Contents
Apigenin
Apigenin also called trihydroxyflavone (4′,5,7-trihydroxyflavone) is a plant-derived flavonoid or polyphenol found in vegetables, fruits and beverages such as parsley, celery, onions, grapes, apples, strawberries, oranges, chamomile tea and red wine 1, 2. It is generally accepted that people cannot synthesize apigenin but can only obtain it from food 3. Fresh parsley had the highest flavone concentrations of the fresh foods, with ≤1484 mg apigenin/100 g 4. Apigenin has many potential physiological functions including antioxidant anti-inflammatory, anticancer effects, lowering blood pressure, anti-genotoxic, antiallergic, neuroprotective, cardioprotective, antibacterial and antiviral properties and low toxicity 5, 6, 7, 8, 9, 10, 11, 12. In test tube studies, apigenin has been demonstrated to show broad anti-cancer effects in various types of cancer cells, including colorectal cancer, breast cancer, liver cancer, lung cancer, melanoma, prostate cancer and osteosarcoma 13, 14, 15, 16, 17, 18. In test tube studies, apigenin inhibits cancer cell proliferation by triggering cell apoptosis, inducing autophagy and modulating the cell cycle 19. Apigenin also decreases cancer cell motility and inhibits cancer cell migration and invasion 19. Recently, apigenin was reported to show anti-cancer activities by stimulating an immune response 20. Future human clinical trials are needed to evaluate and confirm the therapeutic as well as the preventive potential of apigenin.
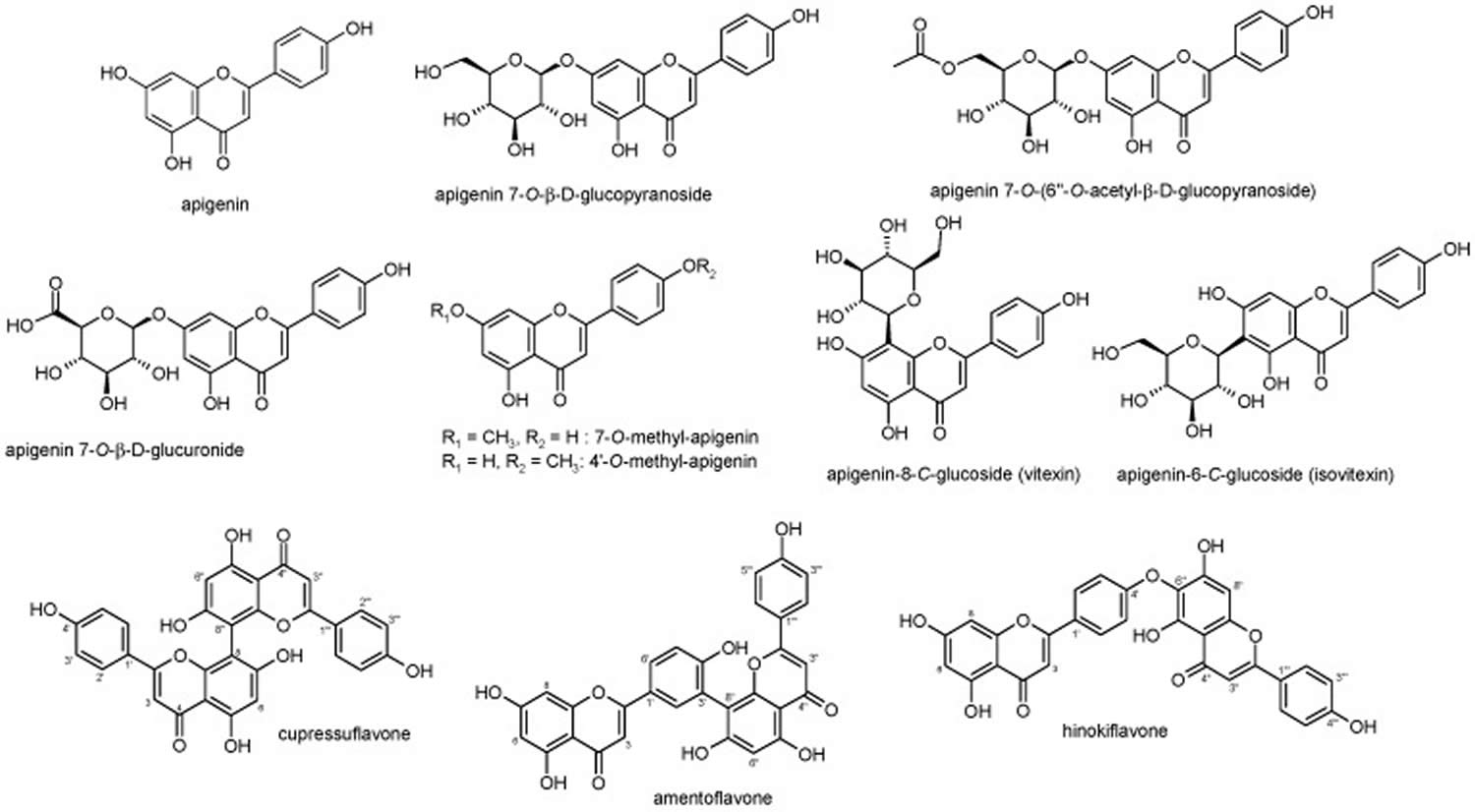
Apigenin because of its low lipid (0.001–1.63 mg/mL) and water (2.16 μg/mL) solubility, it can be deactivated in the acidic environment of the gastrointestinal tract, leading to lower bioavailability, which limits its potential use in healthcare products and functional foods 21, 22, 23. The bioavailability of apigenin also depends on its bioaccessibility, which refers to the extraction of apigenin from the food matrix during gastrointestinal digestion and the transformation into compounds available for absorption 24. Glycosides of apigenin survive acid hydrolysis in the stomach, and it goes to the duodenum unbroken (see Figure 2). Apigenin can be found in various plants as an aglycone or as a number of apigenin glycosides. Apigenin-7-O-glucoside, apigenin-6-C-glucoside (isovitexin), apigenin-8-C-glucoside (vitexin), api-genin-7-O-neohesperidoside (rhoifolin), and apigenin-6-C-glucoside-8-C-arabinoside are the common apigenin glycosides 25. The glycosylated forms of apigenin (e.g., 7-O-glucoside, 6-C-glucoside, or 8-C-glucoside) are broken down by beta-glucosidases in the stomach and small intestine to generate free apigenin 26. Eubacterium ramulus and Bacteroides distasonis have been identified as the main bacterial species essential for the biotransformation of 7-glycosides into free apigenin (aglycone apigenin) 27, 28. Apigenin taken orally is absorbed into the body with an availability of approximately 30% higher in the colon (40%) and lower in the terminal ileum (21%) via passive transport and independently of concentration 26.
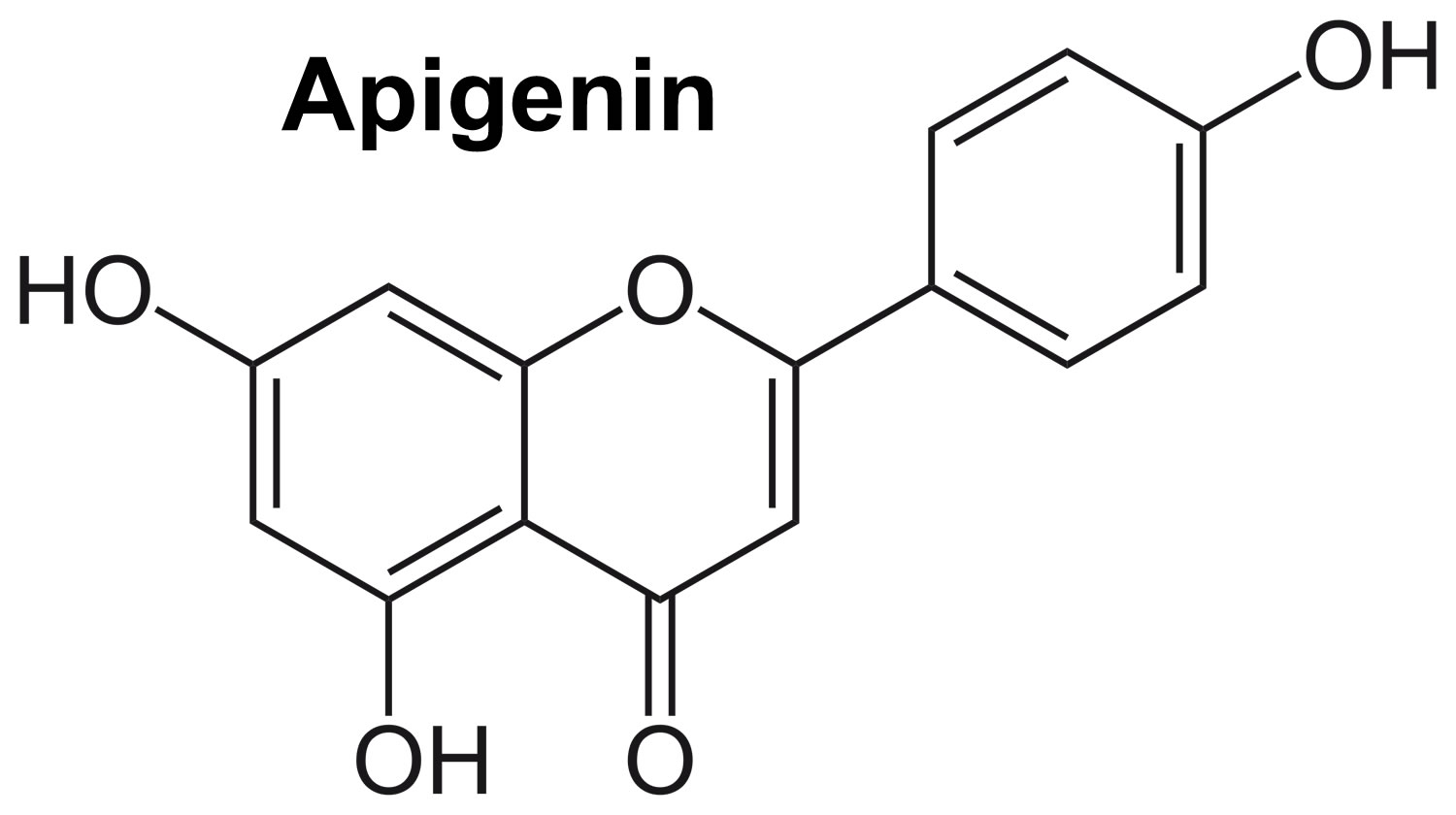
Figure 1. Apigenin chemical structure
[Source 29 ]Figure 2. Apigenin and its glycosides
Footnotes: In nature, apigenin is typically found in a glycosylated form, with the tricyclic core structure linked to a sugar moiety through hydroxyl groups (O-glycosides) or directly to carbon (C-glycosides). The common apigenin glycosides are apiin, apigenin-7-O-glucoside, apigenin-8-C-glucoside (vitexin), apigenin-6-C-glucoside (isovitexin), apigenin-7-O-neohesperidoside (rhoifolin), and apigenin-6-C-glucoside 8-C-arabinoside (schaftoside) 30, 31, 32.
[Source 1 ]Figure 3. Apigenin health benefits
[Source 19 ]Apigenin benefits
Apigenin has many potential physiological functions including antioxidant anti-inflammatory, anticancer effects, lowering blood pressure, anti-genotoxic, antiallergic, neuroprotective, cardioprotective, antibacterial and antiviral properties and low toxicity 5, 6, 7, 8, 9, 10, 11, 12. In test tube studies, apigenin has been demonstrated to show broad anti-cancer effects in various types of cancer cells, including colorectal cancer, breast cancer, liver cancer, lung cancer, melanoma, prostate cancer and osteosarcoma 13, 14, 15, 16, 17, 18. In test tube studies, apigenin inhibits cancer cell proliferation by triggering cell apoptosis, inducing autophagy and modulating the cell cycle 19. Apigenin also decreases cancer cell motility and inhibits cancer cell migration and invasion 19. Recently, apigenin was reported to show anti-cancer activities by stimulating an immune response 20. In addition, apigenin has anti-allergy ability, regulates blood lipids, prevents cardiovascular diseases, and can be used as a natural pigment in the food industry 33, 34, 35. Flavonoid-rich diet is inversely associated with cancer risk 36, 37, 38, 39, 40, 41.
Chamomile tea, which is rich in apigenin, has been used as a traditional medicine for relieving indigestion or gastritis 42. Chamomile is also used in mouth rinse, skin care products, and vapor inhalant to reduce inflammation 42. There are two types of chamomile: the German chamomile and the Roman chamomile, with the former more common as a dietary supplement 43.
Anti-cancer effects
Owing to the potent anti-cancer effects of apigenin on different types of human cancer cells, including colon, bladder, breast, skin, prostate, and liver cancer cells, apigenin has the potential broad application in cancer prevention and treatment 44, 45. A large number of experiments in test tubes or in animals have confirmed the biological effects of apigenin, showing that it has good anti-cancer activity 46.
Some possible mechanisms involved in the anti-cancer properties of apigenin include down-regulation of NF-κB pathway, inactivation of various kinases, and modulation of proteasomal degradation of the HER-2/neu proteins 47. It has been confirmed that apigenin is a selective protein kinase CK2 inhibitor, and evidenced by study results showing an increased apigenin-induced cell death rate in CK2α-high acute myeloid leukemia cells than that in CK2α-low acute myeloid leukemia cells 48. Furthermore, apigenin has been reported to trigger cell cycle arrest and promote and activate apoptosis in cancerous cells. Additionally, NF-κB was inactivated via apigenin inhibition of the Akt signaling-associated protein expression and p65 phosphorylation 49. Moreover, apigenin exerted chemopreventive effects on cancer cells by regulating the expression of antioxidant enzymes and the accumulation of reactive oxygen species (ROS) in lung cancer cells 50. Further studies have reported that apigenin triggers apoptosis via the tumor necrosis factor (TNF) receptor, activating ligand receptor (TRAIL-R)-mediated caspase-dependent cell death pathways in tumor cells 51. These findings suggest that combining apigenin with chemotherapy drugs may enhance cytotoxicity against cancer cells.
Ferroptosis is a new form of cell death described by Dixon et al. in 2012 52 and is characterized by glutathione consumption and lipid peroxide accumulation. An increase in endoplasmic reticulum stress, suppression of the cystine/glutamate antiporter, and activation of mitogen-activated protein kinases (MAPK) and mitochondrial voltage-dependent anion channels contribute to this process 53. An increasing number of studies have shown that ferroptosis has a highly complex relationship with cancer and could be an innovative treatment option for cancer 54. Consequently, it is necessary to conduct clinical trials of ferroptosis-inducing medicines for cancer treatment. Interestingly, several studies have reported that apigenin induces ferroptosis and kills tumor cells 55. According to Adham et al. 56 , the treatment of the multiple myeloma cell line NCI-H929 with apigenin resulted in ferroptosis, autophagy, apoptosis, and cell cycle arrest. However, apigenin cytotoxicity was completely ameliorated by the ferroptosis inhibitor, ferrostatin-1 56 . In a study by Liu et al. 57, mesoporous magnetic nanosystems were developed for the delivery of apigenin, and it was found that the typical characteristics of ferroptosis included cellular lipid peroxidation levels and ROS levels in A549 cells were significantly increased using the targeted apigenin-loaded Fe2O3/Fe3O4@mSiO2-HA nanocomposite delivery system and the underlying mechanisms were mainly upregulation of ferroptosis associated genes COX2 and p53 and downregulation of GPX4 and FTH1.
Antioxidant activity
Apigenin is an antioxidant that quenches singlet oxygen and scavenges of free radicals 58. After the body absorbs oxygen, the oxygen can quickly interact with negatively charged ions (anions) to form superoxide anion radicals, which are then converted into free radicals, including hydroxyl (OH•), superoxide (O2•−), nitric oxide (NO•), nitrogen dioxide (NO2•), peroxyl (ROO•), and lipid peroxyl (LOO•) 59, 60. An excess of free radicals and oxidants give rise to a phenomenon known as oxidative stress (OS), a deleterious process that can lead to cell damage and endogenous dysfunction 61. Long-term oxidative stress can accelerate aging and lead to several chronic disorders. Many oxidants, such as hydrogen peroxide, nitric oxide, metal ion and glutamic acid, have been proven to induce cell dysfunction and diseases 62. Large amounts of published studies have concentrated on the beneficial function of apigenin on oxidative stress-induced progressive diseases such as cancer, neurodegenerative diseases, cardiovascular disease, liver injury, and diabetes mellitus, both in test tube and animal studies 55. In general, apigenin can improve cell viability and/or reduce tissue damage by increasing the resistance to oxidative stress inducers. It has been found that the key to the protective activity of apigenin is its ability to scavenge endogenous reactive oxygen species (ROS) and reduce malondialdehyde (MDA) levels. Further studies report, that apigenin reduced reactive oxygen species (ROS) and malondialdehyde (MDA) levels, thereby enhancing antioxidant enzyme activities, such as those of superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), as well as the upregulation of antioxidant response proteins, such as nuclear factor erythroid 2-related factor 2 (Nrf2) and AMP-activated protein kinase (AMPK) 63, 64. Overall, apigenin can be considered a novel antioxidant that can decrease the risk of oxidative stress-induced disorders 3.
Anti-inflammatory activity
In recent test tube and animal studies, there has been increasing interest in the anti-inflammatory activities of apigenin 65. In a test tube study, apigenin prevented the injury response of lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells by enhancing the reduction of nitric oxide (NO) 66. In these processes, apigenin decreased the levels of pro-inflammatory cytokines TNF-α, IL-18, and IL-6, and downregulated the expression of enzymes (COX-2 and iNOS), as well as reducing the intracellular reactive oxygen species (ROS) production. It has been shown that apigenin can inhibit the activity of intracellular cell adhesion molecules (ICAMS), monocyte inflammatory protein (MIP-1α), and monocyte chemotactic protein (MCP-1α) inhibitors induced by lipopolysaccharide (LPS) in an animal study of mouse leukocytes, resulting in an anti-inflammatory response 67. The downregulation of these pro-inflammatory factors by apigenin may be due to action of some transcriptional factors and kinases, such as extracellular signal-regulated kinases (ERK), NF-kB, and mitogen-activated protein kinase (MAPK) 68.
Glial cells, such as microglia and astrocytes, mediate neuroinflammation which is triggered by the activation of the innate immune system in the brain to cope with inflammation 69. Microglia and astrocytes can be activated by exogenous infection or irritation, releasing inflammatory cytokines to magnify neuroinflammation, leading to enhanced or prolonged brain pathology. Therefore, it is helpful to reduce this inflammation to combat neurodegenerative diseases. The anti-inflammatory properties of apigenin were observed in BV2 microglia stimulated by lipopolysaccharide (LPS), as proven by the activation of GSK-3β/Nrf2 signaling pathway that attenuated the expression of IL-6, IL-1β, and TNF-α 70. Apigenin-induced transformation of IBA1-positive cells into the amoebic phenotype was observed in isolated rat microglial cultures and was related to an increase in the expression of the activated M1 spectral markers OX-42 and iNOS and decreased expression of the M2 spectral marker CD206 71. Taken together, these results demonstrate that apigenin has anti-inflammatory and neuroprotective properties and could serve as a neuroimmunomodulatory agent 72.
Antibacterial activities
The antibacterial potential of apigenin has been tested against many bacteria species and various strains within the same species. Broth microdilution and agar dilution methods are the most popular methods in which the minimal inhibitory concentrations (MICs) are determined as the lowest concentration of treatment that showed no growth after incubation 73, 74, 75, 76. Apigenin could not inhibit the growth of Staphylococcus aureus (8325-4, ATCC 29213, wood 46, and BAA-1717) 76. Despite the reported lack of activity against S. aureus, apigenin was found to decrease the level of alpha-hemolysin at low concentrations in a concentration-dependent manner in Staphylococcus aureus culture supernatants 76. Alpha-hemolysin is a pore-forming cytotoxin that is secreted by most Staphylococcus aureus strains, essential for the pathogenesis of Staphylococcus aureus pneumonia 76. Apigenin protected the adenocarcinomic human alveolar basal epithelial cells (A549 cells) from α-hemolysin-mediated injury in the A549 cells and S. aureus co-culture system 76. Therefore, the protective effect apigenin did not come from a reduction in bacterial quantity, but more likely the altered cell physiology. What is more promising is that apigenin alleviated injury of the lung tissue and decreased cytokine levels in the bronchoalveolar lavage fluid in the mouse model of S. aureus pneumonia 76. When apigenin was applied with LysGH15, the lysin derived from phage GH15 with high efficiency and a broad lytic spectrum against MRSA, synergism was observed using a mouse S. aureus pneumonia model 77.
Contrasting results have been observed on apigenin’s effect on Helicobacter pylori in test tube study. While one study saw no inhibitory effect against thirteen randomly selected clinical strains of H. pylori from antral biopsies and a reference strain H. pylori ATCC 43504 78, apigenin showed moderate antibacterial activity in another, with a MIC of 25 μg/mL against both H. pylori SS1 and H. pylori ATCC 43504 79. An animal experiment using Mongolian gerbils, apigenin treatments (30–60 mg/kg body weight/day) effectively decreased H. pylori-induced atrophic gastritis and N′-methyl-N’-nitro-N-nitroso-guanidine (MNNG)-induced dysplasia/gastric cancer rates 80. The dose of 60 mg apigenin/kg bodyweight/day significantly decreased H. pylori colonization and H. pylori-induced histological changes of neutrophil and monocyte infiltrations and atrophic gastritis 80.
Apigenin anti-anxiety effects
Some preliminary studies suggest that a chamomile dietary supplement rich in apigenin might be helpful for generalized anxiety disorder (GAD). Researchers conducted a randomized, double-blind, placebo-controlled trial to test the effects of chamomile extract in patients diagnosed with mild to moderate generalized anxiety disorder (GAD). Compared with placebo, chamomile was associated with a clinically meaningful and statistically significant greater reduction in mean Hamilton Anxiety Rating (HAM-A) scores 81. Although the researchers suggest that other chamomile species, preparations, and formulations might produce different results 82. It has been shown that apigenin and other constituents of chamomile bind to benzodiazepine receptors and reduce GABA-activated activity and may produce anxiolytic activity 82.
A randomized, long-term, clinical trial of the application of 500 mg three times per day of chamomile extract in the treatment of generalized anxiety disorder (GAD) was conducted by Mao and others 83. For the whole study period, chamomile extract treatment was administered to eligible participants with a DSM-4 Axis-I diagnosis of generalized anxiety disorder by 12 weeks of open label therapy 83. After that, 93 treatment responders were randomized in a double-blind, controlled trial to receive 26 weeks of either continuation of chamomile or placebo 83. Responders treated with chamomile, maintained significantly lower anxiety disorder symptoms than the placebo group. At the same time, chamomile participants showed reduced body weight and mean arterial blood pressure. Chamomile was safe and significantly reduced moderate-to-severe generalized anxiety disorder symptoms. Amsterdam et al. 84 used chamomile extract in the treatment of generalized anxiety disorder in a randomized, double-blind, placebo-controlled trial. Chamomile extract was standardized to a content of 1.2% apigenin. The volunteers had anxiety with co-morbid depression, or anxiety with past history of depression, or anxiety with no current or past depression. The results showed a significantly greater reduction in total Hamilton Depression Rating Scale (HAM-D) scores for chamomile, suggesting that German chamomile (Matricaria chamomilla) can exert an antidepressant effect 84. The unclear antidepressant action was attributed to chamomile’s flavonoids, possibly by modulation of noradrenaline, dopamine (DA), and serotonin (5-HT) neurotransmission.
Depression
A study of the behavioral effects of acute administration of apigenin and chrysin, contained in German chamomile (Matricaria chamomilla) and in purple passionflower (Passiflora incarnata), has been conducted in rats 85. The results indicated that both apigenin and chrysin were able to reduce locomotor activity when injected in rats at a minimal effective dose of 25 mg/kg 85. The sedative effect could not be associated with an interaction with GABA–benzodiazepine receptors, since it was not counteracted by the benzodiazepine antagonist flumazenil 85. Apigenin reduced GABA (gamma-aminobutyric acid)-activated Cl− currents in a dose-dependent fashion. The effect was blocked by co-application of Ro 15-1788, a specific benzodiazepine receptor antagonist. Accordingly, apigenin reduced the latency in the onset of picrotoxin-induced convulsions. Moreover, apigenin injected intraperitoneally in rats reduced locomotor activity, but did not demonstrate anxiolytic or myorelaxant activity 86.
Weng et al. 87 studied the influence on depressive-like mice induced by chronic corticosterone treatment with 20 mg/kg or 40 mg/kg apigenin doses as well as fluoxetine (20 mg/kg). Behavioral tests showed that apigenin reversed the reduction of sucrose preference and the elevation of immobility time 87. In addition, the corticosterone-treated mice supplemented with apigenin ameliorated the decrease in hippocampal brain-derived neurotrophic factor (BDNF) levels underlining its antidepressant action by upregulation of BDNF 87.
Nakazawa et al. 88 found an antidepressant-like activity of apigenin on norepinephrine (NE) and dopamine (DA) turnover in the amygdala and hypothalamus in mice. Han et al. 89 evaluated the effect of apigenin isolated from Cayratia japonica (Japanese Cayratia herb) on monoamine oxidase (MAO) inhibition. Apigenin inhibited both MAO-A and MOA-B; the median inhibitory concentration (IC50) of MAO-A was 1.7 μM and for MAO-B it was 12.8 μM. Chaurasiya and colleagues 90 showed how inhibition of MAO-A by apigenin from propolis was 1.7-fold more selective than MAO-B. According to Lorenzo et al. 91, apigenin increases noradrenaline activity in an isolated rat atria model, at the same time inhibiting MAO activity in rat atria homogenates. On the other hand, Morita et al. 92 found that apigenin stimulated the uptake of L-tyrosine, a noradrenaline precursor. Yi and colleagues 93 in their work on antidepressant and neurochemical effects of citrus-associated apigenin found reduced immobility during the forced swim test, reversed chronic mild stress (CMT)-induced reduction in sucrose intake in rats, lowered stress-induced alterations in 5-HT, DA, and reversed forced swim test-induced increases in hypothalamic‒pituitary‒adrenocortical axis activity. What is more, it is believed that inflammation may contribute to the pathophysiology of depression.
In the study conducted by Li and others 94, the effects of apigenin on lipopolysaccharide (LPS)-induced depressive-like behavior in mice model were examined. Tested animals were pre-treated with 25 mg/kg or 50 mg/kg of apigenin or 20 mg/kg of fluoxetine once daily for seven days 94. The use of apigenin prevented the abnormal behavior induced by LPS, attenuated production of pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). What is more, the authors found that apigenin suppressed inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression. Apigenin, at a dose of 50 mg/kg, reversed the depressive-like behavior induced by tumor necrosis factor-α without altering the locomotor activity 94. They concluded that, due to its anti-inflammatory properties, apigenin is characterized by antidepressant-like properties in LPS-treated mice 94.
Insomnia (problem falling and staying asleep)
Insomnia is a prevalent sleep disorder that can profoundly impact a person’s health and well-being 95. Chamomile flower extract, with more than 2.5 mg of apigenin, was examined for its preliminary efficacy and safety for improving sleep and daytime symptoms in patients with chronic insomnia. Thirty-four adults aged 18–65 years with primary insomnia (DSM-4 criteria) lasting more than six months, with total daily sleep time less than 6.5 h, took part in research carried out by Zick et al. 96. They found no significant differences between groups in changes in sleep diary measures, including total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings 96. It should be highlighted that chamomile did cause a modest improvement in daytime functioning. The authors concluded that chamomile treatment could provide modest benefits in terms of daytime functioning and mixed benefits in terms of sleep diary measures 96.
Due to the fact that both insomnia and depression are central nervous system (CNS)-related diseases, the factor affecting the effectiveness of bioactive compounds is blood‒brain barrier (BBB) penetration. The role of the BBB is to provide nutrients for the brain as well as regulate the brain microenvironment for neuronal functions 97. Consequently, blood‒brain barrier (BBB) protects the central nervous system from compounds that can negatively affect the function of the CNS. Some studies have demonstrated that flavonoids can easily penetrate the BBB 98. According to Yang et al. 99, the permeation order of the flavonoids is genistein > isoliquiritigenin > apigenin > puercetin > kaempferol > hesperidin > rutin > quercetin, where genistein is characterized by the highest permeation. Therefore, apigenin may have direct, positive effects on diseases such as Alzheimer’s disease or insomnia.
Apigenin in cosmetics
Apigenin strongly absorbs UV-B rays (wavelengths between 280 and 320 nm), and can be used in sunscreen cosmetics 3. However, the absorption of apigenin in UV-A (wavelengths between 320 and 400 nm) is small, so low concentrations of apigenin can also be used as a skin darkening agent in tanning oils 100. Apigenin has been used in cosmetics as a pigment stabilizer at a recommended concentration of 1% 3. Apigenin can also be used in creams and in combination with vitamins C, B12, B6, and B1 and is usually mixed with plant essential oils such as chamomile, calendula, and almond oil. High concentrations of apigenin can inhibit the activity of melanocytes 101; can be added to sunscreen, face cream, essence, toner, facial masks, and other cosmetics; and can also be used in shampoos and conditioners.
Apigenin has strong antioxidant properties, a strong trapping capacity for various oxygen-containing free radicals and can prevent the oxidative degradation of oil 102. Apigenin also has anti-inflammatory activity and can prevent skin problems, such as bullous pemphigoid, keratosis, and incomplete keratosis. In addition, apigenin relieves itchy scalps and can be used in hair care products.
Apigenin in beverages
It is well known that deep formulations containing apigenin have been developed by more and more commercial companies for application in the research and development of various energy drinks, including composite fruit drinks, breakfast fortified drinks, and sports drinks. Apigenin energy drinks have anti-fatigue, cooling, and refreshing properties, because apigenin fights inflammation and oxidative stress after a short period of intense exercise 103.
Apigenin in food processing
Apigenin can be used as a food additive and colorant in the food processing industry. Apigenin has been assessed as safe and effective and its use as an ingredient in food has been approved 104, 105. As a natural pigment, apigenin can replace nitrites, ensure food safety, and can be used for corrosion prevention and coloring of biscuits, jellies, and meat products. Apigenin is also used as a coloring agent in pastries, ice-creams, and confectionary 3.
Apigenin food sources
Apigenin is abundant in vegetables, fruits and beverages, such as parsley, vine spinach, celery, celery seed, green celery heart, Chinese celery, onions, grapes, apples, strawberries, oranges, herbs (chamomile, thyme, oregano, basil), and plant-based beverages (tea, beer, and wine) 106, 107. Other plants in which apigenin has been identified include red and white sorghum, rutabagas, oranges, kumquats, onions, wheat sprouts, tea, and cilantro 42, 108. The richest sources of apigenin are the dried forms of celery, oregano, and parsley. According to reports, dried parsley contains the highest concentration of apigenin (45,035 μg/g) that far exceeds any other vegetables or herbs 109. The dried chamomile flowers contain 3,000 to 5,000 μg/g of apigenin. The celery seeds contain 786.5 μg/g while the Chinese celery contains 240.2 μg/g of apigenin 109. The vine spinach contains 622 μg/g of apigenin.
Common fruits like grapefruit, oranges, beverages made from plants, vegetables like onions, chamomile, parsley etc., wheat sprouts, tea, and various seasonings all contain a lot of apigenin 109. The dried flowers of the chamomile plant, Matricaria chamomilla, are also source of apigenin used in herbal teas 110. Chamomile tea, high in apigenin content, is one of the most common sources of apigenin intake from a single ingredient 111. Other species, most of which are used in traditional herbal or alternative medicines, have also been found to contain apigenin as an active bioactive molecule, including propolis (a resinous mixture that honey bees produce by mixing saliva and beeswax with exudate gathered from tree buds, sap flows, or other botanical sources), Combretum erythrophyllum (river bushwillow), Gentiana veitchiorum, Marrubium globosum, and Portulaca oleracea L.
Table 1. Apigenin in fruits and vegetables
| Food (serving size, g) | Flavone | Flavone, mg/100 g fresh weight | Flavone, mg/serving |
|---|---|---|---|
| Spinach (200 g) | Apigenin | <0.12 | <1 |
| Luteolin | <0.13–6.6 | <1–13 | |
| Parsley (5 g) | Apigenin | <2.44–1484 | <1–74 |
| Luteolin | <0.44–22 | <1–1 | |
| Celery stalks (200 g) | Apigenin | 1.3–10.8 | 3–22 |
| Luteolin | <1.2–2.2 | <2–4 | |
| Celery hearts (200 g) | Apigenin | 19.1 | 38 |
| Luteolin | 3.5 | 7 | |
| Chinese celery (200 g) | Apigenin | 24 | 48 |
| Luteolin | 34.9 | 70 | |
| Lettuce (100 g) | Apigenin | <0.75–2.7 | <1–3 |
| Luteolin | <1.26–8.8 | <1–9 | |
| Artichoke heads (200 g) | Apigenin/apigenin glycosides | 3.9–18.9 | 8–38 |
| Luteolin/luteolin glycosides | 2.3–7.5 | 5–15 | |
| Chicory leaves (100 g) | Apigenin/apigenin O-glycosides | <0.1–68 | <1–68 |
| Luteolin/luteolin O-glycosides | <0.1–333 | <1–333 | |
| Rutabaga (200 g) | Apigenin | <0.1–15.4 | <1–31 |
| Luteolin | <0.1 | <1 | |
| Chinese cabbage (200 g) | Apigenin | <0.1–4.5 | <1–9 |
| Luteolin | <0.1–1.2 | <1–2 | |
| Peas (100 g) | Apigenin | <0.12–17.6 | <1–18 |
| Luteolin | <0.1–0.4 | <1 | |
| Black olives (50 g) | Apigenin | 6.5 | 3 |
| Luteolin | 3.2–17.5 | 2–9 | |
| Olive oil (15 g) | Apigenin | <0.1–0.2 | <1 |
| Luteolin | <0.1–0.7 | <1 | |
| Grapefruit (200 g) | Apigenin/apigenin glycosides | <0.35–4.9 | <1–10 |
| Luteolin | <1.2–1.4 | <1–3 | |
| Kumquat (100 g) | Apigenin glycosides | 21.9 | 22 |
| Honey (20 g) | Apigenin | <0.1–29.3 | <1–6 |
| Luteolin | <0.49–3.2 | <1–1 |
Apigenin digestion and absorption
The bioactivity of apigenin primarily originates from the release from raw food materials. In food and herbal sources, the active apigenin is found in the form of various sugar moieties. During digestion, apigenin glycosides survive in the stomach through acid hydrolysis and enter the duodenum unchanged 113. The location and degree of glycosylation affect apigenin absorption in the gastrointestinal tract 114. The distribution of the enzymes needed to generate bioactive apigenin and the characteristics of the linked sugar molecule affect the next step of digestion and absorption. Different cells metabolize apigenin intracellularly via enzymes present in the brush border epithelium 115. However, nondigestible apigenin glycosides require extracellular deglycosylation, which can be performed by bacteria and their associated enzymes that exist in the colon. Microbial alpha-glucosidase can facilitate the digestion of apigenin which is attached to rhamnose, whereas human beta-glucosidase cannot 114. Hanske et al. 116 studied the deglycosylation of apigenin-7-glucoside by human intestinal microorganisms in rats and found that it promoted the biological activity of apigenin to a great extent.
It has been demonstrated that 5–10% of apigenin can be absorbed after the consumption of polyphenols 117. The absorption of apigenin is mainly mediated by the gastrointestinal tract (GIT) prior to its entry into the bloodstream and liver. A study using a rat intestinal irrigation model showed that apigenin was immediately absorbed by the intestine after aglycone apigenin administration 118. There are various absorption routes for apigenin in different parts of the intestine. In the jejunum and duodenum, active and passive vehicle-mediated saturation mechanisms stimulate the absorption of apigenin, while in the ileum and colon absorption occurs via passive transport. However, reports on the absorption rate of apigenin are inconsistent 119. A study by Gradolatto et al. 120 was consistent with the fact that apigenin is slowly absorbed and metabolized after oral administration, and even slowly eliminated (detected in blood circulation after 24 h). However, another study conducted by Chen et al. 121 reported a high absorption rate of apigenin after oral administration to rats (detected in blood circulation after 3.9 h). A possible explanation for the difference in these findings is that the animal strains used in the two studies were different, the former using SD rats and the latter using Wistar rats 121, 122. It has been proved that animal strain difference is one of the main factors affecting the pharmacokinetic performance Barr JT, Tran TB, Rock BM, Wahlstrom JL, Dahal UP. Strain-dependent variability of early discovery small molecule pharmacokinetics in mice: does strain matter? Drug Metab Dispos. (2020) 48:613–21. doi: 10.1124/dmd.120.090621. Therefore, the absorption rate of apigenin requires further investigation.
Human organs and tissues swiftly distribute apigenin after it is absorbed by the body, and numerous studies have demonstrated the presence of apigenin in the serum, lung, kidney, brain, thyroid, ovary, womb, intestine, and liver 3. In addition, after apigenin intake by the body, it can also be found in the urine and feces 123. The blood and liver have been shown to have considerably higher apigenin content. Meyer et al. 124 studied the relationship between dietary intake of apigenin and circulating levels in a range of healthy human volunteers (ages 21–41 years, mean body mass index 23.9 kg/m²). The results showed that systemic levels of apigenin following the ingestion of apigenin-rich parsley (mean intake: 149.5 ± 35.2 g) peaked at about 7 hour after oral intake, resulting in a mean apigenin serum concentration of 127.0 ± 24.3 nM (34.3 ± 6.57 ng/mL) 124. The concentration of apigenin in tissues is determined by the expression and genetic variants of lipoprotein receptors and cholesterol carriers, which affect its accumulation in target organs 125.
Apigenin bioavailability
The bioactivity of apigenin depends primarily on its bioavailability after digestion and absorption 126. After intestinal digestion and absorption, apigenin is converted into glucuronic acid conjugates and secreted back into the lumen of the gut, decreasing net absorption. Furthermore, conjugated apigenin may also be transported through the efflux transporters multidrug resistance protein-1 (also referred to as P-gp, ABCB1, and CD243) and multidrug resistance-associated protein-2 (also referred to as ABCC2 and CMOAT), the abundances of which could be significantly changed under different disease states 127. Additionally, apigenin can be modified by methylation, sulfation, and glucuronidation, all of which affect its bioactivity and distribution 128. Bioavailability is defined as the percentage of the provided chemical which can be absorbed and used for storage or physiological functions, and it is closely linked to bioaccessibility 129. Bioavailability refers to the conversion of apigenin from the food matrix to mixed micelles during digestion and rendering it available for intestinal absorption.
As a bioactive compound, the bioefficacy of apigenin is affected by many factors that include molecular structure, digestibility, food matrix, bioaccessibility, and transporter and metabolizing enzyme availability 130. Published studies have confirmed that apigenin has a low solubility in fat and water, with the solubility of 2.16 μg/mL and 0.001–1.63 mg/mL in water and non-polar solvents, respectively 131. Due to the low oral bioavailability of apigenin, its clinical applications are limited. One of the core factors regulating the bioavailability of apigenin is its transformation in the intestinal mucosa to a large-molecular-weight glucuronic acid glycosidic conjugate 3. Therefore, the net absorption of apigenin is severely reduced in the intestinal lumen 115. It has been reported that both the bioavailability and distribution of apigenin can also be affected by formation of conjugates by methylation, sulfation, and glucuronidation. An intestinal epithelial metabolism model simulated by Caco-2 monolayers demonstrated that apigenin can be translated into a glucuronic acid conjugate by uridine 5′-diphosphate glucuronic acid transferase metabolism in the intestinal epithelium. It was further demonstrated that apigenin exhibited an apparent permeability coefficient (Papp) ranging between 10 to 5 cm/s, suggesting that apigenin has high hydrophilicity and good intestinal absorption 132. UDP-glucuronic acid transferase (UGT) plays a significant role in accelerating apigenin metabolism rate of in the intestinal tract 133. Gut-secreted apigenin in Gunn rats increases the levels of the UGT1A isoform, enhancing its properties through hepatic anion efflux transporters for efficient metabolism and compensatory regulation of intestinal UGT2B, thereby limiting its bioavailability and increasing its disposition.
Apigenin dosage
For general health needs, multiple daily servings of fruits and vegetables can provide adequate amounts of apigenin, which is estimated to be less than 5 mg/day 29, 134. Apigenin is sufficiently bioavailable through dietary sources. In contrast, apigenin that’s been isolated from its food source is rarely stable enough to be absorbed by the body 135.
Is apigenin safe?
Apigenin is considered safe, but at high doses it can trigger muscle relaxation and sedation 136, 137. Taking apigenin glucosides through a daily diet can hardly reach the therapeutic doses used in clinical trial 30 and has not been reported to be harmful. However, safety concerns may arise with a higher level of apigenin exposure such as taking apigenin from dietary supplements or pharmaceutical sources.
Apigenin is known for its low toxicity 42, 138, 139, 140. Therefore, the consumption of flavonoids has been assumed to be safe.
Results of an Ames test, one of the oldest methods employed for in vitro testing of carcinogenicity using Salmonella strains 138, showed that apigenin was not mutagenic or toxic when tested alone 141. Not only was apigenin not mutagenic 142, it protected against multiple genotoxic agents, such as sodium azide, 9-amino acridine 138, 143. Apigenin prevented the reverted mutations as a result of sodium azide exposure using histidine auxotroph S. typhimurium TA100 strain and the hindrance percent of apigenin was 98.17% 144. Apigenin-7-O-glucoside inhibited sodium azide mutagenicity in S. typhimurium TA1535 at 6.67 nM in the top agar when using the layered agar method with an inhibition rate of 27.2% and 9-aminoacridine in S. typhimurium TA1537 at 3.33 nM with an inhibition rate of 91.1%. In the yeast deletion assay using mutagens ethyl methanesulfonate and acridine, the inhibition rates were from 4% to 57.7% 143.
Apart from mutagenesis, hemolytic activity is another measurement of safety. Hemolytic tests showed that the percent hemolysis of apigenin was below the permissible limit of 5% after 30 min treatment. Being hemocompatible indicates that apigenin is safe for intravenous application, and suggests that apigenin is non-toxic in mammalian systems 145. Apigenin was able to significantly attenuate the hemolytic activity of the purified Pneumolysin, the pore-forming toxin secreted by S. pneumoniae, in a concentration-dependent manner 146.
Apigenin side effects
There is little evidence to suggest that apigenin causes adverse effects when consumed as part of a normal diet 147. No toxicity has been reported as a result of apigenin intake from food sources 148, 149. It should be noted, when apigenin dosages exceed typical dietary intake to an extreme (30–100 mg/kg of body weight), sedation has been reported as a side effect 150.
- Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, Antolak H, Azzini E, Setzer WN, Martins N. The Therapeutic Potential of Apigenin. Int J Mol Sci. 2019 Mar 15;20(6):1305. doi: 10.3390/ijms20061305[↩][↩]
- Jang JY, Sung B, Kim ND. Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin. Int J Mol Sci. 2022 Mar 29;23(7):3757. doi: 10.3390/ijms23073757[↩]
- Chen P, Chen F, Guo Z, Lei J, Zhou B. Recent advancement in bioeffect, metabolism, stability, and delivery systems of apigenin, a natural flavonoid compound: challenges and perspectives. Front Nutr. 2023 Jul 26;10:1221227. doi: 10.3389/fnut.2023.1221227[↩][↩][↩][↩][↩][↩][↩]
- Mattila P, Astola J, Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J Agric Food Chem. 2000 Dec;48(12):5834-41. doi: 10.1021/jf000661f[↩]
- Kashyap P, Shikha D, Thakur M, Aneja A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J Food Biochem. 2022 Apr;46(4):e13950. doi: 10.1111/jfbc.13950[↩][↩]
- Birt DF, Walker B, Tibbels MG, Bresnick E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis. 1986;7:959–963. doi: 10.1093/carcin/7.6.959[↩][↩]
- Papay ZE, Kosa A, Boddi B, Merchant Z, Saleem IY, Zariwala MG, Klebovich I, Somavarapu S, Antal I. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J Aerosol Med Pulm Drug Deliv. 2017;30:274–288. doi: 10.1089/jamp.2016.1316[↩][↩]
- Wang YC, Huang KM. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem Toxicol. 2013;53:376–383. doi: 10.1016/j.fct.2012.12.018[↩][↩]
- Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50. doi: 10.1186/s13578-017-0179-x[↩][↩]
- Pápay Z.E., Kósa A., Böddi B., Merchant Z., Saleem I.Y., Zariwala M.G., Klebovich I., Somavarapu S., Antal I. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm. Drug Deliv. 2017;30:274–288. doi: 10.1089/jamp.2016.1316[↩][↩]
- Zhu ZY, Gao T, Huang Y, Xue J, Xie ML. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lalpha in rats. Food Funct. 2016;7:1992–1998. doi: 10.1039/C5FO01464F[↩][↩]
- Ozcelik B, Kartal M, Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 2011;49:396–402. doi: 10.3109/13880209.2010.519390[↩][↩]
- Huang C, Wei YX, Shen MC, Tu YH, Wang CC, Huang HC. Chrysin, abundant in Morinda citrifolia fruit water-EtOAc extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J Agric Food Chem. 2016;64:4235–4245. doi: 10.1021/acs.jafc.6b00766[↩][↩]
- Lee YM, Lee G, Oh TI, Kim BM, Shim DW, Lee KH, Kim YJ, Lim BO, Lim JH. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol. 2016;48:399–408. doi: 10.3892/ijo.2015.3243[↩][↩]
- Zhao G, Han X, Cheng W, Ni J, Zhang Y, Lin J, Song Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol Rep. 2017;37:2277–2285. doi: 10.3892/or.2017.5450[↩][↩]
- Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474[↩][↩]
- Angulo P, Kaushik G, Subramaniam D, Dandawate P, Neville K, Chastain K, Anant S. Natural compounds targeting major cell signaling pathways: a novel paradigm for osteosarcoma therapy. J Hematol Oncol. 2017;10:10. doi: 10.1186/s13045-016-0373-z[↩][↩]
- Xu M, Wang S, Song YU, Yao J, Huang K, Zhu X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol Lett. 2016 May;11(5):3075-3080. doi: 10.3892/ol.2016.4331[↩][↩]
- Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017 Oct 5;7:50. doi: 10.1186/s13578-017-0179-x[↩][↩][↩][↩][↩]
- Cardenas H, Arango D, Nicholas C, Duarte S, Nuovo GJ, He W, Voss OH, Gonzalez-Mejia ME, Guttridge DC, Grotewold E, Doseff AI. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-kappaB activity, halting leukocyte infiltration and restoring normal metabolic function. Int J Mol Sci. 2016;17:323. doi: 10.3390/ijms17030323[↩][↩]
- Adel, M, Zahmatkeshan, M, Akbarzadeh, A, Rabiee, N, Ahmadi, S, Keyhanvar, P, et al. Chemotherapeutic effects of Apigenin in breast cancer: preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol Rep (Amst). (2022) 34:e00730. doi: 10.1016/j.btre.2022.e00730[↩]
- Kashyap D., Sharma A., Tuli H.S., Sak K., Garg V.K., Buttar H.S., Setzer W.N., Sethi G. Apigenin: A Natural Bioactive Flavone-Type Molecule with Promising Therapeutic Function. J. Funct. Foods. 2018;48:457–471. doi: 10.1016/j.jff.2018.07.037[↩]
- Nabavi S., Habtemariam S., Daglia M., Nabavi S. Apigenin and Breast Cancers: From Chemistry to Medicine. Anticancer Agents Med. Chem. 2015;15:728–735. doi: 10.2174/1871520615666150304120643[↩]
- Rein M.J., Renouf M., Cruz-Hernandez C., Actis-Goretta L., Thakkar S.K., da Silva Pinto M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013;75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x[↩]
- Thomas, S. D.; Jha, N. K.; Jha, S. K.; Sadek, B.; Ojha, S. Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin. Nutrients. 2023, 15(2), 385. DOI: 10.3390/nu15020385[↩]
- Fossatelli L, Maroccia Z, Fiorentini C, Bonucci M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. Int J Mol Sci. 2023 Dec 23;25(1):251. doi: 10.3390/ijms25010251[↩][↩]
- Schneider H., Blaut M. Anaerobic Degradation of Flavonoids by Eubacterium Ramulus. Arch. Microbiol. 2000;173:71–75. doi: 10.1007/s002030050010[↩]
- Al-Ishaq R.K., Liskova A., Kubatka P., Büsselberg D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers. 2021;13:3934. doi: 10.3390/cancers13163934[↩]
- Wang M, Firrman J, Liu L, Yam K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res Int. 2019 Oct 16;2019:7010467. doi: 10.1155/2019/7010467[↩][↩]
- Tang D., Chen K., Huang L., Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opinion on Drug Metabolism & Toxicology. 2017;13(3):323–330. doi: 10.1080/17425255.2017.1251903[↩][↩]
- Lefort É. C., Blay J. Apigenin and its impact on gastrointestinal cancers. Molecular Nutrition & Food Research. 2013;57(1):126–144. doi: 10.1002/mnfr.201200424[↩]
- Simirgiotis M. J., Schmeda-Hirschmann G., Bórquez J., Kennelly E. J. The Passiflora tripartita (banana passion) fruit: a source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Molecules. 2013;18(2):1672–1692. doi: 10.3390/molecules18021672[↩]
- Gangwar, V, Garg, A, Lomore, K, Korla, K, Bhat, SS, Rao, RP, et al. Immunomodulatory effects of a concoction of natural bioactive compounds-mechanistic insights. Biomedicine. (2021) 9:1522. doi: 10.3390/biomedicines9111522[↩]
- Wang, X, Li, J, Zhao, D, and Li, J. Therapeutic and preventive effects of apigenin in cerebral ischemia: a review. Food Funct. (2022) 13:11425–37. doi: 10.1039/D2FO02599J[↩]
- Xu, Y, Li, X, and Wang, H. Protective roles of Apigenin against Cardiometabolic diseases: a systematic review. Front Nutr. (2022) 9:875826. doi: 10.3389/fnut.2022.875826[↩]
- Heederik D., Kromhout H., Burema J., Biersteker K., Kromhout D. Occupational Exposure and 25-Year Incidence Rate of Non-Specific Lung Disease: The Zutphen Study. International Journal of Epidemiology. 1990;19(4):945–952. doi: 10.1093/ije/19.4.945[↩]
- Knekt P., J rvinen R., Sepp nen R., et al. Dietary Flavonoids and the Risk of Lung Cancer and Other Malignant Neoplasms. American Journal of Epidemiology. 1997;146(3):223–230. doi: 10.1093/oxfordjournals.aje.a009257[↩]
- Hertog M. G., Feskens E. J., Hollman P. C., Katan M. B., Kromhout D. Dietary flavonoids and cancer risk in the Zutphen elderly study. Nutrition and Cancer. 1994;22(2):175–184. doi: 10.1080/01635589409514342[↩]
- Bosetti C., Spertini L., Parpinel M., et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomarkers & Prevention. 2005;14:805–808. doi: 10.1158/1055-9965.EPI-04-0838[↩]
- Hoensch H., Groh B., Edler L., Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World Journal of Gastroenterology. 2008;14(14):2187–2193. doi: 10.3748/wjg.14.2187[↩]
- Rossi M., Negri E., Lagiou P., et al. Flavonoids and ovarian cancer risk: A case-control study in Italy. International Journal of Cancer. 2008;123(4):895–898. doi: 10.1002/ijc.23549[↩]
- Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (Review) International Journal of Oncology. 2007;30(1):233–245. doi: 10.3892/ijo.30.1.233[↩][↩][↩][↩]
- Vogiatzoglou A., Mulligan A. A., Lentjes M. A., et al. Flavonoid Intake in European Adults (18 to 64 Years) PLoS ONE. 2015;10(5):p. e0128132. doi: 10.1371/journal.pone.0128132[↩]
- Ginwala R, Bhavsar R, Moore P, Bernui M, Singh N, Bearoff F, Nagarkatti M, Khan ZK, Jain P. Apigenin Modulates Dendritic Cell Activities and Curbs Inflammation Via RelB Inhibition in the Context of Neuroinflammatory Diseases. J Neuroimmune Pharmacol. 2021 Jun;16(2):403-424. doi: 10.1007/s11481-020-09933-8[↩]
- Singh D, Gupta M, Sarwat M, Siddique HR. Apigenin in cancer prevention and therapy: A systematic review and meta-analysis of animal models. Crit Rev Oncol Hematol. 2022 Aug;176:103751. doi: 10.1016/j.critrevonc.2022.103751[↩]
- Zhou Y, Yu Y, Lv H, Zhang H, Liang T, Zhou G, Huang L, Tian Y, Liang W. Apigenin in cancer therapy: From mechanism of action to nano-therapeutic agent. Food Chem Toxicol. 2022 Oct;168:113385. doi: 10.1016/j.fct.2022.113385[↩]
- Tong, J, Shen, Y, Zhang, Z, Hu, Y, Zhang, X, and Han, L. Apigenin inhibits epithelial-mesenchymal transition of human colon cancer cells through NF-κB/snail signaling pathway. Biosci Rep. (2019) 39:452. doi: 10.1042/BSR20190452[↩]
- Nelson, N, Szekeres, K, Iclozan, C, Rivera, IO, McGill, A, Johnson, G, et al. Apigenin: selective CK2 inhibitor increases Ikaros expression and improves T cell homeostasis and function in murine pancreatic cancer. PLoS One. (2017) 12:e0170197. doi: 10.1371/journal.pone.0170197[↩]
- Nicholas, C, Batra, S, Vargo, MA, Voss, OH, Gavrilin, MA, Wewers, MD, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. (2007) 179:7121–7. doi: 10.4049/jimmunol.179.10.7121[↩]
- Zheng, S, Cao, P, Yin, Z, Wang, X, Chen, Y, Yu, M, et al. Apigenin protects mice against 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced cholestasis. Food Funct. (2021) 12:2323–34. doi: 10.1039/D0FO02910F[↩]
- Chan, LP, Chou, TH, Ding, HY, Chen, PR, Chiang, FY, Kuo, PL, et al. Apigenin induces apoptosis via tumor necrosis factor receptor-and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta. (2012) 1820:1081–91. doi: 10.1016/j.bbagen.2012.04.013[↩]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060-72. doi: 10.1016/j.cell.2012.03.042[↩]
- Chen, X, Kang, R, Kroemer, G, and Tang, D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. (2021) 218:e20210518. doi: 10.1084/jem.20210518[↩]
- Koppula, P, Zhuang, L, and Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. (2021) 12:599–620. doi: 10.1007/s13238-020-00789-5[↩]
- Jang, JY, Sung, B, and Kim, ND. Role of induced programmed cell death in the Chemopreventive potential of Apigenin. Int J Mol Sci. (2022) 23:3757. doi: 10.3390/ijms23073757[↩][↩]
- Adham, AN, Abdelfatah, S, Naqishbandi, AM, Mahmoud, N, and Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine. (2021) 80:153371. doi: 10.1016/j.phymed.2020.153371[↩][↩]
- Liu, R, Rong, G, Liu, Y, Huang, W, He, D, and Lu, R. Delivery of apigenin-loaded magnetic Fe2O3/Fe3O4@mSiO2 nanocomposites to A549 cells and their antitumor mechanism. Mater Sci Eng C Mater Biol Appl. (2021) 120:111719. doi: 10.1016/j.msec.2020.111719[↩]
- Lefort, ÉC, and Blay, J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. (2013) 57:126–44. doi: 10.1002/mnfr.201200424[↩]
- Pápay, ZE, Kállai-Szabó, N, Balogh, E, Ludányi, K, Klebovich, I, and Antal, I. Controlled release Oral delivery of Apigenin containing pellets with antioxidant activity. Curr Drug Deliv. (2017) 14:145–54. doi: 10.2174/1567201813666160602193047[↩]
- Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K. Reactive oxygen species – sources, functions, oxidative damage. Pol Merkur Lekarski. 2020 Apr 22;48(284):124-127. https://medpress.com.pl/pubmed.php?article=284124[↩]
- Di Meo, S, and Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxidative Med Cell Longev. (2020) 2020:9829176. doi: 10.1155/2020/9829176[↩]
- Cyr, AR, Huckaby, LV, Shiva, SS, and Zuckerbraun, BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. (2020) 36:307–21. doi: 10.1016/j.ccc.2019.12.009[↩]
- Thiruvengadam, M, Venkidasamy, B, Subramanian, U, Samynathan, R, Ali Shariati, M, Rebezov, M, et al. Bioactive compounds in oxidative stress-mediated diseases: targeting the NRF2/ARE signaling pathway and epigenetic regulation. Antioxidants (Basel). (2021) 10:1859. doi: 10.3390/antiox10121859[↩]
- Yi, YS. Regulatory roles of flavonoids on Inflammasome activation during inflammatory responses. Mol Nutr Food Res. (2018) 62:e1800147. doi: 10.1002/mnfr.201800147[↩]
- Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. (2022) 27:2901. doi: 10.3390/molecules27092901[↩]
- Park CH, Min SY, Yu HW, Kim K, Kim S, Lee HJ, et al.. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci. (2020) 21:4620. doi: 10.3390/ijms21134620[↩]
- Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res. (2007) 30:1318–27. doi: 10.1007/BF02980273[↩]
- Patel M, Singh S. Apigenin Attenuates Functional and Structural Alterations via Targeting NF-kB/Nrf2 Signaling Pathway in LPS-Induced Parkinsonism in Experimental Rats : Apigenin Attenuates LPS-Induced Parkinsonism in Experimental Rats. Neurotox Res. (2022) 40:941–60. doi: 10.1007/s12640-022-00521-7[↩]
- Al-Ghraiybah NF, Wang J, Alkhalifa AE, Roberts AB, Raj R, Yang E, et al.. Glial cell-mediated Neuroinflammation in Alzheimer’s disease. Int J Mol Sci. (2022) 23:10572. doi: 10.3390/ijms231810572[↩]
- Chen P, Huo X, Liu W, Li K, Sun Z, Tian J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol Immunotoxicol. (2020) 42:9–16. doi: 10.1080/08923973.2019.1688345[↩]
- Chumsakul O, Wakayama K, Tsuhako A, Baba Y, Takai Y, Kurose T, et al.. Apigenin regulates activation of microglia and counteracts retinal degeneration. J Ocul Pharmacol Ther. (2020) 36:311–9. doi: 10.1089/jop.2019.0163[↩]
- Coelho PLC, Amparo JAO, da Silva AB, da Silva KC, Braga-de-Souza S, Barbosa PR, et al.. Apigenin from Croton betulaster Müll restores the immune profile of microglia against glioma cells. Phytother Res. (2019) 33:3191–202. doi: 10.1002/ptr.6491[↩]
- Özçelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceutical Biology. 2011;49(4):396–402. doi: 10.3109/13880209.2010.519390[↩]
- Cushnie T. T., Hamilton V. E., Lamb A. J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiological Research. 2003;158(4):281–289. doi: 10.1078/0944-5013-00206[↩]
- Mamadalieva N. Z., Herrmann F., El-Readi M. Z., et al. Flavonoids in Scutellaria immaculata and S. ramosissima (Lamiaceae) and their biological activity. Journal of Pharmacy and Pharmacology. 2011;63(10):1346–1357. doi: 10.1111/j.2042-7158.2011.01336.x[↩]
- Dong J., Qiu J., Wang J. Apigenin alleviates the symptoms of Staphylococcus aureus pneumonia by inhibiting the production of alpha-hemolysin. FEMS Microbiology Letters. 2013;338(2):124–131. doi: 10.1111/1574-6968.12040[↩][↩][↩][↩][↩][↩]
- Xia F., Li X., Wang B., et al. Combination Therapy of LysGH15 and Apigenin as a New Strategy for Treating Pneumonia Caused by Staphylococcus aureus. Applied and Environmental Microbiology. 2015;82(1):87–94. doi: 10.1128/AEM.02581-15[↩]
- Konstantinopoulou M., Karioti A., Skaltsas S., Skaltsa H. Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori activity. Journal of Natural Products. 2003;66(5):699–702. doi: 10.1021/np020472m[↩]
- Wu D., Kong Y., Han C., et al. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. International Journal of Antimicrobial Agents. 2008;32(5):421–426. doi: 10.1016/j.ijantimicag.2008.06.010[↩]
- Kuo C.-H., Weng B.-C., Wu C.-C., Yang S.-F., Wu D.-C., Wang Y.-C. Apigenin has anti-atrophic gastritis and anti-gastric cancer progression effects in Helicobacter pylori-infected Mongolian gerbils. Journal of Ethnopharmacology. 2014;151(3):1031–1039. doi: 10.1016/j.jep.2013.11.040[↩][↩]
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009 Aug;29(4):378-82. doi: 10.1097/JCP.0b013e3181ac935c[↩]
- Amsterdam J. D., Li Y., Soeller I., Rockwell K., Mao J. J., Shults J. A Randomized, Double-Blind, Placebo-Controlled Trial of Oral Matricaria recutita (Chamomile) Extract Therapy for Generalized Anxiety Disorder. Journal of Clinical Psychopharmacology. 2009;29(4):378–382. doi: 10.1097/JCP.0b013e3181ac935c[↩][↩]
- Mao J.J., Xie S.X., Keefe J.R., Soeller I., Li Q.S., Amsterdam J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine. 2016;23:1735–1742. doi: 10.1016/j.phymed.2016.10.012[↩][↩][↩]
- Amsterdam JD, Shults J, Soeller I, Mao JJ, Rockwell K, Newberg AB. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012 Sep-Oct;18(5):44-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3600408[↩][↩]
- Zanoli P., Avallone R., Baraldi M. Behavioural characterization of the flavonoids apigenin and crysin. Fitoterapia. 2000;71:S117–S123. doi: 10.1016/S0367-326X(00)00186-6[↩][↩][↩]
- Avallone R., Zanoli P., Puia G., Kleinschnitz M., Shreiner P., Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59:1387–1394. doi: 10.1016/S0006-2952(00)00264-1[↩]
- Weng L., Guo X., Li Y., Yang X., Han Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016;774:50–54. doi: 10.1016/j.ejphar.2016.01.015[↩][↩][↩]
- Nakazawa T., Yasuda T., Ueda J., Ohsawa K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474[↩]
- Han X.H., Hong S.S., Hwang J.S., Lee M.K., Hwang B.Y., Ro J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharmacal Res. 2007;30:13. doi: 10.1007/BF02977772[↩]
- Chaurasiya N., Ibrahim M., Muhammad I., Walker L., Tekwani B. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules. 2014;19:18936–18952. doi: 10.3390/molecules191118936[↩]
- Lorenzo P.S., Rubio M.C., Medina J.H., Adler-Graschinsky E. Involvement of monoamine oxidase and noradrenaline uptake in the positive chronotropic effects of apigenin in rat atria. Eur. J. Pharmacol. 1996;312:203–207. doi: 10.1016/0014-2999(96)00486-4[↩]
- Morita K., Hamano S., Oka M., Teraoka K. Stimulatory actions of bioflavonoids on tyrosine uptake into cultured bovine adrenal chromaffin cells. Biochem. Biophys. Res. Commun. 1990;171:1199–1204. doi: 10.1016/0006-291X(90)90812-2[↩]
- Yi L.T., Li J.M., Li Y.C., Pan Y., Xu Q., Kong L. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008;82:741–751. doi: 10.1016/j.lfs.2008.01.007[↩]
- Li R., Zhao D., Qu R., Fu Q., Ma S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015;594:17–22. doi: 10.1016/j.neulet.2015.03.040[↩][↩][↩][↩]
- Leach M.J., Page A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015;24:1–12. doi: 10.1016/j.smrv.2014.12.003[↩]
- Zick S.M., Wright B.D., Sen A., Arnedt J.T. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: A randomized placebo-controlled pilot study. BMC Complement. Altern. Med. 2011;11:78. doi: 10.1186/1472-6882-11-78[↩][↩][↩]
- Campos-Bedolla P., Walter F.R., Veszelka S., Deli M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014;45:610–638. doi: 10.1016/j.arcmed.2014.11.018[↩]
- Végh K., Riethmüller E., Hosszú L., Darcsi A., Müller J., Alberti Á., Tóth A., Béni S., Könczöl Á., Balogh G.T., et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018;149:488–493. doi: 10.1016/j.jpba.2017.11.029[↩]
- Yang Y., Bai L., Li X., Xiong J., Xu P., Guo C., Xue M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. In Vitro. 2014;28:388–396. doi: 10.1016/j.tiv.2013.12.002[↩]
- Jangdey MS, Kaur CD, Saraf S. Efficacy of Concanavalin-a conjugated nanotransfersomal gel of apigenin for enhanced targeted delivery of UV induced skin malignant melanoma. Artif Cells Nanomed Biotechnol. (2019) 47:904–16. doi: 10.1080/21691401.2019.1578784[↩]
- Zhang B, Wang J, Zhao G, Lin M, Lang Y, Zhang D, et al.. Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones. (2020) 25:277–85. doi: 10.1007/s12192-020-01071-7[↩]
- Vergani L, Vecchione G, Baldini F, Grasselli E, Voci A, Portincasa P, et al.. Polyphenolic extract attenuates fatty acid-induced steatosis and oxidative stress in hepatic and endothelial cells. Eur J Nutr. (2018) 57:1793–805. doi: 10.1007/s00394-017-1464-5[↩]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al.. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. (2006) 54:9966–77. doi: 10.1021/jf061478a[↩]
- Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. (2005) 24:376–84. doi: 10.1080/07315724.2005.10719488[↩]
- Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandström B, et al.. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. (1999) 81:447–55. doi: 10.1017/S000711459900080X[↩]
- Hostetler G.L., Ralston R.A., Schwartz S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017;8:423–435. doi: 10.3945/an.116.012948[↩]
- Bhagwat S., Haytowitz D. B., Holden J. M. USDA Database for the Flavonoid Content of Selected Foods. U.S. Department of Argiculture. 2011:1–156.[↩]
- Lee W., Chen W., Wang C., Lin W., Tseng T. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and β4 integrin function in MDA-MB-231 breast cancer cells. Toxicology and Applied Pharmacology. 2008;226(2):178–191. doi: 10.1016/j.taap.2007.09.013[↩]
- Mushtaq, Zarina & Sadeer, Nabeelah & Hussain, Muzzamal & Mahwish, & Alsagaby, Suliman & Imran, Muhammad & Mumtaz, Tamseela & Umar, Maryam & Tauseef, Ambreen & Alabdulmonem, Waleed & Tufail, Tabussam & Al Jbawi, Entessar & Mahomoodally, Fawzi. (2023). Therapeutical properties of apigenin: a review on the experimental evidence and basic mechanisms. International Journal of Food Properties. 26. 1914-1939. 10.1080/10942912.2023.2236329[↩][↩][↩]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3(6), 423–446. DOI: 10.1007/s40495-017-0113-2[↩]
- McKay D. L., Blumberg J. B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytotherapy Research. 2006;20(7):519–530. doi: 10.1002/ptr.1900[↩]
- Hostetler GL, Ralston RA, Schwartz SJ. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv Nutr. 2017 May 15;8(3):423-435. doi: 10.3945/an.116.012948[↩]
- Kashyap P, Shikha D, Thakur M, Aneja A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: a review. J Food Biochem. (2022) 46:e13950. doi: 10.1111/jfbc.13950[↩]
- Tang D, Chen K, Huang L, Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin Drug Metab Toxicol. (2017) 13:323–30. doi: 10.1080/17425255.2017.1251903[↩][↩]
- Wang M, Firrman J, Liu L, Yam K. A review on flavonoid Apigenin: dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed Res Int. (2019) 2019:1–18. doi: 10.1155/2019/7010467[↩][↩]
- Hanske L, Loh G, Sczesny S, Blaut M, Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr. (2009) 139:1095–102. doi: 10.3945/jn.108.102814[↩]
- Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. (2013) 24:1415–22. doi: 10.1016/j.jnutbio.2013.05.001[↩]
- Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. (2002) 30:370–7. doi: 10.1124/dmd.30.4.370[↩]
- Zhang J, Liu D, Huang Y, Gao Y, Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int J Pharm. (2012) 436:311–7. doi: 10.1016/j.ijpharm.2012.07.002[↩]
- Gradolatto A, Basly JP, Berges R, Teyssier C, Chagnon MC, Siess MH, et al.. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos. (2005) 33:49–54. doi: 10.1124/dmd.104.000893[↩]
- Chen T, Li LP, Lu XY, Jiang HD, Zeng S. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J Agric Food Chem. (2007) 55:273–7. doi: 10.1021/jf062088r[↩][↩]
- Barr JT, Tran TB, Rock BM, Wahlstrom JL, Dahal UP. Strain-dependent variability of early discovery small molecule pharmacokinetics in mice: does strain matter? Drug Metab Dispos. (2020) 48:613–21. doi: 10.1124/dmd.120.090621[↩]
- Lefort ÉC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. (2013) 57:126–44. doi: 10.1002/mnfr.201200424[↩]
- Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metabol. (2006) 50:167–72. doi: 10.1159/000090736[↩][↩]
- Gonzales GB, Smagghe G, Grootaert C, Zotti M, Raes K, Van Camp J. Flavonoid interactions during digestion, absorption, distribution and metabolism: a sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab Rev. (2015) 47:175–90. doi: 10.3109/03602532.2014.1003649[↩]
- Wan L, Guo C, Yu Q, Li Y, Wang X, Wang X, et al.. Quantitative determination of apigenin and its metabolism in rat plasma after intravenous bolus administration by HPLC coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. (2007) 855:286–9. doi: 10.1016/j.jchromb.2007.05.007[↩]
- Michaelis M, Rothweiler F, Nerreter T, Sharifi M, Ghafourian T, Cinatl J. Karanjin interferes with ABCB1, ABCC1, and ABCG2. J Pharm Pharm Sci. (2014) 17:92–105. doi: 10.18433/J3BW2S[↩]
- Braidot E, Zancani M, Petrussa E, Peresson C, Bertolini A, Patui S, et al.. Transport and accumulation of flavonoids in grapevine(Vitis vinifera L.). Plant Signal Behav. (2008) 3:626–32. doi: 10.4161/psb.3.9.6686[↩]
- Catelli Rocha Torres L, de Oliveira G, Sartori A, de Souza Silva AP, Matias de Alencar S. Bioaccessibility and uptake/epithelial transport of vitamin E: discoveries and challenges of in vitro and ex vivo assays. Food Res Int. (2022) 162:112143. doi: 10.1016/j.foodres.2022.112143[↩]
- Kashyap P, Anand S, Thakur A. Evaluation of antioxidant and antimicrobial activity of Rhododendron arboreum flowers extract. Int J Food Ferment Technol. (2017) 7:123–8. doi: 10.5958/2277-9396.2017.00013.7[↩]
- Zhang DY, Zu YG, Fu YJ, Luo M, Wang W, Gu CB, et al.. Enzyme pretreatment and negative pressure cavitation extraction of genistein and apigenin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.] and the evaluation of antioxidant activity[J]. Industrial Crops Products. (2012) 37:311–20. doi: 10.1016/j.indcrop.2011.12.026[↩]
- Ng SP, Wong KY, Zhang L, Zuo Z, Lin G. Evaluation of the first-pass glucuronidation of selected flavones in gut by Caco-2 monolayer model. J Pharm Pharm Sci. 2004 Dec 20;8(1):1-9.[↩]
- Wang SW, Kulkarni KH, Tang L, Wang JR, Yin T, Daidoji T, et al.. Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities. J Pharmacol Exp Ther. (2009) 329:1023–31. doi: 10.1124/jpet.108.147371[↩]
- Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010 Jan;103(2):249-55. doi: 10.1017/S000711450999170X[↩]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004 May;79(5):727-47. doi: 10.1093/ajcn/79.5.727[↩]
- Shakeri F., Boskabady M.H. A review of the relaxant effect of various medicinal plants on tracheal smooth muscle, their possible mechanism(s) and potency. J. Ethnopharmacol. 2015;175:528–548. doi: 10.1016/j.jep.2015.10.017[↩]
- Ross J.A., Kasum C.M. Dietary flavonoids: Bioavailability metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957[↩]
- Ali F., Rahul ., Naz F., Jyoti S., Siddique Y. H. Health functionality of apigenin: A review. International Journal of Food Properties. 2016;20(6):1197–1238. doi: 10.1080/10942912.2016.1207188[↩][↩][↩]
- Xu H., Lee S. F. Activity of plant flavonoids against antibiotic‐resistant bacteria. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2001;15(1):39–43. doi: 10.1002/1099-1573(200102)15:1<39::AID-PTR684>3.0.CO;2-R[↩]
- Way T.-D., Kao M.-C., Lin J.-K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. The Journal of Biological Chemistry. 2004;279(6):4479–4489. doi: 10.1074/jbc.m305529200[↩]
- Birt D. F., Walker B., Tibbels M. G., Bresnick E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis. 1986;7(6):959–963. doi: 10.1093/carcin/7.6.959[↩]
- Czeczot H., Tudek B., Kusztelak J., et al. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutation Research – Genetic Toxicology and Environmental Mutagenesis. 1990;240(3):209–216. doi: 10.1016/0165-1218(90)90060-F[↩]
- Gulluce M., Orhan F., Yanmis D., Arasoglu T., Guvenalp Z., Demirezer L. O. Isolation of a flavonoid, apigenin 7-O-glucoside, from Mentha longifolia (L.) Hudson subspecies longifolia and its genotoxic potency. Toxicology & Industrial Health. 2015;31(9):831–840. doi: 10.1177/0748233713475511[↩][↩]
- Hashemi M, Nouri Long M, Entezari M, Nafisi S, Nowroozii H. Anti-mutagenic and pro-apoptotic effects of apigenin on human chronic lymphocytic leukemia cells. Acta Med Iran. 2010 Sep-Oct;48(5):283-8.[↩]
- Banerjee K., Banerjee S., Das S., Mandal M. Probing the potential of apigenin liposomes in enhancing bacterial membrane perturbation and integrity loss. Journal of Colloid and Interface Science. 2015;453:48–59. doi: 10.1016/j.jcis.2015.04.030[↩]
- Song M., Li L., Li M., Cha Y., Deng X., Wang J. Apigenin protects mice from pneumococcal pneumonia by inhibiting the cytolytic activity of pneumolysin. Fitoterapia. 2016;115:31–36. doi: 10.1016/j.fitote.2016.09.017[↩]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010 Jun;27(6):962-78. doi: 10.1007/s11095-010-0089-7[↩]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19-34. doi: 10.1146/annurev.nutr.22.111401.144957[↩]
- Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999 Dec;31 Suppl:S75-80. doi: 10.1080/10715769900301351[↩]
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, Medina JH, Paladini AC. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995 Jun;61(3):213-6. doi: 10.1055/s-2006-958058[↩]