Contents
- What is amyloidosis
What is amyloidosis
Amyloidosis is a rare disease that occurs when amyloid proteins are deposited in tissues and organs – by extracellular and/or intracellular deposition 1. The amyloid proteins (insoluble abnormal amyloid fibrils) then alter the normal function of tissues. Amyloid proteins are abnormal proteins that the body cannot break down and recycle, as it does with normal proteins. When amyloid proteins clump together, they form amyloid deposits. The buildup of these deposits damages a person’s organs and tissues. Amyloidosis can affect different organs and tissues in different people and can affect more than one organ at the same time.
Amyloidosis most frequently affects:
- the kidneys,
- heart,
- nervous system,
- liver, and
- digestive tract.
The symptoms and severity of amyloidosis depend on the organs and tissues affected.
There’s no cure for amyloidosis. But treatments can help you manage your symptoms and limit the production of amyloid protein.
Only 10% of amyloidosis deposits consist of components such as glycosaminoglycans (GAGs), apolipoprotein-E (apoE), and serum amyloid P-component (SAP), while nearly 90% of the deposits consist of amyloid fibrils that are formed by the aggregation of misfolded proteins. These proteins either arise from proteins expressed by cells at the deposition site (localized), or they precipitate systemically after production at a local site (systemic) 2. In humans, about 23 different unrelated proteins are known to form amyloid fibrils in vivo 3.
Many mechanisms of protein function contribute to amyloidogenesis, including “nonphysiologic proteolysis, defective or absent physiologic proteolysis, mutations involving changes in thermodynamic or kinetic properties, and pathways that are yet to be defined” 3.
Amyloidosis Classification
Amyloid is now classified chemically. The amyloidoses are referred to with a capital A (for amyloid) followed by an abbreviation for the fibril protein. For example, in most cases formerly called primary amyloidosis and in myeloma-associated amyloidosis, the fibril protein is an immunoglobulin light chain or light chain fragment (abbreviated L); thus, patients with these amyloidoses are now said to have light chain amyloidosis (AL). Names such as AL describe the protein (light chain), but not necessarily the clinical phenotype 4.
Similarly, in most cases previously termed senile cardiac amyloidosis and in many cases previously termed familial amyloid polyneuropathy (FAP), the fibrils consist of the transport protein transthyretin (TTR); these diseases are now collectively termed ATTR.
Proteins that form amyloid fibrils differ in size, function, amino acid sequence, and native structure but become insoluble aggregates that are similar in structure and properties. Protein misfolding results in the formation of fibrils that show a common beta-sheet pattern on x-ray diffraction.
In theory, misfolded amyloid proteins can be attributed to infectious sources (prions), de novo gene mutations, errors in transcription, errors in translation, errors in post-translational modification, or protein transport. For example, in ATTR, 100 different points of single mutations, double mutations, or deletions in the TTR gene and several different phenotypes of FAP have been documented 5. Twenty-three different fibril proteins are described in human amyloidosis, with variable clinical features.
The major types of human amyloid are outlined and discussed individually in the table below. Great importance is now placed on appropriate and timely typing and classification of each type of amyloid-related disease, to focus therapy and plan appropriate monitoring 6. The common clinical amyloid entities are AL, AA, ATTR, and Aβ2M types 6.
Table 1. Human Amyloidoses Classification
| Type | Fibril Protein | Main Clinical Settings |
| Systemic | Immunoglobulin light chains | Plasma cell disorders |
| Transthyretin | Familial amyloidosis, senile cardiac amyloidosis | |
| A amyloidosis | Inflammation-associated amyloidosis, familial Mediterranean fever | |
| Beta2 -microglobulin | Dialysis-associated amyloidosis | |
| Immunoglobulin heavy chains | Systemic amyloidosis | |
| Hereditary | Fibrinogen alpha chain | Familial systemic amyloidosis |
| Apolipoprotein AI | Familial systemic amyloidosis | |
| Apolipoprotein AII | Familial systemic amyloidosis | |
| Lysozyme | Familial systemic amyloidosis | |
| Central nervous system | Beta protein precursor | Alzheimer syndrome, Down syndrome, hereditary cerebral hemorrhage with amyloidosis (Dutch) |
| Prion protein | Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease, fatal familial insomnia, kuru | |
| Cystatin C | Hereditary cerebral hemorrhage with amyloidosis (Icelandic) | |
| ABri precursor protein | Familial dementia (British) | |
| ADan precursor protein | Familial dementia (Danish) | |
| Ocular | Gelsolin | Familial amyloidosis (Finnish) |
| Lactoferrin | Familial corneal amyloidosis | |
| Keratoepithelin | Familial corneal dystrophies | |
| Localized | Calcitonin | Medullary thyroid carcinoma |
| Amylin* | Insulinoma, type 2 diabetes | |
| Atrial natriuretic factor amyloidosis | Isolated atrial amyloidosis | |
| Prolactin | Pituitary amyloid | |
| Keratin | Cutaneous amyloidosis | |
| Medin | Aortic amyloidosis in elderly people | |
| *Islet amyloid polypeptide amyloidosis. |
Systemic Amyloidoses
A Amyloidosis (AA)
The precursor protein is a normal-sequence apo-SAA (serum amyloid A protein) now called “A”, which is an acute phase reactant produced mainly in the liver in response to multiple cytokines 2. “A” protein circulates in the serum bound to high-density lipoprotein.
AA occurs in various chronic inflammatory disorders, chronic local or systemic microbial infections, and occasionally with neoplasms; it was formerly termed secondary amyloidosis.. Worldwide, AA is the most common systemic amyloidosis, although the frequency has been shown to vary significantly in different ethnic groups 8. Typical organs involved include the kidney, liver, and spleen.
Some of the conditions associated with A Amyloidosis (AA) include the following:
- Rheumatoid arthritis (RA) 9
- Alzheimer disease 10
- Multiple myeloma 11
- Juvenile idiopathic arthritis 12
- Ankylosing spondylitis 13
- Psoriasis and psoriatic arthritis 14
- Still disease 15
- Behçet syndrome 16
- Familial Mediterranean fever 17
- Crohn disease 18
- Leprosy 19
- Osteomyelitis 20
- Tuberculosis 21
- Chronic bronchiectasis 22
- Castleman disease 23
- Hodgkin disease and non-Hodgkin lymphoma 24
- Renal cell carcinoma 25
- Gastrointestinal, lung, or urogenital carcinoma 26
- Cryopyrin-associated periodic syndromes 27
Therapy has traditionally been aimed at the underlying inflammatory condition to reduce the production of the precursor amyloid protein, SAA. Disease-modifying antirheumatic drugs (DMARDs) such as colchicine, a microtubule inhibitor and weak immunosuppressant, can prevent secondary renal failure due to amyloid deposition specifically in familial Mediterranean fever.
Newer therapies have become more targeted to avoid the cytotoxicity of older agents (eg, chlorambucil, cyclophosphamide). The SAA amyloid seen in Cryopyrin-associated periodic syndromes was reduced with a biologic interleukin (IL)–1β trap called rilonacept.
Tumor necrosis factor–alpha (TNF-alpha) is also thought to be involved in amyloid deposition 28. Aggressive use of newer biologic therapies for RA, such as etanercept (a TNF-alpha blocker), and tocilizumab 29 have been used to decrease the concentration of SAA, serum creatinine, creatinine clearance, and proteinuria in renal AA associated with rheumatoid arthritis 30. One study compared etanercept with tocilizumab and showed that IL-6 inhibition may be a more effective therapy 31.
Additionally, SAA isoforms have been studied using high-resolution 2-dimensional gel electrophoresis and peptide mapping by reverse-phase chromatography, electrospray ionization tandem mass spectrometry, and genetic analysis down to the post-translational modification level. SAA is coded by 4 genes: SAA1, AAA2, SAA3, and SAA4. The SAA1 gene contributes to most of the deposits and contains a single nucleotide polymorphism that defines at least 3 haplotypes. The saa1.3 allele was found to be a risk factor and a poor prognostic indicator in Japanese RA patients. Genetic analysis has proved useful in selecting patients for biologic therapy and for predicting outcome 32. Early treatment is essential to prevent the long-term sequelae of AA 33.
AL Amyloidosis (Light chain amyloidosis or AL)
The precursor protein is a clonal immunoglobulin light chain or light chain fragment. Light chain amyloidosis is a monoclonal plasma cell disorder closely related to multiple myeloma, as some patients fulfill diagnostic criteria for multiple myeloma. Typical organs involved include the heart, kidney, peripheral nervous system, gastrointestinal tract, respiratory tract, and nearly any other organ. Light chain amyloidosis includes former designations of primary amyloidosis and myeloma-associated amyloidosis.
Treatment usually mirrors the management of multiple myeloma (ie, chemotherapy). Selected patients have received benefit from high-dose melphalan and autologous stem-cell transplantation, with reports of prolonged survival in some studies.
The most current guidelines recommend high-dose steroids for isolated organ involvement, but transplantation should be considered early 34. Any transplantation should also be followed by high-dose intravenous melphalan supported with stem-cell transplantation to try to prevent future amyloid deposition in the transplanted organ 34.
Other agents used in light chain amyloidosis have included bortezomib, rituximab, immunomodulatory agents, and standard-dose alkylating agents (eg, melphalan, cyclophosphamide), thalidomide, and lenalidomide 35. Bortezomib is a proteasome inhibitor that is well tolerated in multiple myeloma 36. Patients younger than 65 years may be candidates for stem cell transplantation with melphalan and dexamethasone or thalidomide-cyclophosphamide-dexamethasone regimens 34.
The response is then classified as either partial, complete, or no response. If a partial or complete response is achieved, patients are closely monitored 34. Imaging and some biomarkers like N-terminal pro-brain natriuretic peptide (NT-proBNP), B-type natriuretic peptide (BNP), troponin, and free light-chain concentration can be useful in gauging clinical response, especially in cardiac amyloid disease 37. If no response is seen, the patient becomes a candidate for novel agents that usually include alkylating agents combined with novel agents such as lenalidomide 38.
Heavy chain amyloidosis (AH amyloidosis)
In a few cases, immunoglobulin chain amyloidosis fibrils contain only heavy-chain sequences rather than light-chain sequences, and the disease is termed AH amyloidosis rather than AL amyloidosis. Electron microscopy may be helpful in the detection of small deposits and in the differentiation of amyloid from other types of renal fibrillar deposits 39.
Transthyretin amyloidosis
The precursor protein is the normal- or mutant-sequence transport protein transthyretin (TTR), a transport protein synthesized in the liver and choroid plexus. TTR is a tetramer of 4 identical subunits of 127 amino acids each. Normal-sequence transport protein transthyretin forms amyloid deposits in the cardiac ventricles of elderly people (ie, >70 y); this disease was also termed senile cardiac amyloidosis. The prevalence of transport protein transthyretin cardiac amyloidosis increases progressively with age, affecting 25% or more of the population older than 90 years. Normal-sequence transport protein transthyretin amyloidosis (ATTR) can be an incidental autopsy finding, or it can cause clinical symptoms (eg, heart failure, arrhythmias) 40.
Point mutations in transport protein transthyretin increase the tendency of transport protein transthyretin to form amyloid. Amyloidogenic transport protein transthyretin mutations are inherited as an autosomal dominant disease with variable penetrance. More than 100 amyloidogenic transport protein transthyretin mutations are known, but many remain unknown 41. The most prevalent TTR mutations are TTR Val30Met (common in Portugal, Japan, and Sweden), and TTR Val122Ile (carried by 3.9% of African Americans).
Amyloidogenic transport protein transthyretin mutations cause deposits primarily in the peripheral nerves, heart, gastrointestinal tract, and vitreous. The presentation of ATTR is fairly nonspecific, but signs and symptoms typical of chronic heart failure, polyneuropathy, and carpal tunnel syndrome occur commonly 42.
Treatment for mutant-sequence amyloidogenic ATTR is liver transplantation or supportive care. Liver transplantation should be performed in Val30Met patients as early as possible as it removes the main source of mutant transport protein transthyretin and dramatically reduces the progression of neuropathy (up to 70%) and can double the median survival. The US Food and Drug Administration (FDA) has not approved any pharmacological treatments for ATTR, but tafamidis, diflunisal, patisiran, revusiran, and tolcapone have demonstrated some benefit. For normal-sequence amyloidogenic ATTR, the treatment is supportive care.
Beta2-microglobulin amyloidosis ( Abeta2M)
The precursor protein is a normal beta2 -microglobulin (β2 M), which is the light-chain component of the major histocompatibility complex (MHC). In the clinical setting, β2 M is associated with patients on dialysis and, rarely, patients with renal failure who are not on dialysis.
β2 M is normally catabolized in the kidney after it is displaced from the MHC-I heavy chain in the proximal tubules, but in patients with decreased clearance the serum level of the β2 M can be more than 60 times the normal level 43. In patients with renal failure, the protein accumulates in the serum, leading to secondary osteoarticular destruction and dialysis-related amyloidosis (DRA) 44. Aβ2 M commonly is associated with deposits in the carpal ligaments, synovium, and bone, resulting in carpal tunnel syndrome, destructive arthropathy, bone cysts, and fractures. Other organs involved include the heart, gastrointestinal tract, liver, lungs, prostate, adrenals, and tongue.
Conventional dialysis membranes do not remove β2 M, but new techniques are now being used to improve hemodialysis β2 M removal. Traut et al 45 reported that patients using polyamide high-flux membranes had lower β2 M concentrations compared with those patients on low-flux dialyzers. They postulated that the difference was mediated by an increase in β2 M mRNA, lower concentrations of β2 M released from the blood cells, and or better β2 M clearance in patients treated with high-flux dialyzers 45. Yamamoto et al 46 have investigated Lixelle adsorbent columns that remove serum β2 M safely in dialysis patients and significantly improved quality of life, strength, C-reactive protein levels, and β2 M concentration.
Treatment also includes renal transplantation, which may arrest amyloid progression. For details, see Dialysis-Related Beta-2m Amyloidosis.
Cryopyrin-associated periodic syndrome (CAPS)-associated amyloidosis
Types of CAPS include the following:
- Familial cold autoinflammatory syndrome (FCAS)
- Muckle-Wells syndrome (MWS)
- Neonatal-onset multisystem inflammatory disease (NOMID)
These disorders are typically associated with heterozygous mutations in the NLRP3 (CIAS1) gene, which encodes the cryopyrin (NALP3) protein, and are inherited in an autosomal dominant manner 47. The inflammation in CAPS is driven by excessive release of interleukin (IL)–1β. [51] IL-1β release is normally regulated by an intracellular protein complex known as the inflammasome that maps to a gene sequence called NLRP3. Mutations in NLRP3 may cause an aberrant cryopyrin protein inside the inflammasome, leading to the release of too much IL-1β and subsequent multisystem inflammation.
Secondary to increased IL-1β, CAPS patients have chronically elevated levels of acute-phase reactants, especially serum amyloid A (SAA) and high sensitivity C-reactive protein (hsCRP) 48. With elevated SAA coupled with multisystem cytokine dysregulation, multisystem amyloid deposition can be severe, with the most feared complication being renal failure. By blocking the action of IL-1β or down-regulating its production, inflammation and therefore amyloid deposition can be reduced 49.
In a randomized double-blind CAPS therapy trial, the soluble decoy receptor rilonacept was shown to provide rapid and profound symptom improvement, in addition to improvement in measures of inflammation such as hsCRP and SAA levels 49. In the second part of the study, continued treatment with rilonacept maintained improvements, where disease activity worsened with discontinuation of the drug 49.
Canakinumab is a competing human immunoglobulin G (IgG) monoclonal antibody that also targets IL-1β and has shown efficacy in autoinflammatory conditions that, if untreated, are fatal by age 20 years in about 20% of individuals 27. Both agents have the potential to cause infections, but they are usually mild and treatable.
Muckle-Wells syndrome
Muckle-Wells syndrome is another autoinflammatory syndrome secondary to a mutation in the CIAS gene encoding cryopyrin, a component of the inflammasome that regulates the processing of IL-1β. Patients commonly experience sensorineural hearing loss and other neuropathies 50. The IL-1β receptor antagonist anakinra has been shown to improve the signs and symptoms in Muckle-Wells syndrome by decreasing serum (hsCRP and SAA) and cytokines such as IL-6, IL-8, IL-12, and IL-1β. In some cases, it improved sensory deafness, as well as the laboratory values for markers of inflammation Muckle-Wells syndrome 51.
Hereditary Renal Amyloidoses
Hereditary amyloidoses encompass a group of conditions that each are related to mutations in a specific protein. The most common form is transthyretin amyloidosis (usually neuropathic), but non-neuropathic amyloidoses are likely the result of abnormalities in lysozyme, fibrinogen, alpha-chain, or apolipoprotein A-I and A-II 52. Consider these diseases when a renal biopsy demonstrates amyloid deposition and when they are likely diagnoses (rather than light chain amyloidosis [AL] or A amyloidosis [AA]) because the family history suggests an autosomal dominant disease. Again, the definitive diagnosis is made using immunohistologic staining of the biopsy material with antibodies specific for the candidate amyloid precursor proteins. Clinical correlation is required to diagnose amyloid types, even if a hereditary form is detected by amyloid protein typing 53.
Apolipoprotein AI amyloidosis (apoAI) is an autosomal dominant amyloidosis caused by point mutations in the apoAI gene. Usually, this amyloidosis is a prominent renal amyloid but can also form in many locations. ApoAI (likely of normal sequence) is the fibril precursor in localized amyloid plaques in the aortae of elderly people. ApoAI can present either as a nonhereditary form with wild-type protein deposits in atherosclerotic plaques or as a hereditary form due to germline mutations in the apoA1 gene 54. Currently, more than 50 apoAI variants are known and 13 are associated with amyloidosis 54. As more gene locations are found, the clinical phenotypes are slowly being elucidated.
Fibrinogen amyloidosis (AFib) is an autosomal dominant amyloidosis caused by point mutations in the fibrinogen alpha chain gene. If DNA sequences indicate a mutant amyloid precursor protein, protein analysis of the deposits must provide the definitive evidence in laboratories with sophisticated methods 53.
Lysozyme amyloidosis (ALys) is an autosomal dominant amyloidosis caused by point mutations in the lysozyme gene.
Apolipoprotein AII amyloidosis (AapoAII) is an autosomal dominant amyloidosis caused by point mutations in the apoAII gene. The 2 kindreds described with this disorder have each carried a point mutation in the stop codon, leading to production of an abnormally long protein.
Central nervous system amyloidoses
Beta protein amyloid
The amyloid beta precursor protein (AβPP), which is a transmembrane glycoprotein, is the precursor protein in beta protein amyloid (A). Three distinct clinical settings are as follows:
- Alzheimer disease has a normal-sequence protein, except in some cases of familial Alzheimer disease, in which mutant beta protein is inherited in an autosomal dominant manner.
- Down syndrome has a normal-sequence protein that forms amyloids in most patients by the fifth decade of life.
- Hereditary cerebral hemorrhage with amyloidosis (HCHWA), Dutch type, is inherited in an autosomal dominant manner. The beta protein contains a point mutation. These patients typically present with cerebral hemorrhage followed by dementia.
The accumulation of amyloid-β peptide (Aβ) in the brain both in the form of plaques in the cerebral cortex and in blood vessel as cerebral amyloid angiopathy (CAA) causes progressive cognitive decline. Researchers have used immunization strategies to neutralize amyloid fibrils before deposition. Experimental models and human clinical trials have shown that accumulation of Aβ plaques can be reversed by immunotherapy. Aβ immunization results in solubilization of plaque Aβ42 which, at least in part, exits the brain via the perivascular pathway, causing a transient increase in the severity of CAA. The extent to which these vascular alterations following Aβ immunization in Alzheimer disease are reflected in changes in cognitive function remains to be determined 55.
Newer research has focused on finding a way to prevent the parent molecules from fragmenting and then aggregating to form toxic oligomers. Aβ peptide is known as a factor in the pathology of Alzheimer disease. Aβ aggregation is dependent on monomer concentration, nucleus formation, fibril elongation, and fibril fragmentation. Cellular, kinetic, and radiolabeling experiments have demonstrated that secondary nucleation occurs on the surface of only specific types of Aβ fibrils. The work focused on Aβ42, which could be a target molecule for future therapies to prevent nucleation events, oligomer formation rates, and neurotoxic effects 56.
Other target fibrils with secondary amyloidogenic nucleation potential are also being investigated across large-scale comparative data sets 57.
Prion protein amyloidosis (APrP)
The precursor protein in APrP is a prion protein, which is a plasma membrane glycoprotein. The etiology is either infectious (ie, kuru) and transmissible spongiform encephalitis (TSE) or genetic (ie, Creutzfeldt-Jakob disease [CJD], Gerstmann-Sträussler-Scheinker [GSS] syndrome, fatal familial insomnia [FFI]). The infectious prion protein is a homologous protein encoded by a host chromosomal gene, which induces a conformational change in a native protease-sensitive protein, increasing the content of beta-pleated sheets. The accumulation of these beta-pleated sheets renders the protein protease-resistant and therefore amyloidogenic 58. Patients with TSE, CJD, GSS, and FFI carry autosomal dominant amyloidogenic mutations in the prion protein gene; therefore, the amyloidosis forms even in the absence of an infectious trigger.
Similar infectious animal disorders include scrapie in sheep and goats and bovine spongiform encephalitis (ie, mad cow disease).
Cystatin C amyloidosis
The precursor protein in cystatin C amyloidosis (ACys) is cystatin C, which is a cysteine protease inhibitor that contains a point mutation. This condition is clinically termed Hereditary cerebral hemorrhage with amyloidosis (HCHWA), Icelandic type.
ACys is autosomal dominant. Clinical presentation includes multiple strokes and mental status changes beginning in the second or third decade of life. Many of the patients die by age 40 years. This disease is documented in a 7-generation pedigree in northwest Iceland. The pathogenesis is one of mutant cystatin that is widely distributed in tissues, but fibrils form only in the cerebral vessels; therefore, local conditions must play a role in fibril formation.
Non-amyloid beta cerebral amyloidosis (chromosome 13 dementias)
Two syndromes (British and Danish familial dementia) that share many aspects of clinical Alzheimer disease have been identified. Findings include the presence of neurofibrillary tangles, parenchymal preamyloid and amyloid deposits, cerebral amyloid angiopathy, and amyloid-associated proteins. Both conditions have been linked to specific mutations on chromosome 13; they cause abnormally long protein products (ABri and ADan) that ultimately result in different amyloid fibrils.
Other localized amyloidoses
Gelsolin amyloidosis
This disorders was first reported in 1969 and found to be heritable in a Finnish family in an autosomal dominant fashion. A flurry of research uncovered the molecular dysfunction of the disease but a treatment approach has yet to be devised. Gelsolin amyloidosis has now been described in countries all over the world and is often undiagnosed or misdiagnosed 59.
Amyloid fibrils include a gelsolin fragment that contains a point mutation. Two amyloidogenic gelsolin mutations are described. “A G654A or G654T DNA mutation in the gelsolin coding area (q32–34) of chromosome 9, which changes an aspartate at position 187 in the gelsolin protein to an asparagine or tyrosine (D187N/Y) residue respectively. These mutations lead to gelsolin fragment formation and amyloidogenesis” 59.
The precursor protein in gelsolin amyloidosis (AGel) is the ubiquitous actin-modulating protein gelsolin, but the mutated form lacks a crucial calcium binding site, allowing it to unfold and expose the amino acid chain to proteolysis while processed in the Golgi and again after exocytosis, forming an 8- and 5-kd amyloidogenic fragment 60. When the gelsolin gene is transcribed, some is spliced to form cytoplasm and some is secreted from the cell, but only the secreted forms deposit as amyloid secondary to faulty post-translational modification of gelsolin 61. Mass spectrometric-based proteomic analysis and immunohistochemical staining can reliably differentiate amyloid as gelsolin type 62.
Most early symptoms are ocular; thus, the ophthalmologist is crucial in early diagnosis. Clinical characteristics include slowly progressive cranial neuropathies, distal peripheral neuropathy, and lattice corneal dystrophy. Gelsolin gene sequencing is the only confirmatory test, but tissue samples can also be stained with tagged, commercially available gelsolin antibodies to provide a solid pathology diagnosis 63. An extremely detailed review of this condition, along with the recent scientific developments, was published by Solomon et al in 2012 59.
Atrial natriuretic factor amyloidosis
The precursor protein is atrial natriuretic factor (ANF), a hormone controlling salt and water homeostasis that is synthesized by the cardiac atria. Amyloid deposits are localized to the cardiac atria. This condition is highly prevalent in elderly people and generally is of little clinical significance. ANF amyloidosis (AANF) is most common in patients with long-standing congestive heart failure, presumably because of persistent ANF production. Proper diagnosis requires extensive testing, including paraffin blocks of tissue for molecular typing by liquid chromatography-tandem mass spectrometry and observation of Congo red staining on affected atrial tissues 64. No known relation exists to the amyloidoses that involve the cardiac ventricles (ie, AL, ATTR).
Keratoepithelin amyloidosis and lactoferrin amyloidosis
Point mutations occur in a gene termed BIGH3, which encodes keratoepithelin and leads to autosomal dominant corneal dystrophies characterized by the accumulation of corneal amyloid. Some BIGH3 mutations cause amyloid deposits, and others cause nonfibrillar corneal deposits. Another protein, lactoferrin, is also reported as the major fibril protein in familial subepithelial corneal amyloidosis. The relationship between keratoepithelin and lactoferrin in familial corneal amyloidosis is not yet clear.
Calcitonin amyloid
In calcitonin amyloid (ACal), the precursor protein is calcitonin, a calcium regulatory hormone synthesized by the thyroid. Patients with medullary carcinoma of the thyroid may develop localized amyloid deposition in the tumors, consisting of normal-sequence procalcitonin (ACal). The presumed pathogenesis is increased local calcitonin production, leading to a sufficiently high local concentration of the peptide and causing polymerization and fibril formation.
Islet amyloid polypeptide amyloidosis
In islet amyloid polypeptide amyloidosis (AIAPP), the precursor protein is an islet amyloid polypeptide (IAPP), also known as amylin. IAPP is a protein secreted by the islet beta cells that are stored with insulin in the secretory granules and released in concert with insulin. Normally, IAPP modulates insulin activity in skeletal muscle, influencing energy homeostasis, satiety, blood glucose levels, adiposity, and even body weight 65. IAPP amyloid is found in insulinomas and in the pancreas of many patients with diabetes mellitus type 2 (DM2). IAPP could be toxic at the competitive or non-competitive level. IAPP analogs are now being investigated in the treatment of DM2 and obesity 65.
Prolactin amyloid
In prolactin amyloid (Apro), prolactin or prolactin fragments are found in the pituitary amyloid. This condition is often observed in elderly people and has also been reported in an amyloidoma in a patient with a prolactin-producing pituitary tumor. The spherical amyloid deposits have a characteristic MRI appearance that differs from common pituitary adenomas 66.
Keratin amyloid
Some forms of cutaneous amyloid react with antikeratin antibodies. The identity of the fibrils is not chemically confirmed in keratin amyloid (Aker). Aker is often described as primary cutaneous amyloidosis and is extremely rare 67.
Medin amyloid
Aortic medial amyloid occurs in most people older than 60 years. Medin amyloid (AMed) is derived from a proteolytic fragment of lactadherin, a glycoprotein expressed by mammary epithelium.
Nonfibrillar Components of Amyloid
All types of amyloid deposits contain not only the major fibrillar component (solubility in water, buffers of low ionic strength), but also nonfibrillar components that are soluble in conventional ionic-strength buffers. The role of the minor components in amyloid deposition is not clear. These components do not appear to be absolutely required for fibril formation, but they may enhance fibril formation or stabilize formed fibrils.
The nonfibrillar components, contained in all types of amyloid, are discussed below. However, note that other components found in some types of amyloid include complement components, proteases, and membrane constituents.
Pentagonal component
Pentagonal (P) component comprises approximately 5% of the total protein in amyloid deposits. This component is derived from the circulating serum amyloid P (SAP) component, which behaves as an acute-phase reactant. The P component is one of the pentraxin group of proteins, with homology to C-reactive protein. In experimental animals, amyloid deposition is slowed without the P component.
Radiolabeled material homes to amyloid deposits; therefore, this component can be used in amyloid scans to localize and quantify amyloidosis and to monitor therapy response. Radiolabeled P component scanning has proven clinically useful in England, where the technology was developed, but it is available in only a few centers worldwide.
Apolipoprotein E
Apolipoprotein E (apoE) is found in all types of amyloid deposits.
One allele, ApoE4, increases the risk for beta protein deposition, which is associated with Alzheimer disease. ApoE4 as a risk factor for other forms of amyloidosis is controversial.
The role of apoE in amyloid formation is not known.
Glycosaminoglycans (GAGs)
GAGs are heteropolysaccharides composed of long, unbranched polysaccharides that contain a repeating disaccharide unit. These proteoglycans are basement membrane components intimately associated with all types of tissue amyloid deposits. Amyloidotic organs contain increased amounts of GAGs, which may be tightly bound to amyloid fibrils. Heparan sulfate and dermatan sulfate are the GAGs most often associated with amyloidosis.
Heparan sulfate and dermatan sulfate have an unknown role in amyloidogenesis. Studies of A amyloidosis (AA) and light chain amyloidosis (AL) amyloid have shown marked restriction of the heterogeneity of the glycosaminoglycan chains, suggesting that particular subclasses of heparan and dermatan sulfates are involved.
Compounds that bind to heparan sulfate proteoglycans (eg, anionic sulfonates) decrease fibril deposition in murine models of AA and have been suggested as potential therapeutic agents.
Mechanisms of Amyloid Formation
Amyloid protein structures
In all forms of amyloidosis, the cell secretes the precursor protein in a soluble form that becomes insoluble at some tissue site, compromising organ function. All the amyloid precursor proteins are relatively small (ie, molecular weights 4000-25,000) and do not share any amino acid sequence homology. The secondary protein structures of most soluble precursor proteins (except for SAA and chromosomal prion protein [Prpc]) have substantial beta-pleated sheet structure, while extensive beta-sheet structure occurs in all of the deposited fibrils.
In some cases, hereditary abnormalities (primarily point mutations or polymorphisms) in the precursor proteins are present (eg, lysozyme, fibrinogen, cystatin C, gelsolin). In other cases, fibrils form from normal-sequence molecules (eg, AL, β2 M). In other cases, normal-sequence proteins can form amyloid, but mutations underlying inflammatory milieu accelerate the process (eg, TTR, beta protein precursor or CAPS).
Deposition location
In localized amyloidoses, the deposits form close to the precursor synthesis site; however, in systemic amyloidoses, the deposits may form either locally or at a distance from the precursor-producing cells. Amyloid deposits primarily are extracellular, but reports exist of fibrillar structures within macrophages and plasma cells.
Proteolysis and protein fragments
In some types of amyloidosis (eg, always in AA, often in AL and ATTR), the amyloid precursors undergo proteolysis, which may enhance folding into an amyloidogenic structural intermediate. In addition, some of the amyloidoses may have a normal proteolytic process that is disturbed, yielding a high concentration of an amyloidogenic intermediate. For example, it was shown that the mast cells of allergic responses may also participate in the development of secondary or amyloid AA in chronic inflammatory conditions. Mast cells hasten the partial degradation of the SAA protein that can produce highly amyloidogenic N-terminal fragments of SAA. However, factors that lead to different organ tropisms for the different amyloidoses are still largely unknown.
Whether the proteolysis occurs before or after tissue deposition is unclear in patients in whom beta protein fragments are observed in tissue deposits. In some types of amyloid (eg, AL, Aβ, ATTR), nonfibrillar forms of the same molecules can accumulate before fibril formation; thus, nonfibrillar deposits, in some cases, may represent intermediate deposition.
Amyloidosis symptoms
You may not experience signs and symptoms of amyloidosis until the condition is advanced. When signs and symptoms are evident, they depend on which of your organs are affected.
Signs and symptoms of amyloidosis may include:
- Swelling of your ankles and legs
- Severe fatigue and weakness
- Shortness of breath
- Numbness, tingling or pain in your hands or feet, especially pain in your wrist (carpal tunnel syndrome)
- Diarrhea, possibly with blood, or constipation
- Unintentional, significant weight loss
- An enlarged tongue
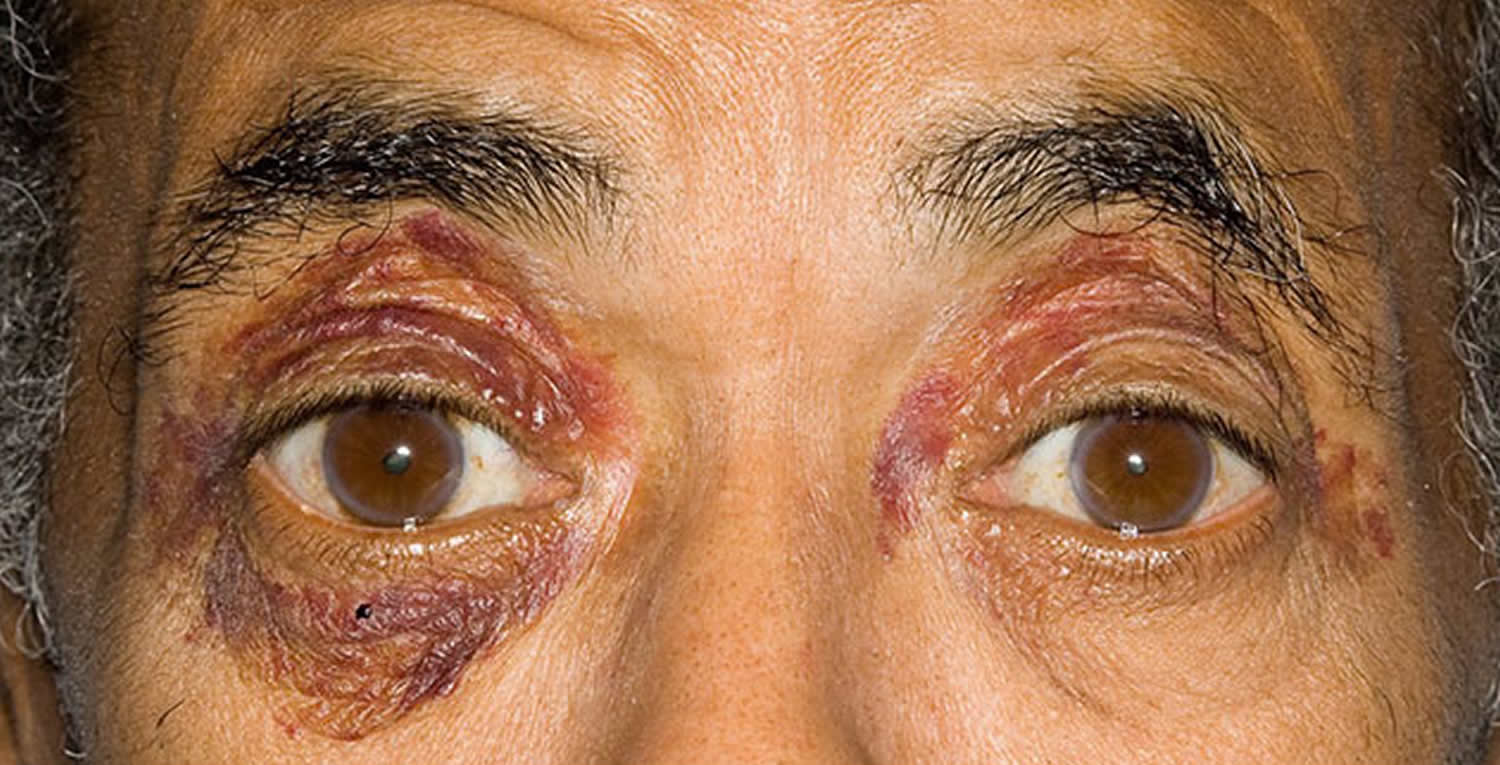
- Skin changes, such as thickening or easy bruising, and purplish patches around the eyes
- An irregular heartbeat
- Difficulty swallowing
Amyloidosis causes
In general, amyloidosis is caused by the buildup of an abnormal protein called amyloid. Amyloid is produced in your bone marrow and can be deposited in any tissue or organ. The specific cause of your condition depends on the type of amyloidosis you have.
There are several types of amyloidosis, including:
- AL amyloidosis (immunoglobulin light chain amyloidosis) is the most common type and can affect your heart, kidneys, skin, nerves and liver. Previously known as primary amyloidosis, AL amyloidosis occurs when your bone marrow produces abnormal antibodies that can’t be broken down. The antibodies are deposited in your tissues as amyloid, interfering with normal function.
- AA amyloidosis mostly affects your kidneys but occasionally your digestive tract, liver or heart. It was previously known as secondary amyloidosis. It occurs along with chronic infectious or inflammatory diseases, such as rheumatoid arthritis or inflammatory bowel disease.
- Hereditary amyloidosis (familial amyloidosis) is an inherited disorder that often affects the liver, nerves, heart and kidneys. Many different types of gene abnormalities present at birth are associated with an increased risk of amyloid disease. The type and location of an amyloid gene abnormality can affect the risk of certain complications, the age at which symptoms first appear, and the way the disease progresses over time.
- Dialysis-related amyloidosis develops when proteins in blood are deposited in joints and tendons — causing pain, stiffness and fluid in the joints, as well as carpal tunnel syndrome. This type generally affects people on long-term dialysis.
Risk factors for amyloidosis
Anyone can develop amyloidosis. Factors that increase your risk include:
- Age. Most people diagnosed with AL amyloidosis, the most common type, are between ages 60 and 70, although earlier onset occurs.
- Sex. Nearly 70 percent of people with AL amyloidosis are men.
- Other diseases. Having a chronic infectious or inflammatory disease increases your risk of AA amyloidosis.
- Family history. Some types of amyloidosis are hereditary.
- Kidney dialysis. Dialysis can’t always remove large proteins from the blood. If you’re on dialysis, abnormal proteins can build up in your blood and eventually be deposited in tissue. This condition is less common with modern dialysis techniques.
- Race. People of African descent appear to be at higher risk of carrying a genetic mutation associated with the type of amyloidosis that can harm the heart.
Complications of amyloidosis
The potential complications of amyloidosis depend on which organs the amyloid deposits affect. Amyloidosis can seriously damage your:
- Kidneys. Amyloid can harm the kidneys’ filtering system, causing protein to leak from your blood into your urine. The kidneys’ ability to remove waste products from your body is lowered, which may eventually lead to kidney failure.
- Heart. Amyloid reduces your heart’s ability to fill with blood between heartbeats. Less blood is pumped with each beat, and you may experience shortness of breath. If amyloidosis affects your heart’s electrical system, your heart rhythm may be disturbed.
- Nervous system. You may experience pain, numbness or tingling of the fingers or numbness, lack of feeling or a burning sensation in your toes or the soles of your feet. If amyloid affects the nerves that control your bowel function, you may experience periods of alternating constipation and diarrhea.
If the condition affects nerves that control blood pressure, you may experience dizziness or near fainting when standing too quickly.
Diagnosis of Amyloidosis
Amyloidosis is often overlooked because the signs and symptoms can mimic those of more-common diseases. Diagnosis as early as possible can help prevent further organ damage. Precise diagnosis is important because treatment varies greatly, depending on your specific condition.
Your doctor is likely to start with a thorough medical history and physical exam. After that, you may have:
- Laboratory tests. Your blood and urine may be analyzed for abnormal protein that can indicate amyloidosis. Depending on your signs and symptoms, you may also have thyroid and liver function tests.
- Biopsy. A tissue sample may be taken and checked for signs of amyloidosis. The biopsy may be taken from your abdominal fat, bone marrow, or an organ such as your liver or kidney. Tissue analysis can help determine the type of amyloid deposit.
- Imaging tests. Images of the organs affected by amyloidosis can help establish the extent of your disease. Echocardiogram may be used to assess the size and functioning of your heart. Other imaging tests can evaluate the extent of amyloidosis in your liver or spleen.
Amyloidosis is diagnosed when Congo red–binding material is demonstrated in a biopsy specimen. For example, in cardiac amyloidosis, the definitive diagnosis of the type of amyloid can be made using an endomyocardial biopsy specimen, with Congo red and immunologic staining of the tissue sample. Alternatively, when noninvasive testing suggests cardiac amyloidosis, studying a subcutaneous fat aspiration instead of endomyocardial biopsy, thereby avoiding an invasive procedure, often provides a specific diagnosis. Because different types of amyloidosis require different approaches to treatment, determining only that a patient has a diagnosis of amyloidosis is no longer adequate. A clinical situation may suggest the type of amyloidosis, but the diagnosis generally must be confirmed by immunostaining a biopsy specimen. Antibodies against the major amyloid fibril precursors are commercially available. For example, AL, ATTR, and Aβ2 M can present as carpal tunnel syndrome or gastrointestinal amyloidosis, but each has a different etiology and requires a different treatment approach.
Similarly, determining whether the amyloid is of the AL or ATTR type is often difficult in patients with cardiac amyloidosis, because the clinical picture usually is similar. Without immunostaining to identify the type of deposited protein, an incorrect diagnosis can lead to ineffective and, perhaps, harmful treatment. Be wary of drawing diagnostic conclusions from indirect tests (eg, monoclonal serum proteins) because the results of these presumptive diagnostic tests can be misleading; for example, monoclonal serum immunoglobulins are common in patients older than 70 years, but the most common form of cardiac amyloidosis is derived from TTR.
New adjuncts to traditional laboratory testing are now available. The serum free light chain test is commercially available and can aid in screening, diagnosis, prognosis, and treatment monitoring by detecting detects low concentrations of free light chains and can measure the ratio of kappa chains to lambda chains 68.
Organ biopsies
It is important to recognize that not all biopsy sites offer the same sensitivity. The best sites from which to obtain a biopsy specimen in systemic amyloidosis are the abdominal fat pad and rectal mucosa (approaching 90% sensitivity for fat pad and 73-84% for rectal mucosa) 69. While some imaging modalities can strongly suggest amyloidosis, a tissue sample showing birefringent material is still the criterion standard. This is definitely the case in terms of cardiac specific amyloid. While, endomyocardial biopsy is the best confirmatory test for local cardiac amyloid deposition, it can be very risk averse and requires a center of excellence with the full complement of immunohistochemical and molecular-based testing 70.
When the subcutaneous fat aspiration biopsy (the least invasive biopsy site) does not provide information to reach a firm diagnosis, biopsy specimens can be obtained from other organs. In addition, an advantage to performing a biopsy of an involved organ (eg, kidney, heart) is that it definitively establishes a cause-and-effect relationship between the organ dysfunction and amyloid deposition.
Other sites that are often sampled but have poor sensitivity for the diagnosis of amyloid include the salivary glands, skin, tongue, gingiva, stomach, and bone marrow. A recent study suggests that the duodenum may be the most sensitive biopsy site compared with the gingiva, esophagus, or gastric antrum in AA renal disease 71.
Treatment of Amyloidosis
There’s no cure for amyloidosis. But treatment can help manage signs and symptoms and limit further production of amyloid protein. Specific treatments depend on the type of amyloidosis and target the source of the amyloid production.
AL amyloidosis. Many of the same chemotherapy medications that treat multiple myeloma are used in AL amyloidosis to stop the growth of abnormal cells that produce amyloid.
Autologous blood stem cell transplant (ASCT) offers an additional treatment option in some cases. This procedure involves collecting your own stem cells from your blood and storing them for a short time while you have high-dose chemotherapy. The stem cells are then returned to your body via a vein.
ASCT is most appropriate for people whose disease isn’t advanced and whose heart isn’t greatly affected.
AA amyloidosis. Treatments target the underlying condition — for example, an anti-inflammatory medication to treat rheumatoid arthritis.
Hereditary amyloidosis. Liver transplantation may be an option because the protein that causes this form of amyloidosis is made in the liver.
Dialysis-related amyloidosis. Treatments include changing your mode of dialysis or having a kidney transplant. Beta-2m-adsorbing columns for dialysis – β2-m–adsorbing columns have demonstrated some benefit in dialysis-related amyloidosis. This addition to long-term dialysis may reduce inflammation and accumulation of fibrils without major adverse effects 72.
Doxycycline. A pilot study demonstrated reduction in arthralgia and increased range of motion with doxycycline treatment. The authors theorize that doxycycline inhibits β2-microglobulin fibrillogenesis and inhibits the accumulation in bone architecture. Their trial monitored long-term dialysis patients with severe amyloid arthropathy. Using a low dose of 100 mg of doxycycline daily, they were able to provide pain reduction, increased range of motion, and no adverse effects at 5 months 73.
Immunotherapy. Aβ immunotherapies have shown some promise in the treatment of Alzheimer disease. Agents with great potential include the passive monoclonal antibodies bapineuzumab 46 and solanezumab 74. However, both of these humanized monoclonal antibodies have failed to provide meaningful cognitive changes in clinical trials 75. Further studies using this strategy are ongoing.
Several other monoclonal antibodies to target Aβ deposition include “PF-04360365 (ponezumab), which targets the free C-terminus of Aβ, and specifically Aβ34–41; MABT5102A, which binds to Aβ monomers, oligomers, and fibrils with equally high affinity; GSK933776A, which, similar to bapineuzumab, targets the N-terminal sequence of Aβ; BAN2401, which targets Aβ protofibrils; and gantenerumab, which targets the N-terminus and central portion of Aβ” 76.
Gantenerumab
Gantenerumab is the first fully human monoclonal antibody that binds regions of Aβ configuration not present in the structure of the native monomeric Aβ. Therefore, gantenerumab preferentially binds to aggregated Aβ 77. Phase II and III clinical trials have shown disease-modifying potential in Alzheimer disease, with reductions in brain amyloid as measured by carbon 11 [11 C]–labeled Pittsburgh compound B PET but no meaningful neurocognitive improvements. It was very interesting that “live-cell imaging indicated that a clearance of fluorescent-labeled gantenerumab bound to amyloid deposits occurred in a dose-dependent manner within hours via active intracellular uptake by brain-activated microglia adjacent to amyloid plaques” 76. This approach may continue to yield more clinically significant results in the future.
Tafamidis
Transthyretin amyloidosis is related to abnormal conformational changes and transthyretin aggregation leading to severe neuropathy. After learning the structure and function of transthyretin, researchers developed a molecule and later a drug that could stabilized the native tetrameric state of transthyretin 78.
A phase II, open-label, single-treatment arm evaluated the pharmacodynamics, efficacy, and safety of this drug, called tafamidis, in patients with non-Val30Met transthyretin (TTR) amyloidosis. Twenty-one patients with 8 different non-Val30Met mutations received daily oral tafamidis, and many experienced improved quality of life measures, while pro-BNP and echocardiographic features improved 79. Another study showed that long-term use continued to stabilize TTR at 30 months. In a cross-over scheme, patients previously have given placebo were able to slow their neurologic decline, proving that earlier treatment is better 80. The success of tafamidis in a subset of amyloidosis is very encouraging for future molecular targets.
Tocilizumab
A subset of patients with amyloidosis (AA) in the setting of arthritis were monitored for benefits from the IL-6 receptor antibody tocilizumab. After a year of therapy with the standard regimen for rheumatoid arthritis (RA) (8 mg/kg 4 days per weeks) a significant reduction in renal dysfunction, serum AA concentration, and urinary protein secretion was observed in conjunction with reduced disease activity. These patients had been previously treated with etanercept without these benefits. This study again highlights the importance of treating the underlying condition, but certain biologic targets may have more downstream effects on amyloidogenesis.
Other
Other innovative medicines (RNA interference, antisense oligonucleotides) have been developed to block hepatic production of both mutant and wild type TTR (noxious in late-onset forms of NAH after age 50 y), and to remove amyloid deposits (monoclonal anti-SAP) 81. Clinical trials should first include patients with late-onset FAP or non-met30 TTR familial amyloid polyneuropathy who are less responsive to LT7 and patients in whom tafamidis is ineffective or inappropriate. Initial and periodic cardiac assessment is necessary, as cardiac impairment is inevitable and largely responsible for mortality. Symptomatic treatment is crucial to improve these patients’ quality of life.
Supportive care for Amyloidosis
To manage ongoing signs and symptoms of amyloidosis, your doctor also may recommend:
- Pain medication
- Fluid retention medication (diuretic) and a low-salt diet
- Blood-thinning medication
- Medication to control your heart rate.
For example, cardiac amyloidosis requires judicious use of rate and rhythm control agents to prevent fatal arrhythmias. Amyloid can infiltrate the conduction system and the coronary arteries 82. Clues to aid in early detection are the presence of cardiac arrhythmia with early heart failure evidenced by abnormal echocardiogram 83. Certain medications such as digoxin or calcium channel blockers are dangerous to the already-hindered conduction system in cardiac amyloid. In addition, higher doses of angiotensin-converting enzyme inhibitors can also foster dangerous hypotension and are not recommended 84. Beta-blockers are relatively contraindicated in cardiac amyloidosis and are associated with a higher death rate 85. Amiodarone (200 mg orally 5 days per week), however, prevents arrhythmias and should be considered first and continued indefinitely 86.
Lifestyle and home remedies for amyloidosis
These tips can help you live with amyloidosis:
- Pace yourself. If you feel short of breath, take a break. You’ll need to avoid strenuous activities, but you may be able to continue normal daily activities, such as going to work. Talk to your doctor about an appropriate level of activity for you.
- Eat a balanced diet. Good nutrition is important to provide your body with adequate energy. Follow a low-salt diet if your doctor recommends it.
Coping and support for amyloidosis
A diagnosis of amyloidosis can be extremely challenging. Here are some suggestions that may make dealing with amyloidosis easier:
- Find someone to talk with. You may feel comfortable discussing your feelings with a friend or family member, or you might prefer meeting with a formal support group.
- Set reasonable goals. Having goals helps you feel in control and can give you a sense of purpose. Choose goals you can reach.
- Amyloidosis & Kidney Disease. https://www.niddk.nih.gov/health-information/kidney-disease/amyloidosis[↩]
- Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, et al. A primer of amyloid nomenclature. Amyloid. 2007 Sep. 14(3):179-83.[↩][↩]
- Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004 Jan. 16(1):67-75.[↩][↩]
- Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, et al. A primer of amyloid nomenclature. Amyloid. 2007 Sep. 14(3):179-83.[↩]
- Ando Y, Ueda M. Novel methods for detecting amyloidogenic proteins in transthyretin related amyloidosis. Front Biosci. 2008. 13:5548-58.[↩]
- Hazenberg BP. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013 May. 39(2):323-45.[↩][↩]
- https://emedicine.medscape.com/article/335414-overview[↩]
- Buck FS, Koss MN, Sherrod AE, Wu A, Takahashi M. Ethnic distribution of amyloidosis: an autopsy study. Mod Pathol. 1989 Jul. 2(4):372-7.[↩]
- Shin JK, Jung YH, Bae MN, Baek IW, Kim KJ, Cho CS. Successful treatment of protein-losing enteropathy due to AA amyloidosis with octreotide in a patient with rheumatoid arthritis. Mod Rheumatol. 2013 Mar. 23(2):406-11.[↩]
- Tam JH, Pasternak SH. Amyloid and Alzheimer’s disease: inside and out. Can J Neurol Sci. 2012 May. 39(3):286-98.[↩]
- Basha HI, Raj E, Bachuwa G. Cardiac amyloidosis masquerading as biventricular hypertrophy in a patient with multiple myeloma. BMJ Case Rep. 2013 Jul 29. 2013[↩]
- Saha A, Chopra Y, Theis JD, Vrana JA, Sethi S. AA amyloidosis associated with systemic-onset juvenile idiopathic arthritis. Am J Kidney Dis. 2013 Oct. 62(4):834-8.[↩]
- Cammelli D. [Extra-articular manifestations of seronegative spondylarthritis]. Recenti Prog Med. 2006 May. 97(5):280-9.[↩]
- Bergis M, Dega H, Planquois V, Benichou O, Dubertret L. [Amyloidosis complicating psoriatic arthritis]. Ann Dermatol Venereol. 2003 Nov. 130(11):1039-42.[↩]
- Kishida D, Okuda Y, Onishi M, Takebayashi M, Matoba K, Jouyama K. Successful tocilizumab treatment in a patient with adult-onset Still’s disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol. 2011 Apr. 21(2):215-8.[↩]
- Erten S, Perçinel S, Olmez U, Ensari A, Düzgün N. Behçet’s disease associated with diarrhea and secondary amyloidosis. Turk J Gastroenterol. 2011 Feb. 22(1):106-7.[↩]
- Lane T, Loeffler JM, Rowczenio DM, Gilbertson JA, Bybee A, Russell TL. AA amyloidosis complicating the hereditary periodic fever syndromes. Arthritis Rheum. 2013 Apr. 65(4):1116-21.[↩]
- Denis MA, Cosyns JP, Persu A, Dewit O, de Galocsy C, Hoang P. Control of AA amyloidosis complicating Crohn’s disease: a clinico-pathological study. Eur J Clin Invest. 2013 Mar. 43(3):292-301.[↩]
- Silva Júnior GB, Barbosa OA, Barros Rde M, Carvalho Pdos R, Mendoza TR, Barreto DM. [Amyloidosis and end-stage renal disease associated with leprosy]. Rev Soc Bras Med Trop. 2010 Jul-Aug. 43(4):474-6.[↩]
- Neugebauer C, Graf R. [Expert opinion problems in the evaluation of osteomyelitis]. Orthopade. 2004 May. 33(5):603-11; quiz 612.[↩]
- Lekpa FK, Ndongo S, Pouye A, Tiendrebeogo JW, Ndao AC, Ka MM. [Amyloidosis in sub-Saharan Africa]. Med Sante Trop. 2012 Jul-Sep. 22(3):275-8.[↩]
- Akçay S, Akman B, Ozdemir H, Eyüboglu FO, Karacan O, Ozdemir N. Bronchiectasis-related amyloidosis as a cause of chronic renal failure. Ren Fail. 2002 Nov. 24(6):815-23.[↩]
- Caro-Cuenca MT, Ortega-Salas R, Espinosa-Hernández M. Renal AA amyloidosis in a Castleman’s disease patient. Nefrologia. 2012. 32(5):699-700.[↩]
- Caro-Cuenca MT, Ortega-Salas R, Espinosa-Hernández M. Renal AA amyloidosis in a Castleman’s disease patient. Blood. 2013 Jul 18. 122(3):458-9.[↩]
- Nobata H, Suga N, Itoh A, Miura N, Kitagawa W, Morita H, et al. Systemic AA amyloidosis in a patient with lung metastasis from renal cell carcinoma. Amyloid. 2012 Dec. 19(4):197-200.[↩]
- Rivera R, Kaul V, DeCross A, Whitney-Miller C. Primary gastric amyloidosis presenting as an isolated gastric mass. Gastrointest Endosc. 2012 Jul. 76(1):186-7.[↩]
- Yokota S, Kikuchi M, Nozawa T, Kizawa T, Kanetaka T, Miyamae T, et al. [An approach to the patients with cryopyrin-associated periodic syndrome (CAPS) : a new biologic response modifier, canakinumab]. Nihon Rinsho Meneki Gakkai Kaishi. 2012. 35(1):23-9.[↩][↩]
- Smith GR, Tymms KE, Falk M. Etanercept treatment of renal amyloidosis complicating rheumatoid arthritis. Intern Med J. 2004 Sep-Oct. 34(9-10):570-2.[↩]
- Miyagawa I, Nakayamada S, Saito K, Hanami K, Nawata M, Sawamukai N. Study on the safety and efficacy of tocilizumab, an anti-IL-6 receptor antibody, in patients with rheumatoid arthritis complicated with AA amyloidosis. Mod Rheumatol. 2013 Oct 21.[↩]
- Nakamura T, Higashi S, Tomoda K, Tsukano M, Baba S. Efficacy of etanercept in patients with AA amyloidosis secondary to rheumatoid arthritis. Clin Exp Rheumatol. 2007 Jul-Aug. 25(4):518-22.[↩]
- Okuda Y, Ohnishi M, Matoba K, Jouyama K, Yamada A, Sawada N, et al. Comparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseases. Mod Rheumatol. 2014 Jan. 24(1):137-43.[↩]
- Nakamura T. Clinical strategies for amyloid A amyloidosis secondary to rheumatoid arthritis. Mod Rheumatol. 2008. 18(2):109-18.[↩]
- Lane T, Loeffler JM, Rowczenio DM, Gilbertson JA, Bybee A, Russell TL, et al. AA amyloidosis complicating the hereditary periodic fever syndromes. Arthritis Rheum. 2013 Apr. 65(4):1116-21.[↩]
- Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011 May 10. 29(14):1924-33.[↩][↩][↩][↩]
- Sissoko M, Sanchorawala V, Seldin D, Sworder B, Angelino K, Broce M, et al. Clinical presentation and treatment responses in IgM-related AL amyloidosis. Amyloid. 2015. 22 (4):229-35.[↩]
- Kastritis E, Anagnostopoulos A, Roussou M, Toumanidis S, Pamboukas C, Migkou M, et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007 Oct. 92(10):1351-8.[↩]
- Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007 Jan 15. 109(2):457-64.[↩]
- Dispenzieri A, Gertz MA, Hayman SR, et al. A phase II study of pomalidomide and dexamethasone in previously treated light-chain (AL) amyloidosis. J Clin Oncol. 2010. 28:579s.[↩]
- Picken MM. Immunoglobulin light and heavy chain amyloidosis AL/AH: renal pathology and differential diagnosis. Contrib Nephrol. 2007. 153:135-55.[↩]
- Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al. Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study. Circulation. 2016 Jan 19. 133 (3):282-90.[↩]
- Planté-Bordeneuve V. [The diagnosis and management of familial amyloid polyneuropathy]. Rev Neurol (Paris). 2006 Nov. 162(11):1138-46.[↩]
- Adams D, Suhr OB, Hund E, Obici L, Tournev I, Campistol JM, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016 Feb. 29 Suppl 1:S14-26.[↩]
- Floege J, Bartsch A, Schulze M, Shaldon S, Koch KM, Smeby LC. Clearance and synthesis rates of beta 2-microglobulin in patients undergoing hemodialysis and in normal subjects. J Lab Clin Med. 1991 Aug. 118(2):153-65.[↩]
- Otsubo S, Kimata N, Okutsu I, Oshikawa K, Ueda S, Sugimoto H, et al. Characteristics of dialysis-related amyloidosis in patients on haemodialysis therapy for more than 30 years. Nephrol Dial Transplant. 2009 May. 24(5):1593-8.[↩]
- Traut M, Haufe CC, Eismann U, Deppisch RM, Stein G, Wolf G. Increased binding of beta-2-microglobulin to blood cells in dialysis patients treated with high-flux dialyzers compared with low-flux membranes contributed to reduced beta-2-microglobulin concentrations. Results of a cross-over study. Blood Purif. 2007. 25(5-6):432-40.[↩][↩]
- Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002 Oct. 3(10):824-8.[↩][↩]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001 Nov. 29(3):301-5.[↩]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006 Aug 10. 355(6):581-92.[↩]
- Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008 Aug. 58(8):2443-52.[↩][↩][↩]
- Stew BT, Fishpool SJ, Owens D, Quine S. Muckle-Wells syndrome: a treatable cause of congenital sensorineural hearing loss. B-ENT. 2013. 9(2):161-3.[↩]
- Yamazaki T, Masumoto J, Agematsu K, Sawai N, Kobayashi S, Shigemura T, et al. Anakinra improves sensory deafness in a Japanese patient with Muckle-Wells syndrome, possibly by inhibiting the cryopyrin inflammasome. Arthritis Rheum. 2008 Mar. 58(3):864-8.[↩]
- Granel B, Valleix S, Serratrice J, Chérin P, Texeira A, Disdier P, et al. Lysozyme amyloidosis: report of 4 cases and a review of the literature. Medicine (Baltimore). 2006 Jan. 85(1):66-73.[↩]
- Picken MM, Linke RP. Nephrotic syndrome due to an amyloidogenic mutation in fibrinogen A alpha chain. J Am Soc Nephrol. 2009 Aug. 20(8):1681-5.[↩][↩]
- Eriksson M, Schönland S, Yumlu S, Hegenbart U, von Hutten H, Gioeva Z, et al. Hereditary apolipoprotein AI-associated amyloidosis in surgical pathology specimens: identification of three novel mutations in the APOA1 gene. J Mol Diagn. 2009 May. 11(3):257-62.[↩][↩]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008 Dec. 131:3299-310.[↩]
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L. Proliferation of amyloid-ß42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A. 2013 Jun 11. 110(24):9758-63.[↩]
- Emily M, Talvas A, Delamarche C. MetAmyl: a METa-predictor for AMYLoid proteins. PLoS One. 2013. 8(11):e79722.[↩]
- Rezaei H. Prion protein oligomerization. Curr Alzheimer Res. 2008 Dec. 5(6):572-8.[↩]
- Solomon JP, Page LJ, Balch WE, Kelly JW. Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit Rev Biochem Mol Biol. 2012 May-Jun. 47(3):282-96.[↩][↩][↩]
- Chen CD, Huff ME, Matteson J, Page L, Phillips R, Kelly JW. Furin initiates gelsolin familial amyloidosis in the Golgi through a defect in Ca(2+) stabilization. EMBO J. 2001 Nov 15. 20(22):6277-87.[↩]
- Kangas H, Paunio T, Kalkkinen N, Jalanko A, Peltonen L. In vitro expression analysis shows that the secretory form of gelsolin is the sole source of amyloid in gelsolin-related amyloidosis. Hum Mol Genet. 1996 Sep. 5(9):1237-43.[↩]
- Ida CM, Yan X, Jentoft ME, Kip NS, Scheithauer BW, Morris JM. Pituicytoma with gelsolin amyloid deposition. Endocr Pathol. 2013 Sep. 24(3):149-55.[↩]
- Haverland N, Pottiez G, Wiederin J, Ciborowski P. Immunoreactivity of anti-gelsolin antibodies: implications for biomarker validation. J Transl Med. 2010 Dec 20. 8:137.[↩]
- Podduturi V, Armstrong DR, Hitchcock MA, Roberts WC, Guileyardo JM. Isolated atrial amyloidosis and the importance of molecular classification. Proc (Bayl Univ Med Cent). 2013 Oct. 26(4):387-9.[↩]
- Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013 Apr 17. 587(8):1119-27.[↩][↩]
- Levine SN, Ishaq S, Nanda A, Wilson JD, Gonzalez-Toledo E. Occurrence of extensive spherical amyloid deposits in a prolactin-secreting pituitary macroadenoma: a radiologic-pathologic correlation. Ann Diagn Pathol. 2013 Aug. 17(4):361-6.[↩]
- Wenson SF, Jessup CJ, Johnson MM, Cohen LM, Mahmoodi M. Primary cutaneous amyloidosis of the external ear: a clinicopathological and immunohistochemical study of 17 cases. J Cutan Pathol. 2012 Feb. 39(2):263-9.[↩]
- Rao M, Lamont JL, Chan J, Concannon TW, Comenzo R, Ratichek SJ, et al. Serum Free Light Chain Analysis for the Diagnosis, Management, and Prognosis of Plasma Cell Dyscrasias: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 73 [Internet]. 2012 Sep.[↩]
- West S, Singleton JD. Rheumatology Secrets. 2nd ed. Philadelpha, PA: Hanley and Belfus; 2003. 519.[↩]
- Thiene G, Bruneval P, Veinot J, Leone O. Diagnostic use of the endomyocardial biopsy: a consensus statement. Virchows Arch. 2013 Jul. 463(1):1-5.[↩]
- Yilmaz M, Unsal A, Sokmen M, Harmankaya O, Alkim C, Kabukcuoglu F, et al. Duodenal biopsy for diagnosis of renal involvement in amyloidosis. Clin Nephrol. 2012 Feb. 77(2):114-8.[↩]
- Yamamoto Y, Hirawa N, Yamaguchi S, Ogawa N, Takeda H, Shibuya K, et al. Long-term efficacy and safety of the small-sized ß2-microglobulin adsorption column for dialysis-related amyloidosis. Ther Apher Dial. 2011 Oct. 15(5):466-74.[↩]
- Montagna G, Cazzulani B, Obici L, Uggetti C, Giorgetti S, Porcari R. Benefit of doxycycline treatment on articular disability caused by dialysis related amyloidosis. Amyloid. 2013 Sep. 20(3):173-8.[↩]
- Senior K. Dosing in phase II trial of Alzheimer’s vaccine suspended. Lancet Neurol. 2002 May. 1(1):3.[↩]
- Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013 Jul. 9(4):438-44.[↩]
- Novakovic D, Feligioni M, Scaccianoce S, Caruso A, Piccinin S, Schepisi C, et al. Profile of gantenerumab and its potential in the treatment of Alzheimer’s disease. Drug Des Devel Ther. 2013. 7:1359-64.[↩][↩]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, et al. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009 Aug 26. 29(34):10582-7.[↩]
- Bannykh SI, Balch WE, Kelly JW, Page LJ, Shelton GD. Formation of gelsolin amyloid fibrils in the rough endoplasmic reticulum of skeletal muscle in the gelsolin mouse model of inclusion body myositis: comparative analysis to human sporadic inclusion body myositis. Ultrastruct Pathol. 2013 Oct. 37(5):304-11.[↩]
- Merlini G, Planté-Bordeneuve V, Judge DP, Schmidt H, Obici L, Perlini S, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013 Dec. 6(6):1011-20.[↩]
- Coelho T, Maia LF, da Silva AM, Cruz MW, Planté-Bordeneuve V, Suhr OB. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013 Nov. 260(11):2802-14.[↩]
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016 Jun 25. 387 (10038):2641-54.[↩]
- Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007 Nov 27. 50(22):2101-10.[↩]
- Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984 Jan. 3(1):107-13.[↩]
- Desport E, Bridoux F, Sirac C, Delbes S, Bender S, Fernandez B, et al. Al amyloidosis. Orphanet J Rare Dis. 2012 Aug 21. 7:54.[↩]
- Soni A, LeLorier P. Sudden death in nondilated cardiomyopathies: pathophysiology and prevention. Curr Heart Fail Rep. 2005 Sep. 2(3):118-23.[↩]
- Palladini G, Perfetti V, Obici L, Caccialanza R, Semino A, Adami F. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004 Apr 15. 103(8):2936-8.[↩]