Contents
What is ZIFT
ZIFT is short for zygote intrafallopian transfer, and is also called tubal embryo transfer. ZIFT technique differs from gamete intrafallopian transfer (GIFT) in that fertilization takes place in the lab rather than the fallopian tube, but is similar in that the fertilized egg is transferred to the tube rather than the uterus. This procedure also requires a laparoscopy. Today, ZIFT (zygote intrafallopian transfer) comprises less than 1% of assisted reproductive technology (ART) procedures performed in the United States.
With ZIFT procedure, fertilization occurs in the laboratory. Then the very young embryo is transferred to the fallopian tube instead of the uterus. ZIFT procedure was primarily advocated for non tubal factor infertility, offers the appropriate physiological environment for the growing zygote/embryo (avoiding the in vitro culture systems), with an optimal synchronization between embryonic and endometrial development. Moreover, since ZIFT prevents intrauterine manipulation and the consequent possible embryo expulsion secondary to sub-endometrial/myometrial contractions, ZIFT was also offered to patients with repeated implantation failure 1.
Gamete intrafallopian transfer (GIFT) is similar to in-vitro fertilization (IVF), but the gametes (egg and sperm) are transferred to the woman’s fallopian tubes rather than her uterus, and fertilization takes place in the tubes rather than in the lab. Another difference is that laparoscopy, a surgical procedure, is necessary to transfer the sperm and egg to the tubes. GIFT (gamete intrafallopian transfer) is an option only for women who have normal fallopian tubes. Some couples may consider GIFT for religious reasons because eggs are not fertilized outside the body. One limitation of GIFT (gamete intrafallopian transfer) is that fertilization cannot be confirmed as with IVF. Today, GIFT comprises less than 1% of assisted reproductive technology (ART) procedures performed in the United States.
In-vitro fertilization (IVF) is a method of assisted reproduction in which a man’s sperm and a woman’s eggs are combined outside of the body in a laboratory dish. One or more fertilized eggs (embryos) may be transferred into the woman’s uterus, where they may implant in the uterine lining and develop. Excess embryos may be cryopreserved (frozen) for future use. Initially, IVF was used to treat women with blocked, damaged, or absent fallopian tubes. Today, IVF is used to treat many causes of infertility, such as endometriosis and male factor, or when a couple’s infertility is unexplained. The basic steps in an IVF treatment cycle are ovarian stimulation, egg retrieval, fertilization, embryo culture, and embryo transfer. These are discussed in the following sections.
Since ZIFT is supposed to improve the physiological environment for the growing zygote/embryo and to offer optimal conditions for embryonic development, those who might benefit from ZIFT procedure are patients with poor in-vitro embryo development 1. The observation by Levran et al. 2, together with their later study 3 that demonstrated significantly higher implantation and pregnancy rate in patients with repeated implantation failure undergoing ZIFT as compared to extended culture with blastocyst stage transfer, actually contradict the aforementioned assumption.
Levran et al. 2 evaluated the efficacy of ZIFT on implantation and pregnancy rates in patients with repeated failure of implantation in IVF-embryo transfer cycles. Data on 70 patients who underwent 92 ZIFT cycles were compared to a control group consisting of patients with the same selection criteria who underwent an additional standard in IVF-embryo transfer cycle during the same time period. The implantation and pregnancy rates (8.7%% and 34.2%, respectively) were significantly higher in the ZIFT group than in the control group. Moreover, in contrary to other authors observation, patients who persistently had low-quality embryos in previous IVF-embryo transfer cycles had relatively low pregnancy rates per transfer in the ZIFT cycles, as compared to those with better quality embryos.
In contrary to Levran et al. 2, Aslan et al. 4 failed to demonstrate significant benefits from ZIFT procedure over IVF-embryo transfer in patients with repeated implantation failure, observation that was in agreement with Habana and Palter 5, who conducted a meta-analysis, demonstrating similar pregnancy and implantation rates in ZIFT and IVF-embryo transfer groups. These observations probably reflect a selection bias, resulting from the nowadays no established criteria for ZIFT.
In a recently published updated data from the same group, Weissman et al. 6 have summarized their experience with 280 laparoscopic ZIFT. They achieved 96 clinical pregnancies per attempt (34.3%) and 72 live births (25.7%). However, when referring to the issue of when and under which circumstances should ZIFT be offered to patients with repeated implantation failure? they could not provide any prerequisite criteria. They concluded that whenever a thorough evaluation revealed no clear and treatable reason and the following therapeutic interventions i.e. mechanical irritation of the endometrium; preimplantation genetic screening; assisted hatching; co-culture; intracytoplasmic morphologically selected sperm injection; and blastocyst transfer failed to achieve pregnancy, ZIFT could be the next step.
According to Gat et al. 1, ZIFT should be offered to young repeated failure of implantation patients (≤31 yrs), who underwent ≤6 cycle attempts, and who achieved over eight 2 pronuclei embryos with low (≤0.4) ratio of number of top-quality embryo to total 2 pronuclei embryos in their previous IVF-embryo transfer cycle. Moreover, in those destined for a ZIFT cycle, only those with >7 2 pronuclei embryo should undergo a transfer of at least five 2 pronuclei embryos, otherwise, a trans cervical embryo transfer should be undertaken. However, further large prospective studies are needed to elucidate the role of ZIFT approach in patients with repeated IVF failures patients and to identify the specific characteristics of women that will aid both fertility specialists’ counseling and their patients in adjusting the appropriate infertility treatment.
Figure 1. ZIFT procedure
ZIFT procedure
The basic steps in an IVF treatment cycle are ovarian stimulation, egg retrieval, fertilization, embryo culture, and embryo transfer. These are discussed in the following sections.
Ovarian stimulation
During ovarian stimulation, also known as ovulation induction, medications or “fertility drugs,” are used to stimulate multiple eggs to grow in the ovaries rather than the single egg that normally develops each month. Multiple eggs are stimulated because some eggs will not fertilize or develop normally after fertilization.
Medications for ovarian stimulation
- Human Menopausal Gonadotropin (hMG)
- Follicle-Stimulating Hormone (FSH)
- Luteinizing Hormone (LH) (used in conjuction with FSH)
- Human Chorionic gonadotropin (hCG)
- Clomiphene citrate
- Letrozole
Medications to prevent premature ovulation
- Gonadotropin-releasing hormone (GnRH) agonists
- Gonadotropin-releasing hormone (GnRH) antagonists
Clomiphene citrate and letrozole are administered orally while the other medications listed are given by injection. These oral medications are less potent than injectable medications and are not as commonly used in assisted reproductive technology (ART) cycles. There is no evidence that one injectable medication is superior to any other. Timing is crucial in an IVF cycle. The ovaries are evaluated during treatment with vaginal ultrasound examinations to monitor the development of ovarian follicles. Blood samples are drawn to measure the response to ovarian stimulation medications. Normally, estrogen levels increase as the follicles develop, and progesterone levels are low until after ovulation.
Using ultrasound examinations and blood testing, the physician can determine when the follicles are ready for egg retrieval. Generally, 8 to 14 days of stimulation are required. When the follicles are ready, human chorionic gonadotropin (hCG) or other medications are given. The hCG replaces the woman’s natural luteinizing hormone (LH) surge and causes the final stage of egg maturation so the eggs are capable of being fertilized. The eggs are retrieved before ovulation occurs, usually 34 to 36 hours after the hCG injection is given.
Up to 20% of cycles may be cancelled prior to egg retrieval. IVF cycles may be cancelled for a variety of reasons, usually due to an inadequate number of follicles developing. Cancellation rates due to low response to the ovulation drugs increase with a woman’s age, especially after age 35. When cycles are cancelled due to a poor response, alternate drug strategies may be helpful to promote a better response in a future attempt. Occasionally, a cycle may be cancelled to reduce the risk of ovarian hyperstimulation syndrome. Treatment with a gonadotropin-releasing hormone (GnRH) agonist or antagonist reduces the possibility of premature luteinizing hormone (LH) surges from the pituitary gland, and thereby reduces the risk of premature ovulation. However, luteinizing hormone (LH) surges and ovulation occur prematurely in a small percentage of ART cycles despite the use of these drugs. When this occurs, since it is unknown when the LH surges began and eggs will mature, the cycle is usually cancelled. Collection of eggs from the peritoneal cavity after ovulation is not efficient.
Egg retrieval
Egg retrieval is usually accomplished by transvaginal ultrasound aspiration, a minor surgical procedure that can be performed in the physician’s office or an outpatient center. Some form of pain medication is generally administered. An ultrasound probe is inserted into the vagina to identify the follicles, and a needle is guided through the vagina and into the follicles (Figure 2).
The eggs are aspirated (removed) from the follicles through the needle connected to a suction device. Removal of multiple eggs can usually be completed in less than 30 minutes. Some women experience cramping on the day of the retrieval, but this sensation usually subsides by the next day. Feelings of fullness and/or pressure may last for several weeks following the procedure because the ovaries remain enlarged. In some circumstances, one or both ovaries may not be accessible by transvaginal ultrasound.
Laparoscopy may then be used to retrieve the eggs using a small telescope placed in the umbilicus.
Figure 2. Egg retrieval
Donor Sperm, Eggs, and Embryos
IVF may be performed with a couple’s own eggs and sperm or with donor eggs and sperm, or both. A couple may choose to use a donor if there is a problem with their own sperm or eggs, or if they have a genetic disease that could be passed on to a child. Donors may be known or anonymous. In most cases, donor sperm is obtained from a sperm bank. Both sperm and egg donors undergo extensive medical and genetic screening, as well as testing for infectious diseases. Sexually transmitted disease screening and testing for both sperm and egg donation are highly regulated by the U.S. Food and Drug Adminstration (FDA).
Donor sperm is frozen and quarantined for six months, the donor is re-tested for infectious diseases including the human immunodeficiency virus (HIV), and sperm are only released for use if all tests are negative. Donor sperm may be used for insemination or in an ART cycle. Unlike intrauterine insemination cycles, the use of frozen sperm in IVF cycles does not lower the chance of pregnancy.
Donor eggs are an option for women with a uterus who are unlikely or unable to conceive with their own eggs. Egg donors undergo much the same medical and genetic screening as sperm donors. Until recently, it has not been possible to freeze and quarantine eggs like sperm. Recent advances in oocyte freezing, though, have made this a possibility, and there are a few companies and clinics that are using such an approach. The egg donor may be chosen by the infertile couple or the ART program. Egg donors assume more risk and inconvenience than sperm donors. In the United States, egg donors selected by ART programs generally receive monetary compensation for their participation. Egg donation is more complex than sperm donation and is done as part of an IVF procedure. The egg donor must undergo ovarian stimulation and egg retrieval. During this time, the recipient (the woman who will receive the eggs after they are fertilized) receives hormonal medications to prepare her uterus for implantation. After the retrieval, the donor’s eggs are fertilized by sperm from the recipient’s partner and transferred to the recipient’s uterus. The recipient will not be genetically related to the child, but she is a biologic parent in the sense that she will carry the pregnancy and give birth. Egg donation is expensive because donor selection, screening, and treatment add additional costs to the IVF procedure. However, the relatively high live birth rate for egg donation, over 50% nationally, provides many couples with their best chance for success. Overall, donor eggs are used in nearly 10% of all ART cycles in the United States.
In some cases, when both the man and woman are infertile, both donor sperm and eggs have been used. Donor embryos may also be used in these cases. Some IVF programs allow couples to donate their unused frozen embryos to other infertile couples. Appropriate screening of the individuals whose genetic embryos are used should adhere to federal and state guidelines. The use of donor sperm, eggs, or embryos is a complicated issue that has lifelong implications. Talking with a trained counselor who understands donor issues can be very helpful in the decision-making process. Many programs have a mental health professional on staff or the physician may recommend one. If a couple knows the donor, their physician may suggest that both the couple and the donor speak with a counselor and an attorney. Some states require and most IVF centers recommend an attorney to file paperwork for the couple with the court when donor gametes or embryos are used.
Fertilization and Embryo Culture
After the eggs are retrieved, they are examined in the laboratory for maturity and quality. Mature eggs are placed in an IVF culture medium and transferred to an incubator to await fertilization by the sperm.
Sperm is separated from semen usually obtained by masturbation or in a special condom used during intercourse. Alternatively, sperm may be obtained from the testicle, epididymis, or vas deferens from men whose semen is void of sperm either due to an obstruction or lack of production.
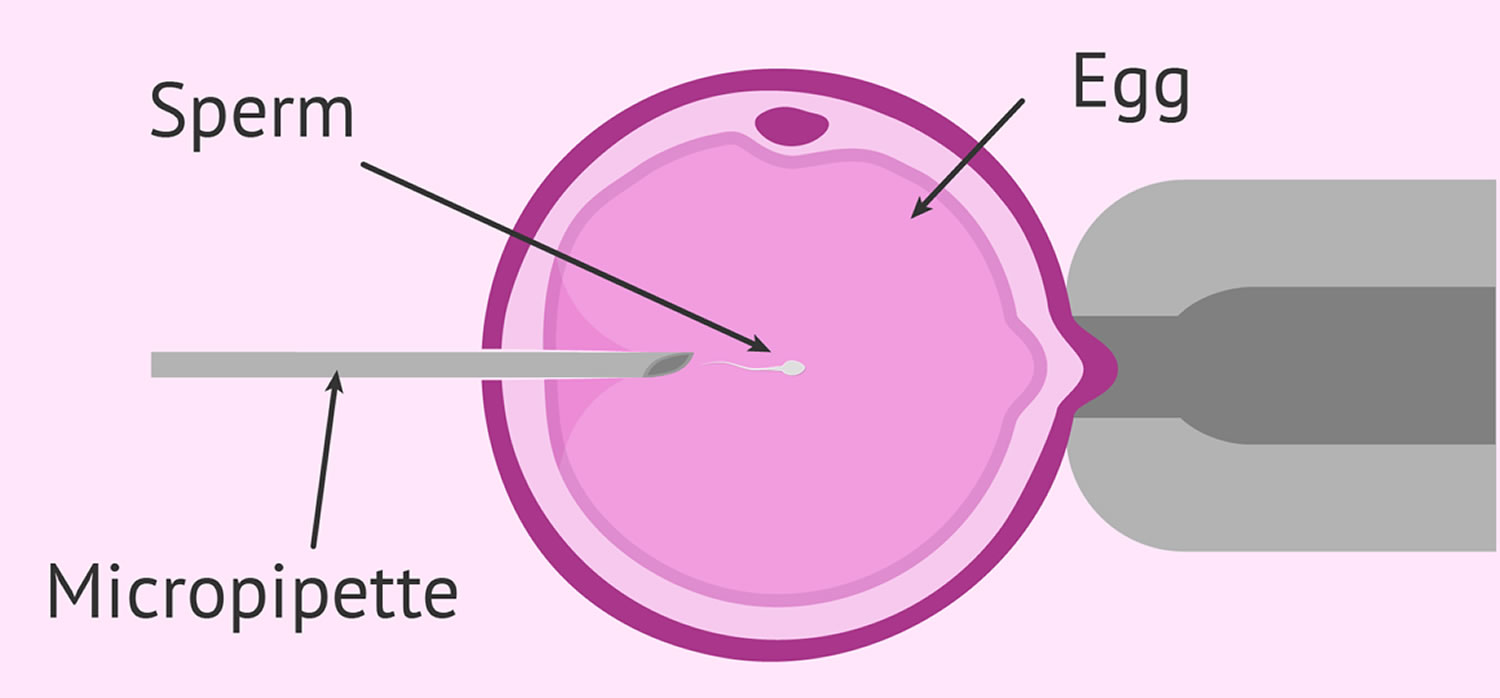
Fertilization may be accomplished by insemination, where motile sperm are placed together with the oocytes and incubated overnight or by intracytoplasmic sperm injection (ICSI), where a single sperm is directly injected into each mature egg (Figure 3). In the United States, ICSI is performed in approximately 60% of ART cycles. ICSI is usually performed when there is a likelihood of reduced fertilization (e.g., poor semen quality, history of failed fertilization in a prior IVF cycle). Overall, pregnancy and delivery rates with ICSI are similar to the rates seen with traditional IVF. Genetic counseling is advisable before ICSI if inherited abnormalities are identified that may be passed from father to son.
Visualization of two pronuclei the following day confirms fertilization of the egg. One pronucleus is derived from the egg and one from the sperm. Usually 65% to 75% of mature eggs will fertilize after insemination or ICSI. Lower rates may occur if the sperm and/or egg quality are poor. Occasionally, fertilization does not occur at all, even if ICSI was used. Two days after the egg retrieval, the fertilized egg has divided to become a 2- to 4-cell embryo.
By the third day, a normally developing embryo will contain approximately 6 to 10 cells. By the fifth day, a fluid cavity forms in the embryo, and the placenta and fetal tissues begin to separate. An embryo at this stage is called a blastocyst. Embryos may be transferred to the uterus at any time between one and six days after the egg retrieval. If successful development continues in the uterus, the embryo hatches from the surrounding zona pellucida and implants into the lining of the uterus approximately 6 to 10 days after the egg retrieval.
Assisted hatching is a micromanipulation procedure in which a hole is made in the zona pellucida just prior to embryo transfer to facilitate hatching of the embryo. Although assisted hatching has not been demonstrated definitively to improve live birth rates, assisted hatching may be used for older women or couples who have had unsuccessful prior IVF attempts. There is no clear benefot of assisted hatching to improve pregnancy or live birth rates in other groups of IVF patients.
Preimplantation genetic diagnosis (PGD) is performed at some centers to screen for inherited diseases. In preimplantation genetic diagnosis, one or two cells are removed from the developing embryo and tested for a specific genetic disease. Embryos that do not have the gene associated with the disease are selected for transfer to the uterus.
These procedures require specialized equipment and experience together with IVF (in a couple who may otherwise not need IVF to conceive). Some couples, especially those who are carriers of genetic diseases, consider embryo screening beneficial in reducing the risk of having an affected child. While preimplantation genetic diagnosis can reduce the likelihood of conceiving a pregnancy with an affected child, it cannot eliminate the risk. Confirmation with chorionic villus sampling (CVS), amniocentesis, or other testing is still necessary.
Figure 3. Intracytoplasmic sperm injection (ICSI)
Embryo transfer
The next step in the IVF process is the embryo transfer. No anesthesia is necessary, although some women may wish to have a mild sedative. The physician identifies the cervix using a vaginal speculum. One or more embryos suspended in a drop of culture medium are drawn into a transfer catheter (a long, thin sterile tube) with a syringe on one end. The physician gently guides the tip of the transfer catheter through the cervix and places the fluid containing the embryos either into the uterine cavity or into the fallopian tube in the case of ZIFT or GIFT. The procedure is usually painless, although some women experience mild cramping.
The maximum number of embryos transferred is based on the patient’s age and other individual patient and embryo characteristics. Since each embryo has a fair probability of implantation and development, the number of embryos to be transferred should be determined for each patient, taking into account the odds of achieving a pregnancy based on the number of embryos transferred weighed against the risk of multiple gestation. These guidelines have been effective in helping U.S. ART programs maintain their high success rates while significantly decreasing the number of high-order multiple pregnancies (triplets and higher). The reproductive endocrinologist or embryologist will discuss this with the patient prior to the transfer.
Cryopreservation
Extra embryos remaining after the embryo transfer may be cryopreserved (frozen) for future transfer. Cryopreservation makes future ART cycles simpler, less expensive, and less invasive than the initial IVF cycle, since the woman does not require ovarian stimulation or egg retrieval. Once frozen, embryos may be stored for prolonged periods, and live births have been reported using embryos that have been frozen for almost 20 years. However, not all embryos survive the freezing and thawing process, and the live birth rate is lower with cryopreserved embryo transfer. Couples should decide if they are going to cryopreserve extra embryos before undergoing IVF. There are two methods used to cryopreserve embryos: conventional (slow) freezing and “vitrification” or fast freezing. Your center will determine which method is best to use based on their experience and the developmental stage at which the embryos are frozen. Although some reports claim that vitrification may have higher success rates after thawing/warming, this is not the case at all centers.
It should also be noted that more and more ART centers are cryopreserving oocytes (eggs) prior to fertilization. This is done most commonly in young women who are about to undergo treatments or procedures that may affect their future fertility, such as chemotherapy for cancer. However, it is also used for couples who do not wish to freeze embryos because of concerns over their survival during freezing and thawing or the dilemma of what to do with remaining embryos after they have completed their families. Clinic success rates may vary.
Finally, it should be noted that although there are theoretical risks, freezing of sperm, eggs, and embryos is very safe. There have been no documented cases of infectious disease transmission, nor do the risks of birth defects, chromosomal anomalies, or pregnancy complications appear to be increased compared with using fresh sperm, eggs, or embryos.
- Gat I, Levron J, Yerushalmi G, Dor J, Brengauz M, Orvieto R. Should zygote intrafallopian transfer be offered to all patients with unexplained repeated in-vitro fertilization cycle failures?. J Ovarian Res. 2014;7:7. Published 2014 Jan 20. doi:10.1186/1757-2215-7-7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3922731[↩][↩][↩]
- Levran D, Mashiach S, Dor J, Levron J, Farhi J. Zygote intrafallopian transfer may improve pregnancy rate in patients with repeated failure of implantation. Fertil Steril. 1998;69:26–30. doi: 10.1016/S0015-0282(97)00452-4[↩][↩][↩]
- Levran D, Farhi J, Nahum H, Royburt M, Glezerman M, Weissman A. Prospective evaluation of blastocyst stage transfer vs. zygote intrafallopian tube transfer in patients with repeated implantation failure. Fertil Steril. 2002;77:971–977. doi: 10.1016/S0015-0282(02)03080-7[↩]
- Aslan D, Elizur SE, Levron J, Shulman A, Lerner-Geva L, Bider D, Dor J. Comparison of zygote intrafallopian tube transfer and transcervical uterine embryo transfer in patients with repeated implantation failure. Eur J Obstet Gynecol Reprod Biol. 2005;122:191–194. doi: 10.1016/j.ejogrb.2005.05.005[↩]
- Habana A, Palter SF. Is tubal embryo transfer of any value? A meta analysis and comparison with the society for assisted reproductive technology database. Fertil Steril. 2001;76:286–293. doi: 10.1016/S0015-0282(01)01879-9[↩]
- Weissman A, Horowitz E, Ravhon A, Nahum H, Golan A, Levran D. Zygote intrafallopian transfer among patients with repeated implantation failure. Int J Gynaecol Obstet. 2013;120:70–73. doi: 10.1016/j.ijgo.2012.07.018[↩]