Contents
What is black cohosh
Black cohosh also known as Cimicifuga racemosa Nuttal or Actaea racemosa L (family Ranunculaceae) is a flowering plant, a member of the buttercup family, native to North America. Other, mostly historical, names for this herb include snakeroot, black bugbane, rattleweed, macrotys, and rheumatism weed 1. Black cohosh is a coarse, perennial, woodland herb with large compound leaves, and a thick, knotted, rhizome system 2. The underground stems and roots/rhizomes of black cohosh have been used traditionally by Native Americans (Penobscot, Winnebago and Dakota) to treat coughs, colds, constipation, fatigue, malaria 3, impaired kidney function 3, sore throat, rheumatism, malaise, menstrual irregularities and childbirth and to stimulate lactation and a variety of conditions related to women’s health 4. Since 1832 a hydroalcoholic extract was described as treatment for pain and inflammation in endometriosis and dysmenorrhea 5; and a fluid extract was listed in the US National Formulary from 1840 until 1946 6; and was a major constituent of the once popular patent medicine “Lydia Pinkham’s Vegetable Compound” used for treatment of “painful complaints and weakness” in females 7. Because of the risks associated with hormone replacement therapy, black cohosh preparations have become popular dietary supplements among women seeking natural treatments for menopausal complaints, because of its purported estrogenic activity 8. Currently, women use black cohosh as a dietary supplement for menopausal symptoms, including hot flashes (also called hot flushes) and night sweats (together known as vasomotor symptoms), vaginal dryness, heart palpitations, tinnitus, vertigo, sleep disturbances, nervousness, and irritability 9. Menopause, which typically occurs in women at about 51 years of age, is the cessation of menstruation and the end of a woman’s reproductive period 9. Black cohosh has also been used as a dietary supplement for other conditions, including menstrual cramps and premenstrual syndrome (PMS), and to induce labor 10. The part of the black cohosh plant used in herbal preparations is the root or rhizome (underground stem). The underground stems and root of black cohosh are used fresh or dried to make tea, capsules, pills, or liquid extracts.
Black cohosh key facts
- There is currently insufficient evidence to support the use of black cohosh for menopausal symptoms 9. Black cohosh has been studied for menopause symptoms in people, but most of the studies were not of the highest quality. Therefore, knowledge of the effects of black cohosh is limited. However, there is adequate justification for conducting further studies in this area. The uncertain quality of identified trials highlights the need for improved reporting of study methods, particularly with regards to allocation concealment and the handling of incomplete outcome data. The effect of black cohosh on other important outcomes, such as health-related quality of life, sexuality, bone health, night sweats and cost-effectiveness also warrants further investigation.
- Studies that tested black cohosh for menopause symptoms have had inconsistent results. The overall evidence is insufficient to support using black cohosh for this purpose.
- There are not enough reliable data to show whether black cohosh is effective for other uses.
The German Commission E (a German’s equivalent of the U.S. Food and Drug Administration [FDA]) has approved black cohosh for the treatment of premenstrual syndrome, dysmenorrhea, and menopausal symptoms 11. Although there is little data regarding the precise rate of use among breast cancer patients, black cohosh remains one of the most controversial natural therapies used by this patient population because of its purported phytoestrogenic activity as a selective estrogen receptor modulator (SERM)–like agent 12. In theory, phytoestrogens possess amphoteric effects on the estrogen receptor (ER). Under conditions of estrogen excess, phytoestrogens may act as estrogen antagonists through competitive inhibition of the estrogen receptor, only stimulating it weakly. Under conditions of low estrogen, phytoestrogens may act as weak agonists 13. In cases where black cohosh contributes to a net estrogenic effect, its use may result in deleterious effects on breast cancer risk or recurrence 14. This is of particular concern among women undergoing antiestrogen therapy. Given these conflicting data and the potential for harm, there is an urgent need for a synthesis of available evidence pertaining to the use of black cohosh and its impact on breast cancer risk. A systematic review by Walji et al. 15 suggested that black cohosh has a high safety profile for use by cancer patients, however, this review has not been updated to include evidence since 2007. Another systematic review done in 2013 12 suggests current evidence does not support an association between use of black cohosh and increased risk of breast cancer. That review 12 suggests that black cohosh has limited estrogenic activity. Black cohosh does not appear to possess classical estrogenic activity, as measured by breast and uterine tissue proliferation, but may possibly have nonclassical activities as seen by its effects on bone metabolism. The review shows that black cohosh has no consistent pattern of influence on serum hormone levels [estradiol, luteinizing hormone (LH), or follicle-stimulating hormone (FSH)] or the following estrogen responsive tissues: endometrial tissue, breast tissue, or vaginal tissues 12. Black cohosh does seem to stimulate bone formation and may inhibit bone breakdown in women with high bone turnover 12. In terms of breast cancer risk of 4 studies examining the impact on breast cancer risk, 2 studies found no significant association 16, 17, and 2 reported an inverse relationship such that black cohosh use was associated with significantly reduced risk of primary breast cancer incidence or breast cancer recurrence, including the study of black cohosh combined with tamoxifen 18, 19. With respect to hot flashes, current evidence is conflicting, with 2 placebo-controlled studies showing no significant effects 20, 21, and 1 study comparing black cohosh plus tamoxifen to tamoxifen alone showing benefit 19. A large placebo effect due to expectation bias may be at play, a real possibility especially given the importance of subjective outcomes in these studies; in addition, one study showing benefits between groups was an open study design, which may introduce bias 22. However, equally plausible is that black cohosh does have some utility in patient with hot flashes.
The popularity of the black cohosh has led to extensive phytochemical and biological investigations, including numerous clinical trials, some of which date back to the 1950s. Despite such extensive research, the clinical efficacy of black cohosh products remains controversial 8. Early studies suggested that black cohosh extracts were effective in reducing the frequency and intensity of hot flashes among premenopausal and postmenopausal women 23, while several recent trials including a Phase II double-blind placebo-controlled trial conducted at the NIH Center for Botanical Dietary Supplements Research demonstrated no vasomotor symptoms benefits 24. Clinical efficacy is not the only controversy surrounding black cohosh. The question of long-term safety of black cohosh came to light after initial reports of liver failure associated with the use of black cohosh had appeared in the literature 25 The concern was serious enough to warrant two workshops organized by the NIH Office of Dietary Supplements to discuss issues related to safety of black cohosh. A recent review sponsored by the US Pharmacopoeia summarized 30 cases of liver damage associated with black cohosh use and recommended that black cohosh products carry a cautionary statement that they may adversely affect the liver 26. In contrast, several recent reviews of randomized controlled clinical trials concluded that there is no evidence of hepatotoxicity 27 and recent in vitro and animal studies seem to support this conclusion 28.
Figure 1. Black cohosh
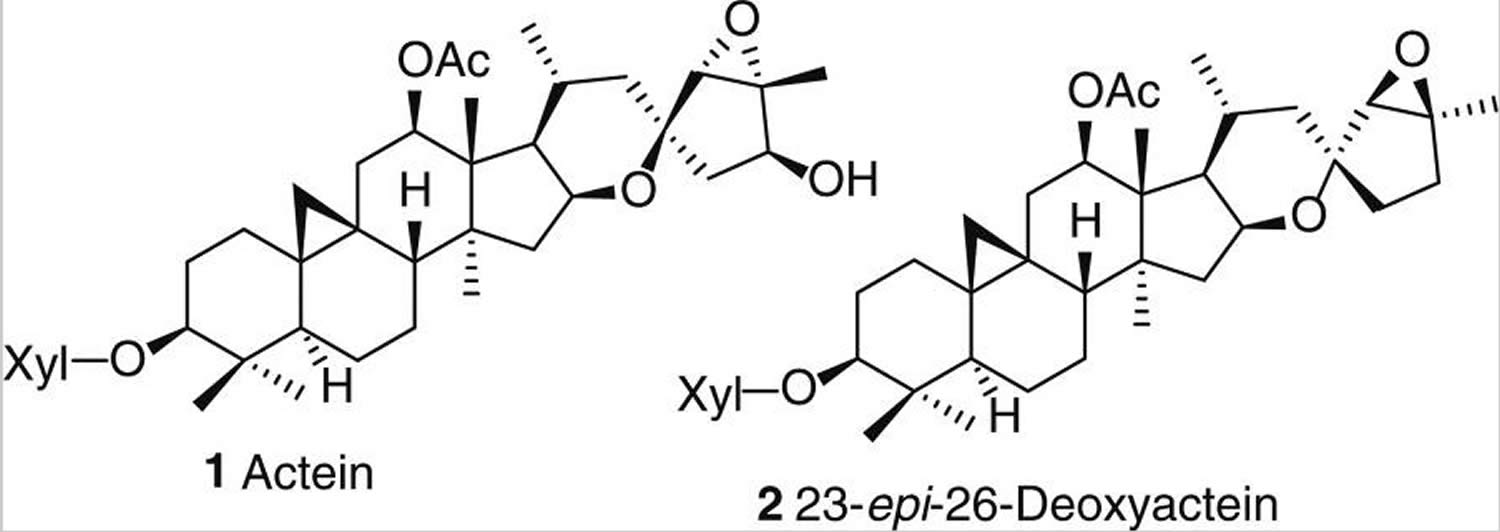
When it comes to phytochemical investigations, the past several decades of research have focused almost exclusively on two abundant classes of compounds: triterpene glycosides and phenolic acids. Triterpene glycosides represent the major constituents of all hydroalcoholic black cohosh extracts and have been extensively studied from both the phytochemical and biological side. More than 40 triterpenes have been isolated and structurally characterized to date 29. They represent a particular analytical challenge due to their close structural similarities and overall complexity of distinguishing these structures, which include numerous stereocenters. The most abundant triterpenes, particularly actein (1) and 23-epi-26-deoxyactein (2) [see Figure 2 below), are often used as markers for the standardization of black cohosh preparations 30. Extensive reviews on the chemistry, rational naming system, and biological activities of the triterpenes have been published recently 31.
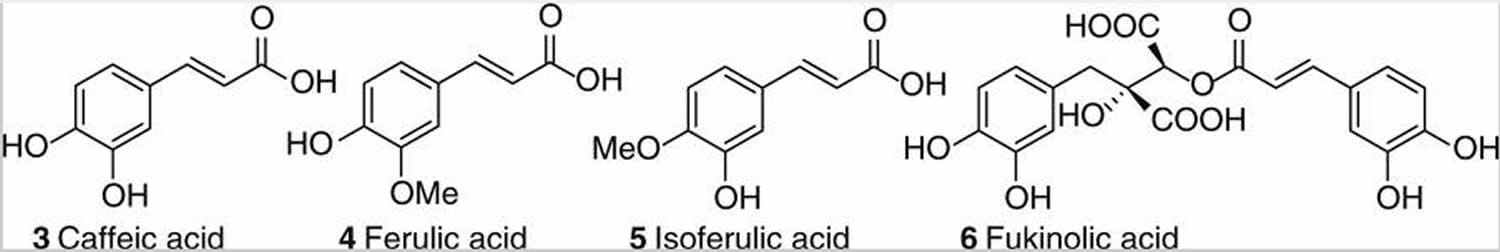
The major phenolic constituents of black cohosh are the hydroxycinnamic acids, caffeic acid (3), ferulic acid (4), and isoferulic acid (5), as well as their condensation products with glycoloyl phenylpropanoids, commonly known as cimicifugic acids [e.g., fukinolic acid (6)] see Figure 3. Numerous members of this class have also been isolated and fully characterized, and a rational naming system has been proposed 32.
Figure 2. Active compounds from black cohosh
Figure 3. Phenolic constituents of black cohosh
Figure 4. Alkaloids from black cohosh
Black cohosh supplements
Preparations of black cohosh are made from its roots and rhizomes (underground stems). They are sold as dietary supplements in such forms as powdered whole herb, liquid extracts, and dried extracts in pill form.
Available preparations vary considerably in their chemical composition, in part because the compounds in black cohosh that may be responsible for any relief of menopausal symptoms are not known. Substances in black cohosh that may account for its activity include triterpene glycosides such as actein, 23-epi-26-deoxyactein, and cimicifugoside; resins, such as cimicifugin; and aromatic acid derivatives such as caffeic, isoferulic, and fukinolic acids 33.
Products containing black cohosh extract are frequently standardized to provide at least 1 mg triterpene glycosides per daily dose 34. Remifemin, a commercial black cohosh product used in several studies included in a 2012 Cochrane review, is an extract currently standardized to be equivalent to 40 mg black cohosh root/rhizome (extracted with isopropyl alcohol) per daily dose of two tablets, but it is not standardized to triterpene glycoside content. The product has been on the market for years and has been reformulated over time 34.
What is blue cohosh
Blue cohosh (Caulophyllum thalictroides) is a native plant grown in eastern United States. As a dietary supplement, blue cohosh is used as an antispasmodic, emenagogue (menstrual flow stimulator), parturifacient (labor inducer), and abortifacient 35. In 1999, it was estimated that 64% of American midwives used blue cohosh to induce labor 36. Neonates born to women that have taken Blue cohosh (Caulophyllum thalictroides) tincture/dietary supplements may suffer perinatal stroke, acute myocardial infarction, congestive heart failure, neonatal shock, and multiple organ injury 35. The side effects associated with blue cohosh administration include diarrhea, increases in blood pressure and blood sugar, and stomach cramps 35.
While blue cohosh is known to produce a variety of bioactive natural products, toxic alkaloids (e.g., N-methylcytisine) have been considered the most likely cause of intoxication events 37. Blue cohosh (Caulophyllum thalictroides) methanol extract exhibited mitochondriotoxic activity.
Black cohosh for hot flashes and menopausal symptoms
Menopause represents the cessation of menstruation and the end of the reproductive period; this typically occurs around 51 years of age 38. Perimenopause is the period of transition to menopause, defined by irregular menstruation within the previous 12 months. Postmenopause is defined as the absence of menstruation for more than 12 months 38. The events leading to menopause are attributed to a reduction in ovarian activity, which may stem from a physiological or iatrogenic (medically induced) cause. Physiological menopause occurs when the ageing ovaries become less responsive to follicle-stimulating hormone (FSH) and luteinising hormone (LH), resulting in fewer ovulations and decreasing amounts of circulating progesterone and oestrogen. Iatrogenic menopause results from medical intervention, such as oophorectomy (removal of the ovaries), chemotherapy and pelvic irradiation 38. While the severity of symptoms of iatrogenic menopause is somewhat greater than physiological menopause, the types of symptoms reported are similar, with the most common manifestations including vasomotor symptoms (i.e. hot flushes and sweating), vulvovaginal atrophic symptoms (i.e. vaginal atrophy, vaginal dryness) and impaired sexual function 39. The average duration of these symptoms is 3.5 years 40, although symptom duration can range anywhere from five months to 10 years, with the severity of these manifestations varying from mild to severe. Postmenopausal women are also at increased risk of osteoporosis 39, with the risk escalating with increasing age. This perimenopausal period may be also associated with a decline in quality of life 41. In fact, perimenopausal women report a significant decline in perceived physical health and a marginally significant decline in psychosomatic domains (i.e. nervous and emotional state, self confidence, work life, ability to make decisions and ability to concentrate) when compared to premenopausal women 42.
Studies using various designs since the 1950s have attempted to determine whether black cohosh affects menopausal symptoms 43. Complicating efforts to understand the efficacy of black cohosh for treating menopausal symptoms is the wide variation in the chemical compositions of formulations. Black cohosh’s active ingredients and potential mechanism(s) of action are unknown. Studies have found varying results for the plant’s effects on human physiology as to whether, for example, it raises the body’s levels of estrogen, luteinizing hormone (LH), or follicle-stimulating hormone (FSH), which are all present in lower levels in menopausal women than in premenopausal women 44. It is not clear whether black cohosh affects the structure and activity of vaginal and uterine tissues 45. Some researchers believe that black cohosh might exert its effects through a brain-related action, such as moduation of serotonergic pathways, or through its potential ability to act as an antioxidant, anti-inflammatory, or selective estrogen receptor modulator 45.
Two high-quality randomized controlled trials investigating black cohosh for menopausal symptoms are described here. One, published in 2006, assigned 351 women aged 45–55 years experiencing daytime hot flashes and night sweats into one of five groups to take one of the following 46:
- 160 mg/day black cohosh (70% ethanolic extract standardized to contain 2.5% triterpene glycosides)
- A multibotanical preparation containing 200 mg black cohosh along with Siberian ginseng, dong quai, and other ingredients
- The same multibotanical preparation plus two daily servings of soy foods providing 12-20 g soy protein
- Hormone therapy (estrogen with or without progesterone)
- A placebo
After 3, 6, and 12 months of supplementation or placebo, the number and intensity of hot flashes and night sweats did not differ between the herbal-intervention groups and the placebo group, with one exception. At 12 months, participants consuming the multibotanical preparation plus soy foods had significantly worse symptom intensity than those consuming the placebo.
Another randomized controlled trial published in 2009 assigned 88 perimenopausal and postmenopausal women (mean age 53 years; 55% from underrepresented minority groups) who were experiencing at least 35 hot flashes and night sweats per week into one of four groups to take one of the following 47:
- 128 mg/day black cohosh (75% ethanolic extract standardized to contain 5.7% triterpene glycosides)
- 398 mg/day red clover (ethanolic extract of the aerial parts standardized to 120 mg isoflavones)
- Hormone therapy (estrogen and progesterone)
- A placebo
After 3, 6, 9, and 12 months of supplementation or placebo, the number of vasomotor symptoms declined significantly in all groups. However, there were no statistically significant differences between the black cohosh and red clover groups compared to placebo, with one exception. The black cohosh group showed worse symptom intensity at 6 and 9 months. This study also investigated secondary endpoints such as somatic symptoms (e.g., insomnia and fatigue), mood changes (e.g., depression and anxiety), and sexual dysfunction (e.g., vaginal dryness). For most of these outcomes, no significant differences were observed between any of the treatment groups at any time.
A 2012 Cochrane review evaluated 16 randomized clinical trials on the effectiveness of black cohosh in reducing menopausal symptoms, including hot flushes, night sweats, vaginal dryness, and combinations of symptoms measured by validated rating scales 9. The 16 included trials randomized a total of 2,027 women (mean age 50.5 to 56.4 years), and their samples ranged from 23 to 351 participants. Study durations were 8 to 54 weeks, with a mean duration of 22.8 weeks. Participants received a daily dose of various formulations of 8 to 160 mg/day black cohosh extract, with a median dose of 40 mg/day. In some cases, the authors of the original study reports indicated that the extract they used came from the root/rhizome, they had extracted the product using isopropyl alcohol or ethanol, and/or they had standardized the extract to contain a specific amount of triterpene glycosides. The studies were highly heterogeneous with respect to such factors as design, duration, type and amount of black cohosh used, and main findings. The review’s authors concluded that there was “insufficient evidence” from these trials “to either support or oppose the use of black cohosh for menopausal symptoms” 9.
A 2016 systematic review and meta-analysis of randomized clinical trials examined four studies of herbal and plant-based therapies that included black cohosh to treat menopausal symptoms 48. The trials randomized a total of 511 women to a daily dose of various formulations of 6.5 to 160 mg/day black cohosh extract or placebo. There were no significant associations between supplementation with black cohosh and reduction in the number of vasomotor symptoms, such as hot flashes. Furthermore, there were no beneficial associations between black cohosh use and relief of menopausal symptoms using self-reported rating scales.
The American College of Obstetricians and Gynecologists, in its 2015 clinical guidelines for managing menopausal symptoms, concluded that “data do not show that” herbal dietary supplements like black cohosh “are efficacious for the treatment of vasomotor symptoms” 49. The North American Menopause Society advises clinicians against recommending herbal therapies such as black cohosh because “they are unlikely to be beneficial” (italics in original) in alleviating vasomotor symptoms 50.
The 2012 Cochrane review found adequate justification for conducting further studies on black cohosh’s use to treat menopausal symptoms 9. Its authors recommended that researchers conduct higher-quality trials with larger samples and provide more details about their experimental protocols. Others have recommended that researchers should completely and comprehensively describe the black cohosh preparation they used so that other researchers could use the same or similar products 51. It is also important to independently analyze and verify the product’s composition to ensure its identity and quality 52.
Black cohosh dosage
The dose of black cohosh has been reported as ranging from 500-1000 mg daily, for treatment of menopause related disorders like anxiety, depression, flashes and myalgia 53. In that clinical study, the researchers regularly exclude from the treatment patients affected by cancer of sexual organs: breast, ovaries, uterus and hypophysis, unless already treated with conventional therapy from more than 7 years and considered recovered 53.
Black cohosh side effects
The safety of long-term use of black cohosh is uncertain. Clinical trials using various black cohosh preparations to treat menopausal symptoms have shown that its use is associated with a low incidence of adverse effects. The most commonly reported side effects are gastrointestinal upset and rashes, both of which are mild and transient 1. Other reported adverse effects in clinical trials have included breast pain/enlargement, infection, vaginal bleeding/spotting, and musculoskeletal complaints, although their incidence was similar in women taking black cohosh and those taking placebo 9. Most studies have examined black cohosh use for short periods, typically 6 months or less, so no published studies have assessed the long-term safety of black cohosh in humans.
The safety of black cohosh has become controversial recently following several case reports of liver toxicity 54. In July 2006 the European Medicines Agency and the Committee on Herbal Medicinal Products released a public statement 55 of case reports of hepatotoxicity (liver injuries) in patients using black cohosh rhizome. Following review of all available data, the European Medicines Agency and the Committee on Herbal Medicinal Products considered that there is a potential connection between herbal medicinal products containing black cohosh rhizome and hepatotoxicity on the base of 42 case reports of hepatotoxicity, collected from European National Competent Authorities (34 cases) as well as literature case reports (8 cases) 55. Of these, only 16 cases were considered sufficiently documented to allow the Committee to assess if use of black cohosh rhizome could be linked to the liver injuries. As a result of the assessment, five cases were excluded and seven cases were considered unlikely to be related. In the remaining only four cases (two autoimmune hepatitis, one hepatocellular liver injury and one fulminant hepatic failure), there was a temporal association. So there are very few cases well documented and few data available, for a concrete decision about its suspected hepatotoxicity; and in many reports black cohosh extracts were mixed with many other substances so that was impossible to get any reliable reference to the assumption of black cohosh.
Because black cohosh extracts are sold as over-the-counter integrators, Italian sanitary authorities decided a precautionary withdrawal from the national market, that later has been reversed, and stringent label warnings have been introduced for black cohosh extracts in United Kingdom. So actually there are some concerns about black cohosh extracts safety especially in patients suffering of liver disease.
Across the world, reports have described at least 83 cases of liver damage—including hepatitis, liver failure, elevated liver enzymes, and assorted other liver injuries—associated with black cohosh use 56. However, there is no evidence of a causal relationship. It is possible that at least some reported cases of hepatotoxicity were due to impurities, adulterants, or incorrect Acteae species in the black cohosh products used. However, no one independently analyzed these products to confirm the existence of these problems 57.
In 2007, the Australian Department of Health began requiring that products containing black cohosh carry the following label statement: “Warning: Black cohosh may harm the liver in some individuals. Use under the supervision of a healthcare professional” 58. In 2008, the U.S. Pharmacopeia (a nonprofit standard-setting organization for foods and drugs) recommended labeling black cohosh products with the following cautionary statement: “Discontinue use and consult a healthcare practitioner if you have a liver disorder or develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice” 59. However, the U.S. Food and Drug Administration does not require such a warning on black cohosh product labels.
The American Herbal Products Association recommends that pregnant women not take black cohosh except under the supervision of their healthcare provider because studies have not rigorously evaluated its use during pregnancy 1. The U.S. Pharmacopeia advises that individuals with liver disorders should also avoid black cohosh 59. It adds that users who develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice, while taking the supplement should discontinue use and contact their doctor.
Safety of black cohosh in humans
In clinical trials only minor adverse side effects have been reported, including nausea, vomiting, head-aches and dizziness: a review of eight clinical trials concluded that extracts of the rhizome of black cohosh might be a safe alternative for women seeking alternative estrogen replacement therapy 60.
- In clinical trials, people have taken black cohosh for as long as 12 months with no serious harmful effects. The only reported side effects were minor problems such as upset stomach or rashes.
- Some commercial black cohosh products have been found to contain the wrong herb or to contain mixtures of black cohosh and other herbs that are not listed on the label.
- Cases of liver damage—some of them very serious—have been reported in people taking commercial black cohosh products. These problems are rare, and it is uncertain whether black cohosh was responsible for them. Nevertheless, people with liver disorders should consult a health care provider before taking black cohosh products, and anyone who develops symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice, while taking black cohosh should stop using it and consult a health care provider.
- The risk of interactions between black cohosh and medicines appears to be small.
- It’s not clear if black cohosh is safe for women who have had hormone-sensitive conditions such as breast cancer or for pregnant women or nursing mothers.
- Black cohosh should not be confused with blue cohosh (Caulophyllum thalictroides), which has different effects and may not be safe. Black cohosh has sometimes been used with blue cohosh to stimulate labor, but this use was linked to severe adverse effects in at least one newborn.
Black Cohosh also contains several catechols, such as caffeic acid, piscidic acid and fukiic acid esters that exhibit some antioxidant properties, including fukinolic acid, cimicifugic acid A and cimicifugic acid B 61. Such catechols could be of significant concern in toxicology because of the possibility that they could be activated, either metabolically or chemically, to electrophilic quinones. The potential of such quinones to cause toxicity and carcinogenesis is well documented, and can occur via arylation of cellular proteins and DNA or redox cycling leading to the formation of reactive oxygen species such as the hydroxyl radical 62. Nevertheless has been shown in six perimenopausal women after administration of a single dose of either 32, 64 or 128 mg of black cohosh that no corresponding mercapturic acids were found in the urine 63. In a previous study, potential toxicity was suspected because catechols from Black Cohosh are activated to quinoid metabolites, but catechols are not absorbed across the bowel.
In a recent trial on 351 randomized women, placebo controlled, Black Cohosh both used as single substance and mixed in multi herbs remedies after 12 month of treatment, did not show any effect on lipids, glucose, insulin and fibrinogen 64.
Instead an important issue about safety is probably the interaction with synthetic drugs because of the interference with the metabolic pathway of cytochrome P4503A4 65, a potential cause of important adverse reactions, especially in patients assuming multi drug regimen therapies as confirmed by a recent work in which ethanol and isopropanol extract induced inhibition of CYP1A2, 2C9, 2D6, 3A4.
To be added a case of cutaneous pseudolymphoma in a patient assuming a commercial extract of black cohosh has been reported recently 66; and a case of muscle damage with asthenia, high levels of creatine phosphokinase and lactate dehydrogenase following assumption of a dietary supplement derived from black cohosh has also been reported 67.
Interactions with tamoxifen and aromatase Inhibitors
The potential for interactions with tamoxifen, in particular aromatase inhibitors, must also be considered. Five studies in our review (4 trials and 1 cohort study) included patients who were receiving both tamoxifen and/or raloxifene 19. None of the trials reported the impact of the combined therapy on risk of recurrence; however, the cohort study by Henneicke-von Zepelin et al. 19 suggested that taking black cohosh reduced risk of recurrence by 25% in the treatment group, 35.8% of which was taking tamoxifen 19. No consistent serious adverse events related to the combination of black cohosh and tamoxifen was reported by any of the trials 20.
An animal study comparing the antitumor effects of formestane with or without the addition of 60 mg/kg isopropanolic black cohosh extract found that the addition of black cohosh had no effect on formestane-induced tumor reduction or reduction of serum estrogen levels 68. A second study examining black cohosh with tamoxifen in a model of endometrial cancer found similar results; unlike the endometrial estrogen agonist tamoxifen, “black cohosh did not further growth or metastasizing potential of the primary tumor” 69. There were no detectable supportive or antagonistic effects between the 2 treatments 69.
In humans, one study reported a statistically significant inhibition of CYP 2D6 by black cohosh 70. This study used a very high dose of black cohosh (>1000 mg), however, and the magnitude of the effect seen (approximately 7%) “did not appear to be clinically relevant” 71, which cast some doubt on its clinical applicability. Other studies failed to confirm this effect 72. Nonetheless, this finding is worth noting, given that tamoxifen, a selective estrogen receptor modulator, is primarily metabolized by CYP 2D6 73. Other inhibitors of CYP 2D6, such as selective serotonin reuptake inhibitors have been shown to reduce serum levels of tamoxifen’s active metabolites, notably endoxifen, by up to 50% 73. Theoretically, black cohosh might have lesser such effects, though this has not been directly studied. Black cohosh does not appear to affect the following enzymes: CYP 1A2, CYP 2E1, CYP 3A4, CYP 3A5, or Pgp 74. Conversely, the aromatase inhibitor anastrozole, which is primarily used in postmenopausal women 75, is metabolized primarly by CYP 3A4 and also to some extent by CYP 3A5, CYP 2C8, and UGT1A4 76 suggesting that this drug is less likely to be affected by a pharmacokinetic interaction with black cohosh. The other third generation aromatase inhibitors including letrozole and exemestane are also not affected by CYP 2D6 77.
- Gardner Z, McGuffin M, eds. American Herbal Products Association’s botanical safety handbook. Second ed. Boca Raton, FL: CRC Press; 2013.[↩][↩][↩]
- Compton JA, Culham A, Jury SL. Reclassification of Actea to include Cimicifuga and Soulica (Ranunculaceae) pyhlogeny inferred from morphology, nrDNA, ITS and cpDNA trnl-F sequence variation. Taxon. 1998;47:593–635.[↩]
- Blumenthal M. The ABC Clinical Guide to Herbs. Austin: American Botanical Council, 2003.[↩][↩]
- McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern Ther Health Med. 2001;7:93–100.[↩]
- Foster S. Black cohosh: Cimicifuga racemosa: a literature review. Herbalgram. 1999;45:35–49.[↩]
- Black cohosh (Actaea/Cimicifuga racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Mahady GB. Treat Endocrinol. 2005; 4(3):177-84. https://www.ncbi.nlm.nih.gov/pubmed/15898823/[↩]
- Firenzuoli F, Gori L, Roberti di Sarsina P. Black Cohosh Hepatic Safety: Follow-Up of 107 Patients Consuming a Special Cimicifuga racemosa rhizome Herbal Extract and Review of Literature. Evidence-based Complementary and Alternative Medicine : eCAM. 2011;2011:821392. doi:10.1093/ecam/nen009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3110476/[↩]
- Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol Res. 2008;58:8–14. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0025895/[↩][↩]
- Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No.: CD007244. DOI: 10.1002/14651858.CD007244.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007244.pub2/full[↩][↩][↩][↩][↩][↩][↩]
- Black Cohosh. https://nccih.nih.gov/health/blackcohosh/ataglance.htm[↩]
- Blumenthal M, ed. Klein S, Rister RS, trans. German Commission E Monographs: Therapeutic Monographs on Medicinal Plants for Human Use. Austin, TX: American Botanical Council;1998.[↩]
- Black Cohosh and Breast Cancer: A Systematic Review. Integrative Cancer Therapies February 25, 2013; Vol 13, Issue 1, pp. 12 – 29. http://journals.sagepub.com/doi/10.1177/1534735413477191[↩][↩][↩][↩][↩]
- Wuttke W, Seidlová-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44(suppl 1):S67-S77.[↩]
- Koos RD. Minireview: putting physiology back into estrogens’ mechanism of action. Endocrinology. 2011;152:4481-4488.[↩]
- Walji R, Boon H, Guns E, Oneschuk D, Younus J. Black cohosh (Cimicifuga racemosa [L.] Nutt.): safety and efficacy for cancer patients. Support Care Cancer. 2007;15:913-921[↩]
- Brasky TM, Lampe JW, Potter JD, Patterson RE, White E. Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:1696-1708.[↩]
- Obi N, Chang-Claude J, Berger J, . The use of herbal preparations to alleviate climacteric disorders and risk of postmenopausal breast cancer in a German case-control study. Cancer Epidemiol Biomarkers Prev. 2009;18:2207-2213.[↩]
- Rebbeck TR, Troxel AB, Norman S, . A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer. 2007;120:1523-1528.[↩]
- Henneicke-von Zepelin HH, Meden H, Kostev K, Schröder-Bernhardi D, Stammwitz U, Becher H. Isopropanolic black cohosh extract and recurrence-free survival after breast cancer. Int J Clin Pharmacol Ther. 2007;45:143-154.[↩][↩][↩][↩][↩]
- Pockaj BA, Gallagher JG, Loprinzi CL, . Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol. 2006;24:2836-2841.[↩][↩]
- Jacobson JS, Troxel AB, Evans J, . Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739-2745.[↩]
- Hernández Muñoz G, Pluchino S. Cimicifuga racemosa for the treatment of hot flushes in women surviving breast cancer. Maturitas. 2003;44(suppl 1):59-65.[↩]
- Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke-von Zepelin HH. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;105:1074–1083[↩]
- Geller SE, Shulman LP, van Breemen RB, Banuvar S, Zhou Y, Epstein G, Hedayat S, Nikolic D, Krause EC, Piersen CE, Bolton JL, Pauli GF, Farnsworth NR. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16:1156–1166[↩]
- Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust. 2002;177:440–443.[↩]
- Mahady GB, Low Dog T, Barrett ML, Chavez ML, Gardiner P, Ko R, Marles RJ, Pellicore LS, Giancaspro GI, Sarma DN. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause. 2008;15:628–638.[↩]
- Teschke R. Black cohosh and suspected hepatotoxicity: inconsistencies, confounding variables, and prospective use of a diagnostic causality algorithm. A critical review. Menopause. 2010;17:426–440.[↩]
- Huang Y, Jiang B, Nuntanakorn P, Kennelly EJ, Shord S, Lawal TO, Mahady GB. Fukinolic acid derivatives and triterpene glycosides from black cohosh inhibit CYP isozymes, but are not cytotoxic to Hep-G2 cells in vitro. Curr Drug Saf. 2010;5:118–124[↩]
- Li JX, Yu ZY. Cimicifugae rhizoma: from origins, bioactive constituents to clinical outcomes. Curr Med Chem. 2006;13:2927–2951.[↩]
- Cicek SS, Aberham A, Ganzera M, Stuppner H. Quantitative analysis of cycloartane glycosides in black cohosh rhizomes and dietary supplements by RRLC-ELSD and RRLC-qTOF-MS. Anal Bioanal Chem. 2011;400:2597–2605.[↩]
- Qiu F, Imai A, McAlpine JB, Lankin DC, Burton I, Karakach T, Farnsworth NR, Chen SN, Pauli GF. Dereplication, residual complexity, and rational naming: the case of the Actaea triterpenes. J Nat Prod. 2012;75:432–443. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3392135/[↩]
- Godecke T, Nikolic D, Lankin DC, Chen SN, Powell SL, Dietz B, Bolton JL, van Breemen RB, Farnsworth NR, Pauli GF. Phytochemistry of cimicifugic acids and associated bases in Cimicifuga racemosa root extracts. Phytochem Anal. 2009;20:120–133[↩]
- Kruse SO, Lohning A, Pauli GF, Winterhoff H, Nahrstedt A. Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med 1999;65:763-4[↩]
- ConsumerLab.com. Product review: menopause supplements (soy and red clover isoflavones, black cohosh) and progesterone creams. 2016. https://www.consumerlab.com/reviews/soy-%20%20isoflavones_red-clover_black-cohosh_supplements/phytoestrogens[↩][↩]
- Dugoua JJ, Perri D, Seely D, Mills E, Koren G. Can J Clin Pharmacol. 2008;15:e66–e73. https://www.ncbi.nlm.nih.gov/pubmed/18204101[↩][↩][↩]
- McFarlin BL, Gibson MH, O’Rear J, Harman P. J Nurse Midwifery. 1999;44:205–216. https://www.ncbi.nlm.nih.gov/pubmed/10380441[↩]
- Rader JI, Pawar RS. Anal Bioanal Chem. 2013;405:4409–4417. https://www.ncbi.nlm.nih.gov/pubmed/23420136[↩]
- Porter RS, Kaplan JL. The Merck Manual Online. Whitehouse Station, New Jersey: Merck Research Laboratories, 2011.[↩][↩][↩]
- Corwin EJ. Handbook of Pathophysiology. Philadelphia: Lippincott, Williams & Wilkins, 2008.[↩][↩]
- McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. American Journal of Human Biology 1992;4(1):37-46.[↩]
- Blumel JE, Castelo-Branco C, Binfa L, Gramegna G, Tacla X, Aracena B, et al. Quality of life after the menopause: a population study. Maturitas 2000;34(1):17-23.[↩]
- Mishra G, Kuh D. Perceived change in quality of life during the menopause. Social Science & Medicine 2006;62(1):93-102.[↩]
- Fabricant DS, Krause EC, Farnsworth NR. Black cohosh. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of dietary supplements. Second ed. New York: Informa Healthcare;2010:60-74.[↩]
- Liske E, Hanggi W, Henneicke-von Zepelin HH, Boblitz N, Wustenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med 2002;11:163-74[↩]
- The North American Menopause Society. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015;22:1155-72[↩][↩]
- Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med 2006;145:869-79. http://annals.org/aim/fullarticle/731061/treatment-vasomotor-symptoms-menopause-black-cohosh-multibotanicals-soy-hormone-therapy[↩]
- Geller SE, Shulman LP, van Breemen RB, Banuvar S, Zhou Y, Epstein G, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 2009;16:1156-66. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2783540/[↩]
- Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA 2016;315:2554-63. https://jamanetwork.com/journals/jama/fullarticle/2529629[↩]
- ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 2014;123:202-16. https://www.ncbi.nlm.nih.gov/pubmed/24463691[↩]
- The North American Menopause Society. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015;22:1155-72. https://www.ncbi.nlm.nih.gov/pubmed/26382310[↩]
- Swanson CA. Suggested guidelines for articles about botanical dietary supplements. Am J Clin Nutr 2002;75:8-10. https://academic.oup.com/ajcn/article/75/1/8/4689239[↩]
- Office of Dietary Supplements, National Institutes of Health. https://ods.od.nih.gov/Research/AMRMProgramWebsite.aspx[↩]
- Firenzuoli F, Gori L, Roberti di Sarsina P. Black Cohosh Hepatic Safety: Follow-Up of 107 Patients Consuming a Special Cimicifuga racemosa rhizome Herbal Extract and Review of Literature. Evidence-based Complementary and Alternative Medicine : eCAM. 2011;2011:821392. doi:10.1093/ecam/nen009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3110476/[↩][↩]
- Teschke R. Black cohosh and suspected hepatotoxicity: inconsistencies, confounding variables, and prospective use of a diagnostic causality algorithm: a critical review. Menopause. 2010;17:426-440.[↩]
- Assessment of case reports connected to herbal medicinal products containing cimicifugae racemose rhizoma (black cohosh, root). Doc. Ref.: EMEA/269259/2006. 2007, http://www.ema.europa.eu/[↩][↩]
- Teschke R, Schwarzenboeck A, Schmidt-Taenzer W, Wolff A, Hennermann KH. Herb induced liver injury presumably caused by black cohosh: a survey of initially purported cases and herbal quality specifications. Ann Hepatol 2011;10:249-59.[↩]
- Health Canada. Black cohosh products and liver toxicity: update. 2010.[↩]
- Black cohosh (Cimicifuga racemosa). New labelling requirements and consumer information for medicines containing Black cohosh. https://www.tga.gov.au/alert/black-cohosh-cimicifuga-racemosa[↩]
- Mahady GB, Low Dog T, Barrett ML, Chavez ML, Gardiner P, Ko R, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008;15:628-38. https://www.ncbi.nlm.nih.gov/pubmed/18340277[↩][↩]
- Liberman S. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. Journal of Women’s Health. 1998;7:525–529.[↩]
- Takahira M, Kusano A, Shibano M, Kusano G, Miyase T. Piscidic acid and fukiic acid esters from Cimicifuga simplex. Phytochemistry. 1998;49(7):2115–2119.[↩]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chemical Research in Toxicology. 2000;13(3):135–160.[↩]
- Johnson BM, Van Breemen RB. In vitro formation of quinoid metabolites of the dietary supplement Cimicifuga racemosa (black cohosh) Chemical Research in Toxicology. 2003;16(7):838–846.[↩]
- Spangler L, Newton KM, Grothaus LC, Reed SD, Ehrlich K, LaCroix AZ. The effects of black cohosh therapies on lipids, fibrinogen, glucose and insulin. Maturitas. 2007;57(2):195–204.[↩]
- Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 inhibitors from the black cohosh (Cimicifuga racemosa) Evidence-Based Complementary and Alternative Medicine. 2005;2(2):223–226.[↩]
- Meyer S, Vogt T, Obermann EC, Landthaler M, Karrer S. Cutaneous pseudolymphoma induced by Cimicifuga racemosa . Dermatology. 2006;214(1):94–96.[↩]
- Minciullo PL, Saija A, Patafi M, Marotta G, Ferlazzo B, Gangemi S. Muscle damage induced by black cohosh (Cimicifuga racemosa) Phytomedicine. 2006;13(1-2):115–118.[↩]
- Nisslein T, Freudenstein J. Coadministration of the aromatase inhibitor formestane and an isopropanolic extract of black cohosh in a rat model of chemically induced mammary carcinoma. Planta Medica. 2007;73:318-322.[↩]
- Nisslein T, Freudenstein J. Concomitant administration of an isopropanolic extract of black cohosh and tamoxifen in the in vivo tumor model of implanted RUCA-I rat endometrial adenocarcinoma cells. Toxicol Lett. 2004;150:271-275.[↩][↩]
- Shord SS, Shah K, Lukose A. Drug-botanical interactions: a review of the laboratory, animal, and human data for 8 common botanicals. Integr Cancer Ther. 2009;8:208-227.[↩]
- Gurley BJ, Gardner SF, Hubbard MA, . In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77:415-426[↩]
- Gurley BJ, Swain A, Hubbard MA, . Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John’s wort, and Echinacea. Mol Nutr Food Res. 2008;52:755-763.[↩]
- Tamoxifen and CYP 2D6 inhibitors: caution. Prescrire Int. 2011;20:182-184.[↩][↩]
- Gurley B, Hubbard MA, Williams DK, . Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J Clin Pharmacol. 2006;46:201-213.[↩]
- Cuzick J, Sestak I, Baum M, . Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135-1141.[↩]
- Kamdem LK, Liu Y, Stearns V, . In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010;70:854-869.[↩]
- Choueiri TK, Alemany CA, Abou-Jawde RM, Budd GT. Role of aromatase inhibitors in the treatment of breast cancer. Clin Ther. 2004;26:1199-1214.[↩]