Contents
What is hypothalamus
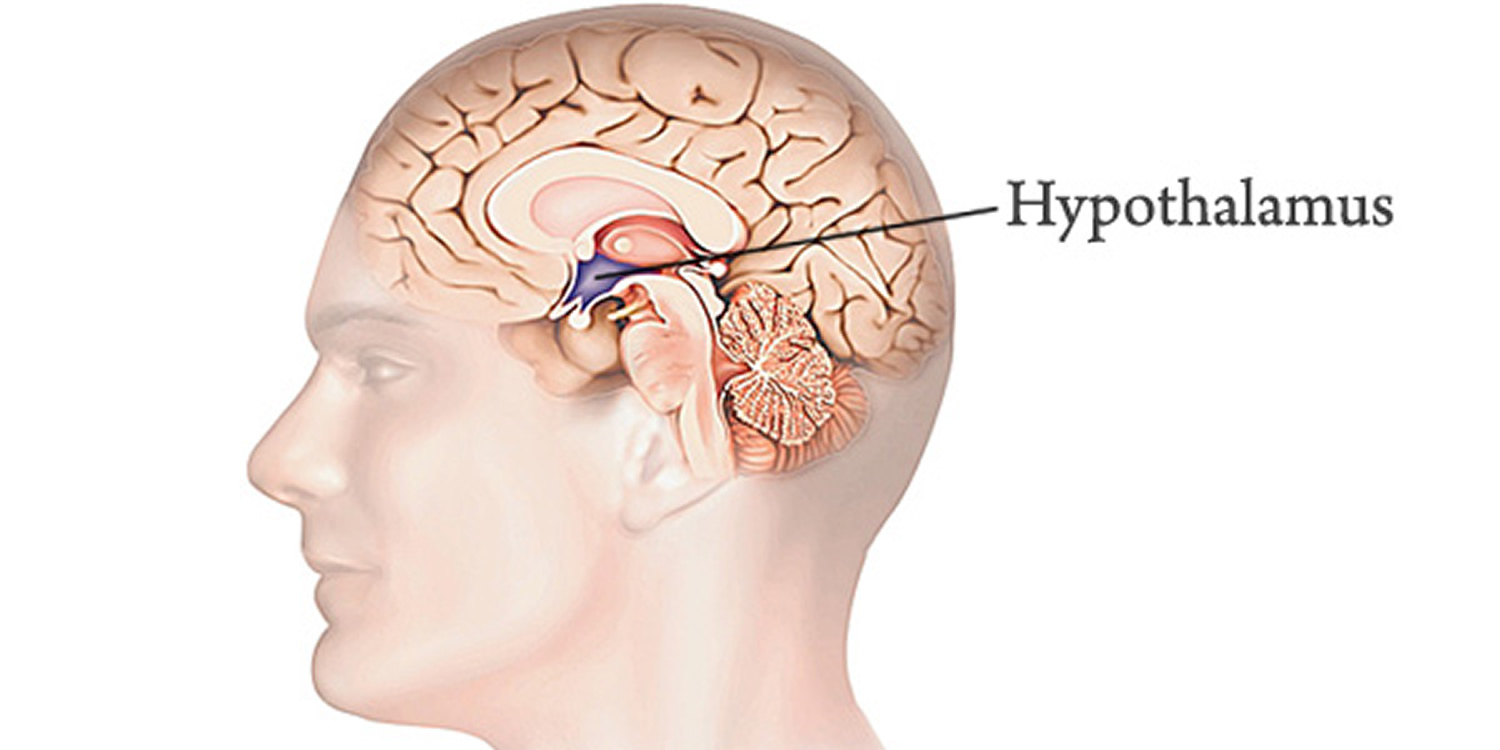
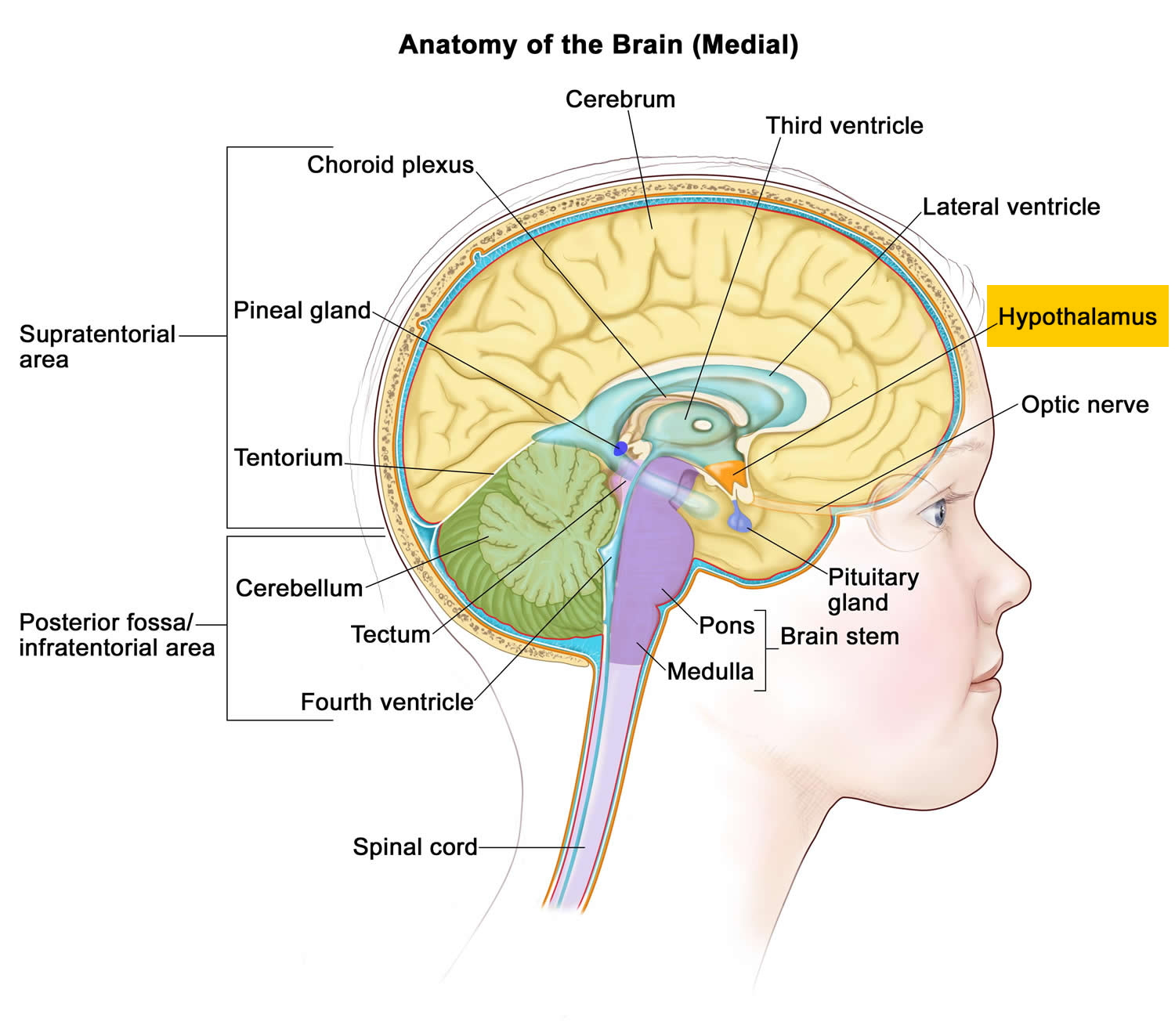
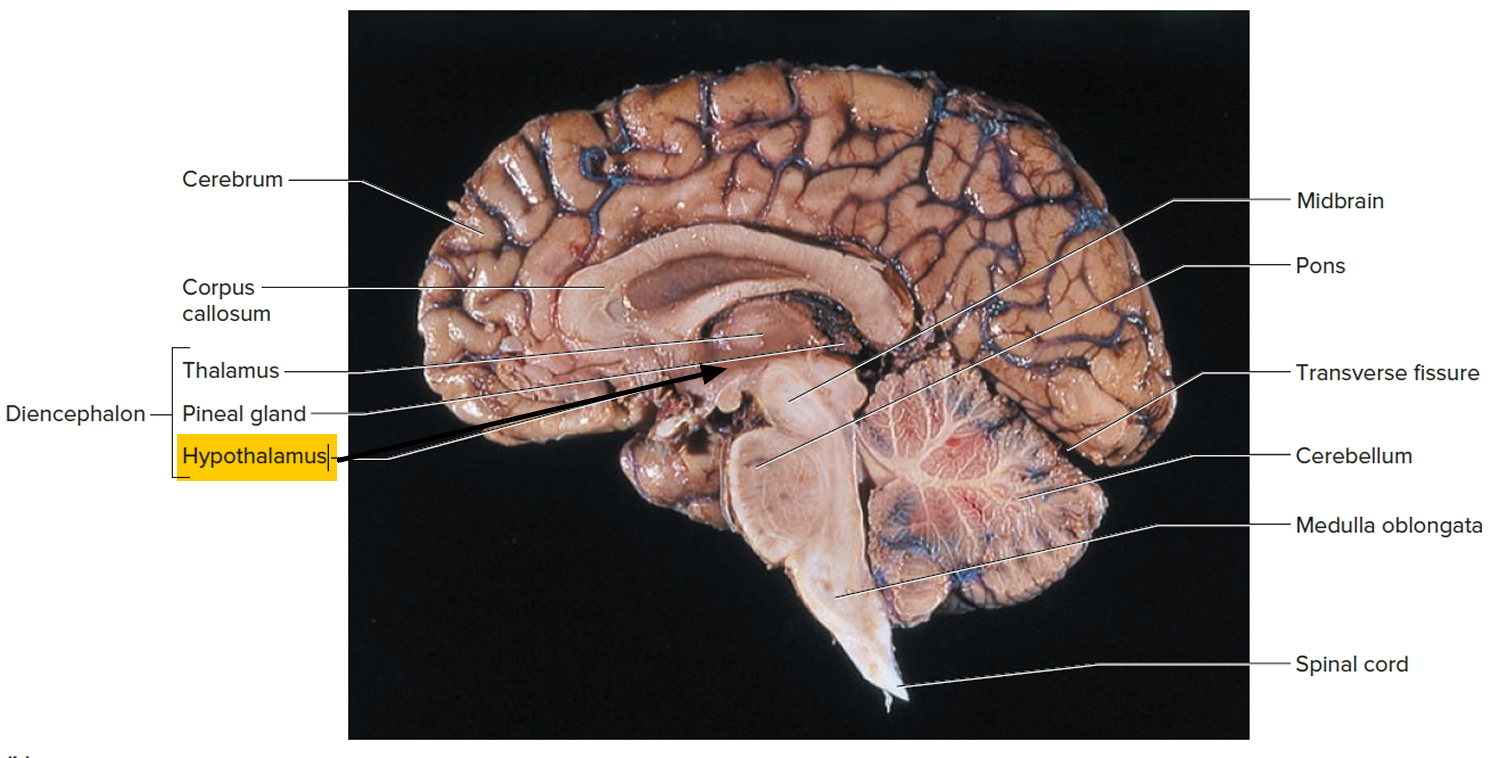
The hypothalamus (“below the thalamus”) is the inferior portion of the diencephalon. Projecting inferiorly from the hypothalamus is the pituitary gland (Figure 1) and the hypothalamus occupies approximately 2 per cent of the brain volume. The hypothalamus is situated in a strategic position at the crossroad of four systems, neurovegetative, neuroendocrine, limbic, and optic 1.
The hypothalamus forms the inferolateral walls of the third ventricle. On the underside of the brain, the hypothalamus lies between the optic chiasma (point of crossover of cranial nerves II, the optic nerves) and the posterior border of the mammillary bodies, rounded bumps that bulge from the hypothalamic floor (mammillary = “little breast”).
The hypothalamus, like the thalamus, contains about a dozen brain nuclei of gray matter. Despite its relatively small size (roughly that of a thumbnail or an almond), functionally, the hypothalamus is the main visceral control center of the body, regulating many activities of the visceral organs.
The hypothalamus is the main point of interaction for the body’s two physical control systems: the nervous system, which transmits information in the form of minute electrical impulses, and the endocrine system, which brings about changes of state through the release of chemical factors. It is the hypothalamus that first detects crucial changes in the body and responds by stimulating various glands and organs to release hormones.
The hypothalamus is composed of a number of cell groups and can be distinguished into three structurally distinct parts, namely, anterior, middle and posterior regions. These regions are alternately known as the supraoptic, tuberal and mammillary, respectively. Some less anatomically distinct areas can also be found in this brain structure. All these parts are collectively responsible for the production of different essential hormones and chemical substances that control and regulate the functioning of various organs in your body.
- Anterior Component
It is also known as supraoptic region. As the very name suggests, the supraoptic division is located above the optic chiasm where the most prominent nuclei include paraventricular and supraoptic. Other less prominent nuclei are: preoptic, medial preoptic, anterior hypothalamic and suprachiasmatic. These nuclei are collectively involved in the secretion of hormones, including oxytocin, vasopressin (ADH), corticotropin releasing hormone (CRH) and somatostatin. It is this region where some of the important body functions are accomplished, such as circadian rhythms, thermoregulation, panting, sweating and differential development between sexes.
- Middle or Tuberal Component
Located at the level of tuber cinereum, the tuberal region is further divided into two parts: medial and lateral. Ventromedial nucleus, the largest and most prominent of the nuclei present in the region, is responsible for shaping and controlling eating habits. Some other functions, like the regulation of blood pressure, heart rate, satiety and gastrointestinal stimulation also fall under the domain of tuberal region.
- Posterior Component
The posterior component is composed of medial and lateral areas. Medial area contains two types of hypothalamic nuclei: mammillary and posterior. These nuclei control the functions, like memory, blood pressure, shivering, energy balance, feeding, sleep, arousal and learning.
Figure 1. Hypothalamus
Figure 2. Parts of Hypothalamus
Hypothalamus function
The hypothalamus functions include the following:
- Control of the autonomic nervous system. At the autonomic level, the hypothalamus stimulates smooth muscle (which lines the blood vessels, stomach, and intestines) and receives sensory impulses from these areas. Thus it controls the heart rate and blood pressure, the passage of food through the alimentary canal, the secretion from sweat glands and salivary glands and contraction of the bladder and many other visceral activities. The hypothalamus
exerts its control over visceral functions by relaying its instructions through the periaqueductal gray matter of the midbrain and the reticular formation of the brain stem, which then carry out those instructions.
- Regulation of body temperature. The body’s thermostat is in the hypothalamus. In the hypothalamus are neurons that monitor body temperature at the surface through nerve endings in the skin and other neurons that monitor the blood flowing through this part of the brain itself, as an indicator of core body temperature. The front part of the hypothalamus contains neurons that act to lower body temperature by relaxing smooth muscle in the blood vessels, which causes them to dilate and increases the rate of heat loss from the skin. Through its neurons associated with the sweat glands of the skin, the hypothalamus can also promote heat loss by increasing the rate of perspiration. In opposite conditions, when the body’s temperature falls below the (rather narrow) ideal range, a portion of the hypothalamus directs the contraction of blood vessels, slows the rate of heat loss, and causes the onset of shivering (which produces a small amount of heat). Hypothalamic centers also induce fever.
- Regulation of hunger and thirst sensations. The hypothalamus is the control center for the stimuli that underlie eating and drinking. By sensing the concentrations of nutrients and salts in the blood, certain hypothalamic neurons mediate feelings of hunger and thirst and thus aid in maintaining the proper concentrations of these substances. The sensations that you interpret as hunger arise partly from a degree of emptiness in the stomach and partly from a drop in the level of two substances: glucose circulating in the blood and a hormone that the intestine produces shortly after the intake of food. Receptors for this hormone gauge how far digestion has proceeded since the last meal. This system is not a simple “on” switch for hunger, however: another portion of the hypothalamus, when stimulated, actively inhibits eating by promoting a feeling of satiety. In experimental animals, damage to this portion of the brain is associated with continued excessive eating, eventually leading to obesity.
- Regulation of sleep-wake cycles. Acting with other brain regions, the hypothalamus helps regulate the complex phenomenon of sleep. The suprachiasmatic nucleus is the body’s biological clock. It generates the daily circadian rhythms and synchronizes these cycles in response to dark-light information sensed via the optic nerve. In response to such signals, the preoptic nucleus induces sleep. Electrical stimulation of a portion of the hypothalamus has been shown to induce sleep in experimental animals, although the mechanism by which this works is not yet known. Other hypothalamic nuclei near the mammillary body mediate arousal from sleep. Furthermore, the hypothalamus forms part of the reticular activating system, the physical basis for that hard-to-define state known as consciousness.
- Control of the endocrine system. The hypothalamus controls the secretion of hormones by the pituitary gland, which in turn influences the activity of many other endocrine organs.
- Control of emotional responses. The hypothalamus lies at the center of the emotional part of the brain, the limbic system. Regions involved in pleasure, rage, and fear are located in the hypothalamus. The hypothalamus is the brain’s intermediary for translating emotion into physical response. When strong feelings (rage, fear, pleasure, excitement) are generated in the mind, whether by external stimuli or by the action of thoughts, the cerebral cortex transmits impulses to the hypothalamus; the hypothalamus may then send signals for physiological changes through the autonomic nervous system and through the release of hormones from the pituitary. Physical signs of fear or excitement, such as a racing heartbeat, shallow breathing, and perhaps a clenched “gut feeling,” all originate here.
- Control of motivational behavior. The hypothalamus controls behavior that is rewarding. For example, the hypothalamus influences your motivation for feeding, thereby determining how much you eat, and also influences sex drive and sexual behavior.
- Formation of memory. The brain nucleus in the mammillary body receives many inputs from the major memory processing structure of the cerebrum, the hippocampal formation.
Lesions of the hypothalamus cause disorders in visceral functions and in emotions. Thus, injuries to the hypothalamus can result in severe weight loss or obesity, sleep disturbances, dehydration, and a broad range of emotional disorders.
In all, the hypothalamus is a richly complex cubic centimeter of vital connections, which will continue to reward close study for some time to come. Because of its unique position as a midpost between thought and feeling and between conscious act and autonomic function, a thorough understanding of its workings should tell us much about the earliest history and development of the human animal.
Summary of the hypothalamus functions:
- Heart rate and arterial blood pressure
- Body temperature
- Water and electrolyte balance
- Control of hunger and body weight
- Control of movements and glandular secretions of the stomach and intestines
- Production of hormones that stimulate the pituitary gland to secrete pituitary hormones
- Sleep and wakefulness
- Memory.
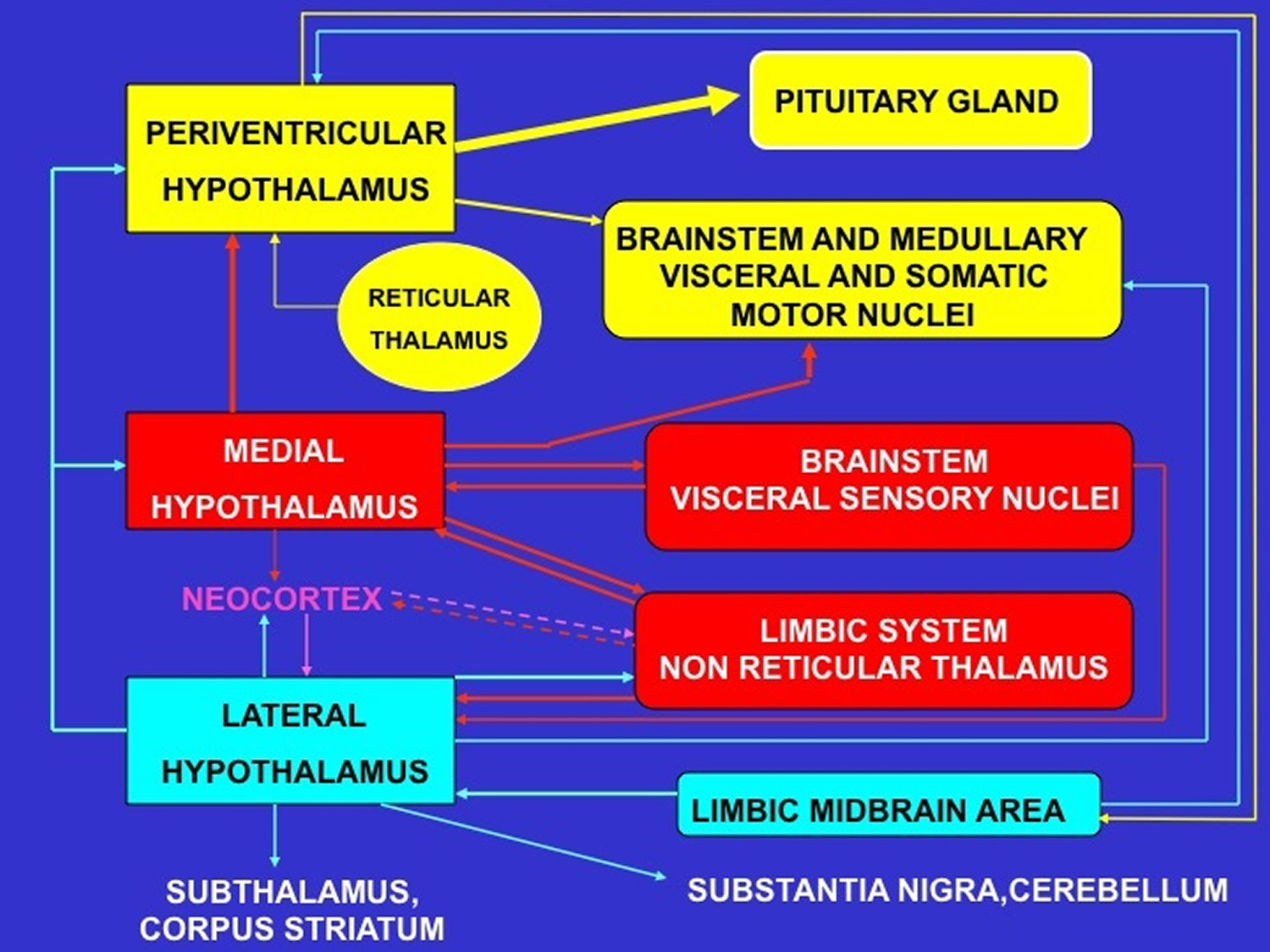
Figure 4. Schematic representation of the major neural pathways connecting the periventricular, medial and lateral hypothalamic subdivisions with the rest of the brain
Appetite control and hypothalamus
The hypothalamus is a major integrator of hormonal and nutrient-induced signals of hunger and satiety with the aim of regulating energy stores and food intakes. The central role of the hypothalamus in the control of feeding has emerged in the past century from lesioning studies. Indeed, various lesions of the ventromedial hypothalamus were shown to cause hyperphagia and obesity 2 while lesions of the lateral hypothalamus caused reduced food intake and leanness 3. These studies indicated that key hypothalamic areas activate responses to promote negative energy balance (i.e., reduced food intake and increased energy expenditure) and decreased nutrient availability (reduced endogenous glucose production). Accordingly, impaired responses or a sort of resistance to afferent input from these hormonal or nutrient-related signals would be predicted to favor weight gain and insulin resistance and may contribute to the development of obesity and type 2 diabetes 4. Many reports have been focused on the identification of hypothalamic pathways that control energy but recent evidence suggests, however, that in addition to playing a critical role in the regulation of energy homeostasis, the central nervous system also control peripheral metabolisms such as glucose metabolism via hypothalamic sensors detecting nutrients availability.
Mechanisms of appetite control became a public health focus because of its numerous clinical implications of obesity, currently reaching world epidemic levels. Weight loss is one of the main therapeutic goals to decrease the related morbidity and mortality of obesity. The neurobehavioral aspects of obesity are complex and poorly understood, food sensing and craving are currently major areas of research. The neurologic component of food regulation is centered on the hypothalamus 5. Recently, functional changes in brain activities in response to food have been seen in humans by using functional magnetic resonance imaging (fMRI) 6. These data emphasize the major regulatory action of central metabolic sensing in the regulation of body weight, however, the modifications of brain structure and function during morbid obesity are still poorly realized. Recently, Thaler et al. 7 reported hypothalamic lesions associated with obesity that could impact the efficiency of deep brain stimulation. Five hypothalamic nuclei are known to be involved in appetite control.8, 9, 10, 11 : The lateral nucleus, the ventromedial and dorsomedial nuclei, the paraventricular nucleus and the arcuate or infundibular nucleus. Although clinical benefit of chronic electric stimulation of lateral hypothalamus is still unproved in humans 12, recent animals studies have demonstrated weight control using electric modulation of the ventromedial nucleus 9, 10, 11. Studies of the hypothalamic stimulation to control food intake failed to control a patient’s weight but suggested the perifornixial stimulation may improve memory processing 12.
Visual system and hypothalamus
The retino-hypothalamus tract is the main connection between the eye and the hypothalamus, a direct afferent pathway from the retina, going through the optic chiasma. In mammalians, axons of photosensitive retinal ganglion cells, expressing melanopsin, project to the suprachiasmatic nucleus 13. Retinal projections to nonvisual centers have been identified in animals, with equally crossed and uncrossed hypothalamic fiber that represent 5% of retinal fibers 14; the axonal targets are the following hypothalamic nuclei: The supra chiasmatic nucleus, the supra optic nucleus, and the sub periventricular area; the olivary pretectal nucleus, the intergeniculate leaflets of the geniculate nuclei, the medial amygdala, the lateral habenula, the nucleus posterior limitans of the thalamus, the superior colliculus and the periaqueductal gray. Sadun and Schaechter 15 described first in human the retino-hypothalamus tract, which penetrates the hypothalamus into the suprachiasmatic nucleus, ending locally and in the paraventricular nucleus 16. The suprachiasmatic nucleus is well-known as the hypothalamic clock 15, synchronizing several biorhythms such as sleep-arousal and food intake; in rats the ventromedial and the lateral nuclei also have circadian rhythms 17.
Limbic system and hypothalamus
Two groups of hypothalamic nuclei are directly involved in the limbic circuitry, the preoptic and the mammillary. Broadly the preoptic connects the frontal lobe, the thalamo-tegmental region, the septum, the lenticular nucleus, the substantia innominata of Reichert, and the anterior perforate; mainly through the basal forebrain bundle, the ansa lenticularis, and the radiate system; the medial nucleus of the preoptic nucleus being in continuity with the nucleus of the stria terminalis 18. The mammillary nuclei participate to the limbic circuitry through the fornix and the mammillo-thalamic bundle. The fornix emerges from the hippocampus and terminates anteriorly and laterally, into the ipsilateral mammillary body; the precommissural fornix could be continuous with the diagonal band of Broca 19. The mammillo-thalamic bundle terminates into the ipsilateral anterior nucleus of the thalamus, from which neuronal relays go to the cingulum.
From a white matter tract point of view, the stria medullaris of the thalamus connects the epithalamus or habenula, with the preoptic and septal regions, and also possibly with the nucleus of Meynert. The basal forebrain bundle, bidirectional, links the upper brain stem, mostly the tegmentum with the anterolateral hypothalamus, the olfactory region, the septum, the nucleus accumbens, the amygdala, and the substantia innominata of Reichert. The diagonal band of Broca connects the septal region, the anterior perforate region and the olfactory area with the amygdala, and possibly the lateral hypothalamus. In rats, hypothalamic stimulation enhances hippocampal plasticity. In humans, deep brain stimulation of the lateral hypothalamus close to the fornix seems to improve memory processing 12, 20.
Hypothalamus hormones
Control of the endocrine system occurs in three ways: (a) The hypothalamus and anterior pituitary stimulate other endocrine glands; (b) the nervous system stimulates a gland directly; or (c) changes in the level of a substance in the blood stimulates a gland directly.
Hypothalamus Hormones
Thus far, 7 physiologically important hypothalamic neurohormones have been identified (see Table 1: Hypothalamic Neurohormones). Except for the biogenic amine dopamine, all are small peptides. Several are produced in the periphery as well as in the hypothalamus and function in local paracrine systems, especially in the gastrointestinal tract. Vasoactive intestinal peptide, which also stimulates the release of prolactin, is one.
Neurohormones may control the release of multiple pituitary hormones. Regulation of most anterior pituitary hormones depends on stimulatory signals from the hypothalamus; the exception is prolactin, which is regulated by inhibitory stimuli. If the pituitary stalk (which connects the pituitary to the hypothalamus) is severed, prolactin release increases, whereas release of all other anterior pituitary hormones decreases.
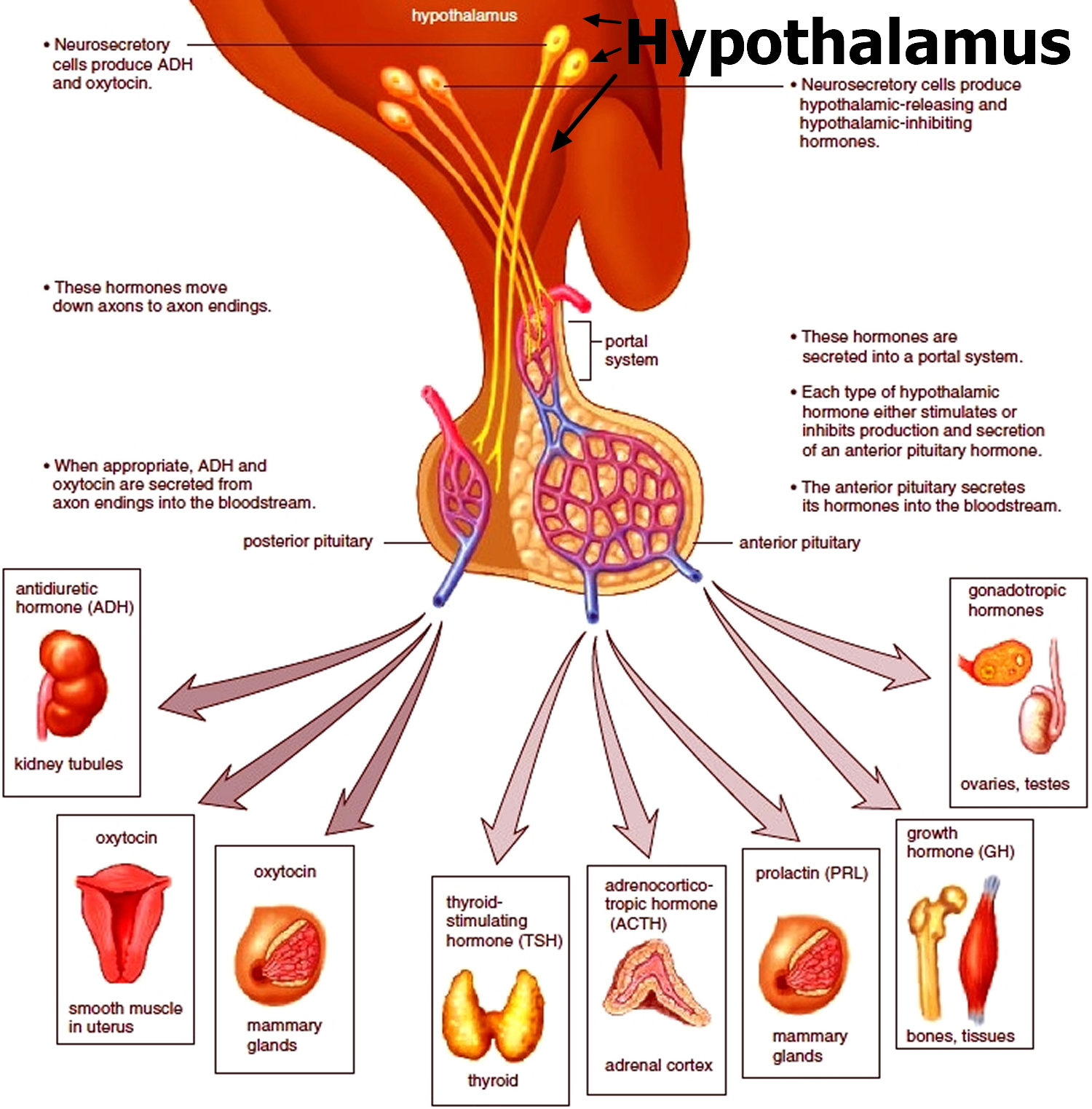
Two other important hormones are secreted by the hypothalamic region of the diencephalon, the anti-diuretic hormone (ADH or Vasopressin) and oxytocin. Having its role in the regulation of water content in the body, the release of anti-diuretic hormone (ADH) facilitates the absorption of water in the nephrons of kidney. Oxytocin, on the other hand, plays a role in the sexual reproduction in human beings and other mammals, particularly child birth and ejection of milk from mammary glands.
Many hypothalamic abnormalities (including tumors and encephalitis and other inflammatory lesions) can alter the release of hypothalamic neurohormones. Because neurohormones are synthesized in different centers within the hypothalamus, some disorders affect only one neuropeptide, whereas others affect several. The result can be undersecretion or oversecretion of neurohormones. Clinical syndromes that result from the ensuing pituitary hormone dysfunction (eg, diabetes insipidus, acromegaly and, hypopituitarism.) are discussed in Pituitary Gland Disorders.
Table 1. Hypothalamic Neurohormones
Neurohormone | Anterior Pituitary Hormones Affected | Effect |
Corticotropin-releasing hormone | ACTH | Stimulate |
Dopamine | Prolactin LH
FSH
TSH | Inhibit Inhibit
Inhibit
Inhibit |
| Gonadotropin-releasing hormone | LH
FSH | Stimulate*
Stimulate* |
| Growth hormone–releasing hormone | GH | Stimulate |
| Prolactin-releasing hormone | Prolactin | Stimulate |
| Somatostatin | GH
TSH | Inhibit
Inhibit |
| Thyrotropin-releasing hormone | TSH
Prolactin | Stimulate
Stimulate |
*Under physiologic conditions and when administered exogenously in intermittent pulses. Continuous infusion inhibits the release of LH and FSH. | ||
ACTH = adrenocorticotropic hormone (corticotropin); FSH = follicle-stimulating hormone; GH = growth hormone; LH = luteinizing hormone; TSH = thyroid-stimulating hormone. | ||
- Regulation of Pituitary Hormones
The hypothalamus controls the secretion of hormones from the pituitary gland (hypophysis) which is an important part of the brain and master secretory organ in your body. The pituitary gland (hypophysis) is located at the base of the brain, where a pituitary stalk (infundibulum) attaches it to the hypothalamus. The pituitary gland is about 1 centimeter in diameter and consists of an anterior pituitary or anterior lobe, and a posterior pituitary, or posterior lobe. Pituitary is associated with the release of eight essential hormones in the body; two of which are synthesized by the hypothalamus, while the remaining six are produced locally. Some of the other endocrine and exocrine functions of hypothalamus include the control of autonomic nervous system, maintenance of homeostasis, balance of emotions, regulation of hunger and thirst and check over the thermostat of the body.
Hypothalamic-Pituitary Relationships
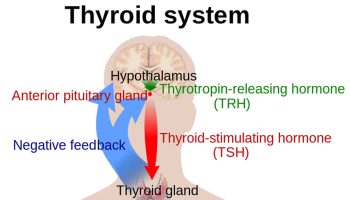
Peripheral endocrine organ functions are controlled to varying degrees by pituitary hormones. Some functions (eg, secretion of insulin by the pancreas, primarily controlled by the blood glucose level) are controlled to a minimal extent, whereas many (eg, secretion of thyroid or gonadal hormones) are controlled to a great extent. Secretion of pituitary hormones is controlled by the hypothalamus.
The interaction between the hypothalamus and pituitary (hypothalamic-pituitary axis) is a feedback control system 21. The hypothalamus receives input from virtually all other areas of the brain and uses it to provide input to the pituitary gland. In response, the pituitary gland releases various hormones that stimulate certain endocrine glands throughout the body. Changes in circulating levels of hormones produced by these endocrine glands are detected by the hypothalamus, which then increases or decreases its stimulation of the pituitary gland to maintain homeostasis.
The hypothalamus modulates the activities of the anterior and posterior lobes of the pituitary gland in different ways. Neurohormones synthesized in the hypothalamus reach the anterior pituitary (adenohypophysis) through a specialized portal vascular system and regulate synthesis and release of the 6 major peptide hormones of the anterior pituitary (see Figure 5). These anterior pituitary hormones regulate peripheral endocrine glands (the thyroid, adrenals, and gonads) as well as growth and lactation. No direct neural connection exists between the hypothalamus and the anterior pituitary. In contrast, the posterior pituitary (neurohypophysis) comprises axons originating from neuronal cell bodies located in the hypothalamus. These axons serve as storage sites for 2 peptide hormones, vasopressin (antidiuretic hormone) and oxytocin, synthesized in the hypothalamus; these hormones act in the periphery to regulate water balance, milk ejection, and uterine contraction.
Virtually all hormones produced by the hypothalamus and the pituitary are released in a pulsatile fashion; periods of such release are interspersed with periods of inactivity. Some hormones (eg, adrenocorticotropic hormone [ACTH], growth hormone, prolactin) have definite circadian rhythms; others (eg, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle) have month-long rhythms with superimposed circadian rhythms.
Figure 5. The hypothalamus and pituitary gland (anterior and posterior) endocrine pathways and target organs
Hypothalamus disorders
Many hypothalamic abnormalities (including tumors and encephalitis and other inflammatory lesions) can alter the release of hypothalamic neurohormones. Because neurohormones are synthesized in different centers within the hypothalamus, some disorders affect only one neuropeptide, whereas others affect several. The result can be undersecretion or oversecretion of neurohormones. Clinical syndromes that result from the ensuing pituitary hormone dysfunction (eg, diabetes insipidus, acromegaly and, hypopituitarism.) are discussed in Pituitary Gland Disorders.
Hypopituitarianism is another ailment of brain in which pituitary gland and hypothalamus are integrated with each other, thus inhibiting the hormonal secretions of hypophysis and disturbing all the vital functions of the body that are regulated by the chemical substances produced therein. In this case, your health care provider may suggest hormonal replacement therapy as the disease results in the hormonal insufficiency in the body.
- Lemaire J-J, Nezzar H, Sakka L, et al. Maps of the adult human hypothalamus. Surgical Neurology International. 2013;4(Suppl 3):S156-S163. doi:10.4103/2152-7806.110667. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3654779/[↩]
- Card JP. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. Hypothalamic Structure-Function Relationships; pp. 57–64.[↩]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–34. https://www.ncbi.nlm.nih.gov/pubmed/20687206[↩]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885915/[↩]
- Young JK, Stanton GB. A three-dimensional reconstruction of the human hypothalamus. Brain Res Bull. 1994;35:323–7. https://www.ncbi.nlm.nih.gov/pubmed/7850481[↩]
- van de Sande-Lee S, Pereira FR, Cintra DE, Fernandes PT, Cardoso AR, Garlipp CR, et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes. 2011;60:1699–704. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114393/[↩]
- Foster RG, Bellingham J. Inner retinal photoreceptors (IRPs) in mammals and teleost fish. Photochem Photobiol Sci. 2004;3:617–27. https://www.ncbi.nlm.nih.gov/pubmed/15170494[↩]
- [↩]
- Card JP, Rinaman L. Encyclopedia of the Human Brain. New York: Academic Press; 2002. Hypothalamus; pp. 525–35.[↩][↩]
- Fontaine D, Lanteri-Minet M, Ouchchane L, Lazorthes Y, Mertens P, Blond S, et al. Anatomical location of effective deep brain stimulation electrodes in chronic cluster headache. Brain. 2010;133:1214–23. https://www.ncbi.nlm.nih.gov/pubmed/20237130[↩][↩]
- Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat. 2003;26:253–70. https://www.ncbi.nlm.nih.gov/pubmed/14729128[↩][↩]
- Swaab DF. The Primate Nervous System, Part I. Vol. 13. Philadelphia: Elsevier; 1997. Chapter II Neurobiology and neuropathology of the human hypothalamus; pp. 39–137.[↩][↩][↩]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–41. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2577158/[↩]
- Sexton T, Buhr E, Van Gelder RN. Melanopsin and mechanisms of non-visual ocular photoreception. J Biol Chem. 2012;287:1649–56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3265846/[↩]
- Sadun AA, Schaechter JD, Smith LE. A retinohypothalamic pathway in man: light mediation of circadian rhythms. Brain Res. 1984;302:371–7. https://www.ncbi.nlm.nih.gov/pubmed/6733517[↩][↩]
- Duvernoy H, Cabanis EA, Iba-Zizen MT, Tamraz J, Guyot J. Le cerveau humain: Surfaces, coupes sériées tridimentionelles et IRM. Paris, Berlin, Heidelberg, New York, Londres, Tokyo, Hong Kong, Bracelone Budapest: Springer-Verlag; 1992.[↩]
- Hendrickson AE. Electron microscopic distrubition of axoplasmic transport. J Comp Neurol. 1972;144:381–97. https://www.ncbi.nlm.nih.gov/pubmed/4116068[↩]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. https://www.ncbi.nlm.nih.gov/pubmed/4116104[↩]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. https://www.ncbi.nlm.nih.gov/pubmed/11834835[↩]
- Koizumi K, Nishino H. Circadian and other rhythmic activity of neurones in the ventromedial nuclei and lateral hypothalamic area. J Physiol (Lond) 1976;263:331–56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1307706/[↩]
- Merck Sharp & Dohme Corp. Merck Manual. Overview of the Endocrine System. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/principles-of-endocrinology/overview-of-the-endocrine-system.[↩]