Contents
- What is acetaminophen
- How does acetaminophen work?
- Acetaminophen in pregnancy
- How should acetaminophen be used?
- Acetaminophen (paracetamol) side effects
- Acetaminophen (paracetamol) Contraindications
- Acetaminophen (paracetamol) Poisoning
- Acetaminophen (paracetamol) poisoing treatment
- Acetaminophen (paracetamol) overdose
- Acetaminophen (paracetamol) overdose prognosis
- Who can take acetaminophen?
- Acetaminophen (paracetamol) dosage
What is acetaminophen
Acetaminophen commonly known as Tylenol, Panadol, paracetamol or N-acetyl-para-aminophenol (APAP) is a medicine used to reduce pain (analgesic) and fever (antipyretic) 1, 2, 3, 4. Acetaminophen has been available as an over-the-counter (OTC) medicine in the United States since 1960 and more than 25 billion doses are sold yearly 3. Acetaminophen is in a class of medications called analgesics (pain relievers) and antipyretics (fever reducers). Acetaminophen works by changing the way your body senses pain and by cooling your body. However, the precise mechanism of action for acetaminophen remains unclear to date. Acetaminophen is a commonly used medicine to relieve mild or moderate pain, such as muscle aches, osteoarthritis (arthritis caused by the breakdown of the lining of the joints), backaches, menstrual periods, toothaches, colds, sore throats or sprains, reactions to vaccinations (shots) and reduce a high temperature (fever) caused by illnesses such as colds and flu. Acetaminophen is often recommended as one of the first treatments for pain and fever, as it’s safe for most people to take and side effects are rare. Acetaminophen can be bought over-the-counter (OTC) in pharmacies, supermarkets and other shops; it is included in many prescription and over-the-counter (OTC) products. In fact, over 60 million Americans use acetaminophen weekly, often unknowingly, due to its presence in various combination products, particularly those containing opioids and diphenhydramine 5, 6, 7.
Acetaminophen doesn’t treat the cause of your pain, it just eases the feeling of pain. You can take acetaminophen for:
- mild to moderate pain, for example backache, headache, migraine, muscle strains, period pain, menstrual cramps, toothache and aches and pains due to colds and flu
- fever (high temperature)
- osteoarthritis and other painful, non-inflammatory conditions.
Acetaminophen (paracetamol) comes in the following forms:
- Tablets
- Capsules
- Suppositories
- Soluble powders
- Liquids
Acetaminophen (paracetamol) is made by lots of different companies and sold using different names. It’s also often combined with other medicines and found in cold and flu medicines and headache tablets. This makes it easy to take too much acetaminophen (paracetamol) by mistake. This can be dangerous and may cause death.
Modified release acetaminophen (paracetamol)
Modified release acetaminophen (paracetamol) has a higher dose of acetaminophen (paracetamol) than standard acetaminophen (paracetamol) tablets. This can also be called:
- controlled release acetaminophen (paracetamol)
- extended-release acetaminophen (paracetamol)
- slow-release acetaminophen (paracetamol)
- sustained release acetaminophen (paracetamol)
Modified release acetaminophen (paracetamol) is often used to help manage pain associated with osteoarthritis. Modified release acetaminophen (paracetamol) is released into your body more slowly than normal acetaminophen (paracetamol) products. It is designed to be taken less often than normal acetaminophen (paracetamol). You take a dose every 8 hours (3 times a day).
Acetaminophen (paracetamol) has weak anti-inflammatory properties and is used as a common analgesic (pain reliever), taking acetaminophen (paracetamol) begins to ease pain and lower a high temperature about 30 minutes after a dose is taken. Its effects usually last for about 4 hours. The recommended acetaminophen (paracetamol) oral dose is 660 to 1000 mg every 4 to 6 hours, but should not to exceed 3 grams per day. Multiple generic formulations of acetaminophen are available (e.g., Tylenol, Anacin Aspirin Free, Feverall, Neopap, Panadol and Tempra) in capsules or tablets of 330 or 500 mg each. Liquid formulations for children are available in concentrations that vary from 15 to 100 mg/mL; the dosage in children should be carefully chosen and kept to less than 75 mg/kg/day. In addition, acetaminophen is a frequent component in many over-the-counter and prescription combinations with decongestants and/or antihistamines for cold and allergy symptoms, or as a sleeping aid and with other analgesics (such as oxycodone, hydrocodone, dilaudid and codeine) for moderate-to-severe forms of pain.
Harmless at low doses, acetaminophen (paracetamol) has the potential to damage your liver and kidney when taken as an overdose and can cause acute liver injury and death from acute liver failure. Even in therapeutic doses, acetaminophen can cause transient serum aminotransferase elevations (liver enzymes). Acetaminophen has been the leading cause of acute liver failure in the United States since 1998 and requires a warning about the liver toxic risks 8. Acetaminophen (paracetamol) toxicity may result from a single toxic dose, from repeated ingestion of large doses of acetaminophen (e.g., 7.5 g to 10 g daily for 1 to 2 days), or from chronic ingestion of the drug 9. Dose-dependent, liver necrosis is the most serious acute toxic effect associated with overdosage and is potentially fatal 10, 9. Acetaminophen toxicity is the second most common cause of liver transplantation worldwide and the most common cause of liver failure in the United States 2. Acetaminophen (paracetamol) toxicity is responsible for 56,000 emergency department visits and 2600 hospitalizations, acetaminophen poisoning causes 500 deaths annually in the United States 11, 2. Notably, around 50% of these poisonings are unintentional, often resulting from patients misinterpreting dosing instructions or unknowingly consuming multiple acetaminophen-containing products 2.
The only approved antidote for acetaminophen overdose and toxicity is N-acetylcysteine (NAC) 12, 2. N-acetylcysteine (NAC) acts as a precursor to glutathione synthesis, aiding in restoring intracellular glutathione stores to neutralize the N-acetyl-p-benzoquinone imine (NAPQI) compound, directly inactivating NAPQI (N-acetyl-p-benzoquinone imine). N-acetylcysteine (NAC) can be administered orally or via the IV route. The IV administration of N-acetylcysteine (NAC) is typically preferred because vomiting is common with acetaminophen overdose. N-acetylcysteine (NAC) administration follows a 20-hour IV or 72-hour oral protocol, and clinicians must monitor aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels during treatment 13, 2.
It’s important to note that the majority of patients who overdose on acetaminophen do not exhibit symptoms in the initial hours after ingesting toxic doses of acetaminophen. During this early period, symptoms may be limited to abdominal pain and nausea, persisting for the first 12 to 24 hours. Although these symptoms may alleviate between 24 and 72 hours, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations may remain abnormal. Patients presenting more than 24 hours after ingesting toxic doses of acetaminophen may manifest symptoms including nausea, vomiting, jaundice, abdominal pain, and hypotension. The management of these patients may involve interventions such as airway management, IV fluids, vasopressors, and addressing symptoms such as cerebral edema as they arise.
A recent consensus statement published by America’s Poison Centers, American Academy of Clinical Toxicology, American College of Medical Toxicology, and Canadian Association of Poison Control Centers addresses acetaminophen toxicity. N-acetylcysteine (NAC) is administered through oral or IV routes, with the initial dose administered promptly upon identifying the need for treatment. The panel recommends a regimen providing a minimum of N-acetylcysteine (NAC) 300 mg/kg, either orally or IV, within the initial 20 to 24 hours of treatment. Nevertheless, the comparative effectiveness of various regimens still requires evaluation.
The guidelines emphasize the importance of continuous assessment and caution against prematurely discontinuing treatment. Notably, a common clinical error involves administering N-acetylcysteine (NAC) for 20 or 21 hours and then discontinuing without reassessing the patient. The panel chose to refine the Rumack-Matthew nomogram by retaining only the lines indicating clinical action. In this refined approach, the blood concentration of APAP is directly plotted on the nomogram, and N-acetylcysteine (NAC) is administered to patients whose concentration exceeds the treatment line. Stopping criteria for N-acetylcysteine (NAC) include an acetaminophen concentration below 10 μg/dL, an INR level below 2, normal levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), or a decrease in AST/ALT by 25% to 50%, provided the patient is clinically stable 14.
High-risk ingestion involves the consumption of at least 30 g of acetaminophen or an acetaminophen concentration surpassing the high-risk line on the Rumack-Matthew nomogram. These cases are managed similarly to other acetaminophen overdoses, with consideration for the extended administration of activated charcoal, especially if the ingestion occurred more than 4 hours prior due to prolonged absorption. In addition, consultation with a clinical toxicologist may be required for an increased N-acetylcysteine (NAC) dosage. In managing repeated supratherapeutic ingestion in 24 hours, unlike acute ingestion cases, treatment is based on signs and symptoms. If the acetaminophen concentration exceeds 20 μg/mL or AST levels or ALT are abnormal, N-acetylcysteine (NAC) should be administered until the established stopping criteria are met.
Extended-release acetaminophen products, intended for 8-hour use in the United States or Canada, are managed similarly to other acetaminophen products. Activated charcoal may continue to be effective for longer than 4 hours after ingestion, especially when evidence indicates ongoing absorption, such as an increasing acetaminophen concentration. N-acetylcysteine (NAC) is required if the acetaminophen concentration from samples drawn 4 to 24 hours after ingestion surpasses the nomogram treatment line. In cases where the concentration from samples drawn 4 to 12 hours after ingestion falls below the treatment line but remains above 10 μg/mL, a follow-up measurement should be taken 4 to 6 hours after the initial assessment.
In simultaneous ingestion with anticholinergic or opioid agonists, the concern is the potential for delays or prolongation of acetaminophen absorption. The management approach aligns with that of other acetaminophen products. If the initial acetaminophen concentration measured 4 to 24 hours after ingestion is 10 μg/mL or lower, further measurements are unnecessary, and N-acetylcysteine (NAC) treatment is not required. Conversely, if any concentration exceeds the treatment line, N-acetylcysteine (NAC) is indicated.
If the acetaminophen concentration measured within the same time frame falls between 10 μg/mL and the treatment line on the revised nomogram, and clinical signs indicating anticholinergic or opioid toxicity are present, a reevaluation should be scheduled 4 to 6 hours following the initial measurement. Notably, the dosing and duration of N-acetylcysteine (NAC) treatment strictly follow the established standard protocol for acetaminophen ingestions. The panel recommends hemodialysis with N-acetylcysteine (NAC) in massive acetaminophen toxicity with a concentration exceeding 900 μg/mL, accompanied by acidosis or altered consciousness 14.
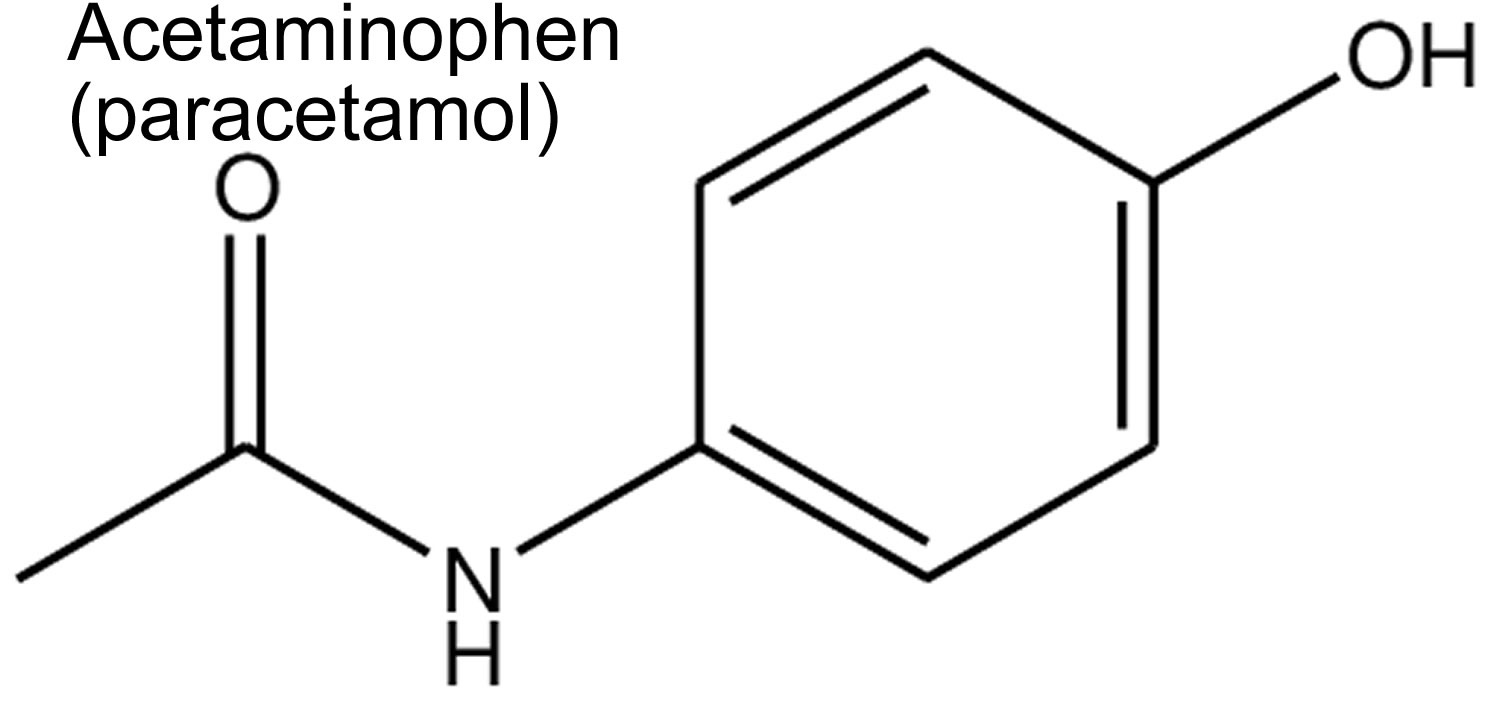
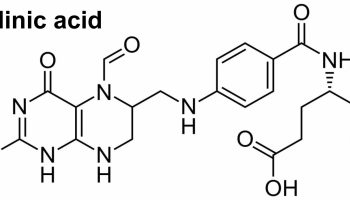
Figure 1. Acetaminophen (paracetamol) chemical structure
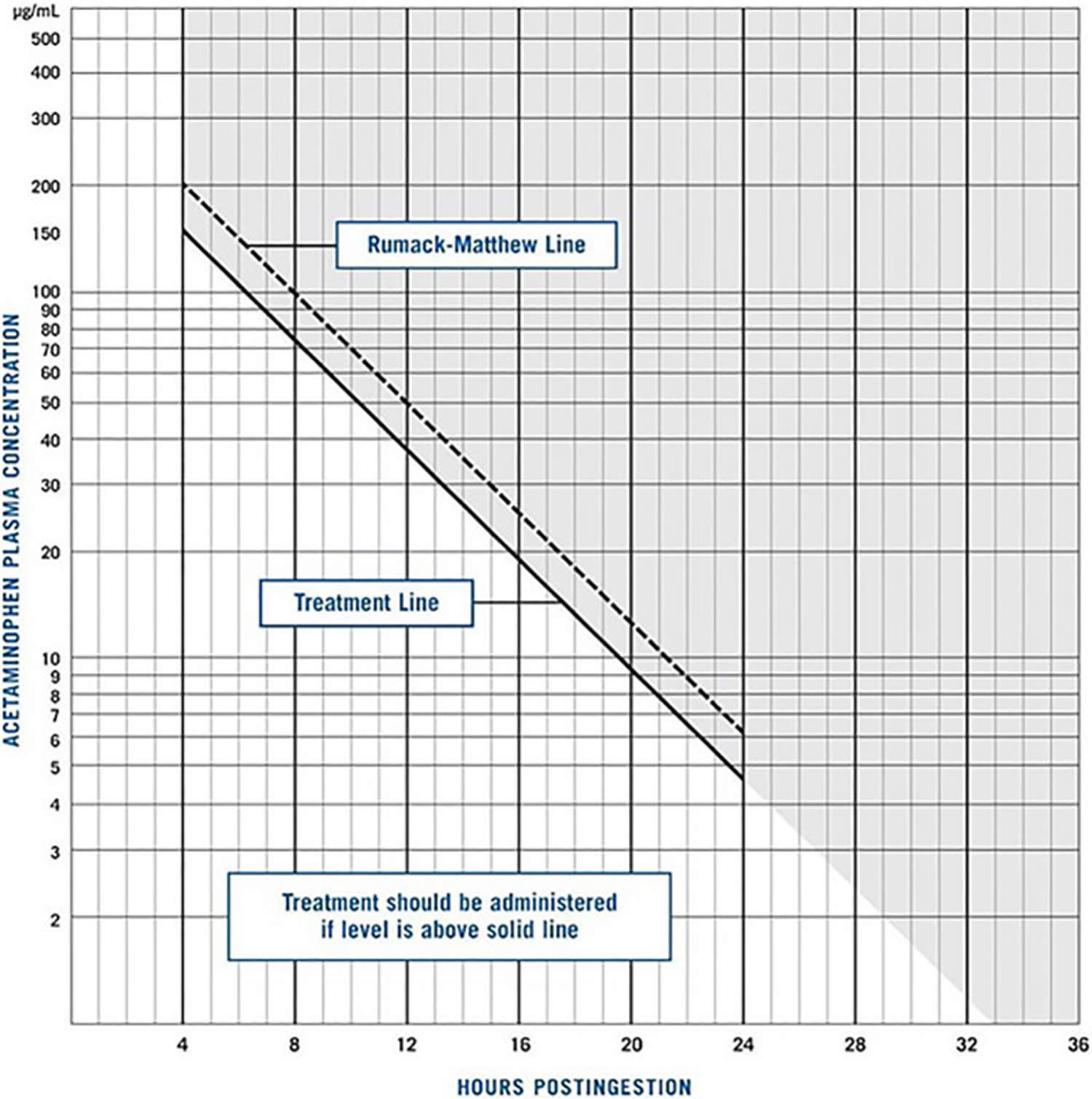
[Source 15 ]Figure 2. Rumack Matthew Nomogram
Footnotes: The Rumack–Matthew nomogram, first published in 1975, was developed to estimate the likelihood of liver injury due to acetaminophen toxicity for patients with a single ingestion at a known time. To use the Rumack–Matthew nomogram, the patient’s plasma acetaminophen concentration and the time interval since ingestion are plotted. If the resulting point is above and to the right of the sloping Rumack–Matthew line, liver injury is likely to result and the use of acetylcysteine is indicated. If the point is below and to the left of the Rumack–Matthew line, liver injury is unlikely. Patients with repeated supratherapeutic acetaminophen ingestion, or with an unknown time of acetaminophen ingestion, cannot be evaluated with the use of the Rumack–Matthew nomogram.
[Source 16 ]Is it okay to drink alcohol when I’m taking acetaminophen (paracetamol)?
Drinking a small amount of alcohol is unlikely to be harmful if you are taking acetaminophen (paracetamol).
Is acetaminophen ibuprofen?
No. Ibuprofen belongs to one of a group of painkillers called non-steroidal anti-inflammatory drugs (NSAIDs). Ibuprofen is better for reducing inflammation (redness and swelling), including teething and toothache.
Whereas acetaminophen is usually best for most types of pain, including headache and stomach ache.
Acetaminophen and ibuprofen are similar strengths, but they work in different ways. So acetaminophen is better for some types of pain than ibuprofen.
Do not give ibuprofen and paracetamol together, though. Instead, if you’ve given acetaminophen to your child and they’re still feverish or in pain when the next dose is due, you could try ibuprofen instead.
Don’t take more than the maximum daily dose of either medicine. See your doctor if you’ve tried both ibuprofen and acetaminophen and they haven’t helped.
Taking too much acetaminophen can cause liver damage, sometimes serious enough to require liver transplantation or cause death. You might accidentally take too much acetaminophen if you do not follow the directions on the prescription or package label carefully, or if you take more than one product that contains acetaminophen.
To be sure that you take acetaminophen safely, you should:
- Not take more than one product that contains acetaminophen at a time. Read the labels of all the prescription and nonprescription medications you are taking to see if they contain acetaminophen. Be aware that abbreviations such as APAP, AC, Acetaminophen, Acetaminoph, Acetaminop, Acetamin, or Acetam. may be written on the label in place of the word acetaminophen. Ask your doctor or pharmacist if you don’t know if a medication that you are taking contains acetaminophen.
- Take acetaminophen exactly as directed on the prescription or package label. Do not take more acetaminophen or take it more often than directed, even if you still have fever or pain. Ask your doctor or pharmacist if you do not know how much medication to take or how often to take your medication. Call your doctor if you still have pain or fever after taking your medication as directed.
- Be aware that you should not take more than 4000 mg of acetaminophen per day. If you need to take more than one product that contains acetaminophen, it may be difficult for you to calculate the total amount of acetaminophen you are taking. Ask your doctor or pharmacist to help you.
- Tell your doctor if you have or have ever had liver disease.
- Not take acetaminophen if you drink three or more alcoholic drinks every day. Talk to your doctor about the safe use of alcohol while you are taking acetaminophen.
stop taking your medication and call your doctor right away if you think you have taken too much acetaminophen, even if you feel well.
Talk to your pharmacist or doctor if you have questions about the safe use of acetaminophen or acetaminophen-containing products.
How does acetaminophen work?
Acetaminophen (paracetamol) is a p-aminophenol derivative, an odorless compound with a slightly bitter taste with analgesic and anti-fever activities that seems to work by blocking chemical messengers in the brain that tell you that you have pain. However, the precise mechanism of action for acetaminophen remains unclear to date. There is a hypothesis that acetaminophen inhibits a different variant of cyclooxygenase (COX) enzyme, also known as COX-3 (cyclooxygenase 3), but this event remains unconfirmed in human studies 17. Nonetheless, the diminished activity of the cyclooxygenase (COX) pathway leads to decreased prostaglandin synthesis in the central nervous system (brain and spinal cord), thus inducing analgesia (serotonergic inhibitory pathways) and antipyretic effect (hypothalamic heat-regulating center) 18, 19, 1, 20, 17, 21.
However, more recent research suggests two other pathways. Acetaminophen (paracetamol) inhibits the synthesis of 2-arachidonoyl-glycerol (2-AG), an endocannabinoid, possibly in circuits permissive for pain 22. In addition, researchers discovered that acetaminophen (paracetamol) metabolite, N-arachidonoylphenolamine (AM404), acts on sodium channels peripherally 23, 24. N-arachidonoylphenolamine (AM404) exerts analgesic effects through dual modulation of glutamatergic synaptic transmission within the spinal cord dorsal horn. This involves facilitating spontaneous transmission and inhibiting C-fiber–evoked transmission, achieved via activation of transient receptor potential vanilloid subtype-1 (TRPV1) receptors 25.
Acetaminophen (paracetamol) is linked to psychological symptoms, such as decreased positive empathy, emotional reactivity, and social pain with regular use, possibly linked to lower physical pain 26, 27, 28.
Pharmacokinetics
Absorption: Oral acetaminophen is rapidly and efficiently absorbed from the gastrointestinal tract, achieving peak plasma concentrations within 30 to 60 minutes. Intravenous (IV) administration of acetaminophen resulted in immediate and higher peak plasma concentrations. The rectal route is preferred for administration to bypass first-pass metabolism, especially in unconscious patients and children. This approach offers an alternative to parenteral administration, thereby mitigating gastric irritation and enabling efficient absorption due to the rich vascular supply in the rectum. Absorption in the upper rectum guides medications into the portal circulation through the superior hemorrhoidal vein, whereas lower rectal absorption results in direct entry into the systemic circulation 29.
Distribution: Acetaminophen exhibits low plasma protein binding of 10% to 25% and shows extensive distribution throughout the body, excluding fat tissue 30.
Metabolism: Acetaminophen undergoes primarily liver metabolism via first-order kinetics, utilizing 3 distinct pathways—conjugation with glucuronide, conjugation with sulfate, and oxidation facilitated by the cytochrome P450 enzyme system, predominantly CYP2E1. CYP3A4 plays a limited role in acetaminophen metabolism. This process forms a reactive intermediate metabolite known as N-acetyl-p-benzoquinone imine (NAPQI). At therapeutic doses, N-acetyl-p-benzoquinone imine (NAPQI) swiftly combines with glutathione, subsequently undergoing further metabolism to generate cysteine and mercapturic acid conjugates 31.
Elimination: Most acetaminophen metabolites are excreted in the urine, with less than 5% appearing as unconjugated or free acetaminophen. Over 90% of the administered dose is eliminated within 24 hours.
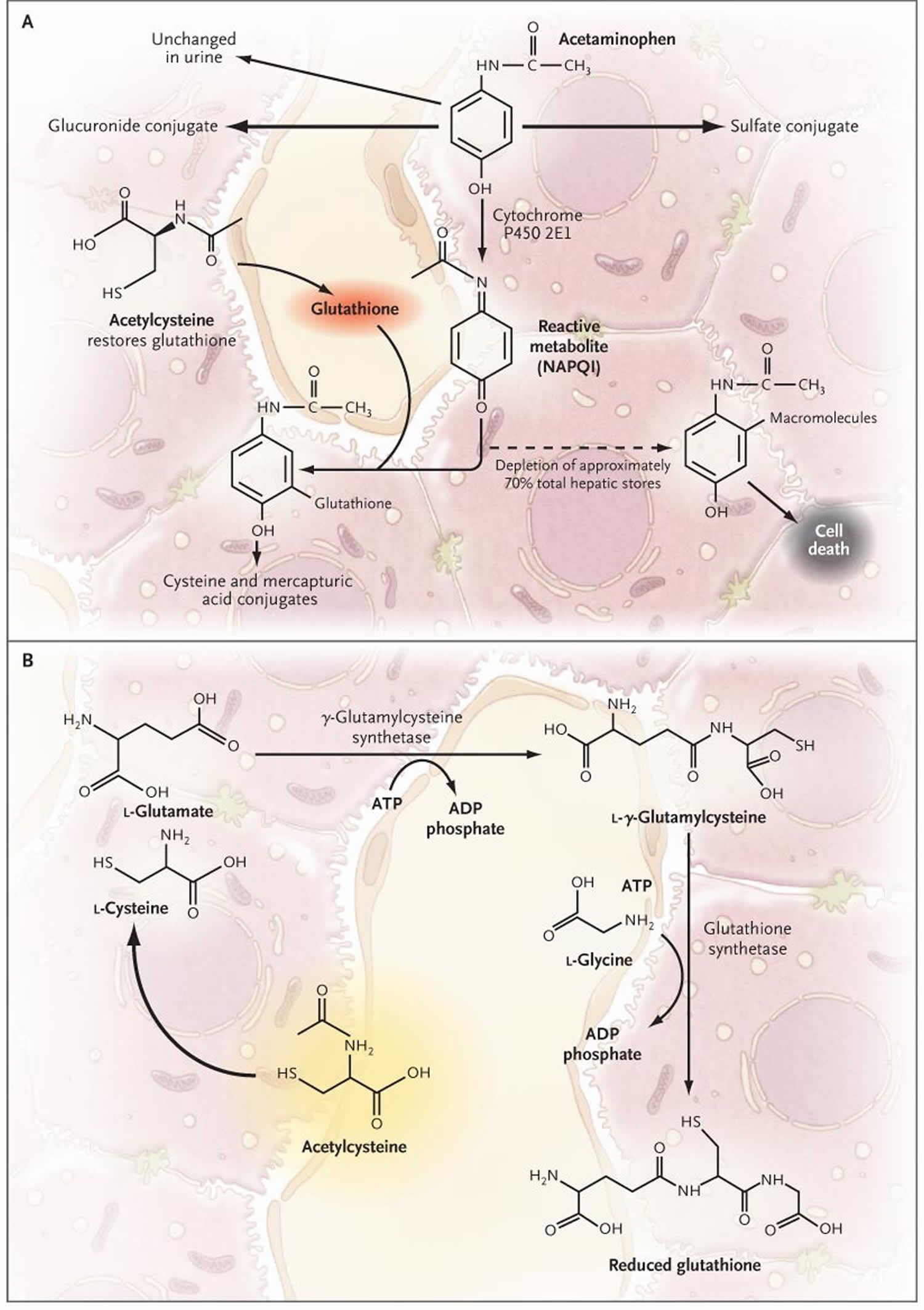
Figure 3. Acetaminophen metabolism
Footnotes: The primary pathways for acetaminophen metabolism (Panel A) are glucuronidation and sulfation to nontoxic metabolites. Approximately 5% of a therapeutic dose is metabolized by cytochrome P450 2E1 to the electrophile N-acetyl-p-benzoquinone imine (NAPQI). NAPQI (N-acetyl-p-benzoquinone imine) is extremely toxic to the liver. Ordinarily, NAPQI is rapidly detoxified by interaction with glutathione to form cysteine and mercapturic acid conjugates. Overdoses of acetaminophen (either a single large ingestion or repeated supra-therapeutic ingestion) can deplete hepatic glutathione stores and allow liver injury to occur 32. If glutathione is depleted, NAPQI (N-acetyl-p-benzoquinone imine) interacts with various macromolecules, leading to hepatocyte injury and death. Glutathione is synthesized from the amino acids cysteine, glutamate, and glycine by means of the pathway shown in Panel B. Glutamate and glycine are present in abundance in hepatocytes; the availability of cysteine is the rate-limiting factor in glutathione synthesis. However, cysteine itself is not well absorbed after oral administration. Acetylcysteine also known as N-acetylcysteine (NAC), in contrast, is readily absorbed and rapidly enters cells, where it is hydrolyzed to cysteine, thus providing the limiting substrate for glutathione synthesis. N-acetylcysteine (NAC) prevents hepatic injury primarily by restoring hepatic glutathione (Panel B) 33.
[Source 34 ]Acetaminophen in pregnancy
Observational studies have associated prenatal acetaminophen (paracetamol) exposure with potential reproductive and neurobehavioral effects, including the risks of cryptorchidism, attention-deficit/hyperactivity disorder (ADHD), and autism spectrum disorder (ASD) with prenatal acetaminophen (paracetamol) exposure 35, 36. Pregnant women are advised to exercise caution when using acetaminophen early in pregnancy due to emerging evidence suggesting that in-utero exposure to acetaminophen may elevate the risk of neurological, reproductive, and urogenital disorders in the fetus 37. A study by Alemany et al 37 investigated prenatal and postnatal acetaminophen exposure in relation to Autism Spectrum Conditions (ASC) and attention-deficit/hyperactivity disorder (ADHD). Acetaminophen exposure was assessed in 73,881 mother-child pairs through questionnaires or interviews. Children aged 4 to 12 exhibiting symptoms of Autism Spectrum Conditions (ASC) or ADHD were evaluated using well-documented instruments. The study found that children prenatally exposed to acetaminophen had an elevated risk of Autism Spectrum Conditions (ASC) (19%) or ADHD (21%), manifesting as borderline or clinical symptoms 37. However, postnatal acetaminophen exposure did not show an association with Autism Spectrum Conditions (ASC) or ADHD symptoms.
In 2021, a group of scientists and doctors published a statement in Nature Reviews Endocrinology that urged the Food and Drug Administration (FDA) to review guidelines for maternal use of acetaminophen (paracetamol) during pregnancy 35. In their review, maternal and perinatal use of acetaminophen (paracetamol) was strongly associated with neurodevelopmental disorders, including autism spectrum disorder (ASD) and ADHD, in 26 out of 29 observational studies. These studies collected data from 220,000 mother-child pairs, spanning multiple regions worldwide. Beyond neurodevelopmental disorders, the article revealed that acetaminophen (paracetamol) was correlated with significant abnormalities in motor and reproductive development.
In 2020, Johns Hopkins University researchers evaluated members of the Boston Birth Cohort by analyzing biological samples of 996 mother-child dyads, focusing on the presence of acetaminophen and its byproducts in umbilical cord blood samples, dividing them into groups of low, medium, and high exposure 36. Maternal age, ethnicity, stress, alcohol and illicit drug usage, education, body mass index (BMI), fever during pregnancy, and other covariates were controlled during the study. The researchers findings revealed a notable association between in utero acetaminophen exposure and an increased risk of ADHD and ASD in childhood 36. Additionally, children in the high-exposure group were 3.62 times more likely to be diagnosed with autism and 2.86 times more likely to be diagnosed with ADHD compared to the low-exposure group 36. One limitation of Boston Birth Cohort study is that cord samples were collected at birth, which may not be representative of overall exposure. Nonetheless, the perinatal period is a markedly critical period for development of behavioral abnormalities in animal studies 38.
A second foundational study was published in 2020 using acetaminophen levels in meconium, the first stools of newborns (sticky, tar-like, dark green or black stool composed of cells, intestinal secretions, and bile that accumulates in the fetus’s intestines during development) 39. The meconium reflects the accumulation of drug exposure in neonates for the last two-thirds of pregnancy 40. Meconium samples were collected from 345 children of the Canadian Birth Cohort, and their acetaminophen (paracetamol) contents were quantified 39. At ages six and seven, it was determined whether a child had received an ADHD diagnosis. When children reached ages 9–11, magnetic resonance imaging (MRI) scans were conducted to assess brain connectivity in three networks associated with ADHD: (1) salience/cingulo-opercular, (2) central executive/frontoparietal, and (3) the default mode networks. Only 48 children were included in the MRI results as this step was ongoing at the time of publication. The results showed that, compared to those with no acetaminophen (paracetamol) exposure, infants with high exposure were found to be 4.1 times as likely to be diagnosed with ADHD. Furthermore, those with detectable acetaminophen (paracetamol) levels in meconium had decreased connectivity in the three networks, with lower levels of connectivity correlating with more pronounced symptoms of ADHD.
Not all studies came to this conclusion. In 2024, a large Swedish birth cohort was studied for a potential causal association between acetaminophen (paracetamol) and ASD/ADHD 41. Researchers primarily measured exposure based on whether mothers used any acetaminophen in pregnancy. Their secondary exposure metric was obtained via quantification of acetaminophen (paracetamol) dosage in prescription dispensations during pregnancy. Of the nearly 2.5 million children in the cohort, 185,909 were exposed to acetaminophen (paracetamol). The prevalence of autism among children without acetaminophen (paracetamol) exposure to those with exposure was 1.33% and 1.55% respectively with a hazard ratio of 1.05. Likewise, the rate of ADHD among children without acetaminophen (paracetamol) exposure to those with exposure was 2.46% and 2.87% respectively with a hazard ratio of 1.07. To put this in perspective, the hazard ratio of a child developing neuropsychiatric disorders because of long term prenatal opioid exposure of 60 days or more is 1.95 42. Nonetheless, researchers used a sibling control analysis to account for familial confounders, such as parents’ autistic traits. One potential downside of sibling comparison studies is that they eliminate potential mediators shared between families that interact with acetaminophen 43. No association between acetaminophen (paracetamol) exposure and ASD/ADHD was found with the analysis.
However, this study has two limitations that impact its ability to quantify acetaminophen (paracetamol) dosage 41. First, the primary exposure metric in this study was the ever-use of acetaminophen. This neglects to quantify dosage. Second, because its secondary exposure metric quantified acetaminophen (paracetamol) use through prescriptions, it does not account for over-the-counter acetaminophen (paracetamol) use. Fifty-four percent of pregnant women use over-the-counter acetaminophen, according to one Iowa-based study 44. Furthermore, past research shows that prescriptions do not always reflect actual use. The mean implementation adherence among pregnant women was 72% in one study, and another study found that prescription guidelines compliance among Danish pregnant women was 43% 45, 46. With these two limitations, the study cannot accurately estimate acetaminophen (paracetamol) usage. Longitudinal studies must quantify acetaminophen (paracetamol) usage since a critical point of exposure may exist 47, 41. Although this study explores confounders through sibling control analyses, the lack of acetaminophen (paracetamol) dose quantification hampers its ability to establish the presence of absence of a correlation between the two variables.
Other studies have utilized sibling control analyses and came to varying conclusions. One found that maternal acetaminophen (paracetamol) usage for over 28 days correlated with ASD/ADHD but found no correlation with a sibling control analyses 48. Another study also found that maternal acetaminophen (paracetamol) usage for over 28 days correlated with ASD/ADHD in a three-year follow up, confirmed by the sibling control analyses 49.
In recent years, evidence has accumulated suggesting that the use of acetaminophen by pregnant women may be associated with an increased risk of neurological conditions such as autism and ADHD in children. Some studies have described that the risk may be most pronounced when acetaminophen is taken chronically throughout pregnancy to childbirth. These concerns may be magnified by the fact that a very young child’s liver may still be developing and thus a child’s ability to metabolize the drug may be limited.
To be clear, while an association between acetaminophen and autism has been described in many studies, a causal relationship has not been established and there are contrary studies in the scientific literature. The association is an ongoing area of scientific debate and clinicians should be aware of the issue in their clinical decision-making, especially given that most short-term fevers in pregnant women and young children do not require medication.
In the spirit of patient safety and prudent medicine, clinicians should consider minimizing the use of acetaminophen during pregnancy for routine low-grade fevers. This consideration should also be balanced with the fact that acetaminophen is the safest over-the-counter alternative in pregnancy among all analgesics and antipyretics; aspirin and ibuprofen have well-documented adverse impacts on the fetus.
Source: https://www.fda.gov/media/188843/download?attachment
The U.S. Food and Drug Administration today initiated the process for a label change for acetaminophen (Tylenol and similar products) to reflect evidence suggesting that the use of acetaminophen by pregnant women may be associated with an increased risk of neurological conditions such as autism and ADHD in children. The agency also issued a related letter alerting physicians nationwide.
The FDA is taking action to make parents and doctors aware of a considerable body of evidence about potential risks associated with acetaminophen, said FDA Commissioner Marty Makary, M.D., M.P.H. Even with this body of evidence, the choice still belongs with parents. The precautionary principle may lead many to avoid using acetaminophen during pregnancy, especially since most low-grade fevers don’t require treatment. It remains reasonable, however, for pregnant women to use acetaminophen in certain scenarios.
Evidence in recent years has suggested a correlation between acetaminophen use during pregnancy and subsequent diagnosis of conditions like autism and ADHD. Multiple large-scale cohort studies, including the Nurses’ Health Study II and the Boston Birth Cohort, find this association. Some studies have described that the risk may be most pronounced when acetaminophen is taken chronically throughout pregnancy.
It is important to note that while an association between acetaminophen and neurological conditions has been described in many studies, a causal relationship has not been established and there are contrary studies in the scientific literature. It is also noted that acetaminophen is the only over-the-counter drug approved for use to treat fevers during pregnancy, and high fevers in pregnant women can pose a risk to their children. Additionally, aspirin and ibuprofen have well-documented adverse impacts on the fetus.
One study of over 24,000 pregnancies with self-reported acetaminophen use reported an increased chance for pregnancy-related complications, including preterm delivery (birth before week 37) and low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) 50. However, there were flaws in the design of this study, including the reason a woman was taking a pain medication was not accounted for 50. These pregnancy-related problems were not found in a report that reviewed the medical literature with acetaminophen use in over 39,000 pregnancies 50.
Some studies have suggested that taking acetaminophen daily or most days during the second half of pregnancy could slightly increase the chance of wheezing or asthma in children 50. However, other factors might be the reason for the development of asthma in the child, such as the illness the parent has, or the reason why the parent needs to use acetaminophen during their pregnancy, and not the acetaminophen itself.
Taking too much acetaminophen can cause liver damage, kidney damage, and anemia (low iron in the blood) in a woman who is pregnant. It has also been reported to cause the same problems in the baby 50.
Does taking acetaminophen in pregnancy affect future behavior or learning for the child?
There have been studies that noted a possible link between the use of acetaminophen in pregnancy and mild developmental delay including language delay and hyperactivity 50. This link was stronger when acetaminophen was used for 28 days or more during pregnancy. Another study showed a weak link between acetaminophen use between 18-32 weeks of pregnancy and hyperactivity and attention problems. However, this link was only seen in children under the age of 7 years. It is not clear if these findings are related to acetaminophen or to other factors.
Evidence in recent years has suggested a correlation between acetaminophen use during pregnancy and subsequent diagnosis of conditions like autism and ADHD 51. Multiple large-scale cohort studies, including the Nurses’ Health Study II 52 and the Boston Birth Cohort 36, find this association. Some studies have described that the risk may be most pronounced when acetaminophen is taken chronically throughout pregnancy.
It is important to note that while an association between acetaminophen and neurological conditions has been described in many studies, a causal relationship has not been established and there are contrary studies in the scientific literature 51. It is also noted that acetaminophen is the only over-the-counter drug approved for use to treat fevers during pregnancy, and high fevers in pregnant women can pose a risk to their children. Additionally, aspirin and ibuprofen have well-documented adverse impacts on the fetus.
One study looked at the reported use of acetaminophen during pregnancy and then evaluated the exposed children at 4 years of age 53.
Acetaminophen is available to be administered orally (tablet, capsule, syrup, oral solution, or suspension), rectally (rectal suppository), or intravenously (IV) 1, 54.
- Oral acetaminophen (paracetamol): Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension.
- Rectal acetaminophen (paracetamol): Acetaminophen is available as a rectal suppository for adult and pediatric patients.
- IV acetaminophen (paracetamol): Acetaminophen is also available as an IV infusion for administration.
Tompkins et al 55 conducted a literature review on the effectiveness of intravenous (IV) acetaminophen (paracetamol) in postoperative pain control. The investigators found a lack of evidence supporting the efficacy of IV acetaminophen (paracetamol) when compared to oral or rectal acetaminophen (paracetamol), opioid analgesics, NSAIDs, or placebo across various surgical procedures, including abdominal, gynecological, genitourinary, orthopedic, neurosurgical, cardiac, and renal surgeries 55. The investigators conclude that intravenous (IV) acetaminophen (paracetamol) offers limited clinical benefits compared to oral or rectal administration.
Establishing the maximum daily allowable dosage of acetaminophen (paracetamol) involves considering all modes of administration, including IV, oral, and rectal, as well as all formulations containing acetaminophen (paracetamol).
Adults and Adolescents Dosages
- Adults and adolescents (13 or older) with a body weight of ≥50 kg: The recommended dosage of acetaminophen (paracetamol) is 1000 mg every 6 hours or 650 mg every 4 hours. The maximum single dose should not exceed 1000 mg, and the minimum dosing interval is 4 hours. Notably, the maximum daily dosage of acetaminophen (paracetamol) should not exceed 4000 mg.
- Adults and adolescents (13 or older) with a body weight <50 kg: The recommended dosage of acetaminophen (paracetamol) is 12.5 mg/kg every 4 hours or 15 mg/kg every 6 hours. The maximum single dose should not exceed 15 mg/kg, and the minimum dosing interval is 4 hours. In addition, it is essential to adhere to a maximum daily dosage of acetaminophen (paracetamol) not exceeding 75 mg/kg, up to a maximum of 3750 mg.
Specific Patient Populations
Liver impairment: Acetaminophen is contraindicated in cases of active liver disease or severe hepatic impairment. Caution is advised for patients with mild hepatic impairment, necessitating a reduced total daily dosage of acetaminophen (paracetamol) and regular monitoring of liver function.
Kidney impairment: In severe renal impairment (creatinine clearance ≤30 mL/min), extending dosing intervals and reducing the total daily dosage of acetaminophen (paracetamol) may be advisable.
Breastfeeding considerations: Acetaminophen is suitable for pain relief and fever reduction in breastfeeding mothers. The levels detected in breast milk are significantly lower than typical infant doses, and there are infrequent reports of adverse effects in breastfed infants 35.
Children aged 2 to 12: The recommended dosage for children aged 2 to 12 is 12.5 mg/kg every 4 hours or 15 mg/kg every 6 hours. The maximum single dose should not exceed 15 mg/kg, and the maximum daily dosage of acetaminophen (paracetamol) is 75 mg/kg.
Neonates: The recommended dosage for premature children born at ≥32 weeks gestational age or less than 28 days old is 12.5 mg/kg every 6 hours, with a maximum recommended daily dosage of acetaminophen (paracetamol) set at 50 mg/kg.
Infants: Infants aged 29 days to 2 years are typically administered a dosage of 15 mg/kg every 6 hours, with a maximum daily dosage of acetaminophen (paracetamol) not exceeding 60 mg/kg.
Older patients: As per the American Geriatric Society, the recommended acetaminophen (paracetamol) dosage is 325 to 500 mg every 4 hours or 500 to 1000 mg every 6 hours, with a typical maximum daily dosage of 4 g. In individuals with liver impairment or a history of alcohol misuse, it is advisable to reduce the maximum dose by 50% to 75% 56.
How should acetaminophen be used?
Acetaminophen comes as a tablet, chewable tablet, capsule, suspension or solution (liquid), extended-release (long-acting) tablet, and orally disintegrating tablet (tablet that dissolves quickly in the mouth), to take by mouth, with or without food. Acetaminophen also comes as a suppository to use rectally. Acetaminophen is available without a prescription, but your doctor may prescribe acetaminophen to treat certain conditions. Follow the directions on the package or prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand.
If you are giving acetaminophen to your child, read the package label carefully to make sure that it is the right product for the age of the child. Do not give children acetaminophen products that are made for adults. Some products for adults and older children may contain too much acetaminophen for a younger child. Check the package label to find out how much medication the child needs. If you know how much your child weighs, give the dose that matches that weight on the chart. If you don’t know your child’s weight, give the dose that matches your child’s age. Ask your child’s doctor if you don’t know how much medication to give your child.
Acetaminophen comes in combination with other medications to treat cough and cold symptoms. Ask your doctor or pharmacist for advice on which product is best for your symptoms. Check nonprescription cough and cold product labels carefully before using two or more products at the same time. These products may contain the same active ingredient(s) and taking them together could cause you to receive an overdose. This is especially important if you will be giving cough and cold medications to a child.
Swallow the extended-release tablets whole; do not split, chew, crush, or dissolve them.
Place the orally disintegrating tablet (‘Meltaways’) in your mouth and allow to dissolve or chew it before swallowing.
Shake the suspension well before each use to mix the medication evenly. Always use the measuring cup or syringe provided by the manufacturer to measure each dose of the solution or suspension. Do not switch dosing devices between different products; always use the device that comes in the product packaging.
Taking acetaminophen with other medicines, food and alcohol
Acetaminophen can react unpredictably with certain other medications. This can affect how well either medicine works and might increase the risk of side effects.
It may not be safe to take acetaminophen at the same time as:
- other products containing tylenol – including combination products where tylenol is one of the ingredients
- carbamazepine – used to treat epilepsy and some types of pain
- colestyramine – used to reduce itchiness caused by primary biliary cirrhosis (a type of liver disease)
- imatinib and busulfan – used to treat certain types of cancer
- ketoconazole – a type of antifungal medicine
- lixisenatide – used to treat type 2 diabetes
- metoclopramide – used to relieve nausea and vomiting
- phenobarbital, phenytoin and primidone – used to control seizures
- warfarin – used to prevent blood clots. Prolonged oral administration of acetaminophen at 4000 mg (4 g)/day has been associated with an elevated international normalized ratio (INR) in patients receiving warfarin. Due to the lack of studies evaluating short-term acetaminophen use with oral anticoagulants, increased frequency of INR (international normalized ratio) monitoring may be advisable in these situations.
Check the leaflet that comes with your medicine to see if it can be taken with acetaminophen. Ask a pharmacist or doctor if you’re not sure.
There are no known problems caused by taking acetaminophen with any specific foods or by drinking moderate amounts of alcohol while taking acetaminophen. However, chronic alcohol misuse increases the risk of acetaminophen toxicity by inducing CYP2E1, reducing hepatic glutathione (GSH) levels, and impairing N-acetyl-p-benzoquinoneimine (NAPQI) detoxification. Moreover, it may decrease glucuronidation, enhance oxidation, cause hepatocyte membrane disruptions, and reduce biliary excretion 57.
Acetaminophen (paracetamol) side effects
Acetaminophen (paracetamol) is a safe and effective medication when used correctly. Documented adverse effects depend on the route of administration. If acetaminophen (paracetamol) is administered orally or rectally, acetaminophen may cause any of the following 4, 1, 2, 58:
- Skin rash or hypersensitivity reactions (toxic epidermal necrolysis, acute generalized exanthematous pustulosis, and Stevens-Johnson syndrome)
- Hematological: anemia, leukopenia, neutropenia, pancytopenia
- Kidney toxicity (nephrotoxicity) characterized by elevations in blood urea nitrogen (BUN) and creatinine
- Metabolic and electrolyte disorders, which may manifest as decreased serum bicarbonate, reduced concentrations of sodium and calcium, hyperammonemia, hyperchloremia, hyperuricemia, increased serum glucose, and elevated levels of bilirubin and alkaline phosphatase
- Decreased serum bicarbonate
- Hyponatremia
- Hypocalcemia
- Hyperammonemia
- Hyperchloremia
- Hyperuricemia
- Hyperglycemia
- Hyperbilirubinemia
- Elevated alkaline phosphatase.
- Liver damage (hepatotoxicity) and kidney damage if you take too much (overdose) – this can be fatal in severe cases.
If acetaminophen (paracetamol) is administered intravenously, adverse effects include nausea, vomiting, itch, constipation, and abdominal pain 1. For children, regardless of the route of administration, the most common adverse reactions are nausea, vomiting, agitation, constipation, itch, and atelectasis (collapse of a lung or part of a lung) 4.
Rare but serious acetaminophen (paracetamol) adverse effects include hypersensitivity, anaphylactic reactions, and severe and fatal skin reactions. These include toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis, and Stevens-Johnson syndrome (SJS) 59. In addition, there have been reported instances of a rare pneumonia resulting from drug-induced lung injury (DILI) due to acetaminophen 60.
MacIntyre et al 61 conducted a double-blind, placebo-controlled, crossover clinical trial to investigate the effects of oral acetaminophen on daytime systolic blood pressure. 110 subjects were randomized to receive 1 g of acetaminophen 4 times a day or a placebo for 2 weeks, followed by a 2-week washout period before receiving the alternative treatment 61. Daytime systolic blood pressure was assessed in subjects who received either acetaminophen or a placebo. The investigators observed a 5-mmHg increase in daytime systolic blood pressure among those who received a daily dose of 4 g of acetaminophen 61. As a result, the researchers concluded that the use of high-dose acetaminophen in hypertensive patients could potentially elevate their risk of cardiovascular disease 61.
Speak to a pharmacist or doctor if you develop any troublesome side effects that you think could be caused by acetaminophen (paracetamol).
Some side effects of acetaminophen (paracetamol) can be serious. If you experience any of the following symptoms, stop taking acetaminophen (paracetamol) and call your doctor immediately or get emergency medical attention:
- getting a skin rash that may include itchy, red, swollen, blistered or peeling skin
- rash
- hives
- itching
- swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
- hoarseness
- difficulty breathing or swallowing
Acetaminophen (paracetamol) may cause other side effects. Call your doctor if you have any unusual problems while you are taking acetaminophen (paracetamol).
Human Exposure and Toxicity
Nausea, vomiting, and abdominal pain usually occur within 2-3 hours after ingestion of toxic doses of the drug acetaminophen. In severe poisoning, central nervous system (CNS) stimulation, excitement, and delirium may occur initially. This may be followed by central nervous system (CNS) depression, stupor, hypothermia, marked prostration, rapid shallow breathing, rapid weak irregular pulse, low blood pressure, and circulatory failure. When an individual has ingested a toxic dose of acetaminophen, the individual should be hospitalized for several days of observation, even if there are no apparent ill effects, because maximum liver damage and/or cardiotoxic effects usually do not become apparent until 2-4 days after ingestion of the drug. Other symptoms of acute poisoning include cerebral edema and nonspecific myocardial depression. Vascular collapse results from the relative hypoxia and from a central depressant action that occurs only with massive doses. Shock may develop if vasodilation is marked. Fatal seizures may occur. Coma usually precedes death, which may occur suddenly or may be delayed for several days. Biopsy of the liver reveals centralobular necrosis with sparing of the periportal area. There have been reports of acute myocardial necrosis and pericarditis in individuals with acetaminophen poisoning. Hypoglycemia, which can progress to coma have been reported in patients ingesting toxic doses of acetaminophen. Low prothrombin levels and thrombocytopenia have been reported in patients with acetaminophen poisoning. Skin reactions of an erythematous or urticarial nature which may be accompanied by fever and oral mucosal lesions also have been reported. For use anytime during pregnancy, 781 exposures were recorded, and possible associations with congenital dislocation of the hip (eight cases) and clubfoot (six cases) were found. There is inadequate evidence in humans for the carcinogenicity of acetaminophen.
Some evidence of increased risk of neurodevelopmental disorders (e.g., attention deficit hyperactivity disorder [ADHD]), respiratory illness (e.g., asthma) and reproductive toxicity (e.g., androgen disruption) has been suggested in epidemiologic studies 36, 52, 62, 58. However, extrapolating causation from pharmaco-epidemiological studies to humans is tricky considering various confounders and biases inherent in the study design. Associations seen in clinical cohort studies need clarification with randomized clinical trials, which would be difficult to perform ethically in pregnant populations. The mechanism by which acetaminophen or its metabolites affect neurological development, asthma, or endocrine/reproductive toxicity is poorly understood. It is important to factor in the risk of untreated febrile illness in mother and child when evaluating risks and benefits of using this drug. There are no controlled data in human pregnancy.
According to published animal studies, this drug may cause reduced fertility in both males and females described as decreased testicular weights, reduced spermatogenesis, reduced fertility; and reduced implantation sites, respectively.
Evidence for Carcinogenicity
There is inadequate evidence in humans for the carcinogenicity of paracetamol. There is inadequate evidence in experimental animals for the carcinogenicity of paracetamol 63. Overall evaluation: Paracetamol is not classifiable as to its carcinogenicity to humans (Group 3) 63.
Animal Toxicity Studies
Concern has been raised over chemical-induced disruption of ovary development during fetal life resulting in long-lasting consequences only manifesting themselves much later during adulthood. A growing body of evidence suggests that prenatal exposure to the mild analgesic acetaminophen/paracetamol can cause such a scenario 64. In a review of three recent reports that collectively indicate that prenatal exposure in a period of 13.5 days post coitum in both rats and mouse can result in reduced female reproductive health. The combined data show that the exposure results in the reduction of primordial follicles, irregular menstrual cycle, premature absence of corpus luteum, as well as reduced fertility, resembling premature ovarian insufficiency syndrome in humans that is linked to premature menopause 64. This could especially affect the Western parts of the world, where the age for childbirth is continuously being increased and acetaminophen is recommended during pregnancy for pain and fever 64. The study authors highlight an urgent need for more studies to verify these data including both experimental and epidemiological approaches 64.
There is inadequate evidence in experimental animals for the carcinogenicity of acetaminophen. In rats fasted 24 hours and given a single dose of acetaminophen (2 g/kg) by gavage, liver necrosis around the central vein was noted at 9-12 hours and was much more extensive at 24 hours after treatment. In mice after dietary exposure to acetaminophen up to 6400 mg/kg daily for 13 weeks hepatotoxicity, organ weight changes and deaths were observed. Cats are particularly susceptible to acetaminophen intoxication, developing more diffuse liver changes, while hepatic centrilobular lesions found in dogs. High doses of acetaminophen caused testicular atrophy and delay in spermiogenesis in mice. Furthermore, reductions in the fertility and neonatal survival in mice were seen in the F0 generation and decreases in F1 pup weights were found at acetaminophen dose 1430 mg/kg. Acetaminophen was not mutagenic in Salmonella typhimurium assay with or without metabolic activation in six strains: TA1535, TA1537, TA1538, TA100, TA97 and TA98. In vitro and animal data indicate that small quantities of acetaminophen are metabolized by a cytochrome P-450 microsomal enzyme to a reactive intermediate metabolite (N-acetyl-p-benzoquinoneimine, N-acetylimidoquinone, NAPQI) which is further metabolized via conjugation with glutathione and ultimately excreted in urine as a mercapturic acid. It has been suggested that this intermediate metabolite is responsible for acetaminophen-induced liver necrosis in cases of overdose. Excipients found in liquid formulations of acetaminophen may decrease its liver toxicity.
Ecotoxicity Studies
Daphnia magna was the most susceptible among the test organisms to the environmental effects of acetaminophen. Acetaminophen has recently been identified as a promising snake toxicant to reduce brown tree snake populations on Guam, while posing only the minimal risks to non-target rodents, cats, pigs and birds.
Acetaminophen (paracetamol) Contraindications
Contraindications to using acetaminophen (paracetamol) include hypersensitivity to acetaminophen, severe liver impairment, or severe active liver disease 59.
Hepatotoxicity (liver toxicity): Acetaminophen (paracetamol) use has been associated with liver failure, occasionally resulting in liver transplants or fatalities. The hepatotoxicity observed with acetaminophen use usually corresponds to high doses that surpass the recommended maximum dose 65, 66. Hepatotoxicity may be associated with the consumption of multiple drug products containing acetaminophen as an ingredient. Liver damage has also been documented in patients with chronic acetaminophen dosing.
Dosing errors: A notable FDA-box warning underscores the importance of preventing dosing errors, especially when administering acetaminophen to children. In addition, it underscores the necessity of ensuring that the total daily dose of acetaminophen does not exceed the recommended maximum when accounting for all medications containing acetaminophen.
Although these effects, warnings, and associations have been documented, acetaminophen (paracetamol) remains a safe and effective medication when used accurately. The current manufacturer dose recommendation is restricted to between 3 and 3.25 g in 24 hours, depending on the formulation. However, toxicity is rare at doses less than 200 mg/kg for a child or 150 mg/kg for adults.
Acetaminophen (paracetamol) Poisoning
Acetaminophen (paracetamol) toxicity may result from a single toxic dose, from repeated ingestion of large doses of acetaminophen (e.g., 7.5 g to 10 g daily for 1 to 2 days), or from chronic ingestion of the drug 9. Dose-dependent, liver necrosis is the most serious acute toxic effect associated with overdosage and is potentially fatal 10, 9. Acetaminophen toxicity is the second most common cause of liver transplantation worldwide and the most common cause of liver failure in the United States 2. Acetaminophen (paracetamol) toxicity is responsible for 56,000 emergency department visits and 2600 hospitalizations, acetaminophen poisoning causes 500 deaths annually in the United States 11, 2. Notably, around 50% of these poisonings are unintentional, often resulting from patients misinterpreting dosing instructions or unknowingly consuming multiple acetaminophen-containing products 2.
If left untreated, acetaminophen (paracetamol) toxicity can lead to both fatal and non-fatal liver necrosis 2. Timely intervention is essential in preventing fulminant liver failure and the need for liver transplantation. Activated charcoal should be administered within the first hour and N-acetylcysteine within the first 8 hours. The most reliable predictor of toxicity is correlating the time of ingestion with the serum acetaminophen concentration using the Revised Rumack-Matthew nomogram. Patients whose levels fall above the treatment line at 4 hours require N-acetylcysteine. A striking 50% of cases result from unintentional overdoses, highlighting a crucial need for healthcare professionals to properly educate patients regarding the proper dosing of acetaminophen and its presence in prescribed and over-the-counter preparations.
The mortality associated with acetaminophen (paracetamol) overdose is low if recognized and treated within the first 8 hours 2, 5, 7. Factors such as alcohol use, genetics, age, medications, herbal supplements, and nutritional status can enhance acetaminophen’s ability to damage the liver 2. In addition to liver failure, affected patients may experience kidney failure as a consequence of acetaminophen toxicity 2.
Nausea, vomiting, and abdominal pain usually occur within 2-3 hours after ingestion of toxic doses of acetaminophen. Unlike aspirin, acetaminophen does not usually cause acid/base changes in toxic doses 9. In severe poisoning, central nervous system (brain and spinal cord) stimulation, excitement, and delirium may occur initially. This may be followed by central nervous system depression; stupor; hypothermia; marked prostration; rapid, shallow breathing; rapid, weak, irregular pulse; low blood pressure; and circulatory failure. Vascular collapse results from the relative hypoxia and from a central depressant action that occurs only with massive doses. Shock may develop if vasodilation is marked. Fatal asphyxial seizures may occur. Coma usually precedes death, which may occur suddenly or may be delayed for several days 9.
Acetaminophen (paracetamol) toxicity usually involves 4 phases 9:
- Anorexia, nausea, vomiting, malaise, and diaphoresis (which inappropriately may prompt administration of additional acetaminophen);
- Resolution of phase-1 manifestations and replacement with right upper quadrant pain or tenderness, liver enlargement, elevated bilirubin and hepatic enzyme concentrations, prolongation of prothrombin time, and occasionally oliguria;
- Anorexia, nausea, vomiting, and malaise recur (usually 3-5 days after initial symptom onset) and signs of hepatic failure (e.g., jaundice, hypoglycemia, coagulopathy, encephalopathy) and possibly renal failure and cardiomyopathy develop; and
- Recovery or progression to fatal complete liver failure.
Three hundred and seven cases of liver injury associated with acetaminophen use were reported to the US Food and Drug Administration (FDA) from January 1998 to July 2001 67. Sixty percent of these adverse events were categorized as severe life-threatening injury with liver failure (category 4); 40% of patients died. Review of these case reports indicates that use of higher than recommended daily dosages of acetaminophen results in adverse hepatotoxic effects more often than use of recommended dosages 67.
The Rocky Mountain Poison and Drug Center 68 reported the results of a nationwide study on acetaminophen overdose during pregnancy involving 113 women. Of the 60 cases that had appropriate laboratory and pregnancy outcome data, 19 occurred in the 1st trimester, 22 during the 2nd trimester, and 19 during the 3rd trimester. In those cases with a potentially toxic serum level of acetaminophen, early treatment with N-acetylcysteine (NAC) was statistically associated with an improved pregnancy outcome by lessening the incidence of spontaneous abortion and fetal death 68. Only one congenital anomaly was observed in the series and that involved a 3rd trimester overdose with nontoxic maternal acetaminophen serum levels 68.
Very high levels of acetaminophen can cause lactic acidosis and altered mental status by uncertain mechanisms, probably involving mitochondrial dysfunctin 69. Symptoms of acute acetaminophen poisoning both metabolic acidosis and metabolic alkalosis have been noted; cerebral edema & nonspecific myocardial depression have also occurred 70. Biopsy of the liver reveals centralobular necrosis with sparing of the periportal area 70.
Low prothrombin levels have been reported in patients with acetaminophen poisoning and in one patient fatal GI hemorrhage was attributed to hypoprothrombinemia. Thrombocytopenia also has been reported. Toxic doses of p-aminophenol derivatives may produce skin reactions of an erythematous or urticarial nature which may be accompanied by fever and oral mucosal lesions 71.
Eighty-eight patients with acetaminophen-induced acute liver failure were recruited 72. Control groups included patients with nonacetaminophen-induced acute liver failure (n = 13), nonhepatic multiple organ failure (n = 28), chronic liver disease (n = 19), and healthy controls (n = 11). Total and caspase-cleaved cytokeratin-18 (M65 and M30) measured at admission and sequentially on days 3, 7, and 10 following admission. Levels were also determined from hepatic vein, portal vein, and systemic arterial blood in seven patients undergoing transplantation. Protein arrays of liver homogenates from patients with acetaminophen-induced acute liver failure were assessed for apoptosis-associated proteins, and histological assessment of liver tissue was performed. Admission M30 levels were significantly elevated in acetaminophen-induced acute liver failure and non-acetaminophen induced acute liver failure patients compared with multiple organ failure, chronic liver disease, and healthy controls. Admission M30 levels correlated with outcome with area under receiver operating characteristic of 0.755. Peak levels in patients with acute liver failure were seen at admission then fell significantly but did not normalize over 10 days. A negative gradient of M30 from the portal to hepatic vein was demonstrated in patients with acetaminophen-induced acute liver failure at the time of liver transplant. Analysis of protein array data demonstrated lower apoptosis-associated protein and higher catalase concentrations in acetaminophen-induced acute liver failure compared with controls. Explant histological analysis revealed evidence of cellular proliferation with an absence of histological evidence of apoptosis. Hepatocellular apoptosis occurs in the early phases of human acetaminophen-induced acute liver failure, peaking on day 1 of hospital admission, and correlates strongly with poor outcome. Hepatic regenerative/tissue repair responses prevail during the later stages of acute liver failure where elevated levels of M30 are likely to reflect epithelial cell death in extrahepatic organs 72.
While acetaminophen (paracetamol) has few side effects when used in therapeutic doses, recent reports suggest that its standard use can result in severe hypersensitivity reactions including Stevens Johnson syndrome and toxic epidermal necrolysis (TEN). Both of these syndromes can be life-threatening and both may be accompanied by evidence of liver injury. The U.S. Food and Drug Administration (FDA) is informing the public that acetaminophen has been associated with a risk of rare but serious skin reactions. These skin reactions, known as Stevens Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and acute generalized exanthematous pustulosis (AGEP), can be fatal 73. However, the liver involvement is usually mild and marked only by asymptomatic mild-to-moderate elevations in serum aminotransferase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Reddening of the skin, rash, blisters, and detachment of the upper surface of the skin can occur with the use of drug products that contain acetaminophen. These reactions can occur with first-time use of acetaminophen or at any time while it is being taken. Anyone who develops a skin rash or reaction while using acetaminophen or any other pain reliever/fever reducer should stop the drug and seek medical attention right away. Anyone who has experienced a serious skin reaction with acetaminophen should not take the drug again and should contact their health care professional to discuss alternative pain relievers/fever reducers. Health care professionals should be aware of this rare risk and consider acetaminophen, along with other drugs already known to have such an association, when assessing patients with potentially drug-induced skin reactions.
Acetaminophen (paracetamol) Liver toxicity
Chronic therapy with acetaminophen (paracetamol) in doses of 4 grams daily has been found to lead to transient elevations in liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), levels in a proportion of subjects, generally starting after 3 to 7 days, and with peak values rising above 3-fold elevated in 39% of persons. These elevations are generally asymptomatic and resolve rapidly with stopping therapy or reducing the dosage, and in some instances resolve even with continuation at full dose.
The best known form of liver toxicity from acetaminophen is an acute, serious hepatocellular injury as a result of intentional or unintentional overdose. The injury is due to a direct, toxic effect of the high doses of acetaminophen. Acetaminophen hepatotoxicity most commonly arises after a suicide attempt using more than 7.5 grams (generally more than 15 grams) as a single overdose. Liver injury generally starts 24 to 72 hours after the ingestion with marked elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (often to above 2000 U/L), followed at 48 to 96 hours by clinical symptoms: jaundice, confusion, hepatic failure and in some instances death. Evidence of kidney insufficiency is also common. Serum aminotransferase levels fall promptly and recovery is rapid if the injury is not too severe. Similar injury can occur with high therapeutic or supratherapeutic doses of acetaminophen given over several days for treatment of pain and not as a purposeful suicidal overdose. This form of acetaminophen liver toxicity is referred to as accidental or unintentional overdose, and usually occurs in patients who have been fasting, or are critically ill with a concurrent illness, alcoholism or malnutrition, or have preexisting chronic liver disease. Some cases of unintentional overdose occur in patients taking acetaminophen in combinations with controlled substances (oxycodone, codeine), who take more than recommended amounts over several days in attempts to control pain or withdrawal symptoms. Instances of unintentional overdose in children are often due to errors in calculating the correct dosage or use of adult sized tablets instead of child or infant formulations. Because acetaminophen is present in many products, both by prescription and over-the-counter, another problem occurs when a patient ingests full or high doses of several products unaware that several contain acetaminophen.
The mechanism of acetaminophen (paracetamol) liver toxicity has been extensively analyzed in humans and in animal models. Acetaminophen (paracetamol) is largely converted to nontoxic glucuronate or sulfate conjugates and secreted in the urine. A minor amount of acetaminophen (paracetamol) is metabolized via the cytochrome P450 system to intermediates that can be toxic, particularly N-acetyl-p-benzoquinoneimine (NAPQI). Ordinarily, N-acetyl-p-benzoquinoneimine (NAPQI) is rapidly conjugated in the liver to reduced glutathione, detoxified and secreted by your kidney. If levels of glutathione are low or the cytochrome P450 system pathway is overwhelmed by high doses of acetaminophen (paracetamol), the reactive intermediate, N-acetyl-p-benzoquinoneimine (NAPQI), accumulates in the liver and binds to intracellular macromolecules that can lead to cell injury, usually through apoptotic pathways 74, 75. When there is a shortage of glutathione, N-acetyl-p-benzoquinoneimine (NAPQI) concentrations increase and as a reactive intermediate, it can react with essential cellular macromolecules such as proteins, lipids, and nucleic acids. This interaction can result in centrilobular (zone 3) hepatic injury and hepatocellular death, along with the potential for nephrotoxicity. Factors that increase the metabolism of acetaminophen through the cytochrome P450 system (certain drugs, chronic alcohol use) or that decrease the availability of glutathione (fasting, malnutrition, alcoholism) can predispose to acetaminophen toxicity. Factors that affect downstream toxicity of acetaminophen metabolic intermediates may also affect toxicity. These factors are important in designing therapies for acetaminophen liver toxicity.
Acetaminophen (paracetamol) poisoing treatment
The treatment of acetaminophen (paracetamol) poisoning is dependent on the timing of drug ingestion. If the patient presents within 1 hour of ingestion, gastrointestinal decontamination should be attempted with activated charcoal 2. Some studies suggest using activated charcoal if the patient presents within 4 hours of ingestion, as it has been shown to decrease acetaminophen absorption, reduce the need for N-acetylcysteine, and lower the risk of liver injury when administered within 4 hours of ingestion 76, 77, 78, 79. Use beyond 1 hour is most effective with large ingestions or with ingestion of an extended-release form or any medication that can delay gastric emptying. Contraindications to activated charcoal include gastrointestinal obstruction and an unprotected airway. Orogastric lavage or whole bowel irrigation is not recommended 80, 81, 82.
Acetaminophen (paracetamol) poisoing evaluation
The diagnosis of acetaminophen toxicity begins with obtaining a serum acetaminophen level, regardless of symptoms. If the timing of ingestion is unclear, an acetaminophen level should be obtained at the time of presentation and again 4 hours after the first dose. Additional recommended investigations include:
- Serum aminotransferase levels
- PT and international normalized ratio (INR)
- Electrolytes
- Blood urea nitrogen and creatinine
- Serum total bilirubin concentration
- Amylase
- Urinalysis
- Human chorionic gonadotropin in women of childbearing age
- Arterial or venous blood gas and serum lactate in critically ill patients or those with altered mental status
- Screening of blood and urine for other ingested drugs
- Electrocardiogram in patients with an intentional overdose
- Salicylate level
- Computed tomography scan of the head in the presence of altered mental status
Evaluation After Ingestion of Immediate-Release Acetaminophen 83, 84, 85:
- Obtain a serum acetaminophen level 4 hours after swallowing the first dose.
- Obtain serum acetaminophen level immediately if the ingestion was over 4 hours before presentation.
- Determine the need for N-acetylcysteine based on the Revised Rumack-Matthew nomogram.
- Administer empiric N-acetylcysteine if ingestion occurred more than 8 hours before presentation or the results of the serum levels exceed 8 hours after the first dose.
If the timing of ingestion is unknown, obtain the following immediately:
- Serum acetaminophen level
- Electrolytes
- Blood urea nitrogen and creatinine
- Serum total bilirubin concentration
- Serum transaminases
A nondetectable serum acetaminophen level drawn between 2 and 4 hours on an assay that detects acetaminophen at a minimum level of 10 mcg/mL likely excludes toxicity.
Evaluation After Ingestion of Extended-Release Formulation or Co-Ingestion with Anticholinergics or Opioids
An extended-release formulation of acetaminophen is labeled for use every 8 hours or more. Clinicians use the Revised Rumack-Matthew nomogram to assess patients who ingest an extended-release formulation or co-ingest anticholinergic or opioid medications. If the initial 4-hour acetaminophen level is ≤10 mcg/mL, a repeat level is unnecessary. If the acetaminophen level between 4 and 12 hours is below a level indicating the need for acetylcysteine but >10 mcg/mL (66 µmol/L), another acetaminophen level should be obtained in 4 to 6 hours.
Evaluation After Repeated Supratherapeutic Ingestion
Repeated supratherapeutic ingestions are multiple ingestions that occur over more than 24 hours. Serum concentrations are generally at therapeutic levels, and using the Revised Rumack-Matthew nomogram is not helpful in this setting. The decision to initiate N-acetylcysteine therapy should be based on a thorough history, physical examination, and laboratory evaluation. Serum acetaminophen, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels should be obtained if a patient with suspected repeated supratherapeutic ingestions meets any of the following criteria:
- Ingested more than 6 g/d or 150 mg/kg/d over 24 to 48 hours
- Ingested more than 4 g/d or 100 mg/kg/d for longer than 48 hours
- Presents with abdominal pain or right upper quadrant tenderness, nausea, vomiting, jaundice, altered mental status, or appears acutely ill
Four to Eight Hours After Ingestion
The 4-hour serum acetaminophen (paracetamol) level should be plotted on the Revised Rumack-Matthew nomogram. If the patient’s serum acetaminophen (paracetamol) level falls at or above the treatment line, N-acetylcysteine (NAC) administration is necessary. The treatment threshold is 150 mcg/mL or 990 µmol/L at 4 hours and 4.69 mcg/mL or 31.3 µmol/L at 24 hours 2. These levels place the patient at possible risk for liver toxicity, and treatment with N-acetylcysteine (NAC) is standard. Patients who have ingested an extended-release formulation or co-ingested an opiate or anticholinergic medication may need a repeat acetaminophen level in 4 to 6 hours 2.
More than Eight Hours After Ingestion
In cases of suspected acute ingestion of acetaminophen (paracetamol) exceeding 150 mg/kg or a total dose of 7.5 g, and when serum acetaminophen levels are unavailable until after 8 hours from ingestion, healthcare professionals should initiate N-acetylcysteine (NAC) therapy while awaiting acetaminophen levels 2. Beyond 8 hours, the risk of liver injury increases as the time to administration of N-acetylcysteine (NAC) increases 2. Discontinuing N-acetylcysteine is appropriate when acetaminophen levels fall below the treatment line on the Revised Rumack-Matthew nomogram and liver function tests are normal.
Patients with repeated supratherapeutic ingestions with a serum acetaminophen concentration ≥20 mcg/mL or 132 µmol/L or elevated aminotransferases warrant N-acetylcysteine (NAC). Clinicians should administer N-acetylcysteine to patients with any evidence of liver injury and a history of acetaminophen ingestion. Patients can still benefit from N-acetylcysteine up to 24 hours after the initial ingestion or until acetaminophen is no longer detectable in the serum. N-acetylcysteine is an antioxidant that diminishes hepatic necrosis, decreases neutrophil infiltration, improves microcirculatory blood flow, and increases tissue oxygen delivery. Hemodialysis can also be an effective treatment, especially with concurrent kidney failure.
Acetaminophen, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and INR (International Normalised Ratio) should be measured every 12 hours following the initiation of N-acetylcysteine 14. If the aspartate aminotransferase (AST) or alanine aminotransferase (ALT) exceeds 1000 IU/L, encephalopathy should be monitored, and serum bicarbonate, glucose, and creatinine should be measured every 12 hours 2.
Dialysis may be necessary if the patient develops acute kidney injury 2. Indications for dialysis include refractory fluid overload, severe hyperkalemia, severe metabolic acidosis, and uremia. Dose adjustments for patients with alcohol use disorder or those who are chronically ill are not required. Dialysis is considered safe for use during pregnancy 2.
High-Risk Ingestion
High-risk ingestion consists of acetaminophen (paracetamol) dose of more than 30g. These patients may require intubation to protect their airway, intravenous fluids and vasopressors, and bicarbonate for metabolic acidosis 2. Patients with a high-risk ingestion of acetaminophen (paracetamol) more than 30g are at an increased risk of developing fulminant liver failure. Activated charcoal should be administered even if beyond 4 hours, and N-acetylcysteine (NAC) should be initiated immediately 2. In addition to N-acetylcysteine, hemodialysis is indicated in patients with an acetaminophen concentration >900 mcg/mL.
N-acetylcysteine (NAC) antidote
N-acetylcysteine (NAC) protects against liver toxicity if administered within 8 hours of acetaminophen ingestion 2. The effectiveness remains unchanged whether initiated between 0 and 4 hours or 4 and 8 hours 2. N-acetylcysteine acts by preventing the binding of N-acetyl-p-benzoquinoneimine (NAPQI) to liver macromolecules by acting as a substitute for glutathione, serving as a precursor for sulfate, and reducing N-acetyl-p-benzoquinoneimine (NAPQI) back to acetaminophen. In addition, in patients with acetaminophen-induced liver failure, N-acetylcysteine (NAC) improves hemodynamics and oxygen use 86, increases clearance of indocyanine green (a measure of hepatic clearance) 87, and decreases cerebral edema 88. The exact mechanism of these effects is not clear, but it may involve scavenging of free radicals or changes in hepatic blood flow 86, 89.
Indications for N-acetylcysteine include:
- A serum acetaminophen (paracetamol) level within the toxic range according to the Revised Rumack-Matthew nomogram
- Acetaminophen (paracetamol) level greater than 10 mcg/mL with an unknown time of ingestion
- A dose of acetaminophen (paracetamol) >140 mg/kg ingested more than 8 hours before presentation
- Abnormal labs with ingestion >24 hours before presentation
- Ingestion with any evidence of liver injury 90.
Either oral or intravenous (IV) N-acetylcysteine (NAC) is acceptable. Intravenous N-acetylcysteine is preferred for patients with intractable vomiting, those who refuse oral intake, those at risk for aspiration, or those who are pregnant or have fulminant liver failure. The IV N-acetylcysteine (NAC) may decrease the length of the hospital stay and may be tolerated better by the patient, as the oral form tastes and smells like rotten eggs. The oral N-acetylcysteine (NAC) also requires 18 doses given 4 hours apart, with the total treatment time being 72 hours. In comparison, the IV N-acetylcysteine (NAC) requires only 20 hours of treatment.
- Oral N-acetylcysteine: The oral regimen consists of 18 doses administered 4 hours apart, resulting in a total treatment time of 72 hours. The protocol begins with administering a 140 mg/kg oral loading dose followed by 70 mg/kg every 4 hours until achieving the stop criteria. Patients weighing over 100 kg receive a maximum 100 mg/kg dose.
- Intravenous N-acetylcysteine: Approximately 10% to 20% of patients may experience nonallergic or non–IgE-mediated anaphylactic reactions to N-acetylcysteine with the IV form within the first 5 hours.
- Two IV N-acetylcysteine (NAC) protocols are available:
- 21-hour N-acetylcysteine (NAC) intravenous protocol
- An initial loading dose of 150 mg/kg IV is administered over 60 minutes.
- Next, a dose of 50 mg/kg is administered over 4 hours or 12.5 mg/kg/h IV for 4 hours.
- Finally, a dose of 100 mg/kg is administered over 16 hours or 6.25 mg/kg/h IV for 16 hours.
- 21-hour N-acetylcysteine (NAC) intravenous protocol
- Two IV N-acetylcysteine (NAC) protocols are available:
For children weighing <40 kg, the N-acetylcysteine (NAC) dose remains the same, but a different diluent protocol prevents fluid overload and excess free water, leading to hyponatremia, seizures, and death.
- Simplified 20-hour N-acetylcysteine (NAC) intravenous protocol
- This protocol combines the first 2 steps of the 21-hour protocol.
- A dose of 50 mg/kg is administered over 4 hours for a total of 200 mg/kg over 4 hours.
- Next, a dose of 6.25 mg/kg is administered over 16 hours for 100 mg/kg over 16 hours.
Studies reveal that the 20-hour N-acetylcysteine (NAC) intravenous protocol reduces the risk of non–IgE-mediated anaphylactic reactions to N-acetylcysteine by 50% 91.
In cases of high-risk ingestions, where reports indicate liver injury with ingestions exceeding 50 g or serum acetaminophen concentrations surpassing 500 mg/L or 3300 µmol/L, some clinicians suggest administering a higher dose of N-acetylcysteine. Consulting a poison center or medical toxicologist is advisable. If not available, a reasonable alternative is to increase the final infusion rate of the 21-hour protocol to 12.5 mg/kg/h. Patients undergoing hemodialysis do not need an adjustment of oral N-acetylcysteine, but IV N-acetylcysteine infusion rates are 12.5 mg/kg/h during hemodialysis.
A case report of oral acetaminophen toxicity in a term newborn infant successfully treated with a 20 hr intravenous N-acetylcysteine infusion protocol without any adverse effects. This case report supports the use of N-acetylcysteine to treat neonatal acetaminophen toxicity 92.
A 3.5 yr old girl with an upper respiratory infection died of an acetaminophen overdose 93. When the child’s temperature remained elevated after treatment with 120 mg every 4 hr for 3 doses, dosage was increased to 720 mg every 3 hr. Over the next 24 hr the patient received 5.04 g 93.
A 63 yr old man with acute psittacosis had severe hepatic damage after ingesting about 10 g acetaminophen over a 48 hr period. Transaminase levels showed striking elevation, with a serum glutamic-oxaloacetic transaminase level of over 15000 iu/L, and decreased rapidly, consistent with toxic insult. The liver showed severe central necrosis at autopsy 94.
An 18-year-old woman, gravida 1, presented at 33 weeks’ gestation with signs and symptoms consistent with acute fatty liver of pregnancy and fetal death. Markedly elevated transaminases prompted a search for other etiologies, and acetaminophen toxicity was diagnosed. Liver biopsy revealed acute fatty liver of pregnancy and toxin-induced injury consistent with acetaminophen use. The patient’s condition deteriorated, resulting in fulminant hepatic failure and requiring postpartum orthotopic liver transplantation. The combination of acute fatty liver of pregnancy and acetaminophen toxicity resulted in acute liver failure 95.
A case involving a 22-year-old woman in her 31st week of pregnancy who consumed a 15 g dose of acetaminophen, followed by a 50 g dose 1 week later is reported 68. Fetal distress was observed 16 hours after the second overdose, as evidenced by complete lack of fetal movements and breathing, a marked decrease in fetal heart rate beat-to-beat variability with no accelerations, and a falling baseline rate. Because of the fetal condition, labor was induced (cesarean section was excluded because of the mothers incipient hepatic failure). Eighty-four hours after the overdose, a 2198 g female infant was delivered with an Apgar scores at 1 and 5 min of 9 and 10, respectively. Except for hypoglycemia, mild respiratory disease, and mild jaundice, the newborn did well. Liver enzymes were always within normal range, and the jaundice was compatible with immaturity. Acetaminophen was not detected in the cord blood. Follow-up examinations of the infant at 6 weeks and again at 6 months were normal. Protection against serious or permanent liver damage was probably afforded by the prompt administration of iv N-acetylcysteine 68.
N-acetylcysteine (NAC) side effects
N-acetylcysteine (NAC) has an unpleasant smell and taste, and vomiting is common with oral administration. However, in a large study at a poison center, only 5% of patients ultimately required intravenous therapy because they could not tolerate the oral agent 96. In adults, even very high doses of oral N-acetylcysteine (NAC) are not associated with severe toxic effects 97.
The most commonly reported adverse effects of intravenous N-acetylcysteine (NAC) are anaphylactoid reactions, including rash, pruritus, angioedema, bronchospasm, tachycardia, and hypotension. Kerr et al 98 reported that approximately 15% of patients who were treated with intravenous N-acetylcysteine (NAC) had an anaphylactoid reaction within 2 hours after the initial infusion and that increasing the infusion time from 15 to 60 minutes did not alter the rate of adverse events. Other common adverse effects included vomiting and flushing. However, administration of the drug was discontinued in only 2% of patients because of an adverse reaction. Retrospective studies identified adverse effects in approximately 5% of cases 96, 99.
Recommendations for the treatment of adverse effects during N-acetylcysteine (NAC) therapy have been proposed 100. According to these recommendations, no treatment is necessary for flushing alone. Patients with urticaria should be treated with diphenhydramine. Those with angioedema, hypotension, or respiratory symptoms (e.g., bronchospasm) should be treated with diphenhydramine, corticosteroids, and bronchodilators for bronchospasm. The N-acetylcysteine (NAC) infusion should be stopped, but it can be restarted at a slower rate 1 hour after the administration of diphenhydramine if symptoms do not recur 100. Alternatively, patients with severe symptoms who do not have liver failure can be treated with oral N-acetylcysteine (NAC).
The most severe adverse effects occur with erroneous dosing of intravenous N-acetylcysteine (NAC) in children. These effects include cerebral edema 101, 102 and hyponatremia (due to administration in 5% dextrose) 103. There are rare reports of deaths due to anaphylactoid reactions 104, 105.
N-acetylcysteine therapy Stopping Criteria
The definitive duration of N-acetylcysteine therapy is a matter of controversy. Although no universally accepted N-acetylcysteine therapy stopping criteria exist, a universally accepted parameter is that patients should receive a minimum of 300 mg/kg over 20 to 24 hours 2. The decision to discontinue N-acetylcysteine should be based on laboratory evidence of improvement, rather than solely on the duration of therapy 14. A recent consensus statement recommends continuing N-acetylcysteine at a rate of at least 6.25 mg/kg/h or 70 mg/kg every 4 hours orally until the serum acetaminophen level is less than 10 mcg/mL, INR <2.0, the ALT and AST are normal for the patient or have decreased a minimum of 25% to 50% from their peak levels, and the patient is clinically well 14. N-acetylcysteine should be continued past 72 hours if there is a fulminant hepatic failure until the patient receives a liver transplant, recovers, or dies 106, 107.
Acetaminophen (paracetamol) overdose
In case of acetaminophen overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call your local emergency services number.
This is a free and confidential service. All local poison control centers in the United States use this national number. You should call if you have any questions about poisoning or poison prevention. You can call 24 hours a day, 7 days a week.
Can you overdose on acetaminophen?
Yes. Taking too much acetaminophen, known as an overdose, can be very dangerous.
If you’ve taken more than the recommended maximum dose, go to your nearest accident and emergency (A&E) department as soon as possible.
It can be helpful to take any remaining medicine and the box or leaflet with you to a hospital accident and emergency department if you can.
Some people feel sick, vomit or have abdominal (tummy) pain after taking too much tylenol, but often there are no obvious symptoms at first.
- Go to a hospital accident and emergency department even if you’re feeling well.
When an individual has ingested a toxic dose of acetaminophen, the individual should be hospitalized for several days of observation, even if there are no apparent ill effects, because maximum liver damage usually does not become apparent until 2-4 days after ingestion of the drug 71. Transient azotemia and renal tubular necrosis have been reported in patients with acetaminophen poisoning; renal failure is often associated with fatality. There have been reports of acute myocardial necrosis and pericarditis in individuals with acetaminophen poisoning. Maximum cardiotoxic effects of these drugs appear to be delayed in a manner similar to hepatotoxic effects. Hypoglycemia, which can progress to coma, and metabolic acidosis have been reported in patients ingesting toxic doses of acetaminophen and cerebral edema occurred in one patient 71.
In acute acetaminophen overdosage, dose-dependent, potentially fatal liver necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur. Plasma acetaminophen levels > 300 ug/mL at 4 hours after oral ingestion were associated with hepatic damage in 90% of patients; minimal hepatic damage is anticipated if plasma levels at 4 hours are < 150 ug/mL or < 37.5 ug/mL at 12 hours after ingestion. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion 108.
Acetaminophen (paracetamol) overdose prognosis
In acute acetaminophen overdosage, the incidence of liver damage for patients treated with N-acetylcysteine (NAC) within 8 hours of acetaminophen ingestion is less than 10%, but it increases to approximately 40% when delayed beyond 16 hours 2. When treated promptly, the chance of dying (mortality) associated with acetaminophen toxicity is less than 2% 2. However, if patients present late and develop severe liver failure, mortality is high. Approximately 1% to 3% of patients with severe liver failure require liver transplantation 76, 109, 110. In general, children younger than 6 have a better prognosis than adults, primarily due to their greater capacity to detoxify acetaminophen.
In patients with fulminant liver failure, the modified King’s College Criteria helps to determine prognosis and provide insight as to which patients should receive care in a facility that offers liver transplants. The prognosis is poor without a liver transplant if the patient meets any of the following criteria:
- Arterial lactate >3.5 mmol/L after at least 1 L of early fluid resuscitation
- Arterial lactate >3.0 mmol/L after adequate fluid resuscitation and restoration of tissue perfusion
- Arterial pH <7.3
- Grade III or IV encephalopathy with both a PT greater than 100 s and serum creatinine greater than 3.4 mg/dL or 300 µmol/L.
If a patient exhibits a progressive increase in aminotransferase levels and worsening coagulopathy, encephalopathy, or multisystem organ failure despite N-acetylcysteine treatment, consulting a liver transplant team is necessary. Consultation with a medical toxicologist or poison center is recommended for patients with high-risk ingestions or patients who do not fit within the typical diagnostic or treatment protocols.
- Gerriets V, Anderson J, Patel P, et al. Acetaminophen. [Updated 2024 Jan 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482369[↩][↩][↩][↩][↩]
- Agrawal S, Murray BP, Khazaeni B. Acetaminophen Toxicity. [Updated 2025 Apr 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441917[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Acetaminophen. [Updated 2016 Jan 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548162[↩][↩]
- Queremel Milani DA, Davis DD. Pain Management Medications. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560692[↩][↩][↩]
- Athersuch TJ, Antoine DJ, Boobis AR, Coen M, Daly AK, Possamai L, Nicholson JK, Wilson ID. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: a perspective. Toxicol Res (Camb). 2018 Mar 6;7(3):347-357. doi: 10.1039/c7tx00340d[↩][↩]
- Jasani B, Weisz DE, McNamara PJ. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin Perinatol. 2018 Jun;42(4):243-252. doi: 10.1053/j.semperi.2018.05.007[↩]
- Rajaram P, Subramanian R. Management of Acute Liver Failure in the Intensive Care Unit Setting. Clin Liver Dis. 2018 May;22(2):403-408. doi: 10.1016/j.cld.2018.01.013[↩][↩]
- FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit[↩]
- American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2209[↩][↩][↩][↩][↩][↩][↩]
- Wang X, Wu Q, Liu A, Anadón A, Rodríguez JL, Martínez-Larrañaga MR, Yuan Z, Martínez MA. Paracetamol: overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab Rev. 2017 Nov;49(4):395-437. doi: 10.1080/03602532.2017.1354014[↩][↩]
- Lee WM. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J Hepatol. 2017 Dec;67(6):1324-1331. doi: 10.1016/j.jhep.2017.07.005[↩][↩]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988 Dec 15;319(24):1557-62. doi: 10.1056/NEJM198812153192401[↩]
- Prescott LF. Treatment of severe acetaminophen poisoning with intravenous acetylcysteine. Arch Intern Med. 1981 Feb 23;141(3 Spec No):386-9. doi: 10.1001/archinte.141.3.386[↩]
- Dart RC, Mullins ME, Matoushek T, Ruha AM, Burns MM, Simone K, Beuhler MC, Heard KJ, Mazer-Amirshahi M, Stork CM, Varney SM, Funk AR, Cantrell LF, Cole JB, Banner W, Stolbach AI, Hendrickson RG, Lucyk SN, Sivilotti MLA, Su MK, Nelson LS, Rumack BH. Management of Acetaminophen Poisoning in the US and Canada: A Consensus Statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. doi: 10.1001/jamanetworkopen.2023.27739. Erratum in: JAMA Netw Open. 2023 Sep 5;6(9):e2337926. doi: 10.1001/jamanetworkopen.2023.37926[↩][↩][↩][↩][↩]
- Design synthesis and crystallization of acetaminophen. J. Chem. Bio. Phy. Sci. Sec. A, November 2016 – January 2017; Vol.7 No.1; 218-230. https://www.researchgate.net/publication/312848234_Design_synthesis_and_crystallization_of_acetaminophen[↩]
- Haider MA, Gheit YS, Nagi T, Vallejo C, Suarez ZK, Hernandez OL, Gaisinskaya P, Markwart N. Severe Acetaminophen Toxicity From the Use of Oxycodone-Acetaminophen With Normal Liver Function Tests and a Completely Asymptomatic Course of Hospitalization. ACG Case Rep J. 2023 Aug 17;10(8):e01126. doi: 10.14309/crj.0000000000001126[↩]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13926-31. doi: 10.1073/pnas.162468699[↩][↩]
- Ghanem CI, Pérez MJ, Manautou JE, Mottino AD. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol Res. 2016 Jul;109:119-31. doi: 10.1016/j.phrs.2016.02.020[↩]
- Jóźwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm. 2014 Jan-Feb;71(1):11-23. https://www.ptfarm.pl/pub/File/Acta_Poloniae/2014/1/011.pdf[↩]
- Finnerup NB. Nonnarcotic Methods of Pain Management. N Engl J Med. 2019 Jun 20;380(25):2440-2448. doi: 10.1056/NEJMra1807061[↩]
- Botting R, Ayoub SS. COX-3 and the mechanism of action of paracetamol/acetaminophen. Prostaglandins Leukot Essent Fatty Acids. 2005 Feb;72(2):85-7. doi: 10.1016/j.plefa.2004.10.005[↩]
- Dvorakova M., Bosquez-Berger T., Billingsley J., Murataeva N., Woodward T., Leishman E., Zimmowitch A., Gibson A., Wager-Miller J., Cai R., et al. Acetaminophen Inhibits Diacylglycerol Lipase Synthesis of 2-Arachidonoyl Glycerol: Implications for Nociception. Cell Rep. Med. 2025;6:102139. doi: 10.1016/j.xcrm.2025.102139[↩]
- Maatuf Y., Kushnir Y., Nemirovski A., Ghantous M., Iskimov A., Binshtok A.M., Priel A. The Analgesic Paracetamol Metabolite AM404 Acts Peripherally to Directly Inhibit Sodium Channels. Proc. Natl. Acad. Sci. USA. 2025;122:e2413811122. doi: 10.1073/pnas.2413811122[↩]
- Ohashi N., Kohno T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020;11:580289. doi: 10.3389/fphar.2020.580289[↩]
- Ohashi N, Uta D, Sasaki M, Ohashi M, Kamiya Y, Kohno T. Acetaminophen Metabolite N-Acylphenolamine Induces Analgesia via Transient Receptor Potential Vanilloid 1 Receptors Expressed on the Primary Afferent Terminals of C-fibers in the Spinal Dorsal Horn. Anesthesiology. 2017 Aug;127(2):355-371. doi: 10.1097/ALN.0000000000001700[↩]
- Durso G.R.O., Luttrell A., Way B.M. Over-the-Counter Relief from Pains and Pleasures Alike: Acetaminophen Blunts Evaluation Sensitivity to Both Negative and Positive Stimuli. Psychol. Sci. 2015;26:750–758. doi: 10.1177/0956797615570366[↩]
- Mischkowski D., Crocker J., Way B.M. A Social Analgesic? Acetaminophen (Paracetamol) Reduces Positive Empathy. Front. Psychol. 2019;10:538. doi: 10.3389/fpsyg.2019.00538[↩]
- Slavich G.M., Shields G.S., Deal B.D., Gregory A., Toussaint L.L. Alleviating Social Pain: A Double-Blind, Randomized, Placebo-Controlled Trial of Forgiveness and Acetaminophen. Ann. Behav. Med. 2019;53:1045–1054. doi: 10.1093/abm/kaz015[↩]
- Mahajan L, Mittal V, Gupta R, Chhabra H, Vidhan J, Kaur A. Study to Compare the Effect of Oral, Rectal, and Intravenous Infusion of Paracetamol for Postoperative Analgesia in Women Undergoing Cesarean Section Under Spinal Anesthesia. Anesth Essays Res. 2017 Jul-Sep;11(3):594-598. doi: 10.4103/0259-1162.206872[↩]
- Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. 2015 Aug;25(8):416-26. doi: 10.1097/FPC.0000000000000150[↩]
- Li Y, Hong X, Liang L, Wang X, Ladd-Acosta C. Association between acetaminophen metabolites and CYP2E1 DNA methylation level in neonate cord blood in the Boston Birth Cohort. Clin Epigenetics. 2023 Aug 18;15(1):132. doi: 10.1186/s13148-023-01551-4[↩]
- Potter WZ, Thorgeirsson SS, Jollow DJ, Mitchell JR. Acetaminophen-induced hepatic necrosis. V. Correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology. 1974;12:129–43. doi: 10.1159/000136531[↩]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211-7.[↩]
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008 Jul 17;359(3):285-92. doi: 10.1056/NEJMct0708278[↩]
- Bauer AZ, Swan SH, Kriebel D, Liew Z, Taylor HS, Bornehag CG, Andrade AM, Olsen J, Jensen RH, Mitchell RT, Skakkebaek NE, Jégou B, Kristensen DM. Paracetamol use during pregnancy – a call for precautionary action. Nat Rev Endocrinol. 2021 Dec;17(12):757-766. doi: 10.1038/s41574-021-00553-7[↩][↩][↩]
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, Riley A, Pearson C, Zuckerman B, Wang X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020 Feb 1;77(2):180-189. doi: 10.1001/jamapsychiatry.2019.3259[↩][↩][↩][↩][↩][↩]
- Alemany S, Avella-García C, Liew Z, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur J Epidemiol. 2021 Oct;36(10):993-1004. doi: 10.1007/s10654-021-00754-4[↩][↩][↩]
- Philippot G., Gordh T., Fredriksson A., Viberg H. Adult Neurobehavioral Alterations in Male and Female Mice Following Developmental Exposure to Paracetamol (Acetaminophen): Characterization of a Critical Period. J. Appl. Toxicol. 2017;37:1174–1181. doi: 10.1002/jat.3473[↩]
- Baker B.H., Lugo-Candelas C., Wu H., Laue H.E., Boivin A., Gillet V., Aw N., Rahman T., Lepage J., Whittingstall K., et al. Association of Prenatal Acetaminophen Exposure Measured in Meconium with Risk of Attention-Deficit/Hyperactivity Disorder Mediated by Frontoparietal Network Brain Connectivity. JAMA Pediatr. 2020;174:1073–1081. doi: 10.1001/jamapediatrics.2020.3080[↩][↩]
- Ostrea E.M., Brady M., Gause S., Raymundo A.L., Stevens M. Drug Screening of Newborns by Meconium Analysis: A Large-Scale, Prospective, Epidemiologic Study. Pediatrics. 1992;89:107–113. doi: 10.1542/peds.89.1.107[↩]
- Ahlqvist V.H., Sjöqvist H., Dalman C., Karlsson H., Stephansson O., Johansson S., Magnusson C., Gardner R.M., Lee B.K. Acetaminophen Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability. JAMA—J. Am. Med. Assoc. 2024;331:1205–1214. doi: 10.1001/jama.2024.3172[↩][↩][↩]
- Kang J., Kim H.J., Kim T., Lee H., Kim M., Lee S.W., Kim M.S., Koyanagi A., Smith L., Fond G., et al. Prenatal Opioid Exposure and Subsequent Risk of Neuropsychiatric Disorders in Children: Nationwide Birth Cohort Study in South Korea. BMJ. 2024;385:e077664. doi: 10.1136/bmj-2023-077664[↩]
- Prada D., Ritz B., Bauer A.Z., Baccarelli A.A. Evaluation of the Evidence on Acetaminophen Use and Neurodevelopmental Disorders using the Navigation Guide Methodology. Environ. Health. 2025;24:56. doi: 10.1186/s12940-025-01208-0[↩]
- Vignato J., Mehner B., Negrete A., Segre L.S. Over-the-Counter Pain Medication Use During Pregnancy. MCN—Am. J. Matern. Child Nurs. 2023;48:209–214. doi: 10.1097/NMC.0000000000000929[↩]
- Korte B.A.C., Smeets N.J.L., Colbers A., Bemt B.J.F., Gelder M.M.H.J. Adherence to Prescription Medication During Pregnancy: Do Pregnant Women Use Pharmacological Treatment as Prescribed? Br. J. Clin. Pharmacol. 2023;89:1521–1531. doi: 10.1111/bcp.15609[↩]
- Olesen C., Søndergaard C., Thrane N., Nielsen G.L., de Jong-van den Berg L., Olsen J. Do Pregnant Women Report Use of Dispensed Medications? Epidemiology. 2001;12:497–501. doi: 10.1097/00001648-200109000-00006[↩]
- Society for Maternal-Fetal Medicine Prenatal Acetaminophen Use and Outcomes in Children. Am. J. Obstet. Gynecol. 2017;216:B14–B15. doi: 10.1016/j.ajog.2017.01.021[↩]
- Gustavson K., Ystrom E., Ask H., Ask Torvik F., Hornig M., Susser E., Lipkin W.I., Lupattelli A., Stoltenberg C., Magnus P., et al. Acetaminophen Use During Pregnancy and Offspring Attention Deficit Hyperactivity Disorder—A Longitudinal Sibling Control Study. JCPP Adv. 2021;1:e12020. doi: 10.1002/jcv2.12020[↩]
- Brandlistuen R.E., Ystrom E., Nulman I., Koren G., Nordeng H. Prenatal Paracetamol Exposure and Child Neurodevelopment: A Sibling-Controlled Cohort Study. Int. J. Epidemiol. 2013;42:1702–1713. doi: 10.1093/ije/dyt183[↩]
- Acetaminophen (Paracetamol). https://mothertobaby.org/fact-sheets/acetaminophen-pregnancy[↩][↩][↩][↩][↩][↩]
- FDA Responds to Evidence of Possible Association Between Autism and Acetaminophen Use During Pregnancy. https://www.fda.gov/news-events/press-announcements/fda-responds-evidence-possible-association-between-autism-and-acetaminophen-use-during-pregnancy[↩][↩]
- Liew Z, Kioumourtzoglou MA, Roberts AL, O’Reilly ÉJ, Ascherio A, Weisskopf MG. Use of Negative Control Exposure Analysis to Evaluate Confounding: An Example of Acetaminophen Exposure and Attention-Deficit/Hyperactivity Disorder in Nurses’ Health Study II. Am J Epidemiol. 2019 Apr 1;188(4):768-775. doi: 10.1093/aje/kwy288[↩][↩]
- Acetaminophen (Paracetamol). https://mothertobaby.org/fact-sheets/acetaminophen-pregnancy). This study did not find an increased chance for harmful effects on the children’s I.Q., learning, or development ((Acetaminophen (Paracetamol). https://mothertobaby.org/fact-sheets/acetaminophen-pregnancy).
Can I take acetaminophen if I’m breastfeeding?
Acetaminophen is found in low levels in breast milk. When needed, it is given to infants at higher doses than they would get from breast milk. Negative effects in exposed newborns are rare. Be sure to talk to your doctor about all your breastfeeding questions.
Who can take acetaminophen?
Take acetaminophen only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. Liver damage can occur if large amounts of acetaminophen are taken for a long time.
If you are taking acetaminophen (Tylenol) or paracetamol (Panadol) without the advice of your doctor, carefully read the package label and follow the dosing instructions. Talk to your doctor if you have any questions.
If you’re not sure whether you can take acetaminophen (Tylenol) or paracetamol (Panadol), check the leaflet that comes with it or ask your pharmacist or doctor for advice.
Always get advice before taking acetaminophen if you:
- have liver or kidney problems
- have problems with alcohol, such as long-term alcohol misuse
- are very underweight
- are taking other medications
Don’t take acetaminophen if you’ve had an allergic reaction to acetaminophen (paracetamol) in the past.
Before taking acetaminophen
- tell your doctor and pharmacist if you are allergic to acetaminophen, any other medications, or any of the ingredients in the product. Ask your pharmacist or check the label on the package for a list of ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, or herbal products you are taking or plan to take. Be sure to mention anticoagulants (‘blood thinners’) such as warfarin (Coumadin); isoniazid (INH); certain medications for seizures including carbamazepine (Tegretol), phenobarbital, and phenytoin (Dilantin); medications for pain, fever, coughs, and colds; and phenothiazines (medications for mental illness and nausea). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have ever developed a rash after taking acetaminophen.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking acetaminophen, call your doctor.
if you drink three or more alcoholic beverages every day, do not take acetaminophen. Ask your doctor or pharmacist about the safe use of alcoholic beverages while taking acetaminophen. - you should know that combination acetaminophen products for cough and colds that contain nasal decongestants, antihistamines, cough suppressants, and expectorants should not be used in children younger than 2 years of age. Use of these medications in young children can cause serious and life-threatening effects or death. In children 2 through 11 years of age, combination cough and cold products should be used carefully and only according to the directions on the label.
- if you have phenylketonuria (PKU, an inherited condition in which a special diet must be followed to prevent mental retardation), you should know that some brands of acetaminophen chewable tablets may be sweetened with aspartame. a source of phenylalanine.
Acetaminophen (paracetamol) dosage
The recommended dose of acetaminophen (paracetamol) for adults is 650 mg to 1000 mg every 4 to 6 hours, a maximum of 4 grams/day. In children, the recommended acetaminophen (paracetamol) dose is 15 mg/kg every 6 hours, up to 60 mg/kg/day ((Agrawal S, Murray BP, Khazaeni B. Acetaminophen Toxicity. [Updated 2025 Apr 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441917[↩]
- Bannwarth B, Péhourcq F. Bases pharmacologiques de l’emploi du paracétamol: aspects pharmacocinétiques et pharmacodynamiques [Pharmacologic basis for using paracetamol: pharmacokinetic and pharmacodynamic issues]. Drugs. 2003;63 Spec No 2:5-13. French.[↩]
- Tompkins DM, DiPasquale A, Segovia M, Cohn SM. Review of Intravenous Acetaminophen for Analgesia in the Postoperative Setting. Am Surg. 2021 Nov;87(11):1809-1822. doi: 10.1177/0003134821989056[↩][↩]
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009 Aug;57(8):1331-46. doi: 10.1111/j.1532-5415.2009.02376.x[↩]
- Caparrotta TM, Antoine DJ, Dear JW. Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature. Eur J Clin Pharmacol. 2018 Feb;74(2):147-160. doi: 10.1007/s00228-017-2356-6[↩]
- McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol – a review. Br J Clin Pharmacol. 2018 Oct;84(10):2218-2230. doi: 10.1111/bcp.13656[↩][↩]
- Popiołek I, Piotrowicz-Wójcik K, Porebski G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007⁻2018. Pharmacy (Basel). 2019 Jan 17;7(1):12. doi: 10.3390/pharmacy7010012[↩][↩]
- Fujii M, Kenzaka T. Drug-induced lung injury caused by acetaminophen in a Japanese woman: A case report. World J Clin Cases. 2022 Sep 26;10(27):9936-9944. doi: 10.12998/wjcc.v10.i27.9936[↩]
- MacIntyre IM, Turtle EJ, Farrah TE, Graham C, Dear JW, Webb DJ; PATH-BP (Paracetamol in Hypertension–Blood Pressure) Investigators*. Regular Acetaminophen Use and Blood Pressure in People With Hypertension: The PATH-BP Trial. Circulation. 2022 Feb 8;145(6):416-423. doi: 10.1161/CIRCULATIONAHA.121.056015[↩][↩][↩][↩]
- Stergiakouli E, Thapar A, Davey Smith G. Association of Acetaminophen Use During Pregnancy With Behavioral Problems in Childhood: Evidence Against Confounding. JAMA Pediatr. 2016 Oct 1;170(10):964-970. doi: 10.1001/jamapediatrics.2016.1775[↩]
- Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. http://monographs.iarc.fr/ENG/Classification/index.php[↩][↩]
- Is exposure during pregnancy to acetaminophen/paracetamol disrupting female reproductive development? Endocr Connect. 2018, Jan; 7(1):149-158.[↩][↩][↩][↩]
- Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40(1):3-20. doi: 10.1081/clt-120002882[↩]
- Chiew AL, Buckley NA. Acetaminophen Poisoning. Crit Care Clin. 2021 Jul;37(3):543-561. doi: 10.1016/j.ccc.2021.03.005[↩]
- American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2211[↩][↩]
- Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Nineth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2011, p. 9[↩][↩][↩][↩][↩]
- OLSON, K.R. (Ed). Poisoning and Drug Overdose, Sixth Edition. McGraw-Hill, New York, NY 2012, p. 69[↩]
- Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 704[↩][↩]
- American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2210[↩][↩][↩]
- Possamai LA et al; Crit Care Med 41 (11): 2543-50; 2013[↩][↩]
- US FDA; FDA Drug Safety Communication: FDA Warns of Rare but Serious Skin Reactions with the Pain Reliever/Fever Reducer Acetaminophen. https://www.fda.gov/drugs/drugsafety/ucm363041.htm[↩]
- Hung O, Nelson L. Acetaminophen. In: Tintinalli J, editor. Tintinalli’s emergency medicine. 7th ed. Toronto, ON: McGraw Hill; 2011. pp. 1246–52.[↩]
- Ogilvie JD, Rieder MJ, Lim R. Acetaminophen overdose in children. CMAJ. 2012 Sep 18;184(13):1492-6. doi: 10.1503/cmaj.111338[↩]
- Chiew AL, Gluud C, Brok J, Buckley NA. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018 Feb 23;2(2):CD003328. doi: 10.1002/14651858.CD003328.pub3[↩][↩]
- Underhill TJ, Greene MK, Dove AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Arch Emerg Med. 1990 Sep;7(3):148-54. doi: 10.1136/emj.7.3.148[↩]
- Spiller HA, Krenzelok EP, Grande GA, Safir EF, Diamond JJ. A prospective evaluation of the effect of activated charcoal before oral N-acetylcysteine in acetaminophen overdose. Ann Emerg Med. 1994 Mar;23(3):519-23. doi: 10.1016/s0196-0644(94)70071-0[↩]
- Buckley NA, Whyte IM, O’Connell DL, Dawson AH. Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose. J Toxicol Clin Toxicol. 1999;37(6):753-7. doi: 10.1081/clt-100102452[↩]
- Alempijevic T, Zec S, Milosavljevic T. Drug-induced liver injury: Do we know everything? World J Hepatol. 2017 Apr 8;9(10):491-502. doi: 10.4254/wjh.v9.i10.491[↩]
- Janssen J, Singh-Saluja S. How much did you take? Reviewing acetaminophen toxicity. Can Fam Physician. 2015 Apr;61(4):347-9. https://pmc.ncbi.nlm.nih.gov/articles/PMC4396760[↩]
- Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014 Oct 11;14(10):e23539. doi: 10.5812/hepatmon.23539[↩]
- McGill MR, Jaeschke H. Biomarkers of drug-induced liver injury: progress and utility in research, medicine, and regulation. Expert Rev Mol Diagn. 2018 Sep;18(9):797-807. doi: 10.1080/14737159.2018.1508998[↩]
- Radke JB, Algren DA, Chenoweth JA, Owen KP, Ford JB, Albertson TE, Sutter ME. Transaminase and Creatine Kinase Ratios for Differentiating Delayed Acetaminophen Overdose from Rhabdomyolysis. West J Emerg Med. 2018 Jul;19(4):731-736. doi: 10.5811/westjem.2018.3.37076[↩]
- Levine M, Stellpflug SJ, Pizon AF, Peak DA, Villano J, Wiegand T, Dib C, Thomas SH. Hypoglycemia and lactic acidosis outperform King’s College criteria for predicting death or transplant in acetaminophen toxic patients. Clin Toxicol (Phila). 2018 Jul;56(7):622-625. doi: 10.1080/15563650.2017.1420193[↩]
- Harrison PM, Wendon JA, Gimson AES, Alexander GJM, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–7. doi: 10.1056/NEJM199106273242604[↩][↩]
- Devlin J, Ellis AE, McPeake J, Heaton N, Wendon JA, Williams R. N-acetyl-cysteine improves indocyanine green extraction and oxygen transport during hepatic dysfunction. Crit Care Med. 1997;25:236–42. doi: 10.1097/00003246-199702000-00007[↩]
- Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303:1026–9. doi: 10.1136/bmj.303.6809.1026[↩]
- Jones AL. Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol. 1998;36:277–85. doi: 10.3109/15563659809028022[↩]
- Fukumoto M. [Are nomograms available for the treatment of acetaminophen poisoning? Limitation of Rumack & Matthew nomogram for evaluation of hepatotoxicity]. Chudoku Kenkyu. 2010 Jun;23(2):111-5. Japanese.[↩]
- Wong A, Graudins A. Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol (Phila). 2016;54(2):115-9. doi: 10.3109/15563650.2015.1115055[↩]
- Walls L et al; J Perinatol 27 (2): 133-5; 2007[↩]
- Nogen A, Bremmer J; J Pediatr 92 (MAY): 832; 1978[↩][↩]
- Davis AM et al; Am J Med 74 (2): 349; 1983[↩]
- Gill EJ t al; J Reprod Med 47 (7): 584-6; 2002[↩]
- Yip L, Dart RC, Hurlbut KM. Intravenous administration of oral N-acetylcysteine. Crit Care Med. 1998;26:40–3. doi: 10.1097/00003246-199801000-00014[↩][↩]
- Miller LF, Rumack BH. Clinical safety of high oral doses of acetylcysteine. Semin Oncol. 1983 Mar;10(1 Suppl 1):76-85.[↩]
- Kerr F, Dawson A, Whyte IM, et al. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402–8. doi: 10.1016/j.annemergmed.2004.08.040[↩]
- Kao LW, Kirk MA, Furbee RB, Mehta NH, Skinner JR, Brizendine EJ. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann Emerg Med. 2003;42:741–50. doi: 10.1016/s0196-0644(03)00508-0[↩]
- Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med. 1998;31:710–5. doi: 10.1016/s0196-0644(98)70229-x[↩][↩]
- Bailey B, Blais R, Letarte A. Status epilepticus after a massive intravenous N-acetylcysteine overdose leading to intracranial hypertension and death. Ann Emerg Med. 2004;44:401–6. doi: 10.1016/j.annemergmed.2004.05.014[↩]
- Hershkovitz E, Shorer Z, Levitas A, Tal A. Status epilepticus following intravenous N-acetylcysteine therapy. Isr J Med Sci. 1996 Nov;32(11):1102-4.[↩]
- Sung L, Simons JA, Dayneka NL. Dilution of intravenous N-acetylcysteine as a cause of hyponatremia. Pediatrics. 1997;100:389–91. doi: 10.1542/peds.100.3.389[↩]
- Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19:594–5. doi: 10.1136/emj.19.6.594[↩]
- Reynard K, Riley A, Walker BE. Respiratory arrest after N-acetylcysteine for paracetamol overdose. Lancet. 1992;340:675. doi: 10.1016/0140-6736(92)92211-w[↩]
- Muñoz Romo R, M Borobia Pérez A, A Muñoz M, Carballo Cardona C, Cobo Mora J, Carcas Sansuán AJ. Efficient diagnosis and treatment of acute paracetamol poisoning: cost-effectiveness analysis of approaches based on a hospital toxicovigilance program. Emergencias. 2018 Jun;30(3):169-176. English, Spanish. https://revistaemergencias.org/wp-content/uploads/2023/08/Emergencias-2018_30_3_169-176-176.pdf[↩]
- Yesil Y, Ozdemir AA. Evaluation of the children with acute acetaminophen overdose and intravenous N-acetylcysteine treatment. Pak J Med Sci. 2018 May-Jun;34(3):590-594. doi: 10.12669/pjms.343.14937[↩]
- US Natl Inst Health; DailyMed. Current Medication Information for OFIRMEV (acetaminophen) injection, solution (October 2013). Available from, as of March 6, 2014[↩]
- Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol. 2016 Jun 28;4(2):131-42. doi: 10.14218/JCTH.2015.00052[↩]
- Frischknecht J. Order in the house. Setting standards for treatment of acetaminophen toxicity. Adv NPs PAs. 2013 Mar;4(3):34-6.[↩]