Contents
What is uremia
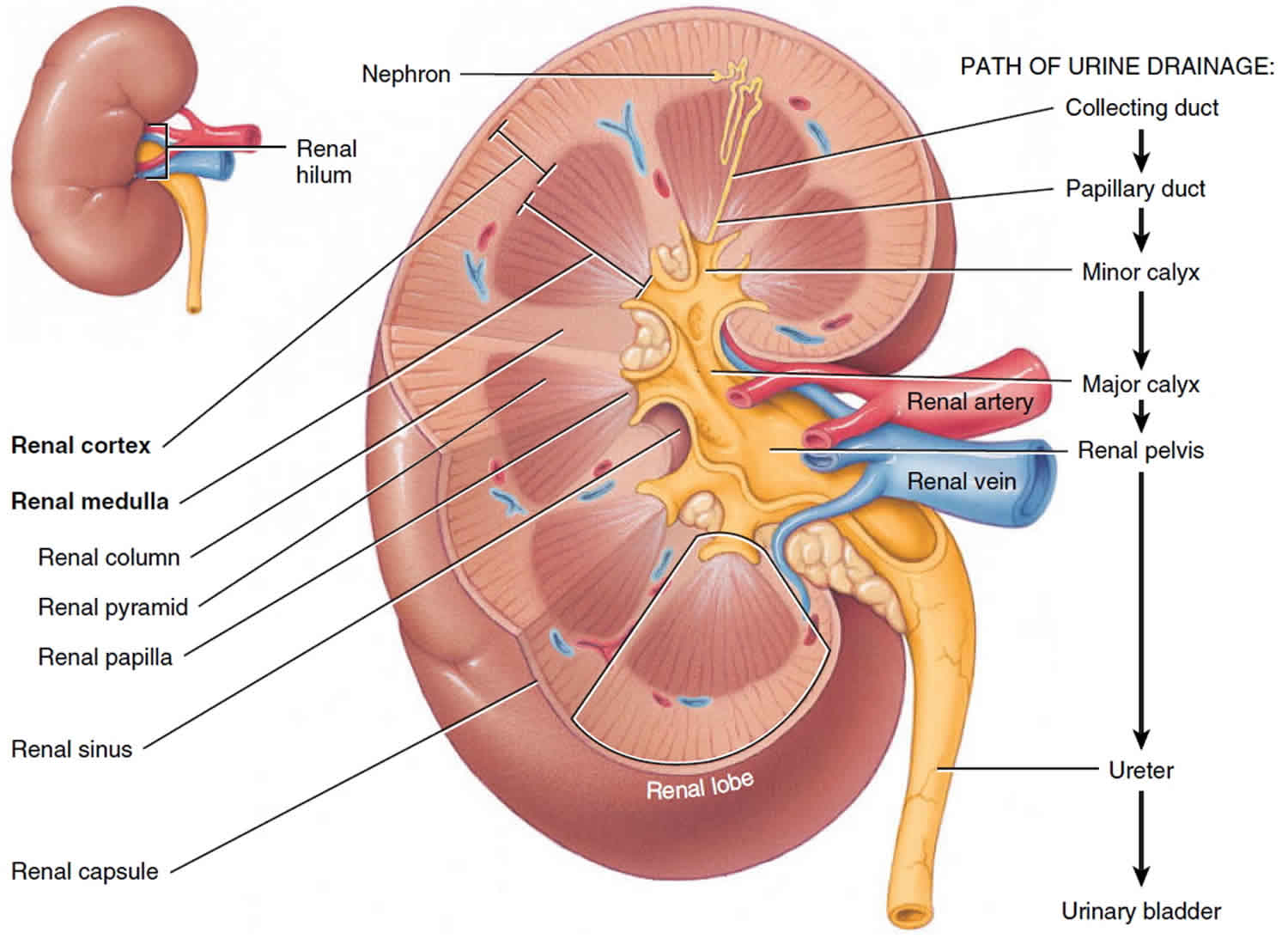
Uremia is a serious clinical condition associated with worsening kidney function and it is characterized by fluid, electrolyte, and hormone imbalances in addition to metabolic abnormalities 1. The literal meaning of uremia is “urine in the blood,” and the condition develops most commonly in the setting of chronic and end-stage renal disease (ESRD), but may also occur as a result of acute kidney injury.
Putative uremic toxins include parathyroid hormone, macroglobulin, advanced glycosylation end products, and beta2 microglobulin, though no specific uremic toxin has been identified as responsible for all clinical manifestations of uremia 2.
There are many symptoms of uremia that occur as kidney function declines. Before severe uremia develops, treatments such as dialysis and transplantation are usually needed. The timing of dialysis will depend on your symptoms and varies from person to person.
Uremia causes
Kidney disease can result from some conditions ranging from primary renal disorders, for example IgA nephropathy, focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, polycystic kidney disease to systemic disorders that can lead to renal damage. Systematic disorders can include diabetes mellitus, lupus, multiple myeloma, amyloidosis, Goodpasture disease, Thrombotic thrombocytopenic purpura, or hemolytic uremic syndrome.
The leading cause of end-stage renal disease (ESRD) in the United States is diabetes. Additional causes, listed in order of decreasing incidence, include:
- Hypertension,
- Glomerulonephritis,
- Interstitial disease,
- Cystitis, and
- Neoplasms.
Uremia may also result from acute kidney injury if the injury involves a sudden increase in urea or creatinine 3.
It is difficult to determine the exact prevalence of uremia in the United States because patients with end-stage renal disease (ESRD) typically begin dialysis before the development of uremic symptoms. Uremic symptoms typically arise once creatinine clearance is less than 10mL/min or 15mL/min in the case of diabetic patients.
There are approximately 354 out of a million individuals diagnosed with the end-stage renal disease each year. This number continues to rise as the life expectancy of those with end-stage renal disease (ESRD) is increasing. Improved survival in patients with diabetes or cardiovascular disease, in addition to increased access to renal therapy, has resulted in the highest increase in the incidence of end-stage renal disease (ESRD) in patients aged 75 years or above. On the other hand, the number of individuals under the age of 60 with end-stage renal disease (ESRD) is declining, except for African American or Native American patients with diabetic end-stage renal disease (ESRD).
The majority of patients with end-stage renal disease (ESRD) are Caucasian (59.8%), the remainder is African American (33.2%), Asian (3.6%) or Native American (1.6%). The incidence of end-stage renal disease (ESRD) among black individuals, however, is 3.7 times higher than it is among the white population. Similarly, the incidence among Native Americans is 1.8 times greater than it is among whites.
Additionally, minority populations tend to initiate dialysis care at a later point in the course of renal disease, usually once there is already a significant decline in glomerular filtration rate (GFR). It is unknown, however, whether racial or ethnic background has an effect on predisposition to the development of uremic symptoms.
Men are 1.2 times more likely than women to develop end-stage renal disease (ESRD), though women are 1.7 times more likely to delay initiation of dialysis. Women are also more prone to development of uremic symptoms at lower creatinine levels, due to the decreased amount of muscle mass and baseline serum creatinine levels that they have 4.
Uremia pathophysiology
When the kidneys are not functioning properly, dysfunction can occur in acid-base homeostasis, fluid and electrolyte regulation, hormone production and secretion, and waste elimination. Altogether, these abnormalities can result in metabolic disturbances and ultimately conditions such as anemia, hypothyroidism, hypertension, acidemia, hyperkalemia, and malnutrition.
Anemia associated with kidney disease is typically normocytic, normochromic, and hyperproliferative. It occurs as a result of decreased erythropoietin production by the failing kidneys. This is associated with a glomerular filtration rate (GFR) of less than 50 mL/min (unless the patient has diabetes, then they may have anemia at GFR less than 60mL/min) or when serum creatinine is greater than 2 mg/mL.
Additional factors associated with chronic kidney disease alone may additionally contribute to the development of anemia. These include iron or vitamin deficiencies, hyperparathyroidism, hypothyroidism, or a decreased lifespan of red blood cells.
The buildup of uremic toxins in the blood may additionally contribute to the development of coagulopathy as a result of reduced platelet adhesion to the vascular endothelial wall, increased platelet turnover, and a slightly reduced absolute number of platelets. A common finding in patients with end-stage renal disease (ESRD) is bleeding diathesis which is the increased susceptibility to bleeding and hemorrhage.
Another major metabolic complication associated with uremia and end-stage renal disease (ESRD) is acidosis because renal tubular cells are the primary regulators of acid-base homeostasis in the body. As kidney failure progresses, there is decreased secretion of hydrogen ions and impaired excretion of ammonium, and eventually buildup of phosphate and additional organic acids (e.g., lactic acid, sulfuric acid, hippuric acid). In turn, the resulting increased anion-gap metabolic acidosis may lead to hyperventilation, lethargy, anorexia, muscle weakness, and congestive heart failure (due to a decreased cardiac response).
Hyperkalemia may also occur in the setting of both acute or chronic renal failure. This condition becomes a medical emergency when serum potassium reaches a level greater than 6.5 mEq/L. This level may be exacerbated with excessive potassium intake or use of certain medications (e.g., potassium-sparing diuretics, angiotensin-converting enzymes (ACE) inhibitors, angiotensin-receptor blockers, beta blockers, NSAIDs). Acidosis resulting from renal failure may additionally contribute to the development of hyperkalemia.
Hypocalcemia, hyperphosphatemia, and elevated parathyroid hormone levels may additionally occur as a result of renal failure. Hypocalcemia occurs due to decreased production of active vitamin D (1,25 dihydroxyvitamin D) which is responsible for gastrointestinal (GI) absorption of calcium and phosphorus and suppression of parathyroid hormone excretion. Hyperphosphatemia occurs because of impaired phosphate excretion in the setting of renal failure. Both hypocalcemia and hyperphosphatemia stimulate hypertrophy of the parathyroid gland and resultant increased production and secretion of parathyroid hormone. Altogether, these changes in calcium metabolism can result in osteodystrophy (renal bone disease) and may lead to calcium deposition throughout the body (i.e., metastatic calcification).
Declining renal function can result in decreased insulin clearance, necessitating a decrease in dosage of antihyperglycemic medications to avoid hypoglycemia. Uremia may also lead to impotence in men or infertility (e.g., anovulation, amenorrhea) in women as a result of dysfunctional reproductive hormone regulation.
The buildup of uremic toxins may also contribute to uremic pericarditis, and pericardial effusions leading to abnormalities in cardiac function. Together with metastatic calcification as a result of declining renal function, these may contribute to worsening of underlying valvular dysfunction or suppression of myocardial contractility 5.
Uremia signs and symptoms
Uremia signs and symptoms may include:
- Weight loss
- Fatigue
- Weakness
- Nausea
- Vomiting
- Bad taste in the mouth
- Loss of appetite
- Restless legs
- Shortness of breath
- Forgetfulness
- Leg cramps
- Difficulty sleeping
- Itching
- Cold intolerance
- Chest pain
- Skin color changes
- Easy bruising
- Decreased sexual desire
- Swelling in ankles and legs
Symptomatic uremia tends to occur once creatinine clearance decreases below 10 mL/min unless kidney failure develops acutely, in which case, some patients may become symptomatic at higher clearance rates.
Patients presenting with uremia typically complain of nausea, vomiting, fatigue, anorexia, weight loss, muscle cramps, pruritus, or changes in mental status. The clinical presentation of uremia can be explained by the metabolic disturbances associated with the condition.
Fatigue as a result of anemia is considered one of the major components of the uremic syndrome.
Patients with a history of diabetes may report improved glycemic control but are at a greater risk of developing hypoglycemic episodes as kidney function worsens.
Hypertension, atherosclerosis, valvular stenosis and insufficiency, chronic heart failure, and angina may all develop as a result of a buildup of uremic toxins and metastatic calcification associated with uremia and end-stage renal disease (ESRD).
Occult gastrointestinal bleeding as a result of platelet abnormalities may present with nausea or vomiting. Uremic fetor, ammonia or urine-like odor of the breath, may also occur in uremic patients.
Uremia complications
Uremia complications:
- Hyperpigmented skin
- Severe itching
- Pericarditis plus effusion
- Pulmonary edema
- Heart valve calcification
Uremic Encephalopathy occurs in patients with acute or chronic renal failure, once the estimated GFR (eGFR) declines and stays below 15 mL/min. It is important to recognize the signs and symptoms early, as untreated uremic encephalopathy can progress to coma, while symptoms are easily reversible with dialysis. Early symptoms of uremic encephalopathy include nausea, anorexia, restlessness, drowsiness, and slowing of concentration and cognitive functions. As uremic encephalopathy progresses, patients typically become more disoriented, confused, and may exhibit bizarre behavior and emotional instability. Eventually, severe uremic encephalopathy will result in stupor and coma. Physical examination may reveal altered mental status, signs of cranial nerve involvement (e.g., nystagmus), or papilledema. Patients may additionally display hyperreflexia, clonus or asterixis, and eventually, coma.
A patient with uremic encephalopathy should improve clinically, following initiation of dialysis. However, electroencephalographic (EEG) findings such as slowing or loss of alpha frequency waves, disorganized signals, slow background activity with intermittent bursts of theta and delta waves may not improve instantly. Improvement may take several months, and one may not ever return completely back to normal. Treating uremic encephalopathy involves addressing many of the same parameters as are addressed when treating any patient with ESRD for example, correcting associated anemia, regulation of calcium or phosphate imbalances, monitoring the adequacy of dialysis.
Uremia diagnosis
A diagnosis of renal failure is based on abnormalities in GFR or creatinine clearance 6.
It is important to determine whether a patient presenting with uremic symptoms is experiencing acute or chronic renal failure, as acute kidney injury is reversible. Laboratory studies to evaluate for abnormalities in hemoglobin, calcium, phosphate, parathyroid hormone, albumin, potassium, and bicarbonate in addition to urinalysis (with microscopic examination) will help point towards any potential abnormalities.
A 24-hour urine collection may provide insight to both GFR and creatinine clearance, though this method is both burdensome and often inaccurate. Alternatively, a nuclear medicine radioisotope (iothalamate) clearance assay may be used to measure GFR. However, this test is also time-consuming and expensive relative to the Cockcroft-Gault formula
Creatinine clearance = Sex times [(140 – Age) / (serum creatinine)] times (weight / 72)
The Modification of Diet in Renal Disease formula [(GFR (mL/min/1.73 m) = 175 x (S) times (Age) times (0.742 if female) or times (1.212 if African American)] that are often used instead.
Creatinine clearance = (U x V) / P
- C = Creatinine clearance, U = urinary concentration, V = urinary flow rate (volume/time i.e., ml/min), and P = plasma concentration
The National Kidney Foundation recommends using the CKD-EPI Creatinine Equation (2009) to estimate glomerular filtration rate (GFR):
CKD-EPI Creatinine Equation (2009) 7
Expressed as a single equation:
eGFR = 141 x min(SCr/κ, 1)α X max(SCr /κ, 1)-1.209 X 0.993Age X 1.018 [if female] X 1.159 [if Black]
Abbreviations / Units
- eGFR (estimated glomerular filtration rate) = mL/min/1.73 m²
- SCr (standardized serum creatinine) = mg/dL
- κ = 0.7 (females) or 0.9 (males)
- α = -0.329 (females) or -0.411 (males)
- min = indicates the minimum of SCr/κ or 1
- max = indicates the maximum of SCr/κ or 1
- age = years
If you have had a recent creatinine or cystatin C measurement, you can calculate the eGFR by using one of the calculators for people 19 years of age or older on the National Kidney Foundation (NKF) web site.
An online adult GFR calculator is available here (https://www.kidney.org/professionals/KDOQI/gfr_calculator)
For children and teens younger than 19 the online children GFR calculator is available here (https://www.kidney.org/professionals/KDOQI/gfr_calculatorPed)
CKD-EPI Creatinine-Cystatin Equation 2012 8:
eGFR = 135 × min(SCr/κ, 1)α X max(SCr/κ, 1)-0.601 X min(Scys/0.8, 1)-0.375 X max(Scys/0.8, 1)-0.711 X 0.995Age X 0.969 [if female] X 1.08 [if black]
Abbreviations / Units
- eGFR (estimated glomerular filtration rate) = mL/min/1.73 m2
- SCr (serum creatinine) = mg/dL
- Scys (standardized serum cystatin C) = mg/l
- κ = 0.7 (females) or 0.9 (males)
- α = -0.248 (females) or -0.207 (males)
- min(SCr/κ or 1) = indicates the minimum of SCr/κ or 1
- max(SCr/κ or 1) = indicates the maximum of SCr/κ or 1
- min(Scys/0.8, 1) = indicates the minimum of Scys/0.8, 1
- max(Scys/0.8, 1) = indicates the maximum of Scys/0.8, 1
- age = years
According to the National Kidney Foundation, the newer CKD-EPI equations may be useful for assessing kidney function in people who have differences in diet, such as vegans, very high or very low muscle mass (e.g., body builders or those with muscle-wasting diseases), or for those who have changing muscle mass. They also may be useful for identifying people diagnosed with chronic kidney disease who have the highest risk of complications.
As per the National Kidney Foundation, patients presenting with chronic kidney disease are staged based on the estimated GFR (creatinine clearance) as calculated by the Modification of Diet in Renal Disease formula.
- Stage 1 – normal GFR (90 mL/min or greater)
- Stage 2 – mildly reduced GFR (60 mL/min to 90 mL/min)
- Stage 3 – moderately reduced GFR (30 mL/min to 59 mL/min)
- Stage 4 – severely reduced GFR (15 mL/min to -29 mL/min)
- Stage 5 – end-stage renal disease (ESRD) (GFR < 15 mL/min or patient is on dialysis)
A renal ultrasound may be useful to determine the size and shape of the kidneys, and to evaluate for hydronephrosis or ureteral and/or bladder obstruction. This may occur as a result of kidney stones, neurologic abnormalities, trauma, pregnancy, prostate enlargement, retroperitoneal fibrosis, abdominal tumors (secondary to cervical or prostate cancers) or additional structural abnormalities. Early diabetic nephropathy, multiple myeloma, polycystic kidney diseases, and glomerulonephritis associated with human immunodeficiency virus (HIV) are all associated with enlarged kidneys on ultrasound. Smaller kidneys are indicative of more chronic, irreversible changes as a result of long-standing kidney disease, ischemic nephropathy, or hypertensive nephrosclerosis.
If a patient presents with significant alterations in mental status, a brain computed tomography (CT) scan may be warranted. Uremic patients with a blood urea nitrogen (BUN) level greater than 150 mg/dL to 200 mg/dL are also at an increased risk of developing spontaneous subdural hematomas. Given the increased risk of bleeding and hemorrhage in uremia (especially in the setting of a fall or trauma), a CT scan of both the brain and abdomen may additionally be considered. An abdominal CT scan might help further elucidate the underlying cause of hydronephrosis if it was found on ultrasound without any obvious etiology.
Finally, magnetic resonance imaging (MRI) may be considered to assess for renal artery stenosis or thrombosis, or aortic and renal artery dissection- all potentially reversible causes of renal failure.
A renal biopsy may be helpful in determining reversibility or treatability of the renal injury, and may ultimately be required to make an accurate diagnosis of acute kidney injury or chronic kidney disease. However, a biopsy should not be performed in the case of small kidneys because of the associated comorbidities and increased risk of bleeding.
Uremia treatment
Dialysis is indicated in a patient with symptomatic uremia (e.g., nausea, vomiting, hyperkalemia, metabolic acidosis) that is not treatable my medical means, and should be initiated as soon as possible, regardless of the patient’s GFR 9.
Patients presenting with a uremic emergency (e.g., hyperkalemia, acidosis, symptomatic pericardial effusion, or uremic encephalopathy) require emergent dialysis which should be initiated gently to avoid dialysis disequilibrium syndrome (neurologic symptoms secondary to cerebral edema occurring during or shortly after the initiation of dialysis).
Ultimately, the best renal replacement therapy is renal transplantation, although practitioners may also consider long-term hemodialysis and peritoneal dialysis. Renal transplantation is associated with improvements in both survival and quality of life, and should be considered early (before the need for dialysis) as the waiting list for transplantation is often longer than two to three years.
Iron replacement should be initiated in patients with anemia of chronic kidney disease and underlying iron deficiency (as long as serum ferritin is greater than 100 mcg/mL). This can be done with dialysis treatments, or as oral therapy, if dialysis has not yet been initiated. Erythropoietic stimulating agents, such as erythropoietin or darbepoetin, may additionally be used in low doses (due to the increased risk of cardiovascular mortality) once hemoglobin levels reach below 10 g/dL.
Hyperparathyroidism and associated or isolated hypocalcemia and hyperphosphatemia can be treated with oral calcium carbonate or calcium acetate, oral vitamin D therapy, and oral phosphate binders (e.g., calcium carbonate, calcium acetate, sevelamer or lanthanum carbonate).
A dietitian should be consulted if dietary alterations are being considered. Patients with chronic kidney disease should reduce potassium, phosphate and sodium intake to 2 g to 3 g, 2 g, and 2 g per day of each, respectively. Though there is some conflicting evidence regarding protein intake in patients with kidney failure, the current low-protein diet recommendations before initiation of dialysis are 0.8 g to 1 g of protein/kg of weight per day with an added gram of protein for each gram of protein lost in the urine in patients with nephrotic syndrome.
A low-protein diet is not recommended in patients with advanced uremia or malnutrition, as this type of diet can result in worsening of malnutrition and has been associated with increased risk of mortality with the initiation of dialysis.
Patients with a creatinine clearance of less than 20 mL/min should avoid excessive potassium intake and use certain medications with caution (e.g., potassium-sparing diuretics, angiotensin-converting enzymes (ACE) inhibitors, angiotensin-receptor blockers, beta blockers, NSAIDs).
Due to the buildup of uremic toxins and potentially increased risk of bleeding and hemorrhage, extra care needs to be taken when prescribing oral anticoagulants or antiplatelet medications to patients who have end-stage renal disease (ESRD).
Finally, nephrotoxic medications (e.g., NSAIDs, aminoglycoside antibiotics) should be avoided in all patients with renal disease. To avoid nephrotoxicity, N-acetylcysteine may be administered before administration of intravenous contrast for radiologic imaging, although alternative modes of imaging like MRI should be considered in these patients, to avoid the risk of acute kidney injury altogether 10.
Uremia prognosis
In general, the prognosis for patients with uremia is poor unless they are treated with renal replacement therapy such as transplantation or dialysis. When the cause of uremia is a reversible cause, the prognosis is better than in patients with an irreversible cause. Uremic patients require frequent admission to the hospitals and have high morbidity and mortality without treatment. While dialysis has improved treatment, vascular access is still a major problem in the long run. In addition, there are not enough kidney donors. Patients with uremia are also at a high risk for adverse cardiac events and stroke compared to the general population. Finally, the cost of care for a dialysis patient is prohibitively expensive costing the healthcare system billions of dollars each year 11.
- Foris LA, Bashir K. Uremia. [Updated 2019 Jan 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441859[↩]
- Tsai HM. Atypical Hemolytic Uremic Syndrome: Beyond Hemolysis and Uremia. Am. J. Med. 2019 Feb;132(2):161-167[↩]
- Martins JM, Magriço R. Uremic Frost in End-Stage Renal Disease. N. Engl. J. Med. 2018 Aug 16;379(7):669[↩]
- Gedney N, Kalantar-Zadeh K. Dialysis Patient-Centeredness and Precision Medicine: Focus on Incremental Home Hemodialysis and Preserving Residual Kidney Function. Semin. Nephrol. 2018 Jul;38(4):426-432[↩]
- Raghavan R, Eknoyan G. Uremia: A historical reappraisal of what happened . Clin. Nephrol. 2018 May;89(5):305-313[↩]
- Massy ZA, Liabeuf S. Middle-Molecule Uremic Toxins and Outcomes in Chronic Kidney Disease. Contrib Nephrol. 2017;191:8-17.[↩]
- CKD-EPI Creatinine Equation 2009. https://www.kidney.org/content/ckd-epi-creatinine-equation-2009[↩]
- CKD-EPI Creatinine-Cystatin Equation 2012. https://www.kidney.org/content/ckd-epi-creatinine-cystatin-equation-2012[↩]
- Leong SC, Sao JN, Taussig A, Plummer NS, Meyer TW, Sirich TL. Residual Function Effectively Controls Plasma Concentrations of Secreted Solutes in Patients on Twice Weekly Hemodialysis. J. Am. Soc. Nephrol. 2018 Jul;29(7):1992-1999[↩]
- Leurs P, Machowska A, Lindholm B. Timing of dialysis initiation: when to start? Which treatment? J Ren Nutr. 2015 Mar;25(2):238-41[↩]
- Vanholder R, Van Laecke S, Glorieux G, Verbeke F, Castillo-Rodriguez E, Ortiz A. Deleting Death and Dialysis: Conservative Care of Cardio-Vascular Risk and Kidney Function Loss in Chronic Kidney Disease (CKD). Toxins (Basel). 2018 Jun 12;10, 6[↩]