Contents
- Trypanosomiasis
- African Trypanosomiasis
- African sleeping sickness transmission cycle
- African sleeping sickness statistics
- Major human epidemics
- African Trypanosomiasis causes
- Risk factors for getting African trypanosomiasis
- African Trypanosomiasis symptoms
- African trypanosomiasis complications
- African Trypanosomiasis diagnosis
- African Trypanosomiasis treatment
- African sleeping sickness antiparasitic
- African sleeping sickness prognosis
- American Trypanosomiasis
- American trypanosomiasis transmission
- Who is more likely to develop American trypanosomiasis?
- If I have American trypanosomiasis (Chagas disease), should my family members be tested for the infection?
- What should I do if I think I have American trypanosomiasis (Chagas disease)?
- In what parts of the world is American trypanosomiasis (Chagas disease) found?
- I plan to travel to a rural area of Latin America that might have American trypanosomiasis (Chagas disease). How can I protect myself from this infection?
- American trypanosomiasis signs and symptoms
- American trypanosomiasis diagnosis
- American trypanosomiasis treatment
- American trypanosomiasis transmission
Trypanosomiasis
Trypanosomiasis is a disease usually referring to African human trypanosomiasis also known as “sleeping sickness” or “African sleeping sickness“. The African trypanosomiasis is caused by the parasites Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense and the tsetse fly (Glossina species) transmits the African Trypanosomiasis 1. Tsetse flies are found in woodland and savannah areas and they bite during daylight hours. Travelers to urban areas are not at risk. The persons most likely to be exposed to the infection are tourists, hunters, and others working in or visiting game parks. Villagers with infected cattle herds are also at risk. African Trypanosomiasis is distinct from American Trypanosomiasis or Chagas disease, which is caused by Trypanosoma cruzi and transmitted by the Reduuvid insect vector 2. Both African Trypanosomiasis and American Trypanosomiasis have unique epidemiologic and clinical characteristics.
African trypanosomiasis is seen in up to 30 countries across sub-Saharan Africa, and over 7000 cases of the disease were reported in 2012 3. The parasite and subsequent disease are classically broken up into west and east African variants. The west/central African form is caused by Trypanosoma brucei gambiense. It is often chronic and deadly if untreated. The east/southern African infection caused by Trypanosoma brucei rhodesiense and in addition to humans is often found in cattle 1. The vast majority of reported infections are caused by Trypanosoma brucei gambiense. Geographical distribution has demonstrated progressive overlap as Trypanosoma brucei rhodesiense has moved northwest 4.
African trypanosomiasis is mainly seen in rural communities and impoverished areas. This distribution is underreported, and although the World Health Organization (WHO) has attempted to re-institute control programs, not all countries report disease or implement these measures.
The chronic form of African trypanosomiasis from Trypanosoma brucei gambiense is rare in short-term tourists and visitors but is seen in refugees and immigrants. In contrast Trypanosoma brucei rhodesiense has been seen in tourists to East Africa, mainly in Tanzania 4.
Longitudinally, the WHO’s goal is to eliminate African trypanosomiasis by 2020. Multiple screening methods, therapeutic delivery plans, and disease reporting programs are being implemented in an attempt to make this a reality.
American Trypanosomiasis also known as Chagas disease, is a potentially life-threatening zoonotic disease caused by the parasite Trypanosoma cruzi. American trypanosomiasis or Chagas disease is an illness that can cause serious heart and stomach problems. American Trypanosomiasis or Chagas disease is usually spread by infected blood-sucking bugs called triatomine bugs. They are also known as “kissing bugs” because they often bite people’s faces. When one of these bugs bites you, it leaves behind infected waste. You can become infected if you rub the waste in your eyes or nose, the bite wound, or a cut. American trypanosomiasis or Chagas disease can also spread through contaminated food, a blood transfusion, a donated organ, or from the pregnant parent to the baby during pregnancy.
American trypanosomiasis or Chagas disease, although originally discovered in 1909, is still a major cause of morbidity and mortality in endemic Central and South America, Trinidad, and the southern United States where poverty is widespread. with up to 10 million individuals are thought to be infected worldwide. It is estimated that as many as 8 million people living in Mexico, Central America, and South America have American Trypanosomiasis (Chagas disease), most of whom do not know they are infected. While the United States is not an endemic area, American trypanosomiasis or Chagas disease has been seen in the southern states such as Texas and Arizona 5. The majority of these cases are thought to be secondary to the recent large-scale immigration of Latin Americans into non-endemic areas. American trypanosomiasis or Chagas disease in Europe and Australia may reflect this pattern as well 6. If untreated, American Trypanosomiasis (Chagas disease) is lifelong infection and can cause life threatening intestinal and heart problems such as 7:
- An serious arrhythmia (a problem with the rate or rhythm of your heartbeat) that can cause sudden death
- An enlarged heart that doesn’t pump blood well
- Problems with digestion and bowel movements
- An increased chance of having a stroke.
Figure 1. Tsetse fly (genus Glossina)
Trypanosomiasis pathophysiology
The vector tsetse fly, Glossina, carries the trypanosome within the midgut after a blood meal. These protozoa then migrate to the salivary glands of the fly whereby they can be transmitted during the next feeding. After inoculation within the host, the parasite can live freely within the bloodstream and evade mammalian host defenses through variable surface glycoproteins 8. The slender form secretes bloodstream stage-specific variable surface glycoproteins to evade the host immune system, and it is in this form that the organism proliferates. As the parasite population increases, a morphologic stumpy form with division arrest occurs. It is in this stage it may be transmitted to another tsetse fly from the mammalian host. Within the new tsetse vector and, after this stumpy stage, the organism progresses to a procyclic form whereby variable surface glycoproteins is lost, and the organism is established again in the fly midgut. Cell division is again arrested, and they migrate to the salivary glands as epimastigote forms. These transition from another proliferative stage to non-proliferative form where they again re-acquire variable surface glycoproteins and are now capable to re-infect a new mammal at the next blood meal 9.
The vector, the Reduviid bug, also called the kissing bug, is primarily a nocturnal insect. At the time of feeding the insect deposits feces through breaks in the skin that contain Trypanosoma cruzi. Less common modes of transmission include the ingestion of contaminated food, congenital transmission, or transmission through contaminated blood or tissue 5. As blood transfusions historically have been a major route of infection in endemic areas, blood donated is often routinely screened.
Trypanosomiasis signs and symptoms
Clinical disease has 2 stages. These are characterized by an early/first hemolymphatic stage and late /second meningoencephalitis stage with an invasion of the central nervous system (CNS).
The earliest manifestation of disease is a cutaneous chancre at the site of inoculation. This however only occurs rarely in patients with Trypanosoma brucei gambiense and infrequently (19%) with those infected by Trypanosoma brucei rhodesiense. Systemic symptoms develop after that with intermittent fever, headache, pruritus, and lymphadenopathy 10. The lymphadenopathy may be particularly conspicuous in the posterior triangle of the neck and has received the eponym “Winterbottom’s sign” 11. Fevers often persist from a day to a week and are separated by afebrile intervals of days to months. Undulating fevers reflect parasites multiplying within the blood. Less frequently hepatosplenomegaly may occur in the early stage. In the late/second stage, CNS symptoms manifest as sleep disturbances or neuropsychiatric disorders. A sleep disorder is the most common symptom of the second stage, and it is from this that the term “African sleeping sickness” was ascribed. Sleep problems are further described as dysregulation of sleep/wake cycles and fragmentation of sleep. Previously inversion of sleep patterns was reported. Additional symptoms include tremor, weakness, paralysis, dyskinesia, or chorea-athetosis. Parkinsonian hypertonia and abnormal reflexes may be seen. Psychiatric changes such as aggression, apathy, psychosis, or irritability may present.
Other organ systems can be involved. Similar to the trypanosome disease of Trypanosoma cruzi the heart may be affected, albeit less frequently, with African trypanosomiasis. Trypanosoma brucei gambiense presents as a prolonged QTc, repolarization abnormalities, or low voltage. These are usually not of clinical significance. Trypanosoma brucei rhodesiense may cause severe pericarditis or myopericarditis.
The thyroid and adrenal cortex may be involved with either hyperfunction or hypofunction. Both are more pronounced with infections caused by Trypanosoma brucei rhodesiense.
Notably, disease in non-native individuals (i.e., travelers or tourists) may be different with cutaneous chancre and a trypanosomal rash much more frequently observed. Additionally, internal involvement is much more pronounced as well.
The clinical end point from either subgroup of Trypanosoma brucei results in coma and death if untreated. Death occurs more rapidly with infections from Trypanosoma brucei rhodesiense often occurring within weeks to months, and later with Trypanosoma brucei gambiense at an average time of 3 years from inoculation 10.
Chagas disease has acute, indeterminate, and chronic stages. Acutely infected patients often are asymptomatic or have mild non-specific febrile illness. Symptoms may include fever, chills, gastrointestinal manifestations, lymphadenopathy, hepatosplenomegaly, or a mixture of cutaneous manifestations. A chagoma is an indurated, erythematous, papule or nodule that occurs at the site of inoculation. This may occur weeks after initial infection. Romana’s sign is classically associated with acute Chagas disease and is characterized by eyelid and periocular edema secondary to parasite deposition into the conjunctiva. Schizotrypanides is a term used to describe a diffuse morbilliform eruption during acute infection and is seen in a minority of infected patients 6.
The indeterminate stage of Chagas disease reflects a host immune response and a decrease in parasite burden. This occurs over months after infection. At this time antibodies to Trypanosoma cruzi are present and clinical disease is absent.
The most devastating stage of the disease is the chronic stage. Up to a third of patients with Chagas disease progress to this stage which exhibits cardiac conduction abnormalities, dilated congestive heart failure, or thromboembolic events. Heart failure often shows aneurysmal dilation of the left ventricle, and the most common conduction defect is a right bundle branch block with or without an anterior fascicular block. Gastrointestinal (GI) involvement occurs in a minority of infected patients, however, of those, the most common manifestation is megaesophagus from damage to autonomic ganglia with subsequent achalasia, dysphagia, weight loss, or recurrent aspiration. Finally, patients with the disease who become immunocompromised may experience reactivation. This may present as recurrence of fever and cutaneous erythematous nodules or plaques along with meningoencephalitis 6.
Trypanosomiasis diagnosis
Before disease onset, screening tests using a card agglutination test have can be implemented. The card agglutination test is a serologic test that utilizes capillary blood, plasma, or serum dilutions. The latter is more specific. This test employs antigens from Trypanosoma brucei gambiense and has a variable sensitivity of near 90%. While some sources boast a negative predictive value of equal to 99% 12, the low prevalence in even endemic areas (less than 5%) causes the positive predictive value to remain low, and thus, the test cannot be used for confirmation of disease 4. Other sensitive serologic tests such as immunofluorescence or enzyme-linked immunosorbent assays are used in non-endemic areas as a screening tool among those in whom it is clinically warranted.
Diagnosis of active disease relies on the constellation of clinical history, exposure to endemic areas, direct visualization of organisms, and adjunctive serologic tests. Microscopic examination of blood, lymph node aspirate, or cerebrospinal fluid (CSF) may yield parasites. Thin and thick blood films have low sensitivity, and miniature anion-exchange centrifugation technique and/or capillary tube centrifugation should be used to increase yield 12. Other than as clinically determined, differentiation between the 2 stages of disease occurs through an examination of the CSF. The number of parasites may be low regardless of stage, and therefore, the WHO criteria allow a white blood cell count (WBC) of greater than 5 cells per microliter or the presence of trypanosomes to be supportive of late disease 12.
Diagnosis of Trypanosoma cruzi is usually established by a history of exposure to an endemic area and direct observation of trypomastigotes in Giemsa-stained wet mounts of blood or CSF. Organisms may be seen in pericardial fluid, bone marrow, brain, skin, or other infected tissues. Polymerase chain reaction (PCR) is the most sensitive mode of detection, but often serologic assays are used to confirm the disease 2.
Trypanosomiasis treatment
Treatment for Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are different, and additionally, treatment is dependent on the stage of infection.
For the first-stage of the disease caused by Trypanosoma brucei gambiense pentamidine is the first-line therapy. Route of administration is intramuscular for one week or intravenous with normal saline over 2 hours. Three injections versus prolonged therapy may be equally effective. Once an involvement of CSF is detected pentamidine is no longer effective. Side effects of pentamidine include injection site reactions, abdominal pain, and hypoglycemia. More serious adverse events seen with pentamidine in the treatment of other diseases include leukopenia, thrombocytopenia, hyperkalemia, and QT-prolongation.
Second stage disease caused by Trypanosoma brucei gambiense includes eflornithine or melarsoprol. Eflornithine has been shown to be superior in reducing mortality as compared to melarsoprol and is, therefore, the preferred medication for second-stage disease. Combination of nifurtimox and eflornithine reduces dosage and cost of therapy 13. Adverse reactions are similar to eflornithine and include pancytopenia, GI distress, and convulsions.
The first stage of disease caused by Trypanosoma brucei rhodesiense is treated with suramin. Although also effective for disease caused by Trypanosoma brucei gambiense the high prevalence of Onchocerca in those areas, and the risk of severe allergic reaction with suramin prohibits use in west and central Africa. For Trypanosoma brucei rhodesiense, first-stage disease suramin is used for up to 30 days. Notably, this medication is rapidly degraded in the air and needs to be injected immediately after dilution with distilled water. Adverse reactions with suramin include hypersensitivity reactions, nephrotoxicity, peripheral neuropathy, bone marrow toxicity, and subsequent agranulocytosis and/or thrombocytopenia 4.
Trypanosoma brucei rhodesiense second-stage disease can be treated with melarsoprol. Adverse reactions may be severe including an encephalopathic syndrome in up to 8% of patients. Dexamethasone and diazepam are used for this. Skin reactions including pruritus and maculopapular rashes are common with bullous lesions occurring rarely. Motor and sensorial neuropathies may occur 13.
Treatment for Chagas disease is unique in that 2 nitroheterocyclic compounds are used. These are benznidazole and nifurtimox, and the former is preferred secondary to its favorable side effect profile. Dosing of benznidazole is 5 mg/kg per day for 60 days, and nifurtimox is given 8 to 10 mg/kg per day in adults, 12.5 mg/kg per day in adolescents, and 15 to 20 mg/kg per day in children, all with a duration of 90 to 120 days. Side effects of benznidazole include photosensitivity, severe exfoliative dermatitis, peripheral neuropathy, and bone marrow suppression. This necessitates a complete metabolic panel, hepatic function panel, and complete blood count with the latter repeated every two weeks while therapy is given. Side effects with nifurtimox include gastrointestinal distress, peripheral neuropathy, and mood disturbances. Complete blood count, hepatic function panel, and a complete metabolic panel (CMP) should be obtained with peripheral neuropathy assessment every 2 weeks while on treatment 14.
Trypanosomiasis prognosis
Prognosis of untreated disease is dismal with death being invariable 15. Early treatment has dramatically reduced mortality rates but a delay in diagnosis may be fatal. When melarsoprol was the only therapeutic option mortality was higher with 4 % to 12% of deaths occurring from treatment alone 11.
Among patients with Chagas disease, cardiomyopathy may occur in up to a third of infected patients and be devastating. Megaesophagus occurs less frequently, but when present, contributes tremendous morbidity. Treatment of the acute disease may result in a cure rate up to 80% and treatment with benznidazole may reduce the occurrence of ECG abnormalities and serological titers. The success of treating patients with chronic stage Chagas disease is uncertain 14.
African Trypanosomiasis
African trypanosomiasis also known as African sleeping sickness or human African trypanosomiasis, is an infectious disease is caused by the parasites Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense 1. Without treatment, African sleeping sickness is considered fatal 16. African trypanosomiasis is transmitted by the tsetse fly (Glossina species), which is found only in sub-Saharan Africa and is endemic in 36 sub-Saharan African countries where the tsetse flies that transmit the disease live in rural environments 17. Tsetse flies are found in woodland and savannah areas and they bite during daylight hours. Travelers to urban areas are not at risk. The persons most likely to be exposed to the infection are tourists, hunters, and others working in or visiting game parks. Villagers with infected cattle herds are also at risk. African Trypanosomiasis is different from American Trypanosomiasis or Chagas disease that occurs mainly in Latin America, which is caused by Trypanosoma cruzi and transmitted by the Reduuvid insect vector 2.
There are two types of Human African trypanosomiasis (African sleeping sickness); each named for the region of Africa in which it was found historically and depending on the subspecies of the parasite involved 18:
- Trypanosoma brucei gambiense the parasite that cause West African trypanosomiasis or Gambian sleeping sickness is found in 24 countries in west and central Africa and currently accounts for 95% of reported cases of African sleeping sickness, causes a chronic infection and is endemic across Central and West Africa 19. A person gets West African trypanosomiasis through the bite of an infected tsetse fly. Occasionally a pregnant woman may pass the infection to her baby. In theory, the infection can be transmitted through a blood transfusion, but such cases rarely have been documented. A person can be infected for months or even years without major signs or symptoms of the disease. When more evident symptoms emerge, the patient is often already in an advanced disease stage where the central nervous system (brain and spinal cord) is affected. In recent years, 7,000-10,000 new cases of West African trypanosomiasis have been reported to the World Health Organization (WHO) annually. However, many cases are not recognized or reported and the true number of annual cases is likely to be higher. Cases of West African trypanosomiasis imported into the United States are extremely rare.
- Trypanosoma brucei rhodesiense the parasite that cause East African trypanosomiasis, which is also carried by the tsetse fly, is found in 13 countries in eastern and southern Africa. Nowadays, East African trypanosomiasis represents under 5% of reported African sleeping sickness cases and causes an acute infection. Each year, a few hundred cases of East African trypanosomiasis are reported to the World Health Organization (WHO). However, many cases are not recognized or reported and the true number of new cases is higher. Since 1967, 40 cases of East African trypanosomiasis have been diagnosed or treated in the United States, all among individuals who had traveled to eastern Africa. First signs and symptoms are observed a few months or weeks after infection. The disease develops rapidly and invades the central nervous system. Only Uganda presents both forms of the disease, but in separate zones.
African trypanosomiasis is seen in up to 30 countries across sub-Saharan Africa, and over 7000 cases of the disease were reported in 2012 3. The parasite and subsequent disease are classically broken up into west and east African variants. The west/central African form is caused by Trypanosoma brucei gambiense. It is often chronic and deadly if untreated. The east/southern African infection caused by Trypanosoma brucei rhodesiense and in addition to humans is often found in cattle 1. The vast majority of reported infections are caused by Trypanosoma brucei gambiense. Geographical distribution has demonstrated progressive overlap as Trypanosoma brucei rhodesiense has moved northwest 4.
African trypanosomiasis is mainly seen in rural communities and impoverished areas. This distribution is underreported, and although the World Health Organization (WHO) has attempted to re-institute control programs, not all countries report disease or implement these measures.
The chronic form of African trypanosomiasis from Trypanosoma brucei gambiense is rare in short-term tourists and visitors but is seen in refugees and immigrants. In contrast Trypanosoma brucei rhodesiense has been seen in tourists to East Africa, mainly in Tanzania 4.
Longitudinally, the WHO’s goal is to eliminate African trypanosomiasis by 2020. Multiple screening methods, therapeutic delivery plans, and disease reporting programs are being implemented in an attempt to make this a reality.
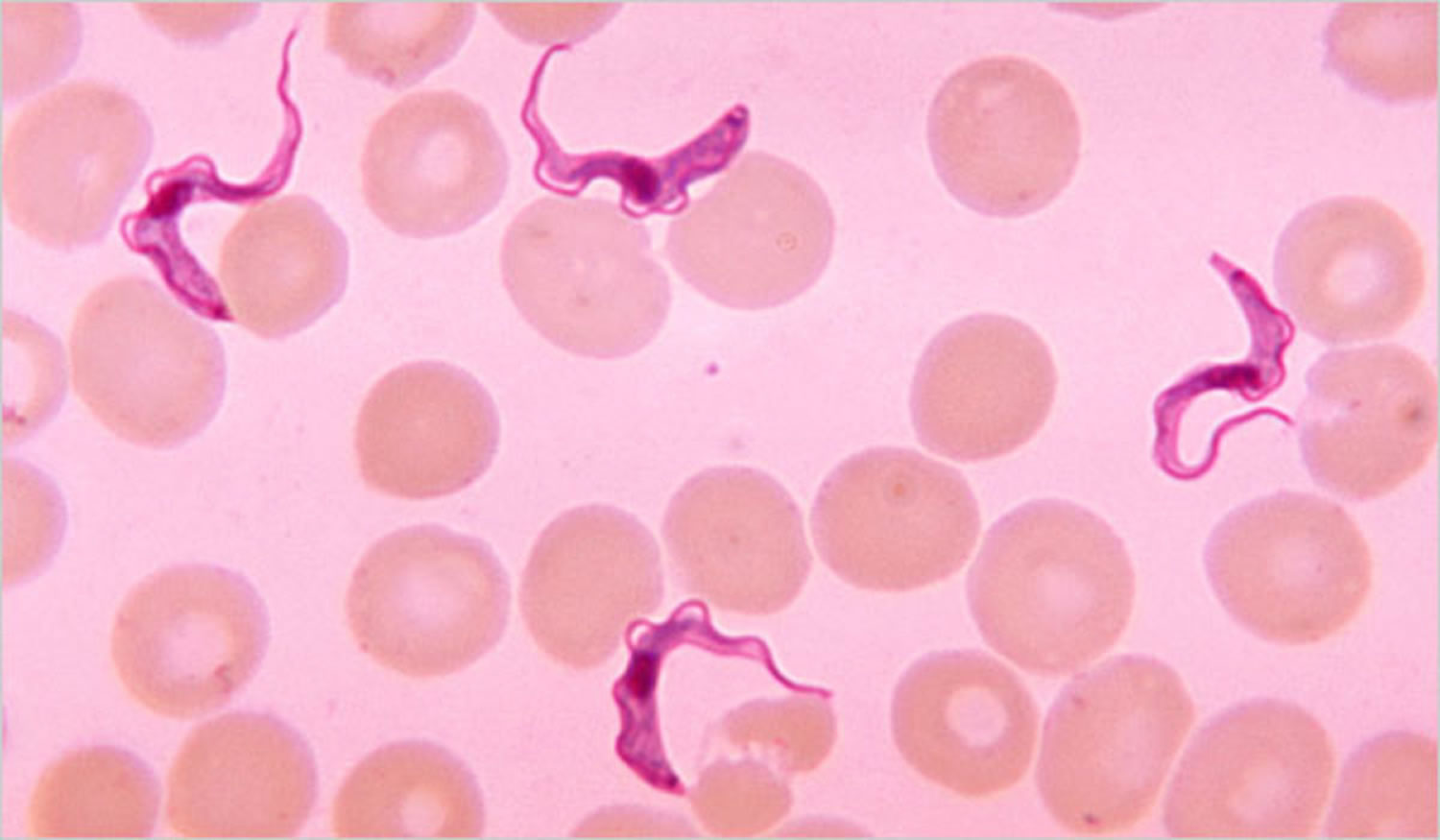
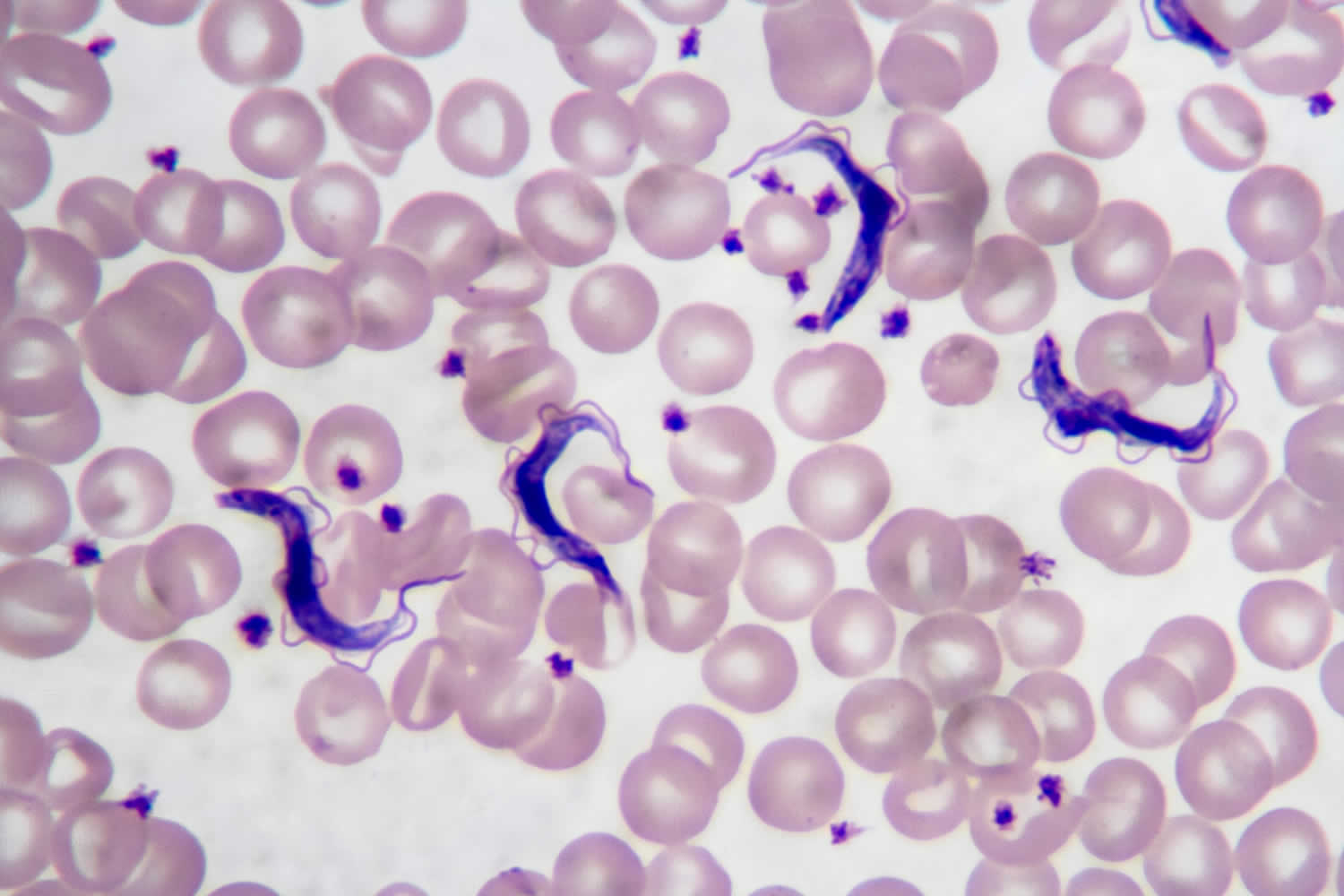
Figure 2. Human African trypanosomiasis (Trypanosoma brucei parasite)
Footnote: Trypanosoma brucei parasites found in a blood smear
East african sleeping sickness
East African trypanosomiasis is caused by the parasite Trypanosoma brucei rhodesiense, which is carried by the tsetse fly. Each year, a few hundred cases of East African trypanosomiasis are reported to the World Health Organization (WHO). However, many cases are not recognized or reported and the true number of new cases is higher. Since 1967, 40 cases of East African trypanosomiasis have been diagnosed or treated in the United States, all among individuals who had traveled to eastern Africa.
A person will get East African trypanosomiasis if he or she is bitten by a tsetse fly infected with the Trypanosoma brucei rhodesiense parasite. The proportion of tsetse flies that are infected with this parasite is low. The tsetse fly is found only in rural Africa. East African trypanosomiasis is found in parts of Eastern and Southeastern Africa. More than 95% of cases are reported from Uganda, Tanzania, Malawi, and Zambia.
East African trypanosomiasis affected individuals typically present after inoculation with a painful eschar and a rapidly progressing illness marked by fevers, rash, fatigue, and myalgias. Within a few weeks to months, the disease progresses to the second stage, with symptoms identical to that of African sleeping sickness Trypanosoma brucei gambiense but with a much-accelerated course that quickly leads to death.
West african sleeping sickness
West African trypanosomiasis also called Gambian sleeping sickness, is caused by a parasite called Trypanosoma brucei gambiense carried by the tsetse fly. In recent years, 7,000-10,000 new cases of West African trypanosomiasis have been reported to the World Health Organization (WHO) annually. However, many cases are not recognized or reported and the true number of annual cases is likely to be higher. Cases of West African trypanosomiasis imported into the United States are extremely rare.
West African trypanosomiasis can be contracted in parts of central Africa and in a few areas of West Africa. Most of the reported cases are found in central Africa (Democratic Republic of Congo, Angola, Sudan, Central African Republic, Republic of Congo, Chad, and northern Uganda). Although the infection is not found in the United States, historically, it has been a serious public health problem in some regions of sub-Saharan Africa. Currently, about 10,000 new cases each year are reported to the World Health organization; however, it is believed that many cases go undiagnosed and unreported. African Trypanosomiasis is considered a neglected tropical disease and is curable with medication, but is fatal if not treated 20.
A person gets West African trypanosomiasis through the bite of an infected tsetse fly. Occasionally a pregnant woman may pass the infection to her baby. In theory, the infection can be transmitted through a blood transfusion, but such cases rarely have been documented.
West African trypanosomiasis initial stage often presents with a painless eschar at the point of infection, though this is often not recalled due to a prolonged asymptomatic course. Patients often have an indolent first stage marked by posterior cervical lymphadenopathy (Winterbottom’s sign), headaches, malaise, and arthralgias. As the disease progresses to the second stage, patients will develop somnolence, fatigue, neurological deficits, tremors, ataxia, seizures, comas, and eventually death.
Who is at risk for contracting West African trypanosomiasis?
The tsetse flies that transmit West African trypanosomiasis are found only in rural areas. Travelers to urban areas are not at risk. The flies bite during daylight hours. They inhabit forests and areas of thick vegetation along rivers and waterholes. Even in areas where the disease is present, most flies are not infected with this parasite, so the risk of infection increases with the number of times a person is bitten by the tsetse fly. Therefore, tourists are not at great risk for contracting West African trypanosomiasis unless they are traveling and spending long periods of time in rural areas of central Africa where the disease is present.
How can I prevent African trypanosomiasis and other insect bites?
- Wear protective clothing, including long-sleeved shirts and pants. The tsetse fly can bite through thin fabrics, so clothing should be made of medium-weight material.
- Wear neutral-colored clothing. The tsetse fly is attracted to bright colors and very dark colors.
- Inspect vehicles for tsetse flies before entering. The tsetse fly is attracted to moving vehicles.
- Avoid bushes. The tsetse fly is less active during the hottest period of the day. It rests in bushes but will bite if disturbed.
- Use insect repellant. Though insect repellants have not proven effective in preventing tsetse fly bites, they are effective in preventing other insects from biting and causing illness.
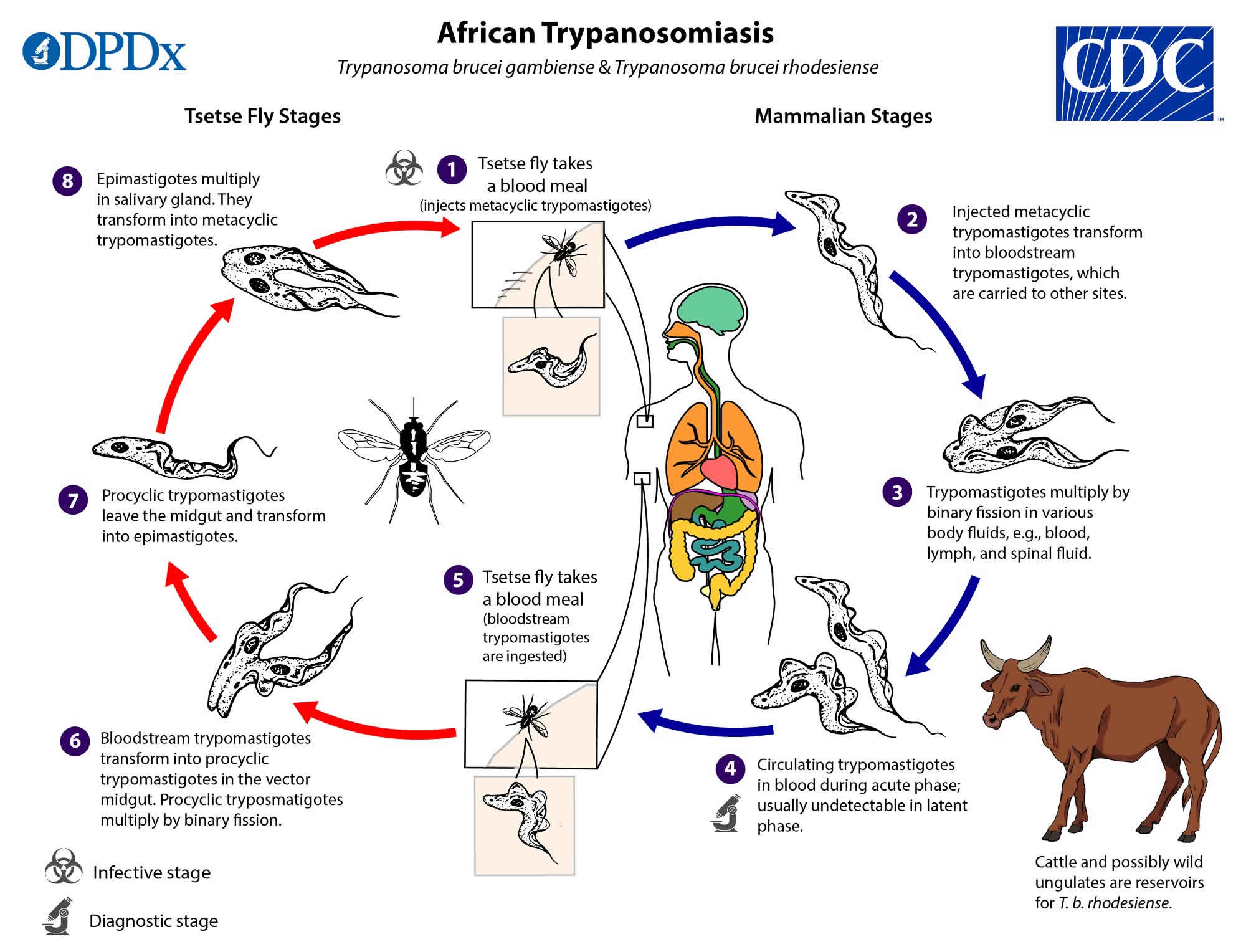
African sleeping sickness transmission cycle
In both forms of human African trypanosomiasis, infection occurrence depends on the interaction of three elements within a particular environment 19:
- The mammalian reservoirs of parasites (human or animal) that can be also the host suffering from the disease, and which are influenced by their behavioral interactions with the environment;
- Tsetse flies or Glossina species as cyclical vectors for transmission that are fully dependent on environmental factors; and
- The pathogenic parasite, the trypanosome.
Due to reasons that are not always well known, but that are related to the interactions between these three elements, the transmission of African sleeping sickness is confined in areas with quite clear spatial limits, beyond which the disease does not occur. This limited space is called a “focus” 21.
Figure 3. African trypanosomiasis life cycle
African sleeping sickness statistics
African trypanosomiasis can occur at any age and can affect all races 22. Human African sleeping sickness threatens millions of people in 36 countries in sub-Saharan Africa 18. Many of the affected populations live in remote rural areas with limited access to adequate health services, which complicates the surveillance and therefore the diagnosis and treatment of cases. In addition, displacement of populations, war and poverty are important factors that facilitate transmission 18.
- In 1998, almost 40 000 cases were reported, but estimates were that 300 000 cases were undiagnosed and therefore untreated.
- During the last epidemic the prevalence reached 50% in several villages in Angola, the Democratic Republic of the Congo, and South Sudan. Sleeping sickness was the first or second greatest cause of mortality in those communities, even ahead of HIV/AIDS.
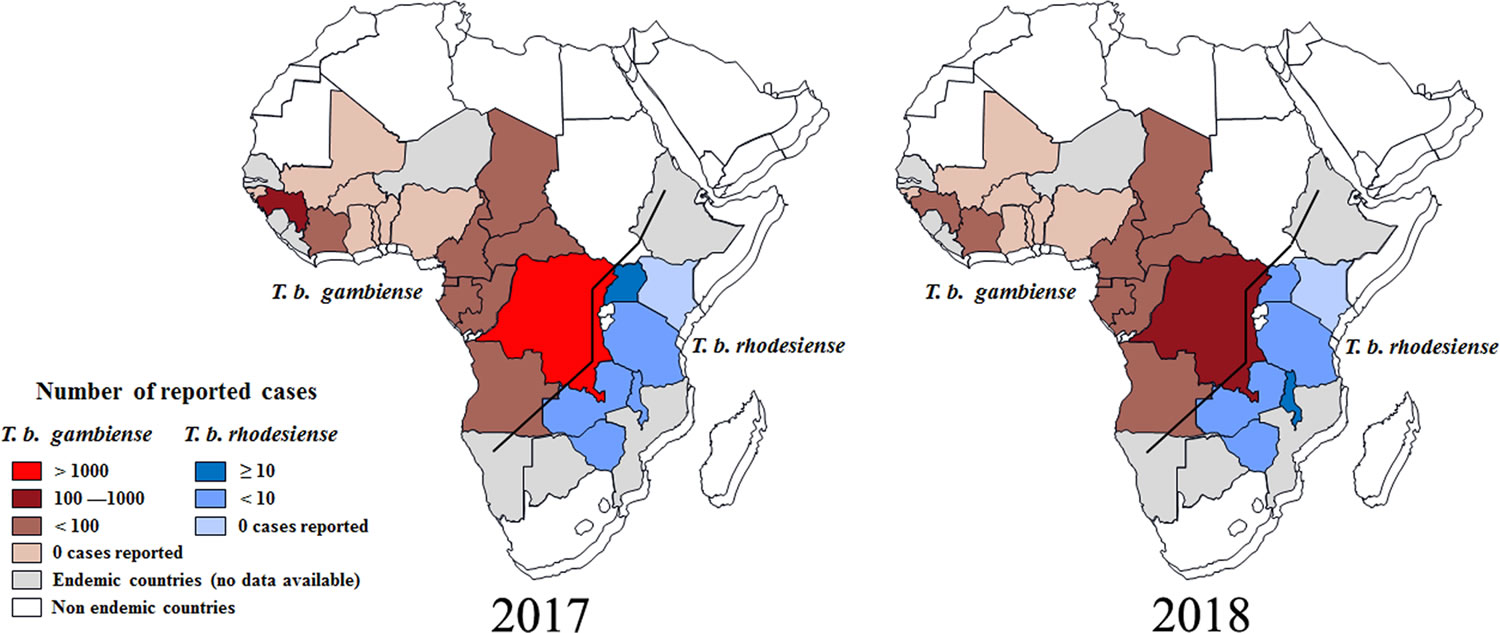
- In 2009, after continued control efforts, the number of cases reported dropped below 10,000 (9,878) for the first time in 50 years. This decline in number of cases has continued with 992 new cases reported in 2019, the lowest level since the start of systematic global data-collection 80 years ago. The estimated population at risk is 65 million people.
- In the last 10 years, over 70% of reported African sleeping sickness cases occurred in the Democratic Republic of the Congo.
- Angola, Cameroon, Central African Republic, Chad, Congo, Guinea, Malawi, South Sudan and Zambia declared between 10 and 100 new cases in 2019, while Côte d’Ivoire, Equatorial Guinea, Gabon, Uganda, United Republic of Tanzania and Zimbabwe declared between 1 and 10 new cases.
- Countries such as Burkina Faso, Ghana, Kenya and Nigeria, have reported sporadic cases in the last 10 years.
- Countries like Benin, Botswana, Burundi, Ethiopia, Gambia, Guinea Bissau, Liberia, Mali, Mozambique, Namibia, Niger, Rwanda, Senegal, Sierra Leone, Swaziland and Togo have not reported any new cases for over a decade. Transmission of African trypanosomiasis seems to have stopped in some of these countries but there are still some areas where it is difficult to assess the exact situation because the unstable social circumstances and/or difficult accessibility hinder surveillance and diagnostic activities.
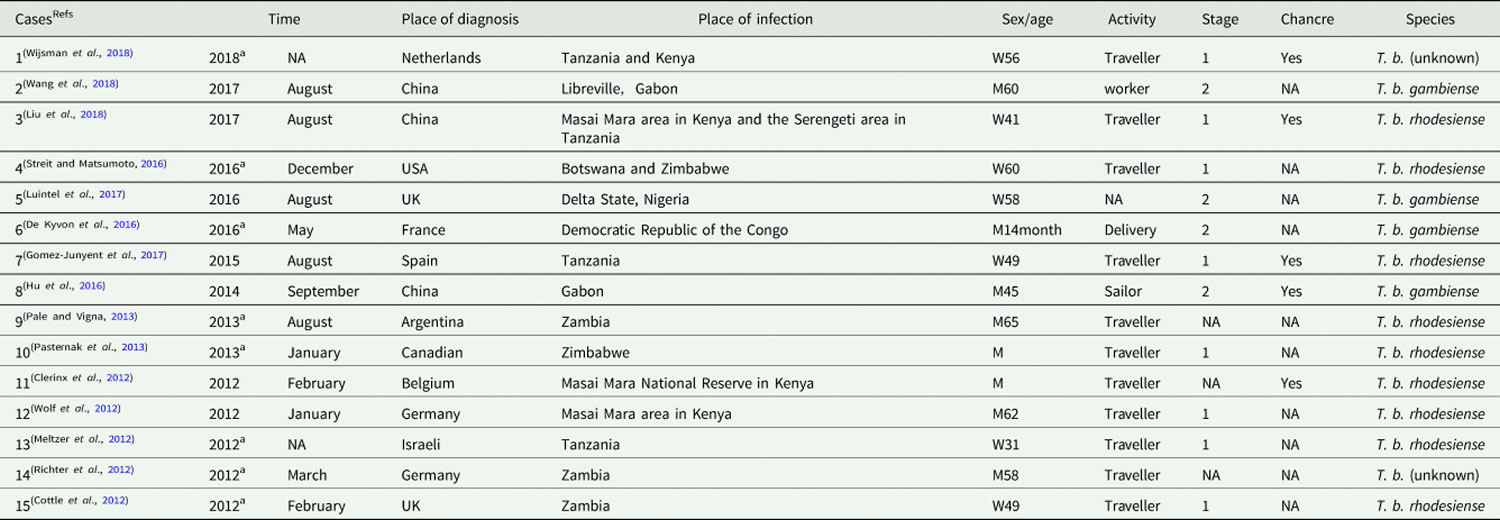
Table 1. Cases of human African trypanosomiasis recorded in non-endemic countries from 2011 to 2018
Footnotes: a Time refers to the published date when the diagnostic date is unavailable.
NA = Information is unavailable on the case report.
[Source 23 ]Figure 4. Distribution of human African trypanosomiasis in endemic countries (2017 and 2018)
[Source 23 ]Major human epidemics
There have been several epidemics in Africa over the last century:
- one between 1896 and 1906, mostly in Uganda and the Congo Basin;
- one in 1920 in a number of African countries; and
- the most recent epidemic started in 1970 and lasted until the late 1990s.
The 1920 epidemic was controlled thanks to mobile teams which carried out the screening of millions of people at risk. By the mid-1960s, the disease was under control with less than 5000 cases reported in the whole continent. After this success, surveillance was relaxed, and the disease reappeared, reaching epidemic proportions in several regions by 1970. The efforts of World Health Organization (WHO), national control programmes, bilateral cooperation and nongovernmental organizations (NGOs) during the 1990s and early 21st century reversed the curve.
Since the number of new human African trypanosomiasis cases reported between 2000 and 2012 dropped significantly as a result of international coordinated efforts, the WHO neglected tropical diseases road map targeted its elimination as a public health problem by 2020 and interruption of transmission (zero cases) for 2030.
African Trypanosomiasis causes
African sleeping sickness is mostly transmitted through the bite of an infected tsetse fly but there are other ways in which people are infected:
- Mother-to-child infection: the trypanosome can cross the placenta and infect the fetus.
- Mechanical transmission through other blood-sucking insects is possible, however, it is difficult to assess its epidemiological impact.
- Accidental infections have occurred in laboratories due to pricks with contaminated needles.
- Transmission of the parasite through sexual contact has been reported.
Once the tsetse fly ingests the trypanosomes, they multiply and develop into epimastigotes. Humans are infected after the bite from a tsetse fly. The injected parasites then rapidly divide in the bloodstream and lymphatics. Eventually, the parasite enters the central nervous system (brain and spinal cord) and causes neurological and behavioral symptoms.
In the first stage, the trypanosomes multiply in subcutaneous tissues, blood and lymph. This is also called hemo-lymphatic stage, which entails bouts of fever, headaches, enlarged lymph nodes, joint pains and itching
In the second stage the parasites cross the blood-brain barrier to infect the central nervous system (CNS). This is known as the neurological or meningo-encephalic stage. In general this is when more obvious signs and symptoms of the disease appear: changes of behaviour, confusion, sensory disturbances and poor coordination. Disturbance of the sleep cycle, which gives the disease its name, is an important feature. Without treatment, sleeping sickness is considered fatal although cases of healthy carriers have been reported.
The trypanosomes evade the host’s immune system because of extensive antigenic variation of the glycoproteins located on the surface of the parasite. During this time, the parasites invade almost every organ in the body.
Some individuals may develop a severe hypersensitivity reaction to the parasite that leads to itching, swelling, and edema.
In the liver, there may be portal infiltration and fatty degeneration.
When the heart is invaded, arrhythmias may develop leading to death.
When the brain is involved, it may lead to meningoencephalitis, bleeding, edema, and granulomatous lesions.
Risk factors for getting African trypanosomiasis
The risk factors for the transmission of African sleeping sickness are determined by the increases in the possibility with which humans come into contact with a tsetse fly, and these factors are thus related to the site of contact between the tsetse fly and the human, and the intensity and frequency of this contact. In rhodesiense African sleeping sickness, these risk factors are also related to the presence of nonhuman reservoirs.
Gambiense human African trypanosomiasis
The risk factors for gambiense African sleeping sickness vary according to the different environmental settings and the characteristics of the vector in the different biotopes, and the activities carried out by human beings in the biotopes occupied by the tsetse flies.
- In humid forest, the tsetse flies are widely distributed, and human–fly contact is related to activities such as hunting, fetching firewood, timber-related activities, and forest clearing for farming 24.
- In the woodland savanna and riverine forest galleries, the fly is found close to rivers and streams. The risk of transmission has been associated with activities that have been developed along these water bodies, such as fetching water, washing clothing or food (cassava), the artisanal extraction of palm oil, brewing, gold and diamond mining, and fishing 25. The risk of transmission increases when tsetse habitats are restricted, for example, during the dry season.
- In the transitional vegetation between forest and woodland savanna, the islands of vegetation provide a suitable habitat for tsetse, and these locations act as points from which hosts are sighted. These areas are often used for farming, making this activity a risk factor in these areas 26.
- In the mangrove areas, tsetse flies find a favorable habitat where a high risk of transmission is associated with fishing and crustacean collection, but the parasite is also found in cleared areas used for rice cultivation. Pirogue jetties and fishing encampments are areas where human–fly contact can be intense 27.
- Coca, coffee, and also mango and banana plantations, where the original forest has been replaced, are also suitable habitats for tsetse flies, and these areas are related to transmission in plantation workers 28.
- Gambiense African sleeping sickness is considered a rural disease, but transmission has also been occasionally observed in urban settings. Nevertheless, transmission in urban areas is associated with travels to neighboring rural areas for cultivating fields, or it may occur in suburban outskirts closer to transitional vegetation areas, where agricultural activities are possible; these areas constitute a suitable tsetse habitat with few alternative hosts 29.
It has been described that certain protective immunity exists against new infections in humans after suffering from the disease 30.
The risk associated with age- and sex-related factors pertains to the activities and behaviors made by the different age and sex groups. In general, gambiense African sleeping sickness is predominately a disease of adults, mainly affecting young adults, as this is the group that is most involved in productive activities that facilitate contact with the vector 31. Children are usually less affected than adults, but in some areas (such as mangroves), teens present a higher rate of infection related to fishing and leisure activities in water areas.109,110 In areas where at-risk activities include mining, hunting, or fishing, the prevalence is higher in males. In transitional vegetation areas where the risk of infection is associated with agriculture and domestic activities at bodies of water, similar prevalence rates have been found in males and females 32.
The clustering of cases and some familial aggregation has been described, as the risk of gambiense African sleeping sickness for a child significantly increased when the mother also had African sleeping sickness 33. It has been suggested that familial clustering was a consequence of similar exposure to the vector and shared behavioral risk factors, rather than of genetic susceptibility 34.
The risk of gambiense African sleeping sickness infection in short-term travelers from nonendemic areas is very low, as tourists rarely visit the rural areas where gambiense African sleeping sickness is endemic. Gambiense African sleeping sickness cases that are occasionally diagnosed in nonendemic countries are mainly seen in immigrants and expatriate residents living in at risk areas for extended periods 35.
Rhodesiense human African trypanosomiasis
The two different settings described for rhodesiense African sleeping sickness mean different situations of risk for contracting the disease.
- In the areas where wildlife is the main reservoir, the main risk factors are associated with entry into areas that are usually restricted and where wildlife is preserved (national parks and game reserves). A high density of tsetse flies in these areas increases this risk. Exposure is related to the movement of humans, animals, and tsetse flies out of or into reserves, especially during specific seasons of the year. Populations living in the periphery of game reserves and national parks are the ones at the highest risk 36. The exposure to rhodesiense African sleeping sickness in this area involves wildlife conservation activities (for example, rangers and park wardens), hunting and poaching, fishing, honey and firewood collection, and visitors of national parks (tourists) 37. The movement of livestock for grazing in these areas is another risk factor for herdsmen 38; there is also the potential that this risk could be transferred to other areas where the cattle is moving.
- In the areas where livestock is the main reservoir, activities linked to cattle raising increase this risk, but the general population living in these areas is also exposed. Those living on the periphery of villages or near the cattle markets are most at risk 37.
With respect to age, those at highest risk for rhodesiense African sleeping sickness are in the working-age group, which is comprised of individuals venturing into tsetse habitats 37. It is assumed that their contact with flies occurs when the humans enter into the woodland habitat of the flies, but recent studies have found the frequent peridomestic presence of some tsetse species, and even tsetse biting humans inside buildings 39, but transmission still mainly occurs in more tsetse-suitable habitats. As is the case in gambiense African sleeping sickness, the sex-based risks are related to the specific activities and behaviors engaged upon by the members of each sex; in general, rhodesiense African sleeping sickness is predominately a disease of males 37. Familial aggregation also occurs in rhodesiense African sleeping sickness 37, which is most likely related to the common exposure of vectors, especially since there are shared behavioral and spatial risk factors among members of a household.
Given that rhodesiense African sleeping sickness is an acute disease, it is possible to observe a certain seasonal variation in transmission that is linked to the density of Glossina: The peak in the Glossina population densities is usually seen after the rainy season, and the peak in the human cases can be detected in 1–3 months after the rainy season.119
The risk of acquiring rhodesiense African sleeping sickness for short-term travelers from nonendemic areas is low, but this risk factor is more important than that of developing gambiense African sleeping sickness, as tourists commonly visit natural areas where rhodesiense African sleeping sickness is transmitted 35. Cases of rhodesiense African sleeping sickness in travelers can occasionally appear in clusters of people who have visited common areas 40.
African Trypanosomiasis symptoms
The clinical course of human African trypanosomiasis has two stages. In the first stage, the parasite is found in the peripheral circulation, but it has not yet invaded the central nervous system. Once the parasite crosses the blood-brain barrier and infects the central nervous system, the disease enters the second stage. The subspecies that cause African trypanosomiasis have different rates of disease progression, and the clinical features depend on which form of the parasite (Trypanosoma brucei rhodesiense or Trypanosoma brucei gambiense) is causing the infection. However, infection with either form will eventually lead to coma and death if not treated.
African sleeping sickness general symptoms include:
- Mood changes, anxiety
- Fever, sweating
- Headache
- Weakness
- Insomnia at night
- Sleepiness during the day (may be uncontrollable)
- Swollen lymph nodes all over the body
- Swollen, red, painful nodule at site of the fly bite
Trypanosoma brucei rhodesiense infection (East African sleeping sickness) progresses rapidly. In some patients, a large sore (a chancre) will develop at the site of the tsetse bite. Most patients develop fever, headache, muscle and joint aches, and enlarged lymph nodes within 1-2 weeks of the infective bite. Some people develop a rash. After a few weeks of infection, the parasite invades the central nervous system and eventually causes mental deterioration and other neurologic problems. Death ensues usually within months.
Trypanosoma brucei gambiense infection (West African sleeping sickness) progresses more slowly. At first, there may be only mild symptoms. Infected persons may have intermittent fevers, headaches, muscle and joint aches, and malaise. Itching of the skin, swollen lymph nodes, and weight loss can occur. Usually, after 1-2 years, there is evidence of central nervous system involvement, with personality changes, daytime sleepiness with nighttime sleep disturbance, and progressive confusion. Other neurologic signs, such as partial paralysis or problems with balance or walking may occur, as well as hormonal imbalances. The course of untreated infection rarely lasts longer than 6-7 years and more often kills in about 3 years.
West African trypanosomiasis signs and symptoms
West African trypanosomiasis symptoms may be minimal or intermittent during the first months of infection. They are usually apparent within a few months to a year after getting an infected tsetse fly bite.
Occasionally, within 1 to 3 weeks, the infective bite develops into a red sore, also called a chancre. Several weeks to months later, other symptoms of sleeping sickness occur. These include fever, rash, swelling of the face and hands, headaches, fatigue, aching muscles and joints, itching skin, and swollen lymph nodes. Weight loss occurs as the illness progresses. Progressive confusion, personality changes, daytime sleepiness with nighttime sleep disturbances, and other neurologic problems occur after the infection has invaded the central nervous system. These symptoms become worse as the illness progresses. If left untreated, death will eventually occur after several years of infection.
East African trypanosomiasis signs and symptoms
East African trypanosomiasis symptoms usually develop within 1 to 3 weeks after an infective tsetse fly bite. A bite by the tsetse fly is often painful and can develop into a red sore, also called a chancre. Fever, severe headaches, irritability, extreme fatigue, swollen lymph nodes, and aching muscles and joints are common symptoms of sleeping sickness. Some people develop a skin rash. Progressive confusion, personality changes, and other neurologic problems occur after infection has invaded the central nervous system. If left untreated, infection becomes worse and death will occur within months.
African trypanosomiasis complications
African sleeping sickness complications include:
- Injury related to falling asleep while driving or during other activities
- Gradual damage to the nervous system
- Uncontrollable sleep as the disease gets worse
- Coma
African Trypanosomiasis diagnosis
If you suspect that you may have West African trypanosomiasis, see your health care provider who will order several tests to look for the parasite. Common tests include examination of blood samples and a spinal tap. Your physician may also take a sample of fluid from swollen lymph nodes.
African sleeping sickness Trypanosoma brucei gambiense can be screened for with the card agglutination trypanosoma test (CATT), a study that examines serum for antigen and that carries a sensitivity of 91% and specificity of 97% 41. Both species can also be identified with Giemsa-stained blood, lymph node aspirates, and cerebrospinal fluid (CSF) fluid. All patients with suspected African sleeping sickness should be screened for central nervous system (CNS) involvement with a lumbar puncture; CSF fluid should be tested for trypanosomes, leukocytosis, and trypanosome IgM. In the second-stage infections, the number of parasites in congestive heart failure (CHF) can be very low. World Health Organization (WHO) diagnostic criteria for suspected second stage trypanosomiasis, therefore, consists of either the presence of trypanosomes in CSF fluid or greater than 5 white blood cells (WBCs) per microliter of fluid in a suspected case.
In many hospitals, in Africa, a blood smear is often done as it will reveal the mobile trypanosomes. Blood smears are often positive in early disease when the number of circulating parasites is very high. The parasite load in Trypanosoma brucei rhodesiense infection is substantially higher than the level in Trypanosoma brucei gambiense infection.
Trypanosoma brucei rhodesiense parasites can easily be found in blood. They can also be found in lymph node fluid or in fluid or biopsy of a chancre. Serologic testing is not widely available and is not used in the diagnosis, since microscopic detection of the parasite is straightforward.
For East African trypanosomiasis, a skin biopsy may be done if you have a chancre.
Lymph node aspiration is sometimes done to identify the parasite and may yield positive results.
CT scan and MRI of the brain frequently reveal massive cerebral edema and enhancement of the white matter.
The classic method for diagnosing Trypanosoma brucei gambiense infection is by microscopic examination of lymph node aspirate, usually from a posterior cervical node. It is often difficult to detect Trypanosoma brucei gambiense in blood. Concentration techniques and serial examinations are frequently needed. Serologic testing is available outside the U.S. for Trypanosoma brucei gambiense; however, it normally is used for screening purposes only and the definitive diagnosis rests on microscopic observation of the parasite.
All patients diagnosed with African trypanosomiasis must have their cerebrospinal fluid examined to determine whether there is involvement of the central nervous system, since the choice of treatment drug(s) will depend on the disease stage. The World Health Organization criteria for central nervous system involvement include increased protein in cerebrospinal fluid and a white cell count of more than 5. Trypanosomes can often be observed in cerebrospinal fluid in persons with second stage infection.
African Trypanosomiasis treatment
African trypanosomiasis treatment depends on the form of the disease (Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense) and the disease stage (i.e. whether the central nervous system has been invaded by the parasite). The earlier the African trypanosomiasis is identified, the better the prospect of a cure. The early stage management requires treatment of fever and malaise. Close monitoring of the central nervous system status is necessary. Sometimes, patients may require intubation and mechanical ventilation as they can not maintain a patent airway 42.
There is no test of cure for African trypanosomiasis. Patients should be followed up to 24 months with a lumbar puncture every 6 months (or sooner, if symptoms return) for 2 years after treatment to detect a relapse should it occur, as parasites may remain viable for long periods and reproduce the disease months after treatment.
Treatment success in the second stage depends on drugs that cross the blood-brain barrier to reach the parasite.
New treatment guidelines for gambiense human African trypanosmiasis were issued by World Health Organization (WHO) in 2019. In total six different drugs are used for the treatment of sleeping sickness. These drugs are donated to WHO by manufacturers and distributed free of charge to disease endemic countries.
Pentamidine, which is the recommended drug for first stage Trypanosoma brucei gambiense infection, is widely available in the U.S. The other drugs (suramin, melarsoprol, eflornithine, and nifurtimox) used to treat African trypanosomiasis are available in the U.S. only from the Centers for Disease Control and Prevention (CDC). Physicians can consult with CDC staff for advice on diagnosis and management and to obtain otherwise unavailable treatment drug.
Pentamidine, given by intravenous infusion over 2 hours or by intramuscular injection, is used to treat first stage Trypanosoma brucei gambiense infection. It is generally well tolerated, but adverse reactions of hypoglycemia, injection site pain, diarrhea, nausea and vomiting occur. Suramin is used to treat first stage Trypanosoma brucei rhodesiense. Suramin is also effective against Trypanosoma brucei gambiense, but it is not often used because severe reactions occur in persons who are co-infected with Onchocerca volvulus. Adverse reactions to suramin are frequent, but usually mild and reversible. These include drug rash, nephrotoxicity, and peripheral neuropathy. In rare instances, suramin administration results in a hypersensitivity reaction, and, for this reason, a small test dose is usually given prior to the full first dose.
Second stage Trypanosoma brucei gambiense is treated with eflornithine, which is given in 4 intravenous infusions daily for 14 days. Adverse effects of eflornithine include bone marrow suppression, gastrointestinal symptoms, and seizures. Eflornithine is highly effective, but the difficulty in administering 4 infusions daily in rural African facilities has led to the use of eflornithine (dosed less frequently) in combination with nifurtimox. The efficacy of the combination regimen appears to be at least as high as eflornithine monotherapy. Eflornithine is not effective against Trypanosoma brucei rhodesiense and it is not recommended for treating the East African form of the disease. Melarsoprol, an organoarsenic compound, is the only drug available for treating second stage Trypanosoma brucei rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. An encephalopathic reaction occurs in 5-10% of patients with a case-fatality rate of approximately 50% when it occurs. Prednisolone is often given to patients who are being treated with melarsoprol to reduce the risk of encephalopathy. Other adverse reactions observed with melarsoprol include skin reactions, gastrointestinal upset, and peripheral neuropathy. Intravenous injections of melarsoprol are painful and can cause phlebitis. The drug is administered by use of lengthy and complicated dosing schedules, however, an abbreviated 10-day regimen appears promising.
African sleeping sickness antiparasitic
Table 2. African Trypanosomiasis treatment
| Species | Drug of choice | Adult Dosage | Pediatric Dosage |
|---|---|---|---|
| Trypanosoma brucei rhodesiense, hemolymphatic stage | Suramin1 | 1 gm IV on days 1, 3, 7 ,14, and 212 | 20 mg/kg IV on days 1, 3, 7, 14, and 213 |
| Trypanosoma brucei rhodesiense, CNS involvement | Melarsoprol4 | 2-3.6 mg/kg/day IV x 3 days.5 After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. | 2-3.6 mg/kg/day IV x 3 days.5 After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. |
| Trypanosoma brucei gambiense, Hemolymphatic stage | Pentamidine6 | 4 mg/kg/day IM or IV x 7-10 days | 4 mg/kg/day IM or IV x 7-10 days |
| Trypanosoma brucei gambiense, CNS involvement | Eflornithine7 | 400 mg/kg/day in 4 doses x 14 days | 400 mg/kg/day in 4 doses x 14 days |
Footnotes:
- Pentamidine is also effective against Trypanosoma brucei rhodesiense in the hemolymphatic stage, but suramin may have somewhat higher efficacy.
- A test dose of 100 mg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- A test dose of 2 mg/kg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- Corticosteroids have been used to prevent melarsoprol encephalopathy. The dose of melarsoprol is progressively increased during the first series.
- Suramin is also effective against Trypanosoma brucei gambiense in the hemolymphatic stage.
- Eflornithine (400 mg/kg/d IV in 2 doses x 7 days) given in combination with oral nifurtimox (15 mg/kg/d x 10 days) is also highly effective against
- Trypanosoma brucei gambiense with CNS involvement (Priotto G et al. Lancet 2009:374; 56-64). Nifurtimox is not FDA-approved for this indication.
Drugs used in the treatment of first stage
- Pentamidine: discovered in 1940, used for the treatment of the first stage of Trypanosoma brucei gambiense sleeping sickness. Despite non-negligible undesirable effects, it is in general well tolerated by patients.
- Suramin: discovered in 1920, used for the treatment of the first stage of Trypanosoma brucei rhodesiense. It provokes certain undesirable effects, including nephrotoxicity and allergic reactions.
Pentamidine, which is the recommended drug for first stage Trypanosoma brucei gambiense infection, is widely available in the U.S. The other drugs (suramin, melarsoprol, eflornithine, and nifurtimox) used to treat African trypanosomiasis are available in the U.S. only from the Centers for Disease Control and Prevention (CDC). Physicians can consult with CDC staff for advice on diagnosis and management and to obtain otherwise unavailable treatment drug.
Pentamidine, given by intravenous infusion over 2 hours or by intramuscular injection, is used to treat first stage Trypanosoma brucei gambiense infection. It is generally well tolerated, but adverse reactions of hypoglycemia, injection site pain, diarrhea, nausea and vomiting occur. Suramin is used to treat first stage Trypanosoma brucei rhodesiense. Suramin is also effective against Trypanosoma brucei gambiense, but it is not often used because severe reactions occur in persons who are co-infected with Onchocerca volvulus. Adverse reactions to suramin are frequent, but usually mild and reversible. These include drug rash, nephrotoxicity, and peripheral neuropathy. In rare instances, suramin administration results in a hypersensitivity reaction, and, for this reason, a small test dose is usually given prior to the full first dose.
Drugs used in the treatment of second stage
- Melarsoprol: discovered in 1949, it is used for the treatment of both gambiense and rhodesiense infections. It is derived from arsenic and has many undesirable side effects, the most dramatic of which is reactive encephalopathy (encephalopathic syndrome) which can be fatal (3% to 10%). It is currently recommended as first-line treatment for the rhodesiense form, but rarely used in the gambiense form.
- Eflornithine: much less toxic than melarsoprol, registered in 1990 is only effective against T.b. gambiense. It is generally used in combination with nifurtimox (as part of the Nifurtimox-eflornithine combination therapy, NECT) but can be used also as monotherapy. The regimen is complex and cumbersome to apply.
- Nifurtimox: The Nifurtimox-eflornithine combination therapy, NECT, was introduced in 2009. It simplifies the use of eflornithine by reducing the duration of treatment and the number of IV perfusions, but unfortunately it has not been studied for Trypanosoma brucei rhodesiense. Nifurtimox is registered for the treatment of American trypanosomiasis but not for human African trypanosomiasis. Both drugs are provided free of charge by WHO to endemic countries with a kit containing all the material needed for its administration.
For treatment advice and to obtain suramin, melarsoprol, or eflornithine, physicians should contact the Centers for Disease Control and Prevention (CDC) Division of Parasitic Diseases and Malaria.
Second stage Trypanosoma brucei gambiense is treated with eflornithine. Eflornithine is available for human use in the United States through the Centers for Disease Control and Prevention (CDC). Eflornithine is given in 4 intravenous infusions daily for 14 days. Adverse effects of eflornithine include bone marrow suppression, gastrointestinal symptoms, and seizures. Eflornithine is highly effective, but the difficulty in administering 4 infusions daily in rural African facilities has led to the use of eflornithine (dosed less frequently) in combination with nifurtimox. The efficacy of the combination regimen appears to be at least as high as eflornithine monotherapy. Eflornithine is not effective against Trypanosoma brucei rhodesiense and it is not recommended for treating the East African form of the disease. Melarsoprol, an organoarsenic compound, is the only drug available for treating second stage Trypanosoma brucei rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. An encephalopathic reaction occurs in 5-10% of patients with a case-fatality rate of approximately 50% when it occurs. Prednisolone is often given to patients who are being treated with melarsoprol to reduce the risk of encephalopathy. Other adverse reactions observed with melarsoprol include skin reactions, gastrointestinal upset, and peripheral neuropathy. Intravenous injections of melarsoprol are painful and can cause phlebitis. The drug is administered by use of lengthy and complicated dosing schedules, however, an abbreviated 10-day regimen appears promising.
Eflornithine
- Note on treatment in pregnancy: Data on the use of eflornithine in pregnant women are limited, and risk to the embryo-fetus is unknown. Eflornithine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Note on treatment in breastfeeding: It is not known whether eflornithine is excreted in breast milk. Eflornithine should be used with caution in breast-feeding women.
- Note on treatment in children: The safety of eflornithine in children has not been established. Eflornithine is not approved by the Food and Drug Administration (FDA) for use in pediatric patients. Eflornithine is listed for the treatment of 1st stage African trypanosomiasis in Trypanosoma brucei gambiense infection on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
Pentamidine
- Note on treatment in pregnancy: Pentamidine is in pregnancy category C. Data on the use of pentamidine in pregnant women are limited, and risk to the embryo-fetus is unknown. Pentamidine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Note on treatment in breastfeeding: It is not known whether pentamidine is excreted in breast milk. The World Health Organization (WHO) classifies pentamidine as compatible with breast-feeding, although data on the use of pentamidine during lactation are limited. Pentamidine should be used during lactation only if the potential benefit of therapy to the mother justifies the potential risk to the infant.
- Note on treatment in children: Intravenous and intramuscular pentamidine have a similar safety profile in children age 4 months and older as in adults. Pentamidine is listed as a medicine for the treatment of 1st stage African trypanosomiasis infection (Trypanosoma brucei gambiense) on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
Drugs used in the treatment of both stages
Fexinidazole is an oral treatment for gambiense human African trypanosomiasis. It was included in 2019 in the WHO Essential medicines list and WHO human African Trypanosomiasis treatment guidelines. This molecule is indicated as first line for first stage and non-severe second stage. It should be administered within 30 minutes after a solid meal and under supervision of trained medical staff. Currently a clinical trial for its use in rhodesiense African sleeping sickness is ongoing.
African sleeping sickness prognosis
If the African sleeping sickness is treated during the early stage, recovery is possible in most patients 41. However, if the patient presents with stage 2 disease, the central nervous system (brain and spinal cord) involvement usually is fatal. Without treatment, death can occur within 6 months from cardiac failure or from Trypanosoma brucei rhodesiense infection itself.
Today, the cure rate with the drug melarsoprol is more than 90% 16.
American Trypanosomiasis
American Trypanosomiasis also known as Chagas disease, is a potentially life-threatening zoonotic disease caused by the parasite Trypanosoma cruzi. Chagas disease is named after the Brazilian physician Carlos Chagas, who discovered the disease in 1909. American trypanosomiasis or Chagas disease is an illness that can cause serious heart and stomach problems. American Trypanosomiasis or Chagas disease is usually spread by infected blood-sucking bugs called triatomine bugs. They are also known as “kissing bugs” because they often bite people’s faces. When one of these bugs bites you, it leaves behind infected waste. You can become infected if you rub the waste in your eyes or nose, the bite wound, or a cut. American trypanosomiasis or Chagas disease can also spread through contaminated food, a blood transfusion, a donated organ, or from the pregnant parent to the baby during pregnancy.
American Trypanosomiasis (Chagas disease) is most commonly seen in Central and South America, Trinidad, and the southern United States where poverty is widespread. However, it is less common outside of rural areas where vectors are commonly found in rustic housing. It is estimated that as many as 8 million people living in Mexico, Central America, and South America have American Trypanosomiasis (Chagas disease), most of whom do not know they are infected. If untreated, infection is lifelong and can be life threatening.
The impact of Chagas disease is not limited to only rural areas of Latin America in which vectorborne transmission (diseases transmitted by insects) occurs. Large-scale population movements from rural to urban areas of Latin America and to other regions of the world have increased the geographic distribution and changed the epidemiology of American Trypanosomiasis (Chagas disease). In the United States and in other regions where American Trypanosomiasis (Chagas disease) is now found but is not endemic, control strategies should focus on preventing transmission from blood transfusion, organ transplantation, and mother-to-baby (congenital transmission).
American trypanosomiasis (Chagas disease) can be transmitted through vertical transmission between mother and fetus or via contact with contaminated feces/urine of the reduviid bug (triatomine bug, kissing bug) and hence serves as the intermediate host for the parasite 43. Other modes of transmission include transfusion of blood products, transplant of the infected organ, or by consumption of infected food or drinks. Major complications of this disease includes cardiomegaly, gastrointestinal disease, and in some case peripheral neuropathy 44.
Figure 5. American Trypanosomiasis (Trypanosoma cruzi parasite)
Figure 6. Triatomine bugs
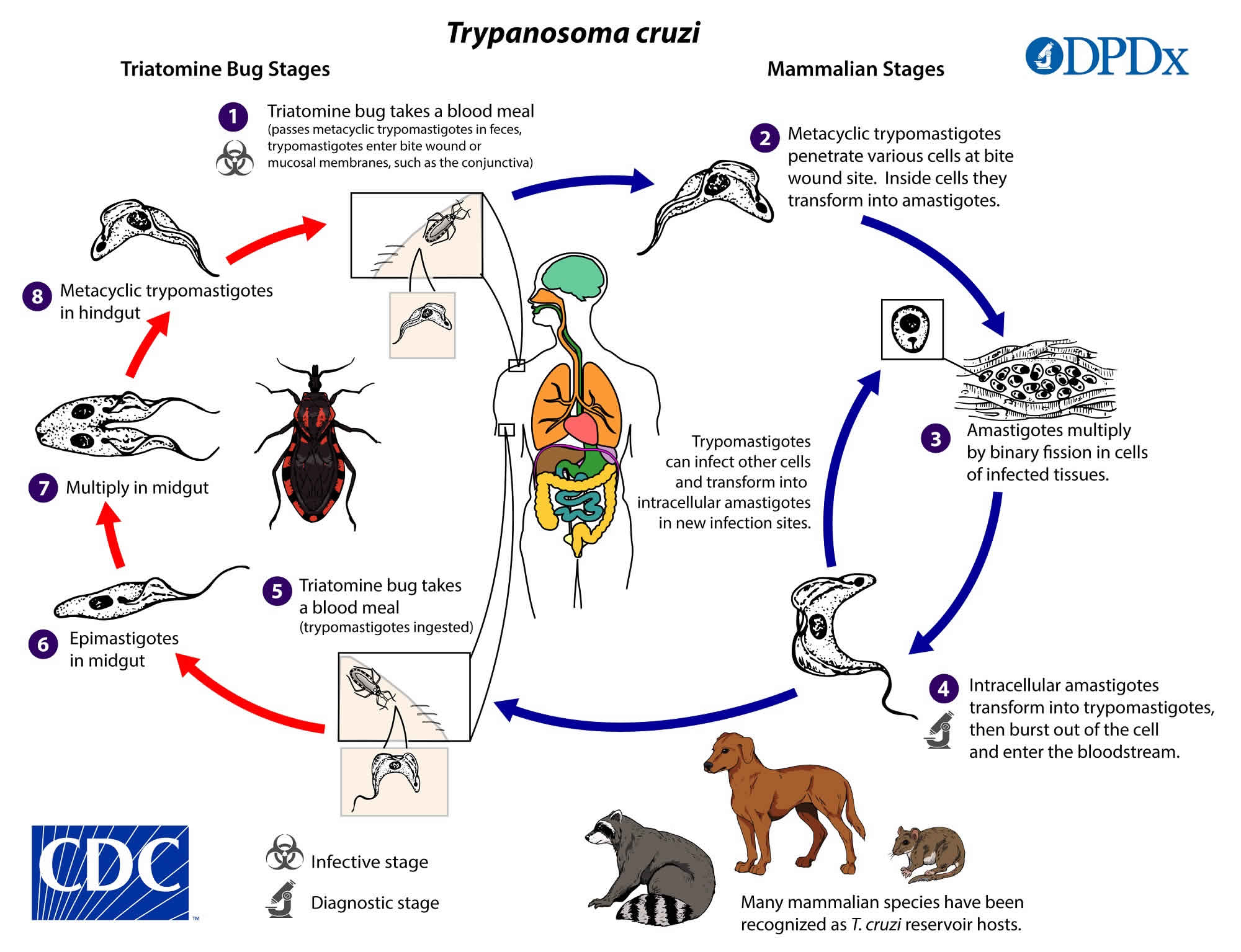
Figure 7. American trypanosomiasis life cycle
American trypanosomiasis transmission
People can become infected in several ways. In areas where American trypanosomiasis (Chagas disease) is common, the main way is through vectorborne transmission. The insect vectors are called triatomine bugs also called “kissing bugs”, cone-nosed bugs, and blood suckers. These blood-sucking bugs get infected with Trypanosoma cruzi by biting an infected animal or person. Once infected, the bugs pass the parasites in their feces. The bugs are found in houses made from materials such as mud, adobe, straw, and palm thatch. During the day, the bugs hide in crevices in the walls and roofs. During the night, when the inhabitants are sleeping, the bugs emerge. Because they tend to bite people’s faces, triatomine bugs are also known as “kissing bugs”. After they bite and ingest blood, they defecate (poop) on the person. The person can become infected if Trypanosoma cruzi parasites in the bug feces enter the body through mucous membranes or breaks in the skin. The unsuspecting, sleeping person may accidentally scratch or rub the feces into the bite wound, eyes, or mouth.
Currently, there are 11 different species of the triatomine bug 45. The most common species in the southern United States are Triatoma sanguisuga and Triatoma gerstaeckeri. The main vectors for Mexico, Central, and South America are Rhodnius prolixus and Triatoma dimidiata. Both sexes of reduviid bug take its blood meal at night, but female bugs must take blood meals to lay their eggs. As it takes the meal or even after the meal, they defecate and expels parasites in the feces or urine near mucous membranes, commonly the mouth or eyes. The parasite then enters through the mucous membrane, or through an open bite wound. Feces of infected reduviid bugs carry the largest number of the infectious trypomastigote form 46.
People also can become infected through:
- Congenital transmission (from a pregnant woman to her baby);
- Blood transfusions;
- Organ transplantation;
- Consumption of uncooked food that is contaminated with feces (poop) from infected triatomine bugs; and
- Accidental laboratory exposure.
It is generally considered safe to breastfeed even if the mother has Chagas disease. However, if the mother has cracked nipples or blood in the breast milk, she should pump and discard the milk until the nipples heal and the bleeding resolves.
American trypanosomiasis (Chagas disease) is not transmitted from person-to-person like a cold or the flu or through casual contact with infected people or animals.
Who is more likely to develop American trypanosomiasis?
Kissing bugs can be found throughout the Americas, but they are more common in certain areas. The people who are most at risk for American trypanosomiasis or Chagas disease:
- Live in rural areas of Latin America
- Have seen the bugs, especially in those areas
- Have stayed in a house with a thatched roof or with walls that have cracks or crevices.
If I have American trypanosomiasis (Chagas disease), should my family members be tested for the infection?
Possibly. They should be tested if they:
- Could have become infected the same way that you did, for example, by vectorborne transmission;
- Are your children and were born after you were infected; or if
- There are other reasons to think that they might have Chagas disease.
What should I do if I think I have American trypanosomiasis (Chagas disease)?
You should discuss your concerns with your healthcare provider, who will examine you and ask you questions (for example, about your health and where you have lived). American trypanosomiasis or Chagas disease is diagnosed by blood tests. If you have Chagas disease, you should have a heart tracing test (electrocardiogram [ECG]), even if you feel fine. You might be referred to a specialist for more tests and treatment.
In what parts of the world is American trypanosomiasis (Chagas disease) found?
People who have American trypanosomiasis or Chagas disease can be found anywhere in the world. However, transmission of the disease by kissing bugs (vectorborne transmission), only occurs in the Americas. Most people with Chagas disease became infected in rural areas of Mexico, Central America, and South America. In some regions of Latin America, efforts to eliminate kissing bugs, called vector control programs, have succeeded in stopping this type of disease spread. Vectorborne transmission does not occur in the Caribbean (for example, in Puerto Rico or Cuba). Rare vectorborne cases of Chagas disease have been noted in the southern United States.
I plan to travel to a rural area of Latin America that might have American trypanosomiasis (Chagas disease). How can I protect myself from this infection?
No drugs or vaccines for preventing American trypanosomiasis or Chagas disease are currently available. Travelers who sleep indoors, in well-constructed facilities (for example, air-conditioned or screened hotel rooms), are at low risk for exposure to infected triatomine bugs that usually live in poor-quality dwellings and are most active at night. Preventive measures include spraying infested dwellings with long-lasting insecticides, using bed nets treated with long-lasting insecticides, wearing protective clothing, and applying insect repellent to exposed skin. Travelers should observe food and beverage precautions and avoid consuming salads, uncooked vegetables, unpeeled fruits, and unpasteurized fruit juices.
American trypanosomiasis signs and symptoms
Much of the clinical information about Chagas disease comes from experience with people who became infected as children through contact with triatomines. The severity and course of an individual infection can vary based on a number of factors, including the age at which a person became infected, the way in which a person acquired the infection, or the particular strain of the Trypanosoma cruzi parasite.
There are two phases of Chagas disease: the acute phase and the chronic phase. Both phases can be symptom free or life threatening.
Acute phase
During this phase, which lasts for the first few weeks or months infection, a person may have no symptoms or mild ones, such as fever, fatigue, body aches, headache, rash, loss of appetite, diarrhea, and vomiting. Because these symptoms are similar to those of other illnesses, most people do not know their illness is from infection with the Trypanosoma cruzi parasite.
However, a doctor may be able to pick up other signs of infection, including mild enlargement of the liver or spleen, swollen glands, or swelling at the site of the bite (called a chagoma), where the parasite entered the body. Some people with acute phase infection may have swelling of the eyelids on the side of the face near the bite wound or where the bug poop was accidentally rubbed into the eye, called Romaña’s sign. Even if a person develops symptoms during the acute phase, they usually feel well within a few weeks or months but if the person is not treated with antiparasitic medication, the infection remains in the body. Rarely, young children (less than 5%) die from severe inflammation and infection of the heart muscle (myocarditis) or brain (meningoencephalitis). The acute phase also can be severe in people with weakened immune systems, such as patients taking chemotherapy or those with advanced HIV infection.
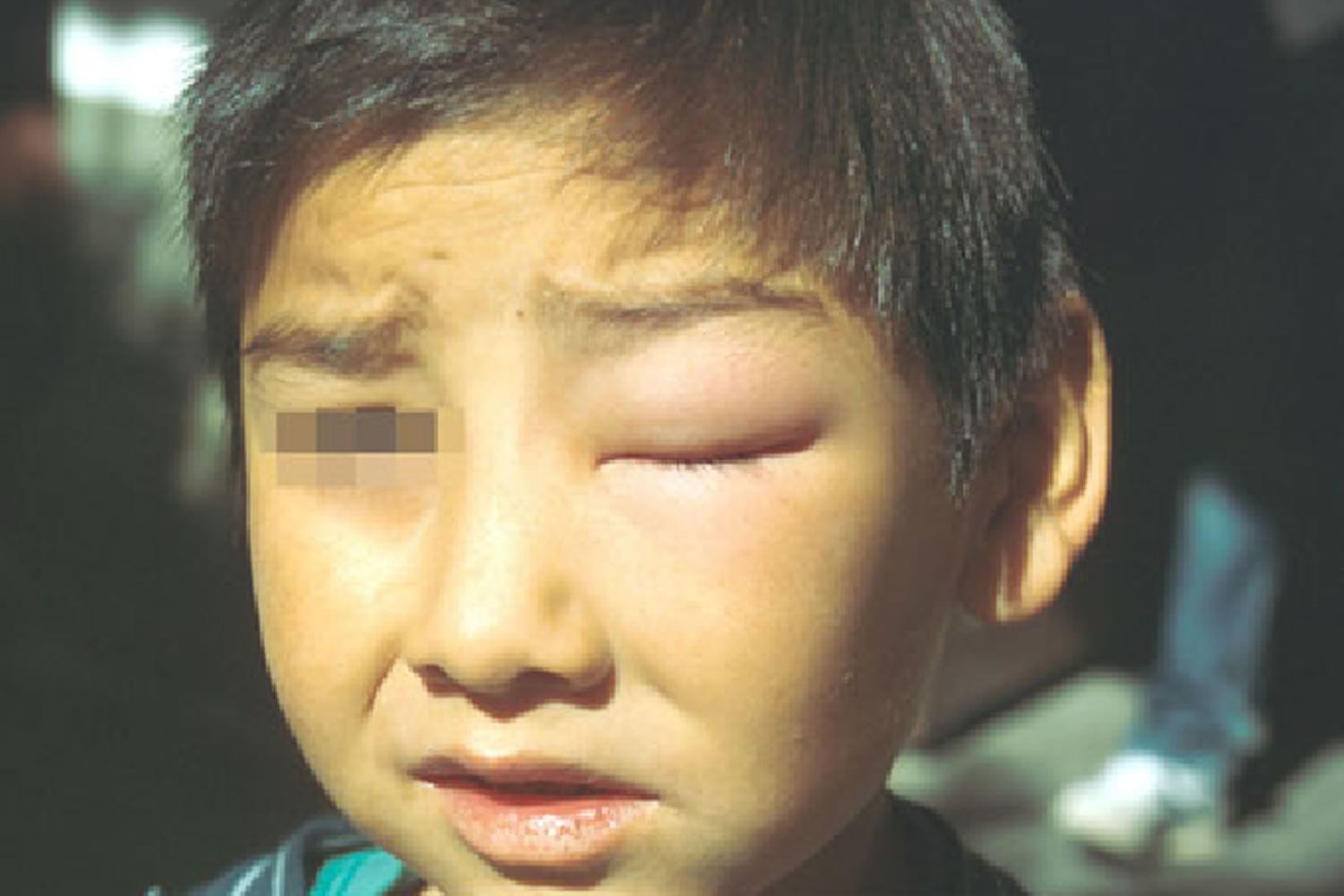
Romaña’s sign is a unilateral painless periorbital swelling of the child’s eyelid, is a marker of acute Chagas disease 47. Swelling is due to Trypanosoma cruzi infecting the eyelid when bug feces are accidentally rubbed into the eye, or because the bite wound was on the same side of the child’s face as the swelling.
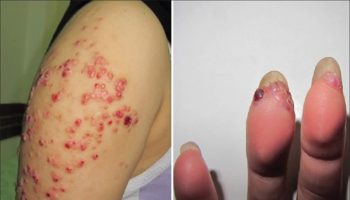
Figure 8. Romana’s sign (a chagoma found over the eyelid, is a marker of acute Chagas disease infection)
Footnote: Romaña’s sign, a unilateral painless periorbital swelling of the child’s eyelid, is a marker of acute Chagas disease. Swelling is due to Trypanosoma cruzi infecting the eyelid when bug feces are accidentally rubbed into the eye, or because the bite wound was on the same side of the child’s face as the swelling.
[Source 48 ]Chronic phase
During the chronic phase, which can last for decades or even for the entirety of someone’s lifetime, most people have no symptoms. Approximately 20–30 percent of infected people develop
- Cardiac complications, which can include an enlarged heart, heart failure, altered heart rate or rhythm, and cardiac arrest (sudden death); and/or
- Gastrointestinal complications, which can include an enlarged esophagus (megaesophagus) or colon (megacolon) and can lead to difficulties with eating or pooping.
American trypanosomiasis diagnosis
During the acute phase of infection, parasites may be seen circulating in the blood. The diagnosis of Chagas disease can be made by observation of the parasite in a blood smear by microscopic examination. A thick and thin blood smear are made and stained for visualization of parasites. However, levels may decrease within 90 days of infection and may be undetectable by microscopy, as the sensitivity of the test decreases and as the disease progresses from acute to chronic. Polymerase chain reaction (PCR) is another diagnostic tool that may be used during the acute phase, monitoring for acute infection in organ transplant recipients or following accidental exposures. PCR assays can demonstrate positive results days to weeks before a peripheral blood smear detects circulating trypomastigotes. Detection of IgG antibodies to T. cruzi can be used to demonstrate chronic infections 49.
Diagnosis of chronic Chagas disease is made after consideration of the patient’s clinical findings, as well as by the likelihood of being infected, such as having lived in a country where Chagas disease is common. Diagnosis is generally made by testing for parasite specific antibodies.
A chest x-ray may show heart involvement with cardiomegaly.
ECG findings may include intraventricular blocks, particularly a right bundle branch block and/or on the left the anterior fascicular block and diffuse ST-T changes.
American trypanosomiasis treatment
Two approaches to therapy, that can be life-saving include:
- Antiparasitic treatment, to kill the parasite; and
- Symptomatic treatment, to manage the symptoms and signs of infection.
Antiparasitic treatment is most effective early in the course of infection but is not limited to cases in the acute phase. In the United States, there are two types of treatments available for the American trypanosomiasis or Chagas disease. Benznidazole is approved by FDA for use in children 2–12 years of age and is commercially available at https://www.benznidazoletablets.com/en/. Nifurtimox (Lampit) is FDA approved for treatment of children from birth to age younger than 18 years and is commercially available for pharmacies to purchase from several drug wholesalers. Treatments with nifurtimox and benznidazole have more than 80% success rate during acute phase; but, has no effect on the amastigote stage 45. Your health-care provider can talk with Centers for Disease Control and Prevention (CDC) staff about whether and how you should be treated. Most people do not need to be hospitalized during treatment.
Supportive therapy for the chronic phase is dependent on which organ system is involved. Symptomatic treatment may help people who have cardiac or gastrointestinal problems from Chagas disease. For example, pacemakers and medications for irregular heartbeats may be life saving for some patients with chronic cardiac disease.
- Sutherland CS, Yukich J, Goeree R, Tediosi F. A literature review of economic evaluations for a neglected tropical disease: human African trypanosomiasis (“sleeping sickness”). PLoS Negl Trop Dis. 2015 Feb;9(2):e0003397[↩][↩][↩][↩]
- Hemmige V, Tanowitz H, Sethi A. Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. Int. J. Dermatol. 2012 May;51(5):501-8.[↩][↩][↩]
- World Health Organization. Control and surveillance of human African trypanosomiasis. World Health Organ Tech Rep Ser. 2013;(984):1-237.[↩][↩]
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010 Jan 09;375(9709):148-59[↩][↩][↩][↩][↩][↩]
- Garcia MN, Woc-Colburn L, Aguilar D, Hotez PJ, Murray KO. Historical Perspectives on the Epidemiology of Human Chagas Disease in Texas and Recommendations for Enhanced Understanding of Clinical Chagas Disease in the Southern United States. PLoS Negl Trop Dis. 2015 Nov;9(11):e0003981[↩][↩]
- Hemmige V, Tanowitz H, Sethi A. Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. Int. J. Dermatol. 2012 May;51(5):501-8[↩][↩][↩]
- Chagas Disease. https://medlineplus.gov/chagasdisease.html[↩]
- Maxfield L, Bermudez R. Trypanosomiasis (Trypansomiasis) [Updated 2018 Dec 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535413[↩]
- Matthews KR. The developmental cell biology of Trypanosoma brucei. J. Cell. Sci. 2005 Jan 15;118(Pt 2):283-90[↩]
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010 Jan 09;375(9709):148-59.[↩][↩]
- Stich A, Abel PM, Krishna S. Human African trypanosomiasis. BMJ. 2002 Jul 27;325(7357):203-6[↩][↩]
- Bonnet J, Boudot C, Courtioux B. Overview of the Diagnostic Methods Used in the Field for Human African Trypanosomiasis: What Could Change in the Next Years? Biomed Res Int. 2015;2015:583262[↩][↩][↩]
- Simarro PP, Franco J, Diarra A, Postigo JA, Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012 Jun;139(7):842-6[↩][↩]
- Sales Junior PA, Molina I, Fonseca Murta SM, Sánchez-Montalvá A, Salvador F, Corrêa-Oliveira R, Carneiro CM. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017 Nov;97(5):1289-1303[↩][↩]
- Bonnet J, Boudot C, Courtioux B. Overview of the Diagnostic Methods Used in the Field for Human African Trypanosomiasis: What Could Change in the Next Years? Biomed Res Int. 2015;2015:583262.[↩]
- Kazumba, L. M., Kaka, J. T., Ngoyi, D. M., & Tshala-Katumbay, D. (2018). Mortality trends and risk factors in advanced stage-2 Human African Trypanosomiasis: A critical appraisal of 23 years of experience in the Democratic Republic of Congo. PLoS neglected tropical diseases, 12(6), e0006504. https://doi.org/10.1371/journal.pntd.0006504[↩][↩]
- Simarro, P. P., Cecchi, G., Paone, M., Franco, J. R., Diarra, A., Ruiz, J. A., Fèvre, E. M., Courtin, F., Mattioli, R. C., & Jannin, J. G. (2010). The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. International journal of health geographics, 9, 57. https://doi.org/10.1186/1476-072X-9-57[↩]
- Trypanosomiasis, human African (sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness[↩][↩][↩]
- Franco, J. R., Simarro, P. P., Diarra, A., & Jannin, J. G. (2014). Epidemiology of human African trypanosomiasis. Clinical epidemiology, 6, 257–275. https://doi.org/10.2147/CLEP.S39728[↩][↩]
- Bentley SJ, Jamabo M, Boshoff A. The Hsp70/J-protein machinery of the African trypanosome, Trypanosoma brucei. Cell Stress Chaperones. 2019 Jan;24(1):125-148[↩]
- Epidemiology and control of African trypanosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1986;739:1-127.[↩]
- Kame-Ngasse, G. I., Njiokou, F., Melachio-Tanekou, T. T., Farikou, O., Simo, G., & Geiger, A. (2018). Prevalence of symbionts and trypanosome infections in tsetse flies of two villages of the “Faro and Déo” division of the Adamawa region of Cameroon. BMC microbiology, 18(Suppl 1), 159. https://doi.org/10.1186/s12866-018-1286-5[↩]
- Gao, J., Qian, Z., Hide, G., Lai, D., Lun, Z., & Wu, Z. (2020). Human African trypanosomiasis: The current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology, 147(9), 922-931. doi:10.1017/S0031182020000645[↩][↩]
- Grebaut P, Bodo JM, Assona A, Foumane Ngane V, Njiokou F, Ollivier G, Soula G, Laveissiere C. Recherche des facteurs de risque de la trypanosomose humaine africaine dans le foyer de Bipindi au Cameroun [Risk factors for human African trypanosomiasis in the Bipindi region of Cameroon]. Med Trop (Mars). 2001;61(4-5):377-83. French.[↩]
- Kohagne, T. L., M’eyi, M. P., Kamkuimo, R. G., Kaba, D., Louis, J. F., & Mimpfoundi, R. (2011). Transmission of human African trypanosomiasis in the Komo-Mondah focus, Gabon. The Pan African medical journal, 8, 36. https://doi.org/10.4314/pamj.v8i1.71151[↩]
- Moore A, Richer M, Enrile M, Losio E, Roberts J, Levy D. Resurgence of sleeping sickness in Tambura County, Sudan. Am J Trop Med Hyg. 1999 Aug;61(2):315-8. doi: 10.4269/ajtmh.1999.61.315[↩]
- Courtin F, Jamonneau V, Camara M, Camara O, Coulibaly B, Diarra A, Solano P, Bucheton B. A geographical approach to identify sleeping sickness risk factors in a mangrove ecosystem. Trop Med Int Health. 2010 Aug;15(8):881-9. doi: 10.1111/j.1365-3156.2010.02559.x[↩]
- Meda AH, Laveissiere C, De Muynck A, Doua F, Diallo PB. Les facteurs de risque de la trypanosomiase humaine africaine dans les foyers endemiques de Cote d’Ivoire [Risk factors for human African trypanosomiasis in the endemic foci of Ivory Coast]. Med Trop (Mars). 1993 Jan-Mar;53(1):83-92. French.[↩]
- Grébaut P, Bena JM, Manzambi EZ, Mansinsa P, Khande V, Ollivier G, Cuny G, Simo G. Characterization of sleeping sickness transmission sites in rural and periurban areas of Kinshasa (République Démocratique du Congo). Vector Borne Zoonotic Dis. 2009 Dec;9(6):631-6. doi: 10.1089/vbz.2008.0118[↩]
- Fischer A, Simarro PP, Franco JR, Becher H, Steverding D. Increased trypanolytic activity in sera of sleeping sickness patients after chemotherapy. Trop Med Int Health. 2001 Dec;6(12):1070-4. doi: 10.1046/j.1365-3156.2001.00805.x[↩]
- Paquet C, Castilla J, Mbulamberi D, Beaulieu MF, Gastellu Etchegorry MG, Moren A. La trypanosomiase a Trypanosoma brucei gambiense dans le foyer du nord-ouest de l’Ouganda. Bilan de 5 années de lutte (1987-1991) [Trypanosomiasis from Trypanosoma brucei gambiense in the center of north-west Uganda. Evaluation of 5 years of control (1987-1991)]. Bull Soc Pathol Exot. 1995;88(1):38-41. French.[↩]
- Abel PM, Kiala G, Lôa V, Behrend M, Musolf J, Fleischmann H, Théophile J, Krishna S, Stich A. Retaking sleeping sickness control in Angola. Trop Med Int Health. 2004 Jan;9(1):141-8. doi: 10.1046/j.1365-3156.2003.01152.x. Erratum in: Trop Med Int Health. 2004 Feb;9(2):314.[↩]
- Pépin J, Méda HA. The epidemiology and control of human African trypanosomiasis. Adv Parasitol. 2001;49:71-132. doi: 10.1016/s0065-308x(01)49038-5[↩]
- Khonde N, Pépin J, Niyonsenga T, De Wals P. Familial aggregation of Trypanosoma brucei gambiense trypanosomiasis in a very high incidence community in Zaire. Trans R Soc Trop Med Hyg. 1997 Sep-Oct;91(5):521-4. doi: 10.1016/s0035-9203(97)90008-0[↩]
- Simarro PP, Franco JR, Cecchi G, Paone M, Diarra A, Ruiz Postigo JA, Jannin JG. Human African trypanosomiasis in non-endemic countries (2000-2010). J Travel Med. 2012 Jan-Feb;19(1):44-53. doi: 10.1111/j.1708-8305.2011.00576.x. Epub 2011 Dec 8. Erratum in: J Travel Med. 2012 Mar-Apr;19(2):135.[↩][↩]
- Kinung’hi SM, Malele II, Kibona SN, et al. Knowledge, attitudes and practices on tsetse and sleeping sickness among communities living in and around Serengeti National Park, Tanzania. Tanzan Health Res Bull. 2006;8(3):168–172.[↩]
- Zoller, T., Fèvre, E. M., Welburn, S. C., Odiit, M., & Coleman, P. G. (2008). Analysis of risk factors for T. brucei rhodesiense sleeping sickness within villages in south-east Uganda. BMC infectious diseases, 8, 88. https://doi.org/10.1186/1471-2334-8-88[↩][↩][↩][↩][↩]
- Allsopp R. (1972). The role of game animals in the maintenance of endemic and enzootic trypanosomiases in the Lambwe Valley, South Nyanza District, Kenya. Bulletin of the World Health Organization, 47(6), 735–746.[↩]
- Vale, G. A., Chamisa, A., Mangwiro, C., & Torr, S. J. (2013). A neglected aspect of the epidemiology of sleeping sickness: the propensity of the tsetse fly vector to enter houses. PLoS neglected tropical diseases, 7(2), e2086. https://doi.org/10.1371/journal.pntd.0002086[↩]
- Jelinek, T., Bisoffi, Z., Bonazzi, L., van Thiel, P., Bronner, U., de Frey, A., Gundersen, S. G., McWhinney, P., Ripamonti, D., & European Network on Imported Infectious Disease Surveillance (2002). Cluster of African trypanosomiasis in travelers to Tanzanian national parks. Emerging infectious diseases, 8(6), 634–635. https://doi.org/10.3201/eid0806.010432[↩]
- Dunn N, Wang S, Adigun R. African Trypanosomiasis. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519580[↩][↩]
- Ferrins, L., Sharma, A., Thomas, S. M., Mehta, N., Erath, J., Tanghe, S., Leed, S. E., Rodriguez, A., Mensa-Wilmot, K., Sciotti, R. J., Gillingwater, K., & Pollastri, M. P. (2018). Anilinoquinoline based inhibitors of trypanosomatid proliferation. PLoS neglected tropical diseases, 12(11), e0006834. https://doi.org/10.1371/journal.pntd.0006834[↩]
- Nguyen T, Waseem M. Chagas Disease (American Trypanosomiasis) [Updated 2019 Mar 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459272[↩]
- Echeverria LE, Morillo CA. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. North Am. 2019 Mar;33(1):119-134.[↩]
- Nguyen T, Waseem M. Chagas Disease. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459272[↩][↩]
- Padilla N A, Moncayo AL, Keil CB, Grijalva MJ, Villacís AG. Life Cycle, Feeding, and Defecation Patterns of Triatoma carrioni (Hemiptera: Reduviidae), Under Laboratory Conditions. J Med Entomol. 2019 Apr 16;56(3):617-624. doi: 10.1093/jme/tjz004[↩]
- Patel S, Sethi A. Imported tropical diseases. Dermatol Ther. 2009 Nov-Dec;22(6):538-49. doi: 10.1111/j.1529-8019.2009.01275.x[↩]
- Muñoz-Saravia SG, Haberland A, Wallukat G, Schimke I. Chronic Chagas’ heart disease: a disease on its way to becoming a worldwide health problem: epidemiology, etiopathology, treatment, pathogenesis and laboratory medicine. Heart Fail Rev. 2012 Jan;17(1):45-64. doi: 10.1007/s10741-010-9211-5[↩]
- Mayta H, Romero YK, Pando A, Verastegui M, Tinajeros F, Bozo R, Henderson-Frost J, Colanzi R, Flores J, Lerner R, Bern C, Gilman RH., Chagas Working Group in Perú and Bolivia. Improved DNA extraction technique from clot for the diagnosis of Chagas disease. PLoS Negl Trop Dis. 2019 Jan;13(1):e0007024[↩]