Contents
What is Diamond Blackfan anemia
Diamond-Blackfan anemia is a rare inherited blood disorder that is characterized by a failure of the bone marrow to produce red blood cells 1. This failure causes Diamond-Blackfan anemia patients to become severely anemic. Symptoms may include a shortage of red blood cells (anemia), physical abnormalities such as small head size (microcephaly) characteristic facial features, cleft palate, cleft lip, short and webbed neck, small shoulder blades, and defects of the hands (mostly of the thumbs), as well as defects of the genitalia, urinary tract, eyes and heart. In some cases there is also short stature.
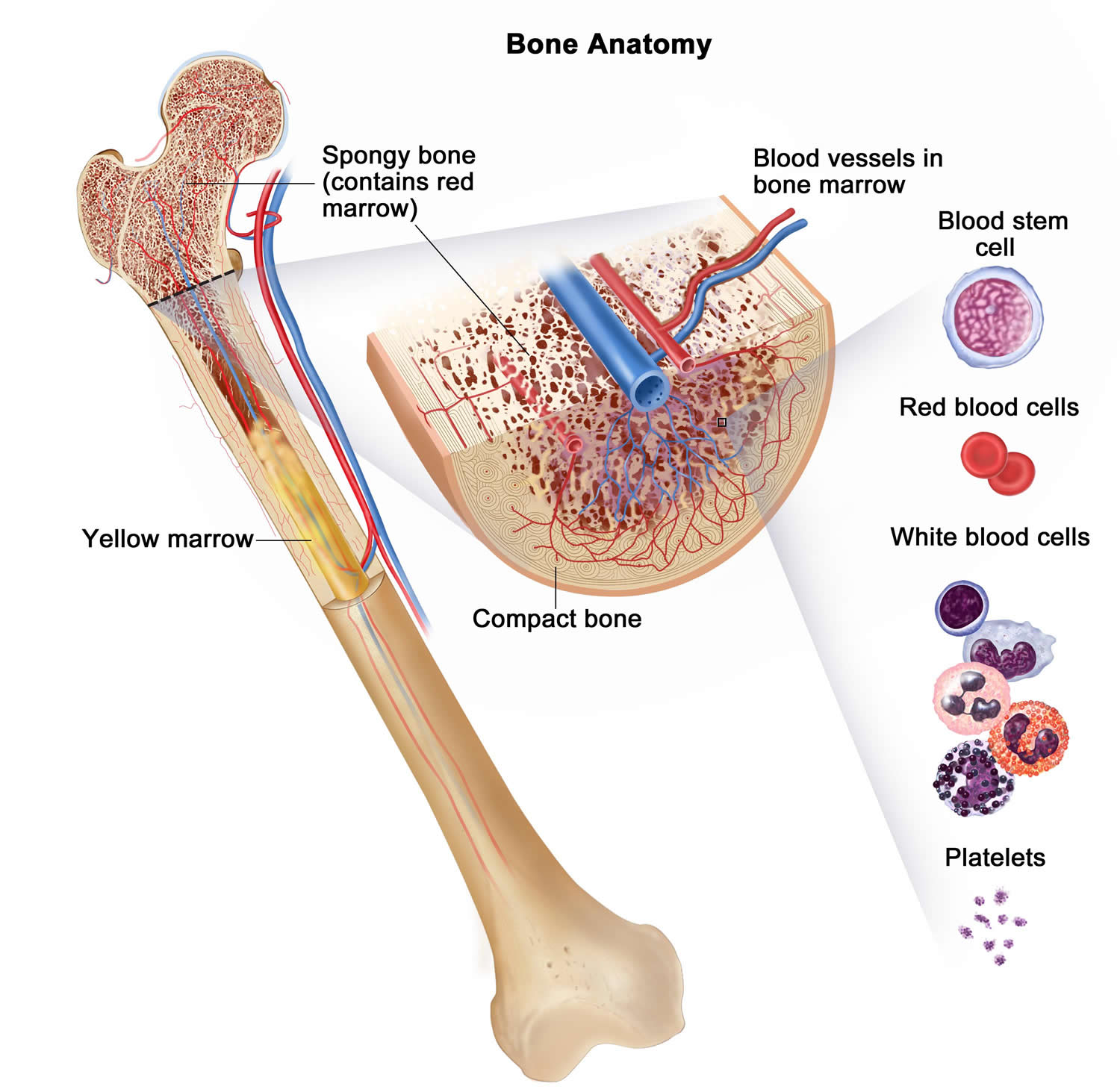
The major function of bone marrow is to produce new blood cells. In Diamond-Blackfan anemia, the bone marrow malfunctions and fails to make enough red blood cells, which carry oxygen to the body’s tissues. The resulting shortage of red blood cells (anemia) usually becomes apparent during the first year of life. Symptoms of anemia include fatigue, weakness, and an abnormally pale appearance (pallor).
The Diamond-Blackfan anemia was named for Dr. Louis K. Diamond and Dr. Kenneth D. Blackfan, the first doctors who documented cases of the disease in the 1930s.
Diamond-Blackfan anemia affects approximately 5 to 7 per million liveborn infants worldwide. There are about 25-35 new cases of Diamond-Blackfan anemia per year in the United States and Canada. Diamond-Blackfan anemia affects both boys and girls equally. It occurs in every ethnic group. Children usually appear to first be affected at 2 months of age with a range from birth to 6 years, although a few adults have been diagnosed. More than 90% of the patients present during the first year of life. The diagnosis is generally made at 12 weeks, or 3 months, of age with a range from birth to adulthood.
People with Diamond-Blackfan anemia have an increased risk of several serious complications related to their malfunctioning bone marrow. Specifically, they have a higher-than-average chance of developing myelodysplastic syndrome (MDS), which is a disorder in which immature blood cells fail to develop normally. Affected individuals also have an increased risk of developing certain cancers, including a cancer of blood-forming tissue known as acute myeloid leukemia (AML) and a type of bone cancer called osteosarcoma.
Approximately half of individuals with Diamond-Blackfan anemia have physical abnormalities. They may have an unusually small head size (microcephaly) and a low frontal hairline, along with distinctive facial features such as wide-set eyes (hypertelorism); droopy eyelids (ptosis); a broad, flat bridge of the nose; small, low-set ears; and a small lower jaw (micrognathia). Affected individuals may also have an opening in the roof of the mouth (cleft palate) with or without a split in the upper lip (cleft lip). They may have a short, webbed neck; shoulder blades which are smaller and higher than usual; and abnormalities of their hands, most commonly malformed or absent thumbs. About one-third of affected individuals have slow growth leading to short stature.
Other features of Diamond-Blackfan anemia may include eye problems such as clouding of the lens of the eyes (cataracts), increased pressure in the eyes (glaucoma), or eyes that do not look in the same direction (strabismus). Affected individuals may also have kidney abnormalities; structural defects of the heart; and, in males, the opening of the urethra on the underside of the penis (hypospadias).
The severity of Diamond-Blackfan anemia may vary, even within the same family. Increasingly, individuals with “non-classical” Diamond-Blackfan anemia have been identified. This form of the disorder typically has less severe symptoms that may include mild anemia beginning in adulthood.
Other Names for Diamond-Blackfan anemia
- Aase-Smith syndrome II
- Aase syndrome
- BDA
- BDS
- Blackfan-Diamond disease
- Blackfan-Diamond syndrome
- chronic congenital agenerative anemia
- congenital erythroid hypoplastic anemia
- congenital hypoplastic anemia of Blackfan and Diamond
- congenital pure red cell anemia
- congenital pure red cell aplasia
- DBA
- erythrogenesis imperfecta
- hypoplastic congenital anemia
- inherited erythroblastopenia
- pure hereditary red cell aplasia
Figure 1. Bone marrow anatomy
Diamond-Blackfan anemia genetic changes
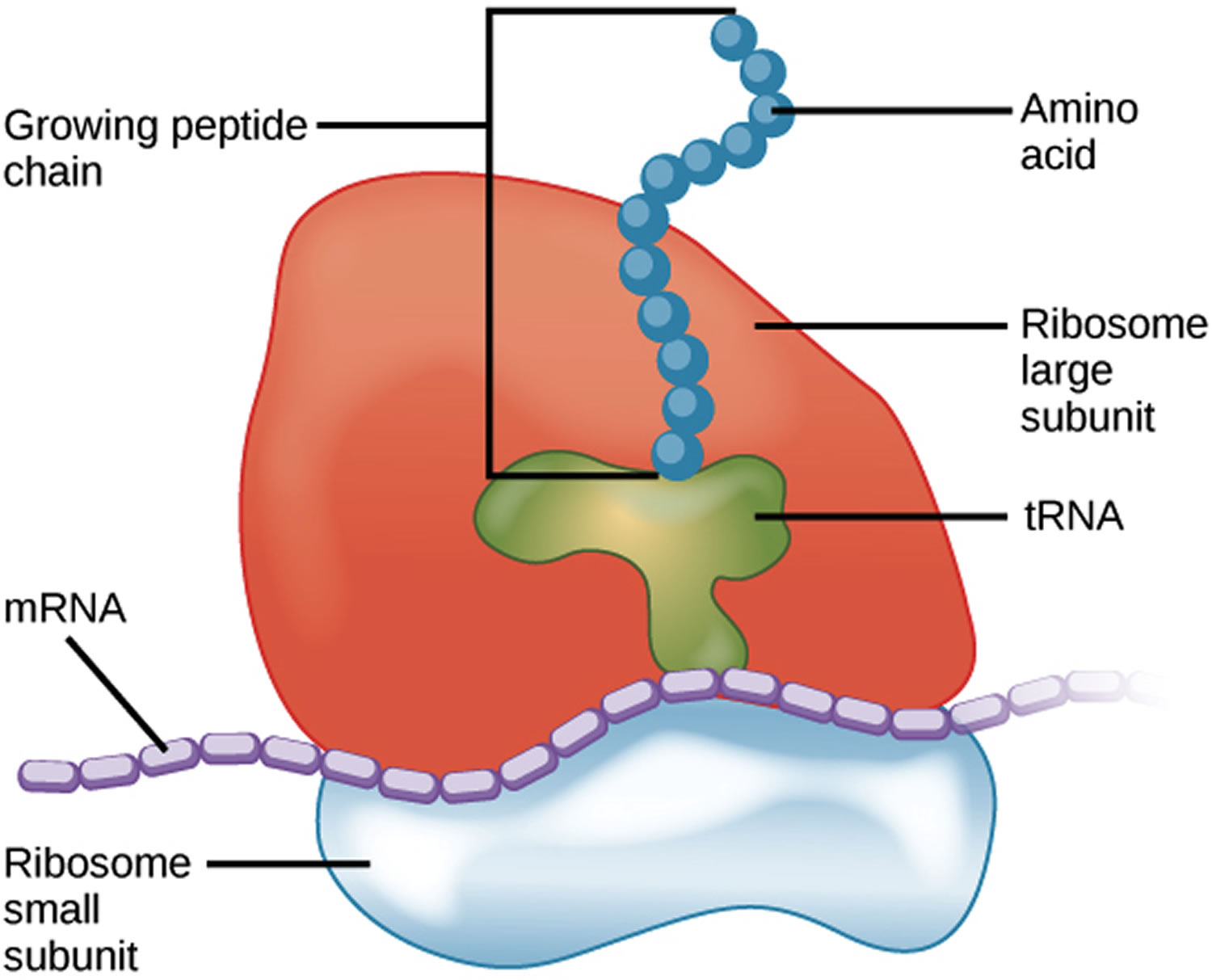
Diamond-Blackfan anemia is caused by mutations in several genes, some of which have been identified and some of which have not. Identified genes include but are not limited to: RPS19, RPL5, RPS10, RPL11, RPL35A, RPS7, RPS17, RPS24, RPS26 and GATA1 genes. These genes provide instructions for making several of the approximately 80 different ribosomal proteins, which are components of cellular structures called ribosomes. Ribosomes process the cell’s genetic instructions to create proteins.
Each ribosome is made up of two parts (subunits) called the large and small subunits. The RPL5, RPL11, and RPL35A genes provide instructions for making ribosomal proteins that are among those found in the large subunit. The ribosomal proteins produced from the RPS7, RPS10, RPS17, RPS19, RPS24, and RPS26 genes are among those found in the small subunit.
Figure 2. Ribosome
The specific functions of each ribosomal protein within these subunits are unclear. Some ribosomal proteins are involved in the assembly or stability of ribosomes. Others help carry out the ribosome’s main function of building new proteins. Studies suggest that some ribosomal proteins may have other functions, such as participating in chemical signaling pathways within the cell, regulating cell division, and controlling the self-destruction of cells (apoptosis).
Mutations in any of the genes listed above are believed to affect the stability or function of the ribosomal proteins. Studies indicate that a shortage of functioning ribosomal proteins may increase the self-destruction of blood-forming cells in the bone marrow, resulting in anemia. Abnormal regulation of cell division or inappropriate triggering of apoptosis may contribute to the other health problems that affect some people with Diamond-Blackfan anemia.
Approximately 25 percent of individuals with Diamond-Blackfan anemia have identified mutations in the RPS19 gene. About another 25 to 35 percent of individuals with this disorder have identified mutations in the RPL5, RPL11, RPL35A, RPS7, RPS10, RPS17, RPS24, or RPS26 genes. In the remaining 40 to 50 percent of cases, the cause of the condition is unknown. Researchers suspect that other genes may also be associated with Diamond-Blackfan anemia.
Different subtypes exist and are divided based on the specific gene mutated; however, they have similar features. Patients with mutations in the RPL5 gene have more serious symptoms and about 45% have cleft palate and are smaller than average size. Patients with mutations in the RPL11 gene have thumb anomalies more frequently than people with the other types. Mutations in the GATA1 gene are associated with severe anemia 2. Most cases are isolated, but about 45% of people with Diamond-Blackfan anemia inherit this condition from a parent. Inheritance is typically autosomal dominant , but can rarely be X-linked 2.
According to the mutated gene people may have some differences in their symptoms 3:
- People who have mutation in the RPL5 gene appear to have more severe problems than people with mutations in the RPL11 and RPS19 genes.
- People with mutations in the RPL5 gene have more chances of having cleft lip and/or cleft palate defects.
- People with mutations in the RPL11 gene have more thumb abnormalities
- People with mutations in the GATA1 gene may have a more severe anemia.
In about 30% of people diagnosed with Diamond-Blackfan anemia no mutation is found in any of the known DBA-linked genes 4.
Diamond-Blackfan anemia Inheritance Pattern
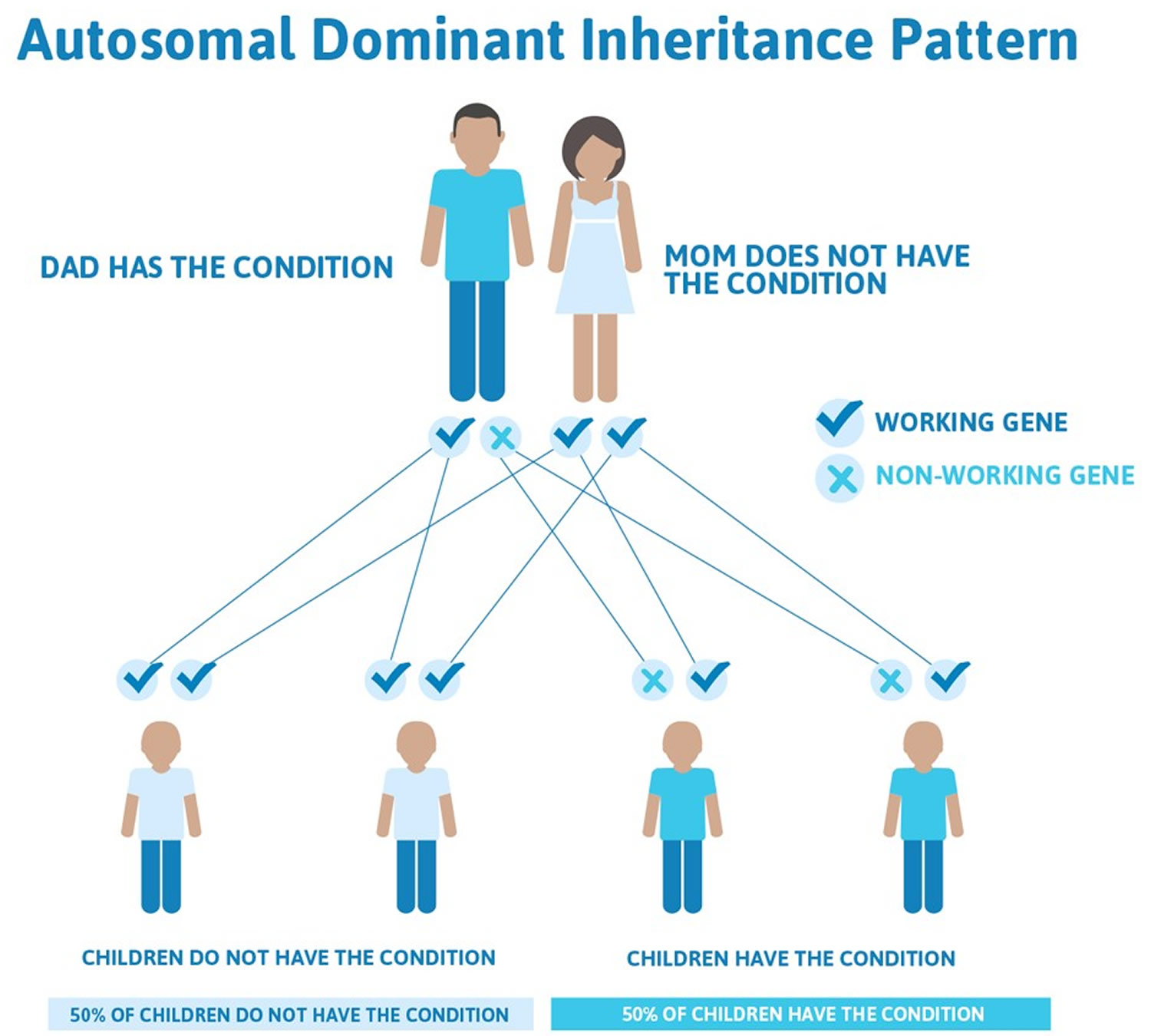
Diamond-Blackfan anemia is most commonly inherited in an autosomal dominant manner. This means that to be affected, a person only needs a change (mutation) in one copy of the mutated gene in each cell to cause the disorder. A person with Diamond-Blackfan anemia has a 50% chance with each pregnancy of passing along the mutated gene to his or her child.
In approximately 45% of affected people have inherited the mutation from a parent and about 55% have a new (de novo) mutation, where the anemia appears for the first time in the family and there are not other cases in the family. People with Diamond-Blackfan anemia may not appear to have a family history of the condition if relatives have very mild signs and symptoms 2.
In rare cases, when caused by mutations in the GATA1 and in the TSR2 gene, Diamond-Blackfan anemia can be inherited in an X-linked manner. In these cases, a if a man have a mutated copy of one of these genes he will be affected; a woman who have an abnormal copy is known as “carrier” but do not have the disease. Carries have a 50% chance of transmitting the mutated copy to each of her daughters or sons in each pregnancy: All of her sons who inherit the mutated copy will have the disease and all her daughters with the mutated copy will be carriers 2.
Figure 3. Diamond-Blackfan anemia inheritance pattern
Diamond-Blackfan anemia diagnosis
Making a diagnosis for a genetic or rare disease can often be challenging. Healthcare professionals typically look at a person’s medical history, symptoms, physical exam, and laboratory test results in order to make a diagnosis. The following resources provide information relating to diagnosis and testing for this condition. If you have questions about getting a diagnosis, you should contact a healthcare professional.
Several tests may be used to tell if a person has Diamond-Blackfan anemia. One test your doctor can perform is called a bone marrow aspirate. This is where a needle is inserted into the bone and a small amount of bone marrow fluid is taken out and studied under a microscope. You may also have blood tests to see if there is a genetic basis for Diamond-Blackfan anemia or certain chemical abnormalities linked to Diamond-Blackfan anemia.
Diamond-Blackfan anemia life expectancy
A “remission” is defined as a stable hemoglobin adequate for age, maintained for at least six months, without any corticosteroids, transfusions, or other therapy.
Approximately 20% of those affected with Diamond-Blackfan anemia have a chance of going into spontaneous remission, with 77% of these patients remitting during the first decade of life. Many of these patients have sustained remissions.
It is possible to go into and out of remission at any point of your life.
Remissions can occur following both steroid and/or transfusion therapies. Diamond-Blackfan anemia patients who are in remission are able to maintain acceptable hemoglobins without steroids and/or transfusions.
Cancer Epidemiology
The DBAR collaborated with the National Cancer Institute to confirm Diamond-Blackfan anemia as a cancer predisposition syndrome with a cumulative incidence of cancer in 22% of patients by age 46 years. A review of the literature reports cases of leukemia and solid tumors in Diamond-Blackfan anemia patients. One important feature of Diamond-Blackfan anemia -associated cancers is that they present at a younger age than these cancers are usually found. Thus careful analysis of DBA patients and their families is essential to defining the cancer risk in this population.
The following cancers have been observed in patients that were enrolled in the DBAR 5:
- Myelodysplastic Syndrome (ages 2y, 17y, 45y, 51y)
- Osteogenic Sarcoma (ages 4y, 13y, 22y)
- Soft Tissue Sarcoma (age 30y)
- Acute Myelogenous Leukemia (age 44y, 45y)
- Breast Cancer (ages 34y, 43y)
- Colon Cancer (ages 34y, 43y,49y)
- Oral Squamous Cell Carcinoma (age 69y)
- Vaginal Squamous Cell Carcinoma (age 45y)
- Rectal Cancer (age 28y)
- Uterine Cancer (age 64y)
- Cervical Cancer (age 27y)
- Testicular Cancer (age 62y)
- Lung Cancer (age 21y)
- Melanoma (age 50y)
- Non Hodgkin Lymphoma (age 41y)
- Basal Cell Cancer (age 30y)
Diamond-Blackfan anemia signs and symptoms
People with Diamond-Blackfan anemia have symptoms common to all other types of anemia, including pale skin, sleepiness, rapid heartbeat, and heart murmurs. In some cases there are no obvious physical signs of Diamond-Blackfan anemia. About one-quarter of people with Diamond-Blackfan anemia have abnormal features involving the face, head, and hands, especially the thumbs. They may also have heart and kidney defects. Many children are short for their age and may start puberty later than normal.
Diamond-Blackfan anemia treatment
Some people have such mild signs and symptoms that they do not require treatment.
To treat very low red blood cell counts in Diamond-Blackfan anemia patients, the two common options for treating Diamond-Blackfan anemia are corticosteroids and blood transfusions. Bone marrow/stem cell transplantation may also be considered. Some children need no specific therapy. Your doctor will recommend the best treatment for you.
In people who require treatment it may include:
- Corticosteroids: Corticosteroid treatment is recommended in children over 1 year of age; this treatment can initially improve the red blood count in approximately 80% of people with Diamond-Blackfan anemia. Prednisone initial dose is 2 mg / kg / day given orally once a day, at morning time. After a month, if there is no improvment after a month the corticosteroids are tapered-of and suspended
- Blood transfusions, which are given along with the corticosteroids or in people who do not get better with corticosteroids
- Bone marrow/stem cell transplantation: It is the only curative treatment for the anemia; however, patients should continue to be followed because they are at increased risk for leukemia and cancer. Results are better for children younger than ten years of age if transplanted using an Human Leukocyte Antigen (HLA)-matched sibling.
What is corticosteroid treatment?
Corticosteroids are drugs used to treat many medical conditions. One type of corticosteroid is called oral prednisone, one of the most successful treatments for children with Diamond-Blackfan anemia.
Side effects of corticosteroid treatment
Major side effects when these drugs are used in high doses for a long time include weight gain, water and salt retention, high blood pressure, muscle weakness, osteoporosis (brittle bones occasionally leading to fractures), wounds that won’t heal, headaches, growth problems, eye diseases such as cataracts and glaucoma, and the disruption of hormones that regulate normal body functions, including diabetes. Patients on these drugs should be watched carefully.
What is a blood transfusion?
In a blood transfusion, a person receives healthy red blood cells from another person. Transfusions may be needed every 3-5 weeks.
Do blood transfusions have any complications?
Sometimes patients can develop transfusion reactions with fever and rash. Medication may be given before the next transfusion to help prevent these symptoms. Red cell transfusions can also cause a build-up of extra iron in the body which can harm the heart and/or liver, cause diabetes, or slow down normal growth. The amount of iron must be regularly checked. If iron levels are too high, your doctor may recommend drugs to remove excess iron in body tissues. This process is called chelation therapy. People getting transfusions should avoid iron supplements.
What are bone marrow transplantation and peripheral blood stem cell transplantation?
Bone marrow/stem cell transplantation replaces a patient’s bone marrow/stem cells with those from a healthy, matching donor.
Bone marrow transplantation (BMT) and peripheral blood stem cell transplantation are procedures that restore stem cells that have been destroyed by high doses of chemotherapy and/or radiation therapy. There are three types of transplants:
- In autologous transplants, patients receive their own stem cells.
- In syngeneic transplants, patients receive stem cells from their identical twin.
- In allogeneic transplants, patients receive stem cells from their brother, sister, or parent. A person who is not related to the patient (an unrelated donor) also may be used.
Stem cell transplantation (SCT), also known as bone marrow or cord blood or peripheral blood stem cell transplantation (depending on the donor source), is curative in Diamond-Blackfan anemia. However, the role of transplantation for patients with Diamond-Blackfan anemia remains complex and controversial. As of the last published analysis, most of the sibling transplants used chemotherapy alone as a conditioning regimen, while most of the alternative donor (mismatched family or unrelated donor) transplants used a combination of chemotherapy with radiation therapy for pre-transplant conditioning. Data from the DBAR show overall survival of 77% for allogeneic sibling stem cell transplantation (94% for allogeneic sibling stem cell transplantation age 9 years and less) and 36% for alternative donor stem cell transplantation (86% for alternative stem cell transplantation done after 2000).
What you should consider before stem cell transplantation/bone marrow transplant
Decide what your reasons are for transplant. Is it because you want it? Are you sick and tired of transfusion and chelation or steroid therapies enough that it is affecting your quality of life? Or is it because you need it? Maybe you have developed antibodies, making it impossible to find a compatible blood donor and are resistant to steroids. Maybe you have developed aplastic anemia or myelodysplastic syndrome (MDS) – which are other bone marrow failure syndromes affecting red cells, white cells and platelets. Maybe steroids do not work and you also have the hemochromatosis gene (which makes you load iron even if not transfused).
Risks vs. benefits. The benefits must outweigh the risks.
Risks:
Death may occur due to complications including: GVH, rejection, infection.
Graft vs. Host Disease (GVHD) – the donor cells can actually attack different parts of the recipient’s body, the body’s natural defense tries to fight the donor marrow, as it is seen as “foreign.” Skin – GVH can cause a rash, discoloration, peeling and sloughing. Gastrointestinal – can cause the GI tract (from the mouth to the anus) to slough off causing sores and diarrhea.
Rejection – your own immune system is strong enough to reject the donor cells, this happens sometimes with “mini transplant.”
Infection – may be severe, even life threatening, if you get something as simple as a cold or virus. Even your food needs to be well cooked, no fresh fruits or vegetables, no fast food, until the immune system comes completely back to normal.
Cancer – Diamond-Blackfan anemia has a risk of cancer to begin with, even if it is a small risk. The transplant requires chemotherapy, which in itself can actually cause possible cancer in the future.
Infertility – Chemotherapy can cause the inability of the reproductive organs to work correctly.
Return of Diamond-Blackfan anemia -This can happen with a related donor who has “silent” Diamond-Blackfan anemia. That is, they have the same gene as the patient, but never knew because they never had anemia or congenital anomalies which sometimes go along with Diamond-Blackfan anemia. This is why the donor needs to be carefully screened.
Benefits:
A successful transplant eliminates the need for transfusion and steroids for treatment of anemia in the future. It does not eliminate the 50% possibility of passing it on to your children or the other risks associated with Diamond-Blackfan anemia. Diamond-Blackfan anemia is in all your genes. Transplant “fixes” the bone marrow production of red blood cells, but does NOT “cure” all aspects of Diamond-Blackfan anemia.
Are there other treatment options for Diamond-Blackfan anemia?
Other treatment options are being studied but to date none work as well as corticosteroids or transfusion therapy. The goal is to one day find a safe, reliable cure, possibly using gene therapy. But this is still many years away.
- Diamond-Blackfan anemia. https://rarediseases.info.nih.gov/diseases/6274/diamond-blackfan-anemia[↩]
- Clinton C, Gazda HT. Diamond-Blackfan Anemia. 2009 Jun 25 [Updated 2016 Apr 7]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7047/[↩][↩][↩][↩]
- Diamond-Blackfan anemia 6. http://omim.org/entry/612561[↩]
- Da Costa L, O’Donohue MF, van Dooijeweert B et al. Molecular approaches to diagnose Diamond-Blackfan anemia: The EuroDBA experience. Eur J Med Genet. October 26, 2017; 1769-7212(17):30505-0. https://www.ncbi.nlm.nih.gov/pubmed/29081386[↩]
- DBAR Findings. http://dbafoundation.org/learn-more/dbar-findings/[↩]