What is camphor
Camphor occurs naturally in the fragrant camphor tree (Cinnamomum camphora) that has been traditionally obtained through the distillation of the wood of the camphor tree and can also be man-made using mainly turpentine as a starting material 1. Camphor is a major essential oil component of many aromatic plant species, as it is biosynthetically synthesized. Camphor, in its pure form, has a penetrating odor and exists as colorless or white crystals. Camphor is volatile, reactive and flammable. Camphor dissolves in water.
The fragrant camphor tree and its products, such as camphor oil, have been used since ancient times. The camphor tree was used as a fragrant wood in Babylon and Egypt. Camphor was used as a fumigant during the Black Death in Europe and considered as a valuable ingredient in both perfume and embalming fluid. Camphor is used as a plasticizer or cellulose nitrate, moth repellant, in varnish, chemical intermediate, mildew proofings, lacquers, insecticides, other explosives 2, preservative, cosmetic ingredient, pyrotechnics and anti-infection agent 3. Camphor is common in many liniments. Camphor is used in perfuming industrial products. Camphor has also been widely used as a fragrance in cosmetics, as a food flavorant, as a common ingredient in household cleaners, as well as in topically applied analgesics and rubefacients for the treatment of minor muscle aches and pains.
Camphor exhibits a number of biological properties such as insecticidal, antimicrobial 4, 5, antiviral, anticoccidial, anti-nociceptive, anticancer and antitussive activities, in addition to its use as a skin penetration enhancer. However, camphor is a very toxic substance and numerous cases of camphor poisoning have been documented 6. Poisoning in adults and children can occur from swallowing, breathing, or dermal contact with preparations containing camphor. Camphor poisoning can result in nervous system and kidney effects. Other symptoms include colic, nausea, vomiting, diarrhea, anxiety, delirium, convulsions, seizures, coma, or rarely, death. In response to a series of poisoning cases of unintentional ingestion of camphor medicinal products, the U.S. Food and Drug Administration in 1982 restricted camphor content in these products to less than 11%. The American Conference of Governmental Industrial Hygienists 7 determined that camphor was “Not Classifiable as a Human Carcinogen”. This is based on an absence of human data and reports of no tumor responses in animal studies following application to or under, the skin.
Camphor acts as a counter-irritant, rubefacient and mild analgesic and is included in liniments for relief of fibrositis, neuralgia and similar conditions. A rubefacient is a substance for skin application that produces redness of the skin e.g. by causing dilation of the capillaries and an increase in blood circulation. By ingestion camphor has irritant and carminative properties (relieving flatulence or prevent formation of gas in the gastrointestinal tract) and has been used as a mild expectorant and to relieve griping. Camphor has been used as a circulatory and respiratory stimulant (as a solution in oil given subcutaneously or intramuscularly), this use is considered hazardous. It has been used in combination with menthol and chenodeoxycholic acid to aid dispersal of bile duct stones, although this is no longer recommended 8.
In response to several toxicity reports, the United States Food and Drug Administration evaluated the efficacy and toxicity of camphor-containing products. A limit of 11% in consumer products was set in 1983 and products labeled as camphorated oil, camphor oil, camphor liniment and camphorated liniment were banned completely 9. However in most countries, especially in developing countries, medicaments containing camphor as high as 20% are easily available. According to 2001 data from the American Association of Poison Control Centers Toxic Exposure Surveillance System (TESS), 8,505 exposures to camphor products were reported, many of which resulted in mild symptoms with 89 moderate to severe outcomes but no fatalities 10.
Dietary exposure to camphor arises from the consumption of foods flavored by using either herbs (e.g. basil, coriander, marjoram, rosemary, sage), their essential oils or the chemically defined flavoring substance d-camphor 11. Camphor is easily absorbed in the gastrointestinal tract. The major metabolic pathway is the oxidation to 5- and 3-hydroxycamphor, followed by conjugation and excretion. Camphor did not show mutagenic activity in Salmonella typhimurium strains and did not induce chromosome aberrations in vitro with and without metabolic activation. There was no evidence of reproductive and developmental toxicity after oral administration to rats and rabbits. The available data on toxicity of camphor are limited and thus a tolerable daily intake (TDI) cannot be derived. However, based on the available toxicity data and the European Food Safety Authority (EFSA) Panel´s conservative estimate of chronic exposure (15 mg/day equivalent to 250 μg/kg body weight (bw)/day) calculated using the maximum limits suggested by the Council of Europe, the European Food Safety Authority (EFSA) Panel considered that there would be no safety concern regarding chronic toxicity.
The reported acute toxicity data on adults and children arise mostly from accidental ingestion of camphor-containing medications. The probable lethal oral bolus dose has been reported to be in the range of 50 to 500 mg/kg body weight. No acute toxicity was reported after doses lower than 2 mg/kg body weight and clinically insignificant signs of toxicity may be seen in sensitive individuals at doses of 5 mg/kg body weight and higher, whereas clinically manifest toxicity in sensitive persons would require doses higher than 30 mg/kg body weight. Potential acute exposure related to the consumption of large amounts of certain foods on a single day was estimated by the European Food Safety Authority (EFSA) Panel for several age groups. It was lowest in adults (from 0.14 to 0.34 mg/kg body weight according to the food commodity) and highest in children under 6 (from 0.41 to 0.83 mg/kg body weight according to the food commodity). The commodity leading to the highest potential acute exposure was fresh cheese in all age groups.
The acute exposure estimates for children and adults are about 60-120 times and 150-360 times, respectively, lower than the probable lowest lethal oral bolus dose of 50 mg/kg body weight. The acute exposure estimates for children and adults are about 2-5 times and 6-14 times, respectively, lower than the dose of 2 mg/kg body weight below which no acute effects have been reported in human case studies. Although these margins might appear to be low, the large number of cases describing the dose-response relationship suggests that the data sufficiently cover inter-individual variability in sensitivity. Therefore, the European Food Safety Authority (EFSA) Panel concluded that it is unlikely that acute effects may occur in relation to consumption of foods providing less than 2mg/kg body weight in one large portion.
However, maximum permitted levels for d-camphor are not currently set in the European Union legislation and there is uncertainty on its actual upper use levels in foods and beverages currently on the market and on the high consumption of food flavored with d-camphor all over Europe. The European Food Safety Authority (EFSA) Panel therefore suggests that maximum limits should be set to ensure that exposure to camphor does not exceed 2 mg/kg body weight on a single day in any age group 11.
Camphor is rapidly oxidized to campherols (2-hydroxycamphor and 3-hydroxycamphor), and then conjugated in the liver to the glucuronide form 12. Camphor-related metabolites are relatively fat-soluble and may accumulate in fatty tissue. Campherol conjugated to glucuronic acid is eliminated mainly in the urine as an inactive compound 12. Trace amounts are eliminated by the lungs.
The majority of drugs administered to humans and animals are eliminated by a combination of hepatic metabolism and renal excretion 13. In the human body, camphor is oxygenated to alcohol campherol and then conjugated with glucuronic acid in the liver to become soluble in water before being excreted in the urine. Following oral ingestion, high concentrations of camphor have been detected in the fetal brain, liver, kidney, blood, as well as in amniotic fluid 14. The exact mechanism of camphor-induced toxicity has not been completely elucidated, but a study by Park et al. 15 indicated camphor specifically inhibits the nicotinic acethylcholine receptors (nAChRs), thereby causing inhibition of catecholamine secretion. This inhibition may be one cause of the toxicity as nicotinic acethylcholine receptors are known to play a major role at neuromuscular junctions 15. Camphor ingestion may lead to abortion as it crosses the placenta and fetuses lack the enzymes to hydroxylate and conjugate with glucuronic acid 16. No teratogenicity was noted during the foetal period of organogenesis after oral (+)-camphor administration to pregnant rats and rabbits at doses up to 1,000 mg/kg bodyweight/day and 681 mg/kg bodyweight/day respectively. However, the high dose of 1,000 mg/kg body weight/day caused toxic symptoms including clonic convulsions, pilo-erection and reduced motility in rats, but no retardations or malformations were observed. In the rabbit model a high dose of 681 mg/kg body weight/day resulted in reduced body weight gain and food consumption, but no retardations or malformations were observed 17.
As mentioned previously, camphor is a major component of many aromatic plant species. Millet et al. 18 investigated the toxicity of some commercial essential oils including sage (Salvia officinalis), hyssop (Hyssopus officinalis), thuja (Thuja occidentalis) and cedar (Juniperus and Cupressus spp.). For sage (Salvia officinalis) oil, 3.2 g/kg of sage oil caused tonic-clonic convulsions in unanesthetized rats resulting in death. It was determined that the toxicity of sage (Salvia officinalis) oil appeared to be related to the presence of camphor 18.
Physical Properties and Sources of Camphor
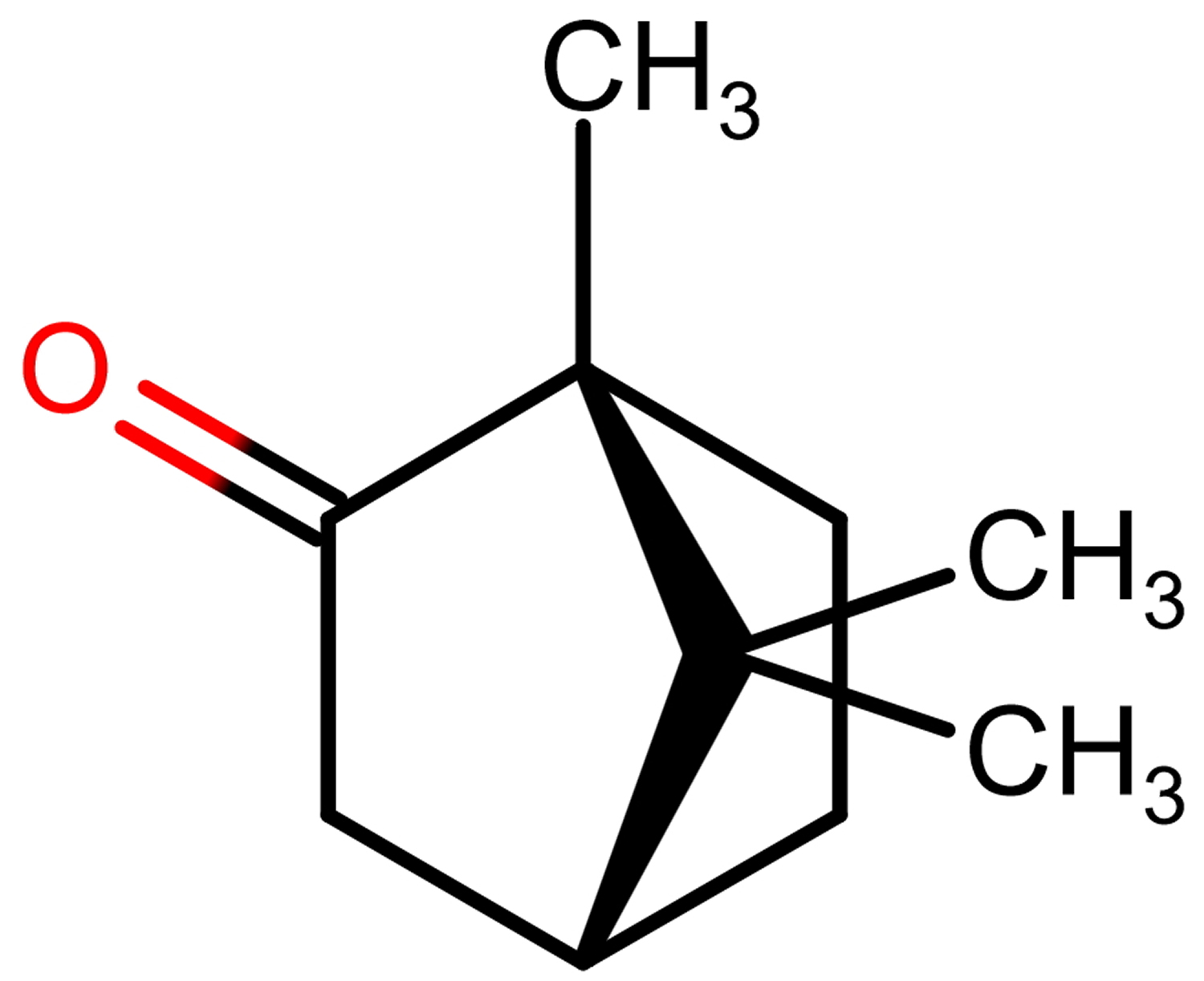
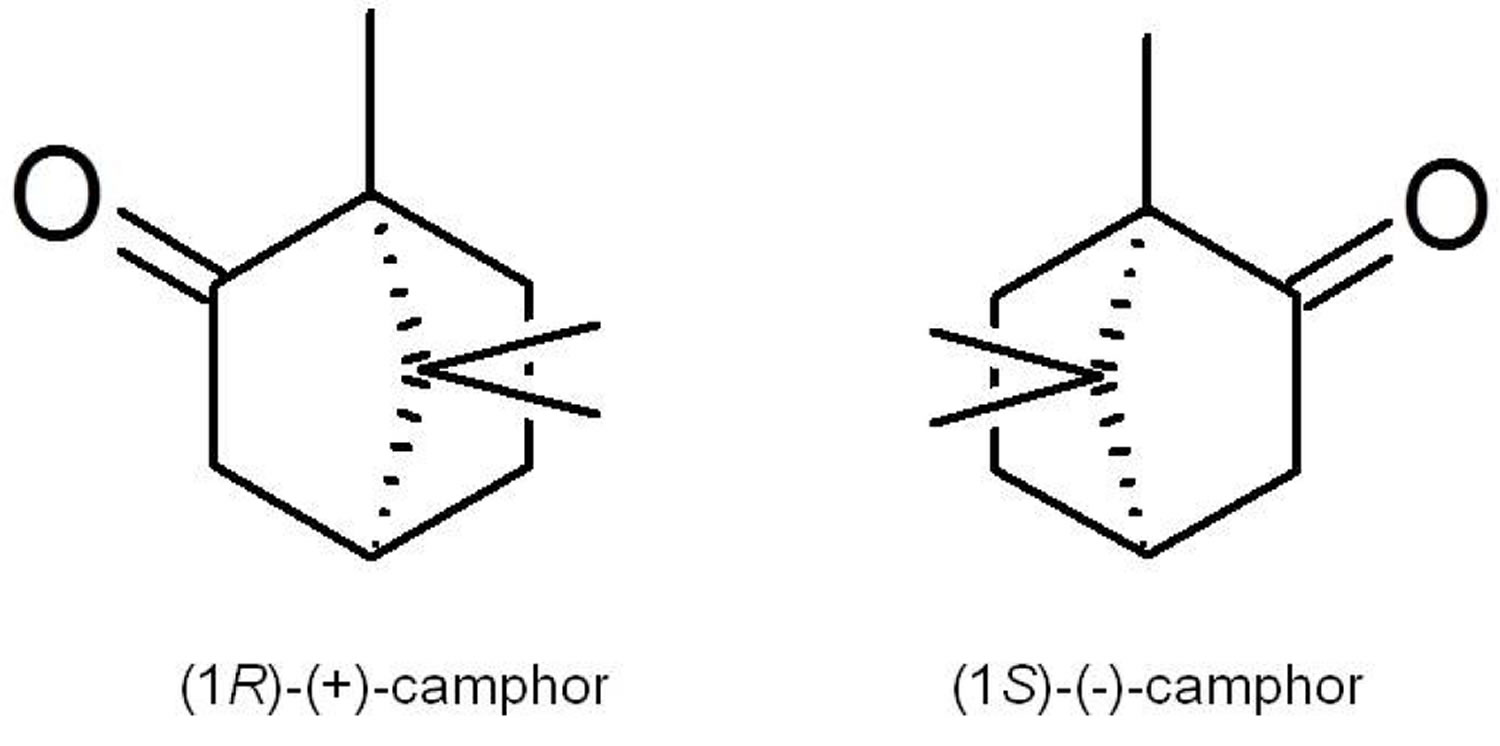
Camphor is a waxy, white or transparent solid with a strong aromatic odor 19 which sublimates at room temperature and melts at 180 °C 20. Camphor is practically insoluble in water, but soluble in alcohol, ether, chloroform and other organic solvents. It is a terpenoid (1,7,7-trimethylbicyclo[2.2.1]-2-heptanone) with a chemical formula of C10H16O and exists in two enantiomeric forms: (1S)-(−)-and (1R)-(+)-camphor (Figure 1). These two enantiomers have a similar camphoraceous odor, but how the stereochemistry impacts on the biological activity is still unknown 21. Synthetic camphor is synthesized mainly from α-pinene obtained from turpentine oil, whilst natural camphor, i.e., (+)-camphor, is obtained through distillation of the wood from the camphor laurel tree (Cinnamomum camphora) found especially in Borneo and Taiwan; the Borneo camphor tree (Dryobalanops aromatica) and the East African camphorwood tree (Ocotea usambarensis) (see Figure 2A). In Asia, a major source of camphor is camphor basil (Ocimum kilimandscharicum). Camphor is also present as a major essential oil component of many aromatic plant species 22.
Figure 1. Camphor
Note: The chemical structure of the (1R)-(+) and (1S)-(−) enantiomers of camphor.
[Sources 6 and 21]The fragrant camphor tree, Cinnamomum camphora (L.) J. Presl (Lauraceae), occurs naturally in Asian countries including Japan, Taiwan and China, but has been naturalised in other parts of the World. The tree is large with pale brown bark, dark green to yellowish leaves (Figure 2A) and small white flowers followed by small purple berries. All the plant parts have the distinctive, easy-to-recognise camphoraceous odor. The essential oil is distilled from the wood (Figure 2B) which yields the active ingredient (1R)-(+)-camphor, i.e., natural camphor 23.
Figure 2. Cinnamomum camphora tree, wood and seeds
[Source 1]Biosynthesis and Chemical Synthesis of Camphor
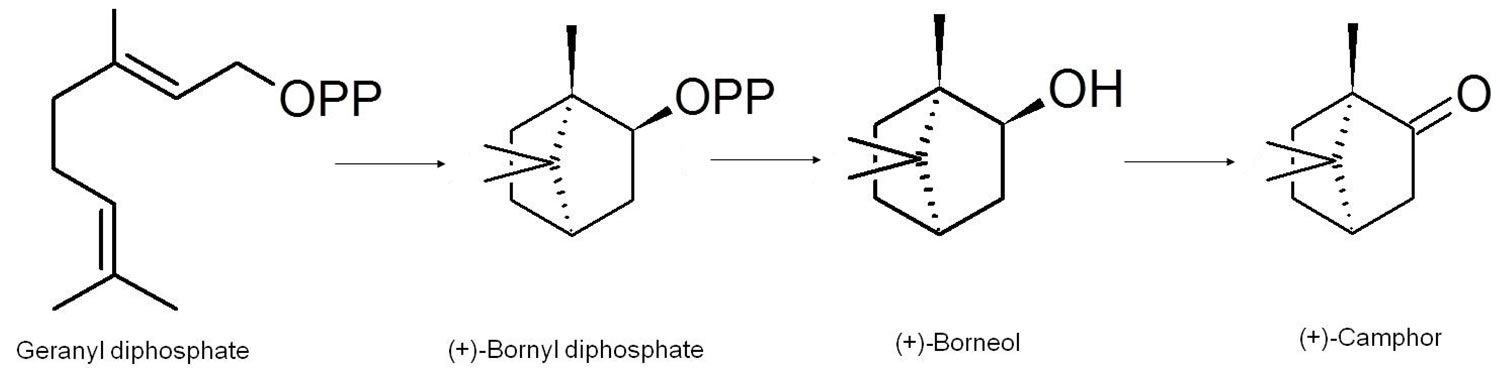
The biosynthesis of camphor was extensively investigated and elucidated by Croteau et al. 24 in their work on Salvia officinalis 24. After several elegant experiments they determined the basic biosynthesis of camphor (Scheme 1) and the enzymes involved to be as follows: Camphor is biosynthetically produced in plants by way of the biotransformation of the starting material geranyl diphosphate which is the preferred substrate. Cyclisation of geranyl diphosphate, by the enzyme (+)-bornyl diphosphate synthase yields (+)-bornyl diphosphate. (+)-Bornyl diphosphate is then hydrolysed to (+)-borneol through the action of bornyl-diphosphate diphosphatase. The last step is catalysed by (+)-borneol dehydrogenase as it oxidises (+)-borneol to (+)-camphor 24.
The synthetic production of camphor involves using turpentine oil as a starting material. Turpentine is used as the source of α-pinene through a distillation process; α-pinene is converted into camphene through the catalysis of a strong acid with acetic acid as the solvent; the camphene then undergoes Wagner-Meerwein rearrangement into the isobornyl cation, which is captured by acetate; the isobornyl acetate subsequently formed is hydrolysed to isoborneol, which is finally converted to camphor through dehydrogenation. The synthetic route from α-pinene produces a racemic mixture, i.e., a 1:1 ratio of (−) and (+)-camphor.
Figure 3. Biosynthesis of camphor
[Source 25]Camphor uses
Camphor has a long-valued history for its extensive and diverse uses in the East: the Chinese used camphor as a circulatory stimulant and analeptic (tending to restore a person’s health or strength; restorative), whilst the Japanese used it in torch-light material and added small quantities to fireworks to make them brighter 2. Camphor was used as a fumigant during the Black Death, a plague that spread through Europe in the 14th century, as well as during outbreaks of smallpox and cholera. Rosewater together with camphor as a perfume ingredient was sprinkled over corpses before shrouding 26. In India, camphor is commonly burnt in temples during religious rituals because unlike any other aromatic smoke, camphoric fumes are non-irritant to eyes 20. Camphor has been widely used as a fragrance in cosmetics, as a flavoring food additive and as a preservative in confectionary goods; in homes it is commonly used as an insect repellent, a plasticiser and as an intermediate in the synthesis of aroma chemicals 20, 27. Camphor is one of the most well-known and widespread commercially important aroma chemicals, with an annual market value of US$ 80–100 million 28.
Camphor exhibits several biological properties such as antimicrobial 4, 5, antiviral and antitussive effects 29, 30, 31. Camphor is a common ingredient in modern medicine in topically applied analgesics and rubefacients for treatment of minor muscle aches and pains and it is reported that camphor has been used to relieve pain caused by breast engorgement by intramuscular injections 32. It has been applied as a topical anti-infective and anti-pruritic and internally as a stimulant and carminative 23. However, camphor is poisonous when ingested and can cause seizures, confusion, irritability and neuromuscular hyperactivity. The lethal dose in humans is reported to be 50–500 mg per kg bodyweight 33.
What is camphor used for
Camphor is a solid, translucent, white crystal with penetrating aromatic odor used as a rubefacient/counter-irritant medication. Camphor is used exclusively because of its local effects. When rubbed on the skin, it acts as a rubefacient and causes localized vasodilatation (mediated by way of an axon reflex), which gives feelings of comfort and warmth. As an anti-pruritic gent, when applied gently on the skin, it may create a feeling of coolness, and a mild, local anesthetic effect, which may be followed by numbness. In dermatology, when it is applied as lotion (0.1 to 3%), camphor is an anti-pruritic and surface anesthetic (when applied gently, it creates a feeling of coolness). Camphor is also used in liniments as a counter-irritant for fibromyalgia, neuralgia, and similar conditions.
Camphor is used:
- as a rubefacient. As with all rubefacients, it should not be applied to abraded, irritated skin.
- as a plasticizer for cellulose esters and ethers
- in the manufacture of plastics (especially celluloid)
- in lacquers and varnishes
- in explosives and pyrotechnics
- as a moth repellent
- in the manufacture of cymene
- as a preservative in pharmaceuticals and cosmetics.
When camphor is applied on the skin, it is analgesic. It is also used in liniments as a counter-irritant in fibrositis, neuralgia, and similar conditions.
In dermatology, when it is applied as lotion (0.1 to 3%), it is an anti-pruritic and surface anaesthetic (when applied gently, it creates a feeling of coolness).
In dentistry, it is prepared with parachlorophenol 35% (and 65% camphor) and used as an antibacterial for infected root canals.
Taken internally, it is an irritant and carminative. It has been used as a mild expectorant and to relieve griping (abdominal discomfort) (this use is now discouraged because of toxicity).
Camphor was formerly administered as a solution in oil by subcutaneous or intramuscular injection to act as a circulatory and respiratory stimulant, but there is no evidence of its value for this purpose 34.
According to the Dutch Information Medicamentorum (1986), camphor is used:
- For pruritus: lotion with 1 to 70 mg/g
- For muscular pains: oil with 40 to 250 mg/g or alcohol solution with 100 mg/ml
- For colds: chest liniment, with 20 to 100 mg/g; nose ointment, with 20 to 50 mg/g: nose drops/spray, with 0.15 mg/ml.
When ingested in small amounts, it creates feelings of warmth and comfort in the stomach, but given in large doses it acts as an irritant.
Camphor is no longer used as a pesticide in the US. Other uses of camphor include insect repellant use (particularly to control clothes moths); cosmetic ingredient.
- It is dangerous to place camphor into infants’ nostrils, since it can cause instant collapse 35.
Antibacterial and Antifungal Activities
Plants have been a valuable source of natural products for maintaining human health and the use of plant compounds for their antimicrobial activity has gradually increased worldwide. Numerous investigations have shown various essential oils of several species containing camphor as the major components, exhibited antimicrobial activity. The composition of essential oil from the aerial parts of sweet wormwood (Artemisia annua) includes camphor (44%), germacrene D (16%), trans-pinocarveol (11%), β-selinene (9%), β-caryophyllene (9%) and artemisia ketone (3%). Significant activity of the essential oil was noted against the Gram-positive bacteria, Enterococcus hirae, as well as against the fungi Candida albicans and Saccharomyces cerevisiae using the liquid diffusion method 29.
Some studies found camphor as a single compound only exhibited weak antimicrobial activity 36. Greek sage (Salvia fruticosa) essential oil, containing camphor as the main component, exhibited poor activity against all of the bacteria tested (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus, Rhizobium leguminosarum and Bacillus subtilis) 37. Santoyo et al. 38 investigated the antimicrobial activity of rosemary essential oil obtained via supercritical fluid extraction and molecules including camphor against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger by the disc diffusion and broth dilution methods. The test samples were active against all the test organisms with the most susceptible organism being Staphylococcus aureus and the least susceptible Aspergillus niger. The standards exhibit activity against all the micro-organisms tested in the order of effectiveness: borneol > camphor > verbenone. In another study, a yarrow variety (Achillea sintenisii) was found to contain camphor (14.8%) as its major component. The essential oil was further fractionated and the antibacterial and antifungal activities determined against a variety of micro-organisms. Data analysis revealed camphor to be the more active compound together with 1,8-cineole, as notable activity against Candida albicans and Candida krusei was reported. The fractions showed the same or higher activity than the neat essential oil in the majority of cases 39. Mevy et al. 40 confirmed that elemol, 1,8-cineole, camphor and p-cymene can be considered as the principal antimicrobial components of tea bush (Lippia chevalieri) oil. The antimicrobial activity of rosemary (Rosmarinus officinalis) and several other oils against organisms implicated in meat spoilage was investigated by Ouattara et al. 41. In a 1/100 dilution, rosemary (Rosmarinus officinalis) oil, containing mainly camphor, was one of the most efficient antibacterial oils having antibacterial activity against two Gram-negative (Pseudomonas fluorescens and Serratia liquefaciens) and four Gram-positive (Brochothrix thermosphacta, Carnobacterium piscicola, Lactobacillus curvatus, and Lactobacillus sake) bacteria 41. The essential oils of Salvia macrochlamys and decorative sage (Salvia recognita), rich in camphor (11% and 42%, respectively), exhibited no antimicrobial activity against methicillin-resistant Staphylococcus aureus, Mycobacterium intracellulare, Cryptococcus neoformans and Aspergillus fumigatus, but moderate antifungal activity against Colletotrichum acutatum, Colletotrichum fragariae, and Colletotrichum gloeosporoides was noted at a concentration of 200 µg/mL. However, in the same study, (−)-camphor and (+)-camphor tested singularly exhibited no activity against the test bacteria and fungi 42. Viljoen et al. 4 determined, using time-kill studies, that a synergistic antimicrobial effect occurs between 1,8-cineole and (−)-camphor. These two compounds are the two major essential oil components of the mountain daisy (Osmitopsis asteriscoides). The study showed that both (+)-camphor and (−)-camphor have negligible antifungal activity on C. albicans, whereas in the case of (−)-camphor combined with 1,8-cineole, a total reduction of colony forming units (CFUs) was observed at 15 min. It was deduced therefore that camphor may act in a synergistic manner with other essential oil components possessing antimicrobial activity 4.
Viral diseases are an increasing health concern worldwide and there has been an intensive search for more effective but less toxic antivirals than those currently used. Aromatic plants, especially their essential oils, are known to exhibit antiviral properties. Sivropoulou et al. 37 investigated the antimicrobial, cytotoxic and antiviral activities of Greek sage (Salvia fruticosa) essential oil. The results demonstrated the essential oil of Greek sage (Salvia fruticosa) and its four main components (1,8-cineole, α- and β-thujone, and camphor) exhibited high levels of virucidal activity against herpes simplex virus type-1 (HSV-1), however, this positive effect was accompanied by cytotoxic activity against African Green Monkey kidney (Vero) cells. Lavender cotton (Santolina insularis) essential oil, which is rich in camphor, deactivated herpes simplex type-1 (HSV-1) and type-2 (HSV-2) in vitro using plaque reduction assays with an IC50 value of 0.88 µg/mL for HSV-1 and 0.7 µg/mL for HSV-2. Reduction of plaque formation assays showed inhibition of cell-to-cell transmission of both HSV-1 and HSV-2 43.
Antitussive Activity
Coughing is a very common clinical symptom with largely ineffective current therapies. Aromatic vapors have been widely used in the symptomatic treatment of upper respiratory tract infections due to their known antitussive effects. Burrow and co-workers 44 investigated the effects of camphor vapor on nasal resistance to airflow and nasal sensation of airflow. Inhalation of camphor had no effect on nasal resistance to airflow, but a cold sensation in the nose with the sensation of improved airflow was described. The results indicated that camphor stimulated cold receptors in the nose 44. Laude et al. 45 reported the action of camphor on the cough reflex in conscious guinea-pigs. Three concentrations (50, 133 and 500 mg/L) of camphor vapor were administered and 500 mg/L camphor significantly reduced (33%) cough frequency. An increase in cough latency coincided with the reduction in cough frequency. Further studies revealed camphor activated cold receptors now identified as TRPM8, the minty-cool ion channel, but the mechanism whereby TRPM8 activation inhibits cough is still not understood 46. In another study, camphor was used to synthesize camphor lactam (α-camphidone) by treatment with hydroxylamine-O-sulfonic acid and glacial acetic acid with a Beckmann-like rearrangement in structure. Both camphor and camphor lactam were tested for their antitussive activity in guinea-pigs with citric-acid induced cough. It was noted that this minor modification in chemical structure significantly increased cough latency whilst reducing cough frequency. In addition, prior exposure to the camphor lactam at concentrations of 125, 250, and 500 μg/L had a higher inhibitory cough response compared to camphor 47.
Anti-Nociceptive Activity
Camphor has an extensive history in its use as a topical analgesic in balms and liniments. In 1990, Green 48 found camphor to be a relatively weak sensory irritant having a modest excitatory effect on thermosensitive (and perhaps nociceptive) cutaneous fibers. Xu et al. 49 further investigated the mechanism of camphor anti-nociceptive (analgesic) activity and reported that camphor activated and de-sensitized the capsaicin receptor (TRPV1) whilst inhibiting the garlic receptor (TRPA1). Both are part of the recently elucidated transient receptor potential (TRP) superfamily, a group of structurally similar ion channels, heavily expressed in nociceptive sensory neurons. Therefore, it is possible that the analgesic effects of camphor may be due to de-sensitisation of TRPV1 and blocking of TRPA1 49. The pain-relieving effects of California sagebrush (Artemisia californica), containing the two major compounds 1,8-cineole (24%) and camphor (18%), were reviewed. Anecdotal use reported successful pain relief in all cases for patients suffering from lower back pain, arthritis, bruises, muscle and ligaments strains, broken bones and even cancer. An alcoholic liniment provided rapid pain relief lasting 2–3 hours with an onset of action of 20 min. The analgesic activity of terpenoids is as a result of binding to TRPV1, TRPV3 and TRPM8 receptors. Camphor is a known agonist of TRPV2, TRPA1 as well as TRPV1 quickly deactivating TRP channels resulting in long-term pain relief 50.
Antimutagenic and Anticancer Activity
Few animal studies demonstrating the potential of camphor in the treatment of cancer have been conducted, but those undertaken included improvement of immune function 51, enhancement of enzymatic breakdown of carcinogens 52 and the increased susceptibility of cancer cells to radiation 53. Goel et al. 54 demonstrated that camphor had a radiomodifying effect. An increase in the frequency of sister-chromatid exchanges in mice bone marrow cells occurs after exposure to gamma radiation, but after a single dose of camphor, administered at 0.5 µM/g bodyweight, this frequency was significantly low. Kanematsu and Shibata 55 reported on possible DNA damage as shown by a positive result of the rec-assay using two strains of Bacillus subtilis. Camphor, often used in endodontic formulations, presented a positive result in the “rec-assay”, showing that camphor may cause genetic toxicity in cells, however, drugs showing positive results do not necessarily cause tumour formation. This shows that more studies on the genotoxicity of camphor are required and that camphor should be used with care. Cultivated sage (Salvia officinalis) rich in camphor reduced UV-induced mutagenesis when screened with the repair-proficient strain, and had no effect on spontaneous mutation frequency in mismatch repair-deficient strains. It also enhanced the formation of Lac+ recombinants, but not as a consequence of SOS induction. This result suggested a protective effect through enhanced re-combinational repair 56. In a subsequent study, Vuković-Gacić et al. 57 investigated the inhibitory potential of cultivated sage essential oil and its monoterpenes on UV-induced mutations tested with SY252 and D7. Camphor showed antimutagenic effects at very low concentrations compared with other monoterpenes screened (about 40% reduction of UV-induced revertant at 0.5 and 1 μg/plate), although higher concentrations failed to increase antimutagenic effects. Nikolić et al. 58 demonstrated that camphor can reduce UV/4NQO mutagenesis in the NER+, but not the NER− strain of Escherichia coli and increased spontaneous and UV-induced recombination in recA730 and recA+ cells. Low doses of camphor are antigenotoxic against 4NQO in mammalian cells and stimulate DNA repair, acting as a bioantimutagen. De-Oliveira et al. 59 hypothesised based on previous findings how the genotoxicity of mutagens may be modulated through cytochrome P4502B subfamily enzyme inhibition. In a study including various monoterpenes using pentoxyresorufin-O-depenthylase (PROD) as a model substrate for cytochrome P4502B1-enzymes, camphor was found to have an inhibitory effect on the PROD enzyme with an IC50 value of 7.89 μM. Through this mechanism of action it is possible for camphor to be considered antimutagenic 59, but more studies are required.
Insecticidal Activity
Certain currently used synthetic pesticides threaten the integrity of the earth’s ozone layer and other environmental buffers, therefore alternatives to these commercial chemicals are urgently needed 60. Essential oils are considered good candidates because of their low toxicity to mammals, high volatility, ready availability in tropical countries and economical viability 61. Monoterpenoids believed to aid plants in chemical defence against phytophagous insects are capable of toxic interference with the biochemical and physiological functions of herbivorous insects 62. Numerous studies have indicated that camphor has insect repellent activity against stored-product pests. Using contact toxicity, grain treatment and repellency assays, the essential oil of camphor basil (Ocimum kilimandscharicum) and its major component, camphor, were investigated against four beetles. The doses of 100 mg/filter paper and 100 µg/insect induced over 93% and 100% mortalities in Sitophilus granarius, Sitophilus zeamais and Prostephanus truncatus, but only 70% and 100% mortality in Tribolium castaneum after 24 hour exposure. Development of eggs and immature stages within grain kernels, as well as progeny emergence, was completely inhibited in camphor-treated grain 63. Bekele and Hassanali 64 revealed the insecticidal activity of camphor basil (O. kilimandscharicum) against Rhyzopertha dominica and S. zeamais was due to camphor and the combined effects of different components, but camphor had no effect on the rice weevil (Sitophilus oryzae) with an LC50 of greater than100 μL/L. Another report indicated that camphor, as a pure compound, showed contact and fumigant activity against Sitophilus oryzae and Rhyzopertha dominica, but had no effect on Tribolium castaneum after 24 hours exposure at a dose of 0.1 μL/720 mL volume 65. Liska et al. 66 found camphor exhibited the highest mortality (78.5%) just after 24 h at the highest tested dose (10.0 μL/adult) for contact toxicity; for fumigant toxicity, camphor at its highest dose (120 μL/350 mL vol.) caused 93.5% mortality. These results were in agreement with an earlier report by Qiantai and Yongcheng 67, who observed that camphor, as the major isolate from essential oils of Chinese cinnamon (Cinnamomum cassia), Chinese star anise (Illicium verum) and camphor laurel (Cinnamomum camphora), showed contact efficacy against the lesser grain borer (Rhyzopertha dominica) and the maize weevil (Sitophlus zeamais). Insect repellent activity, rather than insecticidal activity, against the confused flour beetle (Tribolium castaneum) was noted. Exposure of the newly-laid eggs of the pulse beetle (Callosobruchus chinensis) to concentrations of 0, 12, 24, 48, and 96 ppm of camphor crystals in air-tight containers for one and four weeks resulted in 65.0% to zero (0%) hatching. Postembryonic development was affected when the newly-hatched larvae penetrated into the cowpea seeds; very few adults emerged from the infested seeds exposed to camphor 68.
Camphor was the most effective of the tested monoterpenoids to prevent the multi-colored Asian lady beetle, Harmonia axyridis (Pallas), from overwintering in buildings as determined by the olfactometer bioassay 69. It also exhibited fumigation toxicity against false powder post beetle (Dinoderus bifloveatus) after 48 hours exposure 70. Several monoterpenes (e.g., 1,8-cineole, citronellol, α and β-pinene, linalool, isopinocamphone, camphor) tested against cattle-tick (Boophilus microplus) larvae and camphor, in a 60 min-period, was lethal to 100% of the larvae 71. Blattella germanica (German cockroach) is one of the most important pests of the indoor environment, a major source of allergens and a potential carrier of faecal pathogens 72. Using the filter-paper contact toxicity bioassay, both (1R)-(+)-camphor and (1S)-(−)-camphor were toxic against female B. germanica with LD50 values of 0.10 mg/cm2 and 0.13 mg/cm2, respectively. There was no significant difference in toxicity between (1R)-(+)-camphor and (1S)-(−)-camphor 73.
Mosquitoes are known disease vectors of malaria, hemorrhagic dengue and yellow fever, in addition to being considered a nuisance 74. Most commercial mosquito repellents contain DEET (N,N-diethyl-m-methylbenzamide), but this compound exerts toxic reactions and may damage plastic, synthetic fabrics and painted surfaces 75. Bioassays conducted on a number of essential oils showed repellency against mosquitoes attributed to the presence of its main compounds. However, it was noted that synergy between the essential oil components may lead to an increased repellant response; therefore, the neat oil may be more effective compared to the individual components 76. A review published in 2011 noted 144 patent inventions containing plant essential oils for mosquito repellency. These patents, mostly from Asian countries, listed various essential oil components including amongst many others citronella (Cymbopogon spp.) and eucalyptus (Eucalyptus spp.), whilst camphor (Cinnamomum camphora) was mentioned in >10% of these patents 74. As a major component of the essential oil of aromatic plants, camphor has shown repellency against Anopheles culicifacies, Culex quinquefasciatus 77, Anopheles gambiae and Anopheles funestus 78. It is evident that camphor has great potential for development as an alternative green commercial insect repellent to replace the harmful synthetic agents currently in use.
Cardiovascular Effects
For centuries, camphor has been used for the stimulation of heart and peripheral circulation. Osborne 79 reported that in cardiac failure and collapsed conditions characterized by cold skin, a feeble pulse and failing heart, the subcutaneous injection of camphor in sterile oil caused the surface of the skin to become flushed, dilated the peripheral blood vessels and improved the whole circulation. The results of controlled clinical studies on the cardiovascular effects of (+)-camphor have been published 80. Belz and Loew 81 investigated the effects of (+)-camphor (extracted from fresh Crataegus berries) in orthostatic hypotension using independent, double-blind, randomised, placebo-controlled studies. It was determined that (+)-camphor, as well as the extract from fresh hawthorn (Crataegus) berries, contributed to the pressoric effects with (+)-camphor inducing the initial rapid effect and the extract is responsible for the long-lasting effect 81.
Camphor as a Potential Skin Penetration Enhancer
It has been suggested that terpenes, present in plant essential oils, are clinically acceptable skin penetration enhancers 82. Previous studies also indicated menthol in combination with camphor enhanced the skin penetration of methyl salicylate and inhibited both the in vivo and in vitro hydrolysis of methyl salicylate to salicylic acid 83. The efficacy of some terpenes on skin permeation of tea catechins and theophylline were systemically evaluated using a series of in vitro and in vivo methods 84. It was found that all the evaluated terpenes had significant effects on the (+)-catechin delivery relative to the control. The rank order of enhancement was α-terpineol ≥ menthone >linalool > 1,8-cineole > farnesol ≥ fenchone > cymene ≥ nerolidol > (+)-limonene > camphor. Camphor and fenchone showed the least enhancement amongst the oxygen-containing monoterpenes, which may be related to their bicyclic structure.
Ramesh et al. 85 reported the flux of carvedilol obtained from solutions containing camphor, transcutol, d-limonene, carvone, labrasol and menthol were 7.81, 7.26, 6.52, 5.91, 4.21 and 2.28 times higher respectively, than that observed with the control, using excised rat abdominal skin mounted in Franz diffusion cells. Camphor showed maximum permeation and basil oil (Ocimum basilicum) containing methyl chavicol, eugenol, linalool, camphor and methyl cinnamate showed potential in vitro penetration enhancement of labetolol hydrochloride 86. In a recent study, camphor was found to markedly prevent the permeation of benzocaine across the skin while promoting skin accumulation after 12 hours 87.
Other Applications
Interestingly, according to Iranian folk medicine, camphor was used both as an aphrodisiac and antiaphrodisiac agent. The effect of camphor on the sexual activity of male rats was investigated by Jamshidzadeh et al. 88, by measuring the parameters mount latency and frequency as well as intromission latency and frequency. The results indicated enhanced sexual desire and performance when camphor was administered at 50 mg/kg. It was speculated that the effects of camphor may be due to modulation of the sympathetic nervous system, or its effect on serum testosterone levels 88. Camphor could also have an effect on the reproductive function of the testes in mice, as it was revealed that administration of camphor to young male mice may result in vascularisation and proliferation of sexual cells which can affect maturation of seminiferous tubules 89.
Sweet wormwood (Artemisia annua) leaves and chemical constituents, including camphor, were investigated for its activity against coccidian parasites. A 5% dried leaf supplement addition to chick feed for 3 weeks resulted in infection protection against Eimeria tenella but not Eimeria acervulina or Eimeria maxima. Camphor fed at 119 ppm protected weight gains and protected against E. tenella and E. acervulina 90.
The essential oil of absinthe wormwood (Artemisia absinthium), containing 27.40% camphor, showed activity against promastigote (MIC 0.0097 μL/mL) and axenic amastigote forms (EC50 0.24 nL/mL) of both Leishmania aethiopica and Leishmania donovani. It also showed a weak haemolytic effect with a slightly decreased selectivity index (SI = 0.8) against the THP-1 cell line. This study demonstrated the potential use of Artemisia absinthium oil as source of an innovative agent for the treatment of leishmaniasis 91.
Allelopathic Activity
Allelopathy is the interaction of one plant with another through the release of biochemical compounds into the environment and can be indirect, direct, harmful or beneficial. Allelopathy is a biological phenomenon by which an organism produces one or more biochemicals that influence the germination, growth, survival, and reproduction of other organisms. Allelopathy can occur through several mechanisms including decay with or without micro-organisms, excretion, exudation, volatilisation and leaching. Allellochemicals can be present in any plant part as well as in the surrounding soil and are mostly secondary metabolites and include alkaloids, phenyl propanes, naphthaquinones, steroids and terpenoids amongst others. Allelopathic activity can lead to suppression of growth of one plant by another 92. In a significant study by Schenk 92 the allelopathic influence of the camphor laurel tree (Cinnamomum camphora) was investigated on the seedling growth of fifty-two plant species and twenty-seven soil algal populations. The leaves had a direct allelopathic effect by significantly delaying germination and causing a reduction of radicle and shoot length, leaf area and leaf number, specifically in species associated with camphor laurel (Cinnamomum camphora) communities. In addition, many of the soil algal species disappeared from the soil or exhibited reduced vigour, therefore the allelopathic effect may also be indirect as soil algae are necessary for soil wettability, moisture retention and seed germination enhancement. The persistence and dominance of camphor laurel trees (Cinnamomum camphora) may also be enhanced as the allelopathic activity reduced the competitiveness of surrounding vegetations 92. In another study, the chemical release from leaf powder of the camphor laurel tree (C. camphora) was studied by monitoring soil and air concentrations of (+)-camphor. The growth of the receiver plant—rice seedlings—was inhibited when planted in soil which contained the leaf powder. (+)-Camphor was detected in this soil as well as the soil water and it was therefore determined to be the main phytotoxic allelochemical responsible for the growth suppression 93. The allelopathic activity of camphor and other monoterpenes were studied by determining the antigerminative ability in radish (Raphanus sativus) and garden cress (Lepidium sativum) seeds 120 hours after sowing. The radish (Raphanus sativus) seeds were found to be more sensitive than the garden cress (Lepidium sativum) seeds and at 10−3 M, camphor significantly inhibited the germination of radish (Raphanus sativus) seedlings by 44% and affected the radicle growth of garden cress (Lepidium sativum) seeds. From this study, the authors concluded that monoterpenes such as camphor, which exhibits phytotoxic activity, are therefore potential bio-herbicides which could be developed into natural pesticides 94.
Camphor toxicity
The toxicity of camphor has been well-documented. The ingestion of 3.5 g of camphor can cause death, whilst 2.0 g causes toxic effects in adults leading to congestion of the gastrointestinal tract, kidney and brain; the immediate collapse of an infant has been reported after the application of a small dose to the nostrils 35. Toxic effects appear after the ingestion of approximately 2 g (lethal dose adults: 4 g, children: 0.5-1 g, infants: 70 mg/kg of pure camphor). The main target organs of camphor exposure are the CNS (central nervous system – brain and spinal cord) and kidneys. Convulsions, depression, apnea, asystole, gastric irritation, colic, nausea, vomiting, diarrhea, anxiety, excitement, delirium, and severe post-convulsive coma may occur after oral intake of camphor. The symptoms may appear 5 to 90 minutes after ingestion depending on the product ingested (solid or liquid). Poisoning by camphor is associated with an initial excitatory phase, with vomiting, diarrhea and excitement, followed by CNS depression and death. In humans, the characteristic symptoms of camphor poisoning after ingestion are nausea, vomiting, headache, dizziness, muscular excitability causing tremor and twitching, convulsions and delirium depending on the dosage. In a severe overdose, status epilepticus persisting for several hours occurs, ultimately causing coma and death by asphyxia or exhaustion 95. Camphor inhalation may cause irritation of the mucous membranes above 2 ppm and respiratory depression and apnoea may occur. Camphor can also cause skin and eye irritation on contact. Inhalation and skin exposure may result in acute poisoning depending on the dose with symptoms described above. After ingestion, rapid onset of vomiting improves the prognosis but simultaneous intake of alcohol, or oily solutions, enhance the absorption of camphor and therefore the toxic effect. Camphor poisoning is treated symptomatically as no antidote is known 95.
Acute camphor toxicity begins with nausea and vomiting and quickly progresses to CNS (central nervous system – brain and spinal cord) depression, seizures, respiratory failure, and death from respiratory arrest or status epilepticus 96. Symptoms of camphor toxicity usually begin 5 to 90 minutes after ingestion and are often abrupt in onset. Spontaneous emesis, with the odor of camphor readily apparent, typically occurs first. CNS stimulation ensues with restlessness, confusion, delirium, and increased muscular activity. Severe toxicity may include seizures, apnea, and coma. Death results from respiratory depression or status epilepticus 97.
Camphor administered in doses of 60 mg-4 g was reported to cause flickering, darkening or veiling of vision along with noises in the ears. Corneal erosions have been reported in association with the use of inhalant capsules containing camphor 98.
Chronic ingestion of camphor can cause a variety of symptoms clinically similar to Reye’s syndrome. In chronic ingestion, CNS findings may or may not be present, depending on the dosage. Gastrointestinal symptoms may include nausea, vomiting, epigastric pain, and hepatic enzymes elevation. Pathologic hepatic changes often include such findings as granulomatous hepatitis and fatty metamorphosis 99.
Heavy exposures (concentrations not specified) to camphor are reported to cause nausea, anxiety, dizziness, confusion, headache, twitching of facial muscles, spasticity, and with severe poisoning convulsions and coma. Camphor may be expected to be irritating to the eye, but no serious injuries have been noted 100.
There have been reports of instant collapse in infants after camphor has been applied to their nostrils 35. Camphor is irritating to the eyes, skin and mucous membranes. When camphor is applied on the skin, it is analgesic. Taken internally, it is an irritant and relieves flatulence or prevent formation of gas in the gastrointestinal tract (SRP: an agent used to reduce gas in the gastrointestinal tract). Camphor has been used as a mild expectorant (a medicine which aid in the clearance of mucus from the airways, lungs, bronchi, and trachea – used to treat coughs). Camphor is a CNS (central nervous system – brain and spinal cord) stimulant whose effects range from mild excitation to grand-mal convulsions or status epilepticus. These effects result from excitation of the cerebrum and lower structures of the CNS. Gastric irritation, together with cortical and medullary stimulation, frequently causes vomiting and diarrhea. It is not clear whether camphor toxicity is due to the parent compound, a metabolite (secondary alcohols, including borneol and isomers of hydroxy-camphor), or both.
Inhalation of concentrations above 2 ppm irritates the nose and throat (mucous membranes). Respiratory depression and apnea may occur. Very large exposures will cause the same clinical features as ingestion 101.
With chronic dermal exposure, systemic effects and contact dermatitis can occur as well as significant allergic responses. Ocular exposure results primarily in irritation only, although oral intake has been associated with visual problems 97.
Central serous chorioretinopathy of the right eye was reported in a 50-year old female of Chinese origin who had used Chinese herbal medicinal patches containing camphor as the main ingredient for more than 20 years 102.
Camphor is not a human carcinogen, and the topical use of camphorated oil in pregnancy was not associated with teratogenic effects. However, camphor ingestion may lead to abortion and/or a death of the fetus because camphor crosses the placenta and fetuses lack the enzymes needed to hydroxylate and conjugate with glucuronic acid. Camphor crosses the placenta and has been implicated in fetal and neonatal death. It has been used to induce abortions. Camphor poisoning during pregnancy was reported in four cases and, in each case, camphorated oil was mistaken for castor oil. The topical use of camphorated oil in pregnancy was not associated with teratogenic effects 101.
Animal studies
A study investigating the toxicological effects of camphor on the rabbit (Oryctolagus cuniculus) kidney involved the oral administration of different concentrations of camphor solution for a period of ten days, which resulted in mild edema, glomerulonephritis, glomerular lobulations, tubular necrosis and congestion of the blood cells. Histologically, camphor administration distorted and disrupted the cytoarchitecture of the kidney 103.
Carcinogenicity tests in animals have been negative. Neuronal necrosis produced experimentally in mice by administration of multiple doses. In developmental studies, D-camphor elicited no evidence of teratogenicity when administered orally during the fetal period of organogenesis to pregnant rats at doses up to 1000 mg/kg body weight/day, and to pregnant rabbits at doses up to 681 mg/kg body weight/day. Camphor is not mutagenic with the Ames test but sister chromatid exchange has been reported in mice given 80 mg/kg doses of camphor intraperitoneal, demonstrating possible genotoxicity.
Summary
Camphor is a multipurpose molecule with a most diverse range of applications, ranging from being used to treat medical conditions in humans to being used as a natural poison to kill insects, which seems divergent. In fact, the toxicity of camphor in humans remains a cause for concern as many cases of accidental poisoning, with serious symptoms, have occurred. However not only pure camphor should be considered, it is important to remember many products, plants and essential oils contain camphor. The overwhelmingly distinct aroma of camphor has led to its wide use in ointments and inhalants, particularly as an adjunct to treat the common cold. Scientifically, numerous biological activities have been attributed to camphor including antibacterial, antifungal, antimutagenic, antitussive and insecticidal properties, but it is important to note that bioactivity was determined in many cases using an essential oil rich in camphor and not pure camphor. Due to the high percentage of camphor, these activities may be incorrectly attributed to camphor, whilst synergism seems much more likely as was shown in the example of 1,8-cineole and (−)-camphor. Other studies showed pure camphor did not possess the same activity as the neat essential oil. Clearly, if these properties are to be confirmed, further research on camphor alone needs to follow up on these essential oil studies. In addition to its many medicinal uses, camphor is a useful molecule in chemical reactions where it is used extensively as a catalyst and has served as a chiral starting material and auxiliary. It is evident from this review that camphor is a most versatile molecule with a multitude of applications.
- Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18(5), 5434-5454; doi:10.3390/molecules18055434 http://www.mdpi.com/1420-3049/18/5/5434/htm[↩][↩]
- Hattori, A. Camphor in the Edo era fireworks. Yakushiqaku Zasshi 2001, 36, 27–31.[↩][↩]
- Sax NI and Lewis RJ. 1987. Hawley’s Condensed Chemical Dictionary. 11th edition. Van Nostrand Reinhold Co, New York.[↩]
- Viljoen, A.; van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Baser, H.; van Wyk, B. Osmitopsis astericoides (Asteraceae)—The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143.[↩][↩][↩][↩]
- Hammerschmidt, F.J.; Clark, A.M.; Soliman, F.M.; El-Kashoury, E.S.; Abd El-Kawy, M.M.; El-Fishawy, A.M. Chemical composition and antimicrobial activity of essential oils of Jasonia candicans and J. montana. Planta Med. 1993, 59, 68–70.[↩][↩]
- https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~786BzE:3[↩][↩]
- ACGIH 2001[↩]
- Reynolds JEF ( Editor). 1989. Martindale The Extra Pharmacopeia. 29th edition. The Pharmaceutical Press, London.[↩]
- Theis, J.G.; Koren, G. Camphorated oil: Still endangering the lives of Canadian children. CMAJ 1995, 152, 1821–1824.[↩]
- Manoguerra, A.S.; Erdman, A.R.; Wax, P.M.; Nelson, L.S.; Caravati, E.M.; Cobaugh, D.J.; Chyka, P.A.; Olson, K.R.; Booze, L.L.; Woolf, A.D.; et al. Camphor Poisoning: An evidence-based practice guideline for out-of-hospital management. Clin. Toxicol. 2006, 44, 357–370.[↩]
- Camphor in flavourings and other food ingredients with flavouring properties. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission 2008. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2008.729[↩][↩]
- Kresel JJ (1982) Camphor. Clin Toxic Rev, 4(7): l.[↩][↩]
- Katzung, G.B. Basic and Clinical Pharmacology, 7th ed.; Appleton and Lange: Stamford, CT, USA, 1998; pp. 372–375.[↩]
- Gosselin, R.E.; Smith, R.P.; Hodge, H.C. Clinical Toxicology of Commercial Products, 5th ed.; Williams and Wilkins: Baltimore, MD, USA, 1984.[↩]
- Park, T.; Seo, H.; Kang, B.; Kim, K. Noncompetitive inhibition by camphor of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2001, 61, 787–793.[↩][↩]
- Riggs, J.; Hamilton, R.; Homel, S.; McCabe, J. Camphorated oil intoxication in pregnancy; report of a case. Obstet. Gynecol. 1965, 25, 255–258.[↩]
- Leuschner, J. Reproductive toxicity studies of d-camphor in rats and rabbits. Arzneim. Forsch. 1997, 47, 124–128.[↩]
- Millet, Y.; Jouglard, J.; Steinmetz, M.D.; Tognetti, P.; Joanny, P.; Arditti, J. Toxicity of some essential plant oils. Clinical and experimental study. Clin. Toxicol. 1981, 18, 1485–1498.[↩][↩]
- Mann, J.C.; Hobbs, J.B.; Banthorpe, D.V.; Harborne, J.B. Natural Products: Their Chemistry and Biological Significance; Longman Scientific & Technical: Harlow, Essex, UK, 1994; pp. 309–311.[↩]
- Kumar, M.; Ando, Y. Single-wall and multi-wall carbon nanotubes from camphor-a botanical hydrocarbon. Diamond Relat. Mater. 2003, 12, 1845–1850.[↩][↩][↩]
- Nandi, N. Study of chiral recognition of model peptides and odorants: Carvone and camphor. Curr. Sci. 2005, 88, 1929–1937.[↩][↩]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4104.[↩]
- Van Wyk, B.E.; van Oudtshoorn, B.; Gericke, N. Medicinal plants of South Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2009; p. 92.[↩][↩]
- Croteau , R.; Karp, F. Biosythesis of monoterpenes: Hydrolysis of bornyl pyrophosphate, an essential step in camphor biosynthesis, and hydrolysis of geranyl pyrophosphate, the acyclic precursor of camphor, by enzymes from sage (Salvia officinalis). Arch. Biochem. Biophys. 1979, 198, 523–532.[↩][↩][↩]
- Croteau, R.; Felton, M.; Karp, F.; Kjonaas, R. Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis). Plant Phys. 1981, 67, 820–824[↩]
- Donkin, R.A. Dragon’s brain Perfume: An Historical Geography of Camphor; Koninklijke Brill: Leiden, The Netherlands, 1999; p. 141.[↩]
- Gomes-Carneiro, M.R.; Felzenszwalb, I.; Paumgartten, F.J. Mutagenicity testing (+/−)-camphor, 1,8-cineole, citral, citronellal, (−)-menthol and terpineol with the Salmonella/microsome assay. Mutat. Res. 1998, 416, 129–136.[↩]
- Liu, W. Terpenes: The expansion of chiral pool. In Handbook of Chiral Chemicals, 2nd ed.; Ager, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 65.[↩]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535.[↩][↩]
- Tirillini, B.; Velasquez, E.R.; Pellegrino, R. Chemical composition and antimicrobial activity of essential oil of Piper angustifolium. Planta Med. 1996, 62, 372–373[↩]
- Kamdem, D.P.; Gage, D.A. Chemical composition of essential oil from the root bark of Sassafras albidum. Planta Med. 1995, 61, 574–575.[↩]
- Philpott, N.W. Intramuscular Injections of camphor in the treatment of engorgement of the breasts. CMAC 1929, 20, 494–495.[↩]
- Liebelt, E.L.; Shannon, M.W. Small doses, big problems: A selected review of highly toxic common medications. Pediatr. Emerg. Care 1993, 19, 292–297.[↩]
- Reynolds EF (ed) (1982). Martindale, The Extra Pharmacopoeia, 28th ed, p 35l. London, Pharmaceutical Press.[↩]
- Arena, J.M. Poisoning: Toxicology, Symptoms, Treatments, 4th ed.; CC. Thomas: Springfield, IL, USA, 1979.[↩][↩][↩]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224.[↩]
- Sivropoulou, A.; Nikolaou, C.; Papanikolaou, E.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial, cytotoxic and antiviral activities of Salvia fruticosa essential oil. J. Agric. Food Chem. 1997, 45, 3197–3201.[↩][↩]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibañez, E.; Señoráns, F.J.; Reglero, G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 2005, 68, 790–795.[↩]
- Sökmen, A.; Vardar-Ünlü, G.; Polissiou, M.; Daferera, D.; Sökmen, M.; Dönmez, E. Antimicrobial activity of essential oil and methanol extracts of Achillea sintenisii Hub. Mor. (Asteraceae). Phytother. Res. 2003, 17, 1005–1010.[↩]
- Mevy, J.P.; Bessiere, J.M.; Dherbomez, M.; Millogo, J.; Viano., J. Chemical composition and some biological activities of the volatile oils of a chemotype of Lippia chevalieri Moldenke. Food Chem. 2007, 101, 682–685.[↩]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.P.; Bégin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162.[↩][↩]
- Tabanca, N.; Demirci, B.; Başer, K.H.C.; Aytac, Z.; Ekici, M.; Khan, S.I.; Jacob, M.R.; Wedge, D.E. Chemical composition and antifungal activity of Salvia macrochlamys and Salvia recognita essential oils. J. Agric. Food Chem. 2006, 54, 6593–6597.[↩]
- De Logu, A.; Loy, G.; Pellerano, M.L.; Bonsiqnore, L.; Schivo, M.L. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antiviral Res. 2000, 48, 177–185.[↩]
- Burrow, A.; Eccles, R.; Jones, A.S. The effects of camphor, eucalyptus and menthol vapour on nasal resistance to airflow and nasal sensation. Acta Otolaryngol. 1983, 96, 157–161[↩][↩]
- Laude, E.A.; Morice, A.H.; Grattan, T.J. The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm. Pharmacol. 1994, 7, 179–184.[↩]
- McKemy, D.D. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 2005, 1, 16.[↩]
- Kumar, N.; Nepali, K.; Sapra, S.; Bijjem, K.R.V.; Kumar, R.; Suri, O.P.; Dhar, K.L. Effect of nitrogen insertion on the antitussive properties of menthol and camphor. Med. Chem. Res. 2012, 21, 531–537.[↩]
- Green, B.G. Sensory characteristics of camphor. J. Invest. Dermatol. 1990, 94, 662–666.[↩]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937.[↩][↩]
- Adams, J.D., Jr. The use of California sagebrush (Artemisia californica) liniment to control pain. Pharmaceuticals 2012, 5, 1045–1053.[↩]
- Ghanta, V.K.; Hiramoto, N.S.; Solvason, H.B.; Tyring, S.K.; Spector, N.H.; Hiramoto, R.N. Conditioned enhancement of natural killer cell activity, but not interferon, with camphor or saccharin-LiCl conditioned stimulus. J. Neurosci. Res. 1987, 18, 10–15.[↩]
- Banerjee, S.; Welsch, C.W.; Rao, A.R. Modulatory influence of camphor on the activities of hepatic carcinogen metabolizing enzymes and the levels of hepatic and extrahepatic reduced glutathione in mice. Cancer Lett. 1995, 88, 163–169.[↩]
- Goel, H.C.; Roa, A.R. Radiosensitizing effect of camphor on transplantable mammary adenocarcinoma in mice. Cancer Lett. 1988, 43, 21–27.[↩]
- Goel, H.C.; Singh, S.; Singh, S.P. Radiomodifying influence of camphor on sister-chromatid exchange induction in mouse bone marrow. Mutat. Res. 1989, 224, 157–160[↩]
- Kanematsu, N.; Shibata, K.I. Investigation of DNA reactivity of endodontic agents by rec-assay. Gifu Shika Gakkai Zasshi 1990, 17, 592–597.[↩]
- Simić, D.; Vuković-Gacić, B.; Knezević-Vukcević, J. Detection of natural bioantimutagens and their mechanisms of action with bacterial assay-system. Mutat. Res. 1998, 402, 51–57.[↩]
- Vuković-Gacić, B.; Nikcević1, S.; Berić-Bjedova, T.; Knezević-Vukcević, J.; Simić, D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem. Toxicol. 2006, 44, 1730–1738.[↩]
- Nikolić, B.; Mitić-Ćulafić, D.; Vuković-Gacić, B.; Knezević-Vukcević, J. Modulation of genotoxicity and DNA repair by plant monoterpenes camphor, eucalyptol and thujone in Escherichia coli and mammalian cells. Food Chem. Toxicol. 2011, 49, 2035–2045.[↩]
- De-Oliveira, A.C.; Ribeiro-Pintob, L.F.; Paumgartten, F.J.R. In vitro inhibition of CYP2B1 monooxygenase by beta-myrcene and other monoterpenoid compounds. Toxicol. Lett. 1997, 92, 39–46.[↩][↩]
- Methyl bromide technical options committee (MBTOC): Assessment of alternatives to methyl bromide. Nairobi, Kenya, United Nations Environment Programme, Ozone Secretariat. 1998. http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MBTOC/MBTOC-Assesment-Report-2010.pdf[↩]
- Silva, W.J.; Dória, G.A.A.; Maia, R.T.; Nunes, R.S.; Carvalho, G.A.; Blank, A.F.; Alves, P.B.; Marçal, R.M.; Cavalcanti, S.C.H. Effects of essential oils on Aedes aegypti larvae: Alternatives to environmentally safe insecticides. Bioresour. Technol. 2008, 99, 3251–3255.[↩]
- Brattsten, L.B. Cytochrome P-450 involvement in the interactions between plant terpenes and insect herbivores. In Plant Resistance to Insects; Hedin, P.A., Ed.; ACS (American Chemical Society): Washington, DC, USA, 1983; pp. 173–195.[↩]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, A.J.; Hassanali, A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest Manag. 1998, 44, 203–209.[↩]
- Bekele, J.; Hassanali, A. Blend effects in the toxicity of the essential oil constituents of Ocimum kilimandscharicum and Ocimum kenyense (Labiateae) on two post-harvest insect pests. Phytochemistry 2001, 57, 385–391.[↩]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2006, 43, 349–355.[↩]
- Liska, A.; Rozman, V.; Kalinovic, I.; Ivecic, M.; Balicevic, R. Contact and fumigant activity of 1,8-cineole, eugenol and camphor against Tribolium castaneum (Herbst). 2010, 425, 716–720.[↩]
- Qiantai, L.; Yongcheng, S. Studies on effect of several plant materials against stored grain insects. In Proceedings of the Seventh International Conference on Stored-Product Protection; Zuxun, J., Quan, L., Yongsheng, L., Xianchang, T., Lianghua, G., Eds.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1998; Volume 1, pp. 836–844.[↩]
- Abivardi, C.; Zareh, N. Effect of camphor on embryonic and postembryonic development of Callosobruchus chinensis. J. Econ. Entomol. 1977, 70, 818–820.[↩]
- Riddick, E.W.; Aldrich, J.R.; de Milo, A.; Davis, J.C. Potential for modifying the behavior of the multi-colored Asian lady beetle (Coleoptera: Coccinellidae) with plant-derived natural products. Ann. Entomol. Soc. Am. 2000, 93, 1314–1321.[↩]
- Ojimelukwe, P.C.; Adler, C. Toxicity and repellent effects of eugenol, thymol, linalool, menthol and other pure compounds on Dinoderus bifloveatus (Coleoptera: Bostrichidae). J. Sustain. Agric. Environ. 2000, 2, 47–54.[↩]
- Prates, H.T.; Leite, R.C.; Craveiro, A.A.; Oliveira, A.B. Identification of some chemical components of the essential oil from molasses grass (Melinis minutiflora Beauv.) and their activity against cattle-tick (Boophilus microplus). J. Braz. Chem. Soc. 1998, 9, 993–997.[↩]
- Arlian, L.G. Arthropod allergens and human health. Annu. Rev. Entomol. 2002, 47, 395–433.[↩]
- Jung, W.C.; Jang, Y.S.; Hieu, T.T.; Lee, C.K.; Ahn, Y.J. Toxicity of Myristica fragrans seed compounds against B. germanica (Dictyoptera: Blattellidae). J. Med. Entomol. 2007, 44, 524–529.[↩]
- Pohlit, A.M.; Lopes, N.P.; Gama, R.A.; Tadei, W.P.; de Andrade Neto, V.F. Patent literature on mosquito repellent inventions which contain plant essential oils—A review. Planta Med. 2011, 77, 598–617.[↩][↩]
- Briassoulis, G.; Narlioglou, M.; Hatzis, T. Toxic encephalopathy associated with use of DEET insect repellents: A case analysis of its toxicity in children. Hum. Exp. Toxicol. 2001, 20, 8–14.[↩]
- Gillij, Y.G.; Gleiser, R.M.; Zygadlo, J.A. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour. Technol. 2008, 99, 2507–2515.[↩]
- Ansari, M.A.; Razdan, R.K. Relative efficacy of various oils in repelling mosquitoes. Indian J. Malariol. 1995, 32, 104–111.[↩]
- Seyoum, A.; Killeen, G.F.; Kabiru, E.W.; Knolls, B.G.J.; Hassanali, A. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in western Kenya. Trop. Med. Int. Health 2003, 8, 1005–1011.[↩]
- Osborne, O.T. Camphor and strychnine as cardiac stimulants. JAMA 1928, 90, 403.[↩]
- Belz, G.G.; Breithaupt-Grögler, K.; Butzer, R.; Herrmann, V.; Malerczyk, C.; Mang, C.; Roll, S. Klinische pharmakologie von D-Campher. In Phytopharmaka VI; Rietbrock, N., Ed.; Steinkopff Verlag: Darmstadt, Germany, 2000; pp. 21–28.[↩]
- Belz, G.G.; Loew, D. Dose-response related efficacy in orthostatic hypotension of a fixed combination of D-camphor and an extract from fresh Crataegus berries and the contribution of the single components. Phytomedicine 2003, 10 (Suppl. 4), 61–67.[↩][↩]
- Williams, A.C.; Barry, B.W. Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharmaceut. Res. 1991, 8, 17–24.[↩]
- Yano, T.; Kanetake, T.; Saita, M.; Noda, K. Effects of l-menthol and dl-camphor on the penetration and hydrolysis of methyl salicylate in hairless mouse skin. J. Pharmacobiodyn 1991, 14, 663–669.[↩]
- Fang, J.Y.; Tsai, T.H.; Lin, Y.Y.; Wong, W.W.; Wang, M.N.; Huang, J.F. Transdermal delivery of tea catechins and theophylline enhanced by terpenes: A mechanistic study. Biol. Pharm. Bull. 2007, 30, 343–349.[↩]
- Ramesh, G.; Vamshi, V.Y.; Kishan, V.; Madhusudan, R.Y. Studies on the influence of penetration enhancers on in vitro permeation of carvedilol across rat abdominal skin. Curr. Trends Biotechnol. Pharm. 2007, 1, 62–69.[↩]
- Jain, R.; Aqil, M.; Ahad, A.; Ali, A.; Khar, R.K. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Develop. Ind. Pharm. 2008, 34, 384–389.[↩]
- Liu, H.; Zhou, Y.; Sun, Y.; Sheng, X.; Zhang, J.; Zhang, Z.; Ding, J. Effect of menthol and camphor on permeation of compound diphenhydramine cream in vitro. Cent. South Pharm. 2011, 2, 5[↩]
- Jamshidzadeh, A.; Sajedianfard, J.; Nekooeian, A.A.; Tavakoli, F.; Omrani, G.H. Effects of Camphor on Sexual Behaviors in Male Rats. IJPS 2006, 2, 209–214.[↩][↩]
- Nikravesh, M.R.; Jalali, M. The effect of camphor on the male mice reproductive system. Urol. J. 2004, 1, 268–272.[↩]
- Allen, P.C.; Lydon, J.; Danforth, H.D. Effects of components of Artemisia annua on coccidia infections in chickens. Poult Sci. 1997, 76, 1156–1163.[↩]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodivers. 2011, 8, 614–623.[↩]
- Schenk, J.R. Phytochemistry, allelopathy and the capability attributes of camphor laurel (Cinnamomum camphora (L.) Ness & Eberm.). Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2009.[↩][↩][↩]
- Okamoto, Y.; Yamahi, K.; Kobayashi, K. Allelopathic activity of camphor released from camphor tree (Cinnamomum camphora). Allelopathy J. 2011, 27, 123–132.[↩]
- De Martino, L.; Mancini, E.; de Almeida, L.F.R.; de Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637.[↩]
- International program on chemical safety (IPCS) INCHEM. Camphor. http://www.inchem.org/documents/pims/pharm/camphor.htm[↩][↩]
- Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1053[↩]
- Ford MD, Delaney KA, Ling LJ, Erickson T; Clinical Toxicology. W.B. Saunders Company., Philadelphia, PA. 2001, p. 339[↩][↩]
- National Poisons Information Service Center, United Kingdom; Poisons Information Monograph: Camphor. March 1996[↩]
- Ford MD, Delaney KA, Ling LJ, Erickson T; Clinical Toxicology. W.B. Saunders Company., Philadelphia, PA. 2001, p. 339 Chronic ingestion339[↩]
- American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2[↩]
- IPCS; Poisons Information Monograph 095: Camphor. Date of last update: May 1989[↩][↩]
- Kahook, M.Y.; Thomas, S.A.; Ciardella, A.P. Central serous chorioretinopathy associated with chronic dermal camphor application. Internet J. Ophthalmol. Vis. Sci. 2007, 4, 2.[↩]
- Enaibe, B.; Eweka, A.; Adjene, J. Toxicological effects of camphor administration on the histology of the kidney of the rabbit (Oryctolagus cuniculus). Internet J. Toxicol. 2008, 5.[↩]