiamine deficiency because ethanol reduces gastrointestinal absorption of thiamine, thiamine stores in the liver, and thiamine phosphorylation 62, 158. Also, people with alcoholism tend to have inadequate intakes of essential nutrients, including thiamine.
Older adults
Up to 20%–30% of older adults have laboratory indicators that suggest some degree of thiamine deficiency 61, 77. Possible reasons include low dietary intakes, a combination of chronic diseases, concomitant use of multiple medications, and low absorption of thiamin as a natural result of aging 159, 160. Some small studies have found that the risk of deficiency is particularly high in elderly people who reside in an institution 161, 162.
People with HIV/AIDS
People with HIV infection have an increased risk of thiamin deficiency and its sequelae, including beriberi and Wernicke-Korsakoff syndrome 55, 163. Autopsies of 380 people with AIDS found that almost 10% had Wernicke’s encephalopathy 164, and some experts believe that thiamin deficiency is underdiagnosed in this population 165. The association between thiamin deficiency and HIV/AIDS is probably due to malnutrition as a result of the catabolic state associated with AIDS 70.
People with diabetes
Some small studies have found that thiamine levels in plasma are up to 76% lower in people with type 1 diabetes than in healthy volunteers and 50%–75% lower in people with type 2 diabetes 166, 167. Other studies have shown a higher risk of thiamine deficiency in people with type 1 and/or type 2 diabetes based on tests of erythrocyte transketolase activity 168, 169. These lower thiamine levels might be due to increases in clearance of thiamin by the kidneys. The relevance of these effects to clinical prognosis or outcomes is not known.
People who have undergone weight loss surgery
Weight loss surgery also called bariatric surgery for weight loss is associated with some risks, including severe thiamine deficiency due to malabsorption that can lead to beriberi or Wernicke’s encephalopathy 70. A 2008 literature review identified 84 cases of Wernicke’s encephalopathy after bariatric surgery (primarily gastric bypass surgery) between 1991 and 2008 170. About half of these patients experienced long-lasting neurologic impairments. Micronutrient supplements that include thiamine are almost always recommended for patients following weight loss surgery to avoid deficiencies 171.
Wernicke’s encephalopathy pathophysiology
Brain atrophy associated with Wernicke-Korsakoff Syndrome occurs most predominantly at the mammillary bodies, though it can also occur in other places, including the dorsomedial thalami, the periaqueductal gray, the walls of the third ventricle, and the tectal plate 24. This is best visualized by increased signal intensity on T2/FLAIR on MRI. In addition to the damage seen in these areas, there may be damage to the cortex, although this may be due to the direct toxic effects of alcohol as opposed to thiamine deficiency 24.
The amnesia associated with Wernicke-Korsakoff syndrome is a result of atrophy of the structures of the diencephalon (thalamus, hypothalamus, and mammillary bodies) and is similar to amnesia that is present as a result of damage to the medial temporal lobe 24. It has been argued that the memory impairment can occur as a result of damage along any part of the mammillothalamic tract, which may explain how Wernicke-Korsakoff Syndrome can develop in patients with damage exclusively to either the thalamus or the mammillary bodies 24.
The ocular motor lesions are due to damage to the abducens nuclei and eye movement centers in the midbrain/pons 24. Ataxia is due to damage in the superior vermis 24.
Early changes of astrocytes and microglia have been reported in experimental thiamine deficiency 172 and Wernicke encephalopathy 173. In thiamine deficiency, the earliest biochemical change is the decrease of α-ketoglutarate-dehydrogenase activity (α-KGDH) in astrocytes 174. Astrocyte dysfunction in thiamine deficiency involves a loss of glutamate transporters and other astrocyte-specific proteins which together contribute to focal neuronal injury in terms of neural cell excitotoxicity caused by extracellular build-up of glutamate. A reduction in the thiamine-dependent activity of transketolase leads to a lower use of glucose and oxidative stress secondary to endothelial cell dysfunction. This produces cytotoxic and vasogenic edema firstly in astrocytes, then in neurons along with disruption of the blood–brain barrier 174, 175 and local petechial hemorrhages 176 in brain areas that are specifically vulnerable to thiamine deficiency 177, 178, 179. Subsequently, neuronal DNA fragmentation and lactic acidosis occur in astrocytes and neurons, leading to necrosis and irreversible structural damage 174.
Other studies have focused on inflammation with microglia hyperactivity and pro-inflammatory cytokines in the cellular response to thiamine deficiency 180, 181, which might be an explanation for the focal neuronal loss in thiamine deficiency 172. The function of microglia is normally protective, but defensive features can turn neurotoxic and cause neuronal injury through ongoing microglial overstimulation once microglia cells are no longer inhibited by cholinergic neurons, as described by Van Gool and colleagues 36. From a pathophysiological point of view, thiamine seems to be involved in acetylcholinergic synaptic transmission 182, 174, as thiamine deficiency may cause decreased bioavailability of acetylcholine 183. This may be due to low acetylation rates in acetylcholine production or to selective vulnerability of cholinergic neurons, but the exact underlying mechanisms remain unclear 184, 185.
Wernicke’s encephalopathy prevention
Not drinking alcohol or drinking in moderation and getting enough nutrition reduce the risk of developing Wernicke’s encephalopathy or Wernicke-Korsakoff syndrome. If a heavy drinker will not quit, thiamine supplements and a good diet may reduce the chance of getting this condition, but the risk is not eliminated.
Wernicke’s encephalopathy symptoms
The symptoms of Wernicke’s encephalopathy usually come on quite quickly. Symptoms can vary, but often include:
- problems with the eyes (ophthalmoplegia), such as jerky movements (nystagmus) or double vision (diplopia)
- problems with balance, such as when someones tries to stand
- problems with movement, such as difficulty walking normally (ataxic gait)
- problems with the mind (acute altered mental status), such as feeling disoriented or drowsy
- confusion and loss of mental activity that can progress to coma and death.
Other less common symptoms of Wernicke’s encephalopathy include:
- weakness of the arms and legs
- rapid heartbeat
- low blood pressure on standing (known as postural hypotension)
- alcohol withdrawal
Symptoms of Korsakoff syndrome:
- Inability to form new memories
- Loss of memory, can be severe
- Making up stories (confabulation)
- Seeing or hearing things that are not really there (hallucinations)
Wernicke’s encephalopathy should be suspected in any patient with chronic alcohol abuse or any form of malnutrition and any of the following: acute altered mental status, ophthalmoplegia, ataxic gait, delirium, and hypotension. The classic triad of Wernicke’s encephalopathy is altered mental status, ataxic gait, and ophthalmoplegia 186. The diagnosis is made based on clinical presentation, and a definitive diagnosis is complicated as the clinical triad may not be present in up to 90% of patients.
The hallmark sign of Wernicke encephalopathy is ocular abnormalities especially nystagmus. Other oculomotor symptoms include cranial nerve involvement of oculomotor, abducens, and vestibular nuclei causing conjugate gaze palsies. Pupillary sluggishness, ptosis, and anisocoria are also common.
Gait ataxia is also a significant finding in Wernicke’s encephalopathy where patients will present with a broad-based gait. Also, gait can worsen, and in many cases, patients are unable to walk. Physical examination may include a complete neurological exam with cerebellar testing. Disorientation and altered sensorium characterize encephalopathy. Some patient can present with hyperactive delirium secondary to possible alcohol withdrawal symptoms alongside Wernicke encephalopathy. Less than 5% of patients with Wernicke encephalopathy can present with the severely depressed level of consciousness that will eventually lead to coma and death. Some other warning signs could include hyperthermia and hypotension. The patient could also present with peripheral neuropathy and commonly includes the lower extremity, and an examination would reveal distal sensory loss.
Wernicke’s encephalopathy should be a consideration in a patient with long term malnutrition and episodes of confusion and altered mental status. Over the past few decades, bariatric surgery has been associated with Wernicke’s encephalopathy and malnutrition; the key reason is that after surgery, there is limited intake of food and the stores of thiamine are rapidly depleted.
Many people with Wernicke’s syndrome go on to develop symptoms of Korsakoff syndrome, such as severe short-term memory loss, and trouble forming new memories and learning new things.
If you think that you, or someone you know, may have Wernicke’s encephalopathy, it’s very important to get medical help straight away.
Comorbidities
In patients with alcohol-related Wernicke-Korsakoff syndrome, depression was reported in 18% of the patients, psychotic disorders and hallucinosis in 7%, personality disorders in 16%, and other psychiatric disorders in 7% of the patients 187. Psychiatric conditions such as schizophrenia, pervasive developmental disorder, and schizotypal personality disorders may have been missed until the patient receives medical attention due to the consequences of vitamin deficiencies. Somatic comorbidities are a serious problem in patients with Wernicke-Korsakoff syndrome, and include among others chronic obstructive pulmonary disease (in 34.4%), liver cirrhosis (26.9%), peripheral arterial disease (22.9%), stroke (22.6%), diabetes mellitus (21.8%), epilepsy (13.2%), myocardial infarction (12.3%), and Barrett esophagus (2.3%), according to data of Wernicke-Korsakoff patients in residential care 188. In a study of Novo-Veleiro and colleagues 189, patients with alcohol related Wernicke encephalopathy and liver disease presented more frequently with tremor (26.5% versus 14.0%), flapping (10.5% versus 3.3%), and hallucinations (35.2% versus 21.3%) than those without alcoholic liver disease. Of the patients with suspected Korsakoff syndrome, who were admitted to a diagnostic center, new malignancies were diagnosed in 87/389 (22.4%) patients, including tumors of mouth, throat and esophagus, lung cancer, and colon cancer, median 3 years after admission 188. Importantly, pain perception in Korsakoff syndrome may be disturbed, probably due to higher pain thresholds 190. Consequently, the alerting signals of pain may be less well perceived by Korsakoff patients, which may cause a delay in help-seeking behavior.
Wernicke’s encephalopathy complications
Wernicke’s encephalopathy complications include:
- Neurological injury
- Ataxia
- Korsakoff syndrome
- Ophthalmoplegia
- Heart failure
- Lactic acidosis
Refeeding syndrome
The refeeding syndrome is characterized by water-electrolyte imbalance, in particular hypophosphatemia, hypokalemia and hypomagnesemia, glucose intolerance, manifestation of thiamine deficiency, and fluid overload 191. Reintroducing carbohydrates or administering glucose without the addition of thiamine can be a risk factor for the development of Wernicke encephalopathy in already depleted thiamine stores 192. Criteria for a high risk of developing refeeding syndrome are found in the American Society for Parenteral and Enteral Nutrition (ASPEN) Consensus Recommendations for Refeeding Syndrome 193.
Wernicke encephalopathy may be precipitated by a refeeding syndrome within 2 to 3 days in a hospital 194, as most thiamine deficient patients have not been eating properly for months and sometimes have not eaten at all for several days or weeks 187. These patients should be gradually reintroduced to caloric intake in consultation with a dietitian. They should also be carefully monitored during the first days of admission, including serial electrolyte and blood glucose checks 195.
If there are no abnormal laboratory values for phosphate, calcium, potassium, magnesium and glucose (glucose curves), monitoring in the context of refeeding syndrome is stopped after 72 hour 196.
Wernicke’s encephalopathy diagnosis
The diagnosis of Wernicke encephalopathy or Wernicke-Korsakoff syndrome is made by the history and clinical findings supplemented with lab studies showing thiamine deficiency. Importantly, initial serum thiamine concentrations can be normal despite the clinical signs of Wernicke encephalopathy 16.
A physical examination of the nervous and muscular system may show signs of damage to many nerve systems:
- Changes in walking
- Coordination problems
- Abnormal eye movement
- Drooping of the eyelids
- Decreased or abnormal reflexes
- Fast pulse (heart rate)
- Low blood pressure
- Low body temperature
- Muscle weakness and atrophy (loss of tissue mass)
- Problems with walk (gait) and coordination.
For practical reasons, replacing thiamine as an initial test may be most feasible 197. If the patient responds to thiamine administration, it is safe to assume that a measure of thiamine deficiency was responsible for the signs and symptoms 197. Thiamine is not toxic in high levels, which means that this route carries little risk. In addition, time is saved in treating the patient and money is saved in testing. However, although observation of a patient’s clinical response to thiamine administration remains the easiest, least expensive form of testing, clinicians usually miss the subclinical forms of thiamine deficiency 197.
The diagnosis of Wernicke’s encephalopathy is a histopathological diagnosis, made after a postmortem examination of the brain 198, 199, 200.
The Caine criteria have been described to have a sensitivity or 85% and specificity of 100% for Wernicke encephalopathy, in the alcoholic population 38, 39. Because of the strong overlap, the Caine criteria can be used to help screen for Korsakoff syndrome as well.
Caine criteria for the diagnosis of Wernicke encephalopathy in chronic alcoholics 38. When 2 out of these 4 criteria apply, the clinical diagnosis of Wernicke encephalopathy is made 39, 38:
- Dietary deficiencies
- Undernutrition (body mass index <2 SD below normal)
- A history of grossly impaired dietary intake
- An abnormal thiamine status
- Oculomotor abnormalities
- Ophthalmoplegia
- Nystagmus
- Gaze palsy
- Cerebellar dysfunction
- Unsteadiness or ataxia
- Abnormalities of past pointing
- Dysdiadokokinesia
- Impaired heel-shin testing
- Either an altered mental state OR mild memory impairment
- Altered mental status
- Disorientation in two of three fields
- Confused
- An abnormal digit span
- Comatose
- Mild memory impairment
- Failure to remember two or more words in the four-item memory test
- Impairment on more elaborate neuropsychological tests of memory function
- Altered mental status
The following tests are used to check a person’s nutrition level:
- Serum albumin (relates to person’s general nutrition)
- Transketolase activity in red blood cells (reduced in people with thiamine deficiency). The serum level of thiamin is not a reliable indicator of thiamin status. However, thiamin function can be measured by red blood cells transketolase activity (erythrocytes transketolase activity or ETKA). Red blood cells transketolase activity (EKTA) of 0 to 15% is considered adequate, 15 to 25% is considered a moderate risk, and 25% or higher is considered high risk for thiamin deficiency 201, 202.
- Serum vitamin B1 or thiamine levels. Direct measurement of erythrocyte thiamin pyrophosphate (TPP) can be done using whole-blood testing and has more sensitivity, specificity, precision, and robustness 201. The concentration of TPP in the whole blood ranges from 70 to 180 nmol/L 8.
- Urine tests to see if thiamine is passing through the urine. Urinary thiamine excretion can be used to measure adequate dietary intake. However, it does not reflect the thiamine stores in the body. In adults, urinary thiamine excretion of <100 mcg/day suggests inadequate intake, and less than 40 mcg/day suggests thiamin deficiency 71.
- Liver enzymes may be high in people with a history of long-term alcohol abuse.
A brain magnetic resonance imaging (MRI) may show changes in the tissue of the brain. In magnetic resonance imaging (MRI) during the early Wernicke phase of the disorder, Sullivan and Pfefferbaum 34, 33 have shown an altered signal in various components of the limbic, circuits including the paraventricular regions of the thalamus, the hypothalamus, mammillary bodies, the periaqueductal region, the floor of the fourth ventricle and midline cerebellum. The sensitivity of MRI in detecting Wernicke encephalopathy is only 53%, with a specificity of 93% 34. On diffusion tensor imaging (DTI), Segobin and colleagues 31 found that the anterior thalamic nuclei were mainly connected with the hippocampi (84% of parcellations) with a significantly reduced connectivity in Korsakoff patients. The medial dorsal nuclei were mainly connected with frontal-executive brain regions (70% of parcellations) with reductions in both Korsakoff and non-Korsakoff alcoholics, which was associated with atrophy of mediodorsal nuclei. On FDG-PET (18-fluoro-deoxy-glucose positron emission tomography), Reed and colleagues 33, 203 showed metabolic changes in the thalami and mammillary bodies, and also in surrounding tissue, namely the hypothalamus, a small portion of the basal forebrain, and the retrosplenium, all components of the anterograde memory (limbic) circuitry. But if Wernicke-Korsakoff syndrome is suspected, treatment should start immediately. Usually a brain MRI exam is not needed.
Wernicke’s encephalopathy treatment
Current guidelines on managing Wernicke’s encephalopathy
- Clinical diagnosis of Wernicke’s encephalopathy should be considered in alcoholics if the individual has a dietary deficiency, cerebellar dysfunction, eye signs, and altered mental status
- Total thiamine levels in the blood should be measured before treatment
- MRI can be used to support the clinical diagnosis of Wernicke’s encephalopathy
- Thiamine should be given IV
- After weight loss surgery, follow thiamine levels and supplement with thiamine for at least 6 months
The aim of treatment is prompt and quick correction of the thiamine deficiency in the brain. Wernicke’s encephalopathy is a medical emergency and considered a reversible condition, therefore, requiring immediate emergent attention although the onset of the disease may be acute or chronic. Parenteral administration of thiamine is most effective and provides for rapid administration, however, in some cases, there are persistent neurological deficits, and the acute condition can progress to chronic Korsakoff syndrome.
The preferred dose of thiamine treatment for Wernicke encephalopathy may be as high as 500 mg intravenous thiamine given three times daily for 3 to 5 days, followed by intravenous thiamine, 250 mg/day for 3 to 5 days or until the symptoms disappear, and then further treatment with oral thiamine, 100 mg/day 45, 5. The rationale for using three times daily dosing of intravenous thiamine in acute presentations is based on the short half-life of intravenous thiamine (96 minutes) and the slow, carrier mediated process of thiamine transport across the blood–brain barrier 4. A single intravenous dose of thiamine is less likely to achieve sufficient brain tissue levels, and the bioavailability of oral thiamine hydrochloride is only 3.7% to 5.3% 45.
All malnourished patient may need higher doses of thiamine 5. Thiamine is generally administered before or together with glucose solutions because the glucose oxidation can decrease thiamine levels thereby exacerbating the neurological symptoms of Wernicke encephalopathy 5. Following a review of 19 papers, it has been recommended not to delay in correcting hypoglycemia 204. There are suggestions that prolonged and not acute replacement of glucose without thiamine supplementation increased the risk of Wernicke encephalopathy.
Patients with magnesium deficiency should also be treated as this can result in reduced recovery from Wernicke encephalopathy especially in patients with alcoholism 205, 206, 207. Thiamine is dependent on magnesium for its role in metabolizing glucose in the energy generating processes of the pentose phosphate pathway and the Krebs cycle in the mitochondria of the cells. Consequently, thiamine supplementation may be ineffective if existing or developing magnesium deficiencies are not corrected at the same time. Seizures may occur during both Wernicke encephalopathy 208 and alcohol withdrawal 209, and are also associated with magnesium deficiency 210. In patients with alcohol withdrawal symptoms, most showed low serum magnesium concentrations and were still under-supplemented during follow-up appointments 209. The infrequency of magnesium supplementation in the context of low serum magnesium concentrations may reflect a lack of clinician awareness regarding the clinical significance and prevalence of magnesium deficiency in patients with alcohol use 209. Furthermore, apart from alcohol abuse, malnutrition, or refeeding syndrome, hypomagnesemia may exist due to adverse side effects of proton pump inhibitors and whether or not it is combined with diuretics. For instance, in somatic comorbidity of alcohol-related esophageal peptic ulcers and ascites due to liver cirrhosis.
For Korsakoff syndrome high dose thiamine at 500 mg to 1500 mg, IV, three times daily for at least 3 days 39. Electrolyte abnormalities should be corrected and fluids replaced. In particular, magnesium requires replacement, as thiamine-dependent enzymes cannot operate in a magnesium-deficient state. Many patients will present needing glucose replacement as well, and traditionally it was thought that replacing glucose before thiamine could exacerbate the patient’s symptoms 211.
Thiamine, even at high concentrations, is not toxic in a person with normal kidney function 212. No cases of thiamine toxicity have been reported from the use of thiamine at the dosages indicated, even in patients in critical condition 212.
Once clinical symptoms improve, thiamine supplementation can switch to the oral route with a dose range of 50 to 100 mg per day 60. It is equally important in individuals with thiamine deficiency to require other nutrient supplementation such as magnesium, vitamin B2 (riboflavin), vitamin B3 (nicotinamide), vitamin B6 (pyridoxine), vitamin B12, vitamin C, potassium, and phosphate 70.
Support for cardiac function is necessary in cases of wet beriberi, because lack of cardiac support leads to low-output cardiac failure when the thiamine deficiency is corrected 212.
Follow-up care until delivery of current pregnancy, intensive care for advanced cardiomyopathy, definitive care for hyperthyroidism, or further workup of intestinal derangement may be warranted in patients with thiamine deficiency 212.
Blood tests may be repeated after the treatment is started. These tests will show how well you are responding to the medicine.
After the acute phase of vitamin and electrolyte replacement, there is mounting evidence that memory rehabilitation is beneficial in Korsakoff syndrome. Declarative memory (“knowing what”) seems to be most affected in Korsakoff syndrome, leading to many patients requiring lifelong care. Because procedural learning (“knowing how”) seems to remain somewhat maintained in Korsakoff syndrome, memory rehabilitation focussed in this area has shown promising outcomes. There has been some success in small Korsakoff syndrome patient populations in learning procedures and improving their autonomy 213.
Monitoring and special care may be needed if the person is:
- In a coma
- Lethargic
- Unconscious
Thiamine (vitamin B1) is usually given by injection into a vein or a muscle as soon as possible. This may improve symptoms of:
- Confusion or delirium
- Difficulties with vision and eye movement
- Lack of muscle coordination
Stopping alcohol use can prevent more loss of brain function and damage to nerves. A well-balanced, nourishing diet can help, but it is not a substitute for stopping alcohol use.
Unfortunately, unlike Wernicke encephalopathy, Wernicke-Korsakoff syndrome is a long-term disorder which is often progressive 79. It is very rare for the individual with Wernicke-Korsakoff syndrome to recover fully even with aggressive treatment 79. After thiamine treatment, the symptoms of encephalopathy will improve in 5 to 12 days. The patient should be offered oral thiamine and consulted for rehabilitation and treatment of other comorbid conditions. Most patients with Wernicke-Korsakoff syndrome need long-term care in a chronic care facility. Their prognosis is guarded.
Wernicke’s encephalopathy prognosis
Wernicke’s encephalopathy is a serious life-threatening disorder with enormous disability. Even when the condition is managed with thiamine, the global confusion usually improves rapidly, but the ataxia and ophthalmoplegia may persist for some time. While thiamine can induce partial improvement, the neuropsychological deficits persist in many cases. Patients who have minimal or neurological signs have the best outcomes with thiamine supplement. The confusional state usually improves when IV thiamine is administered but the learning and memory deficits only improve partially. A small number of patients fail to have any improvement and may develop Korsakoff psychosis, which often requires long-term institutionalization. Very few individuals recover at this point. Of these, less than 10% will recover to be discharged from long-term care. A significant number of patients will have long-term neurological deficits like ataxia, nystagmus and Korsakoff syndrome, which seriously diminishes the quality of life. Unfortunately, there are no long-term follow-up studies and anecdotal reports indicate that many of these patients do die prematurely 214.
In the initial Wernicke phase, twenty-five of 468 patients (5.3%) with Wernicke encephalopathy died during hospitalization. The causes of death were cancer, cardiac arrest, infections, and head injury 49. In a tertiary hospital study of Sanvisens and colleagues 215, the median survival time in 51 patients with Wernicke encephalopathy was 8.0 years and mortality was associated with infection, cancer, or malnutrition. Two-thirds of the patients continued alcohol use after discharge, of whom 6% presented with a subsequent Wernicke episode 215. In a national population-based register study by Palm and colleagues 216, the median survival in Wernicke-Korsakoff syndrome and alcohol-related dementia was 10.7 years and 5.9 years, respectively. The main causes of death of people with a diagnosis of Wernicke-Korsakoff syndrome were diseases of the circulatory system (24.0%), neoplasms (16.4%), diseases of the digestive system (16.0%), mental and behavioral disorders (13.3%), and accidents, suicides and other external causes (12.1%) 216. The main causes of death in 138/349 (39.5%) patients receiving residential Korsakoff care were cancer (in 40.6%), infections (26.8%), and sudden death (8.0%). The median survival was 5.8 years after the initial hospitalization because of Wernicke encephalopathy 188.
Wernicke-Korsakoff syndrome recovery
Approximately 25% of patients with Wernicke-Korsakoff syndrome require long-term institutionalization. The patients that depend on long-term care often have one or more comorbidities (somatic and psychiatric).
Mental Status Complications
Global confusion often resolves gradually after treatment.
One in five patients who demonstrate signs of the amnestic state after initiating treatment will have a complete recovery. The remaining patients will have varying degrees of persistent learning and memory impairments.
Maximum recovery may take years and is dependent on abstinence from alcohol.
Ataxic Complications
Approximately half of the patients with ataxic symptoms will recover completely. The other half will have an incomplete recovery, with a residual slow, shuffling, wide-based gait, and the inability to tandem walk.
Vestibular dysfunction improves in about half of all patients.
Ocular Complications
After starting treatment, patients typically recover from Wernicke-Korsakoff syndrome in a predictable pattern. Improvement of ocular abnormalities is the most dramatic, usually occurring within hours after thiamine administration.
If the ocular abnormality does not improve after administrating thiamine, the diagnosis of Wernicke-Korsakoff syndrome should be reevaluated.
Vertical nystagmus may persist for months while the fine horizontal nystagmus can persist indefinitely.
Sixth nerve palsies, ptosis, and vertical-gaze palsies all typically recover completely.
Mortality
Mortality usually occurs secondary to infections and hepatic failure, but some deaths are attributable to defects of prolonged thiamine deficiency.
The mortality rate is 10% to 15% in severe cases.
- Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin Proc. 2019 Jun;94(6):1065-1072. doi: 10.1016/j.mayocp.2019.02.018[↩][↩][↩][↩]
- Firdous U, Sharara HA, Nahia FA, Al Saqqa M, Musaed S: Wernicke′s encephalopathy and hyperemesis gravidarum. Int J Nutr Pharmacol Neurol Dis. 2013, 3:142-145. 10.4103/2231-0738.112841[↩]
- Žigrai, M., Smetanová, V., Gmitterová, K. et al. Wernicke encephalopathy—a rare complication of hyperemesis gravidarum. Eur J Clin Nutr 74, 663–665 (2020). https://doi.org/10.1038/s41430-020-0592-9[↩]
- Wilson RB. Pathophysiology, prevention, and treatment of beriberi after gastric surgery. Nutr Rev. 2020 Dec 1;78(12):1015-1029. doi: 10.1093/nutrit/nuaa004[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Vasan S, Kumar A. Wernicke Encephalopathy. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470344[↩][↩][↩][↩][↩][↩]
- Kumar N. Neurologic presentations of nutritional deficiencies. Neurol Clin. 2010 Feb;28(1):107-70. doi: 10.1016/j.ncl.2009.09.006[↩]
- Kantor S, Prakash S, Chandwani J, Gokhale A, Sarma K, Albahrani MJ. Wernicke’s encephalopathy following hyperemesis gravidarum. Indian J Crit Care Med. 2014 Mar;18(3):164-6. doi: 10.4103/0972-5229.128706[↩]
- Frank, L.L. (2015), Thiamin in Clinical Practice. Journal of Parenteral and Enteral Nutrition, 39: 503-520. https://doi.org/10.1177/0148607114565245[↩][↩]
- Isenberg-Grzeda E., Alici Y., Hatzoglou V., Nelson C., Breitbart W. Nonalcoholic thiamine-related encephalopathy (Wernicke-Korsakoff syndrome) among inpatients with cancer: A series of 18 cases. Psychosomatics. 2016;57:71–81. doi: 10.1016/j.psym.2015.10.001[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Vitamin B. [Updated 2021 May 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548710[↩]
- Shabbir S, Tong O, Gluck L, Robbins M. Convergence Spasm in Wernicke Encephalopathy. Neurohospitalist. 2018 Jan;8(1):NP1-NP2. doi: 10.1177/1941874417690668[↩]
- Tang L, Alsulaim HA, Canner JK, Prokopowicz GP, Steele KE. Prevalence and predictors of postoperative thiamine deficiency after vertical sleeve gastrectomy. Surg Obes Relat Dis. 2018 Jul;14(7):943-950. doi: 10.1016/j.soard.2018.03.024[↩]
- Oudman, E., Wijnia, J.W., Oey, M.J., van Dam, M.J. and Postma, A. (2018), Preventing Wernicke’s encephalopathy in anorexia nervosa: A systematic review. Psychiatry Clin. Neurosci., 72: 774-779. https://doi.org/10.1111/pcn.12735[↩]
- Ahmed S, Ahmed D, Abo Salah S, Mathew J, Yousaf Z. Wernicke’s Encephalopathy Associated With Transient Gestational Hyperthyroidism and Hyperemesis Gravidarum. Cureus. 2020 Aug 25;12(8):e10012. doi: 10.7759/cureus.10012[↩]
- Ropper AH, Samuels MA, Klein JPS. Adams and Victor’s Principles of Neurology. 11th ed. McGrawHill Education; 2019.[↩]
- Wijnia JW. A Clinician’s View of Wernicke-Korsakoff Syndrome. J Clin Med. 2022 Nov 15;11(22):6755. doi: 10.3390/jcm11226755[↩][↩][↩][↩][↩]
- Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60. doi:10.1136/jnnp.62.1.51[↩]
- Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017 Nov 27;13:2875-2890. doi: 10.2147/NDT.S130078[↩]
- Wernicke-Korsakoff Syndrome Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Wernicke-Korsakoff-Syndrome-Information-Page[↩]
- Wernicke-Korsakoff syndrome. https://medlineplus.gov/ency/article/000771.htm[↩][↩][↩][↩][↩]
- Vogrig A, Zanoni T, Moretto G. Nystagmus and Lower Extremity Hyperalgesia After Colectomy. JAMA. 2016 Oct 11;316(14):1488-1489. doi: 10.1001/jama.2016.13658[↩][↩]
- Zuccoli G, Santa Cruz D, Bertolini M, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol 2009;30:171–6. https://doi.org/10.3174/ajnr.A1280[↩]
- Allan D. Thomson and others, THE ROYAL COLLEGE OF PHYSICIANS REPORT ON ALCOHOL: GUIDELINES FOR MANAGING WERNICKE’S ENCEPHALOPATHY IN THE ACCIDENT AND EMERGENCY DEPARTMENT, Alcohol and Alcoholism, Volume 37, Issue 6, November 2002, Pages 513–521, https://doi.org/10.1093/alcalc/37.6.513[↩]
- Akhouri S, Kuhn J, Newton EJ. Wernicke-Korsakoff Syndrome. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430729[↩][↩][↩][↩][↩][↩][↩]
- Polegato, B.F., Pereira, A.G., Azevedo, P.S., Costa, N.A., Zornoff, L.A.M., Paiva, S.A.R. and Minicucci, M.F. (2019), Role of Thiamin in Health and Disease. Nutrition in Clinical Practice, 34: 558-564. https://doi.org/10.1002/ncp.10234[↩][↩]
- Rosenbaum M. Change diagnosis to “alcohol withdrawal delirium”? Am. J. Psychiatry. 2003;160:1357–1358. doi: 10.1176/appi.ajp.160.7.1357-a[↩]
- Wernicke-Korsakoff Syndrome. https://www.ninds.nih.gov/health-information/disorders/wernicke-korsakoff-syndrome[↩][↩]
- Arts NJM, Walvoort SJW, Kessels RPC: Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017, 13:2875-2890. 10.2147/NDT.S130078[↩]
- Wijnia J.W., Goossensen A. Cerebellar neurocognition and Korsakoff’s syndrome: An hypothesis. Med. Hypotheses. 2010;75:266–268. doi: 10.1016/j.mehy.2010.02.035[↩]
- Segobin S., Ritz L., Lannuzel C., Boudehent C., Vabret F., Eustache F., Beaunieux H., Pitel A.L. Integrity of white matter microstructure in alcoholics with and without Korsakoff’s syndrome. Hum. Brain Mapp. 2015;36:2795–2808. doi: 10.1002/hbm.22808[↩]
- Segobin S., Laniepce A., Ritz L., Lannuzel C., Boudehent C., Cabé N., Urso L., Vabret F., Eustache F., Beaunieux H., et al. Dissociating thalamic alterations in alcohol use disorder defines specificity of Korsakoff’s syndrome. Brain. 2019;142:1458–1470. doi: 10.1093/brain/awz056[↩][↩]
- Kopelman M.D., Thomson A.D., Guerrini I., Marshall E.J. The Korsakoff syndrome: Clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148–154. doi: 10.1093/alcalc/agn118[↩][↩][↩]
- Kopelman M.D. What is the Korsakoff syndrome?—A paper in tribute to Prof Alwyn Lishman. Cogn. Neuropsychiatry. 2022;27:296–313. doi: 10.1080/13546805.2022.2067472[↩][↩][↩]
- Sullivan E.V., Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–165. doi: 10.1093/alcalc/agn103[↩][↩][↩]
- Wijnia J.W., Oudman E. Biomarkers of delirium as a clue to diagnosis and pathogenesis of Wernicke-Korsakoff syndrome. Eur. J. Neurol. 2013;20:1531–1538. doi: 10.1111/ene.12217[↩]
- Van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: When cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2[↩][↩]
- Thomson A.D., Cook C.C., Touquet R., Henry J.A., Royal College of Physicians, London The Royal College of Physicians report on alcohol: Guidelines for managing Wernicke’s encephalopathy in the Accident and Emergency Department. Alcohol Alcohol. 2002;37:513–521. doi: 10.1093/alcalc/37.6.513[↩]
- Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997 Jan;62(1):51-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC486695/pdf/jnnpsyc00001-0059.pdf[↩][↩][↩][↩][↩][↩]
- Sharp CS, Wilson MP, Nordstrom K. Psychiatric Emergencies for Clinicians: Emergency Department Management of Wernicke-Korsakoff Syndrome. J Emerg Med. 2016 Oct;51(4):401-404. doi: 10.1016/j.jemermed.2016.05.044[↩][↩][↩][↩][↩][↩]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Arlington, VA, USA: 2013. pp. 490–491. DSM-5.[↩]
- Shimamura A.P., Janowsky J.S., Squire L.R. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8[↩]
- Joyce E.M., Robbins T.W. Frontal lobe function in Korsakoff and non-Korsakoff alcoholics: Planning and spatial working memory. Neuropsychologia. 1991;29:709–723. doi: 10.1016/0028-3932(91)90067-I[↩]
- Schnider A. Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat. Rev. Neurosci. 2003;4:662–671. doi: 10.1038/nrn1179[↩]
- Hammoud N., Jimenez-Shahed J. Chronic neurologic effects of alcohol. Clin. Liver Dis. 2019;23:141–155. doi: 10.1016/j.cld.2018.09.010[↩]
- Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017 May;13(5):727-741. doi: 10.1016/j.soard.2016.12.018[↩][↩][↩][↩]
- Victor, M. V., Adams, R. C. and Collins, G. H. (1989) The Wernicke– Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. F. A. Davis, Philadelphia.[↩]
- Cook, C. C. H., Hallwood, P. M. and Thomson, A. D. (1998) B-vitamin deficiency and neuro-psychiatric syndromes in alcohol misuse. Alcohol and Alcoholism 33,317–336.[↩]
- Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875-2890.[↩]
- Chamorro A.J., Rosón-Hernández B., Medina-García J.A., Muga-Bustamante R., Fernández-Solá J., Martín-González M.C., Seco-Hernández E., Novo-Veleiro I., Suárez-Cuervo C., Mateos-Díaz A.M., et al. Differences between alcoholic and nonalcoholic patients with Wernicke encephalopathy: A multicenter observational study. Mayo Clin. Proc. 2017;92:899–907. doi: 10.1016/j.mayocp.2017.02.019[↩][↩][↩]
- Wijnia J.W., Oudman E., van Gool W.A., Wierdsma A.I., Bresser E.L., Bakker J., van de Wiel A., Mulder C.L. Severe infections are common in thiamine deficiency and may be related to cognitive outcomes: A cohort study of 68 patients with Wernicke-Korsakoff syndrome. Psychosomatics. 2016;57:624–633. doi: 10.1016/j.psym.2016.06.004[↩][↩]
- Kopelman M.D. What is the Korsakoff syndrome?—A paper in tribute to Prof Alwyn Lishman. Cogn. Neuropsychiatry. 2022;27:296–313. doi: 10.1080/13546805.2022.206747[↩]
- Oudman E., Wijnia J.W., Oey M.J., van Dam M., Postma A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J. Neurol. Sci. 2021;426:117482. doi: 10.1016/j.jns.2021.117482[↩][↩]
- Oudman E., Wijnia J.W., van Dam M., Biter L.U., Postma A. Preventing Wernicke encephalopathy after bariatric surgery. Obes. Surg. 2018;28:2060–2068. doi: 10.1007/s11695-018-3262-4[↩]
- Wernicke encephalopathy. Tatsuya Fujikawa, Yuka Sogabe. CMAJ Feb 2020, 192 (6) E143; DOI: 10.1503/cmaj.190998[↩]
- Said HM. Thiamin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:748-53.[↩][↩][↩][↩][↩][↩][↩][↩]
- Sharma S, Sheehy T, Kolonel LN. Ethnic differences in grains consumption and their contribution to intake of B-vitamins: results of the Multiethnic Cohort Study. Nutr J. 2013 May 20;12:65. doi: 10.1186/1475-2891-12-65[↩][↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩][↩][↩]
- Wooley, J.A. (2008), Characteristics of Thiamin and Its Relevance to the Management of Heart Failure. Nutr Clin Pract, 23: 487-493. https://doi.org/10.1177/0884533608323430[↩]
- Saldanha LG, Dwyer JT, Bailen RA. Modernization of the National Institutes of Health Dietary Supplement Label Database. J Food Compost Anal. 2021 Sep;102:104058. doi: 10.1016/j.jfca.2021.104058[↩]
- Sriram, K., Manzanares, W. and Joseph, K. (2012), Thiamine in Nutrition Therapy. Nutrition in Clinical Practice, 27: 41-50. https://doi.org/10.1177/0884533611426149[↩][↩][↩][↩]
- Bettendorff L. Thiamin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:261-79.[↩][↩][↩][↩]
- Bemeur C, Butterworth RF. Thiamin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:317-24.[↩][↩][↩][↩][↩][↩][↩]
- Thiamine. Monograph. Altern Med Rev. 2003 Feb;8(1):59-62.[↩]
- Tallaksen CM, Sande A, Bøhmer T, Bell H, Karlsen J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur J Clin Pharmacol. 1993;44(1):73-8. doi: 10.1007/BF00315284[↩]
- Lonsdale D. A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evid Based Complement Alternat Med. 2006 Mar;3(1):49-59. doi: 10.1093/ecam/nek009[↩]
- Bettendorff, L. and Wins, P. (2009), Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. The FEBS Journal, 276: 2917-2925. https://doi.org/10.1111/j.1742-4658.2009.07019.x[↩]
- Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014 Feb 11;53(5):821-35. doi: 10.1021/bi401618y[↩]
- Nabokina SM, Said HM. A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012 Aug 1;303(3):G389-95. doi: 10.1152/ajpgi.00151.2012[↩]
- Lonsdale D. Thiamin. Adv Food Nutr Res. 2018;83:1-56. doi: 10.1016/bs.afnr.2017.11.001[↩]
- Thiamin. https://ods.od.nih.gov/factsheets/Thiamin-HealthProfessional[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Gibson GE, Blass JP. Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid Redox Signal. 2007 Oct;9(10):1605-19. doi: 10.1089/ars.2007.1766[↩][↩]
- Thiamin. https://lpi.oregonstate.edu/mic/vitamins/thiamin[↩][↩]
- Thiamin. https://ods.od.nih.gov/factsheets/Thiamin-Consumer[↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998[↩][↩]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2013-2014. 2012. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/[↩]
- The USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.[↩][↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/[↩]
- Gossman WG, Newton EJ. Wernicke-Korsakoff Syndrome. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430729[↩][↩][↩]
- World Health Organization, United Nations High Commissioner for Refugees. Thiamine deficiency and its prevention and control in major emergencies. https://apps.who.int/iris/bitstream/handle/10665/66139/WHO_NHD_99.13.pdf[↩][↩][↩][↩][↩][↩][↩]
- Lakhani SV, Shah HN, Alexander K, Finelli FC, Kirkpatrick JR, Koch TR. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res. 2008 May;28(5):293-8. doi: 10.1016/j.nutres.2008.03.002[↩][↩]
- Shah HN, Bal BS, Finelli FC, Koch TR. Constipation in patients with thiamine deficiency after Roux-en-Y gastric bypass surgery. Digestion. 2013;88(2):119-24. doi: 10.1159/000353245[↩][↩][↩]
- Thornalley P.J., Babaei-Jadidi R., Al Ali H., Rabbani N., Antonysunil A., Larkin J., Ahmed A., Rayman G., Bodmer C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164–2170. doi: 10.1007/s00125-007-0771-4[↩]
- Larkin J.R., Zhang F., Godfrey L., Molostvov G., Molostvov G., Zehnder D., Rabbani N., Thornalley P.J. Glucose-induced down regulation of thiamine transporters in the kidney proximal tubular epithelium produces thiamine insufficiency in diabetes. PLoS ONE. 2012;7:e53175. doi: 10.1371/journal.pone.0053175[↩]
- DeWardener H.E., Lennox B. Cerebral beriberi (Wernicke’s encephalopathy); review of 52 cases in a Singapore prisoner-of-war hospital. Lancet. 1947;1:11–17. doi: 10.1016/S0140-6736(47)91272-5[↩]
- Thomson A.D., Cook C.C., Guerrini I., Sheedy D., Harper C., Marshall E.J. Wernicke’s encephalopathy: ‘Plus ça change, plus c’est la même chose’ Alcohol Alcohol. 2008;43:180–186. doi: 10.1093/alcalc/agm149[↩]
- Oudman E., Wijnia J.W., Oey M., van Dam M., Painter R.C., Postma A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;236:84–93. doi: 10.1016/j.ejogrb.2019.03.006[↩]
- DiNicolantonio, J.J., Niazi, A.K., Lavie, C.J., O’Keefe, J.H. and Ventura, H.O. (2013), Thiamine Supplementation for the Treatment of Heart Failure: A Review of the Literature. Congest Heart Fail, 19: 214-222. https://doi.org/10.1111/chf.12037[↩][↩][↩][↩]
- Brown, G. (2014), Defects of thiamine transport and metabolism. J Inherit Metab Dis, 37: 577-585. https://doi.org/10.1007/s10545-014-9712-9[↩][↩]
- Beriberi. https://medlineplus.gov/ency/article/000339.htm[↩][↩]
- Rindi G. Thiamin. In: Ziegler E, Filer L, eds. Present Knowledge in Nutrition. Washington D.C.: ILSI Press; 1996:160-166.[↩]
- Arnold D. British India and the “beriberi problem”, 1798-1942. Med Hist. 2010 Jul;54(3):295-314. doi: 10.1017/s0025727300004622[↩][↩]
- Baeltz B. Ueber das Verhältniss der multiplen peripherischen Neuritis zur Beriberi (Panneuritis endemica) [in German]. Z Klin Med. 1882;4:616–617.[↩]
- Truswell, A.S. (2000), Australian experience with the Wernicke–Korsakoff syndrome. Addiction, 95: 829-832. https://doi.org/10.1046/j.1360-0443.2000.9568291.x[↩]
- Beriberi (Thiamine Deficiency). https://emedicine.medscape.com/article/116930-overview[↩][↩]
- Greenspon J, Perrone EE, Alaish SM. Shoshin beriberi mimicking central line sepsis in a child with short bowel syndrome. World J Pediatr. 2010 Nov;6(4):366-8. doi: 10.1007/s12519-010-0022-5[↩]
- Saad L, Silva LF, Banzato CE, Dantas CR, Garcia C Jr. Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. J Med Case Rep. 2010 Jul 20;4:217. doi: 10.1186/1752-1947-4-217[↩]
- Jung ES, Kwon O, Lee SH, Lee KB, Kim JH, Yoon SH, Kim GM, Jeung HC, Rha SY. Wernicke’s encephalopathy in advanced gastric cancer. Cancer Res Treat. 2010 Jun;42(2):77-81. doi: 10.4143/crt.2010.42.2.77[↩]
- Becker DA, Balcer LJ, Galetta SL. The Neurological Complications of Nutritional Deficiency following Bariatric Surgery. J Obes. 2012;2012:608534. doi: 10.1155/2012/608534[↩]
- Nath A, Tran T, Shope TR, Koch TR. Prevalence of clinical thiamine deficiency in individuals with medically complicated obesity. Nutr Res. 2017 Jan;37:29-36. doi: 10.1016/j.nutres.2016.11.012[↩]
- Oudman E, Wijnia JW, Oey M, van Dam M, Painter RC, Postma A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2019 May;236:84-93. doi: 10.1016/j.ejogrb.2019.03.006[↩]
- Meggs WJ, Lee SK, Parker-Cote JN. Wernicke encephalopathy associated with hyperemesis gravidarum. Am J Emerg Med. 2020 Mar;38(3):690.e3-690.e5. doi: 10.1016/j.ajem.2019.09.012[↩]
- Sequeira Lopes da Silva JT, Almaraz Velarde R, Olgado Ferrero F, Robles Marcos M, Pérez Civantos D, Ramírez Moreno JM, Luengo Pérez LM. Wernicke’s encephalopathy induced by total parental nutrition. Nutr Hosp. 2010 Nov-Dec;25(6):1034-6.[↩]
- Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke’s syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009 Feb;25(2):142-6. doi: 10.1016/j.nut.2008.08.003[↩]
- Zastre JA, Sweet RL, Hanberry BS, Ye S. Linking vitamin B1 with cancer cell metabolism. Cancer Metab. 2013 Jul 24;1(1):16. doi: 10.1186/2049-3002-1-16[↩]
- Thurnham DI, Cathcart AE, Livingstone MB. A retrospective investigation of thiamin and energy intakes following an outbreak of beriberi in The Gambia. Nutrients. 2011 Jan;3(1):135-51. doi: 10.3390/nu3010135[↩][↩]
- Seligmann H, Levi R, Konijn AM, Prokocimer M. Thiamine deficiency in patients with B-chronic lymphocytic leukaemia: a pilot study. Postgrad Med J. 2001 Sep;77(911):582-5. doi: 10.1136/pmj.77.911.582[↩]
- Koike H, Misu K, Hattori N, Ito S, Ichimura M, Ito H, Hirayama M, Nagamatsu M, Sasaki I, Sobue G. Postgastrectomy polyneuropathy with thiamine deficiency. J Neurol Neurosurg Psychiatry. 2001 Sep;71(3):357-62. doi: 10.1136/jnnp.71.3.357[↩]
- Spinazzi M, Angelini C, Patrini C. Subacute sensory ataxia and optic neuropathy with thiamine deficiency. Nat Rev Neurol. 2010 May;6(5):288-93. doi: 10.1038/nrneurol.2010.16[↩]
- Sobue G, Li M, Terao S, Aoki S, Ichimura M, Ieda T, Doyu M, Yasuda T, Hashizume Y, Mitsuma T. Axonal pathology in Japanese Guillain-Barré syndrome: a study of 15 autopsied cases. Neurology. 1997 Jun;48(6):1694-700. doi: 10.1212/wnl.48.6.1694[↩]
- Ohnishi A, Tsuji S, Igisu H, Murai Y, Goto I, Kuroiwa Y, Tsujihata M, Takamori M. Beriberi neuropathy. Morphometric study of sural nerve. J Neurol Sci. 1980 Mar;45(2-3):177-90. doi: 10.1016/0022-510x(80)90164-1[↩]
- Koike, H., Iijima, M., Sugiura, M., Mori, K., Hattori, N., Ito, H., Hirayama, M. and Sobue, G. (2003), Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol., 54: 19-29. https://doi.org/10.1002/ana.10550[↩]
- Khounnorath S, Chamberlain K, Taylor AM, Soukaloun D, Mayxay M, Lee SJ, Phengdy B, Luangxay K, Sisouk K, Soumphonphakdy B, Latsavong K, Akkhavong K, White NJ, Newton PN. Clinically unapparent infantile thiamin deficiency in Vientiane, Laos. PLoS Negl Trop Dis. 2011 Feb 22;5(2):e969. doi: 10.1371/journal.pntd.0000969[↩]
- Chiappetta S, Stein J. Refeeding Syndrome: An Important Complication Following Obesity Surgery. Obes Facts. 2016;9(1):12-6. doi: 10.1159/000442534[↩]
- Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010 Feb;26(2):156-67. doi: 10.1016/j.nut.2009.11.017[↩]
- Marinella MA. Refeeding syndrome: implications for the inpatient rehabilitation unit. Am J Phys Med Rehabil. 2004 Jan;83(1):65-8. doi: 10.1097/01.PHM.0000104666.88102.99[↩]
- Raziel A. Thiamine deficiency after bariatric surgery may lead to Wernicke encephalopathy. Isr Med Assoc J. 2012 Nov;14(11):692-4[↩]
- Krishna S, Taylor AM, Supanaranond W, Pukrittayakamee S, ter Kuile F, Tawfiq KM, Holloway PA, White NJ. Thiamine deficiency and malaria in adults from southeast Asia. Lancet. 1999 Feb 13;353(9152):546-9. doi: 10.1016/s0140-6736(98)06316-8[↩]
- Mayxay M, Taylor AM, Khanthavong M, Keola S, Pongvongsa T, Phompida S, Phetsouvanh R, White NJ, Newton PN. Thiamin deficiency and uncomplicated falciparum malaria in Laos. Trop Med Int Health. 2007 Mar;12(3):363-9. doi: 10.1111/j.1365-3156.2006.01804.x[↩]
- Müri RM, Von Overbeck J, Furrer J, Ballmer PE. Thiamin deficiency in HIV-positive patients: evaluation by erythrocyte transketolase activity and thiamin pyrophosphate effect. Clin Nutr. 1999 Dec;18(6):375-8. doi: 10.1016/s0261-5614(99)80019-3[↩]
- Tanphaichitr V. Thiamin. In: Shils M, ed. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:381-389.[↩][↩]
- Stanga Z, Brunner A, Leuenberger M, Grimble RF, Shenkin A, Allison SP, Lobo DN. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008 Jun;62(6):687-94. doi: 10.1038/sj.ejcn.1602854[↩]
- Vimokesant SL, Hilker DM, Nakornchai S, Rungruangsak K, Dhanamitta S. Effects of betel nut and fermented fish on the thiamin status of northeastern Thais. Am J Clin Nutr. 1975 Dec;28(12):1458-63. doi: 10.1093/ajcn/28.12.1458[↩]
- Ventura A, Mafe MC, Bourguet M, Tornero C. Wernicke’s encephalopathy secondary to hyperthyroidism and ingestion of thiaminase-rich products. Neurologia. 2013 May;28(4):257-9. English, Spanish. doi: 10.1016/j.nrl.2012.01.005[↩]
- Bettendorff L. Thiamin. In: Erdman Jr. JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames: Wiley-Blackwell; 2012:261-279.[↩]
- Soukaloun D, Lee SJ, Chamberlain K, Taylor AM, Mayxay M, Sisouk K, Soumphonphakdy B, Latsavong K, Akkhavong K, Phommachanh D, Sengmeuang V, Luangxay K, McDonagh T, White NJ, Newton PN. Erythrocyte transketolase activity, markers of cardiac dysfunction and the diagnosis of infantile beriberi. PLoS Negl Trop Dis. 2011 Feb 22;5(2):e971. doi: 10.1371/journal.pntd.0000971[↩]
- Earl JW, McCleary BV. Mystery of the poisoned expedition. Nature. 1994 Apr 21;368(6473):683-4. doi: 10.1038/368683a0[↩]
- MATSUKAWA D, CHANG S, MISAWA H, FUJIMIYA M, KOBAYASHI N, HORIKAWA Y, TAKATO K. Studies on the thiamine deficiency due to bacterial thiaminase. I. Investigations on intestinal contents. J Vitaminol (Kyoto). 1954 Jul 10;1(1):43-8. doi: 10.5925/jnsv1954.1.43[↩]
- Nishimune T, Watanabe Y, Okazaki H, Akai H. Thiamin is decomposed due to Anaphe spp. entomophagy in seasonal ataxia patients in Nigeria. J Nutr. 2000 Jun;130(6):1625-8. doi: 10.1093/jn/130.6.1625[↩]
- Vimokesant, S., Kunjara, S., Rungruangsak, K., Nakornchai, S. and Panijpan, B. (1982), BERIBERI CAUSED BY ANTITHIAMIN FACTORS IN FOOD AND ITS PREVENTION. Annals of the New York Academy of Sciences, 378: 123-136. https://doi.org/10.1111/j.1749-6632.1982.tb31191.x[↩]
- Bender DA. Nutritional Biochemistry of the Vitamins. 1st ed. Cambridge, UK: Cambridge University Press; 2003.[↩][↩]
- Carvajal-Moreno M. Mycotoxins that affect the human cardiovascular system. J Biomol Res Ther. 2015;4:2.[↩]
- Rosa CA, Keller KM, Oliveira AA, Almeida TX, Keller LA, Marassi AC, Kruger CD, Deveza MV, Monteiro BS, Nunes LM, Astoreca A, Cavaglieri LR, Direito GM, Eifert EC, Lima TA, Modernell KG, Nunes FI, Garcia AM, Luz MS, Oliveira DC. Production of citreoviridin by Penicillium citreonigrum strains associated with rice consumption and beriberi cases in the Maranhão State, Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010 Feb;27(2):241-8. doi: 10.1080/19440040903289712[↩]
- Sulfiting agents: affirmation of GRAS status. Fed Regis. 1988;53:51065–51084.[↩]
- Sulfites – USA. https://farrp.unl.edu/sulfites-usa[↩]
- https://www.fda.gov/media/80337/download[↩]
- https://www.fda.gov/media/136310/download[↩]
- Suter PM, Haller J, Hany A, Vetter W. Diuretic use: a risk for subclinical thiamine deficiency in elderly patients. J Nutr Health Aging. 2000;4(2):69-71.[↩]
- Rieck J, Halkin H, Almog S, Seligman H, Lubetsky A, Olchovsky D, Ezra D. Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers. J Lab Clin Med. 1999 Sep;134(3):238-43. doi: 10.1016/s0022-2143(99)90203-2[↩]
- Sica, D.A. (2007), Loop Diuretic Therapy, Thiamine Balance, and Heart Failure. Congestive Heart Failure, 13: 244-247. https://doi.org/10.1111/j.1527-5299.2007.06260.x[↩]
- Zenuk C, Healey J, Donnelly J, Vaillancourt R, Almalki Y, Smith S. Thiamine deficiency in congestive heart failure patients receiving long term furosemide therapy. Can J Clin Pharmacol. 2003 Winter;10(4):184-8.[↩]
- Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995 May;98(5):485-90. doi: 10.1016/s0002-9343(99)80349-0[↩]
- Misumida N, Umeda H, Iwase M. Shoshin beriberi induced by long-term administration of diuretics: a case report. Case Rep Cardiol. 2014;2014:878915. doi: 10.1155/2014/878915[↩]
- Hung SC, Hung SH, Tarng DC, Yang WC, Chen TW, Huang TP. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2001 Nov;38(5):941-7. doi: 10.1053/ajkd.2001.28578[↩]
- Wilcox CS. Do diuretics cause thiamine deficiency? J Lab Clin Med. 1999 Sep;134(3):192-3. doi: 10.1016/s0022-2143(99)90198-1[↩]
- Nixon PF, Diefenbach RJ, Duggleby RG. Inhibition of transketolase and pyruvate decarboxylase by omeprazole. Biochem Pharmacol. 1992 Jul 7;44(1):177-9. doi: 10.1016/0006-2952(92)90053-l[↩][↩]
- Toh JW, Ong E, Wilson R. Hypomagnesaemia associated with long-term use of proton pump inhibitors. Gastroenterol Rep (Oxf). 2015 Aug;3(3):243-53. doi: 10.1093/gastro/gou054[↩][↩]
- Basu TK, Aksoy M, Dickerson JW. Effects of 5-fluorouracil on the thiamin status of adult female rats. Chemotherapy. 1979;25(2):70-6. doi: 10.1159/000237825[↩]
- Cho KP, Lee JS, Seong JS, Woo YM, Cho YJ, Jeong BJ, Sohn JH, Kim SJ. [Two cases of Wernicke´s encephalopathy that developed during total parenteral nutrition in colon cancer patients treated with 5-fluorouracil-based chemotherapy]. Korean J Gastroenterol. 2014 Sep 25;64(3):158-63. Korean. doi: 10.4166/kjg.2014.64.3.158[↩]
- Koenig H, Patel A. Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate. Arch Neurol. 1970 Aug;23(2):155-60. doi: 10.1001/archneur.1970.00480260061008[↩]
- Arellano M, Malet-Martino M, Martino R, Gires P. The anti-cancer drug 5-fluorouracil is metabolized by the isolated perfused rat liver and in rats into highly toxic fluoroacetate. Br J Cancer. 1998;77(1):79-86. doi: 10.1038/bjc.1998.12[↩]
- Schümann K. Interactions between drugs and vitamins at advanced age. Int J Vitam Nutr Res. 1999;69:173–178. doi:10.1024/0300-9831.69.3.173[↩]
- Chen L, Shu Y, Liang X, Chen EC, Yee SW, Zur AA, Li S, Xu L, Keshari KR, Lin MJ, Chien HC, Zhang Y, Morrissey KM, Liu J, Ostrem J, Younger NS, Kurhanewicz J, Shokat KM, Ashrafi K, Giacomini KM. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc Natl Acad Sci U S A. 2014 Jul 8;111(27):9983-8. doi: 10.1073/pnas.1314939111[↩]
- Liang X, Chien HC, Yee SW, Giacomini MM, Chen EC, Piao M, Hao J, Twelves J, Lepist EI, Ray AS, Giacomini KM. Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol Pharm. 2015 Dec 7;12(12):4301-10. doi: 10.1021/acs.molpharmaceut.5b00501[↩]
- Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G23-31. doi: 10.1152/ajpgi.00132.2010[↩]
- Salem A, Roland BC. Small intestinal bacterial overgrowth (SIBO). J Gastroint Dig Syst. 2014;4:225.[↩]
- Pelton R, LaValle JB, Hawkins E, Krinsky DL, eds. Drug-Induced Nutrient Depletion Handbook. Hudson, OH: Wolters Kluwer Clinical Drug Information (Lexicomp; );1999:258.[↩]
- ROBERTA AGABIO, THIAMINE ADMINISTRATION IN ALCOHOL-DEPENDENT PATIENTS, Alcohol and Alcoholism, Volume 40, Issue 2, March/April 2005, Pages 155–156, https://doi.org/10.1093/alcalc/agh106[↩]
- Vognar, L. and Stoukides, J. (2009), The Role of Low Plasma Thiamin Levels in Cognitively Impaired Elderly Patients Presenting with Acute Behavioral Disturbances. Journal of the American Geriatrics Society, 57: 2166-2168. https://doi.org/10.1111/j.1532-5415.2009.02542.x[↩]
- T J Wilkinson, H C Hanger, P M George, R Sainsbury, Is thiamine deficiency in elderly people related to age or co-morbidity?, Age and Ageing, Volume 29, Issue 2, March 2000, Pages 111–116, https://doi.org/10.1093/ageing/29.2.111[↩]
- Ito Y, Yamanaka K, Susaki H, Igata A. A cross-investigation between thiamin deficiency and the physical condition of elderly people who require nursing care. J Nutr Sci Vitaminol (Tokyo). 2012;58(3):210-6. doi: 10.3177/jnsv.58.210[↩]
- NICHOLAS P. O’ROURKE, VALDA W. BUNKER, ANITA J. THOMAS, PAUL M. FINGLAS, ANGELA L. BAILEY, BARBARA E. CLAYTON, Thiamine Status of Healthy and Institutionalized Elderly Subjects: Analysis of Dietary Intake and Biochemical Indices, Age and Ageing, Volume 19, Issue 5, September 1990, Pages 325–329, https://doi.org/10.1093/ageing/19.5.325[↩]
- Lu’o’ng KV, Nguyễn LT. The role of thiamine in cancer: possible genetic and cellular signaling mechanisms. Cancer Genomics Proteomics. 2013 Jul-Aug;10(4):169-85.[↩]
- Boldorini R, Vago L, Lechi A, Tedeschi F, Trabattoni GR. Wernicke’s encephalopathy: occurrence and pathological aspects in a series of 400 AIDS patients. Acta Biomed Ateneo Parmense. 1992;63(1-2):43-9.[↩]
- Larsen TR, Dragu D, Williams M. Wernicke’s Encephalopathy: An Unusual Consequence of the Acquired Immune Deficiency Syndrome-Case Report and Literature Review. Case Rep Med. 2013;2013:709474. doi: 10.1155/2013/709474[↩]
- Al-Attas OS, Al-Daghri NM, Alfadda AA, Abd-Alrahman SH, Sabico S. Blood thiamine and its phosphate esters as measured by high-performance liquid chromatography: levels and associations in diabetes mellitus patients with varying degrees of microalbuminuria. J Endocrinol Invest. 2012 Dec;35(11):951-6. doi: 10.3275/8126[↩]
- Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007 Oct;50(10):2164-70. doi: 10.1007/s00125-007-0771-4[↩]
- Jermendy G. Evaluating thiamine deficiency in patients with diabetes. Diab Vasc Dis Res. 2006 Sep;3(2):120-1. doi: 10.3132/dvdr.2006.014[↩]
- Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol (Tokyo). 1987 Dec;33(6):421-30. doi: 10.3177/jnsv.33.421[↩]
- Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008 Nov;248(5):714-20. doi: 10.1097/SLA.0b013e3181884308[↩]
- Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009 Oct;56(5):1105-21. doi: 10.1016/j.pcl.2009.07.002[↩]
- Todd K.G., Butterworth R.F. Early microglial response in experimental thiamine deficiency: An immunohistochemical analysis. Glia. 1999;25:190–198. doi: 10.1002/(SICI)1098-1136(19990115)25:2<190::AID-GLIA9>3.0.CO;2-B[↩][↩]
- Victor M., Adams R.D., Collins G. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. 2nd ed. F.A. Davis Co.; Philadelphia, PA, USA: 1989. ISBN 13: 9780803689213[↩]
- Sechi G., Serra A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7[↩][↩][↩][↩]
- Calingasan N.Y., Baker H., Sheu K.F., Gibson G.E. Blood-brain barrier abnormalities in vulnerable brain regions during thiamine deficiency. Exp. Neurol. 1995;134:64–72. doi: 10.1006/exnr.1995.1037[↩]
- Escourolle R., Poirere J. Manuel Élémentaire de Neuropathology [SYNOPSIS of Neuropathology] Mason; Paris, France: 1977.[↩]
- Desjardins P., Butterworth R.F. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol. Neurobiol. 2005;31:17–25. doi: 10.1385/MN:31:1-3:017[↩]
- Kohnke S., Meek C.L. Don’t seek, don’t find: The diagnostic challenge of Wernicke’s encephalopathy. Ann. Clin. Biochem. 2021;58:38–46. doi: 10.1177/0004563220939604[↩]
- Hazell A.S. Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem. Int. 2009;55:129–135. doi: 10.1016/j.neuint.2009.02.020[↩]
- Wang D., Hazell A.S. Microglial activation is a major contributor to neurologic dysfunction in thiamine deficiency. Biochem. Biophys. Res. Commun. 2010;402:123–128. doi: 10.1016/j.bbrc.2010.09.128[↩]
- Abdou E., Hazell A.S. Thiamine deficiency: An update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem. Res. 2015;40:353–361. doi: 10.1007/s11064-014-1430-z[↩]
- Iwata H. Possible role of thiamine in the nervous system. Trends Pharmacol. Sci. 1982;4:171–173. doi: 10.1016/0165-6147(82)91074-4[↩]
- Vorhees C.V., Schmidt D.E., Barrett R.J., Schenker S. Effects of thiamin deficiency on acetylcholine levels and utilization in vivo in rat brain. J. Nutr. 1977;107:1902–1908. doi: 10.1093/jn/107.10.1902[↩]
- zutowicz A., Bielarczyk H., Ronowska A., Gul-Hinc S., Klimaszewska-Łata J., Dyś A., Zyśk M., Pawełczyk T. Intracellular redistribution of acetyl-CoA, the pivotal point in differential susceptibility of cholinergic neurons and glial cells to neurodegenerative signals. Biochem. Soc. Trans. 2014;42:1101–1106. doi: 10.1042/BST20140078[↩]
- Mkrtchyan G., Aleshin V., Parkhomenko Y., Kaehne T., Di Salvo M.L., Parroni A., Contestabile R., Vovk A., Bettendorff L., Bunik V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci. Rep. 2015;5:12583. doi: 10.1038/srep12583[↩]
- Vasan S, Kumar A. Wernicke Encephalopathy. [Updated 2019 Nov 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470344[↩]
- Wijnia J.W., van de Wetering B.J., Zwart E., Nieuwenhuis K.G., Goossensen M.A. Evolution of Wernicke-Korsakoff syndrome in self-neglecting alcoholics: Preliminary results of relation with Wernicke-delirium and diabetes mellitus. Am. J. Addict. 2012;21:104–110. doi: 10.1111/j.1521-0391.2011.00199.x[↩][↩]
- Wijnia J.W., Oudman E., Wierdsma A.I., Oey M.J., Bongers J., Postma A. Vitamin D supplementation after malnutrition associated with time-related increase of cancer diagnoses: A cohort study of 389 patients with Wernicke-Korsakoff syndrome. Nutrition. 2019;66:166–172. doi: 10.1016/j.nut.2019.05.008[↩][↩][↩]
- Novo-Veleiro I., Herrera-Flores J., Rosón-Hernández B., Medina-García J.A., Muga R., Fernández-Solá J., Martín-González M.C., Seco-Hernández E., Suárez-Cuervo C., Mateos-Díaz A.M., et al. Alcoholic liver disease among patients with Wernicke encephalopathy: A multicenter observational study. Drug Alcohol Depend. 2022;230:109186. doi: 10.1016/j.drugalcdep.2021.109186[↩]
- Wijnia JW, Oudman E, Batjes DM, Brouwer BA, Oey M, Postma A. Korsakoff syndrome and altered pain perception: a search of underlying neural mechanisms. Scand J Pain. 2022 Sep 20;23(2):424-432. doi: 10.1515/sjpain-2022-0053[↩]
- Mehanna H.M., Moledina J., Travis J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ. 2008;336:1495–1498. doi: 10.1136/bmj.a301[↩]
- Schabelman E., Kuo D. Glucose before thiamine for Wernicke encephalopathy: A literature review. J. Emerg. Med. 2012;42:488–494. doi: 10.1016/j.jemermed.2011.05.076[↩]
- Da Silva J.S., Seres D.S., Sabino K., Adams S.C., Berdahl G.J., Citty S.W., Cober M.P., Evans D.C., Greaves J.R., Gura K.M., et al. Parenteral Nutrition Safety and Clinical Practice Committees, American Society for Parenteral and Enteral Nutrition. ASPEN Consensus recommendations for refeeding syndrome. Nutr. Clin. Pract. 2020;35:178–195. doi: 10.1002/ncp.10474[↩]
- Hershkowitz E., Reshef A., Munich O., Yosefi B., Markel A. Thiamine deficiency in self-induced refeeding syndrome, an undetected and potentially lethal condition. Case Rep. Med. 2014;2014:605707. doi: 10.1155/2014/605707[↩]
- The British Association for Parenteral and Enteral Nutrition (BAPEN) Refeeding Syndrome: Identification of Those at Risk. 2012. https://www.bapen.org.uk/pdfs/decision-trees/refeeding-syndrome.pdf[↩]
- Steenhagen E. Richtlijn Refeeding Syndroom [Guideline Refeeding Syndrome] Dietetics Department, University Medical Center Utrecht; Utrecht, The Netherlands: 2015. https://assets-eu-01.kc-usercontent.com/546dd520-97db-01b7-154d-79bb6d950a2d/dadb2dd4-db12-4e58-81a9-17c07cabb2cf/Richtlijn-refeeding.pdf[↩]
- Beriberi (Thiamine Deficiency) Workup. https://emedicine.medscape.com/article/116930-workup[↩][↩][↩]
- Colmant H. Enzephalopathien Bei Chronischem Alkoholismus. Stuttgart: Enke; 1965.[↩]
- Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986 Apr;49(4):341-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1028756/pdf/jnnpsyc00096-0001.pdf[↩]
- Torvik A. Wernicke’s encephalopathy–prevalence and clinical spectrum. Alcohol Alcohol Suppl. 1991;1:381-4.[↩]
- Talwar D, Davidson H, Cooney J, St JO’Reilly D. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000 May;46(5):704-10.[↩][↩]
- Bates CJ. Vitamin analysis. Ann Clin Biochem. 1997 Nov;34 ( Pt 6):599-626. doi: 10.1177/000456329703400604[↩]
- Reed L.J., Lasserson D., Marsden P., Stanhope N., Stevens T., Bello F., Kingsley D., Colchester A., Kopelman M.D. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/S0010-9452(08)70876-1[↩]
- Schabelman E, Kuo D. Glucose before thiamine for Wernicke encephalopathy: a literature review. J Emerg Med. 2012 Apr;42(4):488-94. doi: 10.1016/j.jemermed.2011.05.076[↩]
- Onishi H, Ishida M, Uchida N, Shintani D, Nishikawa T, Hasegawa K, Fujiwara K, Akechi T. Subclinical thiamine deficiency identified by preoperative evaluation in an ovarian cancer patient: Diagnosis and the need for preoperative thiamine measurement. Palliat Support Care. 2019 Oct;17(5):609-610. doi: 10.1017/S1478951518000615[↩]
- Johnson JM, Fox V. Beyond Thiamine: Treatment for Cognitive Impairment in Korsakoff’s Syndrome. Psychosomatics. 2018 Jul-Aug;59(4):311-317. doi: 10.1016/j.psym.2018.03.011[↩]
- Oudman E, Wijnia JW, van Dam M, Biter LU, Postma A. Preventing Wernicke Encephalopathy After Bariatric Surgery. Obes Surg. 2018 Jul;28(7):2060-2068. doi: 10.1007/s11695-018-3262-4[↩]
- Ghosh R., Mandal A., Roy D., Chatterjee S., Ghosh M.K., Dubey S., Lahiri D., Finsterer J., Ray B.K. Seizure as a presenting manifestation of Wernicke’s encephalopathy induced by hyperemesis gravidarum. J. Fam. Med. Prim. Care. 2021;10:567–571. doi: 10.4103/jfmpc.jfmpc_1466_20[↩]
- Maguire D., Talwar D., Burns A., Catchpole A., Stefanowicz F., Robson G., Ross D.P., Young D., Ireland A., Forrest E., et al. A prospective evaluation of thiamine and magnesium status in relation to clinicopathological characteristics and 1-year mortality in patients with alcohol withdrawal syndrome. J. Transl. Med. 2019;17:384. doi: 10.1186/s12967-019-02141-w[↩][↩][↩]
- Van Laecke S. Hypomagnesemia and hypermagnesemia. Acta Clin. Belg. 2019;74:41–47. doi: 10.1080/17843286.2018.1516173[↩]
- Covell T, Siddiqui W. Korsakoff Syndrome. [Updated 2023 Jan 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539854[↩]
- Beriberi (Thiamine Deficiency) Treatment & Management. https://emedicine.medscape.com/article/116930-treatment[↩][↩][↩][↩]
- Oudman E, Nijboer TC, Postma A, Wijnia JW, Van der Stigchel S. Procedural Learning and Memory Rehabilitation in Korsakoff’s Syndrome – a Review of the Literature. Neuropsychol Rev. 2015 Jun;25(2):134-48. doi: 10.1007/s11065-015-9288-7[↩]
- Nakamura ZM, Tatreau JR, Rosenstein DL, Park EM. Clinical Characteristics and Outcomes Associated With High-Dose Intravenous Thiamine Administration in Patients With Encephalopathy. Psychosomatics. 2018 Jul – Aug;59(4):379-387.[↩]
- Sanvisens A., Zuluaga P., Fuster D., Rivas I., Tor J., Marcos M., Chamorro A.J., Muga R. Long-term mortality of patients with an alcohol-related Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2017;52:466–471. doi: 10.1093/alcalc/agx013[↩][↩]
- Palm A., Vataja R., Talaslahti T., Ginters M., Kautiainen H., Elonheimo H., Suvisaari J., Lindberg N., Koponen H. Incidence and mortality of alcohol-related dementia and Wernicke-Korsakoff syndrome: A nationwide register study. Int. J. Geriatr. Psychiatry. 2022;37:e5775. doi: 10.1002/gps.5775[↩][↩]