What is cassia
Cassia (Cinnamomum cassia Presl) commonly known as Chinese cassia or Chinese cinnamon, is one of several species of Cinnamomum from Lauraceae family used primarily for their aromatic bark. The dried stem bark of Cinnamomum cassia, i.e., cassia bark, is an important spice ingredient in food and cosmetic industry, in the United States, United Kingdom, and India, Chinese cassia is the most common type of cinnamon used. Cinnamomum cassia buds are also used as a spice, especially in India, and were once used by the ancient Romans. Cassia bark is also considered to have medicinal properties, such as antimicrobial 1, anti-cancer 2, 3, 4, anti-inflammatory 5 and anti-diabetic properties 6, 7. Cinnamomum cassia extract has anti-invasion and anti-migration effects of highly metastatic human lung carcinoma cells, while cinnamomum cassia extract exerts no cytotoxicity of normal human lung cell lines as a combination medication with adjuvant chemotherapy on human lung cancer patients 8. In addition, the methanol extract of Cinnamomum cassia twigs was found to possess tyrosinase inhibitory activity 9. Tyrosinase is known to be a key enzyme in melanin biosynthesis, involved in determining the color of mammalian skin and hair. Various dermatological disorders, such as melasma, age spots and sites of actinic damage, arise from the accumulation of an excessive level of epidermal pigmentation 10. The twigs of cassia is popularly used in China to treat inflammatory processes, pain, menstrual disorders, hypertension, fever etc 11.
Hyperuricemia is a metabolic disease characterized by elevated blood uric acid levels 12. Hyperuricemia results from increased production or impaired excretion of uric acid 13 and elevated uric acid levels cause accumulation of urate crystals in joints and the kidneys, leading to gout and gouty arthritis 14. It was also recently shown that hyperuricemia is associated with hyperlipidemia, hypertension, cardiovascular diseases, and diabetes 15. Therefore, uric acid level regulation may play an important role in the prevention and treatment of various diseases, including hyperuricemia. Uric acid is a final product of purine catabolism and is produced via the catalytic activities of xanthine oxidase, a rate-limiting enzyme responsible for converting hypoxanthine to xanthine, which is subsequently converted to uric acid 16.
It has been reported that Cinnamomum indicum flower and Cinnamomum cassia bark have served as the main ingredients in several prescriptions designed to treat hyperuricemia and gout in traditional Chinese medicine 17. According to a recent report, in a test tube study of traditional Chinese medicinal plants, methanol extracts of Cinnamomum indicum flower and Cinnamomum cassia bark inhibited xanthine oxidase activity 18, 19. Oil extracts from Cinnamomum indicum flower, Cinnamomum cassia bark, and Cinnamomum osmophloeum leaves reduced serum uric acid levels in potassium oxonate-induced hyperuricemic animals 20. These findings indicate that Cinnamomum indicum flower and Cinnamomum cassia bark have antihyperuricemic effects.
Cassia is an evergreen tree originating in southern China, and widely cultivated there and elsewhere in southern and eastern Asia (India, Indonesia, Laos, Malaysia, Taiwan, Thailand, and Vietnam) 1. The cassia tree grows to 10–15 m tall, with greyish bark and hard, elongated leaves that are 10–15 cm long and have a decidedly reddish color when young.
Chinese cassia is a close relative to Ceylon cinnamon (Cinnamomum verum), Saigon cinnamon (Cinnamomum loureiroi), also known as “Vietnamese cinnamon”, Indonesian cinnamon (Cinnamomum burmannii), also called “korintje”, and Malabar cinnamon (Cinnamomum citriodorum) from Sri Lanka. In “all five species”, the dried cassia bark is used as a spice. Chinese cassia’s flavor is less delicate than that of Ceylon cinnamon. Its bark is thicker, more difficult to crush, and has a rougher texture than that of Ceylon cinnamon.
Figure 1. Cassia bark (Cinnamomum cassia Presl)
Figure 2. Cinnamomum cassia tree
It is known that plant essential oil has various functional properties, such as a pleasant aroma, insect and animal repellant, as well as inhibitory effects against microorganisms. The essential oil from the relative cinnamon species, Cinnamomum zeylanicum Blume, has been reported to show anti-tyrosinase activity, and cinnamaldehyde was found to be mainly responsible for this inhibition effect 21. The Cinnamomum cassia essential oil) has hypouricemic 22 and antifungal activities 22. A number of tyrosinase inhibitors from both natural and synthetic sources that inhibited monophenolase, diphenolase or both of these activities have been identified to date 23. These tyrosinase inhibitors include plant polyphenols and aldehydes, fungal metabolites, derivatives of natural compounds, and synthetics origins. The tyrosinase inhibitory constituents of the essential oil extracted from Cinnamomum cassia 9 and relative cinnamon species, Cinnamomum zeylanicum 21 have been well documented. Cinnamaldehyde was found to be the major constitute of the essential oil. However, the inhibitory pattern of cinnamaldehyde isolated from Cinnamomum cassia 5, Cinnamomum zeylanicum 21, olive oil (Kubo and Kinst-Hori 1999) and the root of Pulsatilla cernua was rather controvesial. Cinnamaldehyde from Cinnamomum cassia was a competitive tyrosinase inhibitor whereas those from Cinnamomum zeylanicum, olive oil and and the root of Pulsatilla cernua was a noncompetitive tyrosinase inhibitor.
Cassia vs Cinnamon
Cinnamon is a spice obtained from the inner bark of several tree species from the genus Cinnamomum. Cinnamomum verum or Sri Lanka or Ceylon cinnamon is sometimes considered to be “true cinnamon”, but most cinnamon in international commerce is derived from related species, also referred to as “cassia” 24. In 2016, Indonesia and China produced 75% of the world’s supply of cinnamon.
Cinnamomum verum (formerly Cinnamomum zeylanicum), “true cinnamon” or Sri Lanka or Ceylon cinnamon, is a small evergreen tropical tree in the Lauraceae (laurel family) that originated in Sri Lanka and is one of several Cinnamomum species that produce the commercially important spice known as cinnamon. Although Cinnamomum cassia (Cinnamomum aromaticum), which is less expensive and has a stronger flavor, is often marketed as “cinnamon,” Cinnamomum verum is generally considered to have a more delicate flavor that is more suitable for desserts.
The names “cinnamon” and “cassia” cause considerable confusion, as they are often used interchangeably. In the U.S., the spice produced from the dried, ground bark of any of Cinnamomum species is referred to as “cinnamon,” without distinguishing among species. In addition, “cinnamon” may also refer to the spice obtained from the aromatic bark of an unrelated species, Canella winterana (in the Canellaceae). When the spice is sold in bark form, rather than ground, Cinnamomum verum (Sri Lanka or Ceylon cinnamon) can be distinguished from Cinnamomum cassia (Cinnamomum aromaticum) because it comes in tight rolls (quills) rather than in looser flakes with curled edges. It can be distinguished from the related Indonesian cinnamon (Cinnamomum burmanii) by the quills having many soft layers, which can easily be ground in a coffee grinder, as opposed neat quills composed of a single extremely hard layer.
The Cinnamomum verum tree grows to around 10 m (30 ft), and has leathery leaves, usually opposite, that are lanceolate to ovate, 11 to 16 cm (4.5 to 6.25 in) long, with pointed tips. The inconspicuous yellow flowers, which are tubular with 6 lobes, grow in panicles (clusters) that are as long as the leaves. The fruit is a small, fleshy berry, 1 to 1.5 cm (0.25 to 0.5 in) long, that ripens to black, partly surrounded by a cup-like perianth (developed from the outer parts of the flower).
The spice form of cinnamon is obtained by removing the outer bark of the tree, and scraping from it the inner bark, which is dried and ground into power. Cultivated trees may also be coppiced (cut back to encourage shoot development), so that the coppiced shoots can be harvested. Cinnamon oil is steam distilled from the leaves and twigs.
Cinnamon from various species has been used as a spice since ancient times. It is widely used to flavor baked goods, puddings and other desserts, and candies, as well as soups and stews, curries, meat and poultry dishes, and pickles. Cinnamon is also used to flavor beverages, including teas and mulled wine.
FAO estimates that total commercial production of all forms of cinnamon (derived from several species of Cinnamomum, including Cinnamomum cassia (Cinnamomum aromaticum), as well as canella (Canella winterana) was 155,000 metric tons, harvested from 186,000 hectares. China, Indonesia, Sri Lanka, and Vietnam together produced around 98% of the world’s total.
Cassia essential oil
The essential oil extracted by steam distillation from the stem bark of Cinnamomum cassia Presl was quantitatively analyzed using gas chromatography coupled with mass spectrometry 25. The 16 constituent compounds identified, along with the retention times and Kovats indices, are listed in Table 1. The results showed that the two major constituents of Cinnamomum cassia essential oil were cis-2-methoxycinnamic acid (43.06%) and cinnamaldehyde (42.37%) and that the minor compounds were o-methoxycinnamaldehyde (5.11%), 1,2-dimethoxy-4-(3-methoxy-1-propenyl) benzene (2.05%), cinnamyl acetate (1.83%) and other compounds (1.25~0.16%) in that study 25. For comparison, a previous study reported that cinnamaldehyde (92.2%) was the most plentiful constituent in the Cinnamomum cassia essential oil 26. Different extraction processes and assay methods could have contributed to differences in cinnamaldehyde levels of Cinnamomum cassia essential oils 27. Cinnamaldehyde (77.1%) was also found to be the major constituent of volatile oil of the bark of Cinnamomum zeylanicum 21. Several studies have demonstrated that cinnamaldehyde has antimicrobial 28, antimutagenic (Shaughnessy et al. 2001), antitumorigenic (Ka et al. 2003) and immunomodulatory activities (Koh et al. 1998); furthermore, cinnamaldehyde is considered to possess tyrosinase-inhibitory effects with IC50 values from 0.52~0.97 mM (Lee 2002; Lee et al. 2000; Ngoc et al. 2009). Lee (2002) reported that 2-methoxycinnamic acid that had been isolated from Pulsatilla cernua root was a potent noncompetitive inhibitor of mushroom tyrosinase with an IC50 value of 0.34 mM. In addition, benzaldehyde, one of the flavor compounds characterized in anise oil, showed potent tyrosinase inhibitory activity with an IC50 of 0.82 mM (Kubo and Kinst-Hori 1998). Because the Cinnamomum cassia essential oil contained major in cinnamaldehyde and cis-2-methoxycinnamic acid, and trace readings of benzaldehyde. Furthermore, cinnamic aldehyde, the major constituent of leaf essential oil from Cinnamomum cassia, has been demonstrated to exhibit antibacteria activities, anti-lipopolysaccharide-induced NF-κB transcriptional activities 29. Cinnamic aldehyde which has α, β unsaturated carbonyl moiety exerted suppressive effect on toll-like-receptor-4- (TLR4-) mediated signaling 30. Cinnamomum cassia essential oil could inhibit cell proliferation and induce apoptosis in human oral squamous cell carcinoma HSC-3 cells 31.
Coumarins posses strong anticoagulant properties and can have potentially toxic effects on the liver 32. The coumarin content in Ceylon cinnamon (“true cinnamon”) is negligible and is not known to cause detrimental health effects, whereas the coumarin level in Cinnamomum cassia is much higher and can cause health risks if consumed in larger quantities on a regular basis 33. Several countries have restricted the regular usage of Cinnamomum cassia as a result of this potential health hazard associated with high levels of coumarins 33.
Table 1. Analysis of the Cinnamomum cassia Presl essential oil
| No | Name | Rta | KIb | Area |
|---|---|---|---|---|
| 1 | Benzaldehyde | 7.58 | 982 | 0.42 |
| 2 | 2,2,4,6,6-Pentamethylheptane | 7.84 | 983 | 0.21 |
| 3 | 2,5,9-Trimethyldecane | 9.10 | 1121 | 0.49 |
| 4 | 2,5-Dimethylundecane | 9.49 | 1136 | 0.33 |
| 5 | Phenylethyl alcohol | 11.79 | 1175 | 0.29 |
| 6 | Cinnamaldehyde | 16.57 | 1189 | 42.37 |
| 7 | 3,4-Dimethoxyphenethyl alcohol | 17.78 | 1514 | 0.79 |
| 8 | Germacrene D | 18.97 | 1515 | 0.32 |
| 9 | cis-2-Methoxycinnamic acid | 19.69 | 1546 | 43.06 |
| 10 | Cinnamyl acetate | 20.88 | 1589 | 1.83 |
| 11 | Coumarin | 20.99 | 1623 | 1.25 |
| 12 | o-Methoxycinnamaldehyde | 22.70 | 1745 | 5.11 |
| 13 | trans-Caryophyllene | 22.95 | 1832 | 0.43 |
| 14 | 1,2-Dimethoxy-4-(3-methoxy-1-propenyl) benzene | 24.11 | 1946 | 2.05 |
| 15 | 2-Ethyl-5-propylphenol | 24.78 | 1993 | 0.21 |
| 16 | β-Phenethyl cinnamate | 32.01 | 2041 | 0.16 |
Note: aRetention time (min); bKovats index.
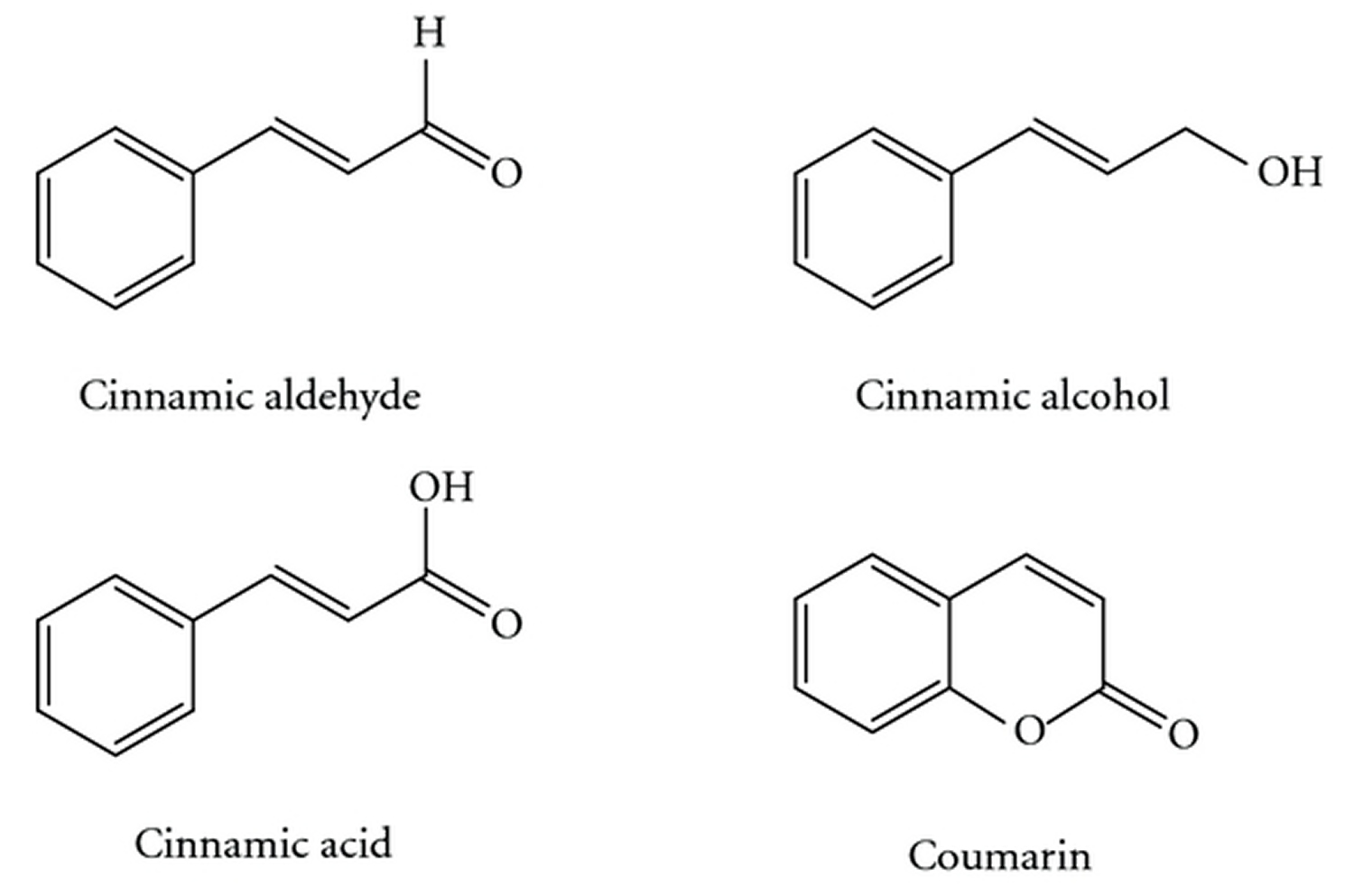
[Source 25]Figure 3. Cinnamomum cassia chemical compounds (cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin)
[Source 34]Cassia Oil Toxicity
Studies have shown that the median lethal dose value (LD50 – is the amount of the substance required (usually per body weight) to kill 50% of the test population) of orally administered cinnamon oil in mouse 2670mg/kg body weight 35, cinnamon oil in rats 2800mg/kg body weight 35, cinnamon oil on rabbit skin 320mg/kg body weight 35, cinnamon oil mouse intra-peritoneum 500mg/kg body weight 35, cinnamaldehyde in animals is 1850 ± 37 mg/kg body weight 36. TOXICITY RATINGS: 3 = MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 g/kg body weight; between 1 oz and 1 pt (or 1 lb) for 70 kg person (150 LB) 37. Previous studies on Cinnamomum cassia have not documented any probable adverse effects related to the regular use of cinnamon in humans, in spite of Cinnamomum cassia containing potentially hepatotoxic coumarin levels 38, 39.
In a 12-week rat feeding study on a mixture of cinnamyl compounds there was slight retardation of growth of males and lowering of food utilization in both sexes at 90 mg/kg body-weight/day. In another study lasting for 16 weeks groups of 10 male and 10 female rats were fed diets containing 0, 0.1, 0.25 and 1.0 per cent. of aldehyde. At the highest level there was slight swelling of hepatic cells and some hyperkeratosis of the epithelium of the forestomach 40.
A 7 1/2 yr old boy ingested 2 oz.(about 60 mL) of cinnamon oil and immediately felt a burning sensation in his mouth, chest, and stomach lasting for about 15 minutes 41. He then developed double vision, dizziness, vomiting, and collapse. Following ipecac and activated charcoal he developed diarrhea, more vomiting, dizziness, abdominal cramps, and burning in the rectal area. The white blood count was 29,800/cu mm. Gastrointestinal symptoms and sleepiness persisted for 5 hours 41.
Three cases of acute contact sensitivity to a new toothpaster (Close-Up). In all cases there was positive reaction to 0.5% cinnamon oil in petrolatum 42.
The European Food Safety Authority (EFSA) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) concludes that: cinnamaldehyde is safe at the maximum use level of 125 mg/kg complete feed for salmonids, veal calves and dogs, and at 25 mg/kg for the remaining target species 43.
- Growth-Inhibiting Effects of Cinnamomum cassia Bark-Derived Materials on Human Intestinal Bacteria. Lee HS, Ahn YJ. J Agric Food Chem. 1998 Jan 19; 46(1):8-12. https://www.ncbi.nlm.nih.gov/pubmed/10554188/[↩][↩]
- Park GH, Song HM, Park SB, et al. Cytotoxic activity of the twigs of Cinnamomum cassia through the suppression of cell proliferation and the induction of apoptosis in human colorectal cancer cells. BMC Complementary and Alternative Medicine. 2018;18:28. doi:10.1186/s12906-018-2096-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5858136/[↩]
- Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, Lee KT. Cancer Lett. 2003 Jul 10; 196(2):143-52. https://www.ncbi.nlm.nih.gov/pubmed/12860272/[↩]
- Extract prepared from the bark of Cinnamomum cassia Blume prevents glutamate-induced neuronal death in cultured cerebellar granule cells. Shimada Y, Goto H, Kogure T, Kohta K, Shintani T, Itoh T, Terasawa K. Phytother Res. 2000 Sep; 14(6):466-8. https://www.ncbi.nlm.nih.gov/pubmed/10960905/[↩]
- Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. Lee HS, Kim BS, Kim MK. J Agric Food Chem. 2002 Dec 18; 50(26):7700-3. https://www.ncbi.nlm.nih.gov/pubmed/12475291/[↩][↩]
- Khan A., Safdar M., Ali Khan M. M., Khattak K. N., Anderson R. A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. http://care.diabetesjournals.org/content/26/12/3215.long[↩]
- Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Verspohl EJ, Bauer K, Neddermann E. Phytother Res. 2005 Mar; 19(3):203-6. https://www.ncbi.nlm.nih.gov/pubmed/15934022/[↩]
- Wu H-C, Horng C-T, Lee Y-L, et al. Cinnamomum Cassia Extracts Suppress Human Lung Cancer Cells Invasion by Reducing u-PA/MMP Expression through the FAK to ERK Pathways. International Journal of Medical Sciences. 2018;15(2):115-123. doi:10.7150/ijms.22293. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5765724/[↩]
- Tyrosinase-inhibitory constituents from the twigs of Cinnamomum cassia. Ngoc TM, Lee I, Ha do T, Kim H, Min B, Bae K. J Nat Prod. 2009 Jun; 72(6):1205-8. https://www.ncbi.nlm.nih.gov/pubmed/19555125/[↩][↩]
- Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Kim YJ, Uyama H. Cell Mol Life Sci. 2005 Aug; 62(15):1707-23. https://link.springer.com/article/10.1007%2Fs00018-005-5054-y[↩]
- The essential oil from the twigs of Cinnamomum cassia Presl alleviates pain and inflammation in mice. J Ethnopharmacol. 2016 Dec 24;194:904-912. doi: 10.1016/j.jep.2016.10.064. Epub 2016 Oct 22. https://www.sciencedirect.com/science/article/pii/S0378874116314921[↩]
- Boffetta P., Nordenvall C., Nyren O., Ye W. A prospective study of gout and cancer. European Journal of Cancer Prevention. 2009;18(2):127–132. doi: 10.1097/CEJ.0b013e328313631a https://www.ncbi.nlm.nih.gov/pubmed/19337060[↩]
- Choi H. K., Mount D. B., Reginato A. M. American physiological, S., pathogenesis of gout. Annals of Internal Medicine. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009 https://www.ncbi.nlm.nih.gov/pubmed/16204163[↩]
- Huang J., Wang S., Zhu M., Chen J., Zhu X. Effects of genistein, apigenin, quercetin, rutin and astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food and Chemical Toxicology. 2011;49(9):1943–1947. doi: 10.1016/j.fct.2011.04.029 https://www.ncbi.nlm.nih.gov/pubmed/21600261[↩]
- Dalbeth N., So A. Hyperuricaemia and gout: State of the art and future perspectives. Annals of the Rheumatic Diseases. 2010;69(10):1738–1743. doi: 10.1136/ard.2010.136218 https://www.ncbi.nlm.nih.gov/pubmed/20858623[↩]
- Fukunari A., Okamoto K., Nishino T., et al. Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: a potent xanthine oxidoreductase inhibitor with hepatic excretion. Journal of Pharmacology and Experimental Therapeutics. 2004;311(2):519–528. doi: 10.1124/jpet.104.070433 http://jpet.aspetjournals.org/content/311/2/519.long[↩]
- Zhao X., Zhu J. X., Mo S. F., Pan Y., Kong L. D. Effects of cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. Journal of Ethnopharmacology. 2006;103(3):357–365. doi: 10.1016/j.jep.2005.08.040 https://www.ncbi.nlm.nih.gov/pubmed/16182482[↩]
- Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Ngoc TM, Khoi NM, Ha do T, Nhiem NX, Tai BH, Don DV, Luong HV, Son DC, Bae K. Bioorg Med Chem Lett. 2012 Jul 15; 22(14):4625-8. https://www.ncbi.nlm.nih.gov/pubmed/22677314/[↩]
- Kong L. D., Cai Y., Huang W. W., Cheng C. H. K., Tan R. X. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. Journal of Ethnopharmacology. 2000;73(1-2):199–207. doi: 10.1016/s0378-8741(00)00305-6 https://www.ncbi.nlm.nih.gov/pubmed/11025157[↩]
- Honda S., Kawamoto S., Tanaka H., et al. Administered chrysanthemum flower oil attenuates hyperuricemia: mechanism of action as revealed by DNA microarray analysis. Bioscience, Biotechnology and Biochemistry. 2014;78(4):655–661. doi: 10.1080/09168451.2014.890028 https://www.ncbi.nlm.nih.gov/pubmed/25036964[↩]
- Supercritical CO2 extract of Cinnamomum zeylanicum: chemical characterization and antityrosinase activity. Marongiu B, Piras A, Porcedda S, Tuveri E, Sanjust E, Meli M, Sollai F, Zucca P, Rescigno A. J Agric Food Chem. 2007 Nov 28; 55(24):10022-7. https://www.ncbi.nlm.nih.gov/pubmed/17966976/[↩][↩][↩][↩]
- Effects of cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. Zhao X, Zhu JX, Mo SF, Pan Y, Kong LD. J Ethnopharmacol. 2006 Feb 20; 103(3):357-65. https://www.ncbi.nlm.nih.gov/pubmed/16182482/[↩][↩]
- Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Kim YJ, Uyama H. Cell Mol Life Sci. 2005 Aug; 62(15):1707-23. https://www.ncbi.nlm.nih.gov/pubmed/15968468/[↩]
- Cinnamomum verum. http://www.eol.org/pages/490672/overview[↩]
- Chang C-T, Chang W-L, Hsu J-C, Shih Y, Chou S-T. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Botanical Studies. 2013;54:10. doi:10.1186/1999-3110-54-10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5432840/[↩][↩][↩]
- Potentiation of antifungal activity of amphotericin B by essential oil from Cinnamomum cassia. Giordani R, Regli P, Kaloustian J, Portugal H. Phytother Res. 2006 Jan; 20(1):58-61. https://www.ncbi.nlm.nih.gov/pubmed/16397923/[↩]
- From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. Dugoua JJ, Seely D, Perri D, Cooley K, Forelli T, Mills E, Koren G. Can J Physiol Pharmacol. 2007 Sep; 85(9):837-47. https://www.ncbi.nlm.nih.gov/pubmed/18066129/[↩]
- Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE. Am J Chin Med. 2006; 34(3):511-22. https://www.ncbi.nlm.nih.gov/pubmed/16710900/[↩]
- Huang B, Yuan HD, Kim DY, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. Journal of Agricultural and Food Chemistry. 2011;59(8):3666–3673 https://www.ncbi.nlm.nih.gov/pubmed/21401097[↩]
- Youn HS, Lee JK, Choi YJ, et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochemical Pharmacology. 2008;75(2):494–502. https://www.ncbi.nlm.nih.gov/pubmed/17920563[↩]
- Cinnamomum cassia essential oil and its major constituent cinnamaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Chang WL, Cheng FC, Wang SP, Chou ST, Shih Y. Environ Toxicol. 2017 Feb; 32(2):456-468. https://www.ncbi.nlm.nih.gov/pubmed/26919256/[↩]
- Coumarin-induced hepatic necrosis. Ghosh P, Markin RS, Sorrell MF. Am J Gastroenterol. 1997 Feb; 92(2):348-9. https://www.ncbi.nlm.nih.gov/pubmed/9040223/[↩]
- Coumarin and cinnamaldehyde in cinnamon marketed in Italy: a natural chemical hazard? Lungarini S, Aureli F, Coni E. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008 Nov; 25(11):1297-305. https://www.ncbi.nlm.nih.gov/pubmed/19680836/[↩][↩]
- Liao J-C, Deng J-S, Chiu C-S, et al. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo . Evidence-based Complementary and Alternative Medicine : eCAM. 2012;2012:429320. doi:10.1155/2012/429320. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3318905/[↩]
- Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 680[↩][↩][↩][↩]
- Subash Babu P, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde—a potential antidiabetic agent. Phytomedicine. 2007;14(1):15–22. doi: 10.1016/j.phymed.2006.11.005. https://www.ncbi.nlm.nih.gov/pubmed/17140783[↩]
- Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-39[↩]
- Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–8. doi: 10.2337/diacare.26.12.3215 https://www.ncbi.nlm.nih.gov/pubmed/14633804[↩]
- Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22(5):507–12. doi: 10.3122/jabfm.2009.05.080093 http://www.jabfm.org/content/22/5/507.long[↩]
- Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W. I., Taylor, J. M., Long., E. L., Nelson, A. A. & Brouwer, J. B. (1967) Fd Cosmet. Toxicol., 5, (2), 141[↩]
- Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1872[↩][↩]
- MILLARD LG; BR MED J 1(5854) 676, 1973[↩]
- Safety and efficacy of aryl-substituted primary alcohol, aldehyde, acid, ester and acetal derivatives belonging to chemical group 22 when used as flavourings for all animal species. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2017.4672[↩]