Contents
Aortic valve disease
Aortic valve disease is a condition in which the valve between the main pumping chamber of your heart (left ventricle) and the main artery to your body (aorta) doesn’t work properly. Aortic valve disease may be a condition present at birth (congenital heart disease), or it may result from other causes.
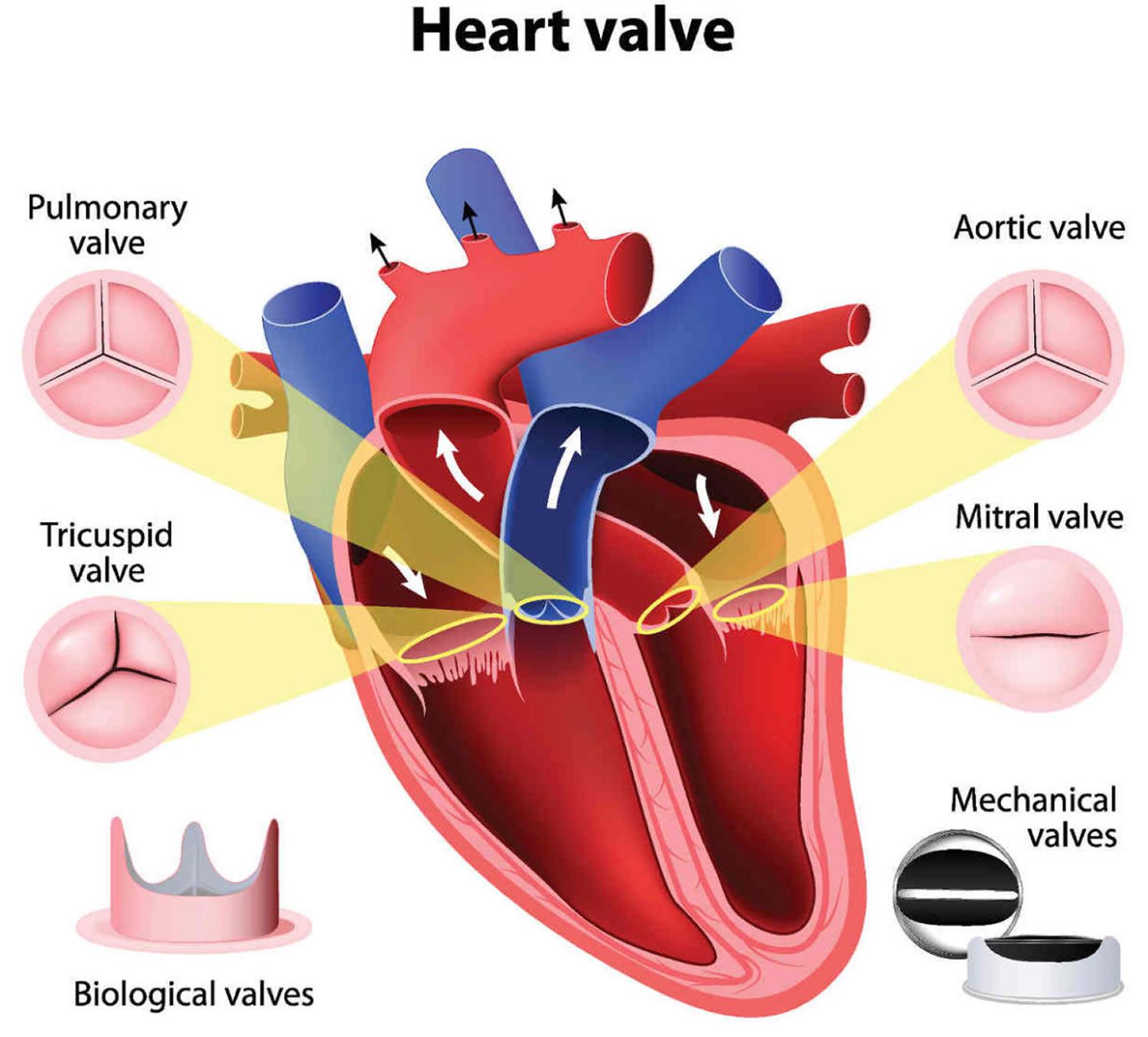
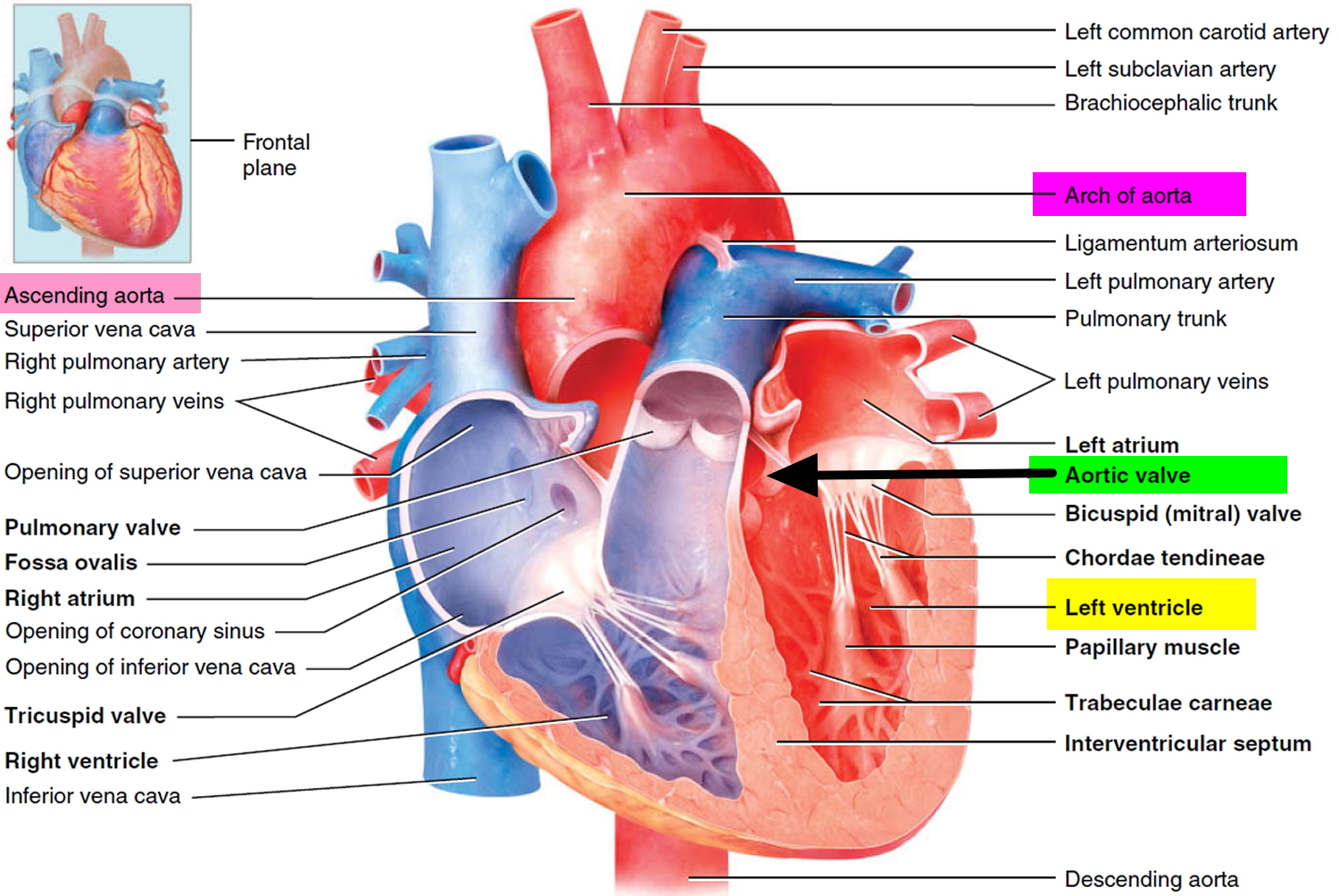
Chambers and valves of the heart
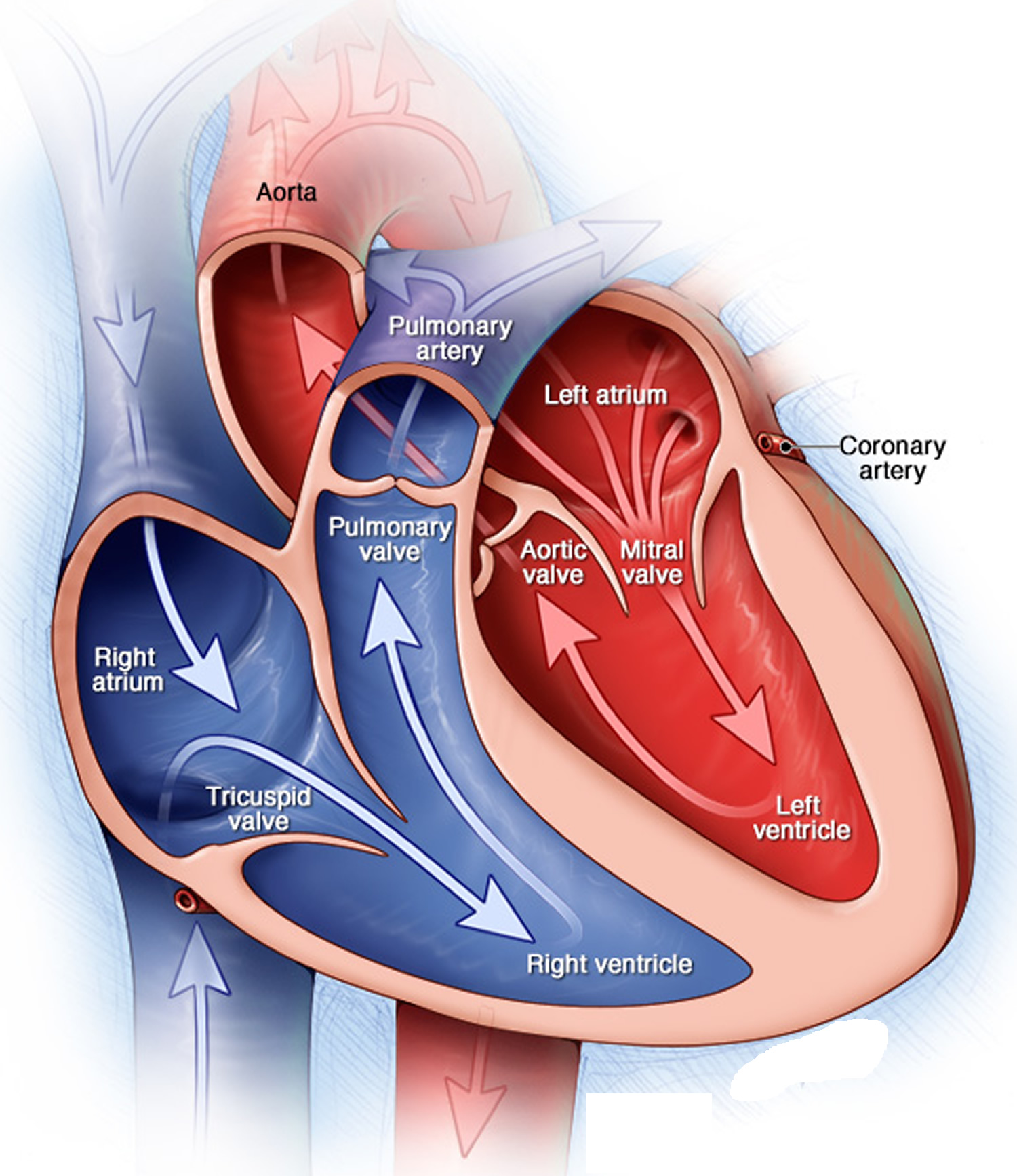
A normal heart has two upper and two lower chambers. The upper chambers, the right and left atria, receive incoming blood. The lower chambers, the more muscular right and left ventricles, pump blood out of your heart. The heart valves, which keep blood flowing in the right direction, are gates at the chamber openings.
Your heart has four valves that keep blood flowing in the correct direction. These valves include the mitral valve, tricuspid valve, pulmonary valve and aortic valve. Each valve has flaps (cusps or leaflets) that open and close once during each heartbeat. Sometimes, the valves don’t open or close properly, disrupting the blood flow through your heart and potentially impairing the ability to pump blood to your body.
There are two main types of aortic valve disease:
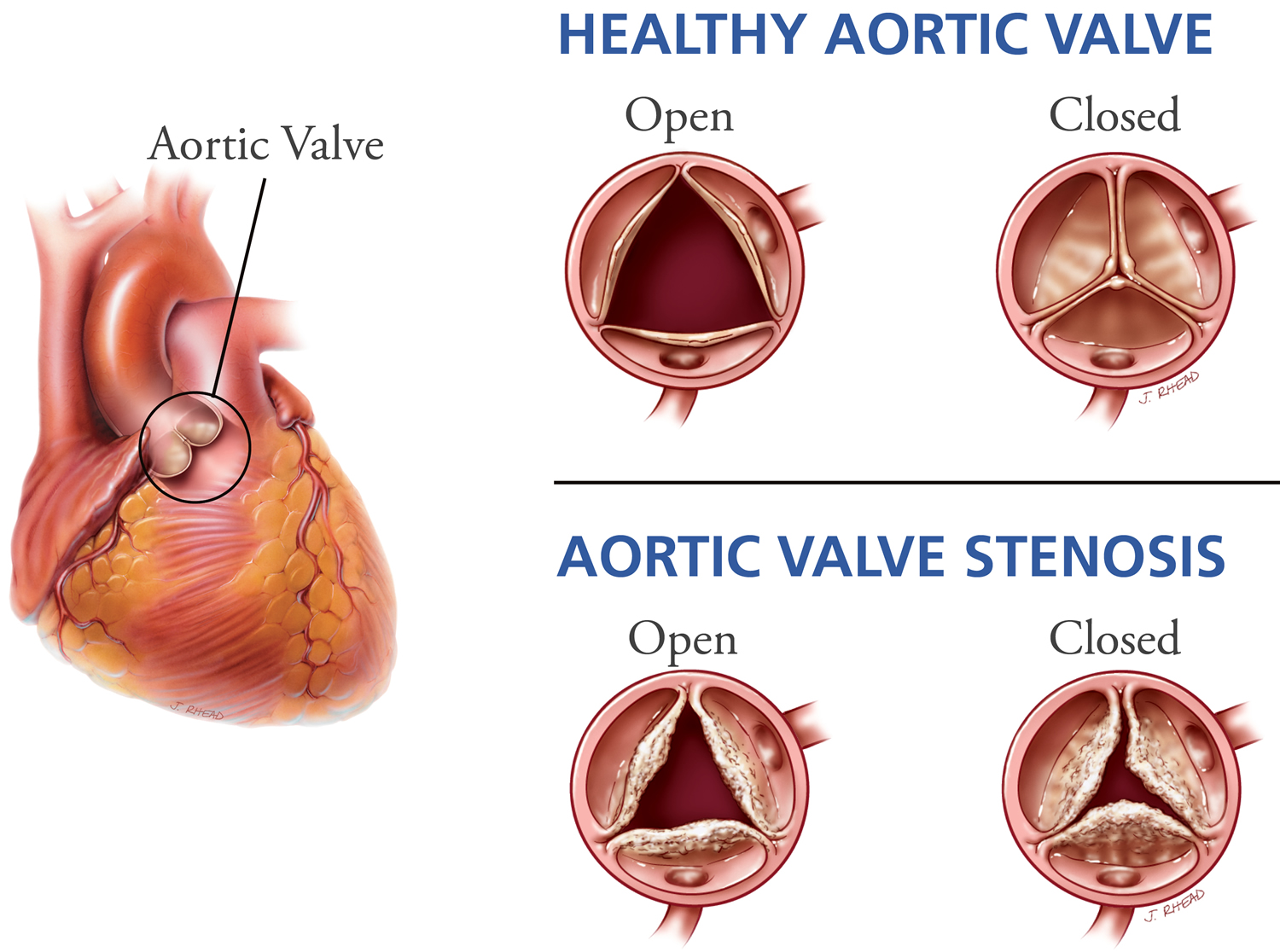
- Aortic valve stenosis
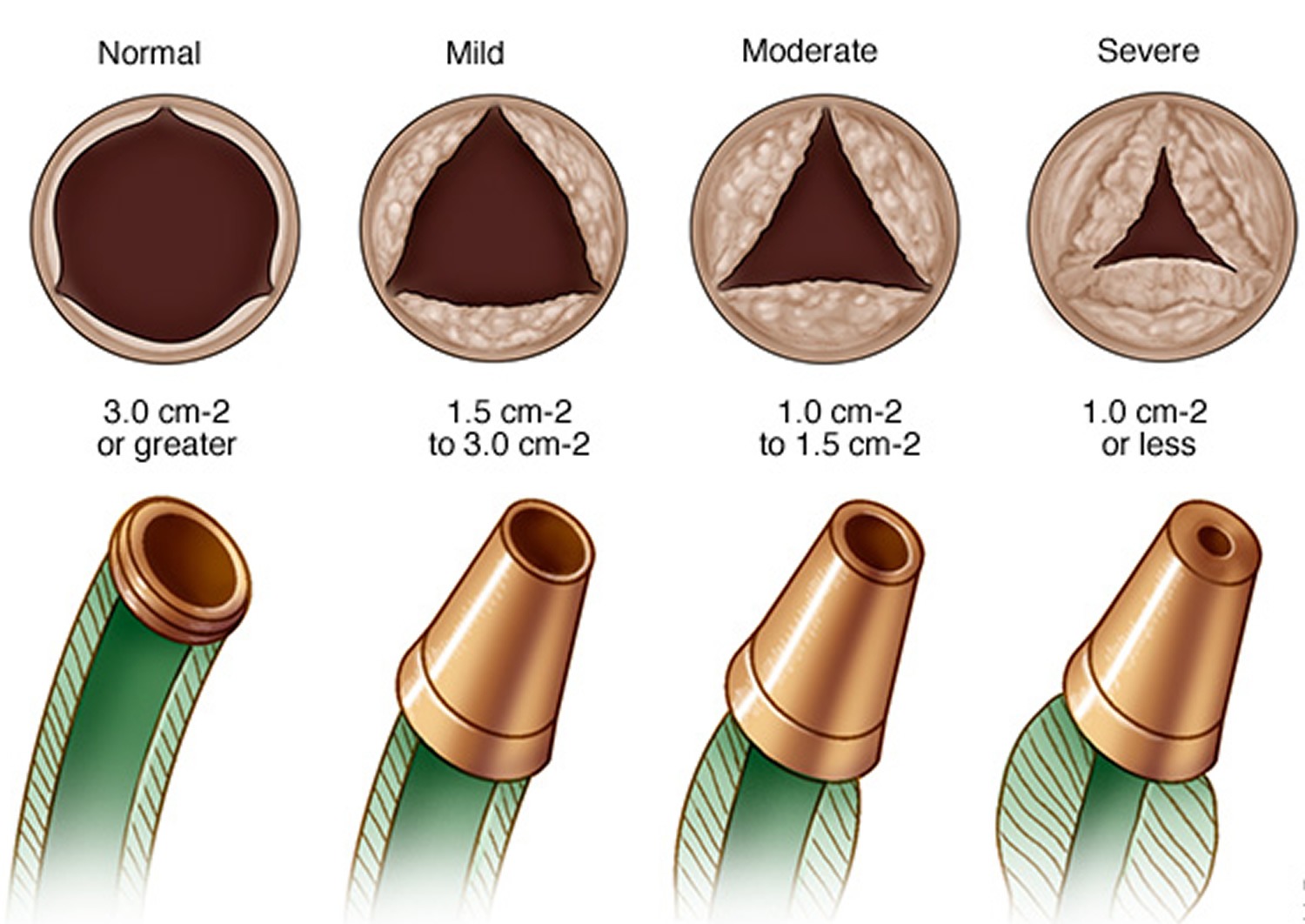
In this condition, the flaps (cusps) of the aortic valve may become thickened and stiff, or they may fuse together (see Figure 2). This causes narrowing of the aortic valve opening. The narrowed valve isn’t able to open fully, which reduces or blocks blood flow from your heart into your aorta and the rest of your body.
- Aortic valve regurgitation
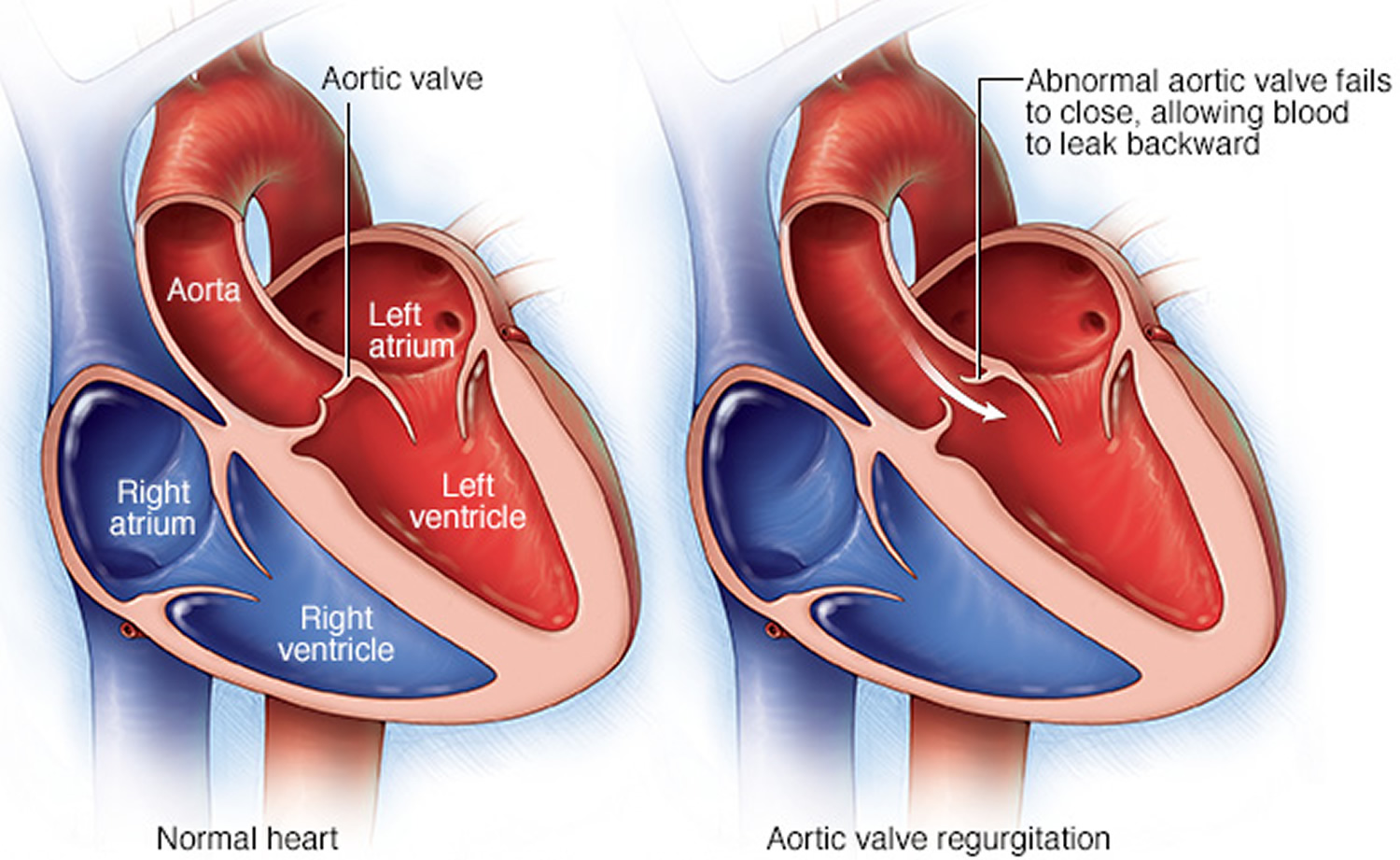
In this condition, the aortic valve doesn’t close properly, causing blood to flow backward into the left ventricle (see Figure 3).
Your treatment depends on the type and severity of your aortic valve disease. In some cases you may need surgery to repair or replace the aortic valve.
Figure 1. Aortic valve
Figure 2. Chambers and valves of the heart
Figure 3. Aortic valve stenosis
Figure 4. Aortic valve regurgitation
Calcific aortic valve disease
Calcific aortic valve disease is the most common valvular heart disease in the aging population of the developed world with projected disease burden expected to increase from 2.5 million in 2000 to 4.5 million in 2030 1. In calcific aortic valve disease, the leaflets become thick, stiff, scarred and calcified, often covered with nodules on the surface facing the aorta. In general, symptoms of calcific aortic valve disease are minimal even as the valve narrows into “aortic stenosis” where pressure overload leads to progressive left ventricular hypertrophy and life-threatening symptoms – angina and/or syncope. This hemodynamic catastrophe results in heart failure and without intervention, death within months to years. The mainstay of treatment for calcific aortic valve disease is surgical aortic valve replacement with a mechanical or bioprosthetic valve 2. Limitations include increased complications due to anticoagulation and the need for reoperation due to the limited lifespan of prosthetic valves 3. Transcatheter aortic valve replacement (TAVR) is currently reserved for patients with excess operative risk, but its indications are now expanding due to the “minimally invasive” nature of TAVR compared to surgical aortic valve replacement 4. If transcatheter aortic valve replacement (TAVR) becomes more routine, some hope it will reduce the need for repeat surgery. Tissue engineered valve replacement, particularly in pediatric patients, holds great potential, but remains elusive after years of effort. Currently, there are no medical therapies for calcific aortic valve disease as alternatives to surgery, other than for complications, such as antibiotics for infections and vasodilators for acute decompensation.
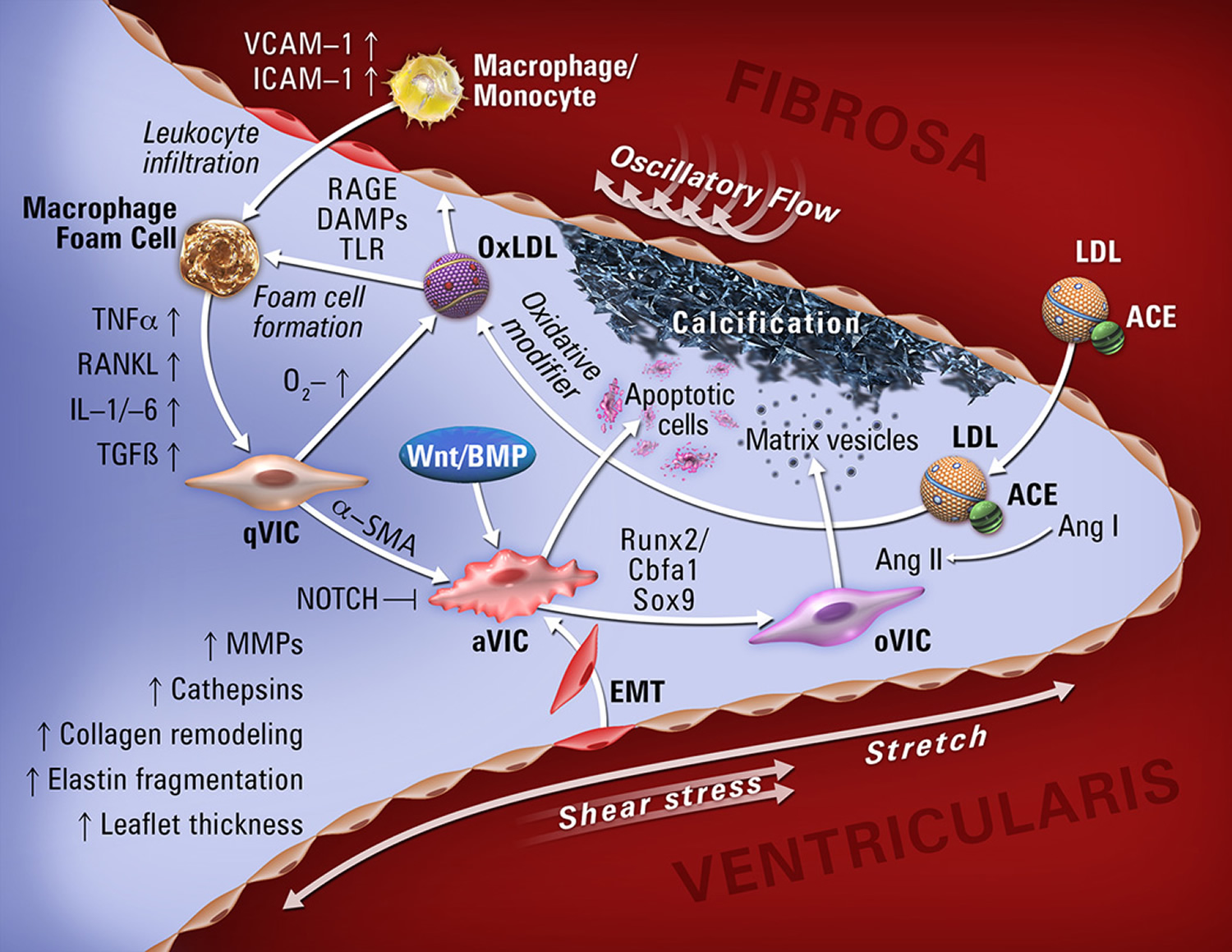
Research now implicates osteogenic processes as key mechanisms in calcific aortic valve disease (Figure 5) 5, opening two general directions for development of new therapies: 1) inhibition of pro-calcific regulators and 2) induction of pro-resorptive regulators in aortic valve tissue. Tissue targeting may be essential to avoid damage to skeletal bone; for example, if osteoclasts or nanoparticles carrying osteoclastic differentiation factors could be targeted to valve tissue, they may reverse calcific aortic valve disease 6. However, even if successful, mineral resorption may leave behind damaged leaflets. Molecular imaging of these events may provide biomarkers for clinical and research monitoring far in advance of critical stenosis. Preventive measures that do not require tissue targeting are under consideration, including modification of various factors (dietary phosphorous, vitamins K and D, fetuin, warfarin, bisphosphonates, anti-inflammatory agents, osteopontin, mineralocorticoids, antioxidants, estrogen) and conditions (uremia, metabolic syndrome, hyperglycemia, hyperlipidemia, hypertension). Based on the association of calcific aortic valve disease with hyperlipidemia and atherosclerosis, clinical trials of “statins” were undertaken, but no benefit seen 7. It remains to be determined whether they may have benefit in earlier disease or in patients with hyperlipidemia. Additionally, while atherosclerosis and calcific aortic valve disease have similar risk factors, they are somewhat poor predictors of calcific aortic valve disease, suggesting that an additional set of unknown factors may contribute to the onset and progression of valve disease.
Whether calcific aortic valve disease is a manifestation of an inflammatory disease or whether inflammation is an associated process occurring secondarily in injured and repairing tissue is not entirely known. Thus, an area of interest remains in investigating whether anti-inflammatory agents may prevent and/or reduce the extent of calcification and osteogenesis, which is fundamentally triggered by inflammatory mediators. To this end, patients with mild-moderate calcific aortic valve disease and coronary artery disease with either diabetes or metabolic syndrome will be evaluated in the ongoing Cardiovascular Inflammation Reduction Trial 8 to examine whether treatment with low dose methotrexate, an effective anti-inflammatory drug, affects progression of calcific aortic valve disease. Evaluation of new therapeutic approaches will require systematically-collected, detailed knowledge of the processes of calcific aortic valve disease that include analysis of normal and diseased human aortic valve tissue, development of suitable animal models, functional assays, and clinical and population studies.
Several large population cohorts have sought to identify the key epidemiological factors contributing to calcific aortic valve disease. The Cardiovascular Health Study, focusing on risk factors for coronary artery disease, identified age, male gender and lipoprotein (a) levels as showing the strongest correlates of prevalent aortic valve sclerosis and stenosis; hypertension, tobacco use and LDL cholesterol levels gave weaker signals 9. Advanced age remains the leading risk factor for calcific aortic valve disease. In all age categories aortic valve sclerosis and stenosis were more prevalent in male subjects compared with women 10. The Multi-Ethic Study of Atherosclerosis 11 demonstrated that the prevalence of calcific aortic valve disease was highest in non-Hispanic whites, followed by Hispanics and African Americans, and confirmed a significant role for age and male gender with the development of calcific aortic valve disease 12. By comparison, impaired kidney function is only modestly associated with aortic valve calcification 13. Metabolic syndrome was independently associated with the development of calcific aortic valve disease 14, potentially due to similar factors participating in their pathogenesis, including circulating ox-LDL and small dense LDL particle phenotype. In addition, metabolic syndrome was independently associated with the progression of aortic stenosis 15. A polymorphism in the LPA gene associated with serum apo(a) peptide levels has been identified as critical in the pathogenesis of calcific aortic valve disease 16. In addition, recent investigations have identified Lp-PLA2 expression in stenotic aortic valves and vulnerable plaques. Lp-PLA2 produced within the aortic valve hydrolyzes oxidized phospholipids from lipoproteins which have proinflammatory effects that promote valve mineralization 17. Thus this lipoprotein may mediate unique aspects of atherosclerosis and calcific aortic valve disease.
Figure 5. Molecular, cellular, and biomechanical mechanisms in calcific aortic valve disease
Note: Abbreviations: qVIC, quiescent valve interstitial cell; aVIC, activated valve interstitial cell; oVIC, osteogenic valve interstitial cell; EMT, endothelial-to-mesenchymal transition; MV, matrix vesicles. VCAM-1, vascular adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1; TNF-α, tumor necrosis factor-α; RANKL, receptor activator of nuclear factor kappa-B ligand; IL1/-6, interleukin1/-6; TGF-β, transforming growth factor-β; BMP, bone morphogenic protein; Runx2, runt-related transcription factor 2; ACE, angiotensin-converting enzyme; AngI/II, angiotensinI/II; LDL, low-density lipoprotein.
Bicuspid aortic valve disease
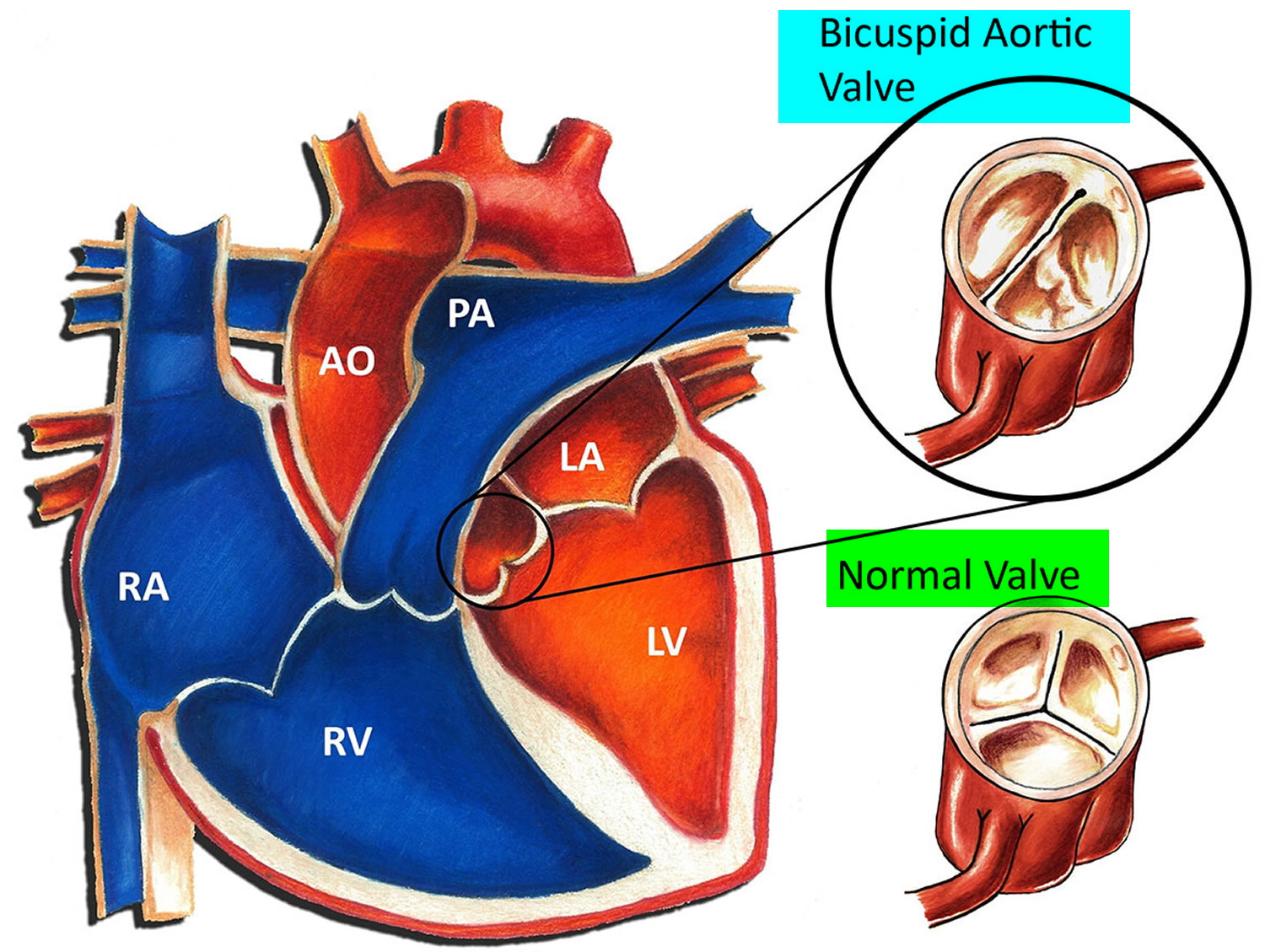
Some people are born with a bicuspid aortic valve (congenital aortic valve abnormality), in which the aortic valve — located between the lower left heart chamber (left ventricle) and the main artery that leads to the body (aorta) — has only two (bicuspid) cusps instead of three. People may also be born with one (unicuspid) or four (quadricuspid) cusps, but these are rare.
A bicuspid aortic valve may cause the heart’s aortic valve to narrow (aortic valve stenosis). This narrowing prevents the valve from opening fully, which reduces or blocks blood flow from the heart to the body. In some cases, the aortic valve doesn’t close tightly, causing blood to leak backward into the left ventricle (aortic valve regurgitation). Most people with a bicuspid aortic valve aren’t affected by valve problems until they’re adults, and some may not be affected until they’re older adults. Some children with bicuspid aortic valves may have valve problems.
Some people with a bicuspid aortic valve may have an enlarged aorta — the main blood vessel leading from the heart. There is also an increased risk of aortic dissection.
Figure 6. Bicuspid aortic valve
Aortic valve disease complications
Aortic valve disease can cause complications, including:
- Heart failure
- Stroke
- Blood clots
- Heart rhythm abnormalities
- Death
Aortic valve disease symptoms
Some people with aortic valve disease may not experience symptoms for many years. Signs and symptoms of aortic valve disease may include:
- Abnormal heart sound (heart murmur) heard through a stethoscope
- Shortness of breath, particularly when you have been very active or when you lie down
- Dizziness
- Fainting
- Chest pain or tightness
- Irregular heartbeat
- Fatigue after being active or having less ability to be active
- Not eating enough (mainly in children with aortic valve stenosis)
- Not gaining enough weight (mainly in children with aortic valve stenosis)
- Swelling of the ankles and feet
Bicuspid aortic valve disease symptoms
Early on, these symptoms may be noticeable only when exercising, but as the disease progresses, you could experience shortness of breath with minimal or no activity.
Some patients will be unable to sleep flat in bed or may awaken short of breath. Another potential symptom is swollen feet, particularly in the late afternoon or early evening.
Aortic valve disease causes
In aortic valve disease, the aortic valve between the lower left heart chamber (left ventricle) and the main artery that delivers blood from the heart to the body (aorta) doesn’t work properly. It may not be closing properly, which causes blood to leak backward to the left ventricle (regurgitation), or the valve may be narrowed (stenosis).
Aortic valve disease may be caused by a heart defect present at birth (congenital). It can also be caused by other conditions, including age-related changes to the heart, infections, high blood pressure or injury to the heart.
The most common congenital aortic valve abnormality, called a bicuspid aortic valve, occurs when the valve has only two leaflets (bicuspid) instead of three (tricuspid). This prevents the valve from opening or closing completely. Although the abnormality has been there since birth, symptoms may not be felt until adulthood. Your physician may be able to hear distinctive murmurs (abnormal sounds that the doctor can hear with his/her stethoscope) when listening to your heart.
Acquired aortic valve disease occurs because your valve simply wears out over time, and usually happens as you age. Calcium collects on the valve and can cause the leaflets to stiffen and narrow, which limits their motion.
If the aorta—the main blood vessel coming out of the heart—is diseased, this also can lead to problems with the aortic valve.
A faulty or failing aortic valve may cause symptoms such as shortness of breath, chest pain, and dizziness or loss of consciousness (passing out). These symptoms are due to the heart having to work harder because of the narrowed or leaky valve.
Risk factors of aortic valve disease
Risk factors of aortic valve disease include:
- Older age
- Certain heart conditions present at birth (congenital heart disease)
- History of infections that can affect the heart
- Chronic kidney disease
- History of radiation therapy to the chest
Aortic valve disease diagnosis
To diagnose aortic valve disease, your doctor may review your signs and symptoms, discuss your medical history, and conduct a physical examination. Your doctor may listen to your heart with a stethoscope to determine if you have a heart murmur that may indicate an aortic valve condition. A doctor trained in heart disease (cardiologist) may evaluate you.
Your doctor may order several tests to diagnose your condition, including:
- Echocardiogram. This test uses sound waves to provide video images of your heart in motion. During this test, specialists hold a wandlike device (transducer) on your chest. Doctors may use this test to evaluate your heart chambers, the aortic valve and the blood flow through your heart. This test can help doctors closely look at the condition of the aortic valve, and the cause and severity of your condition. It can also help doctors determine if you have additional heart valve conditions. Doctors may also use a 3-D echocardiogram. Doctors may conduct another type of echocardiogram called a transesophageal echocardiogram to get a closer look at the aortic valve. In this test, a small transducer attached to the end of a tube is inserted down the tube leading from your mouth to your stomach (esophagus).
- Electrocardiogram (ECG). In this test, wires (electrodes) attached to pads on your skin measure the electrical activity of your heart. An ECG can detect enlarged chambers of your heart, heart disease and abnormal heart rhythms.
- Chest X-ray. A chest X-ray can help your doctor determine whether the heart is enlarged, which can indicate certain types of aortic valve disease. It can also show whether you have an enlarged blood vessel (aorta) leading from the heart or any calcium buildup on the aortic valve. A chest X-ray can also help doctors determine the condition of your lungs.
- Cardiac computerized tomography (CT) scan. A cardiac CT scan uses a series of X-rays to create detailed images of your heart and heart valves. Doctors may use this imaging technique to measure the size of your aorta and look at your aortic valve more closely.
- Cardiac MRI. A cardiac MRI uses magnetic fields and radio waves to create detailed images of your heart. This test may be used to determine the severity of your condition and evaluate the size of the aorta.
- Exercise tests or stress tests. Exercise tests help doctors see whether you have signs and symptoms of aortic valve disease during physical activity, and these tests can help determine the severity of your condition. If you are unable to exercise, medications that have similar effects as exercise on your heart may be used.
- Cardiac catheterization. This test isn’t often used to diagnose aortic valve disease, but it may be used if other tests aren’t able to diagnose the condition or to determine its severity.
In this procedure, a doctor threads a thin tube (catheter) through a blood vessel in your arm or groin to an artery in your heart and injects dye through the catheter to make the artery visible on an X-ray. This provides your doctor with a detailed picture of your heart arteries and how your heart functions. It can also measure the pressure inside the heart chambers.
Aortic valve disease treatment
Treatment for aortic valve disease depends on the severity of your condition, whether or not you’re experiencing signs and symptoms, and if your condition is getting worse.
If your symptoms are mild or you aren’t experiencing symptoms, your doctor may monitor your condition with regular follow-up appointments. Your doctor may recommend you make healthy lifestyle changes and take medications to treat symptoms or reduce the risk of complications.
You may eventually need surgery to repair or replace the diseased aortic valve. In some cases, your doctor may recommend surgery even if you aren’t experiencing symptoms. If you’re having another heart surgery, doctors may perform aortic valve surgery at the same time.
If you have aortic valve disease, consider being evaluated and treated at a medical center with a multidisciplinary team of cardiologists and other doctors and medical staff trained and experienced in evaluating and treating heart valve disease. This team can work closely with you to determine the most appropriate treatment for your condition.
Surgery to repair or replace an aortic valve is usually performed through a cut (incision) in the chest. In some cases, doctors may perform minimally invasive heart surgery, which involves the use of smaller incisions than those used in open-heart surgery.
Surgery options include:
Aortic valve repair
To repair an aortic valve, surgeons may conduct several different types of repair, including separating valve flaps (cusps) that have fused, removing excess valve tissue so that the cusps can close tightly, or patching holes in a valve.
Doctors may conduct a procedure using a long, thin tube (catheter) to repair a valve with a narrowed opening (aortic valve stenosis). In this procedure, called balloon valvuloplasty, a doctor inserts a catheter with a balloon on the tip into an artery in your arm or groin and guides it to the aortic valve. A doctor then inflates the balloon, which expands the opening of the valve. The balloon is then deflated, and the catheter and balloon are removed.
The procedure can treat aortic valve stenosis in infants and children. However, the valve tends to narrow again in adults who’ve had the procedure, so it’s usually only performed in adults who are too ill for surgery or who are waiting for a valve replacement, as they typically need additional procedures to treat the narrowed valve over time.
Doctors may also use a catheter procedure to insert a plug or device to repair a leaking replacement aortic valve.
Aortic valve replacement
Aortic valve replacement is often needed to treat aortic valve disease. In aortic valve replacement, your surgeon removes the damaged valve and replaces it with a mechanical valve or a valve made from cow, pig or human heart tissue (biological tissue valve). Another type of biological tissue valve replacement that uses your own pulmonary valve is sometimes possible.
Surgical aortic valve replacement
There are two valve options for aortic valve replacement – mechanical valves (metal) or biological valves (tissue).
Biological valves are most commonly made from animal tissue. Biological valves are less likely to cause blood clots, but they also are less durable than mechanical valves. Biological tissue valves degenerate over time and may eventually need to be replaced in the future.
The principal advantage of mechanical valves is their durability—they do not wear out; however, blood tends to clot on mechanical valves, so patients must take blood thinning medication (anticoagulants) for the rest of their lives to prevent blood clots. There is also a small risk of stroke due to blood clotting. Your doctor will discuss with you the benefits and risks of each type of valve and discuss which valve may be appropriate for you.
Regardless of which type of valve you choose, there are two different surgical approaches that can be utilized: traditional aortic valve replacement or minimally invasive.
During traditional aortic valve replacement, the cardiothoracic surgeon makes a 6- to 8-inch incision down the center of your breastbone (sternum) to open the chest, providing direct access to your heart. In minimally invasive surgery, the cardiothoracic surgeon makes a 2- to 4-inch, J-shaped incision that opens part of your chest. This can potentially reduce hospital stay.
Minimally invasive aortic valve replacement is not appropriate for all patients, but your cardiothoracic surgeon will review the recommended approach to surgery that is safest for you based on your individual symptoms and circumstances.
Transcatheter aortic valve replacement
Doctors may perform a less invasive procedure called transcatheter aortic valve replacement (TAVR) to replace a narrowed aortic valve. Transcatheter aortic valve replacement (TAVR) may be an option for people who are considered to be at intermediate or high risk of complications from surgical aortic valve replacement.
In transcatheter aortic valve replacement (TAVR), doctors insert a catheter in your leg or chest and guide it to your heart. A replacement valve is then inserted through the catheter and guided to your heart. A balloon may expand the valve, or some valves can self-expand. When the valve is implanted, doctors remove the catheter from your blood vessel.
Doctors may also conduct a catheter procedure to insert a replacement valve into a failing biological tissue valve that is no longer working properly. Other catheter procedures to repair or replace aortic valves continue to be researched.
Figure 7. Transcatheter aortic valve replacement (TAVR)
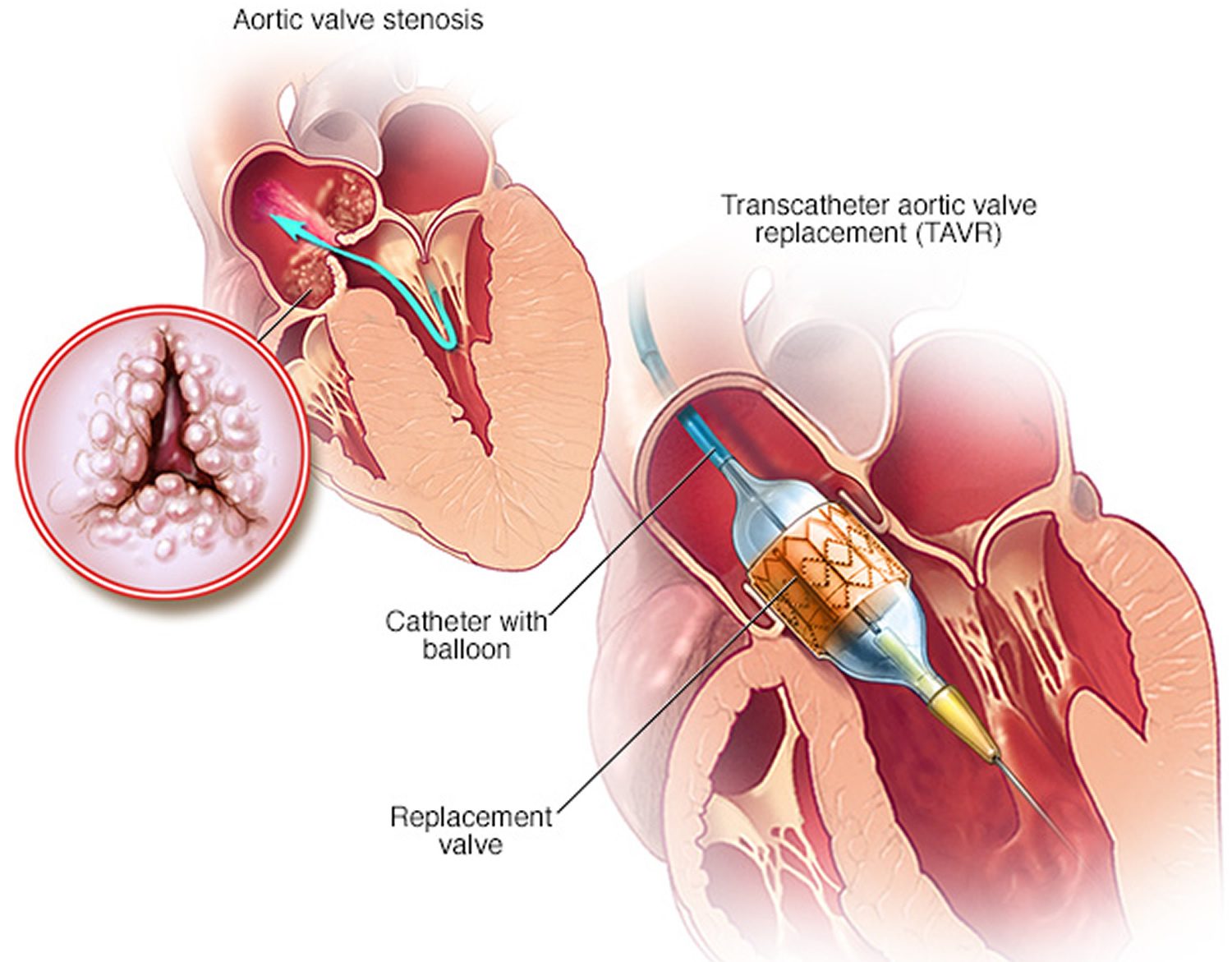 Note: Transcatheter aortic valve replacement (TAVR) is a minimally invasive procedure to replace a narrowed aortic valve that fails to open properly (aortic valve stenosis). In this procedure, doctors insert a catheter in your leg or chest and guide it to your heart. A replacement valve is inserted through the catheter and guided to your heart. A balloon is expanded to press the valve into place. Some transcatheter aortic valve replacement (TAVR) valves are self-expanding.
Note: Transcatheter aortic valve replacement (TAVR) is a minimally invasive procedure to replace a narrowed aortic valve that fails to open properly (aortic valve stenosis). In this procedure, doctors insert a catheter in your leg or chest and guide it to your heart. A replacement valve is inserted through the catheter and guided to your heart. A balloon is expanded to press the valve into place. Some transcatheter aortic valve replacement (TAVR) valves are self-expanding.
Bicuspid aortic valve disease and exercise
Physical exercise has many benefits and should be a regular part of almost anyone’s life. That includes most people with congenital heart disease.
- If you have a severely obstructed valve, vigorous exercise is not a good idea. Your cardiologist may tell you to limit your activity if this is the case. Ask your cardiologist about your exercise limits.
There is no proven link between exercise and harmful outcome from aortic valve stenosis causing an enlarged aorta, but many physicians feel that such patients shouldn’t engage in strenuous exercise, particularly activity that involves straining or grunting like heavy weight lifting 18.
- It’s likely that for most patients, the benefits of exercise outweigh the perceived risks. Low-intensity activity is still preferred. If you have any questions about the appropriateness of exercise for you, talk to your doctor.
If you’ve been inactive for a long time and want to start a regular exercise routine, it’s often wise to talk with your doctor about how to get started safely. Your doctor may recommend an exercise test which can provide you with guidelines for exercise.
Physical activities for children
If the aortic valve is abnormally formed but has no important obstruction or leak, your child may not need any special precautions regarding physical activities and may be able to participate in normal activities without increased risk. Some children with obstruction, leak or heart muscle abnormalities may have to limit how much they do some kinds of exercise. Check with your child’s pediatric cardiologist about this.
What types and how much?
The best and safest types of exercise are “aerobic” activities. These increase the heart rate and make you breath heavily. Examples include brisk walking, swimming, biking, jogging, rowing, cross-country skiing, hiking or stair climbing. Team or court sports such as basketball, soccer, football, tennis, squash and volleyball are also aerobic activities.
A good rule of thumb is to increase your activity so you breathe hard and fast but can still carry on a conversation with someone. If you can speak in full sentences but still feel your heart pounding, you’re likely benefiting from a safe level of activity.
Often patients are trained to check their heart rate during or immediately after activity. Their target heart rate is 70-80 percent of their predicted maximal heart rate (defined as 220 minus age).
It’s best to avoid activities that cause grunting or straining (medically referred to as a “valsalva maneuver”). This happens when a person bears down against a closed throat to increase the strength of arm or abdominal muscles. There’s often a tendency to do this when lifting heavy weights, doing sit-ups, push-ups or chin-ups, etc., but it may be harmful. Straining causes a sudden rise in blood pressure, which adds strain on the heart; it increases the pressure in the lungs, which can affect blood flow from the body into the lungs; and it often means there’s more force on the chest wall, and many congenital heart patients have surgical scars in the chest that can be damaged, particularly in the first year after surgery.
Intensely physical sports such as football, boxing or hockey may increase the chance for injury and unnecessary strain on the cardiovascular system.
Any amount of activity is better than none, and the more physically active a person is, the greater the anticipated cardiovascular benefit. Guidelines for the general population suggest at least 30 minutes of dedicated aerobic activity a day for five or more days a week. This is a good target for congenital heart patients too. If it seems like too much, start with a more modest goal and build from there.
Lifestyle and home remedies
You’ll have regular follow-up appointments with your doctor to monitor your condition.
If you have aortic valve disease, here are some steps that may help you cope:
- Take medications as prescribed. Take your medications as directed by your doctor.
- Get support. Having support from your family and friends can help you cope with your condition. Ask your doctor about support groups that may be helpful.
- Stay active. Aim to stay physically active. Your doctor may give you recommendations about how much and what type of exercise is appropriate for you.
Your doctor may suggest you incorporate several heart-healthy lifestyle changes into your life, including:
- Eating a heart-healthy diet. Eat a variety of fruits and vegetables, low-fat or fat-free dairy products, poultry, fish, and whole grains. Avoid saturated and trans fat, and excess salt and sugar.
- Maintaining a healthy weight. Aim to keep a healthy weight. If you’re overweight or obese, your doctor may recommend that you lose weight.
- Getting regular physical activity. Aim to include about 30 minutes of physical activity, such as brisk walks, into your daily fitness routine.
- Managing stress. Find ways to help manage your stress, such as through relaxation activities, meditation, physical activity, and spending time with family and friends.
- Avoiding tobacco. If you smoke, quit. Ask your doctor about resources to help you quit smoking. Joining a support group may be helpful.
For women with aortic valve disease, it’s important to talk with your doctor before you become pregnant. Your doctor can discuss with you which medications you can safely take, and whether you may need a procedure to treat your valve condition prior to pregnancy.
You’ll likely require close monitoring by your doctor during pregnancy. Doctors may recommend that women with severe valve conditions avoid pregnancy to avoid the risk of complications.
- Aikawa E, Schoen FJ. In: Calcific and degenerative heart valve disease, in Cellular and molecular pathobiology of cardiovascular disease. Stone J, Homeister JM, Willis MS, editors. Elsevier; 2014. pp. 161–181.[↩]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja NJ, Sundt TM, Thomas J. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practical guidelines. Circulation. 2014;129:2440–2492. http://circ.ahajournals.org/content/129/23/2440.long[↩]
- Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res. 2013;113:223–237. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4013234/[↩]
- Desai CS, Roselli EE, Svensson LG, Bonow RO. Transcatheter aortic valve replacement: Current status and future directions. Semin Thoracic Surg. 2013;25:193–196. https://www.ncbi.nlm.nih.gov/pubmed/24331140[↩]
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process. Circulation. 2011;124:1783–1791. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3306614[↩]
- Rementer CW, Wu M, Buranaphatthana W, Yang HY, Scatena M, Giachelli CM. An inducible, ligand-independent receptor activator of NF-kB gene to control osteoclast differentiation from monocytic precursors. PLoS One. 2013;8:e84465. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3874012/[↩]
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. http://circ.ahajournals.org/content/121/2/306.long[↩]
- Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3888829/[↩]
- Arsenault BJ, Boekholdt SM, Dube MP, Rheaume E, Wareham NJ, Khaw KT, Sandhu MS, Tardif JC. Lipoprotein(a) levels, genotype and incident aortic valve stenosis. Circ Cardiovasc Genet, 2014;7:304–310. http://circgenetics.ahajournals.org/content/7/3/304.long[↩]
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. 1997;29:630–634. https://www.ncbi.nlm.nih.gov/pubmed/9060903[↩]
- Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O’Brien KD, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;198:104–114. https://www.ncbi.nlm.nih.gov/pubmed/17950742[↩]
- Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O’Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2829478/[↩]
- Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, Takasu J, Detrano R, O’Brien KD. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–420 https://www.ncbi.nlm.nih.gov/pubmed/17720520[↩]
- Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O’Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–819. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2661576[↩]
- Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP, Arsenault M, Couet J, Pibarot P. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–2236. https://www.ncbi.nlm.nih.gov/pubmed/16750688[↩]
- Kamstrup P, Tybjaerg-Hansen A, Nordestgaard B. Elevated liproprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. https://www.ncbi.nlm.nih.gov/pubmed/24161338[↩]
- Mahmut A, Boulanger M, El Husseini D, Fournier D, Bouchareb R, Despres J, Pibarot P, Bosse Y, Mathieu P. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease. J Am Coll Cardiol. 2014;63:460–469. https://www.ncbi.nlm.nih.gov/pubmed/24161325[↩]
- Congenital Heart Defects and Physical Activity. http://www.heart.org/HEARTORG/Conditions/CongenitalHeartDefects/CareTreatmentforCongenitalHeartDefects/Congenital-Heart-Defects-and-Physical-Activity_UCM_307738_Article.jsp[↩]