Contents
- What is arteriovenous malformation
- Arteriovenous malformation symptoms
- What are the health consequences of arteriovenous malformations?
- What causes vascular lesions in the brain and spinal cord?
- How are arteriovenous malformations and other vascular lesions diagnosed?
- Arteriovenous malformation treatment
- What is an avm in the brain
- Types of brain arteriovenous malformations
- Brain arteriovenous malformation signs and symptoms
- Brain arteriovenous malformation causes
- Risk factors for brain arteriovenous malformation
- Brain arteriovenous malformation complications
- How are cerebral arteriovenous malformations diagnosed?
- Cerebral arteriovenous malformation treatment
- Pulmonary arteriovenous malformation
What is arteriovenous malformation
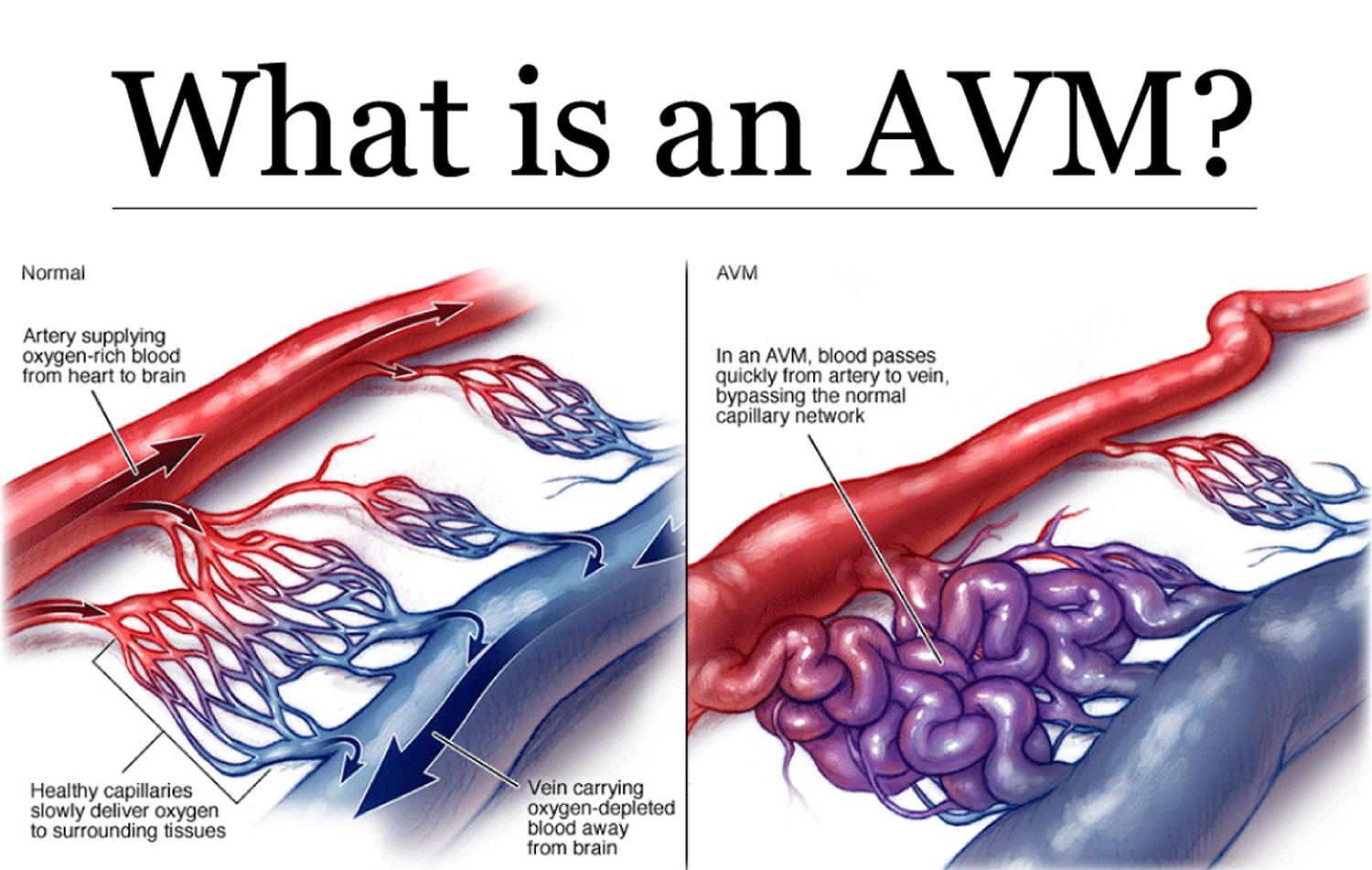
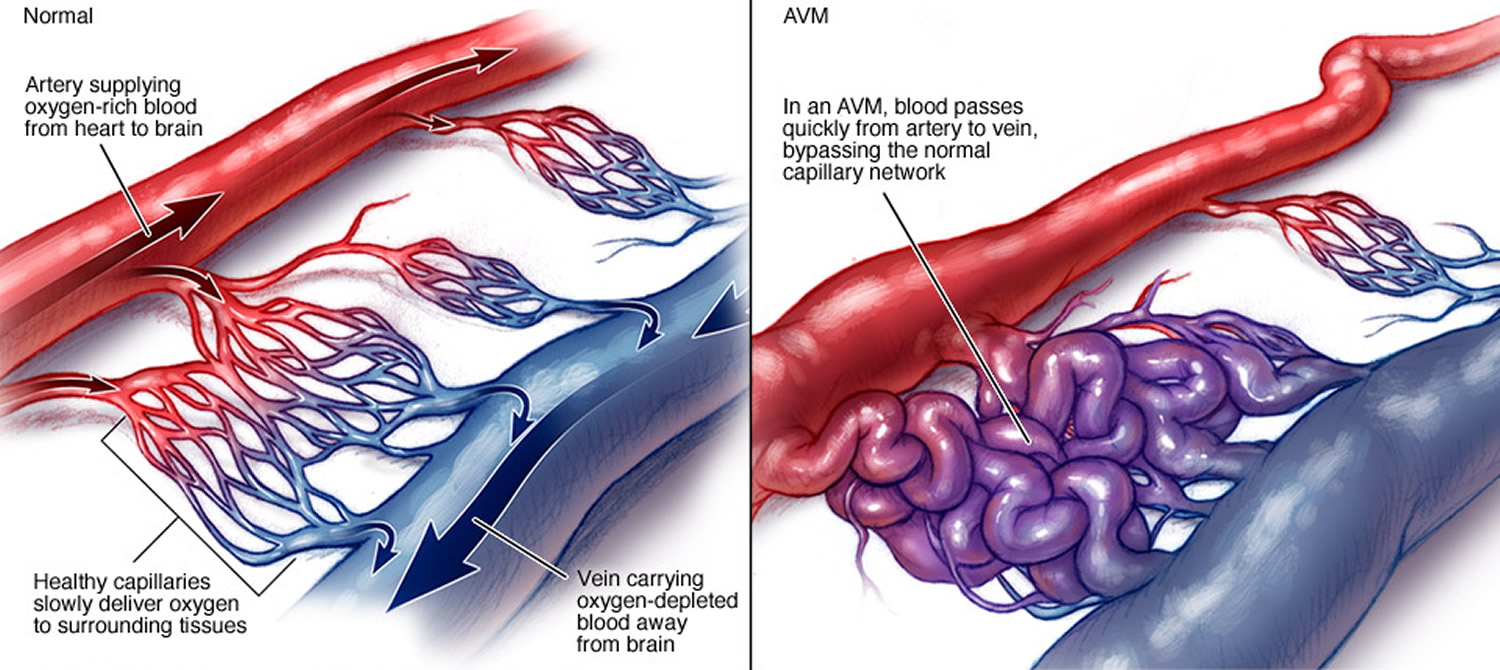
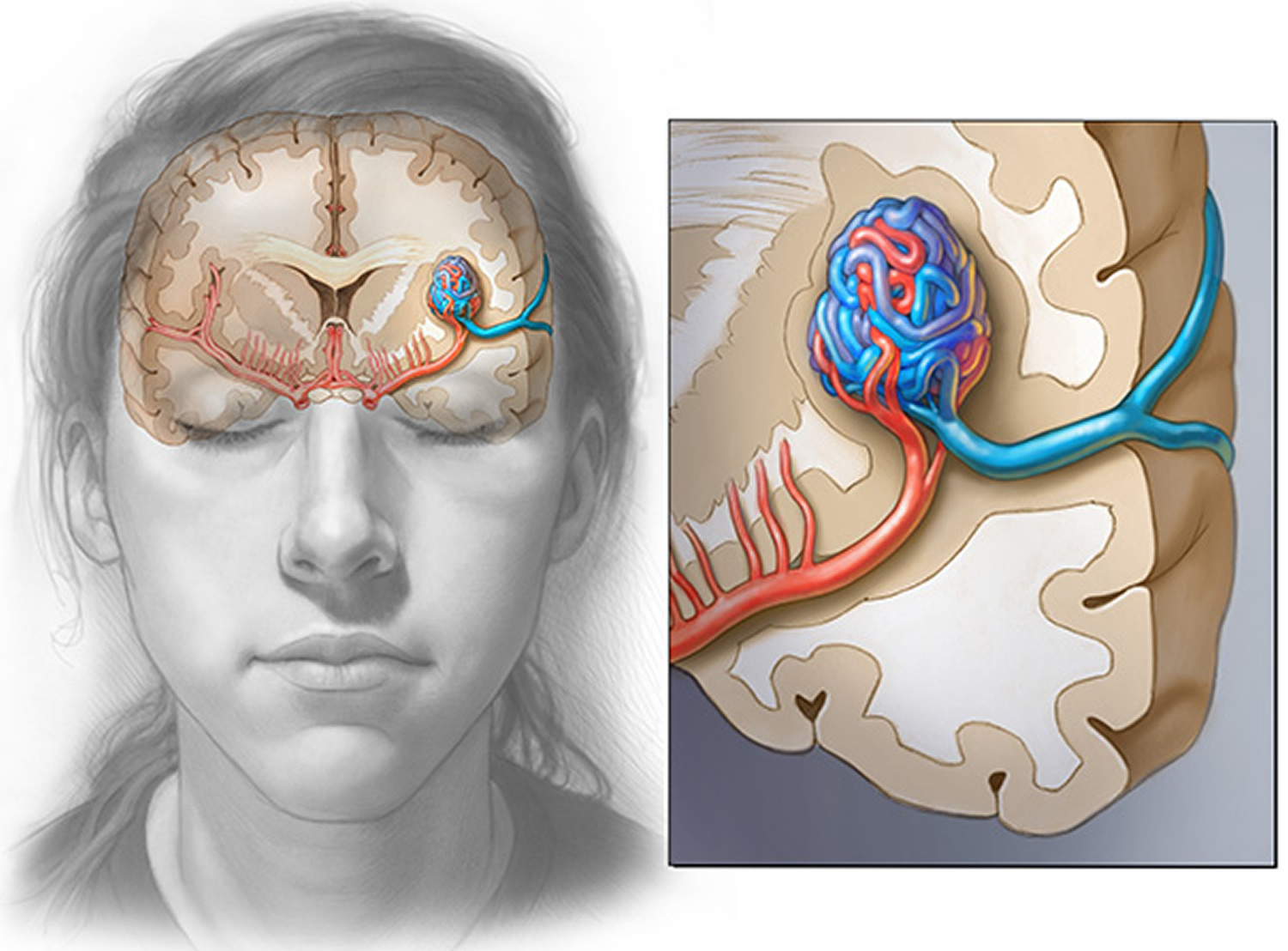
Arteriovenous malformations (AVMs) are abnormal, snarled tangles of blood vessels that cause multiple irregular connections between the arteries and veins. These arteriovenous malformations most often occur in the spinal cord and in any part of the brain or on its surface, but can develop elsewhere in the body. Normally, arteries carry blood containing oxygen from the heart to the brain, and veins carry blood with less oxygen away from the brain and back to the heart. But in an arteriovenous malformation, the absence of capillaries—a network of small blood vessels that connect arteries to veins and deliver oxygen to cells—creates a shortcut for blood to pass directly from arteries to veins and bypass tissue, which can lead to tissue damage and the death of nerve cells and other cells. Over time, some arteriovenous malformations become progressively larger as the amount of blood flow increases.
Figure 1. Arteriovenous malformation – in an arteriovenous malformation (AVM), blood passes quickly from the artery to vein, disrupting the normal blood flow and depriving the surrounding tissues of oxygen
Most people with brain or spinal cord arteriovenous malformations have few, if any, major symptoms. Sometimes they can cause seizures or headaches.
Patients with arteriovenous malformation located in and around the spinal cord can develop many neurological problems. Some problems include, weakness, pain, difficulty walking, paralysis, and even death.
Arteriovenous malformations are rare. The cause is not known, but they seem to develop during pregnancy or soon after birth. Doctors use imaging tests to detect them.
The greatest danger for a arteriovenous malformation is hemorrhage.
In some cases, a weakened blood vessel may burst, spilling blood into the brain (hemorrhage) that can cause stroke and brain damage. Other neurological problems include headache, weakness, seizures, pain, and problems with speech, vision, or movement. In most cases, people with neurological arteriovenous malformations experience few, if any, significant symptoms.
It is unclear why arteriovenous malformations form. Most often arteriovenous malformations are congenital, but they can appear sporadically. In some cases the arteriovenous malformation may be inherited, but it is more likely that other inherited conditions increase the risk of having an arteriovenous malformation. The malformations tend to be discovered only incidentally, usually during treatment for an unrelated disorder or at autopsy. It is estimated that brain arteriovenous malformations occur in less than one percent of the general population; each year about one percent of those with arteriovenous malformations will die as a direct result of the arteriovenous malformation.
Treatment options depend on the type of arteriovenous malformation, its location, noticeable symptoms, and the general health condition of the individual at the time of treatment.
The treatment is aimed at stopping the neurologic problems from worsening and possibly correcting the existing problems. There are two commonly used treatments for arteriovenous malformations, surgery and embolization (blocking off of blood flow to the arteriovenous malformation). However, researchers have limited experience treating these conditions because they are rare. In addition, it has been difficult to classify different kinds of arteriovenous malformations and to develop new treatments for them.
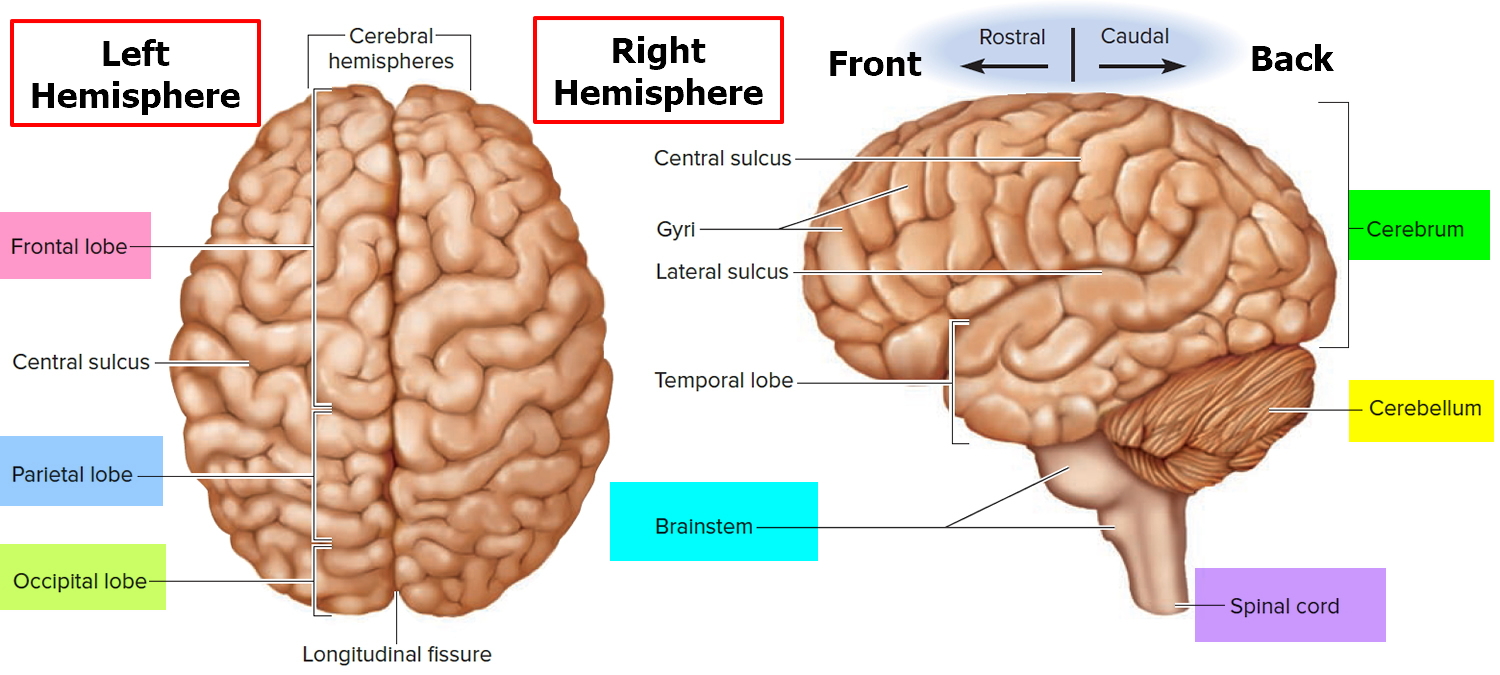
Figure 2. Human brain
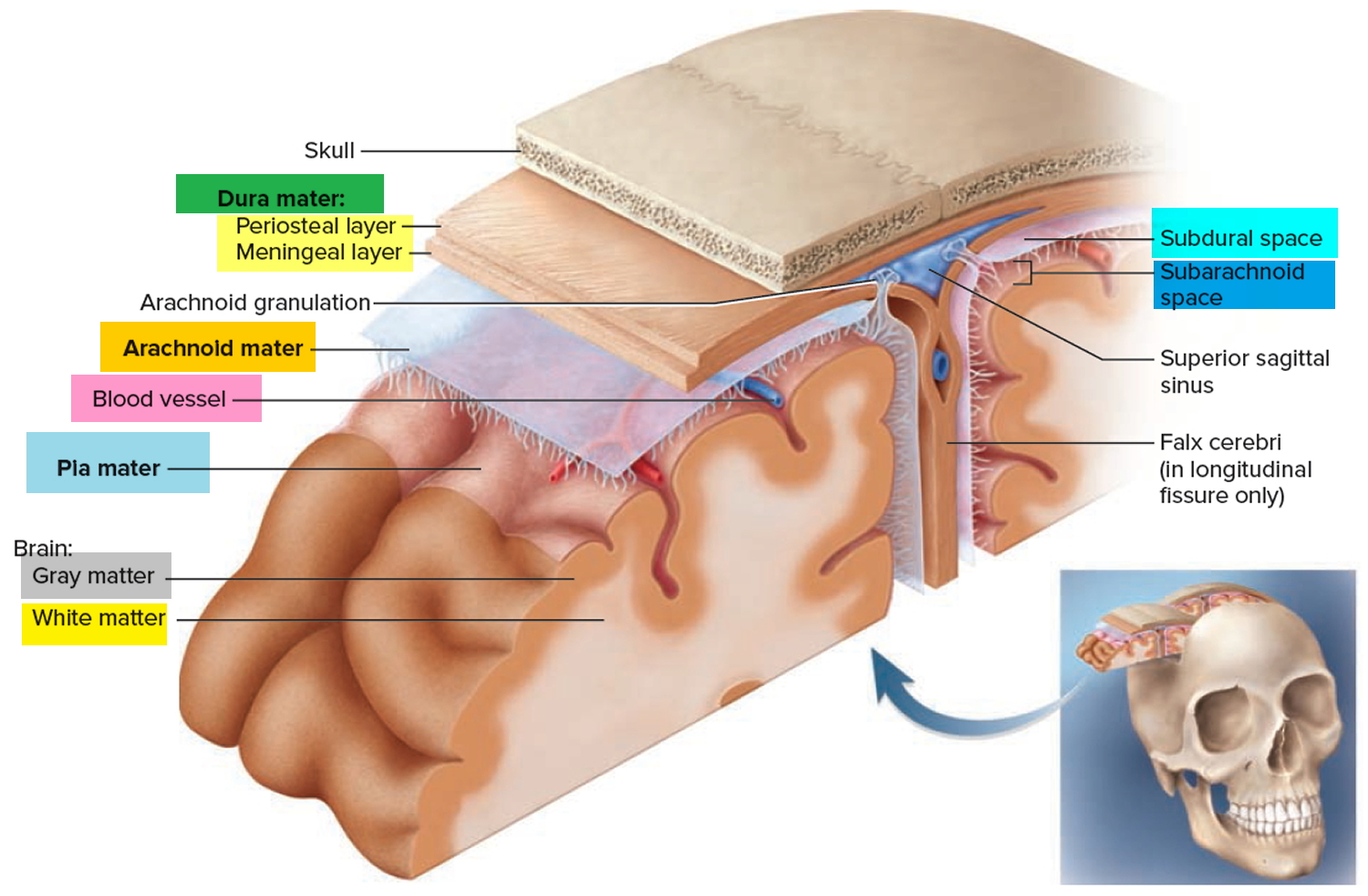
Figure 3. Meninges of the brain
Where do neurological arteriovenous malformations tend to form?
Arteriovenous malformations can form virtually anywhere in the brain or spinal cord—wherever arteries and veins exist. Some are formed from blood vessels located in the dura mater or in the pia mater, the outermost and innermost, respectively, of the three membranes surrounding the brain and spinal cord (see Figure 2 above). The third membrane, called the arachnoid, lacks blood vessels. Arteriovenous malformations of the dura mater affect the function of the spinal cord by transmitting excess pressure to the venous system of the spinal cord. Arteriovenous malformations of the spinal cord affect the function of the spinal cord by hemorrhage, by reducing blood flow to the spinal cord, or by causing excess venous pressure. Spinal arteriovenous malformations frequently cause attacks of sudden, severe back pain, often concentrated at the roots of nerve fibers where they exit the vertebrae, with pain that is similar to that caused by a herniated (slipped) disk. These lesions also can cause sensory disturbances, muscle weakness, or paralysis in the parts of the body served by the spinal cord or the damaged nerve fibers. A spinal cord arteriovenous malformation can lead to degeneration of the nerve fibers within the spinal cord below the level of the lesion, causing widespread paralysis in parts of the body controlled by those nerve fibers.
Arteriovenous malformations on the surface of the cerebral hemispheres—the uppermost portions of the brain—exert pressure on the cerebral cortex, the brain’s “gray matter.” Depending on their location, these arteriovenous malformations may damage portions of the cerebral cortex involved with thinking, speaking, understanding language, hearing, taste, touch, or initiating and controlling voluntary movements. arteriovenous malformations located on the frontal lobe close to the optic nerve or on the occipital lobe (the rear portion of the cerebrum where images are processed) may cause a variety of visual disturbances.
Arteriovenous malformations also can form from blood vessels located deep inside the interior of the cerebrum (the main portion of the brain). These arteriovenous malformations may compromise the functions of three vital structures: the thalamus, which transmits nerve signals between the spinal cord and upper regions of the brain; the basal ganglia surrounding the thalamus, which coordinate complex movements and plays a role in learning and memory; and the hippocampus, which plays a major role in memory.
Arteriovenous malformations can affect other parts of the brain besides the cerebrum. The hindbrain is formed from two major structures: the cerebellum, which is nestled under the rear portion of the cerebrum, and the brain stem, which serves as the bridge linking the upper portions of the brain with the spinal cord. These structures control finely coordinated movements, maintain balance, and regulate some functions of internal organs, including those of the heart and lungs. arteriovenous malformation damage to these parts of the hindbrain can result in dizziness, giddiness, vomiting, a loss of the ability to coordinate complex movements such as walking, or uncontrollable muscle tremors.
How do arteriovenous malformations damage the brain and spinal cord?
Arteriovenous malformations damage the brain or spinal cord through three basic mechanisms by:
- Reducing the amount of oxygen reaching neurological tissues;
- Causing bleeding (hemorrhage) into surrounding tissues; and
- Compressing or displacing parts of the brain or spinal cord.
- Arteriovenous malformations affect oxygen delivery to the brain or spinal cord by altering normal patterns of blood flow using the arteries, veins, and capillaries. In arteriovenous malformations arteries pump blood directly into veins through a passageway called a fistula. Since the network of capillaries is bypassed, the rate of blood flow is uncontrolled and too rapid to allow oxygen to be dispersed to surrounding tissues. As a result, the cells that make up these tissues become oxygen-depleted and begin to deteriorate, sometimes dying off completely.
- This abnormally rapid rate of blood flow frequently causes blood pressure inside the vessels located in the central portion of an arteriovenous malformation directly adjacent to the fistula—an area doctors refer to as the nidus—to rise to dangerously high levels. The arteries feeding blood into the arteriovenous malformation often become swollen and distorted; the veins that drain blood away from it often become abnormally constricted (a condition called stenosis). Also, the walls of the involved arteries and veins are often abnormally thin and weak. Aneurysms—balloon-like bulges in blood vessel walls that are susceptible to rupture—may develop in association with approximately half of all neurological arteriovenous malformations due to this structural weakness.
- Bleeding into the brain, called intracranial hemorrhage, can result from the combination of high internal pressure and vessel wall weakness. Such hemorrhages are often microscopic in size (called microbleeds), causing limited damage and few significant symptoms. Generally, microbleeds do not have short-term consequences on brain function, but microbleeds over time can lead to an increased risk of dementia and cognitive disruption. Even many nonsymptomatic arteriovenous malformations show evidence of past bleeding. But massive hemorrhages can occur if the physical stresses caused by extremely high blood pressure, rapid blood flow rates, and vessel wall weakness are great enough. If a large enough volume of blood escapes from a ruptured arteriovenous malformation into the surrounding brain, the result can be a catastrophic stroke. arteriovenous malformations account for approximately two percent of all hemorrhagic strokes that occur each year.
- Even in the absence of bleeding or significant oxygen depletion, large arteriovenous malformations can damage the brain or spinal cord simply by their presence. They can range in size from a fraction of an inch to more than 2.5 inches in diameter, depending on the number and size of the blood vessels making up the lesion. The larger the lesion, the greater the amount of pressure it exerts on surrounding brain or spinal cord structures. The largest lesions may compress several inches of the spinal cord or distort the shape of an entire hemisphere of the brain. Such massive arteriovenous malformations can constrict the flow of cerebrospinal fluid—a clear liquid that normally nourishes and protects the brain and spinal cord—by distorting or closing the passageways and open chambers (ventricles) inside the brain that allow this fluid to circulate freely. As cerebrospinal fluid accumulates, hydrocephalus results. This fluid buildup further increases the amount of pressure on fragile neurological structures, adding to the damage caused by the arteriovenous malformation itself.
Arteriovenous malformation symptoms
Symptoms can vary greatly in severity; in some people the severity of symptoms becomes debilitating or even life-threatening.
Seizures and headaches that may be severe are the most generalized symptoms of arteriovenous malformations, but no particular type of seizure or headache pattern has been identified. Seizures can be focal (meaning they involve a small part of the brain) or generalized (widespread), involving convulsions, a loss of control over movement, or a change in a person’s level of consciousness. Headaches can vary greatly in frequency, duration, and intensity, sometimes becoming as severe as migraines. Pain may be on either one side of the head or on both sides. Sometimes a headache consistently affecting one side of the head may be closely linked to the site of an arteriovenous malformation. Most often, the location of the pain is not specific to the malformation and may encompass most of the head.
Arteriovenous malformations also can cause a wide range of more specific neurological symptoms that vary from person to person, depending primarily upon the location of the arteriovenous malformation. Such symptoms may include:
- muscle weakness or paralysis in one part of the body
- a loss of coordination (ataxia) that can lead to such problems as gait disturbances
- difficulties carrying out tasks that require planning (apraxia)
- back pain or weakness in the lower extremities caused by a spinal arteriovenous malformation
- dizziness
- visual problems such as a loss of part of the visual field, inability to control eye movement, or swelling of a part of the optic nerve
- difficulty speaking or understanding language (aphasia)
- abnormal sensations such as numbness, tingling, or spontaneous pain
- memory deficits
- confusion, hallucinations, or dementia.
Arteriovenous malformations may also cause subtle learning or behavioral disorders in some people during their childhood or adolescence, long before more obvious symptoms become evident.
Symptoms caused by arteriovenous malformations can appear at any age. Because the abnormalities tend to result from a slow buildup of neurological damage over time, they are most often noticed when people are in their twenties or older. If arteriovenous malformations do not become symptomatic by the time people reach their late forties or early fifties, they tend to remain stable and are less likely to produce symptoms. Some pregnant women may experience a sudden onset or worsening of symptoms due to accompanying cardiovascular changes, especially increases in blood volume and blood pressure.
Although most neurological arteriovenous malformations have very few, if any, significant symptoms, one particularly severe type of arteriovenous malformation causes symptoms to appear at, or very soon after, birth. Called a vein of Galen defect after the major blood vessel involved, this lesion is located deep inside the brain. It is frequently associated with hydrocephalus (an accumulation of fluid within certain spaces in the brain, often with visible enlargement of the head), swollen veins visible on the scalp, seizures, failure to thrive, and congestive heart failure. Children born with this condition who survive past infancy often remain developmentally impaired.
What are the health consequences of arteriovenous malformations?
The greatest potential danger posed by arteriovenous malformations is hemorrhage. Most episodes of bleeding remain undetected at the time they occur because they are not severe enough to cause significant neurological damage. But massive, even fatal, bleeding episodes do occur. Whenever an arteriovenous malformation is detected, the individual should be carefully and consistently monitored for any signs of instability that may indicate an increased risk of hemorrhage.
A few physical characteristics appear to indicate a greater-than-usual likelihood of clinically significant hemorrhage:
- Smaller arteriovenous malformations have a greater likelihood of bleeding than do larger ones.
- Impaired drainage by unusually narrow or deeply situated veins increases the chances of hemorrhage.
- Pregnancy appears to increase the likelihood of clinically significant hemorrhage, mainly because of increases in blood pressure and blood volume.
- Arteriovenous malformations that have hemorrhaged once are about nine times more likely to bleed again during the first year after the initial hemorrhage than are lesions that have never bled.
The damaging effects of a hemorrhage are related to lesion location. Bleeding from arteriovenous malformations located deep inside the interior tissues, or parenchyma, of the brain typically causes more severe neurological damage than does hemorrhage by lesions that have formed in the dural or pial membranes or on the surface of the brain or spinal cord. Deeply located bleeding is usually referred to as an intracerebral or parenchymal hemorrhage; bleeding within the membranes or on the surface of the brain is known as subdural or subarachnoid hemorrhage. Therefore, location is an important factor to consider when weighing the relative risks surgery to treat arteriovenous malformations.
What causes vascular lesions in the brain and spinal cord?
The cause of vascular anomalies of the central nervous system is not yet well understood. Scientists believe the anomalies most often result from mistakes that occur during embryonic or fetal development. These mistakes may be linked to genetic mutations in some cases. A few types of vascular malformations are known to be hereditary and thus are known to have a genetic basis. Some evidence also suggests that at least some of these lesions are acquired later in life as a result of injury to the central nervous system.
During fetal development, new blood vessels continuously form and then disappear as the human body changes and grows. These changes in the body’s vascular map continue after birth and are controlled by angiogenic factors, chemicals produced by the body that stimulate new blood vessel formation and growth. Researchers have identified changes in the chemical structures of various angiogenic factors in some people who have arteriovenous malformations or other vascular abnormalities of the central nervous system. However, it is not yet clear how these chemical changes actually cause changes in blood vessel structure.
By studying patterns of occurrence in families, researchers have established that one type of cavernous malformation involving multiple lesion formation is caused by a genetic mutation in chromosome 7. This genetic mutation appears in many ethnic groups, but it is especially frequent in a large population of Hispanic Americans living in the Southwest; these individuals share a common ancestor in whom the genetic change occurred. Some other types of vascular defects of the central nervous system are part of larger medical syndromes known to be hereditary. They include hereditary hemorrhagic telangiectasia, Sturge-Weber syndrome, and Klippel-Trenaunay syndrome.
How are arteriovenous malformations and other vascular lesions diagnosed?
One of the more distinctive signs clinicians use to diagnose an arteriovenous malformation is an auditory phenomenon called a bruit—a rhythmic, whooshing sound caused by excessively rapid blood flow through the arteries and veins of an arteriovenous malformation. The sound is similar to that made by a torrent of water rushing through a narrow pipe. A bruit can sometimes become a symptom when it is especially severe. When audible to individuals, the bruit may compromise hearing, disturb sleep, or cause significant psychological distress.
An array of imaging technologies can be used to uncover the presence of arteriovenous malformations. Cerebral angiography, also called cerebral arteriography, provides the most accurate pictures of blood vessel structure in brain arteriovenous malformations. A special water-soluble dye, called a contrast agent, is injected into an artery and highlights the structure of blood vessels so that it can be seen on X-rays. CT scans (computed axial tomography) use X-rays to create an image of the head, brain, or spinal cord and are especially useful in revealing the presence of hemorrhage. MRI (magnetic resonance imaging) uses magnetic fields and radio waves to create detailed images that can show subtle changes in neurological tissues. Magnetic resonance angiography (MRA) can record the pattern and velocity of blood flow through vascular lesions as well as the flow of cerebrospinal fluid throughout the brain and spinal cord. Transcranial Doppler ultrasound can diagnose medium-size to large arteriovenous malformations and also detect the presence and extent of hemorrhage. It evaluates blood flow through the brain by directing high-frequency sound waves through the skull at particular arteries. The resulting sound wave signals that bounce back from blood cells are interpreted by a computer to make an image of the velocity of blood flow.
Arteriovenous malformation treatment
There are several options for treating arteriovenous malformations. Although medication can often lessen general symptoms such as headache, back pain, and seizures caused by arteriovenous malformations and other vascular lesions, the definitive treatment for arteriovenous malformations is either surgery or focused radiation therapy. Venous malformations and capillary telangiectases rarely require surgery. Cavernous malformations are usually well defined enough for surgical removal, but surgery on these lesions is less common than for arteriovenous malformations because they do not pose the same risk of hemorrhage.
Because so many variables are involved in treating arteriovenous malformations, doctors must assess the danger posed to individuals largely on a case-by-case basis. A hemorrhage from an untreated arteriovenous malformation can cause serious neurological deficits or death, leading many clinicians to recommend surgical intervention whenever the physical characteristics of an arteriovenous malformation appear to indicate a greater-than-usual likelihood of significant bleeding and subsequent neurological damage. However, surgery on any part of the central nervous system carries some risk of serious complications or death. There is no easy formula that can allow physicians and individuals to reach a decision on the best course of therapy.
An arteriovenous malformation grading system developed in the mid-1980s can help health care professionals estimate the risk of surgery based on the size of the arteriovenous malformation, location in the brain and surrounding tissue involvement, and any leakage.
Three surgical options are used to treat arteriovenous malformations: conventional surgery, endovascular embolization, and radiosurgery. The choice of treatment depends largely on the size and location of an arteriovenous malformation. Endovascular embolization and radiosurgery are less invasive than conventional surgery and offer safer treatment options for some arteriovenous malformations located deep inside the brain.
- Conventional surgery involves entering the brain or spinal cord and removing the central portion of the arteriovenous malformation, including the fistula, while causing as little damage as possible to surrounding neurological structures. This surgery is most appropriate when an arteriovenous malformation is located in a superficial portion of the brain or spinal cord and is relatively small in size. arteriovenous malformations located deep inside the brain generally cannot be approached through conventional surgical techniques because there is too great a possibility that functionally important brain tissue will be damaged or destroyed.
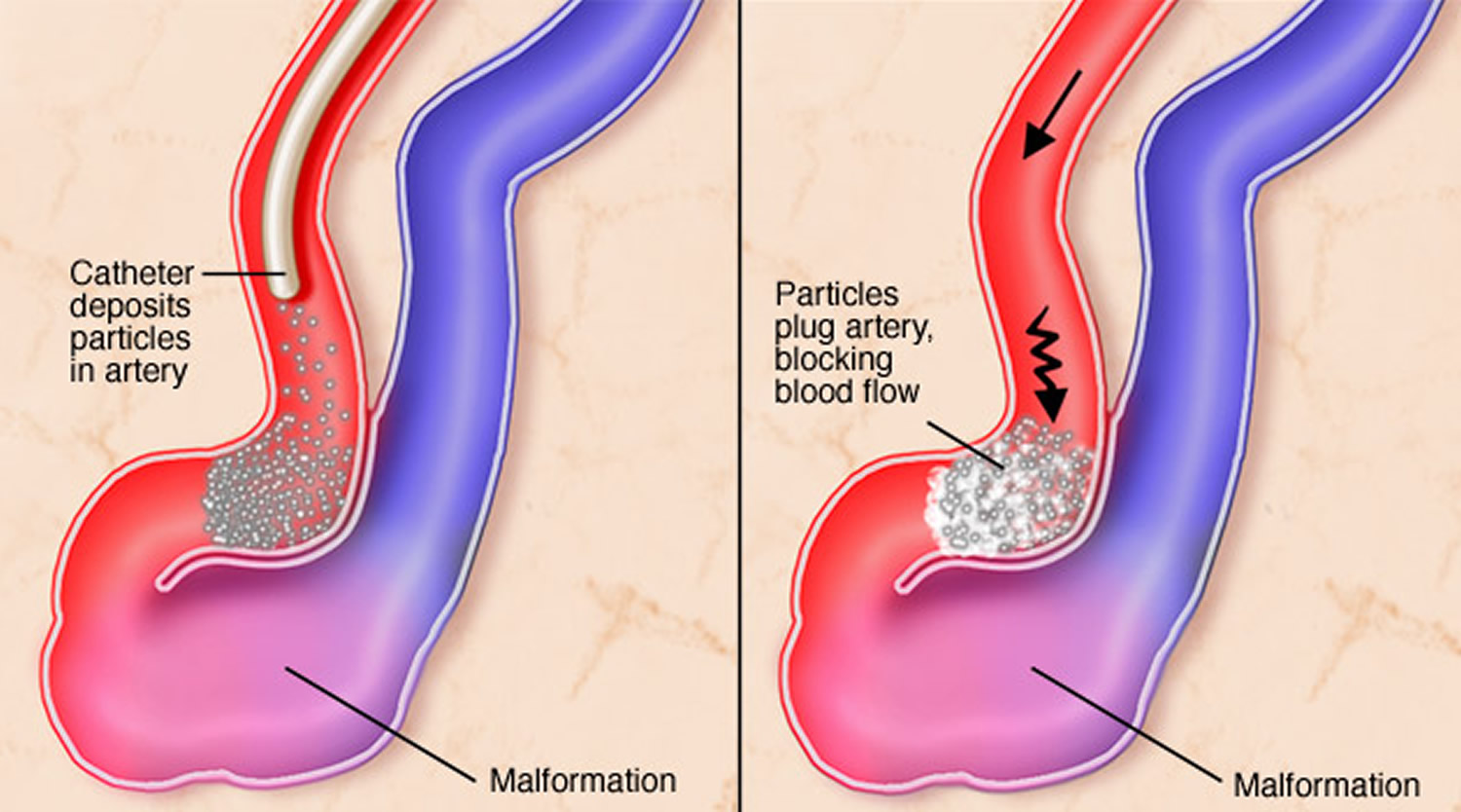
- In endovascular embolization the surgeon guides a catheter though the arterial network until the tip reaches the site of the arteriovenous malformation. The surgeon then injects a substance (such as fast-drying glue-like substances, fibered titanium coils, and tiny balloons) that will travel through blood vessels and create an artificial blood clot in the center of an arteriovenous malformation. Since embolization usually does not permanently obliterate the arteriovenous malformation, it is usually used as an adjunct to surgery or to radiosurgery to reduce the blood flow through the arteriovenous malformation and make the surgery safer.
- Radiosurgery is an even less invasive therapeutic approach often used to treat small arteriovenous malformations that haven’t ruptured. A beam of highly focused radiation is aimed directly on the arteriovenous malformation and damages the walls of the blood vessels making up the lesion. Over the course of the next several months, the irradiated vessels gradually degenerate and eventually close, leading to the resolution of the arteriovenous malformation.
Embolization frequently proves incomplete or temporary, although new embolization materials have led to improved results. Radiosurgery often has incomplete results as well, particularly when an arteriovenous malformation is large, and it poses the additional risk of radiation damage to surrounding normal tissues. Even when successful, complete closure of an arteriovenous malformation takes place over the course of many months following radiosurgery. During that period, the risk of hemorrhage is still present. However, both techniques can treat deeply situated arteriovenous malformations that had previously been inaccessible. And in many individuals, staged embolization followed by conventional surgical removal or by radiosurgery is now performed, resulting in further reductions in death and complication rates.
What is an avm in the brain
Like an arteriovenous malformation in other parts of your body, a brain arteriovenous malformation is a tangle of abnormal blood vessels connecting arteries and veins in the brain. In a brain arteriovenous malformation, blood passes directly from your arteries to your veins via abnormal vessels. This disrupts the normal process of how blood circulates through your brain.
Brain arteriovenous malformations occur in less than 1 percent of the general population. It’s estimated that about one in 2,000–5,000 people may have an arteriovenous malformation. arteriovenous malformations are more common in males than in females.
Some people with brain arteriovenous malformations experience signs and symptoms, such as headache or seizures. arteriovenous malformations are commonly found after a brain scan for another health issue or after the blood vessels rupture and cause bleeding in the brain (hemorrhage).
Once diagnosed, a brain arteriovenous malformation can often be treated successfully to prevent complications, such as brain damage or stroke.
Figure 4. Brain arteriovenous malformation
Types of brain arteriovenous malformations
All blood vessel malformations involving the brain and its surrounding structures are commonly referred to as arteriovenous malformations. But several types exist:
- True arteriovenous malformation. This is the most common brain vascular malformation. It consists of a tangle of abnormal vessels connecting arteries and veins with no normal intervening brain tissue.
- Occult or cryptic arteriovenous malformation or cavernous malformations. This is a vascular malformation in the brain that doesn’t actively divert large amounts of blood. It may bleed and often produce seizures.
- Venous malformation. This is an abnormality only of the veins. The veins are either enlarged or appear in abnormal locations within the brain.
- Hemangioma. These are abnormal blood vessel structures usually found at the surface of the brain and on the skin or facial structures. These represent large and abnormal pockets of blood within normal tissue planes of the body.
- Dural fistula. The covering of the brain is called the “dura mater.” An abnormal connection between blood vessels that involve only this covering is called a dural fistula. Dural fistulas can occur in any part of the brain covering. Three kinds of dural fistulas are:
- Dural carotid cavernous sinus fistula. These occur behind the eye and usually cause symptoms because they divert too much blood toward the eye. Patients have eye swelling, decreased vision, redness and congestion of the eye. They often can hear a “swishing” noise.
- Transverse-Sigmoid sinus dural fistula. These occur behind the ear. Patients usually complain of hearing a continuous noise (bruit) that occurs with each heartbeat, local pain behind the ear, headaches and neck pain.
- Sagittal sinus and scalp dural fistula. These occur toward the top of the head. Patients complain of noise (bruit), headaches, and pain near the top of the head; they may have prominent blood vessels on the scalp and above the ear.
What is the best treatment for a dural fistula?
The best treatment is usually endovascular surgical blocking of the abnormal connections that have caused the fistula. This involves guiding small tubes (catheters) inside the blood vessel with X-ray guidance and blocking off the abnormal connections. Depending on the location and size, many of these can be treated and cured by these less invasive endovascular techniques.
Why do brain arteriovenous malformations occur?
Doctors don’t know why arteriovenous malformations occur. Brain arteriovenous malformations are usually congenital, meaning someone is born with one. But they’re usually not hereditary. People probably don’t inherit an arteriovenous malformation from their parents, and they probably won’t pass one on to their children.
Where do brain arteriovenous malformations occur?
Brain arteriovenous malformations can occur anywhere within the brain or on its covering. This includes the four major lobes of the front part of the brain (frontal, parietal, temporal, occipital), the back part of the brain (cerebellum), the brainstem, or the ventricles (deep spaces within the brain that produce and circulate the cerebrospinal fluid).
Do brain arteriovenous malformations change or grow?
Most arteriovenous malformations don’t grow or change much, although the vessels involved may dilate (widen). Some arteriovenous malformations may shrink due to clots in part of the arteriovenous malformation. Some may enlarge to redirect blood in adjacent vessels toward an arteriovenous malformation.
Brain arteriovenous malformation signs and symptoms
A brain arteriovenous malformation may not cause any signs or symptoms until the arteriovenous malformation ruptures, resulting in bleeding in the brain (hemorrhage). In about half of all brain arteriovenous malformations, hemorrhage is the first sign.
But some people with brain arteriovenous malformation may experience signs and symptoms other than bleeding related to the arteriovenous malformation.
Symptoms may vary depending on where the arteriovenous malformation is located:
- More than 50 percent of patients with an arteriovenous malformation have an intracranial hemorrhage.
- Among arteriovenous malformation patients, 20 percent to 25 percent have focal or generalized seizures.
- Patients may have localized pain in the head due to increased blood flow around an arteriovenous malformation.
- Fifteen percent may have difficulty with movement, speech and vision.
In people without hemorrhage, signs and symptoms of a brain arteriovenous malformation may include:
- Seizures
- Headache or pain in one area of the head
- Muscle weakness or numbness in one part of the body
Some people may experience more-serious neurological signs and symptoms, depending on the location of the arteriovenous malformation, including:
- Severe headache
- Weakness, numbness or paralysis
- Vision loss
- Difficulty speaking
- Confusion or inability to understand others
- Severe unsteadiness
Symptoms may begin at any age but usually emerge between ages 10 and 40. Brain arteriovenous malformations can damage brain tissue over time. The effects slowly build up and often cause symptoms in early adulthood.
Once you reach middle age, however, brain arteriovenous malformations tend to remain stable and are less likely to cause symptoms.
Some pregnant women may have worsened symptoms due to changes in blood volume and blood pressure.
One severe type of brain arteriovenous malformation, called a vein of Galen defect, causes signs and symptoms that emerge soon or immediately after birth. The major blood vessel involved in this type of brain arteriovenous malformation can cause fluid to build up in the brain and the head to swell. Signs and symptoms include swollen veins that are visible on the scalp, seizures, failure to thrive and congestive heart failure.
Brain arteriovenous malformation causes
The cause of brain arteriovenous malformation is unknown, but researchers believe most brain arteriovenous malformations emerge during fetal development.
Normally, your heart sends oxygen-rich blood to your brain through arteries. The arteries slow blood flow by passing it through a series of progressively smaller networks of blood vessels, ending with the smallest blood vessels (capillaries). The capillaries slowly deliver oxygen through their thin, porous walls to the surrounding brain tissue.
The oxygen-depleted blood then passes into small blood vessels and then into larger veins that drain the blood from your brain, returning it to your heart and lungs to get more oxygen.
The arteries and veins in an arteriovenous malformation lack this supporting network of smaller blood vessels and capillaries. Instead, the abnormal connection causes blood to flow quickly and directly from your arteries to your veins, bypassing the surrounding tissues.
Risk factors for brain arteriovenous malformation
Anyone can be born with a brain arteriovenous malformation, but these factors may be a risk:
- Being male. arteriovenous malformations are more common in males.
- Having a family history. Cases of arteriovenous malformations in families have been reported, but it’s unclear if there’s a certain genetic factor or if the cases are only coincidental. It’s also possible to inherit other medical conditions that predispose you to having vascular malformations such as arteriovenous malformations.
Brain arteriovenous malformation complications
Complications of a brain arteriovenous malformation include:
- Bleeding in the brain (hemorrhage). An arteriovenous malformation puts extreme pressure on the walls of the affected arteries and veins, causing them to become thin or weak. This may result in the arteriovenous malformation rupturing and bleeding into the brain (a hemorrhage). This risk of a brain arteriovenous malformation bleeding ranges around 2 percent each year. The risk of hemorrhage may be higher for certain types of arteriovenous malformations, or if you have experienced previous arteriovenous malformation ruptures. Some hemorrhages associated with arteriovenous malformations go undetected because they cause no major brain damage or symptoms, but potentially life-threatening bleeding episodes may occur. Brain arteriovenous malformations account for about 2 percent of all hemorrhagic strokes each year and are often the cause of hemorrhage in children and young adults who experience brain hemorrhage.
- Reduced oxygen to brain tissue. With an arteriovenous malformation, blood bypasses the network of capillaries and flows directly from arteries to veins. Blood rushes quickly through the altered path because it isn’t slowed down by channels of smaller blood vessels. Surrounding brain tissues can’t easily absorb oxygen from the fast-flowing blood. Without enough oxygen, brain tissues weaken or may die off completely. This results in stroke-like symptoms, such as difficulty speaking, weakness, numbness, vision loss or severe unsteadiness.
- Thin or weak blood vessels. An arteriovenous malformation puts extreme pressure on the thin and weak walls of the blood vessels. A bulge in a blood vessel wall (aneurysm) may develop and become susceptible to rupture.
- Brain damage. As you grow, your body may recruit more arteries to supply blood to the fast-flowing arteriovenous malformation. As a result, some arteriovenous malformations may get bigger and displace or compress portions of the brain. This may prevent protective fluids from flowing freely around the hemispheres of the brain. If fluid builds up, it can push brain tissue up against the skull (hydrocephalus).
Figure 5. Intracerebral hemorrhage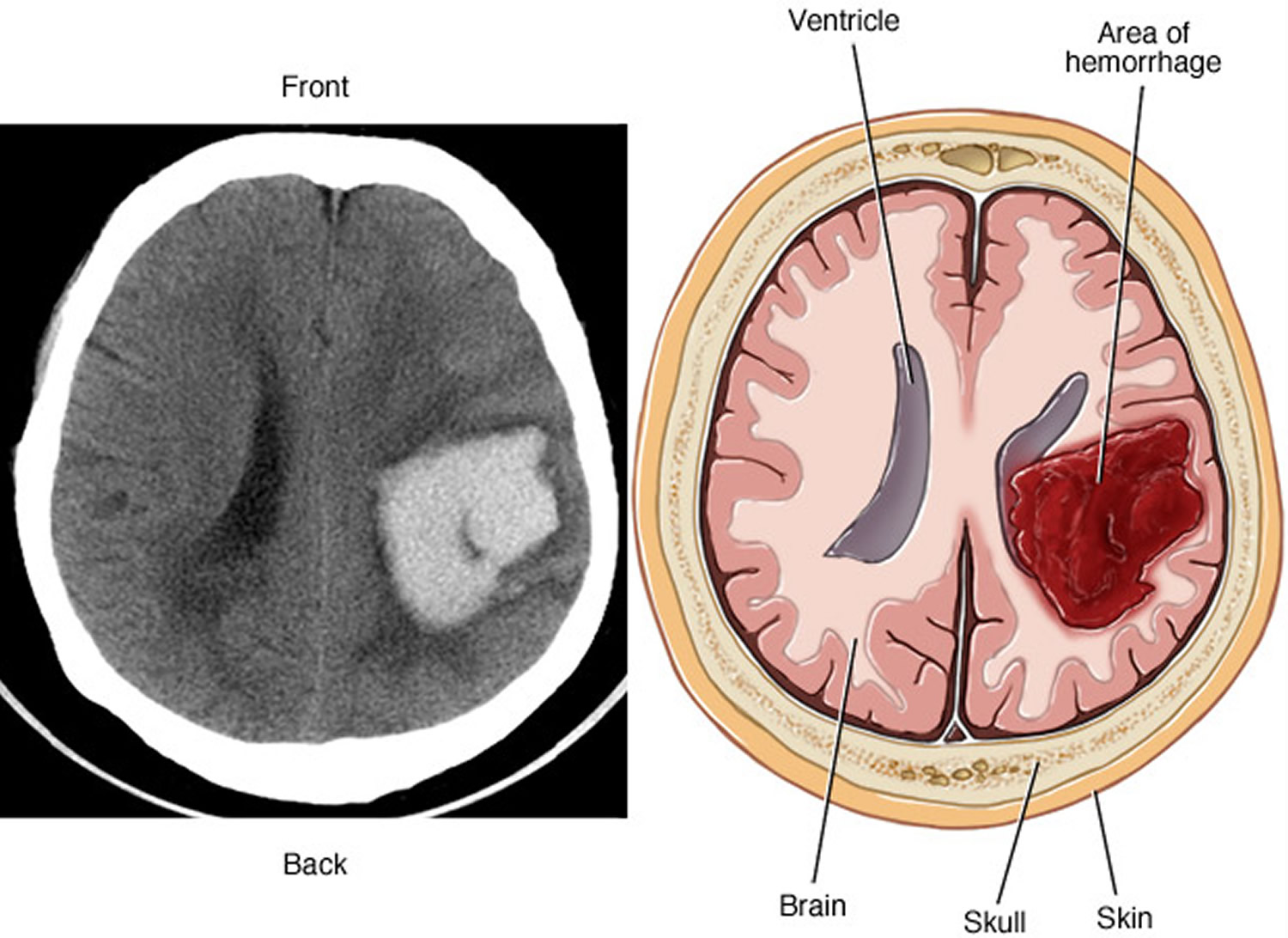
Note: A brain arteriovenous malformation may cause bleeding in the brain (hemorrhage), which can damage the surrounding brain tissue, as shown by this CT scan (left) and illustration (right) of an intracerebral hemorrhage.
What causes brain arteriovenous malformations to bleed?
A brain arteriovenous malformation contains abnormal and therefore, “weakened” blood vessels that direct blood away from normal brain tissue. These abnormal and weak blood vessels dilate over time. Eventually they may burst from the high pressure of blood flow from the arteries, causing bleeding into the brain.
What are the chances of a brain arteriovenous malformation bleeding?
The chance of a brain arteriovenous malformation bleeding is 1 percent to 3 percent per year. Over 15 years, the total chance of an arteriovenous malformation bleeding into the brain — causing brain damage and stroke — is 25 percent.
Does one bleed increase the chance of a second bleed?
The risk of recurrent intracranial bleeding is slightly higher for a short time after the first bleed. In two studies, the risk during the first year after initial bleeding was 6 percent and then dropped to the baseline rate. In another study, the risk of recurrence during the first year was 17.9 percent. The risk of recurrent bleeding may be even higher in the first year after the second bleed and has been reported to be 25 percent during that year. People who are between 11 to 35 years old and who have an arteriovenous malformation are at a slightly higher risk of bleeding.
What can happen if a brain arteriovenous malformation causes a bleed?
The risk of death related to each bleed is 10 percent to 15 percent. The chance of permanent brain damage is 20 percent to 30 percent. Each time blood leaks into the brain, normal brain tissue is damaged. This results in loss of normal function, which may be temporary or permanent. Some possible symptoms include arm or leg weakness/paralysis, or difficulty with speech, vision or memory. The amount of brain damage depends on how much blood has leaked from the arteriovenous malformation.
What functions does an arteriovenous malformation affect?
The functions of the lobes of the brain is very complicated as all lobes have some overlapping functions so that there is communication between them in order for all processes to integrate information. At a basic level, the main functions of the brain lobes are below:
- The frontal lobe functions to process motor (movements), and frontal eye fields, regulates personality and articulation (and other aspects) of speech.
- The parietal lobe functions to process sensory information, such as interpretation of pain and temperature, light touch, vibration and more.
- The temporal lobe functions to process things related to hearing, memory, learning and receptive speech.
- The occipital lobe functions to process things related to vision.
How are cerebral arteriovenous malformations diagnosed?
To diagnose a brain AVM, your neurologist will review your symptoms and conduct a physical examination.
Your doctor may order one or more tests to diagnose your condition. Radiologists trained in brain and nervous system imaging (neuroradiologists) usually conduct imaging tests.
Most arteriovenous malformations are detected with either a computed tomography (CT) brain scan or a magnetic resonance imaging (MRI) brain scan. These tests are very good at detecting brain arteriovenous malformations. They also provide information about the location and size of the arteriovenous malformation and whether it may have bled.
A doctor may also perform a cerebral angiogram. This test involves inserting a catheter (small tube) through an artery in the leg (groin). Then it’s guided into each of the vessels in the neck going to the brain, and a contrast material (dye) is injected and pictures are taken of all the blood vessels in the brain. For any type of treatment involving an arteriovenous malformation, an angiogram may be needed to better identify the type of arteriovenous malformation.
Figure 6. Brain angiogram showing brain arteriovenous malformation (AVM)
Cerebral arteriovenous malformation treatment
There are several potential treatment options for brain arteriovenous malformation. The main goal of treatment is to prevent hemorrhage, but treatment to control seizures or other neurological complications also may be considered.
If you have few or no symptoms or if your arteriovenous malformation is in an area of your brain that’s hard to treat, your doctor may prefer to monitor your condition with regular checkups.
Your doctor will determine the most appropriate treatment for your condition, depending on your age, health, and the size and location of the abnormal blood vessels.
- Medical therapy. If there are no symptoms or almost none, or if an arteriovenous malformation is in an area of the brain that can’t be easily treated, conservative medical management may be indicated. If possible, a person with an arteriovenous malformation should avoid any activities that may excessively elevate blood pressure, such as heavy lifting or straining, and avoid blood thinners like warfarin. A person with an arteriovenous malformation should have regular checkups with a neurologist or neurosurgeon.
Surgery is the most common treatment for brain arteriovenous malformations. The best treatment depends on the symptoms the patient is having, what type of arteriovenous malformation is present and the arteriovenous malformation’s size and location.
There are three different surgical options for treating arteriovenous malformations:
- Surgical removal (resection). If an arteriovenous malformation has bled and/or is in an area that can be easily operated upon, then surgical removal may be recommended. The patient is put to sleep with anesthesia, a portion of the skull is removed, and the arteriovenous malformation is surgically removed. With the help of a high-powered microscope, the surgeon seals off the arteriovenous malformation with special clips and carefully removes it from surrounding brain tissue. The surgeon then reattaches the skull bone and closes the incision in your scalp. Resection is usually done when the arteriovenous malformation can be removed with little risk of hemorrhage or seizures. arteriovenous malformations that are in deep brain regions carry a higher risk of complications. In these cases, your doctor may recommend other treatments.When the arteriovenous malformation is completely taken out, the possibility of any further bleeding should be eliminated.
- Stereotactic radiosurgery. An arteriovenous malformation that’s not too large, but is in an area that’s difficult to reach by regular surgery, may be treated with stereotactic radiosurgery. In this procedure, a cerebral angiogram is done to localize the arteriovenous malformation. Focused-beam high energy sources are then concentrated on the brain arteriovenous malformation to produce direct damage to the vessels that will cause a scar and allow the arteriovenous malformation to “clot off.” This treatment is most appropriate for small AVMs that are difficult to remove with conventional surgery and for those that haven’t caused a life-threatening hemorrhage.
- Interventional neuroradiology/endovascular embolization. It may be possible to treat part or all of the arteriovenous malformation by placing a catheter (small tube) inside the blood vessels that supply the arteriovenous malformation and blocking off the abnormal blood vessels with various materials. These include liquid tissue adhesives (glues), micro coils, particles and other materials used to stop blood flowing to the arteriovenous malformation. Endovascular embolization is less invasive than traditional surgery. It may be performed alone, but is frequently used prior to other surgical treatments to make the procedure safer by reducing the size of the arteriovenous malformation or the likelihood of bleeding. In some large brain arteriovenous malformations, endovascular embolization may be used to reduce stroke-like symptoms by redirecting blood back to normal brain tissue.
Figure 7. Endovascular embolization
Potential future treatments
Researchers are currently studying ways to better predict the risk of hemorrhage in people with brain arteriovenous malformation to better guide treatment decisions. For example, high blood pressure within the arteriovenous malformation and hereditary syndromes associated with neurological issues may play a role.
Innovations in imaging technology, such as 3-D imaging, functional imaging and brain tract mapping also are being evaluated and have the potential to improve surgical precision and safety in removing brain arteriovenous malformations and preserving surrounding vessels.
In addition, ongoing advances in embolization, radiosurgery and microsurgery techniques are making previously inoperable brain arteriovenous malformations more accessible and safer for surgical removal.
What factors influence whether an arteriovenous malformation should be treated?
In general, an arteriovenous malformation may be considered for treatment if it has bled, if it’s in an area of the brain that can be easily treated and if it’s not too large.
What is the best treatment for an arteriovenous malformation?
It depends on what type it is, the symptoms it may be causing and its location and size.
Coping and support
Learning that you have a brain arteriovenous malformation can be frightening. It can make you feel like you have little control over your health. But you can take steps to cope with the emotions that accompany your diagnosis and recovery. Consider trying to:
- Learn enough about brain arteriovenous malformation to make informed decisions about your care. Ask your doctor about the size and location of your brain arteriovenous malformation and how that affects your treatment options. As you learn more about brain arteriovenous malformations, you may become more confident in making treatment decisions.
- Accept your emotions. Complications of brain arteriovenous malformation, such as hemorrhage and stroke, can cause emotional problems as well as physical ones. Recognize that emotions may be hard to control, and some emotional and mood changes may be caused by the injury itself as well as coming to terms with the diagnosis.
- Keep friends and family close. Keeping your close relationships strong will help you during your recovery. Friends and family can provide the practical support you’ll need, like accompanying you to doctors’ appointments, and serve as emotional support.
Find someone to talk with. Find a good listener who is willing to listen to you talk about your hopes and fears. This may be a friend or family member. The concern and understanding of a counselor, medical social worker, clergy member or support group also may be helpful.
Ask your doctor about support groups in your area. Or check your phone book, library or a national organization, such as the American Stroke Association or the Aneurysm and arteriovenous malformation Foundation.
Pulmonary arteriovenous malformation
Pulmonary arteriovenous malformations are usually congenital (present at birth) but can also rarely be acquired. Pulmonary arteriovenous malformations are anomalous direct communications between the branches of pulmonary artery and vein through a thin-walled aneurysmal sac without intervening capillary 1. Pulmonary arteriovenous malformation is also known as pulmonary arteriovenous fistula, pulmonary arteriovenous aneurisms and pulmonary hemangioma. Pulmonary arteriovenous malformation may occur as an isolated anomaly or in association with hereditary hemorrhagic telangiectasia 2.
Pulmonary arteriovenous malformation can be simple or complex, then primary or secondary, large or small. Simple pulmonary arteriovenous malformation are characterized by direct communication between one pulmonary artery and one pulmonary vein, and recommendation therapy for this type of pulmonary arteriovenous malformation is transcatheter embolisation 3. Complex pulmonary arteriovenous malformation has two or more different segmental arteries supplying the aneurismal sac and one or two draining veins. Simple pulmonary arteriovenous malformation occurs in about 80% and complex in 20% 4. The golden standard for diagnosis of complex pulmonary arteriovenous malformation is pulmonary arteriography 5.
Primary or congenital pulmonary arteriovenous malformation are more often, and secondary or acquired are caused by infection and occur due to hyperplastic changes in bronchial arteries or abnormal communication between pulmonary artery and vein (tuberculosis, actinomycosis, schistosomiasis) metastatic thyroid carcinoma, thoracic trauma, hepatic cirrhosis, mitral stenosis 3 modified Fountain’s operation or iatrogenic.
Due to the size, pulmonary arteriovenous malformation can be divided as small (less than 1 to 5 cm) or big (more than 5 cm) and can fulfill the whole hemithorax.
In most cases, pulmonary arteriovenous malformation are asymptomatic until the four decade of patient’s life 6.
Abnormal communications between blood vessels of the lung may also be found in a variety of acquired conditions. Right-to-left shunting as a result of communications between pulmonary arteries and pulmonary veins has been reported in hepatic cirrhosis 7, and less commonly in schistosomiasis 8, mitral stenosis 9, trauma 9, actinomycosis 9, Fanconi’s syndrome 10, and metastatic thyroid carcinoma 11. Communications between bronchial arteries and pulmonary arteries, causing left-to-right shunt, can develop in chronic inflammatory conditions such as bronchiectasis 12.
Incidence of pulmonary arteriovenous malformation is about 1:50,000 cases 13 and less than 400 cases has been described in the literature. Pulmonary arteriovenous malformation are not a common clinical problem. In an autopsy study in 1953 from Johns Hopkins Hospital 14, only three cases of pulmonary arteriovenous malformation were detected in 15,000 consecutive autopsies. However, it was noted by the same investigators that small pulmonary arteriovenous malformation may easily be missed in routine autopsies. The Mayo Clinic saw 63 cases over 20 yr ending in 1972 15, and 38 cases over 8.5 yr ending in 1981 16, for an annual incidence of 3.2 and 4.5 cases/yr, respectively. Smaller, nontertiary care hospitals might expect to see one case every few years.
Pulmonary arteriovenous malformation occurs twice as often in women as in men, but there is a male predominant in newborns 17. In 53-70% cases has been found in lower lobes, in 75% is unilateral, 36% multiple, and half of multiple lesions has bilateral localization. In 81% cases localization is subpleural 4.
The diagnosis of pulmonary arteriovenous malformation in patients remains a diagnostic challenge to the emergency physician. The most common clinical signs of pulmonary arteriovenous malformation are recurrent episodes of epistaxis (nose bleed) and hemoptysis (coughing up blood), so surgical resection is deemed the best curative option to avoid further episodes and recurrence of hemoptysis. Quite often the diagnosis is established after pathohistological examinations.
Endovascular treatment with metallic coil embolization has been accepted as the standard of care for pulmonary arteriovenous malformation 5. However, recanalization is one of the concerns after embolization because some patients can develop neurologic events 17. Digital subtraction angiography is the most reliable modality for detecting recanalization, but this is an invasive procedure. In recent years, computed tomography (CT) and magnetic resonance imaging (MRI) with or without contrast agents have become important in the evaluation of pulmonary arteriovenous malformation patency due to the non-invasiveness of these techniques 18. However, the direct visualization of recanalization through the coils is difficult with CT and MRI because of the prominent streaks or susceptibility artifacts from the metallic coils. This report describes the non-invasive direct visualization of the recanalization of a pulmonary arteriovenous malformation using ultra-short echo time MRI. We also performed a phantom study to elucidate the capability of ultra-short echo time MRI for the detection of signals in the coil.
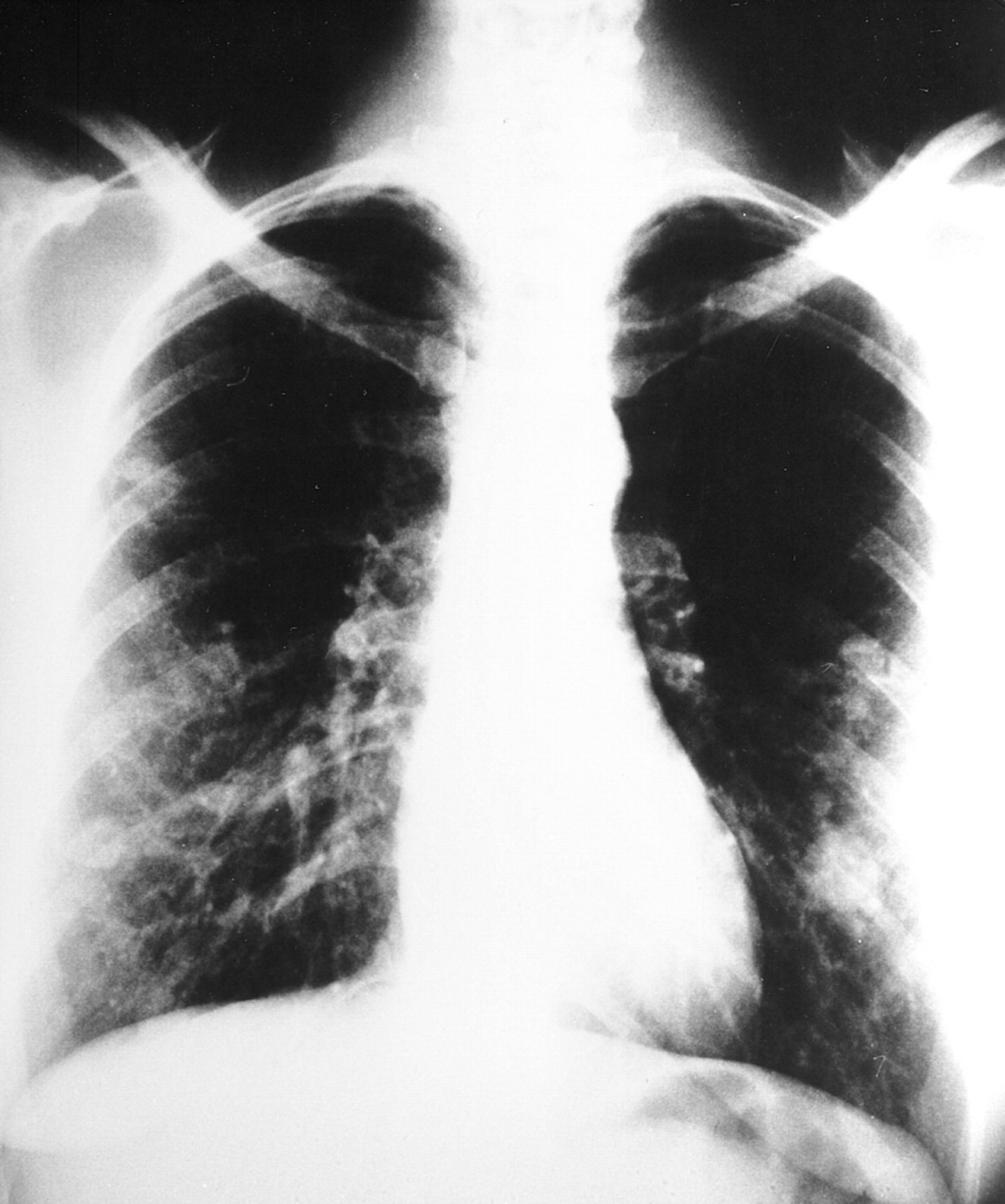
Figure 8. Pulmonary arteriovenous malformation chest x-ray
Note: Plain chest radiograph showing multiple pulmonary arteriovenous malformation in the lower and middle zones of both lungs from a young woman with severe dyspnea and hypoxemia.
Figure 9. Pulmonary arteriovenous malformation CT scans
Note: Note: The chest CT revealed in right upper lobe pulmonary arteriovenous malformation diameter of 5 cm. Feeding artery was the branch of the right pulmonary artery and drainage veins were branches of right upper pulmonary vein.
Pulmonary arteriovenous malformation causes
The etiology of pulmonary arteriovenous malformation is unknown, but recent discoveries about the genetics of hereditary hemorrhagic telangiectasia may be relevant to the etiology of pulmonary arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia—and perhaps in patients without hereditary hemorrhagic telangiectasia. Genetic mapping in the last few years has found that hereditary hemorrhagic telangiectasia is linked to loci on at least three different chromosomes 19. Endoglin, the most abundant transforming growth factor beta (TGF-β) binding protein that is found on endothelial cells, has been identified as the hereditary hemorrhagic telangiectasia gene mapping to locus 9q3, and is presently referred to as the gene for hereditary hemorrhagic telangiectasia 1 19. Thus far, at least 16 novel mutations of the endoglin gene have been discovered in 17 different families 20. Proposed mechanisms by which mutations to the endoglin gene might cause hereditary hemorrhagic telangiectasia 1 have included a dominant negative effect, a two-hit model, and haploinsufficiency, although recent data seem most consistent with haploinsufficiency 20.
Mutations in the gene for activin receptor-like kinase 1 have been mapped to locus 12q and are referred to as the gene for hereditary hemorrhagic telangiectasia 2 21. Activin receptor-like kinase 1 is a type I serine–threonine kinase receptor that can bind TGF-β in the presence of its type II receptor and is expressed predominantly on endothelial cells 22. Thus far, at least 12 novel mutations of the activin receptor-like kinase 1 gene have been discovered in 12 different families 22. All 12 mutations have affected the cytoplasmic kinase region, suggesting that the resultant protein would have decreased kinase activity. As with the endoglin gene, there is evidence to support the dominant negative, two-hit, and haploinsufficiency mechanisms 22. Preliminary data have suggested a third locus for hereditary hemorrhagic telangiectasia at 3p22, where the TGF-β II receptor gene is located 20.
Several reports have suggested a possible correlation between the varied phenotypes of hereditary hemorrhagic telangiectasia and its genetic heterogeneity. In a prospective analysis, Berg and coworkers 23 showed that the incidence of pulmonary arteriovenous malformation was 29.2% in eight families with hereditary hemorrhagic telangiectasia 1 (i.e., those with a linkage to endoglin) versus only 2.9% in eight families in which a linkage to endoglin was excluded. Similarly, Shovlin and coworkers 24 found that 41% of hereditary hemorrhagic telangiectasia patients with endoglin mutations had pulmonary arteriovenous malformation compared with only 14% of hereditary hemorrhagic telangiectasia patients without endoglin mutations. These studies are consistent with the hypothesis that hereditary hemorrhagic telangiectasia families with endoglin mutations have an increased incidence of pulmonary arteriovenous malformation. However, there was no evidence for significant phenotypic variability between the various endoglin mutations—supporting the hypothesis for haploinsufficiency 24.
Therefore, all the known mutations associated with various types of hereditary hemorrhagic telangiectasia result in potential abnormalities in ligands for TGF-β. Transforming growth factor beta family members are involved in a variety of vascular phenomena including angiogenesis, induction of endothelial cell mitogens, and the interplay between cells, matrix, and external factors during the response to vascular insults 22. McAllister and coworkers 20 have postulated that changes in endoglin might result in endothelial cells that would respond abnormally to TGF-β during the process of vascular remodeling, possibly resulting in the formation of arteriovenous malformation 20. Endoglin and activin receptor-like kinase 1 may even act together to alter repair and remodeling of vascular tissue through changes in expression of matrix proteins 21. While intriguing, these hypotheses remain unproven. The reason for the propensity of families with hereditary hemorrhagic telangiectasia 1 to develop pulmonary arteriovenous malformation is also unknown.
The exact pathogenesis of pulmonary arteriovenous malformation is not known either. Some investigators have hypothesized that the cause is a defect in terminal arterial loops which allows dilatation of thin-walled capillary sacs 25. Others have argued that pulmonary arteriovenous malformation are the result of incomplete resorption of the vascular septae that separate the arterial and venous plexuses which normally anastomose during fetal development 26. It has also been suggested that multiple small pulmonary arteriovenous malformation develop as a result of failure of capillary development during fetal development 26. Hales 27 studied the lungs of two patients with multiple 1-12-mm pulmonary telangiectases by injection of the vascular tree with colored vinylite, followed by digestion of lung tissue with acid. Based on a detailed study of the variable morphology of different telangiectases in these two cases, Hales hypothesized that large saccular pulmonary arteriovenous malformation develop by progressive dilation of favored limbs of smaller plexuses. As the favored limbs dilate, the smaller limbs progressively disappear, perhaps due to diversion of blood to the favored limbs. Further enlargement of the favored limbs results in a mass of tortuous loops and U-turns with the formation of multiloculated sacs. Ultimately, the thin vascular walls between these juxtaposed loops may deteriorate or rupture, resulting in formation of a single saccular pulmonary arteriovenous malformation. This latter theory has been challenged by White and coworkers 28, who studied pulmonary arteriovenous malformation architecture with angiography and found that many small pulmonary arteriovenous malformation consisted of a single artery-to-vein connection without an intervening plexus.
Pulmonary arteriovenous malformation signs and symptoms
In most cases, pulmonary arteriovenous malformation are asymptomatic until the four decade of patient’s life 6.
The most common clinical sign of pulmonary arteriovenous malformation is epistaxis. Dispnea (shortness of breath) is the second common clinical sign and is usually present in patients with big or multiple pulmonary arteriovenous malformation. Hemoptysis is the third most common sign, and after that are hypoxia, central cyanosis, clubbing fingers, hematothorax, and brain abscess. About 30-40% patients with pulmonary arteriovenous malformation will have cerebrovascular insult or transitory ischemic attack later in life 29.
One of the hallmarks of pulmonary arteriovenous malformation is orthodeoxia, which is characterized by decreased of oxygen saturation upon standing. It is caused by blood pooling at the lung base, where the most pulmonary arteriovenous malformation is located.
Pulmonary arteriovenous malformation diagnosis
Diagnosis of pulmonary arteriovenous malformation is usually quite difficult, because the first sign can be massive hemoptysis or hematothorax (blood accumulation in the pleural cavity) and the definitive diagnosis will be established on pathohistological examination after surgical intervention. The golden standard for achieving the diagnosis of pulmonary arteriovenous malformation today is pulmonary angiography, and the standard procedure today is helical CT scan 30. Recently, many studies suggest that contrast echocardiography with chest X-ray has 100% sensitivity, negative predictive value; it is cheaper and less invasive, less exposure to radiation and available in smaller medical centers 30.
Differential diagnosis bronchial pulmonary arteriovenous malformation to some other pulmonary arteriovenous malformation is characterized by predominantly male population, right lung localization, solitary lesion and without hereditary component or Rendu-Osler-Weber syndrome 31.
Hereditary hemorrhagic telangiectasia is the most common vascular abnormality in lungs. The classic clinical presentation is epistaxis, dilated blood vessels over lips and fingers and gastrointestinal bleeding. The diagnostic criteria for hereditary hemorrhagic telangiectasia include three of the four following conditions: recurrent epistaxis, telangiectasia elsewhere from the nasal mucosa, autosomal dominant inheritance and visceral involvement 32. Hereditary hemorrhagic telangiectasia is hereditary diseases with gene on the 9th chromosome q33-34. Patients with more than one pulmonary arteriovenous malformation have 90% chances to suffer from hereditary hemorrhagic telangiectasia 29. If the pulmonary arteriovenous malformation is located closer to visceral pleura, then hematothorax is more common clinical presentation, and if it is closer to bronchi, then hemoptysis are more often 31. Patients with pulmonary arteriovenous malformation are recommended to have antibiotics prophylaxis before any surgical or dental procedure 6.
Pulmonary arteriovenous malformation treatment
There are two recommended therapeutic options in treatment of patients with pulmonary arteriovenous malformation: transcatheter embolisation and surgical resection (ligature, excision, segmentectomy, lobectomy, pneumonectomy). Transcatheter embolisation is less invasive technique in treatment of pulmonary arteriovenous malformation with success of 85-98% of cases. In cases of massive hemoptysis or hematothorax, surgery is the therapy of choice, which eradicates the pulmonary arteriovenous malformation, and after the transcatheter embolisation there is a chance of pulmonary arteriovenous malformation revascularization 31.
Surgical resection of pulmonary arteriovenous malformation is recommended for all the patients who can submit general anesthesia, if transcatheter embolisation failed, for patients with neurological complications, newborns or central localization of pulmonary arteriovenous malformation. In patients with non-diagnosed massive hemoptysis, surgical resection has small percentage of perioperative mortality 0-9% 33.
- White RI, Jr, Pollak JS, Wirth JA. Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Interv Radiol 1996; 7: 787–804. https://www.ncbi.nlm.nih.gov/pubmed/8951745[↩]
- Kuhajda I, Milosevic M, Ilincic D, et al. Pulmonary arteriovenous malformation-etiology, clinical four case presentations and review of the literature. Annals of Translational Medicine. 2015;3(12):171. doi:10.3978/j.issn.2305-5839.2015.06.18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4515228/[↩]
- Pelage J, Lagrange C, Chinet T, et al. Embolization of localized pulmonary arteriovenous malformations in adults. J Radiol 2007;88:367-76. https://www.ncbi.nlm.nih.gov/pubmed/17457268[↩][↩]
- Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998;158:643-61. https://www.atsjournals.org/doi/full/10.1164/ajrccm.158.2.9711041[↩][↩]
- Chowdhury UK, Kothari SS, Bishnoi AK, et al. Successful lobectomy for pulmonary arteriovenous malformation causing recurrent massive haemoptysis. Heart Lung Circ 2009;18:135-9. https://www.ncbi.nlm.nih.gov/pubmed/18294908[↩][↩]
- Kessler CS, Leipzig SM. A 24-year-old man with chest pain, hemoptysis, and hypoxia. Am J Emerg Med 2008;26:904-7. https://www.ncbi.nlm.nih.gov/pubmed/18926350[↩][↩][↩]
- Lange P. A., Stoller J. K.The hepatopulmonary syndrome. Ann. Intern. Med.1221995521529[↩]
- de Faria J. L., Czapski J., Ribierto M. O., Leite, de Oliveira D., Penna, Fujioka T., de Ulhoa A. B., CintraCyanosis in Manson’s schistosomiasis: role of pulmonary schistosomatic arteriovenous fistulas. Am. Heart J.541957196204[↩]
- Prager R. L., Laws K. H., Bender H. W.Arteriovenous fistula of the lung. Ann. Thorac. Surg.361983231239[↩][↩][↩]
- Taxman R. M., Halloran M. J., Parker B. M.Multiple pulmonary arteriovenous malformations in association with Fanconi’s syndrome. Chest641973118120[↩]
- Pierce J. A., Reagan W. P., Kimball R. W.Unusual cases of pulmonary arteriovenous fistulas with a note on thyroid carcinoma as a cause. N. Engl. J. Med.181959901907[↩]
- Liebow A. A., Hales M. R., Lindskog G. E.Enlargement of the bronchial arteries and their anastomoses with the pulmonary arteries in bronchiectasis. Am. J. Pathol.251949211231[↩]
- Liu FY, Wang MQ, Fan QS, et al. Endovascular embolization of pulmonary arteriovenous malformations. Chin Med J (Engl) 2010;123:23-8. https://www.ncbi.nlm.nih.gov/pubmed/20137570[↩]
- Sloan R. D., Cooley R. N.Congenital pulmonary arteriovenous aneurysm. A.J.R.701953183210[↩]
- Dines D. E., Arms R. A., Bernatz P. E., Gomes M. R.Pulmonary arteriovenous fistulas. Mayo Clin. Proc.491974460465[↩]
- Dines D. E., Seward J. B., Bernatz P. E.Pulmonary arteriovenous fistula. Mayo Clin. Proc.581983176181[↩]
- Díaz-Aguilera R, Zurera-Tendero LJ, Canis-López M, et al. Embolotherapy of pulmonary arteriovenous malformations: long-term clinical and radiological follow-up. Radiologia 2009;51:85-9. https://www.ncbi.nlm.nih.gov/pubmed/19303485[↩][↩]
- Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med 2004;169:994-1000. https://www.ncbi.nlm.nih.gov/pubmed/14742303[↩]
- McAllister K. A., Lennon F., Bowles-Biesecker B., McKinnon W. C., Helmbold E. A., Markel D. S., Jackson C. E., Guttmacher A. E., Pericak-Vance M. A., Marchuk D. A.Genetic heterogeneity in hereditary haemorrhagic telangiectasia: possible correlation with clinical phenotype. J. Med. Genet.311994927932[↩][↩]
- McAllister K. A., Grogg K. M., Johnson D. W., Gallione C. J., Baldwin M. A., Jackson C. E., Helmbold E. A., Markel D. S., McKinnon W. C., Murrell J., McCormick M. K., Pericak-Vance M. A., Heutink P., Oostra B. A., Haitjema T., Westermann C. J. J., Porteous M. E., Guttmacher A. E., Letarte M., Marchuk D. A.Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet.81994345351[↩][↩][↩][↩][↩]
- Johnson D. W., Berg J. N., Baldwin M. A., Gallione C. J., Marondel I., Yoon S. J., Stenzel T. T., Speer M., Pericak-Vance M. A., Diamond A., Guttmacher A. E., Jackson C. E., Attisano L., Kucherlapati R., Porteous M. E. M., Marchuk D. A.Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet.131996189195[↩][↩]
- Berg J. N., Gallione C. J., Stenzel T. T., Johnson D. W., Allen W. P., Schwartz C. E., Jackson C. E., Porteous M. E. M., Marchuk D. A.The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am. J. Hum. Genet.6119976067[↩][↩][↩][↩]
- Berg J. N., Guttmacher A. E., Marchuk D. A., Porteous M. E. M.Clinical heterogeneity in hereditary haemorrhagic telangiectasia: are pulmonary arteriovenous malformations more common in families linked to endoglin? J. Med. Genet.331996256257[↩]
- Shovlin C. L., Hughes J. M. B., Scot J., Seidman C. E., Seidman J. G.Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet.6119976879[↩][↩]
- Stork W. J.Pulmonary arteriovenous fistulas. A.J.R.741955441454[↩]
- Anabtawi I. N., Ellison R. G., Ellison L. T.Pulmonary arteriovenous aneurysms and fistulas: anatomical variations, embryology, and classification. Ann. Thorac. Surg.11965277285[↩][↩]
- Hales M. R.Multiple small arteriovenous fistulae of the lungs. Am. J. Pathol.321956927943[↩]
- White R. I., Mitchell S. E., Barth K. H., Kaufman S., Kadir S., Chang R., Terry P. B.Angioarchitecture of pulmonary arteriovenous malformations: an important consideration before embolotherapy. A.J.R.1401983681686[↩]
- Borrero CG, Zajko AB. Pulmonary Arteriovenous malformations: clinical features, diagnosis, and treatment. J Radiol Nurs 2006;25:33-7.[↩][↩]
- Liechty KW, Flake AW. Pulmonary vascular malformations. Semin Pediatr Surg 2008;17:9-16. https://www.ncbi.nlm.nih.gov/pubmed/18158137[↩][↩]
- Miyoshi K, Moriyama S, Nawa S. Bronchial arteriovenous malformation with large aneurysm, resected by video-assisted thoracic surgery. Gen Thorac Cardiovasc Surg 2009;57:162-5. https://www.ncbi.nlm.nih.gov/pubmed/19280316[↩][↩][↩]
- Supakul N, Fan R, Karmazyn B. A case report: Pulmonary venous malformation complicated with pulmonary hemorrhage. J Pediatr Surg 2012;47:e35-8. https://www.ncbi.nlm.nih.gov/pubmed/23217914[↩]
- Georghiou GP, Berman M, Vidne BA, et al. Pulmonary arteriovenous malformation treated by lobectomy. Eur J Cardiothorac Surg 2003;24:328-30. https://www.ncbi.nlm.nih.gov/pubmed/12895639[↩]