What is boswellia serrata
Boswellia serrata (also called Frankincense or olibanum) has been used in the traditional herbal formulas to treat inflammatory arthritis in China and India 1. Boswellia serrata contains boswellic acids which have been shown to exhibit anti-inflammatory and antiarthritic properties.
The gum resin extracted from Boswellia serrata has gained considerable attention as a potent anti-inflammatory, anti-arthritic and analgesic agent 2. 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) is the most active compound of Boswellia extract and is a potent inhibitor of 5-lipoxygenase (5-LOX), a key enzyme in the biosynthesis of leukotrienes from arachidonic acid in the cellular inflammatory cascade 3. A number of independent clinical studies support the anti-inflammatory and anti-arthritic properties of Boswellia extracts 4, 5.
In the last decade preparations from the oleogum resin of Boswellia serrata and other Boswellia species, have become more and more popular in some European countries for the treatment of a variety of chronic inflammatory diseases including rheumatoid arthritis, chronic bowel diseases, bronchial asthma, peritumoural brain oedema and others 6. However, studies indicate that upon oral administration, Boswellia extracts exhibit poor intestinal absorption of 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) and poor bioavailability which limits its anti-inflammatory efficacy 7, 8.
Boswellia are moderate-sized flowering plants, including both trees and shrubs, and are native to tropical regions of Africa and Asia. The distributions of the species are primarily associated with the tropics. The greatest diversity of species presently is in Africa and India 9.
Frank (“pure”) incense is the oleogum resin produced in the bark of different Boswellia species belonging to the family of Burseraceae. There are four main species of Boswellia which produce true frankincense. Boswellia sacra (synonyms B. carteri and B. bhaw-dajiana), Boswellia frereana, Boswellia papyrifera, and Boswellia serrata 10 and each type of resin is available in various grades. The grades depend on the time of harvesting, and the resin is hand sorted for quality. The term guggals collectively refers to gum resins.
Table 1. Boswellia serrata (Salai guggal) traditional Indian Ayurvedic Medicine Uses
| Organs and functional systems | Effects |
| Nervous system | Analgesic Mental tonic Stimulation Eye tonic |
| Cardiovascular system | Cardiotonic |
| Gastrointestinal tract | Regulating colour of stool Carminative, stomachic Improving digestion, antidiarrhoeic Improving taste Anthelmintic |
| Urogenital system | Diuretic Aphrodisiac Improving menstruation |
| Fever | Antipyretic |
| Skin | Increases perspiration Wound cleaning |
| Whole organism | Anti-inflammatory Antiseptic Reducing fat Haemostypic Connecting tissue Decreasing Kapha diseases (in Ayurvedic nomenclature) |
Composition of Boswellia Extract Oleogum Resins
More than 200 different compounds were identified in the boswellia extract oleogum resin of different Boswellia species. Main components are volatile oil, pure resin and mucus. The content of these differs from species to species, between different harvestings and different locations. An approximate composition of some oleogum resins is listed in Table 2. The resins of Boswellia species contain pentacyclic and tetracyclic triterpenes. Among the pentacyclic triterpenes, some boswellic acids are mainly responsible for many of the pharmacological effects. Further compounds are tetracyclic triterpenic acids among which tirucallic acids were also shown to be biologically active.
Table 2. Composition of boswellia extract oleogum resin of two different Boswellia species
| Boswellia carteri Birdw. | Boswellia serrata Roxb. | |
| Volatile oil | 5 – 9 % | 7.5 – 9 % to 15 % |
| Pure resin | ≈ 66 % | 55 – 57 % |
| Mucus | ≈ 12 – 20 % | ≈ 23 % |
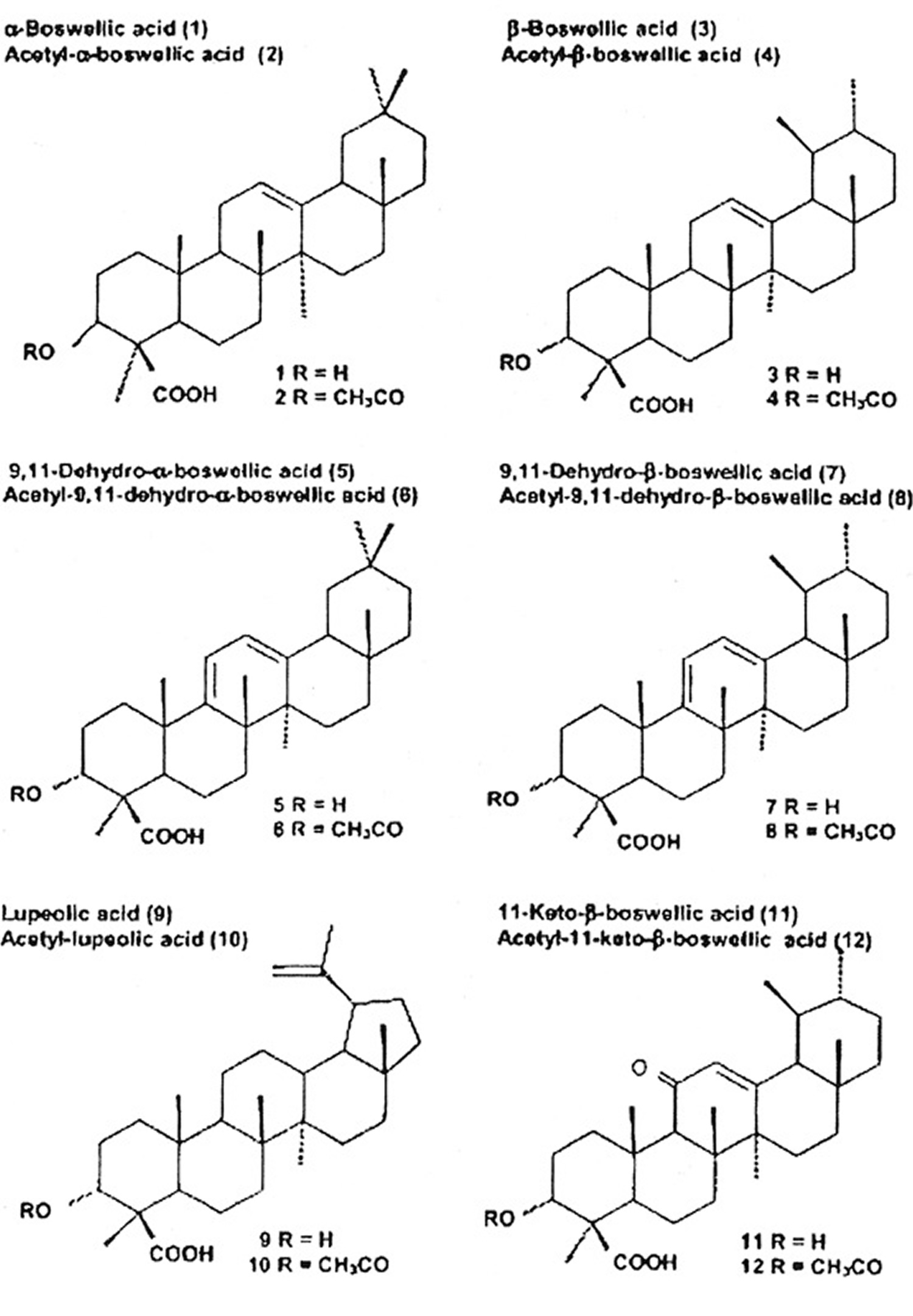
Among the pentacyclic triterpenes, a variety of boswellic acids and other compounds were identified. Some of them are closely related to the pharmacological effects of boswellia serrata. Figure 1 shows their chemical structure and the content in the oleogum resins of boswellia serrata and African species. In 2003, Büchele et al. 12 identified 12 different pentacyclic triterpenes in different samples of Boswellia, i. e., frankincense from India and Africa using an extract. The authors reported marked differences in different species. A striking difference was observed in the content of the main active boswellic acids 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) and 11-keto-β-boswellic acid (KBA). It is evident that the Indian sample contained quite similar amounts of 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) and 11-keto-β-boswellic acid (KBA) whereas the African samples contained less 11-keto-β-boswellic acid (KBA) than 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA). A new pentacyclic triterpene from boswellia serrata, i. e., 3α-acetyl-20(29)-lupene-24-oic acid was recently identified by Beisner et al. 13. Employing a commercial extract from boswellia serrata (H 15 AyurmedicaTM), ∼2.6 mg/100 mg KBA and ∼2.8 mg/100 mg AKBA were detected on average in 11 different lots. Ganzera et al. 14 studied 4 different commercial products containing the oleogum resin of boswellia serrata together with up to 10 other plant extracts. Considering the manufacturer’s dosing recommendations, the daily intake of total boswellic acids varies up to 6-fold (18.49 to 109.62 mg per day). Hamm et al. 15 tested volatile and semi-volatile terpenes from 6 different olibanum samples, i. e., B. carterii, B. sacra, B. serrata, B. papyfera and B. frereana. The chemical composition was different in all species and allowed identification of the taxonomic origin of frankincense samples purchased from various markets.
Among the tetracyclic triterpenes, three tirucallic acids were identified, i. e., 3-oxotirucallic acid, 3-hydroxytirucallic acid and 3-acetoxytirucallic acid, which were also shown to interact with the 5-LO-system 16.
Figure 1. Triterpenic acids present in Frankincense
Boswellia benefits
There are several severe chronic diseases mostly related to autoimmune disorders. Among these are rheumatic diseases, inflammatory bowel diseases, bronchial asthma and others.
In fact, several clinical studies were performed on the effect of boswellia serrata. However, the results published so far must be regarded as inconclusive and early preliminary in character because some were performed in test tubes and in animals order to test theoretical hypothesis that may also be of clinical relevance. Therefore, further studies involving human subjects confirming these findings are necessary.
Rheumatoid Arthritis and Osteoarthritis
In traditional Indian Ayurvedic medicine, Salai guggal is used to treat rheumatoid arthritis. In order to support this traditional use scientifically, Singh and Atal 17 from the Regional Research Laboratory in Jammu, India described for the first time anti-inflammatory properties of Salai guggal ex Boswellia serrata in experimental animals. Further studies with the rat adjuvant arthritis model showed protective effects of Salai guggal and boswellic acids. Classical animal models for testing antiarthritic actions of drugs are formaldehyde- and adjuvant-produced arthritis and the cotton pellet-induced granuloma test. In the study of Singh and Atal 17, arthritis was induced by injecting 0.1 ml of formaldehyde (2 % v/v in normal saline) in the subplantar region on the 1st and 3rd day of experiment. Paw volume was measured before formaldehyde injection and during drug treatment. Drugs were administered orally daily. In a dose range of 50 – 200 mg/kg orally, the alcoholic extract of the oleogum resin of boswellia serrata resulted in marked inhibition of swelling in these rats.
Adjuvant arthritis was caused by injecting 0.05 ml of a (0.5 % w/v) suspension of killed Mycobacterium tuberculosis homogenised in liquid paraffin into the left hind foot. Oral administration of the test drug was started on the day before the injection of Mycobacterium and continued until day 14. Paw volume was measured on alternate days, and percent inhibition was calculated on day 14. In this model, boswellia extract at 100 mg/kg caused a reduction in swelling by 45 %.
In the adjuvant arthritis model, Kesava-Reddy et al. 18 studied the effect of an extract of oleogum resin of boswellia serrata [prepared according to Singh and Atal 17] on urinary excretion of connective tissue metabolites, including hydroxyproline, hexosamine and uronic acid. Compared to controls, the arthritic animals showed increased excretion of these metabolites in the urine. The elevated levels of urinary hydroxyproline (free, total, non-dialysable and dialysable), hexosamine and uronic acid in the arthritic animals were found to be slightly decreased in the acute phase and significantly decreased in the chronic phase of the disease following administration of the drug suggesting a beneficial action.
In another study, Sharma et al. 19 investigated the effect of a boswellia extract on bovine serum albumin (BSA)-induced arthritis in rabbits. Oral administration of the boswellia extract (25, 50 and 100 mg/kg/day) significantly reduced the population of leukocytes in a BSA-injected knee and changed the electrophoretic pattern of the synovial fluid proteins. The local injection of the extract (5, 10 and 20 mg) into the knee 15 min prior to BSA challenge also significantly reduced the infiltration of leukocytes into the knee joint, reduced the infiltration of leukocytes into the pleural cavity and inhibited the migration of PMN in vitro. The leukocyte-inhibitory activity of boswellic acids was not due to a cytotoxic effect and could later be explained by inhibition of leukotriene synthesis and therefore by a failure of the chemotactic action. The antiarthritic action of an acetone extract of Boswellia carterii Birdw. in adjuvant-induced arthritis of Lewin rats has been recently confirmed by Fan et al. 20.
Taken together, these studies suggest an anti-inflammatory action of boswellia extracts in experimental arthritis.
In 2006 a double-blind randomised controlled trial 21 designed to assess the efficacy and safety of 5-Loxin [5-Loxin® is a novel B. serrata extract enriched to 30% AKBA (US Patent publication no.: 2004/0073060A1)] for osteoarthritis of the knee. The 5-Loxin treatment is a gum extract of the ancient herb Boswellia serrata (frankincense), which is enriched with other chemicals to create a compound that inhibits the enzyme 5-lipoxygenase, which is known to be a key enzyme in the inflammatory process.
The trial was carried out in India and 75 people with mild-to-moderate osteoarthritis of the knee were randomised to receive either 100mg (low dose) or 250mg (high dose) of 5-Loxin per day, or identical placebo capsules. Some 236 outpatients were originally selected based on symptoms and signs of osteoarthritis. To be included in the trial, people had to have been suffering knee pain for longer than three months, be between 40 and 80 years old, and score between 4 and 7 on a 10-point visual analogue pain scale once they had stopped taking their usual medication (daily anti-inflammatory drugs or paracetamol) for one week. The researchers excluded all those with inflammatory conditions, such as rheumatoid arthritis, gout, knee injury, need for steroid injection in the past three months, obese people, those with high alcohol intake, or those with medical conditions that may put them at risk from the treatments (e.g. liver or kidney conditions).
The patients completed a baseline questionnaire about their medical history and nutritional status. They were followed up at seven, 30, 60 and 90 days. Pain, stiffness and physical function scores were assessed at each visit, and blood tests for inflammatory markers and urine samples were taken. Based on pain scores, the patients could receive “rescue” anti-inflammatory drugs if they were needed. If these were taken, the patients were advised to discontinue taking the “rescue” treatment three days before each assessment. No other treatments were taken.
At baseline and 90 days, the patients had fluid taken from the knee joint to look at the concentration of matrix metalloproteinase-3 (MMP3) – an enzyme which can break down bone cartilage. Adverse effects were self-reported by the patients. The primary outcome for the study was the difference in pain, stiffness and physical function with 5-Loxin compared with the placebo.
What were the results of the study ?
Seventy patients completed the study. There was significant improvement in pain score with both low- and high-dose 5-Loxin compared with the placebo. Compared with the placebo, low-dose 5-Loxin improved pain score by 49%, 24% and 40% on the three different scales used. However, the 43% improvement in stiffness and 29% improvement in function were not significant when compared with the placebo. In the high-dose group, percentage improvements were greater compared with the placebo across all measures of pain, stiffness and function, and these were significant. Both dose groups showed significant improvement in pain at one week compared with the placebo.
There was no change in MMP3 concentration in the fluid from the knee in the placebo group. However, 5-Loxin significantly reduced MMP3 concentration (by 31% at the low dose and 46% at the high dose). The reduction in MMP3 with high-dose 5-Loxin was also significantly greater than with the low dose. There was no difference in adverse effects seen in the treatment or control groups.
The researchers conclude that 5-Loxin is safe and significantly reduces pain and improves physical functioning in people with osteoarthritis.
What interpretations can we make of this study ?
This is reportedly the first trial of the drug 5-Loxin, and it provides promising results. However, results should be considered preliminary. There are a number of limitations to the interpretation of this study:
- This trial was relatively small, and this may have affected the ability of randomisation to balance the groups for important characteristics. For example, on average those in the high-dose 5-Loxin group were about 6kg lighter, and had a BMI about 3.5 units lower, than those in the other groups.
- The method of how participants were randomly assigned into groups was not described, and some methods of randomisation are not as robust as others.
- The placebo pills were described as being “similar” in appearance, colour and taste to the 5-Loxin pills, but it is not clear if they were similar enough to prevent participants guessing which treatment they were receiving.
- The trial was relatively short. Longer trials will be needed to confirm long-term safety.
In order to confirm efficacy and safety results, further research is needed in larger numbers of people for longer periods of time. Research is also required for people with different characteristics (strict inclusion and exclusion criteria were used here), and for those with osteoarthritis of joints other than the knee. Efficacy compared with the wide range of other medical and surgical treatments used in the treatment of osteoarthritis will also be needed. The possible role of this treatment in arthritic conditions aside from osteoarthritis is not known from this research.
In another double blind randomized clinical study done subsequent to the above 5-Loxin study 22 using a new boswellia extract formulation called Aflapin [which contains Boswellia serrata extract enriched to 20% AKBA and B. serrata non-volatile oil (PCT/IN2009/000505)] in knee osteoarthritis. Sixty eligible osteoarthritis subjects selected through screening were included in the study. The subjects received either 100 mg (n=30) of Aflapin® or placebo (n=30) daily for 30 days. Each subject was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne’s Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 5, 15 and 30. A series of biochemical tests in serum, urine and hematological parameters established the safety of Aflapin. The observations suggest that Aflapin conferred clinically and statistically significant improvements in pain scores and physical function scores in osteoarthritis subjects. Aflapin provided significant improvements in pain score and functional ability in as early as 5 days of treatment. In conclusion, the researchers state that Aflapin is more efficacious as an anti-inflammatory agent compared to the existing Boswellia products, 5-Loxin® and traditional 65% Boswellia extract in alleviating pain, joint stiffness and improving physical functions in degenerative joint disease (osteoarthritis) subjects 22. In comparison with the placebo, at the end of the study, the Aflapin supplemented group showed statistically significant improvements in all pain scores.
Boswellia side effects
During the course of the 30-day Aflapin study 22, no major adverse events were reported. However, nausea and headache were reported as minor adverse events by two subjects during the study; one each from placebo and Aflapin supplemented groups.
One subject from placebo groups was dropped out from the study due to un-availability for the follow up evaluations.
During the course of the 90-day 5-Loxin study 21 period, some minor adverse events were noted: diarrhoea, nausea, abdominal pain, mild fever (up to 37.5°C [99.5°F]) and general weakness. The patients who reported these minor events were distributed evenly throughout the placebo and active treatment groups.
Five patients (one from the low-dose [100 mg 5-Loxin®] group, and two each from placebo and high-dose [250 mg 5-Loxin®] group) were excluded from the study because they were suffering from a nonfatal viral infection during the course of study.
- Chevrier MR, Ryan AE, Lee DY-W, Zhongze M, Wu-Yan Z, Via CS. Boswellia carterii Extract Inhibits TH1 Cytokines and Promotes TH2 Cytokines In Vitro. Clinical and Diagnostic Laboratory Immunology. 2005;12(5):575-580. doi:10.1128/CDLI.12.5.575-580.2005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1112084/[↩]
- Boswellia: an evidence-based systematic review by the Natural Standard Research Collaboration. Basch E, Boon H, Davies-Heerema T, Foppo I, Hashmi S, Hasskarl J, Sollars D, Ulbricht C. J Herb Pharmacother. 2004; 4(3):63-83. https://www.ncbi.nlm.nih.gov/pubmed/15829470/[↩]
- Acetyl-11-keto-beta-boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HP, Safayhi H. Br J Pharmacol. 1996 Feb; 117(4):615-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1909340/[↩]
- Use of complementary and alternative medicine in Germany – a survey of patients with inflammatory bowel disease. Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B. BMC Complement Altern Med. 2006 May 22; 6():19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1539021/[↩]
- Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Phytomedicine. 2003 Jan; 10(1):3-7. https://www.ncbi.nlm.nih.gov/pubmed/12622457/[↩]
- Boswellic Acids in Chronic Inflammatory Diseases. Planta Med 2006; 72(12): 1100-1116. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-2006-947227[↩][↩]
- Metabolism of boswellic acids in vitro and in vivo. Krüger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Müller WE, Karas M, Schubert-Zsilavecz M, Abdel-Tawab M. Drug Metab Dispos. 2008 Jun; 36(6):1135-42. http://dmd.aspetjournals.org/content/36/6/1135.long[↩]
- Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2. Krüger P, Kanzer J, Hummel J, Fricker G, Schubert-Zsilavecz M, Abdel-Tawab M. Eur J Pharm Sci. 2009 Feb 15; 36(2-3):275-84. https://www.ncbi.nlm.nih.gov/pubmed/19010411/[↩]
- Weeks, A., Daly, D.C. and B.B. Simpson. 2005. The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Molecular Phylogenetics and Evolution, 35: 85-101.[↩]
- Frankincense and myrrh. Tucker, A.O. Econ Bot (1986) 40: 425. https://doi.org/10.1007/BF02859654[↩]
- Kreck C, Saller R. Indischer Weihrauch und seine Zubereitungen einschließlich H15 als traditionelles und modernes Therapeutikum. Internist Prax. 1998; 38 857-72.[↩]
- Büchele B, Zugmaier W, Simmet T h. Analysis of pentacyclic triterpenic acids from frankincense gum resins and related phytopharmaceuticals by high-performance liquid chromatography. Identification of lupeolic acid, a novel pentacyclic triterpene. J Chromatogr B. 2003; 791 21-30.[↩]
- Beisner K, Büchele B, Werz U, Simmet T h. Structural analysis of 3-α-acetyl-20(29)-lupene-24-oic acid, a novel pentacyclic triterpene isolated from the gum resin of Boswellia serrata, by NMR spectroscopy. Magn Reson Chem. 2003; 41 629-32.[↩]
- Ganzera M, Khan I A. A reversed phase high performance liquid chromatography method for the analysis of boswellic acids in Boswellia serrata . Planta Med. 2001; 67 778-80[↩]
- Hamm S, Bleton J, Connan J, Tchapla A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry. 2005; 66 1499-514[↩]
- Boden S E, Schweizer S, Bertsche T h, Dufer M, Drews G, Safayhi H. Stimulation of leukotriene synthesis in intact polymorphonuclear cells by the 5-lipoxygenase inhibitor 3-oxotirucallic acid. Mol Pharmacol. 2001; 60 267-73[↩]
- Singh G B, Atal C K. Pharmacology of an extract of salai guggal ex Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986; 18 407-12[↩][↩][↩]
- Kesava Reddy G, Dhar S C, Singh G B. Urinary excretion of connective tissue metabolites under the influence of a new non-steroidal anti-inflammatory agent in adjuvant induced arthritis. Agents Actions. 1987; 22 99-105.[↩]
- Sharma M L, Bani S, Singh G B. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharmacol. 1989; 11 647-52[↩]
- Fan A Y, Lao L, Zhang R X, Wang L B, Lee D Y, Ma Z Z. et al . Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on rats with persistent inflammation. J Altern Complement Med. 2005; 11 323-31[↩]
- Sengupta K, Alluri KV, Satish AR, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Research & Therapy. 2008;10(4):R85. doi:10.1186/ar2461. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2575633/[↩][↩]
- Vishal AA, Mishra A, Raychaudhuri SP. A Double Blind, Randomized, Placebo Controlled Clinical Study Evaluates the Early Efficacy of Aflapin® in Subjects with Osteoarthritis of Knee. International Journal of Medical Sciences. 2011;8(7):615-622. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3198257/[↩][↩][↩]





