Contents
- What is arteriovenous fistula
- Arteriovenous fistula symptoms
- Arteriovenous fistula causes
- Arteriovenous fistula complications
- Arteriovenous fistula diagnosis
- Arteriovenous fistula treatment
- Veins of the Upper Limbs
- Arteriovenous fistula for dialysis
- Dural arteriovenous fistula
- Spinal dural arteriovenous fistula
- Arteriovenous fistula brain
What is arteriovenous fistula
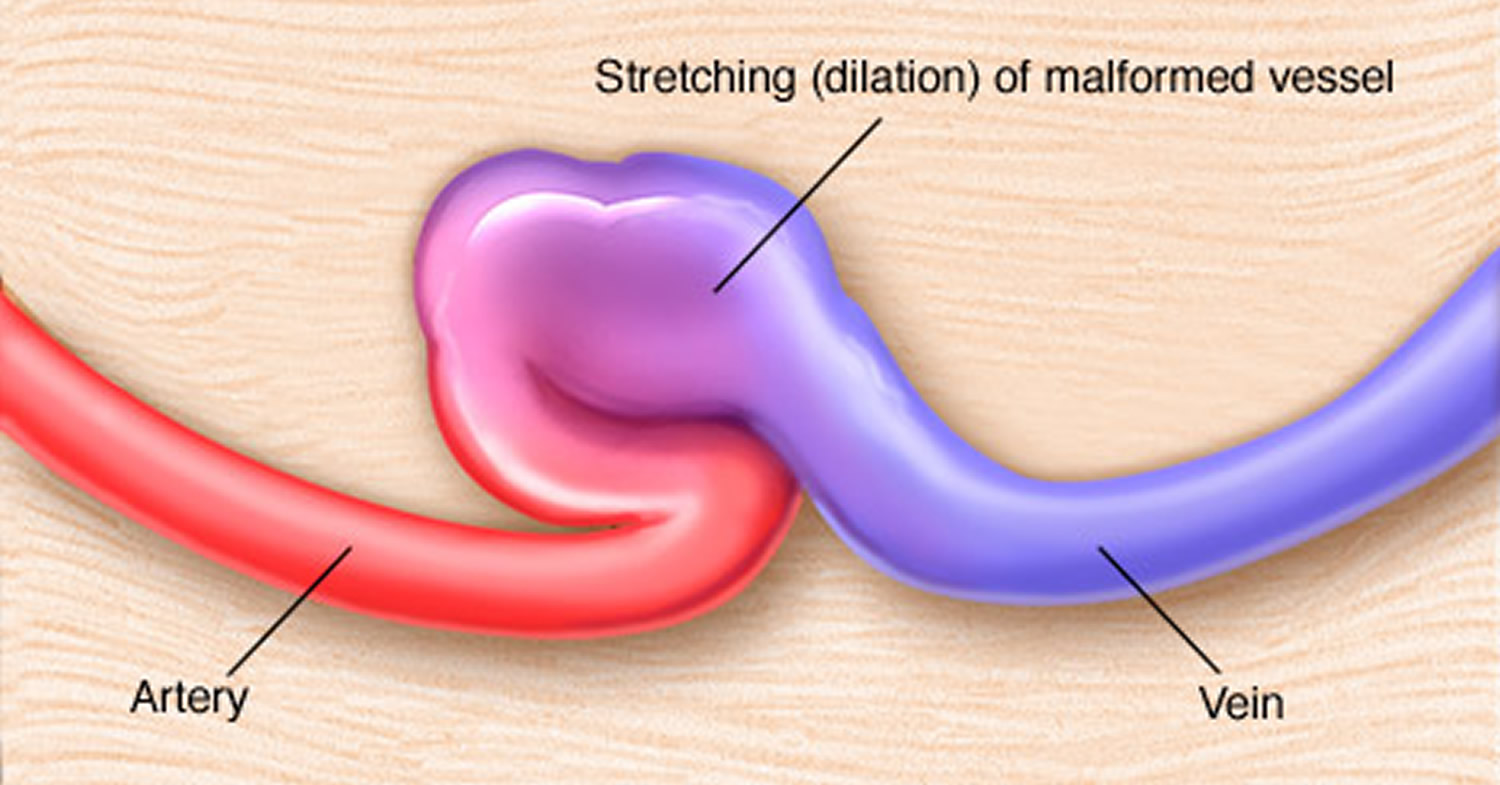
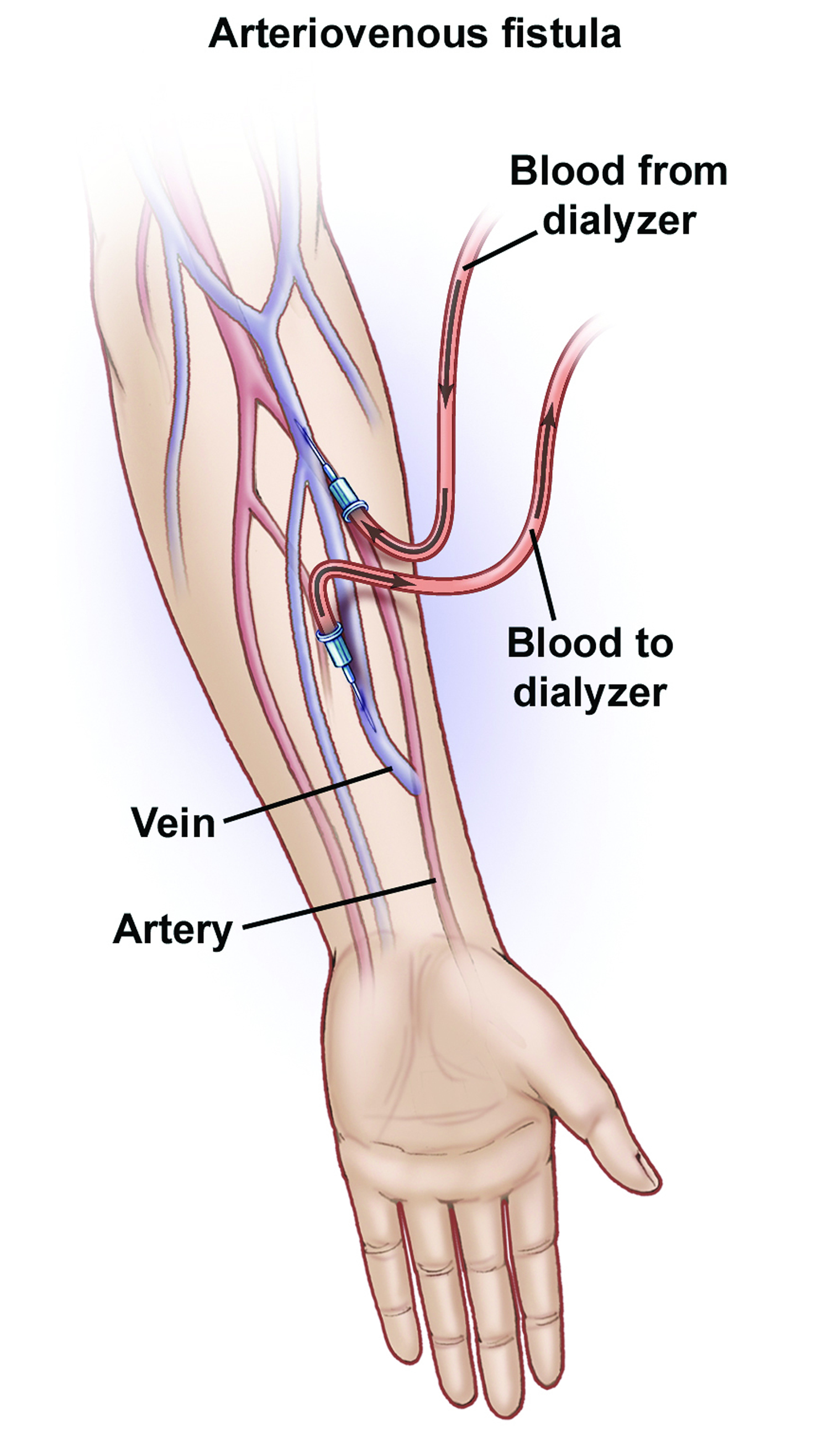
Arteriovenous fistula fistula is an abnormal connection between an artery and a vein. This causes blood to flow directly from the artery into the vein, bypassing the capillaries that are located downstream of the fistula, resulting in a diminished blood supply. Arteriovenous fistula fistulas can occur anywhere in the body, but are common in the legs. Sometimes, an arteriovenous fistula fistula is surgically created for hemodialysis access. Recently, nonsurgical, percutaneous procedures are being used to create arteriovenous fistula fistulas for dialysis access.
A large untreated arteriovenous fistula can lead to serious complications. If you’ve had an arteriovenous fistula created for dialysis, your doctors will monitor you for complications.
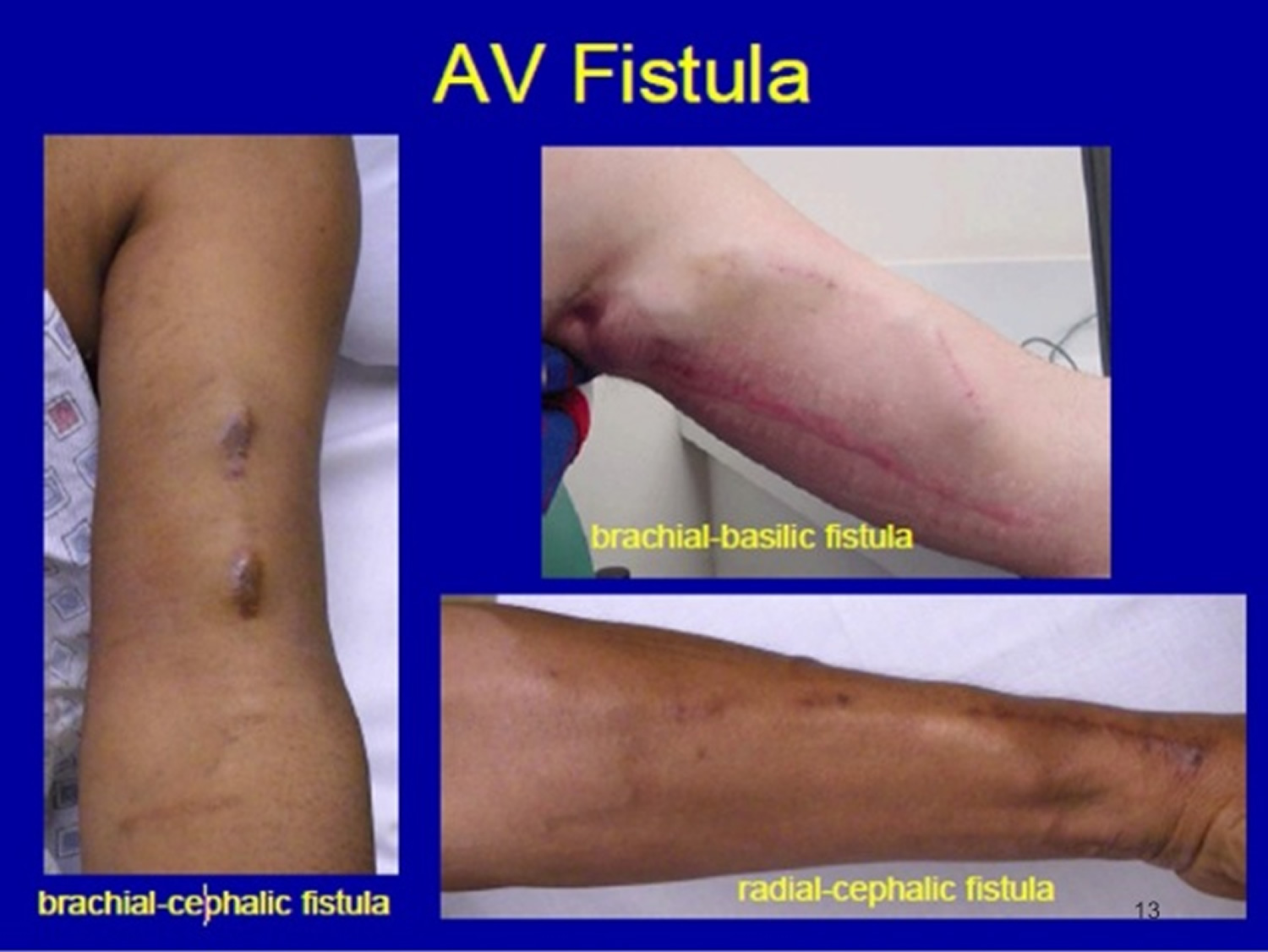
Figure 1. Arteriovenous fistula
Arteriovenous fistula symptoms
Small arteriovenous fistulas in your legs, arms, lungs, kidneys or brain often won’t have any signs or symptoms and usually don’t need treatment other than monitoring by your doctor. Larger arteriovenous fistulas may cause signs and symptoms.
Arteriovenous fistula signs and symptoms may include:
- Purplish, bulging veins that you can see through your skin, similar to varicose veins
- Swelling in the arms or legs
- Decreased blood pressure
- Fatigue
- Heart failure
An arteriovenous fistula in your lungs (pulmonary arteriovenous fistula) is a serious condition and can cause:
- Blueness of the skin
- Clubbing of fingers
- Coughing up blood
An arteriovenous fistula in your gastrointestinal tract can cause bleeding in your digestive tract.
If you have any of these signs and symptoms, and think you might have an arteriovenous fistula, make an appointment to see your doctor. Early detection of an arteriovenous fistula may make your condition easier to treat and may reduce your risk of developing complications, such as blood clots or, in severe cases, heart failure.
Arteriovenous fistula causes
Causes of arteriovenous fistulas include:
- Cardiac catheterization. An arteriovenous fistula may develop as a complication of a procedure called cardiac catheterization. During cardiac catheterization, a long, thin tube called a catheter is inserted in an artery or vein in your groin, neck or arm and threaded through your blood vessels to your heart. If the needle used in the catheterization crosses an artery and vein during your procedure, and the artery is widened (dilated), this can create an arteriovenous fistula. This rarely happens.
- Injuries that pierce the skin. It’s also possible to develop an arteriovenous fistula after a piercing injury, such as a gunshot or stab wound. This may happen if your wound is on a part of your body where a vein and artery are side by side.
- Being born with an arteriovenous fistula. Some people are born with an arteriovenous fistula (congenital). Although the exact reason why isn’t clear, in congenital arteriovenous fistulas the arteries and veins don’t develop properly in the womb.
- Genetic conditions. Arteriovenous fistulas in the lungs (pulmonary arteriovenous fistulas) can be caused by a genetic disease (Osler-Weber-Rendu disease, also known as hereditary hemorrhagic telangiectasia) that causes blood vessels to develop abnormally throughout your body, but especially in the lungs.
- Surgical creation (AV fistula procedure). People who have late-stage kidney failure may also have an arteriovenous fistula surgically created to make it easier to perform dialysis. If a dialysis needle is inserted into a vein too many times, the vein may scar and be destroyed. Creating an arteriovenous fistula widens the vein by connecting it to a nearby artery, making it easier to insert a needle for dialysis and causing blood to flow faster. This AV fistula is usually created in the forearm.
Arteriovenous fistula complications
Left untreated, an arteriovenous fistula can cause complications, some of which can be serious. These include:
- Heart failure. This is the most serious complication of large arteriovenous fistulas. Since your blood flows more quickly through an arteriovenous fistula than it would if your blood flowed through a normal course of arteries, capillaries and veins, your heart pumps harder to compensate for the drop in blood pressure (called high output heart failure). Over time, the increased intensity of your heart’s pumping can weaken your heart muscle, leading to heart failure.
- Blood clots. An arteriovenous fistula in your legs can cause blood clots to form, potentially leading to deep vein thrombosis, a painful and potentially life-threatening condition if the clot travels to your lungs (pulmonary embolism). Depending on where your fistula is, it can lead to a stroke.
- Leg pain. An arteriovenous fistula in your leg can also cause you to develop pain in your legs (claudication), or can worsen pain you already have.
- Bleeding. Arteriovenous malformations may lead to bleeding, including into your gastrointestinal system.
Arteriovenous fistula diagnosis
To diagnose an arteriovenous fistula, your doctor will use a stethoscope to listen to the blood flow through the area where he or she thinks you may have a fistula. The blood flow through an arteriovenous fistula makes a sound similar to clicking or humming machinery (machinery murmur).
If your doctor hears a machinery murmur, you’ll have other tests to confirm that the murmur is caused by an arteriovenous fistula. These can include:
- Duplex ultrasound. Duplex ultrasound is the most effective and common way to check for an arteriovenous fistula in the blood vessels of your legs or arms. In duplex ultrasound, an instrument called a transducer is pressed against your skin over the suspicious area. The transducer produces high-frequency sound waves, which bounce off red blood cells. A duplex ultrasound can estimate how fast blood flows by measuring the rate of change in its pitch (frequency).
- Computerized tomography (CT) angiogram. A CT angiogram allows your doctor to check your arteries to see if blood flow is bypassing the capillaries. You’ll receive an injection of a dye that shows up on CT images, and the doughnut-shaped CT scanner will be moved to take images of the artery your doctor believes is narrowed. The images are then sent to a computer screen for your doctor to view.
- Magnetic resonance angiography (MRA). Your doctor may use an MRA if he or she thinks you may have an arteriovenous fistula in an artery that’s deep under your skin. This test allows your doctor to see the soft tissues in your body. It uses the same technique as magnetic resonance imaging (MRI), but also includes the use of a special dye that helps create images of your blood vessels. During an MRI or MRA, you lie on a table inside a long tube-like machine that produces a magnetic field. An MRI machine uses the magnetic field and radio waves to create pictures of your body’s tissues. Using the images from the test, your doctor may be able to see an arteriovenous fistula.
Arteriovenous fistula treatment
It’s possible your doctor may suggest only monitoring your arteriovenous fistula, especially if it’s small and doesn’t cause any other health problems. Some small arteriovenous fistulas close by themselves without treatment.
If your arteriovenous fistula requires treatment, your doctor may recommend:
- Ultrasound-guided compression. If you have an arteriovenous fistula in your legs and it’s easily visible on ultrasound, treatment with ultrasound-guided compression may be an option for you. In this treatment, an ultrasound probe is used to compress the fistula and block blood flow to the damaged blood vessels. This procedure only takes about 10 minutes. But it only works for about one in three people.
- Catheter embolization. In this procedure, a catheter is inserted in an artery near the site of your arteriovenous fistula. Doctors use X-ray and other imaging techniques to guide the catheter to your fistula, and a small coil or stent is placed at the site of your fistula to reroute your blood flow. Many people who have catheter embolization stay in the hospital for 24 hours or less and can resume all their daily activities within a week.
- Surgery. Large arteriovenous fistulas that can’t be treated with catheter embolization may require surgery. The type of surgery you’ll need depends on the size and location of your arteriovenous fistula.
Veins of the Upper Limbs
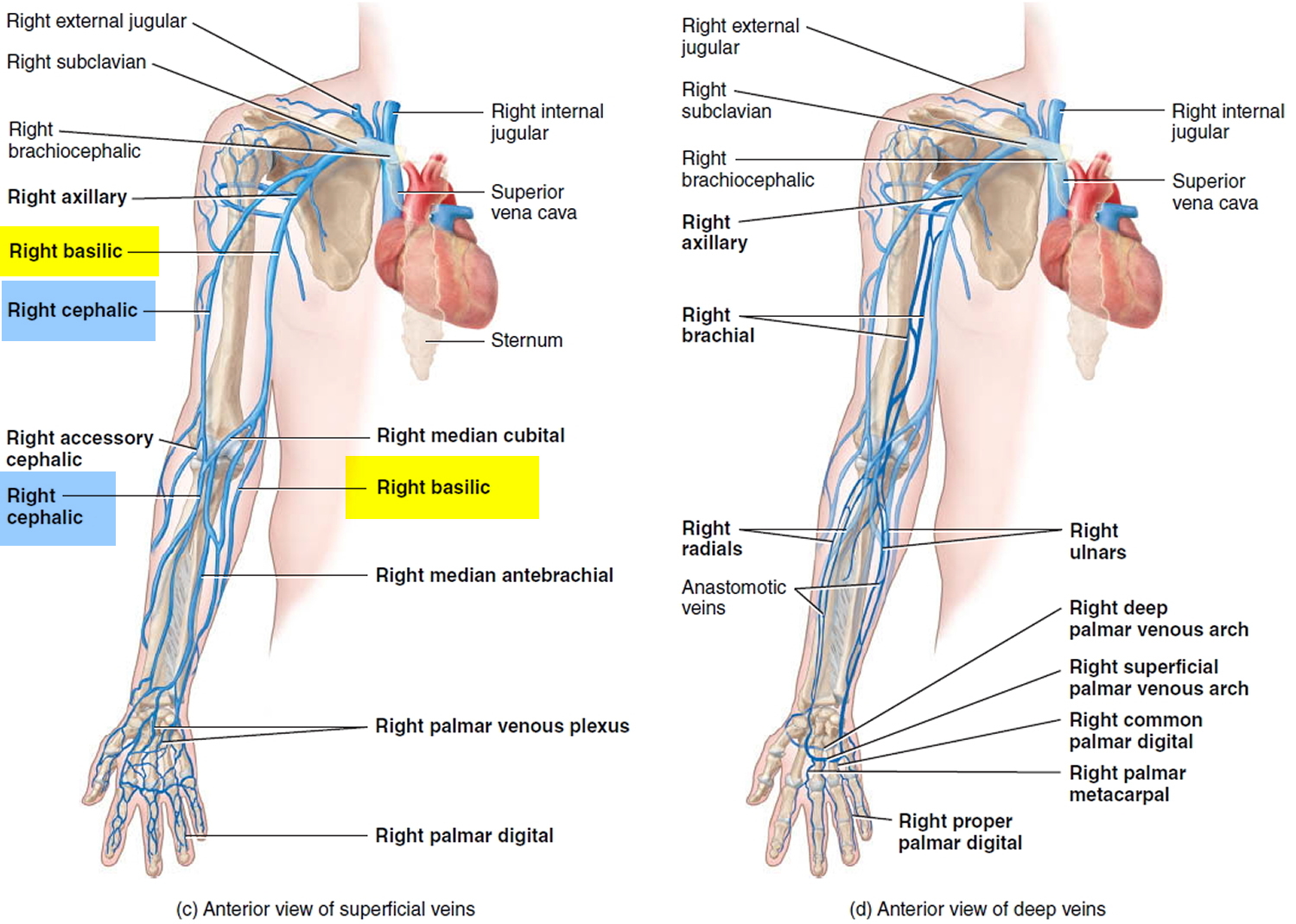
Both superficial and deep veins return blood from the upper limbs to the heart. Superficial veins are located just deep to the skin and are oft en visible. They anastomose extensively with one another and with deep veins, and they do not accompany arteries. Superficial veins are larger than deep veins and return most of the blood from the upper limbs. Deep veins are located deep in the body. They usually accompany arteries and have the same names as the corresponding arteries. Both superficial and deep veins have valves, but valves are more numerous in the deep veins.
Figure 2. Veins of the upper limb
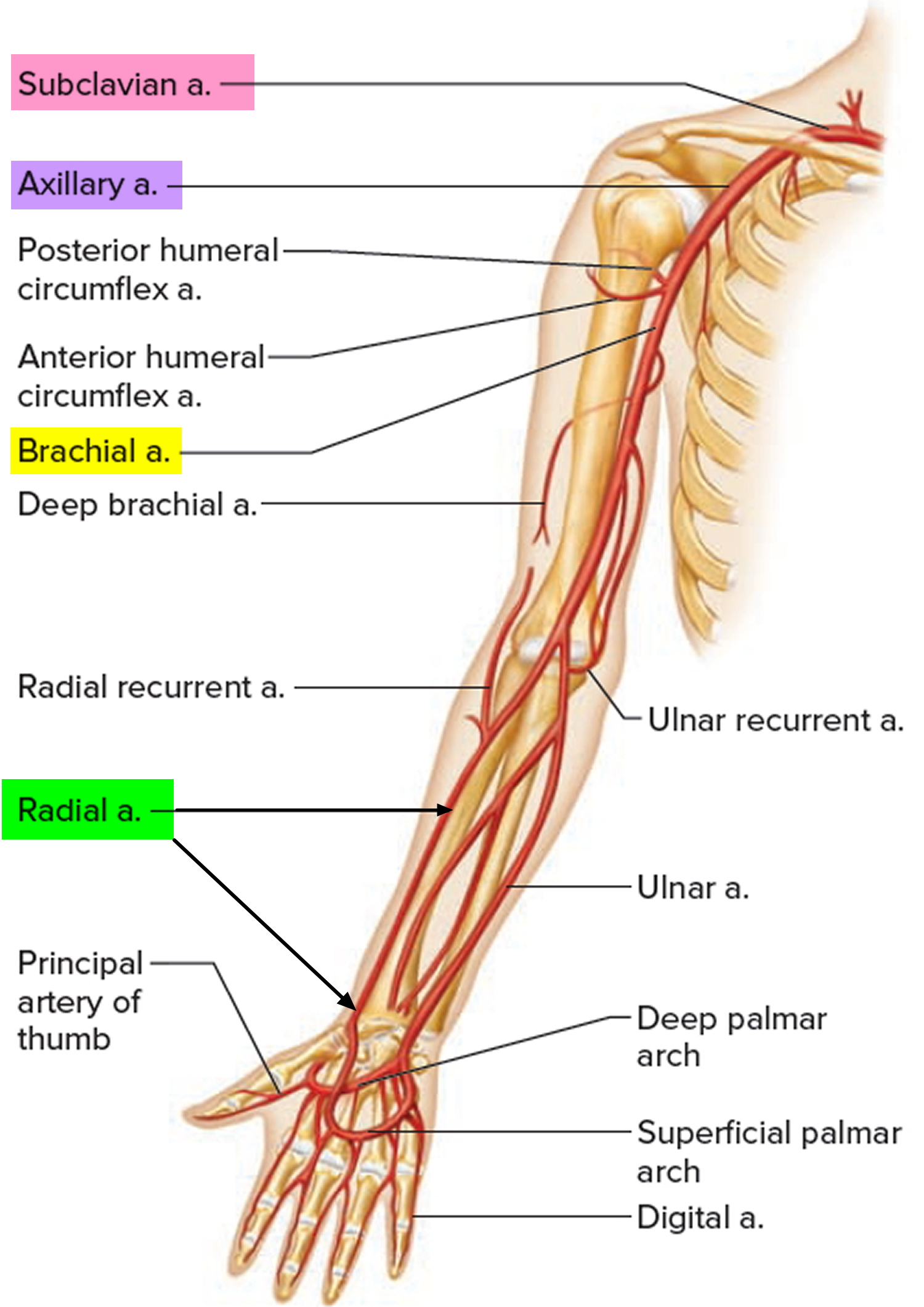
Figure 3. Arteries of the upper limbs
Arteriovenous fistula for dialysis
The superiority of hemodialysis access created by means of an arteriovenous fistula in patients with end stage renal disease has been shown before. Stenosis and thrombosis is less likely to occur in a well-functioning mature arteriovenous fistula when compared to arteriovenous grafts and central venous catheters, resulting in prolonged patency rates for arteriovenous fistulas as has been described 1. Also, arteriovenous fistulas carry a lower risk for infection 2. However, around 20%- 50% of all fistulas fail to mature into a useful hemodialysis access 3.
The preferred location for placing an arteriovenous fistula for the first time is distally at the radius, thus making it possible to place a second fistula proximally if the first one failed to mature. The order of preference for creating an arteriovenous fistula 4:
- Distal Radio-Cephalic (Distal radial artery to cephalic vein)
- Proximal Radio-Cephalic (Proximal radial artery to cephalic vein)
- Brachio-Cephalic (Brachial artery to Cephalic vein)
- Brachio-Basilic (Brachial artery to Basilic vein)
This order is in agreement with the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines 5. However, arteriovenous fistulas created distally at the wrist are less likely to mature compared to proximal arteriovenous fistula, at the same time proximal arteriovenous fistula require less intervention and are likely to last longer 6. The decision of where to create the arteriovenous fistula can be helped by preoperative vascular mapping using ultrasound imaging which is expected to improve chances of creating an arteriovenous fistula that will likely mature into a useful dialysis access 7. Placement of a primary forearm arteriovenous fistula is feasible in 40% to 50%, with an upper arm fistula possible in an additional 25% to 35% of patients 8. An arteriovenous fistula prevalence of ≥ 65% has been recommended in the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines 5 for patients undergoing hemodialysis, this prevalence is currently higher in Europe (67%- 91%) compared to the US (24%- 47%) 9; however, the prevalence of arteriovenous fistulas in the US varies significantly among different dialysis units 10.
Brachiobasilic arteriovenous fistula
The number of patients with end stage renal disease (end stage kidney failure) requiring hemodialysis is steadily rising, a trend that is expected to continue 2. A well-functioning arteriovenous fistula is superior to grafts and central catheters in providing access for hemodialysis efficiently and at the same time with the least rate of access related complications. This has lead vascular surgeons to resort to the basilic vein which by virtue of its anatomical position is less likely to be damaged by repeated cannulation as with the more superficial veins of the arm and forearm. However, a consensus on how to form a brachiobasilic arteriovenous fistula does not exist as some surgeons choose to do this in a one-stage operation, while others prefer a two-stage procedure with the first procedure usually involving making the anastomosis between the basilic vein and the brachial artery, while in the second stage the arterialised vein is mobilised and brought closer to the skin surface to facilitate cannulation for haemodialysis sessions.
Dagher was the first to describe the use of basilic vein to create an arteriovenous fistula in the upper arm between the end of basilic vein and the side of the brachial artery to act as access for long term haemodialysis 11. Since then, the procedure has seen several changes and modifications. Superficialisation of a brachiobasilic fistula to make it more susceptive to cannulation can be achieved either by an elevation technique without mobilisation to bring the vein superficial to the surgically reconstructed deep fascia and subcutaneous tissue in the anatomic location of the basilic vein 12, or by a transposition technique by mobilising the entire length of the basilic vein to position the vein anterolaterally through a subcutaneous flap 13.
Some of the debate surrounding brachiobasilic arteriovenous fistulas has been focused on the decision to choose between one-stage vs the two-stage techniques. The one-stage procedure aims to create a fistula between the basilic vein and the brachial artery in the upper arm in one procedure. This would require a long incision to gain access and mobilise the basilic vein making sure the anastomosis is not placed under tension and no obvious stenosis is present proximally. The main advantage of this technique is the shorter waiting time required to cannulate the fistula. Also the one-stage will prevent the patient from having to undergo another procedure and is more cost effective as hospital resources will be used only once. One of the main disadvantages of this technique is the long incision which will require a longer time to heal and also carries a higher risk for wound-related complications. Also the procedure takes longer and is more demanding 14. Moreover, in a study by Anaya-Ayala et al 15 assessing the anatomy of basilic vein found that only 66% of patients are expected to have a “normal” basilic vein entering one of two paired brachial veins close to the axilla, while up to 34% will have an “abnormal” variant that would negatively influence the newly created fistula maturation.
The two-stage procedure allows the basilic vein to become arterialised and as such, more resistant to torque and will become easier to mobilise in the second procedure as it gets transformed into a bigger and stronger structure. The hope is that operative difficulty and complications would be reduced with improved patency rates 16.
Although more studies seem to favour the 2-stage brachiobasilic arteriovenous fistula approach, evidence in the literature is not sufficient to draw a final conclusion as the difference between the 1-stage and the 2-stage approaches for creation of a brachiobasilic-arteriovenous fistula is not statistically significant in terms of the overall maturation rate and postoperative complications 17. Patency rates (primary, assisted primary and secondary) were comparable in the majority of studies 17. Large randomized properly conducted trials with adequate sub-group analysis are needed before making a final recommendation.
Figure 4. Arteriovenous fistula for dialysis
Dural arteriovenous fistula
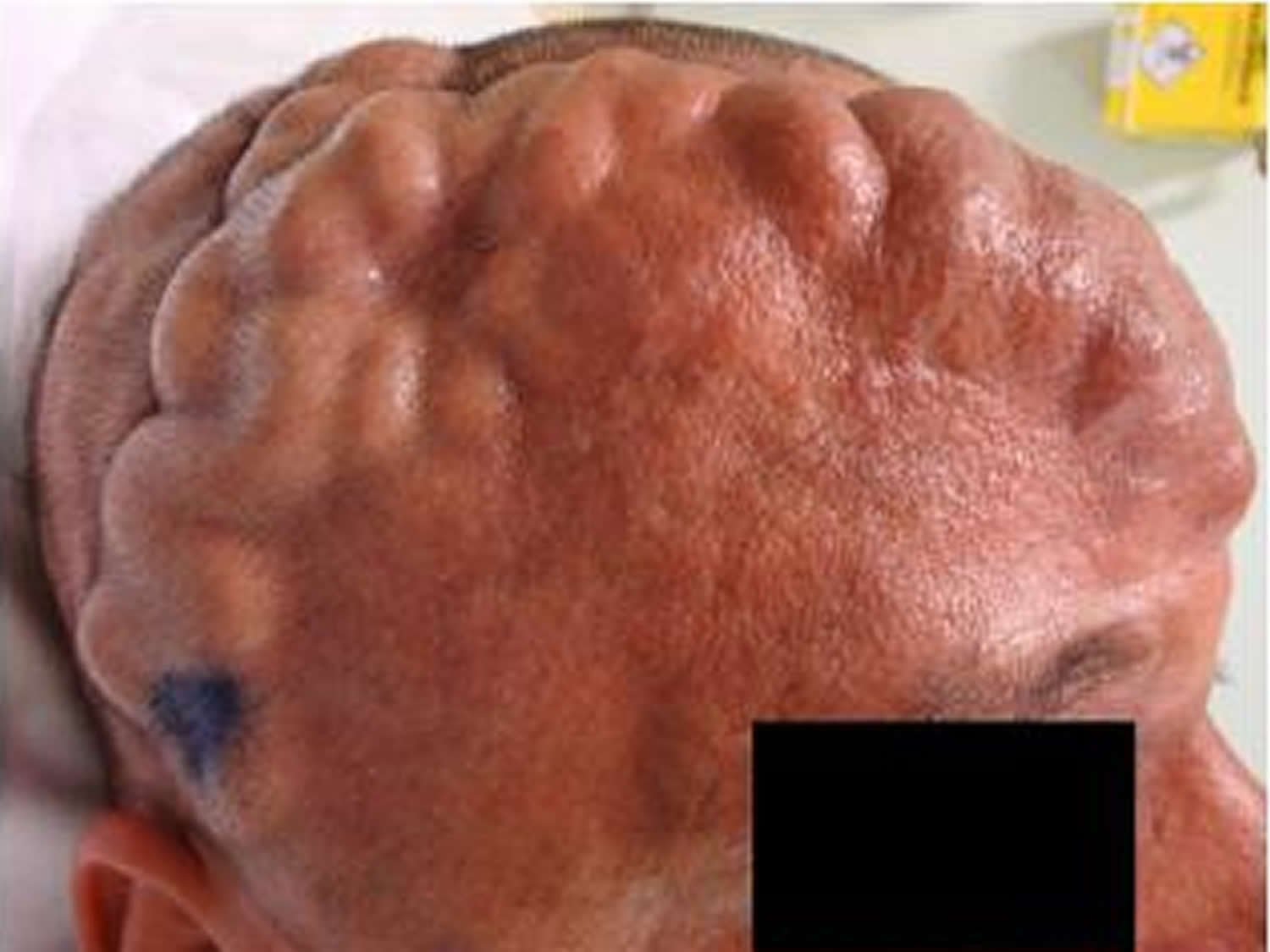
Dural arteriovenous fistula is a rare vascular condition where abnormal connections (fistulas) are made between branches of arteries and veins in the brain or spinal cord covering (dura mater). The dural arteries bring high pressure oxygen-rich blood to the brain and the dural veins take the oxygen-depleted (deoxygenated) low pressure blood back to the heart. A dural arteriovenous fistula causes the high-pressure arterial blood to enter into the veins or sinuses that normally handle low-pressure blood returning to the heart. This can result in ruptures leading to bleeding and brain hemorrhage as well as other neurological issues.
Abnormal passageways between arteries and veins (arteriovenous fistulas) may occur in the brain, spinal cord or other areas of your body.
Dural arteriovenous fistulas tend to occur later in life, and they’re not typically passed on genetically — children aren’t more likely to develop a dural arteriovenous fistula simply because their parent has.
Although some dural arteriovenous fistulas stem from identifiable causes, it’s thought that dural arteriovenous fistulas involving large brain veins usually develop due to narrowing or blockage of one of the brain’s venous sinuses, which normally route circulated blood from the brain back to the heart.
Figure 5. Dural arteriovenous fistula
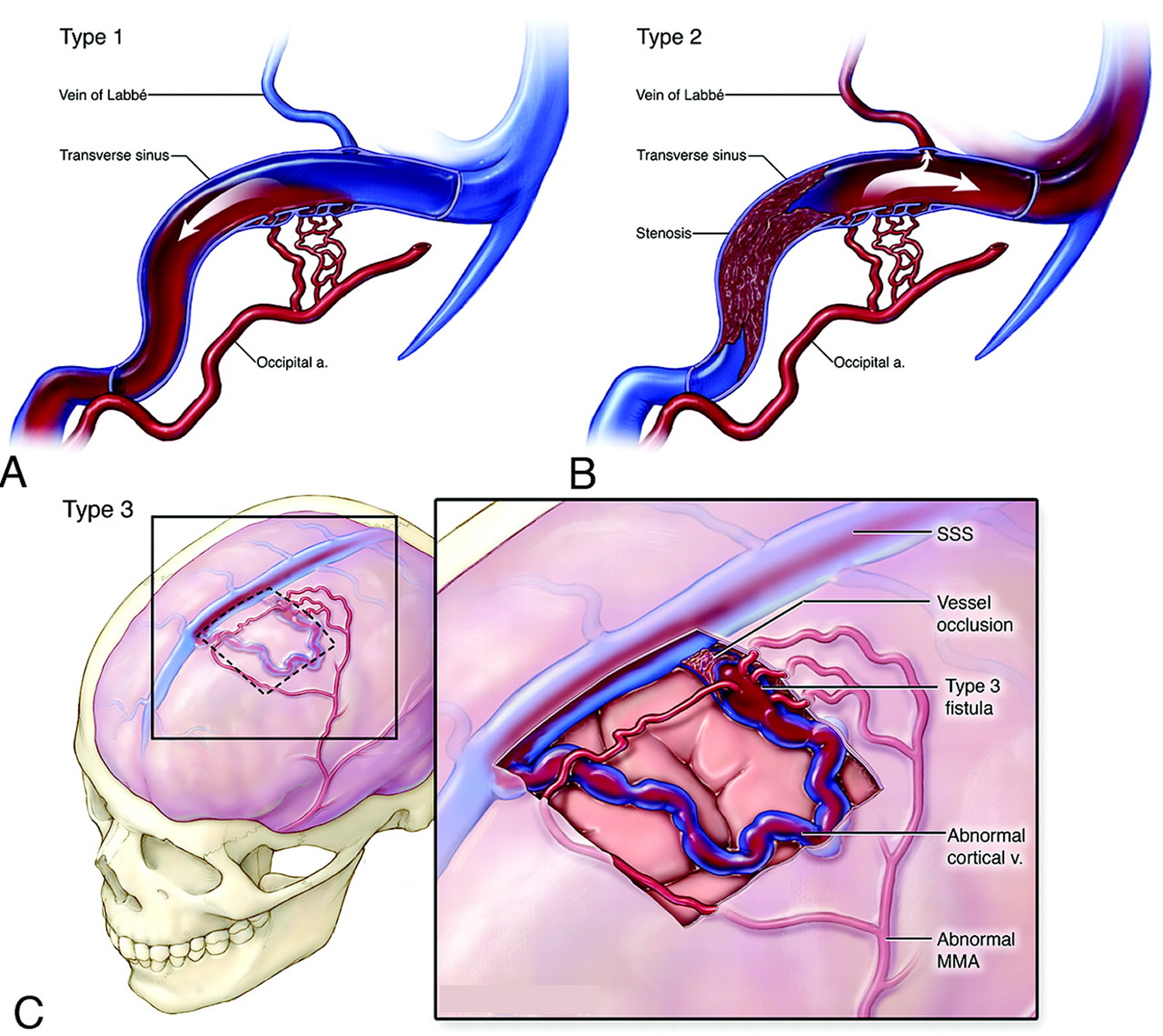 Note: Schematic overview of the Borden system of classification for dural arteriovenous fistulas. A, Type 1 fistula with multiple communications between the occipital artery and transverse sinus. Note antegrade flow and no cortical venous reflux. B, Type 2 lesions are associated with cortical venous reflux. This illustration demonstrates the presence of a transverse sinus fistula with stenosis of the distal transverse and proximal sigmoid sinuses. There is retrograde blood flow into the proximal transverse sinus and the cortical vein. C, Type 3 fistulas represent communication between the meningeal arteries and cortical vein (or an isolated segment of venous sinus). In this schematic drawing, the cortical vein harboring the fistula near the frontal convexity is tortuous and has multifocal stenoses in its pathway.
Note: Schematic overview of the Borden system of classification for dural arteriovenous fistulas. A, Type 1 fistula with multiple communications between the occipital artery and transverse sinus. Note antegrade flow and no cortical venous reflux. B, Type 2 lesions are associated with cortical venous reflux. This illustration demonstrates the presence of a transverse sinus fistula with stenosis of the distal transverse and proximal sigmoid sinuses. There is retrograde blood flow into the proximal transverse sinus and the cortical vein. C, Type 3 fistulas represent communication between the meningeal arteries and cortical vein (or an isolated segment of venous sinus). In this schematic drawing, the cortical vein harboring the fistula near the frontal convexity is tortuous and has multifocal stenoses in its pathway.Figure 6. Traumatic arteriovenous fistula of the superficial temporal artery
Dural arteriovenous fistula symptoms
Some people with a dural arteriovenous fistula may not have any symptoms. However, apparent symptoms can be characterized either as aggressive or benign.
Aggressive dural arteriovenous fistula symptoms can result either from bleeding in the brain (intracerebral hemorrhage) or from neurological effects of non-hemorrhaging neurological deficits.
The symptoms depend on the location and drainage pattern of the dural arteriovenous fistula. Neurological symptoms include:
- Bruit (sound heard due to unusual blood flow)
- Headache
- Pulsatile tinnitus (ringing in the ears)
- Visual difficulty
- Seizures
Bleeding in the brain often causes sudden onset of a headache with varying degrees of neurological disability related to the location and size of the hemorrhage.
By contrast, an non-hemorrhaging neurological deficit usually develops more gradually, over days to weeks, and typically produces a set of symptoms related to its location. These aggressive symptoms can include seizures, speech or language issues, face pain, dementia, Parkinsonism, coordination issues, burning or prickling sensations, weakness, apathy, failure to thrive, and symptoms related to increased pressure such as headaches, nausea and vomiting.
More benign dural arteriovenous fistula symptoms can include both hearing issues (often a bruit behind the ear, also known as pulsatile tinnitus) and vision problems including visual deterioration, eye bulge, swelling in the eye lining, eye-related palsies and cavernous sinus syndrome.
In rare cases, progressive dementia may occur due to venous hypertension.
Make an appointment with a doctor if you develop any signs or symptoms that seem unusual or that worry you.
Seek medical help immediately if you experience any symptoms of seizure, or symptoms that suggest brain hemorrhage, such as:
- Sudden, severe headache
- Nausea
- Vomiting
- Weakness or numbness on one side of the body
- Difficulties in speaking or understanding speech
- Loss of vision
- Double vision
- Balance difficulties
Dural arteriovenous fistula causes
Most dural arteriovenous fistulas have no clear origin, although some result from identifiable causes such as traumatic head injury, infection, previous brain surgery or tumors. Most authorities think that dural arteriovenous fistulas involving the larger brain veins usually arise from progressive narrowing or blockage of one of the brain’s venous sinuses, which route circulated blood from the brain back to the heart.
Risk factors for dural arteriovenous fistula
Genetic risk factors for dural arteriovenous fistulas include those predisposing to vein thrombosis, such as coagulation abnormalities that increase the risk for occlusion of the vein sinuses.
Most frequently, dural arteriovenous fistulas affect people in their late-middle years (roughly from 50 to 60 years old). However, dural arteriovenous fistulas can occur in younger age groups as well, including in children.
Recent evidence does suggest that benign meningeal tumors may also be associated with the development of dural arteriovenous fistulas.
Dural arteriovenous fistula diagnosis
If you have signs or symptoms of a dural arteriovenous fistula (dural arteriovenous fistula), your doctor may recommend that you undergo diagnostic tests, including:
Initial imaging
Initial evaluation typically includes cross-sectional imagery from noncontrast head computerized tomography (CT) and magnetic resonance imaging (MRI).
CT head scans can show fluid buildup caused by heightened cortical vein blood pressure as well as actual bleeding, which may be caused by a dural arteriovenous fistula but occur elsewhere in the brain’s venous system.
MRIs can establish the shape and extent of a dural arteriovenous fistula, detect any micro-hemorrhages (very small bleed locations), and determine the impact of any abnormal blood vessel structures related to the fistula itself.
Angiography
Catheter-based cerebral angiography (also known as digital subtraction angiography) is still the most reliable and definitive tool on dural arteriovenous fistula diagnosis. It’s essential for defining:
- How many fistulae exist and where
- Anatomy of the external carotid arteries and any branches between them and the dura
- Fistula blood vessels’ structure
- Whether cardiovascular disease is also present
- How much narrowing or blockage has occurred in the dural sinus
- Whether any affected veins are dilated and to what extent
Superselective angiography may also be required to identify the area of convergence of the feeding dural arteries and the origin of the draining vein.
Dural arteriovenous fistula treatment
Neurosurgery for dural arteriovenous fistulas
- Endovascular procedures. In an endovascular procedure, your doctor may insert a long, thin tube (catheter) into a blood vessel in your leg or groin and thread it through blood vessels to the dural arteriovenous fistula using X-ray imaging. Your doctor inserts the catheter into the blood vessel that leads to the dural arteriovenous fistula and releases coils or a glue-like substance to block the abnormal connection in the blood vessels.
- Stereotactic radiosurgery. In stereotactic radiosurgery, your doctor uses precisely focused radiation to block the abnormal connection in the blood vessels.
- Surgery. Your surgeon may perform surgery to disconnect the dural arteriovenous fistula.
Spinal dural arteriovenous fistula
Spinal dural arteriovenous fistulas are caused by abnormal blood flow between the dural artery, which supplies the dural root sleeve and adjacent spinal dura, and the medullary vein, which drains the coronal venous plexus 19. This abnormal shunting leads to venous hypertension and the development of clinical symptoms 20. In principle, there are three types of spinal vascular malformation: extradural, dural, and intradural 21. The intradural malformations can be separated into perimedullary fistulas and the real intramedullary arteriovenous malformations (AVMs). Intradural arteriovenous malformations (AVMs) are supplied by spinal-cord supplying arteries, whereas dural malformations are supplied by meningeal arteries as branches of the radicular artery 22. Eighty percent of all spinal arteriovenous malformations (AVMs) are dural fistulas. In contrast to the probably inborn perimedullary fistulas, dural arteriovenous fistulas are most likely acquired 23. The fistula itself is located in the dural layer near the penetration point of the nerve root. Venous drainage runs along the spinal cord veins on the surface of the cord, because the local radicular venous drainage is missing. The high venous pressure is presumed to be the cause of clinical symptoms 23.
The clinical picture presents a slowly progressive myelopathy and/or radiculopathy, with attacks of sudden or fluctuating deterioration up to a transverse lesion 24.
The surgical treatment consists of intradural interruption of the draining vein with coagulation or excision of the dural fistula. Alternatively, endovascular treatment with liquid embolic material is possible 25.
Spinal dural arteriovenous fistula signs and symptoms
The mean duration of symptoms was 15 months (range 4–45 months). Progressive paraparesis was the earliest and most prominent feature in five patients, and monoparesis in three patients. Sensory deficits were the first sign in six patients with dural arteriovenous fistula, and pain was the initial complaint in three patients. One patient initially presented with a bladder disturbance (Table 1).
Table 1. Clinical symptoms of patients with dural arteriovenous fistula, with duration of symptoms in months, initial symptom and symptoms at diagnosis
| No. | Duration | Initial symptom | Symptoms at diagnosis |
| 1 | 28 | Hypesthesia | Sensory transverse lesion L1 with spasticity, ataxia, fasciculations, left-sided |
| 2 | 6 | Back pain | Right-accentuated spastic paraparesis, bladder disturbance, acrodistal sensory deficit, ataxia |
| 3 | 7 | Paraparesis | Flaccid paraplegia, bladder disturbance, no sensory deficit, right-accentuated |
| 4 | 12 | Paraparesis | Paraplegia, sensory transverse lesion L1, bladder disturbance, bilateral |
| 5 | 6 | Bladder dysf. | Flaccid mild paraparesis, sensory transverse lesion L1, bladder disturbance, bilateral, ataxia |
| 6 | 5 | Monoparesis | Flaccid severe right-sided monoparesis, acrodistalsensory deficit |
| 7 | 6 | Ataxia | Flaccid mild paraparesis, sensory deficit L3, bladder disturbance, right-accentuated, ataxia |
| 8 | 8 | Ataxia | Flaccid mild atactic paraparesis, sensory deficit L3, bladder disturbance, right-accentuated |
| 9 | 30 | Sensory deficit | Left-accentuated paraparesis, sensory deficit L1, bladder disturbance, fasciculations |
| 10 | 6 | Sensory deficit | Gait ataxia, sensory transverse lesion T10, bladder disturbance, fasciculations, bilateral |
| 11 | 45 | Spasticity | Spastic atactic paraparesis, sensory transverse lesion T12, bladder disturbance, atrophy, left-accentuated |
| 12 | 13 | Monoparesis | Left-sided mild monoparesis, sensory transverse lesion T12 |
| 13 | 40 | Monoparesis | Spastic severe left-accentuated paraparesis, sensory transverse lesion T10, bladder disturbance, ataxia |
| 14 | 17 | Sensory deficit | Mild left-accentuated paraparesis, sensory transverse lesion T12, bladder disturbance, ataxia |
| 15 | 6 | Back pain | Spastic atactic mild paraparesis, sensory transverse lesion T10, bladder disturbance, atrophy, right-accentuated |
| 16 | 18 | Back pain | Spastic atactic mild paraparesis, sensory deficit L4, bladder disturbance, right-accentuated |
| 17 | 20 | Sensory deficit | Spastic atactic mild paraparesis, sensory deficit L3, bladder disturbance, right-accentuated |
| 18 | 4 | Sensory deficit | Spastic atactic mild paraparesis, sensory transverse lesion T10, bladder disturbance, right-accentuated |
Spinal dural arteriovenous fistula treatment
Although advances toward a better understanding of the pathophysiology of spinal dural arteriovenous fistula have been made, the optimal initial treatment strategy remains a matter of debate. Some clinicians have advocated microsurgical interruption of the fistula as the primary treatment of choice, while others have advocated the use of endovascular embolization 27. Endovascular therapy is less invasive than microsurgery, and it allows both diagnosis and treatment in a single session.
Spinal dural arteriovenous fistula appears to be particularly amenable to endovascular techniques 28. One advantage of endovascular therapy is the ability to diagnose and treat the lesion in a single session; however, more than one session may be necessary for some patients. Although endovascular therapy is potentially less invasive and associated with less morbidity and earlier mobilization than surgery, endovascular therapy has been associated with a lower initial success rate and higher rate of recurrence than microsurgical therapy 29. However, recent reports have shown that liquid adhesive materials, such as N-butyl 2-cyanoacrylate, are superior to polyvinyl alcohol, which showed a recanalization rate of 83%. Another report showed that N-butyl 2-cyanoacrylate embolization was a successful primary treatment in 75-90% of patients with spinal dural arteriovenous fistulas, with a recurrence rate of 15% to 20% 30. In other words, the problem with embolization therapy was that, although the development of embolic materials provided a higher success rate, the long-term recurrence rate was still higher than that observed with microsurgical therapy. However, this study 31 had a longer follow-up period and lower recurrence rate than those of other studies 30.
Several factors need to be considered when selecting endovascular therapy for the treatment of spinal dural arteriovenous fistula. A spinal dural arteriovenous fistula usually consists of multiple dural arterial vessels with a single draining vein 19. Thus, occlusion of a feeding arterial vessel may lead to recanalization or collateral development in the early postoperative period. Recanalization occurred in Patient 7 in this study 31, and N-butyl 2-cyanoacrylate embolization failed to occlude the proximal draining vein. Another important consideration is the identification of patients with conditions that would make them unsuitable for endovascular therapy. Embolization therapy may not be feasible if the arterial feeder is too small to catheterize and arterial damage due to catheter manipulation is likely, as in patients with severe arteriosclerosis, or if the anterior spinal artery of Adamkiewicz and feeding artery of the fistula originate from the same segmental artery 32. Microsurgical obliteration is necessary in such cases. In our study, microsurgery was initially performed in Patient 18 because the anterior spinal artery originated from the same arterial pedicle as the artery feeding the fistula.
Technological advances have made it possible to use a combined approach for the management of spinal dural arteriovenous fistulas. In the this study 31, embolization was performed as the primary treatment modality, and surgery was reserved for patients in whom embolization was deemed dangerous and those in whom embolization had failed or could not be performed. Clinical status improved in 15 patients (83.3%, 15 of 18 patients, 14 embolizations and 1 surgery) during the follow-up period. There were significant differences between functional status at diagnosis and at the last follow-up in the entire patient group 31.
Many reports have stated that failure to occlude the draining vein at the site of the spinal dural arteriovenous fistula is the main cause of spinal dural arteriovenous fistula recurrence 33. Fistula recurrence was observed in 15% of patients in whom N-butyl 2-cyanoacrylate failed to occlude the proximal draining vein 33. Although there are few articles concerning the follow-up treatment after failure of endovascular treatment in patients with spinal Darteriovenous fistulas, the feasibility of filling the draining vein via the microcatheter is considered the main factor for determining the need for repeat embolization therapy 34. However, it is important to consider the fact that repeat embolization therapy is associated with several potential hazards, such as repeat angiography, radiation exposure, risk of inadvertent embolization of the spinal vasculature, and risk of morbidity.
The duration of the follow-up period was less than two years in some patients. Until now, surgery has a higher obliteration rate than endovascular therapy for the treatment of spinal dural arteriovenous fistula. However, the important findings of this study 35 are that the recurrence rate tends to decrease after endovascular therapy when liquid embolization material is used and that endovascular therapy provides a reasonable chance of achieving complete obliteration in most patients. These findings suggest that embolization therapy should be considered as the initial treatment of choice for patients with spinal dural arteriovenous fistulas 35. However, treatment failure of embolization is still higher than that of surgery 26. However, embolization has the advantage of low general morbidity, and may be helpful in patients with reduced physical condition. The chance of complete cure following embolization with a permanent agent is considered to be inferior to surgery. In case of extensive collateral vessels or feeding vessels, the primary option is surgery. Some also recommend surgery as the definitive treatment of dural arteriovenous fistula in recurrent fistulas with collateralization from the opposite side or other levels. Otherwise, an attempt at embolization following diagnostic angiography should be made.
Arteriovenous fistula brain
Brain arteriovenous malformations are complex tangles of abnormal, dilated channels that do not have a typical artery or vein structure 36. They are important risk factors of intracranial hemorrhage, especially in children and young adults 37. The causs is currently not well understood. Prevention of new or recurrent intracranial hemorrhage is the primary rationale to treat arteriovenous malformations, with some combination of resection, embolization and/or radiotherapy. All of these therapies are invasive and associated with considerable side effects 38. Other than nonspecific control of symptomatology, e.g., headache and seizures, no specific medical therapy is available to directly treat arteriovenous malformations or decrease spontaneous rupture risk. About 20% of patients currently are not offered treatment due to excessive risks associated with the treatment 39. There is also considerable controversy regarding unruptured brain arteriovenous malformations being treated using invasive modalities, as treatment risk may outweigh the natural history risk of spontaneous rupture 38. The lack of proper animal models has critically hampered research progress and new therapy development.
Brain arteriovenous malformations are traditionally regarded as congenital lesions, which are thought to arise in the third week of gestation secondary to disordered embryogenesis. Primordial vascular channels fail to differentiate into mature intervening capillaries and veins, and instead create arteriovenous shunts without intervening capillaries 40. However, despite frequent use of prenatal ultrasound, there is remarkably little evidence to show that arteriovenous malformations are congenital lesions arising during embryonic development. In fact, the mean age at presentation (detection) is roughly 40 years of age, with normal distribution. Although it is possible that the lesions uniquely arise prenatally (and a small number do), lacking sufficient data, it would be premature to infer an adequate explanation. There are multiple reports of arteriovenous malformation growing or regressing, and of local arteriovenous malformation regrowth after treatment 41. arteriovenous malformations have been shown to occasionally arise de novo after a normal angiogram and regrow after resection, either de novo from a retained fragment 42 or from a lesion treated with radiotherapy 42. This evidence supports the hypothesis that brain arteriovenous malformation can form postnatally.
More than 95% of brain arteriovenous malformations are sporadic 43. The genesis of brain arteriovenous malformations has been enigmatic. About 5% of brain arteriovenous malformation are due to hereditary hemorrhagic telangiectasia 44, a familial disease characterized by arteriovenous malformations in multiple organs and mucocutaneous telangiectasias (small arteriovenous malformations) 45. The two main subtypes of hereditary hemorrhagic telangiectasia (hereditary hemorrhagic telangiectasia 1 & 2) are caused by mutations in two genes implicated in transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP) canonical signaling pathways: Endoglin (ENG), and Activin-like kinase 1 (ALK1; ACVLR1) 46. As a class, the inherited arteriovenous malformations in hereditary hemorrhagic telangiectasia have some distinguishing morphological features, but are generally similar to the sporadic lesions and cannot be distinguished individually on the basis of their angioarchitecture 47. The prevalence of brain arteriovenous malformation in hereditary hemorrhagic telangiectasia type 1 (ENG-deficient) is 1000-fold higher, and hereditary hemorrhagic telangiectasia type 2 (ALK1-deficient) is 100-fold higher than the prevalence in the general population (10/100,000) 48. Modeling hereditary hemorrhagic telangiectasia type 1 and hereditary hemorrhagic telangiectasia type 2 brain arteriovenous malformations has been fruitful.
Current treatment options for brain arteriovenous malformations are invasive and associated with excessive risk 39. There are no specific medical therapies to treat brain arteriovenous malformations 36.
- Bashar K, Healy D, Browne LD, Kheirelseid EA, Walsh MT, Moloney MC, et al. Role of far infra-red therapy in dialysis arterio-venous fistula maturation and survival: systematic review and meta-analysis. PLoS One. (2014);9: e104931 doi: 10.1371/journal.pone.0104931 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4130633/[↩]
- Frankel A. Temporary access and central venous catheters. Eur J Vasc Endovasc Surg. (2006);31: 417–422. http://www.ejves.com/article/S1078-5884(05)00600-3/fulltext[↩][↩]
- Lynch JR, Mohan S, McClellan WM. Achieving the goal: results from the Fistula First Breakthrough Initiative. Curr Opin Nephrol Hypertens. (2011);20: 583–592. doi: 10.1097/MNH.0b013e32834b33c4 https://www.ncbi.nlm.nih.gov/pubmed/21897231[↩]
- McCann M, Einarsdottir H, Van Waeleghem JP, Murphy F, Sedgewick J. Vascular access management 1: an overview. J Ren Care. (2008);34: 77–84. doi: 10.1111/j.1755-6686.2008.00022.x https://www.ncbi.nlm.nih.gov/pubmed/18498572[↩]
- NKF-KDOQI. 2006. Updates Clinical Practice Guidelines and Recommendations. (2006); pp. https://www.ncbi.nlm.nih.gov/pubmed/17044433[↩][↩]
- Sultan S, Hynes N, Hamada N, Tawfick W. Patients on hemodialysis are better served by a proximal arteriovenous fistula for long-term venous access. Vasc Endovascular Surg. (2012);46: 624–634. doi: 10.1177/1538574412462635 https://www.ncbi.nlm.nih.gov/pubmed/23064823[↩]
- Ilhan G, Esi E, Bozok S, Yurekli I, Ozpak B, Ozelci A, et al. The clinical utility of vascular mapping with Doppler ultrasound prior to arteriovenous fistula construction for hemodialysis access. J Vasc Access. (2013);14: 83–88. doi: 10.5301/jva.5000097 https://www.ncbi.nlm.nih.gov/pubmed/23032950[↩]
- Allon M. Current management of vascular access. Clin J Am Soc Nephrol. (2007);2: 786–800. http://cjasn.asnjournals.org/content/2/4/786.long[↩]
- Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. (2008);23: 3219–3226. doi: 10.1093/ndt/gfn261 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2542410/[↩]
- Allon M. Current management of vascular access. Clin J Am Soc Nephrol. (2007);2: 786–800. http://cjasn.asnjournals.org/content/2/4/786.long [↩]
- Dagher F, Gelber R, Ramos E, Sadler J. The use of basilic vein and brachial artery as an A-V fistula for long term hemodialysis. J Surg Res. (1976);20: 373–376. https://www.ncbi.nlm.nih.gov/pubmed/933493[↩]
- Hossny A. Brachiobasilic arteriovenous fistula: different surgical techniques and their effects on fistula patency and dialysis-related complications. J Vasc Surg. (2003);37: 821–826. https://www.ncbi.nlm.nih.gov/pubmed/12663983[↩]
- Vrakas G, Defigueiredo F, Turner S, Jones C, Taylor J, Calder F. A comparison of the outcomes of one-stage and two-stage brachiobasilic arteriovenous fistulas. J Vasc Surg. (2013);58: 1300–1304. doi: 10.1016/j.jvs.2013.05.030 https://www.ncbi.nlm.nih.gov/pubmed/23810301[↩]
- Koksoy C, Demirci RK, Balci D, Solak T, Kose SK. Brachiobasilic versus brachiocephalic arteriovenous fistula: a prospective randomized study. J Vasc Surg. (2009);49: 171–177 e175 doi: 10.1016/j.jvs.2008.08.002 https://www.ncbi.nlm.nih.gov/pubmed/18945577[↩]
- Anaya-Ayala JE, Younes HK, Kaiser CL, Syed O, Ismail N, Naoum JJ, et al. Prevalence of variant brachial-basilic vein anatomy and implications for vascular access planning. J Vasc Surg. (2011);53: 720–724. doi: 10.1016/j.jvs.2010.09.072 https://www.ncbi.nlm.nih.gov/pubmed/21144691[↩]
- Glickman M. Basilic vein transposition: review of different techniques. J Vasc Access. (2014);15 Suppl 7: S81–84. doi: 10.5301/jva.5000260 https://www.ncbi.nlm.nih.gov/pubmed/24817461[↩]
- Bashar K, Healy DA, Elsheikh S, et al. One-Stage vs. Two-Stage Brachio-Basilic Arteriovenous Fistula for Dialysis Access: A Systematic Review and a Meta-Analysis. Schneditz D, ed. PLoS ONE. 2015;10(3):e0120154. doi:10.1371/journal.pone.0120154. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4353636/[↩][↩]
- Camargo Júnior, Otacílio de, Abreu, Márcia Fayad Marcondes de, Abreu, Guilherme Camargo Gonçalves de, Gabriel, Sthefano Atique, & Silva, Isabella Maria Machado da. (2014). Traumatic arteriovenous fistula of the superficial temporal artery. Jornal Vascular Brasileiro, 13(1), 39-42. https://dx.doi.org/10.1590/jvb.2014.008 http://www.scielo.br/pdf/jvb/v13n1/1677-5449-jvb-13-01-00039.pdf[↩]
- McCutcheon IE, Doppman JL, Oldfield EH. Microvascular anatomy of dural arteriovenous abnormalities of the spine: a microangiographic study. J Neurosurg. 1996;84:215–220. https://www.ncbi.nlm.nih.gov/pubmed/8592223[↩][↩]
- Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989;70:360–370. https://www.ncbi.nlm.nih.gov/pubmed/2492595[↩]
- Anson JA, Spetzler RF (1992) Classification of spinal arteriovenous malformations and implications for treatment. BNI Quarterly 8:2–8[↩]
- Hassler W, Thron A, Grote EH (1989) Hemodynamics of spinal arteriovenous fistulas. J Neurosurg 70:360–370 https://www.ncbi.nlm.nih.gov/pubmed/2492595[↩]
- Partington MD, Rufenacht DA, Marsh WR, Piepgras DG (1992) Cranial and sacral dural arteriovenous fistulas as a cause of myelopathy. J Neurosurg 76:615–622 https://www.ncbi.nlm.nih.gov/pubmed/1545254[↩][↩]
- Biondi A, Merland JJ, Hodes JE, Pruvo JP, Reizine D (1992) Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. AJNR 13:913–922 https://www.ncbi.nlm.nih.gov/pubmed/1590191[↩]
- Westphal M, Koch C (1999) Management of spinal dural arteriovenous fistulae using an interdisciplinary neuroradiological/neurosurgical approach: experience with 47 cases. Neurosurgery 45:451–458 https://www.ncbi.nlm.nih.gov/pubmed/10493366[↩]
- Schick U, Hassler W. Treatment and outcome of spinal dural arteriovenous fistulas. European Spine Journal. 2003;12(4):350-355. doi:10.1007/s00586-002-0487-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3467794/[↩][↩]
- Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995;82:196–200. https://www.ncbi.nlm.nih.gov/pubmed/7815146[↩]
- Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae : results and follow-up. Neurosurgery. 1997;40:675–682. discussion 682-683. https://www.ncbi.nlm.nih.gov/pubmed/9092840[↩]
- Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke. 2002;33:1578–1583. http://stroke.ahajournals.org/content/33/6/1578.long[↩]
- Song JK, Gobin YP, Duckwiler GR, Murayama Y, Frazee JG, Martin NA, et al. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae. AJNR AM J Neuroradiol. 2001;22:40–47. http://www.ajnr.org/content/22/1/40.long[↩][↩]
- Park SB, Han MH, Jahng T-A, Kwon BJ, Chung CK. Spinal Dural Arteriovenous Fistulas: Clinical Experience with Endovascular Treatment as a Primary Therapeutic Modality. Journal of Korean Neurosurgical Society. 2008;44(6):364-369. doi:10.3340/jkns.2008.44.6.364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2615139/[↩][↩][↩][↩]
- Thron A. [Spinal dural arteriovenous fistulas] Radiologe. 2001;41:955–960. https://www.ncbi.nlm.nih.gov/pubmed/11765536[↩]
- Song JK, Vinuela F, Gobin YP, Duckwiler GR, Murayama Y, Kureshi I, et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg. 2001;94:199–204. https://www.ncbi.nlm.nih.gov/pubmed/11302620[↩][↩]
- Jellema K, Sluzewski M, van Rooij WJ, Tijssen CC, Beute GN. Embolization of spinal dural arteriovenous fistulas: importance of occlusion of the draining vein. J Neurosurg Spine. 2005;2:580–583. https://www.ncbi.nlm.nih.gov/pubmed/15945432[↩]
- Park SB, Han MH, Jahng T-A, Kwon BJ, Chung CK. Spinal Dural Arteriovenous Fistulas: Clinical Experience with Endovascular Treatment as a Primary Therapeutic Modality. Journal of Korean Neurosurgical Society. 2008;44(6):364-369. doi:10.3340/jkns.2008.44.6.364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2615139[↩][↩]
- Chen W, Choi E-J, McDougall CM, Su H. Brain Arteriovenous Malformation Modeling, Pathogenesis and Novel Therapeutic Targets. Translational stroke research. 2014;5(3):316-329. doi:10.1007/s12975-014-0343-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4081044/[↩][↩]
- Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863–873. https://www.ncbi.nlm.nih.gov/pubmed/11897302[↩]
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614–621. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4119885/[↩][↩]
- Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98(1):3–7. https://www.ncbi.nlm.nih.gov/pubmed/12546345[↩][↩]
- Rubin D, Santillan A, Greenfield JP, Souweidane M, Riina HA. Surgical management of pediatric cerebral arteriovenous malformations. Childs Nerv Syst. 2010;26(10):1337–1344. https://www.ncbi.nlm.nih.gov/pubmed/20596869[↩]
- Du R, Hashimoto T, Tihan T, Young WL, Perry VH, Lawton MT. Growth and regression of an arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia: case report. J Neurosurg. 2007;106(3):470–477. https://www.ncbi.nlm.nih.gov/pubmed/17367071[↩]
- Klimo P, Jr., Rao G, Brockmeyer D. Pediatric arteriovenous malformations: a 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007;23(1):31–37. https://www.ncbi.nlm.nih.gov/pubmed/17053936[↩][↩]
- Inoue S, Liu W, Inoue K, Mineharu Y, Takenaka K, Yamakawa H, et al. Combination of linkage and association studies for brain arteriovenous malformation. Stroke. 2007;38(4):1368–1370. http://stroke.ahajournals.org/content/38/4/1368.long[↩]
- Bharatha A, Faughnan ME, Kim H, Pourmohamad T, Krings T, Bayrak-Toydemir P, et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke. 2012;43(1):72–78. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3727386/[↩]
- Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990;95(4):422–427. https://www.ncbi.nlm.nih.gov/pubmed/2212727[↩]
- Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–219. https://www.ncbi.nlm.nih.gov/pubmed/20870325[↩]
- Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME, Manzia JL. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000;21(6):1016–1020. http://www.ajnr.org/content/21/6/1016.long[↩]
- Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2640934/[↩]