Contents
- What is a bowel obstruction
- Pathophysiology of bowel obstruction
- Bowel obstruction complications
- What causes bowel obstruction
- Bowel obstruction signs and symptoms
- Bowel obstruction diagnosis
- Bowel obstruction treatment
- Small bowel obstruction
What is a bowel obstruction
Bowel obstruction is a blockage that keeps food or liquid from passing through your small intestine or large intestine (colon). This interruption can occur at any point along the length of the gastrointestinal tract and clinical symptoms often vary based on the level of obstruction, the nature, severity, location and etiology. Bowel obstruction may be functional, due to bowel wall or splanchnic nerve dysfunction (pseudo-bowel obstruction), or mechanical, due to a mechanical barrier. Intestinal obstruction is most commonly caused by intra-abdominal adhesions, malignancy, or intestinal herniation.
Obstruction may occur in the small bowel (small bowel obstruction) or large bowel (large bowel obstruction). Large bowel obstruction or disease states may be associated with or masquerade as small bowel obstruction. Acute functional dilatation of the colon is referred to as “colonic pseudo-obstruction”. Acute functional small bowel dilatation is referred to as “adynamic or paralytic ileus”.
Bowel obstruction may be partial or complete, simple or complicated. Partial bowel obstruction allows some liquid contents and gas to pass through the point of obstruction, whereas complete bowel obstruction impedes passage of all bowel contents. Unlike simple bowel obstruction, complicated bowel obstruction indicates compromise of the circulation to a segment of bowel with resultant ischemia, infarction, and perforation.
Intestinal obstruction accounts for approximately 15 percent of all emergency department visits for acute abdominal pain 1.
Causes of bowel obstruction may include fibrous bands of tissue (adhesions) in the abdomen that form after surgery, an inflamed intestine (Crohn’s disease), infected pouches in your intestine (diverticulitis), hernias and colon cancer.
Complications of intestinal obstruction include bowel ischemia and perforation. Morbidity and mortality associated with intestinal obstruction have declined since the advent of more sophisticated diagnostic tests, but the condition remains a challenging surgical diagnosis.
Without treatment, the blocked parts of the intestine can die, leading to serious problems. However, with prompt medical care, intestinal obstruction often can be successfully treated. Physicians who are treating patients with intestinal obstruction must weigh the risks of surgery with the consequences of inappropriate conservative management.
Because of the serious complications that can develop from intestinal obstruction, seek immediate medical care if you have severe abdominal pain or other symptoms of bowel obstruction.
Pathophysiology of bowel obstruction
The fundamental concerns about intestinal obstruction are its effect on whole body fluid/electrolyte balances and the mechanical effect that increased pressure has on intestinal perfusion. Proximal to the point of obstruction, the intestinal tract dilates as it fills with intestinal secretions and swallowed air 2. Failure of intestinal contents to pass through the intestinal tract leads to a cessation of flatus and bowel movements. Intestinal obstruction can be broadly differentiated into small bowel and large bowel obstruction.
Fluid loss from vomiting (emesis), bowel edema, and loss of absorptive capacity leads to dehydration. Vomiting leads to loss of gastric potassium, hydrogen, and chloride ions, and significant dehydration stimulates renal proximal tubule reabsorption of bicarbonate and loss of chloride, perpetuating the metabolic alkalosis 3. In addition to derangements in fluid and electrolyte balance, intestinal stasis leads to overgrowth of intestinal flora, which may lead to the development of feculent emesis. Additionally, overgrowth of intestinal flora in the small bowel leads to bacterial translocation across the bowel wall 4.
Ongoing dilation of the intestine increases luminal pressures. When luminal pressures exceed venous pressures, loss of venous drainage causes increasing edema and hyperemia of the bowel. This may eventually lead to compromised arterial flow to the bowel, causing ischemia, necrosis, and perforation. A closed-loop obstruction, in which a section of bowel is obstructed proximally and distally, may undergo this process rapidly, with few presenting symptoms. Intestinal volvulus, the prototypical closed-loop obstruction, causes torsion of arterial inflow and venous drainage, and is a surgical emergency.
Bowel obstruction complications
Untreated, intestinal obstruction can cause serious, life-threatening complications, including:
- Tissue death. Intestinal obstruction can cut off the blood supply to part of your intestine. Lack of blood causes the intestinal wall to die. Tissue death can result in a tear (perforation) in the intestinal wall, which can lead to infection.
- Infection. Peritonitis is the medical term for infection in the abdominal cavity. It’s a life-threatening condition that requires immediate medical and often surgical attention.
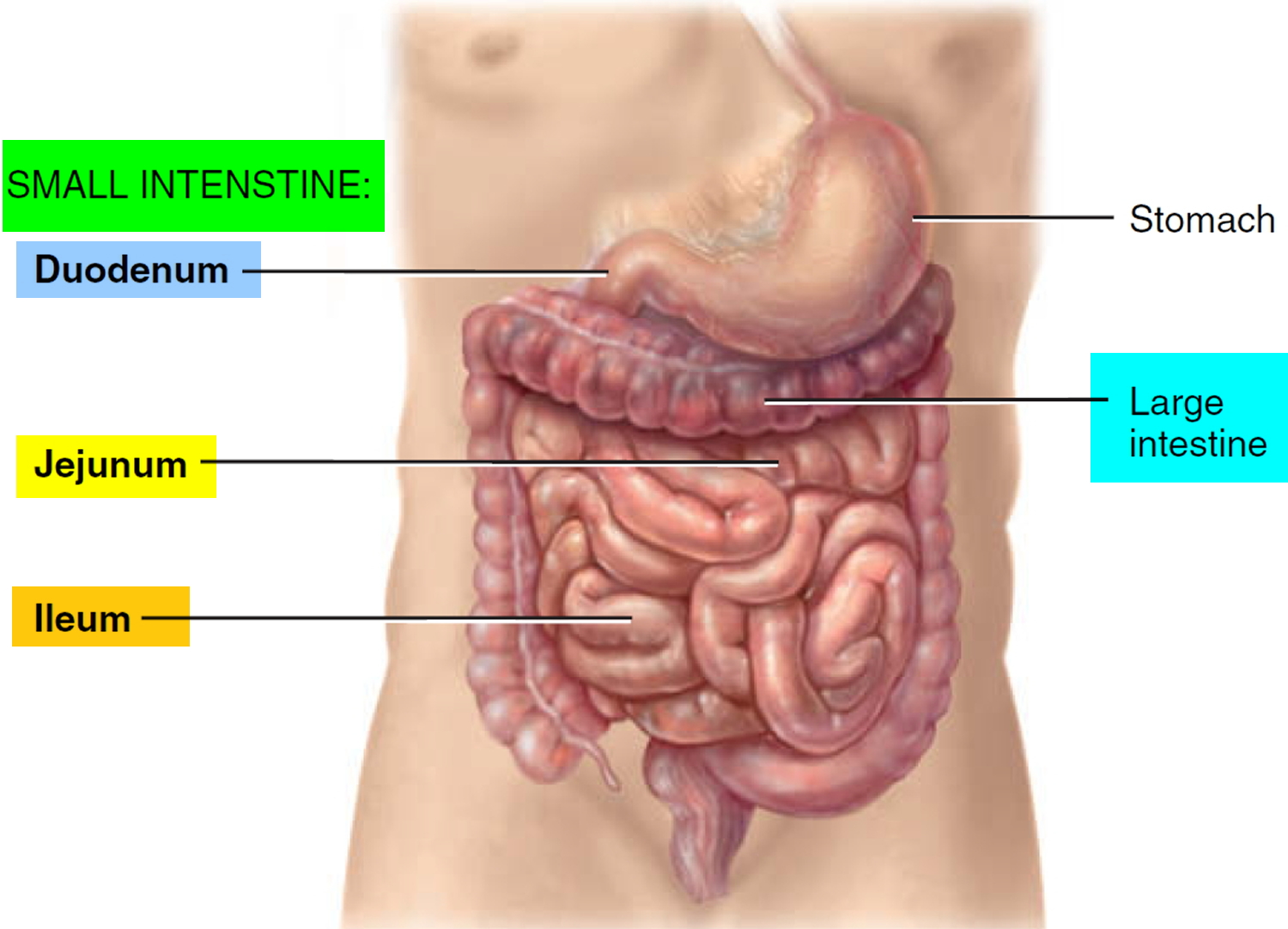
Small Intestine
Most digestion and absorption of nutrients occur in the small intestine. Because of this, its structure is specially adapted for these functions. Its length alone provides a large surface area for digestion and absorption, and that area is further increased by circular folds, villi, and microvilli. The small intestine begins at the pyloric sphincter of the stomach, coils through the central and inferior part of the abdominal cavity, and eventually opens into the large intestine. It averages 2.5 cm (1 in.) in diameter; its length is about 3 m (10 ft ) in a living person.
The small intestine is divided into three regions. The first part of the small intestine is the duodenum, the shortest region, and is retroperitoneal. It starts at
the pyloric sphincter of the stomach and is in the form of a C-shaped tube that extends about 25 cm (10 in.) until it merges with the jejunum. The jejunum is the next portion and is about 1 m (3 ft ) long and extends to the ileum. The final and longest region of the small intestine, the ileum, measures about 2 m (6 ft ) and joins the large intestine at a smooth muscle sphincter called the ileocecal sphincter (valve).
Large Intestine
The large intestine is the last major organ in the alimentary canal (Figure 1). The material that reaches it is a largely digested residue that contains few nutrients. During the 12–24 hours that this residue remains in the large intestine, little additional breakdown of food occurs, except for the small amount of digestion performed by the many bacteria living there. Even though the large intestine absorbs these few remaining nutrients, its main function is to absorb water and electrolytes from the digested mass, resulting in semisolid feces. Propulsion through the large intestine is sluggish and weak, except for mass peristaltic movements, which pass over the colon a few times a day to force the feces powerfully toward the rectum.
The large intestine frames the small intestine on 3½ sides, forming an open rectangle (Figure 1). This organ, which is wider than the small intestine but less than half as long (1.5 meters), has the following subdivisions: cecum, appendix, colon, rectum, and anal canal. Over most of its length, the large intestine exhibits three
special features: teniae coli, haustra, and epiploic appendages. Teniae (taeniae) coli are three longitudinal strips, spaced at equal intervals around the circumference of the cecum and colon. They are thickenings of the longitudinal layer of the muscularis externa, which is thin except at these sites. Because the teniae maintain muscle tone, they cause the large intestine to pucker into sacs, or haustra. Epiploic appendages, also called omental appendices, are fat-filled pouches of visceral peritoneum that hang from the intestine. Their significance is unknown.
The large intestine begins with the saclike cecum (blind pouch) in the right iliac fossa. The opening of the ileum of the small intestine into the cecum’s medial wall is surrounded internally by the ileocecal valve, which is formed by two raised edges of the mucosa. A sphincter in the distal ileum keeps the valve closed until there is food in the stomach, at which time the sphincter reflexively relaxes, opening the valve. As the cecum fills, its walls stretch, pulling the edges of the ileocecal valve together and closing the opening. This action prevents reflux of feces from the cecum back into the ileum.
The appendix is a blind tube that opens into the posteromedial wall of the cecum. Although almost always illustrated as hanging inferiorly, it more often lies “tucked up” posterior to the cecum in the right iliac fossa. The appendix has large masses of lymphoid tissue in its wall. Commonly considered a vestigial organ, current research proposes that the appendix functions as a safe haven for the beneficial bacteria that inhabit the large intestine. According to this theory, beneficial bacteria from the appendix can repopulate the gut following an infectious disease that causes diarrhea and flushes out the intestinal flora.
The colon has several distinct segments. From the cecum, the ascending colon ascends along the right side of the posterior abdominal wall in a secondarily retroperitoneal position and reaches the level of the right kidney, where it makes a rightangle turn, the right colic flexure (also called the hepatic flexure because the liver lies directly superior to it). From this flexure, the transverse colon extends intraperitoneal to the left across the peritoneal cavity. Directly anterior to the spleen, it bends acutely downward at the left colic (splenic) flexure and descends along the left side of the posterior abdominal wall again in a secondarily retroperitoneal position as the descending colon. Inferiorly, the colon becomes intraperitoneal and enters the true pelvis as the S-shaped sigmoid colon.
In the pelvis, the sigmoid colon joins the rectum, which descends along the inferior half of the sacrum in a secondarily retroperitoneal position. The rectum has no teniae coli; its longitudinal muscle layer is complete and well developed, so that it can generate strong contractions for defecation. Even though the word rectum means “straight,” the rectum actually has several tight bends. Internally, these bends are represented as three transverse folds of the rectum, or rectal valves, which prevent feces from being passed along with flatus (gas).
The last subdivision of the large intestine is the anal canal. About 3 cm long, it begins where the rectum passes through the levator ani, the muscle that forms the pelvic floor. A portion of the levator ani is responsible for maintaining the anorectal angle, an acute angle between the anus and the rectum that contributes to fecal continence. The anal canal lies entirely external to the abdominopelvic cavity in the perineum. Internally, the superior half of the anal canal contains longitudinal folds of mucosa, the anal columns. These columns contain the terminal portions of the superior rectal artery and vein (the hemorrhoidal vessels). Neighboring anal columns join each other inferiorly at crescent-shaped transverse folds called anal valves. The pockets just superior to these valves are anal sinuses, which release mucus when they are compressed by feces, providing lubrication that eases fecal passage during defecation. The horizontal line along which the anal valves lie is called the pectinate (“comb-shaped”) line. Because the mucosa superior to this line is innervated by visceral sensory fibers, it is relatively insensitive to pain. Inferior to the pectinate line, however, the mucosa is sensitive to pain because it is innervated by somatic nerves. The wall of the anal canal contains two sphincter muscles: an internal anal sphincter of smooth muscle and an external anal sphincter of skeletal muscle. The former is a thickening of the circular layer of the muscularis, whereas the latter is a distinct muscle. The external sphincter contracts voluntarily to inhibit defecation, whereas the internal sphincter contracts involuntarily, both to prevent feces from leaking from the anus between defecations and to inhibit defecation during emotional stress. During toilet training, children learn to control the external anal sphincter.
Figure 1. Small and large intestines
 What causes bowel obstruction
What causes bowel obstruction
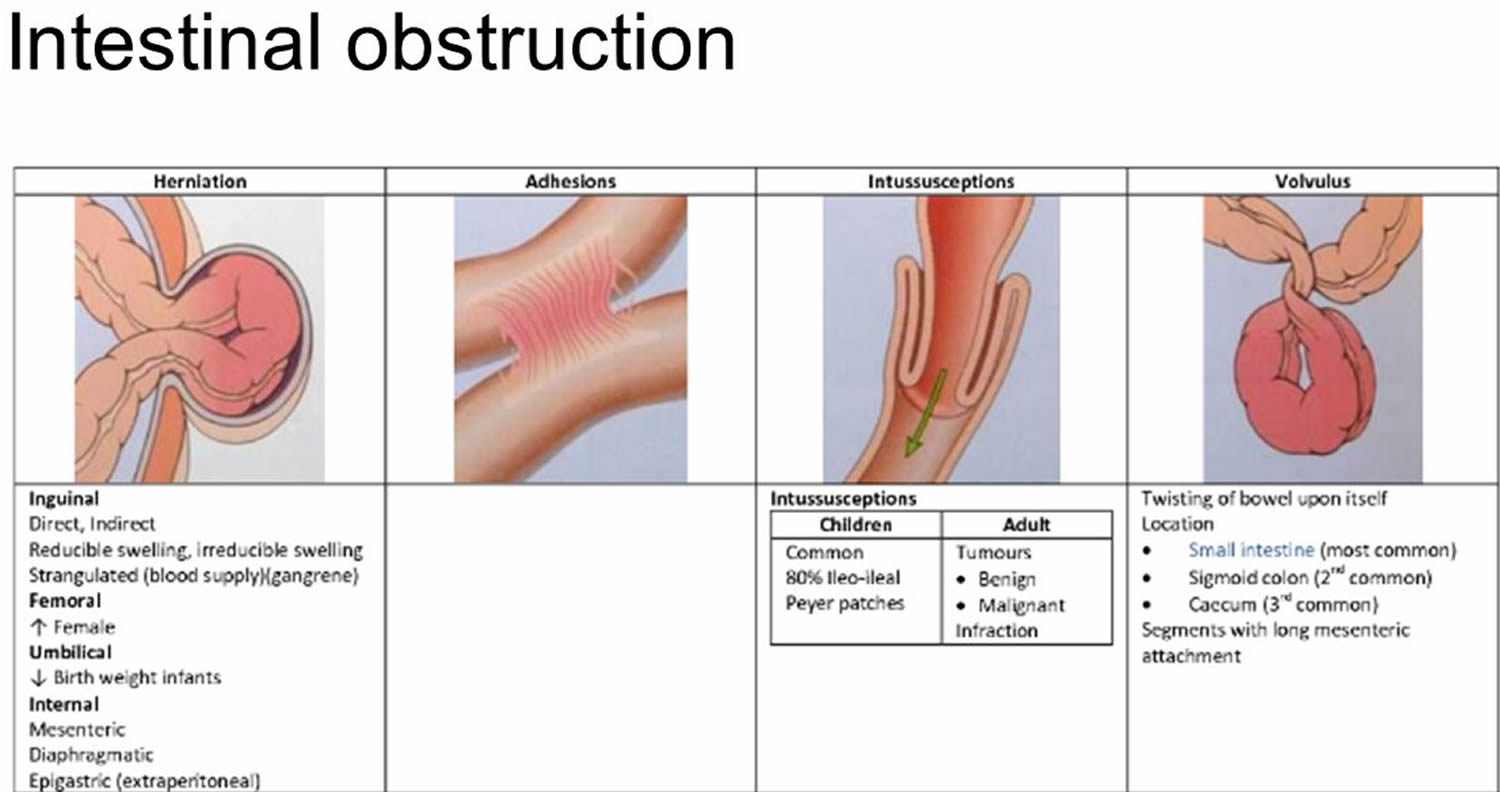
The most common causes of intestinal obstruction include adhesions, neoplasms (tumors) and herniation (Table 1).
The most common causes of intestinal obstruction in adults are:
- Intestinal adhesions (60 percent) — bands of fibrous tissue in the abdominal cavity that can form after abdominal or pelvic surgery
- Colon cancer (20 percent)
Adhesions resulting from prior abdominal surgery are the predominant cause of small bowel obstruction, accounting for approximately 60 percent of cases 5. Lower abdominal surgeries, including appendectomies, colorectal surgery, gynecologic procedures, and hernia repairs, confer a greater risk of adhesive small bowel obstruction. Less common causes of obstruction include intestinal intussusception, volvulus, intra-abdominal abscesses, gallstones, and foreign bodies.
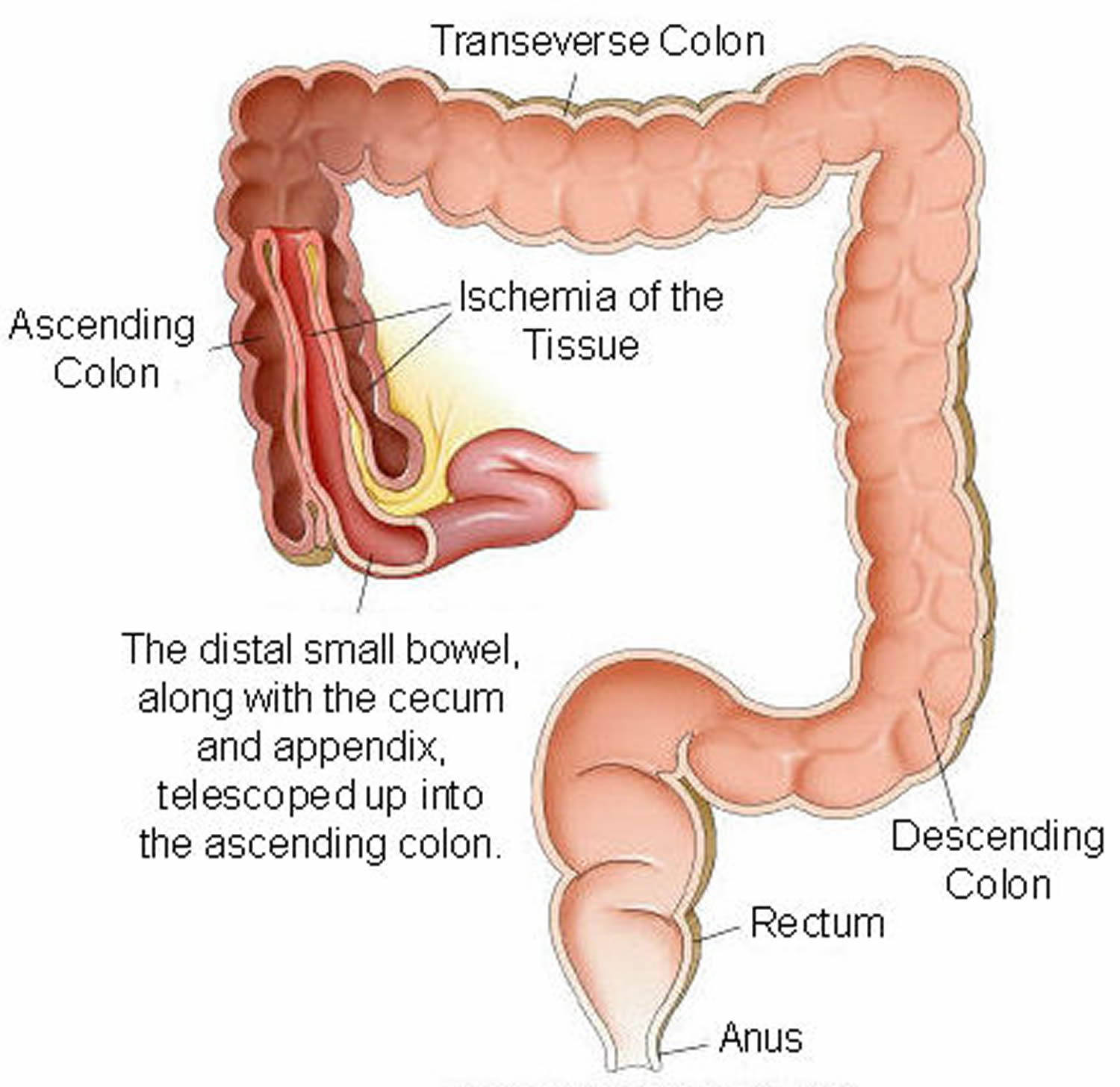
In children, the most common cause of intestinal obstruction is telescoping of the intestine (intussusception) (see Figures 2 and 3).
Other possible causes of intestinal obstruction include:
- Hernias — portions of intestine that protrude into another part of your body
- Inflammatory bowel diseases, such as Crohn’s disease
- Diverticulitis — a condition in which small, bulging pouches (diverticula) in the digestive tract become inflamed or infected
- Twisting of the colon (volvulus)
- Impacted feces
Table 1. Bowel obstruction causes
| Adhesive disease (60 percent) |
| Neoplasm (20 percent) |
| Herniation (10 percent) |
| Inflammatory bowel disease (5 percent) |
| Intussusception (< 5 percent) |
| Volvulus (< 5 percent) |
| Other (< 5 percent) |
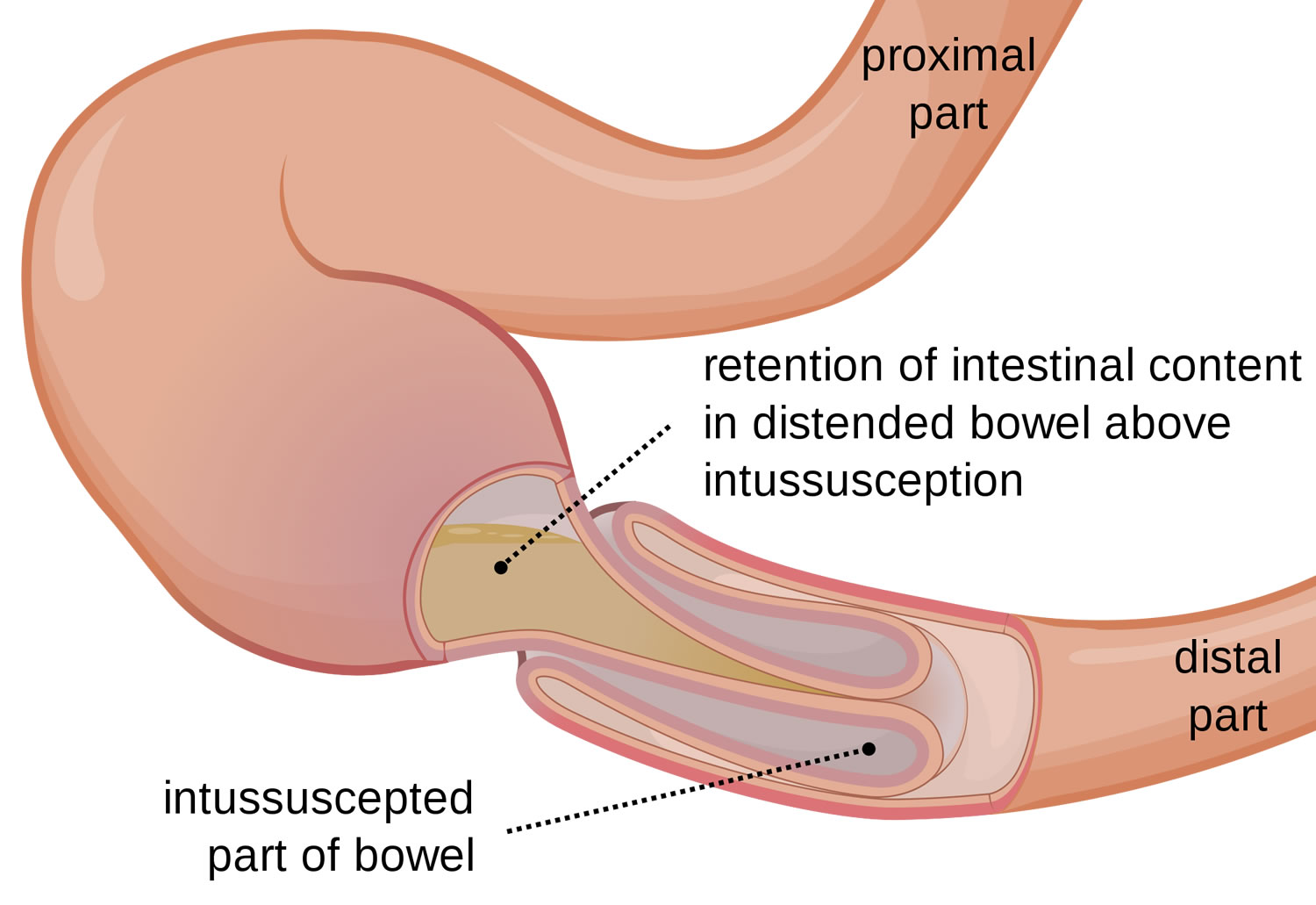
Intussusception
Intussusception is a unique type of bowel obstruction that results from invagination of a segment of bowel into another (see Figures 2 and 3). It may occur anywhere along the gastrointestinal tract distal to the gastric cardia. Intussusception may occur in a downward direction or may be retrograde, and is classified into enteric. The exact mechanism colic and enterocolic of itussuception is not known but an organic lesion, diseased segment of bowel, or an adjacent area of normal bowel may serve as a lead point in initiating the process. Accordingly, intussusception is classified into idiopathic, postoperative, and intussusception due to an organic lesion. In adults, a tumor is the lead point in 80–90% of cases. A Meckel’s diverticulum (see Figure 5) may invaginate into the ileum and sometimes, thence, into the colon 6.
Malignant tumors are being recognized with increasing frequency. A recently recognized subtype is postoperative intussusception. The point of origin of the intussusception is the small bowel and more specifically, the jejunum, particularly proximal jejunum, and dense desmoplastic inflammatory reaction within the mesentery may be the underlying mechanism precipitating the intussusception.
Figure 2. Intussusception
Figure 3. Intussusception of distal small intestine into the cecum (large intestine)
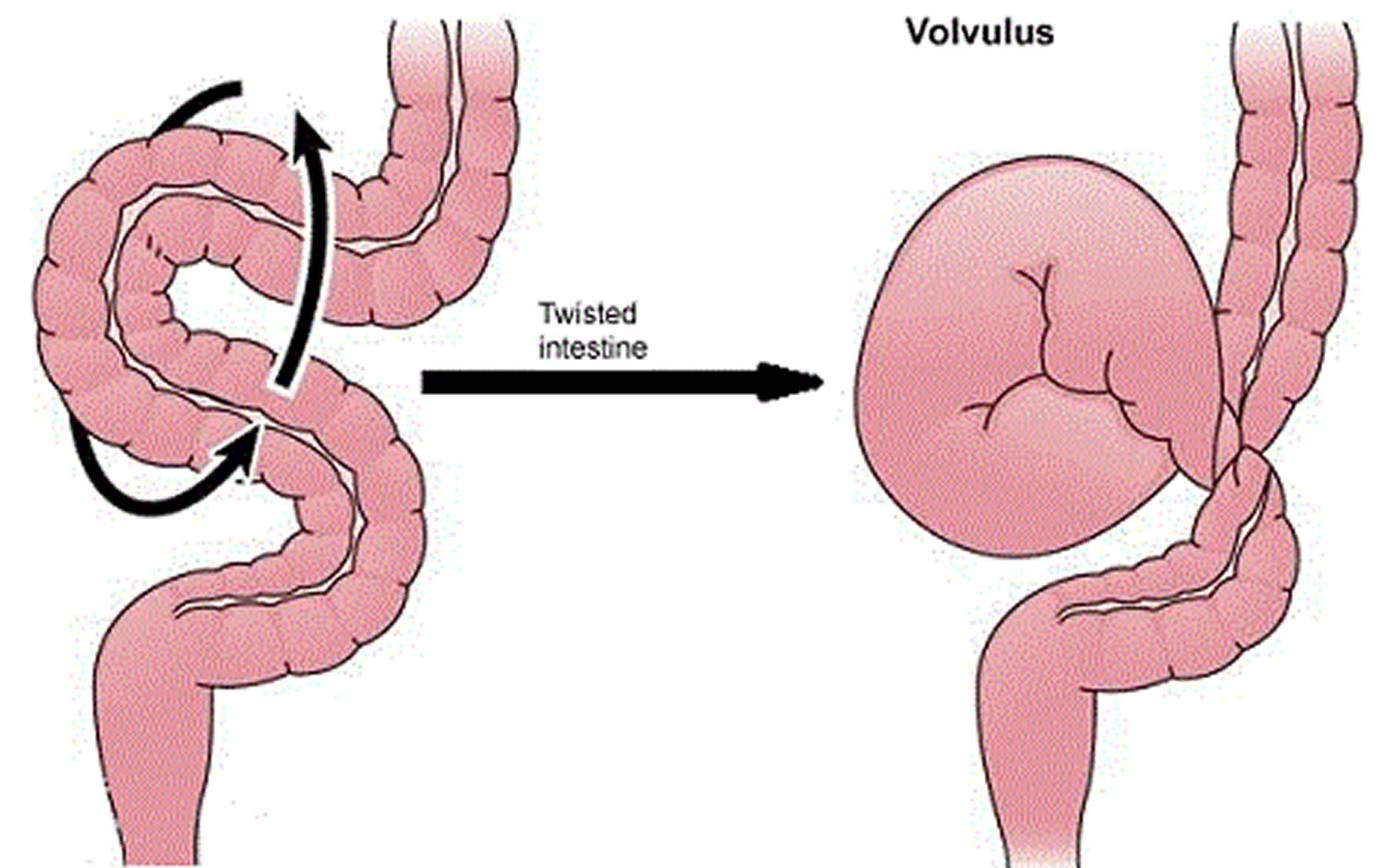
Volvulus
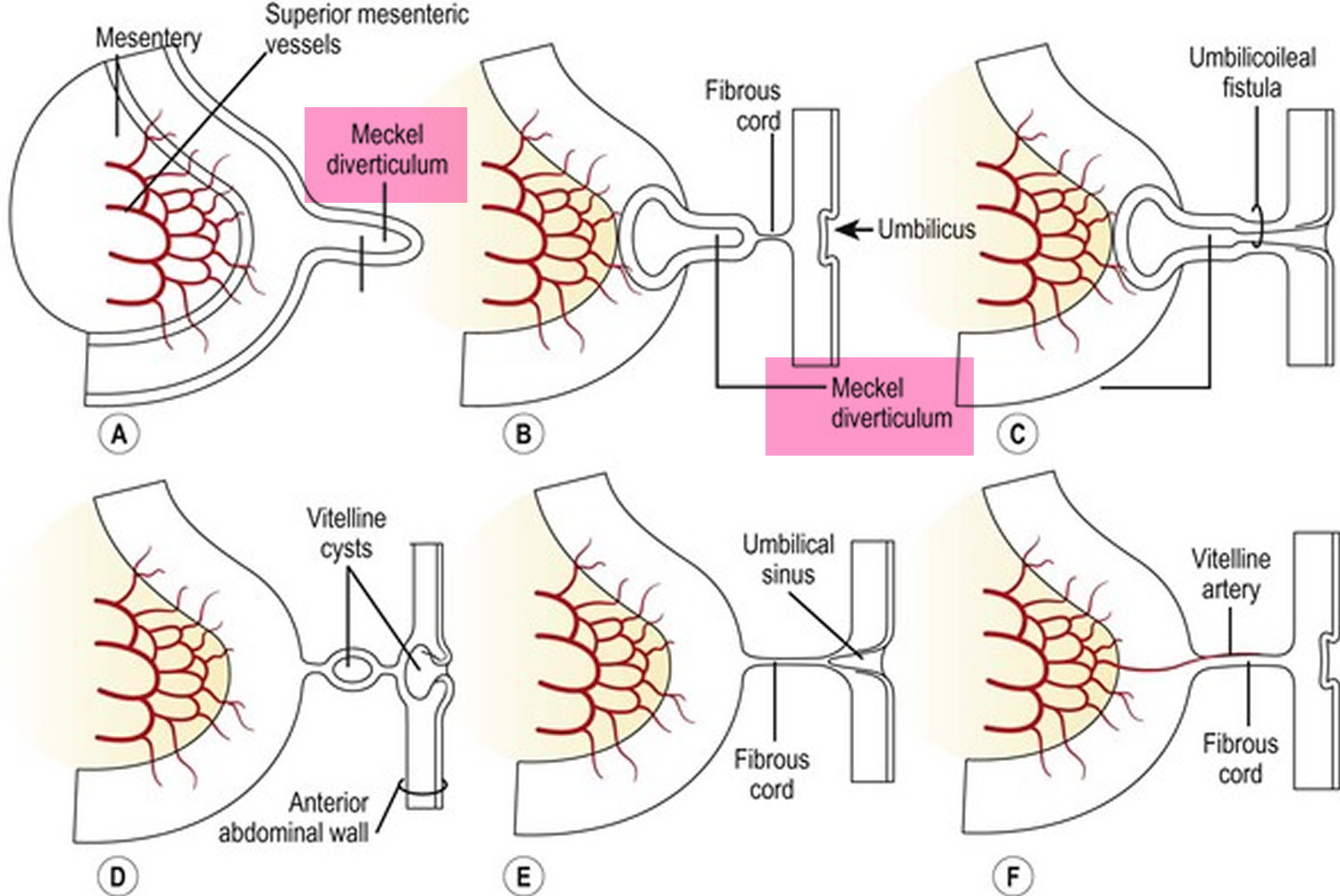
Volvulus is axial twist of the gastrointestinal tract around its mesentery resulting in partial or complete luminal obstruction (closed loop) of the bowel and a variable degree of arterial or venous obstruction (Figure 4). Volvulus commonly occurs in the colon (large intestine) and may affect the stomach or small bowel. Volvulus occurs when the small bowel twists around a Meckel’s diverticulum that is attached by a fibrous cord to the umbilicus 6, or when a closed loop obstruction twists along its long axis. Gallstone ileus is a mechanical bowel obstruction caused by migration of gallstones from the biliary system through a biliary-enteric fistula with impaction within lumen of the bowel. Littre’s hernia is incarcerated Meckel’s diverticulum in an external hernia.
Figure 4. Volvulus
Figure 5. Meckel’s diverticulum
Intestinal Pseudo-obstruction
Intestinal pseudo-obstruction (paralytic ileus) can cause signs and symptoms of bowel obstruction, but doesn’t involve a physical blockage 7. Intestinal pseudo-obstruction is a rare condition with symptoms that resemble those caused by a blockage, or obstruction, of the intestines. In paralytic ileus, muscle or nerve problems disrupt the normal coordinated muscle contractions of the intestines, slowing or stopping the movement of food and fluid through the digestive system.
This condition can occur in people of any age. Some infants are born with congenital intestinal pseudo-obstruction, and some people develop this condition as adults. Intestinal pseudo-obstruction may be acute, occurring suddenly and lasting a short time, or it may be chronic, or long lasting.
Acute colonic pseudo-obstruction, also called Ogilvie syndrome or acute colonic ileus, mostly affects older adults. In this condition, the colon becomes distended, or enlarged, after
- surgery, such as operations to open the abdomen or replace a hip or knee
- injury, such as a hip fracture
- illness, such as a serious infection
Acute large bowel pseudo-obstruction can lead to serious complications. However, people with the condition usually get better with treatment.
What causes intestinal pseudo-obstruction?
Problems with nerves, muscles, or interstitial cells of Cajal cause intestinal pseudo-obstruction. Interstitial cells of Cajal are called “pacemaker” cells because they set the pace of intestinal contractions. These cells convey messages from nerves to muscles.
Problems with nerves, muscles, or interstitial cells of Cajal prevent normal contractions of the intestines and cause problems with the movement of food, fluid, and air through the intestines.
Primary or idiopathic intestinal pseudo-obstruction is intestinal pseudo-obstruction that occurs by itself. In some people with primary intestinal pseudo-obstruction, mutations, or changes, in genes—traits passed from parent to child—cause the condition. However, health care providers do not typically order genetic testing for an intestinal pseudo-obstruction, as they don’t commonly recognize gene mutations as a cause.
Some people have duplications or deletions of genetic material in the FLNA gene. Researchers believe that these genetic changes may impair the function of a protein, causing problems with the nerve cells in the intestines.1 As a result, the nerves cannot work with the intestinal muscles to produce normal contractions that move food, fluid, and air through the digestive tract. Also, these genetic changes may account for some of the other signs and symptoms that can occur with intestinal pseudo-obstruction, such as bladder symptoms and muscle weakness.
A condition called mitochondrial neurogastrointestinal encephalopathy may also cause primary intestinal pseudo-obstruction. In people with this condition, mitochondria—structures in cells that produce energy—do not function normally. Mitochondrial neurogastrointestinal encephalopathy can also cause other symptoms, such as problems with nerves in the limbs and changes in the brain.
Secondary intestinal pseudo-obstruction develops as a complication of another medical condition. Causes of secondary intestinal pseudo-obstruction include:
- Abdominal or pelvic surgery
- Diseases that affect muscles and nerves, such as lupus erythematosus, scleroderma, and Parkinson’s disease
- Infections
- Certain medications that affect muscles and nerves, including tricyclic antidepressants, such as amitriptyline and imipramine (Tofranil), and opioid pain medications, such as those containing hydrocodone (Vicodin) and oxycodone (Oxycontin)
- Radiation to the abdomen
- Certain cancers, including lung cancer
What are the symptoms of intestinal pseudo-obstruction?
Intestinal pseudo-obstruction symptoms may include:
- abdominal swelling or bloating, also called distension
- abdominal pain
- nausea
- vomiting
- constipation
- diarrhea
Over time, the condition can cause malnutrition, bacterial overgrowth in the intestines, and weight loss. Malnutrition is a condition that develops when the body does not get the right amount of the vitamins, minerals, and other nutrients it needs to maintain healthy tissues and organ function.
Some people develop problems with their esophagus, stomach, or bladder.
How is intestinal pseudo-obstruction diagnosed?
To diagnose intestinal pseudo-obstruction, a health care provider may suggest the person consult a gastroenterologist—a doctor who specializes in digestive diseases. A health care provider will perform a physical exam; take a complete medical history, imaging studies, and a biopsy; and perform blood tests. A health care provider may order other tests to confirm the diagnosis. The health care provider also will look for the cause of the condition, such as an underlying illness.
Intestinal pseudo-obstruction can be difficult to diagnose, especially primary intestinal pseudo-obstruction. As a result, a correct diagnosis may take a long time.
Physical Exam
A physical exam is one of the first things a health care provider may do to help diagnose intestinal pseudo-obstruction. During a physical exam, a health care provider usually:
- examines a person’s body
- uses a stethoscope to listen to bodily sounds
- taps on specific areas of the person’s body
Medical History
The health care provider will ask a person to provide a medical and family history to help diagnose intestinal pseudo-obstruction.
A health care provider may order the following imaging studies:
- Abdominal x-ray. An x-ray is a picture recorded on film or a computer that a technician takes using low-level radiation.
- Upper GI series. A health care provider may order an upper GI series to look at the small intestine. A person should not eat or drink for 8 hours before the procedure, if possible. During the procedure, the person will stand or sit in front of an x-ray machine and drink barium, a chalky liquid. Infants lie on a table and the technician will give them barium through a tiny tube placed in the nose that runs into the stomach. Barium coats the lining of the small intestine, making signs of obstruction show up more clearly on x-rays.
- Lower GI series. A health care provider may order a lower GI series, an x-ray exam to look at the large intestine. For the test, the person will lie on a table while the health care provider inserts a flexible tube into the person’s anus. The health care provider will fill the large intestine with barium, making signs of underlying problems show up more clearly on x-rays. The test can show problems with the large intestine that are causing the person’s symptoms. The health care provider may ask the person to follow a clear liquid diet for 1 to 3 days before the procedure. A person may need to use a laxative or an enema before the test. A laxative is medication that loosens stool and increases bowel movements. An enema involves flushing water or laxative into the anus using a special squirt bottle.
- Computerized tomography (CT) scan. CT scans use a combination of x-rays and computer technology to create images.
- Upper GI endoscopy. This procedure involves using an endoscope—a small, flexible tube with a light—to see the upper GI tract, which includes the esophagus, stomach, and duodenum. A gastroenterologist performs the test at a hospital or an outpatient center. The gastroenterologist carefully feeds the endoscope down the esophagus and into the stomach and duodenum. A small camera mounted on the endoscope transmits a video image to a monitor, allowing close examination of the intestinal lining. A health care provider may give a person a liquid anesthetic to gargle or may spray anesthetic on the back of the person’s throat. A health care provider will place an intravenous (IV) needle in a vein in the arm to administer sedation. Sedatives help patients stay relaxed and comfortable. This test can show blockages or other conditions in the upper small intestine. A gastroenterologist may obtain a biopsy of the lining of the small intestine during an upper GI endoscopy.
Biopsy
A gastroenterologist can obtain a biopsy of the intestinal wall during endoscopy or during surgery, if the person has surgery for intestinal pseudo-obstruction and the cause is unknown. If the health care provider needs to examine the nerves in the intestinal wall, a deeper biopsy, which a gastroenterologist can typically obtain only during surgery, is necessary.
A biopsy is a procedure that involves taking a piece of the intestinal wall tissue for examination with a microscope. A health care provider performs the biopsy in a hospital and uses light sedation and local anesthetic; the health care provider uses general anesthesia if performing the biopsy during surgery. A pathologist—a doctor who specializes in diagnosing diseases—examines the intestinal tissue in a lab. Diagnosing problems in the nerve pathways of the intestinal tissue requires special techniques that are not widely available.
A health care provider can also use a biopsy obtained during endoscopy to rule out celiac disease. Celiac disease is an autoimmune disorder in which people cannot tolerate gluten because it damages the lining of their small intestine and prevents absorption of nutrients. Gluten is a protein found in wheat, rye, and barley and in products such as vitamin and nutrient supplements, lip balms, and certain medications.
Blood Tests
A blood test involves drawing blood at a health care provider’s office or a commercial facility and sending the sample to a lab for analysis. The blood test can show the presence of other diseases or conditions that may be causing a person’s symptoms. The blood test also can show levels of essential vitamins and minerals to help detect malnutrition.
Manometry
Manometry is a test that measures muscle pressure and movements in the GI tract, such as how well the smooth muscles of the stomach and small intestine contract and relax. A gastroenterologist performs the test at a hospital or an outpatient center. While the person is under sedation, a health care provider places a thin tube, or manometry tube, into the stomach and moves it down into the small intestine. A gastroenterologist may use an endoscope to place this tube. A health care provider will move the person to a manometry room and connect the manometry tube to a computer. When the person wakes up from sedation, the computer records the pressure inside the intestine while the person is fasting and after the person has eaten a meal. Manometry can confirm the diagnosis of intestinal pseudo-obstruction and show the extent of the condition.
Gastric Emptying Tests
Gastric emptying tests can show if a disorder called gastroparesis is causing a person’s symptoms. People with gastroparesis, which literally refers to a paralyzed stomach, have severely delayed gastric emptying, or the delayed movement of food from the stomach to the small intestine. Some patients with intestinal pseudo-obstruction also have gastroparesis.
Types of gastric emptying tests include the following:
- Gastric emptying scintigraphy. This test involves eating a bland meal—such as eggs or an egg substitute—that contains a small amount of radioactive material. A specially trained technician performs the test in a radiology center or hospital, and a radiologist interprets the results; the person does not need anesthesia. An external camera scans the abdomen to show where the radioactive material is located. The radiologist is then able to measure the rate of gastric emptying at 1, 2, 3, and 4 hours after the meal. Normal values depend on the composition of the meal. With some meals, if more than 10 percent of the meal is still in the stomach at 4 hours, a health care provider confirms the diagnosis of gastroparesis. Obtaining scans for 4 hours after the meal is essential. When the technician only obtains scans 1 to 2 hours after the meal, the results are often unreliable.
- Breath test. With this test, the person eats a meal containing a small amount of nonradioactive material. Then, the health care provider takes breath samples over a period of several hours to measure the amount of nonradioactive material in the exhaled breath. The results allow the health care provider to calculate how fast the stomach is emptying.
- SmartPill. The SmartPill is a small electronic device in capsule form. The SmartPill test is available at specialized outpatient centers. The person swallows the device so that it can move through the entire digestive tract and send information to a cell-phone-sized receiver worn around the person’s waist or neck. The recorded information provides details about how quickly food travels through each part of the digestive tract.
Intestinal pseudo-obstruction treatment
A health care provider will treat intestinal pseudo-obstruction with nutritional support, medications, and, in some cases, decompression. Rarely, a person will need surgery. If an illness, a medication, or both cause intestinal pseudo-obstruction, a health care provider will treat the underlying illness, stop the medication, or do both.
Nutritional Support
People with intestinal pseudo-obstruction often need nutritional support to prevent malnutrition and weight loss. Enteral nutrition provides liquid food through a feeding tube inserted through the nose into the stomach or placed directly into the stomach or small intestine. A health care provider inserts the feeding tube, sometimes using x-ray or endoscopy for guidance, and teaches the person how to care for the tube after returning home. Enteral nutrition is sufficient for most people with intestinal pseudo-obstruction. In a severe case, a person may need IV feeding, also called parenteral nutrition, which provides liquid food through a tube placed in a vein.
Enteral nutrition is possible because the intestinal lining is normal in most people with intestinal pseudo-obstruction. Enteral nutrition is preferred over parenteral nutrition because it has a much lower risk of complications.
Medications
A health care provider prescribes medications to treat the different symptoms and complications of intestinal pseudo-obstruction, such as
- antibiotics to treat bacterial infections
- pain medication, which should be used sparingly, if at all, because most pain medications delay intestinal transit
- medication to make intestinal muscles contract
- antinausea medications
- antidiarrheal medications
- laxatives
Decompression
A person with acute colonic pseudo-obstruction and a greatly enlarged colon who does not respond to medications may need a procedure, called decompression, to remove gas from the colon. A gastroenterologist can perform the procedure in a hospital or an outpatient center. The gastroenterologist may choose to decompress the colon by using colonoscopy. During colonoscopy, the gastroenterologist inserts a flexible tube into the colon through the anus. A health care provider gives the person a light sedative, and possibly pain medication, to relax. If the person requires long-term decompression, the gastroenterologist also can decompress the colon through a surgical opening in the cecum. In this case, the health care provider gives the person local anesthesia.
Surgery
In severe cases of intestinal pseudo-obstruction, a person may need surgery to remove part of the intestine. However, surgery should be performed rarely, if at all, because intestinal pseudo-obstruction is a generalized disorder that typically affects the entire intestine. Removing part of the intestine cannot cure the disease.
A surgeon—a doctor who specializes in surgery—will perform the surgery at a hospital; a person will need general anesthesia. A few highly specialized treatment centers offer small intestine transplantation. A health care provider may recommend small intestine transplantation when all other treatments have failed.
Eating, Diet, and Nutrition
Researchers have not found that eating, diet, and nutrition play a role in causing or preventing intestinal pseudo-obstruction. Following special diets usually does not help improve the disorder. However, eating frequent, small meals with pureed foods or liquids may ease digestion. Vitamin and trace mineral supplements may help a person who is malnourished.
Risk factors for bowel obstruction
Diseases and conditions that can increase your risk of intestinal obstruction include:
- Abdominal or pelvic surgery, which often causes adhesions — a common intestinal obstruction
- Crohn’s disease, which can cause the intestine’s walls to thicken, narrowing the passageway
- Cancer in your abdomen, especially if you’ve had surgery to remove an abdominal tumor or radiation therapy.
Bowel obstruction signs and symptoms
Signs and symptoms of intestinal obstruction include:
- Crampy or colicky abdominal pain that comes and goes
- Loss of appetite
- Constipation
- Nausea and Vomiting
- Inability to have a bowel movement or pass gas
- Swelling of the abdomen.
Bowel obstruction diagnosis
Patients should be asked about their history of abdominal neoplasia (cancer), hernia or hernia repair, and inflammatory bowel disease, because these conditions increase the risk of bowel obstruction. The hallmarks of bowel obstruction include colicky abdominal pain, nausea and vomiting, abdominal distension, and a cessation of flatus and bowel movements. It is important to differentiate between true mechanical obstruction and other causes of these symptoms (Table 2). Distal obstructions allow for a greater intestinal reservoir, with pain and distension more marked than emesis, whereas patients with proximal obstructions may have minimal abdominal distension but marked emesis. The presence of hypotension and tachycardia is an indication of severe dehydration. Abdominal palpation may reveal a distended, tympanitic abdomen; however, this finding may not be present in patients with early or proximal obstruction. Auscultation in patients with early obstruction reveals high-pitched bowel sounds, whereas those with late obstruction may present with minimal bowel sounds as the intestinal tract becomes hypotonic.
Table 2. Differential Diagnosis of Abdominal Pain, Distension, Nausea, and Cessation of Flatus and Bowel Movements
| Alternate diagnosis | Clues |
|---|---|
| Ascites | Acute liver failure, history of hepatitis or alcoholism |
| Medications (e.g., tricyclic antidepressants, narcotics) | Review of medications; diagnosis of exclusion |
| Mesenteric ischemia | History of peripheral vascular disease, hypercoagulable state, or postprandial abdominal angina; recent use of vasopressors |
| Perforated viscus/intra-abdominal sepsis | Fever, leukocytosis, acute abdomen, free air on imaging |
| Postoperative paralytic ileus | Recent abdominal surgery with no postoperative flatus or bowel movement |
| Pseudo-obstruction (Ogilvie syndrome) | Acutely dilated large intestine, history of intestinal dysmotility, diabetes mellitus, scleroderma |
Tests and procedures used to diagnose bowel obstruction include:
- Physical exam. Your doctor will ask about your medical history and your symptoms. He or she will also do a physical exam to assess your situation. The doctor may suspect intestinal obstruction if your abdomen is swollen or tender or if there’s a lump in your abdomen. He or she may listen for bowel sounds with a stethoscope.
- Laboratory tests. Laboratory evaluation of patients with suspected bowel obstruction should include a complete blood count and metabolic panel. Hypokalemic, hypochloremic metabolic alkalosis may be noted in patients with severe emesis. Elevated blood urea nitrogen levels are consistent with dehydration, and hemoglobin and hematocrit levels may be increased. The white blood cell count may be elevated if intestinal bacteria translocate into the bloodstream, causing the systemic inflammatory response syndrome or sepsis. The development of metabolic acidosis, especially in a patient with an increasing serum lactate level, may signal bowel ischemia.
- X-ray. To confirm a diagnosis of intestinal obstruction, your doctor may recommend an abdominal X-ray. However, some intestinal obstructions can’t be seen using standard X-rays.
- Computerized tomography (CT). A CT scan combines a series of X-ray images taken from different angles to produce cross-sectional images. These images are more detailed than a standard X-ray, and are more likely to show an intestinal obstruction.
- Ultrasound. When an intestinal obstruction occurs in children, ultrasound is often the preferred type of imaging. In youngsters with an intussusception, an ultrasound will typically show a “bull’s-eye,” representing the intestine coiled within the intestine.
- Air or barium enema. An air or barium enema is basically enhanced imaging of the colon that may be done for certain suspected causes of obstruction. During the procedure, the doctor will insert air or liquid barium into the colon through the rectum. For intussusception in children, an air or barium enema can actually fix the problem most of the time, and no further treatment is needed.
Imaging Tests
The initial evaluation of patients with clinical signs and symptoms of intestinal obstruction should include plain upright abdominal radiography. Radiography can quickly determine if intestinal perforation has occurred; free air can be seen above the liver in upright films or left lateral decubitus films. Radiography accurately diagnoses intestinal obstruction in approximately 60 percent of cases and its positive predictive value approaches 80 percent in patients with high-grade intestinal obstruction. However, plain abdominal films can appear normal in early obstruction and in high jejunal or duodenal obstruction. Therefore, when clinical suspicion for obstruction is high or persists despite negative initial radiography, non-contrast computed tomography (CT) should be ordered.
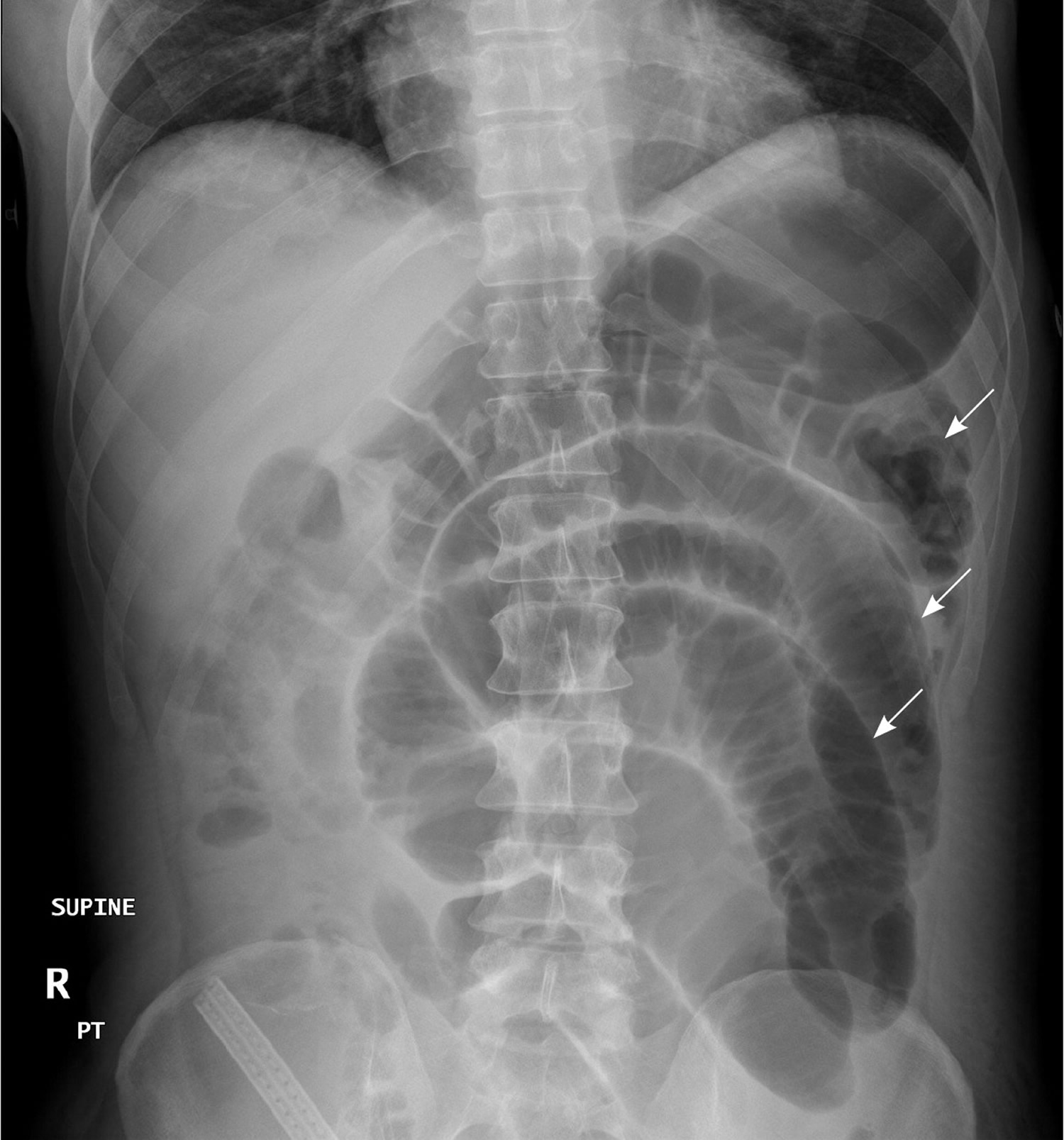
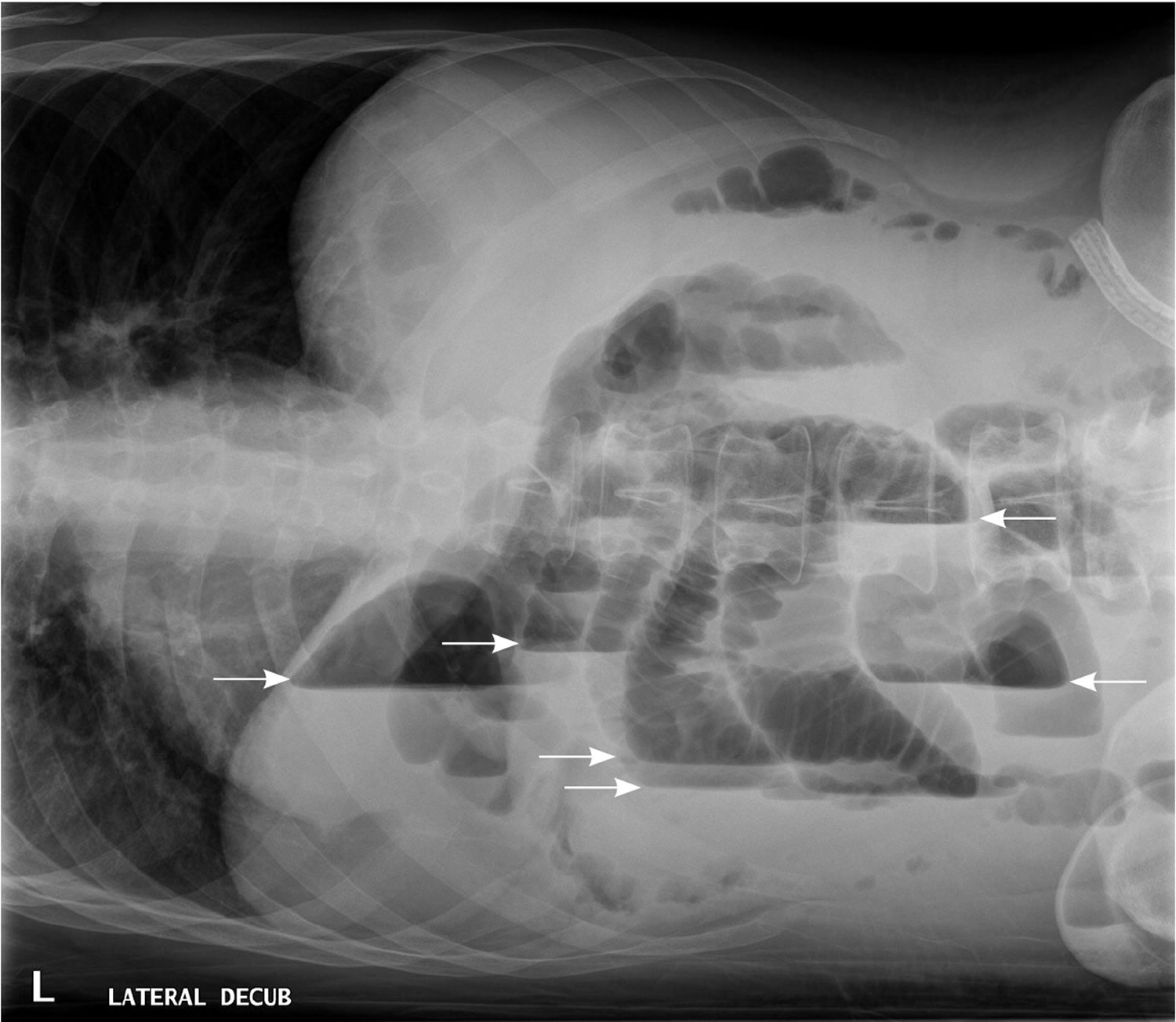
In patients with small bowel obstruction, supine views show dilation of multiple loops of small bowel, with a paucity of air in the large bowel (Figure 6). Those with large bowel obstruction may have dilation of the colon, with decompressed small bowel in the setting of a competent ileocecal valve. Upright or lateral decubitus films may show laddering air fluid levels (Figure 7). These findings, in conjunction with a lack of air and stool in the distal colon and rectum, are highly suggestive of mechanical intestinal obstruction.
Figure 6. X-ray abdomen – Supine view of the abdomen in a patient with small bowel obstruction. Dilated loops of small bowel are visible (arrows).
Figure 7. X-ray abdomen – Lateral decubitus view of the abdomen, showing air-fluid levels consistent with large bowel obstruction (arrows).
CT Scan
Computed Tomography is appropriate for further evaluation of patients with suspected intestinal obstruction in whom clinical examination and radiography do not yield a definitive diagnosis. CT is sensitive for detection of high-grade obstruction (up to 90 percent in some series) 8 and has the additional benefit of defining the cause and level of obstruction in most patients 9. In addition, CT can identify emergent causes of intestinal obstruction, such as volvulus or intestinal strangulation.
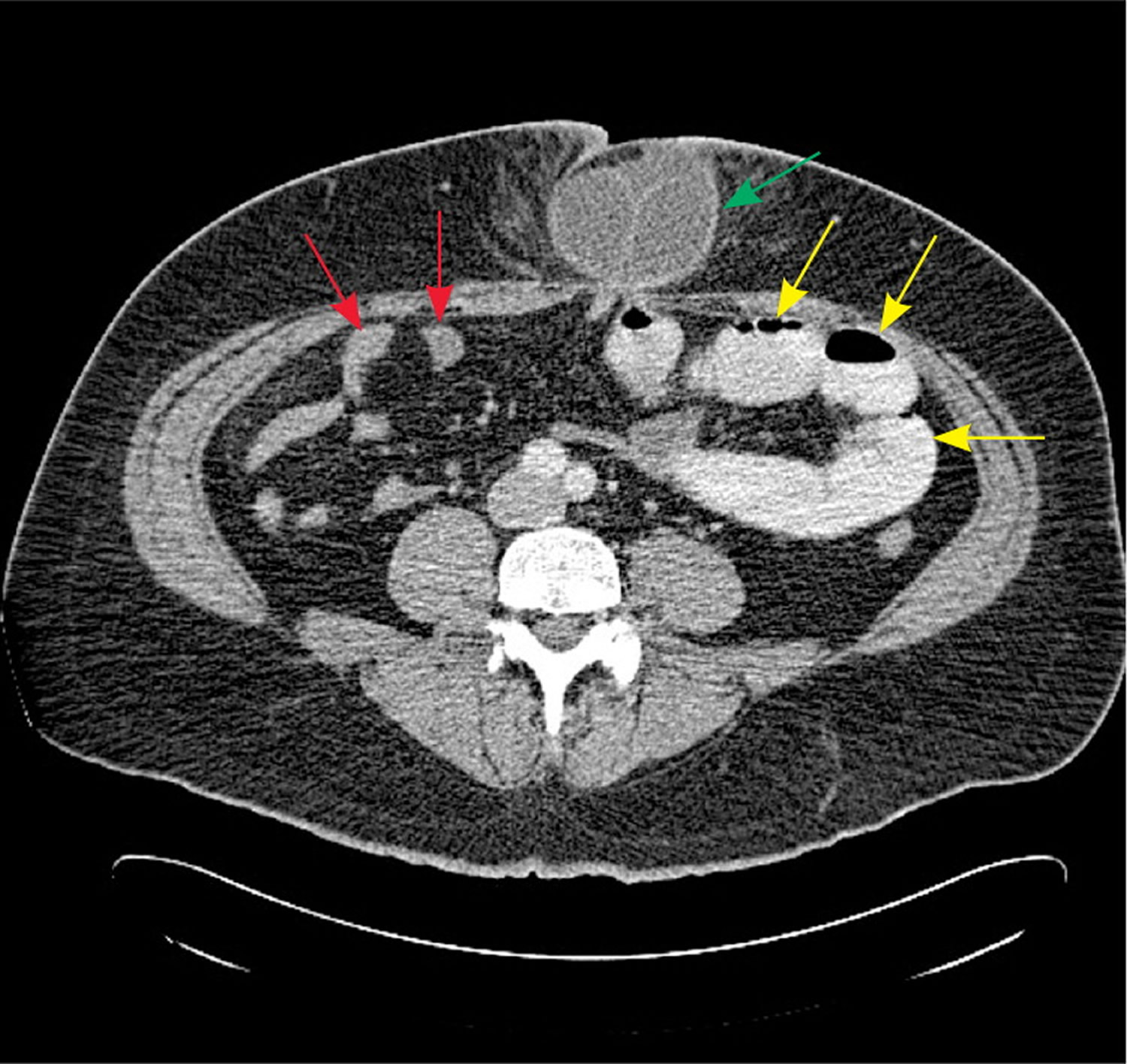
CT findings in patients with bowel obstruction include dilated loops of bowel proximal to the site of obstruction, with distally decompressed bowel. The presence of a discrete transition point helps guide operative planning (Figure 8). Absence of contrast material in the rectum is also an important sign of complete obstruction. For this reason, rectal administration of contrast material should be avoided. A C-loop of distended bowel with radial mesenteric vessels with medial conversion is highly suspicious for intestinal volvulus. Thickened intestinal walls and poor flow of contrast material into a section of bowel suggests ischemia, whereas pneumatosis intestinalis, free intra-peritoneal air, and mesenteric fat stranding suggest necrosis and perforation.
Although CT is highly sensitive and specific for high-grade bowel obstruction, its value diminishes in patients with partial obstruction. In these patients, oral contrast material may be seen traversing the length of the intestine to the rectum, with no discrete area of transition. Fluoroscopy may be of greater value in confirming the diagnosis.
The American College of Radiology recommends non-contrast CT as the initial imaging modality of choice 10. However, because most causes of small bowel obstruction will have systemic manifestations or fail to resolve—necessitating operative intervention—the additional diagnostic value of CT compared with radiography is limited. Radiation exposure is also significant. Therefore, in most patients, CT should be ordered when the diagnosis is in doubt, when there is no surgical history or hernias to explain the etiology, or when there is a high index of suspicion for complete or high-grade obstruction.
Figure 8. CT scan bowel obstruction
Note: Axial computed tomography scan showing dilated, contrast-filled loops of bowel on the patient’s left (yellow arrows), with decompressed distal small bowel on the patient’s right (red arrows). The cause of obstruction, an incarcerated umbilical hernia, can also be seen (green arrow), with proximally dilated bowel entering the hernia and decompressed bowel exiting the hernia.
Contrast fluoroscopy
Contrast studies, such as a small bowel follow-through, can be helpful in the diagnosis of a partial intestinal obstruction in patients with high clinical suspicion and in clinically stable patients in whom initial conservative management was not effective 11. The use of water-soluble contrast material is not only diagnostic, but may also be therapeutic in patients with partial small-bowel obstruction. A randomized controlled trial of 124 patients showed a 74 percent reduction in the need for surgical intervention in patients receiving gastrografin fluoroscopy within 24 hours of initial presentation 12. Contrast fluoroscopy may also be useful in determining the need for surgery; the presence of contrast material in the rectum within 24 hours of administration has a 97 percent sensitivity for spontaneous resolution of intestinal obstruction 13.
There are several variations of contrast fluoroscopy. In the small-bowel follow-through study, the patient drinks contrast material, then serial abdominal radiographs are taken to visualize the passage of contrast through the intestinal tract. Enteroclysis involves naso- or oro-duodenal intubation, followed by the instillation of contrast material directly into the small bowel. Although this study has superior sensitivity compared with small-bowel follow-through 14, it is more labor-intensive and is rarely performed. Rectal fluoroscopy can be helpful in determining the site of a suspected large bowel obstruction.
Ultrasound
In patients with high-grade obstruction, ultrasound evaluation of the abdomen has high sensitivity for intestinal obstruction, approaching 85 percent 15. However, because of the wide availability of CT, it has largely replaced ultrasonography as the first-line investigation in stable patients with suspected intestinal obstruction. Ultrasonography remains a valuable investigation for unstable patients with an ambiguous diagnosis and in patients for whom radiation exposure is contraindicated, such as pregnant women.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) may be more sensitive than CT in the evaluation of bowel obstruction 16. MRI enteroclysis, which involves intubation of the duodenum and infusion of contrast material directly into the small bowel, can more reliably determine the location and cause of obstruction 17. However, because of the ease and cost-effectiveness of abdominal CT, MRI remains an investigational or adjunctive imaging modality for intestinal obstruction.
Bowel obstruction treatment
Treatment for intestinal obstruction depends on the cause of your condition, but generally requires hospitalization.
Management of intestinal obstruction is directed at correcting physiologic derangements caused by the obstruction, bowel rest, and removing the source of obstruction. The former is addressed by intravenous fluid resuscitation with isotonic fluid. The use of a bladder catheter to closely monitor urine output is the minimum requirement for gauging the adequacy of resuscitation; other invasive measures, such as arterial canalization or central venous pressure monitoring, can be used as the clinical situation warrants. Antibiotics are used to treat intestinal overgrowth of bacteria and translocation across the bowel wall 18. The presence of fever and leukocytosis should prompt inclusion of antibiotics in the initial treatment regimen. Antibiotics should have coverage against gram-negative organisms and anaerobes, and the choice of a specific agent should be determined by local susceptibility and availability. Aggressive replacement of electrolytes is recommended after adequate renal function is confirmed.
Hospitalization to stabilize your condition
When you arrive at the hospital, the doctors will first work to stabilize you so that you can undergo treatment. This process may include:
- Placing an intravenous (IV) line into a vein in your arm so that fluids can be given
- Putting a nasogastric tube through your nose and into your stomach to suck out air and fluid and relieve abdominal swelling
- Placing a thin, flexible tube (catheter) into your bladder to drain urine and collect it for testing.
Treatment of stable patients with bowel obstruction and a history of abdominal surgery presents a challenge. Conservative management of a high-grade bowel obstruction should be attempted initially, using intestinal intubation and decompression, aggressive intravenous rehydration, and antibiotics.
Caution should be used when clinical and radiologic evidence suggest complete obstruction, because the use of intestinal stimulation can exacerbate the obstruction and precipitate intestinal ischemia.
Conservative management is successful in 40 to 70 percent of clinically stable patients, with a higher success rate in those with partial obstruction 19. Although conservative management is associated with shorter initial hospitalization (4.9 versus 12 days), there is also a higher rate of eventual recurrence (40.5 versus 26.8 percent) 20. With conservative management, resolution generally occurs within 24 to 48 hours. Beyond this time frame, the risk of complications, including vascular compromise, increases. If intestinal obstruction is not resolved with conservative management, surgical evaluation is required 21.
Treating intussusception
A barium or air enema is used both as a diagnostic procedure and a treatment for children with intussusception. If an enema works, further treatment is usually not necessary.
Due to tumors accounting for majority of cases of adults with intussusception, treatment of intussusception in adults is surgical without attempts at hydrostatic reduction. Optimal surgical procedure depends on the anatomic location, present of a lead point, and local factors, such as edema, inflammation, and ischemia of involved bowel. While resection is the treatment of colic andenterocolic intussusception, the choice in enteric type i.e. attempt at operative reduction vs. resection without attempt at reduction, depends on presence of underlying lesion, chances the lesion is malignant, and viability of involved bowel.
Treatment for partial obstruction
If you have an obstruction in which some food and fluid can still get through (partial obstruction), you may not need further treatment after you’ve been stabilized. Your doctor may recommend a special low-fiber diet that is easier for your partially blocked intestine to process. If the obstruction does not clear on its own, you may need surgery to relieve the obstruction.
The inclusion of oral magnesium hydroxide, simethicone, and probiotics decreased the length of hospitalization in a randomized controlled trial of 144 patients with partial small bowel obstructions (number needed to treat = 7) 22.
Treatment for complete obstruction
If nothing is able to pass through your intestine, you’ll usually need surgery to relieve the blockage. The procedure you have will depend on what’s causing the obstruction and which part of your intestine is affected. Surgery typically involves removing the obstruction, as well as any section of your intestine that has died or is damaged.
Alternatively, your doctor may recommend treating the obstruction with a self-expanding metal stent. The wire mesh tube is inserted into your colon via an endoscope passed through your mouth or colon. It forces open the colon so that the obstruction can clear.
Stents are generally used to treat people with colon cancer or to provide temporary relief in people for whom emergency surgery is too risky. You may still need surgery, once your condition is stable.
Treatment for pseudo-obstruction
If your doctor determines that your signs and symptoms are caused by pseudo-obstruction (paralytic ileus), he or she may monitor your condition for a day or two in the hospital, and treat the cause if it’s known. Paralytic ileus can get better on its own. In the meantime, you’ll likely be given food through a nasal tube or an IV to prevent malnutrition.
If paralytic ileus doesn’t improve on its own, your doctor may prescribe medication that causes muscle contractions, which can help move food and fluids through your intestines. If paralytic ileus is caused by an illness or medication, the doctor will treat the underlying illness or stop the medication. Rarely, surgery may be needed to remove part of the intestine.
In cases where the colon is enlarged, a treatment called decompression may provide relief. Decompression can be done with colonoscopy, a procedure in which a thin tube is inserted into your anus and guided into the colon. Decompression can also be done through surgery.
Bowel obstruction surgery
The decision to perform surgery for intestinal obstruction can be difficult. Peritonitis, clinical instability, or unexplained leukocytosis or acidosis are concerning for abdominal sepsis, intestinal ischemia, or perforation; these findings mandate immediate surgical exploration. Patients with an obstruction that resolves after reduction of a hernia should be scheduled for elective hernia repair, whereas immediate surgery is required in patients with an irreducible or strangulated hernia. Stable patients with a history of abdominal malignancy or high suspicion for malignancy should be thoroughly evaluated for optimal surgical planning. Abdominal malignancy can be treated with primary resection and reconstruction or palliative diversion, or placement of venting and feeding tubes.
Small bowel obstruction
Small intestinal pseudo-obstruction describes a clinical syndrome characterized by manifestations of mechanical small bowel obstruction in the absence of an obstructive lesion. A multitude of conditions cause functional bowel obstruction. Mechanical small bowel obstruction may be due to a luminal, mural, or extra-mural mechanical barrier. Mechanical small bowel obstruction may be proximal (high small bowel obstruction) or distal (low small bowel obstruction), closed loop or open-ended obstruction. In closed loop obstruction the lumen of the bowel is occluded at two points thus preventing prograde and retrograde movement of bowel contents. In open-ended obstruction a one-point obstruction interferes with the prograde propulsion of bowel contents.
Acute small bowel obstruction results in local as well as systemic physiologic and pathologic derangements. Significant partial or complete obstruction is associated with increased incidence of migrating clustered contractions proximal to the site of obstruction. Such contractions are associated with abdominal cramps. With partial obstruction migrating clustered contractionspropel intraluminal contents and allow them to pass distal to the point of obstruction. With complete unrelieved obstruction, bowel contents fail to pass distally, with resultant progressive accumulation of intraluminal fluids and distention of the proximal bowel. This eventually initiates retrograde giant contractions in the small bowel as the first phase of vomiting. In adynamic ileus migratory motor complexes (contractions initiated in the stomach and proximal small bowel almost simultaneously and propagate distally to clear the intestine of secretions and debris) and fed contractions (intermittent and irregular contractions that provide mixing and slow distal propulsion) are inhibited.
As intraluminal pressure in the bowel proximal to the obstruction increases, venous flow in the bowel wall and adjacent mesentery decreases, and ceases if pressure reaches systolic pressure. Blood flow to the mucosa decreases, followed by capillary rupture and hemorrhagic infiltration. A twist of the mesentery or direct pressure on the mesenteric vessels results in venous and/or arterial occlusion. Intestinal epithelium is very vulnerable to anoxia and is the first to suffer necrosis. Perforation may occur as a result of ischemic or pressure necrosis. Pressure necrosis may occur at site where a tight band adhesion passes across a loop of bowel, or where an impacted gall stone or fecoloma produces stercoral ulceration and subsequent perforation. In simple obstruction the bowel proximal to the obstruction appears heavy, edematous, and even cyanosed. In advanced cases serosal tears appear at the antimesenteric border of the bowel.
Acute small bowel obstruction results in volume depletion and electrolyte disturbances. Intestinal contents are cut off from the absorptive surface of the colon. Further loss of volume occurs as bowel contents stagnate in the dilated loops of obstructed bowel, lost through vomiting, or sequestrated in the bowel wall or peritoneal cavity. Water loss is accompanied by electrolyte loss, and depending upon the level of obstruction specific electrolyte concentration changes. As intraluminal pressure increases, absorption of water and sodium decreases and luminal secretion of water, sodium, and potassium increases. In addition there is edema of the bowel wall and leakage of proteins. With strangulation, protein and electrolyte rich exudate accumulate in the peritoneal cavity, and with infarction sequestration of blood in bowel wall occurs. The peritoneal fluid exudate changes from plasma-like clear fluid, to bloody, then foul dark exudate. There is also change in the ecology of bacterial population with increase fecal type of bacterial colonies in the bowel proximal to the obstruction and altered proximal-to-distal gradient change in bacterial flora. Bacterial breakdown of stagnant bowel contents results in formation of “feculent fluid”. With strangulation physiologic changes are complicated by blood loss in the infarcted bowel, death of tissues, gut translocation of bacteria and toxins 23, and the final insult of perforation.
Small bowel obstruction causes
Table 3. Causes of acute small bowel obstruction
| Adynamic ileus: commonest causes: | |
| |
| Mechanical small bowel obstruction: | |
| Luminal |
|
| Mural | 1. Meckel’s diverticulum: mechanism of obstruction:
2. Crohn’s disease: mechanism of obstruction:
3. Neoplasm: Leiomyoma is the commonest benign tumor, and carcinoid or adenocarcinoma are the commonest malignant tumors. Mechanism of obstruction:
4. Intussusception:
5. Vovulus: 1.7–5.7%
6. Radiation enteritis, 0.5–5%: mechanism of obstruction:
7. Hematoma 8. Strictures:
|
| Extramural |
|
Small bowel obstruction symptoms
The four cardinal symptoms of bowel obstruction are colicky pain, vomiting, obstipation/absolute constipation, and abdominal distention. Colicky abdominal pain, vomiting, and abdominal distention are commonly seen in small bowel obstruction. The pain is colicky in nature and becomes dull late in the course of small bowel obstruction. Vomiting is a pronounced symptom in high small bowel obstruction. The vomitus is bilious or semi-indigested food in high small bowel obstruction, and feculant in low small bowel obstruction. Obstipation and constipation are present to a variable degree. “Tumbling small bowel obstruction” describes intermittent symptoms of obstruction seen in patients with gallstone ileus. These episodes correspond to stone impaction, subsequent release, and reobstruction. Biliary symptoms are present before the onset of obstruction in 20–56% of cases. Intermittent partial bowel obstructive symptoms are also suggestive of intussusception.
Small bowel obstruction diagnosis
Past surgical and medical history may shed light on etiology of small bowel obstruction. In the absence of prior surgery and any apparent cause, or in presence of clinically confusing clinical picture, intussusception, Meckel’s diverticulum, gall stone ileus, and neoplasms are suspects.
The presence of strangulation/gangrene in small bowel obstruction cannot always be reliably excluded or confirmed even in the hands of the most experienced clinician 25.
Four classical findings are often used as indicators of strangulation:
- Tachycardia (resting heart rate over 100 beats per minute),
- Localized abdominal tenderness or pain,
- Leuckocytosis (white cells above the normal range in the blood) and
- Fever.
The absence of these four signs indicates simple obstruction, the development of any of the indicators raises the index of suspicion of strangulation, and the presence of multiple clinical parameters is correct in 70% of small bowel obstruction with strangulation.
The diagnosis of majority of cases of bowel obstruction can be made based on clinical presentation and initial plain radiograph of the abdomen. Luminal contrast studies, computed tomography (CT scan), and ultrasonography (US) are utilized in select cases. Once the diagnosis of bowel obstruction is entertained, location, severity and etiology are to be determined. Most importantly is the differentiation between simple and complicated obstruction.
Plain radiograph of the abdomen is the most valuable initial diagnostic test in acute small bowel obstruction. This imaging method gives information diagnostic of small bowel obstruction in 50–60% of cases and provide enough information needed for clinical decision making 26 (Table 3). In 20–30% the radiographic findings are equivocal, and in 10–20% are normal. The typical air fluid levels seen in the dilated bowel proximal to the obstruction may be absent in high small bowel obstruction, closed loop obstruction or late obstruction. Low grade obstruction is difficult to assess with plain radiograph of the abdomen.
Intraluminal contrast studies (small bowel follow-through, enteroclysis, barium enema) are utilized in certain clinical situations.
Small bowel follow – through is indicated when: 1) clinical presentation of bowel obstruction is confusing; 2) plain radiograph of the abdomen is non-diagnostic, and 3) response to nonoperative management is inadequate, and more diagnostic accuracy is needed to aid in decision making i.e. to continue with nonoperative treatment or resort to surgical intervention. The study is particularly indicated when a trial of medical treatment is warranted: postoperative or adynamic ileus, partial small bowel obstruction, malignant small bowel obstruction (carcinomatosis, intraabdominal recurrent or metastatic cancer), radiation enteritis, recurrent adhesive small bowel obstruction, and small bowel obstruction in Crohn’s disease. Small bowel follow-through differentiates adynamic ileus from mechanical small bowel obstruction. In adynamic ileus oral contrast moves to colon in 4–6 hrs. In complete mechanical small bowel obstruction contrast shows dilated small bowel and stops at site of obstruction in one hour or less, and in partial small bowel obstruction transit time of the dye is prolonged. In carcinomatosis multiple points of obstruction with pooling of contrast is noted. In gallstone ileus, small bowel follow-through detects the biliary enteric fistula and filling defect (corresponding to the impacted gall stone) in the small bowel. A beak-like point of obstruction or a mass is suggestive of intussusception.
Enteroclysis (small bowel enema) is a barium infusion study that allows close examination of mucosal pattern, distensibility, and motility of individual bowel loops. It is superior to small bowel follow-through and has greater diagnostic yield. Small bowel enema is used when small bowel follow-through is inconclusive for partial small bowel obstruction and is valuable in the diagnosis of tumors, intussusception, strictures, radiation enteritis, and occasionally Crohn’s disease. A “stretched spring” appearance with intermittent large thick concentric rings as opposed to fine rings in close proximity suggest the presence of vascular compromise in intussusception. Small bowel enema can suggest whether a lead point is benign (causing longer and permanent intussusception) or malignant (short and transient intussusception). A combination of thickened valvulae conniventes mucosal folds measuring greater than 2 mm, mural thickening (wall thickness greater than 2 mm when adjacent bowel loops are parallel for at least 4 cm under compression) are the commonest features noted in radiation enteritis. Other findings include, single or multiple stenoses of variable lengths, stenoses at site or origin of sinus or fistula, and adhesions as evidenced by constant angulation of bowel loops and relative fixity within the pelvis. There is also pooling of barium that represent barium-filled, matted loops of terminal ileum in which individual loops are not distinguishable nor are mucosal folds discernable. In Crohn’s disease there is a combination of thickened valvulae conniventes, stenoses, sinuses, fistulae, discrete fissure ulcers, longitudinal ulcers, cobblestoning, skip lesions, and asymmetrical involvement.
Barium enema is not sensitive in the diagnosis of small bowel obstruction except in distal small bowel obstruction where large bowel obstruction masquerades as small bowel obstruction. Barium (or gastrografin, a water soluble hyperosmolar contrast) enema is utilized more frequently in large bowel obstruction to differentiate pseudo-obstruction from mechanical obstruction, confirm the diagnosis of volvulus, and intussusception, and accurately determine site of obstruction.
Ultrasonography (US) is a valuable diagnostic tool in the evaluation of acute abdomen when used selectively. It is useful in the diagnosis of gallstone ileus, intussusception, pelvic disease, and gallbladder disease, and can aid in the exclusion of small bowel obstruction. In gallstone ileus, Ultrasound reveals diseased gall bladder, gas in the gall bladder or bile ducts or both, and fluid filled bowels that can be followed to the stone in the intestine. The presence of stones in the gall bladder will modify the planned operative procedure in the treatment of gallstone ileus. In intussusception, ultrasound reveals the diagnostic “target sign”, a mass with sonolucent periphery (due to edematous bowel) and a strongly hyperechoic center (from compressed center of intussusception). Paralytic ileus is differentiated from mechanical small bowel obstruction by the presence of peristaltic movement that is easily observed by ultrasound. The location of obstruction is determined by analysis of dilated bowel loops in terms of location and valvulae conniventes. Adhesion is considered the cause of small bowel obstruction when there is no apparent cause of obstruction.
Computed tomography (CT scan) is emerging as a valuable tool in the management of bowel obstruction – CT scan is 85–95% accurate in diagnosis of complete small bowel obstruction. It confirms the diagnosis, differentiates between mechanical and functional obstruction, provides information about cause and site of obstruction, and helps differentiate between simple and complicated small bowel obstruction. Furthermore, CT scan can diagnose other disease states 27. Hence CT scan helps in decision making for early surgical intervention, and prevents delay in treatment. CT scan may give false positive results and may be difficult to interpret when colonic abnormalities cause predominantly small bowel dilatation. CT scan is unable to identify location and cause of obstruction accurately in 18% of cases. Furthermore, CT scan cannot predict who will benefit from conservative treatment in cases of partial small bowel obstruction. In these situations small bowel follow-through or small bowel enema are more helpful.
Small bowel obstruction treatment
A three step approach is paramount in the successful management of bowel obstruction: resuscitation, investigation, and definitive therapy.
A suggested approach to the patient with suspected small bowel obstruction is shown in Figure 9.
Figure 9. Management of Small Bowel Obstruction
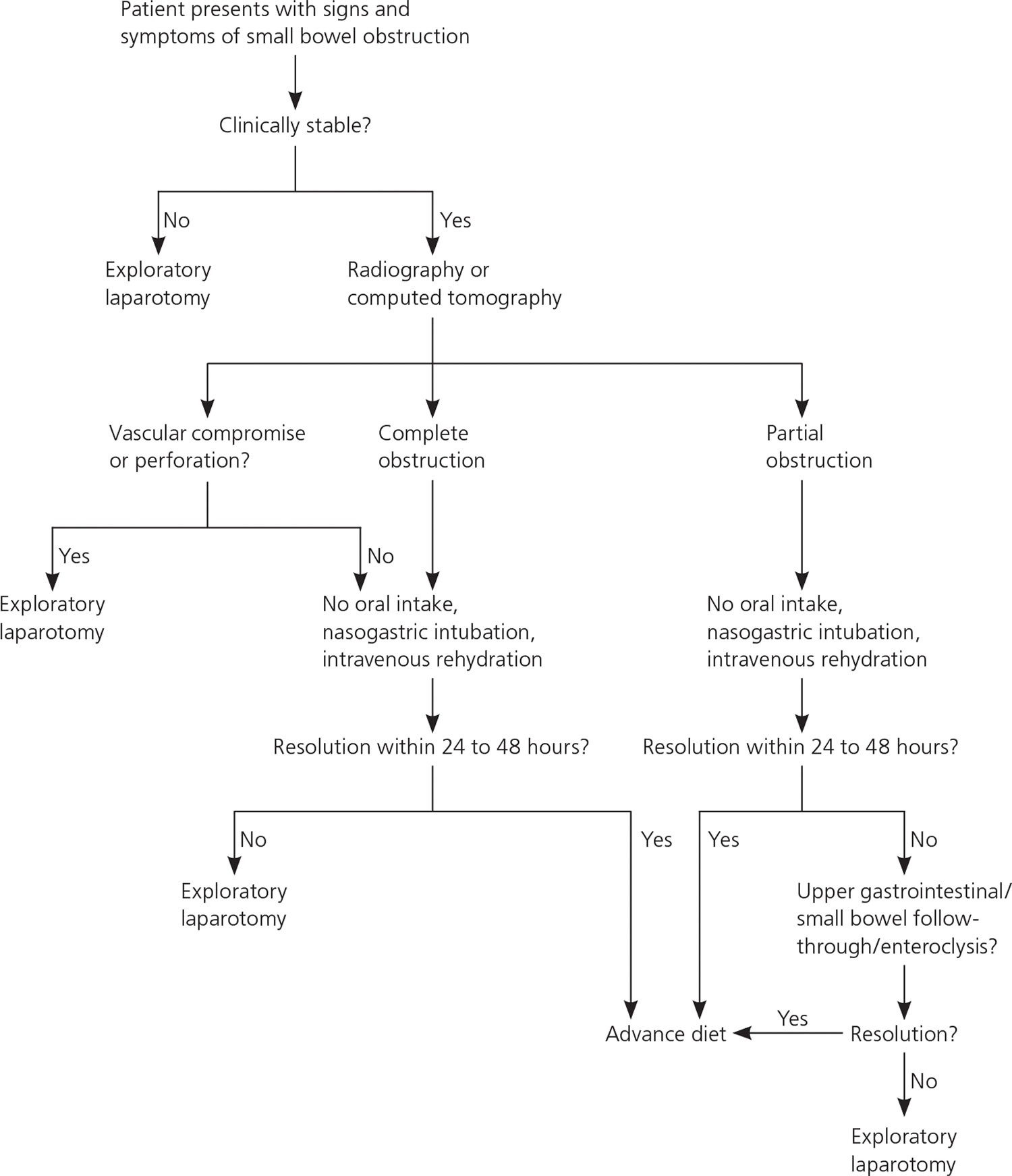 Table 4. Treatment strategies of small bowel obstruction
Table 4. Treatment strategies of small bowel obstruction
| Adhesive obstruction | |
| Partial small bowel obstruction: | Nonoperative management for 24–48 hrs; if no improvement, small bowel follow-through or CT to increase accuracy of diagnosis; laparotomy if radiologic evidence of high grade obstruction, or if clinical, laboratory, or radiologic evidence of strangulation. |
| Complete small bowel obstruction: | Early laparotomy |
| Neoplasm | |
| Primary: | Resection |
| Secondary: | Depending on clinical presentation: laparotomy with limited resection, bypass, gastrostomy, tube jejunostomy or percutaneous gastrostomy |
| Crohn’s disease | |
| Bowel resection | Patients with first time presentation or in the presence of a perforation, phlegmon, or multiple strictures in close proximity. |
| Stricturoplasty | In cases of fibrotic stricture, in the absence of a phlegmon, perforation, or fistula, in patients with previous bowel resections, multiple short strictures, strictures in skip lesions well proximal to resection margin in patients undergoing bowel resection, and duodenal strictures. |
| Gallstone ileus | |
| Relief of small bowel obstruction only |
|
| Relief of small bowel obstruction, cholecystectomy, division of fistula, and common bile duct exploration | |
| Choice based on general condition of patient, extent of inflammation in right upper quadrant, presence bowel perforation. | |
| Radiation enteritis | Bypass or resection depending on operative findings and extent of bowel involvement |
| Meckel’s diverticulum | Diverticulectomy with or without bowel resection depending on state of the diverticulum and adjacent ileum. |
| Intussusception | |
| Enteric |
|
| Ileocolic, colic | Resection |
| Bezoars |
|
| NSAID-stricture | Bowel resection, stricturoplasty, balloon dilation |
Hospitalization to stabilize your condition
Aggressive intravenous fluid therapy and correction of electrolyte imbalance are crucial in the initial management of acute small bowel obstruction. A Foley catheter and occasionally central venous or even a swan ganz catheter are needed to monitor fluid resuscitation. Blood tests identify electrolyte imbalance, elevated leukocyte count, abnormal liver function tests, elevated amylase level, acidosis, anemia, and bleeding tendency. A nasogastric tube allows decompression of the stomach and prevents aspiration. There is no convincing evidence that long intestinal tube is more efficacious than nasogastric tubes in decompression of small bowel obstruction. Plain radiograph of the abdomen is the initial diagnostic test and luminal contrasts tests are used selectively. The indications of CT scan are expanding and benefits of its early utilization are becoming more apparent 28. Repeated examination of the patient during this period of management cannot be overemphasized.
Emergency surgery is indicated in incarcerated external hernia and when there is clinical and radiologic evidence of strangulation, gangrene, or perforation. Otherwise, carefully monitored nonoperative treatment is indicated, at least initially, while specific imaging methods are utilized to identify specific etiology of small bowel obstruction or to monitor progression of small bowel obstruction.
Adhesive small bowel obstruction
With nonoperative treatment, complete small bowel obstruction resolves far less frequently than partial small bowel obstruction, 15–36% vs. 55–75% 25. Surgical intervention is indicated when strangulation is suspected to develop during nonoperative treatment, or when conservative treatment fails. The appearance of the bowel before and after release of adhesion is compared. Vascular compromise is recognized by bluish discoloration of intestinal wall, loss of arterial pulsation, subserosal and mesenteric hemorrhage, and lack of peristalsis. If the bowel loop pinks up, resection is avoided, otherwise resection is indicated.
To prevent subsequent adhesion formation various mechanical and chemical methods have been employed. Mechanical methods include plication (small bowel and mesenteric), and stenting with long intestinal tubes. In addition to failure to prevent re-obstruction, plication is time consuming and tedious, and carries the risk of injury to the bowel or mesenteric vessels. Similarly, long intestinal tubes, in addition to difficulty in positioning distal to ligament of Treitz, are not without complications, and long terms results are not adequately evaluated. Although high dose steroids with or without promethazine, antihistamines, and dextran-70 proved to reduce adhesion formation in animals, the potential for disastrous complications prevented their use in humans. A variety of other chemicals have been used to prevent adhesions with mixed results and associated significant complications. Sodium hyaluronatebased bioabsorbable membrane have been shown to reduce adhesion formation in human, but its effect on intestinal obstruction is yet to be determined 29.
Gallstone ileus
The diagnosis of gallstone ileus is often difficult to make 30. The majority of patients are elderly (average age between 65 and 75 years), and are multimorbid 31. Time from onset of symptoms to surgical intervention is often long and correct diagnosis is made preoperative only in 13–60% of cases. In the small bowel, the site of obstruction is usually the distal ileum, and multiple stones are present in 3–15%.
While extracorporeal shock wave lithotripsy is successful in fragmenting duodenal stone inpaction, treatment of small bowel inpaction is surgical, either enterolithotomy to relieve the obstruction, or one stage procedure i.e. relief of obstruction, cholecystectomy with closure of fistula with or without common bile duct exploration. Following enterolithotomy alone the risk of recurrent obstruction and incidence of cholangitis are low, 5% and 10% respectively. There is increased risk of carcinoma of the gallbladder and a 30% incidence of recurrent biliary pain. Simple enterolithotomy carries a mortality of 11.7% compared to 16.9% for one-stage procedure 32. The most common source of operative morbidity is wound infection occurring in 30–40% ranges from 11 to 75% of cases.
Relief of obstruction i.e. enterolithotomy alone is safe and effective in the treatment of gallstone ileus. The entire bowel and gall bladder are palpated for other gallstones to safe guard against recurrence. Interval cholecystectomy is performed for continued biliary symptoms. In select group of low risk patients, a onestage procedure is appropriate.
Crohn’s disease
Initial treatment with steroids and parenteral nutrition is successful for first time presentation or while patient is on no or minimal medications 33. Patients with recurring obstruction, especially with palpable mass, and while on adequate medical therapy, are candidates for earlier surgical intervention, namely resection. Stricturoplasty is a bowel preserving surgery indicated in a select group of patients.
Early post-operative obstruction
This is defined as small bowel obstruction within 30 days after celiotomy 34. In this clinical situation bowel activity may not return (prolonged ileus) or there is initial temporary return of bowel function. The obstruction is due to adhesions (92%), phlegmon or abscess, intussusception (2.5–4%), or internal hernia. The treatment is conservative in the absence of bowel ischemia or mechanical obstruction. Nasogastric decompression, intravenous fluid therapy, and even parenteral nutrition for up to 10-14 days is indicated if the patient is stable and exhibiting clinical and radiologic improvement continues. After this time further improvement is unlikely and operation should be performed.
Radiation enteritis
Radiation causes actinic damage to intestinal mucosa, connective tissue, and vessels. The small bowel is extremely sensitive to radiation damage. The disease has a progressive nature. The final stage of damage is abnormal bowel, perforation, or stricture formation. Patients become intestinal cripples due to chronic partial intestinal obstruction and malnutrition. In the chronic stage, the serosa of the bowel involved appears thickened, dull, and gray with decreased peristalsis. Normal tissue planes are obliterated, intestines are friable, and fibrosis may be extensive (frozen pelvis). Multiple adhesions exist between damaged loops of bowel and other organs. Local factors in the intestine and mesentery (vascular injury, interstitial infection, and scarring) prevent satisfactory healing and recovery from acute injury making surgical correction hazardous. Although chances of improvement with conservative treatment are high, the relief is not long lasting.
When surgery is indicated, manipulation of the bowel is kept to a minimum and attempts to dissect the damaged bowels loop that are glued together by serositis and fibrosis will result in bowel injury and spillage. Transition from diseased to normal bowel is gradual making it difficult to exclude actinic damage with the naked eye, and the circulation is marginal. The type of surgical management depends on findings at operation i.e. perforation vs. stricture, extent of tissue damage, frozen pelvis, etc. A bypass procedure is safe and effective except in patients with limited involvement of freely mobile bowel where resection is optimal.
Malignant small bowel obstruction
This refers to obstruction occurring after treatment of a primary malignancy. Obstruction is due to benign causes (adhesion, radiation enteritis, internal hernia) occurs in 18–38% of cases. Ten percent to 30% of patients will have relief of obstruction with nonoperative management alone, and about 40% will eventually require surgery. Resolution with nasograstric decompression occurs in 68% of cases and within 3 days. About 35–80% of patients will obtain relief of symptoms with surgery depending on nature of obstruction. Patients presenting in shock, with carcinomatosis, ascites, or palpable mass have a 54% to 100 % mortality. Patients with carcinoma of the ovary, for whom effective chemotherapy is available, have better than average outcome. Bowel strangulation rarely occurs when carcinomatosis is present.
Hence, patients with known cancers should be treated as any other patient presenting with small bowel obstruction, and final decision making regarding surgical intervention must be individualized. Early surgical intervention is indicated in patients with no known recurrence or long interval to the development of small bowel obstruction. In patients with carcinomatosis, ascites, or palpable masses, more prolonged course of nonoperative treatment is justifiable. Surgical intervention is indicated if nasogastric decompression fails or if re-obstruction develops after removal of nasogastric tube. Selection of surgical procedure, resection, bypass, gastrostomy, or tube jejunostomy is based on extent of the disease. Used selectively, percutaneous gastrostomy can improve quality of life.
Intussusception
In adults 85–90% of intussusceptions are associated with a discrete, pathologic process leading the intussusception, and neoplasms account for majority of cases. Malignant lesions are being recognized with increasing frequency. A recently recognized subtype is postoperative intussusception. The point of origin of the intussusception is the small bowel and more specifically, the jejunum, particularly proximal jejunum, and dense desmoplastic inflammatory reaction within the mesentery may be the underlying mechanism precipitating the intussusception.
Treatment of intussusception in adults is surgical without attempts at hydrostatic reduction. Optimal surgical procedure depends on the anatomic location, present of a lead point, and local factors, such as edema, inflammation, and ischemia of involved bowel. While resection is the treatment of colic andenterocolic intussusception, the choice in enteric type i.e. attempt at operative reduction vs. resection without attempt at reduction, depends on presence of underlying lesion, chances the lesion is malignant, and viability of involved bowel.
NSAIDS-induced small bowel obstruction
Chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a wide range of pathology in the stomach, small bowel and colon. In the small bowel NSAIDS induce enteropathy (asymptomatic in 60–70% of patients, or may be associated with low grade protein and blood loss), perforation, ulceration, and stricture formation 35. The strictures are multiple and appear broad based or diaphragm-like that narrow down the lumen to a pinhole resulting in subacute small bowel obstruction. Small intestinal diaphragms are difficult to diagnose. Radiologically nonsteroidal anti-inflammatory drug (NSAID) strictures mimic exaggerated plica circularis and at surgery are difficult to feel. Inflation of the bowel with air facilitates detection of these stricture. Once identified treatment is with intestinal resection or stricturoplasty although balloon dilatation has been reported to be effective.
While conventional laparotomy is routinely performed in the surgical management of the acute abdomen, the indication for laparoscopic approach are evolving 36. Criteria used for laparoscopic management of small bowel obstruction include mild abdominal distention, proximal small bowel obstruction, anticipated single band adhesion, and partial obstruction.
- Irvin TT. Abdominal pain: a surgical audit of 1190 emergency admissions. Br J Surg. 1989;76(11):1121–1125.[↩]
- Wright HK, O’Brien JJ, Tilson MD. Water absorption in experimental closed segment obstruction of the ileum in man. Am J Surg. 1971;121(1):96–99.[↩]
- Wangensteen OH. Understanding the bowel obstruction problem. Am J Surg. 1978;135(2):131–149.[↩]
- Rana SV, Bhardwaj SB. Small intestinal bacterial overgrowth. Scand J Gastroenterol. 2008;43(9):1030–1037.[↩]
- Shelton BK. Intestinal obstruction [published correction appears in AACN Clin Issues. 2000;11(1):following table of contents]. AACN Clin Issues. 1999;10(4):478–491.[↩]
- Cullen J J, Kelly K A. Current management of Meckel’s diverticulum. Advances Surg. (1996);9:207–214. https://www.ncbi.nlm.nih.gov/pubmed/8720004[↩][↩]
- Intestinal Pseudo-obstruction. https://www.niddk.nih.gov/health-information/digestive-diseases/intestinal-pseudo-obstruction[↩]
- Suri S, Gupta S, Sudhakar PJ, Venkataramu NK, Sood B, Wig JD. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40(4):422–428.[↩]
- Furukawa A, Yamasaki M, Furuichi K, et al. Helical CT in the diagnosis of small bowel obstruction. Radiographics. 2001;21(2):341–355.[↩]
- Ros PR, Huprich JE. ACR Appropriateness Criteria on suspected small-bowel obstruction. J Am Coll Radiol. 2006;3(11):838–841.[↩]
- Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1–33.[↩]
- Choi HK, Chu KW, Law WL. Therapeutic value of gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Ann Surg. 2002;236(1):1–6.[↩]
- Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev. 2007;(3):CD004651[↩]
- Dunn JT, Halls JM, Berne TV. Roentgenographic contrast studies in acute small-bowel obstruction. Arch Surg. 1984;119(11):1305–1308.[↩]
- Lim JH, Ko YT, Lee DH, Lee HW, Lim JW. Determining the site and causes of colonic obstruction with sonography. AJR Am J Roentgenol. 1994;163(5):1113–1117.[↩]
- Matsuoka H, Takahara T, Masaki T, Sugiyama M, Hachiya J, Atomi Y. Preoperative evaluation by magnetic resonance imaging in patients with bowel obstruction. Am J Surg. 2002;183(6):614–617.[↩]
- Wiarda BM, Horsthuis K, Dobben AC, et al. Magnetic resonance imaging of the small bowel with the true FISP sequence: intra- and interobserver agreement of enteroclysis and imaging without contrast material. Clin Imaging. 2009;33(4):267–273.[↩]
- Sagar PM, MacFie J, Sedman P, May J, Mancey-Jones B, Johnstone D. Intestinal obstruction promotes gut translocation of bacteria. Dis Colon Rectum. 1995;38(6):640–644.[↩]
- Williams SB, Greenspon J, Young HA, Orkin BA. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005;48(6):1140–1146.[↩]
- Cox MR, Gunn IF, Eastman MC, Hunt RF, Heinz AW. The safety and duration of non-operative treatment for adhesive small bowel obstruction. Aust N Z J Surg. 1993;63(5):367–371.[↩]
- Fevang BT, Jensen D, Svanes K, Viste A. Early operation or conservative management of patients with small bowel obstruction? Eur J Surg. 2002;168(8–9):475–481.[↩]
- Chen SC, Yen ZS, Lee CC, et al. Nonsurgical management of partial adhesive small-bowel obstruction with oral therapy: a randomized controlled trial. CMAJ. 2005;173(10):1165–1169.[↩]
- Sagar P M, MacFie J, Sedman P, May J. Intestinal obstruction promotes gut translocation of bacteria. Dis Colon Rect. (1995);38:640–644. https://www.ncbi.nlm.nih.gov/pubmed/7774478[↩]
- Kulaylat MN, Doerr RJ. Small bowel obstruction. In: Holzheimer RG, Mannick JA, editors. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt; 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6873/[↩][↩]
- Sossa J, Gardner B. Management of patients diagnosed as acute intestinal obstruction secondary to adhesions. Am Surg. (1993);59:125–128. https://www.ncbi.nlm.nih.gov/pubmed/8476142[↩][↩]
- Gupta H, Dupuy D E. Advances in imaging of the acute abdomen. Surg Clin North Am. (1997);77:1245–1262. https://www.ncbi.nlm.nih.gov/pubmed/9431338[↩]
- Megibow A J. Bowel obstruction: Evaluation with CT. Radiol Clin North Am. (1994);32:861– 870. https://www.ncbi.nlm.nih.gov/pubmed/8085000[↩]
- Frager D, Medwid S W, Baer J W, Mollinelli B, Friedman M. CT of small bowel obstruction: Value in establishing the diagnosis and determining the degree and cause. Am J Radiol. (1994);162:37–41. https://www.ncbi.nlm.nih.gov/pubmed/8273686[↩]
- Becker J M, Dayton M T, Fazio V W. et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioabsorbable membrane: A prospective, randomized, doubleblided multicenter study. J Am Coll Surg. (1996);183:297–306. https://www.ncbi.nlm.nih.gov/pubmed/8843257[↩]
- Reisner R M, Cohen J R. Gallstone ileus: A review of 1001 reported cases. Am Surg. (1994);60:441–446. https://www.ncbi.nlm.nih.gov/pubmed/8198337[↩]
- Rodriguez-Sanjuan J C, Casado F, Fernandez M J, Morales D J, Naranjo A. Cholecystectomy and fistula closure versus enterolithotomy alone in gallstone ileus. Br J Surg. (1997);84:634–637. https://www.ncbi.nlm.nih.gov/pubmed/9171749[↩]
- Zuegel N, Hehl A, Lindemann F, Witte J. Advantages of onestage repair in case of gallstone ileus. Hepato-Gastroenterol. (1997);44:59– 62. https://www.ncbi.nlm.nih.gov/pubmed/9058120[↩]
- Alexander-Williams J. Surgical management of small intestinal Crohn’s disease: Resection or stricturoplasty. Seminars Colon Rect Surg. (1994);5:193–198.[↩]
- Seror D, Feigin E, Szold A, Allweis T M, Carmon M, Nissan S, Fruend H R. How conservatively can postoperative small bowel obstruction be treated? Am J Surg. (1993);165:121–126. https://www.ncbi.nlm.nih.gov/pubmed/8418687[↩]
- Bjarnason I, Hayllar J, MacPherson A J, Russell A S. Side effects of nonsteroidal antiinflammatory drugs on the small and large intestine in humans. Gastroenterol. (1993);104:1832–1847. https://www.ncbi.nlm.nih.gov/pubmed/8500743[↩]
- Memon M A, Fitzgibbons R J. The role of minimal access surgery in the acute abdomen. Surg Clinics North Am. (1997);77:1333–1353. https://www.ncbi.nlm.nih.gov/pubmed/9431343[↩]