Contents
- What is juvenile rheumatoid arthritis
- Types of Juvenile Idiopathic Arthritis
- Juvenile rheumatoid arthritis prognosis
- Juvenile rheumatoid arthritis causes
- Complications of juvenile rheumatoid arthritis
- Juvenile rheumatoid arthritis symptoms and signs
- Diagnosis of juvenile rheumatoid arthritis
- Juvenile rheumatoid arthritis treatment
- Treatment of Uveitis
- Medications
- History of Arthritis in 4 or Fewer Joints
- History of Arthritis in 5 or More Joints
- Active Sacroiliac Arthritis
- Systemic Arthritis with Active Systemic Features and without Active Arthritis
- Systemic Arthritis with Active Arthritis and without Active Systemic Features
- Physical Therapies
- Lifestyle and home remedies for juvenile rheumatoid arthritis
- Surgical Treatment
- Coping and support for juvenile rheumatoid arthritis
What is juvenile rheumatoid arthritis
Juvenile rheumatoid arthritis is an older deprecated term that has been replaced by the International League of Associations for Rheumatology 1 as Juvenile Idiopathic Arthritis. Juvenile idiopathic arthritis is a heterogeneous group of conditions which encompasses all forms of arthritis of unknown etiology lasting for at least 6 weeks and with onset before the age of 16 years 2. As a result of the lack of characteristic features, the diagnosis of Juvenile Idiopathic Arthritis is one of exclusion among all possible causes of chronic arthritis in childhood.
Juvenile rheumatoid arthritis causes persistent joint pain, swelling and stiffness. Some children may experience symptoms for only a few months, while others have symptoms for the rest of their lives.
Some types of juvenile rheumatoid arthritis can cause serious complications, such as growth problems and eye inflammation. Treatment of juvenile rheumatoid arthritis focuses on controlling pain, improving function and preventing joint damage.
History findings in children with juvenile idiopathic arthritis may include the following:
- Arthritis present for at least 6 weeks before diagnosis (mandatory for diagnosis of juvenile idiopathic arthritis)
- Either insidious or abrupt disease onset, often with morning stiffness or gelling phenomenon and arthralgia during the day
- Complaints of joint pain or abnormal joint use
- History of school absences or limited ability to participate in physical education classes
- Spiking fevers occurring once or twice each day at about the same time of day
- Evanescent rash on the trunk and extremities
- Psoriasis or more subtle dermatologic manifestations
Physical findings are important to provide criteria for diagnosis and to detect abnormalities suggestive of alternative etiologies, as well as to indicate disease subtypes. Such findings include the following:
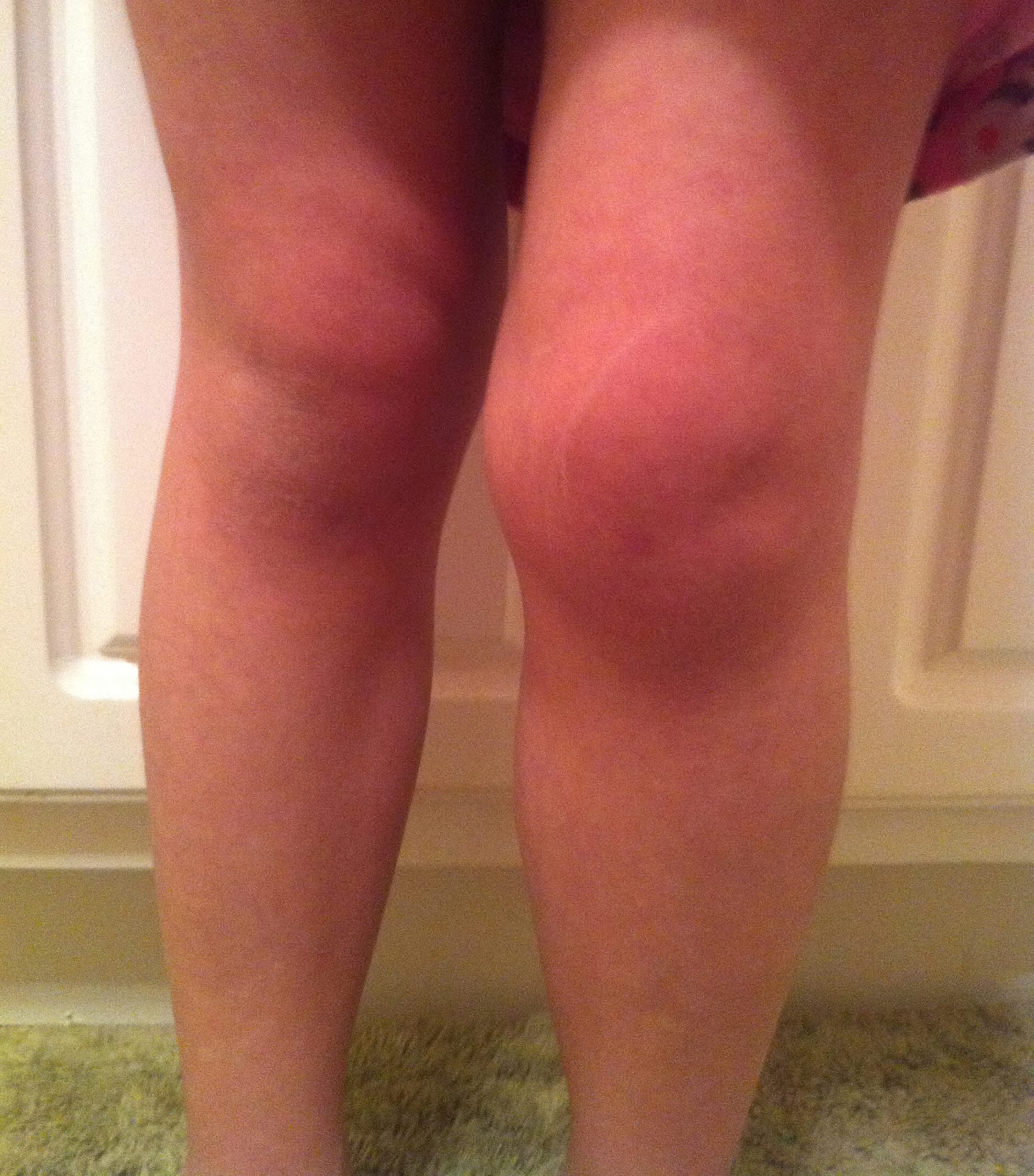
- Arthritis: Defined either as intra-articular swelling on examination or as limitation of joint motion in association with pain, warmth, or erythema of the joint; physical findings in JIA reflect the extent of joint involvement
- Synovitis: Characterized by synovial proliferation and increased joint volume; the joint is held in a position of maximum comfort, and range of motion often is limited only at the extremes
Types of Juvenile Idiopathic Arthritis
Juvenile Idiopathic Arthritis usually appears in kids between 6 months and 16 years old. The first signs often are joint pain or swelling or warm joints. Many rheumatologists (doctors specializing in joint disorders) find that the greater the number of joints affected, the more severe the disease and the less likely that the symptoms will eventually go into total remission. Remission is a medical term for temporary or permanent recovery.
There are seven types of Juvenile Idiopathic Arthritis:
- Systemic Juvenile Idiopathic Arthritis (formerly known as systemic juvenile rheumatoid arthritis). Systemic means the arthritis can affect the whole body, rather than just a specific organ or joint. A child has arthritis with, or that was preceded by, a fever that has lasted for at least 2 weeks. The fever has come and gone, but spiked, or hit its highest temperature, for at least 3 days. The fever occurs with at least one or more of the following:
- Generalized enlargement of the lymph nodes.
- Enlargement of the liver or spleen.
- Inflammation of the lining of the heart (pericarditis) or the lungs (pleuritis).
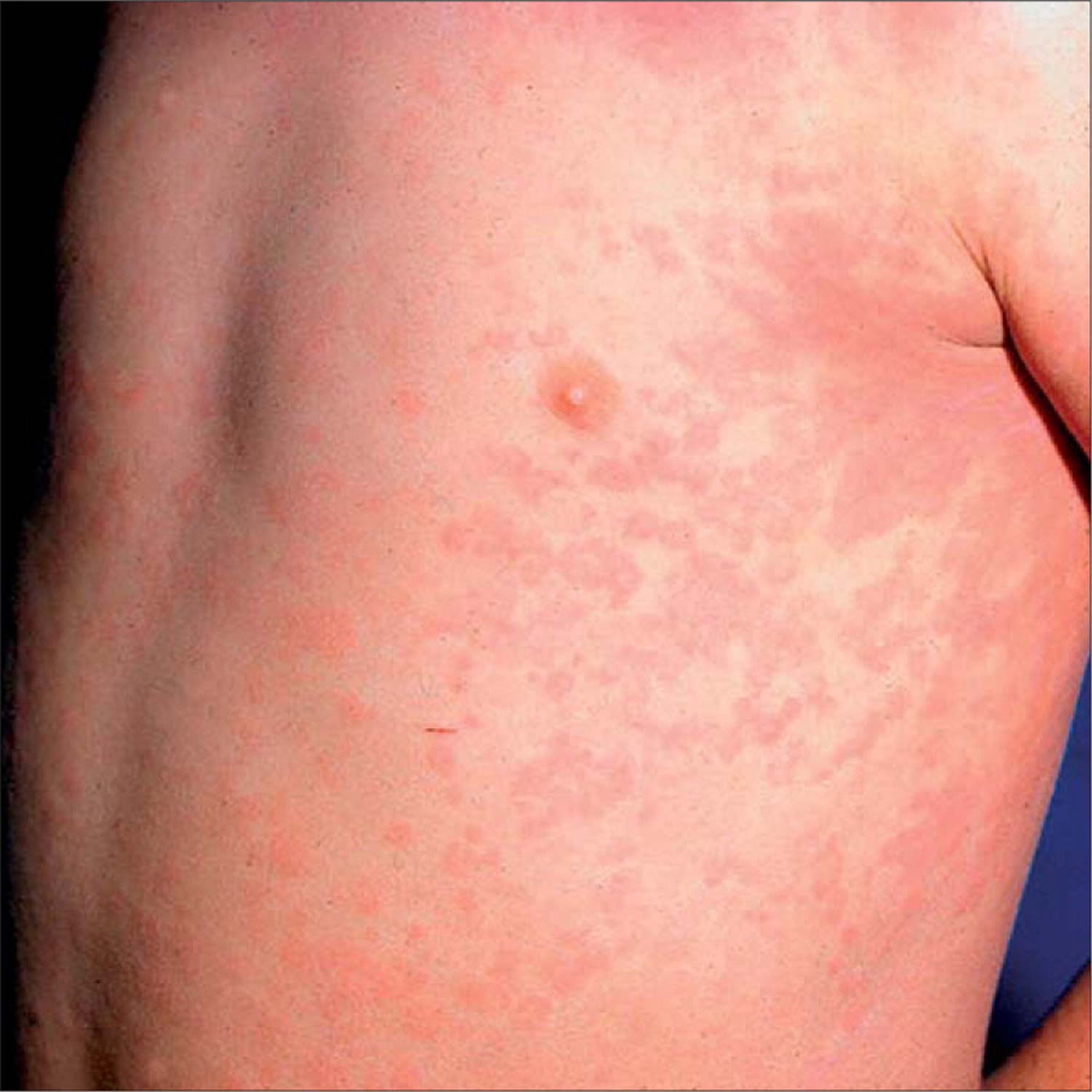
- The characteristic rheumatoid rash, which is flat, pale, pink, and generally not itchy. The individual spots of the rash are usually the size of a quarter or smaller. The rash may suddenly disappear and then quickly appear again. They are present for a few minutes to a few hours, and then disappear without any changes in the skin. Rashes may suddenly appear and disappear, developing in one area and then another.
- Symptoms include high fevers that often increase in the evenings and then may suddenly drop to normal. During the onset of fever, the child may feel very ill, appear pale, or develop a rash. High fevers that tend to increase in the evenings and disappear are characteristic of systemic Juvenile Idiopathic Arthritis. Eventually many of the body’s joints are affected by swelling, pain, and stiffness.
- Oligoarthritis or Oligoarticular juvenile idiopathic arthritis. Affects four or fewer joints, often the knee or ankle. Symptoms include pain, stiffness, or swelling in the joints. The two types of oligoarthritis, persistent and extended, are determined by how many joints are ultimately involved.
- Polyarticular arthritis, rheumatoid factor negative. A child has arthritis in five or more joints during the first 6 months of disease, and all tests for rheumatoid factor (proteins produced by the immune system that can attack healthy tissue, which are commonly found in rheumatoid arthritis and juvenile arthritis) are negative. About 1 in 4 kids and teens with juvenile idiopathic arthritis have polyarthritis, which affects more girls than boys. Symptoms include swelling or pain in five or more joints. The small joints of the hands are affected as well as the weight-bearing joints like the knees, hips, ankles, feet, and neck. A low-grade fever also might develop, as well as bumps or nodules in areas of the body subjected to pressure from sitting or leaning.
- Polyarticular arthritis, rheumatoid factor positive. A child has arthritis in five or more joints during the first six months of the disease. Also, at least two tests for rheumatoid factor, at least three months apart, are positive. This type of juvenile idiopathic arthritis behaves the most like adult rheumatoid arthritis, and kids who have it have a protein called rheumatoid factor (RF) or anti-cyclic citrullinated peptide (CCP antibody) in their blood. Kids with polarticular Juvenile Idiopathic Arthritis are at a higher risk of joint damage with erosions than in the other forms of juvenile idiopathic arthritis.
- Psoriatic arthritis. Kids with this also have the psoriasis rash (a scaly red rash that can start behind the ears, on the eyelids, elbows, knees, or scalp) themselves or a close relative with psoriasis. Their fingernails and toenails might be affected by the condition. A child has both arthritis and psoriasis (a skin disease), or has arthritis and at least two of the following:
- Inflammation and swelling of an entire finger or toe (this is called dactylitis)
- Nail pitting or splitting
- A first-degree relative with psoriasis.
- Enthesitis-related arthritis. The enthesis is the point at which a ligament, tendon, or joint capsule attaches to the bone. If this point becomes inflamed, it can be tender, swollen, and painful with use. Enthesitis-related arthritis includes a special group call juvenile ankylosing spondylitis (where joints of the low back are inflamed) and arthritis associated with inflammatory bowel disease (Crohn’s disease and ulcerative colitis). The most common locations are around the knee and at the Achilles tendon on the back of the ankle. A child is diagnosed with this condition if he or she has both arthritis and inflammation of an enthesitis site, or has either arthritis or enthesitis with at least two of the following:
- Inflammation of the sacroiliac joints (at the bottom of the back) or pain and stiffness in the lumbosacral area (in the lower back).
- A positive blood test for the human leukocyte antigen (HLA) B27 gene.
- Onset of arthritis in males after age six years.
- A first-degree relative diagnosed with ankylosing spondylitis, enthesitis-related arthritis, or inflammation of the sacroiliac joint in association with inflammatory bowel disease or acute inflammation of the eye.
- Undifferentiated arthritis. Arthritis that doesn’t fit into any of the above categories or fits into more than one of the categories.
The first signs of arthritis, which can be subtle or obvious, include limping or a sore wrist, finger, or knee. Joints may suddenly swell and remain enlarged. Stiffness in the neck, hips, or other joints also can occur.
- Inflammation of the iris (the colored area of the eye) may happen with or without active joint symptoms in any type of juvenile rheumatoid arthritis. This inflammation, more likely to happen in girls than boys, is called iridocyclitis, iritis, or uveitis. Kids and teens with juvenile rheumatoid arthritis should see an ophthalmologist (an eye doctor) regularly to check for this.
Appreciation of the different types and their hallmarks is particularly important to accurate diagnosis, which is determined by exclusion of other known disease entities in children with chronic arthritis (more than three months’ duration). Therapy should be directed at the arthritis per se (synovitis), at the extra-articular manifestations, and at the whole child. Salicylates provide the most satisfactory control of the arthritis per se and of the systemic manifestations in most cases. Iridocyclitis should be managed in consultation with an ophthalmologist. Patients should not be regarded as invalids or restricted needlessly. The prognosis for children with juvenile rheumatoid arthritis is good. In most patients, the disease remits without causing permanent joint damage.
Juvenile rheumatoid arthritis prognosis
Children with only a few affected joints may have no symptoms for a long period.
In many children, the disease will become inactive and cause very little joint damage.
The severity of the disease depends on the number of affected joints. It is less likely that symptoms will go away in these cases. These children more often have long-term (chronic) pain, disability, and problems at school. Some children may continue to have arthritis as adults.
Possible Complications of juvenile rheumatoid arthritis
Complications may include:
- Wearing away or destruction of joints (can occur in people with more severe juvenile rheumatoid arthritis)
- Slow rate of growth
- Uneven growth of an arm or leg
- Loss of vision or decreased vision from chronic uveitis (this problem may be severe, even when the arthritis is not very severe)
- Anemia
- Swelling around the heart (pericarditis)
- Long-term (chronic) pain, poor school attendance
Figure 1. Juvenile rheumatoid arthritis
Figure 2. Juvenile rheumatoid arthritis rash
Juvenile rheumatoid arthritis causes
Juvenile rheumatoid arthritis occurs when the body’s immune system attacks its own cells and tissues. It’s unknown why this happens, but both heredity and environment seem to play a role. Certain gene mutations may make a person more susceptible to environmental factors — such as viruses — that may trigger the disease.
Risk factors for juvenile rheumatoid arthritis
Some forms of juvenile rheumatoid arthritis are more common in girls.
Complications of juvenile rheumatoid arthritis
Several serious complications can result from juvenile rheumatoid arthritis. But keeping a careful watch on your child’s condition and seeking appropriate medical attention can greatly reduce the risk of these complications:
Eye problems. Some forms of juvenile rheumatoid arthritis can cause eye inflammation (uveitis). If this condition is left untreated, it may result in cataracts, glaucoma and even blindness.
Eye inflammation frequently occurs without symptoms, so it’s important for children with juvenile rheumatoid arthritis to be examined regularly by an ophthalmologist.
Growth problems. Juvenile rheumatoid arthritis can interfere with your child’s growth and bone development. Some medications used to treat juvenile rheumatoid arthritis, mainly corticosteroids, also can inhibit growth.
Juvenile rheumatoid arthritis symptoms and signs
The most common signs and symptoms of juvenile rheumatoid arthritis are:
- Pain. While your child might not complain of joint pain, you may notice that he or she limps — especially first thing in the morning or after a nap.
- Swelling. Joint swelling is common but is often first noticed in larger joints like the knee.
- Stiffness. You might notice that your child appears clumsier than usual, particularly in the morning or after naps.
Juvenile rheumatoid arthritis can affect one joint or many. In some cases, juvenile rheumatoid arthritis affects the entire body — causing swollen lymph nodes, rashes and fever.
Like other forms of arthritis, juvenile rheumatoid arthritis is characterized by times when symptoms flare up and times when symptoms disappear.
Diagnosis of juvenile rheumatoid arthritis
Diagnosis of juvenile rheumatoid arthritis can be difficult because joint pain can be caused by many different types of problems. The diagnosis of juvenile idiopathic arthritis is based on the history and physical examination findings. No single test can confirm a diagnosis, but tests can help rule out some other conditions that produce similar signs and symptoms. Indeed, all laboratory study findings may be normal in children with this disorder.
However, laboratory studies help to exclude other underlying disorders, classify the type of arthritis, and evaluate for extra-articular manifestations of juvenile idiopathic arthritis. Imaging of affected joints is usually indicated.
When physical findings do not document definite arthritis, further evaluation is warranted. The choice of studies varies on the basis of the specific circumstances.
Blood tests
Some of the most common blood tests for suspected cases of juvenile rheumatoid arthritis include:
- Erythrocyte sedimentation rate (ESR). Sedimentation rate is the speed at which your red blood cells settle to the bottom of a tube of blood. An elevated rate can indicate inflammation. Measuring the ESR may be used to rule out other conditions, to help classify the type of juvenile rheumatoid arthritis and to determine the degree of inflammation.
- C-reactive protein. This blood test also measures levels of general inflammation in the body but on a different scale than the ESR.
- Anti-nuclear antibody (ANA). Anti-nuclear antibodies are proteins commonly produced by the immune systems of people with certain autoimmune diseases, including arthritis.
- Rheumatoid factor. This antibody is commonly found in the blood of children who have rheumatoid arthritis.
- Cyclic citrullinated peptide (CCP). Like the rheumatoid factor, the cyclic citrullinated peptide is another antibody that may be found in the blood of children with rheumatoid arthritis.
In many children with juvenile rheumatoid arthritis, no significant abnormality will be found in these blood tests.
Imaging scans
When only a single joint is affected, radiography is important to exclude other diseases. Basic radiographic changes in juvenile rheumatoid arthritis include the following:
- Soft tissue swelling
- Osteopenia or osteoporosis
- Joint-space narrowing
- Bony erosions
- Intra-articular bony ankylosis
- Periosteitis
- Growth disturbances
- Epiphyseal compression fracture
- Joint subluxation
- Synovial cysts
Imaging may also be used from time to time after the diagnosis to monitor bone development and to detect joint damage.
Other imaging modalities that may be helpful include the following:
- Computed tomography (CT scan)
- Magnetic resonance imaging (MRI)
- Ultrasonography and echocardiography
- Nuclear imaging
Other studies and procedures that may be considered include the following:
- Dual-energy radiographic absorptiometry (DRA)
- Arthrocentesis and synovial biopsy
- Pericardiocentesis
Juvenile rheumatoid arthritis treatment
The ultimate goals in managing juvenile rheumatoid arthritis are to prevent or control joint damage, to prevent loss of function, and to decrease pain 3. These goals are particularly important in juvenile idiopathic arthritis, in which the rate of progression and the onset of debility can be rapid. Juvenile idiopathic arthritis is a chronic disease characterized by periods of remission and flare. Treatment is aimed at inducing remission with the least toxicity from medications with hopes of inducing a permanent remission.
The success of therapy is monitored best with repeated physical examinations and history. The number of joints involved and the duration of morning stiffness should demonstrate continued decrease, with elimination reflecting success. Surgery may be indicated in patients who are unresponsive to medical therapy.
A team-based approach can be helpful. Management may include one or all of the following areas:
- Pharmacologic management consisting of nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), biologic agents 4 and intra-articular and oral steroids
- Psychosocial factors, including counseling for patients and parents
- School performance, such as academic counseling, school-life adjustments, and physical education adjustments
- Nutrition, particularly to address anemia and generalized osteoporosis; often microcytic, anemia is refractive to treatment with iron
- Physical therapy to relieve pain and to address range of motion, muscle strengthening, activities of daily living, and conditioning exercises
- Occupational therapy, including joint protection, a program to relieve pain, range of motion, and attention to activities of daily living
Treatment for juvenile rheumatoid arthritis focuses on helping your child maintain a normal level of physical and social activity. To accomplish this, doctors may use a combination of strategies to relieve pain and swelling, maintain full movement and strength, and prevent complications.
American College of Rheumatology (ACR) criteria for complete remission are as follows 3:
- No inflammatory joint pain
- No morning stiffness
- No fatigue
- No synovitis
- No progression of damage, as determined in sequential radiographic examinations
- No elevation of the ESR and CRP level
The American College of Rheumatology recommends treatment approaches to juvenile rheumatoid arthritis on the basis of the following 5 treatment groups 5:
- A history of arthritis in 4 or fewer joints
- A history of arthritis in 5 or more joints
- Active sacroiliac arthritis
- Systemic arthritis without active arthritis
- Systemic arthritis with active arthritis
Within each group, choice of therapy is guided by the severity of disease activity and the presence or absence of features indicating a poor prognosis.
Within each treatment group, choice of therapy is guided by the severity of disease activity and the presence or absence of features indicating a poor prognosis.
In September 2013, the American College of Rheumatology released updated guidelines for the treatment of systemic juvenile rheumatoid arthritis, which included the medications canakinumab, rilonacept, and tocilizumab 6. These guidelines include the following treatment recommendations:
- For systemic juvenile rheumatoid arthritis with active systemic features and varying degrees of synovitis, initial treatment for most patients should consist of anakinra with systemic glucocorticoids
- For systemic juvenile rheumatoid arthritis without active systemic features and with varying degrees of active synovitis, initial treatment should be methotrexate or leflunomide for an active joint count higher than 4, with a change to abatacept, anakinra, a tumor necrosis factor (TNF)-α inhibitor, or tocilizumab if disease activity continues after 3 months; for patients with 4 or fewer active joints, NSAID monotherapy or intra-articular glucocorticoid injections should be initial treatment
- For systemic juvenile rheumatoid arthritis with features suggesting macrophage activation syndrome (MAS), initial treatment should include anakinra, a calcineurin inhibitor, or systemic glucocorticoid monotherapy for up to 2 weeks.
Treatment of Macrophage Activation Syndrome
Macrophage activation syndrome (MAS) is a rare but important complication of systemic-onset juvenile rheumatoid arthritis in which numbers of all 3 bloodlines become rapidly decreased. Hypofibrinogenemia, thrombocytopenia, and elevated aspartate aminotransferase levels are hallmarks.
Macrophage activation syndrome (MAS) often responds to cyclosporin A, and some case reports have detailed a response to anakinra. Treatment of macrophage activation syndrome (MAS) is a medical emergency and should be performed by physicians familiar with this complication.
Treatment of Uveitis
Uveitis is often asymptomatic. Patients are typically young girls who have positive levels of ANA.
Treatment with topical corticosteroid medication and with mydriatic agents (to prevent closed-angle glaucoma) often can prevent progression of disease to development of calcium deposition in the lens (band keratopathy) and adhesions of the iris to the lens (posterior synechiae), in which an irregular pupillary margin develops. Such complications may herald a chronic active disease in which vision is threatened.
Immunosuppressive agents, such as methotrexate or cyclosporine, may help control chronic uveitis. Infliximab can be effective in some patients who are resistant to immunosuppressive agents.
A multicenter, double-blind, randomized, placebo-controlled trial by Ramanan et al 7 reported that adalimumab plus methotrexate therapy controlled inflammation and was associated with a lower rate of treatment failure than placebo among children and adolescents with active juvenile rheumatoid arthritis-associated uveitis. The study observed 16 treatment failures out of 60 patients (27%) in the adalimumab group compared to 18 treatment failures in 30 patients (60%) in the placebo group. The study also reported a higher rate of adverse events (eg, oropharyngeal pain, cough, arthralgia) in the adalimumab group (10.07 events per patient-year vs. 6.51 events per patient-year) as well as a higher rate of serious adverse events (eg, infections or infestations [0.29 events per patient-year vs. 0.19 events per patient-year]) 7.
Medications
For some children, pain relievers may be the only medication needed. Other children may need help from medications designed to limit the progression of the disease.
Typical medications used for juvenile rheumatoid arthritis include:
Nonsteroidal anti-inflammatory drugs (NSAIDs). These medications, such as ibuprofen (Advil, Motrin IB, others) and naproxen sodium (Aleve), reduce pain and swelling. Stronger NSAIDs are available by prescription. Side effects include stomach upset and liver problems.
Disease-modifying antirheumatic drugs (DMARDs). Doctors use these medications when NSAIDs alone fail to relieve symptoms of joint pain and swelling.
They may be taken in combination with NSAIDs and are used to slow the progress of juvenile rheumatoid arthritis. Commonly used DMARDs for children include methotrexate (Trexall) and leflunomide (Arava).
Side effects may include nausea and liver problems.
Biologic agents. Also known as biologic response modifiers, this newer class of drugs includes tumor necrosis factor (TNF) blockers, such as etanercept (Enbrel) and adalimumab (Humira). These medications can help reduce pain, morning stiffness and swollen joints.
But these types of drugs increase the risk of infections. There may also be a mild increase in the chance of getting some cancers, such as lymphoma.
Other biologic agents work to suppress the immune system, including abatacept (Orencia), rituximab (Rituxin), anakinra (Kineret) and tocilizumab (Actemra).
Corticosteroids. Medications such as prednisone may be used to control symptoms until a DMARD takes effect or to prevent complications, such as inflammation of the sac around the heart (pericarditis).
Corticosteroids may be administered by mouth or by injection directly into a joint. But these drugs can interfere with normal growth and increase susceptibility to infection, so they generally should be used for the shortest possible duration.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs) interfere with prostaglandin synthesis through inhibition of the enzyme cyclooxygenase (COX), thus reducing swelling and pain. NSAIDs are used to treat all subtypes of juvenile rheumatoid arthritis. They may help with pain and decrease swelling. Commonly used NSAIDs include naproxen, ibuprofen, tolmetin, diclofenac, and indomethacin.
- Aspirin is no longer the drug of first choice because of the increased frequency of gastric toxicity and hepatotoxicity when compared to other NSAID medications. The cyclooxygenase 2 (COX-2) inhibitor celecoxib (Celebrex) may have a role in treatment when a bleeding diathesis is a potential problem.
Several dozen NSAIDs are available and can be classified into different groups of compounds. Commonly used NSAIDs include ibuprofen, naproxen, ketoprofen, piroxicam, and diclofenac. Predicting which patient will respond to a particular NSAID is not possible and many children who do not respond to one may benefit by changing to a different NSAID. Indomethacin is particularly effective for fever and pericarditis and is usually preferred for children with active systemic juvenile rheumatoid arthritis.
Meloxicam (Mobic)
Meloxicam is a member of the enolic class of NSAIDs and is structurally related to piroxicam. The pediatric dosage is 0.125 mg/kg/d for patients aged 2 years or older, up to 7.5 mg qd.
Naproxen (Aleve, Naprelan, Naprosyn)
Naproxen is used for analgesic and anti-inflammatory properties, treating arthralgia and arthritis. It inhibits inflammatory reactions and pain by decreasing activity of cyclooxygenase, which is responsible for prostaglandin synthesis. The pediatric dosage is 7-20 mg/kg/d PO divided bid/tid, not to exceed 1 g/d
Ibuprofen (Advil, Motrin)
Ibuprofen inhibits inflammatory reactions and pain by decreasing prostaglandin synthesis. The adult dosage is 400 mg PO q4-6h, 600 mg q6h, or 800 mg q8h while symptoms persist, not to exceed 3.2 g/d; the pediatric dosage is 30-50 mg/kg/d PO divided qid, not to exceed 2.4 g/d.
Diclofenac (Voltaren, Cataflam)
This is one of a series of phenylacetic acids that has demonstrated anti-inflammatory and analgesic properties in pharmacological studies. It is believed to inhibit the enzyme cyclooxygenase, which is essential in the biosynthesis of prostaglandins. Diclofenac can cause hepatotoxicity; hence, liver enzymes should be monitored in the first 8 weeks of treatment. It is absorbed rapidly; metabolism occurs in the liver by demethylation, deacetylation, and glucuronide conjugation. The delayed-release, enteric-coated form is diclofenac sodium, and the immediate-release form is diclofenac potassium.
Celecoxib (Celebrex)
Celecoxib inhibits primarily COX-2. Inhibition of COX-1 may contribute to NSAID gastrointestinal (GI) toxicity. At therapeutic concentrations, COX-1 isoenzyme is not inhibited; thus, incidence of GI toxicity, such as endoscopic peptic ulcers, bleeding ulcers, perforations, and obstructions, may be decreased when compared with nonselective NSAIDs.
Seek the lowest dose for each patient. The adult dosage is 100-200 mg PO bid; the pediatric dosage has not been established for patients younger than 2 years, is 50 mg PO bid for patients 2 years or older whose weight is ≥10 kg to ≤25 kg, and is 100 mg PO bid for patients 2 years or older whose weight is greater than 25 kg.
Tolmetin (Tolectin)
Tolmetin inhibits prostaglandin synthesis by decreasing the activity of the enzyme cyclooxygenase, which in turn decreases formation of prostaglandin precursors. The pediatric dosage is 20 mg/kg/d PO divided tid/qid initially, then 15-30 mg/kg/d, not to exceed 30 mg/kg/d
Indomethacin (Indocin)
Indomethacin is rapidly absorbed, and metabolism occurs in the liver by demethylation, deacetylation, and glucuronide conjugation. It inhibits prostaglandin synthesis. The adult dosage is 25-50 mg PO bid/tid, not to exceed 200 mg/d, and the ER (extended release) product may be administered qd or bid; the pediatric dosage is 1-2 mg/kg/d PO divided bid/qid, not to exceed 4 mg/kg/d or 150-200 mg/d.
Disease-Modifying Antirheumatic Drugs
Disease-modifying antirheumatic drugs (DMARDs) can retard or prevent disease progression and, thus, joint destruction and subsequent loss of function. Successful DMARD therapy may eliminate the need for other anti-inflammatory or analgesic medications; however, until the full action of DMARDs takes effect, anti-inflammatory or analgesic medications may be required as bridging therapy to reduce pain and swelling.
Sulfasalazine (Azulfidine, EN-tabs)
Sulfasalazine decreases the inflammatory response and systemically inhibits prostaglandin synthesis. The pediatric dosage has not been established for patients younger than 6 years; for patients 6 years or older, the typical dose range is 30-50 mg/kg/d; to lessen GI irritation, start at one half to one third of maintenance dose, increasing the dose weekly, not to exceed 2 g/d.
Methotrexate (Rheumatrex, Trexall)
Methotrexate has an unknown mechanism of action in the treatment of inflammatory reactions; it may affect immune function. It ameliorates symptoms of inflammation (eg, pain, swelling, stiffness). Adjust the dose gradually to attain a satisfactory response. Consider SC route for patients who do not respond to PO methotrexate or who have GI intolerance to PO dosing.
The pediatric dosage is 10-25 mg/m2/wk PO/IM/SC as a single dose or divided into 2 doses weekly; many pediatric rheumatologists increase the dose (not to exceed 30 mg/m2, approximately equivalent to 1 mg/kg); administer with folic acid 1-2 mg PO qd or folinic acid 2.5-5 mg PO every wk.
Corticosteroids
Corticosteroids are potent anti-inflammatory drugs used in patients with juvenile rheumatoid arthritis to bridge the time until DMARDs are effective. Adverse events associated with long-term steroid use make dose reductions and cessation important in due course.
Methylprednisolone (Solu-Medrol, Medrol, A-Methapred)
Methylprednisolone decreases inflammation by suppressing migration of polymorphonuclear leukocytes and reversing increased capillary permeability. It is used temporarily for juvenile rheumatoid arthritis until longer-term treatment provides effective relief. The pediatric dosage is 15-30 mg/kg IV qd administered over 30-60 min for 2-3 d.
Prednisone
Prednisone is an immunosuppressant for treatment of juvenile rheumatoid arthritis. It may decrease inflammation by reversing increased capillary permeability and suppressing polymorphonuclear neutrophil activity, and it stabilizes lysosomal membranes and also suppresses lymphocytes and antibody production. The pediatric dosage is 4-5 mg/m2/d PO; alternatively, the dosage is 0.05-2 mg/kg PO divided bid/qid; taper over 2 wk as symptoms resolve and other antirheumatic drugs take effect.
Triamcinolone (Aristospan, Kenalog)
Triamcinolone decreases inflammation by suppressing the migration of polymorphonuclear leukocytes and reversing capillary permeability.
Immunomodulators
The recognition of tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)–1 as central proinflammatory cytokines has led to the development of agents that block these cytokines or their effects. In addition to improving signs and symptoms and quality of life, all biologic agents significantly retard radiographic progression of joint erosions. The TNF blockers, which bind TNF and thus prevent its interaction with its receptors, include etanercept, infliximab, and adalimumab. Consensus statements do not recommend their use until at least one xenobiotic DMARD, usually methotrexate (MTX), has been administered without sufficient success, although one study reported better results with etanercept in patients with less disability and when used before methotrexate 8.
Adverse effects associated with the biologic agents include the generation of antibodies against these compounds, emergence of antinuclear antibodies, occasional drug-induced lupuslike syndromes, and infections. Rarely, demyelinating disorders and bone marrow suppression occur. Acute and chronic infections, demyelinating disorders, class 3 or 4 heart failure, and recent malignancies are contraindications for TNF blockers. Thoroughly searching for latent tuberculosis using chest radiography and/or purified protein derivative testing is recommended before these agents are started 9.
Adalimumab (Humira, Amjevita, adalimumab-atto)
Adalimumab is a recombinant human IgG1 monoclonal antibody that is specific for human TNF. It reduces inflammation and inhibits progression of structural damage. The pediatric dosage has not been established for patients younger than 2 years. For patients age 2 years or older and between 10 kg and just under 15 kg, the dosage is 10 mg SC q2wk; for 15 kg to just under 30 kg, the dosage is 20 mg SC q2wk; and for 30 kg or more, the dosage is 40 mg SC q2wk.
Etanercept (Enbrel)
Etanercept acts by binding and inhibiting TNF, a cytokine that contributes to inflammatory and immune response. The pediatric dosage is not established for patients younger than 4 years. For patients 4-17 years, the dosage is 0.4 mg/kg SC 2 times weekly (administered at least 72-96 h apart), not to exceed 25 mg/dose. For patients older than 17 years, the dosage is administered as in adults.
Abatacept (Orencia)
Abatacept is a selective costimulation modulator that inhibits T-cell activation by binding to CD80 and CED86, thereby blocking CD28 interaction. It is indicated for reducing signs and symptoms of RA, slowing progression of structural damage and improving physical function in adults with moderate-to-severe rheumatoid arthritis who have inadequate response to DMARDs, MTX, or TNF antagonists. It is not recommended for concomitant use with anakinra because of insufficient experience.
The pediatric dosage is not established for patients younger than 6 years. For pediatric patients 6-17 years, the dosage is according to body weight, and the drug is administered on days 1, 15, and 29, then q4wk thereafter; infuse IV over 30 min. For pediatric patients less than or equal to 74 kg, use 10 mg/kg IV; for pediatric patients 75-100 kg, use 750 mg IV; and for pediatric patients heavier than 100 kg, use 1000 mg IV.
Anakinra (Kineret)
Anakinra competitively and selectively inhibits IL-1 binding to type I receptor (IL-1RI). By blocking IL-1 binding, inflammation and pain associated with rheumatoid arthritis are inhibited. It is indicated for rheumatoid arthritis in patients who have failed 1 or more DMARDs. The dose should be administered at approximately the same time every day. The adult dosage is 100 mg SC qd; the pediatric dosage has not been established.
Tocilizumab (Actemra)
Tocilizumab is an IL-6 receptor antagonist that inhibits IL-6 mediated signaling that results in decreased inflammatory cytokine production. It is indicated for systemic JIA and PJIA. The safety and efficacy of tocilizumab has not been established in patients younger than 2 years old.
Canakinumab (Ilaris)
Canakinumab is recombinant, human monoclonal antibody that inhibits interleukin-beta1.
History of Arthritis in 4 or Fewer Joints
According to American College of Rheumatology guidelines, the treatment group that comprises patients who have developed active arthritis in only 4 or fewer joints total throughout their disease course includes patients in the International League of Associations for Rheumatology categories of persistent oligoarthritis, as well as patients with psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis 10.
- In this treatment group, escalation of therapy typically proceeds from NSAIDs to intra-articular glucocorticoid injections to methotrexate to TNF-α inhibitors.
NSAIDs alone may be adequate for patients with involvement of a single joint and other indications of low disease activity (eg, normal inflammatory marker levels); response should be evident within 2 months. For other patients, NSAIDs may be used as adjunctive treatment, as needed.
Intra-articular injections of triamcinolone can be used for any joint involved with active arthritis, and should provide clinical relief for at least 4 months. If so, the injections can be repeated as needed.
Methotrexate can be instituted in patients who fail to show adequate response to NSAIDs and/or joint injections. Alternatively, methotrexate is recommended as initial treatment for patients in this treatment group who have high disease activity and features indicating poor prognosis. In patients with enthesitis-related juvenile rheumatoid arthritis, sulfasalazine rather than methotrexate is recommended for patients who have an inadequate response to joint injection or an adequate trial of NSAIDs.
Patients in this treatment group who fail to respond adequately to joint injections and to 3-6 months (depending on disease characteristics and severity) of methotrexate are candidates for TNF-α treatment. The same is true of patients with enthesitis-related juvenile rheumatoid arthritis who receive sulfasalazine.
History of Arthritis in 5 or More Joints
This group comprises patients who have developed active arthritis in 5 or more joints total throughout throughout their disease course. Patients need not currently have active involvement in 5 or more joints. According to American College of Rheumatology guidelines, this group includes patients with the International League of Associations for Rheumatology categories of extended oligoarthritis, rheumatoid factor (RF) negative and RF-positive polyarthritis, psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis 11.
Treatment in this group places less emphasis on initial NSAIDs. After 1 month of NSAID treatment in patients with low disease activity or 1-2 months in those with moderate disease activity but without poor prognostic features (ie, hip or cervical spine involvement, positive RF or anti-cyclic citrullinated peptide antibodies (CCP), radiographic signs of joint damage), it is appropriate to escalate to methotrexate, plus adjunctive NSAIDs and joint injection as needed.
In patients with moderate disease activity and poor prognostic features, as well as in patients with high disease activity, treatment may start with methotrexate.
Leflunomide may be used as an alternative to methotrexate, after a failed NSAID trial, or as initial treatment in patients with high disease activity and poor prognostic features.
The US Food and Drug Administration (FDA) has approved the interleukin (IL)-6 inhibitor tocilizumab for the treatment of polyarticular juvenile rheumatoid arthritis in children 2 years of age and older with active disease. Tocilizumab can be used alone or in combination with methotrexate 12.
Escalation to a TNF-α inhibitor follows if 3-6 months (depending on disease characteristics and severity) of methotrexate or leflunomide provides inadequate control. Patients who show inadequate response after 3-4 months (depending on disease characteristics and severity) of TNF-α inhibitor treatment can be switched to a different TNF-α inhibitor or to abatacept. If these agents prove inadequate, patients may be started on rituximab; this agent may be most appropriate in patients with RF-positive polyarticular juvenile rheumatoid arthritis.
A study confirmed the acceptable long-term tolerability of etanercept and adalimumab treatment in polyarticular juvenile idiopathic arthritis. However, the authors also add that whether the onset of inflammatory bowel disease and uveitis during etanercept monotherapy is a paradoxical effect or an inadequate response to therapy remains unclear and requires further investigation in this growing cohort 13.
Active Sacroiliac Arthritis
This group includes all patients with clinical and imaging evidence of active sacroiliac arthritis. It may include patients from any of the International League of Associations for Rheumatology juvenile idiopathic arthritis categories.
Use of a TNF-α inhibitor is recommended more readily for patients in this group. A TNF-α inhibitor may be started after failure of an adequate trial of NSAIDs or after 3-6 months (depending on disease characteristics and severity) of methotrexate or sulfasalazine proves inadequate.
Systemic Arthritis with Active Systemic Features and without Active Arthritis
This group includes all patients who fulfill the International League of Associations for Rheumatology criteria for systemic arthritis and who have active fever of systemic juvenile rheumatoid arthritis with or without other systemic features, but without active arthritis 10. A 2-week trials of NSAIDs may be used in patients who have fever and less severe disease, and have had significant active systemic disease for less than 6 months; after that, patients should be started on systemic glucocorticoids, with adjunct NSAIDs as needed.
Patients with high systemic disease activity (eg, significant serositis) may be started on steroids as a first step. There is virtually no published evidence regarding steroid doses or administration routes in this setting.
Patients who sustain or develop active fever while on systemic steroid therapy can be started on anakinra. This agent may be a first choice in patients who have had significant active systemic disease for at least 6 months.
The FDA has approved tocilizumab for the treatment of systemic juvenile rheumatoid arthritis. Clinical trials in children with juvenile rheumatoid arthritis showed significantly fewer disease flares when treated with tocilizumab compared with placebo (26% vs 48%). Additionally, a higher success rate of steroid reduction/discontinuance was achieved in the tocilizumab group (24%) compared with placebo (3%) 14.
The phase III TENDER study demonstrated that tocilizumab was effective in improving the signs and symptoms of systemic juvenile rheumatoid arthritis. After 3 months of treatment, 85% of participants who took tocilizumab had a 30% improvement (juvenile rheumatoid arthritis ACR30+) in the signs and symptoms of systemic juvenile rheumatoid arthritis and absence of fever, compared with 24% of patients who received placebo.
Further data showed 70% of patients on tocilizumab achieved juvenile rheumatoid arthritis ACR70+ and 37% achieved juvenile rheumatoid arthritis ACR90+, compared with 8% and 5% of patients who received placebo, respectively. Additionally, nearly two-thirds of patients in the study were free of rash after 3 months. In this study, the safety profile similar to adults treated with tocilizumab for rheumatoid arthritis 15.
The interleukin-1 β inhibitor canakinumab has also been FDA-approved for systemic juvenile rheumatoid arthritis 16. In the beta-SPECIFIC 1 trial, 84% of patients with systemic juvenile idiopathic arthritis treated with canakinumab experienced at least a 30% improvement in symptoms compared with 10% in the placebo group after 15 days of treatment 17.
In the open-label beta-SPECIFIC 2 trial, 45% of canakinumab-treated patients who were prescribed corticosteroids were able to reduce steroid use, and one-third of patients completely discontinued corticosteroids. Additionally, the canakinumab-treated patients were nearly 3 times less likely to experience a new flare, with 74% remaining flare-free compared to 25% with placebo 17.
Systemic Arthritis with Active Arthritis and without Active Systemic Features
This category includes all patients who fulfill the International League of Associations for Rheumatology criteria for systemic arthritis and who have active arthritis, but who do not have active systemic features 10. Most of these patients are started on NSAIDs while their diagnostic assessment progresses.
NSAID therapy, with intra-articular joint injections as needed, may be adequate for patients with low disease activity who do not have hip involvement or radiographic signs of joint damage. After up to 1 month, however, methotrexate can be added for patients with any degree of disease severity who continue to have active arthritis.
After 3 months of methotrexate therapy, the next step in escalation is to anakinra or a TNF-α inhibitor, although etanercept is less effective in systemic arthritis than in other forms of juvenile rheumatoid arthritis 8. Patients who show inadequate response to TNF-α inhibitor treatment can be started on abatacept.
Physical Therapies
Your doctor may recommend that your child work with a physical therapist to help keep joints flexible and maintain range of motion and muscle tone.
A physical therapist or an occupational therapist may make additional recommendations regarding the best exercise and protective equipment for your child.
A therapist may also recommend that your child make use of joint supports or splints to help protect joints and keep them in a good functional position.
Surgery
In very severe cases of juvenile rheumatoid arthritis, surgery may be needed to improve the position of a joint.
Lifestyle and home remedies for juvenile rheumatoid arthritis
Encourage patients to be as active as possible. Bed rest is not a part of the treatment. In fact, the more active the patient, the better the long-term prognosis. Children may experience increased pain during routine physical activities. As a result, these children must be allowed to self-limit their activities, particularly during physical education classes. A consistent physical therapy program, with attention to stretching exercises, pain modalities, joint protection, and home exercises, can help ensure that patients are as active as possible.
Caregivers can help children learn self-care techniques that help limit the effects of juvenile rheumatoid arthritis. Techniques include:
- Getting regular exercise. Exercise is important because it promotes both muscle strength and joint flexibility. Swimming is an excellent choice because it places minimal stress on joints.
- Applying cold or heat. Stiffness affects many children with juvenile rheumatoid arthritis, particularly in the morning. Although some children respond well to cold packs, most children prefer a hot pack or a hot bath or shower.
- Eating well. Some children with arthritis have poor appetites. Others may gain excess weight due to medications or physical inactivity. A healthy diet can help maintain an appropriate body weight.
Adequate calcium in the diet is important because children with juvenile rheumatoid arthritis are at risk of developing weak bones (osteoporosis) due to the disease, the use of corticosteroids, and decreased physical activity and weight bearing.
Exercise and Other Nonpharmacologic Therapy
Exercise preserves joint range of motion and muscular strength, and it protects joint integrity by providing better shock absorption. Types of exercises that may be advised include a muscle-strengthening program, range-of-motion activity, stretching of deformities, and endurance and recreational exercises. Hydrotherapy is a good form of exercise that helps achieve the aforementioned objectives.
Rarely, children require splinting or serial casting to help decrease contractures in joints unresponsive to medical treatment.
Leg-length discrepancy can result from neovascularization of growth plates of an affected knee. The problem may not be detected in patients with a knee flexion contracture until the contracture is corrected. Treatment consists of a shoe lift on the contralateral side.
In a single-blind, randomized, controlled trial involving 60 children with juvenile rheumatoid arthritis, Coda and colleagues found that the use of inexpensive prefabricated fitted foot orthoses reduced pain and improved quality of life compared with use of sham orthoses. Changes in pain were measured with a visual analogue scale and quality of life was measured with the Pediatric Quality of Life questionnaire. The study included 60 children with lower joint involvement beginning at age 5-18 years, previous failure of orthotic management with no use of orthoses for at least 3 months, the ability to walk 15 meters without assistive devices, and a 6-month history of disease-modifying antirheumatic drugs. At 3- and 6-month follow-ups, children in the fitted orthoses group experienced significantly greater pain reduction and improvements in quality of life than controls.
Surgical Treatment
Advances in medical treatment have reduced the need for surgical intervention in juvenile rheumatoid arthritis. Possible procedures include synovectomy, osteotomy and arthrodesis, and hip and knee replacement.
Synovectomy
Synovectomy is rarely needed, and long-term outcome is poor; however, it may be used in children in whom a single joint or just a few joints are involved and who have very active, proliferative synovitis.
Osteotomy and arthrodesis
Osteotomy and arthrodesis are salvage procedures for patients whose juvenile rheumatoid arthritis is associated with severe joint destruction or deformity.
Arthrodesis is superior to arthroplasty for children who have rheumatic disease in the wrist and fingers and in the ankle.
Total hip and total knee replacements
Total hip and knee replacements provide excellent relief of pain and restore function in a functionally disabled child with debilitating disease.
The role of total hip replacement and total knee replacement in juvenile rheumatoid arthritis is fraught with problems, however. Joint replacement is usually delayed until bone growth has completed, as indicated by epiphyseal closure.
Coping and support for juvenile rheumatoid arthritis
Family members can play critical roles in helping a child cope with juvenile rheumatoid arthritis. As a parent, you may want to try the following:
- Treat your child, as much as possible, like other children in your family.
- Allow your child to express anger about having juvenile rheumatoid arthritis. Explain that the disease isn’t caused by anything he or she did.
- Encourage your child to participate in physical activities, keeping in mind the recommendations of your child’s doctor and physical therapist.
- Discuss your child’s condition and the issues surrounding it with teachers and administrators at his or her school.
- Juvenile rheumatoid arthritis. https://www.ncbi.nlm.nih.gov/medgen/811462[↩]
- Juvenile idiopathic arthritis. Ravelli A, Martini A. Lancet. 2007 Mar 3; 369(9563):767-78. https://www.ncbi.nlm.nih.gov/pubmed/17336654/[↩]
- American College of Rheumatology, Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002 Feb. 46(2):328-46.[↩][↩]
- Tarp S, Amarilyo G, Foeldvari I, Christensen R, Woo JM, Cohen N, et al. Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials. Rheumatology (Oxford). 2015 Nov 30. [↩]
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, Dewitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011 Apr. 63(4):465-82. [↩]
- Ringold S, Weiss PF, Beukelman T, et al. 2013 Update of the 2011 American College of Rheumatology Recommendations for the Treatment of Juvenile Idiopathic Arthritis: Recommendations for the Medical Therapy of Children With Systemic Juvenile Idiopathic Arthritis and Tuberculosis Screening Among Children Receiving Biologic Medications. Arthritis Rheum. Oct 2013. 65 (10):2499–2512.[↩]
- Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med. 2017 Apr 27. 376 (17):1637-1646.[↩][↩]
- Otten MH, Prince FH, Armbrust W, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011 Dec 7. 306(21):2340-7.[↩][↩]
- Actemra (tocilizumab) prescribing information [package insert]. South San Francisco, CA: Genentech, Inc. April 2013.[↩]
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, Dewitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011 Apr. 63(4):465-82.[↩][↩][↩]
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, Dewitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011 Apr. 63(4):465-82.[↩]
- Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=125276[↩]
- Klotsche J, Niewerth M, Haas JP, Huppertz HI, Zink A, Horneff G, et al. Long-term safety of etanercept and adalimumab compared to methotrexate in patients with juvenile idiopathic arthritis (JIA). Ann Rheum Dis. 2015 Apr 29.[↩]
- De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012 Dec 20. 367(25):2385-95.[↩]
- Efficacy and safety of tocilizumab in patients with systemic Juvenile Idiopathic Arthritis (sJIA): 12-week data from the phase 3 TENDER trial. Abstract presented on June 18, 2010.[↩]
- Lowes R. FDA approves Ilaris for rare juvenile arthritis. Medscape Medical News. May 10, 2013.[↩]
- Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012 Dec 20. 367(25):2396-406.[↩][↩]